Abstract
A precious‐metal‐ and Cd‐free photocatalyst system for efficient H2 evolution from aqueous protons with a performance comparable to Cd‐based quantum dots is presented. Rod‐shaped ZnSe nanocrystals (nanorods, NRs) with a Ni(BF4)2 co‐catalyst suspended in aqueous ascorbic acid evolve H2 with an activity up to 54±2 mmol gZnSe −1 h−1 and a quantum yield of 50±4 % (λ=400 nm) under visible light illumination (AM 1.5G, 100 mW cm−2, λ>400 nm). Under simulated full‐spectrum solar irradiation (AM 1.5G, 100 mW cm−2), up to 149±22 mmol gZnSe −1 h−1 is generated. Significant photocorrosion was not noticeable within 40 h and activity was even observed without an added co‐catalyst. The ZnSe NRs can also be used to construct an inexpensive delafossite CuCrO2 photocathode, which does not rely on a sacrificial electron donor. Immobilized ZnSe NRs on CuCrO2 generate photocurrents of around −10 μA cm−2 in an aqueous electrolyte solution (pH 5.5) with a photocurrent onset potential of approximately +0.75 V vs. RHE. This work establishes ZnSe as a state‐of‐the‐art light absorber for photocatalytic and photoelectrochemical H2 generation.
Keywords: delafossite, hydrogen, photocatalysis, photocathode, zinc selenide
Artificial photosynthesis in which solar energy is stored in chemical fuels is a promising strategy for overcoming the temporal mismatch between renewable energy supply and demand.1 H2 is the most prominent example of a solar fuel as it can be generated by photoreduction of aqueous protons by a broad range of photocatalysts.2 Among the most active materials are chalcogenide nanocrystals based on CdS and CdSe.3 Despite the remarkable activities and stabilities shown by these materials,4 the toxicity and carcinogenic nature of cadmium represents a considerable obstacle for their wide‐spread application. Carbon‐based materials, such as carbon nitride,5 carbon dots,6 and conjugated organic polymers7 have recently been introduced as environmentally benign alternatives. While these materials are inexpensive and usually non‐toxic, their performances have yet to match those of Cd‐based photocatalysts to achieve high quantum yields for aqueous H2 production without precious and carcinogenic metals.
Here, we report ZnSe nanorods (NRs) as inexpensive Cd‐free light absorbers for efficient H2 evolution under visible‐light irradiation (Figure 1). The ZnSe NRs exhibit an activity approaching that of Cd‐based materials, even without an added co‐catalyst. Furthermore, we demonstrate that the high activity of the suspended ZnSe nanocrystals under sacrificial conditions can be translated to heterogeneous conditions by assembling a simple, precious‐metal‐free photoelectrode from ZnSe nanocrystals immobilized on p‐type delafossite CuCrO2.
Figure 1.
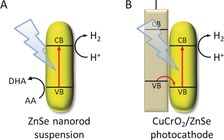
Schematic representation of the reported ZnSe nanorod photocatalyst system and its application for the construction of a noble‐metal‐free photocathode (CB: conduction band, VB: valence band, AA: ascorbic acid, DHA: dehydroascorbic acid).
