Summary
Retinal degenerative diseases (RDs) such as retinitis pigmentosa (RP) are a genetically heterogeneous group of disorders characterized by night blindness and peripheral vision loss, which caused by the dysfunction and death of photoreceptor cells. Although many causative gene mutations have been reported, the final common end stage is photoreceptor cell death. Unfortunately, no effective treatments or therapeutic agents have been discovered. Heat shock protein 70 (HSP70) is highly conserved and has antiapoptotic activities. A few reports have shown that HSP70 plays a role in RDs. Thus, we focused on the role of HSP70 in photoreceptor cell death. Using the N‐methyl‐N‐nitrosourea (MNU)‐induced photoreceptor cell death model in mice, we could examine two stages of the novel cell death mechanism; the early stage, including HSP70 cleavage through protein carbonylation by production of reactive oxygen species, lipid peroxidation and Ca2+ influx/calpain activation, and the late stage of cathepsin and/or caspase activation. The upregulation of intact HSP70 expression by its inducer is likely to protect photoreceptor cells. In this review, we focus on the role of HSP70 and the novel cell death signaling process in RDs. We also describe candidate therapeutic agents for RDs.
Keywords: HSP70 heat shock proteins, Photoreceptor, Protein carbonylation, Retina, Retinitis pigmentosa
Introduction
Retinitis pigmentosa (RP) is one of the major retinal degenerative diseases (RDs), which are caused by photoreceptor cell death 1. At least 50 million people have these diseases, and no effective drugs have been discovered. Animal models of RP have led to a better understanding of the disease pathology and to the development of therapeutic strategies aimed at curing or slowing down the genetic disorder 2. It is not easy to choose an appropriate genetic model for RP because there are many causative genes 3; more than 30 genes and more than 100 rhodopsin mutations are related to RP. Although animal models of RP have a variety of genetic backgrounds (Table 1) 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, the final common end stage of RP is photoreceptor cell death.
Table 1.
Genetic models for RP
| Animal models | Genotypes | Genes | Site of origin | References | |
|---|---|---|---|---|---|
| Mice | Natural | Peripherin‐rds | Peripherin‐rds | Null mutation in the rds/peripherin gene | 4 |
| Rd | Peripherin‐rds | rd/rd (retinal degeneration) mice | 5 | ||
| Rd‐1 | PDE6B | Nonsense mutation in exon 7 of the Pde6b gene in all mouse strains with the rd1 mutation | 6 | ||
| Rd‐4 | – | Inversion encompasses nearly all of Chromosome 4 | 7 | ||
| Rd‐8 | CRB1 | Single base deletion in the Crb1 gene | 8 | ||
| Rd‐10 | PDE6B | Mutation in PDE6b | 9 | ||
| Rd‐12 | RPE65 | Homozygous for the rd12 mutation | 10 | ||
| Rd‐16 | CEP290 | In‐frame deletion in a centrosomal protein CEP290 | 11 | ||
| Transgenic | 307 1‐bp del | Peripherin‐rds | Single base deletion at codon 307 of the rds‐peripherin gene in mice | 12 | |
| C214S | Peripherin‐rds | Peripherin‐rds with the C214S (Cys214–>Ser) missense mutation | 13 | ||
| Crx knockout | Cone‐rod homeobox | Cone‐rod homeobox gene knockout | 14 | ||
| Knockout RPE65 | Rhodopsin | Mice that lack the visual pigment rhodopsin (Rpe65‐/‐) | 15 | ||
| l‐255/256 | Opsin | Mutant opsin gene with a 3‐bp deletion of isoleucine at codon 255/256 | 16 | ||
| L185P/Rom‐1 null | Peripherin‐rds | Doubly heterozygous for a mutation in RDS causing a leucine 185 to proline substitution in rds (L185P) and a null mutation in ROM1 | 17 | ||
| MERTK KO | MERTK | Homozygous for a targeted disruption of the Mer receptor tyrosine kinase gene (mer(kd)) | 18 | ||
| NMF282 | PDE6A | Ethyl nitrosourea (ENU) mutagenesis | 19 | ||
| NMF363 | PDE6A | Ethyl nitrosourea (ENU) mutagenesis | 19 | ||
| P216L | Peripherin‐rds | Proline 216 to leucine (P216L) amino acid substitution in rds/peripherin | 20 | ||
| P23H | Rhodopsin | Missense mutation (P23H) in the rhodopsin gene | 21 | ||
| P347S | Rhodopsin | Rhodopsin, proline‐347 to serine (P347S) mutation | 22 | ||
| Q344ter | Rhodopsin | Heterozygotes with the glutamine‐344‐to‐ter (Q344ter) mutations in the rhodopsin gene (stop codon mutation) | 23 | ||
| Rd12j (NMF137) | PDE6B | Missense point mutation in exon 16 of the beta‐subunit of rod phosphodiesterase gene, (PDE6B) | 9 | ||
| Rpe65−/− | RPE65 | Rpe65‐deficient (KO) | 24 | ||
| Sema4A‐deficient | Sema4A | Sema4A‐deficient | 25 | ||
| Sema4A F350C | Sema4A | Knock‐in mouse lines with corresponding mutations (F350C) in the Sema4A gene | 25 | ||
| Rat | Natural | RCS | MERTK | Small deletion of RCS DNA that disrupts the gene encoding the receptor tyrosine kinase Mertk | 26 |
| Transgenic | P23H | Rhodopsin | Transgenic rat that express P23H rhodopsin | 27 | |
| S334ter | Rhodopsin | Rhodopsin mutation S334ter | 28 | ||
| Chickens | Natural | Rd | GC1 | Null mutation in the photoreceptor guanylate cyclase (GC1) gene | 29 |
| Rdd | PDE6A | Mutation in PDE6A | 30 | ||
N‐Methyl‐N‐nitrosourea (MNU), an alkylating agent, causes photoreceptor cell loss and significantly decreases the outer nuclear layer thickness within 1 week after intraperitoneal injection 31, 32, 33. MNU selectively damages photoreceptor cells; no other retinal cells are TUNEL positive. Thus, we used the MNU model to study the mechanism of photoreceptor cell death.
Heat shock protein 70 (HSP70) plays an important role in protecting cells against various stresses. However, a few reports have shown the effect of HSP70 on photoreceptor cell death in RDs. In the present review, we describe the role of HSP70 in photoreceptor cell death and discuss the possibility of HSP70 inducers as a new therapeutic tool for RDs.
The Mechanisms of MNU‐Induced Photoreceptor Cell Death
Some reports have suggested that MNU induced the generation of free radicals and cell death specifically in retinal photoreceptor cells. Accumulation of 8‐hydroxy‐deoxyguanosine, an indicator of oxidatively damaged DNA, and 4‐hydroxy‐2‐nonenal (4HNE), a reactive aldehyde species generated endogenously from decomposition of hydroperoxide of ω‐6 polyunsaturated fatty acids 34, was detected in MNU‐treated mouse retina 33, 35. MNU also causes a decrease in reduced glutathione, which effectively scavenges free radicals and other reactive oxygen species (ROS) 36, leading to an imbalance between the production of ROS and antioxidants.
