The announcement of a second individual who may have been “cured” of HIV infection – or otherwise placed into durable antiretroviral (ART)-free viral remission – has generated great excitement within the scientific community and among people living with HIV. In a case similar to the first cure (the “Berlin Patient”) [1], an HIV-infected man in London (the “London Patient”) underwent an allogeneic bone marrow transplant for treatment of an underlying cancer [2]. The donor was homozygous for a deletion in the CCR5 gene. As CCR5 is a required co-receptor for most HIV strains, the patient's rebuilt immune system was naturally resistant to HIV infection. Another potential cure using this approach was also recently reported (the “Dusseldorf Patient”) [3].
Antiretroviral therapy (ART) has become increasingly safe and effective, transforming HIV infection into a treatable chronic disease. Given the morbidity and mortality associated with bone marrow transplantation, such an approach could never become widely accessible as a cure for HIV disease. Why then has the announcement of the London Patient generated so much interest?
In large part, the focus on curative interventions for HIV has been motivated by the growing realization that additional approaches will be required to reverse the worldwide HIV epidemic. Not everyone is able to obtain or do well with ART. Due to suboptimal medications, the first generation of treated individuals developed highly resistant isolates requiring complex “salvage” regimens. Those on modern regimens can have side effects that, although subtle, become cumulative over years of therapy. People with well-treated HIV are often burdened with co-morbidities due in part to treatment toxicity and persistent HIV-associated immunologic dysfunction. Stigma is also a profoundly important issue for many and can have detrimental effects on health. Finally, despite massive international investments in testing and treating as many people as possible, up to half of the world's HIV-infected population has a detectable viral load [4]. Accessing and adhering to lifelong therapy is challenging for many. For these individuals, an alternative approach – one that is safe, well-tolerated, accessible, and leads to a cure – would be attractive.
Although bone marrow transplantation is a risky, highly morbid procedure, we believe that the Berlin and London cases provide additional impetus to explore pathways leading to durable ART-free remission of HIV disease. Of paramount importance, we need to find ways to do this safely, inexpensively, and effectively. Possibly, durable remission might be achieved upon provision of novel genetic modifications that suppress the ability of the virus to replicate and/or enhance the antiviral activity of the immune system. Should this route be taken, there are a number of issues to consider: the safety of disrupting/editing human gene functions, the precision with which this can be done, and the efficiency with which cellular targeting and genetic-modifying systems are delivered.
CCR5 is one of the attractive targets for the first generation of genetic-modifying approaches. Nearly 1% of people living in Northern Europe do not express CCR5. Although they may be at higher risk for some viral infections, these individuals have a normal lifespan and generally do well. Theoretically, these individuals may harbor genes whose differential expression compensate for the lack of CCR5, prompting the concern that isolated disruption of CCR5 without these compensatory pathways could be harmful. Long-term safety data with maraviroc, a negative allosteric modulator of the CCR5 receptor, suggest this may not be the case [5], but modification of CCR5 on the genetic level may confer different risks than therapeutic modulation of the receptor itself.
Multiple tools exist which can disrupt CCR5, including transcription activator-like effector nucleases (TALENs), zinc finger nucleases (ZFNs), and clustered regularly interspaced short palindromic repeats CRISPR. ZFNs have already been safely used to disrupt CCR5 ex vivo in circulating T cells [6] and a single infusion of CD4+ T lymphocytes modified using CCR5-specific ZFNs was recently reported to result in delayed rebound, particularly in individuals who were CCR5Δ32 heterozygotes [7]. A controlled study of this approach is ongoing (NCT03666871). The main concern with these technologies will always be the potential for off-target effects.
A major barrier to success is delivery. Ex vivo disruption of stem cells and mature T cells is feasible and likely safe, but short of full ablation followed by autologous transplantation, only a small proportion of cells will be HIV-resistant. Theoretically, if partial engraftment occurs and ART is interrupted, the virus will gradually deplete cells expressing CCR5 while sparing those that lack the receptor. Over time, a fully resistant immune system comparable to the Berlin and London cases may emerge and this may in turn function to generate de novo protective responses against HIV. This approach will take time, necessitate sustained periods of viremia and its associated risks, and may ultimately fail as viruses that enter T cells via another co-receptor (CXCR4) are selected. For this approach to be scalable, CCR5 will need to be edited in vivo, with an as-yet undefined proportion of potential target cells effectively modified before ART is stopped. Accordingly, the main challenge in targeting CCR5 is the current inability to edit genes in vivo.
The promise associated with this approach is not limited to HIV. There is a massive effort underway to determine how best to target selected genetic modifications to discrete cell subpopulations in vivo. Multiple viable approaches exist, including viral vectors and non-viral nanoparticles. Both ex vivo methods for protein replacement and in vivo methods for cell modification are becoming an increasing reality.
Recent advances in sickle cell disease (SCD) provide an early framework for what the future of HIV “cure” might resemble. Studies in SCD patients are investigating autologous transplantation of hematopoietic stem cells transduced with a lentiviral vector aimed at producing anti-sickling hemoglobin (NCT02140554) and efforts to repress a molecular switch regulating fetal hemoglobin production are also underway (NCT03282656) [8]. Similar efforts are ongoing in the context of beta-thalassemia (NCT02906202, NCT03655678). These advances, along with the NIH Somatic Cell Genome Editing program, provide inspiration and perhaps eventually a pathway for in vivo gene editing to disrupt CCR5 in people living with HIV.
