Abstract
Background
Epilepsy is a central nervous system disorder (neurological disorder). Epileptic seizures are the result of excessive and abnormal cortical nerve cell electrical activity in the brain. Despite the development of more than 10 new antiepileptic drugs (AEDs) since the early 2000s, approximately a third of people with epilepsy remain resistant to pharmacotherapy, often requiring treatment with a combination of AEDs. In this review, we summarised the current evidence regarding rufinamide, a novel anticonvulsant medication, which, as a triazole derivative, is structurally unrelated to any other currently used anticonvulsant medication, when used as an add‐on treatment for refractory epilepsy. In January 2009, rufinamide was approved by the US Food and Drug Administration for treatment of children four years of age and older with Lennox‐Gastaut syndrome. It is also approved as an add‐on treatment for adults and adolescents with focal seizures.
Objectives
To evaluate the efficacy and tolerability of rufinamide when used as an add‐on treatment in people with refractory epilepsy.
Search methods
On 2 October 2017, we searched the Cochrane Epilepsy Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO), MEDLINE (Ovid, 1946), ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP). We imposed no language restrictions. We also contacted the manufacturers of rufinamide and authors in the field to identify any relevant unpublished studies.
Selection criteria
Randomised, double‐blind, placebo‐controlled, add‐on trials of rufinamide, recruiting people (of any age or gender) with refractory epilepsy.
Data collection and analysis
Two review authors independently selected trials for inclusion and extracted the relevant data. We assessed the following outcomes: 50% or greater reduction in seizure frequency (primary outcomes); seizure freedom; treatment withdrawal; and adverse effects (secondary outcomes). Primary analyses were intention‐to‐treat (ITT) and we presented summary risk ratios (RR) with 95% confidence intervals (CI). We evaluated dose response in regression models. We carried out a risk of bias assessment for each included study using the Cochrane 'Risk of bias' tool and assessed the overall quality of evidence using the GRADE approach, which we presented in a 'Summary of findings' table.
Main results
The review included six trials, representing 1759 participants. Four trials (1563 participants) included people with uncontrolled focal seizures. Two trials (196 participants) included established Lennox‐Gastaut syndrome. Overall, the age of the adults ranged from 18 to 80 years and the age of the infants ranged from four to 16 years. Baseline phase ranged from 28 to 56 days and double‐blind phases from 84 to 96 days. Five of the six included trials described adequate methods of concealment of randomisation and only three described adequate blinding. All analyses were by ITT. Overall, five studies were at low risk of bias, and one had unclear risk of bias due to lack of reported information around study design. All trials were sponsored by the manufacturer of rufinamide, and therefore, were at high risk of funding bias.
The overall RR for 50% or greater reduction in seizure frequency was 1.79 (95% CI 1.44 to 2.22; 6 RCTs; moderate‐quality evidence) indicating that rufinamide (plus conventional AED) was significantly more effective than placebo (plus conventional AED) in reducing seizure frequency by at least 50%, when added to conventionally used AEDs in people with refractory focal epilepsy. The overall RR for treatment withdrawal (for any reason and due to AED) was 1.83 (95% CI 1.45 to 2.31; 6 RCTs; moderate‐quality evidence) showing that rufinamide was significantly more likely to be withdrawn than placebo. In respect of adverse effects, most were significantly more likely to occur in the rufinamide‐treated group. The adverse events significantly associated with rufinamide were: headache, dizziness, somnolence, vomiting, nausea, fatigue and diplopia. The RRs of these adverse effects were: headache 1.36 (95% Cl 1.08 to 1.69; 3 RCTs; high‐quality evidence); dizziness 2.52 (95% Cl 1.90 to 3.34; 3 RCTs; moderate‐quality evidence); somnolence 1.94 (95% Cl 1.44 to 2.61; 6 RCTs; moderate‐quality evidence); vomiting 2.95 (95% Cl 1.80 to 4.82; 4 RCTs; low‐quality evidence); nausea 1.87 (95% Cl 1.33 to 2.64; 3 RCTs; moderate‐quality evidence); fatigue 1.46 (95% Cl 1.08 to 1.97; 3 RCTs; moderate‐quality evidence); and diplopia 4.60 (95% Cl 2.53 to 8.38; 3 RCTs; low‐quality evidence). There was no important heterogeneity between studies for any of the outcomes. Overall, we assessed the evidence as moderate to low quality, due to potential risk of bias from some studies contributing to the analysis and wide CIs.
Authors' conclusions
In people with drug‐resistant focal epilepsy, rufinamide when used as an add‐on treatment was effective in reducing seizure frequency. However, the trials reviewed were of relatively short duration and provided no evidence for the long‐term use of rufinamide. In the short term, rufinamide as an add‐on was associated with several adverse events. This review focused on the use of rufinamide in drug‐resistant focal epilepsy and the results cannot be generalised to add‐on treatment for generalised epilepsies. Likewise, no inference can be made about the effects of rufinamide when used as monotherapy.
Keywords: Adolescent; Adult; Aged; Aged, 80 and over; Child; Child, Preschool; Female; Humans; Male; Middle Aged; Anticonvulsants; Anticonvulsants/adverse effects; Anticonvulsants/therapeutic use; Drug Resistant Epilepsy; Drug Resistant Epilepsy/drug therapy; Drug Therapy, Combination; Triazoles; Triazoles/adverse effects; Triazoles/therapeutic use
Rufinamide add‐on therapy for refractory epilepsy
Background
Epilepsy is a central nervous system disorder. Most seizures (fits) can be controlled by a single antiepileptic medicine. Unfortunately, some people require more than one antiepileptic medicine to control their seizures (called refractory epilepsy or drug‐resistant epilepsy), especially if these originate from one area of the brain (focal epilepsy), instead of being generalised (involve the entirety of the part of the brain called the cerebral cortex). Rufinamide is a novel anticonvulsant medicine that is structurally unrelated to any other currently used anticonvulsant medicine. In 2009, rufinamide was approved by the US Food and Drug Administration for the treatment of children aged four years and older with Lennox‐Gastaut syndrome (a childhood epilepsy) and then also approved as an 'add‐on' treatment (given in addition to the usual anticonvulsant medicine) for adults and adolescents with focal seizures.
Aim of the review
This review aimed to evaluate the effectiveness and side effects of rufinamide when used as an add‐on treatment for people with drug‐resistant epilepsy.
Results
We found six clinical trials that included in analysis 1759 people with focal epilepsy. These trials were all randomised controlled trials (clinical studies where people were randomly put into one of two or more treatment groups) that compared the antiepileptic drug rufinamide (at doses between 200 mg per day and 3200 mg per day) plus a conventional antiepileptic medicine to a placebo (pretend tablet) plus a conventional epileptic medicine for up to 96 days.
The review found that rufinamide, used in combination with other antiepileptic drugs in people who had drug‐resistant focal epilepsy decreased the frequency of seizures further. The review also showed that rufinamide seemed to be associated with more side effects such as dizziness, tiredness, headache, double vision, nausea and vomiting compared to placebo but more information is needed about some of these events.
The evidence is current to October 2017.
Quality of the evidence
We assessed the trials with regards to risk of bias and quality. Overall, five studies had low risk of bias, and one study had unclear risk of bias due to lack of reported information around study design. All studies were conducted by the pharmaceutical industry. We rated the quality of the evidence as moderate to low as some data were not reported and some information about the trials was unclear. Further trials are needed to assess the long‐term effects of rufinamide, and to compare it with other add‐on drugs. Furthermore, future research should consider rufinamide as add‐on treatment for generalised epilepsies and as a single treatment in focal and generalised epilepsy.
Summary of findings
Summary of findings for the main comparison.
Rufinamide versus placebo for drug‐resistant focal epilepsy
| Rufinamide versus placebo for drug‐resistant focal epilepsy | ||||||
|
Patient or population: people with drug‐resistant focal epilepsy Settings: outpatients Intervention: rufinamide Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Rufinamide | |||||
| 50% or greater reduction in seizure frequency ‐ ITT analysis | Study population |
RR 1.79 (1.44 to 2.22) |
1759 (6 studies) | ⊕⊕⊕⊝ Moderate1 |
RR > 1 indicates outcome is more likely in rufinamide group. | |
| 143 per 1000 | 256 per 1000 (206 to 317) | |||||
| Treatment withdrawal | Study population |
RR 1.83 (1.45 to 2.31) |
1759 (6 studies) | ⊕⊕⊕⊝ Moderate1 |
RR > 1 indicates outcome is more likely in rufinamide group. | |
| 112 per 1000 | 205 per 1000 (162 to 259) | |||||
| Adverse effects: dizziness | Study population |
RR 2.52 (1.90 to 3.34) |
1295 (3 studies) | ⊕⊕⊕⊝ Moderate1 |
RR > 1 indicates outcome is more likely in rufinamide group. | |
| 108 per 1000 | 272 per 1000 (205 to 361) | |||||
| Adverse effects: fatigue | Study population |
RR 1.46 (1.08 to 1.97) |
1295 (3 studies) | ⊕⊕⊕⊝ Moderate1 |
RR > 1 indicates outcome is more likely in rufinamide group. | |
| 112 per 1000 | 164 per 1000 (121 to 221) | |||||
| Adverse effects: headache | Study population |
RR 1.36 (1.08 to 1.69) |
1228 (3 studies) | ⊕⊕⊕⊝ Moderate1 |
RR > 1 indicates outcome is more likely in rufinamide group. | |
| 196 per 1000 | 267 per 1000 (212 to 331) | |||||
| Adverse effects: somnolence | Study population |
RR 1.94 (1.44 to 2.61) |
1759 (6 studies) | ⊕⊕⊕⊝ Moderate1 |
RR > 1 indicates outcome is more likely in rufinamide group. | |
| 82 per 1000 | 159 per 1000 (118 to 214) | |||||
| Adverse effects: nausea | Study population |
RR 1.87 (1.33 to 2.64) |
1295 (3 studies) | ⊕⊕⊕⊝ Moderate1 |
RR > 1 indicates outcome is more likely in rufinamide group. | |
| 82 per 1000 | 153 per 1000 (109 to 216) | |||||
| Adverse effects: vomiting | Study population |
RR 2.95 (1.80 to 4.82) |
777 (4 studies) |
⊕⊕⊝⊝ Low2 |
RR > 1 indicates outcome is more likely in rufinamide group. | |
| 49 per 1000 | 145 per 1000 (88 to 236) | |||||
| Adverse effects: diplopia | Study population |
RR 4.60 (2.53 to 8.38) |
1295 (3 studies) |
⊕⊕⊝⊝ Low2 |
RR > 1 indicates outcome is more likely in rufinamide group. | |
| 24 per 1000 | 110 per 1000 (61 to 201) | |||||
| *The basis for the assumed risk4(e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ITT: intention to treat; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Downgraded once due to risk of bias: unclear methodological information provided for some included studies and all included studies pharmaceutical sponsored.
2. Downgraded once due to imprecision: Wide confidence intervals.
3.Assumed risk is calculated as the event rate in the control group per 1000 people (number of events divided by the number of participants receiving control treatment).
