Abstract
Understanding the neural substrates of depression is crucial for diagnosis and treatment. Here, we review recent studies of functional and effective connectivity in depression, in terms of functional integration in the brain. Findings from these studies, including our own, point to the involvement of at least four networks in patients with depression. Elevated connectivity of a ventral limbic affective network appears to be associated with excessive negative mood (dysphoria) in the patients; decreased connectivity of a frontal‐striatal reward network has been suggested to account for loss of interest, motivation, and pleasure (anhedonia); enhanced default mode network connectivity seems to be associated with depressive rumination; and diminished connectivity of a dorsal cognitive control network is thought to underlie cognitive deficits especially ineffective top‐down control of negative thoughts and emotions in depressed patients. Moreover, the restoration of connectivity of these networks—and corresponding symptom improvement—following antidepressant treatment (including medication, psychotherapy, and brain stimulation techniques) serves as evidence for the crucial role of these networks in the pathophysiology of depression.
Keywords: affective network, cognitive control network, default mode network, depression, reward network
1. INTRODUCTION
Depression is one of the most common psychiatric disorders, with a lifetime prevalence of up to 20% and 30% in men and women, respectively.1 A key step toward developing effective diagnosis and intervention techniques is to uncover the neural substrates of this disorder. For example, which brain systems are associated with affective and cognitive dysfunction in depression? How do distributed regions interact to produce the symptoms of depression? What is the neural mechanism underlying remission following antidepressant treatment? Why is the relapse rate so high in remitted depressed patients? Advances in neuroimaging techniques and brain connectivity analysis are now making it possible to address these questions, thereby tackling one of the greatest mysteries of the human mind.
A growing literature supports the notion that the symptoms of depression are associated with widespread network dysconnectivity rather than the aberrant responses of individual brain regions. Here, we review recent advances in functional magnetic resonance imaging (fMRI) studies that have tried to elucidate the neurobiological underpinnings of depression, from the perspective of functional integration. Depression—frequently seen as withdrawal from the prosocial environment—is characterized by aberrant emotional and affective processing, excessive self‐focus, and diminished cognitive control. To this end, we pay special attention to four core networks that have been implicated in these processes: the affective network (AN), reward network (RN), default mode network (DMN), and cognitive control network (CCN), respectively. First, we briefly summarize brain connectivity analysis methods. Detailed descriptions of the different methods we refer to can be found in Reference 2, 3, 4, 5, 6, 7, 8, 9. We then review findings from recent fMRI studies that have investigated abnormalities in brain connectivity in depression. This is followed by a short discussion on how brain connectivity studies can help with the treatment of the disease. Finally, we suggest that future studies should elucidate the structural and metabolic substrates of depression‐related dysconnectivity and try to develop an extended model of depression for improved diagnosis, treatment, and prevention of the disorder.
2. A BRIEF SUMMARY OF BRAIN CONNECTIVITY ANALYSIS METHODS
Characterizations of brain connectivity include structural connectivity, functional connectivity, and effective connectivity. For the most part, structural connectivity analysis relies on techniques such as diffusion magnetic resonance imaging (dMRI) and tractography, which report the integrity of white matter fiber tracts. The remaining distinction between functional and effective connectivity is important to understand.2, 3, 4 The former refers to (undirected) correlations between the activity of two brain regions, while the latter refers to (directed and usually reciprocal) causal influences among brain regions within a network (Figure 1).
Figure 1.
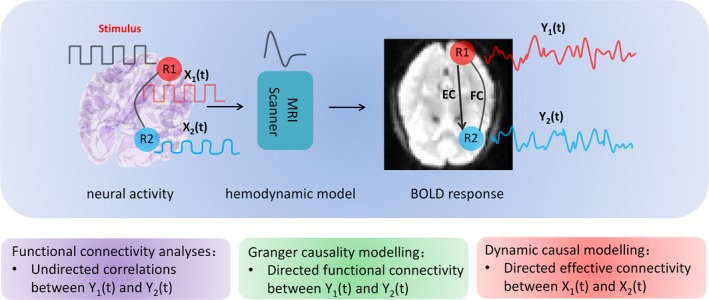
Characterization of different approaches to examine brain connectivity. Experimental inputs usually enter into sensory cortex and cause changes in neuronal activity X1 in the region (R1). Activity in R1 will then be propagated to a second region R2 which is connected to R1 and causes changes in X2. The neuronal activity X1 and X2 are hidden neuronal states because they cannot be observed directly using fMRI. Instead, the BOLD signals recorded in fMRI images are a convolution of the neuronal states with a hemodynamic function. Functional connectivity analyses simply measure the undirected temporal correlations (or statistical dependencies) among observed BOLD signals of different brain regions. Granger causality modeling (GCM) tries to infer directed connectivity using autoregressive models. Strictly speaking, GCM measures directed functional connectivity because it operates on observed hemodynamic (BOLD) responses. In contrast, dynamic causal modeling (DCM) estimates the influence that the neural activity of one brain region exerts on another. FC: functional connectivity; EC: effective connectivity
Specifically, functional connectivity corresponds to the temporal correlations (or statistical dependencies) between the activity of different brain regions.3, 4 It is a simple characterization of brain connectivity and can be measured directly from fMRI data using different methods. The easiest way to measure functional connectivity is to use a seed‐based method. Usually, one extracts the mean time series of a region of interest (ROI) and computes the correlation between the time series of the ROI and all other voxels (or regions) in the brain. The ensuing (thresholded) correlation map represents functional connectivity between the ROI and all other voxels (or regions). Lately, researchers have started to map whole‐brain functional connectivity using fMRI. Usually, the brain is segmented into many (about 100) regions according to a template (eg, the automated anatomical labeling atlas, AAL.10 Whole‐brain functional connectivity can then be summarized with a correlation matrix. The topological properties of the functionally connected networks can then be studied using graph theory approaches. Graphs are constructed to describe the brain networks with the nodes denoting brain regions and the edges denoting significant connections among these regions. Properties such as node degree, efficiency, clustering coefficient, path length, and modularity can be calculated and compared across different groups.11, 12, 13 Finally, independent component analysis (ICA) is widely used to derive coherent patterns or modes of activity from neuroimaging data that correspond to functionally connected brain networks. This sort of characterization decomposes the fMRI images of the whole brain into a series of spatially independent modes or networks.
Unlike functional connectivity, effective connectivity infers directed (ie, causal) interactions within a brain network. Effective connectivity is defined as the influence one neural system exerts on another.2, 4 In the past decade, different approaches to measure effective connectivity such as psychophysiological interaction (PPI) analysis, structural equation modeling (SEM), Granger causality modeling (GCM), and dynamic causal modeling (DCM) have been developed. GCM tries to infer directed connectivity from observed BOLD signals using autoregressive models.5 In contrast, DCM treats the brain as a dynamic system of (unobserved or hidden) neuronal states, which are driven by experimental inputs or endogenous fluctuations to produce BOLD responses.6, 7 DCM estimates neural interactions using state‐space models based on (deterministic or random) differential equations. These equations describe neural dynamics and are supplemented with hemodynamic equations to transform regional neuronal activity into the observed BOLD response (Figure 1).6 Both empirical and simulated data suggest DCM may be more robust than GCM, when estimating directed connectivity.14, 15
3. A NETWORK MODEL OF MAJOR DEPRESSION
Major depressive disorder is characterized by prominent affective disruptions and cognitive impairments. Neuroimaging studies suggested that these deficits may be associated with altered connectivity of four brain networks (Figure 2): Elevated connectivity of a ventral limbic affective network appears to be associated with excessive negative feeling (dysphoria); decreased connectivity of a frontal‐striatal reward network has been suggested to account for loss of interest, motivation, and pleasure (anhedonia); enhanced default mode network connectivity seems to be associated with depressive rumination; and diminished connectivity of a dorsal cognitive control network is thought to underlie cognitive deficits especially ineffective top‐down control of negative thoughts and emotions in depressed patients. In this section, we examine these core networks affected in depression, focusing on the pattern of disruption within each—as related to the symptoms of depression.
