Abstract
Exposure to environmental chemicals such as phthalates has been linked to numerous adverse pregnancy outcomes, potentially through an oxidative stress mediated mechanism. Most research examined urinary 8-iso-prostaglandin F2α (8-iso-PGF2α) as the oxidative stress biomarker. However, 8-iso-PGF2α also originates from enzymatic sources linked to inflammation. Therefore, associations between phthalates and 8-iso-PGF2α could have been misinterpreted. To clarify this, the 8-iso-PGF2α/prostaglandin F2α ratio approach was used to quantitatively distinguish between inflammation or oxidative stress derived 8-iso-PGF2α and estimate their associations with phthalate metabolites in a cohort of 758 pregnant women from The Infant Development and Environment Study (TIDES). Most urinary phthalate metabolites were associated with a significant increase in 8-iso-PGF2α. For example, a 22.4% higher 8-iso-PGF2α concentration (95% confidence interval = 14.4, 30.9) was observed with an interquartile range increase in mono-n-butyl phthalate. For most metabolites, associations were observed solely with oxidative stress derived 8-iso-PGF2α. In contrast, monocarboxy-isononyl phthalate and mono-isononyl phthalate (MNP) were associated with both sources of 8-iso-PGF2α. Metabolites of the phthalate alternative 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH), were only associated with inflammation-derived 8-iso-PGF2α, which is interesting because DINCH metabolites and MNP have structural similarities. In conclusion, phthalates metabolites are not exclusively associated with oxidative stress derived 8-iso-PGF2α. Depending on the metabolite structure, some are also associated with inflammation derived sources, which provides interesting insights in the toxicology of phthalates.
Keywords: 8-iso-PGF2α / PGF2α ratio, F2-isoprostanes, phthalates, phthalate alternatives, oxidative stress biomarkers, inflammation
Graphical Abstract
Introduction
Exposure to some phthalates has been linked to developmental abnormalities as well as complications with pregnancy such as preeclampsia, preterm birth, and others.1–5 One mechanism behind this association has been hypothesized to involve oxidative stress, as numerous studies have shown positive associations between oxidative stress biomarkers levels and urinary phthalate metabolite concentrations.6, 7 Most studies investigating the association between oxidative stress and phthalates have used 8-iso-prostaglandin F2α (8-iso-PGF2α) as their biomarker of oxidative stress. 8-iso-PGF2α is a considered the best marker of oxidative stress, can be measured in many biospecimens and is elevated in a wide range of human medical conditions as well as in response to environmental exposures.8, 9 However, interpretation of elevated 8-iso-PGF2α levels as an indicator solely of oxidative stress is no longer appropriate.10, 11 Generation of 8-iso-PGF2α occurs both through chemical free radical oxidation as well as enzymatic synthesis via the prostaglandin H synthase pathway, which is a hallmark of inflammation.12 Epidemiologic evidence suggests that certain phthalate metabolites may be associated with markers of inflammation such as interleukin-6 (IL-6) in addition to 8-iso-PGF2α.6, 13 Thus, it is important that association between phthalates and oxidative stress as measured by 8-iso-PGF2α be reexamined and the contribution of inflammation be taken into account.
Using a novel measurement strategy, the 8-iso-PGF2α/PGF2α ratio,11 it is possible to distinguish the proportions of 8-iso-PGF2α that are generated by inflammation vs. oxidative stress. By applying this approach to data from The Infant Development and Environment study (TIDES), a cohort of pregnant women, it is now possible to accurately interpret the mechanisms behind associations observed in previous studies of phthalates and 8-iso-PGF2α. Additionally, in this study we were able to explore new metabolites, including those of di(2-ethylhexyl) terephthalate (DEHTP), and 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH, an emerging replacement for phthalates in consumer products) in relation to this endpoint.
Materials and Methods
Study population
TIDES is a prospective cohort of pregnant women recruited between 2010-2012 from four academic prenatal clinics: University of California, San Francisco; University of Rochester Medical Center; University of Minnesota; and University of Washington/Seattle Children’s Hospital. The study design and data collection methods have been previously published.14–16 After meeting the enrollment criteria, at routine prenatal visits, participants completed questionnaires and provided spot urine samples. Visit (and sample collection) occurred at the participant’s convenience and thus occurred at various times throughout the day. Study protocols were approved by the IRBs of each institution as well as the Icahn School of Medicine at Mount Sinai. All participants provided signed informed consent before starting any study activities.
Urinary phthalates and phthalate alternatives metabolite measurements
Urine samples for this part of the study were collected at the third study visit (median gestational age 32 weeks) in phthalate-free polypropylene cups.21 Urine samples were shipped on dry ice for analysis at the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention (CDC).17 The involvement of the CDC did not constitute engagement in human subjects research. Total phthalate and phthalate alternative metabolite concentrations were measured using automated online solid-phase-extraction coupled to isotope-dilution high-performance liquid chromatography tandem mass spectrometry, as described in detail elsewhere.18 Quality control samples were analyzed with each batch with coefficients of variation within the expected range (CV = 3-7%).
Participant samples were analyzed in two sets. One subset (n = 169) had been previously analyzed in the TIDES study and a second subset (n = 593) was analyzed with an expanded phthalate panel for this study in 2017. Depending on the metabolite, the limit of detection (LOD) was between 0.2 and 1.2 ng/mL and all blanks were below the LOD. For statistical analyses, values below the LOD in the first subset were replaced with the LOD divided by the square root of 2, as has been recommended.19 In the second subset, instrument reported values were used even when below LOD, per an update in CDC procedure.
