Abstract
Background
VAR2CSA is the lead antigen for developing a vaccine that would protect pregnant women against placental malaria. A multi-system feasibility study has identified E. coli as a suitable bacterial expression platform allowing the production of recombinant VAR2CSA-DBL1x-2x (PRIMVAC) to envisage a prompt transition to current Good Manufacturing Practice (cGMP) vaccine production.
Methods
Extensive process developments were undertaken to produce cGMP grade PRIMVAC to permit early phase clinical trials. PRIMVAC stability upon storage was assessed over up to 3 years. A broad toxicology investigation was carried out in rats allowing meanwhile the analysis of PRIMVAC immunogenicity.
Findings
We describe the successful cGMP production of 4. 65 g of PRIMVAC. PRIMVAC drug product was stable and potent for up to 3 years upon storage at −20 °C and showed an absence of toxicity in rats. PRIMVAC adjuvanted with Alhydrogel® or GLA-SE was able to generate antibodies able to recognize VAR2CSA expressed at the surface of erythrocytes infected with different strains. These antibodies also inhibit the interaction of the homologous NF54-CSA strain and to a lower extend of heterologous strains to CSA.
Interpretation
This work paved the way for the clinical development of an easily scalable low cost effective vaccine that could protect against placental malaria and prevent an estimated 10,000 maternal and 200,000 infant deaths annually.
Fund
This work was supported by a grant from the Bundesministerium für Bildung und Forschung (BMBF), Germany through Kreditanstalt für Wiederaufbau (KfW) (Reference No: 202060457) and through funding from Irish Aid, Department of Foreign Affairs and Trade, Ireland.
Keywords: Malaria, Plasmodium, Vaccine, VAR2CSA, Placenta
Research in context.
Evidence before this study
Although malaria vaccine has long been a research priority, little progresses were made over the last decades. One major hurdle that could explain the failure of malaria vaccines to date is that the malaria parasite has an extraordinary ability to evade the host immune system. Among emerging strategies, anti-disease vaccines are being explored. Placental malaria is linked to the massive accumulation of Plasmodium falciparum-infected erythrocytes (IEs) and monocytes in the placental intervillous spaces, leading to maternal as well as fetal and infant mortality. Placental parasites bind to chondroitin sulfate A (CSA) to sequester in the placenta, and women become resistant over successive pregnancies as they acquire antibodies that block IEs adhesion to CSA. The P. falciparum-derived protein VAR2CSA has been identified as the main parasite ligand mediating IEs binding to CSA and is the target of antibodies associated to protection. We systematically searched PubMed on January 14th 2019, for original research articles investigating the relationship between VAR2CSA and placental malaria, using the terms “VAR2CSA and pregnancy and malaria” and also VAR2CSA and vaccine, using the terms “VAR2CSA and vaccine”. Our search returned respectively 179 and 116 articles, clearly indicative of a link between VAR2CSA and placental malaria vaccine development. Finally a recent article reporting on a VAR2CSA clinical trial was identified using the terms “VAR2CSA and clinical and First-in-Human”.
Added value of this study
In this manuscript, we report for the first time the successful cGMP production in Escherichia coli of a VAR2CSA-derived vaccine candidate (PRIMVAC). Stability studies demonstrated that PRIMVAC is stable and potent for up to 3 years upon storage at −20 °C. The toxicology investigation carried out in rats showed the absence of PRIMVAC-related toxicity. Furthermore, when injected in rats, PRIMVAC adjuvanted with either Alhydrogel® or GLA-SE generated antibodies able to recognize native VAR2CSA expressed at the surface of erythrocytes infected by different parasite strains (IEs) but also to inhibit the interactions between IEs and the placental ligand CSA.
Implications of the available evidence
Based on the successful PRIMVAC cGMP production, the absence of PRIMVAC-related toxicity and its capacity to generate strain-transcendent reactive and inhibitory antibodies, VAR2CSA has now progressed to phase Ia/Ib clinical evaluation in healthy adult women in France and in Burkina Faso (ClinicalTrials.gov Identifier: NCT02658253).
Alt-text: Unlabelled Box
1. Introduction
According to the World Health Organization (WHO), a total of 219 million cases of malaria occurred globally in 2017 and the disease led to an estimated 435,000 deaths [1]. About 3.1 billion people in 91 countries and territories are at risk of being infected with Plasmodium and developing disease. Every year, 50 million women of which approximately half of them living in sub-Saharan Africa are exposed to the possibility of developing P. falciparum malaria during pregnancy [2]. People living in malaria endemic areas gradually develop, over years of exposure, a protective immunity against the most severe symptoms of the disease with the exception of pregnant women who become susceptible again to severe clinical outcomes during their first pregnancies [3].
Placental malaria (PM) is linked to the massive accumulation of Plasmodium falciparum-infected erythrocytes (IEs) and monocytes in the placental intervillous spaces [4], where a combination of slower blood flow and expression of the chondroitin sulfate A (CSA) [5] provides a new niche for parasites to sequester. This can lead to maternal anaemia and hypertension as well as stillbirth, preterm delivery, intra-uterine growth retardation, low birth-weight as well as post-natal long-lasting consequences on child health [6,7].
Women rapidly acquire immunity against PM after successive pregnancies, the deficit in birthweights in offspring from infected mothers being greater among first-born babies than in second and third-born infants [8,9]. Protection against PM has been linked to the presence of antibodies able to recognize IEs isolated from placentas of women suffering from placental malaria and block their binding to the CSA moiety of chondroitin sulfate proteoglycans (CSPGs) [10,11]. This relatively fast development of clinical protection as well as the demonstration that antibody-mediated inhibition of IEs binding to CSA is an important mechanism of acquired protective immunity [12] has raised the hope that a vaccine against PM could be developed.
The P. falciparum-derived protein VAR2CSA has been identified as the main parasite ligand mediating IEs binding to CSA [[13], [14], [15], [16], [17]]. VAR2CSA is a high molecular weight protein with a 300 kDa extracellular region, organized in 6 Duffy-binding like (DBL) domains and a cystein-rich interdomain region (CIDR) also termed inter-domain 1 (ID1), the N-terminal region ID1-DBL2-ID2 forming the minimal CSA-binding domain [18,19].
Var2csa orthologues are conserved among parasite isolates and strains but the relatively high degree of protein sequence polymorphisms represents a challenge for vaccine design [20]. Nevertheless, several studies demonstrated that immunoglobulins from anti-sera raised against VAR2CSA recombinant proteins were able to recognize IEs from placental origins but also to block their adhesion to CSA [[21], [22], [23], [24]]. Taken together these studies suggest that VAR2CSA expressed by placental parasites possesses conserved antigenic determinants that are the target of antibodies. Multigravid women possess higher levels of antibodies directed against DBL1x-3x than primigravid women highlighting an important role of antibodies targeting the CSA-binding region in the development of immunity against PM [25].
VAR2CSA stands, today, as the lead antigen for developing a vaccine that would protect pregnant women against the severe clinical outcomes of PM. Two placental malaria vaccine candidates (PRIMVAC and PAMVAC) are currently under clinical development [26]. The prophylactic vaccine candidate PRIMVAC is based on a CSA-binding DBL1x-2x multidomain of VAR2CSA from the P. falciparum 3D7 strain (DBL1x-2x (3D7) down-selected from a multi-system expression study [24]). The His-tagged DBL1x-2x (3D7) has been successfully expressed in E.coli SHuffle® in sufficient amount and with a degree of purity compatible for transition to cGMP production [24]. When adjuvanted with Alhydrogel® or Glucopyranosyl Lipid A Adjuvant-Stable Emulsion (GLA-SE), DBL1x-2x (3D7) expressed in E. coli SHuffle® stood up as the best antigen able to generate antibodies that recognize IEs expressing different variants of VAR2CSA and block their adhesion to CSA [24].
In this study, we describe the current good manufacturing practice (cGMP) production of the PRIMVAC drug substance (DS) as well as the stability and potency of the drug product (DP) over time. To comply with criteria compatible with clinical use, the His-tag of the DBL1x-2x (3D7) vaccine candidate was removed. Production of the DS was performed in a 2-phase manufacturing process comprising recombinant protein expression in a bacterial fermentation system (E. coli SHuffle®) followed by 7 steps of purification. A subsequent preclinical safety assessment of the DS was also carried out on small animals (Sprague-Dawley rats) and demonstrated that PRIMVAC adjuvanted either with Alhydrogel® or GLA-SE was well tolerated in rats, with no evidence that it causes systemic toxicity at dose levels similar to those which will be administered in clinical trial settings.
2. Materials and methods
2.1. cGMP production of the PRIMVAC final drug product
2.1.1. Fermentation
After thawing of a master cell bank (MCB) vial, biomass amplification was initiated in two pre-culture steps where E. coli SHuffle® were allowed to grow under shaking at 250 rpm at 30 ± 1 °C in complete Glycerol-Enriched Yeast Extract Culture medium (GY) containing 0.5% glucose and 45 μg/ml kanamycin. The culture was carried on without antibiotics in a bioreactor at 30 ± 1 °C in GY-Mg medium in a final volume of 80 l under controlled pH. When the optical density (OD) reached 9.5 ± 0.5, the induction step was performed in presence of 0 .1mM isopropylthio-β-galactoside (IPTG) at 20 ± 1 °C. After 20 ± 1 h post-induction, cells were harvested and the pellet was stored at −15/−25 °C until lysis.
