Abstract
Specific autoantibodies were assessed among residents of the Navajo Nation in New Mexico chronically exposed to metal mixtures from uranium mine wastes and in drinking water supplies. Age and the extent of exposure to legacy waste from 100 abandoned uranium mine and mill sites were associated with antibodies to denatured DNA, previously known to be an early indicator of medication-induced autoimmunity. Surprisingly, autoantibodies to native DNA and/or chromatin were also linked to environmental exposure, specifically uranium consumption through drinking water for both men and women, while urinary arsenic was negatively associated with these autoantibodies in women. These findings suggest that contaminants derived from uranium mine waste enhanced development of autoantibodies in some individuals, while arsenic may be globally immunosuppressive with gender-specific effects. Specific autoantibodies may be a sensitive indicator of immune perturbation by environmental toxicants, an adverse effect not considered in current drinking water standards or regulatory risk assessment evaluations.
Keywords: autoantibodies, uranium, arsenic, exposure, Navajo Nation
1. INTRODUCTION
The Navajo Nation was heavily mined for uranium from the 1940s through the mid-1980s [1], resulting in a legacy of more than 500 abandoned, non-remediated uranium mines and approximately 1100 associated waste sites [2, 3]. Since the last mine closed over thirty years ago, there has been no evaluation of possible effects of mine waste exposure on community health prior to inception of the Diné Network for Environmental Health (DiNEH) Project, a community-academic partnership from which the data presented here are derived.
Geospatial data and survey responses of 1,304 DiNEH study participants demonstrated that there was increased likelihood of kidney disease for people with exposures during the active mining era and that living close to unremediated uranium mine and waste sites was strongly associated with hypertension and the probability of developing diabetes and chronic kidney disease. [4]. Proximity to abandoned uranium mines also strongly predicted capacity of serum to induce inflammatory biomarkers in cultured endothelial cells [5], consistent with elevated oxidized low-density lipoprotein and other biomarkers of cardiovascular disease in this population [6]. Of possible mechanistic relevance, uranium at 10 μM inhibited poly(ADP-ribose)polymerase-1 and DNA repair in tissue culture cells [7].
Immune system abnormalities such as serum autoantibodies can also be effect biomarkers of heavy metal exposure. Gold miners in Brazil exposed to occupational mercury had increased prevalence of anti-nucleolar and anti-nuclear antibodies (ANA) [8]. ANA determinations were included in the Navajo Area Indian Health Service (NAIHS) contribution to the DiNEH study as part of the Community Uranium Exposure Journey to Healing (CUE-JTH) program. ANA positivity among DiNEH participants was 29% (64/222, unpublished data), double the United States (U.S.) national average in the National Health and Nutrition Examination Survey (NHANES) [9]. This finding motivated evaluation of specific autoantibodies described in the current report.
Because of the large number of described autoantibody specificities [10], testing rationale for specific autoantibody screening can be arbitrary and challenging. In addition, while it is generally presumed that autoantibodies are idiopathic, arising spontaneously in association with autoimmune and several other diseases, long-term treatment with various medications commonly induces anti-denatured (single-stranded) DNA and anti-histone antibodies and occasionally lupus-like symptoms [11]. We hypothesized that such xenobiotic-induced autoantibodies might also develop among DiNEH participants as a consequence of long-term inadvertent ingestion or inhalation of toxicants derived from legacy uranium mine waste. Because the risk of drug-induced autoantibodies increases with both duration and dose of xenobiotic exposure [12], the current study evaluated whether proximity to legacy mines and/or the amount of metal contaminants ingested in drinking water were predictors of these autoantibodies. Several other autoantibodies, commonly associated with idiopathic autoimmune diseases such as systemic lupus erythematosus (SLE) [13], were also evaluated for comparison.
2. METHODS
2.1. Study Population and Biological Samples
From 2002-2013 the DiNEH Project — a partnership of the University of New Mexico (UNM) Health Sciences Center (HSC) Community Environmental Health Program, the Southwest Research and Information Center, and 20 chapters of the Eastern Agency of the Navajo Nation in Southwestern U.S. (Fig. 1) — investigated the contribution of long-term uranium waste exposure on common chronic diseases. All participants enrolled in Phase I were invited to participate in clinical assessments offered by the NAIHS CUE-JTH program, initiated as part of the federal response to Congressional hearings in 2007 into the potential health effects of abandoned uranium mines and waste sites across the Navajo Nation [14]. The study was monitored and approved by the UNM HSC Human Research Protection Office (HRPO# 03-059 and the Navajo Nation Human Research Review Board (NNHRRB NNR.04.145). Bilingual staff communicated with prospective participants with culturally appropriate information about the study, and participants signed informed consent. In addition, the study received explicit community support and documentation from all participating Chapters of the Eastern Agency of the Navajo Nation. Of the original cohort, twenty-percent (N=263) provided blood, and 193 participants provide urine samples at community centers and clinics between June 2010 and May 2011. The demographics of this Phase II group and the original Phase I cohort are shown in Table 1. Biological specimens were processed onsite. After venipuncture, blood was centrifuged at 2500 rpm for 10 minutes, and serum aliquots were stored at −80°C. Urine was collected as spot samples, centrifuged at 500 rpm for 10 minutes, and aliquots of the supernatant were stored at −80°C.
Figure 1.
The DiNEH study recruitment area of the Eastern Agency of the Navajo Nation in Southwestern U.S.
Table 1.