Figure 2.
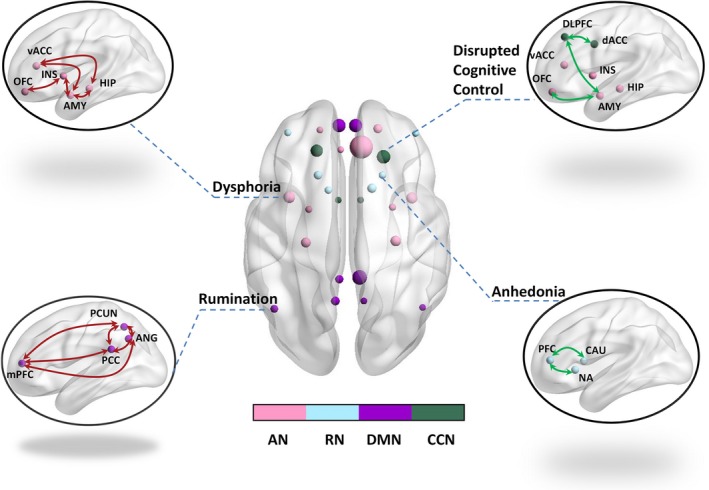
Dysconnectivity and depression. Four networks including the affective network (AN), reward network (RN), default mode network (DMN), and cognitive control network (CCN) have been mainly associated with the neural substrates of depression, with hyperconnectivity (marked in red) of the AN and DMN and attenuated connectivity (marked in green) of the RN and CCN observed in the patients. OFC: orbitofrontal cortex; INS: insula; AMY: amygdala; HIP: hippocampus; vACC: ventral anterior cingulate cortex; mPFC: medial prefrontal cortex; PCC: posterior cingulate cortex; PCUN: precuneus; ANG: Angular; DLPFC: dorsolateral prefrontal cortex; dACC: dorsal anterior cingulate cortex; PFC: prefrontal cortex; CAU: caudate; NA: nucleus accumbens. This figure was prepared with the BrainNet Viewer132
3.1. Elevated affective network connectivity and persistent sad mood
The orbitofrontal cortex (OFC), the affective division of the anterior cingulate cortex (ACC), and limbic regions including the amygdala, hippocampus, and insula form a ventral network which is also known as the brain's affective network (AN).16, 17 Crucially, the AN has been associated with processing and regulation of emotions. Emerging neuroimaging findings suggest an involvement of the AN in the pathophysiology of depression.18, 19 Previous studies have found hyperactivation of the amygdala and subgenual ACC, associated with dysfunctional affective processing in depressed patients. Functional neuroimaging also points to aberrant connectivity within the AN (Figure 2, Table 1), which may underlie emotion dysregulation, a hallmark of depression.
Table 1.
Altered connectivity in brain networks (AN, RN, DMN, and CCN) in patients with depression
| References | Subjects | Paradigms | Methods | Main findings |
|---|---|---|---|---|
| The affective network (AN) | ||||
| Avery et al20 | 20 MDD vs 20 controls |
Resting‐state fMRI; Focused awareness task |
Seed‐based functional connectivity analysis | Increased resting‐state functional connectivity between the dorsal midinsula cortex and the amygdala, subgenual prefrontal cortex, and orbitofrontal cortex |
| Davey et al21 | 18 MDD vs 20 controls | Resting‐state fMRI | Seed‐based functional connectivity analysis | Enhanced functional connectivity between the sgACC and dorsomedial frontal cortex, between the pregenual ACC and left dorsolateral frontal cortex; Reduced connectivity between the pregenual ACC and caudate nucleus |
| Cheng et al22 | 421 MDD vs 488 controls | Resting‐state fMRI | Voxel level functional connectivity analysis | Increased connectivity between the lateral OFC and the precuneus, angular gyrus, and temporal visual cortex |
| Davey et al23 | 56 adolescents, of whom 8 developed depression during a 2‐y follow‐up period | Resting‐state fMRI | Seed‐based functional connectivity analysis | Increased connectivity between the sgACC and amygdala in subjects who developed depression at follow‐up compared with baseline |
| Hamilton et al24 | 16 MDD and 14 controls | Resting‐state fMRI | Granger causality analysis | Increased excitatory influences among limbic and paralimbic regions and inhibitory influences from limbic regions to dorsal cortical regions |
| Admon et al29 | 33 remitted MDD vs 35 controls | Mild psychological stress task | Psychophysical interaction analyses | Enhanced caudate‐amygdala and caudate‐hippocampus connectivity was only observed in remitted MDD subjects during processing of negative stimuli |
| Goulden et al27 | 22 remitted MDD vs 21 controls | Face emotion processing task | Dynamic causal modeling | Reversed pattern of frontotemporal effective connectivity in remitted MDD and controls during processing of happy and sad faces |
| Hamilton et al28 | 14 MDD vs 12 controls | Picture encoding task; Incidental recognition memory task | Psychophysical interaction analysis | Increased memory sensitivity for negative stimuli in the patients which was associated with enhanced amygdala‐hippocampus and amygdala‐caudate‐putamen connectivity |
| The reward network (RN) | ||||
| Cheng et al22 | 421 MDD vs 488 controls | Resting‐state fMRI | Voxel level functional connectivity analysis | Decreased connectivity between the medial orbitofrontal cortex and the parahippocampal gyrus and medial temporal lobe |
| Satterthwaite et al30 | 27 bipolar depression, 25 MDD, and 37 controls |
Resting‐state fMRI; Monetary reward task |
Functional connectivity among 11 nodes | Disrupted resting‐state connectivity of the reward network was correlated with depression severity in both patient groups |
| Meng et al31 | 25 recurrent MDD vs 25 controls | Resting‐state fMRI | Whole‐brain functional connectivity analysis | Abnormal nodal efficiency of the right putamen was associated with the course of depressive episodes |
| Simmons et al37 | 16 patients with MDD and increased appetite, 16 patients with MDD and decreased appetite, and 16 controls |
Resting‐state fMRI; Food/nonfood picture task |
Seed‐based functional connectivity analysis | Patients with increased appetite demonstrated greater activation of the reward regions in response to food pictures; Patients with decreased appetite demonstrated weakened activation of the midinsula; Connectivity of the midinsula and reward regions was correlated with food pleasantness ratings |
| Manelis et al36 | 31 bipolar depression, 39 MDD, and 36 controls | Number guessing reward task | Independent Multiple sample Greedy Equivalence Search (IMaGES); Linear non‐Gaussian Orientation, Fixed Structure (LOFS) algorithms | Win/loss anticipation was characterized differently in patients and controls, with bottom‐up connectivity and top‐down connectivity observed in the MDD patients and controls, respectively |
| Admon et al35 | 26 MDD vs 29 controls | Monetary incentive delay task | Psychophysical interaction analysis | Reduced caudate connectivity to gains and greater caudate connectivity to penalties |
| Heller et al34 | 27 MDD vs 19 controls | Emotion regulation task | Seed‐based functional connectivity analysis | Abnormal connectivity between the left NAcc and left middle frontal gyrus |
| The default mode network (DMN) | ||||
| Greicius et al41 | 28 MDD vs 20 controls | Resting‐state fMRI | Independent component analysis | Increased subgenual cingulate and thalamic connectivity |
| Berman et al42 | 15 MDD vs 15 controls |
Resting‐state fMRI; Short‐term memory task |
Seed‐based functional connectivity analysis | Enhanced subgenual cingulate‐PCC connectivity in the patients was observed at rest but not during performance of the short‐term memory task |
| Li et al43 | 24 MDD vs 29 controls | Resting‐state fMRI | Independent component analysis | The default mode network dissociated into an anterior and another posterior subnetwork; Functional connectivity of both subnetworks was elevated in the patients. |
| Ho et al44 | 26 adolescents with MDD vs 37 controls |
Resting‐state fMRI; Emotion identification task |
Psychophysical interaction analysis | Greater mPFC and PCC connectivity during task performance, as well as increased PCC‐subcallosal cingulate connectivity at rest |
| Belleau et al46 | 16 MDD vs 16 controls |
Self‐focus thought induction task; External‐focus thought induction task |
Seed‐based functional connectivity analysis | Enhanced connectivity of the default mode network and weakened connectivity of the executive and salience network during the external‐focus thought induction task; No significant differences in connectivity were observed between the patients and controls during performance of the self‐focus thought induction task |
| Lemogne et al47 | 15 MDD vs 15 controls | Self‐referential processing task | Seed‐based functional connectivity analysis | Elevated MFC‐dACC and MFC‐DLPFC connectivity |
| Zamoscik et al48 | 29 remitted MDD vs 29 controls |
Resting‐state fMRI; Mood induction task; Rumination phase; Distraction phase |
Seed‐based functional connectivity analysis | Increased connectivity between the PCC and parahippocampal gyri |
| Nixon et al49 | 20 recovered MDD vs 20 controls | Go/No‐Go task | Seed‐based functional connectivity analysis | Increased precuneus connectivity in right dorsomedial prefrontal cortex and right frontal pole |
