Abstract
Rift Valley fever virus (RVFV) causes significant morbidity and mortality in humans and livestock throughout Africa and the Middle East. The clinical disease ranges from mild febrile illness, to hepatitis, retinitis, encephalitis and fatal hemorrhagic fever. RVFV NSs protein has previously been shown to interfere in vitro with the interferon response, and RVFV lacking the NSs protein is attenuated in several animal models. Monocytes and macrophages are key players in the innate immune response via expression of various cytokines and chemokines. Here we demonstrate that wild-type RVFV infection of human monocyte-derived macrophages leads to a productive infection and inhibition of the innate immune response via decreased expression of IFN-α2, IFN-β and TNF-α. Using a recombinant virus lacking the NSs protein, we show that this effect is mediated by the viral NSs protein. Finally, analysis of RVF patient samples demonstrated an association between a pro-inflammatory cytokine response and patient survival.
Keywords: Rift Valley fever virus, RVFV, Interferon, Tumor necrosis factor, Immunity, Cytokine, Monocyte, Macrophage, Survival, Hemorrhagic fever
Background
Rift Valley fever virus (RVFV) is a mosquito-borne hemorrhagic fever virus that causes high morbidity and mortality in humans and livestock. It was first identified in 1931 in Kenya after isolation from a sheep in the Rift Valley (Daubney et al., 1931). The virus has caused disease throughout continental Africa, Madagascar, Yemen and Saudi Arabia (Bird et al., 2009). Recent reports of mosquito vector capacity in North America make this virus not only a scourge on the developing world, but also a potential threat to the US (Turell et al., 2010).
RVFV is a veterinary pathogen that infects cattle, goats, and sheep. Up to 90% mortality has been reported in newborn animals and as high as 30% in adult animals (Swanepoel and Coetzer, 1994). Consistent with its high degree of pathogenicity in juvenile animals, RVFV is also abortigenic; 40–100% of pregnant animals will abort during an outbreak leading to “abortion storms” (Daubney et al., 1931; Swanepoel and Coetzer, 1994). Furthermore, livestock caretakers are exposed to virus in the process of caring for sick and dying animals; both blood and amniotic fluid contain high quantities of virus.
The virus can be transmitted to humans by contact with infected livestock or by the bite of an infected mosquito. Infected individuals typically have a mild disease consisting of fever, malaise, and myalgia. A small percentage of individuals will develop severe disease manifested as hepatitis, encephalitis, retinitis or hemorrhagic fever, which are the hallmarks of fulminant RVFV clinical disease. The overall case fatality is estimated at 0.5–1%. However, in patients whose clinical illness is sufficiently severe to bring them to the attention of medical personnel, case fatality has been reported to be as high as 29%, as was seen in the Kenya 2006–2007 outbreak (Centers for Disease Control and P., 2007). Laboratory findings that are frequently present in RVFV infected patients include leucopenia, thrombocytopenia, and elevated liver transaminases, indicative of the hepatitis that is often associated with infection.
RVFV is a member of the family Bunyaviridae. It is an enveloped virus that has a negative stranded RNA genome consisting of three fragments, aptly named S (small), M (medium), and L (large). The S segment encodes two proteins, a nucleocapsid protein that coats the viral genome in the virion, and a non-structural protein (NSs). The M segment encodes two viral glycoproteins that are expressed on the surface of the virion, and a nonstructural protein (NSm). The L segment encodes the viral RNA polymerase that is responsible for both transcription and replication of the virus (Fields et al., 2007).
The NSs protein is especially interesting in that it is a filamentous nuclear protein expressed by a virus that replicates and assembles in the cytoplasm of infected cells (Yadani et al., 1999). Several investigators have evaluated the role of the NSs protein in altering the host immune response. Initial studies utilized a naturally occurring variant that has a deletion in the S segment such that the NSs protein is truncated, cytoplasmic and rapidly degraded (Muller et al., 1995; Vialat et al., 2000). This variant, known as clone 13, was attenuated in wild type (WT) mice but lethal in IFN α/β receptor deficient mice, and was a potent inducer of Type 1 interferons, unlike the WT virus (Billecocq et al., 2004; Bouloy et al., 2001; Vialat et al., 2000). Clone 13 has also been shown to be immunogenic and protective in sheep (Dungu et al., 2010). More recently, a reverse genetics system has become available for RVFV, thereby facilitating studies of viral pathogenesis (Gerrard et al., 2007; Habjan et al., 2008b; Ikegami et al., 2006). This system has been used to generate viruses with full gene deletions in NSs or mutations of specific regions of the gene. Viruses with whole gene deletions have become live attenuated vaccine candidates since they provided protection in the Wistar-Furth rat model (Bird et al., 2008). This reverse genetics system has proven to be a powerful tool in the study of NSs-mediated pathogenesis.
At the molecular level, the NSs protein interacts with components of the general transcription factor, TFIIH, leading to a generalized down-regulation of host-cell transcription in infected cells (Le May et al., 2004). In addition, specific interactions of NSs with transcription factors YY1 and SAP30 lead to silencing of the IFN-β promoter in mouse fibroblasts (Le May et al., 2008). Via its SAP30 interacting domain, NSs also interacts with pericentromeric chromosomal sequences and causes chromosomal segregation defects in mouse fibroblasts and fetal sheep kidney cells (Mansuroglu et al., 2010). NSs facilitates proteasomal-mediated degradation of PKR, a protein that is important in sensing the presence of dsRNA, shutting down protein synthesis and signaling apoptosis in infected cells (Habjan et al., 2009; Ikegami et al., 2009). The NSs protein has multiple functions in alteration of the innate immune response: generalized and specific transcriptional down-regulation of genes active in innate immunity and targeted degradation of factors involved in the innate immune response. Given the varied spectrum of clinical illness resulting from infection there is clearly a dynamic interplay between the host’s ability to mount an immune response, and these protean viral effects which would seemingly disarm the immune system and make the host vulnerable to disease. Therefore, there is still much to understand about the molecular pathogenesis of RVFV and its interplay with the host immune system.
Macrophages are antigen presenting cells that exist in both circulating and resident populations throughout the body. Upon contact with an antigen, they release cytokines to stimulate recruitment of neutrophils and other immune cells, activate IFN based pathways as part of the innate immune response, and signal T cells and B cells to begin the transition from an innate to an adaptive immune response (Paul, 2008).
