Abstract
According to the social intelligence hypothesis, understanding the challenges faced by social animals is key to understanding the evolution of cognition. In structured social groups, recognising the relationships of others is often important for predicting the outcomes of interactions. Third-party relationship recognition has been widely investigated in primates, but studies of other species are limited. Furthermore, few studies test for third-party relationship recognition in the wild, where cognitive abilities are deployed in response to natural socio-ecological pressures. Here, we used playback experiments to investigate whether wild jackdaws (Corvus monedula) track changes in their own relationships and the relationships of others. Females were presented with ‘infidelity simulations’: playbacks of their male partner copulating with a neighbouring female, and their male neighbour copulating with another female, against a congruent control. Our results showed substantial inter-individual variation in responses, but females did not respond more strongly to infidelity playbacks, indicating that jackdaws may not attend and/or respond to relationship information in this experimental context. Our results highlight the need for further study of relationship recognition and other cognitive traits that facilitate group-living in the wild, particularly in non-primates and in a wider range of social systems.
Subject terms: Animal behaviour, Behavioural ecology
Introduction
The social intelligence hypothesis posits that the sophisticated cognitive abilities seen in some species may have arisen due to the selection pressures associated with group living1,2. Several studies provide support for the social intelligence hypothesis, linking cognitive performance or brain size measures with various aspects of sociality2–7. However, other studies have shown conflicting results8–10 (see11,12 for a detailed discussion) and the social intelligence hypothesis remains controversial. To determine whether social life favours the evolution of associated cognitive abilities, it is necessary to understand how these cognitive abilities help individuals to navigate a dynamically changing social world.
Social species must solve ecological challenges within a social context12,13. In these cases, the ability to recognise other group members and remember past interactions allows individuals to predict (and potentially manipulate) others’ behaviour14. Although obtaining, processing and applying this knowledge is likely to be cognitively demanding5,13,15, individuals who are more socially competent may derive fitness benefits as a result16–18. In social groups where relationships persist over time, being able to track the relationships of other group members can be useful in predicting the outcomes of interactions14. Knowledge of third-party relationships might allow individuals to adjust their own behaviour appropriately to avoid conflict19–22, solicit and provide support during agonistic interactions5,23–27, and take advantage of mating opportunities28. Third-party relationship recognition has been demonstrated in several primate species, originally leading some authors to suggest that this ability may be confined to the primate order29,30. Observations of agonistic interactions indicate that bonnet macaques (Macaca radiata) solicit support from individuals who are higher-ranking than their opponent23 and chimpanzees (Pan troglodytes) will modify their recruitment screams depending on the dominance rank of bystanders26. Playback experiments also provide evidence that primates track third-party relationships. For instance, vervet monkeys Chlorocebus aethiops pygerythrus20 and chacma baboons Papio hamadryas ursinus19 respond to simulated reversals in the existing dominance hierarchy, demonstrating an understanding of the dominance relationships between other group members. Chimpanzees (P. troglodytes) will avoid aggressive individuals who are socially bonded to their former opponent, for several hours following an agonistic encounter21; and male baboons (P. hamadryas ursinus) track consortships between other males and females in order to obtain sneaky matings28. In vervet monkeys (C. aethiops), playbacks of infant distress calls cause nearby females to look towards the infant’s mother, demonstrating recognition of mother-offspring relationships within the social group31.
Few studies have investigated third-party relationship recognition in non-primates, despite many other species living in complex societies where this ability is expected to be useful. For example, hyenas (Crocuta crocuta) live in complex social groups with multiple hierarchically structured matrilines, similar to many primate societies14. Hyenas will join conflicts to support the higher-ranking individual even if the subordinate member of the fighting dyad is more aggressive, implying knowledge of the dominance relationships that exist in the group24 (but see32). Not only is it important to examine a diverse range of species, but also a diversity of social systems – for instance, little is known about the value of third-party relationship recognition in monogamous systems. Among birds, monogamy is the most common social system and has been argued to be central to the evolution of avian cognition5, although little is known about the cognitive demands associated with maintaining long-term pair bonds. Furthermore, many monogamous bird species live in groups and form stable, individualised relationships with others in addition to their breeding partner5. Corvids exhibit this type of social system, and their sophisticated cognitive abilities make them ideal subjects for investigating the evolution of social cognition33–35. Many corvids form long-term pair bonds and live in colonies characterised by strict dominance hierarchies between bonded pairs36. Empirical evidence supports the idea that recognising social relationships is beneficial in corvid colonies35,37. For example, playback experiments show that captive ravens (Corvus corax) respond to dominance rank reversals, both within their own social group and in a neighbouring group22. Furthermore, observations of wild ravens indicate that victims will reduce the frequency of their distress calls during agonistic encounters, if the bonding partner of their aggressor is present in the vicinity; victims also call more frequently when their own kin are nearby27. Anecdotal reports suggest that rooks (Corvus frugilegus) engage in redirected aggression, where individuals are more likely to attack their aggressor’s partner, or the aggressor of their partner, after a fight5. Finally, ravens will intervene in the affiliative interactions of others that appear to be establishing a strong bond, which is likely to require knowledge of the relationships of group members38.
In the only experimental test of third-party relationship recognition in corvids to date, Massen et al.22 found that ravens (C. corax) become stressed and engage in more self-directed behaviour after hearing simulated encounters that violate their expectation of the existing dominance hierarchy within their own colony. Male subjects also exhibited decreased calling and attention behaviour following simulated rank reversals in a neighbouring group, suggesting that ravens deduce third-party relationships by observation alone. However, this study was conducted under controlled conditions using captive individuals, where subjects could observe interactions between conspecifics very frequently. Consequently, it is not clear to what extent these results reflect the cognitive abilities animals employ in the wild, where a greater number of stimuli compete for individual attention15,39,40. Furthermore, most of the research carried out under natural conditions has involved observations of naturally-occurring behaviour, and there is a lack of experimental evidence for third-party relationship recognition in the wild outside the primate order. To this end, a recent study by Pardo et al.41 describes the first experimental field test for third-party relationship recognition in a non-primate. This study found that acorn woodpeckers initiate defensive behaviour more quickly in response to calls from two birds from different social groups, compared to calls of two birds from the same social group, suggesting that individuals recognise group membership outside of their own social group. However, it is not clear to what extent this indicates knowledge of the dyadic relationship between the two callers, or whether it is possible that subjects were responding to the unfamiliar stimulus of two calls occurring together when those calls had only been heard separately in the past. Consequently, much remains to be determined as to the extent of third-party relationship recognition in non-primates in the wild.
