Abstract
Heavy metal pollution continues to be one of the most serious environmental problems which has attracted major global concern. Here, a rapid, high-capacity, yet economical strategy for deep cleaning of heavy metals ions in water is reported based on amidoxime-functionalized macroporous carbon electrode materials. The active sites of our material can be self-refreshed during the electrochemical removal process, which is different from traditional methods. The novel filter device in this work can purify contaminated water very rapidly (3000 L h–1 m–2), and can decrease heavy metal ion concentrations to below 5 ppb with a very short contact time (only 3 s). The original treatment efficiency of the device can be retained even after 1 week of continuous device operation. An extremely high removal capacity of over 2300 mg g–1 can be achieved with 2–3 orders of magnitude higher efficiency than that of surface adsorption-based commercial filters without any decay. Additionally, the cost of energy consumed in our method is lower than $6.67 × 10–3 per ton of wastewater. We envision that this approach can be routinely applied for the rapid, efficient, and thorough removal of heavy metals from both point-of-use water and industrial wastewater.
Short abstract
A rapid, high-capacity, yet economical strategy for deep cleaning of heavy metals in water is reported based on self-refreshed electrode materials.
Introduction
Unlike organic pollutants, heavy metal pollutants in water, including lead, mercury, copper, nickel, chromium, and cadmium, etc., originating from industrial emission such as energy production, mining, electroplating, and microelectronics, do not degrade but instead accumulate in living organisms through the food chain.1−3 After being enriched in the body, even trace heavy metal ions may lead to serious disease, such as itai–itai disease, minamata disease, or even cancer.4−6 Those global risks are constant reminders of the importance of developing efficient and economical methods to remove heavy metals from polluted water.7−12
The approaches for heavy metal ion removal in water mainly involve chemical precipitation,13,14 microbial treatment,15−17 electrodeposition,18−26 and physical/chemical adsorption.27−49Chemical precipitation is a strategy to form a separable solid substance from water. This technique can target very specific components through different reagents to achieve a high degree of selectivity. However, because large amounts of reagents are generally needed, the cost is often very high, and the behavior of dissolution equilibrium limits the depth of treatment. Furthermore, the heavy-metal-containing silt which is generated becomes a further disposal problem. Microbial treatment is a safe, clean, and environmentally friendly technology, especially for heavy metal removal. Nevertheless, the process of treatment is very complicated and involves biosorption, extracellular precipitation, intracellular accumulation, and exocytosis.50 The intracellular accumulation requires energy consumption provided by the metabolic control system of bacteria, and it takes a long period of time to complete, limiting the removal efficiency of the whole process. Electrodeposition is also a general method used to clean industrial wastewater. The strong electric field causes ion migration followed by rapid deposition on electrodes. However, the lack of active sites for ions limits the removal performance and causes more energy waste. Adsorption is another classic and effective approach for processing heavy metal pollution. Numerous novel adsorbent materials for heavy metal removal, such as graphene oxide, carbon nanomaterials, resin, metal–organic frameworks (MOFs), and dendrimers, were reported recently. Unfortunately, adsorption always suffers from some technical bottlenecks: (1) With pore blockage, micropore or mesopore structures are commonly used to increase the surface area, but can easily be fouled and blocked. (2) With finite active sites, after long-term filtration, the surface adsorption sites continue to be occupied, and capacity is reduced. (3) With slow ion diffusion, ions can only be captured when they contact the filter, and ion diffusion rate can largely impact the contact probability. With normal levels of water flow, the ion diffusion rate is so slow that the filter needs a large mass loading to satisfy high treatment rate demands. (4) With chemical/physical adsorption equilibrium issues, different binding energies of functional groups have a great impact on absorption; weaker binding energies facilitate easier desorption. In general, every single technique cannot perfectly satisfy the high demands of water treatment. In this work, we successfully integrate the superiority of each technique above to demonstrate an efficient electrochemical filter device for heavy metal removal. Our approach utilizes a specifically modified macroporous carbon electrode which can perfectly solve the aforementioned issues.
As an electrode substrate, the macroporous carbon felt was used to decrease the pressure drop of flowing water and increase the saturation of physical space to a great extent. As one of the best hydrophilic functional groups, the amidoxime groups modified on the electrode surface largely intensified the adsorption of heavy metals and lowered the impedance of the material when compared with an unmodified electrode. By increasing the driving force of ion migration, the direct current not only enhanced ion transport but also electrochemically reduced the coordinated metal ions to elemental metal, thus releasing active sites and constantly regenerating the filter. The as-designed filtration device was used for the treatment of the simulated water samples and obtained excellent performance. It achieved not only a deposition capacity of over 2300 mg g–1 but also a treatment efficiency of 3000 L h–1 m–2, enabling the removal of heavy metals to concentrations of below 5 ppb (μm L–1) (safe drinking limits51−54) within 3 s. The cost of energy consumed during operation is lower than $6.67× 10–3 per ton which is extremely low (Note S1). These performance parameters demonstrate that this novel filter not only has high efficiency, capacity, and water cleaning power, but also requires low energy costs and is easy to set up.