Animal models and clinical specimens demonstrate positive immunostaining for viral antigen in both the hepatocytes and the resident macrophages of the liver, the Kupffer cells (Kamal, 2009; Shieh et al., 2010; Smith et al., 2010). Circulating white blood cells have also been reported to stain immunopositive in infected goats (Kamal, 2009), and antigen positive dendritic cells have been reported in the spleen of infected mice (Smith et al., 2010). Furthermore, there is microglia proliferation and neuronophagia in the CNS in infected animals (Kamal, 2009). Since the liver and the CNS are main sites for RVFV mediated disease, it is possible that infection of macrophages could represent an important early target and a mechanism for viral spread. Given the known function of the NSs protein in alteration of the innate immune response, we hypothesize that a key component of RVFV pathogenesis is modification of the initiation and/or functionality of the innate immune response in macrophages. To test this hypothesis, we infected human monocyte derived macrophages (MDM) with WT RVFV or recombinant RVFV lacking the NSs gene (ΔNSs RVFV) and assessed replication, virus production, cytopathic effects, and the expression of cytokines under these conditions. Furthermore, data from these experiments, and those done by others with different viral hemorrhagic fever viruses, led us to hypothesize that a pattern of cytokine secretion in infected individuals might be predictive of survival. This hypothesis was supported by analysis of the cytokine expression patterns in human serum samples from the Saudi Arabian 2000–2001 RVFV outbreak.
Results
infection of macrophages with RVFV
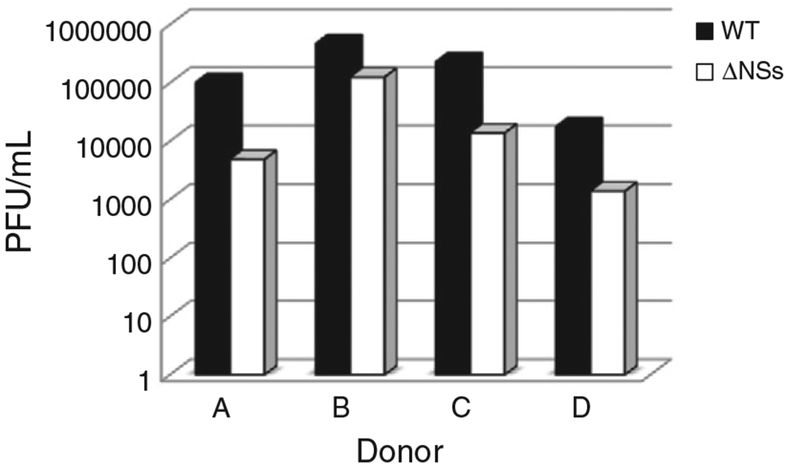
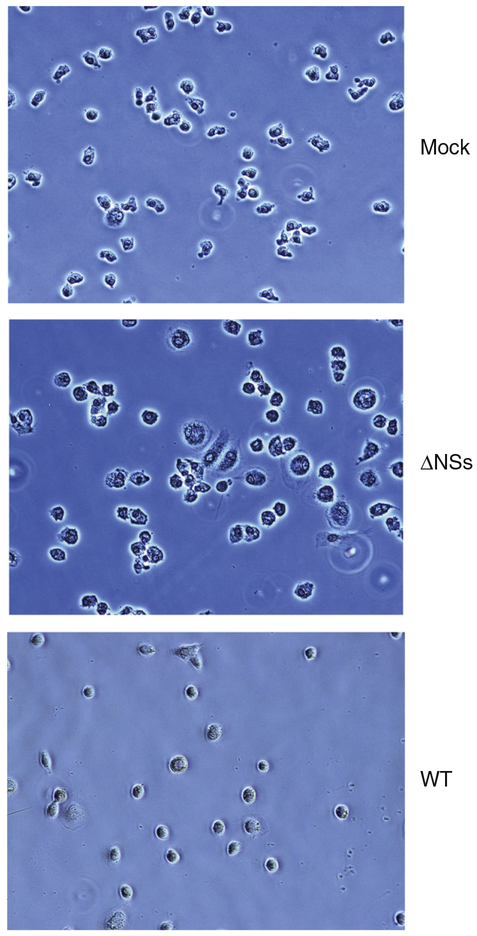
CD14 positive MDM from 4 separate donors were used for these experiments to control for donor-to-donor variability. Cells were infected with either the WT or ΔNSs RVFV and supernatants were analyzed at various times post-infection by plaque assay to quantitate viral production. As indicated in Fig. 1, there was slight donor-to-donor variability in the maximal titers at 24 h post infection (hpi); however, WT virus grew to 0.5 to 1 log higher titers than the ΔNSs virus for each donor. Virus was detected as early as 12 hpi for both WT and ΔNSs viruses, indicating that the ΔNSs virus kinetics were not delayed compared to the WT virus (data not shown). In contrast, infection of Vero cells with WT or ΔNSs RVFV produces equivalent titers (1.5×107 PFU/mL and 4.6×107 PFU/mL respectively). Interestingly, by 48 hpi, there was 80–90% CPE with cell death in the MDM infected with WT virus and little to none in the MDM infected with the ΔNSs virus, and this effect could be seen as early as 12 hpi (Fig. 2). It is also of note in this figure that ΔNSs infected cells have an activated phenotype, being larger and having greater variability in morphology than the mock infected cells. WT infected cells are small and rounded up. By 72 hpi all WT infected cells were dead.
Fig. 1.
RVFV productively infects monocyte derived human macrophages. MDM were infected with WT or ΔNSs RVFV. Supernatants were collected at various times post infection. Supernatants were titered on Vero E6 cells. WT virus (black bars) grew to higher titers than the ΔNSs virus (white bars) on cells from the same donor. Data are presented as PFU/mL at 24 h post infection. 4 different donors are represented in the figure.
Fig. 2.
WT virus causes marked CPE in infected cells while ΔNSs virus does not. MDM were mock-infected, or infected with WT, or ΔNSs RVFV. At 12 hpi cells were photographed under white light using the 20× objective to demonstrate the CPE caused by WT virus.
