Abstract
Moral injury is closely associated with posttraumatic stress disorder (PTSD) and characterized by disturbances in social and moral cognition. Little is known about the neural underpinnings of moral injury, and whether the neural correlates are different between moral injury and PTSD. A sample of 26 U.S. military veterans (two females: 28–55 years old) were investigated to determine how subjective appraisals of morally injurious events measured by Moral Injury Event Scale (MIES) and PTSD symptoms are differentially related to spontaneous fluctuations indexed by amplitude of low frequency fluctuation (ALFF) as well as functional connectivity during resting-state functional magnetic resonance imaging scanning. ALFF in the left inferior parietal lobule (L-IPL) was positively associated with MIES subscores of transgressions, negatively associated with subscores of betrayals, and not related with PTSD symptoms. Moreover, functional connectivity between the L-IPL and bilateral precuneus was positively related with PTSD symptoms and negatively related with MIES total scores. Our results provide the first evidence that morally injurious events and PTSD symptoms have dissociable neural underpinnings, and behaviorally distinct subcomponents of morally injurious events are different in neural responses. The findings increase our knowledge of the neural distinctions between moral injury and PTSD and may contribute to developing nosology and interventions for military veterans afflicted by moral injury.
Keywords: ALFF, functional connectivity, inferior parietal lobe, moral injury, PTSD, resting-state fMRI
1 |. INTRODUCTION
Moral injury refers to disturbances experienced by combat veterans related to guilt, shame, anger, and betrayal arising from violations of their moral code (Bryan et al., 2016; Frankfurt & Frazier, 2016; Jones, 2018; Litz et al., 2009). It may arise from specific acts, such as killing in combat (e.g., killing innocent civilians), but may also be generated by a broader experience that violates deeply held moral and ethical beliefs and expectations. Individual soldiers are left to make sense of their own actions and the actions of others, to integrate those actions with their existing moral and ethical frameworks, and to manage emotional responses prompted by the relative congruence or incongruence between past moral beliefs and recent actions. The inability to integrate long-held ethical worldviews with specific personal actions may lead to ongoing psychological distress manifested by specific behavioral problems (Litz et al., 2009; McClymond & Anthony, 2014).
Both moral injury and military-related posttraumatic stress disorder (PTSD) are associated with consequences of participation in warfare. A required criterion for a PTSD diagnosis is exposure to an event that poses a threat to physical safety; prevailing models of PTSD are predicated on exposure to life-threatening events and are predominantly studied as disorders of fear processing (Milad et al., 2009; Shalev, Liberzon, & Marmar, 2017). Profound moral injury, on the other hand, may be experienced without direct exposure to a personal life threat, and models of moral injury are related to disturbances in social and moral cognition (Frankfurt & Frazier, 2016). Our goal was to investigate the hotly debated comparisons between moral injury and PTSD by probing the relevant neural systems (Bryan et al., 2016; Bryan, Bryan, Roberge, Leifker, & Rozek, 2018; Litz et al., 2009).
The moral injury syndrome and PTSD overlap on several symptoms including anger, depression, anxiety, substance abuse, insomnia, nightmares, suicidal thoughts, shame, and guilt (Frankfurt & Frazier, 2016; however, see Bryan et al., 2018). Although the measurement of PTSD is well-established (Blake et al., 1995; Weathers et al., 2013), the measurement of moral injury syndrome is evolving. The self-report Moral Injury Events Scale (MIES) developed by Nash et al. (2013) is a widely utilized scale measuring both subjective appraisals of exposure to morally injurious events and distress associated with these events. Here, we employed MIES as an initial exploration of neural signals associated with moral injury. The contributory events to MIES are captured by two latent factors: perceived transgressions by self or others (the transgression subscale), and perceived betrayals by others (betrayal subscale). The transgression subscale includes witnessing acts of commission, distress resulting from others’ acts of commission, and perpetration of or distress due to acts of commission/omission. An example would be a soldier who kills an unarmed civilian who was mistakenly believed to be armed. On the other hand, the betrayal subscale measures perceived betrayals by previously trusted military leaders, fellow service members, and nonmilitary others (e.g., a spouse). For instance, a patriotic soldier in a battle may begin to wonder whether the war is not as justified as the leaders have declared.
Both moral transgression (Jones, 2018) and betrayal (Platt, Luoma, & Freyd, 2017) have been linked to feelings of guilt or shame. The release of DSM-5 introduced shame and guilt into the criterion-D symptoms of PTSD (Weathers et al., 2013). Patients with depression, which has high co-occurrence with PTSD and moral injury (Bryan et al., 2018; Nash et al., 2013), also frequently experience symptoms of shame and guilt (Kim, Thibodeau, & Jorgensen, 2011). Shame is associated with the ability to understand the social consequences of one’s own behavior as judged by others (Tangney, Stuewig, & Mashek, 2007). On the other hand, guilt concerns self-perception of one’s own behavior in relation to societal norms or self-imposed standards (Tangney & Dearing, 2002). In this sense, moral injury and PTSD are related with both self-referential processes and theory of mind (ToM). Self-referential processing refers to functions for decoding information about oneself (Northoff & Bermpohl, 2004), while ToM refers to the ability to assign and attribute mental states to both self and others (Baron-Cohen, 1995).
Both self-referential and ToM processes are associated with functions of the default mode network (DMN; Qin & Northoff, 2011), which includes the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), and left inferior parietal lobule and right inferior parietal lobule (L-IPL and R-IPL; Andrews-Hanna, Smallwood, & Spreng, 2014). The DMN is preferentially active when individuals are daydreaming, mind-wandering, engaged in internally focused tasks including retrieving autobiographical memory, envisioning the future, or thinking about others (Buckner, Andrews-Hanna, & Schacter, 2008). The neural correlates of moral processing largely overlap with the DMN (Bastin, Harrison, Davey, Moll, & Whittle, 2016).
Our previous work studying brain responses to guilt scenarios showed that the guilt ratings were positively associated with activations in dorsal mPFC and supramarginal gyrus that is included in the IPL (Morey et al., 2012). Roth, Kaffenberger, Herwig, and Bruhl (2014) investigated the neural correlates of autographical recall about shame and found that shame versus a neutral condition elicited stronger activation in mPFC and PCC as well as weaker activation in IPL. Interestingly, studies on shame and guilt (Bastin et al., 2016) have also reported findings in amygdala and dorsal anterior cingulate cortex (dACC) that are hyperresponsive in PTSD (Hughes & Shin, 2011). For instance, Pulcu et al. (2014) detected increased amygdala response to shame in remitted major depressive disorder. Wagner, N’Diaye, Ethofer, and Vuilleumier (2011) found that guilt in healthy subjects elicited stronger activations in dACC and amygdala than shame. In summary, these previous studies explored the neural correlates of shame and guilt, which are the core components of moral injury (Frankfurt & Frazier, 2016; Jones, 2018). Thus, we hypothesized that morally injurious events measured by moral transgression and betrayal would correlate with brain responses in the DMN, amygdala, and dACC. Given their conceptual differences, we also hypothesized that moral transgression and betrayal would be related to a different brain response in these areas. This knowledge may help us to understand the neural underpinnings of moral injury and PTSD as well as to find potential neural targets for clinical intervention. Our findings may help in the construction of a new and complementary neural model of moral processing.
