Abstract
It is uncertain whether omega‐3 fatty acids are beneficial in statin‐treated patients. Epanova is a mix of omega‐3 free fatty acids, not requiring co‐ingestion with food, which can lower triglycerides by up to 31%. STRENGTH will examine whether Epanova 4 g daily reduces the rate of cardiovascular events in statin‐treated patients with hypertriglyceridemia and low levels of HDL‐C at high risk for developing cardiovascular events. STRENGTH is a randomized, double‐blind, placebo‐controlled trial. Patients had a triglyceride level ≥ 180 to <500 mg/dL and HDL‐C < 42 mg/dL (men) or < 47 mg/dL (women) in the presence of either (1) established atherosclerotic cardiovascular disease, (2) diabetes with one additional risk factor, or (3) were other high‐risk primary prevention patients, based on age and risk factor assessment. Patients should be treated with a statin, for >4 weeks, and have LDL‐C < 100 mg/dL, but were also eligible if LDL‐C was ≥100 mg/dL while on maximum tolerated statin therapy. The study will extend from October 30, 2014 to October 30, 2019. 13 086 patients were randomized to Epanova 4 g or placebo daily in addition to standard medical therapy. The primary efficacy outcome is time to first event of cardiovascular death, myocardial infarction, stroke, coronary revascularization or hospitalization for unstable angina. The trial will continue until 1600 patients reach the primary endpoint, with a median duration of therapy of 3 years. STRENGTH will determine whether Epanova 4 g daily will reduce cardiovascular events in statin‐treated high‐risk patients with hypertriglyceridemia and low HDL‐C levels.
Keywords: cardiovascular risk, clinical trials, non‐HDL cholesterol, omega‐3 fatty acids, triglycerides
1. INTRODUCTION
Randomized controlled trials consistently demonstrate that lowering levels of low‐density lipoprotein cholesterol (LDL‐C) with statins reduce cardiovascular events in high‐risk patients.1 Accordingly, statins have become a cornerstone in treatment guidelines for prevention of atherosclerotic cardiovascular events. Despite widespread statin use, many patients continue to experience cardiovascular events,2 emphasizing the need to identify additional therapeutic targets.
2. TRIGLYCERIDES AND NON‐HDL CHOLESTEROL AS CARDIOVASCULAR TARGETS
Evidence accumulated over many decades implicates triglyceride‐rich lipoproteins in the pathogenesis of atherosclerotic disease.3 Population studies report that triglyceride levels associate with cardiovascular risk.4 Post‐hoc analyses of statin trials demonstrate that on‐treatment triglyceride levels associate with cardiovascular event rates and progression of coronary atherosclerosis, regardless of achieved LDL‐C levels.5 Hypertriglyceridemia accompanies insulin resistance and associated risk factors including hypertension, hyperglycemia, hypercoagulable state and other features of atherogenic dyslipidemia (preponderance of small, dense LDL particles and low levels of HDL‐C), which may contribute to this heightened cardiovascular risk.6 Triglyceride‐rich lipoproteins may directly influence the artery wall. Genetic studies have reported that polymorphisms in factors regulating triglyceride levels,7 including loss of function of apolipoprotein C‐III8 and angiopoietin like protein (ANGPLT),9 associate with higher levels of triglyceride and cardiovascular risk. Preclinical studies have demonstrated that triglyceride‐rich lipoproteins stimulate inflammatory, oxidative, and thrombotic factors implicated in atherosclerosis.10 This is evidenced by animal studies in which genetic manipulation resulting in higher triglyceride levels results in accelerated atherosclerosis.11
Increasing attention has focused on the ability to measure and target the full atherogenic lipoprotein burden. While evidence implicates triglyceride‐rich lipoproteins in atherosclerosis formation and progression, their atherogenicity may result from their ability to deliver cholesterol to the artery wall. Calculation of non‐HDL‐C permits determination of the cholesterol content of all lipoproteins considered to promote atherosclerosis. Reports from population, genetic and clinical trials confirm the association between non‐HDL‐C and cardiovascular risk and a direct relationship between non‐HDL‐C lowering and cardiovascular benefit.12 Given its ability to identify some of the residual risk in statin‐treated patients, non‐HDL‐C has become increasingly integrated into treatment guidelines as both a treatment target and to identify patients who require more aggressive lipid lowering.13 However, studies have demonstrated that only 4% of patients with triglyceride levels greater than 200 mg/dL and at very high risk had an optimal non‐HDL‐C level < 100 mg/dL.14 Since statins produce limited triglyceride lowering, it is likely that many patients will require additional lipid modifying agents to achieve their non‐HDL‐C goal.
