Summary
Motion sickness (MS) is a common physiological response to real or virtual motion. Numerous studies have investigated the neurobiological mechanism and the control measures of MS. This review summarizes the current knowledge about pathogenesis and pathophysiology, prediction, evaluation, and countermeasures of MS. The sensory conflict hypothesis is the most widely accepted theory for MS. Both the hippocampus and vestibular cortex might play a role in forming internal model. The pathophysiology focuses on the visceral afference, thermoregulation and MS‐related neuroendocrine. Single‐nucleotide polymorphisms (SNPs) in some genes and epigenetic modulation might contribute to MS susceptibility and habituation. Questionnaires, heart rate variability (HRV) and electrogastrogram (EGG) are useful for diagnosing and evaluating MS. We also list MS medications to guide clinical practice. Repeated real motion exposure and combined visual‐vestibular interaction training accelerate the progress of habituation. Behavioral and dietary countermeasures, as well as physiotherapy, are also effective in alleviating MS symptoms.
Keywords: Countermeasure, Evaluation, Motion sickness, Pathogenesis, Prediction
Introduction
Motion sickness (MS) is a feeling of unwellness caused by motion, especially during traveling and virtual reality immersion. The main symptoms of MS include autonomic reactions (nausea, vomiting, pallor, sweating, hypersalivation, and stomach awareness) and sopite syndrome referring to drowsiness, lethargy, and persistent fatigue 1. Intact vestibular apparatus and sufficient provocative stimulation are prerequisites for MS. There are great individual differences in MS susceptibility, which is thought to be a result of gene‐environment interaction 2. Although the etiology and precise neurobiological mechanism of MS are still ambiguous, several hypotheses have been proposed in which the sensory conflict hypothesis is the most widely accepted theory. Varieties of countermeasures have been developed and successfully used for decades.
Pathogenesis and Pathophysiology
Sensory Conflict Theory
The “sensory conflict and neural mismatch” theory was originally proposed by Reason and Brand. It is currently accepted for explaining MS 3. MS will develop when mismatches happened between the integrated pattern of sensory information under real motion (e.g., in boats, cars, and airplanes) or virtual environment (e.g., watching 3D video films) and the anticipated “internal model” formed under normal or experienced conditions 4. The physiological significance of “neural mismatch” is to initiate sensory‐motor learning and promote self‐adjustment, ultimately producing MS habituation under novel locomotion environment 5.
Recent studies have added new knowledge to sensory conflict theory. Cullen and his colleagues recently identified sensory conflict neurons in the VN and cerebellum. They found that “vestibular only” (VO) VN neurons and “unimodal” rostral fastigial nucleus (u‐rFN) cerebellar neurons only reacted to passive head movement (exafferent) but not to anticipated active afference (reafference) in primates 6. These sensory conflict neurons may receive inhibitory innervations canceling “reafference” that matches the experience in the “internal model.” As the sensory conflict theory suggests that neural storage of the experienced motion pattern can produce novel “internal model,” it can be presumed that the “exafference” might also be canceled by novel “internal model” established after habituation induced by prolonged or repeated passive motion exposure (Figure 1).
Figure 1.
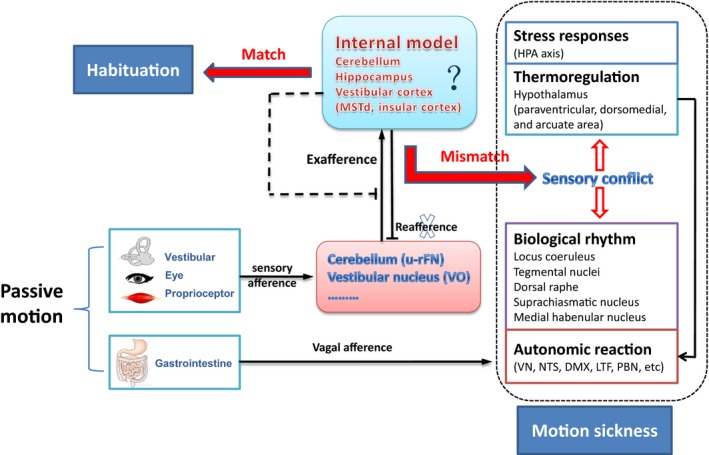
Sensory conflict theory and pathophysiological process of MS.
Several lines of evidence suggest that brain regions involved in space orientation and motion perception (hippocampus and vestibular cortex) are areas where internal model stores 7, 8. As for the hippocampus, forward–backward translocation and passive rotation can induce theta rhythm in dentate gyrus and CA1 regions while lesion in these regions can aggravate MS, suggesting the hippocampal involvement in processing sensory conflict information 9, 10, 11. In the vestibular cortex, electrophysiological experiments showed that bilateral labyrinthectomy significantly decreased the firing rate of neurons in dorsal part of middle superior temporal (MSTd) during physical rotation and translation in the dark, but not in the visual condition 12. During large‐field visual motion stimulation, inhibitory visual‐vestibular interaction was observed in brain regions connected indirectly with MSTd in monkeys 13. A recent fMRI study showed that long‐term spaceflight significantly reduced intrinsic connectivity in insula cortex in a cosmonaut 14. These lines of evidence supported that the vestibular cortex might play a role in visual‐vestibular sensory conflict and possibly in forming “internal model.”
Pathophysiological Mechanisms
Theoretically, activating sensory conflict neurons may trigger autonomic reaction through vestibulo‐autonomic pathways that connect the VN complex with central autonomic regions 15, 16. Yates et al. have confirmed that vestibular system regulates cardiovascular function during movement and changes in posture via vestibulo‐sympathetic reflex 17. Although the contribution of sensory conflict neurons to VN‐autonomic regulation is still ambiguous, downstream pathophysiological mechanisms of MS are updated by recent studies emphasizing visceral vestibular convergence, vestibulo‐thermal regulation, and MS‐related endocrine (Figure 1).
It has been demonstrated that brain regions associated with nausea and vomiting not only receive vestibular afference but also converge gastrointestinal (GI) signal 18, suggesting that visceral mechanosensory input might facilitate VN‐autonomic reaction during MS. Ossenkopp et al. for the first time reported that MS can induce hypothermia which has recently been proved to be caused by increased heat loss resulting from peripheral vasodilatation 19, 20. Ngampramuan et al. proposed that the vestibular thermoregulatory symptoms may serve as a core pathophysiological element of motion‐induced nausea in mammals 21. As body temperature and biorhythms are significantly disrupted by chronic hypergravity and bilateral vestibular loss 22, we speculate that vestibular system might participate in keeping homeostasis during MS via connections with thermal and rhythmic regulation centers (Figure 1).
In addition to autonomic reactions, MS also accompanies stress hormones release and endocrine responses habituated over repeated motion exposure 23. Nevertheless, temporal changes of blood hormones, such as arginine vasopressin (AVP) and adrenocorticotrophic hormone (ACTH), did not synchronize with those of motion‐induced nausea, suggesting that activating hypothalamic–pituitary–adrenal axis might be a general stress response to provocative motion 24. Recently, ghrelin, an endogenous ligand for the growth hormone secretogogue receptor, was observed to be related to acute nausea or vomiting 25. In animals and humans, ghrelin was found to have gastro‐prokinetic activity via facilitating gastric cholinergic activity 25. Our study revealed that plasma ghrelin levels were positively correlated with severe seasickness‐induced autonomic responses in humans (unpublished data), which suggests that gastroenteropancreatic hormones might play a role in MS development. Nevertheless, more detailed evidence is required to verify this hypothesis.
Genetic Contributions
MS is a conserved and a cross‐species phenotype (from fishes, amphibia to mammals) with a heritability around 57–70% in humans 5. Race disparity is also significant. Chinese are more sensitive to MS than Caucasian 2. Finley et al. for the first time reported that a single‐nucleotide polymorphism (SNP) in the α 2‐adrenergic receptor gene correlated with individual differences in autonomic responsiveness to provocative motion and other stressors 26. Recently, a genomewide association study conducted in 80,494 individuals from the 23 and Me database found that 35 SNPs in genes involved in balance function, eye, ear and cranial development, neurological processes, glucose homeostasis, or hypoxia were associated with self‐reported carsickness susceptibility 27. Nevertheless, it has been verified that none of these SNPs is related to vestibular function regulation. It is noteworthy that some SNPs are in or near genes implicated in glucose and insulin homeostasis, which links to our pervious finding that hyperglycemia is related to the GI symptoms of MS both in human and rodents 28. Recent studies have found that SNPs in genes of 5‐Hydroxytryptamine type 3 receptor (5‐HT3), cholinergic muscarinic receptor type 3 (M3 AChR), morphine (μ) opioid receptor, and neurokinin 1 (NK1) receptors are associated with background sensitivity to postoperative and chemotherapy‐induced nausea and vomiting (PINV and CINV) 29. These genetic bases for “final common pathway” of nausea and vomiting may also contribute to MS susceptibility.
Patients with migraine and Meniere's disease are prone to experience MS especially in female patients 30, 31. Mutations in genes related to vasculopathy and cortical spreading depression are responsible for vestibular symptoms and MS hypersusceptibility in migraine patients 32. Previous studies have found sporadic Meniere's disease might be associated with mutations in genes of aquaporins and voltage‐gated potassium channel expressed in the inner ear 33. Given that these genes play important roles in endolymphatic homeostasis, their mutations ought to contribute to subnormal or asymmetrical otolith function associated with MS hypersusceptibility in Meniere patients.
