Abstract
Purpose:
The purpose of this study was to investigate how patient-reported knee function changed over a 2-year period in young athletes after anterior cruciate ligament reconstruction (ACLR) and return-to-sport (RTS), and to determine the impact of clinical measures, after controlling for demographic and surgical covariates.
Methods:
At the time of RTS after primary, unilateral ACLR, the following data were collected in 67 young athletes: Quadriceps (QF), hamstring (HS), and hip abduction (HA) strength; knee range-of-motion, effusion, and anterior laxity; and patient-reported function using the Knee injury and Osteoarthritis Outcome Score (KOOS). At 2 years post-RTS, patient-reported function was reevaluated using the KOOS. Absolute KOOS scores and proportions of participants meeting functional recovery cutoffs were compared between time-points. Multivariable linear regression was used to determine clinical measures at RTS associated with 2-year post-RTS KOOS scores.
Results:
KOOS scores for all subscales were higher at 2 years post-RTS (all p<0.003), and the proportions of participants demonstrating functional recovery were higher at 2 years post-RTS for the KOOS-Symptoms, KOOS-Sport, KOOS-QOL, and all KOOS subscales combined (all p<0.03). After controlling for graft type, clinical measures at RTS associated with higher 2-year post-RTS KOOS scores were: KOOS-Pain (lower HA peak torque); KOOS-Symptoms (higher QF strength symmetry and higher QF peak torque); and KOOS-ADL (lower HA peak torque).
Conclusions:
In this cohort, after controlling for graft type, higher QF strength symmetry, higher involved-limb QF peak torque, and lower involved-limb HA peak torque from the time of RTS were associated with higher function at 2 years post-RTS.
Keywords: ACL Reconstruction, Function, Outcomes
1. INTRODUCTION
Anterior cruciate ligament (ACL) ruptures are devastating injuries that commonly occur in athletes participating in cutting and pivoting sports.[7, 17] While some debate exists regarding the optimal treatment for individuals with ACL injuries,[39] ACL reconstruction (ACLR) is often performed in an effort to restore knee stability.[4, 7] During rehabilitation and at the time of return-to-sport (RTS) clearance after the completion of rehabilitation after ACLR, musculoskeletal impairments are common.[16, 29, 36, 47] Specifically, reported impairments often include deficits in knee range-of-motion (ROM),[36] the presence of knee joint effusion,[29] knee joint anterior laxity,[16] deficits in quadriceps femoris (QF) muscle strength,[47] deficits in hamstring (HS) muscle strength,[53] and deficits in hip muscle strength.[33]
In addition to the presence of musculoskeletal impairments, suboptimal patient-reported knee-related function is observed early after ACLR and over time after the completion of formal rehabilitation. Several patient-reported measures have been validated to measure knee function in individuals after ACLR,[20, 21, 41] and among the most widely used is the Knee injury and Osteoarthritis Outcome Score (KOOS), a self-reported questionnaire with individual subscales evaluating different constructs of knee function.[41, 42, 44] At the time of RTS after ACLR, young athletes (high-school aged; average: 17.4 years old) with and without QF strength asymmetry reported mean KOOS subscale scores ranging from 68.4 to 98.2 (out of a maximum score of 100), with lowest scores for both groups in the KOOS-Sport (79.6, 89.5, respectively) and KOOS-Quality of Life (68.4, 74.4, respectively) subscales.[23] Additionally, several studies have demonstrated that poor self-reported knee function measured by the KOOS persists and may worsen over time for some individuals.[5, 54] At 2 years after ACLR, national registry data showed that nearly 30% of individuals report a KOOS-QOL subscale score of less than 44.[5] In a separate multi-center study, 12% of individuals reported greater than a 10-point worsening in KOOS-Pain scores from 2 years to 6 years post-ACLR.[54]
Several factors after ACLR have been evaluated for their association with longitudinal patient-reported function at various time points, including surgical factors, injury-specific factors, patient and demographic factors, and clinical measures.[12, 49] Surgical factors that have been shown to be associated with lower patient-reported function over time after ACLR include the use of an allograft during ACLR, undergoing menisectomy or meniscus repair, undergoing revision ACLR, and a smaller HS autograft size.[15, 24, 49] Injury-specific factors associated with lower patient-reported function over time after ACLR include the presence of an articular cartilage injury or a meniscus injury.[12, 24, 50] Patient and demographic factors associated with lower patient-reported function over time after ACLR include female sex, higher body mass index, a history of smoking, lower education levels, and weight gain.[12, 15, 24, 49, 50] However, far fewer studies have examined the impact of clinical measures.[35]
Previous research has demonstrated that graft ligamentization and strength recovery often take 2 years or more post-ACLR.[31, 38, 40, 43] Due to the overall lack of knee homeostasis during this time period, understanding functional recovery is important. In addition, given the prevalence of suboptimal knee function in individuals over time after RTS post-ACLR, it is critical to identify clinical measures associated with longitudinal function that may be potentially addressed during post-surgical rehabilitation and considered during plan of care decision-making. However, currently, it is not well understood how knee function changes over time after RTS post-ACLR in young athletes or the impact of clinical measures on knee function. The purpose of this study was to longitudinally investigate how patient-reported knee function changed over the 2 years post-RTS in a cohort of young, active individuals after ACLR, and determine the impact of clinical measures at RTS on patient-reported knee function 2 years later. The primary hypotheses tested were that patient-reported knee function would improve from the time of RTS to 2 years post-RTS, and that, after controlling for important demographic and surgical factors, higher QF strength at RTS, not knee laxity, range of motion or swelling, would be associated with higher patient-reported knee function at 2 years post-RTS.
