Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- AIH
autoimmune hepatitis
- ALT
alanine aminotransferase
- AMA
antimitochondrial antibodies
- AP
alkaline phosphatase
- AST
aspartate aminotransferase
- DILI
drug‐induced liver injury
- ERCP
endoscopic retrograde cholangiopancreatography
- gGT
γ‐glutamyl transpeptidase; LIT, liver injury test
- MELD
Model for End‐Stage Liver Disease
- MRCP
magnetic resonance cholangiopancreatography
- NASH
nonalcoholic steatohepatitis
- PBC
primary biliary cholangitis
- ULN
upper limit of normal
The liver is the largest organ in the body and arguably the most important organ for protein production and detoxification, both of which are facilitated by a myriad of enzymes. Both the detection of enzymes released from liver cells and proteins produced by the liver and released into the blood can be used to analyze liver health.
Standard liver tests (Tables 1 and 2) that assess injury to the liver include alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatases (APs). The excretory function of the liver can be estimated by bilirubin and the metabolic function of the liver by clotting tests and albumin.
Table 1.
Standard Liver Tests, Their Sources of Origin, and Abnormalities
| Parameter | Origin | Associated Disease |
|---|---|---|
| AST | Liver, skeletal muscle, cardiac muscle, red blood cells, brain, pancreas, lungs | Hepatocellular injury of any cause, myopathies, myocardial infarct, hemolysis |
| ALT | Liver, kidneys, skeletal muscle | Hepatocellular injury of any cause, myopathies |
| AP | Liver, bone, placenta, kidneys, intestines | Cholestatic liver disease; sarcoidosis; pregnancy; lymphoma; bone, kidney, and intestinal diseases |
| γ‐Glutamyl transferase | Biliary epithelial cells, kidneys, pancreas, prostate | Biliary or pancreatic disease, myocardial infarct, renal diseases, chronic lung disease, diabetes |
| Conjugated bilirubin | Hemolysis, insufficient excretion from the liver | Severe liver injury from any cause Rotor syndrome, Dubin‐Johnson syndrome |
| Unconjugated bilirubin | Hemolysis | Hemolysis, Gilbert syndrome, Crigler‐Najjar syndrome |
| Albumin | Produced in hepatocytes | Low in nephrotic syndrome, malnutrition, protein‐losing enteropathy |
| Prothrombin time | Clotting factors produced in hepatocytes | Prolonged in liver disease, vitamin K deficiency, fat malabsorption, pancreatic insufficiency |
Table 2.
Elevation of Liver Chemistries With Liver Diseases
| Test | Hepatocellular | Cholestatic | Half‐life (t 1/2) |
|---|---|---|---|
| AST | +++ | N/+ | 17 hours |
| ALT | +++ | N/+ | 47 hours |
| AP | Normal/mild | ++++ | 7 days |
| gGT | ++/+++ | ++++ | 26 days (for abstinence) |
| Total bilirubin | N/++ | N/+++ | Depends on albumin binding |
| Albumin | ++ (chronic) | N | 20 days |
| Prothrombin time | ++ | N |
Tests that describe injury of the liver such as aminotransferases and AP have historically been mislabeled liver injury tests (LIT). In contrast, standard tests such as albumin, bilirubin, and prothrombin time are useful in evaluating liver function.
The pattern of elevation of the different enzymes can be used to discriminate hepatocellular from cholestatic or mixed injury; AST and ALT are more elevated in patients with hepatocellular injury, whereas AP and γ‐glutamyl transpeptidase (gGT) are more elevated in cholestatic injury.
Discrimination of Normal Versus Healthy Values
Normal values for laboratory results are defined as those found in 95% of a population. Thus, 2.5% of a population will be above and below the normal values, respectively. But being outside the normal does not immediately reflect illness; that is, a bilirubin level below normal has no clinical consequences. Contrarily, being within the normal value does not necessarily reflect a healthy state. In that regard it has been suggested to use an upper limit of 19 and 30 U/L for ALT for women and men, respectively,1 to reflect healthy values. This also fits the observed increased mortality in individuals with ALT values that are normal but above the healthy range.2 Thus, liver transaminases likely will be described as healthy (≤19 U/L for women and ≤30 U/L for men) and normal values (i.e., <65 U/L). The normal values will depend on the specific laboratory population, thus limiting standardization.
