Abstract
The pervasive reach of the inflammatory system is evidenced by its involvement in numerous disease states. Cardiovascular disease, marked by high levels of circulating inflammatory mediators, affects an estimated 83.6 million Americans. Similarly, human immunodeficiency virus (HIV) produces a paradoxical state of generalized immune activity despite widespread immunosuppression, and affects 35 million people worldwide. Patients living with HIV (PLWH) suffer from inflammatory conditions, including cardiovascular disease (CVD), at a rate exceeding the general population. In this combined disease state, immune mechanisms that are common to both CVD and HIV may interact to generate a progressive condition that contributes to the exacerbated pathogenesis of the other to the net effect of damage to the brain. In this review, we will outline inflammatory cell mediators that promote cardiovascular risk factors and disease initiation and detail how HIV-related proteins may accelerate this process. Finally, we examine the extent to which these comorbid conditions act as parallel, perpendicular, or progressive sequela of events to generate a neurodegenerative environment, and consider potential strategies that can be implemented to reduce the burden of CVD and inflammation in PLWH.
Keywords: inflammation, HIV, cardiovascular, cerebrovascular, AIDS, immune
INTRODUCTION
The reach of the inflammatory system into all other bodily processes is extraordinary, with evidence of inflammatory components in numerous disease states (Zhang et al., 2013), including marked detrimental effects to brain and behavior (Frank-Cannon et al., 2009; Shalev et al., 2009). Understanding the intricate overlap of inflammation within disease is critical as we have come to learn that inflammation can factor in disease initiation, maintenance, and progression. In a 2014 American Heart Association update, cardiovascular disease (CVD) was estimated to affect 83.6 million Americans and in 2010 it accounted for one of every three deaths (Go et al., 2014). The term ‘inflammation’ casts a long shadow in terms of CVD and contributes to disease initiation and progression from almost every angle (Libby, 2006; Zhang et al., 2013). Furthermore, the contribution of inflammation to the progression of atherosclerosis and cardiovascular events is slow and often silent leading to progressive damage that remains undetected until a subsequent event, such as stroke or heart attack, occurs (Lee et al., 2000; Bernick et al., 2001; Vermeer et al., 2007). This silent and long progression underscores the need for better disease recognition with careful consideration of inflammatory activity and highlights the potential for early intervention and therapeutic options.
The interplay of inflammation and CVD appear to be augmented in the context of human immunodeficiency virus (HIV) infection. Approximately 35 million people are infected with HIV worldwide (www.CDC.gov) and this population is increasing at a steady rate of nearly 50,000 new infections documented each year in the U.S. alone (CDC, 2012). Treatment advances have dramatically improved the prognosis for those infected with HIV. With adequate combination antiretroviral therapy (cART), people living with HIV (PLWH) have a life expectancy close to that of uninfected individuals (Samji et al., 2013), and the number of annual deaths due to acquired immune deficiency syndrome (AIDS) is beginning to decline (Murray et al., 2014). Despite these remarkable treatment advances, PLWH suffer from CVD and other inflammatory conditions more frequently than the general population (Ross et al., 2009; Gutierrez et al., 2013), leading to significant physical and economic burden (Foley et al., 2010). While some of these conditions may stem from side effects of chronic cART (Friis-Møller et al., 2003), HIV appears to generate excessive inflammation and cardiovascular complications independent of treatment (Barbaro et al., 2001; Kim et al., 2003; Singh et al., 2014). Some of the most common cardiovascular comorbidities seen in HIV – dilated cardiomyopathy, atherosclerosis, myocardial infarction, systemic and pulmonary hypertension, thrombosis and cerebrovascular damage (Barbaro et al., 2001) – are seen in both untreated patients and those receiving cART. In fact, elite controllers, defined as HIV infected patients who maintain CD4 counts and exhibit a comparatively slow progression toward AIDS without cART, have an “unexpectedly high degree” of atherosclerosis and an equally elevated degree of monocyte activation even when controlling for cART and CVD risk factors (Pereyra et al., 2012). Though low-grade viral replication may directly contribute to endothelial damage in elite controllers, data from this population illustrate a severe disconnect between CD4 count and coronary health.
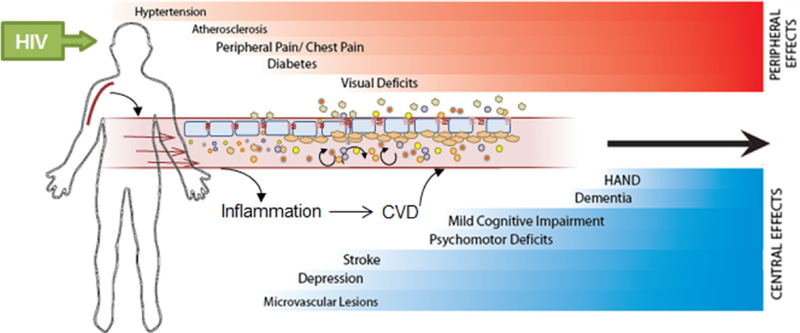
In addition to these more serious cardiovascular events, PLWH might experience somatic symptoms including shortness of breath, chest pain, and fatigue as well as behavioral changes in mood and cognition including comorbid depression and anxiety (Foley et al., 2010; Schroecksnadel and Kurz, 2012) which may be linked to immune activity (see Fig. 1). The pathogenesis of CVD involves disruption of endothelial integrity, a process that both gives rise to, and is fueled further by, inflammatory cascades. This apparent enhancement of immune function is paradoxical in a disease that is known for the generation of immunosuppression (Barbaro et al., 2001); however, other disorders and diseases, such as stroke, also exhibit this shift in immune system function to a paradoxical state which causes harm to the organism while failing to effectively ward off exogenous pathogens (Esmaeili et al., 2012; Nemeth et al., 2014). Although HIV progression leads to immunosuppression, the continuous replication of the virus also causes a state of generalized immune activation as reflected by viral load-dependent increases in the pro-inflammatory cytokines tumor necrosis factor (TNF) and interleukin (IL)-6, as well as inflammatory biomarkers such as neopterin and soluble tumor necrosis factor receptors (sTNF-R75) (Schroecksnadel and Kurz, 2012). Even when viral load is suppressed by cART, treated patients show residual levels of pro-inflammatory cytokines. Growing evidence suggests that this excessive inflammation is partially responsible for the elevated CVD risk seen in HIV.
