Abstract
Background
'Standard Precautions' refers to a system of actions, such as using personal protective equipment or adhering to safe handling of needles, that healthcare workers take to reduce the spread of germs in healthcare settings such as hospitals and nursing homes.
Objectives
To assess the effectiveness of interventions that target healthcare workers to improve adherence to Standard Precautions in patient care.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, LILACS, two other databases, and two trials registers. We applied no language restrictions. The date of the most recent search was 14 February 2017.
Selection criteria
We included randomised trials of individuals, cluster‐randomised trials, non‐randomised trials, controlled before‐after studies, and interrupted time‐series studies that evaluated any intervention to improve adherence to Standard Precautions by any healthcare worker with responsibility for patient care in any hospital, long‐term care or community setting, or artificial setting, such as a classroom or a learning laboratory.
Data collection and analysis
Two review authors independently screened search results, extracted data from eligible trials, and assessed risk of bias for each included study, using standard methodological procedures expected by Cochrane. Because of substantial heterogeneity among interventions and outcome measures, meta‐analysis was not warranted. We used the GRADE approach to assess certainty of evidence and have presented results narratively in 'Summary of findings' tables.
Main results
We included eight studies with a total of 673 participants; three studies were conducted in Asia, two in Europe, two in North America, and one in Australia. Five studies were randomised trials, two were cluster‐randomised trials, and one was a non‐randomised trial. Three studies compared different educational approaches versus no education, one study compared education with visualisation of respiratory particle dispersion versus education alone, two studies compared education with additional infection control support versus no intervention, one study compared peer evaluation versus no intervention, and one study evaluated use of a checklist and coloured cues. We considered all studies to be at high risk of bias with different risks. All eight studies used different measures to assess healthcare workers' adherence to Standard Precautions. Three studies also assessed healthcare workers' knowledge, and one measured rates of colonisation with methicillin‐resistant Staphylococcus aureus (MRSA) among residents and staff of long‐term care facilities. Because of heterogeneity in interventions and outcome measures, we did not conduct a meta‐analysis.
Education may slightly improve both healthcare workers' adherence to Standard Precautions (three studies; four centres) and their level of knowledge (two studies; three centres; low certainty of evidence for both outcomes).
Education with visualisation of respiratory particle dispersion probably improves healthcare workers' use of facial protection but probably leads to little or no difference in knowledge (one study; 20 nurses; moderate certainty of evidence for both outcomes).
Education with additional infection control support may slightly improve healthcare workers' adherence to Standard Precautions (two studies; 44 long‐term care facilities; low certainty of evidence) but probably leads to little or no difference in rates of health care‐associated colonisation with MRSA (one study; 32 long‐term care facilities; moderate certainty of evidence).
Peer evaluation probably improves healthcare workers' adherence to Standard Precautions (one study; one hospital; moderate certainty of evidence).
Checklists and coloured cues probably improve healthcare workers' adherence to Standard Precautions (one study; one hospital; moderate certainty of evidence).
Authors' conclusions
Considerable variation in interventions and in outcome measures used, along with high risk of bias and variability in the certainty of evidence, makes it difficult to draw conclusions about effectiveness of the interventions. This review underlines the need to conduct more robust studies evaluating similar types of interventions and using similar outcome measures.
Plain language summary
Improving healthcare workers' use of Standard Precautions to decrease infection in healthcare settings
What is the aim of this review?
To find out what strategies can be used to improve how well healthcare workers follow a system of actions known as 'Standard Precautions' to decrease infection in healthcare settings.
Key messages
Review authors identified a variety of strategies, most of which involved education of healthcare workers alone or with an additional strategy. It is unclear which strategy or combination of strategies is most effective for improving healthcare workers' adherence to Standard Precautions or their knowledge of Standard Precautions, or for reducing colonisation (potential infection) rates, as we found little evidence; this fact, along with the inconsistency of results, reduced our confidence or certainty about the evidence found.
What was studied in the review?
It is estimated that over four million patients in Europe and 1.7 million in the USA develop an infection each year, and that prevalence is higher in developing countries. Infection is associated with increased length of hospital stay, excess mortality, and billions of dollars in associated hospital costs. Adhering to Standard Precautions, such as using personal protective equipment or following practices for safe handling of needles, can reduce the spread of germs in healthcare settings. The aim of this review was to find out which methods are effective in improving healthcare workers' adherence to Standard Precautions.
What are the main results of the review?
Review authors found eight relevant studies with a total of 673 participants. Three studies were reported from Asia, two from Europe, two from North America, and one from Australia. Intevention strategies consisted of education for healthcare workers, given alone or with other types of education, such as showing how respiratory droplets are spread, or with additional infection control supports. Other intervention strategies were peer evaluation and use of a checklist and coloured cues. All studies used different measures to assess how well healthcare workers followed or adhered to Standard Precautions. Two studies also assessed whether there was any improvement in healthcare workers' knowledge (of Standard Precautions), and one measured rates of colonisation of MRSA (carriage of MRSA with increased potential for infection) among residents and staff of long‐term care facilities
Education showing spread of respiratory droplets, peer evaluation, and use of checklists and coloured cues probably improve adherence to Standard Precautions, and education alone and education with additional infection control support may slightly improve adherence to Standard Precautions.
Education alone may slightly improve knowledge, and education showing spread of respiratory droplets probably leads to little or no difference in knowledge. Education with additional infection control support probably leads to little or no difference in rates of MRSA colonisation.
How up to date is this review?
Review authors searched for studies that had been published up to 14 February 2017.
Summary of findings
Background
Description of the condition
For centuries, healthcare providers, organisations, and governments have been concerned about infection in both community and healthcare settings, but in the past few decades, focus on prevention and control of health care‐associated infections (HAIs) has increased. Global estimates of the prevalence of HAIs are not available, but it has been estimated that over four million patients in Europe and 1.7 million in the USA develop an infection each year, with higher prevalence in developing countries (Allegranzi 2011; WHO 2011). HAIs are associated with increased length of hospital stay, excess mortality, billions of dollars in associated hospital costs, and psychosocial and economic impact on the people involved, as well as on their families and communities (Andersson 2010; WHO 2011).
HAIs can occur when susceptible patients are exposed to infectious micro‐organisms during their stay in a healthcare setting. Patients in hospitals and in long‐term care facilities are frequently more susceptible to infections than those in the community because of their illness, use of immunosuppressive therapy, exposure to invasive procedures, or contact with others who have infections. Infectious agents are most frequently spread by direct contact with contaminated hands, or by indirect contact via contaminated objects, such as patient care equipment, healthcare workers' uniforms, and environmental surfaces (Public Health Agency of Canada 2012; Siegel 2007).
In 1996, the Centers for Disease Control and Prevention in the USA introduced guidelines, called 'Standard Precautions', which summarise strategies to be used to prevent transmission of micro‐organisms in healthcare settings (Siegel 2007). Standard Precautions replaced previously used guidelines such as 'Universal Precautions' (introduced in 1985) and 'Body Substance Isolation' (introduced in 1987). These previously used guidelines had varied in terms of strategies used and conditions to which they applied, whereas Standard Precautions guidelines recommend strategies to be used for all patients at all times in all settings. Standard Precautions guidelines are based on the assumption that all patients carry transmissible micro‐organisms, although patients may be asymptomatic (Siegel 2007).
Standard Precautions include the following strategies (Public Health Agency of Canada 2012; Siegel 2007).
Appropriate hand hygiene (handwashing with soap and water or use of an alcohol‐based hand rub) and appropriate use of gloves to disrupt the spread of micro‐organisms from one patient to another by healthcare workers' contaminated hands.
Use of gowns to disrupt transmission of micro‐organisms carried on healthcare workers' uniforms.
Appropriate cleaning and disinfection of patient care equipment and environment surfaces to reduce transmission by the indirect contact route.
Use of appropriate facial protection, such as masks and goggles or an N95 respirator, to reduce exposure of healthcare workers to infectious agents spread by the droplet or airborne route, respectively.
Management of used needles and other sharp objects to prevent exposure from percutaneous injury.
Management of clinical waste and used linen to reduce environmental contamination.
Cough etiquette to reduce droplet transmission and contamination of the environment
All of these strategies protect patients in the setting and healthcare workers, or both, from exposure to infectious agents.
Standard Precautions guidelines are designed to reduce the potential for transfer of micro‐organisms from one person to another, whether or not a patient is symptomatic. Specific transmission‐based precautions are to be taken when patients are known or suspected to have an infection. Three categories of transmission‐based precautions have been identified: airborne, contact, and droplet. These involve addition of strategies to those of Standard Precautions that are based on the route of transmission of the known or presumed causative micro‐organism (Siegel 2007), and they are used in conjunction with Standard Precautions. Many infections can be managed with Standard Precautions alone and do not require additional precautions (Public Health Agency of Canada 2012).
Standard Precautions have been adopted worldwide (Adebayo 2015), with periodic updates provided since they were first released. In Canada, a similar system, called 'Routine Practices and Additional Precautions', has been in place since 1999 (Public Health Agency of Canada 2012). Although multiple guidelines have been published for control of specific micro‐organisms, such as Clostridium difficile or norovirus, these guidelines have built on, rather than replaced, Standard Precautions.
In spite of widespread adoption of Standard Precautions by organisations, gaps in their implementation by healthcare workers have been noted (Gammon 2008; Powers 2016), and percutaneous injuries from needlesticks and sharps continue to occur (Kevitt 2015). Barriers reported by healthcare workers include inadequate infrastructure such as lack of handwashing facilities; lack of information about transmission; insufficient personal protective equipment (PPE) risk behaviours of workers; and inadequate working conditions (Oliveira 2010; Porto 2016). Therefore, interventions have been devised to promote implementation of Standard Precautions as the basis for infection prevention and control.
Description of the intervention
The Cochrane Effective Practice and Organisation of Care (EPOC) taxonomy consists of four categories by which health system interventions can be classified: delivery arrangements, financial arrangements, governance arrangements, and implementation strategies (EPOC 2015a). Although financial incentive is one type of intervention, delivery arrangements and implementation strategies are most relevant to promoting adherence to Standard Precautions. Interventions related to delivery of care can include providing access to infection prevention and control expertise, or providing and placing materials required to implement Standard Precautions. Implementation strategies can be directed to healthcare organisations, such as strategies to change organisational culture, or they can be directed to healthcare workers. Examples of the latter are audit and feedback, use of reminders and checklists, and education. Educational approaches, such as campaigns, instruction and training, and use of pamphlets or posters, may be targeted to individuals or directed to groups (Huang 2002; Mukti 2000; Wright 1997).
How the intervention might work
Improving access to infection prevention and control expertise can facilitate decision‐making by individual healthcare workers in terms of problem‐solving, and ensuring availability of PPE or adequate housekeeping staff may reduce barriers that prevent optimal adherence to Standard Precautions. Audit and feedback might increase awareness of specific individual behaviours and their consequences and might provide motivation for change, such as inducing shame if individuals do not adhere to guidelines, or pride if adherence is appropriate. Reminders and checklists can prompt healthcare workers to perform required actions at the appropriate time. Educational interventions can increase healthcare workers' knowledge of strategies they should use to reduce transmission of micro‐organisms, when they should use these strategies, and how they can implement them correctly.
Although a previous systematic review examined interventions to improve hand hygiene (Gould 2017), which is one component of Standard Precautions, we have not identified a systematic review of interventions designed to improve adherence to Standard Precautions.
Why it is important to do this review
Standard Precautions form the foundation for infection prevention and control. Because patients without symptoms can carry micro‐organisms, healthcare workers need to take appropriate actions to minimise transfer of those micro‐organisms to other patients or to themselves. Considerable research has focused on interventions to promote hand hygiene (Gould 2017), but researchers have placed much less emphasis on other elements of Standard Precautions. This review should prove useful in providing evidence of the best approach to improve adherence to Standard Precautions during provision of care for healthcare workers working in healthcare settings.
Objectives
To assess the effectiveness of interventions that target healthcare workers to improve adherence to Standard Precautions in patient care.
Methods
Criteria for considering studies for this review
Types of studies
We included the following types of studies when they met explicit entry and quality criteria put forth by the Cochrane Effective Practice and Organisation of Care Group (EPOC).
Randomised trials of individuals and cluster‐randomised trials.
Non‐randomised trials (studies in which investigators use a method that is not random to allocate participants to different groups that are being compared, and follow at least two groups given different interventions).
Controlled before‐after studies (with at least two intervention sites and two control sites).
Interrupted time‐series studies (with at least three observations available before the intervention and another three available after the intervention, and with a clearly defined point in time when the intervention occurred).
See the EPOC definitions of designs (EPOC 2016).
Types of participants
Any healthcare worker including professionals (e.g. doctors, nurses, pharmacists) or other workers (e.g. radiology porter, nursing aide) with responsibility for patient care in any hospital, long‐term care or community setting, or artificial setting, such as a classroom or learning laboratory.
We placed no notable restrictions on the eligibility criteria.
Types of interventions
We considered any intervention intended to improve adherence to Standard Precautions.
Educational interventions, such as distribution of educational materials, educational meetings, or patient‐mediated interventions.
Reminders, including cues or checklists.
Audit and feedback, including peer evaluation.
Financial interventions, such as rewards or benefits or loss thereof, tied to specific actions.
Organisational interventions, such as administrative support or policies, and structural interventions such as changes to the setting/site of service delivery; changes in physical structure, facilities, and equipment; and presence and organisation of quality monitoring mechanisms.
We included studies that evaluated only one component of Standard Precautions such as use of gowns or gloves, and those that evaluated multiple components.
Older studies have examined systems of precautions that existed at the time of the study, rather than Standard Precautions. We also considered studies of interventions intended to improve adherence to universal precautions, category‐specific precautions, body substance isolation precautions, and routine practices and additional precautions. These systems are all sufficiently similar in goals and issues that it is reasonable to assume that interventions for increasing adherence to one system will be relevant for use with another system.
We excluded studies that evaluated only hand hygiene, as another systematic review has covered this topic (Gould 2017). We also excluded studies that evaluated bundles for prevention of specific infections and those that evaluated transmission‐based precautions.
We considered studies that compared interventions against each other or versus no intervention.
Types of outcome measures
We included studies if they addressed the primary outcome.
Primary outcomes
Adherence to Standard Precautions guidelines, as measured by rates of observed Standard Precautions practice (e.g. observed glove use) or a proxy indicator of adherence (e.g. increased application of policy; volume of glove use), or a combination of these. The definition of adherence could vary across studies. Investigators could assess adherence using different observational methods, or they could assess adherence at an individual or organisational level
Secondary outcomes
Health care‐associated infection or colonisation, as measured by rates
Healthcare workers' knowledge about components of Standard Precautions (e.g. about blood‐borne pathogens and components of infection control precautions), as measured by knowledge score on a questionnaire (knowledge tested could vary by study)
Attitude of healthcare workers toward infection control precautions, as measured by attitude score on a questionnaire
Self‐reported behaviours of healthcare workers related to infection control precautions, as measured by a questionnaire
We included studies if they addressed the primary outcome.
Search methods for identification of studies
We searched the Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effects (DARE) for related systematic reviews, and the following databases for primary studies, on 14 February 2017.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) in the Cochrane Library.
Health Technology Assessment Database (HTA; 2016, Issue 4) in the Cochrane Library.
National Health Service Economic Evaluation Database (NHSEED; 2015, Issue 2) in the Cochrane Library.
MEDLINE Ovid (including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations), 1946 to 14 February 2017.
Embase Ovid, 1974 to 14 February 2017.
Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO, 1981 to 14 February 2017.
Latin American and Caribbean Health Sciences database (LILACS), Virtual Health Library (VHL), 1982 to 14 February 2017.
We tested a draft search strategy for MEDLINE by screening selected citations for relevance and validated the strategy by using a selection of exemplar papers on the topic of this review. We translated the MEDLINE strategy for other databases using appropriate syntax and vocabulary for those databases. We applied no date or language limits. We have provided the full search strategies in Appendix 1.
Searching other resources
Grey literature
We conducted a grey literature search to identify studies not indexed in the databases listed above. Sources included the sites listed below.
Open Grey (http://www.opengrey.eu/).
Grey Literature Report (New York Academy of Medicine) (http://greylit.org/).
Agency for Healthcare Research and Quality (AHRQ) (www.ahrq.gov/).
National Institute for Health and Clinical Excellence (NICE) (www.nice.org.uk/).
Trial registries
We searched the following registries for ongoing and completed trials.
International Clinical Trials Registry Platform (ICTRP), World Health Organization (WHO) (http://www.who.int/ictrp/en/).