Selected demographics of the original DiNEH Phase I cohort [4] and of the Phase II group used in the current study
| Phase I | Phase II | |||
|---|---|---|---|---|
| N | % | N | % | |
| Gender: | ||||
| Females | 736 | 56.4 | 151 | 56.5 |
| Males | 568 | 43.6 | 116 | 43.5 |
| All Participants | 1,304 | 100.0 | 267 | 100.0 |
| Age: | ||||
| Mean age, females (yrs) | 51.6 | 55.3 | ||
| Mean age, males (yrs) | 51.4 | 55.1 | ||
| Education: | ||||
| None | 127 | 9.7 | 25 | 9.4 |
| Less than high school | 547 | 41.9 | 94 | 35.2 |
| High school graduate | 317 | 24.3 | 74 | 27.7 |
| Post-high school | 313 | 24.0 | 74 | 27.7 |
| Duration of residency: | ||||
| Lived in current home (yrs) | 32.1 | 32.0 | ||
| Lived in 1 location during life | 870 | 66.7 | 171 | 64.0 |
| Lived in 2 or more locations during life | 415 | 31.8 | 97 | 36.0 |
| Uranium Mining Exposure (M) | ||||
| Worked in uranium mine | 128 | 9.8 | 49 | 18.4 |
| Worked in uranium mill | 24 | 1.8 | 9 | 3.4 |
| Worked in uranium reclamation | 27 | 2.1 | 6 | 2.3 |
| Washed clothing of uranium worker | 275 | 21.1 | 96 | 36.0 |
| Lived in mining camp | 48 | 3.7 | 18 | 6.7 |
| Environmental (E) Legacy Mines Exposure | ||||
| Used mine wastes in construction | 201 | 15.4 | 70 | 26.6 |
| Herded livestock near uranium mines or mills | 165 | 12.7 | 51 | 19.1 |
| Sheltered livestock near uranium mines, mills | 24 | 1.8 | 8 | 3.0 |
| Living near mine waste water | 167 | 12.8 | 62 | 23.2 |
| Playing on uranium tailings | 164 | 12.6 | 52 | 19.5 |
| Playing near uranium mines or mills | 159 | 12.2 | 53 | 19.9 |
2.2. Survey Information
A questionnaire designed to assess environmental exposures was developed by the DiNEH Project staff in partnership with community members following training on research design and basic concepts of exposure and toxicology appropriate to the Navajo culture and language. The survey asked participants about their water and land use, history of potential contact with uranium mines or waste including occupational exposure, as well as personal health histories. Participants who self-reported an autoimmune disease were not excluded from the study because diagnoses could not be confirmed. The complete DiNEH Survey is available in Supplemental Material.
2.3. Environmental Exposures to Uranium Mine Waste
Participants' proximity to 98 uranium mining and 2 milling waste sites was established geospatially by using publicly available mine-location data compiled by the U.S. Environmental Protection Agency (USEPA) and the U.S. Army Corps of Engineers in collaboration with the Navajo Nation Environmental Protection Agency [3]. Graphic Information System "shape" files with latitude-longitude coordinates for uranium waste sites were matched with participants' home locations obtained at the time of in-home surveys using Global Positioning System (GPS) instruments or from rural addressing system maps if GPS coordinates were not available. The distance between each participant’s home and all 100 potential exposure sites was calculated as the distance from participant i to each site j, denoted by d(i,j). In order to estimate a person’s exposure to all sites, a cumulative “proximity” variable (P) was created as the mean of the inverse distance (d) in which P(i) = (1/100) × (sum of 1/d(i,j)). In statistical models a log-transformed proximity variable (LogP) was applied.
The variable “mining era exposure” (M) was created to quantify self-reported mining exposure that occurred during the time of active mining in the study area (1950 -1986). M was estimated by adding the number of affirmative answers to questions (Q., Supplement, DiNEH survey, Section III) about having ever (a) worked in a uranium mine (Q.23); (b) worked in a uranium mill (Q.24); (c) lived in a mining camp (Q.28f); (d) washed or handled the clothes of a uranium worker (Q.28g); and/or (e) worked on reclamation of uranium mine, mill or hauled ore (Q.25). M produced values of 0-5 for each participant.
Exposure involving contact with uranium waste at mine and mill sites after active mining had ceased were defined as “environmental legacy exposure” (E) based on the number of affirmative answers to questions (Supplement, DiNEH survey, Section III) about having ever (a) played on uranium tailings piles or waste dumps (Q.28a); (b) played near or next to a uranium mine, mill or waste dump (Q.28b); (c) drank, waded or came into contact with uranium mine water or waste spills (Q.28c); (d) herded livestock next to a uranium mine, mill or waste dump (Q.28d); (e) sheltered livestock in an abandoned mine (Q.28e); and/or (f) used materials from abandoned mine or mill sites in home construction or around the property (Q.28h). E produced values of 0-6 for each participant (N=263).
2.4. Exposure to Contaminants in Water Sources
Drinking water sources and water quality data were matched to the participants in the DiNEH Phase II biomonitoring program. Survey responses identified 58 different water sources (38 unregulated, see endnote #1, and 20 regulated, including bottled water) used for drinking water by these participants. Standard methods consistent with USEPA procedures for collecting, preserving and shipping samples were used in samples obtained from unregulated water sources [15]. These samples were analyzed for 27 contaminants (largely consisting of metals, metalloids and major ions) at the Carlsbad Environmental Monitoring and Research Laboratory (Carlsbad, NM; 2005-2007) and at the USEPA Region IX lab (Richmond, CA; 2007-2011). Radionuclide analyses were performed at the USEPA Indoor Air and Radiation Laboratory (Las Vegas, NV; 2003-2007) and at the New Mexico State Scientific Laboratory (Albuquerque, NM; 2003-2010) on samples from 30% of unregulated water sources. Five contaminants were assessed in the current study for annual consumption by study participants — uranium, arsenic, nickel, mercury, and total radium (Ra-226 and −228). These were selected based on their elevated prevalence in local water supplies (principally, arsenic, uranium and radium) [15] and on a priori knowledge of their potential immunotoxic effects (principally, mercury and nickel) [16-18]. Multiple samples were available for 13 unregulated water sources; the mean of these replicates, which showed little variability, was used for the current study. Contaminant concentrations in regulated water sources were compiled from annual federally-mandated Consumer Confidence Reports issued by water system operators and from publicly accessible databases managed and maintained by tribal, state and federal regulatory agencies. Chemical composition of bottled water was compiled from online reports of four companies and averaged across all bottled water products.
To address contaminant concentrations reported by laboratories as “non-detects”, the mean of their stated method detection limits (MDLs) was used for each of the five contaminants evaluated in this study. These MDL concentrations were 1.0 microgram per liter (μg/l) for arsenic, 0.094 μg/l for mercury, 0.377 μg/l for nickel, 0.02 picoCuries per liter (pCi/l) for total radium, and 0.38 μg/l for uranium. MDL concentrations were used to establish “baseline” concentrations for the five metals assessed in this study to avoid using zero in annual intake calculations. Estimated annual consumption (EAC) of each contaminant from all drinking water source (s) was calculated for each participant (i) as follows:
A daily drinking water intake of 2 liters was based on USEPA recommendations [19]. Some participants hauled water from, for example, up to four different sources in which case the EAC was calculated as the sum of one-fourth of the concentration from each source. For participants who reported drinking only from a public water system (PWS) the number of water sources used was considered to be one.
