Abstract
Background
Corticosteroids are often preferred over enteral nutrition (EN) as induction therapy for Crohn's disease (CD). Prior meta‐analyses suggest that corticosteroids are superior to EN for induction of remission in CD. Treatment failures in EN trials are often due to poor compliance, with dropouts frequently due to poor acceptance of a nasogastric tube and unpalatable formulations. This systematic review is an update of a previously published Cochrane review.
Objectives
To evaluate the effectiveness and safety of exclusive EN as primary therapy to induce remission in CD and to examine the importance of formula composition on effectiveness.
Search methods
We searched MEDLINE, Embase and CENTRAL from inception to 5 July 2017. We also searched references of retrieved articles and conference abstracts.
Selection criteria
Randomized controlled trials involving patients with active CD were considered for inclusion. Studies comparing one type of EN to another type of EN or conventional corticosteroids were selected for review.
Data collection and analysis
Data were extracted independently by at least two authors. The primary outcome was clinical remission. Secondary outcomes included adverse events, serious adverse events and withdrawal due to adverse events. For dichotomous outcomes, we calculated the risk ratio (RR) and 95% confidence interval (CI). A random‐effects model was used to pool data. We performed intention‐to‐treat and per‐protocol analyses for the primary outcome. Heterogeneity was explored using the Chi2 and I2 statistics. The studies were separated into two comparisons: one EN formulation compared to another EN formulation and EN compared to corticosteroids. Subgroup analyses were based on formula composition and age. Sensitivity analyses included abstract publications and poor quality studies. We used the Cochrane risk of bias tool to assess study quality. We used the GRADE criteria to assess the overall quality of the evidence supporting the primary outcome and selected secondary outcomes.
Main results
Twenty‐seven studies (1,011 participants) were included. Three studies were rated as low risk of bias. Seven studies were rated as high risk of bias and 17 were rated as unclear risk of bias due to insufficient information. Seventeen trials compared different formulations of EN, 13 studies compared one or more elemental formulas to a non‐elemental formula, three studies compared EN diets of similar protein composition but different fat composition, and one study compared non‐elemental diets differing in glutamine enrichment. Meta‐analysis of 11 trials (378 participants) demonstrated no difference in remission rates. Sixty‐four per cent (134/210) of patients in the elemental group achieved remission compared to 62% (105/168) of patients in the non‐elemental group (RR 1.02, 95% CI 0.88 to 1.18; GRADE very low quality). A per‐protocol analysis (346 participants) produced similar results (RR 1.04, 95% CI 0.91 to 1.18). Subgroup analyses performed to evaluate the different types of elemental and non‐elemental diets (elemental, semi‐elemental and polymeric) showed no differences in remission rates. An analysis of 7 trials including 209 patients treated with EN formulas of differing fat content (low fat: < 20 g/1000 kCal versus high fat: > 20 g/1000 kCal) demonstrated no difference in remission rates (RR 1.03; 95% CI 0.85 to 1.26). Very low fat content (< 3 g/1000 kCal) and very low long chain triglycerides demonstrated higher remission rates than higher content EN formulas. There was no difference between elemental and non‐elemental diets in adverse event rates (RR 1.00, 95% CI 0.63 to 1.60; GRADE very low quality), or withdrawals due to adverse events (RR 1.29, 95% CI 0.80 to 2.09; GRADE very low quality). Common adverse events included nausea, vomiting, diarrhea and bloating.
Ten trials compared EN to steroid therapy. Meta‐analysis of eight trials (223 participants) demonstrated no difference in remission rates between EN and steroids. Fifty per cent (111/223) of patients in the EN group achieved remission compared to 72% (133/186) of patients in the steroid group (RR 0.77, 95% CI 0.58 to 1.03; GRADE very low quality). Subgroup analysis by age showed a difference in remission rates for adults but not for children. In adults 45% (87/194) of EN patients achieved remission compared to 73% (116/158) of steroid patients (RR 0.65, 95% CI 0.52 to 0.82; GRADE very low quality). In children, 83% (24/29) of EN patients achieved remission compared to 61% (17/28) of steroid patients (RR 1.35, 95% CI 0.92 to 1.97; GRADE very low quality). A per‐protocol analysis produced similar results (RR 0.93, 95% CI 0.75 to 1.14). The per‐protocol subgroup analysis showed a difference in remission rates for both adults (RR 0.82, 95% CI 0.70 to 0.95) and children (RR 1.43, 95% CI 1.03 to 1.97). There was no difference in adverse event rates (RR 1.39, 95% CI 0.62 to 3.11; GRADE very low quality). However, patients on EN were more likely to withdraw due to adverse events than those on steroid therapy (RR 2.95, 95% CI 1.02 to 8.48; GRADE very low quality). Common adverse events reported in the EN group included heartburn, flatulence, diarrhea and vomiting, and for steroid therapy acne, moon facies, hyperglycemia, muscle weakness and hypoglycemia. The most common reason for withdrawal was inability to tolerate the EN diet.
Authors' conclusions
Very low quality evidence suggests that corticosteroid therapy may be more effective than EN for induction of clinical remission in adults with active CD. Very low quality evidence also suggests that EN may be more effective than steroids for induction of remission in children with active CD. Protein composition does not appear to influence the effectiveness of EN for the treatment of active CD. EN should be considered in pediatric CD patients or in adult patients who can comply with nasogastric tube feeding or perceive the formulations to be palatable, or when steroid side effects are not tolerated or better avoided. Further research is required to confirm the superiority of corticosteroids over EN in adults. Further research is required to confirm the benefit of EN in children. More effort from industry should be taken to develop palatable polymeric formulations that can be delivered without use of a nasogastric tube as this may lead to increased patient adherence with this therapy
Plain language summary
Enteral nutritional therapy for treatment of active Crohn's disease
What is Crohn's disease?
Crohn's disease is a long‐term inflammation of the gastrointestinal (GI) tract, occurring anywhere from mouth to anus. Common symptoms of this condition include abdominal pain, diarrhea and weight loss. When Crohn's disease patients are experiencing symptoms, it is characterized to be 'active' disease. When the symptoms stop, it is called 'remission'.
What is enteral nutrition?
Enteral nutrition is a feeding method where all of a person's daily caloric intake is delivered using the GI tract. An example of this is nasogastric tube feeding, where a tube is inserted through a person's nose to deliver their daily nutritional requirements in liquid form. Enteral nutrition can be a form of nutritional therapy for Crohn's disease patients. Enteral nutrition is categorized into elemental and non‐elemental (semi‐elemental and polymeric) diets. Elemental diets come from amino‐acid sources, whereas non‐elemental diets come from oligopeptide or whole protein sources.
What are corticosteroids?
Corticosteroids are an effective treatment option for active Crohn's disease. These drugs that are taken by mouth and work as immunosuppressants.
What did the researchers investigate?
The researchers studied whether enteral nutrition is better than steroid therapy at producing remission in Crohn's disease patients. In addition, the investigators looked to see if one type of enteral nutrition was better than another (e.g. elemental vs.non‐elemental) at producing remission in Crohn's disease.
What did the researchers find?
The researchers found twenty‐seven studies (1,011 participants) that fulfilled the search criteria. Eleven of these studies, which included 378 patients, compared elemental to non‐elemental diets at producing remission in Crohn's disease. Eight studies, which included 352 patients, investigated enteral nutrition compared to steroid therapy at inducing remission in Crohn's disease. The researchers searched the medical literature extensively up to July 5, 2017.
Very low quality evidence suggests that steroids may be more effective than enteral nutrition for induction of remission in adults with active Crohn's disease. Very low quality evidence also suggests that enteral nutrition may be more effective than steroids for induction of remission in children with active Crohn's disease. There was no difference in remission rates between elemental and non‐elemental diets. An increase in side effects was not seen with elemental diets compared to non‐elemental diets, nor with enteral nutrition compared to steroids. Common side effects experienced with enteral nutrition included vomiting, diarrhea, heartburn and flatulence . Common side effects associated with steroid use included acne, moon facies, muscle weakness, hyperglycemia (high blood sugar) and hypoglycemia (low blood sugar) . Patients on enteral nutrition were more likely to withdraw from the study due to side effects than those on steroids. The most common reason for study withdrawal was inability to tolerate the taste of the enteral nutrition diet. Enteral nutrition should be considered in pediatric Crohn's patients or in adult patients who can comply with nasogastric tube feeding or perceive the formulations to be palatable, or when steroid side effects are not tolerated or better avoided. Further research is required to confirm the superiority of corticosteroids over EN in adults. Further research is required to confirm the benefit of EN in children. More effort from industry should be taken to develop palatable polymeric formulations that can be delivered without use of a nasogastric tube as this may lead to increased patient compliance with this therapy.
Summary of findings
Background
Elemental diets were first used in Crohn's disease to provide preoperative nutritional support. Primary therapeutic efficacy was suspected in an uncontrolled study where patients awaiting surgery appeared to experience improvement in the clinical activity of their inflammatory bowel disease as well as in their nutritional status (Voitk 1973). There is now a vast experience and literature supporting the use of nutritional therapy in Crohn's disease but the mechanism of action and ideal formulation are still unknown. Nutritional therapy is classified by the nitrogen source derived from the amino acid or protein component of the formula. Elemental diets are created by mixing of single amino acids and are entirely antigen free. Oligopeptide or semi‐elemental diets are made by protein hydrolysis and have a mean peptide chain length of four or five amino acids which is too short for antigen recognition or presentation. Polymeric diets contain whole protein from sources such as milk, meat, egg or soy. Polymeric diets can be classified more simply as elemental (amino acid‐based), semi‐elemental (oligopeptide) and polymeric (whole protein) diets.
The use of enteral nutritional therapy to treat active Crohn's disease has been shown to be less effective than conventional corticosteroid therapy in three meta‐analyses published in the mid nineties (Fernandez‐Banares 1995; Griffiths 1995; Messori 1996). This was demonstrated again in previous Cochrane meta‐analyses (Zachos 2001; Zachos 2007), despite differences in criteria for study inclusion. However, in some of the trials evaluating enteral nutrition versus steroids, the steroid‐treated patients sometimes received additional concurrent drug therapy such as sulfasalazine or antibiotics. Exclusion of such trials (Lochs 1991; Malchow 1990), resulted in a lack of superiority of steroids over enteral nutritional therapy, but the sample size was also markedly reduced. One further meta‐analysis showed no difference in effectiveness between enteral nutrition and corticosteroid therapy for the treatment of acute Crohn's disease in children (Heuschkel 2000). This apparent discrepancy may stem from differences involving the study populations (e.g. ages, disease activity, disease duration), interventions (e.g. with or without concurrent 5‐ASA preparations), outcome assessments (disease activity measures), methodology (e.g. sample size, blinding, randomization), and patient compliance (adults may not readily accept nasogastric feeding).
Regardless of whether enteral nutrition therapy is equal or slightly inferior in effectiveness to corticosteroids, its use deserves consideration in both the adult and pediatric population. There is a growing resistance to the frequent use of corticosteroids in children with Crohn's disease due to numerous adverse effects, particularly on growth, bone mineral density and body image. In addition, corticosteroids are not effective therapies for achieving mucosal healing (Landi 1992; Modigliani 1990). Therefore, in some parts of the world such as in Europe, enteral nutrition is presented as first line therapy based on good efficacy data for induction of remission as well as beneficial effects on growth, rapid nutritional restitution, fewer adverse effects and improved quality of life (Afzal 2004). The argument for the use of enteral nutrition as primary therapy for Crohn's disease is further supported by evidence of mucosal healing (Afzal 2004; Fell 2000; Yamamoto 2007), and one study demonstrated significantly higher rates of mucosal healing compared to patients receiving corticosteroids (Berni Canani 2006).
Enteral nutrition as a primary therapy for induction of Crohn's disease is often overlooked as a treatment option in the United States. Despite the evidence supporting its role as a means for induction of remission, only 4% of American gastroenterologists use it regularly to manage mild to moderately active pediatric Crohn's disease (Levine 2003). Reasons for lack of uptake among physicians include concerns about poor acceptability of nasogastric tubes by patients, palatability of formulations, and lack of compliance with a restrictive dietary intervention (Kansal 2013). However, palatable polymeric formulations have been produced recently and oral administration of these formulations has been demonstrated to be as efficacious as feeds delivered by the nasogastric route (Rubio 2011). The ability to use a palatable oral polymeric formulation as a means of induction therapy helps to overcome some of the prior limitations of enteral nutrition treatment. This systematic review and meta‐analysis is an update of a previously published Cochrane review (Zachos 2001; Zachos 2007). Prior versions of this systematic review and meta‐analyses were performed using an intention‐to‐treat analysis, which includes treatment failures who withdrew due to poor acceptance of enteral feeding (Zachos 2001; Zachos 2007). This updated meta‐analysis therefore aimed to also perform a per‐protocol analysis to compare enteral feeding versus corticosteroids for treatment of active Crohn's disease, but exclude treatment failures that occurred on the basis of poor palatability or lack of acceptance of a nasogastric tube, since these limitations may be overcome with use of palatable oral polymeric formulations. This review also provides an update on the existing effectiveness and safety data for both corticosteroids versus enteral nutrition, and for elemental versus non‐elemental feeds (i.e. one form of enteral nutrition versus another) for induction of remission of active Crohn's disease.
Objectives
The primary objectives were to evaluate the effectiveness and safety of exclusive enteral nutrition as primary therapy to induce remission in Crohn's disease and to examine the importance of formula composition on effectiveness.
Methods
Criteria for considering studies for this review
Types of studies
All randomized and quasi‐randomized controlled trials published in any language were considered for inclusion in the review. Trials published in abstract form were included if full details of the protocol and results could be obtained from the authors.
Types of participants
Patients with active Crohn's disease defined by a clinical disease activity index were considered for inclusion.
Types of interventions
Studies that compared the exclusive administration of one type of enteral nutrition (i.e. elemental, semi‐elemental, or polymeric) to another type of enteral nutrition or conventional corticosteroids were considered for inclusion. We excluded the following types of studies: trials allowing oral intake other than clear liquids; trials allowing co‐intervention with antibiotics, or corticosteroids in high or recently increased doses among patients allocated to enteral nutrition; and trials not defining disease activity and remission.
Types of outcome measures
The studies used one of various measures to assess remission: the Crohn's Disease Activity Index (CDAI), van Hees Index, the simple Crohn's Disease Index (or Harvey‐Bradshaw simple CDI), the International Organization of Inflammatory Bowel Disease Index (IOIBD) and the Pediatric Crohn's Disease Activity Index (PCDAI). The definition of remission can vary across studies using the same assessment scale. Thus, the primary outcome was the number of patients achieving remission, as defined by each individual study, and expressed as a percentage of the patients randomized (intention‐to‐treat analysis). Additional analysis was performed on a per‐protocol basis, excluding patients who withdrew due to lack of acceptability of a nasogastric tube for feeding or palatability of the enteral feed. Patients who withdrew for other reasons were still counted as treatment failures. Secondary outcomes included adverse events, withdrawals due to adverse events and serious adverse events.
Search methods for identification of studies
We searched MEDLINE (Ovid) Embase (Ovid) and the Cochrane Library (CENTRAL) from inception to 5 July 2017. The search strategy is reported in Appendix 1.
In addition, a manual search of American Journal of Gastroenterology, Gut, Gastroenterology, Journal of Pediatric Gastroenterology and Nutrition, and Journal of Parenteral and Enteral Nutrition from January 1995 to July 2017 was conducted and included abstracts submitted to major gastroenterologic meetings. Additional citations were sought using references from applicable systematic reviews and studies retrieved from the computerized and manual searches.
