Abstract
Background
Kidney transplantation is the treatment of choice for patients with end‐stage kidney disease. In a previous review we concluded that the routine use of ureteric stents in kidney transplantation reduces the incidence of major urological complications (MUC). Unfortunately, this reduction appears to lead to a concomitant rise in urinary tract infections (UTI). For kidney recipients UTI is now the commonest post‐transplant complication. This represents a considerable risk to the immunosuppressed transplant recipient, particularly in the era of increased immunologically challenging transplants. There are a number of different approaches taken when considering ureteric stenting and these are associated with differing degrees of morbidity and hospital cost.
Objectives
This review aimed to look at the benefits and harms of early versus late removal of the ureteric stent in kidney transplant recipients.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register up to 27 March 2017 through contact with the Information Specialist using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE, and EMBASE; handsearching conference proceedings; and searching the International Clinical Trials Register Search Portal and ClinicalTrials.gov.
Selection criteria
All RCTs and quasi‐RCTs were included in our meta‐analysis. We included recipients of kidney transplants regardless of demography (adults or children) or the type of stent used.
Data collection and analysis
Two authors reviewed the identified studies to ascertain if they met inclusion criteria. We designated removal of a ureteric stent before the third postoperative week (< day 15) or during the index transplant admission as "early" removal. The studies were assessed for quality using the risk of bias tool. The primary outcome of interest was the incidence of MUC. Further outcomes of interest were the incidence of UTI, idiosyncratic stent‐related complications, hospital‐related costs and adverse events. A subgroup analysis was performed examining the difference in complications reported depending on the type of ureteric stent used; bladder indwelling (BI) versus per‐urethral (PU). Statistical analyses were performed using the random effects model and results expressed as relative risk (RR) with 95% confidence intervals (CI).
Main results
Five studies (1127 patients) were included in our analysis. Generally the risk of bias of the included studies was judged low or unclear; they addressed the research question and utilised a prospective randomised design. It is uncertain whether early stent removal verus late stent removal improved the incidence of MUC (5 studies, 1127 participants: RR 1.87, 95% CI 0.61 to 5.71; I2 = 21%; low certainty evidence). The incidence of UTI may be reduced in the early stent removal group (5 studies, 1127 participants: RR 0.49 95% CI 0.30 to 0.81; I2 = 59%; moderate certainty evidence). This possible reduction in the UTI incidence was only apparent if a BI stent was used, (3 studies, 539 participants, RR 0.45 95% CI 0.29 to 0.70; I2 = 13%; moderate certainty evidence). However, if an externalised PU stent was used there was no discernible difference in UTI incidence between the early and late group (2 studies, 588 participants: RR 0.60 95% CI 0.17, 2.03; I2 = 83%; low certainty evidence). Data on health economics and quality of life outcomes were lacking.
Authors' conclusions
Early removal of ureteric stents following kidney transplantation may reduce the incidence of UTI while it uncertain if there is a higher risk of MUC. BI stents are the optimum method for achieving this benefit.
Plain language summary
Early versus late ureteric stent removal after kidney transplantation
What is the issue? The ureter drains urine from the kidney into the bladder and has to be reconnected during kidney transplantation. To protect this new connection the operating surgeon places a plastic stent inside the ureter to help it heal. Routinely this stent would be left in place for up to three months. However, this is associated with an increased risk of urine infection which can be high‐risk for transplant recipients whose immune system is suppressed through anti‐rejection medication. If this stent could be removed earlier then the risk of infection may be reduced but would it be associated with major urological complications e.g. urine leak or obstruction.
What did we do? This study was designed to review all the previously published research in this area to determine the answer to this question. Five studies including 1097 patients were identified.
What did we find? It is uncertain whether the number of major urological complications were different in those patients whose stent was removed early (less than 15 days post‐operatively), when compared with those removed later (more than 15 days post‐operatively). The number of patients suffering from a urinary tract infection may be less in the early removal group ‐ especially if the stent was not exposed to the external environment. The studies identified for this review were generally of poor quality.
Conclusions It is uncertain whether a bladder indwelling ureteric stent that is removed early following kidney transplantation reduces the risk of complications, however it may prevent urine tract infections.
Summary of findings
Summary of findings for the main comparison. Early versus late ureteric stent removal after kidney transplantation.
| Early versus late ureteric stent removal after kidney transplantation | |||||
| Patient or population: kidney transplant recipients Intervention: early ureteric stent removal Comparison: late ureteric stent removal | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with late removal | Risk with early removal | ||||
| Major urological complications: all stents follow‐up range: 3 to 12 months | Study population | RR 1.87 (0.61 to 5.71) | 1127 (5) | ⊕⊕⊕⊕ LOW 1 | |
| 12 per 1,000 | 23 per 1,000 (7 to 69) | ||||
| Major urological complications: bladder indwelling stents follow‐up range: 3 months to 12 months | Study population | RR 1.67 (0.52 to 5.36) | 539 (3) | ⊕⊕⊕⊕ LOW 1 | |
| 15 per 1,000 | 24 per 1,000 (8 to 79) | ||||
| Major urological complications: per‐urethral stents follow‐up range: 3 months to 12 months | Study population | RR 1.51 (0.03 to 74.45) | 588 (2) | ⊕⊕⊕⊕ LOW 1 | |
| 10 per 1,000 | 15 per 1,000 (0 to 732) | ||||
| Urinary tract infection: all stents | Study population | RR 0.49 (0.30 to 0.81) | 1126 (5) | ⊕⊕⊕⊝ MODERATE 1 2 | |
| 185 per 1,000 | 91 per 1,000 (56 to 150) | ||||
| Urinary tract infection: bladder indwelling stents | Study population | RR 0.45 (0.29 to 0.70) | 539 (3) | ⊕⊕⊕⊝ MODERATE 1 2 | |
| 209 per 1,000 | 94 per 1,000 (61 to 146) | ||||
| Urinary tract infection: per‐urethral stents | Study population | RR 0.60 (0.17 to 2.03) | 587 (2) | ⊕⊕⊝⊝ LOW 1 2 | |
| 164 per 1,000 | 98 per 1,000 (28 to 333) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 All studies were unblinded, however, this was unavoidable given the nature of the intervention. The majority of studies provided minimal information on processes of randomisation and allocation
2 Inconsistent definition and variable reporting of urinary tract infection across included studies
Background
Description of the condition
Kidney transplantation is the treatment of choice for patients with end‐stage kidney disease (ESKD). Over the last four decades, surgical techniques have been refined and the majority standardised. In current surgical practice there remains very little variation between centres and surgeons in most aspects of kidney transplant surgery. The anastomosis created between the donor transplant ureter and the bladder remains one aspect of surgical practice where techniques continue to evolve (Nicholson 1991). In a previous review we focused on the role of the ureteric stent and its function in reducing major urological complications (MUC), urinary leak or fistula and ureteric stenosis (Wilson 2013). We concluded that the universal use of stents reduces the incidence of MUC from between 7% and 9%, to 1.5%. Unfortunately, this reduction appears to lead to a concomitant rise in urinary tract infections (UTI) which is offset by the use of antibiotics. In addition, stents are associated with idiosyncratic complications (migration, malposition, haematuria, encrustation, irritative bladder symptoms, and may be forgotten) (Bardapure 2014). More recently there have also been some isolated reports of an association between the use of ureteric stents and the incidence of an opportunistic viral pathogen ‐ BK virus (Siparsky 2011), consequent to its negative effects on distal ureteric motility.
