ABSTRACT
Immune checkpoint modulation in cancer has been demonstrated as a high-value therapeutic strategy in many tumor entities. VISTA is an immune checkpoint receptor regulating T-cell function. To the best of our knowledge, nothing is known about the expression and prognostic impact of VISTA on tumor infiltrating lymphocytes (TILs) in the tumor microenvironment of esophageal adenocarcinoma (EAC). We analyzed in total 393 EACs within a test-cohort (n = 165) and a validation-cohort (n = 228) using a monoclonal antibody (clone D1L2G). These data were statistically correlated with clinical as well as molecular data. 22.2% of the tumor cohort presented with a VISTA expression on TILs. These patients demonstrated an improved median overall survival compared to patients without VISTA expression (202.2 months vs. 21.6 months; p < 0.0001). The favorable outcome of VISTA positive tumors is significant in the entire cohort but mainly driven by the general better prognosis of T1/T2 tumors. However, in the pT1/2 group, VISTA positive tumors show a tremendous survival benefit compared to VISTA negative tumors revealing real long-term survivors in this particular subgroup. The survival difference is independent of the T-stage. This unique characteristic could influence neoadjuvant therapy concepts for EAC, since a profit of therapy could be reduced in the already favorable subgroup of VISTA positive tumors. VISTA emerges as a prognostic biomarker for long-term survival especially in the group of early TNM-stages. Future studies have to show the relevance of VISTA positive TILs within a tumor concerning response to specific immune checkpoint inhibition.
KEYWORDS: Esophageal adenocarcinoma, VISTA, TILs, early tumor stages, prognosis
Introduction
Esophageal cancer is the eighth most common malignant tumor worldwide and the number of incidences of esophageal adenocarcinoma (EAC) is increasing especially in the Western world (see http://www.wcrf.org). The majority of adenocarcinomas arise from Barrett metaplasia due to chronic reflux disease, followed subsequently by an accumulation of different mutations causing genetic instability (Barrett multistep carcinogenesis).1,2 Frequently patients present with a locally advanced tumor stage. Despite improvements in perioperative treatments, the overall survival rates of patients with esophageal adenocarcinoma remains poor.
To evade immune-control, virtually all fully developed tumors are associated with an immunosuppressive tumor microenvironment with elevated levels of tumor associated macrophages (TAMs) and regulatory T-cells (Tregs). The immune-escape of tumor cells itself is facilitated e.g. by a loss of specific antigens and tumor associated immunosuppressive cells preventing T-cell activation. Concurrent, infiltrating effector T-cells develop tolerance against the tumor cells. This is facilitated by co-inhibitory receptors- so called immune checkpoints that are able to modulate the effector T cell function. This has led to numerous studies identifying therapeutically targets for immune checkpoint modulation.
V-domain Ig suppressor of T cell activation (VISTA) is an immune checkpoint receptor expressed on tumor infiltrating T-lymphocytes (TILs) and myeloid cells, leading to suppression of T-cell activation, proliferation and cytokine production.3 The extracellular domain is similar to that of PD-L1, although both proteins interfere with different subsets of T-lymphocytes.
In the here presented study, we analyzed the hypothesis, that elevated numbers of VISTA-positive TILs in esophageal adenocarcinomas are associated with differences in prognosis. Therefore, the number of VISTA positive TILs was assessed on TMAs of two independent cohorts of esophageal adenocarcinoma using immunohistochemistry.
Results
Clinico-pathological and patients characteristics
Patient characteristics are given in Tables 1 and 2. 393 patients with esophageal adenocarcinomas (EAC) that underwent surgical tumor resection were immunohistochemically interpretable on the single-spot and 165 patients on the multi-spot tissue micro array (TMA). Reasons for non-informative cases (35 spots; 8.2%) included a lack of tissue samples or an absence of unequivocal cancer tissue in the TMA spot.
Table 1.
Patient’s characteristics and VISTA expression results on test-cohort (n = 165). Total patient´s numbers do not add to n = 165 due to missing analysable tumor spots on the multi-spot TMA.