Background
Description of the condition
Epilepsy is a central nervous system disorder (neurological disorder). The definition of epilepsy, as recommended by the International League Against Epilepsy (ILAE) Commission on Epidemiology, is as follows: "two or more unprovoked seizures occurring more than 24 hr apart" (ILAE Commission on Epidemiology and Prognosis 1993). Epileptic seizures are the result of excessive and abnormal cortical nerve cell electrical activity in the brain.
Epilepsy imposes a significant clinical, epidemiological and economic burden on societies worldwide. Despite the development of more than 10 new antiepileptic drugs (AEDs) since the early 2000s, approximately a third of people with epilepsy remain resistant to pharmacotherapy, often requiring treatment with a combination of AEDs. The proportion of people with refractory seizures varies in the literature between 6% and 35% (Granata 2009). Therefore, the development of effective new therapies for the treatment of refractory seizures is of considerable importance. Since the late 1990s, the introduction of several new drugs, which often are better tolerated and more manageable than the older ones, has certainly improved our ability to treat people with epilepsy (Panebianco 2015a). Studies have reported that 12% to 17% of treatment‐resistant people become seizure‐free with the addition of a previously untried, in most cases new‐generation, AED (Granata 2009).
Description of the intervention
Rufinamide (1‐(2,6‐difluoro‐phenyl) methyl‐1 hydro‐1,2,3‐triazole‐4 carboxamide) is a novel anticonvulsant medication, which, as a triazole derivative, is structurally unrelated to any other currently used anticonvulsant medications. It was granted orphan drug status for the adjunctive treatment of people with Lennox‐Gastaut syndrome (LGS) in the US in 2004, and was released for use in Europe in 2007. In January 2009, rufinamide was approved by the US Food and Drug Administration for treatment of children aged four years and older with LGS (Coppola 2011; Hsieh 2013). It is also approved as adjunctive treatment for adults and adolescents with focal seizures. There has been renewed interest in the development of newer AEDs, as several of the standard ones are not always effective and are associated with adverse effects. In the first instance, new AEDs are tested in RCTs as an add‐on treatment for people with drug‐resistant focal epilepsy. Once a therapeutic effect is reported by these trials, new AEDs tend to be licenced for add‐on use before monotherapy trials have compared new AEDs versus standard treatments. Placebo‐controlled studies of rufinamide that have provided efficacy data include trials involving people with LGS, people with adult focal‐onset seizures (for both monotherapy and adjunctive therapy), children with focal‐onset seizures in need of adjunctive therapy and people with refractory generalised tonic‐clonic seizures (Biton 2005).
How the intervention might work
The precise mechanisms by which rufinamide exerts its antiepileptic effects are unknown. In vitro studies suggest that a principal mechanism of action is the modulation of activity in sodium channels, particularly prolongation of the inactive state. In cultured cortical neurons from immature rats, rufinamide significantly slowed sodium channel recovery from inactivation after a prolonged prepulse and limited sustained repetitive firing of sodium‐dependent action potentials. Rufinamide has no effect on benzodiazepine or gamma‐aminobutyric acid (GABA) receptors, or on adenosine uptake; it also has no significant interactions with glutamate, adrenergic tryptophan, histamine or muscarinic cholinergic receptors (Wheless 2010). The overall tolerability of rufinamide is good. Most adverse events in clinical trials were mild to moderate, and they were often transient in nature, largely occurring during the titration phase (Wheless 2009).
Why it is important to do this review
The purpose of this review was to report evidence from RCTs on the efficacy and tolerability of rufinamide used as add‐on treatment for people with drug‐resistant epilepsy. This review aimed to address these issues and inform clinical practice and future research.
Objectives
To evaluate the efficacy and tolerability of rufinamide when used as an add‐on treatment in people with refractory epilepsy.
Methods
Criteria for considering studies for this review
Types of studies
Studies were required to meet all the following criteria:
RCTs;
double‐ or single‐blinded trials;
placebo‐controlled, or with an alternative AED or range of rufinamide doses used as controls;
parallel‐group or cross‐over studies;
minimum treatment period of eight weeks.
Types of participants
We considered participants who satisfied both of the following criteria:
any age; and
diagnosis of drug‐resistant epilepsy (i.e. experiencing simple focal, complex focal or secondarily generalised tonic‐clonic seizures).
Types of interventions
Active treatment group, wherein participants received treatment with rufinamide in addition to conventional AED treatment.
Control group(s), wherein participants received a matched placebo/different dose/alternative AED in addition to conventional AED treatment.
Types of outcome measures
Primary outcomes
Fifty percent or greater reduction in seizure frequency: proportion of people with a 50% or greater reduction in seizure frequency during the treatment period compared with the pre‐randomisation baseline period.
Secondary outcomes
Seizure freedom: proportion of people with complete cessation of seizures during the treatment period.
Treatment withdrawal: proportion of people with treatment withdrawn during the course of the treatment period as a measure of 'global effectiveness.' Treatment is likely to be withdrawn due to adverse effects, lack of efficacy or a combination of both, and this is an outcome to which participants make a direct contribution. In trials of shorter duration, it is likely that adverse effects will be the most common reason for withdrawal.
-
Adverse effects: proportion of people who experienced the following adverse effects:
dizziness;
fatigue;
headache;
somnolence;
nausea;
vomiting;
psychiatric adverse effects (anxiety, depression, panic attack, irritability, trouble sleeping, mood or behaviour changes);
loss of appetite;
diplopia;
fever;
loss of co‐ordination;
difficulty walking;
allergic reaction.
Quality of life (QoL): difference between intervention and control group(s) means for QoL measures used in individual studies.
Cognition: difference between intervention and control group(s) means for cognitive assessments used in individual studies.
Mood: difference between intervention and control group(s) means for mood assessments used in individual studies.
Search methods for identification of studies
Electronic searches
We searched the following databases.
Cochrane Epilepsy Group Specialized Register (2 October 2017), using the search strategy set out in Appendix 1.
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 2 October 2017), using the search strategy set out in Appendix 2.
MEDLINE (Ovid, 1946 to 2 October 2017), using the search strategy set out in Appendix 3.
ClinicalTrials.gov (2 October 2017), using the search terms: rufinamide AND epilepsy.
WHO International Clinical Trials Registry Platform (ICTRP, 2 October 2017), using the search terms: rufinamide AND epilepsy.
There were no language restrictions.
Searching other resources
We checked reference lists of retrieved studies for additional reports of relevant studies. We contacted lead study authors for relevant unpublished material. We identified duplicate studies by screening reports according to title, study author names, location and medical institute. We omitted duplicate studies.
We identified any grey literature studies published from 2012 to 2017 by searching:
Zetoc database;
Institute for Scientific Information (ISI) proceedings;
International Bureau for Epilepsy (IBE) congress proceedings database;
ILAE congress proceedings database; and
abstract books of symposia and congresses, meeting abstracts and research reports.
Data collection and analysis
Selection of studies
Two review authors (MP and HP) independently assessed all citations generated by the searches for inclusion. We resolved any disagreements by discussion with a third review author (AGM). Two review authors (MP and HP) independently extracted data and assessed risk of bias; we resolved disagreements by discussion with a third review author (AGM).
Data extraction and management
Two review authors (MP and HP) independently extracted data from each included study. We cross‐checked the data extracted. Review authors discussed disagreements (bringing in a third review author (AG) to arbitrate if need be), documented decisions and, if necessary, contacted trialists for clarification.
We extracted the following information for each trial using a pre‐standardised data extraction form:
Methodological and trial design
Methods of randomisation and allocation concealment.
Method of blinding.
Whether any participants were excluded from reported analyses.
Length of baseline period.
Length of treatment period.
Dose(s) of rufinamide tested.
Participant/demographic information
Total number of participants allocated to each treatment group.
Age/sex.
Number with focal/generalised epilepsy.
Seizure types.
Seizure frequency during the baseline period.
Number of background drugs.
Outcomes
Number of people experiencing each outcome (see Types of outcome measures) per randomly assigned group.
Contacted authors of trials to ask for missing information.
We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We collected characteristics of the included studies in sufficient detail to populate a table of 'Characteristics of included studies'.
Assessment of risk of bias in included studies
Two review authors (MP and HP) independently assessed the risk of bias of each included study. We cross‐checked risk of bias assessments and discussed and resolved any disagreements. We utilised the Cochrane 'Risk of bias' tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We rated included studies as having low, high and unclear risk of bias for six domains applicable to RCTs: randomisation method, allocation concealment, blinding methods, incomplete outcome data, selective outcome reporting and other sources of bias. We made an overall summary judgement of risk of bias for each study per outcome, followed by an overall judgement per outcome across studies. We incorporated risk of bias judgements into the analysis using sensitivity analysis, in that a secondary analysis of the data included only studies rated as having low risk of bias. We presented all results in the 'Results' section of the review. We created Table 1 for outcomes and graded each outcome accordingly using the GRADE approach (Guyatt 2008).
Measures of treatment effect
We presented the primary outcome of seizure reduction as risk ratios (RRs) and 95% confidence intervals (CIs). We presented secondary outcomes, including seizure freedom, treatment withdrawal and adverse effects, as RRs and 95% CIs.
Dealing with missing data
We sought missing data from the study authors. We carried out intention‐to‐treat (ITT), best‐case and worst‐case analyses on the primary outcome for missing data (see Data synthesis). We included all analyses in the main report.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the distribution of important individual participant factors among trials (e.g. age, seizure type, duration of epilepsy, number of AEDs taken at the time of randomisation) and trial factors (e.g. randomisation concealment, blinding, losses to follow‐up). We examined statistical heterogeneity using a Chi2 test and the I2 statistic for heterogeneity and, provided no significant heterogeneity was present (P > 0.10), we employed a fixed‐effect model. In the event that there was heterogeneity, we performed a random‐effects model analysis using the inverse variance method.
Assessment of reporting biases
Protocol versus full study
We requested all protocols from study authors to enable a comparison of outcomes of interest. In the event that a protocol was not available, we investigated outcome reporting bias using the ORBIT matrix system (Kirkham 2010).
Funnel plot
Reporting biases arise when dissemination of research findings is influenced by the nature and direction of results (Higgins 2011; Sterne 2000). We used funnel plots in investigating reporting biases with awareness that they have limited power to detect small‐study effects. We did not use funnel plots for outcomes when 10 or fewer studies were included, or when all studies were of similar size. In other cases, when funnel plots were possible, we sought statistical advice on their interpretation.
Data synthesis
We employed a fixed‐effect model meta‐analyses to synthesise the data. We expected to carry out the following comparisons:
Intervention group versus controls on 50% or greater reduction in seizure frequency.
Intervention group versus controls on seizure freedom.
Intervention group versus controls on treatment withdrawal.
Intervention group versus controls on individual adverse effects.
Intervention group versus controls on QoL.
We stratified each comparison by type of control group, that is, placebo or active control, and types of study characteristics, to ensure the appropriate combination of study data. Our preferred estimator was the Mantel‐Haenszel RR. For the outcomes 50% or greater reduction in seizure frequency, seizure freedom and treatment withdrawal, we used 95% CIs. For individual adverse effects, we used 99% CIs to allow for multiple testing. Our analyses included all participants in the treatment groups to which they were allocated. For the efficacy outcome (50% or greater reduction in seizure frequency), we undertook three analyses.
