Abstract
In the present study we investigated the hepatotoprotective, hepatitis B virus (HBV) inhibitory and hepatic CYP450 enzyme (CYP3A4) modulatory potential of Cyperus rotundus rhizome fractions. The crude ethanol-extract, including different organic and aqueous fractions were tested for in vitro cytoprotection on HepG2 cells (MTT assay), followed by in vivo evaluation in Wistar rats (serum biochemistry and lipid profile). The in vitro anti-HBV activity was tested on HepG2.2.15 cells (HBsAg and HBeAg Elisa). Of these, the n-butanol and aqueous fractions showed the most promising, dose-dependent hepatoprotection in DCFH-injured HepG2 cells. Further, in CCl4-injured rats, oral administration of C. rotundus (100 and 200 mg/kg·bw/day) significantly normalized serum markers of healthy liver function (SGOT, SGPT, GGT, ALP and bilirubin) and lipid profile (cholesterol, HDL, LDL, VLDL, TG and MDA), including tissue NP-SH and TP levels. Compared to other fractions, the ethyl acetate, n-butanol and aqueous fractions exhibited the best inhibitory effects on viral HBsAg and HBeAg secretions in dose- and time-dependent manner. In addition, reporter gene assay (Dual-luciferase) of transfected HepG2 cells showed mild activation of nuclear PXR-mediated CYP3A4 gene by the three active fractions. Taken together, C. rotundus showed very promising hepatoprotective and anti-HBV potential in experimental settings. In addition, this is the first report on modulation of CYP3A4 by C. rotundus that suggests its safe consumption in relation to drug metabolism and efficacy. Our data could therefore, provide the basis for the ethnobotanical medicinal use of C. rotundus in metabolic liver disorder and hepatitis B patients.
Keywords: Cyperus rotundus, Hepatoprotection, Liver diseases, Hepatitis B virus, Anti-HBV, CYP3A4
1. Introduction
Liver diseases, the fifth most common cause of death is one of the major health problems worldwide (Williams, 2006). Liver disorders can be metabolic, occur in response to chemicals or toxins as well as because of infection with hepatotropic viruses like hepatitis B virus (HBV). HBV is responsible for about two billion hepatitis B cases worldwide, and of these, ∼40% of chronic carriers remain at risk to develop fulminant liver failure, cirrhosis or hepatocellular carcinoma (Williams, 2006, Teo and Locarnini, 2010). More than 50% of the world’s population lives in areas where HBV infection is highly endemic that include Asia, the Middle East and Africa (Williams, 2006, Teo and Locarnini, 2010, Torresi, 2008). Unfortunately, all currently approved preventive and therapeutic strategies against HBV have their own limitations. Despite the success of HBV vaccination programs, mutation in HBV surface/envelope gene (antigenic ‘a’ determinant region) can lead to vaccine failure (Teo and Locarnini, 2010, Torresi, 2008). Interferon-α chemotherapy have limited efficacy and a high incidence of adverse effects. Long-term treatment with nucleoside/nucleotide analog-based drugs like, lamivudine and avdifovir eventually leads to the emergence of drug-resistance viral mutants (Lok et al., 2007, Locarnini, 2008). In addition to this, the cost of these anti-HBV agents is too expensive for most developing countries. Therefore, there is an urgent need to search for new complementary and alternative anti-HBV agents with greater efficacy and safety.
Cyperus rotundus (Cyperaceae) is commonly known as nut grass (English) or nagarmotha (Hindi). It is a slender, erect, perennial grass with underground rhizomes which may swell into small, rounded structures (tubers) from which shoots, roots, and further rhizomes arise (Moosavinia and Dore, 1979). The tubers are externally blackish in color and reddish white inside, with a characteristic odor. C. rotundus is thought to be native to Asia, but it is found throughout tropical and subtropical regions around the world. The tubers are the most commonly used plant part, widely used in Indian Ayurveda and traditional medicine as stomachic astringent, sedative, stimulant, vermifuge, diaphoretic, diuretic, analgesic, antispasmodic and carminative (Kokate and Varma, 1982; Rakotonirina et al., 2011, Sonwa and Konig, 2011).
C. rotundus extracts are reported to have several biological activities like, anti-inflammatory (Tandon et al., 2010), in vivo hepatoprotective (Gilani and Janbaz, 1995), antimalarial (Thebtaranonth et al., 1995, Rukunga et al., 2008), antidiarrhoeal (Uddin et al., 2006), antioxidant (Yazdanparast and Ardestani, 2007) antimicrobial (Chen et al., 2011), antiplatelet (Seo et al., 2011), neuroprotective (Kumar et al., 2013), anti-hyperglycemic and anti-nociceptive (Alam et al., 2011, Chaulya et al., 2011). Previous phytochemical screening of C. rotundus showed that it contains many phytoconstituents including flavonoids (Sunil et al., 2011), alkaloids (Sayed et al., 2008) and polyphenols (Zhou and Yin, 2012, Dhillon et al., 1993). Moreover, eight sesquiterpenes, nootkatone, valencene epi-guaidiol, sugebiol, guaidiolA, sugetriol triacetate, cyperenoic acid and cyperotundone were isolated from its rhizomes (Thebtaranonth et al., 1995, Dhillon et al., 1993, Jin et al., 2011, Xu et al., 2009, Tsoyi et al., 2011). Notably, the C. rotundus rhizomes used as Ayurvedic medicine are also beneficial in jaundice and hepatitis B patients (Dr. B. P. Gupta, Aggarwal Hospital, New Delhi, India; personal communication).
The hepatic cytochrome P450 enzyme CYP3A4 is primarily involved in the metabolism of a variety of drugs, xenobiotics, and bioactive phytoproducts wherein its modulation via pregnane X receptor (PXR) is a major cause of adverse effects like drug non-response or organ toxicity (Al-Dosari and Parvez, 2016). In view of this, a good understanding of the therapeutic efficacy and safety of a medicinal herb or product in the modulation of CYP3A4 activity is necessary. With this background information, we therefore, investigated the hepatoprotective, HBV inhibitory and CYP3A4 modulatory activities of C. rotundus rhizomes organic and aqueous fractions.
