Abstract
The discipline of neurotheranostics was forged to improve diagnostic and therapeutic clinical outcomes for neurological disorders. Research was facilitated, in largest measure, by the creation of pharmacologically effective multimodal pharmaceutical formulations. Deployment of neurotheranostic agents could revolutionize staging and improve nervous system disease therapeutic outcomes. However, obstacles in formulation design, drug loading and payload delivery still remain. These will certainly be aided by multidisciplinary basic research and clinical teams with pharmacology, nanotechnology, neuroscience and pharmaceutic expertise. When successful the end results will provide “optimal” therapeutic delivery platforms. The current report reviews an extensive body of knowledge of the natural history, epidemiology, pathogenesis and therapeutics of neurologic disease with an eye on how, when and under what circumstances neurotheranostics will soon be used as personalized medicines for a broad range of neurodegenerative, neuroinflammatory and neuroinfectious diseases.
Keywords: Alzheimer’s disease, Parkinson’s disease, Blood brain barrier, Brain-targeted nanoparticles, Nanomedicine, Neurodegenerative disorders, Neuroimaging, Single photon emission computed tomography, Magnetic resonance imaging, Theranostics, Neurotheranostics
1. Theranostics as personalized medicines
Until recently, the traditional approach to treating medical maladies has been to first make a diagnosis and then to administer a form of therapy. In line with this approach, medical research has primarily been focused on characterizing diseases followed by developing a therapeutic agent effective in treating disease. However, it is clear that this two-part strategy is not always effective for the most devastating of diseases. These are often times heterogenous in clinical presentation and underlying pathology [1]. Because of this fact, many of the so-called best treatments work for only certain affected subpopulations. Additionally, disease progression is also complex making it difficult to design treatments effective at all disease stages [2]. Indeed, the more we understand complex diseases such as cancer, human immunodeficiency virus (HIV), and inflammatory bowel disease amongst others the more apparent it is that a one size fits all approach to treatment is not uniformly effective [3–5].
With these concerns in mind the concept of combining a therapeutic agent with a diagnostic tool to produce personalized treatments was birthed. Protocols emerged that could provide improved prognoses than what is now standard treatments [6]. Personalized medicines based on nuclear and molecular imaging has emerged as a new therapeutic strategy. Such approaches can help physicians in pinpointing precise diagnoses leading to medical or surgical treatments [7]. This growing area of research has been coined ‘theranostics” defined as the combination of diagnostic and therapeutic agents placed into a single platform and enabling both to be delivered together. This allows the diseases to be treated and monitored effectively at the same time [8]. It is also one of the key strategies for the emergence of personalized medicines. Coined by John Funkhouser (Chief Executive Officer of PharmaNetics) in 2002, the term theranostics describes agents employed for combined applications [9]. First, theranostics can be used to identify subgroups of patients with specific clinical profiles likely to respond to specific treatment regimens. In kind the same subgroups like to have adverse reactions to these treatments can also be identified so that the optimal therapies are chosen then administered. Second, disease-combating agents can be used to monitor disease combating responses in real time by diagnostic imaging [10].
Historically, the principle of theranostics was first employed in the 1940s with the imaging and treatment of thyroid cancers using radioactive iodine [11–13]. Similarly, long-acting 131I was used for the therapeutic management of Grave’s disease [12]. With a half-life of 8 days β and γ particles are omitted with each radioactive decay. The γ particles of 131I result in scatter and image blurring. However, 123I can be used solely as a diagnostic tool since it does not emit a β particle. In contrast, 131I collides with atomic nuclei to elicit cell damage that produces a therapeutic effect. As 131I is effective in treatment of thyroid disease, it became the first US Food and Drug Administration (FDA) approved radiopharmaceutical in 1951 [14]. With continued progress in molecular biology and biochemistry the elucidation of functional disease signatures allowed the identification and treatments for a number of cancers [15, 16]. This included recognition of specific surface receptors found to be expressed in abundance on tumor cells [17]. With such information in hand theranostics was applied to identify differences between normal and cancerous cells in order to identify molecular targets for delivery of sensitive and specific cytotoxic payloads for tumor cell elimination [18, 19]. With such molecular targeting agents in hand a plethora of radioactive, fluorescence and paramagnetic imaging agents were subsequently developed for use by positron emission tomography (PET) and single photon emission computed tomography (SPECT/CT), optical imaging and magnetic resonance imaging (MRI) [20–22]. These types of imaging modalities using specific theranostic probes allow the attending physician to visualize the disease target, assess its location and size and determine the best means to control or eliminate it [23–25]. In the larger picture such approaches could be used as a personalized medicine by using the probe to determine in any given patient the use of the therapeutic modality for patient screening. The works could also guide clinical trial enrollment and determine therapeutic effectiveness [26–28].
Advances in radioactive tracers have led to the development of 2D scintigraphy to facilitate the disease localization. This technology greatly improved our understanding of disease markers and laid the groundwork for the advent of 3D and 4D radiographic imaging techniques [29]. An example of the clinical use of such theranostic probes is the targeting of human epidermal growth factor receptor (HER-2) expressed in metastatic breast cancer lesions with poor prognoses [30, 31]. A diagnostic test can identify patients with HER-2 containing tumors. This has enabled imaging guided therapeutics using HER-2 monoclonal antibodies to target then eliminate cancer cells [31, 32]. In 2004, the Pittsburgh compound B (PiB) discovery for PET imaging in amyloid in Alzheimer’s disease (AD) patients led to major advances in bioimaging field [33].
Yet another theranostic is peptide receptor scintigraphy (PRS) and peptide receptor radionuclide therapy (PRRT) used for rapid diagnosis and treatment of pancreatic cancers. This technology came to the fore in the late 1980s when 123I-labeled Tyr3-octroetide was used for the localization of carcinoid tumors, paragangliomas and pancreatic tumors [34]. This was followed by the development of 111In-pentetreotide, which had better sensitivity and specificity and became the first FDA approved in 1994 peptide-based radiopharmaceutical [35, 36]. However, this approach yielded only a modest shrinkage of the tumor and there was an associated risk of development of melody’s plastic syndrome or leukemia [37]. This paved the way for the use of 90Y-Peptide Receptor Radionuclide Therapy (PRRT) and radiolabeled metal DOTA-chelated peptides [38]. However, significant renal uptake of the radionuclide was observed, and further research was needed to identify ways to preclude such events [39]. By the early 1990s, the Erasmus MC lysine-arginine formulation was discovered (Erasmus University Medical Center, Netherlands) and found to impart renal protection [40]. Renal protection with any PRRT with β-emitting radionuclides became a standard protocol since Novartis launched the 90Y-labeled DOTA, Tyr3-octreotide (DOTATOC) in 1997 which was more effective than 111In-pentetreotide although it also led to higher renal toxicity [41, 42]. Around the same time, the first gallium labeled peptide imaging was fashioned by 68Ga-DOTATOC PET [43]. At the turn of the millennium [177Lu-DOTA, Tyr3] octreotate was made then administered in conjunction with adequate amounts to preclude amino acid renal toxicity [44]. It proved to be an effective therapeutic treatment and improved the survival rate of patients in clinical trials and it is presently under review by the FDA and European regulatory agencies. Numerous animal studies with various radionuclides have demonstrated that 90Y-PRRT is more effective with larger tumors than 177Lu-PRRT while the scenario is reversed for small tumor treatments [45]. Researchers have also looked into combination treatment with [177Lu-DOTA, Tyr3] octreotate and a chemotherapeutic agent, which is referred to as peptide receptor chemoradionuclide therapy [46, 47]. Another major innovation in theranostics is NETest, a gene transcript measure [48]. Concurrent advances in biomaterial science has enabled the creation of small nanoparticles capable of possessing both passive imaging agents for MRI or CT, as well active agents for PET or SPECT/CT which creates an imaging agent that can maximize the strengths of each imaging technique while diminishing their inherent weaknesses [49]. Currently several nanoparticle and imaging agent constructs have been FDA approved for various disease treatments.
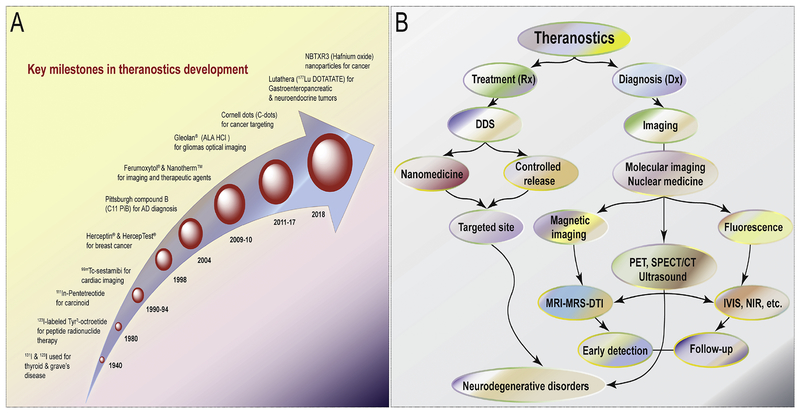
The most common radioisotope used in these nanoparticles is 99mTc, which has a half-life of about six hours before decaying into 99Tc and releasing a 140-keV γ-ray, which makes it ideal for γ-cameras and SPECT/CT imaging [50]. 99mTc has been incorporated into many FDA-approved colloidal nanoparticle platforms. These platforms include, sulfur, albumin, stannous fluoride (SnF2), and rhenium heptasulfide (Re2S7) colloids, which are used mainly for lymphoscintigraphy, gastrointestinal or inflammation imaging [51]. Additionally, nanoparticles based on the superparamagnetic properties of iron oxide have been FDA-approved as excellent MRI contrast agents. These, superparamagnetic iron oxide nanoparticles (SPIONS) have a large magnetic moment that reduces the signal seen on an MRI on T2 and T2*-weighted images [52]. Dextran coated SPIONS have been used for lymph node, perfusion, and mononuclear phagocyte system imaging while carbodextran and polyglucose sorbitol carboxymethylether coated SPIONS are employed for hepatocellular carcinoma and iron-deficiency anemia, respectively [51]. Recently, FDA approved imaging agents and iron replacement therapies based on the nanoparticles are available for clinical applications; Venofer®, Ferrlecit®, INFed®, Dexferrum® Nanotherm™ and Feraheme® [53, 54]. In the future, the pace of more FDA approval of nanoparticle constructs for medical imaging and therapeutic applications should accelerate in development and implementation. The complete historical evaluations of the theranostic discipline are pictured in Fig.1
Fig. 1. An historical overview of theranostics.
(A) Timed events recorded during the development of theranostics until the present. (B) The role of the theranostics in the diagnosis, staging and treatment of neurodegenerative diseases are outlined in this chart. Abbreviations are as follows: DDS; drug delivery system, MRI; magnetic resonance imaging, MRS; magnetic resonance spectroscopy, DTI; diffusion tensor Imaging, PET; positron emission tomography, SPECT CT; single photon emission computed tomography, IVIS; in vivo optical imaging system and NIR; near infrared fluorescence.
As discussed, theranostic approaches have been successfully engaged in treatment of specific types of cancer and have been used effectively to localize and destroy the disease. Without doubt, the intersection between theranostics and personalized medicine is quite clear. Whether cancer, degenerative or infectious diseases theranostic approaches allow a physician to intervene most effectively to combat disease. Successful treatment is dependent on the disease lesion, as demonstrated with certain cancer types, on genetic parameters, and on parameters specific to the disease as well as to monitor disease progression and severity and predict therapeutic responses [55–57]. Thus, the use of the name “personalized” is very much linked to the actual therapeutic index and host factors that would determine efficacy. Indeed, in each and every instance, any implementation of theranostics brings the “concept” of personalized medicine to actual utility and can and often does affect overall morbidity and disease mortality [56]. This is based on the effectiveness of the developed modality to effectively combine therapeutics and diagnostics into single platforms [58, 59]. The unique nature of such an approach is based on specific biological pathways to acquire images for the diagnosis as well as formulations that could be developed that serve to improve delivery of therapeutic agents, ultimately leading to the effectiveness of specific targeted therapies [59–63].
Thus, the overarching concept of theranostics is to deliver medicines at levels capable of eliminating a disease-causing agent, leading to cure. For nearly all applications diagnostic and therapeutic agents are co-delivered in nanoparticles. Thus, nanotechnology has had a dominant role in the field of theranostics in general. A theranostic platform allows encasements of multiple targeting and imaging modalities into a single formulation to optimize biodistribution of active agents [64].
2. Creating biomaterials for disease diagnosis and drug delivery
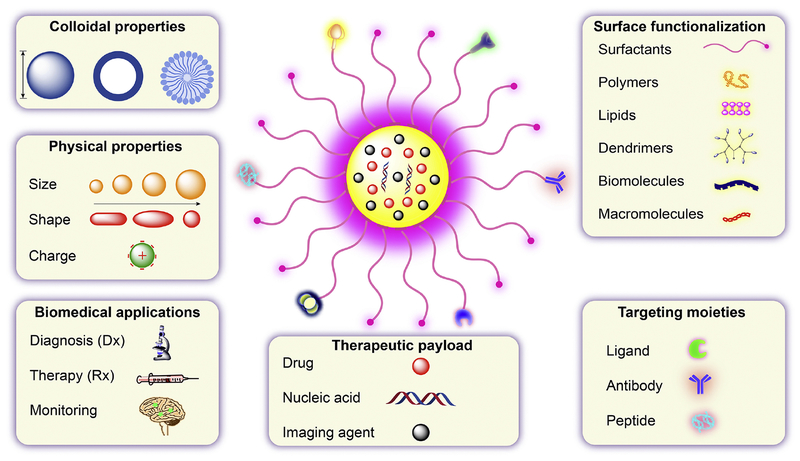
Over the past decade, a vast array of multifunctional nanoparticles “theranostic nanoparticles” have emerged as promising candidates for such biomedical applications due to their physicochemical properties, chemical stability and engineered biocompatibility. As the term “nano” suggests, these particles have at least one dimension less than 1 μm and can be as small as atomic scale lengths of about 0.2 nm [65, 66]. A plethora of theranostic platforms have been explored and developed including polymer-drug conjugates, dendrimers, polymeric particles, magnetic particles, solid lipid particles, gold nanoparticles and carbon nanomaterials [67]. Many nanoparticles such as gold particles, iron oxide particles, and carbon nanotubes have intrinsic theranostic capabilities. Others such as micelles, dendrimers and inorganic nanoparticles can be surface functionalized to express diagnostic properties as well as targeting moieties. Such nanoparticles can be altered to meet any desired physicochemical features. Preparations of aqueous nanosuspensions can be achieved through small molecules, surfactants, macromolecules and polymers [68]. However, nanoparticles are readily taken up by the liver and cleared from the systemic circulation. Therefore, modifications are required to extend drug half-life and circulation times. A modifiable surface can also serve to facilitate particle crossing of the BBB. Additionally, functionalization of nanoparticles with targeting moieties can be explored to deliver a particle to disease relevant cell and tissue sites of injury, inflammation or infection [69–74]. Therefore, the fabrication and development of aqueous-stable, stimuli-responsive, biocompatible, targeted nanoparticles with controllable sizes remains a focus of much research. Such nanoparticles are classified based on their size, shape, chemical properties and surface charge [75, 76]. The selected classes of nanoparticles are illustrated in Fig.2 and discussed below.
Fig. 2. Design, physicochemical properties and applications of multimodal theranostic nanoparticles.
An outline is provided of the physicochemical properties, payload options, imaging agent labeling and surface decoration designed to improve clinical outcomes.
(a). Drug nanocrystals and nanosuspensions for drug delivery.
Aggregation and stability (Ostwald ripening) presents major challenges in the delivery of hydrophobic and lipophilic drugs to disease sites after systemic administration [77]. Formulation of such drugs in forms of drug nanocrystals or nanosuspensions improves their stability and abilities to distribute to tissues of interest [71, 78]. A variety of techniques have been employed for large-scale production of drug nanoparticles including precipitation, high-pressure homogenization, freeze-drying, wet stirring and milling [71, 78–81]. Amphiphilic stabilizers are typically used in the preparation of nanosuspensions stable in an aqueous media [72, 82, 83]. Nanosuspensions can maintain therapeutic efficacy and increase drug half-lives by protecting them from rapid systematic metabolism [84, 85]. Surface modified nanosuspensions with molecules to recognize receptors on the BBB can facilitate outcomes for neurodegenerative diseases [86, 87].
(b). Polymeric nanoparticles for drug delivery.
A wide variety of biocompatible and biodegradable nanoparticles have been fabricated using polymeric entities [88, 89]. Designing nanoplatforms for drug delivery to the nervous system is of pivotal importance. To this end, a variety of polymers have been screened for their suitability for brain delivery applications. These include, but are not limited to, poly(butyl cyanoacrylate) (PBCA), poly(isohexyl cyanoacrylate) (PIHCA), poly(lactic acid) (PLA), poly(glycolic acid) (PGA) or copolymers of poly(lactide-co-glycolide) (PLGA), human serum albumin (HSA) and chitosan. All have proven to be promising nanomaterials for human use due to their unique physicochemical properties, biocompatibility, rapid biodegradability, and ease of drug encapsulation. These polymeric nanoparticles provide a specific set of internal and surface properties which: (i) govern encapsulation interactions in the nanoparticle interior between the polymer and the drug(s), (ii) can be further modified by various surfactants to modulate their interactions with other materials post administration, and (iii) are utilized to anchor targeting ligands, glycoproteins or antibodies. For example, peptide decorated cationic nanogels encapsulating 5′-triphosphates of nucleoside reverse transcriptase inhibitors (NRTIs) were created to target the brain-specific apolipoprotein E receptor [90]. In addition, model fluorescently tagged polystyrene nanoparticles were successfully designed to localize in cells (for example hCMEC/D3) as well as enable CNS delivery [91, 92]. Studies have showed that the particle size and surface functionalization plays an important role in biodistribution following intravenous administration [93]. Polystyrene nanoparticles were further modified by a viral fusion peptide (gH625), which significantly enhanced the nanoparticle permeation across BBB [94]. It has been hypothesized that functionalizing nanoparticles with antibodies against cell surface receptors on brain endothelial cells could facilitate increased penetration of compounds delivered in nanoparticles. In one attempt to improve drug penetration across the BBB, researchers used polysorbate-80 functionalized PLGA nanoparticles covalently linked to a transferrin receptor-targeting antibody (8D3). Transferrin receptors are found in abundance on brain endothelial cells and are responsible for the uptake of iron into the CNS [95]. To demonstrate the enhanced BBB crossing of their particles the researchers studied central analgesia in rodents using a classic test of supraspinal responses to pain, the hot-plate test, in which an animal is placed onto a warm (~54°C) plate and the time until the animal produces a nociceptive response is measured [96]. As a model drug, the researchers used loperamide, a unique morphine-like opioid receptor agonist that, as a substrate for P-glycoprotein efflux transporters, does not accumulate in the CNS [97]. The maximal possible anti-nociceptive effect (MPE, in %) after injection with transferrin targeted, polysorbate 80 functionalized, loperamide loaded, PLGA nanoparticles was approximately 50% better than controls and twice as great as nanoparticles without the 8D3 antibody (~25%). However, researchers did not actually measure drug levels in any tissue during this study so it is not possible to conclude that more drug actually crossed the BBB and accumulated in the CNS [98]. Adding to this confusion is the fact that loperamide has a high affinity for both μ-opioid receptors and peripheral δ-opioid receptors so the increased analgesic effects seen could be mediated through increased peripheral opioid receptor activation [99]. To add further confusion to interpretation of the data, loperamide alone was never used as a control and thus comparison between native loperamide and nanoparticle delivered loperamide was not studied. In fact, the most interesting data from this study was that the greatest MPE was seen after simply injecting loperamide with 15 wt.% of polysorbate 80. This indicates that if loperamide is crossing the BBB in this system, it may be doing so mainly through inactivation of P-glycoprotein efflux pumps by polysorbate 80 [100]. Elsewhere, PLGA nanoparticles modified with a g7 peptide to target high molecular weight drugs for lysosomal storage disorders in the CNS have been developed [101]. Polymers have also been synthesized for efficient gene delivery across the BBB [102]. Dendrimers, another class of ordered, hyperbranched, macromolecular polymers, are also efficient drug delivery vectors for CNS disorders [103, 104]. Studies have shown that fluorescent phosphorus dendrimers can be used for macrophage imaging and diagnosis of spinal cord injuries [105]. Stimuli responsive polymeric nanosystems, known as microbubbles, have gained considerable interest in recent years in the area of image-guided drug delivery vehicles [106–108]. Microbubbles have been found to be efficient delivery vectors, responsive towards focused ultrasound, and enable localized noninvasive imaging of the brain. Unlike other polymeric nanoparticles, they are able to combine various modalities of therapeutics and diagnostics into a single nanosystem [109].
(c). Lipid based theranostic nanoparticles for drug delivery.
Lipid-based nanosystems have been extensively evaluated as nanocarriers for various biomedical applications [110] including treatment of neurodegenerative disorders [111]. Lipids have been known to form multiple varieties of vesicular architectures in aqueous media such as solid lipid nanoparticles (SLNs), monolayer micelles, and bilayer liposomes. SLNs have been successful in delivering various bioactive compounds across the BBB to the desired brain region [112, 113]. Their unique vesicular structural properties, biocompatibility, and stability make them the most obvious choice for delivery of drugs, nucleic acids, and other therapeutic molecules. SLNs have also been widely used as theranostic agents due to their high multi component loading efficiency and ease of functionalization for targeted delivery [114]. SLNs are commonly prepared by hot and cold homogenization techniques. Bae et. al report the preparation of quantum dot incorporated SLNs with a stable low-density lipoprotein core and paclitaxel incorporated shell with electrostatic complexation of siRNA on the surface of the SLNs. The strong fluorescence from the quantum dots enables in vivo visualization [115]. Liposomes are another type of lipid nanoparticles that have high potential for use as theranostic platforms due to their versatility for functionalization and the therapeutic or imaging moiety can be either encapsulated within the hydrophilic core, embedded in the lipophilic bilayer or conjugated to the surface of the liposome. Their small size, hydrophobic and hydrophilic character, biodegradability, biocompatibility, low toxicity and immunogenicity make them effective theranostic platforms [116]. Liposomes are prepared mainly by mechanical dispersions, solvent dispersions and detergent removal. Xu et. al report the preparation of theranostic liposomes (QSC-Lip) integrated with SPIONs, quantum dots (QDs) and the therapeutic peptide cilengitide (CGT) all encapsulated into a PEGylated liposome for dual-image guided cancer surgery. These liposomes were prepared by a process of film hydration followed by sequential extrusion to obtain particle sizes of about 100 nm [117]. For example, a pharmaceutical liposomal formulation loaded with the amphipathic weak base tempamine was developed for treatment of neurodegenerative disorders [118]. While drug-loaded SLNs were designed to reduce amyloid induced oxidative stress in the case of AD by targeting the hippocampus [119], further improvements were made through intranasal delivery of encapsulated SLNs [120]. Both non-targeted and targeted liposomes have proven their enhanced efficacy in delivery of biomolecules to the brain [121]. Non-targeted liposomal nanocarriers are known to successfully encapsulate hydrophobic drugs but fall short in selective localization. These liposomes showed random biodistribution, which led to unwanted accumulation in undesired tissues and development of secondary toxicities. This also results in the requirement of high doses to achieve therapeutically significant drug levels in tissues. Due to these shortcomings and considering the challenge for BBB transport, a wide variety of targeting strategies are being tested for CNS delivery. Small molecule ligands such as glutathione have been explored as targeting molecules conjugated to the liposomal surface [122]. For example, one study demonstrated that glutathione-modified drug-loaded liposomes were specifically taken up by brain capillary endothelial cells [123]. They subsequently crossed the BBB successfully, localized in brain tumor cells, initiated tumor regression and increased the survival rate of the experimental mice. Tissue homing peptides have also been used in modifying the liposomal surface for improved localization across the BBB [124]. Liposomes loaded with the novel peptide H102 exhibited increased brain penetration when delivered through the intranasal route and localized to the hippocampus. These liposomes showed significant neuroprotective effects [125]. In another study, various peptides were synthesized and characterized for use as targeting ligands for drug-loaded liposomes [126]. These liposomes were able to transport the therapeutic load across the BBB and successfully initiated tumor regression of intracranial glioma. A bi-ligand system was also developed by conjugating transferrin to a cationic polypeptide as a targeting moiety for enhanced localization of labeled liposomes containing a plasmid DNA into the brain [127]. These results support the ability of liposomal nanocarriers to carry macromolecules across the BBB. Using a similar approach, investigators used a dually targeted liposomal nanocarrier for delivery of neuroprotective drug to ischemic neurons [128]. A two-step targeting approach was used. In this study a stroke-homing peptide enabled the liposomal carrier to cross the BBB and a second peptide enabled specific targeting of ischemic neurons. A similar design of dual targeting has been successfully explored by many researchers for BBB transport of therapeutic molecules [129]. The combination of multiple targeting platforms and the fabrication of polymericlipoplexes for delivery of neuroprotective and neurotherapeutic agents has been studied [130]. On similar lines, a lipid-protein complex (lipoprotein) was recently developed for combination neuroprotective AD therapies [131].
(d). Inorganic and theranostics nanoparticles.
The nanoplatforms discussed have proven to be very promising as vehicles to transport therapeutic agents across the BBB. They are primarily dependent on optically active fluorescent molecules as imaging modalities. These fluorescent probes are known to be photo-sensitive and chemically-labile, and the images obtained are optically-compromised due to the scattering of light originating from signal in deep-seated tissues. In order to combine delivery, diagnostic, and imaging modalities into a single nanoplatform, inorganic nanoparticles have been studied as promising alternatives [61, 132–134]. Multifunctional theranostics nanoparticles have been designed, for the most part, as image contrast agents to track the progression of disease and drug intervention [59, 135]. New generations of merged particles utilize biocompatible and biodegradable materials and are widely used for concurrent monitoring/imaging of nanotherapeutics [59, 136]. Despite challenges posed by the BBB in brain-targeted therapeutics, inorganic nanoparticles have been developed to facilitate delivery of therapeutic agents to the CNS for a variety of neurodegenerative disorders and tumors [137–139]. Among a wide range of theranostics nanoparticles, magnetic and gold nanoparticles have attracted significant interest in biomedical applications due to their unique abilities to respond to an external magnetic field and high X-ray attenuation coefficients. Due to the colloidal nature of metal nanoparticles, their synthesis has been challenging. Although numerous methods have been used to synthesize magnetic nanoparticles of suitable size and disparity, the most efficient method is the chemical co-precipitation technique of iron salts [140] followed by dispersion in an aqueous phase. However, a major limitation of dispersions is the ability to control the process to produce desired particle size and shape. Newer methods of synthesis have been explored such as the laser ablation method, which is very effective in producing small sized particles [141]. The particles are produced as a fine powder with a particle size of 20–50 nm and a narrow polydispersity. In another study, Dai et. al used an iron precursor to synthesize SPIONS via a polyol process involving high temperature thermal decomposition. These particles were readily dispersible in water with a hydrodynamic diameter of 11.7 nm and a narrow polydipersity [142]. Other types of theranostic particles have been developed that consist of highly magnetic sensitive particles using a multimodal europium doped cobalt ferrite polycaprolactone core synthesized by solvothermal techniques [59, 64]. These particles were coated with a polymer-lipid core shell structure and then functionalized with folic acid to impart macrophage-targeting properties. The particles were further optimized for nuclear imaging by intrinsic labeling with radioactive nuclides, 177Lu and 111In, for rapid assessment of biodistribution and pharmacokinetics of antiretroviral drugs [59]. Gold nanoparticles (GNPs) have also been successfully used for theranostic applications. GNPs are commonly synthesized by chemical treatment of hydrogen tetrachloroaunate [67]. GNPs have a core size of about 1.5 to 10 nm and can be chemically conjugated with drugs and targeting ligands to advance therapeutic capabilities. Chen et al., designed smart theranostic GNPs, wherein doxorubicin was conjugated to the nanoparticles via a gold-sulfide (Au-S) bond [143]. In order to evaluate size-dependent theranostic performances, studies were carried out with insulin-coated GNPs as potential drug carriers across the BBB [144]. Biodistribution and localization of these nanoparticles functionalized for AD was assessed by non-invasive 3D imaging [145].
Neurotheranostics is a new subfield of theranostics that is being developed for neurological disorders. Recent advances in the fields of nanotechnology and bioimaging have enabled the development of a wide array of multifunctional therapeutic platforms capable of transporting drugs across the BBB. For example, attempts were made to functionalize nanoparticles to target specific cell types, releasing drug in a controlled manner, enabling visualization of drug delivery and uncovering altered functional brain states, [146–149]. The field of theranostics, by bridging drug delivery and bioimaging is being applied to identify at risk patients earlier and as such provide more effective treatments. Regenerative, protective, immune modulatory, anti-inflammatory, and imaging agents are readily incorporated in nanoparticles designed to facilitate the delivery of medications to the brain [150]. However, in order to accomplish this the agents must cross the BBB. Based on this need, a variety of colloidal and physical properties along with surface decorations are provided to facilitate drug-encased particle delivery across the BBB (Fig.2). Drug-loaded magnetic nanoparticles for targeting neurodegenerative diseases such as AD and PD have been well studied [151] [152]. Also, optimal magnetic field parameters, which facilitate and dictate crossing of the BBB by these nanoparticles, have been assessed and supported by simulation and mathematical studies [153]. These nanoparticles have also been applied to delivery of RNAi [139]. Magnetic responsive carriers have emerged as the most promising contrast agents for magnetic resonance imaging (MRI). Kirschbaum and colleagues assessed the performance and efficacy of multifunctional, multimodal theranostic magnetic nanoparticles for imaging of immune cells in a murine model of MS [154]. Peptide-targeted, lipid-modified magnetic nanoparticles have also been developed as multimodal-imaging platforms for molecular imaging of glycoproteins in the brain of epileptic rats [155]. Inherent properties of inorganic nanoparticles such as magnetism, photoluminescence and surface plasmon resonance have broadened their application in theranostic particles [156]. Upconverting luminescent tertiary nanoparticles conjugated with 2-dimensional graphene oxide have been developed as biosensors for specific RNA biomarkers of AD [157]. In addition to imaging, in vivo studies have also demonstrated that upconverting luminescent nanoparticles have the potential to capture Cu2+ ions to minimize aggregation of amyloid β (Aβ) proteins in AD [158, 159]. Self-targeting carbon dots have been developed for localization of agents in the brain tissue, and could also be used for non-invasive imaging [160]. Rare earth metal oxides have been reported to not only localizes in the brain tissue, but also can target sub-cellular compartments (mitochondria) in an AD transgenic mouse model [161]. Although there exists a vast pool of functional nanomaterials, promising alternatives include secondary, tertiary, and quaternary nanoparticles, insulator-semiconductor quantum dots, molybdenum disulfide, graphene, cerium oxide, and yttrium oxide. Several have found application in the treatment and diagnostics of AD and PD [157, 162–171]. Among a vast number of suitable options, magnetic nanoparticles, rare earth oxides, semiconductors, and metallic nanoparticles can be used as theranostic nanoparticles [59, 64, 162, 172–174]. By modulating their shape, size and crystal structure they have been successfully used in clinical settings. Despite their encouraging success in the treatment of diseases such as cancer, bone repair, tissue regeneration, cardiac and vascular diseases, diabetes, arthritis, among others, these theranostic nanoparticles face a major challenge for treatment [6, 175]. Current research is focused primarily on brain targeting, localization and brain subregional delivery [176]. While opportunities for neurodegenerative disease treatments [177] the challenge that exists is in bypassing the BBB comprised of sealed cell-to-cell contacts and an extensive network of blood capillaries [178] [179]. For neurodegenerative diseases therapies must include the means to prevent or eliminate protein aggregation that are known to accumulate in brain subregions [180]. The intracellular protein aggregation consists of misfolded tau, α-synuclein, Huntingtin protein and superoxide dismutase-1 (SOD1) in the case of AD, PD, HD and ALS, respectively (Fig.3) [181, 182]. Each of these proteins are actively involved induction of reactive oxygen species (ROS) and pro-inflammatory responses that play key roles in affecting synaptic function and neuronal vitality [183]. Each of these protein aggregates affect glial (microglial, astrocyte and oligodendrocyte) activation responses or induce circulating immunocytes to affect neuronal function [184].
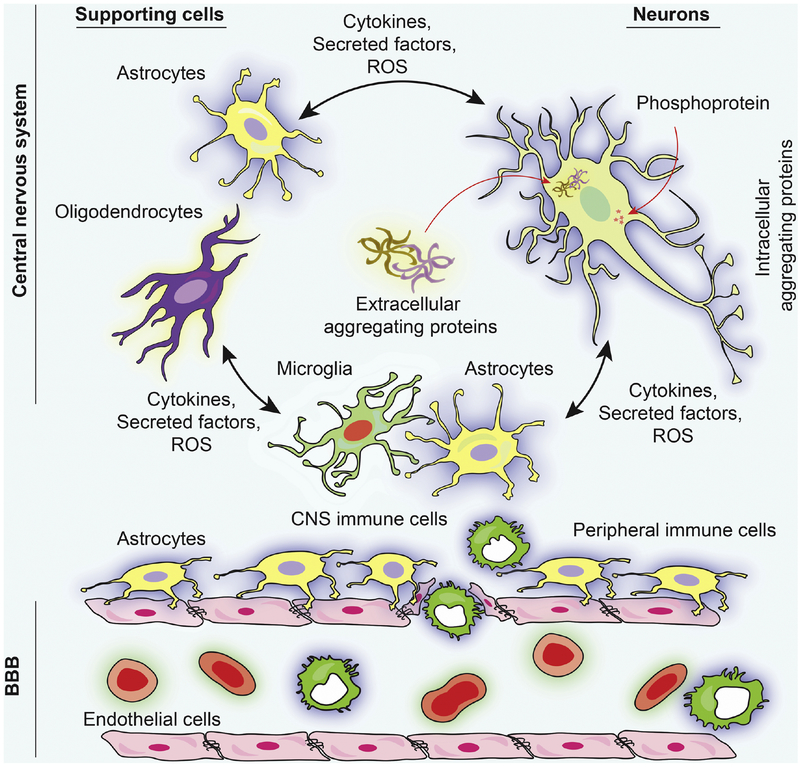
Fig. 3. Molecular mechanisms of neurodegenerative diseases: Role of protein aggregation and neuronal network dysfunction.
Protein aggregates deposited in brain subregions are a common characteristic of neurodegenerative diseases [180]. Extracellular and intracellular protein aggregates are commonly observed. The intracellular protein aggregation consists of (a) tau, (b) α-synuclein, (c) huntingtin protein, (d) SOD1 and (e) self-harm to neurons [181, 182]. Each of these proteins are actively involved with cellular processes that play key roles in affecting microtubule and synaptic function [183]. However, amyloid-β, α-synuclein, and tau are also part of extracellular protein aggregates and stimulate astrocyte and oligodendrocyte responses. These occur with immunocytes to affect neuronal function and vitality [184]. Astrocytes, microglia and oligodendrocyte cytokines and ROS and generate a spectrum of immune cell responses that leads to BBB and neural and glial damage [184, 200]. Schematic illustration concept was adopted from [201].
3. Natural history, pathobiology and therapies for neurodegenerative diseases
Neurodegenerative diseases [for example, AD and PD, MS, HD and ALS], share in common significant morbidities, high prevalence and mortality rate [185–187]. All are incurable and present enormous and still growing medical, social and economic burdens. Unmet needs are considerable. Thus, the need for improved diagnostics and therapeutic options are immediate. The emergence of theranostics (combinations of diagnosis and therapy) for the nervous system (coined neurotheranostics) is timely in serving to fill an important void based on its potential to facilitate improved disease outcomes. Indeed, making precise and timely diagnoses can facilitate early treatment intervention and as such offers hope to patients combating disease associated motor, behavioral, cognitive dysfunctions and an ultimate accelerated and painful death. Notably and in parallel to the suffering lies a prolific financial burden. Indeed, successful therapeutic outcomes would lead to savings of billions of dollars in health care costs that occur with the personal anguish from the disease complex affecting family and friends [188]. Indeed as of 2018 there is essentially nothing the medical industrial complex can offer the patient in affecting the disease itself as treatments remain symptomatic [189, 190].
The challenges in finding improved diagnostic tools and treatments for neurodegenerative diseases are substantive. Indeed, AD and PD are particularly worrisome as they are rapidly increasing in occurrence and frequency. They are both persistent and progressive with links to region specific neuronal impairments and inflammation. Depending on individual disease characteristics both the central and peripheral nervous systems (CNS and PNS) can be engaged and affect cell drop-out [191]. The more common of the two is AD which currently affects approximately 6 million Americans and this number is expected to exceed 14 million by 2050 as the “baby boomers” age [192]. While PD, ALS, HD and MS are less common each still affects large numbers of people. For example, over a million Americans are living with PD [193], 400,000 with MS [194], 30,000 with ALS [195] and another 30,000 with HD [196]. From an economic standpoint alone, the costs associated with caring for patients is staggering. In 2018 medical costs billed to Medicare and Medicaid associated only with AD exceeded $186 billion and accounted for 67% of associated costs. An additional $90 billion of the total costs came from patients or their families. If left unchecked the total medical expenditures (including out of pocket expenses, Medicare and Medicaid) will exceed $1 trillion by 2050 [197, 198].
In many cases, current treatments are inadequate to affect disease progression or even ameliorate symptoms of neurodegeneration. In addition, diagnosis of disease may not occur until such time that the disease course is no longer alterable. The futility of current treatments and diagnostic strategies to combat underlying neurological pathologies demands a solution, as it constitutes an enormous psychological, economic and physical burden on patients and caregivers. In addition to ongoing investigations to uncover the underlying etiology of neurodegenerative diseases, the fight against these diseases is hindered by the blood-brain barrier (BBB) [199]. Because of this anatomical and functional barrier, current attempts to treat neurodegenerative disorders usually involve flooding the peripheral blood system with drug in the hope that a percentage will make its way into the CNS. This approach, though, is inefficient, expensive, and leads to a number of off target toxicities. While blood vessels that vascularize, the nervous system allow tight regulation of the movement of ions, molecules, and cells from the blood to the brain. This controls brain homeostasis and facilitates functional neuronal control while at the same time protecting neural tissue from toxins and pathogens. Changes in BBB biology also underlie disease pathology and progression within the nervous system. The physiological barrier is coordinated by endothelial cells. These form the walls of blood vessels. What regulates BBB function are the vascular, lymphatic immune, glial and neuronal cell interactions and secretory factors. Affecting each or all of these cell populations can regulate BBB function during disease. The end result is BBB and neuronal damage [184, 200] that affects ingress of cells, drug and macromolecules and can be harnessed for therapeutic gain.
3.1. Blood brain barrier (BBB).
The BBB as a concept was first introduced to explain a 19th century observation that basic dyes, when injected into blood, do not enter the brain. It was hypothesized that some barrier existed between the blood and brain. Indeed, in the 1960s the arterioles, venules, and capillaries of the brain were found to differ from those structures located elsewhere in the body in a few important ways. First, tight junctions between blood capillary endothelial cells drastically reduce the space between cells forming a tight wall [202]. Second, the endothelial cells of the brain capillaries have greatly reduced pinocytosis making the uptake of molecules into these cells more challenging [202]. Third, cellular fenestrations and other forms of intracellular gaps are virtually non-existent. These three features ensure that plasma proteins such as albumin do not travel from the blood into the CNS [203]. Selectively located pericytes within the basement membrane also provide support and metabolic functions (Fig. 4) [204, 205]. It is also important to note that the vascular BBB is not the only part of the conceptual BBB. The choroid plexus consists of similarly modified ependymal cells that act as a blood-cerebral spinal fluid barrier [206]. The tanycytic barriers that regulate hormonal passage from the hypothalamus to the blood stream along with specialized barriers in the retina and the cranial/spinal nerves are more recently discovered components of the BBB [207, 208]. The BBB has a complex ultrastructure and is a gatekeeper for outgoing and incoming molecules as well as vitamins, minerals, biomolecules and hormones. It acts as a rate limiting diffusion barrier between the brain and the rest of the body. As such, the BBB is a significant obstacle for entry of any particulate matter into the brain, which makes treatment of neurodegenerative diseases very challenging. Even at high doses, localization of nanoparticles in diseased areas of the brain is minimal. There are several reasons for the limited success rate of nanoparticles targeted to the brain. The most common reasons are: (a) injected nanoparticles undergo modifications by proteins and enzymes and bind to other macromolecules leading to off-site targeting which results in “nonspecific uptake by healthy cells and tissues” [209–211]; (b) complex nanoparticle fluid dynamics in blood vessels [212] and instability of target molecules on nanoparticles [213]; (c) limited number of and variability in cell-targeted biomarkers on diseased cells [214], highly selective barriers [215, 216], and limited selected transporters for specific cells [214]; and (d) biochemical variables, such as pH differences, in tissues and targeted organs [217]. Therefore, a basic understanding of the functional and structural properties of the BBB plays a crucial role in the design of theranostic nanomedicine targeted to the brain [91]. The complex structure of the BBB, therefore, poses a significant obstacle that nanoparticles must overcome to reach sites within the brain [218]. In addition to restricting drug transport, the selective nature of the BBB limits disease diagnosis. This is based on the fact that 100% of the macromolecular drugs and over 98% of the smallmolecule drugs are incapable of crossing the BBB [219, 220]. However, the population of patients with brain disease is increasing and has generated a significant unmet need for the development of nanomedicine platforms that can facilitate drug transport across the BBB and localize the therapeutic molecules effectively to target sites within the CNS. To combat this problem, theranostic nanoplatforms are being evaluated and developed to improve brain delivery [221–223]. These present opportunities for use as a real-time non-invasive bioimaging platform for early diagnostics, evaluation of drug biodistribution and localization and treatments. The multimodal nature of the developed nanoparticles has been used to successfully target brain drug delivery by various researchers [224, 225].