| Gaffrey et al50 | 21 children with a history of preschool depression and 18 controls | Resting‐state fMRI | Seed‐based functional connectivity analysis | Greater PCC connectivity in the sgACC and anterior middle temporal gyrus; Reduced PCC connectivity in the middle temporal gyrus, inferior parietal lobule, and cerebellum |
| Zhu et al54 | 35 MDD vs 35 controls | Resting‐state fMRI | Independent component analysis | Increased connectivity in anterior part and decreased connectivity in posterior part of the default mode network |
| Sambataro et al58 | 20 MDD vs 20 controls | Resting‐state fMRI | Independent component analysis | Increased connectivity within the posterior, ventral subsystems of the DMN; Decreased connectivity from anterior to ventral subsystems. |
| Zhang et al45 | 30 MDD vs 63 controls | Resting‐state fMRI | Whole‐brain functional connectivity analysis | Reduced path length and increased global efficiency; Greater nodal centralities in the hippocampus, inferior parietal, medial frontal, and parietal regions, as well as lower nodal centralities in the occipital, frontal and temporal regions. |
| The cognitive control network (CCN) | ||||
| Vasic et al69 | 14 MDD vs 14 controls | Verbal working memory | Independent component analysis | Decreased connectivity in the ACC, ventrolateral and superior prefrontal cortex, inferior parietal, superior prefrontal, and frontopolar regions; Increased connectivity in the DLPFC and cerebellum |
| Aizenstein et al70 | 13 late‐life depression vs 13 controls | Executive‐control task | Seed‐based functional connectivity analysis | Reduced connectivity between the DLPFC and dACC |
| Kerestes et al72 | 21 MDD vs 21 controls | Resting‐state fMRI | Seed‐based functional connectivity analysis | Elevated connectivity between dorsal caudate nucleus and ventrolateral prefrontal cortex |
| Alexopoulos et al73 | 16 late‐life MDD vs 10 controls | Resting‐state fMRI | Seed‐based functional connectivity analysis | Reduced connectivity of the CCN and increased connectivity of the DMN characterized the patients |
| Stange et al75 | 52 remitted MDD vs 47 controls | Resting‐state fMRI |
Seed‐based functional connectivity analysis; Independent component analysis |
Weakened CCN connectivity pattern seen in the patients was stable over time |
| Anand et al77 | 15 MDD vs 15 controls |
Resting‐state fMRI; Emotion processing task |
Seed‐based functional connectivity analysis | Attenuated connectivity between the ACC and limbic regions both at rest and during task performance |
| Tang et al78 | 28 MDD vs 30 controls | Resting‐state fMRI | Seed‐based functional connectivity analysis | Weakened connectivity between bilateral amygdala and left ventral prefrontal cortex |
| Kong et al79 | 28 MDD vs 30 controls | Emotional face processing task | Seed‐based functional connectivity analysis | Reduced connectivity between the amygdala and left rostral prefrontal cortex in the patients during processing of fear faces |
| Lui et al80 | 28 refractory MDD, 32 nonrefractory MDD, and 48 controls | Resting‐state fMRI | Seed‐based functional connectivity analysis | Attenuated bilateral connectivity in prefrontal‐limbic‐thalamic regions in both patient group |
| Song et al81 | 28 MDD vs 27 controls | Resting‐state fMRI | Voxel‐based eigenvector centrality mapping |
Decreased connectivity in bilateral middle frontal gyrus, insula, hippocampus, amygdala and cerebellum; Increased connectivity in the medial prefrontal cortex; Attenuated frontal–subcortical connection and stronger insula–medial prefrontal cortex connection |
| Carballedo et al82 | 15 MDD vs 15 controls | Face‐matching task | Structural equation modeling | Decreased bilateral connectivity from amygdala to orbitofrontal cortex; Reduced connectivity from right amygdala to ACC, and from ACC to prefrontal cortex |
| Moses‐Kolko et al83 | 14 depressed mothers vs 16 postpartum healthy mothers | Face vs shape matching task | Granger causality analysis | The connection from left dorsomedial prefrontal cortex to amygdala seen in controls was absent in the patients |
| Erk et al84 | 17 MDD vs 17 controls | Emotion regulation task | Psychophysiological interaction analysis | Attenuated prefrontolimbic connectivity during active regulation |
| Grant et al85 | 20 MDD vs 19 controls | Affective variant of the flanker task | Granger causality analysis | Reduced mPFC–amygdala connectivity was only presented in subjects with a history of early life trauma (ELT). For those without ELT, mPFC inhibition of the amygdala was intact |
| Wang et al74 | 18 MDD patients with childhood maltreatment, 20 MDD patients without childhood maltreatment, and 20 controls | Resting‐state fMRI | Whole‐brain functional connectivity analysis | Both patients group demonstrated weakened connectivity in bilateral ventral medial prefrontal cortex/ventral anterior cingulate cortex; Childhood neglect was associated with decreased connectivity of the prefrontal‐limbic‐thalamic‐cerebellar circuitry |
Increased resting‐state interactions between regions of the AN have been consistently reported in depression. The patients showed enhanced functional connectivity between the dorsal midinsula cortex and the amygdala, subgenual prefrontal cortex, and OFC20; between the subgenual ACC and dorsomedial frontal cortex16, 21; between pregenual ACC and left dorsolateral frontal cortex21; and between lateral orbitofrontal cortex and the precuneus, angular gyrus, and temporal visual cortex,22 with connectivity strength positively correlated with illness severity.20, 21, 23 Notably, the strength of the amygdala‐sgACC connectivity was positively correlated with negative affectivity, while an increase in this connection was associated with the onset of depression.23 In addition, enhanced OFC connectivity with the precuneus and angular gyrus was also related to affectively negative sense of the self in the patients.22 Attempts have also been made to determine the directionality of the influences among these regions at rest. Granger causality analysis revealed increased excitatory influences from hippocampus to ventral anterior cingulate cortex and reciprocal interactions between the medial prefrontal cortex and ventral anterior cingulate cortex in major depressive disorder (MDD).24
When presented with sad and happy faces, individuals with depression demonstrated an attentional bias for sad faces,25, 26 whereas healthy controls show a positive bias toward happy faces.26 Related to these findings, an opposite pattern of limbic network connectivity was found during processing of emotional stimuli. Specifically, happy faces modulated bidirectional OFC‐amygdala and OFC‐fusiform gyrus connectivity in depressed subjects. The same pattern of modulation was observed when healthy controls viewed sad faces. Similarly, the connection from the fusiform gyrus to orbitofrontal cortex was modulated when healthy subjects were presented with happy faces and depressed patients were processing sad faces.27 Depressed patients also show increased memory sensitivity for negative information associated with increased amygdala‐hippocampus and amygdala‐caudate‐putamen connectivity.28 Admon et al29 found an increased susceptibility to negative stimuli in remitted patients compared with controls. The increases in cortisol and anxiety levels were higher in the remitted MDD individuals than the controls in a stress task. It is worth noting that elevated caudate‐amygdala and caudate‐hippocampus connectivity during processing of negative stimuli was only seen in remitted subjects, but not the control group.29
3.2. Attenuated frontal‐striatal reward network connectivity and anhedonia
Symptoms such as loss of pleasure, interest, or motivation (anhedonia) are also typical in depression. Evidence from neuroimaging studies suggests that anhedonia seen in the patients may be attributed to diminished interactions in the frontal‐striatal reward network (Figure 2, Table 1). The frontal cortex and striatal regions including the caudate, putmen, and nucleus accumbens form a brain's reward network. Interactions among regions in this network have been shown to be attenuated in patients with depression,22 with reduction in connectivity being in proportion to depression severity.30 Interestingly, nodal efficiency of the right putamen's resting‐state functional connectivity network was associated with the course of depressive episodes—an important predictor of depressive relapse.31 Recently, a study by Felger and colleagues further suggested that anhedonia and hypoconnectvity of the reward network may be caused by elevated inflammation, increased biomarkers of which were seen in depression.32