Description of the intervention
Ureteric stents used in transplantation can be of different lengths (12 cm to 36 cm), calibres (5F to 7F) and designs (percutaneous (PC), per‐urethral (PU), or bladder indwelling (BI)). Most centres have traditionally placed BI stents for a period of four weeks to three months before removal in an operating room using a flexible cystoscope under local anaesthetic, or if it is combined with another procedure such as haemodialysis fistula ligation, under general anaesthetic (Wilson 2013). This approach necessitates a further admission to hospital and hospital costs.
Several approaches have been suggested to maximise the benefit of stents and reduce morbidity, costs or both. One option is to remove the stent before the patient leaves hospital (a period of only one or two weeks) (Indu 2012; Thiyagarajan 2012), another is to use a PC or PU stent which can be removed in the ward or outpatient clinic (Olsburgh 2010). A further option is to tie the BI stent to the urinary catheter (Morris‐Stiff 1998) and remove them simultaneously (week 1). On the basis of these descriptions and standard practices we arbitrarily designated removal of a ureteric stent before the third postoperative week (< day 15) or during the index transplant admission as "early" removal.
How the intervention might work
Ureteric stents seem to reduce MUC in two phases. At initial placement ureteric stents help the surgeon by reducing anatomical kinking and delineating the lumen to aid in suture placement. After implantation, inflammation and oedema can cause obstruction at the anastomosis, and the stent helps urine drain from the kidney into the bladder, reducing intra‐ureteric pressure. This may also aid in preventing Ischaemic‐related necrosis of the distal ureter and subsequent urine leak.
However, as a foreign body, ureteric stents rapidly become colonised with a biofilm of micro‐organisms that may predispose to UTI in the recipient bladder and pyelonephritis due to back flow of urine into the kidney pelvis during bladder detrusor contraction (Waters 2008). In this respect, early removal with the urinary catheter may be considered a significant advantage. PC stents, or PU stents that run beside the urinary catheter, offer the advantage of being able to monitor transplant urine output independently of the native kidney output, thus differentiating between immediate and delayed graft function. This is certainly useful for research studies on ischaemic‐reperfusion injury, but of dubious clinical significance in the short term.
Why it is important to do this review
Live donor kidney transplantation is becoming more widespread as the waiting time for cadaveric transplantation lengthens. As a result ABO‐incompatible transplantation is more common and recipients treated with higher intensity immunosuppression are at increased risk of peri‐operative complications. In one registry review of patients undergoing live donor kidney transplantation, UTI was the most common complication, with an incidence over 30% (Montgomery 2012). Some surgeons believe that the benefit of ureteric stents is only within the first one or two weeks after transplantation, and that leaving them in situ for longer leads to the potential for stent‐related morbidity such as UTI, the possibility of being forgotten, and the risk of severe urosepsis on removing a late encrusted stent at four to six weeks (Bardapure 2014). Other clinicians believe that ischaemic necrosis or stenosis of the ureter is a delayed event and that an indwelling stent can prevent these complications only by being left for longer periods of time.
This review attempted to dissect differences in ureteric morbidity by meta‐analysing data from studies differentiated by the length of time stents were left in situ.
Objectives
This review aimed to look at the benefits and harms of early (before the third postoperative week (< day 15) or during the index transplant admission as "early" removal) versus late removal of the ureteric stent in kidney transplant recipients.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at timing of ureteric stent removal in kidney transplantation.
Types of participants
Inclusion criteria
We included recipients of kidney transplants regardless of demography (adults or children) or the type of stent placed. To adequately assess studies the protocols should include data on the allocation and randomisation status of patients or kidneys with complex urinary tracts (bladder diversion, duplex ureters, en bloc transplants). Multivisceral recipients in whom a kidney is combined with other organs (e.g. liver or pancreas) are also included.
Exclusion criteria
Studies including patients with stenting of ileal conduits or continent urinary diversions were excluded.
Types of interventions
We investigated the timing of stent removal (early versus late) after kidney transplantation. Ureteric stents used in transplantation can be of different lengths (12 cm to 36 cm), calibres (5F to 7F) and designs (PC, PU, BI) (Wilson 2013). This review addressed the question of whether the stent can be removed sooner and reduce morbidity as well as associated hospital costs. We have also attempted to address the following questions.
