Significance Statement
Patients with type 2 diabetes often exhibit salt-sensitive hypertension, but the mechanisms underlying this association remain unclear. One potential link, Kelch-like 3 (KLHL3), regulates BP by targeting the serine/threonine kinases with-no-lysines (WNKs) for degradation, thereby modulating activity of the NaCl cotransporter (NCC). The authors previously found that phosphorylation at serine 433 (S433) in KLHL3 downregulates KLHL3 activity. In this study, they show that protein kinase C–mediated phosphorylation of KLHL3 at S433 inactivates KLHL3 and increases NCC activity in db/db diabetic mice, and they show that an inhibitor of a sodium glucose cotransporter 2 (SGLT2), ipragliflozin, ameliorates this effect. These data indicate a previously unrecognized pathway by which dysregulated glucose metabolism stimulates renal salt reabsorption and provide insights into the mechanism for SGLT2 inhibitors’ cardiorenal protective effect.
Keywords: hyperglycemia, Cell Signaling, diabetes mellitus, distal tubule, Na transport
Visual Abstract
Abstract
Background
Mechanisms underlying the frequent association between salt-sensitive hypertension and type 2 diabetes remain obscure. We previously found that protein kinase C (PKC) activation phosphorylates Kelch-like 3 (KLHL3), an E3 ubiquitin ligase component, at serine 433. We investigated whether impaired KLHL3 activity results in increased renal salt reabsorption via NaCl cotransporter (NCC).
Methods
We used the db/db diabetes mouse model to explore KLHL3′s role in renal salt handling in type 2 diabetes and evaluated mechanisms of KLHL3 dysregulation in cultured cells.
Results
We observed PKC activity in the db/db mouse kidney and phosphorylation of serine 433 in KLHL3 (KLHL3S433-P). This modification prevents binding of with-no-lysine (WNK) kinases; however, total KLHL3 levels were decreased, indicating severely impaired KLHL3 activity. This resulted in WNK accumulation, activating NCC in distal convoluted tubules. Ipragliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, lowered PKC activity in distal convoluted tubule cells and reduced KLHL3S433-P and NCC levels, whereas the thiazolidinedione pioglitazone did not, although the two agents similarly reduced in blood glucose levels. We found that, in human embryonic kidney cells expressing KLHL3 and distal convoluted tubule cells, cellular glucose accumulation increased KLHL3S433-P levels through PKC. Finally, the effect of PKC inhibition in the kidney of db/db mice confirmed PKC’s causal role in KLHL3S433-P and NCC induction.
Conclusions
Dysregulation of KLHL3 is involved in the pathophysiology of type 2 diabetes. These data offer a rationale for use of thiazide in individuals with diabetes and provide insights into the mechanism for cardiorenal protective effects of SGLT2 inhibitors.
Hypertension is one of the important complications in obese diabetic subjects1,2; however, the mechanisms are not fully elucidated. Because a substantial portion of patients with type 2 diabetes mellitus (DM) show hypertension before the onset of albuminuria or decreased GFR,3 the coexistence of hypertension is not solely attributed to the progression of diabetic kidney disease. To elucidate the associated mechanisms, several humoral factors, such as insulin, have been extensively studied.4,5 Indeed, experimental data indicated that insulin together with glucose administration can increase salt reabsorption by modulating Na+ and Cl− transport mechanisms in the kidney.6–9 However, euglycemic hyperinsulinemia does not result in sustained salt retention in humans10,11 or experimental models,12 suggesting the contribution of additional mechanisms. Of note, accumulating data indicated that hyperglycemia is necessary for the antinatriuretic action of insulin.5,13 Nonetheless, the detailed biochemical pathways, whereby the altered glucose metabolism potentiates renal tubular sodium reabsorption, remain unclear. Given the clinical evidence that sodium glucose cotransporter 2 (SGLT2) inhibitors can ameliorate hypertension in type 2 DM,14,15 the pathophysiology of BP elevation in these patients has attracted increasing attention.
NaCl cotransporter (NCC) in the distal convoluted tubule is one of the key mechanisms that tightly regulates BP through controlling renal salt reabsorption.16 The discoveries of causative genes for pseudohypoaldosteronism type II (PHAII; also known as familial hypertensive hyperkalemia or Gordon syndrome; OMIM no. 145260)17–19 over the past decades led to the identification of previously unrecognized pathways that critically regulate NCC activity.20–23 Kelch-like 3 (KLHL3) and cullin-3 are the elements of a really interesting new gene E3 ubiquitin ligase complex that targets the serine/threonine kinases with-no-lysine 1 (WNK1) and WNK4 for degradation.24–26 These kinases, when activated by decreased intracellular Cl− levels,27,28 directly phosphorylate the downstream kinases STE20/SPS1-related proline-alanine–rich protein kinase (SPAK) and oxidative stress–responsive 1 (OSR1).29 SPAK and OSR1, in turn, phosphorylate and activate NCC.30 In PHAII, missense mutations in the Kelch domain of KLHL3 and in an acidic domain of WNK4, both abrogate the binding of KLHL3 and WNK4, resulting in NCC activation via SPAK/OSR1.24–26
Previously, we identified that the phosphorylation of serine 433 (S433) in the Kelch domain (KLHL3S433-P) critically regulates the activity of the ubiquitin ligase composed of KLHL3. Protein kinase C (PKC) directly phosphorylates KLHL3S433, thereby preventing binding and degradation of WNK kinases.31 This pathway is activated by angiotensin II (AngII) through AngII type 1 receptor.31 Given that S433 is frequently mutated in PHAII,18,19 these data indicate that KLHL3 inactivation by either phosphorylation or single-amino acid substitution is sufficient to exert a major effect on BP. Nonetheless, the pathogenic role of KLHL3S433-P in a common disease state is less well understood. In this study, we postulated that the impaired KLHL3 activity results in increased renal salt reabsorption via NCC in a model of obesity and type 2 DM.
