ABSTRACT
Introduction: Our aim was to explore the prognostic value of anthropometric parameters in patients treated with nivolumab for stage IV non-small cell lung cancer (NSCLC).
Methods: We retrospectively included 55 patients with NSCLC treated by nivolumab with a pretreatment 18FDG positron emission tomography coupled with computed tomography (PET/CT). Anthropometric parameters were measured on the CT of PET/CT by in-house software (Anthropometer3D) allowing an automatic multi-slice measurement of Lean Body Mass (LBM), Fat Body Mass (FBM), Muscle Body Mass (MBM), Visceral Fat Mass (VFM) and Sub-cutaneous Fat Mass (SCFM). Clinical and tumor parameters were also retrieved. Receiver operator characteristics (ROC) analysis was performed and overall survival at 1 year was studied using Kaplan–Meier and Cox analysis.
Results: FBM and SCFM were highly correlated (ρ = 0.99). In ROC analysis, only FBM, SCFM, VFM, body mass index (BMI) and metabolic tumor volume (MTV) had an area under the curve (AUC) significantly higher than 0.5. In Kaplan-Meier analysis using medians as cut-offs, prognosis was worse for patients with low SCFM (<5.69 kg/m2; p = 0.04, survivors 41% vs 75%). In Cox univariate analysis using continuous values, BMI (HR = 0.84, p= 0.007), SCFM (HR = 0.75, p = 0.003) and FBM (HR = 0.80, p= 0.004) were significant prognostic factors. In multivariate analysis using clinical parameters (age, gender, WHO performance status, number prior regimens) and SCFM, only SCFM was significantly associated with poor survival (HR = 0.75, p = 0.006).
Conclusions: SCFM is a significant prognosis factor of stage IV NSCLC treated by nivolumab.
KEYWORDS: Body composition, immunotherapy, positron emission tomography, computed tomography, prognostic factor, predictive factor, nuclear medicine, non-small cell lung carcinoma, personalized medicine, nivolumab
Introduction
An estimated 1.8 million new cases of lung cancer occurred in 2012, representing the leading cause of cancer death in developed countries.1 Non-small cell lung cancer (NSCLC) accounts for approximately 85% to 90% of all lung cancers.2 Until recently, effective treatment options were lacking for patients with stage III/IV NSCLC, without actionable driver mutations, who experience disease progression after first-line platinum-based chemotherapy.
Cancers have the ability to disrupt immune response by interfering with adaptive immunity.3,4 Immunotherapy using immune checkpoint inhibitors, notably anti-PD-1 (programmed cell death protein-1) and anti-PDL-1 (PD1 ligand) antibody, has been shown to improve outcome of stage IIIb/IV NSCLC and is a new standard of care.5,6 In this setting, pembrolizumab, nivolumab, and atezolizumab have been approved by the Food and Drug Administration FDA and the European Medicines Agency as first- (pembrolizumab) and second-line treatment.
The Checkmate 0175 and 0576 studies showed the superiority of nivolumab compared to conventional chemotherapy in second-line treatment for NSCLC. The sub-group analysis of these two trials showed high efficacy in patients independent of PDL-1 expression and nivolumab can be prescribed without the determination of PDL-1 status. In contrast, pembrolizumab, according to the Keynote 0107 and Keynote 0248 studies, can only be prescribed if the tumor is positive for PDL-1.
Despite the clinical success of immune checkpoint inhibitors in lung cancer patients, with some durable response, better understanding of determinants affecting response is required.
Some authors9 have shown that tumor burden evaluated on baseline positron emission tomography coupled with computed tomography (PET/CT) is predictive of patient survival. Indeed, in patients managed for stage IV NSCLC, metabolic tumor volume (MTV) and total lesion glycolysis (TLG) appeared significant on overall survival and were more effective than maximal and mean standardized uptake value (SUVmax and SUVmean). Other authors10 confirmed the value of PET/CT volume parameters in patients managed for non-operable stage IIb/III NSCLC.
If tumor burden is an interesting determinant, parameters obtained by determining the patient’s body composition may be even more interesting. Phase I trials11,12 allowed the determination of toxicity profile (for doses ranging from 0.1 to 10 mg/kg) and the effective dose of nivolumab. The pharmacodynamics and pharmacokinetics of nivolumab were evaluated on antibody serum levels and PD-L1 receptor occupancy of circulating TCD3 + lymphocytes. Anti-tumor effects were found to be best at a dose of 3 mg/kg every two weeks. This dose is based on the patient’s weight and does not take into account the patient’s body composition which could impact the bio-availability of nivolumab and therefore modify its effectiveness in breaking the immune inhibition. To support this hypothesis, authors have shown that body mass index (BMI) was higher in melanoma patients treated by anti-PD1 checkpoint inhibitors who had early acute limiting toxicity.13 Although BMI is an interesting parameter to describe the overall mass of patients, it does not describe body composition.
To analyze body composition, we have developed software which allows the automatic and multi-slice measurement of anthropometric parameters on the CT of PET/CT, routinely used for cancer patients. This software uses a multi-atlas segmentation method14 combined with an extrapolation of the body parts outside the field of acquisition.15 Lean Body Mass (LBM), Fat Body Mass (FBM), Muscle Body Mass (MBM), Visceral Fat Mass (VFM) and Subcutaneous Fat Mass (SCFM) can be measured automatically by this software.
The main objective of this study was to evaluate the prognostic value of anthropometric parameters evaluated on the CT of PET/CT by automatic 3D software, in patients treated by nivolumab for metastatic NSCLC. Secondary objectives were to explore the correlation of anthropometric parameters with toxicity and best-observed response.
