Abstract
Bacteria residing in the human gastrointestinal (GI) tract has a symbiotic relationship with its host. Animal models have demonstrated a relationship between exercise and gut microbiota composition. This was the first study to explore the relationship between cardiorespiratory fitness (maximal oxygen consumption, VO2max) and relative gut microbiota composition [Firmicutes to Bacteroidetes ratio (F/B)] in healthy young adults in a free-living environment. Twenty males and 18 females (25.7±2.2 y), who did not take antibiotics in the last 6 months, volunteered for this study. VO2max was measured using a symptom-limited graded treadmill test. Relative microbiota composition was determined by analyzing DNA extracted from stool samples using Quantitative Polymerase Chain Reaction (qPCR) that specifically measured the quantity of a target gene (16s RNA) found in Firmicutes and Bacteroidetes. Relationships between F/B and potentially related dietary, anthropometric, and fitness variables were assessed using correlation analyses with appropriate Bonferroni adjustment (p<0.004). Average F/B ratio in all participants was 0.94±0.03. F/B ratio was significantly correlated to VO2max (r=0.48, p<0.003) but, no other fitness, nutritional intake, or anthropometric variables (p>0.004). VO2max explained ~22% of the variance of an individual’s relative gut bacteria as determined by F/B ratio. These data support animal findings, demonstrating a relationship between relative human gut microbiota composition and cardiorespiratory fitness in healthy young adults. GI bacteria is integral in regulating a myriad of physiological processes, and greater insight regarding ramifications of exercise and nutrition on gut microbial composition may help guide therapies to promote human health.
Keywords: Microbiome, Firmicutes, Bacteroidetes, VO2max, body composition, BodPod, nutrition
INTRODUCTION
The human body contains a vast number of bacteria cells (Sender et al., 2016) and the composition of bacteria in the gut (i.e., gut microbiota) is linked to a variety of physiological functions (Sommer et al., 2013) and diseases, such as obesity (Ley et al., 2006). Firmicutes and Bacteroidetes compose the vast majority of the bacterial species residing in the human gastrointestinal tract, and as such the relative ratio between Firmicutes to Bacteroidetes (F/B) has been used as a measure of gut microbiota health (Koliada et al., 2017). Although obesity has been associated with an elevated F/B (Ley et al., 2006), exercise training has similarly been proven to increase bacterial species within the Firmicutes phyla (Choi, et al., 2013; Queipo-Ortuno et al., 2013), illustrating the lack of consensus on an optimal F/B proportion.
Recent findings suggest a dynamic relationship between gut microbiota and physical activity levels (Allen et al., 2017; Choi et al., 2013; Clarke et al., 2014; Estaki et al., 2016; Evans et al., 2014; Lambert et al., 2015; Petersen et al., 2017; Petriz et al., 2014; Queipo-Ortuno et al., 2013), with professional athletes and higher fit individuals exhibiting more diverse composition compared to their sedentary or less fit counterparts (Clarke et al., 2014; Estaki et al., 2016). Exercise training has further been shown to increase the abundance of bacterial species associated with butyrate production (Allen et al., 2017), including Clostridales, a bacterial order under the Firmicutes phylum. Butyrate production may be important as it is linked to improving the intestinal barrier, which is responsible for sequestering toxic agents and bacteria within the intestine (Peng et al., 2009). Taken together, these findings suggest that exercise may elicit a greater abundance of healthy bacterial species (i.e., butyrate producers) that may help to fortify the intestinal epithelium and prevent the translocation of noxious bacterial species into circulation which would have a wealth of deleterious systemic ramifications.
To the best of our knowledge, no study has examined if F/B is associated with cardiorespiratory fitness, as assessed by maximal oxygen consumption (VO2max), independent from dietary or anthropometric measures. The purpose of this exploratory study was to identify potential relationships among relative gut microbiota composition (F/B) and VO2max, body composition, or dietary intake among healthy young adults in a free-living environment.
MATERIALS AND METHODS
This study was conducted in accordance with the Declaration of Helsinki and approved by San Francisco State University’s Institutional Review Board (approval number X16–67b). Thirty-seven healthy participants (20 males, 17 females; age 25.7±2.2y) provided written informed consent and completed pre-participation, exercise history, and demographics questionnaires (data presented in Table 1). Participants were instructed to follow their normal diet for 7 days and track dietary intake with MyFitnessPal.com (MyFitnessPal Inc., San Francisco, CA). Participants were given a kit for stool collection at home (DNA Genotek Inc., Ontario, Canada) and were instructed on proper use. Participant’s stool samples and dietary logs were given to the researchers and body composition was measured using air displacement plethysmography (BOD POD: Life Measurement, Inc., Concord, CA, USA). Participant’s cardiorespiratory fitness was measured using a symptom-limited maximal graded treadmill exercise test to determine VO2max (Quark CPET: Cosmed, Inc., Rome, Italy). They warmed up (3 minutes of walking, 4.8 km/hr) then started jogging at a self-selected pace for 3 minutes. To obtain VO2max, the treadmill incline was increased by 2% every 2 minutes while keeping the speed constant until either the participant reached volitional exhaustion. To ensure a maximal effort was given, researchers observed a failure for heart rate to increase with additional workload, plateau in VO2, or a Rating of Perceived Exertion (RPE) on the Borg Scale >17.
