Abstract
AIM:
To carry out the comparative nootropic, neuroprotective potentials of two medicinal plant species.
MATERIAL AND METHODS:
For neuroprotective activity; behavior models (elevated plus maze & morris water maze), in vivo antioxidant (superoxide dismutase, catalase, lipid peroxidation & reduced glutathione), inflammatory markers (IL-1β, IL-6 & TNF-α) and acetylcholine esterase (AChE) assessment procedures followed at different dosages i.e. 250 & 500 mg/kg of Evolvulus alsinoides and Centella asiatica ethanolic extracts. At the end of the study, it was performed histopathological analysis of the following organs: brain, heart, liver, and kidney.
RESULTS:
In oral administration of different doses of ethanolic extracts of both medicinal plants i.e. Sco + EEA 250 = 2.49 ± 0.29 , Sco + EEA 500 = 2.67 ± 0.36, Sco + ECA 250 = 2.33 ± 0.17, Sco + ECA 500 = 2.77 ± 0.21, Sco + EEA + ECA 250 = 2.61 ± 0.32 and Sco + EEA + ECA 500 = 2.79 ± 0.16 U/mg of protein respectively against the scopolamine induced group Sco (control) = 5.51 ± 0.35 U/mg of protein extracts shows neuroprotective and nootropic activity with reducing AChE level in the brain homogenate of swiss albino mice.
CONCLUSION:
Since the E. alsinoides & C. asiatica are already used in traditional Indian medicine as the neuroprotective agent and also found promising effects over inflammatory diseases, wound healing, and immunomodulatory activity. The neuroprotective effect of both plants extracts attributed to inhibition of AChE activity and improve the spatial memory formation.
Keywords: Centella asiatica, Evolvulus alsinoides L, Ethanolic extracts, In vivo antioxidant, Neuroprotective, Acetylcholine esterase
Introduction
Alzheimer’s disease is a progressive neurodegenerative disease which is characterised by the loss of learning and memory abilities with ageing [1]. This impairment of memory is correlated with the loss of cholinergic neurons [2], [3]. Learning and memory are complex processes and thought not to be regulated by only one muscarinic receptor subtype; scopolamine a non-selective muscarinic receptor antagonist has been reported to impair both acquisition and long-term memory formation. Scopolamine-induced amnesic animal models are used to screen for drugs that potentially have anti-dementia activities by stimulating the cholinergic system, make them a candidate for the treatment of Alzheimer’s disease [4]. Ayurveda, the Indian system of medicine describe a group of medicinal plants under the category of ‘Medhya Rasayana’, which possess the memory-enhancing effect and facilitate learning acquisition. Medicinal plants are rich sources of important metabolites, which are potential sources of antioxidant, antimicrobial, anti-inflammatory, and anticancer activities [5], [6], [7]. The utilisation of herbal medicine in treating infectious diseases have been practised for 1000s of years and will continue to provide humanity with new remedies [8].
Evolvulus alsinoides L.
Evolvulus alsinoides L. an important medicinal plant is employed for different ailments in India traditionally and grows in the open and grassy places almost throughout India and mostly in the region from India to west Cameroon and widely dispersed elsewhere in tropical Africa and worldwide. Some vernacular names of the plant in India are vishnukranta, vishnugandhiy (sanskrit), shankapushpi (hindi). Evolvulus alsinoides L. is used in Ayurveda as a brain tonic in the treatment of neurodegenerative diseases, asthma and amnesia [9].
Centella asiatica
Centella asiatica is a very important medicinal herb also known as Mandukparni, which has been used as a medicine in the Ayurvedic tradition of India for thousands of years and mentioned in many classical texts of Ayurveda. Centella asiatica (CA) is a rejuvenative nervine recommended for nervous disorders, epilepsy, senility and premature ageing and several other medical conditions. As a brain tonic, it is said to aid intelligence and memory [10]. The extract of Centella asiatica especially from roots and leaves contain a high anti-oxidative activity, which was as good as tocopherol, a natural anti-oxidant, have been reported to play a significant role in wound healing [11]. Centella asiatica extract contains four principle bioactive compounds: asiatic acids, made classic acid, asiaticoside and madecassoside [12], [13], in which asiaticoside was identified as the main active constituent responsible for wound healing.
We are working on the development of anti-dementia natural drug using an in vivo screening of herbal materials due to their safety and cost-effectiveness [14].
Material and Methods
Plant material
Whole plant material of Centella asiatica and Evolvulus alsinoides were collected from village Ramnapur, Varanasi, Uttar Pradesh, India in October 2015 and authentication was done by Department of Botany, Banaras Hindu University, India and also herbarium of Centella asiatica (voucher specimen no. Apia/02/2015) and Evolvulus alsinoides (voucher specimen no. Convolvul./03/2015) of plant was deposited in the Department of Botany, Banaras Hindu University, India.