| VISTA expression surface margin |
VISTA expression infiltration margin |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| low |
high |
low |
high |
|||||||||
| sex | No | % | No | % | No | % | p value | No | % | No | % | p value |
| female | 16 | 9.7% | 13 | 81.3% | 3 | 18.8% | 11 | 73.3% | 4 | 26.7% | ||
| male | 149 | 90.3% | 110 | 77.5% | 32 | 22.5% | 0.508 | 104 | 72.2% | 39 | 27.3% | 0.614 |
| age group | ||||||||||||
| <65 years | 72 | 43.9% | 55 | 78.6% | 15 | 21.4% | 51 | 73.9% | 18 | 26.1% | ||
| >65 years | 92 | 56.1% | 67 | 77.0% | 20 | 23.0% | 0.485 | 63 | 71.6% | 25 | 28.4% | 0.444 |
| tumor stage | ||||||||||||
| pT1 | 49 | 30.1% | 28 | 60.9% | 18 | 39.1% | 29 | 63.0% | 17 | 37.0% | ||
| pT2 | 29 | 17.8% | 18 | 62.1% | 11 | 37.9% | 20 | 69.0% | 9 | 31.0% | ||
| pT3 | 84 | 51.5% | 76 | 93.8% | 5 | 6.2% | 65 | 80.2% | 16 | 19.8% | ||
| pT4 | 1 | 0.6% | 0 | 0.0% | 1 | 100% | <0.0001 | 0 | 0.0% | 1 | 100% | 0.062 |
| lymph node metastasis | ||||||||||||
| pN0 | 63 | 38.7% | 34 | 56.7% | 26 | 43.4% | 35 | 59.3% | 24 | 40.7% | ||
| pN1 | 72 | 44.2% | 65 | 92.9% | 6 | 7.1% | 57 | 81.4% | 13 | 18.6% | ||
| pN2 | 13 | 8% | 10 | 83.3% | 2 | 16.7% | 11 | 84.4% | 2 | 15.4% | ||
| pN3 | 15 | 9.2% | 13 | 86.7% | 2 | 13.3% | <0.0001 | 11 | 73.3% | 4 | 26.4% | 0.030 |
| UICC stage | ||||||||||||
| I | 41 | 26.1% | 21 | 51.2% | 20 | 48.8% | 23 | 57.6% | 17 | 42.5% | ||
| II | 21 | 13.4% | 15 | 71.4% | 6 | 28.6% | 14 | 66.7% | 7 | 33.3% | ||
| III | 75 | 47.8% | 69 | 92.0% | 6 | 8.0% | 60 | 78.9% | 16 | 21.1% | ||
| IV | 20 | 12.7% | 17 | 85.9% | 3 | 15.0% | <0.0001 | 17 | 85.0% | 3 | 15.0% | 0.045 |
Table 2.
Patient’s characteristics, VISTA expression results, HER2- and p53-status on validation cohort (n = 393).
| VISTA Expression |
||||||||
|---|---|---|---|---|---|---|---|---|
| No | % | low | high | p value | ||||
| sex | female | 40 | 10,2% | 27 | 67.5% | 13 | 32.5% | |
| male | 353 | 89,8% | 252 | 71.4% | 101 | 28.6% | 0.364 | |
| age group | <65 yrs | 203 | 51,7% | 141 | 69.3% | 62 | 30.7% | |
| >65 yrs | 190 | 48,3% | 140 | 73.4% | 50 | 26.6% | 0.224 | |
| tumor stage | pT1 | 43 | 10,9% | 22 | 51.2% | 21 | 48.8% | |
| pT 2 | 35 | 8,9% | 27 | 77.1% | 8 | 22.9% | ||
| pT 3 | 305 | 77,6% | 223 | 73.3% | 82 | 26.7% | ||
| pT 4 | 10 | 2,5% | 6 | 60.0% | 4 | 40.0% | 0.017 | |
| lymph node metastasis | pN0 | 148 | 37,7% | 94 | 63.5% | 54 | 36.5% | |
| pN 1 | 155 | 39,4% | 111 | 71.4% | 44 | 28.6% | ||
| pN 2 | 46 | 11,7% | 35 | 76.1% | 11 | 23.9% | ||
| pN 3 | 44 | 11,2% | 39 | 88.6% | 5 | 11.4% | 0.010 | |
| UICC stage | I | 70 | 17,8% | 40 | 57.1% | 30 | 42.9% | |
| II | 70 | 17,8% | 46 | 66.1% | 24 | 33.9% | ||
| III | 160 | 40,7% | 115 | 71.9% | 45 | 28.1% | ||
| IV | 92 | 23,4% | 77 | 83.8% | 15 | 16.2% | 0.008 | |
| TP53 | wildtype | 150 | 41.0% | 108 | 72.2% | 42 | 28.0% | |
| mutation | 216 | 59.0% | 153 | 70.8% | 63 | 29.2% | 0.451 | |
| HER2 | wildtype | 307 | 87.7% | 215 | 70.0% | 92 | 30.0% | |
| amplification | 43 | 12.3% | 33 | 76.7% | 10 | 23.3% | 0.236 | |
VISTA expression (in test and validation cohort)
VISTA immunostaining was localized in the cytoplasm/membrane of tumor infiltrating lymphocytes (Figure 1(a+b)). In total, with the applied scoring system, 22.2% (n = 35) of patients on the multi-spot TMA were considered VISTA positive. In 104 (63.0%) cases we found a heterogeneous expression of VISTA within the 4 spots of the two localizations (surface and invasive margin) (Figure 1(c–f ); Figure 2). On the other hand, the overall expression pattern of the surface compared with the invasive tumor margin showed only a low heterogeneity, thus 18 cases (11.6%) were positive for VISTA on the surface and not on the invasive margin. In cross-table analysis, high amounts of VISTA-positive TILs were correlated with early (pT1/2) tumor stages (p < 0.0001), nodal negative patients (p < 0.001) and early UICC stages (UICC stage I/II) (p = 0.004) (Table 1).
Figure 1.
