Abstract
Throughout their lifetimes, all cells experience force. These forces are sensed by cell surface adhesion receptors, such as the cadherins and integrins. Much attention has focused on identifying how these adhesion receptors transmit force. In contrast, less is known regarding how these force-activated pathways are integrated with other cellular processes. In this review, we describe how cadherins and integrins transmit force, and discuss how these adhesion receptors are linked to cell metabolism. We focus on understanding this connection by highlighting how the cadherins and integrins interact with a master regulator of energy homeostasis, AMP-activated protein kinase (AMPK) and its upstream activator, Liver Kinase B1 (LKB1). We consider why there is a need for force transmission to be coupled to metabolism and highlight the major unanswered questions in the field.
Introduction
Cells respond to numerous forces, such as shear stress, compression, stretching, as well as internally generated tension. These forces are sensed by cell surface adhesion receptors, such as cadherins and integrins, which are physically connected to the cytoskeleton through interactions with actin associated proteins. In response to force, both integrins and cadherins: (1) cluster, (2) recruit a similar repertoire of proteins, and initiate signaling cascades that culminate in activation of the small GTPase, RhoA. RhoA indirectly regulates myosin II activity, which in conjunction with actin filaments, allows cells to respond to mechanical stimuli (Figure 1). This response includes generation of internal contractile forces, reorganization of the actin cytoskeleton, and growth of the associated adhesion complex-a process known as cell stiffening [1, 2].
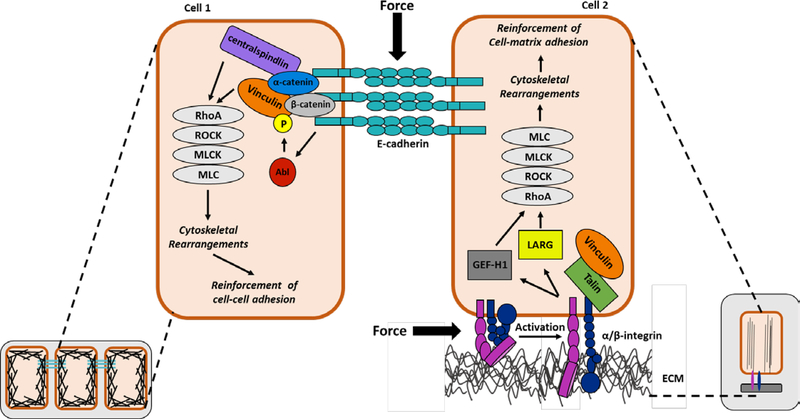
Figure 1: Integrin- and cadherin-mediated mechanotransduction pathways.
At sites of cell-cell contact, the extracellular domains of E-cadherin bind to E-cadherins on neighboring cells to provide strong cell–cell adhesion, while the cytoplasmic domain recruits catenins, which in turn associate with additional cytoskeletal and regulatory proteins, such as vinculin and centralspindlin. In response to force, cadherin induces the activation of Abelson tyrosine kinase (Abl) which leads to the phosphorylation of vinculin at Y822. This signaling event is necessary for the activation of the Rho GTPase pathway, ultimately leading to cadherin-mediated cell stiffening. At cell-matrix adhesions activated integrins interact with the extracellular matrix on the outside of the cell, which triggers activation of intracellular signaling and recruitment of actin binding proteins, such as talin and vinculin. In response to force, the integrins recruit and activate two distinct signaling pathways that trigger recruitment of two RhoA guanine nucleotide exchange factors, LARG and GEF-H1. These integrin-mediated signaling events are critical for the force-induced activation of RhoA and the reinforcement of integrin-actin linkages at the cell-matrix adhesions. Abl=Abelson tyrosine kinase; ROCK=Rho associated protein kinase; MLCK=myosin light chain kinase; MLC=myosin light chain; GEF-H1=guanine nucleotide exchange factor H1; LARG= leukemia-associated Rho-GEF; ECM=extracellular matrix
How the cadherin and integrin adhesion complexes stimulate cell stiffening has been the subject of intense scrutiny. Integrins are heterodimers of alpha and beta subunits that bind to the extracellular matrix on the outside of the cell. On the inside of the cell, the integrin cytoplasmic tails recruit various actin binding proteins, such as talin and vinculin (Figure 1). In response to mechanical force, the integrin tails undergo conformational changes. These changes promote talin binding which in turn stimulates the integrins to adopt an active conformation, associating with the extracellular matrix. Force also causes integrins to stimulate transduction cascades on the inside of the cell. Key among the force-activated cascades is a FAK/Ras/ERK signaling pathway that culminates in activation of the RhoA activator, GEF-H1. In addition, stimulation of a Fyn signaling pathway leads to activation of another RhoA activator, LARG [3]. Both GEF-H1 and LARG are guanine nucleotide exchange factors that promote RhoA activation by stimulating the exchange of GDP for GTP. E-cadherin responds similarly to force by undergoing conformational changes. These rearrangements allow for recruitment of actin binding proteins, such as alpha catenin (Figure 1). Alpha-catenin then binds centralspindlin—a protein complex that links the mitotic spindle to the plasma membranes during cytokinesis [4]. Centralspindlin, in turn, recruits Ect2—a guanine nucleotide exchange factor for RhoA [4]. Taken together these observations indicate that cadherins and integrins respond to force by activating RhoA guanine nucleotide exchange factors, but the repertoire of proteins they use to achieve this response is different. It is important to note that there other GTPase regulatory proteins that have been shown to be regulated by force or whose force sensitivity remains unexplored (reviewed in [5]). Hence it is likely that the list of force-activated Rho regulatory proteins will continue to expand.
