Abstract
BACKGROUND
Black women are more likely to diagnosed at a later stage of breast cancer partly due to barriers to timely screening mammogram, resulting in poorer mortality and survival outcomes. Patient navigation that helps overcome barriers to early detection of breast cancer is an effective intervention in reducing breast cancer disparity. However, the ability to recognize and seek help to overcome barriers may be affected by gendered and racialized social expectations of women.
METHODS
Data from a randomized controlled trial, the Patient Navigation in Medically Underserved Areas (PNMUA) study were used. The likelihood of obtaining follow-up screening mammogram was compared between women who identified one or more barriers and those who did not.
RESULTS
Of the 3,754 women who received the PNMUA navigation intervention, 14% identified one or more barriers, which led to additional navigator contacts. Consequently, those who reported barriers were more likely to obtain a subsequent screening mammogram. Black women, women living in poverty, and women with a higher level of distrust were less likely to report barriers.
CONCLUSIONS
Minority women living in poverty have always been the source of social support for others. But gendered and racialized social expectations may affect ways in which women seek help for their own health needs. A way to improve effectiveness of navigation would be to recognize how minority women’s gender images and expectations could shape how they seek help and support. No barriers does not always mean no problem. Proactive approaches to identify potential barriers may be beneficial.
Keywords: Breast Cancer, Diagnostic Mammgraphy, Barriers, Health Disparity, Gendered Social Norms, Distrust
CONDENSED ABSTRACT
Gendered and racialized social expectations may affect ways in which women seek help for their own health needs. A way to improve effectiveness of navigation would be to recognize how minority women’s gender images and expectations could shape how they seek help and support.
INTRODUCTION
Black women are more likely to diagnosed at a later stage of breast cancer, consequently, mortality and survival outcomes are poorer among black women compare to white women. Many barriers to obtaining screening mammography are shown to contribute to delayed diagnosis of breast cancer. Thus interventions such as patient navigation that aim to help overcome barriers to early detection of breast cancer are shown to be effective in reducing breast cancer disparity.
However, less is known about whether there is difference in the level of ability and or readiness to identify and report barriers. We argue that the ability to recognize and seek help to overcome barriers may determine women’s likelihood of reporting report barriers, consequently receive additional support, which leads to better outcomes. We suggest that medical distrust due to past and current experiences of blacks and social norms and images of women may influence how black women deal with their own needs and interact with healthcare providers.
In this paper, we explore whether women who identified one or more barriers to obtaining mammogram were able to take advantage of more intense patient navigation to achieve adequate e follow-up care. We compare timely follow-up of abnormal screening mammogram among women enrolled in a patient navigation intervention, between those who had no identified barrier and who reported one or more barriers.
Disparities in Breast Cancer Outcomes
More than 230,000 women were diagnosed with invasive breast cancer1 and more than 40,000 women died from breast cancer in 2015.2,3 Although overall breast cancer death rates declined over the past 25 years, recent improvement in breast cancer mortality has not benefited all women equally. Despite the fact that the incidence rate is higher for white women than for black women, breast cancer mortality rate continues to be higher for black women than for white women.4,5
Benefits of screening mammography for women 40 years and older have been well documented.6 Studies have shown that breast cancer mortality can be reduced up to 40 percent with screening mammogram.6 One of the reasons for the reduction in mortality is due to early detection of abnormal changes associated with screening mammogram.7 Late stage diagnosis leads to substantially poorer outcomes: The 5-year relative survival rate for women with early state (Stage I and II) is over 90%; while the 5-year relative survival rate is about 72% for Stage III and only 22% for Stage IV breast cancer.8–11
Late stage diagnosis among black women may in part explain existing racial disparity in breast cancer mortality and survival outcomes.12,13 The Surveillance, Epidemiology, and End Results Program (SEER) 18 registries data between 2004 and 2011 indicated that 37% of breast cancer cases among black women, compared with more than 50% among white women were diagnosed at Stage I. On the other hand, 7.8% of black women and only 4.6 of white women’s breast cancer cases were diagnosed at Stage IV.13
Although screening mammography has been associated with a 44% reduction in risk of late-stage diagnosis and a 30% to 40% reduction in breast cancer mortality,6 actual gains in early detection and survival from screening mammography might be narrower,14 in part, due to substantial over-diagnosis and false positive in screening mammogram results.15 These previous study findings suggest that timely follow-up of abnormal results from screening mammography is key to improving breast cancer outcomes.16,17 Inadequate follow-up of abnormal screening mammogram results, thus, might account for black-white difference in late stage diagnosis of breast cancer.