PC versus BI stents
PU versus PC stents
BI versus PU stents
Types of outcome measures
MUC and UTI are the most important outcomes relevant to this review. MUC is a post‐operative surgical complication usually associated with the vesicoureteric anastomosis. MUC is defined as any urological complication arising within the first 6 months following kidney transplantation that requires an intervention or re‐operation e.g. urinary obstruction, leak, fistula or stenosis. This includes temporary placement of nephrostomy. We also considered:
Stent‐related complications (e.g. irritation, migration, malposition, haematuria, encrustation, irritative bladder symptoms, forgotten stents)
Hospital‐related costs including hospital stay, re‐operation, surgical re‐implantation
Adverse events related to stent removal (urosepsis, haematuria, rare graft loss, BK virus nephropathy)
Graft and patient survival.
Primary outcomes
The primary outcomes of importance were MUC and UTI incidence; and for all included studies, this was the minimum data set accepted.
Secondary outcomes
The secondary outcomes were stent‐related complications, hospital‐related costs and adverse events related to stent removal. The concept of treatment failure is also relevant, where an operatively placed PC or PU stent is replaced with a BI stent during the operation
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 27 March 2017 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that were relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however studies and reviews that included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. There were no non‐English language studies. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data used in the analyses. Where relevant outcomes were only published in earlier versions these data was used. Any discrepancy between published versions has been highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2) and depicted graphically using the RevMan "Risk of bias" tools.
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (MUC, UTI) results are expressed as risk ratio (RR) with 95% confidence intervals (CI). There were no comparative meta‐analysis data using continuous scales of measurement.
Unit of analysis issues
We did not encounter any specific unit of analysis issues; specifically no studies using cluster randomisation or cross‐over allocation.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing corresponding author/s) and any relevant information obtained in this manner was to be included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population was carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward (LOCF)) was to be critically appraised (Higgins 2011). Due to the paucity of data across multiple comparisons "missing data" computations were not considered appropriate.
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. Heterogeneity was then analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). A guide to the interpretation of I2 values is as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a confidence interval for I2) (Higgins 2011).
Assessment of reporting biases
Funnel plots were to be used to assess for the potential existence of small study bias, however there were insufficient studies identified to do this (Higgins 2011).
Data synthesis
Data were pooled using the random‐effects model, but the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity, for example, type of solid organ transplanted and study quality. Heterogeneity among participants could be related to age, gender, co‐morbidities and underlying diseased organ pathology. Heterogeneity in treatments could be related to the type of stent, route of insertion, duration of placement, antibiotic regime, or mechanism of removal.
Adverse effects were tabulated and assessed with descriptive techniques, as they were likely to be different for the various techniques used. Where possible, the risk difference with 95% CI was to be calculated for each adverse effect, either compared to long term stent or to another stent technique. If enough studies were identified we planned to investigate the following clinically relevant subgroup analyses by technique:
PC versus BI stents
PU versus PC stents
BI versus PU stents
Sensitivity analysis
We performed sensitivity analyses in order to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies;
Repeating the analysis taking account of risk of bias, as specified above;
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results;
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication and country.
'Summary of findings' tables
We have presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach GRADE 2008. The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the Table 1.
-
Incidence of MUC
BI stents
PU stents
-
Incidence of UTI
BI stents
PU stents
Results
Description of studies
Results of the search
After searching the Specialised Register we identified 19 records. Five studies (16 records) were included (Gunawansa 2011; Huang 2012; Indu 2012; Parapiboon 2012; TrUST 2017), one study (one record) was excluded (Yari 2014), and two ongoing studies were identified (ACTRN12610000349044; ISRCTN51276329). These ongoing studies will be assessed in a future update of this review (Figure 1). Three of the five authors were contacted for further information regarding study design and results, one author responded to our enquiries.
1.
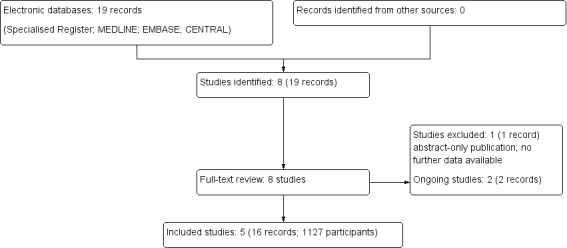
Flow chart of study selection
Included studies
Five RCTs were included in the study with a total of 1127 patients. The studies were heterogeneous in nature, including living and deceased donors, adults and children, and varying definitions of what was defined as 'early' stent removal. This is summarised in detail in the Characteristics of included studies.
Participants
The 1127 patients in the analysis included adult and paediatric kidney transplant recipients. The majority of studies included only adults, while TrUST 2017 included both adults and children. The mean age was 40.4 years in the early removal group and 42.2 years in the late removal group. The type of donor varied: Indu 2012 and Gunawansa 2011 included only live donor recipients; Huang 2012 included only deceased donors; and Parapiboon 2012 and TrUST 2017 included both live and deceased donors. All live donor nephrectomies were laparoscopic.
Interventions
All studies utilised prophylactic double‐J ureteric stents placed intraoperatively. Three studies (Indu 2012; Huang 2012; Parapiboon 2012) preferred the BI stent technique; the stent was removed by flexible cystoscopy at the defined post‐operative date. Two studies (Gunawansa 2011; TrUST 2017) used the PU stent technique; the early removal participants had the stent anchored to the urinary catheter intraoperatively and removed simultaneously on day 7 post‐operatively. The participants in the control arm of these studies received a standard BI stent. The definition of early removal varied considerable between studies; the majority of studies termed early removal at day 7 post‐transplant. However, Huang 2012 study included early removal up to day 21. Unusually, the length of stay in this study was longer than routine, around 3 to 4 weeks. Equally, the author's definition of early removal was longer than our original, day 21 compared to day 15. Despite this discrepancy with our protocol we decided to include this study in the meta‐analysis as the early stent removal time was comparable bearing in mind the relatively increased length of stay and the "intention to treat" fitted with our research question.