ClinicalTrials.gov, US National Institutes of Health (NIH) (http://clinicaltrials.gov/).
We also did the following.
Handsearched journals and available conference proceedings from the UK Hospital Infection Society and the Infection Prevention Society, the American Association of Professionals in Infection Control, the Canadian Community and Hospital Infection Control Association, and Infection Prevention and Control Canada.
Reviewed reference lists of all included studies, relevant systematic reviews, and primary studies.
Contacted authors of relevant studies or reviews to clarify reported published information or to seek unpublished results/data.
Contacted researchers with expertise relevant to the review topic or EPOC interventions.
Conducted cited reference searches for all studies included in citation indexes.
Data collection and analysis
We conducted the review using EPOC methods (EPOC 2013; EPOC 2015b; EPOC 2016).
Selection of studies
Four review authors (RAP, IC, PB, and DM) independently assessed the titles and abstracts of all reports. We obtained full‐text hard copies for studies that met selection criteria and for studies for which review authors had some doubt about whether they fulfilled the selection criteria. We resolved discrepancies via discussion with fourth and fifth review authors (RED and PB).
Data extraction and management
Two review authors (RAP and IC) independently extracted data from each included study. We resolved discrepancies through discussion with a third review author (RED or DM or PB). We used a standard data extraction form to extract the following information: characteristics of the study (design, methods of randomisation); participants; interventions; and outcomes (types of outcome measures, adverse events).
Assessment of risk of bias in included studies
We assessed study quality using the 'Risk of bias' approach for Cochrane reviews (EPOC 2015b; Higgins 2011).
Two review authors (IC and DM) independently assessed risk of bias for each included study using a form with standard criteria described by the EPOC Group (EPOC 2015b). We resolved discrepancies with a third review author (RED). We used the EPOC nine‐point criteria for randomised trials, non‐randomised trials, and controlled before‐after studies to determine the quality of all eligible studies. When studies provided insufficient information, we contacted study authors to request further details. We reported risk of bias for each study in the Characteristics of included studies section. We categorised studies as 'low' risk if we judged all risk of bias criteria to be adequate. We categorised studies as 'moderate' risk if we judged one or two criteria to be inadequate, and as 'high' risk if we judged more than two criteria to be inadequate. We recorded this information for each included trial in 'Risk of bias' tables in Review Manager 5 (RevMan 2014) and summarised the risk of bias for each study in a summary 'Risk of bias' figure and graph. For clarity, we separated the criterion related to blinding into two separate items to distinguish between blinding of participants and blinding of outcome assessment. None of the studies used an interrupted time series design; therefore, we did not need to use the seven‐point criteria for interrupted time series studies.
Measures of treatment effect
We described outcomes using the measures reported in studies. Investigators reported observed adherence to Standard Precautions as the proportion of participants who performed a given task (e.g. hand hygiene, use of PPE, recapping) or as a score on an observation checklist or audit tool. They reported knowledge and attitude as scores on questionnaires, and self‐reported behaviour as a score on a questionnaire or the number of needlestick injuries that had occurred. Trialists described measures of differences as differences in percentage points, in proportions, or in scores. They described rates of methicillin‐resistant Staphylococcus aureus (MRSA) colonisation as proportions of patients or residents who had MRSA colonisation, and they used risk ratio to describe differences in risk between intervention and control groups.
Unit of analysis issues
We assessed whether appropriate analysis was conducted to adjust for clustering and pair‐matching in pair‐matched cluster‐randomised trials. We planned to adjust results using standard approaches to incorporate measures of intracluster correlation coefficients but found that this was not necessary, as we did not conduct a meta‐analysis (Higgins 2011). We reported unit of analysis errors in our qualitative assessment of results.
Dealing with missing data
We were not concerned about missing data, as we did not conduct a meta‐analysis because of heterogeneity in interventions and outcome measures.
Data synthesis
Because of heterogeneity in interventions and outcome measures, a meta‐analysis was not justified. Instead, we present a qualitative assessment of results of all studies, including those with high and variable risk of bias. We have summarised pre‐intervention and post‐intervention results of individual studies in Table 6, Table 7, and Table 8. When study authors did not report differences, we calculated differences using reported data.
1. Results from studies reporting observed adherence to Standard Precautions.
| Study | Comparison | Estimate of adherence | Measure of difference |
| Intervention: education | |||
| Huang 2002 | Randomised trial Intervention: 2‐hour lecture on blood‐borne pathogens and UP, 1‐hour demonstration, and 30‐minute discussion Control: no intervention |
Outcome: observed adherence of individuals using behaviour checklist, reported as % of nurses Intervention group:
Control group:
|
Not reported by researchers Calculated differencesa in adherence in percentage points between pre and post: Intervention group:
Control group:
|
| Mukti 2000 | Non‐randomised trial Intervention: education using academic detailing, with stickers and posters Control: no intervention |
Outcome: observed adherence of individuals using behaviour checklist, reported as median scores (IQR) for: Intervention group:
Control group:
Specific results also reported for pre‐test and post‐test values of specific behaviours (see ‘Measure of difference” column) |
Not reported by researchers Calculated differencesa in total median scores in percentage points between pre and post: Intervention group: 2 points Control group: 0 point Calculated differencesa in median adherence scores in percentage points between pre and post: Intervention group:
Control group:
|
| Wright 1997 | Randomised trial Intervention: computer‐assisted instruction on UP Control: no intervention |
Outcome: observed adherence of individuals using UP Assessment Tool, reported as mean scores (SD) for: Intervention group:
Control group:
|
Difference in mean adherence score (SD) between pre and post: Intervention group: 6.67 (SD: 7.79) Control group: .96 (SD: 3.25) |
| Intervention: education with visualisation | |||
| Carrico 2007 | Randomised trial Intervention: education with visualisation of respiratory droplet dispersion Control: education alone |
Outcome: observed use of mask during clinical interaction with patient with respiratory symptoms, post intervention only, reported as proportion of 42 encounters Intervention group: 74% Control group: 53% Note: not assessed at baseline |
Not reported by researchers Calculated differencesa in use of mask in percentage points between intervention and control groups: 21 points |
| Intervention: education with infection control support | |||
| Baldwin 2010 |
Pair‐matched cluster‐randomised trial Intervention: education plus some staff trained as infection control link workers Control: no intervention |
Researchers did not do a matched analysis. Outcome: mean scores on infection control audit of institutional practices Intervention group:
Control group:
|
Not reported by researchers Calculated differencesa in mean audit score, in percentage points, between scores at baseline and at 12 months: Intervention group: 26 points Control group: 11 points |
| Rao 2009 | Pair‐matched cluster‐randomised trial Intervention: education plus additional 24‐hour telephone infection control support Control: no intervention |
Researchers did not do a matched analysis. Outcome: scores on infection control audit of institutional practices, reported as range of scores across institutions per audit by component: Hand hygiene facilities Intervention group:
Control group:
Environmental cleanliness Intervention group:
Control group:
Disposal of clinical waste Intervention group:
Control group:
|
Mean difference in changes in scores (i.e. final audit score – baseline score) with 95% CI Hand hygiene facilities
Environmental cleanliness
Disposal of clinical waste
|
| Intervention: peer evaluation | |||
| Moongtui 2000 | Randomised trial Intervention: education and utilisation of peer evaluation Control: no intervention |
Outcome: observed adherence of individuals using Modified UP Assessment Tool, reported as mean scores (SD) for: Intervention group:
Control group:
|
Not reported by researchers Calculated differencesa in mean adherence score between pre and end of intervention period, and between pre and 4 weeks post: Intervention group:
Control group:
|
| Intervention: checklist and coloured cues | |||
| Ong 2013 | Randomised trial with cross‐over Group 1: checklist Group 2: coloured cues Group 3: checklist plus coloured cues Group 4: no intervention |
Outcome: observed individual adherence, as % of porters who adhered to recommended practices Full or partial adherence
Adherence to hand hygiene
Adherence to use of gloves
Adherence to use of gown
|
Not reported by researchers Calculated differencesa in mean adherence (full or partial), in percentage points, compared with control:
Calculated differencesa in mean adherence to hand hygiene, in percentage points, compared with control:
Calculated differencesa in mean adherence to glove use, in percentage points, compared with control:
Calculated differencesa in mean adherence to gown use, in percentage points, compared with control:
|
CI: confidence interval; IQR: interquartile range; SD: standard deviation; UP: Universal Precautions.
aWhen researchers did not report differences, review authors calculated differences using data reported by researchers and summarised in the column “Estimate of adherence”.
2. Results from studies reporting knowledge, attitude and self‐reported behaviour.
| Study | Comparison | Estimate of outcome | Measure of difference |
| Intervention: education | |||
| Huang 2002 | Randomised trial Intervention: 2‐hour lecture on blood‐borne pathogens and UP, 1‐hour demonstration, and 30‐minute discussion Control: no intervention |
Outcome: knowledge reported as mean scores (SD) Intervention group:
Control group:
Outcome: self‐reported behaviour reported as mean scores (SD) Intervention group:
Control group:
Outcome: reported numbers of sharps injuries Intervention group:
Control group:
|
Not reported by researchers Calculated differencesa in knowledge scores in percentage points between pre and post: Intervention: 1.45 Control: ‐.14 Calculated differencesa in self‐reported behaviour scores in percentage points between pre and post: Intervention: 12.35 Control: 2.78 Calculated differencesa in reported numbers of sharps injuries between pre and post: Intervention: ‐61 Control: ‐41 |
| Mukti 2000 | Non‐randomised trial Intervention: education using academic detailing, with stickers and posters Control: no intervention |
Outcome: knowledge reported as median scores (IQR) for: Intervention group:
Control group:
Outcome: attitude reported as median scores (IQR) for: Intervention group:
Control group:
|
Not reported by researchers Calculated differencesa in median scores in percentage points between pre and post: Knowledge: Intervention group: 2 Control group: 0 Attitude: Intervention group: 4 Control group: 1 |
| Intervention: education with visualisation | |||
| Carrico 2007 | Randomised trial Intervention: education with visualisation of respiratory droplet dispersion Control: education alone |
Outcome: knowledge reported as mean scores (SD) for: Intervention group:
Control group:
|
Not reported by researchers Calculated differencesa in knowledge scores in percentage points between pre and post: Intervention: 10 Control: 14 |
IQR: interquartile range; SD: standard deviation; UP: Universal Precautions.
aWhen researchers did not report differences, review authors calculated differences using data reported by researchers and summarised in the column “Estimate of outcome”.
3. Results from studies reporting rates of colonisation with MRSA.
| Study | Comparison | Estimate of rates | Measure of difference |
| Baldwin 2010 |
Pair‐matched cluster‐randomised trial Intervention: education plus some staff trained as infection control link workers Control: no intervention |
Researchers did not do a matched analysis. MRSA colonisation in % of staff in intervention group: · Baseline: 1% · At 12 months: 7.3% MRSA colonisation in % of staff in control group: · Baseline: 6% · At 12 months: 4.3% MRSA colonisation in % of residents in intervention group: · Baseline: 17% · At 12 months: 19% MRSA colonisation in % of residents in control group: · Baseline: 17% · At 12 months: 19% |
At 1 year, the risk ratio for colonisation with MRSA among residents in intervention vs control groups was .81 (95% CI .51 to 1.30). Researchers did not provide the risk ratio for colonisation among staff. Calculated differences1 in MRSA colonisation among staff in percentage points between baseline and 12 months: Intervention: +6.3 points Control: ‐2.3 points |
CI: confidence interval; MRSA: methicillin‐resistant Staphylococcus aureus.
aWhen researchers did not report differences, review authors calculated differences using data reported by researchers and summarised in the column “Estimate of outcome”.
'Summary of findings'
We summarised the findings for each intervention strategy using the GRADE approach. Two review authors (DM and RED) independently assessed the certainty of evidence (high, moderate, low, and very low) using the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) for each of the following outcomes to draw conclusions about certainty of the evidence: adherence to Standard Precautions; healthcare workers' knowledge; and rates of health care‐associated colonisation with MRSA (Guyatt 2008). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of interventions and EPOC worksheets (EPOC 2013; Higgins 2011). We resolved disagreements on certainty ratings by discussion and provided justification for decisions to downgrade or upgrade ratings using table footnotes. We used plain language statements to report these findings in the review (EPOC 2013). Completed worksheets can be found in Appendix 2.
Subgroup analysis and investigation of heterogeneity
We did not conduct a meta‐analysis and therefore did not test for statistical heterogeneity nor perform a subgroup analysis.
Results
Description of studies
Details of studies can be found in the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
We identified a total of 6868 unique citations (after removing duplicates) through database searches (see Figure 1). After screening by title, then by abstract, we obtained full‐paper copies for 10 citations that were potentially eligible for inclusion in the review. We excluded two studies as ineligible for the reasons described in the Characteristics of excluded studies tables (Erickson 1996; Gould 1997). The remaining eight studies with a total of 673 participants met minimal methodological requirements (Baldwin 2010; Carrico 2007; Huang 2002; Moongtui 2000; Mukti 2000; Ong 2013; Rao 2009; Wright 1997), and we included them in this review.
1.
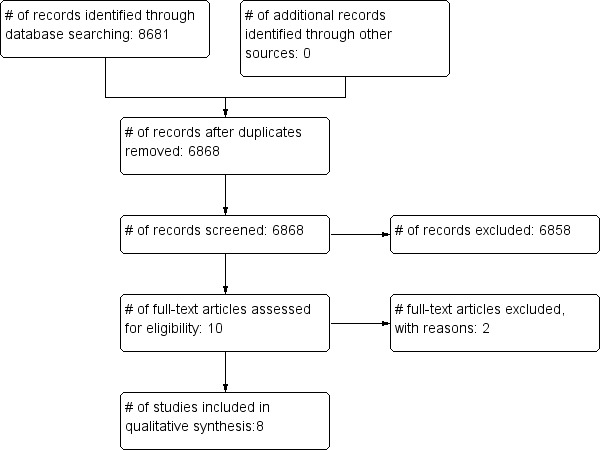
Study flow diagram.
Included studies
We included eight studies with a total of 673 participants (Baldwin 2010; Carrico 2007; Huang 2002; Moongtui 2000; Mukti 2000; Ong 2013; Rao 2009; Wright 1997).
Design of the studies
We classified four included studies as randomised trials (Carrico 2007; Huang 2002; Moongtui 2000; Wright 1997); two as cluster‐randomised trials (both used a pair‐matched design) (Baldwin 2010; Rao 2009); one as a randomised trial with a cross‐over design (Ong 2013); and the other as a non‐randomised trial (Mukti 2000).
Types of study participants
All study participants were healthcare workers, although Baldwin 2010 studied MRSA colonisation among both residents and staff of nursing homes that participated in the study.
Three studies included only registered nurses (RNs) as participants, although they worked on a variety of hospital units in two studies (Huang 2002; Wright 1997), and in the emergency department in one study (Carrico 2007). Moongtui 2000 included RNs, licensed practical nurses (LPNs), and aides from a variety of units. Mukti 2000 included both nurses and doctors from emergency departments, and Baldwin 2010 and Rao 2009 included all nursing home staff. Ong 2013 focused on radiology porters at one hospital.
The mean age of participants ranged from 21.3 to 38 in the three studies reporting mean age (Carrico 2007; Mukti 2000; Wright 1997), and most participants were female in studies reporting the gender of healthcare worker participants (Carrico 2007; Huang 2002; Moongtui 2000; Mukti 2000; Wright 1997).
Five studies involved a single hospital centre, although trials varied in the number and size of included units (Carrico 2007; Huang 2002; Moongtui 2000; Ong 2013; Wright 1997), and Mukti 2000 involved two hospitals. Two studies involved multiple nursing homes (Baldwin 2010; Rao 2009).
Two studies were conducted in the USA (Carrico 2007; Wright 1997), two in the UK (Baldwin 2010; Rao 2009), and one in Australia (Ong 2013). Three studies were conducted in southeast Asia, specifically, in China (Huang 2002), Thailand (Moongtui 2000), and Indonesia (Mukti 2000).
Types of interventions and follow‐up
Three studies focused on education alone and compared education programmes given to intervention groups versus no education or usual practice in control groups (Huang 2002; Mukti 2000; Wright 1997). Education varied in content, delivery, and duration, however. The education programme provided by Huang 2002 consisted of a two‐hour lecture on blood‐borne pathogens and universal precautions, a one‐hour demonstration of universal precautions techniques, and a 30‐minute discussion of blood‐borne pathogens, via multiple media such as pamphlets and DVDs. The other two studies used alternative teaching approaches. Wright 1997 evaluated a computer‐assisted learning programme that was case based; participants were given feedback on their answers as part of the training. Investigators did not specify the duration of the learning programme. Mukti 2000 focussed on academic detailing as a way of delivering personalised education. Two individualised sessions covered principles of universal precautions and how to perform certain procedures, and educators placed stickers and posters on the wall. Control group participants received no intervention.