In the present study, we investigated the relationship between clinical measures of morally injurious events and PTSD symptoms with brain responses measured by spontaneous fluctuation and functional connectivity during resting-state functional magnetic resonance imaging (rs-fMRI) scanning. The rs-fMRI does not measure responses to explicit tasks and is thus convenient for investigating the brain’s functional organization in patients with psychiatric and behavioral disorders. A number of studies have demonstrated that rs-fMRI data predict following behavioral performance in explicit tasks (He et al., 2013; Li et al., 2013; Zou et al., 2013). The spontaneous fluctuations reflect localized neural activity, whereas functional connectivity provides information on correlated activity between two brain regions (Lv et al., 2018). The term “functional” is used because we infer a connection between two regions with temporally correlated activity, rather than evidence of an actual physical (structural) connection. This inference is made by observing increased activity in region 1 over time corresponds to increased activity in region 2 at similar times, and decreased activity in region 1 over time corresponds to decreased activity in region 2 at similar times. This is in contrast to spontaneous fluctuations, which refers to activity in a specific region rather than correlated brain activity between two different regions. The spontaneous fluctuations are measured based on the size (amplitude) of the brain waves (blood–oxygen-level dependent [BOLD] signal) in a specific frequency range. The two methods are complementary to each other and provide a full perspective of the brain responses during rest. Here, we measured the intensity of spontaneous fluctuations in the brain using the amplitude of low frequency fluctuations (ALFF; Zang et al., 2007), which is a common analysis approach for spontaneous neural activity during rs-fMRI and has been widely employed to investigate the neural underpinnings of various psychiatric disorders (Fryer et al., 2015; Zuo et al., 2010). ALFF is positively correlated with other measures of spontaneous fluctuations such as regional homogeneity (Yuan et al., 2013) and regional connectivity (Yu et al., 2013), thus we limited analyses to ALFF for simplicity. Moreover, we measured functional connectivity based on the correlation between the BOLD time course of the seed region and that of all other areas in the brain (Shen, 2015). The functional connectivity method has also been widely used in studies on psychiatric disorders (Lee, Smyser, & Shimony, 2013).
Studies on shame and guilt using rs-fMRI techniques are scarce, while previous work showed that PTSD patients compared to controls are associated with altered spontaneous brain activity during rs-fMRI in several areas including those within DMN such as mPFC, PCC (Wang et al., 2016), and IPL (Disner, Marquardt, Mueller, Burton, & Sponheim, 2018). Moreover, altered resting-state functional connectivity of amygdala was reported in PTSD (Brown et al., 2014), and the functional connectivity patterns in DMN were also found to relate with PTSD symptoms (King et al., 2016; Reuveni et al., 2016). Previous task-based or resting-state fMRI studies have not examined the neural correlates of MIES. Our study was partly hypothesis driven and partly exploratory study. We investigated the neural correlates of MIES and PTSD indexed by either ALFF in regions of interest (ROIs) including the DMN areas as well as amygdala and dACC (Disner et al., 2018) or functional connectivity between these ROIs (seeds) and the rest of the brain. We also examined the relationships between resting-state brain responses and transgression- or betrayal-related subscores of the MIES.
2 |. METHODS AND MATERIALS
2.1 |. Participants and procedure
Detailed demographic and clinical information are described in Table 1. Participants were recruited from Iraq and Afghanistan era military service members in the VA Mid-Atlantic MIRECC Post-Deployment Mental Health Repository (Brancu et al., 2017). The present moral injury study combined data from 26 participants who participated in two postrepository studies of combat-exposed veterans focused on (1) moral injury and (2) rs-fMRI. In study (1), approximately 300 participants completed questionnaire packets by mail that included MIES to assess moral injury (Nash et al., 2013), depressive symptoms using the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996), and combat exposure with the Combat Exposure Scale (CES; Lund, Sipprelle, Foy, & Strachan, 1984). In study (2), participants completed a battery of measures, including determination of PTSD diagnosis using the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995; Weathers et al., 2013) based on symptoms experienced in the past month. Fourteen participants completed DSM-IV and the remaining 12 participants completed DSM-5. To ensure consistency and reliability of CAPS scores between subjects, and because it is possible to “translate” from DSM-5 to DSM-IV but not the reverse, we used established methods for converting the CAPS-5 scores to CAPS-IV scores for the 12 subjects scanned after migrating to CAPS-5 (Supporting Information). The method is adapted from personal communication with Dr. Brian Marx at the National Center for PTSD (Boston, Massachusetts; Weathers, Brian, Matthew, & Paula, 2014). Eleven out of 26 participants were diagnosed with PTSD. Descriptions of the CAPS, MIES, BDI-II, and CES are in the Supporting Information. To be eligible for the present study, participants needed to have deployed to a combat zone and could not have a DSM-IV diagnosis of psychosis. All participants in this study provided verbal informed consent to participate in procedures reviewed and approved by the Institutional Review Boards at Duke University and the Durham VA Medical Center.
TABLE 1.
Demographic and clinical information (N = 26, two females)
| Mean | STD | Range | Max range | |
|---|---|---|---|---|
| Age (years) | 43.5 | 8.8 | 28–55 | NA |
| MIES-transgression | 14.2 | 7.2 | 6–30 | 6–36 |
| MIES-betrayal | 6.3 | 4.5 | 3–18 | 3–18 |
| MIES-total | 20.5 | 10.5 | 9–44 | 9–54 |
| CAPS | 28.5 | 32.9 | 0–100 | 0–136 |
| BDI-II | 12.5 | 14.4 | 0–54 | 0–69 |
| CES | 10.6 | 9.94 | 0–29 | 41 |
Note. MIES-transgression and MIES-betrayal were measured by the Moral Injury Events Scale (MIES; Nash et al., 2013). PTSD symptoms were measured by the Clinician Administered PTSD Scale (CAPS; Weathers et al., 2014). Depressive symptoms were measured by the Beck Depression Inventory-II (Beck et al., 1996). Combat exposure was measured by the Combat Exposure Scale (CES; Lund et al., 1984). STD, standard deviation; Range, range of values in our sample; Max Range, possible range according to the questionnaires and scales.
2.2 |. Brain image acquisition, preprocessing, and ROI selection
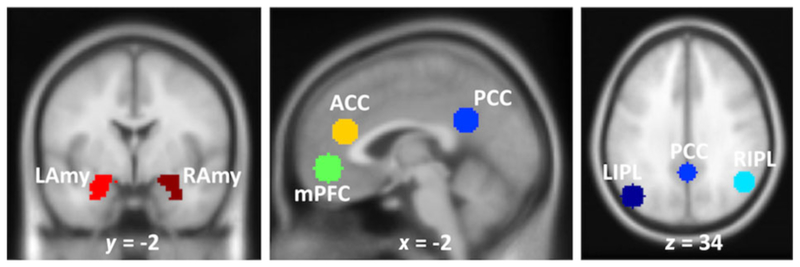
The detailed information of brain image acquisition, preprocessing, and ROI (also seed) selection can be found in the Supporting Information. We here employed seven ROIs: mPFC, PCC, left/right IPL, left/right Amygdala, and dACC, as shown in Figure 1.
FIGURE 1.
