Abstract
Background:
Renal cell carcinoma has become known as a “metabolic disease” owing to the diverse array of metabolic defects and perturbations that occur as a result of the unique genetics that can drive these tumors. Recent attention to this feature of renal cell carcinomas has fueled interest in targeting metabolism as a therapeutic strategy.
Methods:
We conducted a literature search to develop themes around discrete pathways or processes of cellular metabolism, and to provide a framework for understanding emerging therapeutic strategies and to consider future interventions.
Results:
Defects occur in metabolic pathways ranging from glycolysis to mitochondrial function, and impact not only the tumor cell functionality, but also the local environment. We identified opportunities for therapeutic intervention associated with each pathway.
Conclusion:
The metabolism of RCC cells presents a special environment of tumor susceptibilities, with opportunities for novel imaging applications and treatment paradigms that are being tested in monotherapy or as adjuncts to targeted or immune based strategies.
Keywords: renal cell carcinoma, kidney cancer, glycolysis, Krebs cycle, mitochondria, metabolism
INTRODUCTION
The renal cell carcinomas (RCC) represent a diverse set of tumors arising from the kidney cortex, including cancers derived from the proximal and distal portions of the nephron, as well as the collecting duct and renal medulla. This review will limit considerations to the diseases linked with the nephron proper: clear cell, papillary, and chromophobe renal cell carcinoma, which have features that have led investigators to label this cancer as a “metabolic disease”, and one ripe for considerations to apply metabolic pressures for therapeutic advantage1,2.
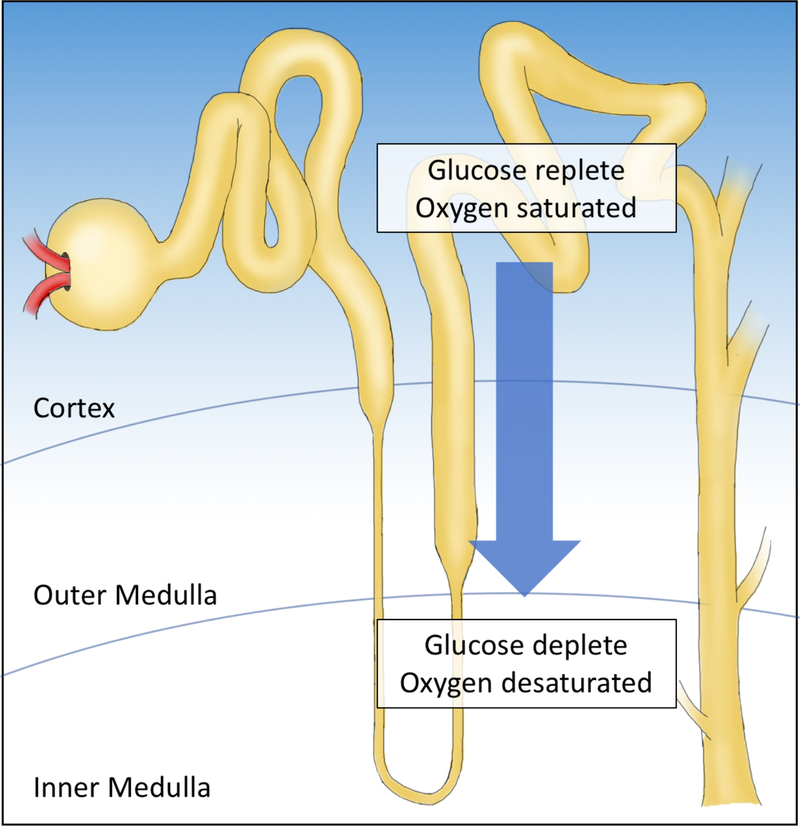
The myriad of metabolic disturbances has invited speculation that the unique environment of the kidney promotes these alterations. The kidney is subject to a remarkably high blood volume, and responds to some of the lowest levels of oxygen in the human body. The kidney is best known for it’s specialized function of filtering waste products and transporting ions for maintaining osmotic homeostasis. In fact, the nephron establishes numerous gradients that regulate and respond to oxygen, glucose, urea, and other critical metabolic features. The gradient of oxygen by kidney nephrons was reported nearly sixty years ago and contributes to a lower urinary oxygen concentration than is even present in renal venous blood3,4. The anatomy of the afferent and efferent vasculature allows for oxygen and glucose levels to remain higher in the cortex. Moreover, the kidney maintains mechanisms that provide for a consistent oxygen level in the proximal and distal portions of the nephron, while the medulla is highly sensitive to changes in blood oxygen levels5 (Figure 1.). Thus, it is plausible that the kidney is a unique tissue to transition to malignancy, and not surprising that metabolic changes dominate the features of this cancer.
Figure 1. Metabolic gradients applied across the nephron.