2. Material and methods
2.1. Plant material
C. rotundus rhizomes were collected from northern India. Authentication of the plant was confirmed by an expert taxonomist at the herbarium of College of Pharmacy, King Saud University, Riyadh, and a voucher specimen was deposited.
2.2. Extraction and preparation C. rotundus fractions
The dried, powdered rhizomes of C. rotundus (100 g) were soaked in 80% aqueous ethanol (Merck, Germany) for two days at 25–30 °C and filtered. Extraction was repeated twice with the same solvent. The extract was collected, passed through Whatman filter paper no.1 (Sigma, USA) and then evaporated using a rotary evaporator (Buchi, Switzerland) under reduced pressure at 40 °C. The obtained greenish brown semi-solid extract (9.38 gm) was suspended in distilled water (200 ml), and then fractionated three times successively with the same volume of hexane (Merck, Germany), chloroform (Merck, Germany), ethyl acetate (Merck, Germany) and aqueous saturated n-butanol (LobaChemie, India) to provide the corresponding extracts. The organic solvents of the fractions were evaporated at reduced pressure using rotatory evaporator. For biological screening, each fraction was dissolved in dimethyl sulfoxide (DMSO, Sigma, USA), and diluted in RPMI 1640 media with the final concentrations (200, 100, 50, 25, 12.5, and 0 µg/ml). The final concentration of DMSO used never exceeded 0.1%, and therefore tolerated by cultured cells.
2.3. Cell cultures and drugs
Human liver carcinoma cell line HepG2 and its derivative HBV reporter line HepG2.2.15 (kind gift of Dr. S. Jameel, International Center for Genetic Engineering & Biotechnology, New Delhi, India). Cells were grown and maintained in RPMI-1640 medium (Gibco, USA), supplemented with 10% heat-inactivated bovine serum (Gibco, USA), 1xpenicillin-streptomycin mix, and 1x sodium pyruvate (HyClone Laboratories, USA) at 37 0C in a humified chamber with 5% CO2 supply. 2,7-Dichlorofluorescein (DCFH; Sigma, USA) was used as an inducer of in vitro hepatotoxicity. The approved nucleoside analog-based anti-HBV drug, lamivudine (3TC; Sigma, USA), was used as standard.
2.4. Cytotoxicity assay of C. rotundus fractions
Hepatotoxicity of the five fractions of C. rotundus rhizomes was tested on HepG2 cells by using MTT cell proliferation assay kits (Tervigen, USA) to determine concentrations (doses) that did not affect the cell viability, and used in subsequent antiviral assays. Briefly, cells (0.5 × 105/100 μl/well) were seeded in flat bottom 96-well tissue culture plates (Corning, USA), and incubated overnight. Next day, the cells were treated (in triplicate) with various doses (12.5, 25, 50, 100 and 200 μg/ml) prepared in culture media, and incubated for 48 h. A set of blank and untreated/negative controls were also included. Briefly, cells were treated with MTT reagent (10 μl/well) and further incubated for 3 h. Upon appearance of purple color, detergent solution (100 μl) was added to each well and further incubated for 1 h. The optical density (OD) was recorded at 570 nm in a microplate reader (BioTek, ELx800). Non-linear regression analysis was performed in Excel software to determine the concentration resulting in 50% cytotoxicity (CC50) using the following equation:
where OD[s], OD[b] and OD[c] are the absorbance of sample, blank and negative control, respectively.
2.5. In vitro hepatoprotective activity of C. rotundus fractions
HepG2 (0.5 × 105/100 μl/well) were seeded in a 96-well flat-bottom plate and grown overnight. The concentration of DCFH that caused 50% inhibition of cell proliferation (IC50: 100 μg/ml), was used as a cytotoxic dose (Al-Yahya et al., 2013, Arbab et al., 2015), prepared in DMSO. The final concentration of DMSO used never exceeded >0.1%, and therefore, were non-cytotoxic. The culture monolayer were replenished with RPMI-1640 containing 100 μg/ml DCFH plus a dose of plant fraction (25, 50, 100 and 200 μg/ml), including untreated as well as DCFH only-treated controls. All samples were in triplicate. The treated cells were incubated for 48 h at 37 °C followed by MTT assay as per the manufacturer’s instruction. The optical density (OD) was recorded at 570 nm in a microplate reader (BioTek, ELx800) and the data analyzed.
2.6. Microscopy
At 24 and 48 h post-treatment, cells were visually monitored for morhphological changes like, lesions of cell membrane and the compactness of cytoplasmic components under an inverted microscope (Optica, Italy) with 200× magnification.
2.7. Animals, experimental design and treatment
Wister rats (males; n = 30; ∼8 wks; ∼200 g) were procured (Experimental Animal Care Center, College of Pharmacy, KSU, Riyadh) and kept in polycarbonate cages in a sterile chamber (12 h dark/light cycle; 25 ± 2 °C) for after acclimatization. Rats were randomized and segregated into different groups (GI-GV; six, each). While GI served as untreated control and fed orally with normal saline (1.0 ml), GII, GIII, GIV and GV received CCl₄ in liquid paraffin (1:1; 1.25 ml/kg⋅bw; i.p.), GIII and GIV were orally fed with the C. rotundus ethanol-extract at 100 and 200 mg/kg⋅bw dose, respectively for three weeks. GV was orally administered with the standard, silymarin (10 mg/kg⋅bw) (Parveen et al., 2011) for three weeks. All animals were cared in compliance with the guidelines of the Ethics Committee of the Experimental Animal Care Society, KSU, Riyadh. Rats were anesthetized with sodium pentobarbital (Sigma–Aldrich, Germany; 50 mg/kg·bw; i.p.), sacrificed by cervical dislocation and blood were collected (21G syringe). The liver tissues were quickly dissected, washed (1× PBS) and preserved.
