Abstract
Background and aims
Our aim is to review the literature and provide guidelines for the assessment of uninvestigated dysphagia.
Methods
A systematic literature search identified studies on dysphagia. The quality of evidence and strength of recommendations were rated according to the Grading of Recommendation Assessment, Development, and Evaluation (GRADE) approach. Statements were discussed and revised via small group meetings, teleconferences, and a web-based platform until consensus was reached by the full group.
Results
The consensus includes 13 statements focused on the role of strategies for the assessment of esophageal dysphagia. In patients presenting with dysphagia, oropharyngeal dysphagia should be identified promptly because of the risk of aspiration. For patients with esophageal dysphagia, history can be used to help differentiate structural from motility disorders and to elicit alarm features. An empiric trial of proton pump inhibitor therapy should be limited to four weeks in patients with esophageal dysphagia who have reflux symptoms and no additional alarm features. For patients with persistent dysphagia, endoscopy, including esophageal biopsy, was recommended over barium esophagram for the assessment of structural and mucosal esophageal disease. Barium esophagram may be useful when the availability of endoscopy is limited. Esophageal manometry was recommended for diagnosis of esophageal motility disorders, and high-resolution was recommended over conventional manometry.
Conclusions
Once oropharyngeal dysphagia is ruled out, patients with symptoms of esophageal dysphagia should be assessed by history and physical examination, followed by endoscopy to identify structural and inflammatory lesions. If these are ruled out, then manometry is recommended for the diagnosis of esophageal dysmotility.
Keywords: deglutition, swallowing, gastroesophageal reflux disease (GERD)
INTRODUCTION
Dysphagia is a common condition affecting about 3% of the adult population (1). It is usually sub-classified into oropharyngeal dysphagia (affecting the mouth and pharynx) and esophageal dysphagia (affecting the esophageal body and esophagogastric junction) (2, 3). Oropharyngeal dysphagia has been described as difficulty initiating a swallow or passing food through the mouth or throat, while esophageal dysphagia is characterized by difficulty transporting material down the esophagus (2). Esophageal dysphagia is usually the result of either structural or inflammatory abnormalities such as strictures, rings, webs, malignancy or esophagitis (e.g., reflux esophagitis or eosinophilic esophagitis, EoE) (4–7), or motility disorders such as achalasia, ineffective esophageal motility (IEM), esophageal spasm or esophagogastric junction (EGJ) outflow obstruction (8–10).
The most recent guidelines on the diagnosis and management of dysphagia from the World Gastroenterology Organization (WGO), provide a practical approach but they did not systematically evaluate the evidence (2). Furthermore, the most recent Canadian guideline for the evaluation of dysphagia was published in 1998 (3).
This consensus guideline was developed using the stringent GRADE (Grading of Recommendation Assessment, Development and Evaluation) method (11) to evaluate the evidence, in order to provide updated evidence-based guidelines for the assessment of uninvestigated dysphagia. This consensus focused on esophageal dysphagia, which accounts for the majority of cases in the general population (1, 4). It is assumed that recommended procedures are conducted by appropriately trained and experienced clinicians, in order to achieve the diagnostic yields suggested by the literature.
METHODS
Scope and Purpose
Participants identified six major topics and discussed 26 specific questions pertaining to the diagnosis of dysphagia. The guideline process was initiated in July 2012 (the consensus group met in a series of teleconferences between September 2013 and February 2015). The process was completed, via discussions by telephone and in person, and through the CAG online platform (see below), without a face-to-face consensus meeting. This process resulted in the 13 statements that were voted on by the members of the committee and are presented in this manuscript. The final document was approved by each member of the consensus group.
Sources and Searches
The consensus group performed a systematic literature search of MEDLINE (from 1946 onward), EMBASE (from 1980 onward), and CENTRAL (Cochrane Central Register of Controlled Trials) up to October 2012, which was subsequently updated to March 2015. Key search terms were deglutition, dysphagia, swallowing, esophagus, oropharyngeal, motility disorders, barium, endoscopy, endosonography, biopsy, gastroesophageal reflux disease (GERD), proton pump inhibitors (PPI), and manometry. The search was limited to human studies and the English language. The strategies used are detailed further in Appendix 1. The searches yielded 11,781 papers, of which the abstracts (when available) were reviewed by two independent committee members assigned to each section, with conflicts resolved by consensus. This resulted in 635 papers being selected for full text review.
Review and Grading of Evidence
Overall quality of evidence for individual statements was assessed using the GRADE method (11) by two non-voting methodologists (GL and FT). QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies) was used to assess the quality of diagnostic accuracy studies (12). The quality of evidence for each statement was graded as high, moderate, low or very low, as described in GRADE (11, 13) and prior CAG consensus documents (14, 15).
Approved product labeling from government regulatory agencies varies from country to country, and while not ignored, recommendations are based on evidence from the literature and consensus discussion, and may not fully reflect the product labeling for a given country.
Consensus Process
The consensus group consisted of nine voting members (eight participants and one chair, LL), including seven gastroenterologists, one speech language pathologist (RM) and one radiologist (NJ) from Canada with expertise in the management of dysphagia.
The CAG facilitated the consensus process via small group meetings, teleconferences and a web-based consensus platform (ECD solutions, Atlanta, Georgia, USA) until consensus on the statements was reached by the full group. The consensus group developed the initial statements, reviewed the results of the literature search (with each article being reviewed by at least two group members) through the web-based platform, and then ‘tagged’ (selected and linked) references deemed relevant to a specific statement. All consensus group members were provided with access to copies of ‘tagged’ references. Using a modified Delphi process (16, 17), anonymous voting was then conducted by participants to determine their level of agreement with the individual statements. The statements were revised via teleconference and emails through three iterations, with accompanying online voting. A statement was accepted if >75% of participants voted five (agree with minor reservation) or six (agree strongly) on a scale of one to six (with one, two, three, and four being disagree strongly, disagree with major reservation, disagree with minor reservation and agree with major reservation, respectively). On the third vote, participants voted on the ‘strength’ of the recommendation, which was accepted with a 51% vote. Consistent with the GRADE system, the strength of each recommendation was classified as strong (“we recommend . . .”) or conditional (“we suggest . . .”). The strength of the recommendation considers risk-benefit balance, patients’ values and preferences, cost and resource allocation, and the quality of the evidence. Therefore, it is possible for a recommendation to be classified as ‘strong’ despite having low-quality evidence to support it, or ‘conditional’ despite the existence of high-quality evidence to support it (18). Based on the GRADE approach, a strong recommendation indicates the statement should be applied in most cases, while a conditional recommendation signifies that clinicians “should recognize that different choices will be appropriate for different patients and that they must help each patient to arrive at a management decision consistent with her or his values and preferences” (18).
The initial manuscript was drafted by the meeting chair, revised and approved by all members of the consensus group, and then made available to all CAG members for comments before submission for publication. As per CAG policy, all participants provided written disclosure of potential conflicts of interest for the 24 months prior to the meeting, which were made available to the other group members.
RECOMMENDATION STATEMENTS
Section 1: Identifying Oropharyngeal Dysphagia in Patients Presenting with Dysphagia
Statement 1.1: In patients presenting with dysphagia, we recommend using presenting symptoms and physical examination as the initial assessment to identify patients with oropharyngeal dysphagia.
• GRADE: Strong recommendation, very low-quality evidence. Vote: agree strongly, 78%; agree with minor reservation, 11%; agree with major reservation, 11%
Statement 1.2: In patients presenting with dysphagia, we recommend prompt identification of those with oropharyngeal dysphagia because of the increased risk of aspiration.
• GRADE: Strong recommendation, low-quality evidence. Vote: agree strongly, 100%
Dysphagia is the sensation that foods and/or liquids are being obstructed or hindered in their passage from the mouth to the stomach. Oropharyngeal dysphagia is described as difficulty initiating a swallow or passing food through the region of the mouth or throat; whereas, esophageal dysphagia refers to difficulty in transferring material down the esophagus in the retrosternal region (2).
Patients with certain diseases or disorders are at increased risk of oropharyngeal dysphagia and a diagnosis of these conditions should direct attention to the possibility of its presence. Some conditions such as stroke; head and neck cancer; surgery to the head, neck or cervical spine; abnormality of cervical spine, (e.g., osteophytes); and abnormality of cranial nerves 5, 7, 9, 10, 11 or 12 are associated with an increased risk of oropharyngeal dysphagia (19). Hence, the presence of these conditions should direct attention to the possible presence of oropharyngeal dysphagia. Oropharyngeal and esophageal dysphagia can occur together in conditions such as progressive neurological disorders (e.g., Parkinson’s disease, myotonic dystrophies), infective disorders (e.g., candida); and collagen vascular disorders (20).