Data collection and analysis
Study selection: The studies (and abstracts when available) retrieved by the search strategy were independently reviewed by at least two investigators (NN, AD or DZ). Study eligibility was determined by discussion and consensus.
Quality Assessment: The quality of included studies was assessed using the Cochrane risk of bias tool (Higgins 2011). We assessed the following domains: methods used for randomization and allocation concealment (selection bias); blinding of participants, personnel and outcome assessors (performance and detection bias); incomplete outcome data (attrition bias); selective reporting of study outcomes and other potential sources of bias. Each potential source of bias was rated as low, high or unclear risk of bias. If an item could not be evaluated due to missing information, it was rated as an unclear risk of bias. The quality of studies was independently assessed by at least two investigators (NN, AD or DZ) with disagreements settled by consensus with all authors. Studies with at least one domain at high risk of bias were excluded on sensitivity analyses.
The GRADE approach was used to evaluate the overall quality of evidence supporting the primary and selected secondary outcomes (Guyatt 2008; Schunemann 2011). The GRADE approach allows overall appraisal of the quality of evidence such that one can determine confidence in how likely the effect estimate reflects the item of interest. Randomized trials start as high quality of evidence, but can be downgraded due to risk of bias, indirectness of evidence, unexplained heterogeneity, imprecision, and publication bias. After consideration of these factors, the overall quality of the evidence was graded as one of:
‐ High ‐ further research is unlikely to change confidence in the estimate of effect;
‐ Moderate ‐ further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate;
‐ Low ‐ further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate; or
‐ Very low ‐ any estimate of effect is very uncertain.
Data extraction: Data extraction forms were developed and data were independently extracted from eligible studies by at least two investigators (NN, AD or DZ). Rereading and discussion resolved any discrepancies in data recorded on the extraction forms. The extracted data included baseline characteristics of patients (i.e. number of patients randomized, age, sex, anatomic distribution of disease, duration of diagnosed disease, disease activity score, and co‐intervention if any); details of formulation and therapeutic administration of enteral nutrition or corticosteroid therapy; details on dropouts/withdrawals; and outcome assessment (i.e. time of assessment, clinical disease activity index used, definition of clinical remission, and remission rates according to the total number of patients randomized). A separate per‐protocol assessment was conducted by removing patients from the analysis who could not tolerate enteral feeding due to palatability of the formulation or non‐acceptance of a nasogastric tube.
Statistical analyses: Data were analyzed using Review Manager (RevMan 5.3.5, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Data were analyzed on an intention‐to‐treat and a per‐protocol basis. For the dichotomous outcomes such as achievement of remission, individual and pooled trial statistics were calculated as risk ratios (RR) with corresponding 95% confidence intervals (CI). A random‐effects model was used to pool data. The results for each analysis were tested for heterogeneity using the Chi2 and I2 statistics.
Studies were separated into two groups: A. one form of enteral nutrition compared to another form of enteral nutrition and B. one form of enteral nutrition compared to corticosteroids. Pre‐specified subgroup analyses included: (i) age (i.e. adults > 18 years versus children < 18 years); (ii) disease duration (i.e. new onset defined as disease duration of < 6 months versus chronic disease defined as disease duration of > 6 months); (iii) disease location (i.e. small bowel, large bowel, or both); (iv) fat composition (i.e. low fat versus high fat content; long chain triglyceride content; or fatty acid content); (v) protein composition (i.e. elemental, semi‐elemental polymeric; or nitrogen source); and (vi) type of carbohydrate source.
Sensitivity analyses were conducted on the basis of: (i) the inclusion of abstracts; and (ii) methodologic quality, excluding studies of lower quality (i.e. at least one aspect of the study considered to be at high risk of bias).
Results
Description of studies
The literature search conducted on July 5, 2017 identified 1135 records. After duplicates were removed, a total of 859 remained for review of titles and abstracts. At least two authors (NN, AD and DZ) independently reviewed the titles and abstracts of these trials. A total of 80 studies were selected for full text review (see Figure 1). Forty reports of 38 studies were excluded (see:Characteristics of excluded studies). Thirty‐four reports of 27 studies (1,011 participants) studies met the pre‐defined inclusion criteria and were included in the review (see Characteristics of included studies). Six ongoing studies were also identified (NCT00265772; NCT01728870; NCT02056418; NCT02231814; NCT02843100; NCT03176875; see Characteristics of ongoing studies).
1.

Study flow diagram.
Enteral nutritional therapy for induction of remission in Crohn's disease Of the 17 trials comparing different formulations of enteral nutrition, 13 compared two or more enteral nutrition formulas based on the nitrogen source. See additional Table 3 for formula composition in studies comparing different forms of enteral nutrition. Of these trials, four compared one or more elemental formulae to a semi‐elemental formula (Mansfield 1995: Middleton 1995; Royall 1994; Sakurai 2002). One of these studies had four arms, three of which were different elemental diets while the fourth arm was a semi‐elemental diet (Middleton 1995). For comparative purposes, the three elemental arms were pooled into one elemental group (standard elemental + long chain triglyceride enriched elemental + medium‐chain triglyceride enriched elemental) for analysis. Seven additional trials compared an elemental to a polymeric formula (Giaffer 1990; Grogan 2012; Kobayashi 1998; Park 1991; Raouf 1991; Rigaud 1991; Verma 2000), while two trials compared a semi‐elemental to a polymeric formula (Griffiths 2000; Seidman 2003). The Griffiths 2000 study, published in abstract form, was only included in the sensitivity analysis. An additional study compared two polymeric diets differing only in glutamine enrichment (Akobeng 2000). This study was not included in the primary analysis but in the subgroup analyses.
1. Formula Composition in Studies Comparing Different Forms of Enteral Nutrition.
| Study | EN type / name | Route | TME (kcal/d) | Ptn (g/1000kcal) | Fat (g/1000kcal) | C (g/1000kcal) |
| Akobeng 2000 | Polymeric glutamine enriched/NS | oral/NG | 2400 | 39.6 | 38.8 | 123 |
| Polymeric/NS | oral/NG/gastrostomy | 2200 | 35.4 | 38.5 | 128.1 | |
| Bamba 2003 | Elemental/Elental (low) | NG | 2400 | 32.85 | 1.3 | 221 |
| Elemental/Elental (medium) | NG | 2400 | 32.85 | 6.9 | 208 | |
| Elemental/Elental (high ) | NG | 2400 | 32.85 | 12.5 | 196 | |
| Gassull 2002 | Polymeric/n9‐rich | oral/NG | 2307 | 54 | 35 | 116 |
| Polymeric/n6‐rich | oral/NG | 2266 | 54 | 35 | 116 | |
| Giaffer 1990 | Elemental/Vivonex | NG | 2500 | 20.5 | 1.44 | 225 |
| Polymeric/ Fortison | NG | 2500 | 40 | 40 | 120 | |
| Grogan 2012 | Elemental/Emsogen | oral/NG | 2106 | 33.07 | 39.23 | 129.2 |
| Polymeric/Alicalm | oral/NG | 2176 | 30.8 | 39.23 | 126.9 | |
| Leiper 2001 | Polymeric (low LCT) | oral (NG if could not tolerate oral) | NS | 39.8 | 38.7 | 122.8 |
| Polymeric (high LCT) | oral (NG if could not tolerate oral) | NS | 39.8 | 38.7 | 122.8 | |
| Mansfield 1995 | Elemental/E028 | NG | 2250 | 26.2 | 17.4 | 184.6 |
| Semi‐elemental/Pepti‐2000 LF liquid | NG | 2250 | 40 | 10 | 187.5 | |
| Middleton 1995 | Elemental/E028 | oral | NS | 26.2 | 17.4 | 184.6 |
| Elemental MCT‐enriched/E028 MCT | oral/NG | NS | 28.2 | 39.5 | 133.2 | |
| Elemental LCT‐enriched/E028 LCT | oral/NG | NS | 25 | 35 | 146 | |
| Semi‐elemental/ Pepdite 2+ | oral | NS | 29.4 | 36.9 | 137.5 | |
| Park 1991 | Elemental/E028 | NG | 2266 | 30 | 16.7 | 194.6 |
| Polymeric/Enteral 400 | NG | 2289 | 28.75 | 39.16 | 144 | |
| Raouf 1991 | Elemental/E028 | oral | 2000 | 126.2 | 17.4 | 184.6 |
| Polymeric | oral | 2000 | 40 | 40 | 120 | |
| Rigaud 1991 | Elemental/Vivonex HN | NG | 2286 | 45 | 0.9 | 203 |
| Polymeric/Ralmentyl | NG | 2311 | 45 | 30 | 137.5 | |
| Polymeric/Nutrison | NG | 2311 | 40 | 39 | 123 | |
| Royall 1994 | Elemental/Vivonex TEN | ND | NS | 38 | 2.8 | 210 |
| Semi‐elemental/Peptamen | ND | NS | 40 | 39 | 127 | |
| Sakurai 2002 | Elemental/Elental | ND | NS | NS | 1.2 | NS |
| Semi‐elemental/Twinline | ND | NS | NS | 27.8 | NS | |
| Verma 2000 | Elemental/NS | NG | 2500 | 53 | 39.5 | 133.2 |
| Polymeric/NS | NG | 2500 | 53 | 39.5 | 133.2 | |
EN = enteral nutrition
NS = not specified
ND = nasoduodenal
NG = nasogastric
TME = total mean energy intake
Ptn = protein
C = carbohydrate
The studies Griffiths 2000, Kobayashi 1998, Pigneur 2014 and Seidman 2003 were not included in the table because the details for formula composition were not available
The remaining three studies used formulas with a similar protein composition and compared two or more enteral nutrition formulas based on fat composition. Two of these trials compared two polymeric diets (Gassull 2002; Leiper 2001) and one compared three elemental diets (Bamba 2003). One trial had a third arm investigating the effect of steroid therapy (Gassull 2002), which is reported below.
Four studies included pediatric participants (Akobeng 2000, Griffiths 2000, Grogan 2012). All of the other studies enrolled adults. Diets were administered by either nasogastric tube feeds or orally. Withdrawals were higher in the patients using oral administration due to unpalatability. The majority of trials described a mixture of participants with new onset disease and chronic disease as well as a mix of disease location (i.e. small bowel, large bowel or both). Disease activity and remission were defined using the CDAI for eight of the studies (Giaffer 1990; Griffiths 2000; Leiper 2001; Mansfield 1995; Rigaud 1991; Royall 1994; Sakurai 2002; Verma 2000), although one study (Royall 1994), used a higher CDAI score of 250 for inclusion criteria as opposed to a CDAI score of 150 or 200 for inclusion in the other studies. The remainder used either the PCDAI (Akobeng 2000; Grogan 2012), the IOIBD plus an elevation of either C‐reactive protein or erythrocyte sedimentation rate (Bamba 2003; Kobayashi 1998), the Van Hees activity index plus abnormal laboratory results (Gassull 2002), or the Harvey‐Bradshaw simple CDI (Middleton 1995, Park 1991, Raouf 1991), with considerable variation in the disease activity and remission criteria among the three studies. Outcomes were assessed at 28 days in eight trials (Akobeng 2000; Bamba 2003; Gassull 2002;Griffiths 2000; Mansfield 1995; Park 1991; Rigaud 1991; Verma 2000), at 21 days in four trials (Middleton 1995, Leiper 2001; Raouf 1991; Royall 1994), at 24 days in one trial (Kobayashi 1998), at 10 days in one trial (Giaffer 1990), and at 42 days in two trials (Sakurai 2002; Grogan 2012).
Enteral nutritional therapy versus steroid therapy for induction of remission in Crohn's disease
Among the ten trials comparing enteral nutrition to steroid therapy (see additional Table 4 for formula composition in studies comparing enteral nutrition to steroids), three were abstract publications included only in the sensitivity analysis (Mantzaris 1996; Pigneur 2014; Seidman 1991). The study participants were adults for all the trials except for two (Borrelli 2006; Terrin 2002). All of the trials used conventional PCDAI or CDAI scores to define disease activity and remission except for two trials, which used the van Hees Activity Index (Gassull 2002; Gonzalez‐Huix 1993). Outcome assessments were made at 70 days for one trial (Borrelli 2006), two months for one trial (Pigneur 2014) 42 days in two trials (Lochs 1991; Malchow 1990), at 28 to 30 days in five trials (Gassull 2002; Gonzalez‐Huix 1993; Lindor 1992; Mantzaris 1996; Terrin 2002) and at 21 days in one trial (Seidman 1991).
2. Formula Composition in Studies Comparing Enteral Nutrition to Steroids.
| Study | EN type/name | Route | TME (kcal/d) | Ptn (g/1000kcal) | Fat (g/1000kcal) | C (g/1000kcal) |
| Borrelli 2006 | Polymeric | NG or oral | 120‐130% of RDI (1500‐3000kcal/d) | 35 | 46.6 | 110 |
| Gonzales‐Huix 1993 | Polymeric/Edanec HN | NG | 2800 | 54.6 | 114.2 | 36.2 |
| Lindor 1992 | Semi‐elemental/Vital HN | NS | 40kcal/kg/d | 42 | 11 | 186 |
| Lochs 1991 | Semi‐elemental/Peptisorb | ND | 35kcal/kg ideal BW | 37.5 | 182.5 | 11.1 |
| Malchow 1990 | Semi‐elemental/DFD | oral | 33kcal/kg/d | 35 | 11.1 | 190 |
| Mantzaris 1996 | Polymeric/Nutrison HE | ND | 1.5L/d | 40 | 39 | 123 |
| Saverymuttu 1985 | Elemental diet (Vivonex) plus oral framycetin, colistin and nystatin | NG | 1800‐2400 ml/d | |||
| Seidman 1991 | Elemental/Vivonex | NG | 1kcal/ml, 50‐80kcal/kg/d | |||
| Terrin 2002 | Elemental diet (Pregomin, Milupa), | NG | 50‐60kcal/kg/d | |||
| LEGEND | EN=enteral nutrition; NS= not specified | ND=nasoduodenal; NG=nasogastric | TME=total mean energy intake; RDI=recommended dietary intake | Ptn=protein | C=carbohydrate |
EN = enteral nutrition
NS = not specified
ND = nasoduodenal
NG = nasogastric
TME = total mean energy intake
RDI = recommended dietary intake
Ptn = protein
C = carbohydrate
The Pigneur 2014 study was not included in the table because the details for formula composition were not available
The diets were administered by nasogastric tubes in all but three trials (Borrelli 2006; Gassull 2002; Malchow 1990). In the trial with two enteral nutrition arms versus a steroid arm (Gassull 2002), participants with mild disease activity were allowed to take the feeds orally, while those with moderate to severe disease received the diet nasogastrically. There was a cumulative withdrawal rate of 26% in those receiving enteral nutrition therapy compared to no withdrawals in the steroid group. For the purpose of analysis, the two groups that received different feeds were combined into one enteral nutrition arm and compared to the steroid arm (Gassull 2002). In the second trial that allowed oral intake of the dietary therapy (Malchow 1990), there was a 39% withdrawal rate in the enteral diet group compared to only 9% in the steroid group. In the pediatric study (Borrelli 2006), the majority of participants took the feed orally, but if they failed adequate oral consumption, nasogastric tube feeds were administered (in 23.5% of subjects). The withdrawal rates were similar in both the enteral nutrition (10.5%) and the steroid arm (11.1%). Borrelli 2006 also differed substantially from all other studies in other respects. It included the longest duration of enteral nutrition therapy (70 days) and all patients were recently diagnosed with Crohn's disease (within 12 weeks from presentation). As a result the patients had not been exposed to any medications other than 5‐aminosalicylates (which were discontinued) prior to entering the study.