Interestingly, there seems to be no substantial difference in mammogram utilization between black and white women. In fact, black women were slightly more likely than white women to obtain a mammogram within past two years.13 These previous study findings indicate that even with equivalent screening rates, inadequate follow-up of abnormal mammogram results can introduce black-white difference in late stage diagnosis of breast cancer.16,17
In addition, women’s socioeconomic status (SES) is shown to increase risk of late stage diagnosis of breast cancer.18–20 While SES seem to have independent effects on breast cancer stage at diagnosis, black women continue to face social and economic inequalities,21,22 thus the interaction between race and SES may produce multiplicative effects on stage at diagnosis.23,24 Some studies show that when other factors were controlled for, racial difference was no longer significant; however, inadequate communication and other logistical barriers were higher among black women than white women.21,25 Thus, racial disparity in breast cancer stage at diagnosis may in part be due to the gap in socioeconomic status between black and white women.
Beyond racial/ethnic and socioeconomic status, Fayanju and colleagues identified that fear of high cost, pain, and potential for bad news were barriers to obtaining mammography.26 Other studies similarly identified financial concerns, perception, fear, discomfort, difficulty accessing transportation, and other life demands as barriers to breast cancer screening27–29.
Patient Navigation
The effectiveness of patient navigation on improving screening and timely follow-up of abnormal mammography has been well documented.30–32 Patient navigators play a significant role in mitigating socioeconomic barriers, inadequate access to care, distrust, and misconceptions about disease or treatment.33–35 Patient navigators improve access to care by assessing barriers and identifying potential solutions to overcome barriers.36 A disproportionate burden of barriers in poor and minority women contributes to delay in follow-up care.37 Women with barriers tend to have a longer time to diagnostic resolution of abnormal mammogram than women without barriers.29,38,39 Furthermore, Tejeda and colleagues found that Hispanic women were more often than black women to report barriers. The authors suggest that this difference between black and Hispanic women may be associated with types of barriers, where Hispanic women compared with black women were more likely to report intrapersonal barriers such as financial problems, transportation, fear, and comorbility.40
However, it is not clear whether there are differences between women who can identify or name potential barriers to timely follow-up and women who are not aware of their barriers. Although it is difficult to find studies examining such differences in individual ability to identify barriers and actively engage in patient navigation interventions, literature concerning the effects of higher patient self-efficacy has shown to improve cancer screening participation.41–43 On the other hand, self-reliance on health-seeking and adherence to treatment seems to suggest that individuals who are more self-reliant are less likely to seek help of others, including treatment for mental health problems.44–46
These study findings raise a question about how the ability to identify barriers are related to follow-up of abnormal mammogram results among navigated women. Not all navigated women would have barriers, and not all women who have barriers would identify/acknowledge them as barriers. In addition, the ability to understand one’s barriers, consequently the level of attention and help these identified barriers generate, would vary even among navigated women. It is also plausible that barriers are not in and of itself a hindrance to timely follow-up, if these barriers can effectively garner help to overcome them. Thus, the ability to identify and recognize barriers might also be an indication of a strength that women possess.
MATERIALS AND METHODS
Setting: Patient Navigation in Medically Underserved Areas
We compared the likelihood of getting one or more follow-up procedures after receiving an abnormal mammogram between women who identified barriers to obtaining mammography, which led to additional navigator contacts, and those who did not have barriers. We used data from the Patient Navigation in Medically Underserved Areas (PNMUA) study which was a patient navigation randomized controlled trial in Chicago, IL.47,48 Participants were recruited from three hospitals on the South Side of Chicago over a three-year period. Participants were women who were referred for a screening mammogram, with a history of benign/normal screening results. Using randomization software, participating women were randomly assigned to the navigation group (n=3,754) and control group (n=5,177). For the purpose of current analysis, we included women who received the navigation intervention. Navigators assessed and helped address potential barriers to mammogram. Dealing with barriers, navigators engaged in addressing financial barriers to treatment, psychosocial support, rescheduling missed appointments, interpersonal factors for treatment refusal, and reviewing treatment options. After the initial contacts prior to the appointment, navigators made additional calls to reassess barriers to adherence to appointment. All participating women were encouraged to contact navigators for help with clinical, barriers, and appointment related questions. Navigators also contacted women who missed appointments to identify and address any barriers specific to these appointments. For women who attended their initial appointment and obtained a normal or benign result, navigators provided phone and mail reminders concerning recommended follow-up screening. For women who received abnormal results, navigators contacted women immediately. Navigators maintained contacts with women until diagnostic resolution was achieved. For women who received a breast cancer diagnosis, women were navigated throughout the treatment process.48
Variables and Analysis
We compared follow-up screening mammogram between women who identified one or more barriers and those who did not. Navigated women were grouped into barrier vs. non-barrier groups. Receipt of follow-screening mammogram was a dichotomous variable (yes vs. no). Demographic characteristics included race/ethnicity (white, black, and Hispanic women), marital status (never married, married and living with partner, divorces, and widowed), and employment status (unemployed, employed, and retired). Race/ethnicity, marital, and employment status were categorical variables, which were used as dummy variables in regression models. Education was a dichotomous variable (high school graduation or more vs. less than high school education). We created a poverty variable using household size and household income, following the 2011 Federal Poverty Level.49 Age was used as a continuous variable.
