Abstract
Background
The high proportion of human cases due to the simian malaria parasite Plasmodium knowlesi in Malaysia is a cause of concern, as they can be severe and even fatal. Merozoite surface protein 7 (MSP7) is a multigene family which forms a non-covalent complex with MSP-1 prior to receptor-ligand recognition in Plasmodium falciparum and thus an important antigen for vaccine development. However, no study has been done in any of the ortholog family members in P. knowlesi from clinical samples. This study investigates the level of polymorphism, haplotypes, and natural selection acting at the pkmsp-7D gene in clinical samples from Malaysia.
Methods
Thirty-six full-length pkmsp7D gene sequences (along with the reference H-strain: PKNH_1266000) obtained from clinical isolates of Malaysia, which were orthologous to pvmsp7H (PVX_082680) were downloaded from public databases. Population genetic, evolutionary and phylogenetic analyses were performed to determine the level of genetic diversity, polymorphism, recombination and natural selection.
Results
Analysis of 36 full-length pkmsp7D sequences identified 147 SNPs (91 non-synonymous and 56 synonymous substitutions). Nucleotide diversity across the full-length gene was higher than its ortholog in Plasmodium vivax (msp7H). Region-wise analysis of the gene indicated that the nucleotide diversity at the central region was very high (π = 0.14) compared to the 5′ and 3′ regions. Most hyper-variable SNPs were detected at the central domain. Multiple test for natural selection indicated the central region was under strong positive natural selection however, the 5′ and 3′ regions were under negative/purifying selection. Evidence of intragenic recombination were detected at the central region of the gene. Phylogenetic analysis using full-length msp7D genes indicated there was no geographical clustering of parasite population.
Conclusions
High genetic diversity with hyper-variable SNPs and strong evidence of positive natural selection at the central region of MSP7D indicated exposure of the region to host immune pressure. Negative selection at the 5′ and the 3′ regions of MSP7D might be because of functional constraints at the unexposed regions during the merozoite invasion process of P. knowlesi. No evidence of geographical clustering among the clinical isolates from Malaysia indicated uniform selection pressure in all populations. These findings highlight the further evaluation of the regions and functional characterization of the protein as a potential blood stage vaccine candidate for P. knowlesi.
Electronic supplementary material
The online version of this article (10.1186/s12936-019-2782-2) contains supplementary material, which is available to authorized users.
Keywords: Plasmodium knowlesi, Merozoite surface protein 7D, Genetic diversity, Positive selection, Haplotypes, Malaysia
Background
Malaria is a major global health problem with high mortality and morbidity rates and thus a significant barrier to the socio-economic development of under-developed countries [1]. Plasmodium knowlesi is a simian malaria parasite which can infect humans and is an emerging infection in Southeast Asian countries [2–6]. Countries in Central Asia and almost all Southeast Asia countries has reported human infections due to P. knowlesi, including Malaysia [4, 7, 8], Singapore [9], Myanmar [10], Vietnam [11], Indonesia [12, 13], Philippines [14], Cambodia [15], India [16] and Thailand [17]. Malaysia being the epicenter of knowlesi malaria, infection accounts up to 90% human infections [4, 8, 18, 19]. Despite the overall reduction in malaria cases around the world, the increasing trend of P. knowlesi cases in Southeast Asia highlights the need for proper and effective elimination measures as well as development of effective vaccines.
Plasmodium knowlesi has a 24-h erythrocytic cycle thus rapid increase in parasitaemia has been associated with the development of severe malaria and also a common cause for severe and fatal malaria in Malaysian Borneo [3, 20–22]. Studies on mitochondrial and ssrRNA genes in P. knowlesi from patients and wild macaques identified two distinct sub-populations which clustered geographically to Peninsular Malaysia and Malaysian Borneo [23]. Additionally, genetic and genomic studies from Malaysia identified 3 distinct sub-populations; two originating from Sarawak and one from Peninsular Malaysia [24–27], highlighting the complexity of infections in humans and the challenges for control and vaccine design.
Development of an effective malaria blood-stage vaccine antigens has focused on selecting antigens that provide long term and strain transcending protection and blocks the invasion of red blood cells (RBCs). Studies have shown that parasite antigens which are recognized by the host’s immune system, accumulate polymorphism to evade host’s defense mechanism and are prime targets for vaccine development. These are achieved through the knowledge of antigen natural selection and the pattern of diversity across geographical areas. Blood-stage antigens in Plasmodium falciparum [e.g. merozoite surface proteins (MSPs), apical membrane antigen 1 (AMA1)] that are under strong positive balancing selection are important targets of such acquired immunity and are supported by antibody inhibition assays in culture as well as naturally acquired antibodies in endemic regions [28, 29]. Several known blood stage antigens (e.g. MSP1, MSP1P and AMA1) have been recently studied in P. knowlesi, but most of the antigens were found to be under the influence of strong negative/purifying selection indicating no role in immune evasion mechanism [30–33]. PfMSP1 has been a major target for anti-malaria vaccine development but recent studies have shown that MSP 6 and 7 forms a non-covalent complex with MSP1 during the invasion process [34]. Disruption of PfMSP7 gene has resulted in partial inhibition of erythrocyte invasion during merozoite stages thus indicating that it is an important molecule [35]. Furthermore, MSP7 binding to P-selectin receptor has suggested its role in modulating disease severity in mice models [36] thereby indicating that immunity induced by MSP7 could potentially disrupt parasite development.
MSP7 is a multi-gene family and the number of paralog members in each species differs [37]. For example, till date, 13 MSP7 members were identified in Plasmodium vivax which are arranged in head-to-tail arrangement in chromosome 12 and are named alphabetically as MSP7A to MSP7M [37]. Three of its members i.e. PvMSP7C, PvMSP7H and PvMSP7I have been found to be highly polymorphic at the central region and under strong positive natural selection in Colombian population indicating it is an important molecule for consideration as a vaccine candidate [38]. Plasmodium falciparum has 8 MSP7 genes and low genetic polymorphism has been observed within the field isolates [39]. Certain MSP7 members forms part of the protein complex interacting with host cells [40] and they are localized at the merozoite surface [41, 42]. Despite the fact that P. knowlesi is phylogenetically closely related to P. vivax and there are five paralog members in pkmsp7 gene family [37], no genetic study has been done to characterize the P. knowlesi MSP7 ortholog members from clinical samples. Thus in this study pkmsp-7D gene which is an ortholog to pvmsp-7H was chosen for genetic analysis.