Results
Patient characteristic
Between February 2015 and October 2017, 234 patients received nivolumab for stage IV NSCLC after at least one chemotherapy session in Pulmonology, Thoracic Oncology, and Respiratory Intensive Care department of Rouen University Hospital. Among them, 55/234 (24%) had pretreatment 18F-FDG PET/CT in our center and were included in this study. PET examinations were performed for 26 patients on a Biograph Sensation 16 HiRes PET/CT device and for 29 patients on a Discovery 710 PET/CT device. The main characteristics of the population are summarized in Table 1.
Table 1.
Patients’ clinical characteristics for the whole population (n = 55).
| Patient characteristics | Number of patients, n = 55, % | |
|---|---|---|
|
Age, years Median Range |
63,5 [37.8–82.4] |
|
|
Sex, n, % Men Women |
41 14 |
75% 25% |
|
Stage at diagnosis, n, % < III IIIA IIIB IV |
5 7 13 30 |
9% 13% 24% 54% |
|
Histology, n, % Adenocarcinoma Squamous cell Adenocarcinoma and squamous cell Undifferentiated carcinoma |
28 21 3 3 |
51% 38% 5.5% 5.5% |
| Cerebral metastasis at diagnosis, n, % | 11 | 20% |
|
Smoking status Non smoker, n, % Active smoker, n, % Former smoker, n, % |
6 19 30 |
11% 34% 55% |
|
WHO status at Nivolumab treatment, n, % 0 1 2 |
14 32 9 |
26% 58% 16% |
|
Number of specific oncological treatments before Nivolumab, n, % 1 2 ≥ 3 |
26 20 9 |
47% 36% 17% |
|
Best response with previous treatment, n, % Complete or partial Stable Progression Unknown |
18 17 19 1 |
33% 31% 34% 2% |
|
Time since the end of the previous treatment, n, % < 3 month 3–6 month > 6 month Unknown |
32 13 9 1 |
58% 24% 16% 2% |
|
Specific oncological treatment(s) before Nivolumab, n, % Platinum-based chemotherapy Pemetrexed Bevacizumab Gemcitabine Paclitaxel Docetaxel Vinorelbine Etoposide Tyrosine kinase inhibitors |
54 27 5 15 26 3 13 2 2 |
98% 49% 9% 27% 47% 5% 24% 4% 4% |
|
Mutational status, n, % EGFR ALK KRAS MET ROS RET BRAF |
13 2 0 8 1 0 1 1 |
24% 4% 0% 15% 2% 0% 2% 2% |
|
PDL-1 status, n, % Positive Negative Unknown |
13 4 38 |
24% 7% 69% |
|
Toxicity, n, % WHO grade 2 Lung Infection Dysthyroidism Asthenia Immunoallergic colitis Acute renal failure Psoriasis Inflammatory arthralgia Gougerot-Sjrögren syndrom WHO grade 3 Immunoallergic colitis Interstitial pneumonia Psoriasis Xerostomia Myositis |
14 4 3 2 1 1 1 1 1 7 3 1 1 1 1 |
25.5% 7.2% 5.5% 3.6% 1.8% 1.8% 1.8% 1.8% 1.8 12.7% 5.5% 1.8% 1.8% 1.8% 1.8% |
WHO: world health organization
The majority of patients were men (75%) and the median age was 63.5 years (37.8–82.4 years). The majority of patients had either WHO grade 0 or 1 (84%), the remaining had WHO grade 2 (16%). Most patients were diagnosed at advanced stages of the disease: 30 at stage IV, 13 at stage IIIb and 12 at stage IIIa or lower. All patients were at stage IV before receiving nivolumab. The most represented tumor histology was adenocarcinoma (28/55 patients). PDL1 status was determined in 17 of 55 patients and was positive in 13/17 (76%).
Nivolumab was proposed as the second therapeutic line for 47% of patients, as third line for 36% and beyond for 17%. At one year after the first course of nivolumab, 32 patients (58%) were still alive.
Survival analysis
A graphical representation of the automatic segmentation of the CT of a patient’s pretreatment PET/CT is displayed in Figure 1.
Figure 1.
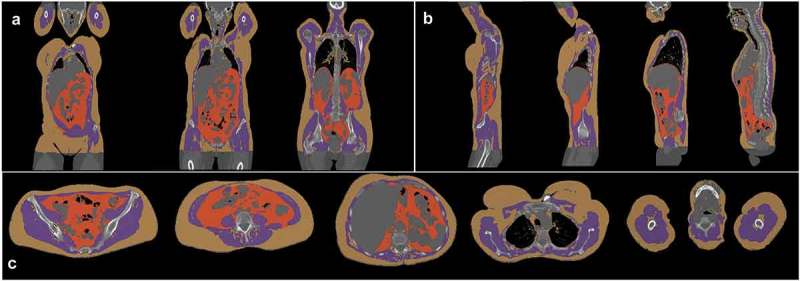
Graphical representation of the automatic segmentation of 18FDG PET/CT by Anthropometer3D with subcutaneous fat (yellow), muscle (purple) and visceral fat (orange) voxels on frontal (a), sagittal (b) and axial (c) views.
Spearman’s correlations are presented in supplemental data 1. FBM and SCFM were highly correlated with Spearman’s coefficient correlation close to 1 (ρ = 0.99). BMI was correlated with FBM, SCFM, and VFM (minimal ρ = 0.76). MBM and LBM were not correlated with the other parameters (maximal ρ = 0.51 between BMI and LBM).
