Abstract
Background
Alcohol dependence is a major public health problem characterized by recidivism, and medical and psychosocial complications. The co‐occurrence of major depression in people entering treatment for alcohol dependence is common, and represents a risk factor for morbidity and mortality, which negatively influences treatment outcomes.
Objectives
To assess the benefits and risks of antidepressants for the treatment of people with co‐occurring depression and alcohol dependence.
Search methods
We searched the Cochrane Drugs and Alcohol Group Specialised Register (via CRSLive), Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase from inception to July 2017. We also searched for ongoing and unpublished studies via ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/).
All searches included non‐English language literature. We handsearched references of topic‐related systematic reviews and the included studies.
Selection criteria
Randomized controlled trials and controlled clinical trials comparing antidepressants alone or in association with other drugs or psychosocial interventions (or both) versus placebo, no treatment, and other pharmacological or psychosocial interventions.
Data collection and analysis
We used standard methodological procedures as expected by Cochrane.
Main results
We included 33 studies in the review (2242 participants). Antidepressants were compared to placebo (22 studies), psychotherapy (two studies), other medications (four studies), or other antidepressants (five studies). The mean duration of the trials was 9.9 weeks (range 3 to 26 weeks). Eighteen studies took place in the USA, 12 in Europe, two in Turkey, and one in Australia. The antidepressant included in most of the trials was sertraline; other medications were amitriptyline, citalopram, desipramine, doxepin, escitalopram, fluoxetine, fluvoxamine, imipramine, mianserin, mirtazepine, nefazodone, paroxetine, tianeptine, venlafaxine, and viloxazine. Eighteen studies were conducted in an outpatient setting, nine in an inpatient setting, and six in both settings. Psychosocial treatment was provided in 18 studies. There was high heterogeneity in the selection of outcomes and the rating systems used for diagnosis and outcome assessment.
Comparing antidepressants to placebo, low‐quality evidence suggested that antidepressants reduced the severity of depression evaluated with interviewer‐rated scales at the end of trial (14 studies, 1074 participants, standardized mean difference (SMD) ‐0.27, 95% confidence interval (CI) ‐0.49 to ‐0.04). However, the difference became non‐significant after the exclusion of studies with a high risk of bias (SMD ‐0.17, 95% CI ‐0.39 to 0.04). In addition, very low‐quality evidence supported the efficacy of antidepressants in increasing the response to the treatment (10 studies, 805 participants, risk ratio (RR) 1.40, 95% Cl 1.08 to 1.82). This result became non‐significant after the exclusion of studies at high risk of bias (RR 1.27, 95% CI 0.96 to 1.68). There was no difference for other relevant outcomes such as the difference between baseline and final score, evaluated using interviewer‐rated scales (5 studies, 447 participants, SMD 0.15, 95% CI ‐0.12 to 0.42).
Moderate‐quality evidence found that antidepressants increased the number of participants abstinent from alcohol during the trial (7 studies, 424 participants, RR 1.71, 95% Cl 1.22 to 2.39) and reduced the number of drinks per drinking days (7 studies, 451 participants, mean difference (MD) ‐1.13 drinks per drinking days, 95% Cl ‐1.79 to ‐0.46). After the exclusion of studies with high risk of bias, the number of abstinent remained higher (RR 1.69, 95% CI 1.18 to 2.43) and the number of drinks per drinking days lower (MD ‐1.21 number of drinks per drinking days, 95% CI ‐1.91 to ‐0.51) among participants who received antidepressants compared to those who received placebo. However, other outcomes such as the rate of abstinent days did not differ between antidepressants and placebo (9 studies, 821 participants, MD 1.34, 95% Cl ‐1.66 to 4.34; low‐quality evidence).
Low‐quality evidence suggested no differences between antidepressants and placebo in the number of dropouts (17 studies, 1159 participants, RR 0.98, 95% Cl 0.79 to 1.22) and adverse events as withdrawal for medical reasons (10 studies, 947 participants, RR 1.15, 95% Cl 0.65 to 2.04).
There were few studies comparing one antidepressant versus another antidepressant or antidepressants versus other interventions, and these had a small sample size and were heterogeneous in terms of the types of interventions that were compared, yielding results that were not informative.
Authors' conclusions
We found low‐quality evidence supporting the clinical use of antidepressants in the treatment of people with co‐occurring depression and alcohol dependence. Antidepressants had positive effects on certain relevant outcomes related to depression and alcohol use but not on other relevant outcomes. Moreover, most of these positive effects were no longer significant when studies with high risk of bias were excluded. Results were limited by the large number of studies showing high or unclear risk of bias and the low number of studies comparing one antidepressant to another or antidepressants to other medication. In people with co‐occurring depression and alcohol dependence, the risk of developing adverse effects appeared to be minimal, especially for the newer classes of antidepressants (such as selective serotonin reuptake inhibitors). According to these results, in people with co‐occurring depression and alcohol dependence, antidepressants may be useful for the treatment of depression, alcohol dependence, or both, although the clinical relevance may be modest.
Plain language summary
Antidepressants for the treatment of people with co‐occurring depression and alcohol dependence
Review question
This review investigated whether antidepressants reduce the severity of depression or alcohol dependence (or both) in people with co‐occurring depression and alcohol dependence.
Background
The co‐occurrence of major depression in people entering treatment for alcohol dependence is common and increases the severity of the condition reducing the effectiveness of treatments. Treatment of these people with medicines is challenging. In this review, we compared the results obtained by people with co‐occurring depression and alcohol dependence treated with antidepressant medicines to those treated with placebo (a sham/pretend treatment) or other treatments.
Search date
The evidence is current up to July 2017.
Study characteristics
We identified 33 medical trials involving 2242 participants: 68% were male, and the mean age was 42 years.
Most studies compared antidepressants to placebo (22 studies), but some compared one antidepressant to antidepressant (five studies), to another type of medicine (four studies), or to psychotherapy (a talking treatment; two studies). The average duration of the trials was 10 weeks (range 3 to 26 weeks). A total of 18 trials took place in the USA, and the others were in Europe, Turkey, and Australia. The antidepressant used in most of the trials was sertraline; the others were: amitriptyline, citalopram, desipramine, doxepin, escitalopram, fluoxetine, fluvoxamine, imipramine, mianserin, mirtazepine, nefazodone, paroxetine, tianeptine, venlafaxine, and viloxazine. The studies used 49 different rating scales and varied in terms of design, quality, participant characteristics, tested medicines, services provided, and treatments administered.
A total of 19 studies reported the source of funding (public funds: six studies; pharmaceutical industry: two studies; both funds: 10 studies).
Only four trials reported a declaration of the authors reporting possible conflicts of interest.
Key results
In the 22 studies comparing antidepressants to placebo, antidepressants may have reduced the severity of depression but we are uncertain whether it increased the number of people with clinical beneficial effects from the reduction of depression severity (response to treatment, i.e. people who halved the severity of depression). However, we found no difference between antidepressants and placebo in other relevant outcomes related to the severity of depression, such as the number of people without depression at the end of the trial (remission).
In addition, we found that the administration of antidepressants probably reduced alcohol consumption evaluated as the number of participants abstinent during the treatment (higher among participants who received antidepressants compared to placebo) and the number of drinks consumed per drinking days (lower among participants who received antidepressants compared to placebo). However, similarly to what we found for the severity of depression, we also observed that the administration of antidepressants did not affect other relevant outcomes related to alcohol dependence, such as the rate of abstinent days, number of heavy drinkers, and time before first relapse.
In terms of safety issues, the rate of people withdrawing from treatment due to side effects (undesirable effects such as dry mouth) may not differ between antidepressants and placebo.
There were few studies comparing one antidepressant to another antidepressant or to other interventions, and these had a small number of participants and the same comparison was not made by more than one study, and were therefore not informative.
Quality of evidence
The quality of the included studies was low or moderate for depression severity, abstinence from alcohol, rate of people withdrawal for medical reasons, and dropouts. In subgroup analyses, in the case of single types of medicines, and comparisons with other medicines, the findings of the review were limited by the small number of available studies.
Authors' conclusions
There is low‐quality evidence supporting the use of antidepressants in the treatment of people with co‐occurring depression and alcohol dependence. Antidepressants have positive effects on certain relevant outcomes related to depression and alcohol use but not on equally relevant other outcomes. However, the risk of developing side effects appears to be minimal, especially for the newer classes of antidepressants.
Summary of findings
Summary of findings for the main comparison. Antidepressants compared to placebo: all studies for the treatment of people with co‐occurring depression and alcohol consumption.
| Antidepressants compared to placebo: all studies | ||||||
|
Patient or population: people with co‐occurring depression and alcohol dependence Settings: unknown Intervention: antidepressants Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Antidepressants | |||||
| Depression severity: final score (interviewer‐rated scales) | ‐ | The mean depression: final score (interviewer‐rated scales) ‐ all studies in the intervention groups was 0.27 standard deviations lower (0.49 lower to 0.04 lower) |
‐ | 1074 (14 studies) | ⊕⊕⊝⊝ Low1,2 | ‐ |
| Response to antidepressive treatment | Study population | RR 1.40 (1.08 to 1.82) | 805 (10 studies) | ⊕⊝⊝⊝ Very low3,4,5 | ‐ | |
| 481 per 1000 | 674 per 1000 (520 to 876) | |||||
| 392 per 1000 | 521 per 1000 (416 to 659) | |||||
| Consumption of alcohol: abstinent days (%) | ‐ | The mean alcohol: abstinent days (%) ‐ all studies in the intervention groups was 1.34 higher (1.66 lower to 4.34 higher) |
‐ | 821 (9 studies) | ⊕⊕⊝⊝ Low6,7 | ‐ |
| Consumption of alcohol: abstinent participants (number) | Study population | RR 1.71 (1.22 to 2.39) | 424 (7 studies) | ⊕⊕⊕⊝ Moderate8,9 | ‐ | |
| 199 per 1000 | 340 per 1000 (243 to 476) | |||||
| 188 per 1000 | 321 per 1000 (229 to 449) | |||||
| Consumption of alcohol: drinks (per drinking days) | ‐ | The mean alcohol: drinks (per drinking days) ‐ all studies in the intervention groups was 1.13 lower (1.79 lower to 0.46 lower) | ‐ | 451 (7 studies) | ⊕⊕⊕⊝ Moderate10 | ‐ |
| Acceptability: dropouts | Study population | RR 0.98 (0.79 to 1.22) | 1159 (17 studies) | ⊕⊕⊝⊝ Low11,12 | ‐ | |
| 334 per 1000 | 328 per 1000 (264 to 408) | |||||
| 307 per 1000 | 301 per 1000 (243 to 375) | |||||
| Tolerability of treatment: withdrawal for medical reasons | Study population | RR 1.15 (0.65 to 2.04) | 947 (10 studies) | ⊕⊕⊝⊝ Low13,14 | ‐ | |
| 69 per 1000 | 80 per 1000 (45 to 141) | |||||
| 32 per 1000 | 37 per 1000 (21 to 65) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Nine studies at unclear risk for selection bias; two studies at high risk and six at unclear risk for performance bias; 13 studies at unclear risk for detection bias (subjective); one study at high risk and two at unclear risk for attrition bias; three studies at high risk and one at unclear risk for reporting bias. 2Significant heterogeneity: Tau² = 0.11; Chi² = 39.01, df = 13 (P = 0.0002); I² = 67%. 3Ten studies at unclear risk for selection bias; one study at high risk and five at unclear risk for performance bias; nine studies at unclear risk for detection bias (subjective); two studies at high risk and two at unclear risk for attrition bias; two studies at high risk and two at unclear risk for reporting bias. 4Significant heterogeneity: Tau² = 0.10; Chi² = 31.63, df = 9 (P = 0.0002); I² = 72%. 5Funnel plot showed asymmetry in favour of 'positive' trials.
6Seven studies with unclear risk of selection bias; one study at high risk and five studies at unclear risk for performance bias; all studies at unclear risk for detection bias (subjective); one study at high risk and two at unclear risk for reporting bias. 7Significant heterogeneity: Tau² = 9.16; Chi² = 39.42, df = 8 (P < 0.00001); I² = 80%. 8Four studies with unclear risk of selection bias; one study at high risk and two studies at unclear risk for performance bias; six studies at unclear risk for detection bias (subjective); one study at high risk and one at unclear risk for reporting bias. 9Total number of events was fewer than 300. 10Five studies with unclear risk of selection bias; one study at high risk and two studies at unclear risk for performance bias; six studies at unclear risk for detection bias (subjective); one study at high risk and two at unclear risk for reporting bias. 11Twelve studies with unclear risk of selection bias; one study at high risk and seven studies at unclear risk for performance bias; 16 studies at unclear risk for detection bias (subjective); four studies at high risk and three studies at unclear risk for attrition bias; five studies at high risk and two at unclear risk for reporting bias. 12Significant heterogeneity: Chi² = 23.80, df = 14 (P = 0.05); I² = 41%. 13Six studies at unclear risk for selection bias; one study at high risk and three at unclear risk for performance bias; nine studies at unclear risk for detection bias (subjective); two studies at high risk and one at unclear risk for attrition bias; three studies at high risk and two at unclear risk for reporting bias. 14Optimal information size not met.
Background
See Appendix 1 for a list of the abbreviations used in this review.
Description of the condition
Major depression disorder and alcohol dependence are among the most prevalent mental disorders worldwide, and their co‐occurrence is common (APA 2013; Grant 1995; Grant 2015; Pettinati 2013). Depression is characterized by a low mood or diminished interest in normal activities on most days, for at least two weeks, as well as other symptoms such as significant weight loss or gain, insomnia or hypersomnia, psychomotor agitation or retardation, fatigue, feelings of guilt or worthlessness, difficulty concentrating, and suicidal ideation (APA 2013). Diagnosis requires the presence of at least five of these symptoms (APA 2000; APA 2013). Alcohol dependence is characterized by bouts of excessive drinking, and inability to control alcohol consumption despite the awareness of its negative consequences (APA 2013). In the last edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5), diagnoses of alcohol dependence and alcohol abuse formerly classified as Alcohol Use Disorders have been replaced by a classification of Alcohol Use Disorder (AUD), which merges their criteria into a single set (APA 2000; APA 2013). AUD diagnosis requires repetitive alcohol‐related problems in at least two out of 11 areas of life as described by a set of criteria that includes 'craving,' which is defined as a strong, obsessive, and irresistible desire to consume alcohol (APA 2013).
Epidemiological studies found that the 12‐month prevalence of depression in the adult population was 5.3% and lifetime prevalence was 13.3% (Hasin 2005), and the 12‐month prevalence of alcohol dependence was 13.9% and lifetime prevalence was 29.1% (Grant 2015). The prevalence of depression has been reported to be higher in people with alcohol dependence than in the general population as well as the prevalence of alcohol dependence in people with depression than in the general population (Regier 1990; Schuckit 1997). Each of these disorders alone is associated with a significant risk of developing the other, and their coexistence is a risk factor for morbidity and mortality, including death from suicide (Schneider 2009; Sher 2005; Wilcox 2004).
The co‐occurrence of depression and alcohol dependence carries potential problems in the diagnostic process (Pettinati 2013; Schuckit 2006). Indeed, depression and alcohol dependence may represent two independent conditions, each requiring to be treated comprehensively (Schuckit 2006). Alternatively, one disorder may influence the development of the second condition. For instance, depression may be the first disorder and is a risk factor for the development of excessive alcohol consumption and the progression to alcohol dependence. In this case, depression is defined as the primary disorder and alcohol dependence is the secondary disorder (Schuckit 2006). However, when the two conditions are of significant duration or severity, both require treatment for as long as is necessary (Schuckit 2006). In contrast, temporary alcohol‐induced depressive symptoms, resulting from the acute alcohol effects of intoxication or withdrawal (APA 2013) tend to spontaneously disappear within approximately one month of alcohol abstinence, without requiring antidepressant therapy (Pettinati 2013; Schuckit 2006).
Description of the intervention
People with alcohol dependence and depression may require different medical treatments depending to the different typology of co‐occurrence. However, pharmacological treatment of people with alcohol dependence and depression constitutes a real challenge (Pettinati 2013).
Except in the case of temporary alcohol‐induced depressive symptoms, people with co‐occurrence of alcohol dependence and depression often receive a combination therapy consisting of medication for the treatment of depression and another for the treatment of alcohol dependence (Pettinati 2013). From a clinical standpoint, this practice is limited by two factors. The first is the extremely low use of medications approved for the treatment of alcohol dependence (Pettinati 2013). One epidemiological study found that less than 10% of people affected by alcohol dependence seek and receive a medical treatment other than 12‐step groups (Grant 2015). The second limitation is the lack of clear evidence of the efficacy of antidepressants in people with alcohol dependence (Pettinati 2013). Usually, depression is considered as a disorder that is amenable to antidepressant treatment (O'Donnell 2011). The most commonly used medications are selective serotonin reuptake inhibitors (SSRIs) and serotonin‐noradrenaline reuptake inhibitors (SNRIs) (O'Donnell 2011). SSRIs and SNRIs are often referred to as second‐generation antidepressants and are considered to be as effective and safer than the older first‐generation antidepressants, such as monoamine oxidase inhibitors (MAOIs), and tricyclic antidepressants (TCAs) (O'Donnell 2011). A common characteristic of antidepressants is that three to four weeks are required following initiation of treatment before a therapeutic response is observed (O'Donnell 2011). In addition, 20% of people with depression may be refractory to multiple antidepressants at adequate doses (O'Donnell 2011).
The efficacy of antidepressants in the treatment of people with alcohol dependence and depression has been investigated in four systematic reviews and meta‐analyses (Foulds 2015; Iovieno 2011; Nunes 2004; Torrens 2005). The first study analyzed 14 trials in which the efficacy of antidepressants was compared to that of placebo in people with dependence on alcohol or other substances of abuse (opioids or cocaine) (Nunes 2004). Among the selected trials, eight specifically investigated the efficacy of antidepressants in people with alcohol dependence and depression (Altamura 1990; Cornelius 1997; Mason 1996; McGrath 1996; Moak 2003; Pettinati 2001a; Roy 1998; Roy‐Byrne 2000). The results of the meta‐analysis revealed that antidepressants had a modest beneficial effect in people with depression who were dependent on alcohol or other substances. The second meta‐analysis compared the efficacy of antidepressants in people who were dependent on substances of abuse, with and without depression (Torrens 2005). Nine studies investigated the efficacy of antidepressants in people with alcohol dependence and depression (Altamura 1990; Cornelius 1997; Gual 2003; Mason 1996; McGrath 1996; Moak 2003; Pettinati 2001a; Roy 1998; Roy‐Byrne 2000). Two of these examined the efficacy of TCAs (Mason 1996; McGrath 1996), five of SSRIs (Cornelius 1997; Gual 2003; Moak 2003; Pettinati 2001a; Roy 1998), one of viloxazine (Altamura 1990), and one of nefazodone (Roy‐Byrne 2000). Although five trials investigated the efficacy of SSRIs, the meta‐analysis did not demonstrate a significant advantage associated with their use but found a significant effect of other antidepressants (Torrens 2005). The third meta‐analysis analyzed 11 trials (Altamura 1990; Cornelius 1997; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; McGrath 1996; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000), and compared the efficacy of antidepressants in people affected by depression or dysthymic disorder (or both) with and without alcohol dependence (Iovieno 2011). The results showed that antidepressants reduced the severity of depression, increasing the rate of responders in people with and without alcohol dependence, with no difference between these two groups; however, there was no effect on the rate of the responders with SSRIs alone. In addition, antidepressant treatment did not reduce alcohol consumption in people with depression and alcohol dependence (Iovieno 2011), which may be explained, at least in part, by the low number of trials reporting data on alcohol consumption. However, there have been conflicting reports on the effects of antidepressants on alcohol consumption in alcohol‐dependent people (Naranjo 2001; Pettinati 2013): while some studies found that antidepressants did not alter consumption (Kranzler 2000; Pettinati 2004), others found that it was significantly reduced (Naranjo 2001), or increased in certain typologies of alcohol‐dependent people (Kranzler 1996; Pettinati 2000). The last meta‐analysis investigated differences in the response to treatment for depression in alcohol‐dependent people according to depression type, independent or alcohol‐induced depression (Foulds 2015). This study analyzed 22 clinical trials of which 13 compared the efficacy of antidepressants to placebo in people with alcohol dependence and depression (Adamson 2015; Altintoprak 2008; Cornelius 1997; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; McGrath 1996; Moak 2003; Muhonen 2008; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000), while the other nine studies investigated the efficacy of other treatments (e.g. psychotherapy or medical management). Two of the 13 former studies were excluded from the meta‐analysis (Altintoprak 2008; Mason 1996); the remaining 11 were divided into two groups, the first comprising trials in which depression was considered to be independent (Cornelius 1997; McGrath 1996; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000), and the second in which depression was considered to be alcohol‐induced or undifferentiated (Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; Pettinati 2001a). The results showed that treatment of depression in alcohol‐dependent people was associated with a large early improvement in the severity of the depression, even when it was independent from drinking, and that the effect of antidepressants was modest but stronger in independent than in alcohol‐induced depression (Foulds 2015).
How the intervention might work
The effect of antidepressants on depression in alcohol‐dependent people could depend on their interference with neurobiological substrates underlying depression, including noradrenaline, dopamine, and serotonin brain circuits (Artigas 2002; Stahl 2003). However, it may also be related to interference with the neurobiological pathways that support alcohol dependence (Carboni 2004; LeMoal 2007; Shirayama 2006). In fact, antidepressants have been proposed for the treatment of alcohol dependence, although their efficacy in this regard remains controversial (Torrens 2005): while some SSRIs have shown positive results in cases of less severe drinking (Pettinati 2001b), others have reported that antidepressants achieved even worse results than placebo (Chick 2004a; Kranzler 1996), especially when treating early‐onset subtypes (Type B, Type II) of alcohol dependence (Babor 1992; Cloninger 1988). Consistent with the heterogeneous origin of depression in alcohol‐dependent people, there are reports of depression symptoms abating spontaneously after alcohol detoxification (Brown 1995; Nunes 2004), and the lack of effect of antidepressants in people who are actively drinking (Pettinati 2004).
Why it is important to do this review
Several Cochrane Reviews on the use of antidepressants for depression are available. However, the generalization of their results to the treatment of people whose depression is complicated by alcohol dependence is limited since it is unknown whether depressive symptoms result from the effects of alcohol or constitute a separate mood disorder (Pettinati 2004). There are no Cochrane Reviews or protocols available on the efficacy of antidepressants in the treatment of people with co‐occurring depression and alcohol dependence, and, although results from the few published reviews (including four meta‐analyses; Foulds 2015; Iovieno 2011; Nunes 2004; Torrens 2005) are suggestive of the efficacy of antidepressants, there are no conclusive results. Therefore, the treatment of a clinical condition associated with significant mortality and morbidity is not yet supported by systematic evaluations of efficacy using rigorous Cochrane methodology. Thus, the evaluation of the efficacy and safety of antidepressants for the treatment of people with co‐occurring depression and alcohol dependence represents a priority.
Objectives
To assess the benefits and risks of antidepressants for the treatment of people with co‐occurring depression and alcohol dependence.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) and controlled clinical trials (CCTs) focused on the use of any antidepressant medication for the treatment of people with co‐occurring depression and alcohol dependence. For cross‐over studies, given possible carry‐over effects and expected dropout rates, we considered only the first period of the trial.
Types of participants
People with co‐occurring depression and alcohol dependence, irrespective of symptom severity. Alcohol dependence and depression were both diagnosed according to standardized criteria such as DSM or equivalent. However, we also accepted trials that did not use explicit diagnostic criteria.
We examined the effect of including people with uncertain diagnoses in the sensitivity analyses. Trials including people with additional diagnoses of dependence by other substances of abuse were also considered eligible. People under 18 years of age and pregnant women were excluded for the substantially different approach to clinical management of these people. People with other comorbid mental health conditions were included and considered in subgroup analysis.
Types of interventions
Experimental intervention
Monoamine oxidase inhibitors (MAOIs): minaprine, moclobemide, phenelzine, selegiline.
Tricyclic antidepressants (TCAs) and TCA‐related antidepressants: amitriptyline, amoxapine, clomipramine, desipramine, dothiepin (also known as dosulepin), doxepin, imipramine, maprotiline, nomifensine, nortriptyline, protriptyline, trimipramine.
Selective serotonin reuptake inhibitors (SSRIs): citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, zimelidine.
Serotonin‐noradrenaline reuptake inhibitors (SNRIs): desvenlafaxine, duloxetine, milnacipran, venlafaxine.
5‐HT2 antagonists: mianserin, mirtazepine, nefazodone, trazodone.
Other antidepressants: agomelatine, bupropion, reboxetine, tianeptine, viloxazine.
These antidepressants may have been administered alone or in combination with other medications for the treatment of alcohol dependence or with any psychosocial intervention.
Control intervention
Placebo.
No intervention.
Other pharmacological interventions.
Any psychosocial intervention.
Types of outcome measures
Primary outcomes
Depression severity measured as group mean scores in continuous: interviewer‐rated scales (e.g. Hamilton Rating Scale for Depression (HRSD)) and self‐administered scales (e.g. Beck Depression Inventory (BDI)).
Response to antidepressive treatment (defined using interviewer‐rated scales as the number of people showing greater than 50% reduction in depression severity from baseline, according to the definition of the study authors, or a 'very much improved' or 'much improved' on the Clinical Global Impression (CGI) improvement scale).
Full remission of depression (defined according to a prespecified score in interviewer‐rated continuous depression scales).
Consumption of alcohol as number of participants who reported use during treatment, or number of participants with positive breath alcohol analysis or urine analyses positive for alcohol, or both.
Liver enzyme levels (alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ‐glutamyltransferase (GGT)).
Acceptability indicated by all‐cause dropouts from the treatment as number of participants who did not complete treatment.
Tolerability of treatment as withdrawal for medical reasons, total number of adverse events, and type of adverse events experienced during treatment.
Suicide and suicide attempts.
Where possible, indices of effectiveness at different time points in the course of treatment were pooled.
Secondary outcomes
Use of other substances of abuse as number of participants who reported use during treatment, or number of participants with urine analyses positive for other substances of abuse, or both; self‐reported quantity and frequency of use of other substances of abuse.
Craving as measured by validated scales (e.g. Brief Substance Craving Scale (BSCS), Visual Analogue Scale (VAS), Obsessive Compulsive Drinking Scale (OCDS)).
Severity of dependence as measured by validated scales (e.g. Addiction Severity Index (ASI), CGI, Severity of Dependence Scale (SDS), Drinker Inventory of Consequences scale (DrInC)).
Psychiatric symptoms/psychological distress diagnosed using standard criteria (e.g. DSM) or measured by validated scales (e.g. Positive and Negative Syndrome Scale (PANSS); Symptoms Check List‐90 (SCL‐90)).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases.
Cochrane Drugs and Alcohol Group (CDAG) Specialised Register (inception to 4 July 2017), using the search strategy outlined in Appendix 2.
Cochrane Central Register of Controlled Trials (CENTRAL) (2017, Issue 7), using the search strategy outlined in Appendix 3.
MEDLINE (via PubMed) (January 1966 to 4 July 2017), using the search strategy outlined in Appendix 4.
Embase (Elsevier, embase.com) (January 1974 to 4 July 2017), using the search strategy outlined in Appendix 5.
We searched for ongoing clinical trials and unpublished trials via Internet searches on the following websites.
ClinicalTrials.gov (www.clinicaltrials.gov) (4 July 2017).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) (4 July 2017).
All searches included non‐English language literature and studies with English abstracts were assessed for inclusion. When considered likely to meet inclusion criteria, studies were translated.
Searching other resources
We searched the reference lists of all relevant papers to identify additional studies, and conference proceedings that were likely to contain trials relevant to the review. We also contacted investigators to seek information about unpublished or incomplete trials.
Data collection and analysis
Selection of studies
Two authors (RA, ET) inspected the search hits by reading titles and abstracts. Two authors (RA, ET) obtained each potentially relevant study identified in the search in full text and independently assessed them for inclusion. We resolved disagreements by discussion or consultation with the third author (PP).
Data extraction and management
Two authors (RA, ET) independently extracted data and used a standardized checklist to collect information on methodology, participants (sociodemographic and clinical information relevant to the review aims), interventions (medications and non‐pharmacological interventions), and primary and secondary outcomes. We resolved disagreements by discussion and, for those that persisted, by consultation with the third author (PP).
Assessment of risk of bias in included studies
Two authors (RA, ET) assessed study quality according to the criteria listed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or consultation with the third author (PP). We assessed the risk of bias for RCTs and CCTs according the five criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The approach recommended for assessing the risk of bias in studies included in a Cochrane Review involves a two‐part tool addressing seven specific domains, namely, sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. The first part of the tool involves describing what was reported to have happened in the study. The second part involves assigning a judgement relating to the risk of bias for that entry in terms of low, high, or unclear risk. To make these judgements, we used the criteria listed in the Cochrane Handbook for Systematic Reviews of Interventions adapted to the addiction field (see Appendix 6).
The domains of sequence generation and allocation concealment (avoidance of selection bias) were addressed in the tool by a single entry for each study.
Blinding of participants and personnel (avoidance of performance bias) was judged to interfere with both subjective and objective outcomes pertaining to the behaviour of participants (such as retention in treatment) and was addressed by a single entry for each study.
Blinding of outcome assessor (avoidance of detection bias) was considered separately for objective outcomes (e.g. retention in treatment, use of substances of abuse measured by breath or urine analysis), and subjective outcomes (e.g. severity of depression, other psychiatric symptoms/psychological distress, severity of dependence).
Incomplete outcome data (avoidance of attrition bias) was considered for all outcomes except for the dropout from the treatment, which is often the primary outcome measure in addiction studies.
To incorporate assessment in the review process, we first plotted intervention effects estimates for different outcomes stratified for risk. If there were differences in results among studies with different risks of bias, we performed a sensitivity analysis excluding studies with high risk of bias in one or more domains.
We also performed subgroup analysis for studies with low and unclear risk of bias.
Measures of treatment effect
We analyzed dichotomous outcomes (e.g. number of participants showing improvement in depression at follow‐up) calculating the risk ratio (RR) for each trial, with the uncertainty of each result expressed as a 95% confidence interval (CI). Continuous outcomes (e.g. severity of depression according to final scores archived in continuous interviewer‐rated scales) were analyzed by calculating the mean difference (MD) with 95% CI, which were calculated by comparing and pooling mean score differences from the end of treatment to baseline for each group. In case of missing data on the standard deviation (SD) of the changes, we used the SD at the end of treatment for each group. We used the standardized mean difference (SMD) when the studies employed different instruments.
Unit of analysis issues
We did not use data presented as a number of positive urine or breath alcohol tests relative to the total number of tests in the experimental and control groups as a measure of substance use. This decision was made because using the number of tests instead of the number of participants as a unit of analysis violates the assumption of the independence of observations. In fact, the results of the tests performed for each participant were not independent.
If multi‐arm studies were included in the meta‐analyses and one arm was considered more than once in the same comparison, we combined groups according to the approaches suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). That is, if one arm (e.g. control group) was compared with different groups in which participants received different doses of the same antidepressant, we combined all the experimental groups (different doses of the same antidepressant) into a single group, which was then compared with the control group. If one arm (e.g. control group) was compared with different experimental groups in which participants received different antidepressants, we planned to split the 'shared' control group into two or more groups with smaller sample sizes, and compared these smaller control groups with the different experimental groups. While this last approach avoided the repeated use of participants in the pooled estimate of treatment effect while retaining information from each arm of the trial, it decreased the precision of the pooled estimate. Both approaches avoided the double counting of participants in the control groups.
Dealing with missing data
We contacted the original investigators to request information on data missing from the studies. In the absence of supplemental data from the study authors, we obtained missing data according to procedures suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Whenever the assumption that random data were missing was supported by available information, we analyzed only available data; on the contrary case, other approaches, such as the last observation carried forward or the assumption that missing data corresponded to poor outcomes, were pursued. To assess the sensitivity of the results to changes made in the assumptions, we carried out a sensitivity analysis. The potential impact of missing data on the findings of the review is addressed in the Discussion.
Assessment of heterogeneity
We analyzed heterogeneity using the I² statistic and the Chi² test. As suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), according to I² values, evidence of heterogeneity may be classified into no important (0% to 40%), moderate (30% to 60%), substantial (50% to 90%), and considerable (75% to 100%). In the present meta‐analysis, we considered the following cut‐off values: I² value 50% or greater; P value for the Chi² test 0.1 or less for significant evidence of heterogeneity.
Assessment of reporting biases
We used a funnel plot (plot of the effect estimate from each study against sample size or effect standard error) to evaluate the potential for bias related to the size of the trials.
Data synthesis
Whenever possible, we combined the outcomes from individual trials in a meta‐analysis (comparing intervention and outcomes between trials) using a fixed‐effect model; when there was significant heterogeneity, we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses for studies with low and unclear risk of bias and for classes of antidepressants. Moreover, we performed subgroup analyses to take into account the following confounders/effect modifiers, when possible.
Setting (inpatient or outpatient treatment).
Starting dose/rate and pattern of dose reduction.
Scheduled duration of treatment.
Severity of depression.
Severity of alcohol dependence.
Being actively drinking.
Length of abstinence.
Other psychiatric comorbidity.
Other pharmacological treatment offered.
Other psychosocial treatment offered.
Sensitivity analysis
To incorporate assessment into the review process, we first plotted intervention effects estimates stratified for risk of bias for each relevant domain. If there were differences in the results among studies at different risks of bias, we performed a sensitivity analysis excluding the studies with a high risk of bias. The effect of including people with uncertain diagnoses was evaluated with the sensitivity analysis; other issues suitable for sensitivity analysis were identified during the review process based on idiosyncrasies of the examined studies.
'Summary of findings' table
We assessed the overall quality of the evidence for the primary outcomes using the GRADE system. The GRADE Working Group developed a system for grading the quality of evidence (GRADE 2004; Guyatt 2008; Guyatt 2011; Schünemann 2006), which takes into account issues related to internal validity and to external validity, such as directness of results. The 'Summary of findings' table presents the main findings of a review in a transparent and simple tabular format. In particular, it provides key information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on the main outcomes.