Spaceflight and microgravity can affect the expression of genes associated with cellular functions 34, 35. Our study also showed that MS susceptible and insusceptible animals have different gene expression profile in the caudal VN after motion stimulation 36. Moreover, for human T‐lymphocyte cells, simulated microgravity exposure could alter the expression of genes involved in DNA methylation and histone modification, inducing DNA hypomethylation and mutational changes 37. These lines of evidence indicate that epigenetic modulations might also contribute to MS susceptibility diversity and MS habituation. In addition, MS susceptibility is also influenced by personal characteristics including trait‐anxiety, aerobic fitness, and hemodynamic as well as age and sex 38. The linkage between genetic and epigenetic basis of these phenotypes and MS merits further investigation.
Prediction and Evaluation
Prevalence Prediction
It has been demonstrated that almost all healthy individuals can obtain MS when exposed to appropriate provocative motion. MS prevalence depends on individual threshold to motion stimulation and varies under different situations, which makes it difficult to predict. Lawther and Griffin established mathematical models with dependence on various vertical motion parameters (acceleration magnitude, frequency, and duration) for predicting incidence of seasickness 39. Perez Arribas and Lopez Pinerio have proposed “sicken passengers ration” which represents variables including ship speed, loading condition, and sea state and includes the effect of passenger behavior and habituation to moving environment 40. These formulas greatly improve ship design to increase the degree of comfort and the work ability on the sea.
Individual Susceptibility Prediction
Birren and Fisher for the first time provided a questionnaire approach to predict seasickness susceptibility 41. Pensacola Motion History Questionnaire (PMHQ) and Reason and Brand MS Susceptibility Questionnaire (MSSQ) were nowadays commonly used in MS studies 42, 43, 44. Golding redesigned a MSSQ‐Short by simplifying the scoring and adding vital items including the demographic (e.g., age, gender, race), the nauseogenic environments avoidance (e.g., cars, planes, video games), and vestibular disorder comorbidities and anthropometric items (e.g., height, body weight, BMI) to increase the reliability and validity 45, 46.
Shupac et al. and other groups assessed vestibular function, such as vestibular‐ocular reflex (VOR), caloric stimulation, and vestibular‐evoked myogenic potential (VEMP), to predict individual MS susceptibility 47, 48, 49, 50. Stoffregen et al. recently proposed the postural instability as a precursor of MS susceptibility 51. Previous studies also demonstrated that computerized dynamic posturography (CDP) data can be used as indicator of seasickness susceptibility and habituation 52, 53. In addition, baseline protein concentration and amylase activity in saliva as well as odor and taster sensitivity were also used as indicators for predicting MS susceptibility in human subjects 54, 55, 56, 57 (Table 1).
Table 1.
Prediction and evaluation for MS
| Category | Description | Application | References |
|---|---|---|---|
| MS Questionnaires | |||
| PMHQ | Coriolis stimulation, very low‐frequency ship motion, and simulator stimulation as scoring keys | Predicting SS susceptibility | 42, 44 |
| MSSQ | Childhood and adults history of transport or entertainment exposure and MS experience | Predicting susceptibility to real motion‐induced MS | 43, 45 |
| Graybiel rating scales | Rating cardinal symptoms including cold sweating, pallor, increases in salivation, drowsiness, headache, pain, and nausea and vomiting | Evaluating MS of all forms | 58 |
| Wiker rating scales | Rating MS by rigging up 28 major, minor, and other symptoms | Evaluating MS of all forms | 44, 59 |
| Kennedy rating scales | Factor analysis of oculomotor, disorientation, and nausea dimensions | Evaluating SS | 60 |
| Muth rating scales | Rating 3 dimensions of nausea including gastrointestinal, somatic, and emotional distress | Assessing MS‐induced nausea | 61 |
| Gianaros rating scales | Rating gastrointestinal, central, peripheral, and sopite‐related dimensions | Multidimensional analysis of MS | 62 |
| Vestibular function | |||
| VOR | Higher gains and lower phase leads | Predicting MS susceptibility; indicator of semicircular canal function | 47, 48 |
| Caloric test | Faster slow‐phase velocity | Predicting MS susceptibility; indicator of semicircular canal function | 165 |
| cVEMPs | Higher threshold; lower peak‐to‐peak amplitude interval | Predicting MS susceptibility; evaluating MS habituation; indicator of saccular function | 49, 50 |
| Physiological indexes | |||
| CDP | Less stability in condition 5 of SOT; decreased MCT strength | Predicting postural instability of MS susceptibles; evaluating MS habituation | 52, 53 |
| Postural dynamics | Greater positional variability; higher temporal dynamics | Predicting postural instability of MS susceptibles | 51 |
| Odors and tastes sensitivity | Sensitive to unpleasant odors (e.g., petrol, leather); sensitive to phenylthiocarbamide tasters | Predicting susceptibility to environment incentives | 56, 57 |
| HRV | Reduction in (HF) high‐frequency component; increment in low‐frequency component (LF) and LF/HF ratio | Evaluating MS‐induced sympathovagal disturbance | 63, 66 |
| EGG | Increased 4–9 cpm activity; absence or reduction of 3 cpm activity | Evaluating MS‐induced gastric response | 50, 64 |
| Core temperature | Reduction in core temperature | Evaluating MS‐induced thermal reaction | 20 |
| Biochemical test | |||
| Stress hormones | Increment in levels of AVP, ACTH, cortisol, beta‐endorphin, etc. after provocative motion stimulation | Evaluating MS‐induced stress | 23, 166 |
| Salivary protein and amylase | High baseline salivary protein concentration; high amylase activity | Preceding MS susceptibility | 54, 55 |
| Fos protein | Increase expression | Indicator of MS‐related neuronal activation; evaluating vestibular habituation | 69, 167 |
| Serum glucose | Elevated after provocative motion stimulation | Indicator of severity of GI symptoms of MS | 28 |
SS, simulator sickness; SOT, sensory organization test; MCT, motor control test.
Diagnosis and Evaluation
MS can be diagnosed according to the manifestations during motion exposure after excluding other pathological disorders. Graybiel et al. and Wiker et al. established two MS severity grading criteria by scoring 7 categories of cardinal signs and symptoms, and 28 major, minor, or other symptoms, respectively 58, 59. Several research groups developed different questionnaires for assessing the multiple dimensions of MS symptoms 60, 61, 62 (Table 1).
Heart rate variability (HRV) and electrogastrogram (EGG) are useful for assessing cardiac sympathovagal interactions and gastric motility during MS, respectively 63, 64. HRV indices might be influenced by motion patterns, intersubject variations, subjects' self‐adjustments, vomiting process, and stress response 65, 66. As for the EGG test, increased 4–9 cpm activity and the absence or decrease of 3 cpm activity may indicate MS‐induced nausea and vomiting, respectively 64. EGG has also been demonstrated to be more sensitive than electroencephalogram, electrocardiogram, and skin conductance in MS evaluation 67.
Fos protein, an indicator of neuronal activity, is considered to be a molecular indicator for MS development and habituation 68, 69. Nevertheless, whether Fos expression can illustrate race and sex difference in MS susceptibility is still unclear. Recently, we found that motion‐induced elevation of serum glucose was significantly related to GI symptoms of MS and might serve as a potential MS marker 28 (Table 1).
Motion sickness Medications
In 1869, the first usage of medications for MS is a combination of chloroform and tincture of belladonna 70. Nowadays, there are at least 9 different kinds of drugs used against MS. Anticholinergics and antihistamines are the most effective MS prophylactics with apparent side effects such as drowsiness and depression. Drug combinations are thus used to increase efficacy and alleviate side effects (Table 2).
Table 2.
