Abstract
Recent studies have suggested that the amount of forage intake by calves around the time of weaning could affect ruminal pH levels. Several studies have also proposed that subacute ruminal acidosis in mature cows is a risk factor for various metabolic disorders and production diseases. In this study, we examined the effects of forage feeding on ruminal pH, ruminal fermentation, rumen lipopolysaccharide (LPS) concentration, plasma metabolites, and hormonal concentrations in calves during pre- and postweaning periods. Sixteen male Holstein calves were used. At 7 wk of age, calves were randomly assigned to one of two dietary treatments: calves in the HAY group (n = 8) were fed starter with forage, and those in the CON group (n = 8) were fed starter without any forage. All calves were weaned at 8 wk of age. The amounts of starter and mixed hay were gradually increased until the end of the experiment (age, 11 wk). Ruminal pH was measured continuously every 10 min using an indwelling sensor. Rumen fluid and peripheral blood samples were obtained prior to morning feedings at −1, 0, 1, and 3 wk after weaning. Compared with the HAY group, in the CON group, the average daily ruminal pH was lower (P < 0.05) and the duration of ruminal pH values below 5.6 was longer (P < 0.05). Regarding ruminal VFA profiles, compared with the HAY group, the CON group had lower (P < 0.05) acetate to propionate ratios at 1 and 3 wk after weaning. Rumen LPS concentrations tended to be higher (P < 0.1) in the CON group than in the HAY group; however, concentrations of LPS-binding protein, haptoglobin, and serum amyloid A in the peripheral blood did not differ significantly. Plasma aspartate aminotransferase and alkaline phosphatase levels were markedly higher (P < 0.05) in the CON group than in the HAY group at 1 and 3 wk after weaning. There was a linear decrease in plasma growth hormone (GH) levels in the CON group after the start of the experiment, and its concentrations were lower (P < 0.05) in the CON group than in the HAY group at 0 and 3 wk after weaning. The results indicated that forage provision during pre- and postweaning periods helped prevent decrease in ruminal pH, change in ruminal fermentation, and liver alteration, and helped maintain plasma GH levels, which suggests that calves around the time of weaning need forage intake with starter to maintain proper metabolic and hormonal functions.
Keywords: hormones, metabolites, rumen lipopolysaccharide concentration, subacute ruminal acidosis, weaning period
INTRODUCTION
Holstein calves are provided with starter feed soon after birth to promote the shift to solid feed and the development of rumen villus (Sander et al., 1959). Starter feed amounts are increased rapidly during weaning and postweaning to avoid growth retardation. In contrast, forage feeding recommendations suggest that <5% total solid feed is supplied during weaning transition (Castells et al., 2013). Recent studies have suggested that the amount of forage intake around the time of weaning can affect ruminal pH levels (Laarman and Oba, 2011; Kim et al., 2016). Thus, the ruminal pH levels of weaning calves may continue to be low if they are exposed to energy-rich rations, resulting in a shortage of forage intake. In dairy cows, subacute ruminal acidosis (SARA) is recognized as the state in which ruminal pH levels are depressed for prolonged periods each day because of a buildup of organic acids in the rumen and decreased rumen buffering (Kleen et al., 2003; Plaizier et al., 2009). Several studies have indicated that SARA could be a cause of serious problems such as decreased DMI (Allen, 2000) and milk production (Enemark, 2008), and diarrhea (Kleen et al., 2003). However, available data conflict with regard to whether SARA induces increased rumen lipopolysaccharide (LPS) and plasma acute phase protein concentrations in steers and dairy cows (Gozho et al., 2005; Khafipour et al., 2009a,b; Li et al., 2012). Additionally, no studies have examined the impact of ruminal pH alternation on rumen LPS concentrations or metabolic or hormonal function in calves. The aim of this study was to determine the effects of forage feeding on ruminal pH, rumen fermentation, rumen LPS concentrations, plasma metabolites, and hormones during pre- and postweaning periods.
MATERIALS AND METHODS
The procedures used in this study were carried out in accordance with the principles and guidelines for animal experimentation issued by the National Institute of Livestock and Grassland Science Animal Care Committee, which were formulated to comply with Japanese regulations (Approval No. A201320).
Animals, Management, and Diets
Sixteen Holstein male calves were used in this study, and calves underwent rumen cannulation surgery at 4 wk of age. Thereafter, they were housed in individual pens bedded with rubber mats, had free access to fresh water, and were fed in two equal portions at 0800 and 1630 hours. Until 7 wk of age, all calves received 600 g 3.6 L−1∙d−1 of milk replacer [CP 24.7%, crude fat (CF) 20.9%; Meiji Feed Co., Ltd., Tokyo, Japan], 1,200 g/d of pelleted calf starter (CP 22.4%, CF 3.7%; Meiji Feed Co., Ltd.), and 200 g/d of mixed hay (orchard and timothy hay; CP 10.8%, NDF 56.6%). The nutrient composition of the diet is shown in Table 1.
Table 1.
Nutrient compositions of the milk replacer, calf starter, and mixed hay fed to calves
| Composition | Milk replacer | Calf starter | Mixed hay1 |
|---|---|---|---|
| DM, % | 96.9 | 88.1 | 83.2 |
| DM basis, % | |||
| CP | 24.7 | 22.4 | 10.8 |
| Crude fat | 20.9 | 3.7 | 1.1 |
| Ash | 5.6 | 5.1 | 8.0 |
| NDF | ND2 | 13.9 | 56.6 |
| ADF | ND | 7.3 | 33.7 |
| Starch | ND | 26.3 | 2.0 |
| Ca | 0.7 | 0.7 | 0.5 |
| P | 0.6 | 0.4 | 0.2 |
1Mixed hay was composed of orchard and timothy hay and fed at its original length (not chopped).