In the kidney, the afferent and efferent vessels are closely associated with the nephron loops, accounting for maintained nutrient and oxygen levels in the cortex, and depleted levels in the medulla.
This review will explore the major sources of metabolic impact in kidney tumors, through the classical biochemistry of cellular metabolism: highlighting features associated with the pseudohypoxia associated with clear cell renal cell carcinoma (ccRCC), disturbances of glucose regulation and glycolysis, factors influencing the Kreb’s cycle in papillary renal cell carcinoma (pRCC), factors that fuel reductive carboxylation, and mitochondrial defects resulting from mutations in electron transport genes occurring in chromophobe renal cell carcinoma (chRCC). Therapeutic strategies are in development around all of these pathways. Finally, the impact on the microenvironment, immune cell functionality, and systemic responses will also be considered.
MOLECULAR GENETICS OF RCC: FIRST STEP IN UNDERSTANDING RCC METABOLISM
Decades of study of the genomics of RCC have shown that kidney cancer is not a single disease6. More recently, high throughput sequencing has confirmed that this is a heterogenous class of diseases7. Work from the Cancer Genome Atlas (TCGA) took up the challenge to tackle separate projects revealing the integrated genetic features of clear cell8, papillary9, and chromophobe10 renal cell carcinomas. Other reviews have detailed the underpinning genetics of these subtypes11.
Not surprisingly, all three reference papers have devoted main sections to the metabolic derangements of the tumors. Recent studies have integrated the data across the RCC types12 to reveal similarities and key differences13, and metabolism pathways emerged again. A new pan-kidney cancer analysis put forward by the TCGA demonstrated mitochondrial activity as a key discriminating feature and reveals ribose sugar metabolism as a feature associated with worse overall prognosis across histologies14.
Consistent with the identification of “Metabolism” as a Hallmark of Cancer by Hanahan and Weinberg15, all histologies of RCC display increases in metabolic activity with disease progression. In particular, one common feature is the invocation of aerobic glycolysis, the “Warburg phenomenon”16. Each RCC is linked to activation of hypoxia response signaling, in the presence of oxygen, creating a classical set-up for this metabolic process. Multiple studies have conducted investigations of metabolic reprogramming, and identified correlation with stage and grade, implying an evolution that involves additional metabolic changes over the natural history of RCC17,18. Linked samples revealing an association between poor transcript-based prognostic scoring and regions displaying higher glucose uptake19. Further, specific metabolic intermediates may promote disease progression, e.g. acetyl-CoA synthetase is associated with cell migration and invasion as a result of changes in cell surface protein expression20.
The TCGA study of nearly 500 ccRCCs showed that high grade, high stage, and low survival was associated with a shift to aerobic glycolysis, dependence on the pentose phosphate shunt and decreased oxidative phosphorylation8. A recent direct examination of the metabolomics of RCC21 using mass spectroscopy for >800 unique metabolites coupled with RNA sequencing revealed the challenges between interpreting transcription level data to understanding the active processes of metabolism. Both progression and metastasis were associated with increases in metabolite levels of glutathione and cysteine/methionine, implicating redox-associated metabolic transitions as critical events in disease progression. Capturing a snapshot of the dynamic processes involved in energy generation and the creation of biomass, further underscored the feature of metabolic processes as a hallmark of this set of cancers.
THE VHL/HIF AXIS AND ccRCC METABOLISM
HIF, Pseudohypoxia, and Glycolysis
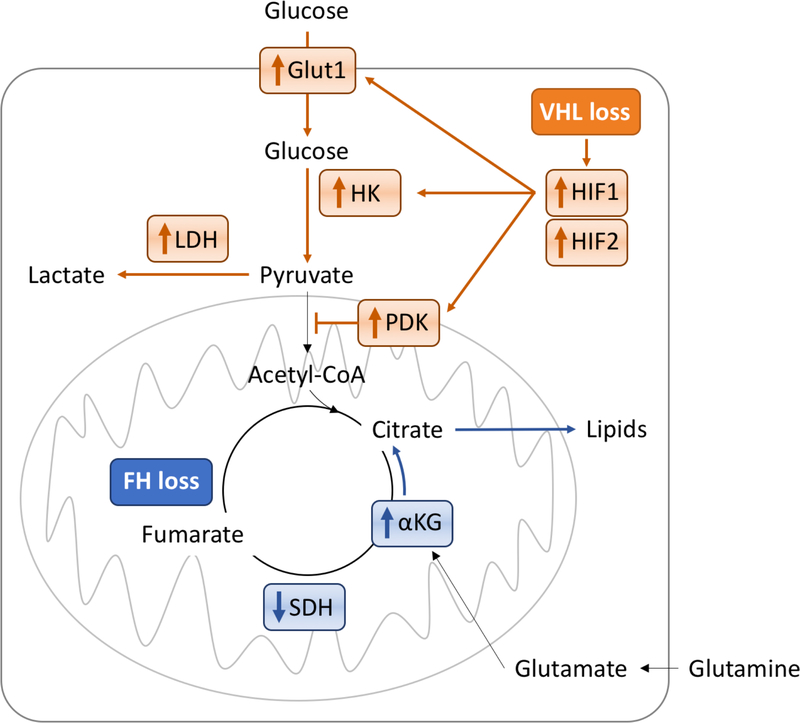