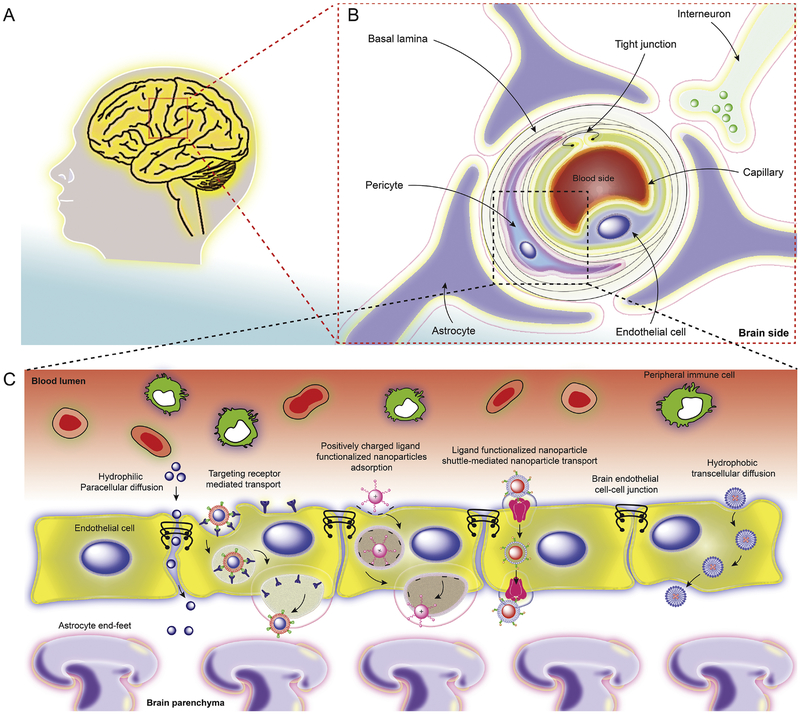
Fig. 4. The structural and functional components of the BBB.
(A) Human brain cross-section and (B) cellular structure, and schematic representation of the BBB including, endothelial cells, astrocytes, tight junctions and transporters. (C) Several putative mechanisms for theranostic nanoparticles trafficking across the BBB. This includes, but is not limited to, passive transport of hydrophilic nanoparticles by paracellular diffusion and limited by endothelial tight junctions. Targeting insulin and transferrin receptors mediates transcytosis by functionalization of nanoparticles with antibody and ligands [73, 236]. Nanoparticles with high positive zeta potential (> 15 mV) show facilitated BBB passage [237–239]. Smaller hydrophobic and lipophilic nanoparticles cross the BBB by diffusion across endothelial cells [240, 241].
The movement of any drug(s) or therapeutic molecules across the BBB can occur through well-described mechanisms that include paracellular and transcellular transport and adsorptive transcytosis. Paracellular transport occurs passively between endothelial cells, utilizing co-transport of small ions and solutes and its rate is dictated by an electrochemical gradient. The presence of tight junctions between the endothelial cells severely limits the passive transport of any nanoscale agents across the BBB. Water-soluble, small molecules, such as carbon dioxide, oxygen, and small lipid soluble molecules can cross the BBB by transcellular diffusion. During active transport across the BBB, the passage of molecules depends largely on the presence and type of transmembrane glycoprotein receptors. This process can facilitate the crossing of nanoparticles, macromolecules, proteins and peptides having high surface charge, polarity, and lipophilicity. Thus, the surface functionality of the nanoparticle, apart from size, will play an important role in identifying the specific receptors and facilitating the passage of any agent across the BBB [144]. A third way for molecules and drugs to cross the BBB is through receptor-mediated endocytosis by the way of caveolae and clathrin-mediated endocytsosis [226, 227]. Caveolae-mediated endocytosis occurs through specialized “microdomains” within the plasma membrane which are referred to as caveolae [228]. Caveolae are small flask-shape pits (approximately 50 nm in diameter) in the membrane that resemble the shape of a cave. The principal membrane components of caveolae are the caveloin proteins whose expression induce and are required for the formation of caveolae [229]. Although the function of these structures has yet to be fully elucidated, but they have been implicated in the endocytosis of certain metabolites [229] [230]. Clathrin-mediated endocytosis is a process by which high-affinity transmembrane receptors and their bound ligands are concentrated into “coated pits” on the plasma membrane. These coated pits are formed by the assembly of cytosolic coat proteins, the main component being the protein, clathrin [231]. These coated pits invaginate and with the help of scission proteins, pinch off to form endocytic vesicles that are encapsulated by the polygonal clathrin coat protein that will carry receptor-ligand complexes into the cell [232, 233]. Some drugs are also transported via ATP-binding cassette transporters (ABCs) [234]. It has been observed that hydrophilic nanoparticles prefer paracellular diffusion via the tight junctions while hydrophobic nanoparticles prefer transcellular transport. Positively charged nanoparticles are transported via negatively charged plasma membrane caveolae of endothelial cells. Some researchers have used negatively charged quantum rods (QRs) for selective targeting of inorganic nanoparticles to neurons. They observed that negatively charged QRs administered at a low concentration (10 nM) interact with the neuronal membrane, whereas positively and neutrally charged QRs never localize to neurons. They proposed that the presence of negatively charged QRs on neuronal cell membranes influences the excitability of neurons by causing an increase in the amplitude and frequency of spontaneous postsynaptic currents at the single cell level and an increase of both the spiking activity and synchronous firing at the neural network level. To this effect, negatively charged QRs of different lengths and diameters were added to primary hippocampal neurons. A fluorescent signal was seen within 10 min of treatment suggesting rapid localization of the QRs to the neuronal cell membrane. Similar results were observed with spherical quantum dots, suggesting a primary role for the negative charge in neuronal localization. Experiments conducted to observe the interaction with neurons showed that nanoparticles interact solely with the neuronal membrane and this interaction is mediated by neuronal spiking activity. It was also shown that negatively charged nanoparticles not only increase the global spiking activity of the network but also the spiking synchronicity of the network, up to a plateau of about −20 mV. It was also noted that negatively charged nanoparticles are able to trigger an overall increase in neuronal and synaptic activity. A theoretical simulation model explained that the potential on the outer neuron surface only retains negative nanoparticles. These observations suggest that electric activity most likely plays a role in the specificity of the nanoparticle–neuron interaction [235]. The nanoparticles, which are functionalized with targeting moieties, initiate the receptor-mediated transport across the BBB [135]. Adsorptive-mediated transcytosis can also facilitate delivery of medicines that are encased in nanoparticles across the BBB. This is notable as the BBB allows binding and uptake of cationic molecules to the luminal surface of endothelial cells. Once binding occurs exocytosis can then be facilitated at the abluminal surface. These possible mechanisms involved in nanoparticle trafficking across the BBB are schematically summarized in Fig.4.
4. Neurotheronostics for degenerative disorders
4.1.1. Alzheimer’s disease (AD) and associated dementias.
In 1906 a German neuropathologist and psychiatrist named Alois Alzheimer gave a lecture at the Southwest German Psychiatrists meeting in Tübingen, Germany. In his talk, he detailed the clinical history of a relatively young patient he had treated. The patient suffered from a chronic, progressive neurological disorder that caused cognitive impairment, hallucinations, delusions, and social deficits. There was rapid loss of memory, disorientation in time and space that progressed to the patient becoming bedridden and incontinent before proceeding to death in just 4.5 years after onset of symptoms [242, 243]. Upon autopsy, a novel observation in the form of neurofibrillary plaques in the neurons of the patient’s cortex was made [244]. Plaques like these had not previously been associated with dementia and from further research a new concept emerged that perhaps senile dementia was a treatable disease instead of a normal unstoppable event associated with aging [243]. As more patients were diagnosed with senile dementia, a greater number were identified with symptoms characteristic of AD.
Today, AD is the most commonly diagnosed neurological disorder affecting millions of people worldwide. In the United States alone, it is estimated that 5.2 million people over the age of 65 have AD [245]. This number is expected to increase drastically by mid-century as more of the “baby-boomer” generation reaches the seventh and eight decades of life [246]. Among developed nations, the chance of being diagnosed with AD doubles every five years after 65 years of age. Further, 10% of people aged 65 or older are affected by some form of dementia and this proportion increases to 33% after age 85 [247]. Around the world, roughly 26 million people were living with AD in 2006 and the number is expected to increase to over 100 million by 2050 [248]. AD and other dementias currently represent an extreme economic burden to society, which will only become more profound over time. In 2018, total healthcare payments related to AD in the US are estimated to be $277 billion dollars [249]. If the number of people with AD quadruples by 2050 as projected, the total costs related to AD care could reach over $1 trillion. It is anticipated that even modest advancements in the preventive and therapeutic strategies could slow the onset and progression of the disease and result in a significant reduction of the economic burden [250]. Early in the disease, symptoms are usually mild and are often overlooked. The most common early signs include a disruption of daily life by memory loss, especially forgetting recently learned information, reduced primacy effect (enhanced ability to recall the first item in a list), and an impaired priming ability (the memory gained from prior exposure to a stimulus) [251]. As the disease progresses, deficits in spoken and written language appear. Patients begin using simpler grammatical structures in their speech as their semantic memory and executive control of verbal fluency deteriorates [252] until mutism and echolalia ensues. Executive dysfunction also manifests relatively early in the disease. Patients have challenges in planning and solving problems, increasing difficulty with familiar tasks, trouble understanding images and spatial relationships, as well as poor judgement [249]. Behavioral symptoms include withdrawal from work and social life, agitation, anxiety, irritability, apathy, increased confusion, wandering, aberrant motor behavior and vocalizations, delusions, hallucinations, dysphoria, and insomnia [253].
The clinical diagnosis of AD relies upon family history, symptoms of dementia, and a plethora of AD imaging and blood biomarkers [254]. Physicians look for pathological levels of Aβ or tau (tubulin-associated unit) protein in cerebrospinal fluid. Additionally, positron emission tomography (PET) is employed to identify amyloid deposits in the brain [255, 256]. However, a definitive diagnosis can only be obtained by post-mortem examination [257]. Detection of extracellular Aβ-peptide fibrils, intracellular neurofibrillary tangles, and high levels of phosphorylated tau protein are needed to fully confirm an AD diagnosis [258]. AD is characterized by the functional and numerical loss of neurons affected by increase in tau protein tangles and loss in neural receptors [259]. These diminish neurotransmission and affect cognitive function [260, 261]. The tangles and the accumulation of amyloid plaques affect neuroinflammation present commonly present in AD brains, which are known to affect memory formation and recall of recent tasks and life events [262]. All are associated with reductions in synaptic proteins in brain subregions, resulting in behavioral impairment associated with changes in the brain’s microenvironment as well as neuronal cytoskeletal changes and cell death [263]. Indeed, the pathological hallmarks of AD are synaptic loss and neuronal loss, decreases in neurotransmitters, and the abundance of extracellular amyloid β-peptide (Aβ) fibrils and intracellular neurofibrillary tangles [264]. An AD neuropathological hallmark is neurofibrillary tangles that include intraneuronal-paired helical filaments of hyperphosphorylated tau and Aβ, a 40–43 amino acids fragment of the amyloid precursor protein (APP) [265, 266]. APP is expressed in the brain, spinal cord, retina, thymus, spleen, all types of muscle, kidney, lung, gut, pancreas, prostate gland and thyroid gland [267, 268]. APP has the characteristics of a cell surface receptor, with the Aβ domain found within the cell membrane. However, the normal function of APP is not completely known, although there is evidence that it plays a role in synaptic formation and plasticity [269]. APP is cleaved by α- or β-secretases, both of which produce an extracellular soluble APP fragment [270]. The α-secretase cleavage site lies within the Aβ domain while the β-secretase cleavage site lies outside this domain. This is an important difference because in the next step, γ-secretase cleaves the α- secretase product into a harmless fragment termed the p3 fragment [271]. However, γ-secretase cleaves the β-secretase product into disordered peptides of 38–42 amino acids collectively referred to as Aβ [272]. The most common isoforms are Aβ40 and Aβ42, which consist of 40 and 42 amino acids respectively. The best evidence for Aβ’s involvement in disease comes from the study of patients with early-onset disease [273, 274]. These patients generally have mutations in one of three genes; APP, PSEN1 or PSEN2 [275]. All three lead to an overproduction Aβ42 which can undergo conformational changes that induce aggregation of soluble peptide fragments into large fibrils that become insoluble plaques [272](Fig.3). Aβ plaque toxicity is mediated by multiple mechanisms that include, but are not limited to, oxidative stress, mitochondrial dysfunction, increased membrane permeability, microglial activation, synaptic dysfunction, and excitotoxicity [276–280]. The Aβ plaques first form in the basal cortex but spread gradually to most associative neocortical regions with the exception of the hippocampus [281]. Sensory and motor areas are generally spared until the very late stages of the disease [260, 266].
In addition to the extracellular Aβ accumulation, AD is characterized by an intracellular accumulation of hyper-phosphorylated tau protein [282]. Tau is a microtubule-associated protein that functions to stabilize neuronal cytoskeleton microtubules and is mainly localized to axons [283]. Hyperphosphorylated tau does not associate with microtubules as strongly as its unphosphorylated counterpart, resulting in destabilization of microtubules [284]. The abnormal phosphorylation of tau makes the protein much more prone to aggregation. However, phosphorylation of tau alone is not enough to induce aggregation indicating that a second insult may work in an additive or synergistic manner with hyperphosphorylation of tau to affect aggregation [285] [286]. Apolipoprotein E (APOE) and its polymorphic alleles are the strongest genetic risk factor for developing the sporadic form of AD [287]. APOE is responsible for lipid and protein homeostasis and is primarily produced by astrocytes and microglia in the brain [288]. APOE was first found to be associated with AD when APOE immunoreactivity was observed in Aβ deposits and tangles [289]. There are 3 common isoforms of APOE; APOE2, APOE3, and APOE4 which differ by only one or two amino acids. The population prevalence of the APOE3 isoform is roughly 78%, with APOE4 at 15% and 7% for APOE2 [290]. Relatively limited studies have been done the role of APOE2 in relation to AD. Most studies indicate that APOE2 is a neuroprotective agent, however, there are studies that indicate the opposite effect. Nagy et al., found that APOE2 provided protection against both amyloid deposition and neurofibrillary tangle formation in AD patients [291]. Similarly studies suggest that APOE2 plays a major role in Aβ clearance and can reduce the risk of cognitive destruction [292]. In contrast, Berlau et al., found that APOE2 expression is related to a decreased risk of dementia but increased AD neuropathology [293]. Population studies suggest a 3-fold increase in the risk of developing AD if one allele of APOE4 is present in an individual’s genome and a 12-fold increase if there are two alleles [294]. Conversely, experiments in mouse models have shown that APOE3 decreases Aβ accumulation relative to whether there are one or two alleles of APOE3 present [295]. In APOE knock-in mice, clearance of Aβ from the CNS to the plasma depends on which alleles are present in the genome. APOE4 isoforms lead to the slowest clearance of Aβ and are dose-dependently associated with increased Aβ deposits [296]. Studies like this indicate that APOE may act as a chaperone for clearing Aβ from the CNS and any changes in the rate of this clearance can be detrimental to cellular physiology.
Currently there are only five medications approved by the United States Food and Drug Administration (FDA) to combat AD. Four are cholinesterase inhibitors (tacrine, donepezil, rivastigmine, galantamine) and the fifth is an N-methyl-D-aspartate receptor antagonist (memantine) [297]. These drugs are short lived, the efficacy varies from person to person, and they cannot stop the progression of neuronal damage and loss [298]. Incidentally, the discovery of new drugs has been challenging. In fact, between 2002–2012 only one drug (memantine), out of 244 drug candidates, successfully completed clinical trials and was granted approval by the FDA [297]. There are many reasons for the very high failure rate. In addition to doubts that animal models faithfully recapitulate AD in humans, most drug candidates have been unable to provide any added benefit over placebo, cause unacceptable toxicities, or fail to cross the BBB well enough to relieve neurological symptoms [299]. Recently, Becker and Greiga published several reviews on flaws in AD clinical trials and provided a detailed rationale for why such failures occurred. First, there are many differences in how studies are conducted among clinical trial centers. Second, there are problems in identifying homogeneous groups and managing large numbers of subjects in a clinical trial. Third, there is a lack of knowledge of the pharmacological effects of new drugs. Fourth, there are flaws in study design, management and methodology, for example, poor design of testable hypotheses that can clearly explain the AD conditions, timing of AD neuropathologies and lack of clinical efficacy of drugs used. Finally, misinterpretation of drug effects can affect the study’s conclusions [300–302].
Nanotheranostics provides an exciting opportunity to overcome these limitations. Research into nanotechnology and targeted drug delivery has revealed some general considerations for getting nanoparticles across the BBB; they should be relatively small (<500 Da), lipid soluble and have a neutral surface charge [303]. Indeed, small lipid molecules are favored for transport. Here we review current research into using nanotheranostic approaches for effective drug release/targeting [304–306], surface-engineered nanoparticles for imaging/diagnosis purposes [307–310], and finally surface-engineered nanoparticles investigated as theranostics (simultaneous drug therapy and imaging for AD [310–312].
4.1.2. AD and theranostics.
(a). AD diagnosis.
The staging and treatment of AD remains limited despite it being the most prevalent neurodegenerative disease [313, 314]. Early diagnosis in AD, before the start of clinical symptoms, is a crucial step in preventing the irreversible neuronal damage that eventually leads to dementia and ultimately death [315, 316]. Presently, two types of approaches are practiced clinically for the diagnosis of AD; first being in vivo brain imaging of brain amyloid, or inflammation [317], and the second being neuropsychological, cognitive, and neurological assays [318, 319] such as measurement of Aβ (specifically Aβ42), total tau or phosphorylated tau levels in cerebrospinal fluid (CSF) [320]. Since there is no single test or method available for the real time detection of AD progression, there is an urgent need for crucial biomedical technology to facilitate rapid detection of specific biomarkers and proteins that demonstrate the location and density of amyloid plaques in the living human brain [321]. Theranostic nanoparticles can serve as nucleation centers for amyloid fibrillation. The interaction of the nanoparticles with amyloid proteins can lead to the formation of intermediate structures that can accelerate or decelerate amyloid fibrillation and hence used as an important device to manipulate fibril formation and provide therapeutic potential. They also have superparamagnetic properties and have been shown to cross the BBB and accumulate in regions of the brain in measurable concentrations [322].
(i). Brain Imaging.
In the last few decades, considerable research has been focused on the development of nanoparticle-based imaging techniques using modalities such as MRI, single-photon emission computed tomography (SPECT) and PET to visualize amyloid plaques in AD patients [317]. Imaging of Aβ and tau pathology with SPECT/CT and MRI has not been well-studied, however, Zhu et al propose that these techniques could greatly improve our understanding of the pathophysiology and treatment of AD which would better guide physicians in the treatment of each patient [323]. PET scanning was recently approved by the FDA for clinical imaging of both Aβ and tau [324], which will contribute significantly to the early diagnosis, differential diagnosis, and the tracking of disease progression during the preclinical, prodromal, and clinical stages of AD. MRI is a key tool in distinguishing between AD and other degenerative causes of dementia [325]. Skaat et. al., recently developed a novel method for selective marking of Aβ40 fibrils by fluorescent-maghemite nanoparticles for early detection of plaques using MRI and fluorescence microscopy for in vivo diagnosis of AD [326]. Similarly, Bingbing et.al., reported a system based on the magnetic properties of iron oxide nanoparticles for MRI detection of amyloid plaques and targeted delivery of AD therapeutic agents. To obtain iron oxide nanoparticles, oleic acid coated magnetic iron oxide particles were synthesized. 1, 2-Distearoyl-sn-glycero-3-phosphoethanolaminepoly (ethylene glycol) (DSPE-PEG)-Congo red and DSPE-PEG-phenylboronic acid were used to improve the biocompatibility of these oleic acid coated nanoparticles via micelle formation. The hydrophilic drug rutin was then grafted onto the surface of the nanoparticles via simple conjugation chemistry. Congo red/rutin-magnetic nanoparticles, when co-administered with mannitol, could penetrate the BBB of APPswe/PS1dE9 transgenic mice and bind to amyloid plaques enabling detection of these plaques by MRI and achieving targeted drug delivery [327]. Investigators used magnetic nanoparticles conjugated with curcumin that specifically bind to amyloid plaques. These nanoparticles were used to visualize amyloid plaques by ex vivo T2*-weighted MRI in Tg2576 mouse brains [328]. Others also developed bovine serum albumin (BSA) coated high magnetic relaxivity nanoparticles and further functionalized them with sialic acid (nanoformulated NBSAx‐Sia) for Aβ imaging [329]. T2*‐weighted MRI showed that NBSAx‐Sia binds with Aβ in a sialic acid dependent manner with high selectivity towards Aβ deposited in neuronal cells. Moreover, investigators used an Aβ oligomer-specific antibody conjugated to nitro-dopamine (nDOPA) and PEG-stabilized 12–16 nm magnetic nanostructures (MNS). MNS–antibody conjugates can detect AD causing toxic oligomers on nerve cell surfaces by MRI [330]. Similarly, Yang et. al., investigated decoration of ultrasmall superparamagnetic iron oxide (USPIO) particles with an Aβ targeting peptide for detection of AD plaques using T2*-weighted microimaging. The T2* values demonstrated significant contrast-injected in APP/PS1 mice compared to control mice after injected with USPIO-Aβ1–42[331]. Recently, other investigators used different types of polymeric and metal ion complexes as MRI contrast agents in AD diagnostics. For example, prepared biodegradable nanocarrier systems made up of poly(n-butyl cyanoacrylate) dextran polymers coated with polysorbate 80 deliver their payloads across the BBB. Whole brain MRI can be used to visualize amyloid plaques in a mouse model of human disease [332]. Investigators developed nanoparticles with ET6–21 (E)-2,2′-[4-(2-(pyrimidin-4-yl)vinyl) phenyl]azanediyl}diethanol) conjugated to detect amyloid pathology in mouse models [333]. Chelated gadolinium and indocyanine green were included in the particles for visualization by MRI and near-infrared microscopy. Their studies demonstrated elevated signal in the brains of mice with amyloid plaques using magnetic resonance imaging (T1-MRI) that was conducted 4 days post-injection. Functional alterations in the AD patient’s brain assessed by SPECT and PET could provide diagnosis that is more efficient at monitoring progression of AD [334]. One can visually detect soluble Aβ by PET (Fig. 5).
Fig. 5. Schematic representation of the clinical role of theranostic nanoparticles.
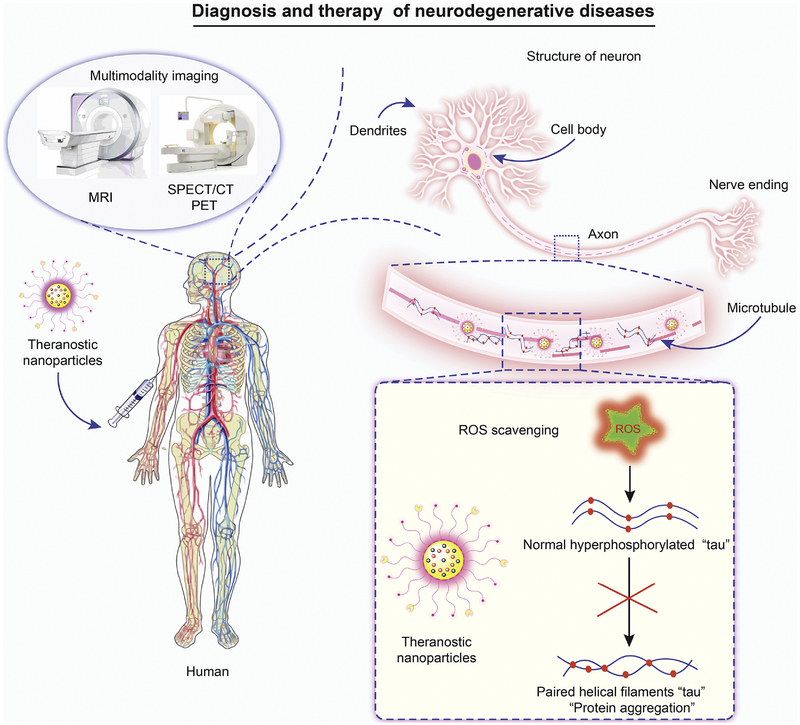
Schematic representation of tau pathogenesis with theranostic nanoparticles: Formation of neurofibrillary tangles by the tau protein in Alzheimer’s disease (AD) tauopathies. In pathological states tau becomes hyperphosphorylated and detaches from microtubules. Phosphorylated tau then aggregates to form paired helical filaments and neurofibrillary tangles. Here multifunctional theranostic nanoparticles injected into an AD patient precisely target hyperphosphorylated tau. Particles can have ROS scavenging, drug release and bioimaging capabilities. These nanoparticles can scavenge ROS to inhibit hyperphosphorylation of tau, aggregation and release drug. This leads to neuroprotection from ROS mediated cell damage.
Images of the brain can visualize disease using PET scans administered with radiolabeled mAb158 [335]. The transferrin receptor antibody facilitated receptormediated transcytosis across the BBB. These investigators observed that the PET signal increased with age and correlated with brain Aβ levels. Similarly, investigators fabricated ultrasmall ceria nanocrystals (CeNCs) and iron oxide nanocrystals (IONCs) based on a multifunctional nanocomposite to target hyperphosphorylated tau protein. These multifunctional nanocomposites were functionalized with amino-T807 and grafted onto the surface of mesoporous silica particles for active hyperphosphorylated tau targeting. This novel tracer was labeled with 68Ga for monitoring tau protein in vivo by MR/PET imaging [336] (Fig. 5). The SPECT images of Aβ plaques in rhesus monkeys using oligoethyleneoxy-99mTc-labeled probes showed significantly enhanced brain uptake [337]. However, SPECT imaging is limited clinically in diagnosing AD compared to PET imaging due to the variable prognostic precision of SPECT images [338]. Development of multi-modal imaging technologies using various particles for design and decoration of targeting ligands will assist in rapid and precise AD diagnosis.
(ii). Disease biomarkers.
Identification of biomarkers for diagnosis of AD and associated dementia have become increasingly important. At present, detection of Aβ1–42, total tau and phospho-tau-181 in CSF by ELISA assay is the most common clinical technique. Sensitivity and specificity of AD diagnosis is made possible by combining all three CSF biomarkers [339]. However, it is a huge challenge to detect and quantify specific biomarkers in CSF and blood using nanoprobe technology. Antibody labeled magnetic nanoparticles is a sensitive and highly specific method for early detection of AD biomarkers. Recently, magnetic biofunctionalized nanoparticles were developed employing antibodies as immunomagnetic reducing reagent against β-amyloid-40 (Aβ-40) [340]. The detection limit for Aβs using the magnetic nanoparticles via immunomagnetic reduction was determined to be ~10 ppt (10 pg/mL). Using this detection system, they showed a significant difference between Aβ-40 and Aβ-42 concentrations in human plasma from normal individuals and AD patients. Similarly, monoclonal anti-tau antibody-coated gold nanoparticles were employed with a two-photon scattering assay to develop a detection method with 16 times greater sensitivity than previously reported detection methods for AD tau protein. The detection limit was as low as 1 pg/mL and orders of magnitude lower than cutoff values (195 pg/mL) for tau protein in CSF [341]. Gold nanoparticle based dot-blot immunoassay was also developed and demonstrated detection of AD related Aβ peptide 1–42 (Aβ1–42) at a concentration as low as 50 pg/mL [342]. A novel immuno-polymerase chain reaction (Nano-iPCR) method was developed where gold nanoparticle-tagged tau-specific monoclonal antibodies and oligonucleotide templates are used to quantitate tau protein in human CSF. Nano-iPCR is more sensitive compared to a commercial ELISA kit [343]. Another novel method used Aβ-targeted fluorescent conjugated liposomes in which the targeting moiety was the highly specific Aβ plaque ligand (methoxy-XO4) [344]. These particles were tested in an AD mouse model (APP/PSEN1 transgenic mice) to determine their ability to bind amyloid plaque deposits. They observed that the particles bound to synthetic Aβ aggregates with greater specificity to the free ligand, and selectively bound Aβ plaque deposits in brain tissue sections with high efficiency. However, most of the reported techniques are generally expensive, laborious with low sensitivity. Currently, electrochemical biosensors are extensively used in clinical diagnosis due to their ease of use, high sensitivity and rapid results [345]. Recently, a shape-code biosensor was made for detection of AD core biomarkers by using localized surface plasmon resonance (LSPR). They determined a detection limit of 34.9 fM for Aβ1–40, 26 fM for Aβ1–42 and 23.6 fM for tau protein corresponding to the ~ 1.0, 2.23 and 3.12 nm of Rayleigh scattering peak shift on a shape-code plasmon system for each biomarker, respectively, in mimicked blood [346]. Another study described a gold-capped nanoparticle LSPR-based immunochip for detection of 10 pg/mL tau in CSF. This technique was much more sensitive compared to ELISA and could analyze up to 300 samples per chip in a single run [347]. Similarly, others used direct label-free detection of 17-beta-hydroxysteroid dehydrogenase types 10 (17β-HSD10) peptide, a mitochondrial enzyme that is involved in AD pathogenesis using a surface plasmon resonance (SPR) biosensor. They used alkylthiolates and amino coupling chemistry for functionalization of Aβ immobilized on the sensor surface or polyclonal antibody against a 17β-HSD10 peptide. The 17β-HSD10-enzyme assay tested in artificial CSF buffer could detect ng/ml levels by high affinity binding of Aβ40 to 17β-HSD10 enzymes [348]. Other investigators used multi-walled carbon nanotubes (MWCNTs) modified with a secondary antibody for detection of tau protein using SPR. They used MWCNTs-antibody conjugate to develop a sandwich-based bioassay with the capability to increase the SPR signal around 100-fold compared to direct detection and conventional unconjugated sandwich assays [349]. The oligomeric forms of Aβ called Aβ-derived diffusible ligands (ADDLs) produced from APP are most toxic to brain neurons [350]. The brain, CSF and blood of AD patients were found to have high levels of ADDLs making it a reliable and noninvasive diagnostic biomarker. The bio-barcode amplification system is illustrated as an example of ADDL detection (Fig.6).
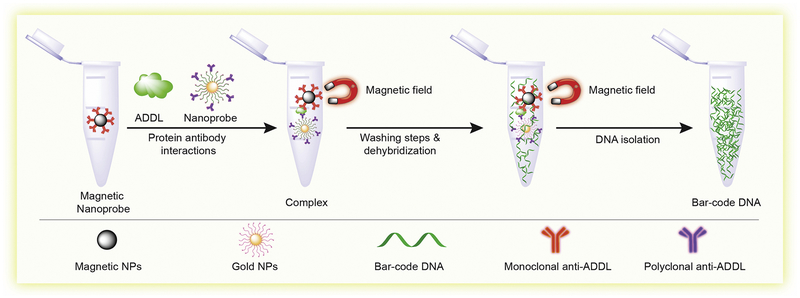
Fig. 6. Bio-barcode amplification.
This assay was developed with the aim to isolate amyloid-β-derived diffusible ligands (ADDLs) concentrated in the CSF. First step, anti ADDLs mAbs were decorated onto magnetic nanoparticles. Second step, double-stranded DNA functionalized gold nanoparticles were allowed to bind the target antigen to create a magnetic nanoparticles complex. Last step, the sandwich complexes were then magnetically separated and collected as barcode DNA (Concept of assay form reference number [351]).
Interestingly, an ultrasensitive bio-barcode assay for measurement of ADDLs concentrations in the CSF was developed. The bio-barcode assay was designed by functionalization of gold nanoparticles and magnetic micro particles with antibodies to ADDLs and used for specific antigen isolation by a sandwich process. They found that ADDLs concentrations for subjects diagnosed with AD were consistently higher than controls. This study was a step towards development of a diagnostic tool based on soluble pathogenic markers for AD [351] (Fig. 6). In a separate study, researchers developed a novel biosensor using an electrochemical redox-generating hydroxyapatite probe to measure the activity of the protease BACE1 (the β-site amyloid precursor protein cleaving enzyme-1) with detection limits as low as 0.1 U/mL BACE1 catalyzes the first step in the synthesis of Aβ peptides that accumulate in the brain in AD [352]. Apart from Aβ, several investigators also looked into using total tau and phospho-tau biomarkers as alternative tools for AD diagnosis. Recently, a highly sensitive and selective assay for acetylcholinesterase (AChE) detection was created based on rhodamine B-modified gold nanoparticles. The uniqueness of this assay was dual readouts by colorimetric and fluorometric techniques. They used the assay to monitor AChE levels in the CSF of transgenic mice with AD, with a detection limit of 0.1 mU/mL. This technology has potential use for early diagnostics and prognostics of AD [353]. Among all the above-mentioned techniques, biosensors have proven to be the most advanced technology for detection of biomarkers, as they are highly sensitive, precise, facilitate rapid analysis over a wide range of concentrations, and enable real time monitoring of specific biomarkers.
(b). AD treatment.
In the present scenario, there are no direct therapy or treatment options available for AD patients. However, cognitive and behavioral symptomatic treatments by using various therapeutic approaches may be realistic. Many investigators are working on alternative treatments to slow and change the progression of the disease and improve the quality of life of people with dementia. Moreover, drug delivery with the help of therapeutic nanoparticles with ultra-small size (1–100 nm) can efficiently pass the BBB [354]. Recently, a “redox silence” approach was employed to treat AD. This was achieved by synthesizing a prototype nanoparticle–chelator conjugate (Nano-N2PY). These particles demonstrated an ability to protect human brain cells from Aβ-related toxicity by Nano-N2PY–chelator conjugates that inhibited Aβ aggregate formation [355]. Similarly, other investigators used graphene oxide nanoparticles (GO) for AD treatment by using heat from NIR laser irradiation to dissociate amyloid aggregates. Further, functionalized GO–thioflavin-S (ThS) showed a robust ability in mouse CSF to dissociate amyloid deposits and thus protect cells from Aβ-related toxicity upon NIR irradiation. Disaggregation of Aβ fibrils was shown by fluorescence [170]. Thioflavin (ThT) and another fluorescent marker NIAD-4 were also used to detect and help in removal of amyloid protein aggregates by NIR [356]. Similarly, graphene quantum dots (GQDs) covalently linked to tramiprosate, exhibited great ability to reverse aggregation of Aβ peptides by breakdown of the β-sheet structure and reduction of Aβ-related cytotoxicity [162]. Fernández et. al., demonstrated that a nanoconjugate composed of magnetic nanoparticles bound to an anti-ferritin antibody, recognized and bound specifically to the ferritin protein and accumulated in the subiculum area in the hippocampus in the brain in an AD mouse model [357]. Delivery of fluorescent carboxyl magnetic Nile Red particles (FMNPs) to the brains of normal mice using a functionalized magnetic field (FMF) composed of positive- and negative-pulsed magnetic fields. Nanoparticles successfully reached the cortex and hippocampus of the brain. Under the same FMF conditions, dextran-coated Fe3O4 magnetic nanoparticles loaded with osmotin (OMNP) were transported into the brains of Aβ1–42-treated mice. Compared to native osmotin, the OMNP potently attenuated Aβ1–42-induced synaptic deficits, Aβ accumulation, BACE-1 expression and tau hyperphosphorylation [358]. Brain-targeting sulfur nanoparticles were also used to check the effect of different particle morphologies on Cu2+-induced Aβ aggregation and neurotoxicity. In vitro results showed that spherical nanoparticles maximally reduced Aβ–Cu2+ complex aggregation and increased cell viability by 92.4% [359]. Cerium oxide nanoparticles also showed a similar effect by formation of amyloid peptides and copper ion complexes and protected against neuronal cytotoxicity [168]. A few other research teams have shown similar results [163, 360]. Another research group successfully engineered selenium nanoparticles (SeNPs) with two targeting peptides for inhibiting Aβ aggregation and crossing the BBB [361]. Curcumin is a yellow-colored, plant-derived, polyphenolic compound that has potent pro-inflammatory and anti-inflammatory activities. Detailed experimental studies in recent years have demonstrated that curcumin can be used as a potential drug in the treatment of AD due to its novel property of disaggregating Aβ plaques. To overcome the poor aqueous solubility of curcumin, a polymeric nanoparticle encapsulated curcumin (NanoCurc™) formulation was developed. Neuronally differentiated human neuroblastoma (SK-N-SH) cells were protected from ROS induced oxidative damage by treatment with a range of concentrations of NanoCurc™. Furthermore, NanoCurc™ was found to protect and preserve the neuronal phenotype from ROS mediated damage in NanoCurc™ treated cells in a dose-dependent manner. In vivo studies in athymic mice showed curcumin levels of about 0.322 ng/mg of brain tissue upon intraperitoneal injection suggesting that this formulation is capable of crossing the BBB. NanoCurc™ may also be able to provide an intracellular protective redox environment. Hence, this study showed that NanoCurc™ treatment has a unique capacity to protect, preserve and rescue human neuronal cells against oxidative damage and can ameliorate ROS-mediated damage in both cell culture and in animal models. This ability of NanoCurc™ to achieve significant intracerebral concentrations and offer neuronal protection against oxidative damage, renders it a unique formulation for the treatment of AD [362]. More recently, Luo et. al., demonstrated the design of a self-destructive nanoparticles that relies on the multifunctional ability of peptide-polymers. Acrylate-modified chitosan particles were linked with two functional peptide analogs. Cytotoxicity assays indicated that these particles were non-toxic at a concentration of 20 ug/ml. The synergistic effects of the two functional peptides, KLVFF (m) and beclin-1(n), were explored at different peptide ratios; it was found that structure M3 with peptide ratios of m= 0.5, n= 0.5 enhanced the anti-Aβ toxicity effect. Thioflavin T fluorescence assay was used to confirm the capturing ability of Aβ42 by the KLVFF peptide on the nanosweeper surface. Transmission electron microscopy (TEM) confirmed the binding effect of M3 particles to Aβ42. To confirm the internalization efficiency of these particles, in vitro studies in N2a cells indicated that the co-assembly of M3 and Aβ42 not only inhibited the Aβ42 fibril formation, but also increased the internalization of Aβ42. Further experiments validated that M3 activated autophagy without blockade of the autophagic flux, suggesting the possibility of autophagic degradation of Aβ42. In vivo studies to assess the BBB crossing efficacy and autophagic activity of these particles confirmed brain accumulation and autophagic activity. To conclude, the results could be compiled to indicate that multifunctional nanosweepers are capable of specifically capturing Aβ, delivering it into cells and promoting its degradation by upregulating autophagy. This novel technology serves as a promising therapeutic agent for the treatment of AD [363]. An alternative method for AD treatment was described in a recently published report by Kuo, et al and showed that rosmarinic acid had strong antioxidant activity against peroxynitrite radicals, which are responsible for increasing the amounts of amyloid fibrils in the brain of AD patients. They further formulated quercetin and rosmarinic acid in liposomes functionalized with phosphatidic acid (PA) and ApoE to prevent cerebral neurodegeneration [364]. QU acts as antioxidant flavonoid that has been shown to protect neurons against oxidative stress and improve cognitive function in AD rat models [365]. PA and ApoE have been shown to improve the in vitro Aβ binding of associated drug carriers [364, 366]. In vivo studies have shown that quercetin and rosmarinic acid-loaded PA liposomes reduced AChE activity, a prominent marker of Aβ plaque formation in the AD brain. It was also found that addition of Tween 80 and APoE led to further inhibition of AChE due to improved BBB penetration and neuronal targeting [364]. Taken together, theranostic approaches are more than capable to solve this protein aggregation problem, provide protection from ROS and neuroregeneration and can also cross the BBB.
Alternative treatments based on gene-silencing antisense therapy for AD have also been developed. Farr et al. designed antisense oligonucleotides that can easily bind with the messenger RNA (mRNA) of specific gene targets and then balanced natural protein to toxic protein ratio for reversal of AD symptoms [367]. They demonstrated the successful use of antisense treatment in an AD mouse model by showing reduction in a glycogen synthase kinase (GSK)-3β (GSK-3β). They observed that after treatment with antisense oligonucleotide (GAO) mice showed improved learning and memory. More recently, DeVos et al., designed and showed successful application of a Tau antisense oligonucleotide (Tau ASO-12) in mice and nonhuman primates [368]. They observed that after treatment with Tau ASO-12, tau levels were decreased in AD mice and brain and spinal cord tau levels were reduced in nonhuman primates. Another alternative treatment is the use of monoclonal antibodies (mAbs) to treat AD by passive immunization. Several studies demonstrated that mAbs are effective at removing toxic Aβ components via microglia, complement activation, prevention of the amyloid cascade and inhibition of neurodegeneration and cognitive impairment [369–371]. Banks et al., developed L11.3 and HyL5 human IgM antibodies to AβP and assessed their ability to cross the BBB in SAMP8 mice a murine model of AD. They observed that L11.3, was effectively taken up into the hippocampus and improved cognition in the aged SAMP8 mouse after direct administration into the brain. These results suggested that L11.3 or other human anti-Aβ antibodies might be effective in the treatment of AD [367]. While there is no direct correlation of treatment with antibodies and clearance of AßP antibodies could reduce the association of AßP with the brain microvasculature [372]. Finke et al., demonstrated that the surface sialic acid modified the monoclonal antibodies (4G8) has high uptake compared to nan-modified in the brain of AD patients [373].
4.2.1. Parkinson’s disease (PD) and associated synucleinopathies.
(a). Parkinson’s disease.
