Abstract
Purpose: The aim of this study was to evaluate the prognostic value of the aspartate transaminase/alanine transaminase (AST/ALT) ratio in a large Chinese cohort surgically treated for localized upper tract urothelial carcinoma (UTUC) using propensity score matching (PSM) analysis.
Methods: Data of 908 consecutive patients with localized UTUC who underwent radical nephroureterectomy (RNU) were retrospectively evaluated. The endpoints of prognosis were progression-free survival (PFS), cancer-specific survival (CSS) and overall survival (OS) after RNU. We compared these endpoints according to the AST/ALT ratio before and after 1:1 PSM. The independent predictors for PFS, CSS and OS were also analyzed.
Results: A high AST/ALT ratio was correlated with unfavorable factors, including elderly age, female gender, history of coronary disease, alcohol and tobacco consumption, lower body mass index, and larger tumor volume. Before PSM, the Kaplan–Meier curves showed significantly poorer survival outcomes in PFS, CSS, and OS (all P<0.001) for patients with high AST/ALT ratios. After PSM, the high AST/ALT ratio group also had significantly inferior survival outcomes in terms of PFS, OS and CSS (all P<0.001). Furthermore, multivariate analyses revealed that the AST/ALT ratio was an independent predictor for PFS, CSS and OS before PSM (PFS hazard ratio [HR] 1.454, P=0.001; CSS HR 2.577, P<0.001; OS HR 1.925, P<0.001) and after PSM (PFS HR 1.711, P<0.001; CSS HR 2.588, P<0.001; OS HR 1.957, P<0.001).
Conclusion: The preoperative AST/ALT ratio can be a convenient and useful prognostic biomarker for patients with localized UTUC.
Keywords: aspartate transaminase, alanine transaminase, upper urinary tract urothelial carcinoma, prognosis
Introduction
Upper tract urothelial carcinoma (UTUC) arises from pyelocaliceal cavities and the ureter and accounts for 5–10% urothelial carcinoma cases.1,2 In Western countries, the incidence is approximately two cases per 100,000 individuals every year.1,3 Radical nephroureterectomy (RNU) with excision of the ureteral orifice and the bladder cuff is the standard care for localized UTUC patients.3 RNU alters renal function and may affect the use of perioperative chemotherapy, therefore, selection of primary therapy, especially consideration of kidney-sparing (KSP) therapy, is important4,5 . For patients with low-risk tumors and two functional kidneys, KSP is recommended the first treatment option. For patients with high-risk cancers, KSP is limited to distal ureteral tumors and imperative cases.1,6 However, risk stratification to discriminate between patients with low- and high-risk tumors remains challenging. According to the recent literature, the neutrophil-lymphocyte ratio, which reflects the inflammatory response, has been confirmed as an independent blood-based prognosticator in UTUC patients.7–9 Nevertheless, knowledge of preoperatively assessable plasma prognostic factors in UTUC remains limited.
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are well-known enzymes that are routinely measured before surgery. The De Ritis ratio (AST/ALT) was originally proposed as a characteristic of viral hepatitis and useful biomarker for other hepatic diseases.10 The AST/ALT ratio was previously demonstrated to be useful prognostic biomarkers in patients with certain types of malignancies.11–14 Recently, Nishikawa M et al, and Lee et al, reported that the AST/ALT ratio is an independent prognostic factor in patients with UTUC treated with surgery.15,16 In their study, patients with a high AST/ALT ratio had inferior survival outcomes. Although their study was valuable for presenting the AST/ALT ratio as a novel prognostic factor for patients with UTUC, they were not able to exclude the effect of alcohol, tobacco consumption and coronary disease on AST/ALT ratio, and they only analyzed a relatively small number of patients.
The aim of this study was to evaluate the prognostic value of AST/ALT ratio in a large Chinese cohort surgically treated for localized UTUC using propensity score matching (PSM) analysis.
Patients and methods
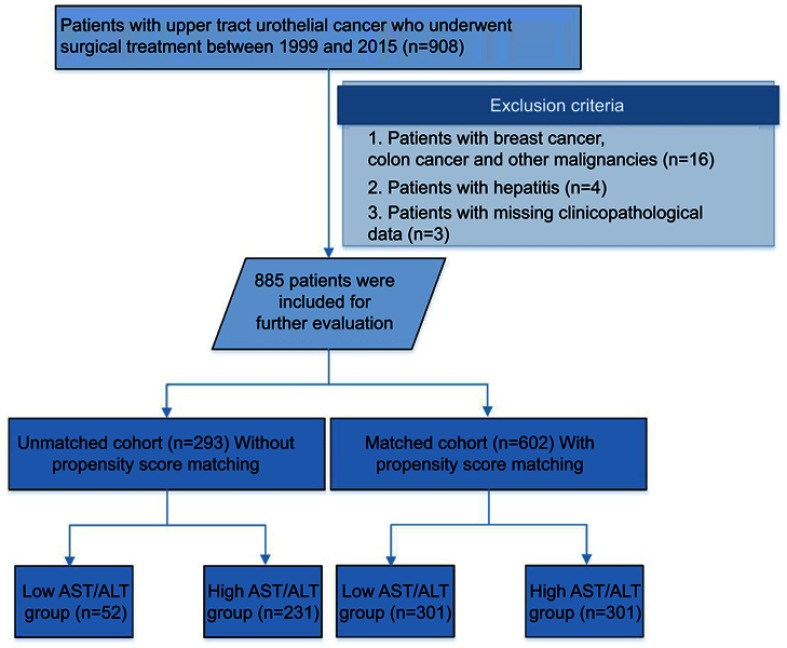
After approval by the internal ethics review board of Peking University First Hospital, we retrospectively collected the records of 908 patients with localized UTUC from March 1999 to January 2015 in Peking University First Hospital. Twenty-three patients were excluded because of accompanying breast cancer, colon cancer or other malignancies (n=16); hepatitis (n=4); or missing clinicopathological data (n=3). In total, 885 patients were included in our study (Figure 1). All patients underwent standard RNU with bladder cuff resection. Routine lymph node dissection was performed when enlarged lymph nodes were found by preoperative image or intraoperative observation. Tumor stage and the histological subtype were assessed according to the 2002 American Joint Committee on Cancer (AJCC) TNM classification. Cellular grade was determined according to the 1973 World Health Organization (WHO) grading system [11]. This study was conducted in accordance with the Declaration of Helsinki, and written informed consent was also obtained from all patients.
Figure 1.
Workflow of this study.
Disease progression was identified on radiological or pathological evidence of local and bladder recurrence, distant metastasis or mortalities from UTUC. Postoperative evaluations were performed in our outpatient or local clinic at 6-month intervals for the first 2 years and yearly thereafter. Cancer-specific survival (CSS) and progression-free survival (PFS) were determined at the last follow-up based on examination results. Overall survival (OS) was determined by review of patient’s medical records and from the Chinese National Statistical Office database.
