Significance
This study illustrates the dynamics of the oral microbiome during long-term starvation. After an initial ecological collapse, only three species were recoverable and displayed significant transcriptional activity: Klebsiella pneumoniae, Klebsiella oxytoca, and Providencia alcalifaciens. Klebsiella spp. are significant human pathogens and are frequently resistant to multiple classes of antibiotics. In addition to its status as a clinical scourge in its own right, K. pneumoniae has emerged as a chief facilitator in the transfer of drug resistance genes from the environment to pathogens. Hospital surfaces contaminated with oral fluids are well-documented sources of outbreaks of drug-resistant Enterobacteriaceae; therefore, the ability of Klebsiella to outcompete its neighbors during starvation and survive long-term in saliva is particularly noteworthy.
Keywords: oral microbiome, microbial ecology, Klebsiella
Abstract
It is well-understood that many bacteria have evolved to survive catastrophic events using a variety of mechanisms, which include expression of stress-response genes, quiescence, necrotrophy, and metabolic advantages obtained through mutation. However, the dynamics of individuals leveraging these abilities to gain a competitive advantage in an ecologically complex setting remain unstudied. In this study, we observed the saliva microbiome throughout the ecological perturbation of long-term starvation, allowing only the species best equipped to access and use the limited resources to survive. During the first several days, the community underwent a death phase that resulted in a ∼50–100-fold reduction in the number of viable cells. Interestingly, after this death phase, only three species, Klebsiella pneumoniae, Klebsiella oxytoca, and Providencia alcalifaciens, all members of the family Enterobacteriaceae, appeared to be transcriptionally active and recoverable. Klebsiella are significant human pathogens, frequently resistant to multiple antibiotics, and recently, ectopic colonization of the gut by oral Klebsiella was documented to induce dysbiosis and inflammation. MetaOmics analyses provided several leads for further investigation regarding the ecological success of the Enterobacteriaceae. The isolates accumulated single nucleotide polymorphisms in known growth advantage in stationary phase alleles and produced natural products closely resembling antimicrobial cyclic depsipeptides. The results presented in this study suggest that pathogenic Enterobacteriaceae persist much longer than their more benign neighbors in the salivary microbiome when faced with starvation. This is particularly significant, given that hospital surfaces contaminated with oral fluids, especially sinks and drains, are well-established sources of outbreaks of drug-resistant Enterobacteriaceae.
Many bacteria are well-equipped to deal with exposure to adverse environmental events such as starvation, oxidative stress, and antimicrobials, as well as fluctuations in temperature, pH, and osmolality. Diverse strategies for coping with these stresses have evolved, including expression of stress response genes (1), quiescence (2), necrotrophy (3), and growth advantages gained through mutation (4). Although these systems are increasingly understood, little is known regarding the dynamics of individual species leveraging these abilities to gain a competitive advantage in an ecologically complex setting. In the case of long-term starvation, the bulk of research on bacterial dynamics and survival mechanisms has been performed using monospecies cultures of Escherichia coli (4–9). In E. coli, populations exhibited a death phase at ∼3 d of starvation, resulting in a loss of >99% of the population (4). This death phase was followed by a long-term stationary phase, in which viable cell counts plateau virtually indefinitely (i.e., for years) (4). During the long-term stationary phase, various subpopulations carrying advantageous mutations (growth advantage in stationary phase [GASP] mutants) arose and came to dominate the culture, displacing their less-fit siblings (4). These mutations frequently resulted in increased ability to catabolize one or more amino acids as a source of carbon and energy (4). Therefore, it was likely that in a complex multispecies community, a succession of species and strains would increase and decrease in relative abundance throughout the course of the experiment, based on which species were most fit in the changing environment.
To our knowledge, there have been no previous reports on the interplay of a complex community of human-associated bacteria subjected to long-term starvation. To begin to address this knowledge gap, the present study monitored a saliva-derived complex oral microbiota during starvation in saline solution and saliva for 100 d. The bacterial community residing in the human oral cavity is a particularly unique microbiota that undergoes cyclical expansions and contractions of microbial diversity as a result of both host food ingestion intervals and hygienic practices, such as brushing and use of mouthwash. These perturbations respectively result in cycles of relative feast or famine and regular killing or removal of large swaths of the microbial population, after which ecological succession begins anew (10). Dental caries and periodontitis represent two extremely prevalent and costly diseases that are now recognized as the result of localized ecological catastrophes of the oral microbiome (11–14). One of the major impediments to the study of complex, human-associated microbial communities is the difficulty cultivating such diverse ecologies in a well-controlled laboratory setting. The oral microbiota was chosen as a model system for this pilot study because of the existence of a well-established in vitro culture system using media that allows for the growth of a diversity of species approaching that of an in vivo human mouth (15).
Results
Long-Term Starvation of a Complex Oral Community Results in a Death Phase, Followed by a Long-Term Stationary Phase.