Regions of interest (ROIs) in the default mode network (DMN). mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; L/R IPL, left/right inferior parietal lobule; ACC, anterior cingulate cortex; L/R Amy, left/right amygdala
2.3 |. Data analytic plan
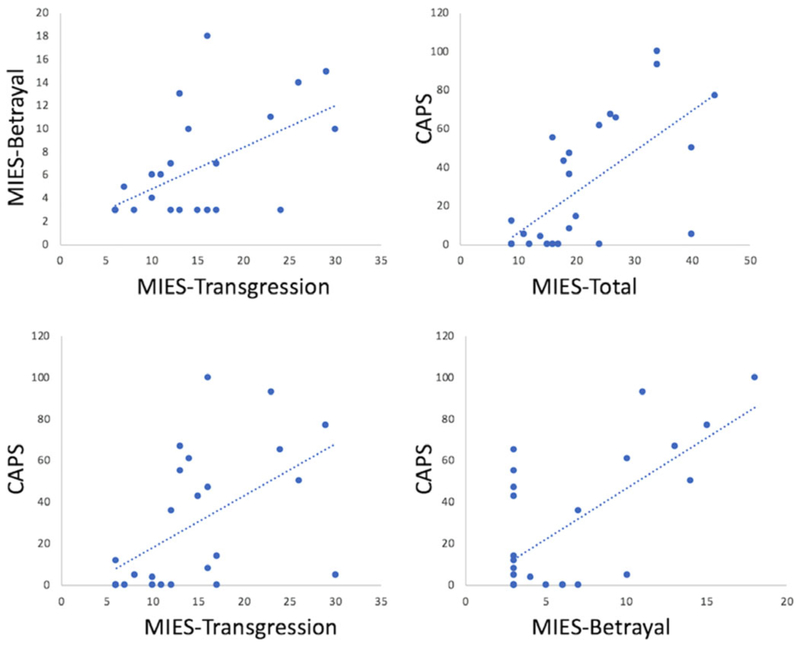
Consistent with previous reports on the relationship between MIES and PTSD measures (Bryan et al., 2016; Nash et al., 2013), the following findings motivated the selection of our statistical models. We found that the CAPS score was positively correlated with MIES-total (R = 0.667, P < 0.001), MIES-transgression (R = 0.546, P = 0.004), and MIES-betrayal (R = 0.672, P < 0.001). MIES-transgression was also found to positively correlated with MIES-betrayal (R = 0.569, P = 0.002). However, as shown in Figure 2, MIES-total, MIES-transgression, and MIES-betrayal are all correlated with the CAPS to varying degrees, but each contributes unique variance to the relationship. This observation motivated us to examine possible dissociations between the neural correlates of related concepts.
FIGURE 2.
Scatter plots showing relationship between MIES and CAPS scores. MIES-total, MIES-transgression, and MIES-betrayal are all correlated with the CAPS to varying degrees, but each contributes unique variance to the relationship
Two statistical models were employed to fully understand the relationship between resting-state brain responses and self-report of morally injurious events as well as PTSD. In both models, age, sex, BDIII, CES scores, and study protocols were entered as covariates of no interest. In Model I, we investigated the neural correlates of either MIES-total (the sum of MIES-transgression and MIES-betrayal scores) or CAPS scores. MIES-total score was listed as a covariate of no interest when studying the neural correlates of CAPS, and CAPS score was a covariate of no interest when studying the neural correlates of MIES-total.
Model I may have overlooked the differences of moral injury sub-scales of transgression and betrayal. To further understand the neural underpinnings of moral injury subscales, Model II was used to investigate the neural correlates of either MIES-transgression, MIES-betrayal, or CAPS scores. Similar to Model I, when investigating the neural correlates of one clinical measure, the other measure served as a covariate of no interest.
To investigate whether the correlations were statistically different, we employed the Williams’s t-test (Weaver & Wuensch, 2013), which is appropriate for the comparison between two nonindependent correlations with a variable in common.
2.4 |. ALFF ROI analyses
The mean ALFF values from each of the seven ROIs were extracted using the MarsBar toolbox (http://marsbar.sourceforge.net). The MATLAB partial correlation function was utilized to control for the effects of covariates of no interest. The FDR method (Benjamini & Hochberg, 1995) was applied to correct for the number of correlations (total number = 14) across seven ROIs and two clinical measures (MIES-total and CAPS) in Model I. It was also employed for the number of correlations (total number = 21) across seven ROIs and three clinical measures (MIES-transgression, MIES-betrayal, and CAPS) in Model II.
2.5 |. ALFF whole-brain analyses
The ROI analyses may have overlooked the MIES- or CAPS-related ALFF changes in regions outside the selected ROIs. We thus employed two multiple regression models (corresponding to Model I and II, respectively) to investigate the relationship between whole-brain voxel-wise ALFF and each of the variables of interest after adjusting for other variables. The whole-brain voxel-wised ALFF analysis was both confirmatory to the ROI analysis and complementary to the ROI analysis. Results were thresholded at P < 0.001 uncorrected and survived P < 0.05 cluster-extent size false discovery rate (FDR) correction.
2.6 |. Seed-based functional connectivity whole-brain analyses
Based on previous neuroimaging studies on moral processing and PTSD, we expected to find MIES- or CAPS-related functional connectivity with the predefined seeds (i.e., aforementioned ROIs). We employed two multiple regression models (corresponding to Model I and II, respectively) to investigate how the functional connectivity between a seed (one of seven ROIs mentioned above) and voxels in the rest of the brain was related with each of the variables of interest after controlling the effects of all the other covariates. Results were thresholded at P < 0.001 uncorrected and survived P < 0.007 (<0.05/7 given that there were seven seeds) cluster-size FDR correction. If a clinical measure was found to significantly correlate with functional connectivity between a seed and a target brain area, further voxelwise analyses were conducted to test whether the other clinical measures also correlated with functional connectivity between the same seed and target pair. The findings were thresholded at P < 0.001 uncorrected and survived P < 0.05 small volume corrected (SVC) within the target area.
3 |. RESULTS
3.1 |. ALFF ROI findings
For Model I, partial correlations between MIES-total or CAPS scores and average ALFF in all of the ROIs are shown in Table 2. No results from Model I survived FDR corrections.
TABLE 2.
Partial correlations between clinical measures and average ALFF in ROIs (Model I)
| ROI | MIES-total | CAPS | ||||
|---|---|---|---|---|---|---|
| R | P | adj_P | R | P | adj_P | |
| mPFC | 0.231 | 0.357 | 0.677 | −0.065 | 0.799 | 0.979 |
| PCC | 0.541 | 0.020† | 0.280 | −0.441 | 0.067 | 0.469 |
| LIPL | 0.224 | 0.371 | 0.677 | −0.217 | 0.387 | 0.677 |
| RIPL | 0.158 | 0.533 | 0.746 | −0.019 | 0.942 | 0.979 |
| dACC | 0.169 | 0.502 | 0.746 | 0.235 | 0.349 | 0.677 |
| LAmy | 0.007 | 0.979 | 0.979 | 0.044 | 0.861 | 0.979 |
| RAmy | −0.334 | 0.176 | 0.616 | 0.384 | 0.115 | 0.537 |
Note. adj_P, adjusted P value through FDR correction. The partial correlation between ALF and one variable of interest was conducted while controlling the effects of all the other variables noninvestigated. mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; L/R IPL, left/right inferior parietal lobule; dACC, dorsal anterior cingulate cortex; L/R Amy, left/right amygdala.
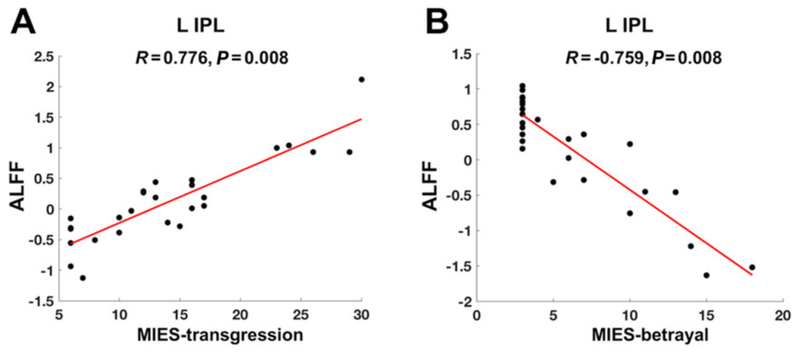
For Model II, partial correlations between MIES-transgression/-betrayal or CAPS scores and average ALFF in ROIs are reported in Table 3. ALFF in L-IPL was positively related with MIES-transgression (R = 0.776, P = 0.008 FDR corrected, Figure 3a), negatively related with MIES-betrayal (R = −0.759, P = 0.008 FDR corrected, Figure 3b), and has no relationship with CAPS scores (R = −0.337, P = 0.615 FDR corrected). Moreover, Williams’s t-tests showed that the ALFF correlation with MIES-transgression was significantly larger than the correlation with MIES-betrayal (t = 8.188, P < 0.001) and the correlation with CAPS (t = 7.852, P < 0.001). The ALFF correlation with MIES-betrayal was not significantly different from the correlation with CAPS (t = −2.090, P = 0.976).
