Abstract
Background
Attention deficit hyperactivity disorder (ADHD) is a developmental condition characterised by symptoms of inattention, hyperactivity and impulsivity, along with deficits in executive function, emotional regulation and motivation. The persistence of ADHD in adulthood is a serious clinical problem.
ADHD significantly affects social interactions, study and employment performance.
Previous studies suggest that cognitive‐behavioural therapy (CBT) could be effective in treating adults with ADHD, especially when combined with pharmacological treatment. CBT aims to change the thoughts and behaviours that reinforce harmful effects of the disorder by teaching people techniques to control the core symptoms. CBT also aims to help people cope with emotions, such as anxiety and depression, and to improve self‐esteem.
Objectives
To assess the effects of cognitive‐behavioural‐based therapy for ADHD in adults.
Search methods
In June 2017, we searched CENTRAL, MEDLINE, Embase, seven other databases and three trials registries. We also checked reference lists, handsearched congress abstracts, and contacted experts and researchers in the field.
Selection criteria
Randomised controlled trials (RCTs) evaluating any form of CBT for adults with ADHD, either as a monotherapy or in conjunction with another treatment, versus one of the following: unspecific control conditions (comprising supportive psychotherapies, no treatment or waiting list) or other specific interventions.
Data collection and analysis
We used the standard methodological procedures suggested by Cochrane.
Main results
We included 14 RCTs (700 participants), 13 of which were conducted in the northern hemisphere and 1 in Australia.
Primary outcomes: ADHD symptoms
CBT versus unspecific control conditions (supportive psychotherapies, waiting list or no treatment)
‐ CBT versus supportive psychotherapies: CBT was more effective than supportive therapy for improving clinician‐reported ADHD symptoms (1 study, 81 participants; low‐quality evidence) but not for self‐reported ADHD symptoms (SMD −0.16, 95% CI −0.52 to 0.19; 2 studies, 122 participants; low‐quality evidence; small effect size).
‐ CBT versus waiting list: CBT led to a larger benefit in clinician‐reported ADHD symptoms (SMD −1.22, 95% CI −2.03 to −0.41; 2 studies, 126 participants; very low‐quality evidence; large effect size). We also found significant differences in favour of CBT for self‐reported ADHD symptoms (SMD −0.84, 95% CI −1.18 to −0.50; 5 studies, 251 participants; moderate‐quality evidence; large effect size).
CBT plus pharmacotherapy versus pharmacotherapy alone: CBT with pharmacotherapy was more effective than pharmacotherapy alone for clinician‐reported core symptoms (SMD −0.80, 95% CI −1.31 to −0.30; 2 studies, 65 participants; very low‐quality evidence; large effect size), self‐reported core symptoms (MD −7.42 points, 95% CI −11.63 points to −3.22 points; 2 studies, 66 participants low‐quality evidence) and self‐reported inattention (1 study, 35 participants).
CBT versus other interventions that included therapeutic ingredients specifically targeted to ADHD: we found a significant difference in favour of CBT for clinician‐reported ADHD symptoms (SMD −0.58, 95% CI −0.98 to −0.17; 2 studies, 97 participants; low‐quality evidence; moderate effect size) and for self‐reported ADHD symptom severity (SMD −0.44, 95% CI −0.88 to −0.01; 4 studies, 156 participants; low‐quality evidence; small effect size).
Secondary outcomes
CBT versus unspecific control conditions: we found differences in favour of CBT compared with waiting‐list control for self‐reported depression (SMD −0.36, 95% CI −0.60 to −0.11; 5 studies, 258 participants; small effect size) and for self‐reported anxiety (SMD −0.45, 95% CI −0.71 to −0.19; 4 studies, 239 participants; small effect size). We also observed differences in favour of CBT for self‐reported state anger (1 study, 43 participants) and self‐reported self‐esteem (1 study 43 participants) compared to waiting list. We found no differences between CBT and supportive therapy (1 study, 81 participants) for self‐rated depression, clinician‐rated anxiety or self‐rated self‐esteem. Additionally, there were no differences between CBT and the waiting list for self‐reported trait anger (1 study, 43 participants) or self‐reported quality of life (SMD 0.21, 95% CI −0.29 to 0.71; 2 studies, 64 participants; small effect size).
CBT plus pharmacotherapy versus pharmacotherapy alone: we found differences in favour of CBT plus pharmacotherapy for the Clinical Global Impression score (MD −0.75 points, 95% CI −1.21 points to −0.30 points; 2 studies, 65 participants), self‐reported depression (MD −6.09 points, 95% CI −9.55 points to −2.63 points; 2 studies, 66 participants) and self‐reported anxiety (SMD −0.58, 95% CI −1.08 to −0.08; 2 studies, 66 participants; moderate effect size). We also observed differences favouring CBT plus pharmacotherapy (1 study, 31 participants) for clinician‐reported depression and clinician‐reported anxiety.
CBT versus other specific interventions: we found no differences for any of the secondary outcomes, such as self‐reported depression and anxiety, and findings on self‐reported quality of life varied across different studies.
Authors' conclusions
There is low‐quality evidence that cognitive‐behavioural‐based treatments may be beneficial for treating adults with ADHD in the short term. Reductions in core symptoms of ADHD were fairly consistent across the different comparisons: in CBT plus pharmacotherapy versus pharmacotherapy alone and in CBT versus waiting list. There is low‐quality evidence that CBT may also improve common secondary disturbances in adults with ADHD, such as depression and anxiety. However, the paucity of long‐term follow‐up data, the heterogeneous nature of the measured outcomes, and the limited geographical location (northern hemisphere and Australia) limit the generalisability of the results. None of the included studies reported severe adverse events, but five participants receiving different modalities of CBT described some type of adverse event, such as distress and anxiety.
Plain language summary
Cognitive‐behavioural therapy for attention deficit hyperactivity disorder (ADHD) in adults
Background
People with ADHD have difficulty paying attention, concentrating, dealing with hyperactivity (e.g. waiting in queues) and acting without thinking (i.e. impulsivity). In adults, ADHD significantly affects social interactions, study and employment performance.
Previous studies suggest that cognitive‐behavioural therapy (CBT) could be effective for treating adults with ADHD, especially when combined with pharmacological (i.e. drug) treatment. CBT aims to change the thoughts and behaviours that reinforce the harmful effects of the disorder by teaching people techniques to control the core symptoms. CBT also aims to help people cope with emotions, such as anxiety and depression, and to improve self‐esteem.
Review question
Does CBT, alone or in combination with pharmacological treatment, reduce the core symptoms of ADHD in adults more than other treatments or no specific treatment?
Search dates
The evidence is current to June 2017.
Study characteristics
We found 14 randomised controlled trials (studies in which participants are randomly assigned to different treatment groups) that described the effects of CBT in 700 adults with ADHD, aged between 18 and 65 years. Thirteen trials took place in the northern hemisphere and one in Australia.
Of the included studies, three compared CBT versus other specific interventions and seven versus unspecific control conditions (unspecific supportive therapy, waiting list or no treatment). Additionally, two compared CBT plus pharmacotherapy versus pharmacotherapy alone. One trial compared CBT to two control groups, one of which was given other specific non‐pharmacological treatment and one of which was a no‐treatment control.
Quality of the evidence
Because of imprecision (i.e. inaccurate results), inconsistency (i.e. results differ across trials) and methodological limitations, we considered the quality of the evidence of the included studies to range from very low to moderate.
Key results
The findings suggest that CBT might improve the core symptoms of ADHD, reducing inattention, hyperactivity and impulsivity.
When combined with pharmacotherapy, there was evidence of an improvement in global functioning (i.e. a person's overall level of functioning in life) and a reduction in depression and anxiety compared to that seen with pharmacotherapy alone.
None of the included studies reported severe adverse events. However, five participants described some type of adverse event, such as distress and anxiety.
Summary of findings
Background
Description of the condition
According to the Diagnostic and Statistical Manual of Mental Disorders, currently in its fifth edition (DSM‐5), attention deficit hyperactivity disorder (ADHD) is a developmental condition characterised by symptoms of inattention, hyperactivity and impulsivity (DSM‐5 2013). Using these criteria, ADHD can be divided into three types: combined type, predominantly inattentive type and predominantly hyperactive‐impulsive type. The International Classification of Diseases (ICD‐10) offers a similar definition for hyperkinetic disorders (WHO 1993), but the required number of symptoms and the age of onset are different. Along with these three main symptomatic clusters, people with ADHD also present with deficits in executive functions, behaviour and emotion regulation, and motivation (Brown 2000; Davidson 2008; Torrente 2011; Wender 2001). There is a high prevalence of comorbid disorders, estimated at 50% to 75% (Kessler 2006), including anxiety, depression and substance abuse (Biederman 1993; Murphy 1996). Epidemiological studies estimate that the prevalence of ADHD is approximately 5% in childhood and around 2.5% in adulthood (Polanczyk 2014; Simon 2009).
Evidence on gender differences in ADHD is controversial. Some authors suggest that there are no differences between females and males (Biederman 2002; Seidman 2006). Other authors, such as Gershon 2002, argue that there are quantitative and qualitative differences in executive functions.
Still 1902 is commonly accredited as the first description of a syndrome in children that included some of the characteristics of ADHD. However, the characterisation of the disorder in adults was more recent, with Adler 2002 attributing it to Wood 1976. Since then, many papers have provided evidence on the diagnostic validity of ADHD in adults (Spencer 1998). The validity of the diagnosis in adulthood is supported by clinical correlates, family history, treatment response and experimental studies (Faraone 2000). Additionally, longitudinal studies have demonstrated the persistence of the disorder in large proportions of adults who were diagnosed with ADHD during childhood (Barkley 1999).
As Brassett‐Harknett 2007 noted, there are diagnostic difficulties with ADHD in adults because the current diagnostic criteria were originally designed for children. ADHD in adulthood has particular characteristics that differ from the syndrome in childhood. For example, hyperactivity tends to decrease in adulthood (Achenbach 1998), with some studies showing that 90% of adults with ADHD present predominantly with inattentive symptoms (Millstein 1997).
Importantly, different authors have recognised the persistence of ADHD in adulthood as a clinical problem with serious health consequences (Davidson 2008; Wilens 2004). Barkley 2008 highlights the severe occupational consequences of the disorder, such as lower occupational status and annual salaries than in a control group, worse employer‐rated job performance, more job dismissals and frequent changes of job. Those who suffer from ADHD are less capable of fulfilling work demands, less likely to be working independently, completing tasks, and getting along well with supervisors, as rated by employers. They have poorer performance at job interviews and find certain tasks at work too difficult. Additionally, Woods 1986 suggests that people with ADHD experience anger dysregulation as a highly associated psychosocial problem. ADHD also carries psychological consequences since repeated life experiences of frustration undermine self‐concept and self‐esteem, leading to the formation of negative beliefs about the self, which, in turn, affect quality of life and emotional adjustment (Torrente 2012).
Description of the intervention
Diverse psychological treatments have been developed for for adults with ADHD in recent years (Knouse 2008; Weiss 2008). Most have been inspired by cognitive‐behavioural therapy (CBT) and designed as adjunct interventions to pharmacological treatment (Safren 2006). As is usual in CBT treatments, the interventions are organised into relatively brief and focused, structured protocols. Most CBT programmes for adults with ADHD take 8 to 12 sessions and can be delivered on an individual or group basis. The main objectives of the treatment are to change the behaviours that reinforce detrimental effects of the disorder by teaching people techniques to control ADHD's core symptoms, improving emotional adjustment, self‐esteem and common comorbid symptoms such as anxiety and depression. Proposed psychotherapeutic techniques include psychoeducation for increasing awareness and understanding of the disorder and cognitive techniques for restructuring the dysfunctional thoughts and maladaptive beliefs that reinforce emotional maladjustment. Finally, behavioural interventions and cognitive remediation methods intend to provide new, healthy, compensatory strategies and skills for deficient attention, executive functioning, impulse control and emotion regulation (Ramsay 2010).
Investigators have applied variants of the classical CBT approach to this population: Hesslinger 2002 and Philipsen 2007 have experimented with dialectical behavioural therapy and Solanto 2010 with meta‐cognitive therapy. These variants emphasise specific types of interventions such as emotion regulation skills in dialectical behavioural therapy and cognitive training methods in meta‐cognitive therapy, but because they share the general model and procedures of CBT, previous, non‐systematic reviews have usually included these methods within the broad spectrum of CBT interventions (Knouse 2008; Weiss 2008). However, no studies have ever directly compared these types of CBTs against each other, so it is unknown if they have different treatment effects. Moreover, comparing CBT versus placebo, waiting list and no treatment could produce different treatment effects for each comparison, and we plan to explore these potential differences in our study.
How the intervention might work
The cognitive‐behavioural approach provides a useful framework for understanding how negative life experiences may reinforce functional impairment and lead to increased emotional disturbance in adults with ADHD. Because of neurobiological deficits in attention, executive function and inhibitory control, failure and underachievement in different domains of function are common occurrences in people with ADHD as they enter adulthood (Barkley 2006a; Biederman 2006). According to the CBT model, such repeated life experiences of frustration undermine self‐concept and self‐esteem, leading to the formation of negative beliefs about the self, which, in turn, favour the expression of negative emotions such as depression and anxiety. Negative self‐beliefs can also lead to the adoption of maladaptive behavioural strategies, including negation, procrastination and extreme avoidance as a means of coping with difficult tasks (Ramsay 2008; Safren 2006; Young 2007a). In addition to emotional disturbances, negative expectations about the future, anticipation of failure and reduced self‐confidence can also affect motivation (Torrente 2011).
The proposed mechanisms of change entail the acquisition of compensatory behavioural and cognitive techniques for improving the core attention and executive deficits of ADHD and modifying distorted negative beliefs to promote emotional maladjustment (Ramsay 2010). CBT programmes are therefore usually organised into several modules with specific techniques for a series of target problems. Most treatments begin with a psychoeducational module in which patients are taught about the disorder and introduced to the rationale for the treatment. This is followed by an organisation module designed to aid the acquisition of different executive techniques such as goal setting, sequencing and prioritising, devising a time schedule, using a calendar or agenda, making 'to do' lists, monitoring progress, and planning breaks and rewards. Patients also learn problem‐solving techniques for articulating problems more clearly, generating a list of potential solutions, evaluating them and finally testing the chosen solution. The distraction management module helps patients to recognise their optimal attention span and organise the tasks according to it, and it introduces skills for dealing with distractions such as writing them down and going back to the task, using cues or alarms, or modifying environmental factors. The impulsivity management module includes strategies for self‐monitoring and self‐control. The self‐monitoring module involves the detection of cues and situations that act as triggers for impulsive behaviour, while self‐control strategies refer to the use of self‐instructions, relaxation techniques or other alternative behaviours. The cognitive restructuring module aims to help patients to become aware of the ideas that reinforce maladaptive behaviours and emotions and replace them with more adaptive thoughts.
Several pilot studies have demonstrated the feasibility and acceptability of the approach (Knouse 2008), and a series of randomised controlled trials have provided evidence for the efficacy of CBT in adults with ADHD (Safren 2005; Safren 2010; Solanto 2010; Stevenson 2002).
Why it is important to do this review
Between 20% and 50% of people with ADHD do not respond to drug treatment (Wilens 2002). Also, pharmacological treatment is frequently associated with relevant side effects in both children and adults (AJCD 2001; Castells 2013; Cunill 2013; Graham 2011; King 2006; Lim 2006; Morton 2000; Perrin 2008; Prescrire 2007). Due to these concerns, it is important to have non‐pharmacological interventions for treating adults with ADHD.
The consequences of ADHD can also have an important and negative impact on different areas of a person's life, such as poor academic performance, deficits in social and occupational functioning, greater job insecurity and a greater number of legal problems (Barkley 2002; Davids 2004). An efficacious psychosocial intervention might be beneficial in one or more of these areas for adults with ADHD.
To our knowledge, three systematic reviews have compared the effects of CBT in adults with ADHD (Jensen 2016; Knouse 2017; Young 2016). However, there are important methodological differences between them, also with respect to our review. Both Jensen 2016 and Young 2016 employed more restrictive criteria for defining CBT treatments that excluded relevant CBT variants such as mindfulness‐based cognitive therapy and dialectical behavioural therapy. Knouse 2017 did not report grades of quality of evidence of the included studies.
Objectives
To assess the effects of cognitive‐behavioural‐based therapy for ADHD in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Adults aged 18 years and above diagnosed with ADHD or hyperkinetic disorder according to the established diagnostic criteria, whose medication was stable (less than 10% change in dose) in the two months prior to the initial evaluation.
Types of interventions
Individual and group treatments of CBT in any of its variants such as standard CBT, dialectical behavioural therapy, meta‐cognitive therapy, or mindfulness‐based cognitive therapy.
All included CBT interventions had to fulfil both of the following criteria.
Treatment was aimed at increasing knowledge on the disorder, identifying and restructuring dysfunctional thinking and maladaptive beliefs, and developing emotional and behavioural compensatory strategies for the core deficits.
The sequence of treatment modules was clearly defined.
We assessed 'CBT as a monotherapy' separately from 'CBT as part of a combined treatment' because the latter may present interactive effects that are not accounted for by any of the interventions alone. We evaluated these as follows.
CBT versus unspecific control conditions (supportive psychotherapies, waiting list or no treatment).
CBT plus pharmacotherapy versus pharmacotherapy alone.
CBT versus other specific interventions (control interventions that include therapeutic ingredients specifically targeted to ADHD).
We did not impose any restriction with regard to the format of the treatment (that is, the duration, quantity and frequency of sessions).
Types of outcome measures
We considered psychometrically validated self‐report measures or those completed by an independent rater or relative.
We present clinical and self‐reported outcomes separately, as do most studies about this topic, because assessing ADHD is more accurate when symptom information comes from more than one source (Barkley 1998a).
We considered the measures as short term (up to 6 months), medium term (6 months to 12 months) and long term (more than 12 months).
We included studies that assessed at least one primary outcome or at least one secondary outcome.
Primary outcomes
We assessed the core symptoms of ADHD (inattention, hyperactivity and impulsivity) as a whole. If the authors of a study reported these symptoms separately, we included the data in the analysis. We assessed the core symptoms using validated measures such as those listed below.
Continuous outcomes (efficacy)
Current Symptoms Scale (Barkley 1998a)
Conners' Adult ADHD Rating Scales ‐ Self‐Report: Long Version (Conners 1999a)
Conners' Adult ADHD Rating Scales ‐ Observer Report (Conners 1999a)
Dichotomous outcomes (safety)
All‐cause treatment discontinuation (proportion of patients randomised who dropped out from the study due to any cause, such as adverse effects of medication)
Secondary outcomes
We assessed the efficacy variables listed below as secondary outcomes. The listed measures are mentioned only as examples, and the list is not exclusive.
Continuous outcomes
-
Psychopathology (depression and anxiety)
Beck Depression Inventory II (Beck 1996)
Beck Anxiety Inventory (Beck 1988)
Hamilton Depression Scale (Hamilton 1960)
Hamilton Anxiety Scale (Hamilton 1959)
State‐Trait Anxiety Inventory (Speilberger 1989)
Anger: State‐Trait Anger Expression Inventory (Spielberger 1988)
Self‐esteem: Rosenberg Self‐Esteem Inventory (Rosenberg 1965a)
Quality of life: Adult Attention‐Deficit/Hyperactivity Disorder Quality‐of‐Life Scale (Brod 2005)
Dichotomous outcomes
Employment status (for example, working/not working, full‐time/part‐time, as defined by the authors of the study)
Considering that self‐ and clinician‐reported core symptoms are the main targets of CBT, we included them in the 'Summary of findings' tables. We prepared these tables using the GRADE methodology (Atkins 2004; Guyatt 2011). To assess the magnitude of effect for continuous outcomes, we used the criteria suggested in section 12.6.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Re‐expressing SMDs using rules of thumb for effect sizes): 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Higgins 2011).
Search methods for identification of studies
We used the following search terms and their synonyms: 'attention deficit disorder with hyperactivity', 'cognitive‐behavioural therapy' and 'adults' We used the Cochrane Highly Sensitive Search Strategy to identify RCTs in MEDLINE (Lefebvre 2011). We modified the search strategy as necessary for other databases.
Electronic searches
We searched the following databases in June 2017. We did not limit the searches by date or language (see search strategies in Appendix 1).
Cochrane Central Register of Controlled Studies (CENTRAL; 2017, Issue 6) in the Cochrane Library, which contains the Cochrane Developmental Psychosocial and Learning Problems Group Specialised Register (searched 13 June 2017).
MEDLINE PubMed, US National Library of Medicine (www.ncbi.nlm.nih.gov/pubmed; 1971 to 13 June 2017).
Embase Elsevier (1974 to 13 June 2017).
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 27 June 2017).
PsycINFO EBSCO (1967 to 15 June 2017).
BIOSIS Previews Web of Science (1926 to 16 June 2017).
Cochrane Database of Systematic Reviews (CDSR; 2017 Issue 6), part of the Cochrane Library (searched 13 June 2017).
Database of Abstracts of Reviews of Effects (DARE; 2015 Issue 2), part of the Cochrane Library (searched 13 June 2017).
LILACS (Latin American and Caribbean Health Sciences Literature; lilacs.bvsalud.org/es; searched 18 June 2017).
Networked Digital Library of Theses and Dissertations (NDLTD; www.ndltd.org/resources; searched 27 June 2017).
ClinicalTrials.gov (clinicaltrials.gov; searched 23 June 2017).
ISRCTN registry BioMed Central (www.isrctn.com; searched 23 June 2017).
World Health Organization International Clinical Trials Registry Portal (WHO ICTRP; apps.who.int/trialsearch; searched 23 June 2017).
Searching other resources
On 17 June 2017, we handsearched the World Congress of Behavioral and Cognitive Therapies from 1995 to 2016, together with the following websites.
Association for Behavioural and Cognitive Therapies (ABCT) Convention, 2008 to 2017 (www.abct.org/Conventions/?m=mConvention&fa=PastFutureConvention).
World Congress on ADHD, organised by the World Federation of ADHD, 2007 to 2017 (www.adhd‐federation.org/congresshistory).
Annual Meeting ‐ American Psychiatric Association (APA), 1973 to 2016 (www.psychiatry.org/psychiatrists/search‐directories‐databases/library‐and‐archive).
We also consulted experts and researchers in the field, including investigators from all review articles and primary studies identified through searches, about ongoing or unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (PL and FT) independently screened the titles and abstracts using the Early Review Organizing Software (EROS) (Ciapponi 2011; Glujovsky 2011; Glujovsky 2010). If it was clear from the title and abstract that the study did not meet the eligibility criteria, we rejected it. If it was not clear, then we obtained the full text of the study, and both review authors independently evaluated the paper using EROS to determine if the study should be included or excluded. If there was disagreement, the review authors tried to solve it by reaching a consensus. In the case that these two review authors could not reach a consensus, a third author (AC) independently assessed the study and resolved the disagreement. We recorded the results of this selection process in a PRISMA diagram (Moher 2009).
Data extraction and management
Two review authors (PL and FT) independently extracted data from each included study and entered the information onto a pro‐forma document designed and piloted for this purpose. We extracted information about the 'Risk of bias' criteria and the methods of participant selection. We also extracted information about the populations, interventions, comparisons, outcomes, outcome data, study designs, gender, comorbidity, severity and baseline symptoms. The two review authors resolved any differences in opinion by consensus. If they were unable to do so, a third review author (AC) was included in the decision process, and all three review authors discussed the issue and made a final decision.
Assessment of risk of bias in included studies
We evaluated the risk of bias in each included trial using the seven criteria described in Table 8.5.d ('Criteria for judging risk of bias in the "Risk of bias" assessment tool') of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (PL and FT) independently assessed each included study as being at low, high or unclear (uncertain) risk of bias for each domain, using EROS software (Ciapponi 2011; Glujovsky 2011; Glujovsky 2010); see Table 4. If there were discrepancies between their assessments, and the two review authors were unable to reach a consensus, a third review author (AC) joined the decision‐making process. All three review authors discussed the issue and made a final decision.
1. Criteria for 'Risk of bias' judgement.
| Criteria | Description |
| Random sequence generation (selection bias ‐ biased allocation to interventions ‐ due to inadequate generation of a randomised sequence) |
|
| Allocation concealment (selection bias ‐ biased allocation to interventions ‐ due to inadequate concealment of allocations prior to assignment) |
|
| Blinding of participants and personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the study) |
|
| Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessors) |
|
| Incomplete outcome data (attrition bias due to the amount, nature or handling of incomplete outcome data) |
|
| Selective reporting (reporting bias due to selective outcome reporting) |
|
| Other bias (other sources of bias not captured by the other domains) |
|
Measures of treatment effect
Continuous data
We calculated mean differences (MD) when studies used the same measure and standardised mean differences (SMD) when studies used different measurement scales, and we present these with 95% confidence intervals (CIs). When necessary, we calculated the effect estimates from the P values, t statistics or other available statistics.
We interpreted the magnitude of effect for the SMD using a rule of thumb where we considered 0.2 as a small effect, 0.5 as a moderate effect, and 0.8 as a large effect (Cohen 1988).
For the studies that reported only change scores, we performed separate analyses from the studies that provided only final values. We combined both values using the generic inverse variance method (Higgins 2011).
In cases in which there were at least two studies pooled for the same comparison of results, we used the median change to facilitate readers' understanding.
We provide a table reporting relative effects in order to allow comparisons of effects of interventions across all outcomes (see Appendix 2). We reported the absolute and relative changes (95% CI) related to the control central estimates of each outcome (negative percentages indicate a reduction of symptoms).
Dichotomous data
We did not find dichotomous data to include in this review (see Lopez 2013; Table 5).
2. Additional methods table.
| Method | Approach |
| Measures of treatment effect |
Dichotomous data We did not find dichotomous data. Should these data become available in future updates of this review, we will calculate the risk ratio (RR) with 95% confidence intervals (CI), as most readers find it easier to understand the RR and 95% CI than the odds ratio and risk difference. |
| Unit of analysis issues |
Cluster‐RCTs We did not find cluster‐RCTs. This design is uncommon in this field. Should such studies become available in the future, and if the investigators report cluster‐randomised trial data as though the randomisation was performed on individuals rather than on clusters, we will request individual participant data to calculate an estimate of the intra‐cluster correlation coefficient (ICC). If the individual participant data are not available, we will obtain external estimates of the ICC from similar studies or available resources (Campbell 2000). Once established, we will use the ICC to re‐analyse the trial data to obtain approximate, correct analyses as described in section 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will combine the effect estimates and their corrected standard errors from cluster‐RCTs with those from parallel‐group designs using the generic inverse variance method (Higgins 2011). If the available information is insufficient to control for clustering in this manner, we will enter the data into Review Manager 5 (RevMan 2014), using individuals as the unit of analysis. We planned to perform sensitivity analyses to assess the potential bias that may occur as a result of the inadequately controlled clustered trials. Additionally, if the ICCs are obtained from external sources, we will perform sensitivity analyses to assess the potential biasing effects of inadequately controlled cluster‐randomised trials (Donner 2001). |
|
Cross‐over trials We did not find cross‐over trials. Should we find these types of studies in future updates of this review, and as the duration of any effect of CBT is unknown, we will use only first‐period data from any cross‐over trials that fit the inclusion criteria to avoid a possible carry‐over effect. | |
| Dealing with missing data | If the studies do not report the standard deviation (SD), we will calculate it from the P values, t values, CIs or standard errors (as described in section 7.7.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011). If this information is not reported or is unattainable, we will impute the SD from the study with the highest SD for that outcome, and assess the effects of this assumption on the analysis by conducting a sensitivity analysis. If the outcome data are reported as a median, a range or as a mean without a variance, we will report the data in additional tables. |
| Sensitivity analysis | We will conduct sensitivity analyses to:
|
CBT: cognitive‐behavioural intervention; CI: confidence interval; ICC: intracluster correlation coefficient; RCT: randomised controlled trial; SD: standard deviation.
See also Lopez 2013.
Unit of analysis issues
For each included study, we determined the appropriateness of the unit of analysis for the unit of randomisation and the design of each study (the number of observations had to match the number of units that were randomised). We expected to find trials with a simple parallel‐group design, with participants randomly allocated as individuals, and a single measurement collected and analysed for each outcome from each participant.