Urinary F2-isoprostane measurements
F2-isoprostane content was measured in third trimester urine samples by the Eicosanoid Core Laboratory at Vanderbilt University Medical Center (Nashville, TN) using established methods.20 Specifically, three compounds 8-iso-prostaglandin F2α (8-iso-PGF2α), prostaglandin F2α (PGF2α) and 2,3-dinor-5,6-dihydro-15-F2t-isoprostane (F2-IsoP-M), a metabolite of 8-iso-PGF2α, were quantified and used as biomarkers in this study. Isotopically labeled standards were employed for each compound to provide the most reliable quantitation. Values below the LOD (0.101 ng/mL) were assigned the value of the LOD divided by the square root of 2. In addition to the measured biomarkers, the origins of 8-iso-PGF2α (chemical and enzymatic lipid peroxidation) are needed to conclusively determine oxidative stress as the source of elevated 8-iso-PGF2α. The sources of 8-iso-PGF2α are derived from the 8-iso-PGF2α / PGF2α ratio as described by van ‘t Erve et al.11 Briefly, when the source of 8-iso-PGF2α is solely from oxidative stress, the urine 8-iso-PGF2α/PGF2α would be near 1; if the source was predominantly enzymatic, the ratio would be near 0. The exact distribution of 8-iso-PGF2α originating from the two pathways (ng/mL 8-iso-PGF2α by chemical lipid peroxidation vs. ng/mL 8-iso-PGF2α by enzymatic lipid peroxidation) is calculated with the custom interface for the R package “Constrained Linear Mixed Effects (CLME)”21 using formulas determined by van ‘t Erve et al.11
Statistics
All measured concentrations of biomarkers of phthalates, phthalate alternatives, and oxidative stress were adjusted for urine dilution by correcting for urinary specific gravity (SG) which was measured within 30 minutes of urine collection using a handheld refractometer.22 Concentrations were corrected using the formula: Mc=M[(1.014-1)/(SG-1)], where 1.014 is the median SG in the TIDES population, M is the biomarker concentration as measured, and Mc is the SG-corrected marker.6
Frequencies and counts were used to describe demographic characteristics of the study population. Distributions of urinary biomarkers were examined using geometric means, geometric standard deviations, and selected percentiles. For all analyses, urinary biomarkers were natural log transformed. Biomarkers detected in <75% of samples were analyzed as dichotomous variables (i.e., detect vs. non-detect). Linear regression models were used to determine unadjusted and adjusted associations and 95% confidence intervals (CI) between each urinary exposure biomarker and each oxidative stress biomarker. P-values <0.05 were considered statistically significant. All beta estimates were standardized to represent the percent change in urinary oxidative stress biomarker in association with an interquartile range (IQR) difference in urinary exposure biomarker concentrations. If the phthalate or DINCH metabolite was treated as detect vs. non-detect then effect estimates represent the percent change in oxidative stress measure in individuals with detectable compared to those with undetectable concentrations.
Maternal age, self-reported pre-pregnancy body mass index (BMI, kg/m2), gestational age at urine sample collection, study center, race/ethnicity, education, household income, current alcohol use reported at the third visit, current smoking reported at the third visit, and prenatal vitamin use reported at the third visit were examined as potential covariates. Covariates that changed point estimates by >10% were retained in final models. We examined effect modification of the phthalate-oxidative stress associations by maternal race/ethnicity and fish oil supplementation (self-reported intake for at least one full week in the third trimester). All statistical analyses were conducted using RStudio Version 1.1.383.
Results
For the present analysis, a total of 758 pregnant women with data on urinary phthalates and DINCH metabolites and urinary oxidative stress biomarkers from the third study visit were included (Table 1). The average age of the study participants was 31.7 years with a pre-pregnancy BMI of 26.1 kg/m2. The majority where White (67.5%), well educated (72.8% graduated college), and had an income of >$25,000 per year (73.1%). Samples were collected at median 32.7 weeks gestation. Most participants reported no smoking (88%) or alcohol use (84.3%) as well as frequent consumption of a prenatal vitamin (86.3%).
Table 1:
Study population demographic characteristics.
| Characteristic | |
|---|---|
| Continuous variables | Mean (SD) |
| Maternal age (years) | 31.67 (5.43) |
| Pre-pregnancy BMI (kg/m2) | 26.10 (6.09) |
| Gestational age at urine collection (weeks) | 32.71 (3.04) |
| Categorical variables | N (%) |
| Race/Ethnicity | |
| White | 513 (67.7) |
| African-American | 101 (13.3) |
| Other | 143 (18.9) |
| Study Center | |
| University of California San Francisco | 213 (28.1) |
| University of Minnesota | 199 (26.3) |
| University of Rochester Medical Center | 204 (26.9) |
| University of Washington | 142 (18.7) |
| Education | |
| <College graduate | 199 (26.2) |
| >=College graduate | 553 (73) |
| Missing | 6 (0.8) |
| Income | |
| <$25k per year | 177 (23.3) |
| $25-75k per year | 199 (26.3) |
| >$75k per year | 356 (47.0) |
| Missing | 26 (3.4) |
| Current smoking | |
| No | 668 (88.1) |
| Yes | 36 (4.8) |
| Missing | 54 (4.1) |
| Current alcohol use | |
| No | 640 (84.4) |
| Yes | 60 (7.9) |
| Missing | 58 (7.7) |
| Prenatal vitamins | |
| No | 71 (9.4) |
| Yes | 654 (86) |
| Missing | 33 (4.6) |
| Body mass index at study visit | |
| <25kg/m2 | 400 (52.8) |
| 25-<30 kg/m2 | 164 (21.6) |
| >30kg/m2 | 160 (21.1) |
| Missing | 34 (4.5) |
| Previous pregnancy | |
| Yes | 456 (60.2) |
| No | 280 (36.9) |
| Missing | 22 (2.9) |
| Total | 758 |
Structures and names for each phthalate and DINCH metabolite measured are presented in Table 2 in order from lowest to highest molecular weight of the parent compound. Distributions of urinary phthalate and DINCH metabolites in this population are shown in Table 3. Correlation between the measured metabolites is shown in Supplemental Table 1 and is highest among metabolites that share a common parent phthalate. Most metabolites had a relatively high detection rate except for the DINCH metabolites, one DEHTP metabolite, and mono-isononyl phthalate (MNP), which were detected in less than 25% of samples. Typically, metabolites of lower molecular weight phthalates were present in higher concentrations than those of higher molecular weight phthalates. Highest concentrations were for mono-ethyl phthalate (MEP), monocarboxyoctyl phthalate (MCOP), and mono-n-butyl phthalate (MBP), with geometric means of 41.7, 15.9 and 9.8 ng/mL, respectively. The two DINCH metabolites (monohydroxy-isononyl ester, 1,2-cyclohexane dicarboxylic acid [MHiNCH] and monocarboxy-isooctyl ester, 1,2-cyclohexane-dicarboxylic acid [MCOCH]) were detected at the lowest frequency and had geometric mean (geometric standard deviation) values of 0.3 ± 2.5 and 0.4 ± 2.0 ng/mL, respectively. The terephthalate metabolite mono(2-ethyl-5-carboxypentyl) terephthalate (MECPTP) had a high detection rate and a geometric mean concentration of 3.2 ng/mL. Concentrations of each phthalate and phthalate alternative metabolite by demographic factors are presented in Supplemental Table 2. In general, higher phthalate metabolite concentrations were observed in urine of women who were African American compared to White or Other race/ethnicity, had a lower education or income level, who smoked or consumed alcohol in pregnancy, and who had a higher BMI. Interestingly, for the DINCH metabolites, the reverse was observed.
