Abstract
Background
We compared two patient-reported outcomes (PROs) of swallowing and their relationship to quality of life (QOL) in long-term oropharyngeal cancer (OPC) survivors.
Methods
The single dysphagia item from the 28-item multi-symptom MD Anderson Symptom Inventory—Head and Neck (MDASI-HN-S) was compared to the dysphagia-specific composite MD Anderson Dysphagia Inventory (MDADI) and the EuroQol visual analogue scale (EQ-VAS) in 714 patients treated 12 months or more prior with definitive radiotherapy. An MDASI-HN-S score ≥6 and MDADI composite score <60 was considered representative of moderate/severe swallowing dysfunction.
Results
Moderate/severe dysphagia was reported by 17% and 16% of respondents by MDASI-HN-S and composite MDADI, respectively. Both swallow PROs were predictive of QOL, with the MDASI-HN-S model being slightly more parsimonious for discrimination of EQ-VAS scores compared to MDADI (BIC 6062 vs 6076). An MDASI-HN-S cut-point score of ≥6 correlated best with EQ-VAS decline (p<0.0001) and was associated with increased radiotherapy dose to several normal surrounding structures.
Conclusion
In this cohort, the single-item MDASI-HN-S performed favorably for discrimination of QOL compared to the multi-item MDADI. A time-efficient model for PRO measurement of swallowing is proposed in which MDADI may be reserved for patients scoring ≥6 on MDASI-HN-S.
Keywords: Dysphagia, patient reported outcomes, oropharynx cancer, radiotherapy, quality of life
CONDENSED ABSTRACT
In a cohort of long-term survivors of oropharyngeal cancer, a single dysphagia item from MDASI-HN (28-item multi-symptom inventory) performed favorably for discrimination of quality of life compared to the multi-item MDADI (20-item swallow QOL). A model of patient-reported outcome measurement of swallowing is proposed in which MDADI may be reserved for those reporting moderate/severe symptoms by a single MDASI-HN dysphagia item.
INTRODUCTION
Definitive radiation therapy (RT) plays an important role in the curative treatment of oropharyngeal cancer (OPC), but carries a risk of long-term toxicity. With an increasing proportion of these cancers related to the human papilloma virus (HPV) that present at an earlier median age of diagnosis and have favorable outcomes compared to non-HPV OPC, there is an enlarging population of long-term survivors in whom late toxicity of treatment is of particular importance [1].
Swallowing dysfunction, or dysphagia, is an important and potentially chronic complication following RT to the oropharynx and has a demonstrated negative impact on quality of life (QOL) [2–4]. Moreover, while highly conformal RT techniques have become standard in the treatment of these cancers with demonstrated improved sparing of the parotid salivary glands and more favorable rates of long-term xerostomia [5], the benefit of conformal treatment with regards to swallowing function is less clear. Dysphagia thus remains commonly cited as a limiting factor in the delivery of curative RT for OPC [6].
Patient-reported outcomes (PRO) questionnaires are increasingly used in clinical practice and research trials to quantify the severity and impact on everyday function of particular symptoms [7]. There exist a number of general multi-symptom questionnaires for patients undergoing treatment for head and neck cancer (HNC), including the MD Anderson Symptom Inventory Head and Neck Module (MDASI-HN) [8–10]. These instruments assess an array of symptoms and toxicities that may co-exist in patients with HNC. For this reason, multi-symptom inventories offer the most efficient method of querying the patients’ perspectives on multiple issues in a single instrument. These instruments, therefore, are attractive for routine use in clinical practice to track symptoms in addition to their value as a research metric. There exist other questionnaires, such as the MD Anderson Dysphagia Inventory (MDADI) [11, 12], that focus solely on a patient’s swallowing function. While these dysphagia-specific questionnaires give a narrower snapshot of a patient’s overall function as compared to the multi-symptom inventories, they generally provide greater detail with regard to the nature of perceived swallowing dysfunction and ways in which it may impact day-to-day life. Yet, the relative performance of dysphagia-specific inventories to single items from multi-symptom scales is not well known. Likewise, the validity of reporting single dysphagia item scores from multi-symptoms inventories is not established. If a single dysphagia item from multi-symptom questionnaires is a valid representation of global patient-reported swallowing outcome, this may offer practitioners and researchers alike a more efficient way to capture PRO data on dysphagia simultaneously with other symptoms from a single instrument.
In addition, while many multi-symptom and dysphagia-specific inventories have been validated for the measurement of treatment-related swallowing dysfunction in patients with HNC, few have established consensus cut-points or meaningful thresholds at which symptoms likely merit further investigation. In this study, we sought to compare the performance of a single dysphagia question from MDASI-HN (MDASI-HN-S) with the composite MDADI score in a large sample of patients with OPC treated with definitive RT.
The specific aims of this study were to:
Examine the performance of a single item dysphagia question from the 28-item multi-symptom instrument (MDASI-HN-S) relative to the widely used dysphagia specific composite MDADI (19-item composite score from 20-item instrument).
Validate clinically meaningful cut-points of the two dysphagia PROs examined in Aim 1 (single item dysphagia MDASI-HN-S and 19-item composite MDADI) by examining performance relative to clinical, QOL, and dosimetric parameters.
Estimate the prevalence of self-reported RAD using PRO cut-points from Aim 2 among a cross-sectional cohort of long-term OPC survivors treated at the University of Texas MD Anderson Cancer Center.
