Abstract
Background
In many countries emergency departments (EDs) are facing an increase in demand for services, long waits, and severe crowding. One response to mitigate overcrowding has been to provide primary care services alongside or within hospital EDs for patients with non‐urgent problems. However, it is unknown how this impacts the quality of patient care and the utilisation of hospital resources, or if it is cost‐effective. This is the first update of the original Cochrane Review published in 2012.
Objectives
To assess the effects of locating primary care professionals in hospital EDs to provide care for patients with non‐urgent health problems, compared with care provided by regularly scheduled emergency physicians (EPs).
Search methods
We searched the Cochrane Central Register of Controlled Trials (the Cochrane Library; 2017, Issue 4), MEDLINE, Embase, CINAHL, PsycINFO, and King's Fund, from inception until 10 May 2017. We searched ClinicalTrials.gov and the WHO ICTRP for registered clinical trials, and screened reference lists of included papers and relevant systematic reviews.
Selection criteria
Randomised trials, non‐randomised trials, controlled before‐after studies, and interrupted time series studies that evaluated the effectiveness of introducing primary care professionals to hospital EDs attending to patients with non‐urgent conditions, as compared to the care provided by regularly scheduled EPs.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We identified four trials (one randomised trial and three non‐randomised trials), one of which is newly identified in this update, involving a total of 11,463 patients, 16 general practitioners (GPs), 9 emergency nurse practitioners (NPs), and 69 EPs. These studies evaluated the effects of introducing GPs or emergency NPs to provide care to patients with non‐urgent problems in the ED, as compared to EPs for outcomes such as resource use. The studies were conducted in Ireland, the UK, and Australia, and had an overall high or unclear risk of bias. The outcomes investigated were similar across studies, and there was considerable variation in the triage system used, the level of expertise and experience of the medical practitioners, and type of hospital (urban teaching, suburban community hospital). Main sources of funding were national or regional health authorities and a medical research funding body.
There was high heterogeneity across studies, which precluded pooling data. It is uncertain whether the intervention reduces time from arrival to clinical assessment and treatment or total length of ED stay (1 study; 260 participants), admissions to hospital, diagnostic tests, treatments given, or consultations or referrals to hospital‐based specialist (3 studies; 11,203 participants), as well as costs (2 studies; 9325 participants), as we assessed the evidence as being of very low‐certainty for all outcomes.
No data were reported on adverse events (such as ED returns and mortality).
Authors' conclusions
We assessed the evidence from the four included studies as of very low‐certainty overall, as the results are inconsistent and safety has not been examined. The evidence is insufficient to draw conclusions for practice or policy regarding the effectiveness and safety of care provided to non‐urgent patients by GPs and NPs versus EPs in the ED to mitigate problems of overcrowding, wait times, and patient flow.
Plain language summary
Primary care professionals providing non‐urgent care in hospital emergency departments
What is the aim of this review?
The aim of this Cochrane Review was to find out whether placing primary care professionals, such as general practitioners, in the hospital emergency department (ED) to provide care for patients with non‐urgent health problems can decrease resource use and costs. We searched for and analysed published and unpublished studies and found four relevant studies. This is the first update of a previously published Cochrane Review.
Key messages
We cannot be sure whether placing primary care professionals in the ED to provide care for patients with non‐urgent problems is as effective or safe as regularly scheduled emergency physician care, as we found little evidence with inconsistent results, which we assessed as of very low certainty. Safety has not been examined.
What was studied in the review?
In many countries, EDs are under a lot of pressure due to high patient attendance, resulting in long waits. One way of solving this problem may be to place primary care professionals in EDs to provide care for patients who do not have problems assessed as urgent at arrival. It has been suggested that this would make emergency physicians more available to provide care to more serious cases, thus decreasing resource use and costs.
What are the main results of the review?
This review included one randomised and three non‐randomised studies, involving a total of 11,463 patients, 16 general practitioners, nine emergency nurse practitioners, and 69 emergency physicians. Studies were conducted in Ireland, the UK, and Australia, with money given by national or regional health authorities and a medical research funding body. We could not combine the results due to differences among the studies. Because the evidence we found was of very low certainty, we cannot be certain if the intervention makes any difference to waiting times or total length of ED stay (1 study; 260 participants), admissions to hospital, diagnostic tests, treatments given, consultations or referrals to hospital‐based specialists (3 studies; 11,203 participants), as well as costs (2 studies; 9325 participants). None of the included studies provided data on adverse events.
How up‐to‐date is this review?
We searched for studies published up to May 2017.
Summary of findings
Summary of findings for the main comparison. Primary care professionals compared with ordinary emergency department physicians for patients with minor injuries and illnesses who attend hospital emergency departments.
| Primary care professionals compared with ordinary emergency department physicians for patients with minor injuries and illnesses who attend hospital emergency departments | ||||
|
Patient or population: patients with minor injuries and illnesses Settings: hospital emergency departments (Ireland, UK, Australia) Intervention: primary care professionals Comparison: ordinary emergency department physicians | ||||
| Outcomes | Relative effect | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Time from arrival to clinical assessment and treatment | MD 2.1 minutes (95% CI ‐4.9 to 9.2) | 260 (1 study) |
⊕⊝⊝⊝1,2 very low |
Expressed in minutes Follow‐up not reported. |
| Total length of ED stay | MD ‐3.2 minutes (95% CI ‐20.2 to 13.8) | 260 (1 study) |
⊕⊝⊝⊝1,2 very low |
Expressed in minutes Follow‐up not reported. |
| Admission to hospital | RR ranged from 0.33 to 1.11 | 11,203 (3 studies) | ⊕⊝⊝⊝ very low3,4,5 | Percentage of patients admitted to hospital from ED Follow‐up: 7 to 15 months |
| Diagnostic tests | RR ranged from 0.35 to 0.96 (laboratory investigations) RR ranged from 0.47 to 1.07 (imaging results) |
11,203 (3 studies) | ⊕⊝⊝⊝ very low1,4,5 | Percentage of patients for whom any blood investigation or imaging results were ordered Follow‐up: 7 to 15 months |
| Treatments given | RR ranged from 0.95 to 1.45 (any prescription) |
11,203 (3 studies) | ⊕⊝⊝⊝ very low1,4,5 | Percentage of patients given medication or prescription Follow‐up: 7 to 15 months |
| Consultations or referrals to hospital‐based specialists | RR ranged from 0.5 to 1.21 | 11,203 (3 studies) | ⊕⊝⊝⊝ very low3,4,5 | Percentage of patients referred to consultants
Follow‐up: 7 to 15 months In Dale 1995, patients referred to on‐call teams were excluded. |
| Costs | Cost reduction associated with the intervention ranged from GBP 60,876 to IEP 95,125. | 9325 (2 studies) |
⊕⊝⊝⊝4,6 very low | Cost in GBP excludes hospital admissions; it is unclear whether cost in IEP includes or excludes hospital admissions. |
| Adverse events | ‐ | ‐ | ‐ | We did not find any study reporting on adverse events. |
|
CI: confidence interval; ED: emergency department; MD: mean difference; RR: risk ratio GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
1We downgraded the evidence due to indirectness. 2We downgraded the evidence two points due to very serious imprecision (very wide confidence intervals including null‐effect and appreciable benefit or harm). 3We downgraded the evidence due to imprecision (wide confidence intervals including null‐effect and appreciable benefit or harm). 4We downgraded the evidence due to trial design (cross‐over of physicians in primary care sessions in Dale 1995 and predictable allocation of patients to either emergency physicians or general practitioners in Murphy 1996 and Gibney 1999). 5We downgraded the evidence due to inconsistency. 6We downgraded the evidence due to risk of bias.
Background
Description of the condition
Emergency departments (EDs) are designed to provide “rapid, high quality, continuously accessible, unscheduled care” for a wide range of acute illnesses and injuries (Ieraci 2000). Many large–volume and urban hospitals in high‐income countries now face rising costs and a crisis in ED overcrowding, a situation in which the demand for services cannot be met in a timely fashion. The cause of ED overcrowding is multifactorial, and can be broken down into input, throughput, and output factors (Asplin 2003). Input factors are those that affect the demand for ED services; throughput factors involve within‐ED management and determine patients' length of ED stay; and output factors involve the efficiency with which patients are discharged or transferred out of the ED for continuing care elsewhere (Asplin 2003).
One of the many possible explanations for overcrowding is the use of EDs for conditions triaged as non‐urgent, an input factor that contributes to increased demand for ED services. Use of the ED for non‐urgent problems that could be cared for in other settings has been described since the 1970s (Lees 1976), and is often labelled by health professionals as 'inappropriate use' (Liggins 1993). The term 'inappropriate use' is complicated by different definitions in the literature and by the fact that even patients with non‐urgent triage can require advanced imaging, consultations, and hospitalisations (Dong 2007). Inappropriate ED use can result in increased health service costs, contribute to overcrowding, and compromise care for true emergencies (Derlet 2000; Jepson 2001; Siddiqui 2002). Inappropriate ED use may also lead to suboptimal care of non‐urgent cases, which are managed hastily and without the benefit of comprehensive, continuous care that could be received in a primary care setting (Carret 2009). The introduction of general practitioners (GPs) and nurse practitioners (NPs) may provide more comprehensive and cost‐ and resource‐effective care for patients with non‐urgent problems in the ED. General practitioners and NPs may also reduce wait times and patient's length of ED stay (by seeing non‐urgent patients quickly and liberating emergency physicians (EPs) to see patients with more urgent problems), thus addressing some throughput and output factors that contribute to overcrowding.
It has been reported that between 6.7% and 89% of ED visits are for non‐urgent problems that could have been looked after in less specialised settings (Carret 2009; Lowy 1994; Murphy 1998; Thompson 2013). This large variation can be explained by a number of factors. First, there is a lack of consistency in the definition of ‘inappropriate use’ (Murphy 1998). Studies may use one or some combination of the following criteria to define inappropriate ED use: number of hours' wait without risk of death; need for tests or treatment; need for hospitalisations; possibility of treatment at other levels of care; hours of observation required; or self perceived urgency (Carret 2009). Second, different triage tools are used across the world, and definitions of non‐urgent triage also vary. Other reasons for the large variation in reported inappropriate use include regional differences in health services, sample population demographics, and the use of different professional groups to determine appropriate use. Inappropriate ED use has been shown to vary across age groups, time of day and day of week, type of disease, region, and socioeconomic status (Bezzina 2005; Carret 2009).
Description of the intervention
Research suggests that patients behave rationally, believing that emergency care is appropriate based on their perception of illness severity, health service availability, and ease of accessibility (Burns 2017; Carret 2009; Parboosingh 1987; Rieffe 1999; Walsh 1995). Moreover, many patients attempt to obtain care in other settings only to end up in the ED after referral there, through advice from others, or lack of access to other timely health care. One response to inappropriate ED use has thus been to provide primary care and community services to which patients can be directed alongside or within hospital EDs. An unpublished report estimates that approximately half of UK hospitals have primary care staff operating within or alongside the ED (Carson 2010). These interventions reflect a trend toward the provision of more comprehensive services in the hospital ED, and aim to provide appropriate services for patients with non‐urgent problems. The co‐location of a primary care out‐of‐hours facility in every ED is a joint recommendation by the College of Emergency Medicine, the Royal College of Physicians, the Royal College of Surgeons, the Royal College of Paediatrics and Child Health, and the NHS Confederation (College of Emergency Medicine 2014).
How the intervention might work
There are different models by which primary care can be introduced to the ED, including primary care services (Carson 2010):
within the ED, whereby patients enter the ED and are triaged into separate streams (broadly speaking urgent versus non‐urgent); the non‐urgent stream is staffed by primary care practitioners;
alongside the ED, whereby primary care is available on‐site, next to the ED, and patients either self select or are redirected from the ED towards the primary care service;
at the front of the ED screening or filtering patients, whereby primary care practitioners are involved in the triage of patients presenting to the ED and may also use the see‐and‐treat model of care for non‐urgent cases or redirect non‐urgent patients;
fully integrated and providing care jointly with ED staff on the full range of primary care and higher acuity emergency cases.
This review focussed on the first two models.
If GPs and NPs provide more efficient and less resource‐intense care than their EP colleagues when managing non‐urgent problems, ED time and resources might be more efficiently targeted towards urgent and potentially life‐threatening cases.
Why it is important to do this review
Overcrowding in EDs occurs throughout the world, and factors associated with crowding vary widely based on country, region, and health systems. The introduction of primary care services within or alongside hospital EDs is one response to this problem; however, it is not known if this intervention results in better care for patients with non‐urgent problems, if it liberates hospital and ED resources to provide better care for more urgent medical problems, if it is a safe strategy, or if it is cost‐effective.
A report commissioned by the UK Department of Health in 2009 examined the impact of introducing primary care services to the ED and concluded that "there is a paucity of evidence on which to base policy and local system design" (Carson 2010). This review strove to establish and identify gaps in the current evidence base for interventions that have introduced primary care professionals into the ED. This is the first update of the original Cochrane Review (Khangura 2012).
Objectives
To assess the effects of locating primary care professionals in hospital EDs to provide care for patients with non‐urgent health problems, compared with care provided by regularly scheduled EPs.
Methods
Criteria for considering studies for this review
Types of studies
We considered individual and cluster randomised trials (RTs), non‐randomised trials, controlled before‐after studies (CBA), and interrupted time series (ITS), which met the quality criteria used by the Cochrane Effective Practice and Organisation of Care (EPOC) Group (EPOC 2017a). Controlled before‐after studies studies were eligible if (1) the pre‐ and postintervention periods were the same in both groups, and (2) if they included a minimum of two intervention and two control sites. We considered ITS studies that reported a clearly defined time point for the intervention and a minimum of three data points both before and after the intervention.
We decided to also include studies that evaluated resource use and cost and that were either conducted concurrently to, or based upon data from, effectiveness studies that met the eligibility criteria above.
Types of participants
Patients who present to hospital EDs with illness or injury conditions suitable for primary care. Primary care‐suitable problems are those that are non‐urgent, self referred, and unlikely to require admission (Bezzina 2005). Furthermore, these problems do not require the specialised services of an ED, such as resuscitative facilities, urgent intervention, rapid and/or complex diagnostic work‐up and could be equally managed in an outpatient primary care setting (Bezzina 2005). Given that what is ‘primary care suitable’ may vary by region, we used the definitions applied in individual studies. We excluded studies comparing triage nurse ordering (Rowe 2011), nurse practitioners for specific problems, or triage liaison physicians to standard care for patients with non‐urgent problems suitable for primary care (Holroyd 2007; Rowe 2011).
Primary care professionals working in hospital EDs. Primary care refers to the health services and health professionals that are the patient’s first point of contact; thus defined it can include GPs, NPs, EPs, optometrists, and dentists. In the context of this review, primary care professionals include any licensed member of an accredited health specialty who normally works in a non‐specialised, outpatient setting to provide continuous “comprehensive care in the sense that only rare or unusual manifestations of ill health are referred elsewhere, and coordination of care such that all facets of care (wherever received) are integrated" (Starfield 1994; Starfield 2001).
Hospital physicians, including residents, senior house officers (SHOs), hospital interns, registrars and consultants (attendings), who work primarily in emergency medicine.
We excluded studies involving dentists and optometrists.
Types of interventions
We included interventions in hospital EDs in which patients who presented with non‐urgent problems were cared for by primary care professionals instead of regularly scheduled EPs. The control group received standard ED care from assigned EPs.
We included all interventions for analysis independent of variations in the type of primary care professional, time of day the patients presented to the ED, or triage criteria used to determine ‘non‐urgent problems'.
A variant of the intervention is where primary care services (e.g. out‐of‐hours GP services) have been established alongside, but not within, a hospital ED. We included these interventions if the newly introduced primary care service and existing hospital ED worked co‐operatively to provide care.
We excluded interventions:
at non‐hospital urgent‐care centres;
in EDs that employed primary care professionals prior to the intervention;
which diverted patients into 'fast track' areas of the ED;
where primary care professionals triaged patients in the ED; and
where primary care professionals cared for both urgent and non‐urgent patients alongside EPs.
Types of outcome measures
Main outcomes
-
Time from arrival to clinical assessment and treatment for:
patients with non‐urgent problems;
patients with urgent problems.
Total length of ED stay (from time of triage/registration to time of admission or discharge)
Admission to hospital
Other outcomes
Diagnostic tests (overall number, cost)
Treatments (e.g. counselling, prescriptions, procedures)
Consultations or referrals to hospital‐based specialists
Arrangement of follow‐up care
Subsequent utilisation of primary care/re‐attendance to the ED
Patient education for self management or appropriate service use
-
Cost comparison of:
diagnostic tests/investigations;
treatment;
referrals.
-
Health outcomes:
mortality;
self reported health status;
adverse events (return visits to the ED or readmissions).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases on 10 May 2017:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4) in the Cochrane Library;
MEDLINE Ovid (including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Versions) (1946 onwards);
Embase Ovid (1974 to 10 May 2017);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1980 onwards);
PsycINFO Ovid (1967 to May Week 1 2017);
Science Citation Index (Web of Knowledge) (citation search for included studies only conducted 11 January 2016).
In addition, we searched:
NHS Economic Evaluation Database (NEED) (www.crd.york.ac.uk/crdweb/);
King's Fund Library Database (kingsfund.koha‐ptfs.eu/).