The ROC curve analysis of the anthropometric parameters for overall survival (OS) are summarized in Table 2 with figures in supplemental data 2. Several indices appear significant as MTV (AUC = 0.68, p= 0.04), BMI before beginning immunotherapy (AUC = 0.71, p= 0.005), SCFM (AUC = 0.72, p = 0.002), FBM (AUC = 0.72, p= 0.003) and VFM (AUC = 0.65, p = 0.03).
Table 2.
Diagnostic performance, clinical and PET metrics, and anthropometric parameters measured on16FDG PET/CT for 1-year overall survival using a ROC analysis.
| Mean Median (± SD) [min-max] |
Cut-off value | AUC | Sensitivity | Specificity | Accuracy | p-value | |
|---|---|---|---|---|---|---|---|
| Age at diagnosis (y) | 62.6 63.5 (± 10.3) [37.8–82.4] |
NA | 0.50 | NA | NA | NA | 0.49 |
| WHO status | 0.71 1 (± 0.60) [0–2] |
NA | 0.58 | NA | NA | NA | 0.13 |
| Number of oncologic treatments before nivolumab | 1.82 2 (± 1.04) [1–5] |
NA | 0.52 | NA | NA | NA | 0.42 |
| SUVmax (g/ml) | 13.1 11.7 (± 5.4) [5.2–35.8] |
NA | 0.54 | NA | NA | NA | 0.30 |
| MTV (cm3) | 97 59 (± 10) [5–503] |
86,3 | 0.68 | 0.65 | 0.75 | 0.71 | 0.01 |
| TLG (g) | 573 302 (± 653) [26–2563] |
NA | 0.61 | NA | NA | NA | 0.083 |
| Weight before treatment (kg) | 74.5 73 (± 14.7) [49–110] |
75 | 0.65 | 0.59 | 0.74 | 0.66 | 0.03 |
| BMI before treatment (kg/m2) | 25.2 24.7 (±3.9) [18.0–34.1] |
25.1 | 0.71 | 0.63 | 0.74 | 0.71 | 0.005 |
| SCFM (kg/m2) | 5.88 5.69 (± 2.76) [0.68–12.70] |
5.0 | 0.72 | 0.78 | 0.61 | 0.73 | 0.002 |
| VFM (kg/m2) | 1.28 1.32 (± 0.61) [0.22–2.88] |
1.38 | 0.65 | 0.63 | 0.74 | 0.67 | 0.03 |
| MBM (kg/m2) | 8.6 9.1 (± 1.23) [6.21–11.69] |
NA | 0.58 | NA | NA | NA | 0.18 |
| LBM (kg/m2) | 18.03 17.85 (± 2.39) [13.09–26.78] |
NA | 0.52 | NA | NA | NA | 0.41 |
| FBM (kg/m2) | 7.16 7.00 (± 3.19) [0.90–14.31] |
5.7 | 0.72 | 0.81 | 0.56 | 0.71 | 0.003 |
BMI: body mass index; FBM: fat body mass; HR: hazard ratio; MBM: muscle body mass; MTV: metabolic tumor volume; SCFM: subcutaneous fat mass; SUV: standardized uptake value; TLG: total lesion glycolysis; VFM: visceral fat mass; WHO: world health organization; AUC: area under the curve; ROC: Receiver Operator Characteristics; y: year; NA: not available
Figure 2 shows Kaplan-Meier analysis with log-rank tests by using medians as cut-offs for MTV, BMI, FBM, and SCFM. Only SCFM (p = 0.04) and VFM (p = 0.08) were found as a significant risk factor for 1-year OS, considering median value of 5.69 kg/m2 and 1.32 kg/m2, respectively. Table 3 shows Cox analysis. In univariate analysis, low BMI, low SCFM, and low FBM were significantly associated with poor survival. In multivariate analysis using clinical parameters (age, gender, WHO performance status, number prior regimens) and SCFM, only low SCFM was significantly associated with poor survival (HR: 0.75, p = 0.006).
Figure 2.
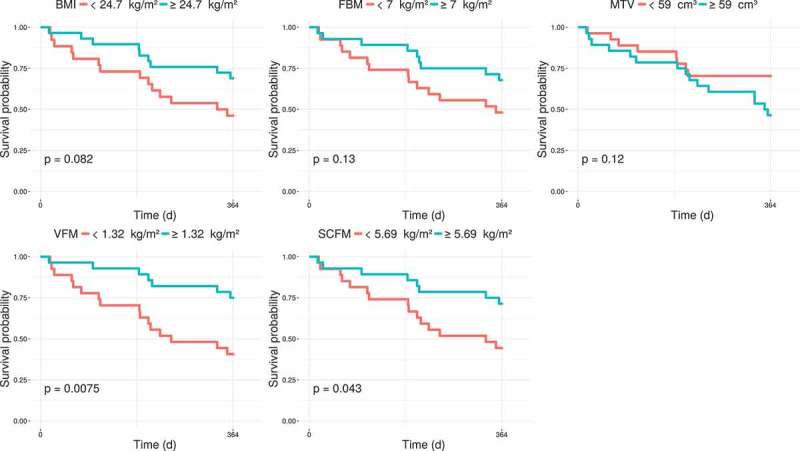
Kaplan-Meier estimates of 1-year overall survival (OS) according to the medians of MTV, BMI, FBM, and SCFM.
Table 3.