Cluster‐RCTs and cross‐over trials
We did not find cluster‐RCTs or cross‐over trials (see Lopez 2013; Table 5).
Multiple treatment groups
For trials with multiple treatment groups, we combined the results across all eligible treatment arms and compared them with the combined results across all eligible control arms, making single, pair‐wise comparisons. Where such a strategy prevented an investigation of the potential sources of heterogeneity, we analysed each treatment arm separately (against a common control group) but divided the sample size of the common comparator groups proportionately across each comparison (Higgins 2011, section 16.5.4). This approach prevented inappropriate double‐counting of individuals.
Dealing with missing data
When necessary, we attempted to contact the corresponding authors of the included studies up to three times to collect any unreported data.
We described missing data and dropouts for each included study in the 'Risk of bias' table (beneath the Characteristics of included studies tables), reporting the reasons for missing data and the number and characteristics of dropouts, and we discussed in the 'Quality of the evidence' section the extent to which the missing data could threaten our results due to attrition bias.
We made no assumptions about loss to follow‐up for continuous data, and we based the analyses on those participants who completed the trial.
See Lopez 2013 and Table 5.
Assessment of heterogeneity
We appraised the extent of clinical heterogeneity among the studies by comparing the distribution of participant characteristics (comorbidity, severity, baseline symptoms, ADHD subtype) and study factors (randomisation, allocation concealment, blinding of outcome assessment, loss to follow‐up, treatment type, type of control group, co‐interventions, different types of outcome measurements). We assessed these variables by subgroup analysis if I2 was more than 30%. Additionally, we deemed a low P value for the Chi2 test (< 0.10) as sufficient reason to explore causes of heterogeneity (Subgroup analysis and investigation of heterogeneity).
We described the statistical heterogeneity of the intervention effects by calculating the I2 statistic and using the Chi2 test. The thresholds used for the interpretation of I2 can be misleading because the importance of inconsistency depends on several factors. We interpreted it as follows.
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: represents considerable heterogeneity.
Assessment of reporting biases
Had there been at least 10 studies in a meta‐analysis, we would have used funnel plots to detect bias. Funnel plot asymmetry can be due to publication bias, but it can also be due to a real relationship between trial size and effect size, such as when larger trials have a lower adherence, and adherence is positively related to effect size. In general, asymmetry may be due to selection biases (publication bias, delayed publication bias, location bias, selective outcome reporting), poor methodological quality leading to spuriously inflated effects in smaller studies (poor methodological design, inadequate analysis, fraud), true heterogeneity or chance (Egger 1997). We used the test proposed by Egger 1997 for continuous outcomes to test for funnel plot asymmetry (Higgins 2011).
Data synthesis
We synthesised the results in a meta‐analysis using Review Manager 5 (RevMan 5) when we considered studies to be sufficiently homogenous in terms of population (regarding sex, age and diagnosis), interventions (comparable modalities of CBT) and comparisons (as a monotherapy or a part of a combined treatment) to avoid clinical heterogeneity, and in terms of outcome measurement methods to avoid methodological heterogeneity (RevMan 2014). Two authors assessed homogeneity independently and solved discrepancies by consensus. In the cases where comparisons had a considerable heterogeneity but the same direction, we present both the global results and the results of each study separately in order to show the range of effects comprised in the comparisons.
We used both a fixed‐effect model and a random‐effects model and compared them to assess the degree of statistical heterogeneity. Because we assumed that clinical heterogeneity was very likely to impact our review results, given the nature of the interventions included, we primarily reported the results of the random‐effects model, regardless of statistical evidence of heterogeneity. We calculated all effects using inverse variance methods. For continuous data, the change in score from baseline to postintervention was the main outcome of interest. We analysed separately continuous data reported as change scores in some studies and as final values in other studies. Additionally, we combined these values using the generic inverse variance method (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
Where it was possible to secure the necessary data, we conducted subgroup analyses, classifying the trials as follows.
Type of ADHD subtype: inattentive, hyperactive‐impulsive or combined type.
Type of control group: other specific treatment or unspecific control conditions (supportive psychotherapies, waiting list or no treatment).
We calculated a pooled effect size for each subgroup.
Sensitivity analysis
We used sensitivity analyses to assess the impact of risk of bias on the results of the primary analyses. For this review, we undertook sensitivity analyses to determine the effect of removing from the analysis: studies with high risk of selection bias (associated with sequence generation or allocation concealment); studies with high risk of performance bias (associated with issues of blinding); and studies with high risk of attrition bias (associated with completeness of data). In addition, we assessed the sensitivity of findings to any imputed data within a study.
We investigated the impact of applying a fixed‐effect model on the results compared to that of a random‐effects model. We also compared the impact of using the odds ratio as an effect measure compared to the risk difference.
Summary of findings table
We prepared a 'Summary of findings' table for our three main comparisons (see Types of interventions) according to GRADE methodology (Atkins 2004; Guyatt 2011), using GRADEpro GDT software (GRADEpro 2015). We included our primary outcome, the core symptoms of ADHD (self‐, clinician‐ or observer‐reported), in the tables.
Two review authors (AC and PL) independently assessed the quality of the evidence as high, moderate, low or very low, downgrading the rating according to the presence of study limitations, including the studies' 'Risk of bias' level; imprecision; inconsistency of results; indirectness of evidence; and likely publication bias.
To assess the magnitude of effect for continuous outcomes, we used the criteria suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect.
Results
Description of studies
Results of the search
Our database searches returned 12,088 records (10,306 unique records). We did not find any relevant records by handsearching sources of congress or conference abstracts. After screening titles and abstracts, we deemed 10,237 records to be irrelevant and retrieved 69 full texts for further scrutiny. Of these 69 reports, 14 studies (from 15 reports) met our predefined inclusion criteria (Criteria for considering studies for this review); we considered one additional reference to be a secondary reference of Stevenson 2002 because the authors used the same data set and reported the same results. We excluded 49 reports as irrelevant (Excluded studies) and identified five ongoing studies. See Figure 1.
1.
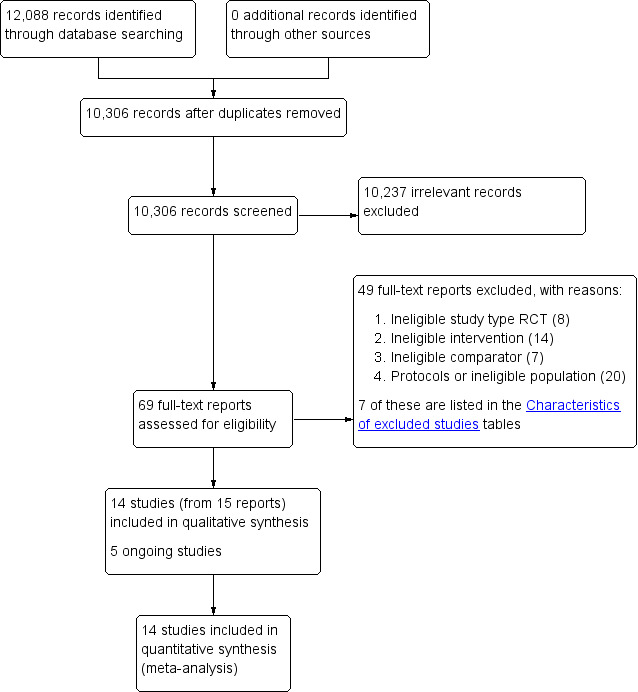
Study flow diagram.
Included studies
Study design
Authors of the 14 included studies described them as RCTs, with a parallel group design, blinded for participants; each lasted 8 to 15 weeks. We did not find any cluster‐RCTs or cross‐over trials.
Setting
Four trials were in outpatients in the USA (Fleming 2015; Safren 2005; Safren 2010; Solanto 2010), and three took place in Sweden (Hirvikoski 2011; Moëll 2015; Pettersson 2017). The seven remaining trials were carried out in: Australia (Stevenson 2002), China (Gu 2017), Finland (Virta 2010), Iceland (Emilsson 2011), the Netherlands (Hepark 2015; Schoenberg 2014), and Spain (Vidal Estrada 2013).
Participants
In total, the 14 trials recruited 700 participants aged 18 to 65 years.
The included trials used three different versions of DSM criteria.
Third Edition‐Revised (DSM‐III‐R 1989), used in one trial (Stevenson 2002);
Fourth Edition (DSM‐IV 1994), used in eight trials (Emilsson 2011; Hirvikoski 2011; Moëll 2015; Safren 2005; Safren 2010; Solanto 2010; Vidal Estrada 2013; Virta 2010); and IV‐Text Revision (DSM‐IV‐TR 2000), used in three trials (Hepark 2015; Pettersson 2017; Schoenberg 2014).
Fifth Edition (DSM‐5 2013) used in two trials (Fleming 2015; Gu 2017).
Intervention and comparisons
All of the trials reported the effects of CBT‐based treatments on adults with ADHD symptoms. We included studies that assessed mindfulness‐based interventions when authors explicitly stated that the treatment included CBT principles or techniques together with mindfulness procedures. We considered meta‐cognitive therapy and dialectical behaviour therapy as variants of CBT. The trials included the following interventions and comparisons.
-
CBT versus unspecific control conditions.
Gu 2017: mindfulness‐based cognitive therapy versus waiting list.
Hepark 2015: mindfulness‐based cognitive therapy versus waiting list.
Hirvikoski 2011: dialectical behavioural therapy‐based skills training versus structured discussion group.
Moëll 2015: CBT‐inspired Internet‐based course with support (Living Smart) versus waiting list.
Pettersson 2017: Internet CBT in a self‐help format (iCBT‐S) versus waiting list, and Internet CBT plus weekly group therapy sessions (iCBT‐G) versus waiting list.
Schoenberg 2014: mindfulness‐based cognitive therapy versus waiting list.
Solanto 2010: meta‐cognitive therapy versus supportive therapy.
Stevenson 2002: standard CBT versus waiting list.
Virta 2010: standard CBT versus no treatment.
-
CBT combined with pharmacotherapy versus pharmacotherapy alone.
-
CBT versus other specific interventions.
Fleming 2015: dialectical behavioural therapy versus a skills handouts control condition.
Safren 2010: standard CBT versus relaxation with educational support.
Vidal Estrada 2013: standard CBT plus limited psychoeducation versus psychoeducation.
Virta 2010: standard CBT versus cognitive training.
Outcome measures
The included trials used a diversity of outcome measures, which made it difficult to make statistical comparisons between treatment regimens. The outcomes were based on clinical assessments by a physician or by self‐report through the use of validated scales.
Primary outcomes
We defined treatment efficacy as an improvement in the core symptoms of ADHD, which was evaluated in terms of specific ADHD symptoms, namely hyperactivity, inattentiveness and impulsivity, using clinical, symptom‐specific scales and scores. The authors used a heterogeneous group of scales for each outcome (see details in Table 6).
3. Primary outcomes: scales used.
| Outcomes | Studies and instruments |
| ADHD symptom severity |
|
| Inattention |
|
| Hyperactivity |
|
| Impulsivity |
|
| Hyperactivity‐impulsivity |
|
| Clinical Global Impression |
|
ADHD: attention deficit hyperactivity disorder; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders ‐ Third Edition ‐ Revised; NIMH: National Institutes of Mental Health.
Secondary outcomes
The authors used a heterogeneous group of scales to assess the secondary outcomes (see details in Table 7).
4. Secondary outcomes: scales used.
| Outcomes | Studies and instruments |
| Depression |
|
| Anxiety |
|
| State anger |
|
| Trait anger |
|
| Self‐esteem |
|
| Quality of life |
|
| Employment status | No study included this outcome. |
ADHD: attention deficit hyperactivity disorder.
Excluded studies
We excluded 49 full‐text reports as ineligible for this review for the following reasons.
Not an RCT (n = 8).
Not CBT (n = 14).
Comparison not considered in this review (n = 7).
Others (protocols, or not ADHD in adults) (n = 20).
We described seven of these studies, which initially seemed to merit inclusion but on closer inspection did not, in the Characteristics of excluded studies tables. We excluded one study because it compared group psychotherapy versus individual psychotherapy, thereby affecting the comparability of the intervention of interest of our review (Philipsen 2015). We excluded two studies because the authors explained that their goal was to assess the efficacy of mindfulness, in Mitchell 2013, and mindfulness plus virtual reality, in Serra‐Pla 2017, without introducing other treatment modalities such as CBT. We excluded four studies because the comparisons used in these studies did not correspond to the comparisons included in our protocol (Cherkasova 2016; Weiss 2012; Young 2015; Young 2017).
Ongoing studies
We also found five ongoing studies (ISRCTN03732556; NCT02463396; NCT02062411; NCT02210728; NCT02829970). See Characteristics of ongoing studies tables.
Risk of bias in included studies
No trial was free from bias across all 'Risk of bias' domains. Authors often described randomisation and allocation concealment processes poorly (See Figure 2 and Figure 3 for 'Risk of bias' graphs). When the authors did not explicitly state the sequence generation method, we asked them for this information through email correspondence (Emilsson 2011; Gu 2017; Hepark 2015; Hirvikoski 2011; Safren 2005; Safren 2010; Schoenberg 2014; Solanto 2010; Stevenson 2002; Vidal Estrada 2013; Virta 2010).
2.
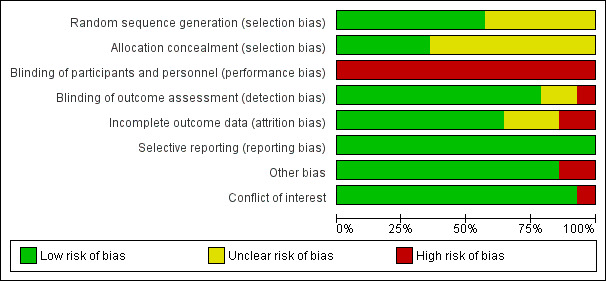
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
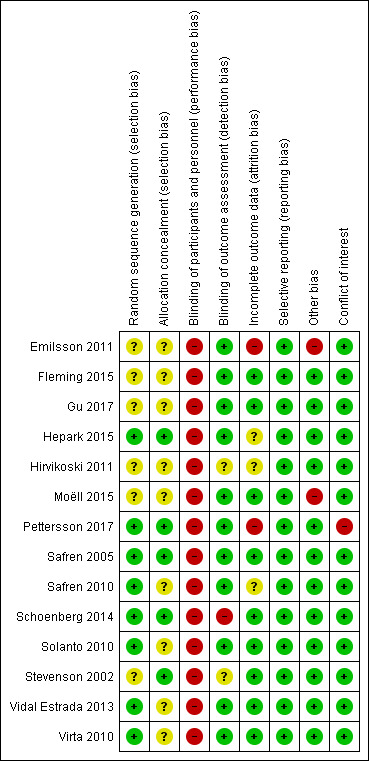
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
All included studies were at high risk of performance bias because it is not possible to blind personnel in psychotherapy. For 10 studies, this was the only domain at high risk of bias.
We considered four studies to be at high risk of bias for another domain. Emilsson 2011 was at high risk of attrition and other bias. Pettersson 2017 was at high risk of attrition bias and had conflicts of interest. These two studies had a higher number of domains with high risk of bias. Additionally, we considered Schoenberg 2014 to be at high risk of detection bias because the outcome assessor was not blinded. Finally, we judged Moëll 2015 to be at high risk of other bias.
Allocation
Sequence generation
Eight trials were at low risk of bias (Hepark 2015; Pettersson 2017; Safren 2005; Safren 2010; Schoenberg 2014; Solanto 2010; Vidal Estrada 2013; Virta 2010). Six trials were at unclear risk of bias because there was no description of the sequence generation process (Emilsson 2011; Fleming 2015; Gu 2017; Hirvikoski 2011; Moëll 2015; Stevenson 2002).
Allocation concealment
Five trials had an adequate description of the allocation concealment process, and we considered them to be at low risk of bias in this domain (Hepark 2015; Pettersson 2017; Safren 2005; Schoenberg 2014; Stevenson 2002).
We considered the remaining nine trials to be at unclear risk of bias. Solanto 2010 affirmed that individuals were stratified by whether or not they were currently receiving medication for ADHD and otherwise randomly assigned to either the CBT or the support group; however, the authors did not describe how they designed this process. Authors of the eight remaining trials provided no information about the randomisation process (Emilsson 2011; Fleming 2015; Gu 2017; Hirvikoski 2011; Moëll 2015; Safren 2010; Vidal Estrada 2013; Virta 2010).
Blinding
Blinding of participants and personnel
We judged all studies to be at high risk of performance bias because, as usual in psychotherapy, it is not possible to blind the personnel (Emilsson 2011; Fleming 2015; Gu 2017; Hepark 2015; Hirvikoski 2011; Moëll 2015; Pettersson 2017; Safren 2005; Safren 2010; Schoenberg 2014; Solanto 2010; Stevenson 2002; Vidal Estrada 2013; Virta 2010).
Blinding of outcome assessment
We considered 11 trials to be at low risk of detection bias because the assessors were blinded to group assignment (Emilsson 2011; Fleming 2015; Gu 2017; Hepark 2015; Moëll 2015; Pettersson 2017; Safren 2005; Safren 2010; Solanto 2010; Vidal Estrada 2013; Virta 2010).
We considered the risk of detection bias to be unclear in two studies because authors did not adequately describe the blinding of the results (Hirvikoski 2011; Stevenson 2002).
Finally, we rated one study, Schoenberg 2014, at high risk of detection bias because the outcome assessor was not blinded.
Incomplete outcome data
We considered nine trials to be at a low risk of attrition bias since the effect size among the missing outcomes was not enough to have a clinically relevant impact on the observed effect size (Fleming 2015; Gu 2017; Moëll 2015; Safren 2005; Schoenberg 2014; Solanto 2010; Stevenson 2002; Vidal Estrada 2013; Virta 2010).
We judged two studies to be at high risk of attrition bias because the dropout rate was around 40% (Emilsson 2011; Pettersson 2017), and the remaining three were at unclear risk of bias: Hepark 2015 and Safren 2010 performed an intention‐to‐treat (ITT) analysis, but there was unbalanced rate of dropouts, and Hirvikoski 2011 performed ITT analysis, but there was an important rate of dropouts (around 20% per group).
Selective reporting
We considered all trials to be free of reporting bias because the published results corresponded to those expected in these types of studies (Emilsson 2011; Fleming 2015; Gu 2017; Hepark 2015; Hirvikoski 2011; Moëll 2015; Pettersson 2017; Safren 2005; Safren 2010; Schoenberg 2014; Solanto 2010; Stevenson 2002; Vidal Estrada 2013; Virta 2010). Five studies prospectively registered the trial, but it was clear that the published reports included all expected outcomes, including those that were pre‐specified (Emilsson 2011; Moëll 2015; Safren 2005; Safren 2010; Solanto 2010); we assessed whether the outcome measures described in the Methods of the paper were reported in the Results section.
Other potential sources of bias
We considered 12 trials to be either free of other potential sources of bias or as being at low risk of other bias (Fleming 2015; Gu 2017; Hepark 2015; Hirvikoski 2011; Pettersson 2017; Safren 2005; Safren 2010; Schoenberg 2014; Solanto 2010; Stevenson 2002; Vidal Estrada 2013; Virta 2010).
We considered two trials to be at high risk of other bias (Emilsson 2011; Moëll 2015). Emilsson 2011 did not ask the participants in either condition to refrain from engaging in other interventions during the study period. In Moëll 2015, the authors reported a lack of confirmed ADHD‐diagnoses for some of the participants, and 12% did not receive an ADHD diagnosis after their previous neuropsychiatric assessment and were thus classified as having sub‐clinical ADHD.
Conflicts of interest
One study had conflicts of interest, and we considered it to be at high risk of bias (Pettersson 2017). We rated all remaining studies at low risk of bias for this domain (Emilsson 2011; Fleming 2015; Gu 2017; Hepark 2015; Hirvikoski 2011; Moëll 2015; Safren 2005; Safren 2010; Schoenberg 2014; Solanto 2010; Stevenson 2002; Vidal Estrada 2013; Virta 2010).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Cognitive‐behavioural interventions versus unspecific control for attention deficit hyperactivity disorder (ADHD) in adults.
| Cognitive‐behavioural interventions versus unspecific control for ADHD in adults | ||||||
| Patient or population: adults with ADHD Setting: ambulatory/hospital (outpatients) Intervention: CBT Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control conditions | Risk with CBT | |||||
| CBT versus supportive therapy | ||||||
| ADHD symptoms: observer‐rated Assessed by: various scales Follow‐up: 12 weeks |
— | The mean ADHD observer‐rated symptoms score in the intervention groups was 0.56 standardised deviations lower (1.01 lower to 0.12 lower) | — | 81 (1 RCT) | ⊕⊕⊝⊝ Lowa | Moderate effect sizeb |
| ADHD symptoms: self‐reported Assessed by: various scales Follow‐up: 12 to 14 weeks |
— | The mean ADHD self‐rated symptoms score in the intervention groups was 0.16 standardised deviations lower (0.52 lower to 0.19 higher) | — | 122 (2 RCTs) | ⊕⊕⊝⊝ Lowc | Small effect sizeb |
| CBT versus waiting list control | ||||||
| ADHD symptoms: observer‐rated Assessed by: various scales Follow‐up: 8 to 12 weeks |
‐ | The mean ADHD self‐rated symptoms score in the intervention groups was 1.22 standardised deviations lower (2.03 lower to 0.41 lower) | — | 126 (2 RCTs) | ⊕⊝⊝⊝ Very lowd | Large effect sizeb |
| ADHD symptoms: self‐reported Assessed by: various scales Follow‐up: 8 to 12 weeks |
— | The mean ADHD self‐rated symptoms score in the intervention groups was 0.84 standardised deviations lower (1.18 lower to 0.50 lower) | — | 251 (5 RCTs) | ⊕⊕⊕⊝ Moderatee | Large effect sizeb |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADHD: attention deficit hyperactivity disorder; CBT: cognitive‐behavioural therapy;CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a We downgraded the quality of evidence due to imprecision (considering the width of the CI), methodological limitations (due to high risk of bias in blinding of participants and personnel), and because the evidence is based on a single study. bTo assess the magnitude of effect for continuous outcomes, we used the criteria suggested by Cohen 1988: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. cWe downgraded the quality of evidence due to imprecision (considering the width of the CI) and methodological limitations (due to high risk of bias in blinding of participants and personnel and five other domains with unclear risk of bias). d We downgraded the quality of evidence due to imprecision (considering the width of the CI), methodological limitations (due to high risk of bias in blinding of participants and personnel and three other domains with unclear risk of bias) and inconsistency (considering the I2 of 74%). The estimates of each study was: Hepark 2015 SMD −0.85 lower (−1.30 lower to −0.40 lower) and Stevenson 2002 SMD −1.68 lower (−2.39 lower to −0.98 lower). e We downgraded the quality of evidence due to methodological limitations (considering that two out of the five studies were at high risk of bias in more than one domain other than blinding of participants and personnel).
Summary of findings 2. Cognitive‐behavioural therapy plus pharmacotherapy versus pharmacotherapy alone for attention deficit hyperactivity disorder (ADHD) in adults.
| Cognitive‐behavioural therapy plus pharmacotherapy versus pharmacotherapy alone for ADHD in adults | ||||||
| Patient or population: adults with ADHD Setting: ambulatory Intervention: CBT plus pharmacotherapy Comparison: pharmacotherapy alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control conditions | Risk with CBT plus pharmacotherapy | |||||
| ADHD symptoms: clinician rated Assessed by: various scales Follow‐up: 8 to 15 weeks |
— | The mean ADHD clinician‐rated symptoms score in the intervention groups was 0.80 standardised deviations lower (1.31 lower to 0.30 lower) | — | 65 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | Large effect sizec |
| ADHD symptoms: self‐reported Assessed by: ADHD Current Symptoms Scale (range 0 (best) to 54 (worst)) Follow‐up: 8 to 15 weeks |
The mean ADHD self‐rated symptoms score in the control groups ranged from 14.75 to 17.22. | The mean ADHD self‐rated symptoms score in the intervention groups was 7.42 lower (11.63 lower to 3.22 lower) | — | 66 (2 RCTs) | ⊕⊕⊝⊝ Lowb | Large effect size |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADHD: attention deficit hyperactivity disorder; CBT: cognitive‐behavioural therapy; CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded the quality of evidence due to methodological limitations (high risk of bias in blinding of participants and personnel, and the fact that Emilsson 2011 had a high risk of bias in three domains in one of the two included studies). bWe downgraded the quality of evidence due to imprecision (considering the width of the CI). cTo assess the magnitude of effect for continuous outcomes, we used the criteria suggested by Cohen 1988: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect.
Summary of findings 3. Cognitive‐behavioural therapy versus other non‐pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in adults.
| Cognitive‐behavioural therapy versus other interventions for ADHD in adults | ||||||
| Patient or population: adults with ADHD Setting: ambulatory/hospital (outpatients) Intervention: CBT Comparison: other specific non‐pharmacological treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control conditions | Risk with CBT | |||||
| ADHD symptoms: clinician‐rated Assessed by: various scales Follow‐up: 10 to 12 weeks |
— | The mean ADHD clinician‐rated symptoms score in the intervention groups was 0.58 standardised deviations lower (0.98 lower to 0.17 lower) | — | 97 (2 RCTs) | ⊕⊕⊝⊝ Lowa | Moderate effect sizeb |
| ADHD symptoms: self‐reported Assessed by: various scales Follow‐up: 8 to 12 weeks |
— | The mean ADHD self‐reported symptoms score in the intervention groups was 0.44 standardised deviations lower (0.88 lower to 0.01 lower) | — | 156 (4 RCTs) | ⊕⊕⊝⊝ Lowa | Small effect sizeb |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADHD: attention deficit hyperactivity disorder; CBT: cognitive‐behavioural therapy; CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a We downgraded the quality of evidence because of imprecision (considering the width of the CI) and methodological limitations (due to the high risk of bias in blinding of participants and personnel and three other domains with unclear risk of bias). bTo assess the magnitude of effect for continuous outcomes, we used the criteria suggested by Cohen 1988: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect.
CBT versus unspecific control conditions
Primary outcomes
ADHD symptoms
Aggregated ADHD symptoms
Three studies evaluated the effect of CBT on observer‐reported ADHD symptoms against unspecific control conditions (Analysis 1.1). Solanto 2010 compared CBT to supportive therapy for this outcome and found a significant effect of treatment (SMD −0.56, 95% CI −1.01 to −0.12; 81 participants; low‐quality evidence; moderate effect size, see Summary of findings table 1). Two studies (126 participants) comparing CBT to waiting list showed significant effects favouring CBT (SMD −1.22, 95% CI −2.03 to −0.41; I2 = 74%; very low‐quality evidence; large effect size; (see Summary of findings table 1): Hepark 2015 (SMD −0.85, 95% CI −1.30 to −0.40; 83 participants) and Stevenson 2002 (SMD −1.68, 95% CI −2.39 to −0.98; 43 participants). Considering that the results of Hepark 2015 and Stevenson 2002 are expressed as SMDs, in line with the methods described in Measures of treatment effect, we present the results of Solanto 2010 as an SMD (SMD −0.56, 95% CI −1.01 to −0.12) in the forest plot because it is not possible to present different statistical measures in the same graphic.
Seven studies assessed the effect of CBT versus unspecific control conditions on self‐reported ADHD symptoms (Analysis 1.2). Two studies (122 participants) compared CBT with supportive therapy (Hirvikoski 2011; Solanto 2010), finding no significant effect of treatment (SMD −0.16, 95% CI −0.52 to 0.19; I2 = 0%; low‐quality evidence; small effect size; see Table 1). Analysis of the five studies (251 participants) that compared CBT to waiting list revealed a significant effect on this outcome favouring CBT (SMD −0.84, 95% CI −1.18 to −0.50; I2 = 38%; Gu 2017; Hepark 2015; Pettersson 2017; Schoenberg 2014; Virta 2010; moderate‐quality evidence; large effect size; see Table 1).
1.2. Analysis.

Comparison 1 CBT vs unspecific control conditions, Outcome 2 ADHD symptoms (self‐reported).