We controlled for cancer history (yes vs. no) and health condition (good vs. poor). Medical distrust and self-efficacy were two scale variables. In addition, we compared two access to care variables: distance to closest clinic and Medically Underserved Area (MUA) designation between the barrier vs. non-barrier groups. Distance to clinic was a continuous variable and MUA designation was a categorical variable (Affluent, poor and MUA, and poor but non-MUA). We used descriptive statistics to compare characteristics of women with identified barriers and women without an identified barrier (Table 1). We then used logical regression models to predict receipt of screening mammogram (Table 2). Finally, we conducted additional analysis to explore factors associated with the likelihood of reporting one or more barriers (Table 3).
TABLE 1.
Sample Characteristics
| Reported barriers
|
|||
|---|---|---|---|
| No | Yes | p | |
|
|
|||
| N = 3,210 | N = 506 | ||
| Any follow-up screening test | 31.2 | 48.4 | <.01 |
| Race/Ethnicity | |||
| Black | 81.3 | 75.7 | |
| Hispanic | 9.5 | 13.1 | <.01 |
| White | 7.6 | 10.2 | |
| Age, mean | 59.3 | 57.7 | <.05 |
| Education < HS | 16.7 | 21.0 | <.05 |
| Living below poverty | 34.9 | 39.3 | n.s. |
| Income <$20K | 38.1 | 41.8 | n.s. |
| Cancer history | 14.3 | 16.0 | n.s. |
| Good health | 75.2 | 69.4 | <.05 |
| Distrust | 30.7 | 30.6 | n.s. |
| Distance to closest clinic | 3.0 | 3.0 | n.s. |
| MUA status | n.s. | ||
| Affluent, not eligible | 23.1 | 23.0 | |
| Poor, MUA | 43.9 | 46.0 | |
| Poor, Non-MUA | 33.0 | 31.0 | |
TABLE 2.
Predicting Barrier Reporting among Navigated Women
| Model | ||||
|---|---|---|---|---|
|
| ||||
| N | I | II | III | IV |
| Black women | 0.72** | 0.69** | 0.63 | 1.91 |
| Distrust | 0.99 | 0.92** | 0.92** | |
| Age | 1.00 | 1.00 | ||
| Living below poverty | 0.42** | 4.69* | ||
| Less than high school | 1.84 | 1.78 | ||
| Cancer history | 1.12 | 1.17 | ||
| Good health | 0.92 | 0.96 | ||
| Black* Poverty | 0.05** | |||
| −2LL | 1351.94 | 1,090.54 | 350.03 | 343.26 |
p<.01;
p<.05
TABLE 3.
Predicting Screening Mammogram Receipt among Navigated Women
| OR | |||
|---|---|---|---|
|
| |||
| I | II | III | |
| Black women | 0.76** | 0.83 | 0.88 |
| Barriers reported | 2.07** | 2.04** | 1.56* |
| Distrust | 1.00 | 1.01 | |
|
| |||
| Age | 1.01* | ||
| Education < HS | 0.70 | ||
| Living below poverty | 0.90 | ||
| Cancer history | 0.33** | ||
| Good health | 1.09 | ||
|
| |||
| −2LL | 1,755.92 | 1253.49 | 646.02 |
p<.01;
p<.05
RESULTS
Sample Characteristics
A total of 3,754 women who received the PNMUA navigation intervention were included in the analysis. Of those, 14% of participating women identified one or more barriers, which led to additional navigator contacts. Bivariate comparison showed that women who identified barriers were significantly more likely to receive a follow-up screening mammogram (Table 1). Black women were less likely to identify barriers, compared with white and Hispanic women. Older women were less likely women who reported to have good health compared with poor health were less likely to report barriers. Women who had less than high school education were more likely and women living below poverty line were more likely to report barriers. Unemployed women were more likely and retired women were less likely, compared with employed women, to report barriers. Women reported to have good health were less likely to report barriers.
There was no statistical difference in marital status, living below poverty, and having cancer history between women who reported barriers and those who did not. There was no statistical difference in neighborhood level access to care variables between women with and without barriers. Finally, medical distrust and self-efficacy scores did not differ by barrier status.
Reporting Barriers
Women who showed a higher level of distrust were less likely to report barriers. Women living below poverty also were less likely to report barriers (Table 3). Finally, to examine how the effect of poverty may differ by race/ethnic group, we introduced a race/ethnicity and poverty interaction term. Probability of reporting barriers, controlling for all other variables, was lower for women living below poverty and for women with a higher level of distrust. In addition, a significant race-poverty interaction was observed. Overall, the probability of reporting barriers decreased as the distrust score increased. In addition, for black women, women living in poverty were less likely to report barriers, while for non-black women, those living in poverty were more likely to report barriers (Figure 1).