When exposed to positive stimuli, depressed patients demonstrated reduced magnitude and duration of positive affect.33 The inability to sustain positive affect has been shown to be associated with reduced frontostriatal connectivity.34 In addition, depressed individuals exhibited lower caudate‐dACC connectivity than the controls in response to monetary gains.35 Win/loss anticipation was mediated through distinct mechanisms in diseased and healthy individuals, with bottom‐up striatal‐frontal connectivity seen in MDD and frontostriatal top‐down connectivity observed in the controls.36 Furthermore, aberrant activation and connectivity of the reward network have also been shown to be associated with depression‐related appetite loss/increase in the patients.37
3.3. Hyperconnectivity of the default mode network and excessive self‐focus
The third system involved in the neural substrates of depression is the task‐negative default mode network (DMN) (Figure 2, Table 1). The DMN mainly encompasses the precuneus, posterior cingulate cortex (PCC), and medial prefrontal cortex (mPFC), as well as the inferior parietal cortex.38, 39 This network is known as a task‐negative network as regions within this network generally demonstrate deactivation during performance of cognitive tasks.39, 40
Enhanced DMN connectivity is marked in depression. An early study conducted by Greicius et al41 reported elevated resting‐state DMN connectivity in patients with depression. Their findings of increased DMN connectivity have been reproduced by several other studies and our analyses.16, 42, 43, 44 In addition, Zhang et al45 reported increased nodal centralities in DMN regions in the patients. Furthermore, depressed subjects also demonstrated enhanced DMN connectivity while being engaged in externally focused thought,46 in an emotion identification task,44 and during self‐referential processing.47 Elevated DMN functional connectivity thus appears to be a robust marker of MDD that is evident even in remitted,48 and recovered state.49 Notably, a history of preschool depression in children may also affect the developmental trajectory of the DMN, with increased PCC functional connectivity in the subgenual and anterior cingulate cortices detected in these individuals.50
The DMN is associated with self‐referential processes,51, 52 which are enhanced in patients with depression. Depressed individuals usually demonstrate maladaptive rumination—the process of repetitively and passively thinking about one's negative feelings, possible causes, and consequences.48, 53 Rumination, the content of which is typically negative, has been shown to predict the onset of depression, prolong the duration, exacerbate negative thinking, and impair problem‐solving.53 Hyperconnectivity of the DMN may represent excessive self‐referential processes and maladaptive rumination in the patients.42, 48, 54, 55 In a study by Berman et al,42 resting‐state functional connectivity, between the posterior cingulate and the subgenual cingulate, correlated positively with rumination scores both in depressed and healthy subjects. In addition, increase in DMN connectivity was seen in the MDD group from unconstrained resting states to induced‐ruminative states.55 Accordingly, stronger DMN connectivity was associated with higher levels of rumination in depression,54 which was also evident in remitted depressed patients.48
3.3.1. DMN subnetworks in depression
Previous studies have also suggested that the DMN may consist of interacting subnetworks.56, 57 Zhu et al54 reported elevated functional connectivity in the anterior division of the DMN in MDD patients to be positively correlated with rumination score. Interestingly, they also found attenuated functional connectivity in the posterior division of the DMN in the patients to be negatively correlated with autobiographical memory scores. In our study, using group ICA to investigate resting‐state functional connectivity in MDD, we found evidence for two dissociable subnetworks in the DMN: an anterior subnetwork which had the highest amplitude in the mPFC, and a posterior subnetwork, which had the highest amplitude in the precuneus.43 Unlike Zhu and colleagues, Sambataro et al58 found increased functional connectivity within posterior, ventral, and core DMN subsystems in patients with MDD. They also reported altered interactions between DMN subsystems in patients.
3.4. Diminished cognitive control network connectivity and impaired top‐down control
In patients with depression, impaired emotion processing is often accompanied by cognitive impairments.59, 60 These impairments can persist even after remission of affective symptoms. Related to these impairments, another brain network has been implicated in the pathophysiology of depression, the so‐called cognitive control network (CCN). This network mainly consists of functionally connected brain regions including the dorsolateral prefrontal cortex (DLPFC), the cognitive subdivision of ACC, and the parietal cortex.61, 62, 63, 64 The CCN is thought to be an executive or control system, responsible for regulating thoughts, and actions in accordance with internal goals.65, 66 Neuroimaging studies have identified coactivation of the CCN during performance of different cognitive tasks. A failure of effective cognitive control over emotional processing is one of the central characteristics of depression.67, 68 Neuroimaging studies seeking to elucidate the neural substrates of depression therefore have identified prominent impairments of the CCN in depression (Figure 2, Table 1).
Dysconnectivity of regions involved in the CCN has been reported in patients with depression during performance of tasks involving working memory,69 executive‐control,70 and affective interference,71 as well as during rest.16, 72, 73, 74 However, the findings have been divergent. Sheline et al,16 using the bilateral DLPFC as a seed region, reported increased resting‐state functional connectivity in the bilateral dorsomedial prefrontal cortex (DMPFC) in depressed subjects. Vasic et al69 observed increased functional connectivity in the left DLPFC during a working memory task in MDD. However, Stange et al75 reported attenuated CCN connectivity which was stable over time in remitted MDD. Aizenstein et al70 reported reduced DLPFC‐dACC functional connectivity on an executive‐control task in patients with late‐life depression (LLD). Children with a parental history of depression are known to be at high risk to develop this disorder. In a recent study, Clasen and the colleagues reported decreased resting‐state functional connectivity within the CCN in depression‐naive adolescent females with a parental history of depression. In addition, severity of the parents’ depression was associated with deficits in functional connectivity of the CCN in their children.76 Neuroimaging studies thus support a link between impairments in the CCN and depression vulnerability even in healthy patients.
Evidence over the years suggests that abnormal top‐down cortical regulation of the limbic systems may also contribute to inefficient emotion regulation in depressed patients. In an early study, Anand and colleagues found that while regions in the affective network showed increased activation, functional connectivity between the ACC and limbic regions was decreased both at rest and during exposure to different stimuli (neutral, positive, and negative pictures) in depressed subjects. This finding may reflect an ineffective regulatory effect of the ACC on the hyperactivation of the limbic system in the patients.77 Additionally, reduced functional connectivity between amygdala and the PFC was found in depressed subjects both at rest and in response to fearful faces.78, 79 In a resting‐state study of mood regulation in refractory and nonrefractory major depression, Lui et al80 found decreased functional connectivity in bilateral prefrontal‐limbic‐thalamic areas in both patient groups. Recently, Song et al81 also reported reduced resting‐state frontal‐subcortical connection. These findings appear to further support a poor top‐down emotional regulation view of depression.