3. CLINICAL TRIALS TARGETING TRIGLYCERIDE‐RICH LIPOPROTEINS
While clinical trials of LDL‐C lowering agents have consistently produced clinical benefit, agents targeting additional lipid parameters have produced variable results. Fibrates are pharmacological agonists of peroxisome proliferator activated receptor (PPAR‐α) that lower triglycerides by up to 25%, modestly raise HDL‐C and reduce small, dense LDL particle levels.15 Cardiovascular outcomes trials of these agents have shown mixed results with evidence of benefit with gemfibrozil,16, 17 but not other agents.18, 19 While triglyceride lowering was not associated with the clinical benefit observed with gemfibrozil, subgroup analyses of all fibrate trials have demonstrated clinical benefit in the subsets of patients with hypertriglyceridemia and low HDL‐C levels at baseline.20 These observations suggest that such patients have potentially modifiable risk and are more likely to benefit from targeted additional therapy. While PPAR‐γ agonists have primarily been used to treat hyperglycemia by improving insulin sensitivity in patients with type 2 diabetes mellitus, these agents similarly demonstrate modest triglyceride lowering, with evidence that lowering the triglyceride/HDL‐C ratio with pioglitazone is independently associated with its ability to slow progression of coronary atherosclerosis.21
Attention has focused on the development of novel PPAR agonists, in an attempt to deliver more potent receptor agonism or activate multiple receptor subclasses. This has proven to be disappointing, due to either evidence of toxicity or clinical futility with PPAR‐γ agonists, novel potent PPAR‐α agents, and dual PPAR‐α/γ agonists in large outcomes trials.22 An ongoing trial of the PPAR‐α agonist, pemafibrate, is evaluating the impact on cardiovascular events in high‐risk patients with concomitant hypertriglyceridemia.23 This study has drawn on prior observations of potentially greater modification of cardiovascular risk in patients with evidence of atherogenic dyslipidemia at baseline. This represents the ability to identify a patient subgroup, which may be more likely to derive clinical benefit from therapy targeting this lipid phenotype. (Figure 1)
Figure 1.
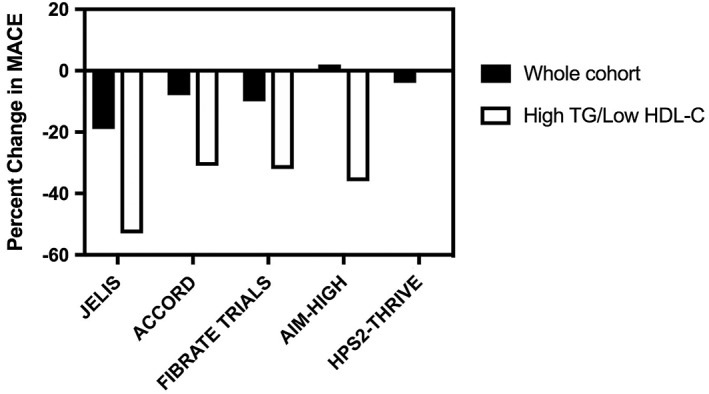
Percentage change in major adverse cardiovascular events (MACE) with therapeutic interventions in both the whole study cohort and patient subgroups with hypertriglyceridemia and low levels of high‐density lipoprotein cholesterol (HDL‐C) at baseline
Niacin is a longstanding lipid modifying strategy with a multitude of effects including lowering levels of triglycerides, LDL‐C, and lipoprotein (a), in addition to raising HDL‐C. While early trials, prior to the statin era, demonstrated clinical benefit with immediate release formulations,24 niacin has proven to be challenging to use in clinical practice due to adverse events and a lack of contemporary evidence of benefit in statin‐treated patients.25 Two large trials of niacin, delivered in either an extended release formulation or in combination with a prostanoid receptor antagonist, both delivery methods intended to improve tolerance, failed to reduce cardiovascular events in statin‐treated patients.26, 27
4. OMEGA‐3 FATTY ACIDS
Considerable evidence has supported a potential role for the polyunsaturated omega‐3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), in protection from cardiovascular disease. Population studies have reported that greater dietary consumption of either fatty fish28 or omega‐3 fatty acids29 are associated with lower rates of cardiovascular events. This observation is further supported by reports that red blood cell levels of EPA and DHA inversely correlate with cardiovascular risk.30 The Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI)‐Prevenzione study provided some data suggesting that administration of low dose of prescription omega‐3 fatty acids was associated with cardiovascular benefit, in the era prior to widespread use of statins.31 In addition to lowering triglyceride and non‐HDL‐C levels, potential cardioprotective effects of omega‐3 fatty acids may derive from favorable effects on inflammation, oxidation, thrombosis, endothelial function, and cardiac arrhythmias.32 A large number of studies have been performed to evaluate the impact of omega‐3 fatty acids on a range of surrogate and clinical cardiovascular measures. These studies have failed to demonstrate consistent evidence of benefit, a conclusion confirmed by recent meta‐analyses of these investigations.33 However, many of these trials involved empirical administration of low doses (eg, 1 g daily) of omega‐3 fatty acids using formulations with variable bioavailability and no real effect on triglycerides, tested in patients regardless of baseline triglyceride levels, and with no evidence that tissue omega‐3 fatty acid content was modified. Average baseline triglyceride levels across these trials were usually around the upper limit of the normal range, and only modestly reduced by treatment. Higher doses of omega‐3 fatty acids have greater lipid effects, whether this translates to an impact on cardiovascular events remains uncertain. Accordingly, the optimal dose and patient subgroup most likely to derive clinical benefit from omega‐3 fatty acids remain uncertain.