Table 1:
Participant anthropometric and demographic characteristics. Values are means ± standard deviations (ranges).
| All Participants (n = 37) | Male (n = 20) | Female (n = 17) | |
|---|---|---|---|
| Anthropometric Measures | |||
| Height (cm) | 170.6 ± 9.9 | 177.6 ± 7.1 | 162.3 ± 5.3 |
| (154.0 – 190.5) | (166.4 – 190.5) | (154.0 – 178.8) | |
| Body Mass (kg) | 69.3 ± 14.0 | 76.2 ± 14.1 | 61.0 ± 8.7 |
| (47.7 – 108.1) | (56.1 – 108.1) | (47.7 – 75.2) | |
| BMI (kg/m2) | 23.7 ± 3.6 | 24.1 ± 4.0 | 23.2 ± 3.0 |
| (17.9 – 31.4) | (17.9 – 31.4) | (18.3 – 28.1) | |
| Body Fat % | 23.1 ± 9.1 | 18.3 ± 9.2 | 28.7 ± 5.2 |
| (7.0 – 38.0) | (7.0 – 37.2) | (21.0 – 38.0) | |
| Fat Mass (kg) | 16.2 ± 8.0 | 14.8 ± 9.9 | 17.7 ± 4.9 |
| (4.1 – 40.2) | (4.1 – 40.2) | (11.3 – 27.0) | |
| Fat Free Mass (kg) | 53.0 ± 11.4 | 61.2 ± 8.0 | 43.3 ± 5.8 |
| (33.7 – 80.1) | (32.3 – 64.0) | (33.7 – 58.1) | |
| VO2max (ml/kg/min) | 46.4 ± 8.0 | 49.6 ± 7.8 | 42.5 ± 6.7 |
| (32.3 – 64.0) | (32.3 – 64.0) | (33.1 – 59.9) | |
| Demographic Characteristics | |||
| Age (years) | 25.7 ± 2.2 | 25.9 ± 2.7 | 25.4 ± 1.8 |
| (22 – 32) | (23 – 32) | (22 – 28) | |
| Ethnicity | |||
| White/Caucasian | 37.8% | 45.0% | 29.4% |
| Hispanic/Latino | 21.6% | 20.0% | 23.5% |
| Asian/Pacific Islander | 21.6% | 10.0% | 35.3% |
| Two Or More Ethnicities | 16.2% | 20.0% | 11.8% |
| Other | 2.7% | 5.0% | 0.0% |
| Education | |||
| Some College | 13.5% | 15.0% | 11.8% |
| Bachelor’s Degree | 70.3% | 65.0% | 76.5% |
| Graduate Degree | 10.8% | 15.0% | 5.9% |
| Doctorate Degree | 5.4% | 5.0% | 5.9% |
Fecal samples were collected using OMNIgene Gut stool collection kits (DNA Genoteck Inc., Onatrio, Canada) which can keep samples stable at room temperature for up to 60 days (Doukhanine et al., 2016). All participants were asked to record the date and time of collection, and samples were returned within 72 hours to ensure DNA extraction was performed within 7 days. This method has proven to deliver high quality DNA without introducing bias for potential downstream applications (Doukhanine et al., 2014).
DNA was extracted from fecal matter using a PowerSoil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA, USA) and yield and purity was determined using a Nanodrop2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The relative F/B for each participant’s sample was determined by real-time quantitative Polymerase Chain Reaction (qPCR) using a StepOnePlus Instrument (Applied Biosystems Inc. USA). Each sample was assayed in triplicate using the FAST SYBR Green Master Mix (Applied Biosystems Inc, USA) using 200 nm of both forward and reverse primers. These primers targeted the 16S rRNA sequences of Firmicutes and Bacteroidetes, primers and cycling conditionings as described previously (Bahl et al., 2012; Guo et al., 2008; Payne et al., 2011). Standard curves were generated from known concentrations of plasmid DNA containing the 16S rRNA sequence of Firmicutes or Bacteroidetes.