Studies also suggest that patients are more accurate when subjectively localizing lesions in the distal rather than the proximal esophagus (21–24). In a prospective radiographic study of 130 patients, 26 were found to have a lower esophageal mucosal ring; 16 (62%) of whom demonstrated marshmallow impaction at the ring, and of these, 12 experienced dysphagia during the study. Of these 12, seven (84%) subjectively reported perceiving bolus sticking in the neck during the radiographic study where no structural pharyngeal or cervical esophagus abnormality was found (21). In another prospective study in 139 patients with dysphagia and an esophageal stricture, 22% of patients localized the level of obstruction exactly, 52% were within +/- 2 cm, and 74% within +/- 4 cm (24). Distal lesions were localized proximally by 15% of patients, but proximal lesions were only localized to the distal esophagus by 5% of patients (24).
The consensus group concluded that when patients indicate that the food is sticking in the retrosternal region, it is likely to be caused by esophageal dysphagia. However, when the patient localizes dysphagia to the sternal notch or the throat, this is unreliable. Because of the increased risk of aspiration, we recommend patients first be investigated for potential oropharyngeal dysphagia.
Although there are no clinical assessment tools that can help differentiate oropharyngeal from esophageal dysphagia, a higher score on the Eating Assessment Tool (EAT) (25) is associated with a higher risk of aspiration in patients presenting with dysphagia (26). In addition, higher scores on the dysphagia-related swallowing quality of life scale (SWAL-QOL) can identify patients with oropharyngeal dysphagia (27). Wet voice quality (WVQ) is frequently used in clinical settings but does not appear to be a reliable indicator of oropharyngeal dysphagia (28). Bedside swallowing tests have been developed to screen for oropharyngeal dysphagia. A systematic review of these tests (29) identified four tests with sensitivity of ≥70% and specificity of ≥60%, including the Toronto bedside swallowing screening test (TOR-BSST©) (30), the volume-viscosity swallowing test (V-VST) (31), the 3-ounce water swallow test (32), and the cough test (33). In neurological patients, these tests have demonstrated utility in identifying oropharyngeal dysphagia (30, 31) and risk of aspiration (32, 33). A prospective, observational study found a significantly lower rate of pneumonia at institutions that used a formal dysphagia screening protocol compared with those that did not (2.4% versus 5.4%; P = 0.0016). This remained significant even after adjusting for stroke severity. However, this study was judged at high risk of bias, since sites with a protocol may have had other characteristics that also had an impact on the development of pneumonia (34). In addition, these tests were not used to differentiate between oropharyngeal and esophageal dysphagia in these studies.
Oropharyngeal dysphagia is associated with high risk of aspiration (26). A history of dysphagia and aspiration increases the risk of pneumonia (35, 36). A systematic review of observational studies in stroke patients reported that dysphagia was associated with an increased risk for pneumonia (RR, 3.17; 95% CI, 2.07–4.87) and an even greater risk for aspiration (RR, 11.56; 95% CI, 3.36–39.77) (35). In a case-control study, elderly patients with oropharyngeal dysphagia had a 12-times greater risk of community-acquired pneumonia (odds ratio [OR], 11.9; 95% CI, 3.03–46.9) compared to those without oropharyngeal dysphagia, in a multivariate analysis that controlled for comorbidities and functionality (36).
The consensus group concluded that available tools, including patient-reported symptoms, physical examination, and bedside screening tests should be used to help identify cases of oropharyngeal dysphagia because of the high risk of aspiration and pneumonia. If oropharyngeal dysphagia is suspected, a referral to a speech language pathologist, or other appropriate specialist, for a clinical assessment (e.g., videofluoroscopic swallowing study) is recommended to confirm the diagnosis and guide management (37).
Section 2: Role of History and Physical Examination in the Assessment of Esophageal Dysphagia
Statement 2.1: In patients with esophageal dysphagia, we recommend history be used to help differentiate structural and motility disorders of the esophagus.
• GRADE: Strong recommendation, moderate quality evidence. Vote: agree strongly, 56%; agree with minor reservation, 33%; disagree with minor reservation, 11%
In observational studies the most common diagnosis in patients with dysphagia was GERD, accounting for about 17% to 28% of cases (4–7). Other less common diagnoses include inflammatory esophagitis (e.g., EoE, upper GI Crohn’s disease), infectious esophagitis, strictures, rings, webs, esophageal cancer and motility disorders (4–7).
Some signs and symptoms have been reported to be associated with structural versus motility disorders (Table 1). In a prospective cohort study, malignancy was more common in patients with shorter duration of dysphagia, while peptic stricture was more frequent in those with longer duration of symptoms (38). There was no difference in the likelihood of malignancy based on the level of dysphagia (pharyngeal level dysphagia 11.9% versus mid-sternal or lower sternal dysphagia 12.4%; P=NS). The strongest predictor of malignancy was duration of dysphagia (< 8 weeks versus > 26 weeks: OR, 11.02; 95% CI, 4.90–24.80; P<0.0001) (38).
Table 1.
Presenting history and symptoms suggestive of structural and motility disorders38, 46, 113
| Structural disorders | Motility disorder | |
|---|---|---|
| Symptoms | • Regular • Short duration, rapid progression • Solid (may progress to liquid) |
• Intermittent • Long duration • Solid and liquid |
| History | • Alarm features o Onset age >50 y o Bleeding o Odynophagia o Weight loss o Vomiting |
• Connective tissue diseases • Diabetes mellitus • Non-cardiac chest pain |
Patients with dysphagia associated with achalasia or esophageal spasm frequently experience symptoms over years including regurgitation, chest pain and weight loss (39–43). Case series have reported that patients with a lower esophageal muscular ring (44), or a Schatzki ring (45), can exhibit features of a ‘motility-like’ condition and experience a long duration of intermittent, non-progressive solid dysphagia.
Conditions such as autoimmune (e.g., lupus, 63–72%) and connective tissue diseases (88%) are frequently associated with esophageal hypomotility; therefore, dysphagia in these patients suggests an esophageal motility disorder (46). EoE is a frequent diagnosis among patients with dysphagia (33–35%) and is more common in patients with a history of food allergies (36.8% versus 9.3%, P<0.001) or asthma (22% versus 9%, P<0.01) compared to those without (47–49).
The consensus group concluded that while there may be some distinguishing clinical features among patients with dysphagia of different etiologies, a standard strategy for investigations should be pursued in all cases, regardless of whether the symptoms suggest a structural or a motility cause. In most circumstances, initial assessment will be conducted to first rule out structural and inflammatory lesions (endoscopy [EGD] and/or barium esophagram), followed by assessment of motility (manometry) if no clear structural or inflammatory lesions has been identified.
Statement 2.2: In patients with esophageal dysphagia, we recommend history and physical examination, including assessment of other alarm features that require urgent investigations to ensure timely referral for appropriate management.
• GRADE: Strong recommendation, low-quality evidence. Vote: agree strongly, 78%; agree with major reservation, 22%
In Canada, the estimated 2017 incidence of new cancer cases was 103,100 cases among males and 103,200 among women. Among these newly diagnosed cancers, esophageal cancer was found to affect 1.7% of men (1,800 cases) and 0.5% of women (530 cases) (50). Among individuals with dysphagia, the rate of esophageal cancer was 5.9% in a retrospective endoscopy database cohort (4), but only 0.6% in a cross-sectional, general population-based cohort (1).
Alarm features, including vomiting, gastrointestinal bleeding, abdominal mass, dysphagia, unexplained weight loss and anemia, have been associated with increased risks of serious diagnoses, including esophageal and other gastric cancers (6, 51–54). Since the risk of esophageal cancers increases with age (50, 55), patients whose age is over 50–55 years is generally also considered as an alarm feature (6, 53, 56).
Almost 60% of patients with dysphagia had additional alarm symptoms, including weight loss (15%), vomiting (15%) and anemia (3.6%) (6). The combination of dysphagia with an additional alarm feature (especially age > 50 years and weight loss) was associated with a significantly increased risk of esophageal cancer (6, 56, 57).
Canadian consensus guidelines on wait times recommended that patients with severe or rapidly progressive dysphagia, or a high likelihood of cancer, be assessed within two weeks (58).
This consensus group concluded that patients with esophageal dysphagia should be assessed for the presence of additional alarm features associated with disorders that have a high risk for poor outcomes, such as GI cancers, so that the appropriate investigations (e.g., endoscopy or imaging studies) can be conducted within the recommended wait time.
Section 3: Role of Barium Contrast Studies in the Evaluation of Esophageal Dysphagia
Statement 3.1: In patients with esophageal dysphagia, we recommend endoscopy over barium esophagram to improve the diagnosis of structural and mucosal esophageal disease.
• GRADE: Strong recommendation, very low-quality evidence. Vote: agree strongly, 78%; agree with minor reservation, 22%
Studies comparing the diagnostic accuracy of endoscopy and barium esophagram are mainly small and retrospective in nature (59–64). Most of these studies used barium swallow as the index test and EGD as the reference standard. However, generally EGD was interpreted with knowledge of the results of the barium swallow, and patients were not randomly selected. The results of these studies are conflicting with some reporting greater accuracy with barium esophagram (59, 63) and others with EGD (60–62, 64).
