Abstract
The HIV-1 trans-activator of transcription (Tat) protein, interacts with psychostimulants to potentiate cocaine-reward in rodents. Sex steroids may protect against Tat-induced deficits. Female GT-tg transgenic mice conditionally-expressed Tat protein targeted to brain via a doxycycline-dependent, GFAP-linked promoter. Mice were tested for cocaine-conditioned place preference (CPP) and cocaine-induced locomotion when in the proestrous (high-hormone) or diestrous (low-hormone) phases of their estrous cycle. Cocaine-CPP was potentiated by Tat induction via 50, 100, or 125 (but not 25) mg/kg doxycycline daily treatment for 7 days. Diestrous mice exposed to Tat protein demonstrated significantly greater cocaine-CPP than did proestrous mice. Tat induction interacted with estrous cycle to decrease acute cocaine-induced locomotion among Tat-induced diestrous mice, but not their uninduced or proestrous counterparts, and attenuated cocaine-sensitization. In a cocaine-challenge, previously cocaine-sensitized mice demonstrated greater cocaine-locomotion over cocaine-naive counterparts and Tat-induction attenuated locomotion. Altogether, data demonstrate Tat and circulating sex steroid influences over cocaine-reward and psychostimulation.
Keywords: Cocaine, conditioned place preference, estrous cycle, human immunodeficiency virus, NeuroAIDS, sensitization, steroid hormones, trans-activator of transcription
INTRODUCTION
Psychostimulant use is associated with the progression of human immunodeficiency virus (HIV) to acquired immune deficiency syndrome (AIDS) [1, 2]. Preclinical and clinical data support the notion that HIV virotoxins influence drug effects once use has been initiated [3, 4], suggesting a dynamic relationship between HIV and drug abuse. In particular, psychostimulants such as cocaine may interact with the HIV-1 viral regulatory protein, trans-activator of transcription (Tat), to potentiate drug effects.
Tat and cocaine both inhibit the dopamine transporter (DAT), potentially promoting synergistic interactions. In rodent models, Tat allosterically inhibits the DAT [5, 6] and modulates DAT cell surface expression (with downregulation observed in rat striatal synaptosomes [5-7] and upregulation demonstrated in mouse midbrain [8]). On the human DAT, mutation of a residue that is integral to Tat-recognition attenuates Tat's inhibitory effects on dopamine (DA) uptake [9]. These inhibitory actions on DAT may underlie functional effects for psychostimulants. In human fetal neuronal cultures, the neurotoxicity of sublethal exposure to the HIV-1 proteins Tat and glycoprotein 120 is potentiated 7-8 fold when the psychostimulants methamphetamine or cocaine are co-administered [10]. Thus, interactions between Tat and psychostimulants may be important contributors to HIV-1 pathology.
In people and animals, psychostimulant use is influenced by gender and hormonal status. Women are less likely than men to use cocaine, but demonstrate more severe behavioral pathology once use is initiated, progressing to cocaine-dependence more quickly and demonstrating more frequent relapse when cessation is attempted [11-14]. Similar effects of psychostimulants are observed among rodents with females demonstrating greater acquisition, selfadministration, and drug reinstatement compared to males [15-18]. Circulating steroids contribute to gender differences as cocaine acquisition and self-administration are greater when estradiol is unopposed in the estrous cycle [19, 20] or is exogenously administered [21-23]. Conversely, progesterone administration attenuates escalation of cocaine self-administration and seeking [24, 25] and antagonizes enhancement by estradiol when co-administered [21, 26]. These findings are of clinical importance given varied literature demonstrating oral progesterone attenuates cocaine craving and subjective pleasurable effects among men and/or women [27-31] (albeit, these effects are not always observed across human samples [32]).
We hypothesized that HIV-1 Tat would interact with cocaine to influence psychostimulant-reward and -locomotor behavior among female mice. Furthermore, we expected these effects to be potentiated at a time in the estrous cycle when potentially-protective sex steroids were at nadir (diestrus) compared to when they were elevated (proestrus). To examine this, we utilized female GFAP/Tat bigenic (GT-tg) mice (originally derived by Dr. Johnny He). GT-tg mice have a GFAP-localized, doxycycline-inducible promoter that drives expression of CNS-targeted Tat1-86 [33]. The copy numbers of the Tat transgene correlate with Tat RNA expressed in this model [33], and doxycycline exposure correlates dose-dependently with Tat protein content in whole brain [34, 35]. Tat expression in this model recapitulates clinical findings of HIV characterized by central macrophage/monocyte infiltration, T-lymphocyte infiltration, neuronal cell death [33], dose-dependent reductions in limbic gray matter density [36], perturbed cognition [34] and affect [35, 37], as well as potentiated locomotion and reward among males administered acute cocaine [38]. Using this model, we have previously demonstrated prophylactic effects of sex steroids over Tat-induced increases in anxiety-like behavior of female mice [37].
MATERIALS AND METHODS
All methods and procedures used were pre-approved by the Institutional Animal Care and Use Committee at the Torrey Pines Institute for Molecular Studies (Port Saint Lucie, FL), and were conducted in accordance with ethical guidelines defined by the National Institutes of Health (NIH Publication No. 85-23).
Subjects and Housing
Female GT-tg mice (N = 264) were generated in the colony in the Torrey Pines Institute for Molecular Studies vivarium (Port Saint Lucie, FL). GT-tg breeders have been previously described [33, 35] and were back-crossed 7 generations onto the C57BL/6J strain. Mice (approximately 70 days of age) were housed 4-5/cage and were maintained in a temperature- and humidity-controlled room on a 12: 12 h light/dark cycle (lights off at 19:00 h) with ad libitum access to food and water.
Determination of Estrous Cycle Phase
Estrous cycle phase was determined as previously described [37]. Given that environmental stimuli can influence estrous cycle synchrony within home-cages [39], the cycle of each mouse was individually assessed daily and manipulations were counterbalanced across home-cages. Briefly, epithelial tissue was collected via vaginal lavage and assessed under a light microscope at 50 X and 200 X magnification. Cycle phases were tracked for 7 consecutive days prior to testing. As in previous studies [37], mice with irregular cycles (< 1-2 proestrous phases) were not tested. Behavioral assessments occurred in the afternoon (~13:00 h) on the day of proestrus (indicated by a majority presence of nucleated epithelial cells, when concentrations of estrogens have peaked and progestogens are rising to peak) or the day of diestrus (indicated by a majority presence of leukocytic cells, when concentrations of estrogens are beginning to rise and progestogens have fallen to nadir) [40-42]. Testing at these times cannot parse the individual effects of estrogens or progestogens but is associated with reduced anxiety-like responding in the proestrous, compared to the diestrous, phases of the cycle [37] demonstrating behavioral differences between high- vs low-hormonal milieux.