The GRADE system uses the following criteria for assigning grades of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
Grading is decreased for the following reasons.
Serious (‐1) or very serious (‐2) study limitation for risk of bias.
Serious (‐1) or very serious (‐2) inconsistency between study results.
Some (‐1) or major (‐2) uncertainty about directness (the correspondence between the population, the intervention, or the outcomes measured in the studies actually found and those under consideration in our systematic review).
Serious (‐1) or very serious (‐2) Imprecision of the pooled estimate.
Strong suspicion of publication bias (‐1).
Results
Description of studies
For substantive descriptions of studies see Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
The searches of the four databases (see Electronic searches) retrieved 8532 records (see Figure 1). Our searches of other resources identified three additional records that appeared to meet the inclusion criteria. Therefore, there was a total of 8535 records.
1.
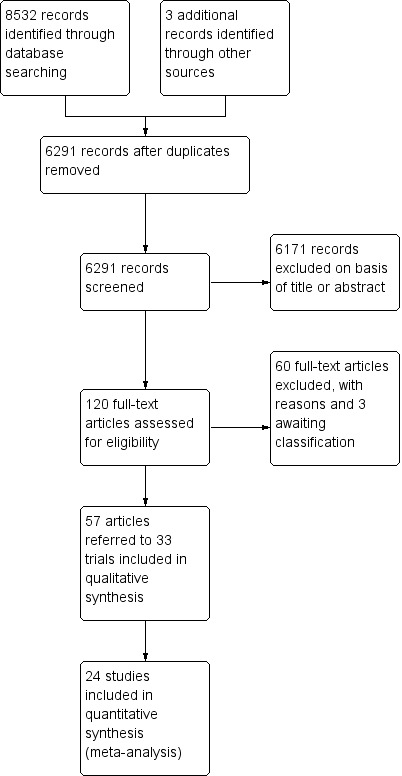
Study flow diagram.
Once duplicates had been removed, there were 6291 records. We excluded 6171 records based on titles and abstracts. We obtained the full text of the remaining 120 records. We excluded 60 records (see Characteristics of excluded studies table). We added three records to the Characteristics of studies awaiting classification table pending information from the authors.
We included 33 studies reported in 57 references. For a further description of our screening process, see the study flow diagram (Figure 1).
Included studies
Thirty‐three studies with 2242 participants met the inclusion criteria (see Characteristics of included studies table).
It was not possible to extract and combine the results of nine studies as their comparisons were not evaluated by more than one study. We extracted data from the other 24 studies (1498 participants) (see Figure 1).
Duration of trials
The mean duration of the trials was 9.9 weeks (range 3 to 26 weeks).
Treatment regimens and setting
Medications evaluated: sertraline (eight studies); amitriptyline (five studies); mirtazapine (four studies); doxepin (three studies); imipramine (three studies); nefazodone, tianeptine, and venlafaxine (two studies each); and citalopram, desimipramine, escitalopram, fluoxetine, fluvoxamine, mianserine, paroxetine, and viloxazine (one study each).
Twenty‐two studies (1438 participants) compared the efficacy of an antidepressant versus placebo, two (60 participants) compared the efficacy of an antidepressant versus psychotherapy, five compared the efficacy of one antidepressant versus another (mirtazapine versus amitriptyline; mirtazapine versus venlafaxine; paroxetine versus amitriptyline; tianeptine versus amitriptyline; tianeptine versus fluvoxamine), four compared the efficacy of antidepressants versus other medications (amitriptyline versus diazepam (one study); doxepin versus diazepam (two studies); escitalopram versus memantine (one study)).
In total, 18 trials were conducted in an outpatient setting, 12 in an inpatient setting, and three trials initially in an inpatient setting and then in an outpatient setting. Eighteen studies took place in the USA, 12 in Europe, two in Turkey, and one in Australia.
Eighteen trials administered psychosocial treatment in conjunction with antidepressants, including cognitive behavioural psychotherapy or relapse prevention therapy (14 trials) and manualized clinical case management or unspecified psychotherapy (four studies).
Studies assessed compliance as the return of unused medications (six studies), trough plasma concentrations (two studies), and use of an electronic monitoring device that recorded the date and time of bottle cap openings (two studies). The remaining 19 studies did not report this information. For more information see Appendix 7.
Rating instruments
The rating instruments used in the included studies are listed in Appendix 8.
Participants
The analysis included 2242 participants affected by alcohol dependence and depression according to DSM criteria (Diagnostic and Statistic Manual of Mental Disorders III ‐ Revised (DSM‐III‐R); Diagnostic and Statistic Manual of Mental Disorders IV ‐ Revised (DSM‐IV‐R)) or other criteria (see Appendix 8).
The sex of 156 participants was unknown; among the remaining 2086 participants, 1425 were men (68.3%), and 661 were women (31.7%). The mean age was 41.7 years (data from 28/33 studies).
Sources of funding
Only 19 trials reported the source of funding for their research. Six trials received funds only from public Institutes; 10 studies were partly supported by both a public institute and a private pharmaceutical company; and two were only partially supported by a private pharmaceutical company.
Declaration of interest
Four trials reported a possible conflict of interest.
Outcomes
For some reported outcomes, it was difficult to make comparisons and pool results due to the different modes of measurement, the selected cut‐off value, and the availability of data from the study or the primary investigator. This was particularly true for use of alcohol and alcohol abstinence, which were expressed in various ways (i.e. rate of drinking days, cumulative number of drinking days, number of drinks per drinking day, weekly number of heavy drinking days, rate of heavy drinkers, number of heavy drinkers, number of participants abstaining during the trial, rate of abstinence days, cumulative abstinence days). Appendix 9 shows the list of outcomes.
Primary outcomes
Depression severity
Twenty‐three studies reported the final score of an interviewer‐rated scale (see Primary outcomes) (Adamson 2015; Altamura 1990; Altintoprak 2008; Butterworth 1971a; Gual 2003; Habrat 2006; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Liappas 2005 arm A; Liappas 2005 arm B; Liappas 2005 arm C; Lôo 1988; Mason 1996; McGrath 1996; Moak 2003; Muhonen 2008; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000; 1796 participants) (see Appendix 8; Appendix 9). Nineteen studies used the HRSD (Altamura 1990; Altintoprak 2008; Gual 2003; Habrat 2006; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Liappas 2005 arm A; Liappas 2005 arm B; Liappas 2005 arm C; Mason 1996; McGrath 1996; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000; 1410 participants); five studies used the Montgomery and Åsberg Depression Rating Scale (MADRS) (Adamson 2015; Gual 2003; Krupitsky 2012; Lôo 1988; Muhonen 2008; 490 participants), and one study used the Brief Psychiatric Rating Scale (BPRS) (Butterworth 1971a; 39 participants). Two studies reported data of two interviewer‐rated scales (MADRS and HRSD) and only included data obtained using the HRSD (Gual 2003; Krupitsky 2012; 143 participants). One study excluded data because they were expressed as medians and interquartile ranges (Mason 1996; 22 participants).
Thirteen studies reported the final score of a self‐administered scale (see Primary outcomes) (Adamson 2015; Butterworth 1971a; Cocchi 1997; Cornelius 2016; Krupitsky 1993 arm A; Krupitsky 1993 arm B; Krupitsky 2012; Lôo 1988; McLean 1986; Moak 2003; Muhonen 2008; Pettinati 2001a; Roy 1998; 825 participants) (see Appendix 8; Appendix 9). Among them, five studies used the BDI scale (Cornelius 2016; Moak 2003; Muhonen 2008; Pettinati 2001a; Roy 1998; 241 participants), one study used the HRSD scale (McLean 1986; 27 participants), two studies used the SCL‐90 scale (Adamson 2015; Lôo 1988; 267 participants), and five studies used the Zung Self‐Assessment Depression Scale (ZUNG) scale (Butterworth 1971a; Cocchi 1997; Krupitsky 1993 arm A; Krupitsky 1993 arm B; Krupitsky 2012; 282 participants). Two studies reported data obtained using the Minnesota Multiphasic Personality Inventory (MMPI) and ZUNG and only included data obtained with ZUNG (Krupitsky 1993 arm A; Krupitsky 1993 arm B; 41 participants).
Six studies reported the difference between the baseline and final score of an interviewer‐rated scale (see Primary outcomes) (Butterworth 1971b; Cornelius 1997; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; Pettinati 2001a; 476 participants) (see Appendix 8; Appendix 9). Five studies used the HRSD (Cornelius 1997; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; Pettinati 2001a; 436 participants), and one trial used the Lehmann Depression Rating Scale (LDRS) (Butterworth 1971b; 40 participants). We excluded data from one study because they were expressed as medians and interquartile ranges (Mason 1996; 28 participants).
Four studies reported the difference between the baseline and final score of a self‐administered scale (see Primary outcomes; Appendix 8; Appendix 9) (Cornelius 1997; Cornelius 2016; McLean 1986; Pettinati 2001a; 129 participants). Among them, three studies used the BDI (Cornelius 1997; Cornelius 2016; Pettinati 2001a; 94 participants), and one study used a self‐rating scale based on the HRSD (McLean 1986; 35 participants).
Response
Fourteen studies reported the response to antidepressive treatment (see Primary outcomes) (Butterworth 1971b; Butterworth 1971a; Gallant 1969 arm a; Gallant 1969 arm b; Gual 2003; Habrat 2006; Kranzler 2006 arm A; Kranzler 2006 arm B; Lôo 1988; Mason 1996; McGrath 1996; Moak 2003; Roy 1998; Roy‐Byrne 2000; 1284 participants) (see Appendix 8; Appendix 9). The studies used different interviewer‐rated scales: five studies used CGI (Butterworth 1971b; Gallant 1969 arm a; Gallant 1969 arm b; Roy 1998; Roy‐Byrne 2000; 240 participants); two studies used MADRS (Gual 2003; Lôo 1988; 212 participants); six studies used HRSD (Habrat 2006; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; McGrath 1996; Moak 2003; 793 participants); and one study used BPRS (Butterworth 1971a; 39 participants). One study reported data obtained using both CGI and HRSD and we only used data obtained using CGI (Roy 1998; 36 participants). One study reported data for significant depression that were converted into response (Moak 2003; 82 participants). Two studies reported response criteria using self‐administered scales; these data were not included in the analyses (Cocchi 1997; Roy 1998).
Remission
Five studies reported remission (see Primary outcomes) (Adamson 2015; Gual 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000; 455 participants) (see Appendix 8; Appendix 9). The studies used different interviewer‐rated scales or different cut‐off values of the same scale: one study used MADRS, final score less than 10 (Adamson 2015; 138 participants), one study used MADRS, final score less than 7 (Gual 2003; 83 participants), one study used HRSD, final score less than 8 (Roy‐Byrne 2000; 64 participants), and two studies used HRSD, final score 9 or less (Pettinati 2010 arm A; Pettinati 2010 arm B; 170 participants). Two studies reported remission criteria using self‐administered scales and we did not include these data in the analyses (Cocchi 1997; McLean 1986).
Alcohol consumption
The studies included in the present meta‐analysis did not report information on alcohol consumption as number of participants who reported use during treatment, or number of participants with positive breath alcohol analysis or urine analyses positive for alcohol (or alcohol consumption and positive breath alcohol analysis or urine analyses) (see Primary outcomes). Conversely, at least two studies reported the following information (see Characteristics of included studies table; Appendix 8; Appendix 9).
Nine studies reported the rate of abstinent days (Adamson 2015; Cornelius 1997; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; McGrath 1996; Moak 2003; Pettinati 2001a; 821 participants).
Eight studies reported the number of abstinent participants during the trials (Cornelius 1997; Hernandez‐Avila 2004; McGrath 1996; Muhonen 2008; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000), 504 participants)
Two studies reported the number of drinking days per week (Cornelius 2016; Hernandez‐Avila 2004; 55 participants).
Seven studies reported the number of drinks per drinking days (Adamson 2015; Cornelius 1997; Cornelius 2016; Hernandez‐Avila 2004; McGrath 1996; Moak 2003; Roy‐Byrne 2000; 451 participants).
Two studies reported the number of drinks per week (Cornelius 2016; Hernandez‐Avila 2004; 55 participants).
Five studies reported the number of heavy drinking days per week (Adamson 2015; Cornelius 1997; Cornelius 2016; Hernandez‐Avila 2004; McGrath 1996; 513 participants) or it was calculated by other outcomes reported by the studies.
Seven studies reported the number of heavy drinkers (Gual 2003; Krupitsky 2012; Mason 1996; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; 459 participants).
Six studies reported the time to the first relapse in days (Cornelius 1997; Gual 2003; Krupitsky 2012; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; 393 participants) or it was calculated by other outcomes reported by the studies.
Liver enzyme levels
The studies included in the present meta‐analysis did not report information on ALT and AST (see Primary outcomes). Two studies reported the final levels of GGT (Hernandez‐Avila 2004; Krupitsky 2012; 101 participants) (see Appendix 8 and Appendix 9).
Three studies reported a global response both in depression and in alcohol consumption (Krupitsky 2012; McGrath 1996; Nunes 1993; 152 participants) (see Appendix 8 and Appendix 9).
Acceptability
Seventeen studies reported acceptability indicated by all‐cause dropouts (see Primary outcomes) (Altamura 1990; Butterworth 1971b; Cornelius 2016; Gallant 1969 arm a; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Mason 1996; McGrath 1996; McLean 1986; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000; 1156 participants) (see Appendix 8; Appendix 9).
Tolerability
Several studies evaluated tolerability of the treatment as number and type of adverse events experienced during the treatment (see Primary outcomes) (see Appendix 8; Appendix 9). Among the different adverse events, the following were reported by at least two studies:
Blurred vision evaluated by two studies (Butterworth 1971a; Roy‐Byrne 2000; 103 participants).
Constipation evaluated by four studies (Altintoprak 2008; Butterworth 1971a; Kranzler 2006 arm A; Roy‐Byrne 2000; 336 participants).
Depression evaluated by two studies (Kranzler 2006 arm A; Moak 2003; 413 participants).
Diarrhoea evaluated by two studies (Gual 2003; Roy‐Byrne 2000; 139 participants).
Dizziness evaluated by three studies (Altintoprak 2008; Gual 2003; Roy‐Byrne 2000; 183 participants).
Dry mouth evaluated by five studies (Altintoprak 2008; Butterworth 1971a; Gallant 1969 arm a; Gallant 1969 arm b; Roy‐Byrne 2000; 286 participants).
Headache evaluated by three studies (Gual 2003; Kranzler 2006 arm A; Roy‐Byrne 2000; 470 participants).
Increase in body weight reported by two studies (Altintoprak 2008; Cornelius 2016; 58 participants).
Insomnia evaluated by four studies (Adamson 2015; Butterworth 1971b; Kranzler 2006 arm A; Roy‐Byrne 2000; 564 participants).
Nausea evaluated by three studies (Adamson 2015; Gual 2003; Roy‐Byrne 2000; 277 participants).
Sedation evaluated by two studies (Altintoprak 2008; Roy‐Byrne 2000; 108 participants).
Total adverse effects evaluated by eight studies (Adamson 2015; Butterworth 1971b; Butterworth 1971a; Gallant 1969 arm a; Gallant 1969 arm b; Habrat 2006; Kranzler 2006 arm A; Krupitsky 2012; 1041 participants).
Total serious adverse events evaluated by seven studies (Adamson 2015; Butterworth 1971b; Cornelius 2016; Kranzler 2006 arm A; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; 774 participants).
Withdrawal for medical reasons evaluated by ten studies (Adamson 2015; Cornelius 1997; Kranzler 2006 arm A; Krupitsky 2012; Mason 1996; McGrath 1996; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000; 947 participants).
Worsening of clinical condition because of relapse evaluated by two studies (Kranzler 2006 arm A; Moak 2003; 413 participants).
Suicide and suicide attempts
Five studies evaluated suicide and suicidal attempts (see Primary outcomes) (Adamson 2015; Cornelius 1997; Habrat 2006; Kranzler 2006 arm A; Moak 2003; 888 participants) (see Appendix 8; Appendix 9).
Secondary outcomes
Participants with substance use disorders were excluded by most studies (see Characteristics of included studies table). Participants with substance use disorders were included by two studies (Adamson 2015; McGrath 1996; 83 participants). Two studies excluded participants with substance use disorders but included participants with abuse of other substances (Cornelius 1997; Roy‐Byrne 2000; 87 participants).
Four studies reported craving (see Secondary outcomes) (Altintoprak 2008; Cornelius 2016; Habrat 2006; Krupitsky 2012; 404 participants). They used different scales. One study used a questionnaire prepared by the authors (Altintoprak 2008; 44 participants). Three studies used the Obsessive‐Compulsive Drinking Scale (OCDS) (Cornelius 2016; Habrat 2006; Krupitsky 2012; 360 participants) (see Appendix 8; Appendix 9). One study used different scales but data obtained only with the OCDS were used (Krupitsky 2012; 60 participants).
Several studies reported severity of alcohol dependence (see Secondary outcomes) but asbaseline characteristics of recruited participants (see Characteristics of included studies table; Appendix 9). Three studies reported final data on the severity of alcohol dependence (see Appendix 8) (Adamson 2015; Hernandez‐Avila 2004; Muhonen 2008; 259 participants). Studies used different interviewer‐rated scales: Alcohol Use Disorders Identification Test (AUDIT) (Muhonen 2008; 80 participants), DrInC (Hernandez‐Avila 2004; 41 participants), and Leeds Dependence Questionnaire (LDQ) (Adamson 2015; 138 participants).
Studies reported baseline characteristics of recruited participants for psychiatric symptoms/psychological distress (see Secondary outcomes; Characteristics of included studies table). Eleven studies reported final score of anxiety severity (Altintoprak 2008; Habrat 2006; Hernandez‐Avila 2004; Krupitsky 1993 arm A; Krupitsky 1993 arm B; Krupitsky 2012; Liappas 2005 arm A; Liappas 2005 arm B; Liappas 2005 arm C; Lôo 1988; Muhonen 2008; 761 participants). Studies used several scales: an interviewer‐rated scale (see Appendix 8): Hamilton Anxiety Rating Scale (HRSA) (Habrat 2006; Krupitsky 2012; Liappas 2005 arm A; Liappas 2005 arm B; Liappas 2005 arm C; Lôo 1988; Muhonen 2008; 615 participants), and three self‐administered scales: Beck Anxiety Inventory (BAI) (Muhonen 2008; 80 participants), MMPI (Krupitsky 1993 arm A; Krupitsky 1993 arm B; 61 participants), and STAI (Altintoprak 2008; Hernandez‐Avila 2004; Krupitsky 1993 arm A; Krupitsky 1993 arm B; Krupitsky 2012; 206 participants).
Comparisons
Antidepressants versus placebo: 22 studies (Adamson 2015; Altamura 1990; Butterworth 1971b; Cornelius 1997; Cornelius 2016; Gallant 1969 arm a; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 1993 arm A; Krupitsky 2012; Mason 1996; McGrath 1996; McLean 1986; Moak 2003; Nunes 1993; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000; 1438 participants).
Antidepressants versus psychotherapy: two studies (Liappas 2005 arm A; Liappas 2005 arm B; 60 participants) compared the efficacy of mirtazapine (Liappas 2005 arm A) or venlafaxine (Liappas 2005 arm B) versus psychotherapy for three weeks. Both studies had a high risk of bias.
Antidepressants versus other medications: four studies compared the efficacy of antidepressants to that of another medication (Butterworth 1971a; Gallant 1969 arm b; Krupitsky 1993 arm B; Muhonen 2008; 228 participants). Of these, one study compared amitriptyline to diazepam (Krupitsky 1993 arm B; 29 participants), two studies doxepin to diazepam (Butterworth 1971a; 39 participants; Gallant 1969 arm b; 71 participants), and one study escitalopram to memantine (Muhonen 2008; 80 participants). In this comparison, we included only the three studies comparing an antidepressant to diazepam (Butterworth 1971a; Gallant 1969 arm b; Krupitsky 1993 arm B; 148 participants). Only the final score of the severity of depression was reported by at least two of these studies.
An antidepressant versus another antidepressant: five studies compared the efficacy of an antidepressant versus another antidepressant (Altintoprak 2008; Cocchi 1997; Habrat 2006; Liappas 2005 arm A; Lôo 1988; 621 participants). Of these, one study compared mirtazapine (up to 60 mg/day) to amitriptyline (up to 150 mg/day) for eight weeks (Altintoprak 2008; 44 participants); one study mirtazapine (up to 60 mg/day) to venlafaxine (up to 300 mg/day) for three weeks (Liappas 2005 arm A; 40 participants); one study paroxetine (20 mg/day) to amitriptyline (25 mg/day) for three to four weeks (Cocchi 1997; 122 participants); one study tianeptine (37.5 mg/day) to amitriptyline (75 mg/day) for four to eight weeks (Lôo 1988; 129 participants); and one study tianeptine (37.5 mg/day) to fluvoxamine (100 mg/day) for six weeks (Habrat 2006; 286 participants). As the same comparison was not made by more than one study, it was not possible to conduct any analyses.
Subgroup analysis
We conducted subgroup analyses only for the comparison between antidepressants and placebo.
Twenty‐two studies reported the setting (see Subgroup analysis and investigation of heterogeneity): 15 studies were conducted in an outpatient setting (Adamson 2015; Cornelius 2016; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Mason 1996; McGrath 1996; Moak 2003; Nunes 1993; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000; 1129 participants), four studies in inpatient setting (Butterworth 1971b; Gallant 1969 arm a; Krupitsky 1993 arm A; McLean 1986; 192 participants), and three studies initially in an inpatient setting and then as outpatients (Altamura 1990; Cornelius 1997; Roy 1998; 117 participants). We investigated the possible role of this confounder factor for each analysis.
Sixteen studies reported the use of a lower starting dose (see Subgroup analysis and investigation of heterogeneity) (Adamson 2015; Butterworth 1971b; Cornelius 1997; Cornelius 2016; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; McGrath 1996; McLean 1986; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000; 1172 participants). No study described a pattern of dose reduction. The possible protective role of a lower starting dose in reducing the risk of appearance of adverse events has not been investigated because there were no differences in number and types of adverse events between antidepressants and placebo.
All studies reported the duration of treatment (see Subgroup analysis and investigation of heterogeneity): 19 studies had a duration of four weeks or greater (Adamson 2015; Altamura 1990; Cornelius 1997; Cornelius 2016; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Mason 1996; McGrath 1996; McLean 1986; Moak 2003; Nunes 1993; Pettinati 2001aPettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000; 1281 participants), and three studies had a duration of less than four weeks (Butterworth 1971b; Gallant 1969 arm a; Krupitsky 1993 arm A; 157 participants). We investigated the possible role of this confounder factor for each analysis.
Fifteen studies evaluated the severity of depression at baseline using an interviewer‐rated scale (Adamson 2015; Altamura 1990; Cornelius 1997; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Mason 1996; McGrath 1996; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000; 1180 participants). One of these studies used the MADRS (Adamson 2015; 138 participants); the other 14 studies used the HRSD (Altamura 1990; Cornelius 1997; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Mason 1996; McGrath 1996; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000; 1042 participants) (see Characteristics of included studies table). According to these values, in five studies, the severity of depression ranged from the absence of depression or mild depression to severe or very severe (Cornelius 1997; Gual 2003; Krupitsky 2012; Mason 1996; McGrath 1996; 291 participants); in other eight studies, the severity of depression ranged from moderate to severe or very severe (Adamson 2015; Hernandez‐Avila 2004; Kranzler 2006 arm A; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000; 720 participants). Only one study included people with very severe depression (Altamura 1990; 30 participants), and another study with mild or moderate depression (Kranzler 2006 arm B; 139 participants). Accordingly, we did not evaluate this possible confounder factor.
Studies were divided according to the typology of depression, into studies with primary depression and with depression induced by alcohol consumption. Eleven studies recruited participants with primary depression (Adamson 2015; Cornelius 1997; Kranzler 2006 arm A; Krupitsky 1993 arm A; McGrath 1996; Moak 2003; Nunes 1993; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998 arm A; Roy‐Byrne 2000; 842 participants); three studies recruited participants with depression induced by alcohol consumption (Kranzler 2006 arm B; Mason 1996; Roy 1998 arm B; 188 participants). The other studies did not report typology of depression and we excluded them from these analyses).
Three studies evaluated the severity of alcohol dependence at baseline using the number of positive diagnostic criteria (Cornelius 1997; Kranzler 2006 arm A; Kranzler 2006 arm B; 379 participants) (see Characteristics of included studies). One study used the DSM‐III criteria and reported a baseline severity ranging from 3.9 to 7.7 positive criteria (Cornelius 1997; 51 participants); the other two studies used the DSM IV criteria and reported a baseline severity ranging from 3.4 to 6.4 (Kranzler 2006 arm A; Kranzler 2006 arm B; 328 participants). Accordingly, it was not feasible to evaluate this possible confounder factor.
Twenty studies reported if participants were actively drinking alcohol at the beginning of the trial (see Subgroup analysis and investigation of heterogeneity): in 12 studies, participants were actively drinking (Adamson 2015; Cornelius 1997; Cornelius 2016; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; McGrath 1996; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000; 986 participants) and in eight studies, participants were not actively drinking (Butterworth 1971b; Gual 2003; Krupitsky 1993 arm A; Krupitsky 2012; Mason 1996; McLean 1986; Nunes 1993; Roy 1998; 346 participants). The other studies did not report this information. We investigated the possible role of this confounder factor for each analysis.
Nine studies reported the length of abstinence (see Subgroup analysis and investigation of heterogeneity) (Butterworth 1971b; Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 1993 arm A; Krupitsky 2012; Mason 1996; Nunes 1993; Roy 1998; 639 participants). Its possible confounder role has not been evaluated because it ranged from a minimum of few days (Butterworth 1971b) to a maximum of 12 weeks (Nunes 1993), also within the same study (Mason 1996).
Fourteen studies used the presence of other psychiatric disorders, including bipolar disorder as an exclusion criterion (see Subgroup analysis and investigation of heterogeneity) (Adamson 2015; Butterworth 1971b; Cornelius 1997; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; McGrath 1996; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998). The majority of the other studies did not report information on possible comorbid psychiatric disorders. Accordingly, it was not feasible to evaluate this possible confounder factor.
Three studies allowed the use of other pharmacological treatments (see Subgroup analysis and investigation of heterogeneity) (Adamson 2015; Butterworth 1971b; McLean 1986). The low number of studies precluded the possibility to evaluate this possible confounder factor.
Fifteen studies offered apsychosocial treatment to participants (see Subgroup analysis and investigation of heterogeneity and Appendix 10) (Adamson 2015; Cornelius 1997; Cornelius 2016; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Mason 1996; McGrath 1996; McLean 1986; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000; 1109 participants). Two studies did not offer a psychosocial treatment to participants (Butterworth 1971b; Krupitsky 1993 arm A; 81 participants), whereas the other studies did not provide information on this issue. We investigated the possible role of this confounder factor for each analysis.
Excluded studies
We excluded 55 published articles for the following reasons: the study design did not meet the inclusion criteria (26 studies); the study relied on the same database as used in another (not included trial; one study); the study population did not meet the inclusion criteria (23 studies); and there was a lack of information (five studies). For details, see Characteristics of excluded studies table.
Risk of bias in included studies
All 33 studies were RCTs.
Allocation
We judged the random sequence generation adequately prevented (i.e. there was a low risk of bias) in 14 studies. For the remaining 19 studies, the risk of selection bias was unclear. There was no study in which the random sequence generation was inadequate (i.e. there was a high risk of bias).
Nine studies had adequately prevented (low risk) allocation concealment. In the other 24 studies, the details provided did not allow a specific evaluation of the procedures adopted to prevent participants and investigators from foreseeing the assignment.
Blinding
We judged the blinding of participants and personnel (performance bias) as low risk in 15 studies, as high risk in seven studies, and as unclear risk in the remaining 11 studies.
We judged the blinding of outcome assessment (detection bias; objective outcomes) as low risk in all 33 studies, whereas blinding of outcome assessment (detection bias; subjective outcomes) was at low risk in two studies, at high risk in six studies, and as unclear risk in the remaining 25 studies.
Incomplete outcome data
The risk of incomplete outcome data (attrition bias) was at low risk in 15 studies, at high risk in 13 studies, and at unclear risk in the remaining five studies.
Selective reporting
We judged missing data on at low risk in 13 studies, high risk in nine studies, and as having unclear risk in the other 11 studies.
2.
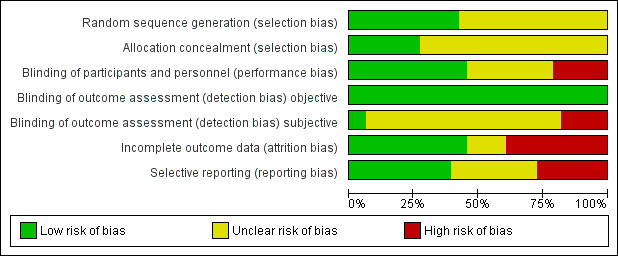
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
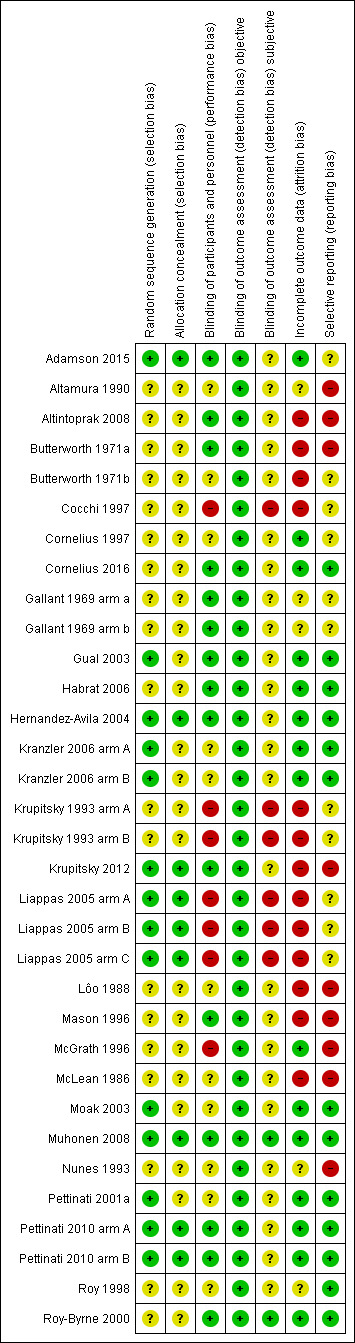
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
We compared quantitative data where at least two of the included studies reported the same outcome measures (see Appendix 8; Appendix 9). For some outcomes, it was impossible to pool data due to variations in the reporting of results, for instance different rating methods and the fact that authors did not identify the data required to proceed with the meta‐analysis. If there was significant heterogeneity, the results of the comparisons were first reported by including all studies, and thereafter by excluding studies with high risk of bias in one or more domains.
Antidepressants versus placebo
Primary outcome: depression severity
Final score (interviewer‐rated scales)
The studies used different interviewer‐rated scales, therefore, we used SMDs (see Appendix 8; Appendix 9). Two studies reported data of two interviewer‐rated scales (MADRS and HRSD) and we included only data obtained using HRSD (Gual 2003; Krupitsky 2012).
All studies
The analysis found low‐quality evidence of a significantly lower final score among participants treated with antidepressants compared to placebo (P = 0.02), with substantial evidence of heterogeneity (14 studies; 1074 participants; SMD ‐0.27, 95% CI ‐0.49 to ‐0.04; Tau² = 0.11; Chi² = 39.01, degrees of freedom (df) = 13 (P = 0.0002); I² = 67%; Analysis 1.1; Table 1; Figure 4) (Adamson 2015; Altamura 1990; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; McGrath 1996; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000).
1.1. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 1 Depression severity: final score (interviewer‐rated scales).
4.
Funnel plot of comparison: 1 Antidepressants versus placebo: all studies, outcome: 1.1 Depression severity: final score (interviewer‐rated scales).
Different classes of antidepressants and single antidepressant
The subgroup analyses found no differences in final score between SSRIs and placebo (10 studies; 881 participants; Analysis 1.1; Figure 4) (Adamson 2015; Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998), and 5‐HT2 antagonists and placebo (2 studies; 97 participants; Analysis 1.1; Figure 4) (Hernandez‐Avila 2004; Roy‐Byrne 2000).
There were no differences in final score between sertraline and placebo (8 studies; 728 participants; analysis not shown) (Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998) and nefazodone and placebo (2 studies; 97 participants ; analysis not shown) (Hernandez‐Avila 2004; Roy‐Byrne 2000).
Confounder factors
The analyses found a significantly lower final score for antidepressants among the studies with a duration four weeks or greater (P = 0.02), with substantial evidence of heterogeneity (14 studies; 1074 participants; SMD ‐0.27, 95% CI ‐0.49 to ‐0.04; Tau² = 0.11; Chi² = 39.01, df = 13 (P = 0.0002); I² = 67%; Analysis 1.1) (Adamson 2015; Altamura 1990; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; McGrath 1996; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000).
There was a significantly lower final score for antidepressants among the studies conducted in inpatient and outpatient settings (P < 0.00001) (2 studies; 63 participants; SMD ‐1.74, 95% CI ‐2.33 to ‐1.15) (Altamura 1990; Roy 1998). One of the studies had a high risk of bias (Altamura 1990).