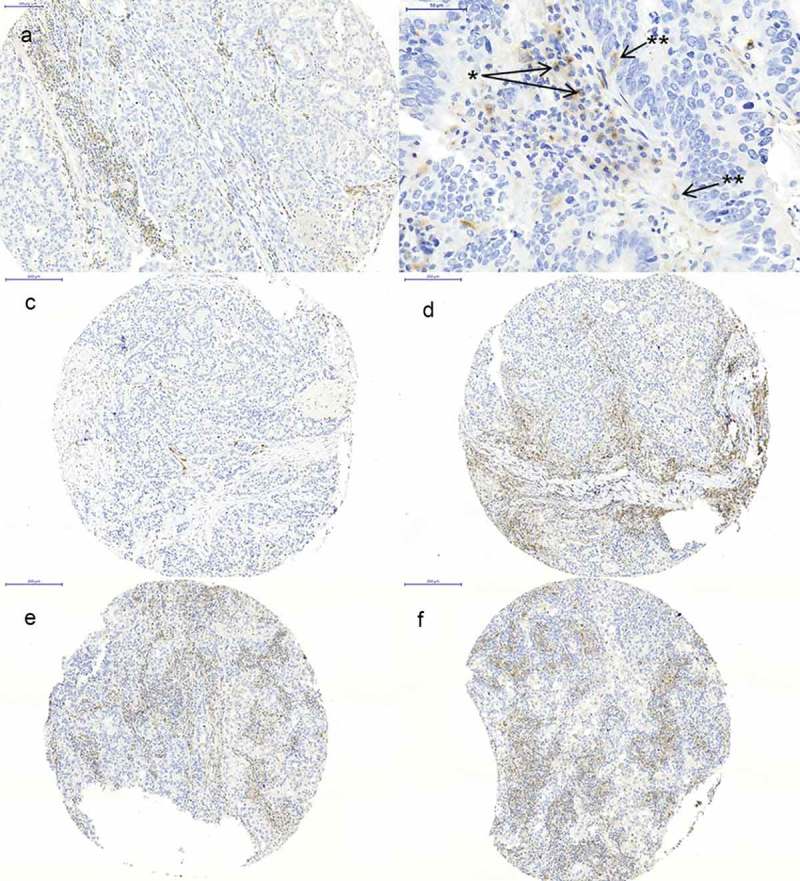
Immunohistochemistry of VISTA. (a) high expression of VISTA on TILs; (b) VISTA expression on lymphocytes (*) and macrophages (**); (c+d) tumor spots of the same tumor showing heterogeneous low and high VISTA expression; (e+f) tumor spots of the same tumor showing homogeneous VISTA expression.
Figure 2.
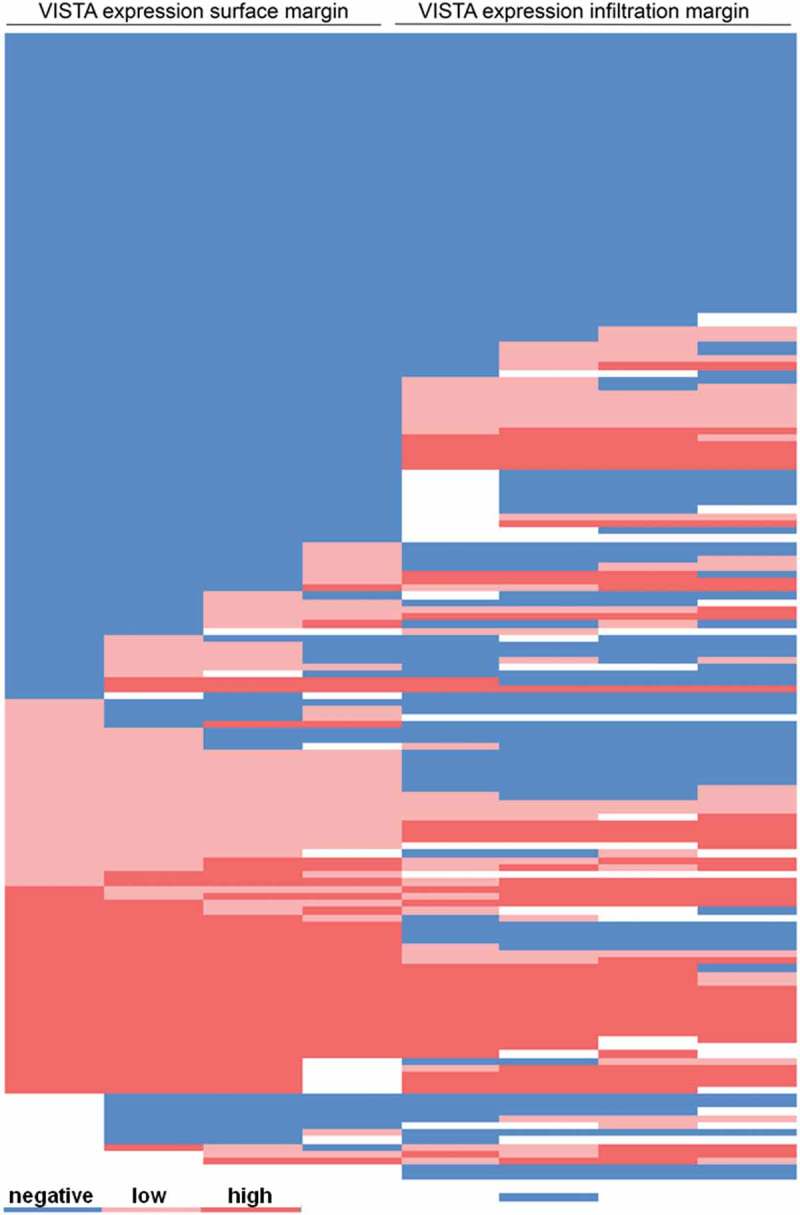
Heat-map of VISTA distribution within the multi spot TMA displaying heterogeneous expression. Each line represents one particular patients, each column represents one spot on the multi-spot TMA. Blue = negative VISTA expression (<1% of TILs), light red = low VISTA expression (1–4% of TILs), dark red = high VISTA expression (>4% of TILs).