2.1.2. Purification
Cells were lysed in lysis buffer (10 mM sodium phosphate, 150 mM NaCl, pH 7.0) and the soluble fraction was first subjected to chromatography on a Heparin HyperD M resin (step 1) packed in a BPG l00/500 column (GE Healthcare life Science) and equilibrated with l0 mM sodium phosphate, 150 mM NaCl, pH 7.0. After soluble fraction passage over the column, sample elution was performed using l0 mM sodium phosphate, 1 M NaCl, pH 7.0. The eluate was then stored at +2/+8 °C up to the next step. The step 2 of the purification process consisted of a chromatography on Hydroxyapatite II, 80 μm resin. The column was equilibrated with 5 mM sodium phosphate, 150 mM NaCl, pH 7.0. The product obtained in step 1 was then loaded on the column and the protein of interest was eluted with 250 mM sodium phosphate, 150 mM NaCl, pH 7.0. The eluate is stored at +2/+8 °C up to the next step. Step 3 consisted in an ultrafiltration procedure realized on 3 Omega T 30 kDa membranes. The product was concentrated and then diafiltered against 25 mM sodium phosphate, 150mM NaCl, pH 6.0. At the end of the diafiltration, the retentate was collected and directly engaged in the SP Sepharose FF step (step 4). The protein of interest was then eluted with a 25 mM sodium phosphate, 235 mM NaCl at pH 6.0. The eluate was stored at +2/+8 °C until the next step. Step 5 consisted in an ultrafiltration procedure realized on Omega T 30 kDa membranes. The product was concentrated and then diafiltered against Phosphate-Buffered Saline (PBS) pH 7.4. The filtered retentate was directly engaged in the Sartobind Q step (step 6). This manufacturing step was performed on Sartobind Q 70 ml membrane (Sartorius). The retentate fraction from step 6 was then loaded on Sartobind Q membrane and the flow through containing the protein of interest was collected and directly engaged in the final filtration step (step 7). The fraction resulting from previous step was filtered through a Sartopore®2150 0 .2 μm filter (Sartorius).
2.2. Western blot
Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on Criterion TGX (Tris-Glycine eXtended) 4–15% gels, after 5 min denaturation at 95 °C in reducing and non-reducing sample buffers. Following electrophoresis, proteins were transferred onto a nitrocellulose membrane which was then blocked with 1% BSA diluted in PBS and incubated with an anti-DBL1x monoclonal antibody. This monoclonal antibody was obtained by fusion of the partner B cell line Sp2/0 with splenocytes from BALB/c mice immunized with the recombinant extracellular region of VAR2CSA (DBL1x-6ε) [17]. Following cell cloning and domain mapping, the monoclonal antibody used in this study was identified as specific for the DBL1x domain of VAR2CSA (3D7). The antigen-antibody complex was then detected by using alkaline phosphatase anti-mouse Immunoglobuline G (IgG) antibody / NBT-BCIP system.
2.3. Toxicity study
The design of the non-clinical toxicology study was performed according to the “Guidelines on the non-clinical evaluation of vaccine adjuvants and adjuvanted vaccines” [27]. Rats were chosen as it is a rodent species in which an immune response to the vaccine antigen VAR2CSA is developed and enhanced by the adjuvants Alhydrogel® and GLA-SE [24] through mechanisms expected to be similar than in humans. Three groups of 15 male and 15 female Sprague-Dawley rats received the vaccine candidate, PRIMVAC, at the dose-level of 110 μg/animal, alone (group 3) or adjuvanted with either Alhydrogel® (Brenntag, Denmark) at 0.85 mg/animal (group 4) or GLA-SE (Infectious Disease Research Institute, USA) at 5 μg/animal (group 5), by intramuscular injection, on Days 1, 15, 29 and 43. Another group of 15 males and 15 females received GLA-SE alone at 5 μg/animal (group 2). A control group of 15 males and 15 females received the control item 0.9% NaCl (group 1) under the same experimental conditions. Two injection sites were used at each treatment, the right and left gastrocnemius muscle on Days 1 and 29 and the right and left quadriceps femoris muscle on Days 15 and 43. Each site was injected with a constant dosage volume of 0.25 ml. At the end of the treatment period, early sacrifice animals were sacrificed on Day 45, 2 days after the last injection, and late sacrifice animals, five surviving animals per sex and group, were kept for a 3-week observation period before sacrifice on Day 65. Blood samples for the determination of anti-PRIMVAC antibodies levels, infected erythrocytes immune recognition assessment and CSA-binding inhibition tests were collected from all animals once before the beginning of the treatment period and from ten surviving animals/group/sex on Day 45 (early sacrifice) and from the remaining five surviving animals/group/sex on Day 65 (late sacrifice). The study was conducted by CitoxLAB (Evreux, France) in compliance with Animal Health regulations (Council Directive No. 2010/63/EU of 22 September 2010 on the protection of animals used for scientific purposes) after approval of CitoxLAB and INSERM ethical committees. Every effort was made to minimize animal suffering.
2.4. Potency study
The potency study aiming to assess the stability of PRIMVAC over-time was performed in mice, a rodent species in which sufficient historical data exist to ensure that an immune response is developed [28]. Groups of 10 BALB/C ByJ mice received the vaccine candidate, PRIMVAC, at the dose-levels of 0.5 μg DP/animal (Group1) or 5 μg DP/animal (group 2) with Alhydrogel® (Brenntag, Denmark) at 0.17 mg/animal, by sub-cutaneous injection, on Days 1 and 29. At the end of the treatment period, animals were sacrificed on Day 39 and blood samples were collected for serum anti-DS antibodies determination. All animal vaccinations were executed in strict accordance with good animal practices, following the EU animal welfare legislation and after approval of the NovasepHenogen (Gosselies, Belgium) and INSERM ethical committees. Every effort was made to minimize animal suffering.
2.5. PRIMVAC-specific IgG titers
Ultra-high binding flat bottom microtiter plates (Immunolon 4HBX 3855) were coated overnight at 4 °C with 1 μg/ml of immunizing antigen in PBS. Plates were then washed 3 times with PBS and blocked for 1 h at 37 °C with dilution buffer (PBS 1% BSA). After blocking solution removing, rat or mouse sera serial dilutions (1/100 to 1/107) were added to the wells and incubated for 1 h at 37 °C with gentle shaking. Plates were washed three times with PBS 1% BSA. One hundred microliters of anti-rat/mouse IgG (Fc-specific) HRP-conjugated antibody (Jackson Immunoresearch) at 1/4000 in dilution buffer were added to each well and incubated for 1 h at 37 °C. Plates were then washed three times with PBS-Tween and 100 μl of TMB (3,3′,5,5′-tetramethylbenzidine) substrate (Biorad) was added per well. Absorbance was measured at 655 nm on a microplate reader. After data plotting and a 4-parameter logistic regression curve fitting, the plasma dilution corresponding to 50% of the maximal OD-value (sigmoid curve plateau) was regarded as the drug specific antibody titer. Mice presenting an anti-DBL1x-2x (3D7) IgG titer ≤1/150 were considered as seroconverted.
2.6. Parasite culture
P. falciparum laboratory adapted parasite strains NF54, FCR3 (IT4) and 7G8 were grown in O+ human erythrocytes in RPMI 1640 medium (Gibco) containing 25 mM Hepes, 2 mM l-glutamine (Gibco) supplemented with 5% Albumax (Gibco), 5% Human serum, 0.1 mM hypoxanthine (Gibco) and 20 μg/ml gentamicin (Gibco) [29]. Parasites were routinely genotyped by PCR using MSP1/MSP2 primers [30] and tested for potential mycoplasma contamination (LookOut Mycoplasma PCR Detection Kit, Sigma). Knob-positive infected erythrocytes were routinely selected by gelatin flotation using Plasmion (Fresenius Kabi) [31]. Erythrocytes infected with NF54, FCR3 (IT4) and 7G8 were selected for the CSA-binding phenotype by multiple panning rounds on CSA (Sigma). These selected populations are referred to as NF54-CSA, FCR3-CSA and 7G8-CSA. Erythrocytes infected with FCR3 were also selected for the CD36-binding phenotype by multiple panning rounds on recombinant CD36 (R&D systems) and are referred to as FCR3-CD36. Erythrocytes infected with trophozoite stage parasites (25 h–30 h post-invasion) were purified from synchronized parasite cultures (3–6% parasitemia) using a VarioMACS system coupled to CS columns (Miltenyi Biotec) as previously described [32]. Var gene transcriptional profiling was routinely performed on NF54-CSA and FCR3-CSA to ensure correct selection of VAR2CSA-expressing infected erythrocytes as well as CSA-binding assays for 7G8-CSA.
2.7. Flow cytometry-based immune recognition assays
Purified infected erythrocytes (trophozoite parasite stage; 25 h–30 h post-invasion) were incubated for 15 min at 37 °C in PBS (Gibco; pH 7.2) supplemented with 1% BSA Fraction V (Roche) and added to a 96-well, round bottom, microplate (200,000 IEs/well). Cells were pelleted by centrifugation at 300 g for 3 min at 37 °C and resuspended in 100 μl of plasma (1:50) diluted in PBS 1% BSA. Rat plasma samples were pre-adsorbed on non-infected red blood cells prior the experiment. After an hour of incubation at 37 °C, cells were washed three times with 200 μl PBS 1% BSA and were then resuspended in 100 μl PBS 1% BSA containing an anti-rat IgG PE-conjugated antibodies (Jackson ImmunoResearch; 1/100). After 45 min of incubation at 37 °C in dark, cells were washed three times with 200 μl PBS and then fixed overnight at 4 °C with 4% paraformaldehyde (PFA) (Electron Microscopy Sciences) diluted in PBS. Before flow cytometry acquisition, cells were washed once with 200 μl PBS 1% BSA and resuspended in PBS supplemented with TO-PRO®-3 (Molecular probes) according to the manufacturer's instructions. Data acquisition was performed using BD FACSCanto™ II flow cytometer (Becton-Dickinson). Infected erythrocytes gating was performed based on their morphological features using the forward (FSC) and side (SSC) scatters. TO-PRO®-3 positive cells (parasite DNA staining) were regarded as infected. Data were then analyzed in FLOWJO 8.1 (Tree Star Inc.) software. Results were expressed as the ratio of the geometrical mean fluorescence intensities (Geo. MFI) in the PE channel of the immune samples over the respective non-immune samples.