2Not determined.
The present study was conducted with 7- to 11-wk-old calves. At 7 wk of age, calves (77.6 ± 4.5 kg; mean ± SE) were offered 300 g 1.8 L−1∙d−1 of milk replacer, and were randomly assigned to one of two dietary treatment groups: HAY group calves (n = 8) were fed 1,600 g/d of calf starter and 400 g/d of mixed hay, and CON group calves (n = 8) were fed 1,600 g/d of calf starter without forage provisions, until the end of the experiment. All calves were weaned at 8 wk of age. Amounts of mixed hay in the HAY group were gradually increased from 600 g/d at 8 wk of age to 800 g/d at 11 wk of age. Amounts of calf starter were also increased from 1,600 g/d at 8 wk of age to 2,400 g/d at 11 wk of age in the HAY group and from 2,200 to 3,200 g/d in the CON group. Both groups were provided with approximately the same amount of DMI of total solid feed throughout the experimental period and were offered a diet equivalent to 100% of their ME and CP requirements according to the Japanese Feeding Standards for dairy cattle (National Agriculture and Food Research Organization, 2017). The total DMI or BW of each calf was recorded daily or weekly.
Rumen Fluid and Blood Sampling
Rumen fluid samples were collected through a rumen cannula prior to morning feedings at 7, 8, 9, and 11 wk of age, namely −1, 0, 1, and 3 wk after weaning, respectively. Rumen fluids were immediately filtered through two layers of sterile cheesecloth. For VFA analysis, 2 mL 25% HO3P in 3 N H2SO4 were added to 10 mL of filtered rumen fluid and stored at −80 °C. Another 2 mL of filtered rumen fluid was centrifuged at 9,000 ×g for 30 min at 4 °C and stored at −80 °C until rumen LPS concentration analyses were performed.
Concurrent with rumen fluid sampling, blood samples were obtained from the jugular vein, and were collected in vacutainer tubes containing sodium heparin (Terumo Corp., Tokyo, Japan) and Na2-EDTA (Terumo Corp.). Blood sample tubes were immediately mixed with 500 Kallikrein Inhibitor Units per milliliter of aprotinin (Sigma-Aldrich Japan, Tokyo, Japan), centrifuged at 2,200 × g for 20 min at 4 °C, and stored at −80 °C until analyzed.
Sample Analysis
Total VFA concentrations and VFA molar proportions were determined by gas chromatography (Model 135; Hitachi Co., Ltd., Tokyo, Japan) using a packed glass column (Thermon-3000 5% SHINCARBON A 60-80; Shinwa Kako Co. Ltd., Kyoto, Japan) as described by Nakamura et al. (2017). Rumen LPS concentrations were determined as described previously by Hirabayashi et al. (2017). Concentrations of plasma total protein, albumin, glucose, triglyceride, inorganic phosphorus, calcium, iron, aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transferase, lactate dehydrogenase (LDH), creatine kinase (CK), and nonesterified fatty acid (NEFA) were measured with an automated analyzer (7070; Hitachi Co., Ltd.). Plasma growth hormone (GH) and insulin concentrations were determined via radioimmunoassay (Kushibiki et al., 2003), and the intra-assay coefficients of measurement variation were 7.2% for GH and 1.9% for insulin. Plasma concentrations of LPS-binding protein (LBP) were measured using a commercially available kit (HK503; HyCult Biotechnology, Uden, the Netherlands). Plasma haptoglobin (Hp) concentrations were analyzed using a commercial assay kit (Cow Haptoglobin ELISA kit; Life Diagnosis Inc., Westchester, USA), and concentrations of serum amyloid A (SAA) were determined using an ELISA kit (Multispecies SAA ELISA kit; Tridelta Development Ltd., Maynooth Country Kildore, Ireland). Concentrations of plasma tumor necrosis factor-α (TNF-α), interleukin-1β, and interferon-γ (IFN-γ) were measured using a sandwich ELISA as previously described by Kushibiki et al. (2006).
Ruminal pH Measurement and Data Analysis
Ruminal pH was measured using an indwelling sensor (YCOW-S; DKK-TOA Yamagata Corp., Yamagata, Japan) that allowed for continuous measurements to be recorded every 10 min during the experimental period. The pH sensor was placed in the ventral sac of the rumen (Figure 1). Ruminal pH data measured 72 h after the sampling day, each week was used to summarize daily minimum, mean, and maximum pH values. In addition, the duration of pH below 5.6 (min/d) and area under pH 5.6 (min × pH/d) were summarized.
Figure 1.
Photograph of the indwelling ruminal pH sensor (160 mm in length and 32 mm in diameter) connected to the cover of the rumen cannula by a cord. The sensor was specifically developed for calf research based on a radio transmission pH measurement system in adults (Sato et al., 2012).
Statistical Analysis
The mixed linear model procedure found in SAS University Edition (SAS Institute, Inc., Cary, NC, USA) was used to determine the effects of treatment on characteristics of total DMI, daily BW gain, ruminal pH, rumen fluid composition, and plasma metabolite composition. The following model was used:
where Yijk is the observation, m is the overall mean, Ti is the effect of treatment, C(T)ij is the random variable of a calf nested in treatment, Wk is the effect of time or sampling week, TWik is the interaction between dietary treatment and time or sampling week, and eijk is the residuals.
