Abstract
High-density lipoprotein (HDL) is a protein-lipid nanoparticle that has predominately been characterized by its cholesterol concentration (HDL-C). Recent studies have challenged the presumed inverse association between HDL-C and cardiovascular events, suggesting a more U-shaped association. This has opened new opportunities to evaluate more novel measures of HDL metabolism, such as HDL particle number (HDL-P) and one of HDL's key functions, cholesterol efflux. Both HDL-P and cholesterol efflux are inversely associated with incident cardiovascular events and may perhaps be better targets for intervention. This review includes recent research on the emerging U-shaped association between HDL-C and cardiovascular events, recent observational studies related to HDL-P, and the effects of established and novel interventions on cholesterol efflux.
Keywords: high-density lipoprotein, HDL, cholesterol efflux capacity, HDL-C, HDL-P
INTRODUCTION
High-density lipoprotein (HDL) is considered “good” cholesterol, but this lipoprotein has numerous functions and features that remain to be understood. Although there are a multitude of HDL-related measures, key features well-studied in humans include cholesterol concentration (HDL-C), HDL particle number (HDL-P), and HDL cholesterol efflux, proposed to reflect HDL's ability to promote cholesterol efflux from cells in vivo (Figure 1). Many reviews discuss the pathobiology of HDL and its functions. However, there have been an increasing number of published studies regarding the role of HDL measures in human populations. This review discusses the evolving paradigm of HDL's role in cardiovascular disease (CVD) and focuses on recent studies related to measures of HDL-C, HDL-P, and cholesterol efflux in human populations.
Figure 1.
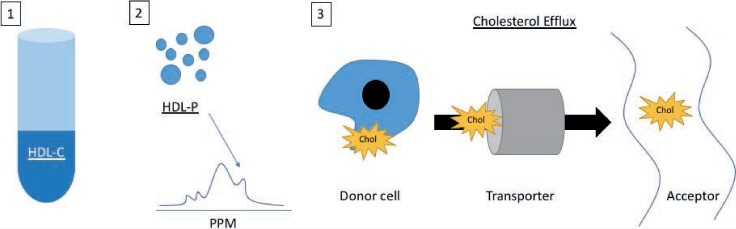
Measurement of high-density lipoproteins (HDL) and HDL function. (1) HDL cholesterol (HDL-C) concentration measurement via assay with centrifugation. (2) HDL particle (HDL-P) count measurement via nuclear magnetic resonance spectroscopy. (3) Cholesterol efflux capacity measures the movement of cholesterol.
HDL-C: EXTREME PLASMA LEVELS AND CVD RISK
Genetic studies have illuminated the degree to which extreme HDL-C levels are genetically determined. Among individuals with extreme values of HDL-C (< 30.9 mg/dL or > 54.1 mg/dL in men and < 38.7 mg/dL or > 69.6 mg/dL in women), more than 30% harbored a genetic variant attributable to either the extreme low or high HDL-C phenotype.1 The relationship between HDL-C and CVD events has typically been described as a linear inverse association within primarily Caucasian cohorts recruited decades ago. Recently, however, other studies have revealed that the association is nonlinear, with extremely high concentrations of HDL-C associated with increased CVD events and mortality.2–4
Extremely Low HDL-C
A major challenge in assessing CVD risk associated with low HDL-C is that low HDL-C is tightly linked to insulin resistance and is part of an atherogenic dyslipidemia phenotype consisting of several lipid derangements. A recent assessment within the Multi-Ethnic Study of Atherosclerosis (MESA) analyzed primary low HDL-C levels, defined as < 40 mg/dL for men and < 50 mg/dL for women, with triglycerides and LDL both less than 100 mg/dL (n = 158/6,814). The study reported that primary low HDL-C was associated with an approximately 2-fold increased risk of incident total coronary heart disease (CHD) (adjusted HR 2.25, 95% CI, 1.20–4.21; P = .011) and CVD (adjusted HR 1.93, 95% CI, 1.11–3.34; P = .020).2,5 With respect to mortality, although large Japanese and Danish cohorts both reported increased all-cause mortality in those with low HDL-C,2,3 primary low HDL-C in the MESA cohort was not associated with all-cause mortality (HR 1.11, 95% CI, 0.67–1.84; P = .69).5 These findings highlight that low HDL-C consistently associates with CVD but does not consistently associate with mortality.