Fig. 1.
Schematic depicts the timeline of somatic symptoms and cardiovascular events which occur as a result of increased inflammatory activation within peripheral and cerebral arteries. In people living with HIV (PLWH), these symptoms and events may be accelerated and exacerbated as a result of HIV-related immune activation. Together, cardiovascular disease (CVD) and HIV pathologies combine to form a progressive disease state with worsening consequences to overall health.
Chronic inflammation and CVD, individually and in combination, catalyze neurodegenerative processes leading to significant impairments in neural function and behavior (Kipnis et al., 2008; Frank-Cannon et al., 2009). Inflammatory responses to an acute stress or injury are often short-lived and contained; however, chronic inflammation stemming from disease or a dysregulated system can lead to a perpetuated inflammatory response which contributes to increased transcription of inflammatory cytokines, accelerated neuronal death, and an unstable blood–brain barrier (BBB; Frank-Cannon et al., 2009; Slavich and Irwin, 2014). Similarly, the role of inflammation in almost all aspects of CVD paves the way for atherosclerosis, increased release of inflammatory markers (Libby, 2006), ischemic events (Blake and Ridker, 2002), depression (Kales et al., 2005; Nemeroff and Goldschmidt-Clermont, 2012), and dementia (Hakim, 2011; Wint, 2011). As discussed below, although initially independent, the effects of chronic inflammation and CVD likely synergistically accelerate the damaging consequences to brain functioning and behavior.
In this review, we discuss the intricate relationship of CVD and inflammation and how HIV pathology interacts with inflammation to influence CVD progression and presentation leading to neural impairment. Specifically, we outline inflammatory cell mediators that promote cardiovascular risk factors and disease initiation and detail how HIV-related proteins may accelerate this process. Finally, we examine the extent to which these comorbid conditions act as parallel, perpendicular, or progressive sequela of events to generate a neurodegenerative environment, and consider potential strategies that can be implemented to reduce the burden of CVD and inflammation in PLWH.
Before understanding the mechanisms by which inflammation, CVD, and HIV may all interact to generate a progressive condition, it is first necessary to establish a framework for consideration by reviewing each of these conditions independently.
PERIPHERAL VASCULAR DISEASE
As a disease fostered in blood vessels, it is important to acknowledge the pervasiveness of vasculature and the inflammatory markers that mediate pathology within. The peripheral vasculature is a dynamic organ system designed to deliver oxygen and nutrients to all systems while maintaining a homeostatic state. The formation of blood vessels begins early in embryonic development with vasculogenesis and angiogenesis promoting blood vessel expansion until vasculature innervates all areas of the body (Liu et al., 2011). Inflammatory cells interact with vasculature from development, aiding in the migration of angioblasts to the sites of vasculogenesis (Schmidt et al., 2007). Once established, vessels of the body are quite ubiquitous, with an estimated length of 100,000 km, of which over 40,000 km are microvessels (Aird, 2005).
Blood vessels allow for trafficking of immune cells throughout the body and several disease states are characterized by flaws in cellular junctions that otherwise maintain a defense against pathogen leakage into the bloodstream. At these tight junctions, adherens, protein complexes that bridge the actin cytoskeletons of neighboring cells, maintain a tight linkage between endothelial cells that line the blood vessels. Within CVD, endothelial cell dysfunction precedes reductions in nitric oxide availability. Nitric oxide, a product of L-arginine and anti-atherosclerotic gas, prevents leukocyte adhesion, cellular migration, and adhesion molecule expression under normal, healthy conditions (Versari et al., 2009). In patients with HIV, systemic oxidative stress emanates from shed HIV-related proteins and contributes to increased activation of thromboxane-prostanoid receptor and endothelin 1 signaling (Wang et al., 2014). Independent of HIV, endothelial cell dysfunction within blood vessels begins the sequelae of events which provide a favorable environment for a pro-inflammatory cardiovascular state.
Several inflammatory cells are involved in the atherosclerotic process once the integrity of the endothelium has been compromised. Leukocytes begin by binding monocytes to the cellular wall. Monocytes, through the ingestion of lipids and lipoproteins, become macrophages which eventually comprise fat streaks and vessel plaques (Willerson and Ridker, 2004). In a feed-forward manner, other inflammatory cells including T cells and mast cells bind the endothelium and release factors which recruit additional inflammatory cells and initiate the migration of cytokines, chemokines, growth factors, and adhesion molecules to the site (Willerson and Ridker, 2004). Some of the most important contributing mediators of CVD initiation include monocytes, macrophages, and cellular adhesion molecules. These factors then release pro-inflammatory cytokines and chemokines, including C-reactive protein (CRP), TNF, IL-1, and IL-6 which aid in the maintenance and progression of CVD.
Monocytes are among the first inflammatory cells at the scene of a lesioned endothelium. Following monocyte differentiation to macrophages, these new cells release interferon-γ (IFN-γ), vascular cell adhesion molecules (VCAM-1), IL-8, IL-6 and matrix metallopeptidase 9 (MMP-9), among other inflammatory activators (Libby, 2006) that promote and participate in the progression of CVD. Illustrating the multi-faceted role of macrophages in the progression of CVD, these cells comprise the main component of ruptured plaques (Willerson and Ridker, 2004) and inflammatory cytokines maintain plaque instability by both preventing the formation of new collagen and promoting the destruction of existing collagen (Libby, 2006). Furthermore, the severity of CVD appears related more to the degree of inflammatory activation than the level of stenosis (Packard and Libby, 2008), suggesting that ongoing cellular activation, likely mediated by macrophages, may serve as a better predictor of CVD events than static disease state.