Univariate and multivariate Cox analysis using continuous values for clinical and significant PET metrics and anthropometric parameters measured on16FDG PET/CT.
| Univariate Cox analysis |
||
|---|---|---|
| HR | p-value | |
| Age | 1.00 | 0.95 |
| Gender | 0.55 | 0.27 |
| WHO status | 1.59 | 0.19 |
| Number prior regimens | 1.0 | 0.94 |
| MTV | 1.00 | 0.021 |
| BMI | 0.84 | 0.007 |
| SCFM | 0.75 | 0.003 |
| FBM | 0.80 | 0.0039 |
| Multivariate Cox analysis | ||
| Age | 1.00 | 0.99 |
| Gender | 1.10 | 0.89 |
| WHO status | 1.66 | 0.19 |
| Number prior regimens | 0.96 | 0.87 |
| SCFM | 0.75 | 0.006 |
BMI: body mass index; FBM: fat body mass; HR hazard ratio; MTV: metabolic tumor volume; SCFM: subcutaneous fat mass; WHO: world health organization;
The results of the five-fold cross-validation (44 turning patients in the training population and 11 turning patients in the testing population) performed by using SCFM are visible in supplemental data 3. All AUCs remained high in this cross-validation, from 0.68 to 0.78 for the training populations and from 0.52 to 0.75 for the testing populations. Moreover, the thresholds for SCFM were relatively stable between the different cross-validations, around 5.0 kg/m2.
Patients’ clinical characteristics classified according to the median of SCFM are presented in supplemental data 4. By using the Wilcoxon test, no significant difference in age, gender, number of oncologic treatments before nivolumab and MTV was observed between the two groups. Only BMI was statistically different between the two groups.
Anti-PD1 toxicity
A total of 21 patients (38%) had toxicity with a WHO grade of 2 or 3. The comparison of parameters between patients with or without toxicity is summarized in Table 4. MTV, BMI, FBM and SCFM were not associated with increased toxicity. However, toxicity with a grade superior to 1 was not statistically correlated to the 1-year survival (Wilcoxon test p-value = 0.33).
Table 4.
Comparison of parameter values according to toxicity and best response observed.
| Toxicity |
Best response observed |
|||||
|---|---|---|---|---|---|---|
| Grade 0–1 Mean (sd) |
Grade 2–3 Mean (sd) |
Wilcoxon test, p-value | Complete and partial response Mean (SD) |
Stability and progression Mean (SD) |
Wilcoxon test, p-value | |
| MTV (cm3) | 117 (121) | 64 (53) | p = 0.1 | 709 (425) | 105 (114) | p = 0.87 |
| BMI (kg/m2) | 24.7 (3.9) | 26.1 (3.6) | p = 0.14 | 25.0 (4.1) | 25.0 (2.9) | p = 0.94 |
| SCFM (kg/m2) | 5.4 (2.7) | 6.6 (2.8) | p = 0.13 | 5.6 (1.9) | 6.0 (3.0) | p = 0.87 |
| FBM (kg/m2) | 6.7 (3.1) | 8.0 (3.2) | p = 0.14 | 6.9 (2.5) | 7.2 (3.4) | P = 0.85 |
BMI: body mass index; FBM: fat body mass; MTV: metabolic tumor volume; SCFM: subcutaneous fat mass; sd: standard deviation
Best response
Partial or complete response was the best response for 13 patients (24%). The comparison of parameters between patients with partial or complete response versus stability or progression as best response is summarized in Table 4. None of the analyzed parameters was associated with the best response observed.
Discussion
Immunotherapy treatments based on PD-1 checkpoint inhibitors, including nivolumab, are game changers in the management of patients with stage IIIb/IV NSCLC.6,11 For better understanding of the determinants affecting response to checkpoint inhibitors, we explored the prognostic value of multiple anthropometric parameters (LBM, FBM, MBM, VFM, and SCFM) measured by 3D automatic software on the pretreatment CT of PET/CT of 55 patients with NSCLC. Other clinical and PET metric parameters, as SUVmax, MTV, TLG, and BMI were also evaluated. For the anthropometric imaging parameters, we found that only FBM and SCFM, both highly correlated (ρ = 0.99), were significant on ROC analysis for overall survival at 1 year. MTV and BMI were also significant. In Kaplan-Meier analysis with log-rank tests by using medians as cut-offs, only SCFM (p = 0.04) and VFM (p = 0.008) were found as significant risk factors. In univariate analysis, low BMI, low SCFM, and low FBM were significantly associated with poor survival. In multivariate Cox analysis using clinical parameters (age, gender, WHO performance status, number prior regimens) and SCFM, only low SCFM was significantly associated with poor survival (HR: 0.75, p = 0.006).
Anthropometric parameters have already been found to be valuable prognostic factors in many cancers. For example, an approximation of MBM determined by using the skeletal muscle area (SMA) assessed by a manual mono-slice segmentation of CT at L3 level has been found to have a prognostic value for head and neck carcinoma,17 esophagogastric junction cancer or upper gastric cancer18 or small cell lung cancer.16 These measurements are however limited by their mono-slice segmentation which are less accurate than a multi-slice segmentation method.14,19,20 Moreover, they are time-consuming for physicians20,21 which restricts their use in clinical routine. BMI, an anthropometric parameter easy to calculate, has also been found to be associated to progression-free survival (PFS) and OS in a retrospective multicohort study of metastatic melanoma treated with targeted therapy and immunotherapy.22 No association was observed with chemotherapy. The prognostic effect of BMI with targeted and immune therapies differed by sex with pronounced inverse associations in males but not females and there was a strong survival advantage associated with obesity in males treated with targeted therapy and with immune therapy.23 Comparable results were observed in a study exploring the association of baseline BMI with OS in 703 metastatic NSCLC treated by Nivolumab and Pembrolizumab with underweight BMI associated with shorter OS (HR: 1.66, p= 0.002) and obese BMI associated with longer OS (HR: 0.75, p= 0.039).24 In another study, authors evaluated whether BMI was associated with the treatment response (PFS and OS) of melanoma patients treated with ipilimumab.24 They found that overweight patients showed a non-significant trend towards longer overall survival (p = 0.056, log-rank test; hazard ratio [HR] = 1.81, Cl 95% = 0.98–3.33), and no difference was found with regard to PFS (p = 0.924, log-rank test; HR = 1.03, CI 95% = 0.62–1.70).24 This discrepancy between OS and PFS is common when evaluating immunotherapy and may possibly be explained by the difficulty of determining a reliable PFS in that setting, and a putative remnant effect of immunotherapy.