Preparation of extracts
The extraction of whole plants was done with Soxhlet method in ethanolic solvents at 72-82°C for 72 hours. The Soxhlet extraction has widely been used for extracting valuable bioactive compounds from various natural sources. It is used as a model for the comparison of new extraction alternatives. A small amount of dry sample is placed in a thimble. The thimble is then placed in distillation flask which contains the solvent of particular interest. After reaching an overflow level, the solution of the thimble-holder is aspirated by a siphon. Siphon unloads the solution back into the distillation flask. This solution carries extracted solutes into the bulk liquid. The solute remains in the distillation flask, and solvent passes back to the solid bed of plant. The processrepeatedly runs until the extraction is completed.
Preparation of extract samples
Ethanolic extracts of C. asiatica (ECA) and E. alsinoides (EEA) were solubilized in distilled water to obtain solutions of 250 and 500 mg/ml [15].
Animals
The experimental Swiss albino mice were issued by Animal house of Institute of Medical Sciences, Banaras Hindu University Varanasi, Uttar Pradesh. Animals were divided into experimental groups, housed in plastic cages and maintained on a 12-hour light and 12-hour dark cycle. They were given standard food and water ad libitum. The Central Animal Ethical Committee of Banaras Hindu University approved all experimental procedures (CAEC/196).
Experimental design and Drug administration
Scopolamine (1.0 mg/kg; dissolved in distilled water) was administered intraperitoneal (i.p.) to the experimental animals. The ethanolic extracts of Evolvulus alsinoides (EEA) and Centella asiatica (ECA) in the following doses: EEA 250 mg/kg/day & 500 mg/kg/day, ECA 250 mg/kg/day & 500 mg/kg/day and combination of EEA + ECA 250 mg/kg/day & 500 mg/kg/day, dissolved in 0.3% CMC and was administered orally (p.o.). Nine groups, each consisting of 6 animals, were included in the study.
Group I (normal) was treated with vehicle daily (0.3% CMC; p.o).
Group II (Sco control) was treated with scopolamine (1.0 mg/kg/day; i.p.).
Group III (Sco + Doz) was treated with a donepezil (1.5 mg/kg/day; i.p.) and scopolamine (1.0 mg/kg/day; i.p.).
Group IV (Sco + EEA 250) was treated with low dose EEA (250 mg/kg/day; p.o.) and scopolamine (1.0 mg/kg/day; i.p.).
Group V (Sco + EEA 500) was treated with high dose of EEA (500 mg/kg/day; p.o.) and scopolamine (1.0 mg/kg/day; i.p.).
Group VI (Sco + ECA 250) was treated with low dose ECA (250 mg/kg/day; p.o.) and scopolamine (1.0 mg/kg/day; i.p.).
Group VII (Sco + ECA 500) was treated with high dose of ECA (500 mg/kg/day; p.o.) and scopolamine (1.0 mg/kg/day; i.p.).
Group VIII (Sco + EEA + ECA 250) was treated with low dose of EEA (250 mg/kg/day; PO) & ECA (250 mg/kg/day; p.o.) and scopolamine (1.0 mg/kg/day; i.p.).
Group IX (Sco + EEA + ECA 500) was treated with high dose of EEA (500 mg/kg/day; p.o.) & ECA (500 mg/kg/day; p.o.) and scopolamine (1.0 mg/kg/day; i.p.).
Behavioural study
Elevated plus maze
The elevated plus maze (EPM) is designed to study the behavioural pattern of experimental animals such as sensitivity to external stimuli (exteroceptive behaviour), anxiety, exploration as well as learning and memory [16], [17]. The EPM consists of four arms (two open and two closed), each 49 cm x 10 cm, with 40 cm high walls in closed arms and the open roof. The whole structure is elevated 50 cm above the ground. On the 10th day, 60 min after the drug treatment, each mice was placed at the end of an open arm, facing away from the central platform. The time is taken by the mice to enter any of the closed arms was recorded and considered as the transfer latency (TL) and served as a parameter for acquisition/ learning. If the mice did not enter into any one of the closed arms within 180 sec, it was gently pushed into one of the two closed arms, and the TL was assigned as 180 sec. For the next 15 sec, the mice were allowed to explore the maze before returning it to its home cage. On the 14th day, TL was recorded again, which served as a parameter for retention of memory. Between each session, the maze was carefully cleaned with 30% ethanol tissue to remove any olfactory cues.