When significant interactions between treatment and sampling week were detected, comparisons between treatments were evaluated using Tukey–Kramer’s test. Least square means and corresponding SEM values were computed and presented. Significance was declared at P < 0.05, and a tendency was identified at P < 0.1.
RESULTS
Calves in both groups were fed all their feed allowance according to the experimental design. Solid and liquid feed intake were equal to the total amount of provisions, and DMI did not differ between the groups throughout the experiment. The daily BW gain in the HAY group did not differ from the CON group in any weekly measurements (data not shown) or the average of the entire experimental period (0.98 ± 0.08 vs. 1.02 ± 0.08 kg/d for the HAY and CON group, respectively). No clinical disorders were observed in the calves during the experiment.
Ruminal pH, VFA Profiles, and LPS concentrations in Rumen Fluid
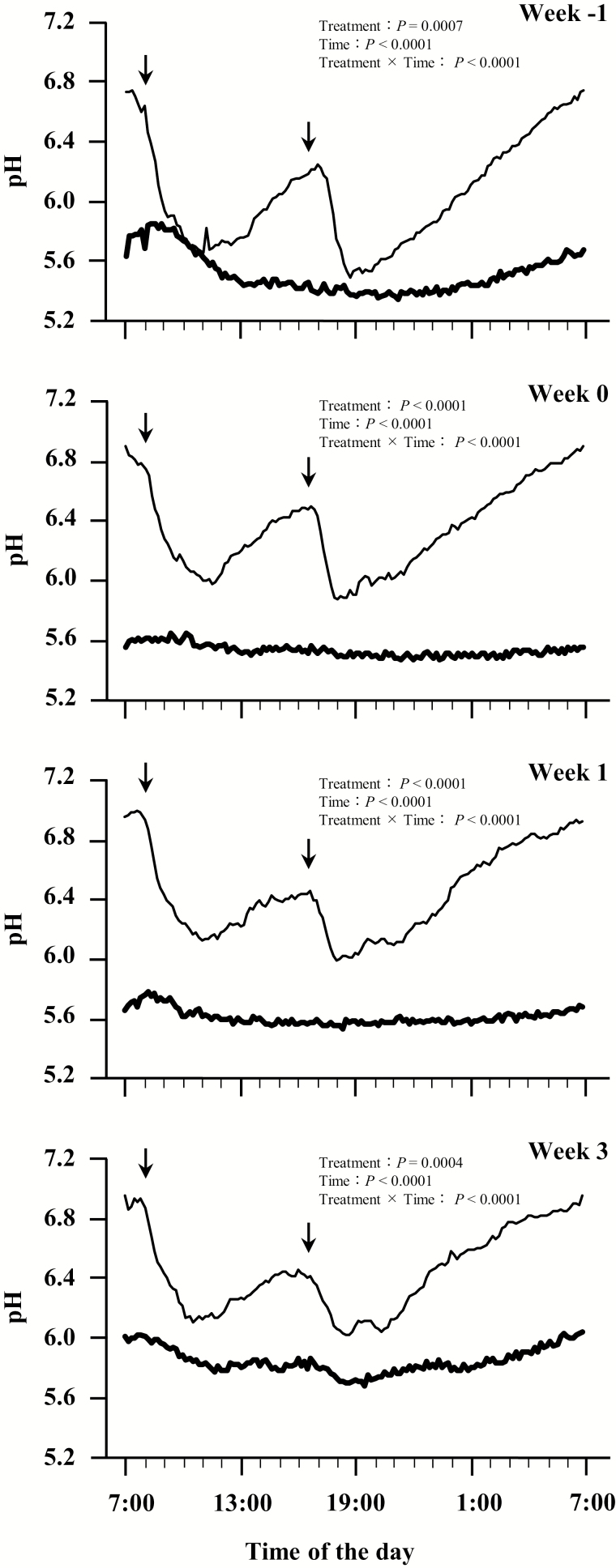
Diurnal changes in ruminal pH are shown in Figure 2. The effects of feeding treatment, time, and the interaction between them on diurnal ruminal pH were significant for all sampling weeks. In the CON group, diurnal changes in ruminal pH were lower (P < 0.05) than in the HAY group during the experimental period. Ruminal pH in the HAY group decreased over a 2 h period after each feeding, and then recovered gradually by the next morning to the same level observed the day before. On the other hand, the CON group calves did not experience diurnal changes in ruminal pH during the experimental period, however, ruminal pH levels remained low (approximately 5.6) in the CON group.
Figure 2.
Diurnal variations of the mean ruminal pH in calves fed with or without forage at −1, 0, 1 and 3 wk after weaning. Values are presented as least square means of eight calves per group. Calves fed starter with forage (HAY group) are represented by a narrow solid line. Calves fed starter without any forage provision (CON group) are represented by a heavy solid line. The arrows show the feeding time at 0800 and 1630 hours. Ruminal pH was measured every 10 min, and the data from 72 h after the sampling day were summarized each week.
Variations in ruminal pH parameters are presented in Table 2. Significant differences were observed between the groups in the mean and maximum daily pH, the duration of pH values below 5.6, and area under pH 5.6. There was a significant treatment × week interaction effect on the mean and maximum daily pH. The mean and maximum daily pH values were significantly lower in the CON group than in the HAY group throughout the study, and there was no difference between the minimum daily pH values of the groups. In the CON group, the duration of pH values below 5.6 was significantly longer than in the HAY group, and the area under pH 5.6 was larger.
Table 2.