Two trials included a third arm of combined steroid plus enteral nutritional therapy (Lindor 1992; Mantzaris 1996). The data from these third arms were not included in the analysis. Two trials allowed the use of similar concurrent therapy in both groups (Gonzalez‐Huix 1993; Lindor 1992). These trials were included despite the use of antibiotics since only one or two patients received antibiotics in each group. The use of concurrent therapy with three grams of sulfasalazine per day in the steroid group was reported in two trials (Lochs 1991; Malchow 1990). These were the only two studies that had more than 100 participants in total (Lochs 1991; Malchow 1990). Malchow 1990 had a large dropout rate in the enteral nutrition arm. The concurrent therapy in the steroid arm, the high dropout rate in the nutritional arm and the relatively large sample sizes of these two studies might contribute substantial bias against enteral nutrition in the overall analysis. For this reason, a further subgroup analysis was conducted post hoc, excluding these two trials which used concurrent therapy only in the steroid group. In contrast, the remaining trials in the post hoc subgroup analysis used similar concurrent therapy in both the enteral nutrition and steroid arms (Gonzalez‐Huix 1993, Lindor 1992).
Risk of bias in included studies
The results of the risk of bias analysis are summarized in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Nine studies were judged to have a low risk of bias for random sequence generation (Borrelli 2006; Gassull 2002; Gonzalez‐Huix 1993; Grogan 2012; Leiper 2001; Mansfield 1995; Rigaud 1991; Royall 1994; Sakurai 2002). Five studies were rated as low risk of bias for allocation concealment (Akobeng 2000; Gassull 2002; Grogan 2012; Leiper 2001; Royall 1994). The rest of the studies were rated as unclear risk of bias due to insufficient information to permit judgment on at least one of the corresponding items.
Blinding
Seven studies were rated as low risk of bias for blinding of participants and personnel (Akobeng 2000; Gassull 2002; Grogan 2012; Leiper 2001; Park 1991; Royall 1994; Verma 2000). Five studies were judged to be at high risk of bias for blinding of participants and personnel (Borrelli 2006; Gonzalez‐Huix 1993; Lindor 1992; Lochs 1991; Raouf 1991). The remaining studies were judged to have an unclear risk of bias for blinding of participants and personnel due to insufficient information to permit a judgement. Five studies were judged to be at low risk of bias for blinding of outcome assessment (Gassull 2002; Leiper 2001; Rigaud 1991; Royall 1994; Terrin 2002). The remaining twenty‐four studies have an unclear risk of bias due to insufficient information to permit judgment on at least one of the corresponding items.
Incomplete outcome data
Twenty‐one studies were rated to be low risk of bias in regards to incomplete outcome data (Akobeng 2000; Borrelli 2006; Gassull 2002; Giaffer 1990; Gonzalez‐Huix 1993; Grogan 2012; Kobayashi 1998; Leiper 2001; Lindor 1992; Lochs 1991; Malchow 1990; Mansfield 1995; Middleton 1995; Park 1991; Raouf 1991; Rigaud 1991; Royall 1994; Sakurai 2002; Saverymuttu 1985; Terrin 2002; Verma 2000). Two studies were rated as high risk of bias (Bamba 2003; Griffiths 2000). The remaining four studies have an unclear risk of bias due to insufficient information to permit judgement on this item (Mantzaris 1996; Pigneur 2014; Seidman 1991; Seidman 2003).
Selective reporting
Twenty‐three studies were rated to have low risk of bias in terms of selective reporting (Akobeng 2000; Bamba 2003; Borrelli 2006; Gassull 2002; Giaffer 1990; Gonzalez‐Huix 1993; Griffiths 2000; Grogan 2012; Kobayashi 1998; Leiper 2001; Lindor 1992; Lochs 1991; Malchow 1990; Mansfield 1995; Middleton 1995; Park 1991; Raouf 1991; Rigaud 1991; Royall 1994; Sakurai 2002; Saverymuttu 1985; Terrin 2002; Verma 2000). Four studies have an unclear risk of bias due to insufficient information to permit judgment on this item (Mantzaris 1996;Pigneur 2014; Seidman 1991; Seidman 2003).
Other potential sources of bias
Twenty‐one studies were rated as low risk of bias for other potential sources of bias (Akobeng 2000; Bamba 2003; Borrelli 2006; Gassull 2002; Giaffer 1990; Gonzalez‐Huix 1993; Grogan 2012; Kobayashi 1998; Leiper 2001; Lindor 1992; Lochs 1991; Malchow 1990; Mansfield 1995; Middleton 1995; Park 1991; Raouf 1991; Rigaud 1991; Royall 1994; Sakurai 2002; Terrin 2002; Verma 2000). One study had a high risk of bias for other bias (Griffiths 2000). Five studies were judged to have an unclear risk of bias for other bias due to insufficient information to permit judgment on this item (Mantzaris 1996; Pigneur 2014; Saverymuttu 1985; Seidman 1991; Seidman 2003).
Effects of interventions
Summary of findings for the main comparison. Elemental compared to non‐elemental enteral feeds for induction of remission in Crohn's disease.
| Elemental compared to non‐elemental enteral feeds for induction of remission in Crohn's disease | ||||||
| Patient or population: induction of remission in Crohn's disease Setting: Intervention: Elemental Comparison: non‐elemental enteral feeds | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with non‐elemental enteral feeds | Risk with Elemental | |||||
| Remission rate ‐ Intention to treat | 625 per 1,000 | 638 per 1,000 (550 to 737) | RR 1.02 (0.88 to 1.18) | 378 (11 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2 | |
| Adverse events | 174 per 1,000 | 176 per 1,000 (108 to 287) | RR 1.01 (0.62 to 1.65) | 323 (9 RCTs) | ⊕⊝⊝⊝ VERY LOW 3,4 | |
| Withdrawal due to adverse events | 173 per 1,000 | 237 per 1,000 (143 to 389) | RR 1.37 (0.83 to 2.25) | 272 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 5,6 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to high or unclear risk of bias. Of 11 studies in the pooled analysis, 2 were rated as low risk of bias, 1 was rated as high risk of bias and 8 were unknown risk of bias
2 Downgraded one level due to sparse data (239 events)
3 Downgraded two levels due to high or unclear risk of bias. Of 9 studies in the pooled analysis, 3 were rated as low risk of bias, 1 was rated as high risk of bias and 5 were rated as unclear risk of bias
4 Downgraded one level due to sparse data (56 events)
5 Downgraded two levels due to unclear risk of bias. Of 7 studies in the pooled analysis, 2 were rated as low risk of bias and 5 were rated as unclear risk of bias
6 Downgraded one level due to sparse data (54 events)
Summary of findings 2. Enteral nutrition compared to corticosteroids for induction of remission in Crohn's disease.
| Enteral nutrition compared to corticosteroids for induction of remission in Crohn's disease | ||||||
| Patient or population: induction of remission in Crohn's disease Setting: Intervention: Enteral nutrition Comparison: corticosteroids | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with corticosteroids | Risk with Enteral nutrition | |||||
| Remission rate ‐ ITT | 715 per 1,000 | 551 per 1,000 (415 to 737) | RR 0.77 (0.58 to 1.03) | 409 (8 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | |
| Remission rate ‐ ITT adult studies | 734 per 1,000 | 477 per 1,000 (382 to 602) | RR 0.65 (0.52 to 0.82) | 352 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 4, 5 | |
| Remission rate ‐ ITT pediatric studies | 607 per 1,000 | 820 per 1,000 (559 to 1,000) | RR 1.35 (0.92 to 1.97) | 57 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 6, 7 | |
| Remission rate ‐ per‐protocol ‐ pediatric studies |
607 per 1,000 | 868 per 1,000 (625 to 1,000) | RR 1.43 (1.03 to 1.97) | 55 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 6, 7 | |
| Adverse events | 159 per 1,000 | 221 per 1,000 (99 to 495) | RR 1.39 (0.62 to 3.11) | 389 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 8, 9, 10 | |
| Withdrawal due to adverse events | 64 per 1,000 | 189 per 1,000 (65 to 544) | RR 2.95 (1.02 to 8.48) | 169 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 11, 12 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; ITT: Intention‐to‐treat | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to high and unclear risk of bias. Of 8 studies in the pooled analysis 1 was rated as low risk of bias, 4 were rated as high risk of bias and 3 were rated as unclear risk of bias.
2 Downgraded one level due to unexplained heterogeneity (I2 = 67%)
3 Downgraded one level due to sparse data (244 events)
4 Downgraded two levels due to high and unclear risk of bias. Of 6 studies in the pooled analysis 1 was rated as low risk of bias, 3 were rated as high risk of bias and 2 were rated as unclear risk of bias
5 Downgraded one level due to sparse data (203 events)
6 Downgraded two levels due to high and unclear risk of bias. Of 2 studies in the pooled analysis 1 was rated as high risk of bias and the other was rated as unclear risk of bias
7 Downgraded one level due to sparse data (41 events)
8 Downgraded two levels due to high and unclear risk of bias. Of 7 studies in the pooled analysis 1 was rated as low risk of bias, 4 were rated as high risk of bias and 2 were rated as unclear risk of bias.
9 Downgraded one level due to unexplained heterogeneity (I2 = 60%)
10 Downgraded one level due to sparse data (82 events)
11 Downgraded two levels due to high or unclear risk of bias. Of three studies in the pooled analysis, 1 was rated as high risk of bias and 2 were rated as unclear risk of bias.
12 Downgraded two levels due to very sparse data (26 events)
Enteral nutritional therapy for induction of remission in Crohn's disease
Clinical remission
A meta‐analysis of eleven trials which included 378 patients (adult and pediatric) treated with an elemental diet or a non‐elemental (semi‐elemental or polymeric) diet for active Crohn's disease demonstrated no statistically significant difference in remission rates among diet formulations. Sixty‐four per cent (134/210) of patients in the elemental diet group entered remission compared to 62% (105/168) in the non‐elemental group (RR 1.02, 95% CI 0.88 to 1.18; GRADE very low quality). No heterogeneity was detected for this comparison (I2 = 0%). Per‐protocol analysis and a sensitivity analysis excluding a high risk of bias study (Raouf 1991), had no effect on the results.
Adverse events
Nine of the studies examining elemental to non‐elemental diets (semi‐elemental or polymeric) reported on adverse events. Meta‐analysis of these nine studies including 320 patients, showed no statistically significant difference in adverse events rates between the two groups. Seventeen per cent (32/185) of participants in the elemental diet group had an adverse event compared to 17% (24/138) in the non‐elemental diet group (RR 1.01, 95% CI 0.62 to 1.65; GRADE very low quality).
Most of the studies did not describe the types of adverse events experienced. However, of those adverse events reported, nausea, vomiting, diarrhea and abdominal bloating were associated with both elemental and non‐elemental diets.
None of these studies reported on the occurrence of serious adverse events.
Withdrawals due to adverse events
Withdrawals due to adverse events were reported in seven studies. Meta‐analysis of these seven studies, which included 272 patients, showed no significant difference in withdrawals due to adverse events. Twenty‐two per cent (35/162) of patients in the elemental diet group withdrew due to an adverse event compared to 17% (19/110) of patients in the non‐elemental diet group (RR 1.37, 95% CI 0.83 to 2.25; GRADE very low quality).
Subgroup analyses: Subgroup analyses were planned a priori to evaluate the influence of disease duration and disease location on remission induction. However, there were inadequate data to allow for these comparisons.
Formula composition:
Protein composition Subgroup analyses were performed to evaluate the different types of diets (elemental, semi‐elemental and polymeric). There was no statistically significant difference in remission rates between trials comparing elemental and polymeric diets [RR 1.06; 95% CI 0.83 to 1.34] (Giaffer 1990; Grogan 2012; Kobayashi 1998; Park 1991; Raouf 1991; Rigaud 1991; Verma 2000) or between trials comparing elemental and semi‐elemental diets (RR 0.99; 95%CI 0.81 to 1.22) (Mansfield 1995; Middleton 1995; Royall 1994; Sakurai 2002). For both comparisons, the statistical test for heterogeneity was not significant. Sensitivity analyses excluding studies at high risk of bias for these two subgroups analyses demonstrated similar results. A sensitivity analysis was conducted including an abstract publication comparing one type of semi‐elemental diet to a polymeric diet in children (Griffiths 2000). The results from the abstract were pooled in the analysis comparing elemental and polymeric diets as one may consider that semi‐elemental diets are more similar in composition to elemental diets as compared to polymeric diets. Therefore, meta‐analysis of eight trials resulted in an increase in the number of participants to 133 in the elemental or semi‐elemental formula groups and 130 in the polymeric group and demonstrated no difference in remission rates between the two cohorts (RR 1.08; 95% CI 0.89 to 1.31). The statistical test for heterogeneity was not significant (I2 = 7%).
All studies compared elemental to non‐elemental diets except one. In this one study, nine patients received a polymeric diet and nine received the same polymeric diet with glutamine enrichment (Akobeng 2000). There was no statistically significant difference in remission rates (RR 0.80; 95% CI 0.31 to 2.04).
Fat content A subgroup analysis was conducted on seven trials comparing low fat (< 20 g fat/1000 kCal) to high fat (>20 g fat/1000 kCal) enteral nutritional therapy including 105 and 104 patients in each group, respectively (Giaffer 1990; Middleton 1995; Park 1991; Raouf 1991; Rigaud 1991; Royall 1994; Sakurai 2002). No statistically significant difference in remission rates was found between the groups (RR 1.03; 95% CI 0.85 to 1.26) and heterogeneity was not demonstrated (i2=12%). Similarly, a subgroup analysis conducted on four trials comparing very low fat (< 3 g fat/1000 kCal) to high fat formulas (Giaffer 1990; Rigaud 1991; Royall 1994; Sakurai 2002), which included 68 patients in each group demonstrated no statistically significant difference in remission rates between the groups (RR 1.11; 95% CI 0.84 to 1.46) and heterogeneity was not demonstrated (I2=32%).
Subgroup analyses were performed on the basis of long chain triglyceride (LCT) content in feeds (in terms of percentage of total energy). The LCT content was classified as low (< 10% LCT) or high (> 10% LCT). Meta‐analysis of six trials (Bamba 2003; Giaffer 1990; Leiper 2001; Rigaud 1991; Raouf 1991; Sakurai 2002) which included 111 patients treated with low LCT content formula and 99 patients treated with a high LCT content formula demonstrated no statistically significant difference in remission rates among diet formulations (RR 1.09; 95% CI 0.80 to 1.49). Statistically significant heterogeneity was not present (I2 = 32%). Further analyses using different cut‐offs of % LCT (e.g. 5%, 15%) did not affect the results.
Further subgroup analyses were conducted in three trials (168 patients) on the basis of n‐6 poly‐unsaturated fatty acid (PUFA) composition (Gassull 2002; Griffiths 2000; Grogan 2012). There was no significant difference in remission rates (RR 1.10, 95% CI 0.70 to 1.73). A subgroup analysis in two trials (97 patients) on the basis of n‐9 mono‐unsaturated fatty acid (MUFA) composition (Gassull 2002; Leiper 2001), found no significant difference in remission rates (RR 0.72, 95% CI 0.22 to 2.36).
Carbohydrate composition When available, the carbohydrate composition was similar between the different formulations and therefore further analyses were not performed.