It’s been over 200 years since the second most common neurodegenerative and most common movement disorder was first described in 1817. James Parkinson, an English surgeon and paleontologist, penned the monograph “An Essay on the Shaking Palsy” where he described “paralysis agitans”, a condition later renamed PD [374, 375]. A century later, in 1912, Frederick Lewis first described the characteristic intraneuronal inclusions (now termed “Lewy Bodies”) found in post-mortem brains of patients who had PD [376]. The disease is also typified histopathologically by specific losses of dopaminergic neurons in the substantia nigra pars compacta leading to profound dopamine deficiency [377, 378]. This progressive loss of dopaminergic neurons leads to profound motor and neurological symptoms, including static tremors, postural imbalance, bradykinesia, muscle rigidity, impaired olfaction, sleep disorders, gastrointestinal problems and cardiovascular dysfunction [379].
PD affects 2–3% of people older than 65 years of age, with men affected more than women [380]. Although the exact pathological mechanism driving Parkinson’s disease is unknown, evidence from population based studies suggest that a variety of genetic and environmental factors contribute to disease progression [381]. Roughly 95% of cases appear to occur sporadically and risk peaks after 65 years of age. The other 5% of cases generally strike before the patient reaches 40 years of age and is termed familial PD. Monogenic mutations have been associated with over 50% of familial and 5% of sporadic PD occurrences [379]. These genes have elucidated key aspects of the pathogenesis of the disease. The first genetic mutation found to be associated with familial PD was a missense mutation in the protein α-synuclein, encoded by the SNCA/PARK1 gene [382]. Importantly, α-synuclein is the main component in Lewy bodies and becomes phosphorylated, which facilitates synuclein fibril uptake by neurons and accelerates the progression of PD [383]. Misfolded, aggregated α-synuclein amongst other proteins, neuroinflammation, glial activation and ROS production can all affect disease progression [384]. The highest risk for developing familial PD occurs in carriers of a genetic mutation in the LRRK2/PARK8 gene, which encodes the Rab GTPase/kinase leucine-rich repeat kinase 2(LRRK2) protein [385]. Other genetic mutations can occur in genes involved in mitochondria quality control (PINK1), ubiquitin ligases (PARK2), endoplasmic/golgi protein sorting (VPS35), antioxidants (DJ-1), glycolipid catabolism (GBA1), P-type ATPases (ATP13A2), and microtubule associated protein tau (MAPT)[386]. The function of these genes and their contribution to PD pathogenesis remain to be fully elucidated.
Similarly, toxins such as herbicides and pesticides, in conjunction with host genetics and aging, are directly implicated as disease inciting events [387, 388]. One such toxin was discovered serendipitously and has led to the development of a very popular mouse model of PD. In 1976 a chemistry student trying to create a synthetic heroin, instead produced 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which is a potent and specific toxin to dopaminergic neurons [389]. Other heroin users made the mistake of using this synthetic drug and quickly developed PD like symptoms. Many of these patients were seen by Dr. William Langston, (a California based movement disorder specialist) who saw the potential for this toxin to create a disease model of PD [390]. The first published report that identified MPTP as the cause of permanent parkinsonism in heroin abusing patients with linked toxicities to the zona compacta of the substantia nigra appeared in the journal Science in 1983 [391]. The link between PD and MPTP was established based on the clinical findings and later substantiated by neuropathology identifying the trademark loss of nigral neurons and degeneration of their terminal connections in the striatum [392, 393]. However, MPTP shows less of an effect in mouse models than primates, although the C57Bl/6 mouse strain is sensitive to MPTP [394]. Another limitation is that this model causes rapid and transient deterioration of dopaminergic neurons and therefore cannot replicate the chronic pathogenesis of PD [395]. Interestingly, MPTP is structurally similar to a variety of known environmental toxins that have been used to generate other toxin-mediated models of PD such as the herbicide paraquat and the insecticide/fish toxin rotenone [396, 397].
Treatments for PD remain symptomatic and none can stop the loss of dopaminergic neurons and associated motor deficits. Initial treatment strategies for dopamine replacement initiated in the 1960s focused on improvement of motor, gait and postural symptoms linked to disease pathophysiology [398]. Levodopa (L-DOPA) is currently the gold standard treatment option for PD and nearly all PD patients will at some point require dopamine replacement by systemic administration of L-DOPA [399]. However, long term L-DOPA treatment is complicated by the development of L-DOPA induced motor complications (i.e. dyskinesias) [400]. Other treatment strategies include catechol-O-methyltransferase inhibitors to prevent peripheral metabolism of dopamine [401], monoamine oxidase type B inhibitors to prevent oxidative stress in glial cells [402], and deep brain stimulation using high-frequency (100–200 Hz) electrical signals to stimulate the subthalamic nucleus [403].
(b). Synucleinopathies.
Incidentally, PD belongs to the group of neurodegenerative disorders referred to as “synucleinopathies” which are characterized by fibrillary aggregates of α-synuclein observed pathologically in the cytoplasm of selective populations of neurons and glia [404]. Apart from PD, synucleinopathies also include dementia with Lewy bodies (DLB), pure autonomic failure (PAF), and multiple system atrophy (MSA) [405–408]. As a group, they clinically have in common chronic and progressive signs and symptoms and include, but are not limited to, decline in motor, cognitive, behavior, and autonomic functions [408]. The nature of disease is dependent on the brain subregions affected and the extent affected neuronal populations are damaged or destroyed [405]. There is clinical overlap between the different disorders making a precise diagnosis difficult [404]. Motor, gait, agility and coordination deficits are dominant symptoms but differ in severity and duration for PD, DLB and MSA [409]. Autonomic dysfunction, operative in PAF is common in PD, MSA and DLB. Symptom onset and severity is most significant in MSA and DLB [404]. These along with visual hallucinations characterized by widespread cortical pathologies and dementia are less common in PD [410, 411]. However, the deposition of aggregates of synuclein in neurons and glia underlies a common pathogenic mechanism amongst all the disorders [412]. Although synuclein plays an important role in disease development the variant symptom complex, prognosis and management that characterize each disorder suggest that the extent, commonality and severity of the synucleinopathy itself may have limited predictive and diagnostic value in staging disease [404, 412, 413].
Theranostic nanoparticles and personalized medicine may be uniquely poised to better treat neurological disorders like PD and even aid in the development of novel strategies for earlier diagnosis and screening [414]. PD is most likely a multifactorial disease with both upstream and downstream pathogenesis that differ from patient to patient and may affect disease outcome. Indeed, PD symptomatology, disease course and neuropathology varies between patients [380]. Nanotheranostics can be developed to differentially screen PD patients for selection of the optimal treatment strategy. Additionally, experimental therapies such as gene therapy, which have shown both promise and failure, are currently delivered via adeno-associated viral vectors or by direct injection into the putamen [415–417]. One way to overcome this limitation would be to package the gene editing technology into lipid nanoparticles capable of crossing the BBB and targeting appropriate cells in the substantia nigra, delivering treatment directly to where it is needed [414].
4.2.2. PD and theranostics.
The molecular changes in PD patients have been well characterized by imaging modalities in clinical practice [418–420]. This technology permits in vivo quantification of critical physiological factors such as gene and protein functions, protein-protein interactions, glucose metabolism [421] and neuroreceptor binding, allowing better understanding of the molecular pathophysiology of PD [418, 422]. A variety of specific imaging agents have been investigated in order to increase the early diagnosis and therapeutic treatment of PD. Diagnosis using PET by injection of radioisotopes bound to specific tracers is a common technique. Antonini et. al., discovered, for the first time, the relationship between striatal DOPA decarboxylase capacity, D2 dopamine receptor binding, and energy metabolism in PD patients by using PET with radioisotope tracers such as 18F-fluorodeoxyglucose (FDG), 6–18F-fluorol-dopa (FDOPA), and 11C-raclopride (RACLO) [420]. Similarly, 123I-ioflupane or DaTSCAN was used for dopamine transporter (DAT) imaging with SPECT for early diagnosis of psychogenic parkinsonism [423, 424]. Nowadays, nanoparticle based novel approaches are used for the diagnosis of PD. Recently, McDonagh et. al., developed manganese oxide nanoparticles functionalized with L‐DOPA that gradually released Mn2+ ions and L‐DOPA. The former moiety gave a positive contrast in MRI and may be useful for the diagnosis and treatment of PD patients [425]. Accumulation of advanced glycation end products in the extracellular space from activated microglial cells is a key factor in pathogenesis of PD and other neurodegenerative diseases [426–428]. Other researchers demonstrated that AGE-albumin could prove useful as a targeting biomarker for theranostic applications [429]. The most common treatment strategy that is employed in PD is the administration of L-DOPA [430]. However, nearly half of the patients who receive this treatment present with complications within the first five years [431]. Presently, nanomedicine has shown promise as a new direction for the better treatment of PD patients. Because of advancements in nanomedicine drugs, research has been focused on the development of therapeutic nanocarriers that can easily cross the BBB. Mead et. al., developed new techniques for delivery of the glial cell-line derived neurotrophic factor (GDNF) by use of MRI guided focused ultrasound (FUS) and brain-penetrating nanoparticles (BPN). These particles carried the GDNF plasmid (~4 kB) for sufficient expression of the therapeutic protein at targeted brain tissue areas where MRI is applied with FUS. This study examined the striatum of a 6-hydroxydopamine (6-OHDA)-induced rat model of PD for up to 10 weeks and the strategy was successful in restoring dopamine levels, dopaminergic neuron density and reversed behavioral indicators of PD-associated motor dysfunction [432]. Niu et. al., also used novel dual targeted magnetic nanoparticles carrying an shRNA plasmid which could interference with α-synuclein synthesis and effectively repair affected brain regions in vivo PD models by inhibiting further apoptosis [139]. Interestingly, PEG–PLGA nanoparticles conjugated with odorrana lectin conjugation were used to develop a nose-to-brain delivery for PD. The therapeutic efficacy of these particles was tested on hemiparkinsonian rats following intranasal administration. The odorrana lectin conjugated to PEG–PLGA increased the brain delivery of nanoparticles and enhanced the therapeutic effects of urocortin peptide loaded nanoparticles in PD [433]. Similarly, lactoferrin-conjugated PEG-PLGA nanoparticles were used to cross the BBB via clathrin-mediated endocytosis. These particles were tested in rats and found to be effective in decreasing striatal lesions [434]. Pahuja et. al., also developed dopamine-loaded PLGA nanoparticles to deliver dopamine to the brain. These particles were shown to successfully cross the BBB and release drug in the brain parenchyma following systemic IV infusion in a 6-OHDA-induced rat model of PD. The investigators further confirmed competency of PLGA nanoparticles in restoring neurobehavioral and neurochemical deficits in parkinsonian rats [435]. Similar findings were observed in another study where pramipexole dihydrochloride loaded chitosan nanoparticles enhanced antioxidant status by increasing superoxide dismutase and catalase activities, and elevating dopamine levels in the brain in a PD rat model [436]. Chung et. al., showed that dextran-coated iron oxide nanoparticles helped to protect dopaminergic neurons damage [437]. Most recently, Hu et. al., developed a technique using plasmid DNA (pDNA)-loaded gold nanoparticle (GNP) composites. These GNPs via neural growth factor receptor-mediated endocytosis were able to suppress the expression of α-synuclein, inhibiting the apoptosis of PC12 cells and substantia nigra and striatal dopaminergic neurons. Further efficacy of GNPs was confirmed in PD rodent models [438]. Oral administration of apocyanin, a plant-derived molecule, has been shown to be effective in preventing early PD-like symptoms in PD mouse models. Apocyanin has antioxidant and NADPH oxidase inhibiting properties and has been studied in pre-clinical models of PD, while its dimer diapocyanin has been shown to have neuroprotective and anti-neuroinflammatory effects [439]. In the same context, treatment with diapocyanin was found to greatly improve locomotor activity, restore dopamine levels and provide protection to dopaminergic neurons against further degeneration in pre-clinical models of PD. Interestingly, it was also shown that early administration of diapocyanin restored neurochemical deficits and halted disease progression in a chronic mouse model of PD [440]. Similarly, resveratrol, a plant-derived polyphenolic compound, has been shown to relieve oxidative stress and mitochondrial dysfunction in PD models [441]. Due to low aqueous solubility and slow dissolution rate, the clinical use of this drug has been greatly limited. However, Palle et. al., examined resveratrol nanoparticles (NRSV) as a novel drug delivery system for improved bioavailability. NRSV were prepared by antisolvent precipitation method and their neuroprotective efficacy was analyzed by behavioral quantifications. Rotarod performance and other test results indicated increased efficiency of NRSV to ameliorate rotenone-induced PD symptoms, behavioral alterations, oxidative stress and mitochondrial dysfunction in rats compared to free resveratrol [442]. Brenza et. al., implemented the neuroprotective properties of apocyanin to synthesize a folic acid (FA) functionalized lipophilic mitochondria-targeted apocyanin (Mito-Apo) nanoparticles. The antioxidant efficacy of these Mito-Apo nanoparticles was tested in primary cortical neurons and the results indicated effective inhibition of H2O2 induced cell-death by FA functionalized Mito-Apo nanoparticles. The neuroprotective effect of these Mito-Apo nanoparticles was studied in mesencephalon-derived neuronal LUHMES cells and it was found that FA functionalized Mito-Apo nanoparticles were effective in protecting against mitochondrial damage and preventing cell death [443]. These results indicated that FA could be used as an effective targeting ligand for neuronal delivery systems. In conclusion, nanotheranostics applications for PD diagnosis and treatment could be improved by using the new-targeted particles with multiple drug payloads.
4.3.1. Prion diseases.
Prion diseases, also known as transmissible spongiform encephalopathies (TSEs), are a unique group of fatal neurodegenerative disorders that include Kuru, Creutzfeldt-Jakob Disease (CJD), Gerstmann-Sträussler-Scheinker, and fatal familial insomnia [444]. Prion diseases can be hereditary, infectious or sporadic [445]. Pathologically, all are marked by spongiform vacuolation, neuronal loss, astrocytosis and misfolded protein aggregation [446]. British sheep farmers reported the first cases of TSEs in the 1750’s, concerned that imported Spanish merino sheep were spreading an unknown disease commonly termed “scrapie” [447]. Scrapie is a fatal, degenerative disease that selectively affects the nervous system of sheep or goats [448]. It is the one of several TSEs related to bovine spongiform encephalopathy (BSE or “mad cow disease”) [449]. The sole diagnostic test that is specific requires brain or lymphoid tissue biopsies making disease detection difficult [450, 451]. Sheep typically live 1 to 6 months after the onset of disease and are commonly found dead. The common signs and symptoms in animals include behavior changes (nervousness or aggression), excessive self-rubbing, and motor dysfunctions that progress to immobility and death [452]. In addition, tremors of the head and neck, “star gazing,” weight loss with change in appetite, wool pulling, and hyperesthesia are common [453]. In humans, broad motor and cognitive symptoms (cerebellar ataxia, oculomotor disturbances, peripheral nerve pain, pyramidal syndrome) followed by dementia are associated with disease and, for example, CJD [454].
For 250 years after the description of the disorder, the nature of the scrapie agent remained unknown [455]. The study of prion biology has led to a new category of infectious and or heredity agents [456]. Prions are the only known example of an infectious and/or heredity pathogen devoid of nucleic acid [457]. As infectious agents were nearly exclusively assumed to be microbial or viral in nature, the lack of identity of microbial nucleic acids in scrapie infected plasma or tissues made agent associations difficult [458]. Indeed, no microbial or viral agents were found at that time. However, biochemical techniques and our understanding of protein biology advanced significantly and finally in the late 1970s and -80s, research intensified after it was discovered that several rare human TSEs were transmissible to non-human primates [459]. This was followed closely by the Mad Cow epidemic in the 1980s [460]. By that time, observations that TSEs could last for years before symptoms appeared, did not incite inflammation, were resistant to formalin fixation, and could not be inactivated by DNases and RNases [461] were made. These findings led Dr. Stanley Pruisner and colleagues to propose that toxic, infectious, misfolded proteins, which he termed “prions” [461], caused TSEs. The idea that an infectious agent could be devoid of nucleic acid was met with a great deal of skepticism at first, but it is now widely accepted that infectious proteins termed “prions” cause TSEs.
Another pivotal discovery was the finding that cells in the brain [462] constitutively expressed the prion protein. Additionally, prion diseases, are unique in that the causative agent acts as a template to change the conformation of recruited benign forms of the agent into the disease state which has a substantially different conformation from that of its precursor, PrPC. Prions diseases are also exceptional in that they can be acquired in several ways either from genetic mutations of the PrPC gene, sporadic conformational changes in the PrPC protein, or through ingestion of the altered PrPSc [463]. Under healthy conditions, the cellular prion protein (PrPC) assumes a three-dimensional conformation composed of mainly α-helices with very few β-sheets. These proteins cause disease when they assume an unnatural conformation that is enriched in β-sheets [464]. The abnormal protein is termed prion protein (PrP)-scrapie or PrPSc. The abnormal PrPSc acts as a corruptive seed or template that can recruit the previously benign PrPC and induce the diseased conformation [465]. The self-propagating mechanism leads to ever increasing amounts of PrPSc in cells. Additionally, PrPSc is very prone to self-aggregation and as these aggregates grow in size they can impair normal cellular functions, activate surrounding microglial cells leading to harmful neuroinflammation, and splinter and spread to other cells, irreversibly leading to widespread neurodegeneration [445].
4.3.2. Prion disease and theranostics.
There is currently no appropriate technology for early diagnosis of prion diseases [466]. These diseases are detected through conventional methods by ex-vivo tissue analysis after noticeable symptomatic signs of the disease in animals. The ex-vivo tissue analysis test includes identification of prion proteins, quantification by ELISA, immunohistochemistry, conformation-dependent immunoassay and cyclic amplification of misfolded protein. These detection assays are time consuming, costly and laborious [467–470]. For example, a serial cyclic amplification of misfolded protein assay cycle requires approximately three days [471]. Thus, a sensitive and time effective technology for detection of prion protein would be helpful in early accurate diagnosis of these diseases.
To date, there are no effective treatments for human prion diseases [466]. Although animal models exist for many prion diseases, none of the proposed anti-prion therapies have shown efficacy in treating CJD-infected mouse models [472]. However, efforts to find a therapeutic intervention have intensified because there is an increased understanding that more common neurodegenerative diseases like AD and PD also proceed through the self-propagation and aggregation of misfolded proteins [473]. The cellular prion protein (PrPSc) is considered the main target of the anti-prion treatments [474]. There are five different types of approaches that have been used to treat prion diseases. These include (a) small molecule inhibitors such as thioflavine, amphotericin B, quinacrine, pentosan, poylsufate, Congo red, anthracyclines, and memantine [475]; (b) immunotherapies by engineering of anti-prion antibodies [476]; (c) prion gene disruption by using a CRISPR/Cas9 system [477]; (d) targeting of the unfolded protein response; and (e) heterologous prion proteins [478].
These therapeutic approaches for longer treatment of prion diseases are not effective. However, in the last few years, theranostics technology has significantly advanced and has facilitated the easy detection of some complex molecular mechanisms of these diseases. This should prove helpful in providing improved early diagnosis and precise treatments for prion diseases [466, 479, 480]. Theranostic nanoparticles such as magnetic and gold nanoparticles were demonstrated to bind with prion proteins and were used for imaging [480, 481]. Recently, Miller et. al., reported that superparamagnetic iron oxide nanoparticles bound to PrPSc molecules efficiently and specifically, permitting magnetic separation of prions from a sample mixture for protein misfolding cyclic amplification reactions to improve detection [466]. Similarly, Irudayaraj’s group demonstrated that streptavidin-conjugated gold-coated magnetic nanoparticles functionalized with biotinylated aptamer could be used for prion protein detections [482]. Another study demonstrated use of silver nanoparticle–aptamer conjugates for anti-prion protein detections [483]. Design of nanoparticle-labeled aptamers linked to PrP antibody was shown to be a major development in the diagnosis of prion diseases. Recently, Zhang et. al., demonstrated detection of prion protein by construction of a simple biosensor that used protein aptamer and gold nanoparticles. The principle of this assay was binding of the prion protein on the target molecule followed by signal amplification by gold nanoparticles and detection using resonance light scattering [484]. Other investigators have reported development of a surface-modified fluorescence nanoparticle labeled antibody assay for the detection of the conserved sequence of PrP. The surface-modified fluorescence nanoparticle produced a signal when it formed a complex with PrP in samples [485]. In short, there is no actual treatment to date for prion diseases. However, Calvo et. al., created a long-circulating PEGylated particle [methoxy poly (ethylene glycol) cyanoacrylate-cohexadecyl cyanoacrylate] (PEG-PHDCA), which demonstrated higher uptake in target tissues of PrP (brain and spleen) in scrapie-infected animals [486]. Thus, these theranostic technologies will improve upon conventional management of prion diseases due to their ability to cross the BBB and their capability to perform simultaneous diagnosis and long-term treatment monitoring options.
4.4.1. Motor neuron diseases (MNDs).
Diseases of motor neurons are often regarded as the most devastating adult onset neurodegenerative disorder and are characterized by loss of upper and lower motor neurons [487]. Upper motor neurons originate in the cortical motor regions and their role is to transfer signals to the lower motor neurons, which are derived from the anterior horn of the spinal column and extend to innervate specific skeletal muscle groups throughout the body at junctions termed as neuromuscular junctions [488]. A motor unit is comprised of a motor neuron, its axon, the neuromuscular junction and all the muscle fibers it innervates [489]. In MNDs, the affected neuronal somas undergo apoptosis and this causes the associated axon to degenerate resulting in the destruction of the neuromuscular junction [490]. This destruction leads to rapidly worsening muscular atrophy in the arms, legs, trunk and bulbar region inevitably leading to paralysis and shortly thereafter, death, often by respiratory failure [487]. The risk of developing MND peaks between age 50 and 75 years and most patients succumb to symptoms within 3–5 years of diagnosis, although a fraction of patients do survive into the second decade after diagnosis [487].
Common MNDs include amyotrophic lateral sclerosis (ALS), primary lateral sclerosis (PLS), progressive muscular atrophy (PMA), progressive bulbar palsy (PBP), and pseudobulbar palsy [491]. Among these, ALS is the most common and is often synonymous with MND [492]. It has a widely diverse clinical presentation and the symptoms depend on which motor neurons are affected. Roughly 60% of patients present with weakness in one or multiple limbs, which could be due to upper motor neuron dysfunction in the motor cortex [493]. Around 30% present with difficulty in swallowing (dysphagia) or pronouncing words (dystharthria), which indicate that the lower motor neurons of the medulla oblongata are primarily affected. Up to 5% of patients present with frontal temporal dementia or respiratory weakness [487]. As the disease progresses, symptoms include cramps, stiffness, shortness of breath, swallowing difficulties, insomnia, loss of appetite, uncontrollable laughing or crying, fasciculation, anxiety, pain, fatigue and depression [494].
There are no treatments available for ALS and other MNDs other than symptomatic relief since like other neurodegenerative disorders its cause and pathophysiology are poorly understood [495]. Identification of causative environmental risk factors have also been ineffective to date. About 90–95% of ALS cases present sporadically with no identifiable genetically inherited component [496]. However, in the other 5–10% of cases, there is a genetic linkage and such research has provided insights into the neurodegenerative processes [497]. Clinically it can be difficult to separate familial from sporadic ALS but advancements made in the fields of genetic sequencing and molecular biology technologies indicate that there are up to 20 genes that are affected which increase susceptibility to disease [498]. The most common of these are SOD1, C9ORF72, TARDBP, FUS, VAPB, and UBQLN2 [499] and inheritance is primarily autosomal dominant [500]. These genes are linked to antioxidant defense, RNA metabolism, protein homeostasis, and cytoskeletal dynamics [501]. SOD1 and C9ORF72 mutations account for the majority of familial ALS but their frequency varies [502]. For example, mutations in C9ORF72 represent the majority of familial ALS cases in Europe (~40%), but in Asia <10% of familial ALS cases can be linked to a mutation in C9ORF72 [503].
In 1993, mutations in the Cu/Zn superoxide dismutase gene (SOD1) were found associated with familial ALS and this discovery led directly to the formation of the first mouse model of human disease. Since that study, over 150 mutations in SOD1 have been described and these contribute to 20% of familial ALS and up to 3% of sporadic ALS [504, 505]. This observation indicated the critical importance of the SOD1 gene encoding a homodimeric metalloenzyme that catalyzes the reaction of toxic O2− into O2 and H2O2 [505]. Indeed, the role of ROS mediated toxicity in neurodegeneration [505] is well studied. Nonetheless, treatments aimed at reducing oxidative stress have not been successful in treating ALS or other motor neuron diseases.
Since the mid-1990s, research has supported a broader role of factors beyond SOD1 for the cause of ALS [505]. Indeed, mutant SOD1 (mutSOD1), for example, does not lose enzymatic activity [506] and a simple knock down of wild type SOD1 fails to cause motor neuron degeneration, at least in mouse models of human disease [507]. Studies have also shown that overexpressing mutSOD1 specifically in neurons is not enough to fully cause disease [508–510]. Some researchers changed their focus to the role of mutSOD1 in non-neuronal cells in the CNS. A study in 2003 found that motor neurons with wild type SOD1 developed ALS type pathology when surrounded by mutSOD1-expressing microglia but neurons expressing mutSOD1 surrounded by wild type microglia appeared normal [511]. Research has shown that microglia harboring mutSOD1 are more activated, produce more ROS, and affect more motor neurons than microglia with wild type SOD1 [512]. In addition, inhibition of the production of cytokines reduces neuron toxicity [513]. Thus mutSOD1 results in overproduction of microglial ROS and apoptosis in nearby neurons [514].
The mechanism that causes the microglia to become activated is debated but post-mortem studies found large aggregates that stain for mutSOD1 and other ALS related proteins in the cytoplasm of affected neurons [515]. This supports the hypothesis that an accumulation of mutSOD1 protein aggregates in diseased neurons may activate microglia, which over produce inflammatory cytokines leading to neurodegeneration in a vicious cycle. However, for C9ORF72, pathogenic expansions in the non-coding region of a G4C2 (GGGGCC) repeat can occur. In healthy individuals, the number of G4C2 repeats is less than 25, but in ALS-affected individuals hundreds or thousands of such repeats are present [516]. This is observed in approximately 40% of familial ALS and even 5–10% of sporadic ALS cases [517]. Due to this mutations’ association with familial and sporadic ALS, extensive research has been done to find out the correlation between repeat expansion mutation and disease. In relation to this mutation, three broad mechanisms have been proposed to explain neuron death. Firstly, the existence of this large repeat in the promoter region leads to down regulation of C9ORF72 and a loss of its cellular functions [518]. Secondly, RNA transcripts with many short repetitive sequences in a row can be trapped in cellular nuclei as they recruit an abnormal amount of cellular machinery leading to “RNA foci” which are accumulations of proteins and RNA transcripts that do not make it to the cytoplasm for translation [519]. These RNA foci have been found in ALS patients harboring this mutation [520]. The third proposed mechanism comes from observations that C9ORF72 mutation carriers show accumulations of dipeptide repeat proteins in their brains and spinal cords [521]. These proteinaceous inclusions have been shown to impair nucleus to cytoplasm transport in affected cells leading to cytotoxicity and cell death [522]. However, more research is required to determine how and to what extent each of these mechanisms may contribute to neuronal death in motor neuron diseases.
4.4.2. Theranostic potential for MND.
There are currently no curative treatments for MND, and there are only a couple of options for palliative care. For over 20 years, the only drug that could effectively slow down the progression of neuromuscular degeneration was riluzole, a glutamate release inhibitor [523]. But at the time of diagnosis, 50% of affected motor neurons are already lost and treatment with riluzole only prolongs life by about 3 months compared to placebo [524]. Finally, in 2015 edaravone, a neuroprotective agent with known free radical scavenging properties, was approved for the treatment of ALS in Japan and South Korea and in 2017 in the United States [525]. The FDA approved this drug for clinical use to treat ALS patients [526]. Originally, edaravone was developed as an intravenous treatment for acute ischemic stroke, but showed promising results in a mouse model of genetic ALS [527]. However, a double blind, placebo controlled phase two study using intravenous edaravone treatment in patients with ALS did not show a significant difference between control and treatment groups, but a post-hoc analysis revealed a select sub-set of patients who may have benefited from the treatment [528]. Based on this data, phase 3 studies were conducted in patients who were recruited under strict criteria. In this 24-week study, the progression of ALS was slowed significantly in patients given edaravone compared to controls [528]. Because of these stringent criteria, less than 10% of ALS patients are expected to benefit from such treatment. It is also worth noting that because of the relatively short duration of phase 3 studies, the efficacy of edaravone in the long-term management of ALS and MNDs is not known [529].
Overall more than 30 agents have been tested to treat or cure MNDs such as ALS, but the only successful treatments are edavarone and riluzole [530]. Hence the research focus has shifted to discovery of biomarkers of MND that could lead to earlier treatment intervention. Such a biomarker would also greatly help the search for new and better medications, as it would provide a real disease-associated end point for clinical trials [531]. The reasons for so many treatment failures include, targeting the wrong target, inability of the drug to cross the BBB and accumulate in the brain at therapeutic concentrations, or because of poor oral bioavailability [532]. There is currently no treatment for ALS or a clear pathway to stop its progression and the inability to make a conclusive diagnosis is a major drawback in designing an effective therapy. Although some drugs have been proposed for the treatment of ALS, their poor bioavailability and in ability to cross biochemical barriers have hindered effective treatment of ALS [533]. Packaging these treatments or prospective treatments into nanoparticle carriers capable of crossing the BBB could prove to be beneficial in the treatment of MNDs and other neurodegenerative illnesses [530]. Recently, Chen et. al., demonstrated the development of calcium phosphate nanoparticles (CaP-lipid NPs) for delivery of SOD1-ASO (superoxide dismutase 1-antisense oligonucleotides) to motor neurons. These particles have uniform spherical core-shell morphology and an average size of 30 nm. In vitro experiments demonstrated that the negatively charged ASO-loaded CaP-lipid NPs could effectively deliver SOD1-targeted ASO into a mouse motor neuron-like cell line (NSC-34) through endocytosis and significantly down-regulated SOD1 expression in HEK293 cells. Further studies showed that ASO-loaded CaP-lipid NPs significantly increased SOD1 knockdown. To assess in vivo delivery of these nanoparticles, empty CaP-lipid NPs were microinjected into live zebrafish larvae. Overall, this study demonstrates that CaP-lipid NPs can circulate freely within the bloodstream following systemic delivery into zebrafish and diffuse throughout the brain and spinal cord after direct injection, suggesting that these nanoparticles could be a useful tool to promote gene delivery for ALS therapy [534].
4.5.1. Huntington’s disease (HD).
In 1872, George Huntington reported the first case of hereditary dementia and chorea symptoms in adults between 30 and 40 years of age in the US. This condition, referred to as Huntington’s disease (HD) [535, 536], is a particularly devastating neurodegenerative disease that affects about 1 in 7,300 individuals in Europe and the Americas [537]. Inherited in an autosomal dominant fashion, patients are generally diagnosed between ages 30 and 45, although there is a juvenile form of the disease in which symptoms appear before 20 years of age. The course of the disease is unstoppable and invariably ends with death of the patient within ~20 years of symptom onset [538, 539]. HD results in a triad of severe physical, cognitive, and physiological abnormalities. Physical symptoms tend to be the most noticeable and include, but are not limited to, dystonia, abnormal eye movements, impaired gait, posture and balance, difficulty in speaking and swallowing, and most commonly involuntary jerking or writhing movements (chorea) [540]. Cognitively, affected individuals show difficulties in organizing or prioritizing tasks, learning and processing thoughts [541, 542], tend to get stuck in loops of thought, behavior or action and lack impulse control. Psychologically, HD leads to depression, social withdrawal insomnia, obsessive-compulsive disorder, mania, or bi-polar disorder [543, 544]. A decade of research has provided key insights into the pathobiology of HD and has generated promising therapeutic targets, but to date no curative treatment exists. Thus, treatment of the disease is limited to attempting to maximize the patient’s quality of life and functional capabilities. The key symptom of HD is involuntary shaking of the arms, shoulders, hips, and head. It also causes a weakening of the thought processes by slowdown of reasoning skills, loss of memory, and difficulty in focusing, common decision-making issues, and unorganized lifestyle [545, 546].
HD is one of nine related conditions termed polyglutamine (polyQ) disorders. PolyQ disorders are monogenic and caused by an expansion of CAG trinucleotides in the affected gene’s coding region that are translated into a long string of glutamine amino acids in the protein product [547]. Notably, all nine disorders primarily affect the CNS with almost no effect on peripheral organs or systems (immune, hematologic, or endocrine). In the case of HD, the affected gene, HTT, is located on chromosome 4 and encodes the protein, huntingtin [548]. The normal function of this protein is currently unknown [549]. However, it is well known that the number of polyQ repeats found near the amino terminus of the protein positively correlates with disease severity and age of onset [550, 551]. Normal huntingtin protein contains between 6 and 35 of such repeats and when expanded to ≥ 40 repeats, the disease phenotype becomes highly penetrant [552, 553]. Generally, one allele of the gene will contain a longer repeat than the other and this allele determines the disease progression and age of onset. The long CAG repeats cause the gene to become “fragile” during meiotic cell division and is almost exclusively passed down through fathers, potentially indicating that CAG expansion occurs during spermatogenesis [554].
While normal huntingtin protein does not have a well-characterized biochemical function, studies have shown that it has critical roles in the early development of the nervous system, the production of brain-derived neurotrophic factor, and in cell adhesion [555, 556]. It is still not completely understood how polyQ expansion leads to a toxic gain-of function in huntingtin protein, though it is known that the protein is susceptible to proteolytic fragmentation [557]. In particular, a small N-terminal fragment is produced from the first exon containing the polyQ repeat sequence [558]. Interestingly, a large body of evidence now shows that HTT exon 1 can readily form a variety of aggregation structures [559, 560] which links HD to other neurological disorders like AD, PD, and ALS.
Fragmentation and aggregation of huntingtin protein forms the foundation of the pathobiology of HD. Fragments of huntingtin protein can be detected in all brain regions of huntingtin mouse models before the appearance of cellular inclusions [561, 562]. The likelihood that the fragments will aggregate and cause cytotoxicity is primarily determined by the expression level of the huntingtin protein [563]. Expression of the protein is highest in CNS tissues, which may explain why the symptoms are primarily CNS related [564].
4.5.2. HD and theranostics.
HD is usually diagnosed based on clinical outcomes from family medical history, genetic testing and neuropsychological examinations. There is no treatment that can suppress symptoms or reverse HD progression [565]. Over the last two decades substantial research efforts have focused on the development of an effective HD treatment. Though some small molecules exhibited potential positive results in animal studies, they were unable to produce the same effect clinically. Tetrabenazine is the only single molecule that has been approved by the FDA for symptomatic treatment of HD-associated chorea [566]. Currently, theranostic nanoparticle-based strategies have played a significant role in the rapid diagnosis and prevention of HD [164, 567–570]. For example poly(trehalose) nanoparticles that can prevent amyloid/polyglutamine aggregation under extra-/intracellular conditions, reduce such aggregation-derived cytotoxicity, and prevent polyglutamine aggregation [568].
While there are no curative treatments for HD, symptomatic medicines can improve some signs of movement and psychiatric disorders. Various attempts have been made to deliver these neuroprotective and/or neuro-corrective therapies to the brain [571, 572]. For example, Pradhan et. al., utilized the protein aggregation inhibiting ability of osmolytes to prepare glutamine/proline-conjugated zwitterionic nanoparticles. Iron oxide nanoparticles were used as the core and coated with a polymeric shell covalently functionalized with glutamine and proline. In vitro studies demonstrated that glutamine and proline can be 1,000–10,000 times more effective in inhibiting protein aggregation when employed in their nanoparticle forms. Further results demonstrated that glutamine-functionalized particles were able to block the aggregation of polyglutamine expanded mutant huntingtin protein in a HD model cell line [573]. Bhatt et. al., fabricated solid lipid nanoparticles for encapsulation and intranasal delivery of rosmarinic acid. Interestingly, rosmaric acid is known to have neuroprotective effects, which are undertaken for the prevention of oxidative stress, and intracellular Ca2+ overload in the neurons [574]. On the other hand, Godinho et. al., followed a neuro-corrective approach via siRNA to silence the mutant protein expression in HD. They encapsulated the nucleic acid in a sugar-based (β-cyclodextrin) nanoparticle and observed encouraging results in a rat brain model [575]. In other studies, Debnath et. al., developed poly(trehalose) nanoparticles that can reduce amyloid-polyglutamine aggregation and aggregation-derived cytotoxicity in the brain of a HD model mouse. These investigators successfully demonstrated the use of trehalose nanoparticles for efficient brain targeting, entry into neuronal cells, and suppression of mutant huntington aggregation [568].
4.6.1. Spinocerebellar ataxia (SCA).
Spinocerebellar ataxia (SCA) is among a class of neurodegenerative disorders that can be described as progressive, degenerative and monogenetic. It has an autosomal dominant pattern of inheritance and affects both males and females equally [576]. There are different types of SCA which are classified based on the mutated gene which is responsible for each specific type. SCA3, referred to as Machado-Joseph disease, is the most common type and the signs and symptoms of most types can develop at any age. The most common symptoms in such patients are difficulty in maintaining coordination and balance, unstable gait, slurred speech, poor hand-eye coordination, dysarthria, dysphagia, vision problems, brisk tendon reflexes, etc. With increasing age, muscle atrophy sets in along with chorea, dystonia, and cognitive impairment, among other symptoms [577]. In the majority of SCA types, the genetic change that causes the condition is CAG repeat expansion. However, there are other repeat expansions as well, such as CTG for SCA8, ATTCT repeat expansion for SCA10 and a point mutation in the PRKCG gene in SCA14 [578, 579]. This is the basis on which the diagnosis of SCA is established. In addition, the use of CT and MRI have revealed cerebellar atrophy and loss of brain stem gray matter while PET demonstrated hypometabolism in individuals with ATXN1 trinucleotide expansion [580, 581]. There is no specific treatment for SCA which relies currently upon rehabilitative management of physical symptoms. Several groups have studied the neuropathology of the disease in order to guide development of new therapeutic approaches. Sandro et. al., developed a lentiviral vector model in the rat to study the pathological features in different regions of the brain with emphasis on the substantia nigra, cortex and striatum. They concluded that delivery of mutant ataxin-3 is a new genetic model of SCA3, which could pave the way for development of effective therapeutic strategies [582]. Daniel et.al have explored the possibility of RNA-targeted therapies in two mouse models (ATXN2-Q127 and BAC-Q72) of SCA2. Their target was the ATXN2 gene, which is associated with protein instability and conformational changes in SCA. Using the antisense oligonucleotide (ASO7), they demonstrated that ASO7 could reduce the expression of ATXN2 mRNA and protein, which in turn delayed the onset of SCA2 in both mouse models [583].
4.6.2. Theranostic potential for SCA.
The pathophysiology of SCA is multifaceted, suggesting a series of possible options that are advisable for symptomatic relief. Neurotransmitters such as serotonin, norepinephrine, acetylcholine, dopamine, and histamine are responsible for natural cerebellar function. These molecules are not used as first-line therapy, however some treatments use small molecules such as riluzole, antiglutaminergics, nicotine receptor agonists, serotonergic therapy, -aminobutyric acid (GABA) therapy and insulin-like growth factor-1 (IGF-1) as second-line treatments [584]. There have been several reports on development of anti-SCA therapeutic agents but these have not translated into clinical applications. Only a few reports have described the use of nanoparticles for treatment and diagnosis of SCA. Interestingly, Malhotra et. al., developed peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery for use in SCA1. The nanoparticles were tested to deliver a functional siRNA against the ataxin-1 gene in an established model of a neurodegenerative SCA1 overexpressing ataxin protein. The results indicated successful suppression of the SCA1 protein following 48 hours of transfection [585]. Prior works employed a gene therapy strategy whereby dopamine D2 receptor was used as a reporter gene and imaged by radioiodinated IBF (5-iodo-7-N-[(1-ethyl-2-pyrrolidinyl)methyl]carboxamide-2,3-dihydrobenzofuran) making it an effective tool for monitoring the progression of SCA [586]. Although there is no effective treatment for SCA, many attempts have been made to deliver the neuroprotective and neurotherapeutic agents packaged in nanocarriers to the affected brain tissue [585, 587, 588]. The development of theranostic nanoparticles for the rapid assessment of disease progression and design of improved treatment would be of great benefit for treatment of this disease.
4.7.1. Spinal muscular atrophy (SMA).
Spinal muscular atrophy is an autosomal recessive neuromuscular disorder which is characterized by degeneration of the alpha motor neurons of the spinal cord anterior horn cells [589]. This in turn results in progressive proximal muscle weakness, atrophy and paralysis. In addition, patients also present with the symptoms such as loss of strength of the respiratory muscles, fasciculations of the tongue, difficulty in maintaining posture and dysphagia [590]. SMA has a relatively high incidence rate of 1 in 6,000–10,000 births and is categorized into types I - IV based on the motor function and age of onset. This incidence rate makes it the second most common autosomal recessive disorder after cystic fibrosis and is almost comparable to Duchenne’s muscular dystrophy and ALS [591]. Unlike most neurologic disorders, there is a specific causative factor for SMA and that is mutations in or deletion of the survival motor neuron (SMN1) gene [592]. This gene is responsible for the formation of the SMN protein and though SMN2, which differs from the former by a single nucleotide, also produces the same protein, it is in very low amounts. The functions carried out by SMN are significant as it facilitates biogenesis and metabolism of various ribonucleoprotein complexes involved in mRNA transport and regulation along with loss of alpha motor neurons of the spinal cord [593–596]. Numerous animal models of SMA have been studied which has provided information about the pathophysiology and molecular pathways of the SMN gene [597]. Although the signs and symptoms provide a clear indication of the condition, diagnosis is confirmed through genetic testing of the SMN gene.