Search strategies are comprised of keywords and controlled vocabulary terms. We applied no language or time limits. Development of the final search strategy was done with the assistance of the EPOC Information Specialist. We included studies regardless of publication status or language of publication. Detailed search strategies are included in Appendix 1.
Searching other resources
We searched the following clinical trials registries on 10 May 2017:
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/);
ClinicalTrials.gov, US National Institutes of Health (clinicaltrials.gov).
One review author (DGB) searched the reference lists of included studies and relevant systematic reviews.
Data collection and analysis
Selection of studies
One review author (DGB) downloaded all titles and abstracts retrieved by the electronic searches to Covidence reference management platform (Covidence 2018), removing duplicates and excluding studies that clearly did not meet the inclusion criteria. One review author (DGB) examined the remaining references and obtained the full text of relevant references. Two review authors (DGB and JKK) independently assessed the eligibility of the full‐text studies. Any disagreements were resolved by discussion.
Data extraction and management
Two review authors (JKK and DGB) independently undertook data extraction using a modified version of the EPOC data extraction form (Appendix 2) (EPOC 2017b). We extracted the following study characteristics.
Methods: study design, number of study centres and location, study setting, withdrawals, date of study, follow‐up.
Participants: number, mean age, age range, sex, severity of condition, diagnostic criteria, inclusion criteria, exclusion criteria, other relevant characteristics.
Interventions: intervention components, comparison, fidelity assessment.
Outcomes: main and other outcomes specified and collected, time points reported.
Notes: funding for trial, notable conflicts of interest of trial authors, ethical approval.
Any disagreements were resolved by discussion between review authors.
Assessment of risk of bias in included studies
Two review authors (JKK and DGB) assessed eligible studies for their risk of bias, in accordance with the Cochrane Handbook for Systematic Reviews of Interventions, Higgins 2011, and the EPOC Risk of Bias Criteria for non‐randomised studies (EPOC 2017c), which included:
sequence generation;
concealment of allocation;
similar baseline outcome measurements;
similar baseline characteristics (for providers and patients);
incomplete outcome data;
blinding of participants, personnel, and outcome assessors;
selective reporting of outcomes;
protection against contamination; and
other sources of bias.
We classified individual studies by risk of bias for each of these criteria as low, unclear, or high risk of bias. Any disagreements were resolved by discussion. Since we identified four studies, we did not assess whether variations in the certainty of the evidence could explain differences in study results.
Measures of treatment effect
We reported postintervention risk ratios (RR) or mean difference (MD) for intervention versus control groups with associated 95% confidence intervals (CI). Postintervention RR were based on raw number of events, adjusted or variable depending on how they were reported. No pre‐intervention data were reported in the included studies. We were not able to combine data due to high levels of statistical heterogeneity, explained by a variety of study designs, interventions, and outcomes. Data are presented in forest plots without a summary estimate, and as a narrative summary.
Unit of analysis issues
We noted that the unit of analysis across all four included studies was the patients. In one study (Dale 1995), the unit of analysis (patients) did not correspond with the unit of allocation (type of physician). A correct analysis for this study adjusting for the unit of allocation would have reduced the precision of the study estimate (larger 95% CI); in the context of a meta‐analysis, this would have reduced the weight given to this study. As we attempted no pooling due to the heterogeneity observed, we decided not to attempt any further adjustment (which would have been based on assumptions of group correlation, as no data on this were reported in the study). We did not identify any ITS designs.
Assessment of heterogeneity
We assessed statistical heterogeneity using I2 and Chi2 tests. Given the limited number of included studies, we did not further explore quantitative assessment for potential sources of heterogeneity. We provided a qualitative assessment of potential sources of heterogeneity in the Discussion.
Data synthesis
High heterogeneity precluded pooling data for outcomes (I2 >= 85%). We have presented the main findings of this review as forest plots without summary estimates. We calculated and reported findings for each outcome as RRs. We could not calculate the relative percent change as planned, as no pre‐intervention data were available. We used Review Manager 5 for all data analyses (RevMan 2011).
'Summary of findings' table and GRADE
Two review authors (JKK and DGB) independently assessed the certainty of the evidence as high, moderate, low, or very low using the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) for each of the following outcomes: time from arrival to clinical assessment and treatment, length of ED stay, admission to hospital, consultations or referrals to hospital‐based specialists, diagnostic tests, treatments given, cost, and adverse events (Guyatt 2008). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2011), the EPOC worksheets (EPOC 2017d), and employed GRADEpro software (GRADEpro GDT). We resolved disagreements on certainty ratings by discussion and provided justification for decisions to down‐ or upgrade the ratings using footnotes in the table and made comments to aid readers' understanding of the review where necessary. We used plain language statements to report these findings in the review (EPOC 2017e). We created a 'Summary of findings' table for the main intervention comparison. We have presented the MD or range of the RR for each outcome across included studies, along with their 95% CI, in the 'Summary of findings' table instead of summary estimates.
Subgroup analysis and investigation of heterogeneity
We had planned the following subgroup analyses, but were unable to perform them due to insufficient data:
patients’ socioeconomic status;
level of primary care health professional training (years in practice or stage of training);
healthcare systems; and
patients' age (0 to 18, 18 to 65, > 65).
Sensitivity analysis
We had planned to conduct sensitivity analyses (using random‐effects versus fixed‐effect model and study quality); however, as we identified only four studies with high heterogeneity for inclusion, we did not pursue this.
Results
Description of studies
See Characteristics of included studies table and Characteristics of excluded studies table.
Results of the search
Bibliographic searches retrieved 4678 records, and screening references of relevant systematic reviews retrieved 16 additional references. Of these 4694 unique references, we short‐listed 124 for full‐text screening, of which 14 were further assessed. We found one eligible study for this update (Jennings 2015), which we added to the three studies identified by the previous version of the review (Khangura 2012). The review includes one randomised trial, Jennings 2015, and three non‐randomised trials (Dale 1995; Gibney 1999; Murphy 1996). See the flow diagram detailing the search results in Figure 1.
1.
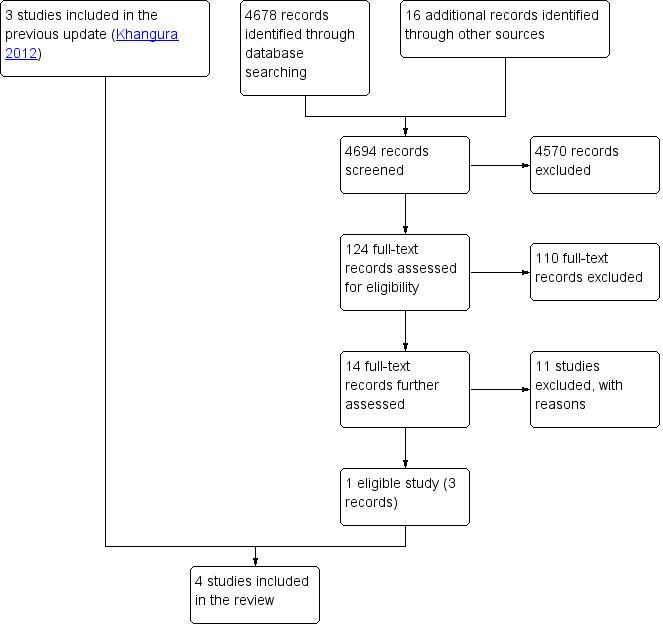
Study flow diagram.
Included studies
We identified four studies for inclusion in the review. The three non‐randomised studies evaluated the effectiveness of introducing GPs into the ED to provide care for patients with “non‐urgent” problems (Dale 1995; Gibney 1999; Murphy 1996). General practitioners working in the ED were supernumerary to the regularly scheduled EPs. These three studies were conducted in Ireland and the UK, where EPs are salaried. The randomised trial assessed the effectiveness of an emergency NP service model for patients who presented to the ED with pain but without immediately life‐threatening conditions. This study was conducted in Australia (Jennings 2015). The studies or the researchers were funded by the Australian National Health and Medical Research Council (Jennings 2015), the UK Department of Health (Murphy 1996), and the King's Fund and regional health authorities in the UK (Dale 1995). One study did not report sources of support (Gibney 1999). We identified no studies conducted in health systems where physicians are reimbursed on a fee‐for‐service basis.
All four trials were single‐site (i.e. one hospital) interventions, with study durations ranging between 7 and 15 months for three studies; one study did not report study duration (Jennings 2015).
Study design and intervention
Three trials were classified as non‐randomised because either (1) the allocation of patients to GPs or EPs was predictable, or (2) there was cross‐over of physicians allocated to primary care sessions (Dale 1995; Gibney 1999; Murphy 1996). The randomised trial was pragmatic, defined by the authors as a trial with limited control over the environment, a flexible intervention, and a heterogeneous sample (Jennings 2015).
Dale 1995 established three blocks of primary care sessions within the ED, to which a GP or an EP was allocated. All patients tagged as 'primary care suitable' during a particular session were seen by the same physician (either GP or EP). Murphy 1996 hired three GPs to work two four‐hour shifts each week alongside EPs, during which non‐urgent patients were allocated to either the GP or EP according to registration time. Gibney 1999 was conducted by the same team as Murphy 1996 and followed a similar design. In Jennings 2015, all eligible participants were randomly allocated to standard ED care, delivered by 17 emergency medicine registrars, or the intervention, staffed by nine emergency NPs. Further details can be found in the Characteristics of included studies table.
Classification of patients: triage methods and definition of non‐urgent patients
The methods to identify non‐urgent patients suitable for primary care differed across the included studies.
In Dale 1995, trained nurses triaged new attendees as either 'primary care' or 'accident and emergency', based on perceived need for care, rather than diagnosis or symptoms. 'Primary care' included self referred, non‐urgent problems that could be managed “in an average local general practice”. Patients referred by their GP, those requiring immediate resuscitation, or those likely to require hospital admission were excluded.
In Murphy 1996, patients were triaged by trained nurses according to the St James triage criteria, which classifies patients as:
life‐threatening;
urgent;
semi‐urgent; and
delay acceptable based on physiological criteria.
Patients in triage categories 3 and 4 were eligible for the study; however, those who were re‐attendees or who were referred by a GP were excluded.
Gibney 1999 used an unstructured triage system executed by untrained receptionists who categorised patients as 'urgent' or 'non‐urgent'. All ambulance patients were excluded from the 'non‐urgent' category. Further details of the criteria used to classify patients were not reported.
In Jennings 2015, trained nurses triaged all patients presenting to the ED using the Australasian Triage Scale (ATS), which is an algorithm with five levels, where each level corresponds to the clinical urgency of the patient's symptoms and indicates the time frame within which the patient should be seen (Jennings 2015). All patients allocated an ATS category 2 to 5 (not immediately life‐threatening) were eligible for the study. Patients with neurovascular compromise, multiple injuries, altered conscious states, and Glasgow Coma Scale greater than 14 were excluded.
Participants and settings
Three of the studies were conducted at major urban teaching hospitals in England (Dale 1995), Ireland (Murphy 1996), and Australia (Jennings 2015). One study was conducted at a small district hospital catering to a mixed urban‐rural population in Ireland (Gibney 1999).
The four included studies involved a total of 11,463 patients, 16 GPs, nine emergency NPs, and 69 EPs (42 senior house officers (SHOs), 25 registrars, and two consultants). General practitioners' experience varied relative to EPs across studies. In Dale 1995, the time since registration was similar for GPs and EPs; in Murphy 1996, GPs had more experience than EPs (seven years versus six months since registration). The level and experience of practitioners in Gibney 1999 was not reported. In Jennings 2015, NPs had a maximum of four years autonomous prescribing experience, while registrars had at least three years of postgraduate experience.
Study populations were similar with respect to age and sex in Dale 1995, Murphy 1996, and Jennings 2015 (not reported in Gibney 1999).
Outcomes
Data were not available for all of the review outcomes outlined in our protocol, such as subsequent utilisation of primary care/re‐attendance to the ED, patient education for self management or appropriate service use (Abi‐Aad 2000). Two of the included studies reported admission to hospital (Gibney 1999; Murphy 1996), and one trial reported total length of ED stay and waiting time (Jennings 2015). Outcomes reported in all three non‐randomised trials were the number of patients: (a) undergoing investigations (laboratory, electrocardiographic, and X‐ray in Dale 1995; any blood or X‐ray in Murphy 1996 and Gibney 1999); (b) receiving prescriptions; and (c) being referred (to consultants in Dale 1995; unspecified referral in the other two papers).
Two of the four included studies provided economic evaluations of the cost‐effectiveness of introducing GPs to the ED, compared with the current standard of care/system with regular ED staff (Dale 1995; Murphy 1996).
Excluded studies
We excluded 20 studies (see Characteristics of excluded studies table). The main reason for exclusion was ineligible study design (7 studies). We excluded other studies due to ineligible intervention or participants.
Risk of bias in included studies
The risk of bias of included studies is described in the 'Risk of bias' table within the Characteristics of included studies table and summarised in Figure 2, Figure 3, and below. The main source of bias across studies related to non‐randomised methods of allocation.
2.
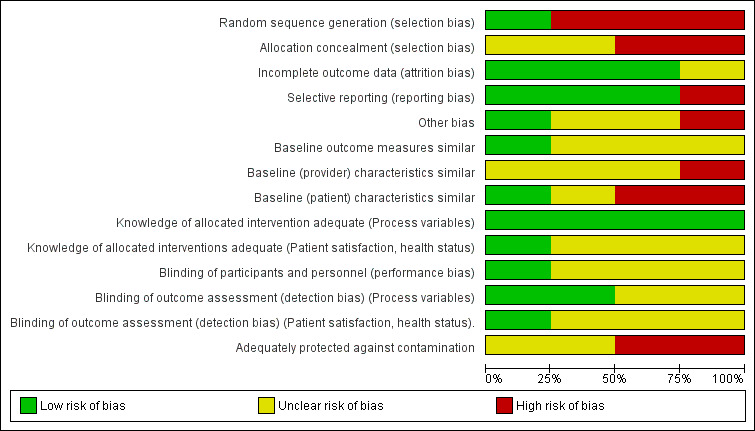
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
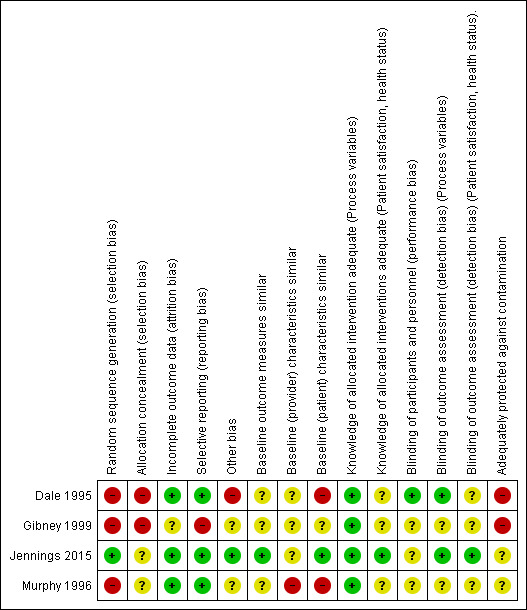
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In one of the included studies the method of sequence generation was random (Jennings 2015). We judged the remaining three studies to have high risk of selection bias due to non‐random allocation. We judged two included studies to be at high risk of bias for allocation concealment (Dale 1995; Gibney 1999), since triage nurses were not blinded to the grade and speciality of the physician providing care for 'non‐urgent ' patients at a particular session, which could have affected the triage and therefore also what type of patients the physician actually saw (i.e. more emergency‐type patients if an EP, and less so if a GP was providing the non‐urgent care). Murphy 1996 did not describe the allocation concealment, therefore we judged the risk of bias as unclear.
Baseline outcome measures
Jennings 2015 reported baseline outcome measures that were similar between groups and was judged to have a low risk of bias; the remaining studies did not and therefore had an unclear risk of bias.
Baseline provider characteristics
Dale 1995, Gibney 1999, and Jennings 2015 did not report any provider characteristics, therefore we judged the risk of bias as unclear. Murphy 1996 reported differences in age and work experience between GPs and EPs, with GPs being older and more experienced, resulting in a high risk of performance bias favouring GPs regarding the number of patients seen in a given time or the types of investigations ordered.
Baseline patient characteristics
In Dale 1995, there were differences in age, presenting complaints, and injury‐related diagnosis with type of doctor seen. Also, in Murphy 1996 there were differences between patients seen by GPs versus EPs for triage 3 (but not triage 4) patients. Hence, the risk of bias due to differences in patient characteristics was high for both of these studies.
We deemed the risk of bias for this item as unclear for Gibney 1999, and low for Jennings 2015, as there were little or no differences between patients.
None of the reported study outcomes adjusted for discrepancies in baseline characteristics.
Blinding
All studies used reliable, objective measures of outcome for investigating differences in processes of care (waiting time, length of ED stay, laboratory investigations, X‐rays, prescriptions, and admissions) between physician groups; risk of detection bias was low for these outcomes.
However, we judged detection bias for referrals as unclear in Murphy 1996 and Gibney 1999 due to a lack of clarity around the definition of referrals and uncertainty as to whether physicians were aware of study outcomes. We assessed Dale 1995 as at low risk of detection bias as physicians were unaware of study outcomes and referrals to outpatient clinics, community/general practice clinics, on‐call specialists teams and scheduled return visits to the ED were all included (Dale 1997).
