Abstract
Cardiovascular disease is the leading cause of death worldwide, and elevated lipid levels is a major contributor. Gene delivery, which involves controlled transfer of nucleic acids into cells and tissues, has been widely used in research to study lipid metabolism and physiology. Several technologies have been developed to somatically overexpress, silence, or disrupt genes in animal models and have greatly advanced our knowledge of metabolism. This is particularly true with regard to the liver, which plays a central role in lipoprotein metabolism and is amenable to many delivery approaches. In addition to basic science applications, many of these delivery technologies have potential as gene therapies for both common and rare lipid disorders. This review discusses three major gene delivery technologies used in lipid research—including adeno-associated viral vector overexpression, antisense oligonucleotides and small interfering RNAs, and the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 genome editing system—and examines their potential therapeutic applications.
Keywords: gene therapy, lipoprotein metabolism, adeno-associated viral vector, antisense oligonucleotides, small interfering RNAs, Clustered Regularly Interspaced Short Palindromic Repeats, CRISPR/Cas9, genome editing
ADENO-ASSOCIATED VIRAL VECTOR OVEREXPRESSION
Viral overexpression is a common approach for investigating candidate genes in lipid and atherosclerosis research.1,2 This is also the underlying principle of additive gene therapy, which seeks to restore activity of a dysfunctional gene by adding an artificial transgene (Figure 1). Adeno-associated viruses (AAVs) are small, nonenveloped, nonintegrating, single-stranded DNA viruses that can package up to 4.9 kb of exogenous DNA.3 Upon delivery, recombinant AAV genomes are converted to double-stranded intermediate and circularize to form episomes, which are maintained extrachromosomally in the nucleus. Relative to adenoviral vectors, AAVs elicit a far milder immune response, allowing for stable transgene expression for months to years.4 Recombinant AAVs can be cross-packaged with capsids from many naturally occurring serotypes or engineered variants, allowing the researcher to restrict or broaden tissue distribution.3 Most AAV capsids have a high liver tropism, making them ideal for delivery to this organ. Thus far, AAV vectors have been used safely in humans in more than 120 clinical gene therapy trials. The first AAV-based gene therapy products were approved for use in humans in 2012 (Europe) and another in 2017 (United States).5,6
Figure 1.
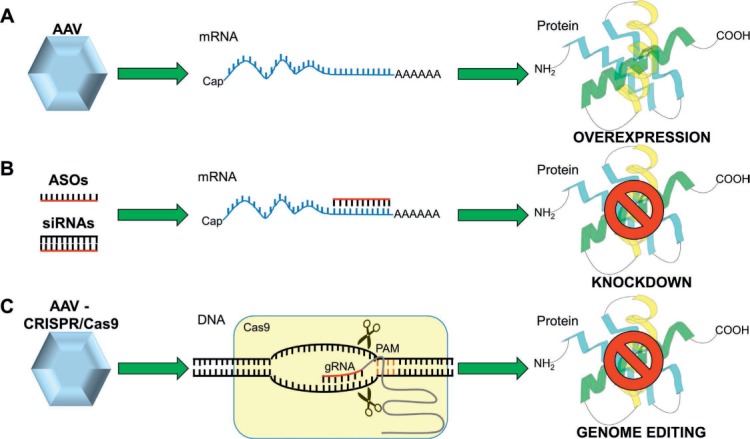
Gene delivery technologies in lipid research and therapies. (A) AAV vectors are commonly used for overexpressing proteins. (B) ASOs and siRNAs are synthetic nucleic acids engineered to hybridize with a target mRNA or pre-mRNA to induce its degradation and silencing. (C) AAV vectors are also used for delivering the CRISPR/Cas9 genome editing system. A gRNA guides the Cas9 nuclease to a complementary genomic site in proximity to a Protospacer Adjacent Motif (PAM). Cas9 induces a DSB causing indel mutations, which can be used to permanently inactivate or knock out the target gene. AAV: adeno-associated virus; ASO: antisense oligonucleotides; siRNAs: small interfering RNAs; CRISPR/Cas9: Clustered Regularly Interspaced Short Palindromic Repeats; gRNA: guide RNA
AAV vectors based on serotype 8 (AAV8) primarily target the liver in mice and have become a mainstay of lipid research. They have been used to overexpress numerous proteins in lipid metabolism, including apolipoproteins, lipases, lipid transfer proteins, lipoprotein receptors, signaling proteins, and several enzymes in lipid synthesis. Delivery of Cre recombinase with AAV8 is rapidly gaining popularity and is viewed by many as superior to albumincre for generating liver-specific knockouts.7 A noteworthy example of the power of liver-directed overexpression involves the use of AAVPCSK9 to induce atherosclerosis. Proprotein convertase subtilisin kexin 9 (PCSK9) is secreted by the liver and promotes degradation of the low-density lipoprotein receptor (LDLR) by preventing recycling to the cell surface.8 Several groups have demonstrated that AAV-based overexpression of PCSK9 gain-of-function variants can be used to model atherosclerosis in mice.1,9 This approach can greatly simplify atherosclerosis studies by avoiding the need to cross mice to Ldlr or Apoe knockout backgrounds.