Methods
Animals
This study was approved by the institutional review board of the Teikyo University Review Board #15–053. db/db mice at 10–14 weeks of age and the control db/+ mice at the same age were obtained from CREA. They were fed ad libitum and housed under a 12-hour light cycle. For some experiments, ipragliflozin was administered to db/db and db/+ mice (provided by Astellas Pharma; 15 mg/kg in a chow). As a control treatment, we chose pioglitazone (Wako; 100 mg/kg chow). Pioglitazone has been shown to effectively reduce blood glucose in this model.32 Where indicated, hydrochlorothiazide (Sigma-Aldrich) was given intraperitoneally at a dose of 25 mg/kg after 7 days of treatment with ipragliflozin or pioglitazone. Urine was collected by using individual metabolic cages, and urinary Na+ levels were directly compared between pre- and post-treatment samples in each mouse. Insulin was administered subcutaneously via osmotic minipump (0.5 U/d) for 7 days. The dosage was determined according to the previous studies on hyperglycemic mice.33 The PKC inhibitor bisindolylmaleimide I hydrochloride (BIM; 4 μg) or vehicle (2.24% DMSO in PBS) was administered intraperitoneally under isoflurane anesthesia once daily for 2 days, and kidneys were removed 3 hours after the injection on day 2. For urinalysis, we injected the same dose of BIM once and collected urine immediately after the injection for 6 hours. The dose of BIM was determined on the basis of the previous report34 and our preliminary dose-finding study; we tested two different doses of BIM (1 and 4 μg/d intraperitoneally for 2 days) and found that the latter efficiently reduced KLHL3S433-P levels in the kidney without apparent toxicity. Candesartan was given orally for 4 weeks (0.005% in a chow).35 Blood samples were collected, and kidneys were removed under isoflurane anesthesia. Blood glucose levels and K+ levels were measured using the iSTAT system (Abbott). Blood glucose >700 mg/dl (which is the upper limit of the measuring range) was regarded as 700 mg/dl. Urine electrolytes were measured by the ion selective electrodes method (SRL).
Western Blot
Western blotting was performed as described previously.31,36 Plasma membrane fraction was purified using the plasma membrane isolation kit (Invent Biotechnologies).37 Enrichment of plasma proteins but not cytoplasmic proteins was validated in the laboratory.38 For total cell lysates, kidneys were homogenized with TNE buffer, except for the detection of WNK1 and WNK4 (for these proteins, lysis buffer containing 250 mM sucrose and 10 mM triethanolamine was used). After the protein quantification, equal amounts of protein were mixed with Laemmli sample buffer. Tubulin and Ponceau S staining was used to monitor the identical loading of different samples. For the Western blotting analysis of anti-KLHL3S433-P, we used mouse monoclonal anti-KLHL3S433-P created and characterized in the laboratory as described previously (in the work by Ishizawa et al.22). Serine mutation at position 433 in KLHL3 completely eliminates the signal in human embryonic kidney (HEK) cells expressing KLHL3, confirming the specificity of the antibody (Supplemental Figure 1 also shows the full-length image of the Western blot analysis using anti-KLHL3S433-P antibody in vivo in the kidney). Because S433 and the surrounding sequences are conserved in KLHL2, the phosphoantibody in theory can also recognize KLHL2 if it is phosphorylated at the corresponding serine. Anti-KLHL3 antibody (Sigma-Aldrich) was characterized as previously described,31 and it was further validated in this study. The antibody was produced using amino acids 2–51 of human KLHL3 as an immunogen, and the sequence identity in this region between KLHL2 and KLHL3 is <50% (Supplemental Figure 2A). In addition, this antibody recognized a strong signal in the cortex but not in the medulla (where KLHL2 is predominantly present),39 confirming that the antibody does not recognize KLHL2 (Supplemental Figure 2B). Other antibodies included anti-Flag (Sigma-Aldrich), anti-tubulin (Sigma-Aldrich), anti-WNK4,22 anti-WNK1 (Cell Signaling Technology), anti-phospho-SPAK/OSR1 (Millipore),40 anti-SPAK (Abcam), anti-NCC,31 anti-NCC phosphorylated at Thr53 (NCCT53-P),41 anti-phospho-PKC (bearing phosphorylation at Ser660 in PKCβII; the antibody also detects phosphorylated PKCα, -βI, -δ, -ε, -η, and -θ; Cell Signaling Technology; we previously confirmed that PKC activation sharply increases the signal detected by this antibody),22 anti-phospho-PKCα/β (bearing phosphorylation at Thr638/641), anti-total PKC, anti-phospho-Akt, and anti-Akt (Cell Signaling Technology).
Immunostaining
Immunofluorescence study was performed as described previously.31,36 For immunostaining of KLHL3S433-P, we used polyclonal rabbit anti-KLHL3S433-P antibodies.31 In some experiments, NCC and KLHL3S433-P were stained in the adjacent sections, because both of the antibodies were made from rabbits. Other antibodies include anti-phospho-PKCα (Abcam) and anti-calbindin D-28-K (Swant).
RNA Extraction and Quantitative RT-PCR
RNA was extracted using a commercially available kit (Qiagen). TaqMan gene expression assays (Thermo Fisher Scientific) were used for the quantitative RT-PCR analysis.
Measurement of Cellular Glucose Content
Intracellular glucose levels in HEK cells and mouse distal convoluted tubule (mDCT) cells (provided by Peter Friedman) were measured by the glucose assay kit (BioVision) and a fluorescence spectrophotometer (F-7000; HITACHI).
Cell Culture, Transient Transfection, and Cell Treatment
HEK cells were incubated in DMEM supplemented with 10% FBS and antibiotics as described previously.42 Transient transfection was carried out using nonliposomal polymer (Mirus Bio).31 mDCT cells were incubated in DMEM/F12 supplemented with 5% FBS and antibiotics.43 The effect of d-glucose on PKC activity was evaluated in accordance with previous studies.44 After incubating with serum-free medium containing 0.5% BSA, 5.6 mM d-glucose, and ITS reagents (GIBCO; which contains insulin at 1.7 mM at final concentration), medium was changed to the DMEM medium containing 5.6 mM d-glucose (low glucose) or 20.6 mM d-glucose (high glucose), and cells were incubated for 3 hours. In some experiments, PKC activity was evaluated by the ELISA-based PKC kinase activity assay (Enzo). As a control experiment, cells were incubated in the presence of 20.6 mM l-glucose (which is not taken up by cells) for 3 hours. When indicated, cells were incubated with 2 μM BIM for 3 hours.
Statistical Analyses
The data are summarized as means±SEM. Unpaired t test was used for comparisons between two groups. The effects of hydrochlorothiazide were evaluated by paired t test in each group. For multiple comparisons, statistical analysis was performed by ANOVA followed by Dunnett post hoc tests (versus db/db group). P values <0.05 was considered statistically significant.
