SUMMARY
Background and purpose
Resveratrol has been regarded as a promising candidate for cancer prevention and treatment. The present study was to investigate the impact of resveratrol on the antitumor effects of temozolomide (TMZ), a standard treatment regiment of glioblastoma (GBM), in vitro and in vivo.
Methods and results
We found that the combination of resveratrol and TMZ significantly resulted in G2/M cell cycle arrest by flow cytometry, triggered a robust increase in expression of astrocyte differentiation marker glial fibrillary acid protein (GFAP), downregulated the expression of matrix metalloproteinase‐9 (MMP‐9) by immunohistochemistry and western blot analysis as well as inhibited cell migration by scratch wound assay. Further study revealed that TMZ in combination with resveratrol remarkably increased reactive oxygen species (ROS) production, which serves as an upstream signal for AMP‐activated protein kinase (AMPK) activation. Subsequently, activated AMPK inhibited mTOR signaling and downregulated antiapoptosis protein Bcl‐2, which was contributed to the additive antiproliferation effects of combination treatment. In an orthotopic xenograft model of GBM, TMZ plus resveratrol treatment significantly reduced the volume of tumor, which was confirmed by decreased expression of Ki‐67, a marker of proliferation index.
Conclusions
Our findings demonstrate for the first time that resveratrol can enhance TMZ‐mediated antitumor effects in GBM in vitro and in vivo, via ROS‐dependent AMPK‐TSC‐mTOR signaling pathway.
Keywords: AMPK, Glioblastoma, mTOR, Resveratrol, Temozolomide
Introduction
Glioblastoma (GBM) is one of the most aggressive solid cancers and the most common primary brain tumor. This tumor is inherently resistant to conventional therapy and the median survival is approximately 14 months. Although standard treatment with surgery, irradiation, and chemotherapy postpones progression and extends survival to some extent, these patients universally recur and unrelentingly result in death [1]. GBM is associated with dismal prognoses because of their ability to infiltrate diffusely into the normal brain parenchyma, are associated with the worst prognoses [2]. Among the chemotherapeutic agents used to treat GBM, alkylating agent temozolomide (TMZ) can cross the blood–brain barrier effectively with few myelocytotoxic effects [3]. However, tumor resistance to TMZ is a common event, for instance, to raise O6‐alkylguanine‐DNA alkyl‐transferase (AGT) levels or to mismatch repair (MMR) deficiency, and strongly affects the rate and durability of clinical response in cancer patients. Moreover, these compounds are also characterized by mutagenic properties favoring the emergence of potentially resistant tumor cell clones [4]. Therefore, improvement of treatment options for patients with GBM is imperative.
Different strategies including combined with other agents to attenuate resistance and increase the efficacy of TMZ have been designed. Among the potential chemosensitizers, the natural agents such as resveratrol, may be an ideal agent for GBM therapy. Resveratrol, a naturally occurring polyphenolic compound, is highly enriched in grapes, peanuts, red wine and existed a wide variety of other food sources [5]. Resveratrol has a low toxicity and can be given in relatively high doses without adverse effects in humans. Emerged evidence suggested that resveratrol might be a potential chemopreventive and/or chemotherapeutic in human cancer [6, 7, 8]. It is reported that resveratrol can act as an antioxidant and antimutagen, mediate anti‐inflammatory effects, inhibit cyclooxygenase and hydroperoxidase functions (antipromotion activity), and induce cell differentiation [antiprogression activity; Ref. 9]. Resveratrol treatment causes alterations in the p53‐responsive genes, p300, Apaf‐1, NF‐κB/p50, p65 and PPAR families of genes [10]. All of these findings suggested that multiple signaling pathways might be responsible for the growth inhibitory effects of resveratrol on the tumor cells [10]. Previous studies also have revealed that resveratrol could promote the sensitivities of tumor cells (including lung carcinoma, acute myeloid leukemia, promyelocytic leukemia, multiple myeloma, prostate cancer, oral epidermoid carcinoma, and pancreatic cancer) to various chemotherapeutic agents such as vincristine, adriamycin, paclitaxel, doxorubicin, and gemcitabine [11, 12, 13, 14, 15, 16]. Therefore, the present study was designed to investigate whether resveratrol can enhance the antitumor effect of TMZ on the human GBM in SHG44 cells and in an intracranial xenograft therapy‐response model, and to demonstrate the involved mechanisms in the additive antitumor effects exerted by TMZ and resveratrol.
Materials and Methods
Materials and Reagents
TMZ was a gift from Tianjin Taslypharmaceutical Co. Ltd. dissolved at 100 mM concentration in dimethylsulfoxide (DMSO; Sigma, St Louis, MO, USA) and kept as stock solutions. Resveratrol, NAC, MTT, and Hoechst 33342 were purchased from Sigma. DMEM and fetal bovine serum (FBS) were purchased from Life Technologies, Inc (Rockville, MD, USA). Antibodies against MMP‐9, Bcl‐2 and Ki‐67 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and the antibodies against phosphorylated mTOR, total‐mTOR, phosphorylated AMPKα, total‐AMPKα were from Cell Signaling Technology (Bevery, MA, USA).
Cell Culture
For the current study, we selected a human GBM cell line SHG44 (WHO grade IV) was supplied by the First Affiliated Hospital of SooChow University. Wild type, TSC−/− mouse embryonic fibroblast (MEF) cells were presented as a gift by Prof. Y. Wan (Department of Biology, Providence College). These cells were cultured in DMEM containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin, and maintained in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Cell Viability Assay (MTT Dye Assay)
Cell viability was measured by the 3‐(4,5‐dimethylthylthiazol‐2‐yl)‐2, 5‐diphenyltetrazolium bromide (MTT) method. In brief, cells were collected and seeded in 96‐well plates at a density of 5×105 cells/cm2. Different seeding densities were optimized at the beginning of the experiments. 20 μL of MTT tetrazolium salt (Sigma) dissolved in Hanks’ balanced solution at a concentration of 5 mg/mL was added to each well with the indicated treatment and incubated in CO2 incubator for 4 h. Finally, the medium was aspirated from each well, and 150 μL of DMSO (Sigma) was added to dissolve formazan crystals, and the absorbance of each well was obtained using a Dynatech MR5000 plate reader at a test wavelength of 490 nm with a reference wavelength of 630 nm. The following formula was used to calculate cell viability: percentage cell viability = (absorbance of the experiment samples/absorbance of the control) × 100.