2. MATERIAL AND METHODS
2.1. Participants.
Participants were included from the larger ACL REconstruction Long-term outcomes in Adolescents and Young adults (ACL-RELAY) Study. The ACL-RELAY study is a prospective, longitudinal cohort study evaluating outcomes after ACLR in young, active individuals (age range 9-25 years at RTS). The ACL-RELAY study collects clinical, functional, biomechanical, and injury data beginning at the time of RTS (baseline visit) through a final visit at 2 years post-RTS. Participants in the ACL-RELAY study are recruited from orthopaedic surgeon practices and physical therapy clinics in the greater Cincinnati/Northern Kentucky (USA) areas. To be included, potential participants must have been previously cleared to RTS by their treating orthopaedic surgeon and rehabilitation specialist and plan to return to regular participation in cutting and pivoting sports (>50 hours per year). Baseline testing at the time of RTS takes place within 4 weeks of each participant’s RTS clearance. Neither rehabilitation nor the decision of RTS clearance (including whether RTS criteria are used) are controlled by the ACL-RELAY study. Participants are included with a variety of graft types, including HS tendon autograft, bone-patellar tendon-bone autograft, and allograft. Potential participants are excluded from the ACL-RELAY study if they have a history of low back pain or lower extremity injury or surgery in either limb (other than their primary ACL injury) requiring the care of a physician in the past year. Additionally, potential participants are excluded from the ACL-RELAY study if they sustained a concomitant knee ligament injury (beyond grade 1 medial collateral ligament injury) with their ACL injury.
Participants in the current work were a subset of individuals from the ACL-RELAY study recruited between 2007 and 2015 and were required to have undergone only primary, unilateral ACLR and to have completed testing at RTS and 2 years after RTS. Participants in the current work were excluded from primary analyses if they sustained a second ACL injury in either limb following testing at RTS. All participants provided informed consent or parental permission and assent (when younger than 18 years old) prior to participation in the ACL-RELAY study. All study procedures were reviewed and approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board.
2.2. Data Collected at the Return-to-Sport (RTS) Study Visit.
At the RTS study visit, clinical measures including QF and HS muscle strength, hip abduction muscle strength, knee range of motion (ROM), knee effusion, and knee laxity were collected.
2.2.1. Muscle strength.
An isokinetic dynamometer (Biodex Medical Systems; Shirley, NY) was used to quantify QF and HS muscle strength at the RTS visit, with the patient positioned in the dynamometer as described previously.[23, 46, 47] Isometric strength testing of the QF muscles was performed during a maximum contraction with the knee at 60° of flexion, maintaining the contraction for 5 seconds.[46, 47] Isokinetic strength testing of the QF and HS muscles was also performed at 180°/second and 300°/second from 90° of knee flexion to full extension.[13, 26] Subjects performed 1 practice trial for each condition on each limb to minimize any learning effect. Subjects then performed 3 trials of isometric testing, 5 trials at 180°/second, and 10 trials at 300°/second on each limb, with verbal encouragement to facilitate maximal effort. The uninvolved limb was always tested first, as this is common in clinical practice. From these trials, peak torque values were calculated for each condition for the involved and uninvolved limbs. Each peak torque value was then normalized to the participant’s body mass (Nm/kg). Measures of symmetry (quantified as LSI) are commonly used to evaluate rehabilitation progression and return-to-activity decision-making after ACLR in clinical practice.[4, 30] As such, LSI were calculated for QF and HS strength measures using Formula 1 below:
| (Formula 1): |
Isometric and isokinetic QF and HS strength testing in this manner demonstrates good reliability in individuals after ACL injury and ACLR, and is able to differentiate asymmetries in strength between involved and uninvolved limbs.[9, 47] Additionally, the literature recommends testing strength after ACLR using both isometric testing and isokinetic testing at varying speeds to assess different muscle performance characteristics of the QF and HS musculature.[13, 26]
To measure hip abduction strength, participants stood on the non-testing limb, positioned in front of an isokinetic dynamometer (Biodex Medical Systems; Shirley, NY) as previously described.[8, 11] Participants placed both hands on top of the dynamometer head for balance and were instructed to avoid excess trunk movement. Following 5 practice trials, participants performed 5 recorded trials at 120°/second, with verbal encouragement to facilitate maximal effort.[8, 11] Peak torque from the 5 trials was recorded for the involved and uninvolved limbs and normalized to body mass (Nm/kg). LSI were calculated for hip abduction peak torques similar to QF and HS strength measures (Formula 1).
2.2.2. Other clinical, demographic, and surgical data.
Knee laxity (mm) was measured using a CompuKT-2000 knee ligament arthrometer (Medmetric Corp.; San Diego, CA) in the anterior direction using 30 pounds of force.[34] Previous work has reported good to excellent reliability assessing anterior laxity using these methods.[34] Knee flexion and extension ROM (degrees) were measured using a goniometer. Knee effusion was evaluated using the stroke test[51] and was documented as either present (score>trace) or absent (score=zero). This assessment of knee effusion demonstrates good reliability.[51] Measures of knee anterior laxity, ROM, and effusion were performed by two experienced sports physical therapists. Pre-injury activity level was evaluated using the Tegner activity scale.[52] Operative reports were reviewed to determine the presence of meniscus injury at the time of ACLR, graft type used during ACLR, and pediatric ACLR modification.
2.3. Data Collected at the RTS and 2 Years Post-RTS Study Visits.
During both study visits, self-reported knee function was assessed using the KOOS. The KOOS is comprised of five subscales covering dimensions including knee pain (KOOS-Pain), knee symptoms (KOOS-Symptoms), activities of daily living (KOOS-ADL), sport and recreational activities (KOOS-Sport), and knee-related quality of life (KOOS-QOL). Questions are scored on a 0-4 scale, each subscale is scored independently, and subscale scores are transformed to a 0-100 score, with 100 indicating no knee-related issues.[41, 42] The KOOS is a valid, reliable, and responsive measure in individuals following ACLR, the minimal detectable change (MDC) ranges from 6.1 to 8.5 points, and the minimal clinically important difference (MCID) values have been suggested to range from 8 to 10 points, depending on the subscale.[41, 42, 44]
2.4. Statistical Analyses.
Statistical analyses were performed using SPSS (Version 22.0; IBM SPSS Statistics; Armonk, NY) and STATA (Version 14.0; StataCorp; College Station, TX) software.