Disaggregated ADHD symptoms
Two studies evaluated the effect of CBT on clinician‐reported inattention separately (Analysis 1.3). One study, Solanto 2010, compared CBT to supportive therapy using the Adult ADHD Investigator Symptom Rating Scale (AISRS) Inattention subscale (range 0 (best) to 27 (worst)) (MD −2.47 points, 95% CI −4.43 points to −0.51 points; 81 participants), and the other study, Hepark 2015, compared CBT to waiting list using Conners' Adult ADHD Rating Scale ‐ Investigator Rated (CAARS‐INV; range 0 (best) to 27 (worst)) (MD −4.10 points, 95% CI −6.00 points to −2.20 points; 83 participants). Both studies showed a significant effect of treatment.
1.3. Analysis.
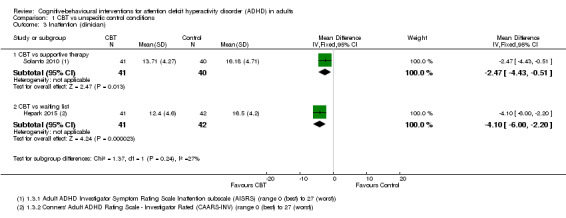
Comparison 1 CBT vs unspecific control conditions, Outcome 3 Inattention (clinician).
Four studies (244 participants) compared the effect of CBT on self‐reported inattention to waiting list (Gu 2017; Hepark 2015; Moëll 2015; Schoenberg 2014). The analysis revealed a significant effect in favour of CBT (SMD −1.10, 95% CI −1.37 to −0.82; I2 = 0%; large effect size; Analysis 1.4).
1.4. Analysis.
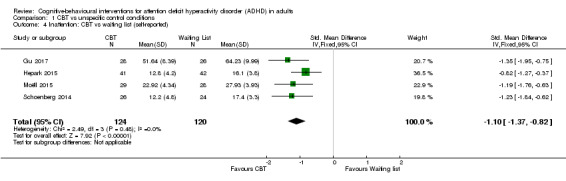
Comparison 1 CBT vs unspecific control conditions, Outcome 4 Inattention: CBT vs waiting list (self‐reported).
Hepark 2015 assessed clinician‐reported hyperactivity‐impulsivity using the CAARS‐INV (range 0 (best) to 27 (worst)) in the comparison of CBT versus waiting list and found a significant effect of treatment on this outcome (MD −2.50 points, 95% CI −4.63 points to −0.37 points; 83 participants; see the illustrative forest plot in Analysis 1.5).
1.5. Analysis.

Comparison 1 CBT vs unspecific control conditions, Outcome 5 Hyperactivity‐impulsivity: CBT vs waiting list (clinician).
Four studies (244 participants) compared CBT to waiting list for self‐reported hyperactivity‐impulsivity (Gu 2017; Hepark 2015; Moëll 2015; Schoenberg 2014), finding a significant effect in favour of CBT (SMD −0.60, 95% CI −0.98 to −0.22; I2 = 53%; moderate effect size; Analysis 1.6).
1.6. Analysis.
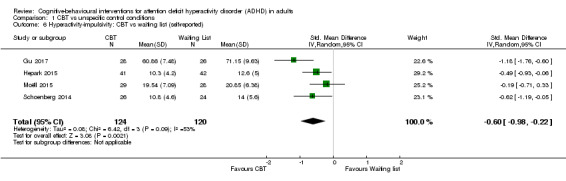
Comparison 1 CBT vs unspecific control conditions, Outcome 6 Hyperactivity‐impulsivity: CBT vs waiting list (self‐reported).
All‐cause treatment discontinuation
Schoenberg 2014 reported excluding two participants because one did not attend the full 12‐week mindfulness‐based cognitive therapy intervention, and the second started extra mindfulness training outside the intervention; nine other participants dropped out of the study due to competing time commitments. Gu 2017 reported that two participants dropped out of mindfulness‐based cognitive therapy after six sessions and did not complete the post‐treatment or follow‐up assessments. There were no dropouts due to adverse events reported for this comparison.
Two of the 21 participants who completed the treatment from a study that evaluated dialectical behavioural therapy reported adverse events to the group leaders at the post‐treatment assessment. Both of them reported anxiety related to separation from the group (Hirvikoski 2011). They had both started pharmacological treatment during the ongoing group treatment. In the control group, two individuals (2/20 who completed the group) reported adverse events to the project leader (TH). These individuals also experienced temporary anxiety due to separation from the group, and they especially missed other participants in the group, rather than group leaders or the sessions themselves. No serious adverse events were reported.
Secondary outcomes
Psychopathology
Depression
Six studies assessed self‐reported depression. One study (81 participants) compared CBT to supportive therapy (Solanto 2010), finding no significant differences (SMD 0.07, 95% CI −0.36 to 0.51; small effect size). The remaining five studies (258 participants) compared CBT to waiting list (Gu 2017; Hepark 2015; Moëll 2015; Pettersson 2017; Virta 2010), finding a significant effect of treatment on self‐reported depression (SMD −0.36, 95% CI −0.60 to −0.11; I2 = 0%; small effect size; see Analysis 1.7).
1.7. Analysis.
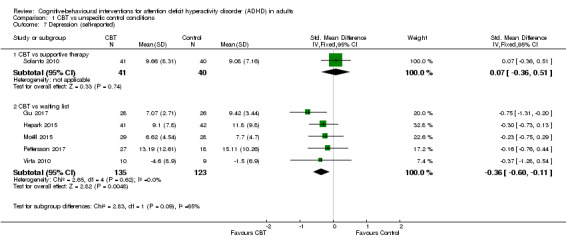
Comparison 1 CBT vs unspecific control conditions, Outcome 7 Depression (self‐reported).
Anxiety
One study (81 participants) assessed clinician‐rated anxiety using the Hamilton Anxiety Scale (HAM‐A; range 0 (best) to 56 (worst)) (Solanto 2010), finding no significant effect of CBT compared to supportive therapy (MD −0.81 points, 95% CI −3.21 points to 1.59 points; see the illustrative forest plot in Analysis 1.8).
1.8. Analysis.
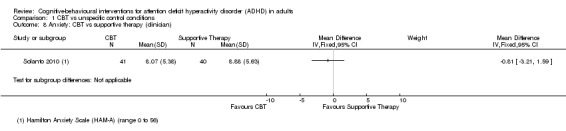
Comparison 1 CBT vs unspecific control conditions, Outcome 8 Anxiety: CBT vs supportive therapy (clinician).
Four studies (239 participants) compared CBT to waiting list for self‐reported anxiety (Gu 2017; Hepark 2015; Moëll 2015; Schoenberg 2014). The analysis revealed a significant effect favouring CBT on this outcome (SMD −0.45, 95% CI −0.71 to −0.19; I2 = 23%; small effect size; Analysis 1.9).
1.9. Analysis.

Comparison 1 CBT vs unspecific control conditions, Outcome 9 Anxiety: CBT vs waiting list (self‐reported).
Anger
One study (43 participants) compared CBT to waiting list using the State‐Trait Anger Expression Inventory (STAXI; range 0 (best) to 66 (worst)) (Stevenson 2002), finding a significant effect of treatment on self‐reported state anger (MD −3.30 points, 95% CI −5.62 points to −0.98 points; see the illustrative forest plot in Analysis 1.10) and a non‐significant effect on self‐reported trait anger (MD −3.80 points, 95% CI −7.63 points to 0.03 points; see the illustrative forest plot in Analysis 1.11).
1.10. Analysis.

Comparison 1 CBT vs unspecific control conditions, Outcome 10 State anger: CBT vs waiting list (self‐reported).
1.11. Analysis.
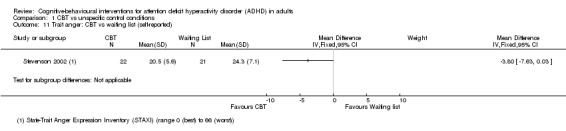
Comparison 1 CBT vs unspecific control conditions, Outcome 11 Trait anger: CBT vs waiting list (self‐reported).
Self‐esteem
Two studies assessed this outcome (Analysis 1.12). One study, Solanto 2010, compared CBT to supportive therapy and found no significant effect of treatment (MD 0.00, 95% CI ‐1.85 to 1.85; 81 participants). The other study, Stevenson 2002, compared CBT to waiting list and found a significant effect favouring CBT (MD 12.40, 95% CI 4.55 to 20.25; 43 participants; large effect size).
1.12. Analysis.
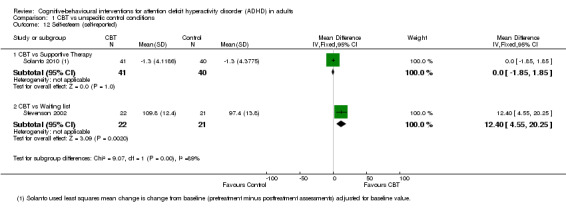
Comparison 1 CBT vs unspecific control conditions, Outcome 12 Self‐esteem (self‐reported).
Quality of life
Two studies (64 participants) evaluated CBT versus waiting list (Pettersson 2017; Virta 2010), finding no significant effect of treatment on self‐reported quality of life (SMD 0.21, 95% CI −0.29 to 0.71; I2 = 0%; small effect size; Analysis 1.13).
1.13. Analysis.
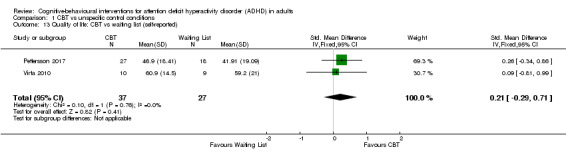
Comparison 1 CBT vs unspecific control conditions, Outcome 13 Quality of life: CBT vs waiting list (self‐reported).
CBT plus pharmacotherapy versus pharmacotherapy alone
Primary outcomes
ADHD symptoms
Aggregated ADHD symptoms
Two studies compared CBT plus pharmacotherapy versus pharmacotherapy alone (Emilsson 2011; Safren 2005). The analysis revealed a significant effect of treatment on clinician‐reported ADHD symptoms (SMD −0.80, 95% CI −1.31 to −0.30; I2 = 0%; 65 participants; Analysis 2.1; very low‐quality evidence; large effect size) and self‐reported ADHD symptoms (MD −7.42 points, 95% CI −11.63 points to −3.22 points; 66 participants; I2 = 0%; Analysis 2.2; low‐quality evidence), as assessed using the Current Symptoms Scale (CSS; range 0 (best) to 54 (worst)). See Table 2.
2.1. Analysis.
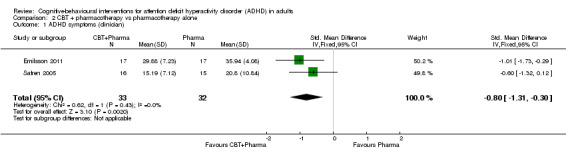
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 1 ADHD symptoms (clinician).
2.2. Analysis.
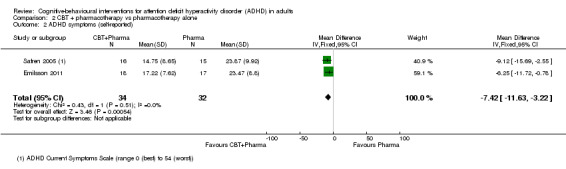
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 2 ADHD symptoms (self‐reported).
Disaggregated ADHD symptoms
Only one study (35 participants) compared CBT plus pharmacotherapy versus pharmacotherapy alone (Emilsson 2011). The study authors found a significant effect of the combined treatment for self‐reported inattention (MD −4.54 points, 95% CI −7.75 points to −1.33 points; see the illustrative forest plot in Analysis 2.3) but no significant effect for self‐reported hyperactivity‐impulsivity (MD −1.70 points, 95% CI −5.29 points to 1.89 points; see the illustrative forest plot in Analysis 2.4), as assessed using the CSS (range 0 (best) to 54 (worst)).
2.3. Analysis.
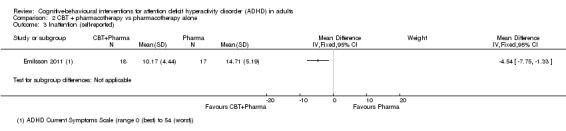
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 3 Inattention (self‐reported).
2.4. Analysis.
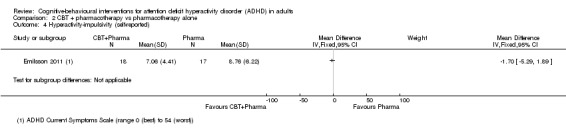
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 4 Hyperactivity‐impulsivity (self‐reported).
All‐cause treatment discontinuation
In one study, Emilsson 2011, one participant in the CBT plus pharmacotherapy condition reported severe distress at the end of treatment due to changes in personal circumstances. This participant then received individual treatment and was not assessed at follow‐up. Emilsson 2011 also described that four participants dropped out during the treatment phase without explanation, one dropped out upon moving out of the area, one due to illness in the family and one due to pregnancy. No dropouts due to adverse events were reported for this comparison, and no other study reported dropouts due to serious adverse events.
Secondary outcomes
Psychopathology
Clinical Global Impression
A meta‐analysis of two studies (65 participants) comparing CBT plus pharmacotherapy versus pharmacotherapy alone revealed a significant effect on the Clinical Global Impression scale (CGI; range 1 (best) to 7 (worst)) in favour of the combined treatment (MD −0.75 points, 95% CI −1.21 points to −0.30 points; Emilsson 2011; Safren 2005; I2 = 0%; Analysis 2.5).
2.5. Analysis.
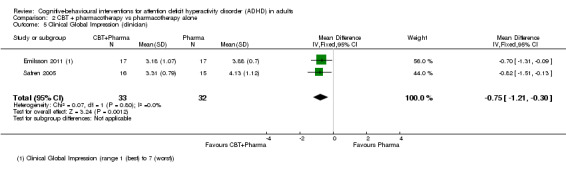
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 5 Clinical Global Impression (clinician).
Depression
Only one study (31 participants) evaluated clinician‐reported depression using the Hamilton Depression Scale (HAM‐D; range 0 (best) to 52 (worst)) (Safren 2005). The comparison of CBT plus pharmacotherapy versus pharmacotherapy alone showed a significant effect of treatment on this outcome (MD −5.56 points, 95% CI −9.71 points to −1.41 points; Analysis 2.6).
2.6. Analysis.
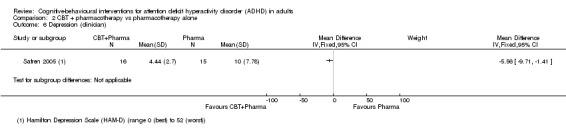
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 6 Depression (clinician).
Two studies (66 participants) assessed self‐reported depression using the Beck Depression Inventory (BDI; range 0 (best) to 63 (worst)) (Emilsson 2011; Safren 2005), reporting a significant effect of CBT plus pharmacotherapy compared to pharmacotherapy alone (MD −6.09 points, 95% CI −9.55 points to −2.63 points; I2 = 0%; Analysis 2.7).
2.7. Analysis.
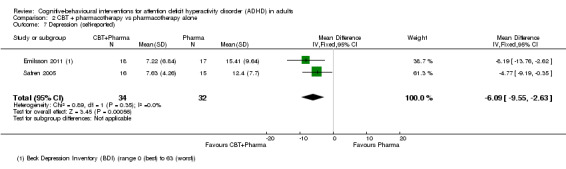
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 7 Depression (self‐reported).
Anxiety
One study (31 participants) evaluated clinician‐reported anxiety using the HAM‐A (range 0 (best) to 56 (worst)) (Safren 2005), finding a significant effect favouring combined CBT plus pharmacotherapy compared to pharmacotherapy alone (MD −5.68 points, 95% CI −10.32 points to −1.04 points; Analysis 2.8).
2.8. Analysis.
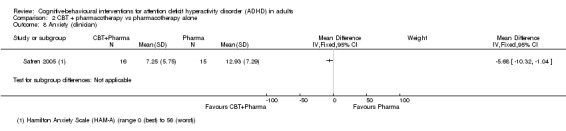
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 8 Anxiety (clinician).
The analysis of self‐reported anxiety in two studies (66 participants) comparing CBT plus pharmacotherapy versus pharmacotherapy alone revealed a significant effect favouring the combined treatment (SMD −0.58, 95% CI −1.08 to −0.08; I2 = 0%; moderate effect size; Emilsson 2011; Safren 2005; Analysis 2.9).
2.9. Analysis.
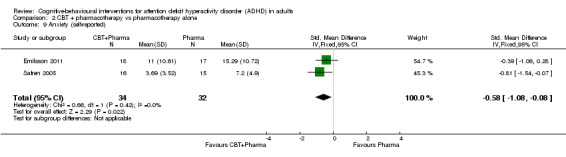
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 9 Anxiety (self‐reported).
No studies reported data on our other secondary outcomes for this comparison: anger, self‐esteem or quality of life.
CBT versus other specific interventions
Primary outcomes
ADHD symptoms
Aggregated ADHD symptoms
Two studies, Safren 2010 and Virta 2010, assessed CBT compared with other specific non‐pharmacological interventions on clinician‐reported ADHD symptoms. The analysis of this comparison revealed a significant effect favouring CBT (SMD −0.58, 95% CI −0.98 to −0.17; 97 participants; I2 = 0%; Analysis 3.1; low‐quality evidence; moderate effect size; Table 3).
3.1. Analysis.
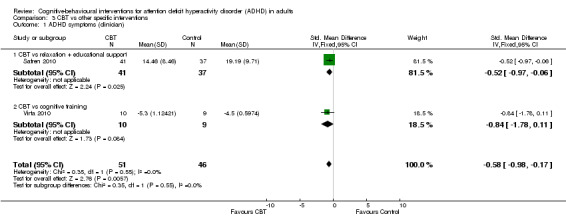
Comparison 3 CBT vs other specific interventions, Outcome 1 ADHD symptoms (clinician).
Four studies (156 participants) evaluated the outcome of CBT on ADHD symptoms through self‐reported measures (Fleming 2015; Safren 2010; Vidal Estrada 2013; Virta 2010). The analysis showed a significant effect of CBT on self‐reported ADHD symptom severity when compared with other specific non‐pharmacological interventions (SMD −0.44, 95% CI −0.88 to −0.01; I2 = 41%; Analysis 3.2; low‐quality evidence; small effect size; Table 3).
3.2. Analysis.
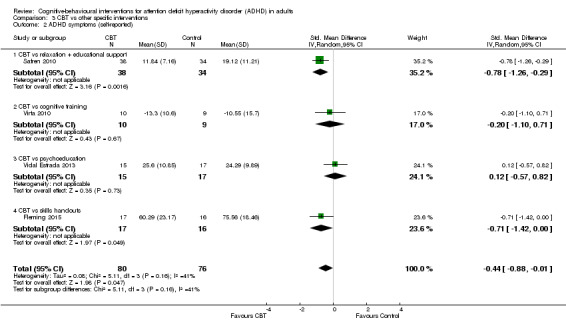
Comparison 3 CBT vs other specific interventions, Outcome 2 ADHD symptoms (self‐reported).
Disaggregated ADHD symptoms
Two studies (65 participants) compared self‐reported inattention symptoms separately (Fleming 2015; Vidal Estrada 2013). This comparison showed no significant differences between CBT and other specific interventions (SMD −0.12, 95% CI −0.61 to 0.37; I2 = 15%; small effect size; Analysis 3.3).
3.3. Analysis.
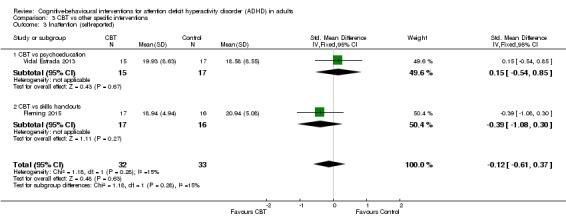
Comparison 3 CBT vs other specific interventions, Outcome 3 Inattention (self‐reported).
Only one study (32 participants) compared CBT to psychoeducation using the Conners' Adult ADHD Rating Scale ‐ Self‐Reported (CAARS‐SR; range 0 (best) to 52 (worst)) (Vidal Estrada 2013), finding no significant effect of treatment for self‐reported hyperactivity (MD 1.72 points, 95% CI −4.41 points to 7.85 points; see the illustrative forest plot in Analysis 3.4) or self‐reported impulsivity (MD 2.84 points, 95% CI −3.26 points to 8.94 points; Analysis 3.5).
3.4. Analysis.
Comparison 3 CBT vs other specific interventions, Outcome 4 Hyperactivity: CBT vs psychoeducation.
3.5. Analysis.
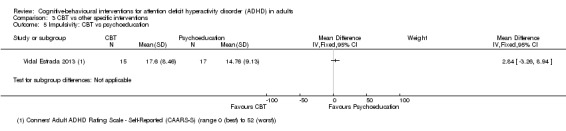
Comparison 3 CBT vs other specific interventions, Outcome 5 Impulsivity: CBT vs psychoeducation.
All‐cause treatment discontinuation
One study, Vidal Estrada 2013, reported that in the psychoeducation group, one participant dropped out after five sessions because of timetable incompatibilities, and one participant was lost to follow‐up because he did not turn up for the post‐treatment assessment. In the CBT group, one participant dropped out because of illness at session six, and three were lost at follow‐up because they missed the post‐treatment evaluation. There were no dropouts due to adverse events reported for this comparison, and no other study reported adverse events for this comparison.
Secondary outcomes
Psychopathology
Clinical Global Impression
Two studies assessed psychopathology using the clinician‐reported CGI scale (range 1 (best) to 7 (worst)). Safren 2010, reported no significant effect when comparing CBT to relaxation plus educational support (MD −0.53 points, 95% CI −1.09 points to 0.03 points; 78 participants), nor did Vidal Estrada 2013, when comparing CBT to psychoeducation (MD 0.18 points, 95% CI −0.19 points to 0.55 points; 32 participants). See Analysis 3.6.
3.6. Analysis.
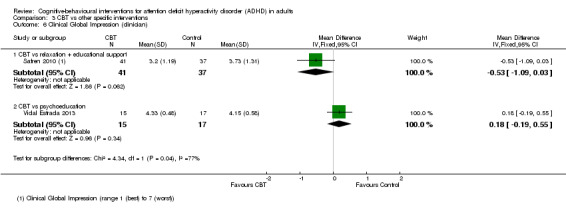
Comparison 3 CBT vs other specific interventions, Outcome 6 Clinical Global Impression (clinician).
One study (32 participants) assessed psychopathology using the self‐reported CGI scale (range 1 (best) to 7 (worst)) but found no significant effect when comparing CBT to psychoeducation (Vidal Estrada 2013): MD 0.29 points, 95% CI −0.32 points to 0.90 points; see the illustrative forest plot in Analysis 3.7).
3.7. Analysis.
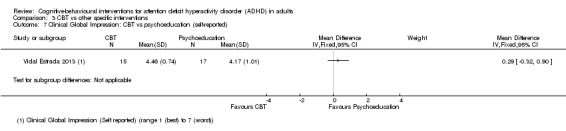
Comparison 3 CBT vs other specific interventions, Outcome 7 Clinical Global Impression: CBT vs psychoeducation (self‐reported).
Depression
Three studies (84 participants) comparing CBT to other specific non‐pharmacological interventions reported non‐significant effects on self‐reported depression (SMD −0.27, 95% CI −0.70 to 0.16; I2 = 0%; small effect size; Fleming 2015; Vidal Estrada 2013; Virta 2010; Analysis 3.8).
3.8. Analysis.
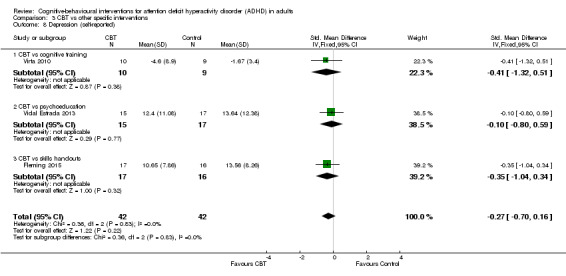
Comparison 3 CBT vs other specific interventions, Outcome 8 Depression (self‐reported).
Anxiety
Two studies (65 participants) comparing CBT to other specific non‐pharmacological interventions reported non‐significant effects of treatment on self‐reported anxiety (SMD −0.46, 95% CI −0.95 to 0.04; I2 = 0%; moderate effect size; Fleming 2015; Vidal Estrada 2013; Analysis 3.9).
3.9. Analysis.
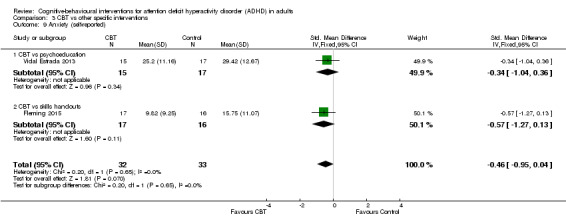
Comparison 3 CBT vs other specific interventions, Outcome 9 Anxiety (self‐reported).
Quality of life
Three studies evaluated this outcome through self‐reported measures. One study, Virta 2010, compared CBT to cognitive training and found a non‐significant effect on self‐reported quality of life (SMD −0.28, 95% CI −1.19 to 0.62; 19 participants; small effect size), as did another study, Vidal Estrada 2013, which compared CBT to psychoeducation (SMD 0.33, 95% CI −0.37 to 1.03; 32 participants; small effect size). In contrast, Fleming 2015, which compared CBT to skills handouts, found a significant effect in favour of CBT (SMD 1.17, 95% CI 0.42 to 1.92; 33 participants; large effect size). See Analysis 3.10.
3.10. Analysis.
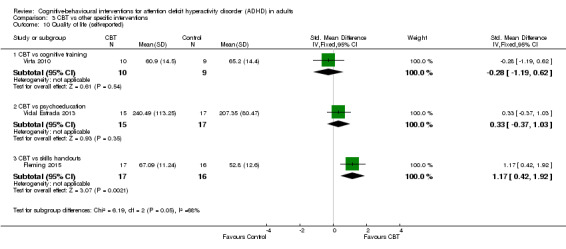
Comparison 3 CBT vs other specific interventions, Outcome 10 Quality of life (self‐reported).
Sensitivity analyses
Two trials presented with a high risk of attrition bias because they registered an important loss of participants in at least one group and/or no imputation (Emilsson 2011; Pettersson 2017).
CBT versus unspecific control conditions
ADHD symptoms (self‐reported)
Excluding Pettersson 2017 from Analysis 1.2.2 (subgroup: CBT versus waiting list) did not affect the conclusion (SMD −0.98, 95% CI −1.27 to −0.69; 4 studies, 206 participants; I2 =0%; large effect size) but did increase the I2 for the subgroup differences (analysis not shown).
Depression (self‐reported)
Excluding Pettersson 2017 from Analysis 1.7.2 (subgroup: CBT versus waiting list) did not affect the conclusion (SMD −0.40, 95% CI −0.67 to −0.12; 4 studies, 213 participants; I2 = 0%; small effect size) but did increase the I2 for the subgroup differences (analyses not shown).
Anxiety (self‐reported)
Excluding Pettersson 2017 from Analysis 1.9 (CBT versus waiting list) did not affect the conclusion (SMD −0.52, 95% CI −0.81 to −0.23; 3 studies, 194 participants; I2 = 24%; moderate effect size; analysis not shown).
Quality of life (self‐reported)
Excluding Pettersson 2017 from Analysis 1.13 (CBT versus waiting list) did not affect the conclusion (MD 1.70 points, 95% CI −14.70 points to 18.10 points; rated on Quality of Life Enjoyment and Satisfaction Questionnaire (higher scores indicate greater enjoyment or satisfaction. As Virta 2010 combined the work and school subscale into a work/study subscale, it is difficult to estimate the range) 1 study, 19 participants; analysis not shown).