TABLE 3.
Partial correlations between clinical measures and average ALFF in ROIs (Model II)
| ROI | MIES-transgression | MIES-betrayal | CAPS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R | P | adj_P | R | P | adj_P | R | P | adj_P | |
| mPFC | 0.314 | 0.220 | 0.462 | −0.103 | 0.695 | 0.859 | −0.060 | 0.818 | 0.904 |
| PCC | 0.337 | 0.186 | 0.462 | 0.468 | 0.058 | 0.269 | −0.459 | 0.064 | 0.269 |
| LIPL | 0.776 | 0.000† | 0.001* | −0.759 | 0.000† | 0.001* | −0.337 | 0.186 | 0.462 |
| RIPL | 0.210 | 0.419 | 0.616 | −0.061 | 0.815 | 0.904 | −0.015 | 0.954 | 0.954 |
| ACC | 0.315 | 0.218 | 0.462 | −0.201 | 0.440 | 0.616 | 0.252 | 0.329 | 0.616 |
| LAmy | −0.135 | 0.606 | 0.795 | 0.207 | 0.425 | 0.616 | 0.040 | 0.880 | 0.924 |
| RAmy | −0.492 | 0.045† | 0.269 | 0.219 | 0.399 | 0.616 | 0.404 | 0.108 | 0.378 |
Note: adj_P, adjusted P value through FDR correction.
Results survived P < 0.05 FDR correction.
P < 0.05 without correction. The partial correlation between ALFF and one variable of interest was conducted while controlling the effects of all the other variables noninvestigated. mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; L/R IPL, left/right inferior parietal lobule; dACC, dorsal anterior cingulate cortex; L/R Amy, left/right amygdala.
FIGURE 3.
ALFF partial correlations. Larger ALFF In the ROI of left inferior parietal lobule (L-IPL) was associated with (a) higher scores of moral injury transgression (MIES-transgression) (R = 0.776, P = 0.008 FDR corrected) and (b) lower scores of moral injury betrayal (MIES-betrayal; R = −0.759, P = 0.008 FDR corrected). The mean ALFF values in the scatter plots are adjusted to regress out the effects of all the other variables noninvestigated
3.2 |. ALFF whole-brain analyses findings
For Model I, MIES-total was negatively related with ALFF in the right posterior insula (maximum effect at x/y/z/ = 38/−20/20, Z-value = 4.57, cluster size = 132 voxels).
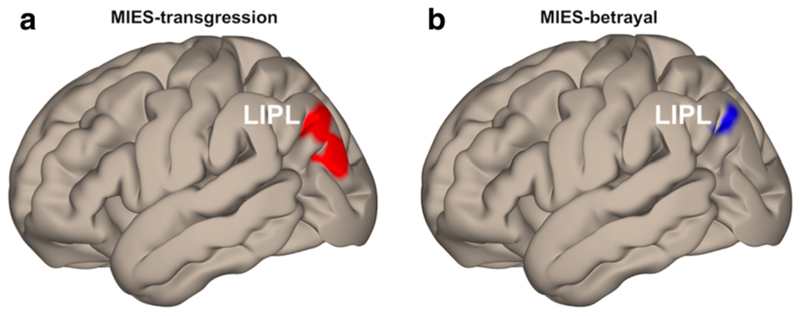
For Model II, as shown in Table 4, MIES-transgression was positively related with ALFF in L-IPL (Figure 4a), and negatively related with ALFF in the right fusiform gyrus and right posterior insula. MIES-betrayal was positively related with ALFF in the left precuneus and negatively related with ALFF in the left angular gyrus within L-IPL (Figure 4b) and right superior parietal lobule.
TABLE 4.
Correlations between whole-brain voxel-wise ALFF and MIES subscales
| MNI | |||||
|---|---|---|---|---|---|
| Area | Size | Z | x | y | z |
| ALFF positively related with MIES-transgression | |||||
| L inferior parietal Lobule (BA19/39) | 361 | 4.39 | −42 | −78 | 38 |
| ALFF negatively related with MIES-transgression | |||||
| R fusiform gyrus (BA18/19) | 173 | 4.00 | 30 | −76 | −16 |
| R posterior insula (BA13) | 109 | 3.93 | 36 | −18 | 20 |
| ALFF positively related with MIES-betrayal | |||||
| L precuneus (BA7) | 112 | 4.22 | −14 | −54 | 54 |
| ALFF negatively related with MIES-betrayal | |||||
| L angular gyrus (BA39) | 135 | 4.30 | −42 | −78 | 40 |
| R superior parietal lobule(BA7) | 128 | 3.80 | 28 | −60 | 62 |
Note. All results were height-thresholded at P < 0.001 and survived P < 0.05 cluster-level FDR correction. BA, Brodmann’s area; Size, number of voxels within the cluster; Z, z value; x/y/z, MNI coordinates. L, left; R, right.
FIGURE 4.
Whole-brain voxelwise ALFF correlations. Larger ALFF in left inferior parietal lobule (LIPL) was associated with (a) higher scores of moral injury transgression (MIES-transgression, maximum effect at x/y/z/ = −42/−78/38) and (b) lower scores of moral injury betrayal (MIES-betrayal, maximum effect at x/y/z/ = −42/−78/40). Results were height-thresholded at P < 0.001 uncorrected and survived P < 0.05 cluster-extent level FDR correction
3.3 |. Seed-based whole-brain functional connectivity analyses results
For Model I, MIES-total was positively correlated with functional connectivity between right amygdala seed and right thalamus (maximum effect at x/y/z/ = 14/−34/6, Z-value = 4.73, cluster size = 214 voxels). No significant relationship was detected between CAPS and functional connectivity to the same seed-target pair.
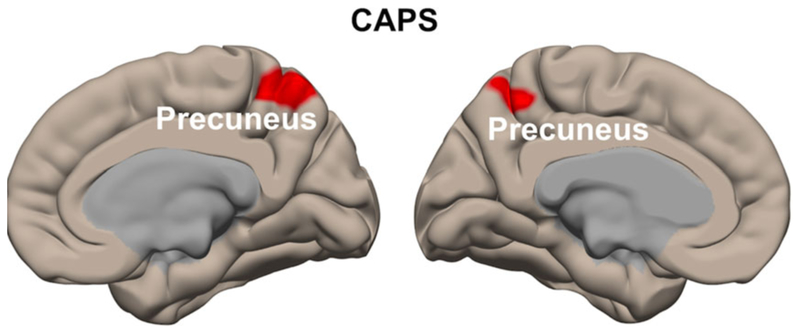
The CAPS was positively correlated with functional connectivity between L-IPL seed and bilateral precuneus (maximum effect at x/y/z/ = −10/−54/52, Z-value = 4.21, cluster size = 461 voxels, Figure 5). Further analyses showed that MIES-total was negatively correlated with functional connectivity to the same seed-target pair (maximum effect at x/y/z/ = −10/−52/52, Z-value = 3.28, cluster size = 8 voxels, SVC).
