Abstract
Background
Ureteral colic is a common reason for patients to seek medical care. Alpha‐blockers are commonly used to improve stone passage through so‐called medical expulsive therapy (MET), but their effectiveness remains controversial. This is an update of a 2014 Cochrane review; since that time, several large randomised controlled trials (RCTs) have been reported, making this update relevant.
Objectives
To assess effects of alpha‐blockers compared with standard therapy for ureteral stones 1 cm or smaller confirmed by imaging in adult patients presenting with symptoms of ureteral stone disease.
Search methods
On 18 November 2017, we searched CENTRAL, MEDLINE Ovid, and Embase. We also searched ClinicalTrials.gov and the WHO Portal/ICTRP to identify all published/unpublished and ongoing trials. We checked all references of included and review articles and conference proceedings for articles relevant to this review. We sent letters to investigators to request information about unpublished or incomplete studies.
Selection criteria
We included RCTs of ureteral stone passage in adult patients that compared alpha‐blockers versus standard therapy.
Data collection and analysis
Two review authors screened studies for inclusion and extracted data using standard methodological procedures. We performed meta‐analysis using a random‐effects model. Primary outcomes were stone clearance and major adverse events; secondary outcomes were stone expulsion time, number of pain episodes, use of diclofenac, hospitalisation, and surgical intervention. We assessed the quality of evidence on a per‐outcome basis using the GRADE approach.
Main results
We included 67 studies with 10,509 participants overall. Of these, 15 studies with 5787 participants used a placebo.
Stone clearance: Based on the overall analysis, treatment with an alpha‐blocker may result in a large increase in stone clearance (risk ratio (RR) 1.45, 95% confidence interval (CI) 1.36 to 1.55; low‐quality evidence). A subset of higher‐quality, placebo‐controlled trials suggest that the likely effect is probably smaller (RR 1.16, 95% CI 1.07 to 1.25; moderate‐quality evidence), corresponding to 116 more (95% CI 51 more to 182 more) stone clearances per 1000 participants.
Major adverse events: Based on the overall analysis, treatment with an alpha‐blocker may have little effect on major adverse events (RR 1.25, 95% CI 0.80 to 1.96; low‐quality evidence). A subset of higher‐quality, placebo‐controlled trials suggest that alpha‐blockers likely increase the risk of major adverse events slightly (RR 2.09, 95% CI 1.13 to 3.86), corresponding to 29 more (95% CI 3 more to 75 more) major adverse events per 1000 participants.
Patients treated with alpha‐blockers may experience shorter stone expulsion times (mean difference (MD) ‐3.40 days, 95% CI ‐4.17 to ‐2.63; low‐quality evidence), may use less diclofenac (MD ‐82.41, 95% CI ‐122.51 to ‐42.31; low‐quality evidence), and likely require fewer hospitalisations (RR 0.51, 95% CI 0.34 to 0.77; moderate‐quality evidence), corresponding to 69 fewer hospitalisations (95% CI 93 fewer to 32 fewer) per 1000 participants. Meanwhile, the need for surgical intervention appears similar (RR 0.74, 95% CI 0.53 to 1.02; low‐quality evidence), corresponding to 28 fewer surgical interventions (95% CI 51 fewer to 2 more) per 1000 participants.
A predefined subgroup analysis (test for subgroup differences; P = 0.002) suggests that effects of alpha‐blockers may vary with stone size, with RR of 1.06 (95% CI 0.98 to 1.15; P = 0.16; I² = 62%) for stones 5 mm or smaller versus 1.45 (95% CI 1.22 to 1.72; P < 0.0001; I² = 59%) for stones larger than 5 mm. We found no evidence suggesting possible subgroup effects based on stone location or alpha‐blocker type.
Authors' conclusions
For patients with ureteral stones, alpha‐blockers likely increase stone clearance but probably also slightly increase the risk of major adverse events. Subgroup analyses suggest that alpha‐blockers may be less effective for smaller (5 mm or smaller) than for larger stones (greater than 5 mm).
Plain language summary
Alpha‐blockers for ureteral stones in adult patients with symptoms of stone disease
Review question
Does medical treatment with alpha‐blockers improve the outcomes of patients with stones stuck in their ureter?
Background
Stones stuck in the ureter, which is the tube that transports urine from the kidney to the bladder, often cause pain and make people see a doctor. Depending on which part of the ureter the stone is stuck in and the size of the stone, it will often pass into the bladder on its own over the course of weeks. If the stone does not come out by itself, people often need to have procedures done to remove the stone.
Alpha‐blockers are medications that relax muscles in the urinary tract and may make the stone pass into the bladder faster. However, they can cause unwanted effects. We updated an existing Cochrane Review from 2014 to look into the effects of alpha‐blockers.
Study characteristics
Based on our latest search of the literature from November 2017, we included 64 studies with 10,509 participants. Of these, 15 studies compared alpha‐blockers with placebo with 5787 participants. A placebo is a pill that looks and tastes exactly like the real medication, so participants did not know what they were getting. These were the higher‐quality studies, which we trusted more.
Key results
Based on the subset of higher‐quality studies that used a placebo, alpha‐blockers likely resulted in more people passing their stones. However, these patients are likely to experience slightly more serious unwanted effects of this medication.
People taking alpha‐blockers may pass their stones in a shorter time, may use less diclofenac (which is a type of pain medication), and are likely to be admitted to the hospital less often. Meanwhile, the need for surgery for their stones was similar.
Upon completing additional analyses, we found that effects of alpha‐blockers may be different in people with small (5 mm or smaller) versus larger (larger than 5 mm) stones. It appears that this medication works better in people with larger stones. We could find no difference in how well alpha‐blockers work, no matter where in the ureter the stone is stuck or what type of alpha‐blocker is used.
Authors' conclusions
For patients with stones stuck in the ureter, alpha‐blockers likely make passing the stone easier but cause slightly more unwanted effects. It appears that alpha‐blockers work better in people with larger (greater than 5 mm) rather than smaller (5 mm or smaller) stones.
Quality of the evidence
The quality of the evidence for most outcomes was moderate or low, meaning that we have moderate or low confidence in most of the reported results.
Summary of findings
Background
Description of the condition
Urinary stone disease refers to the formation of stones or calculi in the urinary tract and is one of the most common reasons for patients to visit a urology practice; it affects about 5% to 10% of the population (Ramello 2000). An even higher frequency of up to 12% has been reported from other parts of the world, and stone disease is rare in only a few geographical areas (e.g. Greenland, coastal areas of Japan) (Tiselius 2003). One study showed an increase in lifetime prevalence of stone disease ranging from 7.14% to 11.62% over a 10‐year period (2000‐2010) (Turney 2011). The incidence and prevalence rates of kidney stones may be affected by genetic, nutritional, and environmental factors. Caucasian males are more likely than Asians, Hispanics, and African Americans to develop urinary stones (Pearle 2007). Besides the probability of stone formation, stone composition and location in the urinary tract may differ among countries (Ramello 2000). Furthermore, in the USA, over 2 million outpatient visits for a primary diagnosis of urinary stones were recorded in 2000 (UDA 2012). Hospital outpatient visits increased by 40% between 1994 and 2000, and physician office visits increased by 43% between 1992 and 2000. Also in the USA, the total estimated annual cost for stone disease was over US $10.3 billion in 2006 ‐ showing an almost five‐fold increase in six years (US $2.1 billion in 2000) and representing a 50% increase since 1994. A further increase in costs to over US $3 billion for emergency department visits was seen in the USA in 2009 (Ghani 2014). This rise could be explained only in part by the increasing prevalence of stone disease (Pearle 2005).
The natural history of urinary stone disease is characterised by specific steps ‐ from formation of Randall’s plaques to development of stones that cause renal or ureteral colic (Matlaga 2007). Symptoms include flank or abdominal pain radiating to the groin or external genitalia. Although some patients with ureteral stones might remain asymptomatic, many have pain and generally seek medical care. An acute episode of colic is the result of a stone entering the ureter and causing an intermittent rise in pressure in the pyelocalyceal system. Spontaneous passage does occur with most of these stones. Stone size and location are the two most important predictors of stone passage (Miller 1999). Passage rates of 68% for stones smaller than 5 mm and 47% for stones greater than 5 mm and up to 10 mm have been reported (Preminger 2007).
Current treatment modalities for ureteral stones include extracorporeal shockwave lithotripsy (ESWL); (flexible or semi‐rigid) ureteroscopy; percutaneous nephrolithotomy; open surgery, laparoscopic surgery, or robot‐assisted surgery; and observation with analgesia with or without adjuvant medications to facilitate stone passage (AUA Guideline 2016 Surgical Management of Stones; EAU guidelines on diagnosis and conservative management of urolithiasis 2016).
According to the guidelines of the European Association of Urology (EAU; EAU guidelines on diagnosis and conservative management of urolithiasis 2016) and the American Urologic Association (AUA; AUA Guideline 2016 Surgical Management of Stones), among patients with a newly diagnosed ureteral stone 10 mm or smaller whose symptoms are controlled, observation with periodic evaluation is an option for initial treatment. These patients may be offered an appropriate medical expulsive therapy to facilitate stone passage during the observation period. However, patients should be informed about the attendant risks of medical expulsive therapy (including associated drug side effects) and should be told that these agents are administered for an 'off‐label' use (Preminger 2007).
Description of the intervention
Different modalities of medical expulsive therapy have been evaluated, including alpha‐blockers, calcium channel blockers, corticosteroids, and combinations of these. However, most experience has been acquired with alpha‐blockers (Hollingsworth 2006; Michel 2006; Singh 2007).
How the intervention might work
Both α‐ and β‐adrenergic receptors are present in the human ureter, although α‐receptors predominate. More specifically, α1‐receptors are important in lower ureteric physiology, and higher densities of α1‐receptors have been discovered in the lower ureters of animals and humans (Nakada 2008). In the ureter, α1‐receptor antagonists inhibit basal tone and decrease peristaltic frequency and amplitude. Consequently, intraureteral pressure might decrease and fluid transport might increase according to some study authors (Morita 1987). These receptors appear to be ideal targets for pharmacotherapy, as they represent the greatest impediment to stone passage (Sterrett 2008). Several trials in the past have assessed effects of alpha‐blockers; most have reported favourable effects on stone clearance. We summarised this information in our previous review (Campschroer 2014).
Why it is important to do this review
Given the high rates of spontaneous passage ureteral stones, a study of medical expulsive therapy is warranted. Effective medical expulsive therapy confers several potential benefits. First, it may decrease the duration of symptoms of ureteral stones and, therefore, the rate of complications such as urinary tract infection (UTI), hydronephrosis, and kidney function impairment. Second, it can potentially decrease the use of more invasive interventions, such as ESWL and ureteroscopy, and therefore may decrease the rate of possible complications associated with these procedures. Last, medical expulsive therapy is likely to spare limited healthcare resources, such as physician time and hospital beds.
Despite the joint Guideline provided by the EAU and the AUA, considerable controversy persists concerning the best treatment approach for ureteral stones and the effectiveness of alpha‐blocker use as medical expulsive therapy. Several published randomised controlled trials (RCTs) on this topic have presented conflicting conclusions. We have prepared an up‐to‐date systematic review of all available data from recent RCTs using the highest methodological standards and have applied the GRADE approach to rate the quality of evidence.