Results
Increased Phosphorylation of KLHL3S433 Results in NCC Activation in the Distal Convoluted Tubules of db/db Mice
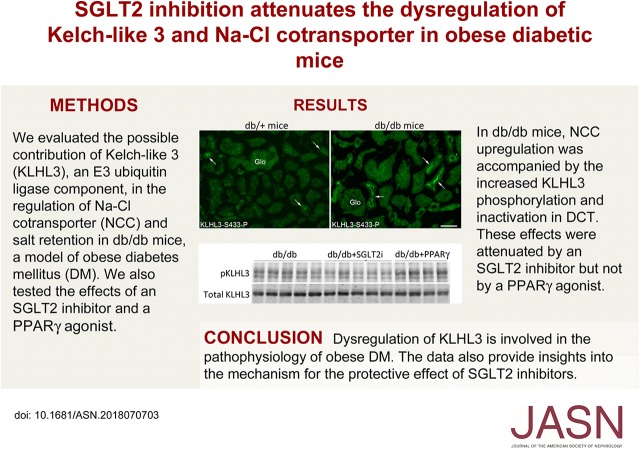
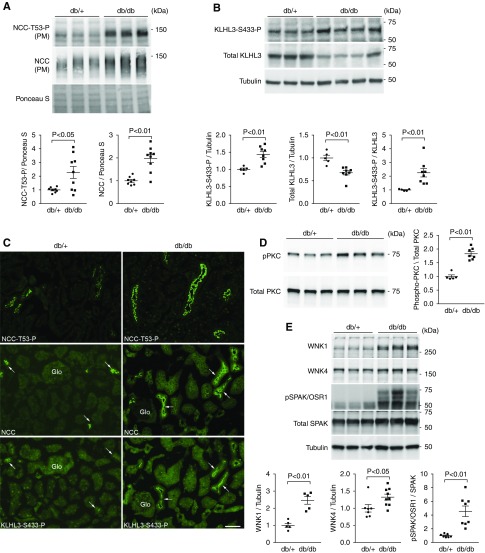
To examine whether the ubiquitin ligase composed of KLHL3 is involved in the pathophysiology of an obese diabetic state, we used db/db mice. Blood glucose levels were significantly higher in db/db mice than in control db/+ mice (617±37 mg/dl in db/db mice versus 202±9 mg/dl in db/+ mice; P<0.01). In accordance with the previous study,45 fasting plasma insulin levels also tended to increase in db/db mice (1.90±0.45 ng/ml in db/db mice versus 0.91±0.08 ng/ml in db/+ mice; P=0.07). We first evaluated the levels of renal NCC by Western blotting in plasma membrane–enriched fraction. The results showed that NCC levels were significantly increased in db/db mice compared with db/+ mice (Figure 1A). We also observed an increase in the active phosphorylated form of NCC (NCCT53-P). We then evaluated KLHL3S433-P and total KLHL3 levels in the kidney. KLHL3S433-P levels were significantly elevated in the kidney of db/db mice (Figure 1B), whereas total KLHL3 levels were significantly decreased. These findings are analogous to our previous observations in K+-depleted mice,22 and they indicate that the activity of the KLHL3-based ubiquitin ligase is suppressed by the combined effects of increased KLHL3S433-P and decreased total KLHL3 levels. However, these observations could not be explained by K+ depletion given that plasma K+ levels were not decreased in this model (5.08±0.24 mEq/L in db/db mice versus 4.72±0.23 mEq/L in db/+ mice; P=0.31).
Figure 1.
Increased phosphorylation of serine 433 in Kelch-like 3 (KLHL3S433-P) is associated with NaCl cotransporter (NCC) activation in the distal convoluted tubules of db/db mice. (A) Expression of NCC in the plasma membrane–enriched fraction (PM) in the kidneys of db/+ and db/db mice in biologic replicates. Dot plot graphs show the results of quantitation. (B) KLHL3S433-P and total KLHL3 levels in the kidneys of db/+ and db/db mice determined by Western blot analysis in biologic replicates. Dot plot graphs show the results of quantitation. (C, top panels) Immunofluorescence microscopy of NCCT53-P in the kidney of db/+ (left panel) and db/db mice (right panel). (C, middle and bottom panels) Serial kidney sections were stained with NCC and KLHL3S433-P with NCC in db/+ and db/db mice, respectively. KLHL3S433-P is predominantly present in the distal convoluted tubule cells (arrows). Glo, glomerulus. Scale bars, 50 μm. (D, upper left panel) Active phosphorylated protein kinase C (PKC) levels in the kidneys of db/+ and db/db mice. (D, lower left panel) Total PKC levels. Blots show biologic replicates. (D, right panel) Dot plot graphs show the results of densitometric quantitation. (E) Levels of with-no-lysine 1 (WNK1), WNK4, and STE20/SPS1-related proline-alanine–rich protein kinase (SPAK)/oxidative stress–responsive 1 (OSR1) phosphorylation in the kidney of db/+ and db/db mice. Blots show biologic replicates. Dot plot graphs show the results of quantitation. Data are expressed as means±SEM.
In the immunofluorescence study, NCCT53-P was increased at the apical membrane of the distal convoluted tubules in db/db mice compared with db/+ mice (Figure 1C, top panels, Supplemental Figure 3A) as expected. Immunostaining using polyclonal anti-KLHL3S433-P antibody revealed that KLHL3S433-P was highly detected in the cortex in db/db mice (Supplemental Figure 3B). At a higher magnification, KLHL3S433-P was present at or near the apical membrane of the renal tubules (Supplemental Figure 3C). To determine in which cells in the kidney KLHL3S433-P is induced, we stained the kidneys with anti-KLHL3S433-P and anti-NCC in serial sections (because both antibodies were made from rabbits). The results showed that KLHL3S433-P signal was mainly localized to the distal convoluted tubule cells that express NCC (Figure 1C, arrows in middle and bottom panels).
Given the evidence that PKC directly phosphorylates KLHL3S433,31 we next evaluated PKC activity in the kidney. Western blot analysis using the antibody against active phosphorylated PKC showed that PKC activity was significantly elevated in db/db mice (1.8-fold increase; P<0.01) (Figure 1D).
Previous studies have shown that WNK1 levels are high in distal convoluted tubule cells.17,46 WNK4 is also enriched in these cells.17,46 If the activity of KLHL3-based ubiquitin ligase is suppressed in the distal convoluted tubules in db/db mice, this should result in the accumulation of the target substrates WNK1 and WNK4. Consistently, we found elevated WNK1 and WNK4 levels in db/db mice (Figure 1E) that resulted in the increased phosphorylation of the downstream target SPAK/OSR1. These data indicate that the KLHL3-based ubiquitin ligase is inactivated in distal convoluted tubule cells of db/db mice, resulting in NCC phosphorylation and activation through WNK and SPAK/OSR1 activation.