There were no differences in final score between antidepressants and placebo among the studies:
without high risk of bias (11 studies; 963 participants) (Adamson 2015; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000);
conducted in an outpatient setting (12 studies; 1011 participants; SMD ‐0.11, 95% CI ‐0.24 to 0.01), with no evidence of heterogeneity (Adamson 2015; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; McGrath 1996; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000);
with primary depression (8 studies; 719 participants; SMD ‐0.14, 95% CI ‐0.28 to 0.01), with no evidence of heterogeneity (Adamson 2015; Kranzler 2006 arm A; McGrath 1996; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998 arm A; Roy‐Byrne 2000);
with secondary depression (2 studies; 160 participants) (Kranzler 2006 arm B; Roy 1998 arm B);
with participants who were actively drinking at the beginning of the trial (10 studies; 913 participants) (Adamson 2015; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; McGrath 1996; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000);
with participants who were not actively drinking at the beginning of the trial, with substantial evidence of heterogeneity (3 studies; 134 participants; SMD ‐0.80, 95% CI ‐1.65 to 0.005; Tau² = 0.42; Chi² = 8.08, df = 2 (P = 0.02); I² = 75%) (Gual 2003; Krupitsky 2012; Roy 1998); and
with psychotherapy (11 studies; 928 participants) (Adamson 2015; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; McGrath 1996; Moak 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000).
Final score (self‐administered scales)
The studies used different interviewer‐rated scales, therefore, we used SMDs. One study reported data obtained using the MMPI and ZUNG and we included only data obtained with ZUNG (Krupitsky 1993 arm A; 41 participants).
All studies
The analysis found no significant difference in final score between antidepressants and placebo with substantial evidence of heterogeneity (8 studies; 373 participants; SMD ‐0.29, 95% CI ‐0.64 to 0.07; Tau² = 0.13; Chi² = 15.94, df = 7 (P = 0.03); I² = 56%; Analysis 1.2) (Adamson 2015; Cornelius 2016; Krupitsky 1993 arm A; Krupitsky 2012; McLean 1986; Moak 2003; Pettinati 2001a; Roy 1998).
1.2. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 2 Depression severity: final score (self‐administered scales).
Different classes of antidepressants and single antidepressant
The subgroup analyses found no difference in final score between SSRIs and placebo (5 studies; 300 participants; Analysis 1.2) (Adamson 2015; Krupitsky 2012; Moak 2003; Pettinati 2001a; Roy 1998), and 5‐HT2 antagonists and placebo (2 studies; 41 participants; Analysis 1.2) (Cornelius 2016; McLean 1986). There were no other comparisons with at least two studies.
There were no differences in final score between sertraline and placebo (3 studies; 147 participants; analysis not shown) (Moak 2003; Pettinati 2001a; Roy 1998). There were no other comparisons with at least two studies.
Confounder factors
There were no differences in final score between antidepressants and placebo among the studies:
without high risk of bias (5 studies; 299 participants) (Adamson 2015; Cornelius 2016; Moak 2003; Pettinati 2001a; Roy 1998);
with a duration of four weeks or greater (6 studies; 259 participants) (Adamson 2015; Cornelius 2016; Krupitsky 2012; McLean 1986; Pettinati 2001a; Roy 1998);
conducted in an outpatient setting (5 studies; 278 participants) (Adamson 2015; Cornelius 2016; Krupitsky 2012; Moak 2003; Pettinati 2001a);
conducted in an inpatient setting (2 studies; 59 participants) (Krupitsky 1993 arm A; McLean 1986);
in which participants were actively drinking at the beginning of the trial (4 studies; 263 participants) (Adamson 2015; Cornelius 2016; Moak 2003; Pettinati 2001a);
with participants who were not actively drinking at the beginning of the trial (4 studies; 110 participants) (Krupitsky 1993 arm A; Krupitsky 2012; McLean 1986; Roy 1998);
with primary depression (4 studies; 267 participants) (Adamson 2015; Krupitsky 1993 arm A; Moak 2003; Roy 1998); and
with psychotherapy (6 studies; 305 participants) (Adamson 2015; Cornelius 2016; Krupitsky 2012; McLean 1986; Moak 2003; Pettinati 2001a). Analyses not shown.
Differences between baseline and final score (interviewer‐rated scales)
The studies used different interviewer‐rated scales, therefore, we used SMDs.
All studies
The analysis found no difference between antidepressants and placebo, with no evidence of heterogeneity (5 studies; 447 participants; SMD 0.15, 95% CI ‐0.12 to 0.42; Analysis 1.3) (Butterworth 1971b; Cornelius 1997; Kranzler 2006 arm A; Kranzler 2006 arm B; Pettinati 2001a).
1.3. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 3 Depression severity: difference between basal and final score (interviewer‐rated scales).
Different classes of antidepressants and single antidepressant
There were no differences between SSRIs and placebo (4 studies; 408 participants; Analysis 1.3) (Cornelius 1997; Kranzler 2006 arm A; Kranzler 2006 arm B; Pettinati 2001a), and sertraline and placebo (3 studies; 357 participants; Analysis 1.3) (Kranzler 2006 arm A; Kranzler 2006 arm B; Pettinati 2001a).
Confounder factors
There were no differences between antidepressants and placebo when other possible confounder factors were examined (high risk of bias, duration of study, typology of depression, typology of setting, being actively drinking at the beginning of the study, and receiving psychotherapy) (analyses not shown).
Differences between baseline and final score (self‐administered scales)
The studies used different interviewer‐rated scales, therefore, we used SMDs.
All studies
The analysis found no difference between antidepressants and placebo, with no evidence of heterogeneity (4 studies; 121 participants; SMD 0.20, 95% CI ‐0.16 to 0.56; Analysis 1.4) (Cornelius 1997; Cornelius 2016; McLean 1986; Pettinati 2001a).
1.4. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 4 Depression severity: difference between basal and final score (self‐administered scales).
Different classes of antidepressants and single antidepressants
There was no difference between SSRIs and placebo (2 studies; 80 participants; Analysis 1.4) (Cornelius 1997; Pettinati 2001a), and 5‐HT2 antagonists and placebo (2 studies; 41 participants; Analysis 1.4) (Cornelius 2016; McLean 1986).
Confounder factors
The analyses found no differences between antidepressants and placebo when possible confounder factors were examined (high risk of bias, duration of study, typology of depression, typology of setting, being actively drinking at the beginning of the study, and receiving psychotherapy) (analyses not shown).
Primary outcome: response to antidepressive treatment
All studies
The analysis found very low‐quality evidence of a significantly higher number of responses among participants who received antidepressants than placebo (P = 0.01), with substantial evidence of heterogeneity (10 studies; 805 participants; RR 1.40, 95% CI 1.08 to 1.82; Tau² = 0.10; Chi² = 31.63, df = 9 (P = 0.0002); I² = 72%; Analysis 1.5; Table 1; Figure 5) (Butterworth 1971b; Gallant 1969 arm a; Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; McGrath 1996; Moak 2003; Roy 1998; Roy‐Byrne 2000).
1.5. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 5 Response to antidepressive treatment.
5.
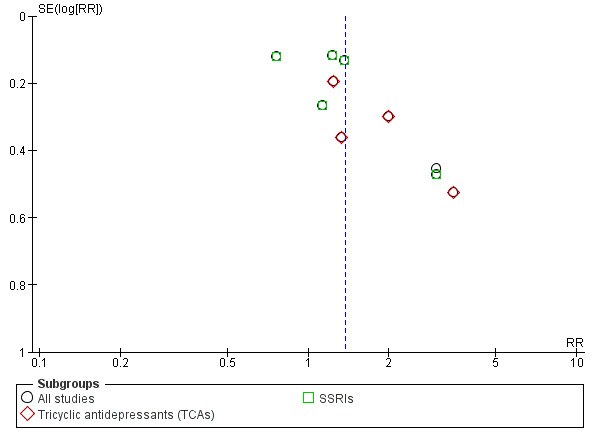
Funnel plot of comparison: 1 Antidepressants versus placebo: all studies, outcome: 1.5 Response to antidepressive treatment.
Different classes of antidepressants and single antidepressant
For the subgroup analyses, the analysis found a significantly higher number of responses among participants who received TCAs compared to placebo (P = 0.02), with no evidence of heterogeneity (4 studies; 212 participants; RR 1.60, 95% CI 1.09 to 2.34; Analysis 1.5; Figure 5) (Butterworth 1971b; Gallant 1969 arm a; Mason 1996; McGrath 1996). However, three of these four studies had a high risk of bias. There were no differences in the number of responses between SSRIs and placebo, with substantial evidence of heterogeneity (5 studies; 529 participants; RR 1.19, 95% CI 0.87 to 1.63; Tau² = 0.09; Chi² = 17.60, df = 4 (P = 0.001); I² = 77%; Analysis 1.5; Figure 5) (Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Moak 2003; Roy 1998).
There was a significantly higher number of responses among participants who received imipramine (P = 0.02), with no evidence of heterogeneity (2 studies; 108 participants; RR 1.68, 95% CI 1.07 to 2.63; analysis not shown) (Butterworth 1971b; McGrath 1996). Both these studies had a high risk of bias. There was no difference between sertraline and placebo in the number of responses, with substantial evidence of heterogeneity (5 studies; 529 participants; RR 1.19, 95% CI 0.87 to 1.63; Tau² = 0.09; Chi² = 17.60, df = 4 (P = 0.001); I² = 77%; Analysis 1.5) (Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Moak 2003; Roy 1998).
Confounder factors
The analysis found a significantly higher number of responses among participants who received antidepressants among the studies: with a duration of four weeks or greater (P = 0.04), with significant evidence of heterogeneity (8 studies; 690 participants; RR 1.40, 95% CI 1.02 to 1.91; Tau² = 0.12; Chi² = 28.51, df = 7 (P = 0.0002); I² = 75%) (Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; McGrath 1996; Moak 2003; Roy 1998; Roy‐Byrne 2000); and with primary depression (P = 0.007), with no evidence of heterogeneity (4 studies; 404 participants; RR 1.36, 95% CI 1.09 to 1.70) (Kranzler 2006 arm A; McGrath 1996; Moak 2003; Roy‐Byrne 2000). One of these studies had a high risk of bias (McGrath 1996). However, the analysis found a significantly higher number of responses among participants who received antidepressants also after the exclusion of this study (P = 0.02), with no evidence of heterogeneity (3 studies; 335 participants; RR 1.40, 95% CI 1.05 to 1.87) (Kranzler 2006 arm A; Moak 2003; Roy‐Byrne 2000).
There were no differences between antidepressants and placebo in the number of responses among the studies:
without high risk of bias, with substantial evidence of heterogeneity (7 studies; 669 participants; RR 1.27, 95% CI 0.96 to 1.68; Tau² = 0.09; Chi² = 23.20, df = 6 (P = 0.0007); I² = 74%) (Gallant 1969 arm a; Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Moak 2003; Roy 1998; Roy‐Byrne 2000);
conducted in an outpatient setting, with substantial evidence of heterogeneity (7 studies; 654 participants; RR 1.30, 95% CI 0.96 to 1.76; Tau² = 0.10; Chi² = 23.69, df = 6 (P = 0.0006); I² = 75%) (Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; McGrath 1996; Moak 2003; Roy‐Byrne 2000);
conducted an inpatient setting (2 studies; 115 participants; RR 1.48, 95% CI 0.94 to 2.32), with no evidence of heterogeneity (Butterworth 1971b; Gallant 1969 arm a);
with a duration less than four weeks (P = 0.09), with no evidence of heterogeneity (2 studies; 115 participants; RR 1.48, 95% CI 0.94 to 2.32) (Butterworth 1971b; Gallant 1969 arm a); and
in which participants received psychotherapy, with substantial evidence of heterogeneity (6 studies; 571 participants; RR 1.35, 95% CI 0.95 to 1.92; Tau² = 0.12; Chi² = 23.85, df = 5 (P = 0.0002); I² = 79%) (Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; McGrath 1996; Moak 2003; Roy‐Byrne 2000);
in which participants were actively drinking at the beginning of the trial (5 studies; 543 participants; RR 1.23, 95% CI 0.88 to 1.72) (Kranzler 2006 arm A; Kranzler 2006 arm B; McGrath 1996; Moak 2003; Roy‐Byrne 2000); and
in which participants were not actively drinking at the beginning of the trial (2 studies; 111 participants; RR 1.81, 95% CI 0.60 to 5.45) (Gual 2003; Mason 1996). Analyses not shown.
Primary outcome: full remission of depression
All studies
The analysis found no significant difference in the number of remissions between antidepressants and placebo, with a substantial evidence of heterogeneity (4 studies; 372 participants; RR 1.19, 95% CI 0.77 to 1.83; Tau² = 0.12; Chi² = 8.82, df = 3 (P = 0.03); I² = 66%; Analysis 1.6) (Adamson 2015; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000).
1.6. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 6 Full remission of depression.
Different classes of antidepressants and single antidepressant
There was no difference in the number of remissions between SSRIs and placebo, with no evidence of heterogeneity (3 studies; 308 participants; RR 1.00, 95% CI 0.74 to 1.36; Tau² = 0.03; Chi² = 3.10, df = 2 (P = 0.21); I² = 35%; Analysis 1.6) (Adamson 2015; Pettinati 2010 arm A; Pettinati 2010 arm B).
There was no difference in the number of remissions between sertraline and placebo, with no evidence of heterogeneity (2 studies; 170 participants; RR 1.18, 95% CI 0.83 to 1.67; Tau² = 0.00; Chi² = 1.07, df = 1 (P = 0.30); I² = 7%; analysis not shown) (Pettinati 2010 arm A; Pettinati 2010 arm B).
Confounder factors
All the studies (4 studies; 372 participants; RR 1.19, 95% CI 0.77 to 1.83; Tau² = 0.12; Chi² = 8.82, df = 3 (P = 0.03); I² = 66%; Analysis 1.6) were without high risk of bias, with a duration of four weeks or greater, conducted in an outpatient setting, with primary depression, with participants who were not actively drinking at the beginning of the trial, and with psychotherapy (Adamson 2015; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000).
Primary outcome: consumption of alcohol
Abstinent days (%)
All studies
There was low‐quality evidence that the rate of abstinent days did not differ between antidepressants and placebo (9 studies; 821 participants; MD 1.34%, 95% CI ‐1.66% to 4.34%; Analysis 1.7; Table 1) (Adamson 2015; Cornelius 1997; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; McGrath 1996; Moak 2003; Pettinati 2001a).
1.7. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 7 Consumption of alcohol: abstinent days (%).
For the five studies where the SDs were not available (Cornelius 1997; Gual 2003; Hernandez‐Avila 2004; McGrath 1996; Pettinati 2001a), we imputed SDs using the mean value of SD calculated from the other four studies (Adamson 2015; Kranzler 2006 arm A; Kranzler 2006 arm B; Moak 2003). Sensitivity analysis, excluding studies without SDs, showed no evidence of difference between antidepressants and placebo (MD ‐1.37 abstinent days, 95% CI ‐3.96 to 1.21).
Different classes of antidepressants and single antidepressant
There were no differences in the rate of abstinent days between SSRIs and placebo (7 studies; 711 participants; analysis not shown) (Adamson 2015; Cornelius 1997; Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Moak 2003; Pettinati 2001a), and sertraline and placebo (5 studies; 522 participants; analysis not shown) (Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Moak 2003; Pettinati 2001a).
Confounder factors
The rate of abstinent days did not differ between antidepressants and placebo when possible confounder factors were examined. Analysis not shown.
Abstinent participants
All studies
The analysis found moderate‐quality evidence of a higher number of abstinents among participants who received antidepressants than placebo (P = 0.002), with no evidence of heterogeneity (7 studies; 424 participants; RR 1.71, 95% CI 1.22 to 2.39; Analysis 1.8; Table 1) (Cornelius 1997; Hernandez‐Avila 2004; McGrath 1996; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000).
1.8. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 8 Consumption of alcohol: abstinent participants (number).
Different classes of antidepressants and single antidepressant
The analyses found a higher number of abstinents among participants who received SSRIs (P = 0.04), with no evidence of heterogeneity (4 studies; 250 participants; RR 1.66, 95% CI 1.02 to 2.68; Analysis 1.8) (Cornelius 1997; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B). There were no differences in the number of abstinents between 5‐HT2 antagonists and placebo (2 studies; 105 participants; Analysis 1.8) (Hernandez‐Avila 2004; Roy‐Byrne 2000).
There were no differences in the number of abstinents between sertraline and placebo (3 studies; 199 participants; analysis not shown) (Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B), and nefazodone and placebo (2 studies; 105 participants; analysis not shown) (Hernandez‐Avila 2004; Roy‐Byrne 2000).
Confounder factors
The analyses found a higher number of abstinents among participants treated with antidepressants among the studies:
without high risk of bias (P = 0.005), with no evidence of heterogeneity (6 studies; 355 participants; RR 1.69, 95% CI 1.18 to 2.43) (Cornelius 1997; Hernandez‐Avila 2004; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000);
with a duration of four weeks or greater (P = 0.002), with no evidence of heterogeneity (7 studies; 424 participants; RR 1.71, 95% CI 1.22 to 2.39) (Cornelius 1997; Hernandez‐Avila 2004; McGrath 1996; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000);
with primary depression (P = 0.002), with no evidence of heterogeneity (5 studies; 354 participants; RR 1.78, 95% CI 1.24 to 2.55) (Cornelius 1997; McGrath 1996; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000);
conducted in an outpatient setting (P = 0.003), with no evidence of heterogeneity (6 studies; 373 participants; RR 1.70, 95% CI 1.20 to 2.41) (Hernandez‐Avila 2004; McGrath 1996; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000);
with participants who were actively drinking at the beginning of the trial (P = 0.002), with no evidence of heterogeneity (7 studies; 424 participants; RR 1.71, 95% CI 1.22 to 2.39) (Cornelius 1997; Hernandez‐Avila 2004; McGrath 1996; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000); and
with psychotherapy (P = 0.002), with no evidence of heterogeneity (7 studies; 424 participants; RR 1.71, 95% CI 1.22 to 2.39) (Cornelius 1997; Hernandez‐Avila 2004; McGrath 1996; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000). Analyses not shown.
Drinking days (per week)
All studies
The analysis found no difference between antidepressants and placebo, with no evidence of heterogeneity (2 studies; 55 participants; MD ‐1.15 days/week, 95% CI ‐2.35 to 0.05; Analysis 1.9) (Cornelius 2016; Hernandez‐Avila 2004).
1.9. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 9 Consumption of alcohol: drinking days (per week).
Different classes of antidepressants and single antidepressant
The analysis found no difference between 5‐HT2 antagonists and placebo, with no evidence of heterogeneity (2 studies; 55 participants; MD ‐1.15 days/week, 95% CI ‐2.35 to 0.05) (Cornelius 2016; Hernandez‐Avila 2004).
Confounder factors
There were no differences between antidepressants and placebo in the number of drinking days per week when possible confounder factors were examined (analyses not shown).
Drinks (per drinking day)
All studies
The analysis found moderate‐quality evidence of a significantly lower number of drinks per drinking days among participants who received antidepressants compared to placebo (P = 0.0009), with no evidence of heterogeneity (7 studies; 451 participants; MD ‐1.13 drinks/drinking day, 95% CI ‐1.79 to ‐0.46; Analysis 1.10; Table 1) (Adamson 2015; Cornelius 1997; Cornelius 2016; Hernandez‐Avila 2004; McGrath 1996; Moak 2003; Roy‐Byrne 2000).
1.10. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 10 Consumption of alcohol: drinks (per drinking days).
Different classes of antidepressants and single antidepressant
The analysis found a significantly lower number of drinks per drinking days among participants who received SSRIs (P = 0.02) (3 studies; 271 participants; MD ‐1.42 drinks/drinking day, 95% CI ‐2.58 to ‐0.26; Analysis 1.10) (Adamson 2015; Cornelius 1997; Moak 2003), and 5‐HT2 antagonists (P = 0.03) (3 studies; 111 participants; MD ‐1.06 drinks/drinking day, 95% CI ‐2.00 to ‐0.11; Analysis 1.10) (Cornelius 2016; Hernandez‐Avila 2004; Roy‐Byrne 2000). The number of drinks per drinking days did not differ between nefazodone and placebo, with no evidence of heterogeneity (2 studies; 97 participants; MD ‐1.14 drinks/drinking day, 95% CI ‐2.30 to 0.03; analysis not shown) (Hernandez‐Avila 2004; Roy‐Byrne 2000).
Confounder factors
The analysis found a significantly lower number of drinks per drinking days among participants treated with antidepressants among the studies:
without high risk of bias (P = 0.0007), with no evidence of heterogeneity (6 studies; 382 participants; MD ‐1.21 drinks/drinking day, 95% CI ‐1.91 to ‐0.51) (Adamson 2015; Cornelius 1997; Cornelius 2016; Hernandez‐Avila 2004; Moak 2003; Roy‐Byrne 2000);
with a duration of four weeks or longer (P = 0.006), with no evidence of heterogeneity (6 studies; 400 participants; MD ‐0.97 drinks/drinking day, 95% CI ‐1.66 to ‐0.28) (Adamson 2015; Cornelius 2016; Hernandez‐Avila 2004; McGrath 1996; Moak 2003; Roy‐Byrne 2000);
with primary depression (P = 0.005), with no evidence of heterogeneity (5 studies; 396 participants; MD ‐1.20 drinks/drinking day, 95% CI ‐2.03 to ‐0.36) (Adamson 2015; Cornelius 1997; McGrath 1996; Moak 2003; Roy‐Byrne 2000);
conducted in an outpatient setting (P = 0.006), with no evidence of heterogeneity (6 studies; 400 participants; MD ‐0.97 drinks/drinking day, 95% CI ‐1.66 to ‐0.28) (Adamson 2015; Cornelius 2016; Hernandez‐Avila 2004; McGrath 1996; Moak 2003; Roy‐Byrne 2000);
with participants who were actively drinking at the beginning of the trial (P = 0.009), with no evidence of heterogeneity (7 studies; 451 participants; MD ‐1.13 drinks/drinking day, 95% CI ‐1.79 to ‐0.46) (Adamson 2015; Cornelius 1997; Cornelius 2016; Hernandez‐Avila 2004; McGrath 1996; Moak 2003; Roy‐Byrne 2000); and
with psychotherapy (P = 0.002), with no evidence of heterogeneity (6 studies; 395 participants; MD ‐1.12 drinks/drinking day, 95% CI ‐1.83 to ‐0.41) (Adamson 2015; Cornelius 1997; Cornelius 2016; Hernandez‐Avila 2004; McGrath 1996; Moak 2003). Analyses not shown.
Drinks (per week)
All studies
The analysis found no difference between antidepressants and placebo, with no evidence of heterogeneity (2 studies; 55 participants; MD ‐5.06 drinks/week, 95% CI ‐12.30 to 2.18; Analysis 1.11) (Cornelius 2016; Hernandez‐Avila 2004).
1.11. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 11 Consumption of alcohol: drinks (per week).
Different classes of antidepressants and single antidepressant
The analysis found no difference between 5‐HT2 antagonists and placebo, with no evidence of heterogeneity (2 studies; 55 participants; Analysis 1.11) (Cornelius 2016; Hernandez‐Avila 2004).
Confounder factors
There were no differences between antidepressants and placebo in the number of drinks per week when possible confounder factors were examined (analyses not shown).
Heavy drinking days (per week)
All studies
The analysis found no difference between antidepressants and placebo, with substantial evidence of heterogeneity (5 studies; 313 participants; MD ‐0.33 heavy drinking days/week, 95% CI ‐0.85 to 0.20; Tau² = 0.25; Chi² = 15.22, df = 4 (P = 0.004); I² = 74%; Analysis 1.12) (Adamson 2015; Cornelius 1997; Cornelius 2016; Hernandez‐Avila 2004; McGrath 1996). Because the SDs were not available for three studies, we used the mean of the SDs of the other studies.
1.12. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 12 Consumption of alcohol: heavy drinking days (per week).
Different classes of antidepressants and single antidepressant
The analysis found no difference between SSRIs and placebo (2 studies; 189 participants; MD ‐0.41 heavy drinking days/week, 95% CI ‐1.09 to 0.27; Analysis 1.12) (Adamson 2015; Cornelius 1997), and 5‐HT2 antagonists and placebo (2 studies; 55 participants; MD ‐0.43 heavy drinking days/week, 95% CI ‐2.09 to 1.22; Analysis 1.12) (Cornelius 2016; Hernandez‐Avila 2004).
Confounder factors
There were no differences between antidepressants and placebo when possible confounder factors were examined (analyses not shown).
Heavy drinkers
All studies
The analysis found no difference between antidepressants and placebo in the number of heavy drinkers, with substantial evidence of heterogeneity (7 studies; 459 participants; RR 0.78, 95% CI 0.57 to 1.07; Tau² = 0.09; Chi² = 15.49, df = 6 (P = 0.02); I² = 61%; Analysis 1.13) (Gual 2003; Krupitsky 2012; Mason 1996; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998).
1.13. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 13 Consumption of alcohol: heavy drinkers (number).
Different classes of antidepressants and single antidepressant
The analysis found no difference in the number of heavy drinkers between SSRIs and placebo (6 studies; 431 participants; RR 0.87, 95% CI 0.69 to 1.11; Analysis 1.13) (Gual 2003; Krupitsky 2012; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998), and sertraline and placebo (5 studies; 371 participants; RR 0.94, 95% CI 0.78 to 1.13; analysis not shown) (Gual 2003; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998).
Confounder factors
There were no differences between antidepressants and placebo when possible confounder factors were examined (analyses not shown).
Time to first relapse (days)
All studies
The analysis found no difference between antidepressants and placebo in the time to the first relapse, with substantial evidence of heterogeneity (6 studies; 348 participants; MD 2.54 days, 95% CI ‐8.79 to 13.87; Tau² = 102.26; Chi² = 13.58, df = 5 (P = 0.02); I² = 63%; Analysis 1.14) (Cornelius 1997; Gual 2003; Krupitsky 2012; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B).
1.14. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 14 Consumption of alcohol: time to first relapse (days).
Different classes of antidepressants and single antidepressant
The analysis found no difference between SSRIs and placebo (6 studies; 348 participants; MD 2.54 days, 95% CI ‐8.79 to 13.87; Analysis 1.14) (Cornelius 1997; Gual 2003; Krupitsky 2012; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B), and sertraline and placebo (4 studies; 282 participants; MD 0.70, 95% CI ‐12.67 to 14.08; analysis not shown) (Gual 2003; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B).
Confounder factors
There were no differences between antidepressants and placebo when the possible confounder factors were examined (analyses not shown).
Primary outcome: liver enzyme levels
GGT
All studies
There was no evidence of a difference between antidepressants and placebo (2 studies; 56 participants; MD ‐8.39 U/L, 95% CI ‐26.47 to 9.68; Analysis 1.15) (Hernandez‐Avila 2004; Krupitsky 2012).
1.15. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 15 Liver enzyme levels: γ‐glutamyltransferase (U/L).
Different classes of antidepressants and single antidepressant
Final levels of GGT were not available for more than one study for any analysis.
Confounder factors
There were no differences between antidepressants and placebo in the final levels of GGT when the possible confounder factors were examined (analyses not shown).
Global response (depression and alcohol consumption)
All studies
The analysis found a higher number of global responses among participants treated with antidepressants than placebo (P = 0.003), with no evidence of heterogeneity (3 studies; 152 participants; RR 2.37, 95% CI 1.34 to 4.19; Analysis 1.16) (Krupitsky 2012; McGrath 1996; Nunes 1993). However, all the three studies had a high risk of bias.
1.16. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 16 Depression and alcohol: global response.
Different classes of antidepressants and single antidepressant
There was a higher number of global responses among participants treated with TCAs compared to placebo (P = 0.03) (2 studies; 92 participants; RR 2.09, 95% CI 1.09 to 4.02; Analysis 1.16) (McGrath 1996; Nunes 1993), and imipramine compared to placebo (P = 0.03) (2 studies; 92 participants; RR 2.09, 95% CI 1.09 to 4.02; analysis not shown) (McGrath 1996; Nunes 1993). However, both these studies had a high risk of bias.
Confounder factors
The analyses found a higher number of global responses among participants treated with antidepressants compared to placebo among the studies:
with a duration of four weeks or greater (P = 0.003), with no evidence of heterogeneity (3 studies; 152 participants; RR 2.37, 95% CI 1.34 to 4.19) (Krupitsky 2012; McGrath 1996; Nunes 1993);
with primary depression (P = 0.03), with no evidence of heterogeneity (2 studies; 92 participants; RR 2.09, 95% CI 1.09 to 4.02) (McGrath 1996; Nunes 1993);
conducted in an outpatient setting (P = 0.003), with no evidence of heterogeneity (3 studies; 152 participants; RR 2.37, 95% CI 1.34 to 4.19) (Krupitsky 2012; McGrath 1996; Nunes 1993);
with participants who were not actively drinking at the beginning of the trial (P = 0.003), with no evidence of heterogeneity (3 studies; 152 participants; RR 2.37, 95% CI 1.34 to 4.19) (Krupitsky 2012; McGrath 1996; Nunes 1993);
with psychotherapy (P = 0.003), with no evidence of heterogeneity (3 studies; 152 participants; RR 2.37, 95% CI 1.34 to 4.19) (Krupitsky 2012; McGrath 1996; Nunes 1993). Analyses not shown. However, all the three studies had a high risk of bias.
Primary outcome: acceptability (all‐cause dropouts)
All studies
The analysis found low‐quality of evidence of no difference in all‐cause dropouts between antidepressants and placebo, with no evidence of heterogeneity (17 studies; 1159 participants; RR 0.98, 95% CI 0.79 to 1.22; Tau² = 0.07; Chi² = 23.80, df = 14 (P = 0.05); I² = 41%; Analysis 1.17; Table 1; Figure 6) (Altamura 1990; Butterworth 1971b; Cornelius 2016; Gallant 1969 arm a; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Mason 1996; McGrath 1996; McLean 1986; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000).
1.17. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 17 Acceptability: dropouts.
6.
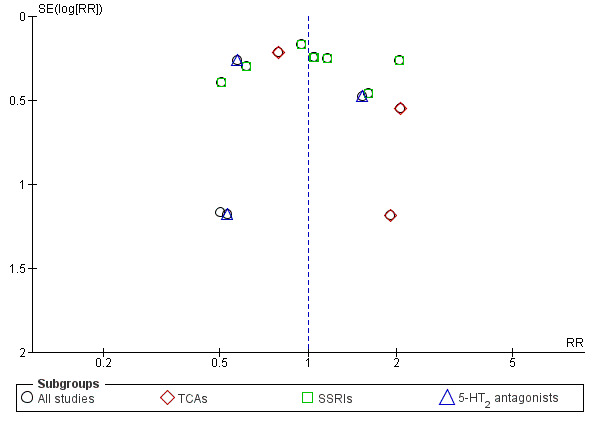
Funnel plot of comparison: 1 Antidepressants versus placebo: all studies, outcome: 1.17 Acceptability: dropouts.
Different classes of antidepressants and single antidepressant
There were no differences in the number of dropouts between:
TCAs and placebo (4 studies; 216 participants; RR 1.21, 95% CI 0.48 to 3.06) (Butterworth 1971b; Gallant 1969 arm a; Mason 1996; McGrath 1996);
SSRIs and placebo (8 studies; 759 participants; RR 1.04, 95% CI 0.79 to 1.36) (Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998);
5‐HT2 antagonists and placebo (4 studies; 154 participants; RR 0.79, 95% CI 0.38 to 1.64; Analysis 1.17; Figure 6) (Cornelius 2016; Hernandez‐Avila 2004; McLean 1986; Roy‐Byrne 2000);
imipramine and placebo (2 studies; 112 participants; RR 2.03, 95% CI 0.76 to 5.41) (Butterworth 1971b; McGrath 1996);
sertraline and placebo (7 studies; 699 participants; RR 1.00, 95% CI 0.76 to 1.33) (Gual 2003; Kranzler 2006 arm A; Kranzler 2006 arm B; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998); and
nefazodone and placebo (2 studies; 105 participants; RR 0.86, 95% CI 0.33 to 2.24) (Hernandez‐Avila 2004; Roy‐Byrne 2000) (not all analyses shown).
Confounder factors
There were no differences between antidepressants and placebo when possible confounder factors were examined (high risk of bias, setting, duration of study, typology of depression, and use of psychotherapy; analyses not shown).
Primary outcome: tolerability of treatment
Withdrawal for medical reasons
All studies
The analysis found low‐quality of evidence of no difference in the number of withdrawals for medical reasons between antidepressants and placebo (10 studies; 947 participants; RR 1.15, 95% CI 0.65 to 2.04; Analysis 1.18; Table 1) (Adamson 2015; Cornelius 1997; Kranzler 2006 arm A; Krupitsky 2012; Mason 1996; McGrath 1996; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000).
1.18. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 18 Tolerability of treatment: adverse events.
Different classes of antidepressants and single antidepressant
There were no differences in the number of withdrawals for medical reasons between SSRIs and placebo (7 studies; 786 participants; RR 1.10, 95% CI 0.52 to 2.32; Analysis 1.18) (Adamson 2015; Cornelius 1997; Kranzler 2006 arm A; Krupitsky 2012; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998), TCAs and placebo (2 studies; 97 participants; RR 0.91, 95% CI 0.10 to 8.41; Analysis 1.18) (Mason 1996; McGrath 1996), and sertraline and placebo (4 studies; 537 participants; RR 0.90, 95% CI 0.35 to 2.34; analysis not shown) (Kranzler 2006 arm A; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998).
Confounder factors
There were no differences between antidepressants and placebo when possible confounder factors were examined. Analyses not shown.
Total adverse events
All studies
The analysis found no difference in the total number of adverse events between antidepressants and placebo, with substantial evidence of heterogeneity (5 studies; 644 participants; RR 1.18, 95% CI 0.97 to 1.44; Tau² = 0.02; Chi² = 13.57, df = 4 (P = 0.009); I² = 71%; Analysis 1.18) (Adamson 2015; Butterworth 1971b; Gallant 1969 arm a; Kranzler 2006 arm A; Krupitsky 2012).
Different classes of antidepressants and single antidepressant
There were no differences between SSRIs and placebo (3 studies; 529 participants; RR 1.06, 95% CI 0.92 to 1.23; Analysis 1.18) (Adamson 2015; Kranzler 2006 arm A; Krupitsky 2012), whereas there was a higher number of total adverse events (P = 0.009) among the studies in which participants received TCAs, with no evidence of heterogeneity (2 studies; 115 participants; RR 1.66, 95% CI 1.13 to 2.42; Analysis 1.18) (Butterworth 1971b; Gallant 1969 arm a). However, one of these studies had a high risk of bias (Butterworth 1971b).