Another protein critical for integrins and cadherins to respond to force is vinculin. Force stimulates vinculin recruitment to and accumulation in cell-cell and cell-matrix adhesions. At these adhesions, vinculin binds actin and bears the force [6], suggesting that vinculin function in cell-cell and cell-matrix adhesions is redundant. However, the behavior of these adhesions is often distinct, suggesting that mechanisms exist to achieve site-specific functions. Insight into how vinculin function can be distinguished is emerging and it is now appreciated that force on E-cadherin stimulates Abelson (Abl) tyrosine kinase to phosphorylate vinculin Y822 [7]. This phosphorylation event is unique to cadherin-mediated mechanotransduction and allows for vinculin binding to β-catenin, specifically recruiting vinculin to the cadherin complexes (but not integrin complexes) and inducing cell stiffening [7]. Recent investigations of the upstream regulators of vinculin Y822 have revealed that the cadherin adhesion complex is coupled to cell metabolism. This review will highlight recent advances in understanding how signals arising from cadherin- and integrin- containing adhesions are linked to the metabolic machinery.
Signal transduction mechanisms for regulating cell metabolism
All organisms need energy to grow, reproduce, maintain homeostasis, and respond to their environments. The preferred energy sources for humans are carbohydrates, fat, and protein. In contrast, cells in culture rely on two primary energy sources: glucose and glutamine [8, 9]. As the glucose concentration decreases, glutamine becomes the sole energy source for cultured cells [9]. Consequently, the effects of glucose deprivation are visible quite quickly; cell cycle arrest begins shortly after glucose starvation [9]. Cultured cells and organisms have evolved mechanisms for stimulating or depressing their metabolic pathways to allow their energy sources to be consumed in quantities that match their energy demands. Indeed, the expression and abundance of metabolic enzymes and regulatory factors are tightly controlled. Post-translational modifications and allosteric effectors confer an additional level of regulation. At the signal transduction level, AMPK is a key regulator of energy metabolism. AMPK is a serine/threonine kinase that is activated when AMP levels are high or in response to physiological stimuli, such as muscle contraction and hormones [10]. Activation of AMPK is further enhanced by phosphorylation of its activation loop by upstream kinases. In mammals, the major upstream kinase phosphorylating AMPK is LKB1 [11]. Once active, AMPK stimulates energy generating processes (glucose uptake and fatty acid oxidation) and decreases energy consuming processes (protein and lipid synthesis) [12]. The ability to monitor the energy status and shift metabolism to maintain homeostasis in cells and organisms has allowed AMPK to emerge as a master regulator of mammalian metabolism.
Links between cell-cell adhesion and AMPK
Links between energy metabolism and cell adhesion have remained largely unexplored. A recent study revealed that treatment of epithelial monolayers with shear stress or application of force directly to E-cadherin stimulates AMPK activation and recruitment to the E-cadherin adhesion complex [13]. AMPK activation, and its localization to cell-cell junctions, requires LKB1 [13]. Furthermore, AMPK is a component of a signal transduction cascade culminating in contractility. In this signal transduction cascade, E-cadherin triggers Abl-mediated phosphorylation of Y822 vinculin leading to RhoA activation and phosphorylation and activation of myosin II (Figure 2). Inhibition of LKB1 or AMPK prevents force- induced Abl activation, vinculin phosphorylation, GTP-loading of RhoA, and myosin II phosphorylation. These observations validate AMPK as an upstream modulator of contractility at cell-cell contacts [13].