CBT plus pharmacotherapy versus pharmacotherapy alone
For Analysis 2.1, when we removed Emilsson 2011 due to its dropout percentage, the difference in favour of CBT and pharmacotherapy treatment became non‐significant (SMD −0.60, 95% CI −1.32 to 0.12; 2 studies, 31 participants; I2 = 0%; large effect size). However, in Analysis 2.2 (MD −9.12 points, 95% CI −15.69 points to −2.55 points; rated on CSS (range 0 (best) to 54 (worst)); 2 studies, 31 participants; I2 = 0%), Analysis 2.5 (MD −0.82 points, 95% CI −1.51 points to −0.13 points; rated on CGI (range 1 (best) to 7 (worst)); 2 studies, 31 participants; I2 = 0%), Analysis 2.7 (MD −4.77 points, 95% CI −9.19 points to −0.35 points; rated on BDI (range 0 (best) to 63 (worst); 2 studies, 31 participants; I2 = 0%) and Analysis 2.9 (SMD −0.81, 95% CI −1.54 to −0.07; 2 studies, 31 participants; I2 = 100%; large effect size), removing this study did not have a significant impact on the results of the comparison (analyses not shown).
Following the criteria specified in the Methods section of our protocol (Lopez 2013), we did not perform an analysis of publication bias because the number of included studies per comparison was less than 10 (see Table 5).
CBT versus other specific interventions
There were not enough trials at high risk of attrition bias to run a sensitivity analysis for this comparison.
Discussion
Summary of main results
We identified 14 RCTs that fulfilled the inclusion criteria of this Cochrane Review (Criteria for considering studies for this review). We considered that Stevenson 2002 was had two relevant study reports; although the authors mentioned the use of two different samples, the reported data coincided exactly. Of the records we excluded, one study reported a comparison between group psychotherapy versus individual psychotherapy, affecting the comparability of the intervention of interest in our review (Philipsen 2015). We excluded four other studies because the employed comparison did not correspond to the comparisons included in our protocol (Cherkasova 2016; Weiss 2012; Young 2015; Young 2017), plus two more because their goals were to assess the efficacy of mindfulness and mindfulness plus virtual reality respectively, without introducing other treatment modalities such as CBT (Mitchell 2013; Serra‐Pla 2017).
The primary outcome of the included trials was the effect of different modalities of CBT‐based treatments on the core symptoms of ADHD in adults. The overall findings of this review suggest that CBT‐based treatments may improve the core symptoms of ADHD as assessed both by clinician‐ and self‐report.
The first main comparison was CBT versus unspecific control conditions. In this analysis, CBT was more effective than a waiting list or supportive therapy for improving observer‐rated core symptoms of ADHD. Additionally, CBT was better than waiting list for reducing self‐reported ADHD symptoms but not more effective than supportive therapy. When analysing symptoms of ADHD separately, we found that CBT was superior to supportive therapy regarding clinician‐reported inattention and superior to the waiting list regarding inattention (clinician‐ and self‐reported) and hyperactivity/impulsivity (clinician‐ and self‐reported). With respect to secondary outcomes, we found significant differences from waiting list in depression (self‐rated), anxiety (self‐rated), state anger (self‐rated) and self‐esteem (self‐rated). There were no differences between CBT and supportive therapy or the waiting list in depression (self‐rated). Additionally, we found no differences between CBT and supportive therapy in anxiety (clinician‐rated) or self‐esteem (self‐rated), or between CBT and waiting list in trait anger (self‐rated), self‐esteem (self‐rated) or quality of life (self‐rated).
The second main comparison was CBT plus pharmacotherapy versus pharmacotherapy alone. The results showed that combined treatment could be more effective than pharmacotherapy alone, not only for improving the core symptoms of ADHD but also for reducing the commonly associated symptoms of depression and anxiety (Emilsson 2011; Safren 2005). However, we found no significant differences in self‐reported hyperactivity‐impulsivity when evaluated separately.
The third main comparison of our review was CBT versus other specific interventions. In this comparison, we found that CBT was more effective than the comparison group in terms of improvement in core ADHD symptoms, in both clinician‐ and self‐reported questionnaires. With further analysis, however, we found no significant differences when separately evaluating self‐reported inattention, hyperactivity and impulsivity. Regarding secondary outcomes, we found no differences in self‐reported depression or anxiety and inconsistent results for self‐reported quality of life.
In the first and the third comparisons described above, and with the exception of Moëll 2015, study authors controlled the proportion of participants who received pharmacological treatment in the different groups.
None of the included studies reported severe adverse events in participants receiving the different modalities of CBT, but five participants described some type of adverse event, such as distress and anxiety.
The sensitivity analysis was limited due to the small number of studies for each outcome. However, the direction of the results was consistent with the primary analysis, except for the comparison between CBT plus pharmacotherapy versus pharmacotherapy alone, for the outcome of clinician‐rated ADHD symptoms.
Overall completeness and applicability of evidence
Our search found studies assessing CBT for adults with ADHD, and their reported results provided findings that could be applicable. However, these studies included heterogeneous clinician‐ and self‐reported measures of ADHD symptoms and associated dimensions. This heterogeneity might have an impact on the interpretation of the results.
Furthermore, our review found few trials, concentrated in the northern hemisphere; there is only one trial from the southern hemisphere and none from low‐ or lower‐middle‐income countries. Because of this, we suggest caution regarding the cross‐cultural applicability of the evidence.
We described the effects of interventions as meta‐analyses when possible and at a study level otherwise.
Quality of the evidence
Important methodological limitations reduced the certainty of the evidence offered by most included trials. Many of the trials were small and included different outcome measures, and selective outcome reporting was occasionally an issue.
We assessed the quality of the evidence using the GRADE approach and presented our ratings in 'Summary of findings' tables (see Table 1; Table 2; Table 3). Considering that self‐ and clinician‐reported core symptoms are the main target of CBT, we included them in the 'Summary of findings' tables.
None of the studies were at high risk of bias for random sequence generation, but six of them were at unclear risk because the authors did not describe the sequence generation process. We were able to record this factor as a low risk only when the study authors responded with more information about this via email.
Similarly, reports did not contain an adequate description of the allocation concealment process. We classified nine studies as being at unclear risk of bias. Five studies were at low risk, and we completed most of the information after contacting the study authors via email.
Due to the inherent characteristics of psychotherapy, we considered all studies to be at high risk of performance bias because it is not possible to blind personnel to this type of intervention.
We considered one trial to be at high risk of detection bias because the study authors described that the outcomes were potentially prone to risk of bias due to lack of blinding of the assessor (Schoenberg 2014).
Additionally, we rated two studies at high risk of attrition bias because the dropout rate was around 40% (Emilsson 2011; Pettersson 2017). Three studies were at unclear risk of bias: Hepark 2015 and Safren 2010 performed an ITT analysis, but the number of dropouts was unbalanced between groups; Hirvikoski 2011 performed an ITT analysis, but there was an important rate of dropouts (around 20% per group).
Five studies prospectively registered the trial and reported all planned outcomes (Emilsson 2011; Moëll 2015; Safren 2005; Safren 2010; Solanto 2010). We considered all studies as free of the risk of reporting bias because the published reports clearly included all expected outcomes.
Two studies presented a high risk of 'other bias' (Emilsson 2011; Moëll 2015). Emilsson 2011 reported not asking participants in either group to refrain from engaging in other interventions during the study period; therefore, they could not establish the impact of a possible second intervention. In Moëll 2015, the study authors did not confirm the diagnosis of ADHD for some of the participants after their previous neuropsychiatric assessment.
We were unable to assess publication bias as planned (Lopez 2013), as there were fewer than 10 included studies per comparison. However, the search strategy was very sensitive, and the field is not so large. Therefore, we consider that there is no evidence of publication bias.
Finally, we considered one study, Pettersson 2017, to be at high risk of bias due to conflicts of interest, because the main author was a partner and shareholder in the company that constructed and owned the rights to the Internet‐based treatment programme, In Focus. This author was also involved in the design and construction of the programme.
Potential biases in the review process
We designed the search process in conjunction with a specialised librarian, and the Cochrane Developmental, Psychosocial and Learning Problems Information Specialist supervised it with the objective of minimising bias in the compilation of potentially relevant references.
In all cases, we repeatedly tried to contact the authors of the included studies by email to obtain more information, when needed. In five cases, we did not receive a reply. The replies we did receive helped us to describe the random allocation process and the random sequence generation.
The high number of outcomes analysed could generate type 1 errors, but the consistency in the direction of most results reduces this possibility.
Finally, we did not identify other significant potential biases in the review process.
Agreements and disagreements with other studies or reviews
According to Chandler 2013, there is a need for a meta‐analysis of CBT versus other psychotherapies. To our knowledge, there are three meta‐analyses of RCTs on psychotherapy for adults with ADHD. In the first one (Jensen 2016), the authors only included studies that evaluated the effects of standard CBT, using a more restrictive criteria for CBT treatments. With respect to search results, these authors found a smaller number of studies, and, after full‐text evaluation, they only included two (Emilsson 2011; Safren 2005). The results of their review are similar to the results of our comparison between CBT with pharmacotherapy versus pharmacotherapy alone (which includes these two studies), except for clinician‐reported ADHD symptoms. This difference might be because Jensen 2016 only reported the data from Safren 2005, leaving out the data from Emilsson 2011. When only considering Safren 2005, the results were not significant, but as a whole (including Emilsson 2011), the results were significant.
The second meta‐analysis reported similar findings even though the eight included studies and comparison groups differed from the present review (Young 2016). The first difference between our reviews was that Young 2016 did not include mindfulness‐based cognitive therapy or dialectical behavioural therapy. Second, those authors defined the control conditions as 'inactive' (waiting list or treatment as usual) or 'active' (alternative treatment to CBT). These categories resemble the ones employed in our review (unspecific control conditions and other specific interventions), but the inclusion of studies was not homologous (i.e. Young 2016 classified supportive therapy as an active control, while we considered it an unspecific control condition). Finally, Young 2016 did not discriminate between studies based on whether or not they employed CBT as an adjunctive treatment to pharmacotherapy. Beyond these differences, the overall findings point to the same conclusions.
The third meta‐analysis included controlled and uncontrolled studies, but the authors meta‐analysed controlled and uncontrolled (pre‐ to‐post) effect sizes separately (Knouse 2017). They also included controlled studies that did not use random assignment. Even though the global results were similar, these authors failed to assess the quality of the evidence, limiting the findings of the review.
Additionally, previous non‐systematic reviews on this topic such as Knouse 2010 and Mongia 2012 showed similar results to our findings.
Our results agree with Antshel 2011 and Ramsay 2007, who stated that CBT plus pharmacotherapy improved treatment outcomes more than medication alone; however, these authors found only limited evidence that CBT was efficacious on its own.
In contrast to Philipsen 2012, our results did not clearly show whether specific treatment for ADHD in adults significantly reduced the commonly associated symptoms (depression, anxiety and anger).
Authors' conclusions
Implications for practice.
Cognitive‐behavioural‐based treatments may be efficacious in the short term for treating the core symptoms of ADHD in adults. Compared with unspecific control conditions and when combined with pharmacotherapy (compared with pharmacotherapy alone), CBT may also improve common comorbid disturbances in adults with ADHD such as depression, anxiety and anger. Additionally, we did not find any severe adverse effects of this psychological treatment.
However, the scarcity of outcomes from long‐term studies, the divergent efficacy measures and designs, and the limited geographical location, mostly confined to high‐income countries in the northern hemisphere, limit the generalisability of the results.
Implications for research.
Further research is needed to precisely determine the efficacy of cognitive‐behavioural therapy for adults with ADHD, particularly in the long term, and, if possible, through multicentre studies that include cost‐effectiveness analyses. Additionally, there is a need to establish consensus‐based standards for study design and efficacy measures of psychotherapy treatments for this population, as the Research Forum on Psychological Treatment of Adults with ADHD noted (Weiss 2008). Although the situation has improved in past decades, it is still necessary to determine which types of psychological treatment are more effective. In particular, according to Philipsen 2012, it is necessary to increase the number of studies that evaluate the effects of therapy on adaptive functioning. We consider that these studies should report participants' employment status and measures of daily functioning.
Acknowledgements
To the members of the Cochrane Developmental, Psychosocial and Learning Problems Editorial Team, for their time dedicated to reviewing and commenting on the draft protocol and review. To Daniel Comandé, for his invaluable collaboration in the search strategy design. To Andrés Rumboll, for his collaboration in reviewing the phrasing of the draft protocol and review. To Marta Roqué Figuls, for her assistance and guidance in the final stages of the full revision.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Database of Systematic Reviews (CDSR) and Database of Abstracts of Reviews of Effectiveness (DARE)
1202 records
1. MeSH descriptor: [Attention Deficit and Disruptive Behavior Disorders] this term only 2. MeSH descriptor: [Attention Deficit Disorder with Hyperactivity] explode all trees 3. Attention Deficit:ti,ab,kw 4. Hyperactivity Disorder*:ti,ab,kw 5. Deficit Disorder*:ti,ab,kw 6. Oppositional Defiant*:ti,ab,kw 7. Defiant Disorder*:ti,ab,kw 8. Disruptive Behavi*:ti,ab,kw 9. ADHD:ti,ab,kw 10. ADDH:ti,ab,kw 11. ADHS:ti,ab,kw 12. AD‐HD:ti,ab,kw 13. HKD:ti,ab,kw 14. Hyperkinetic*:ti,ab,kw 15. Hyperkines*:ti,ab,kw 16. Impulsiv*:ti,ab,kw 17. Inattentiv*:ti,ab,kw 18. Inattention*:ti,ab,kw 19. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 20. MeSH descriptor: [Behavior Therapy] explode all trees 21. MeSH descriptor: [Cognitive Therapy] explode all trees 22. MeSH descriptor: [Psychotherapy, Rational‐Emotive] explode all trees 23. MeSH descriptor: [Imagery (Psychotherapy)] explode all trees 24. MeSH descriptor: [Desensitization, Psychologic] explode all trees 25. MeSH descriptor: [Biofeedback, Psychology] explode all trees 26. Cognitive Behavio*:ti,ab,kw (Word variations have been searched) 27. Cognitive therap*:ti,ab,kw (Word variations have been searched) 28. Metacognitive Therap*:ti,ab,kw (Word variations have been searched) 29. Meta‐Cognitive Therap*:ti,ab,kw (Word variations have been searched) 30. Behavior Therap*:ti,ab,kw (Word variations have been searched) 31. Behavioral Therap*:ti,ab,kw (Word variations have been searched) 32. Behavioral Psychotherap*:ti,ab,kw (Word variations have been searched) 33. Behaviour Therap*:ti,ab,kw (Word variations have been searched) 34. Cognitive Psychotherap*:ti,ab,kw (Word variations have been searched) 35. Behavior Psychotherap*:ti,ab,kw (Word variations have been searched) 36. Behaviour Psychotherap*:ti,ab,kw (Word variations have been searched) 37. Dialectical Behavio*:ti,ab,kw (Word variations have been searched) 38. Rational‐Emotive*:ti,ab,kw (Word variations have been searched) 39. Rational Psychotherap*:ti,ab,kw (Word variations have been searched) 40. Guided Imager*:ti,ab,kw (Word variations have been searched) 41. Reverie Therap*:ti,ab,kw (Word variations have been searched) 42. Imageries:ti,ab,kw (Word variations have been searched) 43. Imagery:ti,ab,kw (Word variations have been searched) 44. False Physiological:ti,ab,kw (Word variations have been searched) 45. Myofeedback*:ti,ab,kw (Word variations have been searched) 46. Psychophysiologic Feedback*:ti,ab,kw (Word variations have been searched) 47. Desensitization*:ti,ab,kw (Word variations have been searched) 48. Desensitisation*:ti,ab,kw (Word variations have been searched) 49. Mindfulness:ti,ab,kw (Word variations have been searched) 50. Biofeedback*:ti,ab,kw (Word variations have been searched) 51. CBT:ti,ab,kw (Word variations have been searched) 52. DBT:ti,ab,kw (Word variations have been searched) 53. #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 54. MeSH descriptor: [Adult] explode all trees 55. Adult*:ti,ab,kw (Word variations have been searched) 56. #54 or #55 57 #19 AND #53 and #56
MEDLINE via PubMed US National Library of Medicine
2186 records
1. Search (Attention Deficit and Disruptive Behavior Disorders[Majr]) 2. Search Attention Deficit Disorder with Hyperactivity[Mesh] 3. Search Attention Deficit[tiab] 4. Search Hyperactivity Disorder*[tiab] 5. Search Deficit Disorder*[tiab] 6. Search Oppositional Defiant*[tiab] 7. Search Defiant Disorder*[tiab] 8. Search Disruptive Behavi*[tiab] 9. Search ADHD[tiab] 10. Search ADDH[tiab] 11. Search ADHS[tiab] 12. Search AD‐HD[tiab] 13. Search HKD[tiab] 14. Search Hyperkinetic*[tiab] 15. Search Hyperkines*[tiab] 16. Search Impulsiv*[tiab] 17. Search Inattentiv*[tiab] 18. Search Inattention*[tiab] 19. Search (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) 20. Search Behavior Therapy[Mesh] 21. Search Cognitive Therapy[Mesh] 22. Search Psychotherapy, Rational‐Emotive[Mesh] 23. Search "Imagery (Psychotherapy)"[Mesh] 24. Search Desensitization, Psychologic[Mesh] 25. Search Biofeedback, Psychology[Mesh] 26. Search Cognitive Behavio*[tiab] 27. Search Cognitive therap*[tiab] 28. Search Metacognitive Therap*[tiab] 29. Search Meta‐Cognitive Therap*[tiab] 30. Search Behavior Therap*[tiab] 31. Search Behavioral Therap*[tiab] 32. Search Behavioral Psychotherap*[tiab] 33. Search Behaviour Therap*[tiab] 34. Search Cognitive Psychotherap*[tiab] 35. Search Behavior Psychotherap*[tiab] 36. Search Behaviour Psychotherap*[tiab] 37. Search Dialectical Behavio*[tiab] 38. Search Rational‐Emotive*[tiab] 39. Search Rational Psychotherap*[tiab] 40. Search Guided Imager*[tiab] 41. Search Reverie Therap*[tiab] 42. Search Imageries[tiab] 43. Search Imagery[tiab] 44. Search False Physiological[tiab] 45. Search Myofeedback*[tiab] 46. Search Psychophysiologic Feedback*[tiab] 47. Search Desensitization*[tiab] 48. Search Desensitisation*[tiab] 49. Search Biofeedback*[tiab] 50. Search CBT[tiab] 51. Search DBT[tiab] 52. Search Mindfulness[tiab] 53. Search (#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52) 54. Search ((Adult[Mesh] OR Adult*[tiab])) 55. Search (#19 AND #53 AND #54)
Embase Elsevier
5483 records
#57 #55 AND #56 #56 'randomized‐controlled‐trial'/de OR 'randomization'/de OR 'controlled‐study'/de OR 'multicenter study'/de OR 'phase‐3‐clinical‐trial'/de OR 'phase‐4‐clinical‐trial'/de OR 'double‐blind‐procedure'/de OR 'single blind‐procedure'/de OR random$:ab,ti OR crossover$:ab,ti OR 'cross over$':ab,ti OR factorial$:ab,ti OR placebo$:ab,ti OR volunteer$:ab,ti OR (singl$:ab,ti OR doubl$:ab,ti OR trebl$:ab,ti OR tripl$:ab,ti AND (blind$:ab,ti OR mask$:ab,ti)) NOT ('animals'/exp NOT ('humans'/exp AND 'animals'/exp)) #55 #53 AND #54 #54 'adult'/exp OR adult$:ab,ti #53 #18 AND #52 #52 #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 #51 dbt:ab,ti #50 cbt:ab,ti #49 biofeedback*:ab,ti #48 'mindfulness':ab,ti #47 desensitisation*:ab,ti #46 desensitization*:ab,ti #45 'psychophysiologic feedback':ab,ti #44 myofeedback*:ab,ti #43 'false physiological':ab,ti #42 'imagery':ab,ti #41 'imageries':ab,ti #40 'reverie therapy':ab,ti #39 'guided imagery':ab,ti OR 'guided imageries':ab,ti #38 'rational psychotherapy':ab,ti OR 'rational psychotherapies':ab,ti #37 'rational‐emotive':ab,ti #36 'dialectical behavior':ab,ti OR 'dialectical behaviour':ab,ti #35 'behaviour psychotherapy':ab,ti OR 'behaviour psychotherapies':ab,ti #34 'behavior psychotherapy':ab,ti OR 'behavior psychotherapies':ab,ti #33 'cognitive psychotherapy':ab,ti OR 'cognitive psychotherapies':ab,ti #32 'behaviour therapy':ab,ti OR 'behaviour therapies':ab,ti #31 'behavioral psychotherapy':ab,ti OR 'behavioral psychotherapies':ab,ti #30 'behavioral therapy':ab,ti OR 'behavioral therapies':ab,ti #29 'behaviour therapy':ab,ti OR 'behaviour therapies':ab,ti #28 'behavior therapy':ab,ti OR 'behavior therapies':ab,ti #27 'meta‐cognitive therapy':ab,ti #26 'metacognitive therapy':ab,ti #25 'cognitive therapy':ab,ti #24 'cognitive behavior':ab,ti OR 'cognitive behaviour':ab,ti #23 'psychophysiology'/exp #22 'desensitization'/exp #21 'guided imagery'/exp #20 'cognitive therapy'/exp #19 'behavior therapy'/exp #18 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 #17 inattention*:ab,ti #16 'inattentiv*':ab,ti #15 'impulsiv*':ab,ti #14 'hyperkines*':ab,ti #13 'hyperkinetic':ab,ti #12 'hkd':ab,ti #11 'ad‐hd':ab,ti #10 'adhs':ab,ti #9 'addh':ab,ti #8 'adhd':ab,ti #7 'disruptive behavior':ab,ti OR 'disruptive behaviour':ab,ti #6 'defiant disorder':ab,ti OR 'defiant disorders':ab,ti #5 'oppositional defiant':ab,ti #4 'deficit disorder':ab,ti OR 'deficit disorders':ab,ti #3 'hyperactivity disorder':ab,ti OR 'hyperactivity disorders':ab,ti #2 'attention deficit':ab,ti #1 'attention deficit disorder'/exp
CINAHL EBSCO
22 records
S1. (MH "Attention Deficit Hyperactivity Disorder") S2. TI Attention Deficit OR AB Attention Deficit S3. TI Hyperactivity Disorder* OR AB Hyperactivity Disorder* S4. TI Deficit Disorder* OR AB Deficit Disorder* S5. TI Oppositional Defiant* OR AB Oppositional Defiant* S6. TI Defiant Disorder* OR AB Defiant Disorder* S7. TI Disruptive Behavi* OR AB Disruptive Behavi* S8. TI ADHD OR AB ADHD S9. TI ADDH OR AB ADDH S10. TI ADHS OR AB ADHS S11. TI AD‐HD OR AB AD‐HD S12. TI HKD OR AB HKD S13. TI Hyperkinetic* OR AB Hyperkinetic* S14. TI Hyperkines* OR AB Hyperkines* S15. TI Impulsiv* OR AB Impulsiv* S16. TI Inattentiv* OR AB Inattentiv* S17. TI Inattention* OR AB Inattention* S18. S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 S19. (MH "Behavior Therapy+") S20. (MH "Cognitive Therapy+") S21. (MH "Psychotherapy, Brief") S22. (MH "Guided Imagery") S23. (MH "Desensitization, Psychologic+") S24. (MH "Biofeedback") S25. TI Cognitive Behavio* OR AB Cognitive Behavio* S26. TI Cognitive therap* OR AB Cognitive therap* S27. TI Metacognitive Therap* OR AB Metacognitive Therap* S28. TI Meta‐Cognitive Therap* OR AB Meta‐Cognitive Therap* S29. TI Behavior Therap* OR AB Behavior Therap* S30. TI Behavioral Therap* OR AB Behavioral Therap* S31. TI Behavioral Psychotherap* OR AB Behavioral Psychotherap* S32. TI Behaviour Therap* OR AB Behaviour Therap* S33. TI Cognitive Psychotherap* OR AB Cognitive Psychotherap* S34. TI Behavior Psychotherap* OR AB Behavior Psychotherap* S35. TI Behaviour Psychotherap* OR AB Behaviour Psychotherap* S36. TI Dialectical Behavio* OR AB Dialectical Behavio* S37. TI Rational‐Emotive* OR AB Rational‐Emotive* S38. TI Rational Psychotherap* OR AB Rational Psychotherap* S39. TI Guided Imager* OR AB Guided Imager* S40. TI Reverie Therap* OR AB Reverie Therap* S41. TI Imageries OR AB Imageries S42. TI Imagery OR AB Imagery S43. TI False Physiological OR AB False Physiological S44. TI Myofeedback* OR AB Myofeedback* S45. TI Psychophysiologic Feedback* OR AB Psychophysiologic Feedback* S46. TI Desensitization* OR AB Desensitization* S47. TI Desensitisation* OR AB Desensitisation* S48. TI Biofeedback* OR AB Biofeedback* S49. TI CBT OR AB CBT S50. TI DBT OR AB DBT S51. TI Mindfulness OR AB Mindfulness S52. S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 S53. S18 AND S52 S54. (MH "Clinical Trials+") S55. PT Clinical trial S56. TX clinic* n1 trial* S57. TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) S58. TX randomi* control* trial* S59. (MH "Random Assignment") S60. TX random* allocat* S61. TX placebo* S62. (MH "Placebos") S63. (MH "Quantitative Studies") S64. TX allocat* random* S65. S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 S66. S53 AND S65
PsycINFO EBSCO
710 records
S1. DE "Attention Deficit Disorder" S2. DE "Attention Deficit Disorder with Hyperactivity" S3. TI Attention Deficit OR AB Attention Deficit S4. TI Hyperactivity Disorder* OR AB Hyperactivity Disorder* S5. TI Deficit Disorder* OR AB Deficit Disorder* S6. TI Oppositional Defiant* OR AB Oppositional Defiant* S7. TI Defiant Disorder* OR AB Defiant Disorder* S8. TI Disruptive Behavi* OR AB Disruptive Behavi* S9. TI ADHD OR AB ADHD S10. TI ADDH OR AB ADDH S11. TI ADHS OR AB ADHS S12. TI AD‐HD OR AB AD‐HD S13. TI HKD OR AB HKD S14. TI Hyperkinetic* OR AB Hyperkinetic* S15. TI Hyperkines* OR AB Hyperkines* S16. TI Impulsiv* OR AB Impulsiv* S17. TI Inattentiv* OR AB Inattentiv* S18. TI Inattention* OR Inattention* S19. S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 S20. DE "Behavior Therapy" OR DE "Aversion Therapy" OR DE "Conversion Therapy" OR DE "Dialectical Behavior Therapy" OR DE "Exposure Therapy" OR DE "Implosive Therapy" OR DE "Reciprocal Inhibition Therapy" OR DE "Response Cost" OR DE "Systematic Desensitization Therapy" S21. DE "Cognitive Therapy" S22. DE "Imagery" OR DE "Conceptual Imagery" OR DE "Spatial Imagery" S23. MM "Biofeedback" S24. TI Cognitive Behavio* OR AB Cognitive Behavio* S25. TI Cognitive therap* OR AB Cognitive therap* S26. TI Metacognitive therap* OR AB Metacognitive therap* S27. TI Meta‐Cognitive Therap* OR AB Meta‐Cognitive Therap* S28. TI Behavior Therap* OR AB Behavior Therap* S29. TI Behavioral Therap$ OR AB Behavioral Therap$ S30. TI Behavioral Psychotherap* OR AB Behavioral Psychotherap* S31. TI Behaviour Therap* OR AB Behaviour Therap* S32. TI Cognitive Psychotherap* OR AB Cognitive Psychotherap* S33. TI Behavior Psychotherap* OR AB Behavior Psychotherap* S34. TI Behaviour Psychotherap* OR AB Behaviour Psychotherap* S35. TI Dialectical Behavio* OR AB Dialectical Behavio* S36. TI Rational‐Emotive OR AB Rational‐Emotive S37. TI Rational Psychotherap* OR AB Rational Psychotherap* S38. TI Guided Imager* OR AB Guided Imager* S39. TI Reverie Therap* OR AB Reverie Therap* S40. TI Imageries OR AB Imageries S41. TI Imagery OR AB Imagery S42. TI False Physiological OR AB False Physiological S43. TI Myofeedback* OR AB Myofeedback* S44. TI Psychophysiologic Feedback* OR AB Psychophysiologic Feedback* S45. TI Desensitization* OR AB Desensitization* S46. TI Desensitisation* OR AB Desensitisation* S47. TI Mindfulness OR AB Mindfulness S48. TI DBT OR AB DBT S49. TI CBT OR AB CBT S50. TI Biofeedback* OR AB Biofeedback* S51. S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 S52. TI Adult$ OR AB Adult$ S53. S19 AND S51 AND S52 S54. MM "Clinical Trials" S55. MD treatment outcome clinical trial S56. MD treatment outcome clinical trial S57. MD treatment outcome clinical trial S58. MD treatment outcome clinical trial S59. (TI Randomized OR TI Randomized OR AB Randomized OR AB Randomised) AND (TI Trial* OR AB Trial*) S60. ( TI single OR TI doubl* OR TI tripl* OR TI Treb* OR AB single OR AB doubl* OR AB tripl* OR AB Treb* ) AND ( TI blind* OR AB blind* OR TI mask* OR AB mask* ) S61. ( TI controlled OR TI clinical OR AB controlled OR AB Clinical ) AND ( TI Trial* OR AB Trial* ) S62. S54 OR S55 OR S56 OR S57 OR S58 S63. S53 AND S59
BIOSIS Previews Web of Science
766 records
1. TS=(Attention Deficit) 2. TS=(Disruptive Behavior) 3. TS=(Hyperactivity) 4. TI=(Attention Deficit) 5. TI=(Disruptive Behavior) 6. TI=(Hyperactivity) 7. TI=(Defiant Disorder*) 8. TI=(Disruptive Behavi*) 9. TI=ADHD 10. TI=(ADDH) 11. TI=(ADHS) 12. TI=(AD‐HD) 13. TI=(Hyperkinetic*) 14. TI=(Hyperkines*) 15. TI=(Impulsiv*) 16. TI=(Inattentiv*) 17. TI=(Inattention*) 18. #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 19. TS=(Behavior Therapy) 20. TS=(Cognitive Therapy) 21. TS=(Imagery) 22. TS=(Desensitization) 23. TS=(Biofeedback) 24. TI=(Cognitive Behavio*) 25. TI=(Metacognitive Therap*) 26. TI=(Meta‐Cognitive Therap*) 27. TI=(Behavior Therap*) 28. TI=(Behavioral Therap*) 29. TI=(Behavioral Psychotherap*) 30. TI=(Behaviour Therap*) 31. TI=(Cognitive Psychotherap*) 32. TI=(Behavior Psychotherap*) 33. TI=(Behaviour Psychotherap*) 34. TI=(Dialectical Behavio*) 35. TI=(Rational‐Emotive*) 36. TI=(Rational Psychotherap*) 37. TI=(Guided Imager*) 38. TI=(Reverie Therap*) 39. TI=(Imageries) 40. TI=(Imagery) 41. TI=(False Physiological) 42. TI=(Myofeedback*) 43. TI=(Psychophysiologic Feedback*) 44. TI=(Desensitization*) 45. TI=(Desensitisation*) 46. TI=(Biofeedback*) 47. TI=(CBT) 48. TI=(DBT) 49. TI=(Mindfulness) 50. #49 OR #48 OR #47 OR #46 OR #45 OR #44 OR #43 OR #42 OR #40 OR #39 OR #38 OR #37 OR #36 OR #35 OR #34 OR #33 OR #32 OR #31 OR #30 OR #29 OR #28 OR #27 OR #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 51. #50 AND #18 52. TS=(Adults) OR TI=(Adult*) 53. #52 AND #51 54. (#53) AND Notas taxonómicas: (Humans)
LILACS (Latin American and Caribbean Health Sciences Literature)
lilacs.bvsalud.org/en
122 records
(MH Attention Deficit and Disruptive Behavior Disorders OR MH Attention Deficit Disorder with Hyperactivity OR Hyperactivity OR Defiant OR Attention OR Disruptive OR Hyperkinetic$ OR Hyperkines$ OR Inattentiv$ OR Inattention$ OR ADHD OR ADDH OR ADHS OR AD‐HD OR HKD) AND (MH Behavior Therapy OR MH Cognitive Therapy OR MH Psychotherapy, Rational‐Emotive OR MH Desensitization, Psychologic OR MH Biofeedback, Psychology OR Cognitive OR Metacognitive OR Behavioral OR Imager$ OR Rational‐Emotive OR Myofeedback OR Desensitization$ OR Desensitisation$ OR Biofeedback$ OR CBT OR DBT OR Mindfulness OR Reverie OR Dialectical) [Words] and (PT Ensayo Clínico Controlado Aleatorio OR PT Ensayo Clínico Controlado OR MH Ensayo Clínico Controlado Aleatorio OR MH Distribución Aleatoria OR MH Método Doble Ciego OR MH Método Simple‐Ciego OR PT Ensayo Clínico OR MH Ensayo Clínico OR (clinical trial OR ensayo clinico OR Ensaio clínico OR ((singl$ OR simpl$ OR doubl$ OR trebl$ OR tripl$)))) [Words]
Networked Digital Library of Theses and Dissertations
407 records
"(attention deficit OR inattention) AND (behavior OR cognitive) AND (trial) AND (adult)"
ClinicalTrials.gov
clinicaltrials.gov
195 records
Attention Deficit OR Disruptive Behavior OR Hyperactivity OR Attention Deficit OR Defiant Disorder OR Hyperkinetic* OR Impulsiv* OR Inattentiv* OR Inattention* | Behavior OR Cognitive OR Desensitization OR Biofeedback OR Metacognitive OR Behavioral OR Dialectical OR Rational‐Emotive OR Rational Psychotherap* OR Imager* OR Reverie OR Myofeedback* OR Psychophysiologic Feedback* OR Mindfulness | Adult, Senior
ISRCTN registry
92 records
"(Condition: Attention Deficit OR Disruptive Behavior OR Hyperactivity OR Attention Deficit OR Defiant Disorder OR Hyperkinetic* OR Impulsiv* OR Inattentiv* OR Inattention* AND Interventions: Behavior OR Cognitive OR Desensitization OR Biofeedback OR Metacognitive OR Behavioral OR Dialectical OR Rational‐Emotive OR Rational Psychotherap* OR Imager* OR Reverie OR Myofeedback* OR Psychophysiologic Feedback* OR Mindfulness)"
World Health Organization International Clinical Trials Registry Portal (WHO ICTRP)
apps.who.int/trialsearch
3 records
(Inattention OR Attention) AND (Behavior OR Cognitive)
Appendix 2. Percentage change in effect sizes
We reported the absolute and relative changes (95% CI) related to the control central estimates of each outcome (negative percentages indicate a reduction of symptoms).