Antimotion sickness medications
| Category | Dosage formation | Usage | Application | References |
|---|---|---|---|---|
| Anticholinergics | ||||
| Scopolamine | p.o. (0.6 mg) | 0.5–1 h before MS, effective within 6 h | Seasickness and experimental MS | 71 * |
| TTS (1.5 mg/patch) | 6–8 h before MS, effective over 72 h | Seasickness, airsickness, ship motion simulator, and experimental MS | 71, * 74, 75 | |
| IN (0.4 mg) | 0.5 h before MS, effective over 6 h | Experimental MS | 76, 77 * | |
| p.o. (0.3 mg) + TTS | 1 h before MS, effective over 72 h | Seasickness | 168 | |
| Zamifenacin | p.o. (20 mg) | 1.5 before MS | Experimental MS | 73 |
| Antihistamines | ||||
| Dimenhydrinate | p.o. (100 mg) | 2 h before MS effective for 8 to 12 h | Seasickness and experimental MS | 84, * 169 |
| CG (3 × 20 mg) | Chewed for 30 min each during MS | Experimental MS | 82 * | |
| Cinnarizine | Oral (30 or 50 mg) | 3 h before MS | Seasickness and flight simulator sickness | 170, 171 |
| Cyclizine (Marezine) | p.o. (50 mg) | 2 h before MS | Experimental MS | 172 |
| Promethazine | p.o. ( 25 or 50 mg) | 2 h before MS, effective within 12 h | Space MS | 91, 173 |
| i.m. (25 or 50 mg) | 1–2 h before MS, effective within 12 h | Space MS, parabolic flight and experimental MS | 92, 174, 175 | |
| Suppository (25 or 50 mg) | 1–2 h before MS, effective within 12 h | Space MS | 175 | |
| Meclizine (Antivert) | p.o. (25 or 50 mg) | 1–2 h before MS, effective within 24 h | Experimental MS | 94, 176 |
| Chlorpheniramine, | p.o. (4 or 12 mg) | 3–4 h before MS | Experimental MS | 83 * |
| Betahistine | p.o. (32 or 48 mg) | 1–2 h before MS | Seasickness and experimental MS | 89 177 |
| Dopamine Antagonists | ||||
| Metoclopramide | i.v. (20 mg) | 15 min after MS initiation | Carsickness | 97 * |
| 5‐HT1B/1D receptor agonist | ||||
| Rizatriptan | p.o. (10 mg) | 2 h before MS | Experimental MS in migraineurs | 103 * |
| Sympathomimetics | ||||
| D‐amphetamine | p.o. (10 mg) | Airsickness | 108 | |
| Neuroleptics | ||||
| Phenytoin | p.o. (200 mg) | 4 h before MS | Seasickness and parabolic flight MS | 117, 118 |
| Baclofen | p.o. (20 mg) | 0.5–1 h before MS | Experimental MS | 116 |
| Calcium channel blocker | ||||
| Flunarizine | – | – | Experimental MS | 125 † |
| μ‐Opiate receptor agonist | ||||
| Loperamide | p.o. (16 mg) | 3 h before MS | Experimental MS | 126 |
| Hormones | ||||
| Dexamethasone | i.v. (0.5 mg) | Every 6 h for 48 h | Experimental MS | 124 |
| Combination | ||||
| Promethazine + d‐amphetamine | p.o. (25 mg+10 mg) | 2 h before MS | Airsickness | 178 |
| Scopolamine + d‐amphetamine | p.o. (0.4–1.2 mg+5 mg) | 0.5–1 h before MS | Parabolic flight MS | 179 |
| Scopolamine + ephedrine |
p.o. (0.3 mg+25 mg) i.m. (0.2 mg+25 mg) |
0.5–1 h before MS or 3 times daily 30 min before MS |
Seasickness and experimental MS Experimental MS |
180
*
181 |
| Chlorpheniramine + ephedrine | p.o. (12 mg+50 mg) | 3–4 h MS | Experimental MS | 83 * |
| Dimenhydrinate + scopolamine | – | – | Air sickness | 182 † |
p.o., per os; TTS, transdermal therapeutic system; IN, intranasal; CG, chewing gum; i.m., intramuscular; i.v., intravenous.
*Randomized control trials.
†Dosage unavailable.
Anticholinergics
Atropine, scopolamine (hyoscine), and hyoscyamine have already been used to treat MS before World War I. A recent cochrane systematic review of 14 randomized controlled trials (RCTs) concluded that scopolamine, the nonselective muscarinic cholinergic receptor (mAChR) antagonist, was more effective than placebo but not superior to antihistamines in preventing MS and was no more likely to induce drowsiness, blurring vision, or dizziness compared to other agents 71. Nevertheless, the precise mAChR subtypes (M1–M5) that serve as the targets of scopolamine is still unclear. As we know that all mAChR subtypes are expressed in the brain, while only M1, M2, and M5 exist in vestibular ganglia and vestibular end organs in humans 72. The M1, M3, and M5 are postsynaptic excitatory receptors; M2 and M4 receptors are inhibitory. Furthermore, selective M3 and M5 antagonist zamifenacin was found to be as effective as scopolamine in preventing MS 73. These lines of evidence suggest that scopolamine might exert its antagonistic effect on peripheral M1 and M5 and/or central M1 and M3 mAChR to prevent MS.
The commonly used dosage forms of scopolamine include oral tablets and liquid, transdermal therapeutic system (TTS), and the intranasal (IN) aerosol (Table 2). The TTS delivering scopolamine to the mastoid area shows a long‐lasting prophylactic effect without psychomotor impairment 74, 75. Noninvasive IN formulation of scopolamine has higher peak plasma concentration and shorter peak time than oral agents 76, 77. In addition, grapefruit juice can increase the bioavailability of orally administrated scopolamine via inhibiting the cytochrome P‐450 3A enzymes which are involved in oxidative demethylation of the scopolamine, while the efficacy of IN and TTS of scopolamine are affected by pH value 78, 79.
Antihistamines
In 1949, Gray and Carliner for the first time discovered that antihistamine dimenhydrinate was effective in preventing seasickness 80. Small RCTs have verified the effectiveness of the first‐generation H1 antihistamines against MS, but the second generations were ineffective 81, 82, 83, 84 (Table 2). Physiological studies suggested that dimenhydrinate, cinnarizine, and meclizine exerted a central action on the medial VN in which high density of H1 and H2 receptor were present 85, 86, while the promethazine had global suppression effect on vestibular system, but all these antihistamines had no effect on the central autonomic regions 87. Betahistine, an H3 receptor antagonist and a weak H1 receptor agonist, is effective in the preventing seasickness and increases tolerability to Coriolis accelerations via reducing histamine release in medial VN 88, 89. Recent studies found that H4 receptors were expressed in rat vestibular ganglia, and H4 receptor antagonists had a pronounced inhibitory effect on primary vestibular neuron activity and significantly alleviated vestibular deficits in rats 90. These results highlighted H4 receptors as potential pharmacological targets for treating MS.
The main dosage forms of antihistamines include oral (all), intramuscular injection (promethazine and cyclizine), suppository (promethazine), chewing gum (dimenhydrinate), and sublingual form (dimenhydrinate) 91. Putcha et al. found that promethazine, as the only drug given by three different routes (orally, intramuscularly, and rectally), was most effective and had minimal side effects when administered intramuscularly in astronauts during space shuttle missions 92. The diphenhydramine chewing gum has been developed to alleviate antihistamine's adverse effects 82, 93. Recently, a new suspension formulation of meclizine was developed with a more rapid effect and higher maximum concentration than marketed oral tablet 94.
Monoamine Antagonists/Agonist
Dopamine D2 and D3 receptors are known to play a role in nausea and emesis. They can alter the amount of cAMP within neurons of the vomiting center via inhibiting adenylate cyclase 95. Competitive D2 receptor antagonist metoclopramide, administered through intravenous or intramuscular injection but not oral route, alleviated overall symptoms and restored gastric emptying after the initiation of MS 96, 97. In addition, orally administered domperidone, a peripherally restricted D2 receptor antagonist and α 1‐adrenoceptor antagonist, failed to prevent spatial disorientation‐induced gastric dysrhythmia and MS symptoms in humans 98, 99 (Table 2). These results suggest that effectiveness of dopamine antagonists may depend on the administration route and timing. Similarly, although the 5‐HT3 receptor antagonists ondansetron are extensively used to prevent and suppress CINV and PONV 100, oral administration of this drug has no preventive effect against seasickness or experimental MS 101, 102. As MS can induce delayed gastric emptying and reduce absorption, oral forms are problematic and injection or transdermal formation is recommended.
Additionally, two double‐blind, placebo‐controlled studies showed that 5‐HT1B/1D receptor agonist rizatriptan prevented the development of MS in migrainous patients 103, 104. The 5‐HT2A antagonist ketanserin significantly suppressed hypergravity‐induced hypophagia in rats, while a 5‐HT1A agonist, 8‐hydroxy‐2‐(di‐n‐propylamino) tetralin hydrobromide (8‐OH‐DPAT), successfully prevented vomiting induced by motion in cats and suncus murinus 105, 106, 107. The precise efficacy of these drugs against MS in humans needs to be verified in the future.
Stimulants and Sedatives
Sympathomimetics d‐amphetamine was found to be highly effective against space MS rather than seasickness 108. Accumulating evidence suggests that d‐amphetamine and ephedrine might counteract the sedative side effects of scopolamine and antihistamines, but at the risk of drug addiction and counterbalancing the vestibular suppression effect (Table 2). Nevertheless, scopolamine used in combination with d‐amphetamine against MS should be cautioned, for scopolamine impairs decision‐making and motivational behavior similar to the effect produced by amphetamine 109. Modafinil, a potential substitute of amphetamine, significantly enhanced the efficacy of scopolamine when used in combination in rodents 110, but failed to prevent MS in humans when used alone 111. Caffeine, a much more commonly used psychostimulant, was found to be effective in counteracting scopolamine‐induced memory impairment in humans and animals 112, 113, while no study has been performed to evaluate efficacy of caffeine in the management of MS alone or in combination with scopolamine and antihistamines. Neuroleptics including barbiturates, diazepam, and baclofen as well as phenytoin were found to be effective in prevention of MS 114, 115, 116, 117, 118 (Table 2).