Hoechst 33342 Staining
To quantify apoptotic cells, cellular monolayer was fixed and stained with Hoechst 33342. Cells with the indicated treatment were fixed with 4% formaldehyde in PBS for 10 min at 4°C. Cells were then incubated with 5 μg/mL Hoechst 33342 to stain the nuclei for 5 min. After washing with PBS, the morphological features of apoptosis (cell shrinkage, chromatin condensation, and fragmentation) were monitored by fluorescence microscopy (Nikon Optical TE2000‐S, Tokyo, Japan), and each treatment was performed in triplicate.
Reactive Oxygen Species (ROS) Production Detection
Formation of ROS was evaluated using 2’, 7’‐dichlorofluorescein diacetate (DCFH‐DA; Sigma). DCFH‐DA enters cells passively and is de‐acetylated by esterase to nonfluorescent DCFH. DCFH reacts with ROS to form DCF, the fluorescent product. DCFH‐DA was dissolved in ethanol at 10 mM and was diluted 500‐fold in DMEM to give DCFH‐DA at 20 μM. Cells were treated with DMEM containing corresponding concentration of resveratrol or TMZ individually and combination for 6 h and then explored to DCFH‐DA for 1 h. The fluorescence was visualized immediately at wavelengths of 485 nm for excitation and 530 nm for emission by a Nikon Optical TE2000‐S (Tokyo, Japan) inverted fluorescence microscope. And the total green fluorescence intensities of every well were quantitated using image‐analysis software (Simple PCI, Hamamatsu City, Japan).
Cell Cycle Analysis
Dissociated SHG44 cells were fixed overnight in 70% ethanol in PBS, resuspended in PBS and treated with 25 μg/mL ribonuclease A (Sigma) for 1 h at room temperature, followed by staining with 50 μg/mL propidium iodide (PI; Sigma). Argon‐ion laser excitation (488 nm) was used to measure PI fluorescence with a 620‐nm bandpass filter. The DNA content was determined by flow cytometry (FACSCalibur). Apoptotic cells were identified by the sub‐G1 phase in the cell‐cycle distribution. For assessment of the apoptotic rate was performed according to the manufacturer's protocol. The apoptotic rate was measured by flow cytometry (FCM) using Cell Quest software.
In Vitro Scratch Wound Assay
Cells (0.25×106) were grown to confluency in 24 well dishes were scratched with a sterile plastic 10‐μL micropipette tip. Cells were rinsed three times in PBS to remove loose and detached cells and images were acquired immediately and 24 h after the scrape. Migration distances were measured using Axiovision software (Zeiss).
Western Blot Analysis
After drug treatment, cells were washed twice with ice‐cold PBS and homogenized in 200 μL lysis buffer. After incubation for 20 min on ice, cell lysates were centrifuged (10,000 × g for 10 min at 4°C) and protein concentration in the extracts was determined by the Bradford assay. Proteins in cell extracts were denatured with SDS sample buffer and separated by 10% or 6% SDS‐PAGE. Proteins were transferred to nitrocellulose membranes using a Bio‐Rad miniprotein‐III wet transfer unit. Nonspecific binding was blocked with 5% nonfat milk dissolved in TBST (pH 7.5, 10 mM Tris–HCl, 150 mM NaCl, and 0.1% Tween‐20) for 1 h at room temperature. The membranes were then incubated overnight at 4°C with individual primary antibodies including anti‐AMPK, anti‐phospho‐AMPK, anti‐total‐mTOR, anti‐phospho‐mTOR, anti‐MMP‐9, anti‐Bcl‐2 and anti‐GFAP. Following three washes with TBST, the membranes were then incubated with the horseradish peroxidase‐conjugated secondary antibodies dilution in TBST containing 5% BSA for 1 h at room temperature. The membranes were then washed thrice with TBST and the protein bands were visualized with the ECL Western blotting detection system according to the manufacturer's instructions.
Fluorescence Assay for Intracellular Presence of GFAP
SHG44 cells were fixed with 4% paraformaldehyde, rinsed in PBS and blocked with 5% BSA for 60 min. Then, cells were incubated in a cocktail solution containing rabbit anti‐GFAP antibody (1:5000; Cell Signaling Technology) at 4°C overnight. The cells were washed in PBS three times and incubated in another cocktail solution containing goat antirabbit FITC (1:200; Chemicon) antibodies at room temperature for 1 h. Cell fluorescence was visualized by fluorescence microscopy (Nikon Optical TE2000‐S) for visualization and photograph.
In Vivo Human Tumor Model and Histopathological Study
Female BALB/cA nude mice were supplied by Shanghai Institute of Materia Medica, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences. The mice were raised in air‐conditioned rooms under controlled lighting (12 h lighting/d) and were fed standard laboratory food and water ad libitum. The animal care and surgery protocols were designed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health, USA.