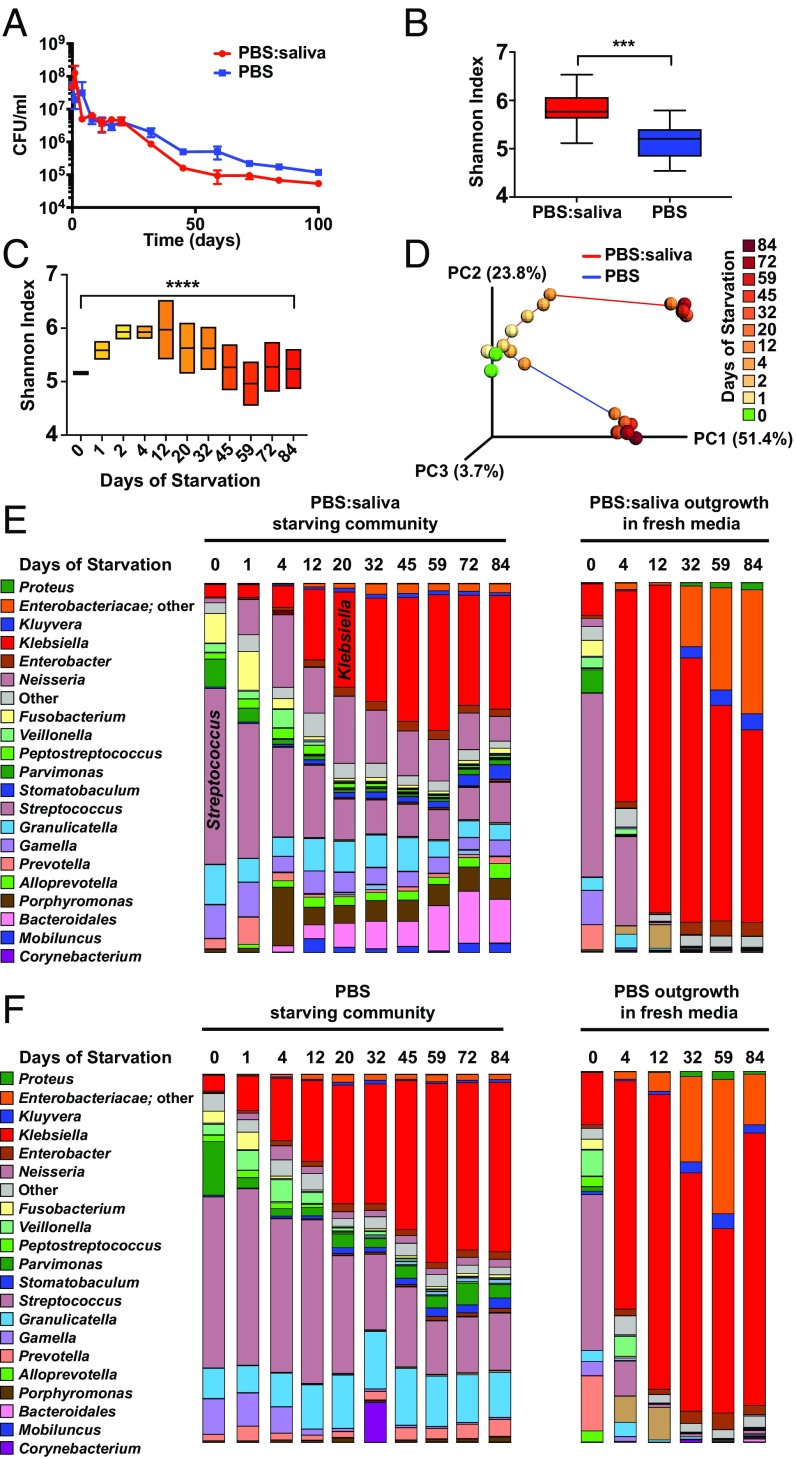
The starting community in this study was derived from a previous study that optimized media (SHI media) and growth conditions to stably maintain the highest diversity of oral bacteria achievable in vitro to date (15). The community was generated from the pooled saliva of six healthy adults, had a microbial diversity approaching that of human oral cavity, and responded to a carbohydrate pulse in a manner similar to in vivo dental plaque (15). After overnight batch growth in SHI media, the community was harvested and subsequently starved in aliquots of either PBS or a 1:1 mixture of PBS and cell-free saliva. Reminiscent of monospecies cultures of E. coli (4), the communities (both in PBS and PBS:saliva) first experienced a death phase with a rapid decrease in colony-forming units per milliliter over time, followed by a stabilization, a long-term stationary phase (Fig. 1A). It is important to note that many taxa that are viable in the liquid culture of the community cannot grow as isolated colonies on solid media, and thus are not measured in Fig. 1A. Therefore, although the colony-forming unit measurement is useful for illustrating the overall trend of viable cells in the community, the results are likely an underestimate, making it critical to complement this assay with mRNA-based taxonomic profiling, as described below, to determine the living members of the community during starvation. Illumina sequencing of 16S rDNA V3-V4 amplicons revealed that alpha diversity was higher in the PBS:saliva community than the PBS community, reflective of the supplementary substrates provided by the sterilized saliva at the start of the starvation (Fig. 1B). Intrasample diversity (alpha diversity) of the community increased across the first several days of starvation and then decreased to levels similar to that of the starting community after day 32 (Fig. 1C). Intercommunity diversity (beta diversity) also shifted over time, particularly during the first 12 d (Fig. 1D), followed by relative stabilization. The presence of saliva in the starvation medium also had an effect on the community, as the two starvation communities separated in Principal Coordinates Analysis (PCoA) space over time (Fig. 1D). There were shifts in the relative abundances of many taxa, with by far the most notable change being an increase in the number of Enterobacteriaceae, particularly Klebsiella, at the expense of Streptococci, which had been the most abundant genera in the starting communities (Fig. 1 E and F). Because the DNA harvested from the starving community was likely to contain the DNA of dead cells, aliquots of the starvation culture from various points were used to inoculate fresh SHI media, and the outgrowth culture was investigated with 16S rDNA sequencing after overnight growth to observe the “live” species from the initial starvation experiment (Fig. 1 E and F). The increase in Enterobacteriaceae 16S rDNA observed during long-term starvation was much more dramatic in the outgrowth communities. After only 4 d of starvation, the proportion of Klebsiella in the communities had increased dramatically, and by day 12, Enterobacteriaceae