2.8. High throughput CSA-binding inhibition assays
Inhibition of infected erythrocytes binding to CSA by rat plasma samples was assessed in a 96-well plate in high throughput format. The assay was performed as described previously. Briefly, MaxiSorp™ high protein-binding capacity polystyrene 96 well ELISA plates (Nunc) were coated with CSA (Sigma) at 1 mg/ml; 100 μl/well or BSA (Roche) at 1%; 100 μl/well diluted in PBS and incubated overnight at 4 °C. Plates were then washed three times with 200 μl RPMI 1640 supplemented with 2% Fetal Bovine Serum (FBS) (Dominique Dutcher). Purified infected erythrocytes (trophozoite parasite stage; 25 h–30 h post-invasion) were pre-incubated with plasma samples diluted in RPMI 2% FCS (1/50) for 1 h at 37 °C. Infected erythrocytes were then added into the pre-coated wells (106 cells; 100 μl/well) and incubated for 1 h at 37 °C. Plates were then washed three times with 200 μl PBS by quick inversion. Infected erythrocytes remaining attached to the surface were lysed by addition of 50 μl 3,3′,5,5′-Tetramethylbenzidine (TMB) (Biorad) followed by 30 s vortexing on a MixMate® mixer (1000 rpm). The blue-colored reaction product resulting from the pseudo-peroxidase activity of the remaining hemoglobin contained in infected erythrocytes absorbs light at 655 nm and the corresponding O.D. can be read using an iMark™ microplate absorbance reader (Biorad). Results were expressed as % of inhibition of the immune samples compared to the respective non-immune samples [% inhibition = 100 − (ODimmune/ODpre-immune)/100].
3. Results
3.1. cGMP production of the PRIMVAC final drug product
The generation of the E. coli strain producing recombinant DBL1x-2x (3D7) (PFL0030c; aa 49–962) was carried out by synthetizing a gene (optimized for bacterial codon usage) coding for the complete open reading frames (ORF) of interest that was subsequently sub-cloned into a proprietary expression plasmid (GTP, Labège, France) modified from the pEX-K vector and compatible for industrial transfer. This plasmid was used to transform E. coli SHuffle® and to create a Research Cell Bank (RCB). The RCB was then expanded to a Master Cell Bank (MCB) in conformity with criteria compatible with clinical use (Supplementary Table 1). Extensive upstream and downstream process development studies including the production of an engineering batch of DBL1x-2x (3D7) have identified the critical process parameters for each step of the drug substance production.
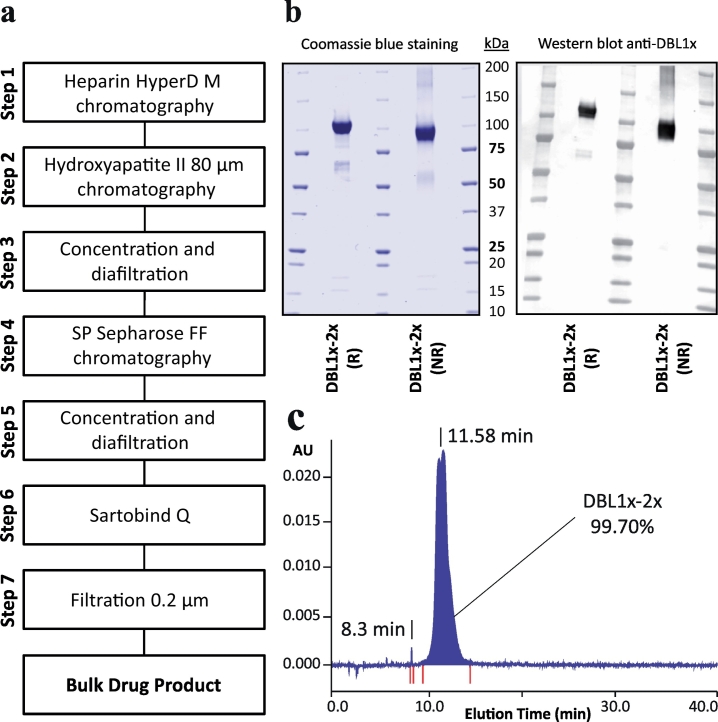
The fermentation production of the DBL1x-2x (3D7) recombinant protein was performed according to the different steps described in Fig. 1A. Following two pre-culture steps in which E. coli SHuffle® were grown at 30 °C in Glucose Yeast (GY) medium, the culture was continued in a bioreactor at 30 °C in GY medium in a final volume of 80 l (Supplementary Fig. 1). The induction of protein expression was performed in presence of isopropylthio-β-galactoside (IPTG) at 20 °C for 20 h. After fermentation, cells from 80 l were harvested and lysed. DBL1x-2x (3D7) was then purified in a 7-step process including 4 chromatographic procedures (Fig. 1A). The Heparin Hyper D-M resin was used as the capture step (step 1). The eluate was passed on a hydroxyapatite II 80 μm resin as the second step of chromatography (step 2) followed by concentration and diafiltration (step 3). Ion exchange chromatography was performed using the SP Sepharose Fast Flow resin (step 4) followed by concentration and diafiltration against the DS final buffer (phosphate-buffered saline (PBS) (step 5). In order to significantly reduce endotoxin content, a Sartobind Q-membrane® purification step (step 6) was implemented as the fourth chromatography procedure. Sterilising grade filtration was performed on Sartopore®2 filters (step 7). The overall process yields 93 mg/l of purified DS DBL1x-2x (3D7). SDS-PAGE analysis of the bulk solution, performed in reducing and non-reducing conditions, revealed a major band at 100 kDa with weak bands at 50–75 kDa and 15–20 kDa (Fig. 1B). Western blot analysis performed using an anti-DBL1x monoclonal antibody confirmed the identity of the protein at 100 kDa and of scarce degradation products at 50–75 kDa. The presence of limited aggregation was also visible in SDS-PAGE under non-reducing conditions.
Fig. 1.
Analysis of the final drug product PRIMVAC. (A) Overview of the PRIMVAC cGMP production process. (B) SDS PAGE under reducing (R) and non-reducing conditions (NR) of the bulk drug product followed by Coomassie Blue staining (left panel) and by western blotting using an anti-DBL1x antibody for specific detection of the protein of interest (right panel). All blots derive from the same experiment and were processed in parallel. (C) Reverse Phase HPLC analysis of PRIMVAC DS. Proteins were injected in aqueous mobile phase on a column providing hydrophobic interactions and eluted with an increasing gradient of organic solvent, thus specifically separated according to their hydrophobicity. Proteins were detected at the end of the column by UV adsorption at 280 nm. DLB1x-2x purity was estimated based on the UV signal area. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The bulk solution of recombinant protein was then diluted with PBS at pH 7.2 to reach a concentration of 335 μg/ml. The solution was filtered through a 0.2 μm-pore filter and aseptically aliquoted in final containers (2R type 1 glass vials) with a filling target of 0 .3ml/vial. Vaccine (PRIMVAC) and quality control (QC) vials were stored at −20 °C.
3.2. Stability over time of the PRIMVAC final drug product
QC vials of the DP were used to test the properties of the PRIMVAC clinical batch. The sterility of the batch was confirmed by an absence of bacterial growth when the vial content was poured on lysogeny broth (LB)-agar petri dishes and incubated for 5 days at 37 °C (pour-plate method) (Table 1) and the levels of bacterial endotoxins that could have resulted from the bacterial production were 30 times (1 IU/dose) lower than the maximal value advocated in the specifications (<30 IU/dose). The low number of sub-visible particles was also within the specification ranges. Protein purity and integrity was assessed by RP-HPLC and SDS-PAGE analysis. RP-HPLC analysis showed a degree of purity of 99.7% for the recombinant protein (main pic) (Fig. 1C). The weak bands observed at 15–20 kDa in SDS-PAGE and not revealed by the anti-DBL1x antibody most likely result from minor impurities co-eluted with the protein of interest. Mass spectrometry analysis identified one of these contaminants as the E.coli-derived protein DPS (DNA-binding Protein from Starved cell).
Table 1.
Stability of the PRIMVAC drug product at −20 °C and 3 0°C.
| −20 °C |
30 °C |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TESTS | Specifications | T0-released batch | 3 months | 6 months | 9 months | 12 months | 18 months | 24 months | 36 months | 7 days |
| Visual appearence | Clear, colorless liquid w/o visible particles | Clear, colorless liquid w/o visible particles | Clear, colorless liquid w/o visible particles | Clear, colorless liquid w/o visible particles | Clear, colorless liquid w/o visible particles | Clear, colorless liquid w/o visible particles | Clear, colorless liquid w/o visible particles | Clear, colorless liquid w/o visible particles | Clear, colorless liquid w/o visible particles | Clear, colorless liquid w/o visible particles |
| pH | 6.5 to 8.0 | 7.1 | 7.2 | 7.2 | 7.2 | 7.3 | 7.2 | 7.2 | 7.2 | 7.2 |
| Profile by SDS-PAGE | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDaa |
| Identity by Western Blot (R and NR conditions) | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa | One major band at 100 kDa |
| Purity by RP-HPLC | Main peak ≥95% | 99.7% | 99.66% | 99.65% | 99.65% | 99.64% | 99.61% | 99.5% | 99.5% | 99.71% |
| Residual DNA | <200 ng/mg | 5 ng/mg | ND | ND | ND | ND | ND | ND | ND | ND |
| Host cell proteins | ≤1% | <1% | ND | ND | ND | ND | ND | ND | ND | ND |
| Protein content by BCA | Between 75 and 125 μg/vial (1 dose/vial) | 101 μg/vial | 90 μg/vial | 87 μg/vial | 87 μg/vial | 88 μg/vial | 83 μg/vial | 83 μg/vial | 80 μg/vial | 86 μg/vial |
| Sterility | Absence of growth | Absence of growth | ND | ND | ND | ND | ND | ND | Absence of growth | ND |
| Bacterial endotoxins | <30 IU/dose | 1 IU/dose | ND | ND | ND | ND | ND | ND | ND | ND |
| Particulate contamination: sub-visible particles | ≥10 μm: ≤6000 particles/vial | ≥10 μm: ≤71 particles/vial | ND | ND | ND | ND | ND | ND | ND | ND |
| ≥25 μm: 3 particles/vial | ≥25 μm: 3 particles/vial | |||||||||
Degradation bands were a bit more intense as compared to that of reference sample (T0). Presence of degradation bands between 25 and 37 kDa.