Outcomes
To investigate for MUC routine imaging (DTPA or ultrasound) was performed by two of the five studies (Indu 2012; TrUST 2017). The other studies investigated for the presence of a MUC if clinically indicated. A UTI was diagnosed based on the presence of bacteriuria on regular routine urine sampling in four studies (Indu 2012; Huang 2012; Parapiboon 2012; TrUST 2017) irrespective of symptoms. The remaining study (Gunawansa 2011) did not describe this approach and did not respond to our request for further information. The more idiosyncratic symptoms caused by ureteric stents (e.g. haematuria, encrustation, migration and irritation) were evaluated by two studies (Huang 2012; TrUST 2017). TrUST 2017 did a more in‐depth analysis on participants quality of life and health status as a result of early stent removal using two separate validated questionnaires. The potential cost‐effective benefits of early stent removal was analysed by Parapiboon 2012.
In summary, the study designs were heterogeneous with varying definitions of early or late stent removal. There was disparity in the type of donor, recipient and length of follow up. Overall studies were of an appropriate randomised controlled design comparing early with late ureteric stent removal. The nature to which the studies focused on our primary outcome, major urological outcomes, varied but all identified this as an important factor for investigation.
Excluded studies
One study was excluded (Yari 2014). This study was excluded as only an abstract was available with very limited information regarding the number of patients in each of the three intervention arms therefore making analysis impossible. The authors did not respond to our attempts at contact for further information.
Risk of bias in included studies
There was a moderate degree of bias across all included studies attributed to varying sources. It is unclear how many studies made an attempt to formally randomise patients using appropriate computer programs and sealed allocation as most studies did not provide any information on these processes. As expected none of the studies attempted to blind participants or personnel to the intervention or to the outcome assessment. The majority of studies detailed complete follow‐up of all participants involved in study, however, only one study include a CONSORT flow diagram (TrUST 2017). There were few published protocols of the studies available for comparison with published data therefore attributing the degree of reporting bias was problematic (Figure 2; Figure 3).
2.
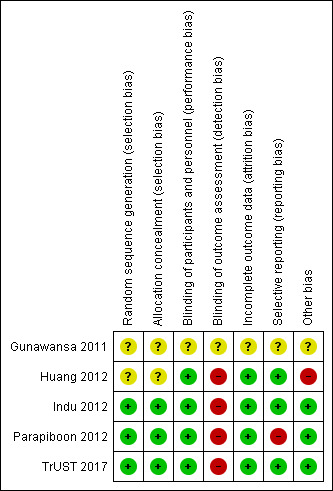
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
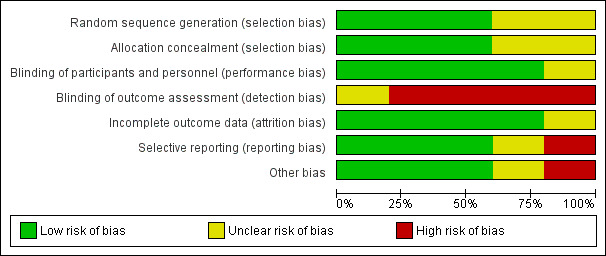
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Gunawansa 2011 and Huang 2012 contained no information and did not respond to further requests for information regarding randomisation and allocation. Three studies (Indu 2012; Parapiboon 2012; TrUST 2017) describe a robust randomisation process.
Indu 2012 randomised using computer‐generated random numbers, placed into sealed opaque envelopes that were opened on day 7 after transplant by nurses on the ward and determined the allocation to either intervention arm. any participant who developed a leak, delayed graft function, or rejection prior to randomisation on day 7 was excluded. Both groups were receiving a BI stent and were not yet randomised to a particular arm therefore these exclusion criteria, although initially appearing unusual actually have no bias effect on outcome.
Parapiboon 2012 described a computer‐generated block of 4 randomisation process, with allocation concealment by sealed opaque envelopes.
TrUST 2017 utilised an online randomisation program which was block stratified for age with randomly varying block sizes. Allocation was revealed to clinicians at the time of randomisation.
Blinding
As expected, none of the studies blinded participants or personnel to their allocated intervention. Equally, none of the studies attempted to blind personnel undertaking outcome assessments. This may represent a high risk area for detection bias as those clinicians caring for participants who were known to still have a ureteric stent in situ may have been more concerned about the risk of UTI and therefore sent urine samples more frequently leading to over diagnosis and treatment of asymptomatic bacteriuria.
Incomplete outcome data
Follow‐up of participants in the included studies was complete for four studies (Huang 2012; Indu 2012; Parapiboon 2012; TrUST 2017). In these studies all patients were accounted for, however, only TrUST 2017 included a CONSORT diagram. Gunawansa 2011 had limited information available from the published abstracts and the authors did not respond to requests for further information.
Selective reporting
The majority of studies included did not have published study protocols available for comparison. TrUST 2017 published a protocol and it appears they have fully reported on all anticipated outcomes. Huang 2012 and Indu 2012 fully reported all outcomes. Gunawansa 2011 had limited information available based from the published abstracts and the author did not respond to requests for further information. Parapiboon 2012 did not report in detail the MUC encountered. These consisted of two patients in each intervention group, but there is no further detail as to the nature of this complication or what they deem to be a MUC. This study has published two papers; one focusing on the incidence of bacteriuria and the other a cost‐benefit analysis. The incidence of UTI data is very detailed and well reported as this was their primary outcome of interest. MUC were not a priority in this study and as such there is very little detail reported on complications encountered potentially resulting in a degree of reporting bias.
Other potential sources of bias
Huang 2012 was judged to be at high risk of other potential bias due to the very long length of stay which may be associated with an increased risk of nosocomial infection. Three studies appeared to be free of other potential sources of bias (Indu 2012; Parapiboon 2012; TrUST 2017), and Gunawansa 2011 was judged unclear as there was insufficient information reported in the conference abstracts.