SHG44 cells (5×105 cells per mouse) in 5 μL of Hanks’ solution were injected intracranially into the right caudate nucleus of 6‐ to 8‐week‐old female nude mice using a screw guide technique as described [17]. Drug administration began on the eighth day after cell grafting and was randomized to treatment groups of eight mice each. Mice were treated daily with oral administration of buffer alone as a control, or administration by oral gavage at 68 mg/kg TMZ, which corresponds to the murine equivalent of the standard clinical dose of 200 mg/m2 and schedule [18], or oral administration of 40 mg/kg resveratrol, or a combination with TMZ and resveratrol. Control buffer solution consisted of 5% DMSO in 0.5% carboxymethylcellulose. Daily assessment of animal skin turgor, feeding behavior, body weight, and appearance was made. Tumor growth was evaluated using magnetic resonance imaging (MRI) on day 28 after tumor implantation, and brains were harvested after intracardiac perfusion with paraformaldehyde and fixed with 10% neutral buffered formalin. Paraffin‐embedded specimens were sectioned 5 μm, these sections were stained with hematoxylin and eosin (H&E) for light microscopic examination. In each coronal section, the area of the entire tumor was measured by tracing the respective tissue boundaries on the computer screen by using the Optical Fractionator method with microbrightfield Stereo‐Investigator software and the volume was determined by multiplying the appropriate area by the section interval thickness. The multiple 5 μm thick sections were deparaffinised and rehydrated using xylene and ethanol and then transferred to the 0.02% Triton for permeabilization. Slides in citrate buffer (pH 6.0) were heated in the steamer for 30 min. After cooling for 30 min and a three 5 min wash in PBS, the slides were incubated in 3% BSA for 30 min. The slides were successively transferred to 3% H2O2 for 10 min a Primary antibody against Ki‐67 and GFAP (1:100, Boehringer Mannheim) was used at overnight at 4°C. Secondary biotinylated antimouse antibody was used at 1:250 dilution for 1 h at room temperature. Ki‐67‐positive cells and GFAP‐positive cells were counted in one of the most strongly staining fields per section (1500–2500 cells/field).
Statistics
The values in the figures are expressed as the mean ± S.E.M. The figures in this study are representative of at least three independent experiments. Values of P < 0.05 were considered as statistically significant with two‐way ANOVA and Student's t‐test.
Results
Combination Treatment of TMZ and Resveratrol exhibits Additive Effect in SHG 44
As shown in Figure 1(A) and (B), TMZ or resveratrol single treatment for 72 h inhibited the growth of SHG44 cells in a concentration‐dependent manner, and the combination produced additive inhibitory effects on the cells growth (Figure 1C).
Figure 1.
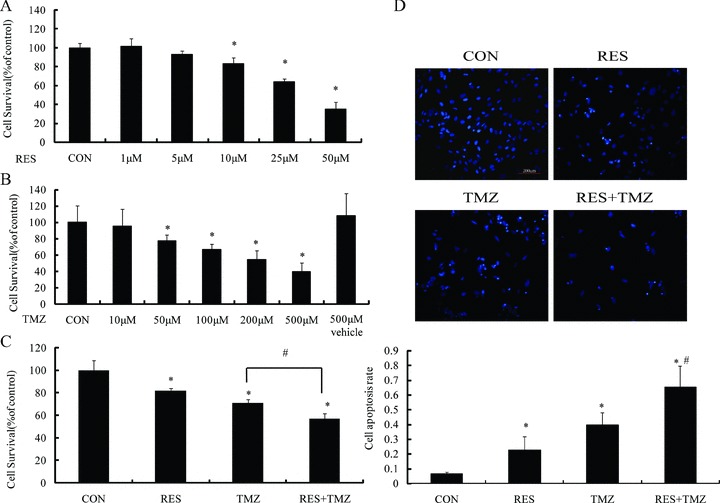
TMZ alone or in combination with resveratrol (RES) induces cell death that is additive in effect. (A) and (B) MTT assay results showed that TMZ and RES decreased the cell viability in a concentration dependent manner after treatment for 72 h, respectively, and the vehicle of 500 μM TMZ (TMZ 500 V) had no effect on the viability of these cells. (C) MTT assay indicated that combination of TMZ and RES had an additive effect on inhibiting cell proliferation. (D) Fluorescence microscopy images of Hoechst 33342‐stained cells showed the appearance of apoptotic morphology in TMZ at 100 μM plus RES at 10 μM for 72 h. Lower histogram: quantitative analysis of condensed nucleus as in above fluorescence images. P represents statistical analysis. *P < 0.05 versus control group; # P < 0.05 versus TMZ.
Furthermore, we investigated whether apoptosis was involved in cell growth inhibition by the treatment, we performed resveratrol, TMZ and the combination on SHG44 cells with Hoechst 33342. As shown in Figure 1(D), untreated SHG44 cells exhibited regular and round shaped nuclei and the condensation and fragmentation of nuclei, the characteristic of apoptotic cells, were evident in the cells treated with resveratrol at 10 μM, TMZ at 100 μM, and the combined for 72 h. In addition, we detected apoptosis increase using FCM analysis in hypodiploid cell populations. The staining of cells with PI was used to distinguish and quantitatively determine the percentage of apoptotic cells (Figure 2B). The percentage of the sub‐G1 fraction, which was indicative of apoptotic cell death in treated cells, increased in an additive‐dependent manner after resveratrol plus TMZ. These results suggested that the additive cytotoxic effect was defined as a significant increase in combination of resveratrol and TMZ in comparison with therapy with either resveratrol or TMZ alone.
Figure 2.
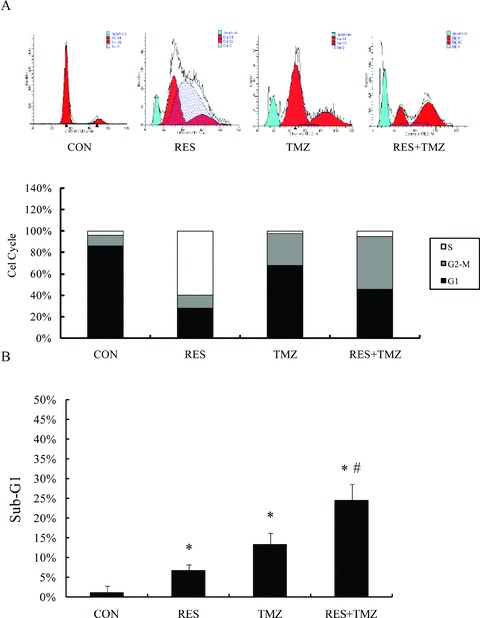
Combination treatment with TMZ and resveratrol (RES) modulates the cell cycle in SHG44 cells. Representative flow‐cytometric histograms of DNA content of TMZ and RES individually and the combination. (A) The treatment of TMZ at 100 μM and RES at 10 μM arrest cell cycle in G2/M phase and S‐phase, respectively. Combination the two agents significantly arrest cell cycle at G2/M phase. (B) The percentage of sub‐G1 phase was measured by flow cytometry. P represents statistical analysis. *P < 0.05 versus control group; # P < 0.05 versus TMZ.