Ipragliflozin, but Not Pioglitazone, Attenuates KLHL3S433-P Induction and NCC Activity in db/db Mice
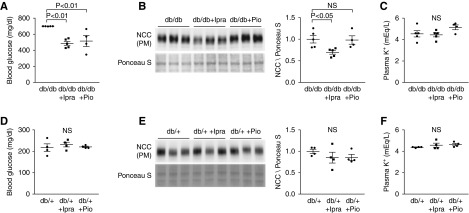
Next, we evaluated the contribution of hyperglycemia on KLHL3S433-P and NCC levels by separately administering two distinct classes of hypoglycemic agents. db/db mice received either ipragliflozin, an SGLT2 inhibitor (15 mg/kg chow; db/db + Ipra), or pioglitazone, a thiazolidinedione (100 mg/kg chow; db/db + Pio) for 7 days, after which we measured NCC levels (we chose this period to avoid any secondary changes, such as decrease or increase in body weight). Both ipragliflozin and pioglitazone significantly ameliorated hyperglycemia in db/db mice to a similar extent (Figure 2A). Interestingly, however, only ipragliflozin significantly reduced NCC levels in the kidney of db/db mice (Figure 2B). Again, there were no differences in plasma K+ levels among the groups (Figure 2C). In addition, these effects of ipragliflozin on NCC were not observed in the control db/+ mice (Figure 2, D–F). We additionally evaluated the effects of insulin infusion on NCC. Insulin administration modestly but significantly reduced blood glucose levels (Supplemental Figure 4A). However, similar to the findings in the db/db + Pio group, insulin did not significantly alter NCC levels (Supplemental Figure 4B).
Figure 2.
Ipragliflozin, but not pioglitazone, reduces NaCl cotransporter (NCC) levels in vivo in db/db mice. (A and D) Blood glucose levels in the indicated groups. In the db/db group, the blood glucose levels >700 mg/dl were regarded as 700 mg/dl. Both ipragliflozin, an sodium glucose cotransporter 2 (SGLT2) inhibitor, and pioglitazone, a Peroxisome Proliferator-Activated Receptor gamma (PPARγ) agonist, effectively reduce blood glucose levels in db/db mice but not in db/+ mice. (B and E) Effects of ipragliflozin and of pioglitazone on NCC levels in the plasma membrane–enriched fraction of the kidneys in indicated animals. Blots show biologic replicates. Dot plot graphs show the results of densitometric quantitation. (C and F) Plasma K+ levels in the indicated groups. Data are expressed as means±SEM; n=5 for db/db and db/db plus ipragliflozin, n=4 for db/db plus pioglitazone, and n=4 for db/+, db/+ plus ipragliflozin, and db/+ plus pioglitazone.
Given that AngII can directly induce NCC in the distal convoluted tubule,31 we evaluated the possible involvement of the renin-angiotensin system in db/db mice. Consistent with the previous studies,47 plasma renin activity was significantly lower in db/db mice (Supplemental Figure 5), and there were no significant differences in plasma renin activity among db/db, db/db + Ipra, and db/db + Pio groups. As an indicator of the intrarenal renin-angiotensin system, we also evaluated angiotensinogen mRNA levels in the kidney.48,49 We found that renal angiotensinogen was increased in db/db mice; however, the expression levels were not reduced by ipragliflozin or pioglitazone (Supplemental Figure 6A). To directly test whether AngII signaling is involved in the NCC upregulation, we administered candesartan,35 the AngII type 1 receptor blocker, to db/db mice. Blood glucose levels were not significantly different between the two groups (600±57 mg/dl in db/db mice versus 641±20 mg/dl in db/db + angiotensin receptor blocker mice; P=0.54). Candesartan did not reduce NCC levels, excluding the possibility that AngII is involved in this mechanism (Supplemental Figure 6B).
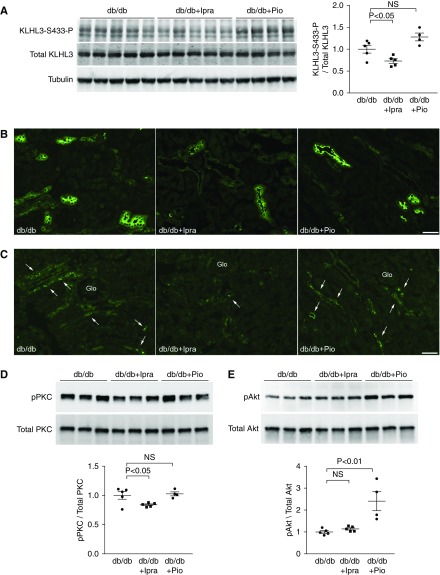
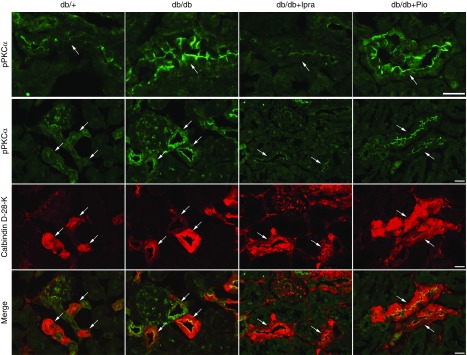
If the distinct effects of ipragliflozin and pioglitazone on NCC are mediated by KLHL3S433-P, its levels should be reduced by ipragliflozin but not by pioglitazone. Indeed, Western blotting showed that KLHL3S433-P levels were significantly reduced in the db/db + Ipra group but not in the db/db + Pio group (Figure 3A). Consistent with the results of Western blotting, immunofluorescence analysis revealed that both NCC and KLHL3S433-P signals were attenuated by ipragliflozin but were not attenuated by pioglitazone (Figure 3, B and C). As upstream signaling, we evaluated PKC levels in the kidney. We found that active phosphorylated PKC levels were attenuated only in the db/db + Ipra group (Figure 3D). In contrast, active phosphorylated Akt levels were not altered by ipragliflozin (Figure 3E). Akt phosphorylation was increased in the db/db + Pio group, likely representing the enhanced insulin signaling in the kidney. To determine the localization of active PKC in vivo in the kidney, we performed an immunofluorescence study. We found that active phosphorylated PKCα levels were mainly increased in the distal convoluted tubules (Figure 4). Moreover, this increase was attenuated by ipragliflozin but was not attenuated by pioglitazone. These data explain the mechanisms for the distinct effect of ipragliflozin and pioglitazone, and they suggest a causal role of PKC in NCC induction in diabetic kidney.
Figure 3.
Ipragliflozin, but not pioglitazone, ameliorates Kelch-like 3 (KLHL3) phosphorylation in db/db mice. (A) Levels of KLHL3S433-P and total KLHL3 in the kidneys were measured the indicated groups. Blots show biologic replicates. Dot plot graphs show the results of quantitation. (B and C) Immunofluorescence microscopy of (B) NaCl cotransporter (NCC) and (C) KLHL3S433-P in the kidney of indicated animals. Glo, glomerulus. (D and E) Effects of ipragliflozin and pioglitazone on the active phosphorylated form of (D) protein kinase C (PKC) and (E) Akt in db/db mice. Dot plot graphs show the results of densitometric quantitation. Data are expressed as means±SEM; n=5 for db/db and db/db plus ipragliflozin, and n=4 for db/db plus pioglitazone.
Figure 4.
Active phosphorylated protein kinase C is increased in distal convoluted tubule cells in db/db mice, and is attenuated by ipragliflozin. Kidneys from indicated mice were sectioned and immunostained with antiphospho-PKCα (green) and anticalbindin D-28-K (red; a distal convoluted tubule marker). Arrows indicate distal convoluted tubule cells. Scale bars, 20 μm.