MATERIALS AND METHODS
Patients
This is a secondary analysis of a cross-sectional survivorship survey. Patients with T1-T4 N0-N3 M0 OPC (per AJCC staging 7th edition) treated with definitive RT at a single institution between January 2000 and April 2014 were sampled from 908 survey respondents (58% response rate). A total of 194 patients were dropped (148 did not complete of one or more of the surveys of interest, 20 did not receive definitive RT as primary modality, one patient had metastatic disease, and 25 received treatment with one year), leaving 714 available for analysis. Tumor subsites included tonsil, base of tongue, soft palate, pharyngeal wall, glossopharyngeal sulcus, and oropharynx NOS. To focus on chronic toxicities, patients were invited to participate if they completed treatment one year or more prior to the survey administration.
Survey administration
Participants were asked to complete the survey once. The survey was administered using an adapted version of Dillman method [13], including: 1) a letter of invitation via the US postal service to eligible patients 2–3 weeks prior to the initial contact, 2) the survey questionnaire sent to all eligible participants via an online server Qualtrics or US postal service, and 3) two reminders sent to non-responders via US postal service at 2–3 weeks and 4–5 weeks after the initial contact. Participants were contacted by multiple modes of communication, including email (among those with addresses on record) via Qualtrics or myMDAnderson (a secure, personalized patient website), and US postal service via first-class mail with a return envelope.
Patient-reported outcomes
The survey included 3 PROs used in this analysis: the multi-symptom MDASI-HN, the multi-item MDADI, and the EuroQoL visual analogue scale (EQ-VAS) questionnaire.
The 28-item MDASI-HN is a multi-symptom inventory that includes 13 core symptom items (e.g., pain, fatigue), each rated for severity from 0 (“not present”) to 10 (“as bad as you can imagine”) as well as 9 disease-specific head and neck symptom severity items (e.g., dysphagia, mucus, voice/speech), and 6 interference items [8]. There are 2 dysphagia related symptom items on the MDASI-HN, (MDASI-HN-S, “your difficulty chewing/swallowing at its worst” and MDASI-HN choke “your coughing/choking (foods/liquids going down the wrong pipe) at its worst”). MDASI-HN-S was selected as the single item dysphagia PRO in this analysis as it represents the more global of the two items, dichotomized for Aims 2 and 3 per published standards [14, 15], with scores 0–5 and 6–10 representing no/mild symptoms and moderate/severe symptoms, respectively.
The MDADI consists of 20 items that assess an individual’s perception of their swallowing ability [11]. MDADI derives a 19-item composite score which summarizes performance across three individual subscales (physical, emotional, and functional) and ranges from 20 (extremely low functioning) to 100 (high functioning). The 19-item composite MDADI score was selected as our MDADI metric in this analysis as it represents the summary measure combining responses from all domains. An MDADI score of 60 has previously been suggested to meaningfully stratify individuals [16–18], and was selected as a cut point to investigate for Aims 2 and 3 in this study.
The EQ-5D questionnaire is a standardized instrument designed to assess the generic status of one’s overall health, and has been widely used to measure health-related QOL in many populations [19]. EQ-5D is separated in two sections. The first includes five questions or dimensions that assess one’s mobility, ability to care for self, level of pain, participation in usual activities, and psychological status. The second section is the visual analogue scale (EQ-VAS) that allows a patient to rate the state of his or her own health on a visual scale from 0 (worst imaginable health state) to 100 (best imaginable health state). Only EQ-VAS estimate of health state were available for this analysis as a surrogate of health-related QOL.
Treatment and planning
While a small subset of patients were treated with 3D conformal therapy (3D-CRT) or proton therapy, the large majority were treated with IMRT. The authors’ institutional IMRT approach from this time period in the treatment of OPC has previously been described in detail [20].
Dosimetric correlates (subset analysis)
Out of 714 patients included in this analysis, a total of 320 treatment plans and dosimetric data were located and restored using Pinnacle 9.6 software (Phillips Medical Systems, Andover, MA). These 320 patients were included in subgroup analysis with PROs as a covariate for validation of the PRO cut points in Aim 2. Planning CT DICOM files were exported into a deformable registration/segmentation software (Velocity AI 3.0.1, Velocity Medical Solutions, Atlanta, GA). Candidate dysphagia-related structures were auto-segmented using a custom region of interest (ROI) library, with dose volume histograms (DVHs) generated for the inferior, middle and superior pharyngeal constrictors (IPC, MPC, and SPC), anterior digastrics (ADM), intrinsic tongue muscles (ITM), mylo/geniohyoid complex (MHM), genioglossus (GGM), cricopharyngeus (CPM), submandibular (SM) and parotid glands (PG), larynx (LX), and esophagus (ESG).
Clinical variables
Clinical variables are listed in Table 1. Patients with unknown HPV status and no history of tobacco use were considered likely HPV positive for the purpose of this analysis. Baseline dysfunction included PEG tube dependence, inability to tolerate solid food, poor airway protection, or impaired mobility of the tongue, soft palate, or true vocal cord at diagnosis, as determined in the medical record by the treating providers.