2.5. Navajo-specific Information
The degree of cultural traditionalism was evaluated based on frequency of Navajo language use at work, at home, and with friends. This variable, N, ranged from 0 to 4 based on self-identification as Navajo and to participant responses to three language usage questions in the DiNEH survey (Supplement, DiNEH survey, Section I, Q.A5 and Q.A6). Language use was a surrogate for traditional cultural practices that might affect exposure patterns.
2.6. Biomonitoring
Urine samples were analyzed for concentration of 4 metals on 193 participants. Frozen samples were transported to the UNM Earth and Planetary Sciences Geo/Analytical Chemistry Laboratory, where inductively coupled plasma mass spectrometry (ICP-MS, Perkin Elmer, Inc., Houston, TX) was used to determine metal concentrations based on internal assay standards. Limits of detection (LOD) in urine for these analyses were 0.15 μg/l for total arsenic, 0.16 μg/l for nickel, 0.09 μg/l for uranium, and 0.10 μg/l for vanadium. Because of the high LOD for uranium in the current study compared to NHANES, a binary detect vs. non-detect variable was employed (1 ≥0.09 μg/1; 0 < 0.09 μg/l) in statistical modeling for uranium, thereby focusing on the upper end of the distribution in the sample. All other metal concentrations were included in the statistical models as continuous variables. Measured concentrations were log transformed to normalize distributions.
2.7. Detection of Specific Autoantibodies
Autoantibodies against histones, denatured DNA (dDNA or single-stranded DNA), chromatin, and native DNA (nDNA or double-stranded DNA) were quantified in 263 participants by in-house enzyme-linked immunosorbent assays (ELISA) [20, 21] using peroxidase-conjugated anti-IgG (SouthernBiotech, Birmingham. AL) as the secondary antibody. Optical densities (OD) beyond the range of direct measurement at 1 hour were extrapolated from the OD value at earlier time-points as previously described [20].
2.8. Numeric Transformation of Antibody Activity
Positive and negative control sera were included in each assay. Correction for inter-assay variation was performed by multiplying each sample’s reactivity by the ratio of the positive control sera reactivity included in each assay to their average reactivities in all assays. After subtracting any background reactivity due to the secondary antibody, all DiNEH samples whose reactivity on each antigen was within that of negative control (normal) sera were considered normal reactivity for the DiNEH samples, which were then used to formally establish the mean and standard deviation assay background for each antigen. For the purpose of statistical analysis elevated reactivity on each antigen was further resolved into four ordinal groups as follows: 0 = <2 SD above the mean; 1 = 2-4 SD above the mean; 2 = 5-9 SD above the mean; 3 ≥ 9 SD above the mean. Autoantibody combinations were used in statistical modeling based on their distinct etiologic categories as follows:
Combination 1: Xenobiotic (such as drug-induced): anti-dDNA and anti-histone antibodies
Combination 2: Idiopathic (such as SLE-associated): anti-chromatin and anti-nDNA antibodies
For each combination, the antibody response variable was defined as the sum of the ordinal values of its two component antibodies.
2.9. Statistical Analyses
Poisson regression models were used to estimate the relationships between each antibody combination (as a count variable) and the exposure variables. Because of the relatively small sample size and potentially large number of variables, Akaike Information Criterion (AIC) was used for model selection in order to minimize information loss associated with stepwise removal of variables that do not have significant power to predict. Modeling was also performed using the predictor variables’ interactions with age and with legacy exposure (E) as modified by the proximity variable (logP), as E was shown to be associated in the Phase 1 study with the likelihood of developing hypertension and multiple chronic diseases including diabetes, kidney, and cardiovascular disease [4], and logP was shown to be a predictor of increased serum inflammatory markers [5]. Exposure survey data (E, M, and P) was missing in several individuals. In order to minimize data dimensionality, modeling was performed separately for participants in which urinary metals data was available (N=193 for “biomonitored” participants) from modeling involving all participants but excluding the urinary metals variables (N=260). Because females are known to have higher risk for autoantibodies [9], modeling was performed using a gender-stratified approach as well as gender-neutral modeling. In addition, modeling was done using the traditional approach of sex as a covariate (Supplement Tables S3 and S4). Modeling was carried out using version 2.12.1 of the statistical software package ‘R' (R Foundation for Statistical Computing, Vienna, Austria; URL:http://www.r-project.org). Reduced models are presented in the Results section only for variables with p<0.05; however, only variables with p-values of ~0.01 or lower are described in detail.
3. RESULTS
3.1. Demographic comparison
The Phase II group of the current study is a 20% subset of the original sample from the Phase I DiNEH Project cohort, but there were no significant differences between the age, sex, educational status and duration of residency between the two samples (Table 1). However, the Phase II group reported a 1.7-fold higher mining work as well as legacy exposure to uranium mine and mill sites compared to the Phase I cohort (Table 1), suggesting selection for a potentially more exposed group. This difference was especially significant for legacy exposure from living near abandoned mine sites based on answers to six survey questions about their activities during this period (p<0.0040, 2-tailed student’s paired t-test).
3.2. Drinking water quality
The participants in Phase II of the DiNEH project drank from 58 different water sources, and water quality data of each source was used to assess annual metals consumption of the 263 participants. For the most part, median and mean concentrations of the five metals selected for this study were well below EPA Safe Drinking Water Act Maximum Contaminant Levels (MCLs) (Table S1). However, 9 water sources (16%, 8 of which were unregulated) had mean concentrations that exceeded MCLs: 2 sources for arsenic, 3 for total radium, and 4 for uranium. Using a stricter criterion, 22 water sources (38%) had mean concentrations >½ MCL for at least one contaminant, and 5 sources (9%) had two or more elevated contaminants. Concentrations of metals in the regulated PWS were generally within USEPA MCLs, but 4 PWS had average total radium concentrations >½ MCL, and one PWS had a 5-year average radium concentration that exceeded the MCL by 10%. One PWS had a 12-year average arsenic level of 70% of the MCL, and another PWS had a 14-year average uranium level of 57% USEPA MCL.