Ruminal pH in calves fed with or without forage at −1, 0, 1, and 3 wk after weaning
| Week −1 | Week 0 | Week 1 | Week 3 | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | HAY1 | CON2 | HAY | CON | HAY | CON | HAY | CON | SEM | Treatment (T) | Week (W) | T × W |
| Ruminal pH | ||||||||||||
| Mean pH | 6.04a | 5.51b | 6.35a | 5.54b | 6.43a | 5.61b | 6.44a | 5.84b | 0.10 | 0.001 | 0.001 | 0.022 |
| Minimum pH | 5.29 | 5.27 | 5.59 | 5.41 | 5.70 | 5.50 | 5.68 | 5.59 | 0.10 | 0.245 | 0.001 | 0.734 |
| Maximum pH | 6.92a | 6.03b | 7.11a | 5.76b | 7.12a | 5.88b | 7.14a | 6.23b | 0.14 | 0.001 | 0.127 | 0.018 |
| Duration3 < pH 5.6, min/d | 320.41 | 985.85 | 66.00 | 885.00 | 44.59 | 584.60 | 38.34 | 487.08 | 147.45 | 0.001 | 0.006 | 0.438 |
| Area < pH 5.6, min × pH/d | 58.65 | 258.15 | 7.63 | 230.59 | 3.56 | 211.45 | 5.61 | 63.21 | 59.41 | 0.012 | 0.031 | 0.328 |
a,bDifferent letters in a row indicate significant effects (P < 0.05) at the same point in a week.
1Calves were fed starter with forage (HAY group).
2Calves were fed starter without any forage provision (CON group).
3If pH was below 5.6, ruminal pH was considered to be below 5.6 for the following 10 min.
Data are presented as least square means and SEM of eight calves per group.
Ruminal pH was measured every 10 min, and the data from 72 h after the sampling day were summarized each week.
No difference in total VFA concentration was detected between the groups (Table 3); however, VFA molar proportions, ruminal acetate and propionate proportions, and acetate to propionate ratios were significantly different between the groups. There were also significant interaction effects on these parameters, except for others proportions. At 1 and 3 wk after weaning, ruminal acetate proportions were significantly lower in the CON group than in the HAY group; however, ruminal propionate proportions were significantly greater in the CON group than in the HAY group. Therefore, acetate to propionate ratios in the CON group were lower (P < 0.05) than those in the HAY group at 1- and 3-wk postweaning.
Table 3.
Rumen fermentation variables and lipopolysaccharide (LPS) concentrations in calves fed with or without forage at −1, 0, 1, and 3 wk after weaning
| Week −1 | Week 0 | Week 1 | Week 3 | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | HAY1 | CON2 | HAY | CON | HAY | CON | HAY | CON | SEM | Treatment (T) | Week (W) | T × W |
| Total VFA, mmol/L | 72.99 | 82.98 | 82.55 | 78.31 | 76.14 | 84.28 | 90.79 | 88.44 | 10.21 | 0.812 | 0.326 | 0.631 |
| VFA proportions, mol/100mol | ||||||||||||
| Acetate | 58.70 | 57.04 | 56.40 | 53.60 | 62.70a | 49.36b | 61.20 a | 50.24b | 1.87 | 0.001 | 0.274 | 0.001 |
| Propionate | 28.98 | 28.19 | 29.16 | 34.54 | 23.81a | 37.15b | 23.26a | 36.63b | 2.40 | 0.009 | 0.337 | 0.001 |
| Butyrate | 7.85a | 10.76b | 8.90 | 7.70 | 8.44 | 7.81 | 10.27 | 8.05 | 0.92 | 0.780 | 0.208 | 0.001 |
| Others3 | 4.47 | 4.03 | 5.54 | 4.17 | 5.05 | 5.69 | 5.27 | 5.12 | 0.45 | 0.466 | 0.021 | 0.070 |
| Acetate/propionate | 2.14 | 2.24 | 2.10 | 1.62 | 2.86a | 1.38b | 2.77a | 1.49b | 0.26 | 0.014 | 0.320 | 0.001 |
| Rumen LPS, EU/mL | 123,995 | 108,694 | 95,698 | 267,703 | 116,739 | 247,327 | 126,758 | 317,188 | 63,331 | 0.081 | 0.245 | 0.196 |
a,bDifferent letters in a row indicate significant effects (P < 0.05) at the same point in a week.
1Calves were fed starter with forage (HAY group).
2Calves were fed starter without any forage provision (CON group).
3Valerate and isovalerate.
Data are presented as least square means and SEM of eight calves per group.
Rumen LPS concentrations in both groups of calves were remarkably high and above 100,000 endotoxin units (EU)/mL during most sampling weeks (Table 3). Concentrations in the CON group tended to be higher (P = 0.08) than those in the HAY group; no significant effect of treatment × week interaction was observed.