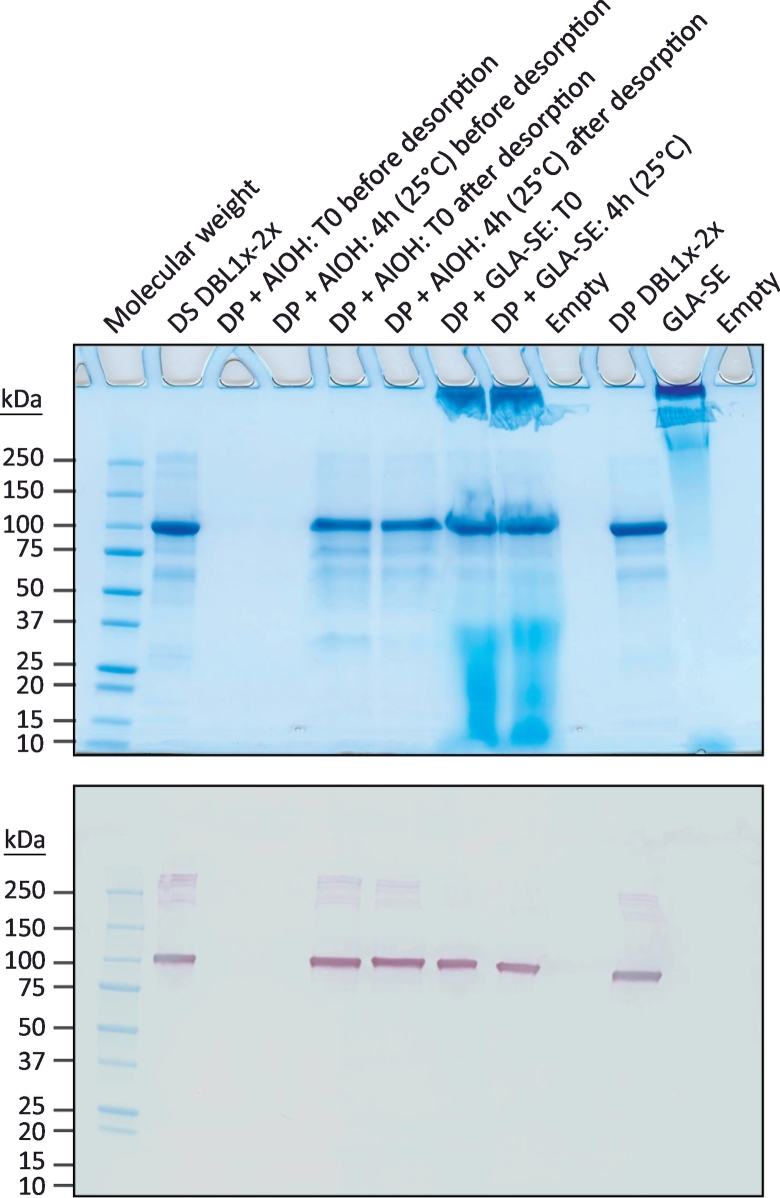
Long-term stability studies were conducted on the DP stored at −20 °C for a period of 3 years (Table 1). As for the released product, protein integrity was assessed by SDS-PAGE analysis coupled to Coomassie Blue staining, by western blotting (reducing/non-reducing conditions) and by RP-HPLC. Using these methods, no significant changes in PRIMVAC profiles were detected over a storage period of 3 years at −20 °C. A slight decrease in protein concentration was observed overtime though meeting the specifications. An accelerated stability study was performed by incubating the QC vials for 7 days at 30 °C (Table 1). SDS-PAGE analysis revealed scarce degradation products at 50–75 kDa, slightly more intense as compared to that of the reference sample (T0). A degradation band between 25 and 37 kDa was also visible. Sample incubation at 30 °C for 7 days was accompanied with a small decrease in protein concentration (−15%), yet again meeting the specifications. Short term stability in presence of adjuvant was also assessed. The DP was formulated with either Alhydrogel® or GLA-SE and DBL1x-2x (3D7) integrity was analyzed after 4 h at 25 °C (Table 2). No changes were detected in the protein profiles after the incubation period but a slight decrease in DBL1x-2x (3D7) concentration was observed after desorption of the drug product from Alhydrogel® (−19%) (Fig. 2).
Table 2.
Stability of the PRIMVAC drug product at 25 °C with adjuvant.
| TESTS | T0 + Alhydrogel® | 4 h + Alhydrogel® | T0 + GLA-SE | 4 h + GLA-SE |
|---|---|---|---|---|
| Visual appearence | White, opalescent w/o visible particles | White, opalescent w/o visible particles | Slightly brownish, opalescent w/o visible particles | Slightly brownish, opalescent w/o visible particles |
| pH | 7.4 | 7.4 | 6.4 | 6.3 |
| Profile by SDS-PAGE | Before desorption: no band | Before desorption: no band | One major band at 100 kDa. Few aggregates. Presence of GLA-SE materials and smears from 37 kDa | One major band at 100 kDa. Few aggregates. Presence of GLA-SE materials and smears from 37 kDa |
| After desorption: One specific band at 100 kDa | After desorption: One specific band at 100 kDa | |||
| Identity by Western Blot (R and NR conditions) | Before desorption: no band | Before desorption: no band | One specific band at 100 kDa | One specific band at 100 kDa |
| After desorption: One specific band at 100 kDa | After desorption: One specific band at 100 kDa | |||
| Protein content by BCA | Before desorption: ND | Before desorption: ND | 398 μg/vial | 398 μg/vial |
| After desorption: 96 μg/vial | After desorption: 78 μg/vial |
Fig. 2.
Characterization of the PRIMVAC vaccine formulation. Stability of PRIMVAC in presence of Alhydrogel® (AlOH) or GLA-SE was assessed at different time points by SDS-PAGE followed by Coomassie Blue staining and western blotting using an anti-DBL1x antibody for specific detection of the protein of interest. All blots derive from the same experiment and were processed in parallel. DBL1x-2x drug substance (DS; engineering batch) and DBL1x-2x drug product (DP; released batch) were used as protein integrity controls. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
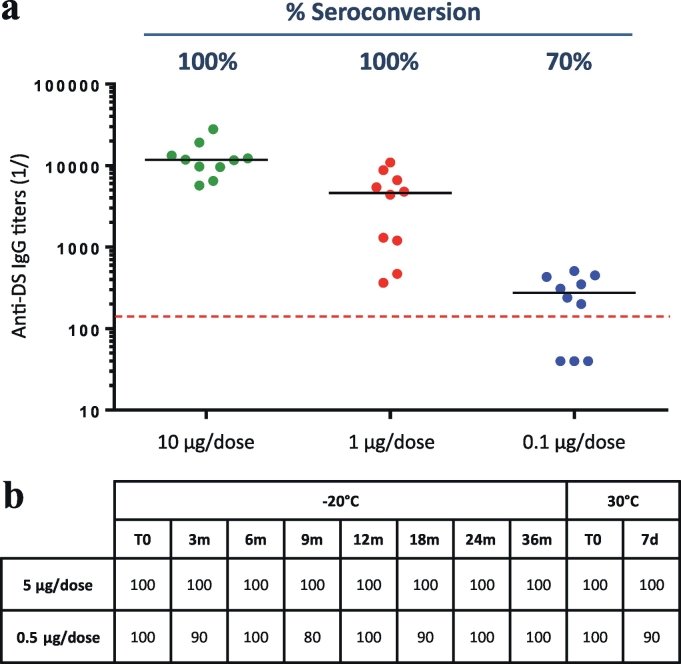
In order to establish an assay to monitor the potency of the PRIMVAC DP over storage time, a pilot study using the DS was undertaken using BALB/C mice. Three groups of 10 mice were immunized with 3 different doses of DS (10, 1, 0.1 μg/injection) formulated with Alhydrogel®. Mice seroconversion was evaluated after two vaccine injections by titrating the serum IgGs specific to the immunizing antigen. Mice with an anti-DBL1x-2x (3D7) IgG titer ≤1/150 were considered seroconverted. Seventy percent of animals belonging to the group immunized with 0.1 μg of DS/dose were seroconverted 10 days after the last injection whereas animals immunized with 1 μg and 10 μg of DS/dose reached a seroconversion plateau (100%) at day 39 (Fig. 3A). These results allowed the selection of two doses of DBL1x-2x (3D7) for performing the potency tests of the PRIMVAC drug product over storage time; 5 μg/dose (seroconversion plateau) and 0.5 μg/dose (close to sub-optimal seroconversion dose).
Fig. 3.