In 1.2% of the analyzed cases we found an expression of VISTA on carcinoma cells.
In the validation cohort (on the single-spot TMA), 114 patients were considered VISTA positive (29.0%). We found comparable results to the multi-spot TMA, especially the strong correlation of VISTA to early tumor stages (pT1/2) (p = 0.017), nodal negative patients (p = 0.010) and UICC stages (UICC stage I and II) (p = 0.008) (Table 2).
VISTA expression reveals long-term survivors in EAC
Prognostic significance with respect to overall survival was seen for VISTA expression as determined by Kaplan-Meier survival analysis. For both, expression on the surface and infiltration margin, VISTA expression was correlated with favorable outcomes in patients with EAC (Figure 3(a,b)). Calculated median overall survival in patients with VISTA expression on the surface margin was 202.2 months (95% confidence interval (CI) 32.6–371.8 months) compared to a median OS of 21.6 months in VISTA negative patients (95%CI 13.3–29.9 months) (p < 0.001). Similar results were found on the invasive margin. The median overall survival in VISTA positive patients was 84.6 months (95%CI 27.2–141.9 months) compared to 22.1 months (95%CI 15.1–29.0 months) in VISTA negative patients (p = 0.024). In subgroup analysis, tumors with VISTA-positive TILs demonstrate a superior overall survival in early tumor stages (pT1/2) compared to patients without VISTA expression on TILs (p < 0.003) (Figure 3(c)). The survival benefit is not seen in higher tumor stages (Figure 3(d)). Due to the fact of highly homogenous distribution of VISTA within the tumor (irrespective of tumor surface or tumor infiltration margin), we do not consider the surface or infiltration margin in the single spot TMA of the validation cohort anymore. We were able to confirm the prognostic power of VISTA on the validation cohort considering 228 additional patients. For the entire validation cohort, VISTA expression was associated with a prolonged overall survival with a median overall survival of 41.9 months (95%CI 18.0–65.9 months) vs. 25.7 months in VISTA negative patients (95% CI 19.1–32.3 months) (p = 0.046) (Figure 3(e)). In subgroup analysis adjusted for tumor stages, similar results as already described for the multi-spot TMA were revealed with a survival benefit especially in the pT1/2 group, where VISTA positive tumors represent real long-term survivors in this particular subgroup (Figure 3(f )). The survival difference is independent of the T-stage (Table 3).
Figure 3.
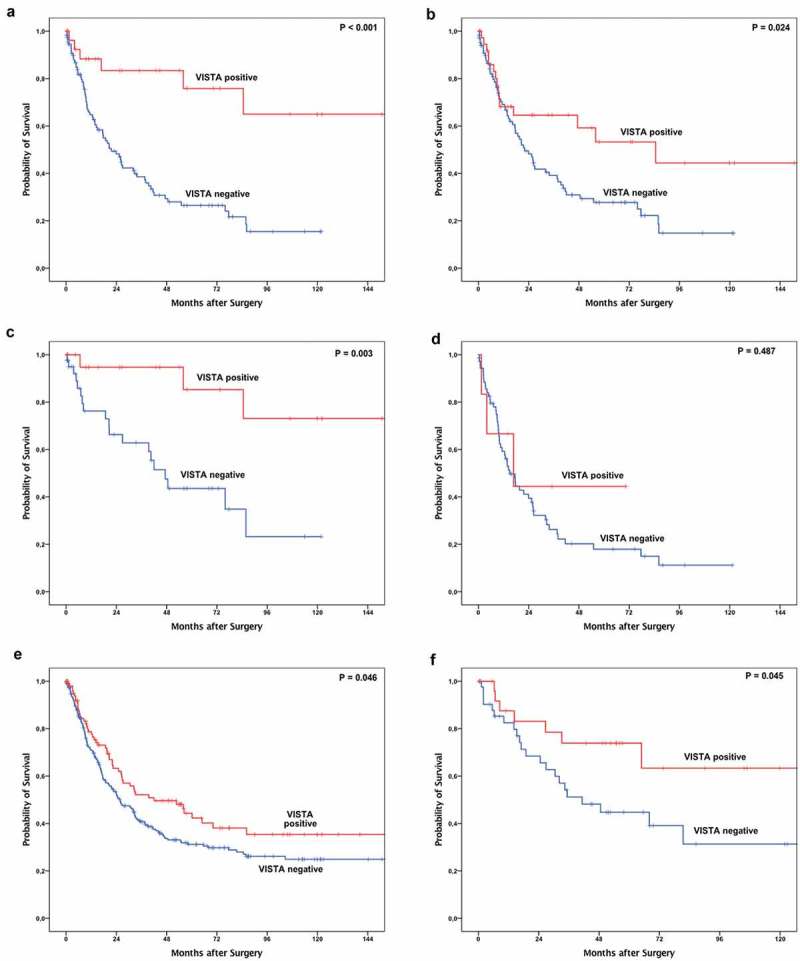
Kaplan-Meier survival analysis of VISTA on the multi spot TMA (n = 165 patients). In both, surface (a) and infiltration zone (b) of the tumor, VISTA expression on tumor infiltrating T-cells (TILs) is correlated with superior overall survival compared to VISTA negative TILs. In subgroup analysis, the survival benefit of VISTA expression reveals in early invasive tumor stages (pT1/2 (c)) and is not detectable in advanced tumor stages (pT3/4 (d)). A difference in overall survival is also detectable in the validation cohort (e) which is predominantly driven by the survival difference in early tumor stages (pT1/2 (f)).