accounted for more than 90% of the relative abundance of the 16S rDNA in the outgrowth communities (Fig. 1 E and F). Although Klebsiella was the genus with the highest relative abundance, species of “unclassified” Enterobacteriaceae (highly likely to be P. alcalifaciens, as described below) increased in relative abundance during the later points of the outgrowth. The community starving in PBS underwent a transition similar to that of the community starving in PBS:saliva, albeit with a reduced abundance of Neisseria and Porphyromonas (Fig. 1F), indicating that components in the filtered saliva made these genera more competitive.
Fig. 1.
Shifts in diversity and relative abundances of constituents of the saliva microbiome during long-term starvation. (A) Number of viable cells in the community, as determined by colony-forming units during starvation in PBS:saliva or PBS over the course of 100 d. (n = 3). (B) Alpha diversity of the PBS:saliva or PBS communities across 84 d of starvation. ***P < 0.001, unpaired t test. (C) Alpha diversity of the PBS:saliva and PBS starvation communities over the course of 84 d of starvation. ****P < 0.0001, Brown-Forsythe test. (D) PCoA plot of unweighted UNIFRAC distances illustrating beta diversity of the PBS:saliva and PBS communities over the course of 84 d of starvation. (E) Relative abundances of bacterial genera in the PBS:saliva starvation experiment, and the starvation community after overnight outgrowth in fresh media, as determined by Illumina sequencing of 16S amplicons. (F) Relative abundances of bacterial genera in the PBS starvation experiment, and the starvation community after overnight outgrowth in fresh media, as determined by Illumina sequencing of 16S amplicons.
After 20 d of Starvation, Only Enterobacteriaceae Are Recoverable on Solid Media.
When plated on SHI media agar, the day 0 community produced colonies with a wide variety of morphologies, reflective of the diversity of species present (Fig. 2). During the death phase, the relative number of the large mucoid colonies obtained increased dramatically, such that by day 20, and all following times, all the colonies obtained were large and mucoid (Fig. 2). A selection of diverse colony morphologies across three times was isolated and identified, using Sanger sequencing of full-length 16S rDNA amplicons (Dataset S1). At day 1, several species of Streptococcus were recovered, as were Gamella sanguinis, Klebsiella pneumoniae, and Klebsiella oxytoca. At day 20, K. pneumoniae, K. oxytoca, Enterobacter homaechei, and Providencia alcalifaciens were the only recoverable species. Finally, at day 84 and day 100, only K. pneumoniae and P. alcalifaciens were recoverable under the conditions tested. This is a particularly interesting result because all the surviving organisms are documented pathogens and are frequently drug resistant (16–18).
Fig. 2.
Colony morphology during long-term starvation. Representative image of the PBS:saliva community on SHI agar after the indicated number of days of long-term starvation.
RNA Sequencing Confirms That Enterobacteriaceae Are the Dominant (and Likely Only Living) Community Members After Long-Term Starvation.