The education programme provided by Carrico 2007 also focussed on classroom training that covered disease transmission, Standard Precautions, and use of PPE. In that study, however, both intervention and control groups received the education, which is different from the approach described in previous studies. In addition, the intervention group received visual demonstration of respiratory particle dispersion. Trial authors did not specify the duration of the education session.
Two studies added infection control support to an education programme. In addition to a two‐hour training session that included lectures and practical demonstrations of hand hygiene and decontamination of equipment and the environment, the intervention group in Baldwin 2010 was assigned a unit‐based infection control link nurse. In Rao 2009, the intervention group received 24‐hour support from an infection control team. In that study, healthcare workers received training related to hand hygiene, environmental cleaning, sharps safety, and disposal of clinical waste. Study authors did not specify the duration of the training session. Both studies were conducted in multiple nursing homes, and the control group in each study received no intervention.
The intervention provided by Moongtui 2000 focussed on peer evaluation. Intervention group participants were given education about peer evaluation and tools they could use. In the intervention phase, participants conducted peer evaluation but did not give feedback to individuals; instead they reported feedback to the unit 11 times over a six‐week period. The control group received no intervention.
Unlike the other studies, the intervention provided by Ong 2013 was not educational. The intervention consisted of a checklist and coloured cues promoting infection control precautions for radiology porters. The study included four groups. Each of two groups evaluated the checklist and cues separately, one group evaluated them together, and the fourth group received no intervention. The same porters were involved in all study groups.
Interventions thus varied across studies. No studies identified the theoretical underpinnings of the intervention, for example, whether it was based on a specific theory or framework for behaviour change. The duration of follow‐up also varied. Two studies reported a follow‐up period of one month (Moongtui 2000; Wright 1997), one a three‐month follow‐up period (Carrico 2007), two a four‐month follow‐up period (Huang 2002; Ong 2013), one a six‐month follow‐up period (Mukti 2000), and one a 12‐month follow‐up period (Baldwin 2010). Rao 2009 did not report any follow‐up period.
Types of outcome measures
Most studies evaluated observed adherence to components of Standard Precautions, but data show variation in what was observed. Carrico 2007 observed use of PPE in clinical interactions with patients who had respiratory symptoms. Huang 2002, Moongtui 2000, Mukti 2000, and Wright 1997 used structured observations to evaluate universal precautions‐related adherence; Moongtui 2000 and Mukti 2000 used variations of the universal precautions assessment tool, and the other researchers used different tools. Ong 2013 assessed the rate of observed adherence with specific infection control precautions when porters transferred patients and measured adherence to the pre‐transfer checklist and reactions to the interventions.
Rather than observations of individual behaviours, two studies assessed institutional adherence to infection control practice standards, using audits and observations of practices within the institution (e.g. environmental cleanliness, hand hygiene facilities) (Baldwin 2010; Rao 2009).
Three studies assessed knowledge via questionnaires (Carrico 2007; Huang 2002; Mukti 2000). Mukti 2000 also assessed attitudes toward infection prevention precautions, as part of the questionnaires. Questionnaires were not standardised and did not focus on identical content. Huang 2002 also used a questionnaire to obtain data on self‐reported behaviours related to universal precautions and asked about the occurrence of sharps injuries.
Only one study collected microbiological data and reported rates of MRSA colonisation among both staff and residents of long‐term care facilities (Baldwin 2010).
Excluded studies
We excluded two studies: Erickson 1996 conducted an interrupted time series design with inadequate data collection points, and Gould 1997 conducted a controlled before‐after study with only a single intervention group and a single control group. See Characteristics of excluded studies.
Risk of bias in included studies
Overall, we considered all included studies to be at high risk of bias. We considered three studies to be at high risk because they had ratings of high risk for two or more of the criteria (Baldwin 2010; Mukti 2000; Ong 2013). Each of the remaining studies had six criteria rated as unclear risk of bias, leading to questions about robustness of the evidence, even though only Moongtui 2000 had one rating of high risk of bias and the others had no criteria rated as high risk (Carrico 2007; Huang 2002; Rao 2009; Wright 1997). The greatest sources of high risk of bias were related to random sequence generation, allocation concealment, and blinding of outcome assessment. See Figure 2 and Figure 3 for results of the 'Risk of bias' assessment.
2.
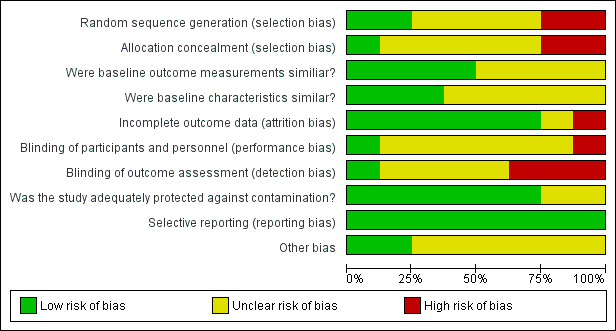
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
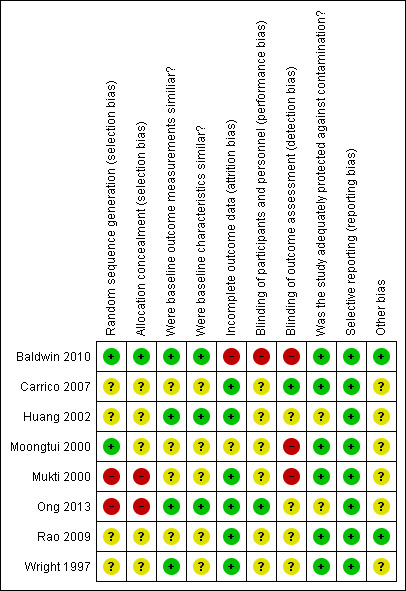
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two criteria are related to allocation: adequacy of random sequence generation and adequacy of allocation concealment. Two studies used appropriate methods of random sequence generation; we therefore classified them as having low risk of bias for this domain. Baldwin 2010 and Moongtui 2000 generated the allocation by Nquery and coin toss, respectively. We classified Baldwin 2010 as having low risk of bias for allocation concealment, but Moongtui 2000 did not describe methods used, and so we classified this study as having unclear risk.
We classified one study as having high risk of bias for random sequence generation. Ong 2013 used a random number generator but had to change methods during the study because of cancellations of transfers and uneven numbers per study group. We therefore categorised this study as having high risk for both items.
Four of the randomised trials did not describe methods used for both generation of allocation sequence and allocation concealment (Carrico 2007; Huang 2002; Rao 2009; Wright 1997), so we classified all of them as having unclear risk of bias for these two domains.
We classified Mukti 2000, a non‐randomised trial, as having high risk of bias for both domains, as per EPOC criteria, because of the study design employed.
Blinding
We considered blinding of participants separately from blinding of outcome assessment. Only one of the eight included studies performed blinding of outcome assessment (Carrico 2007), so we classified this study as having low risk of bias for this domain. Researchers did not report whether participants were blinded, so we classified this study as having unclear risk for this domain.
We identified three studies as having high risk of bias because assessors were not blinded to study groups. In two studies (Baldwin 2010; Moongtui 2000), researchers conducted the outcome assessment, and in Mukti 2000, the trained observer was a senior nurse within the department. Moongtui 2000 and Mukti 2000 did not report blinding of participants, and we classified them as having unclear risk of bias; Baldwin 2010 stated that it was not possible to blind participants to group allocation, and so we classified this trial as having high risk.
Authors of the three remaining studies did not report whether blinding of outcome assessors or of participants had occurred, and so we classified all of them as having unclear risk for both domains (Huang 2002; Rao 2009; Wright 1997).
Ong 2013 reported blinding only of study participants; therefore we classified this study as having low risk of bias. Trial authors did not report blinding of outcome assessors, so we classified this study as having unclear risk of bias for this domain.
Incomplete outcome data
We classified one study as having high risk of bias because of high dropout rates. Baldwin 2010 reported loss of 40.3% and 39.1% in the intervention and control groups, respectively. Moongtui 2000 did not explain the loss of eight participants nor identify the groups they belonged to, and so we classified it as having unclear risk of bias. All remaining studies had minimal losses to follow‐up, and so we classified them as low risk.
Selective reporting
We found no evidence of selective reporting bias in all included studies (Baldwin 2010; Carrico 2007; Huang 2002; Moongtui 2000; Mukti 2000; Ong 2013; Rao 2009; Wright 1997); therefore we judged them as having low risk of bias for this domain.
Other potential sources of bias
We categorised three studies as having low risk of bias because baseline characteristics and outcome measurements were similar (Baldwin 2010; Huang 2002; Ong 2013). We categorised one study as having low risk of bias because baseline outcome measurements were similar; however, the study was at unclear risk of bias regarding similarity of baseline characteristics because study authors did not report baseline characteristics (Wright 1997).
We classified the four remaining studies as having unclear risk of bias related to baseline outcome measurements and baseline characteristics, but for different reasons. In Carrico 2007, study groups had similar knowledge scores at baseline, but researchers did not evaluate use of PPE at baseline, and participants in the intervention group had more years' experience than those in the control group. In Moongtui 2000 and Mukti 2000, control groups had higher adherence rates at baseline, and both trials included more females in the control groups. Mukti 2000 reported significantly more prior training in universal precautions in the intervention group. Rao 2009 had matched nursing homes on the number of residents but described considerable variability in both groups in terms of baseline outcomes and characteristics.
We considered six of the studies to be adequately protected against contamination and at low risk of bias because it was unlikely that control group participants would get the intervention, either because of the nature of the intervention (e.g. computer‐assisted learning), or because participants came from different centres and were unlikely to talk to each other (Baldwin 2010; Carrico 2007; Moongtui 2000; Mukti 2000; Rao 2009; Wright 1997).
We classified two studies as having unclear risk of bias in terms of adequate protection against contamination, but for different reasons. Huang 2002 did not report on possible contamination, but all participants were from the same institution and may have discussed the education provided. We also classified Ong 2013 as having unclear risk of bias because of the potential for porters to remember the checklist, even when they were conducting transfers in the control group.
Six of the studies used direct observation of individual behaviour as an outcome measure, thus we classified them as having unclear risk of bias because of the potential observer effect (Carrico 2007; Huang 2002; Moongtui 2000; Mukti 2000; Ong 2013; Wright 1997). We identified no additional potential sources of bias for Baldwin 2010 or Rao 2009.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
for the main comparison.
| Education compared with no education for Standard Precautions | |||
|
Patient or population: nurses and physicians Settings: acute care hospitals Intervention: education Comparison: no education | |||
| Outcomes | Effects | No. of participants (studies) | Quality of the evidence (GRADE) |
| Observed adherence to Standard Precautions | Adherence improved from baseline in different studies, varying in intervention groups from 6.67 percentage points overall, to mean increases of 8 to 17 points and median increases of 3 to 21 points per specific elements of Standard Precautions. In control groups, changes varied from .97 percentage points overall, to mean differences of ‐2 to +2 points and median differences of ‐4 to +18 points per specific element. | 4 hospitals; 204 nurses, 11 physicians (2 RCTs, 1 NRCT) |
⊕⊕⊝⊝ lowa |
| Knowledge | Calculated differences in knowledge scores were a mean of 1.45 and a median of 2 for intervention groups, and a mean of ‐.14 and a median of 0 for control groups. | 3 hospitals; 144 nurses, 11 physicians (1 RCT, 1 NRCT) |
⊕⊕⊝⊝ lowb |
| Health care‐associated colonisation with MRSA | No studies reported this outcome. | No studies reported this outcome. | No studies reported this outcome. |
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
MRSA: methicillin‐resistant Staphylococcus aureus; NRCT: non‐randomised (controlled) trial; RCT: randomised (controlled) trial.
aEvidence downgraded from high to low certainty owing to non‐randomised evidence (one study); serious risk of bias (one study with three sources of high risk of bias; two studies with six sources of unclear risk of bias); and inconsistency of effect sizes between and within studies.
bEvidence downgraded from high to low certainty owing to non‐randomised evidence (one study), and serious risk of bias (one study with three sources of high risk of bias; one study with six sources of unclear risk of bias).
2.
| Education with visualisation compared with education alone for Standard Precautions | |||
|
Patient or population: nurses Settings: emergency department in acute care hospital Intervention: education with visualisation of respiratory particles Comparison: education without visualisation of respiratory particles | |||
| Outcomes | Effects | No. of participants (studies) | Quality of the evidence (GRADE) |
| Observed adherence to Standard Precautions | Education with visualisation of respiratory particles improved mask use in 74% of encounters with patients with respiratory symptoms compared with mask use in 53% of encounters with nurses who received education without visualisation. | 1 hospital; 20 nurses (1 RCT) |
⊕⊕⊕⊝ moderatea |
| Knowledge | Knowledge scores improved by 10 percentage points for nurses who received education with visualisation of respiratory particles compared with 14 percentage points for nurses who received education without visualisation. | 1 hospital; 20 nurses (1 RCT) |
⊕⊕⊕⊝ moderatea |
| Health care‐associated colonisation with MRSA | No studies reported this outcome. | No studies reported this outcome. | No studies reported this outcome. |
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
MRSA: methicillin‐resistant Staphylococcus aureus; RCT: randomised (controlled) trial.
aEvidence downgraded from high to moderate certainty owing to serious risk of bias (the study has six sources of unclear risk of bias).
3.
| Education with infection control support compared with no intervention for Standard Precautions | |||
|
Patient or population: staff and residents; healthcare organisations Settings: long‐term care facilities Intervention: education with infection control support (link workers or 24‐hour telephone support) Comparison: no intervention | |||
| Outcomes | Effects | No. of participants (studies) | Quality of the evidence (GRADE) |
| Observed adherence to Standard Precautions | Mean differences in audit scores from baseline to final audit varied by study, by practice, and between facilities, with mean differences in total scores of 26 percentage points for intervention groups and 11 points for control groups, and per‐practice differences in scores ranging from 11.7 to 17.5 percentage points for intervention groups and 6.7 to 27.2 points for control groups | 44 long‐term care facilities (2 cluster‐randomised trials) |
⊕⊕⊝⊝ lowa |
| Knowledge | No studies reported this outcome. | No studies reported this outcome. | No studies reported this outcome. |
| Health care‐associated colonisation with MRSA | Data show little or no difference in rates of MRSA among residents or staff in intervention vs control groups at 12 months post intervention compared with baseline. | 32 long‐term care facilities (1 cluster‐randomised trial) |
moderateb |
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
MRSA: methicillin‐resistant Staphylococcus aureus.
aEvidence downgraded from high to low certainty owing to serious risk of bias (one study has three sources of high risk of bias; one study has six sources of unclear risk of bias); inconsistency of effect sizes between and within studies; and imprecision (wide confidence intervals in one study); and no matched analysis was done (both were pair‐matched cluster‐randomised trials).
bEvidence downgraded from high to moderate certainty owing to serious risk of bias (the study has three sources of high risk of bias), and no matched analysis was done (this was a pair‐matched cluster‐randomised trial).
4.
| Peer evaluation compared with no intervention for Standard Precautions | |||
|
Patient or population: nursing staff Settings: acute care hospital Intervention: peer evaluation Comparison: no intervention | |||
| Outcomes | Effects | No. of participants (studies) | Quality of the evidence (GRADE) |
| Observed adherence to Standard Precautions | Scores for adherence to Standard Precautions increased from baseline by 33.5 percentage points at the end of the intervention period and by 24 points 4 weeks post intervention, compared with increases of 3.2 points in the control group at both time points compared with baseline. | 1 hospital; 99 registered nurses, practical nurses, and patient care aides (1 RCT) |
⊕⊕⊕⊝ moderatea |
| Knowledge | No studies reported this outcome. | No studies reported this outcome. | No studies reported this outcome. |
| Health care‐associated colonisation with MRSA | No studies reported this outcome. | No studies reported this outcome. | No studies reported this outcome. |
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
MRSA: methicillin‐resistant Staphylococcus aureus; RCT: randomised (controlled) trial.
aEvidence downgraded from high to moderate certainty owing to serious risk of bias (the study has six sources of unclear risk of bias).