ZnSe is a stable and inexpensive semiconductor with a direct bulk band gap of 2.7 eV,8 which enables absorption of near‐UV and some visible light. The conduction band (CB) is located at around −1.1 V vs. NHE (pH 0),9 providing ample driving force for the reduction of aqueous protons. Despite these favorable properties, ZnSe has received surprisingly little attention for solar fuel generation, unlike its cadmium analogues CdS and CdSe.10 Domen and co‐workers reported ZnSe/copper indium gallium selenide (CIGS) solid solution‐based photocathodes for H2 evolution11 with photocurrents up to 12 mA cm−2 at 0 V vs. the reversible hydrogen electrode (RHE) and onset potentials of +0.89 V vs. RHE.11b However, the complex photocathode assembly required a CdS charge extraction layer and a Pt proton‐reduction catalyst. While a number of reports have demonstrated the application of ZnSe‐based nanomaterials for photocatalytic dye degradation12 and water oxidation,13 only a few examples of Cd‐free ZnSe particles for photocatalytic H2 generation have been reported, all of which show low activity.14
We prepared ZnSe NRs by injecting trioctylphosphine/Se into an octadecane solution of zinc stearate at 300 °C, followed by a 25 min growth period.15 Surface modification of the as‐prepared stearate‐capped ZnSe NRs (ZnSe‐St) was achieved by ligand exchange with mercaptopropionic acid to give water‐soluble NRs (ZnSe‐MPA) and by reactive ligand removal with [Me3O][BF4] to give ligand‐free NRs (ZnSe‐BF4).16 Independent of the surface capping, the NRs are 5.2±0.6 nm in diameter and 30.0±4.8 nm long (aspect ratio 5.8±0.9), as determined from transmission electron microscopy (TEM, Figure S1 in the Supporting Information). Powder X‐ray diffraction (Figure S1 F) shows that the ZnSe NRs are obtained as a mixture of the zinc blende and wurtzite polymorphs, as previously observed with ZnSe nanorods synthesized by hot injection.17 The ZnSe NRs show UV‐visible light absorption up to about 440 nm (Figure S2 A) and two emission maxima separated by 0.097 eV in their photoluminescence (PL) spectra that can be attributed to differences in the band gaps of the two ZnSe polymorphs (Figure S2 B).18 Additional emissions at longer wavelengths likely result from trap states as previously observed with ZnSe nanocrystals.16a PL is reductively quenched by adding ascorbic acid (AA, Figure S2 C, D).
Figure 2 shows that ZnSe NRs are highly active photocatalysts for the reduction of aqueous protons to H2 under visible‐light irradiation (AM 1.5G, 100 mW cm−2, λ>400 nm) in the presence of AA. Under optimized conditions (pH 4.5, 0.4 m AA, 50 mg L−1 ZnSe, see Table S1 and Figure S3 in the Supporting Information for details on optimizing these parameters), ZnSe‐BF4 produced up to 33.6±2.0 mmol gZnSe −1 h−1 (Figure 2 A). To further enhance the photocatalytic activity of the ZnSe NRs, Fe(BF4)2, Co(BF4)2, Ni(BF4)2, and K2PtCl4 were tested as co‐catalysts (Figure 2 A). Ni showed the highest performance increase to 54.3±1.9 mmol gZnSe −1 h−1 at 20 μm, whereas K2PtCl4 quenched the photocatalytic activity almost completely. A low performance of Pt as a co‐catalyst has been previously observed with ligand‐free CdS.19 Pre‐formed Pt nanoparticles showed some activity, but still lower than without a co‐catalyst. Under the same conditions, ligand‐capped ZnSe‐MPA and ZnSe‐St NRs showed a lower H2 generation activity of 45.9±1.4 and 12.1±2.7 mmol gZnSe −1 h−1, respectively (Figure 2 B). This observation agrees with our previous studies, which demonstrated an enhanced H2 evolution activity of CdS nanocrystals upon ligand removal.20 Under simulated full‐spectrum solar irradiation (AM 1.5G, 100 mW cm−2), ZnSe‐BF4 generates up to 149±22 mmol gZnSe −1 h−1 and 95±27 mmol gZnSe −1 h−1 in the presence and absence of Ni(BF4)2, respectively (Figure 2 C). The internal quantum yield (IQE) under monochromatic light (λ=400 nm) was 50.2±3.6 % (35.9±2.6 % external quantum yield, EQE; Supporting Information, Table S2).
Figure 2.