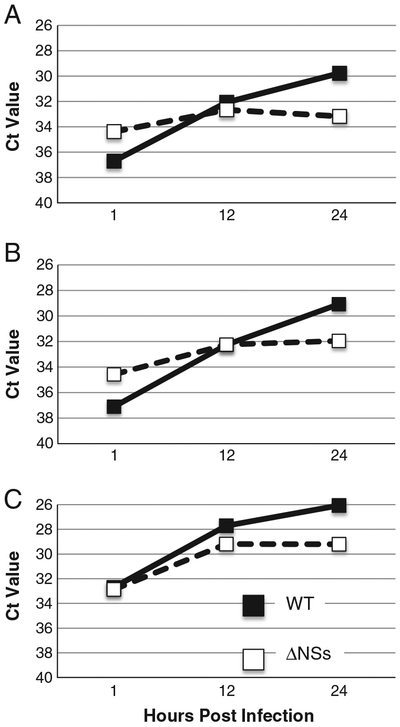
The RNA from WT or ΔNSs RVFV infected MDM from 3 of the donors was analyzed by real time RT-PCR to assess viral replication. Absolute Ct values were corrected by normalization to 18S RNA levels for each sample. Both WT and ΔNSs viruses replicated with similar kinetics; an increase in viral RNA was detected routinely by 12 hpi (Fig. 3). The 1 hpi time point represents the amount of input virus. It is noteworthy that although all experiments were performed with an moi of 5, for the experiments done with donors A and B, there appeared to be more viral RNA present at the 1 h time point for the ΔNSs infected cells. Despite the fact that there was slightly more input RNA, the WT virus still replicated to higher levels than the ΔNSs virus by 24 hpi (Fig. 3). These data demonstrate that primary human MDM are permissive for RVFV infection. Macrophages could be an early and important in vivo target of infection. Given the known role of the NSs protein in immune modulation, we hypothesized that macrophages infected with the ΔNSs virus would exhibit a different pattern of cytokine secretion than those infected with the WT virus.
Fig. 3.
ΔNSs RVFV replicates to lower levels than wild-type RVFV in MDM. MDM were infected with WT or ΔNSs RVFV. RNA was purified from cells at various times post infection and analyzed by real time PCR. WT virus (black squares with solid lines) replicated to higher levels than the ΔNSs virus (white squares with dotted lines) on cells from the same donor. Data are presented as inverse Ct value at various times post infection. 3 different donors are represented in the figure. RNA from the 4th donor was not available for testing.
Cytokine secretion in RVFV infected MDM
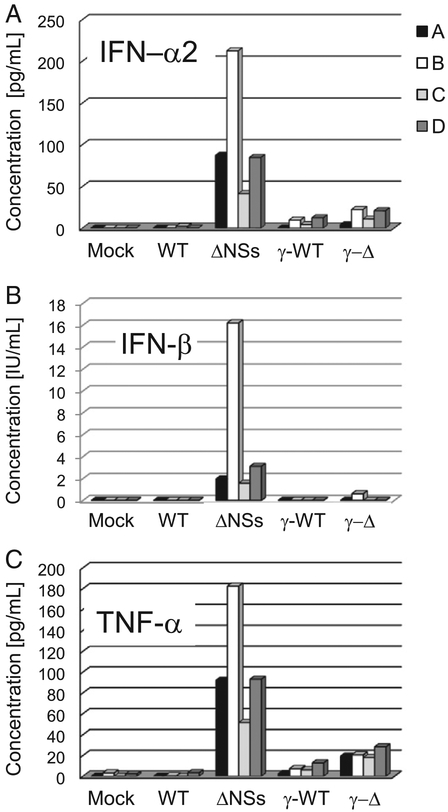
MDM were mock-infected or infected with WT, ΔNSs, γ-WT or γ-ΔNSs virus. Gamma-irradiated (designated by the Greek letter “γ”) control viruses were used to distinguish non-specific cytokine secretion related to supernatant components, including inactivated virions, from those that were a result of active viral infection. Supernantants were collected at 0, 6, 12, 24 and 48 hpi and were analyzed for a selected panel of cytokines. A 12-plex panel of analytes including RANTES, MIP-1α, MIP-1β, 1L-1RA, MCP-1, IP-10, 1L-8, IFN-α2, TNF-α, 1L-12, IL-1β, and 1L-6 were examined on the Luminex platform and IFN-β levels were measured by EL1SA. The most striking results were obtained for IFN-α2 (A), IFN-β (B), and TNF-α (C) (Fig. 4). IFN-α2 is an interferon alpha subtype that has potent antiviral activity in many different responder cell types (Hilkens et al., 2003; Hiscott et al., 1984). IFN-β is another Type 1 interferon that is known to inhibit viral replication and induce apoptosis of virally infected cells (Paul, 2008). TNF-α is a pro-inflammatory cytokine that plays a role in the activation of endothelial gene expression, activation of neutrophils and is a mediator of shock, sepsis, and vascular leakage (Paul, 2008). TNF-α, IFN-α2 and IFN-β were secreted by human MDM that were infected with ANSs virus but not by MDM that were infected with WT virus and only minimally by γ-irradiated viruses. In the ΔNSs virus infected MDM, expression of TNF-α was detectable as early as 6 hpi and IFN-α2 was detectable as early as 12 hpi; only the 24 h data is shown. IFN-β levels were only assessed at the 24 hpi time point.
Fig. 4.
IFN-α2, IFN-β and TNF-α are expressed by MDM upon infection with ΔNSs virus but not WT RVFV virus. MDM were mock-infected, or infected with WT, ΔNSs, γ-WT or γ-ΔNSs RVFV. Supernatants were collected at various times post infection, and levels of cytokines were measured. 4 different donors are represented in the figure (A–D). Data for IFN-α2 (A), IFN-β (B) and TNF-α (C) at the 24 hpi time point are shown.
The secretion of MCP-1, IP-10, RANTES, MIP-1α and MIP-1β did not follow a clear pattern between or among the experimental treatments with the exception that both the mock and WT infected MDM’s always had very low to undetectable cytokine levels. The ΔNSs, γ-WT or γ-ΔNSs infected cells demonstrated varied secretion patterns for these 5 cytokines. This variability in cytokine secretion patterns is likely due to the presence of viral RNA, viral protein, or other activating factors present in the supernatant of the virus preparation used in the inoculum to infect the MDMs. Unlike the data presented earlier for TNF-α, IFN-α2 and IFN-p, these cytokine patterns were not specific to cells infected with replicating virus. IL-8 and IL-1RA were not significantly elevated under any experimental condition except for donor D and donor B respectively, highlighting the importance of using multiple donors in experiments with primary cells (data not shown). Finally, there was no IL-12, IL-Iβ or IL-6 expression detected from any donor regardless of experimental condition.