2.4.1. Change in Knee Function Scores over 2 Years Post-RTS.
KOOS subscale scores at RTS and 2 years post-RTS were compared using Wilcoxon Signed Rank tests. Additionally, KOOS subscale change scores were calculated as: (2-year post-RTS KOOS score – RTS KOOS score). KOOS subscale scores were also dichotomized based on whether participants met cutoffs representing “functional recovery” at both RTS and 2 years post-RTS, as determined from previous work using national registry data.[5] Specific cutoffs for each subscale were as follows: >90 for KOOS-Pain, >84 for KOOS-Symptoms, >91 for KOOS-ADL, >80 for KOOS-Sport, and >81 for KOOS-QOL.[5] The proportions of the cohort that fell above or below these individual and combined functional recovery cutoffs were compared between the time points using McNemar’s tests.
2.4.2. RTS Factors Associated with 2 Year Post-RTS Knee Function.
A linear regression model building approach was used to determine factors from the time of RTS associated with KOOS subscale scores at 2 years post-RTS. Potential RTS independent variables included clinical measures of interest (left column of Table 1). Additionally, demographic and surgical factors were considered as potential covariates during model building (right column of Table 1). To perform model-building for dependent variables of interest (each 2-year post-RTS KOOS subscale score), univariable linear regression models were first fit with each surgical or demographic factor to identify potential covariates to be included in the final models (right column of Table 1). Demographic and surgical factors were considered covariates if their p value was <0.15. These covariates were then forced into the subsequent multivariable models. Following the identification and inclusion of covariates, multivariable linear regression was performed for each 2-year KOOS subscale score. Separate models were fit for each individual RTS clinical measure (left column of Table 1) as independent variables. Multi-collinearity, model diagnostics (linearity; normality; equal variance), and influential observations were then assessed for each final model.
Table 1.
Potential RTS Factors as Independent Variables for Model-Building
| Potential Pool of Independent Variables | |
|---|---|
|
Clinical measures at RTS: • QF strength (isometric and isokinetic; LSI; involved limb peak torque) • HS strength (isometric and isokinetic; LSI; involved limb peak torque) • Hip abduction strength (LSI; involved limb peak torque) • Involved knee ROM (flexion,extension) • Involved knee effusion (0,1) • Involved knee laxity (involved knee,30 pounds) |
Potential demographic and surgical covariates: • Age at RTS • Gender (0,1) • Meniscus injury (0,1) • ACLR graft type (dummy coded) • BMI • Time from ACLR to RTS (time in rehab) • Pediatric ACLR modification (0,1) • Pre-injury activity level |
QF – quadriceps femoris; LSI – limb symmetry index; HS – hamstring; ROM – range of motion; ACLR – anterior cruciate ligament reconstruction; BMI – body mass index
2.4.3. Power Calcuation.
A post-hoc sample size calculation was performed and indicated that, with the 67 included participants and an α level of 0.05, using multiple linear regression analyses (including up to 6 predictors in any final model), 94.2% power was achieved (G Power v 3.1).[19] The effect size was based on a desired correlation coefficient of r=0.5 (r2=0.25).
3. RESULTS
3.1. Demographic Data.
Sixty-seven young, active individuals after ACLR participated at the time of RTS and 2 years post-RTS. Demographic data from the time of RTS for the cohort are shown in Table 2.
Table 2.
Demographic Data at RTS for the Cohort (n=67)
| Variable | |
|---|---|
| Age at RTS*, years | 16.9 ± 3.4 |
| Time from ACLR to Testing Visit at Time of RTS*, months | 7.8 ± 2.1 |
| Tegner Activity Score* | 8.7 ± 1.0 |
| BMI* | 23.9 ± 4.3 |
| Gender, n (percentage) |
46 F (69%) 21 M (31%) |
| Graft Type, n (percentage) |
41 HS (61%) 21 PT (31%) 5 ALLO (8%) |
| Meniscus Injury, n (percentage) |
35 yes (52%) 32 no (48%) |
| Pediatric ACLR Modification, n (percentage) |
8 yes (12%) 59 no (88%) |
Data are presented as mean ± standard deviation; ACLR – anterior cruciate ligament reconstruction; RTS – return-to-sport; BMI – body mass index; F – female; M – male; HS – hamstring autograft; PT – bone-patellar tendon-bone autograft; ALLO – allograft
3.2. Change in Knee Function.
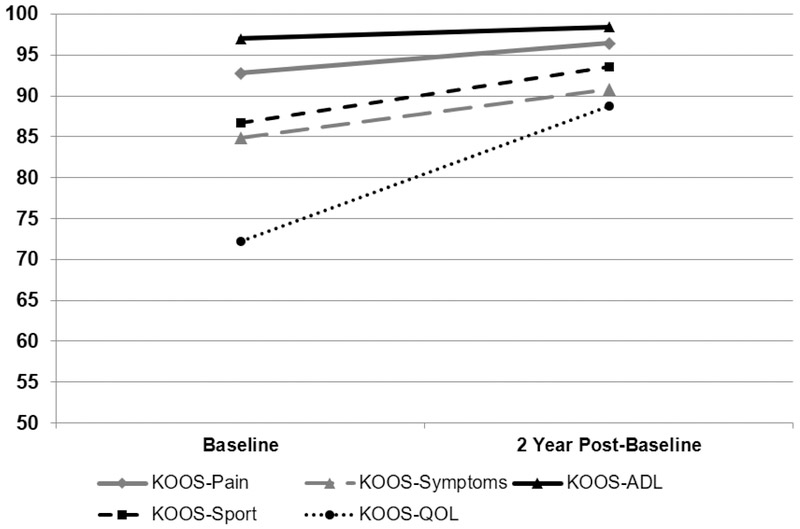
KOOS subscale scores were higher at 2 years post-RTS compared to RTS scores for all KOOS subscales (Wilcoxen Signed Rank tests: all p<0.003; Figure 1). Proportions of participants meeting functional recovery cutoffs at the time of RTS and 2 years post-RTS are shown in Table 3 for each KOOS subscale and all subscales combined. For change scores over the 2 years post-RTS, KOOS scores increased in 53%, 59%, 40%, 51%, and 76% of participants for the KOOS-Pain, KOOS-Symptoms, KOOS-ADL, KOOS-Sport, and KOOS-QOL, respectively.