Extremely High HDL-C
If the association between HDL-C and CVD risk is linear and inverse, one would expect that those with extremely high HDL-C would be the most protected from CVD. However, recent observational data suggest that this may not be the case. A multicohort study in 11,515 men and 12,925 women found that HDL-C values greater than 90 mg/dL in men and greater than 75 mg/dL in women actually plateaued in the hazard ratio for coronary heart disease (CHD) events.4
The Japanese and Danish cohorts also studied extremely high HDL-C values. In the Japanese cohort,3 the highest level of HDL-C (≥ 90 mg/dL) for both men and women was associated with increased risk of mortality from atherosclerotic CVD, CHD, and ischemic stroke (Figure 2). Stratified by sex, both men and women with the highest levels of HDL-C had increased risk of atherosclerotic CVD mortality. In the Danish cohort,2 those with extremely high HDL-C (men ≥ 116 mg/dL, women ≥ 135 mg/dL) had increased cardiovascular mortality (Figure 2). Similar to the Japanese cohort, both men and women with extremely high HDL-C had increased all-cause mortality (Figure 2).
Figure 2.
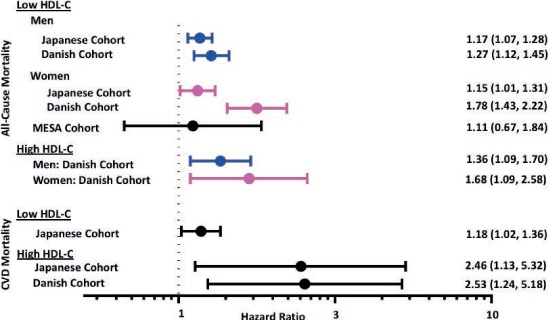
Association of extreme high-density lipoprotein cholesterol (HDL-C) values with mortality. Japanese cohort: Extremely low HDL-C: < 40 mg/dL; extremely high HDL-C ≥ 90 mg/dL. Danish cohort: Extremely low HDL-C: < 39 mg/dL; extremely high HDL-C: men ≥ 116 mg/dL, women ≥ 135 mg/dL.
Optimal HDL-C
The Danish study revealed that a moderate range of HDL-C was associated with the lowest all-cause mortality in both sexes compared to lower and higher HDL-C. For both sexes, there was a U-shaped association between HDL-C and all-cause mortality as well as cardiovascular mortality. In fully adjusted models for men, baseline HDL-C at 73 mg/dL (95% CI, 42–77 mg/dL) was associated with the lowest mortality. In fully adjusted models for women, the lowest mortality was seen at a baseline HDL-C of 93 mg/dL (95% CI, 66–239 mg/dL). These studies, as well as others with similar observations, highlight that in large contemporary cohorts, the association between HDL-C and CVD is inverse and linear at low-to-moderate ranges but becomes nonlinear and plateaus or is directly associated with CVD risk at extremely high HDL-C levels. These studies also raise concern about strategies focused on raising HDL-C as a preventive therapy. Further studies are necessary to understand the relationship between HDL-C and mortality.2
Raising HDL-C: CETP Inhibitors
Cholesteryl ester transfer protein (CETP) inhibitors induce the most potent increases in HDL-C and thus represent the best potential strategy to test whether or not raising HDL-C improves CVD outcomes. Torcetrapib, dalcetrapib, and evacetrapib increased HDL-C by more than 70%, 30%, and 130%, respectively; however, all trials of these drugs were discontinued due to either futility or harm.6–8 The fourth CETP inhibitor studied in outcome-driven trials was anacetrapib. In this trial, anacetrapib increased HDL-C by 104% compared to placebo and was associated with a decreased incidence of major coronary events (10.8% vs 11.8% events; rate ratio of 0.91, 95% CI, 0.85–0.97, P = .004).9 Despite being the first CETP inhibitor to show improved outcomes, subsequent analyses suggested that the benefit of anacetrapib was primarily driven by reducing apolipoprotein B, not by increasing HDL-C.9,10 Given the absence of improved CVD outcomes with potent HDL-C raising and the recent findings that extremely high baseline HDL-C is not cardioprotective and perhaps even harmful, the strategy to raise HDL-C will likely be abandoned at this juncture. This presents the opportunity to pursue other measures of HDL structure and function such as HDL-P and cholesterol efflux.