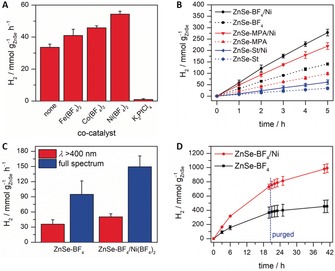
Photocatalytic H2 generation using aqueous ZnSe NRs. A) Ligand‐free ZnSe NRs in the presence of different co‐catalysts (3 h irradiation). B) Effect of the NR capping ligand. C) ZnSe‐BF4 under different irradiation spectra (1 h irradiation). D) Long‐term activity of ZnSe‐BF4 with the photoreactor being purged with N2 after 20 h. The cumulative amount of H2 is shown. Conditions unless stated otherwise: 50 mg L−1 ZnSe NRs, 0.4 m AA, pH 4.5, 20 μm Ni(BF4)2, 25 °C, 100 mW cm−2, AM 1.5G, λ>400 nm.
Long‐term experiments using ZnSe‐BF4 showed that H2 production is sustained over more than 40 h with a gradual decrease in rate (Figure 2 D). This decreasing activity is likely due to accumulation of dehydroascorbic acid (DHA) in solution. Photodegradation of ZnSe is only marginal, since separating ZnSe‐BF4 NRs after 20 h and re‐dispersing them in a fresh AA solution largely restored the activity (some material is lost during separation). In contrast, adding fresh ZnSe NRs had no effect on the activity (Supporting Information, Figure S4). Previous work has shown that the AA oxidation product DHA can inhibit the photocatalytic H2 production.21 UV/Vis spectra before and after prolonged irradiation show no degradation apart from an increase in scattering resulting from particle aggregation (Figure S5). Post‐catalysis TEM confirms the formation of aggregates with aspherical nanocrystalline features (Figure S6). Inductively‐coupled plasma optical emission spectroscopy (ICP‐OES) of ZnSe‐BF4/Ni isolated after 3 h irradiation showed an incorporation of 8.5±2.3 Ni atoms per ZnSe NR (<1 % of total added Ni), suggesting in situ formation of a heterogeneous Ni‐based catalyst on the NR surface.22 No H2 was generated without ZnSe, in the dark, or without an electron donor (Supporting Information, Table S3).
These data demonstrate that ZnSe‐BF4 NRs are efficient and stable light absorbers for aqueous H2 production, considerably outperforming previous Cd‐free ZnSe photocatalysts despite their blue‐shifted absorption spectrum compared to CdS. Previous reports have shown that a Pt/ZnO‐ZnSe nanocomposite generated 3 mmol g−1 h−1 under UV irradiation with 2.54 % EQE,14a and CoP‐decorated ZnSe nanobelts produced <1 mmol g−1 h−1 under visible‐light irradiation.14b However, the photocatalytic activities of ZnSe‐BF4 (33.6±2.0 mmol gZnSe −1 h−1, 25.9±1.2 % EQE) and ZnSe‐BF4/Ni (54.3±1.9 mmol gZnSe −1 h−1, 35.9±2.6 % EQE) approach those of Cd‐based photocatalysts23 such as Cd0.25Zn0.75Se/CoP (45.1 mmol g−1 h−1).14b CdSe quantum dots (QDs) combined with a Ni catalyst were shown to produce H2 with an IQE of 36±10 %.24 Higher performances were reported for CdS with different co‐catalysts25 such as MoS2 (96.7 mmol g−1 h−1, 46.9 % EQE),25a Ni2P (1200 mmol g−1 h−1, 41 % EQE),25b and Pt/PdS (29.2 mmol g−1 h−1, 93 % EQE).25c Without a co‐catalyst, up to 41 mmol g−1 h−1 26 and 2.8 % EQE27 were reported for CdS, and 239 mmol g−1 h−1 28 and 65.7 % EQE29 for CdxZn1−xS. Cd‐free alternatives such as CuInS2‐ZnS,30 carbon nitride,31 conjugated polymers,32 triazine frameworks,33 and polymer dots34 generally show much lower activities, although a recently reported NaCl/KCl‐treated carbon nitride/Pt material achieved up to 60 % EQE.35
Having established a good performance and stability of ZnSe nanorods for photocatalytic H2 production, even in the absence of an added co‐catalyst, we aimed to eliminate the sacrificial electron donor AA. The production of low‐value H2 gas at the expense of a sacrificial electron donor is not sustainable unless the electron donor is freely available, for example by photoreforming waste.19, 36 Instead, a nanocrystal‐sensitized photocathode can be assembled, where the nanocrystal provides electrons for the photocatalysis and a p‐type semiconductor accepts the photogenerated holes, replacing the chemical electron donor. Such systems enable overall water splitting through coupling with a photoanode for water oxidation.37
To this end, we immobilized ZnSe‐BF4 NRs on a CuCrO2 electrode. CuCrO2 is a wide‐band‐gap semiconductor (E g≈3.1 eV), which crystallizes in a delafossite‐type structure. Previous work has shown that modification of CuCrO2 with an organic dye and a nickel bis(diphosphine) catalyst enabled visible‐light‐driven proton reduction in aqueous solution.38 The characteristic high hole mobility, p‐type conductivity, and straightforward synthesis from abundant materials using solution processing techniques make CuCrO2 a suitable candidate for the coupling with ZnSe in a hydrogen‐generating photocathode.