Statistical analysis was performed with IBM SPSS Statistics (version 24, SPSS Inc., Chicago, IL, USA). Only participants who completed all aspects of the investigation were included in the final analyses (n=37) and one individual’s data was dropped because their BMI was >2 SD above the mean. Descriptive statistics were run on all variables and displayed as mean ± SD when applicable. Relationships between relative gut microbiota composition and dietary, anthropometric, and fitness variables were assessed via Pearson’s correlation coefficients (r), with significance set at p<0.004 based on an appropriate Bonferroni adjustment. A linear regression was run to determine the variance between the variables. Analyses were performed on the total sample and separately by gender.
RESULTS
Males had a significantly higher VO2max (p=0.004) and fat free mass percentage (p<0.001) compared to females (anthropometric values displayed in Table 1). Average F/B in all participants was 0.94±0.03 (median and interquartile ranges of bacteria can be found in Table 2) and average VO2max was 46.4 ± 8.0 ml·kg−1min−1. F/B was significantly correlated to VO2max for all participants (r=0.48, p<0.003) as displayed in Figure 1; however, when analyzed separately, neither males (r=0.543, p<0.05) or females (r=0.621, p<0.01) exhibited a statistically significant correlation between F/B and VO2max. No other fitness, nutritional, or anthropometric variables were significantly correlated to F/B as shown in Table 3 (p>0.004). VO2max accounted for approximately 22% of the variance in relative gut microbiota composition.
Table 2:
Phylum Median and interquartile ranges for all participants.
| Firmicutes | 0.0063 | (0.0017–0.0143) |
| Bacteroidetes | 0.0117 | (0.0037–0.0197) |
| F/B Ratio | 0.9216 | (0.6469–1.1966) |
Figure 1:
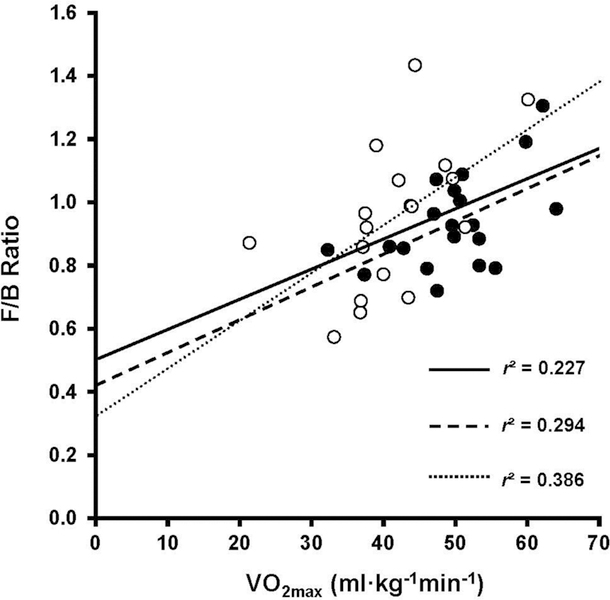
Correlation between Firmicutes to Bacteroidetes ratio (F/B) and cardiorespiratory fitness (maximal oxygen consumption, VO2max) in healthy young adults [Men = black circles; Women = white circles]. Linear regression lines are shown for all participants (solid line), men (dashed line), and women (dotted line).
Table 3:
Correlation matrix among F/B, cardiorespiratory fitness, body composition and, dietary intake.
| Variable | F/B | VO2max
(ml/kg/min) |
Active Min |
MET-Min/Week | Age | BMI (kg/m2) |
Body Fat % |
Protein (g/day) |
Fat (g/day) |
Carbohydrate (g/day) |
Fiber (g/day) |
Coffee (oz/day) |
Alcohol (oz/day) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F/B | - | 0.476* | 0.400 | 0.324 | 0.236 | −0.241 | −0.237 | 0.145 | 0.080 | 0.239 | 0.309 | 0.306 | 0.085 |
| VO2max | - | 0.223 | 0.305 | −0.288 | −0.386* | −0.703* | 0.360 | 0.437 | 0.553* | 0.431 | 0.207 | 0.044 | |
| Active Minutes | - | 0.887* | 0.341 | 0.126 | −0.152 | 0.466* | 0.243 | 0.322 | 0.235 | 0.475* | 0.402 | ||
| MET-Min/Week | - | 0.459* | 0.090 | −.0220 | 0.404 | 0.273 | 0.400 | 0.276 | 0.470* | 0.293 | |||
| Age | - | 0.111 | −0.128 | 0.080 | −0.113 | 0.188 | 0.096 | 0.217 | 0.441 | ||||
| BMI | - | 0.514* | 0.202 | 0.163 | −0.251 | −0.243 | 0.101 | 0.334 | |||||
| Body Fat % | - | −0.447 | −0.460* | −0.611* | −0.360 | −0.163 | −0.044 | ||||||
| Protein | - | 0.660* | 0.502* | 0.336 | 0.296 | 0.375 | |||||||
| Fat | - | 0.534* | 0.378 | 0.495* | 0.167 | ||||||||
| Carbohydrate | - | 0.554* | 0.470* | 0.355 | |||||||||
| Fiber | - | 0.204 | −0.022 | ||||||||||
| Coffee | - | 0.280 | |||||||||||
| Alcohol | - |
p<0.004
Abbreviations: Firmicutes/Bacteriodetes ratio, F/B, maximal oxygen consumption, VO2max, metabolic equivalents, MET; body mass index, BMI.