HDL-P: LINKS TO CARDIOVASCULAR DISEASE AND METABOLIC SYNDROME
HDL particle number is an emerging HDL marker quantified through nuclear magnetic resonance spectroscopy, ion mobility, or 2-dimensional gradient gel electrophoresis.11 Overall, multiple studies have consistently reported that HDL-P is a better predictor of CVD risk than HDL-C.12,13 More recently, a post-hoc analysis of the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial assessed multiple measures of HDL concentrations. HDL-P at baseline and on treatment after 12 months of rosuvastatin (20 mg/d) was inversely associated with incident CVD events (baseline HDL-P: adjusted OR/SD 0.69; 95% CI, 0.56–0.86; P < .001; and on-treatment HDL-P: OR/SD 0.51; 95% CI, 0.33–0.77; P < .001). Comparison of cholesterol efflux, HDL-C, and apolipoprotein AI concentrations with HDL-P showed that HDL-P was the strongest inverse predictor of incident CVD.14
In a multiethnic cohort of the Dallas Heart Study, myeloperoxidase (MPO) indexed to HDL-P was assessed as a metric for HDL oxidative potential. MPO, an enzyme expressed during inflammation, oxidizes HDL by binding to HDL-P and reducing the antioxidant function of HDL. In this study, there was a direct association between increasing quartiles of MPO/HDL-P and CVD. The highest quartile compared to the lowest quartile of MPO/HDL-P associated with a 74% increase in incident atherosclerotic CVD (adjusted HR 1.74, 95% CI, 1.12–2.70) and 91% increase in total incident CVD (adjusted HR 1.91, 95% CI, 1.27–2.85).15
Unlike low plasma levels of HDL-C, which is part of the metabolic syndrome (MetS) profile that is confounded by coexistent insulin resistance, HDL-P levels are not significantly associated with measures of adiposity or insulin resistance. However, a recent report showed that baseline HDL-P levels inversely associate with incident MetS and that this relationship is not attenuated by adjusting for other independent markers of MetS such as visceral fat, triglyceride/HDL-C ratio, body mass index, and homeostatic model assessment of insulin resistance.16 This study reported a 2-fold increase in incident MetS in the lowest quartile of HDL-P (16.11–29.41 mg/dL) and a 32% decrease in risk of incident MetS with one standard deviation increase in HDL-P.
CHOLESTEROL EFFLUX: EFFECTS OF INTERVENTIONS ON HDL FUNCTION
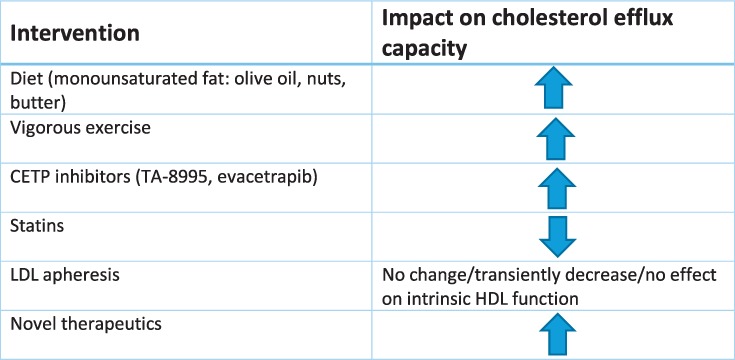
Reverse cholesterol transport (RCT) is considered the most relevant antiatherosclerotic function of HDL. Cholesterol efflux from macrophages is the first critical RCT step and involves movement of cholesterol from cells to primarily apoAI-containing particles via cell-surface lipid transporters such as ATP-binding cassette transporter member 1 (ABCA1), ABCG1, and scavenger receptor B type 1 (SR-B1) as well as by aqueous diffusion.11,17 Measurement of cholesterol efflux capacity (CEC) is not standardized but typically involves quantifying the amount of labeled cholesterol that moves from reference donor cells to apoB-depleted plasma or serum.18 CEC is inversely associated with prevalent and incident atherosclerotic CVD in multiple large cohorts.17,19 Given that HDL-C is not an adequate therapeutic target, attention has increased toward targeting improvement of HDL function as a potential strategy to reduce CV risk, with specific focus on interrogating CEC (Table 1).
Table 1.