Figure 1.
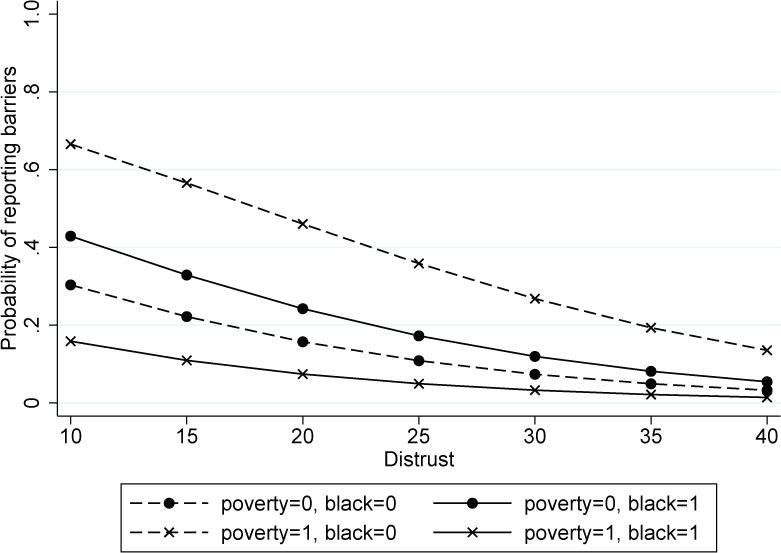
Probability of reporting barriers by distrust
Depending on women’s poverty status and being black compared with other racial/ethnic groups, the degree to which distrust affects reporting barriers differed. Poor non-black women had the highest probability of reporting barriers. The probability of non-poor white women was .08, which increased to .50 for poor white women. Similarly, non-poor Hispanic women’s probability of reporting barriers was .07, which increased to .25 for poor Hispanic women. On the other hand, non-poor black women’s probability of reporting barriers was .12, which then decreased to .04 for poor black women.
Receiving Screening Mammogram
We examined factors explaining the likelihood of obtaining any screening mammogram. First, women who reported barriers were significantly more likely to receive screening mammogram (Table 2). White women compared with black women and older women compared with younger women were more likely to obtain screening mammogram. However, controlling for education, poverty, marital status, and employment status, race/ethnicity was no longer significant. Women living below poverty were less likely to obtain mammograms and retired women compared with unemployed women were less likely to obtain mammograms. Once we introduced cancer history and health condition, only having a barrier was associated with a higher likelihood to obtain a screening mammogram. In addition, women with a history of cancer were less likely to obtain screening mammograms. Distrust and self-efficacy scores were not associated with obtaining mammogram.
Structural equation modeling was used to explain the relationship between reporting barriers and obtaining screening mammogram (Figure 2). Women living below poverty were more likely, but black women living below poverty were less likely, to report barriers. In addition, women with a higher distrust level were less likely to report barriers. Concerning receipt of screening mammograms, women who reported barriers were more likely to obtain screening mammogram. Additionally, women who had a history of cancer were less likely but older women were more likely to obtain screening mammogram.
Figure 2.
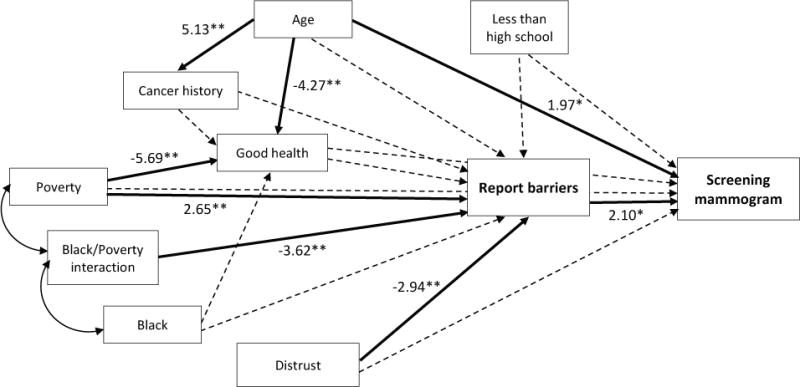
Structural equation model.