The ZnSe nanorods were immobilized by drop‐casting (8 μL cm−2, 1.66 mg mL−1, acetonitrile) directly onto CuCrO2 electrodes (thickness approx. 300 nm, see Figure S7 in the Supporting Information; 13.4 μg ZnSe cm−2). EDX spectra confirmed an even distribution over the electrode surface (Figure S8). UV/Vis spectra of ZnSe‐modified CuCrO2 feature the characteristic absorptions of both CuCrO2 and ZnSe (Figure S9). Linear‐sweep voltammograms and chronoamperograms of ZnSe‐modified electrodes show enhanced photocurrents compared to the bare CuCrO2 electrode, with an onset potential of approximately +0.75 V vs. RHE (Figure 3), indicating the ability of photoexcited ZnSe nanorods to inject holes (E VB,ZnSe=1.6 V vs. RHE) into the valence band of CuCrO2 (E =1.0 V vs. RHE).38 Controlled potential photoelectrolysis (CPPE; Supporting Information, Figure S10) confirmed that the highly reducing CBZnSe electrons are used to reduce aqueous protons to H2. CPPE with a CuCrO2|ZnSe electrode maintained at E app=0 V vs. RHE and illuminated from the front side (100 mW cm−2, AM 1.5G, λ>400 nm) produced 35±7 nmol H2 over the course of 4 h with a Faradaic efficiency (FE) of 7±2 % (Table S5). Bare CuCrO2 produced no detectable H2, confirming the essential role of ZnSe in this system. The high dark current, as previously reported for CuCrO2,38 and dissolved H2 which is not sufficiently accounted for in low current‐generating systems39 both contribute to the modest FE. Adding Ni2+ as a co‐catalyst increases the overall H2 production yield, corresponding well to photocatalysis results (Supporting Information, Figure S11 and Table S5). Incident photon‐to‐current efficiency measurements showed an increased current in the 400–440 nm region for CuCrO2|ZnSe electrodes compared to bare CuCrO2, confirming the role of ZnSe NRs in this photocathode (Figure S12).
Figure 3.
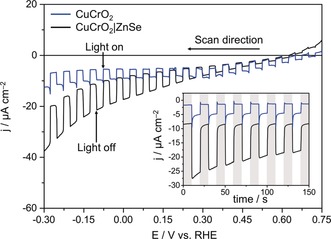
Linear‐sweep voltammograms under chopped light illumination for CuCrO2 (blue) and CuCrO2|ZnSe (black) electrodes, and chronoamperograms (inset) of the same electrodes at E app=0 V vs. RHE. Shading indicates dark chops. Conditions: Aq. Na2SO4 (0.1 m, pH 5.5), room temperature, 100 mW cm−2, AM 1.5G, λ>400 nm, scan rate 5 mV s−1. The photocurrent density was adjusted for an electrode area of 0.25 cm2.