To address this research gap, we conducted an experiment to test whether wild jackdaws (a social corvid, Corvus monedula) track changes in their own relationships and the relationships of other members of their social group. This ability is likely to be useful in jackdaw society: pairs form monogamous bonds and females assume the rank of their male partner in the breeding colony’s strict linear dominance hierarchy42. These hierarchies remain relatively stable over time due to high adult survivorship (c. 80%, although estimates vary) and low rates of ‘divorce’43. For jackdaws, tracking relationships within the colony may allow individuals to avoid conflict with more dominant pairs, especially considering that competition over nest sites can be intense43,44. Relationship tracking may also allow individuals to notice if their partner is engaging in extra-pair copulations. Jackdaws are typically considered to be sexually as well as socially monogamous45,46, with studies to date finding that extra-pair paternity is rare: it has been suggested that the high level of parental investment required to successfully raise offspring may prevent birds from seeking extra-pair copulations46. However, recent findings suggest that extra-pair copulations may not be as uncommon as previously thought47; it may therefore pay females to track their partner’s behaviour.
Following the ‘violation of expectation’ paradigm employed in similar studies20,22,28, we used playback experiments to investigate whether female jackdaws respond to simulations of male infidelity. During mating, including extra-pair copulations, male jackdaws give loud copulation calls48. In a recent study combining acoustic tracking and video surveillance, male jackdaws were recorded emitting copulation calls at the same time as the female was alone on the nest47, suggesting that males do engage in extra-pair copulations and that this should be an ecologically relevant stimulus for the female. Furthermore, in our study population, intruder males are occasionally seen entering nest boxes and attempting to copulate with the incubating female (pers. obs.). Although it is not yet known whether male copulation calls encode information about caller identity, all other jackdaw vocalisations studied to date have been shown to be individually distinct (food calls49, contact calls50 and alarm calls51). Using playbacks of male contact calls and copulation calls in conjunction with female contact calls, we simulated mating events occurring during the egg-laying period of the breeding season, when copulation calls are heard most frequently in the colony (pers. obs.). Contact calls were included to ensure that playback sequences simulated interactions between individuals: contact calls are individually distinctive50 and typically accompany jackdaw copulation events. We used three playback treatments to test whether females track changes in their own relationships and the relationships of other colony members. In the ‘Partner Incongruent’ treatment, the playback simulated the focal female’s partner copulating with a female from a neighbouring nest, and this was expected to elicit a strong response from the focal female. A ‘Neighbour Incongruent’ treatment was designed to test third-party relationship recognition and simulated the male from a neighbouring nest copulating with another female who was not their usual partner. This was predicted to elicit an intermediate response from the focal female, as it violates expectations but does not involve the focal female’s own partner. Using a within-subjects design (Fig. 1), the responses of focal females to both ‘Incongruent’ playbacks were compared to a ‘Congruent’ control predicted to elicit a neutral response (playback of a neighbouring male copulating with their usual partner).
Figure 1.
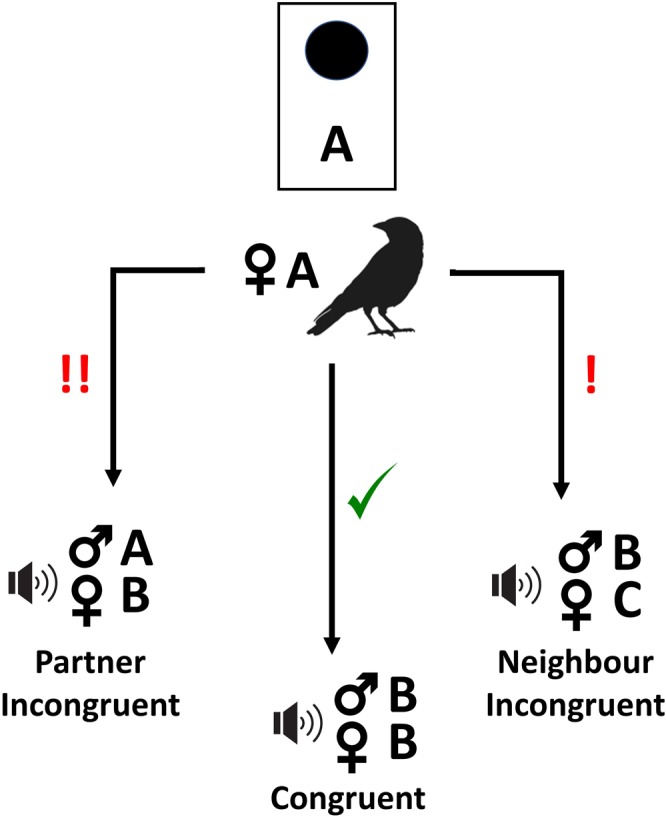
Experimental setup for each nest box. The focal female from nest box A heard three playback presentations. In the ‘Congruent’ control treatment, focal female A heard a playback simulating a copulation event between the neighbouring male from nest box B and the female from nest box B (his usual partner). The focal female (A) was expected to show a weak response to this playback, denoted by a green tick mark. In the ‘Neighbour Incongruent’ treatment, focal female A heard a playback simulating a copulation event between the neighbouring male from nest box B and the female from nest box C (not his usual partner). The focal female (A) was expected to show a stronger response to this playback as it violated expectations, denoted by a red exclamation mark. In the ‘Partner Incongruent’ treatment, the focal female (A) heard a playback simulating a copulation event between her own partner (male from nest box A) with the neighbouring female from nest box B. This playback was expected to elicit the strongest response from focal female A, denoted by two red exclamation marks.
Methods
Ethics Statement
This experiment was carried out with approval from the University of Exeter research ethics committee (2015/974) and following the ASAB Guidelines for the Treatment of Animals in Behavioural Research and Teaching52. Although no birds were handled as part of this study, subjects had been previously captured and ringed by qualified bird ringers licensed by the British Trust for Ornithology and UK Home Office (project licence 30/3261).
Study Population
This experiment was conducted during the 2015–2017 breeding seasons using free-living nest box populations of jackdaws, at three study sites in Cornwall, UK: a village churchyard (Stithians 50°11′26″N, 5°10′51″W; 33 nest boxes), an active farmyard (Pencoose Farm 50°11′56″N, 5°10′9″W; 35 nest boxes), and at the University of Exeter’s Penryn campus (50°17′32″N; 5°11′96″W; 11 nest boxes).