In a prospective study, including 25 patients with dysphagia, in which pathology was used as the reference standard, a correct diagnosis was made in 50% of cases based on radiology, compared to 95.5% of cases based on endoscopy. However, this study may have limited generalizability because all patients were HIV positive and over half had AIDS defining opportunistic infections (64). It should also be noted that all of the barium esophagram studies were performed before the widespread awareness of EoE. Sensitivity and specificity of esophagram for EoE is unknown, however, since biopsy is required for the diagnosis of EoE, it further underscores the advantage of endoscopy over barium studies for diagnosis of dysphagia.
A cost-analysis study found that initial barium esophagram was more costly than initial EGD with therapeutic intent (dilation) for patients with history suggesting benign obstruction ($602 ± 22 versus $515 ± 5, P<0.02), but was less costly than EGD for diagnoses and treatment involving abnormal motility (for achalasia, $67 ± 17 versus $547 ± 21, P<0.001) (65).
Barium esophagram may be useful as the initial test to map the esophagus when the clinician judges the patient to be at high risk of perforation on EGD, such as those with suspected high-risk lesions or strictures high up in the esophagus (66, 67). Performing both tests can provide complementary information in some situations, such as pre-operative assessment of Zenker’s diverticulum (68) or the assessment of suspected achalasia if manometry is not readily available (69).
Statement 3.2: In some patients with esophageal dysphagia, we suggest a barium esophagram when there is limited local access to endoscopy to assess for significant structural lesions and facilitate timely referral to urgent endoscopy and specialist consultation.
• GRADE: Strong recommendation, very low-quality evidence. Vote: agree strongly, 78%; agree with minor reservation, 11%; agree with major reservation, 11%
Studies show that barium esophagram can be sensitive for the detection of rings (62), stenoses (63), dysmotility, hiatal hernia, benign stricture and esophagitis (59), as well as esophageal carcinoma (60). However, in one study, barium esophagram failed to identify about half of proximal stenoses or concentric rings detected on EGD (61). A prospective study, in which pathology was used as the reference standard, reported good specificity, but poor sensitivity of barium esophagram compared to EGD (64).
The consensus group concluded that although EGD is generally preferred (see statement 3.1), in areas of limited availability to EGD, barium esophagram does have diagnostic utility and can be useful in identifying patients who warrant urgent access to EGD.
Statement 3.3: In patients with esophageal dysphagia, we recommend esophageal manometry over barium esophagram to improve the diagnosis of esophageal motility disorders.
• GRADE: Strong recommendation, very low-quality evidence. Vote: agree strongly, 67%; agree with minor reservation, 33%
Prospective studies in consecutive patients (70–74) and retrospective studies (75, 76) have evaluated the diagnostic accuracy of manometry and barium esophagram in patients with dysphagia. In patients with dysphagia, and abnormal manometry studies, barium esophagram often demonstrated poor sensitivity (70, 71, 74–76). Compared to manometry, the sensitivity of barium esophagram was generally greatest for the diagnosis of achalasia, as compared with other esophageal motility disorders (71, 72, 75). In addition, sensitivity improved when nutcracker esophagus and nonspecific esophageal motor disorders were excluded (75).
In some situations, performing both tests can provide complementary information, for example, in patients with EGJ outflow obstruction (Chicago 3 classification) or post-fundoplication dysphagia, barium esophagram can complement manometry in helping to identify functional defects and guide management decisions (77). In addition, in patients with achalasia on manometry, timed barium esophagram can be used to assess the degree of impairment of esophageal emptying (78).
The consensus group agreed that, once structural and inflammatory abnormalities have been ruled out with EGD or barium esophagram, esophageal manometry should be used to assess suspected motility disorders.
Section 4: Role of Empiric Treatment with PPI in Esophageal Dysphagia
Statement 4.1: In patients under 50 years old presenting with esophageal dysphagia and reflux symptoms, and no alarm features to suggest underlying malignancy, we recommend further testing be performed if dysphagia does not resolve completely after a 4-week trial of oral proton pump inhibitor (PPI) given twice daily.
• GRADE: Strong recommendation, very low-quality evidence. Vote: agree strongly, 44%; agree with minor reservation, 66%
An empiric trial of proton pump inhibitor (PPI) therapy is a common strategy in patients presenting with dysphagia and typical GERD symptoms, such as heartburn and regurgitation (4). This strategy is justified by the high rate of GERD or esophagitis in patients with dysphagia. In retrospective database analyses, the most common findings on EGD in patients with dysphagia, were GERD/esophagitis (17%–28%), esophageal strictures (2.6%-41%) and normal esophagus (24%–32%) (4–7). In addition, PPI prescription is the most common treatment after EGD in patients with dysphagia (4), and PPIs have been shown to significantly improve symptoms of dysphagia associated with GERD (79, 80).
The main concern with a strategy of empiric PPI therapy is the potential for missed or delayed cancer diagnoses. In 2017, among Canadian adults, the estimated incidence of new esophageal cancers was 5.7/100,000 (2,300 cases), and of stomach cancers 8.6/100,000 (3,500 cases) (50). However, among patients referred for EGD for evaluation of dysphagia, the rates of esophageal cancer ranged from 3.1 to 8.1% (4–7). Cancer findings were more common in males, patients ages 50–60 years or older, and those with additional alarm features (6, 7, 56, 81).
In population-based studies, the incidence of gastroesophageal cancer was not significantly higher in users of antisecretory medication compared to non-users (82, 83). Data from a Barrett’s esophagus surveillance program showed a lower incidence of dysplasia among patients on a PPI compared to those without prior PPI therapy, suggesting a potential preventive effect for esophageal cancer (84). Although retrospective, a second report which assessed the number of esophageal and gastric cancers diagnosed subsequent to negative findings on an initial EGD found a significantly greater number of delayed diagnosis for gastric adenocarcinoma among PPI-users (versus non-users); however, there was no difference for esophageal adenocarcinoma between groups (85). There is no evidence demonstrating that PPI use prior to EGD would preclude the diagnosis of esophageal cancer in patients presenting with dysphagia.
No study has directly compared the safety and efficacy of an empiric PPI trial first (with EGD for those who do not respond completely) to prompt EGD for all patients (with optional PPI treatment while waiting for the EGD). However, any delay in diagnosis as a result of an empiric PPI trial, as recommended in the statement, would only be four weeks, which would be unlikely to impact cancer outcome. Canadian consensus guidelines on wait times recommend that patients with severe or rapidly progressive dysphagia, or a high likelihood of cancer, be assessed within two weeks (58). However, for patients with stable dysphagia without other alarm features, the wait time for an outpatient EGD from the time of consultation is generally longer than four weeks (86). Therefore, the consensus group recommended empiric PPI therapy and suggested scheduling an EGD, which can be cancelled if symptoms completely resolve to avoid unnecessary investigations.
Section 5: Role of Endoscopy in the Evaluation of Esophageal Dysphagia
Statement 5.1: In patients with persistent esophageal dysphagia, we recommend endoscopy as the initial test to maximize diagnostic yield.
• GRADE: Strong recommendation, very low-quality evidence. Vote: agree strongly, 78%; agree with minor reservation, 22%
Studies comparing the diagnostic accuracy of EGD and barium esophagram were discussed in statement 3.1 (59–64). Results of these studies are conflicting, with some reporting greater accuracy with barium esophagram (59, 63) and others with EGD (60–62, 64). EoE, which is also an increasingly common finding in patients with dysphagia (5, 87, 88), requires EGD with biopsy to establish a diagnosis. Abnormal findings (such as retained food in a dilated esophagus) on EGD may suggest achalasia, which can then be further defined with manometry or barium esophagram (69, 89).
The consensus group concluded that when investigations are considered in patients with persistent dysphagia, EGD should be the initial test, to evaluate inflammatory or structural mucosal lesions.
Statement 5.2: In all patients undergoing endoscopy for esophageal dysphagia, unless there are clear features of erosive reflux esophagitis, we recommend esophageal biopsy be performed to detect mucosal pathology.
• GRADE: Strong recommendation, low-quality evidence. Vote: agree strongly, 78%; agree with minor reservation, 11%; agree with major reservation, 11%.
EoE, generally defined as ≥ 15 (5, 90, 91) or ≥ 20 (92–94) eosinophils per high-powered field, requires EGD with biopsy to make a diagnosis. The prevalence of EoE is about 8–9% among patients with dysphagia (5, 87), compared to about 3.1% in unselected patients (92). Conversely, about 55–73% of patients with EoE have dysphagia (49, 92, 93, 95, 96). The prevalence of EoE has been increasing (5), which cannot entirely be explained by better recognition (97, 98).