| CBT versus unspecific control conditions | |||||||
| Analysis | Name | Control | MD | 95% CI | % change | ||
|
Analysis 1.1 ADHD symptoms (observer) |
1.1.1 | Solanto 2010 | 73.19 | −6.25 | −11.04 | −1.46 | −9% (95% CI −15 to −2) |
| 1.1.2a | Hepark 2015 | 28.00 | −6.5 | −9.77 | −3.23 | −23% (95% CI −35 to −12) | |
| 1.1.2b | Stevenson 2002 | 112.9 | −39.2 | −52.76 | −25.64 | −35% (95% CI −47 to −23) | |
|
Analysis 1.2 ADHD symptoms (self‐reported) |
1.2.1a | Hirvikoski 2011 | 27.28 | −2.81 | −8.02 | 2.4 | −10% (95% CI −29 to 9) |
| 1.2.1b | Solanto 2010 | 76.8 | −1.00 | −6.15 | 4.15 | −1% (95% CI −8 to 5) | |
| 1.2.2a | Gu 2017 | 71.77 | −11.06 | −15.75 | −6.37 | −15% (95% CI −22 to −9) | |
| 1.2.2b | Hepark 2015 | 28.8 | −5.8 | −8.86 | −2.74 | −20% (95% CI −31 to −10) | |
| 1.2.2c | Pettersson 2017 | 29.72 | −2.38 | −8.11 | 3.35 | −8% (95% CI −27 to 11) | |
| 1.2.2d | Schoenberg 2014 | 31.3 | −8.3 | −12.53 | −4.07 | −27% (95% CI −40 to −13) | |
| 1.2.2e | Virta 2010 | −4.6 | −8.7 | −17.47 | 0.07 | 189% (95% CI −2 to 380) | |
|
Analysis 1.3 Inattention (clinician) |
1.3.1 | Solanto 2010 | 16.18 | −2.47 | −4.43 | −0.51 | −15% (95% CI −27 to −3) |
| 1.3.2 | Hepark 2015 | 16.5 | −4.1 | −6.00 | −2.2 | −25% (95% CI −36 to −13) | |
|
Analysis 1.4 Inattention (self‐reported) |
1.4.1a | Gu 2017 | 64.23 | −12.59 | −17.53 | −7.65 | −20% (95% CI −27 to −12) |
| 1.4.1b | Hepark 2015 | 16.1 | −3.3 | −5.02 | −1.58 | −20% (95% CI −31 to −10) | |
| 1.4.2c | Moëll 2015 | 27.93 | −5.01 | −7.16 | −2.86 | −18% (95% CI −26 to −10) | |
| 1.4.2d | Schoenberg 2014 | 17.4 | −5.2 | −7.47 | −2.93 | −30% (95% CI −43 to −17) | |
|
Analysis 1.5 Hyperactivity‐impulsivity (clinician) |
1.5.1 | Hepark 2015 | 11.5 | −2.5 | −4.63 | −0.37 | −22% (95% CI −40 to −3) |
|
Analysis 1.6 Hyperactivity‐impulsivity (self‐reported) |
1.6.1a | Gu 2017 | 71.15 | −10.29 | −14.91 | −5.67 | −14% (95% CI −21 to −8) |
| 1.6.1b | Hepark 2015 | 12.6 | −2.3 | −4.28 | 0.32 | −18% (95% CI −34 to 3) | |
| 1.6.1c | Moëll 2015 | 20.85 | −1.31 | −4.81 | 2.19 | −6% (95% CI −23 to 11) | |
| 1.6.1d | Schoenberg 2014 | 14.00 | −3.2 | −6.05 | −0.35 | −23% (95% CI −43 to −3) | |
|
Analysis 1.7 Depression (self‐reported) |
1.7.1 | Solanto 2010 | 9.08 | 0.58 | −2.8 | 3.96 | 6% (95% CI −31 to 44) |
| 1.7.2a | Gu 2017 | 9.42 | −2.35 | −4.01 | −0.69 | −25% (95% CI −43 to −7) | |
| 1.7.2b | Hepark 2015 | 11.8 | −2.7 | −6.51 | 1.11 | −23% (95% CI −55 to 9) | |
| 1.7.2c | Moëll 2015 | 7.7 | −1.08 | −3.48 | 1.32 | −14% (95% CI −45 to 17) | |
| 1.7.2d | Pettersson 2017 | 15.11 | −1.92 | −8.63 | 4.79 | −13% (95% CI −57 to 32) | |
| 1.7.2e | Virta 2010 | −1.5 | −3.1 | −10.22 | 4.02 | 207% (95% CI −268 to 681) | |
| Analysis 1.8 Anxiety (clinician) | 1.8.1 | Solanto 2010 | 8.88 | −0.81 | −3.21 | 1.59 | −9% (95% CI −36 to 18) |
|
Analysis 1.9 Anxiety (self‐reported) |
1.9.1a | Gu 2017 | 15.08 | −5.58 | −8.97 | −2.19 | −37% (95% CI −59 to −15) |
| 1.9.1b | Hepark 2015 | 90.8 | −12.3 | −22.56 | −2.04 | −14% (95% CI −25 to −2) | |
| 1.9.1c | Moëll 2015 | 8.81 | −0.85 | −2.67 | 0.97 | −10% (95% CI −30 to 11) | |
| 1.9.1d | Pettersson 2017 | 15.22 | −1.55 | −8.04 | 4.94 | −10% (95% CI −53 to 32) | |
| Analysis 1.10 State anger (self‐reported) | 1.10.1 | Stevenson 2002 | 14.00 | −3.3 | −5.62 | −0.98 | −24% (95% CI −40 to −7) |
| Analysis 1.11 Trait anger (self‐reported) | 1.11.1 | Stevenson 2002 | 24.3 | −3.8 | −7.63 | 0.03 | −16% (95% CI −31 to 0) |
|
Analysis 1.12 Self‐steem (self‐reported) |
1.12.1 | Solanto 2010 | −1.3 | 0.00 | −1.85 | 1.85 | 0% (95% CI −142 to 142) |
| 1.12.2 | Stevenson 2002 | 97.4 | 12.4 | 4.55 | 20.25 | 13% (95% CI 5 to 21) | |
|
Analysis 1.13 Qualfeity of life (self‐reported) |
1.13.1a | Pettersson 2017 | 41.91 | 4.99 | −6.23 | 16.21 | 12% (95% CI −15 to 39) |
| 1.13.1b | Virta 2010 | 59.2 | 1.7 | −14.7 | 18.1 | 3% (95% CI −25 to 31) | |
| CBT plus pharmacotherapy versus pharmacotherapy alone | |||||||
| Analysis | Name | Control | MD | 95% CI | % change | ||
|
Analysis 2.1 ADHD symptoms (clinician) |
2.1a | Emilsson 2011 | 35.94 | −6.06 | −10.01 | −2.11 | −17% (95% CI −28 to −6) |
| 2.1b | Safren 2005 | 20.8 | −5.61 | −12.11 | 0.89 | −27% (95% CI −58 to 4) | |
|
Analysis 2.2 ADHD symptoms (self‐reported) |
2.2a | Emilsson 2011 | 23.47 | −6.25 | −11.72 | −0.78 | −27% (95% CI −50 to −3) |
| 2.2b | Safren 2005 | 23.87 | −9.12 | −15.69 | −2.55 | −38% (95% CI −66 to −11) | |
| Analysis 2.3 Inattention (self‐reported) | 2.3 | Emilsson 2011 | 14.71 | −4.54 | −7.75 | −1.33 | −31% (95% CI −53 to −9) |
|
Analysis 2.4 Hyperactivity‐impulsivity (self‐reported) |
2.4 | Emilsson 2011 | 8.76 | −1.7 | −5.29 | 1.89 | −19% (95% CI −60 to 22) |
|
Analysis 2.5 Clinical Global Impression (clinician) |
2.5a | Emilsson 2011 | 3.88 | −0.7 | −1.31 | −0.09 | −18% (95% CI −34 to −2) |
| 2.5b | Safren 2005 | 4.13 | −0.82 | −1.51 | −0.13 | −20% (95% CI −37 to −3) | |
| Analysis 2.6 Depression (clinician) | 2.6 | Safren 2005 | 10.00 | −5.56 | −9.71 | −1.41 | −56% (95% CI −97 to −14) |
|
Analysis 2.7 Depression (self‐reported) |
2.7a | Emilsson 2011 | 15.41 | −8.19 | −13.76 | −2.62 | −53% (95% CI −89 to −17) |
| 2.7b | Safren 2005 | 12.4 | −4.77 | −9.19 | −0.35 | −38% (95% CI −74 to −3) | |
| Analysis 2.8 Anxiety (clinician) | 2.8 | Safren 2005 | 12.93 | −5.68 | −10.32 | −1.04 | −44% (95% CI −80 to −8) |
| Analysis 2.9 Anxiety (self‐reported) | 2.9a | Emilsson 2011 | 15.29 | −4.29 | −11.36 | 2.78 | −28% (95% CI −74 to 18) |
| 2.9b | Safren 2005 | 7.2 | −3.51 | −6.53 | −0.49 | −49% (95% CI −91 to −7) | |
| CBT versus other specific interventions | |||||||
| Analysis | Name | Control | MD | 95% CI | % change | ||
|
Analysis 3.1 ADHD symptoms (clinician) |
3.1.1 | Safren 2010 | 19.19 | −4.73 | −8.79 | −0.67 | −25 (95% CI −46 to −3) |
| 3.1.2 | Virta 2010 | −4.50 | −0.8 | −1.6 | 0 | 18% (95% CI 0 to 36) | |
|
Analysis 3.2 ADHD symptoms (self‐reported) |
3.2.1 | Safren 2010 | 19.12 | −7.28 | −11.68 | −2.88 | −38% (95% CI −61 to −15) |
| 3.2.2 | Virta 2010 | −10.55 | −2.75 | −14.93 | 9.43 | 26% (95% CI −89 to 142) | |
| 3.2.3 | Vidal Estrada 2013 | 24.29 | 1.31 | −5.92 | 8.54 | 5% (95% CI −24 to 35) | |
| 3.2.4 | Fleming 2015 | 75.56 | −15.27 | −29.52 | −1.02 | −20% (95% CI −39 to −1) | |
|
Analysis 3.3 Inattention (self‐reported) |
3.3.1 | Vidal Estrada 2013 | 18.58 | 1.35 | −4.62 | 7.32 | 7% (95% CI −25 to 39) |
| 3.3.2 | Fleming 2015 | 20.94 | −2 | −5.42 | 1.42 | −10% (95% CI −26 to 7) | |
| Analysis 3.4 Hyperactivity | 3.4.1 | Vidal Estrada 2013 | 13.88 | 1.72 | −4.41 | 7.85 | 12% (95% CI −32 to 57) |
| Analysis 3.5 Impulsivity | 3.5.1 | Vidal Estrada 2013 | 14.76 | 2.84 | −3.26 | 8.94 | 19% (95% CI −22 to 61) |
|
Analysis 3.6 Clinical Global Impression (clinician) |
3.6.1 | Safren 2010 | 3.73 | −0.53 | −1.09 | 0.03 | −14% (95% CI −29 to −1) |
| 3.6.2 | Vidal Estrada 2013 | 4.15 | 0.18 | −0.19 | 0.55 | 4% (95% CI −5 to 13) | |
|
Analysis 3.7 Clinical Global Impression (self‐reported) |
3.7.1 | Vidal Estrada 2013 | 4.17 | 0.29 | −0.32 | 0.9 | 7% (95% CI −8 to 22) |
|
Analysis 3.8 Depression (self‐reported) |
3.8.1 | Virta 2010 | −1.67 | −2.93 | −8.88 | 3.02 | 175% (95% CI −181 to 532) |
| 3.8.2 | Vidal Estrada 2013 | 13.64 | −1.24 | −9.37 | 6.89 | −9% (95% CI −69 to 51) | |
| 3.8.3 | Fleming 2015 | 13.56 | −2.91 | −8.42 | 2.6 | −21 (95% CI −62 to 19) | |
|
Analysis 3.9 Anxiety (self‐reported) |
3.9.1 | Vidal Estrada 2013 | 29.42 | −4.22 | −12.48 | 4.04 | −14% (95% CI −42 to 14) |
| 3.9.2 | Fleming 2015 | 15.75 | −5.93 | −12.91 | 1.05 | −38% (95% CI −82 to 7) | |
|
Analysis 3.10 Qualfeity of life (self‐reported) |
3.10.1 | Virta 2010 | 65.20 | −4.3 | −17.31 | 8.71 | −7% (95% CI −27 to 13) |
| 3.10.2 | Vidal Estrada 2013 | 207.35 | 33.14 | −35.76 | 102.04 | 16% (95% CI −17 to 49) | |
| 3.10.3 | Fleming 2015 | 52.8 | 14.29 | 6.13 | 22.45 | 27% (95% CI 12 to 43) | |
1.1. Analysis.
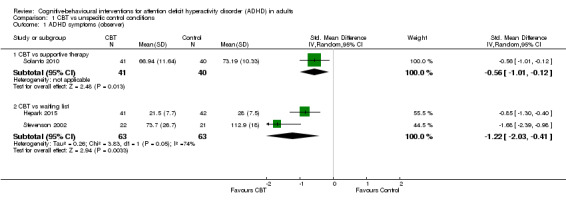
Comparison 1 CBT vs unspecific control conditions, Outcome 1 ADHD symptoms (observer).
Data and analyses
Comparison 1. CBT vs unspecific control conditions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADHD symptoms (observer) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 CBT vs supportive therapy | 1 | 81 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.01, ‐0.12] |
| 1.2 CBT vs waiting list | 2 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.03, ‐0.41] |
| 2 ADHD symptoms (self‐reported) | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 CBT vs supportive therapy | 2 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.52, 0.19] |
| 2.2 CBT vs waiting list | 5 | 251 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.18, ‐0.50] |
| 3 Inattention (clinician) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 CBT vs supportive therapy | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐2.47 [‐4.43, ‐0.51] |
| 3.2 CBT vs waiting list | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐4.1 [‐4.00, ‐2.20] |
| 4 Inattention: CBT vs waiting list (self‐reported) | 4 | 244 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.37, ‐0.82] |
| 5 Hyperactivity‐impulsivity: CBT vs waiting list (clinician) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Hyperactivity‐impulsivity: CBT vs waiting list (self‐reported) | 4 | 244 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.98, ‐0.22] |
| 7 Depression (self‐reported) | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 CBT vs supportive therapy | 1 | 81 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.36, 0.51] |
| 7.2 CBT vs waiting list | 5 | 258 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.60, ‐0.11] |
| 8 Anxiety: CBT vs supportive therapy (clinician) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Anxiety: CBT vs waiting list (self‐reported) | 4 | 239 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.71, ‐0.19] |
| 10 State anger: CBT vs waiting list (self‐reported) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11 Trait anger: CBT vs waiting list (self‐reported) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12 Self‐esteem (self‐reported) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 CBT vs Supportive Therapy | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.85, 1.85] |
| 12.2 CBT vs Waiting list | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 12.40 [4.55, 20.25] |
| 13 Quality of life: CBT vs waiting list (self‐reported) | 2 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.29, 0.71] |
Comparison 2. CBT + pharmacotherapy vs pharmacotherapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADHD symptoms (clinician) | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.31, ‐0.30] |
| 2 ADHD symptoms (self‐reported) | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐7.42 [‐11.63, ‐3.22] |
| 3 Inattention (self‐reported) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Hyperactivity‐impulsivity (self‐reported) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Clinical Global Impression (clinician) | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.21, ‐0.30] |
| 6 Depression (clinician) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Depression (self‐reported) | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐6.09 [‐9.55, ‐2.63] |
| 8 Anxiety (clinician) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Anxiety (self‐reported) | 2 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐1.08, ‐0.08] |
Comparison 3. CBT vs other specific interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADHD symptoms (clinician) | 2 | 97 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.98, ‐0.17] |
| 1.1 CBT vs relaxation + educational support | 1 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.97, ‐0.06] |
| 1.2 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.78, 0.11] |
| 2 ADHD symptoms (self‐reported) | 4 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.88, ‐0.01] |
| 2.1 CBT vs relaxation + educational support | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.26, ‐0.29] |
| 2.2 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.10, 0.71] |
| 2.3 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.57, 0.82] |
| 2.4 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.42, ‐0.00] |
| 3 Inattention (self‐reported) | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.61, 0.37] |
| 3.1 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.54, 0.85] |
| 3.2 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.08, 0.30] |
| 4 Hyperactivity: CBT vs psychoeducation | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Impulsivity: CBT vs psychoeducation | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Clinical Global Impression (clinician) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 CBT vs relaxation + educational support | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.09, 0.03] |
| 6.2 CBT vs psychoeducation | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.19, 0.55] |
| 7 Clinical Global Impression: CBT vs psychoeducation (self‐reported) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Depression (self‐reported) | 3 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.70, 0.16] |
| 8.1 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.32, 0.51] |
| 8.2 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.80, 0.59] |
| 8.3 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐1.04, 0.34] |
| 9 Anxiety (self‐reported) | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.95, 0.04] |
| 9.1 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.04, 0.36] |
| 9.2 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐1.27, 0.13] |
| 10 Quality of life (self‐reported) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐1.19, 0.62] |
| 10.2 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.37, 1.03] |
| 10.3 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.17 [0.42, 1.92] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Emilsson 2011.
| Methods | Randomised controlled trial | |
| Participants |
Country: Iceland Setting: ambulatory Age: adults; specific ages not given Sample size: 54 Sex: 34 women, 20 men Inclusion criteria: clinical diagnosis of ADHD; and stable on prescribed ADHD medication for at least a month Exclusion criteria: severe mental illness; active drug abuse; verbal intelligence quotient (IQ) estimated from clinical records to be below 85; and no valid ADHD diagnosis or not prescribed/taking ADHD medication |
|
| Interventions |
Intervention: CBT (15 sessions total, twice weekly, each lasting 90 minutes) + pharmacotherapy (n = 27) Control: treatment as usual (n = 27) Methylphenidate dosages ranged between 18‐180 mg, with a mean dosage of 60.5 mg at baseline. By the end of treatment, the dosage range was 36‐162 mg, with a mean dosage of 62.5 mg. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | We contacted authors to get the information about random sequence generation and allocation concealment that we included in this table (Young 2014 [pers comm]). Study start date: not specified Study end date: not specified Funding source: Support for the study was received from research grants awarded by RANNIS the Icelandic Centre for Research (Nr. 080443022), the Landspitali Science Fund, and Janssen‐Cilag, Iceland. Declarations of interest: Brynjar Emilsson, Jon F Sigurdsson, Gisli Baldursson, Emil Einarsson and Halldora Olafsdottir declare that they have no competing interests. Susan Young has been a consultant for Janssen‐Cilag, Eli‐Lilly and Shire. She has given educational talks at meetings sponsored by Janssen‐Cilag, Shire, Novatis, Eli‐Lilly and Flynn‐Pharma and has received research grants from Janssen‐Cilag, Eli‐Lilly and Shire. Susan Young is a consultant for the Cognitive Centre of Canada and is co‐author of 'R&R2 for ADHD Youths and Adults'. Gisli Baldursson has been a consultant for Eli‐Lilly and given educational talks at meetings sponsored by Janssen‐Cilag and Shire. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The independent evaluators were psychiatrists who were blind to the treatment condition." |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Comment: the proportion of dropouts was 37% in both groups. Quote: "Missing values were not imputed because the ANCOVA calculates outcome whilst adjusting for all baseline data. Between group effect sizes for the outcome assessments were measured using Cohen's d using unadjusted means for the dependent variables and SD pooled for unequal group sizes. Fisher's exact test was used to compare proportions of medication changes. Since this study follows an ITT protocol, statistical analysis of the outcome variables were completed for all participants regardless of medication changes." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | High risk | Quote: "The participants in both conditions were not asked to refrain from engaging in other interventions during the study period." |
| Conflict of interest | Low risk | Comment: no evidence of conflicts of interest |
Fleming 2015.
| Methods | Randomised controlled trial | |
| Participants |
Country: USA Setting: ambulatory Age: adults (18 to 24 years old) Sample size: 33 Sex: 14 women, 19 men Inclusion criteria: currently enrolled undergraduate students; meeting criteria for ADHD in adulthood, including symptom onset by age 12 and functional impairment in multiple domains Exclusion criteria: current substance abuse/dependence; or active suicidal ideation, major depressive episode, and history of psychotic disorder, bipolar disorder, or pervasive developmental disorder. |
|
| Interventions |
Intervention: dialectical behaviour therapy (DBT) (8 weekly 90 min group sessions focused on skills acquisition and strengthening, and 7 weekly 10‐15 min individual coaching phone calls focused on skills generalisation) (n = 17; 12 participants with pharmacotherapy and 5 without pharmacotherapy) Control: skills handouts control condition (34 pages of skills handouts, drawn from a manual for treatment of adults with ADHD and designed to reflect publicly available self‐help materials for ADHD) (n = 16; 13 participants with pharmacotherapy and 3 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. |
|
| Outcomes |
Primary outcomes
|
|
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: University of Washington – Robert C Bolles Doctoral Research Fellowship Declarations of interest: the author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blinding personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: participants were assessed by an interviewer who was blind to participant condition. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The intent‐to‐treat sample included 17 and 16 participants in the DBT group skills training and self‐guided SH, respectively. One participant dropped out of DBT after four sessions and did not complete the post‐treatment or follow‐up assessments; all other participants completed treatment and the three study assessments. Missing data from this participant were imputed conservatively using the last observation carried forward (LOCF) method ... Two participants receiving DBT and one receiving SH had substantial ADHD medication changes during the study (> 25% change in dose or change in medication type). One participant in each treatment condition met four (rather than five) ADHD inattentive symptom criteria. All analyses were conducted with and without medication changes, and with and without participants who did not meet full DSM‐V criteria. The pattern of results did not differ; thus, results from the full intent‐to‐treat sample are reported." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Quote: "This study cannot rule out therapist effects or non‐specific factors of group psychotherapy, although the latter concern is mitigated by the fact that response rates with SH approximate those of supportive group psychotherapy or similar control conditions in previous trials for adults with ADHD." |
| Conflict of interest | Low risk | Quote: "The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article." |
Gu 2017.