Resveratrol in Combination with TMZ Induces the G2/M Arrest in SHG44 Cells
To evaluate the effect of TMZ and resveratrol on the cell‐cycle progression, SHG44 cells were stained with propidium iodide and DNA concentration in single cells was determined by FCM (Figure 2A). Treatment with TMZ at 100 μM strongly induced the cell cycle arrest in G2/M phase at 36.67%, whereas resveratrol at 10 μM led to the accumulation of the S‐phase cell population at 59.69%. Notably, the combined treatment resulted in the most pronounced effect in the G2/M phase arrest at 61.62% in SHG44 cells.
Combination Treatment with Resveratrol and TMZ Inhibits Cell Migration
To examine the effect of the combination of resveratrol and TMZ on the cell migration during 72 h, in vitro scratch assay was performed by Live Imaging (Figures 3A, B and S1). At the beginning of 24 h, there was no difference between TMZ‐treated groups and combination‐treated groups in cell migration. However, during the last 24 h, resveratrol cooperated with TMZ has better effective inhibitory effects compared to TMZ alone.
Figure 3.
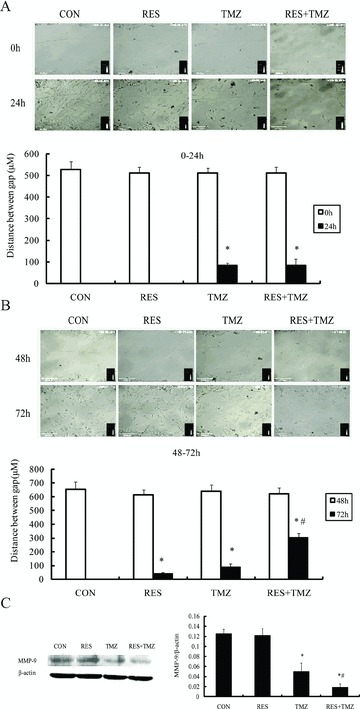
Combination of TMZ and resveratrol (RES) prevented cell migration and inhibited cell metastasis. Representative pictures of SHG44 cells were at 0 h and 48 h after treatment (when a wound was made), and 24 h later, respectively. SHG44 cells subjected to in vitro scratch assay. The migration ability was estimated quantitatively by measuring the remaining area between the two edges of the wound. (A) At the beginning of 24 h, the gap distance was reduced to 1 μM in control groups and RES‐treated groups, and the gap distance of the TMZ group and the combined group was 83.2 ± 12.8 μM and 84.4 ± 26.4 μM, respectively (P= 0.8676). (B) During the last 24 h, the gap distance was reduced to 1 μM under control conditions, and after the treatment of RES and TMZ alone, the gap distance was reduced to 41.7 ± 8.8 and 87.3 ± 21.3 μM, indicating the partial inhibition of cell migration, and the most inhibitory effect after combination treatment was 304.1 ± 31.7 μM. The covered distance was estimated in five different areas of the culture, in three independent experiments. (C) Cells were incubated with indicated the treatment of TMZ at 100 μM and RES at 10 μM for 72 h, and then, cell proteins were obtained and analyzed with anti‐MMP‐9 antibodies by Western blot. The combination treatment significantly down regulated the expression of MMP‐9. *P < 0.05 versus control group (CON); #P < 0.05 versus TMZ.
In addition, as shown in Figure 3(C), compared to TMZ alone treatment, TMZ combined with resveratrol produced additive inhibitory effects on the expression of MMP‐9, which was contributed to the inhibitory effects on cell migration.
Combination of Resveratrol and TMZ Induces Cell Differentiation
GFAP is a reliable marker of differentiated astrocytes, which is frequently lost with increasing grade of malignancy, suggesting that GFAP is important for maintaining glial cell morphology or regulating astrocytoma cell growth [19]. To investigate whether combination of TMZ with resveratrol was able to induce differentiation in GBM cells, GFAP expression was assessed. As shown in Figure 4(A), TMZ or resveratrol increased the expressions of GFAP to 315.65% and 145.03%, respectively. Resveratrol combined with TMZ robustly upregulated the expressions of GFAP to 434.66% (P < 0.01), which was confirmed by western blotting and in the orthotropic model by immunohistochemisty (Figure 4B and C). These results suggested that combination can promote the tumor cell differentiation.
Figure 4.
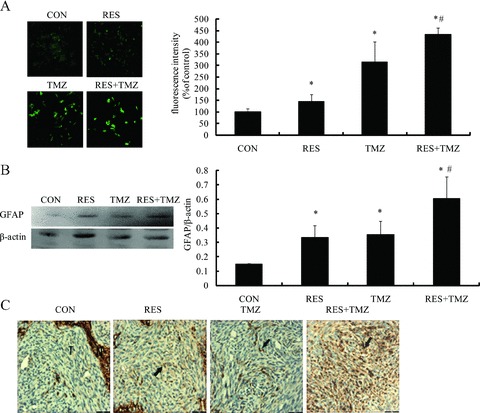
Differentiation effects on the treatment of TMZ and resveratrol (RES). SHG44 cells were treated with either TMZ at 100 μM or RES at 10 μM and the combination for 72h, the expression of GFAP was detected with fluorescence microscopy images (A) and Western blot (B), respectively. (C) In nude model, the GFAP expression (arrow) was by immunohistochemisty after the combination of TMZ (68 mg/kg × 3 d) and RES (40 mg/kg, daily). (T: tumor; N: normal brain). *P < 0.05 versus control group (CON);# P < 0.01 versus TMZ group.
Resveratrol Enhances TMZ Induced Inhibition of the Growth of Orthotropic Implanted GBM in Nude Mice
We further investigated the efficacy and toxicity of TMZ and resveratrol as single agents and in combination against GBM model in vivo. SHG44 cells were implanted into the right caudate nucleus of nude mice. On the eighth day, we randomized animals into four groups and treated them with 68 mg/kg TMZ for 3 days, or 40 mg/kg resveratrol daily, or combination treatment. The mice were sacrificed on the 28th day after tumor cells injection.