Effects of interventions
See: Table 1
Major urological complications
It is uncertain whether early versus late stent removal makes any difference to MUC (Analysis 1.1 (5 studies, 1127 participants): RR 1.87, 95% CI 0.61 to 5.71; I2 = 21%; low certainty evidence). Heterogeneity between studies was deemed to be low.
1.1. Analysis.
Comparison 1 Major urological complications, Outcome 1 Major urological complications.
There was little or no difference in MUC when either BI stents (Analysis 1.1.1 (3 studies, 539 participants): RR 1.67, 95% CI 0.52 to 5.36; participants = 539; studies = 3; I2 = 0%) or PU stents (Analysis 1.1.2 (2 studies, 588 participants): RR 1.51, 95% CI 0.03 to 74.45; participants = 588; studies = 2; I2 = 78%) where used (test for subgroup differences: Chi² = 0.00, df = 1 (P = 0.96), I2 = 0%).
Urinary tract infection
The incidence of UTI varied greatly between studies, ranging from 2.2% to 73%. Early stent removal may reduce the number of UTI compared to late removal (Analysis 2.1 (5 studies, 1126 participants, RR 0.49 95% CI 0.30 to 0.81; I2 = 59%; moderate certainty evidence). These findings are within a markedly heterogeneous group, where the incidence and definition of UTI was very variable. The test for heterogeneity was moderate.
2.1. Analysis.
Comparison 2 Urinary tract infection, Outcome 1 Urinary tract infection.
Patients were probably less likely to develop a UTI with early removal compared to late removal of a BI stent (Analysis 2.1.1 (3 studies, 539 participants): RR 0.45 95% CI 0.29 to 0.70; I2 = 13%; moderate certainty evidence). There was little or no difference in UTI with early versus late PU stent removal (Analysis 2.1.2 (2 studies, 588 participants): RR 0.60, 95% CI 0.17 to 2.03; I2 = 83%; low certainty evidence). Of note, there was substantial heterogeneity with PU stents.
Minor stent‐related complications
Only Huang 2012, Indu 2012 and TrUST 2017 examined minor stent‐related complications in more detail (e.g. haematuria, encrustation, migration). Huang 2012 found these complications were significantly more likely to occur in the late stent removal group. For example, irritative symptoms were experienced in 42/186 patients in the late group compared to 16/179 in the early group (P = 0.001). This study also reported 3 cases of 'forgotten stents' that resulted in removal at a much later date (12 weeks). Indu 2012 examined the incidence of stent migration, breakage and haematuria and found no cases in either the early or late stent removal group. In TrUST 2017, the late stent removal group experienced more pain (0/80 in early group versus 4/126 in late group; P = 0.259), more episodes of haematuria (0/80 early versus 2/126 in the late group; P = 0.666), and more episodes of migration (0/80 early versus 3/126 in the late group; P = 0.409). The TrUST 2017 investigators also evaluated participants health status and quality of life using FAIT‐U and EQ‐5D questionnaires. They found no difference at week one post‐transplant, however, by week six the health status scores (FAIT‐U) were significantly better in those patients who had their ureteric stent removed early (P = 0.012).
Cost‐effectiveness
Only Parapiboon 2012 examined the cost effectiveness of early stent removal. In this study (intention‐to‐treat analysis) patients whose stent was removed at seven days were significantly less likely to develop a UTI (15/37, 40.5% versus 27/37, 72.9%; P = 0.004). According to figures from their centre, the mean hospital cost, including accommodation, investigations and treatment, for patients with a UTI was significantly higher than those without a UTI (11,890 USD versus 6897 USD, P < 0.001). The mean cost of early ureteric stent removal was lower than routine removal (8792 USD versus 11,182 USD; P = 0.06). With early ureteric stent removal the authors estimated a saving of 2390 USD per kidney transplant recipient.
Discussion
Summary of main results
Universal use of ureteric stents in kidney transplantation has significantly reduced the incidence of MUC (Wilson 2013). However, they are associated with other risks such as UTI, haematuria, encrustation and irritative bladder symptoms. These risks are likely to increase in incidence the longer the stent is in place. It is uncertain whether ureteric stents can be safely removed at an earlier time point than traditionally accepted without any increase in risk of MUC (RR 1.87, 95% CI 0.61 to 5.71). There may be a reduction in the incidence of UTI with early stent removal (RR 0.49 95% CI 0.30 to 0.81). The incidence of UTI in the late stent removal group from this set of studies is directly comparable to the summative stented cohort from the meta‐analysis by Wilson 2013.
Our analysis also identified that the associated reduction in UTI incidence was only seen BI stents (RR 0.45, 95% CI 0.29 to 0.70). In those studies where the ureteric stent was tied to the urinary catheter the benefit of early stent removal was lost (RR 0.60 95% CI 0.17 to 2.03). This may be due to the externalisation of the indwelling stent providing an easy track for antimicrobial colonisation. TrUST 2017, which utilised the PU stent method in their early removal arm, reported reasonably high treatment failure rate using this technique (15). This was reported as due to technical difficulties attaching the stent to catheter. This resulted in conversion of a PU stent to a BI stent and these stents were subsequently removed at the later time point six weeks post‐operatively (Table 2).
1. Reported adverse events.
| Study ID | Adverse events |
| Gunawansa 2011 | Two patients in the late group required re‐stenting due to ureteric stenosis |
| Huang 2012 | Three patients in the late group had forgotten stents that were subsequently removed at 12 weeks |
| Indu 2012 | Six patients in the early and 5 patients in the late group had acute rejection that required intervention |
| Parapiboon 2012 | No adverse events reported |
| TrUST 2017 | Sixteen patients did not receive their allocated treatment as there were technical difficulties attaching the stent to the catheter. In the early removal group, 1 patient's stent removal was delayed by 1 day because the urethral catheter balloon needed percutaneous needle puncture due to the stent suture There were 5 complications in patients who had early stent removal and these were all related to the percutaneous technique used in which the stent was tied to the catheter |
One study reporting cost effectiveness estimated a saving of 2390 USD per patient with early stent removal.