Blood Metabolites and Hormones
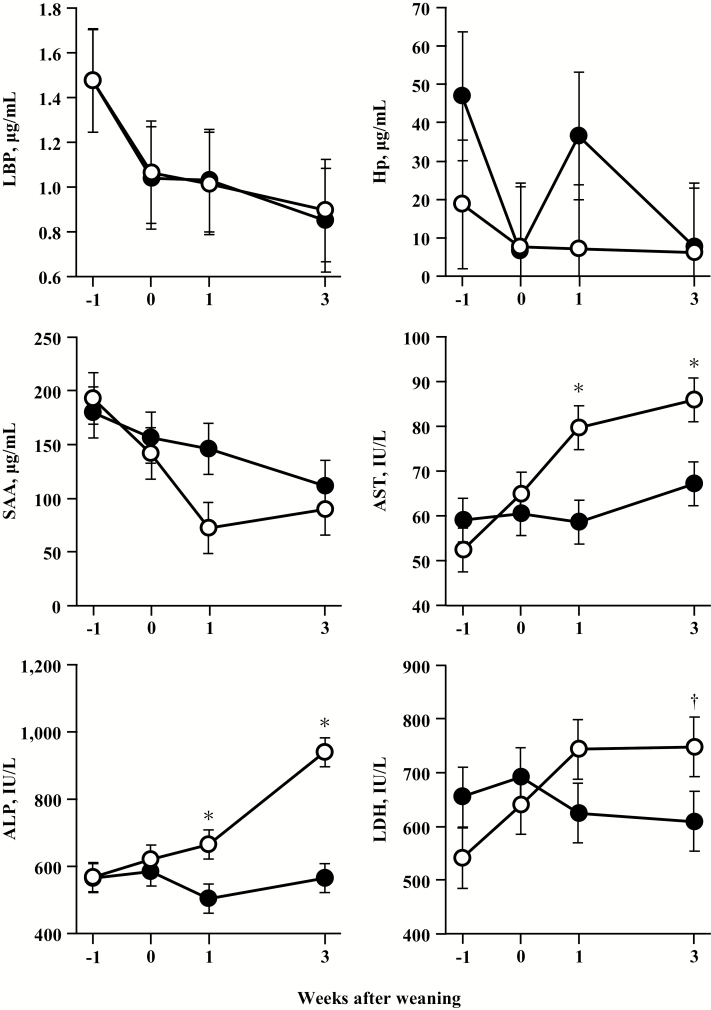
Figure 3 shows changes in plasma concentrations of acute phase proteins, AST, ALP, and LDH in calves fed starter with or without forage. Plasma LBP, Hp, and SAA concentrations were not affected by forage provisions. There were no significant interaction effects on plasma acute phase protein concentrations. A significant difference in plasma ALP concentration was observed between the groups and a tendency towards higher plasma AST concentration in the CON group was observed (P < 0.1). Furthermore, the effects of the treatment × week interaction on plasma ALP and AST concentrations were significant. Plasma AST and ALP concentrations were markedly higher (P < 0.05) in the CON group relative to the HAY group 1 and 3 wk after weaning. Plasma LDH concentrations were not significantly different between the groups, but a significant interaction effect was found. Plasma LDH concentration in the CON group tended to be higher (P = 0.09) than in the HAY group at 3 wk after weaning. Plasma concentration of CK in both groups remained in the normal range, and no differences between groups were observed (data not shown). Plasma levels of TNF-α, IL-1β, and IFN-γ in both groups were not affected by dietary treatment (data not shown).
Figure 3.
Plasma concentrations of LPS-binding protein (LBP), haptoglobin (Hp), serum amyloid A (SAA), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) in calves fed with or without forage at −1, 0, 1, and 3 wk after weaning. Each symbol is expressed as least squares means and SEM of eight calves per group. Closed circles represent calves fed starter with forage (HAY group), and open circles represent calves fed starter without any forage provision (CON group). *P < 0.05 indicate significant differences and †P < 0.1 show a tendency toward significance between the groups at the same point in a week.
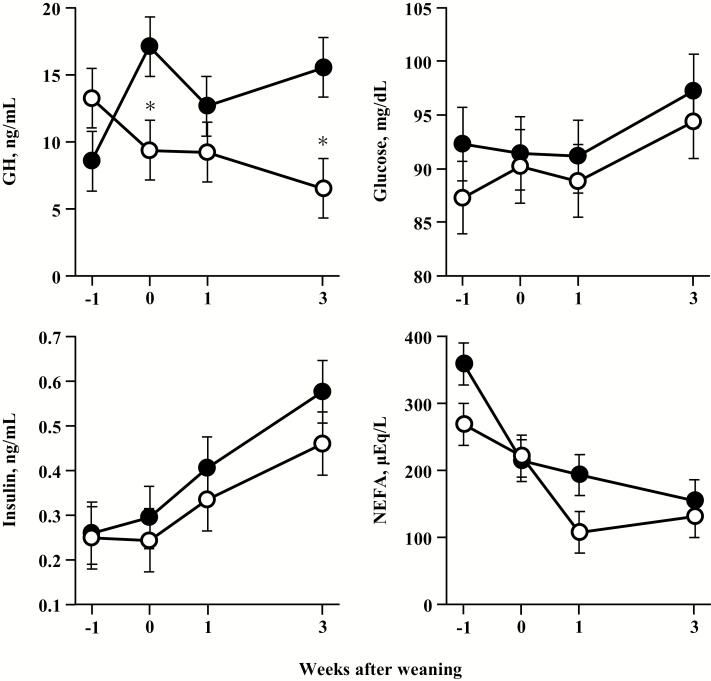
Figure 4 shows changes in the plasma concentrations of glucose, insulin, GH, and NEFA in calves fed starter with or without forage. The plasma GH level in the CON group declined linearly after the start of the experiment. On the contrary, plasma GH concentrations in the HAY group gradually increased 3 wk after weaning. Significant differences in plasma GH concentrations were therefore detected between the groups, and those of the CON group were lower (P < 0.05) at 0 and 3 wk after weaning than those of the HAY group. There was a significant effect of treatment × week interaction on plasma GH concentrations. The difference between the groups was not significant for plasma glucose and insulin, however, plasma NEFA concentration tended to be higher (P = 0.07) in the HAY group than in the CON group. There were no significant interaction effects on plasma glucose, insulin, or NEFA concentrations. No differences were detected between the other metabolites and minerals measured in the two groups (data not shown).
Figure 4.