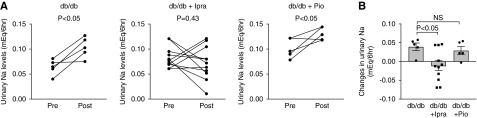
To confirm the biochemical analysis on NCC levels, we evaluated the natriuretic response to thiazide as an indicator of NCC activity. We administered hydrochlorothiazide (25 mg/kg body wt) after 7 days of treatment with ipragliflozin or pioglitazone, and we collected urine for 6 hours after the injection using individual metabolic cages. db/db mice showed significant increases in urinary Na+ levels after hydrochlorothiazide injection, confirming the natriuretic effect (Figure 5). Importantly, the db/db + Pio group also showed significant increase in urinary Na+ levels, whereas there were no significant changes in urinary Na+ levels in the db/db + Ipra group before and after hydrochlorothiazide injection. The increase in urinary Na+ excretion after hydrochlorothiazide administration was significantly greater in the db/db group than in the db/db + Ipra group (Figure 5). These data are consistent with our data showing that ipragliflozin, but not pioglitazone, attenuated NCC levels in db/db mice.
Figure 5.
NaCl cotransporter (NCC) activity is increased in db/db mice and is attenuated by ipragliflozin. (A) Indicated groups received hydrochlorothiazide (an NCC inhibitor; 25 mg/kg body wt), and the amount of urinary Na+ excretion was compared before and after hydrochlorothiazide injection. (B) Changes in urinary Na+ excretion before and after hydrochlorothiazide administration in indicated groups. Data are expressed as means±SEM.
Role of Intracellular Glucose in PKC and KLHL3S433-P Induction
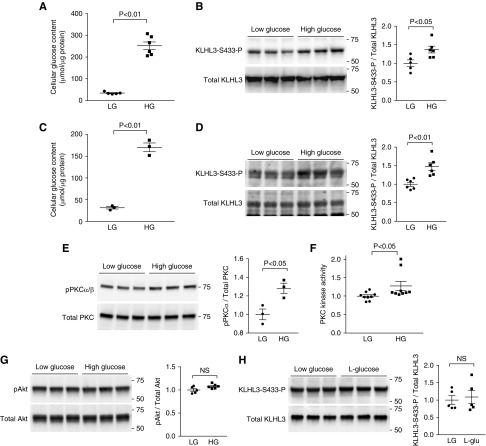
We next evaluated the mechanism for the PKC and KLHL3S433-P induction in a diabetic state. Previous studies indicate that the elevated intracellular glucose levels result in the accumulation of the glucose metabolic intermediates glyceralaldehyde-3-phosphate and glycerol-3 phosphate, which stimulate de novo diacylglycerol synthesis and PKC activity.50–52 Therefore, we determined whether glucose loading can directly induce PKC and KLHL3S433-P in cultured cells. To test this, HEK cells expressing KLHL3 were incubated in the low– or high–d-glucose medium for 3 hours.44 We then measured KLHL3S433-P and total KLHL3 levels by Western blotting. We found that high-glucose loading and the resultant intracellular glucose accumulation (Figure 6A) caused a significant increase in KLHL3S433-P levels (Figure 6B). To test the effect of glucose on endogenous KLHL3, we incubated mDCT cells43 in the high–d-glucose medium for 3 hours. Consistent with the findings in HEK cells, high-glucose loading increased cellular glucose content (Figure 6C) and KLHL3S433-P levels (Figure 6D).
Figure 6.
High-glucose (HG) loading induces Kelch-like 3 (KLHL3) phosphorylation. (A) Cellular glucose levels in human embryotic kidney (HEK) cells exposed to low-glucose (LG) and HG media for 3 hours. (B) Effects of HG on KLHL3S433-P levels in the HEK cells expressing KLHL3 in biologic replicates. Dot plot graphs show the results of densitometric quantitation. (C) Cellular glucose levels in mouse distal convoluted tubule (mDCT) cells exposed to LG and HG media for 3 hours. (D) Effects of HG on KLHL3S433-P levels in the mDCT cells. Dot plot graphs show the results of densitometric quantitation. (E) Active phosphorylated levels of protein kinase Cα/β (PKCα/β) in biologic replicates. (F) PKC activity was evaluated by ELISA in HEK cells exposed to LG and HG for 3 hours. (G) Levels of active phosphorylated levels of Akt in biologic replicates. Dot plot graphs show the results of densitometric quantitation. (H) Effects of l-glucose (20.6 mM) on KLHL3S433-P levels in the HEK cells expressing KLHL3 in biologic replicates. Dot plot graphs show the results of densitometric quantitation. Data are expressed as means±SEM.
We next tested the possible involvement of PKC. We found that the high glucose caused the increase in active phosphorylated PKCα/β (Figure 6E) in HEK cells. The glucose-induced PKC activation was also confirmed in the ELISA-based PKC kinase activity assay (Figure 6F). By contrast, active phosphorylated Akt levels were not different between the two groups in this condition (Figure 6G). As an osmotic control, we also incubated the cells with 20.6 mM l-glucose, which did not alter KLHL3S433-P levels (Figure 6H).