DISCUSSION
This was the first investigation to determine if there was a relationship between VO2max and F/B in healthy young adults in a free-living environment. We found that VO2max was associated with an increase in F/B and accounted for a significant portion of the variance (~22%). No other variables (i.e., diet, body composition, or other fitness measures) were significantly correlated to relative gut microbiota composition among these healthy young adults.
The observed relationship between cardiorespiratory fitness and gut microbiota composition supports previous research suggesting that exercise training may promote an increase in bacterial strains in the Firmicutes phylum (Allen et al., 2017; Estaki et al., 2016). These findings aid in attempting to understand the intricate relationship between physical activity, gut microbial composition, and overall organism health. As microbial transplantation has proven to help restore health status in disease populations (Smith et al., 2013), the growing body of literature on physical activity and gut health may help to inform future bacterial therapeutic interventions attempting to restore microbial homeostasis and preserve physiological systems (e.g., aging skeletal muscle size and function) (Grosicki et al., 2017).
In agreement with previous findings by Finucane et al., (2014), we observed no association between body composition, or Body Mass Index (BMI) and F/B (Finucane et al., 2014). However, these findings contradict those in a classic study by Ley and colleagues (Ley et al., 2006). This difference may be explained by inter-study participant differences; our participants exhibited healthy body compositions (BMI = 23.7±3.6), while all participants in the Ley study were obese as defined by a BMI over 30. As obesity is strongly associated with metabolic derangement, the relationship between BMI and F/B observed by Ley et al. may be related to dysfunctional interactions between gut microbiota and bioenergetic pathways (Musso et al., 2011).
Moreover, we did not find an association between macronutrient intake and F/B. A potential explanation for this was the relatively low fat (average of 78±27 g of fat consumed per day) and homogenous macronutrient intake by our participants, as fat intake has been proposed to contribute to microbial dysbiosis in animal models (Martinez et al., 2017; Murphy et al., 2015). Thus, the association between macronutrient intake and F/B observed in previous reports may be a product of diets that are high in fat (≥ 60%) compared to the diets consumed by our healthy young subjects (~36%).
A couple limitations of this investigation should be noted. First, several variables in this study were obtained through subjective self-reports, such as the dietary intake. Objective measures of dietary intake should be used in future studies to more reliably establish the presence or absence of the relationships among gut microbiota, fitness, and nutrition. Second, this study did not utilize a randomized control trial, so it cannot be concluded that the relationships presented here are a product of exercise training or that the observed microbial characteristics predispose someone to achieving a higher fitness level.
Recent findings have demonstrated the importance of gut microbiota in various physiological systems, highlighting the need for continued exploration of the relationship between physical activity and gut health. Our results are consistent with recent reports (Allen et al., 2017; Estaki et al., 2016) suggesting that exercise training may elicit favorable shifts in gut microbial composition in young healthy adults. Gut microbiota is integral in regulating a myriad of physiological processes, and greater insight regarding the beneficial effects of exercise training for gut microbial composition may help guide therapies to promote human health.
Acknowledgements
This work was partially funded by the award of a National Institutes of Health (NIH) grant to LM-M: UL1 GM118985.
Footnotes
Declaration
The authors report no conflicts of interest associated with this manuscript.