Plasma concentrations of growth hormone (GH), glucose, insulin, and nonesterified fatty acid (NEFA) in calves fed with or without forage at −1, 0, 1, and 3 wk after weaning. Each symbol is expressed as least squares means and SEM of eight calves per group. Closed circles represent calves fed starter with forage (HAY group), and open circles represent calves fed starter without any forage provision (CON group). *P < 0.05 indicates significant differences between the groups at the same point in a week.
DISCUSSION
Weaning is a substantial stress for calves (Carroll et al., 2009; Lambertz et al., 2015), which can cause lower weight gains (Lambertz et al., 2015) and the occurrence of infectious diseases (Callan et al., 2002; Snowder, 2009). Generally, calf starters with high palatability and nutrition are fed excessively to promote the shift from liquid to solid feed and to avoid growth retardation. However, as the amount of starter feed is increased, forage intake decreases. Low forage intake during weaning transition is also associated with a decline in ruminal pH (Laarman and Oba, 2011). Moreover, calves fed starter without any forage provisions displayed low ruminal pH levels on a daily basis during pre- and postweaning periods (Kim et al., 2016). In mature cows, several studies proposed that SARA is a risk factor for various metabolic disorders (Nocek, 1997; Kleen et al., 2003; Stone, 2004) and production diseases (Kleen et al., 2003; Enemark, 2008; Plaizier et al., 2009). However, no studies have yet examined the effects of forage feeding during the pre- and postweaning periods on rumen fermentation and on the concentrations of metabolites and hormones in plasma.
In our study, ruminal pH levels in the HAY group showed diurnal changes in response to feeding. In contrast, ruminal pH levels in the CON group did not exhibit these diurnal changes, but pH levels remained low. Ruminal pH variation in the CON group was similar to that found in a previous study (Kim et al., 2016). In mature cows, SARA is defined as the maintenance of a ruminal pH below 5.6 for more than 180 min per day (Gozho et al., 2005); however, the standard for calves has not been defined. When the definition of SARA in mature cows was applied to our study, SARA was found in calves in the CON group throughout the experiment. The duration of ruminal pH values below 5.6 in the HAY group was above 180 min/d before weaning, and it notably decreased after weaning. Our data for ruminal pH in the HAY group might have been influenced by increased forage intake. During the weaning transition, a negative correlation between the amount of forage intake and the duration of ruminal pH values below 5.6 was associated with increased rumination time and the stimulation of saliva secretion (Laarman and Oba, 2011); this finding suggests that it is also applicable in the postweaning period.
In the present study, the total VFA concentration in the CON group did not differ from that of the HAY group. Previous studies in calves showed that total VFA concentrations were not increased by the increased consumption of calf starter (Kim et al., 2016) or changes in dietary starch concentration (Laarman et al, 2012), but ruminal pH levels decreased. Briefly, decreased ruminal pH is not always followed by high total VFA concentrations in rumen fluid, and our results for VFA concentrations in the CON group are consistent with previous studies. The acetate proportion of the ruminal VFA molar proportions was lower (P < 0.05), and the propionate proportion was greater (P < 0.05) in the CON group than in the HAY group. Consequently, the acetate-to-propionate ratio in the CON group was significantly lower than that of the HAY group. When highly fermentable carbohydrates, such as concentrated feed, were given excessively to dairy Holstein cows, higher propionate proportions, and lower acetate-to-propionate ratios were observed than in control cows (Khafipour et al., 2009a; Li et al., 2012). In the CON group, it is likely that the increased starter intake during the weaning period affected the propionate proportion, causing the lower acetate-to-propionate ratio.
Rumen LPS concentrations in the CON group tended to be higher (P < 0.1) than in the HAY group. Previous studies suggested that persistent low ruminal pH was related to high rumen LPS concentrations in adult steers and lactating cows (Gozho et al., 2005; Khafipour et al., 2009a). Another study suggested that rumen LPS concentrations in lactating cows had marked individual differences, and these differences were especially prominent when cows were fed high-energy diets (Hirabayashi et al., 2017). High levels of rumen LPS concentrations in the CON group (P < 0.1) might be connected to decreased ruminal pH values. However, a statistical difference between rumen LPS concentrations in the two groups was not detected because individual differences in both groups were considerable. In the HAY group, rumen LPS concentrations also remained at high levels (above 100,000 EU/mL). These values were equivalent to, or greater than, the levels observed in lactating and nonlactating Holstein cows with induced SARA (Khafipour et al., 2009b; Li et al., 2012). To our knowledge, this is the first report of rumen LPS concentrations during pre- and postweaning periods. The ruminal bacterial flora of Holstein calves at 2 d old was at the same density as in mature cattle (Minato et al., 1992). In contrast, fluid flow from rumen to reticulum is slower in calves than in adults, because the ruminal fractional passage rate is positively correlated with the solid feed intake and rumen volume (Berends et al., 2015). Therefore, the higher base value of the rumen LPS concentration in our experiment calves, compared with other reports on cattle, may have resulted from the stagnation of the rumen. However, the actual mechanism remains to be determined.
Plasma AST and ALP concentrations in the CON group were significantly greater than in the HAY group after weaning. On the other hand, plasma CK concentrations in the CON group did not differ from those in the HAY group. These data suggest that the concentrations observed in the CON group calves were caused by inflammation in the liver. However, there was no difference between the groups with regard to plasma concentrations of acute phase proteins and cytokines. In induced SARA experiments using lactating cows, increased rumen LPS concentrations were not always accompanied by increased blood concentrations of acute phase proteins (Khafipour et al., 2009b; Li et al., 2012; Chiquette et al., 2015). Although the report revealed increased plasma AST concentrations in heifers with induced SARA (Marchesini et al., 2013), there is little information about the changes in plasma hepatic enzymes when rumen LPS concentrations increase.