Results and Discussion
Schematic of the Filtration Device and Preparation of Electrode Material
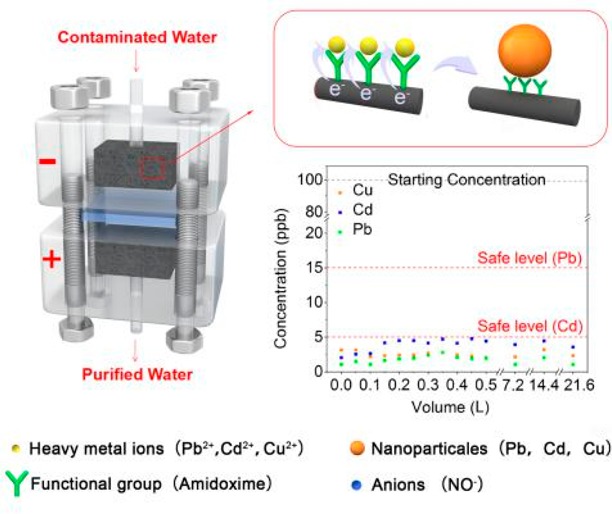
We design an electronic device loaded by polyamidoxime-modified carbon felt (CF) to deal with heavy metals in flowing water for drinking or other demands (Scheme 1a and Figure S1). As shown in Scheme 1b, a direct-current (DC) two-electrode device was built with the contaminated water flowing through the positive and negative CF electrodes which operate under an external electrical bias. Aided by the external electric field, the migration, fixation, reduction, and aggregation of heavy metals in polluted water occur. The water purification processes can be explained through the following four steps. (1) The external electric field drives the migration of the cations to the surface of the cathode to form the double layer (DL). (2) The amidoxime groups are deprotonated to enable their ion coordination behavior (Scheme 1c). The heavy metal ions located at the inner layer of the DL become tightly captured. (3) The chelated cations were then reduced to their metallic state by electron injection. As additional heavy metal ions are reduced at the electrode surface, the deposited metals grow into nanoparticles. (4) After the metal ions are reduced, their previously occupied active sites are set free and can be reused to accept new cations. This electrochemical method, through the combination of the electric-field-driven effect and functional group fixation, can not only achieve a rapid treatment speed but also maintain its treatment efficiency because of the renewable active sites. We adopted an amidoxime group for functionalizing the carbon felt electrodes because of its superior adsorption ability for metal ions resulting from their coordination active sites. As schematically shown in Scheme 1c, the stable effect of the five-membered rings allows the metal to be intensely chelated through transformation from the imino-hydroxylamine form to the amino-oxime form.55−57Figure 1a–c shows the scanning electron microscopy (SEM) images of the carbon felt electrodes with and without amidoxime functionalization, along with the photograph of carbon felt fully dipped in water. The amidoxime group functionalization successfully makes the CF electrodes very hydrophilic with strong ion coordination activity. As demonstrated in Figure 1a, for the sample without modification, a gas layer would form on the surface due to the well-known hydrophobicity of bare carbon materials. The CF electrode, however, can be easily wetted by water after modification.
Scheme 1. Working Principle of the Water Filtration Device.
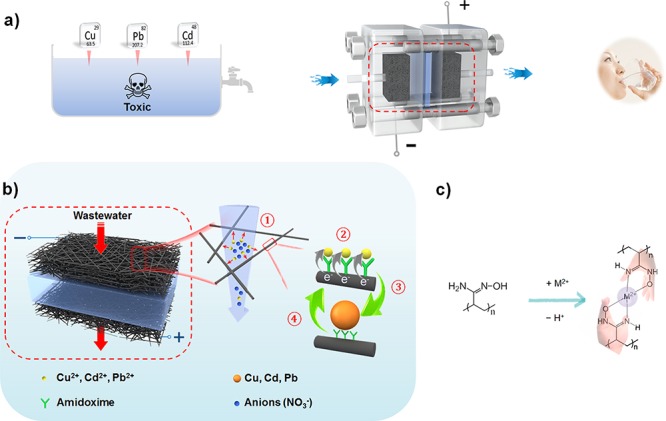
(a) The contaminated water vertically passes through the electrified polymer-modified carbon felt, and directly exits as drinkable water. (b) Schematic of heavy metal ions removal: ① Migration of metal ions by electric field. ② Capture of metal ions by functional groups. ③ Metal ion reduction. ④ Active site refreshment. (c) The chelation mode of amidoxime with metal ions.
Figure 1.
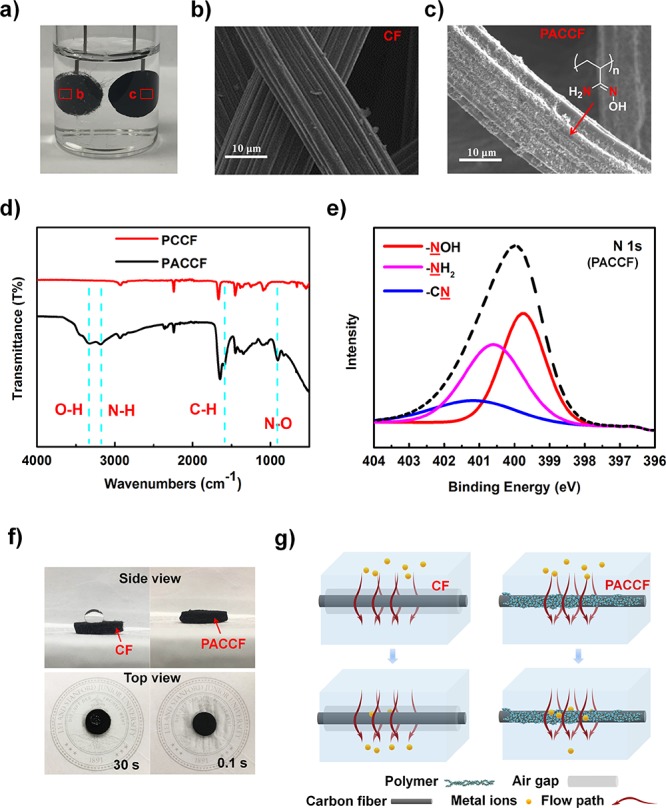
Characterization of the filtration electrode. (a) Hydrophilicity test in water. The blank CF was covered by a gas film. (b, c) SEM images of a single fiber of Blank CF and PACCF, respectively. (d) XPS spectra (N 1s) of PACCF. The red line is attributed to −NOH at 399.8 eV; the pink line is attributed to −NH2 at 400.6 eV; the blue line belongs to −CN at 401.2 eV. (e) Fourier transform infrared spectroscopy (FTIR) spectrum of materials coated with PAN before and after amidoximation reaction. Four blue dashed lines mark the new signals after reaction. (f) Permeability test of water droplet (side view). The droplet can instantly pass through the PACCF and wet the tissue printed logo (top view). (g) Schematic of metal ions in flowing water to explain the hydrophilic effect.
