Abstract
Background
Collagenous colitis is a cause of chronic diarrhea. This updated review was performed to identify therapies for collagenous colitis that have been assessed in randomized controlled trials (RCTs).
Objectives
The primary objective was to assess the benefits and harms of treatments for collagenous colitis.
Search methods
We searched CENTRAL, the Cochrane IBD Group Specialized Register, MEDLINE and EMBASE from inception to 7 November 2016.
Selection criteria
We included RCTs comparing a therapy with placebo or active comparator for the treatment of active or quiescent collagenous colitis.
Data collection and analysis
Data were independently extracted by two authors. The primary outcome was clinical response or maintenance of response as defined by the included studies. Secondary outcome measures included histological response, quality of life and the occurrence of adverse events. Risk ratios (RR) and 95% confidence intervals (CI) were calculated for dichotomous outcomes. The Cochrane risk of bias tool was used to assess bias. The overall quality of the evidence was assessed using the GRADE criteria.
Main results
Twelve RCTs (476 participants) were included. These studies assessed bismuth subsalicylate, Boswellia serrata extract, mesalamine, cholestyramine, probiotics, prednisolone and budesonide therapy. Four studies were low risk of bias. One study assessing mesalamine and cholestyramine was judged to be high risk of bias due to no blinding. The other studies had an unclear risk of bias for random sequence generation (five studies) allocation concealment (six studies), blinding (one study), incomplete outcome data (one study) and selective outcome reporting (one study). Clinical response occurred in 100% (4/4) of patients who received bismuth subsalicylate (nine 262 mg tablets daily for 8 weeks) compared to 0% (0/5) of patients who received placebo (1 study; 9 participants; RR 10.80, 95% CI 0.75 to 155.93; GRADE = very low). Clinical response occurred in 44% (7/16) of patients who received Boswellia serrata extract (three 400 mg/day capsules for 8 weeks) compared to 27% (4/15) of patients who received placebo (1 study; 31 participants; RR 1.64, 95% CI 0.60 to 4.49; GRADE = low). Clinical response occurred in 80% (24/30) of budesonide patients compared to 44% (11/25) of mesalamine patients (1 study; 55 participants; RR 1.82, 95% CI 1.13 to 2.93; GRADE = low). Histological response was observed in 87% (26/30) of budesonide patients compared to 44% (11/25) of mesalamine patients (1 study, 55 participants; RR 1.97, 95% CI 1.24 to 3.13; GRADE = low). There was no difference between the two treatments with respect to adverse events (RR 0.69, 95% CI 0.43 to 1.10; GRADE = low), withdrawals due to adverse events (RR 0.09, 95% CI 0.01 to 1.65; GRADE = low) and serious adverse events (RR 0.12, 95% CI 0.01 to 2.21; GRADE = low). Clinical response occurred in 44% (11/25) of mesalamine patients (3 g/day) compared to 59% (22/37) of placebo patients (1 study; 62 participants; RR 0.74, 95% CI 0.44 to 1.24; GRADE = low). Histological response was observed in 44% (11/25) and 51% (19/37) of patients receiving mesalamine and placebo, respectively (1 study; 62 participants; RR 0.86, 95% CI 0.50 to 1.47; GRADE = low). There was no difference between the two treatments with respect to adverse events (RR 1.26, 95% CI 0.84 to 1.88; GRADE = low), withdrawals due to adverse events (RR 5.92, 95% CI 0.70 to 49.90; GRADE = low) and serious adverse events (RR 4.44, 95% CI 0.49 to 40.29; GRADE = low). Clinical response occurred in 63% (5/8) of prednisolone (50 mg/day for 2 weeks) patients compared to 0% (0/3) of placebo patients (1 study, 11 participants; RR 4.89, 95% CI 0.35 to 68.83; GRADE = very low). Clinical response occurred in 29% (6/21) of patients who received probiotics (2 capsules containing 0.5 x 1010 CFU each of L. acidophilus LA‐5 and B. animalis subsp. lactis strain BB‐12 twice daily for 12 weeks) compared to 13% (1/8) of placebo patients (1 study, 29 participants, RR 2.29, 95% CI 0.32 to 16.13; GRADE = very low). Clinical response occurred in 73% (8/11) of patients who received mesalamine (800 mg three times daily) compared to 100% (12/12) of patients who received mesalamine + cholestyramine (4 g daily) (1 study, 23 participants; RR 0.74, 95% CI 0.50 to 1.08; GRADE = very low). Clinical response occurred in 81% (38/47) of patients who received budesonide (9 mg daily in a tapering schedule for 6 to 8 weeks) compared to 17% (8/47) of placebo patients (3 studies; 94 participants; RR 4.56, 95% CI 2.43 to 8.55; GRADE = low). Histological response was higher in budesonide participants (72%, 34/47) compared to placebo (17%, 8/47) (RR 4.15, 95% CI 2.25 to 7.66; GRADE = low). Clinical response was maintained in 68% (57/84) of budesonide patients compared to 20% (18/88) of placebo patients (3 studies, 172 participants, RR 3.30 95% CI 2.13 to 5.09; GRADE = low). Histological response was maintained in 48% (19/40) of budesonide patients compared to 15% (6/40) of placebo patients (2 studies; 80 participants; RR 3.17, 95% CI 1.44 to 6.95; GRADE = very low). No difference was found between budesonide and placebo for adverse events (5 studies; 290 participants; RR 1.18, o95% CI 0.92 to 1.51; GRADE = low), withdrawals due to adverse events (5 studies, 290 participants; RR 0.97, 95% CI 0.43 to 2.17; GRADE = very low) or serious adverse events (4 studies, 175 participants; RR 1.11, 95% CI 0.15 to 8.01; GRADE = very low). Adverse effects reported in the budesonide studies include nausea, vomiting, neck pain, abdominal pain, excessive sweating and headache. Adverse effects reported in the mesalamine studies included nausea and skin rash. Adverse effects in the prednisolone study included abdominal pain, headache, sleep disturbance, mood change and weight gain.
Authors' conclusions
Low quality evidence suggests that budesonide may be effective for inducing and maintaining clinical and histological response in patients with collagenous colitis. We are uncertain about the benefits and harms of therapy with bismuth subsalicylate, Boswellia serrata extract, mesalamine with or without cholestramine, prednisolone and probiotics. These agents and other therapies require further study.
Plain language summary
Treatments for collagenous colitis
What is collagenous colitis?
Collagenous colitis is a type of microscopic colitis, a condition characterized by chronic watery non‐bloody diarrhea. People with collagenous colitis have a normal appearing bowel when assessed by an endoscope (a camera used to look at the bowel); but have microscopic inflammation of the bowel when assessed by a biopsy (a tissue sample taken during endoscopy). The cause of this disorder is unknown.
What treatments have been tried for lymphocytic colitis?
Budesonide, mesalamine, cholestyramine, Boswellia serrata extract, probiotics, prednisolone and Pepto‐Bismol® have been studied as treatment for collagenous colitis. Budesonide is an immunosuppressive steroid drug that is quickly metabolized by the liver resulting in reduced steroid‐related side‐effects. Prednisolone is a steroid drug used to treat inflammation. Mesalamine (also known as 5‐ASA) is an anti‐inflammatory drug. Cholestyramine is a drug that helps the body remove bile acids. Pepto‐Bismol®, is an antacid medication used to treat discomforts of the stomach and gastrointestinal tract. Boswellia serrata extract is a herbal extract. Probiotics are found in yogurt or dietary supplements and contain potentially beneficial bacteria or yeast.
What did the researchers investigate?
The researchers investigated whether these treatments improve symptoms (e.g. diarrhea) or microscopic inflammation of collagenous colitis and whether any side effects (harms) result from treatment. The researchers searched the medical literature extensively up to 7 November 2016.
What did the researchers find?
Twelve studies (476 participants) were identified. Four studies were high quality. One study assessing mesalamine and cholestyramine was judged to be low quality and the other studies were judged to be of unclear quality due to poor reporting of methods.
Diarrhea resolved in 100% (4/4) of Pepto‐Bismol® (nine 262 mg tablets daily for 8 weeks) participants compared to 0% (0/5) of placebo participants (1 study; very low quality evidence). Diarrhea resolved in 44% (7/16) of Boswellia serrata participants (three 400 mg/day capsules for 8 weeks) compared to 27% (4/15) of placebo participants (1 study; low‐quality evidence). Diarrhea resolved in 80% (24/30) of budesonide participants compared to 44% (11/25) of mesalamine participants (1 study; low‐quality evidence). There was no difference between the two treatments with respect to side effects. Diarrhea resolved in 44% (11/25) of mesalamine (3 g/day) participants compared to 59% (22/37) of placebo participants (1 study; low‐quality evidence). There was no difference between the two treatments with respect to side effects. Diarrhea resolved in 63% (5/8) of prednisolone (50 mg/day for 2 weeks) participants compared to 0% (0/3) of placebo participants (1 study, low‐quality evidence). Diarrhea resolved in 29% (6/21) of participants who received probiotics (2 capsules containing probiotics twice daily for 12 weeks) compared to 13% (1/8) of placebo participants (1 study, very low‐quality evidence). Diarrhea resolved in 73% (8/11) of mesalamine (800 mg three times daily) participants compared to 100% (12/12) of mesalamine + cholestyramine participants (4 g daily) (1 study, very low‐quality evidence). Diarrhea resolved in 81% (38/47) of budesonide (9 mg daily for 6‐8 weeks) participants compared to 17% (8/47) of placebo participants (3 studies; low‐quality evidence). Improvement in microscopic inflammation occurred in 72% (34/47) of budesonide participants compared to 17% (8/47) placebo participants (low‐quality evidence). Resolution of diarrhea was maintained over 6 months in 68% (57/84) of budesonide participants compared to 20% (18/88) of placebo participants (3 studies, low‐quality evidence). Improvement in microscopic inflammation was maintained in 48% (19/40) of budesonide participants compared to 15% (6/40) of placebo participants (2 studies; very low‐quality evidence). No difference was found between budesonide and placebo for side effects (low‐quality evidence) or serious side effects (very low‐quality evidence). Side effects reported in the budesonide studies include nausea, vomiting, neck pain, abdominal pain, excessive sweating and headache. Side effects reported in the mesalamine studies included nausea and skin rash. Side effects in the prednisolone study included abdominal pain, headache, sleep disturbance, mood change and weight gain.
In conclusion, low quality evidence suggests that budesonide may be an effective therapy for active and inactive collagenous colitis. Due to small sample sizes and low study quality we are uncertain about the benefits and harms of therapy with Pepto‐Bismol®, Boswellia serrata extract, mesalamine with or without cholestramine, prednisolone and probiotics. These agents and other therapies require further study.
Summary of findings
Background
Collagenous colitis is a cause of chronic diarrhea. Together with lymphocytic colitis, it falls under the more general heading 'microscopic colitis', an appropriately descriptive name given the normal radiologic and colonoscopic appearance but abnormal histologic appearance of the colon in these disorders. The etiology and pathogenesis of collagenous colitis are unknown. Treatment has been based mainly on anecdotal evidence. The literature includes uncontrolled reports on treatment of one or both of these disorders with traditional corticosteroids (oral, intravenous, or topical), budesonide, bismuth subsalicylate, 5‐ASA compounds, azathioprine/6‐mercaptopurine, methotrexate, cyclosporine, probiotics, antibiotics, cholestyramine/colestipol, octreotide, antihistamines, ketotifen, verapamil, pentoxifylline, antidiarrheal agents, bulking agents, spasmolytics, dietary modification, and surgery (Table 9). It is difficult to draw firm conclusions about treatment efficacy from these uncontrolled studies. Randomized controlled studies provide better evidence for the effectiveness of therapeutic interventions in patients with collagenous colitis. A systematic review of the available randomized controlled studies was undertaken to evaluate the available evidence. This review is an update of a previously published Cochrane systematic review (Chande 2002; Chande 2003a; Chande 2003b; Chande 2004a; Chande 2004b; Chande 2005; Chande 2006; Chande 2008).
1. Unblinded studies of therapies for collagenous colitis.
| Therapy | References |
| 5‐ASA compounds | Weidner 1984, Farah 1985, Giardiello 1987, Wang 1987, Jessurun 1987, Eckstein 1988, Mason 1988, Rokkas 1988, O'Mahony 1990, Gubbins 1991, Giardiello 1991, Carpenter 1992, Fasoli 1994, Katanuma 1995, Bohr 1996, Goff 1997, Mullhaupt 1998, Wang 1999, Bonner 2000, Fielder 2001, Pardi 2001, Kimble 2001, Bozdech 2001, Abdo 2002, Fernandez 2003, Honkoop 2003, Randall 2003, Buchman 2004, Mowat 2005, Fekih 2006, Roe 2006, Madisch 2006, Narvaez 2006, de la Iglesia 2007, Ekiz 2007, Freeman 2007, Koch 2007, Halsey 2007, Rubio‐Tapia 2007 |
| Antibiotics | Mogensen 1984, Wang 1987, Puri 1994, Pimental 1995, Bohr 1996, Mullhaupt 1998, Swensson 1999, Honkoop 2001, Madisch 2006 |
| Antihistamine | Benchimol 2007 |
| Azathioprine/6‐mercaptopurine | Goff 1997, Pardi 2001, Roe 2006, Wickbom 2006 |
| Bismuth subsalicylate | Girard 1987, Fine 1998, Bohr 1999, Bozdech 2001, Buchman 2004, Madisch 2006, Chande 2007, Rubio‐Tapia 2007 |
| Budesonide | Van Gossum 1998, Delarive 1998, Lanyi 1999, Tromm 1999, Bohr 1999, Mueller‐Wittlic 2000, Bajor 2003, Fernandez 2003, Honkoop 2003, Buchman 2004, Hawkins 2004, Barta 2005, Bajor 2006, Roe 2006, Wickbom 2006, Freeman 2006, Hilmer 2006, Chopra 2006, Kiesslich 2006, de la Iglesia 2007, Freeman 2007, Brar 2007 |
| Cholestyramine/colestipol | Andersen 1993, Bohr 1996, Ung 2000, Fernandez 2003, Baert 2004, Mahmoud 2005, Hilmer 2006 |
| Cyclosporine | Eijsbouts 1995, Roe 2006 |
| Dietary modification | Fekih 2006 |
| Elemental diet | Teahon 1994 |
| Ketotifen | Marshall 1998, Benchimol 2007 |
| Methotrexate | Bhullar 1996, Hillman 2001, Riddell 2007 |
| Octreotide | Fisher 1996, Goff 1997 |
| Pentoxifylline | Peterson 1996, Williams 1998 |
| Probiotics | Tromm 2004 |
| Steroids, intravenous | Pardi 2001, Buchman 2004 |
| Steroids, oral | Palmer 1986, Hamilton 1986, Giardiello 1987, Wang 1987, Jessurun 1987, O'Mahony 1990, Sloth 1991, Giardiello 1991, Carpenter 1992, Fasoli 1994, Pimental 1995, Katanuma 1995, Bohr 1996, Goff 1997, Duncan 1997, Wang 1999, Castellano 1999, Swensson 1999, Bonner 2000, Fielder 2001, Persoz 2001, Honkoop 2001, Abdo 2002, Fernandez 2003, Honkoop 2003, Buchman 2004, Mowat 2005, O'Beirne 2005, Taha 2006, Madisch 2006, Narvaez 2006, Rubio‐Tapia 2007 |
| Steroids, topical | Wang 1987, Mason 1988 |
| Surgery | Jarnerot 1995, Alikhan 1997, Munch 2005, Shen 2006, Davis 2007 |
| Symptomatic therapy: antidiarrheal agents, bulking agents, spasmolytics | Bamford 1982, Eaves 1983, Giardiello 1987, Wang 1987, Gubbins 1991, Pimental 1995, Katanuma 1995, Bohr 1996, Goff 1997, Mullhaupt 1998, Wang 1999, Fielder 2001, Abdo 2002, Honkoop 2003, Mowat 2005, Smith 2005, Fekih 2006, Hilmer 2006, Madisch 2006, Ekiz 2007, Khawaja 2007, Halsey 2007 |
| Verapamil | Scheidler 2001 |
Objectives
The primary objective was to assess the benefits and harms of treatments for patients with collagenous colitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials were considered for inclusion.
Types of participants
Patients with biopsy‐proven collagenous colitis were considered for inclusion. For trials assessing induction of response patients were required to have clinically active collagenous colitis at the time of randomization. For trials assessing maintenance of response symptoms needed to be quiescent at the time of randomization. Patients with a diagnosis of microscopic colitis were included only if biopsies revealed a thickened subepithelial collagen band.
Types of interventions
Randomized trials comparing a medical therapy to placebo or an active comparator for treatment of collagenous colitis were considered for inclusion in the review.
Types of outcome measures
For studies assessing treatment of active disease, the primary outcome measure was the number of patients with a clinical response expressed as a percentage of patients randomized (intention‐to‐treat analysis). Clinical response was defined as decreased fecal frequency or stool weight or both. Secondary outcome measures included histological response, effect on quality of life as measured by a validated instrument, and occurrence of adverse events.
For studies assessing maintenance of response, the primary outcome measure was the number of patients with a maintained clinical response expressed as a percentage of patients randomized (intention‐to‐treat analysis). Clinical response was defined as a lack of clinical relapse. Secondary outcome measures included maintenance of histological response, time to relapse, effect on quality of life as measured by a validated instrument, and occurrence of adverse events.
Search methods for identification of studies
We searched the following databases from inception to 7 November 2016:
MEDLINE (Ovid);
EMBASE (Ovid);
Cochrane Central Register of Controlled Trials; and
The Cochrane IBD Inflammatory Bowel Disease and Functional Bowel Disorders Review Group Specialized Trials Register.
The electronic search strategies are described in Appendix 1.
Data collection and analysis
All publications identified by the search strategy were assessed independently by two authors (TSK and TMN or PHP), and relevant studies were selected according to the inclusion criteria. Any disagreement among authors was resolved by consensus or by consulting a third author (JKM). Studies published in abstract form only were included only if the authors could be contacted for further information.
Two authors (TSK and TMN or PHP) independently extracted data using a data extraction form. Any disagreement among authors was resolved by consensus or by consulting a third author (JKM).
Outcome data were extracted from the original research articles and converted into 2x2 tables. In cross‐over studies, only data from the first arm were included. All data were analyzed on an intention‐to‐treat basis, and treated dichotomously. Data were combined for analysis if they assessed the same treatments with the same comparator, and had similar definitions of outcome measures (determined by consensus). We calculated the risk ratio (RR) and 95% confidence intervals (95% CI) for dichotomous outcomes .
Other information extracted from the studies included:
a. Study characteristics and design; b. Characteristics of patients; c. Inclusion and exclusion criteria; d. Interventions; and e. Outcomes scoring methods.
The presence of heterogeneity among studies was assessed using the Chi2test. As the Chi2 chi‐square test has low power in the situation of a meta‐analysis, when trials have small sample size or are few in number, a P value of 0.10 was regarded as statistically significant.
Two authors (TSK and TMN or PHP) independently assessed study quality using the Cochrane risk of bias tool (Higgins 2011), which assesses:
Random sequence generation;
Allocation concealment;
Blinding of participants, personnel and assessment of outcome;
Incomplete outcome data;
Selective reporting; and
Other biases.
Each category was evaluated as low, high or unclear risk of bias and support for each judgment justification was provided in the Characteristics of included studies section. Any disagreement among authors was resolved by consensus or by consulting a third author (JKM).
GRADE Analysis
The overall quality of the evidence supporting the outcomes reported in this review was evaluated using the GRADE approach (Guyatt 2008; Schünemann 2011). In this approach outcome data were rated high, moderate, low or very low. Outcome data from randomized controlled trials begins as high quality but it can be downgraded based on a number of criteria. These criteria are:
Risk of bias in the included studies;
Indirect evidence (by comparison, population, setting);
Inconsistency (unexplained heterogeneity);
Imprecise results (i.e. sparse data, wide confidence intervals); and
Likelihood of publication bias.