Three studies provided self reported patient satisfaction and health status outcomes (Dale 1995; Jennings 2015; Murphy 1996); we judged risk of detection bias as unclear for these outcomes. Gibney 1999 did not present any self reported outcomes.
Performance bias was low in Dale 1995, as neither GPs, EPs, nor nurses were aware of study objectives or whether any particular primary care session was part of the study sample. The risk of performance bias for outcome assessment was also low for Jennings 2015. In Murphy 1996 and Gibney 1999 it was unclear if personnel were blinded to the study objectives or to the outcomes being assessed.
Incomplete outcome data
Dale 1995, Murphy 1996, and Jennings 2015 reported missing data (due to incomplete or missing records). The number of missing records was small relative to the overall sample size, hence we assessed the risk of bias due to incomplete outcome data as low for these three studies. The risk of bias due to incomplete outcome data was unclear in Gibney 1999 because of limited reporting of outcomes and no mention of missing data.
Selective reporting
We judged the risk of selective outcome reporting to be low in three studies (Dale 1995; Jennings 2015; Murphy 1996), where results for all outcomes mentioned in the methods section were reported. Gibney 1999 was a brief report, and was judged as at high risk for selective outcome reporting, as it is possible that the outcome data reported in the publication did not include all the outcomes measured in the study.
Other potential sources of bias
A potential source of bias in Dale 1995 and Murphy 1996 was the difference in number of hours worked by GPs versus EPs. General practitioners had limited numbers of shifts per week (range 6 to 9 hours per week across studies), while there were no restrictions on the number of shifts or hours worked by ED staff. This difference in ED work hours and experience could have created a performance bias affecting the number of patients seen, physicians' attitudes towards patients and their practice patterns when deciding on investigations, prescriptions, referrals, or admissions.
We assessed the risk of bias in Gibney 1999 as unclear due to lack of detailed information reported. We identified no other potential sources of bias for Jennings 2015, which we thus assessed as at low risk of bias.
Effects of interventions
See: Table 1
Meta‐analysis for process outcomes (diagnostic investigations, admissions, and referrals) had very high statistical heterogeneity, with I2 values greater than 85%, and these analyses were not retained. See Table 1 and Table 2 for a summary of the results.
1. Results summary.
|
Dale 1995 (N = 4641) |
Murphy 1996 (N = 4684) |
Gibney 1999 (N = 1878) |
|
| Laboratory investigations ordered | RR 0.22, 95% CI 0.14 to 0.33 | RR 0.35, 95% CI 0.29 to 0.42 | RR 0.96, 95% CI 0.76 to 1.2 |
| X‐rays ordered | RR 0.47, 95% CI 0.41 to 0.54 | RR 0.77, 95% CI 0.72 to 0.83 | RR 1.07, 95% CI 0.99 to 1.15 |
| Admissions | RR 0.33, 95% CI 0.19 to 0.58 | RR 0.45, 95% CI 0.36 to 0.56 | RR 1.11, 95% CI 0.70 to 1.76 |
| Referrals to specialists | RR 0.50, 95% CI 0.39 to 0.63 | RR 0.66, 95% CI 0.60 to 0.73 | RR 1.21, 95% CI 1.09 to 1.33 |
| Prescriptions | RR 0.95, 95% CI 0.88 to 1.03 | RR 1.45, 95% CI 1.35 to 1.56 | RR 1.12, 95% CI 1.01 to 1.23 |
CI: confidence interval; RR: risk ratio
Main outcomes
Time from arrival to clinical assessment and treatment
One study assessed time from arrival to clinical assessment and treatment, showing little or no difference between participants allocated to NPs or EP medical care (mean difference (MD) 2.1 minutes, 95% confidence interval (CI) ‐4.9 to 9.2) (Jennings 2015). It is uncertain whether the intervention reduces time from arrival to clinical assessment and treatment (1 study; 260 participants; very low‐certainty evidence).
Total length of ED stay
One study assessed total length of ED stay, showing little or no difference between participants allocated to NPs or EP for total length of ED stay (MD ‐3.2 minutes, 95% CI ‐20.2 to 13.8) (Jennings 2015). It is uncertain whether the intervention reduces total length of ED stay (1 study; 260 participants; very low‐certainty evidence).
Admission to hospital
General practitioners admitted fewer non‐urgent patients to hospital than EPs in two studies: risk ratio (RR) 0.33 (95% CI 0.19 to 0.58) in Dale 1995; and RR 0.45 (95% CI 0.36 to 0.56) in Murphy 1996. In Gibney 1999, there was little or no difference between the proportion of admissions made by each type of physician (RR 1.11, 95% CI 0.70 to 1.76; Analysis 1.1) (Figure 4). It is uncertain whether the intervention reduces admissions to hospital (3 studies; 11,203 participants; very low‐certainty evidence).
1.1. Analysis.
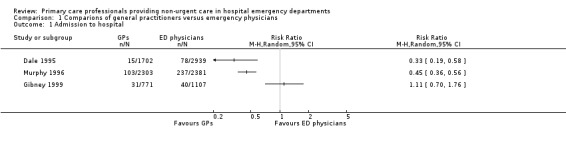
Comparison 1 Comparions of general practitioners versus emergency physicians, Outcome 1 Admission to hospital.
4.
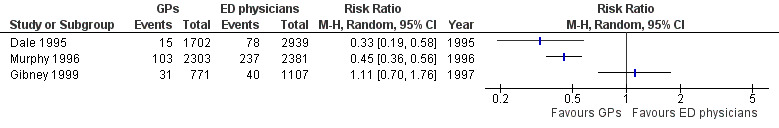
Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.1 Admissions.
Other outcomes
Diagnostic tests
Any investigations
Two studies reported the proportion of patients for whom any investigation was ordered (see Analysis 1.2; Figure 5) (Gibney 1999; Murphy 1996). The direction of effect in the two studies differed, with results in one study suggesting that GPs ordered fewer investigations than regularly scheduled EPs (RR 0.76, 95% CI 0.72 to 0.80) (Murphy 1996), and the second study reporting little or no difference between groups (RR 1.06, 95% CI 1.00 to 1.13) (Gibney 1999).
1.2. Analysis.
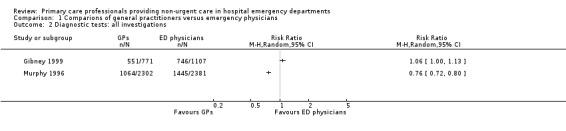
Comparison 1 Comparions of general practitioners versus emergency physicians, Outcome 2 Diagnostic tests: all investigations.
5.

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.2 All investigations.
Laboratory investigations
The results for laboratory investigations ordered (see Analysis 1.3; Figure 6) suggest that sessional GPs, defined as GPs who work as locum or salaried GPs, order fewer blood tests than regularly scheduled EPs, as the direction of effect across all studies was consistent. The size of the effect was similar in Dale 1995 (RR 0.22, 95% CI 0.14 to 0.33) and Murphy 1996 (RR 0.35, 95% CI 0.29 to 0.42). In Gibney 1999 this was less certain, as the effect size was smaller and confidence intervals crossed the line of no effect (RR 0.96, 95% CI 0.76 to 1.21). It is uncertain whether the intervention reduces laboratory investigations (3 studies; 11,203 participants; very low‐certainty evidence).
1.3. Analysis.
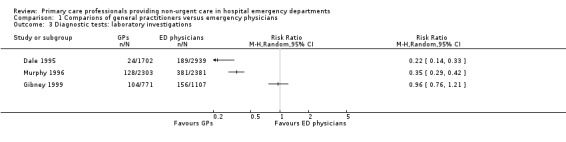
Comparison 1 Comparions of general practitioners versus emergency physicians, Outcome 3 Diagnostic tests: laboratory investigations.
6.
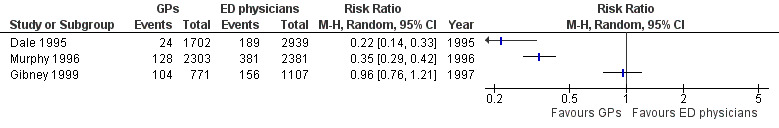
Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.3 Laboratory investigations.
Imaging results
The results for imaging results ordered (see Analysis 1.4; Figure 7) showed that GPs ordered fewer X‐rays than EPs in two studies (RR 0.47, 95% CI 0.41 to 0.54 in Dale 1995; and RR 0.77, 95% CI 0.72 to 0.83 in Murphy 1996); however, data from Gibney 1999 did not support this, with a RR of 1.07, 95% CI 0.99 to 1.15. It is uncertain whether the intervention reduces the number of X‐rays ordered (3 studies; 11,203 participants; very low‐certainty evidence).
1.4. Analysis.
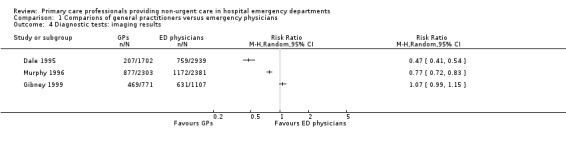
Comparison 1 Comparions of general practitioners versus emergency physicians, Outcome 4 Diagnostic tests: imaging results.
7.
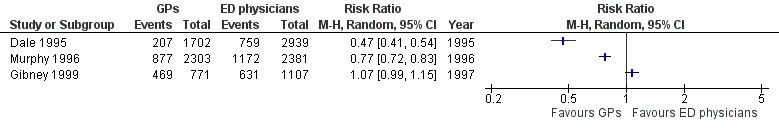
Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.4 Imaging results.
Treatments given
Any prescription (treatments)
As illustrated in Analysis 1.5 (Figure 8), there was little or no difference in prescribing behaviours between sessional GPs and regularly scheduled EPs in two studies: RR 0.95 (95% CI 0.88 to 1.03) in Dale 1995; and RR 1.12 (95% CI 1.01 to 1.23) in Gibney 1999. One study showed that GPs prescribed more than EPs (RR 1.45, 95% CI 1.35 to 1.56) (Murphy 1996). It is uncertain whether the intervention reduces treatments given (3 studies; 11,203 participants; very low‐certainty evidence).
1.5. Analysis.
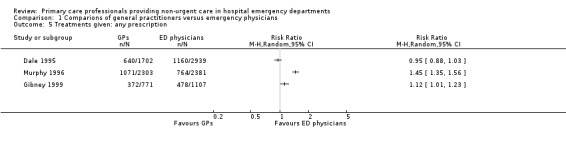
Comparison 1 Comparions of general practitioners versus emergency physicians, Outcome 5 Treatments given: any prescription.
8.
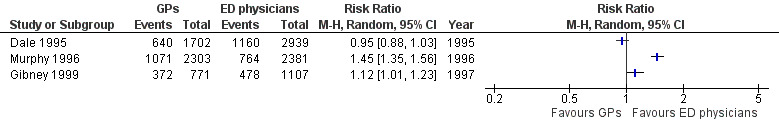
Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.5 Any prescription.
Consultations or referrals to hospital‐based specialists
Two studies found that GPs made fewer referrals to hospital specialists or consultants: RR 0.50 (95% CI 0.39 to 0.63) in Dale 1995; and RR 0.66 (95% CI 0.60 to 0.73) in Murphy 1996. Gibney 1999 reported a greater number of referrals made by GPs than EPs (RR 1.21, 95% CI 1.09 to 1.33). See Analysis 1.6 (Figure 9). It is uncertain whether the intervention reduces consultations or referrals to hospital‐based specialists (3 studies; 11,203 participants; very low‐certainty evidence).
1.6. Analysis.
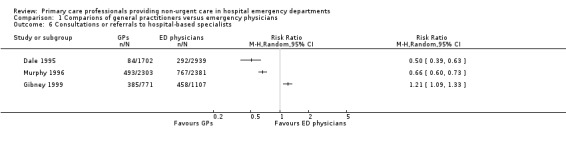
Comparison 1 Comparions of general practitioners versus emergency physicians, Outcome 6 Consultations or referrals to hospital‐based specialists.
9.
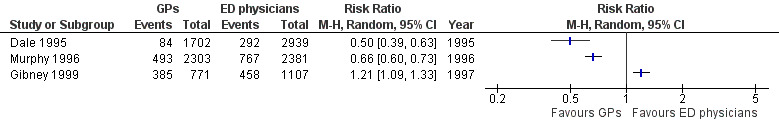
Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.6 Referrals.
Arrangement of follow‐up care
We did not find any study reporting on arrangement of follow‐up care.
Subsequent utilisation of primary care/re‐attendance to the ED
Murphy 1996 found little or no difference in ED re‐attendance rate by patients seen by GPs versus EPs, with 17% (95% CI 15.7% to 18.8%) of patients seen by a GP, and 18% (95% CI 16.3% to 19.5%) of patients seen by an EP re‐attending the ED for the same problem within 30 days of index visit.
Neither Dale 1995 nor Murphy 1996 reported differences in rates of general practice use across groups. In Murphy 1996, 25% (95% CI 17.9% to 31.1%) of study patients seen by a GP, and 22% (95% CI 13.7% to 30.4%) seen by an EP attended a general practice for the same complaint within 30 days of their index ED visit. The Dale 1995 study looked at general practice use in the 7 to 10 days following patients' index visit and reported that 20% (95% CI 14.9% to 25.1%), 18% (95% CI 13.3% to 22.5%), and 21% (95% CI 10.5% to 31.7%) of patients seen by GPs, SHOs, and registrars respectively consulted a GP or nurse practitioner in that time.
Patient education for self management or appropriate service use
We did not find any study reporting on patient education for self management or appropriate service use.
Costs
Dale 1995 reported that employing GPs to attend to primary care patients in the ED between 10 a.m. and 9 p.m. saved a total of GBP 60,876 at 1991 costs when admission costs were excluded, and GBP ˜150,000 when the cost of admissions was included.
Murphy 1996 provided a limited cost comparison for process variables used by GPs versus regularly scheduled EPs and estimated a total savings of IEP 95,125 by employing GPs. It is unclear whether this included the cost of admissions. It is uncertain whether the intervention reduces costs (2 studies; 9325 participants; very low‐certainty evidence).
Health outcomes
We did not find any study reporting on mortality or adverse events.
Only self reported outcome data on patient satisfaction and health status were available in two of the included studies. The type of physician seen made little or no difference for health status scores in Dale 1995 or Murphy 1996. In Dale 1995, self reported health status (n = 563) one week after attending the ED showed that the proportion of patients who were "recovered or improving" was 85.5% of GP patients versus 85.7% of EP patients. In Murphy 1996, 83.4% of patients seen by the GP in the ED were “cured” or “improved” compared to 87.4% of patients who saw ED staff one month after attending the ED.
A sub‐sample of patients were administered questionnaires in Dale 1995 (N = 565) and Murphy 1996 (N = 435 with 74% response rate). Dale 1995 reported high satisfaction ratings (> 71%) amongst the 565 people sampled, with little or no difference across GPs, SHOs, and registrars. Murphy 1996 also reported little or no difference in patient satisfaction between GPs or EPs.
Discussion
Summary of main results
This review included one randomised and three non‐randomised trials evaluating the effectiveness of employing emergency NPs, Jennings 2015, or sessional GPs, Dale 1995, Gibney 1999, Murphy 1996, in EDs to provide care for patients with non‐urgent problems. It is uncertain whether the intervention reduces time from arrival to clinical assessment and treatment, total length of ED stay (1 study; 260 participants), admissions to hospital, diagnostic tests, treatments given, or consultations or referrals to hospital‐based specialist (3 studies; 11,203 participants; very low‐certainty evidence), as well as costs (2 studies; 9325 participants; very low‐certainty evidence). No data were available on mortality or adverse events. Results were inconsistent across studies.
Overall completeness and applicability of evidence
The three non‐randomised studies were conducted in the UK or Ireland between 1993 and 1999, whereas the randomised trial was conducted in Australia in 2014, which may limit the generalisability of results to other countries. Data on the proportion of non‐urgent visits to the ED in these studies would be of interest, especially given the different financial structures in the UK and Ireland at the time the studies were conducted; these data were not available for comparison across all three studies conducted in the 1990s, plus the Australian study was conducted several years later and assessed the role of NPs, as opposed to GPs. In the UK’s national health system, GP and ED visits are available free of charge. The two studies conducted in Ireland, Murphy 1996 and Gibney 1999, were undertaken at a time when the Irish health system was a mix of public (˜85%) and private, in which approximately two‐thirds of patients paid a fee for GP and ED visits (Murphy 1996). Ireland has since adopted a publicly funded health system with the introduction of the Health Act in 2004 (Health Act 2004). Australia has an universal healthcare system that covers approximately 75% of GP costs and all ED costs for citizens who are covered by Medicare. The results of this review may not be applicable in countries with different healthcare structures.
Two major differences that make meaningful comparisons of EDs across studies and centres challenging are variations in: (1) the type of physicians who normally staff EDs; and (2) the triage definitions of 'urgent' and 'non‐urgent'. In major urban centres in many countries such as Canada and the USA, consultants in emergency medicine provide ED coverage every hour of every day. In contrast, the majority of the EPs in the included studies were senior house officers and registrars, who in North America would be considered trainee doctors and would not be categorised as EPs. Additionally, the lack of consensus on triage categories and definitions of non‐urgent primary care‐suitable problems make meaningful comparisons across studies difficult, since patients who classify as 'non‐urgent' at one centre may be triaged as 'urgent' at another.