5.
| Checklist and coloured cues compared with no intervention for Standard Precautions | |||
|
Patient or population: radiology porters conducting transfers of patients Settings: acute care hospital Intervention: checklist, coloured cues, or both Comparison: no intervention | |||
| Outcomes | Effect | No. of participants (studies) | Quality of the evidence (GRADE) |
| Observed adherence to Standard Precautions | Compared with the control group, adherence scores increased in all groups (checklist, coloured cues, and both) by 33 to 36 percentage points in total score, 33 to 36 points for glove use, 5 to 10 points for hand hygiene, and 1 to 13 points for gown use. Data show no consistency in terms of which group had the highest scores. | 1 hospital; 11 radiology porters conducting 300 transfers (1 RCT) |
⊕⊕⊕⊝ moderatea |
| Knowledge | No studies reported this outcome. | No studies reported this outcome. | No studies reported this outcome. |
| Health care‐associated colonisation with MRSA | No studies reported this outcome. | No studies reported this outcome. | No studies reported this outcome. |
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
MRSA: methicillin‐resistant Staphylococcus aureus; RCT: randomised (controlled) trial.
aEvidence downgraded from high to moderate certainty owing to serious risk of bias (the study has two sources of high risk of bias), and because of important inconsistency in effect sizes.
See Table 1, Table 2, Table 3, Table 4, and Table 5. We have provided details of key results for each primary and secondary outcome in Table 6Table 7 and Table 8.
Education versus no intervention
Education may slightly improve healthcare workers' adherence to Standard Precautions (three studies; four centres) and knowledge (two studies; three centres) based on evidence of low certainty for both outcomes. Included studies did not measure or report rates of health care‐associated colonisation with MRSA. See Table 1.
Two randomised trials ‐ Huang 2002,Wright 1997 ‐ and one non‐randomised trial ‐ Mukti 2000 ‐ evaluated educational interventions and compared them with no intervention. The content and delivery of educational programmes differed. All three trials reported observed adherence of individuals to elements of universal precautions ‐ the system that preceded Standard Precautions ‐ but reported outcomes in different ways; we have summarised detailed results in Table 6. As shown in Table 1, adherence improved from baseline in all intervention groups, and control groups showed smaller differences as well as some negative changes. Data show variation in differences by specific element (e.g. hand hygiene, glove use), and researchers did not report the same descriptive statistics (mean or median). The two studies that assessed knowledge found almost no difference in knowledge scores between groups (Huang 2002; .Mukti 2000).
Adherence improved from baseline across studies, varying in intervention groups from 6.67 percentage points overall to mean increases of 8 to 17 points and median increases of 3 to 21 points per specific element of Standard Precautions. In control groups, changes varied from .97 percentage points overall to mean differences of ‐2 to +2 points and median differences of ‐4 to +18 points per specific element.
Wright 1997 evaluated computer‐assisted instruction and reported mean scores on the Universal Precautions Assessment tool. They found a small increase of 6.67 percentage points in scores of the intervention group and almost no change (.97 percentage points) in scores of the control group. Mukti 2000 reported median scores on a different behaviour observation checklist and found a very small increase of 2 percentage points in median total scores in the intervention group and no difference in the control group. However, data show greater increases in the intervention group, ranging from 3 to 21 percentage points in subscores for glove use, hand hygiene, use of disinfectant, and proper disposal compared with changes in the control group (‐4 to 8 points). Both intervention and control groups had similar differences in scores related to recapping ‐ 19 and 18 points, respectively. Huang 2002 evaluated a group session that included lecture, demonstration, and discussion, and reported the proportion of nurses who complied with recommended behaviours during the observation period. They did not report whether nurses performed the behaviour each time the behaviour was required. Greater increases of 8 to 17 percentage points were seen in the proportion of nurses in the intervention group who adhered to recommended behaviours post intervention with hand hygiene and glove use compared with baseline; data show a decrease of 20 points in recapping. In the control group, changes in the proportion of nurses who adhered to these behaviours ranged from ‐2 to 2 points.
Huang 2002 also reported on changes in knowledge and self‐reported behaviours. As summarized in Table 7, investigators reported an increase of 1.45 points in mean knowledge score and 12.35 points in self‐reported behaviour among those in the intervention group, compared with a decrease in knowledge score of .14 points and an increase of 2.38 points in self‐reported behaviour in the control group. Both groups reported decreased numbers of self‐reported sharps injuries: a decrease of 61 injuries in the intervention group and 41 injuries in the control group. Mukti 2000 similarly reported a very small increase in the median knowledge score of 2 percentage points in the intervention group and no change in the control group. That study described an increase of 4 percentage points in attitude score in the intervention group and 1 percentage point in the control group.
Education plus visualisation of aerosolised particles versus only education
Education with visualisation of respiratory particle dispersion probably improves healthcare workers' use of facial protection but probably leads to little or no difference in healthcare workers' knowledge (one study; 20 nurses; moderate certainty of evidence for both outcomes). Included studies did not measure or report rates of health care‐associated colonisation with MRSA. See Table 2.
Carrico 2007 evaluated the effect of adding visualisation of respiratory droplet dispersion to education. Investigators assessed staff in the emergency department in terms of use of masks when a patient presented with respiratory symptoms, and considered both use of mask by the staff member and use of mask by the patient as appropriate. Researchers performed no baseline assessment. Overall, use of the mask improved in the group that received education with visualisation, but knowledge scores did not improve compared with the control group
Table 6 and Table 7 summarise details of results related to observed adherence and knowledge, respectively. A total of 74% of those who had education with visualisation used a mask in the clinical encounter compared with 53% of those who received only education without visualisation. Investigators also evaluated knowledge demonstrated in a post‐test compared with a pre‐test and found a greater increase of 14 percentage points in the knowledge score of staff in the control group compared with an increase of 10 percentage points among those in the intervention group.
Education with infection control support versus no intervention
Education with additional infection control support may slightly improve healthcare workers' adherence to Standard Precautions (two studies; 44 long‐term care facilities; low certainty of evidence) but probably leads to little or no difference in rates of health care‐associated colonisation with MRSA (one study; 32 long‐term care facilities; moderate certainty of evidence). Included studies did not measure or report knowledge. See Table 3.
Two pair‐matched cluster‐randomised trials evaluated the addition of infection control support to education; Baldwin 2010 used infection control link nurses, and Rao 2009 provided 24‐hour telephone support. Control groups received no intervention. Both studies reported adherence at the level of the institution rather than at an individual level in terms of audits of different recommended practices. The authors of both studies did not use a matched analysis as appropriate for the design. Overall, data show improvements in practice, with considerable variation between long‐term care facilities and by type of practice audited.
We have summarised key results in Table 6. Baldwin 2010 reported a greater increase in the mean audit score in the intervention group ‐ from 56% at baseline to 82% at one year ‐ compared with the control group ‐ with 53% at baseline and 64% at 12 months. Rao 2009 reported a range of scores rather than a mean score across institutions, by specific practices. Data show considerable variation. For example, at the final audit, 67% to 100% of intervention institutions were adherent with hand hygiene facilities, and 54% to 96% with environmental cleanliness, compared with 68% to 96% and 77% to 96% of control institutions, respectively. Changes in adherence to environmental cleanliness recommendations were greater among controls than among those given the intervention (mean difference (MD) 10.5 percentage points, 95% confidence interval (CI) ‐18 to 39) but were less common among controls in relation to hand hygiene facilities (MD ‐4.5 points, 95% CI ‐29.1 to 20.1).
Baldwin 2010 also assessed rates of MRSA colonisation among staff and residents (Table 8), reporting similar colonisation rates in intervention and control groups at three, six, and 12 months. At 12 months post intervention compared with baseline, the risk ratio for colonisation among residents in intervention versus control groups was .81 (95% CI .51 to 1.30). In both groups, colonisation occurred in 17% at baseline and in 19% at 12 months post intervention. However, data show a slight decrease in MRSA colonisation among staff in both groups ‐ from 10% at baseline to 7.3% at 12 months in the intervention group, and from 8.5% at baseline to 4.3% at 12 months in the control group.
Peer evaluation
Peer evaluation probably improves healthcare workers' adherence to Standard Precautions (one study; one hospital; moderate certainty of evidence). Included studies did not measure or report knowledge and rates of health care‐associated colonisation with MRSA. See Table 4.
Moongtui 2000, a randomised trial, focussed on peer evaluation. Investigators trained staff in peer evaluation and gave them tools to use; they monitored adherence and provided feedback at the unit, not individual, level. Data showing mean scores on a modified Universal Precautions Assessment tool showed overall larger increases in observed adherence in the intervention group than in the control group.
As shown in Table 6, Moongtui 2000 found an increase of 33.5 percentage points in scores of the intervention group between end of the intervention period and baseline, and an increase of 24 percentage points between the post‐test period (four weeks after completion of the intervention) and baseline. In comparison, data show only a very small increase of 3.2 percentage points in scores of the control group at both time points compared with baseline.
Checklist and coloured cues
Checklists and coloured cues probably improve healthcare workers' adherence to Standard Precautions (one study; one hospital; moderate certainty of evidence). Included studies did not measure or report knowledge and rates of health care‐associated colonisation with MRSA. See Table 5.
Ong 2013, a randomised trial with cross‐over, evaluated effects of checklists and coloured cues on radiology porters’ observed adherence with hand hygiene, glove use, and gown use, and overall adherence with infection control recommendations. Overall, both interventions led to improved adherence.
We have provided detailed results in Table 6. Compared with the control group, data show improved adherence scores across study groups of 33 to 36 percentage points for overall adherence, and specifically for use of gloves. Mean adherence was greater for hand hygiene by 10 percentage points in the checklist group and by 1 percentage point for gown use compared with the control group, and increases were 7 and 13 points, respectively, in the coloured cues group, and 5 and 6 points, respectively, in the group using the checklist and coloured cues at the same time.
Discussion
Summary of main results
In summary, eight studies met review inclusion criteria. Investigators evaluated five different types of interventions, three of which included education. All studies reported adherence to Standard Precautions as an outcome, although investigators used different measures. Three studies reported knowledge as an outcome, and one reported methicillin‐resistant Staphylococcus aureus (MRSA) colonisation rates.
Observed adherence to elements of Standard Precautions increased in both individuals and organisations. However, trials reported considerable variation in baseline adherence and extent of changes, both between and within studies, as well by the specific behaviour assessed.
Moderate‐certainty evidence shows that education showing respiratory particle dispersion (one study), peer evaluation (one study), and use of checklists and coloured cues (one study) probably improved adherence to Standard Precatuions. In comparison, low‐certainty evidence suggests that education alone (three studies) and education with additional infection control support (two studies) may have slightly improved adherence to Standard Precautions.
Low‐certainty evidence suggests that education alone may slightly improve knowledge. In comparison, moderate‐certainty evidence shows that education showing respiratory particle dispersion probably leads to little or no difference in knowledge.
Moderate‐certainty evidence shows that education with additional infection control support probably leads to little or no difference in rates of MRSA colonisation.
We were unable to undertake a meta‐analysis because of the heterogeneity of interventions and outcome measures reported. Because of this heterogeneity, in combination with high risk of bias and few studies evaluating a specific intervention, it is difficult to draw a clear conclusion about the effectiveness of different interventions. In summary, it appears that interventions do promote adherence to Standard Precautions, but further research is warranted to determine which interventions are most effective.
Overall completeness and applicability of evidence
We performed a comprehensive search of the literature to identify the best available clinical evidence to answer our question, “What is the effectiveness of interventions to improve adherence to Standard Precautions in patient care?". Therefore we are confident that we have mapped all studies’ reported effectiveness of interventions to improve adherence to Standard Precautions in patient care. However, we noted considerable heterogeneity in terms of details of interventions and outcome measures. Furthermore, as discussed in the next section, the certainty of evidence was low to moderate. With few studies evaluating similar interventions in similar ways, we found insufficient evidence on which to base a conclusion about the most effective strategies or recommendations to improve adherence to Standard Precautions.
Although much effort has been placed on interventions to promote hand hygiene (Gould 2017), as well as on bundles of interventions to reduce specific types of infections, limited research has been conducted to explore the topic of promoting adherence to Standard Precautions. Application of transmission‐based precautions and use of bundles of interventions to reduce specific types of infection are implemented in conjunction with, not in place of, Standard Precautions. Furthermore, Standard Precautions will reduce transmission of infectious agents when healthcare workers are not aware of the presence of infectious agents (e.g. when the patient is asymptomatic, when infection has not been diagnosed), so it is imperative that healthcare workers adhere to Standard Precautions. Although it is not yet clear which interventions are most effective, the evidence presented in this review is applicable to practice worldwide.
Certainty of the evidence
Overall, we found a limited body of evidence for any given intervention, with only one to three studies evaluating each intervention. Certainty of evidence ranged from low to moderate. For all interventions, we downgraded the certainty of evidence because of serious risk of bias. We considered all studies to be at high risk of bias ‐ three because they had ratings of high risk of bias for two or more criteria, and the rest because they had ratings of unclear risk of bias for six out of ten criteria. Researchers could have addressed risk of bias related to allocation at the design stage, and could have addressed other risks at the reporting stage.
All studies with observed adherence had unclear risk of bias owing to the presence of the observer. Many studies did not report on blinding; although blinding of participants or observers may not always have been feasible, reporting on what was done would have allowed clearer assessment of risk of bias. The same is true for including reporting of baseline characteristics or outcomes, which was not always done. We attempted to make contact but were unable to obtain information from trial authors that would have allowed us to rate risk as other than unclear.
We also downgraded certainty of evidence for some interventions, in addition to risk of bias, because of non‐randomised evidence (one study), inconsistency (two studies), or imprecision (one study). Seven of the studies were randomised trials; we downgraded the certainty of evidence for education because of one non‐randomised trial and because of important inconsistency in results. For education with additional infection control support, we downgraded the certainty of evidence because of important inconsistency and imprecision in results.
Additional studies of specific interventions, with clearer reporting to allow for a robust assessment of risk of bias, would enhance the body of evidence and the certainty of evidence on effectiveness of strategies to promote adherence to Standard Precautions.
Potential biases in the review process
The main potential source of bias in the review process is that we were unable to obtain further data from the authors of each included study to be able to clarify risk of bias in each study. Our review methods followed EPOC guidelines and were unlikely to have introduced bias.
Agreements and disagreements with other studies or reviews
We found no other reviews looking at the effectiveness of interventions to promote Standard Precautions. In a recent review, Picheansanthian 2015 examined issues related to glove use but did not evaluate interventions to promote glove use. They concluded that further research is needed to identify strategies to promote appropriate glove use. Porto 2016 conducted an integrative literature review and summarised factors contributing to low adherence to Standard Precautions but did not address interventions to promote adherence. Hessels 2016 conducted a systematic review on the relationship between patient safety climate and adherence to Standard Precautions and found a correlation between the two, but did not assess strategies to promote a patient safety climate or adherence.
In a systematic review, Gould 2017 found that combinations of strategies recommended by the World Health Organization (WHO) and performance feedback may slightly improve compliance with hand hygiene recommendations and may reduce infection rates (low certainty of evidence). Education and cues may also slightly improve hand hygiene compliance (low certainty of evidence), and placement of alcohol‐based hand rub close to the point of care probably slightly improves compliance (moderate certainty of evidence). Review authors recommended further methodologically robust research to evaluate which interventions or combinations of interventions are most effective in promoting compliance with hand hygiene recommendations. In our review, we found that education alone or provided with additional infection control support may slightly improve adherence (low certainty of evidence), and education with visualisation of respiratory particles, use of cues and checklists, and peer evaluation probably improves adherence (moderate certainty of evidence).
The current literature, although limited, focusses on compliance rates or reasons for adherence. Intervention studies for promotion of adherence to Standard Precautions are far fewer than those conducted to promote hand hygiene. Issues related to promoting adherence may be similar, however, so exploration is warranted, to see if lessons learned from promotion of hand hygiene can be applied to promote adherence to Standard Precautions.
Authors' conclusions
Implications for practice.
Standard Precautions guidelines form the foundation for infection prevention and control to reduce transmission of micro‐organisms to other patients or to healthcare workers. Non‐adherence to different elements of Standard Precautions, such as glove use or sharps safety, has been identified as a concern, justifying the need to take action. The evidence is unclear however as to which interventions should be recommended to promote adherence. Peer evaluation, education with visualisation of respiratory particles, and use of checklists and coloured cues probably promote improved adherence (moderate certainty of evidence), and education alone or provided with additional infection control support may slightly improve adherence (low certainty of evidence). Because of the important role that Standard Precautions can play in reducing transmission, it is logical for organisations to assess adherence and contributing factors locally, and to develop, implement, and evaluate interventions relevant to their needs.