Studies of directed functional and effective connectivity have further confirmed a diminished top‐down cortical control of the limbic systems in depressed patients. Using structural equation modeling, Carballedo et al82 found lower bilateral effective connectivity from the amygdala to OFC in major depression. A recent study compared activity and effective connectivity in postpartum healthy and depressed mothers, when subjects responded to negative emotional faces.83 Using Granger causality mapping, the authors studied the top‐down regulation of the amygdala by the dorsomedial prefrontal cortex. They found a significant effective connection from the left dorsomedial prefrontal cortex to the left amygdala in healthy controls, but this connection was absent in depressed subjects.83 In a separate study using PPI analysis, Erk and colleagues observed reduced amygdala‐DLPFC connectivity in depressed patients during active emotion regulation.84 However, a GCM study showed that only MDD subjects with a history of early life trauma (ELT) presented reduced mPFC‐amygdala connectivity. In non‐ELT exposed patients, mPFC inhibition of the amygdala was intact.85
4. BRAIN CONNECTIVITY AND TREATMENT OF DEPRESSION
In addition to providing a better understanding of the neural substrates of depression, brain connectivity analyses have also helped with the treatment of the disease. fMRI studies have reported partially restored brain connectivity in keeping with improvement in depressive symptoms in the patients after treatment. Notably, pretreatment brain connectivity patterns were shown to be able to predict the outcomes of antidepressant treatment. Responders and nonresponders were characterized by distinct connectivity patterns. Interestingly, although brain stimulation techniques adopted in the treatment of depression targeted a single brain region, the therapeutic effects seem to be mediated by the connections from the target to distributed regions or brain networks. Brain connectivity studies thus allow the identification of the optimal stimulation sites (Figure 3).
Figure 3.
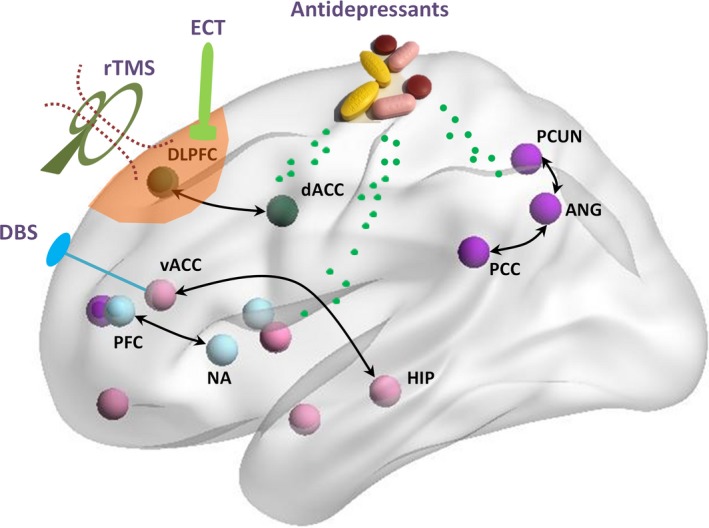
Brain effects of antidepressant treatment. A large part of aberrant connections reported in the patients have been shown to be normalized after treatment with antidepressants, psychotherapy, repetitive transcranial magnetic stimulation (rTMS), deep brain stimulation (DBS), and electroconvulsive therapy (ECT). This figure was prepared with the BrainNet Viewer132
4.1. Normalization of aberrant brain connectivity after antidepressant treatment
An important question of interest to researchers and psychiatrists is whether normalization of aberrant brain connectivity would accompany improvement in depressive symptoms after antidepressant treatment. Studies of depression have reported restored connectivity of the AN,19, 86 RN,87, 88 DMN,19, 89, 90, 91, 92, 93, 94 and CCN95 in the patients following antidepressant treatment. A variety of treatments have targeted the AN and RN in depression. Connectivity of the subcallosal cingulate cortex with limbic regions was reduced after electroconvulsive therapy (ECT) treatment.86 Even administration of a single dose of ketamine (0.5 mg kg−1) resulted in increased neural responses and connectivity of the right caudate during positive emotion perception in patients with treatment‐resistant major depressive disorder.88 In addition, enhancement of dopaminergic transmission in the reward network through amisulpride potentiated diminished corticostriatal connectivity,96 while treatment‐induced increases in network connectivity were associated with gains in positive affect in depressed patients.94 Abnormal connectivity of the DMN has also been modulated by antidepressants and transcutaneous vagus nerve stimulation (tVNS).43, 91 Given the central role of the CCN in the neurobiology of depression, its response to antidepressant treatments has been studied frequently, revealing increased post‐treatment ACC connectivity.97, 98
4.2. Prediction of treatment outcomes
The outcomes of antidepressant treatment vary largely among patients, thereby yielding responders, and nonresponders. Brain connectivity patterns have been shown to be able to predict treatment outcomes with quite high sensitivity and specificity.99 Baseline degree centrality of the posterior default mode network was associated with changes in depression severity after 2 weeks of medication.100 Pretreatment connectivity of the OFC, insula, and RN has been shown to predict response to psychotherapy.101, 102 Compared with responders, nonresponders of dorsomedial prefrontal repetitive transcranial magnetic stimulation (rTMS) were characterized by more severe pretreatment anhedonia symptoms and lower connectivity of the RN.103 Higher baseline sgACC connectivity was associated with greater TMS‐induced clinical improvement.92, 104 Furthermore, two resting‐state networks centered in the dorsomedial prefrontal cortex and ACC have been found to predict the outcome of ECT in treatment‐resistant patients.99 In addition, low pretreatment CCN functional connectivity was associated with low remission rate and residual symptoms when patients with late‐life depression were treated with escitalopram.73 Notably, it has been shown recently that resting‐state functional connectivity of the subcallosal cingulate cortex with left anterior ventrolateral prefrontal cortex/insula, the dorsal midbrain, and the left ventromedial prefrontal cortex may be capable of guiding treatment choice. Specifically, positive summed connectivity scores for these three regions were associated with remission to CBT, while negative summed connectivity was associated with better treatment outcomes to medication. These findings are of particular importance in the identification of the most effective treatment option that an individual patient is likely to benefit from.
4.3. Identification of optimal stimulation sites
Brain stimulation techniques such as deep brain stimulation(DBS)and TMS aim to normalize aberrant brain activity in depressed subjects by applying electrical or magnetic stimulation to specific regions. Such therapy alternatives have been shown to be effective in treatment‐resistant depression.105 DBS initially targeted the sgACC to restore hyperactivity of this region observed in the patients, while the first applications of rTMS targeted the DLPFC which demonstrated hypoactivity.106 However, the clinical efficacy of these traditional protocols still needs to be improved as the response and remission rates are relatively low. Attempts thus have been made to apply rTMS over targets beyond the DLPFC. New advances in neuroimaging studies of depression, MRI‐guided rTMS, as well as the introduction of coils with the capacity to stimulate deep structures, have helped improve the identification of optimal stimulation sites. rTMS targeting other core regions whose connectivity has been shown to be disrupted in depression such as the DMPFC,103, 107, 108, 109 OFC,110, 111 ACC,112 has demonstrated apparent therapeutic effectiveness. Although commonly applied to single brain regions, the effects of DBS and TMS are mediated via distributed networks. Notably, the efficacy of the rTMS was associated with the connectivity profile of the targets.103, 104, 113 Responders and nonresponders to DMPFC‐rTMS had distinct connectivity patterns of the reward network,103 while DLPFC‐rTMS targets that demonstrated stronger anti‐correlation with subgenual cingulate cortex were found to be more effective than others.113 Neuroimaging studies thus not only provide important insights into our understanding of the pathophysiology of depression, but also facilitate the identification of the optimal stimulation sites for the treatment of the disease.
5. FUTURE STEPS
We review recent studies of functional and effective connectivity in depression. The findings above present an emerging picture of four aberrant networks in depression; namely, abnormal connectivity within the AN, RN, DMN, and CCN. However, the interactions between different networks may be disrupted as well.24, 47, 114, 115 Recent meta‐analysis studies have revealed increased functional connectivity of the AN (subgenual prefrontal cortex)115 and the CCN114, 115 with the DMN in MDD. In fact, Sheline et al16 found a bilateral region in the dorsomedial prefrontal cortex, which they termed the dorsal nexus, consistently showing increased functional connectivity with the AN, DMN, and CCN in depression. Later, Perrin et al95 reported reduced connectivity of the dorsal nexus and an improvement in symptoms in depressed patients following treatment with ECT. There is further evidence showing that TMS targeting the DLPFC (a component of the CCN) modulated functional connectivity of the DMN.92 These findings suggest that depression may not only be associated with abnormal interactions between different brain regions within the same neural circuit, but also abnormal interactions between distributed brain networks. Future studies should aim to integrate these core networks and their contributions toward developing an extended model of depression for improved diagnosis, treatment, and prevention of the disorder.