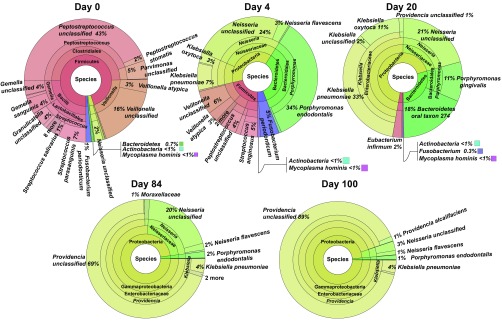
To obtain a finer resolution of the viable and actively transcribing species, shotgun sequencing of cDNA from the transcriptome of the community at five points during the long-term starvation was performed, followed by Metaphlan2 analysis to calculate the relative abundances of taxa (19) (Fig. 3). In the PBS:saliva starvation community, Firmicutes, namely, the genera Streptococcus, Gamella, Peptostreptococcus, and Veillonella, represented >90% of the RNA at day 0. At day 4, there was a diversity of RNA from Streptococcus, Peptostreptococcus, Neisseria, Veillonella, Klebsiella, Porphyromonas, and Fusobacterium. By day 20, Klebsiella increased in number such that the genus represented 46% of all RNA. This increase was largely at the expense of Firmicutes, which decreased to <3% of RNA. By day 84, Providencia accounted for 69% of the RNA, whereas Neisseria accounted for 20%. Ultimately, after 100 d of starvation, Providencia accounted for 90% of the RNA, indicating that it was the major living organism remaining in the community. As with the 16S rDNA profiling, the changes in abundance of taxa in the PBS community were similar to the major trends of the PBS:saliva community. Dataset S2 contains Krona (20) visualizations of Metaphlan2 analysis of the transcriptome from both PBS:saliva and PBS communities, as well as Metaphlan2 analysis of metagenomes (DNA) from both communities. Collectively, the above analyses indicate that species other than Enterobacteriaceae quickly lose viability and are no longer transcriptionally active after ∼10 d of long-term starvation. K. pneumoniae quickly becomes the most abundant member of the community, based on RNA, early during the long-term stationary phase, but is overtaken by P. alcalifaciens by days 84 and 100. Notably, ∼10 d is also the length of time required for starved cultures of E. coli to develop GASP mutations that allow them to displace wild-type siblings (4).
Fig. 3.
Relative abundances of actively transcribing taxa in the PBS:saliva long-term starvation community. At the indicated day based on Metaphlan2 analysis of mRNA.
Whole-Genome Sequencing of Enterobacteriaceae Isolates Reveals Single Nucleotide Polymorphism Accumulation During Starvation.
DNA obtained from Enterobacteriaceae isolates from days 0, 20, and 84 (Dataset S1) was subjected to Illumina shotgun sequencing. Genomes were assembled from each point and compared with the earliest available point for each species (in the PBS community). There were six nonsynonymous single nucleotide polymorphisms (SNPs) observed in the K. pneumoniae and P. alcalifaciens strains that increased in prevalence to >95%, in both PBS and PBS:saliva long-term starvation communities (Dataset S3). In K. pneumoniae, these SNPs occurred in the topB topoisomerase, the allS_2 LysR family transcriptional regulator, the glyS glycine tRNA ligase, and the lamB_1 maltoporin. Most intriguingly, both topoisomerase and LysR-type regulators were previously identified as GASP alleles, although the mechanism of the contribution of mutations in these genes to the GASP phenotype remains unknown (6, 21). Maltoporin, an outer membrane transporter of maltose, has been shown to be critical during adaptation to new growth conditions (22), and thus likely represents a GASP allele. In P. alcalifaciens, two SNPs that increased in frequency during long-term starvation were located in the tamB component of the TAM translocation and assembly module and in Toxin B (toxB). Interestingly, tamB mutants have significantly decreased virulence in K. pneumoniae and Salmonella enterica (23, 24). Although it is currently unclear whether these SNPs contributed to the success of these Enterobacteriaceae during long-term starvation, the fact that these variants increased in frequency several times, independently, highlights a need for further investigation.
Enterobacteriaceae Exhibit Shifts in the Transcriptome During Long-Term Starvation.
To obtain information about transcriptional activity among the Enterobacteriaceae during the long-term starvation, the cDNA Illumina sequencing reads were mapped to the assembled genomes of K. pneumoniae, K. oxytoca, and P. alcalifaciens. Genes that were differentially abundant between day 0 and subsequent times were identified using DeSeq2 (Dataset S4). Gene ontology terms for each differentially abundant gene were summarized and visualized using Revigo (25) and a custom R script (SI Appendix, Fig. S1). Eleven, 14, 15, and 5 nonredundant biological processes exhibited increased expression at days 4, 20, 84, and 100 compared with day 0, respectively. Meanwhile, 22, 10, 20, and 40 nonredundant biological processes exhibited decreased expression on days 4, 20, 84, and 100, respectively. Interestingly, day 20, the point at which Enterobacteriaceae had become the most abundant members of the community, was the only observed point at which the number of biological processes with increased expression exceeded the number of biological processes with decreased expression. Negative regulation of flagellum motility, negative regulation of biofilm formation, and positive regulation of carbohydrate metabolic processes were the biological processes with the most highly elevated expression at days 20, 84, and 100 compared with day 0. By day 100, these three biological processes, along with positive regulation of catalytic activity, and oxidation-reduction process were the only biological processes that were up-regulated compared with day 0. This may suggest that the Enterobacteriaceae may be attempting to conserve energy and passively transport to a location with a novel food source. Xylose metabolism, valine biosynthetic process, and xylose transport were the three most highly up-regulated biological pathways at day 4, indicating that these pathways may be important to the Enterobacteriaceae early during long-term starvation, and that they may play a role in adapting to new conditions. Collectively, pathway analysis provides leads for further research into the mechanism behind the success of the Enterobacteriaceae during long-term starvation.