However, by using the whole-body weight, BMI can be non-sufficient to describe the body composition. Though high BMI can be linked to a high FBM but also to a low FBM if the LBM is important, notably for muscular patient. Using a software to determine the body composition on medical images generated in clinical routine seems therefore useful.
The software we have developed allows the automatic multi-slice measurement of multiple anthropometric parameters on the CT of PET/CT, the wide field of acquisition of this examination (generally from the eyes to the ischium) being exploited to get accurate measurements.15,25
Rather than a reflection of the patient’s physical condition, better represented by MBM, we hypothesize that the prognostic value of FBM (and also SCFM, as they are highly correlated) is related to the pharmacodynamics of nivolumab. Regarding the pharmacokinetics and bioavailability of nivolumab, phase I trials11,12 have demonstrated a profile of PD1 receptor occupancy on circulating CD3 + T cells comparable to the different doses (0.3 to 10 mg/kg). The usual dose (3 mg/kg every 14 days), until very recently, was found to be the most effective in patients treated for NSCLC, with no benefit from an increased dose of 10 mg/kg. The spectrum, frequency, and severity of treatment-related adverse events were similar across the tested dose levels. More recently, authors reported the preparation and in-vivo evaluation of 89Zr labeled nivolumab in healthy non-human primates.26 In vivo PET imaging with 89Zr-nivolumab was performed with tracer only, or carrier-added (1 and 3 mg/kg of nivolumab). Bio-distribution of 89Zr-nivolumab in adipose tissue was low, with uptake unaffected by the addition of 1 or 3 mg/kg of unlabelled nivolumab, and this could be one explanation for the poor prognosis observed in patients with low FBM. Indeed, for treatments as chemotherapy where dose calculation is based on the patient’s body weight (including fat) and where bio-distribution includes only little or no adipose tissue, patients with low FBM receive a relatively low dose of treatment in non-fat tissue, including the tumor target. Conversely, patients with high FBM, receive a relatively high dose in non-fat tissue, including improved exposure to tumor targets. Such an effect is also observed for patients who have a18F-FDG PET as dose calculation is based on the patient’s weight and bio-distribution excludes adipose tissue: lean tissue activity is higher in patients with relatively high FBM (notably due to obesity) compared to patient’s with low FBM.15
Moreover, therapeutic antibodies have been shown to accumulate in fat tissue. In a study exploring the biodistribution of four therapeutic antibodies (lumretuzumab, MMOT0530A, bevacizumab, and trastuzumab) labeled by radioactive 89Zr, Bensch et al. found that an estimated total amount of tracer accumulated in fat tissue ranged between 1.56% and 18.95% of the injected dose, depending on the physique of the patient.27 A similar biodistribution was found for the four antibodies, probably because of their similar molecular structure, binding characteristics, and catabolic pathways.27 Hence, despite low accumulation per gram of tissue, fat can influence the overall distribution of therapeutic antibodies.27 This “storage effect” of the adipose tissue may contribute to our results. Other explanations of the role of SCFM and FBM could be linked to adipokines and obesity-related inflammation. Frasca et al. have shown recently that adipocytes in the human obese SCFM release several pro-inflammatory cytokines and chemokines, which contribute to the establishment and maintenance of local and systemic inflammation.28 This phenomenon could potentiate the effect of immunotherapy and explain why FBM and SCFM, which are highly correlated (ρ = 0.99), are both prognosis factors for OS. However, there is still a critical need to better understand inflammation that occurs in obesity and how it may impact treatment response.29
Very recently, authors,30 in a quantitative clinical pharmacological study, assessed the benefit-risk profile of nivolumab at a 240 mg/14 day flat dose. Flat dosing is already implemented for several cancer immunotherapy antibodies as pertuzumab, pembrolizumab, and atelozizumab. A flat dose of 240 mg was selected by multiplying the initial 3 mg/kg dose by the median body weight of patients in the nivolumab clinical program, to achieve a high degree of overlap in exposure. A flat dose was expected to lead to higher exposure in lighter weight patients. Since this study, a flat dose has been adopted as the standard dose of nivolumab for NSCLC, melanoma, renal cell carcinoma and urothelial cancer in the United States of America. Flat dosing allows rapid preparation of infusion and reduces the risk of dosing error. However, considering a cost-effectiveness approach, dose adjustment based on anthropometric analysis of the patient could be a parameter of interest.
As secondary endpoints, we explored the correlation of significant anthropometric parameters with toxicity and best-observed response. Heidelberger et al.13 found that BMI was significantly correlated with toxicity, with a higher mean BMI (27.9 versus 24.7 kg/m2) for patients with early acute limiting toxicity. In their retrospective study, they investigated whether body composition was associated with early acute limiting toxicity in 68 patients with advanced melanoma receiving either Nivolumab or Pembrolizumab. Body composition was evaluated by assessing muscle tissue areas on CT-scan images at L3 level. A total of 38 (56%) patients had a BMI ≥ 25 kg/m2 and 11 (16%) over 30, while 13 (19%) had both sarcopenia and a BMI ≥ 25 kg/m2. Eleven patients (16%) experienced early toxicities (four Nivolumab and seven Pembrolizumab). Age, weight, lean body mass, and muscle loss were not associated with increased toxicity. However, patients with increased BMI experienced more toxicity and the correlation was even stronger among female patients combining both BMI ≥ 25 kg/m2 (overweight) and sarcopenia (muscle loss): occurrence of early toxicity was 50% vs. 7.7% (p = 0.01, odds ratio: 12; 95% CI: 1.4–103). In our study, however, no anthropometric parameters were statistically significant. This result could be explained by the fact that toxicity was not statistically correlated to the 1-year survival in our study.29.