Implications for research.
This review underlines the need to conduct well‐designed trials to evaluate the effects of interventions. More robust studies evaluating similar types of interventions, using similar outcome measures, and addressing methodological limitations such as random allocation and blinding, would allow comparison across studies and pooling of results, so that conclusions can be drawn that are based on a stronger body of evidence. As the Standard Precautions document has a variety of components, standardised measures of adherence are needed (de Carvalho 2013). Better reporting of methods would allow clearer assessment of risks of bias, further enhancing confidence in conclusions. Continued research on understanding behaviour change issues would allow development of interventions with a clearer theoretical rationale. Lessons learned from promoting hand hygiene can be applied in promoting adherence to Standard Precautions, with relevant interventions evaluated for effectiveness.
Acknowledgements
We would like to thank Pierre Durieux, Alain D. Mayhew, Paul Miller, and Julia Worswick, (Cochrane Effective Practice and Organisation of Care Group) for editorial support and guidance provided during preparation of this review.
We would also like to thank Signe Flottorp, Virginia Mumford, and other external referees for feedback provided on the draft of the review, which proved helpful in strengthening the final version.
National Institute for Health Research, via Cochrane Infrastructure funding to the Effective Practice and Organisation of Care Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Search strategies
MEDLINE (Ovid)
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
| No. | Search terms | Results |
| 1 | universal precautions/ | 1561 |
| 2 | ((routine practice? or standard or universal or transmission‐based or isolation) and precaution?).ti. | 563 |
| 3 | ((standard or universal or transmission‐based or isolation) adj4 precaution?).ab. | 2117 |
| 4 | ((airborn* or bacteria* or barrier? or blood* or body substanc* or body fluid? or contact or droplet* or hai or infection? or infectious or nosocomial) and precaution?).ti. | 417 |
| 5 | ((airborn* or bacteria* or barrier? or blood* or body substanc* or body fluid? or contact or droplet* or hai or infection? or infectious or nosocomial or transmission‐based) adj5 precaution?).ab. | 1934 |
| 6 | ((mask? or glove? or gown?) and precaution?).ti. or ((mask? or glove? or gown?) adj5 precaution?).ab. | 113 |
| 7 | body substance? isolation?.ti,ab. | 34 |
| 8 | ((icu or intensive care unit?) and precaution?).ti. | 15 |
| 9 | ((aseptic or sterile) and precaution?).ti. or ((aseptic or sterile) adj5 precaution?).ab. | 228 |
| 10 | (precaution? adj4 (communication? or sign? or signage or notif*)).ti,ab. | 39 |
| 11 | ((infection? or infectious) and (bundle? or bundling)).ti. or ((infection? or infectious) adj5 (bundle? or bundling)).ab. | 279 |
| 12 | (((central line adj3 infection?) or (catheter* adj3 infection?) or (ventilator* adj3 infection?) or nosocomial or hospital acquired infection? or health care associated infection? or healthcare associated infection? or cross infection) and (best practice or bundle? or checklist? or (clinical adj2 (pathway? or protocol)) or collaborativ* or communication? or compliance or coordinated or cross‐disciplin* or decreas* or educational or (education adj3 (continuing or staff or resident? or physician? or nurse or nurses)) or evidence or guideline? or handoff? or impact or implement* or initiative? or intervention or interdisciplin* or inter‐disciplin* or "length of stay" or multidimensional or multi‐dimensional or mutlidisciplin* or multi‐disciplin* or multifacet* or multi‐facet* or multimodal or multi‐modal or ((patient or care or icu or ward? or surgic*) adj3 transfer?) or prevent or preventing or professional development or program? or programme or programmes or promote or promoting or protocol? or quality improvement or reminder? or stewardship or strategies or strategy or team? or workshop)).ti. | 1507 |
| 13 | or/1‐12 | 6568 |
| 14 | cross infection/pc or pneumonia, ventilator‐associated/pc | 21562 |
| 15 | bacteremia/pc | 2095 |
| 16 | staphylococcal infections/pc | 5099 |
| 17 | ((mrsa or methicil* resistant or bacteremia) and (prevent* or reducing or reduce?)).ti. | 623 |
| 18 | or/14‐17 | 26648 |
| 19 | cross infection/ or pneumonia, ventilator‐associated/ or surgical wound infection/ | 82138 |
| 20 | infectious disease transmission, professional‐to‐patient/ | 1636 |
| 21 | ((hospital? or hospital acquired) adj4 infection?).ti,ab. | 14394 |
| 22 | (hospital* and infection?).ti,hw. | 51030 |
| 23 | (cross infection? or hai or nosocomial*).ti,ab. | 31268 |
| 24 | ((central line? or ventilator?) adj4 infection?).ti,ab. | 1423 |
| 25 | ((health care or healthcare or icu or care unit or care units or ward or wards or ((surgical or intensive care) adj2 (unit? or department?))) adj4 infection?).ti,ab. | 9013 |
| 26 | methicillin‐resistant staphylococcus aureus/ or bacteremia/ | 30805 |
| 27 | ((mrsa or methicil* resistant or bacterimia) adj4 (prevent* or reducing or reduce?)).ab. | 1107 |
| 28 | ((surgery or surgical or postop* or post‐operat*) adj4 infection?).ti,ab. | 28776 |
| 29 | or/19‐28 | 174247 |
| 30 | *catheter‐related infections/ or *prosthesis‐related infections/ or exp *sepsis/ | 81801 |
| 31 | exp *catheterization/ae, co, mo | 21260 |
| 32 | exp *catheterization/ and (infection? or infectious).ti,hw. | 6893 |
| 33 | (catheter* adj3 infection?).ti,ab. | 5893 |
| 34 | (sepsis or septic shock or blood* infection? or blood poisoning or bacter?emia* or endotox?emia*).ti,ab. | 121943 |
| 35 | or/30‐34 | 180325 |
| 36 | infection control/ or antisepsis/ or asepsis/ or blood safety/ or infection control, dental/ or patient isolation/ or quarantine/ or sterilization/ or disinfection/ | 56803 |
| 37 | infection control.ab. | 13043 |
| 38 | ((infection? adj2 control*) or blood safety or (antisepsis or asepsis or sterili?ation or disinfect*)).ti. | 26123 |
| 39 | ((antisepsis or asepsis or sterili?ation or disinfect*) adj7 (procedur* or process or processes or strategy or strategies or strategi? or guideline? or protocol? or pathway? or policy or policies or checklist? or check‐list?)).ab. | 4519 |
| 40 | protective devices/ or eye protective devices/ or masks/ or protective clothing/ or gloves, protective/ or gloves, surgical/ or respiratory protective devices/ | 22019 |
| 41 | (((scrubs or mask or masks or gown or gowns or glove or gloves or gloved or goggle?) adj4 (protect* or infection? or infectious)) or ((eye or eyes or clothing or uniform? or respiratory or equipment) adj2 protective)).ti,ab. | 5960 |
| 42 | isolation room?.ti,ab. | 346 |
| 43 | ((reduce? or reducing or disrupt*) adj2 (transmission? or spread or spreading)).ti,ab. | 6889 |
| 44 | or/36‐43 | 108232 |
| 45 | exp hospital units/ or exp hospitals/ or inpatients/ | 336368 |
| 46 | health facilities/ or academic medical centers/ or exp hospitals, teaching/ or exp outpatient clinics, hospital/ or surgicenters/ or birthing centers/ or dental facilities/ or dental clinics/ or dental offices/ | 95877 |
| 47 | exp hospital departments/ | 161688 |
| 48 | (hospital? or hospitali?ed or ward or wards or (care adj2 unit) or (care adj2 units)).ti. or hospital?.jn,hw. | 646296 |
| 49 | or/45‐48 | 743661 |
| 50 | (best practice or bundle? or checklist? or (clinical adj2 (pathway? or protocol)) or collaborativ* or communication? or compliance or coordinated or cross‐disciplin* or decreas* or educational or (education adj3 (continuing or staff or resident? or physician? or nurse or nurses)) or evidence or guideline? or handoff? or impact or implement* or initiative? or intervention or interdisciplin* or inter‐disciplin* or "length of stay" or multidimensional or multi‐dimensional or mutlidisciplin* or multi‐disciplin* or multifacet* or multi‐facet* or multimodal or multi‐modal or ((patient or care or icu or ward? or surgic*) adj3 transfer?) or prevent or preventing or professional development or program? or programme or programmes or promote or promoting or protocol? or quality improvement or reminder? or stewardship or strategies or strategy or team? or workshop).ti. | 1114542 |
| 51 | (continuing adj2 education*).hw. | 58858 |
| 52 | quality assurance, health care/ or benchmarking/ or total quality management/ | 73942 |
| 53 | ((quality adj2 (assurance or circle or circles or improv* or management)) or benchmarking).ti,ab. | 121000 |
| 54 | impact.ti. | 163029 |
| 55 | (incentive? or complex intervention? or ((physician? or staff) adj3 behavio?r?) or practice pattern? or ((policy or practice?) adj2 (chang* or influenc* or impact))).ti,ab. | 53365 |
| 56 | physician's practice patterns/ or nurse's practice patterns/ | 50458 |
| 57 | or/50‐56 | 1366469 |
| 58 | (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti. | 1105610 |
| 59 | exp animals/ not humans.sh. | 4325671 |
| 60 | 58 not 59 | 1020499 |
| 61 | intervention?.ti. or (intervention? adj6 (clinician? or collaborat* or community or complex or design* or doctor? or educational or family doctor? or family physician? or family practitioner? or financial or gp or general practice? or hospital? or impact? or improv* or individuali?e? or individuali?ing or interdisciplin* or multicomponent or multi‐component or multidisciplin* or multi‐disciplin* or multifacet* or multi‐facet* or multimodal* or multi‐modal* or personali?e? or personali?ing or pharmacies or pharmacist? or pharmacy or physician? or practitioner? or prescrib* or prescription? or primary care or professional* or provider? or regulatory or regulatory or tailor* or target* or team* or usual care)).ab. | 229149 |
| 62 | (pre‐intervention? or preintervention? or "pre intervention?" or post‐intervention? or postintervention? or "post intervention?").ti,ab. | 16560 |
| 63 | (hospital* or patient?).hw. and (study or studies or care or health* or practitioner? or provider? or physician? or nurse? or nursing or doctor?).ti,hw. | 829009 |
| 64 | demonstration project?.ti,ab. | 2244 |
| 65 | (pre‐post or "pre test*" or pretest* or posttest* or "post test*" or (pre adj5 post)).ti,ab. | 91329 |
| 66 | (pre‐workshop or post‐workshop or (before adj3 workshop) or (after adj3 workshop)).ti,ab. | 879 |
| 67 | trial.ti. or ((study adj3 aim?) or "our study").ab. | 888767 |
| 68 | (before adj10 (after or during)).ti,ab. | 425883 |
| 69 | ("quasi‐experiment*" or quasiexperiment* or "quasi random*" or quasirandom* or "quasi control*" or quasicontrol* or ((quasi* or experimental) adj3 (method* or study or trial or design*))).ti,ab,hw. | 126715 |
| 70 | ("time series" adj2 interrupt*).ti,ab,hw. | 1764 |
| 71 | (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month* or hour? or day? or "more than")).ab. | 13341 |
| 72 | pilot.ti. | 54389 |
| 73 | pilot projects/ | 100119 |
| 74 | (clinical trial or controlled clinical trial or multicenter study).pt. | 697743 |
| 75 | (multicentre or multicenter or multi‐centre or multi‐center).ti. | 40225 |
| 76 | random*.ti,ab. or controlled.ti. | 972768 |
| 77 | (control adj3 (area or cohort? or compare? or condition or design or group? or intervention? or participant? or study)).ab. not (controlled clinical trial or randomized controlled trial).pt. | 519521 |
| 78 | (control year? or experimental year? or (control period? or experimental period?)).ti,ab. | 15245 |
| 79 | evaluation studies as topic/ or prospective studies/ or retrospective studies/ | 1160535 |
| 80 | (utili?ation or programme or programmes).ti. | 65967 |
| 81 | (during adj5 period).ti,ab. | 353154 |
| 82 | ((strategy or strategies) adj2 (improv* or education*)).ti,ab. | 26440 |
| 83 | (purpose adj3 study).ab. | 289197 |
| 84 | "comment on".cm. or review.pt. or (review not "peer review*").ti. or randomized controlled trial.pt. | 3470719 |
| 85 | (rat or rats or cow or cows or chicken? or horse or horses or mice or mouse or bovine or animal?).ti,hw. or veterinar*.ti,ab,hw. | 6127437 |
| 86 | exp animals/ not humans.sh. | 4325671 |
| 87 | (or/61‐83) not (or/84‐86) | 3509565 |
| 88 | 18 and 57 | 5246 |
| 89 | 29 and 44 and 57 | 4582 |
| 90 | 35 and 44 and 49 | 1604 |
| 91 | 44 and 49 and 57 | 3637 |
| 92 | ((or/13,88‐91) and 60) or (13 and 87) | 3256 |
EMBASE (Ovid)
EMBASE <1974 to 2017 February 13>
| No. | Search terms | Results |
| 1 | ((routine practice? or standard or universal or transmission‐based or isolation) and precaution?).ti. | 644 |
| 2 | ((standard or universal or transmission‐based or isolation) adj4 precaution?).ab. | 2703 |
| 3 | ((airborn* or bacteria* or barrier? or blood* or body substanc* or body fluid? or contact or droplet* or hai or infection? or infectious or nosocomial) and precaution?).ti. | 490 |
| 4 | ((airborn* or bacteria* or barrier? or blood* or body substanc* or body fluid? or contact or droplet* or hai or infection? or infectious or nosocomial or transmission‐based) adj5 precaution?).ab. | 2740 |
| 5 | ((mask? or glove? or gown?) and precaution?).ti. or ((mask? or glove? or gown?) adj5 precaution?).ab. | 136 |
| 6 | body substance? isolation?.ti,ab. | 37 |
| 7 | ((icu or intensive care unit?) and precaution?).ti. | 24 |
| 8 | ((aseptic or sterile) and precaution?).ti. or ((aseptic or sterile) adj5 precaution?).ab. | 435 |
| 9 | (precaution? adj4 (communication? or sign? or signage or notif*)).ti,ab. | 64 |
| 10 | ((infection? or infectious) and (bundle? or bundling)).ti. or ((infection? or infectious) adj5 (bundle? or bundling)).ab. | 543 |
| 11 | (((central line adj3 infection?) or (catheter* adj3 infection?) or (ventilator* adj3 infection?) or nosocomial or hospital acquired infection? or health care associated infection? or healthcare associated infection? or cross infection) and (best practice or bundle? or checklist? or (clinical adj2 (pathway? or protocol)) or collaborativ* or communication? or compliance or coordinated or cross‐disciplin* or decreas* or educational or (education adj3 (continuing or staff or resident? or physician? or nurse or nurses)) or evidence or guideline? or handoff? or impact or implement* or initiative? or intervention or interdisciplin* or inter‐disciplin* or "length of stay" or multidimensional or multi‐dimensional or mutlidisciplin* or multi‐disciplin* or multifacet* or multi‐facet* or multimodal or multi‐modal or ((patient or care or icu or ward? or surgic*) adj3 transfer?) or prevent or preventing or professional development or program? or programme or programmes or promote or promoting or protocol? or quality improvement or reminder? or stewardship or strategies or strategy or team? or workshop)).ti. | 1981 |
| 12 | or/1‐11 | 7575 |
| 13 | infection control/ | 80510 |
| 14 | *infection prevention/ | 9546 |
| 15 | infection control.ti. | 5791 |
| 16 | or/13‐15 | 89827 |
| 17 | *cross infection/ | 13452 |
| 18 | healthcare associated infection/ | 3334 |
| 19 | (cross infection? or ((central line adj3 infection?) or (catheter* adj3 infection?) or (ventilator* adj3 infection?) or nosocomial or hospital acquired infection? or healthcare associated infection? or health care associated infection?)).ti. | 16482 |
| 20 | or/17‐19 | 29373 |
| 21 | (precaution? or bundle?).ti. | 15005 |
| 22 | (precaution? adj3 (comply* or complian* or observe? or observence or observing)).ab. | 568 |
| 23 | (infection adj3 (bundle? or guideline? or protocol? or collaborat* or evidence based)).ti,ab. | 2673 |
| 24 | ((practice? or procedure? or care) adj3 bundle?).ab. | 1480 |
| 25 | personal protective equipment.ti,ab. | 2104 |
| 26 | or/21‐25 | 20884 |
| 27 | *practice guideline/ and ((adhere* or effectiveness or evidence based or impact or implement* or quality).ti. or (adherence or evidence based or implement* or (quality adj3 (care or improv?ment)) or (impact adj4 (care or process or quality))).ab.) | 14472 |
| 28 | clinical pathway/ or *clinical protocol/ or *good clinical practice/ or nursing care plan/ or nursing protocol/ or clinical handover/ or "change of shift report"/ | 17681 |
| 29 | (practice? adj2 (protocol? or pathway?)).ti,ab. | 1243 |
| 30 | (guideline? adj3 (adher* or comply* or complian* or implement* or quality or impact or effect*)).ti,ab. | 29925 |
| 31 | or/27‐30 | 57695 |
| 32 | (checklist? or collaborat* or compliance or comply* or compliant or (continuing adj2 education*) or educational or impact or implementation or improve? or improving or improvement or incentive? or infrastructure? or innovative or interdisciplin* or inter‐disciplin* or multifacet* or multi‐facet* or organi?ational or prevent* or program? or programme or programmes or reduce? or reducing or reminder? or standardi*ed or team).ti. | 1295725 |
| 33 | intervention.ti. | 86936 |
| 34 | (collaborat* or (continuing adj2 education*) or educational or infrastructure? or implementation or innovative or interdisciplin* or inter‐disciplin* or multifacet* or multi‐facet* or organi?ational* or team).ab. | 699653 |