Table 3.
Multivariate cox-regression model for early invasive tumor stages (pT1 and pT2). HR = hazard ratio.
| 95% confidence interval |
||||
|---|---|---|---|---|
| HR | lower | upper | p value | |
| sex (male vs. female) | 1.421 | 0.377 | 5.357 | 0.603 |
| age group (<65 vs. >65) | 1.569 | 0.576 | 4.271 | 0.378 |
| tumor stage (pT1 vs. pT2) | 1.426 | 0.536 | 3.794 | 0.477 |
| lymph node metastasis (pN0 vs. pN+) | 2.751 | 0.923 | 8.2 | 0.069 |
| VISTA expression | 0.207 | 0.055 | 0.783 | 0.02 |
Correlation of VISTA expression in subgroups of tils
To visualize which subtypes of TILs express VISTA we performed double stain immunofluorescence with VISTA and CD4, CD8 and CD68. A semiquantitative analysis was performed on 40 VISTA positive cases correlating VISTA and CD4, CD8 and CD68. VISTA showed a predominant co-expression with CD68, and in the subgroup of TILs a co-expression with CD4 in most cases (Figure 4). No reliable co-expression with CD8 was detectable.
Figure 4.
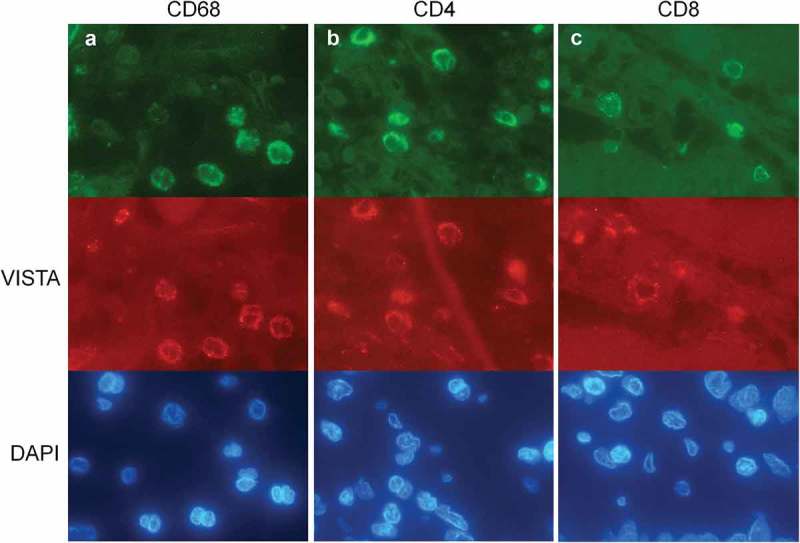
Double staining immunofluorescence of VISTA (red signals) and CD68, CD4 and CD8 (green signals) with counterstaining of the nuclei with DAPI (blue signals). The photos (a-c) represent one double staining each with different fluorescence filters. (a) VISTA and CD68 show a coexpression representing myeloid origin; (b) VISTA and CD4 show a strong coexpression within the VISTA positive TILs; (c) No correlation of VISTA and CD8 was seen.
Correlation of VISTA expression with other biomarkers of the tumor-microenvironment
We correlated the VISTA expression on TILs with the expression pattern of PD-L1 (on tumor cells), LAG3, CD3 and CD8 on TILs (these data currently under review). In the cohort of EAC LAG3 is associated with a better outcome, whereas PD-L1 showed no prognostic impact. We were not able to find any statistically relevant correlation of the aforementioned markers in the present patient’s cohort. No survival differences were observed for patients with low or high CD3 expression with respect to VISTA expression (Supplementary Figure 1).
VISTA expression and TP53 and HER2 status
TP53 mutation and HER2 amplification/expression status was available for the entire patient’s cohort (data not shown). Within the VISTA positive group 63 patients showed a TP53 mutation (60.0%) and 42 patients (40.0%) were TP53 wild-type tumors (p = 0.451). Similar results were found for HER2 amplification. In tumors with high VISTA expression 10 patients showed HER2 amplification, but a correlation via cross-table analysis did not reveal a significant association between VISTA and HER2 amplification (p = 0.236).