FIGURE 5.
Whole-brain seed-based functional connectivity correlations. CAPS was positively correlated with functional connectivity between L-IPL seed and left precuneus (maximum effect at x/y/z/ = −10/−54/52) as well as right precuneus (maximum effect at x/y/z/ = 6/−50/58). The MIES-total was negatively correlated with functional connectivity to the same seed-target pair
For Model II, MIES-transgression was positively correlated with functional connectivity between left amygdala seed and left fusiform gyrus (maximum effect at x/y/z/ = −34/−42/−30, Z-value = 4.56, cluster size = 250 voxels). Further analyses showed that both MIES-betrayal (maximum effect at x/y/z/ = −34/−40/−32, Z-value = 4.11, cluster size = 20 voxels, SVC) and CAPS (maximum effect at x/y/z/ = −42/−54/−26, Z-value = 3.92, cluster size = 40 voxels, SVC) were negatively correlated with functional connectivity for the same seed-target pair.
The CAPS was positively correlated with functional connectivity between L-IPL seed and left precuneus (maximum effect at x/y/z/ = −10/−54/52, Z-value = 4.08, cluster size = 212 voxels) and right precuneus (maximum effect at x/y/z/ = 6/−50/58, Z-value = 3.84, cluster size = 210 voxels). Neither MIES-transgression nor MIES-betrayal was found to correlate with functional connectivity between L-IPL seed and precuneus.
4 |. DISCUSSION
The present study examines the neural correlates of MIES and CAPS, as well as the neural correlates of two MIES subscales, transgression, and betrayal, in combat veterans. We found that ALFF in the L-IPL was positively related with MIES-transgression, negatively associated with MIES-betrayal, and had no relationship with CAPS. Functional connectivity between L-IPL and bilateral precuneus was positively related with CAPS and negatively related with total scores of MIES. These results mark the L-IPL as a locus of dissociable neural correlates between MIES and CAPS, as well as a location of distinct brain responses to MIES score from transgressive acts and MIES score from betrayal.
Our most interesting finding is the different brain response patterns between MIES and CAPS scores. The existing literature finds that moral injury and PTSD are highly correlated constructs (Frankfurt & Frazier, 2016; Nash et al., 2013). These studies found that PTSD can be elicited not only by a close brush with death or serious injury, but also non-A1 stressors (Weathers & Keane, 2007) such as some morally injurious events (Nash et al., 2013). However, Bryan et al. (2016) recently reported a three-factor solution to MIES scores, which includes transgressions by others, transgressions by self, and betrayal. They found that the posttraumatic stress positively predicted transgression by others and betrayal, and negatively predicted transgression by self (although nonsignificant). Their results suggest that the subscores of MIES are differentially associated with PTSD. More recent finding by Bryan et al. (2018) utilizing exploratory structural equation modeling show that PTSD is uniquely characterized by startle reflex, memory loss, and self-reported flashbacks, whereas moral injury is uniquely characterized by guilt, shame, anhedonia, and social alienation. Their behavioral findings are consistent with our brain imaging results—neural correlates of morally injurious events and symptoms as measured specifically by MIES and its subscales may be differentiated from the neural correlates of PTSD as measured by the CAPS.
Our findings suggest that different brain response patterns in brain areas such as L-IPL may underlie the distinct and related constructs of moral injury and PTSD. The IPL serves as a major hub for integrating multisensory information inputs for comprehension and manipulation (Tomasi & Volkow, 2011). It is also an important component of both the DMN (Andrews-Hanna et al., 2014) that plays a role in internally directed or self-generated thoughts and the ToM regions, which infer the mental states of others (Mar, 2011). This area robustly activates during response to moral dilemmas, violations of moral principles, and making moral decisions (Heekeren et al., 2005). The bilateral angular gyrus, in close proximity to the IPL, was found to be more active in the moral-personal condition of pushing a stranger off a bridge to stop a trolley from killing five people, than when switching the tracks of a trolley to kill one person instead of five (Greene, Sommerville, Nystrom, Darley, & Cohen, 2001). These previous studies highlight the role of IPL in social cognition and moral processing, which are consistent with the present findings that show morally injurious events are correlated with resting-state brain responses in the L-IPL. Moreover, our findings also imply that the MIES reflects individual differences in moral processing, because the content of MIES items refers to moral evaluations of self and others’ behaviors. A veteran, who has witnessed moral transgressions committed by others, will interpret these events from the point of view of the self. This requires both interpreting the thoughts and feelings of others, as well as their interpretation in relation to one’s own experiences. A veteran, who has committed self-transgressions (ostensibly perpetrated against other individuals), may be occupied by thoughts about how these might be viewed by others, particularly fellow veterans. The ALFF activation we found in the ToM and self-referential processing regions is consistent with these interpretations. By contrast, we did not find any significant relationship between ALFF in IPL and CAPS scores. This negative finding is inconsistent with a recent meta-analysis reporting that spontaneous brain activity in the L-IPL is positively correlated with PTSD symptom severity (Disner et al., 2018). One potential explanation is that the aforementioned meta-analysis that is based on PTSD did not elaborate on the potential biases incurred by moral injury, shame, guilt, anger, or disrupted social cognition that are often accompanied by PTSD symptoms (Bryan et al., 2016, 2018; Litz et al., 2009). The exact role of resting-state spontaneous fluctuations in L-IPL still needs further investigation in PTSD. On the other hand, Bryan et al. (2018) confirmed that both guilt and shame are associated with moral injury but not PTSD, although they have been added into the DSM-5 diagnosis of PTSD (Weathers et al., 2013). It is possible that ALFF in L-IPL is associated with the neural processing of shame or guilt, although our present results do not necessarily support this conclusion given that we did not specifically examine the neural correlates of shame or guilt. This hypothesis is attractive because it is consistent with previous studies. For instance, the ratings of guilt to scenarios were reported to positively relate with brain activity in the left posterior superior temporal sulcus (Takahashi et al., 2004) and the left supramarginal gyrus (Morey et al., 2012), which are both in close proximity to L-IPL. In addition, both shame and guilt elicit activation in the left superior temporal gyrus, which is close to L-IPL (Michl et al., 2014). The exact role of resting-state spontaneous fluctuations in L-IPL still needs further investigation in PTSD and moral injury.
It is also interesting that ALFF in L-IPL was positively correlated with transgression scores and negatively correlated with betrayal scores. This finding suggests distinct neural underpinnings in L-IPL between perceived transgression and betrayal, consistent with a previous behavioral study (Nash et al., 2013) that dissociated the two latent factors in veterans suffering from moral injury. However, it is hard to determine the exact alteration in neural processing in IPL solely based on ALFF, given the complicated relationship between resting-state brain responses and task-related brain activations. First, stronger ALFF may be related to larger task-related activation in some areas, but smaller activation in other areas (Zou et al., 2013). Second, larger ALFF does not necessarily represent more efficient processing but a compensatory effect for deficits in patients with specific disorders (Tan et al., 2016). Third, beyond social cognition, the IPL is associated with semantic processing, number processing, memory retrieval, spatial attention, and reasoning (Seghier, 2013). Resting-state data cannot differentiate multiple functions in the same area and therefore the exact role of L-IPL in moral injury needs to be clarified with task-based neuroimaging studies. The IPL is more active when engaged in tasks evaluating moral dilemmas (Greene et al., 2001), but less active when posed with moral conflicts as compared to analogous nonmoral scenarios (Borg, Hynes, Van Horn, Grafton, & Sinnott-Armstrong, 2006). A recent study on moral transgression in healthy participants by Crockett, Siegel, Kurth-Nelson, Dayan, and Dolan (2017) developed a task paradigm in which nonclinical participants made decisions whether to accrue monetary benefits by inflicting pain on others. Future neuroimaging studies employing this paradigm or similar tasks may help to directly investigate the neural responses to moral dilemmas and social decisions in people suffering from moral injury.