Promising early data suggest that durable HIV remission might be achieved with genetic manipulations carried out in vivo. Studies in humanized mice using adeno-associated virus (AAV)-delivered CRISPR targeting HIV proviral DNA resulted in reduction of replication competent virus in some animals without off-target effects [9]. The recently reported “Miami Monkey” achieved durable HIV remission through the AAV-based delivery of broadly neutralizing antibodies (bNAbs) against HIV [10]. These preliminary studies reflect a trend toward genetic modification as a new tool that, with time, will hopefully become both feasible and safe.
Most resources aimed at developing a cure are now devoted to “shock and kill,” “block and lock,” and immunotherapies with the goal of generating a remission. The clinical course of the Berlin Patient suggested that CCR5 disruption may be curative and the London case is consistent with this hypothesis. One can imagine a future in which an HIV-infected individual is treated with several injections: one introducing long-acting injectable ART to suppress viral replication and another delivering genetic modifications that permanently protect the host immune system, e.g. by repressing CCR5 expression on target cells, providing bNAbs, and/or otherwise inducing a durable state of ART-free viral remission.
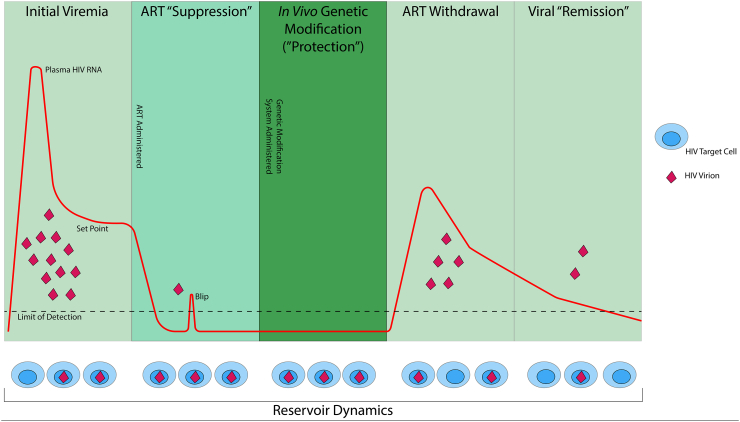
With further refinement, this approach – “suppression and protection” – might prevent post-ART viral rebound and result in a safe, scalable state of durable viral remission for those living with HIV who cannot access or are not able to remain on ART (see Fig. 1).
Fig. 1.
Schematic of a possible approach to inducing HIV “remission.” Panels show the stages of HIV infection and treatment discussed in the article. During or after initial viremia and establishment of the reservoir, ART is initiated and results in virologic suppression with occasional “blips.” Genetic modifications are then delivered in vivo through one of several possible approaches (e.g., CCR5 or proviral editing, provision of bNAbs and/or immunomodulatory agents, induction of a durable antiviral adaptive immune response, etc.), thereby altering the host immune system so that it is resistant to and/or active against HIV. As ART is withdrawn, viral replication may resume but would be disrupted by the absence of permissive target cells, the reduction of cells producing replication-competent virus, and/or the presence of other immune functions facilitating virologic control; durable ART-free viral remission may thereafter ensue.
Contributions
All authors contributed equally to writing and editing the manuscript.
Acknowledgement
The authors would like to thank our colleague, Dr. Dan Wattendorf, Director of Innovative Technology Solutions at the Bill & Melinda Gates Foundation, for his input to the concepts outlined in this paper.
Contributor Information
Steven G. Deeks, Email: sdeeks@php.ucsf.edu.
Joseph M. McCune, Email: mike.mccune@gatesfoundation.org.
References
- 1.Hutter G., Nowak D., Mossner M. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R.K., Abdul-Jawad S., McCoy L.E. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature. 2019 doi: 10.1038/s41586-019-1027-4. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen B.O., Knops E., Lübke N. Conference on retroviruses and opportunistic infections. Seattle, Washington. 2019. Analytic treatment interruption (ATI) after allogeneic CCR5-D32 HSCT for AML in 2013. [Google Scholar]
- 4.Joint United Nations Programme on HIV/AIDS (UNAIDS) 2018. Miles to go—Closing gaps, breaking barriers, righting injustices. [Google Scholar]
- 5.Gulick R.M., Fatkenheuer G., Burnside R. Five-year safety evaluation of maraviroc in HIV-1-infected treatment-experienced patients. J Acquir Immune Defic Syndr. 2014;65(1):78–81. doi: 10.1097/QAI.0b013e3182a7a97a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tebas P., Stein D., Tang W.W. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tebas P., Jadlowsky J., Shaw P. Conference on retroviruses and opportunistic infections. Seattle, Washington. 2019. Delayed viral rebound during ATI after infusion of CCR5 ZFN-treated CD4 T cells. [Google Scholar]
- 8.Esrick E.B., Brendel C., Manis J.P. Flipping the switch: initial results of genetic targeting of the Fetal to adult globin switch in sickle cell patients. Blood. 2018;132(Suppl. 1):1023. [Google Scholar]
- 9.Khalili K., Gendelman H.E. Conference on retroviruses and opportunistic infections. Seattle, Washington. 2019. Combination of CRISPR and LASER ART prevents HIV rebound in humanized mice. [Google Scholar]
- 10.Martinez-Navio J.M., Fuchs S.P., Pantry S.N. Adeno-associated virus delivery of anti-HIV monoclonal antibodies can drive long-term virologic suppression. Immunity. 2019;50(3) doi: 10.1016/j.immuni.2019.02.005. [567-75 e5] [DOI] [PMC free article] [PubMed] [Google Scholar]