Confounder factors
There was a higher number of total adverse events among participants treated with antidepressants for studies with a duration less than four weeks (P = 0.009) (2 studies; 115 participants; RR 1.66, 95% CI 1.13 to 2.42; analysis not shown) (Butterworth 1971b; Gallant 1969 arm a), and conducted in an inpatient setting (P = 0.009) (2 studies; 115 participants; RR 1.66, 95% CI 1.13 to 2.42; analysis not shown) (Butterworth 1971b; Gallant 1969 arm a). However, one of these studies had a high risk of bias (Butterworth 1971b). There were no differences between antidepressants and placebo when the other possible confounder factors were examined.
Other observations
All studies
Seven single adverse events were investigated by more than one study: dry mouth, insomnia, headache, dizziness, diarrhoea, nausea, and constipation. There was a higher number of episodes of insomnia among participants who received antidepressants compared to those who received placebo (P = 0.04) (4 studies; 564 participants; RR 1.69, 95% CI 1.02 to 2.77; Analysis 1.18) (Adamson 2015; Butterworth 1971b; Kranzler 2006 arm A; Roy‐Byrne 2000). There was no difference between antidepressants and placebo for the other adverse events.
Different classes of antidepressants and single antidepressant
The analyses found a higher number of episodes of insomnia among participants treated with SSRIs (P = 0.04) (2 studies; 469 participants; RR 1.75, 95% CI 1.04 to 2.96; Analysis 1.18) (Adamson 2015; Kranzler 2006 arm A). Neither study had a high risk of bias.
There was no difference between antidepressants and placebo in the occurrence of headache for SSRIs (2 studies; 414 participants; Analysis 1.18) (Gual 2003; Kranzler 2006 arm A), or sertraline (2 studies; 414 participants; analysis not shown) (Gual 2003; Kranzler 2006 arm A).
The analyses found no differences between SSRIs and placebo in the occurrence of nausea (2 studies; 221 participants; Analysis 1.18) (Adamson 2015; Gual 2003). The other adverse events were not reported by more than one study of each class of antidepressants.
Confounder factors
The numbers of episodes of insomnia and dry mouth did not differ between antidepressants and placebo when possible confounder factors were examined (analysis not shown).
Total serious adverse events
All studies
The analysis found no difference in the number of total serious adverse events between antidepressants and placebo, with no evidence of heterogeneity (7 studies; 774 participants; RR 1.22, 95% CI 0.80 to 1.86; Analysis 1.18) (Adamson 2015; Butterworth 1971b; Cornelius 2016; Kranzler 2006 arm A; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B).
Different classes of antidepressants and single antidepressant
The analyses found no differences between antidepressants and placebo in total serious adverse events for SSRIs (5 studies; 721 participants; Analysis 1.18) (Adamson 2015; Kranzler 2006 arm A; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B), or sertraline (4 studies; 583 participants; analysis not shown) (Kranzler 2006 arm A; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B).
Confounder factors
There were no differences between antidepressants and placebo when the role of all the possible confounder factors was investigated (analyses not shown).
Single serious adverse event
All studies
The analysis found no difference between antidepressants and placebo regarding the two single serious adverse events investigated by more than one study: worsening of clinical condition because of relapse and depression (Analysis 1.18).
Different classes of antidepressants and single antidepressant
The analysis found no difference when the studies were analyzed according to the different classes of antidepressants and when the role of all the possible confounder factors was investigated (analyses not shown).
Primary outcome: suicide and suicidal attempts
All studies
The analysis found no difference between antidepressants and placebo, with no evidence of heterogeneity (4 studies; 602 participants; RR 1.33, 95% CI 0.23 to 7.61; Analysis 1.19) (Adamson 2015; Cornelius 1997; Kranzler 2006 arm A; Moak 2003). None of these studies had a high risk of bias.
1.19. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 19 Suicide attempts.
Different classes of antidepressants and single antidepressant
The analyses found no difference in the number of suicide attempts between antidepressants and placebo for SSRIs (4 studies; 602 participants; Analysis 1.19) (Adamson 2015; Cornelius 1997; Kranzler 2006 arm A; Moak 2003), or sertraline (2 studies; 413 participants; analysis not shown) (Kranzler 2006 arm A; Moak 2003).
Confounder factors
There were no differences between antidepressants and placebo when confounder factors were examined (analyses not shown).
Secondary outcome: craving
All studies
The analysis found no significant difference between antidepressants and placebo (2 studies; 29 participants; MD 1.00 cravings for alcohol, 95% CI ‐3.27 to 5.27; Analysis 1.20) (Cornelius 2016; Krupitsky 2012).
1.20. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 20 Secondary outcomes: craving.
Different classes of antidepressants and single antidepressant
Measures on craving for alcohol were not available for more than one study for any analysis.
Confounder factors
The analyses found no significant differences between antidepressants and placebo among the studies conducted in an outpatient setting and with psychotherapy (2 studies; 29 participants; analysis not shown) (Cornelius 2016; Krupitsky 2012).
Secondary outcome: severity of alcohol dependence
Because different scales were used, we used SMDs (see Appendix 8; Appendix 9).
All studies
The analysis found no significant difference between antidepressants and placebo (2 studies; 168 participants; SMD ‐0.14, 95% CI ‐0.44 to 0.17; Analysis 1.21) (Adamson 2015; Hernandez‐Avila 2004).
1.21. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 21 Secondary outcomes: severity of dependence.
Different classes of antidepressants and single antidepressant
Measures on severity of alcohol dependence were not available for more than one study for any analysis.
Confounder factors
Both the two studies (168 participants; Adamson 2015; Hernandez‐Avila 2004) were without high risk of bias, conducted in an outpatient setting, with a duration of four weeks or greater, in which participants were actively drinking at the beginning of the trial, and with psychotherapy.
Secondary outcome: psychiatric symptoms/psychological distress
All studies
Final score of anxiety severity using an interviewer‐rated scale was not available for more than one study. The analysis found a significantly lower value among participants using a self‐administered scale (P = 0.002), with no evidence of heterogeneity (3 studies; 97 participants; MD ‐6.31 points, 95% CI ‐10.33 to ‐2.28; Chi² = 0.18, df = 2 (P = 0.91); I² = 0%; Analysis 1.22) (Hernandez‐Avila 2004; Krupitsky 1993 arm A; Krupitsky 2012). However, two of these studies had a high risk of bias (Krupitsky 1993 arm A; Krupitsky 2012).
1.22. Analysis.
Comparison 1 Antidepressants versus placebo: all studies, Outcome 22 Secondary outcomes: severity of anxiety.
Different classes of antidepressants and single antidepressant
Data were not available for more than one study for any analysis.
Confounder factors
The analyses found a significantly lower value among participants who received antidepressants than placebo (P = 0.02) among the studies conducted in an outpatient setting, with psychotherapy, with a duration of four weeks or greater, with no evidence of heterogeneity (2 studies; 56 participants; MD ‐5.78, 95% CI ‐10.50 to ‐1.06; Chi² = 0.01, df = 1 (P = 0.94); I² = 0%; analysis not shown) (Hernandez‐Avila 2004; Krupitsky 2012), and in which participants were not actively drinking at the beginning of the trial, with no evidence of heterogeneity (2 studies; 56 participants; MD ‐6.73, 95% CI ‐12.48 to ‐0.98; Chi² = 0.14, df = 1 (P = 0.71); I² = 0%; analysis not shown) (Krupitsky 1993 arm A; Krupitsky 2012).
Antidepressants versus other medications
Data were not available for more than one study for any analysis.
Antidepressants versus psychotherapy
Depression severity: final score
The analysis found no difference between antidepressants and psychotherapy, with no evidence of heterogeneity (2 studies; 60 participants; MD ‐2.61 points, 95% CI ‐6.92 to 1.70; Analysis 2.1) (Liappas 2005 arm A; Liappas 2005 arm B).
2.1. Analysis.
Comparison 2 Antidepressants versus psychotherapy, Outcome 1 Depression severity: final score.
Global assessment: final score
Participants who received antidepressants achieved lower final scores (P = 0.01) than participants who received psychotherapy, with no evidence of heterogeneity (2 studies; 60 participants; MD 5.92 points, 95% CI 1.30 to 10.54; Analysis 2.2) (Liappas 2005 arm A; Liappas 2005 arm B). Both studies had a high risk of bias.
2.2. Analysis.
Comparison 2 Antidepressants versus psychotherapy, Outcome 2 Global assessment: final score.
Acceptability: all‐causes dropouts
The analysis found no difference between antidepressants and psychotherapy, with no evidence of heterogeneity (2 studies; 68 participants; RR 1.43, 95% CI 0.31 to 6.54; Analysis 2.3) (Liappas 2005 arm A; Liappas 2005 arm B).
2.3. Analysis.
Comparison 2 Antidepressants versus psychotherapy, Outcome 3 Acceptability: dropouts.
Discussion
Summary of main results
A total of 33 trials (2242 participants) met the inclusion criteria: among them, 22 studies (1438 participants) compared an antidepressant to placebo; five (621 participants) compared one antidepressant to another; and four (228 participants) compared the efficacy of an antidepressant to another medication. Two studies (60 participants) compared the efficacy of an antidepressant to psychotherapy.
The antidepressants considered in the studies were: amitriptyline (five studies); citalopram (one study); desimipramine (one study); doxepin (three studies); escitalopram (one study), fluoxetine (one study); fluvoxamine (one study); imipramine (three studies); mianserine (one study); mirtazapine (four studies); nefazodone (two studies); paroxetine (one study); sertraline (eight studies); tianeptine (two studies); venlafaxine (two studies); and vilofaxine (one study).
Comparing antidepressants to placebo, we found low‐quality evidence that antidepressants reduced the severity of depression evaluated using a continuous outcome (i.e. final score in interviewer‐rated scales) and very low‐evidence using a dichotomous outcome (i.e. response). We found no difference between antidepressants and placebo excluding studies with high risk of bias. In addition, we found no difference for other relevant outcomes such as the difference between baseline and final score.
Regarding alcohol consumption, we found moderate‐quality evidence that antidepressants increased with respect to placebo, the number of participants abstaining during the trial and reduced the number of drinks per drinking day. However, we found no differences between antidepressants and placebo for other relevant outcomes such as the rate of abstinent days (low‐quality evidence).
With regards to acceptability and tolerability, we found low‐quality evidence that antidepressants did not increase the rate of dropouts or withdrawals for medical reasons when compared with placebo.
Considering confounders/moderators, trials lasting more than four weeks or including only people with primary major depression showed no impact on the efficacy of antidepressants in reducing the severity of depression at the end of treatment and rate of response. The other confounders/moderators (typology of depression, setting, and psychotherapy) did not have a substantial impact on these results, which were often limited by the small number of included studies in the subgroup and confounders/moderators analyses.
There were few studies comparing one antidepressant versus another or antidepressants versus other interventions, and these had a small sample size and were heterogeneous in terms of the types of interventions that were compared, yielding results that were not informative.
Overall completeness and applicability of evidence
Besides the limits in external validity due to the general requirements of RCTs (strict inclusion criteria, highly homogeneous study groups, limitations in dose adjustment, etc.), the types of participants (adults with co‐occurring depression and alcohol dependence) are quite representative of the general target population. Moreover, interventions (antidepressant dosages), settings, and outcomes investigated (dropouts, alcohol use, adverse events, depression severity changes) are important to patients, practitioners, and decision makers and are relevant to the context of current practice.
In contrast to other reviews in the field of addiction, for which a large majority of studies were conducted in the USA, more than one third of the studies included in this review were conducted in other countries. This is an important issue in terms of generalizability of the evidence, because different social contexts can influence depression severity and alcohol dependence and availability to enter a clinical trial; also, different clinical contexts can influence the selection of participants and the results of the treatment, acting as an effect modifier in the estimation of efficacy of treatment.
Quality of the evidence
For the evaluation of the quality of the evidence, we collected supplementary information from the authors of the primary studies, mainly because some features of the study design were omitted from trial reports or data on outcomes were lacking. Some outcome measures were obtained according to the Cochrane Handbook for Systematic Reviews of Interventions suggested procedures from available values (Higgins 2011). In one case (Analysis 1.7), missing SDs were imputed as the mean of SDs of the other studies included in the comparison. In this case, a sensitivity analysis was carried out to assess how the results were sensitive to changes.
Moreover, the assumption that missing data correspond to poor outcome was made choosing dropout as a primary outcome. Another approach, as the last observation carried forward was not followed since included studies with missing data did not provide multiple point data. In the end, availability of data on primary outcomes varied from 72.7% (16 of 22 RCTs) for dropouts to 9.5% (2 of 22 RCTs) for the GGT values.
The poor reporting of study design was mainly for the methods used for generating random sequences and allocation concealment, with more than two‐thirds of trials at unclear risk for selection bias. Moreover, 25% of the studies had a high risk of attrition and reporting bias. Finally, about half of the studies were at high or unclear risk of performance bias and almost all at unclear risk of detection bias.
Overall, the quality of evidence was moderate for people abstinent during the trial and for the number of drinks per drinking day; low for the severity of depression measured at the end of the trial, for the rate of abstinent days, dropouts, and adverse events; and very low for the ascertainment of the response to the treatment.
Potential biases in the review process
We did not find any unpublished studies despite a significant effort in contacting all the first authors of the included studies and the search of conference proceedings.
The possibility of publication bias was inspected by funnel plot only for the comparison 'Antidepressants versus placebo' and for the outcomes, severity of depression at the end of treatment (Figure 4), rate of responses (Figure 5), and dropouts (Figure 6), because in the other comparisons and for the other outcomes there were too few studies to make the funnel plot informative. One of the three funnel plots (dropouts) showed asymmetry suggesting publication bias in favour of studies with positive results (Figure 6).
We acknowledge that the funnel plot should be seen as a generic mean of displaying small‐study effects, so that asymmetry could be due to publication bias, but also to other reasons such as greater risk of bias of smaller studies, inclusion of a more restrictive and thus responsive population, or merely by the play of chance. The majority of the studies included in this review had a small sample size (fewer than 100 participants). GRADE guidelines suggest that authors of systematic reviews should suspect publication bias when studies are uniformly small (Guyatt 2011).
Agreements and disagreements with other studies or reviews
Four previous non‐Cochrane systematic reviews evaluating pooled data with meta‐analyses have been identified (Foulds 2015; Iovieno 2011; Nunes 2004; Torrens 2005). These reviews were carried out on the basis of pre‐established criteria for searching literature, selecting studies, and assessing their risk of bias. The first review evaluated the efficacy of depression treatment in people with substance‐use disorders, but eight studies specifically investigated the efficacy of antidepressants in people with co‐occurring alcohol dependence and depression (Nunes 2004). This review concluded that antidepressants exert a modest beneficial effect for people with co‐occurring depression and substance‐use disorders, including a positive impact on alcohol consumption.
The second meta‐analysis investigated the efficacy of antidepressants in people with substance‐use disorder with and without depression (Torrens 2005). Among the selected trials, nine studies specifically investigated the efficacy of antidepressants in people with co‐occurring alcohol dependence and depression. The results of the meta‐analysis failed to demonstrate any impact of antidepressants on alcohol consumption or a significant advantage for the use of SSRIs for the treatment of depression while giving indication for a possible effect of other antidepressants (Torrens 2005).
The third meta‐analysis evaluated the efficacy of antidepressants in participants with substance‐use disorder with depression or dysthymic disorder (Iovieno 2011). The results of the meta‐analysis (11 trials) showed that antidepressants are more efficacious than placebo in this population. This review did not consider the impact of antidepressants on alcohol consumption.
The fourth meta‐analysis investigated possible differences in response to a treatment for depression in alcohol‐dependent participants according to the different typology of depression, divided into independent and alcohol‐induced depression (Foulds 2015). This study selected 22 clinical trials of which 13 evaluated the efficacy of antidepressants compared to placebo in people with alcohol dependence and depression. The meta‐analysis was conducted on 11 trials. The results showed that, globally, treatment of depression in alcohol‐dependent people is associated with a large early improvement in depression severity, even when depression is independent from drinking, with a stronger effect in independent depression than in alcohol‐induced depression (Foulds 2015). In this review, the effect of antidepressants on alcohol consumption was not considered.
The first three reviews were not specifically designed for evaluating the efficacy and safety of antidepressants for alcohol‐dependent people. The fourth review adopted very selective inclusion criteria (studies reporting data over at least eight weeks; studies reporting a change in depression scales). All the reviews did not apply stringent Cochrane criteria for systematic reviews and did not consider dropouts and adverse effects as a primary outcome.
Compared to the previous reviews, our review adopted Cochrane criteria, included relevant variables among the primary outcomes such as severity depression, alcohol consumption, dropout rate, and adverse events. Unfortunately, the results obtained, despite based on more than 30 RCTs and 2000 participants, did not resolve the uncertainty coming from the previous reviews. According to our results, compared to placebo, antidepressants exert some positive effects both on depression and alcohol dependence. However, these effects may only have modest impact on both depression and alcohol‐related outcomes. Considering depression severity, the positive effects were limited to only some outcomes (i.e. depression severity evaluated with interviewer‐rated scales at the end of trial and response to treatment) and were no longer significant when studies at high risk of bias were excluded. Similarly, the positive effects observed considering potential confounders (i.e. for depression severity, duration of treatment and setting; for response to treatment, classes of antidepressants, and duration of treatment) were no longer significant when studies at high risk of bias were excluded. Only for primary depression, the positive effects were still present after the exclusion of studies at high risk of bias.
The positive effects also had a modest impact on alcohol consumption. Indeed, antidepressants improve only some outcomes (i.e. the number of abstinent participants and the number of drinks per drinking days) and not others (e.g. the rate of abstinent days). However, the positive effects shown by antidepressants were still evident when studies at high risk of bias are excluded both in the entire sample of participants and when they are divided into subgroups according potential confounders (outpatient setting, duration of treatment greater than four weeks, primary depression, receiving psychotherapy, and being actively drinking at the begging of treatment) and different classes of antidepressants (i.e. SSRIs).
Authors' conclusions
Implications for practice.
We found low‐quality evidence supporting the clinical use of antidepressants in the treatment of people with co‐occurring depression and alcohol dependence. Low‐quality evidence suggests that antidepressants have positive effects on certain outcomes related to depression (severity of depression at the end of the trial and response to treatment) and moderate‐quality evidence in certain outcomes related to alcohol use (number of abstinent participants during the trial and number of drinks per drinking day). However, we found no differences in other relevant outcomes, such as the difference between the baseline and final score for depression severity and rate of abstinent days for alcohol consumption. Moreover, the positive findings for antidepressants on the outcomes related to depression are no longer significant when studies with high risk of bias are excluded. In contrast, low‐quality evidence shows that antidepressants have good acceptability and tolerability, with no significant differences compared to placebo in all‐cause dropouts, withdrawals for medical reasons, and total adverse events.
According to our results, in people with co‐occurring depression and alcohol dependence, antidepressants may be useful for the treatment of depression, alcohol dependence, or both disorders, although the clinical relevance may be modest. Results are also limited by the large number of studies showing high or unclear risk of bias and by the low number of studies comparing one antidepressant to another or antidepressants to other medications. In people with co‐occurring depression and alcohol dependence, the risk of developing adverse effects appears to be minimal, especially for the newer classes of antidepressants (such as selective serotonin reuptake inhibitors).
Implications for research.
Further research is required to strengthen evidence on the efficacy of antidepressants in the treatment of people with co‐occurring depression and alcohol dependence. In implementing new trials on the topic, specific attention should be paid to the main methodological challenges associated with addiction research, particularly the rate of dropouts and related handling of missing data, the validation of self‐reported measures by objective measures, the choice of outcomes and related measures to allow comparison of results between studies, and, more generally, the stricter adherence to methodological standards of reporting, as outlined in the CONSORT statement (Moher 2001).
Looking at the literature on the management of depression in alcohol‐dependent people, factors that influence the research on the efficacy of pharmacological interventions include the uncertainty of diagnosis of depression in people affected by alcohol dependence or substance‐use disorder (or both) (Nunes 2004; Torrens 2005; Nunes 2006), the timeframe necessary for antidepressants to have full effect, and the inadequate reporting of associated psychosocial interventions. Regarding these issues, the adoption of strict operationalized criteria should be encouraged.
Acknowledgements
We thank the team of the Cochrane Drugs and Alcohol Group (CDAG, Rome, Italy) for their support in the preparation and conduct of this review, especially Zuzana Mitrova for help with the search strategy and Laura Amato who commented on and amended the review text. We also thank Professor Evgeny M Krupitsy, Laboratory of Clinical Psychopharmacology of Addictions, Department of Addictions, Bekhterev Research Psychoneurological Institute, St Petersburg, Russia for the contribution of unreported data and information*. *Authors of primary studies not mentioned in this section were not contacted, as all relevant data and information were available from the trial publication, or they were unable to provide requested data or information.
Appendices
Appendix 1. Abbreviations
ADS: Alcohol Dependence Scale
ALT: alanine aminotransferase
ASI: Addiction Severity Index
AST: aspartate aminotransferase
AUD: Alcohol Use Disorder
AUDIT: Alcohol Use Disorders Identification Test
BAI: Beck Anxiety Inventory
BDI: Beck Depression Inventory
BPRS: Brief Psychiatric Rating Scale
BSCS: Brief Substance Craving Scale
CBT: cognitive behavioural therapy
CCT: controlled clinical trial
CDAG: Cochrane Drugs and Alcohol Group
CDT: carbohydrate deficient transferrin
CGI: Clinical Global Impression scale
CI: confidence intervals
CIWA‐Ar: Clinical Institute Withdrawal Assessment for Alcohol scale, Revised
ADS: Alcohol Dependence Scale
ALT: alanine aminotransferase
ASI: Addiction Severity Index
AST: aspartate aminotransferase
AUD: Alcohol Use Disorder
AUDIT: Alcohol Use Disorders Identification Test
BAI: Beck Anxiety Inventory
BDI: Beck Depression Inventory
BPRS: Brief Psychiatric Rating Scale
BSCS: Brief Substance Craving Scale
CBT: cognitive behavioural therapy
CCT: controlled clinical trial
CDAG: Cochrane Drugs and Alcohol Group
CDT: carbohydrate deficient transferrin
CGI: Clinical Global Impression scale
CI: confidence intervals
CIWA‐Ar: Clinical Institute Withdrawal Assessment for Alcohol scale, Revised
DBI: Drinking Behaviour Interview
DOTES: Dosage Record and Treatment Emergent Symptoms Scale
DrInC: Drinker Inventory of Consequences scale
DSM: Diagnostic and Statistic Manual of Mental Disorders
ECT: electroconvulsive therapy
GAD: generalized anxiety disorder
GAF: General Assessment of Functioning scale
GAS: Global Assessment Scale
GGT: γ‐glutamyltransferase
HRSA: Hamilton Anxiety Rating Scale
HRSD: Hamilton Depression Rating Scale
HSCL: Hopkins Symptom Checklist
ICD: International Classification of Diseases
LDQ: Leeds Dependence Questionnaire
LDRS: Lehmann Depression Rating Scale
MADRS: Montgomery and Åsberg Depression Rating Scale
MAOI: monoamine oxidase inhibitor
MAST: Michigan Alcoholism Screening Test
MD: mean difference
MET: motivational enhancement therapy
MINI: Mini International Neuropsychiatric Interview
MMPI: Minnesota Multiphasic Personality Inventory
MMSE: Mini‐Mental State Examination
OCDS: Obsessive‐Compulsive Drinking Scale
PACS: Penn Alcohol Craving scale
PANSS: Positive and Negative Syndrome Scale
PRISM: Psychiatric Rating Instrument for Mental Disorders and Substance Abuse
PSQI: Pittsburgh Sleep Quality Index
RCT: randomized controlled trial
RR: risk ratio
STAI: State Trait Anxiety Inventory
SAFTEE: Systematic Assessment for Treatment of Emergent Events
SCID: Structured Clinical Interview for DSM
SCL‐90: Symptom Check List‐90
SD: standard deviation
SDS: Severity of Dependence Scale
SF‐36: 36‐item Short Form (health‐related quality of life questionnaire)
SMD: standardized mean difference
SNRI: serotonin‐noradrenaline reuptake inhibitor
SOFAS: Social and Occupational Functioning Assessment Scale
SSRI: selective serotonin reuptake inhibitor
SUD: substance‐use disorder
TCA: tricyclic antidepressant
TLFB: timeline follow‐back
UKU: Udvalg for Kliniske Undersogelser Side Effect Rating Scale
VAS: Visual Analogue Scale
WHO: World Health Organization
ZUNG: Zung Self‐Assessment Depression Scale
Appendix 2. Cochrane Drug and Alcohol Group Specialised Register search strategy
CDAG Specialised register (via CRSLive)
4 July 2017 (197 hits)
1. (antidepressant*) AND (INREGISTER)
2. (citalopram OR escitalopram OR paroxetine OR fluoxetine OR fluvoxamine OR sertraline OR trazodone OR nefazodone OR venlafaxine OR desvenlafaxine OR duloxetine OR reboxetine OR bupropion OR amoxapine OR amitriptyline OR maprotiline OR nortriptyline OR desipramine OR trimipramine OR imipramine OR protriptyline OR doxepin OR clomipramine OR mirtazapine OR mianserin OR moclobemide OR phenelzine OR tranylcypromine OR agomelatine OR Acetylcarnitine OR Alaproclate OR Amersergide OR Amiflamine OR Amineptine OR Amisulpride OR Befloxatone OR Benactyzine OR Brofaromine OR Butriptyline OR Caroxazone OR Chlorpoxiten OR Cilosamine OR Cimoxatone OR Clorgyline OR Clorimipramine OR Clovoxamine OR Deanol OR Demexiptiline OR Deprenyl OR Dibenzipin OR Diclofensine OR Dothiepin OR Etoperidone OR Femoxetine OR Fluotracen OR Fluparoxan OR Idazoxan OR Iprindole OR Iproniazid OR isocarboxazid OR Litoxetine OR Lofepramine OR Medifoxamine OR Melitracen OR Metapramine OR Milnacipran OR Minaprine OR Nialamide OR Nomifensine OR Noxiptiline OR Opipramol OR Oxaflozane OR Oxaprotiline OR Pargyline OR Piribedil OR Pirlindole OR Pivagabine OR Prosulpride OR Protriptyline OR Quinupramine OR Rolipram OR SSRI OR Setiptiline OR Sulpiride OR Teniloxine OR Tetrindole OR Thiazesim OR Thozalinone OR Tianeptine OR Toloxatone OR Tomoxetine OR Viloxazine OR Viqualine OR Zimeldine) AND (INREGISTER)
3. #1 OR #2
4. (alcohol:TI) AND (INREGISTER)
5. (alcohol:AB) AND (INREGISTER)
6. (alcohol*:XDI) AND (INREGISTER)
7. #4 OR #5 OR #6
8. #3 AND #7
Appendix 3. CENTRAL search strategy
CENTRAL (via onlinelibrary.wiley.com)
2017, Issue 7 (695 hits)
1. MeSH descriptor: (Alcohol‐Related Disorders) explode all trees
2. ((alcohol) near (dependen* or disorder* or drink* or misuse or abuse* or consumption)):ti,ab,kw
3. alcohol*:ti,ab,kw
4. MeSH descriptor: (Drinking Behavior) explode all trees
5. #1 or #2 or #3 or #4
6. MeSH descriptor: (Antidepressive Agents) explode all trees
7. anti next depres*:ti,ab,kw
8. Antidepress* or "Monoamine Oxidase Inhibitors" or "Selective Serotonin Reuptake Inhibitors" or "Tricyclic Drugs" or acetylcarnitine or agomelatine or alaproclate or amesergide or amiflamine or amineptine or amitriptyline or amoxapine or befloxatone or benactyzine or brofaromine or bupropion or butriptyline or caroxazone or chlorproxithene or cilobamine or cimoxatone or citalopram or clomipramine or clorgyline or chlorimipramine or clovoxamine or deanol or demexiptiline or deprenyl or desipramine or dibenzepin or diclofensine or dothiepin or doxepin or duloxetine or escitalopram or etoperidone or femoxetine or fluotracen or fluoxetine or fluparoxan or fluvoxamine or idazoxan or imipramine or Iprindol* or iproniazid or isocarboxazid or Litoxetin* or Lofepramin* or Maprotilin* or Medifoxamin* or melitracene or Metapramin* or mianserin or milnacipran or Minaprin* or Mirtazapin* or Moclobemid* or Nefazodon* or Nialamid* or Nomifensin* or Nortriptylin* or Noxiptilin* or opipramol or Oxaflozan* or Oxaprotilin* or Pargylin* or Paroxetin* or Phenelzin* or piribedil or Pirlindol* or Pivagabin* or Prosulprid* or Protriptylin* or Quinupramin* or Reboxetin* or rolipram or selegiline or Sertralin* or Setiptilin* or teniloxazine or Tetrindol* or thiazesim or Thozalinon* or Tianeptin* or Toloxaton* or Tomoxetin* or Tranylcypromin* or Trazodon* or Trimipramin* or Venlafaxin* or Viloxazin* or Viqualin* or Zimeldin*:ti,ab,kw
9. #6 or #7 or #8
10. #5 and #9
Appendix 4. MEDLINE search strategy
MEDLINE (via PubMed)
4 July 2017 (4174 hits)
1. Alcohol‐Related Disorders(MeSH)
2. ((alcohol) AND (dependen* OR disorder* OR drink* OR misuse OR abuse* OR consumption))
3. alcohol* (tiab)
4. Drinking behaviour(MeSH)
5. #1 OR #2 OR #3 OR #4
6. Antidepressive Agents(MeSH)
7. anti‐depres*(tiab)
8. Antidepress* OR "Monoamine Oxidase Inhibitors" OR "Selective Serotonin Reuptake Inhibitors" OR "Tricyclic Drugs" OR acetylcarnitine OR agomelatine OR alaproclate OR amesergide OR amiflamine OR amineptine OR amitriptyline OR amoxapine OR befloxatone OR benactyzine OR brofaromine OR bupropion OR butriptyline OR caroxazone OR chlorproxithene OR cilobamine OR cimoxatone OR citalopram OR clomipramine OR clorgyline OR chlorimipramine OR clovoxamine OR deanol OR demexiptiline OR deprenyl OR desipramine OR dibenzepin OR diclofensine OR dothiepin OR doxepin OR duloxetine OR escitalopram OR etoperidone OR femoxetine OR fluotracen OR fluoxetine OR fluparoxan OR fluvoxamine OR idazoxan OR imipramine OR Iprindol* OR iproniazid OR isocarboxazid OR Litoxetin* OR Lofepramin* OR Maprotilin* OR Medifoxamin* OR melitracene OR Metapramin* OR mianserin OR milnacipran OR Minaprin* OR Mirtazapin* OR Moclobemid* OR Nefazodon* OR Nialamid* OR Nomifensin* OR Nortriptylin* OR Noxiptilin* OR opipramol OR Oxaflozan* OR Oxaprotilin* OR Pargylin* OR Paroxetin* OR Phenelzin* OR piribedil OR Pirlindol* OR Pivagabin* OR Prosulprid* OR Protriptylin* OR Quinupramin* OR Reboxetin* OR rolipram OR selegiline OR Sertralin* OR Setiptilin* OR teniloxazine OR Tetrindol* OR thiazesim OR Thozalinon* OR Tianeptin* OR Toloxaton* OR Tomoxetin* OR Tranylcypromin* OR Trazodon* OR Trimipramin* OR Venlafaxin* OR Viloxazin* OR Viqualin* OR Zimeldin*
9. #6 OR #7 OR #8
10. randomized controlled trial(pt)
11. controlled clinical trial(pt)
12. randomized(tiab)
13. placebo(tiab)
14. drug therapy(sh)
15. randomly(tiab)
16. trial(tiab)
17. groups(tiab)
18. #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17
19. animals(mh) NOT humans(mh)
20. #18 NOT #19
21. #5 AND #9 AND #20
Appendix 5. Embase search strategy
Embase (via embase.com)
4 July 2017 (3466 hits)
exp alcoholism'/exp OR alcohol NEAR/6 (dependen* OR disorder* OR drink* OR misuse OR abuse* OR consumption) OR alcohol*:ab,ti OR 'drinking behavior'/exp AND ('clinical trial'/exp OR 'controlled clinical trial'/exp OR 'crossover procedure'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'randomized controlled trial'/exp OR placebo:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti)) AND ('antidepressant agent'/exp OR antidepress*:ab,ti OR antidepress* OR 'monoamine oxidase inhibitors'/exp OR 'selective serotonin reuptake inhibitors' OR 'tricyclic drugs' OR 'acetylcarnitine'/exp OR 'agomelatine'/exp OR 'alaproclate'/exp OR amersergide OR 'amiflamine'/exp OR 'amineptine'/exp OR 'amitriptyline'/exp OR 'amoxapine'/exp OR 'befloxatone'/exp OR 'benactyzine'/exp OR 'brofaromine'/exp OR 'bupropion'/exp OR 'butriptyline'/exp OR caroxazone OR chlorpoxiten OR cilosamine OR 'cimoxatone'/exp OR 'citalopram'/exp OR 'clomipramine'/exp OR 'clorgyline'/exp OR clorimipramine OR 'clovoxamine'/exp OR 'deanol'/exp OR 'demexiptiline'/exp OR 'deprenyl'/exp OR 'desipramine'/exp OR dibenzipin OR 'diclofensine'/exp OR 'dothiepin'/exp OR 'doxepin'/exp OR 'duloxetine'/exp OR 'escitalopram'/exp OR 'etoperidone'/exp OR 'femoxetine'/exp OR fluotracen OR 'fluoxetine'/exp OR 'fluparoxan'/exp OR 'fluvoxamine'/exp OR 'idazoxan'/exp OR 'imipramine'/exp OR iprindol* OR 'iproniazid'/exp OR 'isocarboxazid'/exp OR litoxetin* OR lofepramin* OR maprotilin* OR medifoxamin* OR 'melitracen'/exp OR metapramin* OR 'mianserin'/exp OR 'milnacipran'/exp OR minaprin* OR mirtazapin* OR moclobemid* OR nefazodon* OR nialamid* OR nomifensin* OR nortriptylin* OR noxiptilin* OR 'opipramol'/exp OR oxaflozan* OR oxaprotilin* OR pargylin* OR paroxetin* OR phenelzin* OR 'piribedil'/exp OR pirlindol* OR pivagabin* OR prosulprid* OR protriptylin* OR quinupramin* OR reboxetin* OR 'rolipram'/exp OR seleginine OR sertralin* OR setiptilin* OR teniloxine OR tetrindol* OR thiazesim OR thozalinon* OR tianeptin* OR toloxaton* OR tomoxetin* OR tranylcypromin* OR trazodon* OR viqualin* OR zimeldin*)
Appendix 6. Criteria for risk of bias assessment
| Item | Judgement | Description |
| 1. Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization. |
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention. | |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk. | |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because 1 of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomization); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| High risk | Investigators enrolling participants could possibly foresee assignments because 1 of the following methods was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear risk | Insufficient information to permit judgement of low or high risk. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement. | |
| 3. Blinding of participants and providers (performance bias) | Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding. Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding. Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 4. Blinding of outcome assessor (detection bias) Objective outcomes |
Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding. Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding. Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 5. Blinding of outcome assessor (detection bias) Subjective outcomes |
Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding. Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 6. Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or dropout |
Low risk | No missing outcome data. Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias). Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size. Missing data have been imputed using appropriate methods. All randomized participants are reported/analyzed in the group they were allocated to by randomization irrespective of non‐compliance and cointerventions (intention to treat). |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size. 'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomization. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomized not stated, no reasons for missing data provided; number of dropouts not reported for each group). | |
| 7. Selective reporting (reporting bias) | Low risk | Study protocol is available and all of study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. Study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| High risk | Not all of study's prespecified primary outcomes have been reported. One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified. One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect). One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. Study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. |
Appendix 7. Treatment regimens in included studies
Antidepressants versus placebo
22 studies compared the efficacy of an antidepressant versus placebo (Adamson 2015; Altamura 1990; Butterworth 1971b; Cornelius 1997; Cornelius 2016; Gallant 1969 arm a; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 1993 arm A; Krupitsky 2012; Mason 1996; McGrath 1996; McLean 1986; Moak 2003; Nunes 1993; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000; 1438 participants).