Figure 1.
Comparison of KOOS Subscale Scores at RTS and 2 Years Post-RTS (n=67)
Data are mean values for the cohort (n=67); KOOS – Knee Injury and Osteoarthritis Outcome Score; ADL – activities of daily living; QOL – quality of life
Table 3.
Comparison of Proportions Meeting KOOS Functional Recovery Cutoffs at the Time of RTS and 2 Years Post-RTS (n=67)
| Variable | RTS | 2 Years Post-RTS | P-value* |
|---|---|---|---|
| KOOS-Pain | 73.1% (n=49) | 86.6% (n=58) | 0.057 |
| KOOS-Symptoms | 59.7% (n=40) | 77.6% (n=52) | 0.027 |
| KOOS-ADL | 86.6% (n=58) | 95.5% (n=64) | 0.063 |
| KOOS-Sport | 65.7% (n=44) | 83.6% (n=56) | 0.003 |
| KOOS-QOL | 26.9% (n=18) | 71.6% (n=48) | <0.001 |
| All KOOS Subscales | 25.4% (n=17) | 59.7% (n=40) | 0.024 |
KOOS – Knee Injury and Osteoarthritis Outcome Score; ADL – activities of daily living; QOL – quality of life; RTS – return-to-sport;
P-value from McNemar’s test
3.3. Regression Results.
Due to small group size, individuals with allograft ACLR (n=5) were excluded from regression analyses. Graft type was coded as 0 (HS autograft) and 1 (bone-patellar tendon-bone autograft). Graft type was the only significant potential demographic or surgical covariate identified. Specifically, HS autograft ACLR was associated with higher 2-year post-RTS function for the KOOS-Pain, KOOS-Symptoms, KOOS-ADL, and KOOS-QOL (Table 4). After controlling for graft type in the final models, clinical measures at RTS associated with higher 2-year post-RTS function were as follows for each KOOS subscale final multivariable model: KOOS-Pain (lower involved limb hip abduction strength; final model R2 = 13.9%); KOOS-Symptoms (Model 1: higher isokinetic QF strength symmetry at 300°/second; final model R2 = 14.3%; Model 2: higher involved limb isokinetic QF peak torque at 300°/second; final model R2 = 10.9%); and KOOS-ADL (lower involved limb hip abduction strength; final model R2 = 12.5%) (Table 4). There were no RTS factors (clinical measures or covariates) associated with KOOS-Sport scores at 2 years post-RTS.
Table 4.
Multivariable Regression Results for 2-Year Post-RTS KOOS Scores
| Predictor Variables | Beta Coefficients (95% CI) |
P Value | Model R2 Value | |
|---|---|---|---|---|
| 2-Year KOOS-Pain | -Graft Type (1=PT, 0=HS) | −3.10 (−6.10, −0.10) | 0.043 | 13.9% |
| -Involved limb hip abduction strength | −8.86 (−17.45, −0.27) | 0.043 | ||
| 2-Year KOOS-Symptoms |
Model 1: -Graft Type (1=PT, 0=HS) |
−7.78 (−13.67, −1.89) | 0.011 | 14.3% |
| -Isokinetic QF LSI at 300°/second | 0.34 (0.09, 0.59) | 0.008 | ||
|
Model 2: -Graft Type (1=PT, 0=HS) |
−5.01 (−10.41, 0.39) | 0.068 | 10.9% | |
| -Involved limb isokinetic QF peak torque at 300°/second | 17.60 (1.63, 33.56) | 0.031 | ||
| 2-Year KOOS-ADL | -Graft Type (1=PT, 0=HS) | −1.83 (−3.85, 0.18) | 0.074 | 12.5% |
| -Involved limb hip abduction strength | −5.94 (−11.72, −0.17) | 0.044 | ||
| 2-Year KOOS-QOL | -Graft Type (1=PT, 0=HS) | −9.11 (−16.70, −1.52) | 0.019 | 8.8% |
RTS – return-to-sport; KOOS – Knee Injury and Osteoarthritis Outcome Score; ADL – activities of daily living; QOL – quality of life; CI – confidence interval; PT – patellar tendon; HS – hamstring; QF – quadriceps femoris; LSI – limb symmetry index
4. DISCUSSION
A primary finding from the current study was that mean values for each KOOS subscale were higher at 2 years post-RTS than RTS, and the proportions of participants reporting KOOS values above literature-reported functional recovery cutoffs[5] improved for 3 of the 5 KOOS subscales (KOOS-Symptoms, KOOS-Sport, KOOS-QOL). Another key finding was that higher RTS isokinetic QF strength symmetry and involved limb isokinetic QF peak torque tested at 300°/second were associated with higher KOOS-Symptoms scores at 2 years post-RTS. The use of a HS autograft during ACLR, a covariate in the final models, was associated with higher KOOS-Pain, KOOS-Symptoms, KOOS-ADL, and KOOS-QOL scores at 2 years post-RTS. In addition, lower involved limb hip abduction peak torque values at RTS were associated with higher KOOS-Pain and KOOS-ADL scores at 2 years post-RTS.