In this study, 36 pkmsp-7D full-length sequences (32 clinical isolates and 4 laboratory lines of Malaysia) were obtained from published databases based on the ortholog gene sequence in P. vivax msp-7H (PVX_082680) [37, 43]. The level of sequence diversity, natural selection using full-length genes as well at each of the three regions of the gene (5′, 3′ and the central region) were determined (along with the H-strain) of Malaysia. The information obtained from this study will be helpful for designing functional studies and future rational design of a vaccine against P. knowlesi.
Methods
pkmsp-7D sequence data
Based on the identification of ortholog members in Plasmodium species [37], the pkmsp-7D sequences were downloaded for 36 isolates (32 clinical and 4 long term isolated lines) originating from Kapit, Betong and Sarikei in Malaysian Borneo and Peninsular Malaysia along with the H-strain (PKNH_1266000, old ID; PK13_3510c) (Additional file 1) [24]. The sequence data with accession numbers are given in Additional file 1. Signal peptide for the full-length pkmsp-7D was predicted using Signal IP 3.0 and the transmembrane regions using the Phobious prediction software [44, 45]. The PkMSP7D regions were characterized based on the published ortholog of PvMSP7H (PVX_082680).
Sequence diversity and natural selection
Sequence diversity (π), defined as the average number of nucleotide differences per site between two sequences within the sequences, was determined by DnaSP v5.10 software [46]. Number of polymorphic sites, number of synonymous and non-synonymous substitutions, haplotype diversity (Hd), the number of haplotypes (h) within the pkmsp7D sequences were also determined by DnaSP software.
To investigate departure from neutrality, Tajima’s D analysis was conducted [47]. Under neutrality, Tajimas’D is expected to be 0. Significantly, positive Tajima’s D values indicate recent population bottleneck or balancing selection, whereas negative values suggest population expansion or negative selection. Natural selection was estimated using the modified Nei-Gojobori method to calculate the average number of non-synonymous (dN) and synonymous (dS) substitutions. Difference between dN and dS were determined by applying codon-based Z-test (P < 0.05) in MEGA software v.5 with 1000 bootstrap replications [48]. Additionally, to test for natural selection in inter-species level, the robust McDonald and Kreitman (MK) test were also performed with the closest msp7orthologs in both P. vivax (PVX_082680) and Plasmodium cynomolgi (PCYB_122830) as outgroups individually using DnaSP v5.10 software.
Analysis of recombination and linkage disequilibrium
Analysis of the minimum number of recombination events (Rm) within the pkmsp7D genes was performed using DnaSP software v5.10 software [46]. Linkage disequilibrium (LD) is the non-random association of sequences at two or more loci. Linkage disequilibrium index, r2 (square of the correlation coefficient of allelic states at each pair of loci) against nucleotide distance were plotted for pkmsp7D sequences across the gene using DnaSP 5.10 software. A statistical significance test was conducted using Fisher’s exact test and ɤ2 test departures from randomness and the value of r2 ranges from 0 to 1 in the software.
Phylogenetic analysis
Phylogenetic analysis was conducted using deduced amino acid sequences from 32 PkMSP7 full-length sequences from Malaysian Borneo, 5 laboratory lines from Peninsular Malaysia; reference H-strain (PKNH_1266000), the Malayan Strain (PKNOH_S09532900), MR4H strain, Philippine strain and the Hackeri strain along with other P. knowlesi MSP7 paralog members PkMSP7A (PKNH_1266300), PkMSP7E (PKNH_1265900) and PkMSP7C (PKNH_1266100) were included. Other ortholog members of P. vivax MSP7H (PVX_082680), MSP7E (PVX_082665) and Plasmodium coatneyi (PCOAH_00042440) were also included for comparative analysis. The Maximum likelihood (ML) method based on Poisson correction model was used as described in MEGA 5.0 with 1000 bootstrap replicates to test the robustness of the trees.