Table 1:
Patient and disease characteristics, treatment details, and follow up data
| Proportion of patients by MDASI-HN-S | Proportion of patients by composite MDADI | ||||||
|---|---|---|---|---|---|---|---|
| All patients (%) | 0–5 | 6–10 | p value | <60 | ≥60 | p value | |
| All patients | 714 (100%) | 0.17 | 0.83 | N/A | 0.16 | 0.84 | N/A |
| Gender | 0.854 | 0.381 | |||||
| Male | 602 (84.3%) | 0.83 | 0.17 | 0.15 | 0.85 | ||
| Female | 112 (15.7%) | 0.84 | 0.16 | 0.19 | 0.81 | ||
| HPV* | 0.536 | 0.102 | |||||
| Negative | 217 (30.4%) | 0.82 | 0.18 | 0.19 | 0.81 | ||
| Positive | 497 (69.6%) | 0.84 | 0.16 | 0.15 | 0.85 | ||
| Smoking† | 0.190 | 0.036 | |||||
| No | 327 (45.8%) | 0.85 | 0.15 | 0.13 | 0.87 | ||
| Yes | 387 (54.2%) | 0.82 | 0.18 | 0.19 | 0.81 | ||
| Baseline dysfunction‡ | 0.008 | 0.001 | |||||
| No | 677 (94.8%) | 0.84 | 0.16 | 0.15 | 0.85 | ||
| Yes | 37 (5.2%) | 0.68 | 0.32 | 0.35 | 0.65 | ||
| Tumor subsite | 0.560 | 0.658 | |||||
| Tonsil | 326 (45.7%) | 0.85 | 0.15 | 0.15 | 0.85 | ||
| Base of tongue | 354 (49.6%) | 0.82 | 0.18 | 0.17 | 0.83 | ||
| Other | 34 (4.8%) | 0.82 | 0.18 | 0.15 | 0.85 | ||
| Tumor staging | <0.001 | <0.002 | |||||
| T1 | 257 (36.1%) | 0.86 | 0.14 | 0.11 | 0.89 | ||
| T2 | 280 (39.3%) | 0.86 | 0.14 | 0.13 | 0.87 | ||
| T3 | 106 (14.9%) | 0.79 | 0.21 | 0.21 | 0.79 | ||
| T4 | 69 (9.7%) | 0.65 | 0.35 | 0.38 | 0.62 | ||
| Nodal staging | 0.014 | 0.001 | |||||
| N0 | 65 (9.0%) | 0.78 | 0.22 | 0.19 | 0.81 | ||
| N1 | 100 (14.0%) | 0.85 | 0.15 | 0.11 | 0.89 | ||
| N2a | 89 (12.5%) | 0.79 | 0.21 | 0.11 | 0.89 | ||
| N2b | 319 (44.7%) | 0.88 | 0.12 | 0.13 | 0.87 | ||
| N2c | 120 (16.8%) | 0.76 | 0.24 | 0.26 | 0.74 | ||
| N3 | 22 (3.1%) | 0.77 | 0.23 | 0.36 | 0.64 | ||
| Radiation modality | <0.001 | <0.001 | |||||
| 3D-CRT | 42 (5.9%) | 0.52 | 0.48 | 0.45 | 0.55 | ||
| IMRT | 656 (91.9%) | 0.85 | 0.15 | 0.14 | 0.86 | ||
| Proton | 16 (2.2%) | 1.00 | 0.00 | 0.06 | 0.94 | ||
| Radiation schedule | |||||||
| Standard fractionation | 632 (90.5%) | 0.84 | 0.16 | 0.024 | 0.14 | 0.86 | 0.006 |
| Accelerated fractionation | 66 (9.5%) | 0.73 | 0.27 | 0.27 | 0.73 | ||
| Concurrent therapy | |||||||
| None | 340 (47.6%) | 0.89 | 0.11 | <0.001 | 0.12 | 0.88 | <0.001 |
| Platinum chemotherapy | 251 (35.2%) | 0.76 | 0.24 | 0.24 | 0.76 | ||
| Cetuximab | 123 (17.2%) | 0.84 | 0.16 | 0.10 | 0.89 | ||
| Induction chemotherapy | |||||||
| No | 487 (68.2%) | 0.83 | 0.17 | 0.541 | 0.14 | 0.86 | 0.010 |
| Yes | 227 (31.8%) | 0.85 | 0.15 | 0.21 | 0.79 | ||
| Salvage neck dissection | |||||||
| No | 540 (75.6%) | 0.83 | 0.17 | 0.815 | 0.15 | 0.85 | 0.315 |
| Yes | 174 (24.4%) | 0.84 | 0.16 | 0.18 | 0.82 | ||
| Feeding tube placement | |||||||
| No | 377 (52.8%) | 0.90 | 0.10 | <0.001 | 0.09 | 0.91 | <0.001 |
| Yes | 337 (47.2%) | 0.76 | 0.10 | 0.24 | 0.76 | ||
| Swallowing exercises§ | |||||||
| Partial or full adherence | 226 (48.7%) | 0.83 | 0.17 | 0.906 | 0.15 | 0.85 | 0.458 |
| Minimal or no adherence | 238 (51.3%) | 0.83 | 0.17 | 0.18 | 0.82 | ||
Patients with unknown HPV and no history of smoking considered HPV positive
Any history of smoking at time of diagnosis
Baseline dysfunction as reported by patient
Adherence assessed at time of follow up
Statistical analysis
Summary statistics, chi-squared, and t-tests were used to describe clinical characteristics. Correlations between MDASI-HN-S, composite MDADI, and EQ-VAS were assessed using Spearman’s correlation coefficient. The Bayesian information criteria (BIC) were used to test the discriminant ability of MDASI-HN-S and composite MDADI for QOL as measured by EQ-VAS. This method for use of BIC in predicting model performance has been described previously [14].