Overall completeness and applicability of evidence
This review identified only a small number of studies for which limited information was available despite contacting the authors directly. All of the included studies provided information regarding our primary outcome of interest, MUC, and the secondary outcome UTI. Only three studies provided further information regarding other stent associated complications and, although not statistically significant, two of these studies noted a reduction in pain, haematuria, migration and encrustation of stents if they were removed early. Also of note, there are a number of ongoing studies which were not included and may provide more important information in the future (ACTRN12610000349044; ISRCTN51276329).
Our sensitivity analysis did not reveal any untoward influence on effect size when taking into account the filters described earlier in our methods section; excluding unpublished studies, excluding the largest studies, excluding studies with aberrant diagnostic criteria and excluding studies with a different language of publication. We also examined the data using a "worst‐case" scenario approach and this revealed that our conclusion is robust enough to withstand wide variations in data.
Quality of the evidence
The studies included in the review were generally of poor quality, with only three studies reporting a robust randomisation process (Indu 2012; Parapiboon 2012; TrUST 2017). With the limited information available it was difficult to assess to risk of bias for a few of the studies and these were assumed to be high risk. Due to the nature of the intervention blinding was not possible but this is unlikely to have affected outcome. Across included studies there is a relatively short follow‐up period, median four months, but this is still likely to have captured the outcomes of concern, MUC and UTI.
There was a substantial degree of heterogeneity within the studies when examining UTI incidence, due to the differences in each individual study's definition of UTI. Some studies included all bacterial urinary colonisation irrespective of symptoms and others only included symptomatic patients. However, when investigating an immunosuppressed transplant recipient population any degree of bacteriuria is significant to warrant concern and therefore a change to practice, in this case earlier stent removal, which can minimise this risk, is of benefit.
Potential biases in the review process
In conducting a meta‐analysis there is an inherent risk of publication bias due to the retrospective nature of the search. To minimise this risk we searched multiple databases without language restriction and utilised the Cochrane Kidney and Transplant Specialised Register to gain access to reports of studies only presented at conferences and meetings. The data presented is up to date as of March 2017 and the ongoing studies discovered in the search were still unpublished prior to our publication. However, in an attempt to minimise publication bias, we have included studies only published as a conference abstract which have not been through a robust peer review process. Four of the five studies included were published in peer reviewed journals. The studies included overall have a moderate degree of bias which we have attempted to minimise through developing a detailed protocol for analysis prior to commencing this study.
Agreements and disagreements with other studies or reviews
To our knowledge there are no other meta‐analyses or systematic reviews addressing this issue.
Authors' conclusions
Implications for practice.
It is uncertain whether early removal of ureteric stents following kidney transplantation is associated with a higher risk of MUC and may reduce the incidence of UTI in an immunosuppressed patient population. This benefit is only realised if the ureteric stent is BI as opposed to externalised and attached to the patient catheter.
Implications for research.
A cost‐benefit analysis would be valuable further research when considering early stent removal. It would interesting to include patient quality of life questionnaires as irritative bladder symptoms are a considerable source of patient complaint often ignored as a necessary evil by clinicians. Early removal would minimise this discomfort to patients and decrease the disruption and cost of a return appointment for stent removal at a later date. This has been addressed in TrUST 2017 but needs wider validation. We need more evidence to conclude exactly which early technique is better, BI versus PU versus PC, as there were a limited number of studies in each of these arms. It would also be beneficial to understand potential categories of patients in which early removal is not advised due to an inherent increased risk of MUC.
Acknowledgements
The authors would like to acknowledge the help and support given by Cochrane Kidney and Transplant Group and the referees for their comments and feedback. The research was funded by the National Institute for Health Research Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or NHSBT.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Major urological complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major urological complications | 5 | 1127 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.61, 5.71] |
| 1.1 Bladder indwelling stents | 3 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.52, 5.36] |
| 1.2 Per‐urethral stents | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.03, 74.45] |
Comparison 2. Urinary tract infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Urinary tract infection | 5 | 1126 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.81] |
| 1.1 Bladder indwelling stents | 3 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.29, 0.70] |
| 1.2 Per‐urethral stents | 2 | 587 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.17, 2.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gunawansa 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Huang 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Other information
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement "...were randomly assigned to two groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding but unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for: ‐ 3 patients forgot to return for stent removal at 6 weeks and it was removed at 12 weeks ‐ 4 patients removed from analysis (2 in each group) due to stent migration |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Very long length of stay 3 to 4 weeks, maybe associated with increased risk of nosocomial infection |
Indu 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Other information
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer generated random numbers created by study coordinator |
| Allocation concealment (selection bias) | Low risk | Allocation kept in sealed opaque envelopes until opened on day 7 by ward nurses |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding but unlikely to impact outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, however CONSORT diagram not included |
| Selective reporting (reporting bias) | Low risk | No study protocol data but all reported outcomes are accounted for |
| Other bias | Low risk | The study appears to be free of other biases |
Parapiboon 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Other information
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block of 4 |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding not possible but unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No evidence of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for but no CONSORT diagram |
| Selective reporting (reporting bias) | High risk | No information on what type of urological complications were encountered |
| Other bias | Low risk | The study appears to be free of other biases |
TrUST 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Other information
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online computer generated randomisation process, block stratified with randomly varying block sizes |
| Allocation concealment (selection bias) | Low risk | Allocation revealed to clinicians at time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding not possible but unlikely to affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CONSORT diagram included detailing full follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | The study appears to be free of other biases |
BI ‐ bladder indwelling; CIT ‐ cold ischaemic time; DGF ‐ delayed graft function; DPTA ‐ diethylenetriaminepentaacetic acid; ESKD ‐ end‐stage kidney disease; IQR ‐ interquartile range; IV ‐ intravenous; M/F ‐ male/female; MSU ‐ midstream urine; mTORi ‐ mammalian target of rapamycin inhibitor; MUC ‐ major urological complications; PC ‐ percutaneous; PU ‐ per‐urethral; PP ‐ per protocol; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; USS ‐ urinary ultrasound; UTI ‐ urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Yari 2014 | Not enough information included in abstract available regarding numbers of patients in intervention arms therefore unable to include in analysis. Authors did not respond to our contact for more information. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12610000349044.