Clinicopathological and laboratory data were reviewed and collected from an electronic database of the patients’ medical records. As a single-center study, bias is inevitable. To minimize the selection bias, the patient variables were adjusted using 1:1 PSM.
Statistical analysis
All statistical analyses were performed using the SPSS software package (SPSS 22.0, Chicago, IL, USA). All P-values were two-sided, and a P-value <0.05 indicated statistical significance.
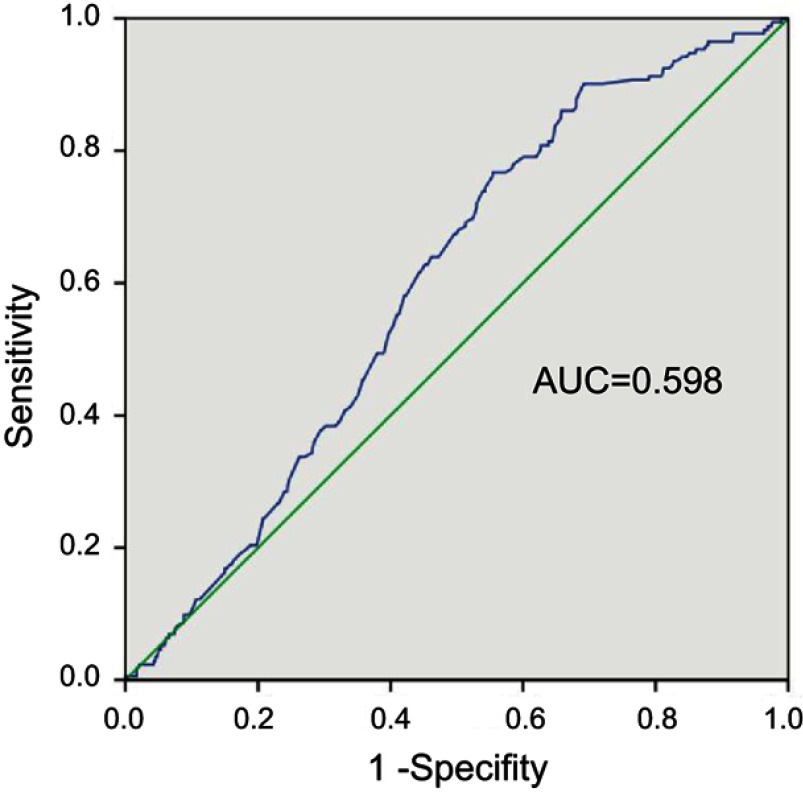
To determine the optimum threshold for the AST/ALT ratio, the receiver-operating curve of AST/ALT for CSS was analyzed. Given that an AST/ALT ratio of 1.23 had a maximum Youden index value, the cut-off value for AST/ALT was set as 1.23. Therefore, in this study, patients with an AST/ALT ratio ≥1.23 were defined as the high AST/ALT group (n=532), while the remaining patients (AST/ALT <1.23) were assigned to the low AST/ALT group (n=353).
Several preoperative characteristics, such as age, gender, body mass index (BMI), tumor size history of coronary disease, alcohol and tobacco consumption, were significantly different between the low and high AST/ALT patients cohort. These characteristics might have important effects on AST/ALT levels. To minimize bias, we performed PSM. The propensity scores were estimated using non-parsimonious multivariate logistic regression according to preoperative characteristics, such as age, gender, BMI, tumor size, and history of coronary disease, alcohol and tobacco consumption. Further, postoperative characteristics, such as pathological stage (T and N) and cellular grade, were also included in PSM because these variables are different between the low and high AST/ALT groups and play important roles for predicting prognosis of UTUC patients. In total, 301 patients with a high AST/ALT ratio were matched to 301 patients with a low AST/ALT ratio in a 1:1 ratio using the nearest-neighbor method with a caliber of 0 (Figure 1). After PSM, the differences of peri-operative characters between the low and high AST/ALT groups were acceptable.
The differences in peri-operative characteristics were determined by independent t-tests for continuous variables (age and BMI exhibit normal distributions in each group) and chi-squared tests for categorical variables. Kaplan–Meier analyses with log-rank tests were used to compare survival outcomes. Univariate and multivariate Cox regression tests were used to reveal the predictors of postoperative survival outcomes.
Results
Patient characteristics
According to the maximum Youden index value, the cutoff value for the AST/ALT ratio was set at 1.23 (Figure 2). Patients with an AST/ALT ratio <1.23 were assigned to the low AST/ALT group, and those with an AST/ALT ratio ≥1.23 were included in the high AST/ALT group. Of the 885 UTUC patients, 532 (60.1%) had a high AST/ALT ratio. The patient characteristics before PSM are summarized in Table 1, and the patient characteristics after PSM are summarized in Table 2. The patients’ median age was 69 years (interquartile range [IQR], 61–75) with a median AST/ALT of 1.36 (IQR, 1.06–1.73) and a median follow-up duration of 61.0 months (IQR, 38–102). Patients in the high AST/ALT group were significantly older (P<0.001); were predominantly female (P<0.001); were more likely to have a history of coronary disease (P=0.060), alcohol consumption (P=0.009) and tobacco use (P=0.014); had a lower BMI (P<0.001); and had a larger tumor volume (P=0.011). Although the difference is not statistically significant, patients in the high AST/ALT group tended to have a positive pathological N stage (7.5% vs 6.2%) and advanced cellular grade (≥G3, 43.4% vs 38.5%) compared with the low AST/ALT group.
Figure 2.
Receiver operating characteristic analysis for cancer-specific survival of the 885 patients. Area under the curve (AUC) of preoperative AST/ALT ratio was 0.598.
Table 1.