Results
Polymorphism within full-length pkmsp7D genes and natural selection
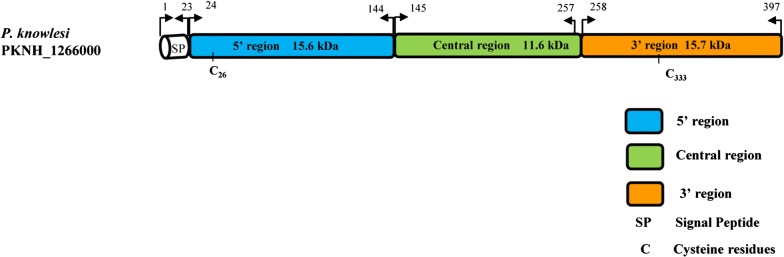
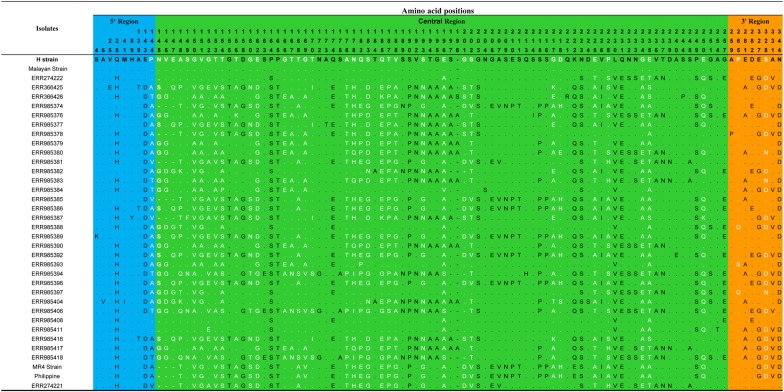
The Signal IP server detected a signal peptide in between amino acid positions 22 and 23 of the PkMSP7D protein (Additional file 2) however, no transmembrane region was detected (data not shown). Alignment and comparison of the amino acid sequences of the full-length P. knowlesi H reference strain MSP7D sequence with P. vivax MSP7H Sal-1 (PVX_082680) reference strain showed 60.4% identity and the next nearest PvMSP7 ortholog gene was E which had 47.3% identity. The amino acid identity within the PkMSP7 paralog members (A, C, D and E) were in the range of 17–26%. The schematic representation of PkMSP7D protein with demarcated regions are shown in Fig. 1. The two cysteine residues at the 5′ and 3′ regions were found to be conserved all the 36 sequences. Within the full-length pkmsp7D sequences (n = 36), there were 203 (17.13%) polymorphic sites leading to 34 haplotypes (Table 1). Of the 203 SNPs, 157 were parsimony informative sites, 46 were singleton variable sites. Among the 157 parsimony informative sites, 131 were two variant, 23 were three variants and 3 were four variants. Parsimony informative sites with 3 or 4 variants led to highly variable non-synonymous substitutions within the central region (Fig. 2). Overall, within the full-length gene, there were 147 SNPs which could be analyzed by DnaSP (91 non-synonymous substitutions and 56 synonymous substitutions) (Table 2) and 75 non-synonymous complex codons (which were highly variable within the central region) were excluded from analysis by the DnaSP software. The overall nucleotide diversity was π = 0.052 ± SD 0.002 which was higher than its ortholog in P. vivax msp7H [38] and the haplotype diversity was 0.998 ± SD 0.002 (Table 1). Sliding window analysis of diversity indicated that the highest diversity was at the central region of the gene (Fig. 3a). The sliding window analysis of Tajimas’D indicated the central region of the gene had the highest D values (Fig. 3b). Interestingly, the 3′ region also had some SNPs, which had positive D values (Fig. 3b). The natural selection analysis of the full-length pkmsp7D genes indicated dN − dS = − 2.1, however, Tajimas’ D and Fu and Li’s D* and F* values were positive (Table 1). The robust inter-species McDonald-Kreitman test indicated a positive selection when P. vivax (NI = 1.25, P > 0.1) and P. cynomolgi (NI = 1.38, P > 0.1) were used as out-groups but were not significant (Table 3). Nucleotide polymorphisms across the full-length gene with hyper-variable SNPs within the sequences are shown in Additional file 3.
Fig. 1.
Schematic diagram of PKMSP7 protein based on the H-Strain (PKNH_1266000). Each box in the schematic diagram is representative of the regions found in PkMSP7 and their respective amino acid positions. The central region is represented in green and blue and orange region represents the 5′ and the 3′ regions respectively. The two 6-Cys residues found within the protein are marked along with approximate molecular weight of each region. Signal peptide is abbreviated as (SP) and cysteine residues as C
Table 1.
Estimates of nucleotide diversity, natural selection, haplotype diversity and neutrality indices of pkmsp7
| Region | No. samples | SNPs | No. haplotype | Diversity ± SD | dN − dS | Codon-based z test | Taj D | Fu and Li’s D* | Fu and Li’s F* | |
|---|---|---|---|---|---|---|---|---|---|---|
| Haplotype | Nucleotide | |||||||||
| Full-length | 36 | 203 | 34 | 0.998 ± 0.007 | 0.052 ± 0.002 | − 2.0 | P > 0.1 | 0.295 | 0.190 | 0.270 |
| 5′ region | 28 | 30 | 0.992 ± 0.009 | 0.010 ± 0.001 | − 3.0 | P > 0.1 | − 1.25 | − 2.24 | − 2.26 | |
| Central region | 151 | 32 | 0.995 ± 0.008 | 0.1495 ± 0.006 | 1.84 | P < 0.05 | 0.673 | 0.896 | 0.973 | |
| 3′ region | 32 | 30 | 0.990 ± 0.010 | 0.017 ± 0.0007 | − 2.68 | P < 0.01 | − 0.275 | − 1.027 | − 0.917 | |
SNPs single nucleotide polymorphisms, SD standard deviation
Fig. 2.
Amino acid polymorphism within 36 full-length PkMSP7D. The number above each amino acid denotes the position of the amino acid based on the H-strain. Hyper-variable amino acids are highlighted in white. The blue, green and orange shaded regions represent the 5′ region, central region and the 3′ region of the PkMSP7D protein respectively. The dots represents identical amino acids and dashes represent a deletion
Table 2.
Synonymous and non-synonymous sites within pkmsp7D
| Location | n | SNPs | Syn. | Non-syn. |
|---|---|---|---|---|
| Full-length | 36 | 147 | 56 | 91 |
| 5′ region | 28 | 18 | 10 | |
| Central region | 89 | 16 | 73 | |
| 3′ region | 30 | 22 | 8 |
SNPs single nucleotide polymorphisms, Syn. synonymous substitutions, Non-syn. nonsynonymous substitutions, n number of samples
Fig. 3.