Recent cardiovascular outcomes trials have evaluated the impact of omega‐3 fatty acids in the setting of contemporary therapy for high‐risk patients. Results from the Japan Eicosapentanoic acid Lipid Intervention Study (JELIS) showed that administration of EPA 1.8 g/day resulted in a 19% reduction in cardiovascular events in statin‐treated patients. This benefit was observed in patients with relatively normal triglyceride levels. However, the subgroup of patients with baseline triglyceride levels greater than 150 mg/dL and HDL‐C < 40 mg/dL achieved a 53% reduction in cardiovascular events with EPA.34 Subsequent investigation demonstrated lower cardiovascular event rates in those patients with higher plasma EPA concentrations.35
A number of ongoing studies are attempting to evaluate the impact of omega‐3 fatty acids on cardiovascular events. The Vitamin D and Omega‐3 Trial (VITAL) is evaluating the impact of omega‐3 fatty acids on cardiovascular events and cancer in a general population of more than 25 000 individuals,36 while A Study of Cardiovascular Events in Diabetes (ASCEND) is comparing administration of omega‐3 fatty acids or placebo in patients with diabetes without clinically manifest cardiovascular disease.37 Both of these studies involve administration of a relatively low dose (1 g per day) of omega‐3 fatty acids. Icosapent ethyl is a purified ethyl ester of EPA, shown to have favorable effects on triglycerides and associated lipid parameters. The Reduction of Cardiovascular Events with Icosapent Ethyl‐Intervention Trial (REDUCE‐IT) is currently investigating the impact of icosapent 4 g/day compared with mineral oil control on cardiovascular events in high risk, statin‐treated patients with triglyceride levels between 200 and < 500 mg/dL.38
5. EPANOVA
Epanova is a formulation of omega‐3 fatty acid that has undergone an additional manufacturing step to hydrolyze and distill the ethyl esters into omega‐3 free fatty acids (omega‐3 carboxylic acids) with a final concentration of 75% EPA and DHA. Given the free fatty acid formulation, Epanova does not require hydrolysis by pancreatic lipase that conventional omega‐3 ethyl ester do to maximize intestinal absorption. This greater bioavailability is supported by a lack of dependency on meal fat content. Accordingly, studies of this agent have the opportunity to provide a major change from prior studies of omega‐3 fatty acid preparations in patients at high risk of cardiovascular events. This may be particularly important in patients with severe hypertriglyceridemia, in whom fat intake restriction is often employed, with potential implications for absorption of omega‐3 ethyl ester formulations. The ability to administer Epanova with greater bioavailability than many prior omega‐3 formulations has the potential to result in higher plasma EPA/DHA concentrations.
Open label administration of Epanova increased trough plasma EPA levels by 351% compared with baseline. The Epanova for Lowering Very High Triglycerides (EVOLVE) study compared the effects of treatment with Epanova 2‐4 g/day with olive oil control for 12 weeks in patients with triglyceride levels between 500 and 2000 mg/dL. Plasma levels of EPA increased by 267% to 406% and DHA by 57% to 72% with Epanova and were associated with lowering of triglycerides by 26% to 31% and non‐HDL‐C by 7.6% to 9.6%.39 Epanova combined with a Statin in Patients with Hypertriglyceridemia to Reduce Non‐HDL Cholesterol (ESPRIT) evaluated the impact of Epanova 2 to 4 g/day compared with olive oil for 6 weeks in high cardiovascular risk patients with baseline fasting triglyceride levels between 200 and 500 mg/dL. In this study, Epanova reduced triglyceride levels by 15% to 21% and non‐HDL‐C by up to 6.9%.40 Any increase in LDL‐C with omega‐3 fatty acids is modest and not thought to adversely impact cardiovascular risk, particularly in the setting of triglyceride and non‐HDL‐C lowering. These trials demonstrated that Epanova administration was well tolerated without any observed concerning side effects, with the most common adverse event being higher incidences of diarrhea, nausea and eructation.