Chemicals
Chemicals, obtained from Sigma-Aldrich (St. Louis, MO), were dissolved in sterile 0.9% saline and diluted to concentration (0.1 ml volume administered per 10 g body weight). Doxycycline hyclate was administered once daily for 7 consecutive days. Studies of doxycycline dose (0, 25, 50, 100, or 125 mg/kg, i.p.) and treatment duration (0, 1, 3, 5, 7, or 14 days) have revealed exposure-dependent elevation of central HIV-1 Tat protein in the GT-tg bigenic brain [34, 35] commensurate with behavioral [34, 35, 37, 38] and neurodegenerative [36] changes. Among male GT-tg mice, Tat-induction via this doxycycline regimen has been demonstrated to potentiate the rewarding and acute locomotor effects of cocaine (10 mg/kg) [38], prompting the assessment at these doses for interactions with hormonal fluctuations as described below.
Cocaine-Conditioned Place Preference
Mice were conditioned in an unbiased, counterbalanced CPP paradigm modified from previous methods [43, 44]. Briefly, the amount of time subjects spent in each of three compartments (two cue-differentiated outer compartments: 25 × 25 × 25 cm, separated by a middle compartment: 8.5 × 25 × 25 cm, each tracking movement via infrared beams; San Diego Instruments, San Diego, CA) was measured over a 30 min testing period for a pre-CPP evaluation prior to cocaine-conditioning. Over the following two days, cocaine placeconditioning was achieved each day by restricting mice to a pre-determined compartment for 30 min following saline administration (saline-associated side) and, 4 h later, restricting mice to the opposing compartment for 30 min following cocaine (10 mg/kg, s.c.) administration (cocaine-associated side). The cocaine-associated side was counterbalanced across experimental groups to obviate confounds associated with side-preference. Post-CPP evaluation was then conducted on the next day of proestrus or diestrus (whichever occurred first). Data are expressed as the difference in time (s) + SEM spent on the cocaine- vs saline- associated chamber. As such, positive values indicate a greater preference for cocaine compared to saline, whereas negative values would indicate an aversion to cocaine.
Cocaine-Induced Locomotion
Following administration of saline or cocaine (10 mg/kg, i.p.), mice were placed in the lower-left corner of a square Plexiglas box (46 x 46 x 30 cm) and allowed to explore for 5 min intervals (30 min total for acute cocaine experiments and 5 min/trial for cocaine sensitization experiments). Movement was monitored and digitally-encoded by a Noldus (Leesburg, VA) EthovisionPro3 image capture software package. The distance (cm) traveled was utilized as an index of locomotor behavior as previously described [37, 38].
Statistical Analyses
Behavioral dependent measures for pre- and post-CPP, and ambulations in the CPP appartus, were assessed via separate two-way analyses of variance (ANOVAs) with Tat-induction status (uninduced with saline or induced with doxycycline, 25-125 mg/kg) and hormone condition (proestrus or diestrus) as between-subjects factors. Cocaine challenge was assessed via three-way ANOVA with cocaine status (previously cocaine-naive or previously cocaine-sensitized) as an additional between-subjects factor to Tat induction status and hormone condition. Cumulative cocaine-induced locomotion in the Noldus apparatus (distance traveled, cm) was assessed in two experiments via separate repeated measures ANOVAs. In the acute cocaine-administration experiment, Tat-induction status (induced or uninduced) and hormone condition (proestrus or diestrus) were utilized as between-subjects factors with time (0-30 min) as the within-subjects factor. In the cocaine-sensitization experiment, Tat-induction status (induced or uninduced) and cocaine status (saline or cocaine 10 mg/kg) were utilized as the between-subjects factors with trial (baseline-cocaine trial#4) as the within-subjects factor. Fisher’s Protected Least Significant Difference post-hoc tests determined group differences following main effects. Interactions were delineated via simple main effects and main effect contrasts with alpha controlled for multiple comparisons. Analyses were considered significant when p < 0.05.
RESULTS
HIV-1 Tat and Estrous Cycle Phase Influence Cocaine-Reward
To assess the influence of HIV-1 Tat protein and estrous cycle on cocaine-reward, mice had their estrous cycles tracked for one week (days 1-7) prior to undergoing a week-long induction of CNS-expressed Tat protein (via daily administration of doxycycline, 25-125 mg/kg, i.p., days 8-14), or were left as uninduced negative controls via daily administration of saline (i.p., days 8-14; (Fig. 1A). Consistent with prior reports [37], doxycycline did not interrupt estrous cycling (all mice demonstrated 1-2 proestrous smears over the 7 days). All mice (Tat-induced and uninduced) were preference-tested in the CPP apparatus on day 15 (open triangle) and underwent 2 days of cocaine-conditioning on days 16 and 17 (squares; (Fig. 1A). Mice had their estrous cycles assessed over the next 7 days and were preference tested (triangles) on the next day of proestrus or diestrus (whichever occurred first over days 18-24; (Fig. 1A). The mean time from the end of conditioning to final CPP testing was 4 ± 0.1 days.