Intercellular Adhesion Molecule 1 (ICAM-1) and VCAM-1 proteins belong to the immunoglobulin superfamily and are expressed at low levels continually and upregulated by cytokines following immune stimulation, specifically through the increased transcription of TNF, IL-1, and nuclear factor-kappaB (NF-κB). VCAM-1 mediates tethering and rolling of monocytes and lymphocytes at lesion prone areas while ICAM-1 mediates arrest and the adhesion of cells to the endothelium. Model animal systems have helped to better define the roles of VCAM-1 and ICAM-1 in CVD. For example, atherosclerotic lesions occur in areas of blood vessels that experience disrupted or turbulent blood flow. Laminar flow prevents the adhesion of such inflammatory cells and results in elongated endothelial cells - an atheroprotective state (Libby, 2006). Turbulent flow allows for cellular adhesion and the increased expression of both VCAM-1 and ICAM-1. Interestingly, work in ApoE–/– mice, which develop spontaneous atherosclerosis characterized by high cholesterol levels, demonstrates the degree of atherosclerotic damage to be related to the expression of VCAM-1 only (Nakashima et al., 1998). ICAM-1, on the other hand, binds in response to shear stress of vessels, independent of atherosclerotic mechanisms. Future work targeting VCAM-1 activity may prove useful toward the slowing of CVD pathologies.
Accumulating evidence suggests that at multiple steps of the above-mentioned disease pathogenesis, HIV infection and HIV-related inflammation accelerate or compound processes that eventually lead to CVD. For example, direct viral infection of endothelial cells lining the liver, umbilical veins, bone-marrow stromal, or cerebral microvessels exacerbate initial endothelial damage observed in CVD (Gresele et al., 2012), which may both accelerate vascular damage as well as deplete the capacity for endothelial repair. In fact, PLWH have more circulating endothelial cells and endothelial-derived microparticles – which reflect endothelial damage and CVD risk – and fewer endothelial progenitor cells (EPCs), indicating impaired endothelial repair and reduced protection from CVD risk (Lopez et al., 2012). Furthermore, colony-forming-unit EPCs are depleted in cART-naïve infected patients and are shown to be particularly susceptible to direct HIV infection (Teofili et al., 2010). In addition, low CD4 cell counts correlate with reduced endothelial function as measured by flow-mediated dilation (Ho et al., 2012). Beyond the virus, cART itself has been linked to low levels of EPCs and worsening endothelial function (Gupta et al., 2012; Gómez-Garre et al., 2013), although short-term cART use did not have deleterious endothelial effects in one study (Francisci et al., 2009). HIV also strongly influences other key players responsible for the pathogenesis of CVD such as monocyte trafficking (Kim et al., 2003) and adhesion molecules. For example, whereas the HIV protein Tat upregulated the expression of adhesion molecules VCAM-1 and ICAM-1 and thereby increased monocyte adhesion (Song et al., 2007), the viral envelope protein gp120 reduced endothelial nitric oxide synthase by increasing ICAM-1. Finally, soluble VCAM-1 consistently correlated with multiple pro-inflammatory cytokines and neopterin, an inflammatory marker, in PLWH (Syed et al., 2013). Soluble VCAM-1 and TNF were in turn associated with internal carotid artery intima media thickness (IMT), a reliable marker for atherosclerosis (Ross et al., 2009).
Collectively, the available literature demonstrates that activation of inflammatory mediators and impaired endothelial cell health are consistent factors in the progression of both CVD and HIV within the periphery. The interaction of these factors within blood vessels of the periphery, while proximally deleterious, also facilitates migration and entry of inflammatory molecules into the brain. Within the brain, inflammatory activity mediates similar processes in the periphery; however, in the cerebral compartment the consequences precipitate alterations in behavior and cognition.
CEREBROVASCULAR DISEASE
Cardiovascular processes are not unique to the periphery. Vascular disease in the brain is linked to a greater risk of silent strokes, focal ischemic stroke (Chen et al., 2010), an increased risk of depression (Kales et al., 2005; Santos et al., 2009) and increased incidence of mild-cognitive impairment (Grau-Olivares and Arboix, 2009), dementia (Knopman, 2007), and Alzheimer’s disease (Farkas and Luiten, 2001; Purandare et al., 2012). Similar to peripheral arterial disease, cerebrovascular disease symptoms include thickening of arterial walls, microvascular lesions, and microembolic stroke (Farkas and Luiten, 2001; Vermeer et al., 2007; Chen et al., 2010). Reports of cerebrovascular disease are varied, ranging from 27% to 87% of the population over age 65 (Wong et al., 2002), underscoring the need to improve the understanding of the pathology and diagnosis of the disease.
According to the “Data collection on Adverse events of Anti-HIV Drugs” (D:A:D) study, a large prospective multi-cohort study, HIV positivity confers an increased risk for both cardiovascular and cerebrovascular disease (Friis-Møller et al., 2003). Furthermore, an additive effect of cerebrovascular disease and HIV in the brain contributes to cognitive decline, as both affect cognition as a common target. Although HIV does not readily infect neurons, it is capable of infiltrating through the BBB to cause a variety of neurotoxic consequences in the brain including excitotoxicity and excessive immune activation (Berman and Eugenin, 2012). Another such consequence is the establishment of reservoirs, or pools of latent HIV infectivity, in microglial cells, the brain’s resident macrophage (Kumar et al., 2014; Le Douce et al., 2014). Microglia and macrophages play prominent roles in the advancement of HIV to the brain (Surdo et al., 2013); however, due to the relative resistance of microglia/macrophages to HIV-induced apoptosis, these reservoirs are extremely difficult to eradicate (Kumar et al., 2014). In addition, it has recently been established that HIV can infect astrocytes and although astrocytes do not support viral replication, infection impairs their function (Churchill et al., 2014). Furthermore, several proteins involved in the progression of both HIV in the brain and inflammation contributing to cardiac pathology are housed in these reservoir cells (Le Douce et al., 2014).
