Supplemental digital content is available in the text.
Key Words: BLOOD VOLUME, CO-REBREATHING, AEROBIC CAPACITY, ADOLESCENTS, MATURATION, TALENT IDENTIFICATION
ABSTRACT
Purpose
It is unknown, whether endurance training stimulates hemoglobin mass (Hbmass) and maximal oxygen uptake (V˙O2max) increases during late adolescence. Therefore, this study assessed the influence of endurance training on Hbmass, blood volume parameters, and V˙O2max in endurance athletes and control subjects from age 16 to 19 yr.
Methods
Hemoglobin mass, blood volume parameters, V˙O2max and anthropometric parameters were measured in male elite endurance athletes from age 16 to 19 yr in 6-month intervals (n = 10), as well as in age-matched male controls (n = 12).
Results
Neither the level of Hbmass per lean body mass (LBM) (P = 0.80) nor the development of Hbmass during the 3 yr (P = 0.97) differed between athletes and controls. Hbmass at age 16 yr was 13.24 ± 0.89 g·kg−1 LBM and increased by 0.74 ± 0.58 g·kg−1 LBM (P < 0.01) from age 16 to 19 yr. There was a high correlation between Hbmass at age 16 and 19 yr (r = 0.77; P < 0.001). Plasma volume, blood volume, and V˙O2max were higher in athletes compared to controls (P < 0.05). Blood volume and V˙O2max increased with age (P < 0.01, similarly in both groups).
Conclusions
Endurance training volumes do not explain individual differences in Hbmass levels nor Hbmass and V˙O2max development in the age period from 16 to 19 yr. The higher V˙O2max levels of athletes may be partially explained by training-induced higher plasma and blood volumes, as well as other training adaptations. Since Hbmass at age 16 yr varies substantially and the development of Hbmass in late adolescence is comparably small and not influenced by endurance training, Hbmass at age 16 yr is an important predictor for Hbmass at adult age and possibly for the aptitude for high-level endurance performance.
Total hemoglobin mass (Hbmass) and blood volume (BV) determine, to a large extent, the oxygen transport capacity of the blood and, consequently, maximal oxygen uptake (V˙O2max) (1). It is well known that elite adult endurance athletes are characterized by having up to ~40% higher levels of Hbmass and BV than untrained subjects (2–5), and there exists a strong relationship between Hbmass and V˙O2max (3,6) as well as between Hbmass and endurance performance (7) even in groups of highly trained endurance athletes. Although endurance training from 6 wk up to 9 months in untrained or moderately trained subjects commonly comprises a 5% to 10% increase in Hbmass (8–11), it seems that sea-level endurance training in highly trained adult endurance athletes exerts no (6,12) or only small (~3%) effects on Hbmass (13,14). Observed training effects in untrained subjects cannot explain the large differences in Hbmass between adult endurance athletes and sedentary subjects. Hence, the question whether the higher Hbmass level in adult endurance athletes is due to several years of endurance training from childhood and adolescence to adulthood, better genetic dispositions, or a combination of both, has yet to be determined.
Although the considerable influence of a good genetic disposition is supported by study results showing a high V˙O2max by virtue of a naturally high Hbmass in adults with no training history (15), the influence of several years of endurance training from adolescence to adulthood is still not entirely clear. From recent cross-sectional data (5,16–18), it can be concluded that endurance-trained children and adolescent endurance-trained athletes have ~15% to 35% lower Hbmass than adult athletes. There seems to be an increase of body weight-related Hbmass with maturation from 9.6 g·kg−1 in 9.7-yr-old children (17) to 10.6 g·kg−1 in 13.8-yr-old cyclists (19) to 12.0–12.4 g·kg−1 in endurance-trained male adolescents at age 16 yr (5,18). An upregulation of testosterone levels in boys during adolescence is a likely explanation of this training-independent increase, because there exists a close relationship between androgen levels and hemoglobin (Hb) concentration in puberty (20). Since Hbmass levels of male endurance athletes at age 16 yr are still lower than the measured 14.6 g·kg−1 in adult endurance athletes (5), it has been suggested that the age period from 16 to 20 yr is probably a sensitive phase to elevate Hbmass and, consequently, red blood cell volume (RBCV) with endurance training (5,18).
However, no data is available for the effects of endurance training on the evolution of Hbmass at this age. From existing investigations with younger endurance athletes, it can be concluded that endurance training had no “additional” effect on the evolution of Hbmass in adolescence. Eriksson (21) reported a 9% increase of absolute Hbmass in 11- to 13-yr-old boys after 16 wk of training, but the effects vanished when Hbmass was corrected for physical growth. Also, no effect of endurance training on the evolution of Hbmass was reported after 12 months of training in cyclists age 11 to 15 yr (16) or after 18 months of training in endurance-trained athletes age 15 to 17 yr (18).