The modification processes of carbon felt is relatively strict in terms of the conductivity, loading mass, and uniformity but easily prepared. Commercial CF with pore sizes of tens to hundreds of micrometers and a connected network structure is an ideal substrate as a filter. Polyacrylonitrile (PAN) and Super P (active carbon) were mixed with DMF to get a PAN/C slurry and was coated on the surface of CF to get PAN/C@CF (PCCF). After reaction with hydroxylamine hydrochloride, most of the nitrile groups are replaced by amidoxime groups,58−60 forming the modified electrode, herein referred to as PAN-ami/C@CF (PACCF). As seen by SEM, this amidoxime-modified CF consists of fibers with 20 μm diameter that were coated with a very thin polymer layer (Figure 1c). The activated carbon nanoparticles bound by the polymer on the surface of the fiber are around 30 nm in size (Figure S2) and can offset the decrease of conductivity from the polymer shell while also enhancing the electrode surface area. FTIR was employed to confirm the presence of the amidoxime group based on the enhanced peak at 2242.36 cm–1 corresponding to the C≡N bond of PCCF (Figure 1d). After the amidoximation reaction, many new peaks appeared at 905.42, 1595.32, 3183.42, and 3327.09 cm–1 in the spectra of PACCF, which correspond to N—O, C=N, N—H, and O—H, respectively. In the X-ray photoelectron spectroscopy spectra (Figure 1e) of PACCF, three N 1s peaks are clearly visible, with the percentage contribution of the peaks of 16.73% (—CN), 42.48% (—NH2), and 40.79% (—NOH), reflecting a ∼70% conversion of nitrile groups to amidoxime groups.
As mentioned above, the polymer coating can largely improve the hydrophilicity of the carbon fiber surface and their conductivity in water. To support this, the electrodes before and after coating were tested as shown in Figure 1f and Figure S3. When a water droplet fell onto the top face of the materials, the unmodified commercial CF could hold the droplet on the surface for over 30 s while PACCF was soaked instantly (less than 0.1 s), and the droplet wet the tissue below. This hydrophilicity arises from the full coverage of the modified surface of the carbon fibers fully by amino and oxime groups which can form hydrogen-bonds with H2O molecules. On the unmodified CF, the hydrophobic surface leads to the air gap around the fiber when in contact with water, which will prevent the metal ions from contacting the electrode surface, allowing the contaminated water to pass through the filter without removing heavy metal ions. In contrast, after coating, most cations can directly contact the electrode surface and be chelated by amidoxime groups, reduced, and electrocrystallized in situ as metal (Figure 1g). Because the polymer is nonconductive, after polymer coating, the electrode conductivity would decrease largely. However, for bare CF in water, the air gap also prevents electron transfer and lowers the effective electrode conductivity. After coating the CF with the PAN-ami/C shell, not only is the air gap removed but the conductivity is also improved through the presence of carbon black nanoparticles. As a result, in the electrical impedance test, the resistance of CF was 10 times higher than that of modified CF (Figure S4).
In consideration of their high toxicity and broad health impact, 100 ppb copper, cadmium, and lead ions were used to simulate contaminated water which was used to evaluate the performance of the filtration device. Cadmium ion accumulation causes lung cancer, osteomalacia, and proteinuria after long-term bodily absorption.61 Lead exposure can result in anemia, encephalopathy, and nephropathy.62 Exceeding safe limits of copper can also induce necrotizing hepatitis and hemolytic anemia.63 These three heavy metal ions are common elements present in industrial sewage discharge. As such, we chose them as typical examples of heavy metal ions for the purification experiments. According to the World Health Organization (WHO) standard, the safety limits for copper, cadmium, and lead are 1.3, 0.005, and 0.015 ppm, respectively. At these concentrations, the solutions have no visible difference when compared to deionized water but still pose risks to body health. Under 10 V of direct current (DC), the contaminated water passes through our filtration device continuously with a flow rate of 5 mL min–1. After filtration, the concentrations of these three metal ions are decreased to below 5 ppb, which is below WHO safe levels. We also demonstrated the filter material works for Hg+ removal as well (Figure S5).
Performance of the Electrode Material and Assembly Device
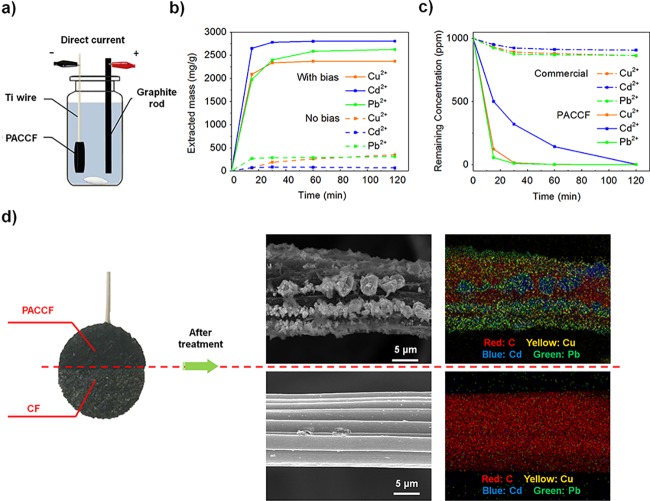
There are four important performance metrics for water filters: capacity, efficiency, stability, and cost. Here, the capacity of the materials is the maximum extraction mass of metal ions from contaminated water, which is one of the most important performance parameters to estimate the operation life of the filter. The stirring system shown in Figure 2a was employed to test this maximum extraction capability of the coated carbon materials (PACCF) under higher ion concentrations. PACCF was used as the working electrode, and a graphite rod served as the counter electrode. The target water used here had initial concentrations each of Cu2+, Cd2+, and Pb2+ of ∼1000 ppm (Figure S6a–c). Figure 2b illustrates the removal curve of the heavy meals with and without external bias. Over 99.9% of heavy metal ions can be removed by the electrode (1 cm2 × 0.318 cm) from 15 mL of the 1000 ppm simulated water after 2 h of electrodeposition. In terms of the activated mass loading of PAN-ami, the total extracted mass can be over 2300 mg g–1 (Cu, 2300 mg g–1; Cd, 2600 mg g–1; and Pb, 2800 mg g–1) of filter (Figure 2b). The PACCF filter is saturated from only physical absorption (no bias) at a capacity of ∼240 mg g–1, representing a removal efficiency of ∼4% for a 1000 ppm solution as compared to 99.9% removal for the case with bias. Combining the above data of the extraction mass with and without bias using the same amount of filter in Figure 2b, it is clear that the filters under bias significantly outperform those without bias. In the comparison with commercial filters made from activated carbon and ion-exchange resin, our material also achieved a higher removal capability (Figure 2c), strongly indicating the superiority of our proposed electric-field-driven chemical adsorption/deposition method relative to traditional adsorption. To obtain further proof, a half-modified electrode (CF and PACCF) was tested in a 1000 ppm mixed contaminated water solution under stirring as shown in Figure 2d. After removal for 2 h, the SEM and EDX images of fibers on each half showed that many particles deposited on the surface of PACCF while there was nearly nothing deposited on the bare CF. This behavior can be mainly attributed to the hydrophilic surface and chelation groups. The similar experiment illustrated in Figure S7 also suggests that the PAN-amidoxime/Au can effectively extract more heavy metals with bias than that of a bare Au substrate.