Several previous longitudinal studies have evaluated longitudinal patient-reported function using the KOOS after ACLR. Ahlden and colleagues[1] evaluated national registry data of 2-year post-ACLR KOOS scores in approximately 18,000 patients and determined that mean scores ranged from 61.6 (KOOS-QOL score) to 92.6 (KOOS-ADL score).[1] In a similar study from the Norwegian Knee Ligament Registry, mean KOOS scores at 2 years after ACLR in individuals post-ACLR ranged from 75 (KOOS-QOL score) to 97 (KOOS-ADL).[27] In the current study, mean 2-year post-RTS KOOS scores ranged from 88.7 (KOOS-QOL) to 98.4 (KOOS-ADL), notably higher than those reported in the previous studies.[1, 27] These differences may be accounted for by the difference in age between the cohorts in the Ahlden study[1] (average age at the time of ACLR of 25.3 years and 27.8 years for females and males, respectively) and the LaPrade study[27] (average age of 28.7 years at the time of ACLR) and the cohort in the current study (16.9 years old at the time of RTS clearance after ACLR). For the KOOS, the MCID values range from 8 and 10 points, depending on the subscale.[42] While all KOOS subscale scores at 2 years post-RTS were statistically higher than at the RTS visit in the current study, only the improvement in the KOOS-QOL subscale exceeded the MCID value.[42] This finding may bring into question the clinical relevance of the improvements in the other KOOS subscales over time in our cohort. In addition, the proportions of participants meeting functional recovery cutoffs for the KOOS at 2 years post-RTS in the current study ranged from 71.2% (KOOS-QOL) to 95.5% (KOOS-ADL) for individual subscales, with only 59.1% meeting functional recovery cutoffs for all KOOS subscales. Barenius and colleagues[5] found that only 19% of individuals met all functional recovery cutoffs at 2 years post-ACLR. Again, the notable difference in the proportions meeting overall functional recovery cutoffs may be explained by the young and active nature of the cohort in the current study compared to previous cohorts.[5]
To our knowledge, the findings of the current study are one of the first to comprehensively examine the longitudinal impact of clinical measures, patient demographic data, and surgical data on patient-reported function in young athletes after ACLR. Undergoing ACLR with a patellar tendon autograft (as compared to HS autograft) was consistently associated with lower function, and was included as a covariate. Regarding graft type, metaanalysis data from 2 previous studies with large sample sizes[32, 55] demonstrated that individuals who underwent ACLR with a patellar tendon autograft as compared to HS tendon autograft demonstrated higher amounts of knee pain.[32, 55] Additionally, previous work has also shown that undergoing ACLR with HS tendon autograft was positively associated with functional recovery on the KOOS at 2 years post-ACLR, when compared to patellar tendon autograft.[5] Similarly, in the current study, undergoing ACLR with a patellar tendon autograft was associated with lower 2-year post-RTS scores (indicating worse function) on the KOOS-Pain, KOOS-Symptoms, KOOS-ADL, and KOOS-QOL. Taken together, these findings suggest that individuals with patellar tendon may be at higher risk for decreased longitudinal knee function. However, it is important to also recognize that individuals that undergo ACLR with a HS autograft consistently demonstrate higher graft failure rates compared to patellar tendon autografts; when examining data from large national registry databases and meta-analyses.[37, 45] Thus, given that both of these graft type groups demonstrate different suboptimal outcomes over time, there may be a need to modify rehabilitation to be more specific to the ACLR graft type to help to promote better outcomes over time.
Multiple cross-sectional studies have established that QF strength is consistently associated with higher patient-reported function, both at the time of RTS[47, 56] and at 3 years[25] and 4 years[14] after ACLR. However, few studies have prospectively examined the relationship between early strength at the time of RTS clearance and patient-reported function over time after ACLR. Nawasreh and colleagues[35] demonstrated that patients after ACLR that passed a battery of RTS criteria (including QF strength symmetry) 6 months after ACLR were more likely to demonstrate normal knee function, while those that did not pass the RTS criteria were more likely to demonstrate impaired knee function.[35] Additionally, other recent work has demonstrated that young athletes after ACLR with QF strength asymmetry at the time of RTS demonstrated decreased knee-related function one year later, when compared to those with more symmetric QF strength.[22] To our knowledge, the current study is one of few to examine the relationship between muscle strength and longitudinal knee function, after also controlling for relevant demographic and surgical covariates. The current study demonstrated that, after controlling for ACLR graft type, higher isokinetic QF strength symmetry and higher involved limb QF peak torque tested at 300°/second were associated with higher KOOS-Symptoms scores at 2 years post-RTS. Interestingly, QF peak torque and symmetry tested in isometric fashion or isokinetic fashion at 180°/second were not associated with KOOS scores at 2 years post-RTS.
Of note, the current study found that lower involved limb hip abduction strength was associated with higher KOOS scores at 2 years post-RTS. A previous study by Bell and colleagues[6] found that individuals post-ACLR with QF strength deficits demonstrated higher hip extension strength, in a seemingly compensatory-type pattern. In light of this, it is possible that those in the current study with higher QF strength or symmetry (associated with better KOOS scores) may have relied less on their hip muscles; potentially leading to the inverse association observed between hip abduction strength and 2-year post-RTS KOOS scores. Additionally, the method of testing hip abduction strength (in standing) requires bilateral hip strength and proximal control to stabilize the pelvis during testing. Further study of the interactions among hip strength, QF strength, and functional recovery is likely needed in this patient population. Lastly, in the current study, there were no associations observed between RTS knee ROM, presence of effusion, or anterior knee laxity and 2-year post-RTS knee function on the KOOS. Previous work has identified that lack of full knee ROM is a risk factor for osteoarthritis development after ACLR.[48] However, longer follow-up periods may be needed to identify associations between these specific early knee-related impairments and decreased knee-related function.