Potency of PRIMVAC over storage time. (A) Dose determination. Three groups of 10 mice received 2 injections (Day 1 and Day 29) of PRIMVAC adjuvanted with Alhydrogel® at 3 different dosage (10, 1, 0.1 μg/injection). Anti-drug substance (DS) antibody titers (IgG) were determined by ELISA 10 days after the last injection. Black horizontal lines represent the median specific IgG titer for a given group. Mice presenting an anti-DS IgG titer ≤1/150 were considered as seroconverted (above the red dashed line). (B) Potency over time. Twenty groups of 10 mice were immunized according to the same protocol used for dose determination with either 5 or 0.5 μg of PRIMVAC stored at −20 °C for up to 36 months or incubated at 30 °C for 7 days. Group seroconversion rates reflecting PRIMVAC potency were determined for each storage time points. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The acceptance criterion for the potency of PRIMVAC formulated with Alhydrogel® was then defined as a minimum of 80% of seroconverted animals for at least one group of mice injected twice with either 0.5 μg or 5 μg/dose. Injection of PRIMVAC formulated with Alhydrogel® at 5 μg/dose allowed the seroconversion of 100% of the mice at all time points and up to 36 months in the long term storage study for the DP stored at −20 °C and for the DP incubated at 30 °C for 7 days. In the same line, 0.5 μg of DP stored at −20 °C for up to 3 years seroconverted at least 80% of the animals (Fig. 3B). Injection of 0.5 μg/dose of PRIMVAC incubated for 7 days at 30 °C (accelerated stability) led to similar results, showing at least 90% of seroconversion in the respective mice groups. Taken together these results indicated that the cGMP-produced PRIMVAC is stable upon storage at −20 °C and suitable for early-phase clinical testing.
3.3. Toxicity study
The objective of this study was to evaluate the potential toxicity of PRIMVAC in rats following four intramuscular administrations every 2 weeks of 110 μg of DS adjuvanted or not with GLA-SE or Alhydrogel®, a dosage above the maximal amount that will be used in humans. On completion of the treatment period, designated animals were sacrificed after the last injection (early sacrifice) or after a 3-week treatment-free period (late sacrifice) in order to evaluate the reversibility of any findings and potential delayed adverse effects. The treatment groups are depicted in Table 3.
Table 3.
Treatment groups.
| Groups | Treatment | Number of animals | Nominal dose-level (/animal) |
|---|---|---|---|
| 1 | 0.9% NaCl | 16 males | 0 |
| 15 females | |||
| 2 | GLA-SE | 15 males | 5 μg |
| 15 females | |||
| 3 | PRIMVAC | 15 males | 110 μg |
| 15 females | |||
| 4 | PRIMVAC + Alhydrogel® | 17 males | 110 μg + 0.85 mg |
| 15 females | |||
| 5 | PRIMVAC + GLA-SE | 15 males | 110 μg + 5 μg |
| 15 females |
Three unscheduled deaths occurred and were considered accidental without relationship to the treatment administrated. A male from the control group (Group 1) was found dead on Day 16 and two males in the PRIMVAC adjuvanted with Alhydrogel® group died under anesthesia before the injection on Day 15. At pathology investigations, marked congestion and moderate edema were seen in the lungs of the control male. This edema may be the cause of the death. There were no histopathological changes which could explain the death of the two males in the PRIMVAC adjuvanted with Alhydrogel® group which died under anesthesia. The major factor contributing to death was considered to be anesthesia.
Clinical observations during the study are presented in Table 4. No clinical signs indicative of systemic toxicity were observed. The findings recorded during the study, i.e. hunched posture, thin appearance, chromorhynorrhea, shortened tail, soiled coat, eye opacity, and skin lesions outside the application sites (thinning of hair, alopecia and scabs), were present both in control and treated animals and/or were reported sporadically in only a few animals and/or are routinely observed in rats of this strain and age. Therefore, they were considered to be unrelated to the treatment administered. Furthermore, ophthalmological examinations performed on all animals before the beginning of the treatment period and before sacrifice did not reveal any PRIMVAC-related effect.
Table 4.
Clinical observations.
| Observations | Sex | TREATMENT |
||||
|---|---|---|---|---|---|---|
| Group 1 |
Group 2 |
Group 3 |
Group 4 |
Group 5 |
||
| 0.9% NaCl | GLA-SE | PRIMVAC | PRIMVAC + Alhydrogel® | PRIMVAC + GLA-SE | ||
| Observation period: Days 1–45 (early and late sacrifice animals) | ||||||
| Thin appearance | M | – | – | – | – | – |
| F | – | – | – | 1/15(7%) | – | |
| Hunched posture | M | – | – | – | – | |
| F | – | – | – | 1/15 (7%) | – | |
| Thinning of hair | M | – | – | – | – | |
| F | 2/15 (13%) | – | – | – | 2/15 (13%) | |
| Shortened tail | M | 1/16 (6%) | – | – | – | – |
| F | – | – | – | – | – | |
| Alopecia/right forelimb | M | 3/16 (19%) | 1/15 (7%) | 1/15 (7%) | – | 1/15 (7%) |
| F | 2/15 (13%) | – | – | – | – | |
| Alopecia/left forelimb | M | 3/16 (19%) | 1/15 (7%) | – | – | 1/15 (7%) |
| F | 2/13 (13%) | – | – | |||
| Scabs | M | 1/16 (6%)1 | – | – | – | – |
| F | – | 1/15 (7%)2 | 1/15 (7%)3 | – | – | |
| Eye opacity | M | 1/16 (6%)5 | – | – | – | – |
| F | – | – | – | – | – | |
| Chromo-rhynorrhea | M | – | 1/15 (7%) | – | – | 1/15 (7%) |
| F | – | – | 1/15 (7%) | – | – | |
| Soiled coat | M | 1/16 (6%) | – | – | – | – |
| F | – | – | – | – | – | |
| Observation period: Days 45–65 (late sacrifice animals only) | ||||||
| Thinning of hair | M | – | 1/5 (20%) | – | – | – |
| F | – | – | – | – | 2/5 (40%) | |
| Alopecia/right forelimb | M | – | – | – | – | – |
| F | 1/5 (20%) | – | – | – | – | |
| Alopecia/left forelimb | M | – | – | – | – | – |
| F | 1/5 (20%) | – | – | 1/5 (20%) | – | |
| Scabs | M | – | 1/5 (20%) | – | – | – |
| F | – | – | – | – | – | |
| Eye opacity | M | 1/5 (20%)5 | – | – | – | – |
| F | – | – | – | – | – | |
Location of scabs: 1 on tongue, 2 on tail, 3 on neck - dorsal region, 4 back - thoracic region.
Eye opacity: 5 left eye only.
– = not observed.
Body weight evolution was considered to be unaffected in all groups. Some statistically significant differences between control and treated groups were nevertheless recorded during the study and were considered to be of no toxicological importance as they were occasional and of low magnitude. No relevant effects on food consumption were observed in any group. Mean body temperature was slightly increased at 4 h after treatment in males receiving PRIMVAC adjuvanted with GLA-SE, or GLA-SE alone (Supplementary Table 2). No increase in mean body temperature was observed in female rats.
3.4. Local reactions
Local reactions at the injection sites mainly consisted of increase in size (corresponding to swelling) in all groups, with a higher incidence in both sexes in the groups treated with PRIMVAC adjuvanted with GLA-SE or Alhydrogel®, and a lower incidence (but slightly higher than in controls) in animals treated with PRIMVAC or GLA-SE alone (Supplementary Table 3). This finding generally appeared no later than 2 days (on average) after each injection and persisted for a few days. After the last treatment, a greater incidence was recorded in all groups. Males and females were generally similarly affected, but after the last treatment, females treated with PRIMVAC alone were more impacted than males, and females treated with PRIMVAC adjuvanted with Alhydrogel® showed nodosities after the 1st injection rather than increased size. Erythema was recorded in all groups, except controls, with a similar low incidence. This finding generally appeared no later than 2 days (on average) after each injection and persisted for a few days.
3.5. Haematology and blood biochemistry investigations
At haematology investigations, changes (comparison with mean control values) were observed mostly in females treated with PRIMVAC adjuvanted with Alhydrogel® or GLA-SE and in those treated with GLA-SE alone (Supplementary Tables 4–5). These changes consisted of increased neutrophil, eosinophil, basophil, lymphocyte, large unstained cell and monocyte counts, leading to higher leucocyte count in females on Day 3 (around +50%). This change was still present on Day 45, with a higher severity in the PRIMVAC+GLA-SE group (+90% vs controls). The same variations were noted in males, but to a lesser extent. Fibrinogen level was higher in both sexes in the PRIMVAC adjuvanted with GLA-SE and GLA-SE groups (between +73 and + 95%) and to a lesser extent in the PRIMVAC adjuvanted with Alhydrogel® group (between +22 to +28%). This change was similarly noted in both sexes on Day 45. This was accompanied by increased plasma alpha-2 macroglobulin levels in both sexes treated with PRIMVAC adjuvanted with GLA-SE, or GLA-SE alone (Supplementary Table 6). The severity of the change was higher on Day 3 (25- to 42-fold) than on Day 45 (6- to 21-fold) and was more pronounced in the PRIMVAC adjuvanted with GLA-SE group. Reversibility of these observations was noted at the end of the 3-week treatment-free period. All these changes were attributed to inflammatory responses at injection sites. No effects were reported for the blood biochemistry parameters.
3.6. Functional characterization of PRIMVAC-induced antibodies in rats
3.6.1. Immune recognition of VAR2CSA selected IEs
To conduct the toxicity study, different groups of rats received 4 injections (Days 1, 15, 29 and 43) of 110 μg of PRIMVAC alone or formulated with adjuvants (Table 3). Sera samples of these animals collected at Day 45 and Day 65 were then used in a parallel study to assess the immunogenicity of the cGMP-produced PRIMVAC and the functional features of the induced antibodies (IgGs).
Relatively high PRIMVAC-specific antibody titers were observed at Day 45 and Day 65 in the PRIMVAC alone group highlighting the intrinsic immunogenic properties of DBL1x-2x (3D7). IgG titers increased when PRIMVAC was formulated with Alhydrogel® or GLA-SE to reach very elevated values (≥ 1/819200) at Day 65 (Table 5).
Table 5.