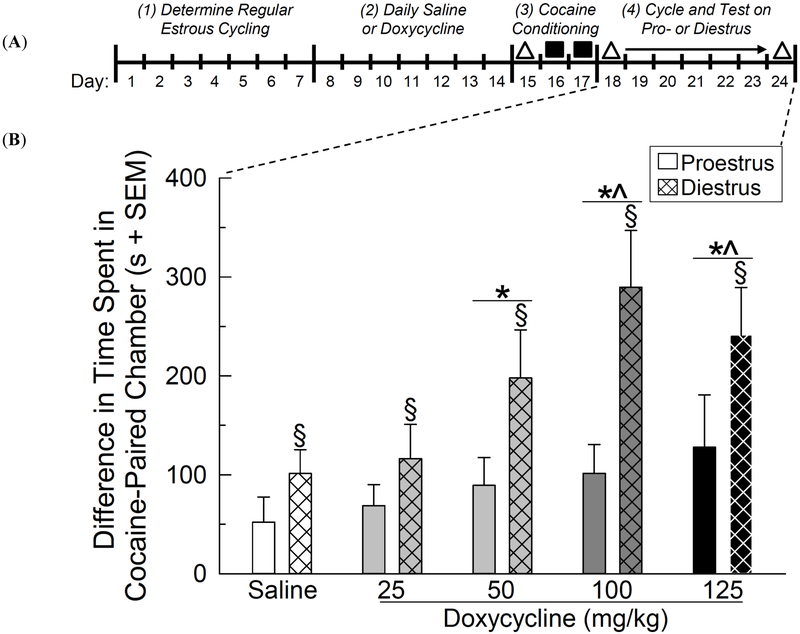
Fig. (1).
(A) Time-course schematic of conditioned place preference (CPP) experiment. Across days 1-7, the estrous cycle of GT-tg female mice was assessed daily. Mice received control saline (0.9%, i.p.) or had central HIV-1 Tat protein induced via administration of doxycycline (25-125 mg/kg, i.p.) daily on days 8-14. Mice were preference tested (triangle on day 15) in the CPP apparatus before conditioning with cocaine (10 mg/kg, s.c.; squares on days 16-17). Post-conditioning preference testing occurred once per animal (over days 18-24; triangles) on the next occurrence of proestrus or diestrus (whichever occurred first). (B) The difference in time (s + SEM) spent in the cocaine-paired chamber following cocaine-CPP among proestrous or diestrous GT-tg female mice (n = 16-20/group) that had HIV-1 Tat induced or were uninduced controls. § significant main effect for diestrous mice to differ from proestrous mice. * significant main effect to differ from uninduced saline-controls. ^ significant main effect to differ from Tat-induced mice administered Dox, 25 mg/kg, (repeated measures ANOVA, p < 0.05).
Exposure to HIV-1 Tat protein potentiated cocaine-CPP in a manner dependent on the dose of doxycycline used for induction [F(4,160) = 3.73, p < 0.05], and cocaine-CPP was greater among diestrous, compared to proestrous, mice [F(1,160) = 16.81, p < 0.05] (Fig. 1B). All groups demonstrated a positive CPP for cocaine; however, compared to saline administration, cocaine-CPP was significantly greater when Tat protein was induced with doxycycline at 50 (p < 0.05), 100 (p = 0.002), or 125 (p = 0.005) mg/kg (Fig. 1B). Tat-induction via doxycycline at 100 (p = 0.01) or 125 (p = 0.02) mg/kg significantly potentiated cocaine-CPP beyond that observed at 25 mg/kg (which itself did not differ from control saline; (Fig. 1B). No effects of Tat-induction were observed on pre-conditioning chamber preferences (Table 1, top) or post-conditioning ambulations (calculated by horizontal beam-breaks in the CPP apparatus; Table 1, bottom).
Table 1.
The difference in time spent in the cocaine-paired chamber prior to conditioned place preference (CPP) and the total ambulations (horizontal beam-breaks) in the CPP apparatus following conditioning among proestrous or diestrous GT-tg female mice (n = 16-20/group) that were administered vehicle saline (0.9 %, i.p., for 7 days) as uninduced controls or doxycycline (DOX; 25 - 125 mg/kg, i.p., 7 days) to induce HIV-1 Tat1-86 expression.
| Proestrus | Diestrus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Saline | DOX 25 mg/kg |
DOX 50 mg/kg |
DOX 100 mg/kg |
DOX 125 mg/kg |
Saline | DOX 25 mg/kg |
DOX 50 mg/kg |
DOX 100 mg/kg |
DOX 125 mg/kg |
|
| Pre-CPP Time Difference in Cocaine-Chamber (s ± SEM) | −2 ± 43 | −16 ± 50 | 10 ± 52 | −68 ± 67 | −89 ± 52 | −6 ± 44 | −36 ± 54 | −41 ± 66 | 24 ± 38 | −14 ± 57 |
| Total Ambulations in CPP Apparatus (mean ± SEM) | 1472 ± 32 | 1452 ± 62 | 1351 ± 68 | 1437 ± 139 | 1373 ± 66 | 1504 ± 67 | 1383 ± 32 | 1326 ± 60 | 1321 ± 130 | 1384 ± 57 |
Tat-Induction Interacts with the Estrous Cycle to Suppress Acute Cocaine-Potentiated Locomotion
To assess the influence of HIV-1 Tat and estrous cycle on cocaine-induced locomotion, the estrous cycles of all mice were tracked for one week (days 1-7), followed by administration of control saline or doxycycline for 7 days (days 8-14) at the previously optimal dose (100 mg/kg; dosing previously shown to optimally express Tat protein in whole brain [34, 35]; (Fig. 2A). Estrous cycles were assessed over the next 7 days and all mice were motor tested (closed circles) on the next day of proestrus or diestrus (whichever occurred first over days 15-21; (Fig. 2A). The mean time from the end of treatment to motor testing was 3 ± 0.4 days. On the day of testing, proestrous or diestrous mice were acutely administered saline (0.9 %, i.p.), had motor activity assessed for 15 min, then were acutely administered cocaine (10 mg/kg, i.p.), and had motor activity assessed for the remaining 15 min (Fig.2A).
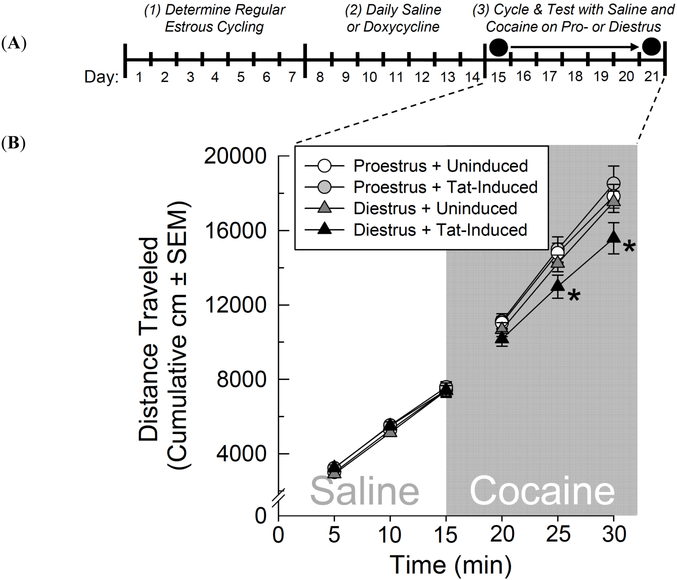
Fig. (2).