Natural Products Analysis Indicates Generation of Cyclic Depsipeptides by Enterobacteriaceae.
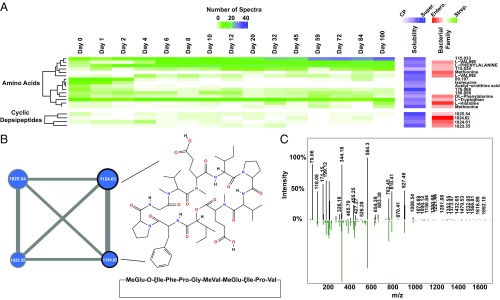
To explore the dynamics of the small molecules produced by the communities during long-term starvation, harvested cells, as well as culture supernatants, of both the community and the isolated strains were analyzed by liquid chromatography mass spectrometry, followed by spectral analysis using Global Natural Products Social Molecular Networking (GNPS) (26). As is the present case with most metabolomics analysis, the majority of MS spectra were unannotated/unknown, with only ∼40% of the spectral clusters mapping to known annotations. Twenty-five unknown spectral clusters were assigned a putative molecule class based on molecular networking analysis. The complete molecular network was visualized using Cytoscape (27) and is available in the SI Appendix, Fig. S3. A large number of the MS spectra that networked with known lipid spectra were found only in the cell pellets, congruent with the concept that these are membrane-associated molecules and are unlikely to be secreted. There were a large number of MS spectra that only appeared in the community, either because the species synthesizing them were not species that were isolated or because isolates that were analyzed did not make the natural products in a single-species culture. There were more spectra associated with the Enterobacteriaceae species than the Streptococci, which could be expected given that the Enterobacteriaceae quickly increased in relative abundance in the communities during starvation. Interestingly, valine was significantly more abundant at later points (Fig. 4A and SI Appendix, Fig. S2), in agreement with up-regulation of transcription of valine biosynthetic processes among the surviving Enterobacteriaceae (SI Appendix, Fig. S1; day 20). The GNPS Dereplicator identified two spectral clusters with a GNPS library hit to a cyclic depsipeptide with a parent mass of 1,124.6 and a cyclic peptide sequence of MeGlu-O-ξIle-Phe-Pro-Gly-MeVal-MeGlu-ξIle-Pro-Val (Fig. 4 B and C). Cyclic depsipeptides are a fascinating class of small molecules that frequently have antimicrobial and/or anticancer activity and are the subject of ongoing research (28–32). These two spectral clusters networked with two other unidentified spectral clusters with a parent mass of 1,025.5, which may indicate loss of a valine residue from the structure of the known cyclic depsipeptide (Fig. 4B). All four cyclic depsipeptide spectral clusters appeared to be associated with Enterobacteriaceae, based on the number of spectra associated with single-species isolates. This family of molecules also appeared in the community samples during early times, when the main shift in species abundance was occurring (∼day 4; Fig. 4A). These molecules may be bactericidal compounds secreted by the Enterobacteriaceae to kill the neighboring species during starvation for use in necrotrophy, and further study of these compounds is warranted.
Fig. 4.
Accumulation of valine and production of cyclic depsipeptides during long-term starvation. (A) Heat map illustrating the number of liquid chromatography mass spectrometry spectra associated with the indicated spectral cluster after the indicated number of days of starvation. Spectral clusters are grouped into molecule classes (e.g., amino acids) based on molecular networking to GNPS library hits. Row clustering within molecule classes was performed using a Pearson similarity distance matrix. Spectral clusters are named using the GNPS library hit where applicable; otherwise, clusters are named using the liquid chromatography mass spectrometry parent mass. The Solubility column indicates the percent of the spectra (0–100%) within a cluster that were associated with the samples extracted from the culture supernatant (Super.) versus the cell pellet (CP). The Bacterial Family column indicates the percentage of spectra within a cluster that were associated with samples extracted from isolates of Streptococcaceae (Strep.) versus Enterobacteriaceae (Entero.). Only amino acids and cyclic depsipeptides are shown here; the full heat map with all spectral clusters is presented in the SI Appendix, Fig. S2. (B) Molecular subnetwork of the four cyclic depsipeptide spectral clusters. Node size corresponds to the total number of spectra in each cluster. Node label indicates parent mass. Edge width indicates the cosine score between spectral cluster nodes. Black outlines denote nodes which mapped to the GNPS library hit, cyclo-MeGlu-O-ξIle-Phe-Pro-Gly-MeVal-MeGlu-ξIle-Pro-Val, with indicated chemical structure. The complete molecular network is available in the SI Appendix, Fig. S3. (C) Comparison of MS/MS spectra from spectral cluster with parent mass 1,124.61 (black bars) to GNPS library spectrum for cyclo-MeGlu-O-ξIle-Phe-Pro-Gly-MeVal-MeGlu-ξIle-Pro-Val (green bars).