In contrast to the abovementioned investigations, Prommer et al. (17) very recently showed an effect of the activity level in children on the development of Hbmass (7% increase after 2.5 yr with >4 h of training per week) independent of physical growth. However, these findings are, on the one hand, based on young (preadolescent) children and, on the other hand, are equivocal to the training effect, since a longitudinal control group was missing.
The aim of the present study was, therefore, to investigate the influence of endurance training on Hbmass, BV parameters, and V˙O2max in adolescent elite endurance athletes and age-matched non–endurance-trained control subjects from 16 to 19 yr of age.
MATERIALS AND METHODS
Subjects
Ten male adolescent endurance athletes (five XC-skiers and five triathletes) and a group of 12 age-matched, healthy, nonsmoking, and non–endurance-trained male subjects participated in the present study. As there are no Junior National Teams at age 16 yr in XC-skiing and triathlon, the inclusion criterion for athletes was a national top 15 overall ranking in either XC-skiing or triathlon in the season preceding the study period. A maximum of either 2 h endurance training per week or 3 h of team sports (disregarding school sport lessons) were set as upper limits for control subjects.
The study was approved by the Regional Ethic Committee in Berne, Switzerland (KEK-BE 019/08) and was carried out according to the recommendations of the Helsinki Declaration. All subjects and parents gave their written consent before any testing.
Study design
Hbmass, BV, V˙O2max, anthropometric characteristics, and several venous blood parameters were assessed in all subjects at seven time points in 6-month intervals, resulting in a monitoring phase of 3 yr. The two visits per year took place in May and November, with athletes starting at the beginning of the off-season period (XC-skiers in May and triathletes in November), whereas controls were all measured for the first time in May or June. Before the first visit, subjects were required to complete a questionnaire for the assessment of the training load in the last 3 months. Throughout the entire study, both athletes and control subjects completed training log books for the assessment of the weekly endurance training volumes, excluding school sport lessons. Subjects had neither conducted altitude training 3 months before any testing nor donated blood during the study period. Subjects were asked to avoid performing strenuous exercise within 24 h of the measurements. All tests were carried out in Magglingen (Switzerland) at an altitude of 950 m.
Determination of hemoglobin mass and BV parameters
Hbmass was measured and calculated using a slightly modified version of the optimized CO- rebreathing procedure by Schmidt and Prommer (22), as described in detail elsewhere (5). Briefly, a bolus of pure CO (CO doses were determined to be 1.2 mL·kg−1 for the athletes and 1.0 mL·kg−1 for the controls) was inhaled and capillary blood samples (35 μL) were taken from an earlobe and analyzed for percent carboxyhemoglobin (%HbCO) using a diode array spectrophotometer (ABL 800flex; Radiometer A/S, Copenhagen, Denmark) both before the inhalation of the CO bolus and at intervals of 6 and 8 min after. All CO rebreathing procedures were conducted by the same experienced investigator to avoid additional intertester variability. A typical error (TE) between 1.1% and 1.4% is observed in our laboratory from duplicate measurements of Hbmass with the method described (23).
RBCV, BV, and plasma volume (PV) were calculated from Hbmass using venous Hb concentration and venous hematocrit (Hct) (see Burge and Skinner for detailed description (24)):
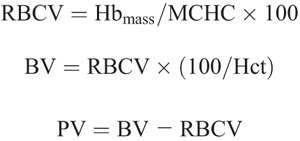
where MCHC is the mean corpuscular Hb concentration; Hct, hematocrit corrected to whole body Hct by the body/venous hematocrit ratio of 0.91.
Measurement of aerobic capacity (V˙O2max test)
The laboratory graded exercise tests to determine V˙O2max were conducted on a treadmill (Model Venus, h/p/cosmos Sports & Medical GmbH, Traunstein, Germany) with an incline set at 4° throughout the test and with continuous measurement of V˙O2 using a breath by breath open-circuit system (Oxycon Pro, Erich Jaeger GmbH, Hoechberg, Germany). After a 5-min warm-up jog, control subjects began running at 7 km·h−1, and athletes ran at 9 km·h−1. The speed was increased by 1 km·h−1 every minute for the first 3 min of the test, and thereafter, by 0.5 km·h−1 every 30 s until exhaustion. The V˙O2max protocols were designed to induce exhaustion in the subjects after 5 to 9 min. The criteria of a plateau in oxygen uptake, a RER value of ≥1.10, and a heart rate close to the age-predicted maximum were used to determine whether the subjects reached V˙O2max (25). V˙O2max was determined as the highest value averaged over 30 s. Heart rate was continuously registered with a Polar HR-monitoring system (Polar S610i; Polar Electro Oy, Kempele, Finland).