** p<.01; * p<.05
DISCUSSION
We found that women who reported having barriers were more likely to obtain follow-up screening mammograms. At first glance, this finding might contradict what we conventionally conceptualize as barriers to timely cancer screening. In general, literature on effects of barriers and screening mammograms considers barriers as simply “barriers”. However, we argue that who and how a woman may recognize and report barriers that, then, will bring about additional support to resolve barriers. This also means that when a woman reports that she does not have barriers, it may mean that perceptions of barriers may be influenced by their beliefs about health and self-efficacy in order to identify and report a barrier.26,50 There may be many reasons why this could happen. For one, some scholars have explored how minority women, particularly black women may have the “superwomen” ideal.51 The image of “strong black woman”, while strength, affects how black women deal with stress, life demands, and health behavior.52–55
Gender, race/ethnicity, and class interact in creating images of ideal woman. Minority women living in poverty have always been the source of social support for others.56–58 But when it comes to healthcare access and adherence, women may be disadvantaged because of their role as caretaker.59,60 Representation of women embedded in sociocultural affects ways in which women deal with barriers and access to care. Patient navigation is known to be effective in improving breast cancer screening and timely follow-up of abnormal mammogram.29,61–63 One of the ways to improve effectiveness of navigation would be to train navigators to recognize how minority women’s gender images and expectations could shape how they seek help and support. Considering that the primary role of patient navigators is to help patients overcome barriers to care,64 we will need to pay more attention to helping navigators assist their patients in recognizing their barriers and the importance of seeking out support.
Our study also found that women with a higher level of distrust were less likely to report barriers. This finding confirms previous studies that document the negative effects of distrust of the healthcare system on access to care and care engagement.65–67 Our finding is interesting because it could explain a mechanism as to how distrust may reduce access to care. Perhaps women who do not trust the health care system may not feel comfortable telling care providers about their barriers and facilitators that could potentially affect their healthcare behavior.
Finally, we found that the effect of poverty on the likelihood of reporting barriers differed by race/ethnicity. For non-black women, poor women were more likely but for black women, poor women were less likely to report barriers. We argue that there is no reason to think that poverty does not affect black women as much as non-black women, in fact, if anything, the opposite is true. Thus, this finding is consistent with our approach to the previous two points: black women’s racialized ideal of strong women and high distrust is due to their current and historical experiences. Poverty may increase barriers to screening mammogram for all, but minority women may have different ways of dealing with the issues they face. Clearly, it is not about race/ethnicity itself, but life experiences and perceptions that influence health behavior. Individuals living in poverty may experience more barriers to access to care due to their economic conditions. In addition, women who are poor have limited social support network ties, in part because their network members are also equally poor. Consequently, poor women tend to have limited access to care and care utilization. Beyond economic difficulties, women are often caretakers and source of social support for others, but because of their expected gender role, women may have difficulty expressing their own need for support. Additional research that explores the role of social networks among health communication of perceived barriers to mammography screening among underserved minority women may also identify other areas for intervention development.
Race/ethnicity and class shape expectations, norms, and ideal images of women. Women, particularly poor minority women, may struggle with an additional burden of highly gendered and racialized caregiving role that does not necessarily return social support. Patient navigation, while a highly effective intervention to reduce disparities in health, needs to pay special attention to how gender and race/ethnicity shapes experiences of minority women that affect ways in which women mobilize formal and informal support for their own health.
Acknowledgments
FUNDING SUPPORT:
This work was supported in part by grant 3P60 MD003424-03S1 from the National Institute on Minority Health and Health Disparities (NIMHD) (Sage Kim).
Footnotes
CONFLICT OF INTEREST DISCLOSURES:
The authors made no disclosures.
AUTHOR CONTRIBUTIONS:
Sage Kim conceptualized and designed the study, conducted data collection and initial statistical analyses, wrote the initial draft, and reviewed and revised the article. Anne Elizabeth Glassgow carried out literature review and revised the manuscript. Karriem Watson contributed to conceptualization and revised the manuscript. Yamile Molina coordinated data and created variables. Elizabeth Calhoun designed the overall parent study and revised the manuscript.
All authors approved the final version as submitted and agreed to be accountable for all aspects of the work.
Contributor Information
Sage Kim, University of Illinois at Chicago, School of Public Health, Division of Health Policy and Administration. 1603 W. Taylor St. Chicago IL, 60612.
Anne Elizabeth Glassgow, University of Illinois at Chicago, College of Medicine. 1747 W Roosevelt Rd, Chicago, IL 60608.
Karriem Watson, University of Illinois at Chicago, Cancer Center. 818 South Wolcott Avenue, # 404 SRH, Chicago, IL, 60612.
Yamile Molina, University of Illinois at Chicago, School of Public Health, Division of Community Health Science. 1603 W. Taylor St. Chicago IL, 60612.
Elizabeth Calhoun, University of Arizona, Health Sciences. 1501 N Campbell Ave, Tucson, AZ 85724.
References
- 1.American Cancer Society. Breast Cancer: Facts & Figures, 2015-2016. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 2.Surveillance E, End Results Program . Cancer statistics factsheets: breast cancer. Institute NC; Bethesda, MD: 2015. [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Breast Cancer Statistics. 2017 https://www.cdc.gov/cancer/breast/statistics/index.htm.
- 4.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA: A Cancer Journal for Clinicians. 2017;67(6):439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Breast Cancer Statistics, 2017: Black/white mortality differences closing in several states. 2017 http://pressroom.cancer.org/BreastCancerStats2017.