Body weight was monitored serially to measure the tolerability of the regimens tested in all mice. As shown in Figure 5(A), all the nude mice were slightly decreased in body weight after the implantation, and gradually recover. In placebo group and the resveratrol single group, their body weight returned to pretreatment levels on the 28th day, however, treated with the combination of TMZ and resveratrol group and TMZ alone group all had a decrease in body weight, and on the fourth day after the initiation of treatment was decreased to 17% and 14%, respectively, and there was no difference between the combination group and the TMZ single group. Importantly, none of these animals died early during experiment. The results indicated that the combination therapy did not increase in toxicity compare to the TMZ alone.
Figure 5.
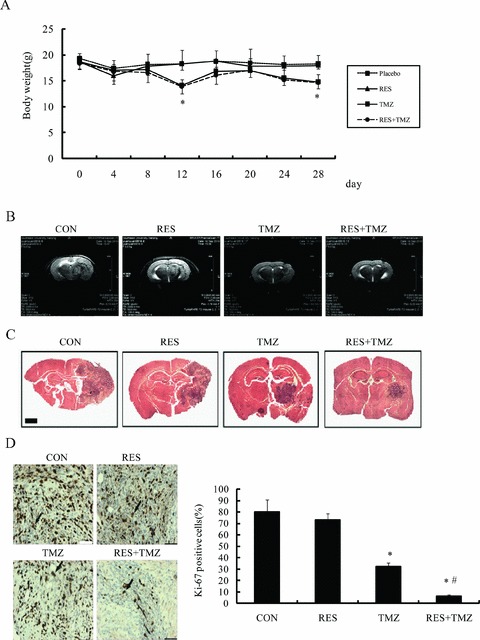
TMZ combined with resveratrol (RES) in GBM orthotopic xenografts. (A) Mice with established orthotopic xenografts from SHG44 were randomized to therapy with the indicated placebo (0.5% carboxymethylcellulose), TMZ (68 mg/kg × 3 d), RES (40 mg/kg, daily) and the combination, change in body weight for mice from the experiment treated with placebo, RES, TMZ, or combination. (B) Representative tumor volumes of orthotopic nude mice were evaluated by magnetic resonance images (MRI). (C) Pathologic analysis of mouse brains. Low (2×), intermediate power images of the tumor area in four different animals. Intracranial tumors were identified in coronal brain sections by H&E staining. (D) Immunohistochemical staining for Ki‐67 (×400). Brown nuclear staining for Ki‐67 indicated proliferating cells. The percentage of Ki‐67‐positive cells in treated tumors. Combination treatment of TMZ and RES showed significant effect in vivo. P values are comparing an indicated treatment, change in body weight for mice from the experiment treated with placebo, RES, TMZ, or combination. *P < 0.005 versus placebo group (CON); # P < 0.001 versus TMZ.
Moreover, MRI was used to evaluate the volume of tumor in the brain performed before sacrificed (Figure 5B). The results showed an evident increase in tumor volume in control and in resveratrol group, suggesting that resveratrol alone did not significantly inhibit the growth of the tumor (P > 0.05 vs. CON). Combined with TMZ could significantly reduced the tumor volume than TMZ alone (P < 0.05). The results of the pathologic examination were in accordance with measurement of the MRI. Representative brain specimens in untreated animals and in those receiving the treatment were shown in Figure 5(C).
The mice were sacrificed and tumors tissues were committed histological analysis for cell proliferation by Ki‐67 immunostaining. The results revealed that Ki‐67 staining index of tumor cells was significantly reduced in mice treated with TMZ (P= 0.0019 vs. CON). Although resveratrol did not decrease the Ki‐67‐positive cells compared with control group, TMZ plus resveratrol remarkably decreased the Ki‐67 staining index, which was significantly lower than that of mice treated by TMZ alone (P= 0.0087; Figure 5D).
Combination of TMZ and Resveratrol increases ROS Generation, and Activates AMPK Signaling Pathway
As shown in Figure 6(A), TMZ or resveratrol alone could induce ROS production in SHG44 cells, though the combination treatment caused significantly increases compared to TMZ. NAC, an antioxidant, prevented the combined treatment‐induced inhibition of cell proliferation (Figure 6B).
Figure 6.