Playback Experiments
Audio Recordings
Nest boxes occupied by breeding jackdaws were fitted with hidden CCTV cameras early in the nest-building phase (late March-early April). A subset of nest boxes selected for this experiment were also fitted with lapel microphones (n = 30). Focal nest boxes were selected with at least one marked individual, and with at least two nearby neighbouring pairs (within 50 m). This was to ensure that neighbours’ contact and copulation calls used in playbacks would be familiar and ecologically relevant stimuli for the focal female.
Audio recordings were made early in the morning (start time: 0700–0900) during late March and early April, when birds were engaged in nest building and copulation. Video recordings were made with digital video recorders (JXD 990) and audio recordings made with multitrack PCM recorders (Olympus LS-100 & Tascam DR-100MKII). Recordings were made daily as required to obtain the necessary vocalisations for use in playback experiments. Each recording ran for 3.5 hours. For some subjects, copulation and contact calls were extracted from recordings obtained during previous seasons (2013–2015) using an identical protocol.
Call extraction
Clear exemplars of contact calls and copulation calls with minimal background noise were extracted from nest box audio recordings and normalised for amplitude using Audacity (www.audacityteam.org). The context of vocalisations and the identity of the caller were ascertained using nest box videos collected alongside the audio recordings. In cases where females vocalised during copulation, female calls were removed from the audio track, leaving only the male copulation call. Extracted calls were arranged into playback files containing a male contact call, followed by a female contact call, followed by a male copulation call, to simulate a copulation event (see Fig. S1 in Supplementary Material). Calls occurred at 2 s intervals to simulate natural calling, and male copulation calls varied in length – this variation was retained to avoid excessively editing the acoustic stimulus and potentially altering important aspects of call structure, but playback duration was later controlled for statistically (see Statistical Analysis). Because of the limited number of suitable copulation call recordings, and the variation in copulation call duration within and between males, some copulation calls appeared in multiple playback trials. Focal females heard the same copulation call from the male neighbour in the Congruent and Neighbour Incongruent treatments, to ensure consistency across the experiment and minimise the potential confounding effects of call duration. Contact calls were not repeated across playback trials.
Experimental Design
This experiment followed a repeat measures design with each focal female (Female A) being assigned three playback files (one for each of the experimental treatments) as follows:
Congruent treatment: Neighbour Male B ‘copulating’ with Neighbour Female B.
Partner incongruent treatment: Partner Male A ‘copulating’ with Neighbour Female B.
Neighbour incongruent treatment: Neighbour Male B ‘copulating’ with Neighbour Female C (Fig. 1).
All experimental trials occurred soon after eggs were laid by the focal female, when females were motivated to remain in the nest box but copulation calls were still being heard frequently around the nesting colony. The order in which focal females received each playback treatment was counterbalanced as far as possible, to ensure a matched design across the experiment. At least 24 hours elapsed between trials for a given focal nest box. All trials were carried out between 09:00 and 18:30, to coincide with peak activity times of the birds44.
We carried out 28 trials across three sites in 2015–2017, at 10 focal nest boxes (two trials were discarded due to camera failure). This was the maximum sample size that could be achieved in this case, due to the limited number of nest boxes with at least two close neighbours and the difficulties in obtaining enough calls from these pairs. All females were colour-ringed, except one bird whose partner was colour-ringed enabling identification of individuals at the nest box. Trials were not carried out in the same area of the colony in the same year. In cases where trials were carried out in the same area in subsequent years, neighbouring birds from previous years were not included in the experiment as focal individuals.
Experimental trials
Prior to trials, a remote-controlled FoxPro Fury 2 loudspeaker (disguised with vegetation to avoid any neophobic responses) was attached to a tripod and placed approximately two-thirds of the distance between the focal nest and the neighbour nest (mean distance 13.6 m between focal nest box and loudspeaker, range 8–21 m). The loudspeaker was set up in the same location for all trials at a nest box. Video recording equipment was also set up (DVR JXD 990) to record female behaviour inside the focal nest box and neighbouring nest box.
Following setup, the experimenter returned to a concealed location a minimum of 50 m away. Playbacks only occurred after the focal female had remained undisturbed in the nest box for at least 5 minutes (no disturbance outside the nest box, female had not left the box or appeared at nest box entrance), and at least 5 minutes following the most recent visit by the male. A baseline period of at least 20 minutes elapsed between the female’s first return to the nest box and presentation of the playback stimulus, to allow focal pairs to return to normal behaviour after setting up equipment.
Behavioural Analysis
Footage of focal females was analysed using BORIS53. The frequency and duration of behaviours exhibited by the focal female were recorded for the 2-minute period following the start of each playback presentation. These included: (i) categorical primary response to playback (looking at the nest box entrance, peeking out of the nest box, or leaving the nest box); (ii) time spent looking at the nest box entrance and peeking out of the nest box. All playbacks were conducted at least 5 minutes after the last visit by the male. There were 5 instances where males returned to the nest box in the two minutes following the playback, and in these cases all female behaviours occurring during and after the male’s visit were discounted.
Twenty percent of videos were analysed by a second coder who was blind to treatment. Inter-rater reliability was analysed using a two-way intraclass correlation coefficient (ICC) and indicated a high level of agreement between coders for all behaviours analysed (time spent looking at entrance in the post-playback period: ICC = 0.98, p < 0.001; time spent peeking in the post-playback period: ICC = 0.87, p = 0.006. In all cases, both coders agreed on the categorical primary response to the playback).
Statistical Analysis
All analyses were carried out in R v3.4.354 with models were built using lme455 and ordinal56. Model plots were examined to ensure that assumptions were met (homogeneity and normality of residuals), and minimum adequate models were obtained via log-likelihood ratio tests.
Behavioural response to playback
In all cases females looked towards the entrance in response to the playback, but some individuals subsequently went on to peek out of the nest box entrance or leave the nest box. The extent of female response was analysed using a cumulative link mixed model (CLMM) using female behaviour (LOOK/PEEK/EXIT) as an ordinal response term. In the model, leaving the nest box was considered the strongest response to the playback (EXIT = 3), followed by peeking out of the nest box from a standing position (PEEK = 2), with looking at the nest box entrance from a seated position taken to be the weakest response (LOOK = 1). Treatment (congruent, partner incongruent or neighbour incongruent) and trial number (1–3) were included as fixed effects and female ID as a random term. The effect of female identity on response was analysed using log-likelihood comparison between the minimal model and a cumulative link model without the random factor57. Four trials were excluded from the analysis as the male returned to the nest box prior to the end of the playback, likely influencing female response.