Morris water maze
The Morris water maze (MWM) [18], [19] test was performed to assess the learning and spatial memory of the experimental animals. The advantage of MWM over other models of learning and memory lies in the fact that no motivational stimuli such as food and water deprivation, electrical stimulation and buzzer sounds are applied. The apparatus consists of a circular pool (45 cm in height and 100 cm in diameter) with a featureless inner surface. The pool was filled with opaque water, maintained at the temperature of 22 ± 2°C, to a height of 30 cm, and was divided into four quadrants of equal area, marked by different visual cues. A platform (29 cm X 6 cm) was placed one centimetre below the level of water at the centre of one of the four quadrants, which was considered as the target quadrant. The position of the platform was kept unaltered throughout the experiment. The MWM test was performed on the 10th day after drug administration was started. On the first experimental day, the mice were allowed to acclimatise in the pool and swim for 120 sec without the platform. During the next four consecutive days, each animal received four learning trials of 120 sec with an inter-trial interval of 60 sec.
For each learning trial, the mice were placed in the water facing the pool wall diagonally opposite to the quadrant in which the platform was kept. The time taken by the animal to locate the submerged platform was recorded as Escape latency time (ELT) for each trial. If the animal were unable to locate the platform within 120 sec, it was directed to the platform and allowed to rest there for 60 sec, and in this case, the ELT was recorded as 120 sec. These sessions were recorded as hidden platform trials for acquisition test. On the 14th day after the learning trial session, the platform was removed from the pool, and the mice were subjected to a Probe trial session to assess memory retention. Each mouse was placed into the water diagonally opposite to the target quadrant, and for 60 sec was allowed to swim and find the quadrant in which the platform was previously placed. The swimming time of the animal to reach the target quadrant was recorded as probe trial memory retention test.
Preparation of Brain homogenate
On the terminal experimental day, animals were euthanized, and the whole brains of all mice were isolated after performing cardiac perfusion with normal saline for biochemical estimations. Then further rinsed in ice-cold isotonic saline and were homogenized with ice-cold 0.1 M phosphate buffer saline (pH 7.4) to form 10% w/v homogenates. These homogenates were then further centrifuged at -4°C (10,000 rpm; C-24 cooling centrifuge instrument, Model no. C-24 Remi, India) for 15 min and the supernatant was used for estimation of biochemical parameters [20].
In vivo antioxidants assessment
Assessment of Superoxide dismutase activity
Every 3 ml of reaction mixture contained 2.8 ml of potassium phosphate buffer (0.1 M, pH 7.4), 0.1 ml of brain homogenate and 0.1 ml of pyrogallol solution (2.6 mM in 10 mM HCl). The change in absorbance was recorded at 325 nm for 5 min with 30 sec. Interval. One unit of SOD is equivalent to the amount of enzyme required to cause 50% inhibition of pyrogallol autoxidation per 3 ml of the assay mixture [21].
Assessment of Catalase activity
The reaction mixture contained 2.0 ml of diluted homogenate in 0.1 M phosphate buffer (enzyme extract). The reaction was started by adding 1.0 ml of 200 mM H2O2. The decrease in OD per min was recorded against the blank (all the reagents except enzyme extract) for 3 min at 240 nm at intervals of 15 sec. CAT activity was expressed as U/mg protein [22].
Assessment of Lipid peroxidation activity
To 100 μl of tissue homogenate, 1.5 ml of 10% TCA solution was added. After 10 min, centrifuge at 5000 rpm for 10 min. The supernatant was separated and mixed with 1.5 ml of TBA. The tubes were kept in boiling water bath for 30 min to complete reaction and were cooled under tap water. The absorbance of the sample at 535 nm against distilled water [23].
Assessment of Reduced glutathione (GSH) activity
The GSH assays were performed as described by Smith I K et al., [24]. In GSH assay, 3 ml of reaction mixture consisted of 2.9 ml of 5, 5-dithiobis (2-nitrobenzoate) (DTNB) prepared in potassium phosphate buffer (0.1 M, pH 7.4) and 0.1 ml of tissue homogenate. The reaction mixture was incubated at 37°C for 15 min, and the absorbance was recorded at 412 nm, and the results were expressed as GSH/mg protein.
Analysis of IL-1β, IL-6 and TNF-α in the brain
The levels of IL-1β, IL-6, and TNF-α in the brain homogenates were determined using commercial (Elabscience) ELISA kits according to the manufacturer’s instructions. The levels of these cytokines in the brain tissues were normalized to the protein content.
AChE estimation
AChE activity was estimated in the whole brain homogenates according to Ellman’s method [25]. Briefly, the brain homogenate was incubated for 5 min with 2.7 ml of phosphate buffer and 0.1 ml of 5, 5-dithiobis (2-nitrobenzoate) (DTNB). Further, 0.1 ml of freshly prepared acetylcholine iodide (pH 8) was added, and the change in absorbance was recorded at 412 nm.
Statistical analysis
The relative haematological and biochemical data were expressed as the mean ± standard error of the mean (SEM). Data were submitted to analysis of variance (one-way ANOVA) followed by Dunnett multiple comparison tests. The results were expressed as mean ± SEM. The software GraphPad Prism 6.0 (GraphPad Software, USA) was used for statistical analysis. P < 0.05 were considered statistically significant.