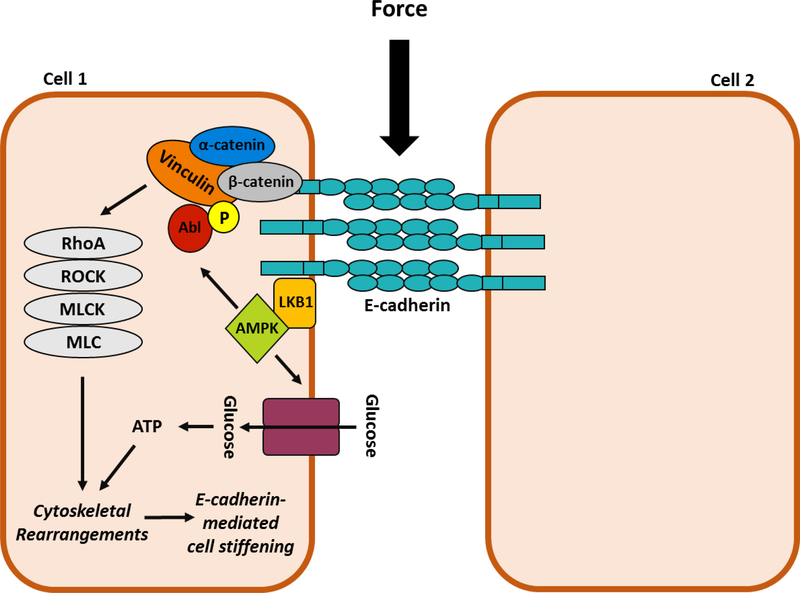
Figure 2: E-cadherin-mediated force transmission stimulates AMPK culminating in energy production.
Force on E-cadherin triggers the recruitment LKB1 and the subsequent activation of AMPK. Activate AMPK has two effects. First, it stimulates a signal transduction cascade that includes Abl-mediated phosphorylation of Y822 vinculin (yellow circle) and the subsequent activation of a RhoA-ROCK-MLCK pathway that leads to increased MLC phosphorylation and elevated contractility. Second, AMPK stimulates glucose uptake and oxidation to ATP to provide energy to allow the actin cytoskeleton to reorganized. Both signaling events are necessary for cell stiffening. LKB1=liver kinase B1; AMPK=AMP- activated protein kinase; Abl=Abelson tyrosine kinase; ROCK=Rho associated protein kinase; MLCK=myosin light chain kinase; MLC=myosin light chain.
While we highlight the role of AMPK in E-cadherin mechanotransduction, other evidence links AMPK and LKB1 to E-cadherin. First, AMPK activators suppress the loss of E-cadherin and subsequent cell-cell adhesion that accompanies the transition of cancer cells from an epithelial to mesenchymal phenotype [14–18]. Second, AMPK is required to maintain two E-cadherin dependent processes in epithelial cells—(1) polarity and (2) barrier function [19–21]. In further support of role, AMPK is now generally appreciated to maintain cell-cell contacts in tissues, including the brain, heart, intestine, and kidney [22–26]. Third, the idea that AMPK lies downstream of E-cadherin is also supported by studies of the role of folliculin—a protein associated with the Birt-Hogg-Dube—a disease characterized by lung collapse and tumor formations in the kidney, colon and skin. Folliculin binds AMPK [27], and disease mutations in the folliculin gene produce truncated proteins that do not bind AMPK [28]. Interestingly, AMPK cannot be activated in epithelial cells in the absence of folliculin. The mechanism for the lack of AMPK activity is not well understood but is linked to changes in E-cadherin expression and localization to cell-cell adhesions. Also of note, deletion of folliculin in the lung epithelium leads to cellular apoptosis, alveolar enlargement, and impaired alveolar epithelial barrier function [29]. All of these events are linked to aberrant force transmission, suggesting the folliculin studies may warrant reconsideration in light of the newer studies. Taken together, the observations establish AMPK and LKB1 are bonafide E-cadherin effectors.