2.8. Estimation of liver enzymes, total protein and lipid profiling
Levels of serum glutamate oxaloacetate transaminase (SGOT), glutamate pyruvate transaminase (SGPT), alkaline phosphatase (ALP), γ-glutamyl transferase (GGT) and bilirubin (BIL) were determined using Reflotron kits (Roche Diagnostics, Germany) and Reflotron Plus Analyzer (Woodley Equipment Co., Ltd., UK). Serum total cholesterol (TC), triglycerides (TG), high-density lipoproteins (HDL) were determined using Reflotron Kits and Plus Analyzer. Very low-density lipoproteins [VLDL = TG/5] and low-density lipoproteins [LDL = TC − (VLDL + HDL)] were calculated. Serum total protein (TP; rescent Diagnostics Kit, Jeddah, Saudi Arabia) was estimated: TP = [ODsample/ODstandard] × Concentration of standard.
2.9. Determination of liver malondialdehyde and nonprotein sulfhydryls
Liver tissue malondialdehyde (MDA) was determined as described elsewhere (Utley et al., 1967). Briefly, tissues were homogenized (Potter-Elvehjem Type-C Homogenizer) in ice-cold 0.15 M KCl. The absorbance (λ = 532 nm) was read and the MDA content (nmol/g wet tissue) was calculated with reference to a standard curve of MDA solution. The nonprotein sulfhydryls (NP-SH) concentrations were measured as described elsewhere (Sedlak and Lindsay, 1986). Briefly, tissues were homogenized in ice-cold 0.02 mM EDTA and the absorbance (λ = 412 nm) was measured after adding 5,5′dithio-bis(2-nitrobenzoic acid).
2.10. Dose-dependent analysis of HBsAg expression in treated cells
HepG2.2.15 cells were seeded in 96 well plates (0.5 × 105/well) and incubated overnight at 37 °C. Next day, the culture media were replaced with the various doses (12.5, 25, 50 and 100 μg/ml) of test samples and controls, and incubated for 48 h. The culture supernatants of each sample were collected and stored at −20 °C for further analysis. The secreted viral HBsAg in the culture supernatants were analyzed by enzyme immunoassays (ELISA) kit (MonolisaHBsAg ULTRA, BioRad, USA) according to the manufacturer’s instructions. The absorbance (OD) was recorded using microplate reader (BioTek, ELx800), and analyzed as per BioRad manual. Non-linear regression analysis was performed using Excel software to determine the concentration (dose) resulting in 50% inhibition (IC50) of HBsAg secretion.
2.11. Time-course analysis of HBsAg inhibition
Based on the dose-dependent inhibition results, further antiviral investigation of the active fractions was subjected to time-course (day 1, 3 and 5) analysis. The HBsAg expressions study was performed by treating cells with the single-dose (100 μg/ml), as determined by the IC50 values.
2.12. Time-course analysis of HBeAg inhibition
C. rotundus fractions showing the most promising inhibitory effects on HBsAg secretion were further subjected to time-course (day 1, 3 and 5) analysis of HBeAg expression at 100 μg/ml dose. The ELISA was carried out on culture media, using HBeAg/Anti-HBe Elisa Kit (DIASource, Belgium) as per the manufacturer’s manual.
2.13. PXR-mediated CYP3A4 modulation assay of C. rotundus fractions
The PXR-dependent CYP3A4 activation properties of C. rotundus ethyl acetate, n-butanol and aqueous fractions were assessed in HepG2 cells co-transfected with luciferase reporter plasmids pCDG-hPXR and pGL3-CYP3A4-XREM (400 ng each) as well as Renilla-luciferase plasmid pRL (200 ng; internal control) as described previously (Al-Dosari and Parvez, 2018). In brief, 24 h post-transfection, cells were treated with the three fractions (50 μg/ml), including rifampicin (10 μM) and Dodonea angustifolia total ethanol-extract (50 μg/ml) as positive and DMSO (0.1%) as negative controls. The levels of luciferase expressions were measured in all triplicated cell-lysates on day 2 (Dual-Luciferase Reporter Assay System; Promega, USA) and fold-expression (luminescence) of CYP3A4 in relation to negative control was determined.
2.14. Statistical analysis
Results were expressed as mean ± S.E.M. Total variation present in a set of data was estimated by one-way analysis of variance (ANOVA) followed by Dunnet’s-test. P < 0.01 was considered significant.
3. Results
3.1. Hepatocyte proliferative and growth stimulatory effect of n-butanol and aqueous fractions
DCFH (100 μg/ml) showed considerable cytotoxic effect on the HepG2 cells as reflected by altered morphology compared to untreated cells. Interestingly, the DCFH-treated cells supplemented with n-butanol and aqueous fractions (200 and 100 μg/ml, respectively) were morphologically different from the DCFH-treated cells but comparable to untreated cells (data not shown).
3.2. Hepatoprotective potential against DCFH-toxicity
In vitro hepatoprotective effect of C. rotundus fractions against DCFH-induced hepatotoxicity was investigated on cultured cells using MTT assay. Of the five fractions evaluated, only fractions showed significant hepatoprotective activities.
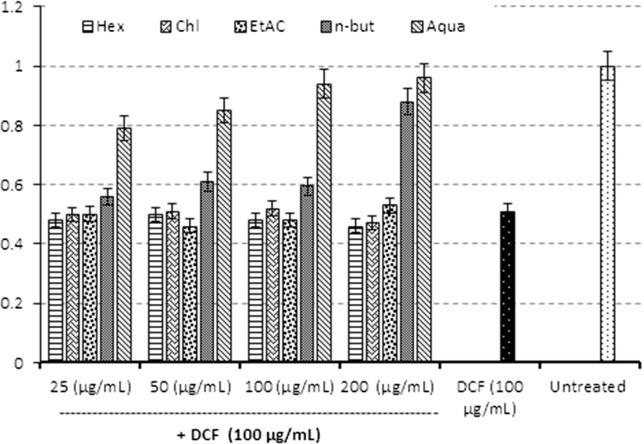
DCFH-toxicated cells were recovered to about 88% and 96% upon treatment with 200 μg/ml of n-butanol and aqueous fractions, respectively (Fig. 1).