Behavioral and therapeutic interventions that affect cholesterol efflux capacity. CETP: cholesteryl ester transfer protein; LDL: low-density lipoprotein; HDL: high-density lipoprotein
Diet
Three recent trials have studied the impact of specific fats on CEC. In the Prevention with Mediterranean Diet (PREDIMED) trial, individuals at high risk for CVD were included in a 1-year intervention of two types of Mediterranean diets compared to a low-fat control diet. Both interventions significantly increased CEC compared to baseline but not compared to the low-fat control diet.20 Another study evaluated the impact of specific fats on CEC in individuals with abdominal obesity. Patients were randomized to a 4-week diet rich in saturated fats, cheese, or butter or rich in unsaturated fats, refined olive oil (monounsaturated), or corn oil (polyunsaturated), with the control being a lower fat and carbohydrate diet.21 Compared to the control diet, there was a 3.3% and 3.8% increase in CEC in the butter and monounsaturated diets but not for the cheese or polyunsaturated diets. The butter diet also significantly increased LDL compared to the low-carb, low-fat control. Lastly, a third trial comparing consumption of three whole eggs daily for 12 weeks versus egg substitute reported a 2.4% increase in CEC compared to baseline in the whole-egg group, with no change in the egg substitute group.22
Exercise
Two randomized clinical trials evaluated the effects of different levels of exercise on measures of HDL function over a 6-month period. The Exercise Dose-Response Effects in Prediabetes (STRRIDE-PD) study involved four exercise regimens (low-amount/moderate intensity, high-amount/moderate intensity, high-amount/vigorous intensity, diet change and low-amount/moderate intensity) and the Examination of Mechanisms (E-MECHANIC) of Exercise-Induced Weight Compensation trial involved two exercise regimens (800–1000 kcal/wk or 2000–2500 kcal/wk, both with target heart rate 65–80% peak oxygen uptake). Both studies included sedentary overweight individuals without CVD or diabetes. The highest-intensity exercise group in STRRIDE-PD significantly increased radiolabeled CEC (+ 6.2%; standard error of mean 0.06) in adjusted models compared to the other exercise groups, but it did not include a control group. The higher-intensity exercise group in E-MECHANIC also significantly increased radiolabeled CEC by 5.7% (95% CI, 1.2–10.2%) compared to the control group, but this association was attenuated after adjusting for HDL-C. The boron-dipyrromethene labeled CEC was not affected by exercise training.23
Another study evaluated the effect of lifestyle modification, diet, and exercise on CEC in men with abdominal obesity and dyslipidemia. After 1 year of counseling with both a kinesiologist and dietician and a minimum of 160 minutes of weekly vigorous activity (50–80% maximum heart rate), there was a noted increase in CEC as well as decreased caloric intake and improved dietary quality.24 In addition, this lifestyle modification led to an overall improvement in cardiometabolic risk profile as well as a significant increase in CEC by 14.1% and 3.4% from either macrophages (J774) or hepatocellular carcinoma cells (HepG2), respectively. There was no change in CEC in the control group after 1 year compared to baseline. Another prospective study of overweight or obese women on a 6-month low-fat and decreased total energy diet with low-intensity exercise resulted in weight loss of 2.2 ± 3.9 kg; however, this was not associated with a significant change in CEC despite a decrease in HDL-C, suggesting again a discordance between HDL-C and HDL functional measurements as a response to therapy.25
In two high-risk secondary prevention groups, the effects of exercise on CEC were mixed. Individuals with peripheral artery disease were randomized into three study arms with exercise interventions, including 3×/wk at near-maximum tolerance of leg symptoms, 3×/wk lower-extremity resistance training with increasing resistance, and a control of no exercise training but 11 nutritional sessions over 6 months.26 There was no association with CEC changes among the three study arms. Another study of patients with acute coronary syndrome who underwent percutaneous coronary intervention compared those who completed cardiac rehab (38.9 mean sessions) versus those who dropped out (13.5 mean sessions).27 There was a significant increase in CEC in the cardiac rehab group compared to baseline levels but not compared to the control group participants who dropped out of rehab.
Collectively, these studies of lifestyle interventions reflect varied effects on CEC depending on the type of intervention, study population, and comparator group. Further studies will need to standardize the intervention and control groups to better ascertain the effects of diet and exercise on HDL function.
MEDICATIONS
Cholesteryl Ester Transfer Protein Inhibitors
New therapeutics for inhibiting CETP in general increase CEC. A phase 2 randomized control trial of a new CETP inhibitor, TA-8995, included study arms of placebo, 1 mg, 5 mg, and 10 mg daily. Total CEC, non–ABCA1-specific CEC, and ABCA1-specific CEC all significantly increased in a dose-dependent fashion, ranging from a 15% to 72% increase in CEC.28 A phase 2 trial testing 12 weeks of evacetrapib monotherapy29 also demonstrated increases in total CEC and non–ABCA1-specific CEC in a dose-dependent manner. ABCA1-specific CEC increased in a non–dose-dependent manner. Both studies also noted a significant increase in pre-beta1, which correlated with the increase in total and ABCA1-specific CEC, although the mechanism by which CETP inhibition increases pre-beta1 levels remains unclear.28,29 A pharmacogenomic effect on CEC has been suggested for the CETP inhibitor dalcetrapib. A post-hoc analysis of dalcetrapib in the main outcomes trial (DAL-OUTCOMES) found a 22.3% increase in CEC with the protective AA genotype compared to 7.8% and 7.4% increases for GG and AG genotypes, respectively.30 A second outcomes trial currently underway is testing the effects of dalcetrapib on CVD events by genotype.31
Statins
The effects of statins on CEC have been inconsistent. In the phase 2 trial of evacetrapib, study arms included statin monotherapy and statin combined with evacetrapib.29 Rosuvastatin and simvastatin significantly decreased total CEC and ABCA1-specific CEC, but atorvastatin had no effect on CEC. The combination of statin with evacetrapib blunted the increase in CEC observed with evacetrapib monotherapy. Similarly, the combination of rosuvastatin with 10 mg TA-8995 increased CEC by 67% compared to a 72% increase with 10 mg TA-8995 monotherapy.28
A post-hoc analysis of the JUPITER trial evaluated the association between CEC and incident cardiovascular disease on statin therapy and determined that rosuvastatin therapy did not change CEC after 12 months.14 Intriguingly, although baseline CEC was not associated with incident CVD (OR/SD 0.89, 95% CI, 0.72–1.10, P = .28), CEC after 12 months of rosuvastatin therapy was inversely associated with incident CVD (OR/SD 0.62, 95% CI, 0.42–0.92, P = .02). These discordant findings remain unexplained but suggest some inconsistency in the association between CEC and outcomes within the context of lipid-modifying trials.