None of the parameters predicting survival was statistically linked to the best response observed (complete or partial response vs stability or progression). This could be explained by the difficulty in assessing effectively the response of immunotherapy by imaging, notably due to atypical responses. In a study based on survival analyses, the RECIST 1.1 evaluation underestimated the benefit of immune checkpoint inhibitors in 11% of patients in response.31 This is somewhat consistent with the study of Heidelberg et al. where no significant correlation was observed between early toxicity and treatment response.13
The main limitation of our study is the single center and retrospective nature of the analysis. These results have to be confirmed on other clinical databases, and physiopathological processes have to be explored in preclinical and clinical studies as a better understanding of the intersection between obesity and immunotherapy outcomes is critical to the field. If confirmed, our results could pave the way for a prospective clinical test comparing patients receiving nivolumab treatment whose dose is adapted to body weight (or flat dose according to recent recommendations) to patients receiving an immunotherapy dose adjusted to their body composition, in particular to FBM and SCFM.
Materials and methods
Population
This single-center, retrospective study was conducted in the Nuclear Medicine department of Henri Becquerel Cancer Center, Rouen, France and in the Pulmonology, Thoracic Oncology, and Respiratory Intensive Care department of Rouen University Hospital, Rouen, France. Medical image analysis was performed in collaboration with the QuantIF-LITIS laboratory, University of Rouen, France.
This non-interventional study complies with the Declaration of Helsinki. The protocol has been approved by the institutional review board and registered as n° 1809B. Patients were informed about the use of anonymized data for research and their right to oppose this use.
Inclusion criteria were all new patients with a histologically or cytologically confirmed NSCLC at stage IV according to the seventh edition lung cancer stage classification, IASLC 200932 and referred between February 2015 and May 2017 for a 18F-fluorodeoxyglucose (18F-FDG) PET/CT no more than three months before second-line (or more) treatment with nivolumab.
Patients were treated in the Pulmonology, Thoracic Oncology, and Respiratory Intensive Care department of Rouen University Hospital and had a standard dose of 3 mg/kg of body weight every 14 days.
Endpoints and assessments
The following baseline clinical data were collected: age, sex, World Health Organization (WHO) performance status, and baseline clinical anthropometric parameters (weight, size, BMI). PDL-1 status was determined and considered positive if >1% of cells expressed the marker in immunohistochemistry. Tumor characteristics as histology, molecular biology and TNM at baseline were collected. Standard PET/CT metrics describing tumor burden and activity (SUVmax, MTV, TLG) were also retrieved using PET VCAR semi-automatic software of AW server 3.2 (General Electric®, Milwaukee, USA) for segmentation.
All patients had 18F-FDG PET/CT before starting immunotherapy. PET/CT was acquired from the mid-thigh toward the base of the skull on either a Biograph Sensation 16 HiRes device (Siemens®, Knoxville, USA) or a Discovery PET/CT 710 device (General Electric®, Milwaukee, USA). Patients fasted for 6 h before the acquisition, then 3.5 to 4.5 MBq/kg of 18F-FDG were injected after 30 min of rest. Sixty minutes later, acquisitions began with a CT scan in the craniocaudal direction with free breathing and no contrast enhancement. For patients with a BMI <30 kg/m2, CT voltage was 100 kV; otherwise, CT voltage was 120 kV. CT milliampere-second (mAs) were automatically regulated by the manufacturer’s dose reduction software based on a Noise Index. This yielded a mean effective mAs of 89.1 ± 6.7. All images were resized to achieve uniform voxel size (1.36 x 1.36 × 5 mm3).
The primary endpoint was overall survival at one year, defined as time from the beginning of immunotherapy to death or last follow-up with maximal follow-up of one year.
Secondary endpoints were correlation with toxicity (WHO grade ≥ 2 vs grade < 2) retrieved from clinical follow-up and best-observed response (complete and partial responses vs stability and progression) retrieved from routine follow-up and using RECIST 1.1 criteria.33
Anthropometric parameters
Anthropometrics parameters were extracted by Anthropometer3D34 software which automatically measures parameters LBM, FBM, MBM, VFM and SCFM (in kg) on the CT of PET/CT. This software exploits the wide field of acquisition (minimum from the ischium to the eyes) generally available with this type of examination to perform multi-atlas segmentation based on 30 manually segmented CT atlases.14 Body parts below the ischium and above the eyes are estimated by using extrapolation factors15 for two tissues of interest (kmuscle for muscles and kfat for fat) calculated on CT atlases as the mean ratio of whole-body voxels of fat (or muscle) divided by the numbers of voxels of fat (or muscle) between the ischium and the eyes.
This segmentation generates three types of mask from the ischium to the eyes: the first for body shape, the second for abdominal cavity and the third for muscles. From the three masks fat voxels, visceral fat voxels and muscles voxels are extracted from the ischium to the eyes by using Hounsfield Unit (HU) thresholds of −190 to −30 for fat voxels and −29 to +150 for muscle voxels.35 Then, MBM, FBM, LBM, VFM, and SCFM are calculated as follows:
With Nmuscle and Nfat, the number of voxels of muscle and fat, respectively, obtained on the truncated CT, W the patient’s weight in g, Vvoxel the volume of one voxel (in ml), density of muscle (ρmuscle) was equal to 1.06 g/ml36 and density of fat (ρfat) was equal to 0.923 g/ml.35 The segmentations were visually checked without modification by a single physician (PD). All values measured by the software were divided by the square of the patient’s body height (m2) to use the same unit as the BMI.