Intraperitoneal injection of MNU induces the accumulation of intracellular Ca2+ in the retina and increases calpain activation, as measured by α‐spectrin proteolysis products, which leads to photoreceptor cell death 37, 38.
MNU‐induced photoreceptor cell loss is caused by a decrease in antiapoptotic Bcl‐2 protein, an increase in proapoptotic Bax protein, and the activation of caspase cascades 39, 40. Caspase‐3, caspase‐6, and caspase‐8 activities were increased within 3 days after MNU injection.
Although such molecular mechanisms of the MNU‐induced photoreceptor cell loss have been described, the total process of the cell death signaling remains obscure. Elucidation of the key molecule that connects these molecular mechanisms is necessary to clarify the photoreceptor cell death signaling process.
Early and Late Stages of MNU‐Induced Photoreceptor Cell Death Processes
The HSP70 family is a family of conserved and ubiquitously expressed heat shock proteins. HSP70 is a central component of the cellular network of molecular chaperones and folding catalysts and protects cells from various stresses. Although HSP70 immunoreactivity is localized in the outer nuclear layer and the inner segments of the retina 41, a few studies have reported the role of HSP70 in RDs. Thus, we investigated the role of HSP70 on MNU‐induced photoreceptor cell death 33. Under pathological conditions of neuronal tissues, such as glaucoma and ischemic/reperfusion of the hippocampus, HSP70 is a common substrate of calpain 42. Carbonylated HSP70 by 4HNE is much more vulnerable to calpain cleavage 43. We found that the levels of 4HNE were clearly increased in MNU‐injected mouse retina. 4HNE is highly reactive and may be considered as a secondary toxic messenger that disseminates and augments initial free radical events 44, 45. Upon the reaction with protein, 4HNE specifically reacts with nucleophilic amino acids, such as cysteine, histidine, and lysine to form their Michael addition adducts possessing carbonyl functionality 46, 47. Thus, HSP70 may be carbonylated by the accumulated 4HNE in MNU‐treated mouse retina. In addition, we confirmed that HSP70 was rapidly and calpain‐dependently cleaved after MNU treatment. Our results indicate that HSP70 cleavage might be involved in both oxidative stress and Ca2+/calpain‐mediated photoreceptor cell loss. Calpain‐mediated cleavage of HSP70 leads to lysosomal rupture and cell death through cathepsin because HSP70 stabilizes lysosomal membranes 48; this process is known as the calpain–cathepsin hypothesis 49, 50. On the other hand, HSP70 protects against neuronal apoptosis through the inhibition of caspase‐dependent apoptosis 51, 52. Thus, caspase‐dependent apoptosis occurs in downstream of HSP70 cleavage. Together, our findings suggest that cleavage of HSP70 is a key event that connects the mechanisms of MNU‐induced photoreceptor cell death (Figure 1). Focusing on HSP70, the MNU‐induced cell death signaling process can be divided into early and late stages. We defined the early stage as HSP70 cleavage through protein carbonylation by oxidative stress, 4HNE production, and Ca2+/calpain activation. The late stage includes the events after HSP70 cleavage, including cathepsin and/or caspase activation.
Figure 1.
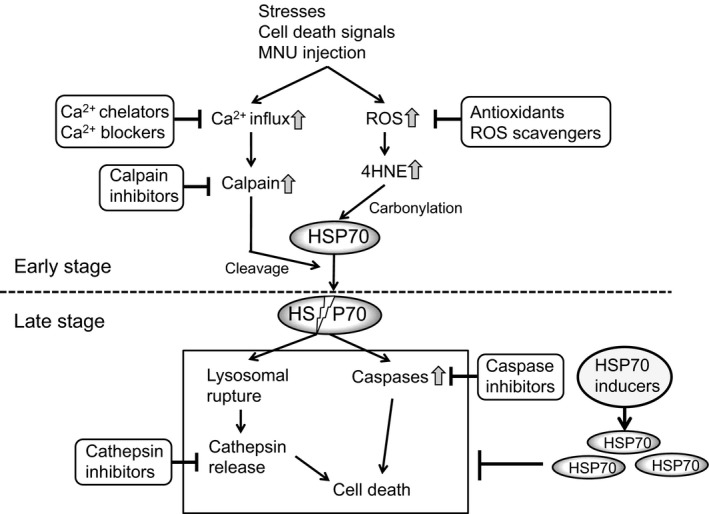
The mechanism of MNU‐induced photoreceptor cell death and the candidate therapeutic agents for RDs.
HSP70 Induction Prevented Photoreceptor Cell Death by MNU
To determine whether HSP70 could protect photoreceptor cell death by MNU, we used valproic acid (VPA), a well‐known HSP70 inducer 53. VPA significantly inhibited MNU‐induced retinal thinning and TUNEL‐positive photoreceptor cell number through HSP70 induction. Coadministration of VPA and HSP inhibitor abolished the protective effect of HSP70; thus, HSP70 plays a crucial role in the protection of photoreceptor cells. Calpain inhibitor also protects photoreceptor cells because of the suppression of HSP70 cleavage. VPA failed to protect HSP70 from MNU‐induced cleavage, but increased the expression levels of intact HSP70. Both VPA and calpain inhibitor completely blocked caspase‐3 activation by MNU. In addition, we previously reported that geranylgeranylacetone (GGA), another HSP70 inducer, also attenuated the photoreceptor cell death by MNU through HSP70 induction 33, 54. Thus, inhibition of HSP70 cleavage or induction of intact HSP70 may be possible therapeutic approaches for preventing photoreceptor cell death.
Cytoprotective Effects of HSP70
The photoreceptor cell layer is the primary site of HSP70 synthesis in the retina, and hyperthermia‐induced HSP70 in the photoreceptor layer prevents retinal photic injury 55, 56. In the retinal detachment‐induced retinal degeneration model, abolishment of HSP70 induction using HSP70−/− mice directly exacerbated photoreceptor apoptosis 57. Furthermore, HSP990, a HSP70 inducer, enhanced visual function and delayed photoreceptor degeneration in a rhodopsin mutation rat model 58. These results are in accordance with previous in vitro and in vivo studies that showed that abolishment of the HSP70 cytoprotective effect augments the initiation of the apoptotic cascade 59, 60, 61. Even in the CNS model, previous studies have reported extensive neuronal damage in HSP70−/− mice after ischemic brain injury, in which the neuronal expression of HSP70 can be regarded as a molecularly defined penumbra of protein denaturation 62. Thus, HSP70 overexpression directly increased the neuronal viability in various CNS degeneration models 63, 64. These reports showed that HSP70 directly prevented photoreceptor cell death in both genetic RP models and acquired models.
Candidate Therapeutic Agents for RDs
Based on the total image of photoreceptor cell death mechanism that we proposed, protein carbonylation by oxidative stress, Ca2+‐dependent protease activation, apoptosis‐related molecules, and HSP70 cleavage are involved in MNU‐induced photoreceptor cell death. The final common end stage of various pathogenic mechanisms in RDs is photoreceptor cell death. Therefore, protection of photoreceptor cells may be a useful therapeutic strategy for RDs. Some of the candidate therapeutic agents for RDs are listed in Table 2 [33, 35, 37, 39, 54, 58, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121]; these agents include therapeutic agents against the early stage of photoreceptor cell death (antioxidants, ROS scavengers, Ca2+ antagonists and calpain inhibitors), therapeutic agents against the late stage of photoreceptor cell death (cathepsin inhibitors and caspase inhibitors), and HSP70 inducers.