Given the evolving epidemiology of CEC in atherosclerotic CVD, interest has increased in prebeta-1, the primary determinant of ABCA1-specific efflux. Statin therapy significantly reduces the plasma level of prebeta-1. In a 6-week study evaluating multiple statins,32 the decrease in prebeta-1 levels was similar among the statin therapies (17.9% reduction) and was associated with a decrease in plasma triglyceride levels but not LDL-C levels. None of the statins changed plasma HDL-C levels significantly.
LDL Apheresis
Among individuals with familial hypercholesterolemia (FH), treatment may include LDL-apheresis—intentional removal of apoB-containing lipoproteins but also removal of all lipoproteins, including HDL-C. The studies that reported on HDL-C and apoAI levels were consistent: HDL-C and apoAI were reduced after apheresis.33,34 However, this effect was transient since the levels normalized 2 days after apheresis.34
Investigations of the relationship between CEC and LDL-apheresis have had mixed results. Studies of homozygous and heterozygous FH revealed no change in CEC after apheresis.33,35 Another cohort of individuals with FH and familial hyperlipidemia showed a reduction in SR-B1-mediated and aqueous diffusion-mediated efflux immediately after apheresis, although this reduction was transient and recovered within 2 days after apheresis.34 However, ABCA1-mediated CEC was immediately and persistently reduced at the 2-day measurement. Studies of this cohort also revealed that macrophage cholesterol-loading capacity was lower immediately and 2 days after apheresis than prior to apheresis. In contrast, a study including FH individuals with the LDLR gene defect found decreased CEC in whole plasma (−15%) without affecting the intrinsic function of HDL particles to mediate cholesterol efflux or delivery of cholesteryl ester to hepatic cells.36
Novel Therapeutics
Current therapies that affect HDL-C levels—specifically niacin, fibrates, estrogen, and CETP inhibitors—have had limited efficacy in improving CVD outcomes. Recent observational studies suggesting that functions related to RCT may better predict CVD events have focused on the development of novel therapies that directly interrogate some aspect of RCT. Most of these therapeutic developments have centered on the primary role of apolipoprotein AI in the structure and function of HDL particles and the impact on RCT promotion and atherosclerosis prevention.
A number of oral and intravenous apoAI mimetic peptide formulations have been developed and studied in animal models and have shown improvements in cholesterol efflux, inflammation, and oxidation. Unfortunately, none have entered larger-scale human studies thus far. In contrast, three intravenous apoAI infusion formulations have been studied in human trials to date. Apo AIMilano was developed as a therapy based on the rationale that carriers with this mutation demonstrated protection from CVD despite very low HDL-C levels. The initial apo AIMilano formulation, ETC-216, was studied in a phase 2 trial of patients treated for acute coronary syndrome and suggested improvement in coronary atherosclerosis by intravascular ultrasound (IVUS).37 Due to serious side effects with ETC-216, another formulation (MDCO-216) was developed and tested in a randomized controlled trial of 122 acute coronary syndrome patients. After 5 weekly infusions, there was no difference from placebo in IVUS-determined plaque characteristics.38 No further development of MDCO-216 is planned.