6. STRENGTH TRIAL OBJECTIVES AND DESIGN
A Long‐Term Outcomes Study to Assess Statin Residual Risk Reduction with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia (STRENGTH: clinicaltrials.gov NCT02104817) tests the hypothesis that administration of EPA/DHA at a higher dose than typically employed in clinical trials in addition to conventional medical therapy affects risk for cardiovascular events in patients with high vascular risk (Figure 2). This is a randomized, double‐blind, placebo‐controlled, event‐driven trial. Patients are treated with Epanova 4 g or matching corn oil placebo capsules once daily. Corn oil, rather than liquid paraffin or mineral oil, was chosen due to a lower incidence of gastrointestinal side effects, and to provide appropriate caloric control. Corn oil may slightly modify lipid levels, including small reductions in non‐HDL‐C.41, 42 The trial will continue until 1600 primary endpoints are positively adjudicated. The expected median duration of the trial is 3 years with a maximum duration of 5 years.
Figure 2.
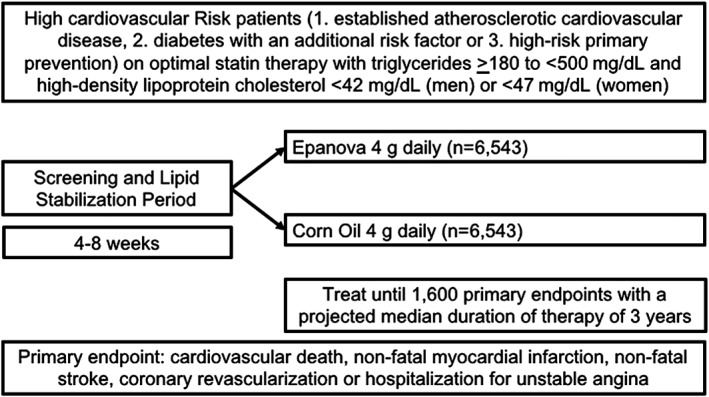
Study design features of the STRENGTH trial
7. STUDY ORGANIZATION
The trial is funded by AstraZeneca Pharmaceuticals AB (Sodertalje, Sweden) and coordinated by the Cleveland Clinic Coordinating Center for Clinical Research (C5 Research, Cleveland, OH). IQVIA (Durham, NC) serves as the contract research organization and provides data and site management. Academic members of the Executive Committee designed the trial in collaboration with the sponsor. The Steering Committee, consisting of the Executive Steering Committee and physician National Coordinators from each country, is responsible for scientific and medical conduct of the study and presentation and publication of the trial results. A copy of the complete final locked database will be transferred to the Executive Steering Committee for independent statistical analyses of study results for the purpose of scientific presentation and publication. An independent Data Monitoring Committee (DMC), supported by an independent data analysis center at Statistics Collaborative, Inc. (Washington DC) monitors the safety of the study and has access to unblinded data. The appropriate national and institutional regulatory and ethical boards approved the protocol, and all patients provided written informed consent. The authors are responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
8. PATIENT POPULATION
A total of 13 086 patients have been enrolled at 675 sites in 22 countries. The study population (Tables 1 and 2) consists of patients ≥18 years of age on a stable diet and treated with a statin for at least 4 weeks prior to randomization with evidence of:
LDL‐C < 100 mg/dL, but subjects were also eligible if LDL‐C was ≥100 mg/dL if they were being treated with high‐intensity or maximally tolerated statin doses, with or without ezetimibe.
Atherogenic dyslipidemia, defined as triglyceride levels ≥180 mg/dL and <500 mg/dL and HDL‐C <42 mg/dL for men or <47 mg/dL for women, measured after an overnight fast.
- Considered to be at high risk for a future cardiovascular event, defined by the presence of:
- Established atherosclerotic cardiovascular disease involving the coronary, peripheral, carotid, or aortic vascular territories (secondary prevention).
- Type 1 or 2 diabetes mellitus with age ≥40 years for men and ≥50 years for women with at least one of the additional following risk factors: chronic cigarette smoking, hypertension, high C‐reactive protein (hs‐CRP) ≥ 2 mg/L, or micro‐albuminuria (urinary albumin: creatinine ratio > 30 mg/g).