Discussion
In a large set of 393 patients with EAC we report the expression of VISTA positive tumor associated lymphocytes (TILs) evaluating the level of heterogeneity and distribution within the tumor. In the patient cohorts, we are able to show a significantly favorable outcome for VISTA positive tumors in pT1/T2 stages and find generally a lower level of VISTA expression in pT3/T4 tumor samples. Furthermore, we do not find any correlation of VISTA with important molecular alterations like TP53 mutational status and HER2 amplification status, as well as with further important biomarkers of the tumor microenvironment like the number of T-cells (CD3) and previously examined LAG3 expression on TILs (Data are currently under revision).
We created a multi-spot TMA considering two different tumor localizations (surface, infiltration margin) as a test cohort, where we were able to prove a low heterogeneity of VISTA expression within the tumor. There is a consistent pattern between the tumor surface and the infiltration margin indicating that EAC samples taken by endoscopic tumor biopsy can represent overall tumor VISTA expression. Furthermore, the absence of significant heterogeneity was one reason to create a single-spot TMA with 228 additional patients as a validation cohort.
To the best of our knowledge we are the first describing the expression pattern of VISTA and its prognostic impact in EAC. Previously, Böger et al.4 examined the role of VISTA in gastric cancer. Interestingly they found comparable results considering the cumulative distribution of VISTA in T1/T2 stages in opposite to T3/T4 – they describe a significant decrease of VISTA positive TILs between the pT2 to the pT3 stage.
In the analyzed cohort, VISTA expression significantly represents a positive prognostic marker in the subgroup of pT1/T2 stage tumors. In addition, the favorable outcome of VISTA positive tumors is even significant in the entire cohort but mainly driven by the general better prognosis of pT1/T2 tumors (VISTA – positive TILs in higher levels in T1/T2 than in T3/T4 stages). However, in the pT1/2 group, VISTA positive tumors show a tremendous survival benefit compared to VISTA negative tumors revealing real long-term survivors in this particular subgroup. The survival difference is independent of the T-stage. This unique characteristic could influence neoadjuvant therapy concepts for EAC, since a profit of therapy could be reduced in the already favorable subgroup of VISTA positive tumors. Further research in this context might contribute to build cohorts of profiting versus non-profiting groups of patients.
Nevertheless, the significantly lower expression of VISTA in pT3/T4 tumor stages remains cryptic. On the one hand, it is conceivable that changes in tumor biology are responsible for a loss/reduced amounts of VISTA-positive TILs in locally advanced tumors; on the other hand VISTA itself could even influence invasive tumor growth.
The exact physiological mechanism of action for VISTA is still obscure. It is assumed to suppress T-cell activity and serves as an immune checkpoint.3 It might therefore play a role in the immune evasion of tumors. Some studies could provide evidence for this assumption. In oral squamous cell cancer, high VISTA expression in combination with low CD8 expression is associated with poor prognosis and lymph node metastases.5 In mouse models, VISTA blockade impaired tumor growth by attenuating the tumor microenvironment.3,6 In the here presented study VISTA expression was a significant and independently positive prognostic marker in the subgroup of pT1/T2 stage tumors. In a previous study of gastric cancer, VISTA expression was predominantly seen in pT1/T2-stages, although it did not correlate with prognosis.
While VISTA is displayed by antigen-presenting cells (APCs) and T-cells, Böger et al.4 showed an expression on gastric carcinoma cells in 8.8%. In the cohort of esophageal adenocarcinoma, VISTA is only rarely present on carcinoma cells (1.2%).
The immune regulating functions of VISTA suggest that an elevated expression is attended by a highly inflamed tumor microenvironment. In opposite to this and previous results for PD-L1 in EAC, we were able to show an independent expression of VISTA concerning the number of T-cells (CD3).7 We found a predominant co-expression of VISTA and CD68, underlining the known expression on myeloid cells.8 On T-cells we were able to find a strong and convincing co-expression of VISTA and CD4-positive T-cells. We did not see a persuading co-expression with CD8-positive T-cells. This is in keeping with known data showing VISTA on CD8-positive T-cells to be expressed in a lower frequency and intensity, so we were probably not able to measure a discrete expression reliably by immunofluorescence.9,10 The functional impact of VISTA-positive CD4-positive T-cells is not clear yet, but there is evidence for VISTA regulating T-cell function both as a ligand and receptor.8,10,11 Anyway, we did not find any correlation with other checkpoint-markers (PD-L1, LAG3) in this cohort indicating that VISTA might function in a more independently manner. The exploration of alternative mechanisms of action, besides regulatory effects on inflammatory microenvironment, might lead to a better understanding of immunomodulation via VISTA in EAC.
Our study has a few limitations. The study is retrospective and a selection bias cannot be excluded. For example, we were not able to test patients who received neoadjuvant treatment and showed a complete tumor response. Functional data on the exact effect of VISTA-expression in EAC could unfortunately not be compiled on formalin fixed paraffin embedded (FFPE) tumor specimen. Our results need to be confirmed by further studies.