3.3. Consumption of metal contaminants in drinking water
Estimated annual consumption of the five metals is shown in Fig. 2 for each participant. Three participants had EACs greater than the USEPA MCL extrapolated to one year for arsenic, seven for total radium, and four for uranium (Table S2). No participant had an EAC exceeding the annualized MCL for Hg or Ni.
Figure 2.
Distribution of estimated annual consumption of five metals among 263 DiNEH Project Phase II participants (Ra measured in nanocuries). The horizontal lines mark the US EPA regulatory limits for each contaminant (Maximum Contaminant Level (MCL)) for public water systems extrapolated to 1-year consumption. MCL for nickel was withdrawn by USEPA in 1995 but used here as a reference value.
3.4. Prevalence of specific autoantibodies
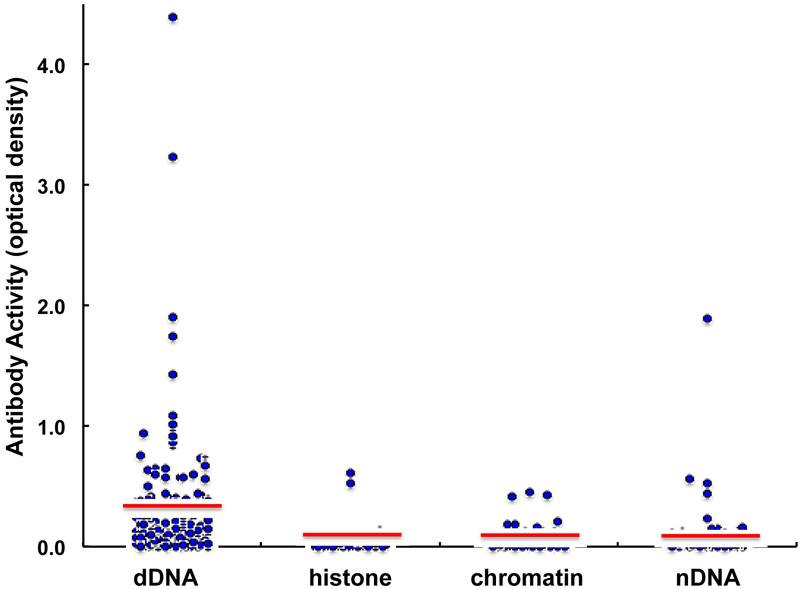
Of the 263 individuals, one or more of the four specific autoantibodies tested were detected in 54 people (21%) (Fig. 3). Forty-two of these individuals had elevated anti-dDNA antibody, which was the only detectable antibody in 32 participants (59% of people with any antibody). Nine of the anti-dDNA positive participants also had anti-nDNA antibodies, representing 75% of the anti-nDNA positives and consistent with the capacity of denatured DNA to spontaneously form double-stranded-like structures to which anti-nDNA antibodies can bind [22]. Of the 12 participants who had anti-nDNA, in only one person was this the sole antibody. In contrast, anti-chromatin was the only reactivity in 2 of 5 females and in 4 of 5 males, presumably reflecting the restricted specificity of these antibodies for histone-DNA complexes [23]. Except for anti-chromatin, more women had elevated reactivity, with a female:male ratio of 2.2 for anti-dDNA, 4.0 for anti-histone, and 3.0 for anti-nDNA.
Figure 3.
Autoantibody reactivities in 263 DiNEH serum samples. For each antibody, the horizontal line demarcates 2 standard deviations above the mean of 76% of DiNEH samples within the range of normal serum binding to each antigen.
3.5. Autoantibody Association with Environmental Exposure
Elevated Combination 1 autoantibodies (anti-dDNA and/or anti-histone autoantibodies) were found in 13.2% of males and 21.5% of females. Age and legacy environmental mine waste exposure (E) were independent predictors of elevated Combination 1 antibodies in males at p-values as low as 10−2 to <10−4 with both the complete data set (Table 2A1, N=113) and the smaller set (Table 2B1, N=89) restricted to men that had urine biomonitoring data as covariables. Age and E also predicted Combination 1 antibodies in women when second order interactions involving age and proximity to mines were included for the entire female sample (Table 2A2, N=147) as well as among women with biomonitoring results (Table 2B2, N=104). Proximity to mines also predicted Combination 1 antibodies when the sample with both sexes combined was used in modeling (p=0.0065) (Table 2A2). Several other correlates appeared in males or females in either positive or negative directions, but these were generally weak and inconsistent. Surprisingly, a history of occupational mining (M) was a significant negative predictor of Combination 1 antibodies, especially with males and gender-neutral modeling involving second order interactions (p=0.0058) (Table 2A2). Hg consumption was a negative predictor of Combination 1 antibodies in females, especially interactions in modeling of the biomonitored samples (p<0.0048) (Table 2B2) but was not significant when all participants were included in the analysis, and urinary Hg did not appear as a significant variable. Ni consumption was a strong predictor of Combination 1 antibodies in women using interactions in modeling the biomonitored samples (p<0.0091) (Table 2B2) as well as with genders-combined models (p<0.0036) (Table 2B2), although urinary Ni levels were not. However, an interaction between urinary Ni and age was a significant negative predictor in women (p<0.0039) (Table 2B2) as well as with genders-combined modeling (p<0.0018), suggesting that the association between Combination 1 antibodies and ingested Ni is skewed toward younger women.
Table 2.