CER-001 is a complex of recombinant apoAI and phospholipids similar to pre-beta HDL and can rapidly mobilize cholesterol in the HDL fraction. Although CER-001 increased in ABCA1-mediated cholesterol efflux, high doses actually suppressed this effect. In the randomized, placebo-controlled CARAT study, CER-001 at 3 mg/kg given over 9 weeks to 301 patients after acute coronary syndrome had no effect on IVUS-determined plaque characteristics.39 Current studies are focused on the effects of CER-001 on carotid atherosclerosis in patients with familial hypoalphalipoproteinemia.40
CSL-112 is a reconstituted particle consisting of native apoAI and phospholipids. This formulation is associated with marked increases in pre-beta HDL and ABCA1-mediated cholesterol efflux. In the AEGIS-I phase 2b trial of more than 1,200 patients with recent myocardial infarction, 4 weekly infusions of CSL-112 were safe and well-tolerated and enhanced cholesterol efflux.41 The large phase 3 AEGIS-II randomized controlled trial is ongoing and testing whether CSL-112 will reduce cardiovascular events in patients post acute coronary syndrome, with an anticipated completion date of 2022.42
CONCLUSION
The role of HDL as a CVD biomarker continues to evolve. Instead of a linear inverse association with CVD, plasma HDL-C concentrations exhibit a U-shaped association with high risk at the extremes of plasma HDL-C concentrations. CETP inhibitors are the most potent enhancers of HDL-C levels but have largely failed to improve outcomes, suggesting that HDL-C is not a valid therapeutic target. Further, HDL-P may be a better marker of cardiovascular risk than HDL-C, and current evidence suggests that HDL-P is a promising therapeutic target. Finally, various interventions affecting HDL metabolism have positive and negative effects on cholesterol efflux capacity. A variety of diet interventions, vigorous exercise, and CETP inhibitors increase CEC, while statins decrease or blunt changes in CEC. Future studies may help determine if specifically targeting cholesterol efflux will decrease CVD risk and whether novel biomarkers reflecting HDL particle concentration, composition, and function may better predict CVD risk and response to therapy.
KEY POINTS
High-density lipoprotein cholesterol (HDL-C) appears to have a U-shaped association with increased cardiovascular risk at extreme levels.
HDL particle number may be a better marker of cardiovascular risk than HDL-C.
Interventions that increase cholesterol efflux capacity (CEC) include dietary interventions, vigorous exercise, and cholesteryl ester transfer protein inhibitors, whereas statins decrease or blunt CEC.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1.Dron JS, Wang J, Low-Kam C et al. Polygenic determinants in extremes of high-density lipoprotein cholesterol. J Lipid Res. 2017 Nov;58(11):2162–70. doi: 10.1194/jlr.M079822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017 Aug 21;38(32):2478–86. doi: 10.1093/eurheartj/ehx163. [DOI] [PubMed] [Google Scholar]
- 3.Hirata A, Sugiyama D, Watanabe M et alAssociation of extremely high levels of high-density lipoprotein cholesterol with cardiovascular mortality in a pooled analysis of 9 cohort studies including 43,407 individuals: The EPOCH-JAPAN study. J Clin Lipidol. 2018 May-Jun;12(3):674–84.e5. doi: 10.1016/j.jacl.2018.01.014. .; EPOCH-JAPAN Research Group. [DOI] [PubMed] [Google Scholar]
- 4.Wilkins JT, Ning H, Stone NJ et al. Coronary heart disease risks associated with high levels of HDL cholesterol. J Am Heart Assoc. 2014 Mar 13;3(2) doi: 10.1161/JAHA.113.000519. e000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed HM, Miller M, Nasir K et al. Primary Low Level of High-Density Lipoprotein Cholesterol and Risks of Coronary Heart Disease, Cardiovascular Disease, and Death: Results From the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2016 May 15;183(10):875–83. doi: 10.1093/aje/kwv305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz GG, Olsson AG, Abt M et alEffects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012 Nov 29;367(22):2089–99. doi: 10.1056/NEJMoa1206797. .; dal-OUTCOMES Investigators. [DOI] [PubMed] [Google Scholar]
- 7.Barter PJ, Caulfield M, Eriksson M et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007 Nov 22;357(21):2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 8.Lincoff AM, Nicholls SJ, Riesmeyer JS et alEvacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N Engl J Med. 2017 May 18;376(20):1933–42. doi: 10.1056/NEJMoa1609581. .; ACCELERATE Investigators. [DOI] [PubMed] [Google Scholar]