| 35 | (incentive? or complex intervention? or ((physician? or staff) adj3 behavio?r?) or practice pattern? or ((policy or practice?) adj2 (chang* or influenc* or impact))).ti,ab. | 66618 |
| 36 | *continuing education/ or *professional development/ | 11516 |
| 37 | *quality control/ or *medical audit/ or *quality circle/ or *total quality management/ or quality control procedures/ | 72811 |
| 38 | ((quality adj2 (assurance or circle or circles or improv* or management)) or benchmarking).ti,ab. | 169355 |
| 39 | impact.ti. | 228823 |
| 40 | or/32‐39 | 2088625 |
| 41 | exp *ward/ | 80872 |
| 42 | "hospital subdivisions and components"/ or delivery room/ or dental clinic/ or hospital bed/ or hospital department/ or hospital laboratory/ or hospital pharmacy/ or operating room/ or recovery room/ | 105772 |
| 43 | *health care personnel/ | 26158 |
| 44 | (ward or wards or operating room? or (hospital adj3 (unit or units or department?)) or icu or ((emergency or burn or burns or intensive care or stroke or surgical or surgery) adj2 (unit or units or department?)) or (care unit or care units) or staff or person?el).ti. | 141409 |
| 45 | or/41‐44 | 280817 |
| 46 | (random* or placebo* or double‐blind*).tw. | 1292108 |
| 47 | (randomized controlled trial/ or multicenter study/) not 46 | 175177 |
| 48 | or/46‐47 | 1467285 |
| 49 | pretest posttest control group design/ or comparative effectiveness/ or quasi experimental study/ or pilot study/ or intervention study/ | 206437 |
| 50 | 12 | 7575 |
| 51 | and/16,20 | 7230 |
| 52 | 20 and (or/26,31,40) | 6934 |
| 53 | 20 and 45 | 4638 |
| 54 | (or/50‐53) and 48 | 1279 |
| 55 | ((or/50‐53) and 49) not 54 | 313 |
| 56 | intervention?.ti. or (intervention? adj6 (clinician? or collaborat* or community or complex or design* or doctor? or educational or family doctor? or family physician? or family practitioner? or financial or gp or general practice? or hospital? or impact? or improv* or individuali?e? or individuali?ing or interdisciplin* or multicomponent or multi‐component or multidisciplin* or multi‐disciplin* or multifacet* or multi‐facet* or multimodal* or multi‐modal* or personali?e? or personali?ing or pharmacies or pharmacist? or pharmacy or physician? or practitioner? or prescrib* or prescription? or primary care or professional* or provider? or regulatory or regulatory or tailor* or target* or team* or usual care)).ab. | 298473 |
| 57 | (pre‐intervention? or preintervention? or "pre intervention?" or post‐intervention? or postintervention? or "post intervention?").ti,ab. | 22492 |
| 58 | (hospital* or patient?).hw. and (study or studies or care or health* or practitioner? or provider? or physician? or nurse? or nursing or doctor?).ti,hw. | 3425598 |
| 59 | demonstration project?.ti,ab. | 2691 |
| 60 | (pre‐post or "pre test*" or pretest* or posttest* or "post test*" or (pre adj5 post)).ti,ab. | 143168 |
| 61 | (pre‐workshop or post‐workshop or (before adj3 workshop) or (after adj3 workshop)).ti,ab. | 1308 |
| 62 | trial.ti. or ((study adj3 aim?) or "our study").ab. | 1284893 |
| 63 | (before adj10 (after or during)).ti,ab. | 563568 |
| 64 | (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month* or hour? or day? or "more than")).ab. | 19192 |
| 65 | pilot.ti. or (pilot adj (project? or study or trial)).ab. | 123160 |
| 66 | (multicentre or multicenter or multi‐centre or multi‐center).ti. | 58753 |
| 67 | random*.ti,ab. or controlled.ti. | 1240766 |
| 68 | (control adj3 (area or cohort? or compare? or condition or design or group? or intervention? or participant? or study)).ab. | 816313 |
| 69 | ((evaluation or prospective or retrospective) adj study).ti,ab. | 324646 |
| 70 | (utili?ation or programme or programmes).ti. | 82357 |
| 71 | (during adj5 period).ti,ab. | 469193 |
| 72 | ((strategy or strategies) adj2 (improv* or education*)).ti,ab. | 33643 |
| 73 | *experimental design/ or *pilot study/ or quasi experimental study/ | 37343 |
| 74 | ("quasi‐experiment*" or quasiexperiment* or "quasi random*" or quasirandom* or "quasi control*" or quasicontrol* or ((quasi* or experimental) adj3 (method* or study or trial or design*))).ti,ab. | 144420 |
| 75 | ("time series" adj2 interrupt*).ti,ab. | 2015 |
| 76 | or/56‐75 | 6612935 |
| 77 | (rat or rats or cow or cows or chicken? or horse or horses or mice or mouse or bovine or animal?).ti. | 1683512 |
| 78 | (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) and (human/ or normal human/ or human cell/) | 18530697 |
| 79 | (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not 78 | 5938259 |
| 80 | 76 not (or/77,79) | 5869282 |
| 81 | ((or/50‐53) and 48) not (or/77,79) | 1258 |
| 82 | ((or/50‐53) and 49) not 81 | 313 |
| 83 | (50 and 40 and 80) not (or/81‐82) | 2368 |
| 84 | 81 or 82 or 83 | 3939 |
The Cochrane Library
| No. | Search terms | Results |
| #1 | [mh "universal precautions"] | 15 |
| #2 | ((routine practice? or standard or universal or transmission‐based or isolation) and precaution?):ti | 12 |
| #3 | ((standard or universal or transmission‐based or isolation) near/4 precaution?):ab | 51 |
| #4 | ((airborn* or bacteria* or barrier? or blood* or body substanc* or body fluid? or contact or droplet* or hai or infection? or infectious or nosocomial) and precaution?):ti | 18 |
| #5 | ((airborn* or bacteria* or barrier? or blood* or body substanc* or body fluid? or contact or droplet* or hai or infection? or infectious or nosocomial or transmission‐based) near/5 precaution?):ab | 40 |
| #6 | ((mask? or glove? or gown?) and precaution?):ti or ((mask? or glove? or gown?) near/5 precaution?):ab | 7 |
| #7 | body substance? isolation?:ti,ab | 0 |
| #8 | ((icu or intensive care unit?) and precaution?):ti | 0 |
| #9 | ((aseptic or sterile) and precaution?):ti or ((aseptic or sterile) near/5 precaution?):ab | 19 |
| #10 | (precaution? near/4 (communication? or sign? or signage or notif*)):ti,ab | 0 |
| #11 | ((infection? or infectious) and (bundle? or bundling)):ti or ((infection? or infectious) near/5 (bundle? or bundling)):ab | 9 |
| #12 | {or #1‐#11} | 117 |
| #13 | [mh "cross infection"/PC] or [mh "pneumonia, ventilator‐associated"/PC] | 887 |
| #14 | [mh bacteremia/PC] | 293 |
| #15 | [mh "methicillin‐resistant staphylococcus aureus"/PC] | 252 |
| #16 | ((mrsa or methicil* resistant or bacteremia) and (prevent* or reducing or reduce?)):ti | 78 |
| #17 | {or #13‐#16} | 1382 |
| #18 | [mh "cross infection"] or [mh "pneumonia, ventilator‐associated"] or [mh "surgical wound infection"] | 4593 |
| #19 | [mh "infectious disease transmission, professional‐to‐patient"] | 33 |
| #20 | ((hospital? or hospital acquired) near/4 infection?):ti,ab | 107 |
| #21 | (hospital* and infection?):ti,kw | 1463 |
| #22 | ("cross infection?" or hai or nosocomial*):ti,ab | 1423 |
| #23 | (("central line?" or ventilator?) near/4 infection?):ti,ab | 1 |
| #24 | ((health care or healthcare or icu or care unit or care units or ward or wards or ((surgical or intensive care) near/2 (unit? or department?))) near/4 infection?):ti,ab | 252 |
| #25 | [mh "methicillin‐resistant staphylococcus aureus"] or [mh bacteremia] | 1075 |
| #26 | ((mrsa or methicil* resistant or bacterimia) near/4 (prevent* or reducing or reduce?)):ab | 45 |
| #27 | ((surgery or surgical or postop* or post‐operat*) near/4 infection?):ti,ab | 1469 |
| #28 | {or #18‐#27} | 8543 |
| #29 | [mh "catheter‐related infections"] or [mh "prosthesis‐related infections"] or [mh sepsis] | 3910 |
| #30 | [mh catheterization/AE,CO,MO] | 2671 |
| #31 | [mh catheterization] and (infection? or infectious):ti,kw | 589 |
| #32 | (catheter* near/3 infection?):ti,ab | 397 |
| #33 | (sepsis or septic shock or blood* infection? or blood poisoning or bacter?emia* or endotox?emia*):ti,ab | 8186 |
| #34 | {or #29‐#33} | 12736 |
| #35 | [mh "infection control"] or [mh antisepsis] or [mh asepsis] or [mh "blood safety"] or [mh "infection control, dental"] or [mh "patient isolation"] or [mh quarantine] or [mh sterilization] or [mh disinfection] | 1333 |
| #36 | infection control:ab | 10663 |
| #37 | ((infection? near/2 control*) or blood safety or (antisepsis or asepsis or sterili?ation or disinfect*)):ti | 1037 |
| #38 | ((antisepsis or asepsis or sterili?ation or disinfect*) near/7 (procedur* or process or processes or strategy or strategies or strategi? or guideline? or protocol? or pathway? or policy or policies or checklist? or check‐list?)):ab | 116 |
| #39 | [mh "protective devices"] or [mh "eye protective devices"] or [mh masks] or [mh "protective clothing"] or [mh "gloves, protective"] or [mh "gloves, surgical"] or [mh "respiratory protective devices"] | 2411 |
| #40 | (((scrubs or mask or masks or gown or gowns or glove or gloves or gloved or goggle?) near/4 (protect* or infection? or infectious)) or ((eye or eyes or clothing or uniform? or respiratory or equipment) near/2 protective)):ti,ab | 269 |
| #41 | isolation room?:ti,ab | 11 |
| #42 | ((reduce? or reducing or disrupt*) near/2 (transmission? or spread or spreading)):ti,ab | 31 |
| #43 | {or #35‐#42} | 14998 |
| #44 | [mh "hospital units"] or [mh hospitals] or [mh inpatients] | 7922 |
| #45 | [mh "health facilities"] or [mh "academic medical centers"] or [mh "hospitals, teaching"] or [mh "outpatient clinics, hospital"] or [mh surgicenters] or [mh "birthing centers"] or [mh "dental facilities"] or [mh "dental clinics"] or [mh "dental offices"] | 13944 |
| #46 | [mh "hospital departments"] | 3539 |
| #47 | (hospital? or hospitali?ed or ward or wards or (care near/2 unit) or (care near/2 units)):ti or hospital?:so,kw | 7673 |
| #48 | {or #44‐#47} | 18167 |
| #49 | (collaborat* or compliance or comply* or compliant or (continuing near/2 education*) or educational or infrastructure? or implementation or innovative or interdisciplin* or inter‐disciplin* or multifacet* or multi‐facet* or organi?ational or program? or programme or programmes or standardi*ed or team):ti,ab | 82415 |
| #50 | (continuing near/2 education*):kw | 1229 |
| #51 | [mh "quality assurance, health care"] or [mh benchmarking] or [mh "total quality management"] | 3999 |
| #52 | ((quality near/2 (assurance or circle or circles or improv* or management)) or benchmarking):ti,ab | 10395 |
| #53 | impact:ti | 17633 |
| #54 | (incentive? or "complex intervention?" or ((physician? or staff) near/3 behavio?r?) or "practice pattern?" or ((policy or practice?) near/2 (chang* or influenc* or impact))):ti,ab | 2035 |
| #55 | [mh "physician's practice patterns"] or [mh "nurse's practice patterns"] | 1363 |
| #56 | {or #49‐#55} | 109150 |
| #57 | #17 and #56 | 200 |
| #58 | #28 and #43 and #56 | 318 |
| #59 | #34 and #43 and #48 | 174 |
| #60 | #43 and #48 and #56 | 231 |
| #61 | {or #12, #57‐#60} | 762 |
CINAHL (EBSCO)
| No. | Search terms | Results |
| S1 | (MH "Universal Precautions") | 1,350 |
| S2 | TI (routine practice* or standard or universal or transmission‐based or isolation OR ICU OR intensive care) AND TI (precaution OR precautions) | 458 |
| S3 | AB (standard N4 precaution) | 241 |
| S4 | AB (standard N4 precaution) or (universal N4 precaution) or (transmission‐based N4 precaution) or (isolation N4 precaution) | 1,970 |
| S5 | TI (airborn* or bacteria* or barrier* or blood* or body substanc* or body fluid* or contact or droplet* or HAI or infection* or infectious or nosocomial*) AND precaution* | 1,108 |
| S6 | AB ((airborn* or bacteria* or barrier? or blood* or body substanc* or body fluid? or contact or droplet* or hai or infection? or infectious or nosocomial or transmission‐based) N5 precaution?) | 457 |
| S7 | TI (mask* or glove or gloves or goggle or goggles or eye cover*) AND precaution | 69 |
| S8 | TI body substance isolation | 8 |
| S9 | AB body substance isolation | 10 |
| S10 | AB airborne AND precaution | 64 |
| S11 | AB (infection n5 bundle*) or (infectious n5 bundle*) or (infection* n5 bundling) or (infectious n5 bundling) | 87 |
| S12 | TI (infection n5 bundle*) or (infectious n5 bundle*) or (infection* n5 bundling) or (infectious n5 bundling) | 44 |
| S13 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 | 2,656 |
| S14 | ((MH "Cross Infection") OR (MH "Catheter‐Related Infections+") OR (MH "Pneumonia, Ventilator‐Associated")) AND (TI (compliance or prevent* or recommend* or guideline* or intervention* or collaborat* or interdisciplin* or multidisciplin* or multi‐disciplin* or bundle* or program or programme or programmes) OR AB (staff compliance or collaborat* or interdisciplin* or multidisciplin* or multi‐disciplin* or bundle*)) | 3,986 |
| S15 | (MH "Disease Transmission, Professional‐to‐Patient") | 402 |
| S16 | (MH "Cross Infection/PC") OR (MH "Cross Infection") OR (MH "Catheter‐Related Infections+") OR (MH "Pneumonia, Ventilator‐Associated") | 22,467 |
| S17 | (MH "Catheter‐Related Infections+/PC") | 2,493 |
| S18 | (MH "Pneumonia, Ventilator‐Associated/PC") | 1,041 |
| S19 | S15 OR S16 OR S17 OR S18 | 22,714 |
| S20 | TI ("infection control") OR AB ("infection control") | 7,278 |
| S21 | TI (best practice or bundle* or checklist* or (clinical N2 (pathway* or protocol)) or collaborativ* or communication* or compliance or coordinated or cross‐disciplin* or decreas* or educational or (education N3 (continuing or staff or resident* or physician* or nurse or nurses)) or evidence or guideline* or handoff* or impact or implement* or initiative* or intervention or interdisciplin* or inter‐disciplin* or "length of stay" or multidimensional or multi‐dimensional or mutlidisciplin* or multi‐disciplin* or multifacet* or multi‐facet* or multimodal or multi‐modal or ((patient or care or icu or ward* or surgic*) N3 transfer*) or prevent or preventing or professional development or program* or programme or programmes or promote or promoting or protocol* or quality improvement or reminder* or stewardship or strategies or strategy or team* or workshop) or AB (best practice or bundle or bundles or bundled or checklist* or educational or (clinical N2 (pathway* or protocol)) or collaborativ* or evidence‐based or (guideline* N2 (adher* or impact or implement*)) or implementation or initiative* or interdisciplin* or inter‐disciplin* or multidimensional or multi‐dimensional or mutlidisciplin* or multi‐disciplin* or multifacet* or multi‐facet* or multimodal or multi‐modal or "professional development" or "quality improvement" or stewardship or team‐based or workshop*) | 428,439 |
| S22 | S13 OR S14 | 6,365 |
| S23 | S19 AND S20 | 2,690 |
| S24 | S22 OR S23 | 8,266 |
| S25 | S21 AND S24 | 3,836 |
| S26 | PT randomized controlled trial | 30,868 |
| S27 | PT clinical trial | 52,904 |
| S28 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) | 120,165 |
| S29 | (MH "Clinical Trials+") | 140,807 |
| S30 | (MH "Random Assignment") | 34,317 |
| S31 | S26 OR S27 OR S28 OR S29 OR S30 | 205,903 |
| S32 | PT randomized controlled trial | 30,868 |
| S33 | PT clinical trial | 52,904 |
| S34 | PT research | 995,969 |
| S35 | (MH "Randomized Controlled Trials") | 30,208 |
| S36 | (MH "Clinical Trials") | 87,600 |
| S37 | (MH "Intervention Trials") | 6,173 |
| S38 | (MH "Nonrandomized Trials") | 183 |
| S39 | (MH "Experimental Studies") | 15,245 |
| S40 | (MH "Pretest‐Posttest Design+") | 28,032 |
| S41 | (MH "Quasi‐Experimental Studies+") | 8,877 |
| S42 | (MH "Multicenter Studies") | 21,695 |
| S43 | (MH "Health Services Research") | 7,568 |
| S44 | TI (randomis* or randomiz* or randomly) OR AB (randomis* or randomiz* or randomly) | 120,165 |
| S45 | TI (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) OR AB (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) | 811,751 |
| S46 | S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 | 1,349,318 |
| S47 | S31 OR S46 | 1,351,036 |
| S48 | S25 AND S47 | 2,315 |
ClinicalTrials.gov
barrier precautions OR universal precaution* OR standard precaution* OR transmission based precaution* OR isolation precaution* OR body substance isolation
WHO International Clinical Trials Registry Platform (ICTRP)
standard precaution* universal precaution* transmission based precaution* isolation precaution*
Appendix 2. Worksheet for assessing the certainty of evidence across studies
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness² | Imprecision | Other² | Certainty (overall score)4 |
|
Intervention: education vs no education Outcome: rates of observed adherence to Standard Precautions | |||||||
| 3 | 2 RCTs, 1 NRCT (3) |
Serious risk of bias (‐.5) |
Important inconsistency in effect sizes (‐.5) |
No serious indirectness | No serious imprecision | None |
Low (2) |
|
Intervention: education vs no education Outcome: knowledge | |||||||
| 2 | 1 RCT, 1 NRCT (3) |
Serious risk of bias (‐.5) |
No important inconsistency in effect sizes |
No serious indirectness | No serious imprecision | None |
Low (2.5) |
|
Intervention: education with visualisation vs no visualisation Outcome: rates of observed adherence to Standard Precautions | |||||||
| 1 | 1 RCT (4) |
Serious risk of bias (‐.5) |
No important inconsistency in effect sizes |
No serious indirectness | No serious imprecision | None |
Moderate (3.5) |
|