1. Primary (ITT analysis): participants not completing follow‐up or with inadequate seizure data were assumed non‐responders. To test the effect of this assumption, we undertook the following sensitivity analyses. We performed ITT analysis when this was reported by the included studies.
a) Worst‐case analysis: participants not completing follow‐up or with inadequate seizure data were assumed to be non‐responders in the intervention group, and responders in the placebo group.
b) Best‐case analysis: participants not completing follow‐up or with inadequate seizure data were assumed to be responders in the intervention group, and non‐responders in the placebo group.
We investigated effects of rufinamide at ranging between doses between 200 mg per day and 3200 mg per day. When trials compared more than one dose, doses were pooled and a comparison of rufinamide versus control was made.
We summarised selected models by expected response rates and expected differences in response rates by dose level compared with placebo.
Subgroup analysis and investigation of heterogeneity
We analysed different adverse effects separately. We aimed to assess clinical heterogeneity by comparing the distribution of important individual participant factors among trials (e.g. age, seizure type, duration of epilepsy, number of AEDs taken at the time of randomisation) and trial factors (e.g. randomisation concealment, blinding, losses to follow‐up).
Sensitivity analysis
We intended to carry out a sensitivity analysis if we found peculiarities between study quality, characteristics of participants, interventions and outcomes (assessment of risk of bias in included studies). We also reported the analysis for all studies and then compared it with an analysis including only studies at low risk of bias.
Results
Description of studies
Results of the search
The search revealed 137 records identified. After duplicates were removed, 95 records remained and we screened all for inclusion in the review. We excluded 58 due to irrelevance leaving 37 full‐text articles to be assessed for eligibility. Following this, we excluded 13 studies (see Figure 1 and Characteristics of excluded studies table for reasons of exclusion). A total of 24 studies were included in the review, six of which were included in meta‐analyses and 18 were linked to included studies. We identified three conference abstracts and contacted the authors of these studies for more information, providing their contact details were available (see Figure 1 and Characteristics of included studies table).
Figure 1.
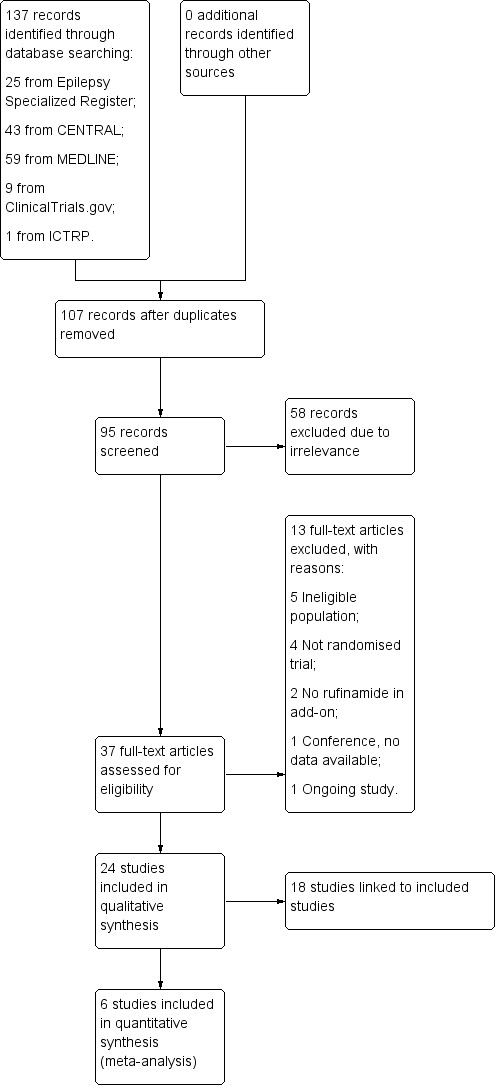
Study flow diagram.
Included studies
Overall, two RCTs compared rufinamide to a placebo in infants and adults aged four to 63 years (Glauser 2008; Ohtsuka 2014), two RCTs that examined rufinamide versus placebo in adolescents and adults aged 12 to 80 years (Biton 2011; Elger 2010), one study that examined rufinamide versus placebo in children aged four to 16 years (Glauser 2005), and one trial comparing rufinamide versus placebo in adults aged over 16 years (Brodie 2009). All the trials were sponsored by Eisai pharmaceutical company and one of these conducted by Novartis (Glauser 2008). In all trials, participants were eligible to take part in the double‐blind period of the trials if they had uncontrolled focal seizures with or without secondary generalisation (Biton 2011; Brodie 2009; Elger 2010; Glauser 2005), or with established LGS (Glauser 2008; Ohtsuka 2014), and were currently taking one to two or up to three AEDs. See Characteristics of included studies table.
One parallel, multi‐centre trial had a pre‐randomisation period of 56 days and a treatment period of 96 days (12‐day titration period followed by 84‐day maintenance phase), randomising 176 participants to rufinamide and 181 to placebo (Biton 2011). Participants were aged 12 to 80 years with inadequately controlled focal‐onset seizures with or without secondary generalisation.
One multi‐centre, parallel trial enrolled 313 adults aged over 16 years with refractory focal seizures (Brodie 2009). It randomised 156 participants to rufinamide 3200 mg and 157 to placebo. This trial had an eight‐week baseline phase and 13‐week treatment phase (two‐week titration period followed by 11‐week maintenance phase).
One multi‐centre parallel trial enrolled 647 adolescents and adults aged 15 to 65 years with refractory focal seizures with or without secondary generalisation. It randomised them to one of five treatment arms: rufinamide 200 mg (127 participants), rufinamide 400 mg (125 participants), rufinamide 800 mg (129 participants), rufinamide 1600 mg (133 participants) or placebo (133 participants) (Elger 2010). The baseline phase was 12 weeks followed by a treatment phase of 12 weeks.
One multi‐centre, parallel study included 268 children aged four to 16 years with refractory focal seizures. It had two treatment arms: rufinamide 45 mg/kg (135 participants) or placebo (133 participants) (Glauser 2005). The baseline period was 56 days and the treatment period was 91 days (14‐day titration phase followed by 77‐day maintenance phase).
One parallel study randomised 139 participants (but one of these did not received medication) aged four to 30 years with diagnosis of LGS (Glauser 2008). It consisted of two treatment arms including rufinamide 45 mg/kg (dose by bodyweight: 1000 mg, 1800 mg, 2400 mg and 3200 mg) per day (74 participants) or placebo (64 participants). This trial had a baseline phase of 28 days and a double‐blind treatment phase of 84 days.
One multi‐centre, parallel trial in Japan included 59 participants (but one participant in the rufinamide group was excluded from the efficacy analysis due to inappropriate diagnosis) aged four to 30 years with LGS (Ohtsuka 2014). It had two treatment arms: rufinamide 45 mg/kg (28 participants) (dose by bodyweight: 1000 mg, 1800 mg, 2400 mg and 3200 mg) or placebo arm (30 participants). This trial had a four‐week baseline phase, 12‐week treatment phase (two‐week titration followed by 10‐week maintenance phase) and a further phase which was either a follow‐up visit or entry into an open‐label extension.
Overall, the six RCTs recruited 1759 participants and between them tested rufinamide doses of 200 mg, 400 mg, 800 mg, 1000 mg, 1600 mg, 1800 mg, 2400 mg and 3200 mg per day. For further information on each trial, see the Characteristics of included studies table.
Excluded studies
We excluded 13 RCTs for the following reasons: five studies had ineligible populations (Biton 2005; Critchley 2005; Madeddu 2013; Palhagen 2001; Xu 2016), four studies were not RCTs (Benedict 2010; Kim 2012; Kluger 2007; Knupp 2016), two studies had no rufinamide in add‐on (Lesser 2005; Todorov 2005), and one study was conference proceedings and no data were available (Racine 2000). In addition, we found one ongoing study (Arzimanoglou 2016).
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of the 'Risk of bias' in each included study. We allocated each study an overall rating for risk of bias. All studies included in the review were individually rated as either low risk of bias or unclear risk of bias. See below for specific domain ratings.
Figure 2.
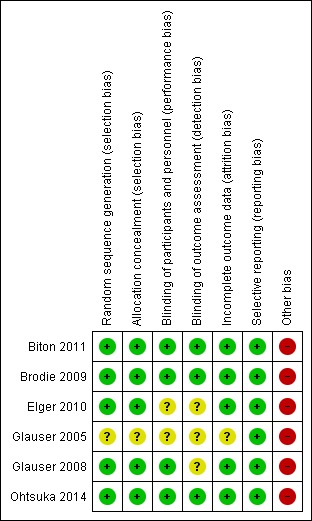
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Figure 3.
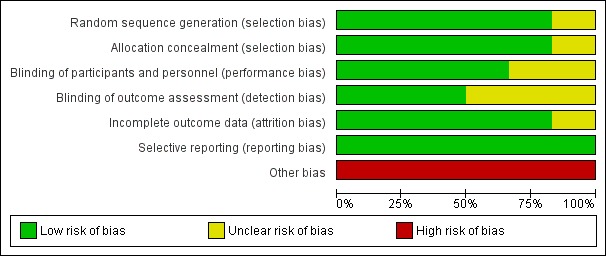
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
In five trials, we rated the methods by which allocation was concealed at low risk of bias (Biton 2011; Brodie 2009; Elger 2010; Glauser 2008; Ohtsuka 2014). One trial did not provide information and was at unclear risk of bias for this domain (Glauser 2005). For sequence generation, we rated five studies at low risk of bias due to the use of a computer‐generated randomisation schedule or random permuted blocks (Biton 2011; Brodie 2009; Elger 2010; Glauser 2008; Ohtsuka 2014). We rated one study at unclear risk of bias due to a lack of details on the methods used (Glauser 2005).
Blinding
We rated the blinding of participants and personnel at low risk of bias in four studies (Biton 2011; Brodie 2009; Glauser 2008; Ohtsuka 2014); there were no details for remaining two studies rated at unclear risk of bias (Elger 2010; Glauser 2005). Blinding of the outcome assessor was difficult to judge due to the lack of detail in three trials (Elger 2010; Glauser 2005; Glauser 2008), and therefore we rated these as unclear in terms of bias. We rated the other three studies at low risk of bias for outcome assessor (Biton 2011; Brodie 2009; Ohtsuka 2014). In five trials (Biton 2011; Brodie 2009; Elger 2010; Glauser 2005; Ohtsuka 2014) blinding was achieved by using identical medication within the rufinamide and placebo groups.
Incomplete outcome data
We rated five studies as low risk of bias for attrition bias due to the ITT analyses undertaken by the study authors (Biton 2011; Brodie 2009; Elger 2010; Glauser 2008; Ohtsuka 2014). One study was at unclear risk of bias (Glauser 2005).
Selective reporting
We requested the protocols for all included studies to compare a priori methods and outcomes to the published report, but these were unavailable. We rated all included studies at low risk of bias for this domain as there was no suspicion of selective outcome reporting bias: all expected outcomes were reported in each of the publications.
Other potential sources of bias
All the studies were sponsored by Eisai Inc., the manufacturers of the rufinamide, and therefore, we rated all studies as having a high risk of funding bias. There was no evidence of further bias in any of the included studies.
Effects of interventions
See: Table 1
See Summary of main results for the main comparison rufinamide versus placebo for refractory epilepsy.