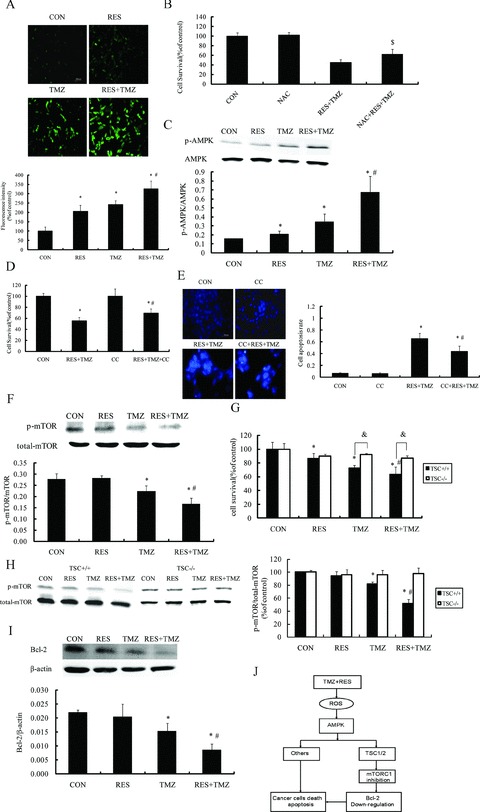
Combination with TMZ and resveratrol (RES)‐induced production of ROS and mediated AMPK signaling in SHG44 cells. (A) DCFH‐DA fluorescence (green) imaging of ROS in SHG44 cells. Cells were labeled with DCFH‐DA 10 mM and then treated with indicated combination of TMZ and RES for 6 h. (B) Antioxidant (NAC) inhibited the antitumor effect of combination with TMZ and RES. MTT assay showed pretreatment with NAC (1 mM) for 1 h inhibited the combination therapy‐induced cell viability decline. (C) Cells were incubated with indicated the treatment of TMZ at 100 μM and RES at 10 μM for 72 h, and then, cell proteins were obtained and analyzed with anti‐phospho‐AMPK and anti‐AMPK antibodies by Western blot. The combination of TMZ and RES significantly increased the phosphorylation of AMPK. AMPK inhibitor Compoud C (CC) partly abolished the antitumor effect of TMZ plus RES. MTT assay showed pretreatment with CC 1 μM partly reduced the combinational therapy induced the antigrowth (D) and apoptosis effect (E). (F) mTOR signaling in SHG44 cells remained responsive to the combination of TMZ and RES. mTOR activity is phosphorylated at a lowest level following the combination treatment. TSC was involved in mTOR signaling pathway in response to the treatment. Wild‐type and TSC−/− MEF cells were treated with indicated treatment for 72 h and then cellular viability was detected with MTT assay. Notably, compared to TEC−/− MEF cells, the combination TMZ and RES markedly decreased the cellular viability in wild‐type MEF cells (G). Wild‐type and TSC−/− MEF cells were incubated with the combination for 72 h, and then, cell proteins were obtained and analyzed with the expression of mTOR by Western blot (H). (I) The combination of TMZ and RES significantly inhibited the expression of Bcl‐2 in SHG44. (J) Schematic model for the mechanisms of the combination with TMZ and RES exerted antitumor effect. The combinational treatment induced ROS generation, thereby activated the proapoptotic stress kinase AMPK via TSC, AMPK inhibited the mTOR activation, which subsequently mediated the antitumor effects, and eventually triggered apoptosis. The results are representative of more than three independent experiments. *P < 0.05 versus control group (CON); # P < 0.05 versus TMZ;$ P < 0.05 versus RES+TMZ. & P < 0.05 TSC+/+ versus TSC−/−.
Previous studies have identified ROS as AMPK activators [20, 21, 22], so we next tested AMPK activity. We found that TMZ plus resveratrol could significantly activate AMPK (Figure 6C). AMPK inhibitor compound C (CC) abolished combination treatment inhibited cell proliferation and induced cell apoptosis (Figure 6D and E). So, our results suggested that ROS‐induced AMPK activation was involved in the mechanism of additive antitumor effects of combination of TMZ and resveratrol in SHG44 cells.
As we know, mTOR has been recognized as one of the major downstream targets of the AMPK pathway [23, 24] and the antitumor activity of AMPK has been attributed to its inhibitory action on the mTOR survival pathway [25]. We measured the phosphorylation status of mTOR activity with resveratrol, TMZ and the combination. As shown in Figure 6(F), TMZ can evidently decreased the mTOR activity, and combined with resveratrol exerted more significant inhibitory effect on the mTOR activity. Previous studies have shown that activation of AMPK inhibits mTOR, primarily through phosphorylating and activating TSC, the upstream inhibitor protein of mTOR, which effects protein synthesis and cell growth [26]. In TSC−/− MEF cells, the combination of TMZ and resveratrol‐induced proliferation and mTOR inhibitory effects were almost reversed (Figure 6G and H), indicating that AMPK inhibits mTOR in a TSC‐dependent manner. In addition, mTOR is well‐known to regulate ribosomal gene expression by increasing translation initiation. Among the genes that are regulated by mTOR, antiapoptotic protein Bcl‐2 has been implicated in the resistance of tumor cells to the proapoptotic effects [27, 28]. Here, we found that the combination treatment downregulated the expression level of Bcl‐2 in SHG44 cells (Figure 6I). Taken together, we suggested that the combination of resveratrol and TMZ‐induced AMPK activation, and then inhibited mTOR signaling in a TSC‐dependent manner. Consequently, mTOR inhibition caused Bcl‐2 downregulation and resulted in cell apoptosis in GBM cells (Figure 6J).
Discussion
In this study, we describe the potential therapeutic effect of resveratrol and TMZ in the treatment of human GBM. The additive cytotoxicity elicited by this combination leads to a significant induction of apoptosis, differentiation and inhibition of metastasis.
The precise mechanism, by which resveratrol and TMZ potentiate each other, is not totally understood. We find that in vitro, AMPK is responsible for the cytotoxicity‐induced in response to the TMZ treatment, and this is particularly true for the combination group. ROS can cause cell death. In our study, it was shown that the combination of TMZ and resveratrol‐induced cell apoptosis that was coupled with quick accumulation of ROS and the antioxidant NAC prevented the treatment‐induced decline in cell viability. Some studies had indicated that ROS could serve as a signaling molecule to activate AMPK, which inhibits cell proliferation and tumorigenesis. Accumulating evidence indicates that AMPK is a central metabolic switch found in all eukaryotes that governs glucose and lipid metabolism in response to alterations in nutrients and intracellular energy levels [29, 30, 31, 32]. Cancer cells have characteristic metabolic changes compared with normal cells and AMPK, being a key metabolic regulator, may regulate the switch. Recently, reports have indicated that AMPK may be a beneficial target for cancer treatment, and AMPK may inhibit tumorigenesis through regulation of cell growth, proliferation, autophagy, stress response, and cell polarity [33]. Therefore, we explored whether the combined treatment could trigger the AMPK activation. We found that TMZ combined resveratrol‐induced AMPK activation in SHG44 cells. Inhibition of AMPK by pharmacological (CC) partly blocked combination treatment‐induced antiproliferation and antiapoptosis effects. Thus, it is possible that AMPK might be involved in TMZ combined with resveratrol induced antitumor effects. Bring this to mind, resveratrol enhances the antitumor effects of TMZ in SHG44 cells might depend on ROS induced AMPK activation.