Causal Role of PKC in KLHL3S433-P Induction and NCC Activation in the Kidney of db/db Mice
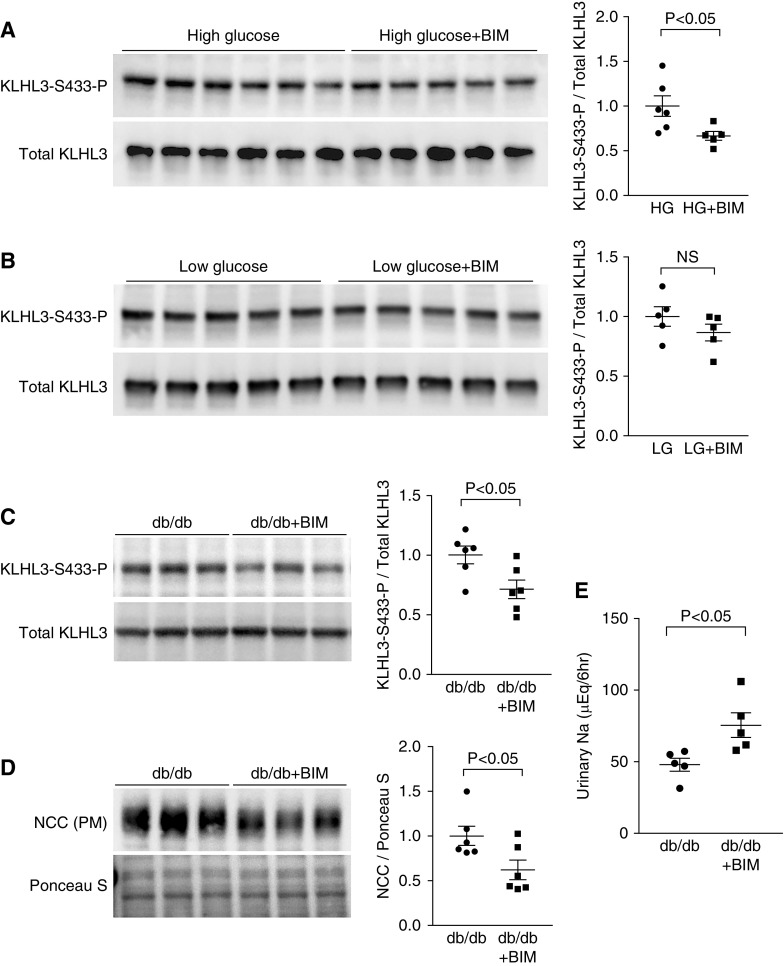
We finally tested the causal role of PKC in the dysregulation of KLHL3S433-P and NCC in db/db mice. For this purpose, we performed both cell culture and in vivo experiments. In vitro, HEK cells expressing KLHL3 were incubated with BIM, the PKC inhibitor, in a high-glucose medium. In vivo, db/db mice received intraperitoneal injection of BIM (4 μg) or vehicle once daily for 2 days, and we analyzed KLHL3S433-P and NCC levels in the kidney. In the cultured cells incubated with the high-glucose medium, we found that the incubation with BIM significantly reduced KLHL3S433-P levels (Figure 7A). These effects were not observed in cells incubated with the low-glucose medium (Figure 7B). Consistently, in vivo experiments showed that KLHL3S433-P levels in the db/db mice kidney were also attenuated by BIM administration (Figure 7C). Moreover, NCC levels in the plasma membrane–enriched fraction were reduced in db/db mice receiving BIM (Figure 7D). In contrast, BIM had no effects on NCC levels in db/+ mice (Supplemental Figure 7A). Blood glucose and plasma K+ levels were similar between the two groups (blood glucose: 679±13 mg/dl in db/db mice versus 668±18 mg/dl in the db/db + BIM group; P=0.61; plasma K+: 4.37±0.07 mEq/L in db/db mice versus 4.55±0.26 mEq/L in db/db + BIM mice; P=0.51).
Figure 7.
Protein kinase C (PKC) is involved in the dysregulation of Kelch-like 3 (KLHL3) and NaCl cotransporter (NCC) in db/db mice. (A) Effects of bisindolylmaleimide I hydrochloride (BIM), the PKC inhibitor, on high glucose (HG)–induced KLHL3S433-P in human embryotic kidney (HEK) cells. Blots show biologic replicates. Dot plot graphs show the results of densitometric quantitation. (B) Effects of BIM on KLHL3S433-P levels in HEK cells incubated in a low-glucose (LG) medium. Blots show biologic replicates. Dot plot graphs show the results of densitometric quantitation. (C) Effects of BIM on KLHL3S433-P levels in the kidneys of db/db mice. Blots show biologic replicates. Dot plot graphs show the results of densitometric quantitation. (D) Effects of BIM on NCC in the plasma membrane–enriched fraction (PM) of the kidneys in db/db mice. Blots show biologic replicates. Dot plot graphs show the results of densitometric quantitation. (E) Effects of BIM on urinary Na+ levels in the db/db mice. Data are expressed as means±SEM.
To show the functional significance of reduced NCC abundance by BIM, we collected urine for 6 hours immediately after BIM injection using individual metabolic cages. We found that urinary Na+ levels were significantly increased by BIM in db/db mice (Figure 7E). In contrast, db/+ mice did not show significant natriuretic response after BIM administration (Supplemental Figure 7B). These results indicate that KLHL3S433-P induction by PKC causes NCC activation and sodium retention in obese diabetic mice.
Discussion
Previous studies showed that hyperglycemia and activation of the glycolytic pathway result in the accumulation of intermediate metabolites that stimulate de novo diacylglycerol synthesis and PKC activation.50,51 Activated PKC, in turn, induces pathogenic consequences in various tissues.50,51 Nonetheless, the role of the PKC pathway in the increased renal salt reabsorption in type 2 DM remained undetermined. Previously, we showed that PKC phosphorylates KLHL3S433 and inactivates the ubiquitin ligase composed of KLHL3, resulting in increased salt reabsorption.31 This study links these observations and shows the pathway in which the dysregulation of glucose metabolism triggers salt retention in the kidney. In addition to the proposed mechanism, it is possible that PKC phosphorylation and activation of WNKs act in parallel to increase NCC levels in the diabetic kidney.53
These data also provide insights into the mechanisms for the recently described protective effects of SGLT2 inhibitors.14,15 We found that PKC activity, KLHL3S433-P, and NCC levels were all lowered by ipragliflozin but not by pioglitazone, despite the fact that both reagents effectively reduced blood glucose. These observations can be explained by the different mechanisms of action of the two hypoglycemic agents (Figure 8). Pioglitazone, a thiazolidinedione, improves glycemic control by acting as an insulin sensitizer and potentiating cellular glucose uptake,54,55 whereas ipragliflozin, an SGLT2 inhibitor, inhibits glucose reabsorption in proximal tubules. We infer that SGLT2 inhibitors might be favorable in attenuating pathologic PKC activity in the diabetic kidney, because they ameliorate hyperglycemia without potentiating cellular glucose uptake and glucose toxicity. It would be interesting to evaluate metabolic changes in vivo in the distal convoluted tubules in response to the different hypoglycemic agents in a future study.
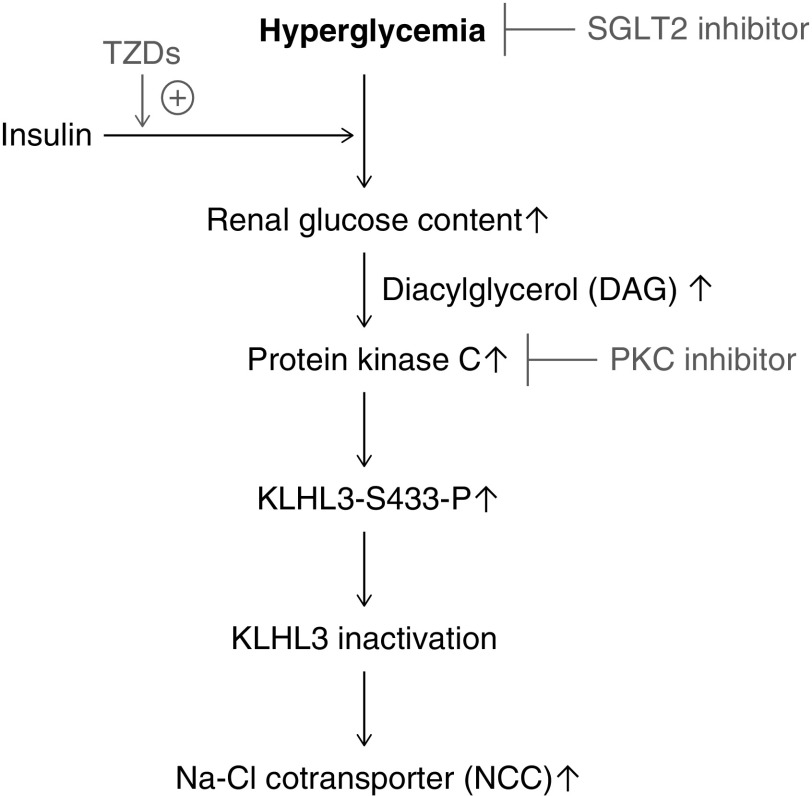
Figure 8.