H2‐generating QD‐sensitized photocathodes in the absence of a co‐catalyst have shown photocurrents of −60 μA cm−2 at 0.3 V vs. RHE with mercaptoacetic‐acid‐modified CdSe on NiO40 and −180 μA cm−2 at 0.5 V vs. RHE using a phenothiazine hole‐accepting ligand with CdSe on NiO.41 CuCrO2|ZnSe photoelectrodes generated −10 μA cm−2 at 0 V vs. RHE, comparable to the photocurrents observed with a molecular dye/catalyst assembly.38 The low photocurrent can be partly attributed to low light absorption, but the dominant limiting factor is likely a non‐ideal interface between CuCrO2 and the ZnSe NRs. This results in high charge recombination, limiting the number of electrons available for catalysis. Adding a H2 evolution co‐catalyst therefore only results in a small activity enhancement. Although this performance does not match that of the corresponding Cd‐based systems yet, it does demonstrate that the ZnSe NR photocatalyst can operate in the absence of a sacrificial reagent and in a photoelectrochemical cell. We expect future improvements for the integration of ZnSe into electrodes through CuCrO2 nanostructuring and ligand engineering to improve the CuCrO2/ZnSe interface,40, 41, 42 alternative assembly methods,43 and the integration of molecular catalysts,44 especially for CO2 reduction,16a making use of the highly reducing CB of ZnSe.
In summary, we have demonstrated that ZnSe nanorods are efficient light‐absorbers for solar‐driven H2 production, even without an added hydrogen‐evolution co‐catalyst. Their performance already approaches that of Cd‐containing quantum dots without exhibiting their carcinogenicity, highlighting the potential of designing novel inorganic materials for efficient photocatalysis. We showed that the ZnSe nanorods can also be integrated into photoelectrochemical cells, which paves the way to closed‐cycle solar fuel synthesis and we also envision its use in organic photoredox catalysis and photoreforming of waste and pollutants in future development.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This work was supported by the Christian Doppler Research Association (Austrian Federal Ministry of Science, Research and Economy and the National Foundation for Research, Technology and Development), the OMV Group (M.F.K., C.D.S., and E.R.), the EPSRC NanoDTC in Cambridge (EP/L015978/1; E.R. and C.E.C.), an EPSRC Underpinning Multi‐User Equipment Grant (EP/P030467/1), the Erasmus+ program (D.W.), the Erasmus program (A.S.) and the World Premier International Research Center Initiative, MEXT, Japan (K.L.O.). We thank Dr. Jane Leung, Miss Taylor Uekert, and Dr. Nikolay Kornienko for helpful discussions, and Dr. Heather Greer for help with the TEM measurements.
M. F. Kuehnel, C. E. Creissen, C. D. Sahm, D. Wielend, A. Schlosser, K. L. Orchard, E. Reisner, Angew. Chem. Int. Ed. 2019, 58, 5059.
Contributor Information
Dr. Moritz F. Kuehnel, http://www‐reisner.ch.cam.ac.uk.
Prof. Erwin Reisner, Email: reisner@ch.cam.ac.uk.
References
- 1. Tachibana Y., Vayssieres L., Durrant J. R., Nat. Photonics 2012, 6, 511–518. [Google Scholar]
- 2. Chen S., Takata T., Domen K., Nat. Rev. Mats. 2017, 2, 17050. [Google Scholar]
- 3. Li X.-B., Tung C.-H., Wu L.-Z., Nat. Rev. Chem. 2018, 2, 160–173. [Google Scholar]
- 4. Wakerley D. W., Ly K. H., Kornienko N., Orchard K. L., Kuehnel M. F., Reisner E., Chem. Eur. J. 2018, 24, 18385–18388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ong W.-J., Tan L.-L., Ng Y. H., Yong S.-T., Chai S.-P., Chem. Rev. 2016, 116, 7159–7329. [DOI] [PubMed] [Google Scholar]
- 6. Hutton G. A. M., Martindale B. C. M., Reisner E., Chem. Soc. Rev. 2017, 46, 6111–6123. [DOI] [PubMed] [Google Scholar]
- 7. Wang L., Zhang Y., Chen L., Xu H., Xiong Y., Adv. Mater. 2018, 30, 1801955. [DOI] [PubMed] [Google Scholar]
- 8. Ebina A., Fukunaga E., Takahashi T., Phys. Rev. B 1974, 10, 2495–2500. [Google Scholar]
- 9. Kaneko H., Minegishi T., Nakabayashi M., Shibata N., Kuang Y., Yamada T., Domen K., Adv. Funct. Mater. 2016, 26, 4570–4577. [Google Scholar]
- 10. Xu Y., Huang Y., Zhang B., Inorg. Chem. Front. 2016, 3, 591–615. [Google Scholar]
- 11.