The two largest included studies (each N > 4000) were conducted at major urban teaching centres (Dale 1995; Murphy 1996). Their results may not be applicable in other healthcare settings (e.g. rural or community hospitals), which are often staffed by GPs. Patient case‐mix may also vary between healthcare settings, which could help explain (in addition to the selection bias) why the results in Gibney 1999, which was conducted at a community hospital, differed consistently across outcomes from the two studies conducted at urban teaching hospitals (Dale 1995; Murphy 1996). There was also some debate on whether the NPs recruited by Jennings 2015 would qualify as primary care professionals, as although they catered to the primary care needs of patients who could not see a GP due to undersupply, they were integrated in a specialised tertiary ED setting.
Finally, the demographics of patients attending any ED are variable across centres, reflecting local socioeconomic factors, health status, and accessibility of primary care services. The type and number of non‐urgent problems that present to a particular centre will vary, and the results from these studies may not be applicable at EDs that cater to a patient population with a different set of non‐urgent problems.
Certainty of the evidence
We identified few studies, which limits the applicability of the study findings given the wide variation in the functions of EDs and healthcare systems. The overall strength of the evidence was weak as assessed with the GRADE approach (Guyatt 2008), with very low certainty of evidence for all outcomes. This was primarily because three of the included studies were non‐randomised trials, and the only randomised trial was very small, with very serious imprecision. We recognise that randomised trials are costly and difficult to conduct in the busy, unpredictable setting of an ED without encumbering ED staff or limiting patient flow; however, innovative trial methods (e.g. cluster or step‐wedge designs) are possible alternatives. The non‐randomised studies included in this review were large (total N = 11,203) and pragmatically designed to limit risk of bias; however, due to the loss of randomisation arising from cross‐over of physicians in Dale 1995 and the predictable allocation of patients to EPs or GPs in Murphy 1996 and Gibney 1999, we were unable to classify them as low risk. We also downgraded the certainty of the evidence for imprecise or inconsistent effects, as illustrated by the high heterogeneity across studies (I2 > 86%). The high heterogeneity may have resulted from differences in study design (e.g. the method of allocation), triage criteria used, healthcare systems, medical practitioner experience, outcome measurements (e.g. laboratory investigations versus haematology and biochemistry), or how events were reported. Finally, reporting bias due to the limited information reported lowered the certainty of evidence of one study (Gibney 1999). Combining data for meta‐analysis for each outcome was not possible because of high heterogeneity across studies.
Potential biases in the review process
The search strategy was developed with experienced information technologists and was designed to maximise sensitivity (detection of relevant research) at the expense of specificity (excluding irrelevant research). We also handsearched conference abstracts from emergency medicine conferences from the last three years, which should have reduced the likelihood of missing relevant studies. Previous research in this field has demonstrated publication bias (positive results published more often than negative results), and the authors recognise that negative results likely exist (Ospina 2006). Another potential bias in systematic reviews is selection bias. Attempts were made to avoid selection bias through independent identification of studies for inclusion, data extraction, 'Risk of bias' assessment, and grading by two or more review authors.
Agreements and disagreements with other studies or reviews
Previous reviews of this topic also reported weak evidence, suggesting cost‐benefits of employing primary care professionals in the ED, and conflicting evidence on resource utilisation with respect to investigations, prescriptions issued, or referrals made (Carson 2010; Cooke 2004; Ramlakhan 2016; Roberts 1998; Turner 2015; Winters 2009). They often included retrospective and observational study designs. None of these reviews provided a formal 'Risk of bias' assessment of included studies.
Authors' conclusions
Implications for practice.
There are few implications for practice based on the currently available evidence.
We found very weak evidence that the introduction of primary care professionals to the emergency department (ED) does not modify patients' subsequent use of primary care or the ED.
We found very weak evidence to suggest that general practitioners (GPs) and nurse practitioners (NPs) may use less resources to treat non‐urgent patients in the ED than emergency physicians (EPs), and thus that employing sessional primary care providers may introduce cost‐savings to EDs. However, it is unclear if less resource utilisation translates into safe care and improved outcomes for patients. The degree to which resource utilisation is influenced by practitioners' level of experience is also unknown, and GP or NP experience relative to EPs varied across the four included studies. Furthermore, cost‐savings will vary in individual healthcare settings and may depend on, for example, the magnitude of the salary difference between primary care and ED practitioners and the relative productivity of each.
Non‐urgent use of the ED has been hypothesised to contribute to long wait times and overcrowding in the ED (Carret 2009; Derlet 2000; Jepson 2001; Liggins 1993). There is insufficient evidence in this review for decision makers to evaluate the full impact of employing GPs in the ED to care for non‐urgent patients and the resulting effect on wait times and overcrowding, as current research has not addressed health outcomes and safety, which are important considerations. Important safety outcomes for which there is no evidence include mortality and re‐attendance. Provider satisfaction has not been examined, and introducing GPs to the ED may not be viable if the intervention is not welcome by EPs, or if GPs are not willing to work in ED settings. In Murphy 1996, three GPs left the study and had to be replaced; the reasons they left were not provided.
It may be noted that the benefit of providing primary care services within the ED may extend beyond cost‐ and resource‐savings, and may be greatest in settings where access to primary care is limited or costly for patients, or a larger proportion of ED visits are for non‐urgent problems. For example, additional benefits may arise when primary care and emergency staff work together through the exchange of ideas across disciplines (Chew‐Graham 2004).
Implications for research.
Three of the four studies included in this review were conducted more than 15 years ago. We identified one small recent randomised trial, although concerns regarding inappropriate ED use and overcrowding appear frequently in the emergency literature. This likely reflects the difficulty of designing and carrying out randomised trials in the busy emergency setting. Factors to consider include an unpredictable workload, that randomisation must be designed so as not to prolong wait times, and that health system‐wide changes may have an impact on the intervention (e.g. pay‐for‐performance, accountability, additional beds, time targets, etc.).
Design
Further research is needed, as evidence of resource and cost‐savings in itself is insufficient for health authorities to decide whether to employ GPs or NPs in the ED. Future studies may wish to investigate whether providing primary care in EDs generates more demand and increases the use of EDs for non‐urgent problems. The effect on wait times, adverse effects, mortality, and patient outcomes is extremely important and has not yet been thoroughly studied. Additional outcomes that are important to consider include the use of evidence‐based care by practitioners and patient safety outcomes.
Future studies should maximise the number of practitioners to reduce the effect of individual practitioners on outcomes. In addition, the methodological quality of the studies designed to evaluate the intervention could be improved by: triaging patients using a standard tool; using concealed allocation to randomise patients to see the EP or GP (e.g. using a black box from which the patients' charts were selected in the case of Murphy 1996 and Gibney 1999); or by randomising days of service prior to physician allocation, rather than selecting days of service post hoc (Holroyd 2007). That way, the length of ED stay, costs, and adverse effects of the intervention can be compared. In order to facilitate comparisons across future studies, researchers need to reach a consensus on the definition of 'primary care‐suitable problems' tailored to an ED.
Reporting
Adequate reporting of the implementation of the intervention is an additional area that requires attention to allow readers to evaluate the applicability of study findings to their own centres. In addition, the lack of consensus on methods of triage across different healthcare systems means that future studies should provide detailed descriptions of the triage criteria and methods used.
Studies must report fidelity of the intervention in order to determine the role of non‐adherence to the protocol may have on the outcomes. For example, when the allocation to the GP is overridden by staff, the reason and frequency should be documented. In addition, when patients referred to the GP are sent back to the regularly scheduled EP, the reasons and frequency need to be documented. Finally, scheduling and attendance by GPs for their shifts should be documented. The failure of an intervention may relate as much to the fidelity of the implementation as to the intervention itself.
Future studies should also aim to include descriptions of the:
pre‐intervention outcome data;
proportion of ED attenders classified as non‐urgent to allow comparisons across studies;
patient characteristics for all groups;
fidelity of the implementation;
hospital characteristics (catchment size, type (teaching or community), location (urban or rural));
medical provider characteristics (age, experience, level of expertise).
What's new
| Date | Event | Description |
|---|---|---|
| 12 December 2017 | New search has been performed | This is the first update of the Cochrane Review published in 2012. We updated the searches to May 2017 and the methods to comply with Cochrane's MECIR standards. We added a new author. |
| 12 December 2017 | New citation required but conclusions have not changed | We found one new study; the review now includes four studies. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 11, 2012
| Date | Event | Description |
|---|---|---|
| 4 October 2011 | Amended | Updated protocol |
| 18 July 2011 | Feedback has been incorporated | Authors added, feedback incorporated. |
| 12 November 2008 | Amended | Converted to new review format |
Acknowledgements
We thank Paul Miller (Information Specialist for the Cochrane Effective Practice and Organisation of Care Group (EPOC)), for his input on the search strategy and for running the searches; Julia Worswick (EPOC's Managing Editor) for her support and feedback during the editorial stages; Luciana Ballini (EPOC's Contact Editor) for her feedback; and Gerrard Abi‐Aad, Lucy Johnson, Nick Mays, and Emilie Roberts, who registered and wrote the original protocol in 2000.
National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Effective Practice and Organisation of Care Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Search strategies
MEDLINE (Ovid)
Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid MEDLINE and Versions
| 1 | Emergency Medical Services/ |
| 2 | Emergency Service, Hospital/ or Trauma centers/ |
| 3 | Triage/ |
| 4 | (emergency adj2 (care or healthcare or department? or unit or units or room? or treatment?)).ti,ab. |
| 5 | ("accident and emergency" or "accident & emergency" or emergency service?).ti,ab. |
| 6 | (trauma adj2 (centre or centres or center or centers or department? or unit or units)).ti,ab. |
| 7 | (triage adj2 (centre or centres or center or centers or department? or unit or units)).ti,ab. |
| 8 | (emergency adj2 visit?).ti,ab. |
| 9 | (urgent adj2 (care or healthcare or health care)).ti,ab. |
| 10 | ((semiurgent or semi‐urgent or nonemergen$ or non‐emergen$) adj2 (treatment? or care or visit?)).ti,ab. |
| 11 | ((emergency or non‐emergency or nonemergency or urgent or non‐urgent or nonurgent or semi‐urgent or semiurgent) adj2 patient?).ti,ab. |
| 12 | or/1‐11 |
| 13 | general practitioners/ or physicians, family/ or physicians, primary care/ |
| 14 | allied health personnel/ or community health aides/ or nurses' aides/ or psychiatric aides/ or pharmacists' aides/ or physician assistants/ or ophthalmic assistants/ or pediatric assistants/ |
| 15 | Nurse Practitioners/ |
| 16 | After‐Hours Care/ |
| 17 | ((general or family) adj3 (practitioner? or physician? or doctor?)).ti,ab. |
| 18 | (nurse practitioner? or nurse specialist?).ti,ab. |
| 19 | ("out of hours" or after hours).ti,ab. |
| 20 | or/13‐19 |
| 21 | 12 and 20 |
| 22 | ((community or primary health$ or primary care) adj2 (nurse or nurses or nursing staff or nursing personnel$)).ti,ab. and (Emergency Service, Hospital/ or Trauma centers/) |
| 23 | Community.ti,hw. and (Emergency Service, Hospital/ or Trauma centers/) |
| 24 | 21 or 22 or 23 |
| 25 | randomized controlled trial.pt. |
| 26 | controlled clinical trial.pt. |
| 27 | randomized.ab. |
| 28 | placebo.ab. |
| 29 | clinical trials as topic/ |
| 30 | randomly.ab. |
| 31 | trial.ti. |
| 32 | intervention*.ti. |
| 33 | (intervention* adj6 (clinician* or collaborat* or community or complex or DESIGN* or doctor* or educational or family doctor* or family physician* or family practitioner* or financial or GP or general practice* or hospital* or impact* or improv* or individuali?e* or individuali?ing or interdisciplin* or multicomponent or multi‐component or multidisciplin* or multi‐disciplin* or multifacet* or multi‐facet* or multimodal* or multi‐modal* or personali?e* or personali?ing or pharmacies or pharmacist* or pharmacy or physician* or practitioner* or prescrib* or prescription* or primary care or professional* or provider* or regulatory or regulatory or tailor* or target* or team* or usual care)).ab. |
| 34 | (collaborativ* or collaboration* or tailored or personali?ed).ti,ab. |
| 35 | (exp hospitals/ or exp Hospitalization/ or exp Patients/ or exp Nurses/ or exp Nursing/) and (study.ti. or evaluation studies as topic/) |
| 36 | demonstration project*.ti,ab. |
| 37 | (pre‐post or "pre test*" or pretest* or posttest* or "post test*" or (pre adj5 post)).ti,ab. |
| 38 | (pre‐workshop or post‐workshop or (before adj3 workshop) or (after adj3 workshop)).ti,ab. |
| 39 | ((study adj3 aim?) or "our study").ab. |
| 40 | (before adj10 (after or during)).ti,ab. |
| 41 | ("quasi‐experiment*" or quasiexperiment* or "quasi random*" or quasirandom* or "quasi control*" or quasicontrol* or ((quasi* or experimental) adj3 (method* or study or trial or design*))).ti,ab,hw. |
| 42 | ("time series" adj2 interrupt*).ti,ab,hw. |
| 43 | (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month* or hour? or day? or "more than")).ab. |
| 44 | pilot.ti. |
| 45 | Pilot projects/ |
| 46 | clinical trial.pt. |
| 47 | multicenter study.pt. |
| 48 | (multicentre or multicenter or multi‐centre or multi‐center).ti. |
| 49 | random*.ti,ab. or controlled.ti. |
| 50 | (control adj3 (area or cohort? or compar? or condition or group? or intervention? or participant? or study)).ab. |
| 51 | 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 |
| 52 | exp animals/ not humans/ |
| 53 | "comment on".cm. or systematic review.ti. or literature review.ti. or editorial.pt. or letter.pt. or meta‐analysis.pt. or news.pt. or review.pt. |
| 54 | 52 or 53 |
| 55 | 51 not 54 |
| 56 | 24 and 55 |
| 57 | (2011* or 2012* or 2013* or 2014* or 2015* or 2016* or 2017*).dc,dp,ed,ep,yr. |
| 58 | 56 and 57 |
Embase (OVID)
Embase <1974 to 2017 May 10>
| 1 | *emergency ward/ |
| 2 | *emergency health service/ |
| 3 | Triage/ |
| 4 | (emergency adj2 (care or healthcare or department? or unit or units or room? or treatment?)).ti,ab. |
| 5 | ("accident and emergency" or "accident & emergency" or emergency service?).ti,ab. |
| 6 | (trauma adj2 (centre or centres or center or centers or department? or unit or units)).ti,ab. |
| 7 | (triage adj2 (centre or centres or center or centers or department? or unit or units)).ti,ab. |
| 8 | (emergency adj2 visit?).ti,ab. |
| 9 | (urgent adj2 (care or healthcare or health care)).ti,ab. |
| 10 | ((semiurgent or semi‐urgent or nonemergen$ or non‐emergen$) adj2 (treatment? or care or visit?)).ti,ab. |
| 11 | ((emergency or non‐emergency or nonemergency or urgent or non‐urgent or nonurgent or semi‐urgent or semiurgent) adj2 patient?).ti,ab. |
| 12 | or/1‐11 |
| 13 | general practitioner/ |
| 14 | health auxiliary/ or mental health care personnel/ or paramedical personnel/ or occupational therapist/ or occupational therapy assistant/ or ophthalmic technologist/ or pharmacist/ or pharmacy technician/ or physiotherapist/ or physiotherapist assistant/ or radiological technologist/ |
| 15 | advanced practice nurse/ or clinical nurse specialist/ |
| 16 | nurse practitioner/ |
| 17 | ((general or family) adj3 (practitioner? or physician? or doctor?)).ti,ab. |
| 18 | (nurse practitioner? or nurse specialist?).ti,ab. |
| 19 | ("out of hours" or after hours).ti,ab. |
| 20 | or/13‐19 |
| 21 | 12 and 20 |
| 22 | ((community or primary health$ or primary care) adj2 (nurse or nurses or nursing staff or nursing personnel$)).ti,ab. and (emergency health service/ or emergency ward/) |
| 23 | Community.ti,hw. and (emergency health service/ or emergency ward/) |
| 24 | emergency nurse practitioner/ |
| 25 | 21 or 22 or 23 or 24 |
| 26 | randomized controlled trial/ |
| 27 | crossover‐procedure/ |
| 28 | double‐blind procedure/ |
| 29 | single‐blind procedure/ |