Aside from our ROI results, we also found that the MIES-transgression score was negatively related with ALFF in the right fusiform gyrus and right posterior insula, whereas MIES-betrayal score was positively related with ALFF in the left precuneus and negatively related with ALFF in the right superior parietal lobule. The fusiform gyrus plays a crucial role in processing facial stimuli (Kanwisher & Yovel, 2006); the insula cortex is engaged during emotional and empathic processes (Pascual, Rodrigues, & Gallardo-Pujol, 2013). The precuneus and superior parietal lobule are either a part of, or close to, DMN (Andrews-Hanna et al., 2014) and ToM regions (Mar, 2011), which play an important role in processing information about sense of self and others. Our findings support the assertion that the individual differences in experiencing morally injurious events are associated with different brain responses in different brain areas related to social cognition and emotion processing.
Besides the ALFF findings, we found that functional connectivity between L-IPL and bilateral precuneus was positively related with PTSD symptoms and negatively associated with MIES total scores, providing further support of the neural dissociation between subjective self-appraisals of exposure to morally injurious events and PTSD symptoms. The IPL-precuneus functional connections have been reported in previous rs-fMRI studies (Igelstrom, Webb, & Graziano, 2015). Task-based studies have also documented the coactivations of IPL and precuneus in attention, self-perception, introspection and memory, and social cognition (Schurz, Radua, Aichhorn, Richlan, & Perner, 2014). It is possible that moral injury and PTSD are different in a few of these cognitive functions. We also found that MIES-total score was positively correlated with functional connectivity between the right amygdala seed and right thalamus. Moral transgression and betrayal are associated with harm that can be imposed by conspecifics, such as aggressive attack, social exclusion, and reputation damage (Frankfurt & Frazier, 2016), which are all related with extreme negative emotions. The thalamus projects to limbic subcortical structures, particularly the amygdala and ventral striatum, and hence is widely involved in affective processing (Pessoa, 2017; Vertes, Linley, & Hoover, 2015). It is possible that the amygdala–thalamus functional connection detected in our study plays roles in regulating the affective processing associated with moral transgression and betrayal. Interestingly, the amygdala–thalamus circuit has been reported to play an essential role in both the establishment of fear memory and the expression of fear responses (Penzo et al., 2015). However, we did not find a significant relationship between this circuit and PTSD. This distinction suggests that the thalamus–amygdala circuit may involve subcomponents in response to fear and social harm, respectively, and that resting-state functional connectivity might be insensitive to the individual differences in fear processing. The functional connectivity between left amygdala seed and left fusiform gyrus was positively correlated with MIES-transgression and negatively correlated with MIES-betrayal and CAPS scores. A previous study found that functional connectivity between left amygdala and face-related areas including fusiform gyrus was correlated with the subjective threat rating for faces (Miyahara, Harada, Ruffman, Sadato, & Iidaka, 2013), and patients with social anxiety disorder showed positive correlation between anxiety severity and amygdala–fusiform functional connectivity in response to fearful faces (Frick, Howner, Fischer, Kristiansson, & Furmark, 2013). It is possible that MIES-transgression versus MIES-betrayal and CAPS scores are associated with different socioemotional information processing. These hypotheses will need to be tested in future studies.
There are a few limitations in the present study. First, the correlation and regression models utilized here may overlook the nonlinear relationships between clinical measures and brain responses. Future studies aimed at dissociating the neural correlates of moral injury and PTSD may consider comparing four groups of participants: (a) PTSD without moral injury, (b) controls exposed to life-threatening events without PTSD or moral injury, (c) high scores in moral injury without PTSD, and (d) low scores in moral injury without PTSD. Thus, the contrast between group 1 and 2 will unveil the neural correlates of PTSD, whereas the comparison between group 3 and 4 will uncover the neural underpinnings of moral injury. Second, the MIES-transgression subscore includes a mix of exposures (questions 1, 3, and 5 of the MIES) and symptoms (questions 2, 4, and 6 of the MIES), whereas the MIES-betrayal subscore pertains only to symptoms. It is unclear whether the neural correlates that we observed stemmed from exposures or symptoms of MIES-transgression. Indeed, our analysis (see Supporting Information) demonstrated that ALFF in the left IPL was positively correlated with both the symptoms of (R = 0.780, P = 0.001) and exposure to (R = 0.706, P = 0.011) moral transgression, which makes it challenging to investigate their differences. Perhaps new questionnaires that are currently under development to assess moral injury will more effectively dissociate these phenomena. We know for instance that trauma exposure and PTSD symptoms are generally highly correlated, but we also know that among certain individuals and groups (e.g., resilient individuals) that trauma exposure and PTSD symptoms are relatively weakly correlated. Third, recent work by Bryan et al. (2016) used explanatory factor analysis to show that MIES-transgression could be subdivided into two subscales, transgressions by others (transgressions-others) was related to posttraumatic stress, whereas transgressions by self (transgressions-self) was accompanied with hopelessness, pessimism, and anger. We explored transgression-self and transgression-others associated neural correlates and found that ALFF in the left IPL was positively related to transgressions-self but not transgressions-others (see Supporting Information). Future studies on moral injury with an improved classification will help to delineate the neurobiological subtypes of moral injury. Fourth, morally injurious experiences are often accompanied with different negative emotions such as shame and guilt (Nash et al., 2013). Anger is possibly a more prominent emotional consequence of transgressions-self and betrayal-related moral injury (Bryan et al., 2016). Future studies should clarify the emotion-specific neural processing in moral injury. Fifth, the CAPS is a clinician-administered questionnaire, whereas the MIES is a self-report questionnaire. This difference might contribute to disparate neural findings in theory, but we have no plausible explanation to account for distinct neural responses related to the method of gathering data (clinician- vs. self-administered). Future work investigating the neural correlates of symptoms of either moral injury or PTSD will help to delineate the underpinnings of the two clinically overlapping syndromes in overlapping populations.
In conclusion, we found that CAPS and MIES sub-scales, that is, transgression and betrayal, are dissociated by ALFF in the L-IPL. Moreover, CAPS and MIES total scores are differentiated by the functional connectivity between L-IPL and precuneus. Our findings significantly enhance our understanding of the neural correlates of moral injury vis-à-vis PTSD, and shed light on neural targets for potential clinical interventions. Knowledge of relevant targets could help predict, guide selection, or monitor treatment response of psychotherapy, pharmacotherapy, or brain stimulation, which may be optimally suited for individual patients. To the best of our knowledge, this is the first publication uncovering the neural correlates associated with moral injury, and the first study that documents the neural differences between moral injury events and PTSD. The shame- and guilt-related disturbances in moral injury offer a complementary model that extends prevailing fear and threat models of PTSD. Expanding our investigation into the neuroscience of moral processing may open new avenues of research that enrich our understanding of PTSD beyond the existing fear-based models (Pitman et al., 2012).
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the U.S. Department of Veterans Affairs (VA) Mid-Atlantic Mental Illness Research, Education, and Clinical Center (MIRECC) core funds and financial support by the Duke Health Scholars Award to Dr. Morey. Dr. Sun was supported by the NIH (5R01-NS086885) and Mid-Atlantic MIRECC pilot research funds. We thank Mira Brancu PhD and Kevin LaBar PhD for their helpful comments and suggestions in the early versions of the manuscript.