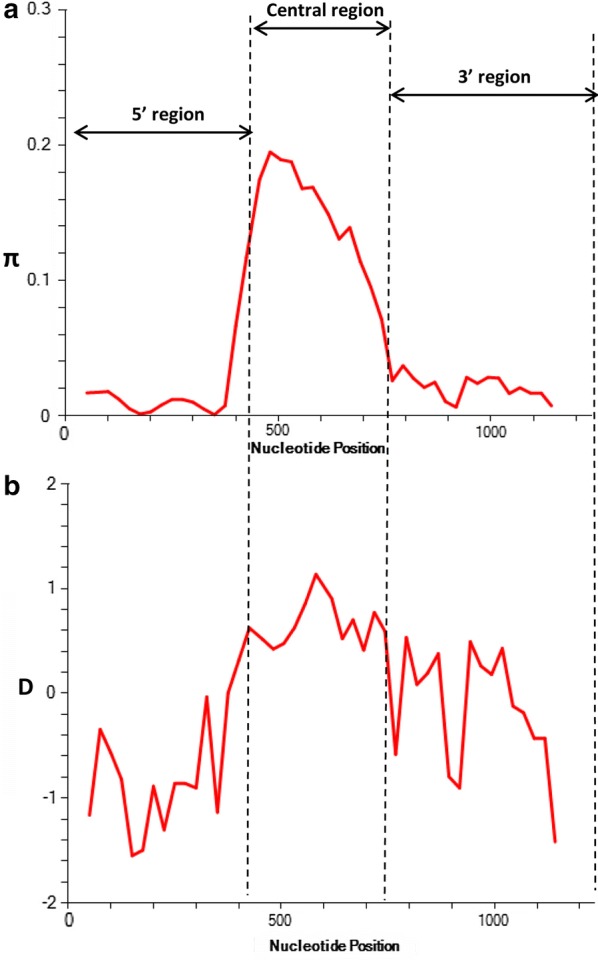
a Graphical representation of nucleotide diversity (π) within 36 full-length pkmsp7D genes from Malaysia. The pkmsp7D regions are marked as dashed line based on the diversity. b Graphical representation of Tajima’s D value across the msp7D gene
Table 3.
McDonald–Kreitman tests on msp7D of Plasmodium knowlesi and its regions with P. vivax and P. cynomolgi orthologs as outgroup species
| MSP7 | Polymorphic changes within P. knowlesi | Fixed differences between species | Neutrality index | |||||
|---|---|---|---|---|---|---|---|---|
| Syn | NonSyn | Pk vs Pv | Pk vs Pcy | Pk vs Pv | Pk vs Pcy | |||
| Syn | NonSyn | Syn | NonSyn | |||||
| Full-length | 56 | 91 | 145 | 188 | 112 | 132 | 1.25 | 1.38 |
| 5′ region | 18 | 10 | 47 | 51 | 52 | 51 | 0.51 | 0.56 |
| Central region | 16 | 73 | 31 | 57 | 15 | 30 | 2.49* | 2.28* |
| 3′ region | 22 | 8 | 67 | 78 | 54 | 61 | 0.31¥ | 0.32¥ |
Syn synonymous sites, NonSyn non synonymous sites, Pk Plasmodium knowlesi, Pv Plasmodium vivax, Pcy Plasmodium cynomolgi
* Fisher’s exact test P-value < 0.05; ¥ Fisher’s exact test P-value < 0.01
Region-wise analysis of diversity and natural selection
Based on the sequence alignment of pkmsp7D and the diversity pattern across the full-length gene (Fig. 3a), three regions were defined; 5′ region (from amino acid 1 to 144), central region (from amino acid 145 to 257) and the 3′ region (from amino acid 258 to 397). Nucleotide diversity at the 5′ and the 3′ regions were of similar levels (π = 0.010–0.017) (Table 1). The number of haplotypes in both the regions were 30 with similar levels of haplotype diversities (Hd = 0.992–0.990) (Table 1). Within the 5′ region, there were 28 SNPs (18 synonymous and 10 non-synonymous substitutions) and the 3′ region had 30 SNPs (22 synonymous and 8 non-synonymous substitutions) (Table 2).
The central region constituted the highest number of SNPs (151) leading to 32 haplotypes (Hd = 0.995) and the nucleotide diversity was very high (π = 0.149) compared to the other regions (Table 1). Of the 151 SNPs, 89 SNPs (16 synonymous and 73 non-synonymous substitutions) could be analysed using DnaSP software. Size variations within the msp7D genes were observed due insertion/deletion at two specific locations within the central domain; 6 nucleotides deletion (from 435 to 440 nt) and 6 to 12 nucleotides insertion/deletion (from 590 to 601 nt) in some isolates (Additional file 3). Twenty-three tri to tetra morphic nucleotides (hyper-variable SNPs) were observed within the central region (Additional file 3).
To determine whether natural selection contributes to the polymorphism in the 3′, 5′ and the central regions of pkmsp7D gene, the difference of (dN − dS) and codon-based Z test was evaluated independently for each region. The negative values at both the 5′ and 3′ region obtained indicated negative/purifying selection (Table 1). The 3′ region was under strong negative selection pressure (dN − dS = 2.68, P < 0.01). Tajimas’ D test, Fu and Li’s D* and F* test were all negative implying negative/purifying selection due to functional constraints at both these regions (Table 1). Negative selection was also evident as there were an excess of synonymous substitutions in both these regions (Table 2). Additionally, the robust MK test, which compares natural selection in the inter-species level, also showed significant values for both P. vivax and P. cynomolgi as outgroup sequences for the 3′ region (Table 3). Though MK did not yield statistically significant results for the 5′ region, the results were indicative of a negative selection.
The test results for natural selection for the highly diverse central region of pkmsp7D indicated that the region was under the influence of strong positive/balancing selection. The difference of dN − dS = 1.84 (P < 0.03) and Tajimas’ D, Fu and Li’s D* and F* values were also positive (Table 1). This was also evident due the presence of excess of non-synonymous substitutions within the region (nsSNPs = 73, sSNPs = 16) (Table 2). MK test, which also showed significant values when both P. vivax (NI = 2.49, P <0.05) and P. cynomolgi (NI = 2.28, P <0.05) were used as outgroup sequences for the central region (Table 3).