(A) Time-course schematic of acute cocaine-induced locomotion experiment. GT-tg mice had their estrous cycle tracked (days 1-7). Mice received control saline (0.9%, i.p.) or had central HIV-1 Tat protein induced via administration of an optimal doxycycline dose (100 mg/kg, i.p.; days 8-14). Mice were tested only once for locomotor behavior in an open field (circles), after receiving saline (at min 0) followed by cocaine (10 mg/kg, i.p. at min 15), on the next occurrence of proestrus or diestrus (whichever occurred first over days 15-21). (B) The cumulative distance traveled (cm ± SEM) in an open field among uninduced or Tat-induced GT-tg female mice (n = 6-8/group) on days of proestrus or diestrus administered saline prior to cocaine. * significant interaction wherein cocaine significantly increased cumulative locomotion amongst all groups except Tat-induced diestrous mice, (repeated measures ANOVA, p < 0.05).
Induction of HIV-1 Tat protein, estrous cycle phase, and time-course significantly interacted to influence cumulative locomotor behavior [F(5,130) = 2.72, p < 0.05] (Fig. 2B). Groups did not differ following saline administration; however, Tat-induced diestrous mice demonstrated a significant attenuation of cocaine-induced locomotion 10 min following cocaine administration (compared to uninduced or induced proestrous mice, p = 0.03-0.04) and 15 min following cocaine administration (compared to any other group, p < 0.01 - 0.05; (Fig. 2B).
Sensitization to Cocaine-Potentiated Locomotion is Attenuated by Tat-Induction Among Pro- and Diestrous Mice
Estrous cycle influenced the initial locomotor response to cocaine; however, it was not known whether endogenous fluctuations in cycle would influence Tat/cocaine interactions once exposure was sensitized. After a one week assessment of estrous cycle (days 1-7), mice underwent a baseline motor test on day 8 (open circle; (Fig. 3A). After one week of Tat-induction via optimal doxycycline exposure (100 mg/kg, i.p., days 9-15), mice underwent a postinduction motor test on day 16 to ensure that nonspecific effects of doxycycline did not account for variance in behavior (open circle; (Fig. 3A). Mice were then administered saline (0.9 %) or cocaine (10 mg/kg, i.p.) and given a test of locomotion once daily for 4 consecutive days to assess cocaine locomotor-sensitization (closed circles on days 17-20; (Fig. 3A). Mice were rested for one week (days 21-27), and then had their estrous cycles assessed over the next 7 days (days 28-34) prior to receiving a cocaine challenge and locomotor test (closed circles) on the next day of proestrus or diestrus (whichever occurred first; (Fig. 3A). The mean time from the beginning of cycling to cocaine challenge was 2 ± 0.2 days.
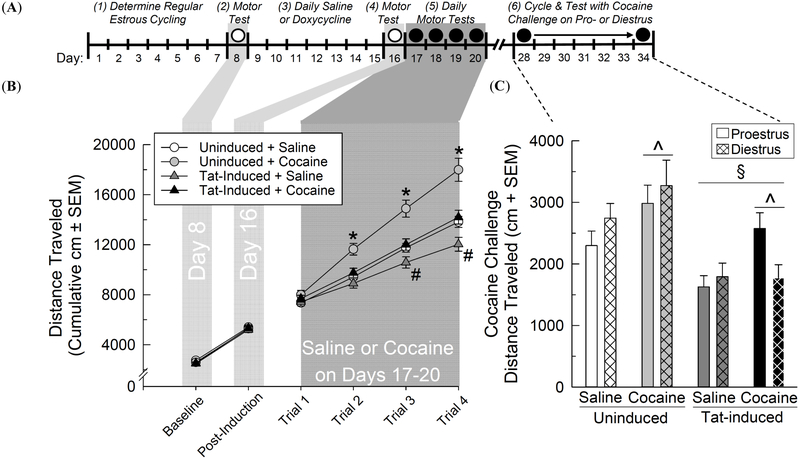
Fig. (3).
(A) Time-course schematic of cocaine locomotor sensitization experiment. GT-tg mice had their estrous cycle tracked (days 1-7). Baseline motor behavior was assessed in an open field (open circle; day 8). Mice received control saline (0.9%, i.p.) or had central HIV-1 Tat protein induced via administration of an optimal doxycycline dose (100 mg/kg, i.p.; days 9-15). Motor behavior was assessed following induction (open circle; day 16). Mice were tested daily for locomotor response to saline or cocaine (10 mg/kg, i.p.; closed circles, days 17-20). After one week, locomotor response to a cocaine challenge was assessed amongst all groups on the next occurrence of proestrus or diestrus (whichever occurred first over days 28-34). (B) The cumulative distance traveled (cm ± SEM) in an open field among uninduced or Tat-induced GT-tg female mice (n = 16/group) administered saline and/or cocaine. * significant interaction wherein uninduced mice exposed to cocaine differ from other groups. # significant interaction wherein Tat-induced controls differ from other groups, (repeated measure ANOVA, p < 0.05). (C) The total distance traveled (cm + SEM) in an open field after a cocaine challenge when saline- or cocaine-exposed mice (n = 8/group) were on diestrus or proestrus. ^ significant main effect for cocaine-exposed mice to differ from saline-exposed controls. § significant main effects for Tat-induced mice to differ from uninduced controls, (three-way ANOVA, p < 0.05).
Tat induction, cocaine administration, and trial condition significantly interacted to influence cumulative locomotor behavior [F(5,300) = 3.82, p < 0.05] (Fig. 3B). Motor behavior did not significantly differ among groups at baseline (day 8), immediately post-Tat-induction (day 16), or on the first trial of saline or cocaine-sensitization (day 17; (Fig. 3B). However, uninduced cocaine-administered mice demonstrated significantly more cumulative distance traveled over cocaine trials 2 - 4 (days 18-20) than any other group (p < 0.0001 - 0.0006; (Fig. 3B). Only on cocaine trials 3 and 4 (days 19 and 20) did Tat-induced cocaine-administered mice significantly demonstrate more cumulative distance traveled than their respective Tat-induced saline-administered controls (p = 0.02 - 0.04; (Fig. 3B). Uninduced and Tat-induced saline-administered controls did not significantly differ from one another on any trial (Fig. 3B).