There are several limitations that should be considered from the current study. First, only one measure of knee-related function (KOOS) was examined over the 2 years post-RTS in this cohort, and other validated measures of knee function may have provided additional insight into functional recovery and associated clinical impairments from the time of RTS. Specifically, several other validated measures of patient-reported function and knee functional performance measures are commonly used in studies evaluating outcomes in individuals after ACLR including the IKDC subjective knee form,[20] the Knee Outcome Survey-Activities of Daily Living Score,[21] and single leg hop test batteries.[9] Secondly, the surgical and injury factors evaluated as potential covariates for patient-reported function included ACLR graft type, the presence of meniscus injury, and a pediatric ACLR modification. Previous studies have demonstrated that additional concomitant injuries along with a primary ACL injury, including cartilage lesions and bone bruises, were related to poorer KOOS scores over 2 to 6 years after ACLR.[12, 28] Including additional surgery or injury information in the current study may have yielded different results. Thirdly, given the relatively low R2 values observed in our 2-year KOOS score models, other clinical measures at the time of RTS not measured in the current study (other measures of muscle performance; psychological variables) may have also been associated with KOOS scores over time after ACLR. Previous work has shown that asymmetries in the rate of QF muscle torque development may persist longer than asymmetries in peak torque[2] and may be more strongly associated with patient-reported function early after ACLR.[18] However, the longitudinal association between rate of QF muscle torque development and patient-reported function is not currently understood. Additionally, while fear of reinjury and psychological readiness are important factors related to the ability to successfully RTS[3] and to function after ACLR,[10] these measures were not considered as potential independent variables in the current study. Fourthly, the longitudinal cohort design of the current study did not allow for any control over the participation in activities that may have affected patient-reported outcomes over the 2 years after RTS, including self-performed strengthening programs. Fifthly, a portion of participants in this longitudinal cohort study sustain second ACL injuries prior to 2 years post-RTS and were excluded from the current analyses. Because the current analyses examine associations between clinical measures at the time of RTS and KOOS scores at 2 years post-RTS, the associations observed may not be reflective of the general population of young, active individuals after ACLR. Lastly, the nature of the participants in the current cohort being young and athletic may also limit the external validity and generalizability of the findings of this study to all individuals after ACLR.
5. CONCLUSIONS
Despite a mean improvement in KOOS scores, some young athletes continue to demonstrate suboptimal patient-reported function and not meet functional recovery cutoffs at 2 years post-RTS. In this cohort, after controlling for graft type, higher QF strength symmetry, higher involved limb QF peak torque, and lower involved limb hip abduction peak torque from the time of RTS were associated with higher patient-reported function at 2 years post-RTS.
6. ACKNOWLEDGEMENTS
The authors thank the staff of the Division of Sports Medicine and the Sports and Orthopaedic Team in the Division of Occupational Therapy and Physical Therapy for their contribution to this work.
FUNDING
This work was funded by support from the National Institutes of Health grant F32-AR055844, the National Football League Charities Medical Research Grants 2007, 2008, 2009, 2011, the Sports Physical Therapy Section Legacy Fund grant 2015, and the Foundation for Physical Therapy Promotion of Doctoral Studies (PODS) II Scholarship. The funding agencies had no role in the study design, collection, analysis, nor interpretation of the data presented. Additionally, the funding agencies had no involvement in the writing of the manuscript, nor the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors have no other interests to declare.
ETHICAL APPROVAL OF RESEARCH ON HUMANS
The Institutional Review Board at Cincinnati Children’s Hospital Medical Center (Cincinnati, Ohio, USA) approved the protocol for this study (Project 2008-0514).
REFERENCES
- 1.Ahlden M, Samuelsson K, Sernert N, Forssblad M, Karlsson J, Kartus J (2012) The Swedish National Anterior Cruciate Ligament Register: a report on baseline variables and outcomes of surgery for almost 18,000 patients. Am J Sports Med 40:2230–2235 [DOI] [PubMed] [Google Scholar]
- 2.Angelozzi M, Madama M, Corsica C, Calvisi V, Properzi G, McCaw ST, et al. (2012) Rate of force development as an adjunctive outcome measure for return-to-sport decisions after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 42:772–780 [DOI] [PubMed] [Google Scholar]
- 3.Ardern CL, Osterberg A, Tagesson S, Gauffin H, Webster KE, Kvist J (2014) The impact of psychological readiness to return to sport and recreational activities after anterior cruciate ligament reconstruction. Br J Sports Med; 10.1136/bjsports-2014-093842 [DOI] [PubMed] [Google Scholar]
- 4.Barber-Westin SD, Noyes FR (2011) Factors used to determine return to unrestricted sports activities after anterior cruciate ligament reconstruction. Arthroscopy 27:1697–1705 [DOI] [PubMed] [Google Scholar]
- 5.Barenius B, Forssblad M, Engstrom B, Eriksson K (2013) Functional recovery after anterior cruciate ligament reconstruction, a study of health-related quality of life based on the Swedish National Knee Ligament Register. Knee Surg Sports Traumatol Arthrosc 21:914–927 [DOI] [PubMed] [Google Scholar]
- 6.Bell DR, Trigsted SM, Post EG, Walden CE (2016) Hip Strength in Patients with Quadriceps Strength Deficits after ACL Reconstruction. Med Sci Sports Exerc 48:1886–1892 [DOI] [PubMed] [Google Scholar]