In summary, combining the studies performed so far provides the opportunity to produce an overall effect estimate of alpha‐blockers as medical expulsive therapy for ureteral stones. The direction and magnitude of this effect can be used to guide decisions about clinical practice.
This current Cochrane Review presents an update of the previous Cochrane Review (Campschroer 2014). Since the last search was conducted (9 July 2012), several new studies have been published, making an update of this Cochrane Review necessary.
Objectives
To assess effects of alpha‐blockers compared with standard therapy for ureteral stones 1 cm or smaller confirmed by imaging in adult patients presenting with symptoms of ureteral stone disease.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs and quasi‐RCTs (trials in which investigators allocated participants to treatment using alternation, alternate medical records, date of birth, or other predictable methods) undertaken to investigate alpha‐blockers for the treatment of adult patients with ureteral stones. We included eligible studies regardless of their publication status or language of publication.
Types of participants
Inclusion criteria
Adult patients (aged 18 years or older)
Symptoms of ureteral stones including flank or abdominal pain, possibly radiating to the groin or external genitalia
Diagnosis confirmed upon imaging (e.g. plain film of the kidney, ureter, and bladder (KUB); computed tomography (CT); intravenous pyelography (IVP); ultrasonography (US))
Single stone measuring 10 mm or smaller
Exclusion criteria
Evidence of UTI or hydronephrosis with complicated factors (e.g. sepsis, uncontrollable pain, deterioration of renal function)
Kidney or ureteral abnormalities (e.g. single kidney, ureteral malformation)
Pregnant or lactating women
Bilateral stones
Taking an alpha‐blocker or a calcium channel blocker, or having allergies to these medications
Types of interventions
Interventions include medical expulsive therapy with alpha‐blockers to treat patients with ureteral stones versus one of two comparators: (1) standard therapy (e.g. non‐steroidal anti‐inflammatory drugs (NSAIDs), corticosteroids, antispasmodics), or (2) placebo. When one study assessed multiple alpha‐blockers, we combined arms for the comparison of alpha‐blockers versus standard therapy or placebo. We conducted subgroup analyses to explore whether effects differ among various alpha‐blockers.
We excluded studies in which researchers evaluated alpha‐blockers as an adjuvant to surgery or lithotripsy. If patients used other medication such as anticholinergics or antispasmodics as an adjuvant to trial medication, we evaluated only comparable groups (e.g. patients in a standard therapy group using anticholinergics vs patients in the trial group using anticholinergics combined with trial medication).
Types of outcome measures
Primary outcomes
Stone clearance (dichotomous outcome defined as percentage of participants identified as stone‐free by diagnostic imaging on the last day of the study trial, or participants who were symptom‐free at the end of the trial, or participants who had expelled their stone and brought it with them to the follow‐up visit)
Major adverse events (dichotomous outcome defined as the number of participants who experienced orthostatic hypotension, collapse, syncope, palpitations, or tachycardia)
Secondary outcomes
Stone expulsion time (continuous outcome defined as days from start of inclusion until stone expulsion)
Pain episodes (continuous outcome defined as number of pain episodes)
Diclofenac use (continuous outcome defined as cumulative dosage of diclofenac in milligrams)
Hospitalisation (dichotomous outcome defined as percentage of participants needing hospital admission because of uncontrollable pain or complicating factors such as UTI or sepsis)
Surgical intervention (dichotomous outcome defined as the number of participants who require surgical intervention as the result of uncontrolled pain and/or hydronephrosis within the trial period)
Main outcomes for 'Summary of findings' tables
We present 'Summary of findings' tables to report the following outcomes listed according to priority.
Comparison 1 (Analysis 1): alpha‐blockers versus standard therapy or placebo (Table 1).
Summary of findings for the main comparison. Alpha‐blockers compared with standard therapy for ureteral stones.
| Alpha‐blockers compared with standard therapy for ureteral stones | |||||
|
Patient or population: adult patients presenting with symptoms of ureteral stone disease Setting: single or multicenter Intervention: alpha‐blocker Comparison: standard therapy | |||||
| Outcomes | No. of participants (studies) Follow‐up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with standard therapy | Risk difference with alpha‐blockers | ||||
| Stone clearance | 10509 (67 RCTs) | ⊕⊕⊝⊝ LOWa,b,c | RR 1.45 (1.36 to 1.55) | Study population | |
| 619 per 1000 | 278 more per 1000 (223 more to 340 more) | ||||
| Major adverse events | 3124 (18 RCTs) | ⊕⊕⊝⊝ LOWa,d | RR 1.25 (0.80 to 1.96) | Study population | |
| 20 per 1000 | 5 more per 1000 (4 fewer to 19 more) | ||||
| Stone expulsion time | 6031 (37 RCTs) | ⊕⊕⊝⊝ LOWa,c,e | ‐ | MD 3.4 lower (4.17 lower to 2.63 lower) | |
| Pain episodes | 1363 (15 RCTs) | ⊕⊕⊝⊝ LOWa,c,f | ‐ | MD 0.66 lower (0.91 lower to 0.42 lower) | |
| Dose of diclofenac | 4373 (14 RCTs) | ⊕⊕⊝⊝ LOWa,c,g | ‐ | MD 82.41 mg lower (122.51 lower to 42.31 lower) | |
| Hospitalisation | 1876 (13 RCTs) | ⊕⊕⊕⊝ MODERATEa | RR 0.51 (0.34 to 0.77) | Study population | |
| 141 per 1000 | 69 fewer per 1000 (93 fewer to 32 fewer) | ||||
| Surgical intervention | 3292 (19 RCTs) | ⊕⊕⊝⊝ LOWa,d | RR 0.74 (0.53 to 1.02) | Study population | |
| 109 per 1000 | 28 fewer per 1000 (51 fewer to 2 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
aMost studies were rated as having high or unclear risk of bias.
bClinically important heterogeneity with I² of 76%; provided rationale for downgrading together with suspected publication bias.
cPublication bias suspected given funnel plot asymmetry.
dConfidence interval consistent; no effect and clinically important harm.
eClinically important heterogeneity with I² of 94%; provided rationale for downgrading together with suspected publication bias.
fClinically important heterogeneity with I² of 80%; provided rationale for downgrading together with suspected publication bias.
gClinically important heterogeneity with I² of 100%; provided rationale for downgrading together with suspected publication bias.
1.1 Stone clearance.
1.2 Major adverse events.
1.3 Stone expulsion time.
1.4 Pain episodes.
1.5 Dose of diclofenac [mg].
1.6 Hospitalisation.
1.7 Surgical intervention.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group Specialized Register for the first version of this Cochrane Review through contact with the Trial Search Co‐ordinator using search terms relevant to this review (search date 9 July 2012). TC and XZ updated the search on 18 November 2017, by using the following sources.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017 November 18) in the Cochrane Library.
MEDLINE (via Ovid 1946 to 18 November 2017).
MEDLINE In‐Process & Other Non‐Indexed Citations (18 November 2017).
Embase (via Ovid 1980 to 18 November 2017).
PubMed (inception to November 2017) (18 November 2017).
International Clinical Trials Registry Platform (ICTRP) search portal (WHO ICTRP) and ClinicalTrials.gov (18 November 2017).
Proceedings of major (mainly renal and stone disease) conferences from 2005 to November 2017: EAU (European Association of Urology); AUA (American Urological Association); ESD (Experts in Stone Disease); WCE (World Congress of Endourology); and SIU (Société Internationale d'Urologie).
We imposed no restrictions, for example, on language or publication status for the searches described above.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of urology textbooks, review articles, and relevant studies. Furthermore, review authors scrutinised the reference lists of identified relevant studies for additional citations.
Letters sent to request information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
We used reference management software to identify and remove duplicate records (Endnote 2016). Two review authors (TC and XZ) independently assessed the titles and abstract of records identified in the search against the predefined inclusion criteria to determine which studies should be assessed in the full‐text evaluation. The same review authors (TC and XZ) investigated all records included in the title/abstract screening as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies, in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved discrepancies through discussion or arbitration by a third review author (TL). If resolution of a disagreement was not possible, we designated the study as 'Awaiting classification' and contacted the study authors for clarification. We documented in Characteristics of excluded studies tables reasons for exclusion of studies that may have reasonably been expected to be included in the review.
We summarised in Characteristics of included studies tables relevant study characteristics, including sample size, gender of participants, stone size, and stone location. We have shown the flow of literature through the assessment process in a PRISMA flowchart (Figure 1).
1.
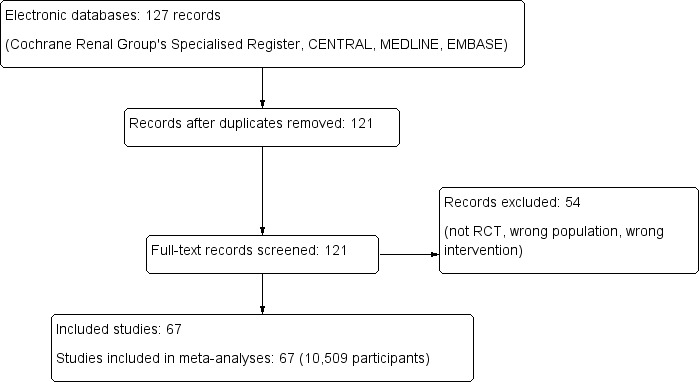
Study flow diagram.
Data extraction and management
Two review authors (TC and XZ) independently carried out data extraction using standard data extraction forms. We tested the forms before implementation in the review. We translated studies reported in non‐English language journals before assessment. In the case that more than one publication of the same study existed, we grouped reports together and included the publication with the most complete data. If outcome data were available at multiple time points during follow‐up of one trial, we extracted solely outcomes with the longest follow‐up. We highlighted any discrepancies between published versions. We resolved disagreements by consulting with another review author (TL). For dichotomous outcomes, we extracted the number of participants with the specific outcome and the denominator of the total participants for whom the outcome was assessed at longest follow‐up. For continuous outcomes, we extracted the number of participants for whom the outcome was measured and determined the mean value and standard deviation (SD) for each outcome. We requested through written correspondence any further information required from the original author(s) and included in the review any relevant information obtained in this manner. When original trial author(s) did not respond to this correspondence, we excluded that study from the review.
Assessment of risk of bias in included studies
Review authors (TC and XZ) independently assessed the risk of bias of each included study. We resolved disagreements by consensus or by consultation with a third review author (TL).
Using the Cochrane 'Risk of bias' assessment tool (Higgins 2011), we assessed risk of bias using the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other sources of bias.
We judged risk of bias domains as 'low risk', 'high risk', or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We have presented 'Risk of bias' summary figures to illustrate these findings (Figure 2 and Figure 3).
2.