Why do cell adhesion molecules activate AMPK?
In response to force, E-cadherin activates AMPK culminating in the uptake of glucose and its oxidation to ATP. Many cellular processes require ATP, and older work suggests the actin cytoskeleton is one of them. Indeed, studies of resting platelets indicate approximately 50% of the total ATP consumed in cells is needed to support actin cytoskeleton [30]. It could be argued that this estimate might be too high as platelets have a higher turnover of cytoplasmic ATP than most cells. However, similar studies of live neurons verified that 50% of the cytoplasmic ATP is needed to support the actin cytoskeleton[31]. The latter study went a step further to demonstrate the requirement for ATP is independent of the energy used by the Na+-K+-ATPase—a major energy consumer in ionic homeostasis [31]. All of these measurements were made in cells in culture which are not expected to be dramatically rearranging their actin cytoskeletons. In contrast, force triggers robust actin cytoskeletal polymerization and rearrangements [32, 33]. Hence, cells under force require vast amounts of ATP to support the polymerization and rearrangement of actin and the amount required is likely to be higher than the estimates from platelets and neurons suggest.
Estimates for the amount of energy the actin cytoskeleton consumes to respond to force are not available. However, epithelial cells exposed to shear stress exhibit a 3.8-fold increase in actin deposition in cell-cell junctions [13]. This increase in F-actin enrichment requires AMPK. Inhibiting either AMPK or the AMPK-derived energy prevents cells from reinforcing their actin cytoskeletons [13]. Thus, epithelial cells under force activate AMPK to intensify their metabolism to provide the energy necessary to allow for F-actin reinforcement at cell-cell junctions.
It is tempting to speculate that the AMPK-derived ATP could also be used to support the sliding of myosin along actin filaments. In non-muscle, mammalian cells, myosin II is the major isoform. Upon phosphorylation at serine 19, myosin II generates force by binding to and sliding along actin filaments—a process that requires the hydrolysis of ATP. In muscle cells, vast amounts of ATP are needed to support contraction. However, myosin is unlikely to be a major energy drain in non-muscle cells as they contain very little myosin and far less of it is bound to actin filaments. Estimates of the molar ratio of actin to myosin in muscle cells are 6:1. In stark contrast, this molar ratio increases to 100:1 in non-muscle cells [34]. Hence, the sliding of non-muscle myosin II along actin filaments is not expected to represent a major energy drain in non-muscle cells.
It is also plausible that AMPK has effects independent of its ability to stimulate energy production. AMPK is a serine/threonine kinase that can phosphorylate many targets. Some of these targets are regulators of RhoA or myosin—two proteins critical for responding to force. For example, in a dividing cell, myosin II and actin accumulate midway between the poles of the spindle and align into a contractile ring which generates the constricting force to separate one cell into two cells. Both active AMPK and serine 19 phosphorylated myosin II localize to the mitotic spindle [35]. AMPK depletion reduces the amount of phosphorylated myosin associated with the spindle pole and decreases spindle alignment. In addition, there is some evidence that indicates AMPK directly phosphorylates myosin regulatory light chains [19]. However, this work has been called into question because the myosin light chain regulatory subunits do not have a consensus phosphorylation site for AMPK [36]. Furthermore, other studies reveal that the commercially available recombinant AMPK used to demonstrate direct phosphorylation of the myosin light chain regulatory subunits is contaminated with other kinases. In support of this notion, pure AMPK did not efficiently phosphorylate myosin light chains [36]. Alternatively, AMPK could control the dephosphorylation of the myosin regulatory light chains. An AMPK-related kinase, known as NUAK1, phosphorylates and inactivates the myosin phosphatase, suggesting that AMPK could perform a similar type of regulation [37]. Finally, it is equally plausible that AMPK affects myosin by modulating the function of its upstream activators. In support of this possibility, LKB1, an upstream AMPK activator, binds to the guanine nucleotide exchange factor, p114RhoGEF [38], and AMPK phosphorylate RhoA at Ser188, thereby reducing Rho-Rock signaling [39]. Taken together these observations indicate that AMPK directly or indirectly modulates the phosphorylation of myosin II.