Results
Behavioral study
Elevated plus maze
The elevated plus maze (EPM) was used to study the behavioral pattern of mice such as anxiety, exploration as well as learning and memory and results are shown in Figure 1. At the days 1st, 6th, 10th and 14th with transfer latency time (sec) for Sco + ECA 250 = 23 ± 0.7, 19 ± 2.27, 18 ± 0.67, 16 ± 0.73; Sco + ECA 500 = 27 ± 2.39, 26 ± 0.79, 20 ± 0.97, 17 ± 0.97; Sco + EEA + ECA 250 = 20 ± 1.73, 18 ± 1.32, 18 ± 0.51, 16 ± 0.72 groups have shown good activity in respect to Sco (control) = 45 ± 2.1, 48 ± 2.1, 50 ± 2.3, 52 ± 2.3 group and Sco + Doz (standard)= 22 ± 1.28, 20 ± 2.86, 18 ± 2.3, 14 ± 2.52 group.
Figure 1.
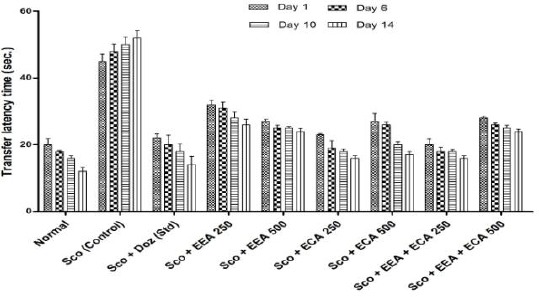
Transfer latency time for all groups in elevated plus maze behavioral study. Data expressed as mean ± sem, n = 6. P < 0.01, compared with the Sco group and P < 0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post –ANOVA test
Morris water maze
The Escape Latency Time (ELT) was measured to assess spatial memory in mice, and the results are shown in Figure 2. On 1st and 6th day and we found that there was no significant difference in ELT in all mice groups. On the 10th and 14th day all plant extract treated groups were found to improve the spatial memory in a dose-dependent manner with a significant (P < 0.01).
Figure 2.
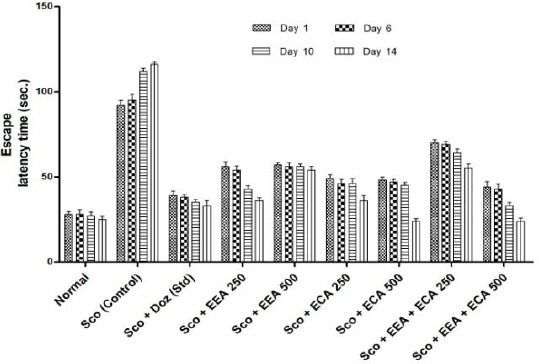
Escape Latency Time (ELT) for all groups in morris water maze behavioral study. Data expressed as mean ± sem, n = 6. P < 0.01, compared with the Sco group and P < 0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test
On 14th day plant extracts treatment groups significantly reduced the ELT at Sco + EEA 250 = 56 ± 2.88, 54 ± 2.3, 43 ± 1.85, 36 ± 1.72; Sco + ECA 250 = 49 ± 2.33, 46 ± 2.5, 46 ± 2.88, 36 ± 2.98; Sco + ECA 500 = 48 ± 1.95, 47 ± 1.45, 45 ± 1.73, 24 ± 1.5; Sco + EEA + ECA 500 = 44 ± 3.2, 43 ± 2.66, 33 ± 1.85, 24 ± 1.76 & Sco + Doz (standard) = 39 ± 2.66, 38 ± 1.62, 35 ± 1.73, 33 ± 2.88 and showed significantly improvement in spatial memory on both the days during treatment regimen (P < 0.01) in comparison with Scopolamine treated mice group Sco (control) = 92 ± 2.8, 95 ± 3.4, 112 ± 1.76, 116 ± 1.45.
In vivo antioxidant activity
Super oxide Dismutase (SOD)
SOD is the major antioxidant enzyme scavenging the free radicals generated during oxidative stress were estimated and results are showed in Figure 3. The Sco + EEA 250 = 146 ± 6.01, Sco + EEA 500 = 148.66 ± 5.23 & Sco + ECA 250 = 148.89 ± 6.63 treatment groups significantly increased the SOD activity at all doses levels (P < 0.01) except Sco + ECA 500 = 141.13 ± 5.18, Sco + EEA + ECA 250 = 141.63 ± 6.11 and Sco + EEA + ECA 500 = 131.55 ± 4.25 found not significant in comparison with Sco (Control) = 119.78 ± 4.57 treated mice group.
Figure 3.