Enterobacteriaceae Increase in Abundance During Starvation in Additional Communities from Individual Donors.
To ensure that the phenomenon of the Enterobacteriaceae species becoming the dominant, living members of the starving community was not unique to this consortium of bacteria, the same long-term starvation experiment (using PBS as starvation media) was performed on five additional salivary communities, each isolated from the saliva of a single, healthy individual. 16S rDNA PCR-denaturing gradient gel electrophoresis was used to monitor taxonomic profiles of the five communities during the first 20 d of starvation (SI Appendix, Fig. S4). The denaturing gradient gel electrophoresis profiles of Community numbers 1 and 5 contained bands representing Enterobacteriaceae, as determined by Sanger sequencing of the DNA contained within the excised band. Most importantly, the density of the Enterobacteriaceae bands increased during starvation, concurrent with a decrease in the density of most other bands within the denaturing gradient gel electrophoresis profile. This finding signifies an increase in the relative abundance of Enterobacteriaceae at the expense of other taxa, indicating that the phenomenon observed in the main experiment was not exclusive to that starting community of bacteria. The other three communities did not appear to have significant numbers of Enterobacteriaceae at any point (SI Appendix, Fig. S4).
Discussion
This study provides an account of a complex, human-associated microbial community experiencing the ecological perturbation of long-term starvation. The finding that Klebsiella and Providencia species were the apparent sole survivors in a community after long-term starvation is significant and highly intriguing. K. pneumoniae, K. oxytoca, and P. alcalifaciens are all members of the Enterobacteriaceae family of Proteobacteria. K. pneumoniae is a significant pathogen and represents the ‘K’ in the ESCKAPE pathogens, a group of organisms frequently resistant to multiple antibiotics (16). K. oxytoca and P. alcalifaciens are also opportunistic pathogens and are frequently drug resistant (17, 18). Oral K. pneumoniae was recently shown to induce inflammation and dysbiosis in the gut after ectopic colonization, and it was hypothesized that the oral cavity provides a reservoir for would-be intestinal pathogens, such as Klebsiella (33). Furthermore, aside from being a substantial pathogen in its own right, evidence is accumulating that K. pneumoniae serves as a key trafficker of drug resistance loci from the environment to human pathogens (34).
The mechanisms employed by these Enterobacteriaceae to outlast their neighbors during long-term starvation await investigation. The Enterobacteriaceae encode among the largest genomes in the oral microbiome (35) and, as such, have added metabolic flexibility compared with Streptococci and other common constituents of the oral cavity (36). It is likely that during long-term starvation, species with reduced genomes have less metabolic flexibility and are at a significant disadvantage to Enterobacteriaceae (37). Klebsiella are diazotrophs, and all Enterobacteriaceae are capable of using nitrate, S-oxides, and N-oxides as terminal electron acceptors (36). Thus, the concept that these abilities were advantageous during long-term starvation remains an attractive hypothesis. In addition, the MetaOmics analyses performed in this study provided several additional hypotheses for further investigation. The increased abundance of SNPs in several genes in K. pneumoniae and P. alcalifaciens may represent GASP mutations, which were originally discovered in E. coli, another member of the family Enterobacteriaceae (4). Overall analysis of the Enterobacteriaceae transcriptome indicated that the species may be attempting to conserve energy and use passive transport to locate a novel food source. Meanwhile, several intriguing cyclic depsipeptides may have been employed by the Enterobacteriaceae to kill their neighbors.
The results presented here also illustrate the value of RNA-based detection methods. Although a large number of taxa were present at all points, according to sequencing of 16S amplicons, as well as metagenomes, sequencing of mRNA revealed a much more drastic reduction of species during and after the death phase. This loss of diversity was also reflected in the sequencing of the outgrowth communities and the plating assay, from which only the three Enterobacteriaceae species were recoverable at day 20 under the conditions tested, despite the presence of DNA from a multitude of species in the community at that time. Because some bacteria are known to enter a viable but not culturable state during adverse growth conditions, the lack of taxonomic diversity at the transcriptional level during the later points of starvation serves as an important validation that the colony-forming units per milliliter and recoverable species on solid media reported here are not largely underestimated.