Fig. 1.
MTT cell proliferation assay. Hepatoprotective effect of C. rotundus rhizome fractions: hexane (Hex), chloroform (Chl), ethyl acetate (EtAc), n-butanol (n-but), and aqueous (aqua) against DCFH-induced toxicity of HepG2 cells. Values (Y-axis): means of three determinations.
3.3. In vivo therapeutic effects of C. rotundus on rat liver markers
The administration of CCl4 drastically elevated SGOT, SGPT, GGT ALP, bilirubin cholesterol, TG, LDL, VLDL, NP-SH, TP and reduced HDL and MDA levels compared to the GI rats (Table 1), indicating liver injury. Treatment with C. rodendus (100 and 200 mg/kg·bw) and silymarin (10 mg/kg·bw) significantly normalized these parameters as compared to the GII control (Table 1).
Table 1.
Therapeutic effect of C. rotundus against CCl4-induced hepatotoxicity related parameters in rats.
| Liver function parameters | GI | GII | GIII | GIV | GV |
|---|---|---|---|---|---|
| SGOT (U/l) | 112.24 ± 5.31 | 254.58 ± 8.33*** | 236.23 ± 7.36 b | 207.87 ± 6.87b | 142.31 ± 6.00**b |
| SGPT (U/l) | 27.78 ± 2.20 | 239.27 ± 9.62***a | 189.12 ± 7.12*b | 162.13 ± 9.17***b | 87.23 ± 4.31***b |
| ALP (U/l) | 331.59 ± 13.8 | 521.23 ± 13.7***a | 481.21 ± 6.12*b | 433.65 ± 12.31**b | 376.07 ± 7.62***b |
| GGT (U/l) | 5.17 ± 0.32 | 13.27 ± 0.98***a | 12.18 ± 0.49b | 9.52 ± 0.32**b | 6.15 ± 0.28***b |
| BIL (mg/dl) | 0.73 ± 0.01 | 2.32 ± 0.08***a | 1.78 ± 0.05*b | 1.27 ± 0.06***b | 97.26 ± 0.06***b |
| TC (mg/dl) | 124.76 ± 3.9 | 217.00 ± 4.53***a | 181.03 ± 6.63**b | 164.81 ± 6.16*** | 138.43 ± 4.88***b |
| TG (mg/dl) | 63.13 ± 2.74 | 163.06 ± 4.61***a | 128.54 ± 3.8***b | 116.23 ± 3.75***b | 108.21 ± 5.26***b |
| HDL (mg/dl) | 48.58 ± 2.40 | 24.05 ± 1.79*** | 26.13 ± 1.22b | 37.52 ± 1.78**b | 41.04 ± 2.97**b |
| LDL (mg/dl) | 51.67 ± 3.22 | 154.18 ± 4.28***a | 128.40 ± 7.50*b | 108.27 ± 7.60**b | 78.25 ± 5.98***b |
| VLDL (mg/dl) | 12.76 ± 0.54 | 33.78 ± 0.92***a | 24.08 ± 0.76***b | 18.23 ± 0.75***b | 16.57 ± 1.05***b |
| TP (g/l) | 109.74 ± 2.8 | 51.27 ± 1.82***a | 54.32 ± 2.81b | 71.36 ± 2.64***b | 96.38 ± 4.08***b |
| MDA (nmol/g) | 0.52 ± 0.02 | 5.13 ± 0.29***a | 4.33 ± 0.23b | 2.87 ± 0.1***b | 1.70 ± 0.16***b |
| NP-SH (nmol/g) | 7.16 ± 0.53 | 3.54 ± 0.44***a | 4.88 ± 0.35**b | 5.74 ± 0.31**b | 6.68 ± 0.31***b |
All values represent mean ± SEM. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ANOVA, followed by Dunnett’s multiple comparison test. aAs compared with control group (GI); bAs compared with CCl4-only group (GII). Group I (GI): Untreated control, fed with normal saline (1.0 ml); Group II (GII): CCl₄ (1:1; 1.25 ml/kg·bw; i.p.) treated control; Group III (GIII): Fed with the C. rotundus extract (100 mg/kg·bw) and CCl4; Group 1V (GIV): Fed with the C. rotundus extract (200 mg/kg·bw) and CCl4; Group V (GV): Positive control, supplemented with standard, silymarin (10 mg/kg·bw) and CCl4.
3.4. Dose-dependent inhibition of HBsAg expressions by ethyl acetate, n-butanol and aqueous fractions
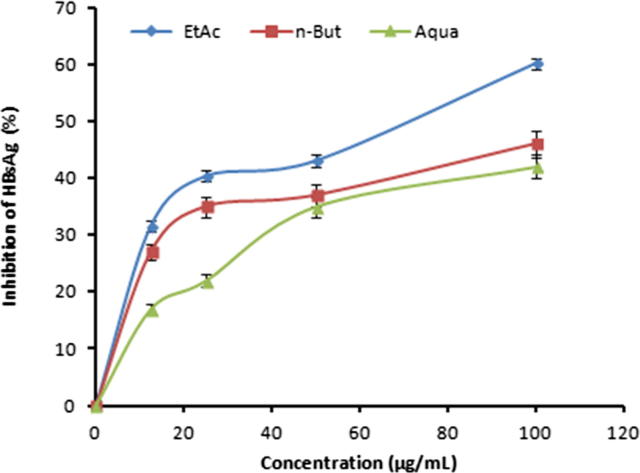
C. rotundus fractions were tested for anti-HBV activities by measuring the expression levels of viral antigens with reference to untreated controls in the culture supernatants. At 48 h post-treatment, while the hexane and chloroform fractions did not inhibit HBsAg expressions, the ethyl acetate, n-butanol and aqueous fractions showed dose- dependent inhibition with IC50 values 64.24, 94.86 and 107.81, respectively. At a higher dose, inhibitions were by 60.27%, 46.87 and 42.76%, respectively (Fig. 2). Therapeutic index (TI) of the three active fractions, were calculated (Table 2).