These ratings correspond to various levels of confidence:
High quality ‐ more research is not likely to alter the finding;
Moderate quality ‐ more research is likely to alter the finding;
Low quality ‐ more research is very likely to alter the finding; or
Very low quality ‐ we are very uncertain about this finding (Guyatt 2008; Schünemann 2011).
All data were analyzed on an intention‐to‐treat basis, and treated dichotomously. Data were combined for analysis if the studies assessed the same treatments with the same comparator, and had similar definitions of outcome measures (determined by consensus). For pooled data, summary test statistics were derived using the RR and corresponding 95% confidence interval. A fixed‐effect model was used for pooling of data when statistical heterogeneity was not present. When statistical heterogeneity was present a random‐effects model was used. If heterogeneity was substantially high (I2 > 75%) and a single study that was causing the heterogeneity was identified, it was excluded from pooled meta‐analysis.
Results
Description of studies
Results of the search
A literature search conducted on 7 November 2016 identified 390 studies. After duplicates were removed a total of 204 studies remained for review of titles and abstracts. Thirty‐nine reports of interventions for collagenous colitis were selected for full text review (Figure 1). Six studies were excluded for not meeting the inclusion criteria (See Characteristics of excluded studies). The remaining 33 reports from 12 studies were evaluated for qualitative analysis and 13 studies underwent quantitative analysis.
1.
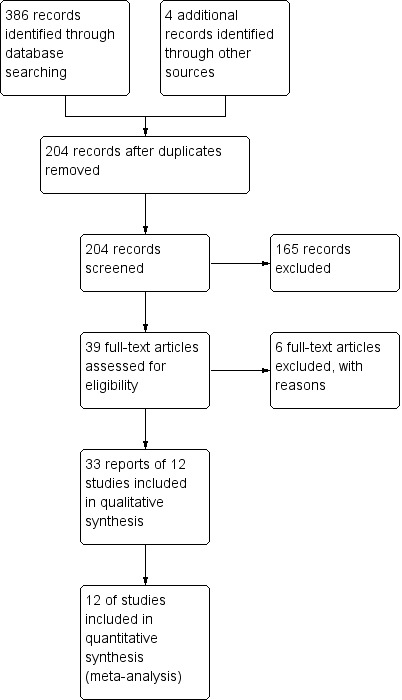
Study flow diagram.
Included studies
Twelve randomized controlled trials (476 participants with collagenous colitis) that met the inclusion criteria were identified (Baert 2002; Bonderup 2003; Bonderup 2009; Calabrese 2007; Fine 1999; Madisch 2007; Miehlke 2002; Miehlke 2008; Miehlke 2014; Munch 2016; Munck 2003; Wildt 2006). Eight of these studies assessed induction of clinical response by comparing an active treatment to placebo: Fine 1999 studied bismuth subsalicylate (published in abstract form only); Madisch 2007 studied Boswellia serrata extract; Wildt 2006 studied probiotics; Munck 2003 studied prednisolone; Baert 2002, Miehlke 2002, and Bonderup 2003 studied budesonide. Miehlke 2014 randomized patients with active disease to three treatment arms: budesonide, mesalamine and placebo. One study (Calabrese 2007) compared mesalamine to mesalamine plus cholestyramine for inducing clinical response in patients with active disease. Three studies compared budesonide to placebo for maintenance of clinical response in patients with quiescent disease (Bonderup 2009, Miehlke 2008; Munch 2016). See Characteristics of included studies.
Baert 2002 performed a prospective, double‐blind, placebo‐controlled clinical trial at Belgian universities and peripheral centres to determine the clinical and histological response of collagenous colitis to budesonide versus placebo over an 8 week trial, with an 8 week treatment‐free follow‐up for responders or an 8 week open‐label budesonide trial for non‐responders. Twenty‐eight patients with established collagenous colitis and chronic symptoms for at least eight weeks were randomized to budesonide 9 mg/day (3 Budenofalk 3 mg capsules with pH‐modified release; n =14) or placebo (n = 14). With the exception of controlled gastroesophageal reflux disease and celiac disease on a long‐term gluten‐free diet, patients with significant gastrointestinal disease were excluded. All other medications were halted and given an appropriate wash out time. After eight weeks, patients were evaluated clinically and histologically for response (clinical: reduction of stool frequency in last week of treatment by at least 50%; histological: statistically significant reduction of the infiltrate in the lamina propria or a significant reduction in the mean thickness of the collagen band). Secondary outcomes included abdominal pain, stool consistency score, patient's general well‐being, time to remission, safety, and long‐term clinical effects of budesonide including relapse rates after weaning or discontinuing budesonide. Clinical response was observed in 57% (8/14) of those taking budesonide compared to 21% (3/14) of those in the placebo arm. Complete histologic response (significant reduction of the infiltrate in the lamina propria) was observed in 9/14 in the budesonide group, with 4/14 reaching partial response compared to only 4/14 achieving partial response and 8 others achieving no response in the placebo group.
Bonderup 2003 conducted a randomized, double blind, placebo‐controlled trial of budesonide (tapering doses over 8 weeks; 9 mg/day for 4 weeks, 6 mg/day for 2 weeks and 3 mg/day for 2 weeks plus 8 week treatment‐free follow‐up) versus matched placebo to determine the effect on clinical response in patients (n = 20, 16 females) aged > 18 years with clinically and histologically confirmed active collagenous colitis (clinical: > 4 stools/day and/or stool weight > 200 g/day averaged over 3 days pre‐treatment; negative stool samples for pathogens, parasites, and ova; histological: collagen layer > 7 um; inflammation was graded on a scale (0 to 3) independently by 2 pathologists). Pateints were excluded from study if they had other chronic gastrointestinal diseases; clinically significant renal or hepatic disease; been treated with anti‐inflammatory drugs (aminosalicylates, corticosteroids, azathioprine) in the previous 3 months; or were pregnant or breast feeding. Outcomes evaluated were clinical response (reduction of stool frequency and/or stool weight by > 50%) and histological response (decrease in inflammation grade or reduction in thickness of the collagen layer). All 10 patients randomized to budesonide achieved a clinical response compared to only 2 in the placebo arm, The budesonide group also had a significant reduction in inflammation compared to the control group.
Bonderup 2009 completed a randomized, double‐blind, placebo‐controlled, multi‐centre study in Denmark to evaluate the ability of budesonide to induce and maintain remission in patients aged > 18 years with clinically and histologically confirmed active collagenous colitis plus negative fecal cultures for intestinal pathogens. Clinically active collagenous colitis was defined as > 3 stools/day over 3 days registration and histologically active was subepithelial collagen layer with a thickness > 10 um, inflammation of the lamina propria, and a lymphocytic infiltrate of the epithelium. Patients were excluded if they had been treated with salazopyrine, 5‐aminosalicylic acid, budesonide or a systemic glucocorticoid within 3 months of trial enrolment or treated with ketoconazole during the 7 days before random selection. Other exclusionary criteria were other chronic gastrointestinal diseases (including celiac disease), clinically relevant impairment of kidney or liver function, previous intestinal resection or stoma. Fourty‐two patients were treated with 9 mg/day budesonide for 6 weeks in an open‐label induction phase and the 34 patients who achieved remission were then randomized to 6 mg/day budesonide or matched placebo for 24 weeks. Those still in remission after 24 weeks were followed for an additional 24 weeks after treatment was ceased. If patients relapsed during maintenance or follow‐up, they were offered treatment with open‐label budesonide (9 mg/day for 6 weeks, followed by budesonide 6 mg/day for 24 weeks). The primary outcome was the proportion of patients maintaining remission after 24 weeks of therapy (budesonide 6 mg/day or matched placebo). Clinical remission was defined as mean stool frequency of < 3 per day. Other outcome measures included: fecal weight (g/day), safety data, maintained histological response (collagen layer <10 um and inflammation score <1), the time to relapse and the rate of relapse after stopping treatment. After 24 weeks of maintenance therapy, 13/17 patients (76.5%) and 2/17 patients (12%) in the budesonide and placebo arms, respectively, were still clinical in remission. Twenty‐one patients underwent repeat colonoscopy/sigmoidoscopy with biopsy (n = 10 in budesonide and n = 11 in placebo), the budesonide group demonstrated significant histological improvement, which was not observed in the placebo arm.
Calabrese 2007 used an open‐label, randomized trial to evaluate the efficacy of mesalazine (800 mg by mouth three times daily) or mesalazine (800 mg by mouth three times daily) + cholestyramine (4 g by mouth once daily) at inducing clinical response over a treatment period of six months in patients with microscopic (lymphocytic or collagenous) colitis. Of the 819 patients that presented to clinic and received a colonoscopy because of chronic watery diarrhoea, 64 were diagnosed with microscopic colitis (23 with collagenous colitis and 41 with lymphocytic colitis), and were then enrolled in the study. Diagnostic criteria included the presence of chronic or recurrent non‐bloody diarrhea (clinical) and increased chronic inflammatory infiltrate (plasma cells, lymphocytes, eosinophils) in the lamina propria; increased number of intraepithelial lymphocytes, damage to surface epithelium, with flattening of epithelial cells and/or epithelial loss and detachment and minimal crypt architecture distortion; specific to the diagnosis of collagenous colitis was a subepithelial collagen band >10 um thick, which entraps superficial capillaries, with an irregular lacy appearance at the lower edge of the basement membrane (histological). Patients were excluded if there was a clear correlation between symptoms and consumption of drugs (e.g. NSAIDS, ticlopidine, PPI). The primary outcomes were clinical (complete response was complete resolution of diarrhoea or partial response was improvement without resolution of diarrhoea) and histological (normalization of histologic pattern) response at 6 months. Secondary outcomes included: adverse events; and days to remission or relapse, as well as various lab data (routine blood biochemistry and hematological counts, C‐reactive protein, antinuclear antibodies blood assay, serum T4 and thyroid stimulating hormone; IgA‐IgG antigliadin, antiendomysium, IgG anti tTG antibody blood assays; and parasitic‐bacterial, fecal‐stool, and hemo‐occult test. A 24‐month follow‐up with coloscopies and biopsies, annually was also performed. In patients relapsed during follow‐up, they were offered a second round of 6 month‐therapy. Relapse was defined as stool frequency of >3 soft or liquid stools per day. At 6 months, 20 (91.3%) patients with CC (12 in the mesalazine + cholestyramine arm and eight mesalazine, P<0.01) were in remission.
Fine 1999 conducted a randomized, double‐blind, placebo controlled trial of bismuth salicylate for the treatment of microscopic colitis over an eight week study. Fourteen patients (11 females, aged 35 to 78 years; 9 with thickened subepithelial collagen, 5 without) were randomized, half and half, to receive bismuth subsalicylate (nine 262 mg chewable tablets/ per day in 3 divided doses) versus placebo (identically coloured and flavoured sucrose tablets). Outcomes were based on clinical and histological comparisons; "48 hour fecal weight and consistency, and distal colonic histology (from 16 biopsies obtained by flexible sigmoidoscopy)" were assessed pre and post therapy; patients also kept a journal of stool frequency and consistency. The patients in the placebo group were crossed over to active treatment while blinding was maintained at the end of 8 weeks for an 8 week course of bismuth salicylate. All 7 patients receiving bismuth salicylate achieved decreased stool weight/frequency and improved consistency over the 8 weeks; however changes in the placebo group were "absent or marginal". Once crossed over to active therapy, the placebo group experienced the same improvements.
Madisch 2007 completed a randomized, placebo‐controlled, double‐blind study at multiple German centres to evaluate the clinical response of Boswellia serrata extract on patients with collagenous colitis compared to placebo over 6 weeks. Thirty‐one patients (aged 18 to 80 years) with clinically and histologically confirmed collagenous colitis ("at least five liquid or soft stools per day on average per week, and a complete colonoscopy performed within the last 4 weeks before randomization") were randomized to receive Boswellia serrata extract (three 400 mg/day; n = 16) or identically matched placebo (n = 15). Patients were excluded in they had received budesonide, salicylates, steroids, prokinetics, antibiotics, ketoconazole, or non‐steroidal anti‐inflammatory drugs within four weeks of randomization or if they had other endoscopically or histologically verified causes for diarrhea, infectious diarrhea, previous colonic surgery, or known intolerance to Boswellia serrata extract or were pregnant or lactating. The primary endpoint was clinical remission after 6 weeks (stool frequency of < 3 per day); secondary outcomes included histological improvements and quality of life measures. "Patients who did not respond to treatment after 6 weeks were individually unblinded. If they were in the active treatment group, they were judged as treatment failure. If they were in the placebo group, crossover therapy with open‐labelled BSE 400 mg, given orally three times daily was offered." Intention to treat analysis demonstrated no significant effect of Boswellia serrata extract compared to placebo on achieving clinical remission, 43.8% vs 26.7%, respectively, P =0.25). Compliance and safety data were also collected.
Miehlke 2002 performed a randomized, double‐blind, placebo‐controlled study was conducted between April 1999 and December 2000 at 35 centres to evaluate the efficacy of oral budesonide (9 mg/day) at inducing clinical remission and improving histology of patients with clinically and histologically active, confirmed collagenous colitis ("at least five liquid or soft stools per day on average per week, and a complete colonoscopy performed within the last 4 weeks before randomization"). Patients were excluded if they had evidence of infectious diarrhea (from culture or biopsy), any other endoscopic or histologic findings (polyps 2 cm, tumors, Crohn’s disease, ulcerative colitis, ischemic colitis) which may have caused diarrhea, known intolerance to budesonide, pregnancy, lactation, or prior partial colonic resection, or if they had received treatment with budesonide, salicylates, steroids, prokinetics, antibiotics, ketoconazole, or non‐steroidal anti‐inflammatory drugs within 4 weeks before randomization. Fifty‐one patients meeting the inclusion criteria were randomized to budesonide (n = 26) or identically matched placebo (n = 25) for 6 weeks. Outcomes were proportion of patients achieving clinical remission or histological improvement after 6 weeks, with clinical remission defined as average of < 3 soft stools per day during the last week of treatment and histologically defined as change of 2 of 3 of the following parameters: collagen band thickness no more than 10 um or reduced to 50% compared to baseline; improvement of inflammation of the lamina propria; improvement of degeneration of surface epithelium. Patients who did not respond to treatment after 6 weeks were unblinded. If they were in the active treatment group, they were judged as treatment failure. If they were in the placebo group, crossover therapy with open‐label budesonide, 9 mg/day po for another 6 weeks. The study reported that 20/26 (76.9%) and 3/25 (12%) patients achieved clinical remission after 6 weeks in the budesonide and placebo groups, respectively. Histological improvement was observed in 14/25 in the budesonide arm compared to only 1/25 in the placebo arm. Sixteen patients who failed the placebo arm entered the cross‐over study, 13 achieved clinical remission on open‐label budesonide.
The Miehlke 2008 study was a randomized, double‐blind, placebo‐controlled trial conducted between April 2004 and March 2007 at 38 centres to evaluate the efficacy of budesonide at inducing remission (9 mg/day for 6 weeks) and maintaining remission (6 mg/day for 6 months) in patients aged >18 years with symptomatic (clinically) and histologically (subepithelial collagen band > 10 um; inflammatory infiltrate in the lamina propria) proven active collagenous colitis. Clinically active defined as ">3 watery/loose stools per day on ≥ 4 of the previous 7 days and had a history of diarrhoea for ≥ 4 weeks." Patients were excluded if they had infectious causes for diarrhoea; other inflammatory bowel diseases; history of colonic surgery; celiac disease; malignancies; severe concomitant (organ) diseases that would interfere with the study; at time of inclusion, were being treated 5‐aminosalicylates, salicylates (except in doses ≤165 mg for cardiovascular prophylaxis), systemic steroids, antibiotics, or NSAIDs (including selective cyclo‐oxygenase‐2 inhibitors); used of budesonide within the 2 weeks prior to enrolment; known intolerance to budesonide; drug and/or alcohol abuse or were pregnant or lactating. The induction phase had 48 patients who all received 9 mg/day po qd budesonide for 6 weeks; those in remission after 6 weeks were randomized to 6 mg/day po qd budesonide (n = 23) or identically matched placebo (n = 23) for 6 months. Primary endpoint was cumulative rate of relapse at the end of 6 months (maintenance phase); remission had been induced during the 6 week induction phase. Relapse was defined as > 3 stools per day on ≥ 4 consecutive days. Relapse rates were determined from daily patient diaries. Secondary outcomes were time to relapse during maintenance therapy; the proportions of patients with clinical remission after 6 weeks’ induction therapy and after 2 and 4 months of maintenance therapy; HRQOL outcomes; and changes in histologic variables after 6 months’ maintenance therapy ("thickness of the collagen band (>10 or <10 µm); inflammation of the lamina propria (infiltration with lymphocytes and plasma cells; absent, mild, moderate, or severe); and degeneration of the surface epithelium (absent, or present)"). Histologic improvement defined as improvement in ≥ 2 variables versus baseline. Safety and tolerability assessments were also performed. At the end of 6 months of maintenance therapy, the cumulative rate of relapse for budesonide maintenance therapy versus placebo was (6/23 [26%] and 15/23 [65%], respectively; P= 0.022.
Miehlke 2014 conducted an 8 week randomized, double‐blind, double‐dummy, placebo‐controlled, comparative phase‐3 trial at 31 hospitals and private clinics in various European countries.The study was to compare the efficacy of budesonide (9 mg/day, n = 30) versus mesalamine (3 g/day, n = 25) versus placebo (n = 37) at inducing clinical and histological remission in patients (n = 92; aged 18‐80) with active collagenous colitis (>4 watery or soft stools on ≥4 days and >3 stools/day in the week prior to baseline; patients must have also had chronic diarrhoea for ≥3 months prior to baseline and have had a colonoscopy within 4 months of baseline; confirmed collagenous colitis with subepithelial collagenous band > 10 um and degeneration of the surface epithelium). Patients were then followed for a 16 week treatment‐free phase to determine maintenance of clinical response. Exclusion criteria included: "other significant colonic diseases (i.e. polyps >2 cm, tumors, Crohn’s disease, ulcerative colitis, ischemic colitis), partial colonic resection, infectious diarrhea, celiac disease (blood tests and/or duodenal histology required), diarrhea caused by other organic diseases of the gastrointestinal tract, treatment with budesonide, Boswellia serrata extract, salicylates, steroids, antibiotics, cholestyramine, nonsteroidal anti‐inflammatory, or other immunosuppressant drugs within the last 4 weeks before baseline, malignant disease, severe comorbidity, abnormal hepatic function or liver cirrhosis, renal insufficiency, active peptic ulcer disease, known intolerance or resistance to study drugs, pregnancy, or breast‐feeding." All medications take for 8 weeks if responsive. If unresponsive after 4 weeks, or relapsed in the 16 week treatment‐free follow‐up, patient's removed from study arm and received 9 mg/day of budesonide for the remaining 4 weeks. Primary and secondary outcomes were evaluated at each interim visit (remission phase: 2, 4, 6, 8 weeks; follow‐up phase: 8 and 16 weeks). Primary outcomes were: clinical remission defined as ≤3 stools/day in the week before the visit and/or histological remission defined as "collagen band thickness 10 mm and no inflammation of the lamina propria with neutrophilic and eosinophilic granulocytes." Secondary outcomes were clinical remission according to the Hjortswang‐Criteria of disease activity (mean <3 stools per day, with <1 watery stool per day), "time to remission, number of watery and solid stools per week, abdominal pain, histopathology, tolerability and safety, symptom relapse during treatment‐free follow‐up, and response to open‐label budesonide." Overall budesonide demonstrated the highest efficacy at achieving clinical remission (80%) compared to 44% of those taking mesalamine and 59.5% of those receiving placebo.