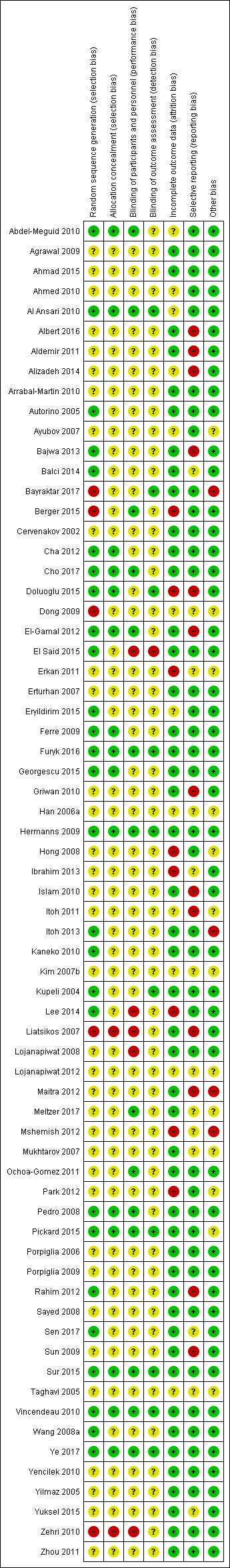
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
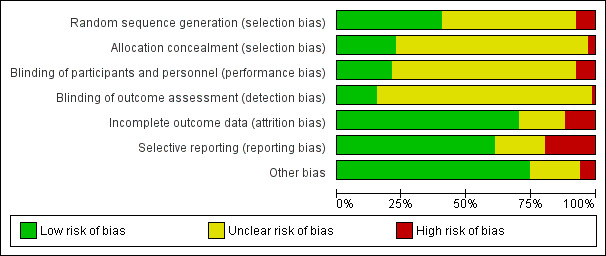
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment), we evaluated the risk of bias separately for each outcome, and we grouped outcomes according to whether they were measured subjectively or objectively when reporting our findings in the 'Risk of bias' tables.
We also assessed attrition bias (incomplete outcome data) on an outcome‐specific basis and grouped outcomes with like judgements when reporting our findings in the 'Risk of bias' tables.
We further summarised risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome.
For assessment of performance bias, we judged all outcomes to be equally susceptible to bias.
For assessment of detection bias, we defined the following endpoints as susceptible to bias (subjective).
Stone clearance.
Major adverse events.
Stone expulsion time.
Pain episodes.
For assessment of detection bias, we defined the following endpoints as not susceptible to bias (objective).
Dose of diclofenac.
Hospitalisation.
Surgical intervention.
We assessed risk of bias of included studies using the bias assessment tool provided by Higgins 2011 (see Appendix 2).
Measures of treatment effect
We analysed all data using Review Manager version 5.3.
For dichotomous outcomes (stone clearance, major adverse events, hospitalisation, surgical intervention), we expressed results as risk ratios (RRs) with 95% confidence intervals (CIs). When we used continuous scales of measurement to assess effects of treatment (stone expulsion time, pain episodes, dose of diclofenac), we used mean differences (MDs) with 95% CIs if the same scales were used.
Unit of analysis issues
The unit of analysis is the study. In studies that included more than two intervention groups (e.g. tamsulosin and alfuzosin, different dosages of the same alpha‐blocker), we collapsed the separate treatment arms into one for our main analysis. If intervention groups could not be separated, or if it was unclear whether studies from the same author with a trial period in the same year had overlapping participants, we asked trial authors to provide us the exact data separately. When we received no reaction or a denial on our request, we had to exclude the study.
Dealing with missing data
We did not impute any data, and we did not have sufficient information to perform an intention‐to‐treat analysis for all trials. We took this into account when we assessed risk of bias.
We requested by written or electronic correspondence any further information or clarification required from trial authors, and we included in the review relevant data obtained in this manner.
Assessment of heterogeneity
We identified heterogeneity (inconsistency) through visual inspection of forest plots to assess the extent of overlap of CIs, and we used the I² statistic to quantify inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). We interpreted I² as follows.
0% to 40%: may not be important.
30% to 60%: may indicate moderate heterogeneity.
50% to 90%: may indicate substantial heterogeneity.
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We attempted to obtain study protocols to assess studies for selective outcome reporting. If we included 10 or more studies investigating a particular outcome, we used funnel plots to assess small‐study effects. Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials), and publication bias. We therefore interpreted results with caution (Sterne 2001).
Data synthesis
For dichotomous outcomes, we used the Mantel‐Haenszel method; for continuous outcomes, we used the inverse variance method; and for time‐to‐event outcomes, we used the generic inverse variance method. We used Review Manager software to perform analyses (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analyses to explore possible sources of heterogeneity (e.g. participants, interventions).
Stone size (stones measuring 5 mm or smaller vs stones measuring 6 to 10 mm).
Stone location (distal ureter stones vs mid or proximal ureter stones).
Type of alpha‐blocker.
We used this rationale in performing the subgroup analyses mentioned above because previous studies suggested possible subgroup effects (Campschroer 2014; Hollingsworth 2016; Preminger 2007).
We limited outcomes for subgroup analyses to data available from the included studies (i.e. analyses were performed only when stratification according to our subgroups was provided).
Sensitivity analysis
We conducted the following sensitivity analyses to explore possible sources of heterogeneity in study design.
Solely placebo‐controlled trials, excluding trials with standard therapy as the control group.
Solely high‐quality trials, excluding trials with high risk of bias.
'Summary of findings' tables and application of GRADE
We presented the overall quality of evidence for each outcome according to the GRADE approach, which takes into account five criteria related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity (e.g. directness of results) (Guyatt 2008). Two review authors (TC and XZ) independently rated the quality of evidence for each outcome as 'high', 'moderate', 'low', or 'very low', and resolved discrepancies by consensus, or, if needed, by arbitration provided by a third review author (TL). We presented a summary of the evidence for main outcomes in a 'Summary of findings' table, which provides key information about the best estimate of the magnitude of effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of overall confidence in effect estimates for each outcome (Guyatt 2008; Schünemann 2006).
Results
Description of studies
Results of the search
Initially, we found 127 records through database searching and identified no additional records through other sources. On the basis of our criteria, we identified 121 potentially relevant titles and retrieved the full text of these articles for further evaluation (Figure 1).
Included studies
Finally, we included 67 studies in this review (Characteristics of included studies).
Most studies (52 of 67) compared a standard therapy group versus an alpha‐blocker group. Standard therapy consisted of appropriate hydration and use of NSAIDs, corticosteroids, or antispasmodics. Fifteen of the 67 studies compared alpha‐blocker versus placebo. Ten of the 67 studies were carried out in a multi‐centre setting (Cho 2017; Dong 2009; Furyk 2016; Lee 2014; Lojanapiwat 2008; Meltzer 2017; Pickard 2015; Sur 2015; Vincendeau 2010; Ye 2017). Sample size varied from 30 to 3450 and mean or median age from 32 to 56 years, and the duration of follow‐up ranged from one week to eight weeks.
Some studies had multiple intervention arms (Agrawal 2009; Ahmed 2010; Albert 2016; Aldemir 2011; Balci 2014; Cha 2012; Dong 2009; El‐Gamal 2012; Erkan 2011; Erturhan 2007; Georgescu 2015; Hong 2008; Ibrahim 2013; Islam 2010; Kupeli 2004; Liatsikos 2007; Lojanapiwat 2008; Maitra 2012; Mshemish 2012; Mukhtarov 2007; Pickard 2015; Porpiglia 2006; Sen 2017; Taghavi 2005; Wang 2008a; Yilmaz 2005; Zhou 2011). Ten studies compared different alpha‐blockers, such as tamsulosin (0.4 mg), alfuzosin (10 mg), doxazosin (4 or 8 mg), terazosin (2 or 5 mg), naftopidil (10 mg), or silodosin (8 mg), with each other and with a standard therapy or placebo (Agrawal 2009; Ahmed 2010; Albert 2016; Cha 2012; Georgescu 2015; Ibrahim 2013; Mshemish 2012; Wang 2008a; Yilmaz 2005; Zhou 2011). Lojanapiwat 2008 and Cha 2012 used different dosages of tamsulosin (0.2 and 0.4 mg). Six studies used tamsulosin 0.2 mg (Cha 2012; Dong 2009; Han 2006a; Kaneko 2010; Kim 2007b; Lojanapiwat 2008).
Excluded studies
We excluded 54 studies (Characteristics of excluded studies).
Investigators in four studies gave participants calcium channel blockers, not alpha‐blockers (Borghi 1994; Cooper 2000; Porpiglia 2000; Skrekas 2003). Five studies treated participants with invasive treatment modalities (ureteral stent positioning, ESWL, or ureterorenoscopy with lithotripsy) (Agarwal 2009; Damiano 2008; Deliveliotis 2006; John 2010; Wang 2009c). We excluded eight studies because they included participants with ureteral stones larger than 10 mm, or with multiple stones (Aravinthan 2012; Avdoshin 2005; Dellabella 2003; Dellabella 2005; Gandhi 2013; Khawaja 2005; Mohseni 2006; Resim 2005). We could not compare study groups from 14 studies for this review, or investigators used no standard therapy/placebo (Cuni 2013; Dellabella 2005a; Gupta 2013; Gupta Shyam 2014; Gurbuz 2011; Imperatore 2014; Jayant 2014; Kumar 2014; Kumar 2015; Lu 2012; Morozumi 2013; Salem 2015; Shabana 2015; Tsuzaka 2011). We excluded nine studies because of study design (no RCTs) (Brausi 2015; Hwang 2012; Itano 2012; Loftus 2015; Moon 2015; Multescu 2014; Nasim 2014; Reddy 2016; Resorlu 2011; Shah 2013; Tchey 2011; Tsuzaka 2011; Vavassori 2012). Other reasons for exclusion were enrolment of children (Aydogdu 2009; Sameer 2014), insufficient recruitment (ISRCTN24675122), addition of corticosteroid drugs to tamsulosin (Dellabella 2005a), inclusion of patients with pyelonephritis (Chau 2011), and insufficient primary and secondary outcome data or data that could not be interpreted (Bak 2007; Bhat 2015; Haxhiu 2014; Ohgaki 2010; Ramesh 2015; Su 2016; Sumer 2012).
Risk of bias in included studies
Allocation
Of the 67 studies, 36 and 50 studies described methods of sequence generation and allocation, respectively, with unclear risk of selection bias. Twenty‐six and 15 studies had low risk of bias, respectively. We judged five and two studies as having high risk of bias, respectively.
Blinding
In total, we judged 48 and 56 studies as having unclear risk of performance and detection bias, respectively. Fourteen and 10 studies had low risk of bias, respectively. We judged five studies and one study as having high risk of bias, respectively.
Incomplete outcome data
In total, we judged 47 studies as having low risk of attrition bias. Twelve studies had unclear risk of bias. Eight studies had high risk of bias.
Selective reporting
The funnel plot as shown in Figure 4 was asymmetrical. Of the 67 included studies, we judged 42 as having low risk of reporting bias. Twelve studies had unclear risk of bias. Thirteen studies had high risk of bias.
4.
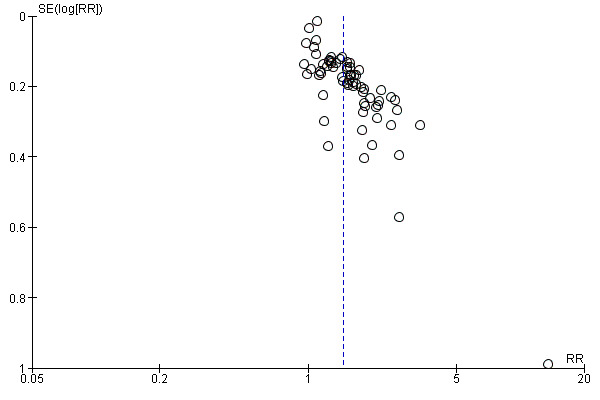
Funnel plot of comparison: 1 Alpha‐blocker versus standard therapy or placebo, outcome: 1.1 Stone clearance.