Time spent looking and peeking following playback
For the two-minute period following the start of the playback, the time that each female spent looking at the nest box entrance and/or peeking out of the nest box was analysed using a general linear mixed model (GLMM) with a Gaussian error distribution. Treatment (congruent, partner incongruent or neighbour incongruent), trial number (1–3) and length of playback were included as fixed effects with focal female ID as a random term. Of the 28 trials, 6 were discarded as the male returned to the nest within two minutes of the playback. One focal female responded to the playback by leaving the box immediately in all three trials, and these were likewise excluded from the analysis. An influential data point was also removed from the model following examination of Cook’s distances: in this case, the focal female spent the full two-minute period looking at the nest box entrance, but was also facing the nest box entrance when the playback started (and therefore may not have represented a reliable response to the playback).
Results
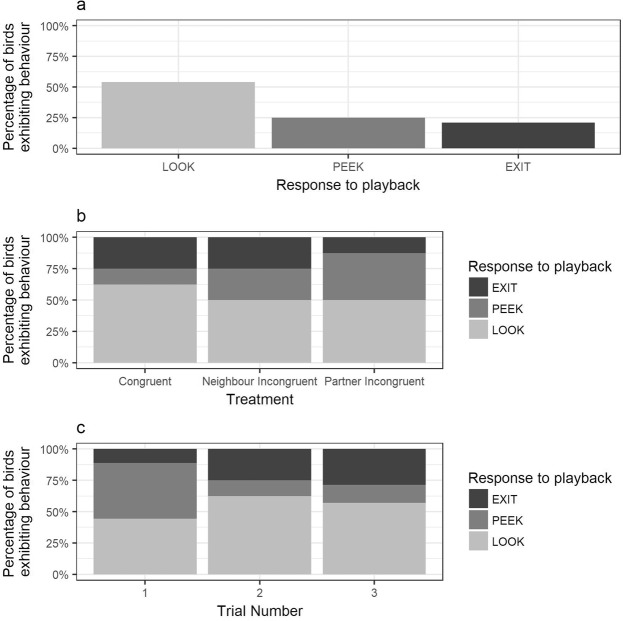
In all cases, females showed some form of response to the playback. These responses ranged from looking at the nest box entrance from a seated position during incubation (“LOOK”, 54% of cases), moving to look out of the nest box entrance (“PEEK”, 25% of cases) and leaving the nest box (“EXIT”, 21% of cases) (see Fig. 2). On no occasion did females vocalise in response to the playback. During the post-playback observation period, there were two occasions when an intruding male (not the focal female’s partner) entered the nest box and attempted to copulate with the focal female. These incidents both occurred during the first trial at the nest boxes in question and approximately half an hour after the playback presentation; once following a ‘Partner Incongruent’ playback (2015) and once following a ‘Congruent’ playback (2017). Intrusions by other males were not observed during any other trials, either before or after the playback presentation.
Figure 2.
Barplots showing behavioural responses of females to playback treatments: looking at the nest box entrance from an incubating position (LOOK, light grey bars); peeking out of the nest box entrance from a standing position (PEEK, mid-grey bars), and leaving the nest box (EXIT, dark grey bars). (a) Percentage of females exhibiting each response across all trials; (b) percentage of females exhibiting each behaviour by treatment (congruent, neighbour incongruent, partner incongruent); (c) percentage of females exhibiting each behaviour by trial number (1–3, treatment presentations counterbalanced across trials).
Behavioural response to playback
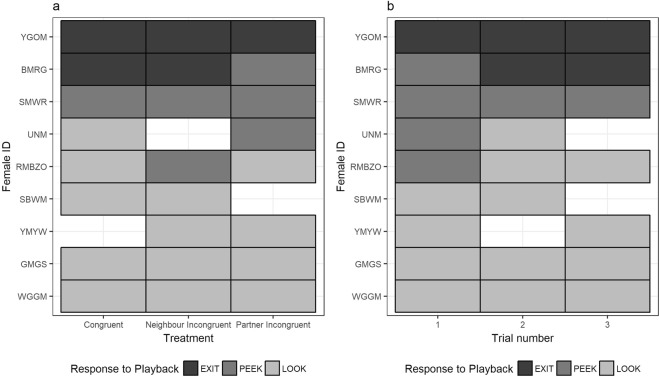
Females responded to playbacks by looking at the nest box entrance (LOOK), peeking out of the entrance from a standing position (PEEK) or leaving the nest box (EXIT). However, the likelihood of females exhibiting these behaviours was similar across treatment groups (CLMM: X2 = 1.21, df = 2, p = 0.55) and was not influenced by trial order (CLMM: X2 = 0.40, df = 2, p = 0.82) (Fig. 2, Table 1). Instead, response to playbacks was strongly influenced by the identity of the female (CLM: X2 = 12.3, df = 1, p < 0.001). For example, females that left the box in one trial were more likely to do so in subsequent trials (Fig. 3, Table 1).
Table 1.
Output of CLMM investigating the effect of treatment (congruent, neighbour incongruent, partner incongruent) and trial number (1–3) on the ordinal response of females to the playback (LOOK = looking at nest box entrance, PEEK = peeking out of nest box entrance, EXIT = leaving the nest box).
| Model parameters | β | SE | z-value | p-value |
|---|---|---|---|---|
| Threshold (response) | ||||
| LOOK|PEEK | 1.47 | 2.74 | 0.54 | |
| PEEK|EXIT | 6.31 | 2.74 | 2.30 | |
| Treatment | ||||
| Congruent (reference) | ||||
| Neighbour incongruent | 1.56 | 1.80 | 0.86 | 0.39 |
| Partner incongruent | −0.13 | 1.63 | −0.08 | 0.94 |
| Trial number | ||||
| Trial 1 (reference) | ||||
| Trial 2 | −0.53 | 1.62 | −0.33 | 0.74 |
| Trial 3 | −0.22 | 1.66 | −0.13 | 0.89 |
| Random effects | Variance | SE | ||
| Female ID | 31.89 | 5.65 | ||
Congruent treatment and Trial 1 are the reference levels, n = 24 observations of 9 females. Values shown from full model, statistically significant effects are given in italics.
Figure 3.