Recombination and Linkage disequilibrium (LD) analysis
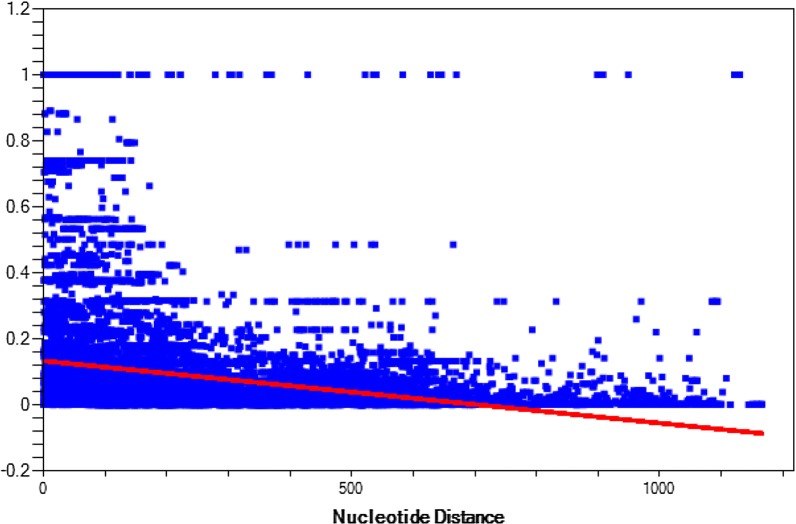
Analysis of recombination within the aligned pkmsp7D sequences estimated a minimum number of 29 recombination events (Rm) indicating intragenic recombination could be a factor adding to the extensive diversity at the central domain of the gene. LD analysis across the full-length Pkmsp7D genes showed the relationship between nucleotide distance and R2 index and the decline in regression trace line indicated that intragenic recombination might exist within the msp7D genes in P. knowlesi (Fig. 4).
Fig. 4.
Linkage disequilibrium across the full-length pkmsp7D genes from Malaysia. Relationship between nucleotide distance and R2 index and the decline in regression trace line indicated intragenic recombination within P. knowlesi isolates
Phylogenetic analysis
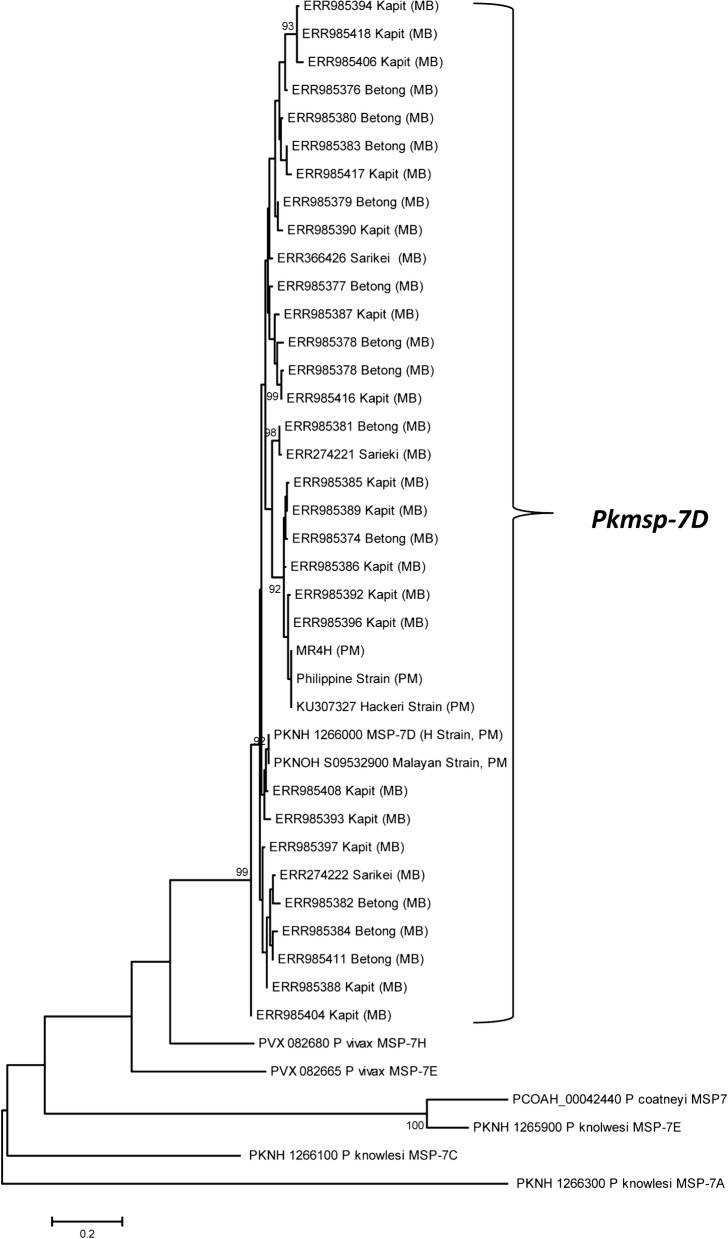
Phylogenetic analysis of the 37 full-length PkMSP7D amino acid sequences indicated that there was no geographical clustering of knowlesi sequences (Fig. 4). The PkMSP7D sequences were found to be more closely related to P. vivax MSP7H compared to its paralogs in P. knowlesi and ortholog in P. vivax and P. coatneyi. The H-strain and the Malayan strain clustered together along with sequences from Malaysian Borneo. The Philippine, MR4 and the Hackeri Strains formed a separate group along with Malaysian Borneo isolates. The distinct sub-populations observed in other invasion genes of P. knowlesi like MSP1P, DBPRII, NBPXA and TRAP was not observed in MSP7D [23, 25–27, 31, 49] (Fig. 5).
Fig. 5.
Phylogenetic relationship of deduced amino acid sequences of PkMSP7D from Sarawak, Malaysia and its paralog members PkMSP7A (PKNH_1266300), PkMSP7E (PKNH_1265900) and PkMSP7C (PKNH_1266100) and ortholog members of Plasmodium vivax MSP7H (PVX_082680) and Plasmodium coatneyi (PCOAH_00042440). The tree was constructed using Maximum Likelihood (ML) method based on Poisson correction model was used as described in MEGA 5.0 with 1000 bootstrap replicates to test the robustness of the trees. MB and PM indicates samples from Malaysian Borneo and Peninsular Malaysia respectively
Discussion
The merozoite surface protein (MSP7) gene family is a group of surface proteins which are involved in the initial interaction with MSP1 through a complex formation at the merozoite surface during the invasion process and orthologs and paralog members have been identified across Plasmodium species [37, 43]. In P. falciparum, MSP7 is expressed during schizont stages and undergoes two step proteolytic processing similar to MSP1 and disruption of the MSP7 gene leads to invasion impairment indicating the molecule is essential for the parasite [35]. Considering previous studies on MSP7 members in P. vivax (i.e. PvMSP7C, MSP7E, PvMSP7H and PvMSP7I) the central region were found to be under the influence of strong positive natural selection and extensive diversity indicating host immune pressure [38, 50]. Extensive functional and field-based studies have been conducted on P. falciparum and P. vivax MSP7 gene family members, but no studies have been done on P. knowlesi. Thus, this study was conducted to genetically characterize the MSP7D genes from clinical isolates of Malaysia, which is the closest ortholog of P. vivax MSP7H.