Other potential sources of bias
In total, we considered 50 studies as having low risk of other potential sources of bias. Thirteen studies had unclear risk of bias. We judged four studies as having high risk of bias.
Effects of interventions
Summary of findings 2. Alpha‐blockers compared with placebo for ureteral stones.
| Alpha‐blockers compared with placebo for ureteral stones | |||||
|
Patient or population: adult patients presenting with symptoms of ureteral stone disease Setting: single or multicenter Intervention: alpha‐blocker Comparison: placebo | |||||
| Outcomes | No. of participants (studies) Follow‐up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk difference with alpha‐blockers | ||||
| Stone clearance | 5787 (15 RCTs) | ⊕⊕⊕⊝ MODERATEa | RR 1.16 (1.07 to 1.25) | Study population | |
| 728 per 1000 | 116 more per 1000 (51 more to 182 more) | ||||
| Major adverse events | 1650 (10 RCTs) | ⊕⊕⊕⊝ MODERATEb | RR 2.09 (1.13 to 3.86) | Study population | |
| 26 per 1000 | 29 more per 1000 (3 more to 75 more) | ||||
| Stone expulsion time | 3240 (7 RCTs) | ⊕⊕⊝⊝ LOWc,d | ‐ | MD 1.98 lower (3.71 lower to 0.24 lower) | |
| Pain episodes | 215 (2 RCTs) | ⊕⊕⊝⊝ LOWc,e | ‐ | MD 0.39 lower (1.07 lower to 0.29 higher) | |
| Dose of diclofenac (mg) | 3576 (4 RCTs) | ⊕⊕⊝⊝ LOWd,f | ‐ | MD 126.32 lower (181.73 lower to 70.9 lower) | |
| Hospitalisation | 500 (2 RCTs) | ⊕⊕⊕⊝ MODERATEe | RR 0.84 (0.48 to 1.47) | Study population | |
| 96 per 1000 | 15 fewer per 1000 (50 fewer to 45 more) | ||||
| Surgical intervention | 1458 (5 RCTs) | ⊕⊕⊕⊕ HIGH | RR 0.93 (0.70 to 1.24) | Study population | |
| 127 per 1000 | 9 fewer per 1000 (38 fewer to 30 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded owing to inconsistency (high heterogeneity with I² of 68%).
bDowngraded owing to imprecision (wide confidence interval consistent with negligible to substantial harm).
cDowngraded owing to inconsistency (heterogeneity with I² of 57%).
dDowngraded owing to imprecision (wide confidence interval; wide confidence interval consistent with large to negligible benefit).
eDowngraded owing to imprecision (wide confidence interval consistent with no effect and small benefit).
fDowngraded owing to inconsistency (high heterogeneity with I² of 90%).
1. Alpha‐blockers versus standard therapy or placebo
Primary outcomes
1.1 Stone clearance
A total of 67 RCTs with 10.509 participants showed that treatment with an alpha‐blocker may result in a large increase in stone clearance (Analysis 1.1; RR 1.45, 95% CI 1.36 to 1.55; P < 0.00001; I² = 76%; low‐quality evidence); this corresponds to 278 more (95% CI 223 more to 340 more) stone clearances per 1000. We downgraded the quality of evidence for study limitations, inconsistency, and concerns about publication bias.
1.1. Analysis.
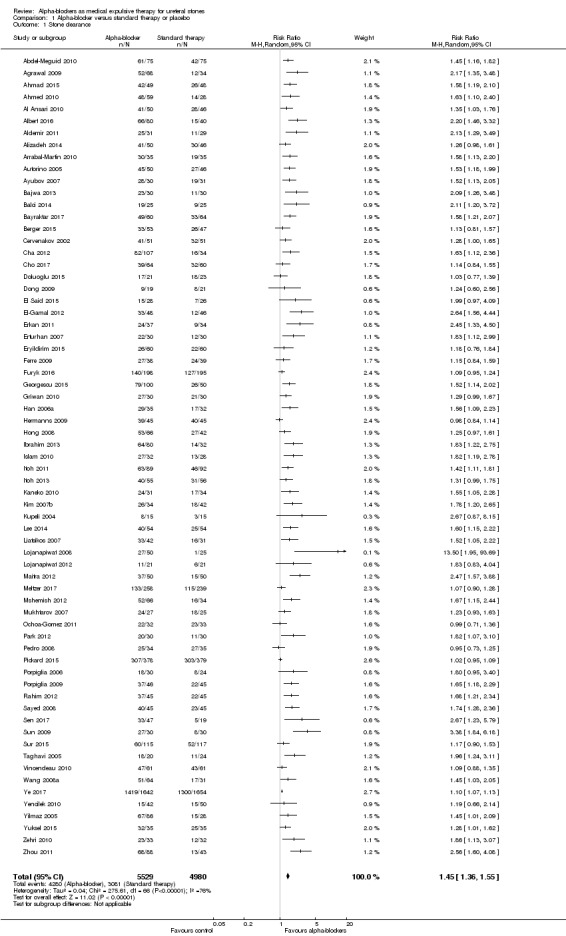
Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 1 Stone clearance.
1.2 Major adverse events
Eighteen studies with 3124 participants described major adverse events. Treatment with an alpha‐blocker may have little effect on major adverse events (Analysis 1.2; RR 1.25, 95% CI 0.80 to 1.96; P = 0.33; I² = 0%; low‐quality evidence); this corresponds to 5 more (95% CI 4 fewer to 19 more) major adverse events per 1000. We downgraded the quality of evidence for study limitations and imprecision.
1.2. Analysis.
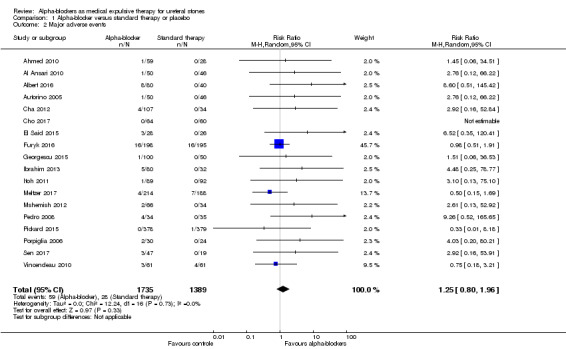
Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 2 Major adverse events.
Seven studies reported discontinuation of treatment due to adverse events (Hermanns 2009; Itoh 2011; Itoh 2013; Pedro 2008; Sen 2017; Sur 2015; Vincendeau 2010). In some studies, discontinuation of treatment due to adverse events was unclear (Meltzer 2017; Pickard 2015).
In total, 59 major adverse events occurred in 3124 participants receiving alpha‐blockers, and 28 major adverse events were reported in 1329 participants receiving standard therapy or placebo.
Eleven of 1735 participants (0.6%) in the alpha‐blocker group discontinued treatment within the study because of major adverse events, as compared with 1 of 1389 (0.07%) in the control group.
Secondary outcomes
1.3 Stone expulsion time
A total of 37 studies with 6031 participants described stone expulsion time. Treatment with an alpha blocker may substantially reduce the time to stone passage (Analysis 1.3; MD ‐3.40 days, 95% CI ‐4.17 to ‐2.63; P < 0.00001; I² = 94%; low‐quality evidence). We downgraded the quality of evidence for study limitations, inconsistency, and concerns about publication bias.
1.3. Analysis.

Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 3 Stone expulsion time.
1.4 Pain episodes
A total of 15 studies with 1363 participants reported the number of pain episodes. Treatment with an alpha‐blocker may provide a small reduction in the number of pain episodes (Analysis 1.4; MD ‐0.66, 95% CI ‐0.91 to ‐0.42; P < 0.00001; I² = 80%; low‐quality evidence). We downgraded the quality of evidence for study limitations, inconsistency, and concerns about publication bias.
1.4. Analysis.
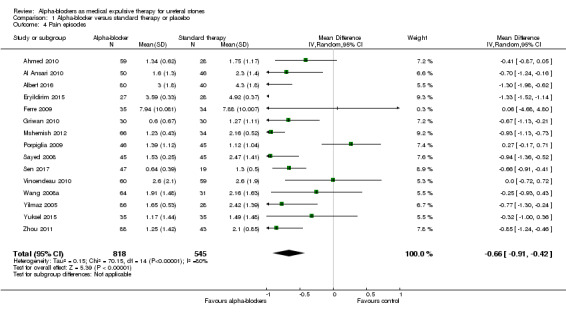
Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 4 Pain episodes.
1.5 Dose of diclofenac
On the basis of 14 studies with 4373 participants, we are uncertain whether treatment with an alpha‐blocker reduces the use of diclofenac (Analysis 1.5; MD ‐82.41, 95% CI ‐122.51 to ‐42.31; P < 0.00001; I² = 100%; low‐quality evidence). It is notable that the dose of diclofenac in the standard therapy group varied largely among studies (from 15 mg to 1405 mg). We downgraded the quality of evidence for study limitations, inconsistency, and concerns about publication bias.
1.5. Analysis.
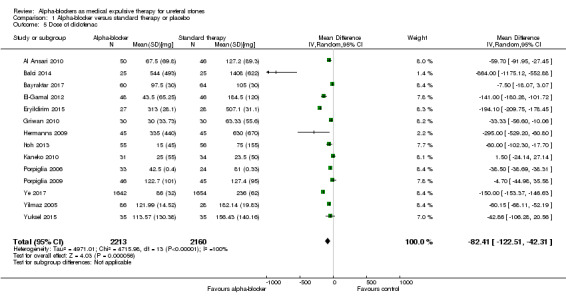
Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 5 Dose of diclofenac.
1.6 Hospitalisation
Findings of 13 studies with 1876 participants show that treatment with an alpha‐blocker may reduce the need for hospitalisation (Analysis 1.6; RR 0.51, 95% CI 0.34 to 0.77; P = 0.001; I² = 40%; moderate‐quality evidence). This corresponds to 69 fewer (95% CI 93 fewer to 32 fewer) hospitalisations per 1000. We downgraded the quality of evidence for study limitations.
1.6. Analysis.
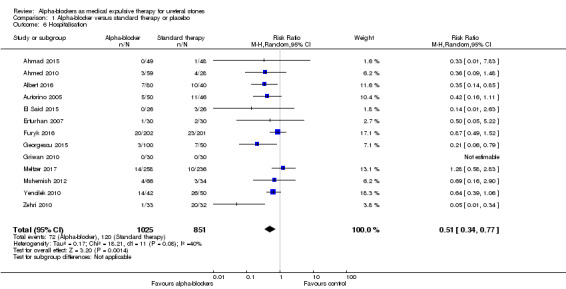
Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 6 Hospitalisation.
1.7 Surgical intervention
A total of 19 studies with 3292 participants reported that treatment with an alpha‐blocker may have little effect on the need for surgical intervention (Analysis 1.7; RR 0.74, 95% CI 0.53 to 1.02; P = 0.07; I² = 37%; low‐quality evidence). This corresponds to 28 fewer (95% CI 51 fewer to 2 more) surgical interventions per 1000. We downgraded the quality of evidence for study limitations and imprecision.