Funding information
Dr. Sun reports grants from NIH, grants from Mid-Atlantic MIRECC, during the conduct of the study. Dr. Phillips has nothing to disclose. Dr. Mulready has nothing to disclose. Dr. Zablonski has nothing to disclose. Dr. Jessica Turner has nothing to disclose. Dr. Matthew Turner has nothing to disclose. Dr. McClymond has nothing to disclose. Dr. Morey reports grants from U.S. Department of Veterans Affairs (VA) MIRECC, grants from Duke University, during the conduct of the study.
Footnotes
DISCLOSURES
The authors have no conflict of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Year in Cognitive Neuroscience, 1316, 29–52. 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S (1995). Mindblindness—An essay on autism and theory of mind. Cambridge, MA: Bradford Book. [Google Scholar]
- Bastin C, Harrison BJ, Davey CG, Moll J, & Whittle S (2016). Feelings of shame, embarrassment and guilt and their neural correlates: A systematic review. Neuroscience & Biobehavioral Reviews, 71, 455–471. 10.1016/j.neubiorev.2016.09.019 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corp. [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate—A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological, 57(1), 289–300. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. [DOI] [PubMed] [Google Scholar]
- Borg JS, Hynes C, Van Horn J, Grafton S, & Sinnott-Armstrong W (2006). Consequences, action, and intention as factors in moral judgments: An fMRI investigation. Journal of Cognitive Neuroscience, 18(5), 803–817. 10.1162/jocn.2006.18.5.803 [DOI] [PubMed] [Google Scholar]
- Brancu M, Wagner HR, Morey RA, Beckham JC, Calhoun PS, Tupler LA, … Workgrp VM-AM (2017). The Post-Deployment Mental Health (PDMH) study and repository: A multi-site study of US Afghanistan and Iraq era veterans. International Journal of Methods in Psychiatric Research, 26(3). 10.1002/mpr.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VM, Labar KS, Haswell CC, Gold AL, McCarthy G, Morey RA, & Workgrp M-AM (2014). Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in post-traumatic stress disorder. Neuropsychopharmacology, 39(2), 351–359. 10.1038/npp.2013.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan CJ, Bryan AO, Anestis MD, Anestis JC, Green BA, Etienne N, … Ray-Sannerud B (2016). Measuring moral injury: Psychometric properties of the moral injury events scale in two military samples. Assessment, 23(5), 557–570. 10.1177/1073191115590855 [DOI] [PubMed] [Google Scholar]
- Bryan CJ, Bryan AO, Roberge E, Leifker FR, & Rozek DC (2018). Moral injury, posttraumatic stress disorder, and suicidal behavior among national guard personnel. Psychological Trauma-Theory Research Practice and Policy, 10(1), 36–45. 10.1037/tra0000290 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network—Anatomy, function, and relevance to disease. Year in Cognitive Neuroscience 2008, 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Siegel JZ, Kurth-Nelson Z, Dayan P, & Dolan RJ (2017). Moral transgressions corrupt neural representations of value. Nature Neuroscience, 20(6), 879–885. 10.1038/nn.4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG, Marquardt CA, Mueller BA, Burton PC, & Sponheim SR (2018). Spontaneous neural activity differences in post-traumatic stress disorder: A quantitative resting-state meta-analysis and fMRI validation. Human Brain Mapping, 39(2), 837–850. 10.1002/hbm.23886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfurt S, & Frazier P (2016). A review of research on moral injury in combat veterans. Military Psychology, 28(5), 318–330. 10.1037/mil0000132 [DOI] [Google Scholar]
- Frick A, Howner K, Fischer H, Kristiansson M, & Furmark T (2013). Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Translational Psychiatry, 3, e312 10.1038/tp.2013.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Roach BJ, Ford JM, Turner JA, van Erp TGM, Voyvodic J, … Mathalon DH (2015). Relating intrinsic low-frequency BOLD cortical oscillations to cognition in schizophrenia. Neuropsychopharmacology, 40(12), 2705–2714. 10.1038/npp.2015.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, & Cohen JD (2001). An fMRI investigation of emotional engagement in moral judgment. Science, 293(5537), 2105–2108. 10.1126/science.1062872 [DOI] [PubMed] [Google Scholar]
- He Z, Deng W, Li M, Chen Z, Jiang L, Wang Q, … Li T (2013). Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychological Medicine, 43(4), 769–780. 10.1017/S0033291712001638 [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Prehn K, Schwintowski HP, & Villringer A (2005). Influence of bodily harm on neural correlates of semantic and moral decision-making. Neuroimage, 24(3), 887–897. 10.1016/j.neuroimage.2004.09.026 [DOI] [PubMed] [Google Scholar]
- Hughes KC, & Shin LM (2011). Functional neuroimaging studies of post-traumatic stress disorder. Expert Review of Neurotherapeutics, 11(2), 275–285. 10.1586/Ern.10.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelstrom KM, Webb TW, & Graziano MSA (2015). Neural processes in the human temporoparietal cortex separated by localized independent component analysis. Journal of Neuroscience, 35(25), 9432–9445. 10.1523/Jneurosci.0551-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E (2018). Moral injury in time of war. Lancet, 391(10132), 1766–1767. 10.1016/S0140-6736(18)30946-2 [DOI] [PubMed] [Google Scholar]
- Kanwisher N, & Yovel G (2006). The fusiform face area: A cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society B-Biological Sciences, 361(1476), 2109–2128. 10.1098/rstb.2006.1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Thibodeau R, & Jorgensen RS (2011). Shame, guilt, and depressive symptoms: A meta-analytic review. Psychological Bulletin, 137(1), 68–96. 10.1037/a0021466 [DOI] [PubMed] [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, … Liberzon I (2016). Altered default mode network (DMN) resting state functional connectivity following a mindfulness-based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depression and Anxiety, 33(4), 289–299. 10.1002/da.22481 [DOI] [PubMed] [Google Scholar]
- Lee MH, Smyser CD, & Shimony JS (2013). Resting-state fMRI: A review of methods and clinical applications. American Journal of Neuroradiology, 34(10), 1866–1872. 10.3174/ajnr.A3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Ma N, Liu Y, He XS, Sun DL, Fu XM, … Zhang DR (2013). Resting-state functional connectivity predicts impulsivity in economic decision-making. Journal of Neuroscience, 33(11), 4886–4895. 10.1523/Jneurosci.1342-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litz BT, Stein N, Delaney E, Lebowitz L, Nash WP, Silva C, & Maguen S (2009). Moral injury and moral repair in war veterans: A preliminary model and intervention strategy. Clinical Psychology Review, 29(8), 695–706. 10.1016/j.cpr.2009.07.003 [DOI] [PubMed] [Google Scholar]
- Lund M, Sipprelle C, Foy D, & Strachan A (1984). The combat exposure scale—A systematic assessment of trauma in the Vietnam-war. Journal of Clinical Psychology, 40(6), 1323–1328. [DOI] [PubMed] [Google Scholar]
- Lv H, Wang Z, Tong E, Williams LM, Zaharchuk G, Zeineh M, … Wintermark M (2018). Resting-state functional MRI: Everything that non-experts have always wanted to know. American Journal of Neuroradiology, 39(8), 1390–1399. 10.3174/ajnr.A5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar RA (2011). The neural bases of social cognition and story comprehension. Annual Review of Psychology, 62, 103–134. 10.1146/annurev-psych-120709-145406 [DOI] [PubMed] [Google Scholar]
- McClymond K, & Anthony FL (2014). Moral injury: A case study in religion & violence In Ricci GR (Ed.), Faith, war, and violence (ch. 10). New York, NY: Routledge. [Google Scholar]