Plasma GH concentrations in the CON group were significantly lower than in the HAY group. Previous studies indicated that increased ruminal propionate proportions restrained plasma GH concentrations in sheep (Matsunaga et al., 1997). Additionally, decreased levels of plasma GH in sheep were maintained in a dose-dependent manner associated with the proportion of concentrate in the diet (Hagino et al., 2005). In the present investigation, the ruminal propionate proportion in the CON group was greater than that of the HAY group. It is possible that the decreased plasma GH concentrations in the CON group were related to the restriction of forage provisions and changes in ruminal VFA molar proportions.
In conclusion, we demonstrated that forage feeding with starter during pre- and postweaning periods helped prevent depression of ruminal pH, changes in rumen fermentation, and liver alterations, and maintained plasma GH levels in calves. Our results suggest that calves need forage intake with starter around the time of weaning to maintain their metabolic and hormonal functions. However, the long-term effects of nutritional management or SARA during such periods on future growth responses or on general health require further investigation.
Conflict of interest statement. None declared.
Footnotes
This research was supported by a KAKENHI Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (grant no. 18K05961). The authors gratefully acknowledge the technical support of Toshihiro Ichijo (Iwate University, Iwate), Atsushi Kimura (Iwate Prefectural Federation of Agricultural Mutual Aid Association, Iwate), Kentaro Ikuta (Awaji Agricultural Technology Center, Hyogo), and Fuminori Terada (Tohoku University, Miyagi) as well as the laboratory staff of the Cooperative Department of Veterinary Medicine at Iwate University (Iwate).
LITERATURE CITED
- Allen M. S. 2000. Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J. Dairy Sci. 83:1598–1624. doi:10.3168/jds.S0022-0302(00)75030-2. [DOI] [PubMed] [Google Scholar]
- Berends H., J. J. van den Borne N. Stockhofe-Zurwieden M. S. Gilbert T. Zandstra W. F. Pellikaan C. G. van Reenen E. A. Bokkers, and Gerrits W. J.. 2015. Effects of solid feed level and roughage-to-concentrate ratio on ruminal drinking and passage kinetics of milk replacer, concentrates, and roughage in veal calves. J. Dairy Sci. 98:5621–5629. doi:10.3168/jds.2015-9367. [DOI] [PubMed] [Google Scholar]
- Callan R. J., and Garry F. B.. 2002. Biosecurity and bovine respiratory disease. Vet. Clin. North Am. Food Anim. Pract. 18:57–77. doi:10.1016/S0749-0720(02)00004-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J. A., J. D. Arthington, and Chase C. C. Jr. 2009. Early weaning alters the acute-phase reaction to an endotoxin challenge in beef calves. J. Anim. Sci. 87:4167–4172. doi:10.2527/jas.2009-2016. [DOI] [PubMed] [Google Scholar]
- Castells L., A. Bach A. Aris, and Terré M.. 2013. Effects of forage provision to young calves on rumen fermentation and development of the gastrointestinal tract. J. Dairy Sci. 96:5226–5236. doi:10.3168/jds.2012-6419. [DOI] [PubMed] [Google Scholar]
- Chiquette J., J. Lagrost C. L. Girard G. Talbot S. Li J. C. Plaizier, and Hindrichsen I. K.. 2015. Efficacy of the direct-fed microbial Enterococcus faecium alone or in combination with Saccharomyces cerevisiae or Lactococcus lactis during induced subacute ruminal acidosis. J. Dairy Sci. 98:190–203. doi:10.3168/jds.2014-8219. [DOI] [PubMed] [Google Scholar]
- Enemark J. M. D. 2008. The monitoring, prevention and treatment of sub-acute ruminal acidosis (SARA): a review. Vet. J. 176:32–43. doi:10.1016/j.tvjl.12.021 [DOI] [PubMed] [Google Scholar]
- Gozho G. N., J. C. Plaizier D. O. Krause A. D. Kennedy, and Wittenberg K. M.. 2005. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J. Dairy Sci. 88:1399–1403. doi:10.3168/jds.S0022-0302(05)72807-1. [DOI] [PubMed] [Google Scholar]
- Hagino A., Inomata E., Sato T., Ohtomo Y., Sasaki Y., and Obara Y.. 2005. Effect in sheep of dietary concentrate content on secretion of growth hormone, insulin and insulin-like growth factor-І after feeding. Anim. Sci. J. 76:55–63. doi:10.1111/j.1740-0929.2005.00238.x [Google Scholar]
- Hirabayashi H., K. Kawashima T. Okimura A. Tateno A. Suzuki S. Asakuma N. Isobe T. Obitsu S. Kushibiki, and Sugino T.. 2017. Effect of nutrient levels during the far-off period on postpartum productivity in dairy cows. Anim. Sci. J. 88:1162–1170. doi:10.1111/asj.12743. [DOI] [PubMed] [Google Scholar]
- Khafipour E., Krause D. O., and Plaizier J. C.. 2009a. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 92:1060–1070. doi:10.3168/jds.2008-1389 [DOI] [PubMed] [Google Scholar]