1.7. Analysis.
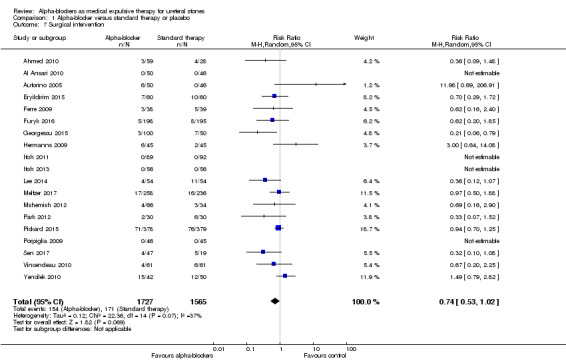
Comparison 1 Alpha‐blocker versus standard therapy or placebo, Outcome 7 Surgical intervention.
Subgroup analyses
Stone size
2.1 Stone clearance
We compared outcomes of participants with stones measuring 5 mm or smaller (14 studies, 2622 participants) versus outcomes of those with stones bigger than 5 mm (10 studies, 2887 participants; Analysis 2.1). We found an RR of 1.06 (95% CI 0.98 to 1.15; P = 0.16; I² = 62%) versus 1.45 (95% CI 1.22 to 1.72; P < 0.0001; I² = 59%), respectively. The test for interaction was suggestive of a possible subgroup effect (Chi² = 9.96, P = 0.002; I² = 90%). Therefore, alpha‐blockers may have little effect on stone clearance in participants with stones measuring 5 mm or smaller, resulting in 48 more (95% CI 16 fewer to 121 more) stone clearances per 1000, but may substantially increase stone clearance in participants with stones bigger than 5 mm, which corresponds to 302 more (95% CI 148 more to 483 more) stone clearances per 1000. We rated the quality of evidence as moderate upon downgrading for study limitations and imprecision.
2.1. Analysis.
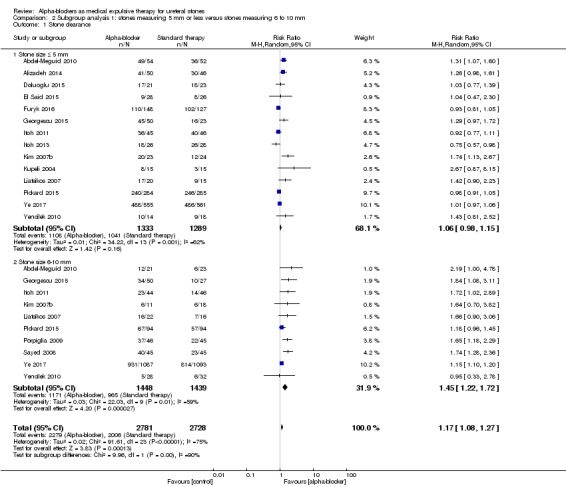
Comparison 2 Subgroup analysis 1: stones measuring 5 mm or less versus stones measuring 6 to 10 mm, Outcome 1 Stone clearance.
2.2 Stone expulsion time
Stratified by stone size, participants with stones measuring 5 mm or smaller did benefit from using alpha‐blockers compared with standard therapy or placebo in terms of stone expulsion time (Analysis 2.2; six studies, 1264 participants) (MD ‐1.07, 95% CI ‐1.99 to ‐0.15; P = 0.02; I² = 60%). The quality of evidence was low upon downgrading for risk of bias, inconsistency, and imprecision. For stones bigger than 5 mm, the alpha‐blocker group had shorter stone expulsion time (Analysis 2.2; four studies, 1884 participants) (MD ‐5.99, 95% CI ‐7.16 to ‐4.82; P < 0.00001; I² = 77%). The quality of evidence was low upon downgrading for risk of bias, inconsistency, and imprecision.
2.2. Analysis.
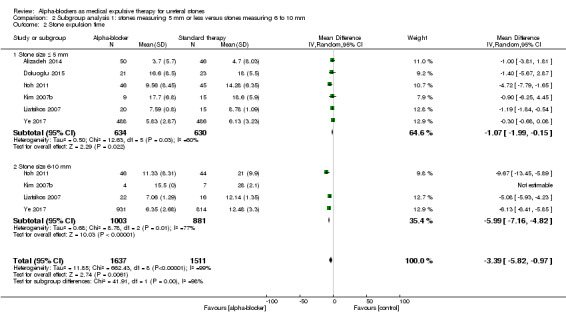
Comparison 2 Subgroup analysis 1: stones measuring 5 mm or less versus stones measuring 6 to 10 mm, Outcome 2 Stone expulsion time.
The subgroup interaction test did not indicate a difference in effect for stone expulsion time based on stone size (Chi² = 41.91, P < 0.00001; I² = 97.6%).
2.3 Other outcomes
We found no studies that reported the outcomes of major adverse events, pain episodes, dose of diclofenac, hospitalisation, and surgical intervention that permitted an analysis stratified by stone size.
Stone location
3.1 Stone clearance
For participants with distal ureteral stones, stone clearance may be improved (Analysis 3.1; 57 studies, 8576 participants) (RR 1.46, 95% CI 1.36 to 1.57; P < 0.00001; I² = 77%). The quality of evidence was low upon downgrading for risk of bias, inconsistency, and publication bias.
3.1. Analysis.
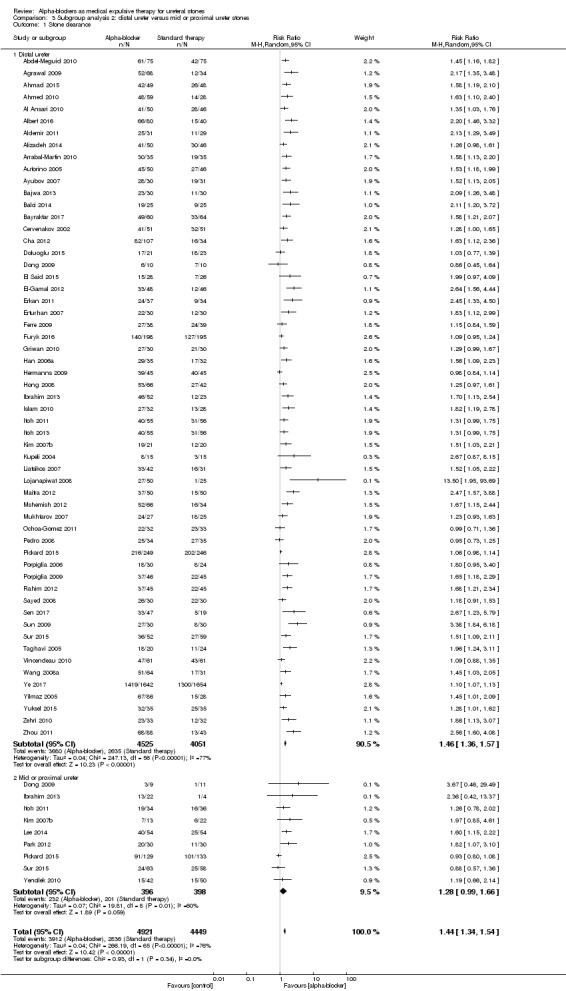
Comparison 3 Subgroup analysis 2: distal ureter versus mid or proximal ureter stones, Outcome 1 Stone clearance.
For participants with mid and proximal ureteral stones, stone clearance was similar (Analysis 3.1; nine studies, 794 participants) (RR 1.28, 95% CI 0.99 to 1.66; P = 0.06; I² = 60%). The quality of evidence was low upon downgrading for risk of bias, inconsistency, and imprecision.
Tests for subgroup differences were not statistically significant (Chi² = 0.93, I² = 0%, P = 0.34).
3.2 Stone expulsion time
Participants with distal ureteral stones may benefit from using alpha‐blockers compared with standard therapy or placebo in terms of stone expulsion time (Analysis 3.2; 32 studies, 5453 participants) (MD ‐3.43, 95% CI ‐4.26 to ‐2.60; P < 0.0001; I² = 95%). The quality of evidence was low upon downgrading for risk of bias, inconsistency, and imprecision. For mid or proximal ureteral stones, the alpha‐blocker group did not show shorter stone expulsion time (Analysis 3.2; two studies, 146 participants) (MD ‐8.64, 95% CI ‐19.75 to 2.48; P = 0.13; I² = 96%). The quality of evidence was moderate upon downgrading for risk of bias and imprecision.
3.2. Analysis.
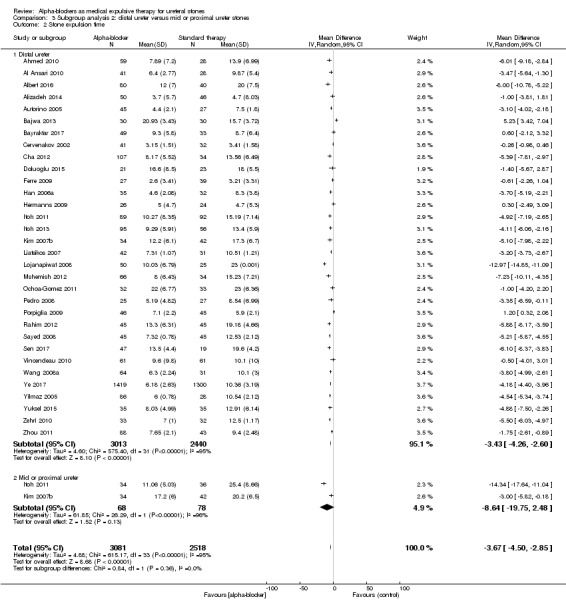
Comparison 3 Subgroup analysis 2: distal ureter versus mid or proximal ureter stones, Outcome 2 Stone expulsion time.
The subgroup interaction test indicated a difference in effect for stone expulsion time based on stone location (Chi² = 0.84, P = 0.36, I² = 0%).
3.3 Other outcomes
We found no studies that reported the outcomes of major adverse events, pain episodes, dose of diclofenac, hospitalisation, and surgical intervention that permitted an analysis stratified by stone location.
Type of alpha‐blocker
4.1 Stone clearance
We performed a subgroup analysis based on types of alpha‐blockers used; these included tamsulosin, alfusozin, terazosin, naftopidil, and silodosin (Analysis 4.1). All types of alpha‐blockers improved stone clearance. The subgroup test for interaction did not show significance (Chi² = 1.44, I² = 0%, P = 0.92).