To identify the possible score of the MDASI-HN-S and composite MDADI at which a detrimental poor QOL could be reported, we used the modified Breiman recursive partitioning analysis (RPA). Training, test and validation sets for optimization of the MDASI-swallowing score RPA were conducted using MDASI-HN-S as a continuous variable. The RPA (decision tree-based partitioning) was performed with 20% verification “holdback” and a minimum split size of 15% per split/partition. A total of 571 patients were including in the training set and 143 in the validation set. Post hoc K-fold cross validation (n = 10) was performed to evaluate for over-fitting. Regression analysis was used to confirm the threshold effect size. The ROI mean dose differentials were then stratified by the derived MDASI-HN-S and composite MDADI scores cut-points as a measure of validity to represent dose-dependent thresholds of swallowing muscle (region) injury.
A p value ≤0.05 was considered to be statistically significant, with statistical tests based on a two-sided significance level. Data analysis was performed using STATA/IC statistical software (version 12.1: STATA, College Station, TX), MatLab (R2011a, Mathwork, Natick, MA), JMP (version 12Pro, SAS Institute, Cary, NC), and IBM SPSS (version 22.0, Chicago, IL).
RESULTS
Patient and treatment characteristics
A total of 714 survey respondents with a mean survival time at survey response of 6.7 years (range 1.5 years to 15.6 years) were included in this analysis. Patient and disease characteristics, treatment details, and follow up data are summarized in Tables 1 and 2.
Table 2:
Patient and disease characteristics, treatment details, and follow up data
| Mean value by MDASI-HN-S | Mean value by composite MDADI | ||||||
|---|---|---|---|---|---|---|---|
| 0–5 | 6–10 | p value | <60 | ≥60 | p value | ||
| Age (years) | |||||||
| Mean | 56.5 | 56.4 | 56.4 | 0.939 | 56.5 | 56.4 | 0.907 |
| Radiation dose (Gray) | |||||||
| Mean | 68.2 | 68.1 | 68.7 | 0.017 | 68.8 | 68.0 | 0.003 |
| BED with time | |||||||
| Mean | 55.3 | 54.5 | 58.6 | 0.085 | 59.7 | 54.3 | 0.003 |
| Radiation fractions | |||||||
| Mean | 32.5 | 32.3 | 33.3 | 0.001 | 33.5 | 32.3 | 0.000 |
| Time since treatment (years) | |||||||
| Mean | 6.7 | 6.6 | 7.4 | 0.026 | 7.4 | 6.6 | 0.027 |
| Weight change* | |||||||
| Mean | −7.2 | −6.2 | −11.8 | <0.001 | −10.9 | −6.5 | <0.001 |
| EQ-5D VAS | |||||||
| Mean | 80.2 | 83.5 | 63.4 | <0.001 | 64.5 | 83.2 | <0.001 |
*Weight change from diagnosis to time of survey
Patient-reported dysphagia per MDASI-HN-S and MDADI and QOL
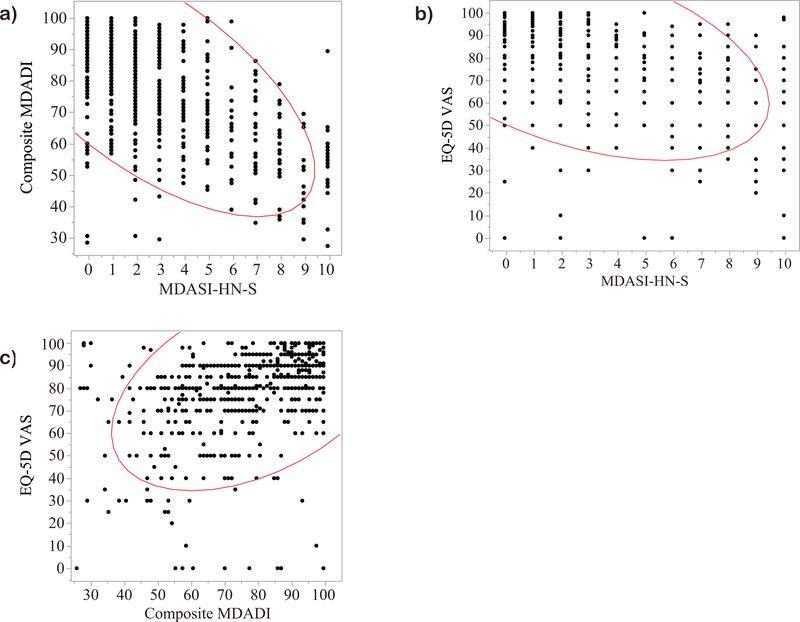
There was a strong inverse relationship between MDASI-HN-S and composite MDADI scores, indicating that both tools capture a similar symptom burden of dysphagia (Spearman’s p = −0.65, p<0.0001) (Figure 1a). Bivariate analyses showed an expected inverse relationship between EQ-VAS and MDASI-HN-S (Spearman’s p = −0.48, p<0.0001) (Figure 1b) and similarly a direct relationship between EQ-VAS and composite MDADI (Spearman’s p = 0.50, p<0.0001) with similar effect size between each dysphagia PRO and QOL (per EQ-VAS) (Figure 1c).
Figure 1:
Bivariate analyses demonstrating a) an inverse relationship between composite MDADI and MDASI-HN-S (Spearman’s p = p = −0.65, p<0.0001), b) an inverse relationship between EQ-5D VAS and MDASI-HN-S (Spearman’s p = −0.48, p<0.0001) and c) a positive relationship between EQ-5D VAS and composite MDADI (Spearman’s p = 0.50, p<0.0001).