Figure 2.
(a) Stirring system for capacity tests (high concentration). (b) Electric field effect on capacity of PACCF in the stirring system with ∼1000 ppm Pb2+, Cd2+, Cu2+ contaminated water, respectively. (99.9% removal within 2 h. The electrode size is 1 cm2 × 3.18 cm. The polymer loading weights are 5.9, 5.5, and 5.8 mg.) (c) Effects of different methods on capacity of the PACCF and commercial filter (mainly contains active carbon and resin) of the same size. (d) A half-modified electrode with PACCF and bare CF was used to treat the contaminated water (Pb2+, Cd2+, Cu2+ mixture, 1000 ppm each). The SEM and EDX images of two different fibers showed that the PACCF half removed significantly more heavy metals.
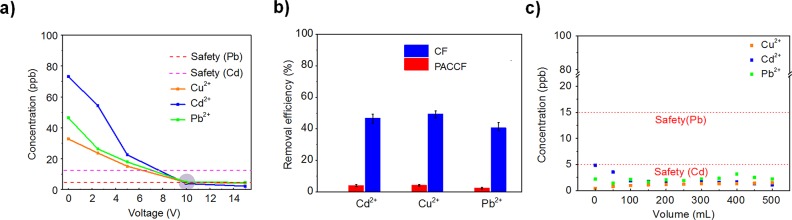
To find the optimal filter voltage and flow rate for purifying water to meet safety standards, a series of removal experiments for ∼100 ppb contaminated water were conducted. The flowing system is shown in Figure S1. First, the voltage was fixed at 0 V with the DC electrical source, and the flow rate was adjusted to 5, 10, 15, and 25 mL min–1. The final concentration of the metal ions in the test solution was then tested by inductively coupled plasma mass spectroscopy (ICP-MS) after filtration. The voltage was then varied to different values of 2.5, 5, 10, and 15 V, and the remaining concentration of metal ions was tested for each solution flow rate. Figure 3a and Figure S6d–f show the final concentrations of Cu2+, Cd2+, and Pb2+ after filtering the ∼100 ppb contaminated water with various filter voltage biases. Higher removal efficiency was achieved for all ions with higher bias voltages and lower flow rates. A higher voltage can accelerate the ion migration by providing a much stronger electric field, while slow flow of incoming solution can increase the residence time of ions near the filter to increase possibility of ion capture. However, a voltage above 10 V is not preferred due to unwanted side reactions like water splitting; this would strongly hinder the electrochemical deposition process due to the energy consumption by side reactions. Finally, a flow rate of 5 mL min–1 and a voltage of 10 V were found to be optimal for achieving safe water with the lowest cost through this electrochemical filtration process. We also compare the removal efficiency for the commercial CF with our developed material. It can be seen that the commercial CF achieves a limited efficiency with 50∼60% heavy metal removal, which is significantly lower than that of PACCF (over 95%). Our results suggest the importance and effectiveness of the modification of CF (Figure 3b).
Figure 3.
(a) Optimization test of flowing water device on voltage and flow rate (100 ppb of starting concentration, 5 mL min–1 of flow rate). (b) Flowing water treatment comparison between CF and PACCF in the same optimum condition (100 ppb of starting concentration, 5 mL min–1 of flow rate, 10 V). (c) Remaining concentrations for long-term flowing with single ion (Cu2+, Cd2+, Pb2+) simulated water (∼100 ppb). The heavy metal ion concentration in the output water is below the drinking safety level (100 ppb of starting concentration, 5 mL min–1 of flow rate, 10 V). (Note: each point of 0 mL is tested on the first 5 mL of filtrated water by ICP-MS.)
Due to the large capacity of the filters, this device also has outstanding long-term stability. To test the stability of the device, contaminated water (∼100 ppb Cu2+, Cd2+, Pb2+) was pumped through the device at 5 mL min–1 until 500 mL of contaminated water is purified with a filter bias of 10 V. The remaining metal ion concentration collected at intervals of 50 mL is shown in Figure 3c. In the single-ion solution systems, Cu2+ was reduced to ∼2.5 ppb, Cd2+ to ∼1.6 ppb, and Pb2+ to ∼1 ppb, with consistent values over the entire volume of water. In the mixed-ions system (Figure 4b), the remaining concentrations of these three ions after purification are ∼3.9 ppb (Cu2+), ∼2.5 ppb (Cd2+), and ∼1.8 ppb (Pb2+), respectively. Over the entire test period, the removal efficiency remained stable with metal ion concentrations of under 5 ppb, indicating that the output water is safe for drinking according to WHO standards. An important aspect to note for this system is the exceptional levels of cadmium removal. Cadmium has the lowest limit for drinking safety, and thus Cd levels are the limiting factor for developing effective filtration. In this case, PAN-amidoxime nonselectively exhibits strong coordination reactions with most heavy metal ions, and thus the filtering efficiency is about the same for all three metals. Longer-term filtration experiments were also performed for 3 days (Figure 4b). On the third day, even after around 21.6 L of mixed-ions contaminated water had been treated by the 1 cm2 area and 0.318 cm thick filter, the output water still had safe metal ion concentrations of 3.3 ppb Cu2+, 4.5 ppb Cd2+, and 4.2 ppb Pb2+. This exceptional performance indicates that the device can maintain its high heavy-metal-removal efficiency over a long time and purify a large quantity of water.
Figure 4.