Venous blood sampling and analysis
Venous blood was sampled on the subjects’ arrival at the institute. After 15 min of rest in the supine position, two blood samples (4 mL for EDTA blood, 5 mL for blood serum) were drawn from the antecubital vein. Hemoglobin, Hct, and percent of reticulocytes were measured with automated hematology analyzers (ADVIA 120; Siemens Healthcare Diagnostics GmbH, Eschborn, Germany or Sysmex XE5000; Sysmex Corporation, Kobe, Japan). Soluble transferrin receptors were quantified with a biochemistry analyzer (Olympus AU 2700; Olympus Medical System Corporation, Tokyo, Japan). Serum erythropoietin (Immulite 2000; Siemens Healthcare Systems) and serum ferritin (Ftn) (ADVIA Centaur, Siemens Healthcare Systems) were measured with two different automated immunoassay systems.
Anthropometric measurements
Height, body mass, percent body fat, and lean body mass (LBM) of the subjects were assessed on all test days. Seven skinfold measurements (chest/pectoral, midaxillary, suprailiac, abdominal, triceps, subscapular, and thigh) were performed, and the percentage of body fat was calculated using the equations of Jackson and Pollock for body density (26) and, subsequently, the age-specific formulas of Heyward and Stolarczyk for percent body fat (27). All anthropometrical measurements were made by the same experienced investigator.
Assessment of biological age
The biological maturity status was estimated at the start of the study with a somatic method that compares the present stature with the projected adult stature (19).
Data analysis
All cardiovascular variables were scaled to LBM to account for general anthropometric growth in the observed age range and to correct for different amounts of body fat (17,28).
We performed linear mixed-effects analyses using the statistical programming language R (R 3.3.1, R Core Team, Vienna, Austria) with the lme4 package (29). We modeled the dependence of the anthropometric and cardiovascular parameters from the fixed effects age and group, with random effects for individual subjects (intercept and correlated slope for the effect of age). Models were constructed by sequentially adding fixed effects if justified by likelihood ratio tests (α < 0.05). Absolute and percent body fat as well as blood Ftn were log-transformed for homoscedasticity of the model residuals (assessed by visual inspections for all models).
Bootstrap sampling (n = 1000) was used to calculate confidence limits for the fixed effects. Linear regression was used to determine the Pearson product correlation between Hbmass at age 16 yr and Hbmass at age 19 yr. Values are reported as mean ± SD. The analysis code is available as Supplemental Digital Content (see File, Supplemental Digital Content 1, Data analysis code, http://links.lww.com/MSS/B475).
RESULTS
Hbmass, BV parameters, and V˙O2max
Hbmass did not differ between athletes and controls (P = 0.80; Table 1). The modeled intercept for Hbmass at age 16 yr was 13.24 ± 0.89 g·kg−1 LBM and Hbmass increased from age 16 to 19 yr by 0.74 ± 0.58 g·kg−1 LBM (P < 0.01; Figure 1). The development of Hbmass during the 3 yr did not differ between athletes and controls (P = 0.97). Hbmass at age 16 yr was highly correlated with Hbmass at age 19 yr (r = 0.77, P < 0.001). Plasma volume did not change with age (P = 0.85) but was higher in athletes compared to controls (P < 0.01). Blood volume and V˙O2max both increased from 16 to 19 yr of age in both athletes and controls (BV, 5.6 ± 2.9 mL·kg−1; LBM, P < 0.01; V˙O2max, 4.0 ± 2.4 mL·min−1·kg−1 LBM; P < 0.01) and were higher in athletes compared with controls (P = 0.02 and P < 0.01, respectively). There was no difference in the increase in BV (P = 0.09) and V˙O2max (P = 0.96) between the groups.
TABLE 1.
Hbmass, BV parameters, and V˙O2max.
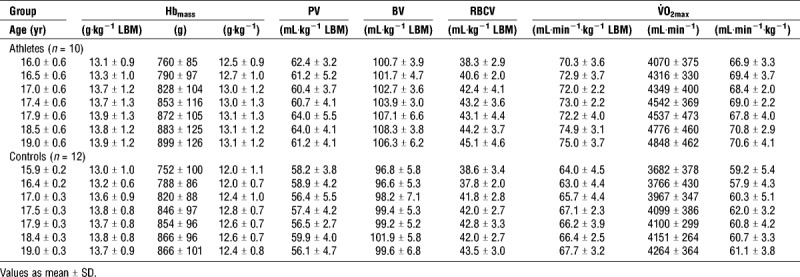
FIGURE 1.