AMPK activation is involved in tumor cell death or inhibiting tumor cell growth by several mechanisms, among which, the most common one is activation of tuberous sclerosis complex 2 (TSC2), which then inhibits mTORC1 signaling and protein synthesis [34, 35]. Studies have placed TSC1/2 as a modulator between PI3K/Akt and mTOR. The regulation of TSC and mTOR by AMPK has special implications because the PI3K‐Akt signaling pathway is constitutively active in many cancers, including GBMs, which leads to inhibition of TSC, activation of mTOR signaling, and an increased in protein synthesis [36]. Here, we showed that TMZ and resveratrol treatment‐induced activation of AMPK inhibited mTOR activation in vitro. Knockout TSC reversed the inhibitory effects of the combined treatment on mTOR activation and cell proliferation. Based on these findings, we suggested that activation of AMPK by the TMZ combined resveratrol inhibited mTOR activation in a TSC‐dependent manner, which caused cell death/apoptosis probably by downregulating proapoptosis protein Bcl‐2 (Figure 6I).
mTOR, a highly conserved and ubiquitously expressed serine‐threonine kinase, has been intensely studied for over a decade as a central regulator of cell growth, proliferation, differentiation, and survival [37]. GFAP as a reliable marker of differentiated astrocytes is frequently lost with increasing grade of malignancy, suggesting that GFAP is important for maintaining glial cell morphology or regulating astrocytoma cell growth [19]. Inducing differentiation is one of the crucial therapeutic methods in cancer treatment. In our study, combination of TMZ and resveratrol could significantly upregulate the expression of GFAP, indicating that the combined treatment can induce cell differentiation.
Recent data also have shown that mTOR plays a critical role in the regulation of tumor cell motility and cancer metastasis [38]. The matrix metalloproteinases (MMPs), particularly MMP‐9, and the angiogenesis process, are attractive pharmaceutical targets for the treatment of cancer. The expression of MMP‐9 is associated with metastasis of various human cancers [39, 40]. Hiratsuka et al. [41] reports implicate MMP‐9 as a rate‐limiting extracellular protease involved in cell migration across basement membranes. Some reports also reveal that MMP‐9 is necessary for secondary malignant cell growth, dependent upon the presence and activation of the VEGF‐VEGFR signaling cascade [42, 43]. In this study, we found that the combination of TMZ and resveratrol significantly inhibited cell migration by the scratch assay and reduce the expression of MMP‐9. Thus, the combination of TMZ and resveratrol could inhibit cancer metastasis through decreasing in MMP‐9 expression, which might be due to regulating mTOR activation.
In summary, the present study found that resveratrol enhanced the antitumor effects of TMZ in GBM in vitro and in vivo, in which ROS‐dependent AMPK signal pathway was involved. Activation of AMPK contributes to cell apoptosis, probably by inhibiting mTOR signaling as well as altering cell cycle, cell differentiation and the expression level of apoptosis‐related protein Bcl‐2.
Conflicts of Interest
The authors declare no conflict of interest.
Supporting information
Figure S1: Combination of TMZ and resveratrol (RES)prevented cell migration.To examine the effect on the cellmigration in vitro scratch assay by either TMZ, RES or thecombination treatment during 0–72 h by Live Imaging.Representative videos of SHG44 cells were at 0 h and 48 h aftertreatment (when a wound was made) during 24 h, respectively. Themigration ability was estimated quantitatively by measuring theremaining area between the two edges of the wound.
Supporting info item
Acknowledgment
The study was supported by the grant from the Jiangsu Province Science Foundation of China (Z201006).
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group, Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 2. Louis DN, Holland EC, Cairncross JG. Glioma classification: A molecular reappraisal. Am J Pathol 2001;159:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uzzaman M, Keller G, Germano IM. Enhanced proapoptotic effects of tumor necrosis factor‐related apoptosis‐inducing ligand on temozolomide resistant glioma cells. J Neurosurg 2007;106:646–651. [DOI] [PubMed] [Google Scholar]
- 4. Tentori L, Graziani G. Recent approaches to improve the antitumor efficacy of Temozolomide. Curr Med Chem 2009;16:245–257. [DOI] [PubMed] [Google Scholar]
- 5. Gagliano N, Moscheni C, Torri C, et al Effect of resveratrol on matrix metalloproteinase‐2 (MMP‐2) and secreted protein acidic and rich in cysteine (SPARC) on human cultured glioblastoma cells. Biomed Pharmacother 2005;59:359–364. [DOI] [PubMed] [Google Scholar]
- 6. Boocock DJ, Faust GE, Patel KR, et al Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev 2007;16:1246–1252. [DOI] [PubMed] [Google Scholar]
- 7. Almeida L, Vaz‐da‐Silva M, Falcão A, et al Pharmacokinetic and safety profile of trans‐resvertral in a rising multiple‐dose study in healthy volunteers. Mol Nutr Food Res 2009;53(Suppl 1):S7–S15. [DOI] [PubMed] [Google Scholar]
- 8. Nunes T, Almeida L, Rocha JF, et al Pharmacokinetics of trans‐resveratrol following repeated administration in healthy elderly and young subjects. J Clin Pharmacol 2009;49:1477–1482. [DOI] [PubMed] [Google Scholar]
- 9. Jang M, Cai L, Udeani GO, et al Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997;275:218–220. [DOI] [PubMed] [Google Scholar]
- 10. Narayanan BA, Narayanan NK, Stoner GD, et al Interactive gene expression pattern in prostate cancer cells exposed to phenolic antioxidants. Life Sci 2002;70:1821–1839. [DOI] [PubMed] [Google Scholar]
- 11. Gupta SC, Kannappan R, Reuter S, et al Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci 2011;1215:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhardwaj A, Sethi G, Vadhan‐Raj S, et al Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down‐regulation of STAT3 and nuclear factor kappa B‐regulated anti‐apoptotic and cell survival gene products in human multiple myeloma cells. Blood 2007;109:2293–2302. [DOI] [PubMed] [Google Scholar]