Tile plots showing responses of focal females to the three playbacks, according to (a) treatment (Congruent, Neighbour Incongruent, Partner Incongruent) and (b) trial number (1–3). Female ID (y-axis) shows colour-ring combinations of focal females. Tile colour corresponds to the behavioural response of the female to the playback: looking at the nest box entrance from an incubating position (LOOK, light grey bars); peeking out of the nest box entrance from a standing position (PEEK, mid-grey bars), and leaving the nest box (EXIT, dark grey bars). Blank tiles represent trials where a reliable measure of females’ initial response to the playback could not be obtained.
Time spent looking/peeking following playback
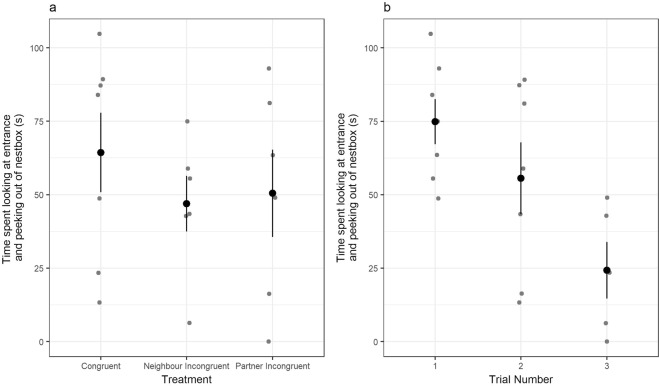
In the two-minutes following the start of the playback, females spent an average of 54 s (±7.3 s) either looking at or peeking out of the nest box entrance. The length of time that females spent looking at or out of the nest box entrance did not differ between treatments (GLMM: X2 = 0.58, df = 2, p = 0.75), and was not influenced by the duration of the playback (GLMM: X2 = 1.12, df = 1, p = 0.29). However, females spent less time looking and peeking following playbacks as trials progressed (GLMM: X2 = 10.13, df = 2, p = 0.006) (Fig. 4, Table 2).
Figure 4.
Plots showing the time spent by focal females looking at the nest box entrance or peeking out of the nest box entrance in the two minutes post-playback, by (a) treatment (congruent, neighbour incongruent, partner incongruent) and (b) trial number (1–3). Grey points represent individual data points (n = 19 observations of 8 females), and black points with error bars denote mean and standard error.
Table 2.
Output of GLMM investigating time spent looking at nest box entrance and peeking out of nest box entrance in the two minutes following the start of playback. Full model includes treatment, trial order and playback duration as fixed effects (statistically significant effects given in italics).
| Fixed effect | β | SE | t-value | |
|---|---|---|---|---|
| Full model | ||||
| Intercept | 101.46 | 22.97 | 4.42 | |
| Treatment | Congruent (reference) | |||
| Neighbour incongruent | −7.27 | 13.0 | −0.56 | |
| Partner incongruent | −9.59 | 13.12 | −0.73 | |
| Trial order | Trial 1 (reference) | |||
| Trial 2 | −16.72 | 12.31 | −1.36 | |
| Trial 3 | −47.20 | 13.55 | −3.48 | |
| Playback duration | −1.33 | 1.24 | −1.08 | |
Congruent treatment and Trial 1 are the reference levels, n = 19 observations of 8 females.
Discussion
We found no effect of treatment on jackdaws’ responses to playbacks, with females behaving in a similar manner following simulations of their partner’s infidelity, their neighbour’s infidelity and a congruent control. There were no significant differences in females’ initial response (looking at the nest box entrance, peeking out of the nest box entrance or leaving the nest box) or the duration of the response (time spent looking at, or out of, the nest box entrance). However, females appear to habituate to playbacks over time, as the length of time females spent investigating the stimulus (looking at or out of the nest box entrance) decreased over successive trials.
Each subject’s initial response to the playback (looking at the nest box entrance, peeking out of the nest box entrance or leaving the nest box) was strongly influenced by the identity of the individual. Inter-individual variation between females was significant, with females’ response during their first trial strongly predicting their response during subsequent trials, regardless of treatment. In terms of the duration of this response (time spent looking at/out of the nest box entrance), none of the test subjects behaved as predicted: we found no evidence of a stronger response to the ‘Partner Incongruent’ or ‘Neighbour Incongruent’ treatments compared to the ‘Congruent’ control. Two females looked/peeked for longer following the infidelity simulation of their partner compared to the control playback, but this may be because these subjects heard their partner’s infidelity simulation first. Overall, these results suggest that individual variation likely plays an important role in influencing subjects’ responses in these types of experiments, yet these individual differences are rarely examined or discussed explicitly in studies of cognition58–60.
Although these results do not provide any evidence that jackdaws track their own relationships and the relationships of others in their social group, this does not necessarily imply that jackdaws are incapable of third-party relationship recognition. Instead, it may be that birds simply failed to demonstrate this ability within the context of our experimental setup. The fact that females failed to respond to simulations of their own partner’s infidelity, as well as the infidelity of a male neighbour, is consistent with this possibility. There are several potential explanations as to why female responses did not differ between experimental treatments. Firstly, the experiment was carried out during an ecologically relevant period when birds were copulating at a high rate compared to other stages in the breeding attempt. It may be that if copulation calls are heard frequently around the colonies at this time, individuals attend to (or ignore) all copulation calls equally. Moreover, it is possible that females do not discriminate between the copulation calls of individual males (although jackdaw contact calls are individually distinct50, and were included in playback sequences to simulate interactions between individuals). Furthermore, if extra-pair copulations are extremely rare45,46, females may not perceive the playback stimulus as an ‘infidelity’. However, recent evidence47 and observations of intruder males in our own study population suggest that extra-pair copulations in jackdaws may occur more commonly than previously thought. For this reason, it seems that it would be beneficial for females to notice when their partner is copulating with another female. If females do perceive the playback stimulus as an ‘infidelity’, perhaps there is no advantage to females in acting on this information (e.g. by leaving the nest to gather more information, or to retaliate against their unfaithful partner61). In a similar experiment, Crockford et al.28 found that subordinate male baboons respond to playbacks of female copulation calls that were indicative of a recent consortship having ended, as these cues provide highly relevant information which may allow them to gain ‘sneaky’ matings. In our study it is possible that, if there is no direct fitness benefit to females, the social information indicating male infidelity is not attended to or acted upon to the same extent. The fact that nest intrusions occurred following two of the playback presentations (where another male entered the focal nest box and attempted to copulate with the resident female) raises the possibility that male jackdaws may eavesdrop on copulation events in a similar way to baboons28. Finally, if male infidelity does not reduce subsequent paternal care, there may be little cost to their female partner. Given the high degree of social monogamy in this species46, it may be that male extra-pair copulation does not merit a response from females. It would be interesting to determine whether male extra-pair copulation behaviour, or playback simulations of male infidelity, influence female behaviour over the long term (e.g. in terms of mate choice, see62).