AAV vectors are also the leading technology in tissue-directed gene therapy for lipid disorders. Lipoprotein lipase is a key enzyme in the catabolism of chylomicrons that hydrolyzes triglycerides (TG). Lipoprotein lipase (LPL) deficiency is a rare inherited disease characterized by the accumulation of chylomicrons in plasma, severe hypertriglyceridemia, and episodes of life-threatening pancreatitis. Glybera (uniQure N.V.) is an AAV1 vector designed for direct intramuscular delivery of the human gain-of-function LPL gene variant S447X.10 Clinical trials demonstrated that Glybera was associated with a lower incidence of pancreatitis in patients with LPL deficiency.5,11 This was the first gene replacement therapy to receive regulatory approval in Europe in 2012. However, since Glybera is locally injected and targets only a small proportion of skeletal muscle in the body, it provides limited restoration of LPL activity. This product was recently withdrawn from the market because of inadequate long-term efficacy and lack of commercial viability. Nonetheless, this was a major step forward for AAV vectors, demonstrating safety in humans and a clear path for regulatory approval.
Familial hypercholesterolemia (FH) is an autosomal-dominant disease characterized by high plasma LDL levels and premature cardiovascular disease (CVD).12 It is most frequently caused by loss-of-function mutations in the LDLR gene, resulting in impaired clearance of apolipoprotein B (apoB)-containing lipoproteins. Functional replacement of LDLR has long been a desired treatment strategy for patients with homozygous FH (HoFH).13 The first clinical trial of FH gene therapy involved ex vivo retroviral LDLR transduction of hepatocytes from patients with HoFH, followed by reimplantation of the cells.14 However, engraftment of the hepatocytes was inefficient and without significant lipid improvements, discouraging further follow-up. Since then, multiple preclinical studies have been performed using an AAV8 vector with the liver-specific thyroxine-binding globulin promoter to express the human LDLR cDNA. These studies demonstrated efficient hepatocyte transduction, sustained LDL lowering, and protection from atherosclerosis,15–17 thus providing the scientific and regulatory support for a phase 1/2 clinical trial in humans that is currently in progress, with results expected in 2019.13
ANTISENSE OLIGONUCLEOTIDES AND SMALL INTERFERING RNAS
Antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs) are synthetic nucleic acids that are commonly used for silencing gene expression in the liver (Figure 1).18 ASOs are short, single-stranded DNA sequences (20–30 oligonucleotides) engineered to hybridize with a target mRNA or pre-mRNA.18 The ASO-mRNA annealing results in the recruitment of a ribonuclease (RNase H) that cleaves the targeted mRNA, leading to its degradation.19 Small interfering RNAs are short, double-stranded RNA sequences (21–23 oligonucleotides) that cause sequence-specific degradation of mRNA through the RNA interference (RNAi) process.18 ASOs have been used extensively by lipid researchers to probe basic biology. Several chemical modifications of ASOs and siRNAs exist that can both improve their stability as well as specific delivery.20 In particular, polyethylene glycol (PEG)-coated lipid nanoparticles and trivalent N-acetylgalactosamine conjugates are respectively used for enhancing the delivery and uptake of oligonucleotide therapeutics to the liver, minimizing off-tissue effects.20,21 These two technologies have also been used for preclinical knockdown of several proteins involved in lipoprotein metabolism, including apoB100, apoCIII, angiopoietin-like 3 (ANGPTL3), lipoprotein(a), and PCSK9. However, all oligonucleotide therapies require careful evaluation of possible off-tissue effects as well as potential adverse events arising from sequence-related off-target silencing and immune activation.