Table 2.
Candidate therapeutic agents for RDs
| Roles | Compounds | References | Roles | Compounds | References |
|---|---|---|---|---|---|
| ROS scavenger | 5‐S‐GAD | 65 | HSP70 inducer | 17‐AAG | 92 |
| /Antioxidant | Alpha lipoic acid | 66 | 17‐DMAG | 92 | |
| Astaxanthin | 67 | 2‐Cyclopenten‐1‐one | 93 | ||
| Carnosic acid | 68 | Alkannin | 94 | ||
| Curcumin | 69 | Arimoclomol | 95 | ||
| DHA | 70 | Bicyclol | 96 | ||
| Edaravone | 35 | Bimoclomol | 97 | ||
| Green tea extract | 71 | Carbenoxolone | 98 | ||
| Lutein | 72 | CdCl2 | 99 | ||
| Melatonin | 73 | Celastrol | 100 | ||
| N‐acetylcysteine | 74 | Curcumin | 101 | ||
| Unoprostone | 75 | Eupalinolide A/B | 102 | ||
| FLZ | 103 | ||||
| Ca chelator/ | 2‐APB | 76 | Geldanamycin | 104 | |
| /Ca blocker | BAPTA‐AM | 77 | GGA | 54 | |
| Diltiazem | 78 | Glucuronic acid | 105 | ||
| Flunarizine | 79 | HSP990 | 58 | ||
| Nicardipine | 80 | KU‐32 | 106 | ||
| Nilvadipine | 81 | Linolenic acid | 107 | ||
| Nimodipine | 36 | MG132 | 99 | ||
| Paeoniflorin | 108 | ||||
| Calpain inhibitor | ALLN | 82 | Polaprezinc | 109 | |
| Calpastatin | 83 | Prostaglandin A1 | 110 | ||
| Calpeptin | 77 | Radicicol | 111 | ||
| CYLA | 84 | Rebamipide | 112 | ||
| MDL28170 | 85 | Resveratrol | 113 | ||
| MG132 | 86 | Safrole oxide | 114 | ||
| PD150606 | 82 | Sodium butyrate | 115 | ||
| SJA6017 | 87 | Sodium fluoride | 116 | ||
| SNJ‐1945 | 37 | Sodium salicylate | 117 | ||
| TRC051384 | 118 | ||||
| Cathepsin inhibitor | CA‐074Me | 88 | Tributyltin | 119 | |
| E‐64 | 89 | VPA | 33 | ||
| Z‐FA‐FMK | 90 | YC‐1 | 120 | ||
| Z‐FY(t‐Bu)‐DMK | 91 | Zinc | 121 | ||
| Caspases inhibitor | Ac‐DEVD‐CHO | 39 |
As therapeutic agents against the early stage of photoreceptor cell death, antioxidants and ROS scavengers could prevent HSP70 carbonylation through reduction of 4HNE production. In MNU‐injected mouse retina, edaravone, a ROS scavenger, can reduce 4HNE generation and the number of TUNEL‐positive cells 35. Polyphenols, such as curcumin and green tea extract, also reduced the number of MNU‐induced TUNEL‐positive photoreceptor cells 69, 71. The Ca2+ antagonist and calpain inhibitors could also protect photoreceptor cells via the inhibition of HSP70 cleavage. Nimodipine, a Ca2+ blocker, inhibits MNU‐induced photoreceptor cell apoptosis and protects retinal function 36. A calpain inhibitor, SNJ‐1945, restored photoreceptor cell autophagy and photoreceptor cell death in MNU‐treated mice 37, 38.
Furthermore, as therapeutic agents against the late stage of photoreceptor cell death, both inhibitors of cathepsin and caspases could suppress photoreceptor cell death. Caspase inhibitor was shown to suppress retinal apoptosis in MNU‐treated rats 39.
In addition to these existing therapies, we further propose that HSP70 inducers could be novel therapeutic agents to prevent photoreceptor cell death in RDs. Many different chemicals have been reported as HSP70 inducers, including arimoclomol 95, celastrol 100, eupalinolide A/B 102, paeoniflorin 108, and radicicol 111 (Table 2). Drug repositioning is the process of developing new indications for existing drugs or biologics. Some antiulcer agents, such as carbenoxolone 98, polaprezinc 109, and rebamipide 112, induce HSP70 expression in various human tissues. Thus, the drug repositioning approach by HSP70 inducers could be an effective way to develop new therapeutic agents for RDs.
Similar Mechanisms of Photoreceptor Cell Death between MNU and RP Models: Early Stage
Although the MNU‐induced photoreceptor cell death model is different from the genetic RP model, the two models appear to share similar mechanisms of photoreceptor cell death. The eye has 3‐ to 4‐fold higher oxygen consumption relative to brain tissue and, consequently, has a higher exposure to ROS such as hydrogen peroxide, hydroxyl radicals, and superoxide anions. Oxidative stress is involved in the pathogenesis of a number of diseases including neurodegenerative disorders such as RP 35, 122. Orally administered N‐acetylcysteine reduced photoreceptor cell death and preserved cone function by reducing oxidative damage in two models of RP, rd1, and rd10 mice, which have a mutation in the rod photoreceptor‐specific cGMP phosphodiesterase (PDE) subunit 74. In addition, coexpression of superoxide dismutase 2 and catalase in the mitochondria of photoreceptors strongly promotes cell survival and the maintenance of photoreceptor function in rd10 mice 123. In some RP models, accumulation of 4HNE was detected in photoreceptor cells 124, 125. Therefore, oxidative stress plays a pivotal role in genetic RP models of retinal photoreceptor degradation.
Under pathological conditions, like those in rd1 mice, intracellular Ca2+ levels significantly increase in photoreceptor cells, even before the detection of apoptotic cells 126. Increased photoreceptor cell death in the rd10 mouse retina is associated with Ca2+ overload and calpain activation, which both occur prior to signs of cell degeneration 127. Mitochondrial calpain may activate apoptosis‐inducing factor to induce photoreceptor apoptosis in Royal College of Surgeon (RCS) rats, a natural model of recessively inherited RDs that has a disrupted gene for the receptor tyrosine kinase 82, 128, and rhodopsin transgenic rats 129. μ‐Calpain contributed to the activation of Bax and apoptosis‐inducing factor nuclear translocation in rd1, P23H (missense mutation in the rhodopsin gene), and rhodopsin knockout retinas 130. The Ca2+ antagonist nilvadipine preserved retinal morphology and electroretinogram responses in RCS rats through the upregulation of fibroblast growth factor‐2 and antiapoptotic molecules in the retina 131. A small clinical trial revealed that nilvadipine retarded the progression of central visual field defects in RP 132. In addition to Ca2+ antagonists, calpain inhibitors can attenuate photoreceptor cell death. Mitochondrial μ‐calpain inhibitor prevents photoreceptor cell death in RCS rats 128. In rd1 mice, a highly specific calpain inhibitor, calpastatin, reduced photoreceptor cell death 133. Therefore, Ca2+‐dependent calpain activation may play an important role in RP and even in the MNU‐induced photoreceptor cell death model.