- Michl P, Meindl T, Meister F, Born C, Engel RR, Reiser M, & Hennig-Fast K (2014). Neurobiological underpinnings of shame and guilt: A pilot fMRI study. Social Cognitive and Affective Neuroscience, 9(2), 150–157. 10.1093/scan/nss114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, … Rauch SL (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry, 66(12), 1075–1082. 10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara M, Harada T, Ruffman T, Sadato N, & Iidaka T (2013). Functional connectivity between amygdala and facial regions involved in recognition of facial threat. Social Cognitive and Affective Neuroscience, 8(2), 181–189. 10.1093/scan/nsr085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, McCarthy G, Selgrade ES, Seth S, Nasser JD, & LaBar KS (2012). Neural systems for guilt from actions affecting self versus others. Neuroimage, 60(1), 683–692. 10.1016/j.neuroimage.2011.12.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash WP, Carper TLM, Mills MA, Au T, Goldsmith A, & Litz BT (2013). Psychometric evaluation of the moral injury events scale. Military Medicine, 178(6), 646–652. 10.7205/Milmed-D-13-00017 [DOI] [PubMed] [Google Scholar]
- Northoff G, & Bermpohl F (2004). Cortical midline structures and the self. Trends in Cognitive Sciences, 8(3), 102–107. 10.1016/j.tics.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Pascual L, Rodrigues P, & Gallardo-Pujol D (2013). How does morality work in the brain? A functional and structural perspective of moral behavior. Frontiers in Integrative Neuroscience, 7, 65 10.3389/fnint.2013.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, … Li B (2015). The paraventricular thalamus controls a central amygdala fear circuit. Nature, 519(7544), 455–459. 10.1038/nature13978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L (2017). A network model of the emotional brain. Trends in Cognitive Sciences, 21(5), 357–371. 10.1016/j.tics.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, … Liberzon I (2012). Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience, 13(11), 769–787. 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt MG, Luoma JB, & Freyd JJ (2017). Shame and dissociation in survivors of high and low betrayal trauma. Journal of Aggression Maltreatment & Trauma, 26(1), 34–49. 10.1080/10926771.2016.1228020 [DOI] [Google Scholar]
- Pulcu E, Lythe K, Elliott R, Green S, Moll J, Deakin JFW, & Zahn R (2014). Increased amygdala response to shame in remitted major depressive disorder. Plos One, 9(1), e86900 10.1371/journal.pone.0086900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin PM, & Northoff G (2011). How is our self related to midline regions and the default-mode network? Neuroimage, 57(3), 1221–1233. 10.1016/j.neuroimage.2011.05.028 [DOI] [PubMed] [Google Scholar]
- Reuveni I, Bonne O, Giesser R, Shragai T, Lazarovits G, Isserles M, … Levin N (2016). Anatomical and functional connectivity in the default mode network of post-traumatic stress disorder patients after civilian and military-related trauma. Human Brain Mapping, 37(2), 589–599. 10.1002/hbm.23051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth L, Kaffenberger T, Herwig U, & Bruhl AB (2014). Brain activation associated with pride and shame. Neuropsychobiology, 69(2), 95–106. 10.1159/000358090 [DOI] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, & Perner J (2014). Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 42, 9–34. 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Seghier ML (2013). The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist, 19(1), 43–61. 10.1177/1073858412440596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev A, Liberzon I, & Marmar C (2017). Post-traumatic stress disorder. New England Journal of Medicine, 376(25), 2459–2469. 10.1056/NEJMra1612499 [DOI] [PubMed] [Google Scholar]
- Shen HH (2015). Core concept: Resting-state connectivity. Proceedings of the National Academy of Sciences of the United States of America, 112(46), 14115–14116. 10.1073/pnas.1518785112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, & Okubo Y (2004). Brain activation associated with evaluative processes of guilt and embarrassment: An fMRI study. Neuroimage, 23(3), 967–974. 10.1016/j.neuroimage.2004.07.054 [DOI] [PubMed] [Google Scholar]
- Tan G, Huang X, Zhang Y, Wu AH, Zhong YL, Wu K, … Shao Y (2016). A functional MRI study of altered spontaneous brain activity pattern in patients with congenital comitant strabismus using amplitude of low-frequency fluctuation. Neuropsychiatric Disease and Treatment, 12, 1243–1249. 10.2147/Ndt.S104756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney JP, & Dearing RL (2002). Shame and guilt. New York, NY: The Guilford Press. [Google Scholar]
- Tangney JP, Stuewig J, & Mashek DJ (2007). Moral emotions and moral behavior. Annual Review of Psychology, 58, 345–372. 10.1146/annurev.psych.56.091103.070145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, & Volkow ND (2011). Association between functional connectivity hubs and brain networks. Cerebral Cortex, 21(9), 2003–2013. 10.1093/cercor/bhq268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, & Hoover WB (2015). Limbic circuitry of the midline thalamus. Neuroscience and Biobehavioral Reviews, 54, 89–107. 10.1016/j.neubiorev.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, N’Diaye K, Ethofer T, & Vuilleumier P (2011). Guilt-specific processing in the prefrontal cortex. Cerebral Cortex, 21(11), 2461–2470. 10.1093/cercor/bhr016 [DOI] [PubMed] [Google Scholar]
- Wang T, Liu J, Zhang JR, Zhan W, Li L, Wu M, … Gong QY (2016). Altered resting-state functional activity in posttraumatic stress disorder: A quantitative meta-analysis. Scientific Reports, 6, 27131 10.1038/srep27131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM (2013). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). Washington, DC: US Department of Veterans Affairs; Retrieved from www.ptsd.va.gov [Google Scholar]
- Weathers FW, Brian PM, Matthew JF, & Paula PS (2014). Posttraumatic stress disorder in DSM-5: New criteria, new measures, and implications for assessment. Psychological Injury and Law, 7(2), 93–107. [Google Scholar]
- Weathers FW, & Keane TM (2007). The Criterion A problem revisited: Controversies and challenges in defining and measuring psychological trauma. Journal of Traumatic Stress, 20(2), 107–121. 10.1002/jts.20210 [DOI] [PubMed] [Google Scholar]
- Weaver B, & Wuensch KL (2013). SPSS and SAS programs for comparing Pearson correlations and OLS regression coefficients. Behavior Research Methods, 45(3), 880–895. 10.3758/s13428-012-0289-7 [DOI] [PubMed] [Google Scholar]
- Yu QB, Sui J, Liu JY, Plis SM, Kiehl KA, Pearlson G, & Calhoun VD (2013). Disrupted correlation between low frequency power and connectivity strength of resting state brain networks in schizophrenia. Schizophrenia Research, 143(1), 165–171. 10.1016/j.schres.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Di X, Kim EH, Barik S, Rypma B, & Biswal BB (2013). Regional homogeneity of resting-state fMRI contributes to both neurovascular and task activation variations. Magnetic Resonance Imaging, 31(9), 1492–1500. 10.1016/j.mri.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, … Wang YF (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain & Development, 29(2), 83–91. 10.1016/j.braindev.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Zou QH, Ross TJ, Gu H, Geng XJ, Zuo XN, Hong LE, … Yang YH (2013). Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Human Brain Mapping, 34(12), 3204–3215. 10.1002/hbm.22136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, … Milham MP (2010). The oscillating brain: Complex and reliable. Neuroimage, 49(2), 1432–1445. 10.1016/j.neuroimage.2009.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.