Recent studies confirmed a structural basis for the altered functional integration seen in depression. Studies using dMRI have demonstrated disrupted white matter integrity and/or structural connectivity in the patients.116, 117, 118, 119, 120 In addition, topological organization of white matter networks was also impaired in the patients.121, 122 Future studies may need to further elucidate how changes in structural changes may relate to functional dysconnectivity in widely distributed networks. Furthermore, the pathophysiology of dysfunctional integration or disconnection in depression may rest on a failure to contextualize interregional coupling; for example, aberrant neuromodulation of synaptic efficacy may be an important etiological factor. One important candidate for this sort of pathophysiology is the neuromodulatory effect of neurotransmitters such as serotonin. Indeed, the imaging literature—using positron emission tomography and radio‐ligand binding—points to an abnormality of 5HT neurotransmission, at the level of transporter availability, (5HT1‐A) receptor binding, etc.123, 124 Furthermore, secondary or complementary changes in metabotropic glutamate receptor function may be intimately involved (or respond) to the synaptic pathophysiology that underlies functional disconnections. This is suggested by imaging studies that show, for example, reduced glutamate receptor 5 (mGluR5) density in major depression and response to antidepressant treatment.125, 126
It is worth emphasizing that although the interactions among different brain regions have been demonstrated to fluctuate over time,127, 128, 129, 130 the majority of functional and effective connectivity studies on depression have treated the brain as a stationary system and calculated the averaged functional or effective connectivity over the whole session which generally last for 5‐10 minutes. Investigating the dynamics of functional interactions among distributed systems may be critically important to concisely delineate the neural mechanisms of the diseases. In a recent study on patients with schizophrenia, the authors reported that transient states of dysconnectivity could only be captured by dynamic connectivity analyses, but not traditional static functional network connectivity analyses.131 Future studies on depression utilizing dynamic functional or even effective connectivity analyses may provide a better understanding of the etiology of depression.
6. CONCLUSION
In conclusion, we have reviewed an overwhelming amount of evidence based upon studies of functional and effective connectivity that implicate key modes or intrinsic brain networks in depression. The functional anatomy of these modes fits comfortably with the psychopathology of depression; namely, depressive rumination, a failure of emotion regulation, and difficulties with top‐down or executive control. The fact that the implicit functional disconnection shows systematic changes with therapeutic interventions lends further support to the notion that depression is linked to a functional disintegration or disconnection within and between intrinsic brain networks.
DISCLOSURES
This manuscript has not been published nor is it being considered for publication elsewhere. The authors report no biomedical financial interests or potential conflict of interests.
ACKNOWLEDGMENTS
We acknowledge the support of the National Natural Science Foundation of China (81301199, 61420106001, 81630032, 81230035), National Key Technologies R & D Program of China (2015BAI13B00, 2015BAI13B01), National Clinical Research Center on Mental Disorders(2015BAI13B02), NLM Family Foundation and Wellcome Trust Principal Research Fellowship (Ref: 088130/Z/09/Z).
Li B‐J, Friston K, Mody M, Wang H‐N, Lu H‐B, Hu D‐W. A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci Ther. 2018;24:1004–1019. 10.1111/cns.12998
Contributor Information
Hong‐Bing Lu, Email: luhb@fmmu.edu.cn.
De‐Wen Hu, Email: dwhu@nudt.edu.cn.
REFERENCES
- 1. Kruijshaar ME, Barendregt J, Vos T, et al. Lifetime prevalence estimates of major depression: an indirect estimation method and a quantification of recall bias. Eur J Epidemiol. 2005;20:103‐111. [DOI] [PubMed] [Google Scholar]
- 2. Friston KJ, Frith CD, Frackowiak RSJ. Time‐dependent changes in effective connectivity measured with PET. Hum Brain Mapp. 1993a;1:69‐80. [Google Scholar]
- 3. Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal‐component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993b;13:5‐14. [DOI] [PubMed] [Google Scholar]
- 4. Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56‐78. [Google Scholar]
- 5. Goebel R, Roebroeck A, Kim DS, Formisano E. Investigating directed cortical interactions in time‐resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn Reson Imaging. 2003;21:1251‐1261. [DOI] [PubMed] [Google Scholar]
- 6. Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273‐1302. [DOI] [PubMed] [Google Scholar]
- 7. Li B, Daunizeau J, Stephan KE, et al. Generalised filtering and stochastic DCM for fMRI. NeuroImage. 2011;58:442‐457. [DOI] [PubMed] [Google Scholar]
- 8. van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting‐state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519‐534. [DOI] [PubMed] [Google Scholar]
- 9. He Y, Evans A. Graph theoretical modeling of brain connectivity. Curr Opin Neurol. 2010;23:341‐350. [DOI] [PubMed] [Google Scholar]
- 10. Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage. 2002;15:273‐289. [DOI] [PubMed] [Google Scholar]
- 11. Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biol Psychiatry. 2015;77:223‐235. [DOI] [PubMed] [Google Scholar]
- 12. Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336‐349. [DOI] [PubMed] [Google Scholar]
- 13. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186‐198. [DOI] [PubMed] [Google Scholar]
- 14. David O, Guillemain I, Saillet S, et al. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biol. 2008;6:2683‐2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friston KJ, Bastos AM, Oswal A, et al. Granger causality revisited. NeuroImage. 2014;101:796‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sheline YI, Price JL, Yan Z, Mintun MA. Resting‐state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010;107:11020‐11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCarthy H, Skokauskas N, Mulligan A, et al. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention‐deficit/hyperactivity disorder in childhood. JAMA Psychiatry. 2013;70:1329‐1337. [DOI] [PubMed] [Google Scholar]
- 18. Sudheimer K, Keller J, Gomez R, et al. Decreased hypothalamic functional connectivity with subgenual cortex in psychotic major depression. Neuropsychopharmacology. 2015;40:849‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang L, Xia M, Li K, et al. The effects of antidepressant treatment on resting‐state functional brain networks in patients with major depressive disorder. Hum Brain Mapp. 2015;36:768‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Avery JA, Drevets WC, Moseman SE, et al. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry. 2014;76:258‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davey CG, Harrison BJ, Yucel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med. 2012;42:2071‐2081. [DOI] [PubMed] [Google Scholar]
- 22. Cheng W, Rolls ET, Qiu J, et al. Medial reward and lateral non‐reward orbitofrontal cortex circuits change in opposite directions in depression. Brain. 2016;139(Pt 12):3296‐3309. [DOI] [PubMed] [Google Scholar]
- 23. Davey CG, Whittle S, Harrison BJ, et al. Functional brain‐imaging correlates of negative affectivity and the onset of first‐episode depression. Psychol Med. 2015;45:1001‐1009. [DOI] [PubMed] [Google Scholar]
- 24. Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting‐state fMRI time‐series data. Mol Psychiatry. 2011;16:763‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113:121‐135. [DOI] [PubMed] [Google Scholar]
- 26. Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 2007;116:80‐85. [DOI] [PubMed] [Google Scholar]
- 27. Goulden N, McKie S, Thomas EJ, et al. Reversed frontotemporal connectivity during emotional face processing in remitted depression. Biol Psychiatry. 2012;72:604‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008;63:1155‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Admon R, Holsen LM, Aizley H, et al. Striatal hypersensitivity during stress in remitted individuals with recurrent depression. Biol Psychiatry. 2015a;78:67‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Satterthwaite TD, Kable JW, Vandekar L, et al. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology. 2015;40:2258‐2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meng C, Brandl F, Tahmasian M, et al. Aberrant topology of striatum's connectivity is associated with the number of episodes in depression. Brain. 2014;137(Pt 2):598‐609. [DOI] [PubMed] [Google Scholar]