The data were not heterogeneous and we performed no 'best case' or 'worst case' analyses.
Fifty percent or greater reduction in seizure frequency
Six RCTs (1759 participants) reported 50% or greater or greater reduction in seizure frequency. A Chi2 test for heterogeneity in a response to rufinamide showed no significant heterogeneity between trials (Chi2 = 5.76, degrees of freedom (df) = 5, P = 0.33). Those participants allocated rufinamide were significantly more likely to achieve 50% or greater reduction in seizure frequency (223/1067 participants with rufinamide versus 99/692 participants with placebo). Using the fixed‐effect model, the overall RR was 1.79 (95% CI 1.44 to 2.22; Analysis 1.1; Figure 4, moderate quality evidence).
Analysis 1.1.
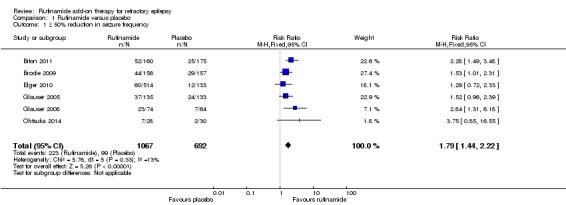
Comparison 1 Rufinamide versus placebo, Outcome 1 ≥ 50% reduction in seizure frequency.
Figure 4.
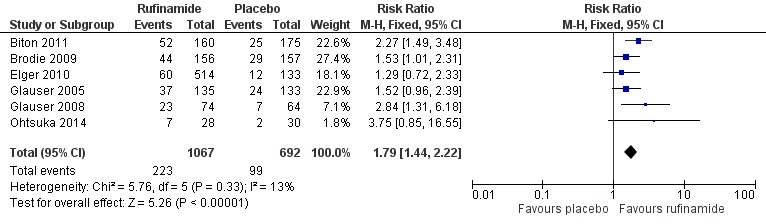
Rufinamide versus placebo, outcome: 1.1 50% reduction in seizure frequency.
Seizure freedom
Data from only one study (73 participants) reported seizure freedom. There was seizure freedom in 6/44 participants with rufinamide versus 3/29 participants with placebo; RR 1.32, 95% CI 0.36 to 4.86; Analysis 1.2, moderate quality evidence).
Analysis 1.2.
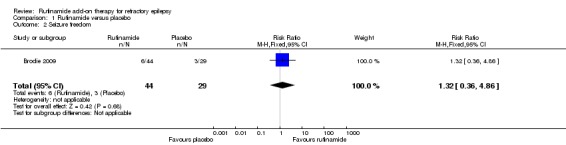
Comparison 1 Rufinamide versus placebo, Outcome 2 Seizure freedom.
Treatment withdrawal (any reason and due to adverse effects)
Six RCTs (1759 participants) reported treatment withdrawal. A Chi2 test for heterogeneity suggested no significant statistical heterogeneity between trials (Chi2 = 5.85, df = 5, P = 0.32). Participants allocated rufinamide were significantly more likely to withdraw from treatment (247/1067 participants with rufinamide versus 78/692 participants with placebo). The overall RR for withdrawal for any reason and due to AED was 1.83 (95% CI 1.45 to 2.31; Analysis 1.3; Figure 5, moderate quality evidence).
Analysis 1.3.
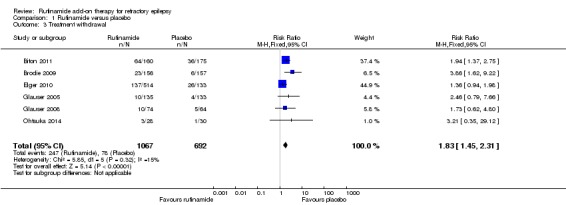
Comparison 1 Rufinamide versus placebo, Outcome 3 Treatment withdrawal.
Figure 5.
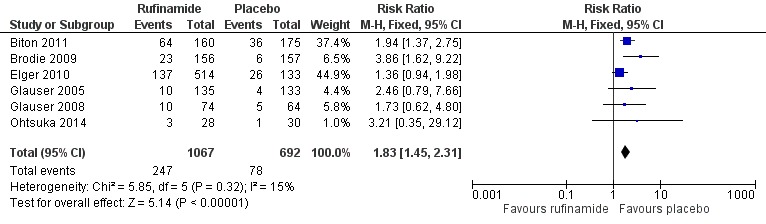
Forest plot of comparison: 1 Rufinamide versus placebo, outcome: 1.3 Treatment withdrawal.
Adverse effects
The RRs for more common adverse effects were as follows (no significant statistical heterogeneity between trials); (Analysis 1.4; Figure 6).
Analysis 1.4.
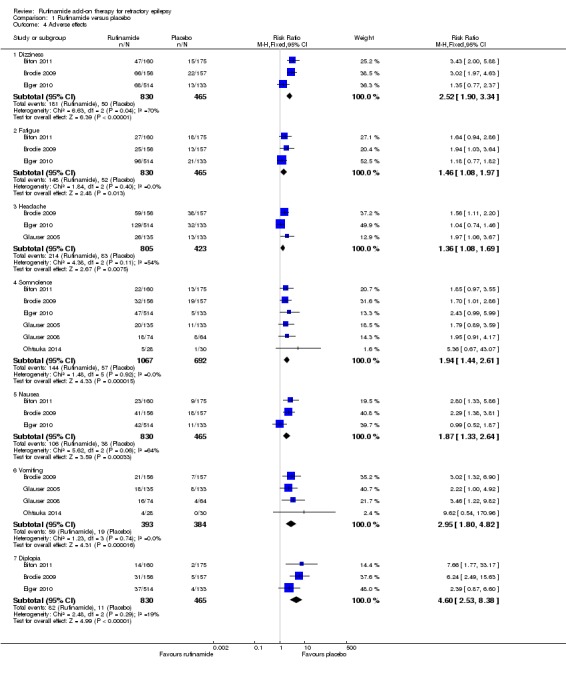
Comparison 1 Rufinamide versus placebo, Outcome 4 Adverse effects.
Figure 6.
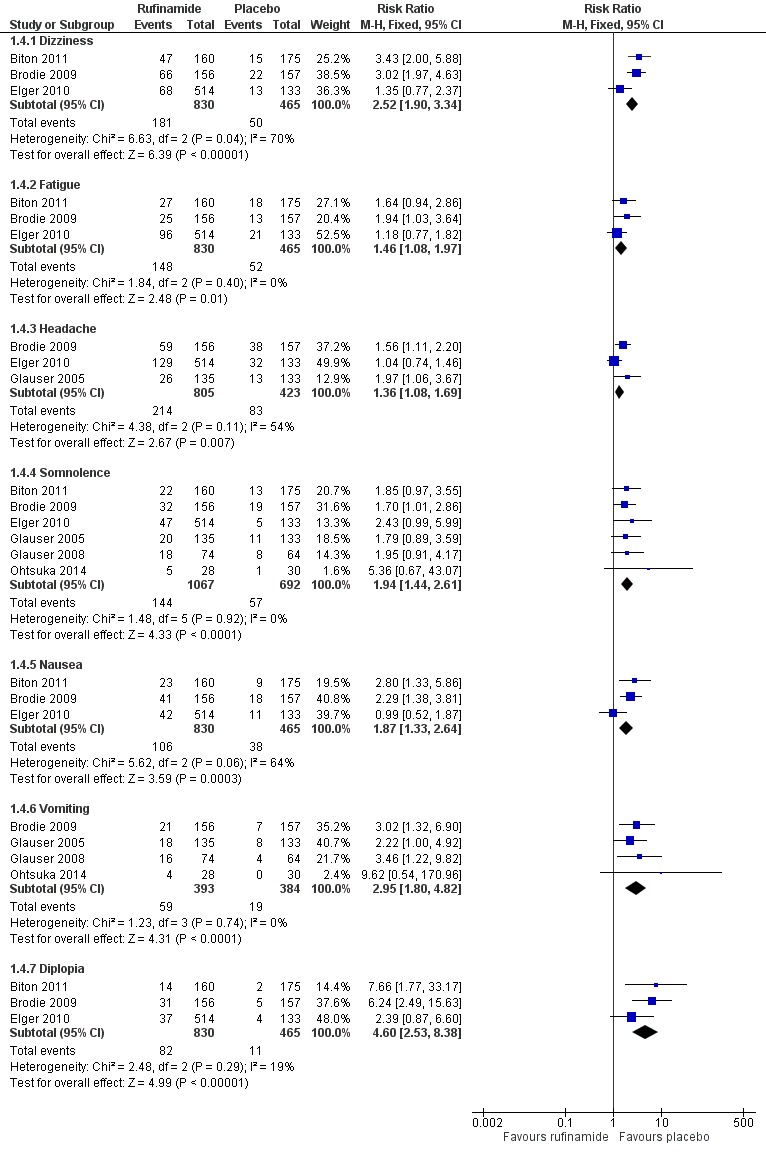
Rufinamide versus placebo: 1 Rufinamide versus placebo: 1.4 Adverse effects.
Dizziness (three RCTs, 1295 participants) in 181/830 participants with rufinamide versus 50/465 participants with placebo (RR 2.52, 99% CI 1.90 to 3.34, moderate quality evidence).
Fatigue (three RCTs, 1295 participants) in 148/830 participants with rufinamide versus 52/465 participants with placebo (RR 1.46 99% CI 1.08 to 1.97, moderate quality evidence).
Headache (three RCTs, 1228 participants) in 214/805 participants with rufinamide versus 83/423 participants with placebo (RR 1.36, 99% CI 1.08 to 1.69, moderate quality evidence).
Somnolence (six RCTs, 1759 participants) in 144/1067 participants with rufinamide versus 57/692 participants with placebo (RR 1.94, 99% CI 1.44 to 2.61, moderate quality evidence).
Nausea (three RCTs, 1295 participants) in 106/830 participants with rufinamide versus 38/465 participants with placebo (RR 1.87, 99% CI 1.33 to 2.64, moderate quality evidence).
Vomiting (four RCTs, 777 participants) in 59/393 participants with rufinamide versus 19/384 participants with placebo (RR 2.95, 99% CI 1.80 to 4.82, low quality evidence).
Diplopia (three RCTs, 1295 participants) in 82/830 participants with rufinamide versus 11/465 participants with placebo (RR 4.60, 99% CI 2.53 to 8.38, low quality evidence).
Only one RCT reported the remaining adverse effects.
Psychiatric adverse effects (anxiety, depression, panic attack, irritability, trouble sleeping, mood or behaviour changes).
Loss of appetite.
Fever.
Loss of co‐ordination.
Discussion
Summary of main results
The review included six trials, with 1759 participants included in the analysis. Four trials (1563 participants) included people with uncontrolled focal seizures. Two trials (196 participants) included established LGS. Overall, adults were aged from 18 to 80 years, and infants were aged from four to 16 years. The baseline phase in all trials ranged from 28 to 56 days, and the treatment phase from 84 to 96 days. Five of the six included trials described adequate methods of concealment of randomisation. Three studies reported effective blinding. All analyses were by ITT. The manufacturer of rufinamide, Eisai Inc., sponsored all trials, and therefore, we rated them at high risk of bias. Overall, five studies were at low risk of bias, and one had unclear risk of bias due to lack of reported information around study design.