4.7.2. SMA and theranostics.
Most of the current treatment options for SMA are designed to improve the muscle weakness and general health of the patient or to provide orthopedic support [598, 599]. Currently, the only approved medication for treatment of SMA is the antisense oligonucleotide nusinersen [600]. Since there is lack of a specific strategy for clinical therapy for SMA, various researchers are exploring nanoplatforms for delivery of interfering or silencing nucleic acids for identification and suppression of mutant protein expression. Shi et. al., studied the combinatorial effects of elevated ROS and lack of SOD1 and have attempted to identify molecular targets involved in SMA in mouse models [601]. As an attempt towards targeted SMA therapeutics, Fazel et. al., reported a synthetic peptide capable of crossing the BBB and delivering the splice-switching phosphorodiamidate morpholino oligonucleotide which is targeted towards the motor neurons in the brain [602]. Although various treatment modalities are being explored, gene therapy promises to be the most viable option through insertion of a whole gene or cDNA sequence into the genome of patients with SMA [603]. The diagnosis of SMA uses the McDonald criteria which is based upon clinical, laboratory and radiological evidence of lesions in different brain regions and at different times [604]. Some of the most commonly employed diagnostic tools are neuroimaging-using MRI with gadolinium used as a contrast agent, analysis of CSF for oligoclonal bands of IgG and sensory and visual evoked potentials [605, 606]. Recently, gold nanoparticles were used as a colorimetric-based technique for diagnosis of SMA [607]. Taking a cue from this diagnostic tool, it would be advantageous to combine theranostics with gene therapy, as it will be able to directly monitor gene expression and assess therapeutic efficiency simultaneously.
4.8.1. Multiple sclerosis (MS).
MS is a chronic inflammatory condition whereby the immune cells attack the nervous system resulting in demyelization and destruction of myelinated axons in the central nervous system. The hallmark of this condition is the formation of plaques consisting of demyelinated nerve cells in the nervous system. MS is also known as disseminated sclerosis and encephalomyelitis and is a progressive autoimmune disorder resulting in impaired and delayed nerve signaling [608–610]. The symptoms are normally found to develop between the second and fourth decade of life and affect about 2.5 million people worldwide [611]. The etiology of MS is still not well understood, but a number of theories as to its cause have been proposed, including a combination of genetics, immune infections, and environmental factors such as sunlight, UV radiation, Epstein-Barr virus, and vitamin D deficiency. [612, 613]. These factors result in an adverse immune response and damage to the myelin sheath. However, the role of genetics is undeniable as there is a 20- to 40-fold increased risk of MS in first-degree relatives of patients. HLA-DRB1*1501 haplotype is the primary genetic factor which increases the risk of MS susceptibility and is present in about 20–30% of the individuals [614]. There is marked gender preponderance in MS as women are found to be affected twice as often as men except in the primary progressive form of MS. Geographically, MS is more prevalent in northern parts of Europe and North America [611]. Depending on the cause and progression of the disease, MS is classified into four groups such as relapsing-remitting MS, primary progressive MS, secondary progressive MS and progressive-relapsing MS. Among all the groups, the first category is the most common and accounts for about 85% of MS patients [615]. MS is a persistent disease, which relapses at intermittent periods and results in diffuse changes in the gray and white matter. In order to study the immunological pathways involved in MS, studies have used experimental autoimmune encephalomyelitis (EAE) animal models. At the same time, studies on the CSF and serum of such patients have revealed an accumulation of iron, which is characteristic of peripheral venous disorders [616–618]. Damage to the myelin sheath occurs as a result of the action of a combination of inflammatory cytokines, proteases and free radicals produced by pro-inflammatory T helper cells, B cells and macrophages [619]. These immunocytes are generated after autoreactive T cells bind to adhesion molecules on the endothelial cells of CNS venules. This in turn enables the cells to cross the BBB with the help of proteases and chemokines [620–622].
4.8.2. MS and theranostics.
There is no cure for MS and therapies are designed to provide symptomatic management and improve the quality of life of the affected patient. However, there are a number of FDA-approved disease-modifying medications, such as interferon beta 1a and 1b, glatiramer acetate and natalizumab, which slow disease progression [623]. Hence, various research groups have explored the use of nanotechnology and nanoparticles for diagnostic as well as therapeutic applications for MS. Several studies have confirmed that dimethyl fumarate (DMF) has immune modulatory characteristic by slow down cytokine production. DMF is now available for orally administered FDA approved for first-line monotherapy of MS[624]. Interestingly, Karaborni et. al., developed nanoparticle compositions of DMF which in turn resulted in high concentrations of the mono compound in MS patients [625]. Nanoparticles have also been used for improved neuroimaging through the use of engineered molecular imaging probes. Santamaria and coworkers recently also developed a therapeutic composition consisting of a MS related antigen for treatment [626]. Theranostics platforms can open up a new avenue for perceiving more efficient targeted medication, early diagnosis and precisely monitoring of MS progression. All together theranostics can provide the opportunities to better treatments for MS patients.
5. Therapeutics that facilitate neuroprotection and neuroregeneration
Despite abundant developments in understanding the basic biology of neurodegenerative diseases, there are no current treatments that have produced significant outcomes. Neuroprotection has been widely explored as an effective treatment strategy to prevent neuronal degeneration in diseases like AD, PD and MS. Contribution of oxidative stress to progression of neurodegenerative disease is suggested by numerous studies. Indeed, overproduction of ROS is a common observation in neurodegenerative disease patients and may lead to neuronal loss [627].
Neuroprotection reflects the preservation of neuronal structure by reducing the rate of neuron loss over time. As an intervention it influences the etiology or the pathogenesis of underlying neurodegenerative diseases, thus preventing or delaying the onset or the progression of the disease [628]. Using effective neuroprotection strategies, disease progression can be slowed or prevented to a considerable extent. The primary mechanisms underlying neuroprotection are the same, immaterial of the disease condition. These mechanisms include mitochondrial dysfunction, increased levels of oxidative stress, inflammatory changes, excitotoxicity, and protein accumulation such as aggregation and fibril formation in AD. Most neuroprotective approaches involve suppression of oxidative stress and excitotoxicity which work synergistically to accelerate neuronal cell death and degradation. Oxidative stress can be reduced in neurodegenerative diseases through the use of antioxidant supplements [629, 630] or other “neuroprotective agents” [631]. While some antioxidants can readily cross the blood brain barrier, including vitamins C and E and N-acetylcysteine [632–634], other available antioxidants cannot [635]. To improve antioxidant penetration across the BBB nanoparticle-based delivery of antioxidants or nanoparticles themselves are being developed for neuroprotection [163]. Cerium oxide nanoparticles (CNPs) have been widely explored as therapeutic drug delivery systems due to their antioxidant potential and potential for treating oxidative stress related conditions [636]. CNPs have unique regenerative properties due to the low reduction potential of the co-existing Ce3+ and Ce4+ redox system on their surface, which is an efficient scavenger of free radicals involved in oxidative stress. CNPs are well tolerated in vitro and in vivo, making them suitable for application in neuroprotection and regeneration [167]. To utilize the antioxidant properties of CNPs, Schubert et. al., developed cerium and yttrium oxide nanoparticles. Yttrium nanoparticles were studied due to the high free energy of oxide formation from elemental yttrium. Experimental results showed that cerium and yttrium oxide particles were non-toxic to HT22 neuronal cells and had antioxidant properties that could promote cell growth and survival under oxidative stress conditions. It was noted that the neuroprotective efficacy of these particles was related to their structure and did not depend on their elemental composition [166]. In another study, Das et. al., synthesized ultrafine, non-agglomerated CNPs by a microemulsion process for neuroprotective applications in spinal cord injury and neurodegenerative disease. In vitro studies in a serum-free cell culture model of adult rat spinal cord indicated a significantly higher cell survival in CNP-treated cultures compared to control cultures. Immuno-staining showed significantly higher numbers of neuronal cells in CNP-treated cultures compared to control cultures. The reversible nature of the Ce3+/Ce4+ redox system on the surface of the CNPs confers an autocatalytic property onto these particles. A hydrogen peroxide-induced oxidative injury model utilizing the adult spinal cord model system was used to demonstrate the autocatalytic property of CNPs. The results indicated that the CNP-treated cultures had a significantly higher peroxide detoxification ability suggesting neuroprotective property. The autocatalytic property of these particles, which enables them to regenerate their anti-oxidant effect, appears to be responsible for their neuroprotective action [637]. CNPs also decrease ischemia through reduction of the levels of 3-nitrotyrosine, a tyrosine modification caused by peroxynitrite radicals. Using an in vitro model of brain ischemia, Estevez et. al., studied the neuroprotective efficacy and extent of cellular localization of CNPs in a rat model of ischemia. Investigators were demonstrated the primary underlying mechanism by which CNPs exert their antioxidant effect involves a significant reduction in peroxynitrile, which in turn is responsible for the formation of 3-nitrotyrosine [165]. More recently researchers found that epigallocatechin-3-gallate (EGCG), a polyphenolic compound present in green tea, has potent antioxidant activity [638]. Several studies have been carried out to examine the interaction of EGCG with a large number of amyloid-forming proteins such as Aβ, α-synuclein, transthyretin, and huntingtin, all of which are involved in neurodegeneration [639–646]. EGCG is a promising drug delivery system as it is able to redirect the amyloid formation pathways and promote the assembly of low-toxicity aggregates to a certain extent [647, 648]. However, EGCG has poor aqueous solubility, which limits its bioavailability and efficiency of targeted cellular uptake. Selenium (Se) is a trace element that is essential to the system and plays a significant role in assisting cells to resist oxidative damage. Se is present biologically in the form of selenoproteins, which play important roles in cellular redox regulation, detoxification and immune system protection [649]. Due to its high expression in the brain, it is thought that Se may be responsible for neuroprotection via antioxidation [650]. In order to utilize the therapeutic potential of EGCG and Se, EGCG-stabilized SeNPs (EGCG@Se) were synthesized. These particles were then coated with Tet-1 peptide due to the affinity and binding characteristics of Tet-1 peptide to neurons (Tet-1 EGCG@SeNPs). In vitro results indicated that the introduction of nanoparticles into Aβ substantially inhibits Aβ aggregation. Cytotoxicity and ultra-structural analysis assays show significant evidence of the ability of Tet-1 EGCG@SeNPs to effectively mitigate Aβ fibrillation. Studies performed to assess the disaggregation of preformed Aβ fibrils by Tet-1 EGCG@SeNPs, suggested that these nanoparticles are able to successfully convert β-sheet-rich fibrils into amorphous aggregates. It was also found that these nanoparticles were capable of transforming Aβ fibrils into structures with reduced ROS producing capability. Hence, these results suggest that Tet-1 EGCG@SeNPs are promising candidates for Aβ disaggregation and can mitigate Aβ fibrillation. These results suggest that EGCG and Se have the potential to serve as effective therapeutic agents in the treatment of AD [651]. In other studies, Esteves et. al., used retinoic acid (RA) loaded nanoparticles as a neuroprotective drug delivery system for an in vivo PD model [652]. RA is important for midbrain dopaminergic (mDA) neurons because its receptors and RA-synthesizing enzymes are abundantly expressed in these neurons and their target regions. In vitro and in vivo studies in a mouse model of PD showed significant neuroprotective effects upon intra-striatal injections of RA nanoparticles (RA-NPs) via decreased levels of Nurr1 mRNA expression. These RA-NPs were also found to support dopaminergic neuronal projections present in the striatum [652]. Future studies will require illuminating the neurological mechanism of action of the theranostic nanoparticles and other agents. The combination of theranostic nanoparticles with other antioxidants demonstrates a favorable course of action as it prevents events that lead to neurodegenerative injury.
5. Therapeutics that facilitate neuroprotection and neuroregeneration
With the surge in research and development, nanoparticles have increasingly been featured in the diagnosis, therapy and monitoring of disease progression in humans. Animal models have shown that nanoparticles such as zinc oxide and iron oxide are able to translocate into the brain by virtue of their size and ability to be surface modified depending on the purpose for which it was fabricated [653–655]. Since the delivery of drugs into the CNS through the BBB is hindered due to various factors, coated nanoparticles such as PEG, transferrin, thiamine, glutathione, and others have proven to be ideal choices as drug carriers [656–659]. Nanoparticles have been extensively used in the development of nanoscaffolds for neuronal growth; carbon nanotubes have proven to be particularly effective in this application [660]. Apart from therapeutic applications, nanoparticles are also used for diagnostic purposes such as nanoelectromechanical systems for the assessment of parameters such as intracranial pressure and cerebrospinal fluid [661]. Although, the application of nanoparticles as contrast agents in medical imaging technologies has shown extraordinary progress further development is needed to enable their use as early diagnostic tools [64, 69, 174, 662, 663].
Despite the fact that these properties of nanoparticles make them an attractive choice as drug carriers into the CNS, there is an undeniable risk of toxicity, which in turn could result in neurotoxic defects [664]. Hirst et. al., had studied the biodistribution and the anti-oxidant effects of CNPs and they found CNPs induced liver toxicity in mice [169]. Similarly, Repar et. al., observed that citrate coated silver nanoparticles (AgSCs) were toxic to human embryonic stem cell-derived neurons and astrocytes [665]. Morphological and biochemical assays indicated that AgSCs reduced neurite outgrowth, decreased postsynaptic density protein 95 and synaptophysin expression, and induced neurodegeneration via the glycogen synthase kinase-3 and caspase pathways. Results also showed that at low concentrations, AgSCs promote astrogenesis, increasing the astrocyte/neuron ratio. Though there has been no direct correlation between nanoparticles and CNS diseases in humans, studies in mice indicate the potential for neurotoxic effects [665]. Mirasattari et. al., reported that repeated oral ingestion of silver nanoparticles led to the development of myoclonic status epilepticus in a 71-year-old male [666]. There have also been reports that nanoparticle exposure in mothers may be harmful to fetal development [667]. Interestingly, Shimizu et. al., observed toxicity of titanium oxide nanoparticles on mice brain development associated genes[667]. Similarly, studies by Mohammadipour, et al. indicated that titanium oxide nanoparticles impaired memory and learning in fetal mice [668]. In addition, zinc oxide nanoparticles induced necrosis and apoptosis in murine macrophages through p47phox and NADPH oxidase regulated ROS formation [669]. These studies together indicate that, although nanoparticles have a host of advantages, significant research still needs to be done to ensure that neurotoxicity is negated.
6. Bioimaging
Macrophage (cell)-based imaging is of increasing interest for diagnostic as well as therapeutic applications, through their ability to deliver nanometer sized contrast and therapeutic agents to disease sites for theranostics [670]. In fact, macrophage based theranostic platforms have emerged as promising bioimaging tools for overcoming limitations in assessment of drug pharmacokinetic and biodistribution [2, 671, 672]. Many physicochemical factors are important for nanoparticulate drug biodistribution, such as particle surface charge, size, shape, hydrophobicity, surface functionalization or composition of coating material, and protein binding ability [673, 674]. Current studies have shown the effects of nanoparticle size and shape on macrophage uptake [675, 676]. These sizes and shapes define precise biological topographies in the interaction with macrophage receptors [676]. Macrophages phagocytose large amounts of nanoparticles and store them in subcellular compartments for long-term systemic circulation and depot formation in reticuloendothelial organs [212, 677–679].
Macrophages can also release metallic nanoparticles from their intracellular compartments into plasma (reverse metabolism) in response to systemic requirements [64, 174]. In short, metal nanoparticles are rapidly taken up by blood circulating monocytes and establish drug depots in the reticuloendothelial system (spleen, lymph nodes and liver), creating flexibility for imaging these tissues for rapid and real-time assessment of biodistribution of drug or biomolecules [670, 676] (Fig.7). Imaging nanoparticles loaded in macrophages provide a tool of choice to monitor the participation of macrophages in inflammatory processes of various diseases and release particles at sites of inflammation. Macrophage-loaded nanoparticles retained within reticuloendothelial system organs can be easily detected by modern imaging techniques (MRI, [64, 174], CT, PET [670], SPECT [684] and fluorescence [685, 686].
Fig. 7. Schematic illustration of theranostic nanoparticle biodistribution.
Biodistribution of multifunctional theranostic nanoparticles loaded in cells. Metal nanoparticle-loaded macrophages are shown here. Nanoparticle loaded macrophages are transported through the intestinal epithelium and are capable of systematic biodistribution to organs susceptible to infection including lung, liver, spleen and lymph nodes. The ultimate fate of theranostic nanoparticles depends on their specific physicochemical properties, targeting moieties on nanoparticles, and the route of administration, as well as altered biochemical processes in disease states [678, 680–683].
Macrophage-based imaging has unlocked new horizons for diagnosis and therapeutics, as well as predictions of disease progression and design of personalized therapeutic approaches [687]. Two major classes of nanoparticles have been reported for cell based bioimaging applications, based on particle type; (i) inorganic nanoparticles such as metal (silver, gold) [688], metal oxides (iron oxide and cobalt ferrite) [689], rare earth doped nanoparticles, metal-doped silica nanoparticles [174, 688], gadolinium [690] semiconductor nanocrystals (e.g. quantum dots (QDs) and more recently up-conversion nanoparticles [688, 691], and (ii) organic nanoparticles (e.g. dye labeled polymeric as well lipid particles and dye labeled drug nanocrystals) [680, 692, 693].
Magnetic nanoparticles (MNPs) have attracted extensive attention due to their widespread applications that include magnetic separation, magnetic hyperthermia, and use as contrast agents for MRI [682, 683, 690]. In particular, iron oxide nanoparticles (IONPs) have attracted extensive interest due to their superparamagnetic properties [682, 683, 690]. IONPs with diameters between 1 to 100 nm are most widely used in biological systems [694, 695] and are mainly in the forms of magnetite (Fe3O4) and its oxidized derivative maghemite (γ-Fe2O3) [674]. Other types of MNPs that are used in bioimaging include other forms of ferrite [64, 174], dysprosium, manganese [690, 696, 697], neodymium [698], and gadolinium [699]. More recently, MNPs have been modified with the core shell structure to enhance their biocompatibility and ease attachment of targeting moieties to trace disease progression as well as therapeutic applications. Silica coated cobalt ferrite nanoparticles have been applied as multimodal probes for tracking antiretroviral drug biodistribution, utilizing macrophages as carrier vehicles [64, 174]. After intravenous administration of MNPs to animals, they are taken up by macrophages via clathrin-mediated endocytosis in addition to phagocytosis and macropinocytosis [700]. The uptake of MNPs by macrophages creates a unique in vivo tool by which imaging techniques can be used for screening of macrophage involvement in inflammatory processes, such as HIV infection, HIV-1 associated encephalitis [701, 702], bacterial infections [703], CNS diseases [704] [705], tumors [706, 707] and atherosclerosis [708, 709]. Our laboratory has recently reported the development of europium-doped cobalt ferrite nanoparticles for rapid assessment of drug biodistribution by MRI and SPECT/CT in rodents and non-human primates [59, 64]. Moreover, by using folic acid and alendronate functionalized IONPs, tagged macrophages were visualized in vitro and in vivo by MRI after their injection in mice [69]. Recently, PEG-coated IONPs in bone marrow-derived M1 and M2 macrophages were assessed for uptake, labeling efficiency, biocompatibility, and in vivo MRI detection. The authors reported that carboxylate modified IONPs showed higher uptake compared to PEGylated IONPs in M2 subsets [710]. A similar study demonstrated that IONPs used as MRI contrast agents undergo a specific mechanism of chemically activated macrophage uptake by using competition experiments with specific ligands of scavenger receptors SRA-I/II [711]. In addition, IONPs have been used to track the progression of atherosclerosis based on macrophage imaging in rabbits [712]. Lipinski et. al., used gadolinium-containing lipid-based nanoparticles for MRI detection of high-risk human plaques prior to use of an atherothrombotic by targeting macrophage-specific (CD36) [713]. Bagalkot et. al., have shown lipid–latex hybrid nanoparticles targeting, controlled drug release and imaging by MRI of M1 macrophages for inflammation in atherosclerosis [714]. For ischemic stroke, Wiart et. al., reported in vivo magnetic labeling of macrophages and use of MRI to assess macrophage involvements in post-ischemic inflammatory responses [715]. In a separate study, the migration of iron-labeled polarized M1 and M2 macrophages to the lung in a lipopolysaccharide-induced chronic obstructive pulmonary disease animal model and the polarization state of the macrophages once they reached the sites of lung inflammation were assessed. Biodistribution of iron labeled macrophages in abdominal organs and homing to the site of inflammation in the lung was tracked by MRI and used to visualize inflammation in the lungs [716]. Daldrup-Link, et. al. showed by MRI that IONPs are preferentially phagocytosed by tumor-associated macrophages but not by malignant tumor cells [707]. Based on their observation, the authors concluded that IONPs might serve as a new cancer biomarker for long-term prognosis and for evaluation of new immune-targeted therapies [707]. Such non-invasive MRI of TAM using imaging nanoparticles has a huge potential for designing treatment regimens and monitoring effectiveness of chemotherapy, radiation and immunotherapy for cancer patients with primary and metastatic tumors. Dousset et. al., reported IONP-loaded macrophage imaging for assessment of allergic encephalomyelitis by MRI and found a low MRI signal intensity related to IONP-loaded macrophages in the CNS in all animals [717]. Representative schematic of cell based multimodal nanoparticles bio imaging were shown in Fig.8.
Fig. 8. Cell-based bioimaging.
Schematic illustration of multimodal cell based bioimaging. Theranostic nanoparticles loaded into cells can be monitored for brain distribution by using SPECT/CT, PET, and MRI.
Unlike MRI-based imaging technologies, nuclear imaging modalities such as SPECT and PET, are highly sensitive, have unlimited tissue penetration depth and are quantitative [684, 718–720]. These qualities of nuclear imaging have enabled scientists to design nanoparticles that can diagnose disease, track therapeutic efficacy and deliver large payloads of therapeutics to disease sites using macrophages in vivo [708, 721]. Commonly used isotopes for PET include 18F, 64Cu, 68Ga, 11C, 86Y, 124I, and 89Zr [721–726]. The decay of these isotopes results in the emission of a positron. In tissues, these positrons travel a short distance before they collide with an electron resulting in the emission of two 511 keV photons 180 degrees apart from one another. A PET scanner can detect the emissions “coincident” in time and thus determine the location of the radionuclide [727].
Yet another dual modality platform for macrophage targeted USPIO core particles were produced. The particles were coated in aminated and carboxylated PEG, functionalized with diethylenetriaminepentacetate acid, and targeted using Annexin-V that can be imaged by SPECT and MRI. The combination of the two modalities resulted in an imaging platform that combines the high resolution of MRI with the high specificity of SPECT imaging [728, 729]. Venneti et. al., used PET imaging with the 11C–labeled R-enantiomer form of PK11195 ([11C](R)-PK11195) to detect brain macrophages in simian immunodeficiency virus-infected rhesus macaques. They found that increased PK11195 binding in vivo and in postmortem brain tissue correlated with the relative abundance of macrophages but not astrocytes (Fig. 8). PET [11C](R)-PK11195 imaging can be used to detect the presence of macrophages in simian immunodeficiency virus encephalitis in vivo and may be useful in predicting the development of human immunodeficiency virus encephalitis [730]. This type of bioimaging technology may be help in for identification of macrophage subpopulations at disease sites and quantification, rapid assessment of pharmacokinetics and drug biodistribution, design of future therapy and real time monitoring of clinical outcomes of patients after treatment with therapeutic agents [59].
7. Personalized medicine and its perspectives
Incomplete and incorrect understanding of neurodegenerative diseases is one of the reasons for failure of current lines of treatment. In the development of a treatment strategy for these disorders, the ability of the therapeutic molecules to cross the BBB is of paramount importance. Nanotechnology-based alternative therapeutic approaches present more promise than the conventional treatment strategies, however, their potential has yet to be fully explored. There is ample evidence in support of the ability of nanotechnology to deliver and localize therapeutically significant amounts of drug, however, no products have yet been approved for clinical use. This may be due in part to that although they show enhanced performances; they also encounter similar difficulties in trafficking across the BBB for effective treatment as do conventional therapies. This increases the complexity of development of these approaches and efficient management of the neurodegenerative diseases. It is has been established that the behavior and biodistribution of these nanocarriers largely depend on their surface characteristics. Thus, newer molecules and approaches are required to tailor the particle surface to achieve selective brain localization and minimal clearance by other organs. These tailorable surfaces can ensure the enhanced adhesion of these nanocarriers to the BBB and their subsequent brain influx. A better understanding of the molecular expression on the endothelial lining of the BBB would in the effective development and utilization of this strategy. The nanocarriers developed on a laboratory scale face difficulty in their large-scale fabrication, which in turn compromises the architectural integrity, stability and shelf life of the formulations. Another key factor to maintaining their shelf life is the fragility and reduced activity of the targeting molecules, including antibodies, which are employed both during production and usage.
Personalized medicine afforded by neurotheranostics technology may enable delivery of effective treatments with reduced side effects through the design of treatment strategies for individual patients. Recent advancements in multimodal nanoparticles design and delivery technologies may promote molecular and nuclear imaging as clinical tools for rapid diagnosis and therapeutics for NDs. The complex structure of the blood brain barrier has limited therenostics use for personalized treatment, thus, it may be some time before bench-scale technology of neurotheranostics can be adapted for clinical applications.
8. Summary and conclusions
Despite considerable advances in the understanding and treatment of diseases of the CNS, development of clinically significant treatment faces many challenges. Limitations in correct and timely diagnosis of disease and delivery of neurotherapeutic drugs to the brain are responsible for the high morbidity and mortality rates in patients suffering from neurodegenerative disorders. Moreover, most of the neurotherapeutic and neuroprotective molecules suffer from poor aqueous stability, which results in low bioavailability and limited clinical utility. While the BBB imposes limitations to the delivery of high concentrations of drugs to the brain, the liver plays a major role in their rapid clearance from the systemic circulation. Thus, many potential therapeutic molecules fail to reach their molecular targets in the brain, limiting their clinical efficiency. Recent years have seen an exponential increase in attempts to develop effective strategies to cross the BBB and deliver the therapeutic molecule to the site of interest. This review presents a consolidated clinical scenario of various neurodegenerative diseases with focus on the current status of research in the field of theranostics for brain delivery. In addition, the molecular details in disease progression and mechanisms of BBB transport have also been discussed.
A promising approach to enhance the delivery of effective drug levels to the brain is the use of colloidal nanocarriers such as nanocrystals, polymeric nanoparticles, conjugate inorganic nanoparticles, oligosaccharides, and lipids. While movement of naked therapeutic molecules across the BBB faces severe restrictions, transport and targeted delivery of nanocarrier-mediated delivery has shown great potential as a viable therapeutic strategy. These nanocarriers can be loaded with drugs, peptides, nucleic acids, and other neurotherapeutic molecules and can facilitate their transport across the BBB. These nanocarriers can also successfully protect their therapeutic payload from the external environment, where its deactivation or degradation occurs. They have been engineered using various strategies to modify their physicochemical properties and their fate in vivo. They have been decorated by various ligands and antibodies, which have been reported to enhance binding to the cerebrovascular endothelium and enable penetration across the BBB in order to specifically, target the brain tissue and enhance localized drug levels. It should be noted that both the surface properties of these nanocarriers and their targeting specificities are essential in combination to achieve an effective targeted brain delivery. Finally, the accessibility of targeting ligands, their systemic circulation time, and their localization efficiency all play important roles in successful targeting of these nanocarriers.
9. Acknowledgements.
This work was supported, in part, by the University of Nebraska Foundation, which includes individual donations from Dr. Carol Swarts, Harriet Singer and Frances and Louie Blumkin and National Institutes of Health grants P01 MH64570, RO1 MH104147, P01 DA028555, R01 NS36126, P01 NS31492, 2R01 NS034239, P01 NS43985, P30 MH062261 and R01 AG043540.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. References
- [1].Nie S, Xing Y, Kim GJ, Simons JW, Nanotechnology applications in cancer, Annual review of biomedical engineering, 9 (2007) 257–288. [DOI] [PubMed] [Google Scholar]
- [2].Xie J, Lee S, Chen X, Nanoparticle-based theranostic agents, Advanced drug delivery reviews, 62 (2010) 1064–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ross JS, Fletcher JA, The HER-2/neu Oncogene in Breast Cancer: Prognostic Factor, Predictive Factor, and Target for Therapy, The Oncologist, 3 (1998) 237–252. [PubMed] [Google Scholar]
- [4].Lukashov VV, Goudsmit J, HIV heterogeneity and disease progression in AIDS: a model of continuous virus adaptation, AIDS (London, England), 12 Suppl A (1998) S43–52. [PubMed] [Google Scholar]
- [5].Bouzid D, Kammoun A, Amouri A, Mahfoudh N, Haddouk S, Tahri N, Makni H, Masmoudi H, Inflammatory bowel disease: susceptibility and disease heterogeneity revealed by human leukocyte antigen genotyping, Genetic testing and molecular biomarkers, 16 (2012) 482–487. [DOI] [PubMed] [Google Scholar]
- [6].Kelkar SS, Reineke TM, Theranostics: Combining Imaging and Therapy, Bioconjugate Chemistry, 22 (2011) 1879–1903. [DOI] [PubMed] [Google Scholar]
- [7].Blau R, Epshtein Y, Pisarevsky E, Tiram G, Israeli Dangoor S, Yeini E, Krivitsky A, Eldar-Boock A, Ben-Shushan D, Gibori H, Scomparin A, Green O, Ben-Nun Y, Merquiol E, Doron H, Blum G, Erez N, Grossman R, Ram Z, Shabat D, Satchi-Fainaro R, Image-guided surgery using near-infrared Turn-ON fluorescent nanoprobes for precise detection of tumor margins, Theranostics, 8 (2018) 3437–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Janib SM, Moses AS, MacKay JA, Imaging and drug delivery using theranostic nanoparticles, Advanced drug delivery reviews, 62 (2010) 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pene F, Courtine E, Cariou A, Mira JP, Toward theragnostics, Critical care medicine, 37 (2009) S50–58. [DOI] [PubMed] [Google Scholar]
- [10].Ozdemir V, Williams-Jones B, Glatt SJ, Tsuang MT, Lohr JB, Reist C, Shifting emphasis from pharmacogenomics to theragnostics, Nature Biotechnology, 24 (2006) 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chapman EM, History of the discovery and early use of radioactive iodine, JAMA, 250 (1983) 2042–2044. [PubMed] [Google Scholar]
- [12].Mumtaz M, Lin LS, Hui KC, Mohd Khir AS, Radioiodine I-131 for the therapy of graves’ disease, The Malaysian journal of medical sciences : MJMS, 16 (2009) 25–33. [PMC free article] [PubMed] [Google Scholar]
- [13].Silberstein EB, Radioiodine: The Classic Theranostic Agent, Seminars in Nuclear Medicine, 42 (2012) 164–170. [DOI] [PubMed] [Google Scholar]
- [14].Pryma DA, Mandel SJ, Radioiodine Therapy for Thyroid Cancer in the Era of Risk Stratification and Alternative Targeted Therapies, Journal of Nuclear Medicine, 55 (2014) 1485–1491. [DOI] [PubMed] [Google Scholar]
- [15].Shukla HD, Comprehensive Analysis of Cancer-Proteogenome to Identify Biomarkers for the Early Diagnosis and Prognosis of Cancer, Proteomes, 5 (2017) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shruthi BS, Vinodhkumar P, Selvamani, Proteomics: A new perspective for cancer, Advanced biomedical research, 5 (2016) 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Conn EM, Madsen MA, Cravatt BF, Ruf W, Deryugina EI, Quigley JP, Cell surface proteomics identifies molecules functionally linked to tumor cell intravasation, The Journal of biological chemistry, 283 (2008) 26518–26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chapman S, Dobrovolskaia M, Farahani K, Goodwin A, Joshi A, Lee H, Meade T, Pomper M, Ptak K, Rao J, Singh R, Sridhar S, Stern S, Wang A, Weaver JB, Woloschak G, Yang L, Nanoparticles for cancer imaging: The good, the bad, and the promise, Nano today, 8 (2013) 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chinen AB, Guan CM, Ferrer JR, Barnaby SN, Merkel TJ, Mirkin CA, Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence, Chemical Reviews, 115 (2015) 10530–10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cormode DP, Skajaa T, Fayad ZA, Mulder WJ, Nanotechnology in medical imaging: probe design and applications, Arteriosclerosis, thrombosis, and vascular biology, 29 (2009) 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Goel S, England CG, Chen F, Cai W, Positron emission tomography and nanotechnology: A dynamic duo for cancer theranostics, Advanced drug delivery reviews, 113 (2017) 157–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Agdeppa ED, Spilker ME, A review of imaging agent development, The AAPS journal, 11 (2009) 286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Toy R, Bauer L, Hoimes C, Ghaghada KB, Karathanasis E, Targeted nanotechnology for cancer imaging, Advanced drug delivery reviews, 76 (2014) 79–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Savla R, Minko T, Nanoparticle design considerations for molecular imaging of apoptosis: Diagnostic, prognostic, and therapeutic value, Advanced drug delivery reviews, 113 (2017) 122–140. [DOI] [PubMed] [Google Scholar]
- [25].Sikkandhar MG, Nedumaran AM, Ravichandar R, Singh S, Santhakumar I, Goh ZC, Mishra S, Archunan G, Gulyas B, Padmanabhan P, Theranostic Probes for Targeting Tumor Microenvironment: An Overview, International journal of molecular sciences, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yaari Z, da Silva D, Zinger A, Goldman E, Kajal A, Tshuva R, Barak E, Dahan N, Hershkovitz D, Goldfeder M, Roitman JS, Schroeder A, Theranostic barcoded nanoparticles for personalized cancer medicine, Nature Communications, 7 (2016) 13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].E.S.o. Radiology, Medical imaging in personalised medicine: a white paper of the research committee of the European Society of Radiology (ESR), Insights into Imaging, 6 (2015) 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Personalized medicine: identifying the appropriate patient through biomarkers in oncology, P & T : a peer-reviewed journal for formulary management, 36 (2011) 3–10. [PMC free article] [PubMed] [Google Scholar]
- [29].Portnow LH, Vaillancourt DE, Okun MS, The history of cerebral PET scanning, From physiology to cutting-edge technology, 80 (2013) 952–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Truffi M, Colombo M, Sorrentino L, Pandolfi L, Mazzucchelli S, Pappalardo F, Pacini C, Allevi R, Bonizzi A, Corsi F, Prosperi D, Multivalent exposure of trastuzumab on iron oxide nanoparticles improves antitumor potential and reduces resistance in HER2-positive breast cancer cells, Scientific Reports, 8 (2018) 6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Henry KE, Ulaner GA, Lewis JS, Human Epidermal Growth Factor Receptor 2-Targeted PET/Single-Photon Emission Computed Tomography Imaging of Breast Cancer: Noninvasive Measurement of a Biomarker Integral to Tumor Treatment and Prognosis, PET Clinics, 12 (2017) 269–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Massicano AVF, Marquez-Nostra BV, Lapi SE, Targeting HER2 in Nuclear Medicine for Imaging and Therapy, Molecular Imaging, 17 (2018) 1536012117745386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang G-F, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B, Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B, Annals of Neurology, 55 (2004) 306–319. [DOI] [PubMed] [Google Scholar]