Human serum cytokine analysis
After demonstrating that the macrophage is a susceptible cell type and that the virus is able to alter the innate immune response in macrophages, it followed that we might expect a perturbation of the cytokine response in severely infected individuals. We hypothesized that a suppression of the pro-inflammatory innate immune response by WT RVFV could play a role in viral pathogenesis. In order to test this hypothesis, we utilized human serum samples from the RVFV outbreak that occurred in Saudi Arabia in 2000–2001. The clinical and epidemiological data from his out-break have been published (Madani et al., 2003). We were able to identify 26 samples from 26 different patients for which there was sufficient sample and for which the clinical outcome was known. Of the 26 cases, 6 were fatal and 20 were non-fatal. All patients were hospitalized and exhibited fever and gastrointestinal symptoms (nausea, vomiting or diarrhea), 7 had jaundice, 3 had bleeding manifestations, 7 had CNS disturbances and none had vision changes. The samples were collected at the time of presentation and were from 1 to 14 days post onset of symptoms. There were 20 male and 6 female patients ranging in age from 17 to 90 years with an average age of 52 years. Key mean laboratory values in these patients are presented in Table 1. The patients all demonstrated clinical and laboratory findings typical for severe RVFV disease. The fatal cases had significantly more thrombocytopenia, coagulopathy and transaminase elevation, as has been previously reported (Madani et al., 2003).
Table 1.
Laboratory characteristics of selected RVFV patients. The 26 patients for whom serum was available for cytokine analysis had the laboratory findings indicated in the table. The noted laboratory value was not necessarily always available for each patient so the number of samples that were used in calculating the mean for the fatal and non-fatal cases respectively are noted next to the analyte. F = fatal (6 total), NF = non-fatal (20 total). WBC = white blood cell count, Hbg = hemoglobin, AST = aspartate aminotransferase, ALT = alanine aminotransferase, PT = prothrombin time, PTT = partial thromboplastin time, CI = confidence interval.
| Analyte | F, NF | Mean fatal | CI | Mean non-fatal | CI | nl rangea |
|---|---|---|---|---|---|---|
| Platelets | 5, 18 | 36 | 17–55 | 110 | 81–139 | 150–300×103/μL |
| WBC | 5, 17 | 11.6 | 4.8–18.4 | 8.8 | 3–14.6 | 4.5–11×103 cell/μL |
| Hgb | 5, 18 | 10 | 5.8–14.2 | 10.5 | 9.1–11.9 | 12–17 g/dL |
| AST | 5, 19 | 16,926 | 5082–28,770 | 1101 | −75–2277 | 10–30 U/L |
| ALT | 5, 19 | 7000 | 4046–9954 | 732 | 319–1145 | 7–40 U/L |
| PT | 4, 6 | 39.9 | 29.8–50.0 | 17.0 | 15.6–18.4 | 11–16 s |
| PTT | 4, 6 | 66.9 | 47.3–86.5 | 41.9 | 30.7–56.1 | 25–35 s |
Goldman: Cecil Medicine 23rd ed. (Cecil et al., 2008).
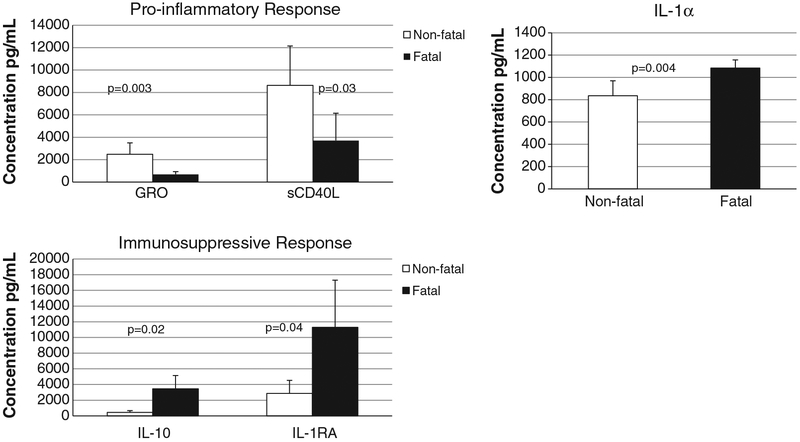
Patient serum samples were analyzed in duplicate using a large multiplex assay to determine the concentration of 39 different cytokines: EGF, Eotaxin, FGF-2, Flt-3 ligand, Fractalkine, G-CSF, GM-CSF, GRO, IFN-α2, IFN-γ, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IL-1RA, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IP-10, MCP-1, MCP-3, MDC (CCL22), MIP-1α, MIP-1β, TGF-α, TNF-α, TNF-β, VEGF, sCD40L, and sIL-2Rα. There were no detectable levels of TNF-α or IFN-α2 in the samples from fatal or non-fatal cases. However there were 5 cytokines that demonstrated a statistically significant difference (p<0.05) between fatal and non-fatal cases by a two-sample T test (Fig. 5). Two pro-inflammatory cytokines, sCD40L (a mediator of B cell activation) and GRO (a mediator of neutrophil activation), were elevated in non-fatal cases as compared to fatal cases.
Fig. 5.
A pro-inflammatory response is associated with survival in human serum samples from the Saudi 2000–2001 outbreak. Serum samples from patients with known clinical outcome were analyzed in duplicate for various cytokines. The 5 cytokines that demonstrated statistical significance between fatal and non-fatal cases are shown. Two pro-inflammatory cytokines were elevated in non-fatal cases (white bars) and two immunosuppressive cytokines were elevated in fatal cases (black bars). The pro-inflammatory cytokine IL-1α is shown separately. Confidence interval is indicated by error bars and p values are noted.
IL-1RA is the receptor antagonist for IL-1, a potent pro-inflammatory pyrogen, so therefore IL-1RA has immunosuppressive properties. IL-1RA binds to the IL-1 receptor with high affinity and prevents receptor dimerization and downstream signaling (Paul, 2008). IL-1RA levels in fatal cases were a log higher than IL-1α levels. Additionally, IL-1RA levels were significantly higher in fatal vs non-fatal cases, leading to an overall immunosuppressive effect in fatal cases.