- 6.Helvie M, Chang J, Hendrick RE, Banerjee M. Reduction in late-stage breast cancer incidence in the mammography era: Implications for overdiagnosis of invasive cancer. Cancer. 2014;120(17):2649–2656. doi: 10.1002/cncr.28784. [DOI] [PubMed] [Google Scholar]
- 7.Morrell S, Taylor R, Roder D, Robson B, Gregory M, Cfaig K. Mammography service screening and breast cancer mortality in New Zealand: a National Cohort Study 1999–2011. Br J Cancer. 2017;116(6):828–839. doi: 10.1038/bjc.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Cancer Society. Breast Cancer Survival Rates. 2018 [Google Scholar]
- 9.Taplin SH, Ichikawa L, Yood MU, et al. Reason for Late-Stage Breast Cancer: Absence of Screening or Detection, or Breakdown in Follow-up? Journal of the National Cancer Institute. 2004;96(20):1518–1527. doi: 10.1093/jnci/djh284. [DOI] [PubMed] [Google Scholar]
- 10.Caplan L. Delay in Breast Cancer: Implications for Stage at Diagnosis and Survival. Front Public Health. 2014;2(87):1–5. doi: 10.3389/fpubh.2014.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Cancer Society. Understanding a breast cancer diagnosis. 2017 https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html.
- 12.Daly B, Olopade O. A Perfect Storm: How Tumor Biology, Genomics, and Health Care Delivery Patterns Collide to Create a Racial Survival Disparity in Breast Cancer and Proposed Interventions for Change. CA: Cancer J Clin. 2015;65:221–238. doi: 10.3322/caac.21271. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal J, Ginsburg O, Rochon P, Sun P, Narod S. Differences in Breast Cancer Stage at Diagnosis and Cancer-Specific Survival by Race and Ethnicity in the United States. Journal of American Medical Association. 2015;313(2):165–173. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 14.Bleyer A, Welch G. Effect of Three Decades of Screening Mammography on Breast-Cancer Incidence. New England Journal of Medicine. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 15.Juhl Jørgensen K, Keen J, Gøtzsche P. Is Mammographic Screening Justifiable Considering Its Substantial Overdiagnosis Rate and Minor Effect on Mortality? Radiology. 2011;260(3):621–627. doi: 10.1148/radiol.11110210. [DOI] [PubMed] [Google Scholar]
- 16.Wujcik D, Shyr Y, Li M, et al. Delay in Diagnostic Testing After Abnormal Mammography in Low-Income Women. Oncol Nurs Forum. 2009;36(6):709–715. doi: 10.1188/09.ONF.709-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Press R, Carrasquillo O, Sciacca RR, Giardina E-GV. Racial/Ethnic Disparities in Time to Follow-Up after an Abnormal Mammogram. Journal of Women’s Health. 2008;17(6):923–930. doi: 10.1089/jwh.2007.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Cancer Institute. About cancer health disparities. Institute NC; Bethesda, MD: 2015. [Google Scholar]
- 19.Clegg L, Reichman M, Miller B, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes & Control. 2009;20(4):1–28. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Booth C, Li G, Zhang-Salomons J, Mackillop W. The Impact of Socioeconomic Status on Stage of Cancer at Diagnosis and Survival. Cancer. 2010;116(17):4160–4167. doi: 10.1002/cncr.25427. [DOI] [PubMed] [Google Scholar]
- 21.Jones BA, Dailey A, Calvocoressi L, et al. Inadequate Follow-up of Abnormal Screening Mammograms: Findings From the Race Differences in Screening Mammography Process Study (United States) Cancer Causes and Control. 2005;16:809–821. doi: 10.1007/s10552-005-2905-7. [DOI] [PubMed] [Google Scholar]
- 22.Williams D, Priest N, Anderson N. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychology. 2016;35(4):407–411. doi: 10.1037/hea0000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz A, Mullings L. Gender, race, class, & health: Intersectional approaches. San Francisco, CA: Jossey-Bass; 2006. [Google Scholar]
- 24.Williams D, Mohammed S, Leavell J, Collins C. Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities. Annals of the New York Academy of Sciences. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strzelczyk J, Dignan M. Disparities in adherence to recommended follow up on screening mammography: interaction of sociodemographic factors. Ethnicity & Disease. 2002;12(1):77–86. [PubMed] [Google Scholar]