Table 2:
Structures of phthalate and phthalate replacement metabolites and their respective parent compounds.
| Parent | Structure | Metabolite | |
|---|---|---|---|
| Phthalates | Di-ethyl phthalate (DEP) |  |
Mono-ethyl phthalate (MEP) |
| Di-isobutyl phthalate (DiBP) | 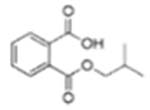 |
Mono-isobutyl phthalate (MiBP) | |
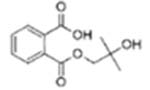 |
Mono-hydroxyisobutyl phthalate (MHiBP) | ||
| Di-n-butyl phthalate (DBP) | 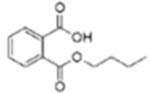 |
Mono-n-butyl phthalate (MBP) | |
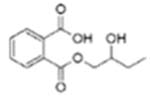 |
Mono(3-hydroxybutyl) phthalate (MHBP) | ||
| Benzylbutyl phthalate (BzBP) | 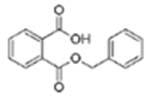 |
Monobenzyl phthalate (MBzP) | |
| Di(2-ethylhexyl) phthalate (DEHP or DOP) | 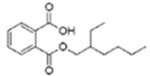 |
Mono(2-ethylhexyl) phthalate (MEHP) | |
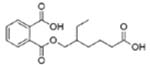 |
Mono(2-ethyl-5-carboxypentyl) phthalate (MECPP) | ||
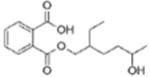 |
Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) | ||
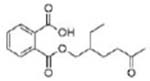 |
Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) | ||
| Di(2-ethylhexyl) terephthalate (DEHTP) | 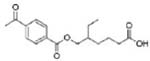 |
Mono(2-ethyl-5-carboxypentyl) terephthalate (MECPTP) | |
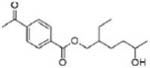 |
Mono-2-ethyl-5-hydroxyhexyl terephthalate (MEHHTP) | ||
| Di-n-octyl phthalate (DNOP) and others | 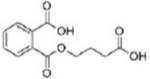 |
Mono (3-carboxypropyl) phthalate (MCPP) | |
| Di-isononyl phthalate (DNP or DiNP) | 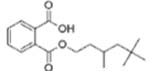 |
Mono-isononyl phthalate (MNP) | |
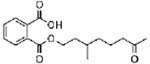 |
monooxoisononyl phthalate (MONP) | ||
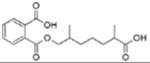 |
Monocarboxyoctyl phthalate (MCOP) | ||
| Di-isodecyl phthalate (DDP or DiDP) | 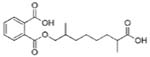 |
Monocarboxy-isononyl phthalate (MCNP) | |
| Phthalate Alternatives | 1,2-Cyclohexane dicarboxylic acid, diisononyl ester (DINCH) | 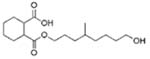 |
Monohydroxy-isononyl ester, 1,2-cyclohexane dicarboxylic acid (MHiNCH) |
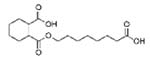 |
Monocarboxy-isooctyl ester, 1,2-cyclohexane-dicarboxylic acid (MCOCH) |
Table 3:
Distributions of specific gravity-corrected urinary phthalate and DINCH metabolites in ng/mL.a
| Percentiles | ||||||||
|---|---|---|---|---|---|---|---|---|
| LOD | %<LOD | GM (GSD) | N | 25th | 50th | 75th | 95th | |
| Phthalate metabolites | ||||||||
| MEP | 1.2 | 0 | 41.7 (4.6) | 756 | 13.9 | 34.3 | 103 | 642 |
| MBP | 0.4 | 0 | 9.8 (2.7) | 756 | 5.8 | 10.5 | 17 | 42.9 |
| MHBP | 0.4 | 13.6 | 1.1 (2.7) | 592 | 0.6 | 1.01 | 1.8 | 5.3 |
| MBzP | 0.3 | 0.8 | 4.8 (3.3) | 756 | 2.2 | 4.82 | 11.1 | 36.3 |
| MiBP | 0.8 | 0.9 | 7.2 (2.6) | 756 | 4.1 | 6.91 | 11.9 | 34.1 |
| MHiBP | 0.4 | 1.0 | 3.1 (2.4) | 592 | 1.9 | 2.91 | 4.73 | 13 |
| MECPP | 0.4 | 0 | 12.0 (2.2) | 756 | 7.4 | 11.4 | 17.7 | 51.3 |
| MEHHP | 0.4 | 0.1 | 6.7 (2.5) | 756 | 4.0 | 6.89 | 10.9 | 31.0 |
| MEOHP | 0.2 | 0.1 | 5.2 (2.5) | 756 | 3.0 | 5.42 | 8.63 | 22.4 |
| MEHP | 0.8 | 17.1 | 1.9 (2.7) | 756 | 1.1 | 1.91 | 3.28 | 9.3 |
| ΣDEHP | - | - | 0.1 (2.3) | 756 | 0.05 | 0.09 | 0.14 | 0.4 |
| MECPTP | 0.2 | 1.4 | 3.2 (4.3) | 592 | 1.3 | 2.66 | 6.6 | 49.6 |
| MEHHTP | 0.4 | 31.4 | 0.8 (3.4) | 592 | 0.4 | 0.7 | 1.5 | 8.5 |
| MCPP | 0.4 | 5.2 | 2.3 (3.8) | 756 | 1.0 | 1.83 | 4.44 | 31.6 |
| MCOP | 0.3 | 0 | 15.9 (3.3) | 756 | 6.6 | 13.1 | 34.1 | 129 |
| MONP | 0.4 | 0.7 | 5.3 (3.8) | 592 | 2.1 | 3.86 | 11.3 | 78.5 |
| MNP | 0.9 | 43.2 | 1.4 (3.8) | 592 | 0.5 | 1.11 | 2.66 | 18.8 |
| MCNP | 0.2 | 0 | 2.8 (2.7) | 756 | 1.4 | 2.33 | 4.2 | 18.9 |
| DINCH metabolites | ||||||||
| MHiNCH | 0.4 | 63.6 | 0.3 (2.5) | 756 | 0.2 | 0.3 | 0.5 | 1.3 |
| MCOCH | 0.5 | 67.7 | 0.4 (2.0) | 592 | 0.3 | 0.36 | 0.6 | 1.2 |
| Measured outcome biomarkers | ||||||||
| 8-iso-PGF2α | 1.0 (1.8) | 759 | 0.7 | 1 | 1.4 | 2.4 | ||
| PGF2α | 2.0 (2.1) | 759 | 1.3 | 2 | 3.2 | 7 | ||
| F2-IsoP-M | 0.6 (1.6) | 759 | 0.5 | 0.6 | 0.9 | 1.4 | ||
| Derived outcome biomarkers | ||||||||
| Enzymatic fraction of 8-iso-PGF2α | 0.2 (6.3) | 759 | 0.1 | 0.3 | 0.5 | 0.9 | ||
| Chemical fraction of 8-iso-PGF2α | 0.6 (2.2) | 759 | 0.4 | 0.6 | 1 | 2.1 | ||
Units for ∑DEHP are in nmol/ml. Abbreviations: DINCH, LOD, limit of detection; GM, geometric mean; GSD, geometric standard deviation.