Other Drugs
Clinical studies have demonstrated that powdered ginger was as effective as other anti‐emetics in reducing the incidence of nausea and vomiting caused by traveling, while exploratory experimental studies had controversial outcomes possibly due to different stimulation patterns and evaluation methods used 119, 120. Chinese medicinal compound recipe composed of ginger, pogostemonis herba, and radix aucklandiae and an ancient prescription Pingandan are also found to be effective against MS in animals 121, 122. Our study revealed that ginsenosides combined with dexamethasone can significantly increase tolerance to acceleration in rats 123, consisting with early findings that dexamethasone can reduce susceptibility to space MS in humans 124 (Table 2). Flunarizine, a calcium channel blocker, was shown to be a peripherally acting labyrinthine suppressant. It was effective in preventing MS without central depressive side effects 125. A placebo‐controlled, crossover study showed that the peripheral acting μ‐opiate agonist loperamide attenuated vertical axis rotation‐induced nausea in humans 126. The NK1 receptor antagonist aprepitant is successfully used for preventing acute and delayed CINV 127. The NK1 receptor antagonists are also effective against MS‐induced emesis in animals but not in humans 128, 129, 130. Recent findings have demonstrated that MS is associated with impaired endocannabinoid activity 123, 131, 132. CB1 receptor agonist (∆9‐tetrahydrocannabinol, ∆9‐THC) and antagonist (cannabidiolic acid) were observed to inhibit emesis induced by motion in suncus murinus via different neural mechanism 133, 134.
Nonpharmacological Countermeasures
Habituation Training
Transient MS habituation can be induced in animals and humans by repeated or prolonged motion stimulation and may generally last for several weeks 69, 135, 136. The habituation acquired under particular stimulus conditions is normally highly specific, while the time‐course of habituation acquirement for linear acceleration is quite different from that for angular acceleration in humans 137. Repeated exposure may produce more sufficient habituation than single prolonged stimulation, but desensitization to one provocative motion could not be transferred to a more severe motion stimulus 138. Thus, the objective of habituation training is to reproduce the sensory conflict as close as possible to the provocative environment. For instance, horizontal suspension, parabolic flight, and neutral buoyancy simulation have been used as microgravity simulation methods for astronaut training 139, 140. Recent studies have demonstrated that preflight virtual reality training is also effective against space MS and disorientation 141. As sufficient activation of vestibular system is the prerequisite to produce novel “internal model,” anti‐MS drugs are not recommended during MS habituation training process 137, 142.
Compared with conventional ground‐based training procedures using revolving chair, winding stair, idler wheel, and swing, combined visual‐vestibular habituation training was more effective and can produce long‐term effect against travel‐induced MS for up to 18 weeks in susceptible subjects 143, 144. Recent prospective studies also showed that optokinetic training comprising vertical, horizontal, and torsional movements of frontly projected bright spots can reconstitute the effects of swell encountered at sea and appears to be an effective training modality for the prevention of disabling seasickness 145. In addition, the pseudorandom galvanic vestibular stimulation (GVS) is expected to be used in astronaut training against landing sickness, as it accurately replicated the postural instability, locomotor impairment, and reduced dynamic visual acuity observed in astronauts after return from space 146.
Behavioral and other Countermeasures
Forward‐looking vision on the distant horizon is effective in alleviating MS symptoms via matching visual and vestibular information in subjects exposed to simulated ship motion 147. Controlled breathing is also beneficial for managing MS symptoms and promoting habituation 148, 149. Nevertheless, breathing supplemental oxygen had no advantage over breathing air in reducing MS in healthy adults 150. MS symptoms can be alleviated by autogenic‐feedback training exercise for autonomic responses control as well as the manipulations to enhance predictability and positive expectancy 151, 152, 153. Recently, smoking deprivation, pleasant music, and odors as well as head vibration and mental distraction have been found to be effective in reducing MS symptoms 154, 155, 156, 157.
High sodium and energy dense or low vitamin A, vitamin C, and iron diets as well as high frequency of meals in previous 24 h increased the airsickness occurrences in pilots 158. A protein‐predominant beverage taken 5 or 30 min before optokinetic stimulation was found to be effective in suppressing gastric tachyarrhythmia and MS symptoms 159. A recent double‐blind, placebo‐controlled crossover study found that vitamin C was effective in suppressing symptoms of seasickness, particularly in youngsters 160.
Acupuncture at the P6 or Neiguan point to treat nausea and vomiting has been practiced in China for many years, but it is still controversial whether SeaBand or ReliefBand designed for acupressure or electrostimulation at P6 are effective in MS treatment 161, 162. Transcutaneous electrical nerve stimulation of the posterior neck and the right Zusanli acupoint was found to be effective in reducing simulator sickness symptoms and alleviating cognitive impairment 163. Recently, stroboscopic illumination at 8 herts, by ambient strobe light or by liquid crystal display shutter glasses, reduced the severity of MS symptoms and improved the performance on the vigilance task in soldiers exposed to a nauseogenic flight in a helicopter 164.
Conclusion
This study reviews the progress of sensory conflict theory, vestibular homeostasis regulation and genetic basis of MS. It also summarizes prediction and evaluation, and available countermeasures. In sensory conflict theory, the “sensory conflict neurons” remain activated and ultimately disrupt homeostasis and trigger MS responses if the provocative motion signal or reafference information mismatches the “internal model.” The heredity of MS susceptibility involves genetic and epigenetic regulation on genes participating in cellular metabolism, autonomic regulation, and vestibular function and development. Several methods are used for MS prediction and evaluation, but specific indicator is scarce. The efficacy of anti‐MS medications depends on dosage forms and time of administration. Novel drugs in development show no remarkable advantages over traditional medications such as anticholinergics and antihistamines. Visual‐vestibular habituation training is the most effective nonpharmacological prophylaxis. Other measures such as acupuncture and stroboscopic illumination could be substitutes for medications when side effects are unacceptable.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
This study is supported by grants from the National Natural Science Foundation of China (81272178) and the Natural Science Foundation of Shanghai (12ZR1437300).
References
- 1. Lackner JR. Motion sickness: More than nausea and vomiting. Exp Brain Res 2014;232:2493–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Golding JF. Motion sickness susceptibility. Auton Neurosci 2006;129:67–76. [DOI] [PubMed] [Google Scholar]
- 3. Reason JT. Motion sickness adaptation: A neural mismatch model. J R Soc Med 1978;71:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tal D, Wiener G, Shupak A. Mal de debarquement, motion sickness and the effect of an artificial horizon. J Vestib Res 2014;24:17–23. [DOI] [PubMed] [Google Scholar]
- 5. Oman CM. Are evolutionary hypotheses for motion sickness “just‐so” stories? J Vestib Res 2012;22:117–127. [DOI] [PubMed] [Google Scholar]
- 6. Carriot J, Brooks JX, Cullen KE. Multimodal integration of self‐motion cues in the vestibular system: Active versus passive translations. J Neurosci 2013;33:19555–19566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oman CM, Cullen KE. Brainstem processing of vestibular sensory exafference: Implications for motion sickness etiology. Exp Brain Res 2014;232:2483–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cullen KE. The vestibular system: Multimodal integration and encoding of self‐motion for motor control. Trends Neurosci 2012;35:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aitake M, Hori E, Matsumoto J, et al. Sensory mismatch induces autonomic responses associated with hippocampal theta waves in rats. Behav Brain Res 2011;220:244–253. [DOI] [PubMed] [Google Scholar]