Fig. 2.
Dose-dependent anti-HBV activities of C. rotundus rhizome fractions at non-cytotoxic doses. ELISA showing inhibitions of HBsAg expression by Ethyl acetate (EtAc), n-butanol (n-But) and aqueous (Aqua) fractions only. Values (Y-axis): means of three determinations.
Table 2.
Determination of C. rotundus fractions cytotoxicity (CC50), anti-HBV activity (IC50) and their corresponding therapeutic index (TI).
| C. rotundus fraction | CC50 | IC50 | TI |
|---|---|---|---|
| Hexane | 389.5 | NA | ND |
| Chloroform | 360.5 | NA | ND |
| Ethyl acetate | 470 | 46.2 | 10.17 |
| n-Butanol | 827.6 | 94.8 | 8.72 |
| Aqueous | 783.5 | 107.8 | 7.26 |
NA: no activity; ND: not determined.
3.5. Time-course inhibition of HBsAg expressions by ethyl acetate, n-butanol and aqueous fractions
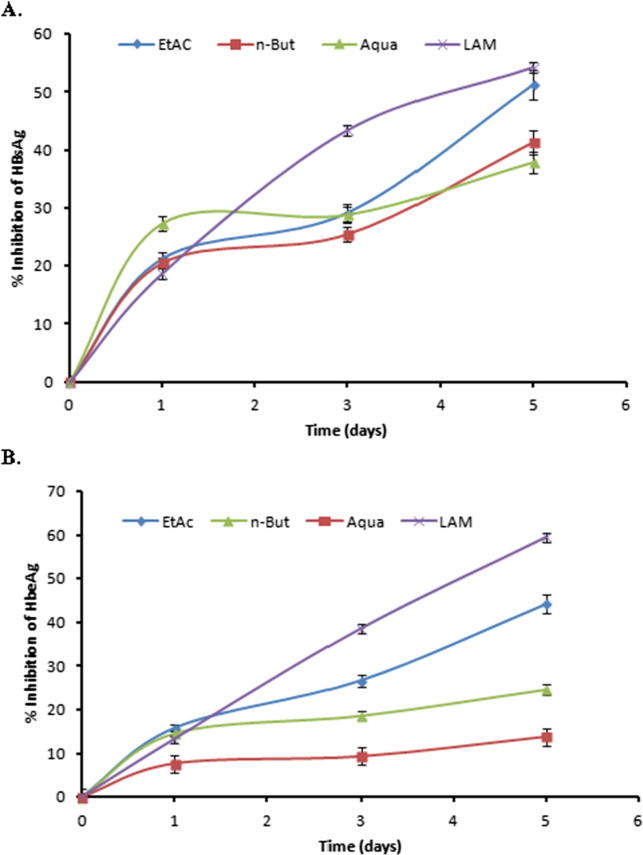
The three fractions showing dose-dependent anti-HBV activity were further evaluated in a time-course study, using 100 μg/ml doses for five days. Compared to days 1 and 5 post-treatment, HBsAg production was inhibited up to ∼50% and ∼40% by ethyl acetate and n-butanol fractions, respectively on day 5 (Fig. 3A). Because treatment beyond day 5 did not show any significant difference, and prolonged continuation of culture resulted in cell overgrowth and death (data not shown), the study was terminated at day 5.
Fig. 3.
Time-course anti-HBV activities of C. rotundus ethyl acetate (EtAc), n-butanol (n-But) and aqueous (Aqua) fractions (100 μg/ml, each). ELISA showing inhibitions of (A) HBsAg expression, and (B) HBeAg expression in HepG2.2.15 culture supernatants at days 1, 3, and 5. Lamivudine (LAM; 2.0 μM) used as reference anti-HBV drug. Values (Y-axis): means of three determinations.
3.6. Down regulation of virus replication by ethyl acetate, n-butanol and aqueous fractions
The HBV ‘e’ (HBe) is a processed secretory antigen of viral ‘pre-Core’ protein that is co-translated with ‘Core’ by a bicistronic subgenomic-RNA. Therefore, production of HBeAg is a marker of active viral DNA replication in infected cells. This resembles human immunodeficiency virus (HIV)-p24 antigen wherein Elisa is a valid tool to monitor HIV replication. Therefore, the three fractions: ethyl acetate, n-butanol and aqueous that showed the best anti-HBV effects were subjected to time-course HBeAg analysis of the culture supernatants. The three fractions showed down regulation of virus replication in a time-dependent manner. At day 5 post-treatment, 100 μg/ml of ethyl acetate and n-butanol fractions suppressed HBV replication by 44.14% and 24.70%, respectively (Fig. 3B).