- 9.Bowman L, Hopewell JC, Chen F et alEffects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N Engl J Med. 2017 Sep 28;377(13):1217–27. doi: 10.1056/NEJMoa1706444. .; HPS3/TIMI55–REVEAL Collaborative Group. [DOI] [PubMed] [Google Scholar]
- 10.Ference BA, Kastelein JJP, Ginsberg HN et al. Association of Genetic Variants Related to CETP Inhibitors and Statins With Lipoprotein Levels and Cardiovascular Risk. JAMA. 2017 Sep 12;318(10):947–56. doi: 10.1001/jama.2017.11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenson RS, Davidson MH, Le NA, Burkle J, Pourfarzib R. Underappreciated opportunities for high-density lipoprotein particles in risk stratification and potential targets of therapy. Cardiovasc Drugs Ther. 2015 Feb;29(1):41–50. doi: 10.1007/s10557-014-6567-0. [DOI] [PubMed] [Google Scholar]
- 12.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013 Sep 10;128(11):1189–97. doi: 10.1161/CIRCULATIONAHA.113.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackey RH, Greenland P, Goff DC, Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2012 Aug 7;60(6):508–16. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khera AV, Demler OV, Adelman SJ et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events: An Analysis From the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) Circulation. 2017 Jun 20;135(25):2494–504. doi: 10.1161/CIRCULATIONAHA.116.025678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khine HW, Teiber JF, Haley RW, Khera A, Ayers CR, Rohatgi A. Association of the serum myeloperoxidase/high-density lipoprotein particle ratio and incident cardiovascular events in a multi-ethnic population: Observations from the Dallas Heart Study. Atherosclerosis. 2017 Aug;263:156–62. doi: 10.1016/j.atherosclerosis.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mani P, Ren HY, Neeland IJ et al. The association between HDL particle concentration and incident metabolic syndrome in the multi-ethnic Dallas Heart Study. Diabetes Metab Syndr. 2017 Nov;11(Suppl 1):S175–S179. doi: 10.1016/j.dsx.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khera AV, Cuchel M, de la Llera-Moya M et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011 Jan 13;364(2):127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohatgi A. High-Density Lipoprotein Function Measurement in Human Studies: Focus on Cholesterol Efflux Capacity. Prog Cardiovasc Dis. 2015 Jul-Aug;58(1):32–40. doi: 10.1016/j.pcad.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohatgi A, Khera A, Berry JD et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014 Dec 18;371(25):2383–93. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernáez Á, Castañer O, Elosua R et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals: A Randomized Controlled Trial. Circulation. 2017 Feb 14;135(7):633–43. doi: 10.1161/CIRCULATIONAHA.116.023712. [DOI] [PubMed] [Google Scholar]
- 21.Brassard D, Arsenault BJ, Boyer M et al. Saturated Fats from Butter but Not from Cheese Increase HDL-Mediated Cholesterol Efflux Capacity from J774 Macrophages in Men and Women with Abdominal Obesity. J Nutr. 2018 Apr 1;148(4):573–80. doi: 10.1093/jn/nxy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen CJ, Blesso CN, Lee J et al. Egg consumption modulates HDL lipid composition and increases the cholesterol-accepting capacity of serum in metabolic syndrome. Lipids. 2013 Jun;48(6):557–67. doi: 10.1007/s11745-013-3780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarzynski MA, Ruiz-Ramie JJ, Barber JL et al. Effects of Increasing Exercise Intensity and Dose on Multiple Measures of HDL (High-Density Lipoprotein) Function. Arterioscler Thromb Vasc Biol. 2018 Apr;38(4):943–52. doi: 10.1161/ATVBAHA.117.310307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyer M, Mitchell PL, Poirier DP et al. Impact of a one-year lifestyle modification program on cholesterol efflux capacities in men with abdominal obesity and dyslipidemia. Am J Physiol Endocrinol Metab. 2018 Oct 1;315(4):E460–E468. doi: 10.1152/ajpendo.00127.2018. [DOI] [PubMed] [Google Scholar]
- 25.Aicher BO, Haser EK, Freeman LA et al. Diet-induced weight loss in over-weight or obese women and changes in high-density lipoprotein levels and function. Obesity (Silver Spring) 2012 Oct;20(10):2057–62. doi: 10.1038/oby.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albaghdadi MS, Wang Z, Gao Y, Mutharasan RK, Wilkins J. High-Density Lipoprotein Subfractions and Cholesterol Efflux Capacity Are Not Affected by Supervised Exercise but Are Associated with Baseline Interleukin-6 in Patients with Peripheral Artery Disease. Front Cardiovasc Med. 2017 Mar 2;4:9. doi: 10.3389/fcvm.