| 30 | (random$ or factorial$ or crossover$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).ti,ab. |
| 31 | or/26‐30 |
| 32 | intervention*.ti. |
| 33 | (intervention* adj6 (clinician* or collaborat* or community or complex or DESIGN* or doctor* or educational or family doctor* or family physician* or family practitioner* or financial or GP or general practice* or hospital* or impact* or improv* or individuali?e* or individuali?ing or interdisciplin* or multicomponent or multi‐component or multidisciplin* or multi‐disciplin* or multifacet* or multi‐facet* or multimodal* or multi‐modal* or personali?e* or personali?ing or pharmacies or pharmacist* or pharmacy or physician* or practitioner* or prescrib* or prescription* or primary care or professional* or provider* or regulatory or regulatory or tailor* or target* or team* or usual care)).ab. |
| 34 | (collaborativ* or collaboration* or tailored or personali?ed).ti,ab. |
| 35 | demonstration project*.ti,ab. |
| 36 | (pre‐post or "pre test*" or pretest* or posttest* or "post test*" or (pre adj5 post)).ti,ab. |
| 37 | (pre‐workshop or post‐workshop or (before adj3 workshop) or (after adj3 workshop)).ti,ab. |
| 38 | ((study adj3 aim?) or "our study").ab. |
| 39 | (before adj10 (after or during)).ti,ab. |
| 40 | ("quasi‐experiment*" or quasiexperiment* or "quasi random*" or quasirandom* or "quasi control*" or quasicontrol* or ((quasi* or experimental) adj3 (method* or study or trial or design*))).ti,ab,hw. |
| 41 | ("time series" adj2 interrupt*).ti,ab. |
| 42 | (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month* or hour? or day? or "more than")).ab. |
| 43 | pilot.ti. |
| 44 | *experimental design/ or *pilot study/ or quasi experimental study/ |
| 45 | (multicentre or multicenter or multi‐centre or multi‐center).ti. |
| 46 | random*.ti,ab. or controlled.ti. |
| 47 | (control adj3 (area or cohort? or compar? or condition or group? or intervention? or participant? or study)).ab. |
| 48 | or/32‐47 |
| 49 | 31 or 48 |
| 50 | (animal model? or animal experiment? or animal study? or animal trial? or canine or feline or bovine or cow or cows or mice or dog? or cat or cats or rabbit? or rat or rats or veterinar$).ti. or (animal or veterinary).hw. |
| 51 | (editorial or letter or note or "review" or trade or survey).pt. |
| 52 | meta‐analysis/ or systematic review/ or "literature review".ti. or "systematic review".ti. or (meta‐analy$ or metaanalyt$).ti. |
| 53 | 50 or 51 or 52 |
| 54 | 49 not 53 |
| 55 | 25 and 54 |
| 56 | (2011* or 2012* or 2013* or 2014* or 2015* or 2016* or 2017*).dp,dd,yr,em. |
| 57 | 55 and 56 |
Cochrane (Wiley)
| #1 | [mh "Emergency Medical Services"] |
| #2 | [mh "Emergency Service, Hospital"] |
| #3 | [mh Triage] |
| #4 | (emergency near/2 (care or healthcare or department* or unit or units or room* or treatment*)):ti,ab,kw |
| #5 | ("accident and emergency" or "accident & emergency" or emergency service*):ti,ab,kw |
| #6 | (trauma near/2 (centre or centres or center or centers or department* or unit or units)):ti,ab,kw |
| #7 | (triage near/2 (centre or centres or center or centers or department* or unit or units)):ti,ab,kw |
| #8 | (emergency near/2 visit*):ti,ab,kw |
| #9 | (urgent near/2 (care or healthcare or health care)):ti,ab,kw |
| #10 | ((semiurgent or semi‐urgent or nonemergen* or non‐emergen*) near/2 (treatment* or care or visit*)):ti,ab,kw |
| #11 | ((emergency or non‐emergency or nonemergency or urgent or non‐urgent or nonurgent or semi‐urgent or semiurgent) near/2 patient*):ti,ab,kw |
| #12 | {or #1‐#11} |
| #13 | [mh "Physicians, Family"] |
| #14 | [mh "Allied Health Personnel"] |
| #15 | [mh "Nurse Practitioners"] |
| #16 | ((general or family) near/3 (practitioner* or physician* or doctor*)):ti,ab,kw |
| #17 | (nurse practitioner* or nurse specialist*):ti,ab,kw |
| #18 | ("out of hours"):ti,ab,kw |
| #19 | [mh "After‐Hours Care"] |
| #20 | ((community or primary health* or primary care) near/2 (nurse or nurses or nursing staff or nursing personnel*)):ti,ab,kw |
| #21 | {or #13‐#20} |
| #22 | #12 and #21 |
PsycINFO (OVID)
PsycINFO 1967 to May Week 1 2017
| 1 | *emergency services/ |
| 2 | (emergency adj2 (care or healthcare or department? or unit or units or room? or treatment?)).ti,ab. |
| 3 | ("accident and emergency" or "accident & emergency" or emergency service?).ti,ab. |
| 4 | (trauma adj2 (centre or centres or center or centers or department? or unit or units)).ti,ab. |
| 5 | (triage adj2 (centre or centres or center or centers or department? or unit or units)).ti,ab. |
| 6 | (emergency adj2 visit?).ti,ab. |
| 7 | (urgent adj2 (care or healthcare or health care)).ti,ab. |
| 8 | ((semiurgent or semi‐urgent or nonemergen$ or non‐emergen$) adj2 (treatment? or care or visit?)).ti,ab. |
| 9 | ((emergency or non‐emergency or nonemergency or urgent or non‐urgent or nonurgent or semi‐urgent or semiurgent) adj2 patient?).ti,ab. |
| 10 | or/1‐9 |
| 11 | family physicians/ or general practitioners/ |
| 12 | exp mental health personnel/ |
| 13 | ((general or family) adj3 (practitioner? or physician? or doctor?)).ti,ab. |
| 14 | (nurse practitioner? or nurse specialist? or nurse aide? or nurse auxilliar$).ti,ab. |
| 15 | ((community or primary health$ or primary care) adj2 (nurse or nurses or nursing staff or nursing personnel$)).ti,ab. |
| 16 | (((allied health or paramedical or auxilliary) adj2 (staff or personnel)) or (pharmacist$ or pharmacy technician$ or pharmacy aide$)).ti,ab. |
| 17 | (((mental health or psychiatric) adj2 (nurse$ or staff or personnel)) or psychiatrist$).ti,ab. |
| 18 | ("out of hours" or after hours).ti,ab. |
| 19 | or/11‐18 |
| 20 | 10 and 19 |
| 21 | (abstract collection or bibliography or chapter or "column/opinion" or "comment/reply" or editorial or letter or obituary or publication information or reprint or review‐book or review‐media or review‐software & other or reviews).dt. |
| 22 | ("literature review" or "systematic review" or (meta‐analy$ or metaanalyt$)).ti. |
| 23 | 21 or 22 |
| 24 | 20 not 23 |
CINAHL (EBSCO)
| S1 | (MH "Emergency Service+") |
| S2 | TI ((emergen* or trauma or triage or urgent or nonurgent or semiurgent or semi‐urgent or nonemergen*) n2 (care or healthcare or department* or unit or units or treatment* or visit or visits)) or AB ((emergency or trauma or triage) n2 (care or healthcare or department* or unit or units or treatment* or visit or visits or patient*)) |
| S3 | TI ("accident and emergency" or "accident & emergency" or emergency service*) or AB ("accident and emergency" or "accident & emergency" or emergency service*) |
| S4 | MH Triage |
| S5 | S1 OR S2 OR S3 OR S4 |
| S6 | MH "Physicians, Family" |
| S7 | (MH "Nurse Practitioners") OR (MH "Clinical Nurse Specialists") OR (MH "Family Nurse Practitioners") OR (MH "Pediatric Nurse Practitioners") OR (MH "Acute Care Nurse Practitioners") OR (MH "Adult Nurse Practitioners") OR (MH "Gerontologic Nurse Practitioners") OR (MH "OB‐GYN Nurse Practitioners") |
| S8 | (MH "Clinical Nurse Specialists") |
| S9 | (MH "Allied Health Personnel") OR (MH "Emergency Medical Technicians") OR (MH "Medical Assistants") OR (MH "Ophthalmic Technologists") OR (MH "Orthopedic Technologists") OR (MH "Pharmacy Technicians") OR (MH "Community Health Workers") OR (MH "Mental Health Personnel+") OR (MH "Pharmacists") OR (MH "Rural Health Personnel") OR (MH "Nursing Assistants") OR (MH "Psychiatric Technicians") |
| S10 | TI (nurse practitioner* or nurse specialist*) or AB (nurse practitioner* or nurse specialist*) |
| S11 | TI ("out of hours" or afterhours care or after hours care) or AB ("out of hours" or afterhours care or after hours care) |
| S12 | S6 OR S7 OR S8 OR S9 OR S10 OR S11 |
| S13 | S5 AND S12 |
| S14 | (MH "Emergency Nurse Practitioners") |
| S15 | TI ((community or primary health or primary care) n2 (nurse or nurses or nursing staff or nursing personnel*)) or AB ((community or primary health or primary care) n2 (nurse or nurses or nursing staff or nursing personnel*)) and ((MH "Emergency Service") OR (MH "Trauma Centers")) |
| S16 | (TI community or MW community) and ((MH "Emergency Service") OR (MH "Trauma Centers")) |
| S17 | S13 OR S14 OR S15 Or S16 |
| S18 | PT randomized controlled trial |
| S19 | PT clinical trial |
| S20 | PT research |
| S21 | (MH "Randomized Controlled Trials") |
| S22 | (MH "Clinical Trials") |
| S23 | (MH "Intervention Trials") |
| S24 | (MH "Nonrandomized Trials") |
| S25 | (MH "Experimental Studies") |
| S26 | (MH "Pretest‐Posttest Design+") |
| S27 | (MH "Quasi‐Experimental Studies+") |
| S28 | (MH "Multicenter Studies") |
| S29 | (MH "Health Services Research") |
| S30 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) |
| S31 | TI (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) OR AB (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) |
| S32 | S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 |
| S33 | S17 AND S32 |
ClinicalTrials.gov
| conditions: | emergency OR "out of hours" OR "after hours" OR accident OR urgent |
| interventions: | ("primary care" OR "general practitioner" OR "family physician") |
WHO ICTRP
| emergency AND primary care |
| emergency AND general practitioner |
| emergency AND family physician |
| out of hours AND primary care |
| out of hours AND general practitioner |
| out of hours AND family physician |
| after hours AND primary care |
| after hours AND general practitioner |
| after hours AND family physician |
| accident AND primary care |
| accident AND general practitioner |
| accident AND family physician |
| urgent AND primary care |
| urgent AND general practitioner |
| urgent AND family physician |
Appendix 2. Data extraction form
| Study ID No. | 1st Author | Year | Contact Email or No. |
| |
|||
| Title: | |||
| Reviewer: | Date: | ||
ELIGIBILITY CRITERIA
A. Intervention :
| Population | |
| Intervention | |
| Comparison | |
| Outcomes | |
| Location |
B. Study Design is one of the following; please record the corresponding number in the box.
1. Randomized control trial (RCT)
2. Controlled Before‐After (CBA) with
3. Interrupted Time Series (ITS) where the
4. Qualitative
5. Other _______________________ (not to be included in review).
If the study is either a CBA or RCT, does it meet the following EPOC criteria? [yes or no]
If a Controlled Before‐After (CBA), design includes:
o Contemporaneous data collection & minimum 2 control and intervention sites
o Choice of control site / activity appropriate for question asked
o A second comparison site
If an Interrupted Time Series (ITS) study, design includes:
o Intervention occurs at a clearly defined point in time and there are
o Minimum of 3 data points before and 3 after intervention
_____No _____Yes Excel Code: 0 no; 1 yes
C. Studies must meet the following methodological criteria for inclusion:
(a) study includes objective measurement(s) of outcomes.
| Done | (e.g. drug levels a by a test, performance of providers against pre‐set criteria, number of tests ordered, number of c‐sections performed etc.). Outcome measures like provider or patient satisfaction included if assessed using a questionnaire with known reliability and validity. | |
| Not clear | the paper should be discussed with the contact editor for the review before data extraction is undertaken | |
| Not done | (e.g. self‐reported data, measurement of attitudes, beliefs, perceptions or satisfaction) |
(b) Relevant and interpretable data is presented or obtainable
| Done | |
| Not clear | |
| Not done |
| If criteria B and C above are met, continue. Otherwise, provide reason for exclusion: |
INTERVENTIONS
3.1.Type of Intervention(s).
3.2. Triage method used (include def’n or criteria used for ‘primary care suitable’ cases::
3.3. Describe the study control group(s):
Characteristics of Intervention(s):
| Who is delivering intervention? | |
| Skill type and level of training of health care providers | |
| Number of staff | |
| Setting (e.g. inside the A&E? Elsewhere in hospital?) | |
| Goal(s) of intervention: Highlight as many reasons as applicable to the study. | 1. Decrease costs 2. Decrease wait times 3. Decrease health resource utilisation 4. Improve quality of care 5. Measure patient satisfaction 6. Measure provider satisfaction 7. Other (list below): |
| Source of funding: | |
| Conflicts of interest? | |
3.4 Study Timing
| When (historically) study took place (Eg. 1990‐92) | |
| Intervention timing: (E.g. Which days? How many hours? How long?) | |
| Length of time underway (weeks): | |
| Duration of pre‐intervention data collection (weeks): | |
| Duration post‐intervention follow‐up period |
PARTICIPANT CHARACTERISTICS
(a) hospital characteristics / Setting
| Country: | |
| City: | |
| Provide any hospital characteristics, such as: § Rural vs urban § Size (# beds) § Average bed occupancy rate § Average no. visits per year § Academic status (teaching vs non‐teaching) |
|
| Hospital ownership: 1. Public or state owned, 2. private, 3. foreign owned, 4. other (provide), 5. not clear |
|
| Type of hospital: 1. military 2. civic 3. not applicable 4. not clear |
|
| Hospital scope 1. full service – i.e. tertiary hospital with access to most specialties 2. limited service – few specialists available 3. other 4. not clear |
|
| System of finance for primary care visits: 1. universal public 2. private insurance 3. patients out of pocket 4. other (specify) 5. not clear |
|
| System of finance for emergency visits: 1. universal public 2. private insurance 3. patient pays user fees or co‐payments 4. not clear |
|
| System of remuneration for health care providers in A&E: 1. fee per shift or hours worked 2. captitation 3. salary |
(b) Provider characteristics
| Group | Profession (nurse, GP, A&E doctor etc) | Level of training (junior doctor, resident, etc) | Time since graduation (i.e. years in practice) |
| Intervention | |
||
| Control | |
(b) participant (patient) characteristics
| Group | Age (mean, median, range) | Sex (% female) |
Ethnicity (breakdown by %) |
Clinical characteristics * | Other Information |
| Intervention | |
||||
| Control | |
* if study provides breakdown of patients by triage category or by types of problems, include this here
(c) Summary of numbers included in the study
| n | Other info? | |
| Patients | |
|
| Providers | |
|
| Practices | |
|
| Hospitals | |
METHODS
| Unit of allocation | |
| Unit of analysis | |
| Power calculation | |
Quality Criteria:
6.4 Risk of bias assessment
(If the trial is an ITS go directly to 6.4.2 for the RoB assessment)
6.4.1 Risk of bias assessment for randomised controlled trials (RCTs), controlled clinical trials (CCTs) and controlled before and after studies (CBAs)
a) Was the allocation sequence adequately generated ?(cut and paste from the paper verbatim)
| Score YES |
If a random component in the sequence generation process is described (e.g. referring to a random numbers table) |
|
| Score NO |
If a non‐random method is used (e.g. performed by date of submission) | |
| Score UNCLEAR |
If not specified in the paper. |
b) Was the allocation adequately concealed?
| Score YES |
If the unit of allocation was by institution, team or professional and allocation was performed at all units at the start of the study; or if the unit of allocation was by patient or episode of care and there was some kind of centralised randomisation scheme; an on‐site computer system or if sealed opaque envelopes were used. | |
| Score NO |
If none of the above mentioned methods were used (or if a CBA) | |
| Score UNCLEAR |
If not specified in the paper. |
Were baseline outcome measurements similar?
| Score YES |
If performance or patient outcomes were measured prior to the intervention, and no important differences were present across study groups | |
| Score NO |
If important differences were present and not adjusted for in analysis.** | |
| Score UNCLEAR |
If RCTs have no baseline measure of outcome** |
d) Were baseline characteristics similar?
| Score YES |
If baseline characteristics of the study and control providers are reported and similar | |
| Score NO |
If there is no report of characteristics in the text or tables or if there are differences between control and intervention providers. | |
| Score UNCLEAR |
If it is not clear in the paper (e.g. characteristics are mentioned in the text but no data were presented) |
e) Were incomplete outcome data adequately addressed?
| Score YES |
If missing outcome variables were unlikely to bias the results (e.g. the proportion of missing data was similar in the intervention and the control group, or the proportion of missing data was less than the effect size, i.e. unlikely to overturn the study results | |
| Score NO |
If missing data was likely to bias the results. | |
| Score UNCLEAR |
If not specified in the paper (Do not assume 100% follow up unless stated explicitly). |
f) Was knowledge of the allocated interventions adequately addressed?*
| Score YES |
If the authors state explicitly that primary outcome variables was assessed blindly, or the outcomes are objective e.g. length of hospital stay. | |
| Score NO |
If the outcomes were not assessed blindly. | |
| Score UNCLEAR |
If not specified in the paper. |
d) Was the study adequately protected against contamination?
| Score YES |
If allocation was by community, institution or practice and it s unlikely that the control group received the intervention. | |
| Score NO |
If it is likely that the control group received the intervention (e.g. if patients rather than professionals were randomised) | |
| Score UNCLEAR |
I professionals were allocated within a clinic or practice and it is possible that communication between intervention and control professionals could have occurred (e.g. physicians within practices were allocated to intervention or control) |
e) Was the study free from selective outcome reporting?
| Score YES |
If there is no evidence that outcomes were selectively reported (e.g. all relevant outcomes in the methods section are reported in the result section) | |
| Score NO |
If some important outcomes are subsequently omitted from the results. | |
| Score UNCLEAR |
If not specified in the paper. |
f) Was the study free from other risks of bias?
| Score YES |
If no evidence of other risks of bias | |
| Score NO |
||
| Score UNCLEAR |
* If some primary outcomes were imbalanced at baseline, assessed blindly or affected by missing data and others were not, each primary outcome can be scored separately.