When cocaine challenge was assessed (on days 28-34), induction of HIV-1 Tat [F(1,56) = 22.21, p < 0.05] and prior cocaine exposure [F(1,56) = 8.18, p < 0.05] significantly influenced locomotor responding to cocaine (Fig. 3C). Mice previously-administered cocaine throughout the sensitization phase traveled a significantly greater distance in response to a cocaine challenge than did mice that had previously received only saline (irrespective of Tat or estrous cycle condition; (Fig. 3C). Mice exposed to Tat protein traveled significantly less in response to a cocaine challenge than did their uninduced counterparts (irrespective of prior cocaine experience or estrous cycle; (Fig. 3C). Tat-induced mice that were proestrous demonstrated a notably greater locomotor response to a cocaine challenge than did their diestrous counterparts (Fig. 3C), but this did not reach statistical significance (p = 0.07, n.s.).
DISCUSSION
The present hypotheses that induction of HIV-1 Tat would interact with cocaine-CPP, psychostimulation, and sensitization were upheld. Consistent with findings in male GT-tg mice [38], Tat protein potentiated cocaine-CPP in an exposure-dependent manner among females. Optimal Tat induction was also observed to attenuate psychomotor stimulation during cocaine sensitization, as well as a cocaine challenge, consistent with findings in ovariectomized female [45] and gonadally-intact male [46] rats. These data also upheld the hypothesis that fluctuations in estrous cycle would influence Tat effects on psychostimulant reward and locomotor behavior. Cocaine-CPP was the greatest amongst diestrous females and this effect was potentiated when Tat was induced in an exposure-dependent manner (with optimal effects at 100 mg/kg/d doxycycline for 7 days). Introducing an acute cocaine challenge following Tat induction attenuated cocaine's psychostimulant effects when females were in the diestrous (but not proestrous) phase of their cycle. Notably, estrous cycle phase did not significantly influence the psychomotor response to a cocaine challenge once cocaine responding was sensitized (albeit, diestrous mice demonstrated a notable, but non-significant, reduction in psychomotor behavior). Together, these data demonstrate that induction of HIV-1 Tat enhances cocaine-CPP and perturbs acute and sensitized psychomotor responding in female mice. Furthermore, these findings demonstrate acute cocaine effects to be greater when endogenous steroids are at nadir but cyclical hormone fluctuations exert less influence over chronic exposure.
Dopaminergic substrates are important clinical targets for the toxic effects of Tat protein, and may have partly influenced cocaine responding in the present study. In people, a progression to AIDS has long been associated with neuronal loss in DA-rich brain regions [47] and neuroleptic drugs that perturb DA function produce Parkinsonian-like symptoms in this population ([reviewed in [4]). Decreases in bioavailable DAT have also been reported among seropositive patients with HIV dementia (but not those without neurologic impairment) [48, 49]. Chronic cocaine use likewise confers dopaminergic dysfunction, reducing striatal DA and DAT expression [50], supporting a point of clinical convergence for cocaine and HIV interaction. Similar findings are observed in non-human primates with simian immunodeficiency virus reducing DA and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the nucleus accumbens [51, 52]. Herein we observed potentiation of CPP to acute cocaine and attenuation of locomotor sensitization in response to chronic cocaine. Importantly, the DA system is not the only target to interact with both Tat and cocaine, particularly given recent demonstrations of L-type Ca2+ channel-mediated synergy between Tat and cocaine to produce excitotoxic effects in cortex [53]. However, Tat-mediated DA dysfunction is presently better characterized in rodent models.
In rats, acute exposure to HIV-1 Tat protein is sufficient to cause persistent changes in central DA metabolism and DAT expression with functional outcomes for cocaine response. Acute intra-accumbal infusion of HIV-1 Tat causes a persistent decrease in the DA metabolites DOPAC and HVA independent of prior cocaine experience, and attenuates KCl-stimulated DA release [46, 54]. In males, cocaine-induced DA efflux is dampened by intra-accumbal Tat administration and is nearly abolished by repeated cocaine administration, supporting the notion of Tat-mediated DAT dysfunction [54]. Even when infused after cocaine sensitization has already occurred, Tat sensitizes DA release and attenuates cocaine-locomotor sensitization [46]. These effects are consistent among females that have been ovariectomized (to eliminate cyclical steroid fluctuations), where intra-accumbal injection of a shorter Tat peptide sequence (Tat1-72) increases psychomotor stimulation by intravenous cocaine acutely, but attenuates locomotor sensitization when cocaine is administered repeatedly [45]. Congruent with these findings, we used gonadally-intact female mice stratified by estrous cycle phase and observed acute cocaine to stimulate locomotion amongst all groups with the exception of diestrous mice, demonstrating an acute interaction with fluctuating steroids. Consistent with prior reports, we observed that exposure to Tat protein attenuates locomotor sensitization arising from repeated administration of cocaine. Investigations using the potent dopaminergic psychostimulant methamphetamine report a 5-fold reduction in striatal DA following Tat injections to the striatum of rats [4]. As such, Tat may act to degrade DA neurons and/or nerve terminal sites of function, including DATs, to acutely potentiate cocaine effects, but attenuate sensitization following repeated cocaine exposure. Toxic effects on neurons and integrated DA systems may have important consequences for addiction and may be involved in mechanisms of steroid-mediated protection.