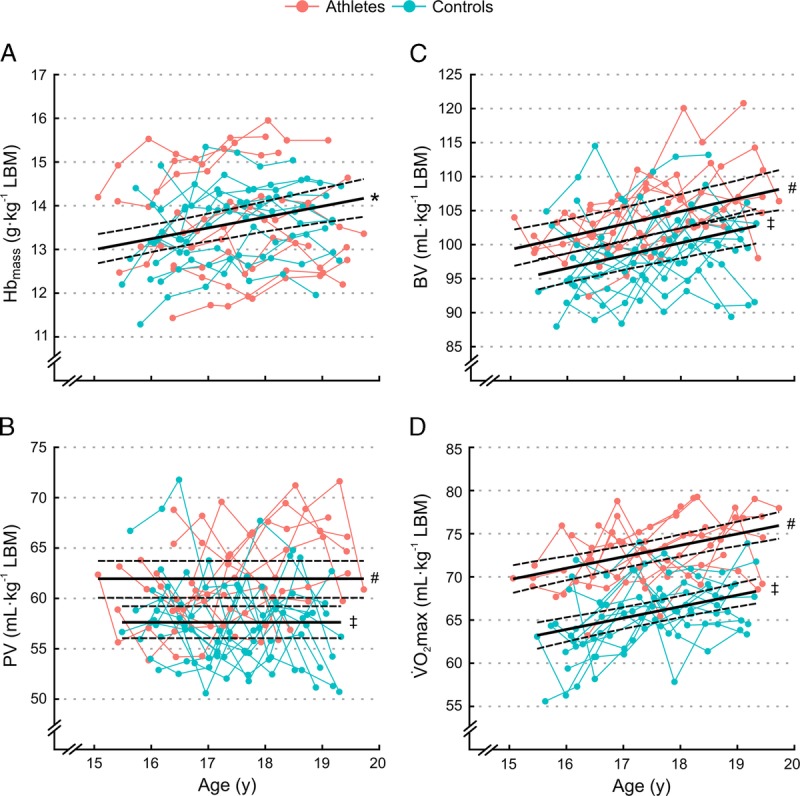
Individual development of (A) Hbmass, (B) PV, (C) BV, and (D) V˙O2max over the 3-yr study period for athletes (red) and controls (blue). Solid lines indicate the fixed effect of age with 90% confidence limits (dashed lines) for either both groups combined (*; combined fixed effect), or for athletes (#), and controls (‡) separately.
The ratio of V˙O2max to BV was 0.68 ± 0.05 min−1, constant over the analyzed age range (P = 0.83) and not different between groups (P = 0.07). By contrast, the ratio of V˙O2max to Hbmass stayed constant with aging (P = 0.68) but was higher in athletes compared to controls (5.4 ± 0.4 mL·min−1·g−1 and 4.9 ± 0.4 mL·min−1·g−1 respectively, P < 0.01), whereas the ratio of V˙O2max to PV increased with age (P < 0.01) but did not differ between groups (P = 0.27).
Peak velocity in the V˙O2max test was higher (P < 0.01) in athletes (15.6 ± 0.5 km·h−1) than controls (13.0 ± 0.8 km·h−1) and did not increase with age (P = 0.67).
Blood parameters
Hb was lower (P = 0.02) in athletes (14.4 ± 1.1 g·dL−1) than controls (15.1 ± 0.7 g·dL−1) at all testing sessions and did not change in both groups over the study period (P = 0.93). Hct was similar between groups (at age 16 yr, athletes: 43.1% ± 3.1%, controls: 44.0% ± 1.1%, P = 0.33) and increased similarly (P = 0.52) in both groups from age 16 to 19 yr by 3.6% ± 1.1% (P < 0.01).
Further, there were no differences between groups in percent reticulocytes (athletes: 0.8% ± 0.3%, controls: 0.8% ± 0.3%, P = 0.95), serum erythropoietin (athletes: 10.9 ± 2.9 U·L−1, controls: 10.6 ± 3.4 U·L−1, P = 0.59), soluble transferrin receptor (athletes: 7.6 ± 1.2 nmol·L−1, controls: 7.4 ± 1.5 nmol·L−1, P = 0.27), or Ftn (athletes, 60 ± 30 μg·L−1; controls, 47 ± 23 μg·L−1, P = 0.25).
Anthropometric characteristics and endurance training volume
Body mass (P = 0.57), LBM (P = 0.89) and height (P = 0.95) did not differ between groups at all testing sessions, but athletes exhibited a lower absolute (P = 0.02) and percent body fat (P < 0.01; Table 2). All anthropometric characteristics increased over the observed age range (all P < 0.01), but the development of all parameters was not different between athletes and controls (all P > 0.38). Further, biological age at the study’s start did not differ between groups (P = 0.63).
TABLE 2.
Anthropometric characteristics and training volume.
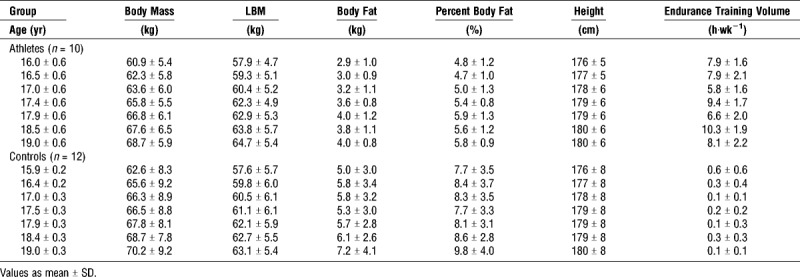
Athletes conducted a higher volume of endurance training than the control group (P < 0.01) and increased the training volume over the observed age range (P = 0.01; Table 2).