Amitriptyline (75 mg/day) or placebo for 3 weeks (Krupitsky 1993 arm A; 41 participants)
Citalopram (up to 60 mg/day) plus naltrexone (up to 100 mg/day) or placebo plus naltrexone (up to 100 mg/day) for 12 weeks (Adamson 2015; 138 participants)
Desipramine (200 mg/day) or placebo for 6 months (Mason 1996; 28 participants)
Doxepin (75 mg/day or 150 mg/day) or placebo for 3 weeks (Gallant 1969 arm a; 76 participants)
Escitalopram (10 mg/day) or placebo for 13 weeks (Krupitsky 2012; 60 participants)
Fluoxetine (20 mg/day) or placebo for 12 weeks (Cornelius 1997; 51 participants)
Imipramine (25 mg/day) or placebo for 3 weeks (Butterworth 1971b; 40 participants)
Imipramine (up to 300 mg/day) or placebo for 12 weeks (McGrath 1996; 69 participants)
Imipramine (dose not available) or placebo for 24 weeks (Nunes 1993; 23 participants)
Mianserin (60 mg/day) or placebo for 4 weeks (McLean 1986; 35 participants)
Mirtazapine (30 mg/day) or placebo for 12 weeks (Cornelius 2016; 14 participants)
Nefazodone (up to 600 mg/day) or placebo for 10 weeks (Hernandez‐Avila 2004; 41 participants)
Nefazodone (up to 500 mg/day) or placebo for 12 weeks (Roy‐Byrne 20000; 64 participants)
Sertraline (up to 150 mg/day) or placebo for 24 weeks (Gual 2003; 83 participants)
Sertraline (200 mg/day) or placebo for 10 weeks (Kranzler 2006 arm A; score HRSD ≥ 17; 189 participants)
Sertraline (200 mg/day) or placebo for 10 weeks (Kranzler 2006 arm B; score HRSD ≤ 16; 139 participants)
Sertraline (200 mg/day) or placebo for 12 weeks (Moak 2003; 82 participants)
Sertraline (up to 200 mg/day) or placebo for 14 weeks (Pettinati 2001a; 29 participants)
Sertraline (200 mg/day) or placebo for 14 weeks (Pettinati 2010 arm A; 79 participants)
Sertraline (200 mg/day) plus naltrexone (100 mg/day) or placebo plus naltrexone for 14 weeks (Pettinati 2010 arm B; 91 participants)
Sertraline (100 mg/day) or placebo for 6 weeks (Roy 1998; 36 participants)
Vilofaxine (400 mg/day) or placebo for 12 weeks (Altamura 1990; 30 participants)
Antidepressants versus psychotherapy
Two studies compared the efficacy of an antidepressant versus psychotherapy (Liappas 2005 arm A; Liappas 2005 arm B; 60 participants).
Mirtazapine (up to 60 mg/day) or psychotherapy for 3 weeks (Liappas 2005 arm A; 30 participants)
Venlafaxine (up to 300 mg/day) or psychotherapy for 3 weeks (Liappas 2005 arm B; 30 participants)
Antidepressants versus other medications
Four studies compared the efficacy of antidepressants to that of other medications (Butterworth 1971a; Gallant 1969 arm b; Krupitsky 1993 arm B; Muhonen 2008; 228 participants).
Amitriptyline (75 mg/day) or diazepam (15 mg/day) for 3 weeks (Krupitsky 1993 arm B; 29 participants)
Doxepin (25 mg/day) or diazepam (5 mg/day) for 3 weeks (Butterworth 1971a; 39 participants)
Doxepin (75 mg/day or 150 mg/day) or diazepam (15 mg/day) for 3 weeks (Gallant 1969 arm b; 71 participants)
Escitalopram (20 mg/day) or memantine (20 mg/day) for 26 weeks (Muhonen 2008; 80 participants)
One antidepressant versus another antidepressant
Five studies compared the efficacy of an antidepressant versus another (Altintoprak 2008; Cocchi 1997; Habrat 2006; Liappas 2005 arm C; Lôo 1988; 621 participants).
Mirtazapine (up to 60 mg/day) or amitriptyline (up to 150 mg/day) for 8 weeks (Altintoprak 2008; 44 participants)
Mirtazapine (up to 60 mg/day) or venlafaxine (up to 300 mg/day) for 3 weeks (Liappas 2005 arm C; 40 participants)
Paroxetine (20 mg/day) or amitriptyline (25 mg/day) for 3‐4 weeks (Cocchi 1997; 122 participants)
Tianeptine (37.5 mg/day) or amitriptyline (75 mg/day) for 4‐8 weeks (Lôo 1988; 129 participants)
Tianeptine (37.5 mg/day) versus fluvoxamine (100 mg/day) for 6 weeks (Habrat 2006; 286 participants)
Appendix 8. Rating instruments utilized
Depression
Diagnostic criteria and interviews
DSM III‐R (APA 1987) utilized in Altamura 1990; Cornelius 1997; Mason 1996; McGrath 1996; Moak 2003; Nunes 1993; Pettinati 2001a; Roy 1998; Roy‐Byrne 2000.
DSM‐IV (APA 1994) utilized in Adamson 2015; Altintoprak 2008; Cocchi 1997; Cornelius 1997; Cornelius 2016; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Pettinati 2010 arm A; Pettinati 2010 arm B.
DSM‐IV‐TR (APA 2000) utilized in Muhonen 2008.
ICD‐10 (WHO 1993) utilized in Gual 2003; Habrat 2006; Krupitsky 2012.
MINI (Sheehan 1998) utilized in Cornelius 2016; Mason 1996.
SCID (Spitzer 1992) utilized in Adamson 2015; Altintoprak 2008; Cornelius 1997; Hernandez‐Avila 2004; Moak 2003; Pettinati 2001a; Roy‐Byrne 2000.
SCID‐P (First 1995) utilized in Muhonen 2008; Pettinati 2010 arm A; Pettinati 2010 arm B.
Severity
Observer‐rated scales
BPRS utilized in Butterworth 1971a; Krupitsky 2012.
CGI (Guy 1975) utilized in Butterworth 1971b; Gallant 1969 arm a; Gallant 1969 arm b; Habrat 2006; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; McGrath 1996; Roy 1998; Roy‐Byrne 2000.
HRSD (Hamilton 1960; Hamilton 1967; 17 items utilized in Altintoprak 2008; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B.
HRSD (Hamilton 1960; 21 items utilized in Altamura 1990; Krupitsky 2012; McGrath 1996; Moak 2003; Nunes 1993; Roy 1998;
HRSD (Hamilton 1960; 24 items utilized in Cornelius 1997; Liappas 2005 arm A; Liappas 2005 arm B; Liappas 2005 arm C; Mason 1996; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B.
HRSD (Hamilton 1960; number of items unknown in Habrat 2006; Lôo 1988; Roy‐Byrne 2000.
LDRS (Rockliff 1971) utilized in Butterworth 1971b.
MADRS (Montgomery 1979) utilized in Adamson 2015; Gual 2003; Krupitsky 2012; Lôo 1988; Muhonen 2008.
PRISM (Hasin 1996) utilized in Moak 2003.
Self‐administered scales
BDI (Beck 1961) utilized in Cornelius 1997; Cornelius 2016; Kranzler 2006 arm A; Kranzler 2006 arm B; Moak 2003; Pettinati 2001a; Roy 1998.
BDI‐II (Beck 1996) utilized in Muhonen 2008.
HRSD as described by Carr 1981 utilized in McLean 1986.
MMPI utilized in Krupitsky 1993 arm A; Krupitsky 1993 arm B.
SCL‐90 (Derogatis 1974) utilized in Adamson 2015; Lôo 1988.
Zung (Zung 1965) utilized in Butterworth 1971a; Cocchi 1997; Krupitsky 1993 arm A; Krupitsky 1993 arm B; Krupitsky 2012.
Remission
Observer‐rated scales and cut‐off values
HRSD (Hamilton 1960); final score < 8 utilized in Roy‐Byrne 2000.
HRSD (Hamilton 1960); final score ≤ 9 utilized in 3 final weeks of treatment in Pettinati 2010 arm A; Pettinati 2010 arm B.
MADRS (Montgomery 1979); final score < 10 utilized in Adamson 2015.
MADRS (Montgomery 1979); final score < 7 utilized in Gual 2003.
Self‐administered scales and cut‐off values
HRSD (Hamilton 1960); final score < 17 utilized in McLean 1986.
Zung (Zung 1965); participants no longer depressed utilized in Cocchi 1997.
These studies were not included in the analysis as they used self‐administered scales.
Response
Observer‐rated scales and cut‐off values
BPRS, marked improvement or moderate improvement in final score utilized in Butterworth 1971a.
CGI (Guy 1975); final score equal to 'Excellent' (very much improved) + 'Good' (improved) utilized in Butterworth 1971b; Gallant 1969 arm a; Gallant 1969 arm b; Roy 1998; Roy‐Byrne 2000.
HRSD (Hamilton 1960; reduction in final score > 50% utilized in Habrat 2006; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; McGrath 1996; Roy 1998.
MADRS (Montgomery 1979); reduction in final score > 50% utilized in Gual 2003; Lôo 1988.
Self‐administered scales and cut‐off values
DBI, Reduction in final score > 50% utilized in Roy 1998.
Zung, participants improved but depressed utilized in Cocchi 1997.
These data were not included in the analysis as a self‐reported scale was used.
Significant depression
Observer‐rated scales and cut‐off values
HRSD (Hamilton 1960); final score ≥ 50% utilized in Moak 2003.
Alcohol dependence and consumption of alcohol
Diagnostic criteria
DSM‐III‐R (APA 1987) utilized in Altamura 1990; Cornelius 1997; Mason 1996; McGrath 1996; Moak 2003; Nunes 1993; Pettinati 2001a; Roy 1998; Roy‐Byrne 2000.
DSM‐IV (APA 1994) utilized in Adamson 2015; Altintoprak 2008; Cocchi 1997; Cornelius 2016; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Liappas 2005 arm A; Liappas 2005 arm B; Liappas 2005 arm C; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B.
DSM‐IV‐TR (APA 2000) utilized in Muhonen 2008.
ICD‐10 (WHO 1993) utilized in Gual 2003; Habrat 2006; Krupitsky 2012.
MINI (Sheehan 1998) utilized in Cornelius 2016.
SCID (Spitzer 1992) utilized in Adamson 2015; Altintoprak 2008; Cornelius 1997; Hernandez‐Avila 2004; Moak 2003; Pettinati 2001a; Roy 1998; Roy‐Byrne 2000.
SCID‐P (First 1995) utilized in Muhonen 2008; Pettinati 2010 arm A; Pettinati 2010 arm B.
Observer‐rated scales
ASI (McLellan 1992) utilized in Cornelius 1997; Krupitsky 2012; Pettinati 2010 arm A; Pettinati 2010 arm B.
CGI (Guy 1975) utilized in Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Roy 1998.
TLFB (Sobell 1992) utilized in Adamson 2015; Cornelius 1997; Cornelius 2016; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Mason 1996; McGrath 1996; Moak 2003; Nunes 1993; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000.
Self‐administered scales
ADS (Skinner 1982; Skinner 1984) utilized in Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; McGrath 1996; Moak 2003.
AUDIT (Saunders 1993) utilized in Muhonen 2008; Roy‐Byrne 2000.
DBI (Shelton 1969) utilized in Altamura 1990.
DrInC (Miller 1995) utilized in Hernandez‐Avila 2004.
LDQ (Raistrick 1994) utilized in Adamson 2015.
MAST (Selzer 1971) utilized in Altintoprak 2008; Nunes 1993.
Alcohol outcomes
Abstinent days per week utilized by Muhonen 2008.
Abstinent patients: percentage of participants abstinent for the treatment period utilized by Muhonen 2008; Pettinati 2010 arm A; Pettinati 2010 arm B.
CDT values utilized in Moak 2003.
Cumulative abstinence duration defined as the number of days of abstinence recorded during the study utilized in Gual 2003.
Heavy drinking days 1 = ≥ 4 drinks per day (women) and ≥ 5 drinks per day (men) utilized by Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Pettinati 2010 arm A; Pettinati 2010 arm B.
Heavy drinking days 2 = ≥ 5 drinks per day utilized by Cornelius 1997; Cornelius 2016.
Heavy drinking days 3 = > 6 drinks per day utilized by Mason 1996.
Heavy drinking days 4 = ≥ 6 oz per day utilized by McGrath 1996.
Heavy drinking days 5 = ≥ 80 g/day (men) and 60 g/day (women) utilized by Adamson 2015.
Heavy drinking days 6 = According to AUDIT‐3 utilized by Muhonen 2008
Relapse 1 = 50 g alcohol per day for at least 3 days per week or 100 g alcohol in a single dose utilized by Gual 2003.
Relapse 2 = ≥ 5 drinks per day utilized by Pettinati 2001a.
Relapse 3 = > 2 heavy‐drinking days (> 6 drinks per day) per week for 2 consecutive weeks or if collateral report of heavy drinking coincided with participant refusal to continue in the trial utilized by Mason 1996.
Relapse 4 = 3 or 4 consecutives heavy drinking days utilized by Krupitsky 2012.
Relapse 5 = A heavy drinking day utilized by Pettinati 2010 arm A; Pettinati 2010 arm B.
Relapse 6 = Failure to meet the response criteria for 2 consecutive weeks utilized by Nunes 1993.
Treatment failure = Occurrence of at least 3 relapses utilized by Gual 2003; Mason 1996.
Liver enzyme levels
γ‐Glutamyltransferase (GGT) levels utilized in Hernandez‐Avila 2004; Krupitsky 2012.
Craving
A 24‐item questionnaire prepared by authors (Altintoprak 2008) utilized in Altintoprak 2008.
OCDS (Anton 1996) utilized in Cornelius 2016; Habrat 2006; Krupitsky 2012; Muhonen 2008.
PACS (Flannery 1999) utilized in Krupitsky 2012.
VAS utilized in Krupitsky 2012.
Global response (depression and alcohol use)
CGI, much improved or very much improved on both depression and on alcohol utilized in McGrath 1996.
Participants rating of much improved in depression and either abstinence or a marked reduction in drinking with minimal functional impairment utilized in Nunes 1993.
Psychiatric symptoms/psychological distress
Anxiety
Observer‐rated scales
HRSA (Hamilton 1959; Hamilton 1969) utilized in Habrat 2006; Krupitsky 2012; Liappas 2005 arm A; Liappas 2005 arm B; Liappas 2005 arm C; Lôo 1988; Muhonen 2008; Roy‐Byrne 2000.
Self‐administered scales
BAI (Beck 1988) utilized in Muhonen 2008.
MMPI utilized in Krupitsky 1993 arm A; Krupitsky 1993 arm B.
STAI (Spielberger 1970; Spielberger 1983) utilized in Altintoprak 2008; Hernandez‐Avila 2004; Krupitsky 1993 arm A; Krupitsky 1993 arm B; Krupitsky 2012.
Cognitive functioning
MMSE (Fillenbaum 1997) utilized in Mason 1996; Muhonen 2008.
Quality of life
SOFAS (Goldman 1992) utilized in Muhonen 2008.
VAS (Scott 1976) utilized in Muhonen 2008.
Quality of sleep
PSQI (Buysse 1989) utilized in Hernandez‐Avila 2004.
Global assessment
BPRS (Overall 1962) utilized in Butterworth 1971a; Krupitsky 2012.
CGI (Busner 2009) utilized in Nunes 1993.
GAF (APA 1994) utilized in Krupitsky 2012.
GAS (Endicott 1976) utilized in Liappas 2005 arm A; Liappas 2005 arm B; Liappas 2005 arm C.
Tolerability and suicide and suicide attempts
DOTES (Guy 1976) utilized in Altamura 1990.
SAFTEE (Levine 1986) utilized in Altintoprak 2008; Cornelius 2016; Hernandez‐Avila 2004.
UKU (Lingjaerde 1987) utilized in Altintoprak 2008; Habrat 2006.
Appendix 9. Outcomes
Primary outcomes
Depression
Final score in interviewer‐rated scales (see Appendix 8) reported by Adamson 2015; Altamura 1990; Altintoprak 2008; Butterworth 1971a; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Lôo 1988; Mason 1996; McGrath 1996; Moak 2003; Muhonen 2008; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000. Three studies reported data obtained using two interviewer‐rated scales (MADRS and HRSD) and only data obtained using HRSD were included (Gual 2003; Krupitsky 2012; Lôo 1988). Data from one study were excluded because they were expressed as medians and interquartile ranges (Mason 1996).
Final score in self‐administered scales (see Appendix 8) reported by Adamson 2015; Butterworth 1971a; Cocchi 1997; Cornelius 2016; Krupitsky 1993 arm A; Krupitsky 1993 arm B; Krupitsky 2012; Lôo 1988; McLean 1986; Moak 2003; Pettinati 2001a; Roy 1998. Two studies reported data obtained using the MMPI and ZUNG and only data obtained with ZUNG were included (Krupitsky 1993 arm A; Krupitsky 1993 arm B).
Differences between baseline and final score in interviewer‐rated scales (see Appendix 8) reported by Butterworth 1971b; Cornelius 1997; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; Pettinati 2001a. Data from one study were excluded because they were expressed as medians and interquartile ranges (Mason 1996).
Differences between baseline and final score in self‐administered scales (see Appendix 8) reported by Cornelius 1997; Cornelius 2016; McLean 1986; Pettinati 2001a.
Response criteria (see Appendix 8) were reported by Butterworth 1971b; Butterworth 1971a; Gallant 1969 arm a; Gallant 1969 arm b; Gual 2003; Habrat 2006; Kranzler 2006 arm A; Kranzler 2006 arm B; Mason 1996; McGrath 1996; Moak 2003; Roy‐Byrne 2000.
Remission criteria (see Appendix 8) were reported by Adamson 2015; Gual 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000.
Consumption of alcohol
Abstinent participants (number) during the trial evaluated by Cornelius 1997; Hernandez‐Avila 2004; McGrath 1996; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy‐Byrne 2000.
Abstinent days (%) evaluated by Adamson 2015; Cornelius 1997; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; McGrath 1996; Moak 2003; Pettinati 2001a.
Abstinent days (cumulative number) evaluated by Gual 2003.
Carbohydrate deficient transferrin (CDT) utilized in Moak 2003.
DrInC score (% of mean reduction) evaluated by Hernandez‐Avila 2004.
Drinking days (cumulative number) during the trial evaluated by Cornelius 1997; Krupitsky 2012.
Drinking days (number per week) evaluated by Cornelius 2016; Hernandez‐Avila 2004.
Drinking days (%) evaluated by Hernandez‐Avila 2004; Krupitsky 2012; McGrath 1996; Pettinati 2001a.
Drinks during the trial (cumulative number) evaluated by Cornelius 1997.
Drinks per drinking day evaluated by Adamson 2015; Cornelius 1997; Cornelius 2016; Hernandez‐Avila 2004; McGrath 1996; Moak 2003; Roy‐Byrne 2000.
Drinks (number per week) evaluated by Cornelius 2016; Hernandez‐Avila 2004.
Global response in depression and in alcohol consumption reported by Krupitsky 2012; McGrath 1996; Nunes 1993.
Heavy drinkers (number) evaluated by Gual 2003; Krupitsky 2012; Mason 1996; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998.
Heavy drinking days (number per week) evaluated by Adamson 2015; Cornelius 1997; Cornelius 2016; Hernandez‐Avila 2004; McGrath 1996.
Heavy drinking days (%) evaluated by Adamson 2015; McGrath 1996.
Time to first heavy drinking (number of weeks) evaluated by Cornelius 1997.
Time to first relapse (in number of days) evaluated by Cornelius 1997; Gual 2003; Krupitsky 2012; Pettinati 2001a; Pettinati 2010 arm A; Pettinati 2010 arm B.
Treatment failure (%) evaluated by Mason 1996.
Liver enzyme levels
Final levels of GGT reported by Hernandez‐Avila 2004; Krupitsky 2012.
Acceptability
Number of dropouts reported by Altamura 1990; Butterworth 1971b; Butterworth 1971a; Cornelius 2016; Gallant 1969 arm a; Gallant 1969 arm b; Gual 2003; Hernandez‐Avila 2004; Kranzler 2006 arm A; Kranzler 2006 arm B; Krupitsky 2012; Liappas 2005 arm A; Liappas 2005 arm B; Liappas 2005 arm C; Mason 1996; McGrath 1996; McLean 1986; Moak 2003; Pettinati 2010 arm A; Roy 1998; Roy‐Byrne 2000.
Tolerability
Abdominal cramps reported by Adamson 2015.
Anxiety evaluated by Roy‐Byrne 2000.
Back pain evaluated by Gual 2003.
Blood in the stool evaluated by Kranzler 2006 arm A.
Blurred vision evaluated by Butterworth 1971a; Roy‐Byrne 2000.
Bodyweight (final values) reported by Altintoprak 2008.
Constipation evaluated by Altintoprak 2008; Butterworth 1971a; Kranzler 2006 arm A; Roy‐Byrne 2000.
Coughing evaluated by Gual 2003.
Depression evaluated by Kranzler 2006 arm A; Moak 2003.
Diarrhoea evaluated by Gual 2003; Roy‐Byrne 2000.
Difficulty sleeping evaluated by Adamson 2015.
Dizziness evaluated by Altintoprak 2008; Gual 2003; Roy‐Byrne 2000.
Drowsiness evaluated by Butterworth 1971a.
Dry mouth evaluated by Altintoprak 2008; Butterworth 1971a; Gallant 1969 arm a; Gallant 1969 arm b; Roy‐Byrne 2000.
Dyspepsia evaluated by Gual 2003.
Fatigue/weakness evaluated by Roy‐Byrne 2000.
Headache evaluated by Gual 2003; Kranzler 2006 arm A; Roy‐Byrne 2000.
Heart palpitations evaluated by Roy‐Byrne 2000.
Increase in bodyweight reported by Altintoprak 2008; Cornelius 2016.
Influenza‐like symptoms evaluated by Gual 2003.
Insomnia evaluated by Adamson 2015; Butterworth 1971b; Kranzler 2006 arm A; Roy‐Byrne 2000.
Low energy evaluated by Adamson 2015.
Nausea evaluated by Adamson 2015; Gual 2003; Roy‐Byrne 2000.
Other adverse effects evaluated by Roy‐Byrne 2000.
Paraesthesia evaluated by Gual 2003.
Poor memory evaluated by Roy‐Byrne 2000.
Procedure (medical/surgical/health service) evaluated by Gual 2003.
Sedation evaluated by Altintoprak 2008; Roy‐Byrne 2000.
Sexual dysfunction evaluated by Roy‐Byrne 2000.
Skin rash evaluated by Roy‐Byrne 2000.
Syncope evaluated by Kranzler 2006 arm A.
Total adverse effects evaluated by Adamson 2015; Butterworth 1971b; Butterworth 1971a; Gallant 1969 arm a; Gallant 1969 arm b; Habrat 2006; Kranzler 2006 arm A; Krupitsky 2012.
Total serious adverse events evaluated by Adamson 2015; Butterworth 1971b; Cornelius 2016; Kranzler 2006 arm A; Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B.
Visual trails evaluated by Roy‐Byrne 2000.
Weight gain reported by Cornelius 2016.
Withdrawal for medical reasons evaluated by Adamson 2015; Cornelius 1997; Kranzler 2006 arm A; Krupitsky 2012; Mason 1996; McGrath 1996; Pettinati 2010 arm A; Pettinati 2010 arm B; Roy 1998; Roy‐Byrne 2000.
Worsening of clinical condition because of relapse evaluated by Kranzler 2006 arm A; Moak 2003.
Suicide and suicide attempts
Suicidal ideation or attempts evaluated by Adamson 2015; Cornelius 1997; Habrat 2006; Kranzler 2006 arm A; Moak 2003.
Secondary outcomes
Use of other substances
Participants with substance use disorders were included by Adamson 2015; McGrath 1996.
Participants with abuse of other substances were included by Cornelius 1997; Roy‐Byrne 2000.
Craving for alcohol
Final score in a self‐administered scale (see Appendix 8) reported by Altintoprak 2008; Cornelius 2016; Habrat 2006; Krupitsky 2012.
Severity of alcohol dependence
Final score in a self‐administered scale a (see Appendix 8) reported by Adamson 2015; Hernandez‐Avila 2004; Muhonen 2008; Roy‐Byrne 2000.
Psychiatric symptoms/psychological distress
Anxiety severity
Final score in an interviewer‐rated scale reported by Habrat 2006; Krupitsky 2012; Liappas 2005 arm A; Liappas 2005 arm B; Liappas 2005 arm C; Lôo 1988; Muhonen 2008.
Final score in a self‐administered scale reported by Altintoprak 2008; Hernandez‐Avila 2004; Krupitsky 1993 arm A; Krupitsky 1993 arm B; Krupitsky 2012; Muhonen 2008.
Sleep quality
Difference between baseline and final score reported by Hernandez‐Avila 2004.
Global assessment
Difference between basal and final score reported by Cornelius 1997.
Final score in a rating scale reported by Krupitsky 2012; Liappas 2005 arm A; Liappas 2005 arm B; Liappas 2005 arm C.
Quality of life
Final score in a rating scale evaluated by Muhonen 2008.
Appendix 10. Psychosocial therapy
CBT (Kadden 1992) utilized by Moak 2003; Pettinati 2010 arm A; Pettinati 2010 arm B.
MET (Miller 1992) utilized by Cornelius 2016.
Data and analyses
Comparison 1. Antidepressants versus placebo: all studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression severity: final score (interviewer‐rated scales) | 14 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All studies | 14 | 1074 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.49, ‐0.04] |
| 1.2 Selective serotonin reuptake inhibitors (SSRIs) | 10 | 881 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.40, 0.07] |
| 1.3 5‐HT2 antagonists | 2 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.69, 0.11] |
| 2 Depression severity: final score (self‐administered scales) | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All studies | 8 | 373 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.64, 0.07] |
| 2.2 SSRIs | 5 | 300 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.69, 0.18] |
| 2.3 5‐HT2 antagonists | 2 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.59, 0.64] |
| 3 Depression severity: difference between basal and final score (interviewer‐rated scales) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 All studies | 5 | 447 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.12, 0.42] |
| 3.2 SSRIs | 4 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.18, 0.32] |
| 4 Depression severity: difference between basal and final score (self‐administered scales) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 All studies | 4 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.16, 0.56] |
| 4.2 SSRIs | 2 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.16, 0.73] |
| 4.3 5‐HT2 antagonists | 2 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.59, 0.64] |
| 5 Response to antidepressive treatment | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 All studies | 10 | 805 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.08, 1.82] |
| 5.2 Tricyclic antidepressants (TCAs) | 4 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.09, 2.34] |
| 5.3 SSRIs | 5 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.87, 1.63] |
| 6 Full remission of depression | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 All studies | 4 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.77, 1.83] |
| 6.2 SSRIs | 3 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.74, 1.36] |
| 7 Consumption of alcohol: abstinent days (%) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 All studies | 9 | 821 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐1.66, 4.34] |
| 7.2 SSRIs | 7 | 711 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐3.20, 2.26] |
| 8 Consumption of alcohol: abstinent participants (number) | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 All studies | 7 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.22, 2.39] |
| 8.2 SSRIs | 4 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.02, 2.68] |
| 8.3 5‐HT2 antagonists | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.77, 3.39] |
| 9 Consumption of alcohol: drinking days (per week) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 All studies | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐2.35, 0.05] |
| 9.2 5‐HT2 antagonists | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐2.35, 0.05] |
| 10 Consumption of alcohol: drinks (per drinking days) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 All studies | 7 | 451 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.79, ‐0.46] |
| 10.2 SSRIs | 3 | 271 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐2.58, ‐0.26] |
| 10.3 5‐HT2 antagonists | 3 | 111 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.00, ‐0.11] |
| 11 Consumption of alcohol: drinks (per week) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 All studies | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐5.06 [‐12.30, 2.18] |
| 11.2 5‐HT2 antagonists | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐5.06 [‐12.30, 2.18] |
| 12 Consumption of alcohol: heavy drinking days (per week) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 All studies | 5 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.85, 0.20] |
| 12.2 SSRIs | 2 | 189 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.09, 0.27] |
| 12.3 5‐HT2 antagonists | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐2.09, 1.22] |
| 13 Consumption of alcohol: heavy drinkers (number) | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 All studies | 7 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.57, 1.07] |
| 13.2 SSRIs | 6 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.11] |
| 13.3 5‐HT2 antagonists | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.68, 4.67] |
| 14 Consumption of alcohol: time to first relapse (days) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 All studies | 6 | 348 | Mean Difference (IV, Random, 95% CI) | 2.54 [‐8.79, 13.87] |
| 14.2 SSRIs | 6 | 348 | Mean Difference (IV, Random, 95% CI) | 2.54 [‐8.79, 13.87] |
| 15 Liver enzyme levels: γ‐glutamyltransferase (U/L) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 All studies | 2 | 56 | Mean Difference (IV, Random, 95% CI) | ‐8.39 [‐26.47, 9.68] |
| 16 Depression and alcohol: global response | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 All studies | 3 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [1.34, 4.19] |
| 16.2 TCAs | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.09, 4.02] |
| 17 Acceptability: dropouts | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 All studies | 17 | 1159 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 17.2 TCAs | 4 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.48, 3.06] |
| 17.3 SSRIs | 8 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.79, 1.36] |
| 17.4 5‐HT2 antagonists | 4 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.38, 1.64] |
| 18 Tolerability of treatment: adverse events | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Withdrawal for medical reasons: all studies | 10 | 947 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.65, 2.04] |
| 18.2 Withdrawal for medical reasons: SSRIs | 7 | 786 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.52, 2.32] |
| 18.3 Withdrawal for medical reasons: TCAs | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.10, 8.41] |
| 18.4 Total adverse events: all studies | 5 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.97, 1.44] |
| 18.5 Total adverse events: TCAs | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.13, 2.42] |
| 18.6 Total adverse events: SSRIs | 3 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.92, 1.23] |
| 18.7 Dry mouth: all studies | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.96, 3.81] |
| 18.8 Insomnia: all studies | 4 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.02, 2.77] |
| 18.9 Insomnia: SSRIs | 2 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.04, 2.96] |
| 18.10 Headache: all studies | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.89, 1.64] |
| 18.11 Headache: SSRIs | 2 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.87, 1.61] |
| 18.12 Dizziness: all studies | 2 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.42, 6.73] |
| 18.13 Diarrhoea: all studies | 2 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.37, 10.22] |
| 18.14 Nausea: all studies | 3 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.66, 3.23] |
| 18.15 Nausea: SSRIs | 2 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.62, 3.35] |
| 18.16 Constipation: all studies | 2 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.19, 15.64] |
| 18.17 Total serious adverse events: all studies | 7 | 774 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.80, 1.86] |
| 18.18 Total serious adverse events: SSRIs | 5 | 721 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.80, 1.86] |
| 18.19 Worsening of clinical condition because of relapse: all studies | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.73, 10.87] |
| 18.20 Worsening of clinical condition because of relapse: SSRIs | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.73, 10.87] |
| 18.21 Depression: all studies | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.30, 17.69] |
| 18.22 Depression: SSRIs | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [0.30, 17.69] |
| 19 Suicide attempts | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 All studies | 4 | 602 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.23, 7.61] |
| 19.2 SSRIs | 4 | 602 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.23, 7.61] |
| 20 Secondary outcomes: craving | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 All studies | 2 | 29 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.27, 5.27] |
| 21 Secondary outcomes: severity of dependence | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1 All studies | 2 | 168 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.44, 0.17] |
| 22 Secondary outcomes: severity of anxiety | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.1 All studies | 3 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐6.31 [‐10.33, ‐2.28] |
Comparison 2. Antidepressants versus psychotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression severity: final score | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐2.61 [‐6.92, 1.70] |
| 2 Global assessment: final score | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 5.92 [1.30, 10.54] |
| 3 Acceptability: dropouts | 2 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.31, 6.54] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adamson 2015.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 138 depressed people with alcohol dependence (56 men and 82 women; mean (± SD) age 43.6 ± 9.1 years). Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy: manualized clinical case management was delivered by experienced addiction clinicians. Scheduled duration of treatment: 12 weeks Sites: 7 addiction clinics spanning urban, provincial, and rural catchments in Australia. Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence:
Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: 47.1% of participants had current anxiety disorder. Other substance‐use disorders: 14.5% of participants had current substance dependence. Other characteristics of study Other pharmacological treatment offered: all participants received naltrexone. Funding sources: study funded by Health Research Council of New Zealand grant HRC 07/138. Declaration of interest: authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using a computer‐generated random number table. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation was conducted by an administrative staff member independent of study investigators or research clinicians, and the allocation sequence record was stored securely. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The investigators, doctors, participants, and any other staff members taking part in the experiment were unaware which of the groups any particular participant belonged to. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were imputed using appropriate methods. |
| Selective reporting (reporting bias) | Unclear risk | Numbers of dropouts per group were missing. |
Altamura 1990.