Links between cell-matrix adhesions and AMPK
AMPK is also emerging as a modulator of integrin-mediated events. AMPK is a component of the integrin adhesome [40]. In addition, AMPK localizes to the leading edge of migrating cells—a locale where integrin function is well characterized [41]. Cells respond to migratory cues by extending a leading edge or protrusion in the direction of the migratory cue. These protrusions contain thin, sheet-like membrane protrusions known as lamellipodia. The lamellipodia are rich in a dense branched network of actin filaments. The leading edge protrudes by polymerizing new actin filaments and disassembling older filaments behind the leading edge. The growing actin filaments are thought to provide the force necessary to protrude and push the cell membrane forward [42]. In addition to actin networks, the leading edge also contains small nascent integrin adhesions. These adhesions turnover rapidly or give rise to focal complexes, which anchor the protrusion to the extracellular matrix behind the leading edge. In turn, the adhesions develop into more mature adhesions known as focal adhesions which assemble from near the front of the cell to its rear. In addition, some cells form fibrillar adhesions—stable and elongated adhesive structures that are not prominent in rapidly migrating cells [43].
Intense effort devoted towards understanding how cells migrate has revealed a role for AMPK. The exact nature of this role remains to be determined. Accumulating evidence indicates that AMPK modulates integrins directly and indirectly through effects on the actin cytoskeleton. The leading edge of migrating cells has increased levels of mitochondria and mitochondrial-derived ATP when compared to the cell body [41]. The increased ATP levels are accompanied by a significantly lower ATP:ADP ratio which triggers activation of AMPK. In this cellular region, active AMPK increases mitochondrial flux, ATP levels, and cytoskeletal dynamics; its inhibition suppresses cell migration and invasion. Another study describes a requirement for AMPK in integrin-mediated events. Indeed, AMPK is required for the reorganization of the actin cytoskeleton that supports monocyte adhesion to adhere to endothelial cells (Figure 3) [44]. Hence, AMPK positively modulates actin dynamics and protrusive events that occur in actively adhering and migrating cells.
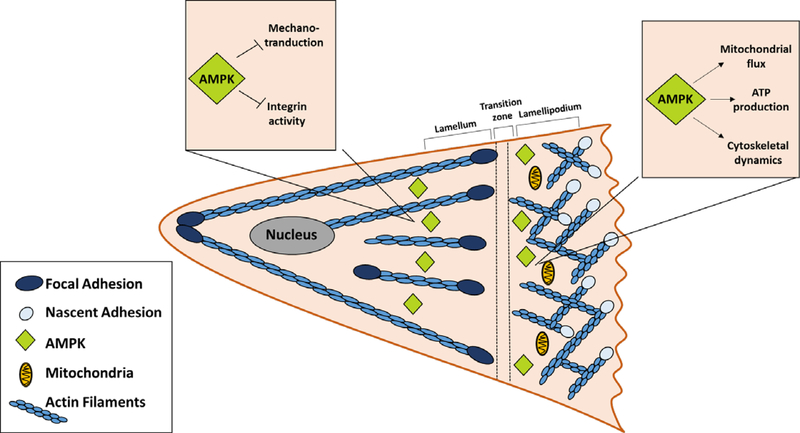
Figure 3: Model for the actions of AMPK in a migrating cell.
The leading edge or lamellipodium of a migrating cell is integrin-rich nascent adhesions and mitochondria. In this region, AMPK stimulates mitochondria flux, ATP production and cytoskeletal dynamics. Behind the leading edge is a transition zone where the nascent adhesions mature or disassemble. Mature focal adhesions are present further back in the cell in the lamellum. In mature adhesions, AMPK inhibits integrin activity and mechanotransduction.