Conclusion
Our study reveals the prognostic significance of VISTA expression in esophageal adenocarcinoma. We find VISTA-positive TILs in a significant number of EAC in this cohort, which correlates with improved overall survival within pT1/T2 stages. Thus, this subgroup might be a promising approach to improve personal targeted treatment decisions and lead to new perspectives on neoadjuvant therapy concepts in EAC.
Methods
Patients and tumor samples
In this retrospective study we analyzed 393 patients with esophageal adenocarcinomas that underwent primary surgical resection or resection after neoadjuvant therapy between 1999 to 2016 at the Department of General, Visceral and Cancer Surgery, University of Cologne, Germany. The recently published criteria for reporting recommendations for tumor marker prognostic studies (REMARK criteria) were followed in this study.12,13
According to the suggestions of the international immuno-oncology working group for assessing tumor infiltrating lymphocytes (TILs) in solid tumors we created a multi spot tissue micro array (TMA) with up to 12 tumor spots as a test-cohort considering formalin fixed and paraffin embedded (FFPE) material of 165 patients with EAC.14 148 patients (89%) of the test-cohort did not receive any neoadjuvant treatment. We considered equally the tumor surface/centre and the tumor infiltration margin where possible. Additionally we created a single spot TMA considering 228 additional patients as a validation-cohort (393 patients in total). The construction of the TMAs was performed as previously described.15,16 In brief, tissue cylinders with a diameter of 1.2 mm each were punched from selected FFPE tumor tissue blocks using a self-constructed semi-automated precision instrument and embedded in empty recipient paraffin blocks. For the multi-spot TMA, up to 8 tumor spots were punched out of the tumor, four spots each from the endoluminal and the invasion front. These data were statistically correlated with molecular data like TP53 mutational and the HER2 amplification status. Four μm sections of the resulting TMA blocks were transferred to an adhesive coated slide system (Instrumedics Inc., Hackensack, NJ) for immunohistochemistry. Standard surgical procedure was laparotomic or laparoscopic gastrolysis and right transthoracic en bloc esophagectomy with intrathoracic esophagogastrostomy including two-field lymphadenectomy of mediastinal and abdominal lymph nodes or transhiatal extended distal esophagectomy with transabdominal intrathoracic or cervical anastomosis as described previously.17 Patients with advanced esophageal cancer (cT3, cNx, M0) received preoperative chemoradiation (5-FU, cisplatin, 40Gy) or chemotherapy. Follow-up data were available for all patients. Depending on the effect of neoadjuvant chemo- or radiochemotherapy, there is a preponderance of minor responders, defined as histopathological residual tumor of ≥10%.18 All procedures followed the national and institutional ethical standards and were in accordance with the relevant version of the Helsinki Declaration. Informed and ethical approved consent (13-091) was obtained from all included patients.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on TMA slides. For VISTA the rabbit IgG monoclonal antibody (D1L2G; dilution 1:100; Cell Signalling Technology, Netherlands) was used. All immunohistochemical stainings were performed using the Leica BOND-MAX stainer (Leica Biosystems, Germany) according to the protocol of the manufacturers. The evaluation of immunohistochemical expression was separately assessed independently by two experienced pathologists (AQ and PL), blinded to clinical data. Discrepant results, which occurred in less than 10% of samples, were resolved by consensus review of the particular tumor spots.
Strategy of evaluation
VISTA is expressed on myeloid cells and lymphocytes. Only the expression on lymphocytes was evaluated. VISTA: <1% of lymphocytes was defined as negative, 1–4% of lymphocytes was assessed as “VISTA low”, >4% of lymphocytes was counted as “VISTA high”.
Concerning the multi-spot TMA four spots of tumor surface and invasive margin each were examined. We built the average of the scores and matched the four samples to one category based on limit values: 0–0.49 = negative, 0.5–1.49 = low, 1.5–2 = high.
(e.g.: VISTA expression in spot 1: 2, spot 2: 1, spot 3: 0, spot 4: 2, average of the spots: 1.25 → category “low”).
Discrepant results were resolved by consensus review.
For the statistical analysis, high VISTA expression was assessed as positive and negative or low expression as negative.
Double staining immunofluorescence
Immunofluorescence was performed on TMA slides. For the immunofluorescence double staining, paraffin sections were deparaffinised and antigens were retrieved with citrat. Slides were incubated with the primary antibody (CD4, Thermo Scientific MS-1528 1:100; CD8, Dako M7103 1:100; CD68 Dako M0876 1:200; VISTA, Cell signalling 649535 1:100) followed by incubation with the appropriate secondary antibodies coupled to Alexa Fluor 488 or Alexa Fluor 594 (Invitrogen) and counterstaining of the nuclei with DAPI (Sigma-Aldrich). Tumor tissue was scanned for VISTA positive cells (red signals) using a 63x objective (DM5500 fluorescent microscope; Leica). VISTA positive cells were counted and a co-expression with CD4, CD8 or CD68 (green signals) was assessed.