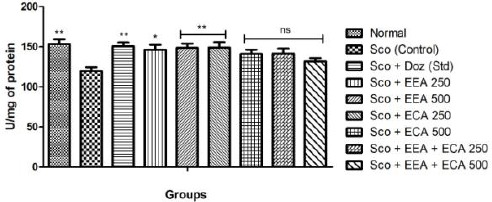
Super oxide dismutase activity in all mice groups show significant increase. Data expressed as mean ± sem, n = 6. P < 0.01, compared with the Sco (control) group and P<0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant)
Sco + Doz (standard) = 150.52 ± 4.61 administration also showed protection from oxidative stress by elevating the SOD activity as compared to Sco (control) mice (P < 0.01).
Catalase
In the all treatment groups; Sco + EEA 500 = 126.8 ± 5.7, Sco + ECA 500 = 126.0 ± 5.2 & Sco + EEA + ECA 500 = 126.7 ± 5.8 & Sco + Doz (standard) = 131.1 ± 4.7 significantly increased the catalase activity (P < 0.01) except Sco + ECA 250 = 103.1 ± 6.6 which found not significant in comparison with Sco (control) = 98.32 ± 5.3 treated mice group (Figure 4).
Figure 4.
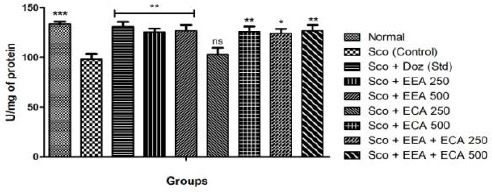
Catalase activity in all mice groups and Data expressed as mean ± sem, n = 6. P < 0.01, compared with the Sco group and P < 0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant)
Lipid Peroxidation
In the all treatment groups; Sco + EEA 250 = 0.48 ± 0.05, Sco + EEA 500 = 0.37 ± 0.03 & Sco + ECA 500 = 0.45 ± 0.02 and Sco + EEA + ECA 250 = 0.6 ± 0.03 significantly increased the LPO activity (P < 0.05) except Sco + ECA 250 = 0.66 ± 0.03 and Sco + EEA + ECA 500 = 0.7 ± 0.03 in comparison with Sco (control) = 0.81 ± 0.06 treated mice group as well as Sco + Doz (standard) = 0.45 ± 0.02 also decreased the elevated MDA levels.
Figure 5.
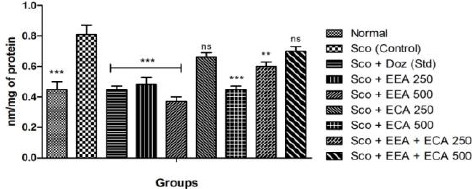
Lipid peroxidation assay in all mice groups showed significant decrease in comparison to Sco group. Data expressed as mean ± sem, n = 6. P < 0.01, compared with the Sco (control) group and P < 0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant)
Reduced glutathione (GSH)
Reduced glutathione (GSH) level were increased after treatment with all the experimental animal groups; Sco + EEA 250 = 4.75 ± 0.47, Sco + EEA 500 = 4.61 ± 0.18, Sco + ECA 500 = 4.26 ± 0.06 & Sco + EEA+ ECA 250 = 4.18 ± 0.09 which found to be significant (P < 0.05) except Sco + ECA 250 = 4.08 ± 0.16 and Sco + EEA + ECA 500 = 4.02 ± 0.11 which found not significant in comparison to Sco (control) = 3.2 ± 0.26 treated group and Sco + Doz (standard) = 4.8 ± 0.15 significantly elevated GSH levels (P < 0.05).
Figure 6.
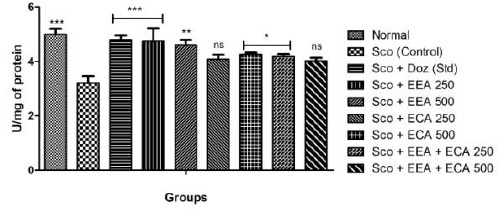
Reduced glutathione activity in all mice groups showed significant increase in comparison to Sco group. Data expressed as mean ± sem, n = 6. P < 0.05, compared with the Sco group and P < 0.05 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant)
Inflammatory markers
IL-1β
The effect of plant extracts treated at different concentrations Sco + EEA 250 = 394.1 ± 23.03, Sco + EEA 500 = 355.5 ± 14.64, Sco + ECA 500 = 387.26 ± 25.3 & Sco + EEA + ECA 250 = 386.21 ± 14.38 & Sco + Doz (standard) = 396.38 ± 15.28 on whole brain markedly decrease the levels of IL-1β when compared with Sco (control) = 485.85 ± 14.76 group (P < 0.01) although Sco + ECA 250 = 405.5 ± 23.16 and Sco + EEA + ECA 500 = 406.38 ± 24.65 groups founds to be not significant.
Figure 7.