Anti-PRIMVAC IgG titers (1/X).
| 0.9% NaCl |
PRIMVAC |
PRIMVAC + Alhydrogel® |
PRIMVAC + GLA-SE |
|||||
|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | |
| Pre-treatment | 20 | 20 | 0 | 0 | 0 | 30 | 0 | 0 |
| Day 45 | 160 | 440 | 158,720 | 204,800 | 634,880 | ≥819,200 | 634,880 | ≥819,200 |
| Day 65 | 70 | 110 | 174,080 | 296,960 | ≥819,200 | ≥819,200 | ≥819,200 | ≥819,200 |
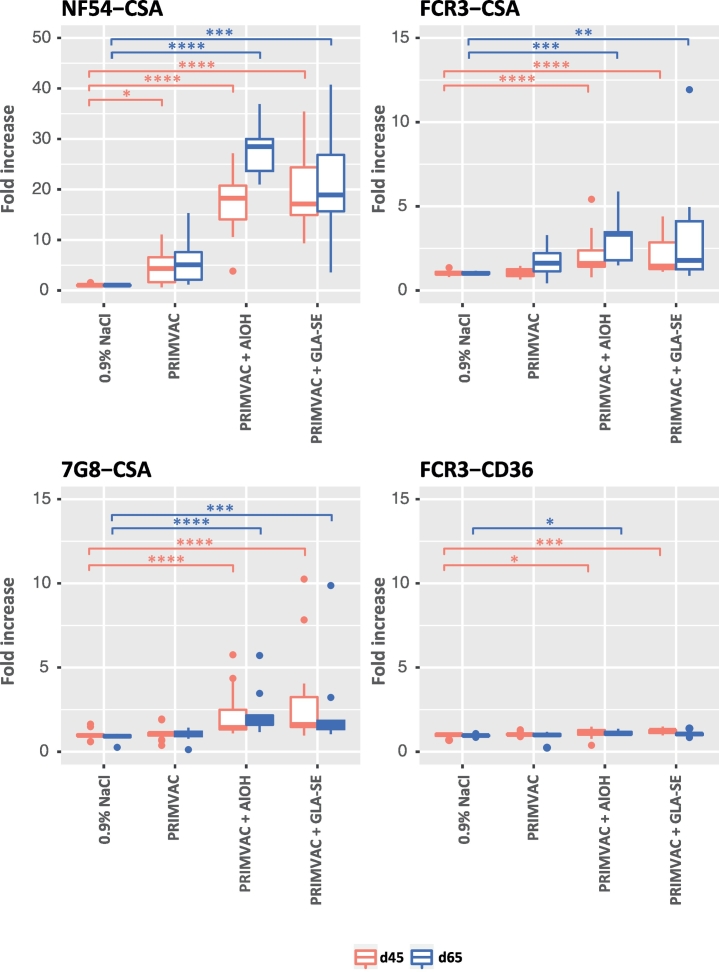
In order to test the capability of the PRIMVAC-induced antibodies to recognize native VAR2CSA from different parasite strains, sera samples were incubated with erythrocytes infected with parasite strains of different genetic background and selected for either CSA-binding or CD36-binding phenotypes. Group comparison analysis (vs NaCl control group) revealed that treatment with PRIMVAC alone was able to generate anti-PRIMVAC antibodies capable to significantly recognize VAR2CSA present at the surface of erythrocytes infected with the autologous parasite strain NF54 (Fig. 4). When adjuvanted with either Alhydrogel® or GLA-SE, PRIMVAC treatment led to antibody responses at Day 45 and Day 65, which strongly recognize NF54-CSA IEs but also cross-react with FCR3-CSA and 7G8-CSA expressing heterologous VAR2CSA variants sharing around 80% identities with the 3D7 sequence (Supplementary Fig. 2). A statistically significant recognition was observed with FCR3-CD36 for the PRIMVAC/Alhydrogel® and PRIMVAC/GLA-SE groups but amplitude of the response (fold increase day 45/Day 65 vs Day 0) was very low (medians ≤1274). These results are in line with those obtained in a second type of statistical analysis, in which the adjuvant effect was assessed (vs PRIMVAC alone). Addition of Alhydrogel® or GLA-SE to PRIMVAC treatment significantly increased both the recognition of native VAR2CSA from the autologous strain NF54 but also the amplitude of cross-reactivity towards FCR3-CSA and 7G8-CSA. No significant differences were observed when comparing Alhydrogel® to GLA-SE (Supplementary Fig. 3).
Fig. 4.
Immune recognition of erythrocytes infected by different parasite strains (NF54, FCR3, and 7G8) selected for different adhesive phenotypes (CSA and CD36) by vaccination-induced antibodies directed towards native VAR2CSA. Infected erythrocytes were incubated with individual rat serum samples diluted 1:50 in PBS 1% BSA. Erythrocyte-bound IgGs were detected using an anti-rat IgG PE-conjugated antibody. Cells were then subjected to flow cytometry analysis. Results are expressed as the fold-increase (FI) in geometrical mean fluorescence intensity (PE) of the immune serum (Day 45 or Day 65) as compared to the respective non-immune serum (Day 0); [FI = MFIgeo immune/MFIgeo pre-immune]. Kruskal-Wallis one-way ANOVA on ranks test was performed for each of the 8 parasite strain/time of sacrifice combinations separately, followed by Dunn's multiple comparison procedure in case of showing differences at a 0.05 significance level. Significant differences against the 0.9% NaCl group are shown: *, p ≤ .05; **, p ≤ .01; ***, p ≤ .001; ****, p ≤ .0001 (45-day groups: 0.9% NaCl and PRIMVAC + GLA-SE, n = 19; PRIMVAC, n = 20; PRIMVAC + AlOH, n = 18. 65-day groups, n = 10).
3.6.2. Inhibition of IEs selected for VAR2CSA expression
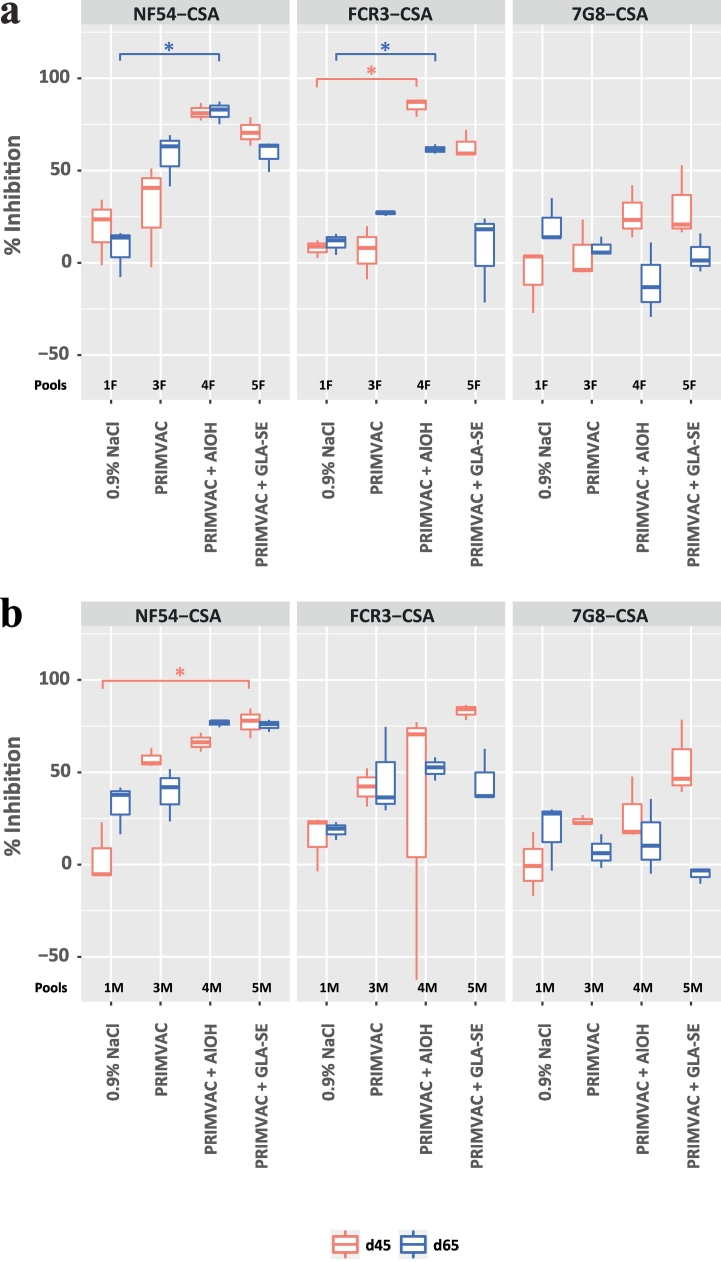
Due to the limited amount of pre-immune serum samples available to perform inhibition assays, sera from each group were pooled with regards to the sex of the animals (≈15 males or females per pool (Supplementary Table 7)). Sera from the PRIMVAC group alone were able to inhibit the interaction between CSA and erythrocytes infected by the autologous strain NF54 and selected for VAR2CSA expression (≈50%) (Fig. 5). The inhibition levels were increased when PRIMVAC was adjuvanted with either Alhydrogel® or GLA-SE to reach up to 80%. When formulated with adjuvants, PRIMVAC treatment was able to generate antibodies able, to some extent, to inhibit the adhesion of IEs expressing other CSA variants, the cross-inhibition towards FCR3-CSA being more important than towards 7G8-CSA IEs.
Fig. 5.