- High‐risk primary prevention defined as age > 50 years for men >60 years for women with at least one of the additional following risk factors: family history of premature CAD, chronic cigarette smoking, hs‐CRP >2.0 mg/L, impaired renal function, or coronary calcium score > 300 Agatston units.
Table 1.
STRENGTH inclusion criteria
|
Table 2.
STRENGTH exclusion criteria
|
At least 50% of randomized patients were required to satisfy criteria for secondary prevention. Patients were permitted to undergo rescreening of lipid parameters on two occasions to evaluate the impact of adjustment of lipid modifying therapy or to discontinue excluded agents. Once patients met all inclusion criteria and no exclusion criteria and agreed to volunteer for trial participation, they were randomized to study treatment within 14 days. The study was executed in accordance with the Declaration of Helsinki, all patients provided written informed consent and the protocol was approved by ethics or institutional review boards for each site.
9. TREATMENT REGIMEN AND FOLLOW‐UP
Patients were randomized to treatment using an interactive voice response system to treatment with Epanova 4 g orally once daily or matching placebo, in a 1:1 fashion, in addition to background therapy that adhered to national treatment guidelines for patients at high risk of a cardiovascular event. Given the free fatty acid formulation, Epanova does not require stringent co‐administration with food, unlike prior studies of alternative fish oil preparations. Following dispensing of study drug, patients were to be seen at clinic visits at 3, 6, and 12 months following randomization and then every 6 months thereafter. A safety visit will be performed approximately 3 weeks after the last dose of study medication. Additional telephone calls to patients will be made on a 3‐month basis commencing at month 9, in order to query about adverse events, clinical endpoints, changes in medications and any issues with study drug.
In addition to evaluation of patients for the incidence of cardiovascular events and adverse events, blood is collected at baseline, during the treatment period and at the end of treatment for assessment of lipids, biochemistry, hs‐CRP, glycemic control, and fatty acid content within both plasma and red blood cells. Blood samples are also collected on a limited number of patients at sites within the United States for examination of exploratory lipid (apoB, apoC‐III) and inflammatory biomarkers, in addition to genetic analyses. Patients who declined to participate in the genetic substudy were not prevented from participation in the overall trial.
While temporary discontinuation of study drug is permitted, every effort is made to recommence therapy as soon as possible. If patients discontinue study drug permanently, they continue to be followed in the trial for all efficacy and safety evaluations, where possible, given the intent to treat design of the study. For patients who are either unable or unwilling to return to the study site for follow‐up, every effort will be undertaken to use alternative approaches to review, including telephone calls or medical record review, in order to promote retention of patients in the study.
10. ENDPOINTS
An independent clinical events committee, blinded to treatment allocation, will adjudicate all reported clinical events. The primary efficacy measure is the time to first occurrence of any component of the composite of cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, coronary revascularization, and hospitalization for unstable angina. Death is defined as cardiovascular unless there is a documented identifiable non‐vascular cause. Myocardial infarction includes types 1 to 5 of the Universal Definition. Stroke is defined as an acute episode of neurological dysfunction caused by vascular injury lasting longer than 24 hours. Elective and emergent coronary revascularization includes both percutaneous and surgical procedures. Hospitalization for unstable angina is defined by symptoms of myocardial ischemia at rest or with increasing frequency without evidence of acute myocardial infarction, but with evidence of either myocardial ischemia on non‐invasive imaging or the presence of severe stenotic disease or thrombus on coronary angiography that is considered to underpin the clinical presentation.
Key secondary endpoints, tested hierarchically, include (i) cardiovascular events (cardiovascular death, non‐fatal myocardial infarction, and non‐fatal stroke), (ii) coronary events (cardiac death, non‐fatal myocardial infarction, coronary revascularization, and hospitalization for unstable angina), and (iii) time to cardiovascular death. Other secondary endpoints include each of the individual components of the primary endpoint and all‐cause death. Tertiary endpoints include (1) new onset atrial fibrillation, (2) total thrombotic events (coronary stent thrombosis and any systemic thromboembolism), and (3) new onset heart failure events. Changes in lipid parameters and standard safety assessments will be performed. There are currently no planned substudies.