It is impossible to consider metabolism without examining the quintessential phenotype of ccRCC; dysregulation of hypoxia signaling via mutations in the VHL gene22. VHL is a key mediator of oxygen sensing, and absence or alteration of this protein is associated with stabilization of hypoxia inducible factor (HIF) family members. In normoxia, the potent transcription factors, HIF1 and HIF2, undergo prolyl hydroxylation which allows the VHL complex to target and degrade HIF. In hypoxia, HIF does not undergo prolyl hydroxylation, the VHL complex cannot target and degrade HIF, and HIF accumulates. However, with mutation (or loss) of VHL, such as in ccRCC, VHL cannot target and degrade HIF, even in normoxia, and HIF accumulates. HIF accumulation then activates the expression of a broad repertoire of genes involved in the hypoxic response pathway, including notable regulators of glucose uptake and glycolytic metabolism23. This pseudohypoxic response is a robust transcriptional activation of key factors, e.g. glucose transporter Glut1, hexokinase, and lactate dehydrogenase. In addition, HIF activates pyruvate dehydrogenase kinase, which results in pyruvate being shunted to lactate production, rather than being converted to acetyl Co-A as a fuel for the Krebs cycle24 (Figure 2).
Figure 2. Glycolysis and Kreb’s cycle are impacted in genetically diverse renal tumors.
Numerous features of renal tumorigenesis impact the flux of glucose to drive the generation of ATP, reducing equivalents, and biomass. Impacting distinct limbs of these central metabolic processes, VHL mutation, associated with ccRCC, drives a classical Warburg phenomenon (effects shown in orange), whereas FH mutation, associated with CIMP type pRCC, uncouples the Krebs cycle to allow for glutaminolysis, reductive carboxylation, and additionally aerobic glycolysis (effects downstream of glycolysis shown in blue).
Li et al25 demonstrated that another key glycolytic enzyme, fructose-1-bisphophatase (FBP1), also plays a critical role in titrating the activation of this pathway. Loss of FBP1 was observed universally in ccRCC tumors, and demonstrated function as a tumor suppressor. In the absence of FBP1, a block on glycolytic flux in renal tubular epithelial cells is released, allowing the Warburg effect to proceed unabated. FBP1 further functions as an inhibitor of HIF nuclear function, such that it’s absence permits a more robust HIF transcriptional response.
Beyond glycolysis: HIF2 emerges as the major driver of ccRCC
Intriguingly, while HIF1 is a major mediator of key glycolytic factors, HIF2 appears to have a larger role in altering lipid metabolism26. HIF2 contributes additionally to serine and one carbon metabolism, a pathway that converts glycolytic intermediates to serine via the rate limiting enzyme PHGDH27. These aspects of cellular metabolism regulated by HIF2 provide unique opportunities for novel imaging strategies or therapeutic interventions.
The growth of VHL mutated cancer cells is dependent on HIF upregulation28. Further dissection of the two dominant HIF isoforms demonstrated that HIF1 had greater impact on both aerobic and anaerobic glycolysis29, and those tumors that displayed exclusively HIF2 upregulation varied in activation of myc and mTOR master regulatory genes30.
MASTER REGULATOR: A TARGET FOR METABOLIC INTERVENTION
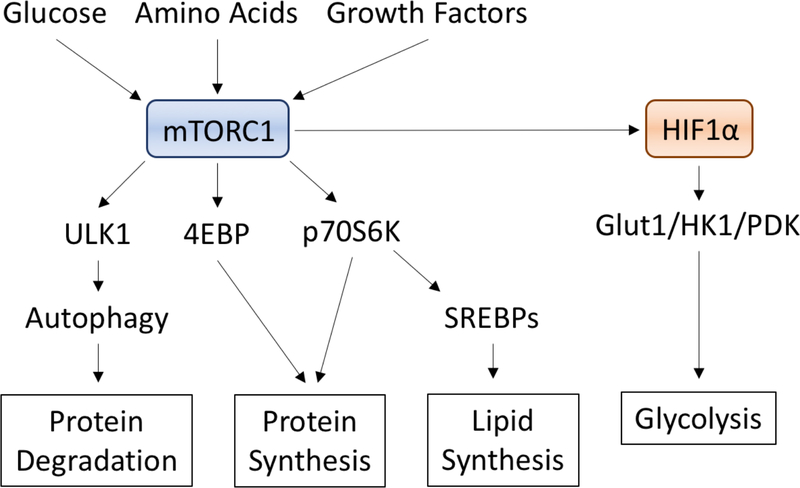
The first metabolic target advanced therapeutically in RCC was mTOR. Multiple lines of evidence implied this was a high value target for RCC: upregulation of phosphor-AKT and phosphor-S6 in a subset of poor risk tumors, mTOR functioning as a regulator of HIF translation (and as a pathway activated as a result of HIF stabilization), and as a master regulator of glycolytic activity (Figure 3). Two drugs were approved targeting mTOR activity, temsirolimus and everolimus, in patients with poor risk or progressive disease31,32. Treatment with everolimus decreases glucose uptake in tumors33. Although the demonstrated benefit of these drugs was brief, certain patients have been observed to demonstrate prolonged response, and harbor mutations in components of the overall mTOR signaling pathway, including PTEN, PIK3CA, and activating mutations in mTOR itself34. These associations are tantalizing to consider, yet remain to be validated as predictive features. Ultimately, rapid reprogramming of signaling pathways and evidence of upregulation of the mTOR Complex I component RAPTOR contributes to resistance mechanisms35.