High glucose-induced protein kinase C activation is involved in the dysregulation of Kelch-like 3 (KLHL3) and NaCl cotransporter (NCC) in the obese diabetic kidney. Hyperglycemia and cellular glucose accumulation can lead to increased protein kinase C (PKC) activity via the increased diacylglycerol (DAG) synthesis. Activated PKC, in turn, phosphorylates and inactivates the ubiquitin ligase component KLHL3 in the distal convoluted tubules, resulting in increased NCC activity and NaCl retention. Sodium glucose cotransporter 2 (SGLT2) inhibitors effectively ameliorate this pathway by reducing blood glucose without stimulating cellular glucose uptake. Insulin and thiazolidinedione (TZD) also ameliorate hyperglycemia but can promote cellular glucose uptake.
In this study, we showed that the PKC/KLHL3/WNK pathway is involved in NCC activation that does not require AngII in db/db mice. Given that KLHL3 and WNKs are also regulated by AngII signaling,31,56 our data indicate that a diabetic state mimics AngII signaling by phosphorylating KLHL3 in distal convoluted tubules, resulting in salt retention. These data also underscore the importance of the KLHL3/WNK system in physiologic and pathologic conditions. Other than the proposed mechanism, it is likely that several other pathways act in parallel.57 Nonetheless, we infer that the activation of NCC by KLHL3S433-P induction has a major effect on renal salt handling in an obese diabetic state given the critical role of KLHL3 in regulating BP homeostasis in humans.18,19
Because S433 and the surrounding sequences in KLHL3 are conserved in KLHL2, it is possible that KLHL2 is also phosphorylated by a similar or related mechanism. The role of KLHL2 phosphorylation in renal medulla and its downstream effectors could be the area of future investigation.
A limitation of the study is that we tested the short-term effects of ipragliflozin. This is because we intended to avoid any secondary changes associated with a longer treatment, such as decrease or increase in body weight, and because clinical studies indicated that SGLT2 inhibitors alter BP at an early stage.14 Although we showed that the administration of BIM reduced KLHL3S433-P and NCC levels in db/db mice, the direct demonstration of the role of PKC in the distal convoluted tubules would require selective knockout of PKC isozymes in this segment. Also, the metabolic changes in the distal convoluted tubules in response to different hypoglycemic agents need additional evaluation. Despite these limitations, this study provides the first in vivo evidence that the ubiquitin ligase component KLHL3 is involved in the pathophysiology of obese DM. These data also provide insights into the mechanisms for the clinically proven but poorly defined protective effects of SGLT2 inhibitors.
Supplementary Material
Acknowledgments
We thank Dr. Peter Friedman and Dr. Tatsuo Shimosawa for providing mouse distal convoluted tubule cells.
This work was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI grants 17K16097 (to K.Ishizawa), 15H02538 (to T.Fujita), and 15H04837 (to S.Shibata) and a Takeda Science Foundation grant (to S.Shibata).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Disclosures
Ipragliflozin was provided by Astellas Pharma. The company had no role in study design, execution of experiments, decision to publish, or preparation of the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018070703/-/DCSupplemental.
Supplemental Figure 1. Characterization of monoclonal KLHL3S433-P antibody.
Supplemental Figure 2. KLHL3 antibody does not recognize KLHL2.
Supplemental Figure 3. Immunofluorescence study using anti-NCCT53-P anti-KLHL3S433-P antibodies in the kidney of db/db mice.
Supplemental Figure 4. Effect of insulin on NCC levels in db/db mice.
Supplemental Figure 5. Evaluation of plasma renin activity in db/+ and db/db mice.
Supplemental Figure 6. Role of angiotensin II signaling in the kidney in the regulation of NCC in db/db mice.
Supplemental Figure 7. Effects of bisindolylmaleimide I in db/+ mice.