Association of Combination 1 antibodies with exposure variables
| A1. Reduced models without interactions: all participants* | ||||||
|---|---|---|---|---|---|---|
| Male (N=113) | Female (N=147) | All (N=260) | ||||
| Parameter | estimate | p-value | estimate | p-value | estimate | p-value |
| age | 0.083 | 0.0000031 | 0.042 | 0.00000064 | ||
| E (legacy mine exp) | 0.38 | 0.010 | 0.14 | 0.038 | ||
| M (mine work) | −0.33 | 0.032 | ||||
| Navajo use | 0.27 | 0.027 | ||||
| A2. Reduced models with second order interactions (involving age and E•P): all participants* | ||||||
| Parameter | Male (N=113) | Female (N=147) | All (N=260) | |||
| estimate | p-value | estimate | p-value | estimate | p-value | |
| age | 0.17 | 0.000056 | 0.053 | 0.023 | 0.031 | 0.0025 |
| E (legacy mine exp) | 0.47 | 0.044 | ||||
| P (proximity) | 66 | 0.010 | 13.9 | 0.0065 | ||
| M (mine work) | −5.5 | 0.028 | −0.45 | 0.0058 | ||
| E:P | −9.5 | −0.014 | −5.5 | 0.0053 | ||
| Navajo use | −0.85 | 0.013 | ||||
| age:M | 0.073 | 0.029 | ||||
| age:E | −0.031 | 0.0058 | ||||
| U consumption | 0.24 | 0.033 | ||||
| B1. Reduced models without interactions: biomonitored participants* | ||||||
| Male (N=89) | Female (N=104) | Both (N=193) | ||||
| Parameter | estimate | p-value | estimate | p-value | estimate | p-value |
| age | 0.24 | 0.00017 | 0.043 | 0.00016 | ||
| E (legacy mine exp) | 0.66 | 0.000099 | 0.21 | 0.0052 | ||
| M (mine work) | −0.73 | 0.033 | ||||
| Navajo use | −1.2 | 0.010 | ||||
| As consumption | 0.89 | 0.031 | ||||
| Hg consumption | −1.1 | 0.029 | ||||
| urinary U | 0.69 | 0.049 | ||||
| B2. Reduced models with second order interactions (involving age and E•P): biomonitored participants* | ||||||
| Male (N=89) | Female (N=104) | Both (N=193) | ||||
| Parameter | estimate | p-value | estimate | p-value | estimate | p-value |
| age | 0.28 | 0.0038 | 0.27 | 0.00045 | ||
| E (legacy mine exp) | 0.55 | 0.049 | ||||
| P (proximity) | 22 | 0.042 | 119 | 0.031 | ||
| Ni consumption | 3.1 | 0.0091 | 3.3 | 0.0036 | ||
| age:P | −2.0 | 0.033 | ||||
| age:urinary Ni | −0.055 | 0.0039 | −0.05 | 0.0018 | ||
| age:urinary U | 0.088 | 0.013 | ||||
| As consumption | 0.74 | 0.027 | ||||
| Hg consumption | −1.3 | 0.0048 | ||||
Only results of statistical analysis with p-values <0.05 are included.
Only 6.1% of males and 8.7% of females had Combination 2 autoantibodies, anti-nDNA and/or anti-chromatin autoantibodies. A consistent predictor of Combination 2 antibodies was uranium consumption. Annual U consumption was an independent predictor in male (p<0.00099), female (p<0.011) and genders-combined modeling without secondary interactions (p<0.000014) (Table 3A1) as well as for male (p<0.032), female (p<0.0086) and genders-combined modeling (p<0.00014) with secondary interactions (Table 3A2). Combination 2 antibodies also correlated with legacy exposure to abandoned uranium mines (E) in the gender-neutral biomonitored participants, especially when interactions with age and proximity were included (p<0.00097) (Table 3B2). However, home proximity to mine sites was an inconsistent predictor of Combination 2 antibodies, in which significant positive association (p=0.0037) required the entire sample and second order interactions (Table 3A2), but the estimates for men compared with women were in opposite directions (Table 3A2). In males, arsenic (As) consumption was positively associated with Combination 2 antibodies (p<0.011), but this association was strongly influenced by age (p<0.0021) (Table 3A2). Among the average age DiNEH study males (55.1 years, Table 1), As consumption from water sources was responsible for an immunosuppressive effect (Estimate = −1.26), suggesting a suppression of these antibodies during aging. Similarly, urinary As was a strong independent negative predictor in females (p<0.0028) and with the entire sample (p<0.000012) (Table 3B1). Compared to Combination 1 antibodies, age alone was a weak predictor of Combination 2 antibodies and only with the entire, gender-neutral model without interactions among biomonitored participants (p= 0.034) (Table 3B1).
Table 3.
Association of Combination 2 antibodies with exposure variables
| A1. Reduced models without interactions: all participants* | ||||||
|---|---|---|---|---|---|---|
| Male (N=113) | Female (N=147) | All (N=260) | ||||
| Parameter | estimate | p-value | estimate | p-value | estimate | p-value |
| E (legacy mine exp) | 0.43 | 0.023 | 0.23 | 0.036 | ||
| P (proximity) | −47 | 0.017 | −15 | 0.043 | ||
| Ni consumption | −0.67 | 0.025 | ||||
| U consumption | 1.2 | 0.00099 | 0.42 | 0.011 | 0.58 | 0.000014 |
| A2. Reduced models with second order interactions (involving age and E•P): all participants* | ||||||
| Male (N=113) | Female (N=147) | All (N=260) | ||||
| Parameter | estimate | p-value | estimate | p-value | estimate | p-value |
| P | −55 | 0.023 | 90 | 0.019 | 143 | 0.0037 |
| M (mine work) | 2.0 | 0.036 | ||||
| age:P | −1.5 | 0.019 | −2.4 | 0.0051 | ||
| E:P | −43 | 0.024 | ||||
| Navajo use | −1.1 | 0.033 | ||||
| age:M | −0.037 | 0.030 | ||||
| As consumption | 5.9 | 0.011 | ||||
| U consumption | 1.5 | 0.032 | 0.47 | 0.0086 | 0.54 | 0.00014 |
| age:As consumption | −0.13 | 0.0021 | ||||
| age:Navajo use | 0.022 | 0.037 | 0.021 | 0.018 | ||
| B1. Reduced models without interactions: biomonitored participants* | ||||||
| Male (N=89) | Female (N=104) | Both (N=193) | ||||
| Parameter | estimate | p-value | estimate | p-value | estimate | p-value |
| age | 0.038 | 0.034 | ||||
| E (legacy mine exp) | 0.38 | 0.011 | ||||
| Hg consumption | 0.76 | 0.042 | ||||
| U consumption | 0.57 | 0.0023 | ||||
| urinary As | −1.1 | 0.0028 | −1.6 | −0.000012 | ||
| B2. Reduced models with second order interactions (involving age and E•P): biomonitored participants* | ||||||
| Male (N=89) | Female (N=104) | Both (N=193) | ||||
| Parameter | estimate | p-value | estimate | p-value | estimate | p-value |
| E (legacy mine exp) | 12 | 0.00097 | ||||
| M | 4.81 | 0.045 | ||||
| P | 255 | 0.031 | ||||
| age:P | −4.8 | 0.0095 | ||||
| E:P | −196 | 0.0032 | ||||
| age:M | −0.091 | 0.042 | ||||
| age:E | −0.18 | 0.0016 | ||||
| U consumption | 0.80 | 0.0093 | ||||
| age:E:P | 3.1 | 0.0035 | ||||
Only results of statistical analysis with p-values <0.05 are included.