11. STATISTICAL CONSIDERATIONS
STRENGTH is an event‐driven clinical trial. The targeted minimum number of primary endpoints is 1600, with patients treated for a projected median of 3 years. With a total of 13 000 patients randomized to Epanova or corn oil placebo, the study has 90% power to detect a 15% or greater relative reduction in hazard for the primary endpoint using log‐ranked test at a two‐sided alpha of 0.05. An annual incidence of the primary composite outcome of 4% per year is assumed, leading to an assumed total study duration of 4.5 years. The enrolment of patients with clinically manifest atherosclerotic cardiovascular disease was specified to comprise at least 50% of the entire study cohort. Sequential efficacy and futility assessments by the unblinded DMC will be performed following positive adjudication of approximately 50% and 75% of the targeted composite primary endpoint events. A group‐sequential design will be used to preserve the overall type 1 error probability of 0.05.
The efficacy analysis data set will be based on the intent‐to‐treat population including all randomized patients. The safety analysis set will include all randomized patients who have received at least one dose of study drug. Patients lost to follow‐up will be censored at the time that they are last known to be event free and those who withdraw consent will be censored at the time of withdrawal. For the primary endpoint, the test of the null hypothesis will be based on the log‐rank test. Contingent on Epanova favorably reducing the primary endpoint, key secondary endpoints will be evaluated in a hierarchical fashion.
12. TRIAL PROGRESS
Enrolment started in October 2014 and was completed on July 12, 2017 when 13 086 patients had been randomized. The DMC has reviewed patient safety and trial conduct during seven meetings through March 2018 and recommended continuation of the trial without modification after each of these reviews.
13. SUMMARY
Greater triglyceride and non‐HDL‐C levels identify statin‐treated patients at higher residual cardiovascular risk. While omega‐3 fatty acids are of interest as a potential preventive therapy, most studies have used suboptimal doses and formulations with variable bioavailability for non‐HDL‐C reduction and failed to evaluate their impact in patients who are most likely to derive potential benefit. STRENGTH will permit the opportunity to determine the effects of administration of Epanova 4 g daily, a much higher dose than evaluated in many studies and with a preparation that does not require co‐administration with food, on atherosclerotic cardiovascular risk in patients with atherogenic dyslipidemia, considered to identify patients with high modifiable risk.
Conflict of interest
Stephen J. Nicholls has received research support: AstraZeneca, Amgen, Anthera, Eli Lilly, Esperion, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi‐Regeneron, and LipoScience and is a consultant for AstraZeneca, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi‐Regeneron, CSL Behring, Esperion, Boehringer Ingelheim. A. Michael Lincoff reports receiving grant support, through a research contract with his institution, from Eli Lilly, AstraZeneca, Roche, CSL Behring, Esperion, and AbbVie. Christie M. Ballantyne received grant/research support (all significant [>$10 000] and paid to institution, not individual) from: Abbott Diagnostic, Amarin, Amgen Inc, Esperion, Ionis, Novartis, Pfizer, Regeneron, Roche Diagnostic, Sanofi‐Synthelabo, NIH, AHA, ADA; and served as a consultant (significant [>$10 000] denoted by *) for: Abbott Diagnostics, Amarin, Amgen Inc, AstraZeneca*, Boehringer Ingelheim*, Eli Lilly, Esperion, Ionis, Matinas BioPharma Inc, Merck*, Novartis, Pfizer*, Regeneron, Roche Diagnostic, Sanofi‐Synthelabo*. Philip J. Barter reports Research Grants received from Merck, Pfizer, honoraria received from Amgen, AstraZeneca, Merck, Pfizer, Sanofi‐Regeneron and is a member of Advisory Boards: Amgen, Merck, Pfizer, Sanofi‐Regeneron. Michael H. Davidson has received consultant and advisory board fees and honoraria and has participated in speaker's bureaus for AstraZeneca, Sanofi, Regeneron, and Amgen. He reports holding equity in Omthera Pharmaceuticals. John J. P. Kastelein received personal consulting fees from: Sanofi, Affiris, Akarna Therapeutics, Amgen Inc, CSL Behring, Regeneron, Staten Biotech, Madrigal, The Medicines Company, Kowa, Lilly, Esperion, Gemphire, Ionis Pharmaceuticals, Akcea Pharmaceuticals. Wolfgang Koenig reports modest consultation fees for advisory board meetings from Novartis, Pfizer, Kowa, Amgen, and AstraZeneca and modest personal fees for lectures from Novartis, Sanofi, and Amgen. Darren K. McGuire reports Clinical trial leadership: AstraZeneca, Sanofi