Clinicopathological characteristics of the entire cohort and subgroups according to AST/ALT ratio (AST/ALT ratio <1.23 are low group, otherwise high group) before propensity score matching
| Characteristics | Entire cohort | Low AST/ALT | High AST/ALT | P-value |
|---|---|---|---|---|
| Number of Subjects | 885 | 353 | 532 | |
| Preoperative characteristics | ||||
| Age, years | 66.98±10.55 | 64.07±10.93 | 69.04±9.60 | <0.001 |
| Aged >65 years | 575 (65.0%) | 187 (53.0%) | 388 (73.0%) | <0.001 |
| Gender (male) | 396 (44.7%) | 195 (55.2%) | 158 (29.7%) | <0.001 |
| BMI | 24.25±3.53 | 25.06±3.45 | 23.70±3.46 | <0.001 |
| Diabetes | 134 (15.1%) | 60 (17.0%) | 74 (13.9%) | 0.126 |
| Hypertension | 333 (37.6%) | 130 (36.8%) | 203 (38.1) | 0.946 |
| Coronary disease | 99 (11.2%) | 30 (8.5%) | 69 (13.0%) | 0.060 |
| Alcohol | 98 (11.1%) | 51 (14.4%) | 47 (8.8%) | 0.009 |
| Tobacco | 138 (15.6%) | 68 (19.3%) | 70 (13.2%) | 0.014 |
| Hydronephrosis | 427 (48.2%) | 158 (44.8%) | 269 (50.6%) | 0.233 |
| Tumor location (pelvis) | 474 (53.6%) | 187 (53.0%) | 287 (53.9%) | 0.776 |
| Tumor size (>5 cm) | 94 (10.6%) | 25 (7.1%) | 69 (13.0%) | 0.011 |
| Postoperative characteristics | ||||
| Pathological T Stage | 0.832 | |||
| Ta | 6 (0.7%) | 1 (0.3%) | 5 (0.9%) | |
| T1 | 322 (36.4%) | 130 (36.8%) | 192 (36.1%) | |
| T2 | 295 (33.3%) | 116 (32.9%) | 179 (33.6%) | |
| T3 | 238 (26.9%) | 96 (27.2%) | 142 (26.7%) | |
| T4 | 24 (2.7%) | 10 (2.8%) | 14 (2.6%) | |
| Pathological N Stage | 0.081 | |||
| N0 | 823 (93.0%) | 331 (93.8%) | 492 (92.5%) | |
| N1 | 57 (6.4%) | 18 (5.1%) | 39 (7.3%) | |
| N2 | 5 (0.6%) | 4 (1.1%) | 1 (0.2%) | |
| Cellular grade | 0.286 | |||
| G1 | 25 (2.8%) | 12 (3.4%) | 13 (2.4%) | |
| G2 | 493 (55.7%) | 205 (58.1%) | 288 (54.1%) | |
| G3 | 367 (41.5%) | 136 (38.5%) | 231 (43.4%) | |
| Lymphatic microvascular invasion | 46 (5.20%) | 17 (4.8%) | 29 (5.5%) | 0.677 |
| Tumor major diameter | 3.46±2.26 | 3.33±2.29 | 3.55±2.23 | 0.160 |
| Tumor necrosis | 114 (12.9%) | 43 (12.2%) | 71 (13.3%) | 0.613 |
| Tumor hemorrhage | 28 (3.2%) | 9 (2.5%) | 19 (3.6%) | 0.395 |
| Tumor architecture (sessile) | 161 (18.2%) | 59 (16.7%) | 102 (19.2%) | 0.587 |
| Squamous metaplasia | 85 (9.6%) | 29 (8.2%) | 56 (10.5%) | 0.253 |
| Sarcomatoid differentiation | 34 (3.8%) | 12 (3.4%) | 22 (4.1%) | 0.577 |
| Glandular differentiation | 39 (4.4%) | 15 (4.2%) | 24 (4.5%) | 0.716 |
Table 2.
Clinicopathological characteristics for the entire cohort and subgroups according to AST/ALT ratio (AST/ALT ratio <1.23 are low group, otherwise high group) after propensity score matching
| Characteristics | Entire cohort | Low AST/ALT | High AST/ALT | P-value |
|---|---|---|---|---|
| Number of subjects | 602 | 301 | 301 | |
| Preoperative characteristics | ||||
| Age, years | 66.77±9.90 | 65.98±9.79 | 67.56±9.96 | 0.051 |
| Aged >65 years | 385 (64.0%) | 181 (60.1%) | 204 (67.8%) | 0.051 |
| Gender (male) | 285 (47.3%) | 147 (48.8%) | 138 (45.8%) | 0.463 |
| Body mass index | 24.58±3.52 | 24.67±3.34 | 24.49±3.69 | 0.516 |
| Diabetes | 99 (16.4%) | 51 (16.9%) | 48 (15.9%) | 0.742 |
| Hypertension | 224 (37.2%) | 114 (37.9%) | 110 (36.5) | 0.736 |
| Coronary disease | 67 (11.1%) | 29 (9.6%) | 38 (12.6%) | 0.243 |
| Alcohol | 6 5(10.8%) | 33 (11.0%) | 32 (10.6%) | 0.896 |
| Tobacco | 94 (15.6%) | 47 (15.6%) | 47 (15.6%) | 1.000 |
| Hydronephrosis | 296 (49.2%) | 142 (47.2%) | 154 (51.2%) | 0.328 |
| Tumor location (pelvis) | 310 (51.5%) | 151 (50.0%) | 159 (52.8%) | 0.514 |
| Tumor size (>5cm) | 88 (14.6%) | 40 (13.3%) | 48 (15.9%) | 0.356 |
| Postoperative characteristics | ||||
| Pathological T Stage | 0.492 | |||
| Ta | 6 (1.0%) | 1 (0.3%) | 5 (1.7%) | |
| T1 | 234 (38.9%) | 115 (38.2%) | 119 (39.5%) | |
| T2 | 196 (32.6%) | 97 (32.2%) | 99 (32.9%) | |
| T3 | 148 (24.6%) | 78 (25.9%) | 70 (23.3%) | |