Finally, IL-10, a cytokine that is well known to be suppressive to the cell-mediated immune response, was elevated in fatal vs. non-fatal cases. In summary, a pro-inflammatory cytokine response was associated with increased survival while actively or passively suppressed cytokine response was associated with increased risk of fatality.
Discussion
Our studies have demonstrated that MDM are permissive for RVFV infection and that infection with WT virus leads to CPE and cell death. Furthermore, we have studied the role of the NSs protein and determined that NSs deficient viruses do not replicate as well as WT RVFV in MDM. Since these two viruses replicate to equivalent levels in Vero cells, which are unable to produce interferon (Desmyter et al., 1968; Emeny and Morgan, 1979), it may be the case that the 1FN response that is stimulated in ΔNSs RVFV infected MDM’s is responsible for the decrease in viral titers and the lack of CPE during infection with this virus.
Macrophages may play a role in the pathogenesis of WT RVFV. An infected macrophage would be unable to signal a pro-inflammatory response secondary to the inhibitory effects of the NSs protein. 1n addition, the intracellular anti-viral mechanisms would be rendered inactive because of NSs mediated inhibition of expression of type 1 1FN’s and virally mediated degradation of PKR. These many effects of the NSs protein could usurp a sentinel cell and convert it into a virus factory. The macrophage might also act as vehicle to transmit the virus to its target organs, the liver and the CNS. There is clearly precedence in the literature for viruses using the monocyte/macrophage to gain entry to the CNS in the case of Hepatitis C virus, Junin virus, Dengue virus, and HIV (Gras and Kaul, 2010; Koenig et al., 1986; Medeot et al., 1995; Miagostovich et al., 1997; Wilkinson et al., 2009). Further studies will need to be done to fully define the role of the macrophage in RVFV in vivo pathogenesis.
Our studies have demonstrated NSs-mediated inhibition of TNF-α, IFN-α2, and IFN-β expression in RVFV infected MDM’s. It was noted that several cytokines were activated by infection with gamma irradiated viruses. This non-specific activation (i.e., did not require viral gene expression or replication) could be secondary to the presence of viral RNA and/or protein in these inoculates or could represent activation by factors that were carried over in the supernatants during virus preparation. It is well known that surrogates for viral RNA such as poly I-C can activate a cytokine response in exposed cells, so these results were not surprising. However, it was quite striking that all of the non-specific activations were significantly diminished by the presence of the NSs protein in cells infected with WT virus. The NSs protein led to a striking, generalized down-regulation of all of the studied cytokines.
IFN-α2, IFN-β and TNF-α were elevated only in cells that were productively infected with ΔNSs virus. Expression of these cytokines required active viral transcription and/or replication. The R1G-1-like RNA helicases, RIG-I and MDA-5, are cytoplasmic viral RNA detector molecules that recognize ssRNA containing a 5′ triphosphate and dsRNA respectively (Paul, 2008). In one study, RIG-I recognized the 5′ triphosphate of a transfected RVFV genome and this led to downstream activation of the IFN-β promoter (Habjan et al., 2008a) This intracellular molecular sensor would be activated during ΔNSs infection of macrophages and initiate the signaling cascade that leads to IRF and NFκB activation and Type 1 IFN and TNF-α transcription respectively. Our findings are consistent with previously published results that demonstrate the importance of the NSs protein in inhibition of Type 1 1FN’s in RVFV infected cells and animals. However, the finding of TNF-α inhibition by NSs is novel, and would be expected given our knowledge of the signaling mechanisms involved during viral infections.
Previous studies of other hemorrhagic fever viruses such as Ebola virus and CCHF virus have demonstrated release of pro-inflammatory cytokines in in vitro cell culture and in animal models and have reported an association between a pro-inflammatory response and increased fatality, when examining clinical specimens (Connolly-Andersen et al., 2009; Ergonul et al., 2006; Gupta et al., 2001; Hutchinson and Rollin, 2007; Papa et al., 2006; Stroher et al., 2001; Villinger et al., 1999). In contrast, Lassa virus appears to down-regulate the immune response; macrophages and dendritic cells are not activated by infection nor do they produce inflammatory cytokines when infected (Baize et al., 2004; Lukashevich et al., 1999). Additionally, activation of dendritic cells or macrophages by poly I-C, LPS or IFN-α prior to infection led to down-regulation of Lassa virus replication (Baize et al., 2006) and we have seen similar results with RVFV (data not shown). In animal studies done in cynomolgus macaques, survival from Lassa virus infection was associated with lower viral loads, faster antibody response, activation of an early type 1 1FN response, high activated monocyte counts and circulating activated T cells (Baize et al., 2009). In clinical samples from Lassa virus infected patients it has been reported that there are lower levels of the pro-inflammatory cytokines IL-8 and IP-10 in fatal cases (Mahanty et al., 2001). Taken together these data support the idea that a critical part of the pathogenesis of Lassa virus is preventing the activation of the immune response. 1t would follow that survival rates are higher in individuals who can activate this response despite virally mediated inhibition. The data that we have presented here using samples from human RVFV cases demonstrated a similar phenomenon, where survival is associated with a robust pro-inflammatory cytokine response.
Our data demonstrating an association between pro-inflammatory cytokines and human survival during RVFV infection is limited by the fact that we only have data for 6 fatal cases. Unfortunately, these types of samples are very difficult to obtain. However, it is striking that we found statistical significance given that our samples were obtained from 1 to 14 days post onset of symptoms. There was no significant difference between the time of presentation of the fatal vs nonfatal cases, (mean of 4.8 days vs 3.75 days; p = 0.41) lending even more credence to our data. All of our cases clearly represented severe disease since they came to the attention of medical personnel and exhibited derangement in their laboratory parameters. We suspect that if we were able to obtain data from mild cases and compare them to severe cases, the cytokine effects that we have seen would be even more pronounced.