Sequence alignment of 36 full-length amino acid sequences of pkmsp7D genes from Malaysia showed that it shares approximately 60.4% sequence identity with its ortholog Pvmsp7H and the overall diversity across the full-length gene was higher than P. vivax [38]. Interestingly, the sequence identity within the PkMSP7 paralog member were low. Analysis of polymorphism across the full-length pkmsp7D gene indicated that only the central region acquired high number of polymorphism (SNPs = 151) compared to the 5′ and 3′ regions. This finding was similar to the diversity in the msp7 members (C, E, H and I) in P. vivax [38, 50]. The genetic diversity at the central region of the gene was 14-fold higher (π = 0.1495) than the 5′ and 3′ region. This was due to mainly two reasons; firstly, due to size variations in some of the clinical isolates in two specific locations within the central region and, second, due the high number of non-synonymous hyper-variable SNP sites (tri-morphic and tetra-morphic). The number of singletons (low-frequency polymorphisms) in each of the regions were high leading a to high number of haplotypes and haplotype diversity (Table 1). Similar hyper-variable sites were also observed in P. vivax msp7 sequences from Thailand [50]. Tests for natural selection (using Taj D, Li and Fu’s D*, F*, dN-dS and MK test) at each region indicated that only the central region was under strong positive selection indicating immune evasion mechanism of parasite at this region, however, the 5′ and the 3′ prime regions are under functional constraints (purifying selection). These regions could potentially be the putative B cell epitope binding regions. This was probably due to the structural conformity attained by the protein during the invasion process and exposure of the central region to host immune system. Indeed, it remains to be proven through functional studies whether PkMSP7D forms a complex with PkMSP1 as observed in PfMSP1-MSP7 complex [34, 42]. A recent genetic and structural study on P. vivax msp7E sequences from Thailand predicted 4–6 α-helical domains at the 5′ and 3′ regions suggesting structural and functional constraints in these domains [50]. Sliding window analysis of Tajimas’D across the full-length Pkmsp7D identified high D values within the central region and a short region within the 3′ region. These regions with elevated D values were probable epitope binding regions.
Previous studies identified two sympatric sub-populations from Sarawak and geographical separation in samples from Peninsular Malaysia [24], however, phylogenetic analysis of the current study did not show any specific separation of the P. knowlesi MSP7D isolates. This is an interesting finding because the pkmsp7D sequences used in this study is from the same genomic data [24]. Bifurcation of trees, indicating dimorphism and strong negative/purifying selection on invasion genes like DBPαII (PkDBPαII) [31], PkNBPXa [26], PkAMA1 [30] and Pk41 [51] have been reported from clinical samples. This probably indicates that Pkmsp7D is not influenced by geographical origin of the parasite and both the parasite sub-populations were under strong host immune pressure at the central region similar to the non-repeat region in Pkcsp from Malaysia [52]. High recombination values and LD analysis indicated that intragenic recombination could play a crucial role in increasing diversity in the central region. These findings will be helpful for future designing functional binding assays to validate the molecule as a vaccine candidate.
Conclusion
This study is the first to investigate genetic diversity and natural selection of pkmsp7D gene from clinical samples from Malaysia. High level of genetic diversity and strong positive selection pressure was observed in the central region of the pkmsp7D gene indicating exposure to host immune pressure. Absence of both dimorphism and geographical clustering within the parasite populations indicated uniform exposure of the central region to host immunity. Future studies should focus on the functional characterization of pkmsp7D as a vaccine candidate.
Additional files
Additional file 1: Table S1. Study samples and origin.
Additional file 2. Signal peptide identifed by Signal IP server.
Additional file 3. Neucleotide polymorphism across the full-length pkmsp7D genes. Hyper-variable SNPs are marked in bold and white and SNPs with deletions marked in red.