This meta‐analysis suggested that rufinamide was more effective than placebo in reducing seizure frequency by at least 50%, when used in combination with other AEDs (from one to three) in people with refractory focal epilepsy. We were unable to examine dose effects in the planned subgroup analyses, but the results suggested increased efficacy with an increased dose. Only one study recruited children; we have no evidence from this review to indicate whether rufinamide is more or less effective in infants and children than in adults. The use of 50% or more reduction in seizure frequency as a measure of efficacy could be criticised, given that seizure freedom would be a more relevant clinical measure. However, only one study reported data on seizure freedom and, therefore, this finding must be interpreted with caution.
Results for treatment withdrawal (any reason and due to adverse effects) showed that rufinamide was significantly more likely to be withdrawn than placebo. In trials of relatively short duration, such as those reviewed here, this is likely to represent problems with tolerability rather than poor seizure control. Most of the adverse effects were significantly more likely to occur in the rufinamide‐treated group.
This review focused on the use of rufinamide in drug‐resistant focal epilepsy and the results cannot be generalised to add‐on treatment for generalised epilepsies. Likewise, no inference can be made about the effects of rufinamide when used as monotherapy.
Review findings were not compared with other published evidence, because we found no previous reviews or published information on this topic.
Overall completeness and applicability of evidence
Caution is required when translating the results of clinical trials into everyday practice, since the participants in trials are a highly selected population who may be better motivated, and are closely followed and monitored; participants who are unco‐operative and non‐compliant, who are likely to have adverse effects and fewer benefits, are excluded. The results of this review cannot be extrapolated to people with generalised epilepsies, about whom there is a great paucity of data. The safety of rufinamide during pregnancy and lactation cannot be ascertained from this review. The duration of the studies included in this review was insufficient to detect changes in cognition, social problems or long‐term adverse effects. Rare phenomena such as habituation and tolerance may not be evident in short‐term trials. The economic aspects of rufinamide therapy also needs to be examined.
Quality of the evidence
Overall, five studies were at low risk of bias and one at unclear risk of bias due to lack of reported information around study design. Only three studies had effective blinding of rufinamide. We rated all the included studies at low risk of bias for incomplete outcome data due to the ITT analyses undertaken by the study authors.
We used the GRADE approach to rate the quality of evidence for each outcome, which is presented in Table 1. For the main outcome of 50% or greater reduction in seizure frequency, the quality of evidence was high (all studies contributed to the analysis). Tolerability outcomes (withdrawal and adverse effects) were at moderate‐to‐low quality due to potential risk of bias from some studies contributing to the analysis and wide CIs.
Potential biases in the review process
Although we requested all protocols, the time frames in which the majority of the studies were conducted made retrieval of all these difficult. This could lead to potential bias through omitted information to which we did not have access.
All studies were sponsored by Eisai Inc., the manufacturers of rufinamide, and this could be a potential source of bias.
Agreements and disagreements with other studies or reviews
We found no reviews or published information on the use of rufinamide as add‐on therapy for refractory epilepsy.
Authors' conclusions
In people with drug‐resistant focal epilepsy, rufinamide when used as an add‐on treatment was effective in reducing seizure frequency. However, the trials reviewed were of relatively short duration, the participants were followed up for three to six months and evidence regarding some adverse events is limited and imprecise.
Further evaluation of rufinamide is required to assess the following effects in the long term:
effects on seizures (in terms of "seizure freedom" together with the proportion of people who have certain percentage reduction in seizure episode, and "seizure‐associated mortality");
adverse effects;
effects on cognition;
effects on quality of life;
health economic effects.
Evaluation of the effects of rufinamide in the following scenarios is also required:
rufinamide for generalised epilepsy;
more trials to assess rufinamide for children and adolescents;
rufinamide compared to other add‐on treatments in drug‐resistant focal epilepsy;
-
rufinamide compared with standard antiepileptic drugs such as:
rufinamide as monotherapy in focal epilepsy, and
rufinamide as monotherapy in generalised epilepsy.
Acknowledgements
This review update was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Epilepsy Specialized Register search strategy
rufinamide OR banzel OR "cgp 33101" OR "e 2080" OR inovelon OR "ruf 331" OR xilep AND INREGISTER
Appendix 2. CENTRAL via CRSO search strategy
#1 ("banzel" OR "cgp 33101" OR "e 2080" OR inovelon OR "ruf 331" OR rufinamide OR "xilep"):TI,AB,KY
#2 * NOT INMEDLINE
#3 #1 AND #2
Appendix 3. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials (Lefebvre 2011).
1. rufinamide.mp.
2. exp Epilepsy/
3. exp Seizures/
4. (epilep$ or seizure$ or convuls$).tw.
5. 2 or 3 or 4
6. exp *Pre‐Eclampsia/ or exp *Eclampsia/
7. 5 not 6
8. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
9. clinical trials as topic.sh.
10. trial.ti.
11. 8 or 9 or 10
12. exp animals/ not humans.sh.
13. 11 not 12
14. 1 and 7 and 13
15. remove duplicates from 14
Data and analyses
Comparison 1.
Rufinamide versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ≥ 50% reduction in seizure frequency | 6 | 1759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.44, 2.22] |
| 2 Seizure freedom | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.36, 4.86] |
| 3 Treatment withdrawal | 6 | 1759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.45, 2.31] |
| 4 Adverse effects | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Dizziness | 3 | 1295 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.90, 3.34] |
| 4.2 Fatigue | 3 | 1295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.08, 1.97] |
| 4.3 Headache | 3 | 1228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.08, 1.69] |
| 4.4 Somnolence | 6 | 1759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.44, 2.61] |
| 4.5 Nausea | 3 | 1295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.33, 2.64] |
| 4.6 Vomiting | 4 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [1.80, 4.82] |
| 4.7 Diplopia | 3 | 1295 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.60 [2.53, 8.38] |
Differences between protocol and review
None.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group multi‐centre study. Number of control centres: 65. Country/location: US and Canada. 2 treatment arms: 1 rufinamide, 1 placebo. |
|
| Participants | Participants: adolescents and adults (aged 12‐80 years) with inadequately controlled focal‐onset seizures. Gender: 46.9% male (rufinamide group 47.7%; placebo group 46.1%). Mean age (years): 37.3 (rufinamide group 36.4; placebo group 38.1). Mean weight (kg): 78.2 (rufinamide group 77.4; placebo group 79.0). Ethnic groups: black 9.3%; white 80.1%, Hispanic 7.6%; other 3.0%. Median number of seizures: 13.3 (rufinamide group 13; placebo group 13.8). Duration of epilepsy: not reported. Inclusion criteria: males or females, aged 12‐80 years, with focal‐onset seizures with or without secondarily generalised seizures; person's seizures inadequately controlled on stable doses of up to 3 concomitantly administered AEDs, with no evidence of AED treatment non‐compliance. Exclusion criteria: known generalised epilepsies or history of status epilepticus or seizure clusters in the past year, or if they required felbamate, vigabatrin or rescue benzodiazepines; moreover, if they had clinically significant medical or psychiatric disease, clinically significant ECG abnormality, or a diagnosis of congenital short QT syndrome, psychogenic seizures in the previous year, history of drug abuse and/or positive finding on urinary drug screening, or a history of alcohol abuse in the past 2 years. Diagnostic criteria: established by clinical history, electroencephalography, and CT/MRI of the brain performed within the last 10 years. Comorbidities: none. Comedications: ≤ 3 AEDs. Total people randomised 357: rufinamide group 176; placebo group 181. One participant was excluded from the analysis because required laboratory assessments were not obtained. 61 people withdrew from study: rufinamide group 37; placebo group 24. |
|
| Interventions | Intervention: rufinamide 3200 mg/day Control: placebo. 2‐phase study: 56‐day baseline/screening phase; and 96‐day treatment phase (12‐day titration period followed by 84‐day maintenance phase). |
|
| Outcomes | Primary outcomes (as stated in publication):
Secondary outcomes (as stated in publication):
|
|
| Notes | Stated aim of study: "This randomized study was conducted to evaluate and confirm the efficacy and safety (seizure control and adverse effects) of rufinamide as adjunctive treatment for refractory focal‐onset seizures." Language of publication: English. Commercial funding: yes. Publication status (peer review journal): yes. Publication status (journal supplement): no. Publication status (abstract): no. Funded by Eisai Medical Research. No conflict of interest. NCT00334958 is linked to this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule using blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Allocated participants to each of the 2 treatment groups in a 1:1 ratio. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant and clinical staff blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported. ITT efficacy analysis employed. |
| Selective reporting (reporting bias) | Low risk | Protocol unavailable but appeared all expected and prespecified outcomes were reported. |
| Other bias | High risk | Sponsored by Eisai Inc., the manufacturer of rufinamide. |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group multi‐centre study. Number of control centres: 48. Country/location: 17 centres in US and 31 international centres in Argentina, Chile, France, UK, Germany, Italy, Russia, Slovakia, South Africa, Spain, Switzerland and Uruguay. 2 treatment arms: 1 rufinamide, 1 placebo. |
|