Intervention: education with visualisation vs no visualisation Outcome: knowledge | |||||||
| 1 | 1 RCT (4) |
Serious risk of bias (‐.5) |
No important inconsistency in effect sizes |
No serious indirectness | No serious imprecision | None |
Moderate (3.5) |
|
Intervention: education with infection control support vs no intervention Outcome: rates of observed adherence to Standard Precautions | |||||||
| 2 | 2 cluster RCTs (4) |
Serious risk of bias (‐.5) |
Important inconsistency in effect sizes (‐.5) |
No serious indirectness | Serious imprecision (‐.5) |
Did not do a matched analysis |
Low (2.5) |
|
Intervention: education with infection control support vs no intervention Outcome: rates of MRSA colonisation | |||||||
| 1 | 1 cluster RCT (4) |
Serious risk of bias (‐.5) |
No important inconsistency in effect sizes |
No serious indirectness | No serious imprecision | Did not do a matched analysis |
Moderate (3.5) |
|
Intervention: peer evaluation vs no intervention Outcome: rates of observed adherence to Standard Precautions | |||||||
| 1 | 1 RCT (4) |
Serious risk of bias (‐.5) |
No important inconsistency in effect sizes |
No serious indirectness | No serious imprecision | None |
Moderate (3.5) |
|
Intervention: checklist and cues vs no intervention Outcome: rates of observed adherence to Standard Precautions | |||||||
| 1 | 1 RCT (4) |
Serious risk of bias (‐.5) |
Important inconsistency in effect sizes (‐.5) |
No serious indirectness | No serious imprecision | None |
Moderate (3) |
| NRCT: non‐randomised (controlled) trial; RCT: randomised (controlled) trial. | |||||||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baldwin 2010.
| Methods | Design of study: randomised trial (cluster) Multi‐centre Period of study: January 2007 to August 2008 Follow‐up: 12 months Setting: Northern Ireland, Ireland, UK |
|
| Participants | N = Nursing homes were randomised to intervention (n = 16) or control (n = 16) with a total of 793 residents (intervention, n = 392 randomised, and n = 234 analysed at 12 months; control, n = 401 randomised, and n = 244 analysed at 12 months) and 338 staff. Before random allocation, nursing homes were matched using baseline data, then 1 nursing home in each pair was randomly allocated to either control or intervention via NQuery. Sex: intervention group, 28% male; control group, 32% male (information related to residents). The gender for staff participants was not reported. Age: intervention group, mean 84 years old; control group, mean 82 years old (information related to residents). The age of staff participants was not reported. Inclusion criteria: all residents aged ≥ 65 years were eligible; nursing home staff (all occupations) Exclusion criteria: terminally ill, those attending on a daycare basis only |
|
| Interventions | The intervention group consisted of a training session that was 2 hours in length and included lectures and DVD presentations. Training sessions also included practical demonstrations of hand hygiene and decontamination of both equipment and the environment. In addition, group members were given their baseline infection control scores and information about how practice could be improved. Some staff were selected to act as infection control link workers. They were given 5 additional hours of training. Their role was to reinforce good infection control. Training sessions were repeated twice (at 3 months and at 6 months). Control sites followed their usual practice and did not receive any training or feedback nor any infection control link workers. |
|
| Outcomes | Investigators collected multiple specimens from both residents and staff and calculated MRSA rates as the primary outcome. The secondary outcome was change in scores on an infection control audit that assessed, via observation, 10 separate types of practice standards. | |
| Notes | Researchers did not perform a matched analysis. Funding source: Health and Social Services Fellowship, Public Health Agency, Northern Ireland Declaration of interest: none declared We contacted the authors on 9 April 2015, to request clarification and received some information from them. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | They used Nquery to randomly allocate 1 of each pair to group. |
| Allocation concealment (selection bias) | Low risk | Nursing homes were allocated to group at the start of the study, following assessment of baseline data. |
| Were baseline outcome measurements similiar? | Low risk | Matched on baseline rates |
| Were baseline characteristics similar? | Low risk | Matched on baseline characteristics |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At 12 months, 40.3% and 39.1% of residents were lost from intervention and control groups, respectively. Researchers could not assess changes in personnel. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | In the discussion, investigators reported that it was not possible to blind participants to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Researchers conducted the audits and were not blinded to group, although they did use another infection control nurse who was blinded to the allocated groups to conduct some of the audits and found those results similar to their own. |
| Was the study adequately protected against contamination? | Low risk | Unlikely control group would get intervention |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Carrico 2007.
| Methods | Design of study: randomised trial Single‐centre Period of study: January to March 2005 Follow‐up: 3 months Setting: University Medical Center, Kentucky, USA |
|
| Participants | N = 20 randomised emergency department registered nurses Sex: Intervention group participants were 100% female and control group participants were 90% female and 10% male. Mean age: Intervention group mean age was 38 years and control group mean age was 37 years. Inclusion criteria: nurses who were employed by the hospital Exclusion criteria: mobile or per diem nurses |
|
| Interventions | Intervention group received standard classroom training with supplemental training that consisted of visual demonstration of respiratory particle dispersion. Control group received standard training classes only related to mechanisms of disease transmission, Standard Precautions, and appropriate use of PPE. |
|
| Outcomes | Knowledge was assessed before classroom training and then on its completion, via a questionnaire; knowledge scores were calculated. During the weeks after training, participants were observed in the clinical setting during interactions with patients who had respiratory symptoms. Two trained observers evaluated use of PPE during the interaction. No baseline assessment of PPE use was performed. | |
| Notes | Funding source: Research Foundation for Prevention of Complications Associated With Health Care Declaration of interest: no information given We contacted the trial authors on 9 April 2015, to request clarification related to sources of bias. We received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation to group was done at the start of the study, but the method was not reported. |
| Were baseline outcome measurements similiar? | Unclear risk | Similar knowledge scores at baseline, but use of PPE not evaluated at baseline |
| Were baseline characteristics similar? | Unclear risk | More experience in the intervention group but not clear what effect this would have |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. Not likely possible to blind participants to group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers were blinded to participants' group assignment. |
| Was the study adequately protected against contamination? | Low risk | Unlikely control group would get intervention |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Potential for observer effect |
Huang 2002.
| Methods | Design of study: randomised trial Single‐centre Period of study: September 2000 and January 2001 Follow‐up: 4 months Setting: Second Xiang Ya Teaching Hospital, Central South University, Changsha, Hunan Province, People's Republic of China, China |
|
| Participants | N = 100 randomised nurses and 98 analysed Intervention group (n = 50 randomised and 49 analysed) vs control group (n = 50 randomised and 49 analysed) Sex: All participants were female. Mean age: In the intervention group, 34.7% were < 25 years old, and in the control group, 42.9% were < 25 years old. Inclusion criteria: nurses from all hospital departments including medical and surgical wards, operating rooms, the central supply room, intensive care units, dialysis centre, and obstetrical and gynaecology wards Exclusion criteria: not reported |
|
| Interventions | Intervention consisted of a 2‐hour lecture on blood‐borne pathogens and universal precautions; a 1‐hour demonstration of universal precautions techniques; and a 30‐minute discussion clarifying risks for blood‐borne pathogen exposure in nursing practice. Materials used were pamphlets, printed materials, slides, photographs, and safety devices. The control group did not receive anything; however after data collection, those nurses also received the educational intervention. |
|
| Outcomes | Nurses' knowledge and behaviour about blood‐borne pathogens and universal precautions via a questionnaire adapted from a 30‐item instrument described (Phipps 2002); self‐reported sharps injury; and behaviour assessment via a behaviour observation checklist. Each nurse was observed for 30 minutes. | |
| Notes | Funding source: Yale‐China Association and Becton Dickinson Global Healthcare Fund Declaration of interest: no information given We contacted trial authors on 22 April 2015, to request clarification related to sources of bias. We received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done at the start of the study but the method was not reported. |
| Were baseline outcome measurements similiar? | Low risk | Similar in both groups |
| Were baseline characteristics similar? | Low risk | Similar baseline characteristics |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant from each group was lost to follow‐up. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Was the study adequately protected against contamination? | Unclear risk | Unlikely control group would get intervention. However, participants were from the same hospital and might have discussed the content with each other. |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Potential for observer effect |
Moongtui 2000.
| Methods | Design of study: randomised trial Single‐centre Period of study: September 1997 to February 1998 The ED, which had 36 available participants and was the largest of the available units, formed 1 group. Healthcare workers from the other 3 units were combined to form the second group, with 55 participants. Follow‐up: 12 weeks Setting: Maharaj Nakom Chiangmai Hospital, a government‐owned tertiary healthcare centre in a city in northern Thailand |
|
| Participants | N = 99 randomised and 91 analysed Sex: F/M total sample, 73/18; intervention group, 18/18; control group, 43/12 Mean age: total sample, 29.9 years old; intervention group, 29.7 years old; control group, 30.0 years old Inclusion criteria: full‐time registered nurses, practical nurses, and patient care aides who had worked in the ED, trauma unit, neurological intensive care unit, and emergency surgical unit for ≥ 1 month Exclusion criteria: worker who declined to sign the consent form |
|
| Interventions | Intervention group (n = 36 healthcare workers in the ED and n = 55 healthcare workers in the neurological ICU, trauma unit, and emergency surgical unit) Intervention group consisted of 4 phases. Phase I (baseline assessment): All participants completed the Modified Beliefs Assessment of Bloodborne Diseases tool to assess knowledge and beliefs about blood‐borne diseases (BBDs) and use of UPs. Phase 2 (baseline observation): All participants were observed for ≥ 1 hour, until a minimum of 15 opportunities to use handwashing or glove wearing had occurred. Phase 3 (intervention‐observation phase): All participants were observed as before. In addition, those in the intervention group were educated about peer evaluation, including goals, benefits, and obstacles of peer evaluation. They then began to implement peer evaluation using the Peer Feedback Assessment Tool. Peer feedback results were posted on the bulletin board on the unit a total of 11 times. Phase 4 (postintervention observation phase, 4 weeks after the intervention was completed): All participants in both groups were again directly observed for UP‐related practices by the investigator, as before. Control group did not receive any intervention. |
|
| Outcomes | Observed UP‐related adherence rates via a modified Universal Precautions Assessment tool; perceived severity of belief about BBDs, perceived benefits of use of UP, perceived barriers to use of UP, cues to UP action, and perceived self‐efficacy were measured in a questionnaire and used as covariates rather than outcomes of interest. | |
| Notes | Funding source: no information given Declaration of interest: no information given We contacted the authors on 22 April 2015, to request clarification related to sources of bias. We received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Done at the start of the study but method was inappropriate |
| Were baseline outcome measurements similiar? | Unclear risk | Control group had higher adherence rates at baseline. |
| Were baseline characteristics similar? | Unclear risk | Similar baseline characteristics; more females in control group but unlikely to make a difference |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss of 8 participants was not explained and the distribution across groups was not identified. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. Unclear if participants knew what group they were in and whether it would make a difference. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The researcher conducted the observations and was not blinded to group. |
| Was the study adequately protected against contamination? | Low risk | Unlikely control group would get intervention |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Potential for observer effect |
Mukti 2000.
| Methods | Design of study: non‐randomised trial Multi‐centre Period of study: not reported Follow‐up: 6 months after the intervention Setting: ED of 2 hospitals (public hospital, Sardjito; private hospital, PKU) in Yogyakarta, Indonesia |
|
| Participants | N = 55 healthcare workers (44 nurses and 11 doctors) (divided, with 35 in the intervention group and 20 in the control group) Sex: 39 female and 16 male Mean age: 32 years old Inclusion criteria: all full‐time health workers in the ED of both hospitals Exclusion criteria: not reported |
|
| Interventions | Intervention group (n = 35) at Sardijto: Participants received an intervention of academic detailing over 2 interviews, in which they discussed principles of UP and how to perform certain procedures safely. Doctors and nurses got the same education, but a senior doctor did the detailing for physicians, and a trained senior nurse did the detailing for nurses. Stickers and posters were used on the walls of the unit to summarise key points about UP. They were changed after 1 month. Control group at PKU (n = 20): no intervention |
|
| Outcomes | Knowledge and attitudes were assessed via an 87‐item questionnaire. A trained nurse observer from the unit observed each participant 3 times over a 30‐minute period and assessed adherence with UP using a checklist. Knowledge and adherence scores were calculated. | |
| Notes | Funding source: World AIDS Foundation Declaration of interest: no information given We contacted trial authors on 22 April 2015, to request clarification related to sources of bias. We received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised trial |
| Allocation concealment (selection bias) | High risk | Non‐randomised trial |
| Were baseline outcome measurements similiar? | Unclear risk | Control group had higher adherence rates at baseline. |
| Were baseline characteristics similar? | Unclear risk | Similar baseline characteristics; more females in control group but unlikely to make a difference. Significantly more intervention group participants had previous training re UP, but it is unclear what difference this would make. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 35 in the intervention group and 19 of 20 in the control group were assessed in the post‐intervention period. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. Unclear if they were blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trained observer was a senior nurse in the department. |
| Was the study adequately protected against contamination? | Low risk | Unlikely control group would get intervention as participants were from different institutions |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Potential for observer effect |
Ong 2013.