TP53 status
The immunohistochemical TP53 status was correlated with the TP53 mutational status using parallel sequencing. A detailed description was recently published (Quaas A et al., Genomic characterization of TP53 wild type esophageal carcinoma, Translational Oncology, in press). In brief, the tumor DNA was extracted, amplified with a customized GeneRead DNAseq Targeted Panel V2 (Qiagen), libraries were constructed and quantified, and exons 5–8 of the TP53 gene were sequenced on the MiSeq (Illumina). A 5% cut-off for variant calls was used and results were only interpreted if the coverage was >200x.
Statistical analysis
Clinical data were collected prospectively according to a standardized protocol. SPSS Statistics for Mac (Version 21, SPSS) was used for statistical analysis. Interdependence between staining and clinical data was calculated using the chi-squared and Fisher’s exact tests, and displayed by cross-tables. Survival curves were plotted using the Kaplan-Meier method and analyzed using the log-rank test. Univariate and multivariate analyses were performed for prognostic factors of overall survival using the Cox regression model. All tests were two-sided. P values <0.05 were considered statistically significant.
Funding Statement
Max Kraemer is supported by the Koeln Fortune Program/Faculty of Medicine,Universität zu Köln, grant number [410/2018].
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Barrett JC. Mechanisms of multistep carcinogenesis and carcinogen risk assessment. Environ Health Perspect. 1993;100:9–20. doi: 10.1289/ehp.931009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fassan M, Cagol M, Pennelli G, Rizzetto C, Giacomelli L, Battaglia G, Zaninotto G, Ancona E, Ruol A, Rugge M.. Programmed cell death 4 protein in oesophageal cancer. Oncol Rep. 2010;24:135–139. [DOI] [PubMed] [Google Scholar]
- 3.Lines JL, Sempere LF, Broughton T, Wang L, Noelle R. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res. 2014;2:510–517. doi: 10.1158/2326-6066.CIR-14-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boger C, Behrens HM, Kruger S, Rocken C. The novel negative checkpoint regulator VISTA is expressed in gastric carcinoma and associated with PD-L1/PD-1: A future perspective for a combined gastric cancer therapy? Oncoimmunology. 2017;6:e1293215. doi: 10.1080/2162402X.2017.1293215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu L, Deng WW, Huang CF, Bu LL, Yu GT, Mao L, Zhang W-F, Liu B, Sun Z-J. Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:627–636. doi: 10.1007/s00262-017-1968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Mercier I, Chen W, Jl L, Day M, Li J, Sergent P, Noelle RJ, Wang L. VISTA regulates the development of protective antitumor immunity. Cancer Res. 2014;74:1933–1944. doi: 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jesinghaus M, Steiger K, Slotta-Huspenina J, Drecoll E, Pfarr N, Meyer P, Konukiewitz B, Bettstetter M, Wieczorek K, Ott K, et al. Increased intraepithelial CD3+ T-lymphocytes and high PD-L1 expression on tumor cells are associated with a favourable prognosis in oesophageal squamous cell carcinoma and allow prognostic immunogenic subgrouping. Oncotarget. 2017;8:46756–46768. doi: 10.18632/oncotarget.18606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O’Connell S, Ceeraz S, Suriawinata AA, Yan S, Ernstoff MS, et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74:1924–1932. doi: 10.1158/0008-5472.CAN-13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowak EC, Lines JL, Varn FS, Deng J, Sarde A, Mabaera R, Kuta A, Le Mercier I, Cheng C, Noelle RJ. Immunoregulatory functions of VISTA. Immunol Rev. 2017;276:66–79. doi: 10.1111/imr.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu W, Hieu T, Malarkannan S, Wang L. The structure, expression, and multifaceted role of immune-checkpoint protein VISTA as a critical regulator of anti-tumor immunity, autoimmunity, and inflammation. Cell Mol Immunol. 2018;15:438–446. doi: 10.1038/cmi.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu L-F, Gondek D, Wang Y, Fava RA, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM; Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumor MARKer prognostic studies (REMARK). Br J Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC Med. 2012;10:51. doi: 10.1186/1741-7015-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: part 2: tils in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24:311–335. doi: 10.1097/PAP.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon R, Mirlacher M, Sauter G. Tissue microarrays. Methods Mol Med. 2005;114:257–268. doi: 10.1385/1-59259-923-0:257. [DOI] [PubMed] [Google Scholar]
- 16.Helbig D, Ihle MA, Putz K, Tantcheva-Poor I, Mauch C, Buttner R, Quaas A. Oncogene and therapeutic target analyzes in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget. 2016;7:21763–21774. doi: 10.18632/oncotarget.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holscher AH, Schneider PM, Gutschow C, Schroder W. Laparoscopic ischemic conditioning of the stomach for oesophageal replacement. Ann Surg. 2007;245:241–246. doi: 10.1097/01.sla.0000245847.40779.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider PM, Metzger R, Schaefer H, Baumgarten F, Vallbohmer D, Brabender J, Wolfgarten E, Bollschweiler E, Baldus SE, Dienes HP, et al. Response evaluation by endoscopy, re-biopsy, and endoscopic ultrasound does not accurately predict histopathologic regression after neoadjuvant chemoradiation for oesophageal cancer. Ann Surg. 2008;248:902–908. doi: 10.1097/SLA.0b013e31818f3afb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