4.1. Analysis.
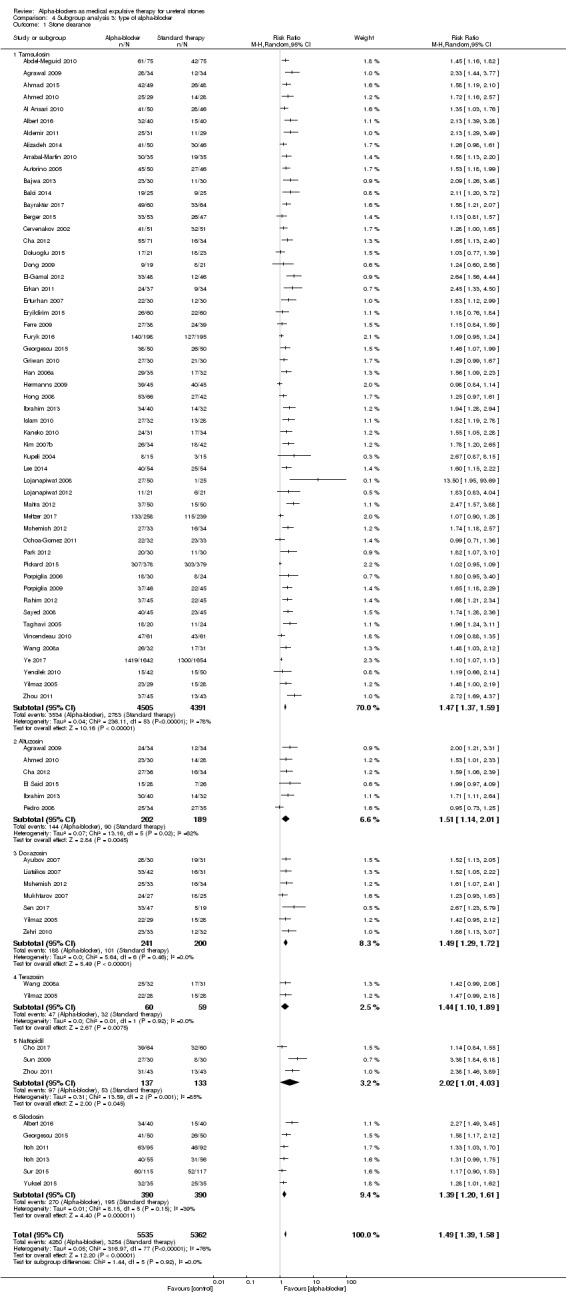
Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 1 Stone clearance.
4.2 Major adverse events
We performed a subgroup analysis based on types of alpha‐blockers used; these included tamsulosin, alfusozin, doxasozin, terazosin, naftopidil, and silodosin (Analysis 4.2). Major adverse events were not increased, except with alfuzosin (five studies, 323 participants) (RR 5.51, 95% CI 1.46 to 20.83; P = 0.01; I² = 0%). However, the subgroup test for interaction did not show significance (Chi² = 5.57, I² = 46.1%, P = 0.13).
4.2. Analysis.

Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 2 Major adverse events.
4.3 Stone expulsion time
We performed a subgroup analysis based on types of alpha‐blockers used; these included tamsulosin, alfusozin, terazosin, naftopidil, and silodosin (Analysis 4.3). Use of an alpha‐blocker consistently shortened time to stone passage. The subgroup test for interaction did not show significance (Chi² = 23.76, I² = 79.0%, P = 0.0002).
4.3. Analysis.
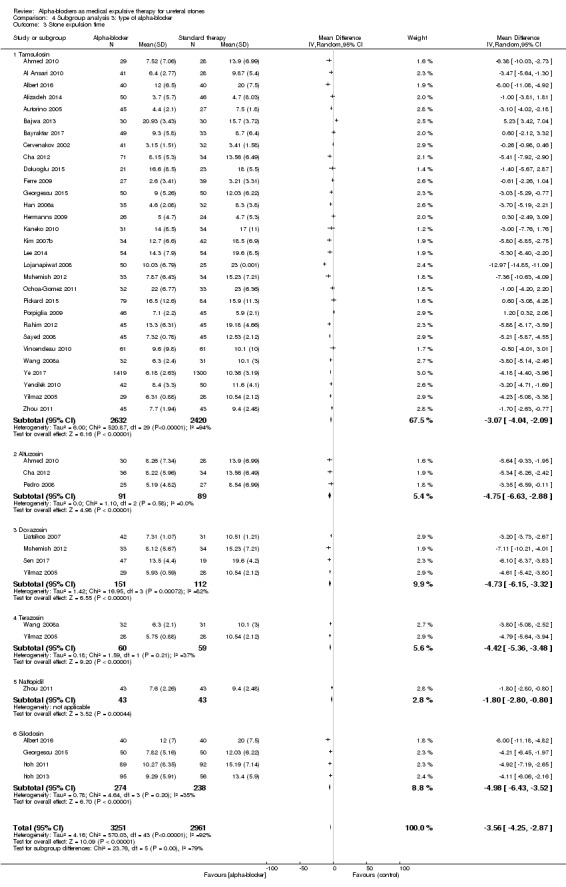
Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 3 Stone expulsion time.
4.4 Pain episodes
We performed a subgroup analysis based on types of alpha‐blockers used; these included tamsulosin, alfusozin, doxasozin, terazosin, naftopidil, and silodosin (Analysis 4.4). Pain episodes were consistently decreased, except with silodosin (two studies, 150 participants) (MD ‐0.89, 95% CI ‐2.05 to 0.26; P = 0.13; I² = 80%). The subgroup test for interaction did not show significance (Chi² = 3.02, I² = 0%, P = 0.70).
4.4. Analysis.
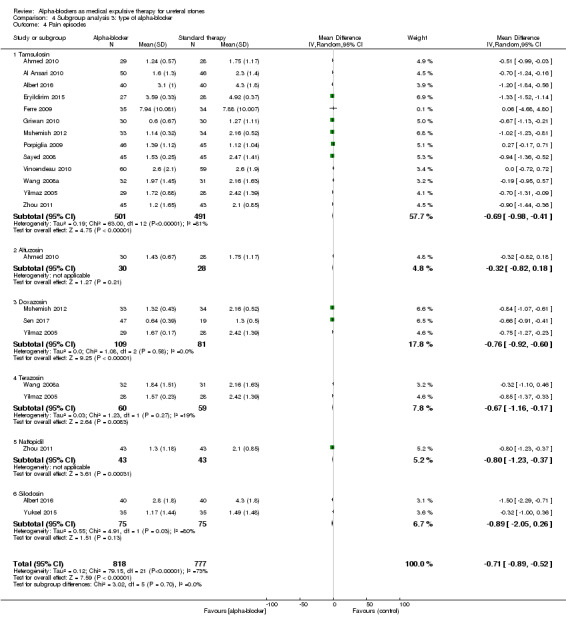
Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 4 Pain episodes.
4.5 Dose of diclofenac (mg)
We performed a subgroup analysis based on types of alpha‐blockers used; these included tamsulosin, doxasozin, terazosin, and silodosin (Analysis 4.5). Diclofenac use was consistently decreased. The subgroup test for interaction did not show significance (Chi² = 1.37, I² = 0%, P = 0.71).
4.5. Analysis.
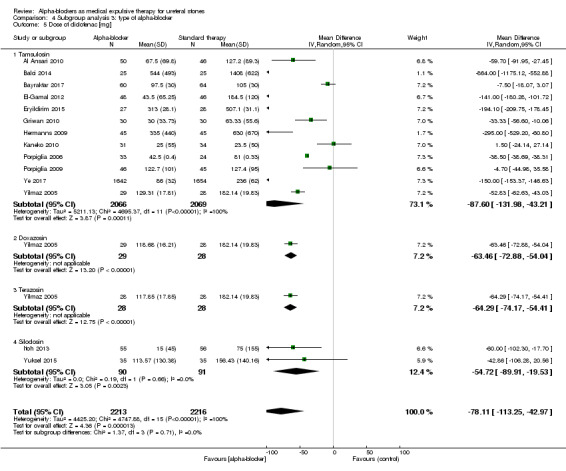
Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 5 Dose of diclofenac [mg].
4.6 Hospitalisation
Tamsulosin versus standard therapy or placebo showed fewer hospitalisations in favour of alpha‐blockers (Analysis 4.6; 11 studies, 1606 participants) (RR 0.57, 95% CI 0.38 to 0.86; P = 0.007; I² = 33%). The risk difference for participants receiving tamsulosin was 58 fewer more (95% CI 19 fewer to 84 fewer) hospitalisations per 1000. The quality of evidence was moderate upon downgrading for risk of bias.
4.6. Analysis.
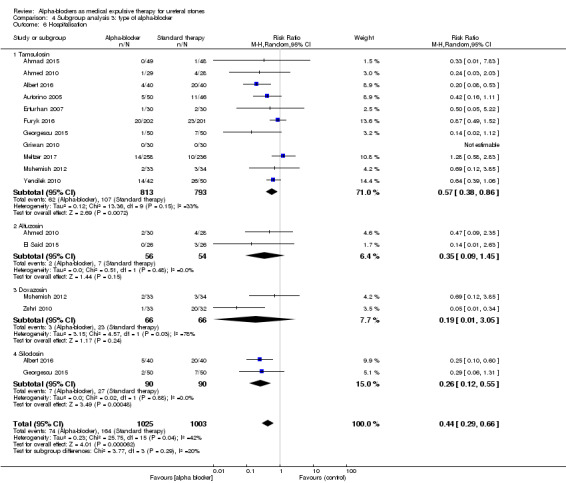
Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 6 Hospitalisation.
Alfuzosin did not show a beneficial effect in the alpha‐blocker group in terms of hospitalisation (Analysis 4.6; two studies, 110 participants) (RR 0.35, 95% CI 0.09 to 1.45; P = 0.15; I² = 0%). The risk difference for participants receiving alfuzosin was 84 fewer (95% CI 118 fewer to 58 more) hospitalisations per 1000. The quality of evidence was low upon downgrading for risk of bias and imprecision.
Use of doxazosin did not result in fewer hospitalisations (Analysis 4.6; two studies, 132 participants) (RR 0.19, 95% CI 0.01 to 3.05; P = 0.24; I² = 78%). The risk difference for participants receiving doxazosin was 282 fewer (95% CI 345 fewer to 714 more) hospitalisations per 1000. The quality of evidence was low upon downgrading for risk of bias, inconsistency, and imprecision.
Silodosin versus standard therapy or placebo showed fewer hospitalisations in the alpha‐blocker group (Analysis 4.6; two studies, 180 participants) (RR 0.26, 95% CI 0.12 to 0.55; P = 0.0005; I² = 0%). The risk difference for participants receiving silodosin was 222 fewer (95% CI 135 fewer to 264 fewer) hospitalisations per 1000. The quality of evidence was moderate upon downgrading for risk of bias.
Testing for subgroup differences was not statistically significant (Chi² = 3.77, I² = 20.4%, P = 0.29).
4.7 Surgical intervention
Use of tamsulosin did not lead to fewer surgical interventions (Analysis 4.7; 16 studies, 2820 participants) (RR 0.82, 95% CI 0.59 to 1.13; P = 0.22; I² = 29%). The risk difference for participants receiving tamsulosin was 21 fewer (95% CI 49 fewer to 15 more) surgical interventions per 1000. The quality of evidence was moderate upon downgrading for risk of bias.
4.7. Analysis.
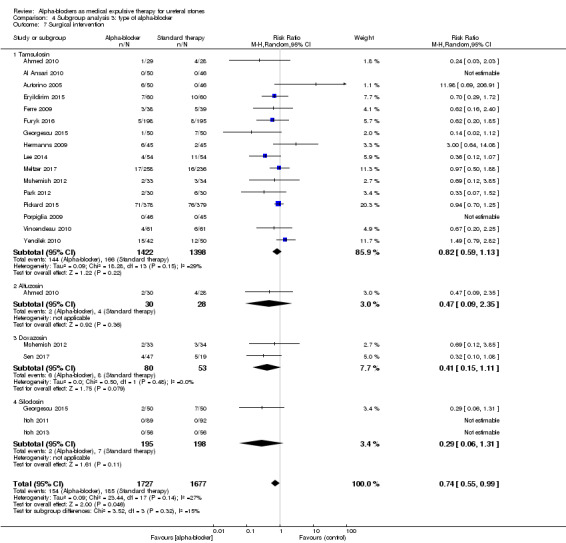
Comparison 4 Subgroup analysis 3: type of alpha‐blocker, Outcome 7 Surgical intervention.