The ability of the Enterobacteriaceae to survive longer than other members of the saliva microbial community may have a great deal of clinical significance. Although the long-term starvation model used in this study is unlikely to simulate the oral cavity, where periods of starvation are much shorter, it is presumably analogous to the succession that occurs when human saliva is deposited in environmental locations not exposed to rapid desiccation. Contaminated hospital surfaces, particularly sinks, have been the source of outbreaks of multidrug-resistant Klebsiella (17, 38, 39). It is therefore easy to imagine a scenario in which Enterobacteriaceae survive for extended periods in mixtures of saliva and water in sinks and drains, where aerosol formation after subsequent use of the sink leads to spread of the infection. This danger is compounded by the frequent horizontal gene transfer of resistance genes employed by Enterobacteriaceae (34, 39). Elucidation of the mechanisms used by the Enterobacteriaceae to survive long-term starvation in a community setting is highly important, and further research is currently in progress.
Materials and Methods
More detailed methods with additional references are available in the SI Appendix. The starting bacterial community (S-mix), derived from the saliva of six healthy subjects, ages 25–35 y, has been described previously (15). After overnight growth in SHI medium in microaerophilic conditions (2%O2, 5%CO2, 93%N2), 1 mL aliquots of S-mix were starved in either 1× PBS or a 1:1 mixture of 1× PBS and cell-free saliva. Colony-forming units per milliliter was determined by growth for 72 h on SHI media agar under microaerophilic conditions. 16S rDNA taxonomic profiling was performed by Illumina sequencing of V2-V4 amplicons, followed by analysis using QIIME. Metagenomes were assembled de novo using SPAdes and aligned using Mauve. Metatranscriptomic reads were mapped to Enterobacteriaceae genomes using Burrows-Wheeler alignment, and genes that were significantly differentially expressed were identified using DeSeq2. Natural products analysis was performed using reverse-phase high-pressure liquid chromatography followed by tandem mass spectrometry, as detailed in the SI Appendix. Raw sequencing data have been deposited in the Sequence Read Archive (SRA) (40, 41).
Supplementary Material
Acknowledgments
The authors thank Roberta Faustoferri for helpful proofreading of the manuscript. This study was supported by National Institutes of Health/National Institute of Dental and Craniofacial Research Grants F32-DE026947 (to J.L.B.), R00-DE024543 (to A.E.), and R01-DE020102 and R01-DE026186 (to X.H., J.S.M., and W.S.).
Footnotes
Conflict of interest statement: J.L.B. is a part-time consultant for uBiome, Inc. W.S. is a part-time chief science officer of C3J Therapeutics, Inc., which has licensed technologies from the University of California Regents that could be indirectly related to this research project.
This article is a PNAS Direct Submission.
Data deposition: The raw sequencing files used in the genomic, metagenomic, and transcriptomic analyses in this study have been deposited in the Sequence Read Archive, https://www.ncbi.nlm.nih.gov/sra [accession nos. PRJNA525688 (genomes) and PRJNA525517 (metagenomes/transcriptomes)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820594116/-/DCSupplemental.
References
- 1.Magnusson LU, Farewell A, Nyström T. ppGpp: A global regulator in Escherichia coli. Trends Microbiol. 2005;13:236–242. doi: 10.1016/j.tim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Rittershaus ES, Baek SH, Sassetti CM. The normalcy of dormancy: Common themes in microbial quiescence. Cell Host Microbe. 2013;13:643–651. doi: 10.1016/j.chom.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claverys JP, Håvarstein LS. Cannibalism and fratricide: Mechanisms and raisons d’être. Nat Rev Microbiol. 2007;5:219–229. doi: 10.1038/nrmicro1613. [DOI] [PubMed] [Google Scholar]
- 4.Finkel SE. Long-term survival during stationary phase: Evolution and the GASP phenotype. Nat Rev Microbiol. 2006;4:113–120. doi: 10.1038/nrmicro1340. [DOI] [PubMed] [Google Scholar]
- 5.Avrani S, Bolotin E, Katz S, Hershberg R. Rapid genetic adaptation during the first four months of survival under resource exhaustion. Mol Biol Evol. 2017;34:1758–1769. doi: 10.1093/molbev/msx118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chib S, Ali F, Seshasayee ASN. Genomewide mutational diversity in Escherichia coli population evolving in prolonged stationary phase. MSphere. 2017;2:e00059-17. doi: 10.1128/mSphere.00059-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jõers A, Tenson T. Growth resumption from stationary phase reveals memory in Escherichia coli cultures. Sci Rep. 2016;6:24055. doi: 10.1038/srep24055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phaiboun A, Zhang Y, Park B, Kim M. Survival kinetics of starving bacteria is biphasic and density-dependent. PLOS Comput Biol. 2015;11:e1004198. doi: 10.1371/journal.pcbi.1004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takano S, Pawlowska BJ, Gudelj I, Yomo T, Tsuru S. Density-dependent recycling promotes the long-term survival of bacterial populations during periods of starvation. MBio. 2017;8:e02336-16. doi: 10.1128/mBio.02336-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: Dynamic communities and host interactions. Nat Rev Microbiol. 2018;16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilian M, et al. The oral microbiome–An update for oral healthcare professionals. Br Dent J. 2016;221:657–666. doi: 10.1038/sj.bdj.2016.865. [DOI] [PubMed] [Google Scholar]