Enteral nutritional therapy versus steroid therapy for induction of remission in Crohn's disease
Clinical remission
A meta‐analysis of eight trials, which included 223 patients (adult and pediatric) treated with enteral nutrition and 186 treated with steroids, yielded no statistically significant difference in remission rates between the two groups. Fifty per cent (111/223) of patients in the enteral nutrition group achieved remission compared to 72% (133/186) of patients in the steroid group (RR 0.77, 95% CI 0.58 to 1.03; GRADE very low quality). Statistically significant heterogeneity was identified (I2 = 67%). Subgroup analysis by age (children versus adults) demonstrated a statistically significant difference favouring steroid therapy over enteral nutrition for inducing remission in Crohn's disease in adults but not children. Forty‐five per cent (87/194) of adult patients in the enteral nutrition group achieved remission compared to 73% (116/158) of the steroid group (RR 0.65, 95% CI 0.52 to 0.82; GRADE very low quality). No significant heterogeneity was demonstrated for this analysis (i2 = 36%). Eighty‐three per cent (24/29) of pediatric patients in the enteral nutrition group achieved remission compared to 61% (17/28) of the steroid group (RR 1.35, 95% CI 0.92 to 1.97; GRADE very low quality). No significant heterogeneity was demonstrated for this analysis (i2 = 14%). When a per‐protocol analysis accounting for withdrawals due to inability to tolerate the nasogastric tube or poor palatability of the formulation was performed, there was no statistically significant difference in overall remission rates seen between patients treated with enteral nutrition or steroids. Sixty‐three per cent (111/176) of patients in the enteral nutrition group achieved remission compared to 72% (133/186) of patients in the steroid group (RR 0.93; 95% CI 0.75 to 1.14). However, there was a statistically significant difference in remission rates for each age‐based subgroup. Fifty‐eight per cent (87/149) of adult patients in the enteral nutrition group achieved remission compared to 73% (116/158) of the steroid group (RR 0.82, 95% CI 0.70 to 0.95). No significant heterogeneity was demonstrated for this analysis (i2 = 0%). Eighty‐nine per cent (24/27) of pediatric patients in the enteral nutrition group achieved remission compared to 61% (17/28) of the steroid group (RR 1.43, 95% CI 1.03 to 1.97; GRADE very low quality). No significant heterogeneity was demonstrated for this analysis (i2 = 0%). In a sensitivity analysis that included two abstract publications, the combined sample sizes increased to 235 in the enteral nutrition group and 195 in the steroid group. There was no statistically significant difference in remission rates (RR 0.85, 95% CI 0.65 to 1.10).
As previously reported, the difficulties in double blinding in trials that compare enteral nutrition to steroids led to a high risk of bias rating for the majority of trials. A sensitivity analysis was performed with the two studies that did not have high risk of bias (Gassull 2002; Malchow 1990), and there was a statistically significant result favouring steroid therapy over enteral nutrition (RR 0.53, 95% CI 0.39 to 0.70). Heterogeneity was not demonstrated for this comparison(I2 = 0%). There were inadequate data from full publications to perform further subgroup analyses by age, disease duration or disease location.
Adverse events
Seven trials (389 patients) comparing enteral nutrition to steroid therapy reported on the occurrence of adverse events. Meta‐analysis of these trials showed no statistically significant difference in adverse event rates between the two groups. Twenty‐five per cent (54/213) of patients in the enteral nutrition group experienced an adverse event compared to 16% (28/176) of patients in the steroid group (RR 1.39, 95% CI 0.62 to 3.11; GRADE very low quality).
While there was no statistical difference in adverse event rates, the types of adverse events experienced amongst the two groups differed. Adverse events experienced in the enteral nutrition group include heartburn, flatulence, diarrhea and vomiting. Whereas in the steroid therapy group,common adverse events experienced are acne, moon facies, hyperglycemia, muscle weakness and hypoglycemia. One patient on steroid therapy reported having an intraabdominal abscess.
None of these studies reported on the occurrence of serious adverse events.
Withdrawal due to adverse events
Only three trials reported on withdrawals due to adverse events (Borrelli 2006; Malchow 1990; Saverymuttu 1985). Meta‐analysis of these studies, which included 169 patients, showed no significant difference in withdrawal due to adverse events. Twenty‐three per cent (21/91) of patients in the enteral nutrition group withdrew due to an adverse event compared to 6% (5/78) of steroid patients (RR 2.95, 95% CI 1.02 to 8.48; GRADE very low quality).
Common reasons cited for withdrawal in the enteral nutrition were unpalatability of diet and non‐tolerance of the nasogastric tube. Some of the reasons given for withdrawal in the steroid therapy group were common side effects associated with steroid use, as described above.
Discussion
Individual studies comparing the effectiveness of enteral nutritional therapy for induction of remission in Crohn's disease have varied considerably in results with remission rates ranging from 20 to 84.2%. This apparent discrepancy may stem from differences involving study populations (e.g. ages, disease activity), interventions (e.g. route of administration), outcome assessments (disease activity measures) and methodology (e.g. sample size, blinding, randomization). This meta‐analysis aimed to pool data from existing randomized controlled studies to examine whether formula composition affected efficacy. No difference in remission rates was observed when different formula compositions were compared by meta‐analytic techniques. Although the choice of activity index utilized in the studies varied, the majority of studies included in the meta‐analysis used a validated index with similar cut‐off values defining inclusion or remission (Sandborn 2002).
Subgroup and sensitivity analyses based on study quality or specific aspects of protein or fat composition did not yield any statistically significant differences in remission rates. No statistically significant differences were seen between diets containing different nitrogen sources, classified simply as elemental (free amino acids), semi‐elemental (oligopeptides) or polymeric (whole protein). Comparisons between any combination of the different protein sources showed no significant difference in remission rates. Similarly, one study comparing polymeric diets differing in glutamine‐enrichment showed no difference in remission rates (Akobeng 2000). A non significant trend favouring very low fat and low LCT content was demonstrated in this review. Although different values for LCT content were evaluated and no statistically significant difference was found, the trend supporting lower LCT content was strongest for the lowest value evaluated (< 5% LCT). However, these results should be interpreted with caution due to statistically significant heterogeneity and small numbers of patients resulting in a lack of statistical power to show a difference should one exist. Several studies have hypothesized that the proportion or type of fat in an enteral feeding can affect the production of pro or anti‐inflammatory mediators (Bamba 2003; Gassull 2002; Leiper 2001; Sakurai 2002). The possibility that fat composition influences immunomodulatory or anti‐inflammatory effect in active Crohn's disease warrants further exploration with larger trials.
Data concerning effectiveness of enteral nutrition based on disease duration or disease distribution were not reported in the majority of studies, so subgroup analyses with respect to disease characteristics could not be performed. Several early reports suggest increased efficacy of enteral nutrition in patients with small bowel involvement and those patients with isolated colonic involvement seemed less likely to benefit from enteral feeding (Afzal 2005b; Wilschanski 1996). However, recent studies suggest disease phenotype may not impact response to enteral nutrition. Buchanan 2009 carefully defined phenotypic classification in 110 patients on enteral nutrition and found no significant differences in remission rates based on disease location. One retrospective study compared remission rates according to route of administration, and incidentally found that the site of disease involvement had no impact on response to nutritional therapy (Rubio 2011). Current recommendations suggest the use of exclusive enteral nutrition as first line therapy to induce remission in children with active luminal Crohn's disease (Ruemmele 2014). There are inadequate data from the trials examined in this meta‐analysis to confirm or refute this recommendation. The recent data demonstrating equal efficacy in colonic disease as compared to small bowel disease also suggests a potential role of enteral nutrition as a means to induce patients with ulcerative colitis and this merits further investigation.
The use of enteral nutritional therapy to treat active Crohn's disease was shown to be less effective than conventional corticosteroid therapy in past meta‐analyses (Fernandez‐Banares 1995; Griffiths 1995; Messori 1996). This updated meta‐analysis confirms this finding in adult populations and suggests that enteral nutrition may be more effective than steroids in pediatric populations. Further research about which form of intervention is most effective in pediatric patients is warranted. On a per‐protocol basis, no statistically significant difference in the overall effect estimate was seen between enteral nutrition and corticosteroid therapy. This suggests that failure of enteral nutrition therapy may be due to difficulty with tolerating nasogastric tube feeding and poorly palatable formulations. For the per‐protocol analysis, steroids were superior to enteral nutrition in adults and enteral nutrition was superior to steroids in children.
This meta‐analysis did not aim to compare the method by which enteral nutrition should be delivered to induce remission. Although polymeric diets are more palatable, failure of enteral nutrition can occur if inadequate oral administration occurs, and the nasogastric route should then be used to optimize compliance and effectiveness (Knight 2005). Although exclusion of a normal diet or nasogastric route of administration may be viewed as barriers to enteral nutritional therapy, even young children can learn to insert the tube for overnight feeds (Sanderson 2005). Recent efforts by industry to develop palatable polymeric formulas may help increase acceptance from patients since the use of nasogastric feeding can be avoided. Various flavouring agents also assist to make these formulas more palatable.
There is substantial variability in use of enteral nutrition in different parts of the world. Whereas 62% of western European gastroenterologists use this therapy routinely for the management of pediatric patients with Crohn’s disease, only 4% of American gastroenterologists regularly use enteral nutrition in their practice (Levine 2003). A barrier to its uptake in the United States is related to personal experience and training of clinicians involved in patient care. Clinicians trained in a centre where enteral nutrition is not used routinely are less likely to adapt it for use in their own practice. Other barriers preventing routine use of enteral nutrition include the lack of a uniform protocol on its use, uncertainty regarding the duration of treatment, uncertainty whether concurrent oral intake should be permitted, and how to reintroduce food once remission has been induced. Immunomodulators or biologic therapies are often used for maintenance of remission in Crohn's disease, but there may be a role for continuing to use enteral nutrition in patients with quiescent disease. Small studies suggest remission could be maintained by delivering a portion of caloric intake via overnight enteral feeding by nasogastric tube and allowing patients to eat normally during the day (Takagi 2006; Wilschanski 1996; Yamamoto 2007). This needs to be confirmed in larger studies and different populations before being adapted.
There are several additional benefits to enteral nutrition that are not examined in this meta‐analysis due to insufficient data. Adverse events often seen in patients using corticosteroids such as stunted growth or osteoporosis are not a concern when using enteral nutrition. Although patients treated with corticosteroids often achieve clinical remission, corticosteroids often fail to induce mucosal healing (Modigliani 1990). Case series have described mucosal healing with enteral nutrition therapy (Fell 2000; Yamamoto 2007). The Borrelli 2006 trial which failed to show a significant difference in remission rates between enteral nutrition and corticosteroids, did demonstrate a significant difference in mucosal healing with enteral nutrition. The goals of treatment of Crohn’s disease have changed in the past decade, with a recent focus on targeting objective improvement in several domains including mucosal healing. In addition to symptom control, this advantage of enteral nutrition also adds further appeal over corticosteroid therapy.
In summary, meta‐analysis of the available trials has not demonstrated any significant benefit based on the formula composition of nutritional therapies. More prospective data are needed to evaluate the effect of disease factors such as disease location and duration on response to therapy. Nevertheless, the effectiveness of enteral nutrition for the induction of remission in Crohn's disease is evident from the remission rates shown in the trials included in this meta‐analysis. The additional benefits for mucosal healing, growth, nutritional status and quality of life should strengthen the argument for considering the use of enteral nutrition as primary therapy in Crohn's disease. Given that no statistically significant difference in remission rates was seen between enteral nutrition and corticosteroid therapy when analyzed on a per‐protocol basis, this suggests a need to focus on methods of increasing compliance with enteral nutrition therapy from patients, including the development of palatable polymeric formulations that can be delivered without use of a nasogastric tube. Further study on the mechanisms by which enteral nutrition works, its use in maintenance of remission, whether it should be combined with drug therapy for induction or maintenance of remission, and the efficacy of enteral nutrition in ulcerative colitis are needed.
Authors' conclusions
Implications for practice.
Very low quality evidence suggests that corticosteroid therapy may be more effective than enteral nutrition for induction of clinical remission in adults with active Crohn's disease. Very low quality evidence also suggests that enteral nutrition may be more effective than steroids in children with active Crohn's disease. Protein composition does not appear to influence the effectiveness of enteral nutrition for the treatment of active Crohn's disease. Enteral nutritional therapy should be considered as therapy for Crohn's disease in certain cases, such as in pediatric patients or in patients who can comply with nasogastric tube feeding or perceive the formulations to be palatable, or when steroid side effects are not tolerated or better avoided.
Implications for research.
Further research is required to confirm the superiority of corticosteroids over enteral nutrition in adults. Further research is required to confirm the benefit of enteral nutrition in children. More effort from industry should be taken to develop palatable polymeric formulations that can be delivered without use of a nasogastric tube as this may lead to increased patient compliance with this therapy..
What's new
| Date | Event | Description |
|---|---|---|
| 1 March 2018 | New citation required and conclusions have changed | Updated review with changes to the conclusions and new authors |
| 5 July 2017 | New search has been performed | New literature search was conducted on 5 July 2017. Four new studies were added to the review |
Acknowledgements
The authors are grateful to Dr. Anne Griffiths for her contribution to the original meta‐analysis on this subject matter for the Cochrane Collaboration. The authors would also like to thank Mr. Byungju Lee for his assistance with the quality assessment and Dr. Latifa Yeung for translation of one study (Kobayashi 1998).
Funding for the Cochrane IBD Group (May 1, 2017 ‐ April 30. 2022) has been provided by Crohn's and Colitis Canada (CCC).
Appendices
Appendix 1. Search strategies
Embase on OVID:
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. crohn*.mp.
22. exp Crohn disease/
23. ileitis.mp.
24. enteritis, regional.mp.
25. or/21‐24
26. 20 and 25
27. enteral nutrition.mp. or exp enteral nutrition/
28. food.mp. or exp food/
29. diet.mp. or exp diet/
30. polymeric diet.mp. or exp polymeric diet/
31. elemental diet.mp. or exp elemental diet/
32. or/27‐31
33. 26 and 32
MEDLINE on OVID:
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. randomized controlled trial/
14. or/1‐13
15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
16. 14 not 15
17. crohn*.mp.
18. exp Crohn disease/
19. ileitis.mp.
20. enteritis, regional.mp.
21. or/17‐20
22. 16 and 21
23. enteral nutrition.mp. or exp enteral nutrition/
24. food.mp. or exp food/
25. diet.mp. or exp diet/
26. polymeric diet.mp. or exp polymeric diet/
27. elemental diet.mp. or exp elemental diet/
28. or/23‐27
29. 22 and 28
CENTRAL
#1. crohn* #2. enteral nutrition or food or diet or polymeric diet or elemental diet #3. #1 and #2
Data and analyses
Comparison 1. Elemental versus non‐elemental enteral feeds.
1.1. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 1 Remission rate ‐ Intention‐to‐treat.
1.2. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 2 Remission rate ‐ Per‐protocol.
1.3. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 3 Remission rate ‐ sensitivity analysis excluding studies at high risk of bias.
1.4. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 4 Remission rate ‐ elemental versus polymeric enteral feeds.
1.5. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 5 Remission rate ‐ elemental versus semi‐elemental.
1.6. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 6 Remission rate ‐ sensitivity analysis elemental vs polymeric, excluding studies at high risk of bias.
1.7. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 7 Remission rate ‐ sensitivity analysis elemental vs semi‐elemental, excluding studies at high risk of bias.
1.8. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 8 Remission rate ‐ elemental vs non‐elemental enteral feeds: sensitivity analysis including abstract.
1.9. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 9 Remission rate ‐ Protein composition: polymeric vs glutamine‐enriched polymeric.
1.10. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 10 Remission rate ‐ Comparison of fat content: very low fat vs high fat.
1.11. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 11 Remission rate ‐ Comparison of fat content: low fat vs high fat.
1.12. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 12 Remission rate ‐Type of fat in non‐elemental feeds: n9 rich feeds.