- [34].Lamberts SWJ, Barker WH, Reubi J-C, Krenning EP, Somatostatin-Receptor Imaging in the Localization of Endocrine Tumors, New England Journal of Medicine, 323 (1990) 1246–1249. [DOI] [PubMed] [Google Scholar]
- [35].Graham MM, Menda Y, Radiopeptide imaging and therapy in the United States, Journal of nuclear medicine : official publication, Society of Nuclear Medicine, 52 Suppl 2 (2011) 56s–63s. [DOI] [PubMed] [Google Scholar]
- [36].Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, van Hagen M, Postema PT, de Jong M, Reubi JC, et al. , Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients, European journal of nuclear medicine, 20 (1993) 716–731. [DOI] [PubMed] [Google Scholar]
- [37].Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, De Jong FH, Christiansen A, Kam BL, De Herder WW, Stridsberg M, Lindemans J, Ensing G, Krenning EP, Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience, Semin Nucl Med, 32 (2002) 110–122. [DOI] [PubMed] [Google Scholar]
- [38].Albert R, Smith-Jones P, Stolz B, Simeon C, Knecht H, Bruns C, Pless J, Direct synthesis of [DOTA-DPhe1]-octreotide and [DOTA-DPhe1,Tyr3]-octreotide (SMT487): two conjugates for systemic delivery of radiotherapeutical nuclides to somatostatin receptor positive tumors in man, Bioorganic & medicinal chemistry letters, 8 (1998) 1207–1210. [DOI] [PubMed] [Google Scholar]
- [39].Hammond PJ, Wade AF, Gwilliam ME, Peters AM, Myers MJ, Gilbey SG, Bloom SR, Calam J, Amino acid infusion blocks renal tubular uptake of an indium-labelled somatostatin analogue, British journal of cancer, 67 (1993) 1437–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Melis M, Krenning EP, Bernard BF, Barone R, Visser TJ, de Jong M, Localisation and mechanism of renal retention of radiolabelled somatostatin analogues, European Journal of Nuclear Medicine and Molecular Imaging, 32 (2005) 1136–1143. [DOI] [PubMed] [Google Scholar]
- [41].Jamar F, Barone R, Mathieu I, Walrand S, Labar D, Carlier P, De Camps J, Schran H, Chen T, Smith MC, Bouterfa H, Valkema R, Krenning EP, Kvols LK, Pauwels S, 86Y-DOTA0-d-Phe1-Tyr3-octreotide (SMT487)—a phase 1 clinical study: pharmacokinetics, biodistribution and renal protective effect of different regimens of amino acid co-infusion, European Journal of Nuclear Medicine and Molecular Imaging, 30 (2003) 510–518. [DOI] [PubMed] [Google Scholar]
- [42].Barone R, Borson-Chazot F, Valkema R, Walrand S, Chauvin F, Gogou L, Kvols LK, Krenning EP, Jamar F, Pauwels S, Patient-Specific Dosimetry in Predicting Renal Toxicity with 90Y-DOTATOC: Relevance of Kidney Volume and Dose Rate in Finding a Dose–Effect Relationship, Journal of Nuclear Medicine, 46 (2005) 99S–106S. [PubMed] [Google Scholar]
- [43].Delaloye AB, Highlights of the Annual Meeting of the European Association of Nuclear Medicine: Copenhagen 1996 Eur J Nuel Med (1997) 24:219–232 [DOI] [PubMed] [Google Scholar]
- [44].Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O’Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E, Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors, New England Journal of Medicine, 376 (2017) 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Seregni E, Maccauro M, Chiesa C, Mariani L, Pascali C, Mazzaferro V, De Braud F, Buzzoni R, Milione M, Lorenzoni A, Bogni A, Coliva A, Vullo SL, Bombardieri E, Treatment with tandem [90Y]DOTA-TATE and [177Lu]DOTA-TATE of neuroendocrine tumours refractory to conventional therapy, European Journal of Nuclear Medicine and Molecular Imaging, 41 (2014) 223–230. [DOI] [PubMed] [Google Scholar]
- [46].van Essen M, Krenning EP, Kam BL, de Herder WW, van Aken MO, Kwekkeboom DJ, Report on short-term side effects of treatments with 177Lu-octreotate in combination with capecitabine in seven patients with gastroenteropancreatic neuroendocrine tumours, Eur J Nucl Med Mol Imaging, 35 (2008) 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Claringbold PG, Turner JH, Pancreatic Neuroendocrine Tumor Control: Durable Objective Response to Combination 177Lu-Octreotate-Capecitabine-Temozolomide Radiopeptide Chemotherapy, Neuroendocrinology, 103 (2016) 432–439. [DOI] [PubMed] [Google Scholar]
- [48].Bodei L, Kidd M, Modlin IM, Severi S, Drozdov I, Nicolini S, Kwekkeboom DJ, Krenning EP, Baum RP, Paganelli G, Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors, Eur J Nucl Med Mol Imaging, 43 (2016) 839–851. [DOI] [PubMed] [Google Scholar]
- [49].Lee SY, Jeon SI, Jung S, Chung IJ, Ahn C-H, Targeted multimodal imaging modalities, Advanced drug delivery reviews, 76 (2014) 60–78. [DOI] [PubMed] [Google Scholar]
- [50].Banerjee S, Ambikalmajan Pillai MR, Ramamoorthy N, Evolution of Tc-99m in diagnostic radiopharmaceuticals, Seminars in Nuclear Medicine, 31 (2001) 260–277. [DOI] [PubMed] [Google Scholar]
- [51].Thakor AS, Jokerst JV, Ghanouni P, Campbell JL, Mittra E, Gambhir SS, Clinically Approved Nanoparticle Imaging Agents, Journal of Nuclear Medicine, 57 (2016) 1833–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tong L, Zhao M, Zhu S, Chen J, Synthesis and application of superparamagnetic iron oxide nanoparticles in targeted therapy and imaging of cancer, Frontiers of Medicine, 5 (2011) 379–387. [DOI] [PubMed] [Google Scholar]
- [53].Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR, Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date, Pharmaceutical Research, 33 (2016) 2373–2387. [DOI] [PubMed] [Google Scholar]
- [54].Kinch MS, Woodard PK, Analysis of FDA-approved imaging agents, Drug Discovery Today, 22 (2017) 1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vats S, Singh M, Siraj S, Singh H, Tandon S, Role of nanotechnology in theranostics and personalized medicines, Journal of Health Research and Reviews, 4 (2017) 1–7. [Google Scholar]
- [56].Jo SD, Ku SH, Won YY, Kim SH, Kwon IC, Targeted Nanotheranostics for Future Personalized Medicine: Recent Progress in Cancer Therapy, Theranostics, 6 (2016) 1362–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ahn BC, Personalized Medicine Based on Theranostic Radioiodine Molecular Imaging for Differentiated Thyroid Cancer, BioMed research international, 2016 (2016) 1680464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jeelani S, Reddy RC, Maheswaran T, Asokan GS, Dany A, Anand B, Theranostics: A treasured tailor for tomorrow, Journal of pharmacy & bioallied sciences, 6 (2014) S6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ottemann BM, Helmink AJ, Zhang W, Mukadam I, Woldstad C, Hilaire JR, Liu Y, McMillan JM, Edagwa BJ, Mosley RL, Garrison JC, Kevadiya BD, Gendelman HE, Bioimaging predictors of rilpivirine biodistribution and antiretroviral activities, Biomaterials, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhou J, Yang Y, Zhang C.-y., Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application, Chemical Reviews, 115 (2015) 11669–11717. [DOI] [PubMed] [Google Scholar]
- [61].Dykman L, Khlebtsov N, Gold nanoparticles in biomedical applications: recent advances and perspectives, Chemical Society Reviews, 41 (2012) 2256–2282. [DOI] [PubMed] [Google Scholar]
- [62].Xiaoming L, Jianrong W, E. AK, Yubo F, Qingling F, Fu‐Zhai C, Fumio W, Current investigations into magnetic nanoparticles for biomedical applications, Journal of Biomedical Materials Research Part A, 104 (2016) 1285–1296. [DOI] [PubMed] [Google Scholar]
- [63].Gao J, Gu H, Xu B, Multifunctional Magnetic Nanoparticles: Design, Synthesis, and Biomedical Applications, Accounts of Chemical Research, 42 (2009) 1097–1107. [DOI] [PubMed] [Google Scholar]
- [64].Kevadiya BD, Woldstad C, Ottemann BM, Dash P, Sajja BR, Lamberty B, Morsey B, Kocher T, Dutta R, Bade AN, Liu Y, Callen SE, Fox HS, Byrareddy SN, McMillan JM, Bronich TK, Edagwa BJ, Boska MD, Gendelman HE, Multimodal Theranostic Nanoformulations Permit Magnetic Resonance Bioimaging of Antiretroviral Drug Particle Tissue-Cell Biodistribution, Theranostics, 8 (2018) 256–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Buzea C, Pacheco II, Robbie K, Nanomaterials and nanoparticles: sources and toxicity, Biointerphases, 2 (2007) Mr17–71. [DOI] [PubMed] [Google Scholar]
- [66].Bleeker EAJ, de Jong WH, Geertsma RE, Groenewold M, Heugens EHW, Koers-Jacquemijns M, van de Meent D, Popma JR, Rietveld AG, Wijnhoven SWP, Cassee FR, Oomen AG, Considerations on the EU definition of a nanomaterial: Science to support policy making, Regulatory Toxicology and Pharmacology, 65 (2013) 119–125. [DOI] [PubMed] [Google Scholar]
- [67].Muthu MS, Leong DT, Mei L, Feng SS, Nanotheranostics - application and further development of nanomedicine strategies for advanced theranostics, Theranostics, 4 (2014) 660–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Thanh NTK, Green LAW, Functionalisation of nanoparticles for biomedical applications, Nano today, 5 (2010) 213–230. [Google Scholar]
- [69].Li T, Gendelman HE, Zhang G, Puligujja P, McMillan JM, Bronich TK, Edagwa B, Liu XM, Boska MD, Magnetic resonance imaging of folic acid-coated magnetite nanoparticles reflects tissue biodistribution of long-acting antiretroviral therapy, Int J Nanomedicine, 10 (2015) 3779–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Martinez-Skinner AL, Arainga MA, Puligujja P, Palandri DL, Baldridge HM, Edagwa BJ, McMillan JM, Mosley RL, Gendelman HE, Cellular Responses and Tissue Depots for Nanoformulated Antiretroviral Therapy, PLoS One, 10 (2015) e0145966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Puligujja P, McMillan J, Kendrick L, Li T, Balkundi S, Smith N, Veerubhotla RS, Edagwa BJ, Kabanov AV, Bronich T, Gendelman HE, Liu XM, Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections, Nanomedicine, 9 (2013) 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhou T, Lin Z, Puligujja P, Palandri D, Hilaire J, Araínga M, Smith N, Gautam N, McMillan J, Alnouti Y, Liu X, Edagwa B, Gendelman HE, Optimizing the preparation and stability of decorated antiretroviral drug nanocrystals, Nanomedicine, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S, Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium, Proceedings of the National Academy of Sciences, 110 (2013) 10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wiley DT, Webster P, Gale A, Davis ME, Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor, Proceedings of the National Academy of Sciences, 110 (2013) 8662–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bhatia S, Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications, Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae, Springer International Publishing, Cham, 2016, pp. 33–93. [Google Scholar]
- [76].Gatoo MA, Naseem S, Arfat MY, Mahmood Dar A, Qasim K, Zubair S, Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations, BioMed research international, 2014 (2014) 498420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lindfors L, Skantze P, Skantze U, Rasmusson M, Zackrisson A, Olsson U, Amorphous Drug Nanosuspensions. 1. Inhibition of Ostwald Ripening, Langmuir, 22 (2006) 906–910. [DOI] [PubMed] [Google Scholar]
- [78].Sillman B, Bade AN, Dash PK, Bhargavan B, Kocher T, Mathews S, Su H, Kanmogne GD, Poluektova LY, Gorantla S, McMillan J, Gautam N, Alnouti Y, Edagwa B, Gendelman HE, Creation of a long-acting nanoformulated dolutegravir, Nature Communications, 9 (2018) 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Miao X, Yang W, Feng T, Lin J, Huang P, Drug nanocrystals for cancer therapy, Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 10 (2018) e1499. [DOI] [PubMed] [Google Scholar]
- [80].Rasenack N, Müller BW, Micron‐Size Drug Particles: Common and Novel Micronization Techniques, Pharmaceutical Development and Technology, 9 (2004) 1–13. [DOI] [PubMed] [Google Scholar]
- [81].Kevadiya B, Barvaliya M, Zhang L, Anovadiya A, Brahmbhatt H, Paul P, Tripathi C, Fenofibrate Nanocrystals Embedded in Oral Strip-Films for Bioavailability Enhancement, Bioengineering, 5 (2018) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Liu Y, Cao X, The origin and function of tumor-associated macrophages, Cell Mol Immunol, 12 (2015) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lu Y, Wang Z.-h., Li T, McNally H, Park K, Sturek M, Development and evaluation of transferrin-stabilized paclitaxel nanocrystal formulation, Journal of Controlled Release, 176 (2014) 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Pawar VK, Singh Y, Meher JG, Gupta S, Chourasia MK, Engineered nanocrystal technology: In-vivo fate, targeting and applications in drug delivery, Journal of Controlled Release, 183 (2014) 51–66. [DOI] [PubMed] [Google Scholar]
- [85].Hollis CP, Weiss HL, Leggas M, Evers BM, Gemeinhart RA, Li T, Biodistribution and bioimaging studies of hybrid paclitaxel nanocrystals: Lessons learned of the EPR effect and image-guided drug delivery, Journal of Controlled Release, 172 (2013) 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Chen C, Wang L, Cao F, Miao X, Chen T, Chang Q, Zheng Y, Formulation of 20(S)-protopanaxadiol nanocrystals to improve oral bioavailability and brain delivery, International Journal of Pharmaceutics, 497 (2016) 239–247. [DOI] [PubMed] [Google Scholar]
- [87].Roma MI, Hocht C, Chiappetta DA, Gennaro SSD, Minoia JM, Bramuglia GF, Rubio MC, Sosnik A, Peroni RN, Tetronic® 904-containing polymeric micelles overcome the overexpression of ABCG2 in the blood–brain barrier of rats and boost the penetration of the antiretroviral efavirenz into the CNS, Nanomedicine, 10 (2015) 2325–2337. [DOI] [PubMed] [Google Scholar]
- [88].Kreuter J, Drug delivery to the central nervous system by polymeric nanoparticles: What do we know?, Advanced drug delivery reviews, 71 (2014) 2–14. [DOI] [PubMed] [Google Scholar]
- [89].Cheng CJ, Tietjen GT, Saucier-Sawyer JK, Saltzman WM, A holistic approach to targeting disease with polymeric nanoparticles, Nature Reviews Drug Discovery, 14 (2015) 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Gerson T, Makarov E, Senanayake TH, Gorantla S, Poluektova LY, Vinogradov SV, Nano-NRTIs demonstrate low neurotoxicity and high antiviral activity against HIV infection in the brain, Nanomedicine: Nanotechnology, Biology and Medicine, 10 (2014) 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bramini M, Ye D, Hallerbach A, Nic Raghnaill M, Salvati A, Åberg C, Dawson KA, Imaging Approach to Mechanistic Study of Nanoparticle Interactions with the Blood–Brain Barrier, ACS Nano, 8 (2014) 4304–4312. [DOI] [PubMed] [Google Scholar]
- [92].Kulkarni SA, Feng S-S, Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery, Pharmaceutical Research, 30 (2013) 2512–2522. [DOI] [PubMed] [Google Scholar]
- [93].Gao K, Jiang X, Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles, International Journal of Pharmaceutics, 310 (2006) 213–219. [DOI] [PubMed] [Google Scholar]
- [94].Guarnieri D, Falanga A, Muscetti O, Tarallo R, Fusco S, Galdiero M, Galdiero S, Netti PA, Shuttle‐Mediated Nanoparticle Delivery to the Blood–Brain Barrier, Small, 9 (2013) 853–862. [DOI] [PubMed] [Google Scholar]
- [95].Cabezón I, Manich G, Martín-Venegas R, Camins A, Pelegrí C, Vilaplana J, Trafficking of Gold Nanoparticles Coated with the 8D3 Anti-Transferrin Receptor Antibody at the Mouse Blood–Brain Barrier, Molecular Pharmaceutics, 12 (2015) 4137–4145. [DOI] [PubMed] [Google Scholar]
- [96].Menéndez L, Lastra A, Hidalgo A.n., Baamonde A, Unilateral hot plate test: a simple and sensitive method for detecting central and peripheral hyperalgesia in mice, Journal of Neuroscience Methods, 113 (2002) 91–97. [DOI] [PubMed] [Google Scholar]
- [97].Montesinos RN, Moulari B, Gromand J, Beduneau A, Lamprecht A, Pellequer Y, Coadministration of P-Glycoprotein Modulators on Loperamide Pharmacokinetics and Brain Distribution, Drug Metabolism and Disposition, 42 (2014) 700–706. [DOI] [PubMed] [Google Scholar]
- [98].Fornaguera C, Dols-Perez A, Calderó G, García-Celma MJ, Camarasa J, Solans C, PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood–brain barrier, Journal of Controlled Release, 211 (2015) 134–143. [DOI] [PubMed] [Google Scholar]
- [99].Shinoda K, Hruby VJ, Porreca F, Antihyperalgesic effects of loperamide in a model of rat neuropathic pain are mediated by peripheral δ-opioid receptors, Neuroscience Letters, 411 (2007) 143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Li-Blatter X, Nervi P, Seelig A, Detergents as intrinsic P-glycoprotein substrates and inhibitors, Biochimica et Biophysica Acta (BBA) - Biomembranes, 1788 (2009) 2335–2344. [DOI] [PubMed] [Google Scholar]
- [101].Salvalaio M, Rigon L, Belletti D, D’Avanzo F, Pederzoli F, Ruozi B, Marin O, Vandelli MA, Forni F, Scarpa M, Tomanin R, Tosi G, Targeted Polymeric Nanoparticles for Brain Delivery of High Molecular Weight Molecules in Lysosomal Storage Disorders, PLOS ONE, 11 (2016) e0156452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kozielski KL, Tzeng SY, Hurtado De Mendoza BA, Green JJ, Bioreducible Cationic Polymer-Based Nanoparticles for Efficient and Environmentally Triggered Cytoplasmic siRNA Delivery to Primary Human Brain Cancer Cells, ACS Nano, 8 (2014) 3232–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Leiro V, Santos SD, Lopes CDF, Pêgo AP, Dendrimers as Powerful Building Blocks in Central Nervous System Disease: Headed for Successful Nanomedicine, Advanced Functional Materials, 28 (2018) 1700313. [Google Scholar]
- [104].Xu L, Zhang H, Wu Y, Dendrimer Advances for the Central Nervous System Delivery of Therapeutics, ACS Chemical Neuroscience, 5 (2014) 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Shakhbazau A, Mishra M, Chu TH, Brideau C, Cummins K, Tsutsui S, Shcharbin D, Majoral JP, Mignani S, Blanchard‐Desce M, Bryszewska M, Yong VW, Stys PK, Minnen J.v., Fluorescent Phosphorus Dendrimer as a Spectral Nanosensor for Macrophage Polarization and Fate Tracking in Spinal Cord Injury, Macromolecular Bioscience, 15 (2015) 1523–1534. [DOI] [PubMed] [Google Scholar]
- [106].Nance E, Timbie K, Miller GW, Song J, Louttit C, Klibanov AL, Shih T-Y, Swaminathan G, Tamargo RJ, Woodworth GF, Hanes J, Price RJ, Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood−brain barrier using MRI-guided focused ultrasound, Journal of Controlled Release, 189 (2014) 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Liu H-L, Fan C-H, Ting C-Y, Yeh C-K, Combining Microbubbles and Ultrasound for Drug Delivery to Brain Tumors: Current Progress and Overview, Theranostics, 4 (2014) 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Aryal M, Arvanitis CD, Alexander PM, McDannold N, Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system, Advanced drug delivery reviews, 72 (2014) 94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Koczera P, Appold L, Shi Y, Liu M, Dasgupta A, Pathak V, Ojha T, Fokong S, Wu Z, van Zandvoort M, Iranzo O, Kuehne AJC, Pich A, Kiessling F, Lammers T, PBCA-based polymeric microbubbles for molecular imaging and drug delivery, Journal of Controlled Release, 259 (2017) 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Allen TM, Cullis PR, Liposomal drug delivery systems: From concept to clinical applications, Advanced drug delivery reviews, 65 (2013) 36–48. [DOI] [PubMed] [Google Scholar]
- [111].J Julia S-U, Jimena C.-F. Fabiola, Dalet F.-G. Eunice, Guadalupe T.-F. Jose, Antonio S.-U. Marvin, Scope of Lipid Nanoparticles in Neuroscience: Impact on the Treatment of Neurodegenerative Diseases, Current Pharmaceutical Design, 23 (2017) 3120–3133. [DOI] [PubMed] [Google Scholar]
- [112].Tapeinos C, Battaglini M, Ciofani G, Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases, Journal of Controlled Release, 264 (2017) 306–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Cacciatore I, Ciulla M, Fornasari E, Marinelli L, Di Stefano A, Solid lipid nanoparticles as a drug delivery system for the treatment of neurodegenerative diseases, Expert Opinion on Drug Delivery, 13 (2016) 1121–1131. [DOI] [PubMed] [Google Scholar]
- [114].Singh I, Swami R, Khan W, Sistla R, Lymphatic system: a prospective area for advanced targeting of particulate drug carriers, Expert Opinion on Drug Delivery, 11 (2014) 211–229. [DOI] [PubMed] [Google Scholar]
- [115].Bae KH, Lee JY, Lee SH, Park TG, Nam YS, Optically Traceable Solid Lipid Nanoparticles Loaded with siRNA and Paclitaxel for Synergistic Chemotherapy with In situ Imaging, Advanced Healthcare Materials, 2 (2013) 576–584. [DOI] [PubMed] [Google Scholar]
- [116].Torchilin VP, Recent advances with liposomes as pharmaceutical carriers, Nature Reviews Drug Discovery, 4 (2005) 145. [DOI] [PubMed] [Google Scholar]
- [117].Xu H-L, Yang J-J, ZhuGe D-L, Lin M-T, Zhu Q-Y, Jin B-H, Tong M-Q, Shen B-X, Xiao J, Zhao Y-Z, Glioma-Targeted Delivery of a Theranostic Liposome Integrated with Quantum Dots, Superparamagnetic Iron Oxide, and Cilengitide for Dual-Imaging Guiding Cancer Surgery, Advanced Healthcare Materials, 7 (2018) 1701130. [DOI] [PubMed] [Google Scholar]
- [118].Kizelsztein YBO, Liposomal formulations comprising an amphipathic weak base like tempamine for treatment of neurodegenerative conditions, Hadasit Medical Research Services and Development Co Yissum Research Development Co of Hebrew University EU, 2004-September-09. [Google Scholar]
- [119].Vedagiri A, Thangarajan S, Mitigating effect of chrysin loaded solid lipid nanoparticles against Amyloid β25–35 induced oxidative stress in rat hippocampal region: An efficient formulation approach for Alzheimer’s disease, Neuropeptides, 58 (2016) 111–125. [DOI] [PubMed] [Google Scholar]
- [120].Rassu G, Soddu E, Posadino AM, Pintus G, Sarmento B, Giunchedi P, Gavini E, Nose-to-brain delivery of BACE1 siRNA loaded in solid lipid nanoparticles for Alzheimer’s therapy, Colloids and Surfaces B: Biointerfaces, 152 (2017) 296–301. [DOI] [PubMed] [Google Scholar]
- [121].Vieira DB, Gamarra LF, Getting into the brain: liposome-based strategies for effective drug delivery across the blood–brain barrier, International Journal of Nanomedicine, 11 (2016) 5381–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lindqvist A, Rip J, van Kregten J, Gaillard PJ, Hammarlund-Udenaes M, In vivo Functional Evaluation of Increased Brain Delivery of the Opioid Peptide DAMGO by Glutathione-PEGylated Liposomes, Pharmaceutical Research, 33 (2016) 177–185. [DOI] [PubMed] [Google Scholar]
- [123].Gaillard PJ, Appeldoorn CCM, Dorland R, van Kregten J, Manca F, Vugts DJ, Windhorst B, van Dongen GAMS, de Vries HE, Maussang D, van Tellingen O, Pharmacokinetics, Brain Delivery, and Efficacy in Brain Tumor-Bearing Mice of Glutathione Pegylated Liposomal Doxorubicin (2B3–101), PLOS ONE, 9 (2014) e82331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kenny GD, Bienemann AS, Tagalakis AD, Pugh JA, Welser K, Campbell F, Tabor AB, Hailes HC, Gill SS, Lythgoe MF, McLeod CW, White EA, Hart SL, Multifunctional receptor-targeted nanocomplexes for the delivery of therapeutic nucleic acids to the Brain, Biomaterials, 34 (2013) 9190–9200. [DOI] [PubMed] [Google Scholar]
- [125].Zheng X, Shao X, Zhang C, Tan Y, Liu Q, Wan X, Zhang Q, Xu S, Jiang X, Intranasal H102 Peptide-Loaded Liposomes for Brain Delivery to Treat Alzheimer’s Disease, Pharmaceutical Research, 32 (2015) 3837–3849. [DOI] [PubMed] [Google Scholar]
- [126].Chen C, Duan Z, Yuan Y, Li R, Pang L, Liang J, Xu X, Wang J, Peptide-22 and Cyclic RGD Functionalized Liposomes for Glioma Targeting Drug Delivery Overcoming BBB and BBTB, ACS Applied Materials & Interfaces, 9 (2017) 5864–5873. [DOI] [PubMed] [Google Scholar]
- [127].Sharma G, Modgil A, Layek B, Arora K, Sun C, Law B, Singh J, Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: Biodistribution and transfection, Journal of Controlled Release, 167 (2013) 1–10. [DOI] [PubMed] [Google Scholar]
- [128].Zhao Y, Jiang Y, Lv W, Wang Z, Lv L, Wang B, Liu X, Liu Y, Hu Q, Sun W, Xu Q, Xin H, Gu Z, Dual targeted nanocarrier for brain ischemic stroke treatment, Journal of Controlled Release, 233 (2016) 64–71. [DOI] [PubMed] [Google Scholar]
- [129].Gao J-Q, Lv Q, Li L-M, Tang X-J, Li F-Z, Hu Y-L, Han M, Glioma targeting and blood–brain barrier penetration by dual-targeting doxorubincin liposomes, Biomaterials, 34 (2013) 5628–5639. [DOI] [PubMed] [Google Scholar]
- [130].Hernando S, Herran E, Figueiro-Silva J, Pedraz JL, Igartua M, Carro E, Hernandez RM, Intranasal Administration of TAT-Conjugated Lipid Nanocarriers Loading GDNF for Parkinson’s Disease, Molecular Neurobiology, 55 (2018) 145–155. [DOI] [PubMed] [Google Scholar]
- [131].Huang M, Hu M, Song Q, Song H, Huang J, Gu X, Wang X, Chen J, Kang T, Feng X, Jiang D, Zheng G, Chen H, Gao X, GM1-Modified Lipoprotein-like Nanoparticle: Multifunctional Nanoplatform for the Combination Therapy of Alzheimer’s Disease, ACS Nano, 9 (2015) 10801–10816. [DOI] [PubMed] [Google Scholar]
- [132].Hong AR, Kim Y, Lee TS, Kim S, Lee K, Kim G, Jang HS, Intense Red-Emitting Upconversion Nanophosphors (800 nm-Driven) with a Core/Double-Shell Structure for Dual-Modal Upconversion Luminescence and Magnetic Resonance in Vivo Imaging Applications, ACS Applied Materials & Interfaces, 10 (2018) 12331–12340. [DOI] [PubMed] [Google Scholar]
- [133].Ehlerding EB, Grodzinski P, Cai W, Liu CH, Big Potential from Small Agents: Nanoparticles for Imaging-Based Companion Diagnostics, ACS Nano, 12 (2018) 2106–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Khlebtsov N, Bogatyrev V, Dykman L, Khlebtsov B, Staroverov S, Shirokov A, Matora L, Khanadeev V, Pylaev T, Tsyganova N, Terentyuk G, Analytical and Theranostic Applications of Gold Nanoparticles and Multifunctional Nanocomposites, Theranostics, 3 (2013) 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L, Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases, Journal of Controlled Release, 235 (2016) 34–47. [DOI] [PubMed] [Google Scholar]
- [136].Agyare EK, Jaruszewski KM, Curran GL, Rosenberg JT, Grant SC, Lowe VJ, Ramakrishnan S, Paravastu AK, Poduslo JF, Kandimalla KK, Engineering theranostic nanovehicles capable of targeting cerebrovascular amyloid deposits, Journal of Controlled Release, 185 (2014) 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Dilnawaz F, Sahoo SK, Therapeutic approaches of magnetic nanoparticles for the central nervous system, Drug Discovery Today, 20 (2015) 1256–1264. [DOI] [PubMed] [Google Scholar]
- [138].Busquets MA, Sabaté R, Estelrich J, Potential applications of magnetic particles to detect and treat Alzheimer’s disease, Nanoscale Research Letters, 9 (2014) 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Niu S, Zhang L-K, Zhang L, Zhuang S, Zhan X, Chen W-Y, Du S, Yin L, You R, Li C-H, Guan Y-Q, Inhibition by Multifunctional Magnetic Nanoparticles Loaded with Alpha-Synuclein RNAi Plasmid in a Parkinson’s Disease Model, Theranostics, 7 (2017) 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN, Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications, Chemical Reviews, 108 (2008) 2064–2110. [DOI] [PubMed] [Google Scholar]
- [141].Amendola V, Riello P, Meneghetti M, Magnetic Nanoparticles of Iron Carbide, Iron Oxide, Iron@Iron Oxide, and Metal Iron Synthesized by Laser Ablation in Organic Solvents, The Journal of Physical Chemistry C, 115 (2011) 5140–5146. [Google Scholar]
- [142].Dai L, Liu Y, Wang Z, Guo F, Shi D, Zhang B, One-pot facile synthesis of PEGylated superparamagnetic iron oxide nanoparticles for MRI contrast enhancement, Materials Science and Engineering: C, 41 (2014) 161–167. [DOI] [PubMed] [Google Scholar]
- [143].Chen W-H, Xu X-D, Jia H-Z, Lei Q, Luo G-F, Cheng S-X, Zhuo R-X, Zhang X-Z, Therapeutic nanomedicine based on dual-intelligent functionalized gold nanoparticles for cancer imaging and therapy in vivo, Biomaterials, 34 (2013) 8798–8807. [DOI] [PubMed] [Google Scholar]
- [144].Betzer O, Shilo M, Opochinsky R, Barnoy E, Motiei M, Okun E, Yadid G, Popovtzer R, The effect of nanoparticle size on the ability to cross the blood–brain barrier: an in vivo study, Nanomedicine, 12 (2017) 1533–1546. [DOI] [PubMed] [Google Scholar]
- [145].Ruff J, Hüwel S, Kogan MJ, Simon U, Galla H-J, The effects of gold nanoparticles functionalized with ß-amyloid specific peptides on an in vitro model of blood-brain barrier, Nanomedicine: Nanotechnology, Biology and Medicine, 13 (2017) 1645–1652. [DOI] [PubMed] [Google Scholar]
- [146].Gao H, Progress and perspectives on targeting nanoparticles for brain drug delivery, Acta Pharmaceutica Sinica. B, 6 (2016) 268–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Li Y, Liu R, Ji W, Li Y, Liu L, Zhang X, Delivery systems for theranostics in neurodegenerative diseases, Nano Research, (2018). [Google Scholar]
- [148].Leyva-Gómez G, Cortés H, Magaña JJ, Leyva-García N, Quintanar-Guerrero D, Florán B, Nanoparticle technology for treatment of Parkinson’s disease: the role of surface phenomena in reaching the brain, Drug Discovery Today, 20 (2015) 824–837. [DOI] [PubMed] [Google Scholar]
- [149].Singh AV, Khare M, Gade WN, Zamboni P, Theranostic Implications of Nanotechnology in Multiple Sclerosis: A Future Perspective, Autoimmune Diseases, 2012 (2012) 160830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Bolognesi ML, Gandini A, Prati F, Uliassi E, From Companion Diagnostics to Theranostics: A New Avenue for Alzheimer’s Disease?, Journal of Medicinal Chemistry, 59 (2016) 7759–7770. [DOI] [PubMed] [Google Scholar]
- [151].Amiri H, Saeidi K, Borhani P, Manafirad A, Ghavami M, Zerbi V, Alzheimer’s Disease: Pathophysiology and Applications of Magnetic Nanoparticles as MRI Theranostic Agents, ACS Chemical Neuroscience, 4 (2013) 1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Ji B, Wang M, Gao D, Xing S, Li L, Liu L, Zhao M, Qi X, Dai K, Combining nanoscale magnetic nimodipine liposomes with magnetic resonance image for Parkinson’s disease targeting therapy, Nanomedicine, 12 (2017) 237–253. [DOI] [PubMed] [Google Scholar]
- [153].Pedram M, Shamloo A, Alasty A, Ghafar-Zadeh E, Optimal Magnetic Field for Crossing Super-Para-Magnetic Nanoparticles through the Brain Blood Barrier: A Computational Approach, Biosensors, 6 (2016) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Kirschbaum K, Sonner JK, Zeller MW, Deumelandt K, Bode J, Sharma R, Krüwel T, Fischer M, Hoffmann A, Costa da Silva M, Muckenthaler MU, Wick W, Tews B, Chen JW, Heiland S, Bendszus M, Platten M, Breckwoldt MO, In vivo nanoparticle imaging of innate immune cells can serve as a marker of disease severity in a model of multiple sclerosis, Proceedings of the National Academy of Sciences, 113 (2016) 13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Yu X, Wang J, Liu J, Shen S, Cao Z, Pan J, Zhou S, Pang Z, Geng D, Zhang J, A multimodal Pepstatin A peptide-based nanoagent for the molecular imaging of P-glycoprotein in the brains of epilepsy rats, Biomaterials, 76 (2016) 173–186. [DOI] [PubMed] [Google Scholar]
- [156].Lécuyer T, Teston E, Ramirez-Garcia G, Maldiney T, Viana B, Seguin J, Mignet N, Scherman D, Richard C, Chemically engineered persistent luminescence nanoprobes for bioimaging, Theranostics, 6 (2016) 2488–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Vilela P, El-Sagheer A, Millar TM, Brown T, Muskens OL, Kanaras AG, Graphene Oxide-Upconversion Nanoparticle Based Optical Sensors for Targeted Detection of mRNA Biomarkers Present in Alzheimer’s Disease and Prostate Cancer, ACS Sensors, 2 (2017) 52–56. [DOI] [PubMed] [Google Scholar]
- [158].Cui Z, Bu W, Fan W, Zhang J, Ni D, Liu Y, Wang J, Liu J, Yao Z, Shi J, Sensitive imaging and effective capture of Cu2+: Towards highly efficient theranostics of Alzheimer’s disease, Biomaterials, 104 (2016) 158–167. [DOI] [PubMed] [Google Scholar]
- [159].Huang P, Wu F, Mao L, Target-Triggered Switching on and off the Luminescence of Lanthanide Coordination Polymer Nanoparticles for Selective and Sensitive Sensing of Copper Ions in Rat Brain, Analytical Chemistry, 87 (2015) 6834–6841. [DOI] [PubMed] [Google Scholar]
- [160].Zheng M, Ruan S, Liu S, Sun T, Qu D, Zhao H, Xie Z, Gao H, Jing X, Sun Z, Self-Targeting Fluorescent Carbon Dots for Diagnosis of Brain Cancer Cells, ACS Nano, 9 (2015) 11455–11461. [DOI] [PubMed] [Google Scholar]
- [161].Kwon HJ, Cha M-Y, Kim D, Kim DK, Soh M, Shin K, Hyeon T, Mook-Jung I, Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease, ACS Nano, 10 (2016) 2860–2870. [DOI] [PubMed] [Google Scholar]
- [162].Liu Y, Xu L-P, Wang Q, Yang B, Zhang X, Synergistic Inhibitory Effect of GQDs–Tramiprosate Covalent Binding on Amyloid Aggregation, ACS Chemical Neuroscience, 9 (2018) 817–823. [DOI] [PubMed] [Google Scholar]
- [163].Naz S, Beach J, Heckert B, Tummala T, Pashchenko O, Banerjee T, Santra S, Cerium oxide nanoparticles: a ‘radical’ approach to neurodegenerative disease treatment, Nanomedicine (Lond), 12 (2017) 545–553. [DOI] [PubMed] [Google Scholar]
- [164].Manne N, Arvapalli R, Nepal N, Rice K, Blough E, Cerium Oxide Nanoparticles Confer Protection against Severe Sepsis Induced Hepatic Inflammation and Injury in Sprague Dawley Rats, The FASEB Journal, 29 (2015) 620.613. [Google Scholar]
- [165].Estevez AY, Pritchard S, Harper K, Aston JW, Lynch A, Lucky JJ, Ludington JS, Chatani P, Mosenthal WP, Leiter JC, Andreescu S, Erlichman JS, Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia, Free Radical Biology and Medicine, 51 (2011) 1155–1163. [DOI] [PubMed] [Google Scholar]
- [166].Schubert D, Dargusch R, Raitano J, Chan S-W, Cerium and yttrium oxide nanoparticles are neuroprotective, Biochemical and Biophysical Research Communications, 342 (2006) 86–91. [DOI] [PubMed] [Google Scholar]
- [167].Das S, Dowding JM, Klump KE, McGinnis JF, Self W, Seal S, Cerium oxide nanoparticles: applications and prospects in nanomedicine, Nanomedicine (Lond), 8 (2013) 1483–1508. [DOI] [PubMed] [Google Scholar]
- [168].Zhao Y, Xu Q, Xu W, Wang D, Tan J, Zhu C, Tan X, Probing the molecular mechanism of cerium oxide nanoparticles in protecting against the neuronal cytotoxicity of A[small beta]1–42 with copper ions, Metallomics, 8 (2016) 644–647. [DOI] [PubMed] [Google Scholar]
- [169].Hirst SM, Karakoti A, Singh S, Self W, Tyler R, Seal S, Reilly CM, Bio‐ distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice, Environmental Toxicology, 28 (2013) 107–118. [DOI] [PubMed] [Google Scholar]
- [170].Li M, Yang X, Ren J, Qu K, Qu X, Using Graphene Oxide High Near‐Infrared Absorbance for Photothermal Treatment of Alzheimer’s Disease, Advanced Materials, 24 (2012) 1722–1728. [DOI] [PubMed] [Google Scholar]
- [171].Li X, Du X, Molybdenum disulfide nanosheets supported Au-Pd bimetallic nanoparticles for non-enzymatic electrochemical sensing of hydrogen peroxide and glucose, Sensors and Actuators B: Chemical, 239 (2017) 536–543. [Google Scholar]
- [172].Khan I, Saeed K, Khan I, Nanoparticles: Properties, applications and toxicities, Arabian Journal of Chemistry, (2017). [Google Scholar]
- [173].Gupta BK, Singh S, Kumar P, Lee Y, Kedawat G, Narayanan TN, Vithayathil SA, Ge L, Zhan X, Gupta S, Martí AA, Vajtai R, Ajayan PM, Kaipparettu BA, Bifunctional Luminomagnetic Rare-Earth Nanorods for High-Contrast Bioimaging Nanoprobes, Scientific Reports, 6 (2016) 32401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kevadiya BD, Bade AN, Woldstad C, Edagwa BJ, McMillan JM, Sajja BR, Boska MD, Gendelman HE, Development of europium doped core-shell silica cobalt ferrite functionalized nanoparticles for magnetic resonance imaging, Acta Biomater, 49 (2017) 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Chen F, Ehlerding EB, Cai W, Theranostic Nanoparticles, Journal of nuclear medicine 55 (2014) 1919–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Sintov AC, Velasco-Aguirre C, Gallardo-Toledo E, Araya E, Kogan MJ, Chapter Six - Metal Nanoparticles as Targeted Carriers Circumventing the Blood–Brain Barrier, in: Al-Jamal KT (Ed.) International Review of Neurobiology, Academic Press; 2016, pp. 199–227. [DOI] [PubMed] [Google Scholar]
- [177].Ahmad J, Akhter S, Rizwanullah M, Khan MA, Pigeon L, Addo RT, Greig NH, Midoux P, Pichon C, Kamal MA, Nanotechnology Based Theranostic Approaches in Alzheimer’s Disease Management: Current Status and Future Perspective, Current Alzheimer research, 14 (2017) 1164–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Shadab AP, Zeenat I, Syed MAZ, Sushma T, Divya V, Gaurav KJ, Adnan A, Nitin J, Jigar RL, Roop KK, Farhan JA, CNS Drug Delivery Systems: Novel Approaches, Recent Patents on Drug Delivery & Formulation, 3 (2009) 71–89. [DOI] [PubMed] [Google Scholar]
- [179].Sweeney MD, Sagare AP, Zlokovic BV, Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders, Nature Reviews Neurology, 14 (2018) 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Lee S-J, Lim H-S, Masliah E, Lee H-J, Protein aggregate spreading in neurodegenerative diseases: Problems and perspectives, Neuroscience Research, 70 (2011) 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Walker LC, LeVine H, Corruption and Spread of Pathogenic Proteins in Neurodegenerative Diseases, The Journal of biological chemistry, 287 (2012) 33109–33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Chánez-Cárdenas ME, Vázquez-Contreras E, The Aggregation of Huntingtin and α-Synuclein, Journal of Biophysics, 2012 (2012) 606172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Barry J, Gu C, Coupling Mechanical Forces to Electrical Signaling: Molecular Motors and the Intracellular Transport of Ion Channels, The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry, 19 (2013) 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Wood LB, Winslow AR, Strasser SD, Systems biology of neurodegenerative diseases, Integrative Biology, 7 (2015) 758–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Gendelman HE, Anantharam V, Bronich T, Ghaisas S, Jin H, Kanthasamy AG, Liu X, McMillan J, Mosley RL, Narasimhan B, Mallapragada SK, Nanoneuromedicines for Degenerative, Inflammatory, and Infectious Nervous System Diseases, Nanomedicine : nanotechnology, biology, and medicine, 11 (2015) 751–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Yacoubian TA, Chapter 1 - Neurodegenerative Disorders: Why Do We Need New Therapies? A2 - Adejare, Adeboye, Drug Discovery Approaches for the Treatment of Neurodegenerative Disorders, Academic Press; 2017, pp. 1–16. [Google Scholar]
- [187].Legname G, Novel Approaches to Diagnosis and Therapy in Neurodegenerative Diseases, Springer International Publishing, Cham, 2015, pp. 155–158. [Google Scholar]
- [188].Agrawal M, Biswas A, Molecular diagnostics of neurodegenerative disorders, Frontiers in Molecular Biosciences, 2 (2015) 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Chen X, Pan W, The Treatment Strategies for Neurodegenerative Diseases by Integrative Medicine, Integrative Medicine International, 1 (2014) 223–225. [Google Scholar]
- [190].Kiaei M, New Hopes and Challenges for Treatment of Neurodegenerative Disorders: Great Opportunities for Young Neuroscientists, Basic and Clinical Neuroscience, 4 (2013) 3–4. [PMC free article] [PubMed] [Google Scholar]
- [191].Amor S, Puentes F, Baker D, van der Valk P, Inflammation in neurodegenerative diseases, Immunology, 129 (2010) 154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease, New England Journal of Medicine, 367 (2012) 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].DeMaagd G, Philip A, Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis, Pharmacy and Therapeutics, 40 (2015) 504–532. [PMC free article] [PubMed] [Google Scholar]
- [194].Dilokthornsakul P, Valuck RJ, Nair KV, Corboy JR, Allen RR, Campbell JD, Multiple sclerosis prevalence in the United States commercially insured population, Neurology, 86 (2016) 1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Petrov D, Mansfield C, Moussy A, Hermine O, ALS Clinical Trials Review: 20 Years of Failure. Are We Any Closer to Registering a New Treatment?, Frontiers in Aging Neuroscience, 9 (2017) 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Roos RAC, Huntington’s disease: a clinical review, Orphanet Journal of Rare Diseases, 5 (2010) 40–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Alzheimer’s Association, in: Williams T (Ed.) News Release, March 20, 2018.