Females showed habituation to playbacks over time, suggesting that there may be aspects of our experimental setup that were incongruent with naturally-occurring copulation events. For example, the timings of calls in the playback sequence may not be a reliable indication of two birds being in close proximity at the same time. Each playback sequence consisted of a male contact call and female contact call, followed by a copulation call from the same male (Fig. S1 in supplementary material). A pause of two seconds occurred between each call, which represents natural calling rates for individual birds (unpublished data). Playbacks were conducted when the area was quiet and no other birds were heard calling, but in busy areas of the colony where calling is generally frequent, it may be that the calls of multiple birds are frequently heard together without any direct interaction between callers. The fact that all playback calls were emitted from the same direction may have provided an additional cue that calls represent a social interaction; on the other hand, call direction may be difficult for a female jackdaw to discern from inside a nest box. Observations of female responses to naturally-occurring copulation events and male infidelity may shed light on why females failed to respond to our playbacks, and would be an important avenue for future study.
It could be that jackdaws are more likely to respond to relationship changes that influence agonistic encounters, such as changes in dominance rank. Jackdaw colonies are structured according to a linear dominance hierarchy, where females assume the rank of their male partner42. Pairs then compete for food and nest sites, with conflict over nesting cavities being particularly intense43,44. Recognising changes in dominance rank may be of fitness relevance to birds in allowing them to gain access to resources whilst avoiding conflicts that are potentially costly. Playback experiments have demonstrated that primates recognise changes in dominance rank19,20, and hyenas also appear to apply knowledge of third-party relationships during agonistic interactions24 (but fail to demonstrate this ability in other contexts32). Unfortunately, jackdaws do not give dominance calls, which would make an experimental test of knowledge of third-party ranks logistically challenging. Other corvids have been shown to respond to simulated changes in dominance rank, both within their own social group and a neighbouring group22. However, this study was conducted in captivity with small groups of birds housed in close proximity. Birds therefore had extensive opportunities to learn about social relationships by observing frequent interactions between all group members; it is currently unknown whether these opportunities occur similarly under natural conditions. Therefore, the extent of third-party relationships knowledge in the wild, and the contexts in which corvids apply this knowledge, remains to be determined.
This study presents one of the first experimental tests of third-party relationship recognition in a non-primate under natural conditions. To date, only one other field experiment has been conducted on birds, and suggests that acorn woodpeckers are aware of which individuals make up neighbouring groups41. However, it is unclear whether the act of calling together in woodpeckers provides any information about the nature of the dyadic relationship between callers. Here, we used copulation calls, which are directed at specific individuals during a specific type of social interaction, to investigate dyadic and third-party relationship representation. We found no evidence that jackdaws track their own relationships and the relationships of other individuals in their social group. However, we cannot rule out that jackdaws possess this ability, as none of the test subjects responded in a manner consistent with the experimental predictions. Moreover, due to the difficulties in obtaining a sufficient number of calls from close neighbours in the experimental colonies, our sample size (n = 10) is modest (see Methods). Our sample size is in line with similar studies of corvids in captivity, both for tests of social cognition and cognitive abilities more generally22,63–67. It could be that under natural conditions, where subjects’ attention is divided and there are more confounding environmental variables, larger sample sizes are required to detect an effect. This emphasises the need to complement research in the laboratory with rigorous field studies addressing questions related to social cognition.
A growing body of research, both observational and experimental, shows that species that live in complex societies possess knowledge of third-party relationships and other socio-cognitive abilities considered to be relatively ‘sophisticated’. To date, many of these studies have been carried out using captive populations, with field studies mostly confined to primates. More studies are needed in a wider range of species and social systems, especially in a field context where findings may be more likely to accurately reflect the cognitive processes animals use to solve real-world socio-ecological challenges39,40. Studies of this kind would make a valuable contribution to our understanding of social cognition in different species, and how these abilities help individuals to navigate a changing social world.
Supplementary information
Acknowledgments
V.E.L. was supported by the Natural Environment Research Council [NE/L002434/1] and A.T. and G.E.M. were supported by a BBSRC David Phillips Fellowship awarded to A.T. (BB/H021817/1 and BB/H021817/2). We are most grateful to the Gluyas family and staff at Pencoose Farm, Odette Eddy and the people of Stithians where the experiment was carried out. Richard Woods, Charlie Savill, Jenny Coomes and several student volunteers helped to collect call recordings, and Lewis Flintham acted as an independent coder for video analysis.
Author Contributions
V.E.L. and A.T. designed the experiment; V.E.L. ran the experimental trials and analysed the data; G.E.M. provided logistical support and maintained field sites/study populations; V.E.L. and A.T. wrote the manuscript.
Data Availability
Data and R scripts associated with this study are available in the Figshare repository (10.6084/m9.figshare.7825943).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43247-x.
References
- 1.Humphrey, N. The social function of intellect. in Growing points in ethology (eds Bateson, P. P. G. & Hinde, R. A.) 303–317 (Cambridge University Press, 1976).