Familial hypercholesterolemia has been aggressively pursued as a candidate for mRNA silencing therapeutics. The proposed mechanisms of cholesterol lowering generally involve inhibition of very low-density lipoprotein (VLDL) production or promotion of LDL clearance by the liver. Mipomersen is an ASO that inhibits the synthesis of apoB100 and is approved as an adjunctive therapy for HoFH.10,22 ApoB100 is the major structural component of VLDL, intermediate-density lipoprotein, and LDL, and its expression is critical for the normal export of TG from the liver.23 In a phase 3 study in patients with HoFH, mipomersen showed significant reductions of LDL-C, non-HDL cholesterol, and apoB lipoproteins.24 In addition, long-term mipomersen treatment has been associated with reduced cardiovascular events in FH patients.25 To date, the use of mipomersen in the United States is only available through the U.S. Food and Drug Administration's (FDA) Risk Evaluation and Mitigation Strategy drug safety program because of the potential for liver toxicity, which may involve on-target effects of apoB inhibition on hepatic fat content.
Inhibition of PCSK9 with monoclonal antibodies has been extremely successful, particularly in the setting of heterozygous FH. Therefore, it is not surprising that several RNA silencing drugs targeting PCSK9 have been pursued. ALN-PCSsc is an siRNA investigational agent that targets PCSK9 and is the only siRNA currently in clinical trials for lipid-related disorders. In a phase 2 clinical study, patients with high baseline LDL-C levels who received ALN-PCSsc demonstrated a significant decrease of plasma circulating PCSK9 and LDL-C levels at 240 days of follow-up.26 These positive results led to phase 2 and 3 clinical studies that are now in progress.27–31
Triglycerides are emerging as an important independent risk factor for CVD, and no currently available drugs substantially reduce this lipid class. ApoCIII is a liver-expressed secreted glycoprotein that binds to apoB-containing lipoproteins. It has been shown to inhibit LPL hydrolysis in vitro32 and interferes with receptor-mediated clearance of TG-rich lipoproteins by the liver.33 Elevated apoCIII levels are a risk factor for CVD, and loss-of-function variants are associated with reduced risk of coronary heart disease.34 Volanesorsen is an ASO that showed dose-dependent and prolonged reduction of circulating apoCIII and TG in multiple preclinical models and in a phase 1 trial.35 Recently, volanesorsen has been reported to reduce TG, abdominal pain, and pancreatitis attacks in patients with familial chylomicronemia syndrome within a phase 3 study.36 This drug was considered by the FDA in September 2018 but was not approved, possibly due to concerns about the risk of thrombocytopenia.
Another promising target for TG lowering is ANGPTL3, a liver-derived secreted protein that raises plasma lipids by inhibiting LPL and preventing hepatic uptake of apoB lipoproteins.37,38 Loss-of-function mutations in the human ANGPTL3 gene have been associated with low plasma LDL-C and TG.39 AKCEAANGPTL3-LRx is an ASO agent targeting ANGPTL3 that was developed to treat HoFH and severe dyslipidemias. Data from a recent phase 1 trial showed that AKCEA-ANGPTL3-LRx strongly reduced plasma levels of circulating ANGPTL3 protein, TG, LDL, VLDL, apoB, and apoCIII after 6 weeks of treatment, without serious adverse events.40 Several phase 2 clinical trials in patients with high TG levels are currently in progress.41–44
GENOME EDITING
The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 genome editing system is derived from a naturally occurring immune system in bacteria.45 This technology consists of an RNA-guided nuclease (Cas9) and a 20- to 23-nucleotide synthetic guide RNA (gRNA) that hybridizes to a complementary target sequence in genomic DNA (Figure 1). Cas9 induces a double-strand break (DSB) that can then be repaired by nonhomologous end-joining (NHEJ) or homology-directed repair (HDR). As the dominant repair pathway in mammalian cells, NHEJ results in insertions and/or deletions of nucleotides (referred as “indels”) that can be used to knock out genes. The HDR pathway uses a DNA template to repair DSB through homologous recombination and is active only in dividing cells. By providing an exogenous donor template with homology to the genome, it may eventually be possible to use CRISPR/Cas9 to correct pathogenic mutations in patients. The theoretical advantage of genome editing over other methods is the ability to make precise, permanent changes at the DNA level with a single delivery.