Similar Mechanisms of Photoreceptor Cell Death between MNU and RP Models: Late Stage
Cathepsin D also contributed to photoreceptor cell death in rd1, P23H, and rhodopsin knockout retina 130. Thus, cathepsin inhibitors may attenuate photoreceptor cell death.
In some RP models, altered expression of apoptosis‐related proteins was also involved in photoreceptor cell death 134. Inhibitors of caspase‐3, caspase‐7, and caspase‐9 also showed neuroprotection of photoreceptors at both the structural and functional levels in rhodopsin transgenic rat models of RP 135. Thus, caspase inhibitors are thought to be effective therapeutic tools for RP.
On the basis of similarities between genetic models of RP and MNU‐induced photoreceptor cell death, the therapeutic agents for MNU‐induced photoreceptor cell death might be effective in genetic models of RP.
Conclusion
In our recent studies, HSP70 carbonylation by 4HNE and its subsequent cleavage by calpain was one of the novel central mechanisms in photoreceptor cell death. In addition, VPA and GGA protected against photoreceptor cell death by MNU via the induction of HSP70 expression 33, 54. Further studies are needed to confirm these possibilities and to clarify the possible mechanism of pathogenesis and interaction between HSP70 cleavage and chronic photoreceptor cell death using a genetic model for RDs. Taken together, HSP70 inducers may be considered as candidate therapeutic agents for the prevention of RDs, such as RP.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Guadagni V, Novelli E, Piano I, Gargini C, Strettoi E. Pharmacological approaches to retinitis pigmentosa: A laboratory perspective. Prog Retin Eye Res 2015;48:62–81. [DOI] [PubMed] [Google Scholar]
- 2. Rivas MA, Vecino E. Animal models and different therapies for treatment of retinitis pigmentosa. Histol Histopathol 2009;24:1295–1322. [DOI] [PubMed] [Google Scholar]
- 3. Rossmiller B, Mao H, Lewin AS. Gene therapy in animal models of autosomal dominant retinitis pigmentosa. Mol Vis 2012;18:2479–2496. [PMC free article] [PubMed] [Google Scholar]
- 4. Cayouette M, Behn D, Sendtner M, Lachapelle P, Gravel C. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci 1998;18:9282–9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP‐phosphodiesterase. Nature 1990;347:677–680. [DOI] [PubMed] [Google Scholar]
- 6. Paquet‐Durand F, Azadi S, Hauck SM, Ueffing M, van Veen T, Ekstrom P. Calpain is activated in degenerating photoreceptors in the rd1 mouse. J Neurochem 2006;96:802–814. [DOI] [PubMed] [Google Scholar]
- 7. Roderick TH, Chang B, Hawes NL, Heckenlively JR. A new dominant retinal degeneration (Rd4) associated with a chromosomal inversion in the mouse. Genomics 1997;42:393–396. [DOI] [PubMed] [Google Scholar]
- 8. Mehalow AK, Kameya S, Smith RS, et al. CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Hum Mol Genet 2003;12:2179–2189. [DOI] [PubMed] [Google Scholar]
- 9. Chang B, Hawes NL, Pardue MT, et al. Two mouse retinal degenerations caused by missense mutations in the beta‐subunit of rod cGMP phosphodiesterase gene. Vision Res 2007;47:624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pang JJ, Chang B, Hawes NL, et al. Retinal degeneration 12 (rd12): A new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA). Mol Vis 2005;11:152–162. [PubMed] [Google Scholar]
- 11. Chang B, Khanna H, Hawes N, et al. In‐frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early‐onset retinal degeneration in the rd16 mouse. Hum Mol Genet 2006;15:1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McNally N, Kenna PF, Rancourt D, et al. Murine model of autosomal dominant retinitis pigmentosa generated by targeted deletion at codon 307 of the rds‐peripherin gene. Hum Mol Genet 2002;11:1005–1016. [DOI] [PubMed] [Google Scholar]
- 13. Stricker HM, Ding XQ, Quiambao A, Fliesler SJ, Naash MI. The Cys214–>Ser mutation in peripherin/rds causes a loss‐of‐function phenotype in transgenic mice. Biochem J 2005;388:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watanabe S, Sanuki R, Ueno S, Koyasu T, Hasegawa T, Furukawa T. Tropisms of AAV for subretinal delivery to the neonatal mouse retina and its application for in vivo rescue of developmental photoreceptor disorders. PLoS ONE 2013;8:e54146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grimm C, Wenzel A, Hafezi F, Yu S, Redmond TM, Reme CE. Protection of Rpe65‐deficient mice identifies rhodopsin as a mediator of light‐induced retinal degeneration. Nat Genet 2000;25:63–66. [DOI] [PubMed] [Google Scholar]
- 16. Penn JS, Li S, Naash MI. Ambient hypoxia reverses retinal vascular attenuation in a transgenic mouse model of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 2000;41:4007–4013. [PubMed] [Google Scholar]
- 17. Kedzierski W, Nusinowitz S, Birch D, et al. Deficiency of rds/peripherin causes photoreceptor death in mouse models of digenic and dominant retinitis pigmentosa. Proc Natl Acad Sci U S A 2001;98:7718–7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duncan JL, LaVail MM, Yasumura D, et al. An RCS‐like retinal dystrophy phenotype in mer knockout mice. Invest Ophthalmol Vis Sci 2003;44:826–838. [DOI] [PubMed] [Google Scholar]
- 19. Sakamoto K, McCluskey M, Wensel TG, Naggert JK, Nishina PM. New mouse models for recessive retinitis pigmentosa caused by mutations in the Pde6a gene. Hum Mol Genet 2009;18:178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kedzierski W, Lloyd M, Birch DG, Bok D, Travis GH. Generation and analysis of transgenic mice expressing P216L‐substituted rds/peripherin in rod photoreceptors. Invest Ophthalmol Vis Sci 1997;38:498–509. [PubMed] [Google Scholar]
- 21. Olsson JE, Gordon JW, Pawlyk BS, et al. Transgenic mice with a rhodopsin mutation (Pro23His): A mouse model of autosomal dominant retinitis pigmentosa. Neuron 1992;9:815–830. [DOI] [PubMed] [Google Scholar]