| T4 | 18 (3.0%) | 10 (3.3%) | 8 (2.7%) | |
| Pathological N Stage | 0.120 | |||
| N0 | 561 (93.2%) | 280 (93.0%) | 281 (93.4%) | |
| N1 | 37 (6.1%) | 17 (5.6%) | 20 (6.6%) | |
| N2 | 4 (0.7%) | 4 (1.3%) | 0 (0.0%) | |
| Cellular grade | 0.653 | |||
| G1 | 15 (2.5%) | 8 (2.7%) | 7 (2.3%) | |
| G2 | 342 (56.8%) | 176 (58.5%) | 166 (55.1%) | |
| G3 | 245 (40.7%) | 117 (38.9%) | 128 (42.5%) | |
| Lymphatic microvascular invasion | 25 (4.15%) | 11 (44.0%) | 14 (56.0%) | 0.540 |
| Tumor major diameter | 3.39±2.26 | 3.33±2.38 | 3.56±2.33 | 0.542 |
| Tumor necrosis | 75 (12.5%) | 39 (13.0%) | 36 (12.0%) | 0.711 |
| Tumor hemorrhage | 19 (3.2%) | 8 (2.7%) | 11 (3.7%) | 0.484 |
| Tumor architecture (sessile) | 109 (18.1%) | 55 (18.3%) | 54 (17.9%) | 0.916 |
| Squamous metaplasia | 51 (8.5%) | 28 (9.3%) | 23 (7.6%) | 0.464 |
| Sarcomatoid differentiation | 26 (4.3%) | 13 (4.3%) | 13 (4.3%) | 1.000 |
| Glandular differentiation | 28 (4.7%) | 14 (4.7%) | 14 (4.7%) | 1.000 |
Survival outcomes
Before PSM
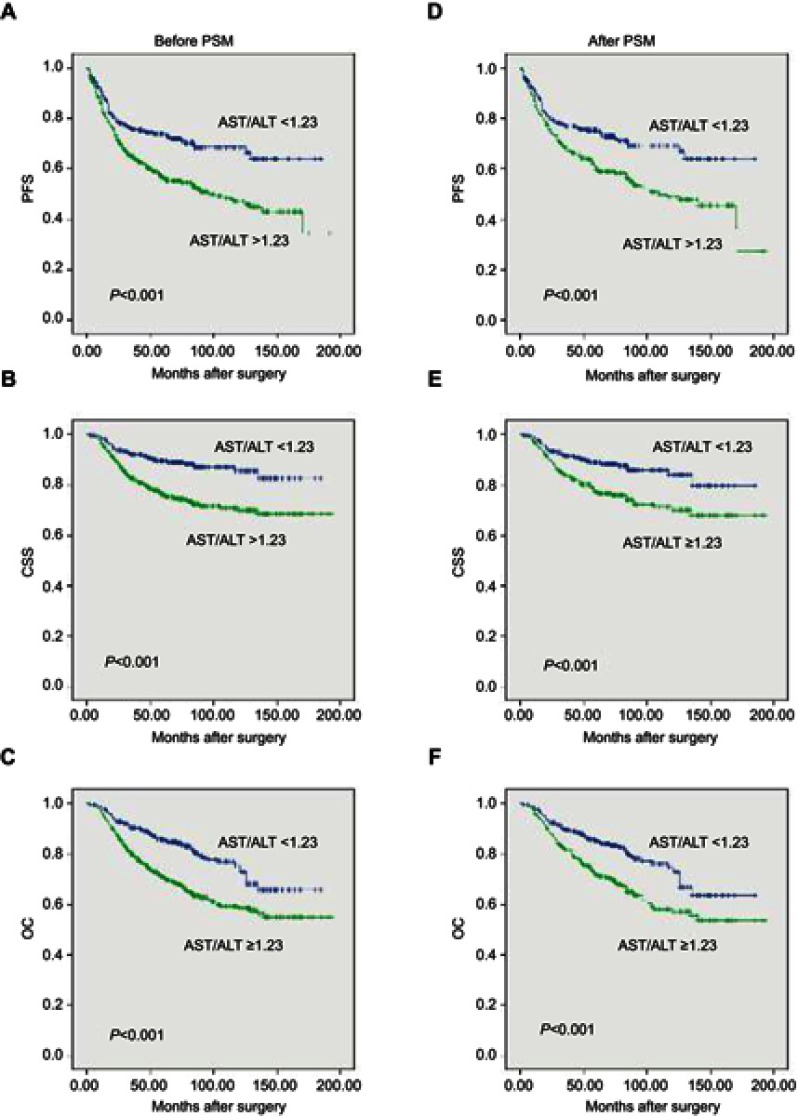
After a median (IQR) of 18 (9–32) months postoperatively, 332 (37.5%) patients experienced disease progression. There were 172 (19.4%) cancer-specific deaths after a median (IQR) of 26 (16.25–45.75) months and 248 (28.0%) overall deaths after a median (IQR) of 32.5 (19.0–57.75) months postoperatively. The Kaplan–Meier curves showed significantly poorer survival outcomes in PFS (P<0.001), CSS (P<0.001) and OS (P<0.001) for patients with high AST/ALT (Figure 3AC). A higher AST/ALT ratio was found to be an independent predictor for PFS (hazard ratio [HR] 1.454, 95% confidence interval [CI] 1.157–1.828, P=0.001), CSS (HR 2.577, 95% CI 1.7145–3.875, P<0.001) and OS (HR 1.925, 95% CI 1.454–2.548, p<0.001) based on multivariate Cox regression analyses (Table 3) when adjusted by clinicopathological parameters, as shown in Table 3. For PFS, patient gender (P=0.009), BMI (P=0.048) and hydronephrosis (P=0.023) were significant copredictors. Moreover, patient gender, pathologic T and N stage, tumor size and tumor hemorrhage were revealed as significant copredictors of postoperative CSS and OS (all P<0.05). Patient age (P=0.003) and tumor glandular differentiation (P=0.003) significantly predicted OS but not CSS (both P>0.05) (Table 3).
Figure 3.
PFS (A), CSS (B) and OS (C) of the 885 patients with clinically localized upper tract urothelial carcinoma according to preoperative AST/ALT ratio before PSM. PFS (D), CSS (E) and OS (F) of the 602 patients with clinically localized upper tract urothelial carcinoma according to preoperative AST/ALT ratio after PSM.
Abbreviations: CSS, cancer-specific survival; OS, overall survival; PFS, progression-free survival; PSM, propensity score matching.
Table 3.
Results of univariate and multivariate cox regression analyses for CSS, OS and PFS before propensity score matching
| Characteristics | CSS | OS | PFS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||
| P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | |
| Aged > 65 years | 0.069 | 1.337 (0.978–1.829) | 0.063 | 1.417 (0.982–2.047) | 0.001 | 1.583 (1.206–2.079) | 0.001 | 1.677 (1.222–2.302) | 0.142 | 1.185 (0.945–1.485) | ||
| Gender (male) | <0.001 | 1.763 (1.320–2.354) | <0.001 | 1.884 (1.355–2.621) | <0.001 | 1.539 (1.208–1.960) | 0.003 | 1.516 (1.151–1.997) | 0.015 | 1.297 (1.051–1.600) | 0.009 | 1.340 (1.076–1.670) |
| BMI | 0.007 | 0.943 (0.904–0.984) | 0.073 | 0.957 (0.913–1.004) | 0.023 | 0.960 (0.926–0.994) | 0.078 | 0.965 (0.928–1.004) | 0.037 | 0.968 (0.938–0.998) | 0.048 | 0.968 (0.938–1.000) |
| Diabetes | 0.937 | 1.016 (0.690–1.496) | 0.814 | 1.040 (0.751–1.439) | 0.604 | 1.077 (0.813–1.429) | ||||||
| Hypertension | 0.152 | 1.239 (0.924–1.662) | 0.012 | 1.369 (1.070–1.750) | 0.860 | 1.020 (0.819–1.270) | ||||||