| Methods | Randomised controlled trial | |
| Participants |
Country: China Setting: ambulatory Age: undergraduate students between the ages of 19 and 24 Sample size: 54 Sex: 24 women, 30 men Inclusion criteria: meeting DSM‐5 criteria for ADHD in adulthood Exclusion criteria: major depressive episode, bipolar disorder, substance abuse/dependence within the last 6 months; actively suicidal ideation; history of psychotic disorder, and learning difficulties or other cognitive impairments |
|
| Interventions |
Intervention: mindfulness‐based cognitive therapy (8 weekly 2.5‐h sessions) (n = 28; 20 participants with pharmacotherapy and 8 without pharmacotherapy) Control: waiting list group (n = 26; 20 participants with pharmacotherapy and 6 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. |
|
| Outcomes |
Primary outcomes
|
|
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: the author(s) received no financial support for the research, authorship or publication of this article. Declarations of interest: the author(s) declared no potential conflicts of interest with respect to the research, authorship or publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: participants were assessed by an interviewer who was blind to participant condition. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The intent‐to‐treat sample consisted of 30 and 26 participants from the MBCT treatment group and [waiting list] control group, respectively. Two participants dropped out of [mindfulness‐based cognitive therapy] after six sessions and did not complete the post‐treatment or follow‐up assessments; all other participants completed treatment and the three study assessments." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Quote: "The majority of the sample was Chinese students who were recruited through general psychology courses." |
| Conflict of interest | Low risk | Comment: the author(s) declared no potential conflicts of interest with respect to the research, authorship or publication of this article. |
Hepark 2015.
| Methods | Randomised controlled trial | |
| Participants |
Country: the Netherlands Setting: ambulatory Age: adults, 18‐65 years old Sample size: 103 Sex: 56 women, 47 men Inclusion criteria: meeting criteria for ADHD in adulthood with all subtypes Exclusion criteria: substance abuse/ dependence within the last 6 months; comorbid psychotic disorders; borderline‐ and/or antisocial personality disorders; learning difficulties; chronic suicidal ideation; and automutilation. |
|
| Interventions |
Intervention: mindfulness‐based cognitive therapy (12 sessions) (n = 55; 33 participants with pharmacotherapy and 22 without pharmacotherapy) Control: waiting list group (n = 48; 26 participants with pharmacotherapy and 22 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: the author(s) received no financial support for the research, authorship, and/or publication of this article. Declarations of interest: the author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Cornelis C. Kan has also been a member of the advisory board and consultancy team of Eli Lilly BV and was a speaker at the Adult‐ADHD Academy of Eli Lilly. The other authors declared that they have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: in this study, randomisation was done by shuffling cards on which an identifier number for each participant was written. |
| Allocation concealment (selection bias) | Low risk | Comment: an independent researcher randomly assigned the participant to the MBCT or control group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The clinical interviews were conducted single blindly by a psychiatrist." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Comment: the proportion of dropouts was 25% for the mindfulness‐based cognitive therapy and 12% for the waiting list group. Quote: "To provide a more conservative estimate of the treatment effect, the authors performed ITT analyses with imputation of missing data according to LOCF. In addition, the smaller sample size at the end of the study might have led to Type II errors, that is, not establishing differences that were present, due to insufficient power." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Quote: "The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Cornelis C Kan has also been a member of the advisory board and consultancy team of Eli Lilly BV and was a speaker at the Adult‐ADHD Academy of Eli Lilly. The other authors declared that they have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article." |
Hirvikoski 2011.
| Methods | Randomised controlled trial | |
| Participants |
Country: Sweden Setting: hospital (outpatients) Age: adults, 18 years old or older Sample size: 51 Sex: 32 women, 19 men Inclusion criteria: ADHD as the main neurodevelopmental diagnosis; if on any psychoactive drug treatment (for ADHD or other diagnoses), the treatment should have been stable for at least three months Exclusion criteria: ongoing substance abuse (during the last 3 months); mental retardation (Intelligence Quotient 70); organic brain injury; autism spectrum disorder; suicidal ideation; with clinically unstable psychosocial circumstances or psychiatric disorders that were of such a severity that participation was impossible, such as being homeless, or having severe depression, psychosis, or bipolar syndrome not under stable pharmacological treatment (judged by a clinical psychologist and a psychiatrist). |
|
| Interventions |
Intervention: dialectical behaviour therapy (DBT) (14 structured sessions) (n = 26; 5 participants with pharmacotherapy and 11 without pharmacotherapy) Control: structured discussion group (14 sessions of loosely structured discussion group) (n = 25; 14 participants with pharmacotherapy and 11 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. |
|
| Outcomes |
Primary outcome
|
|
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: the clinical part of the study was conducted as part of the clinical work at Neuropsychiatric Unit Karolinska, Psychiatry Northwest, Stockholm County Council. The scientific parts of the projects were supported by the foundations Psykiatrifonden and Bror Gadelius Minnesfond. The funding sources had no role in study design, in the collection, analysis or interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. Declarations of interest: all authors declare that they have no conflicts of interest related to this work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the study authors did not describe this aspect. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Comment: the proportion of dropout was 19% for the DBT group and 20% for the structured discussion group. The study authors executed an ITT analysis. Quote: "Although the study plan described on treatment analysis, i.e. analysis of those that completed the treatment staying stable on medication (if they had any), we also wanted to a posteriori explore whether the results would change if those cases who did not fulfill these criteria were included in the analyses (Intention To Treat, ITT, analyses with LOCF, last observation carried forward)." In general well‐being, the effect was significant also in the ITT analyses (P < 0.05). |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Comment: all study authors declared that they have no conflicts of interest related to this work. |
Moëll 2015.
| Methods | Randomised controlled trial | |
| Participants |
Country: Sweden Setting: ambulatory Age: adults over the age of 18 Sample size: 57 Sex: 39 women, 18 men Inclusion criteria: confirmed or probable diagnosis of ADHD; current problems with organising daily activity and inattention defined, as 17 or more points on the ADHD Self‐Report Scale (ASRS; R. C. Kessler et al., 2005) subscale for Inattention (items 1–4 and 7–11); has access to a smart phone (android or Iphone) with Internet access; speaks, writes and reads Swedish; and cannot foresee any practical barriers to participation such as travels or medical operations. Exclusion criteria: high alcohol or drug use assessed by the AUDIT/DUDIT and assessment interview; somatic or psychiatric problems that are directly contraindicated or seriously hamper the implementation of the treatment (e.g. psychotic disorders). |
|
| Interventions |
Intervention: CBT‐inspired Internet‐based course with support (Living Smart) (n = 29). The course consisted of 7 text modules distributed over 6 weeks. The weekly modules taught the use of an online calendar (via computer and smartphone) and applications for reminders and to‐do lists. Furthermore, additional apps were introduced that previously had been shown beneficial for adults with ADHD. Control: waiting list group (n = 28) The authors did not explicitly state how many patients received pharmacological treatment. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: October 2012 Study end date: March 2013 Funding source: Karolinska Institutet Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blind evaluators also assessed improvement in organisation and inattention at post‐treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: the proportion of dropout was 10% for the CBT‐inspired Internet‐based course with support (Living Smart) and 4% for the WL group. Quote: "The analyses were done according to the principles of intent‐to treat. All participants, including those who ended the course prematurely, were asked to fill out the post‐measurement after the 6‐week period of the online course. For all statistical analyses, observed data were used in the primary analyses. To evaluate the effect of missing data, additional sensitivity analyses were performed using last‐observation‐carried forward where the last ASRS‐score of the weekly measures was used to replace missing data at post‐treatment." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | High risk | Comment: the lack of confirmed ADHD‐diagnoses for some of the participants and the fact that 12% did not receive an ADHD diagnose after their previous neuropsychiatric assessment and therefore were classified as sub‐clinical ADHD |
| Conflict of interest | Low risk | Comment: no evidence of conflicts of interest |
Pettersson 2017.
| Methods | Randomised controlled trial | |
| Participants |
Country: Sweden Setting: hospital (outpatients) Age: adults (specific ages not given) Sample size: 45 Sex: 29 women, 16 men Inclusion criteria: having ADHD as the primary diagnosis; having access to a computer and the Internet; and being able to set aside one afternoon a week for group meetings Exclusion criteria: diagnosis of borderline or antisocial personality disorder and bipolar disorder; ongoing substance abuse; suicidal ideation; dyslexia; mental retardation; ongoing psychotherapy |
|
| Interventions |
Intervention:
Both the iCBT‐G and iCBT‐S groups followed the iCBT programme In Focus, developed by the Swedish company Livanda – Internet Clinic, Ltd., in collaboration with the NPC. Control: waiting list group (n = 18; 9 participants with specific pharmacotherapy for ADHD and 9 without specific pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | We could not contact the authors due to incorrect email address. Study start date: not specified Study end date: not specified Funding source: the author(s) disclosed receipt of financial support for the research, authorship, and/or publication of this article: this study was financed by the 'Sjukskrivningsmiljarden', an economic fund established by the Swedish government to encourage Swedish county councils to give higher healthcare priority to sick leave and to develop processes and methods to reduce its frequency. In addition, Kent W Nilsson, as the principal investigator, received research grants from Forskningsrådet för samhällsvetenskap och arbetsliv (FAS), Systembolagets råd för alkoholforskning (SRA), the Swedish Brain Foundation, the Uppsala and Örebro Regional Research Council, Fredrik and Ingrid Thurings Foundation, the County Council of Västmanland, the König‐Söderströmska Foundation, the Swedish Psychiatric Foundation, and Svenska Spel Research Foundation. None of these organisations had a role in the study design, data collection, data analysis, data interpretation, or writing of the report. Declarations of interest: the author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Richard Pettersson is a partner and shareholder in the company Livanda – Internet Clinic, Ltd, that constructed and owns the rights to the Internet‐based treatment programme In Focus. Richard Pettersson was also involved in the design and construction of the programme. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A series of 54 patients were randomised in blocks to one of the study conditions over a period of four semesters (spring 2009 to autumn 2010). The results were kept in sealed envelopes, each coupled to the number in the consecutive series of patients referred to the study and who met the inclusion criteria. Unfortunately, the study had to be adjusted before the planned sample of 54 patients had been recruited because of the referral of fewer patients than expected, as well as limited financial resources and access to personnel. A total of 45 patients had been randomised to the study at the time of adjustment." |
| Allocation concealment (selection bias) | Low risk | Comment: the randomisation protocol was created by an independent statistician. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Independent evaluators, blinded to group assignment, administered the self‐report measures and conducted the semi‐structured interview." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The treatment dropout rate, defined as patients who did not complete all nine treatment modules, was 50% (seven patients) in the iCBT‐G group and 46% (six patients) in the iCBT‐S group. This gave a total dropout rate of 50% in the iCBT‐G group and 77% in the iCBT‐S group." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | High risk | Comment: the principal author was a partner and shareholder in the company Livanda – Internet Clinic, Ltd, that constructed and owns the rights to the Internet‐based treatment programme, In Focus. The principal author was also involved in the design and construction of the programme. |
Safren 2005.
| Methods | Randomised controlled trial | |
| Participants |
Country: USA Setting: ambulatory Age: adults (18‐65 years old) Sample size: 31 Sex: 17 women, 14 men Inclusion criteria: have had a principal diagnosis of Attention‐Deficit Hyperactivity Disorder with external validation of childhood onset and clinical severity of at least a moderate level (Clinical Global Impression; CGI of 4 or above); have been able to give informed consent and comply with study procedures; and have been stabilised on medications for ADHD or related symptoms. Stabilisation on medications was defined as no more than 10% change in medication dose over a 2‐month period with clinical evidence of improvement compared to the patients' unmedicated status. Exclusion criteria: moderate to severe major depression; clinically significant panic disorder; organic mental disorders, psychotic spectrum disorders, bipolar disorders, active substance abuse or dependence (past three months), pervasive developmental disorder; active suicidal ideation; history of cognitive‐behavioral therapy (CBT); estimated or documented verbal intelligence quotient (IQ) of less than 90 |
|
| Interventions |
Intervention: CBT (12‐15 weekly sessions) + continued psychopharmacology (n = 16) Control: continued psychopharmacology alone (n = 15) Dosage, timing of dosage and administration of pharmacotherapy were not specified. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | We contacted authors to get the information about random sequence generation and allocation concealment that we included in this table (Safren 2014 [pers comm]) Study start date: September 2001 Study end date: August 2003 Funding source: this study was supported by grant NIMH 60940 (Steven A Safren, PhD). Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the study authors created a randomisation table in blocks of 2, stratified by severity (CGI scale) and sex. After the person was assessed and the team agreed that they met the inclusion/criteria, they were randomised based on the table. |
| Allocation concealment (selection bias) | Low risk | Comment: the interventionist was blinded to randomisation until the team had met and it was deemed that the person met criteria. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The baseline and outcome assessments consisted of a clinician‐administered interview by an evaluator who was blind to treatment condition, and a battery of self‐report measures". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Comment: no evidence of conflicts of interest. |
Safren 2010.
| Methods | Randomised controlled trial | |
| Participants |
Country: USA Setting: ambulatory Age: adults (18‐65 years old) Sample size: 86 Sex: 38 women, 48 men Inclusion criteria: principal diagnosis of ADHD (with childhood onset); Clinical Global Impression scale score for severity of 3 (mildly ill) or greater; able to provide informed consent and comply with study procedures; and stabilised on psychotropic medications Exclusion criteria: moderate to severe major depression, clinically significant (i.e., Clinical Global Impression scale score for severity 4) panic disorder, organic mental disorders, psychotic spectrum disorders, bipolar disorders, active substance abuse or dependence, mental retardation, or pervasive developmental disorder; active suicidal ideation; history of CBT; antisocial personality disorder or a learning disability that would interfere with treatment |
|
| Interventions |
Intervention: CBT (12 weekly sessions) for Medication‐Treated Adults (n = 43) Control: Relaxation with Educational Support for Medication‐Treated Adults (n = 43) Dosage, timing of dosage and administration of pharmacotherapy were not specified. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | We contacted authors to get the information about random sequence generation and allocation concealment that we included in this table (Safren 2014 [pers comm]) Study start date: September 2004 Study end date: July 2010 Funding source: this study was funded by National Institutes of Health grant 5R01MH69812. Declarations of interest: Drs Safren, Sprich, and Otto reported receiving royalty payments from Oxford University Press. Dr Surman reported receiving research support from Abbott, Alza, Cephalon, Eli Lilly, El Minda the Hildaand Preston Davis Foundation, McNeil, Merck, New River, National Institutes of Health, Organon, Pfizer, Shire, and Takeda; being a speaker for Janssen‐Ortho, McNeil, Novartis, Shire, and MGH Academy/Reed Medical Education (which receives funding from multiple pharmaceutical companies); and being a consultant or advisor for McNeil, Shire, and Takeda. Dr Knouse reported receiving consulting income from Eli Lilly. Dr Otto reported receiving consulting income from Jazz Pharmaceuticals, Organon (Schering‐Plough), Pfizer, and Sanofi‐Aventis; research support from Organon (Schering‐Plough); and royalty payments for use of the SIGH‐A from Lilly. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the method used was coin flip. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding was maintained by having a single independent assessor who would not participate in meetings when cases were discussed. The blinded assessments were conducted by a doctoral‐level clinician with specific training from the Massachusetts General Hospital ADHD programme. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Comment: the proportion of dropout was 5% for CBT and 14% for relaxation with educational support. Quote: "Following intent‐to‐treat principles, data were analysed for all participants regardless of whether they changed their medications postrandomisation, despite the consent and inclusion criteria that specified that only those with a stable regimen of medications with no plans to change should enroll and agree not to do this during the acute treatment period of approximately 15 weeks." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Comment: no evidence of conflicts of interest |
Schoenberg 2014.
| Methods | Randomised controlled trial | |
| Participants |
Country: the Netherlands Setting: ambulatory Age: adults (18 to 65 years old) Sample size: 44 Sex: 23 women, 21 men Inclusion criteria: primary diagnosis of ADHD, DSM‐IV‐TR confirmed by 3 psychiatrists Exclusion criteria: substance abuse/dependence within the last 6 months; co‐morbid psychotic‐, borderline‐, antisocial‐, and behavioural disorders; and learning difficulties |
|
| Interventions |
Intervention: mindfulness‐based cognitive therapy (12 weekly sessions) (n = 24; 15 participants with pharmacotherapy and 9 without pharmacotherapy). Control: waiting list group (n = 20; 16 participants with pharmacotherapy and 4 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. |
|
| Outcomes |
Primary outcome
|
|
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: this research was supported by BrainGain SmartMix Programme of the Netherlands Ministry of Economic Affairs and Netherlands Ministry of Education, Culture and Science. Declarations of interest: all authors declare that they have no conflicts of interest related to this work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: a random number table was used – whether the number was even or odd would dictate allocation to MBCT or WL. |
| Allocation concealment (selection bias) | Low risk | Comment: this procedure was carried out by a member of staff unrelated to the data collection, and was witnessed by the data manager of the project. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the outcomes were potentially prone to risk of bias without blinding of the assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: of the remaining 44 participants, complete clinical data sets were not available for 2 (1 MBCT, 1 WL); in 1 case the baseline, the other the post‐treatment, questionnaires were not completed at the time of testing due to practical/time constraints. |
| Selective reporting (reporting bias) | Low risk | Comment: the study authors reported all proposed outcomes. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Comment: no evidence of conflicts of interest |
Solanto 2010.
| Methods | Randomised controlled trial | |
| Participants |
Country: USA Setting: ambulatory Age: adults (18‐65 years old) Sample size: 88 Sex: 58 women, 30 men Inclusion criteria: DSM‐IV diagnosis of ADHD, predominantly inattentive or combined subtype; stabilised on a given drug for at least 2 months and on a given dose for at least 1 month Exclusion criteria: active substance abuse or dependence; suicidal ideation; overtly hostile or aggressive behaviour likely to alienate group members; 'asocial' characteristics (e.g. pervasive developmental disorder); cognitive disability (estimated intelligence quotient (IQ) < 80); psychosis; borderline personality disorder; Alzheimer's disease or other dementia; overt neurological disorder; and childhood history of abuse or trauma or other severe psychiatric condition that confounded ascertainment of childhood ADHD symptoms. |
|
| Interventions |
Intervention: meta‐cognitive therapy (12‐week manualised meta‐cognitive therapy group intervention; 2‐h sessions) (n = 45; 19 participants with pharmacotherapy and 26 without pharmacotherapy) Control: supportive therapy (12 weeks; 2‐h sessions) (n = 43; 20 participants with pharmacotherapy and 23 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | We contacted authors to get the information about random sequence generation and allocation concealment that we included in this table (Solanto 2014 [pers comm]) Study start date: May 2005 Study end date: October 2008 Funding source: NIMH grant 1R34MH071721 to Dr Solanto Declarations of interest: Dr Solanto has served on the medical advisory board of Shire Pharmaceuticals and has served as a consultant and speaker for Ortho‐McNeil‐Janssen Pharmaceuticals. Dr Abikoff has received research funding from NIMH, the Hughes, Lemberg, and Heckscher Foundations, Ortho‐McNeil, Shire, and Eli Lilly, has served as a consultant to Shire, Eli Lilly, Cephalon, and Novartis, and has a financial interest in the Children's Organizational Skills Scale, published by Multi‐Health Systems. Dr Alvir is an employee of Pfizer. Drs Marks, Wasserstein, Mitchell, and Kofman report no financial relationships with commercial interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: a random number sequence was electronically generated. |
| Allocation concealment (selection bias) | Unclear risk | Comment:participants were stratified by whether or not they were currently receiving medication treatment for ADHD, and otherwise randomly assigned to either the CBT or the support group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: although individuals were, of course, not told to which group they were assigned, most were able to ultimately discern this because of the very different nature of the intervention (i.e. the participants were savvy enough to know that formal CBT is much more structured than a supportive intervention). Also, it is not possible to maintain a blinding of personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: response was assessed via a structured interview completed by an independent (blind) evaluator, and by questionnaires completed by the patient and a significant other, immediately pre‐ and post‐treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The proportion of drop‐out was 11% for Meta‐cognitive therapy and 12% for Supportive Therapy. All data were analysed both with and without non‐completers and medication changers." "The pattern of treatment contrasts indicated that the larger the score at baseline (that is, the more severe the symptoms), the greater the differential improvement observed with meta‐cognitive therapy; this occurred whether the data were analyzed with or without those who did not complete the program and those who made proscribed medication changes (interaction coefficients, 0.66 and 0.72, respectively)." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Quote: "Dr. Solanto is currently on the Medical Advisory Board for Shire Pharmaceuticals. She has previously served as a consultant and speaker for Ortho‐McNeil‐Janssen Pharmaceuticals, Inc. During the past five years, Dr. Abikoff has received research funding from the National Institute of Mental Health, the Hughes, Lemberg and Heckscher Foundations, Ortho‐McNeil, Shire, and Eli Lilly; has consulted to Shire, Eli Lilly, Cephalon, and Novartis; and has a financial interest in the Childrens Organizational Skills Scale, published by Multi‐Health Systems. Drs. Marks, Wasserstein, Mitchell, Alvir, and Kofman have no competing interests." |
Stevenson 2002.
| Methods | Randomised controlled trial | |
| Participants |
Country: Australia Setting: ambulatory Age: adults (21 years old or older) Sample size: 43 Sex: 14 women, 29 men Inclusion criteria: ADHD symptoms from childhood (i.e. a score of 36 or over on the Wender Utah Rating Scale), current endorsement of the DSM‐IIIR criteria for ADHD by the applicant; lifelong history consistent with ADHD; evidence that the symptoms were causing impairment in day‐to‐day functioning; and willingness to participate in a study and sign a consent form Exclusion criteria: no ADHD like symptoms; under 21 years of age; current drug or alcohol problem; history of psychosis; reported involvement in criminal activities; and mental retardation |
|
| Interventions |
Intervention: cognitive remediation programme (intensive format with 8, 2‐h weekly sessions) (n = 22; 13 participants with pharmacotherapy and 9 without pharmacotherapy) Control: waiting list group (n = 21; 11 participants with pharmacotherapy and 10 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. |
|
| Outcomes |
|
|
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: this research was supported by a grant from the Department of Psychology at Sydney University. Declarations of interest: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Low risk | Comment: the study authors used opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: no evidence of other bias |
| Conflict of interest | Low risk | Comment: no evidence of conflicts of interest |
Vidal Estrada 2013.
| Methods | Randomised controlled trial | |
| Participants |
Country: Spain Setting: hospital (outpatients) Age: adults (older than 18 years) Sample size: 32 Sex: 17 women, 15 men Inclusion criteria: meeting DSM‐IV diagnostic criteria for ADHD; aged 18 or older; stable medication prescribed for ≥ 2 months; a minimum score of 24 on the ADHD Rating Scale; minimum score of 4 on the Clinical Global Impression Severity Scale Exclusion criteria: history of substance abuse in the past 6 months or current comorbidity of other axis I or II disorders of DSM‐IV; history of psychiatric comorbidity with non‐stabilised symptoms at the moment of the study were also included. |
|
| Interventions |
Intervention: psychoeducation in medication‐treated adults (12 weeks; 2‐h sessions) (n = 17) Control: CBT in medication‐treated adults (12 weeks; 2‐h sessions) (n = 15) Dosage, timing of dosage and administration of pharmacotherapy were not specified. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: this study was supported by a non‐restricted grant from Departament de Salut, Government of Catalonia, and from ADANA Foundation Declarations of interest: the authors declare no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the participants were randomised using the Statistical Package for the Social Sciences (SPSS) software and were assigned either to psychoeducation or to CBT. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the allocation concealment process was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: self‐report measures and the CGI‐S clinician version were completed at pretreatment (time 1). Outcome measures were repeated at the end of the treatment (time 2). The pretreatment and post‐treatment evaluations were conducted by a psychologist blinded to this study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: the proportion of dropouts was 7% for the the CBT group and 6% for the psychoeducation group. Quote: "Data were analyzed (using SPSS version 20) according to intent‐to‐treat principles using a last observation carried forward procedure." |
| Selective reporting (reporting bias) | Low risk | Comment: the study authors report all proposed outcomes. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Comment: the authors declare no conflict of interest. |
Virta 2010.
| Methods | Randomised controlled trial | |
| Participants |
Country: Finland Setting: ambulatory Age: adults (18–49 years old) Sample size: 29 Sex: 15 women, 14 men Inclusion criteria: ADHD diagnosis made by a physician; no diagnosis of psychosis, severe depression or paranoia; deficits of attention, executive functions or working memory identified in an earlier neuropsychological evaluation; no current alcohol dependency or drug use; not receiving a disability pension; no participation in our previous group rehabilitation study; currently not undergoing any other psychological rehabilitation; no medication or medication that has been stable for at least three months Exclusion criteria: no neuropsychological examination; diagnosis of psychosis, severe depression or paranoia; older age, retired, or current psychological rehabilitation. |
|
| Interventions |
Intervention:
Control: control group (not specified) (n = 10; 7 participants with specific pharmacotherapy for ADHD and 3 without specific pharmacotherapy). Dosage, timing of dosage and administration of pharmacotherapy were not specified. |
|
| Outcomes |
|
|
| Notes | We contacted authors to get the information about random sequence generation and allocation concealment that we included in this table (Virta 2014 [pers comm]). Study start date: not specified Study end date: not specified Funding source: this study was supported by RAY, Finland's Slot Machine Association. Maarit Virta received funding for preparation of this manuscript from the Rinnekoti Research Foundation. Declarations of interest: the first author report no conflicts of interest in this work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the randomisation was done before the study started by raffling/draw lots. The authors had a randomised list of the rehabilitation methods beforehand. They had 4 groups: 1. CBT; 2. computerised training; 3. hypnotherapy; and 4. control. So the list looked like: 2, 1, 1, 3, 4, 1, 4, etc. Then every enrolled participant was assigned to the next group. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the independent evaluator was a clinical psychologist who was blind to the actual study group of participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Comment: the study authors report no conflicts of interest in this work. |
ADHD: attention deficit hyperactivity disorder; ANCOVA: analysis of covariance; ASRS: Adult ADHD Self‐Report Scale; CBT: cognitive‐behavioural therapy; CGI‐S: Clinical Global Impressions ‐ Severity; DBT: dialectical behaviour therapy; DSM: Diagnostic and Statistical Manual of Mental Disorders; ITT: intention‐to‐treat; LOCF: last observation carried forward; MBCT: mindfulness‐based cognitive therapy; SD: standard deviation; SH: skills handout; WL: waiting list.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cherkasova 2016 | The comparison between CBT alone versus CBT combined with medication was not a type of comparison prespecified in our protocol (Lopez 2013). |
| Mitchell 2013 | The goal of the current study was to assess the preliminary efficacy of mindfulness, without introducing other treatment modalities such as CBT. |
| Philipsen 2015 | This study compared group psychotherapy versus individual psychotherapy, affecting the comparability of the intervention of interest for our review. |
| Serra‐Pla 2017 | The goal of the current study was to assess the efficacy of virtual reality and mindfulness, without introducing other treatment modalities such as CBT. |
| Weiss 2012 | The comparison between CBT plus dextroamphetamine versus CBT plus placebo was not a type of comparison prespecified in our protocol (Lopez 2013) |
| Young 2015 | The comparison between medication plus TAU versus medication plus CBT was not a type of comparison prespecified in our protocol (Lopez 2013), due to the fact that TAU was defined by the study authors as receiving usual treatment, which included both pharmacological and non‐pharmacological treatments |
| Young 2017 | The comparison between medication plus TAU versus medication plus CBT was not a type of comparison prespecified in our protocol (Lopez 2013), due to the fact that TAU was defined by the study authors as receiving usual treatment, which included both pharmacological and non‐pharmacological treatments |
CBT: cognitive‐behavioural therapy; TAU: treatment as usual.