One of the first in vivo applications of CRISPR/Cas9 involved the somatic disruption of the Pcsk9 gene in mice. The authors used an adenoviral vector to deliver Streptococcus pyogenes (Sp) Cas9 and a gRNA targeting Pcsk9 to the liver,46 which resulted in a high-rate of NHEJ-derived indels and significant reductions in circulating PCSK9 and plasma cholesterol.A subsequent study by Ran et al. used AAV to deliver Staphylococcus aureus (Sa) Cas9 and a gRNA targeting Pcsk9.47 The authors achieved sustained reductions of PCSK9 protein and plasma cholesterol with the advantage of using a clinically relevant AAV vector for delivery. Jarrett et al. showed that AAV delivery of guide RNAs to the Cas9-transgenic mice could efficiently disrupt the Ldlr and Apob genes.48 Liver-directed editing of Ldlr resulted in severe hypercholesterolemia and atherosclerosis that could be rescued with concomitant deletion of Apob. However, CRISPR-mediated deletion of Apob produced a microvesicular steatosis, highlighting the risk of inhibiting VLDL secretion from the liver as a therapeutic strategy. In follow-up work, the authors generated an all-in-one vector to disrupt the Ldlr gene using the SaCas9 nuclease.49 They showed that a single injection of AAV-CRISPR could produce severe hypercholesterolemia and atherosclerosis that was comparable to AAV-PCSK9 overexpression. These studies show that AAV delivery of CRISPR/Cas9 is an attractive alternative to RNA silencing for loss-of-function studies. Preexisting immunity to SpCas9 has recently been found in humans,50 and it is likely that this will also be the case for SaCas9. Therefore, the targeting specificity as well as immune responses to these bacterially derived nucleases will both be important considerations for therapeutic genome editing applications. Recently, the CRISPR/Cas9 system has been modified by adding a cytosine deaminase domain to catalytically inactive Cas9. This “dead” Cas9 becomes a base editor that can catalyze deamination of cytosine to uracil without generating DSB.51 Uracil is ultimately converted into thymine, thus enabling either the correction of specific mutations or the generation of premature stop codons. Chadwick et al. used adenoviral vectors encoding base editor 3 (BE3) and a gRNA targeting Angptl3 to introduce loss-of-function mutations into Angptl3 genes in the liver.52 The authors reported a 35% base editing efficiency of Angptl3, resulting in significant lowering of plasma ANGPLT3, TG, and cholesterol levels. Recently, base editing has been efficiently used for performing in utero gene editing of the liver, where the tyrosine catabolic pathway was used to confer a selective advantage to base-edited hepatocytes.53 Base editing is innovative because it could allow precise repair of genes in both dividing and nondividing cells, and it avoids potential genotoxicity and insertional mutagenesis that may occur with DSB by CRISPR/Cas9. However, all base editing systems are very large and present significant delivery challenges using AAV vectors. In addition, off-target base editing could in theory be gRNA-independent and therefore more difficult to predict and detect.
In principle, almost any gene that is a candidate for inhibition by RNA silencing could also be therapeutically targeted for permanent deletion with CRISPR/Cas9 or base editing. More precise repair of disease-causing lipid genes must contend with the requirement of active cell division for homology directed repair, which is a major issue for post-mitotic tissues such as liver. In addition, there are numerous challenges to be addressed with regard to delivery, editing efficiency, specificity of genome editing, unwanted side effects of genome editing, and persistent expression of the editing enzymes themselves. Nonetheless, the concept of permanently correcting lipid disorders by modifying the patient's own DNA is inherently exciting and will undoubtedly usher in a new era of precision medicine.