The clinical outcome due to viral and/or HIV protein presence in the central nervous system is a spectrum of disorders collectively known as HIV-1-associated neurocognitive disorders (HAND). While the most severe forms of HAND such as HIV-associated dementia are responsive to cART, its milder forms affecting behavior, cognition, and motor function continue to be prevalent among infected individuals. Importantly, within a cohort of PLWH, cerebrovascular risk correlated with slower processing speed, deficits in learning and memory, and impaired executive functioning, when compared to those without known cerebrovascular risk (Foley et al., 2010). Conversely, cerebrovascular risk markers associated with decreased cognitive function – including IMT and increased ophthalmic artery resistance index (OARI) – are more common in PLWH compared to seronegative controls (Butters et al., 2008; Grima et al., 2012). These data suggest that cerebrovascular disease and HIV each exacerbate the pathogenesis of the other, to the net effect of worse cognitive outcome.
CEREBROVASCULAR IMMUNE MODULATORS
Mechanisms that underlie cerebrovascular processes mirror those of the periphery. Namely, higher levels of inflammation compounded by variations in blood flow promote the accumulation of vascular adhesion molecules, increased permeability of the BBB, cell extravasation into tissues, and the production of additional inflammatory factors. Throughout the body, blood flow and shear stress are driving factors in the localization and adherence of inflammatory cells (Turjman et al., 2014). Nowhere, however, is this more evident than in cases of aneurysm and stroke. Areas of the brain most prone to plaque or emboli formation are those at vessel bifurcations or areas of extreme curvature where blood flow is more frequently turbulent (Turjman et al., 2014). As a highly vascularized organ, the blood vessels of the brain are numerous and vary depending on the structure and metabolic needs of the tissue. Deep subcortical regions of the brain, characterized by terminal processes of microvessels, therefore, are exceedingly susceptible to rupture.
Peripheral inflammation is associated with increased inflammatory activity within the brain, and certain markers, including CRP, are associated with the future risk of stroke events and death (Blake and Ridker, 2002; Drake et al., 2011). Though little is known about how peripheral inflammatory activity contributes to neuropathology within the context of cardiovascular disease, animal models have shed light on the mediators of this relationship (Wohleb et al., 2012, 2013). Inhibition of the IL-1 receptor in a multiple sclerosis mouse model of inflammation markedly reduces overall cytokine activity including levels of VCAM-1, a significant contributor to cardiovascular disease progression (Denes et al., 2012). Similarly, in a ApoE–/– rodent model, diet-induced vascular distress (atheroma) is reduced in both the periphery and the brain following IL-1r ablation (Denes et al., 2012). Finally, IL-1 knockdown in mice reduced stress-induced inflammation and the manifestation of anxiety-like behaviors, again demonstrating the influential role of inflammation within the brain (Wohleb et al., 2014). Within CVD, little evidence supports the direct correlation of central inflammatory markers and functional changes such as cognitive decline; however, cognitive decline in the context of HIV (HAND) is most closely correlated with activated microglia and infiltrating monocytes rather than with other variables such as CNS viral load (Singh et al., 2014).
COMBINED CONSEQUENCES OF CVD AND HIV: WHAT IS THE NATURE OF THE RELATIONSHIP?
Cardiac chambers are a common source of cerebral emboli and result in large vessel stroke (Ogata et al., 2011). Much research to date has focused on strokes on a grand scale; however, strokes affecting arterioles and smaller vessels that feed deep gray matter are more common than classic stroke episodes. For example, it is estimated that five silent (asymptomatic ischemic events of small vessels) occur for every one recognized clinical stroke (Dempsey et al., 2010). In such cases, patient levels of CRP are noted to be higher than patients without silent brain infarction (Hoshi et al., 2005), again implicating inflammatory processes as a mechanism for the formation and likely rupture of microemboli. The downstream consequences of microembolic events are alarming, and much evidence to date supports the role of microvascular ischemia in changes of mood, cognition, and the severity of Alzheimer’s and Parkinson’s disease, to name a few (thoroughly reviewed by Taylor et al. (2013) and Nemeth et al. (2014)).
HIV can incur CVD-associated damages in the brain through several different mechanisms such as disruption of the BBB as well as direct disruption of the cerebrovascular endothelium. The former mechanism compromises BBB integrity through trafficking of infected and activated monocyte-macrophages, thus simultaneously promoting viral dissemination in the CNS (Kanmogne, 2012). Recent literature suggests additional mechanisms by which HIV can cross the BBB including more readily permeable complexes formed between activated platelets and monocytes (Singh et al., 2014). The resulting increase in permeability allows for infiltration of pro-inflammatory cytokines, thereby substantially elevating neuroinflammation. This process may become a feed-forward cycle because HIV likely acts through similar mechanisms as in the periphery to disrupt the endothelium of blood vessels in the brain. At a macro level, HIV can also cause dilatation of cerebral arteries by triggering an outward vascular remodeling of brain arteries (Gutierrez et al., 2013). Although not yet demonstrated in the context of HIV, arterial damage has been linked to changes in behavior, as evidenced from rodent models of both cardiac arrest (Neigh et al., 2009) and acute cerebral ischemia (Nemeth et al., 2012).
In addition to exerting direct effects on central blood vessels, HIV inflicts secondary damages to nearby brain tissue. White matter hyperintensities (WMHs) are lesions in the deep white matter appearing as hyperintensities on T2-weighted magnetic resonance imaging (MRI) scans, and depict areas of demyelination and mild gliosis. WMHs are thought to result from disruption of microvasculature in the brain and are predictive of an increased risk of stroke and other cerebrovascular events (Debette and Markus, 2009). Although WMHs are present in normal aging, an increase in WMHs was significantly more common among PLWH with lower CD4 count (Dooneief et al., 1996), thus suggesting that disease progression exacerbates this brain abnormality. Interestingly, a recent study by McMurtray et al. (2008) showed that among PLWH the presence of moderate WMHs was associated with decreased cortical volumes in the frontal lobes bilaterally, supporting previously determined links between WMHs and poorer performance on neuropsychological tests dependent on frontal lobe functions. Collectively, the research to date suggests that CVD and HIV bidirectionally interact to cause progressive amplification of both conditions to the ultimate deficit of cerebral health and behavior.