Inhibition of infected erythrocytes binding to CSA by vaccination-induced antibodies directed towards native VAR2CSA. NF54-CSA, FCR3-CSA, 7G8-CSA IEs were pre-incubated for 1 h with pools of (A) female or (B) male rat serum diluted 1:50 in PBS 1% BSA. Cells were then transferred into CSA-coated wells and incubated for 1 h, a sufficient time for complete cell sedimentation. CSA-binding inhibition was assessed by relative quantification of IEs remaining bound to the plate surface after washes. For a given condition, the percentage of inhibition was calculated as follow: [% = 100 − (ODimmune/ODpreimmune) × 100]. The OD measures were obtained in duplicate; means were used for calculation. Kruskal-Wallis one-way ANOVA on ranks test was performed for each of the 12 parasite strain/time of sacrifice/sex combinations separately, followed by Dunn's multiple comparison procedure in case of showing differences at a 0.05 significance level. Significant differences against the 0.9% NaCl group are shown: *, p ≤ .05; n = 3.The OD measures were obtained in duplicate; means were used for calculation. Kruskal-Wallis one-way ANOVA on ranks test was performed for each of the 12 parasite strain/time of sacrifice/sex combinations separately, followed by Dunn's multiple comparison procedure in case of showing differences at a 0.05 significance level. Significant differences against the 0.9% NaCl group are shown: *, p ≤ .05; n = 3.
4. Discussion
VAR2CSA stands today as the leading candidate to develop a vaccine that would protect pregnant women living in P. falciparum endemic areas against the severe clinical outcomes associated with PM. In order to progress towards the clinical assessment of such vaccine, a multi-system feasibility study identified a suitable heterologous expression platform to permit a prompt transition of PRIMVAC to cGMP vaccine production [24]. We describe in this study the successful cGMP production of the PRIMVAC vaccine.
An extensive process development was carried out to define optimal upstream and downstream production parameters for PRIMVAC. SDS-PAGE analysis of the final bulk product shows a high purity of the protein of interest reflected by a major band at the expected molecular weight. This was further confirmed by RP-HPLC with a main peak representing 99.7% of the total protein content. A minor contaminant was present around 20 kDa and was identified by mass spectrometry as an E. coli-derived protein (DNA-binding Protein from Starved cell; DPS) (data not shown). The drug product that was filled into sterile glass vials presented the same physico-chemical and biological properties than the drug substance of the bulk and met all the specifications in terms of purity and sterility (Table 1). Stability testing of the clinical PRIMVAC DP showed that the recombinant protein was stable up to 3 years when stored at −20 °C, thus suitable for early phase clinical testing. A slight decrease in protein concentration was noted over time without affecting the SDS-PAGE analysis profiles (no apparent degradation) suggesting that the protein loss could result from a minor precipitation process or from the adsorption of a fraction of the protein content to the glass wall of the storage vials.
Among a panel of different adjuvants tested in a previous study, Alhydrogel® and GLA-SE were determined as the most potent to generate anti-PRIMVAC antibodies capable of cross-reacting with native VAR2CSA from different parasite strains and of inhibiting the interaction between IEs and CSA [24]. The aluminium hydroxide adsorbs protein antigens, thereby ensuring that the inflammatory response to alum at the injection site is directed towards the co-administered antigen [33]. On the other hand, GLA-SE is a stable oil-in-water emulsion containing glucopyranosyl lipid A (GLA), which is a synthetic lipid A and Toll-like receptor (TLR) 4 agonist. PRIMVAC stability was assessed in presence of Alhydrogel® and GLA-SE for 4 h at room temperature (25 °C). Results confirmed that PRIMVAC is stable in the presence of adjuvants within the time frame needed for clinical administration of the vaccine (Fig. 2). Potency assays performed in mice also showed that PRIMVAC was functionally stable over time as injections with 0.5 μg or 5 μg of DP (formulated with Alhydrogel®) consistently seroconverted at least 80% of animals (Fig. 3).
The potential toxicity of PRIMVAC, alone or adjuvanted with Alhydrogel® or GLA-SE, was evaluated in male and female Sprague-Dawley rats. Aluminium hydroxide being the most widely used adjuvant in human vaccines with a well-established safety profile [34], the potential toxicity effect of Alhydrogel® alone was not evaluated in this study. Administration of PRIMVAC alone was clinically well tolerated. Changes were limited to some occasional local reactions at the injection sites (increased size and erythema) and no significant changes in haematology or biochemistry parameters were noted (Table 4 and Supplementary Tables 2–6). Administration of PRIMVAC adjuvanted with GLA-SE, or GLA-SE alone resulted in local reactions at the injection sites, associated with signs of inflammatory reaction, such as increased mean body temperature, higher white blood cell count and fibrinogen level and increased alpha-2 macroglobulin but reversibility was noted at the end of the treatment-free period and no adverse systemic changes were observed. This data are in line with the safety profiles of GLA-SE that has been assessed in human volunteers for a number of candidate vaccines against infectious diseases, such as influenza [35], leishmaniasis [36], schistosomiasis [37], tuberculosis [38]. A recent clinical study performed in malaria-naïve volunteers has demonstrated that a VAR2CSA-based vaccine (PAMVAC) adjuvanted with either Alhydrogel®, GLA-SE or GLA-LSQ was well tolerated, induced antibodies that recognized heterologous VAR2CSA recombinant proteins and inhibit the adhesion of the homologous VAR2CSA expressing FCR3 strain [39]. It is therefore reasonable to presume that PRIMVAC can be safely administered with either Alhydrogel® or GLA-SE as a three-dose course at dose levels up to 110 μg in a phase I clinical trial.
When injected in rats, PRIMVAC adjuvanted with either Alhydrogel® or GLA-SE generate antibodies able to recognize native VAR2CSA expressed at the surface of erythrocytes infected by different parasite strains (Fig. 4) but also to some extent inhibit the interactions between IEs and the placental ligand CSA (Fig. 5). The data presented in this study did not reveal any significant difference between Alhydrogel® and GLA-SE with regards to immunogenicity or to the functionality of vaccine-induced antibodies. Therefore, it was decided to use both of them in the future Phase Ia/Ib clinical trial.
In line with our previous results obtained in animal studies using a VAR2CSA-based His-tagged DBL1x-2x construct [24], we observed a discrepancy between IEs surface reactivity and binding inhibition. Even if the high magnitude of sera reactivity to native VAR2CSA expressed by the autologous parasites NF54 positively correlates with the high CSA-binding inhibition activity against NF54-CSA IEs, the relatively high inhibition observed for FCR3-CSA does not completely align with the modest IEs recognition. This suggests that, among the antibodies able to recognize native VAR2CSA, only a limited amount targets accessible conserved epitopes relevant for CSA-binding inhibition. Low levels of blocking antibodies appear to be sufficient to efficiently inhibit the interaction between FCR3-CSA IEs and CSA (Fig. 5) but the sensitivity limits of the flow cytometry technique does not allow a clear detection of their presence at the cell surface (Fig. 4). The existence of limited amounts of blocking antibodies is also supported by an apparent drop in sera inhibition activity between Day 45 and Day 65 (Fig. 5), whereas surface recognition levels remain unchanged between this two time points. Even if sufficient quantities of anti-VAR2CSA antibodies remain present to recognize IEs two weeks after the last immunization (Day 65), our data seems to indicated that the levels of blocking antibodies have dropped below the threshold needed for efficient CSA-binding inhibition.
Overall, inhibition of adhesion to CSA was relatively high for erythrocytes infected with the homologous parasite strain NF54 and the heterologous strain FCR3. Nevertheless, CSA-binding inhibition for erythrocytes infected with 7G8 was rather weak. The generation of strain-transcending (cross-clade) inhibitory antibodies being a desirable facet for a vaccine against PM, the evaluation of this key element in clinical phase will be of particular interest. Primary efficacy elements showed that PAMVAC vaccination induced antibodies able to inhibit the adhesion of CSA-binding erythrocytes infected with the autologous strain FCR3 [39]. Thus promising, these results call for additional investigations that would assess, in Plasmodium falciparum-exposed individuals, the capacity of vaccination-induced antibodies to react with different native VAR2CSA variants and to inhibit the interaction between CSA and erythrocytes infected with parasites originated from different parts of the world.
In conclusion, this work led to the successful production of around 4. 65 g of cGMP grade PRIMVAC DS. The subsequent DP conditioned in glass vials is stable and potent for up to 3 years upon storage at −20 °C. The present study also revealed an absence of toxicity when PRIMVAC was administered to rats. Furthermore, PRIMVAC in formulation with Alhydrogel® or GLA-SE generate antibodies in rats recognizing native VAR2CSA expressed at the IEs surface and to some extent inhibiting their interaction with CSA. PRIMVAC has now progressed to phase Ia/Ib clinical evaluation in healthy adult women in France and in Burkina Faso (ClinicalTrials.gov Identifier: NCT02658253).
Acknowledgments
Acknowledgments
The authors thank Sophia Hundt, Céline Maleike, Fabrice Somé and María del Mar Castro Noriega from EVI for project management support, Sophie Houard from EVI for scientific advice, Sophie Hallez, Sophie Rousseau and Pasqualina Mazzu from Novasep, Belgium for cGMP lot production and characterization.
Funding sources
This work was supported by a grant from the Bundesministerium für Bildung und Forschung (BMBF), Germany through Kreditanstalt für Wiederaufbau (KfW) (Reference No: 202060457) and through funding from Irish Aid, Department of Foreign Affairs and Trade, Ireland. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
Dr. Guadall has nothing to disclose. Dr. Chêne, Dr. Gangnard, Dr. Gamain reports grants from EVI, during the conduct of the study; Dr. Ginisty reports grants from EVI, grants from INSERM, during the conduct of the study; grants from Pharmaceutical and drug discovery companies outside the submitted work; Dr. Havelange reports grants from Bundesministerium für Bildung und Forschung (BMBF), Germany through Kreditanstalt für Wiederaufbau (KfW), grants from Irish Aid, Department of Foreign Affairs and Trade, Ireland, during the conduct of the study; grants, personal fees, non-financial support and other from European Vaccine Initiative, outside the submitted work; Dr. Viebig and Dr. Leroy reports grants from Bundesministerium für Bildung und Forschung (BMBF), Germany through Kreditanstalt für Wiederaufbau (KfW), grants from Irish Aid, Department of Foreign Affairs and Trade, Ireland, during the conduct of the study; grants, personal fees, non-financial support and other from Pharmaceutical and drug discovery companies, outside the submitted work;
Author contributions
A.C., S.G., H.G. and B.G. performed the experiments. AG performed the statistical analysis. A.C., S.G., A.G., H.G., O.L., N.H., N.K.V. and B.G. analyzed the results. A.C., N.K.V. and B.G. wrote the manuscript. B.G. oversaw all aspects of the study. All authors read and approved the final manuscript.