CONCLUSIONS
Gene transfer technologies are a critical component of lipid research in model organisms (Table 1). Adeno-associated viral vectors are a well-established tool for overexpressing genes, particularly in the liver, in order to study lipid metabolism and physiology. RNA knockdown with ASOs and siRNA has been useful for somatic knockdown of genes in the liver, in many cases bypassing the need for new knockout animals. The emerging field of somatic genome editing with CRISPR/Cas9 provides a useful alternative to RNA knockdown approaches for loss-of-function studies. In addition, these tools are not proprietary and should be accessible to most laboratories competent in basic molecular biology techniques. These same technologies could be harnessed for gene therapy of lipid disorders. Some gene therapy products have already been approved for clinical use, such as Glybera for the treatment of LPL deficiency and Mipomersen for HoFH. Other products in clinical development are showing promising efficacy and tolerability in patients. However, the withdrawal of Glybera and the potential hepatic side effects of mipomersen encourage further optimization of gene therapy products with regard to efficiency and safety as well as consideration of biological mechanisms.
Table 1.
Summary of gene transfer technologies. AAV: adeno-associated virus; ASO: antisense oligonucleotides; siRNAs: small interfering RNAs; CRISPR/Cas9: Clustered Regularly Interspaced Short Palindromic Repeats; NHEJ: nonhomologous end-joining
| APPROACH | TECHNOLOGY | STRENGTHS | WEAKNESSES | POTENTIAL THERAPEUTIC TARGETS |
|---|---|---|---|---|
| Overexpression | AAV | Safe for use in humans Nonreplicating Low risk of insertional mutagenesis Noncytotoxic, modest immune response Efficient transduction of dividing and nondividing cells Strong and sustained transgene expression for months to years |
Limited packaging capacity ~4.9 kb Artificial expression cassettes do not preserve endogenous regulation High frequency of neutralizing antibodies to AAV capsids in humans T-cell responses to capsid managed with immunosuppression |
Pursued LPL LDLR Possible LCAT APOE APOC2 APOA1 LIPA LIPC LDLRAP1 GPIHBP1 |
| Knockdown | ASOs | Efficient knockdown by RNAse H recruitment or translation blocking Splicing modulation by targeting pre-mRNA Chemically modified for improved liver uptake Efficient long-term silencing with weekly or biweekly administration Subcutaneous injection |
Chemical modifications needed to increase nuclease resistance and half-life Possibility of sequence-related off-targets Potential class effects depending on modifications Mild skin reactions |
Pursued APOB APOC3 ANGPTL3 LPA Possible PCSK9 |
| siRNA | Efficient knockdown by RNAi machinery Long-term silencing can be achieved Chemically modified for direct liver uptake Can also be effective at lower doses via lipid nanoparticle delivery |
Possibility of sequence-related off-targets Potential class effects depending on modifications Some formulations require intravenous injection |
Pursued PCSK9 Possible APOB APOC3 ANGPTL3 LPA |
|
| Genome editing | CRISPR/Cas9 | Ease of design and customization High NHEJ-mediated editing efficiency Multiplex genome editing capacity Correct gene dosage Preservation of regulatory elements One-time treatment Permanent correction to patient's own DNA |
Potential off-target activity that requires careful testing Potential unintended consequences at the DSB site (i.e., large insertions/deletions) Low efficiency of HDR-mediated gene correction (restricted to dividing cells) Potential immune response against Cas9-expressing cells |
Possible APOB APOC3 ANGPTL3 PCSK9 LPA |
Although very promising, the long-term efficacy and safety of AAV gene therapy is still being established in humans. There are additional concerns regarding off-target effects of siRNA, ASOs, and CRISPR/Cas9, which has a target specificity based on Watson-Crick base pairing. Improvements in targeting design and off-target prediction, as well as the development of more sensitive sequencing technologies, will help improve the precision of these gene therapy products. Gene transfer technologies have already made invaluable contributions to our understanding of lipid metabolism and physiology. Despite several important challenges, gene therapy represents one of the most promising therapeutic approaches for correction of lipid disorders and CVD risk reduction.
KEY POINTS
Gene transfer technologies are versatile tools for investigating lipid metabolism and physiology and have tremendous potential to treat lipid disorders.
Adeno-associated viral vectors are widely used for overexpressing genes in the liver and are the leading vector for liver-directed gene therapy in humans.
Antisense oligonucleotides and small interfering RNAs are synthetic oligonucleotides that can efficiently silence genes involved in lipoprotein metabolism and cardiovascular disease.