- 11a. Kageshima Y., Minegishi T., Goto Y., Kaneko H., Domen K., Sustainable Energy Fuels 2018, 2, 1957–1965; [Google Scholar]
- 11b. Kaneko H., Minegishi T., Nakabayashi M., Shibata N., Domen K., Angew. Chem. Int. Ed. 2016, 55, 15329–15333. [DOI] [PubMed] [Google Scholar]
- 12.
- 12a. Xiong S., Xi B., Wang C., Xi G., Liu X., Qian Y., Chem. Eur. J. 2007, 13, 7926–7932; [DOI] [PubMed] [Google Scholar]
- 12b. Zhang L., Yang H., Yu J., Shao F., Li L., Zhang F., Zhao H., J. Phys. Chem. C 2009, 113, 5434–5443; [Google Scholar]
- 12c. Yao T., Zhao Q., Qiao Z., Peng F., Wang H., Yu H., Chi C., Yang J., Chem. Eur. J. 2011, 17, 8663–8670; [DOI] [PubMed] [Google Scholar]
- 12d. Chen P., Xiao T.-Y., Li H.-H., Yang J.-J., Wang Z., Yao H.-B., Yu S.-H., ACS Nano 2012, 6, 712–719; [DOI] [PubMed] [Google Scholar]
- 12e. Cho S., Jang J.-W., Lee J. S., Lee K.-H., Nanoscale 2012, 4, 2066–2071; [DOI] [PubMed] [Google Scholar]
- 12f. Liu B., Tian L., Wang Y., ACS Appl. Mater. Interfaces 2013, 5, 8414–8422; [DOI] [PubMed] [Google Scholar]
- 12g. Chen W., Zhang N., Zhang M. Y., Zhang X. T., Gao H., Wen J., CrystEngComm 2014, 16, 1201–1206; [Google Scholar]
- 12h. Tabar M. B., Elahi S. M., Ghoranneviss M., Yousefi R., CrystEngComm 2018, 20, 4590–4599; [Google Scholar]
- 12i. Huang X., Zou Y., Hao J., Jiang J., CrystEngComm 2018, 20, 4020–4024; [Google Scholar]
- 12j. Yousefi R., Azimi H. R., Mahmoudian M. R., Basirun W. J., Appl. Surf. Sci. 2018, 435, 886–893. [Google Scholar]
- 13. Chen D., Zhang H., Li Y., Pang Y., Yin Z., Sun H., Zhang L.-C., Wang S., Saunders M., Barker E., Jia G., Adv. Mater. 2018, 30, 1803351. [DOI] [PubMed] [Google Scholar]
- 14.
- 14a. Shaikh A. F., Arbuj S. S., Tamboli M. S., Naik S. D., Rane S. B., Kale B. B., ChemistrySelect 2017, 2, 9174–9180; [Google Scholar]
- 14b. Qiu B., Zhu Q., Xing M., Zhang J., Chem. Commun. 2017, 53, 897–900. [DOI] [PubMed] [Google Scholar]
- 15. Reiss P., Quemard G., Carayon S., Bleuse J., Chandezon F., Pron A., Mater. Chem. Phys. 2004, 84, 10–13. [Google Scholar]
- 16.