Elevated steroid milieu may confer limited prophylaxis over acute Tat toxicity, perhaps limiting effects on cocaine-reward and psychomotor response. In vitro, sex steroids suppress Tat-mediated HIV replication. Viremia is reduced in peripheral blood mononuclear cells collected from premenopausal women when they are in the high-hormone phase of their menstrual cycle [55]. Similarly, progesterone enhances the efficacy of antiretroviral therapy in acutely infected monocytic cell lines [56]. These effects are shown to be both partially dependent on [56], and independent of [55], Tat-modulation of the HIV LTR, suggesting multiple mechanisms by which sex steroids may interact with HIV. Importantly, sex steroid interactions with Tat activity extend to its neurotoxic effects. Estradiol and other agonists of the β isoform of its cognate receptor inhibit Tat-mediated cell death and pro-apoptotic signaling in cultured rat cortical neurons [57, 58]. In human neuronal cultures, estradiol can ameliorate effects of Tat to synergize with cocaine or methamphetamine, destabilizing mitochondrial membranes and promoting cell death [59]. Limited protection over Tat1-72 effects on human fetal neuronal tissues has also been observed for the steroid hormone precursor cholesterol and progesterone [60]. Notably, estradiol blocks the combined oxidative stress effects of this Tat sequence and glycoprotein 120 and partially reverses the reductions in DA reuptake [61], thereby potentially contributing to protection of Tat-cocaine interactions. However, the identity of the sex steroid mechanisms involved in Tat-protection are only beginning to be understood. The ability of progesterone to block Tat transactivation [56] and to mediate chemokines in peripheral blood mononuclear cells [62, 63] was found to be independent of actions at cognate hormone response elements [56] and traditional progestin receptors, respectively [62, 63]. These data suggest the involvement of nontraditional sites of steroid action to influence Tat effects. In human glia, estradiol also inhibits HIV LTR activity [64], an effect that is enhanced by down-regulation of the α isoform of the estrogen receptor [65], reinforcing the importance of novel steroid signaling. Indirect effects of steroids to attenuate inflammatory signaling must also be considered. Estradiol is demonstrated to block Tat1-72-induced NF-κB activation and upregulation of IL-1β in human endothelial cells [66] as well as downstream MAP kinase-mediated microglial activation [67] which likely diminish neurotoxicity.
Cocaine behavioral sensitization is influenced by natural fluctuations in steroid hormones; however, exogenous steroid administration may have greater efficacy in the amelioration of HIV-1 Tat/cocaine-mediated behavior. In female rats, psychomotor response to repeated cocaine administration is greatest during the estrous cycle when estradiol is expected to be high and unopposed by progesterone [68]. In prior studies, exogenous progesterone (but not estradiol) attenuated cocaine-sensitized stereotypy [68]. Conversely, exogenous estradiol potentiates acute cocaine stereotypies and repeated cocaine-locomotion [69]. Using the GT-tg model, we have previously observed exogenous high-dose progesterone to attenuate Tat-induced impairments in affective behavior to a greater degree than natural steroid fluctuations, or exogenous high-dose estradiol (which impaired affect on its own) [37]. The divergence between exogenously-administered steroids may indicate the importance of the estradiol: progestogen ratio. Manipulating estradiol and progesterone concentrations demonstrates psychomotor activity of acutely administered cocaine to be attenuated when progesterone largely opposes estradiol, but this effect is reversed as estradiol is increased to ~5-fold physiological concentrations [70]. These effects of progesterone may further be influenced by actions of downstream progestogen metabolites, such as allopregnanolone, the biosynthesis of which is altered by cocaine in a dose-dependent manner [71], and which may be required for progesterone's effects to attenuate cocaine-seeking in female rats [72, 73]. Thus, identifying the effects and mechanisms of estradiol/progestogen therapies is warranted for future research into the modulation of drug reward and abuse by HIV.
It needs to be noted that the synergy of Tat and psychostimulants, as well as the efficacy of steroid hormones to influence these effects, may be subject to the HIV subtype under investigation. While our data are largely consistent with preclinical findings across rodent species and Tat sequences (72 or 86 amino acid-long peptides), some of these effects may be expected to differ across HIV clades. In human brain microvascular epithelial cells, clade B Tat was shown to disrupt the blood-brain barrier to a greater extent than clade C Tat; effects that were potentiated by cocaine [74, 75]. As well, HIV replication in peripheral blood mononuclear cells was greater in clades B and C compared to other subtypes, and the efficacy of estradiol or progesterone to attenuate this depended on both HIV clade and whether the cells were obtained from a male or female host [76]. Future investigations will need to parse out the extent to which the important mechanisms for the present effects are conserved across HIV subtypes, and the extent to which prophylactic effects of sex steroids are efficacious across males and females.
CONCLUSION
The present report demonstrates the capacity for CNS-targeted HIV-1 Tat to potentiate cocaine-reward and attenuate cocaine-sensitized locomotor behavior in gonadally-intact female mice. Natural fluctuations in sex steroid milieu interacted with Tat induction such that cocaine's effects on reward and acute psychomotor behavior were greater when potentially-protective sex steroids were at nadir. These data demonstrate the important prophylactic protective effects that sex steroids may confer in Tat-mediated psychostimulant responses.
ACKNOWLEDGEMENTS
We thank Johnny He for the gift of the GT-tg transgenic breeder mice. This work was supported by funding from the National Institute of Mental Health (MH085607 to JPM) and funds from the State of Florida, Executive Office of the Governor’s Department of Economic Opportunity. The sources of funding had no involvement in the planning, execution, or presentation of these data in any manner.
ABBREVIATIONS
- AIDS
Acquired Immune Deficiency Syndrome
- ANOVA
Analysis of variance
- Ca2+
Calcium
- CPP
Conditioned place preference
- DA
Dopamine
- DAT
Dopamine transporter
- DOPAC
3,4-Dihydroxyphenylacetic acid
- DOX
Doxycycline
- GFAP
Glial Fibrillary Acidic Protein
- HIV
Human Immunodeficiency Virus
- HVA
Homovanillic acid
- i.p.
Intraperitoneal
- LTR
Long terminal repeat
- MAP
Mitogen-activated protein
- s.c.
Subcutaneous
- Tat
Trans-activator of transcription
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content presents no conflict of interest.