1.13. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 13 Remission rate ‐ Type of fat in non‐elemental feeds: n6 rich feeds.
1.14. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 14 Remission rate ‐ Comparision of LCT content: low % LCT (<10%) vs high % LCT (>10%).
1.15. Analysis.
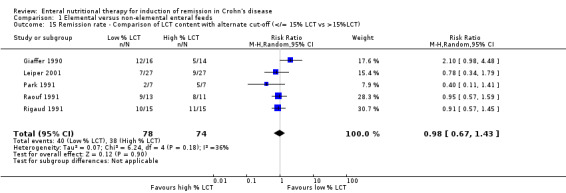
Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 15 Remission rate ‐ Comparison of LCT content with alternate cut‐off (</= 15% LCT vs >15%LCT).
1.16. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 16 Remission rate ‐ Comparison of LCT content with alternate cut‐off (</=5%LCT vs >5% LCT).
1.17. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 17 Adverse events.
1.18. Analysis.

Comparison 1 Elemental versus non‐elemental enteral feeds, Outcome 18 Withdrawal due to adverse events.
Comparison 2. Enteral nutrition versus corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Remission rate ‐ Intention‐to‐treat | 8 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.58, 1.03] |
| 1.1 Pediatric studies | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.92, 1.97] |
| 1.2 Adult studies | 6 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.52, 0.82] |
| 2 Remission rate ‐ Per‐protocol | 8 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.14] |
| 2.1 Pediatric studies | 2 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.03, 1.97] |
| 2.2 Adult studies | 6 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.95] |
| 3 Remission rate ‐ sensitivity analysis including abstracts | 10 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.10] |
| 4 Remission rate ‐ sensitivity analysis excluding studies at high risk of bias | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.39, 0.70] |
| 5 Remission rate ‐ sensitivity analysis excluding trials with concurrent therapy | 4 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.22] |
| 6 Remission rate ‐ published studies only semi‐elemental diet vs steroid | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.50, 0.78] |
| 7 Remission rate ‐ polymeric diet vs steroids | 4 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.50, 1.18] |
| 7.1 Remission rate ‐ published studies only | 3 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.50, 1.32] |
| 7.2 Remission rate ‐ abstracts only | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.35] |
| 8 Remission rate ‐ elemental diet vs steroids | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.69, 2.09] |
| 8.1 Remission rate ‐ published studies only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Remission rate ‐ abstracts only | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.69, 2.09] |
| 9 Adverse events | 7 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.62, 3.11] |
| 10 Withdrawal due to adverse events | 3 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [1.02, 8.48] |
2.1. Analysis.

Comparison 2 Enteral nutrition versus corticosteroids, Outcome 1 Remission rate ‐ Intention‐to‐treat.
2.2. Analysis.

Comparison 2 Enteral nutrition versus corticosteroids, Outcome 2 Remission rate ‐ Per‐protocol.
2.3. Analysis.

Comparison 2 Enteral nutrition versus corticosteroids, Outcome 3 Remission rate ‐ sensitivity analysis including abstracts.
2.4. Analysis.

Comparison 2 Enteral nutrition versus corticosteroids, Outcome 4 Remission rate ‐ sensitivity analysis excluding studies at high risk of bias.
2.5. Analysis.

Comparison 2 Enteral nutrition versus corticosteroids, Outcome 5 Remission rate ‐ sensitivity analysis excluding trials with concurrent therapy.
2.6. Analysis.

Comparison 2 Enteral nutrition versus corticosteroids, Outcome 6 Remission rate ‐ published studies only semi‐elemental diet vs steroid.
2.7. Analysis.

Comparison 2 Enteral nutrition versus corticosteroids, Outcome 7 Remission rate ‐ polymeric diet vs steroids.
2.8. Analysis.
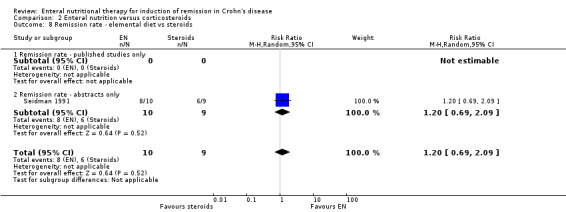
Comparison 2 Enteral nutrition versus corticosteroids, Outcome 8 Remission rate ‐ elemental diet vs steroids.
2.9. Analysis.

Comparison 2 Enteral nutrition versus corticosteroids, Outcome 9 Adverse events.
2.10. Analysis.

Comparison 2 Enteral nutrition versus corticosteroids, Outcome 10 Withdrawal due to adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akobeng 2000.
| Methods | Randomized trial (assignments in sealed envelopes), double‐blind (three‐letter codes) | |
| Participants | 18 children, 10 males and 8 females with PCDAI > 12 | |
| Interventions | 9 patients received the glutamine‐enriched polymeric diet and 9 received the standard polymeric diet | |
| Outcomes | Remission rate defined by PCDAI < 10 after 28 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomisation to one of two treatment diets was performed by assignments in sealed envelopes” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Researchers and patients blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were disclosed and explained in detail and the analyses were performed on an intention to treat basis |
| Selective reporting (reporting bias) | Low risk | Main outcome (remission) and all results were well reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Bamba 2003.
| Methods | Randomized trial (method not specified), not double‐blind | |
| Participants | 28 patients, 17 males and 11 females with IOIBD > 2 with at least one abnormal marker (CRP or ESR) | |
| Interventions | 10 patients received the low‐fat elemental feed, 10 received the medium fat elemental feed and 8 received the high fat elemental feed | |
| Outcomes | Remission rate defined by reduction in IOIBD rating to 0 or 1 with normal CRP or ESR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement for the method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients were followed up at weekly intervals for 4 weeks Losses to follow‐up were fully disclosed and the analysis were performed on a per‐protocol basis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Borrelli 2006.
| Methods | Randomized trial (computer generated), open label | |
| Participants | 37 pediatric patients randomized, 16 male, 22 female with PCDAI >10 [11patients: 5 EN, 6 steroid had PCDAI of 11‐30; 21 patients: 12 in EN group, 9 in steroid group had PCDAI >30} | |
| Interventions | 19 patients received polymeric diet for 10weeks and 12 received oral methylprednisolone at 1.5mg/kg to max of 60mg for 4 weeks then routine wean | |
| Outcomes | Remision rate defined by PCDAI of < 10 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization group assignments were generated using a computer‐generated randomization schedule by an independent statistician; the randomization list was stratified according to the disease location (ileum, ileocolon, and colon) in permutated blocks of 4 or 6 allocated randomly |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement for the method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. Blinding was impossible for the use of nasogastric tube for the polymeric enteral nutrition (PEN) group. Initial hospitalization just for polymeric diet alone group may impact on outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were disclosed and the analyses were conducted using, firstly, a modified intention to treat analysis and, secondly, on a per‐protocol basis. Missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Gassull 2002.
| Methods | Randomized trial (sealed envelopes), double‐blind (diet of same appearance and coded) | |
| Participants | 43 patients, 19 males and 24 females with VHAI>120 and at least 2 abnormal laboratory tests. | |
| Interventions | 20 patients received the n9‐rich polymeric feed and 23 received the n6‐rich polymeric feed | |
| Outcomes | Remission rate defined by VHAI<120 after 28 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned and randomisation was stratified according to the variables |
| Allocation concealment (selection bias) | Low risk | They used the sealed envelopes for each randomization stratum. Enteral diets were identical in appearance and were supplied in identical coded sachets |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The investigator, study staff, and patients were blinded to the diet assignment throughout the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study staff were blinded including staff for the outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcomes. The attrition cases were mentioned and the reason for the withdrawals were well described |
| Selective reporting (reporting bias) | Low risk | Clinical remission criteria, which is the main outcome, was well mentioned. Inclusion and exclusion criteria was well described in a protocol |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Giaffer 1990.
| Methods | Randomized trial (method not specified), not double‐blind | |
| Participants | 30 patients, 8 males and 22 females with CDAI > 150 | |
| Interventions | 16 patients received the elemental diet and 14 received the polymeric diet | |
| Outcomes | Remission rate defined by CDAI <150 after 10 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement for the method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were disclosed and explained in detail |
| Selective reporting (reporting bias) | Low risk | Main outcome (remission) and all the results were well reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Gonzalez‐Huix 1993.
| Methods | Randomized trial (table of random numbers), not double‐blind | |
| Participants | 32 patients, 17 males and 15 females with Van Hees Activity Index (VHAI) > 120 | |
| Interventions | 15 patients received the polymeric diet and 17 received prednisone | |
| Outcomes | Remission rate defined by VHAI < 120 after 28 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned by means of a random number table to one of two treatment groups and stratified randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement for the method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was impossible for the use of nasogastric tube for polymeric enteral nutrition (PEN) group. Insufficient information to permit judgement of treating patients according to a strict protocol such as same admission periods for both groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups (2 among 17 steroid group and 1 among 15 PEN group before four weeks of treatment), with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Clinical remission criteria, which is main outcome, was well mentioned. Exclusion criteria were well described |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Griffiths 2000.
| Methods | Randomized trial (randomization was performed centrally at coordinating center via random number tables), double‐blind (diet of same appearance) | |
| Participants | 84 children (sexes not specified) with CDAI >150 and < 450 | |
| Interventions | 42 patients received the elemental diet and 42 received the polymeric diet | |
| Outcomes | Remission rate defined by CDAI < 150 or CDAI decrease of 40% or 100 points of initial level after 28 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement for the method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up were not explained in detail |
| Selective reporting (reporting bias) | Low risk | Main outcomes (remission and response) were well reported |
| Other bias | High risk | The study may have other sources of bias (EF provided by Abbott, no details on PF) |
Grogan 2012.
| Methods | Randomized trial (sealed envelopes), double‐blind (neither patients or treating physician aware) | |
| Participants | 41 pediatric patients, newly diagnosed Crohn's disease with PCDAI >11 and no isolated colonic disease included | |
| Interventions | 20 patients received the elemental diet and 21 received the polymeric diet | |
| Outcomes | Remission defined by PCDAI < 11 at 6 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization performed and described in detail |
| Allocation concealment (selection bias) | Low risk | Randomization was by numbered, sealed envelope in a 1:1 ratio Concealment of allocation was maintained until after assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The type of formulas were not disclosed either to the patients or the treating physicians |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were disclosed and the analyses were performed on a per‐protocol and intention to treat basis |
| Selective reporting (reporting bias) | Low risk | Clinical remission, which is the main outcome, and all secondary outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kobayashi 1998.
| Methods | Randomized trial (method not specified), not double‐blind | |
| Participants | 19 patients (sexes not specified) with CDAI > 150 | |
| Interventions | 10 patients received the elemental diet and 9 received the polymeric diet | |
| Outcomes | Remission rate defined by CDAI < 150 after 24 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study is described as randomized, but insufficient information provided regarding random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement for the method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data was analyzed as intention to treat and adjusted for withdrawals due to inability to tolerate the feed |
| Selective reporting (reporting bias) | Low risk | Main outcome (remission) and all secondary outcomes were well reported in pre‐specified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Leiper 2001.
| Methods | Randomized trial (randomization table), double blind (diet of same appearance) | |
| Participants | 54 patients (sexes not specified) with CDAI > 200 and CRP > 10 mg/L | |
| Interventions | 27 patients received the low LCT feed and 27 received the high LCT feed | |
| Outcomes | Remission rate defined by CDAI < 150 after 21 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization conducted using randomization tables, by a designated pharmacist who had no other involvement in the trial |
| Allocation concealment (selection bias) | Low risk | Patients were randomized to receive feeds that were identical in color and packaging |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Halfway blinded interim analysis performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data was analyzed as intention to treat and adjusted for withdrawals due to inability to tolerate the feed |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified (primary and secondary) outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lindor 1992.
| Methods | Randomized trial (method not specified), not double‐blind | |
| Participants | 19 patients, 6 males and 13 females with CDAI > 150 | |
| Interventions | 9 received the semi‐elemental diet and 10 received prednisone | |
| Outcomes | Remission rate defined by a CDAI decrease of 100 points or more from initial value (definite remission) after 30 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Further description of allocation is not included |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was impossible for the different form of treatment among three groups and patients in group II, some of them used nasogastric. Therefore, the number of hospital visits would be affected by the use of nasogastric tube |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear information of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were disclosed and the analyses were performed on intention to treat basis |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes were reported and pre‐specified subgroup analysis has been done |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lochs 1991.
| Methods | Randomized trial (method not specified), not double‐blind | |
| Participants | 107 patients, 37 males and 70 females with CDAI > 200 | |
| Interventions | 55 patients received the semi‐elemental diet and 52 received a combination of sulfasalazine and 6‐methyl‐prednisolone | |
| Outcomes | Remission rate defined by a CDAI decrease of at least 100 points or 40% from initial value after 42 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement for the method of concealment is not described or not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was impossible for the use of nasogastric tube for the diet group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were disclosed and explained |
| Selective reporting (reporting bias) | Low risk | Main outcome (remission) and all the results were well reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Malchow 1990.
| Methods | Randomized trial (method not specified), not double‐blind | |
| Participants | 95 patients, 41 males and 54 females with CDAI > 150 | |
| Interventions | 51 patients received the semi‐elemental diet and 44 received a combination of sulfasalazine and 6‐methyl‐prednisolone | |
| Outcomes | Remission rate defined by a decrease in CDAI of at least 100 points or 40 % of the initial value after 42 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | The patients were randomized, but further description of allocation is not included |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was impossible for different form of regimens for two groups. Insufficient information to permit judgement of treating patients according to a strict protocol such as same admission periods for both groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear information of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were disclosed and explained in detail |
| Selective reporting (reporting bias) | Low risk | Main outcome (remission) and all the results were well reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Mansfield 1995.
| Methods | Randomized trial (method not specified), not double‐blind | |
| Participants | 44 patients, 16 males and 28 females with CDAI > 150 | |
| Interventions | 22 patients received the elemental diet and 22 received the semi‐elemental diet | |
| Outcomes | Remission rate defined by CDAI reduction of 100 points or 40% of the initial value after 28 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified for site of disease between the three categories, small bowel, colonic, and both small and large bowel |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement for the method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were disclosed. Among 44 participants, 6 patients withdrew from the study within the first week due to the nasogastric feeding inconvenience Analysis of the results was performed on an intention to treat basis |
| Selective reporting (reporting bias) | Low risk | Main outcome (remission) and all the results were well reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Mantzaris 1996.
| Methods | Randomized trial (method not specified), not double‐blind | |
| Participants | 20 patients (sexes not specified), CDAI for inclusion not specified (presumed to be CDAI >150) | |
| Interventions | 10 patients received the polymeric diet and 10 received prednisolone | |
| Outcomes | Remission rate defined by a CDAI decrease of 100 points or CDAI < 150 after 28 days of treatment | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information about blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information about outcome assessment blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcomes of interest were not pre‐specified |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all results were reported |
| Other bias | Unclear risk | Insufficient information available to evaluate other biases |
Middleton 1995.
| Methods | Randomized trial (method not specified), not double‐blind | |
| Participants | 76 patients (sexes not specified) with Harvey‐ Bradshaw simple CDI > 6 | |
| Interventions | 58 patients received elemental diets ( 17 received the standard elemental diet, 22 received the LCT‐enriched elemental diet, and 19 received the MCT‐enriched elemental diet) and 18 received the semi‐elemental diet | |
| Outcomes | Remission rate defined by Harvey‐Bradshaw simple CDI < 3 after 21 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were disclosed and the reasons for them were described. Exclusion criteria was mentioned. |
| Selective reporting (reporting bias) | Low risk | Main outcome (remission) and all the results were well reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Park 1991.