COMBINED CONSEQUENCES OF INFLAMMATION AND CVD: WHAT IS THE NATURE OF THE RELATIONSHIP?
The interaction of the immune system with cardiovascular processes is complex and often difficult to discern. Patients with elevated markers of inflammation are at an increased risk for both CVD and diabetes (Haffner, 2006), and cardiovascular events produce levels of circulating inflammatory cytokines elevated to a degree that resembles a “sepsis-like” state (Adrie et al., 2002). Atherosclerotic processes stem from inflammatory activation of cellular adhesion molecules, chemokines, and cytokines. These inflammatory molecules trigger oxidative stress and the accumulation of immune cells in perivascular fat and contribute to blood pressure changes and hypertension (Schiffrin, 2014). While the inflammatory ‘activator’ of atherosclerosis remains unclear, many consider inflammation to be a response to injury. These injuries, per se, are triggered by cigarette smoking, hyperglycemia, and hypertension- and themselves comprise inflammatory processes seemingly forming a continuous loop of inflammatory activation and CVD progression (Pearson et al., 2003; Packard and Libby, 2008). Similarly, HIV presents an injury-like stimulus which triggers inflammatory sequela (Schroecksnadel and Kurz, 2012).
Data available currently demonstrate that the influence of HIV infection on CVD and inflammation is twofold: first, HIV directly affects many of the key players involved in CVD pathogenesis at various stages, including endothelial integrity, lipid composition, coagulation, and monocyte trafficking, thus potentiating CVD-triggered inflammation. Second, the generalized state of inflammation caused by the presence of HIV and its gene products potentially perpetuates the CVD-inflammation loop by rendering some of the above-mentioned processes more likely. Even when viral load is suppressed and deleterious immune activation is reduced through the use of cART, both residual low-grade inflammation and toxic effects of cART continue to contribute to this loop. Furthermore, HIV-associated comorbidities such as drug abuse may also independently elevate inflammation and CVD risk. For example, morphine – a substance commonly abused by PLWH – accelerates neuroinflammation through enhancing vascular permeability in brain endothelial cells (Wen et al., 2011).
Research in model animal systems has helped to systematize many aspects of immune-cardiovascular process interactions. Work in a mouse model of cardiac arrest/cardiopulmonary resuscitation (CA/CPR) demonstrates that immune challenge in the form of antigen presentation prior to CA/CPR decreased survival from the procedures and CA/CPR decreased the antibody response to subsequent exposure (Neigh et al., 2005). These findings demonstrate the bidirectional relationship under laboratory conditions: cardiovascular events lead to immune suppression and immune suppression decreases survival following cardiovascular events. Further, a rodent model of microvascular infarction demonstrates that the induction of microvascular damage, independent of classic cardiovascular risk factors, leads to increases in central and peripheral inflammation and deficits in affective behavior (Nemeth et al., 2012) underscoring that inflammatory consequences following a vascular event, alone, are sufficient to impair functionality. Due to limitations in rodent models of HIV (Brehm et al., 2013), comprehensive research on the relationship between inflammation and CVD in the context of HIV has not been completed to date; however, new rodent models are being developed and initial assessments of cardiovascular implications of HIV within rodent models have recently been published (Hansen et al., 2013). Future work should focus on the cerebral response to cerebrovascular challenges in well-accepted rodent models of HIV. Given the progressive interactions between CVD and HIV and the apparently mediating role of inflammation, understanding of the neural response to ischemia in the context of HIV will be of critical importance to adequate treatment of PLWH.
BREAKING THE REVERBERATING LOOPS
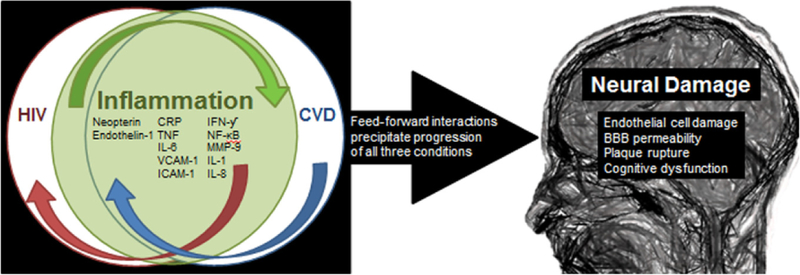
In summary, and to better illustrate the complex relationship of inflammation and CVD, inflammation can be thought of in one of two ways: first, inflammatory mechanisms may serve as the “risk factor” of atherosclerosis and progressive CVD, or alternatively, traditional cardiovascular risk factors may initially trigger the inflammatory response which then serves as a “risk marker” of CVD (Pearson et al., 2003). Though both models include inflammatory activation early in the progression of CVD, the true relationship is likely more entwined with inflammation serving as both a factor and marker of atherosclerotic processes. Once HIV is added into the equation (see Fig. 2), the virus serves as both an inflammatory and cardiovascular challenge leading to a progressive feed-forward relationship in response to the omnipresent virus which appears to have a pervasive influence even with adequate viral control. Left unchecked, this HIV-fueled undercurrent of inflammation and cardiovascular challenge catalyzes a neuroimmune response and potentially inflicts direct neural damage through cerebrovascular compromise. The initiation of neuroinflammation then likely promotes further cerebral viral entry which would lead to additional harboring of the virus and potentially increased replication establishing a third reverberating loop in this scenario. These ongoing interactions, which could each independently alter neural function and behavior, are likely to precipitate profound neural dysfunction and manifest as both affective disturbances and HIV-associated neurocognitive disorders. The progressive nature of these interactions strongly suggests that neural consequences of HIV should be prevented through early intervention focused on reducing chronic inflammation and CVD. The best practices to facilitate this intervention have not yet been established and is an area in critical need of study in order to improve the quality of life of PLWH and reduce co-morbid conditions.