- 10. Zou D, Aitake M, Hori E, et al. Rat hippocampal theta rhythm during sensory mismatch. Hippocampus 2009;19:350–359. [DOI] [PubMed] [Google Scholar]
- 11. Uno A, Takeda N, Horii A, et al. Effects of amygdala or hippocampus lesion on hypergravity‐induced motion sickness in rats. Acta Otolaryngol 2000;120:860–865. [DOI] [PubMed] [Google Scholar]
- 12. Takahashi K, Gu Y, May PJ, et al. Multimodal coding of three‐dimensional rotation and translation in area MSTd: Comparison of visual and vestibular selectivity. J Neurosci 2007;27:9742–9756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen A, DeAngelis GC, Angelaki DE. Convergence of vestibular and visual self‐motion signals in an area of the posterior sylvian fissure. J Neurosci 2011;31:11617–11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Demertzi A, Van Ombergen A, Tomilovskaya E, et al. Cortical reorganization in an astronaut's brain after long‐duration spaceflight. Brain Struct Funct 2015. doi: 10.1007/s00429-015-1054-3 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holstein GR, Friedrich VL Jr, Martinelli GP. Projection neurons of the vestibulo‐sympathetic reflex pathway. J Comp Neurol 2014;522:2053–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holstein GR, Friedrich VL Jr, Kang T, Kukielka E, Martinelli GP. Direct projections from the caudal vestibular nuclei to the ventrolateral medulla in the rat. Neuroscience 2011;175:104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yates BJ, Bolton PS, Macefield VG. Vestibulo‐sympathetic responses. Compr Physiol 2014;4:851–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yates BJ, Catanzaro MF, Miller DJ, McCall AA. Integration of vestibular and emetic gastrointestinal signals that produce nausea and vomiting: Potential contributions to motion sickness. Exp Brain Res 2014;232:2455–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ossenkopp KP, Ossenkopp MD. Animal models of motion sickness: Are nonemetic species an appropriate choice? Physiologist 1985;28:S61–S62. [PubMed] [Google Scholar]
- 20. Nobel G, Tribukait A, Mekjavic IB, Eiken O. Effects of motion sickness on thermoregulatory responses in a thermoneutral air environment. Eur J Appl Physiol 2012;112:1717–1723. [DOI] [PubMed] [Google Scholar]
- 21. Ngampramuan S, Cerri M, Del Vecchio F, et al. Thermoregulatory correlates of nausea in rats and musk shrews. Oncotarget 2014;5:1565–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fuller PM, Jones TA, Jones SM, Fuller CA. Neurovestibular modulation of circadian and homeostatic regulation: Vestibulohypothalamic connection? Proc Natl Acad Sci U S A 2002;99:15723–15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rohleder N, Otto B, Wolf JM, et al. Sex‐specific adaptation of endocrine and inflammatory responses to repeated nauseogenic body rotation. Psychoneuroendocrinology 2006;31:226–236. [DOI] [PubMed] [Google Scholar]
- 24. Otto B, Riepl RL, Klosterhalfen S, Enck P. Endocrine correlates of acute nausea and vomiting. Auton Neurosci 2006;129:17–21. [DOI] [PubMed] [Google Scholar]
- 25. Camilleri M, Papathanasopoulos A, Odunsi ST. Actions and therapeutic pathways of ghrelin for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol 2009;6:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Finley JC Jr, O'Leary M, Wester D, et al. A genetic polymorphism of the alpha2‐adrenergic receptor increases autonomic responses to stress. J Appl Physiol (1985) 2004;96:2231–2239. [DOI] [PubMed] [Google Scholar]
- 27. Hromatka BS, Tung JY, Kiefer AK, et al. Genetic variants associated with motion sickness point to roles for inner ear development, neurological processes and glucose homeostasis. Hum Mol Genet 2015;24:2700–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mo FF, Qin HH, Wang XL, et al. Acute hyperglycemia is related to gastrointestinal symptoms in motion sickness: An experimental study. Physiol Behav 2012;105:394–401. [DOI] [PubMed] [Google Scholar]
- 29. Janicki PK, Sugino S. Genetic factors associated with pharmacotherapy and background sensitivity to postoperative and chemotherapy‐induced nausea and vomiting. Exp Brain Res 2014;232:2613–2625. [DOI] [PubMed] [Google Scholar]
- 30. Persico AM, Verdecchia M, Pinzone V, Guidetti V. Migraine genetics: Current findings and future lines of research. Neurogenetics 2015;16:77–95. [DOI] [PubMed] [Google Scholar]
- 31. Sharon JD, Hullar TE. Motion sensitivity and caloric responsiveness in vestibular migraine and Meniere's disease. Laryngoscope 2014;124:969–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferrari MD, Klever RR, Terwindt GM, Ayata C, van den Maagdenberg AM. Migraine pathophysiology: Lessons from mouse models and human genetics. Lancet Neurol 2015;14:65–80. [DOI] [PubMed] [Google Scholar]
- 33. Chiarella G, Petrolo C, Cassandro E. The genetics of Meniere's disease. Appl Clin Genet 2015;8:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taylor WE, Bhasin S, Lalani R, Datta A, Gonzalez‐Cadavid NF. Alteration of gene expression profiles in skeletal muscle of rats exposed to microgravity during a spaceflight. J Gravit Physiol 2002;9:61–70. [PubMed] [Google Scholar]
- 35. Ward NE, Pellis NR, Risin SA, Risin D. Gene expression alterations in activated human T‐cells induced by modeled microgravity. J Cell Biochem 2006;99:1187–1202. [DOI] [PubMed] [Google Scholar]
- 36. Wang JQ, Qi RR, Zhou W, et al. Differential gene expression profile in the rat caudal vestibular nucleus is associated with individual differences in motion sickness susceptibility. PLoS ONE 2015;10:e0124203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh KP, Kumari R, Dumond JW. Simulated microgravity‐induced epigenetic changes in human lymphocytes. J Cell Biochem 2010;111:123–129. [DOI] [PubMed] [Google Scholar]
- 38. Paillard AC, Quarck G, Paolino F, et al. Motion sickness susceptibility in healthy subjects and vestibular patients: Effects of gender, age and trait‐anxiety. J Vestib Res 2013;23:203–209. [DOI] [PubMed] [Google Scholar]
- 39. Lawther A, Griffin MJ. Prediction of the incidence of motion sickness from the magnitude, frequency, and duration of vertical oscillation. J Acoust Soc Am 1987;82:957–966. [DOI] [PubMed] [Google Scholar]
- 40. Arribas FLP, Pineiro AL. Seasickness prediction in passenger ships at the design stage. Ocean Eng 2007;34:2086–2092. [Google Scholar]
- 41. Birren JE, Fisher MB. Susceptibility to seasickness; a questionnaire approach. J Appl Psychol 1947;31:288–297. [DOI] [PubMed] [Google Scholar]
- 42. Kennedy RS. Motion sickness questionnaire and field independence scores as predictors of success in naval aviation training. Aviat Space Environ Med 1975;46:1349–1352. [PubMed] [Google Scholar]
- 43. Reason JT. Relations between motion sickness susceptibility, the spiral after‐effect and loudness estimation. Br J Psychol 1968;59:385–393. [DOI] [PubMed] [Google Scholar]
- 44. Kennedy RS, Fowlkes JE, Berbaum KS, Lilienthal MG. Use of a motion sickness history questionnaire for prediction of simulator sickness. Aviat Space Environ Med 1992;63:588–593. [PubMed] [Google Scholar]
- 45. Golding JF. Motion sickness susceptibility questionnaire revised and its relationship to other forms of sickness. Brain Res Bull 1998;47:507–516. [DOI] [PubMed] [Google Scholar]
- 46. Lamb S, Kwok KC. MSSQ‐short norms may underestimate highly susceptible individuals: Updating the MSSQ‐short norms. Hum Factors 2015;57:622–633. [DOI] [PubMed] [Google Scholar]
- 47. Nachum Z, Gordon CR, Shahal B, Spitzer O, Shupak A. Active high‐frequency vestibulo‐ocular reflex and seasickness susceptibility. Laryngoscope 2002;112:179–182. [DOI] [PubMed] [Google Scholar]
- 48. Gordon CR, Spitzer O, Doweck I, Shupak A, Gadoth N. The vestibulo‐ocular reflex and seasickness susceptibility. J Vestib Res 1996;6:229–233. [PubMed] [Google Scholar]
- 49. Fowler CG, Sweet A, Steffel E. Effects of motion sickness severity on the vestibular‐evoked myogenic potentials. J Am Acad Audiol 2014;25:814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tal D, Hershkovitz D, Kaminski‐Graif G, et al. Vestibular evoked myogenic potentials and habituation to seasickness. Clin Neurophysiol 2013;124:2445–2449. [DOI] [PubMed] [Google Scholar]
- 51. Varlet M, Bardy BG, Chen FC, Alcantara C, Stoffregen TA. Coupling of postural activity with motion of a ship at sea. Exp Brain Res 2015;233:1607–1616. [DOI] [PubMed] [Google Scholar]
- 52. Shahal B, Nachum Z, Spitzer O, et al. Computerized dynamic posturography and seasickness susceptibility. Laryngoscope 1999;109:1996–2000. [DOI] [PubMed] [Google Scholar]