Characteristics of ongoing studies [ordered by study ID]
ISRCTN03732556.
| Trial name or title | Protocol for a proof of concept randomized controlled trial of cognitive‐behavioural therapy for adult ADHD as a supplement to treatment as usual, compared with treatment as usual alone |
| Methods | Two‐arm randomised controlled trial |
| Participants |
Sample size: 60 participants Inclusion criteria
Exclusion criteria
|
| Interventions | TAU plus 16 sessions of individual CBT vs TAU alone |
| Outcomes |
Primary outcomes, rated by participant (self‐report)
Secondary outcomes, rated by participant (self‐report)
Nominated informant ratings
Independent evaluator ratings
Therapist ratings
Other
|
| Starting date |
ISRCTN register number: ISRCTN03732556, assigned 4 November 2010 Start date: 21 April 2010 End date: 30 April 2014 Recruitment status: no longer recruiting |
| Contact information |
Principal investigator: Dr Antonia J Dittner Email: antonia.dittner@slam.nhs.uk Address: King's College London, King's Health Partners, Behavioural and Developmental Psychiatry Clinical Academic Group, Maudsley Adult ADHD Service, South London and Maudsley NHS Foundation Trust, London, UK |
| Notes |
Funding source: South London and Maudsley NHS Foundation Trust (UK) Declarations of interest: not reported |
NCT02062411.
| Trial name or title | A randomized controlled study of cognitive behavioral therapy for adults with attention deficit disorder |
| Methods | Randomised controlled trial, parallel assignment |
| Participants | 108 participants Inclusion criteria
Exclusion criteria
|
| Interventions | CBT vs CBT plus booster sessions |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | Current Controlled Trials: NCT02062411, date of registration: 12 February 2014 Start date: October 2013 End date: March 2016 Recruitment status: completed |
| Contact information | Dr Fang Huang. Correspondence: qianqiujin@bjmu.edu.cn. Peking University Sixth Hospital/Institute of Mental Health, National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), No. 51, Hua Yuan Bei Lu, Haidian District, Beijing 100191, China. Key Laboratory of Mental Health, Ministry of Health, Peking University, No. 51, Hua Yuan Bei Lu, Haidian District, Beijing 100191, China. |
| Notes |
Funding source: Peking University Sixth Hospital Declarations of interest: not reported |
NCT02210728.
| Trial name or title | Efficacy of cognitive behavioral therapy in treatment of adults with attention deficit hyperactivity disorder |
| Methods | Allocation: randomised Intervention Model: parallel Assignment |
| Participants | This study is ongoing, but not recruiting participants. Inclusion criteria
Exclusion criteria
|
| Interventions | Stimulant medication only versus CBT only vs combined CBT and stimulant medication group |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date |
Start date: April 2006 End date: October 2017 Recruitment status: active, not recruiting |
| Contact information | Dr Lily Hechtman. McGill University Health Center |
| Notes |
Funding source: McGill University Health Center Declarations of interest: not reported |
NCT02463396.
| Trial name or title | Mindfulness based cognitive therapy versus treatment as usual in adults with attention deficit hyperactivity disorder (ADHD) |
| Methods | Multicentre, parallel‐group, randomised controlled trial |
| Participants | 120 adults with ADHD Inclusion criteria
Exclusion criteria
|
| Interventions | Mindfulness‐based cognitive therapy (MBCT) plus TAU or TAU alone |
| Outcomes |
Primary outcome measure will be severity of ADHD symptoms rated by a blinded clinician. Secondary outcome measures will be self‐reported ADHD symptoms, executive functioning, mindfulness skills, self‐compassion, positive mental health and general functioning. In addition, a cost‐effectiveness analysis will be conducted. |
| Starting date |
Start date: September 013 End date: December 2017 Recruitment status: active, not recruiting |
| Contact information | Dr Lotte Janssen. Correspondence: Lotte.Janssen@radboudumc.nl. Department of Psychiatry, Radboud University Medical Center, Nijmegen, the Netherlands |
| Notes |
Funding source: ZonMw grant number: 837001501 Declarations of interest: not reported |
NCT02829970.
| Trial name or title | Behavioral activation to reduce problem alcohol use in college students with ADHD |
| Methods | Allocation: randomised Intervention model: parallel assignment |
| Participants | Estimated enrollment: 80 participants Inclusion criteria
Exclusion criteria
|
| Interventions | SUCCEEDS programme (Psychoeducation, Brief Motivational Interviewing and Behavioral Activation) vs Living a Healthy College Lifestyle (Psychoeducation, Brief Motivational Interviewing and Supportive Counseling) |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date |
Start date: September 2015 End date: September 2018 Recruitment status: recruiting |
| Contact information | Dr Andrea Chronis‐Tuscano (achronis@umd.edu), University of Maryland |
| Notes |
Funding source: University of Maryland Declarations of interest: not reported |
ADHD: attention deficit hyperactivity disorder; AUDIT: Alcohol Use Disorders Identification Test; CBT: cognitive‐behavioural therapy; DSM: Diagnostic and Statistical Manual of Mental Disorders;IQ: intelligence quotient; MBCT: mindfulness‐based cognitive therapy; SD: standard deviation; TAU: treatment as usual.
Differences between protocol and review
-
We pointed out the differences between ICD‐10 and DSM‐5, and we updated the reference about the prevalence of ADHD in childhood.
-
Why it is important to do this review
Where we argued that between 20% and 50% of people with ADHD do not respond to drug treatment, we added a sentence about the side effects of psychopharmacological treatment.
When the protocol was published, no systematic review on this topic existed. At the time of publication of this review, we found three systematic reviews. We added this information.
-
We modified the comparisons that considered CBT was considered as monotherapy to clarify the different types of control groups. In the protocol (Lopez 2013), we considered: "monotherapy (CBT versus control (supportive psychotherapies, placebo interventions, waiting list or no treatment) and CBT versus usual treatment (other specific psychotherapies for ADHD)); and combined therapy (CBT combined with pharmacotherapy versus pharmacotherapy alone)". In the review, we redefined the comparison as follows: CBT versus unspecific control conditions (supportive psychotherapies, waiting list or no treatment); CBT plus pharmacotherapy versus pharmacotherapy alone; CBT versus other specific interventions (control interventions that include therapeutic ingredients specifically targeted to ADHD).
-
To clarify the analysis of the outcomes, we added the following paragraph: "We presented clinical and self‐reported outcomes separately, as do most studies about this topic, because assessing ADHD is more accurate when symptom information comes from more than one source (Barkley 1998a)."
We stated in the protocol that we planned to include studies that assessed at least one primary outcome or secondary outcome (Lopez 2013), but in the full review we clarified that we included studies that assessed at least one primary outcome or at least one secondary outcome.
The safety outcome 'All‐cause treatment discontinuation', which we considered a secondary outcome in the protocol (Lopez 2013), is now considered a primary outcome in the full review.
We included the criteria to assess the magnitude of effect for continuous outcomes using the suggestions in section 12.6.2 'Re‐expressing SMDs using rules of thumb for effect sizes' of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect.
-
We planned to use MEDLINE Ovid but we used MEDLINE PubMed because of the availability of this interface.
We replaced the metaRegister of Controlled Trials (mRCT), which was reported as "under review", with the World Health Organization International Clinical Trials Registry Portal (WHO ICTRP).
-
We added the World Congress of Behavioral and Cognitive Therapies to search for conference abstracts.
-
Assessment of risk of bias in included studies
We expanded the description of the process (see Table 11).
-
If the studies did not report the standard deviation (SD), we planned to calculate it from the P value, t values, CIs or standard errors (as described in section 7.7.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011). If this information was not reported or was unattainable, we planned to impute the SD from the study with the highest SD for that outcome and assess the effects of this assumption on the analysis by conducting a sensitivity analysis. If the outcome data were reported as a median, a range or as a mean without a variance, we planned to report the data in additional tables. Since these situations did not happen, we did not apply these planned approaches.
-
We revised the bands that we reported for I2 from "0% to 30%: might not be important; 30% to 60%: may represent moderate heterogeneity; more than 60%: may represent substantial or considerable heterogeneity" (Lopez 2013), to "0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; and 75% to 100%: represents considerable heterogeneity".
-
We explained the conditions for consideration of homogeneity in more detail.
We also included a new subsection named 'Summary of findings' beneath this section, to explain the criteria we used to rate the quality of evidence according to the GRADE methodology.
-
Subgroup analysis and investigation of heterogeneity
We decided to remove 'type of CBT' as a possible subgroup analysis after the comparisons were redefined (see 'Types of interventions' above).
-
As we did not include cluster‐RCTs, we did not perform sensitivity analyses to assess the potential biasing effects of inadequately controlled cluster‐RCTs (Donner 2001), or the effect of different values of the ICC.
We did not conduct, as planned, a sensitivity analysis comparing the results of the analyses with our imputed 'highest SD' versus analyses that used an SD imputed from the study with the lowest SD.
We did not conduct, as planned, a sensitivity analysis to assess the effects of eventual missing dichotomous data on our primary meta‐analyses by assuming, on the one hand, that all missing data were successes and, on the other hand, that all missing data were failures (best‐ versus worst‐case scenario analyses).
-
While we did not foresee the transformation of the continuous results to relative percentage changes in the protocol, we included it in Appendix 2 to facilitate the readers' understanding.
5. Differences between protocol and full review.
| Section of Review | Protocol | Full review |
| Description of the condition | "The International Classification of Diseases (ICD‐10) offers a similar definition for hyperkinetic disorders (WHO 1993). Along with these three main symptomatic clusters, people with ADHD also present with deficits in executive functions, behaviour and emotion regulation, and motivation (Brown 2000; Wender 2001; Davidson 2008; Torrente 2011). There is a high prevalence of comorbid disorders, estimated at 50% to 75% (Kessler 2006), including anxiety, depression and substance abuse (Biederman 1993; Murphy 1996). Epidemiological studies estimate that the prevalence of ADHD is approximately 5% in childhood (Polanczyk 2007) and approximately 2.5% in adulthood (Simon 2009)." | "The International Classification of Diseases (ICD‐10) offers a similar definition for hyperkinetic disorders (WHO 1993), but the required number of symptoms and the age of onset are different. Along with these three main symptomatic clusters, people with ADHD also present with deficits in executive functions, behaviour and emotion regulation, and motivation (Brown 2000; Davidson 2008; Torrente 2011; Wender 2001). There is a high prevalence of comorbid disorders, estimated at 50% to 75% (Kessler 2006), including anxiety, depression and substance abuse (Biederman 1993; Murphy 1996). Epidemiological studies estimate that the prevalence of ADHD is approximately 5% in childhood and around 2.5% in adulthood (Polanczyk 2014; Simon 2009)." |
| Why it is important to do this review | "Between 20% and 50% of people with ADHD do not respond to drug treatment (Wilens 2002)." | "Between 20% and 50% of people with ADHD do not respond to drug treatment (Wilens 2002). Also, pharmacological treatment is frequently associated with relevant side effects in both children and adults (AJCD 2001; Castells 2013; Cunill 2013; Graham 2011; King 2006; Lim 2006; Morton 2000; Perrin 2008; Prescrire 2007). Due to these concerns, it is important to have non‐pharmacological interventions for treating adults with ADHD." |
| "To date, no systematic review has examined the effects of CBT in adults with ADHD. The growing number of randomised controlled trials assessing the efficacy of CBT for this population (Knouse 2008) suggest that this review is timely." | "To our knowledge, three systematic reviews have compared the effects of CBT in adults with ADHD (Jensen 2016; Knouse 2017; Young 2016). However, there are important methodological differences between them, also with respect to our review. Both Jensen 2016 and Young 2016 employed more restrictive criteria for defining CBT treatments that excluded relevant CBT variants such as mindfulness‐based cognitive therapy and dialectical behavioural therapy. Knouse 2017 did not report grades of quality of evidence of the included studies." | |
| Types of interventions | We considered the following comparisons: Monotherapy
Combined therapy
|
We considered the following comparisons:
|
| Types of outcome measures | We considered psychometrically validated self‐report measures or those completed by an independent rater or relative. The measures were considered short‐ (up to six months), medium‐ (six months to 12 months) and long‐term (more than 12 months)." | "We considered psychometrically validated self‐report measures or those completed by an independent rater or relative. "We presented clinical and self‐reported outcomes separately, as do most studies about this topic, because assessing ADHD is more accurate when symptom information comes from more than one source (Barkley 1998a). "We considered the measures as short term (up to 6 months), medium term (6 months to 12 months) and long term (more than 12 months). "We included studies that assessed at least one primary outcome or at least one secondary outcome." |
| "We will include studies that have assessed at least one primary or secondary outcome." | "We included studies that assessed at least one primary outcome or at least one secondary outcome." | |
| The safety outcome 'All‐cause treatment discontinuation' was considered as secondary outcome | We considered the safety outcome 'All‐cause treatment discontinuation' to be a primary outcome. | |
| Electronic searches | We planned to search Ovid MEDLINE | We used MEDLINE PubMed because of the availability of this interface. |
| Searching other resources | "Additionally, we searched dissertations and abstracts from the following.
|
"Additionally, we searched dissertations and abstracts from the following.
|
| Assessment of risk of bias in included studies | "We independently evaluated the risk of bias using EROS. This process followed the six criteria described in Table 8.5.d, "Criteria for judging risk of bias in the 'Risk of bias' assessment tool" of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (See Table 4)." | "We evaluated the risk of bias in each included trial using the seven criteria described in Table 8.5.d ('Criteria for judging risk of bias in the "Risk of bias' assessment tool") of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (PL and FT) independently assessed each included study as being at low, high or unclear (uncertain) risk of bias for each domain, using EROS software (Ciapponi 2011; Glujovsky 2011; Glujovsky 2010); see Table 4. If there were discrepancies between their assessments, and the two review authors were unable to reach a consensus, a third review author (AC) joined the decision‐making process. All three review authors discussed the issue and made a final decision." |
| Assessment of heterogeneity | The bands that we reported for I2 were:
|
The bands that we reported for I2 were:
|
| Data synthesis | "When we considered studies to be sufficiently homogenous in terms of participants, interventions and outcomes, we synthesised the results in a meta‐analysis using RevMan (RevMan 2014)." | "We synthesised the results in a meta‐analysis using Review Manager 5 (RevMan 5) when we considered studies to be sufficiently homogenous in terms of population (regarding sex, age and diagnosis), interventions (comparable modalities of CBT) and comparisons (as a monotherapy or a part of a combined treatment) to avoid clinical heterogeneity, and in terms of outcome measurement methods to avoid methodological heterogeneity (RevMan 2014). Two authors assessed homogeneity independently and solved discrepancies by consensus." |
| We did not include a subsection about summary of findings | We included a 'Summary of findings table' subsection: "We prepared a 'Summary of findings' table for our three main comparisons (see Types of interventions) according to GRADE methodology (Atkins 2004; Guyatt 2011), using GRADEpro GDT software (GRADEpro 2015). We included our primary outcome, the core symptoms of ADHD (self‐, clinician‐ or observer‐reported), in the tables. "Two review authors (AC and PL) independently assessed the quality of the evidence as high, moderate, low or very low, downgrading the rating according to the presence of the following criteria: study limitations, in which we considered the studies' 'Risk of bias' level; imprecision; inconsistency of results; indirectness of evidence; and likely publication bias. "To assess the magnitude of effect for continuous outcomes, we used the criteria suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect." |
|
| Subgroup analysis and investigation of heterogeneity | We considered the type of CBT as a possible subgroup analysis | We did not include the type of CBT as a possible subgroup analysis after we redefined the comparisons. |
| Sensitivity analysis | For this review, we had planned to undertake sensitivity analyses to determine the effect of restricting the analysis to: "(a) only studies with low risk of selection bias (associated with sequence generation or allocation concealment), (b) only studies with low risk of performance bias (associated with issues of blinding), and (c) only studies with low risk of attrition bias (associated with completeness of data). In addition, we will assess the sensitivity of findings to any imputed data within a study." | "[W]e undertook sensitivity analyses to determine the effect of removing from the analysis: studies with high risk of selection bias (associated with sequence generation or allocation concealment); studies with high risk of performance bias (associated with issues of blinding); and studies with high risk of attrition bias (associated with completeness of data). In addition, we assessed the sensitivity of findings to any imputed data within a study." |
| Effects of interventions | The transformation of the continuous results to relative percentage changes had not been foreseen. | We included the transformation of continuous results to relative percentage changes in Appendix 2. |
We describe all modifications in the Additional Methods Table (Table 11).
Contributions of authors
Pablo López: overall responsibility for the review, protocol writing, trial selection, data extraction and assimilation, statistical analysis and review writing. Fernando Torrente: protocol writing, trial selection, data extraction and assimilation, statistical analysis and review writing. Agustín Ciapponi: protocol writing, trial selection, data extraction and assimilation, statistical analysis and review writing. Alicia Graciela Lischinsky: protocol and review writing. Marcelo Cetkovich‐Bakmas: protocol and review writing. Juan Ignacio Rojas: protocol and review writing. Marina Romano: protocol and review writing. Facundo F Manes: overall supervision of the review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Pablo Luis Lopez ‐ none known. Fernando Manuel Torrente ‐ none known. Agustín Ciapponi ‐ none known. Alicia Graciela Lischinsky received payment as an invited speaker at Ely Lilly and Jannsen Cilag Labs. Marcelo Cetkovich‐Bakmas ‐ none known. Juan Ignacio Rojas ‐ none known. Marina Romano received payment from Novartis Argentina for review preparation and funds for travel/accommodation/meeting expenses from Boehringer‐Ingelheim. MR declares no conflict of interest related to this review. Facundo F Manes ‐ none known.
New
References
References to studies included in this review
Emilsson 2011 {published data only}
- Emilsson B, Gudjonsson G, Sigurdsson JF, Baldursson G, Einarsson E, Olafsdottir H, et al. Cognitive behaviour therapy in medication‐treated adults with ADHD and persistent symptoms: a randomized controlled trial. BMC Psychiatry 2011;11:116. [DOI: 10.1186/1471-244X-11-116; ACTRN12611000533998; PMC3155481; PUBMED: 21787431] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleming 2015 {published data only}
- Fleming AP, McMahon RJ, Moran LR, Peterson AP, Dreessen A. Pilot randomized controlled trial of dialectical behavior therapy group skills training for ADHD among college students. Journal of Attention Disorders 2015;19(3):260‐71. [DOI: 10.1177/1087054714535951; PUBMED: 24874347] [DOI] [PubMed] [Google Scholar]
Gu 2017 {published data only}
Hepark 2015 {published data only}
- Hepark S, Janssen L, Vries A, Schoenberg PLA, Donders R, Kan CC, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. Journal of Attention Disorders 2015 Nov 20 [Epub ahead of print]. [DOI: 10.1177/1087054715613587; PUBMED: 26588940] [DOI] [PubMed]
Hirvikoski 2011 {published data only}
- Hirvikoski T, Waaler E, Alfredsson J, Pihlgren C, Holmström A, Johnson A, et al. Reduced ADHD symptoms in adults with ADHD after structured skills training group: results from a randomized controlled trial. Behaviour Research and Therapy 2011;49(3):175‐85. [DOI: 10.1016/j.brat.2011.01.001; PUBMED: 21295767] [DOI] [PubMed] [Google Scholar]
Moëll 2015 {published data only}
- Moëll B, Kollberg L, Nasri B, Lindefors N, Kaldo V. Living SMART – A randomized controlled trial of a guided online course teaching adults with ADHD or sub‐clinical ADHD to use smartphones to structure their everyday life. Internet Interventions 2015;2(1):24‐31. [DOI: 10.1016/j.invent.2014.11.004] [DOI] [Google Scholar]
Pettersson 2017 {published data only}
Safren 2005 {published data only}
- Safren SA, Otto MW, Sprich S, Winett CL, Wilens TE, Biederman J. Cognitive‐behavioral therapy for ADHD in medication‐treated adults with continued symptoms. Behaviour Research and Therapy 2005;43(7):831‐42. [DOI: 10.1016/j.brat.2004.07.001; PUBMED: 15896281] [DOI] [PubMed] [Google Scholar]
Safren 2010 {published data only}
- Safren SA, Sprich S, Mimiaga MJ, Surman C, Knouse L, Groves M, et al. Cognitive behavioral therapy vs relaxation with educational support for medication‐treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA 2010;304(8):875‐80. [DOI: 10.1001/jama.2010.1192; NCT00118911; PMC3641654; PUBMED: 20736471] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoenberg 2014 {published data only}
- Schoenberg PL, Hepark S, Kan CC, Barendregt HP, Buitelaar JK, Speckens AE. Effects of mindfulness‐based cognitive therapy on neurophysiological correlates of performance monitoring in adult attention‐deficit/hyperactivity disorder. Clinical Neurophysiology 2014;125(7):1407‐16. [DOI: 10.1016/j.clinph.2013.11.031; PUBMED: 24374088] [DOI] [PubMed] [Google Scholar]
Solanto 2010 {published data only}
- Solanto MV, Marks DJ, Wasserstein J, Mitchell, Abikoff H, Alvir JM, et al. Efficacy of meta‐cognitive therapy (MCT) for adult ADHD. American Journal of Psychiatry 2010;167(8):958‐68. [DOI: 10.1176/appi.ajp.2009.09081123; NIHMS439495; PMC3633586] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevenson 2002 {published data only}
- Stevenson CS, Stevenson RJ, Whitmont S. A self‐directed psychosocial intervention with minimal therapist contact for adults with attention deficit hyperactivity disorder. Clinical Psychology & Psychotherapy 2003;10(2):93‐101. [DOI: 10.1002/cpp.356] [DOI] [Google Scholar]
- Stevenson CS, Whitmont S, Bornholt L, Livesey D, Stevenson RJ. A cognitive remediation programme for adults with attention deficit hyperactivity disorder. Australian and New Zealand Journal of Psychiatry 2002;36(5):610‐6. [DOI: 10.1046/j.1440-1614.2002.01052.x; PUBMED: 12225443] [DOI] [PubMed] [Google Scholar]
Vidal Estrada 2013 {published data only}
- Vidal R, Bosch R, Nogueira M, Gómez‐Barros N, Valero S, Palomar G, et al. Psychoeducation for adults with attention deficit hyperactivity disorder vs cognitive behavioral group therapy: a randomized controlled pilot study. Journal of Nervous and Mental Disease 2013;201(10):894‐900. [DOI: 10.1097/NMD.0b013e3182a5c2c5; PUBMED: 24080677] [DOI] [PubMed] [Google Scholar]
Virta 2010 {published data only}
- Virta M, Salakari A, Antila M, Chydenius E, Partinen M, Kaski M, et al. Short cognitive behavioral therapy and cognitive training for adults with ADHD – a randomized controlled pilot study. Neuropsychiatric Disease and Treatment 2010;6(1):443‐53. [DOI: 10.2147/NDT.S11743; PMC2938293; PUBMED: 20856608] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Cherkasova 2016 {published data only}
- Cherkasova MV, French LR, Syer CA, Cousins L, Galina H, Ahmadi‐Kashani Y, et al. Efficacy of cognitive behavioral therapy with and without medication for adults with ADHD: a randomized clinical trial. Journal of Attention Disorders 2016 Oct 6 [Epub ahead of print]. [10.1177/1087054716671197] [DOI] [PubMed]
Mitchell 2013 {published data only}
- Mitchell JT, McIntyre EM, English JS, Dennis MF, Beckham JC, Kollins SH. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning,and emotion dysregulation. Journal of Attention Disorders 2017;21(13):1105‐20. [DOI: 10.1177/1087054713513328; PMC4045650; PUBMED: 4305060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Philipsen 2015 {published data only}
- Philipsen A, Jans T, Graf A, Matthies S, Borel P, Colla M, et al. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention‐deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry 2015;72(12):1199‐210. [DOI: 10.1001/jamapsychiatry.2015.2146; ISRCTN54096201; PUBMED: 26536057] [DOI] [PubMed] [Google Scholar]
Serra‐Pla 2017 {published data only}
- Serra‐Pla JF, Pozuelo M, Richarte V, Corrales M, Ibáñez Pol, Bellina M, et al. Treatment of attention deficit hyperactivity disorder in adults using virtual reality through a mindfulness programme [Tratamiento del trastorno por déficit de atención/hiperactividad en la edad adulta a través de la realidad virtual mediante un programa de mindfulness]. Revista de Neurología 2017;64(s01):S117‐22. [PUBMED: 28256698] [PubMed] [Google Scholar]
Weiss 2012 {published data only}
- Weiss M, Murray C, Wasdell M, Greenfield B, Giles L, Hechtman L. A randomized controlled trial of CBT therapy for adults with ADHD with and without medication. BMC Psychiatry 2012;12:30. [DOI: 10.1186/1471-244X-12-30] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2015 {published data only}
- Young S, Khondoker M, Emilsson B, Sigurdsson JF, Philipp‐Wiegmann F, Baldursson G, et al. Cognitive‐behavioural therapy in medication‐treated adults with attention‐deficit/hyperactivity disorder and co‐morbid psychopathology: a randomized controlled trial using multi‐level analysis. Psychological Medicine 2015;45(13):2793–804. [DOI: 10.1017/S0033291715000756; PMC4595859; PUBMED: 26022103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2017 {published data only}
- Young S, Emilsson B, Sigurdsson JF, Khondoker M, Philipp‐Wiegmann F, Baldursson G, et al. A randomized controlled trial reporting functional outcomes of cognitive‐behavioural therapy in medication‐treated adults with ADHD and comorbid psychopathology. European Archives of Psychiatry and Clinical Neuroscience 2017;267(3):267‐76. [DOI: 10.1007/s00406-016-0735-0; PMC5357275] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN03732556 {published data only}
- Dittner AJ, Rimes KA, Russell AJ, Chalder T. Protocol for a proof of concept randomized controlled trial of cognitive‐behavioural therapy for adult ADHD as a supplement to treatment as usual, compared with treatment as usual alone. BMC Psychiatry 2014;14:248. [DOI: 10.1186/s12888-014-0248-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02062411 {unpublished data only}
- NCT02062411. A randomized controlled study of cognitive behavioral therapy for adults with attention deficit disorder [A randomized controlled study of cognitive behavioral therapy for adults with attention deficit disorder]. clinicaltrials.gov/ct2/show/study/NCT02062411 (first received 12 February 2014).
NCT02210728 {unpublished data only}
- NCT02210728. Efficacy of cognitive behavioral therapy in treatment of adults with attention deficit hyperactivity disorder [Efficacy of cognitive behavioral therapy in treatment of adults with attention deficit hyperactivity disorder]. clinicaltrials.gov/ct2/show/NCT02210728 (first received 31 July 2014).
NCT02463396 {published data only}
- Janssen L, Kan CC, Carpentier PJ, Sizoo B, Hepark S, Grutters J, et al. Mindfulness based cognitive therapy versus treatment as usual in adults with attention deficit hyperactivity disorder (ADHD). BMC Psychiatry 2015;15:216. [DOI: 10.1186/s12888-015-0591-x; PMC4572448] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02829970 {unpublished data only}
- NCT02829970. Helping college students with ADHD lead healthier lifestyles [Behavioral activation to reduce problem alcohol use In college students with ADHD]. clinicaltrials.gov/ct2/show/record/NCT02829970 (first received 7 July 2016).