Figure 3. MTORC1 signaling as a master regulator of metabolic signaling.
MTOR complex 1 intercepts signals from glucose, growth factors, and amino acids to drive protein and lipid processing, in addition to promoting HIF1 translation. Inhibitors of MTORC1 are approved for the treatment of many forms of renal cell carcinoma.
FACTORS REGULATING GLUTAMINOLYSIS
Glutamine biology and a strategy for intervention
Another major nutrient that cancer cells utilize for creation of biomass and to support bioenergetics is glutamine. The Warburg effect drives glycolysis as a result of HIF upregulation and creates a demand for Krebs cycle intermediates which is fueled in part through glutamine uptake and conversion to alpha-ketoglutarate and reductive carboxylation36. These processes proceed unabated upon loss of the tumor suppressor N-myc downstream regulated gene 2 (NDRG2), which normally restrains glycolysis and glutaminolysis processes via inhibition of c-myc expression37. Intriguingly, a mouse model that incorporated n-myc with VHL loss demonstrated glutamine addiction as a driving feature38. The utilization of glutamine by ccRCC appears to vary with the expression of HIF1 or HIF2, and effects multiple functions in the cell, providing for biomass creation as well as suppressing oxidative stress39.
Measuring uptake of glutamine by kidney cancer cells provides an opportunity to expand functional imaging in these tumors40. More importantly, a reliance on glutamine by these cells presents an important opportunity for therapeutic intervention. Glutaminase inhibitors have been developed, and in cell lines these inhibitors negatively impact the cancer cells ability to conduct pyrimidine synthesis41. Clinical trials are ongoing for these agents alone, or in combination with mTOR inhibitors or immunotherapies.
KREB CYCLE ALTERATION: A VIEW TO PAPILLARY RCC
The biochemistry of Krebs cycle impairment
Although pRCC transcriptionally maps to the proximal tubule as it’s site of origin, there are key functional differences in metabolic processes highlighted in this population of cancers. pRCC itself is highly heterogeneous. Type I pRCC driven by MET activity, is relatively indolent. Type II pRCC includes a subset of tumors found to have driver mutations in the gene for the Krebs cycle enzyme, fumarate hydratase (FH)9, and presents with highly aggressive disease42, similar to the germline syndrome of hereditary leiomyomatosis and renal cell carcinoma (HLRCC). These tumors are characterized by impaired oxidative phosphorylation, increased glutaminolysis, and a subsequent Warburg metabolic shift to aerobic glycolysis43 (Figure 2). FH-deficient RCC is dependent on glucose for ATP production needed for the rapid proliferation that characterizes this disease44,45. Fumarate levels are increased in fumarate hydratase-deficient type 2 pRCC. Increased fumarate has been shown to inhibit prolyl hydroxylase46, which results in a VHL-independent dysregulation of HIF degradation in FH-deficient Type 2 papillary RCC. FH-deficient RCC has been shown to be characterized by glutamine dependent reductive carboxylation47, suggesting the possibility of also targeting the glutamine pathway in this type of RCC. A case study of demonstrated evidence of the metabolic reprogramming in the form of intensely FDG avid tumor features48.
A critical component of the cellular program resulting from FH deficiency is activation of the Nrf2/Keap2 pathway49. FH-deficient papillary RCC is characterized by increased ROS50 and Nrf2 enables cells to maintain redox balance to control excessive ROS. FH-deficiency leads to stabilization of Nrf251,52 and induction of stress-response genes and activation of ABL153. Two regulators of Nrf2 activity have been identified as potential targets for treatment of FH deficient tumors: iASPP, a protein that competes with Nrf2 for Keap1 binding54, and Abl tyrosine kinase, which plays a role in Nrf2 nuclear localization53.