REFERENCES
- [1].Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr 2009; 50: 93–9 [DOI] [PubMed] [Google Scholar]
- [2].Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS 1999; 13: 257–62. [DOI] [PubMed] [Google Scholar]
- [3].Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Mol Neurobiol 2011; 44: 102–10. [DOI] [PubMed] [Google Scholar]
- [4].Nath A, Hauser KF, Wojna V, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr 2002; 31: S62–9. [DOI] [PubMed] [Google Scholar]
- [5].Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2β-carbomethoxy-3-β-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther 2009; 329: 1071–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhu J, Ananthan S, Mactutus CF, Booze RM. Recombinant human immunodeficiency virus-1 transactivator of transcription1-86 allosterically modulates dopamine transporter activity. Synapse 2011; 65: 1251–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Midde NM, Gomez AM, Zhu J. HIV-1 Tat protein decreases dopamine transporter cell surface expression and vesicular monoamine transporter-2 function in rat striatal synaptosomes. J Neuroimmune Pharmacol 2012; 7: 629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Perry SW, Barbieri J, Tong N, et al. Human immunodeficiency virus-1 Tat activates calpain proteases via the ryanodine receptor to enhance surface dopamine transporter levels and increase transporter-specific uptake and Vmax. J Neurosci 2010; 30: 14153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Midde NM, Huang X, Gomez AM, et al. Mutation of tyrosine 470 of human dopamine transporter is critical for HIV-1 Tat-induced inhibition of dopamine transport and transporter conformational transitions. J Neuroimmune Pharmacol 2013; 8: 975–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nath A, Anderson C, Jones M, et al. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol 2000; 14: 222–7. [DOI] [PubMed] [Google Scholar]
- [11].Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005; 180: 169–76. [DOI] [PubMed] [Google Scholar]
- [12].Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat 1993; 10: 63–6. [DOI] [PubMed] [Google Scholar]
- [13].McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis 1996; 184: 616–22. [DOI] [PubMed] [Google Scholar]
- [14].Westermeyer J, Kopka S, Nugent S. Course and severity of substance abuse among patients with comorbid major depression. Am J Addict 1997; 6: 284–92. [PubMed] [Google Scholar]
- [15].Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008; 197: 237–46. [DOI] [PubMed] [Google Scholar]
- [16].Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 2000; 148: 196–200. [DOI] [PubMed] [Google Scholar]
- [17].Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 2004; 172: 443–9. [DOI] [PubMed] [Google Scholar]
- [18].Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav 2004; 78: 199–207. [DOI] [PubMed] [Google Scholar]
- [19].Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend 2007; 89: 183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 2000; 152: 132–9. [DOI] [PubMed] [Google Scholar]
- [21].Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology 2006; 31: 129–38. [DOI] [PubMed] [Google Scholar]
- [22].Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 2001; 68: 641–6. [DOI] [PubMed] [Google Scholar]
- [23].Zhao W, Becker JB. Sensitization enhances acquisition of cocaine self-administration in female rats: estradiol further enhances cocaine intake after acquisition. Horm Behav 2010; 58: 8–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology 2009; 34: 343–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol 2007; 15: 461–71. [DOI] [PubMed] [Google Scholar]
- [26].Yang H, Zhao W, Hu M, Becker JB. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behav Brain Res 2007; 184: 174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Evans SM. The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans. Exp Clin Psychopharmacol 2007; 15: 418–26. [DOI] [PubMed] [Google Scholar]
- [28].Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology 2006; 31: 659–74. [DOI] [PubMed] [Google Scholar]
- [29].Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav 2002; 72: 431–5. [DOI] [PubMed] [Google Scholar]
- [30].Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol 1999; 7: 274–83. [DOI] [PubMed] [Google Scholar]
- [31].Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav 2004; 78: 699–705. [DOI] [PubMed] [Google Scholar]
- [32].Reed SC, Evans SM, Bedi G, Rubin E, Foltin RW. The effects of oral micronized progesterone on smoked cocaine self-administration in women. Horm Behav 2011; 59: 227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol 2003; 162: 1693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res 2012; 229: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology (Berl) 2014; 231: 2349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Carey AN, Liu X, Mintzopoulos D, et al. Conditional Tat protein expression in the GT-tg bigenic mouse brain induces gray matter density reductions. Prog Neuropsychopharmacol Biol Psychiatry 2013; 43: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Paris JJ, Fenwick J, McLaughlin JP. Progesterone protects normative anxiety-like responding among ovariectomized female mice that conditionally express the HIV-1 regulatory protein, Tat, in the CNS. Horm Behav 2014; 65: 445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Paris JJ, Carey AN, Shay CF, Gomes SM, He JJ, McLaughlin JP. Effects of conditional central expression of HIV-1 Tat protein to potentiate cocaine-mediated psychostimulation and reward among male mice. Neuropsychopharmacology 2014; 39: 380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marsden HM, Bronson FH. Estrous synchrony in mice: alteration by exposure to male urine. Science 1964; 144: 1469. [DOI] [PubMed] [Google Scholar]
- [40].Michael SD. Plasma prolactin and progesterone during the estrous cycle in the mouse. Proc Soc Exp Biol Med 1976; 153: 254–7. [DOI] [PubMed] [Google Scholar]
- [41].Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia 2006; 47: 1423–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wood PA, Bove K, You S, Chambers A, Hrushesky WJ. Cancer growth and spread are saltatory and phase-locked to the reproductive cycle through mediators of angiogenesis. Mol Cancer Ther 2005; 4: 1065–75. [DOI] [PubMed] [Google Scholar]