Munch 2016 a multi centre, prospective, randomized, placebo‐controlled trial was conducted to examine low‐dose budesonide therapy for maintenance of clinical remission in patients with collagenous colitis. Patients (n = 110) >18 years were eligible if they had: a histologically diagnosed for collagenous colitis, watery diarrhoea for >2 weeks in newly diagnosed collagenous colitis or a prescreening history of clinical relapse for >1 week in patients with previously established collagenous colitis and a mean of ≥3, including a mean of ≥1 watery stool/day, during the week prior to baseline.
The study started with an initial open‐label induction phase with budesonide therapy for 8 weeks to achieve clinical remission of collagenous colitis. During the open‐label induction phase, all patients received a daily budesonide at a dose of 9mg/day for 4 weeks, then 6 mg/day for 2 weeks, followed by alternate doses of 6mg/day and 3 mg/day for the final 2 weeks. The patients who achieved clinical remission during the last week of the open‐label phase were eligible for randomization into a double blind, randomized, placebo‐controlled 12 month phase for maintenance of clinical remission. The patients who achieved clinical remission (92/110) were randomized into the budesonide treatment group (n = 44) continued to receive budesonide of 6mg/day and 3 mg/day on alternate days. The patients randomized to the placebo group received two placebo capsules and one placebo capsule on alternate days, administered once a day. Patients in clinical remission at the end of the double blind phase were followed for a 6 month untreated follow‐up phase. Clinical remission at 1 year was achieved by 27/44 (61.4%) patients in the budesonide treatment group compared to 8/48 (16.7%) of patients in the placebo group.
Munck 2003, a multi‐centre, randomized, double‐blind, placebo‐controlled trial was conducted to examine the ability of prednisolone to induce remission in patients with severe, disabling diarrhoea due to collagenous colitis after a short duration of treatment. Selected patients (n = 12, 11 with collagenous colitis and 1 with lymphocytic colitis) were aged >18 years reporting at least 3 months with diarrhoea without blood or pus and with a stool volume ≥350 g/day or ≥200 g/day and a stool frequency ≥5/day and a histological diagnosis of microscopic colitis. Female patients also needed to use appropriate contraceptive techniques. Patients were diagnosed histologically using a macroscopic normal colonoscopy or sigmoidoscopy plus a normal barium enema and confirmed by an independent pathologist with either lymphocytic colitis or collagenous colitis using the following criteria: “chronic inflammatory infiltrate in the lamina propria and either a lymphocytic infiltration of at least 20% of epithelial crypt cells (lymphocytic colitis) and/or a subepithelial collagen bond >10 µm in a well‐oriented biopsy (collagenous colitis).” Excluded patients: tested positive for pathogenic bacteria or parasites; failed a normal lactose absorption test and vitamin B12 absorption test, or a normal barium follow through; had celiac disease (confirmed with IgG and IgA antigliadin antibodies and antiendomysium antibodies and/or abnormal histology in duodenal biopsies); had bile acid malabsorption and/or no response to cholestyramine, and/or steatorrhoea; had other gastrointestinal diseases or previous gastrointestinal surgery (exception: cholecystectomy); had other serious diseases, abnormal laboratory tests (haematology, renal function, liver enzymes, urinalysis); had been treated with immunosuppressives within 3 months of randomization; or used medicines with known effects on gastrointestinal functioning including anti‐ulcer medication, antacids, antibiotics and NSAIDs. Patients were randomized to prednisolone (n = 9) 50 mg/day po qd for 2 weeks, tapered to 37.5 mg in the 3rd week, or identical placebo tablets (n = 3) for 2 weeks All patients received 12.5 mmol calcium (500 mg)/5 µg vitamin D tablets, twice daily and were not allowed antidiarrhoeal medication. Outcomes were clinical response (remission or effect) after 2 weeks; clinical remission was defined as stool weight ≤ 200 g/day or frequency ≤ 2/day; effect was defined as >50% reduction of either stool frequency or weight. Adverse events were also monitored. Remission and effect were attained by 2/9 and 5/9 respectively in the prednisolone arm and 0/3 in both outcomes in placebo.
Wildt 2006 conducted a randomized, double‐blind, placebo‐controlled trial at 4 centres to evaluate the ability of AB‐Cap‐10 (a mixture of L. acidophilus strain LA‐5 and B animalis subsp. lactis strain BB‐12), a probiotic, to induce clinical response in patients with collagenous colitis over 12 weeks followed by a 5 week follow up. Patients (n = 36) selected were aged ≥18 years with confirmed histological diagnosis of collagenous colitis ("a subepithelial collagen band > 10 um in a well oriented section of the mucosa and inflammation of the lamina propria with infiltration of predominantly lymphocytes and plasma cells") that is active (> 21 liquid or soft stools per week or stool weight of > 200 g/day) and untreated for at least 4 weeks prior to study inclusion. Exclusion criteria included: those who were pregnant or breast feeding; had chronic liver or kidney disease, severe vascular or cardiopulmonary disease, malignancy, immunosuppressive disease or treatment, known inflammatory bowel disease besides collagenous colitis (including celiac disease), evidence of infectious diarrhea, prior gastrointestinal surgery other than appendectomy; or had malabsorption syndromes; or those who were had received treatment with aminosalicylates, antibiotics, cholestyramine, nonsteroidal anti‐inflammatory drugs, and steroids was not allowed 4 weeks prior to study entrance. Patients were randomized in a 2:1 fashion to receive probiotic AB‐Cap‐10, n = 21 (containing 0.5 x 10^10 colony‐forming units of each bacterium, leading to a total delivery of 1 x 10^10 CFU per capsule) and identically matched placebo, n = 8 for 12 weeks with a 5 week follow up. Patients were assessed at weeks ‐1, 0, 4, 6, 12, and 16. "The primary end point was the proportion of patients achieving a reduction in the number of stools per week of at least 50% at week 12 in each treatment arm. Secondary end points were changes in bowel frequency, stool consistency, stool weight, abdominal pain and bloating, histopathology of biopsies from the sigmoid colon, scores in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ), use of antidiarrhoeal medication, and registration of side effects of the probiotic." At week 12 the number of patients achieving at least a 50% reduction in the number of stools per week in the probiotic group was 6/21 (29%) compared to 1/8 (13%) in the placebo group.
Risk of bias in included studies
This risk of bias for the included studies is summarized in Figure 2. The studies included were generally at low risk of bias. Seven studies described the method for used for random sequence generation (Baert 2002; Bonderup 2009; Calabrese 2007; Madisch 2007; Miehlke 2002; Miehlke 2014; Wildt 2006) and thus were rated at low risk of bias for that item . The remaining studies reported that the patients were randomized, but did not describe the method, which resulted in a rating of unclear risk of bias. Six studies reported adequate methods for allocation concealment ( Baert 2002; Bonderup 2003; Bonderup 2009; Madisch 2007; Miehlke 2002; Miehlke 2014), which were rated at low risk of bias; the remainder were unclear risk, with no description provided. Adequate methods for blinding were described in the ten of the studies and these studies were rated as low risk of bias (Bonderup 2003; Bonderup 2009; Fine 1999; Madisch 2007; Miehlke 2002; Miehlke 2008; Miehlke 2014; Munch 2016; Munck 2003; Wildt 2006). One study did not describe methods used for blinding but reported the study was double‐blind and was rated unclear (Baert 2002). One study was open label and was rated at high risk of bias for blinding (Calabrese 2007). Eleven trials were at low risk of bias for incomplete outcome data (Baert 2002; Bonderup 2009; Calabrese 2007; Fine 1999; Madisch 2007; Miehlke 2002; Miehlke 2008; Miehlke 2014; Munch 2016; Munck 2003; Wildt 2006). Bonderup 2003 did not report on how many participants completed the study and did not describe any dropouts or withdrawals resulting in a rating of unclear for incomplete outcome dataCalabrese 2007 did not describe any pre‐specified outcomes in the manuscript and was rated an unclear risk of bias for selective reportingAll included studies were rated at a low risk for other bias (Baert 2002; Bonderup 2003; Bonderup 2009; Calabrese 2007; Fine 1999; Madisch 2007; Miehlke 2002; Miehlke 2008; Miehlke 2014; Munch 2016; Munck 2003; Wildt 2006).
2.
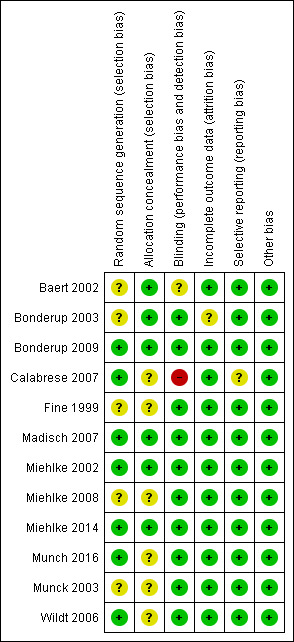
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
Summary of findings for the main comparison. Bismuth subsalicylate versus placebo for treating collagenous colitis.
| Bismuth subsalicylate versus placebo for treating collagenous colitis | ||||||
| Patient or population: Patients with collagenous colitis Setting: Outpatient Intervention: Bismuth subsalicylate Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Bismuth subsalicylate | |||||
| Clinical response | 0 per 10001 | 0 per 1000 (0 to 0) | RR 10.80 (0.75 to 155.93) | 9 (1 RCT) | ⊕⊝⊝⊝ very low2,3 | |
| Histological response | 0 per 10001 | 0 per 1000 (0 to 0) | RR 10.80 (0.75 to 155.93) | 9 (1 RCT) | ⊕⊝⊝⊝ very low2,3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk comes from control arm of the included study. 2 Downgraded two levels due to very sparse data (4 events). 3 Downgraded one level due to unclear risk of bias for random sequence generation and allocation concealment.
Summary of findings 2. Boswellia serrata extract versus placebo for treating collagenous colitis.
| Boswellia serrataextract versus placebo for treating collagenous colitis | ||||||
| Patient or population: Patients with collagenous colitis Setting: Outpatient Intervention:Boswellia serrata extract Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Boswellia serrata extract | |||||
| Clinical response | 267 per 10001 | 437 per 1000 (160 to 1000) | RR 1.64 (0.60 to 4.49) | 31 (1 RCT) | ⊕⊕⊝⊝ low2 | |
| Adverse events | 67 per 10001 | 125 per 1000 (13 to 1000) | RR 1.88 (0.19 to 18.60) | 31 (1 RCT) | ⊕⊕⊝⊝ low3 | |
| Withdrawals due to adverse events | 0 per 10001 | 0 per 1000 (0 to 0) | RR 2.82 (0.12 to 64.39) | 31 (1 RCT) | ⊕⊕⊝⊝ low4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk comes from control arm of the included study. 2 Downgraded two levels due to very sparse data (11 events). 3 Downgraded two levels due to very sparse data and wide confidence interval (3 events). 4 Study had very few events. Downgraded two levels due to very sparse data and wide confidence interval (1 event).
Summary of findings 3. Budesonide versus mesalazine for treating collagenous colitis.
| Budesonide versus mesalazine for treating collagenous colitis | ||||||
| Patient or population: Patients with collagenous colitis Setting: Outpatient Intervention: Budesonide Comparison: Mesalazine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with mesalazine | Risk with Budesonide | |||||
| Clinical response | 440 per 10001 | 801 per 1000 (497 to 1000) | RR 1.82 (1.13 to 2.93) | 55 (1 RCT) | ⊕⊕⊝⊝ low2 | |
| Histological response | 440 per 10001 | 867 per 1000 (546 to 1000) | RR 1.97 (1.24 to 3.13) | 55 (1 RCT) | ⊕⊕⊝⊝ low3 | |
| Adverse events | 680 per 10001 | 469 per 1000 (292 to 748) | RR 0.69 (0.43 to 1.10) | 55 (1 RCT) | ⊕⊕⊝⊝ low4 | |
| Withdrawals due to adverse events | 160 per 10001 | 14 per 1000 (2 to 264) | RR 0.09 (0.01 to 1.65) | 55 (1 RCT) | ⊕⊕⊝⊝ low5 | |
| Serious adverse events | 120 per 10001 | 14 per 1000 (1 to 265) | RR 0.12 (0.01 to 2.21) | 55 (1 RCT) | ⊕⊕⊝⊝ low6 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk comes from control arm of the included study. 2 Downgraded two levels due to very sparse data (35 events). 3 Downgraded two levels due to very sparse data (37 events). 4 Downgraded two levels due to very sparse data (31 events). 5 Downgraded two levels due to very sparse data and wide confidence interval (4 events). 6 Downgraded two levels due to very sparse data and wide confidence intervals (3 events).
Summary of findings 4. Mesalamine versus placebo for treating collagenous colitis.
| Mesalamine versus placebo for treating collagenous colitis | ||||||
| Patient or population: Patients with collagenous colitis Setting: Outpatient Intervention: Mesalamine Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Mesalamine | |||||
| Clinical response | 595 per 10001 | 440 per 1000 (262 to 737) | RR 0.74 (0.44 to 1.24) | 62 (1 RCT) | ⊕⊕⊝⊝ low2 | |
| Histological response | 514 per 10001 | 442 per 1000 (257 to 755) | RR 0.86 (0.50 to 1.47) | 62 (1 RCT) | ⊕⊕⊝⊝ low3 | |
| Adverse events | 541 per 10001 | 681 per 1000 (454 to 1000) | RR 1.26 (0.84 to 1.88) | 62 (1 RCT) | ⊕⊕⊝⊝ low4 | |
| Withdrawals due to adverse events | 27 per 10001 | 160 per 1000 (19 to 1000) | RR 5.92 (0.70 to 49.90) | 62 (1 RCT) | ⊕⊕⊝⊝ low5 | |
| Serious adverse events | 27 per 10001 | 120 per 1000 (13 to 1000) | RR 4.44 (0.49 to 40.29) | 62 (1 RCT) | ⊕⊕⊝⊝ low6 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk comes from control arm of the included study. 2 Downgraded two levels due to very sparse data (33 events). 3 Downgraded two levels due to very sparse data (30 events). 4 Downgraded two levels due to very sparse data (37 events). 5 Downgraded two levels due to very sparse data and wide confidence interval (5 events). 6 Downgraded two levels due to very sparse data and wide confidence interval (4 events).
Summary of findings 5. Mesalazine versus mesalazine + cholestyramine for treating collagenous colitis.
| Mesalazine vs. mesalazine + cholestyramine for treating collagenous colitis | ||||||
| Patient or population: Patients with collagenous colitis Setting: Outpatient Intervention: Mesalazine Comparison: Mesalazine + cholestyramine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with mesalazine + cholestyramine | Risk with Mesalazine | |||||
| Clinical response | 167 per 10001 | 123 per 1000 (83 to 180) | RR 0.74 (0.50 to 1.08) | 23 (1 RCT) | ⊕⊝⊝⊝ very low2,3 | |
| Adverse events | 0 per 10001 | 0 per 1000 (0 to 0) | RR 0.22 (0.01 to 4.07) | 23 (1 RCT) | ⊕⊝⊝⊝ very low2,4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk comes from control arm of the included study. 2 Downgraded one level due to high risk of bias for blinding. 3 Downgraded two levels due to very sparse data (20 events). 4 Downgraded two levels due to very sparse data and wide confidence interval (2 events).
Summary of findings 6. Prednisolone versus placebo for treating collagenous colitis.
| Prednisolone versus placebo for treating collagenous colitis | ||||||
| Patient or population: Patients with collagenous colitis Setting: Outpatient Intervention: Prednisolone Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Prednisolone | |||||
| Clinical response | 0 per 10001 | 0 per 1000 (0 to 0) | RR 4.89 (0.35 to 68.83) | 11 (1 RCT) | ⊕⊝⊝⊝ very low2,3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk comes from control arm of the included study. 2 Downgraded two levels due to very sparse data (5 events). 3 Downgraded one level due to unclear risk of bias for random sequence generation and allocation concealment.
Summary of findings 7. Probiotics versus placebo for treating collagenous colitis.
| Probiotics versus placebo for treating collagenous colitis | ||||||
| Patient or population: Patients with collagenous colitis Setting: Outpatients Intervention: Probiotics Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Probiotics | |||||
| Clinical response | 125 per 10001 | 286 per 1000 (40 to 1000) | RR 2.29 (0.32 to 16.13) | 29 (1 RCT) | ⊕⊝⊝⊝ very low2,3 | |
| Adverse events | 500 per 10001 | 285 per 1000 (110 to 750) | RR 0.57 (0.22 to 1.50) | 29 (1 RCT) | ⊕⊝⊝⊝ very low3,4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk comes from control arm of the included study. 2 Downgraded two levels due to very sparse data and wide confidence interval (7 events). 3 Downgraded one level due to unclear risk of bias for allocation concealment. 4 Downgraded two levels due to very sparse data and wide confidence interval (10 events).
Summary of findings 8. Budesonide versus placebo for treating collagenous colitis.
| Budesonide versus placebo for treating collagenous colitis | ||||||
| Patient or population: Patients with collagenous colitis Setting: Outpatient Intervention: Budesonide Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Budesonide | |||||
| Clinical response sensitivity analysis excluding Miehlke 2014 |
170 per 10001 | 722 per 1000 (388 to 1000) | RR 4.56 (2.43 to 8.55) |
94 (3 RCTs) | ⊕⊕⊝⊝ low2,3 | |
| Histological response sensitivity analysis excluding Miehlke 2014 |
170 per 10001 | 706 per 1000 (383 to 1000) | RR 4.15 (2.25 to 7.66) |
94 (3 RCTs) | ⊕⊕⊝⊝ low3,4 | |
| Maintenance of clinical response | 205 per 10001 | 675 per 1000 (436 to 1000) | RR 3.30 (2.13 to 5.09) | 172 (3 RCTs) | ⊕⊕⊝⊝ low5,6 | |
| Maintenance of histological response | 150 per 10001 | 476 per 1000 (216 to 1000) | RR 3.17 (1.44 to 6.95) | 80 (2 RCTs) | ⊕⊝⊝⊝ very low7,8 | |
| Adverse events | 420 per 10001 | 496 per 1000 (386 to 634) | RR 1.18 (0.92 to 1.51) | 290 (5 RCTs) | ⊕⊕⊕⊝ low6,9 | |
| Withdrawals due to adverse events | 73 per 10001 | 71 per 1000 (31to 158) | RR 0.97 (0.43 to 2.17) | 290 (5 RCTs) | ⊕⊕⊝⊝ very low6,10 | |
| Serious adverse events | 11 per 10001 | 12 per 1000 (2 to 88) | RR 1.11 (0.15 to 8.01) | 175 (4 RCTs) | ⊕⊕⊝⊝ very low11,12 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk comes from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to sparse data (46 events). 3 Downgraded one level due unclear risk of bias for random sequence generation and blinding in one study and random sequence generation and incomplete outcome data in another study in the pooled analysis. 4 Downgraded one level due to sparse data (42 events). 5 Downgraded one level due to sparse data (75 events). 6 Downgraded one level due unclear risk of bias for sequence generation in one study and allocation concealment in two studies in the pooled analysis. 7 Downgraded two levels due to very sparse data (25 events). 8 Downgraded one level due unclear risk of bias for random sequence generation and allocation concealment in one study in the pooled analysis. 9 Downgraded one level due to sparse data (131 events). 10 Downgraded two levels due to very sparse data (21 events). 11 Downgraded two levels due to very sparse data and wide confidence interval (2 events). 12 Downgraded one level due unclear risk of bias for sequence generation in two studies, blinding in one study and allocation concealment in one study in the pooled analysis.
Induction of response
Bismuth subsalicylate versus placebo
Clinical response
In Fine 1999, 100% (4/4) of patients treated with bismuth subsalicylate achieved a clinical response after 8 weeks, compared to 0% (0/5) of patients treated with placebo (RR 10.80, 95% CI 0.75 to 155.93). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to unclear risk of bias (sequence generation and allocation concealment) and very serious imprecision (4 events; See Table 1).
Histological response
In Fine 1999, 100% (4/4) of patients treated with bismuth subsalicylate achieved a histological response after 8 weeks, compared to 0% (0/5) of patients treated with placebo (RR 10.80, 95% CI 0.75 to 155.93). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to unclear risk of bias (sequence generation and allocation concealment) and very serious imprecision (4 events; See Table 1).