Patients with EoE can present with a normal appearing mucosa on EGD (47, 49, 94); therefore, mucosal biopsy is recommended for the assessment of dysphagia. A longitudinal study has showed that education on the need for biopsy can increase detection of EoE by 40% (88). In patients with dysphagia, biopsy is also needed to detect relatively rare conditions such as lymphocytic esophagitis (95), esophageal tuberculosis (99), linitis plastica adenocarcinoma (100), and esophageal lichen planus (101).
Guidelines for the management of EoE, from the American Gastroenterological Association (AGA) (90) and the American College of Gastroenterology (ACG) (102), as well as the recent European guidelines (103), recommend two to four esophageal biopsies from at least two locations (i.e., proximal and distal esophagus) from all patients in whom EoE is being considered, regardless of the gross appearance of the mucosa. When the number of biopsies reaches six to nine, diagnostic sensitivity approaches 100% (104–106).
The consensus group agreed with previous recommendation on the assessment of EoE and recommended multiple biopsies at multiple sites in patients in whom EoE is suspected, even if the mucosa appears to be normal endoscopically.
In patients with typical erosive esophagitis (Los Angeles Classification, Grade B or higher), biopsy findings may be inadequate to confirm the diagnosis of EoE since eosinophils are also found in erosive esophagitis, and dysphagia is frequent in patients with erosive esophagitis. In those patients, the committee suggests to first treat the patient with PPI for at least eight weeks. If dysphagia does not resolve, consider repeat endoscopy with biopsy to assess for the presence of EoE, and in those with severe erosive esophagitis, repeat endoscopy to evaluate for the presence of Barrett’s esophagus.
Section 6: Role of Esophageal Manometry in the Evaluation of Esophageal Dysphagia
Statement 6.1: Esophageal manometry is the gold standard for diagnosing esophageal motility disorders.
• GRADE: Strong recommendation, very low-quality evidence. Vote: agree strongly, 89%; agree with minor reservation, 11%
Statement 6.2: In patients with persistent esophageal dysphagia, after structural and inflammatory causes have been ruled out, we recommend esophageal manometry to evaluate esophageal motility disorders.
• GRADE: Strong recommendation, very low-quality evidence. Vote: agree strongly, 100%
In clinical practice, EGD is generally the first diagnostic test to evaluate structural and inflammatory disorders, followed by manometry when motility disorders are the suspected cause of dysphagia. In patients with dysphagia and a normal EGD assessment, subsequent esophageal manometry had a higher diagnostic yield than barium esophagram (4.6% versus 33.3%) (4). As described in statement 3.3, there is good concordance between manometry and barium esophagram in patients with dysphagia (72, 73), although some studies show good specificity but poor sensitivity of barium esophagram in patients with dysphagia and abnormal manometry studies (70, 71, 74–76).
Manometry has demonstrated clinical utility when used in patients with dysphagia after prior non-diagnostic EGD and/or barium esophagram (8, 9). In a survey of patients who had dysphagia as an indication for manometry, new information was obtained in 90% of patients; the diagnosis was changed in 51% (most common new diagnoses were achalasia, IEM and EGJ outflow obstruction), and management was changed in 69% (8). Similarly, in another observational study, 79.4% of patients with dysphagia had abnormal results on manometry, with the most frequent diagnosis being achalasia (53.7%) (9).
The consensus group concluded that manometry should be performed in patients who continue to have dysphagia once structural and inflammatory (including GERD and EoE) causes have been ruled out by a normal EGD with appropriate biopsies.
Statement 6.3: In patients with dysphagia, we suggest high-resolution esophageal manometry over conventional esophageal manometry to improve diagnostic performance.
• GRADE: Strong recommendation, very low-quality evidence. Vote: agree strongly, 56%; agree with minor reservation, 33%; agree with major reservation, 11%
High resolution esophageal manometry (HREM) has theoretical technologic advantages over conventional manometry in the diagnosis of esophageal motility disorders. Low-quality data suggest HREM can provide more manometric information than conventional manometry and may increase diagnostic yield in patients with dysphagia (107–109) and unselected patients (110, 111). Use of the Chicago Classification with HREM may increase diagnostic accuracy (107, 110) and enhance consistency in reporting.
The consensus group concluded that HREM is preferred, when available. The group also recommended that recordings be interpreted by trained and experienced clinicians and that it is inadequate to rely solely on computer interpretation to ensure diagnostic accuracy.
CONCLUSIONS
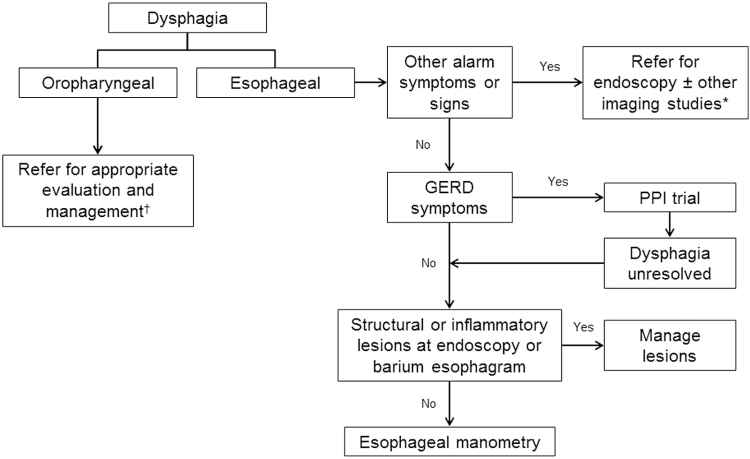
The suggested diagnostic approach to uninvestigated dysphagia is summarized in figure 1. Oropharyngeal dysphagia should be considered first and ruled out in all patients presenting with uninvestigated dysphagia. If oropharyngeal dysphagia is suspected, referral to a speech language pathologist, or other appropriate specialist, for further investigation and management is recommended. Patients with symptoms of esophageal dysphagia should be assessed by history for alarm features that require urgent investigations (e.g., endoscopy or other imaging studies, such as computed tomography or ultrasound). A PPI trial can be considered in patients who are < 50 years old and have no other alarm features. If dysphagia persists after a four-week PPI trial, investigations to assess inflammatory or structural lesions are recommended. Once inflammatory or structural lesions have been ruled out by endoscopy (with biopsy as indicated) and/or barium esophagram, esophageal manometry should be used to evaluate all patients with persistent dysphagia. In a patient with subjective sensation of dysphagia, when thorough investigations have excluded oropharyngeal and esophageal causes, a diagnosis of functional dysphagia should be considered (112).
Figure 1.
Suggested diagnostic approach to uninvestigated dysphagia.
*Imaging studies could include computed tomography (CT) and ultrasound. †Expertise (e.g., speech language pathologist or occupational therapist) and assessments (e.g., videofluoroscopic swallowing study [VFSS] or fiberoptic endoscopic evaluation of swallowing [FEES]) vary depending on regional availability.
Future Directions
In the first iteration of the statements, the committee intended to recommend using symptoms to differentiate oropharyngeal from esophageal dysphagia. In clinical practice, many clinicians have found that “the timing of dysphagia (i.e., immediate vs delayed after the initiation of swallow is completed)” is often helpful in distinguishing between oropharyngeal dysphagia and esophageal dysphagia. After an extensive literature search, we were unable to identify any direct evidence demonstrating that symptoms subjectively reported by patients can help distinguish oropharyngeal from esophageal dysphagia. The lack of data on the sensitivity and specificity of patient-reported symptoms in differentiating the types of dysphagia was identified as a knowledge gap, despite the fact that this is an important first step in the clinical assessment of patients presenting with dysphagia and affects management decisions. Furthermore, because GERD is common in the general population and is frequently associated with dysphagia, there is a need for well- conducted RCTs on the impact of empiric PPI therapy in patients < 50 years old with dysphagia and without other alarm symptoms compared to early EGD. Further studies to assess the diagnostic accuracy of HREM over conventional manometry in the evaluation of patients with different esophageal motility disorders are warranted to determine optimal utilization.
ACKNOWLEDGEMENTS
The consensus group would like to thank the following people for their contributions: Paul Sinclair, Louise Hope and Lesley Marshall (CAG representatives, administrative and technical support, and logistics assistance); Pauline Lavigne and Steven Portelance (unaffiliated, editorial assistance).