**If “UNCLEAR” or “No”, but there is sufficient data in the paper to do an adjusted analysis (e.g. Baseline adjustment analysis or Intention to treat analysis) the criteria should be re scored to “Yes”.
6.4.2 Risk of bias assessment for interrupted time series (ITS) designs
Note: If the ITS study has ignored secular (trend) changes and performed a simple t‐test of the pre versus post intervention periods without further justification, the study should not be included in the review unless reanalysis is possible.
a) Was the intervention independent of other changes? (cut and paste from the paper verbatim)
| Score YES |
If there are compelling arguments that the intervention occurred independently of other changes over time and the outcome was not influenced by other confounding variables/historic events during study period. | |
| Score NO |
If reported that intervention was not independent of other changes in time. If Events/variables identified, note what they are. |
|
| Score UNCLEAR |
If not specified in the paper. |
b) Was the shape of the intervention effects pre‐specified?
| Score YES |
If point of analysis is the point of intervention OR a rational explanation for the shape of intervention effect was given by the author(s). Where appropriate, this should include an explanation if the point of analysis is NOT the point of intervention; | |
| Score NO |
If it is clear that the condition above is not met |
|
| Score UNCLEAR |
If not specified in the paper. |
c) Was the intervention unlikely to affect data collection?
| Score YES |
If reported that intervention itself was unlikely to affect data collection (for example, sources and methods of data collection were the same before and after the intervention. | |
| Score NO |
If the intervention itself was likely to affect data collection (for example, any change in source or method of data collection reported). | |
| Score UNCLEAR |
If not stated in the paper. |
d) Was knowledge of the allocated interventions adequately prevented during the study?***
| Score YES |
If the authors state explicitly that the primary outcome variables were assessed blindly, or the outcomes are objective, e.g. length of hospital stay. Primary outcomes are those variables that correspond to the primary hypothesis or question as defined by the authors. | |
| Score NO |
If the outcomes were not assessed blindly | |
| Score UNCLEAR |
If not specified in the paper |
e) Were incomplete outcome data adequately addressed?***
| Score YES |
If missing outcome measures were unlikely to bias the results (e.g. the proportion of missing data was similar in the pre‐ and post‐intervention periods or the proportion of missing data was less than the effect size i.e. unlikely to overturn the study result) | |
| Score NO |
If missing data was likely to bias the results. | |
| Score UNCLEAR |
If not specified in the paper (Do not assume 100% follow up unless stated explicitly). |
f) Was the study free from selective outcome reporting?
| Score YES |
If there is no evidence that outcomes were selectively reported (e.g. all relevant outcomes in the methods section are reported in the result section) | |
| Score NO |
If some important outcomes are subsequently omitted from the results. | |
| Score UNCLEAR |
If not specified in the paper. |
g). Was the study free from other risks of bias?
| Score YES |
If no evidence of other risks of bias e.g. should consider if seasonality is an issue (i.e. if January to June comprises the pre‐intervention period and July to December the post, could the “seasons’ have caused a spurious effect). | |
| Score NO |
||
| Score UNCLEAR |
*** If some primary outcomes were assessed blindly or affected by missing data and others were not, each primary outcome can be scored separately.
RESULTS
1. Main Outcomes
| Intervention | Control | Notes (SD, CI, other): | |
| Mean time (arrival to assessment) in hours for MINORS | |||
| Mean time (arrival to admission/discharge) in hours for MINORS | |||
| Mean time (arrival to assessment) in hours for MAJORS |
|||
| Mean time (arrival à admission/discharge) in hours for MAJORS | |||
| % of patients admitted to hospital via A&E (number) | |||
| % discharged from ED |
|||
| % left without being seen |
2. Secondary Outcomes
| Intervention | Control | Notes (SD, CI, other): | |
| Diagnostic tests (overall #) | |||
| Diagnostic tests (mean cost in study currency) | |||
| % of patients referred to consultant | |||
| % of patients referred to primary care | |||
| % of patients for whom treatment initiated | |||
| Arrangement of follow‐up care (%) | |||
| % patients who subsequently visit primary care for same problem (w/in 1 mos) | |||
| % patients who reattend A&E for same problem (w/in 1 mos) | |||
| Patient education Provided (%) |
|||
| Adverse outcome: % incorrect treatment |
|||
| Adverse outcome: % death within 1 mos of visit |
3. If reported, economic variables:
| Cost of intervention (US $) | |
| Changes in direct HC costs due to intervention (US $) | |
| Are costs a/w intervention linked to outcomes? |
Cost‐comparison:
| Mean cost of | Intervention | Control | Notes: |
| Diagnostic tests | |||
| Treatment | |||
| Referrals | |||
| Admissions |
OTHER:
Data and analyses
Comparison 1. Comparions of general practitioners versus emergency physicians.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admission to hospital | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Diagnostic tests: all investigations | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Diagnostic tests: laboratory investigations | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Diagnostic tests: imaging results | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Treatments given: any prescription | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Consultations or referrals to hospital‐based specialists | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dale 1995.
| Methods |
Design: non‐randomised trial Timeline: 1 June 1989 to 31 May 1990 (not bank holidays or first 2 weeks of August, February) Duration: 1 year Triage: patients categorised by trained nurses based on perceived need for care as either 'primary care' or 'accident and emergency' Data collection: Data on process and outcome variables (doctor's use of radiology, haematology, chemical pathology and microbiology investigations, items prescribed), referral and discharge decisions were obtained from hospital records and consultation record forms. Patient satisfaction and health status were assessed through a simple questionnaire (administered by phone or through post) to assess (1) self reported recovery in 7 to 10 days subsequent to attending ED and (2) health‐seeking behaviour during this period, including re‐attendance at ED or attendance at own GP surgery. |
|
| Participants |
Intervention group: N = 8 GPs (11 GPs applied, 6 were appointed, 2 left during study and were replaced) Control group: N = 31 EPs (27 senior house officers, 3 registrars, and 1 senior registrar) Provider characteristics: none reported Patients: new ED attendees with 'primary care' suitable problems Total number of patients: N = 4641; intervention group: n = 1702 patients seen by GPs; control group: n = 2939 patients seen by EPs Patient characteristics: Sex: 47.4% female Age: 41.7% 17 to 30 years Duration of complaints: 62.2% problems > 24 hours; 20.8% had previously seen a GP Most common diagnoses: injury and poisoning (44.4%), musculoskeletal diseases (13.7%), non‐specific symptoms and signs (7.0%) Patient characteristics for control and intervention groups not available. Setting: Hospital: one, King’s College Hospital Country: United Kingdom Hospital characteristics (1990 figures): Beds: n/a Teaching hospital, inner city, "multiethnic, socially deprived" Yearly attendance: 70,000 Yearly re‐attendance: n/a |
|
| Interventions |
Intervention: sessional GPs providing care for non‐urgent patients in the ED Control: regularly scheduled EPs providing care for non‐urgent patients in the ED Patients referred by GPs were excluded. Study took place from 1 June 1989 until 31 May 1990 (48 weeks total within 12 months, as bank holidays and the first two weeks of August and February when senior house officers change employment were excluded). Primary care sessions were established within the ED from 10‐1300 h, 14‐1700 h, and 18‐2100 h each day, except weekends when evening sessions were not available (see Figure 2). 1 physician (either a GP or an EP) was allocated to staff each primary care session according to a weekly rota. All patients triaged as 'primary care suitable' during a particular session were seen by the same physician (a GP or an EP). Medical staff knew patients’ triage status, but patients were unaware of their triage status or the type of physician (GP or EP) they were seeing. Both GPs and EPs were encouraged to use a designated consultation room for primary care sessions and were required to complete a consultation record form for each patient seen. Physicians were unaware how this data would be analysed. Each week, a random number table was used to select 2 to 3 daytime and 1 evening weekday sessions and 1 daytime weekend session for inclusion in the study (see Figure 2). Hence 8 to 10 sessions, which included a mix of GP and EP assignments, were selected for inclusion each week; this was done for a total of 48 weeks. Physicians were unaware of which sessions were included in the study and what outcomes were being measured. A total of 419 primary care sessions (215 GP‐ and 204 EP‐staffed sessions) were selected by stratified random sampling for inclusion in the study. Primary care sessions staffed by an EP formed the control group. The study authors noted that there was occasional cross‐over where the allocated physician did not treat primary care patients. This loss of randomisation occurred in both GP‐ and EP‐staffed sessions when the primary care session workload was excessive (to prevent unacceptable wait times) or when EPs were called away to manage urgent patients or to supervise junior physicians in the ED. The frequency and extent with which cross‐over occurred was not reported. To remedy this loss of randomisation, the study authors regrouped patients according to the type of doctor seen and used log‐linear modelling to adjust for confounding factors in their analysis. |
|
| Outcomes |
|
|
| Notes | Funding: Study authors funded by Lambeth Inner City Partnership and the King's Fund; SETRHA Primary Care Development provided additional funding for conducting the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "General practitioners and accident and emergency medical staff were considered as two groups, and each group was allocated two or three weekday sessions running from 1000 to 1300 and 1400 to 1700, one weekday evening session from 1800 to 2100, and one weekend daytime session for each week during the study period... ...weekly rosters stipulated a named doctor with responsibility for primary care patients for every three hour session" and "a random sample of sessions stratified by time of day and day of week was determined by using a table of random numbers. ...Hence, 8‐10 sessions were sampled each week for a total of 48 weeks. The sample of sessions allocated to accident and emergency staff was the same as those described in the accompanying paper." See P.1, Col.2, Para.4. Comment: Primary care sessions selected for inclusion in study were randomly selected using a random number table, however allocation of physicians to selected sessions was not random, but depended on physician availability and scheduling. Also, since nurses performing triage knew if a GP or an EP was seeing the 'non‐urgent' cases, this could affect what type of patients the physician in charge of providing care for the 'non‐urgent' patient group actually saw (i.e. more emergency‐type patients if an EP, and less so if a GP). |
| Allocation concealment (selection bias) | High risk | Quote: "Patients were unaware of their triage status or the grade and specialty of their doctor". See P.1, Col.5, Para.5 Comment: While patients were unaware of whether they were in the intervention (GP) or control (A&E staff) groups, this did not provide adequate allocation concealment; the type of physician providing care for each primary care session was open and not concealed. Importantly, triage nurses were not blinded to the grade and speciality of the physician in charge for providing care for 'non‐urgent' patients, which could have affected the triage and therefore also what type of patients the physician actually saw (i.e. more emergency‐type patients if an EP, and less so if a GP). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Not all records were complete" See P.2, Col.2, Para.2 Comment: Unclear whether missing data was predominantly from control or intervention group, or approximately equal across groups. Given binary outcomes and large samples, proportion of missing data probably less than effect size and low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods section were reported. |
| Other bias | High risk | Quote: "General practitioners worked sessions of only three hours in accident and emergency, compared with senior house officers' and registrars' shifts of up to 11 hours. Duration of shift may affect attitudes to patient care and influence the threshold of initiating referral or investigation." See P.4, Col.2, Para.1 Comments: General practitioners and EPs did not work equal numbers of hours in ED; this imbalance in experience and numbers of patients seen between providers could bias results. |
| Baseline outcome measures similar | Unclear risk | No baseline measure of outcome reported. |
| Baseline (provider) characteristics similar | Unclear risk | Quote: in recruiting GPs, "preference was given, firstly to those who had recently completed training (that is, general practitioners registered for similar numbers of years to the accident and emergency doctors) and, secondly, to those with flexible hours of availability". See P.1, Col.2, Para.3 Comment: This does not tell us what the actual provider characteristics were, only what was aimed for in the recruitment process. Also, no data are presented. |
| Baseline (patient) characteristics similar | High risk | Quote: "Two variables ‐ age and an injury related diagnosis ‐ were found to vary significantly with type of doctor seen. In addition, other variables (such as diagnosis of a mental disorder or a disease of the skin) varied significantly but had small effect sizes." See P.3, Col.2, Para.4, and Table VI. |
| Knowledge of allocated intervention adequate (Process variables) | Low risk | Unclear if outcomes were assessed blindly, but process variables (laboratory and X‐ray investigations, prescriptions, referrals, admissions) were objective. Referrals were defined in the primary author's PhD thesis as outpatient, on‐call team and hospital admissions were all counted as referrals. |
| Knowledge of allocated interventions adequate (Patient satisfaction, health status) | Unclear risk | Questionnaires were administered by standardised telephone interview or post within 7 to 10 days of patients' index visit: "We interviewed the patients again 7‐10 days later by telephone (or sent them a postal questionnaire if they lacked a telephone) about their satisfaction with their assessment and treatment in the department, the extent of their recovery, and the health care they required after attending the department. Responses to questions of satisfaction were recorded on five point Likert scales, ranging from very satisfied to very dissatisfied." See P.1, Col.2, Para.3 (Dale 1996). Comment: Self reported data and unvalidated questionnaire (as per Dale thesis, no validated questionnaires were available at time of study). Unclear if interviewer was blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Neither the general practitioners nor the accident and emergency doctors or nurses were informed about the study objectives or whether any particular session was part of the study sample." See P.1, Col.2, Para.4 "Patients were unaware of their triage status or the grade and speciality of their doctor." See P.427, Col.2, Para.5 Comments: All personnel (GPs, EPs, and nurses) were blinded to the study objectives and whether any particular session was part of the study sample, and the patients were unaware which type of doctor they were seen by. |
| Blinding of outcome assessment (detection bias) (Process variables) | Low risk | Quote: "All doctors...were asked to complete a consultation record form for each patient seen...Doctors remained blind to how data from these forms would be analysed." See P.2, Col.1, Para.3 Comments: Outcomes were objective, and physicians were unaware of what data were being collected for the study. It is unclear if researchers knew which physician saw patients. |
| Blinding of outcome assessment (detection bias) (Patient satisfaction, health status). | Unclear risk | Unclear if outcome assessors for patient satisfaction and health status were blinded |
| Adequately protected against contamination | High risk | Quote: "Although the intention was that all primary care patients would be treated by the allocated doctor, this did not always occur. Firstly, at times when the primary care workload was excessive, other doctors were directed by the nurse performing triage to treat primary care patients to prevent unacceptably long waiting periods from occurring; secondly, registrars in particular were often interrupted from completing primary care sessions by departmental circumstances (such as responding to patients with urgent or life threatening needs or providing advice or supervision to senior house officers). Hence patients were sometimes attended by a non‐allocated doctor, both during sessions originally allocated to a general practitioner and during those allocated to another member of accident and emergency staff." See P.2, Col.1, Para.2 "Since this breakdown of randomisation was not always clearly documented, data for all recorded primary care consultations occurring during the selected sessions were included in the sample, and data on patients were regrouped according to the type of doctor actually seen. The loss of randomisation was allowed for by including confounding factors in the analysis of the data." See P.2, Col.1, Para.2 |
Gibney 1999.
| Methods |
Design: non‐randomised trial Time: March 1996 to September 1996 Duration: 7 months Triage: patients categorised by receptionists with no formal training into "urgent" and "non‐urgent" Data collection: Process data were collected from a review of written patient records. |
|
| Participants |
Intervention group: N = 3 GPs Control group: N = 8 EPs (1 consultant, 2 registrars, 5 senior house officers) Provider characteristics: none reported Patients: all "non‐urgent" and non‐ambulance patients attending the ED; ambulance patients were excluded Total number of patients: N = 1878; intervention group: n = 771 patients seen by GPs; control group: n = 1107 patients seen by EPs Patient characteristics: data no longer available Setting: Hospital: one, James Connolly Memorial Hospital Country: Ireland Hospital characteristics (1996 figures): Beds: 336, small district hospital, urban/rural mix Yearly attendance: 25,047 Yearly re‐attendance: 8213 |
|
| Interventions |
Intervention: sessional GPs providing care for non‐urgent patients in the ED Control: regularly scheduled EPs providing care for non‐urgent patients in the ED (when GP present at the ED) Patients referred by GPs included. Conducted March to September 1996 (7 months). This study was designed by the same author‐group as Murphy 1996. 3 GPs were hired by the hospital to work on a sessional basis. The frequency and duration of GP sessions in the ED were not reported. As in the Murphy 1996 study, non‐urgent patients were allocated to either a GP or an EP in alternating (but not random or consecutive) order according to time of registration. Triage status did not factor into the order in which patients were seen, as only two triage categories were used: "urgent" and "non‐urgent". As in Murphy 1996, the control group comprised non‐urgent patients seen by EPs when a GP was on‐site. |
|
| Outcomes |
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: Allocation of patients "to either GP or A&E staff was the same as our previous study (Murphy 1996) and was performed according to time of registration." See P.1, Col.2, Para.5. Comment: Sequence generation was non‐random; patients were seen in temporal order, and allocation to provider was not necessarily consecutive, depending on the length of previous consultations. |
| Allocation concealment (selection bias) | High risk | Quote: "An unstructured receptionist‐based triage system divides all non‐ambulance patients into two categories: 'urgent' and 'non‐urgent'." See P.1, Col.2, Para.3. Comment: Patient allocation occurred as individuals entered the study (by attending the ED). It is unclear how physician allocation to primary care sessions was performed. It is not specified whether nurses performing triage were blinded; nurses' knowledge of whether a GP or an EP was working could have affected triage and the type of patients that physician working in primary care sessions saw (i.e. more emergency‐type patients if an EP, and less so if a GP). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified in the paper |
| Selective reporting (reporting bias) | High risk | All outcomes mentioned in the text were reported in the results, however the study was designed and carried out by same author‐group as Murphy 1996, and fewer outcomes are reported without explanation. |
| Other bias | Unclear risk | It is probable that GPs and EPs did not work equal numbers of hours in the ED; this imbalance between providers in experience and numbers of patients seen could bias the results. |
| Baseline outcome measures similar | Unclear risk | No baseline measure of outcome reported. |
| Baseline (provider) characteristics similar | Unclear risk | No provider characteristics reported. |
| Baseline (patient) characteristics similar | Unclear risk | Quote: "There were no differences in age, sex, socio‐economic status, registration with a GP or type of presenting complaint between patients seen by a GP or usual A&E staff." See P.1, Col.2, Para.6. Comment: No data on patient characteristics were reported, hence we cannot corroborate that the patient groups seen by GPs or EPs were comparable in terms of duration of complaints, diagnoses, etc. |
| Knowledge of allocated intervention adequate (Process variables) | Low risk | The outcomes are objective. |
| Knowledge of allocated interventions adequate (Patient satisfaction, health status) | Unclear risk | Not specified in the paper |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified in the paper |
| Blinding of outcome assessment (detection bias) (Process variables) | Unclear risk | Unclear if outcomes were assessed blindly, but process variables (laboratory and X‐ray investigations, prescriptions, admissions) were objective. A definition of what constituted referrals in the study was not provided; if only some types of referrals (e.g. to on‐call physicians) were counted, this would not objectively account for the total referrals made (e.g. to non‐physician health professionals) by both intervention and control groups. |
| Blinding of outcome assessment (detection bias) (Patient satisfaction, health status). | Unclear risk | Not specified in the paper |
| Adequately protected against contamination | High risk | Quote: "Study enrolment only occurred when both GPs and usual A&E staff were on duty together." See P.1, Col.2, Para.5. Comments: General practitioners and EPs worked simultaneously in primary care sessions, and overlap and contamination between groups was possible. |
Jennings 2015.