- 26.Fayanju O, Kraenzle S, Drake B, Oka M, Goodman M. Perceived Barriers to Mammography among Underserved Women in a Breast Health Center Outreach Program. American Journal of Surgey. 2014;208(3):425–434. doi: 10.1016/j.amjsurg.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todd A, Stuifergen A. Barriers and Facilitators Related to Breast Cancer Screening. International Journal of MS Care. 2011;13(2):49–56. doi: 10.7224/1537-2073-13.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman HP, Chu KC. Determinants of cancer disparities: barriers to cancer screening, diagnosis, and treatment. Surgical oncology clinics of North America. 2005;14(4):655–669. doi: 10.1016/j.soc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Katz M, Young G, Reiter P, et al. Barriers Reported Among Patients with Breast and Cervical Abnormalities in the Patient Navigation Research Program: Impact on Timely Care. Women’s Health Issues. 2014;24(1):155–162. doi: 10.1016/j.whi.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Percac-Lima S, Ashburner J, McCarthy AM, Piawah S, Atlas S. Patient Navigation to Improve Follow-Up of Abnormal Mammograms Among Disadvantaged Women. Journal of Women’s Health. 2015;24(2):138–143. doi: 10.1089/jwh.2014.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markossian TW, Darnell JS, Calhoun EA. Follow-up and timeliness after an abnormal cancer screening among underserved, urban women in a patient navigation program. Cancer Epidemiology Biomarkers and Prevention. 2012;21(10):1691–1700. doi: 10.1158/1055-9965.EPI-12-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells KJ, Battaglia TA, Dudley DJ, et al. Patient Navigation: State of the Art, or Is It Science? Cancer. 2008;113(8):1999–2010. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina Y, Kim S, Berrios N, Calhoun EA. Medical mistrust and patient satisfaction with mammography: the mediating effects of perceived self‐efficacy among navigated African American women. Health Expectations. 2015;18(6):2941–2950. doi: 10.1111/hex.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freeman H. The Origin, Evolution, and Principles of Patient Navigation. Cancer Epdemiology, Biomarkers & Prevention. 2012;21(10):1–5. doi: 10.1158/1055-9965.EPI-12-0982. [DOI] [PubMed] [Google Scholar]
- 35.Ko N, Darnell J, Calhoun E, et al. Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Research Program. Journal of Clinical Oncology. 2014;32(25):2758–2764. doi: 10.1200/JCO.2013.53.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pratt-Chapman M. Whate does a patient navifator do? Washington, DC: Association of Community Cancer Centers; 2016. [Google Scholar]
- 37.Palmer N, Weaver K, Hauser S, et al. Disparities in barriers to follow-up care between African American and White breast cancer survivors. Supportive Care in Cancer. 2015;23(11):3201–3209. doi: 10.1007/s00520-015-2706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramachandran A, Snyder F, Katz M, et al. Barriers to health care contribute to delays in follow-up among women with abnormal cancer screening: Data from the Patient Navigation Research Program. Cancer. 2015;121(22):4016–4024. doi: 10.1002/cncr.29607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiter P, Katz M, Young G, Paskett E. Predictors of resolution in navigated patients with abnormal cancer screening tests. Journal of Community Supportive Oncology. 2014;12(12):431–438. doi: 10.12788/jcso.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tejeda S, Darnell J, Cho YI, Stolley M, Markossian T, Calhoun E. Patient Barriers to Follow-Up Care for Breast and Cervical Cancer Abnormalities. J Womens Health. 2013;22(6):507–517. doi: 10.1089/jwh.2012.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogedegbe G, Cassells A, Robinson C, et al. Perceptions of Barriers and Facilitators of Cancer Early Detection among Low-Income Minority Women in Community Health Centers. Journal of the National Medical Association. 2005;97(2):162–170. [PMC free article] [PubMed] [Google Scholar]
- 42.Jerome-D’Emilia B, Supplee P. Mammogram Use and Self-Efficacy in an Urban Minority Population. Public Health Nursing. 2015;32(4):287–297. doi: 10.1111/phn.12162. [DOI] [PubMed] [Google Scholar]
- 43.Melvin C, Jefferson M, Rice L, Cartmell K, Halbert H. Predictors of Participation in Mammography Screening among Non-Hispanic Black, Non-Hispanic White, and Hispanic Women. Frontiers in public health. 2016;4(188):1–7. doi: 10.3389/fpubh.2016.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gulliver A, Griffiths K, Christensen H. Perceived barriers and facilitators to mental health help-seeking in young people: a systematic review. BMC Psychiatry. 2010;10(113):1–9. doi: 10.1186/1471-244X-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Labouliere C, Kleinman M, Gould M. When Self-Reliance Is Not Safe: Associations between Reduced Help-Seeking and Subsequent Mental Health Symptoms in Suicidal Adolescents. International Journal of Environmental Research and Public Health. 2015;12(4):3741–3755. doi: 10.3390/ijerph120403741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jennings K, Cheung J, Britt T, et al. How are perceived stigma, self-stigma, and self-reliance related to treatment-seeking? A three-path model. Psychiatric rehabilitation Journal. 2015;38(2):109–116. doi: 10.1037/prj0000138. [DOI] [PubMed] [Google Scholar]