Other studies indicate that AMPK inhibits integrins and cell migration. In support of an inhibitory role, AMPK was identified in an RNAi screen of proteins negatively regulating integrin activity [45]. Subsequent studies confirmed increases in integrin activation, fibrillar adhesion formation and mechanotransduction when AMPK is inhibited [45, 46]. AMPK also modulates actin and microtubule polymerization and decreases the rate and persistence of cell migration by phosphorylating the actin binding proteins VASP [47]and PDlim5, and the microtubule binding protein CLIP-170 [48, 49]. Additionally, AMPK phosphorylates and targets the endosomal trafficking protein—sorting nexin 17 (SNX17)—for degradation, culminating in decreased β1 and β5 integrins at the plasma membrane [50, 51]. Taken together, these observations indicate that AMPK inhibits integrins and integrin-mediated events and are in contrast to the role of AMPK in modulating integrin function in the leading edge of migrating cells
There is a possible explanation for the discrepancy in the requirement for AMPK in integrin-mediated events. The evidence supporting a role for AMPK in cell migration examines AMPK in the leading edge of cells or in actively adhering cells-two settings that require energy intensive, actin polymerization and protrusive formation. In contrast, the data demonstrating AMPK plays an inhibitory role are largely confined to mature focal adhesions and fibrillar adhesions which are absent from the leading edge and are not expected to increase the energy burden. Taken together these findings suggest AMPK positively regulates integrin events and the actin cytoskeleton in the leading edge of cells and inhibits these processes in more distal regions of the cell where the more mature adhesive structures reside. Hence, it is possible that AMPK plays different roles depending on its subcellular localization and its mechanical environment. Different subcellular functions for other proteins have been described in migrating cells. Most well characterized among these is RhoA, which must be inhibited at the leading edge to allow for membrane protrusion and activated at the rear of the cell to promote migration. An alternative possibility is the different cell types contribute to the phenotypic differences. Numerous studies have shown that AMPK does not function similarly, or through identical targets, in different cells [52]. More work is needed to resolve these complexities.
Conclusions and Future Directions
Progress in the field of mechanotransduction has been substantial in the recent years. This work reveals a surprising connection between the adhesive machinery and the enzymes that regulate mammalian metabolism. These new findings precipitated a closer inspection of older work and have revealed that other connections between cell adhesion and cell metabolism exist. Furthermore, it is increasingly apparent that a cells response to force requires energy—with significant levels of energy being used to reinforce the actin cytoskeleton.
With these new findings, exciting questions linking cellular mechanics and metabolism are open for discussion. Key among these questions is whether other cellular processes that involve acute actin polymerization activate AMPK to provide energy to sustain these events. Additionally, it remains unclear how many linkages there are between the adhesive and metabolic machineries. The integrin adhesome contains 43 gene products with metabolic function [40], and the cadherin adhesome contains 52 metabolic proteins [53]. Hence, it is likely that AMPK is only a small perceptible component of pathways that remain to be uncovered. Finally, metabolism is experiencing a renaissance because disturbances in metabolic regulation are increasingly appreciated as a cause of disease. Hence, it will be important to investigate the linkages between cell metabolism and mechanotransduction in diseases such as cancer, diabetes and obesity which are accompanied by losses in cell adhesion and metabolic reprogramming.
Acknowledgements
We thank Peter Rubenstein, Hannah Campbell and Christy Heidema for critical reading of this manuscript. AMS is supported by the Predoctoral Training Grant in the Pharmacological Sciences (T32 GM067795) and an Alfred P. Sloan Fellowship and work in the DeMali laboratory is supported by the National Institutes of Health grant to KAD (GM112805).
References
- 1.Marjoram RJ, Lessey EC, and Burridge K, Regulation of RhoA activity by adhesion molecules and mechanotransduction. Curr Mol Med, 2014. 14(2): p. 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vicente-Manzanares M, et al. , Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol, 2009. 10(11): p. 778–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilluy C, et al. , The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol, 2011. 13(6): p. 722–7.••This manuscript identifies how integrins activate RhoA in a cell under force. The authors identify two signal transduction cascades that are stimulated by force and culminate in activation of GEF-H1 and LARG, two RhoA guanine nucleotide exchange factors.
- 4.Ratheesh A, et al. , Centralspindlin and alpha-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol, 2012. 14(8): p. 818–828.••This manuscript describes how RhoA is activated downstream of E-cadherin. The authors present evidence that alpha catenin, an E-cadherin associate protein, binds to centraspindilin,and centraspindilin recruits Ect2, a RhoA guanine nucleotide exchange factor. Since centraspindilin and Ect2 are associate with cytokinesis, this manuscript provides the first evidence of an extra-mitotic role for these proteins.