- 22. Li T, Snyder WK, Olsson JE, Dryja TP. Transgenic mice carrying the dominant rhodopsin mutation P347S: Evidence for defective vectorial transport of rhodopsin to the outer segments. Proc Natl Acad Sci U S A 1996;93:14176–14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sung CH, Makino C, Baylor D, Nathans J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J Neurosci 1994;14:5818–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11‐cis‐vitamin A in the retinal visual cycle. Nat Genet 1998;20:344–351. [DOI] [PubMed] [Google Scholar]
- 25. Nojima S, Toyofuku T, Kamao H, et al. A point mutation in Semaphorin 4A associates with defective endosomal sorting and causes retinal degeneration. Nat Commun 2013;4:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D'Cruz PM, Yasumura D, Weir J, et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 2000;9:645–651. [DOI] [PubMed] [Google Scholar]
- 27. Machida S, Kondo M, Jamison JA, et al. P23H rhodopsin transgenic rat: Correlation of retinal function with histopathology. Invest Ophthalmol Vis Sci 2000;41:3200–3209. [PubMed] [Google Scholar]
- 28. Green ES, Rendahl KG, Zhou S, et al. Two animal models of retinal degeneration are rescued by recombinant adeno‐associated virus‐mediated production of FGF‐5 and FGF‐18. Mol Ther 2001;3:507–515. [DOI] [PubMed] [Google Scholar]
- 29. Semple‐Rowland SL, Lee NR, Van Hooser JP, Palczewski K, Baehr W. A null mutation in the photoreceptor guanylate cyclase gene causes the retinal degeneration chicken phenotype. Proc Natl Acad Sci U S A 1998;95:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burt DW, Morrice DR, Lester DH, et al. Analysis of the rdd locus in chicken: A model for human retinitis pigmentosa. Mol Vis 2003;9:164–170. [PubMed] [Google Scholar]
- 31. Herrold KM. Pigmentary degeneration of the retina induced by N‐methyl‐N‐nitrosourea. An experimental study in syrian hamsters. Arch Ophthalmol 1967;78:650–653. [DOI] [PubMed] [Google Scholar]
- 32. Tsubura A, Yoshizawa K, Kuwata M, Uehara N. Animal models for retinitis pigmentosa induced by MNU; disease progression, mechanisms and therapeutic trials. Histol Histopathol 2010;25:933–944. [DOI] [PubMed] [Google Scholar]
- 33. Koriyama Y, Sugitani K, Ogai K, Kato S. Heat shock protein 70 induction by valproic acid delays photoreceptor cell death by N‐methyl‐N‐nitrosourea in mice. J Neurochem 2014;130:707–719. [DOI] [PubMed] [Google Scholar]
- 34. Tanito M, Kaidzu S, Anderson RE. Delayed loss of cone and remaining rod photoreceptor cells due to impairment of choroidal circulation after acute light exposure in rats. Invest Ophthalmol Vis Sci 2007;48:1864–1872. [DOI] [PubMed] [Google Scholar]
- 35. Tsuruma K, Yamauchi M, Inokuchi Y, Sugitani S, Shimazawa M, Hara H. Role of oxidative stress in retinal photoreceptor cell death in N‐methyl‐N‐nitrosourea‐treated mice. J Pharmacol Sci 2012;118:351–362. [DOI] [PubMed] [Google Scholar]
- 36. Wang D, Li Y, Wang Z, Sun GY, Zhang QH. Nimodipine rescues N‐methyl‐N‐nitrosourea‐induced retinal degeneration in rats. Pharmacogn Mag 2013;9:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oka T, Nakajima T, Tamada Y, Shearer TR, Azuma M. Contribution of calpains to photoreceptor cell death in N‐methyl‐N‐nitrosourea‐treated rats. Exp Neurol 2007;204:39–48. [DOI] [PubMed] [Google Scholar]
- 38. Kuro M, Yoshizawa K, Uehara N, Miki H, Takahashi K, Tsubura A. Calpain inhibition restores basal autophagy and suppresses MNU‐induced photoreceptor cell death in mice. In Vivo 2011;25:617–623. [PubMed] [Google Scholar]
- 39. Yoshizawa K, Yang J, Senzaki H, et al. Caspase‐3 inhibitor rescues N ‐methyl‐ N ‐nitrosourea‐induced retinal degeneration in Sprague‐Dawley rats. Exp Eye Res 2000;71:629–635. [DOI] [PubMed] [Google Scholar]
- 40. Yoshizawa K, Nambu H, Yang J, et al. Mechanisms of photoreceptor cell apoptosis induced by N‐methyl‐N‐nitrosourea in Sprague‐Dawley rats. Lab Invest 1999;79:1359–1367. [PubMed] [Google Scholar]
- 41. Dean DO, Kent CR, Tytell M. Constitutive and inducible heat shock protein 70 immunoreactivity in the normal rat eye. Invest Ophthalmol Vis Sci 1999;40:2952–2962. [PubMed] [Google Scholar]
- 42. Nakajima E, David LL, Bystrom C, Shearer TR, Azuma M. Calpain‐specific proteolysis in primate retina: Contribution of calpains in cell death. Invest Ophthalmol Vis Sci 2006;47:5469–5475. [DOI] [PubMed] [Google Scholar]
- 43. Sahara S, Yamashima T. Calpain‐mediated Hsp70.1 cleavage in hippocampal CA1 neuronal death. Biochem Biophys Res Commun 2010;393:806–811. [DOI] [PubMed] [Google Scholar]
- 44. Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4‐hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 1991;11:81–128. [DOI] [PubMed] [Google Scholar]
- 45. Uchida K. 4‐Hydroxy‐2‐nonenal: A product and mediator of oxidative stress. Prog Lipid Res 2003;42:318–343. [DOI] [PubMed] [Google Scholar]
- 46. Shimozu Y, Hirano K, Shibata T, Shibata N, Uchida K. 4‐Hydroperoxy‐2‐nonenal is not just an intermediate but a reactive molecule that covalently modifies proteins to generate unique intramolecular oxidation products. J Biol Chem 2011;286:29313–29324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stadtman ER, Berlett BS. Reactive oxygen‐mediated protein oxidation in aging and disease. Drug Metab Rev 1998;30:225–243. [DOI] [PubMed] [Google Scholar]
- 48. Kirkegaard T, Roth AG, Petersen NH, et al. Hsp70 stabilizes lysosomes and reverts Niemann‐Pick disease‐associated lysosomal pathology. Nature 2010;463:549–553. [DOI] [PubMed] [Google Scholar]
- 49. Oikawa S, Yamada T, Minohata T, et al. Proteomic identification of carbonylated proteins in the monkey hippocampus after ischemia‐reperfusion. Free Radic Biol Med 2009;46:1472–1477. [DOI] [PubMed] [Google Scholar]
- 50. Yamashima T. Hsp70.1 and related lysosomal factors for necrotic neuronal death. J Neurochem 2012;120:477–494. [DOI] [PubMed] [Google Scholar]
- 51. Sabirzhanov B, Stoica BA, Hanscom M, Piao CS, Faden AI. Over‐expression of HSP70 attenuates caspase‐dependent and caspase‐independent pathways and inhibits neuronal apoptosis. J Neurochem 2012;123:542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Beere HM, Wolf BB, Cain K, et al. Heat‐shock protein 70 inhibits apoptosis by preventing recruitment of procaspase‐9 to the Apaf‐1 apoptosome. Nat Cell Biol 2000;2:469–475. [DOI] [PubMed] [Google Scholar]