| Trial name or title | Pilot study: prospective randomised controlled trial of ureteric double J stenting with early vs standard stent removal to improve graft and patient outcome and reduce urological complications after renal transplantation |
| Methods | Prospective RCT comparing early removal of ureteric stent at day 4 (attached to catheter) compared to late removal 4‐6 weeks post‐op cystoscopically. |
| Participants | All patients > 16 years on the kidney transplant waiting list at a single centre Exclusion criteria: neurogenic bladder dysfunction or re‐transplant |
| Interventions | Double J stent sutured to urinary catheter and removed simultaneously on day 4 post‐transplant |
| Outcomes | Primary outcome (1): graft outcome assessed using histology from renal biopsy, SCr and eGFR Primary outcome (2): at 12 months post‐transplant patient mortality data will be recorded Secondary outcome: MUC |
| Starting date | 01/05/2010 |
| Contact information | Dr Adam Bartlett, adamb@adhb.govt.nz |
| Notes | No outcome data to be obtained regarding UTI |
ISRCTN51276329.
| Trial name or title | Randomised controlled trial of early versus late ureteric stent removal post kidney transplant |
| Methods | Parallel RCT |
| Participants | Sample size set at 350 based on power calculations. To include all adults receiving at kidney either living or deceased donor |
| Interventions | Group A ‐ removal of ureteric stent on day 6‐8 post‐transplant Group B ‐ removal of ureteric stent during week 4‐6 post‐transplant |
| Outcomes | Primary outcome: composite incidence of UTI and ureteric complications Secondary outcome: incidence of UTI, urine leak, stenosis, patient death, graft loss, surgical complications, immunological complications, readmission and length of stay, medical complications Measure at 3 months post‐transplant |
| Starting date | 1/1/2014 |
| Contact information | Dept of Surgery Addenbrookes |
| Notes |
eGFR ‐ estimated glomerular filtration rate; MUC ‐ major urological complications; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; UTI ‐ urinary tract infection
Differences between protocol and review
There were no identified studies that utilised the PC method of stent placement and therefore this subgroup analysis that was included in the protocol could not be included. Only two studies included examined in any detail the incidence of idiosyncratic stent complications (e.g. bladder irritation, haematuria, encrustation) and therefore a robust meta‐analysis could not be performed.
Contributions of authors
Draft the protocol: CHW, SAH, MLN
Study selection: CHW, SAH, ERT
Extract data from studies: CHW, SAH, ERT
Enter data into RevMan: CHW, SAH, ERT
Carry out the analysis: CHW, SAH, ERT
Interpret the analysis: CHW, SAH, ERT
Draft the final review: CHW, SAH, ERT
Disagreement resolution: MLN
Update the review: CHW, SAH
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute for Health Research Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge, UK.
Newcastle University, UK.
NHS Blood and Transplant (NHSBT), UK.
Declarations of interest
Emily Thompson: none known
Sarah Hosgood: none known
Michael Nicholson: none known
Colin Wilson: none known
New
References
References to studies included in this review
Gunawansa 2011 {published data only}
- Gunawansa N, Cassim R, Abeydeera A, Wijeyaratne M. Early bedside removal versus late cystoscopic removal of ureteric stents following renal transplantation; Does it make a difference? [abstract no: P‐41]. American Journal of Transplantation 2011;11(Suppl 1):73. [EMBASE: 70329005] [Google Scholar]
- Gunawansa N, Cassim R, Abeydheera A, Wijeyaratne M. Early bedside removal versus late cystoscopic removal of ureteric stents following renal transplantation; does it make a difference? [abstract no: P‐241]. Transplant International 2011;24(Suppl 2):288. [EMBASE: 70528137] [Google Scholar]
- Gunawansa N, Wijeyaratne M, Cassim R, Sahabandu C. Early bedside removal versus delayed cystoscopic removal of ureteric stents following live donor renal transplantation: a randomized prospective study [abstract no: O323]. Transplant International 2015;28(Suppl 4):118. [EMBASE: 72111563] [Google Scholar]
Huang 2012 {published data only}
- Huang L, Wang X, Ma Y, Wang J, Tao X, Liao L, et al. A comparative study of 3‐week and 6‐week duration of double‐J stent placement in renal transplant recipients. Urologia Internationalis 2012;89(1):89‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Indu 2012 {published data only}
- Indu KN, Lakshminarayana G, Anil M, Rajesh R, George K, Ginil K, et al. Is early removal of prophylactic ureteric stents beneficial in live donor renal transplantation?. Indian Journal of Nephrology 2012;22(4):275‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parapiboon 2012 {published data only}
- Parapiboon W, Ingsathit A, Disthabanchong S, Nongnuch A, Jearanaipreprem A, Charoenthanakit C, et al. Impact of early ureteric stent removal and cost‐benefit analysis in kidney transplant recipients: results of a randomized controlled study. Transplantation Proceedings 2012;44(3):737‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parapiboon W, Ingsathit A, Jirasiritham S, Sumethkul V. High incidence of bacteriuria in early post‐kidney transplantation; results from a randomized controlled study. Transplantation Proceedings 2012;44(3):734‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parapiboon W, Ingsathit A, Junchotikul P, Wiengpon K, Viseshsindh W, Leenanupunth C, et al. Early ureteric stent removal reduces urinary tract infection in kidney transplant recipients, a randomized controlled trial (EUREKA) [abstract no: O‐155]. Transplant International 2011;24(Suppl 2):43. [EMBASE: 70527232] [Google Scholar]
TrUST 2017 {published data only}
- Olsburgh J. Clinical study protocol: TrUST Transplant Ureteric Stent Trial Protocol version 2. 2010. www.isrctn.com/editorial/retrieveFile/81979356‐1fe4‐4b96‐bc2b‐35b7afa4ffd5/19505 (date accessed 23 August 2017).