- 47.Kim S, Molina Y, Glassgow L, Guadamuz J, Calhoun E. The effects of navigation and types of neighborhoods on timely follow-up of abnormal mammogram among black women. Medical Research Archives. 2016;3:1–17. doi: 10.18103/mra.v0i3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molina Y, Glassgow AE, Kim SJ, et al. Patient Navigation in Medically Underserved Areas study design: A trial with implications for efficacy, effect modification, and full continuum assessment. Contemporary Clinical Trials. 2017;53:29–35. doi: 10.1016/j.cct.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Department of Health and Human Services. Annual Update of the HHS Poverty Guidelines. Federal Register. 2011;76(13):3637–3638. [Google Scholar]
- 50.Jensen J, Ratcliff C, Weaver J, Krakow M, Payton W, Loewen S. Explicating perceived barriers to mammography for the USCREEN project: concerns about breast implants, faith violations, and perceived recommendations. Breast Cancer Research and Treatment. 2015;154(1):201–207. doi: 10.1007/s10549-015-3581-2. [DOI] [PubMed] [Google Scholar]
- 51.Woods-Giscombe C. Superwoman Schema: African American Women’s Views on Stress, Strength, and Health. Oual Health Res. 2010;20(5):668–683. doi: 10.1177/1049732310361892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Black AR, Woods-Giscombe C. Applying the Stress and ‘Strength’ Hypothesis to Black Women’s Breast Cancer Screening Delays. Stress Health. 2012;28(5):389–396. doi: 10.1002/smi.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beauboeuf-Lafontant T. Keeping up appearances, getting fed up: The embodiment of strength among African American women. Meridians: Feminism, Race, Ttransnationalism. 2005;5(2):104–123. [Google Scholar]
- 54.Abrams J. The heart of strenth: The strong black woman schema and cardiovascular disease risk. Richmond, VA: Virginia Commonwealth University; 2015. [Google Scholar]
- 55.Belgrave F, Abrams J, Hood K, Moore M, Nguyen A. Development and Validation of a Preliminary Measure of African American Women’s Gender Role Beliefs. Journal of Black Psychology. 2016;42(4):320–342. doi: 10.1177/0095798415576614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edin K, Lein L. Making Ends Meet: How Single Mothers Survive Welfare and Low-Wage Work. New York, NY: Russell Sage Foundation; 1997. [Google Scholar]
- 57.Cattell V. Poor people, poor places, and poor health: the mediating role of social networks and social capital. Social Science & Medicine. 2001;52:1501–1516. doi: 10.1016/s0277-9536(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 58.Stack C. All our kin: strategies for survival in a black community. New York, NY: Harper and Row; 1974. [Google Scholar]
- 59.Ziersch A, Baum F, MacDougall C, Putland C. Neighbourhood life and social capital: The implications for health. Social Science & Medicine. 2005;60:71–86. doi: 10.1016/j.socscimed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 60.Knowlton A, Hua W, Latkin C. Social support networks and medical service use among HIV-positive injection drug users: Implications to intervention. AIDS Care. 2005;17(4):479–492. doi: 10.1080/0954012051233131314349. [DOI] [PubMed] [Google Scholar]
- 61.Battaglia TA, Roloff K, Posner MA, Freund KM. Improving follow-up to abnormal breast cancer screening in an urban population. A patient navigation intervention. Cancer. 2007;109(2):359–367. doi: 10.1002/cncr.22354. [DOI] [PubMed] [Google Scholar]
- 62.Gotlib Conn L, Hammond Mobilio M, Rotstein OD, Blacker S. Cancer patient experience with navigation service in an urban hospital setting: A qualitative study. European Journal of Cancer Care. 2014;24(1):1–9. doi: 10.1111/ecc.12247. [DOI] [PubMed] [Google Scholar]
- 63.Markossian T, Darnell J, Calhoun E. Follow-up and timeliness after an abnormal cancer screening among underserved, urban women in a patient navigation program. Cancer Epidemiology Biomarkers and Prevention. 2012;21(10):1691–1700. doi: 10.1158/1055-9965.EPI-12-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fowler T, Steakley C, Garcia R, Kwok J, Bennett LM. Reducing Disparities in the Burden of Cancer: The Role of Patient Navigators. PLoS Medicice. 2006;3(7):e193. doi: 10.1371/journal.pmed.0030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shoff C, Yang T-C. Untangling the associations among distrust, race, and neighborhood social environment: A social disorganization perspective. Soc Sci Med. 2012;74(9):1342–1352. doi: 10.1016/j.socscimed.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tse-Chuan Y, I-Chien C, Noah A. Examining the complexity and variation of health care system distrust across neighborhoods: Implications for preventive health care. Res Sociol Health Care. 2015;33:43–66. doi: 10.1108/S0275-495920150000033003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ebony Boulware L, Cooper L, Ratner L, LaVieist T, Powe N. Race and trust in the health care system. Public Health Rep. 2003;118(4):358–365. doi: 10.1016/S0033-3549(04)50262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