The urinary oxidative stress biomarkers were detectable in all study participants (Table 3). In this population of pregnant women, specific gravity corrected PGF2α levels were on average twice as high as the corrected 8-iso-PGF2α levels. The average ratio of 8-iso-PGF2α / PGF2α was 0.47 ± 1.89, which indicates the majority of 8-iso-PGF2α in the urine samples was formed chemically. Associations between the oxidative stress biomarkers and demographic characteristics are presented in Supplemental Table 3 and have been previously discussed for this study population.23
Adjusted associations between the three measured urinary oxidative stress biomarkers and urinary phthalate and DINCH metabolites are presented in Table 4. All phthalates metabolites except for MEP had significant positive associations with 8-iso-PGF2α. Among these, the associations that were greatest in magnitude were observed with mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-isobutyl phthalate (MiBP), and MBP, where an IQR difference in urinary concentration was associated with a 21.5, 17.8 and 15.3% respective elevation in 8-iso-PGF2α levels. Among the phthalates treated dichotomously, the largest magnitude association was observed for MNP with a 19.6% difference in 8-iso-PGF2α levels between detect vs. non-detect individuals. Certain phthalate metabolites were positively associated with PGF2α, specifically MNP (20.3% higher levels with detection) and monocarboxy-isononyl phthalate (MCNP; 13.5% higher levels with an IQR difference). Smaller but still significant associations were observed for MiBP and mono(2-ethylhexyl) phthalate (MEHP). All phthalates metabolites were also associated with an elevation in the 8-iso-PGF2α metabolite (F2-IsoP-M) levels. In comparison to the parent 8-iso-PGF2α, associations between phthalate metabolites and F2-IsoP-M were smaller in magnitude, with larger associations being observed only for MEP and the non-specific phthalate metabolite mono(3-carboxypropyl) phthalate (MCPP). For metabolites of the phthalate alternatives, detection of the two DINCH metabolites (MHiNCH and MCOCH) had large positive associations with PGF2α and F2-IsoP-M. However, no association with 8-iso-PGF2α was observed. For the two DEHTP metabolites, positive associations were observed with all measured biomarkers except between MECPTP and F2-IsoP-M. All unadjusted associations were similar and are presented in Supplemental Table 4.
Table 4:
Adjusteda percent change (95% confidence interval) in urinary 8-iso-PGF2α, PGF2α, and F2-IsoP-M in association with an interquartile range increase in urinary phthalate and DINCH metabolite concentrations.
| 8-iso-PGF2α | PGF2α | F2-IsoP-M | ||||||
|---|---|---|---|---|---|---|---|---|
| Parent | Metabolite | %Δ (95% CI) | p | %Δ (95% CI) | p | %Δ (95% CI) | p | |
| Phthalates | DEP | MEP | 4.48 (−1.1,10.36) | 0.1 | 2.83 (−4.66,10.91) | 0.5 | 5.39 (0.96,10) | 0.02 |
| DBP | MBP | 15.27 (9.62,21.21) | <0.01 | 6.07 (−1.16,13.84) | 0.1 | 9.98 (5.7,14.43) | <0.01 | |
| MHBP | 8.04 (2.39,13.99) | <0.01 | 5.81 (−1.79,14.01) | 0.1 | 4.63 (0.42,9.03) | 0.03 | ||
| BzBP | MBzP | 12.99 (6.67,19.69) | <0.01 | 3.13 (−4.83,11.76) | 0.5 | 10.39 (5.52,15.48) | <0.01 | |
| DiBP | MiBP | 17.79 (12.52,23.31) | <0.01 | 9.29 (2.42,16.62) | <0.01 | 12.06 (8.08,16.18) | <0.01 | |
| MHiBP | 11.96 (6.59,17.61) | <0.01 | 6.02 (−1.06,13.61) | 0.1 | 6.6 (2.63,10.72) | <0.01 | ||
| DEHP | MECPP | 11.68 (6.89,16.69) | <0.01 | 5.01 (−1.25,11.66) | 0.1 | 9.29 (5.59,13.11) | <0.01 | |
| MEHHP | 8.5 (3.84,13.37) | <0.01 | 0.88 (−5.1,7.23) | 0.8 | 6.2 (2.6,9.92) | <0.01 | ||
| MEOHP | 9.42 (4.48,14.6) | <0.01 | 1.49 (−4.84,8.23) | 0.7 | 6.78 (2.97,10.72) | <0.01 | ||
| MEHP | 9.26 (4.32,14.43) | <0.01 | 7.99 (1.29,15.15) | 0.02 | 4.23 (0.5,8.11) | 0.03 | ||
| ΣDEHP | 12.19 (6.63,18.04) | <0.01 | 4.42 (−2.74,12.1) | 0.2 | 9.04 (4.78,13.48) | <0.01 | ||
| DEHTP | MECPTP | 10.63 (5.19,16.34) | <0.01 | 9.31 (1.9,17.26) | 0.01 | 3.35 (−0.59,7.45) | 0.1 | |
| MEHHTP b | 17.74 (6.64,29.99) | <0.01 | 21.68 (6.09,39.57) | <0.01 | 10.32 (2.25,19.02) | 0.01 | ||
| DOP | MCPP | 5.29 (0.37,10.44) | 0.04 | 3.61 (−3.01,10.69) | 0.3 | 5.4 (1.53,9.42) | <0.01 | |
| DNP | MCOP | 13.06 (6.79,19.7) | <0.01 | 7.32 (−0.88,16.19) | 0.08 | 9.38 (4.59,14.39) | <0.01 | |
| MNP* | 11.72 (1.67,22.77) | 0.02 | 20.3 (5.63,37) | <0.01 | 7.3 (−0.17,15.32) | 0.06 | ||
| MONP | 6.62 (0.5,13.12) | 0.03 | 2.61 (−5.47,11.38) | 0.5 | 3.38 (−1.2,8.17) | 0.2 | ||
| DDP | MCNP | 8.75 (4.02,13.7) | <0.01 | 13.54 (6.8,20.71) | <0.01 | 5.99 (2.35,9.76) | <0.01 | |
| Phthalate alternative | DINCH | MHiNCH b | 3.69 (−5.01,13.18) | 0.4 | 27.57 (13.21,43.75) | <0.01 | 16.18 (8.56,24.32) | <0.01 |
| MCOCH b | 9.02 (−1.51,20.67) | 0.1 | 31.81 (14.73,51.44) | <0.01 | 17.26 (8.61,26.6) | <0.01 | ||
Models adjusted for maternal Race/Ethnicity, education, income, and body mass index. Phthalate and DINCH metabolites, oxidative stress biomarkers are corrected for urinary specific gravity.