Additional references
Achenbach 1998
- Achenbach TM, Howell C, McConaughy SH, Stanger C. Six‐year predictors of problems in a national sample: IV. Young adult signs of disturbance. Journal of the American Academy of Child and Adolescent Psychiatry 1998;37(7):718‐27. [DOI: 10.1097/00004583-199807000-00011; PUBMED: 9666627] [DOI] [PubMed] [Google Scholar]
Adler 2002
- Adler LA, Chua HC. Management of ADHD in adults. Journal of Clinical Psychiatry 2002;63(Suppl 12):29‐35. [PUBMED: 12562059] [PubMed] [Google Scholar]
Adler 2003
- Adler LA, Spencer T, Biederman J. Adult ADHD Investigator Symptom Rating Scale–AISRS. Boston (MA); New York (NY): Massachusetts General Hospital; New York University School of Medicine, 2003. [Google Scholar]
AJCD 2001
- America Journal of Clinical Dermatology. Cutaneous drug reaction case reports: from the world literature. American Journal of Clinical Dermatology 2001;2(4):267‐74. [PUBMED: 11817970] [DOI] [PubMed] [Google Scholar]
Amador‐Campos 2014
- Amador‐Campos JA, Gómez‐Benito J, Ramos‐Quiroga JA. The Conners' Adult ADHD Rating Scales ‐‐ short self‐report and observer forms: psychometric properties of the Catalan version. Journal of Attention Disorders 2014;18(8):671‐9. [DOI: 10.1177/1087054712446831; PUBMED: 22771453] [DOI] [PubMed] [Google Scholar]
Antshel 2011
- Antshel KM, Hargrave TM, Simonescu M, Kaul P, Hendricks K, Faraone SV. Advances in understanding and treating ADHD. BMC Medicine 2011;9:72. [DOI: 10.1186/1741-7015-9-72] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Services Research 2004;4(1):38. [DOI: 10.1186/1472-6963-4-38; PMC545647; PUBMED: 15615589] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barkley 1998a
- Barkley RA, Murphy KR. Attention‐Deficit Hyperactivity Disorder: A Clinical Workbook. 2nd Edition. New York (NY): Guilford Press, 1998. [Google Scholar]
Barkley 1998b
- Barkley RA. Attention Deficit‐Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. 2nd Edition. New York (NY): Guilford Press, 1998. [Google Scholar]
Barkley 1999
- Barkley RA. Niños Hiperactivos: Cómo Comprender y Atender sus Necesidades Especiales. Guía Completa del Trastorno por Déficit de Atención con Hiperactividad [Hyperactive Children: How to Understand and Address their Special Needs: Complete Guide to Attention Deficit Hyperactivity Disorder]. Barcelona (ES): Paidós, 1999. [Google Scholar]
Barkley 2002
- Barkley RA. Major life activity and health outcomes associated with attention‐deficit/hyperactivity disorder. Journal of Clinical Psychiatry 2002;63(Suppl 12):10‐5. [PUBMED: 12562056] [PubMed] [Google Scholar]
Barkley 2006a
- Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult outcome of hyperactive children: adaptive functioning in major life areas. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(2):192‐202. [DOI: 10.1097/01.chi.0000189134.97436.e2; PUBMED: 16429090] [DOI] [PubMed] [Google Scholar]
Barkley 2006b
- Barkley RA, Murphy KR. Attention‐Deficit Hyperactivity Disorder: A Clinical Workbook. 3rd Edition. New York (NY): Guilford Press, 2006. [Google Scholar]
Barkley 2008
- Barkley RA, Murphy KR, Fischer M. ADHD in Adults: What the Science Says. New York (NY): Guilford Press, 2008. [Google Scholar]
Barkley 2011
- Barkley RA. Barkley Adult ADHD Rating Scale‐IV (BAARS‐IV). New York (NY): Guilford Press, 2011. [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [PUBMED: 13688369] [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56(6):893–7. [PUBMED: 3204199] [DOI] [PubMed] [Google Scholar]
Beck 1993
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio (TX): Psychological Corporation, 1993. [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory‐II. San Antonio (TX): Psychological Corporation, 1996. [Google Scholar]
Beck 2012
- Beck AT, Steer RA, Järvå H. BAI – Beck Anxiety Inventory: Manual, Svensk Version. Stockholm (SE): Pearson, 2012. [Google Scholar]
Biederman 1993
- Biederman J, Faraone SV, Spencer T, Wilens T, Norman D, Lapey KA, et al. Patterns of psychiatric comorbidity, cognition, and psychosocial functioning in adults with attention deficit hyperactivity disorder. American Journal of Psychiatry 1993;150(12):1792‐8. [DOI: 10.1176/ajp.150.12.1792; PUBMED: 8238632] [DOI] [PubMed] [Google Scholar]
Biederman 2002
- Biederman J, Mick E, Faraone SV, Braaten E, Doyle A, Spencer T, et al. Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. American Journal of Psychiatry 2002;159(1):36‐42. [DOI: 10.1176/appi.ajp.159.1.36; PUBMED: 11772687] [DOI] [PubMed] [Google Scholar]
Biederman 2006
- Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, et al. Young adult outcome of attention deficit hyperactivity disorder: a controlled 10‐year follow‐up study. Psychological Medicine 2006;36(2):167–79. [DOI: 10.1017/S0033291705006410; PUBMED: 16420713] [DOI] [PubMed] [Google Scholar]
Brassett‐Harknett 2007
- Brassett‐Harknett A, Butler N. Attention‐deficit/hyperactivity disorder: an overview of the etiology and a review of the literature relating to the correlates and lifecourse outcomes for men and women. Clinical Psychology Review 2007;27(2):188‐210. [DOI: 10.1016/j.cpr.2005.06.001; PUBMED: 16081194] [DOI] [PubMed] [Google Scholar]
Brod 2005
- Brod M, Perwien A, Adler L, Spencer T, Johnston J. Conceptualization and assessment of quality of life for adults with attention deficit disorder. Primary Psychiatry 2005;12(6):58‐64. [www.researchgate.net/profile/Meryl_Brod/publication/232471361_Conceptualization_and_Assessment_of_Quality_of_Life_for_Adults_with_Attention‐DeficitHyperactivity_Disorder/links/53d26cf00cf220632f3c9993/Conceptualization‐and‐Assessment‐of‐Quality‐of‐Life‐for‐Adults‐with‐Attention‐Deficit‐Hyperactivity‐Disorder.pdf] [Google Scholar]
Brod 2006
- Brod M, Johnston J, Able S, Swindle R. Validation of the adult attention‐deficit/hyperactivity disorder quality‐of‐life scale (AAQoL): a disease‐specific quality‐of‐life measure. Quality of Life Research 2006;15(1):117‐29. [DOI: 10.1007/s11136-005-8325-z; PUBMED: 16411036] [DOI] [PubMed] [Google Scholar]
Brown 1996
- Brown TE. Attention‐Deficit Disorder Scales Manual. San Antonio (TX): Psychological Corporation, 1996. [Google Scholar]
Brown 2000
- Brown TE. Attention Deficit Disorders and Comorbidities in Children, Adolescents and Adults. Washington (DC): American Psychiatric Press, 2000. [Google Scholar]
Campbell 2000
- Campbell M, Grimshaw J, Steen N. Sample size calculations for cluster randomised trials. Changing Professional Practice in Europe Group (EU BIOMED II Concerted Action). Journal of Health Services Research & Policy 2000;5(1):12‐6. [DOI: 10.1177/135581960000500105; 10787581] [DOI] [PubMed] [Google Scholar]
Castells 2013
- Castells X, Cunill R, Capellà D. Treatment discontinuation with methylphenidate in adults with attention deficit hyperactivity disorder: a meta‐analysis of randomized clinical trials. European Journal of Clinical Pharmacology 2013;69(3):347‐56. [DOI: 10.1007/s00228-012-1390-7; PUBMED: 22983311] [DOI] [PubMed] [Google Scholar]
Chandler 2013
- Chandler ML. Psychotherapy for adult attention deficit/hyperactivity disorder: a comparison with cognitive behavior therapy. Journal of Psychiatric and Mental Health Nursing 2013;20(9):814‐20. [DOI: 10.1111/jpm.12023; PUBMED: 23506050] [DOI] [PubMed] [Google Scholar]
Ciapponi 2011
- Ciapponi A, Glujovsky D, Bardach A, García Martí S, Comande D. EROS: A new software for early stage of systematic reviews. HTAi 2011 Conference, 2011 June 27‐29; Rio de Janeiro. 2011.
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioural Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
Conners 1999a
- Conners CK, Erhardt D, Sparrow E. Conners CAARS Adult ADHD Rating Scales: Technical Manual. New York (NY): Multi‐Health Systems Inc., 1999. [Google Scholar]
Conners 1999b
- Conners CK, Erhardt D, Epstein JN, Parker JDA, Sitarenios G, Sparrow E. Self‐ratings of ADHD symptoms in adults I: factor structure and normative data. Journal of Attention Disorders 1999;3(3):141‐51. [DOI: 10.1177/108705479900300303] [DOI] [Google Scholar]
Conners 1999c
- Conners CK, Erhardt D, Sparrow E. Conners' CAARS Adult ADHD Rating Scales: Technical Manual. Toronto (ON): Multi‐Health Systems, 1999. [Google Scholar]
Conners 2008
- Conners CK, Erhardt D, Sparrow EP. Conners’ Adult ADHD Rating Scale ㅡ Self: Screening Version (CAARS‐S:SV). New South Wales (AU): Psychological Assessments, 2008. [Google Scholar]
Cunill 2013
- Cunill R, Castells X, Tobias A, Capellà D. Atomoxetine for attention deficit hyperactivity disorder in the adulthood: a meta‐analysis and meta‐regression. Pharmacoepidemiology and Drug Safety 2013;22(9):961‐9. [DOI: 10.1002/pds.3473; PUBMED: 23813665] [DOI] [PubMed] [Google Scholar]
Davids 2004
- Davids E, Krause DA, Specka M, Gastpar M. Analysis of a special consultation for attention deficit/hyperactivity disorder in adults [Analyse einer Spezialsprechstunde für die Aufmerksamkeitsdefizit‐/Hyperaktivitätsstörung bei Erwachsenen]. Gesundheitswesen 2004;66(7):416–22. [DOI: 10.1055/s-2004-813327; PUBMED: 15314733] [DOI] [PubMed] [Google Scholar]
Davidson 1960
- Davidson HH, Lang G. Children's perceptions of their teachers feelings towards them related to self perception, school achievement and behavior. Journal of Experimental Education 1960;29(2):107‐18. [DOI: 10.1080/00220973.1960.11010675] [DOI] [Google Scholar]
Davidson 2008
- Davidson MA. ADHD in adults: a review of the literature. Journal of Attention Disorders 2008;11(6):628‐41. [DOI: 10.1177/1087054707310878; 18094324] [DOI] [PubMed] [Google Scholar]
Donner 2001
- Donner A, Piaggio G, Villar J. Statistical methods for the meta‐analysis of cluster randomization trials. Statistical Methods in Medical Research 2001;10(5):325‐38. [DOI: 10.1177/096228020101000502; PUBMED: 11697225] [DOI] [PubMed] [Google Scholar]
DSM‐5 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). 5th Edition. Arlington (VA): American Psychiatric Association, 2013. [Google Scholar]
DSM‐III‐R 1989
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐III‐R). 3rd Edition. Washington (DC): American Psychiatric Association, 1989. [Google Scholar]
DSM‐IV 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington (DC): American Psychiatric Association, 1994. [Google Scholar]
DSM‐IV‐TR 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR). 4th Edition. Washington (DC): American Psychiatric Association, 2000. [Google Scholar]
DuPaul 1990
- DuPaul G. The ADHD Rating Scale: normative data, reliability and validity. Unpublished manuscript of the University of Massachussetts Medical School ca 1990.
DuPaul 1998
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale‐IV: Checklists, Norms, and Clinical Interpretation. New York (NY): Guilford Press, 1998. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [9310563; PMC2127453] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endicott 1993
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacology Bulletin 1993;29(2):321‐6. [PUBMED: 8290681] [PubMed] [Google Scholar]
Erhardt 1999
- Erhardt D, Epstein JN, Conners CK, Parker JDA, Sitarenios G. Self‐ratings of ADHD symptoms in adults: II. Reliability, validity, and diagnostic sensitivity. Journal of Attention Disorders 1999;3(3):153‐8. [DOI: 10.1177/108705479900300304] [DOI] [Google Scholar]
Faraone 2000
- Faraone SV, Biederman J, Spencer T, Wilens T, Seidman LJ, Mick E, et al. Attention‐deficit/hyperactivity disorder in adults: an overview. Biological Psychiatry 2000;48(1):9‐20. [PUBMED: 10913503] [DOI] [PubMed] [Google Scholar]
Gershon 2002
- Gershon J. A meta‐analytic review of gender differences in ADHD. Journal of Attention Disorders 2002;5(3):143‐54. [DOI: 10.1177/108705470200500302; PUBMED: 11911007] [DOI] [PubMed] [Google Scholar]
Gittelman 1985
- Gittelman R. Self‐evaluation (teenager's) self‐reports. Psychopharmacology Bulletin 1985;21:925‐6. [Google Scholar]
Glujovsky 2010
- Glujovsky D, Bardach A, García Martí S, Comande D, Ciapponi A. New software for early stage of systematic reviews. Abstracts of the Joint Cochrane and CampbellColloquium; 2010 Oct 18‐22; Keystone (CO). Chichester (UK): Wiley‐Blackwell, John Wiley & Sons, 2010:P95. [DOI: 10.1002/14651858.CD000002; P90] [DOI]
Glujovsky 2011
- Glujovsky D, Bardach A, García Martí S, Comande D, Ciapponi A. EROS: A new software for early stage of systematic reviews. ISPOR 3rd Latin America Conference: Building Networks Across Institutions for Access to Health Care in Latin America; 2011 Sep 8‐10; Mexico City (MX). Mexico City (MX): IPSOR (International Society for Pharmacoecomics and Outcomes Research), 2011:111.
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 12 December 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Graham 2011
- Graham J, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Dittmann RW, et al. European guidelines on managing adverse effects of medication for ADHD. European Child & Adolescent Psychiatry 2011;20(1):17–37. [DOI: 10.1007/s00787-010-0140-6; PMC3012210] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guy 1976
- Guy W, National Institute of Mental health (US), Psychopharmacology Research Branch. Division of Extramural Research Programs. ECDEU Assessment Manual for Psychopharmacology, Revised. Rockville (MD): US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976. [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026; PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32(1):50‐5. [DOI: 10.1111/j.2044-8341.1959.tb00467.x] [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [PMC495331; PUBMED: 14399272] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hesslinger 2002
- Hesslinger B, Tebartz Van Elst L, Nyberg E, Dykierek P, Richter H, Berner M, et al. Psychotherapy of attention deficit hyperactivity disorder in adults ‐‐ a pilot study using a structured skills training program. European Archives of Psychiatry and Clinical Neuroscience 2002;252(4):177‐84. [DOI: 10.1007/s00406-002-0379-0; PUBMED: 12242579] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (Editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jensen 2016
- Jensen CM, Amdisen BL, Jorgensen KJ, Arnfred SM. Cognitive behavioural therapy for ADHD in adults: systematic review and meta‐analyses. Attention Deficit and Hyperactivity Disorders 2016;8(1):3‐11. [DOI: 10.1007/s12402-016-0188-3; PUBMED: 26801998] [DOI] [PubMed] [Google Scholar]
Kaufman 1996
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Kiddie‐SADS‐Present and Lifetime Version (K‐SADS‐PL). Available from www.psychiatry.pitt.edu/sites/default/files/Documents/assessments/ksads‐pl.pdf (accessed 12 December 2017).
Kessler 2005
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, et al. The World Health Organization Adult ADHD Self‐Report Scale (ASRS): a short screening scale for use in the general population. Psychological Medicine 2005;35(2):245‐56. [PUBMED: 15841682] [DOI] [PubMed] [Google Scholar]
Kessler 2006
- Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg P, Hirschfeld RM, et al. Prevalence and effects of mood disorders on work performance in a nationally representative sample of US workers. American Journal of Psychiatry 2006;163(9):1561‐8. [DOI: 10.1176/ajp.2006.163.9.1561; PMC1924724; PUBMED: 16946181] [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2006
- King A, Harris P, Fritzell J, Kurlan R. Syncope in children with Tourette's syndrome treated with guanfacine. Movement Disorders 2006;21(3):419–20. [DOI: 10.1002/mds.20738; 16229000] [DOI] [PubMed] [Google Scholar]
Knouse 2008
- Knouse LE, Cooper‐Vince C, Sprich S, Safren SA. Recent developments in the psychosocial treatment of adult ADHD. Expert Reviews of Neurotherapeutics 2008;8(10):1537‐48. [10.1586/14737175.8.10.1537; NIHMS75098; PMC2628311] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knouse 2010
- Knouse LE, Safren SA. Current status of cognitive behavioral therapy for adult attention‐deficit hyperactivity disorder. Psychiatric Clinics of North America 2010;33(3):497‐509. [DOI: 10.1016/j.psc.2010.04.001; NIHMS213647; PMC2909688] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knouse 2017
- Knouse LE, Teller J, Brooks MA. Meta‐analysis of cognitive–behavioral treatments for adult ADHD. Journal of Consulting and Clinical Psychology 2017;85(7):737‐50. [DOI: 10.1037/ccp0000216; 28504540] [DOI] [PubMed] [Google Scholar]
Landgraf 2007
- Landgraf JM. Monitoring quality of life in adults with ADHD: reliability and validity of a new measure. Journal of Attention Disorders 2007;11(3):351‐62. [DOI: 10.1177/1087054707299400; PUBMED: 17494834] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lim 2006
- Lim JR, Faught PR, Chalasani NP, Molleston JP. Severe liver injury after initiating therapy with atomoxetine in two children. Journal of Pediatrics 2006;148(6):831–4. [DOI: 10.1016/j.jpeds.2006.01.035; PUBMED: 16769398] [DOI] [PubMed] [Google Scholar]
Millstein 1997
- Millstein RB, Wilens TE, Biederman J, Spencer TJ. Presenting ADHD symptoms and subtypes in clinically referred adults with ADHD. Journal of Attention Disorders 1997;2(3):159‐66. [DOI: 10.1177/108705479700200302] [DOI] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mongia 2012
- Mongia M, Hechtman L. Cognitive behavior therapy for adults with attention‐deficit/hyperactivity disorder: a review of recent randomized controlled trials. Current Psychiatry Reports 2012;14(5):561‐7. [DOI: 10.1007/s11920-012-0303-x; PUBMED: 22878974] [DOI] [PubMed] [Google Scholar]
Morton 2000
- Morton WA, Stockton GG. Methylphenidate abuse and psychiatric side effects. Primary Care Companion to The Journal of Clinical Psychiatry 2000;2(5):159‐64. [PMC181133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 1996
- Murphy K, Barkley RA. Attention deficit hyperactivity disorder adults: comorbidities and adaptive impairments. Comprehensive Psychiatry 1996;37(6):393‐401. [PUBMED: 8932963] [DOI] [PubMed] [Google Scholar]
NIMH 1985
- National Institute of Mental Health. CGI (Clinical Global Impression) Scale — NIMH. Psychopharmacology Bulletin 1985;21:839‐44. [Google Scholar]
Perrin 2008
- Perrin JM, Friedman RA, Knilans TK, Black Box Working Group, Section on Cardiology, Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention‐deficit/hyperactivity disorder. Pediatrics 2008;122(2):451–3. [DOI: 10.1542/peds.2008-1573] [DOI] [PubMed] [Google Scholar]
Philipsen 2007
- Philipsen A, Richter H, Peters J, Alm B, Sobanski E, Colla M, et al. Structured group psychotherapy in adults with attention deficit hyperactivity disorder: results of an open multicenter study. Journal of Nervous and Mental Disease 2007;195(12):1013‐9. [DOI: 10.1097/NMD.0b013e31815c088b; PUBMED: 18091195] [DOI] [PubMed] [Google Scholar]
Philipsen 2012
- Philipsen A. Psychotherapy in adult attention deficit hyperactivity disorder: Implications for treatment and research. Expert Review of Neurotherapeutics 2012;12(10):1217‐25. [DOI: 10.1586/ern.12.91; PUBMED: 23082738] [DOI] [PubMed] [Google Scholar]
Polanczyk 2014
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta‐regression analysis. International Journal of Epidemiology 2014;43(2):434‐42. [DOI: 10.1093/ije/dyt261; PMC4817588; PUBMED: 24464188] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prescrire 2007
- Prescrire International. Modafinil: serious skin reactions. Prescrire International 2007;16(88):71. [PUBMED: 17465032] [PubMed] [Google Scholar]
Ramsay 2007
- Ramsay JR. Current status of cognitive‐behavioral therapy as a psychosocial treatment for adult attention‐deficit/hyperactivity disorder. Current Psychiatry Reports 2007;9(5):427‐33. [PUBMED: 17915084] [DOI] [PubMed] [Google Scholar]
Ramsay 2008
- Ramsay JR, Rostain AL. Cognitive‐Behavioral Therapy for Adult ADHD: An Integrative Psychosocial and Medical Approach. New York (NY): Routledge, 2008. [Google Scholar]
Ramsay 2010
- Ramsay JR. CBT for adult ADHD: adaptations and hypothesized mechanisms of change. Journal of Cognitive Psychotherapy 2010;24(1):37‐45. [DOI: 10.1891/0889-8391.24.1.37] [DOI] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenberg 1965a
- Rosenberg M. The association between self‐esteem and anxiety. Journal of Psychiatric Research 1962;1:135‐52. [PUBMED: 13974903] [DOI] [PubMed] [Google Scholar]
Rosenberg 1965b
- Rosenberg M. Society and the Adolescent Self‐Image. Princeton (NJ): Princeton University Press, 1965. [Google Scholar]
Safren 2006
- Safren SA. Cognitive‐behavioral approaches to ADHD treatment in adulthood. Journal of Clinical Psychiatry 2006;67(Suppl 8):46‐50. [PUBMED: 16961430] [PubMed] [Google Scholar]
Safren 2014 [pers comm]
- Safren SA. Random sequence generation and allocation concealment [personal communication]. Email to: S Safren 12 June 2014.
Seidman 2006
- Seidman LJ. Neuropsychological functioning in people with ADHD across the lifespan. Clinical Psychology Review 2006;26(4):446‐85. [DOI: 10.1016/j.cpr.2006.01.004; PUBMED: 16473440] [DOI] [PubMed] [Google Scholar]
Shear 2001
- Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, et al. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH‐A). Depression and Anxiety 2001;13(4):166‐78. [PUBMED: 11413563] [PubMed] [Google Scholar]
Simon 2009
- Simon V, Czobor P, Bálint S, Mészáros A, Bitter I. Prevalence and correlates of adult attention‐deficit hyperactivity disorder: meta‐analysis. British Journal of Psychiatry 2009;194(3):204‐11. [DOI: 10.1192/bjp.bp.107.048827; PUBMED: 19252145] [DOI] [PubMed] [Google Scholar]
Solanto 2014 [pers comm]
- Solanto MV. Random sequence generation and allocation concealment [personal communication]. Email to: M V Solanto 11 June 2014.
Speilberger 1989
- Spielberger CD. State‐Trait Anxiety Inventory: A Comprehensive Bibliography. 2nd Edition. Palo Alto (CA): Consulting Psychologists Press, 1989. [Google Scholar]
Speilberger 1991
- Spielberger CD. State‐Trait Anger Expression Inventory: Revised Research Edition: Professional Manual. Odessa (FL): Psychological Assessment Resources, 1991. [Google Scholar]
Spencer 1998
- Spencer T, Biederman J, Wilens TE, Faraone SV. Adults with attention‐deficit/hyperactivity disorder: a controversial diagnosis. Journal of Clinical Psychiatry 1998;59(S7):59‐68. [PUBMED: 9680054] [PubMed] [Google Scholar]
Spielberger 1986
- Spielberger CD, Gorsuch RL, Lushene RE. STAI: State‐Trait Anxiety Inventory: Manual [STAI: cuestionario de ansiedad estado‐rasgo: Manual]. Madrid (ES): TEA Ediciones, 1986. [Google Scholar]
Spielberger 1988
- Spielberger CD. State‐Trait Anger Expression Inventory: Professional Manual. Odessa (FL): Psychological Assessment resources, 1988. [Google Scholar]
Still 1902
- Still GF. The Goulstonian lectures on some abnormal psychical condition in children. Lancet 1902;159(4104):1163‐8. [DOI: 10.1016/S0140-6736(01)74901-X] [DOI] [Google Scholar]
Torrente 2011
- Torrente F, Lischinsky A, Torralva T, López P, Roca M, Manes F. Not always hyperactive? Elevated apathy scores in adolescents and adults with ADHD. Journal of Attention Disorders 2011;15(7):545‐56. [DOI: 10.1177/1087054709359887; PUBMED: 20207850] [DOI] [PubMed] [Google Scholar]
Torrente 2012
- Torrente F, López P, Alvarez Prado D, Kichic R, Cetkovich‐Bakmas M, Lischinsky A, et al. Dysfunctional cognitions and their emotional, behavioral, and functional correlates in adults with attention deficit hyperactivity disorder (ADHD): is the cognitive‐behavioral model valid?. Journal of Attention Disorders 2014;18(5):412‐24. [DOI: 10.1177/1087054712443153; PUBMED: 22628149] [DOI] [PubMed] [Google Scholar]
Van der Ploeg 2000
- Ploeg HM, Defares PB, Spielberger CD. Handleiding bij de Zelf‐Beoordelings Vragenlijst ZBV. Een Nederlandse bewerking van de Speilberger State‐Trait Anxiety Inventory STAI‐DY [Manual for the Self‐Assessment Questionnaire ZBV. A Dutch Version of the Speilberger State‐Trait Anxiety Inventory STAI‐DY]. Amsterdam (NLD): Pearson Assessment and Information BV, 2000. [Google Scholar]
Virta 2014 [pers comm]
- Virta M. Random sequence generation and allocation concealment [personal communication]. Email to: M Virta 17 June 2014.
Weiss 2008
- Weiss M, Safren SA, Solanto MV, Hechtman L, Rostain AL, Ramsay JR, et al. Research forum on psychological treatment of adults with ADHD. Journal of Attention Disorders 2008;11(6):642‐51. [DOI: 10.1177/1087054708315063; PUBMED: 18417729] [DOI] [PubMed] [Google Scholar]
Wender 2001
- Wender PH, Wolf LE, Wasserstein J. Adults with ADHD. An overview. Annals of the New York Academy of Sciences 2001;931:1‐16. [PUBMED: 11462736] [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders. Diagnostic Criteria for Research. Geneva: World Health Organization, 1993. [Google Scholar]
Wilens 2002
- Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annual Review of Medicine 2002;53:113‐31. [DOI: 10.1146/annurev.med.53.082901.103945; PUBMED: 11818466] [DOI] [PubMed] [Google Scholar]
Wilens 2004
- Wilens TE, Dodson W. A clinical perspective of attention‐deficit/hyperactivity disorder into adulthood. Journal of Clinical Psychiatry 2004;65(10):1301‐11. [PUBMED: 15491232] [DOI] [PubMed] [Google Scholar]
Wood 1976
- Wood DR, Reimherr FW, Wender PH, Johnson GE. Diagnosis and treatment of minimal brain dysfunction in adults: a preliminary report. Archives of General Psychiatry 1976;33(12):1453‐60. [DOI: 10.1001/archpsyc.1976.01770120057005; PUBMED: 793563] [DOI] [PubMed] [Google Scholar]
Woods 1986
- Woods D. The diagnosis and treatment of attention deficit disorder, residual type. Psychiatric Annals 1986;16(1):23‐8. [DOI: 10.3928/0048-5713-19860101-06] [DOI] [Google Scholar]
Young 2007a
- Young S, Bramham J. ADHD in Adults: A Psychological Guide to Practice. Chichester (UK): John Wiley and Sons, 2007. [Google Scholar]
Young 2007b
- Young S, Ross RR. R&R2 for ADHD Youths and Adults: A Prosocial Training Program. Ottawa (ON): Cognitive Centre of Canada, 2007. [Google Scholar]
Young 2014 [pers comm]
- Young S. Random sequence generation and allocation concealment [personal communication]. Email to: Susan Young 18 June 2014.
Young 2016
- Young Z, Moghaddam N, Tickle A. The efficacy of cognitive behavioral therapy for adults with ADHD: a systematic review and meta‐analysis of randomized controlled trials. Journal of Attention Disorders 2016 Aug 22 [Epub ahead of print]. [DOI: 10.1177/1087054716664413; PUBMED: 27554190] [DOI] [PubMed]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [PUBMED: 6880820] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lopez 2013
- Lopez PL, Torrente FM, Ciapponi A, Lischinsky AG, Cetkovich‐Bakmas M, Rojas JI, et al. Cognitive‐behavioural interventions for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010840] [DOI] [PMC free article] [PubMed] [Google Scholar]