Targeting metabolically reprogrammed tumors
CIMP tumors provide possibly the purest form of a metabolically altered, aggressive and highly lethal malignancy in which to apply metabolic interventional therapeutic strategies that attack the Achilles heel, per se55. Judicious application of GLUT1 inhibitors, currently in use as research tools, is considered to have potential in this cancer43. Similarly, glycolytic inhibitors, including 2-deoxyglucose (the tracer molecule widely utilized in trace quantities for FDG-PET scans), a glycolytic poison, has been considered as a potential therapeutic agent. Compassionate use in one patient48 was not successful. Other approaches have been considered, including targeting lactate dehydrogenase and applying strategies that exploit the vulnerability to reactive oxygen species of cells that are unable to generate sufficient reducing equivalents45.
ALTERNATIVE METABOLIC PROGRAMS: IDO-1, TRYPTOPHAN AND KYNURENINE
Other metabolites also play important roles in cellular viability and fitness. Kynurenine is a metabolite of tryptophan, converted through the enzymatic activity of IDO-1, with roles in immune cell fitness via the production of nicotinamide adenine dinucleotide (NAD+). It is elevated nearly 6-fold in ccRCC tissue and promotes RCC cells’ survival, migration, and chemoresistance through interaction with the aryl hydrocarbon receptor, the cofactor for HIF1 and HIF2 transcription factor activity. IDO-1 levels are associated with patient outcome56. Moreover, expression of IDO-1 in other components of the immune microenvironment (endothelial cells), has been associated with response to immune checkpoint blockade57. However, in spite of promising phase II results, the phase III ECHO-301/KEYNOTE-252 trial comparing IDO-1 in combination with pembrolizumab (PD-1 inhibitor) to pembrolizumab alone failed to improve progression free survival.
MITOCHONDRIA, AND THE SPECIAL CASE OF CHROMOPHOBE RCC
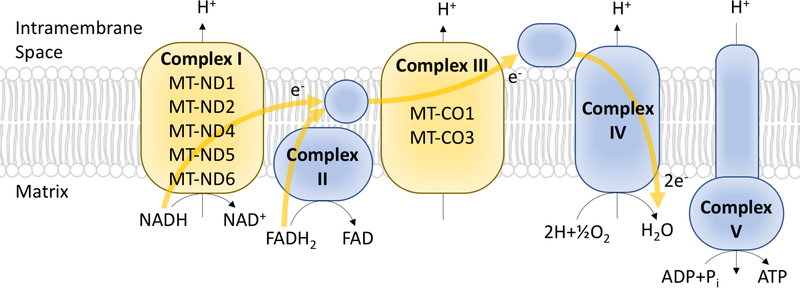
Finally, the mitochondrial electron transport chain plays a role in RCC and potential therapeutic strategies. One feature of chRCC, known as the eosinophilic variant, is recurrent mutations in genes involved in electron transport housed within the mitochondrial genome10 (Figure 4). The effect of these mutations is an accumulation of defective mitochondria, which may be a checkpoint limiting autophagy and inhibiting tumor progression58. The extent to which this activity applies more broadly to other types of tumors is unknown, and has widespread implications. Strategies to overcome mitochondrial deficits in human tumors are still preliminary, but early genetic studies suggest that ATP redistribution and mitochondrial reprogramming can create specific vulnerabilities, as in the case of the NOX4, which is upregulated in response to ATP redistribution, and promotes the stabilization of PKM2, establishing a potential sensitivity to agents that impede NOX4 or PKM2 activity59.
Figure 4. Mutations in the electron transport chain drive mitochondrial dysfunction in chRCC.
Mutations in mitochondrial genes (displayed in yellow) are encountered in the eosinophilic variant forms of chRCC. These mutations disrupt the normal polarization of mitochondria, and result in a compensatory increase in mitochondrial mass.
IMAGING METABOLIC FEATURES
The uptake of glucose by tumors using F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) scanning has long been a tool in the oncologist’s armamentarium that allows for visualizing and characterizing various tumor metabolic attributes. FDG uptake is variable in ccRCC and has never reached approval because of flaws as a detection tool due to heterogenous signal60. However, sources of variability imply potential biomarkers, and the intense avidity of FDG-PET in FH-mutant (CIMP) and other papillary tumors, highlights the distinct metabolic properties of these malignancies48. The metabolic heterogeneity of tumors can be characterized in a variety of ways. This was defined using magnetic resonance imaging (MRI) coupled FDG-PET19, which demonstrated that FDG avidity corresponded with differential patterns of gene expression and expression of metabolic proteins (FBP1).
METABOLISM, ENVIRONMENT, AND OBESITY/INFLAMMATION
Cellular metabolic changes do not happen in isolation, and the metabolic reprogramming events that have been described in this review alter the tumor microenvironment. These changes impact the balance of available nutrients, discarded metabolites, and secreted waste products and other local features, such as pH, oxygen tension, and redox state, that may directly or indirectly impact stromal and immune cells. The specific interactions are only beginning to be appreciated, but likely impact the may begin with tumor initiation. The earliest stages of VHL inactivation in precancerous cells can mount an inflammatory response, which sets the stage for later tumor cell-immune cell.