These metabolites are modeled dichotomously as above or below LOD instead of numerically
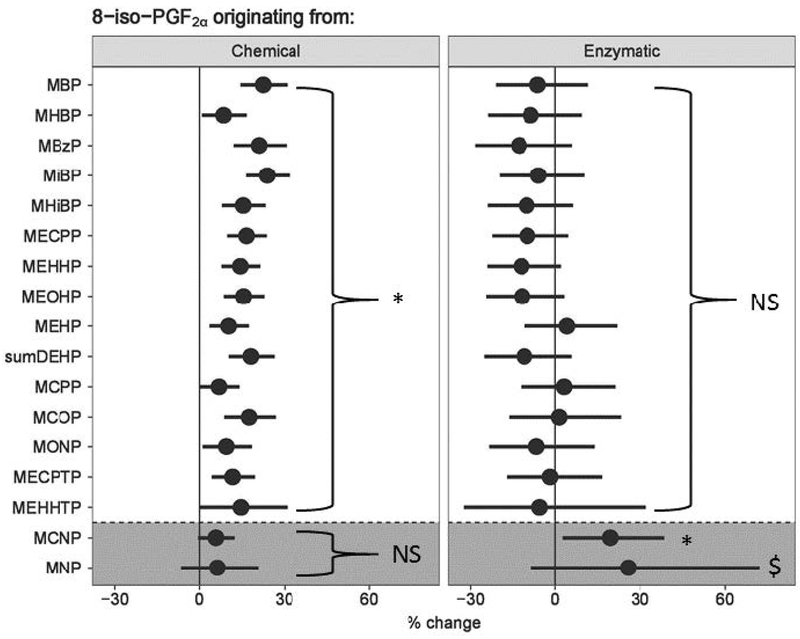
With the significant positive associations observed for certain metabolites with 8-iso-PGF2α as well as PGF2α, it is important to investigate the source of 8-iso-PGF2α generation to mechanistically interpret the findings. The 8-iso-PGF2α / PGF2α ratio was used to distinguish between enzymatic (i.e., PGHS) and chemical (i.e. oxidative stress) sources of 8-iso-PGF2α. Contribution from the chemical source represents traditional oxidation of arachidonic acid by free radicals and/or reactive oxygen species, often referred to as oxidative damage, whereas enzymatic sources are through direct synthesis by PGHS which is induced during inflammation. Distinction between the two sources was determined for only those metabolites which had significant associations with 8-iso-PGF2α in the adjusted linear model, therefore DINCH metabolites and MEP were excluded. Generally, phthalates and DEHTP metabolites had positive associations only with the chemical pathway (6.9 – 23.8% change with an IQR increase in metabolite concentration, Figure 1). Exceptions to this rule were the two phthalate metabolites MCNP and MNP which were associated only with the enzymatic pathway. MNP was only statistically significantly associated with the enzymatic pathway in the unadjusted linear model, and MCNP was significant in both models. No other phthalate or DINCH metabolites were associated with changes in 8-iso-PGF2α derived from the enzymatic pathway. Effect estimates for adjusted and unadjusted associations for all metabolites and the enzymatic and chemical sources of 8-iso-PGF2α are presented in Supplemental Tables 5 and 6, respectively. Lastly, results from models of raw concentrations with the inclusion of specific gravity as a covariate in the model produced similar results (Supplemental Tables 7 and 8).
Figure 1: Percent change (95% confidence interval) in chemical and enzymatic derived 8-iso-PGF2α and PGF2α in association with an interquartile range increase in urinary phthalate metabolite concentration.
Results from linear model adjusted for maternal Race/Ethnicity, education, income, and body mass index. *Denotes significant association after adjustment, p < 0.05. $Denotes significant association only in unadjusted model, p < 0.05
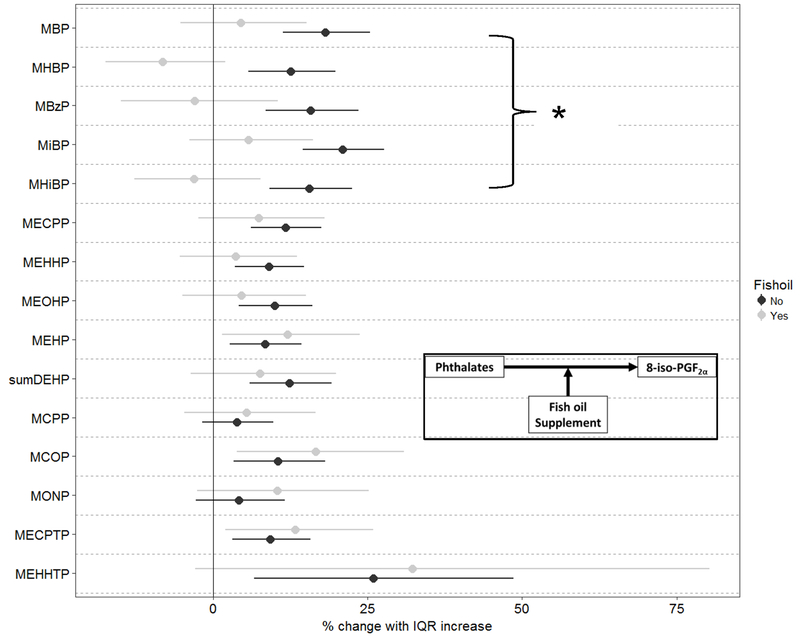
Effect modification of additional variables on the association between phthalate or DINCH metabolites and biomarker concentrations were examined. Analyses were limited to investigating effects on 8-iso-PGF2α and those phthalates or DINCH metabolites that previously showed a significant association. Modification by maternal race/ethnicity was investigated, as levels of urinary oxidative stress biomarkers are notably different in White mothers compared to those of other races/ethnicities. Additionally, consumption of fish oil supplements was investigated as there had been previous reports of potential effects supplementation on 8-iso-PGF2α levels.24 Fish oil supplementation would specifically diminish associations with chemically generated 8-iso-PGF2α through its potential to act as an antioxidant. No effect modification by maternal race/ethnicity was observed (data not shown). However, among the 172 women self-reporting fish oil supplementation, we did note large changes in the associations observed between 8-iso-PGF2α and five phthalate metabolites: MBP, mono(3-hydroxybutyl) phthalate (MHBP), monobenzyl phthalate (MBzP), MiBP, and mono-hydroxyisobutyl phthalate (MHiBP) (Figure 2).
Figure 2: Percent change (95% confidence interval) in 8-iso-PGF2α in association with an interquartile range increase in urinary phthalate metabolite concentration from models stratified by fish oil supplementation.