Authors’ contributions
MAA and FSQ designed the study. MAA retrieved Pkmsp7D gene sequences from genomic databases, performed the genetic analysis. MAA and FQS wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Dr. Syeda Wasfeea Wazid for helping in data retrieval and organizing the data for analysis.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets analysed during the current study were derived from the following public region resources: 10.1371/journal.pone.0121303 and 10.1073/pnas.1509534112.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by the grants from the National Research Foundation of Korea (NRF) (2018R1A2B6003535 and 2018R1A6A1A03025124).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- MSP17
merozoite surface protein 7 paralog
- kDa
kilodalton
References
- 1.WHO . World malaria report. Geneva: World Health Organization; 2016. [Google Scholar]
- 2.White NJ. Plasmodium knowlesi: the fifth human malaria parasite. Clin Infect Dis. 2008;46:172–173. doi: 10.1086/524889. [DOI] [PubMed] [Google Scholar]
- 3.Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165–171. doi: 10.1086/524888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–1024. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- 5.Garnham PCC. Malaria parasites and other haemosporidia. Oxford: Blackwell Scientific Publications; 1966. [Google Scholar]
- 6.Ahmed MA, Cox-Singh J. Plasmodium knowlesi - an emerging pathogen. ISBT Sci Ser. 2015;10(Suppl 1):134–140. doi: 10.1111/voxs.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vythilingam I, Noorazian YM, Huat TC, Jiram AI, Yusri YM, Azahari AH, et al. Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasit Vectors. 2008;1:26. doi: 10.1186/1756-3305-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber BE, William T, Jikal M, Jilip J, Dhararaj P, Menon J, et al. Plasmodium knowlesi malaria in children. Emerg Infect Dis. 2011;17:814–820. doi: 10.3201/eid1705.101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng OT, Ooi EE, Lee CC, Lee PJ, Ng LC, Pei SW, et al. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg Infect Dis. 2008;14:814–816. doi: 10.3201/eid1405.070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang N, Chang Q, Sun X, Lu H, Yin J, Zhang Z, et al. Co-infections with Plasmodium knowlesi and other malaria parasites, Myanmar. Emerg Infect Dis. 2010;16:1476–1478. doi: 10.3201/eid1609.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Eede P, Van HN, Van Overmeir C, Vythilingam I, Duc TN, le Hung X, et al. Human Plasmodium knowlesi infections in young children in central Vietnam. Malar J. 2009;8:249. doi: 10.1186/1475-2875-8-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figtree M, Lee R, Bain L, Kennedy T, Mackertich S, Urban M, et al. Plasmodium knowlesi in human, Indonesian Borneo. Emerg Infect Dis. 2010;16:672–674. doi: 10.3201/eid1604.091624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herdiana H, Irnawati I, Coutrier FN, Munthe A, Mardiati M, Yuniarti T, et al. Two clusters of Plasmodium knowlesi cases in a malaria elimination area, Sabang Municipality, Aceh, Indonesia. Malar J. 2018;17:186. doi: 10.1186/s12936-018-2334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luchavez J, Espino F, Curameng P, Espina R, Bell D, Chiodini P, et al. Human Infections with Plasmodium knowlesi, the Philippines. Emerg Infect Dis. 2008;4:811–813. doi: 10.3201/eid1405.071407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khim N, Siv S, Kim S, Mueller T, Fleischmann E, Singh B, et al. Plasmodium knowlesi infection in humans, Cambodia, 2007–2010. Emerg Infect Dis. 2011;17:1900–1902. doi: 10.3201/eid1710.110355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyagi RK, Das MK, Singh SS, Sharma YD. Discordance in drug resistance-associated mutation patterns in marker genes of Plasmodium falciparum and Plasmodium knowlesi during coinfections. J Antimicrob Chemother. 2013;68:1081–1088. doi: 10.1093/jac/dks508. [DOI] [PubMed] [Google Scholar]
- 17.Sermwittayawong N, Singh B, Nishibuchi M, Sawangjaroen N, Vuddhakul V. Human Plasmodium knowlesi infection in Ranong province, southwestern border of Thailand. Malar J. 2012;11:36. doi: 10.1186/1475-2875-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yusof R, Lau YL, Mahmud R, Fong MY, Jelip J, Ngian HU, et al. High proportion of knowlesi malaria in recent malaria cases in Malaysia. Malar J. 2014;13:168. doi: 10.1186/1475-2875-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajahram GS, Barber BE, William T, Grigg MJ, Menon J, Yeo TW, et al. Falling Plasmodium knowlesi malaria death rate among adults despite rising incidence, Sabah, Malaysia, 2010–2014. Emerg Infect Dis. 2016;22:41–48. doi: 10.3201/eid2201.151305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daneshvar C, Davis TM, Cox-Singh J, Rafa’ee MZ, Zakaria SK, Divis PC, et al. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin Infect Dis. 2009;49:852–860. doi: 10.1086/605439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.William T, Menon J, Rajahram G, Chan L, Ma G, Donaldson S, et al. Severe Plasmodium knowlesi malaria in a tertiary care hospital, Sabah, Malaysia. Emerg Infect Dis. 2011;17:1248–1255. doi: 10.3201/eid1707.101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willmann M, Ahmed A, Siner A, Wong IT, Woon LC, Singh B, et al. Laboratory markers of disease severity in Plasmodium knowlesi infection: a case control study. Malar J. 2012;11:363. doi: 10.1186/1475-2875-11-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yusof R, Ahmed MA, Jelip J, Ngian HU, Mustakim S, Hussin HM, et al. Phylogeographic evidence for 2 genetically distinct zoonotic Plasmodium knowlesi parasites, Malaysia. Emerg Infect Dis. 2016;22:1371–1380. doi: 10.3201/eid2208.151885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assefa S, Lim C, Preston MD, Duffy CW, Nair MB, Adroub SA, et al. Population genomic structure and adaptation in the zoonotic malaria parasite Plasmodium knowlesi. Proc Natl Acad Sci USA. 2015;112:13027–13032. doi: 10.1073/pnas.1509534112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinheiro MM, Ahmed MA, Millar SB, Sanderson T, Otto TD, Lu WC, et al. Plasmodium knowlesi genome sequences from clinical isolates reveal extensive genomic dimorphism. PLoS ONE. 2015;10:e0121303. doi: 10.1371/journal.pone.0121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed MA, Fong MY, Lau YL, Yusof R. Clustering and genetic differentiation of the normocyte binding protein (nbpxa) of Plasmodium knowlesi clinical isolates from Peninsular Malaysia and Malaysia Borneo. Malar J. 2016;15:241. doi: 10.1186/s12936-016-1294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed AM, Pinheiro MM, Divis PC, Siner A, Zainudin R, Wong IT, et al. Disease progression in Plasmodium knowlesi malaria is linked to variation in invasion gene family members. PLoS Negl Trop Dis. 2014;8:e3086. doi: 10.1371/journal.pntd.0003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy MC, Wang J, Zhang Y, Miles AP, Chitsaz F, Saul A, et al. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun. 2002;70:6948–6960. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polley SD, Tetteh KK, Lloyd JM, Akpogheneta OJ, Greenwood BM, Bojang KA, et al. Plasmodium falciparum merozoite surface protein 3 is a target of allele-specific immunity and alleles are maintained by natural selection. J Infect Dis. 2007;195:279–287. doi: 10.1086/509806. [DOI] [PubMed] [Google Scholar]