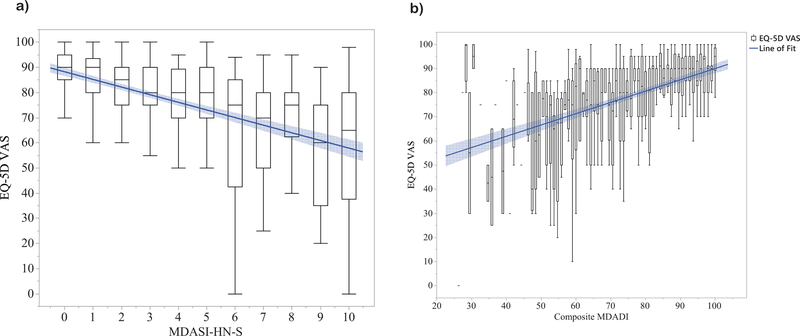
Using the Bayesian information criteria to assess performance of MDASI-HN-S and composite MDADI in predicting EQ-VAS, model performance was superior for MDASI-HN-S compared to MDADI (BIC of 6062 vs 6076). While the difference between MDASI-HN-S and MDADI BIC models is “very strong” (posterior probability >99%), the R-squared for these two models were similar, 0.21 and 0.19, respectively. Thus, while this data suggests improved parsimony for discrimination of QOL-altering swallowing dysfunction for MDASI-HN-S as compared to MDADI, dysphagia symptoms as captured by either tool significantly correlated with QOL scores in similar magnitude. Figure 2 displays EQ-VAS scores as boxplots stratified by MDASI-HN-S (0–10) and by composite MDADI (0–100) groupings with a line of best fit demonstrating that, in general, QOL declines with more significant self-reported dysphagia symptoms.
Figure 2:
EQ-5D VAS scores displayed as boxplots stratified by a) MDASI-HN-S and b) composite MDADI, with lines of best fit shown in blue.
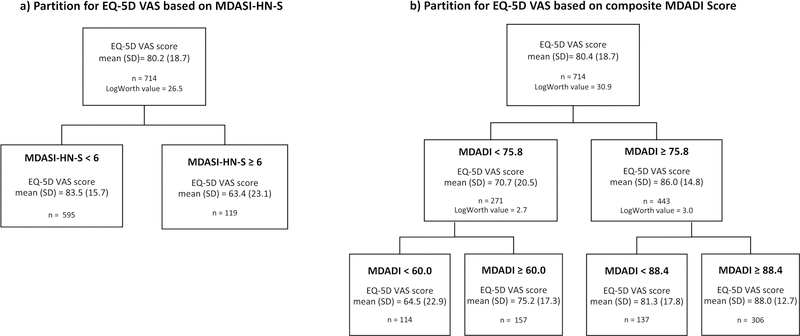
Swallowing PRO cut-point validation by QOL
Using the modified Breiman RPA, an MDASI-HN-S score of ≥6 was verified as the threshold for EQ-VAS decline in the training set (p<.0001)(Figure 3a). These results were maintained after 10-fold cross validation. Assessing this cut-point as a binary split across the entire cohort (n = 714) yielded mean (SD) EQ-VAS scores of 63.4 (23.1) vs. 83.5 (15.7) for those with MDASI-HN-swallowing scores ≥6 (n=119) vs. <6 (n=595), respectively (p < 0.001). A similarly designed modified Breiman RPA for EQ-VAS based on composite MDADI scores demonstrated MDADI cut-points of 60, 75.8, and 88.4 (p<.0001)(Figure 3b). Assessing these across the entire cohort (n=714) yielded mean (SD) EQ-VAS scores of 64.5 (22.9), 75.2 (17.3), 81.3 (17.8), and 88.0 (12.7) for those with composite MDADI scores of <60, ≥60 but <75.8, ≥75.8 but <88.4, and ≥88.4, respectively.
Figure 3:
Modified Breiman RPA for EQ-5D VAS score by dysphagia PRO, showing a) an MDASI-HN-S cut-point of 6 (p<0.0001) and b) composite MDADI cut-points of 60, 75.7, and 88.4 (p<0.0001)
Clinical covariate validation of swallowing PRO cut-points
Moderate/severe dysphagia was reported by 17% and 16% of respondents by MDASI-HN-S ≥6 and composite MDADI <60, respectively. In univariate analysis, patient demographic, disease, and treatment characteristics significantly associated with moderate/severe dysphagia by both MDASI-HN-S and by composite MDADI score included baseline swallowing dysfunction (p=0.008 and p=0.001 for MDASI-HN-S and composite MDADI, respectively), T4 tumor stage (p=<0.001 for both inventories), 3D-CRT (<0.001 for both inventories), accelerated fractionation schedule (p=0.024 and p=0.006), higher radiation dose (p=0.017 and p=0.003) and fraction number (p=0.001 and p<0.001), and concurrent platinum-based chemotherapy (p<0.001 for both inventories). Those with unfavorable MDASI-HN-S and composite MDADI scores displayed a higher rate of feeding tube placement and more significant mean weight loss from baseline to survey response.
Dosimetric validation of PRO thresholds
Moderate/severe symptoms by MDASI-HN-S ≥6 was significantly associated with a higher mean dose to the contralateral ADM (p=0.019), ITM (p=0.035), and LX (p =0.032) (Table3). Similarly, moderate/severe dysphagia by MDADI score < 60 was significantly associated with a higher mean dose to the contralateral ADM (<0.001), ITM (p=0.003), MHM (p=0.028), ESG (p=0.038), LX (p=0.005), and contralateral SM (p=0.010).