For some assessments such as drug safety, the times upper limit of normal (ULN) is established to define safety margins. Substituting the healthy range values for normal range value will therefore need to be carefully addressed in the future.
Mild abnormalities in liver‐related tests may warrant repeat testing before a more extensive workup is initiated. Abnormal liver chemistries may occur in 1% to 4% of the asymptomatic population.3, 21
Markers of Hepatocellular Injury
Transaminases are involved in transferring the amino groups of aspartate and alanine to ketoglutaric acid. Although ALT is more liver specific, elevated ALT levels are also reported in myopathies (Table 3).4, 5
Table 3.
Disease Association According to Aminotransferases Elevation Pattern
| AST Predominant | ALT Predominant |
|---|---|
| Alcohol‐related liver injury | Chronic hepatitis C |
| Cirrhosis | Chronic hepatitis B |
| Hemolysis | Acute viral hepatitis (types A‐E, herpes simplex virus, Epstein‐Barr virus, cytomegalovirus) |
| Myopathy | Steatosis/steatohepatitis |
| Thyroid disease | Hemochromatosis |
| Strenuous exercise | Medications/toxins |
| Autoimmune hepatitis | |
| Wilson's disease | |
| Celiac disease |
Hepatocyte injury results in altered cell membrane permeability causing the excessive leakage of transaminases. Periportal hepatocytes (zone 1) have relatively more ALT, whereas the hepatocytes near the central vein (zone 3) have more AST (Fig. 1). Thus, causes of hepatic inflammation that are predominantly involving zone 1 such as viral and autoimmune hepatitis result in predominantly ALT elevation. In contrast, ischemic or toxic insults are more likely to involve zone 3, causing a predominance of AST elevation. AST/ALT ratio, also known as De Ritis ratio, is useful in assessing various liver diseases.6 In alcoholic hepatitis, AST is usually higher than ALT, with the AST/ALT ratio reaching 2:1. In acute viral hepatitis, ALT levels are usually higher than AST. High AST/ALT ratio (>1.5) in acute viral hepatitis may be indicative of potential fulminant course.9 AST/ALT ratio greater than 1.0 in chronic liver diseases may be indicative of advanced fibrosis.8, 9 AST and ALT are also used together with platelets to assess the likelihood of advanced liver fibrosis and are part of the aspartate aminotransferase‐to‐platelet ratio index (APRI) and FIB‐4 score:
APRI: AST level (/ULN) / platelet counts (109/L) × 100
FIB‐4 score: [age (years) × AST (U/L)]/{platelets (109/L) × [ALT (U/L)]1/2}.
Figure 1.
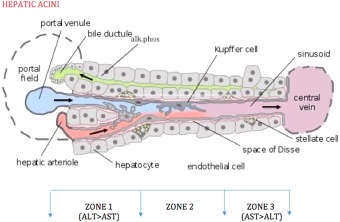
Zone 1 has more ALT than AST, and zone 3 has more AST than ALT. Autoimmune and viral hepatitis predominantly involve zone 1 (ALT > ALT). Ischemic and toxic events, heart failure, and Budd‐Chiari syndrome predominantly involve zone 3 (AST > ALT). AP is mostly present on basolateral membrane of hepatocytes lining the bile canaliculi. Reproduced from PLoS Biology. Copyright 2005, Frevert et al.
Aminotransferases are normal or only mildly elevated in obstructive jaundice except in acute phase of biliary obstruction caused by the passage of gallstone into the common bile duct.10 In this case, aminotransferases may reach values greater than 1000, decreasing quickly, with liver test rapidly evolving into those of typical cholestasis or normalizing completely.