| Participants | Participants: adolescents and adults ≥ 16 years with refractory focal seizures. Gender: 44.4% male (rufinamide group 40.4%; placebo group 48.4%). Mean age (years): 36.8 (rufinamide group 35.8; placebo group 37.9). Mean weight (kg): 74.2 (rufinamide group 73.1; placebo group 75.3). Ethnic groups: black 3.8%; white/Caucasian 85.6%; other 10.5%. Median number of focal seizures: 8.3. Duration of epilepsy: not reported. Inclusion criteria: aged ≥ 16 years, weighed ≥ 18 kg, with focal seizures (including simple focal, complex focal and secondarily generalised seizures), stable dose of 1 or 2 AEDs during 8‐week baseline phase and at least 6 documented focal seizures during 8‐week baseline phase; CT or MRI confirmed absence of progressive brain lesion. Exclusion criteria: treatable cause of seizures (e.g. active infection, neoplasm or metabolic disorder); diagnosis of generalised epilepsy/generalised seizures; history of generalised status epilepticus within 2 months before 8‐week baseline phase; seizures occurring only in a cluster pattern; required use of intermittent benzodiazepines > 2 times per month; evidence or history of clinically significant cardiac, respiratory, hepatic, gastrointestinal, renal, haematological or progressive neurological disorders; clinically significant ECG abnormalities; evidence or history of malignancy; history of psychiatric or mood disorders within the past 6 months requiring medical or electroconvulsive therapy (or both); diagnosis of schizophrenia or psychotic symptomatology; substance abuse (current or historical); history of suicide attempt; prior use of rufinamide; and participation in another clinical trial or use of felbamate within 30 days of 8‐week baseline phase. Diagnostic criteria: EEG or CCTV/EEG with features consistent with a diagnosis of focal seizures. Comorbidities: none. Comedications: ≤ 2 AEDs. Total people randomised 313: rufinamide group 156; placebo group 157. All participants included in analysis. 56 people withdrew from study: rufinamide group 36; placebo group 20. |
|
| Interventions | Intervention: rufinamide 3200 mg/day. Control: placebo. 2‐phase study: 8‐week baseline phase; and 13‐week treatment phase (2‐week titration period followed by 11‐week maintenance phase). |
|
| Outcomes | Primary outcomes (as stated in the publication):
Secondary outcomes (as stated in the publication):
|
|
| Notes | Stated aim of study: "This randomized trial was conducted to evaluate efficacy and safety (seizure control and adverse effects) of rufinamide as adjunctive treatment for refractory focal seizures." Language of publication: English. Commercial funding: yes. Publication status (peer review journal): yes. Publication status (journal supplement): no. Publication status (abstract): no. Funded by Eisai Inc. No conflict of interest. Aldenkamp 2006 (Epilepsia) is a part of this study (AE/ET1 trial) to evaluate the cognitive effects of rufinamide. Brodie 2005 (Epilepsia), Rosenfeld 2006 (Epilepsia) and Vazquez 2000 (Epilepsia) are linked to this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule using blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Allocated participants to each of the 2 treatment groups in a 1:1 ratio. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant and clinical staff blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported. ITT efficacy analysis employed. |
| Selective reporting (reporting bias) | Low risk | Protocol unavailable but appeared all expected and prespecified outcomes were reported. |
| Other bias | High risk | Sponsored by Eisai Inc., the manufacturer of rufinamide. |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group multi‐centre study. Number of control centres: not reported. Country/location: Germany and US. 5 treatment arms: rufinamide 200 mg/day, 400 mg/day, 800 mg/day and 1600 mg/day, and placebo. |
|
| Participants | Participants: adolescents and adults aged 15‐65 years with refractory focal seizures. Gender: 54% males. Mean age (years): 36.1 (rufinamide all doses group 35.8; placebo group 37.3). Mean weight (kg): 72.2 (rufinamide all doses group 71.8; placebo group 74.0). Ethnic groups: not reported. Median number of focal seizures: rufinamide all doses group 11.4; placebo group 11.7. Duration of epilepsy: not reported. Inclusion criteria: inpatients or outpatients, aged 15‐65 years, who had a diagnosis of focal seizures, simple or complex (or both), with or without secondary generalisation, who were receiving stable dosages of 1‐3 AEDs for at least 4 weeks prior to starting the baseline phase and were experiencing ≥ 4 seizures per month during the 6 months prior to the baseline phase. Exclusion criteria: positive pregnancy test, lactation, use of oral/hormonal contraceptives, history of any seizure type other than focal seizure, status epilepticus within 24 months prior to study entry, any degenerative neurological disorder or history of a major psychiatric disorder within 24 months prior to study entry, or a history of suicide attempts or ideation; in addition, clinically relevant abnormalities in screening physical examination or laboratory data; presence of AIDS, acute hepatitis or other clinically relevant medical disorders; alcohol or drug abuse within 12 months prior to study entry; or the use of ethosuximide or felbamate. Comorbidities: none. Comedications: ≤ 3 AEDs. Total people randomised 647: rufinamide 200 mg/day group 127, rufinamide 400 mg/day group 125, rufinamide 800 mg/day group 129, rufinamide 1600 mg/day group 133, and placebo group 133. All participants included in analysis. 93 people withdrew from study: rufinamide 200 mg/day group 16; rufinamide 400 mg/day group 20; rufinamide 800 mg/day group 19; rufinamide 1600 mg/day group 21; and placebo group 17. |
|
| Interventions | Intervention: rufinamide 200 mg/day, rufinamide 400 mg/day, rufinamide 400 mg/day, rufinamide 1600 mg/day. Control: placebo. 2‐phase study: 12‐week baseline phase and 12‐week treatment phase. |
|
| Outcomes | Primary outcomes (as stated in the publication):
Secondary outcomes (as stated in the publication):
|
|
| Notes | Stated aim of study: "This randomized trial was conducted to evaluate efficacy, safety, tolerability (seizure control and adverse effects) and pharmacokinetics of rufinamide as adjunctive treatment for refractory focal seizures." Language of publication: English. Commercial funding: yes. Publication status (peer review journal): yes. Publication status (journal supplement): no. Publication status (abstract): no. Funded by Eisai Inc. No conflict of interest. Elger 2005 (Epilepsia), Elger 2006 (Epilepsia) and Stefan 2000 (Epilepsia) are linked to this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme using block size of 5. |
| Allocation concealment (selection bias) | Low risk | Allocated participants to each of the 5 treatment groups in a 1:1:1:1:1 ratio. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details for blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of outcome assessment blinding not provided. Identical medication with different dosages. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysed on ITT basis. |
| Selective reporting (reporting bias) | Low risk | Data published in full according to protocol. |
| Other bias | High risk | Sponsored by Eisai Inc., the manufacturer of rufinamide. |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group multi‐centre study. Number of control centres: not reported. Country/location: not reported. 2 treatment arms: 1 rufinamide, 1 placebo. |
|
| Participants | Participants: children aged 4‐16 years with refractory focal seizures. Gender: not reported. Mean age: not reported. Ethnic group: not reported. Median number of focal seizures: not reported. Duration of epilepsy: not reported. Inclusion criteria: children aged 4 to 16 years, with a diagnosis of uncontrolled focal seizures who were taking stable dosage of 1 or 2 concomitant AEDs. Exclusion criteria: not reported. Comorbidity: none. Comedication: ≤ 2 AEDs. Total people randomised 269. 1 participant excluded from analysis. 14 people withdrew from study: rufinamide group 10; placebo group 4. |
|
| Interventions | Intervention: rufinamide 45 mg/kg per day. Control: placebo. 2‐phase study: 56‐day baseline/screening phase, and 91‐day treatment phase (14‐day titration phase followed by 77‐day maintenance phase). |
|
| Outcomes | Primary outcomes (as stated in the publication):
Secondary outcomes (as stated in the publication):
|
|
| Notes | Stated aim of study: "This study aimed to assess the efficacy and safety (seizure control and adverse effects) of rufinamide as adjunctive therapy in paediatric patients with inadequately controlled primary focal seizures." Language of publication: English. Commercial funding: yes. Publication status (peer review journal): no. Publication status (journal supplement): no. Publication status (abstract): yes. Supported by Eisai Inc. No conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomisation provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information available to make judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information available to make judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reasons reported for exclusion of 1 participant from analysis. |
| Selective reporting (reporting bias) | Low risk | Appeared all expected and prespecified outcomes were reported. |
| Other bias | High risk | Sponsored by Eisai Inc., the manufacturer of rufinamide. |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group multi‐centre study. Number of control centres: 36. Country/location: Belgium, Brazil, Germany, Hungary, Italy, Norway, Poland, Spain and US. 2 treatment arms: 1 rufinamide, 1 placebo. |
|
| Participants | Participants: aged 4‐30 years with Lennox‐Gastaut syndrome. Gender: 62.3% males (rufinamide group 62.2%; placebo group 62.5%). Median age (years): 12.0 (rufinamide group 13.0; placebo group 10.5%). Median weight (kg): 34.7 (rufinamide group 35.9; placebo group 33.5). Ethnic groups: white 83.3%, black 7.2%, other 9.5%. Median number of seizures: rufinamide group 290; placebo group 205. Duration of epilepsy (years): 7.7 (rufinamide group 7.9; placebo group 7.5). Inclusion criteria: aged 4‐30 years with established Lennox‐Gastaut syndrome; minimum of 90 seizures in month before the 28‐day baseline period; EEG within 6 months of study entry demonstrating a pattern of slow spike‐and‐wave complexes (< 2.5 Hz); weight ≥ 18 kg; fixed‐dose regimen of 1‐3 concomitant AEDs during the baseline period; and CT scan or MRI study confirming absence of a progressive lesion. Exclusion criteria: ≥ 3 AEDs; pregnant or not using adequate contraception; correctable aetiology of their seizures (active infection, neoplasm, metabolic disturbance); history of generalised tonic‐clonic status epilepticus within 30 days before baseline; history of any clinically significant non‐neurological medical condition. Diagnostic criteria: Lennox‐Gastaut syndrome was diagnosed based on a history of tonic or atonic (or both) seizures and atypical absence seizures and slow spike‐and‐wave complex patterns on EEG within 6 months before the baseline period. Comorbidities: none. Comedications: ≤ 2 AEDs. Total people randomised 139 (1 patient was excluded from analysis): rufinamide group 74; placebo group 64. 15 people withdrew from the study: rufinamide group 10; placebo group 5. |
|
| Interventions | Intervention: rufinamide 45 mg/kg (dose by bodyweight: 1000 mg, 1800 mg, 2400 mg and 3200 mg) per day. Control: placebo. 2‐phase study: 28‐day pre‐randomisation baseline phase and 84‐day treatment phase (14‐day titration phase followed by 70‐day maintenance phase). |
|
| Outcomes | Primary outcomes (as stated in the publication):
Secondary outcomes (as stated in the publication):
|
|