RCC tumors display among the highest signals of CD8 T cell infiltration, and intriguingly demonstrate T cells interspersed throughout the tumor, rather than in clusters. A first examination of these tumor infiltrating lymphocytes (TILs) found them to be functionally impaired in response to conventional stimuli, defective in nutrient utilization, and harboring fragmented and hyperpolarized mitochondria61. The metabolic defects of TIL in ccRCC tumors were shown to directly contribute to their impaired function, as bypassing the glycolytic defect and fueling the mitochondria directly allowed partial stimulation. Future strategies that alter T cell metabolism or the microenvironment to improve nutrient conditions for T cells may be successful in impacting the immune cell functional status of TIL.
Discovering the tumor, host, or environmental features that optimize the response to immune therapy will be the next major breakthrough in advancing immune treatments for this disease. Early indicators suggest that T cell exhaustion and anergy in tumors is not permanent62. Consistent with major themes for RCC biology, mTOR and HIF1 are key mediators of immune fate decisions for both CD4 and CD8 T cells63, and both PD-1 and CTLA4 checkpoints can potently suppress T cell metabolism to impair effector function64. Further interrogation of the unique interplay between RCC tumor cells and the immune response will undoubtedly involve metabolic cross talk between these key subsets of the tumor environment.
Obesity, inflammation, and RCC
RCC occurs in a host environment that may also impact the tumor itself. Unlike other neoplasms, RCC has a long history of obesity association with better outcome65–68. This finding has been associated with expression of fatty acid synthase as a possible mechanism, and extends to the biological risk classifications, although a careful assessment across RCC subtypes has not been performed in detail. Given the immune-infiltrated nature of RCC, and recent findings that obesity can enhance immunotherapy in melanoma68, inflammation associated with obesity may enhance intrinsic anti-tumor activities.
CONCLUSIONS, THERAPY, AND SPECIAL CONSIDERATIONS
To summarize all of the ways that renal carcinomas can impact and be impacted by cellular metabolism is beyond the scope of any singular review. RCC tumors define a special case of malignancies for which metabolic features in the tumor cells, the environment, and the immune response figure prominently, and where reprogramming of conventional metabolic processes is a critical part of the cancer picture. The potential opportunity to apply therapeutic strategies to modify metabolic processes, or to take advantage of metabolic vulnerabilities is especially tantalizing. A number of agents are in development that target aspects of metabolism, and a potential benefit is the milder side effect profile, which was observed with the early use of mTOR inhibitors. Further efforts are needed to develop metabolic inhibitors that are active, and not subject to additional reprogramming, and to apply strategies that consider the unique metabolic state of the distinct types of RCC.
TABLE 1.
Current and emerging compounds targeting metabolic pathways in RCC.
| Pharmaceutical | Mechanism of Action | Application | Status |
|---|---|---|---|
| Everolimus | mTOR | Second line | Approved |
| Temsirolimus | mTOR | First line | Approved |
| CB-839 | GLS1 inhibitor | Phase I/II | Investigational |
| WZB117 | Glut-1 inhibitor | Preclinical | Investigational |
| PT2977, PT2385 | HIF-2 inhibitor | Phase II | Investigational |
| Epacadostat | IDO-1 inhibitor | Phase II | Investigational |
| 2-deoxyglucose | Glycolysis inhibitor | Phase I/II | Investigational |
ACKNOWLEDGEMENTS
The authors would like to pay special thanks to Ayaka Sugiura for assistance in generating figures.