DISCUSSION
This study was the first to assess the influence of endurance training on Hbmass and V˙O2max development in adolescent male endurance athletes from age 16 to 19 yr. The major findings of the present study were: 1) Hbmass development was independent from endurance training volume in the age period from 16 to 19 yr in male adolescents; 2) interindividual Hbmass levels at age 16 yr varied substantially more than the individual development of Hbmass over the study period, which suggests Hbmass level at age 16 yr is an important predictor for Hbmass in adulthood; 3) athletes had higher training-induced PV, BV, and V˙O2max than controls; 4) V˙O2max development in the 3-yr study period was independent from training volume.
Hb mass
During the 3-yr study period, absolute Hbmass increased by 18% in athletes and by 15% in controls. When scaling to LBM to account for general anthropometric growth in the observed age range (17) and to correct for different amounts of body fat, relative Hbmass increased in both groups by 6% (0.74 g·kg−1 LBM). Surprisingly, the amount of endurance training influenced neither the initial Hbmass level at age 16 yr nor the development of Hbmass during the study period.
The available data demonstrate that endurance training in late adolescence does not yield the additional stimulating effects on Hbmass that have been proposed for this age (5,17,18). Based on our mixed model, an increase of relative Hbmass from ages 16 to 19 yr can be expected for 90% of the subjects, irrespective of training volume. Increases up to 1.90 g·kg−1 LBM (average increase +2 SD) are possible, but on average, 0.74 g·kg−1 LBM can be expected. In other words, at age 16 yr, adolescent male athletes should already have an Hbmass of more than 14 g·kg−1 LBM to possibly reach levels at adult age of about 15.4 g·kg−1 LBM measured with the same methodology in adult elite endurance athletes (5). Hbmass at age 19 yr was highly correlated with Hbmass at age 16 yr and approximately three quarters of the variance of Hbmass level at age 19 yr can be explained by the initial Hbmass at age 16 yr. Hence, in contrast to our assumption from a cross-sectional study (5), Hbmass at age 16 yr seems to have an important predictive value for Hbmass at the end of adolescence, and due to the stability of Hbmass with sea-level endurance training in adult elite endurance athletes (6,12), also for a high Hbmass at adult age. Consequently, because Hbmass is strongly related to V˙O2max (3,5,6,11,15) as well as to endurance performance (7) in elite athletes, Hbmass at age 16 yr is one possible candidate to estimate the aptitude for high-level endurance sports in adulthood. This is in line with the hypothesis of the prognostic value of Hbmass for talent identification in younger athletes (16,17).
Compared with earlier studies utilizing identical or closely related methods with younger adolescent endurance athletes, our results differ on two points. First, previously reported relative Hbmass levels for adolescent endurance athletes were 10% to 15% higher than in control subjects or nonendurance athletes (16,18). However, higher levels of Hbmass for the endurance athletes in these investigations can most likely be explained by highly unbalanced proportions of male and female subjects, which makes the results prone to misinterpretation. Second, studies investigating the influence of endurance training on Hbmass in adolescent endurance athletes (16,18,21) did not find significant increases in Hbmass beyond the alterations explained by growth and maturation with endurance training. Only one study with preadolescent subjects reported a training effect of up to 7% on Hbmass over 2.5 yr not caused by normal growth mechanisms (17). Unfortunately, no control group was included, and with the results of the present study in mind (6% increase of Hbmass without endurance training over 3 yr), it can be hypothesized that nonactive subjects would have shown a similar increase in Hbmass.
The mechanisms of individually different Hbmass development remain unknown. We cannot determine the degree to which factors relating to erythropoiesis in adolescents and adults—such as varying levels of human growth hormone, insulin-like growth factors, or testosterone (20,30,31)—influenced Hbmass, as we did not conduct any of these measurements. It is assumed that these factors could have influenced initial level variations at age 16 yr, as well as the development of Hbmass during the study period. At the very least, the average increase of relative Hbmass as an effect of aging could be attributed to higher testosterone and human growth hormone levels in this late phase of adolescence, as no relative increase has been observed with younger athletes and control subjects (16,18,21).
Blood volume parameters, and V˙O2max
Results suggest that the main cardiovascular adaptation to endurance training before reaching 16 yr of age and persisting up to 19 yr is an increased PV, and consequently BV. Blood volume increases further from age 16 to 19 yr, irrespective of training volume, due to the increase in Hbmass and RBCV. It has been reported that higher BV and PV reduce cardiovascular and thermoregulatory strain and increase the buffering capacity of the blood, while a higher BV increases venous return and cardiac output (32) and thereby V˙O2max (10,33). These adaptations most likely have a positive effect on endurance performance, as athletes were not endowed with higher levels of Hbmass than their age-matched counterparts but reached significantly higher V˙O2max levels and a higher endurance performance (final speed attained in the performance test on the treadmill). An increased BV due to PV expansion is also typically observed in routine measurements in adult athletes between the off-season and the competition season, indicating a higher endurance performance capacity during the competition season (unpublished results from our laboratory).