- [198].McHale D, Reclassifying neurodegenerative diseases to enable drug development and help patients, Research & Innovation News -Aetionomy, July 1, 2016. [Google Scholar]
- [199].Ramos-Cabrer P, Campos F, Liposomes and nanotechnology in drug development: focus on neurological targets, International Journal of Nanomedicine, 8 (2013) 951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Johnstone M, Gearing AJH, Miller KM, A central role for astrocytes in the inflammatory response to β-amyloid; chemokines, cytokines and reactive oxygen species are produced, Journal of Neuroimmunology, 93 (1999) 182–193. [DOI] [PubMed] [Google Scholar]
- [201].Wood LB, Winslow AR, Strasser SD, Systems biology of neurodegenerative diseases, Integrative biology : quantitative biosciences from nano to macro, 7 (2015) 758–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Zlokovic BV, The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders, Neuron, 57 (2008) 178–201. [DOI] [PubMed] [Google Scholar]
- [203].Banks WA, Developing drugs that can cross the blood-brain barrier: applications to Alzheimer’s disease, BMC Neuroscience, 9 (2008) S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Ballabh P, Braun A, Nedergaard M, The blood–brain barrier: an overview: Structure, regulation, and clinical implications, Neurobiology of Disease, 16 (2004) 1–13. [DOI] [PubMed] [Google Scholar]
- [205].Zhou Y, Peng Z, Seven ES, Leblanc RM, Crossing the blood-brain barrier with nanoparticles, Journal of Controlled Release, 270 (2018) 290–303. [DOI] [PubMed] [Google Scholar]
- [206].Banks WA, Drug delivery to the brain in Alzheimer’s disease: Consideration of the blood–brain barrier, Advanced drug delivery reviews, 64 (2012) 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Rodríguez EM, Blázquez JL, Guerra M, The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: The former opens to the portal blood and the latter to the cerebrospinal fluid, Peptides, 31 (2010) 757–776. [DOI] [PubMed] [Google Scholar]
- [208].Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, Pardridge W, Rosenberg GA, Smith Q, Drewes LR, Strategies to advance translational research into brain barriers, The Lancet Neurology, 7 (2008) 84–96. [DOI] [PubMed] [Google Scholar]
- [209].Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA, What the Cell “Sees” in Bionanoscience, Journal of the American Chemical Society, 132 (2010) 5761–5768. [DOI] [PubMed] [Google Scholar]
- [210].Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE, Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy, Advanced drug delivery reviews, 61 (2009) 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Shah NB, Vercellotti GM, White JG, Fegan A, Wagner CR, Bischof JC, Blood–Nanoparticle Interactions and in Vivo Biodistribution: Impact of Surface PEG and Ligand Properties, Molecular Pharmaceutics, 9 (2012) 2146–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nat Biotechnol, 33 (2015) 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Clark AJ, Davis ME, Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core, Proceedings of the National Academy of Sciences, 112 (2015) 12486–12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Banks WA, Characteristics of compounds that cross the blood-brain barrier, BMC Neurology, 9 (2009) S3–S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Sarin H, Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability, Journal of Angiogenesis Research, 2 (2010) 14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Duvernoy H, Delon S, Vannson JL, The vascularization of the human cerebellar cortex, Brain Research Bulletin, 11 (1983) 419–480. [DOI] [PubMed] [Google Scholar]
- [217].Sharma A, Cornejo C, Mihalic J, Geyh A, Bordelon DE, Korangath P, Westphal F, Gruettner C, Ivkov R, Physical characterization and in vivo organ distribution of coated iron oxide nanoparticles, Scientific Reports, 8 (2018) 4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Krol S, Challenges in drug delivery to the brain: Nature is against us, Journal of Controlled Release, 164 (2012) 145–155. [DOI] [PubMed] [Google Scholar]
- [219].Wohlfart S, Gelperina S, Kreuter J, Transport of drugs across the blood–brain barrier by nanoparticles, Journal of Controlled Release, 161 (2012) 264–273. [DOI] [PubMed] [Google Scholar]
- [220].Pardridge WM, The blood-brain barrier and neurotherapeutics, NeuroRX, 2 (2005) 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Grabrucker AM, Ruozi B, Belletti D, Pederzoli F, Forni F, Vandelli MA, Tosi G, Nanoparticle transport across the blood brain barrier, Tissue Barriers, 4 (2016) e1153568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Shah L, Yadav S, Amiji M, Nanotechnology for CNS delivery of bio-therapeutic agents, Drug Delivery and Translational Research, 3 (2013) 336–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Gomes MJ, Neves J.d., Sarmento B, Nanoparticle-based drug delivery to improve the efficacy of antiretroviral therapy in the central nervous system, International Journal of Nanomedicine, 9 (2014) 1757–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Dube T, Chibh S, Mishra J, Panda JJ, Receptor Targeted Polymeric Nanostructures Capable of Navigating across the Blood-Brain Barrier for Effective Delivery of Neural Therapeutics, ACS Chemical Neuroscience, 8 (2017) 2105–2117. [DOI] [PubMed] [Google Scholar]
- [225].Kabanov AV, Gendelman HE, Nanomedicine in the diagnosis and therapy of neurodegenerative disorders, Progress in polymer science, 32 (2007) 1054–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Wang Z, Caveolae-mediated Delivery of Therapeutic Nanoparticles across Blood-endothelial Barrier, Austin journal of analytical and pharmaceutical chemistry, 1 (2014) 1018. [PMC free article] [PubMed] [Google Scholar]
- [227].Georgieva JV, Kalicharan D, Couraud P-O, Romero IA, Weksler B, Hoekstra D, Zuhorn IS, Surface Characteristics of Nanoparticles Determine Their Intracellular Fate in and Processing by Human Blood–Brain Barrier Endothelial Cells In Vitro, Molecular Therapy, 19 (2011) 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Conner SD, Schmid SL, Regulated portals of entry into the cell, Nature, 422 (2003) 37. [DOI] [PubMed] [Google Scholar]
- [229].Cheng Z-J, Deep Singh R, Marks DL, Pagano RE, Membrane microdomains, caveolae, and caveolar endocytosis of sphingolipids (Review), Molecular Membrane Biology, 23 (2006) 101–110. [DOI] [PubMed] [Google Scholar]
- [230].Wang Z, Tiruppathi C, Minshall RD, Malik AB, Size and Dynamics of Caveolae Studied Using Nanoparticles in Living Endothelial Cells, ACS Nano, 3 (2009) 4110–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Xiao G, Gan LS, Receptor-mediated endocytosis and brain delivery of therapeutic biologics, Int J Cell Biol, 2013 (2013) 703545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Kaksonen M, Roux A, Mechanisms of clathrin-mediated endocytosis, Nature Reviews Molecular Cell Biology, 19 (2018) 313. [DOI] [PubMed] [Google Scholar]
- [233].Traub LM, Regarding the Amazing Choreography of Clathrin Coats, PLOS Biology, 9 (2011) e1001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Löscher W, Potschka H, Blood-brain barrier active efflux transporters: ATP-binding cassette gene family, NeuroRX, 2 (2005) 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Dante S, Petrelli A, Petrini EM, Marotta R, Maccione A, Alabastri A, Quarta A, De Donato F, Ravasenga T, Sathya A, Cingolani R, Proietti Zaccaria R, Berdondini L, Barberis A, Pellegrino T, Selective Targeting of Neurons with Inorganic Nanoparticles: Revealing the Crucial Role of Nanoparticle Surface Charge, ACS Nano, 11 (2017) 6630–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Ulbrich K, Hekmatara T, Herbert E, Kreuter J, Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB), European Journal of Pharmaceutics and Biopharmaceutics, 71 (2009) 251–256. [DOI] [PubMed] [Google Scholar]
- [237].Jallouli Y, Paillard A, Chang J, Sevin E, Betbeder D, Influence of surface charge and inner composition of porous nanoparticles to cross blood–brain barrier in vitro, International Journal of Pharmaceutics, 344 (2007) 103–109. [DOI] [PubMed] [Google Scholar]
- [238].Gao X, Qian J, Zheng S, Changyi Y, Zhang J, Ju S, Zhu J, Li C, Overcoming the Blood–Brain Barrier for Delivering Drugs into the Brain by Using Adenosine Receptor Nanoagonist, ACS Nano, 8 (2014) 3678–3689. [DOI] [PubMed] [Google Scholar]
- [239].Schnyder A, Huwyler J, Drug Transport to Brain with Targeted Liposomes, NeuroRx, 2 (2005) 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Hersh DS, Wadajkar AS, Roberts N, Perez JG, Connolly NP, Frenkel V, Winkles JA, Woodworth GF, Kim AJ, Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier, Current pharmaceutical design, 22 (2016) 1177–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Jain KK, Nanobiotechnology-based strategies for crossing the blood-brain barrier, Nanomedicine (Lond), 7 (2012) 1225–1233. [DOI] [PubMed] [Google Scholar]
- [242].Lohmann E, Guerreiro RJ, Erginel-Unaltuna N, Gurunlian N, Bilgic B, Gurvit H, Hanagasi HA, Luu N, Emre M, Singleton A, Identification of PSEN1 and PSEN2 gene mutations and variants in Turkish dementia patients, Neurobiology of aging, 33 (2012) 1850.e1817–1850.e1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Stelzmann RA, Schnitzlein HN, Murtagh FR, An english translation of alzheimer’s 1907 paper, “über eine eigenartige erkankung der hirnrinde”, Clinical Anatomy, 8 (1995) 429–431. [DOI] [PubMed] [Google Scholar]
- [244].Magalingam KB, Radhakrishnan A, Ping NS, Haleagrahara N, Current Concepts of Neurodegenerative Mechanisms in Alzheimers Disease, BioMed research international, 2018 (2018) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Hebert LE, Weuve J, Scherr PA, Evans DA, Alzheimer disease in the United States (2010–2050) estimated using the 2010 census, Neurology, 80 (2013) 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].New Analysis Shows More Than 28 Million Baby Boomers Will Develop Alzheimer’s Disease; Will Consume Nearly 25% Of Medicare Spending, Alzheimer’s Association newsroom @ AAIC, 202-249-4002, 2015.
- [247].von Strauss E, Viitanen M, De Ronchi D, Winblad B, Fratiglioni L, Aging and the occurrence of dementia: Findings from a population-based cohort with a large sample of nonagenarians, Archives of Neurology, 56 (1999) 587–592. [DOI] [PubMed] [Google Scholar]
- [248].Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM, Forecasting the global burden of Alzheimer’s disease, Alzheimer’s & Dementia, 3 (2007) 186–191. [DOI] [PubMed] [Google Scholar]
- [249].2018 Alzheimer’s disease facts and figures, Alzheimer’s & Dementia, 14 (2018) 367–429. [Google Scholar]
- [250].Qiu C, Kivipelto M, von Strauss E, Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention, Dialogues in Clinical Neuroscience, 11 (2009) 111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Minati L, Edginton T, Bruzzone MG, Giaccone G, Current concepts in Alzheimer’s disease: a multidisciplinary review, Am J Alzheimers Dis Other Demen, 24 (2009) 95–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Groves-Wright K, Neils-Strunjas J, Burnett R, O’Neill MJ, A comparison of verbal and written language in Alzheimer’s disease, Journal of Communication Disorders, 37 (2004) 109–130. [DOI] [PubMed] [Google Scholar]
- [253].Mega MS, Cummings JL, Fiorello T, Gornbein J, The spectrum of behavioral changes in Alzheimer’s disease, Neurology, 46 (1996) 130–135. [DOI] [PubMed] [Google Scholar]
- [254].Hane FT, Robinson M, Lee BY, Bai O, Leonenko Z, Albert MS, Recent Progress in Alzheimer’s Disease Research, Part 3: Diagnosis and Treatment, Journal of Alzheimer’s Disease, 57 (2017) 645–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P, Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS–ADRDA criteria, The Lancet Neurology, 6 (2007) 734–746. [DOI] [PubMed] [Google Scholar]
- [256].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease, Alzheimer’s & Dementia, 7 (2011) 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Kelley BJ, Petersen RC, Alzheimer’s Disease and Mild Cognitive Impairment, Neurologic clinics, 25 (2007) 577–v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ, National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease, Alzheimer’s & dementia : the journal of the Alzheimer’s Association, 8 (2012) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Nisbet RM, Polanco J-C, Ittner LM, Götz J, Tau aggregation and its interplay with amyloid-β, Acta Neuropathologica, 129 (2015) 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Spires-Jones TL, Hyman BT, The intersection of amyloid beta and tau at synapses in Alzheimer’s disease, Neuron, 82 (2014) 756–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Iqbal K, del C. Alonso A, Chen S, Chohan MO, El-Akkad E, Gong C-X, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I, Tau pathology in Alzheimer disease and other tauopathies, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1739 (2005) 198–210. [DOI] [PubMed] [Google Scholar]
- [262].Zhang F, Jiang L, Neuroinflammation in Alzheimer’s disease, Neuropsychiatric Disease and Treatment, 11 (2015) 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Bali J, Halima SB, Felmy B, Goodger Z, Zurbriggen S, Rajendran L, Cellular basis of Alzheimer’s disease, Annals of Indian Academy of Neurology, 13 (2010) S89–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Brion JP, Neurofibrillary tangles and Alzheimer’s disease, Eur Neurol, 40 (1998) 130–140. [DOI] [PubMed] [Google Scholar]
- [265].Murray PS, Kirkwood CM, Gray MC, Fish KN, Ikonomovic MD, Hamilton RL, Kofler JK, Klunk WE, Lopez OL, Sweet RA, Hyperphosphorylated Tau is Elevated in Alzheimer’s Disease with Psychosis, Journal of Alzheimer’s disease : JAD, 39 (2014) 759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT, Neuropathological Alterations in Alzheimer Disease, Cold Spring Harbor Perspectives in Medicine:, 1 (2011) a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Nalivaeva NN, Turner AJ, The amyloid precursor protein: A biochemical enigma in brain development, function and disease, FEBS Letters, 587 (2013) 2046–2054. [DOI] [PubMed] [Google Scholar]
- [268].Dawkins E, Small DH, Insights into the physiological function of the β‐amyloid precursor protein: beyond Alzheimer’s disease, Journal of Neurochemistry, 129 (2014) 756–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ, A Critical Function for β-Amyloid Precursor Protein in Neuronal Migration Revealed by In Utero RNA Interference, The Journal of Neuroscience, 27 (2007) 14459–14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Chow VW, Mattson MP, Wong PC, Gleichmann M, An Overview of APP Processing Enzymes and Products, Neuromolecular medicine, 12 (2010) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Mockett BG, Richter M, Abraham WC, Müller UC, Therapeutic Potential of Secreted Amyloid Precursor Protein APPsα, Frontiers in Molecular Neuroscience, 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Golde TE, Eckman CB, Younkin SG, Biochemical detection of Aβ isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1502 (2000) 172–187. [DOI] [PubMed] [Google Scholar]
- [273].Lucey BP, Bateman RJ, Amyloid-β diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis, Neurobiology of Aging, 35 (2014) S29–S34. [DOI] [PubMed] [Google Scholar]
- [274].Bekris LM, Yu C-E, Bird TD, Tsuang DW, Genetics of Alzheimer Disease, Journal of geriatric psychiatry and neurology, 23 (2010) 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Murphy MP, LeVine H, Alzheimer’s Disease and the β-Amyloid Peptide, Journal of Alzheimer’s disease : JAD, 19 (2010) 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Canevari L, Clark JB, Bates TE, β‐Amyloid fragment 25–35 selectively decreases complex IV activity in isolated mitochondria, FEBS Letters, 457 (1999) 131–134. [DOI] [PubMed] [Google Scholar]
- [277].Lin H, Bhatia R, Lal R, Amyloid beta protein forms ion channels: implications for Alzheimer’s disease pathophysiology, FASEB Journal 15 (2001) 2433–2444. [DOI] [PubMed] [Google Scholar]
- [278].Rosales-Corral S, Tan D-X, Reiter RJ, Valdivia-Velázquez M, Acosta M, nez JP, Ortiz GG, Kinetics of the neuroinflammation-oxidative stress correlation in rat brain following the injection of fibrillar amyloid-β onto the hippocampus in vivo, Journal of Neuroimmunology, 150 (2004) 20–28. [DOI] [PubMed] [Google Scholar]
- [279].Butterfield DA, Reed T, Newman SF, Sultana R, Roles of amyloid β-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment, Free Radical Biology and Medicine, 43 (2007) 658–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Parameshwaran K, Dhanasekaran M, Suppiramaniam V, Amyloid beta peptides and glutamatergic synaptic dysregulation, Experimental Neurology, 210 (2008) 7–13. [DOI] [PubMed] [Google Scholar]
- [281].Li T, Braunstein KE, Zhang J, Lau A, Sibener L, Deeble C, Wong PC, The neuritic plaque facilitates pathological conversion of tau in an Alzheimer’s disease mouse model, Nature Communications, 7 (2016) 12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Rajmohan R, Reddy PH, Amyloid Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons, Journal of Alzheimer’s disease : JAD, 57 (2017) 975–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Simic G, Babic Leko M, Wray S, Harrington C, Delalle I, Jovanov-Milosevic N, Bazadona D, Buee L, de Silva R, Di Giovanni G, Wischik C, Hof PR, Tau Protein Hyperphosphorylation and Aggregation in Alzheimer’s Disease and Other Tauopathies, and Possible Neuroprotective Strategies, Biomolecules, 6 (2016) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Mietelska-Porowska A, Wasik U, Goras M, Filipek A, Niewiadomska G, Tau Protein Modifications and Interactions: Their Role in Function and Dysfunction, International journal of molecular sciences, 15 (2014) 4671–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Jouanne M, Rault S, Voisin-Chiret A-S, Tau protein aggregation in Alzheimer’s disease: An attractive target for the development of novel therapeutic agents, European Journal of Medicinal Chemistry, 139 (2017) 153–167. [DOI] [PubMed] [Google Scholar]
- [286].Wang Y, Mandelkow E, Tau in physiology and pathology, Nature Reviews Neuroscience, 17 (2015) 22. [DOI] [PubMed] [Google Scholar]
- [287].Liu C-C, Kanekiyo T, Xu H, Bu G, Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy, Nature reviews. Neurology, 9 (2013) 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Huang Y, Weisgraber KH, Mucke L, Mahley RW, Apolipoprotein E, Journal of Molecular Neuroscience, 23 (2004) 189–204. [DOI] [PubMed] [Google Scholar]
- [289].Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K, Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease, Brain Research, 541 (1991) 163–166. [DOI] [PubMed] [Google Scholar]
- [290].Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. , Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease, Neurology, 43 (1993) 1467–1472. [DOI] [PubMed] [Google Scholar]
- [291].Nagy ZS, Esiri MM, Jobst KA, Johnston C, Litchfield S, Sim E, Smith AD, Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer’s disease, Neuroscience, 69 (1995) 757–761. [DOI] [PubMed] [Google Scholar]
- [292].Conejero-Goldberg C, Gomar JJ, Bobes-Bascaran T, Hyde TM, Kleinman JE, Herman MM, Chen S, Davies P, Goldberg TE, APOE2 enhances neuroprotection against Alzheimer’s disease through multiple molecular mechanisms, Molecular Psychiatry, 19 (2014) 1243. [DOI] [PubMed] [Google Scholar]
- [293].Berlau DJ, Corrada MM, Head E, Kawas CH, APOE ε2 is associated with intact cognition but increased Alzheimer pathology in the oldest old, Neurology, 72 (2009) 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Farrer LA, Cupples L, Haines JL, et al. , Effects of age, sex, and ethnicity on the association between apolipoprotein e genotype and alzheimer disease: A meta-analysis, JAMA, 278 (1997) 1349–1356. [PubMed] [Google Scholar]
- [295].DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JW, Harmony JAK, Aronow BJ, Bales KR, Paul SM, Holtzman DM, ApoE and Clusterin Cooperatively Suppress Aβ Levels and Deposition: Evidence that ApoE Regulates Extracellular Aβ Metabolism In Vivo, Neuron, 41 (2004) 193–202. [DOI] [PubMed] [Google Scholar]
- [296].Hartmann T, Intracellular biology of Alzheimer’s disease amyloid beta peptide, Eur Arch Psychiatry Clin Neurosci, 249 (1999) 291–298. [DOI] [PubMed] [Google Scholar]
- [297].Cummings JL, Morstorf T, Zhong K, Alzheimer’s disease drug-development pipeline: few candidates, frequent failures, Alzheimer’s Research & Therapy, 6 (2014)37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Dong H, Yuede CM, Coughlan C, Lewis B, Csernansky JG, Effects of Memantine on Neuronal Structure and Conditioned Fear in the Tg2576 Mouse Model of Alzheimer’s Disease, Neuropsychopharmacology, 33 (2008) 3226–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Anderson RM, Hadjichrysanthou C, Evans S, Wong MM, Why do so many clinical trials of therapies for Alzheimer’s disease fail?, The Lancet, 390 (2017) 2327–2329. [DOI] [PubMed] [Google Scholar]
- [300].Robert EB, Nigel HG, Alzheimers Disease Drug Development in 2008 and Beyond: Problems and Opportunities, Current Alzheimer Research, 5 (2008) 346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Becker RE, Greig NH, Giacobini E, Schneider LS, Ferrucci L, A new roadmap for drug development for Alzheimer's disease, Nature Reviews Drug Discovery, 13 (2013) 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Becker RE, Greig NH, Giacobini E, Why do so many drugs for Alzheimer’s disease fail in development? Time for new methods and new practices?, Journal of Alzheimer’s disease : JAD, 15 (2008) 303–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Pardridge WM, Blood-brain barrier drug targeting: the future of brain drug development, Mol Interv, 3 (2003) 90–105, 151. [DOI] [PubMed] [Google Scholar]
- [304].Mulik RS, Mönkkönen J, Juvonen RO, Mahadik KR, Paradkar AR, ApoE3 Mediated Poly(butyl) Cyanoacrylate Nanoparticles Containing Curcumin: Study of Enhanced Activity of Curcumin against Beta Amyloid Induced Cytotoxicity Using In Vitro Cell Culture Model, Molecular Pharmaceutics, 7 (2010) 815–825. [DOI] [PubMed] [Google Scholar]
- [305].Doggui S, Sahni JK, Arseneault M, Dao L, Ramassamy C, Neuronal uptake and neuroprotective effect of curcumin-loaded PLGA nanoparticles on the human SK-NSH cell line, Journal of Alzheimer’s disease : JAD, 30 (2012) 377–392. [DOI] [PubMed] [Google Scholar]
- [306].Mathew A, Fukuda T, Nagaoka Y, Hasumura T, Morimoto H, Yoshida Y, Maekawa T, Venugopal K, Kumar DS, Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease, PLoS One, 7 (2012) e32616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Tokuraku K, Marquardt M, Ikezu T, Real-Time Imaging and Quantification of Amyloid-β Peptide Aggregates by Novel Quantum-Dot Nanoprobes, PLOS ONE, 4 (2010) e8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Zaim WY, S. EM, S. Marcin, E. JI, L. Yongsheng, S. Henrieta, T.C. Ying, A. Gilbert, P. Miguel, D. Karen, W. Thomas, T. DH, Detection of Alzheimer’s amyloid in transgenic mice using magnetic resonance microimaging, Magnetic Resonance in Medicine, 50 (2003) 293–302. [DOI] [PubMed] [Google Scholar]
- [309].Choi J.-s., Choi HJ, Jung DC, Lee J-H, Cheon J, Nanoparticle assisted magnetic resonance imaging of the early reversible stages of amyloid [small beta] self-assembly, Chemical Communications, (2008) 2197–2199. [DOI] [PubMed] [Google Scholar]
- [310].Zhang D, Fa HB, Zhou JT, Li S, Diao XW, Yin W, The detection of β-amyloid plaques in an Alzheimer’s disease rat model with DDNP-SPIO, Clinical Radiology, 70 (2015) 74–80. [DOI] [PubMed] [Google Scholar]
- [311].Jaruszewski KM, Curran GL, Swaminathan SK, Rosenberg JT, Grant SC, Ramakrishnan S, Lowe VJ, Poduslo JF, Kandimalla KK, Multimodal Nanoprobes to target cerebrovascular amyloid in Alzheimer’s disease brain, Biomaterials, 35 (2014) 1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Zhang C, Wan X, Zheng X, Shao X, Liu Q, Zhang Q, Qian Y, Dual-functional nanoparticles targeting amyloid plaques in the brains of Alzheimer’s disease mice, Biomaterials, 35 (2014) 456–465. [DOI] [PubMed] [Google Scholar]
- [313].Yiannopoulou KG, Papageorgiou SG, Current and future treatments for Alzheimer’s disease, Therapeutic Advances in Neurological Disorders, 6 (2013) 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Santiago JA, Bottero V, Potashkin JA, Dissecting the Molecular Mechanisms of Neurodegenerative Diseases through Network Biology, Frontiers in Aging Neuroscience, 9 (2017) 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Khan A, Usman M, Early diagnosis of Alzheimer’s disease using machine learning techniques: A review paper, 2015 7th International Joint Conference on Knowledge Discovery, Knowledge Engineering and Knowledge Management (IC3K), 2015, pp. 380–387. [Google Scholar]
- [316].Shankar GM, Walsh DM, Alzheimer’s disease: synaptic dysfunction and Aβ, Molecular Neurodegeneration, 4 (2009) 48–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Noble JM, Scarmeas N, Application of PET imaging to diagnosis of Alzheimer’s disease and mild cognitive impairment, International review of neurobiology, 84 (2009) 133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM, Nazar MD, Rich SA, Berlau DJ, Peltz CB, Tan MT, Kawas CH, Federoff HJ, Plasma phospholipids identify antecedent memory impairment in older adults, Nature Medicine, 20 (2014) 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Segovia F, Bastin C, Salmon E, Górriz JM, Ramírez J, Phillips C, Combining PET Images and Neuropsychological Test Data for Automatic Diagnosis of Alzheimer’s Disease, PLOS ONE, 9 (2014) e88687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Hampel H, Blennow K, CSF tau and β-amyloid as biomarkers for mild cognitive impairment, Dialogues in Clinical Neuroscience, 6 (2004) 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Perrin RJ, Fagan AM, Holtzman DM, Multi-modal techniques for diagnosis and prognosis of Alzheimer’s disease, Nature, 461 (2009) 916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Álvarez YD, Pellegrotti JV, Stefani FD, Gold Nanoparticles as Nucleation Centers for Amyloid Fibrillation, in: Santamaria F, Peralta XG (Eds.) Use of Nanoparticles in Neuroscience, Springer New York, New York, NY, 2018, pp. 269–291. [Google Scholar]
- [323].Zhu L, Ploessl K, Kung HF, PET/SPECT imaging agents for neurodegenerative diseases, Chemical Society reviews, 43 (2014) 6683–6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Choe YS, Lee K-H, PET Radioligands for Imaging of Tau Pathology: Current Status, Nuclear Medicine and Molecular Imaging, 49 (2015) 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Stoessl AJ, Neuroimaging in the early diagnosis of neurodegenerative disease, Translational Neurodegeneration, 1 (2012) 5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Skaat H, Margel S, Synthesis of fluorescent-maghemite nanoparticles as multimodal imaging agents for amyloid-β fibrils detection and removal by a magnetic field, Biochemical and Biophysical Research Communications, 386 (2009) 645–649. [DOI] [PubMed] [Google Scholar]
- [327].Bingbing H, Fengying D, Zhanming F, Guanghui M, Qunwei T, Xin Z, Nanotheranostics: Congo Red/Rutin‐MNPs with Enhanced Magnetic Resonance Imaging and H2O2‐Responsive Therapy of Alzheimer’s Disease in APPswe/PS1dE9 Transgenic Mice, Advanced Materials, 27 (2015) 5499–5505. [DOI] [PubMed] [Google Scholar]
- [328].Cheng KK, Chan PS, Fan S, Kwan SM, Yeung KL, Wáng Y-XJ, Chow AHL, Wu EX, Baum L, Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI), Biomaterials, 44 (2015) 155–172. [DOI] [PubMed] [Google Scholar]
- [329].Nasr SH, Kouyoumdjian H, Mallett C, Ramadan S, Zhu DC, Shapiro EM, Huang X, Detection of β‐Amyloid by Sialic Acid Coated Bovine Serum Albumin Magnetic Nanoparticles in a Mouse Model of Alzheimer’s Disease, Small, 14 (2018) 1701828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Viola KL, Sbarboro J, Sureka R, De M, Bicca MA, Wang J, Vasavada S, Satpathy S, Wu S, Joshi H, Velasco PT, MacRenaris K, Waters EA, Lu C, Phan J, Lacor P, Prasad P, Dravid VP, Klein WL, Towards non-invasive diagnostic imaging of early-stage Alzheimer’s disease, Nature Nanotechnology, 10 (2014) 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Yang J, Zaim Wadghiri Y, Minh Hoang D, Tsui W, Sun Y, Chung E, Li Y, Wang A, de Leon M, Wisniewski T, Detection of amyloid plaques targeted by USPIOAβ1–42 in Alzheimer’s disease transgenic mice using magnetic resonance microimaging, NeuroImage, 55 (2011) 1600–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Koffie RM, Farrar CT, Saidi L-J, William CM, Hyman BT, Spires-Jones TL, Nanoparticles enhance brain delivery of blood–brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging, Proceedings of the National Academy of Sciences, 108 (2011) 18837–18842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Tanifum EA, Ghaghada K, Vollert C, Head E, Eriksen JL, Annapragada A, A Novel Liposomal Nanoparticle for the Imaging of Amyloid Plaque by Magnetic Resonance Imaging, Journal of Alzheimer’s disease : JAD, 52 (2016) 731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Mosconi L, Berti V, Glodzik L, Pupi A, De Santi S, de Leon MJ, Pre-Clinical Detection of Alzheimer’s Disease Using FDG-PET, with or without Amyloid Imaging, Journal of Alzheimer’s disease : JAD, 20 (2010) 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Sehlin D, Fang XT, Cato L, Antoni G, Lannfelt L, Syvänen S, Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer’s disease, Nature Communications, 7 (2016) 10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Chen Q, Du Y, Zhang K, Liang Z, Li J, Yu H, Ren R, Feng J, Jin Z, Li F, Sun J, Zhou M, He Q, Sun X, Zhang H, Tian M, Ling D, Tau-Targeted Multifunctional Nanocomposite for Combinational Therapy of Alzheimer’s Disease, ACS Nano, 12 (2018) 1321–1338. [DOI] [PubMed] [Google Scholar]
- [337].Zhang X, Hou Y, Peng C, Wang C, Wang X, Liang Z, Lu J, Chen B, Dai J, Liu B, Cui M, Oligoethyleneoxy-Modified 99mTc-Labeled β-Amyloid Imaging Probes with Improved Brain Pharmacokinetics for Single-Photon Emission Computed Tomography, Journal of Medicinal Chemistry, 61 (2018) 1330–1339. [DOI] [PubMed] [Google Scholar]
- [338].Coimbra A, Williams DS, Hostetler ED, The role of MRI and PET/SPECT in Alzheimer’s disease, Curr Top Med Chem, 6 (2006) 629–647. [DOI] [PubMed] [Google Scholar]
- [339].Humpel C, Identifying and validating biomarkers for Alzheimer’s disease, Trends in Biotechnology, 29 (2011) 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Yang C-C, Yang S-Y, Chieh J-J, Horng H-E, Hong C-Y, Yang H-C, Chen KH, Shih BY, Chen T-F, Chiu M-J, Biofunctionalized Magnetic Nanoparticles for Specifically Detecting Biomarkers of Alzheimer’s Disease in Vitro, ACS Chemical Neuroscience, 2 (2011) 500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Neely A, Perry C, Varisli B, Singh AK, Arbneshi T, Senapati D, Kalluri JR, Ray PC, Ultrasensitive and Highly Selective Detection of Alzheimer’s Disease Biomarker Using Two-Photon Rayleigh Scattering Properties of Gold Nanoparticle, ACS Nano, 3 (2009) 2834–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Wang C, Liu D, Wang Z, Gold nanoparticle based dot-blot immunoassay for sensitively detecting Alzheimer’s disease related [small beta]-amyloid peptide, Chemical Communications, 48 (2012) 8392–8394. [DOI] [PubMed] [Google Scholar]
- [343].Stegurová L, Dráberová E, Bartos A, Dráber P, Řípová D, Dráber P, Gold nanoparticle-based immuno-PCR for detection of tau protein in cerebrospinal fluid, Journal of Immunological Methods, 406 (2014) 137–142. [DOI] [PubMed] [Google Scholar]
- [344].Tanifum EA, Dasgupta I, Srivastava M, Bhavane RC, Sun L, Berridge J, Pourgarzham H, Kamath R, Espinosa G, Cook SC, Eriksen JL, Annapragada A, Intravenous delivery of targeted liposomes to amyloid-beta pathology in APP/PSEN1 transgenic mice, PLoS One, 7 (2012) e48515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Shui B, Tao D, Florea A, Cheng J, Zhao Q, Gu Y, Li W, Jaffrezic-Renault N, Mei Y, Guo Z, Biosensors for Alzheimer’s disease biomarker detection: A review, Biochimie, 147 (2018) 13–24. [DOI] [PubMed] [Google Scholar]
- [346].Kim H, Lee JU, Song S, Kim S, Sim SJ, A shape-code nanoplasmonic biosensor for multiplex detection of Alzheimer’s disease biomarkers, Biosensors and Bioelectronics, 101 (2018) 96–102. [DOI] [PubMed] [Google Scholar]
- [347].Vestergaard M.d., Kerman K, Kim D-K, Hiep HM, Tamiya E, Detection of Alzheimer’s tau protein using localised surface plasmon resonance-based immunochip, Talanta, 74 (2008) 1038–1042. [DOI] [PubMed] [Google Scholar]
- [348].Hegnerová K, Bocková M, Vaisocherová H, Krištofiková Z, Říčný J, Řípová D, Homola J, Surface plasmon resonance biosensors for detection of Alzheimer disease biomarker, Sensors and Actuators B: Chemical, 139 (2009) 69–73. [Google Scholar]
- [349].Lisi S, Scarano S, Fedeli S, Pascale E, Cicchi S, Ravelet C, Peyrin E, Minunni M, Toward sensitive immuno-based detection of tau protein by surface plasmon resonance coupled to carbon nanostructures as signal amplifiers, Biosensors and Bioelectronics, 93 (2017) 289–292. [DOI] [PubMed] [Google Scholar]
- [350].Wen J, Fang F, Guo SH, Zhang Y, Peng XL, Sun WM, Wei XR, He JS, Hung T, Amyloid beta-Derived Diffusible Ligands (ADDLs) Induce Abnormal Autophagy Associated with Abeta Aggregation Degree, J Mol Neurosci, 64 (2018) 162–174. [DOI] [PubMed] [Google Scholar]
- [351].Georganopoulou DG, Chang L, Nam J-M, Thaxton CS, Mufson EJ, Klein WL, Mirkin CA, Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease, Proceedings of the National Academy of Sciences of the United States of America, 102 (2005) 2273–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Qu F, Yang M, Rasooly A, Dual Signal Amplification Electrochemical Biosensor for Monitoring the Activity and Inhibition of the Alzheimer’s Related Protease β-Secretase, Analytical Chemistry, 88 (2016) 10559–10565. [DOI] [PubMed] [Google Scholar]
- [353].Liu D, Chen W, Tian Y, He S, Zheng W, Sun J, Wang Z, Jiang X, A Highly Sensitive Gold‐Nanoparticle‐Based Assay for Acetylcholinesterase in Cerebrospinal Fluid of Transgenic Mice with Alzheimer’s Disease, Advanced Healthcare Materials, 1 (2012) 90–95. [DOI] [PubMed] [Google Scholar]
- [354].Leszek J, Md Ashraf G, Tse WH, Zhang J, Gasiorowski K, Avila-Rodriguez MF, Tarasov VV, Barreto GE, Klochkov SG, Bachurin SO, Aliev G, Nanotechnology for Alzheimer Disease, Curr Alzheimer Res, 14 (2017) 1182–1189. [DOI] [PubMed] [Google Scholar]
- [355].Liu G, Men P, Kudo W, Perry G, Smith MA, Nanoparticle–chelator conjugates as inhibitors of amyloid-β aggregation and neurotoxicity: A novel therapeutic approach for Alzheimer disease, Neuroscience Letters, 455 (2009) 187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [356].N. EE, S. Jesse, H. BT, K. WE, B. BJ, S. TM, In Vivo Optical Imaging of Amyloid Aggregates in Brain: Design of Fluorescent Markers, Angewandte Chemie International Edition, 44 (2005) 5452–5456. [DOI] [PubMed] [Google Scholar]
- [357].Fernández T, Martínez-Serrano A, Cussó L, Desco M, Ramos-Gómez M, Functionalization and Characterization of Magnetic Nanoparticles for the Detection of Ferritin Accumulation in Alzheimer’s Disease, ACS Chemical Neuroscience, (2018). [DOI] [PubMed] [Google Scholar]
- [358].Amin FU, Hoshiar AK, Do TD, Noh Y, Shah SA, Khan MS, Yoon J, Kim MO, Osmotin-loaded magnetic nanoparticles with electromagnetic guidance for the treatment of Alzheimer’s disease, Nanoscale, 9 (2017) 10619–10632. [DOI] [PubMed] [Google Scholar]
- [359].Sun J, Xie W, Zhu X, Xu M, Liu J, Sulfur Nanoparticles with Novel Morphologies Coupled with Brain-Targeting Peptides RVG as a New Type of Inhibitor Against Metal-Induced Aβ Aggregation, ACS Chemical Neuroscience, 9 (2018) 749–761. [DOI] [PubMed] [Google Scholar]
- [360].Heckman KL, DeCoteau W, Estevez A, Reed KJ, Costanzo W, Sanford D, Leiter JC, Clauss J, Knapp K, Gomez C, Mullen P, Rathbun E, Prime K, Marini J, Patchefsky J, Patchefsky AS, Hailstone RK, Erlichman JS, Custom Cerium Oxide Nanoparticles Protect against a Free Radical Mediated Autoimmune Degenerative Disease in the Brain, ACS Nano, 7 (2013) 10582–10596. [DOI] [PubMed] [Google Scholar]
- [361].Yang L, Sun J, Xie W, Liu Y, Liu J, Dual-functional selenium nanoparticles bind to and inhibit amyloid [small beta] fiber formation in Alzheimer’s disease, Journal of Materials Chemistry B, 5 (2017) 5954–5967. [DOI] [PubMed] [Google Scholar]
- [362].Ray B, Bisht S, Maitra A, Maitra A, Lahiri DK, Neuroprotective and Neurorescue Effects of a Novel Polymeric Nanoparticle Formulation of Curcumin (NanoCurc™) in the Neuronal Cell Culture and Animal Model: Implications for Alzheimer’s Disease, Journal of Alzheimer’s Disease, 23 (2011) 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Luo Q, Lin Y-X, Yang P-P, Wang Y, Qi G-B, Qiao Z-Y, Li B-N, Zhang K, Zhang J-P, Wang L, Wang H, A self-destructive nanosweeper that captures and clears amyloid β-peptides, Nature Communications, 9 (2018) 1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Kuo Y-C, Chen IY, Rajesh R, Use of functionalized liposomes loaded with antioxidants to permeate the blood–brain barrier and inhibit β-amyloid-induced neurodegeneration in the brain, Journal of the Taiwan Institute of Chemical Engineers, (2018). [Google Scholar]
- [365].Costa LG, Garrick JM, Roquè PJ, Pellacani C, Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More, Oxidative Medicine and Cellular Longevity, 2016 (2016) 2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Bana L, Minniti S, Salvati E, Sesana S, Zambelli V, Cagnotto A, Orlando A, Cazzaniga E, Zwart R, Scheper W, Masserini M, Re F, Liposomes bi-functionalized with phosphatidic acid and an ApoE-derived peptide affect Aβ aggregation features and cross the blood–brain-barrier: Implications for therapy of Alzheimer disease, Nanomedicine: Nanotechnology, Biology and Medicine, 10 (2014) 1583–1590. [DOI] [PubMed] [Google Scholar]
- [367].Farr SA, Ripley JL, Sultana R, Zhang Z, Niehoff ML, Platt TL, Murphy MP, Morley JE, Kumar V, Butterfield DA, Antisense oligonucleotide against GSK-3β in brain of SAMP8 mice improves learning and memory and decreases oxidative stress: Involvement of transcription factor Nrf2 and implications for Alzheimer disease, Free Radical Biology and Medicine, 67 (2014) 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].DeVos SL, Miller RL, Schoch KM, Holmes BB, Kebodeaux CS, Wegener AJ, Chen G, Shen T, Tran H, Nichols B, Zanardi TA, Kordasiewicz HB, Swayze EE, Bennett CF, Diamond MI, Miller TM, Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy, Science Translational Medicine, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [369].van Dyck CH, Anti-Amyloid-β Monoclonal Antibodies for Alzheimer’s Disease: Pitfalls and Promise, Biological Psychiatry, 83 (2018) 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Prins ND, Scheltens P, Treating Alzheimer’s disease with monoclonal antibodies: current status and outlook for the future, Alzheimer’s Research & Therapy, 5 (2013) 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Banks WA, Farr SA, Morley JE, Wolf KM, Geylis V, Steinitz M, Anti-amyloid beta protein antibody passage across the blood–brain barrier in the SAMP8 mouse model of Alzheimer’s disease: An age-related selective uptake with reversal of learning impairment, Experimental Neurology, 206 (2007) 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [372].Banks WA, Pagliari P, Nakaoke R, Morley JE, Effects of a behaviorally active antibody on the brain uptake and clearance of amyloid beta proteins, Peptides, 26 (2005) 287–294. [DOI] [PubMed] [Google Scholar]
- [373].Finke JM, Ayres KR, Brisbin RP, Hill HA, Wing EE, Banks WA, Antibody blood-brain barrier efflux is modulated by glycan modification, Biochimica et Biophysica Acta (BBA) - General Subjects, 1861 (2017) 2228–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Parkinson James, An Essay on the Shaking Palsy, The Journal of Neuropsychiatry and Clinical Neurosciences, 14 (2002) 223–236. [DOI] [PubMed] [Google Scholar]
- [375].Pearce JM, Aspects of the history of Parkinson’s disease, Journal of Neurology, Neurosurgery, and Psychiatry, 52 (1989) 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Zeng X-S, Geng W-S, Jia J-J, Chen L, Zhang P-P, Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease, Frontiers in Aging Neuroscience, 10 (2018) 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Surmeier DJ, Guzman JN, Sanchez-Padilla J, Goldberg JA, Chapter 4 - What causes the death of dopaminergic neurons in Parkinson’s disease?, in: Björklund A, Cenci MA (Eds.) Progress in Brain Research, Elsevier 2010, pp. 59–77. [DOI] [PubMed] [Google Scholar]