Results from linear model adjusted for maternal Race/Ethnicity, education, income, and body mass index. *Denotes significant difference between groups, p < 0.05, from linear adjusted model with an interaction term between fish oil supplementation and exposure.
Discussion
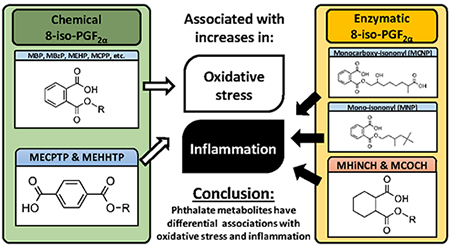
In this study, the association between urinary concentrations of metabolites of phthalates and DINCH in pregnant women and urinary F2-isoprostane levels were reinterpreted using the novel 8-iso-PGF2α/PGF2α ratio approach. The results show that most phthalates are indeed associated with the chemical pathways specifically reported before. However, certain phthalate and phthalate alternative metabolites with a specific structure were associated with the enzymatic pathway and not the chemical as had been previously assumed. This suggests that there is a structure-dependent relationship driving different mechanisms of toxicity for phthalates and phthalate alternative metabolites, and there is not simply a general rule for this class of compounds.
The distribution of phthalate and DINCH metabolites in this population were overall similar to previously reported values in pregnant as well as non-pregnant women. For the most concentrated metabolites MEP and MCOP, levels were higher than the National Health and Nutrition Examination Survey (NHANES) reported values and levels of MBP were slightly lower (geometric means of 41.7, 15.9 and 9.8 ng/mL compared to 56.7, 6.3, and 11.1 ng/mL, respectively).25 The two DINCH metabolites, MHINCH and MCOCH, were detected at the lowest frequency (36% and 32%, respectively) and had geometric mean values of 0.3 and 0.4 ng/mL, respectively. Low detection rates (56% and 30%, respectively) as well as low mean values (0.4 and 2.7 ng/mL, respectively) were similarly reported previously in NHANES.25
In previous reports, most urinary phthalate metabolites, except for metabolites of low molecular weight phthalates like MEP, have been associated with higher levels of 8-iso-PGF2α in both pregnant and non-pregnant women as well as in men and children.6, 7, 26–28 The associations with 8-iso-PGF2α reported in this work are comparable to other studies in pregnant women, although slightly smaller in magnitude.6, 7, 26 This is most likely due to the change in analytical technique used (GC/MS in the present study compared to enzyme linked immunosorbent assay [ELISA]). Due to antibody cross-reactivity with PGF2α, the ELISA assay tends to overestimate the amount of 8-iso-PGF2α, and if the urinary phthalate metabolite is associated with both 8-iso-PGF2α and PGF2α then the association would consequently be overestimated. This does not happen when 8-iso-PGF2α is measured by GC/MS and is able to precisely identify 8-iso-PGF2α.
The phthalate and DINCH metabolites MNP, MHBP, MHiBP, MHiNCH, and MCOCH have only been studied in relation to 8-iso-PGF2α in a small study of non-pregnant women.28 Wu et al. observed associations between MHiBP and MNP and elevated 8-iso-PGF2α in that study, but no associations were observed for MHiNCH, MHBP, or MCOCH. Similarly, we also observed positive associations for MHiBP and MNP but not for MHiNCH and MCOCH. However, we did observe a positive association with MHBP. The differences between the two studies likely arise from using different methodologies and sample sizes but could also be attributed to the difference in pregnancy status.
This study is the first to report on associations with MECPTP and mono-2-ethyl-5-hydroxyhexyl terephthalate (MEHHTP), metabolites of DEHTP, which is used as a replacement for di(2-ethylhexyl) phthalate (DEHP). Much like DEHP metabolites, the DEHTP metabolites are also associated with 8-iso-PGF2α as well as the 8-iso-PGF2α metabolite. This study is also the first to report on the associations between phthalates metabolites and PGF2α which independently is an indicator of inflammation.
In addition, we explored the sources of the higher levels of 8-iso-PGF2α by employing the new ratio approach to quantify the chemical vs. enzymatic (i.e., oxidative stress vs. inflammation derived) sources of 8-iso-PGF2α. It is important to know the origins of 8-iso-PGF2α because each distinct pathway is associated with different secondary health outcomes. In addition, misclassifying sources can lead to the design of ineffective strategies to diminish toxicities associated with high exposure to phthalates. Even though the equations designed to quantify the amounts of chemical and enzymatic lipid peroxidation were derived from in vitro experiments and later confirmed in animal and human studies,10, 11, 29 there is no reason to expect that the underlying chemistry and enzymes operate differently in pregnant women than in other human or animal populations. In addition, the chemistry is not altered for different biospecimens with the exception of serum, in which enzymatic F2-isoprostanes are formed ex vivo, tainting the results.30 For most phthalates, associations in this population were only observed with chemically derived 8-iso-PGF2α suggesting an association between phthalate concentration and oxidative stress. Notable exceptions are MCNP and MNP which both are only association with the prostaglandin synthase derived 8-iso-PGF2α, i.e., inflammation-derived. Both are high molecular weight isomeric phthalates. The association between MNP and inflammation can be substantiated by its structural similarities to DINCH which can be synthesized by hydrogenation of DiNP (the parent compound of MNP). The two DINCH metabolites are strongly associated with PGF2α and therefore it is plausible that a structure-dependent relationship exists where the isononyl group of both compounds could be inducing inflammation. The higher levels of inflammatory markers may furthermore be linked to hypertrophy of the thyroid observed in general toxicity studies on DINCH;31–33 thyroid hypertrophy has been shown to increase prostaglandin H synthase activity.34
There is experimental evidence behind our findings of diverse structure-dependent effect. Specific phthalates have been shown to induce oxidative stress by activating Kupffer cells, increasing the production of free radicals in vivo.35 However, there is also evidence that certain phthalates metabolites, such as MCNP and some of the DEHP metabolites, are associated with increases in inflammation markers such as IL-6 in pregnant women.6 The ability to differentially induce these two enzymes through a structure activity / receptor driven mechanism would explain the diverse effects of the phthalate and phthalate alternative metabolites. It is therefore important that the sources of 8-iso-PGF2α be distinguished before changes in 8-iso-PGF2α can be mechanistically interpreted.