Aminotransferases levels also vary with age, sex, race, and body mass index.11 Levels are found to be higher in obese patients and lower in dialysis patients,12 whereas ALT levels are noted to decline with weight loss.13 AST levels are 15% higher in African American males as compared with Caucasians.11 Some individuals may have asymptomatic AST elevation caused by a defect in clearance of the enzyme.14 Transaminases levels can be very high in patients with acute viral hepatitis, drug‐induced liver injury, hepatic ischemia, and Budd‐Chiari syndrome (Fig. 2). In asymptomatic patients with no underlying disease, mild aminotransferase elevation for more than 6 months warrants further investigation.22
Figure 2.
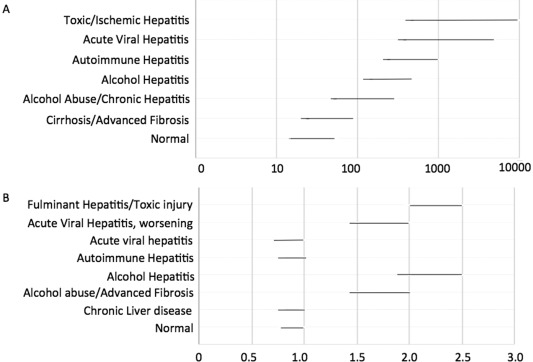
Typical AST elevation and De Ritis ratios for different kinds of liver diseases.
Figure 3.
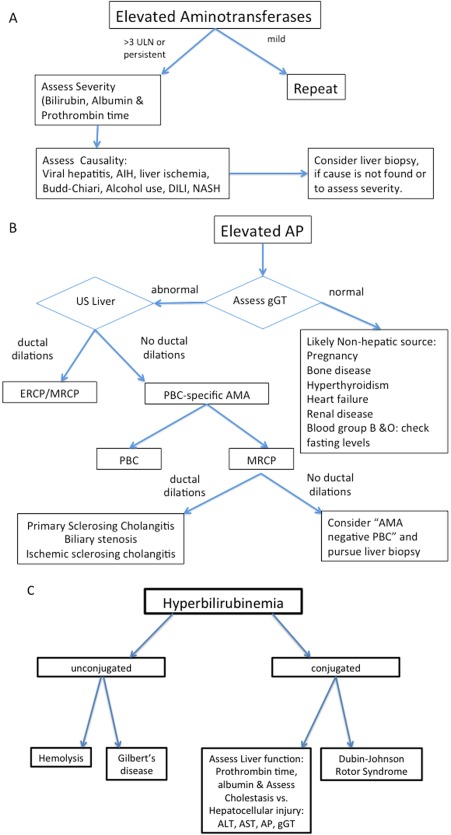
Algorithms for evaluation of elevated aminotransferases (A), AP (B), and bilirubin (C), respectively. Abbreviations: AIH, autoimmune hepatitis; AMA, antimitochondrial antibodies; DILI, drug‐induced liver injury; ERCP, endoscopic retrograde cholangiopancreatography; MRCP, magnetic resonance cholangiopancreatography; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cholangitis.
Markers of Cholestasis
AP is the standard liver test reflecting cholestasis and can be complemented by gGT. gGT is part of a typical liver panel in some countries, whereas in the United States the standard liver test usually includes only AST, ALT, and AP. Because gGT is diffusely located in endoplasmic reticulum of bile ductal cells, its elevation is less specific for cholestasis but supports the suspicion that an elevated AP is liver derived as opposed to being of extrahepatic origin (Tables 1 and 4).15
Table 4.