| Methods | Randomized trial (method not specified), double‐blind (food reservoir covered with large plastic bag and tape) | |
| Participants | 14 patients, 1 male and 13 females with Harvey‐Bradshaw simple CDI > 2 | |
| Interventions | 7 patients received the elemental diet and 7 received the polymeric diet | |
| Outcomes | Remission rate defined by Harvey‐Bradshaw simple CDI < 2 after 28 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | The participants were randomized, but further description of allocation is not included |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nuclear medicine specialists were blinded for scoring results of nuclear scans, but the information to permit judgement of blinding of a clinical scoring assessment was insufficient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were disclosed with the same reason in both groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Pigneur 2014.
| Methods | Randomized controlled trial (method not specified) | |
| Participants | 19 patients (ages 6 through 17) with active Crohn's disease | |
| Interventions | 13 patients received exclusive enteral nutrition (EEN) and 6 patients received steroids | |
| Outcomes | Clinical remission was defined by HBI <5 after two months of treatment | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgment about blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment about outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment |
| Other bias | Unclear risk | Insufficient information |
Raouf 1991.
| Methods | Randomized trial (method not specified), not double‐blind | |
| Participants | 24 patients (ages and sexes not specified) with Harvey‐Bradshaw simple CDI | |
| Interventions | 13 patients received the elemental diet and 11 received the polymeric diet | |
| Outcomes | Remission rate defined by Harvey‐Bradshaw simple CDI < 4 after 21 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement for the method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, even though patients not treated openly, each group had been flavoured differently |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reason for attrition was described and exclusion criteria were explained for this cross over study |
| Selective reporting (reporting bias) | Low risk | Clinical remission criteria, which is main outcome, was well mentioned |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rigaud 1991.
| Methods | Randomized trial (random tables), not double‐blind | |
| Participants | 30 patients, 18 males and 12 females with CDAI > 150 and < 450 | |
| Interventions | 15 patients received the elemental diet and 15 received the polymeric diet | |
| Outcomes | Remission rate defined by CDAI < 150 after 28 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized into EED or PFD groups according to random tables |
| Allocation concealment (selection bias) | Unclear risk | There was no description about concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The endoscopists were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals. Exclusions criteria was well explained |
| Selective reporting (reporting bias) | Low risk | Clinical remission criteria, which is main outcome, was well mentioned. Every outcome results were well reported . |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Royall 1994.
| Methods | Randomized trial (table of random numbers), double‐blind (coded diets and same appearance) | |
| Participants | 40 patients, 23 males and 17 females with CDAI > 250 | |
| Interventions | 19 patients received the elemental diet and 21 received the semi‐elemental diet | |
| Outcomes | Remission rate defined by CDAI < 150 after 21 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables were used for randomization |
| Allocation concealment (selection bias) | Low risk | Diets premixed by nutritional dept and sent directly to ward in the same cartons for patient to start |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the patients and the evaluating persons were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical assessment was done by at least two physicians (attending physician and a physician who did not participate in primary care) independently |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up (only one patient after five days) were disclosed and explained in detail |
| Selective reporting (reporting bias) | Low risk | Main outcome (remission) and all the results were well reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sakurai 2002.
| Methods | Randomized trial (table of random numbers), not double‐blind | |
| Participants | 36 patients, 30 males and 6 females with CDAI > 150 | |
| Interventions | 18 patients received the elemental diet and 18 received the non‐elemental diet | |
| Outcomes | Remission rate defined by CDAI decrease of at least 40% or by 100 or more after 42 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were assigned to a group according to a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | 37 patients were randomized, but further description of allocation is not included |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear information of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient was excluded from the analysis but it is unlikely that this affected results |
| Selective reporting (reporting bias) | Low risk | All of the study’s pre‐specified outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Saverymuttu 1985.
| Methods | Restricted randomisation (method not described) | |
| Participants | 37 patients with symptoms of active Crohn's disease. 5 patients were intolerant of the elemental diet regime and were withdrawn from the trial within 72 hours. Restricted randomisation was completed so that 16 patients (six men and ten women) were randomised to receive the steroid treatment and 16 patients (six men and ten women) received the elemental diet plus non‐absorbable antibiotics | |
| Interventions | 16 patients received 0.5 mg/kg/day prednisolone and a normal diet and 16 patients received an elemental diet (Vivonex, 1800‐2400 ml/day via fine bore nasogastric tube) plus oral framycetin (500 mg 4 times daily), colistin (1.5 mega U four times daily), and nystatin (1 mega U four times daily). Patients previously taking salazopyrine at the time of development of active symptoms stayed on the medication | |
| Outcomes | Remission rate was defined by CDAI, after ten days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Restricted randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | Further description of allocation is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No clear information of blinding of outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
Seidman 1991.
| Methods | Randomized trial (method not specified), not double‐blind | |
| Participants | 19 children (sexes not specified) with 150 > CDAI < 450 | |
| Interventions | 10 patients received the elemental diet and 9 received prednisone | |
| Outcomes | Remission rate defined by CDAI < 150 after 21 days of treatment | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information about blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information about outcome assessment blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcomes of interest were not pre‐specified |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all results were reported |
| Other bias | Unclear risk | Insufficient information available to evaluate other biases |
Seidman 2003.
| Methods | Randomised controlled trial | |
| Participants | Pediatric patients with active CD | |
| Interventions | Participants were randomised to receive either a n‐3 PUFA‐enriched semi‐elemental diet (n‐3 SED) or a polymeric formula (PF) | |
| Outcomes | Remission rate was defined by CDAI < 150 or > 75 point decrease vs. baseline after 28 days of treatment | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement for the method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insuficcient information to permit judgment of outcome assessment blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information about if all pre‐specified outcomes were reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all results were reported |
| Other bias | Unclear risk | Insufficient information available to evaluate other biases |
Terrin 2002.
| Methods | Randomised controlled trial | |
| Participants | 20 children (ages ranging from 7 to 17 years old) with active Crohn's disease | |
| Interventions | Participants were randomized to receive either nutritional therapy of hydrolyzed formula or corticosteroids for 8 weeks | |
| Outcomes | Remission was defined using PCDAI < 10 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The physician who performed the clinical evaluation and the pediatric gastroenterologist who performed the endoscopy were unaware of the therapeutic allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Verma 2000.
| Methods | Randomized trial (method not specified), double‐blind (coded diets and similar in appearance) | |
| Participants | 21 patients, 8 males and 13 females with CDAI > 150 | |
| Interventions | 10 patients received the elemental diet and 11 received the polymeric diet | |
| Outcomes | Remission rate defined by CDAI < 150 or CDAI decrease of 100 points from baseline level after 28 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described or not described in sufficient detail to allow a definite judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition of two cases due to intolerance of nasogastric tube was mentioned. The exclusion criteria were mentioned |
| Selective reporting (reporting bias) | Low risk | The published reports include all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Raouf: There was no specific entry cut‐off score for disease activity defined in this study. However, the disease activity in all patients ranged from 8‐24 which was greater than the disease activity entry criteria defined by Park and Middleton who also used the Harvey Bradshaw simple CDI. Therefore, the Raouf study was included in the review Lindor 1992: Definite remission (as defined above) was the outcome used for the analysis; probable remission (CDAI decrease of 50 points or more from initial value) was not used as an outcome for the analysis.
PCDAI = Pediatric Corhn's disease activity index
CRP = C‐reactive protein
ESR = erythrocyte sedimentation rate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afzal 2005a | Not randomised controlled trial |
| Akobeng 2002 | Both groups received polymeric diets |
| Berni Canani 2006 | Retrospective study |
| Buchanan 2009 | Not randomised controlled trial ‐ cohort study |
| Chiang 2009 | Retrospective, no control group |
| Day 2006 | Retrospective, lack of comparison group |
| Engelman 1993 | The number of patients achieving remission was not specified Abstract, therefore no quality score |
| Gassull 2000 | Unable to calculate remission rate by intention‐to‐treat analysis because the number of patients randomized per group was not specified |
| Giffin 2014 | Abstract, retrospective |
| Goncalves 2014 | Retrospective |
| Gorard 1993 | The number of patients achieving remission was not specified |
| Grover 2012 | Abstract, lack of control group, details of enteric feed not provided |
| Gunasekera 2012 | Elemental diet compared to sham diet |
| Hirakawa 1993 | Not randomised controlled trial |
| Johnson 2006 | Comparison group allowed 50% of nutrition from regular diet. |
| Jones 1987 | Compared enteral nutrition and total parenteral nutrition |
| Klerkus 2013 | Not randomized |
| Levine 2014 | Not randomized or quasi‐randomized |
| Ludvigsson 2004 | Concurrent therapy with high dose steroids (7 patients received > 0.5 mg/kg prednisolone) in enteral nutrition group was allowed |
| Middleton 1991 | Abstract, later published in full article [Middleton (a)] |
| Munkholm Larsen 1989 | Compared elemental diet to placebo diet |
| Navas Lopez 2008 | Lack of control group |
| O'Morain 1984 | The disease activity and the remission criteria were not defined |
| Otley 2015 | Not randomised controlled trial ‐ retrospective cohort study |
| Papadopoulou 1995 | Not randomised controlled trial |
| Rabast 1986 | Both groups received elemental diets |
| Riordan 1993 | Patients who are in remission are randomised. Trial studying the maintenance of remission, not the induction of remission |
| Ruuska 1994 | The disease activity and the remission criteria were not defined, and the number of patients achieving remission and the time of outcome assessment were not specified |
| Sanderson 1987 | The number of patients achieving remission was not specified and the remission criteria were not defined |
| Seidman 1993 | The number of patients achieving remission was not specified. Abstract, therefore no quality score |
| Sigall‐Boneh 2014 | Allowed concurrent oral intake with enteral feed, no control group |
| Sokulmez 2014 | Compared elemental diet to placebo diet |
| Soo 2013 | Retrospective |
| Thomas 1993 | The number of patients achieving remission was not specified and the remission criteria were not defined |
| Ueki 1994 | The number of patients achieving remission was not specified |
| Watanabe 2010 | Retrospective, no remission data specified |
| Zoli 1996 | Abstract, later published in full article (Zoli 1997) |
| Zoli 1997 | The disease activity and the remission criteria were not defined |
Characteristics of ongoing studies [ordered by study ID]
NCT00265772.
| Trial name or title | Phase IV Study Comparing a Nutritional Anti‐Inflammatory Treatment to Steroids for Pediatric Crohn's Disease ‐ the Molecular Basis |
| Methods | Randomised controlled trial |
| Participants | Pediatric patients ages 6 to 18 with active Crohn's disease |
| Interventions | Enteral nutrition (Modulen) will be given to one group and corticosteroids to another |
| Outcomes | Clinical remission as defined by HBI < 5 |
| Starting date | November 2005 |
| Contact information | Frank M Ruemmele, MD PhD, frank.ruemmele@nck.ap‐hop‐paris.fr |
| Notes |
NCT01728870.
| Trial name or title | Comparison of Partial Enteral Nutririon (Modulen) With a Unique Diet to Exclusive Enteral Nutrition (Modulen) for the Treatment of Pediatric Crohn's Disease. A Prospective Randomized Controlled Trial |
| Methods | Prospective randomised controlled trial |
| Participants | Patients aged 4‐18 with a recent diagnosis of Crohn's disease |
| Interventions | One group will receive partial enteral nutrition and limited whole food diet and one group will receive exclusive enteral nutrition over 12 weeks |
| Outcomes | Remission as defined by PCDAI ≤10 or less than 7.5 without height component; response as defined by a drop in PCDAI of 12.5 points |
| Starting date | Janaury 2013 |
| Contact information | Arie Levine, MD, alevine@wolfson.health.gov.il |
| Notes |
NCT02056418.
| Trial name or title | A Randomized, Controlled, Single‐blind Study of Effects of Enteral Nutrition and Corticosteroid on Intestinal Flora in Induction Remission of Crohn Disease in Adult |
| Methods | Randomised controlled, single‐blind study |
| Participants | Patients aged 18‐75 with active Crohn's disease |
| Interventions | One group will receive enteral nutrition, one group will receive corticosteroid and one group will serve as the control group consisting of healthy people eating a normal diet |
| Outcomes | CDAI will be monitored at week 6 and change from baseline CDAI every week |
| Starting date | January 2014 |
| Contact information | Zhu Weiming, professor, Jinling Hospital, China |
| Notes |
NCT02231814.
| Trial name or title | Dietary Therapy Using Partial Enteral Nutrition and the Crohn's Disease Exclusion Diet (CDED) for Induction and Maintenance of Remission in Mild to Moderate Crohn's Disease in Adults‐ A Pilot Study |
| Methods | Prospective open‐label randomized controlled pilot trial |
| Participants | Patients between the ages of 18 to 55 with established Crohn's disease |
| Interventions | One group will receive Crohn's disease exclusion diet (CDED) and partial enteral nutrition; the other group will receive the CDED alone |
| Outcomes | Clinical remission as defined by HBI < 5 at week 6 |
| Starting date | August 2014 |
| Contact information | Arie Levine, MD, alevine@wolfson.health.gov.il |
| Notes |
NCT02843100.
| Trial name or title | Modified Exclusive Enteral Nutrition With the Crohn's Disease Exclusion Diet for Induction and Maintenance of Remission and Re‐biosis |
| Methods | Open label randomized controlled pilot study |
| Participants | Pediatric patients between the ages of 8 to 18 with established Crohn's disease |
| Interventions | One group will receive exclusive enteral nutrition followed by partial enteral nutrition along with the Crohn's disease exclusion diet while the other group will receive exclusive enteral nutrition for 12 weeks |
| Outcomes | Clinical remission as defined by PCDAI < 10 at week 14 |
| Starting date | January 2017 |
| Contact information | Arie Levine, MD, alevine@wolfson.health.gov.il |
| Notes |
NCT03176875.
| Trial name or title | Comparison of Partial and Exclusive Enteral Nutrition in the Treatment of Active Childhood‐onset Crohn's Disease |
| Methods | Prospective randomised controlled trial |
| Participants | Pediatric patients between the ages of 4 to 18 with Crohn's disease and young adults between the ages of 18 to 30 with childhood‐onset Crohn's disease |
| Interventions | One group will receive partial enteral nutrition (75% of daily dietary needs) and an antiinflammatory diet (25% of daily dietary needs) for 6 weeks while another group receives exclusive enteral nutrition for 6 weeks |
| Outcomes | Clinical remission as defined by PCDAI < 10 after 6 weeks of therapy |
| Starting date | May 2017 |
| Contact information | Darja Urlep, MD, MSc, darja.urlep@gmail.com |
| Notes |
Differences between protocol and review
This review update includes a PRISMA flow diagram, a risk of bias assessment of included studies (to replace the Jadad scale), a GRADE analysis and Summary of Findings tables, a per‐protocol analysis for the primary outcomes and the inclusion of adverse event outcomes.
Contributions of authors
NN, AD and DZ were responsible for formulating the study question, carrying out the literature search, selecting and reviewing the studies, risk of bias assessment, performing the analyses, and writing the manuscript.
MZ provided methodological expertise, IBD expert opinion, and reviewed the manuscript.
MS, MT and WR provided IBD expert opinion and reviewed the manuscript.
Declarations of interest
Neeraj Narula has no known declarations of interest to declare.
Amit Dhillon has no known declarations of interest to declare.
Dongni Zhang has no known declarations of interest to declare.
Mary Sherlock has served as an advisory board member for Abbvie and Jannsen and received travel expenses from Abbvie to attend an IBD meeting in 2015.