Somatic genome editing with CRISPR/Cas9 is a promising approach for selectively inactivating or correcting genes in the liver.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1.Roche-Molina M, Sanz-Rosa D, Cruz FM et al. Induction of sustained hyper-cholesterolemia by single adeno-associated virus-mediated gene transfer of mutant hPCSK9. Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):50–9. doi: 10.1161/ATVBAHA.114.303617. [DOI] [PubMed] [Google Scholar]
- 2.Lagor WR, Johnston JC, Lock M, Vandenberghe LH, Rader DJ. Adeno-associated viruses as liver-directed gene delivery vehicles: focus on lipoprotein metabolism. Methods Mol Biol. 2013;1027:273–307. doi: 10.1007/978-1-60327-369-5_13. [DOI] [PubMed] [Google Scholar]
- 3.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012 Apr;20(4):699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitajima K, Marchadier DH, Miller GC, Gao GP, Wilson JM, Rader DJ. Complete prevention of atherosclerosis in apoE-deficient mice by hepatic human apoE gene transfer with adeno-associated virus serotypes 7 and 8. Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1852–7. doi: 10.1161/01.ATV.0000231520.26490.54. [DOI] [PubMed] [Google Scholar]
- 5.Gaudet D, Méthot J, Déry S et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013 Apr;20(4):361–9. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dias MF, Joo K, Kemp JA et al. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog Retin Eye Res. 2018 Mar;63:107–131. doi: 10.1016/j.preteyeres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Bauer RC, Sasaki M, Cohen DM et al. Tribbles-1 regulates hepatic lipogenesis through posttranscriptional regulation of C/EBPα. J Clin Invest. 2015 Oct 1;125(10):3809–18. doi: 10.1172/JCI77095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidah NG, Awan Z, Chretien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res. 2014 Mar 14;114(6):1022–36. doi: 10.1161/CIRCRESAHA.114.301621. [DOI] [PubMed] [Google Scholar]
- 9.Bjørklund MM, Hollensen AK, Hagensen MK et al. Induction of atherosclerosis in mice and hamsters without germline genetic engineering. Circ Res. 2014 May 23;114(11):1684–9. doi: 10.1161/CIRCRESAHA.114.302937. [DOI] [PubMed] [Google Scholar]
- 10.Gaudet D, Brisson D. Gene-based therapies in lipidology: current status and future challenges. Curr Opin Lipidol. 2015 Dec;26(6):553–65. doi: 10.1097/MOL.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 11.Gaudet D, Stroes ES, Méthot J et al. Long-Term Retrospective Analysis of Gene Therapy with Alipogene Tiparvovec and Its Effect on Lipoprotein Lipase Deficiency-Induced Pancreatitis. Hum Gene Ther. 2016 Nov;27(11):916–25. doi: 10.1089/hum.2015.158. [DOI] [PubMed] [Google Scholar]
- 12.Defesche JC, Gidding SS, Harada-Shiba M, Hegele RA, Santos RD, Wierzbicki AS. Familial hypercholesterolaemia. Nat Rev Dis Primers. 2017 Dec 7;3 doi: 10.1038/nrdp.2017.93. 17093. [DOI] [PubMed] [Google Scholar]
- 13.Ajufo E, Cuchel M. Recent Developments in Gene Therapy for Homozygous Familial Hypercholesterolemia. Curr Atheroscler Rep. 2016 May;18(5):22. doi: 10.1007/s11883-016-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossman M, Rader DJ, Muller DW et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995 Nov;1(11):1148–54. doi: 10.1038/nm1195-1148. [DOI] [PubMed] [Google Scholar]
- 15.Lebherz C, Gao G, Louboutin JP, Millar J, Rader D, Wilson JM. Gene therapy with novel adeno-associated virus vectors substantially diminishes atherosclerosis in a murine model of familial hypercholesterolemia. J Gene Med. 2004 Jun;6(6):663–72. doi: 10.1002/jgm.554. [DOI] [PubMed] [Google Scholar]