- 5.Lawson CD and Burridge K, The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases, 2014. 5: p. e27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bays JL and DeMali KA, Vinculin in cell-cell and cell-matrix adhesions. Cell Mol Life Sci, 2017. 74(16): p. 2999–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bays JL, et al. , Vinculin phosphorylation differentially regulates mechanotransduction at cell-cell and cell-matrix adhesions. J Cell Biol, 2014. 205(2): p. 251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nash RW, McKay BS, and Burke JM, The response of cultured human retinal pigment epithelium to hypoxia: a comparison to other cell types. Invest Ophthalmol Vis Sci, 1994. 35(6): p. 2850–6. [PubMed] [Google Scholar]
- 9.Wood JP, et al. , Energy substrate requirements of rat retinal pigmented epithelial cells in culture: relative importance of glucose, amino acids, and monocarboxylates. Invest Ophthalmol Vis Sci, 2004. 45(4): p. 1272–80. [DOI] [PubMed] [Google Scholar]
- 10.Gowans GJ and Hardie DG, AMPK: a cellular energy sensor primarily regulated by AMP. Biochem Soc Trans, 2014. 42(1): p. 71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woods A, et al. , LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol, 2003. 13(22): p. 2004–8. [DOI] [PubMed] [Google Scholar]
- 12.Hardie DG, AMPK--sensing energy while talking to other signaling pathways. Cell Metab, 2014. 20(6): p. 939–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bays JL, et al. , Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat Cell Biol, 2017. 19(6): p. 724–731.••This manuscript seeks to understand if an how mechanotransduction and cell metabolism are coupled. The authors demonstrate that force on E-cadherin activates AMPK. The force-activated AMPK stimulates RhoA-mediated contractility and reinforcement of the actin cytoskeleton. In addition, AMPK provides energy for these events by stimulating the uptake and oxidation of glucose.
- 14.Matoba R, et al. , Suppressive effect of AMP-activated protein kinase on the epithelial-mesenchymal transition in retinal pigment epithelial cells. PLoS One, 2017. 12(7): p. e0181481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JH, et al. , AMP-activated protein kinase inhibits TGF-beta-, angiotensin II-, aldosterone-, high glucose-, and albumin-induced epithelial-mesenchymal transition. Am J Physiol Renal Physiol, 2013. 304(6): p. F686–97. [DOI] [PubMed] [Google Scholar]
- 16.Chou CC, et al. , AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a signaling axis. Cancer Res, 2014. 74(17): p. 4783–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee P, et al. , Metformin mediated reversal of epithelial to mesenchymal transition is triggered by epigenetic changes in E-cadherin promoter. J Mol Med (Berl), 2016. 94(12): p. 1397–1409. [DOI] [PubMed] [Google Scholar]
- 18.Thakur S, et al. , Activation of AMP-activated protein kinase prevents TGF-beta1-induced epithelial-mesenchymal transition and myofibroblast activation. Am J Pathol, 2015. 185(8): p. 2168–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JH, et al. , Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature, 2007. 447(7147): p. 1017–20. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, et al. , AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci U S A, 2006. 103(46): p. 17272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng B and Cantley LC, Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci U S A, 2007. 104(3): p. 819–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castanares-Zapatero D, et al. , Connection between cardiac vascular permeability, myocardial edema, and inflammation during sepsis: role of the alpha1AMP-activated protein kinase isoform. Crit Care Med, 2013. 41(12): p. e411–22. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, et al. , Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J Neuroinflammation, 2014. 11: p. 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takata F, et al. , Metformin induces up-regulation of blood-brain barrier functions by activating AMP-activated protein kinase in rat brain microvascular endothelial cells. Biochem Biophys Res Commun, 2013. 433(4): p. 586–90. [DOI] [PubMed] [Google Scholar]
- 25.Seo-Mayer PW, et al. , Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am J Physiol Renal Physiol, 2011. 301(6): p. F1346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spruss A, et al. , Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab Invest, 2012. 92(7): p. 1020–32. [DOI] [PubMed] [Google Scholar]