- 32. Felger JC, Li Z, Haroon E, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Horner MS, Siegle GJ, Schwartz RM, et al. C'mon get happy: reduced magnitude and duration of response during a positive‐affect induction in depression. Depress Anxiety. 2014;31:952‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heller AS, Johnstone T, Shackman AJ, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto‐striatal brain activation. Proc Natl Acad Sci USA. 2009;106:22445‐22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Admon R, Nickerson LD, Dillon DG, et al. Dissociable cortico‐striatal connectivity abnormalities in major depression in response to monetary gains and penalties. Psychol Med. 2015b;45:121‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Manelis A, Almeida JR, Stiffler R, et al. Anticipation‐related brain connectivity in bipolar and unipolar depression: a graph theory approach. Brain. 2016;139(Pt 9):2554‐2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simmons WK, Burrows K, Avery JA, et al. Depression‐related increases and decreases in appetite: dissociable patterns of aberrant activity in reward and interoceptive neurocircuitry. Am J Psychiatry. 2016;173:418‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648‐663. [DOI] [PubMed] [Google Scholar]
- 39. Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Greicius MD, Flores BH, Menon V, et al. Resting‐state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Berman MG, Peltier S, Nee DE, et al. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li B, Liu L, Friston KJ, et al. A treatment‐resistant default mode subnetwork in major depression. Biol Psychiatry. 2013;74:48‐54. [DOI] [PubMed] [Google Scholar]
- 44. Ho TC, Connolly CG, Henje Blom E, et al. Emotion‐dependent functional connectivity of the default mode network in adolescent depression. Biol Psychiatry. 2015;78:635‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang J, Wang J, Wu Q, et al. Disrupted brain connectivity networks in drug‐naive, first‐episode major depressive disorder. Biol Psychiatry. 2011;70:334‐342. [DOI] [PubMed] [Google Scholar]
- 46. Belleau EL, Taubitz LE, Larson CL. Imbalance of default mode and regulatory networks during externally focused processing in depression. Soc Cogn Affect Neurosci. 2015;10:744‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lemogne C, le Bastard G, Mayberg H, et al. In search of the depressive self: extended medial prefrontal network during self‐referential processing in major depression. Soc Cogn Affect Neurosci. 2009;4:305‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zamoscik V, Huffziger S, Ebner‐Priemer U, Kuehner C, Kirsch P. Increased involvement of the parahippocampal gyri in a sad mood predicts future depressive symptoms. Soc Cogn Affect Neurosci. 2014;9:2034‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nixon NL, Liddle PF, Nixon E, et al. Biological vulnerability to depression: linked structural and functional brain network findings. Br J Psychiatry. 2014;204:283‐289. [DOI] [PubMed] [Google Scholar]
- 50. Gaffrey MS, Luby JL, Botteron K, Repovs G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. J Child Psychol Psychiatry. 2012;53:964‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Broyd SJ, Demanuele C, Debener S, et al. Default‐mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279‐296. [DOI] [PubMed] [Google Scholar]
- 52. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self‐referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259‐4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nolen‐Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspect Psychol Sci. 2008;3:400‐424. [DOI] [PubMed] [Google Scholar]
- 54. Zhu X, Wang X, Xiao J, et al. Evidence of a dissociation pattern in resting‐state default mode network connectivity in first‐episode, treatment‐naive major depression patients. Biol Psychiatry. 2012;71:611‐617. [DOI] [PubMed] [Google Scholar]
- 55. Berman MG, Misic B, Buschkuehl M, et al. Does resting‐state connectivity reflect depressive rumination? A tale of two analyses NeuroImage. 2014;103:267‐279. [DOI] [PubMed] [Google Scholar]
- 56. Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011;31:4407‐4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sambataro F, Wolf ND, Pennuto M, Vasic N, Wolf RC. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychol Med. 2014;44:2041‐2051. [DOI] [PubMed] [Google Scholar]
- 59. Oral E, Canpolat S, Yildirim S, et al. Cognitive functions and serum levels of brain‐derived neurotrophic factor in patients with major depressive disorder. Brain Res Bull. 2012;88:454‐459. [DOI] [PubMed] [Google Scholar]
- 60. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta‐analysis and review. Psychol Bull. 2013;139:81‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343‐360. [DOI] [PubMed] [Google Scholar]
- 62. Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073‐11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328‐3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Niendam TA, Laird AR, Ray KL, et al. Meta‐analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci. 2012;16:106‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. De Baene W, Brass M. Switch probability context (in)sensitivity within the cognitive control network. NeuroImage. 2013;77:207‐214. [DOI] [PubMed] [Google Scholar]
- 67. Wolkenstein L, Plewnia C. Amelioration of cognitive control in depression by transcranial direct current stimulation. Biol Psychiatry. 2013;73:646‐651. [DOI] [PubMed] [Google Scholar]
- 68. Goeleven E, De Raedt R, Baert S, Koster EH. Deficient inhibition of emotional information in depression. J Affect Disord. 2006;93:149‐157. [DOI] [PubMed] [Google Scholar]
- 69. Vasic N, Walter H, Sambataro F, Wolf RC. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychol Med. 2009;39:977‐987. [DOI] [PubMed] [Google Scholar]
- 70. Aizenstein HJ, Butters MA, Wu M, et al. Altered functioning of the executive control circuit in late‐life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17:30‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kaiser RH, Andrews‐Hanna JR, Spielberg JM, et al. Distracted and down: neural mechanisms of affective interference in subclinical depression. Soc Cogn Affect Neurosci. 2015a;10:654‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kerestes R, Harrison BJ, Dandash O, et al. Specific functional connectivity alterations of the dorsal striatum in young people with depression. Neuroimage Clin. 2015;7:266‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Alexopoulos GS, Hoptman MJ, Kanellopoulos D, et al. Functional connectivity in the cognitive control network and the default mode network in late‐life depression. J Affect Disord. 2012;139:56‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang L, Dai Z, Peng H, et al. Overlapping and segregated resting‐state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum Brain Mapp. 2014;35:1154‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stange JP, Bessette KL, Jenkins LM, et al. Attenuated intrinsic connectivity within cognitive control network among individuals with remitted depression: temporal stability and association with negative cognitive styles. Hum Brain Mapp. 2017;38:2939‐2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Clasen PC, Beevers CG, Mumford JA, Schnyer DM. Cognitive control network connectivity in adolescent women with and without a parental history of depression. Dev Cogn Neurosci. 2014;7:13‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005a;57:1079‐1088. [DOI] [PubMed] [Google Scholar]
- 78. Tang Y, Kong L, Wu F, et al. Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment‐naive patients with major depressive disorder: a resting‐state functional magnetic resonance imaging study. Psychol Med. 2013;43:1921‐1927. [DOI] [PubMed] [Google Scholar]
- 79. Kong L, Chen K, Tang Y, et al. Functional connectivity between the amygdala and prefrontal cortex in medication‐naive individuals with major depressive disorder. J Psychiatry Neurosci. 2013;38:417‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lui S, Wu Q, Qiu L, et al. Resting‐state functional connectivity in treatment‐resistant depression. Am J Psychiatry. 2011;168:642‐648. [DOI] [PubMed] [Google Scholar]
- 81. Song Z, Zhang M, Huang P. Aberrant emotion networks in early major depressive disorder patients: an eigenvector centrality mapping study. Transl Psychiatry. 2016;6:e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Carballedo A, Scheuerecker J, Meisenzahl E, et al. Functional connectivity of emotional processing in depression. J Affect Disord. 2011;134:272‐279. [DOI] [PubMed] [Google Scholar]