Aventis, Janssen, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Lexicon, Eisai, GlaxoSmithKline, Esperion; Consultancy: AstraZeneca, Sanofi Aventis, Lilly US, Boehringer Ingelheim, Merck & Co, Pfizer, Novo Nordisk and Metavant. Dariush Mozaffarian reports personal fees from Acasti Pharma, AstraZeneca, GOED, DSM, Nutrition Impact, Pollock Communications, Bunge, and Indigo Agriculture; royalties from UpToDate for online medical chapters; scientific advisory board, Omada Health and Elysium Health; and research funding from the National Institutes of Health and the Gates Foundation; all outside the present work. Terje R. Pedersen reports grants and personal fees from Merck, Pfizer, and Amgen. Paul M. Ridke has received research grant support from Kowa, Novartis, and Pfizer and served as a consultant to AstraZeneca during the course of this trial. Kausik Ray reports grants and/or personal fees from Pfizer, MSD, AstraZeneca, Sanofi, Aegerion, Regeneron, Abbvie, Kowa, Cerenis, Medicines Company, Lilly, Esperion, Amgen, Cipla, Algorithm, Takeda, Boehringer Ingelheim, and Novo Nordisk within the last 12 months outside of the submitted work. Björn W. Karlson and Torbjörn Lundström are employees of AstraZeneca Pharmaceuticals. Steven E. Nissen reports that the Cleveland Clinic Center for Clinical Research has received funding to perform clinical trials from Abbvie, AstraZeneca, Amgen Inc, Cerenis, Eli Lilly, Esperion, Pfizer, The Medicines Company, Takeda, and Orexigen. Dr. Nissen is involved in these clinical trials, but receives no personal remuneration for his participation. Dr. Nissen consults for many pharmaceutical companies, but requires them to donate all honoraria or consulting fees directly to charity so that he receives neither income nor a tax deduction. Dianna Bash and Kathy Wolski have no industry relationships to disclose.
Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega‐3 carboxylic acids in statin‐treated patients with high levels of triglycerides and low levels of high‐density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41:1281–1288. 10.1002/clc.23055
Funding information AstraZeneca Pharmaceuticals
REFERENCES
- 1. Cholesterol Treatment Trialists , Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46:1225‐1228. [DOI] [PubMed] [Google Scholar]
- 3. Triglyceride Coronary Disease Genetics , Emerging Risk Factors , Sarwar N, et al. Triglyceride‐mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118:2047‐2056. [DOI] [PubMed] [Google Scholar]
- 5. Puri R, Nissen SE, Shao M, et al. Non‐HDL cholesterol and triglycerides: implications for coronary atheroma progression and clinical events. Arterioscler Thromb Vasc Biol. 2016;36:2220‐2228. [DOI] [PubMed] [Google Scholar]
- 6. Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495‐506. [DOI] [PubMed] [Google Scholar]
- 7. Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hdl Working Group of the Exome Sequencing Project NHL , Blood I, et al. Loss‐of‐function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stitziel NO, Khera AV, Wang X, et al. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol. 2017;69:2054‐2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626‐635. [DOI] [PubMed] [Google Scholar]
- 11. Mansouri RM, Bauge E, Gervois P, et al. Atheroprotective effect of human apolipoprotein A5 in a mouse model of mixed dyslipidemia. Circ Res. 2008;103:450‐453. [DOI] [PubMed] [Google Scholar]
- 12. Boekholdt SM, Arsenault BJ, Mora S, et al. Association of LDL cholesterol, non‐HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta‐analysis. JAMA. 2012;307:1302‐1309. [DOI] [PubMed] [Google Scholar]
- 13. Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient‐centered management of dyslipidemia: part 1‐‐full report. J Clin Lipidol. 2015;9:129‐169. [DOI] [PubMed] [Google Scholar]
- 14. Davidson MH, Maki KC, Pearson TA, et al. Results of the National Cholesterol Education (NCEP) program evaluation ProjecT utilizing novel E‐technology (NEPTUNE) II survey and implications for treatment under the recent NCEP writing group recommendations. Am J Cardiol. 2005;96:556‐563. [DOI] [PubMed] [Google Scholar]
- 15. Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088‐2093. [DOI] [PubMed] [Google Scholar]
- 16. Bloomfield Rubins H, Davenport J, Babikian V, et al. Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: the veterans affairs HDL intervention trial (VA‐HIT). Circulation. 2001;103:2828‐2833. [DOI] [PubMed] [Google Scholar]
- 17. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high‐density lipoprotein cholesterol. Veterans affairs high‐density lipoprotein cholesterol intervention trial study group. N Engl J Med. 1999;341:410‐418. [DOI] [PubMed] [Google Scholar]