| Methods | Randomized, placebo‐controlled, double‐blind trial | |
| Participants | 30 people with alcohol dependence with dysthymia (24 men and 6 women; mean (± SD) age: 44.5 ± 2.6 years). Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available. |
|
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 12 weeks Site: 1 centre, Department of Clinical Psychiatry, Policlinico, Milan, Italy Setting: inpatient setting for first 4 weeks, then outpatients for following 8 weeks. Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: information not available Dropouts Adverse effects: information not available |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: information not available Other substance‐use disorders: participants with other substance‐use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: information not available Funding sources: information not available Declaration of interest: information not available Other information Standard errors were converted into SDs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated but no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Method to account for missing data not described. Intention‐to‐treat approach not reported. No high numbers of dropouts or unbalanced between groups. |
| Selective reporting (reporting bias) | High risk | DBI and DOTES scores are reported in 2 figures (figures 3 and 4 of the publication) in which the titles of the y axis do not correspond to those reported in the legends and in the text, and the positions of the points do not correspond to the values indicated in the y axes. Accordingly, these results were not included in the present meta‐analysis. |
Altintoprak 2008.
| Methods | Double‐blind, randomized, comparative trial | |
| Participants | 44 depressed people with alcohol dependence (number of men and women: information not available; mean age: information not available). Sociodemographic characteristics available only for 36 participants (20 mirtazapine, 16 amitriptyline). Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy: information not available. Scheduled duration of treatment: 8 weeks. Site: Ege University School of Medicine Hospital, Specialized Addiction Unit, Izmir, Turkey Setting: inpatient Route of administration: orally Starting dose:
Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: data not available Alcohol craving:
Dropouts: data not available Adverse effects:
Bodyweight:
Anxiety:
|
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Global assessment: information not available. Weight (mean ± SD):
Other psychiatric comorbidity: participants with other psychiatric disorders were excluded. Other substance use disorders: participants with other substance use disorders were excluded. Other characteristics of study Other pharmacological treatment: no other pharmacological treatment was allowed. Funding sources: not available. Declaration of interest: not available. Other information After their inclusion in study, participants were admitted at a specialized department for alcohol detoxification on an inpatient basis. Alcohol consumption was prohibited during hospitalization and people who consumed alcohol during study were excluded from study. At end of alcohol detoxification treatment (approximately 10‐14 days), people were included in study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind stated. Medication and placebo prepared to appear identical ("Both the clinicians and patients were blind to the treatment. Drugs were given in identical‐looking opaque capsules"). |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat approach not used ("Dropouts were not included in the analysis due to missing data"). People who consumed alcohol during study were excluded by study. |
| Selective reporting (reporting bias) | High risk | People who consumed alcohol during study were excluded by study. |
Butterworth 1971a.
| Methods | Randomized, double‐blind, comparative trial | |
| Participants | 39 people with alcohol dependence (all men; mean age: information not available) with a significant degree of anxious‐depressive symptomatology. Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available |
|
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 3 weeks Site: Alcoholism Treatment Service of East Louisiana State Hospital, Mandeville, LA, USA Setting: inpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: data not available Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: information not available Other substance‐use disorders: information not available Other characteristics of study Other pharmacological treatment: other concomitant therapy not allowed Funding source: medications were supplied by Laboratories of Pfizer Inc. Declaration of interest: information not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind stated. Medications prepared to appear identical. Evaluations conducted by 2 independent investigators and the results pooled. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information on dropouts provided. Methods applied to account for missing data not described. Intention‐to‐treat approach not reported. |
| Selective reporting (reporting bias) | High risk | No information on dropouts provided. Methods applied to account for missing data not described. Intention‐to‐treat approach not reported. |
Butterworth 1971b.
| Methods | Randomized, placebo‐controlled, double‐blind trial | |
| Participants | 40 depressed people with alcohol dependence (all men; mean age: majority aged 31‐50 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available |
|
| Interventions | Drugs:
Psychotherapy: none Scheduled duration of treatment: 3 weeks Site: Alcoholic Treatment Service, East Louisiana State Hospital, Jackson, LA, USA Setting: inpatients for first 3‐4 days for treatment of alcohol withdrawal, then 3 weeks for trial Route of administration: orally Starting dose:
Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: data not available Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: information not available. Other substance‐use disorders: information not available. Other characteristics of study Other pharmacological treatment offered: participants received pharmacological treatment to control the acute symptoms of alcohol withdrawal for 3‐4 days. Funding sources: information not available Declaration of interest: information not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind stated. Medication and placebo prepared to appear identical. No specific reference made to blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Methods applied to account for missing data not described. Intention‐to‐treat approach not reported. People who left study were replaced by other people ("One patients taking imipramine left the hospital ... and global evaluation were omitted. Two additional patients left without permission just after entering the trial, and were therefore replaced in the study. One had received six doses of imipramine and the other one placebo"). |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement. |
Cocchi 1997.
| Methods | Randomized comparative trial | |
| Participants | 122 depressed people with alcohol dependence (95 men and 27 women; mean age: 42 years) Inclusion criteria:
Exclusion criteria: information not available Participants with bipolar disorder: information not available |
|
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 3‐4 weeks Site: Alcohol Unit, casa di Cura Villa Silvia per malattie nervose e mentali, Senigallia, Italy Setting: inpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression
Alcohol dependence: data not available Dropouts: data not available Adverse effects: data not available |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence: data not available Other psychiatric comorbidity: information not available Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment: information not available Funding sources: information not available Declaration of interest: information not available Other information Data on response and remission were excluded because evaluated using a self‐administered scale. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on the design (double‐blind or open trial), on the preparation and appearance of medications, and on blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on study design (double‐blind or open trial). |
| Blinding of outcome assessment (detection bias) subjective | High risk | No information on study design (double‐blind or open trial). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Methods applied to account for missing data. Intention‐to‐treat approach not reported. No high number of dropouts or unbalanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement. |
Cornelius 1997.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 51 depressed people with alcohol dependence (26 men and 25 women; age (mean ± SD) = 34.8 ± 10.2 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 12 weeks Site: Western Psychiatric Institute and Clinic of the University of Pittsburgh, Pittsburgh, USA Setting: inpatients for first 2 weeks of abstinence, then outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence:
Global assessment:
Dropouts: information not available Adverse effects: information not available |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance‐use disorders: participants with substance‐use disorders were excluded. Abuse of other substances was not an exclusionary criterion, provided that alcohol was the main substance of abuse. Other characteristics of study Other pharmacological treatment: other pharmacological treatments were not allowed. Funding sources: work was supported by the National Institute on Alcohol Abuse and Alcoholism (grants AA09127 and AA10523), and by the Mental Health Clinical Research Center, Rockville, MD (grant MH30915). Declarations of interest: information not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization balanced for gender and race. It was not reported whether a computer‐generated list was used. |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement. Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Medications were administered in identical opaque capsules. Substantial blood levels of fluoxetine were observed in more than 99% of participants assigned to fluoxetine. Not reported if blood analyses were made also to participants who received placebo. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis and last point carried forward analysis applied. |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement. |
Cornelius 2016.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 14 depressed people with alcohol dependence (10 men and 4 women; mean age = 41.3 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available |
|
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 12 weeks Site: University of Pittsburgh, Western Psychiatric Institute and Clinic, Pittsburgh, USA Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence:
Craving for alcohol:
Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment: participants did not receive other pharmacological treatments. Funding sources: study received grants from the National Institute on Alcohol Abuse and Alcoholism (R21 AA022123, R21 AA022863, R01 AA013370, R01 AA015173, K24 AA15320) and from the National Institute on Drug Abuse (R01 DA019142, P50 DA05605, K02 DA017822). Declarations of interest: information not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement of low or high risk. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medications were identical in appearance (identical‐looking opaque capsules). |
| Blinding of outcome assessment (detection bias) objective | Low risk | Medications were identical in appearance (identical‐looking opaque capsules). |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat approach reported. |
| Selective reporting (reporting bias) | Low risk | No dropouts |
Gallant 1969 arm a.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 76 people with alcohol dependence (all men; mean age = 42 years) in association with a predominant chronic anxiety or depressive reaction. Inclusion criteria: information not available Exclusion criteria:
Participants with bipolar disorder: information not available |
|
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 3 weeks Site: Alcoholism Treatment Service of Southeast Louisiana Hospital, Mandeville, LA, USA Setting: inpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: data not available Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants included inGallant 1969 arm a; Gallant 1969 arm b Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were included. Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment offered: information not available Funding source: the project was partially supported by PHS Grant MH‐03701‐08, Psychopharmacology Research Branch, NIMH. Declarations of interest: information not available Other information In the original study, 100 participants were divided into 4 groups:
In the present meta‐analysis, participants were divided into 2 substudies:
The first arm (Gallant 1969 arm a) was included in the 'Effects of interventions: Antidepressants versus placebo' comparison and the second arm (Gallant 1969 arm b) in the 'Effects of interventions: Antidepressants versus other medications' comparison. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement of low or high risk. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medications were identical in appearance and were coded in accordance with double‐blind procedure to ensure that all personal involved in project remained blind as to which group any given participant belonged. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Methods applied to account for missing data not described. Intention‐to‐treat approach not reported. However, there were no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. However, there were no dropouts. |
Gallant 1969 arm b.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 71 people with alcohol dependence (all men; mean age: 42 years) in association with a predominant chronic anxiety or depressive reaction (diagnosis of depression was uncertain). Inclusion criteria: information not available Exclusion criteria:
Participants with bipolar disorder: information not available |
|
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 3 weeks Site: Alcoholism Treatment Service of Southeast Louisiana Hospital, Mandeville, LA, USA Setting: inpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: data not available (probably because of inpatient setting). Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants included inGallant 1969 arm a; Gallant 1969 arm b Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were included. Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment offered: information not available Funding source: the project was partially supported by PHS Grant MH‐03701‐08, Psychopharmacology Research Branch, NIMH. Declarations of interest: information not available Other information In the original study, 100 patients were divided into 4 groups:
In the present meta‐analysis, participants were divided into 2 substudies:
The first arm (Gallant 1969 arm a) was included in the 'Effects of interventions: Antidepressants versus placebo' comparison and the second arm (Gallant 1969 arm b) in the 'Effects of interventions: Antidepressants versus other medications' comparison. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information on sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medications were identical in appearance and were coded in accordance with double‐blind procedure to ensure that all personal involved in project remained blind as to which group any given participant belonged. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Methods applied to account for missing data not described. Intention‐to‐treat approach not reported. However, there were no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
Gual 2003.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 83 depressed people with alcohol dependence (44 men and 39 women; mean age = 47 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 24 weeks Site: Alcohol Unit of the Hospital ‘Clínico y Provincial' in Barcelona, Spain Setting: outpatients Route of administration: orally Starting dose: 50 mg/day Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence:
Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: people with other mental disorders were excluded. Other substance‐use disorders: people with substance‐use disorders were excluded. Other characteristics of study Other pharmacological treatment: other pharmacological treatments were not allowed. Funding source: information not available Declarations of interest: information not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation stated. The investigator did not have access to the randomization code. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching packets containing placebo were provided for all possible sertraline dose progressions, so that titration could be performed double blind. Medication was dispensed in bottles with MEMS caps, which contain an electronic monitoring device that records the date and time of bottle cap openings (Aprex Corp, San Diego, CA). |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat method utilized in all statistical analyses. |
| Selective reporting (reporting bias) | Low risk | For alcohol consumption data, participants with missing assessments at last observation were treated as non‐abstinent. For depression scale scores, missing data were handled on the principle of last observation carried forward. |
Habrat 2006.
| Methods | Randomized, double‐blind, comparative trial | |
| Participants | 286 depressed people with alcohol dependence (222 men and 64 women; mean age: information not available). Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were included. |
|
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 6 weeks (responders were proposed to continue the same treatment up to 12 weeks). Site: Department of Substance Use Prevention and Treatment, Institute of Psychiatry and Neurology, Warsaw, Poland Setting: outpatients Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: data not available Craving for alcohol:
Anxiety:
Dropout Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Craving for alcohol:
Anxiety:
Other psychiatric comorbidity: information not available Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment: other pharmacological treatments were not allowed. Funding source: information not available Declarations of interest: information not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured. Both drugs were blinded to participants. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only full analysis set were evaluated by the present meta‐analysis. |
| Selective reporting (reporting bias) | Low risk | Only full analysis set were evaluated by the present meta‐analysis. |
Hernandez‐Avila 2004.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 41 depressed people with alcohol dependence (20 men and 21 women; age (mean ± SD): 42.9 ± 8.6 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 10 weeks Site: Alcohol Research Center, Department of Psychiatry, University of Connecticut School of Medicine, Farmington, CT, USA Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence:
Anxiety:
Sleep quality:
Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Quality of sleep
Other psychiatric comorbidity: participants with other mental disorders were included. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding sources: study supported by NIH Grants P50‐AA03510, K24‐AA13736, and M01‐RR06192 and the Bristol‐Myers Squibb Co. Declarations of interest: information not available Other information After a baseline assessment, participants entered a 1‐week single‐blind placebo treatment, followed by random assignment to nefazodone or placebo groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to treatment groups used an urn randomization procedure, which balanced group assignment on sex, age, educational level, percentage of heavy drinking days, and severity of depressive symptoms at the time of the initial assessment. |
| Allocation concealment (selection bias) | Low risk | Method of concealment not reported but unlikely that selection bias was introduced. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information provided on blinding of assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information provided on blinding of assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Mixed model analysis used to examine variables measured at each visit during study. This procedure allows the inclusion of all cases (41 participants) by estimating individual trajectories even when other data points are missing because of participant attrition. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
Kranzler 2006 arm A.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 189 depressed people with alcohol dependence (123 men; 66 women; age (mean ± SD): placebo = 44.0 ± 8.0 years; sertraline = 41.7 ± 9.4 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy: at each study visit, participants received supportive therapy delivered according to a manual developed specifically for study consisting in:
Scheduled duration of treatment: for up to 10 weeks Sites: 13 investigative sites in USA Setting: outpatients Route of administration: orally Starting dose: 50 mg/day Pattern of dose reduction:
|
|
| Outcomes | Depression:
Alcohol dependence:
Dropout Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: supported by Pfizer Pharmaceuticals. Manuscript preparation supported by NIH grant K24 AA13736. Declarations of interest: information not available. Other information In the original study, 328 participants were divided into the 2 groups on whether initially elevated HRSD score declined with cessation of heavy drinking:
In the present meta‐analysis, participants were divided into 2 substudies:
Both the substudies (Kranzler 2006 arm A; Kranzler 2006 arm B) were included in the 'Effects of interventions: Antidepressants versus placebo' comparison. Unfortunately, the original study did not report the adverse events for the single substudies. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization schedule. |
| Allocation concealment (selection bias) | Unclear risk | Assignment to medication group was done according to a computer‐generated randomization schedule for groups A and B, with the medication groups within each stratum balanced for recent outpatient/inpatient status. Despite random assignment, participants who received placebo were older, reported more drinks per week during the pretreatment period, and had higher CGI depression scores at baseline. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All analyses used an intention‐to‐treat approach. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. However, some of them were reported only as a % reduction (e.g. BDI score). |
Kranzler 2006 arm B.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 139 depressed people with alcohol dependence (86 men and 53 women; age (mean ± SD): placebo = 42.9 ± 9.2 years; sertraline = 41.8 ± 9.4 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy: At each study visit, participants received supportive therapy delivered according to a manual developed specifically for study consisting of:
Scheduled duration of treatment: up to 10 weeks Sites: 13 investigative sites in the USA Setting: outpatients Route of administration: orally Starting dose: 50 mg/day Pattern of dose reduction:
|
|
| Outcomes | Depression:
Alcohol dependence:
Dropout Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: study supported by Pfizer Pharmaceuticals. Manuscript preparation supported by NIH grant K24 AA13736. Declarations of interest: information not available Other information In the original study, 328 participants were divided into the 2 groups on whether initially elevated HRSD score declined with cessation of heavy drinking:
In the present meta‐analysis, participants were divided into 2 substudies:
Both the substudies (Kranzler 2006 arm A; Kranzler 2006 arm B) were included in the 'Effects of interventions: Antidepressants versus placebo' comparison. Unfortunately, the original study did not report the adverse events for the single substudies. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization schedule reported. |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment was not described. Despite random assignment, participants who received placebo were older, reported more drinks per week during the pretreatment period, and had higher CGI depression scores at baseline. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All analyses used an intention‐to‐treat approach. Weekly comparisons including only participants for whom data were available from that visit, whereas end of study analyses used last observation carried forward analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. However, some of them were reported only as a % reduction (e.g. BDI score). |
Krupitsky 1993 arm A.
| Methods | Randomized, placebo‐controlled trial | |
| Participants | 41 people with alcohol dependence (number of men and women not available; mean age = 36‐37 years) with non‐severe affective disorders Inclusion criteria: information not available Exclusion criteria: information not available Participants with bipolar disorder: information not available |
|
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 3 weeks Site: Russia Setting: inpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: no information provided Anxiety:
Dropouts: data not available Adverse effects: information not provided |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Other psychiatric comorbidity: information not available Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment offered: other treatments were not administrated during study Funding sources: information not available. Other information In the original study, 90 people with alcohol dependence were randomly divided into 4 groups:
In the present meta‐analysis, study was divided into 2 substudies:
The first substudy (Krupitsky 1993 arm A) was included in the 'Effects of interventions: Antidepressants versus placebo' comparison and the second substudy (Krupitsky 1993 arm B) in the 'Antidepressants versus other medications' comparison |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported if it was a double‐ or single‐blind study. |
| Blinding of outcome assessment (detection bias) objective | Low risk | Not reported if it was a double‐ or single‐blind study. |
| Blinding of outcome assessment (detection bias) subjective | High risk | Not reported if it was a double‐ or single‐blind study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of dropouts not reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
Krupitsky 1993 arm B.
| Methods | Randomized, controlled trial | |
| Participants | 38 people with alcohol dependence (number of men and women not available; mean age = 36‐37 years) with affective disorders not severe Inclusion criteria: information not available Exclusion criteria: information not available Participants with bipolar disorder: information not available |
|
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 3 weeks Site: Russia Setting: inpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: no information provided Anxiety:
Dropouts: data not available Adverse effects: data not available |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Other psychiatric comorbidity: information not available Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment offered: other treatments were not administrated during study Funding sources: information not available Other information In the original study, 90 people with alcohol dependence were randomly divided into 4 groups:
In the present meta‐analysis, study was divided into 2 substudies:
The first substudy (Krupitsky 1993 arm A) was included in the 'Effects of interventions: Antidepressants versus placebo' comparison and the second substudy (Krupitsky 1993 arm B) in the 'Antidepressants versus other medications' comparison. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported if it was a double‐ or single‐blind study. |
| Blinding of outcome assessment (detection bias) objective | Low risk | Not reported if it was a double‐ or single‐blind study. |
| Blinding of outcome assessment (detection bias) subjective | High risk | Not reported if it was a double‐ or single‐blind study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of dropouts not reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
Krupitsky 2012.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 60 depressed people with alcohol dependence (47 men and 13 women; age (mean ± SD): escitalopram = 43.9 ± 1.1 years; placebo = 40.9 ± 1.3 years). Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 13 weeks Site: a single‐site at the Department of Narcology (Addiction Psychiatry) of the Bekhterev PsychoNeurological Research Institute, St Petersburg, Russia Setting: outpatients Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence:
Craving for alcohol:
Global response:
Anxiety:
Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: information not available. Declarations of interest: information not available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by means of generation random numbers in Excel. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered drug containers of identical appearance. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The investigators, doctors, participants, and any other staff members taking part in the experiment were unaware which of the groups any particular person belonged to. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Methods: participants who relapsed to heavy drinking were excluded from study. Results: the relatively small number of alcohol consumption days in the groups was due to participants who relapsed being excluded from trial. |
| Selective reporting (reporting bias) | High risk | Methods: participants who relapsed to heavy drinking were excluded from study. Results: the relatively small number of alcohol consumption days in the groups was due to participants who relapsed being excluded from trial. |
Liappas 2005 arm A.
| Methods | Randomized, single‐blind, comparative trial | |
| Participants | 30 people with alcohol dependence of an original group constituted by 60 participants (41 men and 19 women; mean age: 47 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Treatment:
Psychotherapy: cognitive behavioural psychotherapy administered in individual sessions and family interventions, twice a week Scheduled duration of treatment: 3 weeks Site: Drug and Alcohol Addiction Clinic, Athens University Psychiatric Clinic, Eginition Hospital, Athens, Greece Setting: inpatients for 1 week, then residential treatment Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: no information available Anxiety:
Global assessment:
Dropouts Adverse effects: data not available |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance‐use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: information not available Declarations of interest: information not available Other information In the original study, 60 participants were included into 4 groups:
Participants but not clinicians were blind to group status In the present meta‐analysis, we divided the control group (only psychotherapy) into 2 smaller groups, and compared these 2 smaller groups to the 2 antidepressants, using 3 subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random sequence generation was used ("At the end of the first week, individuals were randomly/electronically allocated to one of the three groups"). |
| Allocation concealment (selection bias) | Low risk | Computer sequence of allocation used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind study ("Patients but not clinicians were blind to group status"). |
| Blinding of outcome assessment (detection bias) objective | Low risk | Single‐blind study ("Patients but not clinicians were blind to group status"). |
| Blinding of outcome assessment (detection bias) subjective | High risk | Single‐blind study ("Patients but not clinicians were blind to group status"). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were not included in the analysis due to missing data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
Liappas 2005 arm B.
| Methods | Randomized, single‐blind, comparative trial | |
| Participants | 30 people with alcohol dependence of an original group constituted by 60 participants (41 men and 19 women; mean age: 47 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Treatment:
Psychotherapy: cognitive behavioural psychotherapy was administered in individual sessions and family interventions, twice a week Scheduled duration of treatment: 3 weeks Site: Drug and Alcohol Addiction Clinic, Athens University Psychiatric Clinic, Eginition Hospital, Athens, Greece Setting: inpatients for 1 week, then residential treatment Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: no information available Anxiety:
Global assessment:
Dropouts Adverse effects: data not available |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance‐use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: information not available Declarations of interest: information not available Other information In the original study, 60 participants were included into 4 groups:
Participants but not clinicians were blind to group status In the present meta‐analysis, we divided the control group (only psychotherapy) into 2 smaller groups, and compared these 2 smaller groups to the 2 antidepressants, using 3 subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random sequence generation reported ("At the end of the first week, individuals were randomly/electronically allocated to one of the three groups") |
| Allocation concealment (selection bias) | Low risk | Computer sequence of allocation reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind study ("Patients but not clinicians were blind to group status"). |
| Blinding of outcome assessment (detection bias) objective | Low risk | Single‐blind study ("Patients but not clinicians were blind to group status"). |
| Blinding of outcome assessment (detection bias) subjective | High risk | Single‐blind study ("Patients but not clinicians were blind to group status"). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were not included in the analysis due to missing data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
Liappas 2005 arm C.
| Methods | Randomized, single‐blind, comparative trial | |
| Participants | 40 people with alcohol dependence of an original group constituted by 60 participants (41 men and 19 women; mean age: 47 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy: cognitive behavioural psychotherapy was administered in individual sessions and family interventions, twice a week. Scheduled duration of treatment: 3 weeks Site: Drug and Alcohol Addiction Clinic, Athens University Psychiatric Clinic, Eginition Hospital, Athens, Greece Setting: inpatients for 1 week, then residential treatment Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: no information available Anxiety:
Global assessment:
Dropouts Adverse effects: data not available |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Anxiety:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: information not available Declarations of interest: information not available Other information In the original study, 60 participants were included into 4 groups:
Participants but not clinicians were blind to group status. In the present meta‐analysis, we divided the control group (only psychotherapy) into 2 smaller groups, and compared these 2 smaller groups to the 2 antidepressants, using 3 subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random sequence generation reported ("At the end of the first week, individuals were randomly/electronically allocated to one of the three groups") |
| Allocation concealment (selection bias) | Low risk | Computer sequence of allocation reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind study, and the outcomes are likely to be influenced by lack of blinding ("Patients but not clinicians were blind to group status"). |
| Blinding of outcome assessment (detection bias) objective | Low risk | Single‐blind study, and the outcomes are likely to be influenced by lack of blinding ("Patients but not clinicians were blind to group status"). |
| Blinding of outcome assessment (detection bias) subjective | High risk | Single‐blind study, and the outcomes are likely to be influenced by lack of blinding ("Patients but not clinicians were blind to group status"). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts were not included in the analysis due to missing data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
Lôo 1988.
| Methods | Randomized, double‐blind, comparative trial | |
| Participants | 129 people with alcohol dependence with depression or dysthymic disorder (111 men and 18 women; mean age: approximately 38 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available |
|
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 4‐8 weeks (depending on the centre concerned) Sites: 7 centres, in France Setting: unclear Route of administration: orally Starting dose: tianeptine = 37.5 mg/day; amitriptyline = 75.0 mg/day Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: data not available Anxiety:
Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: information not available Other substance use disorders: information not available Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were allowed. Funding source: information not available Declarations of interest: information not available Other information In the original study, the duration of the trial was 4‐8 weeks depending on the centre concerned. In the present meta‐analysis, only data of 4 weeks were analyzed. Before the onset of the trial, participants received a pretreatment with a placebo for 3‐10 days to screen out placebo‐responder participants. After this period, participants received tianeptine or amitriptyline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind stated. Medication and placebo prepared to appear identical. No specific reference made to blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis not used. However, the numbers of dropouts were low (10/64, 12/65) and were not unbalanced between groups. |
| Selective reporting (reporting bias) | High risk | Several data were missing (final MADRS score for amitriptyline, adverse effects, and alcohol consumption). |
Mason 1996.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 28 depressed people with alcohol dependence (24 men and 4 women; mean age = desimipramine: 36.0 ± 22 years; placebo = 41.0 ± 15.5 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available |
|
| Interventions | Drugs:
Psychotherapy: participants were encouraged to participate in Alcoholics Anonymous and any other psychosocial treatments. Scheduled duration of treatment: 6 months Sites: Department of the New York (NY) Hospital‐Cornell Medical Center, and the University of Miami (FL), School of Medicine, USA Setting: outpatients Route of administration: orally Starting dose: medication was prescribed in divided doses for the first week, then changed to bedtime dosing Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence:
Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: grants from the National Institute on Alcohol Abuse and Alcoholism (AA06866 and AA08111) Declarations of interest: information not available Other information Final HRSD score and difference between baseline and final HRSD score were excluded because they were expressed as medians and interquartile ranges. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind stated and blinding of key study personnel ensured by sentences of the evaluation of plasma desipramine concentration. "Plasma desipramine concentration was assessed and results were reviewed by a physician not involved in patient ratings to verify compliance and make dose recommendations. Equivalent dosing instructions were given by the nonblinded physician to blinded therapists for placebo‐treated patients to preserve the double‐blind study design." |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention to treat was not used. Participants who relapsed, who demonstrated non‐compliance, or who did not improve were removed from study. |
| Selective reporting (reporting bias) | High risk | Participants who relapsed, who demonstrated non‐compliance, or who did not improve were removed from study. |
McGrath 1996.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 69 depressed people with alcohol dependence (34 men and 35 women; age (mean ± SD): imipramine = 37.4 ± 6.7 years; placebo = information not available (report stated "10.6 ± 9.1")). Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 12 weeks Site: Depression Evaluation Service, New York State Psychiatric Institute, New York, NY, USA Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence:
Global response Dropouts Adverse effects: data not available |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: history of hypomania was not exclusionary. Other substance‐use disorders: history of current abuse of other substances was not exclusionary, provided that alcohol was clearly the main substance of abuse. Other characteristics of study Other pharmacological treatment offered: information not available Funding source: grants from the National Institute on Alcohol Abuse and Alcoholism (AA9539), the state of New York, and the Mental Health Clinical Research Center (NIMH 30906). Medications were supplied by Ciba‐Geigy Corp. Declarations of interest: information not available Other information Eligible participants were given single‐blind placebo for 1 week. Participants whose depression was not rated 'much improved' or 'very much improved' on the improvement item of the CGI scale for depression were randomized to receive placebo or imipramine. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double‐blind stated and medications prepared to appear identical. No specific reference made to blinding of participants and personnel. Plasma dosage of imipramine performed but no information on blinding of personnel provided. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analyses used. "Ratings from the last observation were carried forward for those subjects who did not complete all 12 weeks of treatment. Intention‐to‐treat analyses included all randomized patients and carried last observation forward for dropouts and those who completed less than 12 weeks." |
| Selective reporting (reporting bias) | High risk | Treatment outcomes were reported only for completers. |
McLean 1986.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 35 people with alcohol dependence (number of men and women: not available; mean age: information not available) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder: information not available |
|
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 4 weeks Site: Alcoholism Treatment Unit, Mapperley Hospital, Nottingham, UK Setting: inpatients Route of administration: orally Starting dose:
Pattern of dose reduction:
|
|
| Outcomes | Depression:
Alcohol dependence: data not available Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: sociopathic personality disorder was present in 6 participants; 3 were considered to have significant anxiety; there were no diagnoses of schizophrenia or psychotic illness. Other substance‐use disorders: information not available Other characteristics of study Other pharmacological treatment offered:
Funding source: Bencard. Declarations of interest: information not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation stated. No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind stated. No information on blinding of participants and personnel and on the appearance of medications. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis not used. However, the number of dropouts was low and balanced between groups. |
| Selective reporting (reporting bias) | High risk | Data of participants who dropped out of study were not included in results. |
Moak 2003.
| Methods | Randomized, placebo‐controlled trial | |
| Participants | 82 depressed people with alcohol dependence (50 men and 32 women; age (mean ± SD): sertraline = 41 ± 11 years; placebo = 42 ± 10 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy:
Scheduled duration of treatment: 12 weeks Site: Alcohol Research Center, Center for Drug and Alcohol Programs, Charleston, SC, USA Setting: outpatients Route of administration: orally Starting dose: 50 mg/day Pattern of dose reduction: titrated back down 50 mg over 7‐day period |
|
| Outcomes | Depression:
Alcohol dependence:
Dropout Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: National Institute on Alcohol Abuse and Alcoholism (grant AA10476). Pfizer supplied study drug and matched placebo. Declarations of interest: information not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomization used. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo‐controlled medication design applied. Medications dispensed in identically tablets. No further details provided on blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information provided on blinding of assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information provided on blinding of assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis used. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
Muhonen 2008.