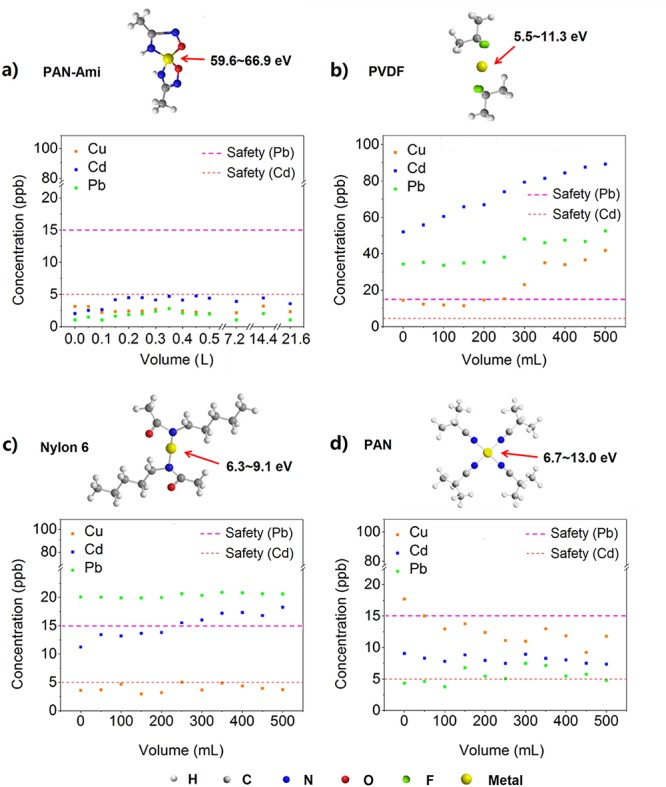
Tests of long-term flowing performance on different polymer-modified CF with mixed-ions contaminated water and DFT calculation ranges of their binding energy with metal ions. (a–d) Binding modes with metal ions and performances of PACCF, PVDF/C@CF, nylon-6/C@CF, and PCCF for mixed contaminated water.
The extracted electrodeposited metal species were further characterized by scanning electron microscope, X-ray photoelectron spectroscopy, and X-ray powder diffraction. As the SEM images show in Figure S8a, after 10 min of treatment in the stirring system, the metal ions (1000 ppm) from the test water were electrochemically deposited as nanoparticles in different morphologies on the electrode surface. These electrodeposits on the surface of the filtration electrode (Figure S8b,c) were identified to be metallic Cu, Cd, and Pb (JCPDS 85-1326, 65-1183, and 65-2873) by X-ray Diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) method. The copper deposition forms ball-like clusters, with each ball surrounded by large amounts of small nanocubes (about 50 nm). The cadmium and lead deposits are both nanosheets, with cadmium forming stacked sheets and lead being more well-dispersed. After long-term treatment (21.6 L, 100 ppb) of mixed water, the similar morphologies still can be found in the SEM images (Figure S9), but with higher degrees of crystallization.
Heavy metals have high toxicity and biological accumulation, and thus we have focused on developing an energy-efficient strategy to solve water pollution issues. There are significant economic benefits in recovering heavy metal ions from wastewater due to their unique physical and chemical characteristics. Therefore, it is necessary to exploit a new way to extract heavy metal ions separately. Thus, after different attempts, we successfully use an AC–DC combination method to remove the ions step by step (Figure S10). This direct separation and recovery of heavy metal ions has great value on resource recycling as compared with traditional treatments.
DFT Calculation of Binding Energy
For this filtration device, there are two important driving forces behind the filtration process. First, the applied bias generates an electric field which causes metal ion migration and deposition on the filter. Additionally, the polymer with many amidoxime functional groups strongly chelates the heavy metal ions to prevent them from being washed away in the flow of water. The polymer shell can make the electrode inert to prevent water splitting at low ionic strengths.
To prove the strong binding ability of PAN-ami with metal ions, a series of density functional theory (DFT) calculations for the adsorption of heavy metal ions (Cu2+, Cd2+, Pb2+) have been performed for the monomers of various polymers (PAN-ami, PAN, PVDF, and nylon-6), as shown in Figure 4 and Table S1. The full geometry optimizations, energetic calculations, and Mulliken population analyses were carried out by the DMol3 package. In all electron calculations by the DMol3 program, the density functional of generalized gradient corrected (GGA) with the Perdew–Burke–Ernzerhof (PBE) was adopted. For comparison, geometries and binding energies of the composite systems for the adsorptions of heavy metals on the polymers have been investigated.
To evaluate the interactions of heavy metals with polymers, the binding energies (Eb) were calculated by
where E(Mx+), E(P), and E(nP + Mx+) are the energies of the heavy metal ion (Mx+), monomer of each polymer (P), and the total energy of the complex systems of Mx+ adsorbed by relevant monomers, respectively.
The DFT calculation showed that the monomer of PAN-ami is over 5–10 times stronger than the others when binding with heavy metal ions as Cu2+, Cd2+, and Pd2+. Accordingly, these numbers explain the remarkable performance of PAN-ami for heavy metal ion adsorption. For monomers of PVDF, there are no strong binding sites such as coordination sites exposed to the cations. The nylon-6 monomer has a coordination site after deprotonation of the amide group, but this amide coordination bond formed is weaker than that of the nitrile group on PAN. The amidoxime group can coordinate with cations to form stable pentacyclic compounds, suggesting that this coordination bond should be stronger than other kinds of monodentate groups as compared in Table S1. Experimentally, we found that none of these polymers (PVDF, nylon-6, PAN) were able to match the performance of PAN-ami in removing metal ions to safe drinking levels (Figure 4b–d).
Conclusions
In summary, we provided a highly stable and efficient heavy metal ion removal method for drinking water with low cost. The working electrode combines the advantages of the carbon electrode and amidoxime-functionalized polymer. The carbon felt with macropores can greatly lower the pressure drop compared with that of conventional filtration membranes. The polymer with amidoxime groups can coordinate metal cations strongly. The chelated ions can be then reduced to their metallic state, releasing the coordination sites for new ions, which greatly enhances the stability and efficiency of the filter. The high electric field draws cations to the electrode surface and electrodeposits them, while only drawing 1∼2 mA cm–2 of current density. Our device can remove trace concentrations of metal ions from water continually with high long-term stability. The filter capacity has a high capacity of over 2300 mg g–1 filter while continuously providing safe drinking water or even other high demands. Furthermore, the cost of energy consumed during operation of this device is lower than $6.67 × 10–3 per ton of water filtered. Thus, we expect that this work could provide a new thought to the next generation of the water purification industry.