- 16a. Kuehnel M. F., Sahm C. D., Neri G., Lee J. R., Orchard K. L., Cowan A. J., Reisner E., Chem. Sci. 2018, 9, 2501–2509; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16b. Rosen E. L., Buonsanti R., Llordes A., Sawvel A. M., Milliron D. J., Helms B. A., Angew. Chem. Int. Ed. 2012, 51, 684–689. [DOI] [PubMed] [Google Scholar]
- 17. Cozzoli P. D., Manna L., Curri M. L., Kudera S., Giannini C., Striccoli M., Agostiano A., Chem. Mater. 2005, 17, 1296–1306. [Google Scholar]
- 18. Yeh C.-Y., Wei S.-H., Zunger A., Phys. Rev. B 1994, 50, 2715–2718. [DOI] [PubMed] [Google Scholar]
- 19. Wakerley D. W., Kuehnel M. F., Orchard K. L., Ly K. H., Rosser T. E., Reisner E., Nat. Energy 2017, 2, 17021. [Google Scholar]
- 20. Chang C. M., Orchard K. L., Martindale B. C. M., Reisner E., J. Mater. Chem. A 2016, 4, 2856–2862. [Google Scholar]
- 21.
- 21a. Guttentag M., Rodenberg A., Kopelent R., Probst B., Buchwalder C., Brandstätter M., Hamm P., Alberto R., Eur. J. Inorg. Chem. 2012, 59–64; [Google Scholar]
- 21b. Martindale B. C. M., Joliat E., Bachmann C., Alberto R., Reisner E., Angew. Chem. Int. Ed. 2016, 55, 9402–9406. [DOI] [PubMed] [Google Scholar]
- 22. Simon T., Bouchonville N., Berr M. J., Vaneski A., Adrović A., Volbers D., Wyrwich R., Döblinger M., Susha A. S., Rogach A. L., Jäckel F., Stolarczyk J. K., Feldmann J., Nat. Mater. 2014, 13, 1013–1018. [DOI] [PubMed] [Google Scholar]
- 23. Yuan Y.-J., Chen D., Yu Z.-T., Zou Z.-G., J. Mater. Chem. A 2018, 6, 11606–11630. [Google Scholar]
- 24. Han Z., Qiu F., Eisenberg R., Holland P. L., Krauss T. D., Science 2012, 338, 1321–1324. [DOI] [PubMed] [Google Scholar]
- 25.
- 25a. He J., Chen L., Wang F., Liu Y., Chen P., Au C.-T., Yin S.-F., ChemSusChem 2016, 9, 624–630; [DOI] [PubMed] [Google Scholar]
- 25b. Sun Z., Zheng H., Li J., Du P., Energy Environ. Sci. 2015, 8, 2668–2676; [Google Scholar]
- 25c. Yan H., Yang J., Ma G., Wu G., Zong X., Lei Z., Shi J., Li C., J. Catal. 2009, 266, 165–168; [Google Scholar]
- 25d. Gopannagari M., Kumar D. P., Reddy D. A., Hong S., Song M. I., Kim T. K., J. Catal. 2017, 351, 153–160; [Google Scholar]
- 25e. Sun Z., Yue Q., Li J., Xu J., Zheng H., Du P., J. Mater. Chem. A 2015, 3, 10243–10247. [Google Scholar]
- 26. Xu Y., Zhao W., Xu R., Shi Y., Zhang B., Chem. Commun. 2013, 49, 9803–9805. [DOI] [PubMed] [Google Scholar]
- 27. Li C., Han L., Liu R., Li H., Zhang S., Zhang G., J. Mater. Chem. 2012, 22, 23815–23820. [Google Scholar]
- 28. Jiang D., Sun Z., Jia H., Lu D., Du P., J. Mater. Chem. A 2016, 4, 675–683. [Google Scholar]
- 29. Du H., Liang K., Yuan C.-Z., Guo H.-L., Zhou X., Jiang Y.-F., Xu A.-W., ACS Appl. Mater. Interfaces 2016, 8, 24550–24558. [DOI] [PubMed] [Google Scholar]
- 30. Sandroni M., Gueret R., Wegner K. D., Reiss P., Fortage J., Aldakov D., Collomb M.-N., Energy Environ. Sci. 2018, 11, 1752–1761. [Google Scholar]
- 31.