- 16.Kassim SH, Li H, Bell P et al. Adeno-associated virus serotype 8 gene therapy leads to significant lowering of plasma cholesterol levels in humanized mouse models of homozygous and heterozygous familial hypercholesterolemia. Hum Gene Ther. 2013 Jan;24(1):19–26. doi: 10.1089/hum.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen SJ, Sanmiguel J, Lock M et al. Biodistribution of AAV8 vectors expressing human low-density lipoprotein receptor in a mouse model of homozygous familial hypercholesterolemia. Hum Gene Ther Clin Dev. 2013 Dec;24(4):154–60. doi: 10.1089/humc.2013.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watts JK, Corey DR. Silencing disease genes in the laboratory and the clinic. J Pathol. 2012 Jan;226(2):365–79. doi: 10.1002/path.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-Targeted Therapeutics. Cell Metab. 2018 Apr 3;27(4):714–39. doi: 10.1016/j.cmet.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Prakash TP, Graham MJ, Yu J et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014 Jul;42(13):8796–807. doi: 10.1093/nar/gku531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittrup A, Lieberman J. Knocking down disease: a progress report on siRNA therapeutics. Nat Rev Genet. 2015 Sep;16(9):543–52. doi: 10.1038/nrg3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGowan MP, Tardif JC, Ceska R et al. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049006. e49006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raabe M, Kim E, Véniant M, Nielsen LB, Young SG. Using genetically engineered mice to understand apolipoprotein-B deficiency syndromes in humans. Proc Assoc Am Physicians. 1998 Nov-Dec;110(6):521–30. [PubMed] [Google Scholar]
- 24.Raal FJ, Santos RD, Blom DJ et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010 Mar 20;375(9719):998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 25.Duell PB, Santos RD, Kirwan BA, Witztum JL, Tsimikas S, Kastelein JJP. Long-term mipomersen treatment is associated with a reduction in cardiovascular events in patients with familial hypercholesterolemia. J Clin Lipidol. 2016 Jul-Aug;10(4):1011–21. doi: 10.1016/j.jacl.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Ray KK, Landmesser U, Leiter LA et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N Engl J Med. 2017 Apr 13;376(15):1430–40. doi: 10.1056/NEJMoa1615758. [DOI] [PubMed] [Google Scholar]
- 27.Clinical Trials [Internet] Bethesda, MD: U.S. National Library of Medicine; c2018. Study of ALN-PCSSC in Participants With Homozygous Familial Hypercholesterolemia (HoFH) (ORION-2); 2018 Apr 25 [cited 2018 Oct 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02963311. [Google Scholar]
- 28.Clinical Trials [Internet] Bethesda, MD: U.S. National Library of Medicine; c2018. An Extension Trial of Inclisiran Compared to Evolocumab in Participants With Cardiovascular Disease and High Cholesterol; 2017 Dec 22 [cited 2018 Oct 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT03060577. [Google Scholar]
- 29.Clinical Trials [Internet] Bethesda, MD: U.S. National Library of Medicine; c2018. Inclisiran for Participants With Atherosclerotic Cardiovascular Disease and Elevated Low-density Lipoprotein Cholesterol (ORION-10); 2018 Apr 23 [cited 2018 Oct 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT03399370. [Google Scholar]
- 30.Clinical Trials [Internet] Bethesda, MD: U.S. National Library of Medicine; c2018. Inclisiran for Subjects With ACSVD or ACSVD-Risk Equivalents and Elevated Low-density Lipoprotein Cholesterol (ORION-11); 2018 Apr 26 [cited 2018 Oct 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT03400800. [Google Scholar]
- 31.Clinical Trials [Internet] Bethesda, MD: U.S. National Library of Medicine; c2018. Trial to Evaluate the Effect of Inclisiran Treatment on Low Density Lipoprotein Cholesterol (LDL-C) in Subjects With Heterozygous Familial Hypercholesterolemia (HeFH) (ORION-9); 2018 Apr 9 [cited 2018 Oct 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT03397121. [Google Scholar]
- 32.Wang CS, McConathy WJ, Kloer HU, Alaupovic P. Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C-III. J Clin Invest. 1985 Feb;75(2):384–90. doi: 10.1172/JCI111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordts PL, Nock R, Son NH et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest. 2016 Aug 1;126(8):2855–66. doi: 10.1172/JCI86610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norata GD, Tsimikas S, Pirillo A, Catapano AL. Apolipoprotein C-III: From Pathophysiology to Pharmacology. Trends Pharmacol Sci. 2015 Oct;36(10):675–87. doi: 10.1016/j.tips.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Graham MJ, Lee RG, Bell TA, 3rd et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. 2013 May 24;112(11):1479–90. doi: 10.1161/CIRCRESAHA.111.300367. [DOI] [PubMed] [Google Scholar]