- 13.Bowen WH, Burne RA, Wu H, Koo H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26:229–242. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 15.Edlund A, et al. An in vitro biofilm model system maintaining a highly reproducible species and metabolic diversity approaching that of the human oral microbiome. Microbiome. 2013;1:25. doi: 10.1186/2049-2618-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pendleton JN, Gorman SP, Gilmore BF. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther. 2013;11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 17.Leitner E, et al. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob Agents Chemother. 2015;59:714–716. doi: 10.1128/AAC.04306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, et al. Pathogenic Providencia alcalifaciens strain that causes fatal hemorrhagic pneumonia in piglets. Curr Microbiol. 2014;68:278–284. doi: 10.1007/s00284-013-0470-y. [DOI] [PubMed] [Google Scholar]
- 19.Truong DT, et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods. 2015;12:902–903. doi: 10.1038/nmeth.3589. [DOI] [PubMed] [Google Scholar]
- 20.Ondov BD, Bergman NH, Phillippy AM. Interactive metagenomic visualization in a Web browser. BMC Bioinformatics. 2011;12:385. doi: 10.1186/1471-2105-12-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silby MW, Giddens SR, Mahanty HK. Mutation of a LysR-type regulator of antifungal activity results in a growth advantage in stationary phase phenotype in Pseudomonas aureofaciens PA147-2. Appl Environ Microbiol. 2005;71:569–573. doi: 10.1128/AEM.71.1.569-573.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenyon WJ, Thomas SM, Johnson E, Pallen MJ, Spector MP. Shifts from glucose to certain secondary carbon-sources result in activation of the extracytoplasmic function sigma factor sigmaE in Salmonella enterica serovar Typhimurium. Microbiology. 2005;151:2373–2383. doi: 10.1099/mic.0.27649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Struve C, Forestier C, Krogfelt KA. Application of a novel multi-screening signature-tagged mutagenesis assay for identification of Klebsiella pneumoniae genes essential in colonization and infection. Microbiology. 2003;149:167–176. doi: 10.1099/mic.0.25833-0. [DOI] [PubMed] [Google Scholar]
- 24.Burall LS, et al. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: Identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect Immun. 2004;72:2922–2938. doi: 10.1128/IAI.72.5.2922-2938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat Biotechnol. 2016;34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shannon P, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue Y, et al. Cyanobacteria-derived peptide antibiotics discovered since 2000. Peptides. 2018;107:17–24. doi: 10.1016/j.peptides.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Han B, Goeger D, Maier CS, Gerwick WH. The wewakpeptins, cyclic depsipeptides from a Papua New Guinea collection of the marine cyanobacterium Lyngbya semiplena. J Org Chem. 2005;70:3133–3139. doi: 10.1021/jo0478858. [DOI] [PubMed] [Google Scholar]
- 30.Lemmens-Gruber R, Kamyar MR, Dornetshuber R. Cyclodepsipeptides–Potential drugs and lead compounds in the drug development process. Curr Med Chem. 2009;16:1122–1137. doi: 10.2174/092986709787581761. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y, Phat C, Hong SC. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides. 2017;95:94–105. doi: 10.1016/j.peptides.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Kitagaki J, Shi G, Miyauchi S, Murakami S, Yang Y. Cyclic depsipeptides as potential cancer therapeutics. Anticancer Drugs. 2015;26:259–271. doi: 10.1097/CAD.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 33.Atarashi K, et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017;358:359–365. doi: 10.1126/science.aan4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyres KL, Holt KE. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr Opin Microbiol. 2018;45:131–139. doi: 10.1016/j.mib.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Chen T, et al. The human oral microbiome database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford) 2010;2010:baq013. doi: 10.1093/database/baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winter SE, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339:708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol. 2011;10:13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- 38.Lowe C, et al. Mount Sinai Hospital Infection Control Team Outbreak of extended-spectrum β-lactamase-producing Klebsiella oxytoca infections associated with contaminated handwashing sinks(1) Emerg Infect Dis. 2012;18:1242–1247. doi: 10.3201/eid1808.111268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weingarten RA, et al. NISC Comparative Sequencing Program Genomic analysis of hospital plumbing reveals diverse reservoir of bacterial plasmids conferring carbapenem resistance. MBio. 2018;9:e02011-17. doi: 10.1128/mBio.02011-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker JL. 2019 Klebsiella and Providencia notch a tag-team victory in a battle royale of oral bacteria. Sequence Read Archive (SRA). Available at https://www.ncbi.nlm.nih.gov/sra/PRJNA525688. Deposited March 4, 2019.
- 41.Baker JL. 2019 Klebsiella and Providencia notch a tag-team victory in a battle royale of oral bacteria. Sequence Read Archive (SRA). Available at https://www.ncbi.nlm.nih.gov/sra/PRJNA525517. Deposited March 5, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.