Quality of life
Quality of life was not reported as an outcome measure in Fine 1999.
Adverse events
No adverse events were reported in either the bismuth subsalicylate or the placebo groups in Fine 1999.
Boswellia serrataextract versus placebo
Clinical response
In Madisch 2007, 44% (7/16) of patients treated with Boswellia serrata extract achieved a clinical response after 6 weeks compared to 27% (4/15) of patients treated with placebo (RR 1.64, 95% CI 0.60 to 4.49). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (11 events; See Table 2).
Histological response
In Madisch 2007, there was a slight reduction in the thickness of the subepithelial collagen band and inflammation score in both the Boswellia serrata and placebo groups at the end of 6 weeks of therapy, but no difference compared to baseline or between the groups.
Quality of life
Madisch 2007 used the "SF‐36" survey, a validated 36 item questionnaire measuring both physical and mental components of quality of life at baseline and at the end of 6 weeks of therapy. The mean scores in patients with collagenous colitis were lower at baseline than normal controls. At the end of 6 weeks of therapy, there were no significant changes in quality of life scores in either the Boswellia serrata or placebo groups compared to baseline or between groups.
Adverse events
In Madisch 2007, 12.5% (2/16) of patients treated with Boswellia serrata extract reported an adverse event. Of these, 1 patient withdrew from the trial due to hypoglycemia, dizziness and anorexia. The other developed bacterial enteritis but completed the trial. One of 15 patients (7%) in the placebo group reported an adverse event (eczema and Coxsackie virus infection), but completed the trial. There was no significant different between the groups in adverse events or withdrawals due to adverse events. Twelve per cent (2/16) of patients treated with Boswellia serrata extract had an adverse event compared to 7% (1/15) of patients treated with placebo (RR 1.88, 95% CI 0.19 to 18.60). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (3 events; See Table 2). Six (1/16) of patients treated with Boswellia serrata extract withdrew due to an adverse event compared to 0% (0/15) of patients treated with placebo (RR 2.82, 95% CI 0.12 to 64.39). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (1 event; See Table 2). None of the adverse events were considered serious.
Budesonide versus mesalamine
Clinical response
In Miehlke 2014, 80% (24/30) of patients randomized to receive budesonide and 44% (11/25) of patients randomized to receive mesalamine achieved a clinical response (RR 1.82, 95% CI 1.13 to 2.93). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (35 events; See Table 3).
Histological response
In Miehlke 2014, 87% (26/30) and 45% (11/25) of patients randomized to budesonide and mesalamine, respectively, achieved a histological response (RR 1.97, 95% CI 1.24 to 3.13). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (37 events; See Table 3).
Adverse events
Miehlke 2014 reported adverse event data. Forty‐seven per cent (14/30) of patients on budesonide and 68% (17/25) of patients on mesalamine experienced at least one adverse event (RR 0.69, 95% CI 0.43 to 1.10). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (31 events; See Table 3). Common adverse events reported in this study included headache, nasopharyngitis and dyspepsia. Zero per cent (0/30) of the patients taking budesonide and (4/25) of patients taking mesalamine withdrew due to adverse events (RR 0.09, 95% CI 0.01 to 1.65). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (4 events; See Table 3). Zero per cent (0/30) of patients receiving budesonide experienced a serious adverse event, but 12% (3/25) of patients on mesalamine did (RR 0.12, 95% CI 0.01 to 2.21). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (3 events; See Table 3).
Mesalamine versus placebo
Clinical response
In Miehlke 2014, 44% (11/25) of patients administered mesalamine and 60% (22/37) of patients administered placebo had a clinical response (RR 0.74, 95% CI 0.44 to 1.24). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (33 events; See Table 4).
Histological response
In Miehlke 2014, 45% (11/25) of patients given mesalamine and 50% (19/37) of patients given placebo had a histological response (RR 0.86, 95% CI 0.50 to 1.47). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (30 events; See Table 4).
Adverse events
Miehlke 2014 provided adverse event data. Sixty eight per cent (17/25) and 54% (20/37) of patients given mesalamine and placebo, respectively, experienced at least one adverse event (RR 1.26, 95% CI 0.84 to 1.88). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (37 events; See Table 4). Sixteen per cent (4/25) and 3% (1/37) of patients from the mesalamine and placebo groups, respectively, withdrew due to an adverse event (RR 5.92, 95% CI 0.70 to 49.90). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (5 events; See Table 4). Twelve per cent (3/25) of patients receiving mesalamine experienced a serious adverse event, while 3% (1/37) of patients receiving placebo experienced such an event (RR 4.44, 95% CI 0.49 to 40.29). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to very serious imprecision (4 events; See Table 4).
Mesalamine versus mesalamine + cholestyramine
Clinical response
In Calabrese 2007, 73% (8/11) of patients treated with mesalamine alone achieved a clinical response after 6 months compared to 100% (12/12) of patients treated with mesalamine + cholestyramine (RR 0.74, 95% CI 0.50 to 1.08). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to high risk of bias (blinding) and very serious imprecision (20 events; See Table 5).
Histological response
In Calabrese 2007, 90% (18/20) of patients in the combined mesalamine and mesalamine + cholestyramine groups who underwent a follow up colonoscopy at 6 months had a histological response. It was not clear from the paper in which groups these patients were enrolled and attempts to obtain this information from the authors were unsuccessful.
Quality of life
Quality of life was not reported as an outcome measure in Calabrese 2007.
Adverse events
In Calabrese 2007, the adverse events that were reported were in the mesalamine + cholestyramine groups; nausea was experienced by two participants (RR 0.22 95% CI 0.01 to 4.07). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to high risk of bias (blinding) and very serious imprecision (20 events; See Table 5).
Prednisolone versus placebo
Clinical response
In Munck 2003, 63% (5/8) of patients treated with prednisolone achieved a clinical response after 2 weeks of therapy, compared to 0% (0/3) of patients treated with placebo (RR 4.89, 95% CI 0.35 to 68.83). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to unclear risk of bias (random sequence generation and allocation concealment) and very serious imprecision (5 events; See Table 6).
Histological response
In Munck 2003, no patients underwent follow‐up colonoscopy or sigmoidoscopy to determine histological response to therapy.
Quality of life
Quality of life was not reported as an outcome measure in Munck 2003.
Adverse events
In Munck 2003, typical corticosteroid‐related side effects were common in the prednisolone group, but none were severe enough to cause patient withdrawal from the study. Reported adverse events included abdominal pain, headache, sleep disturbance, mood change, and weight gain.
Probiotics versus placebo
Clinical response
In Wildt 2006, 29% (6/21) of patients treated with probiotics achieved a clinical response after 12 weeks compared to 13% (1/8) of patients treated with placebo (RR 2.29, 95% CI 0.32 to 16.13). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to unclear risk of bias (allocation concealment) and very serious imprecision (7 events; See Table 7).
Histological response
In Wildt 2006, no differences in changes of the histopathological features (thickness of the collagen band, inflammation of the lamina propria, detachment of the surface epithelium) between or within groups were observed after 12 weeks of treatment.
Quality of life
Wildt 2006 used the Short Inflammatory Bowel Disease Questionnaire (SIBDQ), a validated 10‐item questionnaire measuring health related quality of life to measure quality of life at baseline and after 12 weeks of treatment with probiotics or placebo. Scores on the SIBDQ range from 10 to 70 with higher scores corresponding with better quality of life. The median baseline SIBDQ score was 46 in the probiotics group compared to 53.5 in the placebo group. After 12 weeks of treatment the median score was unchanged in the placebo group (53.5 to 59.5) but increased significantly in the probiotics group (46 to 55; P < 0.05). After correction for multiple comparisons this difference was no longer statistically significant.
Adverse events
In Wildt 2006, a variety of mild adverse events were reported by patients in both the probiotic and placebo groups. Gastrointestinal symptoms, including mild worsening of diarrhea (n = 1), abdominal pain and constipation (n = 2), stomach burn (n = 1), nausea (n = 1), and flatulence (n = 1), were considered possibly related to the probiotic treatment. Twenty‐nine per cent (6/21) of participants in the placebo group had an adverse event compared to 50% (4/8) of placebo participants (RR 0.57 95% CI 0.22 to 1.50). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to unclear risk of bias (allocation concealment) and very serious imprecision (10 events; See Table 7). No patients withdrew from the study due to adverse events.
Budesonide versus placebo
Clinical response
A total of 161 patients were enrolled in the four trials (Baert 2002; Miehlke 2002, Miehlke 2014; Bonderup 2003). After 6 to 8 weeks of treatment, pooled analysis revealed 81% (62/77) of patients treated with budesonide achieved a clinical response compared to 36% (30/84) patients treated with placebo (RR 2.98, 95% CI 1.14 to 7.75; random‐effects). This analysis was statistically significant for heterogeneity (P=0.001, I2=81%). Visual inspection of the forest plots revealed an outlier. Sensitivity analysis to exclude Miehlke 2014 from pooled analysis is justified as this study resulted in an abnormally large response rate in the placebo group of 59.5% compared to an average of 19% (12 to 25%) in the other three trials; whereas study method and patient characteristics were comparable within the characteristics described. After exclusion of Miehlke 2014, the I2 statistic decreased to 0%. Eighty‐one per cent (38/47) of budesonide participants achieved clinical response compared to 17% (8/47) of placebo participants (RR 4.56, 95% CI 2.43 to 8.55). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to unclear risk of bias (random sequence generation and blinding in one study and random sequence generation and incomplete outcome data in another study) and serious imprecision (46 events; See Table 7). The number needed to treat to achieve a clinical response to budesonide was 2 patients.
Histological response
The definition of histological response and criteria used to evaluate such response were comparable across the four trials (Baert 2002; Bonderup 2003; Miehlke 2002; Miehlke 2014), although there is consideration for subjective error between the pathologists readings. A quoted description of the criteria and definitions used for the studies are as follows, along with diagnostic criteria described by Baert 2002:
“Diagnosis involved: subepithelial collagen band on a well‐oriented section of the mucosa had the typical feathery appearance of the inferior border and exceeded 10 mm. In addition, an increased mixed inflammatory cell infiltrate in the predominantly mononuclear lamina propria should be present. Other findings may include regenerative epithelial changes with mucin depletion, surface epithelial damage and sloughing, rare infiltration of neutrophils and eosinophils both in the epithelium and the lamina propria.”
Histologic analysis consisted of the evaluation of the thickness of the collagen band (measured as the mean thickness on the well‐oriented section), the degree of infiltration in the lamina propria, and the number of intraepithelial lymphocytes. The infiltrate of the lamina propria was scored semi‐quantitatively as normal, slightly increased, or dense.
Histologic analysis: “One pathologist measured the thickness of the collagen layer.The other pathologist measured the grade of inflammation in the lamina propria semi‐quantitatively on a scale from 0 to 3: 0=no inflammation; 1=mild—that is, inflammatory infiltrate confined to the upper part of the lamina propria; 2=moderate—that is, inflammatory infiltrate extending beyond the base of the crypts; and 3=severe—that is, heavy inflammatory infiltrate occupying the lamina propria and infiltrating the lamina muscularis mucosa.”
Histologic analysis: “On well‐oriented sections in which at least 3 adjacent crypts were cut in their vertical plane, the following parameters were evaluated: thickness of the collagen band (µm), inflammation of the lamina propria (semi‐quantitative score, 0–3), and degeneration of the surface epithelium (present or absent). A collagen band thickness of <10 µm post‐treatment or a reduction of at least 50% compared with baseline was defined as significant reduction. Significant histologic improvement was defined as improvement of at least 2 of the 3 histologic parameters”
Histologic analysis: “On well‐oriented sections in which at least 3 adjacent crypts were cut in their vertical plane, we measured the thickness of the collagen band (µm) and inflammation of the lamina propria (semi‐quantitative score 0‐3). Histologic remission was defined as a collagen band thickness <10 µm and no inflammation of the lamina propria with neutrophilic and eosinophilic granulocytes.”
A pooled analysis of the four studies, which resulted in total of 161 patients with histological remission occurring in 60/77 (78%) and 27/84 (32%) of patients receiving budesonide and placebo, respectively (RR 2.68 95% CI 1.37 to 5.24), which did demonstrate a statistically significant response. As with the clinical response analysis, histological response was statistically significant for heterogeneity (P=0.04, I2=63%). The same rational was applied to histological response after visual inspection of the forest plots and Miehlke 2014 was excluded due to unusually high responses in the placebo group (51% compared to the 4 to 30% reported in the other 3 studies). A sensitivity analysis excluding Miehlke 2014 reduced the I2 statistic to heterogeneity to 32% (P = 0.23), which is no longer significant. Seventy‐two per cent (34/47) of budesonide participants achieved histological response compared to 17% (8/47) of placebo participants (RR 4.15, 95% CI 2.25 to 7.66). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to unclear risk of bias (random sequence generation and blinding in one study) and serious imprecision (42 events; See Table 7).
Quality of life
Miehlke 2002 provided quality of life (reported in Madisch 2005). The validated Gastrointestinal Quality of Life Index (GIQLI) was used to measure quality of life at baseline and after six weeks of treatment with budesonide or placebo. Scores on the GIQLI range from 0 to 144 with higher scores corresponding with better quality of life. A complete quality of life assessment was calculated for 29 trial participants (budesonide: n = 17; placebo: n = 12). At baseline, the mean GIQLI score for the trial participants was low (mean = 76). The mean baseline GIQLI score was 67 in the budesonide group and 86 in the placebo group. After six weeks of treatment the mean GIQLI score remained unchanged in the placebo group (86 to 88) but increased significantly in the budesonide group (67 to 92; P < 0.001). Neither Baert 2002 nor Bonderup 2003 measured quality of life using a validated instrument.
Adverse events
Adverse events for budesonide in the induction and maintenance of response is analysed as a pooled analysis below.
Maintenance of response
Budesonide versus placebo
Maintenance of clinical response
A pooled analysis of three studies showed that 68% (57/84) of patients receiving budesonide maintained remission at their respective study endpoints, whereas only 20% (18/88) of patients receiving placebo maintained remission (RR 3.30 95% CI 2.13 to 5.09). This analysis was not statistically significant for heterogeneity (P=0.21, I2=35%). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to unclear risk of bias (random sequence generation and allocation concealment in one study) and serious imprecision (75 events; See Table 7).
Bonderup 2009 continued to follow patients for an additional 24 weeks after stopping therapy. At the end of this period, 4 of 17 patients (24%; 95% CI 9% to 48%) initially randomized to budesonide and 2 of 17 patients (12%; 95% CI 2% to 36%) initially randomized to placebo maintained their response (P = 0.38). The median time to relapse after stopping 6 weeks of open‐label budesonide treatment was 207 days in the budesonide group compared to 45 days in the placebo group (P < 0.02). The median time to relapse after stopping active treatment (6 + 24 weeks in the budesonide group; 6 weeks in the placebo group) was 40 versus 45 days, respectively (P = NS).
In Miehlke 2008 the mean time to relapse in the budesonide group was 37 days compared to 53 days in the placebo group (P = NS).
Munch 2016 had a six month follow‐up of the patients maintaining remission at treatment cessation. Within the 6 months, only 18% of those originally in remission had maintained it (after 1 year treatment + 6 month follow up) with a median time to relapse after stopping budesonide of 40 (95% CI 27 to 57) days.
Maintenance of histological response
In Bonderup 2009 and Miehlke 2008, 25 patients assigned to budesonide with a maintained clinical response underwent a follow up colonoscopy or sigmoidoscopy at the end of 6 months of treatment. Of these, 19 patients had also maintained their histological response, representing 48% (19/40) of the initial patient cohort randomized to budesonide. In comparison, 19 patients assigned placebo with a maintained clinical response also underwent a follow up colonoscopy or sigmoidoscopy at the end of 6 months of treatment. Six of these patients, representing 15% (6/40) of the initial patient cohort randomized to placebo, had a maintained histological response. The pooled RR for maintenance of histological response was 3.17 (95% CI 1.44 to 6.95). This was not significant for heterogeneity (P=0.60, I2=0%). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to unclear risk of bias (random sequence generation and allocation concealment in one study) and very serious imprecision (25 events; See Table 7).
Quality of life
Quality of life was not reported as an outcome measure in either Bonderup 2009 or Miehlke 2008.
Adverse events
Five out of the seven trials (Bonderup 2009; Miehlke 2002; Miehlke 2008; Miehlke 2014; Munch 2016) reported the proportion of patients experiencing at least one adverse event and four studies reported withdrawals due to adverse events (Bonderup 2009; Miehlke 2002; Miehlke 2008; Miehlke 2014). Four trials (Baert 2002; Bonderup 2009; Miehlke 2008; Miehlke 2014), reported serious adverse events. Baert 2002 reported only minor adverse events related to study medications, but did not report them separately for the budesonide and placebo groups. Bonderup 2003 did not report adverse events as an outcome measure. In Bonderup 2009, 12 of 42 patients reported a mild adverse event during the 6 week open‐label induction period with budesonide. Of these, 1 patient with leg cramps withdrew from the study. In the maintenance phase, 4 of 17 patients treated with budesonide reported mild adverse events. One patient suffered a subarachnoid hemorrhage, not related to the study medication, but leading to study withdrawal. Seven of 17 patients in the placebo group reported mild adverse events. One patient developed depression, not related to the study medication, but leading to study withdrawal. This data was included in the above pooled analysis of adverse events. Adverse events in Miehlke 2002 were more common in patients treated with budesonide (39%) than placebo (12%). Two patients (8%) in the budesonide group (1 with nausea, headache, increase in body weight, and disturbed sleep; the other with upper abdominal discomfort) and 1 patient (4%) in the placebo group (arthralgia) withdrew from the study due to an adverse event. All other events were minor. In Miehlke 2008, 8 patients in each group reported an adverse event. None were thought to be related to the study medication. Three patients in the budesonide group and 1 in the placebo group withdrew due to adverse events. There were no severe adverse events. Miehlke 2014 provided us with their adverse event data. Forty‐seven per cent (14/30) and 54% (20/37) of patients receiving budesonide and placebo, respectively, experienced at least one adverse event. The most common adverse events were headache, nasopharyngitis and dyspepsia. There were no serious adverse events in the budesonide group, but one patient receiving placebo experienced one. No patients receiving budesonide withdrew due to an adverse event, while one patient did so receiving placebo (Miehlke 2014). Munch 2016 reported that adverse drug reactions occurred in 7/44 patients receiving budesonide and 5/48 patients on placebo, but did not describe what type of reaction.
Pooled adverse event data showed no statistically significant difference in adverse event rates between budesonide and placebo. Data were pooled regardless of whether the study was an induction or maintenance trial. Forty‐nine per cent (68/140) of patients given budesonide and 42% (63/150) of patients given placebo experienced at least one adverse event (5 studies, 290 patients, RR 1.18, 95% CI 0.92, 1.51). A GRADE analysis rated the overall quality of the evidence supporting this outcome as low due to unclear risk of bias (random sequence generation and allocation concealment in one study and allocation concealment in another study) and serious imprecision (131 events; See Table 7). Seven per cent (10/140) and 7% (11/150) of patients administered budesonide and placebo, respectively, withdrew due to adverse events (5 studies, 290 patients, RR 0.97, 95% CI 0.43 to 2.17). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to unclear risk of bias (random sequence generation and allocation concealment in one study and allocation concealment in another study) and very serious imprecision (21 events; See Table 7). Serious adverse events were rare, with 1% (1/84) patients receiving budesonide and 1% (1/91) of patients receiving placebo experiencing one (4 studies, 175 patients, RR 1.11, 95% CI 0.15 to 8.01). A GRADE analysis rated the overall quality of the evidence supporting this outcome as very low due to unclear risk of bias (random sequence generation and blinding in one study and random sequence generation and allocation concealment in another study) and very serious imprecision (2 events; See Table 7).