- 7.Beynnon BD, Johnson RJ, Abate JA, Fleming BC, Nichols CE (2005) Treatment of anterior cruciate ligament injuries, part I. Am J Sports Med 33:1579–1602 [DOI] [PubMed] [Google Scholar]
- 8.Brent JL, Myer GD, Ford KR, Paterno MV, Hewett TE (2013) The effect of sex and age on isokinetic hip-abduction torques. J Sport Rehabil 22:41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brosky JA Jr., Nitz AJ, Malone TR, Caborn DN, Rayens MK (1999) Intrarater reliability of selected clinical outcome measures following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 29:39–48 [DOI] [PubMed] [Google Scholar]
- 10.Chmielewski TL, Jones D, Day T, Tillman SM, Lentz TA, George SZ (2008) The association of pain and fear of movement/reinjury with function during anterior cruciate ligament reconstruction rehabilitation. J Orthop Sports Phys Ther 38:746–753 [DOI] [PubMed] [Google Scholar]
- 11.Clagg S, Paterno MV, Hewett TE, Schmitt LC (2015) Performance on the modified star excursion balance test at the time of return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 45:444–452 [DOI] [PubMed] [Google Scholar]
- 12.Cox CL, Huston LJ, Dunn WR, Reinke EK, Nwosu SK, Parker RD, et al. (2014) Are articular cartilage lesions and meniscus tears predictive of IKDC, KOOS, and Marx activity level outcomes after anterior cruciate ligament reconstruction? A 6-year multicenter cohort study. Am J Sports Med 42:1058–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies GJ (2017) Individualizing the return to sports after ACL reconstruction. Operative Techniques in Orthopaedics 70–78 [Google Scholar]
- 14.Davis HC, Troy Blackburn J, Ryan ED, Luc-Harkey BA, Harkey MS, Padua DA, et al. (2017) Quadriceps rate of torque development and disability in individuals with anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 46:52–56 [DOI] [PubMed] [Google Scholar]
- 15.Dunn WR, Spindler KP (2010) Predictors of activity level 2 years after anterior cruciate ligament reconstruction (ACLR): a Multicenter Orthopaedic Outcomes Network (MOON) ACLR cohort study. Am J Sports Med 38:2040–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwillie AD, Shah SS, McHugh MP, Nicholas SJ (2017) The Effect of Postoperative KT-1000 Arthrometer Score on Long-Term Outcome After Anterior Cruciate Ligament Reconstruction. Am J Sports Med; 10.1177/0363546517690525363546517690525 [DOI] [PubMed] [Google Scholar]
- 17.Gottlob CA, Baker CL (2000) Anterior cruciate ligament reconstruction: socioeconomic issues and cost effectiveness. Am J Orthop 29:472–476 [PubMed] [Google Scholar]
- 18.Hsieh CJ, Indelicato PA, Moser MW, Vandenborne K, Chmielewski TL (2015) Speed, not magnitude, of knee extensor torque production is associated with self-reported knee function early after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 23:3214–3220 [DOI] [PubMed] [Google Scholar]
- 19.Hsieh FY, Bloch DA, Larsen MD (1998) A simple method of sample size calculation for linear and logistic regression. Stat Med 17:1623–1634 [DOI] [PubMed] [Google Scholar]
- 20.Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, et al. (2001) Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med 29:600–613 [DOI] [PubMed] [Google Scholar]
- 21.Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD (1998) Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am 80:1132–1145 [DOI] [PubMed] [Google Scholar]
- 22.Ithurburn MP, Altenburger AR, Thomas S, Hewett TE, Paterno MV, Schmitt LC (2018) Young athletes after ACL reconstruction with quadriceps strength asymmetry at the time of return-to-sport demonstrate decreased knee function 1 year later. Knee Surg Sports Traumatol Arthrosc 26:426–433 [DOI] [PubMed] [Google Scholar]
- 23.Ithurburn MP, Paterno MV, Ford KR, Hewett TE, Schmitt LC (2015) Young Athletes With Quadriceps Femoris Strength Asymmetry at Return to Sport After Anterior Cruciate Ligament Reconstruction Demonstrate Asymmetric Single-Leg Drop-Landing Mechanics. Am J Sports Med 43:2727–2737 [DOI] [PubMed] [Google Scholar]
- 24.Jones MH, Spindler KP (2017) Risk factors for Radiographic Joint Space Narrowing and Patient Reported Outcomes of Post-Traumatic Osteoarthritis after ACL Reconstruction: Data from the MOON Cohort. J Orthop Res; 10.1002/jor.23557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuenze C, Hertel J, Saliba S, Diduch DR, Weltman A, Hart JM (2015) Clinical thresholds for quadriceps assessment after anterior cruciate ligament reconstruction. J Sport Rehabil 24:36–46 [DOI] [PubMed] [Google Scholar]
- 26.Kvist J (2004) Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Med 34:269–280 [DOI] [PubMed] [Google Scholar]
- 27.LaPrade CM, Dornan GJ, Granan LP, LaPrade RF, Engebretsen L (2015) Outcomes After Anterior Cruciate Ligament Reconstruction Using the Norwegian Knee Ligament Registry of 4691 Patients: How Does Meniscal Repair or Resection Affect Short-term Outcomes? Am J Sports Med 43:1591–1597 [DOI] [PubMed] [Google Scholar]
- 28.Lattermann C, Jacobs CA, Reinke EK, Scaramuzza EA, Huston LJ, Dunn WR, et al. (2017) Are Bone Bruise Characteristics and Articular Cartilage Pathology Associated with Inferior Outcomes 2 and 6 Years After Anterior Cruciate Ligament Reconstruction? Cartilage 8:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lentz TA, Tillman SM, Indelicato PA, Moser MW, George SZ, Chmielewski TL (2009) Factors associated with function after anterior cruciate ligament reconstruction. Sports Health 1:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logerstedt D, Lynch A, Axe MJ, Snyder-Mackler L (2012) Symmetry restoration and functional recovery before and after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc; 10.1007/s00167-012-1929-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marumo K, Saito M, Yamagishi T, Fujii K (2005) The "ligamentization" process in human anterior cruciate ligament reconstruction with autogenous patellar and hamstring tendons: a biochemical study. Am J Sports Med 33:1166–1173 [DOI] [PubMed] [Google Scholar]