Glossary
Abbreviations:
- AGA
American Gastroenterological Association
- ACG
American College of Gastroenterology
- CAG
Canadian Association of Gastroenterology
- CI
confidence interval
- CPG
clinical practice guideline
- EGJ
esophagogastric junction
- EGD
endoscopy
- EoE
eosinophilic esophagitis
- GRADE
Grading of Recommendation Assessment, Development and Evaluation
- HR
hazard ratio
- HREM
high resolution esophageal manometry
- IEM
ineffective esophageal motility
- LES
lower esophageal sphincter
- OR
odds ratio
- RCT
randomized controlled trial
- RR
relative risk
- SWAL-QOL
swallowing quality of life scale
- TOR-BSST©
Toronto bedside swallowing screening test
- V-VST
viscosity swallowing test
- WGO
World Gastroenterology Organisation
- WVQ
wet voice quality
APPENDIX
Online Appendix 1. Search strategies used for EMBASE and MEDLINE and CENTRAL
(All searches were updated to March 2015)
Statement 1
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <September 2012>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to September 2012>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>, Embase <1974 to 2012 October 04>
Search Strategy:
--------------------------------------------------------------------------------
1 exp Deglutition Disorders/ (74138)
2 exp Deglutition/ (18795)
3 dysphagia.mp. (55554)
4 exp dysphagia/ (74138)
5 exp swallowing/ (18795)
6 swallow*.mp. (46835)
7 1 or 2 or 3 or 4 or 5 or 6 (122463)
8 (esophag* or oesophag*).mp. (346880)
9 oropharyn*.mp. (36252)
10 8 and 9 (3428)
11 7 and 10 (1178)
12 (oral-pharyn* or oropharyn*).mp. (37124)
13 (eso-phag* or oeso-phag* or esophag* or oesophag*).mp. (346882)
14 12 and 13 (3696)
15 7 and 14 (1306)
16 15 not 11 (128)
17 (eso-phag* or oeso-phag* or esophag* or oesophag* or oral-pharyn* or oropharyn*).ti. (179875)
18 (dysphagia or swallowing disorder* or deglutition).ti. (11353)
19 17 and 18 (1911)
20 19 not 11 (1767)
21 16 or 20 (1883)
22 limit 21 to english language [Limit not valid in CCTR,CDSR; records were retained] (1491)
23 remove duplicates from 22 (864)
24 (animals not (humans and animals)).sh. (3699389)
25 (animal not (humans and animal)).sh. (1804242)
26 24 or 25 (5503631)
27 23 not 26 (860)
************************** ************************** ***********
Statement 2
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <September 2012>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to September 2012>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>, Embase <1974 to 2012 October 08>
Search Strategy:
--------------------------------------------------------------------------------
1 Deglutition Disorders/ (47102)
2 Deglutition/ (18798)
3 Esophageal Motility Disorders/ (2370)
4 dysphagia/ (47102)
5 swallowing/ (18798)
6 1 or 2 or 3 or 4 or 5 (62704)
7 exp Medical History Taking/ (185694)
8 exp Physical Examination/ (1119282)
9 exp anamnesis/ (167864)
10 7 or 8 or 9 (1269979)
11 6 and 10 (3562)
12 ((clinical manifestation* or alarm symptom*) adj10 (dysphagia or deglutition or swallowing)).mp. (210)
13 (((patient* adj2 history) or (history adj2 tak*) or (medical history or medical record*)) adj10 (dysphagia or deglutition or swallowing)).mp. (1065)
14 ((historical or physical examination* or anamnesis) adj10 (dysphagia or deglutition or swallowing)).mp. (2024)
15 12 or 13 or 14 (3201)
16 11 or 15 (4759)
17 (diagnos* and (dysphagia or deglutition or swallowing)).ti. (566)
18 16 or 17 (5265)
19 limit 18 to english language [Limit not valid in CCTR,CDSR; records were retained] (4481)
20 remove duplicates from 19 (4051)
21 (animals not (humans and animals)).sh. (3699389)
22 (animal not (humans and animal)).sh. (1804559)
23 21 or 22 (5503948)
24 20 not 23 (3925)
************************** ************************** ***********
Statement 3
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>, Embase <1974 to 2012 September 11>
Search Strategy:
--------------------------------------------------------------------------------
1 *Deglutition Disorders/ (18054)
2 *Deglutition/ (6754)
3 *swallowing/ (6754)
4 swallowing disorder*.ti,ab. (1709)
5 *dysphagia/ (18054)
6 dysphagia.ti,ab. (36959)
7 1 or 2 or 3 or 4 or 5 or 6 (49114)
8 Barium.ti,ab. (37465)
9 Barium meal/ (2373)
10 Barium Sulfate/ (14265)
11 *Barium/ (5809)
12 *Contrast Media/ (47468)
13 contrast media.ti,ab. (20452)
14 *esophagography/ or esophagram.ti,ab. or oesophagram.ti,ab. (2530)
15 8 or 9 or 10 or 11 or 12 or 13 or 14 (107252)
16 7 and 15 (3503)
17 remove duplicates from 16 (2289)
18 limit 17 to english language (1954)
19 limit 18 to humans (1655)
************************** ************************** ***********
Statement 4
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>, Embase <1974 to 2012 September 20>
Search Strategy:
--------------------------------------------------------------------------------
1 Deglutition Disorders/ (46523)
2 Deglutition/ (18527)
3 dysphagia.mp. (54386)
4 dysphagia/ (46523)
5 (swallow$ adj2 disorder*).mp. (2026)
6 1 or 2 or 3 or 4 or 5 (74759)
7 exp Gastroesophageal Reflux/ (60269)
8 exp esophagitis/ (30787)
9 (esophagitis or oesophagitis).mp. (39022)
10 ((gastro adj2 oesophageal adj2 reflux) or (gastro adj2 esophageal adj2 reflux)).tw. (10052)
11 (((gastro-esophageal adj2 reflux) or gastro-oesophageal) adj2 reflux).tw. (10040)
12 (gastro?esophageal adj2 reflux).tw. (29377)
13 *duodenogastric reflux/ (1827)
14 (duodenogastric adj2 reflux).tw. (1860)
15 (acid adj3 reflux).tw. (5366)
16 (GORD or GERD).mp. (13798)
17 (NERD or NORD or EoE).mp. (6344)
18 Esophagitis, Peptic/ (13244)
19 Eosinophilic Esophagitis/ (1681)
20 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 18 or 19 (91647)
21 6 and 20 (10318)
22 exp proton pump inhibitors/ (46911)
23 (proton adj2 pump adj2 inhibitor$).mp. (31325)
24 exp omeprazole/ (32296)
25 omeprazole.tw. (15190)
26 (lansoprazole or lanzoprazole).tw. (4340)
27 esomeprazole.mp. (5070)
28 pantoprazole.mp. (6707)
29 rabeprazole.mp. (4119)
30 tenatoprazole.mp. (69)
31 (Dexlansoprazole or Kapidex or Dexilant).mp. (139)
32 (PPI or PPIs).mp. (19354)
33 proton pumps/ (7948)
34 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 (78730)
35 21 and 34 (1702)
36 remove duplicates from 35 (1379)
37 limit 36 to english language (1241)
************************** ************************** ***********
Statement 5
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <September 2012>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to September 2012>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>, Embase <1974 to 2012 October 05>
Search Strategy:
--------------------------------------------------------------------------------
1 *Deglutition Disorders/ (18143)
2 *Deglutition/ (6790)
3 swallowing disorder*.ti,ab. (1749)
4 *dysphagia/ (18143)
5 dysphagia.ti,ab. (37970)
6 1 or 2 or 3 or 4 or 5 (50193)
7 *Endoscopy/ or *Endoscopy, Gastrointestinal/ or *Endoscopy, Digestive System/ (60272)
8 Endoscopy, Digestive System/mt (1357)
9 Endoscopy, Digestive System/ut (48)
10 Endosonography/mt (2111)
11 Endosonography/ut (50)
12 *Endosonography/ (11771)
13 Esophagoscopy/mt (1766)
14 Esophagoscopy/ut (30)
15 *esophagoscopy/ (7051)
16 Endoscopy, Gastrointestinal/mt (3685)
17 Endoscopy, Gastrointestinal/ut (144)
18 *fiberscope endoscopy/ or *high resolution endoscopy/ or *digestive tract endoscopy/ or *gastrointestinal endoscopy/ (14205)
19 *endoscopic echography/ (6975)
20 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 (80254)
21 6 and 20 (1539)
22 ((endoscopic or endoscopy or endoscope or scope or EGD or endosonograph* or esophagoscop* or oesophagoscop*) adj10 (dysphagia or swallowing disorder* or deglutition)).ti,ab. (2524)
23 21 or 22 (3668)
24 *Biopsy, Fine-Needle/ or *Biopsy/ or *Biopsy, Needle/ (48053)
25 Biopsy/mt, ut (9135)
26 Biopsy, Fine-Needle/mt, ut (1785)
27 Biopsy, Needle/mt, ut (8101)
28 *biopsy technique/ (408)
29 *oral biopsy/ or *biopsy technique/ or *duodenum biopsy/ or *esophagus biopsy/ or *gastrointestinal biopsy/ or *endoscopic biopsy/ (1698)
30 24 or 25 or 26 or 27 or 28 or 29 (57579)
31 6 and 30 (68)
32 ((biopsy or biopsies) adj10 (dysphagia or swallowing disorder* or deglutition)).ti,ab. (301)
33 31 or 32 (359)
34 23 or 33 (3892)
35 limit 34 to english language [Limit not valid in CCTR,CDSR; records were retained] (3053)
36 (animals not (humans and animals)).sh. (3699389)
37 (animal not (humans and animal)).sh. (1804242)
38 36 or 37 (5503631)
39 35 not 38 (3032)
40 remove duplicates from 39 (1955)
************************** ************************** ***********
Statement 6
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <September 2012>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to September 2012>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>, Embase <1974 to 2012 October 08>
Search Strategy:
--------------------------------------------------------------------------------
1 *Deglutition Disorders/ (18150)
2 *Deglutition/ (6790)
3 *Esophageal Motility Disorders/ (1509)
4 dysphagia/ (47102)
5 swallowing disorder*.tw. (1754)
6 dysphagia.tw. (38058)
7 1 or 2 or 3 or 4 or 5 or 6 (68249)
8 *Manometry/ (8575)
9 exp esophagus manometry/ (2303)
10 manometry.tw. (18073)
11 8 or 9 or 10 (25112)
12 7 and 11 (3966)
13 remove duplicates from 12 (2569)
14 limit 13 to english language [Limit not valid in CCTR,CDSR; records were retained] (2155)
15 (animals not (humans and animals)).sh. (3699389)
16 (animal not (humans and animal)).sh. (1804559)
17 15 or 16 (5503948)
18 14 not 17 (2145)
************************** ************************** ***********
Role of the Funding Sources
The CAG funded and administered all aspects of the meeting, with no external funding sources.