4. DISCUSSION
Modeling using all participants as well as gender-stratified modeling showed that a history of exposure to uranium mines, mills or waste dumps contributed to age-dependent development of Combination 1 antibodies, predominately anti-dDNA antibodies. Age may be a surrogate for long-term exposure, suggesting that life-long residency near mine contaminants may promote induction and/or augmentation of these antibodies. Compared to females, males showed a much stronger association of Combination 1 antibodies to legacy mine exposure (E), but its negative association with occupational mining (M) complicates interpretation. Only with females did proximity to mines predict Combination 1 antibodies. While these autoantibodies were more than twice as common in women than men, that both genders showed the same relationship to legacy mine exposure supports a common mechanistic basis for this immune perturbation. Regarding metals in drinking water, only Ni consumption predicted Combination 1 antibodies, and this was observed only with the biomonitored gender-neutral samples and with biomonitored women. That estimates of five metals ingested through drinking water did not generally correlate with these antibodies may suggest that other agent(s) or their interactions in the environment and/or route of entry underlie the source of possible antibody-inducing agents. Because single urine samples were used for biomonitoring metals, their non-correlation with Combination 1 antibodies would not necessarily reflect chronic exposure to individual, multiple, and possibly interacting environmental contaminants over the some >30 year residency in the same home.
While Combination 2 autoantibodies were not as common as Combination 1 antibodies, their detection in any DiNEH participant was surprising because anti-nDNA and anti-chromatin antibodies are generally associated with idiopathic autoimmune disease [13], although anti-chromatin antibodies often develop with symptomatic lupus induced by medications [11]. While legacy exposure to mine waste and proximity were not consistently strong predictors of Combination 2 antibodies, the significant appearance of these immune parameters in both males and females suggests that mine contaminant(s) are possible etiological factors for Combination 2 antibodies as well. Of the five measured metals in water sources, only annual uranium consumption predicted Combination 2 antibodies, and this metal was a strong predictor across all sample groups regardless of sex, suggesting that uranium intake enhanced development of these autoantibodies. These autoantibodies are commonly detected in patients with SLE [13], evocative of the findings of Lu-Fritts, et. al. [24] of a four-fold increase in diagnosed SLE among residents of Ferrnald, OH, who were considered to have historical exposure to uranium due to an ore processing plant in their community. However, reliable clinical information on the participants in the current study was not available. Unlike Combination 1 antibodies, age was not a predictor of Combination 2 antibodies, consistent with the categorization of these antibodies as idiopathic.
Older males showed a negative association with arsenic consumption and Combination 2 antibodies, and urinary As excretion was a negative predictor of Combination 2 antibodies in females. This suggests that As is immunosuppressive for development of Combination 2 autoantibodies. Women may convert As to particularly immunosuppressive metabolites, and long-term exposure of men to As during aging may suppress these autoantibodies. These findings are consistent with the association between urinary As and decreased protective antibody response to mumps vaccine [25] and to challenge with purified protein derivative in BCG-vaccinated Bangledesh children [18], and that sex-specific differences may complicate this effect [26], In direct tests arsenic trioxide inhibited interferon-γ synthesis in vitro by lymphocytes from patients with SLE [27] and prolonged survival and decreased anti-nDNA production in lupus-prone mice [28], possibly by inhibiting inflammasome activation [29].
While retrospective analyses have demonstrated that serum autoantibodies can predate development of SLE [30] or other autoimmune diseases [31] by many years, autoantibodies related to environmental exposure do not necessarily lead to autoimmune disease [32]. Nevertheless, autoantibodies associated with chemicals, radiation, medications, infectious agents, or dietary factors [33] may be a reflection of immune dysregulation, and anti-dDNA and anti-histone antibodies commonly develop in people treated with various medications but who remain asymptomatic [34]. In the current study 16% of the participants had anti-dDNA antibodies, which were associated with legacy exposure to abandoned uranium mines. While it is unlikely that most of these people will develop clinical autoimmune disease [11, 12], these findings suggest anti-dDNA antibodies are a sensitive indicator of the capacity of uranium mine waste to cause immune dysregulation.
There are several limitations in this study. Approximately 20% of the Phase I DiNEH Project cohort volunteered to donate blood and urine samples at 11 community collection and education events spanning 2400 square miles of Northwestern New Mexico. While the demographics of the Phase I and Phase II groups were similar, the participants in Phase II may have been less healthy and/or had potentially more environmental legacy mine exposure. While Phase II participants may, therefore, have been more motivated to contribute to this study, those who developed medical disabilities may have been less willing. In addition, the four metals selected to assess internal exposure are not necessarily the only potential toxicants, and one-time collected urine samples do not principally reflect long-term exposure. Furthermore, only binary assessment of urinary uranium was performed, possibly failing to capture a potential contribution of uranium as a continuous variable as calculated for other metals. While the hypothesis of this project, that specific autoantibodies known to be induced by xenobiotics would be markers for exposure to environmental toxicants, satisfied a fundamental statistical requirement for a meaningful finding [35], many variables as well as their interactions were included as potential autoantibody-affecting factors. Some variables became significant predictors of autoantibodies, but they were not corrected for the number of comparisons performed, the effect magnitude (estimate) varied, and uncertainty metrics (confidence intervals) were not included. These shortcomings are diminished because this was a cross-sectional study and the variables were not considered random and independent and by focusing on p-values = 0.01 or less as suggested [36]. Finally, autoantibodies may appear spontaneously in association with aging [9], there may be a genetic predisposition to autoimmunity in the DiNEH population, and heritable epigenetic changes due to multigenerational mine waste exposure may contribute to autoantibody development.