Because high V˙O2max levels are not only dependent on cardiac output and the oxygen carrying capacity of the blood (i.e., Hbmass) but also on factors like mitochondrial density and capillarization of the muscles (34), it should be obvious that endurance training could have influenced these parameters, and hence, V˙O2max and endurance performance, without recognition in BV parameters and Hbmass.
However, the increases in absolute (athletes, +19%; controls, +16%) and relative V˙O2max (both about 6%) correspond very well with the development of Hbmass and BV but not with that of PV. This suggests that the V˙O2max increase during the 3-yr studied was based at least in part on the higher Hbmass (and hence, BV). This fact is supported by constant ratios for V˙O2max to Hbmass and V˙O2max to BV over the analyzed age range, while the V˙O2max to PV ratio increased with age. The similar increase in relative V˙O2max for athletes and control subjects over the study period surprised us. To our knowledge, this is the first controlled study to show this aspect in adolescents age 16 to 19 yr.
It is suggested that up to 50% of V˙O2max is genetically and familial nongenetically inherited (35,36). Besides these genetically predisposed higher values, the trainability of V˙O2max is also regarded as dependent on yet undetermined inherited characteristics (37,38). These two factors do not seem to be necessarily related, and therefore, it is hypothesized that both a phenotype that is superior with respect to aerobic power and one that is superior with respect to response to endurance training exist (39). Although no detailed genomic signature has been found so far that differentiates endurance athletes from sedentary subjects (40), it can be hypothesized, that an optimal gene–training (environment) interaction plays a preponderant role in the development of high-level endurance athletes. Due to our findings, it can be assumed that a similar model of diverse phenotypes could also be applicable to Hbmass levels and development. On the one hand, Hbmass levels varied substantially among control subjects with low volumes of training, indicating considerably different Hbmass “starting” values, and on the other hand, the development of Hbmass appears to be highly variable, irrespective of subjects’ training volumes.
Strengths and Limitations of the Study
The adolescent athletes were selected on the grounds of competition results at age 16 yr, where the lack of a strong physiological predisposition could be partly compensated by high technical skills, a higher training volume, and a longer training history. In elite athletes, a lack of physiological talent is very unlikely due to the rigorous selection process from adolescent to adult athletes. Consequently, results are usually reported only for subjects with an extremely high aerobic capacity and a high endurance performance. Accordingly, lower Hbmass values of the adolescent athletes compared to Hbmass levels of elite athletes (3,5) could already be explained by selection bias. A replication of the measurements of junior athletes from sports with lower technical demands (e.g., runners), and hence, a higher relevance of physiological characteristics on endurance performance, may have yielded different results.
Although the endurance training volumes of the athletes appear rather low at first glance, it must be considered that only the volume of specific endurance training is reported to focus on the influence of endurance exercise on Hbmass development. Moreover, endurance training volumes are similar to levels found in the study by Ulrich et al. (18) for comparable sports (distance running, canoeing) but considerably higher than in the cyclists (5.9 h·wk−1) from the study of Eastwood et al. (16). We are aware that, for example, swimmers train significantly more (>20 h·wk−1) in the same age spectrum. The chance to discover a training-induced erythropoietic stimulation as reported for untrained subjects (9) might have been greater for such athletes.
Moreover, we were surprised by the relatively high Hbmass and V˙O2max levels of the control subjects. However, it was not a prerequisite that the control subjects were completely untrained. They were allowed to conduct leisure activities; nevertheless, they have fulfilled the inclusion criteria of no more than 2 h of regular endurance training per week. Further, they should have met the characteristics of the athletes (age, low to moderate percentage of body fat) to the extent possible, and been able to complete a maximal exercise test on a treadmill. Therefore, we are convinced that because of our homogenous and very well-matched control group (same biological age at the study’s start and same development of body mass, height, and LBM over the analyzed age range as the athletes), the influence of additional extensive endurance training (in addition to leisure activity) on Hbmass could have been revealed. In addition, similar V˙O2max levels (~58 mL·kg−1·min−1) were observed by Åstrand in untrained adolescent subjects up to 18 yr of age with a similar treadmill protocol (41).
CONCLUSIONS
Our results indicate that endurance training seems to have no additional effect on changes in Hbmass over a 3-yr training period from ages 16 to 19 yr. A combination of high baseline Hbmass values, as a result of optimal gene–environment interaction, and an inherent endowment to increase Hbmass in late adolescence are assumed to be important factors to reach levels of Hbmass as high as those observed in top endurance athletes. Hbmass at age 16 yr seems to be an important predictor for Hbmass in adulthood and consequently one puzzle piece for the aptitude for high-level endurance sports at adult age. Although higher PV and BV induced through training lead to higher V˙O2max levels in athletes, longitudinal results indicate no additional effect of endurance training on the development of V˙O2max from 16 to 19 yr. Which mechanisms lead to an overall increase of relative Hbmass in late adolescence and individual differences in the development of Hbmass at that age still needs to be examined.