| Notes | Stated aim of study: "This randomized trial was conducted to evaluate efficacy and tolerability (seizure control and adverse effects) of rufinamide adjunctive therapy in patients with Lennox‐Gastaut syndrome." Language of publication: English. Commercial funding: yes. Publication status (peer review journal): yes. Publication status (journal supplement): no. Publication status (abstract): no. Sponsored by Eisai Pharmaceutical and conducted by Novartis Pharmaceutical. No conflict of interest. Glauser 2005 (Annals of Neurology), Glauser 2005 (Neurology), Glauser 2007 (Epilepsia), Glauser 2009 (Epilepsia), Kluger 2006 (Epilepsia), Krauss 2007 (Epilepsia), McMurray 2016 (Neurology & Therapy), Striano 2015 (Epilepsia) are linked to this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised in blocks of 4 to receive either rufinamide or matching placebo. |
| Allocation concealment (selection bias) | Low risk | Assigned sequential numbers at each site during the first visit. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical tablets and packaging. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of outcome assessment blinding not provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported. ITT efficacy analysis employed. |
| Selective reporting (reporting bias) | Low risk | Protocol unavailable but appeared all expected and prespecified outcomes were reported. |
| Other bias | High risk | Sponsored by Eisai Inc., the manufacturer of rufinamide. |
| Methods | Randomised, double‐blind, placebo‐controlled, multi‐centre study in Japan. Number of control centres: not reported. Country/location: Japan. 2 treatment arms: 1 rufinamide, 1 placebo. |
|
| Participants | Participants: people aged 4‐30 years with Lennox‐Gastaut syndrome. Gender: 62% males (rufinamide group 60.7%; placebo group 63.3%). Mean age (years): 15 (rufinamide group 16.0; placebo group 13.9). Mean weight (kg): 34.7 (rufinamide group 39.0; placebo group 40.9). Ethnic groups: not reported. Median number of seizures: rufinamide group 253; placebo group 296.7. Duration of epilepsy (years): 9.9 (rufinamide group 10.5; placebo group 9.3 years). Inclusion criteria: aged 4‐30 years, weighing ≥ 15 kg with established Lennox‐Gastaut syndrome. Exclusion criteria: people with experienced tonic‐clonic status epilepticus during baseline period; if they had other clinically severe diseases or ECG/laboratory abnormalities. Diagnostic criteria: Lennox‐Gastaut syndrome diagnosed based on history of tonic or atonic (or both) seizures and atypical absence seizures and slow spike‐and‐wave complex patterns on EEG within 6 months before the baseline period. Comorbidities: none. Comedications: ≤ 3 AEDs. Total people randomised 59: rufinamide group 29; placebo group 30. 1 participant assigned to rufinamide group excluded from analysis. 5 people withdrew from study: rufinamide group 4; placebo group 1. |
|
| Interventions | Intervention: rufinamide 45 mg/kg (dose by bodyweight: 1000 mg, 1800 mg, 2400 mg or 3200 mg) per day. Control: placebo. 2‐phase study: 4‐week baseline phase and 12‐week treatment phase (2‐week titration phase followed by 10‐week maintenance phase). In addition, a 4th phase: either a follow‐up visit or entry into an open‐label extension. |
|
| Outcomes | Primary outcomes (as stated in the publication):
Secondary outcomes (as stated in the publication):
|
|
| Notes | Stated aim of study: "This randomized trial in Japan was conducted to evaluate efficacy and tolerability (seizure control and adverse effects) and pharmacokinetics of rufinamide as an adjunctive therapy in patients with Lennox‐Gastaut syndrome." Language of publication: English. Commercial funding: yes. Publication status (peer review journal): yes. Publication status (journal supplement): no. Publication status (abstract): no. Sponsored by Eisai Pharmaceutical. No conflict of interest. Study design referred to previous trial of rufinamide for Lennox‐Gastaut syndrome (Glauser 2008). NCT01140951 and Ohtsuka 2016 (Epilepsy Research) were linked to this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule that assigned each participant to rufinamide group or placebo group in a 1:1 ratio. |
| Allocation concealment (selection bias) | Low risk | Participants randomised to receive rufinamide or placebo in a 1:1 ratio according to bodyweight. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant and clinical staff blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators blinded. Identical tablets and packaging. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All evaluated on ITT bias. |
| Selective reporting (reporting bias) | Low risk | Appeared all expected and prespecified outcomes were reported. |
| Other bias | High risk | Sponsored by Eisai Inc., the manufacturer of rufinamide. |
AED: antiepileptic drug; CCTV: closed‐circuit television; CT: computer tomography; ECG: electrocardiograph; EEG: electroencephalogram; ITT: intention to treat; MRI: magnetic resonance imaging.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benedict 2010 | Not an RCT. |
| Biton 2005 | Ineligible population (people with primary generalised tonic‐clonic). |
| Critchley 2005 | Ineligible population (healthy people). |
| Kim 2012 | Not an RCT. |
| Kluger 2007 | Not an RCT. |
| Knupp 2016 | Not an RCT. |
| Lesser 2005 | No rufinamide in add‐on. |
| Madeddu 2013 | Ineligible population (people with epileptic encephalopathies secondary to complex brain malformations). |
| Palhagen 2001 | Ineligible population (participants with no refractory epilepsy). |
| Racine 2000 | Conference (4th European Congress on Epileptology): abstract and full paper not available. |
| Todorov 2005 | No rufinamide in add‐on. |
| Xu 2016 | Ineligible population (healthy people). |
RCT: randomised controlled trial;
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Safety and Pharmacokinetic Profile of Rufinamide in Pediatric Patients Aged Less than 4 Years with Lennox‐Gastaut Syndrome: an Interim Analysis from a Multicenter, Randomized, Active‐Controlled, Open‐Label Study. |
| Methods | Randomised, active‐controlled, open‐label multi‐centre study. Number of control centres: 20. Country/location: North America and the EU. 2 treatment arms: 1 rufinamide, 1 any other approved AED. |
| Participants | Participants: children aged 1 to < 4 years. Gender: 63.9% boys; 36.1% girls. Mean age (months): 29.2 (rufinamide group 28.3; any other AED group 31.3). Inclusion criteria: aged 1 to < 4 years; clinical diagnosis of LGS, which might include the presence of multiple types of seizures progressively enriching the clinical picture, a slow background EEG rhythm, slow spike‐wave pattern (< 3 Hz) or the presence of polyspikes, or both. Exclusion criteria: diagnosed with benign myoclonic epilepsy of infancy, atypical benign focal epilepsy (pseudo‐Lennox syndrome), or continuous spike‐waves of slow sleep, as well as other epilepsy syndromes not suggesting the electroclinical profile of children within the LGS spectrum; additionally, children with familial short QT syndrome and with prior treatment with rufinamide. Comedications: ≤ 3 AEDs. Total children randomised 37: rufinamide group 25; any other approved AED group 12. 1 child assigned to any other AED group was excluded from analysis. |
| Interventions | Intervention: rufinamide up to 45 mg/kg per day. Control: any other AED. 2‐phase study: 8‐week pre‐randomisation phase included a screening period and baseline visit, and a 106‐week randomisation phase included titration and maintenance. Interim analysis at 6 months. |
| Outcomes | Primary outcomes:
|
| Starting date | June 2011. |
| Contact information | aarzimanoglou@orange.fr (A. Arzimanoglou). |
| Notes | Funded by Eisai Inc. |
AED: antiepileptic drug; EEG: electroencephalogram; LGS: Lennox‐Gastaut syndrome.
Contributions of authors
MP was primarily responsible for the writing of this review and completing data extraction and 'Risk of bias' assessments.
AGM provided guidance and manuscript feedback.
HP assessed the studies for eligibility, extracted data and assessed risk of bias.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National health Service (NHS) or the Department of Health.
Declarations of interest
MP: none known.
AGM: a consortium of pharmaceutical companies (GSK, Eisai, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to the University of Liverpool. Professor Tony Marson is part funded by National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care North West Coast (NIHR CLAHRC NWC).
HP: none known.
New
References
References to studies included in this review
- Biton V, Krauss G, Vasquez‐Santana B, Bibbiani F, Mann A, Perdomo C, et al. A randomized, double‐blind, placebo‐controlled, parallel‐group study of rufinamide as adjunctive therapy for refractory partial‐onset seizures. Epilepsia 2011;52(2):234‐42. [DOI: 10.1111/j.1528-1167.2010.02729.x; PUBMED: 20887365] [DOI] [PubMed] [Google Scholar]; NCT00334958. Rufinamide Given as Adjunctive Therapy in Patients With Refractory Partial Seizures. https://ClinicalTrials.gov/show/NCT003349582006.
- Aldenkamp AP, Alpherts WCJ. The effect of the new antiepileptic drug rufinamide on cognitive functions. Epilepsia 2006;47(7):1153‐9. [DOI: 10.1111/j.1528-1167.2006.00589.x; ISSN: 0013‐9580; PUBMED: 16886978] [DOI] [PubMed] [Google Scholar]; Brodie MJ, Rosenfeld W, Vasquez B, Sachdeo R, Ko D, Perdomo C, et al. Efficacy and safety of rufinamide as adjunctive therapy in adult patients with inadequately controlled partial seizures. Epilepsia 2005;46 Suppl 8:171, Abstract no: 2.239. 15679497 [Google Scholar]; Brodie MJ, Rosenfeld W, Vazquez B, Sachdeo R, Perdomo C, Mann A, et al. Rufinamide for the adjunctive treatment of partial seizures in adults and adolescents: a randomized placebo‐controlled trial. Epilepsia 2009;50(8):1899‐909. [DOI: 10.1111/j.1528-1167.2009.02160.x; PUBMED: 19490053] [DOI] [PubMed] [Google Scholar]; Rosenfeld W, Brodie M, Vazquez B, Sachdeo R, Ko D, Perdomo C, et al. Efficacy and safety of rufinamide as adjunctive therapy in adult patients with inadequately controlled partial seizures. Epilepsia 2006;47 Suppl 3:140, Abstract no: p538. [Google Scholar]; Vazquez BR, Sachdeo R, Maxoutova AL, Beydoun A, Karolchyk MA, Mesenbrink PG. Efficacy and safety of rufinamide as adjunctive therapy in adult patients with therapy‐resistant partial‐onset seizures. Epilepsia 2000;41 Suppl 7:255, Abstract no: L.09. [Google Scholar]
- Elger C, Stefan H, Perdomo C, Arroyo S. Dose‐range relationships of rufinamide in patients with inadequately controlled partial seizures. Epilepsia 2005;46 Suppl 8:83‐4, Abstract no: 1.201. [Google Scholar]; Elger C, Stefan H, Perdomo C, Arroyo S. Dose‐range relationships of rufinamide in patients with inadequately controlled partial seizures. Epilepsia 2006;47 Suppl 3:140, Abstract no: p539. [Google Scholar]; Elger CE, Stefan H, Mann A, Narurkar M, Sun Y, Perdomo C. A 24‐week multicenter, randomized, double‐blind, parallel‐group, dose ranging study of rufinamide in adults and adolescents with inadequately controlled partial seizures. Epilepsy Research 2010;88(2‐3):255‐63. [DOI: 10.1016/j.eplepsyres.2009.12.003; PUBMED: 20061123] [DOI] [PubMed] [Google Scholar]; Stefan H, Remy C, Guberman A, Thomson A, Arbelaiz R, Karolchyk MA. A multicenter double‐blind randomized placebo‐controlled 5‐arm, parallel‐group trial of rufinamide in patients with therapy‐resistant partial seizures. Epilepsia 2000;41 Suppl Florence:39. 11156510 [Google Scholar]
- Glauser T, Arzimanoglou A, Litzinger M, Frank LM, Vigevano F, Perdomo C. Efficacy and safety of rufinamide as adjunctive therapy for inadequately controlled partial seizures in pediatric patients. Epilepsia 2005;46 Suppl 8:194‐5. [Google Scholar]