3.7. Mild activation of hepatic CYP3A4 by C. rotundus fractions
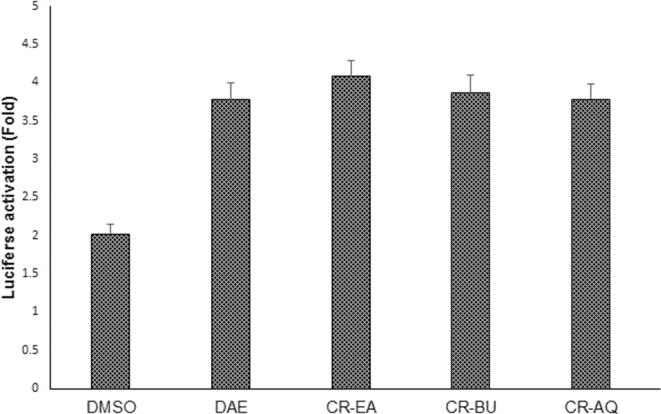
Our dual-luciferase assay showed mild PXR-mediated CYP3A4 activation by the three tested fractions (ethyl acetate, n-butanol and aqueous) of C. rotundus as compared to D. angustifolia activity (Al-Dosari and Parvez, 2018) in HepG2 cells (Fig. 4). This is the first study on modulation of CYP3A4 by C. rotundus, suggesting its safe consumption in relation to drug metabolism.
Fig. 4.
Luciferase reporter-gene assay, showing fold-activation of PXR-mediated CYP3A4 in transfected HepG2 cells, treated with C. rotundus bioactive fractions (CR-EA: ethyl acetate; CR-BU: n-butanol; CR-AQ: aqueous). Rifampicin (10 μM) and Dodonaea angustifolia ethanol-extract (DAE; 50 μg/ml) served as positive controls, and DMSO (0.1%) acted as negative control. Values (Y-axis): means of three determinations.
4. Discussion
Liver disorders, one of the major causes of death can occur in response to chemical toxicity or because of infection with hepatitis viruses. Development of anti-HBV therapies has been impeded until recently by the lack of suitable in vitro as well as in vivo models that could closely mimic natural viral infection (Chen et al., 2011, Seo et al., 2011, Kumar et al., 2013, Alam et al., 2011). Of the several HBV reporter hepatoma cell lines, HepG2.2.15 is widely used to screen and identify potential antiviral therapeutics agents. In recent times, cell culture based in vitro cytotoxicity and hepatoprotective activities of phytoproducts are widely used for primary level screening (Arbab et al., 2015). We therefore, evaluated the hepatoprotective as well as anti-HBV potential of organic and aqueous fractions of C. rotundus rhizome extracts.
The natural or phytoproducts antiviral activities are suggested through direct inhibition, enhancement of host immunity or anti-inflammation and cytoprotecon from oxidative stress or damages. In line with this, the indirect antiviral efficacy of Ampelopsis silica root extract against herpes (Chen and Yang, 1999) and duck hepatitis B (Chen et al., 2000) has been shown via its anti-inflammatory and anti-oxidative activity (Chen et al., 2005). Also, in vitro and in vivo anti-oxidative, hepatoprotective and anti-HBV potential of Acacia mellifera leaves extract has been demonstrated recently (Arbab et al., 2015).
DCFH is generally used to measure in vitro oxidative stress generated by free radicals through the principle of oxidation of DCFH to the fluorescent DCFH (Rota et al., 1999). In the present study, we used DCFH as an inducer of in vitro hepatotoxicity. Of the five fractions tested, n-butanol and aqueous fractions protected the hepatocytes against DCFH-induced injury and caused cell recovery comparable to healthy control. Our finding was in agreement with the reported antioxidant (Yazdanparast and Ardestani, 2007) and hepatoprotective efficacy of C. Rotundus total extract in CCl4-induced hepatic injury in rats (Gilani and Janbaz, 1995, Mohamed, 2015). The observed hepatoprotective effect of C. rotundus against DCFH-cytotoxicity can be attributed to the presence of flavonoids and related phenolic compounds which are well known as antioxidants and free radical scavengers (Sunil et al., 2011, Zhou and Yin, 2012).
In the antiviral evaluations of the different fractions, the ethyl acetate fraction showed very promising anti-HBV activities followed by n-butanol and aqueous fraction. The variable activities shown by the different fractions may be because of the diversity of structure and/or uneven distribution of phytochemical constituents present in these fractions. In the literature, many plant secondary metabolites including, flavonoids, saponins, alkaloids, and lignans have been reported to have promising anti-HBV activities. These compounds differ from one another in mode of action on HBV gene expressions and DNA replication. Of these, the flavonoids and polyphenol reported to have anti-HBV activities include wogonin (Scutellaria radix) (Guo et al., 2007), epigallocatechin gallate (Camellia sinensis) (Huang et al., 2014, Xu et al., 2008), and protocatechuic aldehyde (Salvia miltiorrhiza) (Zhou et al., 2007). Further, atriterpenoidal saponin glycyrrhizin isolated from the roots of Glycyrrhiza galabra and alkaloid oxymatrines howed promising anti-HBV activities (Takahara et al., 1994, Wang et al., 2011). Of the anti-HBV lignans of plant origin, niranthin and hinokinin (Phyllanthus spp.) have been already reported (Takahara et al., 1994, Wang et al., 2011). Very recently, we have shown promising anti-HBV activities of some plant-derived compounds viz., quercetin, baccatin III, psoralen, embelin, menisdaurin, azadirachtin, lupeol, rutin, beta-sitosterol and hesperidin in cultured HepG2.2.15 cells (Parvez et al., 2019). In addition, our hepatic CYP3A4 activation assay of C. rotundus fractions suggested its safe consumption in relation to drug metabolism and efficacy.
5. Conclusions
Our data showed very promising hepatoprotective and anti-HBV potential of C. rotundus rhizome extracts in vitro. Taken together, while the n-butanol and aqueous fractions exhibited hepatoprotective as well as anti-HBV activities, the ethyl acetate fraction showed only antiviral effect. In addition, this is the first report on modulation of CYP3A4 by C. rotundus that suggests its safe consumption. The findings could therefore, provide the basis for the claimed traditional use of C. rotundus for metabolic and HBV infection associated liver diseases. However, further phytochemical, biological and clinical studies are required.
Acknowledgments
Acknowledgement
The authors gratefully acknowledge the Deanship of Scientific Research at King Saud University for funding this research (Research Group Project No. RG-1435-053).