- 53. Marinova Z, Ren M, Wendland JR, et al. Valproic acid induces functional heat‐shock protein 70 via Class I histone deacetylase inhibition in cortical neurons: A potential role of Sp1 acetylation. J Neurochem 2009;111:976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koriyama Y, Ogai K, Sugitani K, Hisano S, Kato S. Geranylgeranylacetone suppresses N‐methyl‐N‐nitrosourea‐induced photoreceptor cell loss in mice. Adv Exp Med Biol 2016;854:237–243. [DOI] [PubMed] [Google Scholar]
- 55. Tytell M, Barbe MF, Brown IR. Induction of heat shock (stress) protein 70 and its mRNA in the normal and light‐damaged rat retina after whole body hyperthermia. J Neurosci Res 1994;38:19–31. [DOI] [PubMed] [Google Scholar]
- 56. Kim JH, Kim JH, Yu YS, Jeong SM, Kim KW. Protective effect of heat shock proteins 70.1 and 70.3 on retinal photic injury after systemic hyperthermia. Korean J Ophthalmol 2005;19:116–121. [DOI] [PubMed] [Google Scholar]
- 57. Kayama M, Nakazawa T, Thanos A, et al. Heat shock protein 70 (HSP70) is critical for the photoreceptor stress response after retinal detachment via modulating anti‐apoptotic Akt kinase. Am J Pathol 2011;178:1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aguila M, Bevilacqua D, McCulley C, et al. Hsp90 inhibition protects against inherited retinal degeneration. Hum Mol Genet 2014;23:2164–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Spencer JP, Rice‐Evans C, Williams RJ. Modulation of pro‐survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J Biol Chem 2003;278:34783–34793. [DOI] [PubMed] [Google Scholar]
- 60. Sharp FR, Zhan X, Liu DZ. Heat shock proteins in the brain: Role of Hsp70, Hsp 27, and HO‐1 (Hsp32) and their therapeutic potential. Transl Stroke Res 2013;4:685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mansilla MJ, Montalban X, Espejo C. Heat shock protein 70: Roles in multiple sclerosis. Mol Med 2012;18:1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee SH, Kwon HM, Kim YJ, Lee KM, Kim M, Yoon BW. Effects of hsp70.1 gene knockout on the mitochondrial apoptotic pathway after focal cerebral ischemia. Stroke 2004;35:2195–2199. [DOI] [PubMed] [Google Scholar]
- 63. Lin PY, Simon SM, Koh WK, Folorunso O, Umbaugh CS, Pierce A. Heat shock factor 1 over‐expression protects against exposure of hydrophobic residues on mutant SOD1 and early mortality in a mouse model of amyotrophic lateral sclerosis. Mol Neurodegener 2013;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hu D, Chen F, Guan C, Yang F, Qu Y. Anti‐hypoxia effect of adenovirus‐mediated expression of heat shock protein 70 (HSP70) on primary cultured neurons. J Neurosci Res 2013;91:1174–1182. [DOI] [PubMed] [Google Scholar]
- 65. Koriyama Y, Ohno M, Kimura T, Kato S. Neuroprotective effects of 5‐S‐GAD against oxidative stress‐induced apoptosis in RGC‐5 cells. Brain Res 2009;1296:187–195. [DOI] [PubMed] [Google Scholar]
- 66. Koriyama Y, Nakayama Y, Matsugo S, Kato S. Protective effect of lipoic acid against oxidative stress is mediated by Keap1/Nrf2‐dependent heme oxygenase‐1 induction in the RGC‐5 cellline. Brain Res 2013;1499:145–157. [DOI] [PubMed] [Google Scholar]
- 67. Otsuka T, Shimazawa M, Nakanishi T, et al. Protective effects of a dietary carotenoid, astaxanthin, against light‐induced retinal damage. J Pharmacol Sci 2013;123:209–218. [DOI] [PubMed] [Google Scholar]
- 68. Rezaie T, McKercher SR, Kosaka K, et al. Protective effect of carnosic acid, a pro‐electrophilic compound, in models of oxidative stress and light‐induced retinal degeneration. Invest Ophthalmol Vis Sci 2012;53:7847–7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Emoto Y, Yoshizawa K, Uehara N, et al. Curcumin suppresses N‐methyl‐N‐nitrosourea‐induced photoreceptor apoptosis in Sprague‐Dawley rats. In Vivo 2013;27:583–590. [PubMed] [Google Scholar]
- 70. Bazan NG. Cell survival matters: Docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci 2006;29:263–271. [DOI] [PubMed] [Google Scholar]
- 71. Emoto Y, Yoshizawa K, Kinoshita Y, et al. Green tea extract suppresses N‐methyl‐N‐nitrosourea‐induced photoreceptor apoptosis in Sprague‐Dawley rats. Graefes Arch Clin Exp Ophthalmol 2014;252:1377–1384. [DOI] [PubMed] [Google Scholar]
- 72. Kiang AS, Humphries MM, Campbell M, Humphries P. Antioxidant therapy for retinal disease. Adv Exp Med Biol 2014;801:783–789. [DOI] [PubMed] [Google Scholar]
- 73. Liang FQ, Aleman TS, Yang Z, Cideciyan AV, Jacobson SG, Bennett J. Melatonin delays photoreceptor degeneration in the rds/rds mouse. NeuroReport 2001;12:1011–1014. [DOI] [PubMed] [Google Scholar]
- 74. Lee SY, Usui S, Zafar AB, et al. N‐Acetylcysteine promotes long‐term survival of cones in a model of retinitis pigmentosa. J Cell Physiol 2011;226:1843–1849. [DOI] [PubMed] [Google Scholar]
- 75. Tsuruma K, Tanaka Y, Shimazawa M, Mashima Y, Hara H. Unoprostone reduces oxidative stress‐ and light‐induced retinal cell death, and phagocytotic dysfunction, by activating BK channels. Mol Vis 2011;17:3556–3565. [PMC free article] [PubMed] [Google Scholar]
- 76. Li J, Wang P, Yu S, Zheng Z, Xu X. Calcium entry mediates hyperglycemia‐induced apoptosis through Ca(2 + )/calmodulin‐dependent kinase II in retinal capillary endothelial cells. Mol Vis 2012;18:2371–2379. [PMC free article] [PubMed] [Google Scholar]
- 77. Kim C, Yun N, Lee YM, et al. Gel‐based protease proteomics for identifying the novel calpain substrates in dopaminergic neuronal cell. J Biol Chem 2013;288:36717–36732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Frasson M, Sahel JA, Fabre M, Simonutti M, Dreyfus H, Picaud S. Retinitis pigmentosa: Rod photoreceptor rescue by a calcium‐channel blocker in the rd mouse. Nat Med 1999;5:1183–1187. [DOI] [PubMed] [Google Scholar]
- 79. Edward DP, Lam TT, Shahinfar S, Li J, Tso MO. Amelioration of light‐induced retinal degeneration by a calcium overload blocker. Flunarizine. Arch Ophthalmol 1991;109:554–562. [DOI] [PubMed] [Google Scholar]