| Methods |
Design: pragmatic randomised trial Time: first participant enrolled February 2014 Duration: not described Triage: participants triaged by trained nurses using the Australasian Triage Scale Data collection: baseline data collected from all consenting participants during enrolment. Pain score reduction reported by the participant, all other outcomes collected from the ED patient information system and electronic health record. |
|
| Participants |
Intervention group: N = 9 emergency NPs Control group: N = 17 emergency medicine registrars Years of postgraduate training (minimum): 3 years Patients: all patients presenting to the ED with "pain" and allocated to the "fast‐track" zone Total number of patients: intervention: 130; control: 128 Patient characteristics: Sex: intervention: 47% female; control: 39% female Age (median): intervention: 33 years; control: 30 years Pain score (median): intervention: 5; control: 5 Setting: Hospital: one, adult tertiary ED Country: Australia ED characteristics (2013 figures): Major urban teaching hospital Yearly attendance: 65,000 |
|
| Interventions |
Intervention: People presenting with pain, who were triaged to fast‐track area (Australasian Triage Scale 2 to 5), were randomly assigned to receive either standard ED medical care or emergency NP care. Control: Care was provided by medical officers with assistance from registered nurses, if required. |
|
| Outcomes |
Primary outcomes: pain score reduction and time to analgesia Secondary outcomes: waiting time, number of patients who did not wait, length of stay in ED, re‐presentations with 48 hours Integrity of the intervention measured through clinicians' use of evidence‐based guidelines for management of knee, ankle, and burns injury. (Outcomes as per the published protocol.) |
|
| Notes | Funding: National Health and Medical Research Council postgraduate scholarship through Queensland University of Technology, Australia (principal investigator) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed with an allocation sequence of four and generated by computer random number generator and then transcribed into opaque sequentially numbered sealed envelopes" (p.775) |
| Allocation concealment (selection bias) | Unclear risk | "Each envelope contained a card with the allocation group recorded and treatment pack. Allocation adhered strictly to the generated sequence and was maintained. Both participants and treating staff were aware of treatment allocation." (p.775) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up; 2 participants allocated to intervention excluded from analysis as consent forms not signed (0.02%). |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the protocol published (primary and secondary outcomes reported separately). |
| Other bias | Low risk | No other risk detected. |
| Baseline outcome measures similar | Low risk | Clinical research assistants used an examination cubicle to recruit and consent patients and collect baseline demographic information. |
| Baseline (provider) characteristics similar | Unclear risk | Not described |
| Baseline (patient) characteristics similar | Low risk | Little or no differences between groups (Table 1) |
| Knowledge of allocated intervention adequate (Process variables) | Low risk | Most outcomes are objective. |
| Knowledge of allocated interventions adequate (Patient satisfaction, health status) | Low risk | Not applicable, not outcomes for this study |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not enough information to ascertain risk of bias |
| Blinding of outcome assessment (detection bias) (Process variables) | Low risk | Primary investigators were blinded to treatment allocation for data analyses. Most outcomes were objective. |
| Blinding of outcome assessment (detection bias) (Patient satisfaction, health status). | Low risk | Not applicable, not outcomes for this study |
| Adequately protected against contamination | Unclear risk | Not enough information to ascertain risk of bias. Both medical officers and NPs worked in fast‐track area at overlapping times. |
Murphy 1996.
| Methods |
Design: non‐randomised study Time: August 1993 to October 1994 Duration: 15 months Triage: Patients triaged by trained nurses based on physiological criteria as (1) life‐threatening, (2) urgent, (3) semi‐urgent, or (4) delay acceptable. Data collection: Process information (investigations, referrals, prescriptions, etc.) was collected from hospital records. The numbers of patients re‐attending the ED within 1 month of the index visit was determined using the hospital's mainframe computer. Patient satisfaction was assessed immediately by a blinded interviewer using the consultation satisfaction questionnaire. Health status was determined 1 month after the initial consultation by means of a simple questionnaire (4 questions) completed by telephone or letter. Marginal (materials and disposables) and total (marginal plus all staff) costs were determined in conjunction with the hospital's finance department and X‐ray and laboratory staff. Costs were calculated for the following: full blood counts; measurements of blood urea and plasma electrolyte concentrations, plasma glucose concentration, and serum amylase activity; sequential multiple analysis with computer (SMAC); and chest, limb, skull, spine, and abdominal radiographs. Based on the hospital admission profile, an estimate of the average cost per admission was also obtained. |
|
| Participants |
Intervention group: N = 5 GPs Age (median): 32 years Years since registration (median): 7 years Control group: N = 13 EPs (1 consultant, 2 registrars, 10 senior house officers) Age (median): 26 years Patients: new ED attendees triaged as "semi‐urgent" or "delay acceptable" Total number of patients: N = 4684; intervention group: n = 2303 patients seen by GPs; control group: n = 2381 patients seen by EPs Patient characteristics: Sex: 41.4% female Age: median 28 to 34 years Years since registration (median): 6 months Duration of complaints: 44% problems > 24 hours; 92.6% registered with GPs (unclear how many saw GP prior to attending) Most common diagnoses: musculoskeletal (50.9%), skin complaints (19.0%), and neurological (8.8%) Setting: Hospital: one, St James' Hospital Country: Ireland Hospital characteristics (1992 figures): Beds: 490, catchment 219,300 people Major teaching hospital Yearly attendance: 40,159 Yearly re‐attendance: 7589 |
|
| Interventions |
Intervention: sessional GPs providing care for non‐urgent patients at hospital ED Control: regularly scheduled EPs providing care for non‐urgent patients when GP present in department Patients referred by GPs (20%) were excluded. The study took place between August 1993 and October 1994 (15 months). 3 GPs were hired to work two 4‐hour shifts each week alongside EPs. During these primary care shifts, non‐urgent patients were allocated to either the GP or EP according to registration time. The control group comprised non‐urgent patients seen by EPs when a GP was on‐site. The allocation of patients was predictable but not necessarily consecutive, as the order in which patients were allocated depended on the length of consultations. In addition to temporal ordering, patients were also ordered by triage category: triage category 3 patients were seen prior to category 4. The GPs and EPs in this study had access to all of the same ED facilities, and patients were unaware what type of physician was treating them. |
|
| Outcomes |
|
|
| Notes | Funding: Department of Health through the General Practice Unit of the Eastern Health Board | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Randomisation of patients to the general practitioner or accident and emergency staff depended on time of registration. Once patients were registered their charts were divided according to triage category on to four separate shelves and then placed in line by strict temporal order. Doctors took the first chart on the triage 3 shelf and continued doing so until the shelf was empty. They then moved to the triage 4 shelf." See P.2, Col.1, Para.3 Comment: Sequence generation was non‐random; patients were seen in temporal order, and allocation to provider was not necessarily consecutive, depending on the length of previous consultations. Although a research nurse was employed to ensure adherence to the temporal order, this open allocation method could be problematic if the triage information recorded on chart influences physician's choice to accept or reject a patient (by waiting for the other physician to take the top chart). For example, GPs investigated fewer semi‐urgent (triage 3) and more delay‐acceptable (triage 4) patients than EPs. See P.3, Table 1:
|
| Allocation concealment (selection bias) | Unclear risk | Quote: "General practitioners...were dressed similarly to the usual staff and patients were unaware that they were being seen by a general practitioner" See P.2, Col.1, Para.2‐3 Comment: Patient allocation occurred as individuals entered the study (by attending the ED) and was carried out by a study researcher and enforced by the triage nursing team. It is unclear whether the same person conducted both steps of the randomisation process. Physicians were not blinded to the triage category of the patients being seen, however patients were probably unaware of the type of physician treating them. It is unclear how physician allocation to primary care sessions was performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The hospital's computer could not locate 83 (2%) of the 4684 patients enrolled in the study. Thirty three had been seen by the general practitioners and fifty by the usual accident and emergency staff." See P.4, Col.2, Para.4 Comment: There were similar numbers of missing records across the 2 groups, and a relatively small portion of data was missing, hence probably low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the text were reported in the results. |
| Other bias | Unclear risk | Quote: Each GP "worked two four hour sessions a week, managing non‐emergency patients". See P.2, Col.1, Para.2. General practitioners and EPs did not work equal numbers of hours in the ED; this imbalance between providers in experience and numbers of patients seen could bias the results. |
| Baseline outcome measures similar | Unclear risk | No baseline measure of outcome reported. |
| Baseline (provider) characteristics similar | High risk | The median age and time since registration were not equal between GPs and EPs. The median age of the 5 GPs employed during the project was 32 years, compared with 26 years for EPs. Similarly, the median time since full registration was 7 years for GPs and 6 months for EPs. See P.3, Col.2, Para.3 This difference in experience between the groups could bias the study outcomes. |
| Baseline (patient) characteristics similar | High risk | Quote: "There were significant differences (in presenting complaints)....between (triage 3) patients seen by the general practitioners and those seen by the usual accident and emergency staff". See P.4, Table 3. "There were no differences between triage 4 patients seen by general practitioners and those seen by the usual accident and emergency staff". See P.3, Col.2, Para.5 Comment: High risk of bias because patient diagnoses in control and intervention groups were not equal. |
| Knowledge of allocated intervention adequate (Process variables) | Low risk | Unclear if outcomes were assessed blindly, but process variables (laboratory and X‐ray investigations, prescriptions, referrals, admissions) were objective. (Referrals were "when a second doctor was formally requested to review a patient and did so". P.2, Col.2, Para.2) |
| Knowledge of allocated interventions adequate (Patient satisfaction, health status) | Unclear risk | Quote: "Patient satisfaction was assessed immediately by a blinded interviewer using the consultation satisfaction questionnaire." See P.2, Col.2, Para.4 "Health status was determined after one month by means of a simple questionnaire completed by telephone or letter". Patient satisfaction was assessed blindly. Unclear if health status was assessed blindly. See P.2, Col.2, Para.4 Comment: Self reported data, and unclear if questionnaires were validated or if health status was assessed blindly. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "General practitioners...had access to the same facilities as the usual medical staff. They were dressed similarly to the usual staff and patients were unaware that they were being seen by a general practitioner". Comment: Patients were unaware of which type of physician they were seeing. It is unclear whether medical practitioners were aware of the study objectives. Knowledge of study objectives may have affected performance (e.g. consciously choosing to order fewer investigations or make more referrals to the community rather than to a second doctor). |
| Blinding of outcome assessment (detection bias) (Process variables) | Unclear risk | It is unclear if outcomes were assessed blindly, but most process measures were objective items such as the number of investigations ordered, prescriptions given, and admissions made. Referrals were only counted in the study if "a second doctor was formally requested to review a patient and did so" (See P.2, Col.2, Para.1). Hence any referrals to community or non‐physician healthcare providers (e.g. community nurses, social workers, mental health professionals) were excluded, and detection bias could have been introduced if physicians were aware of the study definition or outcome; we therefore judged the risk of bias as unclear. |
| Blinding of outcome assessment (detection bias) (Patient satisfaction, health status). | Unclear risk | Quotes: "Patient satisfaction was assessed immediately by a blinded interviewer using the consultation satisfaction questionnaire." See P.2, Col.2, Para.4 "Health status was determined after one month by means of a simple questionnaire completed by telephone or letter". See P.2, Col.2, Para.4 Comment: Satisfaction assessment was blinded, but it is unclear if health status assessments were blinded. |
| Adequately protected against contamination | Unclear risk | Unclear. General practitioners and EPs worked simultaneously in primary care sessions, and overlap and contamination between groups was possible. See P.2, Col.2, Para.2, 4‐6 |
A&E: accident & emergency department; ECG: electrocardiogram; ED: emergency department; EPs: emergency physicians; GPs: general practitioners; NPs: nurse practitioners
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boeke 2010 | Uncontrolled before‐after study |
| Bosmans 2012 | Uncontrolled before‐after study |
| Byrne 2000 | No effectiveness data; satisfaction is the only outcome |
| Colliers 2017 | Ineligible intervention: GPs were located in out‐of‐hospital co‐operatives rather than ED |
| Combs 2006 | Ineligible intervention: establishment of a fast‐track unit staffed by emergency staff |
| Jennings 2008 | Ineligible study design |
| Jimenez 2005 | Non‐randomised study comparing period with GP to period without GP (no pre‐intervention data) |
| Martin 2005 | Uncontrolled before‐after study |
| McClellan 2012 | Nurse practitioners had additional training for specific minor illnesses. |
| Mortimer 2011 | Ineligible professional group (pharmacists) |
| NCT02417181 | Compares physician assistants and GPs |
| Noble 2014 | Ineligible intervention |
| O'Keeffe 2014 | Ineligible professional group (emergency care practitioner) |
| Rhee 1995 | No effectiveness data; satisfaction is the only outcome |
| Sakr 1999 | Ineligible intervention: nurses who already worked in ED, not PC |
| Schulz 2016 | Ineligible study design |
| Steiner 2009 | Ineligible intervention: addition of a "broad‐scope" NP to the ED team, but no comparison with care provided by a PC professional |
| Tsai 2012 | Uncontrolled before‐after study |
| Van Der Biezen 2016 | Compares NPs to GPs, no EPs |
| van der Linden 2010 | Compares ENPs and EPs, no PC professionals |
ED: emergency department; ENP: emergency nurse practitioner; EP: emergency physician; GP: general practitioner; NP: nurse practitioner; PC: primary care
Differences between protocol and review
We edited the order and description of the objectives to reflect the original outcomes defined in the protocol (Abi‐Aad 2000). We included non‐randomised trials after discussion amongst the current author team. We added a 'Summary of findings' table and updated the Methods section to comply with current Methodological Expectations of Cochrane Intervention Reviews (MECIR) standards. Gerrard Abi‐Aad, Lucy Johnson, Nick Mays, and Emilie Roberts left the review author team, and Daniela C Gonçalves‐Bradley, Jaspreet K Khangura, Gerd Flodgren, Rafael Perera, Brian H Rowe, and Sasha Shepperd joined the review author team.
Contributions of authors
DGB and JKK screened references, extracted data, rated the certainty of the evidence and wrote the review. GF, RP, BHR, and SS provided feedback and contributed to the completion of the review.
Sources of support
Internal sources
-
Tier I Canada Research Chair in Evidence‐based Emergency Medicine through the Canadian Institutes of Health Research (CIHR) and the Government of Canada (Ottawa, ON), Canada.
Support provided to BHR to work on this review
External sources
National Institute of Health Research, UK.