Conflicts of interest
The authors have nothing to declare.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohammad K. Parvez, Email: mohkhalid@ksu.edu.sa, khalid_parvez@yahoo.com.
Mohammed S. Al-Dosari, Email: mdosari@ksu.edu.sa.
References
- Al-Dosari M.S., Parvez M.K. Genetic polymorphisms of drug eliminating enzymes and transporters. Biomed. Gen. Genom. 2016;1:44–50. [Google Scholar]
- Al-Dosari M.S., Parvez M.K. Novel plant inducers of PXR-dependent cytochrome P450 3A4 expression in HepG2 cells. Saudi Pharm. J. 2018;26:1069–1072. doi: 10.1016/j.jsps.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M.A., Jahan R., Rahman S., Das A.K., Rahmatullah M. Antinociceptive and anti-hyperglycemic activity of methanol leaf extract of cyperus scariosus. Pakistan J. Pharm. Sci. 2011;24:53–56. [PubMed] [Google Scholar]
- Al-Yahya M., Al-Dosari M.S., Al-Sohaibani M., Al-Said M., Mothana R., Parvez M.K., Rafatullah S. Attenuation of CCl4-induced oxidative stress and hepato-nephrotoxicity by Saudi sidr honey in Rats. Evid.-Based Compl. Alter. Med. 2013;2013:1–10. doi: 10.1155/2013/569037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab A.H., Parvez M.K., Al-Dosari M.S. Hepatoprotective and antiviral efficacy of acacia mellifera leaves fractions against hepatitis B virus. Biomed. Res. Int. 2015;2015:929131. doi: 10.1155/2015/929131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaulya N.C., Haldar P.K., Mukherjee A. Antidiabetic activity of methanol extract of rhizomes of cyperus tegetum roxb. Acta Pol. Pharm. 2011;68:989–992. [PubMed] [Google Scholar]
- Chen Y., Zhao Y.-Y., Wang X.-Y. GC-MS analysis and analgesic activity of essential oil from fresh rhizoma of cyperus rotundus. J. Chin. Med. Mat. 2011;34:1225–1229. [PubMed] [Google Scholar]
- Chen K., Yang Z. The acting part anti-HSV-1 in infected cells of extract from Ampelopsis sinica roots. J. Chin. Drug. 1999;2:225–226. [Google Scholar]
- Chen K., Li H., Chen Y., Zhang C. Inhibition of extracts of Ampelopsis sinica roots on DHB vs. Ag in sera of ducklings. J. Chin. Med. Mater. 2000;23:46–47. [PubMed] [Google Scholar]
- Chen K., Plumb G.W., Bennett R.N., Bao Y. Antioxidant activities of extracts from five anti-viral medicinal plants. J. Ethnopharmacol. 2005;96:201–205. doi: 10.1016/j.jep.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Dhillon R.S., Singh S., Kundra S., Basra A.S. Studies on the chemical-composition and biological-activity of essential oil from cyperus-rotundus linn. Plant Growth Reg. 1993;13:89–93. [Google Scholar]
- Gilani A.U., Janbaz K.H. Studies on protective effect of cyperus scariosus extract on acetaminophen and ccl4-induced hepatotoxicity. Gen. Pharmacol. 1995;26:627–631. doi: 10.1016/0306-3623(94)00200-7. [DOI] [PubMed] [Google Scholar]
- Guo Q.L., Zhao L., You Q.D. Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antivir. Res. 2007;74:16–24. doi: 10.1016/j.antiviral.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Huang H.C., Tao M.H., Hung T.M. (-)-epigallocatechin-3-gallate inhibits entry of hepatitis B virus into hepatocytes. Antiviral Res. 2014;111:100–111. doi: 10.1016/j.antiviral.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Jin J.H., Lee D.U., Kim Y.S., Kim H.P. Anti-allergic activity of sesquiterpenes from the rhizomes of cyperus rotundus. Arch. Pharm. Res. 2011;34:223–228. doi: 10.1007/s12272-011-0207-z. [DOI] [PubMed] [Google Scholar]
- Kokate C.K., Varma K.C. Pharmacological investigations of volatile oil of cyperus eleusinoides kunth effect on central nervous system. Ancient Sci. Life. 1982;1:206–209. [PMC free article] [PubMed] [Google Scholar]
- Kumar H., Tamatam A., Pal A., Khanum F. Neuroprotective effects of cyperus rotundus on sin-1 induced nitric oxide generation and protein nitration: ameliorative effect against apoptosis mediated neuronal cell damage. Neurotoxicology. 2013;34:150–159. doi: 10.1016/j.neuro.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Locarnini S.A. Primary resistance, multidrug resistance, and cross-resistance pathways in HBV as a consequence of treatment failure. Hepatol. Int. 2008;2:147–151. doi: 10.1007/s12072-008-9048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok A.S.F., Zoulim F., Locarnini S.A. Antiviral drug-resistant HBV: Standardization of nomenclature and assays and recommendations for management. Hepatology (Baltimore, Md.) 2007;46:254–265. doi: 10.1002/hep.21698. [DOI] [PubMed] [Google Scholar]
- Moosavinia H., Dore J. Factors affecting glyphosate activity in imperata-cylindrica (l) beau and cyperus-rotundus l.1. Effect of soil-moisture. Weed Res. 1979;19:137–143. [Google Scholar]
- Mohamed G.A. Iridoids and other constituents from cyperus rotundus l. Rhizomes. Bull. Faculty Pharm., Cairo Univ. 2015;53:5–9. [Google Scholar]
- Parveen R., Baboota S., Ali J. Effects of silymarin nanoemulsion against carbon tetrachloride-induced hepatic damage. Arch. Pharmacol. Res. 2011;34:767–774. doi: 10.1007/s12272-011-0510-8. [DOI] [PubMed] [Google Scholar]