- 13. Kubota T, Uemura Y, Kobayashi M, Taguchi H. Combined effects of resveratrol and paclitaxel on lung cancer cells. Anticancer Res 2003;23:4039–4046. [PubMed] [Google Scholar]
- 14. Nigg EA. Centrosome aberrations: Cause or consequence of cancer progression? Nat Rev Cancer 2002;2:815–825. [DOI] [PubMed] [Google Scholar]
- 15. Lee SC, Chan JY, Pervaiz S. Spontaneous and 5‐fluorouracil‐induced centrosome amplification lowers the threshold to resveratrol‐evoked apoptosis in colon cancer cells. Cancer Let 2010;288:6–41. [DOI] [PubMed] [Google Scholar]
- 16. Harikumar KB, Kunnumakkara AB, Sethi G, et al Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mousemodel of human pancreatic cancer. Int J Cancer 2010;127:257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lal S, Lacroix M, Tofilon P, et al An implantable guide screw system for brain tumor studies in small animals. J Neurosurg 2000;92:326–333. [DOI] [PubMed] [Google Scholar]
- 18. Brada M, Judson I, Beale P, et al Phase I dose‐escalation and pharmacokinetic study of temozolomide(SCH 52365) for refractory or relapsing malignancies. Br J Cancer 1999;81:1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kawamata H, Tachibana M, Fujimori T, Imai Y. Differentiation‐inducing therapy for solid tumors. Curr Pharm Des 2006;12:379–385. [DOI] [PubMed] [Google Scholar]
- 20. Kim DS, Woo ER, Chae SW, et al Plantainoside D protects adriamycin‐induced apoptosis in H9c2 cardiac muscle cells via the inhibition of ROS generation and NF‐kappaB activation. Life Sci 2007;80:314–323. [DOI] [PubMed] [Google Scholar]
- 21. Zhang JF, Liu JJ, Lu MQ, et al Rapamycin inhibits cell growth by induction of apoptosis on hepatocellular carcinoma cells in vitro. Transpl Immunol 2007;17:162–168. [DOI] [PubMed] [Google Scholar]
- 22. Kim WH, Lee JW, Suh YH, et al AICAR potentiates ROS production induced by chronic high glucose: Roles of AMPK in pancreatic beta‐cell apoptosis. Cell Signal 2007;19:791–805. [DOI] [PubMed] [Google Scholar]
- 23. Kopelovich L, Fay JR, Sigman CC, Crowell JA. The mammalian target of rapamycin pathway as a potential target for cancer chemoprevention. Cancer Epidemiol Biomarkers Prev 2007;16:1330–1340. [DOI] [PubMed] [Google Scholar]
- 24. Meric‐Bernstam F, Gonzalez‐Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol 2009;27:2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garami A, Zwartkruis FJ, Nobukuni T, et al Insulin activation of Rheb, a mediator of mTOR/S6K/4E‐BP signaling, is inhibited by TSC1 and 2. Mol Cell 2003;11:1457–1466. [DOI] [PubMed] [Google Scholar]
- 26. Guha A, Mukherjee J. Advances in the biology of astrocytomas. Curr Opin Neurol 2004;17:655–662. [DOI] [PubMed] [Google Scholar]
- 27. Zhang JF, Liu JJ, Lu MQ, et al Rapamycin inhibits cell growth by induction of apoptosis on hepatocellular carcinoma cells in vitro. Transpl Immunol 2007;17:162–168. [DOI] [PubMed] [Google Scholar]
- 28. Romano MF, Avellino R, Petrella A, et al Rapamycin inhibits doxorubicin‐induced NF‐kappaB/Rel nuclear activity and enhances the apoptosis of melanoma cells. Eur J Cancer 2004;40:2829–2836. [DOI] [PubMed] [Google Scholar]
- 29. Saha AK, Persons K, Safer JD, et al AMPK regulation of the growth of cultured human keratinocytes. Biochem Biophys Res Commun 2006;349:519–524. [DOI] [PubMed] [Google Scholar]
- 30. Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 2006;574:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu SY, Chan DW, Liu VW, Ngan HY. Inhibition of cervical cancer cell growth through activation of upstream kinases of AMP‐activated protein kinase. Tumour Biol 2009;30:80–85. [DOI] [PubMed] [Google Scholar]
- 32. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003;115:577–590. [DOI] [PubMed] [Google Scholar]
- 33. Wang W, Guan KL. AMP‐activated protein kinase and cancer. Acta Physiol (Oxf) 2009;196:55–63. [DOI] [PubMed] [Google Scholar]
- 34. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003;115:577–590. [DOI] [PubMed] [Google Scholar]
- 35. Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem 2004;279:15719–15722. [DOI] [PubMed] [Google Scholar]
- 36. Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3‐kinase/AKT pathway. Proc Natl Acad Sci U S A 1999;96:4240–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu SY, Chan DW, Liu VW, Ngan HY. Inhibition of cervical cancer cell growth through activation of upstream kinases of AMP‐activated protein kinase. Tumour Biol 2009;30:80–85 [DOI] [PubMed] [Google Scholar]
- 38. Zhou H, Huang S. mTOR signaling in cancer cell motility and tumor metastasis. Crit Rev Eukaryot Gene Expr 2010;20:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee PP, Hwang JJ, Murphy G, Ip MM. Functional significance of MMP‐9 in tumor necrosis factor‐induced proliferation and branching morphogenesis of mammary epithelial cells. Endocrinology 2000;14:3764–3773. [DOI] [PubMed] [Google Scholar]
- 40. Waas ET, Lomme RM, DeGroot J, et al Tissue levels of active matrix metalloproteinase‐2 and ‐9 in colorectal cancer. Br J Cancer 2002;86:1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hiratsuka S, Nakamura K, Iwai S, et al MMP9 induction by vascular endothelial growth factor receptor‐1 is involved in lung‐specific metastasis. Cancer Cell 2002;2:289–300. [DOI] [PubMed] [Google Scholar]
- 42. Bergers G, Brekken R, McMahon G, et al Matrix metalloproteinase‐9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000;2:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP‐9 supplied by bone marrow‐derived cells contributes to skin carcinogenesis. Cell 2000;103:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Combination of TMZ and resveratrol (RES)prevented cell migration.To examine the effect on the cellmigration in vitro scratch assay by either TMZ, RES or thecombination treatment during 0–72 h by Live Imaging.Representative videos of SHG44 cells were at 0 h and 48 h aftertreatment (when a wound was made) during 24 h, respectively. Themigration ability was estimated quantitatively by measuring theremaining area between the two edges of the wound.
Supporting info item