| Coronary disease | 0.164 | 0.702 (0.426–1.156) | 0.989 | 1.003 (0.696–1.445) | 0.937 | 1.013 (0.730–1.406) | ||||||
| Alcohol | 0.852 | 0.957 (0.602–1.521) | 0.649 | 1.090 (0.753–1.577) | 0.248 | 0.810 (0.566–1.159 ) | ||||||
| Tobacco | 0.189 | 1.273 (0.888–1.824) | 0.054 | 1.339 (0.994–1.802) | 0.823 | 0.968 (0.726–1.291) | ||||||
| Hydronephrosis | 0.139 | 1.252 (0.930–1.687) | 0.048 | 1.394 (1.004–1.937) | 0.178 | 1.186 (0.925–1.521) | 0.003 | 1.611 (1.170–2.218) | 0.040 | 1.261 (1.011–1.572) | 0.023 | 1.296 (1.036–1.620) |
| AST/ALT ≥1.23 | <0.001 | 2.339 (1.642–3.331) | <0.001 | 2.599 (1.725–3.913) | <0.001 | 1.925 (1.454–2.548) | <0.001 | 1.842 (1.337–2.540) | <0.001 | 1.726 (1.360–2.190) | 0.001 | 1.454 (1.157–1.828) |
| Tumor location (pelvis) | 0.809 | 0.986 (0.878–1.106) | 0.056 | 1.271 (0.994–1.626) | 0.012 | 1.509 (1.095–2.081) | 0.247 | 0.883 (0.715–1.090) | ||||
| T Stage (≥3) | <0.001 | 3.118 (2.339–4.156) | <0.001 | 2.482 (1.763–3.494) | <0.001 | 2.391 (1.876–3.049) | <0.001 | 2.098 (1.571–2.803) | 0.002 | 1.418 (1.139–1.765) | 0.029 | 1.319 (1.029–1.691) |
| Tumor size (>5cm) | <0.001 | 2.968 (2.114–4.168) | <0.001 | 2.015 (1.391–2.917) | <0.001 | 2.595 (1.911–3.523) | <0.001 | 1.916 (1.374–2.670) | 0.002 | 1.602 (1.195–2.416) | 0.030 | 1.411 (1.034–1.924) |
| N Stage (≥1) | <0.001 | 3.217 (2.176–4.758) | 0.001 | 2.084 (1.345–3.229) | <0.001 | 2.606 (1.817–3.739) | 0.002 | 1.873 (1.255–2.794) | 0.067 | 1.404 (0.976–2.020) | ||
| Cellular grade (≥3) | <0.001 | 1.993 (1.492–2.663) | <0.001 | 1.768 (1.388–2.252) | 0.072 | 1.213 (0.983–1.497) | ||||||
| Lymphatic microvascular invasion | 0.039 | 1.781 (1.031–3.077) | 0.297 | 1.321 (0.783–2.226) | 0.218 | 1.312 (0.851–2.022) | ||||||
| Tumor necrosis | 0.055 | 1.467 (0.992–2.170) | 0.093 | 1.343 (0.952–1.894) | 0.339 | 1.162 (0.854–1.581) | ||||||
| Tumor hemorrhage | 0.016 | 2.049 (1.141–3.679) | 0.010 | 2.273 (1.219–4.239) | 0.017 | 1.849 (1.115–3.067) | 0.004 | 2.197 (1.286–3.754) | 0.340 | 1.286 (0.766–2.158) | ||
| Tumor architecture (sessile) | 0.001 | 1.755 (1.271–2.423) | <0.001 | 1.703 (1.286–2.254) | 0.110 | 1.234 (0.953–1.597) | ||||||
| Squamous metaplasia | 0.022 | 1.652 (1.075–2.539) | 0.016 | 1.590 (1.091–2.316) | 0.793 | 1.049 (0.736–1.495) | ||||||
| Sarcomatoid differentiation | 0.008 | 2.219 (1.236–3.984) | 0.004 | 2.121 (1.279–3.518) | 0.715 | 1.105 (0.647–1.886) | ||||||
| Glandular differentiation | 0.064 | 1.221 (0.988–1.508) | 0.011 | 2.317 (1.210–4.439) | 0.036 | 1.228 (1.013–1.489) | 0.003 | 2.388 (1.355–4.208) | 0.749 | 1.042 (0.809–1.343) | ||
| Laparoscopy | 0.032 | 0.678 (0.476–0.967) | 0.143 | 0.802 (0.598–1.077) | 0.361 | 0.890 (0.693–1.143) | ||||||
Abbreviations: BMI, body mass index; CSS, cancer-specific survival; OS, overall survival; PFS, progression-free survival.
After PSM
At a median (IQR) of 17 (8–31) months postoperatively, 217 patients had disease progression. There were 112 cancer-specific deaths after a median (IQR) of 26 (16–48) months and 159 deaths from all causes after a median (IQR) of 32 (19–63) months postoperatively. The high AST/ALT group also had significantly inferior survival outcomes in terms of PFS, OS and CSS in the propensity score-matched cohort (all P<0.001, Figure 3D–F), as demonstrated by Kaplan–Meier curves. Multivariate Cox regression analyses demonstrated that a high AST/ALT ratio was an independent predicator of PFS (HR 1.711, 95% CI 1.299–2.254), CSS (HR 2.588, 95% CI 1.727–3.877) and OS (HR 1.957, 95% CI 1.409–2.717) (all P<0.001). Moreover, pathological T and N stages were significant copredictors of PFS, CSS and OS (all P<0.05). Patient gender was a significant copredictor of PFS (P=0.037). Patient gender and tumor size were significant copredictors of CSS (both P<0.05). Patient age, tumor location, size and glandular differentiation were significant copredictors of OS (all P<0.05) (Table 4).
Table 4.