DISCUSSION
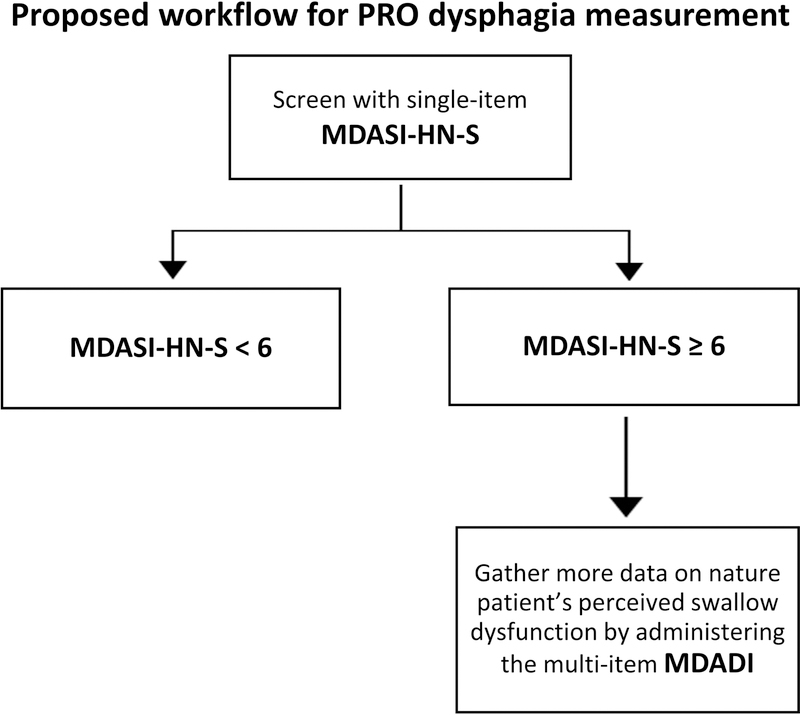
This report demonstrates that patient-reported dysphagia as captured by the single item MDASI-HN-S (from a multi-symptom inventory) performs favorably in distinguishing QOL-altering dysphagia symptoms as compared to the widely used 19-item dysphagia-specific MDADI inventory. Both MDASI-HN-S and composite MDADI models, however, correlated with EQ-VAS scores and similar clinical/dosimetric factors, suggesting that dysphagia symptoms as captured by either inventory are valid measures. Indeed, these results should not imply that one inventory universally replace the other. Rather, they demonstrate the potential role of the single-item MDASI-HN-S as a time-efficient initial screening tool for QOL-altering dysphagia. For patients with an MDASI-HN-S score ≥ 6, MDADI may then be used to explore with greater detail and granularity the patient’s swallowing symptoms (Figure 4).
Figure 4:
Proposed workflow to capture QOL-altering dysphagia in clinic using MDASI-HN-S and MDADI.
With a median follow-up of nearly 7 years, this study highlights that greater than 15% of patients may experience long-term QOL-altering dysfunction in swallowing following treatment. While not analyzed longitudinally in this study, patients have previously demonstrated marked worsening in swallowing function at 6 months to 1 year following definitive IMRT for OPC, with partial recovery by 2 years [16]. By limiting our cohort to those who completed treatment 1 year or more prior, we largely exclude those with acute or sub-acute symptoms of dysphagia that may recover. In our cohort, patients reporting moderate/severe dysphagia tended to have a longer time since treatment interval, which may suggest continued and persistent deterioration in function many years after treatment, or be a surrogate for older and less conformal treatments techniques.
MDASI-HN is a compelling candidate as a screening tool in patients with OPC following RT for reasons beyond only swallowing. It was designed and validated with the aim to assess, in a single instrument, the burden of 22 distinct symptoms specific to oncology patients and HNC treatment [8]. Further, previous analyses similar to the present study have shown single-item MDASI-HN questions to perform favorably to multi-item inventories in predicting the severity of radiation-induced mucositis and xerostomia (compared to the Functional Assessment of Cancer Therapy—Head and Neck (FACT—HN) module and multi-question Xerostomia Questionnaire (XQ), respectively) [14, 21]. Routine use of MDASI-HN following RT for OPC may thus accurately and efficiently screen for an array of common symptoms, after which patients may be triaged to more detailed questionnaires, clinical referrals, imaging tests, or other work up as needed.
If MDASI-HN is to be used as a routine screening tool, clinically relevant single-item cut-points must be established. While these have been explored for other symptoms of MDASI-HN (dry mouth score of ≥6 for xerostomia [14]), a clinically meaningful MDASI-HN-S cut-off point has, to the best of our knowledge, not yet been validated. As no consensus cut-point for MDASI-HN-S has previously been identified, a value of ≥6 to represent moderate/severe symptoms was chosen based on published data of optimal cut-points from composite and other single item MDASI-HN items [14, 15]. By RPA, an MDASI-HN-S cut-point of ≥ 6 was validated to best distinguish QOL-altering dysphagia symptoms as measured by EQ-VAS. Regarding MDADI, a score of 60 has previously been proposed as a clinically meaningful cut-off point [16, 22] and is used as an endpoint for patient reported swallowing dysfunction in NRG HN-002, a recent randomized trial of IMRT in OPC [23]. These findings support this cut-point as RPA confirmed an MDADI score of less than 60 to be associated with worse QOL by EQ-VAS. Two additional MDADI cut-points of 75.8 and 88.4 were found to distinguish QOL, suggesting that further partitioning of MDADI may be of clinical relevance. In a subset analysis exploring dose/PRO correlates, several structures of swallowing were found to correlate with moderate-severe dysphagia by both MDASI-HN-S and composite MDADI per the pre-specified cut-off points of ≥6 and <60, respectively. These relationships further support the validity of the proposed MDADI and MDASI-HN-S cut-points as well the use of self-reported swallowing outcomes as a dose-dependent measure of RT toxicity.