Melody Tondeur has no known declarations of interest to declare.
Walter Reinisch has served as a speaker, a consultant or an advisory board member for Abbott Laboratories, Abbvie, Aesca, Amgen, AM Pharma, Aptalis, Astellas, Astra Zeneca, Avaxia, Bioclinica, Biogen IDEC, Bristol‐Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Galapagos, Genentech, Grünenthal, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Robarts Clinical Trial, Schering‐Plough, Setpointmedical, Shire, Takeda, Therakos, Tigenix, UCB, Vifor, Yakult, Zyngenia, and 4SC.
Mary Zachos has served as an advisory board member for Abbvie, Janssen and Ferring.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Akobeng 2000 {published data only}
- Akobeng AK, Miller V, Stanton J, Elbadri AM, Thomas AG. Double‐blind randomized controlled trial of glutamine‐enriched polymeric diet in the treatment of active Crohn's disease. Journal of Pediatriatric Gastroenterology and Nutrition 2000;30(1):78‐84. [DOI] [PubMed] [Google Scholar]
Bamba 2003 {published data only}
- Bamba T, Shimoyama T, Sasaki M, Tsujikawa T, Fukuda Y, Koganei K, et al. Dietary fat attenuates the benefits of an elemental diet in active Crohn's disease: a randomized, controlled trial. European Journal of Gastroenterology & Hepatology 2003;15(2):151‐7. [DOI] [PubMed] [Google Scholar]
Borrelli 2006 {published data only}
- Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn's disease: a randomized controlled open‐label trial. Clinical Gastroenterology and Hepatology 2006;4(6):744‐53. [DOI] [PubMed] [Google Scholar]
Gassull 2002 {published data only}
- Gassull A, Fernandez‐Banares F, Cabre E, Papo M, Giaffer MH, Sanchez‐Lombrana JL, et al. Fat composition is the differential factor to explain the primary therapeutic effect of enteral nutrition in Crohn's disease. Results of a double‐blind, randomized, multicenter European trial. Gastroenterology 2000;118:A117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassull MA, Fernandez‐Banares F, Cabre E, Papo M, Giaffer MH, Sanchez‐Lombrana JL, et al. Fat composition may be a clue to explain the primary therapeutic effect of enteral nutrition in Crohn's disease: results of a double blind randomised multicentre European trial. Gut 2002;51(2):164‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giaffer 1990 {published data only}
- Giaffer MH, North G, Holdsworth CD. Controlled trial of polymeric versus elemental diet in treatment of active Crohn's disease. Lancet 1990;335(8693):816‐9. [DOI] [PubMed] [Google Scholar]
Gonzalez‐Huix 1993 {published data only}
- Gonzalez‐Huix F, Leon R, Acero D, Esteve M, Figa M, Abad A, et al. Total enteral nutrition with polymeric diets (TENP) as primary treatment in Crohn's disease (CD). Preliminary results of a controlled trial [abstract]. Clinical Nutrition 1991;10:47. [Google Scholar]
- Gonzalez‐Huix F, Leon R, Fernandez‐Banares F, Esteve M, Cabre E, Acero D, et al. Polymeric enteral diets as primary treatment of active Crohn's disease: a prospective steroid controlled trial. Gut 1993;34(6):778‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffiths 2000 {published data only}
- Griffiths AM, Pendley F, Issenman R, Cockram D, Jacobsen K, Kelley MJ, et al. Elemental versus polymeric nutrition as primary therapy of active Crohn's disease: a multi‐centre pediatric randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2000;31(Suppl 2):S75. [Google Scholar]
Grogan 2012 {published data only}
- Grogan JL, Casson DH, Terry A, Burdge GC, El‐Matary W, Dalzell AM. Enteral feeding therapy for newly diagnosed pediatric Crohn's disease: A double‐blind randomized controlled trial with two years follow‐up. Inflammatory Bowel Diseases 2012;18(2):246‐53. [DOI] [PubMed] [Google Scholar]
Kobayashi 1998 {published data only}
- Kobayashi K, Katsumata T, Yokoyama K, Takahashi H, Igarashi M, Saigenji K. A randomized controlled study of total parenteral nutrition and enteral nutrition by elemental and polymeric diet as primary therapy in active phase of Crohn's disease. Nippon Shokakibyo Gakkai Zasshi 1998;95(11):1212‐21. [PubMed] [Google Scholar]
Leiper 2001 {published data only}
- Leiper K, Woolner J, Mullan MM, Parker T, Vliet M, Fear S, et al. A randomised controlled trial of high versus low long chain triglyceride whole protein feed in active Crohn's disease. Gut 2001;49(6):790‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindor 1992 {published data only}
- Lindor KD, Fleming CR, Burnes JU, Nelson JK, Ilstrup DM. A randomized prospective trial comparing a defined formula diet, corticosteroids, and a defined formula diet plus corticosteroids in active Crohn's disease. Mayo Clinic Proceedings 1992;67(4):328‐33. [DOI] [PubMed] [Google Scholar]
Lochs 1991 {published data only}
- Lochs H, Steinhardt HJ, Klaus‐Wentz B, Zeitz M, Vogelsang H, Sommer H, et al. Comparison of enteral nutrition and drug treatment in active Crohn's disease. Results of the European Cooperative Crohn's Disease Study. IV. Gastroenterology 1991;101(4):881‐8. [DOI] [PubMed] [Google Scholar]
Malchow 1990 {published data only}
- Malchow H, Steinhardt HJ, Lorenz‐Meyer H, Strohm WD, Rasmussen S, Sommer H, et al. Feasibility and effectiveness of a defined‐formula diet regimen in treating active Crohn's disease. European Cooperative Crohn's Disease Study III. Scandinavian Journal of Gastroenterology 1990;25(3):235‐44. [PubMed] [Google Scholar]
Mansfield 1995 {published data only}
- Mansfield JC, Giaffer MH, Holdsworth CD. Controlled trial of oligopeptide versus amino acid diet in treatment of active Crohn's disease. Gut 1995;36(1):60‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantzaris 1996 {published data only}
- Mantzaris GJ, Archavlis E, Amberiadis P, Kourtessas D, Triantafullou G. A Prospective Randomized Trial in Active Crohn's Disease Comparing prednisolone, a Polymeric Diet and a Polymeric Diet Plus Prednisolon [abstract]. Gut 1996;39 Suppl 3:A166. [Google Scholar]
- Mantzaris GJ, Archavlis E, Amperiadis P, Kourtessas D, Triantafyllou G. A randomized prospective trial in active Crohn's disease comparing a polymeric diet, prednisolone and a polymeric diet plus prednisolone. Gastroenterology 1996;110(4):A955. [Google Scholar]
Middleton 1995 {published data only}
- Middleton SJ, Rucker JT, Kirby GA, Riordan AM, Hunter JO. Long‐chain triglycerides reduce the efficacy of enteral feeds in patients with active Crohn's disease. Clinical Nutrition 1995;14(4):229‐36. [DOI] [PubMed] [Google Scholar]
Park 1991 {published data only}
- Park RH, Galloway A, Danesh B, Russell RI. Double blind trial comparing elemental and polymeric diet as primary therapy for active Crohn's disease [abstract]. Clinical Nutrition 1989;8:79. [Google Scholar]
- Park RH, Galloway A, Danesh B, Russell RI. Double blind trial comparing elemental and polymeric diet as primary treatment for active Crohns`s disease [abstract]. Gut 1989;30 Suppl 10:A1453. [Google Scholar]
- Park RH, Galloway A, Danesh BJ, Russell RI. Double‐blind controlled trial of elemental and polymeric diets as primary therapy in active Crohn's disease. European Journal of Gastroenterology and Hepatology 1991;3(6):483‐9. [Google Scholar]
Pigneur 2014 {published data only}
- Pigneur B, Garnier‐Lengline H, Lepage P, Schmitz J, Goulet O, Dore J. Effect of exclusive enteral nutrition on the course of CD and intestinal microbiota. Journal of Crohn's and Colitis 2014;8:S433. [Google Scholar]
Raouf 1991 {published data only}
- Raouf AH, Hildrey V, Daniel J, Walker RJ, Krasner N, Elias E, et al. Enteral feeding as sole treatment for Crohn's disease: controlled trial of whole protein versus amino acid based feed and a case study of dietary challenge. Gut 1991;32(6):702‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rigaud 1991 {published data only}
- Rigaud D, Cosnes J, Quintrec Y, Rene E, Gendre JP, Mignon M. Controlled trial comparing two types of enteral nutrition in treatment of active Crohn's disease: elemental versus polymeric diet. Gut 1991;32(12):1492‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Royall 1994 {published data only}
- Royall D, Jeejeebhoy KN, Baker JP, Allard JP, Habal FM, Cunnane SC, et al. Comparison of amino acid versus peptide based enteral diets in active Crohn's disease: clinical and nutritional outcome. Gut 1994;35(6):783‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sakurai 2002 {published data only}
- Sakurai T, Matsui T, Yao T, Takagi Y, Hirai F, Aoyagi K, et al. Short‐term efficacy of enteral nutrition in the treatment of active Crohn's disease: a randomized, controlled trial comparing nutrient formulas. JPEN Journal of Parenteral and Enteral Nutrition 2002;26(2):98‐103. [DOI] [PubMed] [Google Scholar]
Saverymuttu 1985 {published data only}
- Saverymuttu S, Hodgson HJ, Chadwick VS. Controlled trial comparing prednisolone with an elemental diet plus non‐absorbable antibiotics in active Crohn's disease. Gut 1985;26(10):994‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seidman 1991 {published data only}
- Seidman EG, Lohoues MJ, Turgeon J, Bouthillier L, Morin CL. Elemental diet versus prednisone as initial therapy in Crohn's disease: early and long‐term results. Gastroenterology 1991;100:150A. [Google Scholar]
Seidman 2003 {published data only}
- Seidman EG, Bouthillier L, Thibault L, Lepage G, Chartre M, Deslandres C, et al. Clinical & nutritional parameters in Crohn's disease patients treated with either an n‐3 PUFA‐enriched semi‐elemental or a polymeric diet. Results of a randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2003;37(3):393. [Google Scholar]
Terrin 2002 {published data only}
- Terrin G, Canani RB, Ambrosini A, Viola F, Mesquita MB, Nardo G, et al. A semielemental diet (Pregomin) as primary therapy for inducing remission in children with active Crohn's disease. Italian Journal of Pediatrics 2002;28(5):401‐5. [Google Scholar]
Verma 2000 {published data only}
- Verma S, Brown S, Giaffer MH. Elemental versus polymeric diet in treatment of active Crohn's disease: A double‐blind randomised trial. Gut 1997;41(Suppl 3):A226. [DOI] [PubMed] [Google Scholar]
- Verma S, Brown S, Giaffer MH. Randomised double‐blind trial of polymeric versus elemental diet in treatment of active Crohn's disease. Gut 1997;40(Suppl 1):A19. [DOI] [PubMed] [Google Scholar]
- Verma S, Brown S, Kirkwood B, Giaffer MH. Polymeric versus elemental diet as primary treatment in active Crohn's disease: a randomized, double‐blind trial. American Journal of Gastroenterology 2000;95(3):735‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Afzal 2005a {published data only}
- Afzal NA, Davies S, Paintin M, Arnaud‐Battandier F, Walker‐Smith JA, Murch S, et al. Colonic Crohn's disease in children does not respond well to treatment with enteral nutrition if the ileum is not involved. Digestive Diseases and Sciences 2005;50(8):1471‐5. [DOI] [PubMed] [Google Scholar]
Akobeng 2002 {published data only}
- Akobeng AI, Clayton PE, Miller V, Hall CM, Thomas AG. Low serum concentrations of insulin‐like growth factor‐I in children with active Crohn disease: effect of enteral nutritional support and glutamine supplementation. Scandinavian Journal of Gastroenterology 2002;37:1422‐7. [DOI] [PubMed] [Google Scholar]
Berni Canani 2006 {published data only}
- Berni Canani R, Terrin G, Borrelli O, Romano MT, Manguso F, Coruzzo A, et al. Short‐ and long‐term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn's disease. Digestive and Liver Disease 2006;38(6):381‐7. [DOI] [PubMed] [Google Scholar]
Buchanan 2009 {published data only}
- Buchanan E, Gaunt WW, Cardigan T, Garrick V, McGrogan P, Russell RK. The use of exclusive enteral nutrition for induction of remission in children with Crohn's disease demonstrates that disease phenotype does not influence clinical remission. Alimentary Pharmacology & Therapeutics 2009;30(5):501‐7. [DOI] [PubMed] [Google Scholar]
Chiang 2009 {published data only}
- Chiang NYZ, Haslinger T, Richman E, Rhodes JM, Leiper K. Efficacy of enteral feeding with modulen in adults with Crohn's disease: A retrospective study. Gut 2009;58:A97. [Google Scholar]
Day 2006 {published data only}
- Day AS, Whitten KE, Lemberg DA, Clarkson C, Vitug‐Sales M, Jackson R, et al. Exclusive enteral feeding as primary therapy for Crohn's disease in Australian children and adolescents: A feasible and effective approach. Journal of Gastroenterology and Hepatology 2006;21(10):1609‐14. [DOI] [PubMed] [Google Scholar]
Engelman 1993 {published data only}
- Engelman JL, Black L, Murphy GM, Sladen GE. Comparison of a semi‐elemental diet (peptamen) with prednisolone in the primary treatment of active ileal Crohn's disease. Gastroenterology 1993;104:697A. [Google Scholar]
Gassull 2000 {published data only}
- Gassull A, Fernandez‐Banares F, Cabre E, Papo M, Giaffer MH, Sanchez‐Lombrana JL, et al. Fat composition is the differential factor to explain the primary therapeutic effect of enteral nutrition in Crohn's disease. Results of a double‐blind, randomized, multicenter European trial. Gastroenterology 2000;118:A117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giffin 2014 {published data only}
- Giffin N, Grant A, Mahdi G, Rashid M, Otley AR. Exclusive enteral nutrition optimizes growth outcomes versus corticosteroid therapy for pediatric Crohn's disease. Gastroenterology 2014;5(Suppl 1):S‐217. [Google Scholar]
Goncalves 2014 {published data only}
- Goncalves JP, Quaresma L, Rego H, Ratola A, Tavares M, Trindade E, et al. Steroids may reduce the benefit of exclusive enteral feeding in paediatric Crohn's disease. Journal of Crohn's and Colitis 2014;8:S207. [Google Scholar]
Gorard 1993 {published data only}
- Gorard DA, Hunt JB, Payne‐James JJ, Palmer KR, Rees RG, Clark ML, et al. Initial response and subsequent course of Crohn's disease treated with elemental diet or prednisolone. Gut 1993;34(9):1198‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JB, Payne‐James JJ. A randomised controlled trial of elemental diet versus prednisolone in treatment of new and recurrent Crohn's disease [abstract]. Clinical Science 1989;77 Suppl 2:26p. [Google Scholar]
- Hunt JB, Payne‐James JJ, Palmer KR, Kumar PK, Clark M L, Farthing MJ. A randomised controlled trial of elemental diet versus prednisolone in the treatment of new & recurrent Crohns disease [abstract]. Clinical Nutrition 1989;8:80. [Google Scholar]
Grover 2012 {published data only}
- Grover Z, Muir R, Lewindon P. A prospective study of new cases of paediatric Crohn's disease treated with exclusive enteral nutrition: Early clinical, biochemical, mucosal and transmural outcomes. Gastroenterology 2012;142(5 Suppl. 1):S76‐7. [Google Scholar]