Alfuzosin did not result in less surgical interventions (Analysis 4.7; one study, 58 participants) (RR 0.47, 95% CI 0.09 to 2.35, P = 0.36). The risk difference for participants receiving alfuzosin was 76 fewer (95% CI 130 fewer to 193 more) surgical interventions per 1000. The quality of evidence was low upon downgrading for risk of bias and imprecision.
Use of doxazosin did not result in fewer surgical interventions (Analysis 4.7; two studies, 133 participants) (RR 0.41, 95% CI 0.15 to 1.11; P = 0.08; I² = 0%). The risk difference for participants receiving doxazosin was 89 fewer (95% CI 128 fewer to 17 more) surgical interventions per 1000. The quality of evidence was low upon downgrading for risk of bias and imprecision.
Data show no difference in terms of surgical interventions between silodosin versus standard therapy or placebo (Analysis 4.7; three studies, 133 participants) (RR 0.41, 95% CI 0.15 to 1.11; P = 0.11; I² = 27%). The risk difference for participants receiving silodosin was 25 fewer (95% CI 33 fewer to 11 more) surgical interventions per 1000. The quality of evidence was low upon downgrading for risk of bias and imprecision.
Testing for subgroup differences did not show statistical significance (Chi² = 3.52, I² = 14.9%, P = 0.32).
Sensitivity analyses
Alpha‐blockers versus placebo
5.1 Stone clearance
Participants who received alpha‐blockers were more likely to be stone‐free compared with those who received placebo (Analysis 5.1; 15 studies, 5787 participants) (RR 1.16, 95% CI 1.07 to 1.25, P <0.00001; I² = 68%). The risk difference with alpha‐blockers was 116 more (95% CI 51 more to 182 more) stone clearances per 1000. The quality of evidence was moderate upon downgrading for risk of bias and inconsistency.
5.1. Analysis.
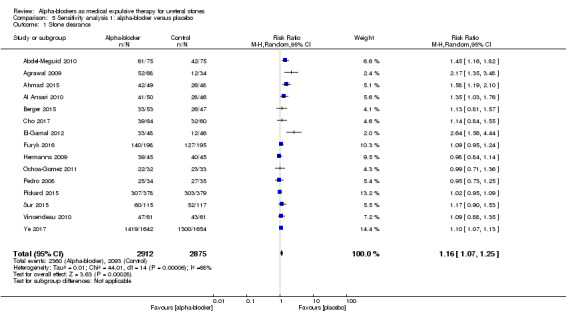
Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 1 Stone clearance.
5.2 Major adverse events
Participants using alpha‐blockers experienced more major adverse events of the medication compared with those given placebo (Analysis 5.2; 10 studies, 1650 participants) (RR 2.09, 95% CI 1.13 to 3.86; P = 0.02; I²= 25%). The risk difference with alpha‐blockers was 29 more (95% CI 3 more to 75 more) major adverse events per 1000. The quality of evidence was moderate upon downgrading for risk of bias and imprecision. For 4 of the 848 (0.47%) participants in the alpha‐blocker group, the major adverse event led to cessation of therapy versus none in the placebo group.
5.2. Analysis.
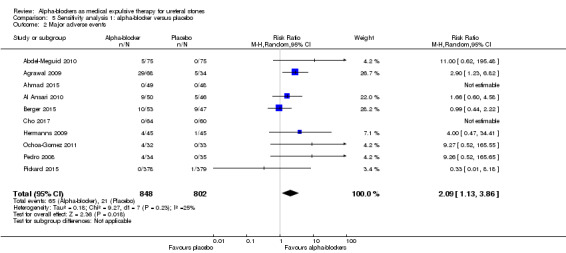
Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 2 Major adverse events.
5.3 Stone expulsion time
Stone expulsion time was shorter among participants using alpha‐blockers compared with those receiving placebo (Analysis 5.3; seven studies, 3240 participants) (MD ‐1.98, 95% CI ‐3.71 to ‐0.24; P = 0.03; I² = 76%). The quality of evidence was low upon downgrading for inconsistency and imprecision.
5.3. Analysis.
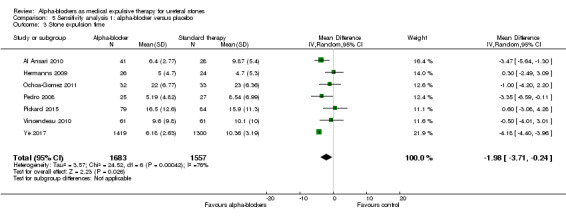
Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 3 Stone expulsion time.
5.4 Pain episodes
The number of pain episodes was not statistically different between participants using alpha‐blockers and those receiving placebo (Analysis 5.4; two studies, 215 participants) (MD ‐0.39, 95% CI ‐1.07 to 0.29; P = 0.13; I² = 57%). The quality of evidence was low upon downgrading for inconsistency and imprecision.
5.4. Analysis.

Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 4 Pain episodes.
5.5 Dose of diclofenac (mg)
The dose of diclofenac used was statistically lower for participants using alpha‐blockers compared with those receiving placebo (Analysis 5.5; four studies, 3576 participants) (MD ‐126.32, 95% CI ‐181.73 to ‐70.90; P < 0.00001; I² = 90%). The quality of evidence was low upon downgrading for risk of bias, inconsistency, and imprecision.
5.5. Analysis.
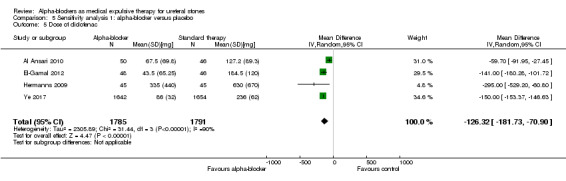
Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 5 Dose of diclofenac.
5.6 Hospitalisation
Hospitalisation was not statistically different between participants using alpha‐blockers and those receiving placebo (Analysis 5.6; two studies, 500 participants) (RR 0.84, 95% CI 0.48 to 1.47; P = 0.55; I² = 0%). The risk difference with alpha‐blockers was 15 fewer (95% CI 50 fewer to 45 more) hospitalisations per 1000. The quality of evidence was moderate upon downgrading for risk of bias and imprecision.
5.6. Analysis.
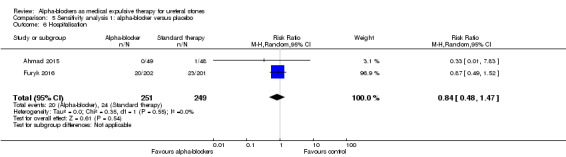
Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 6 Hospitalisation.
5.7 Surgical intervention
Surgical intervention was not statistically different between participants using alpha‐blockers and those receiving placebo (Analysis 5.7; five studies, 1458 participants) (RR 0.93, 95% CI 0.70 to 1.24; P = 0.39; I² = 1%). The risk difference with alpha‐blockers was 9 fewer (95% CI 38 fewer to 30 more) surgical interventions per 1000. The quality of evidence was high.
5.7. Analysis.
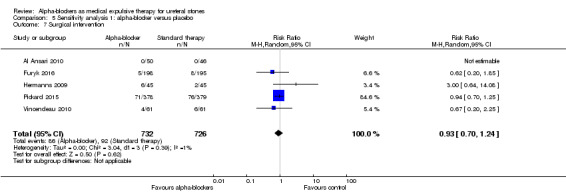
Comparison 5 Sensitivity analysis 1: alpha‐blocker versus placebo, Outcome 7 Surgical intervention.
High‐quality studies
6.1 Stone clearance
Data show statistically significant higher stone clearance between intervention and control groups in the high‐quality sensitivity analysis (Analysis 6.1; five studies, 4133 participants) (RR 1.09, 95% CI 1.06 to 1.13; P < 0.00001; I² = 0%). The risk difference with alpha‐blockers was 68 more (95% CI 45 more to 98 more) stone clearances per 1000. The quality of evidence was high.
6.1. Analysis.
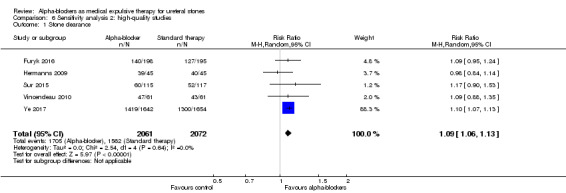
Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 1 Stone clearance.
6.2 Major adverse events
Participants using alpha‐blockers did not experience more major adverse events of the medication compared with those given placebo (Analysis 6.2; two studies, 515 participants) (RR 0.94, 95% CI 0.51 to 1.72; P = 0.84; I²= 0%). The risk difference with alpha‐blockers was 5 fewer (95% CI 38 fewer to 56 more) major adverse events per 1000. The quality of evidence was moderate upon downgrading for imprecision.
6.2. Analysis.
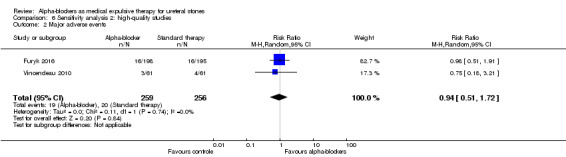
Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 2 Major adverse events.
6.3 Stone expulsion time
Stone expulsion time was not statistically different between participants using alpha‐blockers and those receiving placebo (Analysis 6.3; three studies, 2891 participants) (MD ‐1.72, 95% CI ‐5.13 to 1.68; P = 0.32; I² = 86%). The quality of evidence was low upon downgrading for inconsistency and imprecision.
6.3. Analysis.
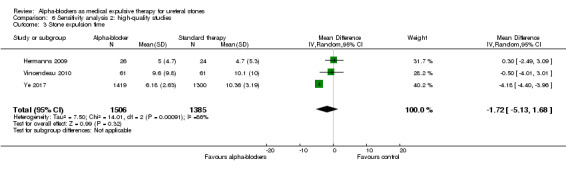
Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 3 Stone expulsion time.
6.4 Pain episodes
The number of pain episodes was not statistically different between participants using alpha‐blockers and those given placebo (Analysis 6.4; one study, 119 participants) (MD 0.00, 95% CI ‐0.72 to 0.72; P = 1). The quality of evidence was moderate upon downgrading for imprecision.
6.4. Analysis.

Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 4 Pain episodes.
6.5 Dose of diclofenac (mg)
The dose of diclofenac used was statistically lower among participants using alpha‐blockers compared with those receiving placebo (Analysis 6.5; two studies, 3386 participants) (MD ‐173.28, 95% CI ‐277.60 to ‐68.95; P = 0.001). The quality of evidence was moderate upon downgrading for imprecision.