Experimental Section
Electrode Modification
A 1.5 mg portion of Super P (carbon blank) with 2 mg of polyacrylonitrile (PAN, average Mw 15 000) were added in 40 mL of DMF and stirred overnight until the slurry became uniform and sticky. The commercial carbon felts were immersed and squeezed in the slurry to disperse the slurry uniformly (PCCF). After doing this, the PCCF was dried at 90 °C for 1 h to remove the solvent. For the amidoximation reaction,40 10 pieces of PCCF were submerged into 20 mL of DI water under a heated water bath at 70 °C. After the temperature stabilized, 1.5 g of Na2CO3 and 2 g of NH2OH·HCl were successively added into the water. After 1.5 h, the reaction is complete. The PACCF pieces are removed and cleaned three times by DI water followed by air drying in a furnace (80 °C) before use.
Material Characterization
Electrode materials are characterized by scanning electron microscopy (SEM, FEI Nova NanoSEM 450), Fourier transform infrared spectroscopy (FTIR, Nicolet iS50), X-ray diffraction (XRD, PANalytical Material Research diffractometer), and X-ray photoelectron spectroscopy (XPS, SSI SProbe XPS spectrometer with Al Kα source). The ion (Cu2+, Cd2+, Pb2+, and Hg2+) concentrations were measured by inductively coupled plasma mass spectrometry (ICP-MS).
Acknowledgments
We acknowledge the Stanford facilities, SNL, SMF, and EMF, for characterization. We also want to thank G. Li for help in ICP-MS measurement.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.9b00130.
Additional characterizations including the demo of the filtration device, XPS characterization, EDX images, schematic comparison, performance control test, capacity test, optimization test, flow test, and cost analysis (PDF)
Author Contributions
§ T.W. and C.L. contributed equally to this work. T.W., C.L., and Y.C. conceived the concept. T.W. synthesized the electrode materials. J.S. made the DFT calculations. B.K. designed the scheme. Y.G., K.L., J.X., and Z.Z. helped the characteristic measurement. T.W. and C.L. analyzed the data. T.W., C.L., and Y.C. cowrote the manuscript. All the authors discussed the whole work.
The authors declare no competing financial interest.
Notes
No unexpected or unusually high safety hazards were encountered.
Supplementary Material
References
- Schwarzenbach R. P.; Egli T.; Hofstetter T. B.; Von Gunten U.; Wehrli B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. 10.1146/annurev-environ-100809-125342. [DOI] [Google Scholar]
- Lu W. Q.; Xie S. H.; Zhou W. S.; Zhang S. H.; Liu A. L. Water pollution and health impact in China: a mini review. Open Environ. Sci. 2008, 2, 1–5. 10.2174/1876325100802010001. [DOI] [Google Scholar]
- Sridhara Chary N.; Kamala C. T.; Samuel Suman Raj D. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008, 69, 513–524. 10.1016/j.ecoenv.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Järup L. Hazards of heavy metal contamination Br. Br. Med. Bull. 2003, 68, 167–182. 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- Forstnerand U.; Wittmann G. T.. Metal pollution in the aquatic environment; Springer, 1981; pp 19–22, 98–100. [Google Scholar]
- Schwarzenbach R. P.; Escher B. I.; Fenner K.; Hofstetter T. B.; Johnson C. A.; Von Gunten U.; Wehrli B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. 10.1126/science.1127291. [DOI] [PubMed] [Google Scholar]
- Shen Y.; Fang Q.; Chen B. Environmental applications of three-dimensional graphene-based macrostructures: adsorption, transformation, and detection. Environ. Sci. Technol. 2015, 49, 67–84. 10.1021/es504421y. [DOI] [PubMed] [Google Scholar]
- Teresa Albelda M.; Frias J. C.; Garcia-Espana E.; Schneider H.-J. Supramolecular complexation for environmental control. Chem. Soc. Rev. 2012, 41, 3859–3877. 10.1039/c2cs35008d. [DOI] [PubMed] [Google Scholar]
- Gupta V. K.; Nayak A.; Agarwal S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environ. Eng. Res. 2015, 20, 1–18. 10.4491/eer.2015.018. [DOI] [Google Scholar]
- Gupta V. K.; Kumar R.; Nayak A.; Saleh T. A.; Barakat M. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv. Colloid Interface Sci. 2013, 193, 24–34. 10.1016/j.cis.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Ahmaruzzaman M.; Gupta V. K. Rice husk and its ash as low cost adsorbents in water and wastewater treatment. Ind. Eng. Chem. Res. 2011, 50, 13589–13613. 10.1021/ie201477c. [DOI] [Google Scholar]
- Gupta V. K.; Saleh T. A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene - An overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. 10.1007/s11356-013-1524-1. [DOI] [PubMed] [Google Scholar]
- Matlock M. M.; Howerton B. S.; Atwood D. A. Chemical precipitation of heavy metals from acid mine drainage. Water Res. 2002, 36, 4757–4764. 10.1016/S0043-1354(02)00149-5. [DOI] [PubMed] [Google Scholar]
- Charerntanyarak L. Heavy metals removal by chemical coagulation and precipitation. Water Sci. Technol. 1999, 39, 135–138. 10.2166/wst.1999.0642. [DOI] [Google Scholar]
- Srivastava N. K.; Majumder C. B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. 10.1016/j.jhazmat.2007.09.101. [DOI] [PubMed] [Google Scholar]
- Ahluwalia S. S.; Goyal D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. 10.1016/j.biortech.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Karimi-Maleh H.; Tahernejad-Javazmi F.; Atar N.; Yola M. L.; Gupta V. K.; Ensafi A. A. A novel DNA biosensor based on a pencil graphite electrode modified with polypyrrole/functionalized multiwalled carbon nanotubes for determination of 6-mercaptopurine anticancer drug. Ind. Eng. Chem. Res. 2015, 54, 3634–3639. 10.1021/ie504438z. [DOI] [Google Scholar]