Discussion
In the past, the treatment of collagenous colitis had been mainly based on small case series and uncontrolled trials utilizing a wide variety of therapeutic choices, many of which have been reported to be effective (Table 9). However, collagenous colitis is a disorder with a variable clinical course, characterized by periods of spontaneous improvement or exacerbation of symptoms, and such uncontrolled studies are subject to bias due to "regression towards the mean". Patients enter these studies when their symptoms are at their worst, and the improvement seen with treatment may simply be due to the spontaneous improvement in their disease. As a result, randomized trials, which eliminate this bias, have been performed. Twelve trials (476 participants with collagenous colitis) assessed bismuth subsalicylate, Boswellia serrata extract, budesonide, mesalamine with or without cholestyramine, probiotics, and prednisolone for induction of response and three trials (172 participants) assessed budesonide for maintenance of response in collagenous colitis. All of these studies have relatively small numbers of subjects. However, the high rates of clinical and histological response, particularly for budesonide, are encouraging in assisting to define effective therapies for this disorder. In addition, budesonide is well‐tolerated and also appears to improve patients' quality of life. The results for budesonide were consistent across the four randomized trials for induction of response and the three randomized trials for maintenance of response, although the GRADE analysis indicates that the overall quality of the evidence is low due to sparse data and unclear risk of bias in some studies.
Induction of response
Bismuth subsalicylate
The Fine 1999 study of bismuth subsalicylate included only nine patients with collagenous colitis. Although treatment with bismuth subsalicylate was effective in achieving clinical and histological responses in this study (both outcomes 100% versus placebo 0%), it is difficult to make any definite conclusions based on such a small number of individuals. The GRADE analysis was very low quality due to very sparse data and unclear risk of bias which indicates that we are very uncertain about the results. Nonetheless, therapy appears to be safe and well‐tolerated in this study and in nonrandomized studies (Table 9). A trial of bismuth subsalicylate, nine 262 mg/day tablets in 3 divided doses for 8 weeks may be reasonable in a patient with collagenous colitis.
Boswellia serrataextract
Madisch 2007 included 31 patients with collagenous colitis in the trial of Boswellia serrata extract. Although more patients in the Boswellia serrata extract group than the placebo group achieved a clinical response (44% versus 27%), there may have been a lack of power to show a statistically significant difference given the small numbers of patients in each group. Boswellia serrata extract may have no effect on colonic histology or quality of life, but may be well‐tolerated. The GRADE analysis was low quality due to very sparse data which indicates that we are uncertain about the benefits and harms of Boswellia serrata extract.
Mesalamine +/‐ cholestyramine
Calabrese 2007 included 23 patients with collagenous colitis in the trial of mesalamine versus mesalamine + cholestyramine. More patients in the combined mesalamine + cholestyramine group than the mesalamine alone group achieved a clinical response (100% versus 73%), but there may have been a lack of power to show a statistically significant difference given the small numbers of patients in each group. A GRADE analysis indicated that the overall quality of the evidence was very low due to very sparse data and high risk of bias in this study. A histological response was achieved in most patients in both groups at the end of treatment. The therapies appear to be well‐tolerated. Quality of life was not reported in this study. This trial was unblinded and there was no placebo group. Some of the measured effect in both groups may have been due to spontaneous improvement of the disease. We are uncertain if mesalamine 800 mg/day three times with or without cholestyramine 4 g/day is effective for treating patients with active collagenous colitis.
Miehlke 2014 examined 92 patients receiving mesalamine alone (n= 25), budesonide alone (n= 30) and placebo (n= 37). Low quality evidence suggests that mesalamine alone may be less effective than budesonide for inducing clinical response in people with collagenous colitis. Low quality evidence suggests that mesalamine may be no more effective than placebo for inducing both clinical and histological response. In fact, the treatment group receiving mesalamine was stopped prematurely due to futility. Overall we are uncertain about the benefits and harms of mesalamine treatment in people with collagenous colitis.
Prednisolone
Munck 2003 included only 11 patients with collagenous colitis in the trial studying prednisolone. Of these, eight were assigned prednisolone and three were assigned placebo. Although there was a trend towards achieving a clinical response in patients on prednisolone compared to placebo (63% versus 0%), there may have been a lack of power to show a difference given the small numbers of patients in each group. Additionally, the two week course of therapy may have been too short to show a benefit with prednisolone, when compared to the 6 to 8 weeks of treatment used in the budesonide trials. Follow‐up colonoscopy was not performed and quality of life was not measured in this study. Typical corticosteroid‐related side effects were common in prednisolone‐treated patients. A GRADE analysis indicates that the overall quality of the evidence is very low due to very sparse data and unclear risk of bias. Overall we are very uncertain about the benefits and harms of prednisolone 50 mg/day for treating collagenous colitis.
Probiotics
Wildt 2006 included 29 patients with collagenous colitis in the trial of probiotics. Probiotics had no statistically significant effect upon clinical (probiotics 29% versus placebo 13%) or histological response. However, there may have been a lack of power to show a difference given the small numbers of patients in each group. Probiotic treatment had no effect on histology or quality of life, but appeared to be well‐tolerated. A GRADE analysis indicates that the overall quality of the evidence is very low due to very sparse data and unclear risk of bias. Overall we are very uncertain about the benefits and harms of probiotics for treating people with collagenous colitis.
Budesonide
A pooled analysis suggests that budesonide may be effective for the treatment of collagenous colitis, with very high clinical (81% compared to 36% for placebo) and histological (ranging from 61% to 100% for budesonide and 4% to 50% for placebo) response rates (Baert 2002; Bonderup 2003; Miehlke 2002; Miehlke 2014). However, a high degree of heterogeneity was detected for this analysis (I2 = 81%). Sensitivity analysis revealed that Miehlke 2014 was an outlier, which after visual inspection of the forest plots can be explained by the unusually high rate of remission in the placebo group compared to the other trials included for that comparison. After excluding Miehlke 2014 the clinical response rate is 17% in the placebo group and 81% in the budesonide group and the I2 value drops to 0%. A GRADE analysis indicates that the overall quality of the evidence for this outcome is low due to sparse data and unclear risk of bias in two studies in the pooled analysis. Low quality evidence also suggests that budesonide may be effective for inducing histological response in people with collagenous colitis. Budesonide may improve patients' quality of life. Miehlke 2002 provides long term follow‐up data showing that clinical relapse may occur after cessation of budesonide therapy. However, re‐initiation of budesonide therapy may be beneficial for patients who experience clinical relapse. Bonderup 2003, Baert 2002 and Miehlke 2002 reported no serious adverse effects. Other uncontrolled studies using budesonide in collagenous colitis (Table 9) have reported this therapy as being generally well‐tolerated. In addition, trials performed in patients with Crohn's disease (Greenberg 1994, Greenberg 1996) report a similar adverse event profile, with less systemic effects than are observed with conventional corticosteroids. Miehlke 2002 used 3 mg/day of budesonide for 6 weeks, while Baert 2002 used a single 9 mg/day dose of budesonide for 8 weeks. Two studies used 9 mg/day of budesonide for 4 weeks (Bonderup 2003) and 8 weeks (Miehlke 2014), 6 mg/day for 2 weeks and 3 mg/day for 2 weeks. Low quality evidence suggests that budesonide 9 mg/day orally or in a tapering course for 6 to 8 weeks may be an effective and well‐tolerated therapy for inducing clinical and histological response and improving quality of life in patients with active collagenous colitis.
Maintenance of response
Budesonide
Bonderup 2009, Miehlke 2008 and Munch 2016 included 172 patients who had achieved a clinical response with open‐label budesonide in their trials of budesonide maintenance therapy of collagenous colitis. At the end of 6 months, more patients assigned to budesonide than placebo had maintained their clinical response (75% versus 25%). Although not all patients underwent a follow‐up colonoscopy or flexible sigmoidoscopy at the end of treatment, more patients assigned to budesonide than placebo had also maintained their histological response (48% versus 15%) (Bonderup 2009; Miehlke 2008). A GRADE analysis indicates that the overall quality of the evidence for these outcomes is low due to sparse data and unclear risk of bias in two studies in the pooled analysis. Munch 2016 showed that after 12 months of study 61% of patients receiving low dose budesonide were still in remission compared to 17% of those in the placebo arm. No serious adverse events due to budesonide occurred, although one patient suffered a sub‐arachnoid haemorrhage in that study arm and had to cease treatment. Quality of life was not reported. After the 6 and 12 months of therapy, Bonderup 2009 and Munch 2016 continued to follow patients in each group for 6 months. Most patients relapsed off treatment. The median time to relapse was not different in the budesonide group compared to the placebo group once the active drug (either open‐label budesonide induction therapy or blinded budesonide maintenance therapy) had been stopped. This suggests that the effect of budesonide at maintaining response is not sustained once the drug is discontinued. Overall low quality evidence suggests that budesonide 6 mg/day may be effective at maintaining both clinical and histological response in patients with active collagenous colitis induced by budesonide, and may be well‐tolerated.
Authors' conclusions
Implications for practice.
Collagenous colitis is a cause of chronic diarrhea. There are numerous case series and anecdotal reports of success with various pharmacological measures for treating collagenous colitis (Table 9). Randomized trials, which provide stronger evidence for efficacy, have also been performed, assessing both induction and maintenance of response. It should be noted that 'response' does not necessarily imply 'remission', and the definitions of response, both clinically and histologically, vary between trials. There are no standardized criteria for either clinical or histological remission in collagenous colitis. Until such criteria are established, no conclusions can be made about the effectiveness of the therapies assessed in these trials for inducing or maintaining a true remission in patients with collagenous colitis. Low quality evidence suggests that budesonide may be effective for inducing and maintaining clinical and histological responses in patients with collagenous colitis. Budesonide may improve quality of life, and appears to be well‐tolerated at least over 6 months of therapy. We are uncertain about the benefits and harms of treatment with bismuth subsalicylate, Boswellia serrata extract, mesalamine with or without cholestramine, prednisolone and probiotics.
Implications for research.
Collagenous colitis is a chronic condition and some patients require long‐term therapy to prevent relapses. The utility of budesonide in this setting requires further investigation.
Although budesonide may be safe and effective for treating collagenous colitis, significant cost and theoretical potential for toxicity with long‐term use warrant consideration of less‐proven interventions. The favourable results in one small trial of bismuth subsalicylate, a safe and inexpensive therapy, justify larger trials with this drug. The trials assessing Boswellia serrata extract and probiotics included small numbers of patients and may have lacked the power necessary to show a difference between active treatment and placebo should one exist. Larger trials of these treatments may be considered. Prednisolone may also warrant further investigation. The trial included here included only a very small number of patients, and may have lacked the power necessary to show a difference between placebo and prednisolone treated patients, should one exist. In addition, the two week course of therapy that was used is relatively short compared to the 6 to 8 weeks of therapy that was used in the bismuth subsalicylate and budesonide studies. It may be that a longer course of treatment than two weeks is necessary to obtain significant clinical improvement. Mesalamine and mesalamine + cholestyramine seem to be effective in treating collagenous colitis. However, neither of these treatments has been assessed in a blinded, placebo‐controlled trial, which would provide better evidence for their effectiveness.
The difference between 'response' and 'remission' in collagenous colitis may be important. Since standardized definitions of clinical and histological remission do not exist, the outcome measures defining response vary between trials of treatments of collagenous colitis, and thus the effectiveness of therapies at inducing and maintaining true disease remission are unknown. However, recently Hjortswang 2009 examined how various symptoms related to health‐related quality of life scales. Based on their analysis, they proposed that remission in collagenous colitis should be defined as less than three stools per day and less than one watery stool per day on average. This has not been validated yet, but this is an area to be addressed with further research.
Additionally, inclusion criteria defining histological features of collagenous colitis and clinical activity vary between trials, so that patients in different trials may not be comparable. If standardized definitions for clinical and histological features, disease activity and remission for collagenous colitis are established, trials studying therapies could use uniform inclusion criteria and outcome measures, allowing more accurate assessment of treatment effectiveness and comparisons between trials.
Lymphocytic colitis is a related but histologically different disorder from collagenous colitis. Some studies in the literature include both these diseases under the broader title 'microscopic colitis' when reporting therapeutic success. Whether or not therapies for collagenous colitis should be offered to patients with lymphocytic colitis is currently being investigated in randomized controlled trials. Budesonide, in particular, may be a promising therapy for lymphocytic colitis (Chande 2017).
What's new
| Date | Event | Description |
|---|---|---|
| 7 November 2016 | New search has been performed | New literature search performed on 7 November 2016. Two new studies were added. |
| 7 November 2016 | New citation required and conclusions have changed | Updated review with changes to conclusions and new authors |
Acknowledgements
Partial funding for the Cochrane IBD Group (April 1, 2016 ‐ March 31, 2018) has been provided by Crohn's and Colitis Canada (CCC).
Appendices
Appendix 1. Electronic Search Strategy
MEDLINE Search Strategy:
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. lymphocytic colitis.mp. or exp lymphocytic colitis/
22. microscopic colitis.mp or exp microscopic colitis/
23. collagenous colitis.mp or exp collagenous colitis/
24. 21 or 22 or 23
25. 20 and 24
= 84
EMBASE Search Strategy:
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. lymphocytic colitis.mp. or exp lymphocytic colitis/
22. microscopic colitis.mp or exp microscopic colitis/
23. collagenous colitis.mp or exp collagenous colitis/
24. 21 or 22 or 23
25. 20 and 24
Cochrane Library Search Strategy:
1. microscopic colitis OR lymphocytic colitis OR collagenous colitis
Cochrane IBD Specialized Register
1. microscopic colitis (ab/ti)
2. lymphocytic colitis (ab/ti)
3. collagenous colitis (ab/ti)
Data and analyses
Comparison 1. Bismuth subsalicylate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Histological response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Withdrawals due to adverse events | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious adverse events | 1 | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
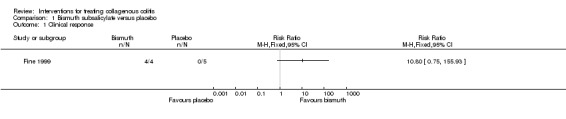
Comparison 1 Bismuth subsalicylate versus placebo, Outcome 1 Clinical response.
1.2. Analysis.
Comparison 1 Bismuth subsalicylate versus placebo, Outcome 2 Histological response.
1.3. Analysis.
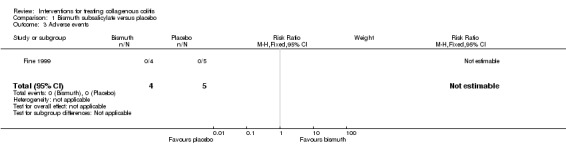
Comparison 1 Bismuth subsalicylate versus placebo, Outcome 3 Adverse events.
1.4. Analysis.
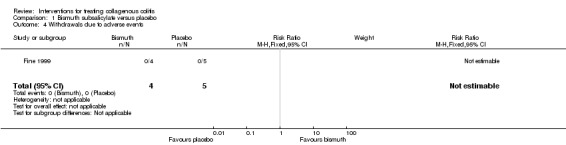
Comparison 1 Bismuth subsalicylate versus placebo, Outcome 4 Withdrawals due to adverse events.
1.5. Analysis.
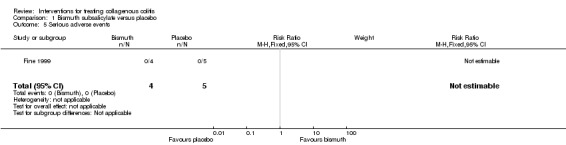
Comparison 1 Bismuth subsalicylate versus placebo, Outcome 5 Serious adverse events.
Comparison 2. Boswellia serrata extract versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
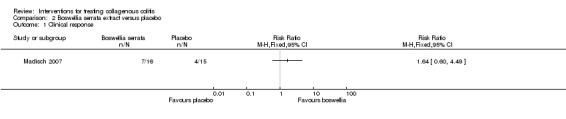
Comparison 2 Boswellia serrata extract versus placebo, Outcome 1 Clinical response.
2.2. Analysis.
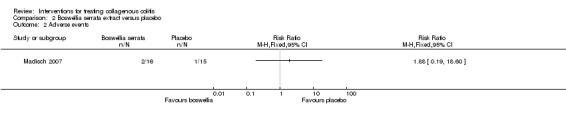
Comparison 2 Boswellia serrata extract versus placebo, Outcome 2 Adverse events.
2.3. Analysis.
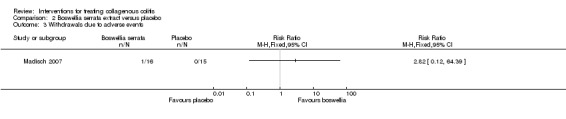
Comparison 2 Boswellia serrata extract versus placebo, Outcome 3 Withdrawals due to adverse events.
Comparison 3. Budesonide versus mesalazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Histological response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
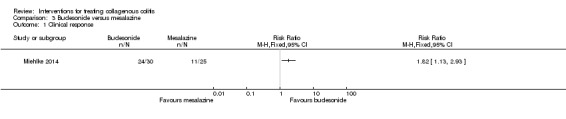
Comparison 3 Budesonide versus mesalazine, Outcome 1 Clinical response.
3.2. Analysis.
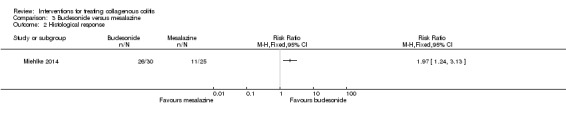
Comparison 3 Budesonide versus mesalazine, Outcome 2 Histological response.
3.3. Analysis.
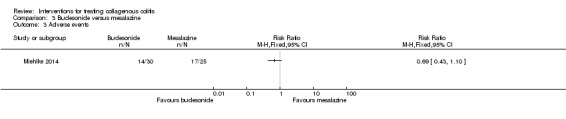
Comparison 3 Budesonide versus mesalazine, Outcome 3 Adverse events.
3.4. Analysis.
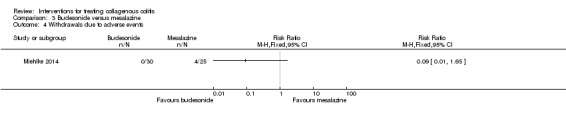
Comparison 3 Budesonide versus mesalazine, Outcome 4 Withdrawals due to adverse events.
3.5. Analysis.
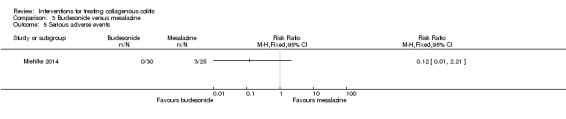
Comparison 3 Budesonide versus mesalazine, Outcome 5 Serious adverse events.
Comparison 4. Mesalamine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Histological response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.
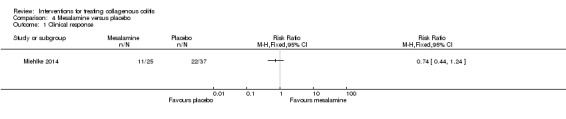
Comparison 4 Mesalamine versus placebo, Outcome 1 Clinical response.
4.2. Analysis.
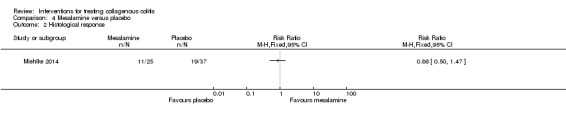
Comparison 4 Mesalamine versus placebo, Outcome 2 Histological response.
4.3. Analysis.
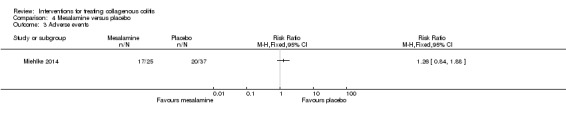
Comparison 4 Mesalamine versus placebo, Outcome 3 Adverse events.