Lastly, we explored effect modification of the association between urinary phthalate metabolites and 8-iso-PGF2α by fish oil supplementation. Fish oil supplements are commonly taken during pregnancy as a source of omega-3 fatty acids such as docosahexaenoic acid and eicosapentaenoic acid to support fetal brain development.36 In addition to being essential for healthy development, omega-3 fatty acids have also been shown, in some cases, to inhibit 8-iso-PGF2α formation, presumably by scavenging free radicals.37 A marked reduction in the association between 8-iso-PGF2α and metabolites of three parent compounds (di-n-butyl phthalate, di-isobutyl phthalate, and benzylbutyl phthalate) was observed for pregnant women supplementing with fish oil. Interestingly, all metabolites from these three parent compounds were associated with higher levels of 8-iso-PGF2α through the chemical pathway specifically. Fish oil contains high concentrations of potential anti-oxidants which would decrease 8-iso-PGF2α formed through the chemical pathway only.24 The effect modification of fish oil supplementation altering the association with the chemical derived 8-iso-PGF2α strengthens the conclusions of the involvement of oxidative stress in the toxicity mechanism of specific phthalates.
There are some limitations to the analysis in this study. Biomarkers were measured in a single urine specimen collected in the third trimester of pregnancy. Given the quick metabolism of phthalates and likely episodic nature of the exposures, concentrations of metabolites in urine represent only recent exposures, whereas urinary 8-iso-PGF2α represents a compounded effect of a much longer duration relative to phthalate metabolites. This could have led to measurement error. Additionally, these findings may not be generalizable outside of this specific population of pregnant women (i.e., to other pregnant women, non-pregnant women, or men). Finally, we did not have complete information on time of day of urine sample collection, which could have led to residual confounding. Despite these limitations, this study has numerous strengths. In addition to a larger sample size compared to previous studies, we measured multiple F2-isoprostane biomarkers and used the ratio approach to better understand the biological mechanisms driving our associations. This approach is currently the most accurate for determining oxidative stress and its origins.11
In summary, we observed that phthalate and phthalate alternative metabolites have associations 8-iso-PGF2α that arise from different pathways, including chemical oxidative stress as well as inflammation. Accurately determining the mechanism behind elevated 8-iso-PGF2α can give us more information to determine the overall toxicological effects of phthalate exposure on human health and provide insights into the design of future intervention strategies to reduce or alleviate toxicity and human health hazards.
Supplementary Material
Acknowledgements
The author gratefully acknowledges the study coordinators: Garry Alcedo, Sarah Caveglia, Alana Cordeiro, and Stacey Moe. We also acknowledge Drs. Stephanie London and Maria Kadiiska for their scientific input and feedback, and Dr. Manori Silva, Ella Samanda, Jim Preau, and Tao Jia for the quantification of the phthalates and DINCH metabolites. This work was supported by the Intramural Research Program, National Institutes of Health, National Institute of Environmental Health Sciences (ZIA103313) and the extramural NIEHS grants R01 ES016863-04 and P30 ES005022.
Abbreviations:
- 8-iso-PGF2α
8-iso-prostaglandin F2α
- PGF2α
prostaglandin F2α
- F2-IsoP-M
2,3-dinor-5,6-dihydro-15-F2t-isoprostane
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Supporting information
Spearman correlation matrix of specific gravity-corrected urinary phthalate and phthalate alternative metabolites; Urinary phthalate and phthalate alternative metabolite geometric means by demographics; Urinary oxidative stress biomarker geometric means by demographics; Unadjusted associations between urinary phthalate and phthalate alternative metabolites and measured oxidative stress biomarkers; Adjusted associations between urinary phthalate and phthalate alternative metabolites and derived oxidative stress biomarkers; Unadjusted associations between urinary phthalate and phthalate alternative metabolites and derived oxidative stress biomarkers; Adjusted association between uncorrected urinary phthalate and phthalate alternative metabolites and measured oxidative stress biomarkers with specific gravity in the model; Adjusted association between uncorrected urinary phthalate and phthalate alternative metabolites and derived oxidative stress biomarkers with specific gravity in the model.
References
- 1.Zhao Y; Shi HJ; Xie CM; Chen J; Laue H; Zhang YH, Prenatal phthalate exposure, infant growth, and global DNA methylation of human placenta. Environ. Mol. Mutagen 2015, 56, (3), 286–92. [DOI] [PubMed] [Google Scholar]
- 2.Kay VR; Chambers C; Foster WG, Reproductive and developmental effects of phthalate diesters in females. Crit. Rev. Toxicol 2013, 43, (3), 200–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantonwine DE; Meeker JD; Ferguson KK; Mukherjee B; Hauser R; McElrath TF, Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environ. Health. Perspect 2016, 124, (10), 1651–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James-Todd TM; Meeker JD; Huang T; Hauser R; Ferguson KK; Rich-Edwards JW; McElrath TF; Seely EW, Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ. Int 2016, 96, 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson KK; McElrath TF; Ko YA; Mukherjee B; Meeker JD, Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ. Int 2014, 70, 118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson KK; Cantonwine DE; Rivera-Gonzalez LO; Loch-Caruso R; Mukherjee B; Anzalota Del Toro LV; Jimenez-Velez B; Calafat AM; Ye X; Alshawabkeh AN; Cordero JF; Meeker JD, Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environ. Sci. Technol 2014, 48, (12), 7018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson KK; McElrath TF; Chen YH; Mukherjee B; Meeker JD, Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ. Health. Perspect 2015, 123, (3), 210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadiiska MB; Gladen BC; Baird DD; Germolec D; Graham LB; Parker CE; Nyska A; Wachsman JT; Ames BN; Basu S; Brot N; Fitzgerald GA; Floyd RA; George M; Heinecke JW; Hatch GE; Hensley K; Lawson JA; Marnett LJ; Morrow JD; Murray DM; Plastaras J; Roberts LJ 2nd; Rokach J; Shigenaga MK; Sohal RS; Sun J; Tice RR; Van Thiel DH; Wellner D; Walter PB; Tomer KB; Mason RP; Barrett JC, Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med 2005, 38, (6), 698–710. [DOI] [PubMed] [Google Scholar]