- 32.Mohtadi NG, Chan DS, Dainty KN, Whelan DB (2011) Patellar tendon versus hamstring tendon autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev; 10.1002/14651858.CD005960.pub2CD005960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouzopoulos G, Siebold R, Tzurbakis M (2015) Hip flexion strength remains decreased in anterior cruciate ligament reconstructed patients at one-year follow up compared to healthy controls. Int Orthop; 10.1007/s00264-014-2662-x [DOI] [PubMed] [Google Scholar]
- 34.Myer GD, Ford KR, Paterno MV, Nick TG, Hewett TE (2008) The effects of generalized joint laxity on risk of anterior cruciate ligament injury in young female athletes. Am J Sports Med 36:1073–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nawasreh Z, Logerstedt D, Cummer K, Axe MJ, Risberg MA, Snyder-Mackler L (2017) Do Patients Failing Return-to-Activity Criteria at 6 Months After Anterior Cruciate Ligament Reconstruction Continue Demonstrating Deficits at 2 Years? Am J Sports Med 45:1037–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noll S, Garrison JC, Bothwell J, Conway JE (2015) Knee Extension Range of Motion at 4 Weeks Is Related to Knee Extension Loss at 12 Weeks After Anterior Cruciate Ligament Reconstruction. Orthop J Sports Med 3:2325967115583632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persson A, Fjeldsgaard K, Gjertsen JE, Kjellsen AB, Engebretsen L, Hole RM, et al. (2014) Increased risk of revision with hamstring tendon grafts compared with patellar tendon grafts after anterior cruciate ligament reconstruction: a study of 12,643 patients from the Norwegian Cruciate Ligament Registry, 2004-2012. Am J Sports Med 42:285–291 [DOI] [PubMed] [Google Scholar]
- 38.R AM, Binzel K, Zhang J, Wei W, M UK, D CF, et al. (2017) ACL graft metabolic activity assessed by (18)FDG PET-MRI. Knee 24:792–797 [DOI] [PubMed] [Google Scholar]
- 39.Ramski DE, Kanj WW, Franklin CC, Baldwin KD, Ganley TJ (2014) Anterior cruciate ligament tears in children and adolescents: a meta-analysis of nonoperative versus operative treatment. Am J Sports Med 42:2769–2776 [DOI] [PubMed] [Google Scholar]
- 40.Roewer BD, Di Stasi SL, Snyder-Mackler L (2011) Quadriceps strength and weight acceptance strategies continue to improve two years after anterior cruciate ligament reconstruction. J Biomech 44:1948–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roos E, Roos H, Lohmander L, Ekdahl C, Beynnon B (1998) Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther 28:88–96 [DOI] [PubMed] [Google Scholar]
- 42.Roos EM, Lohmander LS (2003) The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health and quality of life outcomes 1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rougraff B, Shelbourne KD, Gerth PK, Warner J (1993) Arthroscopic and histologic analysis of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Am J Sports Med 21:277–284 [DOI] [PubMed] [Google Scholar]
- 44.Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M (2011) Knee injury and Osteoarthritis Outcome Score (KOOS); reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. . Osteoarthritis Cartilage 19:406–410 [DOI] [PubMed] [Google Scholar]
- 45.Samuelsen BT, Webster KE, Johnson NR, Hewett TE, Krych AJ (2017) Hamstring Autograft versus Patellar Tendon Autograft for ACL Reconstruction: Is There a Difference in Graft Failure Rate? A Meta-analysis of 47,613 Patients. Clin Orthop Relat Res; 10.1007/s11999-017-5278-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt LC, Paterno MV, Ford KR, Myer GD, Hewett TE (2015) Strength Asymmetry and Landing Mechanics at Return to Sport after Anterior Cruciate Ligament Reconstruction. Med Sci Sports Exerc 47:1426–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitt LC, Paterno MV, Hewett TE (2012) The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 42:750–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shelbourne KD, Urch SE, Gray T, Freeman H (2012) Loss of normal knee motion after anterior cruciate ligament reconstruction is associated with radiographic arthritic changes after surgery. American Journal of Sports Medicine 40:108–113 [DOI] [PubMed] [Google Scholar]
- 49.Spindler KP, Huston LJ, Wright RW, Kaeding CC, Marx RG, Amendola A, et al. (2011) The prognosis and predictors of sports function and activity at minimum 6 years after anterior cruciate ligament reconstruction: a population cohort study. Am J Sports Med 39:348–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spindler KP, Warren TA, Callison JC Jr., Secic M, Fleisch SB, Wright RW (2005) Clinical outcome at a minimum of five years after reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am 87:1673–1679 [DOI] [PubMed] [Google Scholar]
- 51.Sturgill LP, Snyder-Mackler L, Manal TJ, Axe MJ (2009) Interrater reliability of a clinical scale to assess knee joint effusion. J Orthop Sports Phys Ther 39:845–849 [DOI] [PubMed] [Google Scholar]
- 52.Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 43–49 [PubMed] [Google Scholar]
- 53.Vairo GL (2014) Knee flexor strength and endurance profiles after ipsilateral hamstring tendons anterior cruciate ligament reconstruction. Arch Phys Med Rehabil 95:552–561 [DOI] [PubMed] [Google Scholar]
- 54.Wasserstein D, Huston LJ, Nwosu S, Kaeding CC, Parker RD, Wright RW, et al. (2015) KOOS pain as a marker for significant knee pain two and six years after primary ACL reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) prospective longitudinal cohort study. Osteoarthritis Cartilage 23:1674–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q (2015) A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee 22:100–110 [DOI] [PubMed] [Google Scholar]
- 56.Zwolski C, Schmitt LC, Quatman-Yates C, Thomas S, Hewett TE, Paterno MV (2015) The influence of quadriceps strength asymmetry on patient-reported function at time of return to sport after anterior cruciate ligament reconstruction. Am J Sports Med 43:2242–2249 [DOI] [PubMed] [Google Scholar]