Fig. 2.
The synergistic and feed-forward mechanisms of inflammatory activation within both CVD and HIV precipitate both conditions as well as the chronic inflammatory state. All three processes precipitate neural damage through endothelial cell damage, increased permeability of the blood-brain barrier, rupture of inflammatory plaques within the vessels and the development of cognitive impairment, including HIV-1-associated neurocognitive disorders (HAND).
Acknowledgments—
G.N.N. and M.B. partially supported by CNIHR Grant R24-AI067039-06 G.N.N. and C.L.N. supported by American Heart Association Predoctoral Fellowship.
Abbreviations:
- AIDS
acquired immune deficiency syndrome
- BBB
blood-brain barrier
- CA/CPR
cardiac arrest/cardiopulmonary resuscitation
- cART
combination antiretroviral therapy
- CRP
C-reactive protein
- CVD
cardiovascular disease
- EPCs
endothelial progenitor cells
- HAND
HIV-1-associated neurocognitive disorders
- HIV
human immunodeficiency virus
- ICAM-1
intercellular adhesion molecule 1
- IL
interleukin
- IML
intima media thickness
- PLWH
patients living with HIV
- TNF
tumor necrosis factor
- VCAM-1
vascular cell adhesion molecules
- WMHs
white matter hyperintensities
REFERENCES
- Adrie C et al. (2002) Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation 106(5):562–568. [DOI] [PubMed] [Google Scholar]
- Aird WC (2005) Spatial and temporal dynamics of the endothelium. J Thromb Haemostasis 3:1392–1406. [DOI] [PubMed] [Google Scholar]
- Barbaro G, Fisher SD, Lipshultz SE (2001) Pathogenesis of HIV-associated cardiovascular complications. Lancet 1(2): 115–124. [DOI] [PubMed] [Google Scholar]
- Berman J, Eugenin E (2012) Mechanisms of viral and cell entry into the central nervous system. Neurol AIDS:231–345.
- Bernick C, Kuller L, Dulberg C, Longstreth WT, Manolio T, Beauchamp N, Price T (2001) Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology: 105–111. [DOI] [PubMed]
- Blake GJ, Ridker PM (2002) Inflammatory bio-markers and cardiovascular risk prediction. J Int Med 252(4):283–294. [DOI] [PubMed] [Google Scholar]
- Brehm M, Shultz L, Luban J, Greiner D (2013) Overcoming current limitations in humanized mouse research. J Infect Dis(208 suppl. 2):S125–S130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Howard J, Dekosky ST, Becker JT (2008) Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialog Clin Neurosci 10:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2012) Estimated HIV incidence in the United States, 2007–2010
- Chen R-L, Balami JS, Esiri MM, Chen L-K, Buchan AM (2010) Ischemic stroke in the elderly: an overview of evidence. Nat Rev Neurol 6(5):256–265. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Cowley DJ, Wesselingh SL, Gorry PR, Gray LR (2014) HIV-1 transcriptional regulation in the central nervous system and implications for HIV cure research. J Neurovirol 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Markus HS (2009) The clinical importance of white matter hyperintensities on brain magnetic resonance imaging : systematic review and meta-analysis. BMJ:1–9. [DOI] [PMC free article] [PubMed]
- Dempsey RJ, Vemuganti R, Varghese T, Hermann BP (2010) A review of carotid atherosclerosis and vascular cognitive decline: a new understanding of the keys to symptomology. Neurosurgery 67(2):484–493 [discussion 493–4]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Drake C, Stordy J, Chamberlain J, McColl BW, Gram H, Crossman D, Francis S, Allan SM, Rothwell NJ (2012) Interleukin-1 mediates neuroinflammatory changes associated with diet-induced atherosclerosis. J Am Heart Assoc 1(3):e002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooneief GH, Bello JA, Todak GG, Tang MX, Marder KS, Stern Y, Mayeux RP (1996) Serial MRI in HIV infection with and without neurologic impairment. J Neuro-AIDS 1(4):49–57. [DOI] [PubMed] [Google Scholar]
- Drake C et al. (2011) Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun 25(6): 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili A, Dadkhahfar S, Fadakar K, Rezaei N (2012) Post-stroke immunodeficiency: effects of sensitization and tolerization to brain antigens. Int Rev Immunol 31(5):396–409. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG (2001) Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 64(6):575–611. [DOI] [PubMed] [Google Scholar]
- Foley J, Ettenhofer M, Wright MJ, Siddiqi I, Choi M, Thames AD, Mason K, Castellon S, Hinkin CH (2010) Neurocognitive functioning in HIV-1 infection: effects of cerebrovascular risk factors and age. Clin Neuropsychol 24(2):265–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisci D, Giannini S, Baldelli Franco, Leone M, Belfiori B, Guglielmini G, Malincarne L, Gresele Paolo (2009) HIV type 1 infection, and not short-term HAART, induces endothelial dysfunction. AIDS 23(5):589–596. [DOI] [PubMed] [Google Scholar]
- Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Molec Neurodegen 4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis-Møller N et al. (2003) Cardiovascular disease risk factors in HIV patients – association with antiretroviral therapy. Results from the DAD study. AIDS 17(8): 1179–1193. [DOI] [PubMed] [Google Scholar]
- Go AS et al. (2014) Heart disease and stroke statistics - 2014 update: a report from the American Heart Association. Circulation 129(3):e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Garre D, Estrada V, Ortega-Hernández A, Muñoz-Pacheco P, Serrano-Villar S, Avila M, Fuentes-Ferrer M, Tejerina T, Fernández-Cruz A (2013) Association of HIV-infection and antiretroviral therapy with levels of endothelial progenitor cells and subclinical atherosclerosis. J Acquir Immun Defic Synd 62(1):e23–e25. [DOI] [PubMed] [Google Scholar]