Supplementary Material
Acknowledgments
This study was financially supported in part by the Federal Council of Sports, Switzerland.
We thank Prof. Bernard Marti for his vital advice and continuous support. Furthermore, the laboratory assistance of Franziska Gyger and Elisabeth Probst, as well as the skillful technical assistance of Nicole Naef and Beat Müller, are gratefully acknowledged.
The authors declare that they have no conflict of interest. The authors herewith state that the results of the present study do not constitute endorsement by the American College of Sports Medicine and are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
REFERENCES
- 1.Schmidt W, Prommer N. Effects of various training modalities on blood volume. Scand J Med Sci Sports. 2008;18(1 Suppl):57–69. [DOI] [PubMed] [Google Scholar]
- 2.Green HJ, Carter S, Grant S, et al. Vascular volumes and hematology in male and female runners and cyclists. Eur J Appl Physiol. 1999;79:244–50. [DOI] [PubMed] [Google Scholar]
- 3.Heinicke K, Wolfarth B, Winchenbach P, et al. Blood volume and hemoglobin mass in elite athletes of different disciplines. Int J Sports Med. 2001;22(7):504–12. [DOI] [PubMed] [Google Scholar]
- 4.Kjellberg SR, Rudhe U, Sjostrand T. Increase in the amount of Hb and blood volume in connection with physical training. Acta Physiol Scand. 1949;19:147–51. [Google Scholar]
- 5.Steiner T, Wehrlin JP. Does hemoglobin mass increase from age 16 to 21 and 28 in elite endurance athletes? Med Sci Sports Exerc. 2011;43(9):1735–43. [DOI] [PubMed] [Google Scholar]
- 6.Gore CJ, Hahn AG, Burge CM, Telford RD. VO2max and haemoglobin mass of trained athletes during high intensity training. Int J Sports Med. 1997;18(6):477–82. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs RA, Rasmussen P, Siebenmann C, et al. Determinants of time trial performance and maximal incremental exercise in highly trained endurance athletes. J Appl Physiol. 2011;111(5):1422–30. [DOI] [PubMed] [Google Scholar]
- 8.Bonne TC, Doucende G, Fluck D, et al. Phlebotomy eliminates the maximal cardiac output response to six weeks of exercise training. Am J Physiol Regul Integr Comp Physiol. 2014;306(10):R752–60. [DOI] [PubMed] [Google Scholar]
- 9.Montero D, Breenfeldt-Andersen A, Oberholzer L, et al. Erythropoiesis with endurance training: dynamics and mechanisms. Am J Physiol Regul Integr Comp Physiol. 2017;312(6):R894–902. [DOI] [PubMed] [Google Scholar]
- 10.Sawka MN, Convertino VA, Eichner ER, Schnieder SM, Young AJ. Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc. 2000;32(2):332–48. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt W, Prommer N. Impact of alterations in total hemoglobin mass on VO 2max. Exerc Sport Sci Rev. 2010;38(2):68–75. [DOI] [PubMed] [Google Scholar]
- 12.Prommer N, Sottas PE, Schoch C, Schumacher YO, Schmidt W. Total hemoglobin mass—a new parameter to detect blood doping? Med Sci Sports Exerc. 2008;40(12):2112–8. [DOI] [PubMed] [Google Scholar]
- 13.Garvican LA, Martin DT, McDonald W, Gore CJ. Seasonal variation of haemoglobin mass in internationally competitive female road cyclists. Eur J Appl Physiol. 2010;109(2):221–31. [DOI] [PubMed] [Google Scholar]
- 14.Eastwood A, Sharpe K, Bourdon PC, et al. Within-subject variation in hemoglobin mass in elite athletes. Scand J Med Sci Sports. 2012;44(4):725–32. [DOI] [PubMed] [Google Scholar]
- 15.Martino M, Gledhill N, Jamnik V. High VO2max with no history of training is primarily due to high blood volume. Med Sci Sports Exerc. 2002;34(6):966–71. [DOI] [PubMed] [Google Scholar]
- 16.Eastwood A, Bourdon PC, Withers RT, Gore CJ. Longitudinal changes in haemoglobin mass and VO(2max) in adolescents. Eur J Appl Physiol. 2009;105(5):715–21. [DOI] [PubMed] [Google Scholar]
- 17.Prommer N, Wachsmuth N, Thieme I, et al. Influence of endurance training during childhood on total hemoglobin mass. Front Physiol. 2018;9:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulrich G, Bartsch P, Friedmann-Bette B. Total haemoglobin mass and red blood cell profile in endurance-trained and non-endurance-trained adolescent athletes. Eur J Appl Physiol. 2011;111(11):2855–64. [DOI] [PubMed] [Google Scholar]