- 83. Moses‐Kolko EL, Perlman SB, Wisner KL, et al. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167:1373‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Erk S, Mikschl A, Stier S, et al. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010;30:15726‐15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Grant MM, White D, Hadley J, et al. Early life trauma and directional brain connectivity within major depression. Hum Brain Mapp. 2014;35:4815‐4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Argyelan M, Lencz T, Kaliora S, et al. Subgenual cingulate cortical activity predicts the efficacy of electroconvulsive therapy. Transl Psychiatry. 2016;6:e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017;42:1210‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Murrough JW, Collins KA, Fields J, et al. Regulation of neural responses to emotion perception by ketamine in individuals with treatment‐resistant major depressive disorder. Transl Psychiatry. 2015;5:e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Karim HT, Andreescu C, Tudorascu D, et al. Intrinsic functional connectivity in late‐life depression: trajectories over the course of pharmacotherapy in remitters and non‐remitters. Mol Psychiatry. 2017;22:450‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Leaver AM, Espinoza R, Joshi SH, et al. Desynchronization and plasticity of striato‐frontal connectivity in major depressive disorder. Cereb Cortex. 2016;26:4337‐4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79:266‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liston C, Chen AC, Zebley BD, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wu M, Andreescu C, Butters MA, et al. Default‐mode network connectivity and white matter burden in late‐life depression. Psychiatry Res. 2011;194:39‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Heller AS, Johnstone T, Light SN, et al. Relationships between changes in sustained fronto‐striatal connectivity and positive affect in major depression resulting from antidepressant treatment. Am J Psychiatry. 2013;170:197‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Perrin JS, Merz S, Bennett DM, et al. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci USA. 2012;109:5464‐5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Admon R, Kaiser RH, Dillon DG, et al. Dopaminergic enhancement of striatal response to reward in major depression. Am J Psychiatry. 2017;174:378‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Anand A, Li Y, Wang Y, et al. Antidepressant effect on connectivity of the mood‐regulating circuit: an FMRI study. Neuropsychopharmacology. 2005b;30:1334‐1344. [DOI] [PubMed] [Google Scholar]
- 98. Beall EB, Malone DA, Dale RM, et al. Effects of electroconvulsive therapy on brain functional activation and connectivity in depression. J ECT. 2012;28:234‐241. [DOI] [PubMed] [Google Scholar]
- 99. van Waarde JA, Scholte HS, van Oudheusden LJ, et al. A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment‐resistant depression. Mol Psychiatry. 2015;20:609‐614. [DOI] [PubMed] [Google Scholar]
- 100. Shen Y, Yao J, Jiang X, et al. Sub‐hubs of baseline functional brain networks are related to early improvement following two‐week pharmacological therapy for major depressive disorder. Hum Brain Mapp. 2015;36:2915‐2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Crowther A, Smoski MJ, Minkel J, et al. Resting‐state connectivity predictors of response to psychotherapy in major depressive disorder. Neuropsychopharmacology. 2015a;40:1659‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Walsh E, Carl H, Eisenlohr‐Moul T, et al. Attenuation of frontostriatal connectivity during reward processing predicts response to psychotherapy in major depressive disorder. Neuropsychopharmacology. 2017;42:831‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Downar J, Geraci J, Salomons TV, et al. Anhedonia and reward‐circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry. 2014;76:176‐185. [DOI] [PubMed] [Google Scholar]
- 104. Salomons TV, Dunlop K, Kennedy SH, et al. Resting‐state cortico‐thalamic‐striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology. 2014;39:488‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment‐resistant depression. Neuron. 2005;45:651‐660. [DOI] [PubMed] [Google Scholar]
- 106. Downar J, Daskalakis ZJ. New targets for rTMS in depression: a review of convergent evidence. Brain Stimul. 2013;6:231‐240. [DOI] [PubMed] [Google Scholar]
- 107. Sender D, Nazar BP, Baczynski T, et al. Bilateral DMPFC‐rTMS leads to sustained remission in geriatric treatment‐resistant depression: a case report. Psychiatr Danub. 2017;29:218‐220. [DOI] [PubMed] [Google Scholar]
- 108. Bakker N, Shahab S, Giacobbe P, et al. rTMS of the dorsomedial prefrontal cortex for major depression: safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta‐burst stimulation. Brain Stimul. 2015;8:208‐215. [DOI] [PubMed] [Google Scholar]
- 109. Dunlop K, Gaprielian P, Blumberger D, et al. MRI‐guided dmPFC‐rTMS as a treatment for treatment‐resistant major depressive disorder. J Vis Exp. 2015;(102):e53129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fettes P, Peters S, Giacobbe P, Blumberger DM, Downar J. Neural correlates of successful orbitofrontal 1 Hz rTMS following unsuccessful dorsolateral and dorsomedial prefrontal rTMS in major depression: a case report. Brain Stimul. 2017;10:165‐167. [DOI] [PubMed] [Google Scholar]
- 111. Feffer K, Fettes P, Giacobbe P, et al. 1Hz rTMS of the right orbitofrontal cortex for major depression: safety, tolerability and clinical outcomes. Eur Neuropsychopharmacol. 2018;28:109‐117. [DOI] [PubMed] [Google Scholar]
- 112. Kreuzer PM, Schecklmann M, Lehner A, et al. The ACDC pilot trial: targeting the anterior cingulate by double cone coil rTMS for the treatment of depression. Brain Stimul. 2015;8:240‐246. [DOI] [PubMed] [Google Scholar]
- 113. Fox MD, Buckner RL, White MP, Greicius MD, Pascual‐Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kaiser RH, Andrews‐Hanna JR, Wager TD, Pizzagalli DA. Large‐scale network dysfunction in major depressive disorder: a meta‐analysis of resting‐state functional connectivity. JAMA Psychiatry. 2015b;72:603‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default‐mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78:224‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang A, Leow A, Ajilore O, et al. Quantitative tract‐specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology. 2012;37:959‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhang A, Ajilore O, Zhan L, et al. White matter tract integrity of anterior limb of internal capsule in major depression and type 2 diabetes. Neuropsychopharmacology. 2013;38:1451‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Peng HJ, Zheng HR, Ning YP, et al. Abnormalities of cortical‐limbic‐cerebellar white matter networks may contribute to treatment‐resistant depression: a diffusion tensor imaging study. BMC Psychiatry. 2013;13:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Korgaonkar MS, Fornito A, Williams LM, Grieve SM. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol Psychiatry. 2014;76:567‐574. [DOI] [PubMed] [Google Scholar]
- 120. Silver M, Moore CM, Villamarin V, et al. White matter integrity in medication‐free women with peripartum depression: a tract‐based spatial statistics study. Neuropsychopharmacology. 2018;43:1573‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Long Z, Duan X, Wang Y, et al. Disrupted structural connectivity network in treatment‐naive depression. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:18‐26. [DOI] [PubMed] [Google Scholar]
- 122. Qin J, Wei M, Liu H, et al. Abnormal brain anatomical topological organization of the cognitive‐emotional and the frontoparietal circuitry in major depressive disorder. Magn Reson Med. 2014;72:1397‐1407. [DOI] [PubMed] [Google Scholar]
- 123. Parsey RV, Ogden RT, Miller JM, et al. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry. 2010;68:170‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kaufman J, Sullivan GM, Yang J, et al. Quantification of the serotonin 1A receptor using PET: identification of a potential biomarker of major depression in males. Neuropsychopharmacology. 2015;40:1692‐1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Deschwanden A, Karolewicz B, Feyissa AM, et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry. 2011;168:727‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Esterlis I, DellaGioia N, Pietrzak RH, et al. Ketamine‐induced reduction in mGluR5 availability is associated with an antidepressant response: an [(11)C]ABP688 and PET imaging study in depression. Mol Psychiatry. 2018;23:824‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chang C, Glover GH. Time‐frequency dynamics of resting‐state brain connectivity measured with fMRI. NeuroImage. 2010;50:81‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Handwerker DA, Roopchansingh V, Gonzalez‐Castillo J, Bandettini PA. Periodic changes in fMRI connectivity. NeuroImage. 2012;63:1712‐1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Calhoun VD, Miller R, Pearlson G, Adali T. The chronnectome: time‐varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 2014;84:262‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hansen EC, Battaglia D, Spiegler A, Deco G, Jirsa VK. Functional connectivity dynamics: modeling the switching behavior of the resting state. NeuroImage. 2015;105:525‐535. [DOI] [PubMed] [Google Scholar]
- 131. Damaraju E, Allen EA, Belger A, et al. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 2014;5:298‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]