- Allioux F.-M.; Kapruwan P.; Milne N.; Kong L.; Fattaccioli J.; Chen Y.; Dumée L. F. Electro-capture of heavy metal ions with carbon cloth integrated microfluidic devices. Sep. Purif. Technol. 2018, 194, 26–32. 10.1016/j.seppur.2017.10.064. [DOI] [Google Scholar]
- Caprarescu S.; Vaireanu D.; Cojocaru A.; Maior I.; Purcar V. A 3-cell electrodialysis system for the removal of copper ions from electroplating wastewater. Optoelectron. Adv. Mater., Rapid Commun. 2011, 5 (12), 1346–1351. [Google Scholar]
- Gupta V. K.; Nayak A.; Agarwal S.; Singhal B. Recent advances on potentiometric membrane sensors for pharmaceutical analysis. Comb. Chem. High Throughput Screening 2011, 14, 284–302. 10.2174/138620711795222437. [DOI] [PubMed] [Google Scholar]
- Gupta V. K.; Sethi B.; Sharma R. A.; Agarwal S.; Bharti A. Mercury selective potentiometric sensor based on low rim functionalized thiacalix [4]-arene as a cationic receptor. J. Mol. Liq. 2013, 177, 114–118. 10.1016/j.molliq.2012.10.008. [DOI] [Google Scholar]
- Gupta V. K.; Ganjali M. R.; Norouzi P.; Khani H.; Nayak A.; Agarwal S. Electrochemical analysis of some toxic metals by ion selective electrodes. Crit. Rev. Anal. Chem. 2011, 41, 282–313. 10.1080/10408347.2011.589773. [DOI] [PubMed] [Google Scholar]
- Gupta V. K.; Karimi-Maleh H.; Sadegh R. Simultaneous determination of hydroxylamine, phenol and sulfite in water and waste water samples using a voltammetric nanosensor. Int. J. Electrochem. Sci. 2015, 10, 303–316. [Google Scholar]
- Yola M. L.; Gupta V. K.; Eren T.; Sen A. E.; Atar N. A novel electro analytical nanosensor based on graphene oxide/silver nanoparticles for simultaneous determination of quercetin and morin. Electrochim. Acta 2014, 120, 204–211. 10.1016/j.electacta.2013.12.086. [DOI] [Google Scholar]
- Gupta V. K.; Kumar S.; Singh R.; Singh L. P.; Shoora S. K.; Sethi B. Cadmium (II) ion sensing through p-tert-butyl calix[6]arene based potentiometric sensor. J. Mol. Liq. 2014, 195, 65–68. 10.1016/j.molliq.2014.02.001. [DOI] [Google Scholar]
- Jain A. K.; Gupta V. K.; Sahoo B. B.; Singh L. P. Copper (II)-selective electrodes based on macrocyclic compounds. Anal. Proc. 1995, 32, 99–101. 10.1039/ai9953200099. [DOI] [Google Scholar]
- Smara A.; Delimi R.; Poinsignon C.; Sandeaux J. Electroextraction of heavy metals from diluted solutions by a process combining ionexchange resins and membranes. Sep. Purif. Technol. 2005, 44, 271–277. 10.1016/j.seppur.2005.02.003. [DOI] [Google Scholar]
- Bolisetty S.; Mezzenga R. Amyloid-carbon hybrid membranes for universal water purification. Nat. Nanotechnol. 2016, 11, 365–371. 10.1038/nnano.2015.310. [DOI] [PubMed] [Google Scholar]
- Vilela D.; Parmar J.; Zeng Y. F.; Zhao Y. L.; Sanchez S. Graphene-based microbots for toxic heavy metal removal and recovery from water. Nano Lett. 2016, 16, 2860–2866. 10.1021/acs.nanolett.6b00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K. J.; Chen B. L.; Zhu X. Y.; Xing B. S. Aggregation, adsorption, and morphological transformation of graphene oxide in aqueous solutions containing different metal cations. Environ. Sci. Technol. 2016, 50, 11066–11075. 10.1021/acs.est.6b04235. [DOI] [PubMed] [Google Scholar]
- Kressman T. R. E. Ion exchange resin membranes and resin-impregnated filter paper. Nature 1950, 165, 568. 10.1038/165568a0. [DOI] [Google Scholar]
- Li B. Y.; Zhang Y. M.; Ma D. X.; Shi Z.; Ma S. Q. Mercury nano-trap for effective and efficient removal of mercury(II) from aqueous solution. Nat. Commun. 2014, 5, 5537–5543. 10.1038/ncomms6537. [DOI] [PubMed] [Google Scholar]
- Ding S. Y.; Dong M.; Wang Y. W.; Chen Y. T.; Wang H. Z.; Su C. Y.; Wang W. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(II). J. Am. Chem. Soc. 2016, 138, 3031–3037. 10.1021/jacs.5b10754. [DOI] [PubMed] [Google Scholar]
- Yang H. Y.; Han Z. J.; Yu S. F.; Pey K. L.; Ostrikov K.; Karnik R. Carbon nanotube membranes with ultrahigh specific adsorption capacity for water desalination and purification. Nat. Commun. 2013, 4, 2220–2227. 10.1038/ncomms3220. [DOI] [PubMed] [Google Scholar]
- Gupta V. K.; Ali I.; Saleh Y. A.; Siddiqui M. N.; Agarwal S. Chromium removal from water by activated carbon developed from waste rubber tires. Environ. Sci. Pollut. Res. 2013, 20, 1261–1268. 10.1007/s11356-012-0950-9. [DOI] [PubMed] [Google Scholar]
- Gupta V. K.; Agarwal S.; Saleh T. A. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J. Hazard. Mater. 2011, 185, 17–23. 10.1016/j.jhazmat.2010.08.053. [DOI] [PubMed] [Google Scholar]
- Khani H.; Rofouei M. K.; Arab P.; Gupta V. K.; Vafaei Z. Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: Application to potentiometric monitoring of mercury ion(II). J. Hazard. Mater. 2010, 183, 402–409. 10.1016/j.jhazmat.2010.07.039. [DOI] [PubMed] [Google Scholar]
- Gupta V. K.; Mergu N.; Kumawat L. K.; Singh A. K. Selective naked-eye detection of Magnesium (II) ions using a coumarin-derived fluorescent probe. Sens. Actuators, B 2015, 207, 216–223. 10.1016/j.snb.2014.10.044. [DOI] [Google Scholar]
- Srivastava S. K.; Gupta U. K.; Jain S. Determination of lead using a poly(vinyl chloride)-based crown ether membrane. Analyst 1995, 120, 495–498. 10.1039/an9952000495. [DOI] [Google Scholar]
- Srivastava S. K.; Gupta V. K.; Dwivedi M. K.; Jain S. Caesium PVC-crown (dibenzo-24-crown-8) based membrane sensor. Anal. Proc. 1995, 32, 21–23. 10.1039/AI9953200021. [DOI] [Google Scholar]