- 36.Gaudet D, Digenio A, Alexander V et al. The approach study: a randomized, double-blind, placebo-controlled, phase 3 study of volanesorsen administered subcutaneously to patients with familial chylomicronemia syndrome (FCS) Atherosclerosis. 2017 Aug;263:E10. [Google Scholar]
- 37.Lee EC, Desai U, Gololobov G et al. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL) J Biol Chem. 2009 May 15;284(20):13735–45. doi: 10.1074/jbc.M807899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu YX, Redon V, Yu H et al. Role of angiopoietin-like 3 (ANGPTL3) in regulating plasma level of low-density lipoprotein cholesterol. Atherosclerosis. 2018 Jan;268:196–206. doi: 10.1016/j.atherosclerosis.2017.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dewey FE, Gusarova V, Dunbar RL et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N Engl J Med. 2017 Jul 20;377(3):211–21. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham MJ, Lee RG, Brandt TA et al. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N Engl J Med. 2017 Jul 20;377(3):222–32. doi: 10.1056/NEJMoa1701329. [DOI] [PubMed] [Google Scholar]
- 41.Clinical Trials [Internet] Bethesda, MD: U.S. National Library of Medicine; c2018. Study of AKCEA-ANGPTL3-LRX (ISIS 703802) in Patients With With Familial Partial Lipodystrophy (FPL); 2018 Jul 2 {cited 2018 Oct 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT03514420. [Google Scholar]
- 42.Clinical Trials [Internet] Bethesda, MD: U.S. National Library of Medicine; c2018. Study of AKCEA-ANGPTL3-LRX (ISIS 703802) in Patients With Homozygous Familial Hypercholesterolemia (HoFH); 2018 Apr 23 [cited 2018 Oct 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT03455777. [Google Scholar]
- 43.Clinical Trials [Internet] Bethesda, MD: U.S. National Library of Medicine; c2018. Phase 2 Study of AKCEA-ANGPTL3-LRX (ISIS 703802) in Patients With Familial Chylomicronemia Syndrome (FCS); 2018 Aug 1 [cited 2018 Oct 5]. https://clinicaltrials.gov/ct2/show/NCT03360747. [Google Scholar]
- 44.Clinical Trials [Internet] Bethesda, MD: U.S. National Library of Medicine; c2018. Study of ISIS 703802 in Subjects With Hypertriglyceridemia, Type 2 Diabetes Mellitus, and Nonalcoholic Fatty Liver Disease; 2018 Sep 26 [cited 2018 Oct 5]. https://clinicaltrials.gov/ct2/show/NCT03371355. [Google Scholar]
- 45.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012 Aug 17;337(6096):816–21. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding Q, Strong A, Patel KM et al. Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ Res. 2014 Aug 15;115(5):488–92. doi: 10.1161/CIRCRESAHA.115.304351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ran FA, Cong L, Yan WX et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015 Apr 9;520(7546):186–91. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarrett KE, Lee CM, Yeh YH et al. Somatic genome editing with CRISPR/Cas9 generates and corrects a metabolic disease. Sci Rep. 2017 Mar 16;7 doi: 10.1038/srep44624. 44624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarrett KE, Lee C, De Giorgi M et al. Somatic Editing of Ldlr With Adeno-Associated Viral-CRISPR Is an Efficient Tool for Atherosclerosis Research. Arterioscler Thromb Vasc Biol. 2018 Sep;38(9):1997–2006. doi: 10.1161/ATVBAHA.118.311221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner DL, Amini L, Wendering DJ et al. High prevalence of Streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat Med. 2018 Oct 29; doi: 10.1038/s41591-018-0204-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016 May 19;533(7603):420–4. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chadwick AC, Evitt NH, Lv W, Musunuru K. Reduced Blood Lipid Levels With In Vivo CRISPR-Cas9 Base Editing of ANGPTL3. Circulation. 2018 Feb 27;137(9):975–7. doi: 10.1161/CIRCULATIONAHA.117.031335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossidis AC, Stratigis JD, Chadwick AC et al. In utero CRISPR-mediated therapeutic editing of metabolic genes. Nat Med. 2018 Oct;24(10):1513–8. doi: 10.1038/s41591-018-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