Results of univariate and multivariate analyses for CSS, OS and PFS after propensity score matching
| Characteristics | CSS | OS | PFS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||
| P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | |
| Aged >65 years | 0.501 | 1.144 (0.773–1.692) | 0.041 | 1.427 (1.015–2.006) | 0.016 | 1.615 (1.091–2.389) | 0.615 | 0.932 (0.708–1.227) | ||||
| Gender (male) | 0.001 | 1.950 (1.332–2.885) | 0.002 | 1.992 (1.300–3.053) | 0.014 | 1.482 (1.084–2.026) | 0.063 | 1.396 (0.982–1.985) | 0.015 | 1.394 (1.067–1.821) | 0.004 | 1.560 (1.149–2.119) |
| BMI | 0.033 | 0.942 (0.891–0.995) | 0.086 | 0.961 (0.919–1.006) | 0.145 | 0.972 (0.936–1.010) | ||||||
| Diabetes | 0.561 | 0.858 (0.512–1.438) | 0.624 | 0.899 (0.586–1.378) | 0.992 | 0.998 (0.701–1.422) | ||||||
| Hypertension | 0.871 | 1.032 (0.706–1.508) | 0.146 | 1.262 (0.922–1.726) | 0.74 | 0.955 (0.725–1.257) | ||||||
| Coronary disease | 0.975 | 0.991 (0.556–1.767) | 0.560 | 1.147 (0.724–1.815) | 0.829 | 1.046 (0.694–1.576) | ||||||
| Alcohol | 0.780 | 1.086 (0.609–1.937) | 0.640 | 1.121 (0.694–1.811) | 0.406 | 0.826 (0.526–1.296) | 0.070 | 0.624 (0.374–1.040) | ||||
| Tobacco | 0.346 | 1.252 (0.785–1.996) | 0.447 | 1.167 (0.784–1.737) | 0.585 | 0.903 (0.626–1.303) | ||||||
| Hydronephrosis | 0.353 | 1.194 (0.822–1.734) | 0.412 | 1.140 (0.834–1.559) | 0.020 | 1.600 (1.077–2.377) | 0.195 | 1.193 (0.913–1.559) | ||||
| AST/ALT ≥1.23 | <0.001 | 2.313 (1.551–3.450) | <0.001 | 2.977 (1.904–4.653) | <0.001 | 1.942 (1.402–2.689) | <0.001 | 2.049 (1.426–2.945) | <0.001 | 1.684 (1.28–2.216) | <0.001 | 1.834 (1.348–2.496) |
| Tumor location (pelvis) |
0.088 | 1.389 (0.953–2.024) | 0.004 | 1.600 (1.161–2.205) | 0.003 | 1.847 (1.238–2.755) | 0.862 | 0.977 (0.748–1.276) | ||||
| T Stage (≥3) | <0.001 | 3.393 (2.341–4.919) | 0.002 | 2.006 (1.303–3.089) | <0.001 | 2.558 (1.869–3.501) | 0.006 | 1.728 (1.168–2.558) | 0.015 | 1.421 (1.072–1.884) | ||
| Tumor size (>5 cm) | <0.001 | 3.228 (2.141–4.867) | 0.001 | 2.141 (1.353–3.387) | <0.001 | 2.675 (1.849–3.871) | <0.001 | 2.592 (1.669–4.025) | 0.003 | 1.682 (1.199–2.360) | 0.009 | 1.655 (1.133–2.418) |
| N Stage (≥1) | <0.001 | 3.817 (2.352–6.193) | <0.001 | 2.785 (1.631–4.757) | <0.001 | 2.753 (1.739–4.359) | 0.012 | 1.944 (1.159–3.261) | 0.098 | 1.475 (0.931–2.337) | ||
| Cellular grade (≥3) | <0.001 | 2.376 (1.628–3.468) | <0.001 | 1.949 (1.426–2.665) | 0.068 | 1.440 (0.973–2.130) | 0.121 | 1.236 (0.946–1.616) | ||||
| Lymphatic microvascular invasion | 0.077 | 1.916 (0.933–3.936) | 0.403 | 1.355 (0.665–2.761) | 0.511 | 1.226 (0.668–2.250) | ||||||
| Tumor necrosis | 0.156 | 1.440 (0.870–2.386) | 0.150 | 1.376 (0.891–2.125) | 0.075 | 0.591 (0.332–1.053) | 0.352 | 1.204 (0.814–1.782) | ||||
| Tumor hemorrhage | 0.090 | 1.939 (0.902–4.169) | 0.023 | 2.491 (1.137–5.459) | 0.057 | 1.862 (0.981–3.534) | 0.004 | 2.920 (1.417–6.017) | 0.444 | 1.298 (0.666–2.530) | ||
| Tumor architecture (sessile) | <0.001 | 2.268 (1.524–3.375) | <0.001 | 1.935 (1.369–2.735) | 0.112 | 1.298 (0.941–1.790) | ||||||
| Squamous metaplasia | 0.012 | 2.008 (1.164–3.466) | 0.011 | 1.873 (1.157–3.030) | 0.497 | 1.173 (0.740–1.860) | ||||||
| Sarcomatoid differentiation | 0.001 | 2.954 (1.541–5.661) | <0.001 | 2.984 (1.722–5.170) | 0.332 | 1.351 (0.736–2.478) | ||||||
| Glandular differentiation | 0.004 | 2.759 (1.393–5.466) | 0.031 | 2.357 (1.081–5.141) | 0.001 | 2.852 (1.579–5.153) | 0.006 | 2.660 (1.322–5.354) | 0.084 | 1.673 (0.933–3.000) | ||
| Laparoscopy | 0.054 | 0.628 (0.391–1.008) | 0.405 | 0.852 (0.584–1.243) | 0.359 | 0.858 (0.619–1.190) | ||||||
Abbreviations: CSS, cancer-specific survival; OS, overall survival; PFS, progression-free survival; PSM, propensity score matching.
Discussion
The present study showed that UTUC patients with higher AST/ALT ratio were more likely to have a worse prognosis of PFS, CSS and OS based on a large Chinese cohort. A high AST/ALT ratio was independently associated with postoperative PFS, CSS and OS analyzed by multivariate Cox regression. PSM was performed to minimize bias balancing of several perioperative characteristics, such as patient age, gender, BMI, tumor size, history of coronary disease, alcohol and tobacco consumption, pathological stage (T and N) and cellular grade. In a matched cohort of 602 UTUC patients, the aforementioned findings persisted for all three survival endpoints of PFS, CSS and OS. These results suggest that a high AST/ALT ratio is a robust independent predictor for UTUC patients.
Aspartate transaminase (AST) and alanine transaminase (ALT) were both first described by Arthur Karmen and colleagues in 1954.17,18 AST was expressed in liver, heart, skeletal muscle, kidneys, brain and red blood cells. However, ALT was mostly expressed in the liver. Therefore, serum ALT and AST are both biomarkers for liver health, and serum AST in blood tests may also reflect pathology in the heart and muscle.19 In clinic care, serum AST and ALT are well-known biomarkers for identifying liver diseases, such as viral hepatitis and alcohol abuse. AST/ALT ratio or De Ritis ratio was downregulated significantly in viral hepatitis and first introduced by De Ritis and colleagues.20 Moreover, recent studies have shown that the AST/ALT ratio is a significant prognostic predictor for urological cancer patients. Bezan A et al, analyzed 698 patients with nonmetastatic renal cell carcinoma and found that preoperative AST/ALT represents a poor prognostic factor.21 Additionally, a propensity score-matched study in 2,965 patients surgically treated for nonmetastatic RCC suggested that the AST/ALT ratio is an independent predictor of PFS, CSS and OS.13 Recently, Wang H et al, found that a higher AST/ALT ratio could be predictive of worse pathological outcomes and higher biochemical recurrence in localized prostate cancer patients.22 Moreover, high AST/ALT ratios have also been identified as a significant prognostic biomarker in patients with metastatic castration-resistant prostate cancer treated with cabazitaxel.23
The prognostic effects of AST/ALT ratio in UTUC were first introduced by Nishikawa M et al,15 and confirmed by Lee et al,16 Nishikawa M et al, retrospectively analyzed 109 consecutive patients with clinically localized UTUC and demonstrated that the AST/ALT ratio was significantly correlated with several unfavorable parameters, including elderly age, high pathological stage, high cellular grade and lymphovascular invasion. Multivariate analysis found that a high AST/ALT ratio was an independent predicator of extravesical recurrence-free survival. Lee et al, also studied 623 patients with localized UTUC and reported that elevation of AST/ALT ratio was correlated with adverse pathologic events and a significant predictor of worse postoperative survival in patients surgically treated for UTUC. These findings were in accordance with our results. Although these studies were limited by the small number of patients and selection bias, they were valuable for introducing the novel prognostic impact of AST/ALT for the first time. To the best of our knowledge, there are no other studies focusing on the prognostic impact of AST/ALT in patients with UTUC.