- Gupta V. K.; Singh A. K.; Kumawat L. K. Thiazole Schiff base turn-on fluorescent Chemosensor for Al3+ ion. Sens. Actuators, B 2014, 195, 98–108. 10.1016/j.snb.2013.12.092. [DOI] [Google Scholar]
- Srivastava S. K.; Gupta V. K.; Jain S. PVC-based 2, 2, 2-cryptand sensor for zinc ions. Anal. Chem. 1996, 68, 1272–1275. 10.1021/ac9507000. [DOI] [PubMed] [Google Scholar]
- Gupta V. K.; Singh L.; Singh R.; Upadhyay N.; Kaur S.; Sethi B. A novel copper (II) selective sensor based on dimethyl 4, 4’(o-phenylene) bis (3-thioallophanate) in PVC matrix. J. Mol. Liq. 2012, 174, 11–16. 10.1016/j.molliq.2012.07.016. [DOI] [Google Scholar]
- Karthikeyan S.; Gupta V.; Boopathy R.; Titus A.; Sekaran G. A new approach for the degradation of high concentration of aromatic amine by heterocatalytic Fenton oxidation: kinetic and spectroscopic studies. J. Mol. Liq. 2012, 173, 153–163. 10.1016/j.molliq.2012.06.022. [DOI] [Google Scholar]
- Dehghani M. H.; Sanaei D.; Ali I.; Bhatnagar A. Removal of chromium(VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: Kinetic modeling and isotherm studies. J. Mol. Liq. 2016, 215, 671–679. 10.1016/j.molliq.2015.12.057. [DOI] [Google Scholar]
- Asfaram A.; Ghaedi M.; Agarwal S.; Tyagi I.; Gupta V. K. Removal of basic dye Auramine-O by ZnS: Cu nanoparticles loaded on activated carbon: optimization of parameters using response surface methodology with central composite design. RSC Adv. 2015, 5, 18438–18450. 10.1039/C4RA15637D. [DOI] [Google Scholar]
- Gupta V. K.; Atar N.; Yola M. L.; ÜstÜndağ Z.; Uzun L. A novel magnetic Fe@Au core-shell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res. 2014, 48, 210–217. 10.1016/j.watres.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Gupta V. K.; Mergu N.; Kumawat L. K.; Singh A. K. A reversible fluorescence “off-on-off” sensor for sequential detection of aluminum and acetate/fluoride ions. Talanta 2015, 144, 80–89. 10.1016/j.talanta.2015.05.053. [DOI] [PubMed] [Google Scholar]
- Jain A. K.; Gupta V. K.; Singh L. P. Neutral carrier and organic resin based membranes as sensors for uranyl ions. Anal. Proc. 1995, 32, 263–265. 10.1039/ai9953200263. [DOI] [Google Scholar]
- El-sayed M. T. The use of Saccharomyces cerevisiae for removing cadmium (II) from aqueous waste solutions. Afr. J. Microbiol. Res. 2012, 6, 6900–6910. 10.5897/AJMR12.613. [DOI] [Google Scholar]
- The National Primary Drinking Water Regulations (NPDWR), required by the Safe Drinking Water Act (SDWA) in United States.https://www.epa.gov/dwstandardsregulations (accessed May, 2009).
- National Standard of the People’s Republic of China (GB 5749-2006), required by Ministry of Health of China and Standardization Administration of China. https://webstore.ansi.org/standards/spc/gb57492006 (accessed December, 2006).
- Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption, Official Journal of the European Communities.
- Hattori M. The Drinking Water Quality Standards (DWQSs) and problems of odor and taste in water supply. Journal of Japan Association on Odor Environment 2008, 39, 94–101. 10.2171/jao.39.94. [DOI] [Google Scholar]
- Abney C. W.; Mayes R. T.; Piechowicz M.; Lin Z.; Bryantsev V. S.; Veith G. M.; Dai S.; Lin W. XAFS investigation of polyamidoxime-bound uranyl contests the paradigm from small molecule studies. Energy Environ. Sci. 2016, 9, 448–453. 10.1039/C5EE02913A. [DOI] [Google Scholar]
- Ivanov A. S.; Leggett C. J.; Parker B. F.; Zhang Z.; Arnold J.; Dai S.; Abney C. W.; Bryantsev V. S.; Rao L. Origin of the unusually strong and selective binding of vanadium by polyamidoximes in seawater. Nat. Commun. 2017, 8, 1560–1569. 10.1038/s41467-017-01443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efthymiou C. G.; Cuhna-Silva L.; Perlepes S. P.; Brechin E. K.; Inglis R.; Evangelisti M.; Papatriantafyllopoulou C. In search of molecules displaying ferromagnetic exchange: multiple-decker Ni12 and Ni16 complexes from the use of pyridine-2-amidoxime. Dalton Trans. 2016, 45, 17409–17419. 10.1039/C6DT03511F. [DOI] [PubMed] [Google Scholar]
- Zhijiang C.; Jianru J.; Qing Z.; Haizheng Y. Preparation of amidoxime surface-functionalized polyindole (ASFPI) nanofibers for Pb(II) and Cd(II) adsorption from aqueous solutions. RSC Adv. 2015, 5, 82310–82323. 10.1039/C5RA13253C. [DOI] [Google Scholar]
- Saeed K.; Haider S.; Oh T. J.; Park S. Y. Preparation of amidoxime-modified polyacrylonitrile (PAN-oxime) nanofibers and their applications to metal ions adsorption. J. Membr. Sci. 2008, 322, 400–405. 10.1016/j.memsci.2008.05.062. [DOI] [Google Scholar]
- Liu C.; Hsu P.-C.; Xie J.; Zhao J.; Wu T.; Wang H.; Liu W.; Zhang J.; Chu S.; Cui Y. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat. Energy 2017, 2, 17007–17014. 10.1038/nenergy.2017.7. [DOI] [Google Scholar]
- Godt J.; Scheidig F.; Grosse-Siestrup C.; Esche V.; Brandenburg P.; Reich A.; Groneberg D. A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22–27. 10.1186/1745-6673-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchounwou P. B.; Yedjou C. G.; Patlolla A. K.; Sutton D. J. Heavy metal toxicity and the environment. EXS 2012, 101, 133–164. 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern B. R. Essentiality and toxicity in copper health risk assessment: overview, update and regulatory considerations. J. Toxicol. Environ. Health, Part A 2010, 73, 114–127. 10.1080/15287390903337100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.