- 2.Dunbar RIM. The social brain hypothesis. Evol. Anthropol. 1998;6:178–190. doi: 10.1002/(SICI)1520-6505(1998)6:5<178::AID-EVAN5>3.0.CO;2-8. [DOI] [Google Scholar]
- 3.Bond AB, Kamil AC, Balda RP. Social complexity and transitive inference in corvids. Anim. Behav. 2003;65:479–487. doi: 10.1006/anbe.2003.2101. [DOI] [Google Scholar]
- 4.Bond AB, Kamil AC, Balda RP. Serial reversal learning and the evolution of behavioral flexibility in three species of North American corvids (Gymnorhinus cyanocephalus, Nucifraga columbiana, Aphelocoma californica) J. Comp. Psychol. 2007;121:372–379. doi: 10.1037/0735-7036.121.4.372. [DOI] [PubMed] [Google Scholar]
- 5.Emery NJ, Seed AM, von Bayern AMP, Clayton NS. Cognitive adaptations of social bonding in birds. Philos. Trans. R. Soc. B. 2007;362:489–505. doi: 10.1098/rstb.2006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amici F, Aureli F, Call J. Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr. Biol. 2008;18:1415–1419. doi: 10.1016/j.cub.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Ashton BJ, Ridley AR, Edwards EK, Thornton A. Cognitive performance is linked to group size and affects fitness in Australian magpies. Nature. 2018;554:364–367. doi: 10.1038/nature25503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holekamp KE. Questioning the social intelligence hypothesis. Trends Cogn. Sci. 2007;11:65–69. doi: 10.1016/j.tics.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Sayol, F. et al. Environmental variation and the evolution of large brains in birds. Nat. Commun. 7 (2016). [DOI] [PMC free article] [PubMed]
- 10.DeCasien AR, Williams SA, Higham JP. Primate brain size is predicted by diet but not sociality. Nat. Ecol. Evol. 2017;1:1–7. doi: 10.1038/s41559-017-0112. [DOI] [PubMed] [Google Scholar]
- 11.Healy SD, Rowe C. Costs and benefits of evolving a larger brain: Doubts over the evidence that large brains lead to better cognition. Anim. Behav. 2013;86:e1–e3. doi: 10.1016/j.anbehav.2013.05.017. [DOI] [Google Scholar]
- 12.Ashton BJ, Thornton A, Ridley AR. An intraspecific appraisal of the social intelligence hypothesis. Philos. Trans. R. Soc. B. 2018;373:20170288. doi: 10.1098/rstb.2017.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shultz S, Dunbar RIM. The evolution of the social brain: Anthropoid primates contrast with other vertebrates. Proc. R. Soc. B. 2007;274:2429–2436. doi: 10.1098/rspb.2007.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holekamp KE, Sakai ST, Lundrigan BL. Social intelligence in the spotted hyena (Crocuta crocuta) Philos. Trans. R. Soc. B. 2007;362:523–38. doi: 10.1098/rstb.2006.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheney, D. L. & Seyfarth, R. M. Baboon Metaphysics: The Evolution of a Social Mind. (University of Chicago Press, 2008).
- 16.Silk JB, Alberts SC, Altmann J. Social Bonds of Female Baboons. Science. 2003;302:1231–1235. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- 17.Cameron EZ, Setsaas TH, Linklater WL. Social bonds between unrelated females increase reproductive success in feral horses. Proc. Natl. Acad. Sci. 2009;106:13850–13853. doi: 10.1073/pnas.0900639106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell LAD, Tkaczynski PJ, Lehmann J, Mouna M, Majolo B. Social thermoregulation as a potential mechanism linking sociality and fitness: Barbary macaques with more social partners form larger huddles. Sci. Rep. 2018;8:1–8. doi: 10.1038/s41598-017-17765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM. Hierarchical classification by rank and kinship in baboons. Science. 2003;302:1234–1236. doi: 10.1126/science.1087513. [DOI] [PubMed] [Google Scholar]
- 20.Borgeaud C, van de Waal E, Bshary R. Third-party ranks knowledge in wild vervet monkeys (Chlorocebus aethiops pygerythrus) PLoS One. 2013;8:e58562. doi: 10.1371/journal.pone.0058562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittig RM, Crockford C, Langergraber KE, Zuberbühler K. Triadic social interactions operate across time: a field experiment with wild chimpanzees. Proc. R. Soc. B. 2014;281:20133155. doi: 10.1098/rspb.2013.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massen JJM, Pašukonis A, Schmidt J, Bugnyar T. Ravens notice dominance reversals among conspecifics within and outside their social group. Nat. Commun. 2014;5:3679. doi: 10.1038/ncomms4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silk JB. Male bonnet macaques use information about third-party rank relationships to recruit allies. Anim. Behav. 1999;58:45–51. doi: 10.1006/anbe.1999.1129. [DOI] [PubMed] [Google Scholar]
- 24.Engh AL, Siebert ER, Greenberg DA, Holekamp KE. Patterns of alliance formation and postconflict aggression indicate spotted hyaenas recognize third-party relationships. Anim. Behav. 2005;69:209–217. doi: 10.1016/j.anbehav.2004.04.013. [DOI] [Google Scholar]
- 25.Schino G, Tiddi B, Di Sorrentino EP. Simultaneous classification by rank and kinship in Japanese macaques. Anim. Behav. 2006;71:1069–1074. doi: 10.1016/j.anbehav.2005.07.019. [DOI] [Google Scholar]
- 26.Slocombe KE, Zuberbühler K. Chimpanzees modify recruitment screams as a function of audience composition. Proc. Natl. Acad. Sci. 2007;104:17228–17233. doi: 10.1073/pnas.0706741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szipl G, Ringler E, Bugnyar T. Attacked ravens flexibly adjust signalling behaviour according to audience composition. Proc. R. Soc. B. 2018;285:20180375. doi: 10.1098/rspb.2018.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crockford C, Wittig RM, Seyfarth RM, Cheney DL. Baboons eavesdrop to deduce mating opportunities. Anim. Behav. 2007;73:885–890. doi: 10.1016/j.anbehav.2006.10.016. [DOI] [Google Scholar]
- 29.Tomasello, M. & Call, J. Primate Cognition. (Oxford University Press, 1997).