From our present study, the AST/ALT ratio might be a useful preoperative biomarker for risk stratification of UTUC patients. Before surgery, the AST/ALT ratio could contribute to the classification of low-risk and high-risk patients. The optimal treatment option was suggested based on risk stratification of UTUC patients. Several studies reported that neoadjuvant chemotherapy and adjuvant chemotherapy improves the survival of patients with high-risk UTUC.24–26 Recent retrospective studies reported contrary results about whether adjuvant chemotherapy benefits UTUC patient survival; however, conclusions should be discussed in large well-organized prospective studies.16,27,28 Therefore, it is required to identify enhanced patient selection criteria. AST/ALT ratio as a perioperative prognostic predictor for UTUC may help to select candidates with high-risk UTUC for neoadjuvant chemotherapy or adjuvant chemotherapy.
The molecular mechanism that mediates high serum AST/ALT levels remains unclear. However, researchers have found that most cancer cells rely on anaerobic glycolysis to generate the energy needed for cellular survival, growth and metastasis even in the presence of oxygen via a process known as the “Warburg effect”.29–31 Increased anaerobic glycolysis in tumor cells is associated with alterations of nicotinamide adenine dinucleotide (NAD)-related enzymes and glucose transporters in mitochondria.32 High lactate dehydrogenase (LDH) and cytosolic NADH/NAD+ are essential for maintaining this enhanced glycolysis.33 Notably, AST is an important component of a malate-aspartate shuttle pathway that allows NADH/NAD+ conversion.34 Additionally, Oxamate, an LDH inhibitor, could inhibit the proliferation of breast adenocarcinoma cells and SDH-deficient cells in vitro,35,36 and AST overexpression could rescue cell growth in the presence of oxamate.35 Studies have demonstrated that AST and ALT are involved in glutamine metabolism, which is necessary for tumor cells to maintain the biosynthesis of nucleotide and nonessential amino acids.37–39 Taken together, AST and ALT, reflecting tumor metabolism, are possible biomarkers for patient’s prognosis.
Limitations
Although important discoveries were revealed by this study in the largest Chinese center of UTUC patients, there were also some limitations. First, it should be noted that this study is a retrospective single-center study. Thus, we matched the patient variables between the high and low AST/ALT groups using propensity scoring, resulting in groups that were as well adjusted for the analyses as possible. However, research should be performed on a large scale using multicenter prospective validation of UTUC groups. Second, the AST/ALT ratio might have been biased by the presence of all the undetected diseases or conditions (Li et al, medicine or dermatomyositis) that affect serum AST or ALT levels. Third, our study exclusively consisted of Chinese patients; therefore, care must be taken when applying our results in other racial groups. Finally, the mechanism by which AST/ALT affects the survival of patients should be further explored through basic studies of the metabolism of UTUC cells. Despite these limitations, we report that the preoperative serum AST/ALT ratio was correlated with RFS, CSS and OS in patients with UTUC.
Conclusion
This study showed that a high preoperative serum AST/ALT ratio, which reflects tumor metabolism, is an independent risk factor affecting RFS, CSS and OS of patients before and after PSM, thus balancing the difference in important variables that affect prognosis. AST and ALT levels can be easily evaluated and monitored by routine blood tests. This novel biomarker can help predict prognosis and may help select candidates for neoadjuvant chemotherapy or adjuvant chemotherapy for UTUC patients.
Data sharing statement
Supporting data are available from the first author Yifan Li. E-mail: 1165085399@qq.com.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (81772703), the Beijing Natural Science Foundation (L182004, 7152146, 7172219), the Clinical Features Research of Capital (No. Z151100004015173), the Capital Health Research and Development of Special (2016-1-4077), the Fund for Fostering Young Scholars of Peking University Health Science Center (BMU2017PY009), and the Collaborative Research Foundation of Peking University Health Science Center and National Taiwan University, the College of Medicine (BMU20120318). Structured data processsing occurred partially using Medbanks' approach (Medbanks Network Technology Co. Ltd., Beijing, China).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Roupret M, Babjuk M, Comperat E, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73(1):111-122.doi: 10.1016/j.eururo.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Cancer statistics 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3.Baard J, de Bruin DM, Zondervan PJ, Kamphuis G, de la Rosette J, Laguna MP. Diagnostic dilemmas in patients with upper tract urothelial carcinoma. Nat Rev Urol. 2017;14(3):181–191. doi: 10.1038/nrurol.2016.252 [DOI] [PubMed] [Google Scholar]
- 4.Kaag MG, O’Malley RL, O’Malley P, et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol. 2010;58(4):581–587. doi: 10.1016/j.eururo.2010.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silberstein JL, Power NE, Savage C, et al. Renal function and oncologic outcomes of parenchymal sparing ureteral resection versus radical nephroureterectomy for upper tract urothelial carcinoma. J Urol. 2012;187(2):429–434. doi: 10.1016/j.juro.2011.09.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khene ZE, Mathieu R, Kammerer-Jacquet SF, et al. Risk stratification for kidney sparing procedure in upper tract urothelial carcinoma. Transl Androl Urol. 2016;5(5):711–719. doi: 10.21037/tau.2016.09.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalpiaz O, Ehrlich GC, Mannweiler S, et al. Validation of pretreatment neutrophil-lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. BJU Int. 2014;114(3):334–339. doi: 10.1111/bju.12441 [DOI] [PubMed] [Google Scholar]
- 8.Dalpiaz O, Pichler M, Mannweiler S, et al. Validation of the pretreatment derived neutrophil–lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. Br J Cancer. 2014;110(10):2531–2536. doi: 10.1038/bjc.2014.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka N, Kikuchi E, Kanao K, et al. A multi-institutional validation of the prognostic value of the neutrophil-to-lymphocyte ratio for upper tract urothelial carcinoma treated with radical nephroureterectomy. Ann Surg Oncol. 2014;21(12):4041–4048. doi: 10.1245/s10434-014-3830-3 [DOI] [PubMed] [Google Scholar]
- 10.Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117–130. [PMC free article] [PubMed] [Google Scholar]