- 53. Tal D, Bar R, Nachum Z, Gil A, Shupak A. Postural dynamics and habituation to seasickness. Neurosci Lett 2010;479:134–137. [DOI] [PubMed] [Google Scholar]
- 54. Igarashi M, Reschke MF, Henley C, et al. Salivary total protein and experimental Coriolis sickness. Acta Otolaryngol Suppl 1993;504:38–40. [DOI] [PubMed] [Google Scholar]
- 55. Gordon CR, Jackman Y, Ben‐Aryeh H, et al. Salivary secretion and seasickness susceptibility. Aviat Space Environ Med 1992;63:356–359. [PubMed] [Google Scholar]
- 56. Sharma K, Sharma P, Sharma A, Singh G. Phenylthiocarbamide taste perception and susceptibility to motion sickness: Linking higher susceptibility with higher phenylthiocarbamide taste acuity. J Laryngol Otol 2008;122:1064–1073. [DOI] [PubMed] [Google Scholar]
- 57. Paillard A, Jacquot L, Millot JL. Olfactory perception and motion sickness. Chem Senses 2011;36:E35–E36. [Google Scholar]
- 58. Graybiel A, Wood CD, Miller EF, Cramer DB. Diagnostic criteria for grading the severity of acute motion sickness. Aerosp Med 1968;39:453–455. [PubMed] [Google Scholar]
- 59. Wiker SF, Kennedy RS, McCauley ME, Pepper RL. Susceptibility to seasickness: Influence of hull design and steaming direction. Aviat Space Environ Med 1979;50:1046–1051. [PubMed] [Google Scholar]
- 60. Fowlkes JE, Kennedy RS, Hettinger LJ, Harm DL. Changes in the dark focus of accommodation associated with simulator sickness. Aviat Space Environ Med 1993;64:612–618. [PubMed] [Google Scholar]
- 61. Muth ER, Stern RM, Thayer JF, Koch KL. Assessment of the multiple dimensions of nausea: The Nausea Profile (NP). J Psychosom Res 1996;40:511–520. [DOI] [PubMed] [Google Scholar]
- 62. Gianaros PJ, Muth ER, Mordkoff JT, Levine ME, Stern RM. A questionnaire for the assessment of the multiple dimensions of motion sickness. Aviat Space Environ Med 2001;72:115–119. [PMC free article] [PubMed] [Google Scholar]
- 63. Lin CT, Lin CL, Chiu TW, Duann JR, Jung TP. Effect of respiratory modulation on relationship between heart rate variability and motion sickness. Conf Proc IEEE Eng Med Biol Soc 2011;2011:1921–1924. [DOI] [PubMed] [Google Scholar]
- 64. Lang IM, Sarna SK, Shaker R. Gastrointestinal motor and myoelectric correlates of motion sickness. Am J Physiol 1999;277:G642–G652. [DOI] [PubMed] [Google Scholar]
- 65. Lin CL, Jung TP, Chuang SW, et al. Self‐adjustments may account for the contradictory correlations between HRV and motion‐sickness severity. Int J Psychophysiol 2013;87:70–80. [DOI] [PubMed] [Google Scholar]
- 66. Lacount L, Napadow V, Kuo B, et al. Dynamic cardiovagal response to motion sickness: A point‐process heart rate variability study. Comput Cardiol 2009;36:49–52. [PMC free article] [PubMed] [Google Scholar]
- 67. Hu S, McChesney KA, Player KA, et al. Systematic investigation of physiological correlates of motion sickness induced by viewing an optokinetic rotating drum. Aviat Space Environ Med 1999;70:759–765. [PubMed] [Google Scholar]
- 68. Pompeiano O, d'Ascanio P, Balaban E, Centini C, Pompeiano M. Gene expression in autonomic areas of the medulla and the central nucleus of the amygdala in rats during and after space flight. Neuroscience 2004;124:53–69. [DOI] [PubMed] [Google Scholar]
- 69. Cai YL, Wang JQ, Chen XM, et al. Decreased Fos protein expression in rat caudal vestibular nucleus is associated with motion sickness habituation. Neurosci Lett 2010;480:87–91. [DOI] [PubMed] [Google Scholar]
- 70. Schmal F. Neuronal mechanisms and the treatment of motion sickness. Pharmacology 2013;91:229–241. [DOI] [PubMed] [Google Scholar]
- 71. Spinks A, Wasiak J. Scopolamine (hyoscine) for preventing and treating motion sickness. Cochrane Database Syst Rev 2011:CD002851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ishiyama A, Lopez I, Wackym PA. Molecular characterization of muscarinic receptors in the human vestibular periphery. Implications for pharmacotherapy. Am J Otol 1997;18:648–654. [PubMed] [Google Scholar]
- 73. Golding JF, Stott JR. Comparison of the effects of a selective muscarinic receptor antagonist and hyoscine (scopolamine) on motion sickness, skin conductance and heart rate. Br J Clin Pharmacol 1997;43:633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Noy S, Shapira S, Zilbiger A, Ribak J. Transdermal therapeutic system scopolamine (TTSS), dimenhydrinate, and placebo–a comparative study at sea. Aviat Space Environ Med 1984;55:1051–1054. [PubMed] [Google Scholar]
- 75. Gleiter CH, Antonin KH, Bieck PR. Transdermally applied scopolamine does not impair psychomotor performance. Psychopharmacology 1984;83:397–398. [DOI] [PubMed] [Google Scholar]
- 76. Klocker N, Hanschke W, Toussaint S, Verse T. Scopolamine nasal spray in motion sickness: A randomised, controlled, and crossover study for the comparison of two scopolamine nasal sprays with oral dimenhydrinate and placebo. Eur J Pharm Sci 2001;13:227–232. [DOI] [PubMed] [Google Scholar]
- 77. Simmons RG, Phillips JB, Lojewski RA, et al. The efficacy of low‐dose intranasal scopolamine for motion sickness. Aviat Space Environ Med 2010;81:405–412. [DOI] [PubMed] [Google Scholar]
- 78. Ebert U, Oertel R, Kirch W. Influence of grapefruit juice on scopolamine pharmacokinetics and pharmacodynamics in healthy male and female subjects. Int J Clin Pharmacol Ther 2000;38:523–531. [DOI] [PubMed] [Google Scholar]
- 79. Renner UD, Oertel R, Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit 2005;27:655–665. [DOI] [PubMed] [Google Scholar]
- 80. Gay LN, Carliner PE. The prevention and treatment of motion sickness I. seasickness. Science 1949;109:359. [DOI] [PubMed] [Google Scholar]
- 81. Cheung BS, Heskin R, Hofer KD. Failure of cetirizine and fexofenadine to prevent motion sickness. Ann Pharmacother 2003;37:173–177. [DOI] [PubMed] [Google Scholar]
- 82. Seibel K, Schaffler K, Reitmeir P, Golly I. A randomised, placebo‐controlled study comparing two formulations of dimenhydrinate with respect to efficacy in motion sickness and sedation. Arzneimittelforschung 2002;52:529–536. [DOI] [PubMed] [Google Scholar]
- 83. Buckey JC Jr, Alvarenga DL, MacKenzie TA. Chlorpheniramine and ephedrine in combination for motion sickness. J Vestib Res 2007;17:301–311. [PubMed] [Google Scholar]
- 84. Pyykko I, Schalen L, Jantti V. Transdermally administered scopolamine vs. dimenhydrinate. I. Effect on nausea and vertigo in experimentally induced motion sickness. Acta Otolaryngol 1985;99:588–596. [DOI] [PubMed] [Google Scholar]
- 85. Zhou L, Zhou W, Zhang S, et al. Changes in histamine receptors (H1, H2, and H3) expression in rat medial vestibular nucleus and flocculus after unilateral labyrinthectomy: Histamine receptors in vestibular compensation. PLoS ONE 2013;8:e66684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang XY, Yu L, Zhuang QX, et al. Postsynaptic mechanisms underlying the excitatory action of histamine on medial vestibular nucleus neurons in rats. Br J Pharmacol 2013;170:156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Weerts AP, De Meyer G, Pauwels G, et al. Pharmaceutical countermeasures have opposite effects on the utricles and semicircular canals in man. Audiol Neurootol 2012;17:235–242. [DOI] [PubMed] [Google Scholar]
- 88. Wang JJ, Dutia MB. Effects of histamine and betahistine on rat medial vestibular nucleus neurones: Possible mechanism of action of anti‐histaminergic drugs in vertigo and motion sickness. Exp Brain Res 1995;105:18–24. [DOI] [PubMed] [Google Scholar]
- 89. Matsnev EI, Sigaleva EE. Efficacy of histaminergic drugs in experimental motion sickness. J Vestib Res 2007;17:313–321. [PubMed] [Google Scholar]
- 90. Desmadryl G, Gaboyard‐Niay S, Brugeaud A, et al. Histamine H4 receptor antagonists as potent modulators of mammalian vestibular primary neuron excitability. Br J Pharmacol 2012;167:905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Murdin L, Golding J, Bronstein A. Managing motion sickness. BMJ 2011;343:d7430. [DOI] [PubMed] [Google Scholar]
- 92. Putcha L, Berens KL, Marshburn TH, Ortega HJ, Billica RD. Pharmaceutical use by U.S. astronauts on space shuttle missions. Aviat Space Environ Med 1999;70:705–708. [PubMed] [Google Scholar]
- 93. Valoti M, Frosini M, Dragoni S, Fusi F, Sgaragli G. Pharmacokinetics of diphenhydramine in healthy volunteers with a dimenhydrinate 25 mg chewing gum formulation. Methods Find Exp Clin Pharmacol 2003;25:377–381. [DOI] [PubMed] [Google Scholar]
- 94. Wang Z, Lee B, Pearce D, et al. Meclizine metabolism and pharmacokinetics: Formulation on its absorption. J Clin Pharmacol 2012;52:1343–1349. [DOI] [PubMed] [Google Scholar]
- 95. Sanger GJ, Andrews PL. Treatment of nausea and vomiting: Gaps in our knowledge. Auton Neurosci 2006;129:3–16. [DOI] [PubMed] [Google Scholar]