- 27.Baba M, et al. , Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A, 2006. 103(42): p. 15552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Possik E, et al. , Folliculin regulates ampk-dependent autophagy and metabolic stress survival. PLoS Genet, 2014. 10(4): p. e1004273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goncharova EA, et al. , Folliculin controls lung alveolar enlargement and epithelial cell survival through E-cadherin, LKB1, and AMPK. Cell Rep, 2014. 7(2): p. 412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniel JL, et al. , Nucleotide exchange between cytosolic ATP and F-actin-bound ADP may be a major energy-utilizing process in unstimulated platelets. Eur J Biochem, 1986. 156(3): p. 677–84. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein BW and Bamburg JR, Actin-ATP hydrolysis is a major energy drain for neurons. J Neurosci, 2003. 23(1): p. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CS, Tan J, and Tien J, Mechanotransduction at cell-matrix and cell-cell contacts. Annu Rev Biomed Eng, 2004. 6: p. 275–302. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, et al. , Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A, 2010. 107(22): p. 9944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niederman R and Pollard TD, Human platelet myosin. II. In vitro assembly and structure of myosin filaments. J Cell Biol, 1975. 67(1): p. 72–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thaiparambil JT, Eggers CM, and Marcus AI, AMPK regulates mitotic spindle orientation through phosphorylation of myosin regulatory light chain. Mol Cell Biol, 2012. 32(16): p. 3203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bultot L, et al. , Myosin light chains are not a physiological substrate of AMPK in the control of cell structure changes. FEBS Lett, 2009. 583(1): p. 25–8. [DOI] [PubMed] [Google Scholar]
- 37.Zagorska A, et al. , New roles for the LKB1-NUAK pathway in controlling myosin phosphatase complexes and cell adhesion. Sci Signal, 2010. 3(115): p. ra25. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, et al. , LKB1 controls human bronchial epithelial morphogenesis through p114RhoGEF-dependent RhoA activation. Mol Cell Biol, 2013. 33(14): p. 2671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gayard M, et al. , AMPK alpha 1-induced RhoA phosphorylation mediates vasoprotective effect of estradiol. Arterioscler Thromb Vasc Biol, 2011. 31(11): p. 2634–42. [DOI] [PubMed] [Google Scholar]
- 40.Winograd-Katz SE, et al. , The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol, 2014. 15(4): p. 273–88. [DOI] [PubMed] [Google Scholar]
- 41.Cunniff B, et al. , AMPK activity regulates trafficking of mitochondria to the leading edge during cell migration and matrix invasion. Mol Biol Cell, 2016. 27(17): p. 2662–74.•This manuscript investigates the role of mitochondria and AMPK in the leading edge of migrating cells. The authors demonstrate that mitochondria are recruited to the leading edge in migrating cells and AMPK is activated. Evidence is presented demonstrating that AMPK and increased mitochondrial flux are needed for cell migration.
- 42.Mogilner A and Oster G, Cell motility driven by actin polymerization. Biophys J, 1996. 71(6): p. 3030–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons JT, Horwitz AR, and Schwartz MA, Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol, 2010. 11(9): p. 633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang MY, et al. , PKC-dependent human monocyte adhesion requires AMPK and Syk activation. PLoS One, 2012. 7(7): p. e40999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rantala JK, et al. , SHARPIN is an endogenous inhibitor of beta1-integrin activation. Nat Cell Biol, 2011. 13(11): p. 1315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Georgiadou M, et al. , AMPK negatively regulates tensin-dependent integrin activity. J Cell Biol, 2017. 216(4): p. 1107–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blume C, et al. , AMP-activated protein kinase impairs endothelial actin cytoskeleton assembly by phosphorylating vasodilator-stimulated phosphoprotein. J Biol Chem, 2007. 282(7): p. 4601–12. [DOI] [PubMed] [Google Scholar]
- 48.Yan Y, et al. , Augmented AMPK activity inhibits cell migration by phosphorylating the novel substrate Pdlim5. Nat Commun, 2015. 6: p. 6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakano A, et al. , AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol, 2010. 12(6): p. 583–90. [DOI] [PubMed] [Google Scholar]
- 50.Schaffer BE, et al. , Identification of AMPK Phosphorylation Sites Reveals a Network of Proteins Involved in Cell Invasion and Facilitates Large-Scale Substrate Prediction. Cell Metab, 2015. 22(5): p. 907–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bottcher RT, et al. , Sorting nexin 17 prevents lysosomal degradation of beta1 integrins by binding to the beta1-integrin tail. Nat Cell Biol, 2012. 14(6): p. 584–92. [DOI] [PubMed] [Google Scholar]
- 52.Mantovani J and Roy R, Re-evaluating the general(ized) roles of AMPK in cellular metabolism. FEBS Lett, 2011. 585(7): p. 967–72. [DOI] [PubMed] [Google Scholar]
- 53.Toret CP, et al. , A genome-wide screen identifies conserved protein hubs required for cadherin-mediated cell-cell adhesion. J Cell Biol, 2014. 204(2): p. 265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]