- 30.Tomasello M. Uniquely primate, uniquely human. Dev. Sci. 1998;1:1–16. doi: 10.1111/1467-7687.00002. [DOI] [Google Scholar]
- 31.Cheney DL, Seyfarth RM. Vocal recognition in free-ranging vervet monkeys. Anim. Behav. 1980;28:362–367. doi: 10.1016/S0003-3472(80)80044-3. [DOI] [Google Scholar]
- 32.Holekamp KE, et al. Vocal recognition in the spotted hyeana and its possible implications regarding the evolution of intelligence. Anim. Behav. 1999;58:383–395. doi: 10.1006/anbe.1999.1157. [DOI] [PubMed] [Google Scholar]
- 33.Emery NJ, Clayton NS. The mentality of crows: convergent evolution of intelligence in corvids and apes. Science. 2004;306:1903–7. doi: 10.1126/science.1098410. [DOI] [PubMed] [Google Scholar]
- 34.Güntürkün O, Bugnyar T. Cognition without cortex. Trends Cogn. Sci. 2016;20:291–303. doi: 10.1016/j.tics.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Bugnyar T. Social cognition in ravens. Comp. Cogn. Behav. Rev. 2013;8:1–12. doi: 10.3819/ccbr.2013.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clayton NS, Emery NJ. The social life of corvids. Curr. Biol. 2007;17:R652–6. doi: 10.1016/j.cub.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 37.Boucherie, P. H., Loretto, M. C., Massen, J. J. M. & Bugnyar, T. What constitutes “social complexity” and “social intelligence” in birds? Lessons from ravens. Behav. Ecol. Sociobiol. 73 (2019). [DOI] [PMC free article] [PubMed]
- 38.Massen JJM, Szipl G, Spreafico M, Bugnyar T. Ravens intervene in others’ bonding attempts. Curr. Biol. 2014;24:1–4. doi: 10.1016/j.cub.2014.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thornton A, Lukas D. Individual variation in cognitive performance: developmental and evolutionary perspectives. Philos. Trans. R. Soc. B. 2012;367:2773–83. doi: 10.1098/rstb.2012.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pritchard DJ, Hurly TA, Tello-Ramos MC, Healy SD. Why study cognition in the wild (and how to test it)? J. Exp. Anal. Behav. 2016;105:41–55. doi: 10.1002/jeab.195. [DOI] [PubMed] [Google Scholar]
- 41.Pardo MA, et al. Wild acorn woodpeckers recognize associations between individuals in other groups. Proc. R. Soc. B. 2018;285:20181017. doi: 10.1098/rspb.2018.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salomons HM, Dijkstra C, Verhulst S. Strong but variable associations between social dominance and clutch sex ratio in a colonial corvid. Behav. Ecol. 2007;19:417–424. doi: 10.1093/beheco/arm149. [DOI] [Google Scholar]
- 43.Röell AA. Social behaviour of the jackdaw, Corvus monedula, in relation to its niche. Behaviour. 1978;64:1–124. doi: 10.1163/156853978X00459. [DOI] [Google Scholar]
- 44.Henderson IG, Hart PJB. Provisioning, parental investment and reproductive success in jackdaws Corvus monedula. Ornis Scand. 1993;24:142–148. doi: 10.2307/3676364. [DOI] [Google Scholar]
- 45.Liebers D, Peter H-U. Intraspecific interactions in jackdaws Corvus monedula: A field study combined with parentage analysis. Ardea. 1998;86:221–235. [Google Scholar]
- 46.Henderson IG, Hart PJB, Burke T. Strict monogamy in a semi-colonial passerine: the Jackdaw Corvus monedula. J. Avian Biol. 2000;2:177–182. doi: 10.1034/j.1600-048X.2000.310209.x. [DOI] [Google Scholar]
- 47.Gill, L. F. Studying individual vocal communication in group-living songbirds. (Universität Konstanz, 2016).
- 48.Stowell D, Benetos E, Gill LF. On-Bird Sound Recordings: Automatic Acoustic Recognition of Activities and Contexts. IEEE/ACM Trans. Audio Speech Lang. Process. 2017;25:1193–1206. doi: 10.1109/TASLP.2017.2690565. [DOI] [Google Scholar]
- 49.Zandberg L, Jolles JW, Boogert NJ, Thornton A. Jackdaw nestlings can discriminate between conspecific calls but do not beg specifically to their parents. Behav. Ecol. 2014;25:565–573. doi: 10.1093/beheco/aru026. [DOI] [Google Scholar]
- 50.Stowell, D., Morfi, V. & Gill, L. F. Individual identity in songbirds: signal representations and metric learning for locating the information in complex corvid calls. in Proceedings of Interspeech 1–9, 10.21437/Interspeech.2016-465 (2016).
- 51.Woods RD, Kings M, McIvor GE, Thornton A. Caller characteristics influence recruitment to collective anti-predator events in jackdaws. Sci. Rep. 2018;8:7343. doi: 10.1038/s41598-018-25793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 83, 301–309 (2012). [DOI] [PubMed]
- 53.Friard O, Gamba M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016 doi: 10.1111/2041-210X.12584. [DOI] [Google Scholar]
- 54.R Core Team. R: A language and environment for statistical computing, https://www.r-project.org/ (2017).
- 55.Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 56.Christensen, R. H. B. ordinal - Regression Models for Ordinal Data, http://www.cran.r-project.org/package=ordinal/ (2018).
- 57.Christensen, R. H. B. A Tutorial on fitting Cumulative Link Mixed Models with clmm2 from the ordinal Package. Available at, https://cran.r-project.org/web/packages/ordinal/vignettes/clmm2_tutorial.pdf (2015).
- 58.Sih A, Del Giudice M. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Philos. Trans. R. Soc. B. 2012;367:2762–72. doi: 10.1098/rstb.2012.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffin AS, Guillette LM, Healy SD. Cognition and personality: An analysis of an emerging field. Trends Ecol. Evol. 2015;30:207–214. doi: 10.1016/j.tree.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Cauchoix M, et al. The repeatability of cognitive performance. Philos. Trans. R. Soc. B. 2018;373:20170281. doi: 10.1098/rstb.2017.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valera F, Hoi H, Krištín A. Male shrikes punish unfaithful females. Behav. Ecol. 2003;14:403–408. doi: 10.1093/beheco/14.3.403. [DOI] [Google Scholar]
- 62.Mennill DJ, Ratcliffe LM, Boag PT. Female eavesdropping on male song contests in songbirds. Science. 2002;296:873. doi: 10.1126/science.296.5569.873. [DOI] [PubMed] [Google Scholar]
- 63.Paz-y-Miño-C G, Bond AB, Kamil AC, Balda RP. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430:5–8. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- 64.Dally DM, Emery NJ, Clayton NS. Food-caching Western Scrub-Jays keep track of who was watching when. Science. 2006;312:1662–1665. doi: 10.1126/science.1126539. [DOI] [PubMed] [Google Scholar]
- 65.Seed AM, Tebbich S, Emery NJ, Clayton NS. Investigating physical cognition in rooks, Corvus frugilegus. Curr. Biol. 2006;16:697–701. doi: 10.1016/j.cub.2006.02.066. [DOI] [PubMed] [Google Scholar]
- 66.Kondo N, Izawa E-I, Watanabe S. Crows cross-modally recognize group members but not non-group members. Proc. R. Soc. B. 2012;279:1937–42. doi: 10.1098/rspb.2011.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mikolasch S, Kotrschal K, Schloegl C. Transitive inference in jackdaws (Corvus monedula) Behav. Processes. 2013;92:113–7. doi: 10.1016/j.beproc.2012.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and R scripts associated with this study are available in the Figshare repository (10.6084/m9.figshare.7825943).