- [378].Alexander GE, Biology of Parkinson’s disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder, Dialogues in Clinical Neuroscience, 6 (2004) 259–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [379].Ferrer I, Martinez A, Blanco R, Dalfó E, Carmona M, Neuropathology of sporadic Parkinson disease before the appearance of parkinsonism: preclinical Parkinson disease, Journal of Neural Transmission, 118 (2011) 821–839. [DOI] [PubMed] [Google Scholar]
- [380].Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A-E, Lang AE, Parkinson disease, Nature Reviews Disease Primers, 3 (2017) 17013. [DOI] [PubMed] [Google Scholar]
- [381].Ascherio A, Schwarzschild MA, The epidemiology of Parkinson’s disease: risk factors and prevention, The Lancet Neurology, 15 (2016) 1257–1272. [DOI] [PubMed] [Google Scholar]
- [382].Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL, Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease, Science, 276 (1997) 2045–2047. [DOI] [PubMed] [Google Scholar]
- [383].Karampetsou M, Ardah MT, Semitekolou M, Polissidis A, Samiotaki M, Kalomoiri M, Majbour N, Xanthou G, El-Agnaf OMA, Vekrellis K, Phosphorylated exogenous alpha-synuclein fibrils exacerbate pathology and induce neuronal dysfunction in mice, Scientific Reports, 7 (2017) 16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [384].Hoenen C, Gustin A, Birck C, Kirchmeyer M, Beaume N, Felten P, Grandbarbe L, Heuschling P, Heurtaux T, Alpha-Synuclein Proteins Promote Pro-Inflammatory Cascades in Microglia: Stronger Effects of the A53T Mutant, PLOS ONE, 11 (2016) e0162717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [385].Steger M, Diez F, Dhekne HS, Lis P, Nirujogi RS, Karayel O, Tonelli F, Martinez TN, Lorentzen E, Pfeffer SR, Alessi DR, Mann M, Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis, eLife, 6 (2017) e31012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [386].Bekris LM, Mata IF, Zabetian CP, The Genetics of Parkinson Disease, Journal of Geriatric Psychiatry and Neurology, 23 (2010) 228–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [387].Hatcher JM, Pennell KD, Miller GW, Parkinson’s disease and pesticides: a toxicological perspective, Trends in pharmacological sciences, 29 (2008) 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Farrer MJ, Genetics of Parkinson disease: paradigm shifts and future prospects, Nature Reviews Genetics, 7 (2006) 306. [DOI] [PubMed] [Google Scholar]
- [389].Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, Kopin IJ, Chronic Parkinsonism secondary to intravenous injection of meperidine analogues, Psychiatry Res, 1 (1979) 249–254. [DOI] [PubMed] [Google Scholar]
- [390].William Langston J, Forno LS, Rebert CS, Irwin I, Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey, Brain Research, 292 (1984) 390–394. [DOI] [PubMed] [Google Scholar]
- [391].Langston J, Ballard P, Tetrud J, Irwin I, Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis, Science, 219 (1983) 979–980. [DOI] [PubMed] [Google Scholar]
- [392].Burke RE, O’Malley K, Axon Degeneration in Parkinson’s Disease, Experimental neurology, 246 (2013) 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [393].Herkenham M, Little MD, Bankiewicz K, Yang SC, Markey SP, Johannessen JN, Selective retention of MPP+ within the monoaminergic systems of the primate brain following MPTP administration: An in vivo autoradiographic study, Neuroscience, 40 (1991) 133–158. [DOI] [PubMed] [Google Scholar]
- [394].Heikkila R, Hess A, Duvoisin R, Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice, Science, 224 (1984) 1451–1453. [DOI] [PubMed] [Google Scholar]
- [395].Jenner P, Functional models of Parkinson’s disease: a valuable tool in the development of novel therapies, Ann Neurol, 64 Suppl 2 (2008) S16–29. [DOI] [PubMed] [Google Scholar]
- [396].Di Monte D, Sandy MS, Ekström G, Smith MT, Comparative studies on the mechanisms of paraquat and 1-methyl-4-phenylpyridine (MPP+) cytotoxicity, Biochemical and Biophysical Research Communications, 137 (1986) 303–309. [DOI] [PubMed] [Google Scholar]
- [397].Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT, Chronic systemic pesticide exposure reproduces features of Parkinson’s disease, Nature Neuroscience, 3 (2000) 1301. [DOI] [PubMed] [Google Scholar]
- [398].DeMaagd G, Philip A, Part 2: Introduction to the Pharmacotherapy of Parkinson’s Disease, With a Focus on the Use of Dopaminergic Agents, Pharmacy and Therapeutics, 40 (2015) 590–600. [PMC free article] [PubMed] [Google Scholar]
- [399].LeWitt PA, Fahn S, Levodopa therapy for Parkinson disease, A look backward and forward, 86 (2016) S3–S12. [DOI] [PubMed] [Google Scholar]
- [400].Olanow CW, Obeso JA, Stocchi F, Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications, The Lancet Neurology, 5 (2006) 677–687. [DOI] [PubMed] [Google Scholar]
- [401].Müller T, Catechol-O-Methyltransferase Inhibitors in Parkinson’s Disease, Drugs, 75 (2015) 157–174. [DOI] [PubMed] [Google Scholar]
- [402].Schapira AHV, Monoamine Oxidase B Inhibitors for the Treatment of Parkinson’s Disease, CNS Drugs, 25 (2011) 1061–1071. [DOI] [PubMed] [Google Scholar]
- [403].Bronstein JM, Tagliati M, Alterman RL, et al. , Deep brain stimulation for parkinson disease: An expert consensus and review of key issues, Archives of Neurology, 68 (2011) 165–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [404].M. MJ, T. Eduardo, C. Jaume, Clinical overview of the synucleinopathies, Movement Disorders, 18 (2003) 21–27. [DOI] [PubMed] [Google Scholar]
- [405].Jellinger KA, A critical evaluation of current staging of α-synuclein pathology in Lewy body disorders, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1792 (2009) 730–740. [DOI] [PubMed] [Google Scholar]
- [406].Kaufmann H, Norcliffe-Kaufmann L, Palma J-A, Biaggioni I, Low PA, Singer W, Goldstein DS, Peltier AC, Shibao CA, Gibbons CH, Freeman R, Robertson D, The Natural History of Pure Autonomic Failure: a U.S. Prospective Cohort, Annals of neurology, 81 (2017) 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [407].Singer W, Berini SE, Sandroni P, Fealey RD, Coon EA, Suarez MD, Benarroch EE, Low PA, Pure autonomic failure, Predictors of conversion to clinical CNS involvement, 88 (2017) 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [408].Kaufmann H, Biaggioni I, Autonomic failure in neurodegenerative disorders, Semin Neurol, 23 (2003) 351–363. [DOI] [PubMed] [Google Scholar]
- [409].Kao AW, Racine CA, Quitania LC, Kramer JH, Christine CW, Miller BL, Cognitive and Neuropsychiatric Profile of the Synucleinopathies: Parkinson’s Disease, Dementia with Lewy Bodies and Multiple System Atrophy, Alzheimer disease and associated disorders, 23 (2009) 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [410].Blanc F, Colloby SJ, Philippi N, de Pétigny X, Jung B, Demuynck C, Phillipps C, Anthony P, Thomas A, Bing F, Lamy J, Martin-Hunyadi C, O’Brien JT, Cretin B, McKeith I, Armspach J-P, Taylor J-P, Cortical Thickness in Dementia with Lewy Bodies and Alzheimer’s Disease: A Comparison of Prodromal and Dementia Stages, PLOS ONE, 10 (2015) e0127396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [411].Gomperts SN, Lewy Body Dementias: Dementia With Lewy Bodies and Parkinson Disease Dementia, Continuum : Lifelong Learning in Neurology, 22 (2016) 435–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [412].Martí MJ, Tolosa E, Campdelacreu J, Clinical overview of the synucleinopathies, Movement Disorders, 18 (2003) 21–27. [DOI] [PubMed] [Google Scholar]
- [413].Miraglia F, Betti L, Palego L, Giannaccini G, Parkinson’s disease and alpha-synucleinopathies: from arising pathways to therapeutic challenge, Cent Nerv Syst Agents Med Chem, 15 (2015) 109–116. [DOI] [PubMed] [Google Scholar]
- [414].Huang R, Ma H, Guo Y, Liu S, Kuang Y, Shao K, Li J, Liu Y, Han L, Huang S, An S, Ye L, Lou J, Jiang C, Angiopep-Conjugated Nanoparticles for Targeted Long-Term Gene Therapy of Parkinson’s Disease, Pharmaceutical Research, 30 (2013) 2549–2559. [DOI] [PubMed] [Google Scholar]
- [415].K. JH, H. CD, D. Biplob, B. RAE, S. James, G. Mehdi, B. RT, Delivery of neurturin by AAV2 (CERE‐120)‐mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP‐treated monkeys, Annals of Neurology, 60 (2006) 706–715. [DOI] [PubMed] [Google Scholar]
- [416].Bartus RT, Johnson EM, Clinical tests of neurotrophic factors for human neurodegenerative diseases, part 1: Where have we been and what have we learned?, Neurobiology of Disease, 97 (2017) 156–168. [DOI] [PubMed] [Google Scholar]
- [417].Bartus RT, Johnson EM, Clinical tests of neurotrophic factors for human neurodegenerative diseases, part 2: Where do we stand and where must we go next?, Neurobiology of Disease, 97 (2017) 169–178. [DOI] [PubMed] [Google Scholar]
- [418].Loane C, Politis M, Positron emission tomography neuroimaging in Parkinson’s disease, Am J Transl Res, 3 (2011) 323–341. [PMC free article] [PubMed] [Google Scholar]
- [419].Ito K, Morrish PK, Rakshi JS, Uema T, Ashburner J, Bailey DL, Friston KJ, Brooks DJ, Statistical parametric mapping with 18F-dopa PET shows bilaterally reduced striatal and nigral dopaminergic function in early Parkinson’s disease, J Neurol Neurosurg Psychiatry, 66 (1999) 754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [420].Antonini A, Vontobel P, Psylla M, et al. , Complementary positron emission tomographic studies of the striatal dopaminergic system in parkinsons disease, Archives of Neurology, 52 (1995) 1183–1190. [DOI] [PubMed] [Google Scholar]
- [421].Jacobs AH, Kracht LW, Gossmann A, Rüger MA, Thomas AV, Thiel A, Herholz K, Imaging in Neurooncology, NeuroRx, 2 (2005) 333–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [422].Khan NL, Brooks DJ, Pavese N, Sweeney MG, Wood NW, Lees AJ, Piccini P, Progression of nigrostriatal dysfunction in a parkin kindred: an [18F]dopa PET and clinical study, Brain, 125 (2002) 2248–2256. [DOI] [PubMed] [Google Scholar]
- [423].Ba F, Martin WRW, Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice, Parkinsonism & Related Disorders, 21 (2015) 87–94. [DOI] [PubMed] [Google Scholar]
- [424].Catafau AM, Tolosa E, Impact of dopamine transporter SPECT using 123I‐ Ioflupane on diagnosis and management of patients with clinically uncertain parkinsonian syndromes, Movement Disorders, 19 (2004) 1175–1182. [DOI] [PubMed] [Google Scholar]
- [425].McDonagh BH, Singh G, Hak S, Bandyopadhyay S, Augestad IL, Peddis D, Sandvig I, Sandvig A, Glomm WR, L‐DOPA‐Coated Manganese Oxide Nanoparticles as Dual MRI Contrast Agents and Drug‐Delivery Vehicles, Small, 12 (2016) 301–306. [DOI] [PubMed] [Google Scholar]
- [426].Li J, Liu D, Sun L, Lu Y, Zhang Z, Advanced glycation end products and neurodegenerative diseases: Mechanisms and perspective, Journal of the Neurological Sciences, 317 (2012) 1–5. [DOI] [PubMed] [Google Scholar]
- [427].Salahuddin P, Rabbani G, Khan RH, The role of advanced glycation end products in various types of neurodegenerative disease: a therapeutic approach, Cellular & Molecular Biology Letters, 19 (2014) 407–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [428].Byun K, Yoo Y, Son M, Lee J, Jeong G-B, Park YM, Salekdeh GH, Lee B, Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases, Pharmacology & Therapeutics, 177 (2017) 44–55. [DOI] [PubMed] [Google Scholar]
- [429].Bayarsaikhan E, Bayarsaikhan D, Lee J, Son M, Oh S, Moon J, Park H-J, Roshini A, Kim SU, Song B-J, Jo S-M, Byun K, Lee B, Microglial AGE-albumin is critical for neuronal death in Parkinson’s disease: a possible implication for theranostics, International Journal of Nanomedicine, 10 (2015) 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [430].Katzenschlager R, Lees AJ, Treatment of Parkinson’s disease: levodopa as the first choice, Journal of Neurology, 249 (2002) ii19–ii24. [DOI] [PubMed] [Google Scholar]
- [431].Stocchi F, Optimising levodopa therapy for the management of Parkinson’s disease, J Neurol, 252 Suppl 4 (2005) Iv43–iv48. [DOI] [PubMed] [Google Scholar]
- [432].Mead BP, Kim N, Miller GW, Hodges D, Mastorakos P, Klibanov AL, Mandell JW, Hirsh J, Suk JS, Hanes J, Price RJ, Novel Focused Ultrasound Gene Therapy Approach Noninvasively Restores Dopaminergic Neuron Function in a Rat Parkinson’s Disease Model, Nano Letters, 17 (2017) 3533–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [433].Wen Z, Yan Z, Hu K, Pang Z, Cheng X, Guo L, Zhang Q, Jiang X, Fang L, Lai R, Odorranalectin-conjugated nanoparticles: Preparation, brain delivery and pharmacodynamic study on Parkinson’s disease following intranasal administration, Journal of Controlled Release, 151 (2011) 131–138. [DOI] [PubMed] [Google Scholar]
- [434].Hu K, Shi Y, Jiang W, Han J, Huang S, Jiang X, Lactoferrin conjugated PEGPLGA nanoparticles for brain delivery: Preparation, characterization and efficacy in Parkinson’s disease, International Journal of Pharmaceutics, 415 (2011) 273–283. [DOI] [PubMed] [Google Scholar]
- [435].Pahuja R, Seth K, Shukla A, Shukla RK, Bhatnagar P, Chauhan LKS, Saxena PN, Arun J, Chaudhari BP, Patel DK, Singh SP, Shukla R, Khanna VK, Kumar P, Chaturvedi RK, Gupta KC, Trans-Blood Brain Barrier Delivery of Dopamine-Loaded Nanoparticles Reverses Functional Deficits in Parkinsonian Rats, ACS Nano, 9 (2015) 4850–4871. [DOI] [PubMed] [Google Scholar]
- [436].Raj R, Wairkar S, Sridhar V, Gaud R, Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity, International Journal of Biological Macromolecules, 109 (2018) 27–35. [DOI] [PubMed] [Google Scholar]
- [437].Chung TH, Hsu SC, Wu SH, Hsiao JK, Lin CP, Yao M, Huang DM, Dextran-coated iron oxide nanoparticle-improved therapeutic effects of human mesenchymal stem cells in a mouse model of Parkinson’s disease, Nanoscale, 10 (2018) 2998–3007. [DOI] [PubMed] [Google Scholar]
- [438].Hu K, Chen X, Chen W, Zhang L, Li J, Ye J, Zhang Y, Zhang L, Li C-H, Yin L, Guan Y-Q, Neuroprotective effect of gold nanoparticles composites in Parkinson’s disease model, Nanomedicine: Nanotechnology, Biology and Medicine, 14 (2018) 1123–1136. [DOI] [PubMed] [Google Scholar]
- [439].Dranka BP, Gifford A, McAllister D, Zielonka J, Joseph J, O’Hara CL, Stucky CL, Kanthasamy AG, Kalyanaraman B, A novel mitochondrially-targeted apocynin derivative prevents hyposmia and loss of motor function in the leucine-rich repeat kinase 2 (LRRK2R1441G) transgenic mouse model of Parkinson’s disease, Neuroscience Letters, 583 (2014) 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [440].Ghosh A, Kanthasamy A, Joseph J, Anantharam V, Srivastava P, Dranka BP, Kalyanaraman B, Kanthasamy AG, Anti-inflammatory and neuroprotective effects of an orally active apocynin derivative in pre-clinical models of Parkinson’s disease, J Neuroinflammation, 9 (2012) 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [441].Jin F, Wu Q, Lu Y-F, Gong Q-H, Shi J-S, Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats, European Journal of Pharmacology, 600 (2008) 78–82. [DOI] [PubMed] [Google Scholar]
- [442].Palle S, Neerati P, Improved neuroprotective effect of resveratrol nanoparticles as evinced by abrogation of rotenone-induced behavioral deficits and oxidative and mitochondrial dysfunctions in rat model of Parkinson’s disease, Naunyn Schmiedebergs Arch Pharmacol, 391 (2018) 445–453. [DOI] [PubMed] [Google Scholar]
- [443].Brenza TM, Ghaisas S, Ramirez JEV, Harischandra D, Anantharam V, Kalyanaraman B, Kanthasamy AG, Narasimhan B, Neuronal protection against oxidative insult by polyanhydride nanoparticle-based mitochondria-targeted antioxidant therapy, Nanomedicine: Nanotechnology, Biology and Medicine, 13 (2017) 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [444].Imran M, Mahmood S, An overview of human prion diseases, Virology Journal, 8 (2011) 559–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [445].Jucker M, Walker LC, Self-propagation of pathogenic protein aggregates in neurodegenerative diseases, Nature, 501 (2013) 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [446].DeArmond SJ, Discovering the Mechanisms of Neurodegeneration in Prion Diseases, Neurochemical Research, 29 (2004) 1979–1998. [DOI] [PubMed] [Google Scholar]
- [447].Plummer PJG, Scrapie—A Disease of Sheep: A Review of the literature, Canadian Journal of Comparative Medicine and Veterinary Science, 10 (1946) 49–54. [PubMed] [Google Scholar]
- [448].Srithayakumar V, Mitchell GB, White BN, Identification of amino acid variation in the prion protein associated with classical scrapie in Canadian dairy goats, BMC Veterinary Research, 12 (2016) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [449].Liberski PP, Ironside JW, Chapter 23 - Prion Diseases A2 - Zigmond, Michael J, in: Rowland LP, Coyle JT (Eds.) Neurobiology of Brain Disorders, Academic Press, San Diego, 2015, pp. 356–374. [Google Scholar]
- [450].Belay ED, Sejvar JJ, Shieh W-J, Wiersma ST, Zou W-Q, Gambetti P, Hunter S, Maddox RA, Crockett L, Zaki SR, Schonberger LB, Variant Creutzfeldt-Jakob Disease Death, United States, Emerging Infectious Diseases, 11 (2005) 1351–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [451].Barnard G, Hopkins L, Moorthie S, Seilly D, Tonks P, Dabaghian R, Clewley J, Coward J, McConnell I, Direct Detection of Disease Associated Prions in Brain and Lymphoid Tissue Using Antibodies Recognizing the Extreme N Terminus of PrP(C), Prion, 1 (2007) 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [452].Geschwind MD, Prion Diseases, Continuum (Minneapolis, Minn.), 21 (2015) 1612–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [453].Divers TJ, Rebhun’s Diseases of Dairy Cattle, 2nd Edition ed. [Google Scholar]
- [454].Appleby BS, Lyketsos CG, Rapidly progressive dementias and the treatment of human prion diseases, Expert Opinion on Pharmacotherapy, 12 (2011) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [455].Soto C, Prion Hypothesis: The end of the Controversy?, Trends in biochemical sciences, 36 (2011) 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [456].Tuite MF, Serio TR, The prion hypothesis: from biological anomaly to basic regulatory mechanism, Nature reviews. Molecular cell biology, 11 (2010) 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [457].Harris DA, Cellular Biology of Prion Diseases, Clinical Microbiology Reviews, 12 (1999) 429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [458].LeBrun M, Huang H, Li X, Susceptibility of cell substrates to PrP(Sc) infection and safety control measures related to biological and biotherapeutical products, Prion, 2 (2008) 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [459].Gajdusek DC, Unconventional viruses and the origin and disappearance of kuru, Science, 197 (1977) 943–960. [DOI] [PubMed] [Google Scholar]
- [460].Hansel PA, Mad Cow Disease—the OR Connection, AORN Journal, 70 (1999) 224–238. [DOI] [PubMed] [Google Scholar]
- [461].Prusiner SB, Novel proteinaceous infectious particles cause scrapie, Science, 216 (1982) 136–144. [DOI] [PubMed] [Google Scholar]
- [462].Chesebro B, Race R, Wehrly K, Nishio J, Bloom M, Lechner D, Bergstrom S, Robbins K, Mayer L, Keith JM, Garon C, Haase A, Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain, Nature, 315 (1985) 331. [DOI] [PubMed] [Google Scholar]
- [463].Prusiner SB Neurodegenerative Diseases and Prions, New England Journal of Medicine, 344 (2001) 1516–1526. [DOI] [PubMed] [Google Scholar]
- [464].Aguzzi A, Sigurdson C, Heikenwaelder M, Molecular mechanisms of prion pathogenesis, Annu Rev Pathol, 3 (2008) 11–40. [DOI] [PubMed] [Google Scholar]
- [465].Colby DW, Prusiner SB, Prions, Cold Spring Harb Perspect Biol, 3 (2011) a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [466].Miller MB, Supattapone S, Superparamagnetic Nanoparticle Capture of Prions for Amplification, Journal of Virology, 85 (2011) 2813–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [467].Safar JG, Geschwind MD, Deering C, Didorenko S, Sattavat M, Sanchez H, Serban A, Vey M, Baron H, Giles K, Miller BL, DeArmond SJ, Prusiner SB, Diagnosis of human prion disease, Proceedings of the National Academy of Sciences of the United States of America, 102 (2005) 3501–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [468].Madec JY, Groschup MH, Buschmann A, Belli P, Calavas D, Baron T, Sensitivity of the Western blot detection of prion protein PrPres in natural sheep scrapie, Journal of Virological Methods, 75 (1998) 169–177. [DOI] [PubMed] [Google Scholar]
- [469].Saborio GP, Permanne B, Soto C, Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding, Nature, 411 (2001) 810. [DOI] [PubMed] [Google Scholar]
- [470].Shan Z, Yamasaki T, Suzuki A, Hasebe R, Horiuchi M, Establishment of a simple cell-based ELISA for the direct detection of abnormal isoform of prion protein from prion-infected cells without cell lysis and proteinase K treatment, Prion, 10 (2016) 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [471].Chen B, Morales R, Barria MA, Soto C, Estimating prion concentration in fluids and tissues by quantitative PMCA, Nature Methods, 7 (2010) 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [472].Giles K, Olson SH, Prusiner SB, Developing Therapeutics for PrP Prion Diseases, Cold Spring Harb Perspect Med, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [473].Walker LC, Jucker M, Neurodegenerative Diseases: Expanding the Prion Concept, Annual Review of Neuroscience, 38 (2015) 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [474].Forloni G, Artuso V, Roiter I, Morbin M, Tagliavini F, Therapy in prion diseases, Curr Top Med Chem, 13 (2013) 2465–2476. [DOI] [PubMed] [Google Scholar]
- [475].Panegyres PK, Armari E, Therapies for human prion diseases, American Journal of Neurodegenerative Disease, 2 (2013) 176–186. [PMC free article] [PubMed] [Google Scholar]
- [476].Burchell JT, Panegyres PK, Prion diseases: immunotargets and therapy, ImmunoTargets and Therapy, 5 (2016) 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [477].Bevacqua RJ, Fernandez-Martín R, Savy V, Canel NG, Gismondi MI, Kues WA, Carlson DF, Fahrenkrug SC, Niemann H, Taboga OA, Ferraris S, Salamone DF, Efficient edition of the bovine PRNP prion gene in somatic cells and IVF embryos using the CRISPR/Cas9 system, Theriogenology, 86 (2016) 1886–1896.e1881. [DOI] [PubMed] [Google Scholar]
- [478].White MD, Mallucci GR, Therapy for prion diseases: Insights from the use of RNA interference, Prion, 3 (2009) 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [479].Ai Tran HN, Sousa F, Moda F, Mandal S, Chanana M, Vimercati C, Morbin M, Krol S, Tagliavini F, Legname G, A novel class of potential prion drugs: preliminary in vitro and in vivo data for multilayer coated gold nanoparticles, Nanoscale, 2 (2010) 2724–2732. [DOI] [PubMed] [Google Scholar]
- [480].Kouassi GK, Irudayaraj J, A nanoparticle-based immobilization assay for prionkinetics study, Journal of Nanobiotechnology, 4 (2006) 8–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [481].Zhang H-J, Zheng H-Z, Long Y-J, Xiao G-F, Zhang L-Y, Wang Q-L, Gao M, Bai W-J, Gold nanoparticles as a label-free probe for the detection of amyloidogenic protein, Talanta, 89 (2012) 401–406. [DOI] [PubMed] [Google Scholar]
- [482].Kouassi GK, Wang P, Sreevatan S, Irudayaraj J, Aptamer-mediated magnetic and gold-coated magnetic nanoparticles as detection assay for prion protein assessment, Biotechnol Prog, 23 (2007) 1239–1244. [DOI] [PubMed] [Google Scholar]
- [483].Zhan L, Peng L, Huang C-Z, Stable silver nanoparticles–aptamer bioconjugates for cellular prion protein imaging, Chinese Science Bulletin, 59 (2014) 964–970. [Google Scholar]
- [484].Zhang H-J, Lu Y-H, Long Y-J, Wang Q-L, Huang X-X, Zhu R, Wang X-L, Liang L-P, Teng P, Zheng H-Z, An aptamer-functionalized gold nanoparticle biosensor for the detection of prion protein, Analytical Methods, 6 (2014) 2982–2987. [Google Scholar]
- [485].Henry J, Anand A, Chowdhury M, Coté G, Moreira R, Good T, Development of a nanoparticle-based surface-modified fluorescence assay for the detection of prion proteins, Analytical Biochemistry, 334 (2004) 1–8. [DOI] [PubMed] [Google Scholar]
- [486].Calvo P, Gouritin B, Brigger I, Lasmezas C, Deslys J-P, Williams A, Andreux JP, Dormont D, Couvreur P, PEGylated polycyanoacrylate nanoparticles as vector for drug delivery in prion diseases, Journal of Neuroscience Methods, 111 (2001) 151–155. [DOI] [PubMed] [Google Scholar]
- [487].Bäumer D, Talbot K, Turner MR, Advances in motor neurone disease, Journal of the Royal Society of Medicine, 107 (2014) 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [488].Van den Berg-Vos RM, Van den Berg LH, Visser J, de Visser M, Franssen H, Wokke JHJ, The spectrum of lower motor neuronsyndromes, Journal of Neurology, 250 (2003) 1279–1292. [DOI] [PubMed] [Google Scholar]
- [489].Rezania K, Roos RP, Spinal cord: motor neuron diseases, Neurol Clin, 31 (2013) 219–239. [DOI] [PubMed] [Google Scholar]
- [490].Moloney EB, de Winter F, Verhaagen J, ALS as a distal axonopathy: molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease, Frontiers in Neuroscience, 8 (2014) 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [491].Zarei S, Carr K, Reiley L, Diaz K, Guerra O, Altamirano PF, Pagani W, Lodin D, Orozco G, Chinea A, A comprehensive review of amyotrophic lateral sclerosis, Surgical Neurology International, 6 (2015) 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [492].Talbot K, Motor neurone disease, Postgraduate Medical Journal, 78 (2002) 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [493].Wijesekera LC, Leigh PN, Amyotrophic lateral sclerosis, Orphanet Journal of Rare Diseases, 4 (2009) 3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [494].Kübler A, Nijboer F, Mellinger J, Vaughan TM, Pawelzik H, Schalk G, McFarland DJ, Birbaumer N, Wolpaw JR, Patients with ALS can use sensorimotor rhythms to operate a brain-computer interface, Neurology, 64 (2005) 1775–1777. [DOI] [PubMed] [Google Scholar]
- [495].Zoccolella S, Santamato A, Lamberti P, Current and emerging treatments for amyotrophic lateral sclerosis, Neuropsychiatric Disease and Treatment, 5 (2009) 577–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [496].Tang BL, Amyotrophic lateral sclerosis disease modifying therapeutics: a cell biological perspective, Neural Regeneration Research, 12 (2017) 407–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [497].Couthouis J, Raphael AR, Daneshjou R, Gitler AD, Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis, PLoS Genetics, 10 (2014) e1004704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [498].Renton AE, Chiò A, Traynor BJ, State of play in amyotrophic lateral sclerosis genetics, Nature Neuroscience, 17 (2013) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [499].Morgan S, Orrell RW, Pathogenesis of amyotrophic lateral sclerosis, British Medical Bulletin, 119 (2016) 87–98. [DOI] [PubMed] [Google Scholar]
- [500].Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC, Amyotrophic lateral sclerosis, Lancet, 377 (2011) 942–955. [DOI] [PubMed] [Google Scholar]
- [501].Lall D, Baloh RH, Microglia and C9orf72 in neuroinflammation and ALS and frontotemporal dementia, The Journal of Clinical Investigation, 127 (2017) 3250–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [502].Tripolszki K, Csányi B, Nagy D, Ratti A, Tiloca C, Silani V, Kereszty É, Török N, Vécsei L, Engelhardt JI, Klivényi P, Nagy N, Széll M, Genetic analysis of the SOD1 and C9ORF72 genes in Hungarian patients with amyotrophic lateral sclerosis, Neurobiology of Aging, 53 (2017) 195.e191–195.e195. [DOI] [PubMed] [Google Scholar]
- [503].Chi S, Jiang T, Tan L, Yu J-T, Distinct neurological disorders with C9orf72 mutations: genetics, pathogenesis, and therapy, Neuroscience & Biobehavioral Reviews, 66 (2016) 127–142. [DOI] [PubMed] [Google Scholar]
- [504].Andersen PM, Al-Chalabi A, Clinical genetics of amyotrophic lateral sclerosis: what do we really know?, Nature Reviews Neurology, 7 (2011) 603. [DOI] [PubMed] [Google Scholar]
- [505].Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng H-X, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak–Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH Jr, Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis, Nature, 362 (1993) 59. [DOI] [PubMed] [Google Scholar]
- [506].Prudencio M, Borchelt DR, Superoxide dismutase 1 encoding mutations linked to ALS adopts a spectrum of misfolded states, Molecular Neurodegeneration, 6 (2011) 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [507].Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW, Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1, Science, 281 (1998) 1851–1854. [DOI] [PubMed] [Google Scholar]
- [508].Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA, Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment, The Journal of neuroscience, 21 (2001) 3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [509].Lino MM, Schneider C, Caroni P, Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease, The Journal of neuroscience 22 (2002) 4825–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [510].Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW, Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis, Nature Neuroscience, 11 (2008) 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [511].Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillée S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown RH, Julien J-P, Goldstein LSB, Cleveland DW, Wild-Type Nonneuronal Cells Extend Survival of SOD1 Mutant Motor Neurons in ALS Mice, Science, 302 (2003) 113–117. [DOI] [PubMed] [Google Scholar]
- [512].Xiao Q, Zhao W, Beers DR, Yen AA, Xie W, Henkel JS, Appel SH, Mutant SOD1(G93A) microglia are more neurotoxic relative to wild-type microglia, J Neurochem, 102 (2007) 2008–2019. [DOI] [PubMed] [Google Scholar]
- [513].Hall ED, Oostveen JA, Gurney ME, Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS, Glia, 23 (1998) 249–256. [DOI] [PubMed] [Google Scholar]
- [514].Boillée S, Cleveland DW, Revisiting oxidative damage in ALS: microglia, Nox, and mutant SOD1, The Journal of Clinical Investigation, 118 (2008) 474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [515].Pokrishevsky E, Grad LI, Yousefi M, Wang J, Mackenzie IR, Cashman NR, Aberrant localization of FUS and TDP43 is associated with misfolding of SOD1 in amyotrophic lateral sclerosis, PLoS One, 7 (2012) e35050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [516].Ng ASL, Tan E-K, Intermediate C9orf72 alleles in neurological disorders: does size really matter?, Journal of Medical Genetics, 54 (2017) 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [517].DeJesus-Hernandez M, Mackenzie Ian R., Boeve Bradley F., Boxer Adam L., Baker M, Rutherford Nicola J., Nicholson Alexandra M., Finch NiCole A., Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung G.-Yuek R., Karydas A, Seeley William W., Josephs Keith A., Coppola G, Geschwind Daniel H., Wszolek Zbigniew K., Feldman H, Knopman David S., Petersen Ronald C., Miller Bruce L., Dickson Dennis W., Boylan Kevin B., Graff-Radford Neill R., Rademakers R, Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS, Neuron, 72 (2011) 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [518].Ciura S, Lattante S, Ber IL, Latouche M, Tostivint H, Brice A, Kabashi E, Loss of function of C9orf72 causes motor deficits in a zebrafish model of amyotrophic lateral sclerosis, Annals of Neurology, 74 (2013) 180–187. [DOI] [PubMed] [Google Scholar]
- [519].Wojciechowska M, Krzyzosiak WJ, Cellular toxicity of expanded RNA repeats: focus on RNA foci, Hum Mol Genet, 20 (2011) 3811–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [520].Haeusler AR, Donnelly CJ, Periz G, Simko EAJ, Shaw PG, Kim M-S, Maragakis NJ, Troncoso JC, Pandey A, Sattler R, Rothstein JD, Wang J, C9orf72 nucleotide repeat structures initiate molecular cascades of disease, Nature, 507 (2014) 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [521].Mackenzie IR, Arzberger T, Kremmer E, Troost D, Lorenzl S, Mori K, Weng SM, Haass C, Kretzschmar HA, Edbauer D, Neumann M, Dipeptide repeat protein pathology in C9ORF72 mutation cases: clinico-pathological correlations, Acta Neuropathol, 126 (2013) 859–879. [DOI] [PubMed] [Google Scholar]
- [522].Jovičić A, Mertens J, Boeynaems S, Bogaert E, Chai N, Yamada SB, Paul JW Iii, Sun S, Herdy JR, Bieri G, Kramer NJ, Gage FH, Van Den Bosch L, Robberecht W, Gitler AD, Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS, Nature Neuroscience, 18 (2015) 1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [523].Miller RG, Mitchell JD, Moore DH, Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND), Cochrane Database of Systematic Reviews, (2012). [Google Scholar]
- [524].G. Amyotrophic Lateral Sclerosis/Riluzole Study II, Lacomblez L, Bensimon G, Meininger V, Leigh PN, Guillet P, Dose-ranging study of riluzole in amyotrophic lateral sclerosis, The Lancet, 347 (1996) 1425–1431. [DOI] [PubMed] [Google Scholar]
- [525].Takei K, Watanabe K, Yuki S, Akimoto M, Sakata T, Palumbo J, Edaravone and its clinical development for amyotrophic lateral sclerosis, Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 18 (2017) 5–10. [DOI] [PubMed] [Google Scholar]
- [526].Scott A, On the treatment trail for ALS, Nature, 550 (2017) S120. [DOI] [PubMed] [Google Scholar]
- [527].Ito H, Wate R, Zhang J, Ohnishi S, Kaneko S, Ito H, Nakano S, Kusaka H, Treatment with edaravone, initiated at symptom onset, slows motor decline and decreases SOD1 deposition in ALS mice, Experimental Neurology, 213 (2008) 448–455. [DOI] [PubMed] [Google Scholar]
- [528].Abe K, Itoyama Y, Sobue G, Tsuji S, Aoki M, Doyu M, Hamada C, Kondo K, Yoneoka T, Akimoto M, Yoshino H, Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients, Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 15 (2014) 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [529].Hardiman O, van den Berg LH, Edaravone: a new treatment for ALS on the horizon?, The Lancet Neurology, 16 (2017) 490–491. [DOI] [PubMed] [Google Scholar]
- [530].Mazibuko Z, Choonara YE, Kumar P, Du Toit LC, Modi G, Naidoo D, Pillay V, A review of the potential role of nano-enabled drug delivery technologies in amyotrophic lateral sclerosis: lessons learned from other neurodegenerative disorders, J Pharm Sci, 104 (2015) 1213–1229. [DOI] [PubMed] [Google Scholar]
- [531].Wagner J, Biomarkers: Principles, Policies, and Practice, Clinical Pharmacology & Therapeutics, 86 (2009) 3–7. [DOI] [PubMed] [Google Scholar]
- [532].Roney C, Kulkarni P, Arora V, Antich P, Bonte F, Wu A, Mallikarjuana NN, Manohar S, Liang H-F, Kulkarni AR, Sung H-W, Sairam M, Aminabhavi TM, Targeted nanoparticles for drug delivery through the blood–brain barrier for Alzheimer’s disease, Journal of Controlled Release, 108 (2005) 193–214. [DOI] [PubMed] [Google Scholar]
- [533].Jablonski MR, Markandaiah SS, Jacob D, Meng NJ, Li K, Gennaro V, Lepore AC, Trotti D, Pasinelli P, Inhibiting drug efflux transporters improves efficacy of ALS therapeutics, Annals of Clinical and Translational Neurology, 1 (2014) 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [534].Chen L, Watson C, Morsch M, Cole NJ, Chung RS, Saunders DN, Yerbury JJ, Vine KL, Improving the Delivery of SOD1 Antisense Oligonucleotides to Motor Neurons Using Calcium Phosphate-Lipid Nanoparticles, Frontiers in Neuroscience, 11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [535].Ross CA, Tabrizi SJ, Huntington’s disease: from molecular pathogenesis to clinical treatment, The Lancet Neurology, 10 (2011) 83–98. [DOI] [PubMed] [Google Scholar]
- [536].Das S, Bhattacharyya NP, Huntingtin interacting protein HYPK is a negative regulator of heat shock response and is downregulated in models of Huntington’s Disease, Experimental Cell Research, 343 (2016) 107–117. [DOI] [PubMed] [Google Scholar]
- [537].Kay C, Collins J, Miedzybrodzka Z, Wright G, Madore S, Gordon E, Gerry N, Fisher E, Davidson M, Slama R, Hayden M, I2 Huntington’s disease reduced penetrance alleles occur at high frequency and affect age-related increases in prevalence, Journal of Neurology, Neurosurgery & Psychiatry, 87 (2016) A59–A60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [538].Lipe H, Bird T, Late Onset Huntington Disease: Clinical and Genetic Characteristics of 34 Cases, Journal of the neurological sciences, 276 (2009) 159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [539].Chial H, Huntington’s Disease: The Discovery of the Huntingtin Gene, Nature Education (2008). [Google Scholar]
- [540].Shannon KM, Chapter 1 - Huntington’s disease – clinical signs, symptoms, presymptomatic diagnosis, and diagnosis, in: Weiner WJ, Tolosa E (Eds.) Handbook of Clinical Neurology, Elsevier; 2011, pp. 3–13. [DOI] [PubMed] [Google Scholar]
- [541].Rosenblatt A, Ranen N, Nance M, & Paulsen J, A Physician’s Guide to the Management of Huntington’s Disease, 2 ed., New York, NY, USA: HDSA, 1999. [Google Scholar]
- [542].Warby S, Graham R, & Hayden M, Huntington Disease, 2010, April 22. [Google Scholar]
- [543].Rosenblatt A, Neuropsychiatry of Huntington’s disease, Dialogues in Clinical Neuroscience, 9 (2007) 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [544].Raj R, Sidhu BS, Dalla E, Bipolar affective disorder in Huntington’s disease: A neuropsychiatric perspective, Indian Journal of Psychiatry, 57 (2015) 107–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [545].Paulsen JS, Cognitive Impairment in Huntington Disease: Diagnosis and Treatment, Current neurology and neuroscience reports, 11 (2011) 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [546].Stout JC, Rodawalt WC, Siemers ER, Risky decision making in Huntington’s disease, J Int Neuropsychol Soc, 7 (2001) 92–101. [DOI] [PubMed] [Google Scholar]
- [547].Budworth H, McMurray CT, A Brief History of Triplet Repeat Diseases, Methods in molecular biology (Clifton, N.J.), 1010 (2013) 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [548].Ross CA, Tabrizi SJ, Huntington’s disease: from molecular pathogenesis to clinical treatment, The Lancet Neurology, 10 83–98. [DOI] [PubMed] [Google Scholar]
- [549].Schulte J, Littleton JT, The biological function of the Huntingtin protein and its relevance to Huntington’s Disease pathology, Current trends in neurology, 5 (2011) 65–78. [PMC free article] [PubMed] [Google Scholar]
- [550].Menon RP, Nethisinghe S, Faggiano S, Vannocci T, Rezaei H, Pemble S, Sweeney MG, Wood NW, Davis MB, Pastore A, Giunti P, The Role of Interruptions in polyQ in the Pathology of SCA1, PLoS Genetics, 9 (2013) e1003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [551].Kim M, Pathogenic polyglutamine expansion length correlates with polarity of the flanking sequences, Molecular Neurodegeneration, 9 (2014) 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [552].Hendricks AE, Latourelle JC, Lunetta KL, Cupples LA, Wheeler V, MacDonald ME, Gusella JF, Myers RH, Estimating the probability of de novo HD cases from transmissions of expanded penetrant CAG alleles in the Huntington disease gene from male carriers of high normal alleles (27–35 CAG), American Journal of Medical Genetics Part A, 149A (2009) 1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [553].Wexler NS, Huntington’s Disease: Advocacy Driving Science, Annual Review of Medicine, 63 (2012) 1–22. [DOI] [PubMed] [Google Scholar]
- [554].Pearson CE, Slipping while sleeping? Trinucleotide repeat expansions in germ cells, Trends Mol Med, 9 (2003) 490–495. [DOI] [PubMed] [Google Scholar]
- [555].Bathina S, Das UN, Brain-derived neurotrophic factor and its clinical implications, Archives of Medical Science : AMS, 11 (2015) 1164–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [556].Zuccato C, Cattaneo E, Role of brain-derived neurotrophic factor in Huntington’s disease, Progress in Neurobiology, 81 (2007) 294–330. [DOI] [PubMed] [Google Scholar]
- [557].Cisbani G, Cicchetti F, An in vitro perspective on the molecular mechanisms underlying mutant huntingtin protein toxicity, Cell Death & Disease, 3 (2012) e382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [558].Juenemann K, Schipper-Krom S, Wiemhoefer A, Kloss A, Sanz Sanz A, Reits EAJ, Expanded Polyglutamine-containing N-terminal Huntingtin Fragments Are Entirely Degraded by Mammalian Proteasomes, The Journal of biological chemistry, 288 (2013) 27068–27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [559].Arndt JR, Chaibva M, Legleiter J, The emerging role of the first 17 amino acids of huntingtin in Huntington’s disease, Biomolecular concepts, 6 (2015) 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [560].Chen M, Wolynes PG, Aggregation landscapes of Huntingtin exon 1 protein fragments and the critical repeat length for the onset of Huntington’s disease, Proceedings of the National Academy of Sciences of the United States of America, 114 (2017) 4406–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [561].Schilling G, Klevytska A, Tebbenkamp ATN, Juenemann K, Cooper J, Gonzales V, Slunt H, Poirer M, Ross CA, Borchelt DR, Characterization of Huntingtin Pathologic Fragments in Human Huntington Disease, Transgenic Mice, and Cell Models, Journal of Neuropathology & Experimental Neurology, 66 (2007) 313–320. [DOI] [PubMed] [Google Scholar]