Research support for laboratory investigations (Calithera, Inc, Incyte Pharmaceuticals), JC Rathmell
Research support to institution for conduct of clinical trials (Calithera, Inc; Peloton, Inc; Novartis Pharmaceuticals), WK Rathmell
Footnotes
The authors declare the following conflict of interest:
REFERENCES
- 1.Teicher BA, Linehan WM, Helman LJ: Targeting cancer metabolism. Clin Cancer Res 18:5537–45, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudarshan S, Karam JA, Brugarolas J, et al. : Metabolism of kidney cancer: from the lab to clinical practice. Eur Urol 63:244–51, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aukland K: Urine oxygen tension; lack of correlation to some renal functions. Acta Physiol Scand 55:362–75, 1962 [DOI] [PubMed] [Google Scholar]
- 4.Aukland K, Krog J: Renal oxygen tension. Nature 188:671, 1960 [DOI] [PubMed] [Google Scholar]
- 5.Dyson A, Bezemer R, Legrand M, et al. : Microvascular and interstitial oxygen tension in the renal cortex and medulla studied in a 4-h rat model of LPS-induced endotoxemia. Shock 36:83–9, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Schmidt LS, Linehan WM: Genetic predisposition to kidney cancer. Semin Oncol 43:566–574, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brugarolas J: Molecular genetics of clear-cell renal cell carcinoma. J Clin Oncol 32:1968–76, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499:43–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research N, Linehan WM, Spellman PT, et al. : Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med 374:135–45, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis CF, Ricketts CJ, Wang M, et al. : The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 26:319–330, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haake SM, Weyandt JD, Rathmell WK: Insights into the Genetic Basis of the Renal Cell Carcinomas from The Cancer Genome Atlas. Mol Cancer Res 14:589–98, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaeffeler E, Buttner F, Reustle A, et al. : Metabolic and Lipidomic Reprogramming in Renal Cell Carcinoma Subtypes Reflects Regions of Tumor Origin. Eur Urol Focus, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Haake SM, Rathmell WK: Renal cancer subtypes: Should we be lumping or splitting for therapeutic decision making? Cancer 123:200–209, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricketts CJ, De Cubas AA, Fan H, et al. : The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Reports In press, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA: Hallmarks of cancer: the next generation. Cell 144:646–74, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Warburg O: On respiratory impairment in cancer cells. Science 124:269–70, 1956 [PubMed] [Google Scholar]
- 17.Bianchi C, Meregalli C, Bombelli S, et al. : The glucose and lipid metabolism reprogramming is grade-dependent in clear cell renal cell carcinoma primary cultures and is targetable to modulate cell viability and proliferation. Oncotarget 8:113502–113515, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wettersten HI, Hakimi AA, Morin D, et al. : Grade-Dependent Metabolic Reprogramming in Kidney Cancer Revealed by Combined Proteomics and Metabolomics Analysis. Cancer Res 75:2541–52, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks SA, Khandani AH, Fielding JR, et al. : Alternate Metabolic Programs Define Regional Variation of Relevant Biological Features in Renal Cell Carcinoma Progression. Clin Cancer Res 22:2950–9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao L, Guo X, Gui Y: Acetyl-CoA Synthetase 2 Promotes Cell Migration and Invasion of Renal Cell Carcinoma by Upregulating Lysosomal-Associated Membrane Protein 1 Expression. Cell Physiol Biochem 45:984–992, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Hakimi AA, Reznik E, Lee CH, et al. : An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell 29:104–116, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen C, Kaelin WG Jr.: The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol 23:18–25, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu CJ, Wang LY, Chodosh LA, et al. : Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 23:9361–74, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JW, Tchernyshyov I, Semenza GL, et al. : HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3:177–85, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Li B, Qiu B, Lee DS, et al. : Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature 513:251–5, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordan JD, Simon MC: Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev 17:71–7, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshino H, Nohata N, Miyamoto K, et al. : PHGDH as a Key Enzyme for Serine Biosynthesis in HIF2alpha-Targeting Therapy for Renal Cell Carcinoma. Cancer Res 77:6321–6329, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo K, Klco J, Nakamura E, et al. : Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell 1:237–46, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Lum JJ, Bui T, Gruber M, et al. : The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev 21:1037–49, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordan JD, Lal P, Dondeti VR, et al. : HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell 14:435–46, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudes G, Carducci M, Tomczak P, et al. : Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356:2271–81, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Motzer RJ, Escudier B, Oudard S, et al. : Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372:449–56, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Chen JL, Appelbaum DE, Kocherginsky M, et al. : FDG-PET as a predictive biomarker for therapy with everolimus in metastatic renal cell cancer. Cancer Med 2:545–52, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voss MH, Hakimi AA, Pham CG, et al. : Tumor genetic analyses of patients with metastatic renal cell carcinoma and extended benefit from mTOR inhibitor therapy. Clin Cancer Res 20:1955–64, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Earwaker P, Anderson C, Willenbrock F, et al. : RAPTOR up-regulation contributes to resistance of renal cancer cells to PI3K-mTOR inhibition. PLoS One 13:e0191890, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gameiro PA, Yang J, Metelo AM, et al. : In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab 17:372–85, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi W, Xu X, Yan F, et al. : N-Myc downstream-regulated gene 2 restrains glycolysis and glutaminolysis in clear cell renal cell carcinoma. Oncol Lett 14:6881–6887, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shroff EH, Eberlin LS, Dang VM, et al. : MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism. Proc Natl Acad Sci U S A 112:6539–44, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu Aboud O, Habib SL, Trott J, et al. : Glutamine Addiction in Kidney Cancer Suppresses Oxidative Stress and Can Be Exploited for Real-Time Imaging. Cancer Res 77:6746–6758, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Wildt B, Wilhelmus MM, Bijkerk J, et al. : Development of carbon-11 labeled acryl amides for selective PET imaging of active tissue transglutaminase. Nucl Med Biol 43:232–42, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Okazaki A, Gameiro PA, Christodoulou D, et al. : Glutaminase and poly(ADP-ribose) polymerase inhibitors suppress pyrimidine synthesis and VHL-deficient renal cancers. J Clin Invest 127:1631–1645, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merino MJ, Torres-Cabala C, Pinto P, et al. : The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol 31:1578–85, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Linehan WM, Rouault TA: Molecular pathways: Fumarate hydratase-deficient kidney cancer--targeting the Warburg effect in cancer. Clin Cancer Res 19:3345–52, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y, Valera V, Sourbier C, et al. : A novel fumarate hydratase-deficient HLRCC kidney cancer cell line, UOK268: a model of the Warburg effect in cancer. Cancer Genet 205:377–90, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sourbier C, Valera-Romero V, Giubellino A, et al. : Increasing reactive oxygen species as a therapeutic approach to treat hereditary leiomyomatosis and renal cell carcinoma. Cell Cycle 9:4183–9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isaacs JS, Jung YJ, Mole DR, et al. : HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8:143–53, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Mullen AR, Hu Z, Shi X, et al. : Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep 7:1679–1690, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamasaki T, Tran TA, Oz OK, et al. : Exploring a glycolytic inhibitor for the treatment of an FH-deficient type-2 papillary RCC. Nat Rev Urol 8:165–71, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arenas Valencia C, Lopez Kleine L, Pinzon Velasco AM, et al. : Gene expression analysis in peripheral blood cells of patients with hereditary leiomyomatosis and renal cell cancer syndrome (HLRCC): identification of NRF2 pathway activation. Fam Cancer, 2018 [DOI] [PubMed] [Google Scholar]
- 50.Sudarshan S, Sourbier C, Kong HS, et al. : Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1alpha stabilization by glucose-dependent generation of reactive oxygen species. Mol Cell Biol 29:4080–90, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adam J, Hatipoglu E, O’Flaherty L, et al. : Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell 20:524–37, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ooi A, Wong JC, Petillo D, et al. : An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell 20:511–23, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Sourbier C, Ricketts CJ, Matsumoto S, et al. : Targeting ABL1-mediated oxidative stress adaptation in fumarate hydratase-deficient cancer. Cancer Cell 26:840–850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ge W, Zhao K, Wang X, et al. : iASPP Is an Antioxidative Factor and Drives Cancer Growth and Drug Resistance by Competing with Nrf2 for Keap1 Binding. Cancer Cell 32:561–573 e6, 2017 [DOI] [PubMed] [Google Scholar]
- 55.Srinivasan R, Ricketts CJ, Sourbier C, et al. : New strategies in renal cell carcinoma: targeting the genetic and metabolic basis of disease. Clin Cancer Res 21:10–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lucarelli G, Rutigliano M, Ferro M, et al. : Activation of the kynurenine pathway predicts poor outcome in patients with clear cell renal cell carcinoma. Urol Oncol 35:461 e15–461 e27, 2017 [DOI] [PubMed] [Google Scholar]
- 57.Seeber A, Klinglmair G, Fritz J, et al. : High IDO-1 Expression in Tumor Endothelial Cells is Associated with Response to Immunotherapy in Metastatic Renal Cell Carcinoma. Cancer Sci, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joshi S, Tolkunov D, Aviv H, et al. : The Genomic Landscape of Renal Oncocytoma Identifies a Metabolic Barrier to Tumorigenesis. Cell Rep 13:1895–908, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shanmugasundaram K, Nayak BK, Friedrichs WE, et al. : NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat Commun 8:997, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrison MR, George DJ: Better late than early: FDG-PET imaging in metastatic renal cell carcinoma. Clin Cancer Res 17:5841–3, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Siska PJ, Beckermann KE, Mason FM, et al. : Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight 2, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clavijo-Salomon MA, Bergami-Santos PC, JA MB: Immunomonitoring reveals interruption of anergy after vaccination in a case of type-2-papillary renal cell carcinoma. Immunotherapy 9:319–329, 2017 [DOI] [PubMed] [Google Scholar]
- 63.Michalek RD, Gerriets VA, Jacobs SR, et al. : Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186:3299–303, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patsoukis N, Bardhan K, Chatterjee P, et al. : PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 6:6692, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albiges L, Hakimi AA, Xie W, et al. : Body Mass Index and Metastatic Renal Cell Carcinoma: Clinical and Biological Correlations. J Clin Oncol, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haake SM, Brooks SA, Welsh E, et al. : Patients with ClearCode34-identified molecular subtypes of clear cell renal cell carcinoma represent unique populations with distinct comorbidities. Urol Oncol 34:122 e1–7, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hakimi AA, Furberg H, Zabor EC, et al. : An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst 105:1862–70, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McQuade JL, Daniel CR, Hess KR, et al. : Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 19:310–322, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]