| Methods | Randomized, double‐blind, comparative trial | |
| Participants | 80 depressed people with alcohol dependence (44 men and 36 women; age (mean ± SD): memantine = 47.5 ± 8.3 years; escitalopram = 47.9 ± 8.3 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy: no psychosocial intervention was offered. Scheduled duration of treatment: 26 weeks (6 months) Sites: 3 centres, Helsinki, Finland, and Europe. Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence:
Anxiety:
Cognitive functioning:
Quality of life:
Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other medications prescribed by the patient's physician were allowed, except other antidepressants. Funding source: National Public Health Institute, the Finnish Foundation for Alcohol Research and Helsinki Health Center Research. Study medication provided by Lundbeck Oy Ab, Turku, Finland Declaration of interest: no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participants meeting the inclusion criteria were randomly assigned by an independent person to escitalopram or memantine groups using a 1:1 ratio and random permuted blocks (Vassar Statistics randomizing algorithm). |
| Allocation concealment (selection bias) | Low risk | Randomization was concealed until study database was locked on by an independent clinical study monitor. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study medication was double‐dummy packed: participant took 2 tablets every time, 1 of which was the active medicine and 1 was an identical placebo for the second medication. The medication was labelled and controlled by an independent supplier. |
| Blinding of outcome assessment (detection bias) objective | Low risk | Outcome analysis was performed by an independent source. |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Outcome analysis was performed by an independent source. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis used. |
| Selective reporting (reporting bias) | Low risk | Data of all randomized participants were reported except for 1 participant due to an interrupted interview. |
Nunes 1993.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 26 depressed people with alcohol dependence (number of men and women: data not available; mean age: data not available) Inclusion criteria:
Exclusion criteria: information not available Participants with bipolar disorder: information not available |
|
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 6 months Site: Depression Evaluation Service, New York State Psychiatric Institute, New York, NY, USA Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available |
|
| Outcomes | Depression: data not available Alcohol dependence: data not available Global response Dropouts: data not available Adverse effects: data not available |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: information not available. Other substance use disorders: information not available. Other characteristics of study Other pharmacological treatment offered: information not available. Funding sources: supported in part by training grant MH‐15144 from NIMH, grants AA‐07688 and AA‐08030 from the National Institute on Alcohol Abuse and Alcoholism, and Scientist Development Award for Clinicians DA‐00154 from the National Institute on Drug Abuse. CIBA/Geigy provided imipramine and matching placebo. Declarations of interest: information not available Other information Only data of the double‐blind trial were included in the present meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information insufficient to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement. |
| Selective reporting (reporting bias) | High risk | Not all of study's prespecified outcomes were reported. |
Pettinati 2001a.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 29 depressed people with alcohol dependence (number of men and women: data not available; mean age: data not available) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy: weekly individual cognitive‐behavioural therapy Scheduled duration of treatment: 14 weeks Sites: University of Pennsylvania and the Carrier Foundation, USA Setting: outpatients Starting dose: 50 mg/day Pattern of dose reduction: tapering during the last 2 weeks of treatment |
|
| Outcomes | Depression:
Alcohol dependence:
Dropouts: information not available Adverse effects: information not available |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: National Institute on Alcohol Abuse and Alcoholism (R01‐AA09544 and KO2‐AA00239) and by Veteran Affairs Medical Center. Pfizer Inc. provided sertraline and matching placebo. Declarations of interest: information not available Other information In the original study participants were divided into 2 groups:
Participants with lifetime depression were then divided into 2 subgroups:
In the meta‐analysis, we included only participants with current depression. Unfortunately, dropouts and adverse events were not provided for each subgroup. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation referred to 1 randomization schedule for both sites. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind stated and medications prepared to appear identical. No specific reference made to blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis used. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
Pettinati 2010 arm A.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 79 depressed people with alcohol dependence (49 men and 30 women; age (mean ± SD): sertraline = 43.9 ± 11.5 years; placebo = 43.4 ± 8.9 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy: weekly individual CBT Scheduled duration of treatment: 14 weeks Site: University of Pennsylvania Treatment Research Center, USA Setting: outpatients Route of administration: orally Starting dose: 50 mg/day Pattern of dose reduction:
|
|
| Outcomes | Depression:
Alcohol dependence:
Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: National Institute on Alcohol Abuse and Alcoholism (grant R01‐AA09544‐10). Pfizer Inc., USA, Pharmaceutical Group provided sertraline and matching placebo. Declaration of interest: the authors declared the grants received. Other information In the original study participants were divided into 4 groups:
In the meta‐analysis, data were analyzed and included in 2 substudies:
Both the substudies (Pettinati 2010 arm A; Pettinati 2010 arm B) were included in the 'Effects of interventions: Antidepressants versus placebo' comparison. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomization reported. |
| Allocation concealment (selection bias) | Low risk | Urn randomization used to evenly distribute participants across groups using 4 pretreatment variables: gender, regular smoking, HRSD scores at time of randomization, and drinking frequency. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were imputed using appropriate methods. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
Pettinati 2010 arm B.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 91 depressed people with alcohol dependence (57 men and 34 women; age (mean ± SD): sertraline plus naltrexone = 43.4 ± 10.2 years; naltrexone = 42.9 ± 8.1) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy: weekly individual CBT Scheduled duration of treatment: 14 weeks Site: University of Pennsylvania Treatment Research Center, USA Setting: outpatients Route of administration: orally Starting doses:
Pattern of dose reduction:
|
|
| Outcomes | Depression:
Alcohol dependence:
Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Other characteristics of study Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: National Institute on Alcohol Abuse and Alcoholism (grant R01‐AA09544‐10). Pfizer Inc., USA, Pharmaceutical Group provided sertraline and matching placebo. Declaration of interest: the authors declared the grants received. Other information In the original study participants were divided into 4 groups:
In the meta‐analysis, data were analyzed and included in 2 substudies:
Both the substudies (Pettinati 2010 arm A; Pettinati 2010 arm B) were included in the 'Effects of interventions: Antidepressants versus placebo' comparison. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomization. |
| Allocation concealment (selection bias) | Low risk | Urn randomization used to evenly distribute participants across groups using 4 pretreatment variables: gender, regular smoking, HRSD scores at the time of randomization, and drinking frequency. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were imputed using appropriate methods. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
Roy 1998.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 36 depressed people with alcohol dependence (33 men and 3 women; age (mean ± SD): sertraline = 40.5 ± 7.7 years; placebo = 41.2 ± 5.6 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy: information not available Scheduled duration of treatment: 6 weeks Site: Alcoholic Rehabilitation Unit of the Veterans Administration Medical Center, East Orange, New Jersey, USA Setting: inpatients for the first 2 weeks then outpatients for 6 weeks Route of administration: orally Starting dose: information not available Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence: information not available Dropouts Adverse effects: information not available. |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were excluded. Other substance use disorders: participants with substance use disorders were excluded. Abuse of other substances was not an exclusion. Other characteristics of study Other pharmacological treatment offered: participants who had received antidepressant medication in the previous month were excluded. Funding source: information not available Declarations of interest: information not available Other information Final HRSD and BDI scores and number of withdrawal for medical reasons were provided separately for participants with primary depression (arm A; 15 participants) and participants with secondary depression (arm B; 21 participants). For the other outcomes, data were provided for the entire sample of participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and key study personnel Information ensured but information insufficient to permit judgement. |
| Blinding of outcome assessment (detection bias) objective | Low risk | No information on the blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) subjective | Unclear risk | No information on the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis referred to, but for participants who did not complete the study (dropouts), the last observation was not carried forward. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
Roy‐Byrne 2000.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 64 depressed people with alcohol dependence (29 men and 35 women; age (mean ± SD): 40.2 ± 8.2 years) Inclusion criteria:
Exclusion criteria:
Participants with bipolar disorder were excluded. |
|
| Interventions | Drugs:
Psychotherapy: cognitive‐behavioural skills training and psychoeducational group for alcohol‐dependence and depression led by an experienced therapist; group sessions lasted 1 hour once per week. Scheduled duration of treatment: 12 weeks Site: Harborview Medical Center Outpatient Psychiatry Clinic, University of Washington, Seattle, USA Setting: outpatients Route of administration: orally Starting dose:
Pattern of dose reduction: information not available |
|
| Outcomes | Depression:
Alcohol dependence:
Dropouts Adverse effects |
|
| Notes |
Baseline characteristics of participants Depression:
Alcohol dependence:
Other psychiatric comorbidity: participants with other mental disorders were included. Other substance use disorders: participants with substance use disorders were excluded. Other pharmacological treatment offered: other pharmacological treatments were not allowed. Funding source: supported in part by Bristol‐Meyers Squibb. Declarations of interest: information not available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured. |
| Blinding of outcome assessment (detection bias) objective | Low risk | Blinding of outcome assessment ensured. |
| Blinding of outcome assessment (detection bias) subjective | Low risk | Blinding of outcome assessment ensured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were imputed using appropriate methods. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
ADS: Alcohol Dependence Scale; ALT: alanine aminotransferase; AST: aspartate aminotransferase; AUDIT: Alcohol Use Disorders Identification Test; BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; BPRS: Brief Psychiatric Rating Scale; CBT: cognitive behavioural therapy; CGI: Clinical Global Impression scale; DBI: Drinking Behaviour Interview; DrInC: Drinker Inventory of Consequences scale; DOTES: Dosage Record and Treatment Emergent Symptoms Scale; DSM: Diagnostic and Statistic Manual of Mental Disorders; DSM‐III‐R: Diagnostic and Statistic Manual of Mental Disorders III ‐ Revised; DSM‐IV: Diagnostic and Statistic Manual of Mental Disorders ‐ IV; ECT: electroconvulsive therapy; GAF: General Assessment of Functioning scale; GAS: Global Assessment Scale; GGT: γ‐glutamyltransferase; HRSA: Hamilton Rating Scale for Anxiety; HRSD: Hamilton Rating Scale for Depression; ICD: International Classification of Diseases; LDQ: Leeds Dependence Questionnaire; LDRS: Lehmann Depression Rating Scale; MADRS: Montgomery and Åsberg Depression Rating Scale; MAST: Michigan Alcoholism Screening Test; MET: Motivational Enhancement Therapy; MMPI: Minnesota Multiphasic Personality Inventory; MMSE: Mini‐Mental State Examination; OCDS: Obsessive‐Compulsive Drinking Scale; PACS: Penn Alcohol Craving scale; PSQI: Pittsburgh Sleep Quality Index; SCL‐90: Symptom Check List‐90; SD: standard deviation; SOFAS: The Social and Occupational Functioning Assessment Scale; STAI: State Trait Anxiety Inventory; UKU: Udvalg for Kliniske Undersogelser Side Effect Rating Scale; VAS: Visual Analogue Scale; ZUNG: Zung Self‐Assessment Depression Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anthenelli 2014 | Type of participants not in the inclusion criteria: no depression |
| Arnow 2015 | Type of participants not in the inclusion criteria: no alcohol dependence |
| Balaratnasingam 2011 | Data of single group not available |
| Bandati 2013 | Study design not in the inclusion criteria: no control group |
| Batki 2015 | Type of participants and type of intervention not in the inclusion criteria: no depression, no use of antidepressant medications |
| Bowman 1966 | Type of intervention not in the inclusion criteria: no antidepressant medications used |
| Brewer 2015 | Type of participants and type of intervention not in the inclusion criteria: no depression, no alcohol dependence; no antidepressant medications used |
| Brown 2003 | Study design not in the inclusion criteria: no control group |
| Brunelin 2014 | Type of participants and type of intervention not in the inclusion criteria: no alcohol dependence; no antidepressant medications used |
| Charney 2015 | Type of participants not in the inclusion criteria: only 22% of participants had depression (single data of these participants not available) |
| Charnoff 1967 | Type of participants not in the inclusion criteria: no depression |
| Chick 2004b | Type of participants not in the inclusion criteria: no depression |
| Clark 2003 | Study population not in the inclusion criteria: people aged < 18 years |
| Cornelius 1993 | Study design not in the inclusion criteria: no control group |
| Cornelius 2011 | Type of participants not in the inclusion criteria: people aged < 18 years |
| Cornelius 2012 | Study design not in the inclusion criteria: no control group |
| Davis 2005 | Study design not in the inclusion criteria: no control group |
| Desai 1999 | Study design not in the inclusion criteria: no randomised trial |
| Douglas 1996 | Study design not in the inclusion criteria: commentary on Mason 1996 |
| Eriksson 2001 | Type of participants not in the inclusion criteria: no alcohol dependence. Only 73% of recruited participants with alcohol dependence; data of these participants not reported. |
| Farren 1999 | Study design not in the inclusion criteria: case report |
| Foulds 2016 | Commentary |
| García‐Portilla 2005 | Study design not in the inclusion criteria: no control group |
| Glasner‐Edwards 2007 | Type of intervention not in the inclusion criteria: the name of the antidepressant medication used was not indicated |
| Gorelick 1992 | Type of participants not in the inclusion criteria: no alcohol dependence |
| Grelotti 2014 | Type of participants not in the inclusion criteria: no alcohol dependence |
| Han 2013 | Study design not in the inclusion criteria: no control group for the antidepressant. Study compared the efficacy of aripripazole plus escitalopram to escitalopram in reducing the severity of depression and craving for alcohol in people with alcohol dependence and depression. |
| Hautzinger 2005 | Type of participants not in the inclusion criteria: no depression |
| Ionescu 2011 | Lack of information: number of patients for each group; time of collected data (1 month, 3 months, or 6 months?) |
| Ivanets 1998 | Type of participants not in the inclusion criteria: no depression |
| Janiri 1996 | Type of participants not in the inclusion criteria: no depression |
| Janiri 1997 | Type of participants not in the inclusion criteria: no depression |
| Kalyoncu 2007 | Study design not in the inclusion criteria: no control group |
| Kranzler 1995 | Type of participants not in the inclusion criteria: no alcohol dependence. Only 14% of recruited participants with alcohol dependence; data of these participants not reported. |
| Kranzler 2011 | Type of participants not in the inclusion criteria: no depression |
| Krupitsky 2013 | Data on severity of depression, craving for alcohol, and consumption of alcohol were reported to be improved but not provided as score achieved in the different scales used |
| Krystal 2008 | Type of intervention not in the inclusion criteria: the name of antidepressant medications was not indicated. |
| Labbate 2004 | Study design not in the inclusion criteria. No control group for the antidepressant. Study compared the efficacy of sertraline in people with post‐traumatic stress disorder and alcohol dependence with comorbid anxiety or depression to that in people with post‐traumatic stress disorder and alcohol dependence but without comorbid anxiety or depression. |
| Lee 2012 | Study design not in the inclusion criteria: no control group for the antidepressant. Study compared the efficacy of aripripazole plus escitalopram to escitalopram in reducing the severity of depression and craving for alcohol in people with alcohol dependence and depression. |
| Liappas 2004 | Type of participants not in the inclusion criteria: no depression |
| Macher 1991 | Study design not in the inclusion criteria: no control group |
| Malka 1992 | Study design not in the inclusion criteria: no control group |
| Marey 1991 | Study design not in the inclusion criteria: review |
| Mason 1999 | Study design not in the inclusion criteria: no control group for the antidepressant. Study compared the efficacy of naltrexone plus sertraline to sertraline in reducing the severity of depression and alcohol consumption in people with alcohol dependence and depression. |
| Naranjo 1997 | Study design not in the inclusion criteria: statistical elaboration of previous studies |
| Oslin 2005 | Study design not in the inclusion criteria: no control group for the antidepressant. Study compared the efficacy of naltrexone plus sertraline to sertraline in reducing the severity of depression and alcohol consumption in people with alcohol dependence and depression. |
| Overall 1973 | Type of participants not in the inclusion criteria: no depression |
| Powell 1995 | Type of participants not in the inclusion criteria: no depression |
| Ruiz‐Mellott 2005 | Study design not in the inclusion criteria: no control group |
| Saatcioglu 2005 | Study design not in the inclusion criteria: no control group |
| Salloum 2011 | Study design not in the inclusion criteria: no control group |
| Salloum 2013 | Study design not in the inclusion criteria: no control group |
| Schottenfeld 1989 | Study design not in the inclusion criteria: no control group |
| Shaw 1975 | Study design not in the inclusion criteria: no control group for the antidepressant. Study compared the efficacy of chlordiazepoxide plus imipramine to placebo. |
| Witte 2012 | Study design not in the inclusion criteria: no control group for the antidepressant. Study compared the efficacy of acamprosate plus escitalopram to escitalopram in reducing the severity of depression and alcohol consumption in people with alcohol dependence and depression. |
Characteristics of studies awaiting assessment [ordered by study ID]
Petrakis 2013.
| Methods | Requested from the authors |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Schifano 1993.
| Methods | Requested from the authors |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Differences between protocol and review
The present meta‐analysis presents the following differences from the protocol.
Primary and secondary outcomes
In the present review, the number of participants that reported use of alcohol was not used as a primary outcome as we planned in the Protocol (see Primary outcomes). This difference is due to the fact that the studies included did not report the number of participants who used alcohol to descrive alcohol consumption but other outcomes such as rate of drinking days, cumulative number of drinking days, number of drinks per drinking day, weekly number of heavy drinking days, rate of heavy drinkers, number of heavy drinkers, and son on (see Appendix 9). Whenever possible, we combined togethar these outcomes and used them to evaluate the effects of antidepressants in alcohol consumption.
Similarly, in the present meta‐analysis the number of participants that used other substances was not used as a secondary outcome as we planned in the Protocol (see Secondary outcomes). This difference is due to the fact that most of the studies excluded participants with substance use disorders or participants that used other substances of abuse.
Sources of electronic searches
Given that the original protocol was published in 2010, some sections needed updating to fulfill the current methodological guidelines for Cochrane Reviews. In detail, we changed the databases that we planned to search in the protocol, because of lack of access to some of these databases and because of some changes to standard search routines. We were unable to include searches from CINAHL as we lost access to it prior to searching whereas we added EMBASE (embase.com) search. Since the protocol for this review was published, Current Controlled Trials has been replaced by the ISTRCN Registry (isrctn.com), and we have searched ICTRP Registry instead.
'Summary of findings' table
We prepared a 'Summary of findings' table, including assessing the quality of evidence using GRADE (GRADEpro 2014).
Contributions of authors
PP and ET wrote the protocol.
RA and ET inspected the search hits by reading titles and abstracts, obtained the full text, assessed studies for inclusion, and assess study quality.
All three authors resolved disagreements.
RA and ET wrote the results.
PP wrote the discussion.
All three authors read and agree the final version.
Sources of support
Internal sources
Department of Epidemiology, ASL RM/E, Italy.
-
Department of Biomedical Sciences, Section of Neurosciences and Clinical Pharmacology, University of Cagliari, Italy.
Two grants from the University of Cagliari (FIR 2016 and FIR 2017) assigned to Dr Roberta Agabio
External sources
No sources of support supplied
Declarations of interest
RA: no interests to declare relating to this work.
ET: no interests to declare relating to this work.
PP: no interests to declare relating to this work.
New
References
References to studies included in this review
Adamson 2015 {published data only}
- Adamson SJ, Sellman JD, Foulds JA, Frampton CM, Deering D, Dunn A, et al. A randomized trial of combined citalopram and naltrexone for nonabstinent outpatients with co‐occurring alcohol dependence and major depression. Journal of Clinical Psychopharmacology 2015;35(2):143‐9. [DOI] [PubMed] [Google Scholar]
- Foulds JA, Douglas Sellman J, Adamson SJ, Boden JM, Mulder RT, Joyce PR. Depression outcome in alcohol dependent patients: an evaluation of the role of independent and substance‐induced depression and other predictors. Journal of Affective Disorders 2015;174:503‐10. [DOI] [PubMed] [Google Scholar]
- Foulds JA, Ton K, Kennedy MA, Adamson SJ, Mulder RT, Sellman JD. OPRM1 genotype and naltrexone response in depressed alcohol‐dependent patients. Pharmacogenetics and Genomics 2015;25(5):270‐3. [DOI] [PubMed] [Google Scholar]
Altamura 1990 {published data only}
- Altamura AC, Mauri MC, Girardi T, Panetta B. Alcoholism and depression: a placebo controlled study with viloxazine. International Journal of Clinical Pharmacology Research 1990;10(5):293‐8. [PubMed] [Google Scholar]
Altintoprak 2008 {published data only}
- Altintoprak AE, Zorlu N, Coskunol H, Akdeniz F, Kitapcioglu G. Effectiveness and tolerability of mirtazapine and amitriptyline in alcoholic patients with co‐morbid depressive disorder: a randomized, double‐blind study. Human Psychopharmacology 2008;23(4):313‐9. [DOI] [PubMed] [Google Scholar]
Butterworth 1971a {published data only}
- Butterworth AT, Watts RD. Treatment of hospitalized alcoholics with doxepin and diazepam. A controlled study. Quarterly Journal of Studies on Alcohol 1971;32(1):78‐81. [PubMed] [Google Scholar]
Butterworth 1971b {published data only}
- Butterworth AT. Depression associated with alcohol withdrawal. Imipramine therapy compared with placebo. Quarterly Journal of Studies on Alcohol 1971;32(2):343‐8. [PubMed] [Google Scholar]
Cocchi 1997 {published data only}
- Cocchi R. Paroxetine vs amitryptiline in depressed alcoholics. European Neuropsychopharmacology 1997;7(Suppl 2):S254. [Google Scholar]
Cornelius 1997 {published data only}
- Cornelius JC. Fluoxetine versus placebo in depressed alcoholics. Psychopharmacology. Psychopharmacology Bulletin 1994;30(4):661. [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Cornelius MD, Perel JM, Ehler JG, Jarrett PJ, et al. Preliminary report: double‐blind, placebo‐controlled study of fluoxetine in depressed alcoholics. Psychopharmacology Bulletin 1995;31(2):297‐303. [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Ehler JG, Jarrett PJ, Cornelius MD, Black A, et al. Double‐blind fluoxetine in depressed alcoholic smokers. Psychopharmacology Bulletin 1997;33(1):165‐70. [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Ehler JG, Jarrett PJ, Cornelius MD, Perel JM, et al. Fluoxetine in depressed alcoholics. A double‐blind, placebo‐controlled trial. Archives of General Psychiatry 1997;54(8):700‐5. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Haskett RF, Daley DC, Cornelius MD, Thase ME, et al. Fluoxetine versus placebo in depressed alcoholics: a 1‐year follow‐up study. Addictive Behaviors 2000;25(2):307‐10. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Thase ME, Haskett RF, Daley DC, Jones‐Barlock A, et al. Fluoxetine versus placebo in depressed alcoholic cocaine abusers. Psychopharmacology Bulletin 1998;34(1):117‐21. [PubMed] [Google Scholar]
Cornelius 2016 {published data only}
- Cornelius J, Chung T, Douaihy A, Glance J. Mirtazapine pilot trial in youthful MDD/AUD subjects. American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2017;26(3):250‐1. [Google Scholar]
- Cornelius J, Chung T, Douaihy A, Glance J, Kmiec J, Wesesky M, et al. Double‐blind mirtazapine pilot trial in AUD/MDD. Alcoholism, Clinical and Experimental Research 2016;40:233. [Google Scholar]
- Cornelius JR, Chung T, Douaihy AB, Kirisci L, Glance J, Kmiec J, et al. Mirtazapine in comorbid major depression and an alcohol use disorder: a double‐blind placebo‐controlled pilot trial. Psychiatry Research 2016;242:326‐30. [DOI: 10.1016/j.psychres.2016.06.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Chung TA, Wesesky MA, Douaihy A, Fitzgerald D, Kirisci L, et al. Combined young and older adult mirtazapine pilot trials in AUD/MDD. Alcoholism: Clinical and Experimental Research 2017;41:176A. [Google Scholar]
Gallant 1969 arm a {published data only}
- Gallant DM, Bishop MP, Guerrero‐Figueroa R, Selby M, Phillips R. Doxepin versus diazepam: a controlled evaluation in 100 chronic alcoholic patients. Journal of Clinical Pharmacology and New Drugs 1969;9(1):57‐65. [PubMed] [Google Scholar]
Gallant 1969 arm b {published data only}
- Gallant DM, Bishop MP, Guerrero‐Figueroa R, Selby M, Phillips R. Doxepin versus diazepam: a controlled evaluation in 100 chronic alcoholic patients. Journal of Clinical Pharmacology and New Drugs 1969;9(1):57‐65. [PubMed] [Google Scholar]
Gual 2003 {published data only}
- Gual A, Balcells M, Torres M, Madrigal M, Diez T, Serrano L. Sertraline for the prevention of relapse in detoxicated alcohol dependent patients with a comorbid depressive disorder: a randomized controlled trial. Alcohol and Alcoholism (Oxford, Oxfordshire) 2003;38(6):619‐25. [DOI] [PubMed] [Google Scholar]
Habrat 2006 {published data only}
- Habrat B, Zaloga B. A double‐blind controlled study of the efficacy and acceptability of tianeptine in comparison with fluvoxamine in the treatment of depressed alcoholic patients. Psychiatria Polska 2006;40(3):579‐97. [PubMed] [Google Scholar]
Hernandez‐Avila 2004 {published data only}
- Hernandez‐Avila CA, Modesto‐Lowe V, Feinn R, Kranzler HR. Nefazodone treatment of comorbid alcohol dependence and major depression. Alcoholism, Clinical and Experimental Research 2004;28(3):433‐40. [DOI] [PubMed] [Google Scholar]
Kranzler 2006 arm A {published data only}
- Kranzler HR, Mueller T, Cornelius J, Pettinati HM, Moak D, Martin PR, et al. Sertraline treatment of co‐occurring alcohol dependence and major depression. Journal of Clinical Psychopharmacology 2006;26(1):13‐20. [DOI] [PubMed] [Google Scholar]
Kranzler 2006 arm B {published data only}
- Kranzler HR, Mueller T, Cornelius J, Pettinati HM, Moak D, Martin PR, et al. Sertraline treatment of co‐occurring alcohol dependence and major depression. Journal of Clinical Psychopharmacology 2006;26(1):13‐20. [DOI] [PubMed] [Google Scholar]
Krupitsky 1993 arm A {published data only}
- Krupitsky EM, Burakov AM, Grinenko AIa, Borodkin IuS. Effect of pharmacotherapy of affective disorders on the psycho‐semantics of alcoholic patients. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 1995;95(6):67‐71. [PubMed] [Google Scholar]
- Krupitsky EM, Burakov AM, Ivanov VB, Karandashova GF, Lapin IP, Grinenko Ala, et al. The use of baclofen for treating affective disorders in alcoholism. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 1994;94(1):57‐61. [PubMed] [Google Scholar]
- Krupitsky EM, Burakov AM, Ivanov VB, Krandashova GF, Lapin IP, Grinenko AJa, et al. Baclofen administration for the treatment of affective disorders in alcoholic patients. Drug and Alcohol Dependence 1993;33(2):157‐63. [DOI] [PubMed] [Google Scholar]
Krupitsky 1993 arm B {published data only}
- Krupitsky EM, Burakov AM, Grinenko AIa, Borodkin IuS. Effect of pharmacotherapy of affective disorders on the psycho‐semantics of alcoholic patients. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 1995;95(6):67‐71. [PubMed] [Google Scholar]
- Krupitsky EM, Burakov AM, Ivanov VB, Karandashova GF, Lapin IP, Grinenko AIa, et al. The use of baclofen for treating affective disorders in alcoholism. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 1994;94(1):57‐61. [PubMed] [Google Scholar]
- Krupitsky EM, Burakov AM, Ivanov VB, Krandashova GF, Lapin IP, Grinenko AJa, et al. Baclofen administration for the treatment of affective disorders in alcoholic patients. Drug and Alcohol Dependence 1993;33(2):157‐63. [DOI] [PubMed] [Google Scholar]
Krupitsky 2012 {published data only}
- Krupitsky E, Yerish S, Kiselev A. Clinical trial of escitalopram for alcoholism comorbid with affective disorders. Alcoholism, Clinical and Experimental Research 2012;36:297A. [Google Scholar]
- Krupitsky E, Yerish S, Kiselev A. Clinical trial of escitalopram for alcoholism comorbid with affective disorders. European Neuropsychopharmacology 2010;20(Suppl 3):S571. [Google Scholar]
- Krupitsky EM, Yerish SM, Berntsev VA, Kiselev A, Alexandrovsky NA, Torban MN, et al. Double blind placebo controlled randomized clinical trial of escitalopram for alcoholism comorbid with affective disorders (depression and anxiety). ISBRA 2010 Poster Abstracts. 2010:167A.
- Krupitsky EM, Yerish SM, Kiselev AS, Berntsev VA, Alexandrovsky NA, Torban MN, et al. A double blind, placebo controlled, randomized clinical trial of escitalopram for the treatment of affective disorders in alcohol dependent patients in early remission. The International Psychiatry and Behavioral Neurosciences Yearbook. Boutros N. Vol. II, New York (NY): Nova Science Publishers, 2012:239‐56. [Google Scholar]
Liappas 2005 arm A {published data only}
- Liappas J, Paparrigopoulos T, Tzavellas E, Rabavilas A. Mirtazapine and venlafaxine in the management of collateral psychopathology during alcohol detoxification. Progress in Neuro‐psychopharmacology & Biological Psychiatry 2005;29(1):55‐60. [DOI] [PubMed] [Google Scholar]
Liappas 2005 arm B {published data only}
- Liappas J, Paparrigopoulos T, Tzavellas E, Rabavilas A. Mirtazapine and venlafaxine in the management of collateral psychopathology during alcohol detoxification. Progress in Neuro‐psychopharmacology & Biological Psychiatry 2005;29(1):55‐60. [DOI] [PubMed] [Google Scholar]
Liappas 2005 arm C {published data only}
- Liappas J, Paparrigopoulos T, Tzavellas E, Rabavilas A. Mirtazapine and venlafaxine in the management of collateral psychopathology during alcohol detoxification. Progress in Neuro‐psychopharmacology & Biological Psychiatry 2005;29(1):55‐60. [DOI] [PubMed] [Google Scholar]
Lôo 1988 {published data only}
- Lôo H, Malka R, Defrance R, Barrucand D, Benard JY, Niox‐Rivière H, et al. Tianeptine and amitriptyline. Controlled double‐blind trial in depressed alcoholic patients. Neuropsychobiology 1988;19(2):79‐85. [DOI] [PubMed] [Google Scholar]
Mason 1996 {published data only}
- Mason BJ, Kocsis JH. Desipramine treatment of alcoholism. Psychopharmacology Bulletin 1991;27(2):155‐61. [PubMed] [Google Scholar]
- Mason BJ, Kocsis JH, Ritvo EC, Cutler RB. A double‐blind, placebo‐controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. JAMA 1996;275(10):761‐7. [PubMed] [Google Scholar]
McGrath 1996 {published data only}
- McGrath PJ, Nunes EV, Stewart JW, Goldman D, Agosti V, Ocepek‐Welikson K, et al. Imipramine treatment of alcoholics with primary depression: a placebo‐controlled clinical trial. Archives of General Psychiatry 1996;53(3):232‐40. [DOI] [PubMed] [Google Scholar]
- McGrath PJ, Nunes EV, Stewart JW, Ocepek‐Welikson K, Quitkin FM. Antidepressant treatment of primary depression in alcoholics: a placebo‐controlled randomized clinical trial. Psychopharmacology Bulletin. 1995:598.
McLean 1986 {published data only}
- McLean PC, Ancill RJ, Szulecka TK. Mianserin in the treatment of depressive symptoms in alcoholics. A double‐blind placebo controlled study using a computer delivered self‐rating scale. Psychiatria Polska 1986;20(6):417‐27. [PubMed] [Google Scholar]
Moak 2003 {published data only}
- Moak DH, Anton RF, Latham PK, Voronin KE, Waid RL, Durazo‐Arvizu R. Sertraline and cognitive behavioral therapy for depressed alcoholics: results of a placebo‐controlled trial. Journal of Clinical Psychopharmacology 2003;23(6):553‐62. [DOI] [PubMed] [Google Scholar]
Muhonen 2008 {published data only}
- Muhonen LH, Lahti J, Alho H, Lönnqvist J, Haukka J, Saarikoski ST. Serotonin transporter polymorphism as a predictor for escitalopram treatment of major depressive disorder comorbid with alcohol dependence. Psychiatry Research 2011;186(1):53‐7. [DOI] [PubMed] [Google Scholar]
- Muhonen LH, Lahti J, Sinclair D, Lönnqvist J, Alho H. Treatment of alcohol dependence in patients with co‐morbid major depressive disorder ‐ predictors for the outcomes with memantine and escitalopram medication. Substance Abuse Treatment, Prevention, and Policy 2008;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhonen LH, Lönnqvist J, Juva K, Alho H. Double‐blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. Journal of Clinical Psychiatry 2008;69(3):392‐9. [DOI] [PubMed] [Google Scholar]
- Muhonen LH, Lönnqvist J, Lahti J, Alho H. Age at onset of first depressive episode as a predictor for escitalopram treatment of major depression comorbid with alcohol dependence. Psychiatry Research 2009;167(1‐2):115‐22. [DOI] [PubMed] [Google Scholar]
Nunes 1993 {published data only}
- Nunes EV, McGrath PJ, Quitkin FM, Stewart JP, Harrison W, Tricamo E, et al. Imipramine treatment of alcoholism with comorbid depression. American Journal of Psychiatry 1993;150(6):963‐5. [DOI] [PubMed] [Google Scholar]
Pettinati 2001a {published data only}
- Dundon W, Lynch KG, Pettinati HM, Lipkin C. Treatment outcomes in type A and B alcohol dependence 6 months after serotonergic pharmacotherapy. Alcoholism, Clinical and Experimental Research 2004;28(7):1065‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin SC. The efficacy of sertraline treatment for subtypes of alcohol dependence: advancing individualized treatments. Dissertation Abstracts International 2010;71(4‐B2):2333. [Google Scholar]
- Pettinati HM, Dundon W, Lipkin C. Gender differences in response to sertraline pharmacotherapy in Type A alcohol dependence. American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2004;13(3):236‐47. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcoholism, Clinical and Experimental Research 2000;24(7):1041‐9. [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Luck G, Kranzler HR, Rukstalis MR, Cnaan A. Double‐blind clinical trial of sertraline treatment for alcohol dependence. Journal of Clinical Psychopharmacology 2001;21(2):143‐53. [DOI] [PubMed] [Google Scholar]
Pettinati 2010 arm A {published data only}
- NCT00004554. Sertraline for alcohol dependence and depression. clinicaltrials.gov/ct2/show/NCT00004554 Date first received: 7 February 2000.
- Pettinati HM, Leshner AR, Dundon WD. Sertraline treatment in subtypes of alcohol dependence: a replication. Alcoholism: Clinical and Experimental Research 2012;36:245A. [Google Scholar]
- Pettinati HM, Oslin DW, Kampman KM, Dundon WD, Xie H, Gallis TL, et al. A double‐blind, placebo‐controlled trial combining sertraline and naltrexone for treating co‐occurring depression and alcohol dependence. American Journal of Psychiatry 2010;167(6):668‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Mahoney EM, Dundon WD, Pettinati HM. Combination pharmacotherapy sertraline and naltrexone decreases suicidal ideation scores in co‐morbid depressed alcoholics. Alcoholism: Clinical and Experimental Research 2012;36:245A. [Google Scholar]
Pettinati 2010 arm B {published data only}
- NCT00004554. Sertraline for alcohol dependence and depression. clinicaltrials.gov/ct2/show/NCT00004554 Date first received: 7 February 2000.
- Pettinati HM, Leshner AR, Dundon WD. Sertraline treatment in subtypes of alcohol dependence: a replication. Alcoholism: Clinical and Experimental Research 2012;36:245A. [Google Scholar]
- Pettinati HM, Oslin DW, Kampman KM, Dundon WD, Xie H, Gallis TL, et al. A double‐blind, placebo‐controlled trial combining sertraline and naltrexone for treating co‐occurring depression and alcohol dependence. American Journal of Psychiatry 210;167(6):668‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Mahoney EM, Dundon WD, Pettinati HM. Combination pharmacotherapy sertraline and naltrexone decreases suicidal ideation scores in co‐morbid depressed alcoholics. Alcoholism: Clinical and Experimental Research 2012;36:245A. [Google Scholar]
Roy 1998 {published data only}
- Roy A. Placebo‐controlled study of sertraline in depressed recently abstinent alcoholics. Biological Psychiatry 1998;44(7):633‐7. [DOI] [PubMed] [Google Scholar]
- Roy A. Treatment of depressed alcoholics. 150th Annual Meeting of the American Psychiatric Association; 1997 May 17‐22; San Diego (CA). 1997:151.