- 31a. Wang Y., Bayazit M. K., Moniz S. J. A., Ruan Q., Lau C. C., Martsinovich N., Tang J., Energy Environ. Sci. 2017, 10, 1643–1651; [Google Scholar]
- 31b. Rahman M. Z., Tapping P. C., Kee T. W., Smernik R., Spooner N., Moffatt J., Tang Y., Davey K., Qiao S.-Z., Adv. Funct. Mater. 2017, 27, 1702384; [Google Scholar]
- 31c. Indra A., Acharjya A., Menezes P. W., Merschjann C., Hollmann D., Schwarze M., Aktas M., Friedrich A., Lochbrunner S., Thomas A., Driess M., Angew. Chem. Int. Ed. 2017, 56, 1653–1657. [DOI] [PubMed] [Google Scholar]
- 32.
- 32a. Sprick R. S., Bonillo B., Clowes R., Guiglion P., Brownbill N. J., Slater B. J., Blanc F., Zwijnenburg M. A., Adams D. J., Cooper A. I., Angew. Chem. Int. Ed. 2016, 55, 1792–1796; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32b. Woods D. J., Sprick R. S., Smith C. L., Cowan A. J., Cooper A. I., Adv. Energy Mater. 2017, 7, 1700479. [Google Scholar]
- 33.
- 33a. Bi S., Lan Z.-A., Paasch S., Zhang W., He Y., Zhang C., Liu F., Wu D., Zhuang X., Brunner E., Wang X., Zhang F., Adv. Funct. Mater. 2017, 27, 1703146; [Google Scholar]
- 33b. Kuecken S., Acharjya A., Zhi L., Schwarze M., Schomäcker R., Thomas A., Chem. Commun. 2017, 53, 5854–5857. [DOI] [PubMed] [Google Scholar]
- 34. Wang L., Fernández-Terán R., Zhang L., Fernandes D. L. A., Tian L., Chen H., Tian H., Angew. Chem. Int. Ed. 2016, 55, 12306–12310. [DOI] [PubMed] [Google Scholar]
- 35. Zhang G., Lin L., Li G., Zhang Y., Savateev A., Zafeiratos S., Wang X., Antonietti M., Angew. Chem. Int. Ed. 2018, 57, 9372–9376. [DOI] [PubMed] [Google Scholar]
- 36.
- 36a. Uekert T., Kuehnel M. F., Wakerley D. W., Reisner E., Energy Environ. Sci. 2018, 11, 2853–2857; [Google Scholar]
- 36b. Kuehnel M. F., Reisner E., Angew. Chem. Int. Ed. 2018, 57, 3290–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gibson E. A., Chem. Soc. Rev. 2017, 46, 6194–6209. [DOI] [PubMed] [Google Scholar]
- 38. Creissen C. E., Warnan J., Reisner E., Chem. Sci. 2018, 9, 1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Windle C. D., Massin J., Chavarot-Kerlidou M., Artero V., Dalton Trans. 2018, 47, 10509–10516. [DOI] [PubMed] [Google Scholar]
- 40. Liu B., Li X.-B., Gao Y.-J., Li Z.-J., Meng Q.-Y., Tung C.-H., Wu L.-Z., Energy Environ. Sci. 2015, 8, 1443–1449. [Google Scholar]
- 41. Li X.-B., Liu B., Wen M., Gao Y.-J., Wu H.-L., Huang M.-Y., Li Z.-J., Chen B., Tung C.-H., Wu L.-Z., Adv. Sci. 2016, 3, 1500282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abdellah M., Zhang S., Wang M., Hammarström L., ACS Energy Lett. 2017, 2, 2576–2580. [Google Scholar]
- 43. Lv H., Wang C., Li G., Burke R., Krauss T. D., Gao Y., Eisenberg R., Proc. Natl. Acad. Sci. USA 2017, 114, 11297–11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.
- 44a. Meng P., Wang M., Yang Y., Zhang S., Sun L., J. Mater. Chem. A 2015, 3, 18852–18859; [Google Scholar]
- 44b. Kuehnel M. F., Orchard K. L., Dalle K. E., Reisner E., J. Am. Chem. Soc. 2017, 139, 7217–7223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