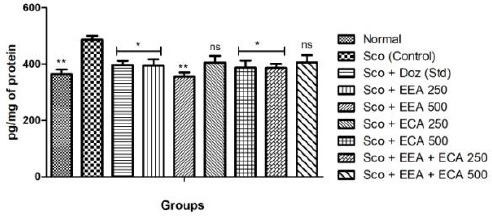
Effect of plant extracts on brain IL-1β levels in Sco induced mice model. Data expressed as mean ± sem, n = 6. P < 0.01, compared with the Sco group and P < 0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P < 0.05, **P < 0.01, ***P < 0.001, ns= not significant)
IL-6
Figure 8 shows the effect of plant extracts on the IL-6 levels in the whole brain and we found that Sco + EEA 500 = 395.54 ± 14.17, Sco + ECA 250 = 398.87 ± 16.25 Sco + ECA 500 = 395.12 ± 23.68 & Sco + EEA + ECA 250 = 413.66 ± 20.26 & Sco + Doz (standard) = 360.54 ± 20.86 except Sco + EEA 250 = 465.75 ± 13.62 all treatment groups markedly decrease the levels of IL-6 when compared with Sco (control) = 541.58 ± 19.82 group.
Figure 8.
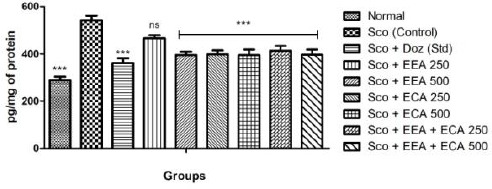
Effect of plant extracts on brain IL-6 levels in Sco induced mice model. Data expressed as mean ± sem, n = 6. P < 0.01, compared with the Sco group and P < 0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant)
TNF-α
Figure 9 shows the effect of plant extracts on the TNF-α level in the whole brain on treatment with Sco + EEA 250 = 112.17 ± 11.52; Sco + EEA 500 = 108.97 ± 12.32; Sco + ECA 250 = 130.12 ± 11.50; Sco + ECA 500 = 176.92 ± 14.72; Sco + EEA + ECA 250 = 109.61 ± 15.86; Sco + EEA + ECA 500 = 107.69 ± 14.63 & Sco + Doz (standard) = 83.97 ± 14.42 subsequently decrease the levels of TNF-α activity when compared with Sco (control) = 233.97 ± 12.4 group.
Figure 9.
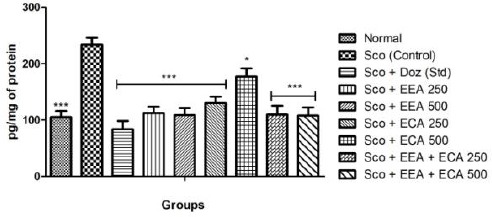
Effect of plant extracts on brain TNF-α levels in Sco induced mice model. Data expressed as mean ± sem, n = 6. P < 0.01, compared with the Sco group and P < 0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant)
Acetylcholine esterase (AChE) activity
Sco administration resulted in significant increase in the brain AChE activity. All the treatment groups Sco + EEA 250 = 2.49 ± 0.29; Sco + EEA 500 = 2.67 ± 0.36; Sco + ECA 250 = 2.33 ± 0.17; Sco + ECA 500 = 2.77 ± 0.21; Sco + EEA + ECA 250 = 2.61 ± 0.32; Sco + EEA + ECA 500 = 2.79 ± 0.16 & Sco + Doz (standard) = 2.74 ± 0.3 significantly inhibit the AChE activity in comparison with Sco (control) = 5.51 ± 0.35 treated mice group.
Figure 10.
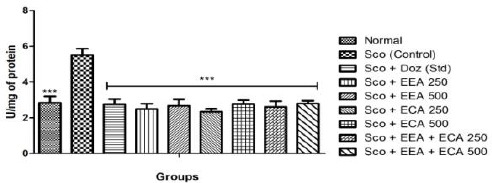
Acetylcholine esterase activity in all mice groups found significant in comparison to Sco group. Data expressed as mean ± sem, n = 6. P < 0.01, compared with the Sco group and P < 0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant)
Discussion
In neurodegeneration, oxidative stress plays an important role in the ageing process, and the brain is highly susceptible to the oxidative imbalance due to its high energy demand, high oxygen consumption [26]. Basal forebrain and amygdala are involved in learning and memory formation and are more vulnerable areas susceptible to oxidative stress [27]. Mitochondrial dysfunction caused by increased reactive oxygen species generation has been involved in ageing and neurodegenerative disorders [28]. In this study, Scopolamine treatment significantly decreased the reactive oxygen species scavenging enzymes activities like superoxide dismutase, catalase and also reduced glutathione levels in brain tissue. Scopolamine treatment also significantly increased lipid peroxidation as compared to the normal group. Both plants extract me. e. EEA, ECA, EEA + ECA treatments were found to significantly elevate the SOD, catalase activity, lipid peroxidation activity and reduced glutathione levels in comparison with Scopolamine group. The expression of cytokine receptors is temporally and spatially regulated in the central nervous system, and they are closely involved in cell proliferation, gliogenesis, neurogenesis, cell migration, apoptosis, and synaptic release of neurotransmitters [29], [30]. The level of cytokines IL-1β, IL-6 and TNF-α are significantly decreased on treatment with plant extracts which increased by Scopolamine administration. The elevated activity of AChE leads to increased degradation of acetylcholine (Ach) neurotransmitter and which in turn declines the ACh pool in the brain which is essential in learning and memory [31]. Scopolamine administration amplifies the AChE activity which is one of the major causes for the cholinergic deficit occurrence after its administration [20]. In this study we found that treatment with both plants ethanolic extracts significantly reduced the AChE activity as compared to the Scopolamine treated mice. It reveals that inhibition of AChE activity by plant extracts have a protective role in acetylcholine degradation and improved the cholinergic neurotransmission. Thus, plant extracts reduced the cholinergic deficits produced by Scopolamine administration resulting in an enhanced neuroprotective effect.