- 80. Takano Y, Ohguro H, Dezawa M, et al. Study of drug effects of calcium channel blockers on retinal degeneration of rd mouse. Biochem Biophys Res Commun 2004;313:1015–1022. [DOI] [PubMed] [Google Scholar]
- 81. Yamazaki H, Ohguro H, Maeda T, et al. Preservation of retinal morphology and functions in royal college surgeons rat by nilvadipine, a Ca(2 + ) antagonist. Invest Ophthalmol Vis Sci 2002;43:919–926. [PubMed] [Google Scholar]
- 82. Mizukoshi S, Nakazawa M, Sato K, Ozaki T, Metoki T, Ishiguro S. Activation of mitochondrial calpain and release of apoptosis‐inducing factor from mitochondria in RCS rat retinal degeneration. Exp Eye Res 2010;91:353–361. [DOI] [PubMed] [Google Scholar]
- 83. Yang J, Weimer RM, Kallop D, et al. Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron 2013;80:1175–1189. [DOI] [PubMed] [Google Scholar]
- 84. David J, Melamud A, Kesner L, et al. A novel calpain inhibitor for treatment of transient retinal ischemia in the rat. NeuroReport 2011;22:633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lee E, Eom JE, Kim HL, et al. Neuroprotective effect of undecylenic acid extracted from Ricinus communis L. through inhibition of mu‐calpain. Eur J Pharm Sci 2012;46:17–25. [DOI] [PubMed] [Google Scholar]
- 86. Tie L, Xu Y, Lin YH, et al. Down‐regulation of brain‐pancreas relative protein in diabetic rats and by high glucose in PC12 cells: Prevention by calpain inhibitors. J Pharmacol Sci 2008;106:28–37. [DOI] [PubMed] [Google Scholar]
- 87. Sharma AK, Rohrer B. Calcium‐induced calpain mediates apoptosis via caspase‐3 in a mouse photoreceptor cell line. J Biol Chem 2004;279:35564–35572. [DOI] [PubMed] [Google Scholar]
- 88. Cho K, Yoon SY, Choi JE, Kang HJ, Jang HY, Kim DH. CA‐074Me, a cathepsin B inhibitor, decreases APP accumulation and protects primary rat cortical neurons treated with okadaic acid. Neurosci Lett 2013;548:222–227. [DOI] [PubMed] [Google Scholar]
- 89. Okubo A, Sameshima M, Unoki K, Uehara F, Bird AC. Ultrastructural changes associated with accumulation of inclusion bodies in rat retinal pigment epithelium. Invest Ophthalmol Vis Sci 2000;41:4305–4312. [PubMed] [Google Scholar]
- 90. Gottron FJ, Ying HS, Choi DW. Caspase inhibition selectively reduces the apoptotic component of oxygen‐glucose deprivation‐induced cortical neuronal cell death. Mol Cell Neurosci 1997;9:159–169. [DOI] [PubMed] [Google Scholar]
- 91. Xiang B, Fei X, Zhuang W, Fang Y, Qin Z, Liang Z. Cathepsin L is involved in 6‐hydroxydopamine induced apoptosis of SH‐SY5Y neuroblastoma cells. Brain Res 2011;1387:29–38. [DOI] [PubMed] [Google Scholar]
- 92. Turturici G, Sconzo G, Geraci F. Hsp70 and its molecular role in nervous system diseases. Biochem Res Int 2011;2011:618127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rossi A, Elia G, Santoro MG. 2‐Cyclopenten‐1‐one, a new inducer of heat shock protein 70 with antiviral activity. J Biol Chem 1996;271:32192–32196. [DOI] [PubMed] [Google Scholar]
- 94. Yoshihisa Y, Hassan MA, Furusawa Y, Tabuchi Y, Kondo T, Shimizu T. Alkannin, HSP70 inducer, protects against UVB‐induced apoptosis in human keratinocytes. PLoS ONE 2012;7:e47903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Parfitt DA, Aguila M, McCulley CH, et al. The heat‐shock response co‐inducer arimoclomol protects against retinal degeneration in rhodopsin retinitis pigmentosa. Cell Death Dis 2014;5:e1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bao XQ, Liu GT. Induction of overexpression of the 27‐ and 70‐kDa heat shock proteins by bicyclol attenuates concanavalin A‐Induced liver injury through suppression of nuclear factor‐kappaB in mice. Mol Pharmacol 2009;75:1180–1188. [DOI] [PubMed] [Google Scholar]
- 97. Hargitai J, Lewis H, Boros I, et al. Bimoclomol, a heat shock protein co‐inducer, acts by the prolonged activation of heat shock factor‐1. Biochem Biophys Res Commun 2003;307:689–695. [DOI] [PubMed] [Google Scholar]
- 98. Nagayama S, Jono H, Suzaki H, et al. Carbenoxolone, a new inducer of heat shock protein 70. Life Sci 2001;69:2867–2873. [DOI] [PubMed] [Google Scholar]
- 99. Wang T, Yu Q, Chen J, Deng B, Qian L, Le Y. PP2A mediated AMPK inhibition promotes HSP70 expression in heat shock response. PLoS ONE 2010;5:e13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kyung H, Kwong JM, Bekerman V, et al. Celastrol supports survival of retinal ganglion cells injured by optic nerve crush. Brain Res 2015;1609:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Xia C, Cai Y, Li S, Yang J, Xiao G. Curcumin increases HSP70 expression in primary rat cortical neuronal apoptosis induced by gp120 V3 loop peptide. Neurochem Res 2015;40:1996–2005. [DOI] [PubMed] [Google Scholar]
- 102. Yamashita Y, Ikeda T, Matsuda M, Maji D, Hoshino T, Mizushima T. Purification and characterization of HSP‐inducers from Eupatorium lindleyanum. Biochem Pharmacol 2012;83:909–922. [DOI] [PubMed] [Google Scholar]
- 103. Kong XC, Zhang D, Qian C, Liu GT, Bao XQ. FLZ, a novel HSP27 and HSP70 inducer, protects SH‐SY5Y cells from apoptosis caused by MPP(+). Brain Res 2011;1383:99–107. [DOI] [PubMed] [Google Scholar]
- 104. Lu A, Ran R, Parmentier‐Batteur S, Nee A, Sharp FR. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem 2002;81:355–364. [DOI] [PubMed] [Google Scholar]
- 105. Kim YM, Kim HJ, Song EJ, Lee KJ. Glucuronic acid is a novel inducer of heat shock response. Mol Cell Biochem 2004;259:23–33. [DOI] [PubMed] [Google Scholar]
- 106. Li C, Ma J, Zhao H, Blagg BS, Dobrowsky RT. Induction of heat shock protein 70 (Hsp70) prevents neuregulin‐induced demyelination by enhancing the proteasomal clearance of c‐Jun. ASN Neuro 2012;4:e00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Polyunsaturated fatty acids induce ischemic and epileptic tolerance. Neuroscience 2002;109:231–241. [DOI] [PubMed] [Google Scholar]