Gunasekera 2012 {published data only}
- Gunasekera AV, Rajendran N, Mendall MA, Kumar D. An igg4 specific food exclusion diet improves both clinical disease activity and quality of life in Crohn's disease; a randomised sham diet controlled study. Gastroenterology 2012;1:S567. [Google Scholar]
Hirakawa 1993 {published data only}
- Hirakawa H, Fukuda Y, Tanida N, Hosomi M, Shimoyama T. Home elemental enteral hyperalimentation (HEEH) for the maintenance of remission in patients with Crohn's disease. Gastroenterologia Japonica 1993;28(3):379‐84. [DOI] [PubMed] [Google Scholar]
Johnson 2006 {published data only}
- Johnson T, Macdonald S, Hill SM, Thomas A, Murphy MS. Treatment of active Crohn's disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut 2006;55(3):356‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1987 {published data only}
- Jones VA. Comparison of total parenteral nutrition and elemental diet in induction of remission of Crohn's disease. Long‐term maintenance of remission by personalized food exclusion diets. Digestive Diseases and Sciences 1987;32(12 Supplement):100S‐7S. [DOI] [PubMed] [Google Scholar]
Klerkus 2013 {published data only}
- Klerkus J, Szymanska S, Szczepanski M, Wiernicka A, Szymanska E, Matuszczyk M, et al. The efficacy of total enteral nutrition in inducing remission and improving nutritional status in children with moderate to severe Crohn's disease. Przegla̜d Gastroenterologiczny 2013;8(1):57‐61. [Google Scholar]
Levine 2014 {published data only}
- Levine A, Turner D, Gik TP, Dias JA, Veres G, Shaoul R, et al. Comparison of outcomes parameters for induction of remission in new onset pediatric Crohn's disease: Evaluation of the Porto IBD group "Growth relapse and outcomes with therapy" (GROWTH‐CD) study. Inflammatory Bowel Diseases 2014;20:278‐85. [DOI] [PubMed] [Google Scholar]
Ludvigsson 2004 {published data only}
- Ludvigsson JF, Krantz M, Bodin L, Stenhammar L, Lindquist B. Elemental versus polymeric enteral nutrition in paediatric Crohn's disease: a multicentre randomized controlled trial. Acta Paediatrica 2004;93(3):327‐35. [PubMed] [Google Scholar]
Middleton 1991 {published data only}
- Middleton SJ, Riordan AM, Hunter JO. Comparison of elemental and peptide‐based diets in the treatment of acute Crohn's disease. Italian Journal of Gastroenterology 1991;23:609. [Google Scholar]
Munkholm Larsen 1989 {published data only}
- Munkholm Larsen P, Rasmussen D, Ronn B, Munck O, Elmgreen J, Binder V. Elemental diet: a therapeutic approach in chronic inflammatory bowel disease. Journal of Internal Medicine 1989;225(5):325‐31. [DOI] [PubMed] [Google Scholar]
Navas Lopez 2008 {published data only}
- Navas Lopez VM, Blasco Alonso J, Sierra Salinas C, Barco Galvez A, Vicioso Recio MI. Efficacy of exclusive enteral feeding as primary therapy for paediatric Crohn's disease. Anales de Pediatria 2008;69(6):506‐14. [DOI] [PubMed] [Google Scholar]
O'Morain 1984 {published data only}
- O'Morain C, Segal AW, Levi AJ. Elemental diet as primary treatment of acute Crohn's disease: a controlled trial. British Medical Journal 1984;288(6434):1859‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Otley 2015 {published data only}
- Otley AR, Grant A, Giffin N, Rashid M, Mahdi G, Limbergen J. Steroids No More! exclusive enteral nutrition therapy in pediatric patients with Crohn's disease results in long‐term avoidance of corticosteroid therapy. Gastroenterology 2015;1:S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papadopoulou 1995 {published data only}
- Papadopoulou A, Rawashdeh MO, Brown GA, McNeish AS, Booth IW. Remission following an elemental diet or prednisolone in Crohn's disease. Acta Paediatrica 1995;84(1):79‐83. [DOI] [PubMed] [Google Scholar]
Rabast 1986 {published data only}
- Rabast U, Heskamp R. Adjuvant therapy with elemental diet in chronic inflammatory intestinal diseases. Comparative study. Deutsche Medizinische Wochenschrift 1986;111(8):293‐7. [DOI] [PubMed] [Google Scholar]
Riordan 1993 {published data only}
- Riordan AM, Hunter JO, Cowan RE, Crampton J R, Davidson AR, Dickinson RJ. Treatment of active Crohn's disease by exclusion diet: East Anglian multicentre controlled trial. Lancet 1993;342:1131‐4. [DOI] [PubMed] [Google Scholar]
Ruuska 1994 {published data only}
- Ruuska T, Savilahti E, Maki M, Ormala T, Visakorpi JK. Exclusive whole protein enteral diet versus prednisolone in the treatment of acute Crohn's disease in children. Journal of Pediatric Gastroenterology and Nutrition 1994;19(2):175‐80. [DOI] [PubMed] [Google Scholar]
Sanderson 1987 {published data only}
- Sanderson IR, Udeen S, Davies PS, Savage MO, Walker‐Smith JA. Remission induced by an elemental diet in small bowel Crohn's disease. Archives of Disease in Childhood 1987;62(2):123‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seidman 1993 {published data only}
- Seidman EG, Grifftiths AM, Jones A, Issenman R. Semi‐elemental diet versus prednisone in pediatric Crohn's disease. Gastroenterology 1993;104:A778. [Google Scholar]
Sigall‐Boneh 2014 {published data only}
- Sigall‐Boheh R, Pfeffer‐Gik T, Segal I, Zangen T, Boaz M, Levine A. Partial enteral nutrition with a Crohn's disease exclusion diet is effective for induction of remission in children and young adults with Crohn's disease. Inflammatory Bowel Diseases 2014;20:1353‐60. [DOI] [PubMed] [Google Scholar]
Sokulmez 2014 {published data only}
- Sökülmez P, Demirba AE, Arslan P, Diibeyaz S. Effects of enteral nutritional support on malnourished patients with inflammatory bowel disease by subjective global assessment. Turkish Journal of Gastroenterology 2014;25(5):493‐507. [DOI] [PubMed] [Google Scholar]
Soo 2013 {published data only}
- Soo J, Malik BA, Turner JM, Persad R, Wine E, Siminoski K, et al. Use of exclusive enteral nutrition is just as effective as corticosteroids in newly diagnosed pediatric Crohn's disease. Digestive Diseases and Sciences 2013;58:3584‐91. [DOI] [PubMed] [Google Scholar]
Thomas 1993 {published data only}
- Thomas AG, Taylor F, Miller V. Dietary intake and nutritional treatment in childhood Crohn's disease. Journal of Pediatric Gastroenterology and Nutrition 1993;17(1):75‐81. [DOI] [PubMed] [Google Scholar]
Ueki 1994 {published data only}
- Ueki M, Matsui T, Yamada M, Sakurai T, Yao T, Okada M, et al. Randomized controlled trial of amino acid based diet versus oligopeptide based diet in enteral nutritional therapy of active Crohn's disease. Nippon Shokakibyo Gakkai Zasshi 1994;91(9):1415‐25. [PubMed] [Google Scholar]
Watanabe 2010 {published data only}
- Watanabe O, Ando T, Ishiguro K, Takahashi H, Ishikawa D, Miyake N, et al. Enteral nutrition decreases hospitalization rate in patients with Crohn's disease. Journal of Gastroenterology and Hepatology 2010;25(Suppl 1):S134‐7. [DOI] [PubMed] [Google Scholar]
Zoli 1996 {published data only}
- Zoli G, Care M, Falco F, Parazza M, Spano C, Gasbarrini G. Effect of oral elemental diet on nutritional status, intestinal permeability and disease activity in Crohn's patients. Gastroenterology 1996;110(4):A1054. [Google Scholar]
Zoli 1997 {published data only}
- Zoli G, Care M, Parazza M, Spano C, Biagi PL, Bernardi M, et al. A randomized controlled study comparing elemental diet and steroid treatment in Crohn's disease. Alimentary Pharmacology and Therapeutics 1997;11(4):735‐40. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00265772 {published data only}
- NCT00265772. Phase IV Study Comparing a Nutritional Anti‐Inflammatory Treatment to Steroids for Pediatric Crohn's Disease ‐ the Molecular Basis. clinicaltrials.gov/show/NCT00265772 (accessed 3 August 2017).
NCT01728870 {published data only}
- NCT01728870. Comparison of Partial Enteral Nutririon (Modulen) With a Unique Diet to Exclusive Enteral Nutrition (Modulen) for the Treatment of Pediatric Crohn's Disease. A Prospective Randomized Controlled Trial. clinicaltrials.gov/show/NCT01728870 (accessed 3 August 2017).
NCT02056418 {published data only}
- NCT02056418. A Randomized, Controlled, Single‐blind Study of Effects of Enteral Nutrition and Corticosteroid on Intestinal Flora in Induction Remission of Crohn Disease in Adult. clinicaltrials.gov/show/NCT02056418 (accessed 23 July 2017).
NCT02231814 {published data only}
- NCT02231814. Pilot Study of Partial Enteral Nutrition With a Unique Diet for the Treatment of Adult Patients With Crohn's Disease. clinicaltrials.gov/show/NCT02231814 (accessed 3 August 2017).
NCT02843100 {published data only}
- NCT02843100. Diet for Induction and Maintenance of Remission and Re‐biosis in Crohn's Disease. clinicaltrials.gov/show/NCT02843100 (accessed 3 August 2017).
NCT03176875 {published data only}
- NCT03176875. Comparison of Partial and Exclusive Enteral Nutrition in the Treatment of Active Childhood‐onset Crohn's Disease. clinicaltrials.gov/show/NCT03176875 accessed 3 bAugust 2017.
Additional references
Afzal 2004
- Afzal NA, Zaag‐Loonen HJ, Arnaud‐Battandier F, Davies S, Murch S, Derkx B, et al. Improvement in quality of life of children with acute Crohn's disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Alimententary Pharmacology & Therapeutics 2004;20(2):167‐72. [DOI] [PubMed] [Google Scholar]
Afzal 2005b
- Afzal NA, Fagbemi A, Arnaud‐Battandier F, Paintin M, Thomson M, Walker‐Smith JA, et al. Enteral nutrition treats children with chronic Crohn's disease more effectively if the ileum is also involved. Archives of Disease in Childhood 2005;86(Suppl 1):A22. [Google Scholar]
Fell 2000
- Fell JM, Paintin M, Arnaud‐Battandier F, Beattie RM, Hollis A, Kitching P, et al. Mucosal healing and a fall in mucosal pro‐inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn's disease. Alimentary & Pharmacologic Therapeutics 2000;14(3):281‐9. [DOI] [PubMed] [Google Scholar]
Fernandez‐Banares 1995
- Fernandez‐Banares F, Cabre E, Esteve‐Comas M, Gassull MA. How effective is enteral nutrition in inducing clinical remission in active Crohn's disease? A meta‐analysis of the randomized clinical trials. JPEN Journal of Parenteral and Enteral Nutrition 1995;19(5):356‐64. [DOI] [PubMed] [Google Scholar]
Griffiths 1995
- Griffiths AM, Ohlsson A, Sherman PM, Sutherland LR. Meta‐analysis of enteral nutrition as a primary treatment of active Crohn's disease. Gastroenterology 1995;108(4):1056‐67. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman, AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heuschkel 2000
- Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral nutrition and corticosteroids in the treatment of acute Crohn's disease in children. Journal of Pediatric Gastroenterology and Nutrition 2000;31(1):8‐15. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Kansal 2013
- Kansal S, Wagner J, Kirkwood CD, Catto‐Smith AG. Enteral nutrition in Crohn's disease: an underused therapy. Gastroenterology Research and Practice 2013;2013:482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knight 2005
- Knight C, El‐Matary W, Spray C, Sandhu BK. Long‐term outcome of nutritional therapy in paediatric Crohn's disease. Clinical Nutrition 2005;24(5):775‐9. [DOI] [PubMed] [Google Scholar]
Landi 1992
- Landi B, Ahn TN, Cortot A, Soule JC, Rene E, Gendre JP, et al. Endoscopic monitoring of Crohn's disease treatment: a prospective, randomized clinical trial. Gastroenterology 1992;102(5):1647‐53. [DOI] [PubMed] [Google Scholar]
Levine 2003
- Levine A, Milo T, Buller H, Markowitz J. Consensus and controversy in the management of pediatric Crohn disease: an international survey. Journal of Pediatric Gastroenterology and Nutrition 2003;36(4):464‐9. [DOI] [PubMed] [Google Scholar]
Messori 1996
- Messori A, Trallori G, D'Albrasio G, Milla M, Vannozzi G, Pacini F. Defined‐formula diets versus steroids in the treatment of active Crohn's disease: a meta‐analysis. Scandinavian Journal of Gastroenterology 1996;31(3):267‐72. [DOI] [PubMed] [Google Scholar]
Modigliani 1990
- Modigliani R, Mary JY, Simon JF, Cortot A, Soule JC, Gendre JP, et al. Clinical, biological and endoscopic picture of attacks of Crohn's disease. Evolution on prednisolone. Gastroenterology 1990;98(4):811‐8. [DOI] [PubMed] [Google Scholar]
Rubio 2011
- Rubio A, Pigneur B, Garnier‐Lengline H, Talbotec C, Schmitz J, Canioni D, et al. The efficacy of exclusive nutritional therapy in paediatric Crohn's disease, comparing fractionated oral vs. continuous enteral feeding. Alimentary Pharmacology & Therapeutics 2011;33(12):1332‐9. [DOI] [PubMed] [Google Scholar]
Ruemmele 2014
- Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. Journal of Crohn's & Colitis 2014;8(10):1179‐207. [DOI] [PubMed] [Google Scholar]
Sandborn 2002
- Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Lofberg R, Modigliani R, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology 2002;122(2):512‐30. [DOI] [PubMed] [Google Scholar]
Sanderson 2005
- Sanderson IR, Croft NM. The anti‐inflammatory effects of enteral nutrition. JPEN Journal of Parenteral and Enteral Nutrition 2005;29(4 Suppl):S134‐8. [DOI] [PubMed] [Google Scholar]
Schunemann 2011
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Takagi 2006
- Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M, et al. Effectiveness of an 'half elemental diet' as maintenance therapy for Crohn's disease: A randomized‐controlled trial. Alimentary & Pharmacology Therapeutics 2006;24(9):1333‐40. [DOI] [PubMed] [Google Scholar]
Voitk 1973
- Voitk AJ, Echave V, Feller JH, Brown RA, Gurd FN. Experience with elemental diet in the treatment of inflammatory bowel disease. Is this a primary therapy?. Archives of Surgery 1973;107(2):329‐33. [DOI] [PubMed] [Google Scholar]
Wilschanski 1996
- Wilschanski M, Sherman P, Pencharz P, Davis L, Corey M, Griffiths A. Supplementary enteral nutrition maintains remission in paediatric Crohn's disease. Gut 1996;38(4):543‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamamoto 2007
- Yamamoto T, Nakahigashi M, Saniabadi AR, Iwata T, Maruyama Y, Umegae S, et al. Impacts of long‐term enteral nutrition on clinical and endoscopic disease activities and mucosal cytokines during remission in patients with Crohn's disease: A prospective study. Inflammatory Bowel Disease 2007;13(12):1493‐1501. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Zachos 2001
- Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for inducing remission of Crohn's disease. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD000542] [DOI] [PubMed] [Google Scholar]
Zachos 2007
- Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD000542.pub2] [DOI] [PubMed] [Google Scholar]