Since the E. alsinoides & C. asiatica are already used in traditional Indian medicine as the neuroprotective agent and also found promising effects over inflammatory diseases, wound healing, and immunomodulatory activity. The neuroprotective effect of both plants extracts attributed to inhibition of AChE activity and improve the spatial memory formation. The neuroprotective activity could be ascribed to extracts strong antioxidant potential, inhibitory role on AChE activity. The research above findings of this study prospect ethanolic extracts of both plants, i.e. EEA, ECA and EEA + ECA extracts as the promising therapeutic candidate for neurodegenerative diseases.
Acknowledgement
This research work was done in collaboration with the Department of Kayachikitsa & Centre of Experimental Medicine & Surgery (CEMS), Institute of medical sciences, Banaras Hindu University, Varanasi. All authors contributed equally.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Terry AV, Jr, Callahan PM, Hall B, Webster SJ. Alzheimer's disease and age-related memory decline (preclinical) Pharmacology Biochemistry and Behavior. 2011;99(2):190–210. doi: 10.1016/j.pbb.2011.02.002. https://doi.org/10.1016/j.pbb.2011.02.002 PMid:21315756 PMCid:PMC3113643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer's disease and the basal forebrain cholinergic system:relations to β-amyloid peptides, cognition, and treatment strategies. Progress in neurobiology. 2002;68(3):209–45. doi: 10.1016/s0301-0082(02)00079-5. https://doi.org/10.1016/S0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 3.Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer's disease:therapeutic implications. Expert review of neurotherapeutics. 2008;8(11):1703–18. doi: 10.1586/14737175.8.11.1703. https://doi.org/10.1586/14737175.8.11.1703 PMid:18986241 PMCid:PMC2631573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrera-Morales W, Mar I, Serrano B, Bermúdez-Rattoni F. Activation of hippocampal postsynaptic muscarinic receptors are involved in long-term spatial memory formation. European Journal of Neuroscience. 2007;25(5):1581–8. doi: 10.1111/j.1460-9568.2007.05391.x. https://doi.org/10.1111/j.1460-9568.2007.05391.x PMid:17355252. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez NE, Tereschuk ML, Abdala LR. Antimicrobial activity of flavonoids in medicinal plants from Tafídel Valle (Tucumain, Argentina) J Ethnopharmacol. 2000;73:317–322. doi: 10.1016/s0378-8741(00)00295-6. https://doi.org/10.1016/S0378-8741(00)00295-6. [DOI] [PubMed] [Google Scholar]
- 6.Polya G. Biochemical targets of plant bioactive compounds:a pharmacological reference guide to sites of action and biological effects. CRC press; 2003. https://doi.org/10.1201/9780203013717 PMid:12901082. [Google Scholar]
- 7.Maruthanila VL, Poornima J, Mirunalini S. Attenuation of carcinogenesis and the mechanism underlying by the influence of indole-3-carbinol and its metabolite 3, 3′-diindolylmethane:a therapeutic marvel. Advances in pharmacological sciences. 2014;2014:1–7. doi: 10.1155/2014/832161. https://doi.org/10.1155/2014/832161 PMid:24982671 PMCid:PMC4060499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cragg GM, Newman DJ. Drug from nature:past achievement, future prospect. Adv Phytomed. 2007;1:23–37. https://doi.org/10.1016/S1572-557X(02)80010-1. [Google Scholar]
- 9.Goyal PR, Singh KP. Shankhpuspi (Evolvulus alsinoides Linn.):a medicinal herb. Int J Mendel. 2005;2:124. [Google Scholar]
- 10.Kartning T. Clinical Application of Centella asiatica (L.) Urb. In: Craker L.E, Simon J.E, editors. Herbs Spices and Medicinal Plants. II. Oryx Press, Phoemix, AZ; 1988. p. 145. [Google Scholar]
- 11.Zainol MK, Abd-Hamid A, Yusof S, Muse R. Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.). Urban. Food Chemistry. 2003;81(4):575–81. https://doi.org/10.1016/S0308-8146(02)00498-3. [Google Scholar]
- 12.Inamdar PK, Yeole RD, Ghogare AB, De Souza NJ. Determination of biologically active constituents in Centella asiatica. Journal of Chromatography A. 1996;742(1-2):127–30. https://doi.org/10.1016/0021-9673(96)00237-3. [Google Scholar]
- 13.Zheng CJ, Qin LP. Chemical components of Centella asiatica and their bioactivities. Journal of Chinese Integrative Medicine. 2007;5:348–351. doi: 10.3736/jcim20070324. https://doi.org/10.3736/jcim20070324. [DOI] [PubMed] [Google Scholar]