5. CONCLUSIONS
Statistical modeling of the current survey and analytic data showed that long-term exposure to abandoned, extant uranium mines was associated with development of autoantibodies. Specifically, [anti-native DNA+anti-chromatin] autoantibodies, generally believed to be idiopathic in origin, showed strong linkage to uranium intake through drinking water in both sexes. However, these autoantibodies showed negative association with ingested arsenic in aging men and with urinary arsenic in women, implying that the autoimmune-enhancing effects of uranium would have been even stronger in the absence of arsenic-mediated immunosuppression. These findings were surprising because most of the water sources were within sanctioned USEPA MCL. However, drinking water standards for uranium contamination are largely based on documented nephrotoxicity [19, 37]; autoimmune-related effects were not considered. Anti-denatured DNA antibodies were predicted by long-term lifestyle exposure to abandoned uranium mines in both sexes and proximity to mines in women as well as by nickel consumption in women. Overall, these findings suggest that low but chronic contaminant exposure and contact with mixed-metal mining waste results in immune dysfunction including autoreactivity. Particular autoantibodies may be sensitive biomarkers for monitoring exposure and potential health effects of environmental toxicants among community members living on tribal lands.
Supplementary Material
HIGHLIGHTS.
Environmental exposure to uranium mines and mill sites and serum autoantibodies were assessed in residents of the Navajo Nation.
Life-long exposure was associated with anti-denatured DNA antibodies, especially in men, while anti-native DNA/-chromatin autoantibodies were linked to uranium consumption through drinking water.
Arsenic consumption through water and urinary arsenic were negative predictors of autoantibodies.
Specific autoantibodies may be of value for monitoring immune-perturbing effects of environmental toxicants.
Acknowledgments:
We thank K. Michael Pollard, Ph.D., The Scripps Research Institute, for critical reading of the manuscript.
Funding: The DiNEH project was supported by the National Institutes of Health [grant numbers NIH/ES-014565; NIH/ES-013208; NIH/ES-012072; 3P20MD004811-02S1; NIH/NCRR-1UL1RR031977]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Endnote
#1: Unlike regulated water sources, an unregulated water source is not tested or treated to reduce contaminant concentrations and does not have to meet primary drinking water standards established by USEPA, the states, or tribes pursuant to the Safe Drinking Water Act.
Financial Disclosure: The authors state that they have no competing financial interests associated with the study and/or the analysis presented herein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- [1].Brugge D, Goble R, The history of uranium mining and the Navajo people, Am J Public Health 92 (2002) 1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Furlow B, Uranium mines: Lung disease and mistrust among Native Americans, Lancet Respir Med 2 (2014) 178–179. [DOI] [PubMed] [Google Scholar]
- [3].Navajo nation AUM screening assessment report and atlas with geospatial data, U.S. Environmental Protection Agency. In: U.S. Army Corps of Engineers Region 9, Federal Register: Office of the Federal Register (2007) 1–198. https://www.epa.gov/sites/production/files/2017-01/documents/navajo_nation_aum_screening_assess_report_atlas_geospatial_data-2007-08.pdf [Google Scholar]
- [4].Hund L, Bedrick EJ, Miller C, Huerta G, Nez T, Ramone S, Shuey C, Cajero M, Lewis J, A Bayesian framework for estimating disease risk due to exposure to uranium mine and mill waste on the Navajo nation, Journal of the Royal Statistical Society Series a-Statistics in Society 178 (2015) 1069–1091. [Google Scholar]
- [5].Harmon ME, Lewis J, Miller C, Hoover J, Ali AS, Shuey C, Cajero M, Lucas S, Zychowski K, Pacheco B, Erdei E, Ramone S, Nez T, Gonzales M, Campen MJ, Residential proximity to abandoned uranium mines and serum inflammatory potential in chronically exposed Navajo communities, J Expo Sci Environ Epidemiol 27 (2017) 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Harmon ME, Campen MJ, Miller C, Shuey C, Cajero M, Lucas S, Pacheco B, Erdei E, Ramone S, Nez T, Lewis J, Associations of circulating oxidized LDL and conventional biomarkers of cardiovascular disease in a cross-sectional study of the Navajo population, PLoS One 11 (2016) e0143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cooper KL, Dashner EJ, Tsosie R, Cho YM, Lewis J, Hudson LG, Inhibition of poly(ADP-ribose)polymerase-1 and DNA repair by uranium, Toxicol Appl Pharmacol 291 (2016) 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gardner RM, Nyland JF, Silva IA, Ventura AM, de Souza JM, Silbergeld EK, Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in amazonian brazil: A cross-sectional study, Environ Res 110 (2010)345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, Jusko TA, Walker NJ, Germolec DR, Whitt IZ, Crockett PW, Pauley BA, Chan JY, Ross SJ, Birnbaum LS, Zeldin DC, Miller FW, Prevalence and sociodemographic correlates of antinuclear antibodies in the United States, Arthritis Rheum 64 (2012) 2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shoenfeld Y, Blank M, Abu-Shakra M, Amital H, Barzilai O, Berkun Y, Bizzaro N, Gilburd B, Zandman-Goddard G, Katz U, Krause I, Langevitz P, Mackay IR, Orbach H, Ram M, Sherer Y, Toubi E, Gershwin ME, The mosaic of autoimmunity: Prediction, autoantibodies, and therapy in autoimmune diseases--2008, Isr Med Assoc J 10 (2008) 13–19. [PubMed] [Google Scholar]
- [11].Rubin RL, Drug-induced lupus, Expert Opin Drug Saf 14 (2015) 361–378. [DOI] [PubMed] [Google Scholar]
- [12].Henningsen NC, Cederberg A, Hanson A, Johansson BW, Effects of long-term treatment with procaine amide. A prospective study with special regard to ANF and SLE in fast and slow acetylators, Acta Med Scand 198 (1975) 475–482. [PubMed] [Google Scholar]