Roy‐Byrne 2000 {published data only}
- Roy‐Byrne PP, Pages KP, Russo JE, Jaffe C, Blume AW, Kingsley E, et al. Nefazodone treatment of major depression in alcohol‐dependent patients: a double‐blind, placebo‐controlled trial. Journal of Clinical Psychopharmacology 2000;20(2):129‐36. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anthenelli 2014 {published data only}
- Anthenelli RM, Blom TJ, Heffner JL, Higley AE, Bekman NM, Doran N, et al. Central serotonergic & peripheral mechanisms underlie sex‐sensitive, stressor‐specific endocrine responses in alcohol dependent subjects and controls. Alcoholism: Clinical and Experimental Research 2014;38:85A. [Google Scholar]
Arnow 2015 {published data only}
- Arnow BA, Blasey C, Williams LM, Palmer DM, Rekshan W, Schatzberg AF, et al. Depression subtypes in predicting antidepressant response: a report from the iSPOT‐D trial. American Journal of Psychiatry 2015;172(8):743‐50. [DOI] [PubMed] [Google Scholar]
Balaratnasingam 2011 {published data only}
- Balaratnasingam S, Janca A. Combining sertraline and naltrexone in the treatment of adults with comorbid depression and alcohol dependence. Current Psychiatry Reports 2011;13(4):245‐7. [DOI] [PubMed] [Google Scholar]
Bandati 2013 {published data only}
- Bandati A, Bandati AO, Albu A. Management of affective disorders in alcohol‐dependent patients. European Neuropsychopharmacology 2013;23:S564‐5. [Google Scholar]
Batki 2015 {published data only}
- Batki S, Pennington D, Meyerhoff D, Spigelman I, Back S, Petrakis I. Pharmacologic therapies for alcohol use disorder and PTSD. Alcoholism, Clinical and Experimental Research 2015;39:289A. [Google Scholar]
Bowman 1966 {published data only}
- Bowman EH, Thimann J. Treatment of alcoholism in the subacute stage. (A study of three active agents). Diseases of the Nervous System 1966;27(5):342‐6. [PubMed] [Google Scholar]
Brewer 2015 {published data only}
- Brewer A, Thompson‐Lake DG, Mahoney JJ, Newton TF, Garza R. Evaluation of lisdexamfetamine alone and lisdexamfetamine + modafinil for cocaine use disorder. Drug and Alcohol Dependence 2015;146:e231. [Google Scholar]
Brown 2003 {published data only}
- Brown ES, Bobadilla L, Nejtek VA, Perantie D, Dhillon H, Frol A. Open‐label nefazodone in patients with a major depressive episode and alcohol dependence. Progress in Neuro‐psychopharmacology & Biological Psychiatry 2003;27(4):681‐5. [DOI] [PubMed] [Google Scholar]
Brunelin 2014 {published data only}
- Brunelin J, Jalenques I, Trojak B, Attal J, Szekely D, Gay A, et al. The efficacy and safety of low frequency repetitive transcranial magnetic stimulation for treatment‐resistant depression: the results from a large multicenter French RCT. Brain Stimulation 2014;7(6):855‐63. [DOI] [PubMed] [Google Scholar]
Charney 2015 {published data only}
- Charney DA, Heath LM, Zikos E, Palacios‐Boix J, Gill KJ. Poorer drinking outcomes with citalopram treatment for alcohol dependence: a randomized, double‐blind, placebo‐controlled trial. Alcoholism, Clinical and Experimental Research 2015;39(9):1756‐65. [DOI] [PubMed] [Google Scholar]
Charnoff 1967 {published data only}
- Charnoff SM. Long‐term treatment of alcoholism with amitriptyline and emylcamate. A double‐blind evaluation. Quarterly Journal of Studies on Alcohol 1967;28(2):289‐94. [PubMed] [Google Scholar]
Chick 2004b {published data only}
- Chick J, Aschauer H, Hornik K, Investigators' Group. Efficacy of fluvoxamine in preventing relapse in alcohol dependence: a one‐year, double‐blind, placebo‐controlled multicentre study with analysis by typology. Drug and Alcohol Dependence 2004;74(1):61‐70. [DOI] [PubMed] [Google Scholar]
Clark 2003 {published data only}
- Clark DB, Wood DS, Cornelius JR, Bukstein OG, Martin CS. Clinical practices in the pharmacological treatment of comorbid psychopathology in adolescents with alcohol use disorders. Journal of Substance Abuse Treatment 2003;25(4):293‐5. [DOI] [PubMed] [Google Scholar]
Cornelius 1993 {published data only}
- Cornelius JR, Salloum IM, Cornelius MD, Perel JM, Thase ME, Ehler JG, et al. Fluoxetine trial in suicidal depressed alcoholics. Psychopharmacology Bulletin 1993;29(2):195‐9. [PubMed] [Google Scholar]
Cornelius 2011 {published data only}
- Cornelius JR, Douaihy A, Bukstein OG, Daley DC, Wood SD, Kelly TM, et al. Evaluation of cognitive behavioral therapy/motivational enhancement therapy (CBT/MET) in a treatment trial of comorbid MDD/AUD adolescents. Addictive Behaviors 2011;36(8):843‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornelius 2012 {published data only}
- Cornelius JR, Douaihy A, Clark DB. Mirtazapine pilot trial in depressed alcoholics. American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2012;21(4):392. [Google Scholar]
- Cornelius JR, Douaihy AB, Clark DB, Chung T, Wood DS, Daley D. Mirtazapine in comorbid major depression and alcohol dependence: an open‐label trial. Journal of Dual Diagnosis 2012;8(3):200‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2005 {published data only}
- Davis LL, Rush JA, Wisniewski SR, Rice K, Cassano P, Jewell ME, et al. Substance use disorder comorbidity in major depressive disorder: an exploratory analysis of the Sequenced Treatment Alternatives to Relieve Depression cohort. Comprehensive Psychiatry 2005;46(2):81‐9. [DOI] [PubMed] [Google Scholar]
- Davis LL, Wisniewski SR, Howland RH, Trivedi MH, Husain MM, Fava M, et al. Does comorbid substance use disorder impair recovery from major depression with SSRI treatment? An analysis of the STAR*D level one treatment outcomes. Drugs and Alcohol Dependence 2010;107(2‐3):161‐70. [DOI] [PubMed] [Google Scholar]
Desai 1999 {published data only}
- Desai N, Losardo M, Farrell D, Penk W, Petrakis I, Rounsaville B. The efficacy of sertraline versus fluoxetine in Veterans comorbid depression and alcohol dependence. Abstract. 1999:45.
Douglas 1996 {published data only}
- Douglas MB, Fleming MF. Treating depression in alcoholics. Journal of Family Practice 1996;42(6):565‐6. [PubMed] [Google Scholar]
Eriksson 2001 {published data only}
- Eriksson M, Berggren U, Blennow K, Fahlke C, Balldin J. Further investigation of citalopram on alcohol consumption in heavy drinkers: responsiveness possibly linked to the DRD2 A2/A2 genotype. Alcohol 2001;24(1):15‐23. [DOI] [PubMed] [Google Scholar]
Farren 1999 {published data only}
- Farren CK, O'Malley SS. Occurrence and management of depression in the context of naltrexone treatment of alcoholism. American Journal of Psychiatry 1999;156(8):1258‐62. [DOI] [PubMed] [Google Scholar]
Foulds 2016 {published data only}
- Foulds JA, Sellman JD, Mulder RT. Antidepressant therapy for depressed patients with an alcohol use disorder. Australian and New Zealand Journal of Psychiatry 2016;50(3):199‐200. [DOI] [PubMed] [Google Scholar]
García‐Portilla 2005 {published data only}
- García‐Portilla MP, Bascarán MT, Saiz PA, Mateos M, González‐Quirós M, Pérez P, et al. Effectiveness of venlafaxine in the treatment of alcohol dependence with comorbid depression. Actas Espanolas de Psiquiatria 2005;33(1):41‐5. [PubMed] [Google Scholar]
Glasner‐Edwards 2007 {published data only}
- Glasner‐Edwards S, Tate SR, McQuaid JR, Cummins K, Granholm E, Brown SA. Mechanisms of action in integrated cognitive‐behavioral treatment versus twelve‐step facilitation for substance‐dependent adults with comorbid major depression. Journal of Studies on Alcohol and Drugs 2007;68(5):663‐72. [DOI] [PubMed] [Google Scholar]
Gorelick 1992 {published data only}
- Gorelick DA, Paredes A. Effect of fluoxetine on alcohol consumption in male alcoholics. Alcoholism, Clinical and Experimental Research 1992;16(2):261‐5. [DOI] [PubMed] [Google Scholar]
Grelotti 2014 {published data only}
- Grelotti DJ, Hammer GP, Dilley JW, Karasic DH, Sorensen JL, Bangsberg DR, et al. Does substance use compromise depression treatment? A randomized trial of homeless HIV+ persons. Topics in Antiviral Medicine 2014;22(e‐1):541‐2. [Google Scholar]
Han 2013 {published data only}
- Han DH, Kim SM, Choi JE, Min KJ, Renshaw PF. Adjunctive aripiprazole therapy with escitalopram in patients with co‐morbid major depressive disorder and alcohol dependence: clinical and neuroimaging evidence. Journal of Psychopharmacology (Oxford, England) 2013;27(3):282‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hautzinger 2005 {published data only}
- Hautzinger M, Wetzel H, Szegedi A, Scheurich A, Lörch B, Singer P, et al. Combination treatment with SSRI and cognitive behavior therapy for relapse prevention of alcohol‐dependent men. Results of a randomized, controlled multicenter therapeutic study. Nervenarzt 2005;76(3):295‐307. [DOI] [PubMed] [Google Scholar]
Ionescu 2011 {published data only}
- Ionescu D, Dehelean C, Funar TS, Dumache R. A comparative evaluation of therapy escitalopram versus memantine in the case of patients with alcohol dependence and co‐morbid major depressive disorder. Toxicology Letters 2011;205:S89. [Google Scholar]
Ivanets 1998 {published data only}
- Ivanets NN. Treatment of alcoholism with antidepressant mianserin. 5th World Congress on Innovations in Psychiatry; 1998 19‐22 May; London. 1998:S27.
- Ivanets NN, Anokhina IP, Kogan BM, Chirko VV, Nebarakova TP, Rusinov AV. The efficacy and mechanisms of action of lerivon in alcoholism. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 1996;96(5):52‐8. [PubMed] [Google Scholar]
Janiri 1996 {published data only}
- Janiri L, Gobbi G, Mannelli P, Pozzi G, Serretti A, Tempesta E. Effects of fluoxetine at antidepressant doses on short‐term outcome of detoxified alcoholics. International Clinical Psychopharmacology 1996;11(2):109‐17. [PubMed] [Google Scholar]
Janiri 1997 {published data only}
- Janiri L, Hadjichristos A, Lombardi U, Rago R, Mannelli P, Tempesta E. SSRIs in alcoholism: fluvoxamine vs fluoxetine in alcoholic outpatients. Sixth World Congress of Biological Psychiatry; 1997 Jun 22‐27; Nice. 1997:35S.
Kalyoncu 2007 {published data only}
- Kalyoncu OA, Mirsal H, Pektas O, Tan D, Beyazyurek M. The efficacy of venlafaxine on depressive symptoms of patients diagnosed with both alcohol use disorder and major depressive disorder. Journal of Dependence 2007;8(2):59‐65. [Google Scholar]
Kranzler 1995 {published data only}
- Kranzler HR, Burleson JA, Korner P, Boca FK, Bohn MJ, Brown J, et al. Placebo‐controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. American Journal of Psychiatry 1995;152(3):391‐7. [DOI] [PubMed] [Google Scholar]
Kranzler 2011 {published data only}
- Kranzler HR, Armeli S, Tennen H. Post‐treatment outcomes in a double‐blind, randomized trial of sertraline for alcohol dependence. Alcoholism, Clinical and Experimental Research 2012;36(4):739‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Tennen H, Covault J, Feinn R, Arias AJ, et al. A double‐blind, randomized trial of sertraline for alcohol dependence: moderation by age of onset [corrected] and 5‐hydroxytryptamine transporter‐linked promoter region genotype. Journal of Clinical Psychopharmacology 2011;31(1):22‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krupitsky 2013 {published data only}
- Krupitsky EM, Yerish SM, Rybakova KV, Kiselev AS. Single blind placebo controlled randomized clinical trial of trazodon for alcoholism comorbid with affective disorders. Alcoholism, Clinical and Experimental Research 2013;37:18A. [Google Scholar]
Krystal 2008 {published data only}
- Krystal JH, Gueorguieva R, Cramer J, Collins J, Rosenheck R, VA CSP No. 425 Study Team. Naltrexone is associated with reduced drinking by alcohol dependent patients receiving antidepressants for mood and anxiety symptoms: results from VA Cooperative Study No. 425, "Naltrexone in the treatment of alcoholism". Alcoholism, Clinical and Experimental Research 2008;32(1):85‐91. [DOI] [PubMed] [Google Scholar]
Labbate 2004 {published data only}
- Labbate LA, Sonne SC, Randal CL, Anton RF, Brady KT. Does comorbid anxiety or depression affect clinical outcomes in patients with post‐traumatic stress disorder and alcohol use disorders?. Comprehensive Psychiatry 2004;45(4):304‐10. [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee Y, Han D, Kim S, Park D, Na C. Augmentation therapy of aripiprazole with escitalopram in patients with co‐occurrence of depression and alcohol dependence. European Neuropsychopharmacology 2012;22:S389. [Google Scholar]
Liappas 2004 {published data only}
- Liappas J, Paparrigopoulos T, Malitas P, Tzavellas E, Christodoulou G. Mirtazapine improves alcohol detoxification. Journal of Psychopharmacology 2004;18(1):88‐93. [DOI] [PubMed] [Google Scholar]
Macher 1991 {published data only}
- Macher JP, Minot R, Duval F, Luthringer R, Toussaint M, Schaltenbrand N. Neuro‐electrophysiologic studies in abstinent and depressed alcoholic patients treated with tianeptine. Presse Medicale 1991;20(37):1853‐7. [PubMed] [Google Scholar]
Malka 1992 {published data only}
- Malka R, Loo H, Ganry H, Souche A, Marey C, Kamoun A. Long‐term administration of tianeptine in depressed patients after alcohol withdrawal. British Journal of Psychiatry 1992;15 Suppl:66‐71. [PubMed] [Google Scholar]
Marey 1991 {published data only}
- Marey C, Ganry H, Delalleau B, Kamoun A. Therapeutic effects of tianeptine in patients with depression and anxiety disorders with or without associated alcoholism. Presse Medicale 1991;20(37):1828‐36. [PubMed] [Google Scholar]
Mason 1999 {published data only}
- Mason BJ, Salvato FR, Cutler RB, Cooper TB, Suckow RF, Williams LD. Naltrexone augmentation of sertraline in depressed alcoholics. 152nd Annual Meeting of the American Psychiatric Association; 1999 May 15‐20; Washington (DC). 1999.
- Mason BJ, Salvato FR, Williams LD, Cutler RB. Naltrexone and/or sertraline in outpatients with alcoholism and depression. 11th European College of Neuropsychopharmacology Congress; 1998 Oct 31‐Nov 4; Paris. 1999.
Naranjo 1997 {published data only}
- Naranjo CA, Bremner KE, Bazoon M, Turksen IB. Using fuzzy logic to predict response to citalopram in alcohol dependence. Clinical Pharmacology and Therapeutics 1997;62(2):209‐24. [DOI] [PubMed] [Google Scholar]
Oslin 2005 {published data only}
- Gopalakrishnan R, Ross J, O'Brien C, Oslin D. Course of late‐life depression with alcoholism following combination therapy. Journal of Studies on Alcohol 2009;70(2):237‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW. Treatment of late‐life depression complicated by alcohol dependence. American Journal of Geriatric Psychiatry 2005;13(6):491‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Overall 1973 {published data only}
- Overall JE, Brown D, Williams JD, Neill LT. Drug treatment of anxiety and depression in detoxified alcoholic patients. Archives of General Psychiatry 1973;29(2):218‐25. [DOI] [PubMed] [Google Scholar]
Powell 1995 {published data only}
- Powell BJ, Campbell JL, Landon JF, Liskow BI, Thomas HM, Nickel EJ, et al. A double‐blind, placebo‐controlled study of nortriptyline and bromocriptine in male alcoholics subtyped by comorbid psychiatric disorders. Alcoholism, Clinical and Experimental Research 1995;19(2):462‐8. [DOI] [PubMed] [Google Scholar]
Ruiz‐Mellott 2005 {published data only}
- Ruiz‐Mellott K, Poland R, Wilkins J. 5‐HTTLPR, antidepressant response, and alcohol use disorder. 158th Annual Meeting of the American Psychiatric Association; 2005 May 21‐26; Atlanta, GA. 2005:28.
Saatcioglu 2005 {published data only}
- Saatcioglu O, Evren C, Erim R, Cakmak D. Venlafaxine treatment in alcohol dependants with comorbid depression. European Neuropsychopharmacology 2005;Suppl 3:S589. [Google Scholar]
Salloum 2011 {published data only}
- Salloum IM, Cornelius JR, Douaihy A, Kirisci L. Gender difference in alcohol treatment response in patients with comorbid alcoholism and depression. Alcoholism, Clinical and Experimental Research 2011;35:19A. [Google Scholar]
Salloum 2013 {published data only}
- Salloum IM, Cornelius JR, Douaihy A, Daley DC. Predicting medication adherence and treatment completion among depressed alcoholics. Alcoholism, Clinical and Experimental Research 2013;37:198A. [Google Scholar]
Schottenfeld 1989 {published data only}
- Schottenfeld RS, O'Malley SS, Smith L, Rounsaville BJ, Jaffe JH. Limitation and potential hazards of MAOI's for the treatment of depressive symptoms in abstinent alcoholics. American Journal of Drug and Alcohol Abuse 1989;15(3):339‐44. [DOI] [PubMed] [Google Scholar]
Shaw 1975 {published data only}
- Shaw JA, Donley P, Morgan DW, Robinson JA. Treatment of depression in alcoholics. American Journal of Psychiatry 1975;132(6):641‐4. [DOI] [PubMed] [Google Scholar]
Witte 2012 {published data only}
- Witte J, Bentley K, Evins AE, Clain AJ, Baer L, Pedrelli P, et al. A randomized, controlled, pilot study of acamprosate added to escitalopram in adults with major depressive disorder and alcohol use disorder. Journal of Clinical Pharmacology 2012;32(6):787‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Petrakis 2013 {published data only}
- Petrakis I, Jane JS, O'Brien E, Keegan K, Ralevski E, Desai N. Effect of depression on outcomes in a clinical trial evaluating antidepressants +/‐ naltrexone for treatment of alcohol dependence and PTSD. Alcoholism, Clinical and Experimental Research 2013;37:19A. [Google Scholar]
- Petrakis IL, Ralevski E, Desai N, Trevisan L, Gueorguieva R, Rounsaville B, et al. Noradrenergic vs serotonergic antidepressant with or without naltrexone for veterans with PTSD and comorbid alcohol dependence. Neuropsychopharmacology 2012;37(4):996‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schifano 1993 {published data only}
- Schifano F, Garofoli F. Dothiepin vs. S‐adenosylmethionine (SAMe) for the treatment of major depression in weaned alcoholics: a pilot study. European Neuropsychopharmacology 1993;3(3):330. [Google Scholar]
Additional references
Anton 1996
- Anton RR, Moak DH, Latham PK. The obsessive compulsive drinking scale: a new method of assessing outcome in alcoholism treatment studies. Archives of General Psychiatry 1996;53:225‐31. [DOI] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington (DC): American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM III‐R). Washington (DC): American Psychiatric Press, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington (DC): American Psychiatric Press, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR). Washington (DC): American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington (VA): American Psychiatric Association, 2013. [Google Scholar]
Artigas 2002
- Artigas F, Nutt DJ, Shelton R. Mechanism of action of antidepressants. Psychopharmacology Bulletin 2002;36(Suppl 2):123‐32. [PubMed] [Google Scholar]
Babor 1992
- Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, et al. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Archives of General Psychiatry 1992;49:599‐608. [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56:893‐7. [DOI] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory: Manual. 2nd Edition. San Antonio (TX): Harcourt Brace, 1996. [Google Scholar]
Brown 1995
- Brown SA, Inaba RK, Gillin JC, Schuckit MA, Stewart MA, Irwin MR. Alcoholism and affective disorder: clinical course of depressive symptoms. American Journal of Psychiatry 1995;152:45‐52. [DOI] [PubMed] [Google Scholar]
Busner 2009
- Busner J, Targum SD, Miller DS. The Clinical Global Impressions scale: errors in understanding and use. Comprehensive Psychiatry 2009;50(3):257‐62. [DOI] [PubMed] [Google Scholar]
Buysse 1989
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28:193‐213. [DOI] [PubMed] [Google Scholar]
Carboni 2004
- Carboni E, Silvagni A. Dopamine reuptake by norepinephrine neurons: exception or rule?. Critical Reviews in Neurobiology 2004;16(1‐2):121‐8. [DOI] [PubMed] [Google Scholar]
Carr 1981
- Carr AC, Ancill RJ, Ghosh A, Margo A. Direct assessment of depression by microcomputer. A feasibility study. Acta Psychiatrica Scandinavica 1981;64(5):415‐22. [DOI] [PubMed] [Google Scholar]
Chick 2004a
- Chick J, Aschauer H, Hornik K. Efficacy of fluvoxamine in preventing relapse in alcohol dependence: a one‐year, double blind, placebo‐controlled Multicentre study with analysis by typology. Drug and Alcohol Dependence 2004;74:61‐70. [DOI] [PubMed] [Google Scholar]
Cloninger 1988
- Cloninger CR, Sigvardsson S, Gilligan SB, Knorring AL, Reich T, Bohman M. Genetic heterogeneity and the classification of alcoholism. Advances in Alcohol and Substance Abuse 1988;7:3‐16. [DOI] [PubMed] [Google Scholar]
Derogatis 1974
- Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self‐report symptom inventory. Behavioral Science 1974;19(1):1‐15. [DOI] [PubMed] [Google Scholar]
Edwards 1976
- Edwards G, Gross MM. Alcohol dependence: provisional description of a clinical syndrome. BMJ 1976;1(6017):1058‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endicott 1976
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scales: a procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry 1976;35:837‐44. [DOI] [PubMed] [Google Scholar]
Fillenbaum 1997
- Fillenbaum GG, Beekly D, Edland SD, Hughes JP, Heyman A, Belle G. Consortium to establish a registry for Alzheimer's disease: development, database structure, and selected findings. Topics in Health Information Management 1997;18(1):47‐58. [PubMed] [Google Scholar]
First 1995
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM‐IV Axis I Disorders, Patient Edition (SCID‐P), version 2. New York, New York State Psychiatric Institute, Biometrics Research 1995.
Flannery 1999
- Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcoholism, Clinical and Experimental Research 1999;23(8):1289‐95. [PubMed] [Google Scholar]
Foulds 2015
- Foulds JA, Adamson SJ, Boden JM, Williman JA, Mulder RT. Depression in patients with alcohol use disorders: systematic review and meta‐analysis of outcomes for independent and substance‐induced disorders. Journal of Affective Disorders 2015;185:47‐59. [DOI] [PubMed] [Google Scholar]
Goldman 1992
- Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM‐IV: a review of measures of social functioning. American Journal of Psychiatry 1992;149(9):1148‐56. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- The GRADE working group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 1995
- Grant BF, Harford TC. Comorbidity between DSM‐IV alcohol use disorders and major depression: results of a national survey. Drug and Alcohol Dependence 1995;39:197‐206. [DOI] [PubMed] [Google Scholar]
Grant 2015
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM‐5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015;72(8):757‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guy 1975
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): National Institute of Mental Health, 1975. [Google Scholar]
Guy 1976
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington (DC): DHEW Publication, 1976. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7560):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64:1277‐82. [DOI] [PubMed] [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32:50‐5. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology and Psychiatry 1960;23:56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 1967
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology 1967;6:278‐96. [DOI] [PubMed] [Google Scholar]
Hamilton 1969
- Hamilton M. Diagnosis and rating of anxiety. British Journal of Psychiatry 1969;Special publication:76‐9. [Google Scholar]
Hasin 1996
- Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental disorders (PRISM): reliability for substance abusers. American Journal of Psychiatry 1996;153(9):1195‐201. [DOI] [PubMed] [Google Scholar]
Hasin 2005
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the national epidemiologic survey on alcoholism and related conditions. Archives of General Psychiatry 2005;62(10):1097‐106. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Iovieno 2011
- Iovieno N, Tedeschini E, Bentley KH, Evins AE, Papakostas GI. Antidepressants for major depressive disorder and dysthymic disorder in patients with comorbid alcohol use disorders: a meta‐analysis of placebo‐controlled randomized trials. Journal of Clinical Psychiatry 2011;72(8):1144‐51. [DOI] [PubMed] [Google Scholar]
Kadden 1992
- Kadden R, Carroll K, Donovan D, Cooney N, Monti P. Cognitive‐behavioral coping skills therapy manual: a clinical research guide for therapists treating individuals with alcohol abuse and dependence. Vol. 3, Washington (DC): NIAAA Project MATCH Monograph Series, 1992. [Google Scholar]
Kranzler 1996
- Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive behavioral therapy in type B alcoholics. Alcoholism, Clinical and Experimental Research 1996;20:1534‐41. [DOI] [PubMed] [Google Scholar]
Kranzler 2000
- Kranzler HR, Modesto‐Lowe V, Kirk J. Naltrexone vs. nefazodone for treatment of alcohol dependence. A placebo‐controlled trial. Neuropsychopharmacology 2000;22(5):493‐503. [DOI] [PubMed] [Google Scholar]
LeMoal 2007
- Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. European Neuropsychopharmacology 2007;17:377‐93. [DOI] [PubMed] [Google Scholar]
Levine 1986
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacology Bulletin 1986;22(2):343‐81. [PubMed] [Google Scholar]
Lingjaerde 1987
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. Acta Psychiatrica Scandinavica 1987;76(suppl 334):1‐96. [DOI] [PubMed] [Google Scholar]
McLellan 1992
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment 1992;9(3):199‐213. [DOI] [PubMed] [Google Scholar]
Miller 1992
- Miller WR, Zweben A, DiClemente CC, Rychtarik RG. Motivational Enhancement Therapy Manual. Vol. 2, Washington (DC): Project MATCH Monograph Series. National Institute on Alcohol Abuse and Alcoholism, 1992. [Google Scholar]
Miller 1995
- Miller WR, Tonigan JS, Longabaugh R. The Drinker Inventory of Consequences (DrInC): an instrument for assessing adverse consequences of alcohol abuse (Project MATCH Monograph Series). NIH Publication No. 95‐3911. Vol. 4, Rockville (MD): US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism, 1995. [Google Scholar]
Moher 2001
- Moher D, Jones A, Lepage L, CONSORT Group (Consolidated Standards for Reporting of Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1992‐5. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Naranjo 2001
- Naranjo CA, Knoke DM. The role of selective serotonin reuptake inhibitors in reducing alcohol consumption. Journal of Clinical Psychiatry 2001;62(Suppl 20):18‐25. [PubMed] [Google Scholar]
Nunes 2004
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta‐analysis. JAMA 2004;29:1887‐96. [DOI] [PubMed] [Google Scholar]
Nunes 2006
- Nunes EV, Rounsaville BJ. Comorbidity of substance use with depression and other mental disorders: from Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV) to DSM‐V. Addiction 2006;101:S89‐96. [DOI] [PubMed] [Google Scholar]
O'Donnell 2011
- O'Donnell JM, Shelton RC. Drug Therapy of Depression and Anxiety Disorders. In: Brunton LL, Chabner BA, Knollmann BC editor(s). Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th Edition. Columbus (OH): McGraw‐Hill, Medical Publishing Division, 2011:397‐415. [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Pettinati 2000
- Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcoholism, Clinical and Experimental Research 2000;24(7):1041‐9. [PubMed] [Google Scholar]
Pettinati 2001b
- Pettinati HM, Volpicelli JR, Luck G, Kranzler HR, Rukstalis MR, Cnaan A. Double‐blind clinical trial of sertraline treatment for alcohol dependence. Journal of Clinical Psychopharmacology 2001;21:143‐53. [DOI] [PubMed] [Google Scholar]
Pettinati 2004
- Pettinati HM. Antidepressant treatment of co‐occurring depression and alcohol dependence. Biological Psychiatry 2004;56:785‐92. [DOI] [PubMed] [Google Scholar]
Pettinati 2013
- Pettinati HM, O'Brien CP, Dundon WD. Current status of co‐occurring mood and substance use disorders: a new therapeutic target. American Journal of Psychiatry 2013;170(1):23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raistrick 1994
- Raistrick D, Bradshaw J, Tober G, Weiner J, Allison J, Healey C. Development of the Leeds Dependence Questionnaire (LDQ): a questionnaire to measure alcohol and opiate dependence in the context of a treatment evaluation package. Addiction 1994;89(5):563‐72. [DOI] [PubMed] [Google Scholar]
Regier 1990
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith D, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. JAMA 1990;264:2511‐8. [PubMed] [Google Scholar]
Rockliff 1971
- Rockliff BW. A brief rating scale for antidepressant drug trials. Comprehensive Psychiatry 1971;12(2):122‐35. [DOI] [PubMed] [Google Scholar]
Saunders 1993
- Saunders JB, Aasland OG, Babor TF, Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption ‐ II. Addiction 1993;88(6):791‐804. [DOI] [PubMed] [Google Scholar]
Schneider 2009
- Schneider B. Substance use disorders and risk for completed suicide. Archives of Suicide Research 2009;13:303‐16. [DOI] [PubMed] [Google Scholar]
Schuckit 1997
- Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL. Comparison of induced and independent major depressive disorders in 2,945 alcoholics. American Journal of Psychiatry 1997;154:948‐57. [DOI] [PubMed] [Google Scholar]
Schuckit 2006
- Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction 2006;101(Suppl 1):76‐78. [DOI] [PubMed] [Google Scholar]
Schünemann 2006
- Schünemann HJ, Jaeschke R, Cook D, Bria W, El‐Solh A, Ernst A, et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006;174:605‐14. [DOI] [PubMed] [Google Scholar]
Scott 1976
- Scott J, Huskisson EC. Graphic representation of pain. Pain 1976;2:175‐84. [PubMed] [Google Scholar]
Selzer 1971
- Selzer M. The Michigan Alcoholism Screening Test (MAST): the quest for a new diagnostic instrument. American Journal of Psychiatry 1971;127:1653‐8. [DOI] [PubMed] [Google Scholar]
Sheehan 1998
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. Journal of Clinical Psychiatry 1998;59(Suppl 20):22‐33. [PubMed] [Google Scholar]
Shelton 1969
- Shelton J, Hollister LE, Gocka EF. The drinking behavior interview (an attempt to quantify alcoholic impairment). Diseases of the Nervous System 1969;30(7):464‐7. [PubMed] [Google Scholar]
Sher 2005
- Sher L, Oquendo MA, Galfalvy HC, Grunebaum MF, Burke AK, Zalsman G, et al. The relationship of aggression to suicidal behavior in depressed patients with a history of alcoholism. Addictive behaviours 2005;30:1144‐53. [DOI] [PubMed] [Google Scholar]
Shirayama 2006
- Shirayama Y, Chaki S. Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Current Neuropharmacology 2006;4(4):277‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skinner 1982
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. Journal of Abnormal Psychology 1982;91:199‐209. [DOI] [PubMed] [Google Scholar]
Skinner 1984
- Skinner HA, Horn JL. Alcohol dependence scale: user's guide. Toronto: Addiction Research Foundation 1984.
Sobell 1992
- Sobell L, Sobell M. Timeline follow‐back: a technique for assessing self‐reported alcohol consumption. In: Litten RZ, Allen JP editor(s). Measuring Alcohol Consumption. Totowa (NJ): The Humana Press, 1992:41‐72. [Google Scholar]
Spielberger 1970
- Spielberger C, Gorsuch A, Lushene R. The State‐Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press, 1970. [Google Scholar]
Spielberger 1983
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. State‐Trait Anxiety Inventory for Adults. Palo Alto (CA): Mind Garden, 1983. [Google Scholar]
Spitzer 1992
- Spitzer RL, Williams BW, Gibbon M, First MB. The Structured Clinical Interview for DSM‐III‐R (SCID). I: History, rationale, and description. Archives of General Psychiatry 1992;49:624‐9. [DOI] [PubMed] [Google Scholar]
Stahl 2003
- Stahl SM, Grady MM. Differences in mechanism of action between current and future antidepressants. Journal of Clinical Psychiatry 2003;64(Suppl 13):13‐7. [PubMed] [Google Scholar]
Torrens 2005
- Torrens M, Fonseca F, Mateu G, Farré M. Efficacy of antidepressants in substance use disorders with and without comorbid depression. A systematic review and meta‐analysis. Drug and Alcohol Dependence 2005;78(1):1‐22. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36‐item Short Form Health Survey I: conceptual framework and item selection. Medical Care 1992;30:473‐83. [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. The ICD 10. The ICD‐10 Classification of Mental and Behavioural Disorders. Diagnostic Criteria for Research. Geneva: WHO, 1993. [Google Scholar]
Wilcox 2004
- Wilcox HC, Conner KR, Caine ED. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug and Alcohol Dependence 2004;76S:11‐9. [DOI] [PubMed] [Google Scholar]
Zung 1965
- Zung WW. A self‐rating depression scale. Archives of General Psychiatry 1965;12:63‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Pani 2010
- Pani PP, Trogu E, Amato L, Davoli M. Antidepressants for the treatment of depression in alcohol dependent individuals. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD008581] [DOI] [Google Scholar]