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koba S, Ayaori M, Uto-Kondo H et al. Beneficial Effects of Exercise-Based Cardiac Rehabilitation on High-Density Lipoprotein-Mediated Cholesterol Efflux Capacity in Patients with Acute Coronary Syndrome. J Atheroscler Thromb. 2016 Jul 1;23(7):865–77. doi: 10.5551/jat.34454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Capelleveen JC, Kastelein JJ, Zwinderman AH et al. Effects of the cholesteryl ester transfer protein inhibitor, TA-8995, on cholesterol efflux capacity and high-density lipoprotein particle subclasses. J Clin Lipidol. 2016 Sep-Oct;10(5):1137–1144.e3. doi: 10.1016/j.jacl.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Nicholls SJ, Ruotolo G, Brewer HB et al. Cholesterol Efflux Capacity and Pre-Beta-1 HDL Concentrations Are Increased in Dyslipidemic Patients Treated With Evacetrapib. J Am Coll Cardiol. 2015 Nov 17;66(20):2201–10. doi: 10.1016/j.jacc.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Tardif JC, Rhainds D, Brodeur M et al. Genotype-Dependent Effects of Dalcetrapib on Cholesterol Efflux and Inflammation: Concordance With Clinical Outcomes. Circ Cardiovasc Genet. 2016 Aug;9(4):340–8. doi: 10.1161/CIRCGENETICS.116.001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinical Trials [Internet] Bethesda, MD: U.S. National Library of Medicine; c2018. Effect of Dalcetrapib vs Placebo on CV Risk in a Genetically Defined Population With a Recent ACS (dal-GenE); 2018 Sep 26 [cited 2018 Oct 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT02525939. [Google Scholar]
- 32.Quinn AG, Schwemberger R, Stock EO et al. Moderate statin treatment reduces prebeta-1 high-density lipoprotein levels in dyslipidemic patients. J Clin Lipidol. 2017 Jul-Aug;11(4):908–14. doi: 10.1016/j.jacl.2017.04.118. [DOI] [PubMed] [Google Scholar]
- 33.Nenseter MS, Narverud I, Graesdal A et al. Cholesterol efflux mediators in homozygous familial hypercholesterolemia patients on low-density lipoprotein apheresis. J Clin Lipidol. 2013 Mar-Apr;7(2):109–16. doi: 10.1016/j.jacl.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Adorni MP, Zimetti F, Puntoni M et al. Cellular cholesterol efflux and cholesterol loading capacity of serum: effects of LDL-apheresis. J Lipid Res. 2012 May;53(5):984–9. doi: 10.1194/jlr.P024810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lappegård KT, Kjellmo CA, Ljunggren S et al. Lipoprotein apheresis affects lipoprotein particle subclasses more efficiently compared to the PCSK9 inhibitor evolocumab, a pilot study. Transfus Apher Sci. 2018 Feb;57(1):91–6. doi: 10.1016/j.transci.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Orsoni A, Villard EF, Bruckert E et al. Impact of LDL apheresis on atheroprotective reverse cholesterol transport pathway in familial hypercholesterolemia. J Lipid Res. 2012 Apr;53(4):767–75. doi: 10.1194/jlr.M024141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nissen SE, Tsunoda T, Tuzcu EM et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003 Nov 5;290(17):2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 38.Nicholls SJ, Puri R, Ballantyne CM et al. Effect of Infusion of High-Density Lipoprotein Mimetic Containing Recombinant Apolipoprotein A-I Milano on Coronary Disease in Patients With an Acute Coronary Syndrome in the MILANO-PILOT Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018 Jul 25; doi: 10.1001/jamacardio.2018.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholls SJ, Andrews J, Kastelein JJP et al. Effect of Serial Infusions of CER-001, a Pre-beta High-Density Lipoprotein Mimetic, on Coronary Atherosclerosis in Patients Following Acute Coronary Syndromes in the CER-001 Atherosclerosis Regression Acute Coronary Syndrome Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018 Jul 25; doi: 10.1001/jamacardio.2018.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clinical Trials [Internet] Bethesda, MD: U.S. National Library of Medicine; c2018. CER-001 Therapy as a Novel Approach to Treat Genetic Orphan Diseases (TANGO); 2017 Mar 20 [cited 2018 Oct 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT02697136. [Google Scholar]
- 41.Michael Gibson C, Korjian S, Tricoci P et al. Safety and Tolerability of CSL112, a Reconstituted, Infusible, Plasma-Derived Apolipoprotein A-I, After Acute Myocardial Infarction: The AEGIS-I Trial (ApoA-I Event Reducing in Ischemic Syndromes I) Circulation. 2016 Dec 13;134(24):1918–30. doi: 10.1161/CIRCULATIONAHA.116.025687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clinical Trials [Internet] Bethesda, MD: U.S. National Library of Medicine; c2018. Study to Investigate CSL112 in Subjects With Acute Coronary Syndrome (AEGIS-II); 2018 Mar 22 [cited 2018 Oct 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT0347322. [Google Scholar]