This study highlights a wide EQ-VAS differential by PRO dysphagia scores (an absolute difference of more than 20 EQ-VAS points between patients scoring <6 vs ≥6 on MDASI-HN-S). While there is an abundance of literature on the clinical relevance and meaning of EQ-VAS scores, there exists no consensus interpretation. Studies, however, consistently demonstrate an association between EQ-VAS and the five EQ-5D domains (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) [24, 25]. Thus, while it is difficulty to encapsulate a person’s perceived quality of life in a single number, such wide differences in EQ-VAS may suggest meaningful differences in overall health, independence in daily activities, and psychosocial functioning. These survey results, therefore, further highlight the large impact of dysphagia symptoms on overall QOL in OPC survivorship.
Several limitations exist in this study, including those inherent to secondary analysis of a cross-sectional survey from a single cancer center, including lack of longitudinal follow up. Second, while this series includes over 700 patients, only 320 DICOM files were attainable for review to correlate PRO responses with dose. Third, dysphagia was determined in this study solely on basis of patient reported outcomes. While an objective measurement such as modified barium swallow or endoscopy may have offered additional useful information, these were not available in a similar time to the survey response. Moreover, we analyzed MDASI-HN-S out of context from its original place as only part of a larger inventory. It is thus possible that responses to a stand-alone single dysphagia would differ than what is observed here. Fourth, while we intended to allow all RT types into this study to enhance generalizability, due to institutional practices in the study period, the large majority received IMRT.
In conclusion, this comparison of a single-item dysphagia question from the multi-symptom MDASI-HN to the dysphagia-specific 20-item MDADI instrument in over 700 patients and with a mean follow up of nearly 7 years marks, to the best of our knowledge, the largest study measuring long-term self-reported swallowing dysfunction following RT for OPC. These results show that although the single item MDASI-HN-S performed slightly better in predicting QOL as compared to the multi-item MDADI score, both models successfully captured QOL-altering dysphagia. Moreover, an MDASI-HN-S score of 6 or greater and an MDADI score less than 60 were associated with worse QOL scores, known clinical risk factors for dysphagia, and higher swallowing region RT dose. A model in which MDADI is reserved for patients scoring ≥6 on MDASI-HN-S may thus be used a time-efficient method to effectively capture patients experiencing QOL-altering dysphagia symptoms following RT for OPC. As additional single item cut-points are explored and validated in future studies, MDASI-HN may become an increasingly valuable clinical tool.
Table 3:
Normal tissue dose by swallowing inventory score
| Mean dose by MDASI-HN-S | Mean dose by composite MDADI | ||||||
|---|---|---|---|---|---|---|---|
| 0–5 | 6–10 | p value | <60 | ≥60 | p value | ||
| Inferior pharyngeal constrictors | 30.9 | 34.8 | 0.160 | 35.8 | 30.8 | 0.076 | |
| Middle pharyngeal constrictors | 56.5 | 57.1 | 0.756 | 57.8 | 56.4 | 0.540 | |
| Superior pharyngeal constrictors | 61.3 | 64.2 | 0.056 | 64.3 | 61.3 | 0.054 | |
| Ipsilateral anterior digastric | 57.2 | 58.5 | 0.413 | 60.1 | 57.0 | 0.060 | |
| Contralateral anterior digastric | 45.3 | 51.2 | 0.019 | 53.7 | 45.1 | <0.001 | |
| Intrinsic tongue muscles | 54.3 | 58.2 | 0.035 | 59.7 | 54.2 | 0.003 | |
| Mylogeniohyoid | 48.3 | 53.3 | 0.095 | 54.8 | 48.1 | 0.028 | |
| Genioglossus | 58.7 | 60.7 | 0.293 | 62.0 | 58.6 | 0.066 | |
| Cricopharyngeal muscle | 18.0 | 19.9 | 0.415 | 20.7 | 17.9 | 0.254 | |
| Ipsilateral parotid gland | 35.6 | 37.9 | 0.253 | 38.1 | 35.6 | 0.342 | |
| Contralateral parotid gland | 20.2 | 24.0 | 0.052 | 24.4 | 20.2 | 0.057 | |
| Ipsilateral submandibular gland | 68.5 | 68.9 | 0.892 | 69.7 | 68.4 | 0.074 | |
| Contralateral submandibular gland | 55.1 | 55.9 | 0.133 | 57.7 | 49.9 | 0.010 | |
| Larynx | 25.5 | 29.9 | 0.032 | 30.1 | 25.4 | 0.005 | |
| Esophagus | 26.8 | 29.0 | 0.277 | 30.8 | 26.6 | 0.038 | |
Acknowledgments
Funding sources and financial disclosures: This research is supported by the Andrew Sabin Family Foundation; Dr. Fuller is a Sabin Family Foundation Fellow. Dr. Fuller receives funding and salary support from the National Institutes of Health (NIH), including: the National Institute for Dental and Craniofacial Research Award (1R01DE025248–01/R56DE025248–01); a National Science Foundation (NSF), Division of Mathematical Sciences, Joint NIH/NSF Initiative on Quantitative Approaches to Biomedical Big Data (QuBBD) Grant (NSF 1557679); the NIH Big Data to Knowledge (BD2K) Program of the National Cancer Institute (NCI) Early Stage Development of Technologies in Biomedical Computing, Informatics, and Big Data Science Award (1R01CA214825–01); NCI Early Phase Clinical Trials in Imaging and Image-Guided Interventions Program (1R01CA218148–01); an NIH/NCI Cancer Center Support Grant (CCSG) Pilot Research Program Award from the UT MD Anderson CCSG Radiation Oncology and Cancer Imaging Program (P30CA016672) and an NIH/NCI Head and Neck Specialized Programs of Research Excellence (SPORE) Developmental Research Program Award (P50 CA097007–10). Dr. Fuller has received direct industry grant support and travel funding from Elekta AB.