- 14.Myers SP, Cheras PA. The other side of the coin:safety of complementary and alternative medicine. Med. J. Aust. 2004;181:222–225. PMid:15310261. [PubMed] [Google Scholar]
- 15.Derelanko M J, Hollinger M A. Handbook of toxicology. 2nd edition. CRC press; 2002. PMid:12454789. [Google Scholar]
- 16.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature protocols. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. https://doi.org/10.1038/nprot.2007.44 PMid:17406592 PMCid:PMC3623971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. https://doi.org/10.1073/pnas.0406344101 PMid:15452348 PMCid:PMC521976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gacar N, Mutlu O, Utkan T, Komsuoglu Celikyurt I, Gocmez SS, Ulak G. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and morris water maze tests in rats. Pharmacol Biochem Behav. 2011;99(3):316–323. doi: 10.1016/j.pbb.2011.05.017. https://doi.org/10.1016/j.pbb.2011.05.017 PMid:21624386. [DOI] [PubMed] [Google Scholar]
- 19.Harrison FE, Hosseini AH, Dawes SM, Weaver S, May JM. Ascorbic acid attenuates scopolamine-induced spatial learning deficits in the water maze. Behavioural brain research. 2009;205(2):550–8. doi: 10.1016/j.bbr.2009.08.017. https://doi.org/10.1016/j.bbr.2009.08.017 PMid:19703495 PMCid:PMC2759855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goverdhan P, Sravanthi A, Mamatha T. Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. International Journal of Alzheimer's Disease. 2012;2012 doi: 10.1155/2012/974013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X. Improved pyrogallol autoxidation method:a reliable and cheap superoxide scavenging assay suitable for all antioxidants. J Agric Food Chem. 2012;60:6418–6424. doi: 10.1021/jf204970r. https://doi.org/10.1021/jf204970r PMid:22656066. [DOI] [PubMed] [Google Scholar]
- 22.Aebi H. Catalase. In: Bergmeyer U, editor. Methods of enzymatic analysis. New York and London: Academic Press; 1974. pp. 673–680. https://doi.org/10.1016/B978-0-12-091302-2.50032-3. [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical biochemistry. 1979;95(2):351–8. doi: 10.1016/0003-2697(79)90738-3. https://doi.org/10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5, 5′-dithiobis (2-nitrobenzoic acid) Analytical biochemistry. 1988;175(2):408–13. doi: 10.1016/0003-2697(88)90564-7. https://doi.org/10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- 25.Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical pharmacology. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. https://doi.org/10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 26.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S. Oxidative damage is the earliest event in Alzheimer disease. Journal of Neuropathology &Experimental Neurology. 2001;60(8):759–67. doi: 10.1093/jnen/60.8.759. https://doi.org/10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 27.Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer's and Parkinson's diseases. Ann NY Acad Sci. 1999;839:154–175. doi: 10.1111/j.1749-6632.1999.tb07824.x. https://doi.org/10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- 28.Swerdlow RH. Brain aging, Alzheimer's disease, and mitochondria. Biochem Biophys Acta. 2011;1812(12):130–1639. doi: 10.1016/j.bbadis.2011.08.012. https://doi.org/10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borsini A, Zunszain PA, Thuret S, Pariante CM. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015;38(3):145–57. doi: 10.1016/j.tins.2014.12.006. https://doi.org/10.1016/j.tins.2014.12.006 PMid:25579391. [DOI] [PubMed] [Google Scholar]
- 30.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64(1):93–109. doi: 10.1016/j.neuron.2009.09.001. https://doi.org/10.1016/j.neuron.2009.09.001 PMid:19840552. [DOI] [PubMed] [Google Scholar]
- 31.Singh M, Kaur M, Kukreja H, Chugh R, Silakari O, Singh D. Acetylcholinesterase inhibitors as Alzheimer therapy:from nerve toxins to neuroprotection. European Journal of Medicinal Chemistry. 2013;70:165–88. doi: 10.1016/j.ejmech.2013.09.050. https://doi.org/10.1016/j.ejmech.2013.09.050 PMid:24148993. [DOI] [PubMed] [Google Scholar]