- Grau-Olivares M, Arboix A (2009) Mild cognitive impairment in stroke patients with ischemic cerebral small-vessel disease: a forerunner of vascular dementia? Exp Rev Neurother 9(8): 1201–1217. [DOI] [PubMed] [Google Scholar]
- Gresele P, Falcinelli E, Sebastiano M, Baldelli F (2012) Endothelial and platelet function alterations in HIV-infected patients. Thromb Res 129(3):301–308. [DOI] [PubMed] [Google Scholar]
- Grima P, Fabbiani M, Ciccarelli N, Tana M, Farina S, Colafigli M, Mondi A, Cauda R, Di Giambenedetto S (2012) Increased ophthalmic artery resistance index is associated with cognitive impairment in HIV-infected patients. J Infect 65(5):439–446. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Shen C, Moe SM, Kamendulis LM, Goldman M, Dubé MP (2012) Worsening endothelial function with efavirenz compared to protease inhibitors: a 12-month prospective study. PloS ONE 7(9):e45716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Elkind MSV, Petito C, Chung DY, Dwork AJ, Marshall RS (2013) The contribution of HIV infection to intracranial arterial remodeling: a pilot study. Neuropathology 33(3):256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner SM (2006) The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol 97(2A):3A–11A. [DOI] [PubMed] [Google Scholar]
- Hakim AM (2011) Depression, strokes and dementia: new biological insights into an unfortunate pathway. Cardiovasc Psychiatr Neurol 2011(Mci):649629. [DOI] [PMC free article] [PubMed]
- Hansen L, Parker I, Sutliff RL, Platt MO, Gleason RL (2013) Endothelial dysfunction, arterial stiffening, and intima-media thickening in large arteries from HIV-1 transgenic mice. Ann Biomed Eng 41 (4):682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JE, Scherzer R, Hecht FM, Maka K, Selby V, Martin JN, Ganz P, Deeks SG, Hsue PY (2012) The association of CD4+ T-cell counts and cardiovascular risk in treated HIV disease. AIDS 26(9):1115–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Kitagawa K, Yamagami H, Furukado S, Hougaku H, Hori M (2005) Relations of serum high-sensitivity C-reactive protein and interleukin-6 levels with silent brain infarction. Stroke 36(4):768–772. [DOI] [PubMed] [Google Scholar]
- Kales HC, Maixner DF, Mellow AM (2005) Cerebrovascular disease and late-life depression. Am J Geriatr Psychiatry 13(2):88–98. [DOI] [PubMed] [Google Scholar]
- Kanmogne G (2012) Monocyte-macrophages and viral central nervous system entry. Neurol AIDS:246–254.
- Kim W, Corey S, Alvarez X, Williams K (2003) Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukocyte Biol 74(5):650–656. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Derecki NC, Yang C, Scrable H (2008) Immunity and cognition: what do age-related dementia, HIV-dementia and “chemo-brain” have in common? Trends Immunol 29(10):455–463. [DOI] [PubMed] [Google Scholar]
- Knopman DS (2007) Cerebrovascular disease and dementia. Br J Radiol 80(Spec No 2):S121–S127. [DOI] [PubMed] [Google Scholar]
- Kumar A, Abbas W, Herbein G (2014) HIV-1 latency in monocytes/macrophages. Viruses 6(4): 1837–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douce V, Cherrier T, Riclet R, Rohr O, Schwartz C (2014) The many lives of CTIP2: from AIDS to cancer and cardiac hypertrophy. J Cell Phys 229(5):533–537. [DOI] [PubMed] [Google Scholar]
- Lee SC, Park SJ, Ki HK, Gwon HC, Chung CS, Byun HS, Shin KJ, Shin MH, Lee WR (2000) Prevalence and risk factors of silent cerebral infarction in apparently normal adults. Hypertension 36(1):73–77. [DOI] [PubMed] [Google Scholar]
- Libby P (2006) Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 83(2):456S–460S. [DOI] [PubMed] [Google Scholar]
- Liu D, Krueger J, Le Noble F (2011) The role of blood flow and microRNAs in blood vessel development. Int J Dev Biol 55(4–5):419–429. [DOI] [PubMed] [Google Scholar]
- Lopez M, Vispo E, Herrero D, Peris A, Corral A, Soriano V, Benito JM (2012) High risk of endothelial dysfunction in HIV individuals may result from deregulation of circulating endothelial cells and endothelial progenitor cells. AIDS Res Hum Retrov 28(7): 656–659. [DOI] [PubMed] [Google Scholar]
- McMurtray A, Nakamoto B, Shikuma C, Valcour V (2008) Cortical atrophy and white matter hyperintensities in HIV: the Hawaii Aging with HIV Cohort Study. J Stroke Cerebrovasc Dis 17(4):212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJL et al. (2014) Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 6736(14): 1–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R (1998) Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vase Biol 18(5):842–851. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Glasper ER, Bilbo SD, Traystman RJ, Courtney DeVries A (2005) Cardiac arrest/cardiopulmonary resuscitation augments cell-mediated immune function and transiently suppresses humoral immune function. J Cereb Blood Flow Metab 25(11): 1424–1432. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Karelina K, Zhang N, Glasper ER, Owens MJ, Plotsky PM, Nemeroff CB, DeVries AC (2009) Cardiac arrest and cardiopulmonary resuscitation dysregulates the hypothalamic–pituitary–adrenal axis. J Cereb Blood Flow Metab 29(10): 1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Goldschmidt-Clermont PJ (2012) Heartache and heartbreak - the link between depression and cardiovascular disease. Nat Rev Cardiol 9(9):526–539. [DOI] [PubMed] [Google Scholar]
- Nemeth CL, Shurte MS, McTigue DM, Nemeroff CB, Neigh GN (2012) Microembolism infarcts lead to delayed changes in affective-like behaviors followed by spatial memory impairment. Behav Brain Res 234(2):259–266. [DOI] [PubMed] [Google Scholar]
- Nemeth CL, Haroon E, Neigh GN (2014) Heartsick: psychiatric and inflammatory implications of cerebromicrovascular disease. Int J Geriatr Psychiatry 29(6):577–585. [DOI] [PubMed] [Google Scholar]