Elucidating the factors that determine why some patients are able to mount a pro-inflammatory response and survive while others do not remains an area for future study. Genetic heterogeneity as the basis of differential susceptibility to RVFV infection has been well established in the rat and mouse model (Anderson et al., 1987; Anderson et al., 1991; do Valle et al., 2010; Peters and Slone, 1982; Ritter et al., 2000). Variable expression of interferon regulated genes were demonstrated recently in mouse embryo fibroblasts (MEFs) from BALB/cByJ mice versus the more susceptible MBT/Pas mouse (do Valle et al., 2010). In this study, the authors also report increased expression of Ifnb1 and Ifna4 transcripts upon infection of MEF’s with a ΔNSs virus as compared to a WT virus. They were examining RNA at very early time points in infection (<9 h) and this might explain why they were able to see some expression of Ifn transcripts in cells infected with WT virus. The known heterogeneity of response to infection with RVFV in humans and animals is consistent with the heterogeneity of response that we saw amongst our four donors. One might predict that donor B, the donor with the highest cytokine levels in our study, would have a better outcome upon infection with RVFV than the other donors.
In reality, it is most likely that a combination of genetic and environmental factors are responsible for disease outcome. While we cannot rule out a specific genetic predisposition to fatal disease in a small proportion of the population, it is more likely that a person’s immune status at the time of infection (e.g. concurrent infections, nutritional status, stress level, etc.) is responsible for the lack of response that leads to a fatal outcome. We would predict that early and vigorous medical intervention, possibly targeting specific virulence factors, such as NSs, could significantly improve disease outcomes by maximizing the response potential of any given human genotype to viral infection.
Materials and methods
Virus and cells
All work with live virus was performed under BSL-4 conditions in a positive pressure suit. RVFV ZH501 (Bird et al., 2007b) or RVFV ΔNSs (Bird et al., 2008) were propagated in Vero E6 cells by infecting at an MOI of 0.1. Supernatants were collected 3 or 4 days post infection, clarified by centrifugation, aliquoted, and stored at −80 °C.
Peripheral blood mononuclear cell pheresis products were obtained from healthy human donors at Emory Hospital. Pheresis products were diluted 1:1 with PBS (without calcium or magnesium), layered onto Histopaque (Sigma) or Ficoll-Paque (GE Healthcare), and mononuclear cells were purified per the manufacturer’s instructions. After purification, cells were washed several times in PBS and resuspended in MACS buffer (Miltenyl Biotech). Magnetically coupled CD14 antibodies (Miltenyl Biotech) were used to selectively purify the CD14 positive cells per the manufacturer’s instructions. CD14 positive cells were stored at −80 °C in freezing medium (90% FBS, 10% DMSO) until use.
Infections
CD14 positive cells were seeded onto 24 well plates in complete media (RPMI with 5% FBS, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 2 mM l-glutamine), and allowed to mature to macrophages by 5 days of adherence in culture. Cells were re-fed with fresh media every 2 days. Cells were then infected at an MOI of 5 with either ZH501, ΔNSs, gamma irradiated viruses (γ-ZH501 or γ-ANSs) that were inactivated by irradiation with 5×106rads, or mock infected with conditioned media. After allowing 1 h for adsorption, the inoculum was removed, cells were washed 3× with PBS, and then re-fed with complete media. At defined times post infection, supernatants were collected, centrifuged to pellet any debris, and stored at −80 °C for future analysis. Cells were lysed in NA lysis buffer (ABI) for RNA purification and stored at −80 °C until purification was performed.
Plaque assays
Vero E6 cells were plated onto 6 well plates at a density of 70%. The following day, supernatants were diluted serially in complete media and 200 μl of each dilution was placed per well in duplicate. Inocula were allowed to adsorb for 1 h with rocking every 15 min to prevent drying. Each well was then overlaid with 3 mL of overlay media (0.6% Seakem ME agarose, 1× EMEM, 10% FBS, 100U/mL of penicillin, 100 μg/mL of streptomycin, and 2 mM l-glutamine) and incubated at 37 °C. After 3 days, cells were fixed in 10% formalin, agarose was removed and monolayers were stained with crystal violet and washed in PBS. Plaques were counted on a white light trans-illuminator.
Real time PCR
RNA was purified from cells that had been lysed in NA lysis buffer (ABI) according to the manufacturer’s instructions. Ten microliters of total RNA was used for an 18S assay (ABI) that allowed for normalization between samples. Twenty microliters of RNA was used in a RVFV assay that has been previously described (Bird et al., 2007a). Reactions were performed on an ABI 7500 real time PCR machine.
Cytokine assays
MDM-culture supernatants were gamma irradiated (5×106 rads) to inactivate infectious materials prior to cytokine analysis. Cytokine assays were performed in duplicate according to the manufacturer’s instructions (Millipore-Milliplex MAP Kit) and analyzed on a Luminex 200 IS platform.
ELISA
The IFN-β ELISA was performed on the same supernatants that were used in the Luminex assays and according to manufacturer’s instructions (Invitrogen).
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The authors thank Kimberly Dodd, Tatyana Kilmova and Drs. Christina Spiropoulou, and David White for critical reading of the manuscript.
Biography
Anita K. McElroy is currently a fellow in the Infectious Disease Division and was formally a resident in the Department of Pediatrics at Emory University. While a resident, she was a participant in the American Board of Pediatrics Integrated Research Pathway. This work was also performed while she was a recipient of the NIH loan repayment program award and the Pediatric Infectious Disease Society-St. Jude Fellowship Award.