References
- 1.Fuller JH: Epidemiology of hypertension associated with diabetes mellitus. Hypertension 7: II3–II7, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Sowers JR, Epstein M, Frohlich ED: Diabetes, hypertension, and cardiovascular disease: An update. Hypertension 37: 1053–1059, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group : Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55: 1832–1839, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Hall JE, Mizelle HL, Hildebrandt DA, Brands MW: Abnormal pressure natriuresis. A cause or a consequence of hypertension? Hypertension 15: 547–559, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Brands MW, Manhiani MM: Sodium-retaining effect of insulin in diabetes. Am J Physiol Regul Integr Comp Physiol 303: R1101–R1109, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Goldberg M, Agus ZS: The effects of glucose and insulin on renal electrolyte transport. J Clin Invest 58: 83–90, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA: Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol 290: F1055–F1064, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Komers R, Rogers S, Oyama TT, Xu B, Yang CL, McCormick J, et al.: Enhanced phosphorylation of Na(+)-Cl- co-transporter in experimental metabolic syndrome: Role of insulin. Clin Sci (Lond) 123: 635–647, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishida H, Sohara E, Nomura N, Chiga M, Alessi DR, Rai T, et al.: Phosphatidylinositol 3-kinase/Akt signaling pathway activates the WNK-OSR1/SPAK-NCC phosphorylation cascade in hyperinsulinemic db/db mice. Hypertension 60: 981–990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsutsu N, Nunoi K, Kodama T, Nomiyama R, Iwase M, Fujishima M: Lack of association between blood pressure and insulin in patients with insulinoma. J Hypertens 8: 479–482, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Fujita N, Baba T, Tomiyama T, Kodama T, Kako N: Hyperinsulinaemia and blood pressure in patients with insulinoma. BMJ 304: 1157, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall JE, Brands MW, Mizelle HL, Gaillard CA, Hildebrandt DA: Chronic intrarenal hyperinsulinemia does not cause hypertension. Am J Physiol 260: F663–F669, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Manhiani MM, Cormican MT, Brands MW: Chronic sodium-retaining action of insulin in diabetic dogs. Am J Physiol Renal Physiol 300: F957–F965, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al.: EMPA-REG OUTCOME Investigators : Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al.: CANVAS Program Collaborative Group : Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017 [DOI] [PubMed] [Google Scholar]
- 16.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al.: 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507–520, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, et al.: Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, et al.: Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482: 98–102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, et al. : KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44: 456–460, 2012 [DOI] [PubMed] [Google Scholar]
- 20.McCormick JA, Yang CL, Zhang C, Davidge B, Blankenstein KI, Terker AS, et al.: Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest 124: 4723–4736, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, et al.: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishizawa K, Xu N, Loffing J, Lifton RP, Fujita T, Uchida S, et al.: Potassium depletion stimulates Na-Cl cotransporter via phosphorylation and inactivation of the ubiquitin ligase Kelch-like 3. Biochem Biophys Res Commun 480: 745–751, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohara E, Uchida S: Kelch-like 3/Cullin 3 ubiquitin ligase complex and WNK signaling in salt-sensitive hypertension and electrolyte disorder. Nephrol Dial Transplant 31: 1417–1424, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, et al.: The CUL3-KLHL3 E3 ligase complex mutated in Gordon’s hypertension syndrome interacts with and ubiquitylates WNK isoforms: Disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J 451: 111–122, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP: Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci U S A 110: 7838–7843, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, et al.: Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Reports 3: 858–868, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ: Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal 7: ra41, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazúa-Valenti S, Chávez-Canales M, Rojas-Vega L, González-Rodríguez X, Vázquez N, Rodríguez-Gama A, et al.: The effect of WNK4 on the Na+-Cl- cotransporter is modulated by intracellular chloride. J Am Soc Nephrol 26: 1781–1786, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitari AC, Deak M, Morrice NA, Alessi DR: The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J 391: 17–24, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, et al.: Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Shibata S, Arroyo JP, Castañeda-Bueno M, Puthumana J, Zhang J, Uchida S, et al.: Angiotensin II signaling via protein kinase C phosphorylates Kelch-like 3, preventing WNK4 degradation. Proc Natl Acad Sci U S A 111: 15556–15561, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marumo T, Yagi S, Kawarazaki W, Nishimoto M, Ayuzawa N, Watanabe A, et al.: Diabetes induces aberrant DNA methylation in the proximal tubules of the kidney. J Am Soc Nephrol 26: 2388–2397, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma K, Jin Y, Guo J, Ziyadeh FN: Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 45: 522–530, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Kim JE, Kang TC: The P2X7 receptor-pannexin-1 complex decreases muscarinic acetylcholine receptor-mediated seizure susceptibility in mice. J Clin Invest 121: 2037–2047, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsukuda K, Mogi M, Li JM, Iwanami J, Min LJ, Sakata A, et al.: Amelioration of cognitive impairment in the type-2 diabetic mouse by the angiotensin II type-1 receptor blocker candesartan. Hypertension 50: 1099–1105, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Shibata S, Rinehart J, Zhang J, Moeckel G, Castañeda-Bueno M, Stiegler AL, et al.: Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab 18: 660–671, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haber PK, Ye M, Wysocki J, Maier C, Haque SK, Batlle D: Angiotensin-converting enzyme 2-independent action of presumed angiotensin-converting enzyme 2 activators: Studies in vivo, ex vivo, and in vitro. Hypertension 63: 774–782, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu N, Hirohama D, Ishizawa K, Chang WX, Shimosawa T, Fujita T, et al.: Hypokalemia and pendrin induction by aldosterone. Hypertension 69: 855–862, 2017 [DOI] [PubMed] [Google Scholar]
- 39.Kasagi Y, Takahashi D, Aida T, Nishida H, Nomura N, Zeniya M, et al.: Impaired degradation of medullary WNK4 in the kidneys of KLHL2 knockout mice. Biochem Biophys Res Commun 487: 368–374, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Roy A, Al-Qusairi L, Donnelly BF, Ronzaud C, Marciszyn AL, Gong F, et al.: Alternatively spliced proline-rich cassettes link WNK1 to aldosterone action. J Clin Invest 125: 3433–3448, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picard N, Trompf K, Yang CL, Miller RL, Carrel M, Loffing-Cueni D, et al.: Protein phosphatase 1 inhibitor-1 deficiency reduces phosphorylation of renal NaCl cotransporter and causes arterial hypotension. J Am Soc Nephrol 25: 511–522, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al.: Modification of mineralocorticoid receptor function by Rac1 GTPase: Implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Magyar CE, White KE, Rojas R, Apodaca G, Friedman PA: Plasma membrane Ca2+-ATPase and NCX1 Na+/Ca2+ exchanger expression in distal convoluted tubule cells. Am J Physiol Renal Physiol 283: F29–F40, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Williams B, Schrier RW: Characterization of glucose-induced in situ protein kinase C activity in cultured vascular smooth muscle cells. Diabetes 41: 1464–1472, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Koranyi L, James D, Mueckler M, Permutt MA: Glucose transporter levels in spontaneously obese (db/db) insulin-resistant mice. J Clin Invest 85: 962–967, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ichihara A, Sakoda M, Kurauchi-Mito A, Nishiyama A, Itoh H: Involvement of receptor-bound prorenin in development of nephropathy in diabetic db/db mice. J Am Soc Hypertens 2: 332–340, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Kobori H, Harrison-Bernard LM, Navar LG: Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol 12: 431–439, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navar LG, Nishiyama A: Why are angiotensin concentrations so high in the kidney? Curr Opin Nephrol Hypertens 13: 107–115, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Sheetz MJ, King GL: Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 288: 2579–2588, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Menne J, Shushakova N, Bartels J, Kiyan Y, Laudeley R, Haller H, et al.: Dual inhibition of classical protein kinase C-α and protein kinase C-β isoforms protects against experimental murine diabetic nephropathy. Diabetes 62: 1167–1174, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castañeda-Bueno M, Arroyo JP, Zhang J, Puthumana J, Yarborough O 3rd, Shibata S, et al.: Phosphorylation by PKC and PKA regulate the kinase activity and downstream signaling of WNK4. Proc Natl Acad Sci U S A 114: E879–E886, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kramer D, Shapiro R, Adler A, Bush E, Rondinone CM: Insulin-sensitizing effect of rosiglitazone (BRL-49653) by regulation of glucose transporters in muscle and fat of Zucker rats. Metabolism 50: 1294–1300, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Defronzo RA: Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58: 773–795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, et al.: Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci U S A 106: 4384–4389, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Shibayama Y, Kobori H, Liu Y, Kobara H, Masaki T, et al.: High glucose augments angiotensinogen in human renal proximal tubular cells through hepatocyte nuclear factor-5. PLoS One 12: e0185600, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.