Research Grants and Clinical Trial Funding
Allergan (CNA), Canada Research Chair in Swallowing Disorders (RM), Janssen (CNA)
Advisory Board
AbbVie (LL, CNA, DA, ML), Allergan (LL, CNA, DA), Takeda (DA), Pendopharm (DA), Pfizer (DA)
Educational Support
Medtronic (LL, CNA), Pfizer (DA)
Speaker’s Bureau
AbbVie (LL, DA), Allergan (LL, CNA, ML), Medtronic (LL, CNA), Pendopharm (CNA) Takeda (DA, ML)
Canadian Association of Gastroenterology Statement
This clinical practice guideline (CPG) for the assessment of uninvestigated esophageal dysphagia was developed under the direction of Dr. Louis Liu, in accordance with the policies and procedures of the Canadian Association of Gastroenterology (CAG) and under the direction of CAG Clinical Affairs. It has been reviewed by the CAG Practice Affairs and Clinical Affairs Committees and the CAG Board of Directors. The CPG was developed following a thorough consideration of medical literature and the best available evidence and clinical experience. It represents the consensus of a Canadian panel comprised of experts on this topic. The CPG aims to provide a reasonable and practical approach to care for specialists and allied health professionals charged with the duty of providing optimal care to patients and families; however, the CPG can be subject to change as scientific knowledge and technology advance and as practice patterns evolve. The CPG is not intended to substitute for physicians’ use of their individual judgment in managing clinical care in consultation with the patient, with appropriate regard to all the individual circumstances of the patient, diagnostic and treatment options available, and available resources. Adherence to these recommendations will not necessarily produce successful outcomes in every case.
CONFLICT OF INTEREST
The authors do not have any industry or government relationships to report (AL, ND, NJ, RM, WP, GL).
Author Contributions
All members of the consensus group (with the exception of GL and FT) reviewed the literature, drafted the statements, and voted on the recommendations. GL and FT assessed the evidence and provided GRADE evaluations. The chair drafted the initial manuscript, which was reviewed, revised, and approved by all members of the consensus group. Subsequently it was made available to all CAG members for comments prior to submission for publication.
References
- 1. Cho SY, Choung RS, Saito YA et al. Prevalence and risk factors for dysphagia: a USA community study. Neurogastroenterol Motil 2015;27:212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malagelada JR, Bazzoli F, Boeckxstaens G et al. World gastroenterology organisation global guidelines: dysphagia--global guidelines and cascades update September 2014. J Clin Gastroenterol 2015;49:370–8. [DOI] [PubMed] [Google Scholar]
- 3. Cockeram AW. Canadian Association of Gastroenterology Practice Guidelines: evaluation of dysphagia. Can J Gastroenterol 1998;12:409–13. [DOI] [PubMed] [Google Scholar]
- 4. Rosenstock A, Kushnir V, Patel A et al. Diagnostic yield in the evaluation of dysphagia [abstract Su1509]. Gastrointest Endosc 2011;73:AB287. [Google Scholar]
- 5. Kidambi T, Toto E, Ho N et al. Temporal trends in the relative prevalence of dysphagia etiologies from 1999–2009. World J Gastroenterol 2012;18:4335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qureshi NA, Hallissey MT, Fielding JW. Outcome of index upper gastrointestinal endoscopy in patients presenting with dysphagia in a tertiary care hospital-A 10 years review. BMC Gastroenterol 2007;7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varadarajulu S, Eloubeidi MA, Patel RS et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005;61:804–8. [DOI] [PubMed] [Google Scholar]
- 8. Lacy BE, Paquette L, Robertson DJ et al. The clinical utility of esophageal manometry. J Clin Gastroenterol 2009;43:809–15. [DOI] [PubMed] [Google Scholar]
- 9. Ciriza de los Rios C, Garcia Menendez L, Diez Hernandez A et al. Role of stationary esophageal manometry in clinical practice. Manometric results in patients with gastroesophageal reflux, dysphagia or non-cardiac chest pain. Rev Esp Enferm Dig 2004;96:606–8. [DOI] [PubMed] [Google Scholar]
- 10. Kahrilas PJ, Bredenoord AJ, Fox M et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guyatt GH, Oxman AD, Vist GE et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whiting PF, Rutjes AW, Westwood ME et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- 13. Sultan S, Falck-Ytter Y, Inadomi JM. The AGA. institute process for developing clinical practice guidelines part one: grading the evidence. Clin Gastroenterol Hepatol 2013;11:329–32. [DOI] [PubMed] [Google Scholar]
- 14. Bressler B, Marshall JK, Bernstein CN et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology 2015;148:1035–58. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen GC, Bernstein CN, Bitton A et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology 2014;146:835–48 e6. [DOI] [PubMed] [Google Scholar]
- 16. Dalkey N. An experimental study of group opinion: the Delphi method. Futures 1969;1:408–26. [Google Scholar]
- 17. Cook DJ, Greengold NL, Ellrodt AG et al. The relation between systematic reviews and practice guidelines. Ann Intern Med 1997;127:210–6. [DOI] [PubMed] [Google Scholar]
- 18. Guyatt GH, Oxman AD, Kunz R et al. Going from evidence to recommendations. BMJ 2008;336:1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shaw SM, Martino R. The normal swallow: muscular and neurophysiological control. Otolaryngol Clin North Am 2013;46:937–56. [DOI] [PubMed] [Google Scholar]
- 20. Clave P, Shaker R. Dysphagia: current reality and scope of the problem. Nat Rev Gastroenterol Hepatol 2015;12:259–70. [DOI] [PubMed] [Google Scholar]
- 21. Smith DF, Ott DJ, Gelfand DW et al. Lower esophageal mucosal ring: correlation of referred symptoms with radiographic findings using a marshmallow bolus. AJR Am J Roentgenol 1998;171:1361–5. [DOI] [PubMed] [Google Scholar]
- 22. Jones B, Donner MW, Rubesin SE et al. Pharyngeal findings in 21 patients with achalasia of the esophagus. Dysphagia 1987;2:87–92. [DOI] [PubMed] [Google Scholar]
- 23. Edwards D. Discriminatory value of symptoms in the differential diagnosis of dysphagia. Clin Gastroenterol 1976;5:49–57. [Google Scholar]
- 24. Wilcox CM, Alexander LN, Clark WS. Localization of an obstructing esophageal lesion. Is the patient accurate?Dig Dis Sci 1995;40:2192–6. [DOI] [PubMed] [Google Scholar]
- 25. Belafsky PC, Mouadeb DA, Rees CJ et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol 2008;117:919–24. [DOI] [PubMed] [Google Scholar]
- 26. Cheney DM, Siddiqui MT, Litts JK et al. The ability of the 10-Item Eating Assessment Tool (EAT-10) to predict aspiration risk in persons with dysphagia. Ann Otol Rhinol Laryngol 2015;124:351–4. [DOI] [PubMed] [Google Scholar]
- 27. McHorney CA, Robbins J, Lomax K et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia 2002;17:97–114. [DOI] [PubMed] [Google Scholar]
- 28. Groves-Wright KJ, Boyce S, Kelchner L. Perception of wet vocal quality in identifying penetration/aspiration during swallowing. J Speech Lang Hear Res 2010;53:620–32. [DOI] [PubMed] [Google Scholar]
- 29. Kertscher B, Speyer R, Palmieri M et al. Bedside screening to detect oropharyngeal dysphagia in patients with neurological disorders: an updated systematic review. Dysphagia 2014;29:204–12. [DOI] [PubMed] [Google Scholar]
- 30. Martino R, Silver F, Teasell R et al. The Toronto Bedside Swallowing Screening Test (TOR-BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke 2009;40:555–61. [DOI] [PubMed] [Google Scholar]
- 31. Clave P, Arreola V, Romea M et al. Accuracy of the volume-viscosity swallow test for clinical screening of oropharyngeal dysphagia and aspiration. Clin Nutr 2008;27:806–15. [DOI] [PubMed] [Google Scholar]