References
- Anderson GW Jr., Slone TW Jr., Peters CJ, 1987. Pathogenesis of Rift Valley fever virus (RVFV) in inbred rats. Microb. Pathog 2, 283–293. [DOI] [PubMed] [Google Scholar]
- Anderson GW Jr., Lee JO, Anderson AO, Powell N, Mangiafico JA, Meadors G, 1991. Efficacy of a Rift Valley fever virus vaccine against an aerosol infection in rats. Vaccine 9, 710–714. [DOI] [PubMed] [Google Scholar]
- Baize S, Kaplon J, Faure C, Pannetier D, Georges-Courbot MC, Deubel V, 2004. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J. Immunol 172, 2861–2869. [DOI] [PubMed] [Google Scholar]
- Baize S, Pannetier D, Faure C, Marianneau P, Marendat I, Georges-Courbot MC, Deubel V, 2006. Role of interferons in the control of Lassa virus replication in human dendritic cells and macrophages. Microbes Infect. 8,1194–1202. [DOI] [PubMed] [Google Scholar]
- Baize S, Marianneau P, Loth P, Reynard S, Journeaux A, Chevallier M, Tordo N, Deubel V, Contamin H, 2009. Early and strong immune responses are associated with control of viral replication and recovery in lassa virus-infected cynomolgus monkeys. J. Virol 83, 5890–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billecocq A, Spiegel M, Vialat P, Kohl A, Weber F, Bouloy M, Haller O, 2004. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J. Virol 78, 9798–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird BH, Bawiec DA, Ksiazek TG, Shoemaker TR, Nichol ST, 2007a. Highly sensitive and broadly reactive quantitative reverse transcription-PCR assay for high-throughput detection of Rift Valley fever virus. J. Clin. Microbiol 45, 3506–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird BH, Khristova ML, Rollin PE, Ksiazek TG, Nichol ST, 2007b. Complete genome analysis of 33 ecologically and biologically diverse Rift Valley fever virus strains reveals widespread virus movement and low genetic diversity due to recent common ancestry. J. Virol 81, 2805–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird BH, Albarino CG, Hartman AL, Erickson BR, Ksiazek TG, Nichol ST, 2008. Rift valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J. Virol 82, 2681–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird BH, Ksiazek TG, Nichol ST, Maclachlan NJ, 2009. Rift Valley fever virus. J. Am. Vet. Med. Assoc 234, 883–893. [DOI] [PubMed] [Google Scholar]
- Bouloy M, Janzen C, Vialat P, Khun H, Pavlovic J, Huerre M, Haller O, 2001. Genetic evidence for an interferon-antagonistic function of rift valley fever virus non-structural protein NSs. J. Virol 75, 1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecil RL, Goldman L, Ausiello DA, 2008. Cecil medicine, 23 rd ed. Saunders Elsevier, Philadelphia. [Google Scholar]
- Centers for Disease Control and P., 2007. Rift Valley fever outbreak—Kenya, November 2006-January 2007: MMWR—Morbidity & Mortality Weekly Report, 56, pp. 73–76. [PubMed] [Google Scholar]
- Connolly-Andersen AM, Douagi I, Kraus AA, Mirazimi A, 2009. Crimean Congo hemorrhagic fever virus infects human monocyte-derived dendritic cells. Virology 390, 157–162. [DOI] [PubMed] [Google Scholar]
- Daubney R, Hudson JR, Garnham PC, 1931. Enzootic hepatitis or Rift Valley fever. An undescribed virus disease of sheep, cattle and man from East Africa. J. Pathol. Bacteriol 34, 545–579. [Google Scholar]
- Desmyter J, Melnick JL, Rawls WE, 1968. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J. Virol 2, 955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Valle TZ, Billecocq A, Guillemot L, Alberts R, Gommet C, Geffers R, Calabrese K, Schughart K, Bouloy M, Montagutelli X, Panthier JJ, 2010. A new mouse model reveals a critical role for host innate immunity in resistance to Rift Valley fever. J. Immunol 185, 6146–6156. [DOI] [PubMed] [Google Scholar]
- Dungu B, Louw I, Lubisi A, Hunter P, von Teichman BF, Bouloy M, 2010. Evaluation of the efficacy and safety of the Rift Valley Fever Clone 13 vaccine in sheep. Vaccine 28, 4581–4587. [DOI] [PubMed] [Google Scholar]
- Emeny JM, Morgan MJ, 1979. Regulation of the interferon system: evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol 43, 247–252. [DOI] [PubMed] [Google Scholar]
- Ergonul O, Tuncbilek S, Baykam N, Celikbas A, Dokuzoguz B, 2006. Evaluation of serum levels of interleukin (IL)-6, IL-10, and tumor necrosis factor-alpha in patients with Crimean-Congo hemorrhagic fever. J. Infect. Dis 193, 941–944. [DOI] [PubMed] [Google Scholar]
- Fields BN, Knipe DM, Howley PM, 2007. Fields’ virology, 5th ed. Wolters kluwer/Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- Gerrard SR, Bird BH, Albarino CG, Nichol ST, 2007. The NSm proteins of Rift Valley fever virus are dispensable for maturation, replication and infection. Virology 359, 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras G, Kaul M, 2010. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M, Mahanty S, Ahmed R, Rollin PE, 2001. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with ebola virus secrete MIP-1alpha and TNF-alpha and inhibit poly-IC-induced IFN-alpha in vitro. Virology 284, 20–25. [DOI] [PubMed] [Google Scholar]
- Habjan M, Andersson I, Klingstrom J, Schumann M, Martin A, Zimmermann P, Wagner V, Pichlmair A, Schneider U, Muhlberger E, Mirazimi A, Weber F, 2008a. Processing of genome 5’ termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS One 3, e2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habjan M, Penski N, Spiegel M, Weber F, 2008b. T7 RNA polymerase-dependent and -independent systems for cDNA-based rescue of Rift Valley fever virus. J. Gen. Virol 89, 2157–2166. [DOI] [PubMed] [Google Scholar]
- Habjan M, Pichlmair A, Elliott RM, Overby AK, Glatter T, Gstaiger M, Superti-Furga G, Unger H, Weber F, 2009. NSs protein of rift valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J. Virol 83, 4365–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilkens CM, Schlaak JF, Kerr IM, 2003. Differential responses to IFN-alpha subtypes in human T cells and dendritic cells. J. Immunol 171, 5255–5263. [DOI] [PubMed] [Google Scholar]
- Hiscott J, Ryals J, Dierks P, Hofmann V, Weissmann C, 1984. The expression of human interferon alpha genes. Philos. Trans. R. Soc. Lond. B Biol. Sci 307,217–226. [DOI] [PubMed] [Google Scholar]