- 108. Yan D, Saito K, Ohmi Y, Fujie N, Ohtsuka K. Paeoniflorin, a novel heat shock protein‐inducing compound. Cell Stress Chaperones 2004;9:378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Odashima M, Otaka M, Jin M, et al. Induction of a 72‐kDa heat‐shock protein in cultured rat gastric mucosal cells and rat gastric mucosa by zinc L‐carnosine. Dig Dis Sci 2002;47:2799–2804. [DOI] [PubMed] [Google Scholar]
- 110. Wang X, Qin ZH, Leng Y, et al. Prostaglandin A1 inhibits rotenone‐induced apoptosis in SH‐SY5Y cells. J Neurochem 2002;83:1094–1102. [DOI] [PubMed] [Google Scholar]
- 111. Miyabara EH, Martin JL, Griffin TM, Moriscot AS, Mestril R. Overexpression of inducible 70‐kDa heat shock protein in mouse attenuates skeletal muscle damage induced by cryolesioning. Am J Physiol Cell Physiol 2006;290:C1128–C1138. [DOI] [PubMed] [Google Scholar]
- 112. Hahm KB, Park IS, Kim YS, et al. Role of rebamipide on induction of heat‐shock proteins and protection against reactive oxygen metabolite‐mediated cell damage in cultured gastric mucosal cells. Free Radic Biol Med 1997;22:711–716. [DOI] [PubMed] [Google Scholar]
- 113. Putics A, Vegh EM, Csermely P, Soti C. Resveratrol induces the heat‐shock response and protects human cells from severe heat stress. Antioxid Redox Signal 2008;10:65–75. [DOI] [PubMed] [Google Scholar]
- 114. Zhao Y, Xin J, Sun C, Zhao B, Zhao J, Su L. Safrole oxide induced neuronal differentiation of rat bone‐marrow mesenchymal stem cells by elevating Hsp70. Gene 2012;509:85–92. [DOI] [PubMed] [Google Scholar]
- 115. Garcia‐Bermejo L, Vilaboa NE, Perez C, Galan A, De Blas E, Aller P. Modulation of heat‐shock protein 70 (HSP70) gene expression by sodium butyrate in U‐937 promonocytic cells: Relationships with differentiation and apoptosis. Exp Cell Res 1997;236:268–274. [DOI] [PubMed] [Google Scholar]
- 116. Cheng TJ, Chen TM, Chen CH, Lai YK. Induction of stress response and differential expression of 70 kDa stress proteins by sodium fluoride in HeLa and rat brain tumor 9L cells. J Cell Biochem 1998;69:221–231. [DOI] [PubMed] [Google Scholar]
- 117. Ishihara K, Horiguchi K, Yamagishi N, Hatayama T. Identification of sodium salicylate as an hsp inducer using a simple screening system for stress response modulators in mammalian cells. Eur J Biochem 2003;270:3461–3468. [DOI] [PubMed] [Google Scholar]
- 118. Mohanan A, Deshpande S, Jamadarkhana PG, et al. Delayed intervention in experimental stroke with TRC051384–a small molecule HSP70 inducer. Neuropharmacology 2011;60:991–999. [DOI] [PubMed] [Google Scholar]
- 119. Zhang H, Liu AY. Tributyltin is a potent inducer of the heat shock response in human diploid fibroblasts. J Cell Physiol 1992;153:460–466. [DOI] [PubMed] [Google Scholar]
- 120. Liu YN, Pan SL, Peng CY, et al. YC‐1 induces heat shock protein 70 expression and prevents oxidized LDL‐mediated apoptosis in vascular smooth muscle cells. Shock 2008;30:274–279. [DOI] [PubMed] [Google Scholar]
- 121. Cheng Y, Liu YF, Liang J. Protective effect of zinc: A potent heat shock protein inducer in cold preservation of rat liver. Hepatobiliary Pancreat Dis Int 2002;1:258–261. [PubMed] [Google Scholar]
- 122. Murakami Y, Ikeda Y, Yoshida N, et al. MutT homolog‐1 attenuates oxidative DNA damage and delays photoreceptor cell death in inherited retinal degeneration. Am J Pathol 2012;181:1378–1386. [DOI] [PubMed] [Google Scholar]
- 123. Usui S, Komeima K, Lee SY, et al. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol Ther 2009;17:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Shen J, Yang X, Dong A, et al. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J Cell Physiol 2005;203:457–464. [DOI] [PubMed] [Google Scholar]
- 125. Tanito M, Haniu H, Elliott MH, Singh AK, Matsumoto H, Anderson RE. Identification of 4‐hydroxynonenal‐modified retinal proteins induced by photooxidative stress prior to retinal degeneration. Free Radic Biol Med 2006;41:1847–1859. [DOI] [PubMed] [Google Scholar]
- 126. Doonan F, Donovan M, Cotter TG. Activation of multiple pathways during photoreceptor apoptosis in the rd mouse. Invest Ophthalmol Vis Sci 2005;46:3530–3538. [DOI] [PubMed] [Google Scholar]
- 127. Rodriguez‐Muela N, Hernandez‐Pinto AM, Serrano‐Puebla A, et al. Lysosomal membrane permeabilization and autophagy blockade contribute to photoreceptor cell death in a mouse model of retinitis pigmentosa. Cell Death Differ 2015;22:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ozaki T, Nakazawa M, Yamashita T, et al. Intravitreal injection or topical eye‐drop application of a mu‐calpain C2L domain peptide protects against photoreceptor cell death in Royal College of Surgeons’ rats, a model of retinitis pigmentosa. Biochim Biophys Acta 2012;1822:1783–1795. [DOI] [PubMed] [Google Scholar]
- 129. Ozaki T, Ishiguro S, Hirano S, et al. Inhibitory peptide of mitochondrial mu‐calpain protects against photoreceptor degeneration in rhodopsin transgenic S334ter and P23H rats. PLoS ONE 2013;8:e71650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Comitato A, Sanges D, Rossi A, Humphries MM, Marigo V. Activation of Bax in three models of retinitis pigmentosa. Invest Ophthalmol Vis Sci 2014;55:3555–3562. [DOI] [PubMed] [Google Scholar]
- 131. Sato M, Ohguro H, Ohguro I, et al. Study of pharmacological effects of nilvadipine on RCS rat retinal degeneration by microarray analysis. Biochem Biophys Res Commun 2003;306:826–831. [DOI] [PubMed] [Google Scholar]
- 132. Nakazawa M, Ohguro H, Takeuchi K, Miyagawa Y, Ito T, Metoki T. Effect of nilvadipine on central visual field in retinitis pigmentosa: A 30‐month clinical trial. Ophthalmologica 2011;225:120–126. [DOI] [PubMed] [Google Scholar]
- 133. Paquet‐Durand F, Sanges D, McCall J, et al. Photoreceptor rescue and toxicity induced by different calpain inhibitors. J Neurochem 2010;115:930–940. [DOI] [PubMed] [Google Scholar]
- 134. Sizova OS, Shinde VM, Lenox AR, Gorbatyuk MS. Modulation of cellular signaling pathways in P23H rhodopsin photoreceptors. Cell Signal 2014;26:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Leonard KC, Petrin D, Coupland SG, et al. XIAP protection of photoreceptors in animal models of retinitis pigmentosa. PLoS ONE 2007;2:e314. [DOI] [PMC free article] [PubMed] [Google Scholar]