- [43].Paris JJ, Reilley KJ, McLaughlin JP. Kappa opioid receptor-mediated disruption of novel object recognition: relevance for psychostimulant treatment. J Addict Res Ther 2011; S4: 007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Aldrich JV, Senadheera SN, Ross NC, et al. Alanine analogues of [D-Trp]CJ-15,208: novel opioid activity profiles and prevention of drug- and stress-induced reinstatement of cocaine-seeking behaviour. Br J Pharmacol 2014; 171: 3212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Harrod SB, Mactutus CF, Fitting S, Hasselrot U, Booze RM. Intra-accumbal Tat1-72 alters acute and sensitized responses to cocaine. Pharmacol Biochem Behav 2008; 90: 723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. Hyperdopaminergic tone in HIV-1 protein treated rats and cocaine sensitization. J Neurochem 2010; 115: 885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R. Nigral degeneration in acquired immune deficiency syndrome (AIDS). Acta Neuropathol 1991; 82: 39–44. [DOI] [PubMed] [Google Scholar]
- [48].Chang L, Wang GJ, Volkow ND, et al. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage 2008; 42: 869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang GJ, Chang L, Volkow ND, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain 2004; 127: 2452–8. [DOI] [PubMed] [Google Scholar]
- [50].Wilson JM, Levey AI, Bergeron C, et al. Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann Neurol 1996; 40: 428–39. [DOI] [PubMed] [Google Scholar]
- [51].Jenuwein M, Scheller C, Neuen-Jacob E, et al. Dopamine deficits and regulation of the cAMP second messenger system in brains of simian immunodeficiency virus-infected rhesus monkeys. J Neurovirol 2004; 10: 163–70. [DOI] [PubMed] [Google Scholar]
- [52].Scheller C, Sopper S, Jenuwein M, et al. Early impairment in dopaminergic neurotransmission in brains of SIV-infected rhesus monkeys due to microglia activation. J Neurochem 2005; 95: 377–87. [DOI] [PubMed] [Google Scholar]
- [53].Napier TC, Chen L, Kashanchi F, Hu XT. Repeated cocaine treatment enhances HIV-1 Tat-induced cortical excitability via over-activation of L-type calcium channels. J Neuroimmune Pharmacol 2014; 9: 354–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. The human immunodeficiency virus-1-associated protein, Tat1-86, impairs dopamine transporters and interacts with cocaine to reduce nerve terminal function: a no-net-flux microdialysis study. Neuroscience 2009; 159: 1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Asin SN, Heimberg AM, Eszterhas SK, Rollenhagen C, Howell AL. Estradiol and progesterone regulate HIV type 1 replication in peripheral blood cells. AIDS Res Hum Retroviruses 2008; 24: 701–16. [DOI] [PubMed] [Google Scholar]
- [56].Lee AW, Mitra D, Laurence J.Interaction of pregnancy steroid hormones and zidovudine in inhibition of HIV type 1 replication in monocytoid and placental Hofbauer cells: implications for the prevention of maternal-fetal transmission of HIV. AIDS Res Hum Retroviruses 1997; 13: 1235–42. [DOI] [PubMed] [Google Scholar]
- [57].Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. ER-β mediates 17β-estradiol attenuation of HIV-1 Tat-induced apoptotic signaling. Synapse 2010; 64: 829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Soy isoflavones genistein and daidzein exert anti-apoptotic actions via a selective ER-mediated mechanism in neurons following HIV-1 Tat(1-86) exposure. PLoS One 2012; 7: e37540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Turchan J, Anderson C, Hauser KF, et al. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci 2001; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kendall SL, Anderson CF, Nath A, et al. Gonadal steroids differentially modulate neurotoxicity of HIV and cocaine: testosterone and ICI 182,780 sensitive mechanism. BMC Neurosci 2005; 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat1-72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse 2006; 59: 51–60. [DOI] [PubMed] [Google Scholar]
- [62].Cabrera-Muñoz E, Fuentes-Romero LL, Zamora-Chávez J, Camacho-Arroyo I, Soto-Ramírez LE. Effects of progesterone on the content of CCR5 and CXCR4 coreceptors in PBMCs of seropositive and exposed but uninfected Mexican women to HIV-1. J Steroid Biochem Mol Biol 2012; 132: 66–72. [DOI] [PubMed] [Google Scholar]
- [63].Cabrera-Muñoz E, Hernández-Hernández OT, Camacho-Arroyo I. Role of estradiol and progesterone in HIV susceptibility and disease progression. Mini Rev Med Chem 2012; 12: 1049–54. [DOI] [PubMed] [Google Scholar]
- [64].Wilson ME, Allred KF, Bisotti AJ, Bruce-Keller A, Chuahan A, Nath A. Estradiol negatively regulates HIV-LTR promoter activity in glial cells. AIDS Res Hum Retroviruses 2006; 22: 350–6. [DOI] [PubMed] [Google Scholar]
- [65].Heron PM, Turchan-Cholewo J, Bruce-Keller AJ, Wilson ME. Estrogen receptor alpha inhibits the estrogen-mediated suppression of HIV transcription in astrocytes: implications for estrogen neuroprotection in HIV dementia. AIDS Res Hum Retroviruses 2009; 25: 1071–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lee YW, Eum SY, Nath A, Toborek M. Estrogen-mediated protection against HIV Tat protein-induced inflammatory pathways in human vascular endothelial cells. Cardiovasc Res 2004; 63: 139–48. [DOI] [PubMed] [Google Scholar]
- [67].Bruce-Keller AJ, Barger SW, Moss NI, Pham JT, Keller JN, Nath A. Pro-inflammatory and pro-oxidant properties of the HIV protein Tat in a microglial cell line: attenuation by 17β-estradiol. J Neurochem 2001; 78: 1315–24. [DOI] [PubMed] [Google Scholar]
- [68].Souza MF, Couto-Pereira NS, Freese L, et al. Behavioral effects of endogenous or exogenous estradiol and progesterone on cocaine sensitization in female rats. Braz J Med Biol Res 2014; 47: 505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Perrotti LI, Russo SJ, Fletcher H, et al. Ovarian hormones modulate cocaine-induced locomotor and stereotypic activity. Ann N Y Acad Sci 2001; 937: 202–16. [DOI] [PubMed] [Google Scholar]
- [70].Niyomchai T, Weirstall K, Jenab S, Quiñones-Jenab V. Coadministration of estrogen and progesterone differentially affects locomotor responses to cocaine in rats. Ethn Dis 2008; 18: S2-51–3. [PubMed] [Google Scholar]
- [71].Kohtz AS, Paris JJ, Frye CA. Low doses of cocaine decrease, and high doses increase, anxiety-like behavior and brain progestogen levels among intact rats. Horm Behav 2010; 57: 474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Anker JJ, Carroll ME. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci Biobehav Rev. 2010; 35: 315–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Anker JJ, Holtz NA, Zlebnik N, Carroll ME. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology (Berl) 2009; 203: 63–72. [DOI] [PubMed] [Google Scholar]
- [74].Gandhi N, Saiyed ZM, Napuri J, et al. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: implications for HIV-1-associated neurocognitive disorder. J Neurovirol 2010; 16: 294–305. [DOI] [PubMed] [Google Scholar]
- [75].Nair MP, Samikkannu T. Differential regulation of neurotoxin in HIV clades: role of cocaine and methamphetamine. Curr HIV Res 2012; 10: 429–34. [DOI] [PubMed] [Google Scholar]
- [76].Ragupathy V, Devadas K, Tang S, et al. Effect of sex steroid hormones on replication and transmission of major HIV subtypes. J Steroid Biochem Mol Biol 2013; 138: 63–71. [DOI] [PubMed] [Google Scholar]