| Methods | Design of study: randomised trial; cross‐over study with randomisation done for transfers instead of radiology porters Four study groups: 2 that each had a single intervention, 1 with both interventions together, and 1 with no intervention Single‐centre Period of study: March 2010 to June 2010 Follow‐up: 4 months Setting: teaching hospital in Australia |
|
| Participants | N = 11 radiology porters observed over 300 transfers randomised (analysed 63 transfers in checklist group, 49 transfers in cue group, 40 transfers in checklist + cue group, and 148 transfers in control group) Sex: not reported Mean age: not reported Inclusion criteria: all radiology porters and transfers between radiology and inpatient wards (with the exception of ED and ICU) Exclusion criteria: radiology porters and transfers between emergency and intensive care units |
|
| Interventions | This study examined the effectiveness of 2 simple interventions with 3 intervention groups (n = 152 transfers) vs control group (n = 148) Interventions consisted of: • a checklist to promote proactive communication • a coloured cue to enhance the prominence of written information |
|
| Outcomes | The primary outcome measure was rate of adherence with infection control precautions by porters when transferring patients between inpatient wards and radiology. Secondary outcome measures included: • adherence to the pre‐transfer checklist • any adverse effects caused by the interventions • participants’ reactions to interventions, assessed through informal interviews Researchers shadowed all transfers (including those involving non‐infectious patients) so participants would not know intent was adherence with infection control precautions. |
|
| Notes | Funding source: Australian Research Council; Australian Government National Health and Medical Research Council Declaration of interest: none declared We contacted trial authors on 22 April 2015 to request clarification related to sources of bias. We received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | They used a computerised random number generator; their unit of randomisation was the episode of care ‐ not the participants. Because of cancellation of transfers, they had to use alternative strategies to ensure balance between groups. |
| Allocation concealment (selection bias) | High risk | Allocation procedures were altered during the course of the study. |
| Were baseline outcome measurements similiar? | Low risk | Same participants in all arms |
| Were baseline characteristics similar? | Low risk | Same participants in all arms |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of missing data |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | They said they had blinded participants to the true intent of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Was the study adequately protected against contamination? | Unclear risk | Same participants in all groups; porters might have remembered the checklist |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Potential for observer effect |
Rao 2009.
| Methods | Design of study: randomised trial (cluster) Multi‐centre Period of study: October 2005 to February 2007 Follow‐up: not reported Setting: nursing homes in South London, London, UK |
|
| Participants | N = 12 nursing homes were randomised ‐ 6 to the intervention group (n = 300 residents) and 6 to the control group (n = 265 residents), via matched pair randomisation Sex: not reported Age: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions |
Intervention group: The intervention used an infection control team to support practice. The team provided training for healthcare workers and other nursing home staff for prevention and control of MRSA, and other common infections. The team also provided general training on infection control including aspects of environmental cleanliness, hand hygiene, sharps safety, and disposal of clinical waste. Those in the intervention group also received personal alcohol‐containing gels to improve hand hygiene. In addition, 24‐hour telephone support was available for management of specific infection control problems. Control group: The control group did not receive any intervention. |
|
| Outcomes | The primary outcome measure was adherence with infection control guidelines set out in the infection control audit tool that assessed multiple practices. Trained observers conducted the observations. | |
| Notes | They did not do a matched analysis. Funding source: Dunhill Medical Trust; Ecolab Declaration of interest: no information given We contacted trial authors on 2 April 2015, to request clarification related to sources of bias. We received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Methods were not reported. |
| Were baseline outcome measurements similiar? | Unclear risk | Matched on number of residents but considerable variability in both groups |
| Were baseline characteristics similar? | Unclear risk | Matched on number of residents but differences in size and configuration, staffing |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All nursing homes completed the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Was the study adequately protected against contamination? | Low risk | Unlikely control group would get intervention, as participants were from different institutions |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Wright 1997.
| Methods | Design of study: randomised trial Single‐centre Period of study: not reported Follow‐up: post‐test data collected over a period of 1 month Setting: an acute care facility in an urban city in Arkansas, providing services to patients experiencing short‐term illness or trauma |
|
| Participants | N = 60 randomised nurses: 30 were randomly assigned to the intervention group (Group A) and 30 to the control group (Group B) Sex: 56 female and 4 male (information retrieved through an unpublished data/thesis) Mean age: 35.6 years Inclusion criteria: nurses selected as study population based on nurses' opportunities to have frequent patient contact and to practice a wide variety of universal precautions‐related behaviours Exclusion criteria: not reported |
|
| Interventions | Intervention group (n = 30): consisted of computer‐assisted instruction related to universal precautions Control group (n = 30): no intervention |
|
| Outcomes | Observation for 1 hour or until participant had 12 opportunities for UP‐related activity. Universal Precautions Assessment Tool was used to document actions. Rate of universal precautions‐related behaviours was calculated. |
|
| Notes | Funding source: no information given Declaration of interest: no information given We contacted trial authors on 22 April 2015, to request clarification related to sources of bias. We received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Were baseline outcome measurements similiar? | Low risk | Similar scores |
| Were baseline characteristics similar? | Unclear risk | They did not report baseline characteristics by group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. It would be difficult to blind participants to inclusion in the intervention group, but the effect is unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Was the study adequately protected against contamination? | Low risk | Unlikely control group would get intervention (computer‐assisted education) |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Unclear risk | Potential for observer effect |
BBD: blood‐borne disease.
ED: emergency department.
ICU: intensive care unit.
MRSA: methicillin‐resistant Staphylococcus aureus.
PPE: personal protective equipment.
UP: universal precautions.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Erickson 1996 | Interrupted time‐series design, with inadequate data collection points before and after intervention |
| Gould 1997 | Controlled before‐after design, with 1 intervention group and 1 control group |
Differences between protocol and review
The protocol identified one primary outcome (observed adherence to Standard Precautions) and one secondary outcome (rates of health care‐associated interventions (HAIs)). As most interventions involved education and knowledge is needed as a precursor to behaviour change, we added the following to our outcomes of interest and reported on them: knowledge; attitude toward infection control precautions; and self‐reported behaviours related to infection control precautions.
We added to the objective the phrase "which target healthcare workers" to clarify the focus.
The protocol identified that we would consider interventions that promoted adherence to transmission‐based precautions. We excluded such studies in this review as they relate to care of patients who have a known or suspected specific infection, which is based on different assumptions than use of Standard Precautions. We focussed on Standard Precautions, as healthcare workers need to be able to apply them and reduce transmission of micro‐organisms, even when they do not know or suspect that a patient has an infection.
We used GRADE in rating the certainty of evidence and developed 'Summary of findings' tables.
An intention‐to‐treat analysis (ITT) is one in which all participants in a trial are analysed according to the intervention to which they were allocated, whether they received the intervention or not. For each trial, we planned to report whether or not investigators stated if the analysis was performed according to the ITT principle. If participants were excluded after allocation, we planned to perform an ITT analysis per worst‐case scenario. It was not possible to conduct this ITT analysis because the data were not provided by the studies, and we could not obtain them from trial authors.
We had planned to assess the likelihood of potential publication bias by using funnel plots if we identified at least eight trials for inclusion in a meta‐analysis. As we did not conduct a meta‐analysis owing to heterogeneity of interventions and outcome measures, we did not assess the likelihood of publication bias.
Similarly, it was not possible to perform subgroup analyses or a sensitivity analysis because of lack of relevant data.
We added a new co‐review author (Rafaela Prata).
Contributions of authors
Conceiving the review: Ione Corrêa (IC) and Regina El Dib (RED).
Co‐ordinating the review: RED and Donna Moralejo (DM).
Writing the review: Rafaela Prata (RP), RED, Pasqual Barretti (PB), DM, and IC.
Serving as guarantor for the review (one author): DM.
Reading and checking the review before submission: RP, RED, DM, PB, and IC.
Sources of support
Internal sources
No source, Brazil.
-
Canada, Other.
No source
External sources
No sources of support supplied
Declarations of interest
Rafaela Prata: none known.
Regina El Dib: none known.
Donna Moralejo: none known.
Pasqual Barretti: none known.
Ione Correa: none known.
New
References
References to studies included in this review
Baldwin 2010 {published data only}
- Baldwin NS, Gilpin D, Tunney MM, Kearney M, Crymble L, Cardwell C, et al. Cluster randomised controlled trial of an infection control education and training intervention programme focusing on methicillin‐resistant Staphylococcus aureus in nursing homes for older people. Journal of Hospital Infection 2010;76(1):36‐41. [DOI] [PubMed] [Google Scholar]
Carrico 2007 {published data only}
- Carrico RM, Coty MB, Goss LK, LaJoie AS. Changing health care worker behavior in relation to respiratory disease transmission with a novel training approach that uses biosimulation. American Journal of Infection Control 2007;35(1):14‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2002 {published data only}
- Huang J, Jiang D, Wang X, Liu Y, Fennie K, Burgess J. Changing knowledge, behavior, and practice related to universal precautions among hospital nurses in China. Journal of Continuing Education in Nursing 2002;33(5):217‐24. [DOI] [PubMed] [Google Scholar]
Moongtui 2000 {published data only}
- Moongtui W. Compliance with components of universal precautions guidelines: using peer feedback program as a cue to action among health care workers in Thailand. University of Alabama; Birmingham; Doctoral thesis 1999.
- Moongtui W, Gauthier DK, Turner JG. Using peer feedback to improve handwashing and glove usage among Thai health care workers. American Journal of Infection Control 2000;28(5):365‐9. [DOI] [PubMed] [Google Scholar]
Mukti 2000 {published data only}
- Mukti AG, Treloar C, Suprawimbarti, Asdie AH, D'Este K, Higginbotham N, et al. A universal precautions education intervention for health workers in Sardjito and PKU Hospital Indonesia. Southeast Asian Journal of Tropical Medicine & Public Health 2000;31(2):405‐11. [PubMed] [Google Scholar]
Ong 2013 {published data only}
- Ong MS, Magrabi F, Post J, Morris S, Westbrook J, Wobcke W. Communication interventions to improve adherence to infection control precautions: a randomised crossover trial. BMC Infectious Diseases 2013;13:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2009 {published data only}
- Gopal RG, Jeanes A, Russell H, Wilson D, Atere‐Roberts E, O'Sullivan D. Effectiveness of short‐term, enhanced, infection control support in improving compliance with infection control guidelines and practice in nursing homes: a cluster randomized trial. Epidemiology & Infection 2009;137(10):1465‐71. [DOI] [PubMed] [Google Scholar]
Wright 1997 {published data only}
- Mitchell BW. The influence of computer assisted instruction on the rate of universal precautions related behaviours. University of Alabama; Birmingham; Doctoral thesis 1995.
- Wright BJ, Turner JG, Daffin P. Effectiveness of computer‐assisted instruction in increasing the rate of universal precautions‐related behaviours. American Journal of Infection Control 1997;25(5):426‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Erickson 1996 {published data only}
- Erickson TB, VanRooyen MJ, Werbiski P, Mycyk M, Levy P. Emergency medicine education intervention in Rwanda. Annals of Emergency Medicine 1996;28(6):648‐51. [DOI] [PubMed] [Google Scholar]
Gould 1997 {published data only}
- Gould D, Chamberlain A. The use of a ward‐based educational teaching package to enhance nurses' compliance with infection control procedures. Journal of Clinical Nursing 1997;6(1):55‐67. [DOI] [PubMed] [Google Scholar]
Additional references
Adebayo 2015
- Adebayo O, Labiran A, Imarhiagbe L. Standard Precautions in clinical practices: a review. International Journal of Health Sciences and Research 2015;5:521‐8. [Google Scholar]
Allegranzi 2011
- Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health‐care‐associated infection in developing countries: systematic review and meta‐analysis. Lancet 2011;377:228‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Andersson 2010
- Andersson AE, Bergh I, Karlsson J, Nilsson K. Patients' experiences of acquiring a deep surgical site infection: an interview study. American Journal of Infection Control 2010;38(9):711‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Carvalho 2013
- Carvalho NP, Nogueira PC, Godoy S, Mendes IA. Measures of knowledge about Standard Precautions: a literature review in nursing. Nurse Education in Practice 2013;13(4):244‐9. [DOI] [PubMed] [Google Scholar]
EPOC 2013
- Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC Resources for Review Authors. Oslo: Norwegian Knowledge Centre for the Health Services. Available from http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors; 2013.
EPOC 2015a
- Effective Practice, Organisation of Care (EPOC). EPOC Taxonomy. Available from https://epoc.cochrane.org/epoc‐taxonomy; 2015.
EPOC 2015b
- Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for Review Authors. Available from http://www.epoc.cochrane.org/epoc‐specific‐resources‐review‐authors; 2015.
EPOC 2016
- Effective Practice, Organisation of Care (EPOC). What study designs should be included in an EPOC review?. EPOC Resources for Review Authors. Oslo: Norwegian Knowledge Centre for the Health Services. Available from http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors; 2016.
Gammon 2008
- Gammon J, Morgan‐Samuel H, Gould D. A review of the evidence for suboptimal compliance of healthcare practitioners to stand/universal infection control precautions. Journal of Clinical Nursing 2008;17(2):157‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gould 2017
- Gould DJ, Moralejo D, Drey N, Chudleigh JH, Taljaard M. Interventions to improve hand hygiene compliance in patient care. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD005186.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt G, Oxman A, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello Y, et al. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hessels 2016
- Hessels AJ, Larson EL. Relationship between patient safety climate and standard precaution adherence: a systematic review of the literature. Journal of Hospital Infection 2016;92(4):349‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kevitt 2015
- Kevitt F, Hayes B. Sharps injuries in a teaching hospital: changes over a decade. Occupational Medicine (London) 2015;65(2):135‐8. [DOI] [PubMed] [Google Scholar]
Oliveira 2010
- Oliveira AC, Cardoso CS, Mascarenhas D. Contact precautions in the intensive care unit: factors that facilitate or hamper [Precauções de contato em unidade de terapia intensiva: fatores facilitadores e dificultadores]. Revista da Escola de Enfermagem da USP 2010;44(1):161‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Phipps 2002
- Phipps W, Honghong W, Min Y, Burgess J, Pellico L, Watkins CW, et al. Risk of medical sharps injuries among Chinese nurses. American Journal of Infection Control 2002;30(5):277‐82. [DOI] [PubMed] [Google Scholar]
Picheansanthian 2015
- Picheansanthian W, Chotibang J. Glove utilization in the prevention of cross transmission: a systematic review. JBI Database of Systematic Reviews and Implementation Reports 2015;13(4):188‐230. [PUBMED: 26447080] [DOI] [PubMed] [Google Scholar]
Porto 2016
- Porto JS, Marziale MH. Reasons and consequences of low adherence to standard precautions by the nursing team. Revista Gaucha de Enfermagem 2016;37(2):e57395. [DOI] [PubMed] [Google Scholar]
Powers 2016
- Powers D, Armellino D, Dolansky M, Fitzpatrick J. Factors influencing nurse compliance with Standard Precautions. American Journal of Infection Control 2016;44(1):4‐7. [DOI] [PubMed] [Google Scholar]
Public Health Agency of Canada 2012
- Public Health Agency of Canada. Routine practices and additional precautions for preventing the transmission of infection in healthcare settings. Ottawa 2012. [PubMed]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Siegel 2007
- Siegel JD, Rhinehart E, Jackson M, Chiarelho L. The Healthcare Infection Control Practices Advisory Committee. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. American Journal of Infection Control 2007;35(10 Suppl 2):S64‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. Report on the burden of healthcare‐associated infection worldwide: a systematic review of the literature, 2011. www.who.int/gpsc/country_work/burden_hcai/en/index.html (accessed 17 September 2013).
References to other published versions of this review
El Dib 2013
- Corrêa I, Moralejo D, Barretti P. El Dib RP. Interventions to improve adherence to guidelines on 'Standard Precautions' for the control of healthcare‐associated infections. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010768] [DOI] [PMC free article] [PubMed] [Google Scholar]