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest.
REFERENCES
- 1.Gillison ML, et al. , Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J Clin Oncol, 2015. 33(29): p. 3235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goepfert RP, et al. , Symptom burden as a driver of decisional regret in long-term oropharyngeal carcinoma survivors. Head Neck, 2017. 39(11): p. 2151–2158. [DOI] [PubMed] [Google Scholar]
- 3.Hunter KU, et al. , Toxicities affecting quality of life after chemo-IMRT of oropharyngeal cancer: prospective study of patient-reported, observer-rated, and objective outcomes. Int J Radiat Oncol Biol Phys, 2013. 85(4): p. 935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langendijk JA, et al. , Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol, 2008. 26(22): p. 3770–6. [DOI] [PubMed] [Google Scholar]
- 5.Nutting CM, et al. , Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol, 2011. 12(2): p. 127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutcheson KA, et al. , Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer, 2012. 118(23): p. 5793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshpande PR, et al. , Patient-reported outcomes: A new era in clinical research. Perspect Clin Res, 2011. 2(4): p. 137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenthal DI, et al. , Measuring head and neck cancer symptom burden: the development and validation of the M. D. Anderson symptom inventory, head and neck module. Head Neck, 2007. 29(10): p. 923–31. [DOI] [PubMed] [Google Scholar]
- 9.Bjordal K, et al. , Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol, 1999. 17(3): p. 1008–19. [DOI] [PubMed] [Google Scholar]
- 10.Murphy BA, et al. , Reliability and validity of the Vanderbilt Head and Neck Symptom Survey: a tool to assess symptom burden in patients treated with chemoradiation. Head Neck, 2010. 32(1): p. 26–37. [DOI] [PubMed] [Google Scholar]
- 11.Chen AY, et al. , The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg, 2001. 127(7): p. 870–6. [PubMed] [Google Scholar]
- 12.McHorney CA, et al. , The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: I. Conceptual foundation and item development. Dysphagia, 2000. 15(3): p. 115–21. [DOI] [PubMed] [Google Scholar]
- 13.Dillman DA, Smyth JD, and Christian LM, Internet, phone, mail, and mixed-mode surveys : the tailored design method 4th edition. ed. 2014, Hoboken: Wiley; xvii, 509 pages. [Google Scholar]
- 14.Kamal M, et al. , Patient reported dry mouth: Instrument comparison and model performance for correlation with quality of life in head and neck cancer survivors. Radiother Oncol, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eraj SA, et al. , Long-term patient reported outcomes following radiation therapy for oropharyngeal cancer: cross-sectional assessment of a prospective symptom survey in patients >/=65 years old. Radiat Oncol, 2017. 12(1): p. 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goepfert RP, et al. , Long-Term, Prospective Performance of the MD Anderson Dysphagia Inventory in “Low-Intermediate Risk” Oropharyngeal Carcinoma After Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys, 2017. 97(4): p. 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen PH, et al. , Prevalence of perceived dysphagia and quality-of-life impairment in a geriatric population. Dysphagia, 2009. 24(1): p. 1–6. [DOI] [PubMed] [Google Scholar]
- 18.Bhide SA, et al. , Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol, 2009. 93(3): p. 539–44. [DOI] [PubMed] [Google Scholar]
- 19.Balestroni G and Bertolotti G, [EuroQol-5D (EQ-5D): an instrument for measuring quality of life]. Monaldi Arch Chest Dis, 2012. 78(3): p. 155–9. [DOI] [PubMed] [Google Scholar]
- 20.Garden AS, et al. , Patterns of disease recurrence following treatment of oropharyngeal cancer with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys, 2013. 85(4): p. 941–7. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal DI, et al. , The M. D. Anderson symptom inventory-head and neck module, a patient-reported outcome instrument, accurately predicts the severity of radiation-induced mucositis. Int J Radiat Oncol Biol Phys, 2008. 72(5): p. 1355–61. [DOI] [PubMed] [Google Scholar]
- 22.Hutcheson KA, et al. , What is a clinically relevant difference in MDADI scores between groups of head and neck cancer patients? Laryngoscope, 2016. 126(5): p. 1108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NRG Oncology. Reduced-Dose Intensity-Modulated Radiation Therapy With or Without Cisplatin in Treating Patients With Advanced Oropharyngeal Cancer Available from: https://clinicaltrials.gov/ct2/show/NCT02254278.
- 24.Jelsma J and Ferguson G, The determinants of self-reported health-related quality of life in a culturally and socially diverse South African community. Bull World Health Organ, 2004. 82(3): p. 206–12. [PMC free article] [PubMed] [Google Scholar]
- 25.Whynes DK, Does the correspondence between EQ-5D health state description and VAS score vary by medical condition? Health Qual Life Outcomes, 2013. 11: p. 155. [DOI] [PMC free article] [PubMed] [Google Scholar]