- Glauser T, Kluger G, Krauss G, Arroyo S. Effects of rufinamide on the frequency of different seizure types in patients with Lennox‐Gastaut syndrome: a long‐term study. Epilepsia 2007;48 Suppl 7:156, Abstract no: p415. [Google Scholar]; Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Rufinamide for generalized seizures associated with Lennox‐Gastaut Syndrome. Neurology 2008;70(21):1950‐8. [DOI: 10.1212/01.wnl.0000303813.95800.0d; PUBMED: 18401024] [DOI] [PubMed] [Google Scholar]; Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Short‐term and long‐term efficacy and safety of rufinamide as adjunctive therapy in patients with inadequately controlled Lennox‐Gastaut syndrome. Annals of neurology 2005;58 Suppl 9:S92, Abstract no: M15. [Google Scholar]; Glauser T, Kluger G, Sachedo R, Krauss G, Perdomo C, Arroyo S. Efficacy and safety of rufinamide adjunctive therapy in patients with Lennox‐Gastaut syndrome (LGS): a multicenter, randomized, double‐blind, placebo‐controlled, parallel trial. Neurology 2005;64(10):1826, Abstract no: LBS.001. [Google Scholar]; Glauser T, Santana BV, Wang W, Narurkar M. Early and sustained response to rufinamide as adjunctive therapy for seizures associated with Lennox‐Gastaut syndrome. Epilepsia 2009;50 Suppl 11:261, Abstract no: 2.229. [Google Scholar]; Kluger G, Glauser T, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Short‐term and long‐term efficacy and safety of rufinamide as adjunctive therapy in patients with inadequately controlled Lennox‐Gastaut syndrome. Epilepsia 2006;47 Suppl 3:139, Abstract no: p537. [Google Scholar]; Krauss GL, Glauser T, Kluger G, Arroyo S. Long‐term safety of rufinamide in patients with Lennox‐Gastaut syndrome. Epilepsia 2007;48 Suppl 6:359, Abstract no: 3.303. 17295631 [Google Scholar]; McMurray R, Striano P. Treatment of Adults with Lennox‐Gastaut Syndrome: Further Analysis of Efficacy and Safety/Tolerability of Rufinamide. Neurology & Therapy 2016;5(1):35‐43. [DOI: 10.1007/s40120-016-0041-9; ISSN: 2193‐8253 2193‐6536; PUBMED: 26861566] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01146951. A Placebo‐Controlled, Double‐Blind Comparative Study of E2080 in Lennox‐Gastaut Syndrome Patients (Study E2080‐J081‐304). https://ClinicalTrials.gov/show/NCT011469512010. ; Ohtsuka Y, Yoshinaga H, Shirasaka Y, Takayama R, Takano H, Iyoda K. Long‐term safety and seizure outcome in Japanese patients with Lennox‐Gastaut syndrome receiving adjunctive rufinamide therapy: An open‐label study following a randomized clinical trial. Epilepsy Research 2016;121:1‐7. [DOI: 10.1016/j.eplepsyres.2016.01.002; PUBMED: 26827266] [DOI] [PubMed] [Google Scholar]; Ohtsuka Y, Yoshinaga H, Shirasaka Y, Takayama R, Takano H, Iyoda K. Rufinamide as an adjunctive therapy for Lennox‐Gastaut syndrome: a randomized double‐blind placebo‐controlled trial in Japan. Epilepsy Research 2014;108(9):1627‐36. [DOI: 10.1016/j.eplepsyres.2014.08.019; PUBMED: 25219353] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Benedict A, Verdian L, Maclaine G. The cost effectiveness of rufinamide in the treatment of Lennox‐Gastaut syndrome in the UK. Pharmacoeconomics 2010;28(3):185‐99. [DOI] [PubMed] [Google Scholar]
- Biton V, Sachdeo R, Rosenfeld W, Schachter S, Perdomo C, Arroyo S. Efficacy and safety of adjunctive rufinamide in patients with inadequately controlled primary generalized tonic‐clonic seizures. Epilepsia 2005;46(8):206. [Google Scholar]
- Critchley D, Fuseau E, Perdomo C, Arroyo S. Pharmacokinetic and pharmacodynamic parameters of adjunctive rufinamide in patients with Lennox‐Gastaut syndrome. Epilepsia 2005;46 Suppl 8:209. [Google Scholar]
- Kim SH, Eun SH, Kang HC, Kwon EJ, Byeon JH, Lee YM. Rufinamide as an adjuvant treatment in children with Lennox‐Gastaut syndrome. Seizure 2012;21(4):288‐91. [DOI] [PubMed] [Google Scholar]
- Kluger G, Bauer B. Role of rufinamide in the management of Lennox‐Gastaut syndrome (childhood epileptic encephalopathy). Neuropsychiatric Disease & Treatment 2007;3(1):3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knupp KG, Leister E, Coryell J, Nickels KC, Ryan N, Juarez‐Colunga E, et al. Pediatric Epilepsy Research Consortium. Response to second treatment after initial failed treatment in a multicenter prospective infantile spasms cohort. Epilepsia 2016;57(11):1834‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser RP, Biton V, Sackellares JC, Ness PC, Perdomo C, Arroyo S. Efficacy and safety of rufinamide monotherapy for treatment of patients with refractory partial seizures. Epilepsia 2005;46 Suppl 8:177‐8. [Google Scholar]
- Madeddu F, Moavero R, Battaglia D, Matricardi S, Coppola G, Balestri M. Efficacy of rufinamide in epileptic encephalopathies secondary to complex brain malformations. Bollettino ‐ Lega Italiana Contro l'Epilessia 2013;145:278‐80. [Google Scholar]
- Palhagen S, Canger R, Henriksen O, Parys JA, Riviere ME, Karolchyk MA. Rufinamide: a double‐blind, placebo‐controlled proof of principle trial in patients with epilepsy. Epilepsy Research 2001;43(2):115‐24. [DOI] [PubMed] [Google Scholar]
- Racine A, Rouan MC, Chang SW, Yeh CM, Karolchyk MA. Population pharmacokinetics and drug‐drug interactions of rufinamide in a multicenter, double‐blind, randomized, placebo‐controlled, 5‐arm parallel trial in patients with partial seizures on up to three concomitant antiepileptic drugs. Epilepsia 2000;41 Suppl:149‐50. [Google Scholar]
- Todorov A, Biton V, Krauss GL, Perdomo C, Arroyo S. Efficacy and safety of high‐versus low‐dose rufinamide monotherapy in patients with inadequately controlled partial seizures. Epilepsia 2005;46 Suppl 8:218‐9. [Google Scholar]
- Xu M, Ni Y, Zhou Y, He X, Li H, Chen H. Pharmacokinetics and tolerability of rufinamide following single and multiple oral doses and effect of food on pharmacokinetics in healthy Chinese subjects. European Journal of Drug Metabolism and Pharmacokinetics 2016;41(5):541‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Arzimanoglou A, Ferreira JA, Satlin A, Mendes S, Williams B, Critchley D, et al. Safety and pharmacokinetic profile of rufinamide in pediatric patients aged less than 4 years with Lennox‐Gastaut syndrome: an interim analysis from a multicenter, randomized, active‐controlled, open‐label study. European Journal of Paediatric Neurology: EJPN 2016;20(3):393‐402. [DOI] [PubMed] [Google Scholar]
Additional references
- Aldenkamp AP, Alpherts WCJ. The effect of the new antiepileptic drug rufinamide on cognitive functions. Epilepsia 2006;47(7):1153‐9. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Rosenfels W, Vasquez B, Sachdeo R, Perdomo C. Efficacy and safety of rufinamide as adjunctive therapy in adult patients with inadequately controlled partial seizures. Epilepsia 2005;46 Suppl 8:171. 15679497 [Google Scholar]
- Coppola G. Update on rufinamide in childhood epilepsy. Neuropsychiatric Disease and Treatment 2011;7:399‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elger C, Stefan H, Perdomo C, Arroyo S. Dose‐range relationships of rufinamide in patients with inadequately controlled partial seizures. Epilepsia 2005;46 Suppl 8:83‐4. [Google Scholar]
- Elger C, Stefan H, Perdomo C, Arroyo S. Dose‐range relationships of rufinamide in patients with inadequately controlled partial seizures. Epilepsia 2006;47 Suppl 3:140. [Google Scholar]
- Glauser T, Kluger G, Krauss G, Arroyo S. Effects of rufinamide on the frequency of different seizure types in patients with Lennox‐Gastaut syndrome: a long‐term study. Epilepsia 2007;48 Suppl 7:156. [Google Scholar]
- Glauser T, Santana BV, Wang W, Narurkar M. Early and sustained response to rufinamide as adjunctive therapy for seizures associated with Lennox‐Gastaut syndrome. Epilepsia 2009;50 Suppl 11:261. [Google Scholar]
- Granata T, Marchi N, Carlton E, Chosh C, Gonzalez‐Martinez J, Alexopoulous A, et al. Management of the patient with medically refractory epilepsy. Expert Review of Neurotherapeutics 2009;9(12):1791‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. the GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Hsieh DT, Thiele EA. Efficacy and safety of rufinamide in pediatric epilepsy. Therapeutic Advances in Neurological Disorders 2013;6(3):189‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILAE Commission on Epidemiology and Prognosis. Guidelines for epidemiologic studies on epilepsy. Epilepsia 1993;34:592‐6. [DOI] [PubMed] [Google Scholar]
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
- Kluger G, Glauser T, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Short‐term and long‐term efficacy and safety of rufinamide as adjunctive therapy in patients with inadequately controlled Lennox‐Gastaut syndrome. Epilepsia 2006;47 Suppl 3:139. [Google Scholar]
- Krauss GL, Glauser T, Kluger G, Arroyo S. Long‐term safety of rufinamide in patients with Lennox‐Gastaut syndrome. Epilepsia 2007;48 Suppl 6:359. 17295631 [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- McMurray R, Striano P. Treatment of adults with Lennox‐Gastaut syndrome: further analysis of efficacy and safety/tolerability of rufinamide. Neurology and Therapy 2016;5(1):35‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbiani F. Rufinamide given as adjunctive therapy in patients with refractory partial seizures. clinicaltrials.gov/show/NCT00334958 Date first registered: 8 June 2006.
- Takano H. A placebo‐controlled, double‐blind comparative study of E2080 in Lennox‐Gastaut syndrome patients. clinicaltrials.gov/show/NCT01146951 Date first registered: 22 June 2010.
- Ohtsuka Y, Yoshinaga H, Shirasaka Y, Takayama R, Takano H, Iyoda K. Long‐term safety and seizure outcome in Japanese patients with Lennox‐Gastaut syndrome receiving adjunctive rufinamide therapy: an open‐label study following a randomized clinical trial. Epilepsy Research 2016;121:1‐7. [DOI] [PubMed] [Google Scholar]
- Panebianco M, Rigby A, Marson AG, Pulman J. Vagus nerve stimulation for partial seizures. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD002896.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld W, Brodie M, Vazquez B, Sachdeo R, Ko D, Perdomo C. Efficacy and safety of rufinamide as adjunctive therapy in adult patients with inadequately controlled partial seizures. Epilepsia 2006;47 Suppl 3:140. [Google Scholar]
- Stefan H, Remy C, Guberman A, Thomson A, Arbelaiz R, Karolchyk MA. A multicenter double‐blind randomized placebo‐controlled 5‐arms, parallel‐group trial of rufinamide in patients with therapy‐resistant partial seizures. Epilepsia 2000;41(Suppl):39. 11156510 [Google Scholar]
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in literature. Journal of Clinical Epidemiology 2000;53(11):1119‐29. [DOI] [PubMed] [Google Scholar]
- Striano P, McMurray R. Rufinamide as adjunctive treatment for adults with Lennox‐Gastaut syndrome: subgroup analysis from a phase III trial. Epilepsia 2015;56(Suppl 1):211. [Google Scholar]
- Vazquez BR, Sachdeo R, Maxoutova AL, Beydoun A, Karolchyk MA, Mesenbrink PG. Efficacy and safety of rufinamide as adjunctive therapy in adult patients with therapy‐resistant partial‐onset seizures. Epilepsia 2000;41(Suppl 7):255. [Google Scholar]
- Wheless J, Conry J, Krauss G, Mann A, LoPresti A, Narurkar M. Safety and tolerability of rufinamide in children with epilepsy: a pooled analysis of seven clinical trials. Journal of Child Neurology 2009;50:1158‐66. [DOI] [PubMed] [Google Scholar]
- Wheless JW, Vazquez B. Rufinamide: a novel broad‐spectrum antiepileptic drug. Epilepsy Currents 2010;10(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Panebianco M, Marson AG. Rufinamide add‐on therapy for refractory epilepsy. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011772] [DOI] [PMC free article] [PubMed] [Google Scholar]