- 18. Effect of fenofibrate on progression of coronary‐artery disease in type 2 diabetes: the diabetes atherosclerosis intervention study, a randomised study. Lancet. 2001;357:905‐910. [PubMed] [Google Scholar]
- 19. Keech A, Simes RJ, Barter P, et al. Effects of long‐term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849‐1861. [DOI] [PubMed] [Google Scholar]
- 20. Lee M, Saver JL, Towfighi A, Chow J, Ovbiagele B. Efficacy of fibrates for cardiovascular risk reduction in persons with atherogenic dyslipidemia: a meta‐analysis. Atherosclerosis. 2011;217:492‐498. [DOI] [PubMed] [Google Scholar]
- 21. Goldberg RB, Kendall DM, Deeg MA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 22. Nicholls SJ, Uno K. Peroxisome proliferator‐activated receptor (PPAR alpha/gamma) agonists as a potential target to reduce cardiovascular risk in diabetes. Diab Vasc Dis Res. 2012;9:89‐94. [DOI] [PubMed] [Google Scholar]
- 23. Ferri N, Corsini A, Sirtori C, Ruscica M. PPAR‐alpha agonists are still on the rise: an update on clinical and experimental findings. Expert Opin Investig Drugs. 2017;26:593‐602. [DOI] [PubMed] [Google Scholar]
- 24. Clofibrate and niacin in coronary heart disease. JAMA. 1975;231:360‐381. [PubMed] [Google Scholar]
- 25. Superko HR, Zhao XQ, Hodis HN, Guyton JR. Niacin and heart disease prevention: engraving its tombstone is a mistake. J Clin Lipidol. 2017;11:1309‐1317. [DOI] [PubMed] [Google Scholar]
- 26. Group HTC , Landray MJ, Haynes R, et al. Effects of extended‐release niacin with laropiprant in high‐risk patients. N Engl J Med. 2014;371:203‐212. [DOI] [PubMed] [Google Scholar]
- 27. Investigators A‐H , Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255‐2267. [DOI] [PubMed] [Google Scholar]
- 28. Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33:2657‐2661. [DOI] [PubMed] [Google Scholar]
- 29. Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med. 1992;200:177‐182. [DOI] [PubMed] [Google Scholar]
- 30. Harris WS, Von Schacky C. The Omega‐3 index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212‐220. [DOI] [PubMed] [Google Scholar]
- 31. Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI‐Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447‐455. [PubMed] [Google Scholar]
- 32. Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega‐3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585‐594. [DOI] [PubMed] [Google Scholar]
- 33. Aung T, Halsey J, Kromhout D, et al. Associations of Omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 2018;3:225‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saito Y, Yokoyama M, Origasa H, et al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub‐analysis of primary prevention cases from the Japan EPA lipid intervention study (JELIS). Atherosclerosis. 2008;200:135‐140. [DOI] [PubMed] [Google Scholar]
- 35. Itakura H, Yokoyama M, Matsuzaki M, et al. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. 2011;18:99‐107. [DOI] [PubMed] [Google Scholar]
- 36. Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the VITamin D and OmegA‐3 TriaL (VITAL). Contemp Clin Trials. 2016;47:235‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bowman L, Mafham M, Stevens W, et al. ASCEND: a study of cardiovascular events iN diabetes: characteristics of a randomized trial of aspirin and of omega‐3 fatty acid supplementation in 15,480 people with diabetes. Am Heart J. 2018;198:135‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhatt DL, Steg PG, Brinton EA, et al. Rationale and design of REDUCE‐IT: reduction of cardiovascular events with Icosapent ethyl‐intervention trial. Clin Cardiol. 2017;40:138‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kastelein JJ, Maki KC, Susekov A, et al. Omega‐3 free fatty acids for the treatment of severe hypertriglyceridemia: the EpanoVa fOr lowering very high triglyceridEs (EVOLVE) trial. J Clin Lipidol. 2014;8:94‐106. [DOI] [PubMed] [Google Scholar]
- 40. Maki KC, Orloff DG, Nicholls SJ, et al. A highly bioavailable omega‐3 free fatty acid formulation improves the cardiovascular risk profile in high‐risk, statin‐treated patients with residual hypertriglyceridemia (the ESPRIT trial). Clin Ther. 2013;35:1400–11 e1‐1400–11 e3. [DOI] [PubMed] [Google Scholar]
- 41. Davidson MH, Stein EA, Bays HE, et al. Efficacy and tolerability of adding prescription omega‐3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8‐week, randomized, double‐blind, placebo‐controlled study. Clin Ther. 2007;29:1354‐1367. [DOI] [PubMed] [Google Scholar]
- 42. Imamura F, Micha R, Wu JH, et al. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose‐insulin homeostasis: a systematic review and meta‐analysis of randomised controlled feeding trials. PLoS Med. 2016;13:e1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]