4.4. Analysis.
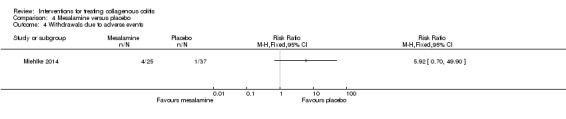
Comparison 4 Mesalamine versus placebo, Outcome 4 Withdrawals due to adverse events.
4.5. Analysis.
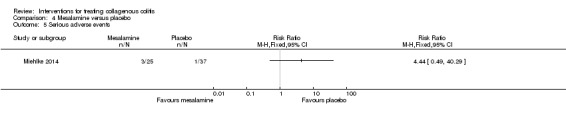
Comparison 4 Mesalamine versus placebo, Outcome 5 Serious adverse events.
Comparison 5. Mesalazine vs. mesalazine + cholestyramine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
5.1. Analysis.
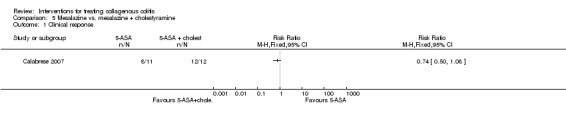
Comparison 5 Mesalazine vs. mesalazine + cholestyramine, Outcome 1 Clinical response.
5.2. Analysis.
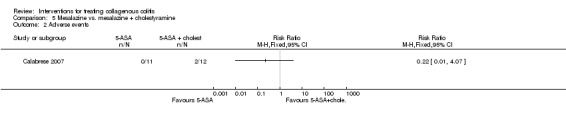
Comparison 5 Mesalazine vs. mesalazine + cholestyramine, Outcome 2 Adverse events.
Comparison 6. Prednisolone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
6.1. Analysis.
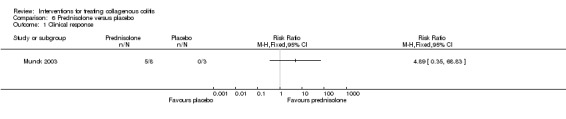
Comparison 6 Prednisolone versus placebo, Outcome 1 Clinical response.
6.2. Analysis.
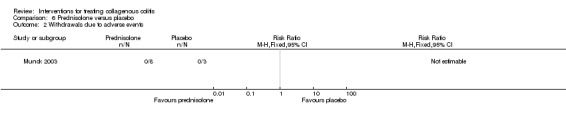
Comparison 6 Prednisolone versus placebo, Outcome 2 Withdrawals due to adverse events.
Comparison 7. Probiotics versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
7.1. Analysis.
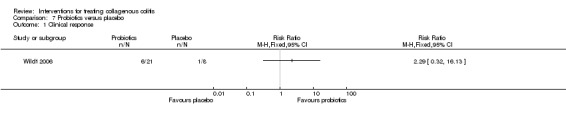
Comparison 7 Probiotics versus placebo, Outcome 1 Clinical response.
7.2. Analysis.
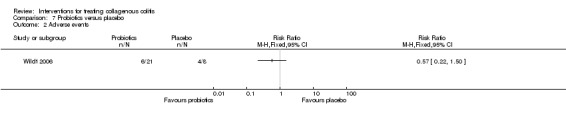
Comparison 7 Probiotics versus placebo, Outcome 2 Adverse events.
7.3. Analysis.
Comparison 7 Probiotics versus placebo, Outcome 3 Withdrawals due to adverse events.
Comparison 8. Budesonide versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response | 4 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [1.14, 7.75] |
| 2 Clinical response sensitivity analysis excluding Miehlke 2014 | 3 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.56 [2.43, 8.55] |
| 3 Histological response | 4 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 2.68 [1.37, 5.24] |
| 4 Histological response sensitivity analysis excluding Miehlke 2014 | 3 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.15 [2.25, 7.66] |
| 5 Maintenance of clinical response | 3 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.30 [2.13, 5.09] |
| 6 Maintenance of histological response | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.17 [1.44, 6.95] |
| 7 Adverse events | 5 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.92, 1.51] |
| 8 Withdrawals due to adverse events | 5 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.43, 2.17] |
| 9 Serious adverse events | 4 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.15, 8.01] |
8.1. Analysis.
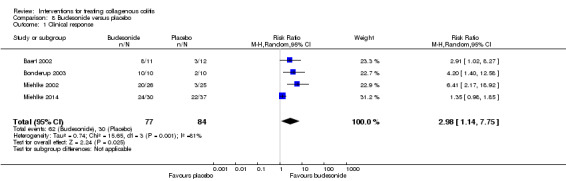
Comparison 8 Budesonide versus placebo, Outcome 1 Clinical response.
8.2. Analysis.
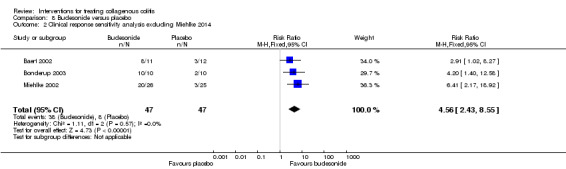
Comparison 8 Budesonide versus placebo, Outcome 2 Clinical response sensitivity analysis excluding Miehlke 2014.
8.3. Analysis.
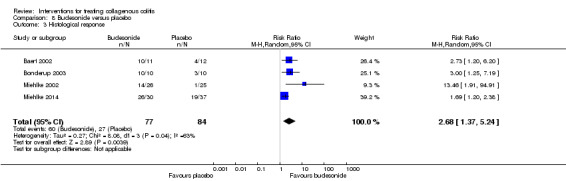
Comparison 8 Budesonide versus placebo, Outcome 3 Histological response.
8.4. Analysis.
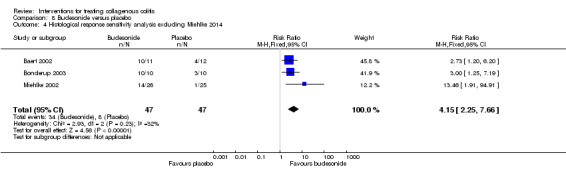
Comparison 8 Budesonide versus placebo, Outcome 4 Histological response sensitivity analysis excluding Miehlke 2014.
8.5. Analysis.
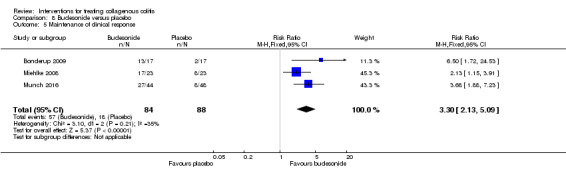
Comparison 8 Budesonide versus placebo, Outcome 5 Maintenance of clinical response.
8.6. Analysis.
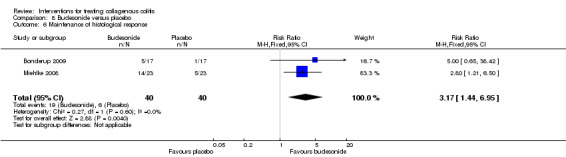
Comparison 8 Budesonide versus placebo, Outcome 6 Maintenance of histological response.
8.7. Analysis.
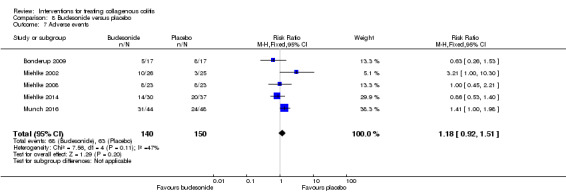
Comparison 8 Budesonide versus placebo, Outcome 7 Adverse events.
8.8. Analysis.
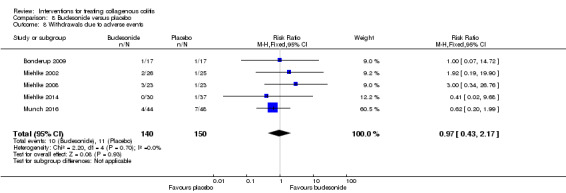
Comparison 8 Budesonide versus placebo, Outcome 8 Withdrawals due to adverse events.
8.9. Analysis.
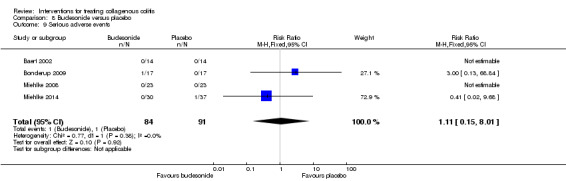
Comparison 8 Budesonide versus placebo, Outcome 9 Serious adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baert 2002.
| Methods | Randomized, double‐blind, placebo‐controlled. Duration of treatment was 8 weeks, plus an 8 week treatment‐free follow‐up, for a total of 16 weeks | |
| Participants | Patients (n = 28) aged > 18 years with clinically and histologically confirmed active collagenous colitis Clinical: minimum 3 semi‐loose or loose stools per day for at least 8 weeks, no other significant cause on history/physical, negative stool examination for pathogens, parasites, and C. difficile toxin and no macroscopic inflammation on colonoscopy (and no other endoscopic findings other than diverticulosis or diminutive polyps). Histological: subepithelial collagen band > 10 um thick and typical feathery appearance of the inferior border; increased mixed inflammatory cell infiltrate in lamina propria. Cases with overlapping features with lymphocytic colitis were allowed if the collagen band was a predominant finding Patients with significant gastrointestinal disease (except controlled gastroesophageal reflux disease and celiac disease on a long‐term gluten‐free diet) were excluded |
|
| Interventions | Budesonide (Budenofalk) 9 mg/day (n = 14) versus placebo (n = 14) for 8 weeks | |
| Outcomes | Proportion of patients achieving clinical and/or histological response Clinical: reduction of stool frequency in last week of treatment by at least 50%. Histological: statistically significant reduction of the infiltrate in the lamina propria and/or a significant reduction in the mean thickness of the collagen band Other end‐points were impact on abdominal pain, stool consistency score, patient's general well‐being, amount of time necessary to induce remission, safety of budesonide, and long‐term clinical effects of budesonide including the relapse rates after weaning or discontinuing budesonide All patients kept a diary throughout the study period. Each patient underwent colonoscopy and standardized biopsy protocol pre‐ and post‐treatment |
|
| Notes | Data from first 8 weeks of the study only were included in the analysis, as this was the duration of treatment with active drug or placebo. Five patients that failed to meet the inclusion criteria after being randomized into the trial (upon review of their stool diaries) were excluded from the analysis. Medications that could possibly affect stool frequency or the natural history of the disease were not allowed during the study and were discontinued (with an appropriate wash‐out period) before inclusion. Other chronic medications were allowed to be continued as long as the intake remained stable throughout the study period. 3 patients (2 placebo) dropped out of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomization was not described |
| Allocation concealment (selection bias) | Low risk | Randomization was done centrally by the company delivering the drugs and placebo |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐blind" Quote: "All biopsies were randomly read by 2 blinded expert pathologists" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All calculations were made on an intention‐to‐treat basis" Quote: "Three patients dropped out of the study (2 placebo), one for noncompliance and 2 because of treatment failure" Intention‐to‐treat was followed for clinical response Intention‐to‐treat was not followed for histologic response (denominators of 13 and 12 for the treatment and placebo group respectively) |
| Selective reporting (reporting bias) | Low risk | All primary outcomes were reported. Time to clinical remission was not directly reported in text, but it was interpretable from one of their published figures (Figure 1) |
| Other bias | Low risk | Study appeared to be free of other forms of bias |
Bonderup 2003.
| Methods | Randomized, double‐blind, placebo‐controlled. Duration of study was 8 weeks. Stool frequency and stool weight was recorded pre‐ and immediately after stopping treatment. All patients underwent sigmoidoscopy with standardized biopsy protocol pre‐ and post‐treatment. Randomization was performed by the drug company. Medication and placebo were delivered prepackaged with consecutive randomized numbers | |
| Participants | Patients (n = 20, 16 females) aged > 18 years with clinically and histologically confirmed active collagenous colitis Clinical: > 4 stools/day and/or stool weight > 200 g/day averaged over 3 days pre‐treatment. Negative stool samples for pathogens, parasites, and ova. Histological: collagen layer > 10 um Inflammation was graded on a scale (0 to 3) independently by 2 pathologists Patients with other chronic gastrointestinal diseases were excluded, as were those with clinically significant renal or hepatic disease, those who had been treated with anti‐inflammatory drugs (aminosalicylates, corticosteroids, azathioprine) in the previous 3 months or were pregnant or breast feeding |
|
| Interventions | Budesonide (9 mg/day for 4 weeks, 6 mg for 2 weeks and 3 mg for 2 weeks) versus placebo for 8 weeks | |
| Outcomes | Primary outcome was the proportion of patients that achieved a clinical or histological response Clinical: reduction of stool frequency and/or stool weight by > 50% Histological: decrease in inflammation grade or reduction in thickness of the collagen layer |
|
| Notes | No antiinflammatory drug treatment was allowed during the study period or for 3 months prior to inclusion. During the study antidiarrheal medications were allowed except during the periods of stool sampling. During these periods no other treatments with effects on the GI tract were allowed. NSAIDS were not permitted, but other chronic medications (e.g. ‐ antihypertensives) were allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in published study |
| Allocation concealment (selection bias) | Low risk | Randomization was performed centrally by the drug company Medication and placebo were delivered prepackaged with consecutive randomized numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" Quote: "The placebo medication was identical in appearance" Quote: "Histopathological evaluation was performed blindly by the two pathologists" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description of dropouts or withdrawals |
| Selective reporting (reporting bias) | Low risk | The primary outcome of clinical remission was reported Histopathological changes were also described |
| Other bias | Low risk | Study appeared to be free of other forms of bias |
Bonderup 2009.
| Methods | Randomized, double‐blind, placebo‐controlled. Randomization was done with a computerized randomization program in blocks of 4 patients Induction: 6 weeks Maintenance: 24 weeks Treatment‐free follow‐up: 24 weeks |
|
| Participants | Patients (n = 42) aged > 18 years with clinically (> 3 stools/day over 3 days registration) and histologically (subepithelial collagen layer with a thickness > 10 um, inflammation of the lamina propria, and a lymphocytic infiltrate of the epithelium) confirmed active collagenous colitis plus negative faecal cultures for intestinal pathogens Induction: n = 42 Maintenance: n = 34, 17 in each arm Follow‐up: n = 15, 13 in the budesonide arm and 2 in the placebo arm Patients were excluded if they had been treated with salazopyrine, 5‐aminosalicylic acid, budesonide or a systemic glucocorticoid within 3 months of trial enrolment or treated with ketoconazole during the 7 days before random selection. Other exclusionary criteria were other chronic gastrointestinal diseases (including celiac disease), clinically relevant impairment of kidney or liver function, previous intestinal resection or stoma |
|
| Interventions | Induction: 6 weeks, open‐label 9 mg/day budesonide, randomized to maintenance or placebo therapy Maintenance: 24 weeks, budesonide 6 mg/day versus placebo Treatment‐free follow‐up: 24 weeks Patients who relapsed during the maintenance or follow‐up were offered treatment with open‐label budesonide (9 mg/day for 6 weeks, followed by budesonide 6 mg/day for 24 weeks) |
|
| Outcomes | Induction: proportion entering clinical/histological remission, randomized to maintenance or placebo therapy after 6 weeks Maintenance: proportion maintaining clinical/histological remission after 24 weeks Treatment‐free follow‐up: proportion maintaining clinical/histological remission 24 weeks Clinical remission was defined as mean stool frequency of < 3 per day *Each patient underwent colonoscopy or sigmoidoscopy pre‐treatment. All were scheduled to undergo sigmoidoscopy at relapse or at the end of treatment, but this was only performed in 21 patients Other outcome measures included: fecal weight (g/day), safety data, maintained histological response (collagen layer <10 um and inflammation score <1), the time to relapse and the rate of relapse after stopping treatment |
|
| Notes | Data from the 24 weeks of the study only were included as the primary outcome measure, as this was the duration of active treatment with budesonide or placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated block randomisation" |
| Allocation concealment (selection bias) | Low risk | Allocation sequence appears to be centrally generated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" Quote: "budesonide 6 mg once a day (2 x 3 mg capsules) or matching placebo" Quote: "blinded follow‐up period (the randomisation code was unbroken until completion of follow‐up, such that neither patients nor physicians knew which treatment the patient had received during maintenance therapy)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All analyses were completed on an intention‐to‐treat basis; premature discontinuation of treatment was considered as relapse in both treatment arms" Quote: "Two patients, one in each group, discontinued maintenance treatment because of adverse events" |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other forms of bias |
Calabrese 2007.
| Methods | Randomized, unblinded, open‐label study | |
| Participants | Patients (n = 819) who presented to clinic, underwent a colonoscopy because of chronic watery diarrhoea and were diagnosed with microscopic colitis (aged 19–68 years; n = 64; 23 with collagenous colitis and 41 with lymphocytic colitis) Clinical components of diagnosis: Chronic or recurrent non‐bloody diarrhea Histological components of diagnosis: Increased chronic inflammatory infiltrate (plasma cells, lymphocytes, eosinophils) in the lamina propria; increased number of intraepithelial lymphocytes, damage to surface epithelium, with flattening of epithelial cells and/or epithelial loss and detachment and minimal crypt architecture distortion; specific to the diagnosis of collagenous colitis was a subepithelial collagen band >10 um thick, which entraps superficial capillaries, with an irregular lacy appearance at the lower edge of the basement membrane "Patients with a clear correlation between symptoms and [consumption] of drugs (e.g. NSAIDS, ticlopidine, PPI) were excluded" |
|
| Interventions | Mesalazine 800 mg po tid (n = 20 with lymphocytic colitis and 11 with collagenous colitis) vs. mesalazine 800 mg po tid + cholestyramine 4 g po od (n = 21 with lymphocytic colitis and 12 with collagenous colitis) for 6 months A 24‐month treatment free follow was also performed A second round of 6 month‐therapy was offered if patients relapsed in follow‐up |
|
| Outcomes | Primary outcomes: Clinical response: "Complete response was complete resolution of diarrhoea. Partial response was improvement but not resolution of diarrhoea Histological response: Normalization of histologic pattern at the end of 6 months Secondary outcomes: 24‐month follow‐up with coloscopies and biopsies, annually; adverse events; and days to remission or relapse, as well as various lab data (routine blood biochemistry and hematological counts, C‐reactive protein, antinuclear antibodies blood assay, serum T4 and thyroid stimulating hormone; IgA‐IgG antigliadin, antiendomysium, IgG anti tTG antibody blood assays; and parasitic‐bacterial, fecal‐stool, and hemo‐occult test |
|
| Notes | "Relapse was defined as stool frequency greater than three soft or liquid stools per day" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed with a computer generated list |
| Allocation concealment (selection bias) | Unclear risk | Not described in published study |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "open‐label" Quote: "All biopsies were analyzed by a single experienced pathologist in a blinded fashion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 of 23 patients with collagenous colitis were lost to follow‐up over a 24 month period The treatment groups and reasons for the missing data were not reported |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were not pre‐specified in the methods section of the manuscript |
| Other bias | Low risk | Study appeared to be free of other forms of bias |
Fine 1999.