- 11.Kimm H, Kim S, Jee SH. The independent effects of cigarette smoking, alcohol consumption, and serum aspartate aminotransferase on the alanine aminotransferase ratio in Korean men for the risk for esophageal cancer. Yonsei Med J. 2010;51(3):310–317. doi: 10.3349/ymj.2010.51.3.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishihara H, Kondo T, Yoshida K, et al. Evaluation of preoperative aspartate transaminase/alanine transaminase ratio as an independent predictive biomarker in patients with metastatic renal cell carcinoma undergoing cytoreductive nephrectomy: a propensity score matching study. Clin Genitourin Cancer. 2017;15(5):598–604. doi: 10.1016/j.clgc.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Lee SE, Byun SS, Kim HH, Kwak C, Hong SK. De Ritis ratio (aspartate transaminase/alanine transaminase ratio) as a significant prognostic factor after surgical treatment in patients with clear-cell localized renal cell carcinoma: a propensity score-matched study. BJU Int. 2017;119(2):261–267. doi: 10.1111/bju.13545 [DOI] [PubMed] [Google Scholar]
- 14.Takenaka Y, Takemoto N, Yasui T, et al. Transaminase activity predicts survival in patients with head and neck cancer. PLoS One. 2016;11(10):e0164057. doi: 10.1371/journal.pone.0164057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishikawa M, Miyake H, Fujisawa M. De Ritis (aspartate transaminase/alanine transaminase) ratio as a significant predictor of recurrence-free survival in patients with upper urinary tract urothelial carcinoma following nephroureterectomy. Urol Oncol. 2016;34(9):417.e9–417.e15. doi: 10.1016/j.urolonc.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Choi YH, Sung HH, et al. De ritis ratio (AST/ALT) as a significant prognostic factor in patients with upper tract urothelial cancer treated with surgery. Clin Genitourin Cancer. 2017;15(3). e379-e385. doi: 10.1016/j.clgc.2016.08.023 [DOI] [PubMed] [Google Scholar]
- 17.Karmen A. A note on the spectrometric assay of glutamic-oxalacetic transaminase in human blood serum. J Clin Invest. 1955;34(1):131–133. [PubMed] [Google Scholar]
- 18.Karmen A, Wroblewski F, Ladue JS. Transaminase activity in human blood. J Clin Invest. 1955;34(1):126–131. doi: 10.1172/JCI103055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladue JS, Wroblewski F, Karmen A. Serum glutamic oxaloacetic transaminase activity in human acute transmural myocardial infarction. Science (New York, NY). 1954;120(3117):497–499. doi: 10.1126/science.120.3117.497 [DOI] [PubMed] [Google Scholar]
- 20.De Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin Chim Acta. 1957;2(1):70–74. doi: 10.1016/0009-8981(57)90027-X [DOI] [PubMed] [Google Scholar]
- 21.Bezan A, Mrsic E, Krieger D, et al. The preoperative AST/ALT (De Ritis) ratio represents a poor prognostic factor in a cohort of patients with nonmetastatic renal cell carcinoma. J Urol. 2015;194(1):30–35. doi: 10.1016/j.juro.2015.01.083 [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Fang K, Zhang J, et al. The significance of De Ritis (aspartate transaminase/alanine transaminase) ratio in predicting pathological outcomes and prognosis in localized prostate cancer patients. Int Urol Nephrol. 2017;49(8):1391–1398. doi: 10.1007/s11255-017-1618-7 [DOI] [PubMed] [Google Scholar]
- 23.Miyake H, Matsushita Y, Watanabe H, et al. Significance of De Ritis (Aspartate Transaminase/Alanine Transaminase) ratio as a significant prognostic but not predictive biomarker in Japanese patients with metastatic castration-resistant prostate cancer treated with cabazitaxel. Anticancer Res. 2018;38(7):4179–4185. doi: 10.21873/anticanres.12711 [DOI] [PubMed] [Google Scholar]
- 24.Porten S, Siefker-Radtke AO, Xiao L, et al. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer. 2014;120(12):1794–1799. doi: 10.1002/cncr.28655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leow JJ, Martin-Doyle W, Fay AP, Choueiri TK, Chang SL, Bellmunt J. A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol. 2014;66(3):529–541. doi: 10.1016/j.eururo.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 26.Fujita K, Taneishi K, Inamoto T, et al. Adjuvant chemotherapy improves survival of patients with high-risk upper urinary tract urothelial carcinoma: a propensity score-matched analysis. BMC Urol. 2017;17(1):110. doi: 10.1186/s12894-017-0305-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Necchi A, Lo Vullo S, Mariani L, et al. Adjuvant chemotherapy after radical nephroureterectomy does not improve survival in patients with upper tract urothelial carcinoma: a joint study by the European Association of Urology-Young Academic Urologists and the Upper Tract Urothelial Carcinoma Collaboration. BJU Int. 2018;121(2):252–259. doi: 10.1111/bju.14020 [DOI] [PubMed] [Google Scholar]
- 28.Seisen T, Krasnow RE, Bellmunt J, et al. Effectiveness of adjuvant chemotherapy after radical nephroureterectomy for locally advanced and/or positive regional lymph node upper tract urothelial carcinoma. J Clin Oncol. 2017;35(8):852–860. doi: 10.1200/JCO.2016.69.4141 [DOI] [PubMed] [Google Scholar]
- 29.Justus CR, Sanderlin EJ, Yang LV. Molecular connections between cancer cell metabolism and the tumor microenvironment. Int J Mol Sci. 2015;16(5):11055–11086. doi: 10.3390/ijms160511055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY). 2009;324(5930):1029–1033. doi: 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Kidney and Renal Pelvis Cancer. Bethesda, MD: National Cancer Institute; 2018. Available from: http://seer.cancer.gov/statfacts/html/kidrp.html. [Google Scholar]
- 32.Dorward A, Sweet S, Moorehead R, Singh G. Mitochondrial contributions to cancer cell physiology: redox balance, cell cycle, and drug resistance. J Bioenerg Biomembr. 1997;29(4):385–392. [DOI] [PubMed] [Google Scholar]
- 33.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9(6):425–434. doi: 10.1016/j.ccr.2006.04.023 [DOI] [PubMed] [Google Scholar]
- 34.Greenhouse WV, Lehninger AL. Occurrence of the malate-aspartate shuttle in various tumor types. Cancer Res. 1976;36(4):1392–1396. [PubMed] [Google Scholar]
- 35.Thornburg JM, Nelson KK, Clem BF, et al. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008;10(5):R84. doi: 10.1186/bcr2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardaci S, Zheng L, MacKay G, et al. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat Cell Biol. 2015;17(10):1317–1326. doi: 10.1038/ncb3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wise DR, DeBerardinis RJ, Mancuso A, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105(48):18782–18787. doi: 10.1073/pnas.0810199105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85. doi: 10.1038/nrc2981 [DOI] [PubMed] [Google Scholar]
- 39.Ellinger JJ, Lewis IA, Markley JL. Role of aminotransferases in glutamate metabolism of human erythrocytes. J Biomol NMR. 2011;49(3–4):221–229. doi: 10.1007/s10858-011-9481-9 [DOI] [PMC free article] [PubMed] [Google Scholar]