Thioredoxin (Trx) is a 12‐kDa protein ubiquitously expressed in all living cells that fulfills a variety of biological functions related to cell proliferation and apoptosis 1. It is characterized by the highly conserved reduction/oxidation (redox)‐active site sequence Trp‐Cys‐Gly‐Pro‐Cys‐Lys. Trx acts as a powerful antioxidant and plays an important role in maintaining critical protein thiols in the reduced state. Trx‐1 has been shown to have neuroprotective effects on both brain ischemia and excitotoxic hippocampal injury in transgenic mice overexpressing human Trx‐1 2.
In the adult brain, new neurons are continuously generated in the subventricular zone (SVZ) and dentate gyrus (DG) and brain insults such as cerebral ischemia, which cause neuronal death, lead to increased neurogenesis in the SVZ and DG. Evidences have shown that these stroke‐induced newborn precursors could migrate into the severely damaged striatum and develop into mature neurons 3. This process is modulated by many factors and signals. It was reported that Trx‐1 could promote neurite growth by activation of transcription factors 4, but the exact effect of Trx‐1 on neurogenesis after ischemia is still unknown.
Focal cerebral ischemia in adult male Sprague‐Dawley rats (weighing 220–240 g) was induced by middle cerebral artery occlusion (MCAO) for 90 min with an intraluminal filament according to the method previously described by Longa 5. Recombinant human Trx‐1 (RhTrx‐1) (200 μg/kg) was administered intraventricularly at 24, 48, and 72 h after reperfusion. The rats were administered 5‐bromo‐2′‐deoxyuridine (BrdU) intraperitoneally 100 mg/kg daily for 5 days at 24 h after reperfusion. At 7 days after reperfusion, Nissl staining in hippocampus showed extensive neuronal loss, but rhTrx‐1 administration could attenuate this injury (Figure 1). This is the first evidence that intraventricularlly administered rhTrx‐1 could exert neuroprotective effect on brain ischemia/reperfusion (I/R) injury in rats.
Figure 1.
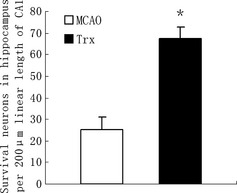
Effect of rhTrx‐1 on hippocampal neurons survival in rats subjected to 7 days of reperfusion after 90‐min middle cerebral artery occlusion (MCAO) (Nissl staining). The survival neurons in MCAO group (n = 5) and rhTrx‐1 treatment group rats (n = 5) were statistically different (P < 0.01). Data were expressed as mean ± SD. Data were analyzed by One‐way ANOVA.
In our study, we also examined the effect of rhTrx‐1 on neurogenesis after brain I/R injury. RhTrx‐1 treatment increased BrdU+ cells nearly 2‐fold in the SVZ compared with I/R group at 7 days (Figure 2A), and this elevation persisted significantly even at 14 days after I/R. Most of these BrdU+ cells were BrdU/Dcx double‐labeled cells. The number of BrdU/NeuN double‐labeled cells also increased after MCAO and Trx‐1 treatment group showed significantly greater numbers of BrdU/NeuN double‐labeled cells in the ipsilateral striatum (ST), especially at 14 days (Figure 2B). This indicated Trx‐1 could promote the newborn neuronal precursors in the SVZ to migrate to the damaged ST and mature into neurons. Our early research had reported that Trx‐1 could inhibit apoptosis by activation of Akt‐dependent signal pathway 6, and this could partly explain the phenomena in our study, as most of the stroke‐induced newborn precursors would die of apoptosis 7. Our results suggest that Trx‐1 could protect brain I/R injury by augmentation of neurogenesis, and administration of Trx‐1 may be a novel therapeutic strategy for the treatment of ischemic stroke.
Figure 2.
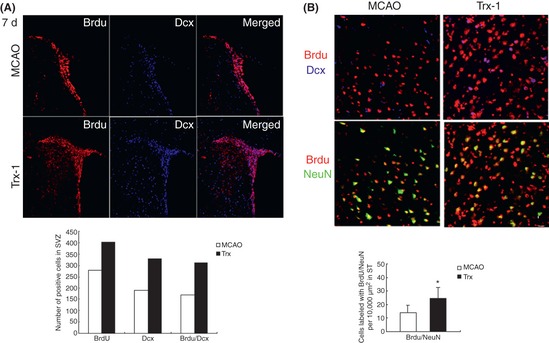
Effect of rhTrx‐1 on neurogenesis after brain I/R injury. (A) Cell proliferation and migration after middle cerebral artery occlusion (MCAO) and rhTrx‐1 (200 μg/kg) treatment at 7 days after reperfusion. The subventircular zone for the evaluation of neuronal precursor cells was stained by BrdU (red) and Dcx (blue). The numbers of BrdU, Dcx and BrdU/Dcx double‐positive cells in rhTrx‐1 treatment group were greater than those in MCAO group (P < 0.01). (B) Cells double‐labeled with BrdU/NeuN in ST increased after MCAO. Trx‐1 treatment augmented the migration and differentiation of these precursor cells at 14 days after reperfusion. Data were expressed as mean ± SD (n = 6 in each group). Data were analyzed by One‐way ANOVA.
Acknowledgments
This study was supported by National Natural Sciences Foundation of China (30801183), Key Clinical Disciplines Program of Chinese Health Ministry and Grants of Health Bureau of Zhejiang Province (2012).
The first two authors contributed equally to this study.
References
- 1. Yamawaki H, Haendeler J, Berk BC. Thioredoxin: A key regulator of cardiovascular homeostasis. Circ Res 2003;93:1029–1033. [DOI] [PubMed] [Google Scholar]
- 2. Takagi Y, Mitsui A, Nishiyama A, et al. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc Natl Acad Sci USA 1999;96:4131–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 2002;8:963–970. [DOI] [PubMed] [Google Scholar]
- 4. Bai J, Nakmura H, Kwon YW, et al. Critical roles of thioredoxin in nerve growth factor‐mediated signal transduction and neurite outgrowth in PC12 cells. J Neurosci 2003;23:503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20:84–91. [DOI] [PubMed] [Google Scholar]
- 6. Zhou F, Gomi M, Fujimoto M, et al. Attenuation of neuronal degeneration in thioredoxin‐1 overexpressing mice after mild focal ischemia. Brain Res 2009;1272:62–70. [DOI] [PubMed] [Google Scholar]
- 7. Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA 2001;98:4710–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]