| Methods | Randomized, double‐blind, placebo‐controlled. Duration of study was 8 weeks. After 8 weeks, placebo group remained blinded and crossed over to active treatment with bismuth salicylate | |
| Participants | Patients (n = 14, split evenly) with microscopic colitis (11F, 3M; aged 35‐78 years; 9 with thickened subepithelial collagen, 5 without) Clinical: 8 weeks of non bloody watery diarrhea (without steatorrhea) and normal endoscopic appearance of the colonic mucosa. Histological (including involvement of the distal colon): excess mononuclear inflammatory cells in the lamina propria and surface epithelium without significant neutrophilia or eosinophilic inflammation, numerous crypt abscesses, or granuloma; and no other evidence of Crohn's disease |
|
| Interventions | Bismuth subsalicylate (nine 262 mg/day chewable tablets in 3 divided doses) versus placebo (identically coloured and flavoured sucrose tablets) for 8 weeks. | |
| Outcomes | "48 hour fecal weight and consistency, and distal colonic histology (from 16 biopsies obtained by flexible sigmoidoscopy)" were assessed pre and post therapy Clinical: improvement of diarrhea to passage of 2 or less formed or semi‐formed stools/day Histological: improvement of histopathology score by at least 50% |
|
| Notes | Only patients with a thickened subepithelial collagen band on biopsy were included (scored as normal, focally thickened, or diffusely thickened). 4 patients with normal thickness of the subepithelial collagen band were excluded from the analysis. Patients were not to take antibiotics or anti‐inflammatory agents for minimum 6 weeks, and not to take antidiarrheals for minimum 2 weeks prior to the beginning of the study Abstract publication For the histological outcome analysis, a histopathology score from 0 to 10 was based on the following parameters: surface epithelium assessed for micro‐ulceration, cell flattening, and mucin depletion (scored: 0 ‐ normal, 1 ‐ moderate, 2 ‐ severe); crypts (scored: 0 ‐ normal, 1 ‐ distorted architecture and/or cryptitis with neutrophils, 2 ‐ containing crypt abscesses); lamina propria cellularity (scored: 0 ‐ normal, 1 ‐ focally increased with neutrophils, mononuclear inflammatory cells, or both, 2 ‐ diffusely increased with neutrophils, mononuclear inflammatory cells, or both); number of intraepithelial lymphocytes within surface epithelium (scored 0 ‐ normal, 1 ‐ moderately increased, 2 ‐ significantly increased); number of intraepithelial lymphocytes within crypt epithelium (scored 0 ‐ normal, 1 ‐ moderately increased, 2 ‐ significantly increased) Additional information provided by author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was performed by pulling pieces of paper out of a sealed box" |
| Allocation concealment (selection bias) | Unclear risk | Not described in abstract publication |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" Quote: "identically coloured and favoured sucrose‐placebo tablets" Quote: "Blind histologic analysis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients taking BSS completed the study; one patient receiving placebo dropped out of the study after 4 weeks" |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other forms of bias |
Madisch 2007.
| Methods | Randomized, double‐blind, placebo‐controlled trial performed at multiple German centres. Duration of study was 6 weeks. Study had potential crossover for the non‐responders in the placebo group | |
| Participants | Patients (n = 31) aged 18‐80 years with clinically and histologically confirmed collagenous colitis ("at least five liquid or soft stools per day on average per week, and a complete colonoscopy performed within the last 4 weeks before randomization") Histological diagnosis made with colonoscopy with biopsy: main criteria was collagen band > 10 um thick Other analyzed criteria included inflammation of lamina propria (semi‐quantitative definition) and degeneration of surface epithelium (qualitative definition) Patients were excluded if they had other endoscopically or histologically verified causes for diarrhea, infectious diarrhea, pregnancy or lactation, previous colonic surgery, or known intolerance to Boswellia serrata extract. Patients who had received therapy within 4 weeks of randomization were also excluded if therapies included budesonide, salicylates, steroids, prokinetics, antibiotics, ketoconazole, or non‐steroidal anti‐inflammatt ory drugs |
|
| Interventions |
Boswellia serrata extract (three 400 mg/day capsules) versus placebo for 6 weeks Cross‐over therapy offered to non‐responders after 6 weeks, open‐labelled BSE 400 mg po t.i.d |
|
| Outcomes | Primary endpoint was clinical remission after 6 weeks (stool frequency of < 3 per day) stool frequency of less than 3 per day Secondary endpoints were histological changes and quality of life Histological (via colonoscopy with biopsy): improvement in baseline parameters Quality of life: assessed with SF‐36 surveys at the beginning and at the end of 6 weeks of therapy "Stool frequency and consistency, intake of study medication, adverse events, and any intake of allowed concomitant medication were assessed by standardized questionnaire" "Patients who did not respond to treatment after 6 weeks were individually unblinded. If they were in the active treatment group, they were judged as treatment failure. If they were in the placebo group, crossover therapy with open‐labelled BSE 400 mg, given orally three times daily was offered" |
|
| Notes | During the first three weeks of treatment loperamide was allowed as rescue medication. "Patients were allowed to use butylscopolamine in case of abdominal pain" Steroids, anti‐inflammatory drugs, immunosuppressives, antibiotics, prokinetics and bismuth compounds were not allowed during the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was via a central computer generated randomization list in groups of four patients |
| Allocation concealment (selection bias) | Low risk | Quote: "central computer‐generated randomization list" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" Quote: "Physicians, patients, and pathologist were blinded to the treatment group. Study medication was provided in identical‐ looking white boxes labelled with consecutive numbers corresponding to the randomization list. In addition, the placebo containers were prepared from the inside to mimic the typical scent of incense to prevent unblinding by the typical odour of BSE." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/31 patients discontinued (4 patients, reasons described) the trial or were lost to follow‐up (1 patient). All 31 patients were included in the intention‐to‐treat analysis, 26 patients were included in the per‐protocol analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other forms of bias |
Miehlke 2002.
| Methods | Randomized, double‐blind, placebo‐controlled performed at 35 centres in Germany (hospitals and private clinics), which used a centrally‐removed pathologist. Duration of study was 6 weeks | |
| Participants | Patients (n = 51) aged 18‐80 years with clinically and histologically confirmed collagenous colitis ("at least five liquid or soft stools per day on average per week, and a complete colonoscopy performed within the last 4 weeks before randomization"). Female patients must also be using appropriate contraception Histological diagnosis made with colonoscopy with biopsy: main criteria was collagen band > 10 um thick by Van Giesen staining. Other analyzed criteria included inflammation of lamina propria (semi‐quantitative definition) and degeneration of surface epithelium (qualitative definition) Patients were excluded if they had evidence of infectious diarrhea (from culture or biopsy), any other endoscopic or histologic findings (polyps 2 cm, tumors, Crohn’s disease, ulcerative colitis, ischemic colitis) which may have caused diarrhea, known intolerance to budesonide, pregnancy, lactation, or prior partial colonic resection, or if they had received treatment with budesonide, salicylates, steroids, prokinetics, antibiotics, ketoconazole, or non‐steroidal anti‐inflammatory drugs within 4 weeks before randomization |
|
| Interventions | Budesonide 9 mg/day (three 3 mg/day tablets once in the morning) versus identically‐matched placebo for 6 weeks Cross‐over therapy offered to non‐responders after 6 weeks, open‐label budesonide, 9 mg/day po for another 6 weeks |
|
| Outcomes | Proportion of patients achieving clinical remission or histological improvement after 6 weeks Clinical remission defined as: average of < 3 soft stools per day during the last week of treatment Histological (via colonoscopy with biopsy): change of 2 of 3 of the following parameters: collagen band thickness no more than 10 um or reduced to 50% compared to baseline; improvement of inflammation of the lamina propria; improvement of degeneration of surface epithelium Patients also recorded daily stool frequency and consistency, "intake of the study medication, any side effects, and any intake of allowed concomitant medication" Patients who did not respond to treatment after 6 weeks were unblinded. If they were in the active treatment group, they were judged as treatment failure. If they were in the placebo group, crossover therapy with open‐label budesonide, 9 mg/day po for another 6 weeks |
|
| Notes | Other therapies for collagenous colitis were discontinued for at least 3 weeks prior to enrolment in the trial. Loperamide was allowed for the first 4 weeks of the trial (used by 4 patients in the placebo group and 2 in the budesonide group), but no anti‐diarrhoeals allowed in the last two weeks. Patients were allowed to use butylscopolamine for abdominal pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomized by groups of 4 patients according to a central computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" Quote: "Active and placebo capsules were identical in appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/51 patients withdrew (3 from placebo and 3 from budesonide) from the trial (reasons described) Both per‐protocol and intention‐to‐treat analysis available For endoscopic investigations per‐protocol analysis used |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other forms of bias |
Miehlke 2008.
| Methods | Randomized, double‐blind, placebo‐controlled performed at 38 centres in Germany | |
| Participants | Patients aged >18 years with symptomatic and histologically proven collagenous colitis Clinically active defined as ">3 watery/loose stools per day on ≥ 4 of the previous 7 days and had a history of diarrhoea for ≥ 4 weeks" Histological requirements: Subepithelial collagen band > 10 um; inflammatory infiltrate in the lamina propria Exclusion criteria: infectious causes for diarrhea; other inflammatory bowel diseases; history of colonic surgery; celiac disease; malignancies; severe concomitant (organ) diseases that would interfere with the study; at time of inclusion, were being treated 5‐aminosalicylates, salicylates (except in doses ≤165 mg for cardiovascular prophylaxis), systemic steroids, antibiotics, or NSAIDs (including selective cyclo‐oxygenase‐2 inhibitors); used of budesonide within the 2 weeks prior to enrolment, known intolerance to budesonide; pregnancy, lactation, drug and/or alcohol abuse Induction phase: n = 48 Maintenance phase: n = 46, split equally to budesonide and placebo |
|
| Interventions | Induction phase: open‐label budesonide 9 mg/day (3 x 3 mg capsules [Entocort CIR capsules]) once/day for 6 weeks (all included patients) Maintenance phase: budesonide 6 mg/day or placebo for 6 months |
|
| Outcomes | Primary endpoint was cumulative rate of relapse at the end of 6 months (maintenance phase); remission had been induced during the 6 week induction phase. Relapse was defined as > 3 stools per day on ≥ 4 consecutive days. Relapse rates were determined from daily patient diaries Secondary outcomes were time to relapse during maintenance therapy; the proportions of patients with clinical remission after 6 weeks’ induction therapy and after 2 and 4 months of maintenance therapy; HRQOL outcomes; and changes in histologic variables after 6 months’ maintenance therapy ("thickness of the collagen band (>10 or <10 µm); inflammation of the lamina propria (infiltration with lymphocytes and plasma cells; absent, mild, moderate, or severe); and degeneration of the surface epithelium (absent, or present)"). Histologic improvement defined as improvement in ≥ 2 variables versus baseline Safety and tolerability assessments were also performed |
|
| Notes | HRQOL was assessed using the validated Medical Outcome Short Form (SF)‐36 questionnaire26 and the Short Inflammatory Bowel Disease Questionnaire (sIBDQ) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described in published study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" Quote: "budesonide and placebo capsules appeared identical and were packaged in identical bottles" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21/46 patients withdrew during the maintenance phase, 17 due to relapse (14 taking placebo and 3 taking budesonide), 4 due to adverse events (1 taking placebo and 3 taking budesonide) Quote: "for the purposes of intention‐to‐treat analysis, patients who withdrew because of adverse events during maintenance therapy were counted as relapses" |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other forms of bias |
Miehlke 2014.
| Methods | Randomized, double‐blind, double‐dummy, placebo‐controlled, comparative phase‐3 trial performed at 31 European centres (hospitals and private clinics) ‐ Germany, Denmark, Lithuania, Spain, and the United Kingdom. Duration of study was 8 weeks | |
| Participants | N = 92 (budesonide n = 30; mesalamine n = 25, placebo n = 37) Inclusion criteria: Clinical: Patients between 18 and 80 years of age with >4 watery or soft stools on ≥4 days and >3 stools/day in the week prior to baseline. Patients must have also had chronic diarrhoea for ≥3 months prior to baseline and have had a colonoscopy within 4 months of baseline Histological: confirmed collagenous colitis with subepithelial collagenous band > 10 um and degeneration of the surface epithelium Exclusion criteria: "other significant colonic diseases (i.e. polyps >2 cm, tumors, Crohn’s disease, ulcerative colitis, ischemic colitis), partial colonic resection, infectious diarrhea, celiac disease (blood tests and/or duodenal histology required), diarrhea caused by other organic diseases of the gastrointestinal tract, treatment with budesonide, Boswellia serrata extract, salicylates, steroids, antibiotics, cholestyramine, nonsteroidal anti‐inflammatory, or other immunosuppressant drugs within the last 4 weeks before baseline, malignant disease, severe comorbidity, abnormal hepatic function or liver cirrhosis, renal insufficiency, active peptic ulcer disease, known intolerance or resistance to study drugs, pregnancy, or breast‐feeding" |
|
| Interventions | Budesonide 9 mg/day ["(3x3 mg pH‐modified release capsules, Budenofalk) 30 minutes before breakfast"] Mesalamine 3 g/day [morning dosage of "sachets each containing 1.5 g mesalamine presented as a granule formulation, Salofalk"] Placebo All medications take for 8 weeks if responsive. If unresponsive after 4 weeks, or relapsed in the 16 week treatment‐free follow‐up, patient's removed from study arm and received 9 mg/day of budesonide for the remaining 4 weeks. |
|
| Outcomes | Measured at each interim visit: 2, 4, 6, 8 weeks; 8 and 16 weeks Primary Outcomes: Clinical: remission defined as ≤3 stools/day in the week before the visit. Histological: measured collagen band thickness (≤10um or 50% reduction), lamina propria inflammation (by scoring), intraepithelial lymphocytes (by scoring) and whether the surface epithelium was degenerated. Improvement was defined as improvement of two of the parameters. Histological remission was defined as "collagen band thickness 10 mm and no inflammation of the lamina propria with neutrophilic and eosinophilic granulocytes." Secondary Outcome: Clinical remission was also evaluated according to Hjortswang‐Criteria of disease activity ("mean <3 stools per day, with <1 watery stool per day)" Also, "time to remission, number of watery and solid stools per week, abdominal pain, histopathology, tolerability and safety, symptom relapse during treatment‐free follow‐up, and response to open‐label budesonide" |
|
| Notes | Relapse was defined as: ">4 watery/soft stools on at least 4 days in the week before the visit and >3 stools per day within the last 7 days before the visit" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers assigning at a 1:1:1 ratio between the 3 arms of the study |
| Allocation concealment (selection bias) | Low risk | Computer generated, random numbers list prepared by a contract research organization that had no clinical involvement with the trial Used medication packed in boxes with consecutive numbers according to the randomization list |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind, double‐dummy" Identical placebo capsules and sachets Single pathologist was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21/92 patients withdrew from the trial prematurely (before 8 weeks). Two taking budesonide, 9 taking mesalamine and 10 taking placebo. Reasons described. 64/92 entered follow up, 16 entered open‐label budesonide. Intention‐to‐treat was followed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Study appeared to be free of other forms of bias |
Munch 2016.
| Methods | An initial 8‐week open‐label induction phase with budesonide therapy to achieve clinical remission was followed by a double‐blind, randomized, placebo‐controlled, parallel‐group, multicentre, 12‐month phase for maintenance of clinical remission. After this there was 6 months of treatment‐free follow‐up | |
| Participants | A total of 148 patients were screened, all age ≥ 18 years Inclusion criteria: 1. Histologically established diagnosis of collagenous colitis, defined as thickened subepithelial collagen layer ≥10 mm on well‐orientated sections, and increased inflammatory cells indicating chronic inflammation in the lamina propria 2. Prescreening history of non‐bloody, watery diarrhea for ≥2 weeks in patients with newly diagnosed collagenous colitis, or a prescreening history of clinical relapse for ≥1 week in patients with previously established collagenous colitis 3. A mean of ≥3 stools/day, including a mean of ≥1 watery stool/day, during the week prior to baseline Exclusion criteria: 1. Diabetes mellitus, infection, glaucoma, tuberculosis, peptic ulcer disease or hypertension if careful medical monitoring was not ensured 2. Established cataract 3. Known hereditary problems of galactose or fructose intolerance, lactase deficiency, increased levels of anti‐transglutaminase 2 antibodies 4. Established osteoporosis with T‐score <−2.5 As per Figure 2: 110 met eligibility criteria and started the open‐label phase. 92 patients had achieved remission during the open‐label phase and were randomized for treatment in the double‐blind phase (44 budesonide, 48 placebo). 43 completed the 12‐month study visit (32 budesonide, 11 placebo). 36 patients at the end of the double‐blind phase (28 budesonide, 8 placebo) entered the follow‐up phase |
|
| Interventions | During the open‐label induction phase, all patients received once‐daily budesonide (Budenofalk 3 mg capsules) at a dose of 9 mg/day for 4 weeks, then 6 mg/day for 2 weeks, followed by alternate daily doses of 6 and 3 mg/day (mean 4.5 mg/day) for the final 2 weeks During the double‐blind phase, the active treatment group received once‐daily budesonide 6 and 3 mg/day on alternate days (mean 4.5 mg/day). The placebo group received two placebo capsules and one placebo capsule on alternate days, administered once daily After the final visit of the double‐blind phase (month 12), there was a 2‐week tapering‐off period, during which patients in the active treatment group received 3 mg/day budesonide for 1 week followed by 3 mg/day budesonide every second day for 1 week. Patients in the placebo group received one placebo capsule on the corresponding days Patients who remained in clinical remission at the end of the double‐blind phase received no further study drug after the 2‐week tapering‐off period During the treatment‐free follow‐up, no intervention was given |
|
| Outcomes | The primary endpoint was the proportion of patients remaining in clinical remission during the 12‐month double‐blind phase, with clinical remission defined as a mean of <3 stools/day, including a mean of <1 watery stool/day over 1 week The main secondary endpoints during the double‐label phase included health‐related quality of life using the Short Health Scale (SHS) and the Psychological General Well‐Being Index (PGWBI) Further secondary endpoints during the double‐blind phase were achievement of histological remission or histological improvement |
|
| Notes | During the entire study period, loperamide, anti‐inflammatory or immunosuppressant drugs were not permitted Prophylactic treatment of osteoporosis with calcium and vitamin D3 was strongly recommended and under the responsibility of the investigator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomization was described as "a computer‐generated randomisation list using randomly permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding is mentioned, but not described in more detail. Placebo capsules were used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Well described patient disposition in Figure 2 and had intention‐to‐treat analysis described. They accounted for attrition/exclusions with reasons given |
| Selective reporting (reporting bias) | Low risk | All primary outcomes were reported. Most secondary outcomes were reported with exception of histological outcomes Safety and adverse‐effect data was also presented, but not described as part of methods section |
| Other bias | Low risk | Study appeared to be free of other forms of bias |
Munck 2003.
| Methods | Randomized, double‐blind, placebo‐controlled, multi‐centred trial | |
| Participants | Patients (n = 12, 11 with collagenous colitis and 1 with lymphocytic colitis) aged >18 years reporting at least 3 months with diarrhoea without blood or pus and with a stool volume ≥350 g/day or ≥200 g/day and a stool frequency ≥5/day and a histological diagnosis of microscopic colitis. Female patients also needed to use appropriate contraceptive techniques Patients were diagnosed histologically using a macroscopic normal colonoscopy or sigmoidoscopy plus a normal barium enema and confirmed by an independent pathologist with either lymphocytic colitis or collagenous colitis using the following criteria: “chronic inflammatory infiltrate in the lamina propria and either a lymphocytic infiltration of at least 20% of epithelial crypt cells (lymphocytic colitis) and/or a subepithelial collagen bond >10 µm in a well‐oriented biopsy (collagenous colitis)” Excluded patients: tested positive for pathogenic bacteria or parasites; failed a normal lactose absorption test and vitamin B12 absorption test, or a normal barium follow through; had celiac disease (confirmed with IgG and IgA antigliadin antibodies and antiendomysium antibodies and/or abnormal histology in duodenal biopsies); had bile acid malabsorption and/or no response to cholestyramine, and/or steatorrhoea; had other gastrointestinal diseases or previous gastrointestinal surgery (exception: cholecystectomy); had other serious diseases, abnormal laboratory tests (haematology, renal function, liver enzymes, urinalysis); had been treated with immunosuppressives within 3 months of randomization; or used medicines with known effects on gastrointestinal functioning including anti‐ulcer medication, antacids, antibiotics and NSAIDs |
|
| Interventions | Prednisolone, n = 9 (50 mg/dayfor 2 weeks, tapered to 37.5 mg/day in third week) versus placebo, n = 3, for 2 weeks. All patients also received Ca 500 mg + vitamin D 5 ug/day | |
| Outcomes | Proportion of patients achieving clinical remission after 2 weeks Clinical remission was defined as stool weight ≤ 200 g/day or frequency ≤ 2/day; effect was defined as >50% reduction of either stool frequency or weight. Side effects were also recorded |
|
| Notes | Inclusion of patients was stopped when planned monitoring indicated that prednisone did not induce remission. Protocol also included a 48‐week azathioprine continuation phase which was closed when it became clear that the calculated number of patients could not be recruited Medications with immunosuppressive effects, antidiarrhoeals or those with known effects on gastrointestinal function were not allowed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described in published study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" Quote: "identical placebo tablets" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data Quote: "All patients complied with and completed the treatment protocol" |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other forms of bias |
Wildt 2006.