- Khafipour E., D. O. Krause, and Plaizier J. C.. 2009b. Alfalfa pellet-induced subacute ruminal acidosis in dairy cows increases bacterial endotoxin in the rumen without causing inflammation. J. Dairy Sci. 92:1712–1724. doi:10.3168/jds.2008-1656. [DOI] [PubMed] [Google Scholar]
- Kim Y. H., R. Nagata N. Ohtani T. Ichijo K. Ikuta, and Sato S.. 2016. Effects of dietary forage and calf starter diet on ruminal pH and bacteria in Holstein calves during weaning transition. Front. Microbiol. 7:1575. doi:10.3389/fmicb.2016.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleen J. L., G. A. Hooijer J. Rehage, and Noordhuizen J. P.. 2003. Subacute ruminal acidosis (SARA): a review. J. Vet. Med. A Physiol. Pathol. Clin. Med. 50:406–414. [DOI] [PubMed] [Google Scholar]
- Kushibiki S., K. Hodate H. Shingu Y. Obara E. Touno M. Shinoda, and Yokomizo Y.. 2003. Metabolic and lactational responses during recombinant bovine tumor necrosis factor-alpha treatment in lactating cows. J. Dairy Sci. 86:819–827. doi:10.3168/jds.S0022-0302(03)73664-9. [DOI] [PubMed] [Google Scholar]
- Kushibiki S., Shingu H., Komatsu T., Itoh F., Kasuya E., Aso H., and Hodate K.. 2006. Effect of recombinant bovine tumor necrosis factor‐α on hormone release in lactating cows. Anim. Sci. J. 77:603–612. doi:10.1111/j.1740-0929.2006.00392.x [Google Scholar]
- Laarman A. H., and Oba M.. 2011. Short communication: effect of calf starter on rumen pH of Holstein dairy calves at weaning. J. Dairy Sci. 94:5661–5664. doi:10.3168/jds.2011-4273. [DOI] [PubMed] [Google Scholar]
- Laarman A. H., T. Sugino, and Oba M.. 2012. Effects of starch content of calf starter on growth and rumen ph in Holstein calves during the weaning transition. J. Dairy Sci. 95:4478–4487. doi:10.3168/jds.2011-4822. [DOI] [PubMed] [Google Scholar]
- Lambertz C., A. Farke-Röver, and Gauly M.. 2015. Effects of sex and age on behavior and weight gain in beef calves after abrupt weaning. Anim. Sci. J. 86:345–350. doi:10.1111/asj.12285. [DOI] [PubMed] [Google Scholar]
- Li S., E. Khafipour D. O. Krause A. Kroeker J. C. Rodriguez-Lecompte G. N. Gozho, and Plaizier J. C.. 2012. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J. Dairy Sci. 95:294–303. doi:10.3168/jds.2011-4447. [DOI] [PubMed] [Google Scholar]
- Marchesini G., R. De Nardi M. Gianesella A. L. Stefani M. Morgante A. Barberio I. Andrighetto, and Segato S.. 2013. Effect of induced ruminal acidosis on blood variables in heifers. BMC Vet. Res. 9:98. doi:10.1186/1746-6148-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga N., T. Goka K. T. Nam S. Oda A. Ohneda, and Sasaki Y.. 1997. Inhibition of GH releasing factor (GRF)-induced GH secretion by intraruminal infusion of volatile fatty acids (VFA) in sheep. Endocr. J. 44: 133–140. doi:10.1507/endocrj.44.133 [DOI] [PubMed] [Google Scholar]
- Minato H., Otsuka M., Shirasaka S., Itabashi H., and Mitsumori M.. 1992. Colonization of microorganisms in the rumen of young calves. J. Gen. Appl. Microbiol. 38: 447–456. doi:10.2323/jgam.38.447 [Google Scholar]
- Nakamura S.-I., Kim Y. H., Takashima K., Kimura A., Nagai K., Ichijo T., and Sato S.. 2017. Composition of the microbiota in forestomach fluids and feces of Japanese Black calves with white scours. J. Anim. Sci. 95:3949–3960. doi:10.2527/jas2017.1431 [DOI] [PubMed] [Google Scholar]
- National Agriculture and Food Research Organization 2017. Japanese Feeding Standard for dairy cattle 2017 edn. Japan Livestock Industry Association, Tokyo, Japan: (in Japanese). [Google Scholar]
- Nocek J. E. 1997. Bovine acidosis: implications on laminitis. J. Dairy Sci. 80:1005–1028. doi:10.3168/jds.S0022-0302(97)76026-0. [DOI] [PubMed] [Google Scholar]
- Plaizier J. C., Krause D. O., Gozho G. N., and McBride B. W.. 2009. Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Vet. J. 176:21–31. doi:10.1016/j.tvil.2007.12.016 [DOI] [PubMed] [Google Scholar]
- Sander E. G., Warner R. G., Harrison H. N., and Loosli H. N.. 1959. The stimulatory effect of sodium butyrate and sodium propionate on the development of rumen mucosa in the young calf. J. Dairy Sci. 42:1600–1605. doi:10.3168/jds.S0022-0302(59)90772–6 [Google Scholar]
- Sato S., H. Mizuguchi K. Ito K. Ikuta A. Kimura, and Okada K.. 2012. Technical note: development and testing of a radio transmission ph measurement system for continuous monitoring of ruminal ph in cows. Prev. Vet. Med. 103:274–279. doi:10.1016/j.prevetmed.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Snowder G. D. 2009. Genetics, environment and bovine respiratory disease. Anim. Health Res. Rev. 10:117–119. doi:10.1017/S1466252309990144 [DOI] [PubMed] [Google Scholar]
- Stone W. C. 2004. Nutritional approaches to minimize subacute ruminal acidosis and laminitis in dairy cattle. J. Dairy Sci. 87:E13–E26. doi:10.3168/jds.S0022-0302(04)70057-0 [Google Scholar]