- [13].Mehra S, Fritzler MJ, The spectrum of anti-chromatin/nucleosome autoantibodies: Independent and interdependent biomarkers of disease, J Immunol Res 2014 (2014) 368274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Health and environmental impacts of uranium contamination in the Navajo nation five-year plan. In: Agency USEP, Federal Register: Office of the Federal Register; (2008). https://www.epa.gov/sites/production/files/2016-06/documents/nn-5-year-plan-june-12.pdf [Google Scholar]
- [15].Hoover J, Gonzales M, Shuey C, Barney Y, Lewis J, Elevated arsenic and uranium concentrations in unregulated water sources on the Navajo nation, USA, Expo Health 9 (2017) 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Haley PJ, Bice DE, Muggenburg BA, Hahn FF, Benjamin SA, Immunopathologic effects of nickel subsulfide on the primate pulmonary immune system, Toxicol Appl Pharmacol 88 (1987) 1–12. [DOI] [PubMed] [Google Scholar]
- [17].Aranyi C, Bradof JN, O'Shea WJ, Graham JA, Miller FJ, Effects of arsenic trioxide inhalation exposure on pulmonary antibacterial defenses in mice, J Toxicol Environ Health 15 (1985) 163–172. [DOI] [PubMed] [Google Scholar]
- [18].Ahmed S, Moore SE, Kippler M, Gardner R, Hawlader MD, Wagatsuma Y, Raqib R, Vahter M, Arsenic exposure and cell-mediated immunity in pre-school children in rural Bangladesh, Toxicol Sci 141 (2014) 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].National primary drinking water regulations; radionuclides; final rule. In: Agency EP, editor., Federal Register: Office of the Federal Register, National Archives and Records Administration; 65 (2000) 76707–76753. [Google Scholar]
- [20].Burlingame RW, Rubin RL, Subnucleosome structures as substrates in enzyme-linked immunosorbent assays, J Immunol Methods 134 (1990) 187–199. [DOI] [PubMed] [Google Scholar]
- [21].Burlingame RW, Rubin RL. Enzyme-linked immunosorbent assays for diagnostically important antinuclear antibodies In: Rose NR, Hamilton RG, Detric B, editors. Manual of clinical laboratory immunology, Washington, D.C: American Society for Microbiology; 2002, p. 951–960. [Google Scholar]
- [22].Ali R, Dersimonian H, Stollar BD, Binding of monoclonal anti-native DNA autoantibodies to DNA of varying size and conformation, Mol Immunol 22 (1985) 1415–1422. [DOI] [PubMed] [Google Scholar]
- [23].Burlingame RW, Boey ML, Starkebaum G, Rubin RL, The central role of chromatin in autoimmune responses to histones and DNA in systemic lupus erythematosus, J Clin Invest 94 (1994) 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lu-Fritts PY, Kottyan LC, James JA, Xie C, Buckholz JM, Pinney SM, Harley JB, Association of systemic lupus erythematosus with uranium exposure in a community living near a uranium-processing plant: A nested case-control study, Arthritis Rheumatol 66 (2014) 3105–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Raqib R, Ahmed S, Ahsan KB, Kippler M, Akhtar E, Roy AK, Lu Y, Arifeen SE, Wagatsuma Y, Vahter M, Humoral immunity in arsenic-exposed children in rural Bangladesh: Total immunoglobulins and vaccine-specific antibodies, Environ Health Perspect 125 (2017) 067006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lindberg AL, Ekstrom EC, Nermell B, Rahman M, Lonnerdal B, Persson LA, Vahter M, Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh, Environ Res 106 (2008) 110–120. [DOI] [PubMed] [Google Scholar]
- [27].Hu H, Chen E, Li Y, Zhu X, Zhang T, Zhu X, Effects of arsenic trioxide on inf-gamma gene expression in MRL/lpr mice and human lupus, Biol Trace Elem Res 184 (2018)391–397. [DOI] [PubMed] [Google Scholar]
- [28].Xia XR, Lin SX, Zhou Y, Effects of arsenic trioxide on the autoimmunity and survival time in BXSB lupus mice, Zhongguo Zhong Xi Yi Jie He Za Zhi 27 (2007) 138–141. [PubMed] [Google Scholar]
- [29].Maier NK, Crown D, Liu J, Leppla SH, Moayeri M, Arsenic trioxide and other arsenical compounds inhibit the NLRP1, NLRP3, and AIP5/NLRC4 inflammasomes, J Immunol 192 (2014) 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB, Development of autoantibodies before the clinical onset of systemic lupus erythematosus, N Engl J Med 349 (2003) 1526–1533. [DOI] [PubMed] [Google Scholar]
- [31].Perez D, Gilburd B, Cabrera-Marante O, Martinez-Flores JA, Serrano M, Naranjo L, Pleguezuelo D, Morillas L, Shovman O, Paz-Artal E, Shoenfeld Y, Serrano A, Predictive autoimmunity using autoantibodies: Screening for anti-nuclear antibodies, Clin Chem Lab Med 56 (2018) 1771–1777. [DOI] [PubMed] [Google Scholar]
- [32].Pollard KM, Environment, autoantibodies, and autoimmunity, Front Immunol 6 (2015) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, De Roos AJ, Epidemiology of environmental exposures and human autoimmune diseases: Findings from a national institute of environmental health sciences expert panel workshop, J Autoimmun 39 (2012) 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rubin RL, Reimer G, McNally EM, Nusinow SR, Searles RP, Tan EM, Procainamide elicits a selective autoantibody immune response, Clin Exp Immunol 63 (1986) 58–67. [PMC free article] [PubMed] [Google Scholar]
- [35].Wasserstein RL, Fazar NA, The ASA's statement on p-values: Context, process, and purpose, American Statistician 70 (2016) 129–131. [Google Scholar]
- [36].Chavalarias D, Wallach JD, Li AH, Ioannidis JP, Evolution of reporting p values in the biomedical literature, 1990–2015, JAMA 315 (2016) 1141–1148. [DOI] [PubMed] [Google Scholar]
- [37].Uranium: Renal (urinary system or kidneys). In: Federal Register, Office of the Federal Register, AfTSaD, editor., Federal Register: Office of the Federal Register, National Archives and Records Administration; (2013) CAS ID #: 7440–61-1. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=440&tid=77 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.