- Patel P, Rebollo‐Mesa I, Banga N, MacDougall I, Webb M, Mamode N, et al. TrUST (Transplant Ureteric Stent Trial): early versus standard removal [abstract]. European Urology Supplements 2014;13(1):e1010. [EMBASE: 71486000] [Google Scholar]
- Patel P, Rebollo‐Mesa I, Banga N, MacDougall I, Webb M, Mamode N, et al. TrUST (Transplant Ureteric Stent Trial): early versus standard removal [abstract]. Journal of Urology 2014;191(4 Suppl 1):e775‐6. [EMBASE: 71402065] [Google Scholar]
- Patel P, Rebollo‐Mesa I, Banga N, Macdougall I, Webb M, Mamode N, et al. TrUST (Transplant Ureteric Stent Trial): early versus standard removal. A randomised controlled trial [abstract]. Transplantation 2014;98(Suppl 1):638. [EMBASE: 71545698] [Google Scholar]
- Patel P, Rebollo‐Mesa I, Ryan E, Sinha MD, Marks SD, Banga N, et al. Prophylactic ureteric stents in renal transplant recipients: a multicenter randomized controlled trial of early versus late removal. American Journal of Transplantation 2017;17(8):2129‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Patel P, Sinha M, Koffman G, Olsburgh J. Transplant Ureteric Stent Trial (TrUST): Early versus standard removal. A randomised controlled trial ‐ pilot data [abstract no: 149]. British Transplantation Society (BTS). 14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
- Patel P, Sinha M, Mamode N, Koffman G, Olsburgh J. Transplant Ureteric Stent Trial (TrUST): Early versus standard removal. a randomised controlled trial‐pilot data [abstract no: RO‐269]. Transplant International 2011;24(Suppl 2):205. [EMBASE: 70527807] [Google Scholar]
- Patel PP, Sinha M, Koffman G, Olsburgh J. TrUST (Transplant Ureteric Stent Trial): early versus standard removal. A randomised controlled trial ‐ the pilot data [abstract no: P101]. BJU International 2011;108(Suppl 1):58. [EMBASE: 70480185] [Google Scholar]
References to studies excluded from this review
Yari 2014 {published data only}
- Yari H, Aliasgari FD, Tara SA, Argani H, Alirezaii A. Benefits and complications of removing the ureteral stent in renal transplantation based on the time interval from transplantation surgery [abstract]. International Journal of Urology 2014;21(Suppl 2):A265. [EMBASE: 71768475] [Google Scholar]
References to ongoing studies
ACTRN12610000349044 {published data only}
- Bartlett A. Pilot study: prospective randomized controlled trial of ureteric JJ stenting with early vs standard stent removal to improve graft and patient outcome and reduce urological complications after renal transplantation. www.anzctr.org.au/ACTRN12610000349044.aspx (first received 30 April 2010).
ISRCTN51276329 {published data only}
- Saeb‐Parsy K. A single centre, open label, randomised controlled study to compare the incidence of urinary tract infections and urological complications among renal transplant recipients who have a ureteric stent removed 6‐8 days versus 4‐6 weeks post renal transplantation. www.isrctn.com/ISRCTN51276329 (first received 26 October 2014).
Additional references
Bardapure 2014
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Montgomery 2012
- Montgomery JR, Berger JC, Warren DS, James NT, Montgomery RA, Segev DL. Outcomes of ABO‐incompatible kidney transplantation in the United States. Transplantation 2012; Vol. 93, issue 6:603‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Morris‐Stiff 1998
- Morris‐Stiff G, Balaji V, Lord RH. Simple technique for non‐operative removal of ureteric stents after renal transplantation. Annals of the Royal College of Surgeons of England 1998; Vol. 80, issue 5:370‐1. [MEDLINE: ] [PMC free article] [PubMed]
Nicholson 1991
- Nicholson ML, Veitch PS, Donnelly PK, Bell PR. Urological complications of renal transplantation: the impact of double J ureteric stents. Annals of the Royal College of Surgeons of England 1991; Vol. 73, issue 5:316‐21. [MEDLINE: ] [PMC free article] [PubMed]
Olsburgh 2010
- Olsburgh J. Clinical study protocol: TrUST Transplant Ureteric Stent Trial Protocol version 2. 2010. www.isrctn.com/editorial/retrieveFile/81979356‐1fe4‐4b96‐bc2b‐35b7afa4ffd5/19505 (accessed 23 August 2017).
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Siparsky 2011
- Siparsky NF, Kushnir LF, Gallichio MH, Conti DJ. Ureteral stents: a risk factor for polyomavirus BK viremia in kidney transplant recipients undergoing protocol screening. Transplantation Proceedings 2011;43(7):2641‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thiyagarajan 2012
- Thiyagarajan UM, Thiyagarajan P, Bagul A, Nicholson ML. Early removal of ureteric stents and its impact on reducing the urinary infection in renal transplantation‐A single centre experience. www.journal‐surgery.net/article/S1743‐9191(12)00583‐3/pdf (accessed 23 August 2017).
Waters 2008
Wilson 2013
- Wilson CH, Rix DA, Manas DM. Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD004925.pub3] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wilson 2015
- Wilson CH, Hosgood SA, Nicholson ML. Early versus late ureteric stent removal after kidney transplantation. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD011455] [DOI] [PMC free article] [PubMed] [Google Scholar]