- [562].Sari Y, Huntington’s Disease: From Mutant Huntingtin Protein to Neurotrophic Factor Therapy, International Journal of Biomedical Science : IJBS, 7 (2011) 89–100. [PMC free article] [PubMed] [Google Scholar]
- [563].Saleh AA, Bhadra AK, Roy I, Cytotoxicity of Mutant Huntingtin Fragment in Yeast Can Be Modulated by the Expression Level of Wild Type Huntingtin Fragment, ACS Chemical Neuroscience, 5 (2014) 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [564].Bhide PG, Day M, Sapp E, Schwarz C, Sheth A, Kim J, Young AB, Penney J, Golden J, Aronin N, DiFiglia M, Expression of Normal and Mutant Huntingtin in the Developing Brain, The Journal of Neuroscience, 16 (1996) 5523–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [565].Frank S, Treatment of Huntington’s Disease, Neurotherapeutics, 11 (2014) 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [566].Videnovic A, Treatment of Huntington Disease, Current treatment options in neurology, 15 (2013) 424–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [567].Valenza M, Chen JY, Di Paolo E, Ruozi B, Belletti D, Ferrari Bardile C, Leoni V, Caccia C, Brilli E, Di Donato S, Boido MM, Vercelli A, Vandelli MA, Forni F, Cepeda C, Levine MS, Tosi G, Cattaneo E, Cholesterol‐loaded nanoparticles ameliorate synaptic and cognitive function in Huntington’s disease mice, EMBO Molecular Medicine, 7 (2015) 1547–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [568].Debnath K, Pradhan N, Singh BK, Jana NR, Jana NR, Poly(trehalose) Nanoparticles Prevent Amyloid Aggregation and Suppress Polyglutamine Aggregation in a Huntington’s Disease Model Mouse, ACS Applied Materials & Interfaces, 9 (2017) 24126–24139. [DOI] [PubMed] [Google Scholar]
- [569].Sandhir R, Yadav A, Mehrotra A, Sunkaria A, Singh A, Sharma S, Curcumin Nanoparticles Attenuate Neurochemical and Neurobehavioral Deficits in Experimental Model of Huntington’s Disease, NeuroMolecular Medicine, 16 (2014) 106–118. [DOI] [PubMed] [Google Scholar]
- [570].Ramachandran S, Thangarajan S, A novel therapeutic application of solid lipid nanoparticles encapsulated thymoquinone (TQ-SLNs) on 3-nitroproponic acid induced Huntington’s disease-like symptoms in wistar rats, Chemico-Biological Interactions, 256 (2016) 25–36. [DOI] [PubMed] [Google Scholar]
- [571].Godinho BMDC, Malhotra M, O’Driscoll CM, Cryan JF, Delivering a disease-modifying treatment for Huntington’s disease, Drug Discovery Today, 20 (2015) 50–64. [DOI] [PubMed] [Google Scholar]
- [572].Abhishek C, Ashu J, Flint BM, Prospects for neuroprotective therapies in prodromal Huntington’s disease, Movement Disorders, 29 (2014) 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [573].Pradhan N, Jana NR, Jana NR, Inhibition of Protein Aggregation by Iron Oxide Nanoparticles Conjugated with Glutamine- and Proline-Based Osmolytes, ACS Applied Nano Materials, 1 (2018) 1094–1103. [Google Scholar]
- [574].Bhatt R, Singh D, Prakash A, Mishra N, Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington’s disease, Drug Delivery, 22 (2015) 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [575].Godinho BMDC, Ogier JR, Darcy R, O’Driscoll CM, Cryan JF, Self-assembling Modified β-Cyclodextrin Nanoparticles as Neuronal siRNA Delivery Vectors: Focus on Huntington’s Disease, Molecular Pharmaceutics, 10 (2013) 640–649. [DOI] [PubMed] [Google Scholar]
- [576].Opal T.A. Puneet, Spinocerebellar Ataxia Type 1, University of Washington, Seattle, 1993–2018. [Google Scholar]
- [577].Rossi M, Perez‐Lloret S, Doldan L, Cerquetti D, Balej J, Vernetti PM, Hawkes M, Cammarota A, Merello M, Autosomal dominant cerebellar ataxias: a systematic review of clinical features, European Journal of Neurology, 21 (2014) 607–615. [DOI] [PubMed] [Google Scholar]
- [578].Choubtum L, Witoonpanich P, Hanchaiphiboolkul S, Bhidayasiri R, Jitkritsadakul O, Pongpakdee S, Wetchaphanphesat S, Boonkongchuen P, Pulkes T, Analysis of SCA8, SCA10, SCA12, SCA17 and SCA19 in patients with unknown spinocerebellar ataxia: a Thai multicentre study, BMC Neurology, 15 (2015) 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [579].Rosenberg RN, Khemani P, Chapter 71 - The Inherited Ataxias, Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease (Fifth Edition), Academic Press, Boston, 2015, pp. 811–832. [Google Scholar]
- [580].Dohlinger S, Hauser TK, Borkert J, Luft AR, Schulz JB, Magnetic resonance imaging in spinocerebellar ataxias, Cerebellum, 7 (2008) 204–214. [DOI] [PubMed] [Google Scholar]
- [581].Öz G, Hutter D, Tkáč I, Clark HB, Gross MD, Jiang H, Eberly LE, Bushara KO, Gomez CM, Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status, Movement disorders : Journal of the Movement Disorder Society, 25 (2010) 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [582].Alves S, Régulier E, Nascimento-Ferreira I, Hassig R, Dufour N, Koeppen A, Carvalho AL, Simões S, de Lima MCP, Brouillet E, Gould VC, Déglon N, de Almeida LP, Striatal and nigral pathology in a lentiviral rat model of Machado-Joseph disease, Human Molecular Genetics, 17 (2008) 2071–2083. [DOI] [PubMed] [Google Scholar]
- [583].Scoles DR, Meera P, Schneider MD, Paul S, Dansithong W, Figueroa KP, Hung G, Rigo F, Bennett CF, Otis TS, Pulst SM, Antisense oligonucleotide therapy for spinocerebellar ataxia type 2, Nature, 544 (2017) 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [584].Harini S, Lynn SV, Treatment Options in Degenerative Cerebellar Ataxia: A Systematic Review, Movement Disorders Clinical Practice, 1 (2014) 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [585].Malhotra M, Tomaro-Duchesneau C, Prakash S, Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases, Biomaterials, 34 (2013) 1270–1280. [DOI] [PubMed] [Google Scholar]
- [586].Shiba K, Ogawa K, Torashima T, Hirai H, Akhter N, Kinuya S, Mori H, Potential usefulness of the D2R reporter gene imaging by IBF as a gene therapy monitoring for cerebellar disorder, Journal of Nuclear Medicine, 48 (2007) 242P. [DOI] [PubMed] [Google Scholar]
- [587].Bonanomi M, Natalello A, Visentin C, Pastori V, Penco A, Cornelli G, Colombo G, Malabarba MG, Doglia SM, Relini A, Regonesi ME, Tortora P, Epigallocatechin-3-gallate and tetracycline differently affect ataxin-3 fibrillogenesis and reduce toxicity in spinocerebellar ataxia type 3 model, Hum Mol Genet, 23 (2014) 6542–6552. [DOI] [PubMed] [Google Scholar]
- [588].Wang Z, Experimental and Clinical Strategies for Treating Spinocerebellar Ataxia Type 3, Neuroscience, 371 (2018) 138–154. [DOI] [PubMed] [Google Scholar]
- [589].Crawford TO, Pardo CA, The Neurobiology of Childhood Spinal Muscular Atrophy, Neurobiology of Disease, 3 (1996) 97–110. [DOI] [PubMed] [Google Scholar]
- [590].D’Amico A, Mercuri E, Tiziano FD, Bertini E, Spinal muscular atrophy, Orphanet Journal of Rare Diseases, 6 (2011) 71–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [591].Ogino S, Leonard DG, Rennert H, Ewens WJ, Wilson RB, Genetic risk assessment in carrier testing for spinal muscular atrophy, Am J Med Genet, 110 (2002) 301–307. [DOI] [PubMed] [Google Scholar]
- [592].Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, Le Paslier D, Frézal J, Cohen D, Weissenbach J, Munnich A, Melki J, Identification and characterization of a spinal muscular atrophy-determining gene, Cell, 80 (1995) 155–165. [DOI] [PubMed] [Google Scholar]
- [593].Pellizzoni L, Chaperoning ribonucleoprotein biogenesis in health and disease, EMBO Rep, 8 (2007) 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [594].Burghes AHM, Beattie CE, Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick?, Nature Reviews Neuroscience, 10 (2009) 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [595].Monani UR, Spinal Muscular Atrophy: A Deficiency in a Ubiquitous Protein; a Motor Neuron-Specific Disease, Neuron, 48 (2005) 885–895. [DOI] [PubMed] [Google Scholar]
- [596].Burnett BG, Crawford TO, Sumner CJ, Emerging treatment options for spinal muscular atrophy, Curr Treat Options Neurol, 11 (2009) 90–101. [DOI] [PubMed] [Google Scholar]
- [597].Schmid A, DiDonato CJ, Animal Models of Spinal Muscular Atrophy, Journal of Child Neurology, 22 (2007) 1004–1012. [DOI] [PubMed] [Google Scholar]
- [598].Arnold WD, Kassar D, Kissel JT, Spinal Muscular Atrophy: Diagnosis and Management in a New Therapeutic Era, Muscle & nerve, 51 (2015) 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [599].Baioni MTC, Ambiel CR, Atrofia muscular espinhal: diagnóstico, tratamento e perspectivas futuras, Jornal de Pediatria, 86 (2010) 261–270. [DOI] [PubMed] [Google Scholar]
- [600].Chiriboga CA, Nusinersen for the treatment of spinal muscular atrophy, Expert Rev Neurother, 17 (2017) 955–962. [DOI] [PubMed] [Google Scholar]
- [601].Shi Y, Ivannikov MV, Walsh ME, Liu Y, Zhang Y, Jaramillo CA, Macleod GT, Van Remmen H, The Lack of CuZnSOD Leads to Impaired Neurotransmitter Release, Neuromuscular Junction Destabilization and Reduced Muscle Strength in Mice, PLOS ONE, 9 (2014) e100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [602].Shabanpoor F, Hammond SM, Abendroth F, Hazell G, Wood MJA, Gait MJ, Identification of a Peptide for Systemic Brain Delivery of a Morpholino Oligonucleotide in Mouse Models of Spinal Muscular Atrophy, Nucleic Acid Therapeutics, 27 (2017) 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [603].Singh J, Salcius M, Liu S-W, Staker BL, Mishra R, Thurmond J, Michaud G, Mattoon DR, Printen J, Christensen J, Bjornsson JM, Pollok BA, Kiledjian M, Stewart L, Jarecki J, Gurney ME, DcpS as a Therapeutic Target for Spinal Muscular Atrophy, ACS Chemical Biology, 3 (2008) 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [604].Fangerau T, Schimrigk S, Haupts M, Kaeder M, Ahle G, Brune N, Klinkenberg K, Kotterba S, Möhring M, Sindern E, Diagnosis of multiple sclerosis: comparison of the Poser criteria and the new McDonald criteria, Acta Neurologica Scandinavica, 109 (2004) 385–389. [DOI] [PubMed] [Google Scholar]
- [605].Ellidag HY, Eren E, Erdogan N, Ture S, Yilmaz N, Comparison of neurophysiological and MRI findings of patients with multiple sclerosis using oligoclonal band technique, Annals of Neurosciences, 20 (2013) 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [606].McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS, Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis, Ann Neurol, 50 (2001) 121–127. [DOI] [PubMed] [Google Scholar]
- [607].Ahmadpour-Yazdi H, Hormozi-Nezhad MR, Abadi AR, Sanati MH, Kazemi B, Colourimetric-based method for the diagnosis of spinal muscular atrophy using gold nanoprobes, IET Nanobiotechnol, 9 (2015) 5–10. [DOI] [PubMed] [Google Scholar]
- [608].Compston A, Coles A, Multiple sclerosis, The Lancet, 372 (2008) 1502–1517. [DOI] [PubMed] [Google Scholar]
- [609].Weinshenker BG, Epidemiology of multiple sclerosis, Neurol Clin, 14 (1996) 291–308. [DOI] [PubMed] [Google Scholar]
- [610].Hauser SL, Goodin DS, Chapter 380. Multiple Sclerosis and Other Demyelinating Diseases, in: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J (Eds.) Harrison’s Principles of Internal Medicine, 18e, The McGraw-Hill Companies, New York, NY, 2012. [Google Scholar]
- [611].Koch-Henriksen N, Sørensen PS, The changing demographic pattern of multiple sclerosis epidemiology, The Lancet Neurology, 9 (2010) 520–532. [DOI] [PubMed] [Google Scholar]
- [612].Loma I, Heyman R, Multiple Sclerosis: Pathogenesis and Treatment, Current Neuropharmacology, 9 (2011) 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [613].Miller DH, Chard DT, Ciccarelli O, Clinically isolated syndromes, The Lancet Neurology, 11 (2012) 157–169. [DOI] [PubMed] [Google Scholar]
- [614].Stephens M, Donnelly P, A Comparison of Bayesian Methods for Haplotype Reconstruction from Population Genotype Data, The American Journal of Human Genetics, 73 (2003) 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [615].Ascherio A, Munger KL, Environmental risk factors for multiple sclerosis. Part I: The role of infection, Annals of Neurology, 61 (2007) 288–299. [DOI] [PubMed] [Google Scholar]
- [616].Singh AV, Zamboni P, Anomalous venous blood flow and iron deposition in multiple sclerosis, Journal of cerebral blood flow and metabolism 29 (2009) 1867–1878. [DOI] [PubMed] [Google Scholar]
- [617].Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H, The relation between inflammation and neurodegeneration in multiple sclerosis brains, Brain, 132 (2009) 1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [618].Katzberg HD, Khan AH, So YT, Assessment: Symptomatic treatment for muscle cramps (an evidence-based review), Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology, 74 (2010) 691–696. [DOI] [PubMed] [Google Scholar]
- [619].Frohman EM, Racke MK, Raine CS, Multiple Sclerosis — The Plaque and Its Pathogenesis, New England Journal of Medicine, 354 (2006) 942–955. [DOI] [PubMed] [Google Scholar]
- [620].Wuest SC, Edwan JH, Martin JF, Han S, Perry JSA, Cartagena CM, Matsuura E, Maric D, Waldmann TA, Bielekova B, A role for interleukin-2 trans-presentation in dendritic cell–mediated T cell activation in humans, as revealed by daclizumab therapy, Nature Medicine, 17 (2011) 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [621].Kaur G, Trowsdale J, Fugger L, Natural killer cells and their receptors in multiple sclerosis, Brain, 136 (2013) 2657–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [622].Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, Martin R, Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand, Nature Medicine, 6 (2000) 1167. [DOI] [PubMed] [Google Scholar]
- [623].Tsang BK, Macdonell R, Multiple sclerosis- diagnosis, management and prognosis, Aust Fam Physician, 40 (2011) 948–955. [PubMed] [Google Scholar]
- [624].Xu Z, Zhang F, Sun F, Gu K, Dong S, He D, Dimethyl fumarate for multiple sclerosis, Cochrane Database of Systematic Reviews, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [625].Karaborni S, Chen Mao, Gwozdz Garry T., Nanoparticle compositions of dimethyl fumarate, in: I. XENOPORT (Ed.) US patent Xenoport, Inc., USA, March/19/2015. [Google Scholar]
- [626].Santamaria P, Nanoparticle compositions for sustained therapy, UTI LP US 2016-November-10.
- [627].Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L, Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications, Oxidative Medicine and Cellular Longevity, 2017 (2017) 2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [628].Shoulson I, Where do we stand on neuroprotection? Where do we go from here?, Mov Disord, 13 Suppl 1 (1998) 46–48. [PubMed] [Google Scholar]
- [629].Albarracin SL, Stab B, Casas Z, Sutachan JJ, Samudio I, Gonzalez J, Gonzalo L, Capani F, Morales L, Barreto GE, Effects of natural antioxidants in neurodegenerative disease, Nutr Neurosci, 15 (2012) 1–9. [DOI] [PubMed] [Google Scholar]
- [630].Rao AV, Balachandran B, Role of oxidative stress and antioxidants in neurodegenerative diseases, Nutr Neurosci, 5 (2002) 291–309. [DOI] [PubMed] [Google Scholar]
- [631].Levi MS, Brimble MA, A review of neuroprotective agents, Curr Med Chem, 11 (2004) 2383–2397. [DOI] [PubMed] [Google Scholar]
- [632].Shahripour R. Bavarsad, Harrigan MR, Alexandrov AV, N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities, Brain and Behavior, 4 (2014) 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [633].Spector R, Johanson CE, REVIEW: Vitamin transport and homeostasis in mammalian brain: focus on Vitamins B and E, Journal of Neurochemistry, 103 (2007) 425–438. [DOI] [PubMed] [Google Scholar]
- [634].Agus DB, Gambhir SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, Golde DW, Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters, The Journal of Clinical Investigation, 100 (1997) 2842–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [635].Merav BS, Yossi GS, Daniel O, Hana P, Ann S, Nili KG, Aari B, Daphne A, Eldad M, A novel thiol antioxidant that crosses the blood brain barrier protects dopaminergic neurons in experimental models of Parkinson’s disease, European Journal of Neuroscience, 21 (2005) 637–646. [DOI] [PubMed] [Google Scholar]
- [636].Caputo F, Mameli M, Sienkiewicz A, Licoccia S, Stellacci F, Ghibelli L, Traversa E, A novel synthetic approach of cerium oxide nanoparticles with improved biomedical activity, Scientific Reports, 7 (2017) 4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [637].Das M, Patil S, Bhargava N, Kang J-F, Riedel LM, Seal S, Hickman JJ, Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons, Biomaterials, 28 (2007) 1918–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [638].Singh NA, Mandal AKA, Khan ZA, Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG), Nutrition Journal, 15 (2016) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [639].Xu Y, Zhang Y, Quan Z, Wong W, Guo J, Zhang R, Yang Q, Dai R, McGeer PL, Qing H, Epigallocatechin Gallate (EGCG) Inhibits Alpha-Synuclein Aggregation: A Potential Agent for Parkinson’s Disease, Neurochem Res, 41 (2016) 2788–2796. [DOI] [PubMed] [Google Scholar]
- [640].Weinreb O, Amit T, Mandel S, Youdim MBH, Neuroprotective molecular mechanisms of (−)-epigallocatechin-3-gallate: a reflective outcome of its antioxidant, iron chelating and neuritogenic properties, Genes & Nutrition, 4 (2009) 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [641].Mähler A, Mandel S, Lorenz M, Ruegg U, Wanker EE, Boschmann M, Paul F, Epigallocatechin-3-gallate: a useful, effective and safe clinical approach for targeted prevention and individualised treatment of neurological diseases?, The EPMA Journal, 4 (2013) 5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [642].Rezai-Zadeh K, Arendash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, Shytle RD, Tan J, Green tea epigallocatechin-3-gallate (EGCG) reduces β-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice, Brain Research, 1214 (2008) 177–187. [DOI] [PubMed] [Google Scholar]
- [643].Cascella M, Bimonte S, Muzio MR, Schiavone V, Cuomo A, The efficacy of Epigallocatechin-3-gallate (green tea) in the treatment of Alzheimer’s disease: an overview of pre-clinical studies and translational perspectives in clinical practice, Infectious Agents and Cancer, 12 (2017) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [644].Nelson F, Isabel C, Rosário DM, Rui V, Margarida B, Guangyue B, João SM, Rosário AM, Binding of epigallocatechin‐3‐gallate to transthyretin modulates its amyloidogenicity, FEBS Letters, 583 (2009) 3569–3576. [DOI] [PubMed] [Google Scholar]
- [645].Ehrnhoefer DE, Duennwald M, Markovic P, Wacker JL, Engemann S, Roark M, Legleiter J, Marsh JL, Thompson LM, Lindquist S, Muchowski PJ, Wanker EE, Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models, Hum Mol Genet, 15 (2006) 2743–2751. [DOI] [PubMed] [Google Scholar]
- [646].Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE, EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity, Proceedings of the National Academy of Sciences, 107 (2010) 7710–7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [647].Smith A, Giunta B, Bickford PC, Fountain M, Tan J, Shytle RD, Nanolipidic particles improve the bioavailability and α-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer’s disease, International Journal of Pharmaceutics, 389 (2010) 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [648].Siddiqui IA, Mukhtar H, Nanochemoprevention by Bioactive Food Components: A Perspective, Pharmaceutical research, 27 (2010) 1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [649].Godoi GL, de Oliveira Porciúncula L, Schulz JF, Kaufmann FN, da Rocha JB, de Souza DOG, Ghisleni G, de Almeida HL, Selenium Compounds Prevent Amyloid β-Peptide Neurotoxicity in Rat Primary Hippocampal Neurons, Neurochemical Research, 38 (2013) 2359–2363. [DOI] [PubMed] [Google Scholar]
- [650].Bellinger FP, Raman AV, Reeves MA, Berry MJ, Regulation and function of selenoproteins in human disease, The Biochemical journal, 422 (2009) 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [651].Zhang J, Zhou X, Yu Q, Yang L, Sun D, Zhou Y, Liu J, Epigallocatechin-3-gallate (EGCG)-Stabilized Selenium Nanoparticles Coated with Tet-1 Peptide To Reduce Amyloid-β Aggregation and Cytotoxicity, ACS Applied Materials & Interfaces, 6 (2014) 8475–8487. [DOI] [PubMed] [Google Scholar]
- [652].Esteves M, Cristóvão AC, Saraiva T, Rocha SM, Baltazar G, Ferreira L, Bernardino L, Retinoic acid-loaded polymeric nanoparticles induce neuroprotection in a mouse model for Parkinson’s disease, Frontiers in Aging Neuroscience, 7 (2015) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [653].Wu J, Ding T, Sun J, Neurotoxic potential of iron oxide nanoparticles in the rat brain striatum and hippocampus, Neurotoxicology, 34 (2013) 243–253. [DOI] [PubMed] [Google Scholar]
- [654].Han D, Tian Y, Zhang T, Ren G, Yang Z, Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in hippocampus of Wistar rats, International Journal of Nanomedicine, 6 (2011) 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [655].Chen J, Dong X, Xin Y, Zhao M, Effects of titanium dioxide nano-particles on growth and some histological parameters of zebrafish (Danio rerio) after a long-term exposure, Aquatic Toxicology, 101 (2011) 493–499. [DOI] [PubMed] [Google Scholar]
- [656].Gref R, Lück M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Müller RH, ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption, Colloids and Surfaces B: Biointerfaces, 18 (2000) 301–313. [DOI] [PubMed] [Google Scholar]
- [657].Chang J, Jallouli Y, Kroubi M, Yuan X.-b., Feng W, Kang C.-s., Pu P.-y., Betbeder D, Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood–brain barrier, International Journal of Pharmaceutics, 379 (2009) 285–292. [DOI] [PubMed] [Google Scholar]
- [658].Oyewumi MO, Liu S, Moscow JA, Mumper RJ, Specific Association of Thiamine-Coated Gadolinium Nanoparticles with Human Breast Cancer Cells Expressing Thiamine Transporters, Bioconjugate Chemistry, 14 (2003) 404–411. [DOI] [PubMed] [Google Scholar]
- [659].Grover A, Hirani A, Pathak Y, Sutariya V, Brain-Targeted Delivery of Docetaxel by Glutathione-Coated Nanoparticles for Brain Cancer, AAPS PharmSciTech, 15 (2014) 1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [660].Fabbro A, Cellot G, Prato M, Ballerini L, Chapter 18 - Interfacing neurons with carbon nanotubes:: (re)engineering neuronal signaling, in: Schouenborg J, Garwicz M, Danielsen N (Eds.) Progress in Brain Research, Elsevier; 2011, pp. 241–252. [DOI] [PubMed] [Google Scholar]
- [661].Ekinci KL, Yakhot V, Rajauria S, Colosqui C, Karabacak DM, High-frequency nanofluidics: a universal formulation of the fluid dynamics of MEMS and NEMS, Lab on a Chip, 10 (2010) 3013–3025. [DOI] [PubMed] [Google Scholar]
- [662].Wang H, Mararenko A, Cao G, Gai Z, Hong K, Banerjee P, Zhou S, Multifunctional 1D Magnetic and Fluorescent Nanoparticle Chains for Enhanced MRI, fluorescent Cell Imaging, And Combined Photothermal/Chemotherapy, ACS Applied Materials & Interfaces, 6 (2014) 15309–15317. [DOI] [PubMed] [Google Scholar]
- [663].Carvalho A, Martins MBF, Corvo ML, Feio G, Enhanced contrast efficiency in MRI by PEGylated magnetoliposomes loaded with PEGylated SPION: Effect of SPION coating and micro-environment, Materials Science and Engineering: C, 43 (2014) 521–526. [DOI] [PubMed] [Google Scholar]
- [664].Medina C, Santos‐Martinez MJ, Radomski A, Corrigan OI, Radomski MW, Nanoparticles: pharmacological and toxicological significance, British Journal of Pharmacology, 150 (2007) 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [665].Repar N, Li H, Aguilar JS, Li QQ, Drobne D, Hong Y, Silver nanoparticles induce neurotoxicity in a human embryonic stem cell-derived neuron and astrocyte network, Nanotoxicology, 12 (2018) 104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [666].Mirsattari SM, Hammond RR, Sharpe MD, Leung FY, Young GB, Myoclonic status epilepticus following repeated oral ingestion of colloidal silver, Neurology, 62 (2004) 1408–1410. [DOI] [PubMed] [Google Scholar]
- [667].Shimizu M, Tainaka H, Oba T, Mizuo K, Umezawa M, Takeda K, Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse, Particle and Fibre Toxicology, 6 (2009) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [668].Mohammadipour A, Fazel A, Haghir H, Motejaded F, Rafatpanah H, Zabihi H, Hosseini M, Bideskan AE, Maternal exposure to titanium dioxide nanoparticles during pregnancy; impaired memory and decreased hippocampal cell proliferation in rat offspring, Environmental Toxicology and Pharmacology, 37 (2014) 617–625. [DOI] [PubMed] [Google Scholar]
- [669].Wilhelmi V, Fischer U, Weighardt H, Schulze-Osthoff K, Nickel C, Stahlmecke B, Kuhlbusch TAJ, Scherbart AM, Esser C, Schins RPF, Albrecht C, Zinc Oxide Nanoparticles Induce Necrosis and Apoptosis in Macrophages in a p47phox- and Nrf2-Independent Manner, PLoS ONE, 8 (2013) e65704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [670].Weissleder R, Nahrendorf M, Pittet MJ, Imaging macrophages with nanoparticles, Nat Mater, 13 (2014) 125–138. [DOI] [PubMed] [Google Scholar]
- [671].Patel SK, Janjic JM, Macrophage targeted theranostics as personalized nanomedicine strategies for inflammatory diseases, Theranostics, 5 (2015) 150–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [672].Chouly C, Pouliquen D, Lucet I, Jeune JJ, Jallet P, Development of superparamagnetic nanoparticles for MRI: effect of particle size, charge and surface nature on biodistribution, Journal of Microencapsulation, 13 (1996) 245–255. [DOI] [PubMed] [Google Scholar]
- [673].Shebl RI, Farouk F, Azzazy HME-S, Effect of Surface Charge and Hydrophobicity Modulation on the Antibacterial and Antibiofilm Potential of Magnetic Iron Nanoparticles, Journal of Nanomaterials, 2017 (2017) 15. [Google Scholar]
- [674].Mohammed L, Gomaa HG, Ragab D, Zhu J, Magnetic nanoparticles for environmental and biomedical applications: A review, Particuology, 30 (2017) 1–14. [Google Scholar]
- [675].Doshi N, Mitragotri S, Macrophages recognize size and shape of their targets, PLoS One, 5 (2010) e10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [676].Nowacek AS, Balkundi S, McMillan J, Roy U, Martinez-Skinner A, Mosley RL, Kanmogne G, Kabanov AV, Bronich T, Gendelman HE, Analyses of nanoformulated antiretroviral drug charge, size, shape and content for uptake, drug release and antiviral activities in human monocyte-derived macrophages, Journal of controlled release 150 (2011) 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [677].MacParland SA, Tsoi KM, Ouyang B, Ma XZ, Manuel J, Fawaz A, Ostrowski MA, Alman BA, Zilman A, Chan WC, McGilvray ID, Phenotype Determines Nanoparticle Uptake by Human Macrophages from Liver and Blood, ACS Nano, 11 (2017) 2428–2443. [DOI] [PubMed] [Google Scholar]
- [678].Batrakova EV, Gendelman HE, Kabanov AV, Cell-mediated drug delivery, Expert Opin Drug Deliv, 8 (2011) 415–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [679].Batrakova EV, Li S, Brynskikh AM, Sharma AK, Li Y, Boska M, Gong N, Mosley RL, Alakhov VY, Gendelman HE, Kabanov AV, Effects of pluronic and doxorubicin on drug uptake, cellular metabolism, apoptosis and tumor inhibition in animal models of MDR cancers, Journal of controlled release 143 (2010) 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [680].McMillan J, Batrakova E, Gendelman HE, Cell delivery of therapeutic nanoparticles, Prog Mol Biol Transl Sci, 104 (2011) 563–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [681].McMillan J, Batrakova E, Gendelman HE, Chapter 14 - Cell Delivery of Therapeutic Nanoparticles, in: Villaverde A (Ed.) Progress in Molecular Biology and Translational Science, Academic Press; 2011, pp. 563–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [682].Singh D, McMillan JM, Liu X-M, Vishwasrao HM, Kabanov AV, Sokolsky-Papkov M, Gendelman HE, Formulation design facilitates magnetic nanoparticle delivery to diseased cells and tissues, Nanomedicine, 9 (2014) 469–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [683].Singh D, McMillan JoEllyn M., Kabanov AV, Sokolsky-Papkov M, Gendelman HE, Bench-to-bedside translation of magnetic nanoparticles, Nanomedicine, 9 (2014) 501–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [684].Ferreira LK, Rondina JM, Kubo R, Ono CR, Leite CC, Smid J, Bottino C, Nitrini R, Busatto GF, Duran FL, Buchpiguel CA, Support vector machine-based classification of neuroimages in Alzheimer’s disease: direct comparison of FDG-PET, rCBF-SPECT and MRI data acquired from the same individuals, Rev Bras Psiquiatr, (2017) 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [685].Vasquez KO, Casavant C, Peterson JD, Quantitative Whole Body Biodistribution of Fluorescent-Labeled Agents by Non-Invasive Tomographic Imaging, PLOS ONE, 6 (2011) e20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [686].Bruckman MA, Randolph LN, VanMeter A, Hern S, Shoffstall AJ, Taurog RE, Steinmetz NF, Biodistribution, pharmacokinetics, and blood compatibility of native and PEGylated tobacco mosaic virus nano-rods and -spheres in mice, Virology, 449 (2014) 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [687].von Roemeling C, Jiang W, Chan CK, Weissman IL, Kim BYS, Breaking Down the Barriers to Precision Cancer Nanomedicine, Trends in Biotechnology, 35 (2017) 159–171. [DOI] [PubMed] [Google Scholar]
- [688].Wolfbeis OS, An overview of nanoparticles commonly used in fluorescent bioimaging, Chem Soc Rev, 44 (2015) 4743–4768. [DOI] [PubMed] [Google Scholar]
- [689].Angelakeris M, Magnetic nanoparticles: A multifunctional vehicle for modern theranostics, Biochimica et Biophysica Acta (BBA) - General Subjects, 1861 (2017) 1642–1651. [DOI] [PubMed] [Google Scholar]
- [690].Estelrich J, Sanchez-Martin MJ, Busquets MA, Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents, Int J Nanomedicine, 10 (2015) 1727–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [691].Chen G, Qiu H, Prasad PN, Chen X, Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics, Chemical Reviews, 114 (2014) 5161–5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [692].Fuhrmann K, Gauthier MA, Leroux J-C, Targeting of Injectable Drug Nanocrystals, Molecular Pharmaceutics, 11 (2014) 1762–1771. [DOI] [PubMed] [Google Scholar]
- [693].Valencia PM, Basto PA, Zhang L, Rhee M, Langer R, Farokhzad OC, Karnik R, Single-Step Assembly of Homogenous Lipid−Polymeric and Lipid−Quantum Dot Nanoparticles Enabled by Microfluidic Rapid Mixing, ACS Nano, 4 (2010) 1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [694].Shen Z, Wu A, Chen X, Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging, Mol Pharm, 14 (2017) 1352–1364. [DOI] [PubMed] [Google Scholar]
- [695].Gupta AK, Gupta M, Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications, Biomaterials, 26 (2005) 3995–4021. [DOI] [PubMed] [Google Scholar]
- [696].Kattel K, Park JY, Xu W, Kim HG, Lee EJ, Bony BA, Heo WC, Jin S, Baeck JS, Chang Y, Kim TJ, Bae JE, Chae KS, Lee GH, Paramagnetic dysprosium oxide nanoparticles and dysprosium hydroxide nanorods as T2 MRI contrast agents, Biomaterials, 33 (2012) 3254–3261. [DOI] [PubMed] [Google Scholar]
- [697].Li J, Wu C, Hou P, Zhang M, Xu K, One-pot preparation of hydrophilic manganese oxide nanoparticles as T1 nano-contrast agent for molecular magnetic resonance imaging of renal carcinoma in vitro and in vivo, Biosensors and Bioelectronics, 102 (2018) 1–8. [DOI] [PubMed] [Google Scholar]
- [698].Mimun LC, Ajithkumar G, Pokhrel M, Yust BG, Elliott ZG, Pedraza F, Dhanale A, Tang L, Lin AL, Dravid VP, Sardar DK, Bimodal Imaging Using Neodymium Doped Gadolinium Fluoride Nanocrystals with Near-Infrared to Near-Infrared Downconversion Luminescence and Magnetic Resonance Properties, J Mater Chem B Mater Biol Med, 1 (2013) 5702–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [699].Reddy LH, Arias JL, Nicolas J, Couvreur P, Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications, Chem Rev, 112 (2012) 5818–5878. [DOI] [PubMed] [Google Scholar]
- [700].Kuhn DA, Vanhecke D, Michen B, Blank F, Gehr P, Petri-Fink A, Rothen-Rutishauser B, Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages, Beilstein Journal of Nanotechnology, 5 (2014) 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [701].Gorantla S, Dou H, Boska M, Destache CJ, Nelson J, Poluektova L, Rabinow BE, Gendelman HE, Mosley RL, Quantitative magnetic resonance and SPECT imaging for macrophage tissue migration and nanoformulated drug delivery, J Leukoc Biol, 80 (2006) 1165–1174. [DOI] [PubMed] [Google Scholar]
- [702].Gorantla S, Poluektova L, Gendelman HE, Rodent models for HIV-associated neurocognitive disorders, Trends in Neurosciences, 35 (2012) 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [703].Eggleston H, Panizzi P, Molecular imaging of bacterial infections in vivo: the discrimination of infection from inflammation, Informatics (MDPI), 1 (2014) 72–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [704].Petry KG, Boiziau C, Dousset V, Brochet B, Magnetic resonance imaging of human brain macrophage infiltration, Neurotherapeutics, 4 (2007) 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [705].Brochet B, Dousset V, Deloire M, Boiziau C, Petry KG, MRI to predict severe tissue damage in inflammatory lesions in animal models of multiple sclerosis, Brain, 131 (2008) e92–e92. [DOI] [PubMed] [Google Scholar]
- [706].Serkova NJ, Nanoparticle-Based Magnetic Resonance Imaging on Tumor-Associated Macrophages and Inflammation, Front Immunol, 8 (2017) 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [707].Daldrup-Link HE, Golovko D, Ruffell B, Denardo DG, Castaneda R, Ansari C, Rao J, Tikhomirov GA, Wendland MF, Corot C, Coussens LM, MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles, Clinical cancer research 17 (2011) 5695–5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [708].Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R, Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis, Circulation, 117 (2008) 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [709].Kratz JD, Chaddha A, Bhattacharjee S, Goonewardena SN, Atherosclerosis and Nanotechnology: Diagnostic and Therapeutic Applications, Cardiovascular drugs and therapyc/sponsored by the International Society of Cardiovascular Pharmacotherapy, 30 (2016) 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [710].Al Faraj A, Preferential magnetic nanoparticle uptake by bone marrow derived macrophages sub-populations: effect of surface coating on polarization, toxicity, and in vivo MRI detection, Journal of Nanoparticle Research, 15 (2013) 1797. [Google Scholar]
- [711].Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Corot C, Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10, Invest Radiol, 39 (2004) 56–63. [DOI] [PubMed] [Google Scholar]
- [712].Morishige K, Kacher DF, Libby P, Josephson L, Ganz P, Weissleder R, Aikawa M, High-resolution magnetic resonance imaging enhanced with superparamagnetic nanoparticles measures macrophage burden in atherosclerosis, Circulation, 122 (2010) 1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [713].Lipinski MJ, Frias JC, Amirbekian V, Briley-Saebo KC, Mani V, Samber D, Abbate A, Aguinaldo JG, Massey D, Fuster V, Vetrovec GW, Fayad ZA, Macrophage-specific lipid-based nanoparticles improve cardiac magnetic resonance detection and characterization of human atherosclerosis, JACC Cardiovasc Imaging, 2 (2009) 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [714].Bagalkot V, Badgeley MA, Kampfrath T, Deiuliis JA, Rajagopalan S, Maiseyeu A, Hybrid nanoparticles improve targeting to inflammatory macrophages through phagocytic signals, Journal of controlled release 217 (2015) 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [715].Wiart M, Davoust N, Pialat JB, Berthezene Y, Nighoghossian N, Magnetic resonance imaging (MRI) of inflammation in stroke, Conf Proc IEEE Eng Med Biol Soc, 2007 (2007) 4316–4319. [DOI] [PubMed] [Google Scholar]
- [716].Al Faraj A, Shaik A. Sultana, Pureza MA, Alnafea M, Halwani R, Preferential macrophage recruitment and polarization in LPS-induced animal model for COPD: noninvasive tracking using MRI, PLoS One, 9 (2014) e90829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [717].Dousset V, Delalande C, Ballarino L, Quesson B, Seilhan D, Coussemacq M, Thiaudiére E, Brochet B, Canioni P, Caillé JM, In vivo macrophage activity imaging in the central nervous system detected by magnetic resonance, Magnetic Resonance in Medicine, 41 (1999) 329–333. [DOI] [PubMed] [Google Scholar]
- [718].Keliher EJ, Ye Y-X, Wojtkiewicz GR, Aguirre AD, Tricot B, Senders ML, Groenen H, Fay F, Perez-Medina C, Calcagno C, Carlucci G, Reiner T, Sun Y, Courties G, Iwamoto Y, Kim H-Y, Wang C, Chen JW, Swirski FK, Wey H-Y, Hooker J, Fayad ZA, Mulder WJM, Weissleder R, Nahrendorf M, Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischaemic heart disease, Nature Communications, 8 (2017) 14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [719].Foss CA, Liu L, Mease RC, Wang H, Pasricha P, Pomper MG, Imaging Macrophage Accumulation in a Murine Model of Chronic Pancreatitis with (125)I-Iodo-DPA-713 SPECT/CT, Journal of Nuclear Medicine, 58 (2017) 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [720].Van De Wiele C, Sathekge M, Maes A, Targeting monocytes and macrophages by means of SPECT and PET, The quarterly journal of nuclear medicine and molecular imaging 58 (2014) 269–275. [PubMed] [Google Scholar]
- [721].Pérez-Medina C, Tang J, Abdel-Atti D, Hogstad B, Merad M, Fisher EA, Fayad ZA, Lewis JS, Mulder WJM, Reiner T, PET Imaging of Tumor-Associated Macrophages with (89)Zr-Labeled High-Density Lipoprotein Nanoparticles, Journal of nuclear medicine, 56 (2015) 1272–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [722].Baek SK, Makkouk AR, Krasieva T, Sun CH, Madsen SJ, Hirschberg H, Photothermal treatment of glioma; an in vitro study of macrophage-mediated delivery of gold nanoshells, J Neurooncol, 104 (2011) 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [723].Stockhofe K, Postema J, Schieferstein H, Ross T, Radiolabeling of Nanoparticles and Polymers for PET Imaging, Pharmaceuticals, 7 (2014) 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [724].Locatelli E, Gil L, Israel LL, Passoni L, Naddaka M, Pucci A, Reese T, Gomez-Vallejo V, Milani P, Matteoli M, Llop J, Lellouche JP, Franchini MC, Biocompatible nanocomposite for PET/MRI hybrid imaging, International Journal of Nanomedicine, 7 (2012) 6021–6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [725].Singh A, Srivastava R, Wali S, Agarwal A, Long term outcome of surgical treatment of fractures of pelvis, Journal of Oral and Maxillofacial Radiology, 1 (2013) 37–42. [Google Scholar]
- [726].Luehmann HP, Pressly ED, Detering L, Wang C, Pierce R, Woodard PK, Gropler RJ, Hawker CJ, Liu Y, PET/CT Imaging of Chemokine Receptor CCR5 in Vascular Injury Model Using Targeted Nanoparticle, Journal of Nuclear Medicine, 55 (2014) 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [727].Farwell MD, Pryma DA, Mankoff DA, PET/CT imaging in cancer: Current applications and future directions, Cancer, 120 (2014) 3433–3445. [DOI] [PubMed] [Google Scholar]
- [728].Cheng D, Li X, Zhang C, Tan H, Wang C, Pang L, Shi H, Detection of Vulnerable Atherosclerosis Plaques with a Dual-Modal Single-Photon-Emission Computed Tomography/Magnetic Resonance Imaging Probe Targeting Apoptotic Macrophages, ACS Applied Materials & Interfaces, 7 (2015) 2847–2855. [DOI] [PubMed] [Google Scholar]
- [729].Cheng D, Li X, Tan H, Meng L, Pang L, Shi H, Comparing 18F-FDG PET and 99mTc-labeled ultrasmall superparamagnetic iron oxide-conjugated annexin V SPECT/CT/MR imaging apoptosis in atherosclerotic plaques, Journal of Nuclear Medicine, 55 (2014) 1705. [Google Scholar]
- [730].Venneti S, Lopresti BJ, Wang G, Bissel SJ, Mathis CA, Meltzer CC, Boada F, Capuano S 3rd, Kress GJ, Davis DK, Ruszkiewicz J, Reynolds IJ, Murphey-Corb M, Trichel AM, Wisniewski SR, Wiley CA, PET imaging of brain macrophages using the peripheral benzodiazepine receptor in a macaque model of neuroAIDS, J Clin Invest, 113 (2004) 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]