- Hutchinson KL, Rollin PE, 2007. Cytokine and chemokine expression in humans infected with Sudan Ebola virus. J. Infect. Dis 196 (Suppl. 2), S357–S363. [DOI] [PubMed] [Google Scholar]
- Ikegami T, Won S, Peters CJ, Makino S, 2006. Rescue of infectious rift valley fever virus entirely from cDNA, analysis of virus lacking the NSs gene, and expression of a foreign gene. J. Virol 80, 2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Narayanan K, Won S, Kamitani W, Peters CJ, Makino S, 2009. Rift Valley fever virus NSs protein promotes post-transcriptional downregulation of protein kinase PKR and inhibits eIF2alpha phosphorylation. PLoS Pathog. 5, e1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal SA, 2009. Pathological studies on postvaccinal reactions of Rift Valley fever in goats. Virol. J 6, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS, 1986. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233, 1089–1093. [DOI] [PubMed] [Google Scholar]
- Le May N, Dubaele S, Proietti De Santis L, Billecocq A, Bouloy M, Egly JM, 2004. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell 116, 541–550. [DOI] [PubMed] [Google Scholar]
- Le May N, Mansuroglu Z, Leger P, Josse T, Blot G, Billecocq A, Flick R, Jacob Y, Bonnefoy E, Bouloy M, 2008. A SAP30 complex inhibits IFN-beta expression in Rift Valley fever virus infected cells. PLoS Pathog. 4, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashevich IS, Maryankova R, Vladyko AS, Nashkevich N, Koleda S, Djavani M, Horejsh D, Voitenok NN, Salvato MS, 1999. Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: different effects on IL-8 and TNF-alpha gene expression. J. Med. Virol 59, 552–560. [PMC free article] [PubMed] [Google Scholar]
- Madani TA, Al-Mazrou YY, Al-Jeffri MH, Mishkhas AA, Al-Rabeah AM, Turkistani AM, Al-Sayed MO, Abodahish AA, Khan AS, Ksiazek TG, Shobokshi O, 2003. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin. Infect. Dis 37,1084–1092 [See comment]. [DOI] [PubMed] [Google Scholar]
- Mahanty S, Bausch DG, Thomas RL, Goba A, Bah A, Peters CJ, Rollin PE, 2001. Low levels of interleukin-8 and interferon-inducible protein-10in serum are associated with fatal infections in acute Lassa fever. J. Infect. Dis 183,1713–1721. [DOI] [PubMed] [Google Scholar]
- Mansuroglu Z, Josse T, Gilleron J, Billecocq A, Leger P, Bouloy M, Bonnefoy E, 2010. Nonstructural NSs protein of rift valley fever virus interacts with pericentromeric DNA sequences of the host cell, inducing chromosome cohesion and segregation defects. J. Virol 84, 928–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeot SI, Contigiani MS, Sabattini MS, Camara A, 1995. The role of mononuclear blood cells in experimental Junin virus spread to the central nervous system. Viral Immunol. 8, 101–108. [DOI] [PubMed] [Google Scholar]
- Miagostovich MP, Ramos RG, Nicol AF, Nogueira RM, Cuzzi-Maya T, Oliveira AV, Marchevsky RS, Mesquita RP, Schatzmayr HG, 1997. Retrospective study on dengue fatal cases. Clin. Neuropathol 16, 204–208. [PubMed] [Google Scholar]
- Muller R, Saluzzo JF, Lopez N, Dreier T, Turell M, Smith J, Bouloy M, 1995. Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am. J. Trop. Med. Hyg 53,405–411. [DOI] [PubMed] [Google Scholar]
- Papa A, Bino S, Velo E, Harxhi A, Kota M, Antoniadis A, 2006. Cytokine levels in Crimean-Congo hemorrhagic fever. J. Clin. Virol 36, 272–276. [DOI] [PubMed] [Google Scholar]
- Paul WE, 2008. Fundamental immunology, 6th ed. Wolters Kluwer / Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- Peters CJ, Slone TW, 1982. Inbred rat strains mimic the disparate human response to Rift Valley fever virus infection. J. Med. Virol 10, 45–54. [DOI] [PubMed] [Google Scholar]
- Ritter M, Bouloy M, Vialat P, Janzen C, Haller O, Frese M, 2000. Resistance to Rift Valley fever virus in Rattus norvegicus: genetic variability within certain ‘inbred’ strains. J. Gen. Virol 81, 2683–2688. [DOI] [PubMed] [Google Scholar]
- Shieh WJ, Paddock CD, Lederman E, Rao CY, Gould LH, Mohamed M, Mosha F, Mghamba J, Bloland P, Njenga MK, Mutonga D, Samuel AA, Guarner J, Breiman RF, Zaki SR, 2010. Pathologic studies on suspect animal and human cases of Rift Valley fever from an outbreak in Eastern Africa, 2006–2007. Am. J. Trop. Med. Hyg 83, 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Steele KE, Shamblin J, Honko A, Johnson J, Reed C, Kennedy M, Chapman JL, Hensley LE, 2010. The pathogenesis of Rift Valley fever virus in the mouse model. Virology 407, 256–267. [DOI] [PubMed] [Google Scholar]
- Stroher U, West E, Bugany H, Klenk HD, Schnittler HJ, Feldmann H, 2001. Infection and activation of monocytes by Marburg and Ebola viruses.J. Virol 75,11025–11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanepoel R, Coetzer JAW., 1994. Rift Valley fever In: Coetzer JAW, T.G., Tutsin RC (Eds.), Infectious Diseases of Livestock with Special References to South Africa. Oxford University Press, Capetown, pp. 688–717. [Google Scholar]
- Turell MJ, Wilson WC, Bennett KE, 2010. Potential for North American mosquitoes (Diptera: Culicidae) to transmit rift valley fever virus. J. Med. Entomol 47, 884–889. [DOI] [PubMed] [Google Scholar]
- Vialat P, Billecocq A, Kohl A, Bouloy M, 2000. The S segment of riftvalley fever phlebo-virus (Bunyaviridae) carries determinants for attenuation and virulence in mice. J. Virol 74, 1538–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villinger F, Rollin PE, Brar SS, Chikkala NF, Winter J, Sundstrom JB, Zaki SR, Swanepoel R, Ansari AA, Peters CJ, 1999. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J. Infect. Dis 179 (Suppl. 1), S188–S191. [DOI] [PubMed] [Google Scholar]
- Wilkinson J, Radkowski M, Laskus T, 2009. Hepatitis C virus neuroinvasion: identification of infected cells. J. Virol 83,1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadani FZ, Kohl A, Prehaud C, Billecocq A, Bouloy M, 1999. The carboxy-terminal acidic domain of Rift Valley Fever virus NSs protein is essential for the formation of filamentous structures but not for the nuclear localization of the protein. J. Virol 73, 5018–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]