This software had previously been validated by a leave-one-out cross-validation on the 30 truncated CTs included as atlases in the software. The measurements of MBM, FBM, LBM, VFM, and SCFM by the software Anthropomer3D were compared to reference standards which were based on the manual segmentation of the corresponding whole-body CT. Correlations were analyzed using intra-class coefficient correlation (ICC) and all ICC were excellent, equal to 0.99 with a minimal value of 95% confidence interval of 0.97. This software is available for clinical studies on the website https://www.anthropometer3d.org.
Statistical analysis
Descriptive statistics of the population and results were performed with continuous variables reported as mean ± standard deviation (SD) and categorical variables as frequencies (percentage). Correlations between each anthropometric parameter were evaluated using Spearman’s correlations. Predictive accuracy of survival by anthropometric parameters was assessed by Receiver Operator Characteristics (ROC) analysis and measured by the Area Under the Curve (AUC). An optimal cut-off value was computed by simultaneously maximizing specificity and sensitivity criteria. Two-sided tests were reported at the 5% level of significance. For parameters with a ROC curve statistically superior to 0.5, Kaplan–Meier method was used to estimate one-year overall survival rates with log-rank test. Univariate and multivariate analysis were performed using a Cox proportional hazards model to test the relationship between study variables and survival rates. Finally, a five-fold cross-validation was performed to check the stability of significant parameter with keeping turning four-fifths of the total population for the training population to determine by ROC analysis AUC and threshold and keeping turning one-fifth of the total population for the testing population.
For significant parameters for survival analysis, Wilcoxon test was used for comparison of continuous variables according to toxicity (WHO grade ≥ 2 vs grade < 2) and best-observed response (complete and partial responses vs stability and progression). All statistical analyses were done on R software version 3.4.2.37
Conclusion
In this study, we have shown that Sub-Cutaneous Fat Mass, automatically measured on a multi-slice basis by 3D automatic software, on pretreatment multi-slice18FDG PET/CT is a prognostic factor of stage IV NSCLC treated by nivolumab. Nevertheless, these results have to be confirmed and the physiopathology should be further explored. One hypothesis is that Sub-Cutaneous Fat Mass is a predictive factor of response to immunotherapy. If this hypothesis is confirmed, it would support the idea of adapting nivolumab dose not on patient’s weight or flat dosing, but on anthropometric parameters measured on medical images.
Funding Statement
No funding to declare for this work.
Acknowledgments
The authors are grateful to Nikki Sabourin-Gibbs for her help in editing the manuscript.
Disclosure statement
No disclosure to report.
Ethical approval
This study was performed in accordance with the Helsinki Declaration and local laws, and the protocol was approved by the Institutional Review Board of Henri Becquerel Centre.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A.. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Riely GJ.. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31(8):992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- 3.Cortinovis DL, Canova S, Abbate M, Colonese F, Bidoli P.. Focus on nivolumab in NSCLC. Front Med (Lausanne). 2016;3:67. doi: 10.3389/fmed.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters S, Kerr KM, Stahel R. PD-1 blockade in advanced NSCLC: a focus on pembrolizumab. Cancer Treat Rev. 2018;62:39–49. doi: 10.1016/j.ctrv.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Molina J, Kim J-H, Arvis CD, Ahn M-J, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 8.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 9.Liao S, Penney BC, Zhang H, Suzuki K, Pu Y. Prognostic value of the quantitative metabolic volumetric measurement on 18F-FDG PET/CT in stage IV nonsurgical small-cell lung cancer. Acad Radiol. 2012;19(1):69–77. doi: 10.1016/j.acra.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Salavati A, Duan F, Snyder BS, Wei B, Houshmand S, Khiewvan B, Opanowski A, Simone CB, Siegel BA, Machtay M, et al. Optimal FDG PET/CT volumetric parameters for risk stratification in patients with locally advanced non-small cell lung cancer: results from the ACRIN 6668/RTOG 0235 trial. Eur J Nucl Med Mol Imaging. 2017;44(12):1969–1983. doi: 10.1007/s00259-017-3753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidelberger V, Goldwasser F, Kramkimel N, Jouinot A, Huillard O, Boudou-Rouquette P, Chanal J, Arrondeau J, Franck N, Alexandre J, et al. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Invest New Drugs. 2017;35(4):436–441. doi: 10.1007/s10637-017-0464-x. [DOI] [PubMed] [Google Scholar]
- 14.Decazes P, Rouquette A, Chetrit A, Vera P, Gardin I. Automatic measurement of the total visceral adipose tissue from computed tomography images by using a multi-atlas segmentation method. J Comput Assist Tomogr. 2018;42(1):139–145. doi: 10.1097/RCT.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 15.Decazes P, Métivier D, Rouquette A, Talbot J-N KK. A method to improve the semiquantification of 18F-FDG uptake: reliability of the estimated lean body mass using the conventional, low-dose CT from PET/CT. J Nucl Med. 2016;57(5):753–758. doi: 10.2967/jnumed.115.164913. [DOI] [PubMed] [Google Scholar]