- Parvez M.K., Rehman M.T., Alam P. Plant-derived antiviral drugs as novel hepatitis B virus inhibitors: cell culture and molecular docking study. Saudi Pharm J. 2019;27:389–400. doi: 10.1016/j.jsps.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakotonirina V.S., Bum E.N., Rakotonirina A., Bopelet M. Sedative properties of the decoction of the rhizome of cyperus articulatus. Fitoterapia. 2011;72:22–29. doi: 10.1016/s0367-326x(00)00243-4. [DOI] [PubMed] [Google Scholar]
- Rota C., Chignell F., Mason R.P. Evidence for free radical formation during the oxidation of 2′-7′-dichlorofluorescin to the fluorescent dye 2′-7′-dichlorofluorescein by horseradish peroxidase: Possible implications for oxidative stress measurements. Free Radic. Biol. Med. 1999;27:873–881. doi: 10.1016/s0891-5849(99)00137-9. [DOI] [PubMed] [Google Scholar]
- Rukunga G.M., Muregi F.W., Omar S.A. Anti-plasmodial activity of the extracts and two sesquiterpenes from cyperus articulatus. Fitoterapia. 2008;79:188–190. doi: 10.1016/j.fitote.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Sayed H.M., Mohamed M.S., Farag S.F. Fructose-amino acid conjugate and other constituents from cyperus rotundus linn. Nat. Prod. Res. 2008;22:1487–1497. doi: 10.1080/14786410802038556. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 1986;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Seo E.J., Lee D.-U., Kwak J.H. Antiplatelet effects of cyperus rotundus and its component (+)-nootkatone. J. Ethnopharmacol. 2011;135:48–54. doi: 10.1016/j.jep.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Sunil A.G., Kesavanarayanan K.S., Kalaivani P. Total oligomeric flavonoids of cyperus rotundus ameliorates neurological deficits, excitotoxicity and behavioral alterations induced by cerebral ischemic-reperfusion injury in rats. Brain Res. Bull. 2011;84:394–405. doi: 10.1016/j.brainresbull.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Sonwa M.M., Konig W.A. Chemical study of the essential oil of cyperus rotundus. Phytochemistry. 2011;58:799–810. doi: 10.1016/s0031-9422(01)00301-6. [DOI] [PubMed] [Google Scholar]
- Takahara T., Watanabe A., Shiraki K. Effects of glycyrrhizin on hepatitis B surface-antigen – a biochemical and morphological-study. J. Hepatol. 1994;21:601–609. doi: 10.1016/s0168-8278(94)80108-8. [DOI] [PubMed] [Google Scholar]
- Tandon S., Inarkar M., Kumar R. Wetland treatment (hssp) of wastewater from a milk-processing unit using bambusa vulgaris, typha latifolia and cyperus rotundus. J. Environ. Sci. Eng. 2010;52:23–26. [PubMed] [Google Scholar]
- Teo C.G., Locarnini S.A. Potential threat of drug-resistant and vaccine-escape hbv mutants to public health. Antivir. Ther. 2010;15:445–449. doi: 10.3851/IMP1556. [DOI] [PubMed] [Google Scholar]
- Thebtaranonth C., Thebtaranonth Y., Wanauppathamkul Y.S., Yuthavong Y. Antimalarial sesquiterpenes from tubers of cyperus rotundus: structure of 10,12-peroxycalamenene, a sesquiterpene endoperoxide. Phytochemistry. 1995;40:125–128. doi: 10.1016/0031-9422(95)00260-e. [DOI] [PubMed] [Google Scholar]
- Torresi J. Hepatitis b antiviral resistance, vaccine escape: two sides of the same coin. Antivir. Ther. 2008;13:337–340. [PubMed] [Google Scholar]
- Tsoyi K., Jang H.J., Lee Y.S. (+)-nootkatone and (+)-valencene from rhizomes of cyperus rotundus increase survival rates in septic mice due to heme oxygenase-1 induction. J. Ethnopharmacol. 2011;137:1311–1317. doi: 10.1016/j.jep.2011.07.062. [DOI] [PubMed] [Google Scholar]
- Uddin S.J., Mondal K., Shilpi J.A., Rahman M.T. Antidiarrhoeal activity of cyperus rotundus. Fitoterapia. 2006;77:134–136. doi: 10.1016/j.fitote.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Utley H.G., Bernheim F., Hochstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 1967;118:29–32. [Google Scholar]
- Wang Y.P., Zhao W., Xue R. Oxymatrine inhibits hepatitis B infection with an advantage of overcoming drug-resistance. Antivir. Res. 2011;89:227–231. doi: 10.1016/j.antiviral.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- Xu J., Wang J., Deng F., Hu Z., Wang H. Green tea extract and its major component epigallocatechin gallate inhibits hepatitis B virus in vitro. Antiviral Res. 2008;78:242–249. doi: 10.1016/j.antiviral.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Xu Y., Zhang H.-W., Wan X.-C., Zou Z.M. Complete assignments of (1)h and (13)c nmr data for two new sesquiterpenes from cyperus rotundus l. Magnetic Reson. Chem. 2009;47:527–531. doi: 10.1002/mrc.2416. [DOI] [PubMed] [Google Scholar]
- Yazdanparast R., Ardestani A. In vitro antioxidant and free radical scavenging activity of cyperus rotundus. J. Med. Food. 2007;10:667–674. doi: 10.1089/jmf.2006.090. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Yin W. Two novel phenolic compounds from the rhizomes of cyperus rotundus linn. Molecules (Basel, Switzerland). 2012;17:2636–12641. doi: 10.3390/molecules171112636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Zhang Y., Ding X.R. Protocatechuic aldehyde inhibits hepatitis B virus replication both in vitro and in vivo. Antivir. Res. 2007;74:59–64. doi: 10.1016/j.antiviral.2006.12.005. [DOI] [PubMed] [Google Scholar]