| Methods | Randomized, double‐blind, placebo‐controlled trial at 4 Danish centres. Randomization was in a 2:1 fashion (probiotic:placebo) | |
| Participants | Patients (n = 36*, therefore n = 29, 2 men) aged ≥18 years with confirmed histological diagnosis of collagenous colitis that is active and untreated for at least 4 weeks prior to study inclusion. Clinically active disease is defined as > 21 liquid or soft stools per week or stool weight of > 200 g/day for at least 4 weeks Histological diagnosis required "a subepithelial collagen band > 10 um in a well oriented section of the mucosa and inflammation of the lamina propria with infiltration of predominantly lymphocytes and plasma cells" Exclusion criteria included: pregnancy or breast feeding, chronic liver or kidney disease, severe vascular or cardiopulmonary disease, malignancy, immunosuppressive disease or treatment, known inflammatory bowel disease besides collagenous colitis (including celiac disease), evidence of infectious diarrhea, prior gastrointestinal surgery other than appendectomy, and malabsorption syndromes. Treatment with aminosalicylates, antibiotics, cholestyramine, nonsteroidal anti‐inflammatory drugs, and steroids was not allowed 4 weeks prior to study entrance |
|
| Interventions | Probiotic (AB‐Cap‐10; two capsules twice daily) or placebo (2 capsules twice daily) for 12 weeks. Loperamide and opioids were allowed during the study | |
| Outcomes | Primary outcome was the proportion of patients with a at least a 50% reduction in the number of stools per week at 12 weeks
Secondary outcomes: changes in bowel frequency, stool consistency, stool weight, abdominal pain and bloating, histopathology scores from biopsies, Short Inflammatory Bowel Disease Questionnaire (SIBDQ) scores, use of antidiarrhoeal medication, and adverse events Histological scores from: significant change in three parameters: reduction of thickness of the collagen band; improvement in the degree of inflammation of the lamina propria; improvement of degeneration of surface epithelium The study period was 17 weeks (12 weeks treatment + 5 weeks follow up) with patients being assessed at weeks ‐1, 0, 4, 6, 12, and 16. All patients kept a diary throughout the study period |
|
| Notes | AB‐Cap‐10 is a mixture of L. acidophilus strain LA‐5 and B. animalis subsp. lactis strain BB‐12. Each capsule contained 0.5 x 10^10 colony‐forming units of each bacterium, leading to a total delivery of 1 x 10^10 CFU per capsule SIBDQ; a 10‐item questionnaire measuring health‐related quality of life [HRQOL] intended for patients with Crohn’s disease and ulcerative colitis *Seven patients that failed to meet the inclusion criteria after being randomized into the trial (six patients had lymphocytic colitis and one had a subepithelial collagen band < 10 um thick) are excluded from the analysis Study enrolment was stopped early due to difficulties recruiting patients |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was completed in blocks of 9 using a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Method of randomization not described in study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" Quote: "Placebo medication (Chr. Hansen A/S) was identical in appearance, size, and taste" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 probiotic treatment patients dropped out because of lack of response Quote: "When data at week 12 were missing because of withdrawals, the last observation was carried forward" |
| Selective reporting (reporting bias) | Low risk | All outcome reported. One post hoc analysis noted |
| Other bias | Low risk | Study appeared to be free of other forms of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Calabrese 2011 | Not a randomized trial. It was a cohort extension trial of another randomized controlled trial |
| Delarive 1998 | Not a randomized trial. It is a case report |
| Gentile 2015 | Not a randomized trial. It also included microscopic colitis patients without specifying for collagenous colitis |
| Mali 2015 | Not a randomized trial. It also included microscopic colitis patients without specifying for collagenous colitis |
| Miehlke2014 | Withdrawal of short‐term budesonide therapy. Examines different outcomes |
| Taheri 2011 | No study data |
Differences between protocol and review
We updated the methods to include a full risk of bias assessment for the included studies. We utilized the GRADE criteria to assess the overall quality of the evidence supporting the primary and secondary outcomes. A PRISMA diagram was used to document the study flow.
Declarations of interest
Tahir S Kafil: None known.
Tran M Nguyen: None known.
Petrease H Patton: None known.
John K MacDonald: None known.
Nilesh Chande has received consulting fees from AbbVie, Janssen, Takeda, and Ferring; and speaker's fees from AbbVie, Janssen, and Actavis. All of these financial activities are outside the submitted work.
John WD McDonald: None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Baert 2002 {published data only}
- Baert F, Schmit A, D'Haens G, Dedeurwaerdere F, Louis E, Cabooter M, et al. Budesonide in collagenous colitis: A prospective double blind placebo controlled trial with histologic follow‐up. Gut 2000;47(Suppl III A250):966. [DOI] [PubMed] [Google Scholar]
- Baert F, Schmit A, D'Haens G, Dedeurwaerdere F, Louis E, Cabooter M, et al. Budesonide in collagenous colitis: a double‐blind placebo‐controlled trial with histologic follow‐up. Gastroenterology 2002;122(1):20‐5. [DOI] [PubMed] [Google Scholar]
Bonderup 2003 {published data only}
- Bonderup OK, Bach‐Hansen J, Madsen P, Vestergaard V, Fallingborg J, Teglbjaerg PS. Expression of inducible nitric oxide synthase (iNOS) mRNA in colonic mucosa in collagenous colitis ‐ Correlation to the histological and clinical activity and the effect of budesonide treatment. Gastroenterology 2004;126(4 Suppl 2):A154‐5. [DOI] [PubMed] [Google Scholar]
- Bonderup OK, Hansen JB, Birket‐Smith L, Vestergaard V, Teglbjaerg PS, Fallingborg J. Budesonide treatment of collagenous colitis: a randomised, double blind, placebo controlled trial with morphometric analysis. Gut 2003;52(2):248‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonderup OK, Hansen JB, Birket‐Smith L, Vestergaard V, Teglbjaerg PS, Fallingborg J. Budesonide treatment of collagenous colitis: a randomised, double‐blind, placebo controlled trial with morphometric analysis. Gut 2003;52(2):248‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonderup OK, Hansen JB, Madsen P, Vestergaard V, Fallingborg J, Teglbjaerg PS. Budesonide treatment and expression of inducible nitric oxide synthase mRNA in colonic mucosa in collagenous colitis. European Journal of Gastroenterology and Hepatology 2006;18(10):1095‐9. [DOI] [PubMed] [Google Scholar]
Bonderup 2009 {published data only}
- Bonderup O, Hansen J, Teglbjaerg P, Christensen L, Fallingborg J. Long‐term budesonide treatment of collagenous colitis ‐ a randomized, double‐blind, placebo‐controlled trial. United European Gastroenterology Week 2007:No. FP‐247. [DOI] [PubMed]
- Bonderup OK, Bach‐Hansen J, Teglbjaerg PS, Christensen LA, Fallingborg J. Long‐Term Budesonide Treatment of Collagenous Colitis ‐ a Randomized, Double Blind, Placebo Controlled Trial. Gastroenterology 2008;134(4 Suppl 1):A487‐8. [DOI] [PubMed] [Google Scholar]
- Bonderup OK, Hansen JB, Teglbjrg PS, Christensen LA, Fallingborg JF. Long‐term budesonide treatment of collagenous colitis: a randomised, double‐blind, placebo‐controlled trial. Gut 2009;58(1):68‐72. [DOI] [PubMed] [Google Scholar]
Calabrese 2007 {published data only}
- Calabrese C, Fabbri A, Areni A, Zahlane D, Scialpi C, Febo G. Mesalazine with or without cholestyramine in the treatment of microscopic colitis: randomized controlled trial. Journal of Gastroenterology and Hepatology 2007;22(6):809‐14. [DOI] [PubMed] [Google Scholar]
Fine 1999 {published data only}
- Fine K, Ogunji F, Lee E, Lafon G, Tanzi M. Randomized, double‐blind, placebo‐controlled trial of bismuth subsalicylate for microscopic colitis. Gastroenterology 1999;116(4):A880. [Google Scholar]
Madisch 2007 {published data only}
- Madisch A, Miehlke S, Eichele E, Bethke B, Mrwa J, Wilhelms G, et al. Boswellia serrata extract for the treatment of collagenous colitis: a randomized double‐blind, placebo‐controlled, multicenter trial. Gastroenterology 2005;128(4 Suppl 2):A581. [DOI] [PubMed] [Google Scholar]
- Madisch A, Miehlke S, Eichele O, Mrwa J, Bethke B, Kuhlisch E, et al. Boswellia serrata extract for the treatment of collagenous colitis. A double‐blind, randomized, placebo‐controlled multicenter trial. International Journal of Colorectal Disease 2007;22(12):1445‐51. [DOI] [PubMed] [Google Scholar]
Miehlke 2002 {published data only}
- Madisch A, Heymer P, Voss C, Wigginghaus B, Bastlein E, Bayerdorffer E, et al. Oral budesonide therapy improves quality of life in patients with collagenous colitis. International Journal of Colorectal Disease 2005;20(4):312‐6. [DOI] [PubMed] [Google Scholar]
- Madisch A, Miehlke S, Heymer P, Bethke B, Baestlein E, Lehn N, et al. Oral budesonide therapy improves quality of life in patients with collagenous colitis‐results of placebo‐controlled, multicenter trial. Gastroenterology 2003;124(4 Suppl. 1):A191. [Google Scholar]
- Miehlke S, Heymer P, Bethke B, Baestlein E, Meier E, Bartram HP, et al. Budesonide treatment for collagenous colitis: a randomized, double‐blind, placebo‐controlled, multicenter trial. Gastroenterology 2002;123(4):978‐84. [DOI] [PubMed] [Google Scholar]
- Miehlke S, Heymer P, Ochsenkuehn T, Baestlein E, Yarian G, Morgner A, et al. Oral budesonide is highly effective in the treatment of collagenous colitis: A randomized, double‐blind, placebo‐controlled, multicenter trial. Gastroenterology 2001;125(5 Suppl. 1):A‐40. [Google Scholar]
- Miehlke S, Madisch A, Voss C, Kuhlisch E, Morgner A, Heymer P, et al. Long‐term follow‐up and predictive factors for clinical relapse in patients with collagenous colitis after induction of remission with budesonide capsules. Gastroenterology 2004;126(4 Suppl 2):A465. [Google Scholar]
- Miehlke S, Madisch A, Voss C, Morgner A, Heymer P, Kuhlisch E, et al. Long‐term follow‐up of collagenous colitis after induction of clinical remission with budesonide. Alimentary Pharmacology and Therapeutics 2005;22(11‐12):1115‐9. [DOI] [PubMed] [Google Scholar]
Miehlke 2008 {published data only}
- Miehlke S, Madisch A, Bethke B, Morgner A, Baretton G, Stolte M. Oral budesonide or maintenance treatment of collagenous colitis: a randomised, placebo‐controlled, double‐blind trial. Gastroenterology 2008;134(4 Suppl. 1):A488‐9. [DOI] [PubMed] [Google Scholar]
- Miehlke S, Madisch A, Bethke B, Morgner A, Kuhlisch E, Henker C, et al. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, placebo controlled, double‐blind trial. Gastroenterology 2008;135:1510‐16. [DOI] [PubMed] [Google Scholar]
- Miehlke S, Madisch A, Bethke B, Morgner A, Kuhlisch E, Henker C, et al. Remission‐maintaining therapy of collagenous colitis with Budesonide ‐ a randomised, double‐blind, placebo‐controlled multi‐centre study. Zeitschrift fur Gastroenterologie 2008;46(9):932. [Google Scholar]
- Miehlke S, Madisch A, Bethke B, Morgner A, Kuhlish E, Henker C, et al. Budesonide for maintenance treatment of collagenous colitis ‐ a randomized, double‐blind, placebo‐controlled trial. United European Gastroenterology Week 2007:No. PS‐M‐13. [DOI] [PubMed]
Miehlke 2014 {published data only}
- Miehlke S, Madisch A, Kupcinskas L, Heptner G, Böhm G, Marks H, et al. Double‐blind, double‐dummy, randomized, placebo‐controlled, multicenter trial of budesonide and mesalamine in collagenous colitis. Gastroenterology May 2012;142(5 (Suppl 1)):S211. [Google Scholar]
- Miehlke S, Madisch A, Kupcinskas L, Petrauskas D, Böhm G, Marks H, et al. Budesonide is more effective than mesalamine or placebo in short‐term treatment of collagenous colitis. Gastroenterology 2014;146(4):1222‐30. [DOI] [PubMed] [Google Scholar]
- Miehlke S, Madisch A, Kupcinskas L, Petrauskas D, Heptner G, Böhm G, et al. European multicenter trial of budesonide and mesalazine for short‐term treatment of active collagenous colitis (BUC60/COC). Journal of Crohn's and Colitis 2013;7:S214. [Google Scholar]
- Mohrbacher R. Double‐blind, double‐dummy, randomized, placebo‐controlled, multicenter trial of budesonide and mesalamine in collagenous colitis. Email Correspondance July 30, 2012.
Munch 2016 {published data only}
- Munch A, Bohr J, Miehlke S, Benoni C, Olesen M, Ost A, et al. Clinical remission and quality of life in collagenous colitis: a one‐year, randomised, placebo‐controlled study with low‐dose budesonide (BUC‐63/ COC). Gastroenterology 2014;146(5 suppl. 1):S586. [Google Scholar]
- Munch A, Bohr J, Miehlke S, Benoni C, Olesen M, Ost A, et al. Low‐dose budesonide maintains clinical remission and quality of life in collagenous colitis over a one year period: results from the randomised, placebo‐controlled BUC‐63/COC trial. Journal of Crohn's and Colitis 2014;8:S52. [Google Scholar]
- Münch A, Bohr J, Miehlke S, Benoni C, Olesen M, Öst A, et al. Low‐dose budesonide for maintenance of clinical remission in collagenous colitis: A randomised, placebo‐controlled, 12‐month trial. Gut 2016;65:47‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munck 2003 {published data only}
- Munck LK, Kjeldsen J, Philipsen E, Fischer Hansen B. Incomplete remission with short‐term prednisolone treatment in collagenous colitis: a randomized study. Scandinavian Journal of Gastroenterology 2003;38(6):606‐10. [DOI] [PubMed] [Google Scholar]
Wildt 2006 {published data only}
- Wildt S, Munck LK, Vinter‐Jensen L, Hansen BF, Nordgaard‐Lassen I, Christensen S, et al. Probiotic treatment of collagenous colitis: a randomized, double‐blind, placebo‐controlled trial with Lactobacillus acidophilus and Bifidobacterium animalis subsp. Lactis. Inflammatory Bowel Diseases 2006;12(5):395‐401. [DOI] [PubMed] [Google Scholar]
- Wildt S, Munck LK, Vinther‐Jensen L, Hansen BF, Nordgaard‐Lassen I, Christensen S, et al. Treatment of collagenous colitis with Lactobacillus acidophilus and Bifidobacterium animalis subsp. Lactis. A randomised, double‐blind, placebo‐controlled trial. United European Gastroenterology Week 2005:OP‐G‐67.
References to studies excluded from this review
Calabrese 2011 {published data only}
- Calabrese C, Gionchetti P, Liguori G, Areni A, Fornarini GS, Campieri M, et al. Clinical course of microscopic colitis in a single‐center cohort study. Journal of Crohn's & Colitis 2011;5(3):218‐21. [DOI] [PubMed] [Google Scholar]
Delarive 1998 {published data only}
- Delarive J, Saraga E, Dorta G, Blum A. Budesonide in the treatment of collagenous colitis. Digestion 1998;59(4):364‐6. [DOI] [PubMed] [Google Scholar]
Gentile 2015 {published data only}
- Gentile NM, Abdalla AA, Khanna S, Smyrk TC, Tremaine WJ, Faubion WA, et al. Outcomes of patients with microscopic colitis treated with corticosteroids: a population‐based study. American Journal of Gastroenterology 2013;108(2):256‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mali 2015 {published data only}
- Mali P, Komanapalli S, Kumar N. Role of maintenance steroids in microscopic colitis. American Journal of Gastroenterology 2015;110:S569. [Google Scholar]
Miehlke2014 {published data only}
- Miehlke S, Hansen JB, Madisch A, Schwarz F, Kuhlisch E, Morgner A, et al. Risk factors for symptom relapse in collagenous colitis after withdrawal of short‐term budesonide therapy. Journal of Crohn's & colitis 2014;8:S215‐6. [DOI] [PubMed] [Google Scholar]
Taheri 2011 {published data only}
- Taheri A, Sadighi A, Nikkhoo B, Farhangi E. Evaluation of effects and complications of probiotics in microscopic colitis, a double blind placebo control clinical trial. Gastroenterology 2011;140(5):S852. [Google Scholar]
Additional references
Chande 2017
- Chande N, Al Yatama N, Bhanji T, Nguyen TM, McDonald JW, MacDonald JK. Interventions for treating lymphocytic colitis. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD006096.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenberg 1994
- Greenberg GR, Feagan BG, Martin F, Sutherland LR, Thomson AB, Williams CN, et al. Oral budesonide for active Crohn's disease. Canadian Inflammatory Bowel Disease Study Group. New England Journal of Medicine 1994;331(13):836‐41. [DOI] [PubMed] [Google Scholar]
Greenberg 1996
- Greenberg GR, Feagan BG, Martin F, Sutherland LR, Thomson AB, Williams CN, et al. Oral budesonide as maintenance treatment for Crohn's disease: a placebo‐controlled, dose‐ranging study. Canadian Inflammatory Bowel Disease Study Group. Gastroenterology 1996;110(1):45‐51. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Hjortswang 2009
- Hjortswang H, Tysk C, Bohr J, Benoni C, Kilander A, Larsson L, et al. Defining clinical criteria for clinical remission and disease activity in collagenous colitis. Inflammatory Bowel Diseases 2009;15(2):1875‐1881. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
References to other published versions of this review
Chande 2002
- Chande N, McDonald JW, MacDonald JK. Interventions for treating collagenous colitis. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003575] [DOI] [PubMed] [Google Scholar]
Chande 2003a
- Chande N, McDonald JW, MacDonald JK. Interventions for treating collagenous colitis. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003575] [DOI] [PubMed] [Google Scholar]
Chande 2003b
- Chande N, McDonald JW, MacDonald JK. Interventions for treating collagenous colitis. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003575] [DOI] [PubMed] [Google Scholar]
Chande 2004a
- Chande N, McDonald JW, MacDonald JK. Interventions for treating collagenous colitis. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003575.pub2] [DOI] [PubMed] [Google Scholar]
Chande 2004b
- Chande N, McDonald JWD, MacDonald JK. Interventions for treating collagenous colitis: A Cochrane Inflammatory Bowel Disease Group systematic review of randomized trials. American Journal of Gastroenterology 2004;99(12):2459‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chande 2005
- Chande N, McDonald JW, MacDonald JK. Interventions for treating collagenous colitis. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003575.pub3] [DOI] [PubMed] [Google Scholar]
Chande 2006
- Chande N, McDonald JW, MacDonald JK. Interventions for treating collagenous colitis. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003575.pub4] [DOI] [PubMed] [Google Scholar]
Chande 2008
- Chande N, McDonald JW, MacDonald JK. Interventions for treating lymphocytic colitis. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD003575.pub5] [DOI] [PubMed] [Google Scholar]