- 16.Kim EY, Kim YS, Park I, Ahn HK, Cho EK, Jeong YM. Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J Thorac Oncol. 2015;10(12):1795–1799. doi: 10.1097/JTO.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 17.Grossberg AJ, Chamchod S, Fuller CD, Mohamed ASR, Heukelom J, Eichelberger H, Kantor ME, Hutcheson KA, Gunn GB, Garden AS, et al. Association of body composition with survival and locoregional control of radiotherapy-treated head and neck squamous cell carcinoma. JAMA Oncol. 2016;2(6):782–789. doi: 10.1001/jamaoncol.2015.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudou K, Saeki H, Nakashima Y, Edahiro K, Korehisa S, Taniguchi D, Tsutsumi R, Nishimura S, Nakaji Y, Akiyama S, et al. Prognostic significance of sarcopenia in patients with esophagogastric junction cancer or upper gastric cancer. Ann Surg Oncol. 2017;24(7):1804–1810. doi: 10.1245/s10434-017-5811-9. [DOI] [PubMed] [Google Scholar]
- 19.Greenfield JR, Samaras K, Chisholm DJ, Campbell LV. Regional intra-subject variability in abdominal adiposity limits usefulness of computed tomography. Obes Res. 2002;10(4):260–265. doi: 10.1038/oby.2002.35. [DOI] [PubMed] [Google Scholar]
- 20.Shen W, Chen J, Gantz M, Velasquez G, Punyanitya M, Heymsfield SB. A single MRI slice does not accurately predict visceral and subcutaneous adipose tissue changes during weight loss. Obesity (Silver Spring). 2012;20(12):2458–2463. doi: 10.1038/oby.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonekamp S, Ghosh P, Crawford S, Solga SF, Horska A, Brancati FL, Diehl AM, Smith S, Clark JM. Quantitative comparison and evaluation of software packages for assessment of abdominal adipose tissue distribution by magnetic resonance imaging. Int J Obes. 2008;32(1):100–111. doi: 10.1038/sj.ijo.0803696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richtig G, Hoeller C, Wolf M, Wolf I, Rainer BM, Schulter G, Richtig M, Grübler MR, Gappmayer A, Haidn T, et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: an observational multi-centre study Haass NK, editor. PLoS One. 2018;13(10):e0204729. doi: 10.1371/journal.pone.0204729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19(3):310–322. doi: 10.1016/S1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhi J, Khozin S, Kuk D, Torres AZ, Sorg R, Lee SE, Miksad RA, Pazdur R, Abernethy AP. Association of baseline body mass index (BMI) with overall survival (OS) in patients (pts) with metastatic non-small cell lung cancer (mNSCLC) treated with nivolumab (N) and pembrolizumab (P). Jco. 2018;36(15_suppl):6553. doi: 10.1200/JCO.2018.36.15_suppl.6553. [DOI] [Google Scholar]
- 25.Chan TSY, Luk T-H, Lau JSM, Khong P-L, Kwong Y-L. Low-dose pembrolizumab for relapsed/refractory Hodgkin lymphoma: high efficacy with minimal toxicity. Ann Hematol. 2017;96(4):647–651. doi: 10.1007/s00277-017-2931-z. [DOI] [PubMed] [Google Scholar]
- 26.Cole EL, Kim J, Donnelly DJ, Smith RA, Cohen D, Lafont V, Morin PE, Huang RY-C, Chow PL, Hayes W, et al. Radiosynthesis and preclinical PET evaluation of 89Zr-nivolumab (BMS-936558) in healthy non-human primates. Bioorg Med Chem. 2017;25(20):5407–5414. doi: 10.1016/j.bmc.2017.07.066. [DOI] [PubMed] [Google Scholar]
- 27.Bensch F, Smeenk MM, van Es SC, de Jong JR, Schröder CP, Oosting SF, Lub-de Hooge MN, Menke-van der Houven van Oordt CW, Brouwers AH, Boellaard R, et al. Comparative biodistribution analysis across four different 89Zr-monoclonal antibody tracers-the first step towards an imaging warehouse. Theranostics. 2018;8(16):4295–4304. doi: 10.7150/thno.26370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frasca D, Diaz A, Romero M, Thaller S, Blomberg BB. Secretion of autoimmune antibodies in the human subcutaneous adipose tissue. PLoS One. 2018;13(5):e0197472. doi: 10.1371/journal.pone.0197472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canter RJ, Le CT, Beerthuijzen JMT, Murphy WJ. Obesity as an immune-modifying factor in cancer immunotherapy. J Leukoc Biol. 2018;104(3):487–497. doi: 10.1002/JLB.5RI1017-401RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Suryawanshi S, Hruska M, Feng Y, Wang X, Shen J, Vezina HE, McHenry MB, Waxman IM, Achanta A, et al. Assessment of nivolumab benefit-risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28(8):2002–2008. doi: 10.1093/annonc/mdx235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, Balleyguier C, Planchard D, Gazzah A, Soria JC, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. doi: 10.1016/j.ejca.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Tanoue LT, Detterbeck FC. New TNM classification for non-small-cell lung cancer. Expert Rev Anticancer Ther. 2009;9(4):413–423. doi: 10.1586/era.09.11. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Decazes P, Tonnelet D, Vera P, Gardin I Anthropometer3D: Automatic Multi-Slice Segmentation Software for the Measurement of Anthropometric Parameters from CT of PET/CT. J Digit Imaging. 2019; Epub ahead of print. doi: 10.1007/s10278-019-00178-3. [DOI] [PMC free article] [PubMed]
- 35.Chowdhury B, Sjöström L, Alpsten M, Kostanty J, Kvist H, Löfgren R. A multicompartment body composition technique based on computerized tomography. Int J Obes Relat Metab Disord. 1994;18:219–234. [PubMed] [Google Scholar]
- 36.Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- 37.Team RDC R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