- 96. Kohl RL. Failure of metoclopramide to control emesis or nausea due to stressful angular or linear acceleration. Aviat Space Environ Med 1987;58:125–131. [PubMed] [Google Scholar]
- 97. Rubio S, Weichenthal L, Andrews J. Motion sickness: Comparison of metoclopramide and diphenhydramine to placebo. Prehosp Disaster Med 2011;26:305–309. [DOI] [PubMed] [Google Scholar]
- 98. Takeda N, Hasegawa S, Morita M, et al. Neuropharmacological mechanisms of emesis. I. Effects of antiemetic drugs on motion‐ and apomorphine‐induced pica in rats. Methods Find Exp Clin Pharmacol 1995;17:589–590. [PubMed] [Google Scholar]
- 99. Kono T, Tokumaru O, Mizumoto C, Tatsuno J, Chen JD. Impaired gastric slow waves induced by spatial disorientation and effect of domperidone. Am J Gastroenterol 1999;94:1224–1229. [DOI] [PubMed] [Google Scholar]
- 100. Carlisle JB, Stevenson CA. Drugs for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev 2006:CD004125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hershkovitz D, Asna N, Shupak A, et al. Ondansetron for the prevention of seasickness in susceptible sailors: An evaluation at sea. Aviat Space Environ Med 2009;80:643–646. [DOI] [PubMed] [Google Scholar]
- 102. Levine ME, Chillas JC, Stern RM, Knox GW. The effects of serotonin (5‐HT3) receptor antagonists on gastric tachyarrhythmia and the symptoms of motion sickness. Aviat Space Environ Med 2000;71:1111–1114. [PubMed] [Google Scholar]
- 103. Furman JM, Marcus DA, Balaban CD. Rizatriptan reduces vestibular‐induced motion sickness in migraineurs. J Headache Pain 2011;12:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Marcus DA, Furman JM. Prevention of motion sickness with rizatriptan: A double‐blind, placebo‐controlled pilot study. Med Sci Monit 2006;12:PI1–PI7. [PubMed] [Google Scholar]
- 105. Abe C, Tanaka K, Iwata C, Morita H. Vestibular‐mediated increase in central serotonin plays an important role in hypergravity‐induced hypophagia in rats. J Appl Physiol (1985) 2010;109:1635–1643. [DOI] [PubMed] [Google Scholar]
- 106. Lucot JB, Crampton GH. 8‐OH‐DPAT suppresses vomiting in the cat elicited by motion, cisplatin or xylazine. Pharmacol Biochem Behav 1989;33:627–631. [DOI] [PubMed] [Google Scholar]
- 107. Brame RE, Lucot JB. Guamanian Suncus murinus responsiveness to emetic stimuli and the antiemetic effects of 8‐OH‐DPAT. Pharmacol Biochem Behav 2011;99:381–384. [DOI] [PubMed] [Google Scholar]
- 108. Wood CD, Kennedy RE, Graybiel A, Trumbull R, Wherry RJ. Clinical effectiveness of anti‐motion‐sickness drugs. Computer review of the literature. JAMA 1966;198:1155–1158. [PubMed] [Google Scholar]
- 109. Silveira MM, Malcolm E, Shoaib M, Winstanley CA. Scopolamine and amphetamine produce similar decision‐making deficits on a rat gambling task via independent pathways. Behav Brain Res 2015;281:86–95. [DOI] [PubMed] [Google Scholar]
- 110. Yu XH, Cai GJ, Liu AJ, Chu ZX, Su DF. A novel animal model for motion sickness and its first application in rodents. Physiol Behav 2007;92:702–707. [DOI] [PubMed] [Google Scholar]
- 111. Hoyt RE, Lawson BD, McGee HA, Strompolis ML, McClellan MA. Modafinil as a potential motion sickness countermeasure. Aviat Space Environ Med 2009;80:709–715. [DOI] [PubMed] [Google Scholar]
- 112. Botton PH, Costa MS, Ardais AP, et al. Caffeine prevents disruption of memory consolidation in the inhibitory avoidance and novel object recognition tasks by scopolamine in adult mice. Behav Brain Res 2010;214:254–259. [DOI] [PubMed] [Google Scholar]
- 113. Riedel W, Hogervorst E, Leboux R, et al. Caffeine attenuates scopolamine‐induced memory impairment in humans. Psychopharmacology 1995;122:158–168. [DOI] [PubMed] [Google Scholar]
- 114. Noble RL. The effect of barbiturates and other substances on motion sickness in dogs. Can J Res E Med Sci 1948;26:283–294. [DOI] [PubMed] [Google Scholar]
- 115. McClure JA, Lycett P, Baskerville JC. Diazepam as an anti‐motion sickness drug. J Otolaryngol 1982;11:253–259. [PubMed] [Google Scholar]
- 116. Cohen B, Dai M, Yakushin SB, Raphan T. Baclofen, motion sickness susceptibility and the neural basis for velocity storage. Prog Brain Res 2008;171:543–553. [DOI] [PubMed] [Google Scholar]
- 117. Chelen W, Kabrisky M, Hatsell C, et al. Use of phenytoin in the prevention of motion sickness. Aviat Space Environ Med 1990;61:1022–1025. [PubMed] [Google Scholar]
- 118. Knox GW, Woodard D, Chelen W, Ferguson R, Johnson L. Phenytoin for motion sickness: Clinical evaluation. Laryngoscope 1994;104:935–939. [DOI] [PubMed] [Google Scholar]
- 119. Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005;12:684–701. [DOI] [PubMed] [Google Scholar]
- 120. Palatty PL, Haniadka R, Valder B, Arora R, Baliga MS. Ginger in the prevention of nausea and vomiting: A review. Crit Rev Food Sci Nutr 2013;53:659–669. [DOI] [PubMed] [Google Scholar]
- 121. Chen Y, Zhang C, Zhang M, Fu X. Three statistical experimental designs for enhancing yield of active compounds from herbal medicines and anti‐motion sickness bioactivity. Pharmacogn Mag 2015;11:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pei JS, Tong BL, Chen KJ, Li CS, Zhang GX. Experimental research on antimotion sickness effects of Chinese medicine “pingandan” pills in cats. Chin Med J (Engl) 1992;105:322–327. [PubMed] [Google Scholar]
- 123. Zheng Y, Wang XL, Mo FF, Li M. Dexamethasone alleviates motion sickness in rats in part by enhancing the endocannabinoid system. Eur J Pharmacol 2014;727:99–105. [DOI] [PubMed] [Google Scholar]
- 124. Kohl RL, MacDonald S. New pharmacologic approaches to the prevention of space/motion sickness. J Clin Pharmacol 1991;31:934–946. [DOI] [PubMed] [Google Scholar]
- 125. Lee JA, Watson LA, Boothby G. Calcium antagonists in the prevention of motion sickness. Aviat Space Environ Med 1986;57:45–48. [PubMed] [Google Scholar]
- 126. Otto B, Riepl RL, Otto C, et al. mu‐Opiate receptor agonists – a new pharmacological approach to prevent motion sickness? Br J Clin Pharmacol 2006;61:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hargreaves R, Ferreira JC, Hughes D, et al. Development of aprepitant, the first neurokinin‐1 receptor antagonist for the prevention of chemotherapy‐induced nausea and vomiting. Ann N Y Acad Sci 2011;1222:40–48. [DOI] [PubMed] [Google Scholar]
- 128. Mathis A, Lee K, Alibhai HI. The use of maropitant to prevent vomiting induced by epidural administration of preservative free morphine through an epidural catheter in a dog. Vet Anaesth Analg 2011;38:516–517. [DOI] [PubMed] [Google Scholar]
- 129. Reid K, Palmer JL, Wright RJ, et al. Comparison of the neurokinin‐1 antagonist GR205171, alone and in combination with the 5‐HT3 antagonist ondansetron, hyoscine and placebo in the prevention of motion‐induced nausea in man. Br J Clin Pharmacol 2000;50:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lucot JB, Obach RS, McLean S, Watson JW. The effect of CP‐99994 on the responses to provocative motion in the cat. Br J Pharmacol 1997;120:116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chouker A, Kaufmann I, Kreth S, et al. Motion sickness, stress and the endocannabinoid system. PLoS ONE 2010;5:e10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Strewe C, Feuerecker M, Nichiporuk I, et al. Effects of parabolic flight and spaceflight on the endocannabinoid system in humans. Rev Neurosci 2012;23:673–680. [DOI] [PubMed] [Google Scholar]
- 133. Cluny NL, Naylor RJ, Whittle BA, Javid FA. The effects of cannabidiol and tetrahydrocannabinol on motion‐induced emesis in Suncus murinus. Basic Clin Pharmacol Toxicol 2008;103:150–156. [DOI] [PubMed] [Google Scholar]
- 134. Bolognini D, Rock EM, Cluny NL, et al. Cannabidiolic acid prevents vomiting in Suncus murinus and nausea‐induced behaviour in rats by enhancing 5‐HT1A receptor activation. Br J Pharmacol 2013;168:1456–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wilpizeski CR, Lowry LD, Miller R. Intensification and habituation of experimental motion sickness in squirrel monkeys by repeated horizontal rotation. Otolaryngol Head Neck Surg 1986;94:628–632. [DOI] [PubMed] [Google Scholar]
- 136. Hu S, Stern RM. The retention of adaptation to motion sickness eliciting stimulation. Aviat Space Environ Med 1999;70:766–768. [PubMed] [Google Scholar]
- 137. Wood CD, Stewart JJ, Wood MJ, et al. Habituation and motion sickness. J Clin Pharmacol 1994;34:628–634. [DOI] [PubMed] [Google Scholar]
- 138. Cheung B, Hofer K. Desensitization to strong vestibular stimuli improves tolerance to simulated aircraft motion. Aviat Space Environ Med 2005;76:1099–1104. [PubMed] [Google Scholar]
- 139. Strauss S. Space medicine at the NASA‐JSC, neutral buoyancy laboratory. Aviat Space Environ Med 2008;79:732–733. [PubMed] [Google Scholar]