Declarations of interest
DGB: none known JKK: none known GF: none known RP: none known BHR: none known SS: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Dale 1995 {published and unpublished data}
- Dale J, Green J, Reid F, Glucksman E, Higgs R. Primary care in the accident and emergency department: II. Comparison of general practitioners and hospital doctors. BMJ 1995;311:427‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J, Lang H, Roberts JA, Green J, Glucksman E. Cost effectiveness of treating primary care patients in accident and emergency: a comparison between general practitioners, senior house officers, and registrars. BMJ 1996;312:1340‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale JR. Primary Care in Accident and Emergency Departments: The Cost Effectiveness and Applicability of a New Model of Care (PhD thesis). London: London School of Hygiene & Tropical Medicine, 1997. [Google Scholar]
Gibney 1999 {published data only (unpublished sought but not used)}
- Gibney D, Murphy AW, Barton D, Byrne C, Smith M, Bury G, et al. Randomised controlled trial of general practitioner versus usual medical care in a suburban accident and emergency department using an informal triage system. British Journal of General Practice 1999;49:43‐4. [PMC free article] [PubMed] [Google Scholar]
Jennings 2015 {published data only}
- Jennings N, Gardner G, O'Reilly G. A protocol for a pragmatic randomized controlled trial evaluating outcomes of emergency nurse practitioner service. Journal of Advanced Nursing 2014;70(9):2140‐8. [DOI: 10.1111/jan.12386] [DOI] [PubMed] [Google Scholar]
- Jennings N, Gardner G, O'Reilly G, Mitra B. Emergency NP model of care in an Australian Emergency Department. Journal for Nurse Practitioners 2015;11(8):774‐81. [Google Scholar]
- Jennings N, Gardner G, O'Reilly G, Mitra B. Evaluating emergency nurse practitioner service effectiveness on achieving timely analgesia: a pragmatic randomized controlled trial. Academic Emergency Medicine 2015;22(6):678‐84. [DOI: 10.1111/acem.12687] [DOI] [PubMed] [Google Scholar]
Murphy 1996 {published data only (unpublished sought but not used)}
- Murphy AW, Bury G, Plunkett PK, Gibney D, Smith M, Mullan E, et al. Randomised controlled trial of general practitioner versus usual medical care in urban accident and emergency department: process, outcome and comparative cost. BMJ 1996;312:1135‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AW, Plunkett PK, Bury G, Leonard C, Walsh J, Lynam F, et al. Effect of patients seeing a general practitioner in accident and emergency on their subsequent re‐attendance: cohort study. BMJ 2000;320:903‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Boeke 2010 {published data only}
- Boeke AJP, Randwijck‐Jacobze ME, Lange‐Klerk EMS, Grol SM, Kramer MHH, Horst HE. Effectiveness of GPs in accident and emergency departments. British Journal of General Practice 2010;60(579):e378‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosmans 2012 {published data only}
- Bosmans JE, Boeke AJ, Randwijck‐Jacobze ME, Grol SM, Kramer MH, Horst HE, et al. Addition of a general practitioner to the accident and emergency department: a cost‐effective innovation in emergency care. Emergency Medical Journal 2012;3:192‐6. [DOI] [PubMed] [Google Scholar]
Byrne 2000 {published data only}
- Byrne G, Richardson M, Brunsdon J, Patel A. Patient satisfaction with emergency nurse practitioners in A&E. Journal of Clinical Nursing 2000;9(1):83‐93. [DOI] [PubMed] [Google Scholar]
Colliers 2017 {published data only}
- Colliers A, Remmen R, Streffer ML, Michiels B, Bartholomeeusen S, Monsieurs KG, et al. Implementation of a general practitioner cooperative adjacent to the emergency department of a hospital increases the caseload for the GPC but not for the emergency department. Acta Clinica Belgica 2017;72(1):49‐54. [DOI: 10.1080/17843286.2016.1245936] [DOI] [PubMed] [Google Scholar]
Combs 2006 {published data only}
- Combs S, Chapman R, Bushby A. Fast track: one hospital's journey. Accident and Emergency Nursing 2006;14(4):197‐203. [DOI] [PubMed] [Google Scholar]
Jennings 2008 {published data only}
- Jennings N, O'Reilly G, Lee G, Cameron P, Free B, Bailey M. Evaluating outcomes of the emergency nurse practitioner role in a major urban emergency department, Melbourne, Australia. Journal of Clinical Nursing 2008;17(8):1044‐50. [DOI] [PubMed] [Google Scholar]
Jimenez 2005 {published data only}
- Jimenez S, Red G, Miro O, Bragulat E, Coll‐Vinent B, Senar E, et al. Effect of the incorporation of a general practitioner on emergency department effectiveness. Medicina Clinica 2005;125(4):132‐7. [DOI] [PubMed] [Google Scholar]
Martin 2005 {published data only}
- Martin R, Considine J. Knowledge and attitudes of ED staff before and after implementation of the emergency nurse practitioner role. Australasian Emergency Nursing Journal 2005;8(3):73‐8. [Google Scholar]
McClellan 2012 {published data only}
- McClellan CM, Cramp F, Powell J, Benger JR. A randomised trial comparing the clinical effectiveness of different emergency department healthcare professionals in soft tissue injury management. BMJ Open 2012;2(6):e001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mortimer 2011 {published data only}
- Mortimer C, Emmerton L, Lum E. The impact of an aged care pharmacist in a department of emergency medicine. Journal of Evaluation in Clinical Practice 2011;17(3):478‐85. [DOI] [PubMed] [Google Scholar]
NCT02417181 {published data only}
- NCT02417181. The (cost‐)effectiveness of physician assistants working at the primary out of hours emergency service. clinicaltrials.gov/ct2/show/NCT02417181 (first received 15 April 2015).
Noble 2014 {published data only}
- Noble AJ, McCrone P, Seed PT, Goldstein LH, Ridsdale L. Clinical‐ and cost‐effectiveness of a nurse led self‐management intervention to reduce emergency visits by people with epilepsy. PLoS ONE 2014;9(6):e90789. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Keeffe 2014 {published data only}
- O'Keeffe C, Mason S, Knowles E. Patient experiences of an extended role in healthcare: comparing emergency care practitioners (ECPs) with usual providers in different emergency and urgent care settings. Emergency Medicine Journal 2014;31(8):673‐4. [DOI: 10.1136/emermed-2013-202415] [DOI] [PubMed] [Google Scholar]
Rhee 1995 {published data only}
- Rhee KJ, Dermyer AL. Patient satisfaction with a nurse practitioner in a university emergency service. Annals of Emergency Medicine 1995;26(2):130‐2. [DOI] [PubMed] [Google Scholar]
Sakr 1999 {published data only}
- Sakr M, Angus J, Perrin J, Nixon C, Nicholl J, Wardrope J. Care of minor injuries by emergency nurse practitioners or junior doctors: a randomised controlled trial. Lancet 1999;354(9187):1321‐6. [DOI] [PubMed] [Google Scholar]
Schulz 2016 {published data only}
- Schulz P, Prescott J, Shifman J, Fiore J, Holland A, Harding P. Comparing patient outcomes for care delivered by advanced musculoskeletal physiotherapists with other health professionals in the emergency department: a pilot study. Australasian Emergency Nursing Journal 2016;19(4):198‐202. [DOI] [PubMed] [Google Scholar]
Steiner 2009 {published data only}
- Steiner IP, Nichols DN, Blitz S, Tapper L, Stagg AP, Sharma L, et al. Impact of a nurse practitioner on patient care in a Canadian emergency department. CJEM 2009;11(3):207‐14. [DOI] [PubMed] [Google Scholar]
Tsai 2012 {published data only}
- Tsai VW, Sharieff GQ, Kanegaye JT, Carlson LA, Harley J. Rapid medical assessment: Improving pediatric emergency department time to provider, length of stay, and left without being seen rates. Pediatric Emergency Care 2012;28(4):345‐6. [DOI: 10.1097/PEC.0b013e31824d9d27] [DOI] [PubMed] [Google Scholar]
Van Der Biezen 2016 {published data only}
- Biezen M, Adang E, Burqt R, Wensing M, Laurant M. The impact of substituting general practitioners with nurse practitioners on resource use, production and health‐care costs during out‐of‐hours: a quasi‐experimental study. BMC Family Practice 2016;17(1):132. [DOI: 10.1186/s12875-016-0528-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Linden 2010 {published data only}
- Linden C, Reijnen R, Vos R. Diagnostic accuracy of emergency nurse practitioners versus physicians related to minor illnesses and injuries. Journal of Emergency Nursing 2010;36(4):311‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Asplin 2003
- Asplin BR, Magid DJ, Rhodes KV, Solberg LI, Lurie N, Camargo CA Jr. A conceptual model of emergency department crowding. Annals of Emergency Medicine 2003;42(2):173‐80. [DOI] [PubMed] [Google Scholar]
Bezzina 2005
- Bezzina AJ, Smith PB, Cromwell D, Eagar K. Primary care patients in the emergency department: who are they? A review of the definition of the 'primary care patient' in the emergency department. Emergency Medicine Australasia 2005;17:472‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burns 2017
- Burns TR. Contributing factors of frequent use of the emergency department: a synthesis. International Emergency Nursing 2017;35:51‐5. [DOI] [PubMed] [Google Scholar]
Carret 2009
- Carret ML, Fassa AC, Domingues MR. Inappropriate use of emergency services: a systematic review of prevalence and associated factors. Cadernos de Saúde Pública 2009;25(1):7‐28. [DOI] [PubMed] [Google Scholar]
Carson 2010
- Carson D, Clay H, Stern R. Primary care and emergency departments. Primary Care Foundation, primarycarefoundation.co.uk/images/PrimaryCareFoundation/Downloading_Reports/Reports_and_Articles/Primary_Care_and_Emergency_Departments/Primary_Care_and_Emergency_Departments_RELEASE.pdf (accessed 05 February 2018).
Chew‐Graham 2004
- ChewGraham C, Rogers A, May C, Sheaf R, Ball E. A new role for the general practitioner? Reframing ‘inappropriate attenders’ to inappropriate services. Primary Health Care Research & Development 2004;5(1):60‐7. [Google Scholar]
College of Emergency Medicine 2014
- College of Emergency Medicine. Acute and emergency care: Prescribing the remedy. rcpch.ac.uk/sites/default/files/page/D___websites__Medicine__collemergencymed2014__Upload__documentz__CEM7884‐Acute%20and%20emergency%20care%20‐%20prescribing%20the%20remedy.pdf (accessed 3 December 2017).
Cooke 2004
- Cooke M, Fisher J, Dale J, Mcleod E, Szczepura A, Walley P, et al. Reducing attendances and waits in emergency departments: A systematic review of present innovations. Warwick (UK): Report to the National Co‐ordinating Centre for NHS Service Delivery and OrganisationR & D (NCCSDO); 2004 January.
Covidence 2018
- Covidence systematic review software. Veritas Health Innovation, Melbourne, Australia. Available at covidence.org (accessed 05 February 2018).
Dale 1997
- Dale J. Primary Care in Accident and Emergency Departments: the cost effectiveness and applicability of a new model of care. London, UK: University of London, 1997. [Google Scholar]
Derlet 2000
- Derlet RW, Richards JR. Crowding in the nation’s emergency departments: complex causes and disturbing effects. Annals of Emergency Medicine 2000;35:63‐7. [DOI] [PubMed] [Google Scholar]
Dong 2007
- Dong SL, Bullard MJ, Meurer DP, Blitz S, Akhmetshin E, Ohinmaa A, et al. Predictive validity of a computerized emergency triage tool. Academic Emergency Medicine 2007;14(1):16‐21. [DOI] [PubMed] [Google Scholar]
EPOC 2017a
- Cochrane Effective Practice, Organisation of Care (EPOC). What study designs should be included in an EPOC review? EPOC Resources for review authors, 2017. epoc.cochrane.org/resources /epoc‐resources‐review‐authors (accessed 03 November 2017).
EPOC 2017b
- Cochrane Effective Practice, Organisation of Care (EPOC). Screening, data extraction and management. EPOC resources for review authors, 2017. epoc.cochrane.org/resources/epoc‐resources‐review‐authors (accessed 03 November 2017).
EPOC 2017c
- Cochrane Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors, 2017. epoc.cochrane.org/resources/epoc‐resources‐review‐authors (accessed 03 November 2017).
EPOC 2017d
- Cochrane Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a 'Summary of findings' table using GRADE. EPOC resources for review authors, 2017. epoc.cochrane.org/resources/epoc‐resources‐review‐authors (accessed 03 November 2017).
EPOC 2017e
- Cochrane Effective Practice, Organisation of Care (EPOC). Reporting the effects of an intervention in EPOC reviews. EPOC resources for review authors, 2017. epoc.cochrane.org/resources/epoc‐resources‐review‐authors (accessed 03 November 2017).
GRADEpro GDT [Computer program]
- GRADE Working Group. GRADEpro GDT. Hamilton (ON): McMaster University, 2017.
Guyatt 2008
- Guyatt G, Oxman A, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello Y, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Health Act 2004
- Irish Statute Book. Health Act 2004. www.irishstatutebook.ie/eli/2004/act/42/enacted/en/index.html (accessed prior to 22 January 2018).
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holroyd 2007
- Holroyd BR, Bullard MJ, Latoszek K, Gordon D, Allen S, Tam S, et al. Impact of a triage liaison physician on Emergency Department overcrowding and throughput: a randomised controlled trial. Academic Emergency Medicine 2007;14:702‐8. [DOI] [PubMed] [Google Scholar]
Ieraci 2000
- Ieraci S, Cunningham P, Talbot‐Stern J, Walker S. Emergency medicine and "acute" general practice: comparing apples with oranges. Australian Health Review 2000;23(2):1252‐61. [PUBMED: 11010567] [DOI] [PubMed] [Google Scholar]
Jepson 2001
- Jepson GMH. How do primary health care systems compare across Western Europe?. Pharmaceutical Journal 2001;267:269‐73. [Google Scholar]
Lees 1976
- Lees RE, Steele R, Spasoff RA. Primary care for nontraumatic illness at the emergency department and the family physician's office. Canadian Medical Association Journal 1976;114:333‐7. [PMC free article] [PubMed] [Google Scholar]
Liggins 1993
- Liggins K. Inappropriate attendance at accident and emergency departments: a literature review. Journal of Advanced Nursing 1993;18(7):1141‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lowy 1994
- Lowy A, Kohler B, Nicholl J. Attendance at accident and emergency departments: unnecessary or inappropriate. Journal of Public Health Medicine 1994;16(2):134‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murphy 1998
- Murphy AW. 'Inappropriate' attenders at accident and emergency departments I: Definition, incidence and reasons for attendance. Journal of Family Practice 1998;15(1):23‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ospina 2006
- Ospina MB, Kelly K, Klassen TP, Rowe BH. Publication bias of randomized controlled trials in emergency medicine. Academic Emergency Medicine 2006;13(1):102‐8. [DOI] [PubMed] [Google Scholar]
Parboosingh 1987
- Parboosingh EJ, Larsen DE. Factors influencing frequency and appropriateness of utilization of the emergency room by the elderly. Medical Care 1987;25(12):1137‐47. [DOI] [PubMed] [Google Scholar]
Ramlakhan 2016
- Ramlakhan S, Mason S, O’Keeffe C, Ramtahal A, Ablard S. Primary care services located with EDs: a review of effectiveness. Emergency Medicine Journal 2016;33:495‐503. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rieffe 1999
- Rieffe C, Oosterveld P, Wijkel D, Wiefferink C. Reasons why patients bypass their GP to visit a hospital emergency department. Accident and Emergency Medicine 1999;7:217‐25. [DOI] [PubMed] [Google Scholar]
Roberts 1998
- Roberts E, Mays N. Can primary care and community‐based models of emergency care substitute for the hospital accident and emergency (A & E) department?. Health Policy 1998;44(3):191‐214. [DOI] [PubMed] [Google Scholar]
Rowe 2011
- Rowe BH, Guo X, Villa‐Roel C, Bullard MJ, Ospina M, Vandermeer B, et al. The role of triage nurse ordering in emergency department overcrowding: a systematic review of the literature. Academic Emergency Medicine 2011;18(12):1349‐57. [DOI: 10.1111/j.1553-2712.2011.01081.x] [DOI] [PubMed] [Google Scholar]
Siddiqui 2002
- Siddiqui S, Ogbeide DO. Utilization of emergency services in a community hospital. Saudi Medical Journal 2002;23(1):69‐72. [PUBMED: 11938367] [PubMed] [Google Scholar]
Starfield 1994
- Starfield B. Is primary care essential?. Lancet 1994;344:1129‐33. [DOI] [PubMed] [Google Scholar]
Starfield 2001
- Starfield B. New paradigms for quality in primary care. British Journal of General Practice 2001;51(465):303‐9. [PMC free article] [PubMed] [Google Scholar]
Thompson 2013
- Thompson MIW, Lasserson D, McCann L, Thompson M, Heneghan C. Suitability of emergency department attenders to be assessed in primary care: survey of general practitioner agreement in a random sample of triage records analysed in a service evaluation project. BMJ Open 2013;3:e003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2015
- Turner J, Coster J, Chambers D, Cantrell A, Phung V‐H, Knowles E, et al. What evidence is there on the effectiveness of different models of delivering urgent care? A rapid review. Health Services and Delivery Research 2015;3(43):hsdr03430. [PubMed] [Google Scholar]
Walsh 1995
- Walsh M. The health belief model and the use of accident and emergency services by the general public. Journal of Advanced Learning 1995;22(4):694‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Winters 2009
- Winters L. Reducing Emergency Admissions to Hospital ‐ Redesign of services. LIverpool (UK): Liverpool Public Health Observatory; 2009 August. Observatory Report Series no 82.
References to other published versions of this review
Abi‐Aad 2000
- Abi‐Aad G, Johnson L, Mays N, Roberts E. Primary and community health care professionals in hospital emergency departments: effects on process and outcome of care and resources. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002097] [DOI] [Google Scholar]
Khangura 2012
- Khangura JK, Flodgren G, Perera R, Rowe BH, Shepperd S. Primary care professionals providing non‐urgent care in hospital emergency departments. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD002097.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