Elevated AP
| Hepatic | Nonhepatic |
|---|---|
| Bile duct obstruction | Bone disease |
| Benign intrahepatic recurrent cholestasis | Pregnancy |
| Primary biliary cholangitis | Chronic renal failure |
| Primary sclerosing cholangitis | Lymphoma and other malignancies |
| Medications | Congestive heart failure |
| Infiltrating diseases of the liver | Childhood growth |
| Sarcoidosis | |
| Hepatic metastasis |
Elderly individuals older than 60 years, especially women, may have a mildly elevated AP.16 Individuals with blood types O and B may have an elevation of the serum AP after eating a fatty meal because of the influx of intestinal AP into circulation.7 AP can also be nonpathologically elevated in children and adolescents undergoing rapid bone growth16 and in women late in normal pregnancies because of the influx of placental AP.17
Markers of Liver Function
Bilirubin, albumin, and prothrombin time are standard tests to evaluate the liver function.
Bilirubin is the result of enzymatic breakdown of heme. Bilirubin is conjugated in the liver, resulting in water solubility. The conjugated bilirubin is then secreted into the bile. In healthy individuals, conjugated bilirubin comprises a small proportion of total bilirubin.18
In adults, unconjugated bilirubin elevation is most often of extrahepatic origin, mainly caused by hemolysis. In the absence of hemolysis, isolated unconjugated hyperbilirubinemia in an otherwise healthy patient should raise the suspicion for Gilbert syndrome. Up to 5% of the population has Gilbert syndrome, which is due to partial defects in uridine 5′‐diphosphate‐glucuronosyltransferase, the enzyme that conjugates bilirubin.19 Crigler‐Najjar syndrome is a rare cause of unconjugated hyperbilirubinemia.
In adults, conjugated hyperbilirubinemia is almost always a sign of biliary obstruction or impaired hepatic function. Two rare hereditary conditions cause defects in the secretory mechanism, Dubin‐Johnson syndrome and Rotor syndrome, which result in elevated conjugated bilirubin.
Total serum bilirubin with increased prothrombin time correlates with poor outcomes in alcoholic hepatitis.18 Both are also critical components of Model for End‐Stage Liver Disease (MELD) score and Child‐Pugh score.
Serum albumin is exclusively synthesized by hepatocytes, but the long half‐life of albumin makes it difficult to interpret in the setting of acute liver injury. In chronic liver disease, albumin is the first of the three standard liver function tests to decline in advancing liver cirrhosis, before increase in bilirubin or prothrombin time. Albumin less than 35 g/dL should raise suspicion for cirrhosis. Differential diagnosis for hypoalbuminemia includes protein malnutrition of any cause, as well as protein‐losing enteropathies, nephrotic syndrome, and chronic infection.
With the exception of factor VIII, all coagulation factors are synthesized in the liver. Because of the short half‐lives of the coagulation factors, these are the best parameters to measure synthetic function of liver in acute conditions. This is most frequently done by prothrombin time determination. Because most clotting factors synthesized in the liver depend on vitamin K, prothrombin time is affected by vitamin K deficiency or use of vitamin K inhibitors. Vitamin K deficiency is seen in patients with chronic cholestasis or fat malabsorption from disease of the pancreas or small bowel. Prothrombin time is a better indicator of hepatic dysfunction than the international normalized ratio (INR),20 despite INR having become a crucial part of the MELD score used for prioritizing liver allocations. In acute and chronic liver disease, prolonged prothrombin time (>5 seconds), which does not respond to parenteral vitamin K, is a poor prognostic sign.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137:1-10. [DOI] [PubMed] [Google Scholar]