Availability of data and material
Materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.03.010.
Appendix A. Supplementary data
Supplementary material
References
- 1.World Malaria Report . World Health Organization; Geneva: 2018. ISBN: 978 92 4 156565 3. [Google Scholar]
- 2.Menendez C. Malaria during pregnancy. Curr Mol Med. 2006 Mar;6(2):269–273. doi: 10.2174/156652406776055186. [DOI] [PubMed] [Google Scholar]
- 3.White N.J., Pukrittayakamee S., Hien T.T., Faiz M.A., Mokuolu O.A., Dondorp A.M. Malaria. Lancet. 2014 Feb 22;383(9918):723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 4.Walter P.R., Garin Y., Blot P. Placental pathologic changes in malaria. A histologic and ultrastructural study. Am J Pathol. 1982 Dec;109(3):330–342. [PMC free article] [PubMed] [Google Scholar]
- 5.Agbor-Enoh S.T., Achur R.N., Valiyaveettil M., Leke R., Taylor D.W., Gowda D.C. Chondroitin sulfate proteoglycan expression and binding of Plasmodium falciparum-infected erythrocytes in the human placenta during pregnancy. Infect Immun. 2003 May;71(5):2455–2461. doi: 10.1128/IAI.71.5.2455-2461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai M., ter Kuile F.O., Nosten F., McGready R., Asamoa K., Brabin B. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007 Feb;7(2):93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 7.Umbers A.J., Aitken E.H., Rogerson S.J. Malaria in pregnancy: small babies, big problem. Trends Parasitol. 2011 Apr;27(4):168–175. doi: 10.1016/j.pt.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Brabin B.J. An analysis of malaria in pregnancy in Africa. Bull World Health Organ. 1983;61(6):1005–1016. [PMC free article] [PubMed] [Google Scholar]
- 9.McGregor I.A., Wilson M.E., Billewicz W.Z. Malaria infection of the placenta in the Gambia, West Africa; its incidence and relationship to stillbirth, birthweight and placental weight. Trans R Soc Trop Med Hyg. 1983;77(2):232–244. doi: 10.1016/0035-9203(83)90081-0. [DOI] [PubMed] [Google Scholar]
- 10.Fried M., Duffy P.E. Maternal malaria and parasite adhesion. J Mol Med (Berl) 1998 Mar;76(3–4):162–171. doi: 10.1007/s001090050205. [DOI] [PubMed] [Google Scholar]
- 11.Fried M., Nosten F., Brockman A., Brabin B.J., Duffy P.E. Maternal antibodies block malaria. Nature. 1998 Oct 29;395(6705):851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 12.Duffy P.E. Maternal immunization and malaria in pregnancy. Vaccine. 2003 Jul 28;21(24):3358–3361. doi: 10.1016/s0264-410x(03)00332-3. [DOI] [PubMed] [Google Scholar]
- 13.Salanti A., Staalsoe T., Lavstsen T., Jensen A.T., Sowa M.P., Arnot D.E. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003 Jul;49(1):179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 14.Salanti A., Dahlback M., Turner L., Nielsen M.A., Barfod L., Magistrado P. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med. 2004 Nov 1;200(9):1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viebig N.K., Gamain B., Scheidig C., Lepolard C., Przyborski J., Lanzer M. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Rep. 2005 Aug;6(8):775–781. doi: 10.1038/sj.embor.7400466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viebig N.K., Levin E., Dechavanne S., Rogerson S.J., Gysin J., Smith J.D. Disruption of var2csa gene impairs placental malaria associated adhesion phenotype. PLoS One. 2007 Sep 19;2(9):e910. doi: 10.1371/journal.pone.0000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivastava A., Gangnard S., Round A., Dechavanne S., Juillerat A., Raynal B. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc Natl Acad Sci U S A. 2010 Mar 16;107(11):4884–4889. doi: 10.1073/pnas.1000951107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clausen T.M., Christoffersen S., Dahlback M., Langkilde A.E., Jensen K.E., Resende M. Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J Biol Chem. 2012 Jul 6;287(28):23332–23345. doi: 10.1074/jbc.M112.348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava A., Gangnard S., Dechavanne S., Amirat F., Lewit Bentley A., Bentley G.A. Var2CSA minimal CSA binding region is located within the N-terminal region. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bockhorst J., Lu F., Janes J.H., Keebler J., Gamain B., Awadalla P. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Mol Biochem Parasitol. 2007 Oct;155(2):103–112. doi: 10.1016/j.molbiopara.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Avril M., Cartwright M.M., Hathaway M.J., Smith J.D. Induction of strain-transcendent antibodies to placental-type isolates with VAR2CSA DBL3 or DBL5 recombinant proteins. Malar J. 2011 Feb 11;10:36. doi: 10.1186/1475-2875-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahlback M., Jorgensen L.M., Nielsen M.A., Clausen T.M., Ditlev S.B., Resende M. The chondroitin sulfate A-binding site of the VAR2CSA protein involves multiple N-terminal domains. J Biol Chem. 2011 May 6;286(18):15908–15917. doi: 10.1074/jbc.M110.191510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen M.A., Resende M., de Jongh W.A., Ditlev S.B., Mordmuller B., Houard S. The influence of sub-unit composition and expression system on the functional antibody response in the development of a VAR2CSA based Plasmodium falciparum placental malaria vaccine. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0135406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chene A., Gangnard S., Dechavanne C., Dechavanne S., Srivastava A., Tetard M. Down-selection of the VAR2CSA DBL1-2 expressed in E. coli as a lead antigen for placental malaria vaccine development. NPJ Vaccines. 2018;3:28. doi: 10.1038/s41541-018-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dechavanne S., Srivastava A., Gangnard S., Nunes-Silva S., Dechavanne C., Fievet N. Parity-dependent recognition of DBL1X-3X suggests an important role of the VAR2CSA high-affinity CSA-binding region in the development of the humoral response against placental malaria. Infect Immun. 2015 Jun;83(6):2466–2474. doi: 10.1128/IAI.03116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chene A., Houard S., Nielsen M.A., Hundt S., D'Alessio F., Sirima S.B. Clinical development of placental malaria vaccines and immunoassays harmonization: a workshop report. Malar J. 2016 Sep 17;15:476. doi: 10.1186/s12936-016-1527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO technical report series, no. 927, annex 2. World Health Organization; Geneva: 2005. WHO guidelines on nonclinical evaluation of vaccine adjuvants and adjuvanted vaccines. [Google Scholar]
- 28.Avril M., Hathaway M.J., Srivastava A., Dechavanne S., Hommel M., Beeson J.G. Antibodies to a full-length VAR2CSA immunogen are broadly strain-transcendent but do not cross-inhibit different placental-type parasite isolates. PLoS One. 2011 Feb 7;6(2) doi: 10.1371/journal.pone.0016622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trager W., Jensen J.B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 30.Snounou G., Zhu X., Siripoon N., Jarra W., Thaithong S., Brown K.N. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans R Soc Trop Med Hyg. 1999 Jul-Aug;93(4):369–374. doi: 10.1016/s0035-9203(99)90120-7. [DOI] [PubMed] [Google Scholar]
- 31.Lelievre J., Berry A., Benoit-Vical F. An alternative method for Plasmodium culture synchronization. Exp Parasitol. 2005 Mar;109(3):195–197. doi: 10.1016/j.exppara.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Staalsoe T., Giha H.A., Dodoo D., Theander T.G., Hviid L. Detection of antibodies to variant antigens on Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry. 1999 Apr 1;35(4):329–336. doi: 10.1002/(sici)1097-0320(19990401)35:4<329::aid-cyto5>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 33.De Gregorio E., Caproni E., Ulmer J.B. Vaccine adjuvants: mode of action. Front Immunol. 2013;4:214. doi: 10.3389/fimmu.2013.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindblad E.B. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004 Oct;82(5):497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 35.Treanor J.J., Essink B., Hull S., Reed S., Izikson R., Patriarca P. Evaluation of safety and immunogenicity of recombinant influenza hemagglutinin (H5/Indonesia/05/2005) formulated with and without a stable oil-in-water emulsion containing glucopyranosyl-lipid a (SE+GLA) adjuvant. Vaccine. 2013 Nov 19;31(48):5760–5765. doi: 10.1016/j.vaccine.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 36.Coler R.N., Duthie M.S., Hofmeyer K.A., Guderian J., Jayashankar L., Vergara J. From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin Transl Immunology. 2015 Apr;4(4):e35. doi: 10.1038/cti.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tendler M., Almeida M., Simpson A. Development of the Brazilian anti schistosomiasis vaccine based on the recombinant fatty acid binding protein Sm14 plus GLA-SE adjuvant. Front Immunol. 2015;6:218. doi: 10.3389/fimmu.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coler R.N., Day T.A., Ellis R., Piazza F.M., Beckmann A.M., Vergara J. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial. NPJ Vaccines. 2018;3:34. doi: 10.1038/s41541-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mordmuller B., Sulyok M., Egger-Adam D., Resende M., de Jongh W.A., Jensen M.H. First-in-human, randomized, double-blind clinical trial of differentially adjuvanted PAMVAC, a vaccine candidate to prevent pregnancy-associated malaria. Clin Infect Dis. 2019 Jan:10. doi: 10.1093/cid/ciy1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.