- 30.Faber BW, Abdul Kadir K, Rodriguez-Garcia R, Remarque EJ, Saul FA, Vulliez-Le Normand B, et al. Low levels of polymorphisms and no evidence for diversifying selection on the Plasmodium knowlesi apical membrane antigen 1 gene. PLoS ONE. 2015;10:e0124400. doi: 10.1371/journal.pone.0124400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fong MY, Lau YL, Chang PY, Anthony CN. Genetic diversity, haplotypes and allele groups of Duffy binding protein (PkDBPalphaII) of Plasmodium knowlesi clinical isolates from Peninsular Malaysia. Parasit Vectors. 2014;7:161. doi: 10.1186/1756-3305-7-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yap NJ, Goh XT, Koehler AV, William T, Yeo TW, Vythilingam I, et al. Genetic diversity in the C-terminus of merozoite surface protein 1 among Plasmodium knowlesi isolates from Selangor and Sabah Borneo, Malaysia. Infect Genet Evol. 2017;54:39–46. doi: 10.1016/j.meegid.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed MA, Fauzi M, Han ET. Genetic diversity and natural selection of Plasmodium knowlesi merozoite surface protein 1 paralog gene in Malaysia. Malar J. 2018;17:115. doi: 10.1186/s12936-018-2256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kauth CW, Woehlbier U, Kern M, Mekonnen Z, Lutz R, Mucke N, et al. Interactions between merozoite surface proteins 1, 6, and 7 of the malaria parasite Plasmodium falciparum. J Biol Chem. 2006;281:31517–31527. doi: 10.1074/jbc.M604641200. [DOI] [PubMed] [Google Scholar]
- 35.Kadekoppala M, O’Donnell RA, Grainger M, Crabb BS, Holder AA. Deletion of the Plasmodium falciparum merozoite surface protein 7 gene impairs parasite invasion of erythrocytes. Eukaryot Cell. 2008;7:2123–2132. doi: 10.1128/EC.00274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perrin AJ, Bartholdson SJ, Wright GJ. P-selectin is a host receptor for Plasmodium MSP7 ligands. Malar J. 2015;14:238. doi: 10.1186/s12936-015-0750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garzon-Ospina D, Cadavid LF, Patarroyo MA. Differential expansion of the merozoite surface protein (msp)-7 gene family in Plasmodium species under a birth-and-death model of evolution. Mol Phylogenet Evol. 2010;55:399–408. doi: 10.1016/j.ympev.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Garzon-Ospina D, Lopez C, Forero-Rodriguez J, Patarroyo MA. Genetic diversity and selection in three Plasmodium vivax merozoite surface protein 7 (Pvmsp-7) genes in a Colombian population. PLoS ONE. 2012;7:e45962. doi: 10.1371/journal.pone.0045962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy SW, Weedall GD, da Silva RL, Polley SD, Ferreira MU. Sequence diversity and evolutionary dynamics of the dimorphic antigen merozoite surface protein-6 and other Msp genes of Plasmodium falciparum. Gene. 2009;443:12–21. doi: 10.1016/j.gene.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Pachebat JA, Ling IT, Grainger M, Trucco C, Howell S, Fernandez-Reyes D, et al. The 22 kDa component of the protein complex on the surface of Plasmodium falciparum merozoites is derived from a larger precursor, merozoite surface protein 7. Mol Biochem Parasitol. 2001;117:83–89. doi: 10.1016/S0166-6851(01)00336-X. [DOI] [PubMed] [Google Scholar]
- 41.Mello K, Daly TM, Long CA, Burns JM, Bergman LW. Members of the merozoite surface protein 7 family with similar expression patterns differ in ability to protect against Plasmodium yoelii malaria. Infect Immun. 2004;72:1010–1018. doi: 10.1128/IAI.72.2.1010-1018.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mello K, Daly TM, Morrisey J, Vaidya AB, Long CA, Bergman LW. A multigene family that interacts with the amino terminus of Plasmodium MSP-1 identified using the yeast two-hybrid system. Eukaryot Cell. 2002;1:915–925. doi: 10.1128/EC.1.6.915-925.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castillo AI, Andreina Pacheco M, Escalante AA. Evolution of the merozoite surface protein 7 (msp7) family in Plasmodium vivax and P. falciparum: a comparative approach. Infect Genet Evol. 2017;50:7–19. doi: 10.1016/j.meegid.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 45.Kall L, Krogh A, Sonnhammer EL. Advantages of combined transmembrane topology and signal peptide prediction—the Phobius web server. Nucleic Acids Res. 2007;35:W429–W432. doi: 10.1093/nar/gkm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 47.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed MA, Lau YL, Quan FS. Diversity and natural selection on the thrombospondin-related adhesive protein (TRAP) gene of Plasmodium knowlesi in Malaysia. Malar J. 2018;17:274. doi: 10.1186/s12936-018-2423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng CW, Putaporntip C, Jongwutiwes S. Polymorphism in merozoite surface protein-7E of Plasmodium vivax in Thailand: natural selection related to protein secondary structure. PLoS ONE. 2018;13:e0196765. doi: 10.1371/journal.pone.0196765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmed MA, Chu KB, Quan FS. The Plasmodium knowlesi Pk41 surface protein diversity, natural selection, sub population and geographical clustering: a 6-cysteine protein family member. PeerJ. 2018;6:e6141. doi: 10.7717/peerj.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fong MY, Ahmed MA, Wong SS, Lau YL, Sitam F. Genetic diversity and natural selection of the Plasmodium knowlesi circumsporozoite protein nonrepeat regions. PLoS ONE. 2015;10:e0137734. doi: 10.1371/journal.pone.0137734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Study samples and origin.
Additional file 2. Signal peptide identifed by Signal IP server.
Additional file 3. Neucleotide polymorphism across the full-length pkmsp7D genes. Hyper-variable SNPs are marked in bold and white and SNPs with deletions marked in red.
Data Availability Statement
The datasets analysed during the current study were derived from the following public region resources: 10.1371/journal.pone.0121303 and 10.1073/pnas.1509534112.