REFERENCES
- Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, … Woods JA. (2017). Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc 10.1249/MSS.0000000000001495 [DOI] [PubMed]
- Bahl MI, Bergstrom A, & Licht TR (2012). Freezing fecal samples prior to DNA extraction affects the Firmicutes to Bacteroidetes ratio determined by downstream quantitative PCR analysis. FEMS Microbiol Lett, 329(2), 193–197. 10.1111/j.1574-6968.2012.02523.x [DOI] [PubMed] [Google Scholar]
- Choi JJ, Eum SY, Rampersaud E, Daunert S, Abreu MT, & Toborek M (2013). Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect, 121(6), 725–730. 10.1289/ehp.1306534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, … Cotter PD. (2014). Exercise and associated dietary extremes impact on gut microbial diversity. Gut, 63(12), 1913–1920. 10.1136/gutjnl-2013-306541 [DOI] [PubMed] [Google Scholar]
- Doukhanine E, Bouevitch A, Pozza L, & Merino C (2014). OMNIgene®·GUT enables reliable collection of high quality fecal samples for gut microbiome studies (2014). (White Paper) Retrieved from http://www.dnagenotek.com/US/pdf/PD-WP-00040.pdf.
- Doukhanine E, Bouevitch A, Brown A, LaVecchia JG, Merino C, & Pozza L (2016). OMNIgene®·GUT stabilizes the microbiome profile at ambient temperature for 60 days and during transport. (White Paper). Retrieved from http://www.dnagenotek.com/US/pdf/PD-WP-00042.pdf.
- Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, … Gibson DL. (2016). Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome, 4(1), 42 10.1186/s40168-016-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, … Ciancio MJ. (2014). Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One, 9(3), e92193 10.1371/journal.pone.0092193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane MM, Sharpton TJ, Laurent TJ, & Pollard KS (2014). A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS One, 9(1), e84689 10.1371/journal.pone.0084689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosicki GJ, Fielding RA, & Lustgarten MS (2017). Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif Tissue Int 10.1007/s00223-017-0345-5 [DOI] [PMC free article] [PubMed]
- Guo X, Xia X, Tang R, Zhou J, Zhao H, & Wang K (2008). Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol, 47(5), 367–373. 10.1111/j.1472-765X.2008.02408.x [DOI] [PubMed] [Google Scholar]
- Kolida A, Syzenko G, Moseiko V, Budosvska L, Puchkov K, Perederiy V, …(2017). Association between body mass index and Firmicutes?Bacteroidetes ratio in an adult Ukrainian popultion. BMC microbiology, 17(1), 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JE, Myslicki JP, Bomhof MR, Belke DD, Shearer J, & Reimer RA (2015). Exercise training modifies gut microbiota in normal and diabetic mice. Appl Physiol Nutr Metab, 40(7), 749–752. 10.1139/apnm-2014-0452 [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, & Gordon JI (2006). Microbial ecology: human gut microbes associated with obesity. Nature, 444(7122), 1022–1023. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- Martinez KB, Leone V, & Chang EB (2017). Western diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut Microbes, 8(2), 130–142. 10.1080/19490976.2016.1270811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EA, Velazquez KT, & Herbert KM (2015). Influence of high-fat diet on gut microbiota: a driving force for chronic disease risk. Curr Opin Clin Nutr Metab Care, 18(5), 515–520. 10.1097/MCO.0000000000000209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G, Gambino R, & Cassader M (2011). Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med, 62, 361–380. 10.1146/annurev-med-012510-175505 [DOI] [PubMed] [Google Scholar]
- Payne AN, Chassard C, Zimmermann M, Muller P, Stinca S, & Lacroix C (2011). The metabolic activity of gut microbiota in obese children is increased compared with normal-weight children and exhibits more exhaustive substrate utilization. Nutr Diabetes, 1, e12 10.1038/nutd.2011.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Li ZR, Green RS, Holzman IR, & Lin J (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr, 139(9), 1619–1625. 10.3945/jn.109.104638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LM, Bautista EJ, Nguyen H, Hanson BM, Chen L, Lek SH, … Weinstock GM. (2017). Community characteristics of the gut microbiomes of competitive cyclists. Microbiome, 5(1), 98 10.1186/s40168-017-0320-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petriz BA, Castro AP, Almeida JA, Gomes CP, Fernandes GR, Kruger RH, … Franco OL. (2014). Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics, 15, 511 10.1186/1471-2164-15-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queipo-Ortuno MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, … Tinahones FJ. (2013). Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One, 8(5), e65465 10.1371/journal.pone.0065465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Fuchs S, & Milo R (2016). Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol, 14(8), e1002533 10.1371/journal.pbio.1002533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F, & Backhed F (2013). The gut microbiota--masters of host development and physiology. Nat Rev Microbiol, 11(4), 227–238. 10.1038/nrmicro2974 [DOI] [PubMed] [Google Scholar]
- Smith MI, Yatsunenko T, Manary MJ,. Trehan I, Mkakosya R, Cheng J, … Liu J. (2013). Gut microbiomes of Malawian tiwn pairs discordant for kwashiorkor. Science, 339(6119), 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]