- 140. De Witt JK, Perusek GP, Lewandowski BE, et al. Locomotion in simulated and real microgravity: Horizontal suspension vs. parabolic flight. Aviat Space Environ Med 2010;81:1092–1099. [DOI] [PubMed] [Google Scholar]
- 141. Chen W, Chao JG, Chen XW, Wang JK, Tan C. Quantitative orientation preference and susceptibility to space motion sickness simulated in a virtual reality environment. Brain Res Bull 2015;113:17–26. [DOI] [PubMed] [Google Scholar]
- 142. Wood CD, Manno JE, Manno BR, Odenheimer RC, Bairnsfather LE. The effect of antimotion sickness drugs on habituation to motion. Aviat Space Environ Med 1986;57:539–542. [PubMed] [Google Scholar]
- 143. Dai M, Raphan T, Cohen B. Prolonged reduction of motion sickness sensitivity by visual‐vestibular interaction. Exp Brain Res 2011;210:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Stroud KJ, Harm DL, Klaus DM. Preflight virtual reality training as a countermeasure for space motion sickness and disorientation. Aviat Space Environ Med 2005;76:352–356. [PubMed] [Google Scholar]
- 145. Ressiot E, Dolz M, Bonne L, Marianowski R. Prospective study on the efficacy of optokinetic training in the treatment of seasickness. Eur Ann Otorhinolaryngol Head Neck Dis 2013;130:263–268. [DOI] [PubMed] [Google Scholar]
- 146. Dilda V, MacDougall HG, Moore ST. Tolerance to extended galvanic vestibular stimulation: Optimal exposure for astronaut training. Aviat Space Environ Med 2011;82:770–774. [DOI] [PubMed] [Google Scholar]
- 147. Tal D, Gonen A, Wiener G, et al. Artificial horizon effects on motion sickness and performance. Otol Neurotol 2012;33:878–885. [DOI] [PubMed] [Google Scholar]
- 148. Stromberg SE, Russell ME, Carlson CR. Diaphragmatic breathing and its effectiveness for the management of motion sickness. Aerosp Med Hum Perform 2015;86:452–457. [DOI] [PubMed] [Google Scholar]
- 149. Yen Pik Sang F, Billar J, Gresty MA, Golding JF. Effect of a novel motion desensitization training regime and controlled breathing on habituation to motion sickness. Percept Mot Skills 2005;101:244–256. [DOI] [PubMed] [Google Scholar]
- 150. Ziavra NV, Yen Pik Sang FD, Golding JF, Bronstein AM, Gresty MA. Effect of breathing supplemental oxygen on motion sickness in healthy adults. Mayo Clin Proc 2003;78:574–578. [DOI] [PubMed] [Google Scholar]
- 151. Cowings PS, Toscano WB. Autogenic‐feedback training exercise is superior to promethazine for control of motion sickness symptoms. J Clin Pharmacol 2000;40:1154–1165. [PubMed] [Google Scholar]
- 152. Levine ME, Stern RM, Koch KL. The effects of manipulating expectations through placebo and nocebo administration on gastric tachyarrhythmia and motion‐induced nausea. Psychosom Med 2006;68:478–486. [DOI] [PubMed] [Google Scholar]
- 153. Levine ME, Stern RM, Koch KL. Enhanced perceptions of control and predictability reduce motion‐induced nausea and gastric dysrhythmia. Exp Brain Res 2014;232:2675–2684. [DOI] [PubMed] [Google Scholar]
- 154. Bos JE. Less sickness with more motion and/or mental distraction. J Vestib Res 2015;25:23–33. [DOI] [PubMed] [Google Scholar]
- 155. Keshavarz B, Hecht H. Pleasant music as a countermeasure against visually induced motion sickness. Appl Ergon 2014;45:521–527. [DOI] [PubMed] [Google Scholar]
- 156. Golding JF, Prosyanikova O, Flynn M, Gresty MA. The effect of smoking nicotine tobacco versus smoking deprivation on motion sickness. Auton Neurosci 2011;160:53–58. [DOI] [PubMed] [Google Scholar]
- 157. Keshavarz B, Stelzmann D, Paillard A, Hecht H. Visually induced motion sickness can be alleviated by pleasant odors. Exp Brain Res 2015;233:1353–1364. [DOI] [PubMed] [Google Scholar]
- 158. Lindseth G, Lindseth PD. The relationship of diet to airsickness. Aviat Space Environ Med 1995;66:537–541. [PubMed] [Google Scholar]
- 159. Williamson MJ, Levine ME, Stern RM. The effect of meals of varying nutritional composition on subjective and physiological markers of nausea in response to optokinetic motion. Digestion 2005;72:254–260. [DOI] [PubMed] [Google Scholar]
- 160. Jarisch R, Weyer D, Ehlert E, et al. Impact of oral vitamin C on histamine levels and seasickness. J Vestib Res 2014;24:281–288. [DOI] [PubMed] [Google Scholar]
- 161. Miller KE, Muth ER. Efficacy of acupressure and acustimulation bands for the prevention of motion sickness. Aviat Space Environ Med 2004;75:227–234. [PubMed] [Google Scholar]
- 162. Stern RM, Jokerst MD, Muth ER, Hollis C. Acupressure relieves the symptoms of motion sickness and reduces abnormal gastric activity. Altern Ther Health Med 2001;7:91–94. [PubMed] [Google Scholar]
- 163. Chu H, Li MH, Huang YC, Lee SY. Simultaneous transcutaneous electrical nerve stimulation mitigates simulator sickness symptoms in healthy adults: A crossover study. BMC Complement Altern Med 2013;13:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Webb CM, Estrada A, Athy JR. Motion sickness prevention by an 8‐Hz stroboscopic environment during air transport. Aviat Space Environ Med 2013;84:177–183. [DOI] [PubMed] [Google Scholar]
- 165. Cui J, Mukai C, Iwase S, et al. Response to vestibular stimulation of sympathetic outflow to muscle in humans. J Auton Nerv Syst 1997;66:154–162. [DOI] [PubMed] [Google Scholar]
- 166. Drummer C, Stromeyer H, Riepl RL, et al. Hormonal changes after parabolic flight: Implications on the development of motion sickness. Aviat Space Environ Med 1990;61:821–828. [PubMed] [Google Scholar]
- 167. Cai YL, Ma WL, Li M, et al. Glutamatergic vestibular neurons express Fos after vestibular stimulation and project to the NTS and the PBN in rats. Neurosci Lett 2007;417:132–137. [DOI] [PubMed] [Google Scholar]
- 168. Nachum Z, Shahal B, Shupak A, et al. Scopolamine bioavailability in combined oral and transdermal delivery. J Pharmacol Exp Ther 2001;296:121–123. [PubMed] [Google Scholar]
- 169. Muth ER, Elkins AN. High dose ondansetron for reducing motion sickness in highly susceptible subjects. Aviat Space Environ Med 2007;78:686–692. [PubMed] [Google Scholar]
- 170. Doweck I, Gordon CR, Spitzer O, Melamed Y, Shupak A. Effect of cinnarizine in the prevention of seasickness. Aviat Space Environ Med 1994;65:606–609. [PubMed] [Google Scholar]
- 171. Lucertini M, Mirante N, Casagrande M, Trivelloni P, Lugli V. The effect of cinnarizine and cocculus indicus on simulator sickness. Physiol Behav 2007;91:180–190. [DOI] [PubMed] [Google Scholar]
- 172. Weinstein SE, Stern RM. Comparison of marezine and dramamine in preventing symptoms of motion sickness. Aviat Space Environ Med 1997;68:890–894. [PubMed] [Google Scholar]
- 173. Gandia P, Saivin S, Le‐Traon AP, Guell A, Houin G. Influence of simulated weightlessness on the intramuscular and oral pharmacokinetics of promethazine in 12 human volunteers. J Clin Pharmacol 2006;46:1008–1016. [DOI] [PubMed] [Google Scholar]
- 174. Cowings PS, Toscano WB, DeRoshia C, Miller NE. Promethazine as a motion sickness treatment: Impact on human performance and mood states. Aviat Space Environ Med 2000;71:1013–1022. [PubMed] [Google Scholar]
- 175. Davis JR, Jennings RT, Beck BG. Comparison of treatment strategies for Space Motion Sickness. Acta Astronaut 1993;29:587–591. [DOI] [PubMed] [Google Scholar]
- 176. Wood CD, Graybiel A. Evaluation of sixteen anti‐motion sickness drugs under controlled laboratory conditions. Aerosp Med 1968;39:1341–1344. [PubMed] [Google Scholar]
- 177. Gordon CR, Doweck I, Nachum Z, et al. Evaluation of betahistine for the prevention of seasickness: Effect on vestibular function, psychomotor performance and efficacy at sea. J Vestib Res 2003;13:103–111. [PubMed] [Google Scholar]
- 178. Weerts A, Pattyn N, Van de Heyning P, Wuyts F. Evaluation of the effects of anti‐motion sickness drugs on subjective sleepiness and cognitive performance of healthy males. J Psychopharmacol 2013;28:655–664. [DOI] [PubMed] [Google Scholar]
- 179. Makowski AL, Lindgren K, Locke JP. Visual side effects of scopolamine/dextroamphetamine among parabolic fliers. Aviat Space Environ Med 2011;82:683–688. [DOI] [PubMed] [Google Scholar]
- 180. Tokola O, Laitinen LA, Aho J, Gothoni G, Vapaatalo H. Drug treatment of motion sickness: Scopolamine alone and combined with ephedrine in real and simulated situations. Aviat Space Environ Med 1984;55:636–641. [PubMed] [Google Scholar]
- 181. Wood CD, Stewart JJ, Wood MJ, Mims M. Effectiveness and duration of intramuscular antimotion sickness medications. J Clin Pharmacol 1992;32:1008–1012. [DOI] [PubMed] [Google Scholar]
- 182. Chinn HI, Strickland BA, Waltrip OH, Gainer SH. Prevention of air sickness by benadryl‐scopolamine mixtures. U S Armed Forces Med J 1951;2:401–404. [PubMed] [Google Scholar]