- 9.van ‘t Erve TJ; Kadiiska MB; London SJ; Mason RP, Classifying oxidative stress by F2-isoprostane levels across human diseases: A meta-analysis. Redox Biol 2017, 12, 582–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van ‘t Erve TJ; Lih FB; Jelsema C; Deterding LJ; Eling TE; Mason RP; Kadiiska MB, Reinterpreting the best biomarker of oxidative stress: The 8-iso-prostaglandin F2alpha/prostaglandin F2alpha ratio shows complex origins of lipid peroxidation biomarkers in animal models. Free Radic. Biol. Med 2016, 95, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van ‘t Erve TJ; Lih FB; Kadiiska MB; Deterding LJ; Eling TE; Mason RP, Reinterpreting the best biomarker of oxidative stress: The 8-iso-PGF2α/PGF2α ratio distinguishes chemical from enzymatic lipid peroxidation. Free Radic. Biol. Med 2015, 83, 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadiiska MB; Gladen BC; Baird DD; Graham LB; Parker CE; Ames BN; Basu S; Fitzgerald GA; Lawson JA; Marnett LJ; Morrow JD; Murray DM; Plastaras J; Roberts LJ II; Rokach J; Shigenaga MK; Sun J; Walter PB; Tomer KB; Barrett JC; Mason RP, Biomarkers of oxidative stress study III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radic. Biol. Med 2005, 38, (6), 711–8. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson KK; McElrath TF; Mukherjee B; Loch-Caruso R; Meeker JD, Associations between Maternal Biomarkers of Phthalate Exposure and Inflammation Using Repeated Measurements across Pregnancy. PloS one 2015, 10, (8), e0135601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swan SH; Sathyanarayana S; Barrett ES; Janssen S; Liu F; Nguyen RH; Redmon JB, First trimester phthalate exposure and anogenital distance in newborns. Hum. Reprod 2015, 30, (4), 963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett ES; Sathyanarayana S; Janssen S; Redmon JB; Nguyen RH; Kobrosly R; Swan SH; Team TS, Environmental health attitudes and behaviors: findings from a large pregnancy cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol 2014, 176, 119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen EM; van’t Erve TJ; Boss J; Sathyanarayana S; Barrett ES; Nguyen RH; Bush NR; Milne GL; McElrath TF; Swan SH Free Radic. Biol. Med Urinary oxidative stress biomarkers and accelerated time to spontaneous delivery. 2019, 130, 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva MJ; Samandar E; Preau JL Jr.; Reidy JA; Needham LL; Calafat AM, Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 2007, 860, (1), 106–12. [DOI] [PubMed] [Google Scholar]
- 18.Serrano SE; Karr CJ; Seixas NS; Nguyen RH; Barrett ES; Janssen S; Redmon B; Swan SH; Sathyanarayana S Int. J. Environ. Res. Public Health. Dietary phthalate exposure in pregnant women and the impact of consumer practices. 2014, 11, (6), 6193–6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornung RW; Reed LD, Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg 1990, 5, (1), 46–51. [Google Scholar]
- 20.Milne GL; Sanchez SC; Musiek ES; Morrow JD, Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat. Protoc 2007, 2, (1), 221–6. [DOI] [PubMed] [Google Scholar]
- 21.Jelsema CM; Peddada SD, CLME: An R Package for Linear Mixed Effects Models under Inequality Constraints. J. Stat. Softw 2016, 75, (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacPherson S; Arbuckle TE; Fisher M, Adjusting urinary chemical biomarkers for hydration status during pregnancy. J. Expo. Sci. Environ. Epidemiol 2018, 28, (5), 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eick SM; Barrett ES; van ‘t Erve TJ; Nguyen RHN; Bush NR; Milne G; Swan SH; Ferguson KK, Association between prenatal psychological stress and oxidative stress during pregnancy. Paediatr. Perinat. Epidemiol 2018, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barden AE; Mori TA; Dunstan JA; Taylor AL; Thornton CA; Croft KD; Beilin LJ; Prescott SL, Fish oil supplementation in pregnancy lowers F-2-isoprostanes in neonates at high risk of atopy. Free Radical Res 2004, 38, (3), 233–239. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. https://www.cdc.gov/exposurereport/ [accessed 02/19/19].
- 26.Holland N; Huen K; Tran V; Street K; Nguyen B; Bradman A; Eskenazi B, Urinary Phthalate Metabolites and Biomarkers of Oxidative Stress in a Mexican-American Cohort: Variability in Early and Late Pregnancy. Toxics 2016, 4, (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kataria A; Levine D; Wertenteil S; Vento S; Xue J; Rajendiran K; Kannan K; Thurman JM; Morrison D; Brody R; Urbina E; Attina T; Trasande L; Trachtman H, Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr. Res 2017, 81, (6), 857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H; Olmsted A; Cantonwine DE; Shahsavari S; Rahil T; Sites C; Pilsner JR, Urinary phthalate and phthalate alternative metabolites and isoprostane among couples undergoing fertility treatment. Env. Res 2017, 153, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van ‘t Erve TJ; Lih FB; Kadiiska MB; Deterding LJ; Mason RP, Elevated plasma 8-iso-prostaglandin F2alpha levels in human smokers originate primarily from enzymatic instead of non-enzymatic lipid peroxidation. Free Radic. Biol. Med 2018, 115,105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Praticò D; Barry OP; Lawson JA; Adiyaman M; Hwang SW; Khanapure SP; Iuliano L; Rokach J; FitzGerald GA, IPF2α-I: an index of lipid peroxidation in humans. Proc. Natl. Acad. Sci. USA 1998, 95, (7), 3449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engel A; Buhrke T; Kasper S; Behr AC; Braeuning A; Jessel S; Seidel A; Volkel W; Lampen A, The urinary metabolites of DINCH have an impact on the activities of the human nuclear receptors ERalpha, ERbeta, AR, PPARalpha and PPARgamma. Toxicol. Lett 2018, 287, 83–91. [DOI] [PubMed] [Google Scholar]
- 32.Albert O; Nardelli TC; Lalancette C; Hales BF; Robaire B, Effects of In Utero and Lactational Exposure to New Generation Green Plasticizers on Adult Male Rats: A Comparative Study With Di(2-Ethylhexyl) Phthalate. Toxicol. Sci 2018, 164, (1), 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David RM; White RD; Larson MJ; Herman JK; Otter R, Toxicity of Hexamoll((R)) DINCH following intravenous administration. Toxicol. Lett 2015, 238, (2),100–9. [DOI] [PubMed] [Google Scholar]
- 34.Smith TJ; Jennings TA; Sciaky D; Cao HJ, Prostaglandin-endoperoxide H Synthase-2 Expression in Human Thyroid Epithelium: evidence for constitutive expression in vivo and in cultured kat-50 cells. J. Biol. Chem 1999, 274, (22), 15622–15632. [DOI] [PubMed] [Google Scholar]
- 35.Rusyn I; Kadiiska MB; Dikalova A; Kono H; Yin M; Tsuchiya K; Mason RP; Peters JM; Gonzalez FJ; Segal BH; Holland SM; Thurman RG, Phthalates rapidly increase production of reactive oxygen species in vivo: role of Kupffer cells. Mol. Pharmacol 2001, 59, (4), 744–50. [DOI] [PubMed] [Google Scholar]
- 36.Agostoni C, Role of long-chain polyunsaturated fatty acids in the first year of life. Journal of pediatric gastroenterology and nutrition 2008, 47 Suppl 2, S41–4. [DOI] [PubMed] [Google Scholar]
- 37.van ‘t Erve TJ, Strategies to decrease oxidative stress biomarker levels in human medical conditions: A meta-analysis on 8-iso-prostaglandin F2alpha. Redox Biol 2018, 17, 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.