- 32. Suiter DM, Leder SB. Clinical utility of the 3-ounce water swallow test. Dysphagia 2008;23:244–50. [DOI] [PubMed] [Google Scholar]
- 33. Wakasugi Y, Tohara H, Hattori F et al. Screening test for silent aspiration at the bedside. Dysphagia 2008;23:364–70. [DOI] [PubMed] [Google Scholar]
- 34. Hinchey JA, Shephard T, Furie K et al. Formal dysphagia screening protocols prevent pneumonia. Stroke 2005;36:1972–6. [DOI] [PubMed] [Google Scholar]
- 35. Martino R, Foley N, Bhogal S et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005;36:2756–63. [DOI] [PubMed] [Google Scholar]
- 36. Almirall J, Rofes L, Serra-Prat M et al. Oropharyngeal dysphagia is a risk factor for community-acquired pneumonia in the elderly. Eur Respir J 2013;41:923–8. [DOI] [PubMed] [Google Scholar]
- 37. Jaffer NM, Ng E, Au FW et al. Fluoroscopic evaluation of oropharyngeal dysphagia: anatomic, technical, and common etiologic factors. AJR Am J Roentgenol 2015;204:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murray IA, Palmer J, Waters C et al. Predictive value of symptoms and demographics in diagnosing malignancy or peptic stricture. World J Gastroenterol 2012;18:4357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khan MQ, AlQaraawi A, Al-Sohaibani F et al. Clinical, endoscopic, and radiologic features of three subtypes of achalasia, classified using high-resolution manometry. Saudi J Gastroenterol 2015;21:152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fisichella PM, Raz D, Palazzo F et al. Clinical, radiological, and manometric profile in 145 patients with untreated achalasia. World J Surg 2008;32:1974–9. [DOI] [PubMed] [Google Scholar]
- 41. Aljebreen AM, Samarkandi S, Al-Harbi T et al. Efficacy of pneumatic dilatation in Saudi achalasia patients. Saudi J Gastroenterol 2014;20:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aghajanzadeh M, Moghadam AD, Hemmati H et al. Results of short- and long-segment cardioesophageal myotomy for achalasia. Saudi J Gastroenterol 2012;18:237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Almansa C, Heckman MG, DeVault KR et al. Esophageal spasm: demographic, clinical, radiographic, and manometric features in 108 patients. Dis Esophagus 2012;25:214–21. [DOI] [PubMed] [Google Scholar]
- 44. Hirano I, Gilliam J, Goyal RK. Clinical and manometric features of the lower esophageal muscular ring. Am J Gastroenterol 2000;95:43–9. [DOI] [PubMed] [Google Scholar]
- 45. Muller M, Gockel I, Hedwig P et al. Is the Schatzki ring a unique esophageal entity?World J Gastroenterol 2011;17:2838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sheehan NJ. Dysphagia and other manifestations of oesophageal involvement in the musculoskeletal diseases. Rheumatology (Oxford) 2008;47:746–52. [DOI] [PubMed] [Google Scholar]
- 47. Mackenzie SH, Go M, Chadwick B et al. Eosinophilic oesophagitis in patients presenting with dysphagia--a prospective analysis. Aliment Pharmacol Ther 2008;28:1140–6. [DOI] [PubMed] [Google Scholar]
- 48. Heerasing N, Lee SY, Alexander S et al. Prevalence of eosinophilic oesophagitis in adults presenting with oesophageal food bolus obstruction. World J Gastrointest Pharmacol Ther 2015;6:244–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Muller S, Puhl S, Vieth M et al. Analysis of symptoms and endoscopic findings in 117 patients with histological diagnoses of eosinophilic esophagitis. Endoscopy 2007;39:339–44. [DOI] [PubMed] [Google Scholar]
- 50. Canadian Cancer Society’s Steering Committee on Cancer Statistics. Canadian Cancer Statistics 2017. Toronto, ON: [Accessed 2017 October 12]; Available from: www.cancer.ca. [Google Scholar]
- 51. Astin MP, Martins T, Welton N et al. Diagnostic value of symptoms of oesophagogastric cancers in primary care: a systematic review and meta-analysis. Br J Gen Pract 2015;65:e677-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fransen GA, Janssen MJ, Muris JW et al. Meta-analysis: the diagnostic value of alarm symptoms for upper gastrointestinal malignancy. Aliment Pharmacol Ther 2004;20:1045–52. [DOI] [PubMed] [Google Scholar]
- 53. Kapoor N, Bassi A, Sturgess R et al. Predictive value of alarm features in a rapid access upper gastrointestinal cancer service. Gut 2005;54:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thomson AB, Barkun AN, Armstrong D et al. The prevalence of clinically significant endoscopic findings in primary care patients with uninvestigated dyspepsia: the Canadian Adult Dyspepsia Empiric Treatment - Prompt Endoscopy (CADET-PE) study. Aliment Pharmacol Ther 2003;17:1481–91. [DOI] [PubMed] [Google Scholar]
- 55. Engel LS, Chow WH, Vaughan TL et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst 2003;95:1404–13. [DOI] [PubMed] [Google Scholar]
- 56. National Collaborating Centre for Cancer. Suspected cancer: recognition and referral, NICE Guideline, No. 12. London: National Institute for Health and Care Excellence (UK); 2015. [updated Jun; Accessed 2016 Nov 28]; Available from: http://www.nice.org.uk/guidance/ng12. [Google Scholar]
- 57. Stapley S, Peters TJ, Neal RD et al. The risk of oesophago-gastric cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer 2013;108:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Paterson WG, Depew WT, Pare P et al. Canadian consensus on medically acceptable wait times for digestive health care. Can J Gastroenterol 2006;20:411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Halpert RD, Feczko PJ, Spickler EM et al. Radiological assessment of dysphagia with endoscopic correlation. Radiology 1985;157:599–602. [DOI] [PubMed] [Google Scholar]
- 60. DiPalma JA, Prechter GC, Brady CE 3rd. X-ray-negative dysphagia: is endoscopy necessary?J Clin Gastroenterol 1984;6:409–11. [DOI] [PubMed] [Google Scholar]
- 61. Potter JW, Saeian K, Staff D et al. Eosinophilic esophagitis in adults: an emerging problem with unique esophageal features. Gastrointest Endosc 2004;59:355–61. [DOI] [PubMed] [Google Scholar]
- 62. Ott DJ, Chen YM, Wu WC et al. Radiographic and endoscopic sensitivity in detecting lower esophageal mucosal ring. AJR Am J Roentgenol 1986;147:261–5. [DOI] [PubMed] [Google Scholar]
- 63. Somers S, Stevenson GW, Thompson G. Comparison of endoscopy and barium swallow with marshmallow in dysphagia. Can Assoc Radiol J 1986;37:73–5. [PubMed] [Google Scholar]
- 64. Connolly GM, Forbes A, Gleeson JA et al. Investigation of upper gastrointestinal symptoms in patients with AIDS. AIDS 1989;3:453–6. [DOI] [PubMed] [Google Scholar]
- 65. Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002;97:2733–7. [DOI] [PubMed] [Google Scholar]
- 66. Zhang X, Wang M, Han H et al. Corrosive induced carcinoma of esophagus after 58 years. Ann Thorac Surg 2012;94:2103–5. [DOI] [PubMed] [Google Scholar]
- 67. Kaplan M, Mutlu EA, Jakate S et al. Endoscopy in eosinophilic esophagitis: “feline” esophagus and perforation risk. Clin Gastroenterol Hepatol 2003;1:433–7. [DOI] [PubMed] [Google Scholar]
- 68. Pomerri F, Costantini M, Dal Bosco C et al. Comparison of preoperative and surgical measurements of Zenker’s diverticulum. Surg Endosc 2012;26:2010–5. [DOI] [PubMed] [Google Scholar]
- 69. Ferri LE, Cools-Lartigue J, Cao J et al. Clinical predictors of achalasia. Dis Esophagus 2010;23:76–81. [DOI] [PubMed] [Google Scholar]
- 70. Davies HA, Evans KT, Butler F et al. Diagnostic value of “bread-barium” swallow in patients with esophageal symptoms. Dig Dis Sci 1983;28:1094–100. [DOI] [PubMed] [Google Scholar]
- 71. Fuller L, Huprich JE, Theisen J et al. Abnormal esophageal body function: radiographic-manometric correlation. Am Surg 1999;65:911–4. [PubMed] [Google Scholar]
- 72. Anumandla A, Hal H, Shi G et al. Dysphagia and reflux: manometry versus barium swallow [abstract]. Am J Gastroenterol 2010;105:S12. [Google Scholar]
- 73. Cho YK, Choi MG, Oh SN et al. Comparison of bolus transit patterns identified by esophageal impedance to barium esophagram in patients with dysphagia. Dis Esophagus 2012;25:17–25. [DOI] [PubMed] [Google Scholar]