- 2. Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004;328:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kundrotas LW, Clement DJ. Serum alanine aminotransferase (ALT) elevation in asymptomatic US Air Force basic trainee blood donors. Dig Dis Sci 1993;38:2145-2150. [DOI] [PubMed] [Google Scholar]
- 4. Lin YC, Lee WT, Huang SF, Young C, Wang PJ, Shen YZ. Persistent hypertransaminasemia as the presenting findings of muscular dystrophy in childhood. Acta Paediatr Taiwan 1999;40:424-429. [PubMed] [Google Scholar]
- 5. Scola RH, Werneck LC, Prevedello DM, Toderke EL, Iwamoto FM. Diagnosis of dermatomyositis and polymyositis: a study of 102 cases. Arq Neuropsiquiatr 2000;58(3B):789-799. [DOI] [PubMed] [Google Scholar]
- 6. De Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis: the transaminase serum activities. J Infect Dis 1957;101:219-223. [DOI] [PubMed] [Google Scholar]
- 7. Matsushita M, Komoda T. [Relationship between the effects of a high‐fat meal and blood group in determination of alkaline phosphatase activity]. Rinsho Byori 2011;59:923-929. [PubMed] [Google Scholar]
- 8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev 2013;34:117-130. [PMC free article] [PubMed] [Google Scholar]
- 9. Botros M, Sikaris KA, Lu ZX, McNeil A. The short term prognostic usefulness of the De Ritis ratio. Clin Biochem Rev 2013;34:S18. [PMC free article] [PubMed] [Google Scholar]
- 10. Anciaux, M. L. , Pelletier, G. , Attali, P. , Meduri, B. , Liguory, C. , & Etienne, J. P. Prospective study of clinical and biochemical features of symptomatic choledocholithiasis. Dig Dis Sci 1986;31:449-453. [DOI] [PubMed] [Google Scholar]
- 11. Siest G, Schiele F, Galteau MM, Panek E, Steinmetz J, Fagnani F, et al. Aspartate aminotransferase and alanine aminotransferase activities in plasma: statistical distributions, individual variations, and reference values. Clin Chem 1975;21:1077-1087. [PubMed] [Google Scholar]
- 12. Yasuda K, Okuda K, Endo N, Ishiwatari Y, Ikeda R, Hayashi H, et al. Hypoaminotransferasemia in patients undergoing long‐term hemodialysis: clinical and biochemical appraisal. Gastroenterology 1995;109:1295-1300. [DOI] [PubMed] [Google Scholar]
- 13. Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology 1990;99:1408-1413. [DOI] [PubMed] [Google Scholar]
- 14. Vajro P, Lofrano MM, Fontanella A, Fortunato G. Immunoglobulin complexed AST (“macro‐AST”) in an asymptomatic child with persistent hyper‐transaminasemia. J Pediatr Gastroenterol Nutr 1992;15:458-460. [DOI] [PubMed] [Google Scholar]
- 15. Levinson M, Holbert J, Blackwell C, Wruble LD. Serum gamma‐glutamyl transpeptidase: its specificity and clinical value. South Med J. 1979;72:837-841. [DOI] [PubMed] [Google Scholar]
- 16. Moss DW. Alkaline phosphatase isoenzymes. Clin Chem 1982;28:2007-2016. [PubMed] [Google Scholar]
- 17. Fishman WH, Bardawil WA, Habib HG, Anstiss CL, Green S. The placental isoenzymes of alkaline phosphatase in sera of normal pregnancy. Am J Clin Pathol 1972;57:65-74. [DOI] [PubMed] [Google Scholar]
- 18. Pratt DS. Evaluation of liver function In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, eds. Harrison's Principles of Internal Medicine, 19th ed New York, NY: McGraw‐Hill; 2015. [Google Scholar]
- 19. Monaghan G, Ryan M, Seddon R, Hume R, Burchell B. Genetic variation in bilirubin UPD‐glucuronosyltransferase gene promoter and Gilbert's syndrome. Lancet 1996;347:578-581. [DOI] [PubMed] [Google Scholar]
- 20. Robert A, Chazouilleres O. Prothrombin time in liver failure: time, ratio, activity percentage, or international normalized ratio? Hepatology 1996;24:1392-1394. [DOI] [PubMed] [Google Scholar]
- 21. Hultcrantz R, Glaumann H, Lindberg G, Nilsson LH. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scand J Gastroenterol 1986;21:109-113. [DOI] [PubMed] [Google Scholar]
- 22. Mathiesen UL, Franzen LE, Fryden A, Foberg U, Bodemar G. The clinical significance of slightly to moderately increased liver transaminase values in asymptomatic patients. Scand J Gastroenterol 1999;34:85-91. [DOI] [PubMed] [Google Scholar]