- 19.Beunen GP, Malina RM, Lefevre J, Claessens AL, Renson R, Simons J. Prediction of adult stature and noninvasive assessment of biological maturation. Med Sci Sports Exerc. 1997;29(2):225–30. [DOI] [PubMed] [Google Scholar]
- 20.Hero M, Wickman S, Hanhijarvi R, Siimes MA, Dunkel L. Pubertal upregulation of erythropoiesis in boys is determined primarily by androgen. J Pediatr. 2005;146(2):245–52. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson BO. Physical training, oxygen supply and muscle metabolism in 11-13-year old boys. Acta Physiol Scand Suppl. 1972;384:1–48. [PubMed] [Google Scholar]
- 22.Schmidt W, Prommer N. The optimised CO-rebreathing method: a new tool to determine total haemoglobin mass routinely. Eur J Appl Physiol. 2005;95(5–6):486–95. [DOI] [PubMed] [Google Scholar]
- 23.Naef N, Steiner T, Wehrlin JP. Replicate measurements of haemoglobin mass during a single day are feasible and precise. Int J Sports Med. 2015;36(5):e19–23. [DOI] [PubMed] [Google Scholar]
- 24.Burge CM, Skinner SL. Determination of hemoglobin mass and blood volume with CO: evaluation and application of a method. J Appl Physiol. 1995;79(2):623–31. [DOI] [PubMed] [Google Scholar]
- 25.Howley ET, Bassett DR, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27(9):1292–301. [PubMed] [Google Scholar]
- 26.Jackson AS, Pollock ML. Practical assessment of body composition. Phys Sportsmed. 1985;13(5):76–90. [DOI] [PubMed] [Google Scholar]
- 27.Heyward VH, Stolarczyk LM. Applied Body Composition Assessment. Champaign (IL): Human Kinetics; 1996. pp. 183–4. [Google Scholar]
- 28.Schumacher YO, Ahlgrim C, Pottgiesser T. Evaluation of anthropometrical reference parameters for hemoglobin mass in endurance athletes. J Sports Med Phys Fitness. 2008;48(4):509–14. [PubMed] [Google Scholar]
- 29.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Software. 2015;67(1):1–48. [Google Scholar]
- 30.Anttila R, Koistinen R, Seppala M, Koistinen H, Siimes MA. Insulin-like growth factor I and insulin-like growth factor binding protein 3 as determinants of blood hemoglobin concentration in healthy subjects. Pediatr Res. 1994;36(6):745–8. [DOI] [PubMed] [Google Scholar]
- 31.Christ ER, Cummings MH, Westwood NB, et al. The importance of growth hormone in the regulation of erythropoiesis, red cell mass, and plasma volume in adults with growth hormone deficiency. J Clin Endocrinol Metab. 1997;82(9):2985–90. [DOI] [PubMed] [Google Scholar]
- 32.Convertino VA. Blood volume response to physical activity and inactivity. Am J Med Sci. 2007;334(1):72–9. [DOI] [PubMed] [Google Scholar]
- 33.Lundby C, Montero D, Joyner M. Biology of VO2 max: looking under the physiology lamp. Acta Physiol (Oxf). 2017;220(2):218–28. [DOI] [PubMed] [Google Scholar]
- 34.Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32(1):70–84. [DOI] [PubMed] [Google Scholar]
- 35.Bouchard C, Daw EW, Rice T, et al. Familial resemblance for VO2max in the sedentary state: the HERITAGE family study. Med Sci Sports Exerc. 1998;30(2):252–8. [DOI] [PubMed] [Google Scholar]
- 36.Fagard R, Bielen E, Amery A. Heritability of aerobic power and anaerobic energy generation during exercise. J Appl Physiol. 1991;70(1):357–62. [DOI] [PubMed] [Google Scholar]
- 37.Bouchard C, An P, Rice T, et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE family study. J Appl Physiol. 1999;87(3):1003–8. [DOI] [PubMed] [Google Scholar]
- 38.Weber G, Kartodihardjo W, Klissouras V. Growth and physical training with reference to heredity. J Appl Physiol. 1976;40(2):211–5. [DOI] [PubMed] [Google Scholar]
- 39.Danis A, Kyriazis Y, Klissouras V. The effect of training in male prepubertal and pubertal monozygotic twins. Eur J Appl Physiol. 2003;89(3–4):309–18. [DOI] [PubMed] [Google Scholar]
- 40.Rankinen T, Fuku N, Wolfarth B, et al. No evidence of a common DNA variant profile specific to world class endurance athletes. PLoS One. 2016;11(1):e0147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Åstrand PO. Experimental Studies of Physical Working Capacity in Relation to Sex and Age. Kopenhagen: Munksgaard; 1952. pp. 23–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