- 74. Nicodeme F, de Ruigh A, Xiao Y et al. A comparison of symptom severity and bolus retention with Chicago classification esophageal pressure topography metrics in patients with achalasia. Clin Gastroenterol Hepatol 2013;11:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ott DJ, Richter JE, Chen YM et al. Esophageal radiography and manometry: correlation in 172 patients with dysphagia. AJR Am J Roentgenol 1987;149:307–11. [DOI] [PubMed] [Google Scholar]
- 76. El-Takli I, O’Brien P, Paterson WG. Clinical diagnosis of achalasia: how reliable is the barium x-ray?Can J Gastroenterol 2006;20:335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tatum RP, Shi G, Manka MA et al. Bolus transit assessed by an esophageal stress test in postfundoplication dysphagia. J Surg Res 2000;91:56–60. [DOI] [PubMed] [Google Scholar]
- 78. Vaezi MF, Baker ME, Richter JE. Assessment of esophageal emptying post-pneumatic dilation: use of the timed barium esophagram. Am J Gastroenterol 1999;94:1802–7. [DOI] [PubMed] [Google Scholar]
- 79. Bhandare B, Satyanarayana V, Pavithra K. A comparative study of the efficacy and safety of dexrabeprazole 10 mg versus rabeprazole 20 mg in the treatment of GERD in a tertiary care hospital. Int J Pharm Sci Rev Res 2014;24:263–5. [Google Scholar]
- 80. Oda K, Iwakiri R, Hara M et al. Dysphagia associated with gastroesophageal reflux disease is improved by proton pump inhibitor. Dig Dis Sci 2005;50:1921–6. [DOI] [PubMed] [Google Scholar]
- 81. Krishnamurthy C, Hilden K, Peterson KA et al. Endoscopic findings in patients presenting with dysphagia: analysis of a national endoscopy database. Dysphagia 2012;27:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lassen A, Hallas J, de Muckadell OB. The risk of missed gastroesophageal cancer diagnoses in users and nonusers of antisecretory medication. Gastroenterology 2005;129:1179–86. [DOI] [PubMed] [Google Scholar]
- 83. Poulsen AH, Christensen S, McLaughlin JK et al. Proton pump inhibitors and risk of gastric cancer: a population-based cohort study. Br J Cancer 2009;100:1503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hillman LC, Chiragakis L, Shadbolt B et al. Effect of proton pump inhibitors on markers of risk for high-grade dysplasia and oesophageal cancer in Barrett’s oesophagus. Aliment Pharmacol Ther 2008;27:321–6. [DOI] [PubMed] [Google Scholar]
- 85. Bramble MG, Suvakovic Z, Hungin AP. Detection of upper gastrointestinal cancer in patients taking antisecretory therapy prior to gastroscopy. Gut 2000;46:464–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Leddin D, Armstrong D, Borgaonkar M et al. The 2012 SAGE wait times program: Survey of Access to GastroEnterology in Canada. Can J Gastroenterol 2013;27:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Foroutan M, Norouzi A, Molaei M et al. Eosinophilic esophagitis in patients with refractory gastroesophageal reflux disease. Dig Dis Sci 2010;55:28–31. [DOI] [PubMed] [Google Scholar]
- 88. Kanakala V, Lamb CA, Haigh C et al. The diagnosis of primary eosinophilic oesophagitis in adults: missed or misinterpreted?Eur J Gastroenterol Hepatol 2010;22:848–55. [DOI] [PubMed] [Google Scholar]
- 89. Cameron AJ, Malcolm A, Prather CM et al. Videoendoscopic diagnosis of esophageal motility disorders. Gastrointest Endosc 1999;49:62–9. [DOI] [PubMed] [Google Scholar]
- 90. Furuta GT, Liacouras CA, Collins MH et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007;133:1342–63. [DOI] [PubMed] [Google Scholar]
- 91. Abe Y, Iijima K, Ohara S et al. Localized esophageal eosinophilia: Is it an early manifestation of eosinophilic esophagitis or a subtype of gastroesophageal reflux disease?Dig Endosc 2014;26:337–43. [DOI] [PubMed] [Google Scholar]
- 92. Kapel R, Gerta R. Clinical presentation of 4905 adults with histopathologic diagnosis of eosinophilic esophagitis [abstract S1091]. Gastroenterology 2010;138:S177. [Google Scholar]
- 93. Veerappan GR, Perry JL, Duncan TJ et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009;7:420–6. [DOI] [PubMed] [Google Scholar]
- 94. Prasad GA, Talley NJ, Romero Y et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol 2007;102:2627–32. [DOI] [PubMed] [Google Scholar]
- 95. Haque S, Genta RM. Lymphocytic oesophagitis: clinicopathological aspects of an emerging condition. Gut 2012;61:1108–14. [DOI] [PubMed] [Google Scholar]
- 96. Dellon ES, Gibbs WB, Fritchie KJ et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2009;7:1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dellon ES, Erichsen R, Baron JA et al. The increasing incidence and prevalence of eosinophilic oesophagitis outpaces changes in endoscopic and biopsy practice: national population-based estimates from Denmark. Aliment Pharmacol Ther 2015;41:662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Giriens B, Yan P, Safroneeva E et al. Escalating incidence of eosinophilic esophagitis in Canton of Vaud, Switzerland, 1993–2013: a population-based study. Allergy 2015;70:1633–9. [DOI] [PubMed] [Google Scholar]
- 99. Gomes J, Antunes A, Carvalho A et al. Dysphagia as a manifestation of esophageal tuberculosis: a report of two cases. J Med Case Rep 2011;5:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Honda M, Izumi Y, Miura A et al. Linitis plastica-type adenocarcinoma of the esophagus: a case report. Esophagus 2010;7:225–9. [Google Scholar]
- 101. Katzka DA, Smyrk TC, Bruce AJ et al. Variations in presentations of esophageal involvement in lichen planus. Clin Gastroenterol Hepatol 2010;8:777–82. [DOI] [PubMed] [Google Scholar]
- 102. Dellon ES, Gonsalves N, Hirano I et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013;108:679–92. [DOI] [PubMed] [Google Scholar]
- 103. Lucendo AJ, Molina-Infante J, Arias A et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J 2017;5:335–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liacouras CA, Spergel JM, Ruchelli E et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol 2005;3:1198–206. [DOI] [PubMed] [Google Scholar]
- 105. Gonsalves N, Policarpio-Nicolas M, Zhang Q et al. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc 2006;64:313–9. [DOI] [PubMed] [Google Scholar]
- 106. Saffari H, Peterson KA, Fang JC et al. Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: Implications for endoscopic biopsy [Letter]. J Allergy Clin Immunol 2012;130:798–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shah N, Lee R. Impact of high resolution manometry (HRM) and the chicago classification on the diagnosis and clinical management of patients with dysphagia [abstract]. Am J Gastroenterol 2010;105:S19. [Google Scholar]
- 108. Narayanan A, Pande G, Siyad I et al. Use of high resolution manometry for the evaluation of dysphagia [abstract T1245]. Gastroenterology 2009;136:A530. [Google Scholar]
- 109. Fox M, Hebbard G, Janiak P et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil 2004;16:533–42. [DOI] [PubMed] [Google Scholar]
- 110. Kindt S, Rommel N, Tack J. Diagnostic (Dis)agreement between conventional manometry criteria and the Chicago classification of esophageal motility disorders in tertiary care patients [abstract 64]. Gastroenterology 2010;138:S13. [Google Scholar]
- 111. Teramoto O, Sobrino-Cossio S, Gollas A et al. High-resolution manometry: Indications and advantages in the clinical practice compared with conventional manometry [abstract 192]. Neurogastroenterol Motil 2009;21:59.18823291 [Google Scholar]
- 112. Aziz Q, Fass R, Gyawali CP et al. Functional esophageal disorders. Gastroenterology 2016;150:1368–79. [DOI] [PubMed] [Google Scholar]
- 113. DeVault KR. Chapter 13: Symptoms of esophageal disease. In: Qayed E, Srinivasan S, Shahnavaz N, (eds). Sleisenger and Fordtran’s Gastrointestinal and Liver Disease Review and Assessment, 10th ed Elsevier Health Sciences: Philadelphia, PA, 2016. [Google Scholar]