- Ogata J, Yamanishi H, Ishibashi-Ueda H (2011) Review: role of cerebral vessels in ischaemic injury of the brain. Neuropathol Appl Neurobiol 37(1)40–55. [DOI] [PubMed] [Google Scholar]
- Packard RRS, Libby P (2008) Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem 54(1):24–38. [DOI] [PubMed] [Google Scholar]
- Pearson TA et al. (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107(3)499–511. [DOI] [PubMed] [Google Scholar]
- Pereyra F, Lo J, Triant V, Wei J, Buzon MJ, Fitch KV, Hwang J, Campbell JH, Burdo TH, Williams KC, Abbara S, Grinspoon S (2012) Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 26:2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purandare N, Burns A, Morris J, Perry EP, Wren J, Mccollum C (2012) Association of cerebral emboli with accelerated cognitive deterioration in Alzheimer’s disease and vascular dementia. Am J Psychiatry 169(3):300–308. [DOI] [PubMed] [Google Scholar]
- Ross AC, Rizk N, O’Riordan MA, Dogra V, El-Bejjani D, Storer N, Harrill D, Tungsiripat M, Adell J, McComsey Ga (2009) Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 49(7): 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samji H et al. (2013) Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS ONE 8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M, Gold G, Kovari E, Herrmann FR, Bozikas VP, Bouras C, Giannakopoulos P (2009) Differential impact of lacunes and microvascular lesions on poststroke depression. Stroke 40(11): 3557–3562. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL (2014) Immune mechanisms in hypertension and vascular injury. Clin Sci 126(4):267–274. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Brixius K, Bloch W (2007) Endothelial precursor cell migration during vasculogenesis. Circ Res 101 (2): 125–136. [DOI] [PubMed] [Google Scholar]
- Schroecksnadel S, Kurz K (2012) Immune activation and neuropsychiatric symptoms in human immunodeficiency virus type 1 infection. Neurobehav HIV Med:1–13.
- Shalev H, Serlin Y, Friedman A (2009) Breaching the blood-brain barrier as a gate to psychiatric disorder. Cardiovasc Psychiatry Neurol 2009:278531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MV, Davidson DC, Jackson JW, Singh VB, Silva J, Ramirez SH, Maggirwar SB (2014) Characterization of platelet-monocyte complexes in HIV-1-infected individuals: possible role in HIV-associated neuroinflammation. J Immunol 192(10)4674–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR (2014) From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 140(3):774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HY, Ryu J, Ju SM, Park LJ, Lee JA, Choi SY, Park J (2007) Extracellular HIV-1 Tat enhances monocyte adhesion by upregulation of ICAM-1 and VCAM-1 gene expression via ROS-dependent NF-kappaB activation in astrocytes. Exp Mol Med 39(1):27–37. [DOI] [PubMed] [Google Scholar]
- Surdo M, Cortese M, Perno F, Aquaro S (2013) NeuroAIDS: virological aspects of HIV infection. J Biol Regul Homeost Agents 2(Suppl.):115–128. [PubMed] [Google Scholar]
- Syed SS, Balluz RS, Kabagambe EK, Meyer WAI, Lukas S, Wilson CM, Kapogiannis BG, Nachman SA, Sleasman JW (2013) Assessment of biomarkers of cardiovascular risk among HIV Type 1-infected adolescents: role of soluble vascular cell adhesion molecule as an early indicator of endothelial inflammation. AIDS Res Hum Retrov 29(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS (2013) The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry: 1–12. [DOI] [PMC free article] [PubMed]
- Teofili L et al. (2010) Endothelial progenitor cell trafficking in human immunodeficiency virus-infected persons. AIDS 24(16): 2443–2450. [DOI] [PubMed] [Google Scholar]
- Turjman AS, Turjman F, Edelman ER (2014) Role of fluid dynamics and inflammation in intracranial aneurysm formation. Circulation 129(3):373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer SE, Longstreth William T, Koudstaal PJ (2007) Silent brain infarcts: a systematic review. Lancet Neurol 6(7):611–619. [DOI] [PubMed] [Google Scholar]
- Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S (2009) Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care 32(suppl. 2):S314–S321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Melancon JK, Verbesey J, Hu H, Liu C, Aslam S, Young M, Wilcox CS (2014) Microvascular endothelial dysfunction and enhanced thromboxane and endothelial contractility in patients with HIV. J AIDS Clin Res 4(12):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Lu Y, Yao H, Buch S (2011) Morphine induces expression of platelet-derived growth factor in human brain microvascular endothelial cells: implication for vascular permeability. PloS ONE 6(6):e21707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerson JT, Ridker PM (2004) Inflammation as a cardiovascular risk factor. Circulation 109(21 suppl. 1):II2–II10. [DOI] [PubMed] [Google Scholar]
- Wint D (2011) Depression: a shared risk factor for cardiovascular and Alzheimer disease. Cleveland Clin J Med 78(suppl. 1):S44–S46. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP (2012) Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology 37(9):1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF (2013) Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 33(34): 13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF (2014) Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci 34(7): 2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BEK, Liao D-P, Hubbard LD, Mosley TH (2002) Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA 288(1):67–74. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Fan H, Xu J, Xiao Y, Xu Y, Li Y, Li X (2013) Network analysis reveals functional cross-links between disease and inflammation genes. Sci Rep 3:3426. [DOI] [PMC free article] [PubMed] [Google Scholar]