6.5. Analysis.
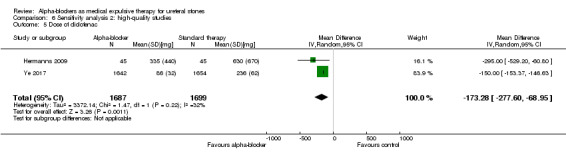
Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 5 Dose of diclofenac.
6.6 Hospitalisation
Hospitalisation was not statistically different between participants using alpha‐blockers and those receiving placebo (Analysis 6.6; one study, 403 participants) (RR 0.87, 95% CI 0.49 to 1.52; P = 0.62). The risk difference with alpha‐blockers was 15 fewer (95% CI 58 fewer to 60 more) hospitalisations per 1000. The quality of evidence was moderate upon downgrading for imprecision.
6.6. Analysis.
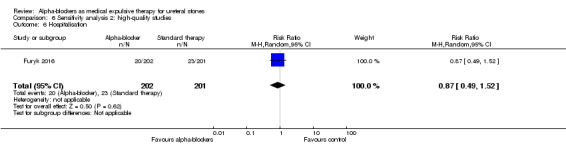
Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 6 Hospitalisation.
6.7 Surgical intervention
Surgical intervention was not statistically different between participants using alpha‐blockers and those receiving placebo (Analysis 6.7; three studies, 605 participants) (RR 0.94, 95% CI 0.38 to 2.32; P = 0.90; I² = 34%). The risk difference with alpha‐blockers was 3 fewer (95% CI 33 fewer to 70 more) surgical interventions per 1000. The quality of evidence was moderate upon downgrading for imprecision.
6.7. Analysis.
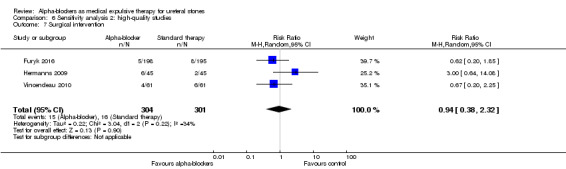
Comparison 6 Sensitivity analysis 2: high‐quality studies, Outcome 7 Surgical intervention.
Discussion
Summary of main results
The main findings of this meta‐analysis are that greater stone clearance can be achieved with medical expulsive therapy with an alpha‐blocker than with standard therapy without an alpha‐blocker (low‐quality evidence). Use of alpha‐blockers does not appear to result in an increase in major adverse events (low‐quality evidence).
Based on a preplanned sensitivity analysis of placebo‐controlled studies only, we found alpha‐blockers to be less effective than was suggested by the overall analysis of all studies; this finding corresponds to greater stone clearance (moderate‐quality evidence) and an increase in major adverse events (moderate‐quality evidence).
By performing a preplanned subgroup analysis, we found support for a possible subgroup effect based on stone size; alpha‐blockers may provide clinically meaningful improvement in stone clearance among patients with stones measuring 6 to 10 mm, but not among patients with stones measuring 5 mm or less. We did not find evidence of a subgroup effect based on stone location or type of alpha‐blocker.
For secondary outcomes, treatment with alpha‐blockers appears to shorten the time to stone expulsion and reduce the number of pain episodes, use of diclofenac, and the need for hospitalisation (very low‐ to moderate‐quality evidence). Our subgroup analyses showed a possible subgroup effect of stone size based on testing for interaction suggesting a larger effect in stones measuring 6 to 10 mm in size. For stone location and type of alpha‐blocker, the test for subgroup differences did not show statistical significance.
Overall completeness and applicability of evidence
For this review, we performed an extensive search: Two of three review authors independently extracted and managed trial data and resolved disagreements in consultation with the third review author. To collect additional potentially useful journal articles, we had non‐English language journals translated, and we asked authors of studies with missing data to deliver these data by written correspondence.
We assessed risk of bias using Cochrane's 'Risk of bias' assessment tool and assessed the quality of evidence (see below) through the GRADE approach. It should be noted that on the basis of funnel plot asymmetry, we suspected publication bias for several outcomes, which led to downgrading of evidence quality.
Follow‐up involved radiological examination in 50 of 67 studies (74.6%). Four studies (6.3%) did not perform radiological assessment, and in 13 (20.3%) studies, it was unclear whether this was done. We believe that radiological confirmation is warranted to assess stone clearance, although review of images by (especially not‐blinded) radiologists may have introduced bias.
Because studies were conducted in a wide variety of countries worldwide over several continents (i.e. North America, Europe, and Asia), the results of this review are probably applicable worldwide.
Furthermore, we want to point out the potential issue of incomplete reporting of major adverse events.
Quality of the evidence
We used GRADE to assess the quality of evidence on a per‐outcome basis and frequently lowered the quality of evidence. The main issues that lowered our confidence in estimates of effect were study limitations, specifically unclear allocation concealment, lack of blinding resulting in performance and detection bias, and potential attrition bias.
Other issues that prompted frequent downgrading were clinically important inconsistency in study results, imprecision, and concerns over potential publication bias in the light of observed funnel plot asymmetry.
Last, most studies did not prospectively stratify for clinically important subgroups at the time of randomisation; therefore it is unclear whether prognostic balance existed. Therefore, the results of these secondary analyses must be interpreted with caution.
Potential biases in the review process
We searched with no language restrictions. However, despite our best efforts, which included contacting the principal investigators of existing studies, we may have missed some studies that were published in non‐indexed journals or were not published.
We investigated reporting bias using funnel plots, which showed asymmetry for stone clearance and major adverse events (Figure 4; Figure 5; Figure 6). We performed sensitivity analyses after excluding non‐placebo‐controlled trials and still found a favourable effect of alpha‐blockers on stone clearance when we suspected publication bias.
5.
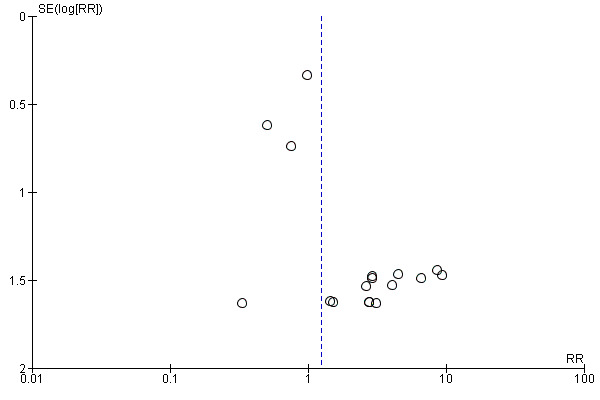
Funnel plot of comparison: 1 Alpha‐blocker versus standard therapy or placebo, outcome: 1.2 Major adverse events.
6.
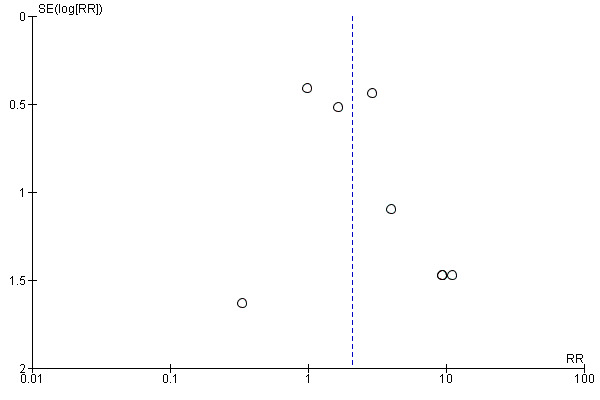
Funnel plot of comparison 2: Alpha‐blocker versus placebo, outcome: 2.2 Major adverse events.
Agreements and disagreements with other studies or reviews
Although many systematic reviews have examined this topic, very few have been conducted with the same methodological rigour that is standard for Cochrane Reviews. This review is the most up‐to‐date and includes the unpublished and relatively large study that was presented by Meltzer and colleagues at AUA 2017 (Meltzer 2017).
Most earlier trials examining alpha‐blockers for ureteral stones conclude that stone clearance and stone expulsion time are improved. From 2013 to 2015, multiple meta‐analyses were performed for different alpha‐blockers (tamsulosin, silodosin, and alfuzosin; Fan 2013; Huang 2015; Liu 2015, respectively) and those results were similar to findings discussed in the present review. In a meta‐analysis performed in 2016 by Hollingsworth and colleagues (Hollingsworth 2016), review authors came to similar principal findings as ours, stating that alpha‐blockers should be considered for use in individuals with ureteral stones. The Hollingsworth meta‐analysis included some multi‐centre placebo‐controlled randomised controlled trials (RCTs) that did not demonstrate beneficial effects of alpha‐blockers on stone clearance when compared with placebo (Furyk 2016; Pickard 2015; Sur 2015). We included these studies in the current meta‐analysis as well. Two of these trials involved a large percentage of individuals with small stones (75% of patients taking alpha‐blockers had stones measuring 5 mm or smaller) (Furyk 2016; Pickard 2015). However, because smaller stones are more likely to pass spontaneously (even without medical expulsive therapy), this fact could have influenced the overall effect noted in these studies (i.e. the potential benefits of medical expulsive therapy may have been diluted by the inclusion of smaller stones in these two studies). Closer analysis of the data of Furyk and colleagues reveals a favourable effect in the tamsulosin group versus the placebo group for stones measuring 5 to 10 mm. The present review endorses this finding, as included trials reported significant effects of alpha‐blockers in individuals with larger stones (6 mm or bigger) in terms of stone clearance and possible differences in effect detected by subgroup interaction testing.
The SUSPEND trial by Pickard and colleagues was designed to assess the clinical effectiveness of medical expulsive therapy rather than therapeutic efficacy, as radiological assessment was not a primary endpoint (Pickard 2015). Although these trial authors concluded that medical expulsive therapy is not effective in reducing the need for intervention at four weeks, differences in stone clearance were attenuated for stones larger than 5 mm, favouring tamsulosin as compared with nifedipine and placebo (71.3%, 61.7%, and 60.6%, respectively). Moreover, the response rate for both 4‐week (62%) and 12‐week questionnaires (49%) was considerably lower than that noted in primary outcome participation but could influence secondary outcome results. Investigators in the SUSPEND trial did not monitor medication adherence, and this could raise some concerns. Results of the SUSPEND trial may have led to changes in clinical practice in some countries. However, this is not consistent with findings derived from our meta‐analysis, which demonstrated a persistent beneficial effect of alpha‐blockers for distal ureteral stones larger than 5 mm. Consistent with the SUSPEND trial, data show no difference in surgical interventions between groups. In line with findings of the SUSPEND trial, a very small proportion of participants receiving alpha‐blockers experienced major adverse events, and even a smaller proportion discontinued treatment because of this.
Sur and colleagues found a positive effect of alpha‐blockers for individuals with distal ureteral stones upon performing a subgroup analysis (Sur 2015). This is in line with the findings of the present review (i.e. that the greatest effect of alpha‐blockers can be effectuated in the distal part of the ureter and can be achieved in patients with stones measuring 6 to 10 mm).
More recently, an abstract was presented at AUA 2017 that reported on a trial included in our meta‐analysis (Meltzer 2017). These trial authors also failed to demonstrate a favourable effect of alpha‐blockers. However, investigators assessed stone clearance through telephone calls and by participant reporting. About 50% of participants underwent follow‐up computed tomography (CT), revealing no difference in stone clearance between groups. In this trial, about 75% of participants had stones smaller than 5 mm; no results from any subgroup analyses based on stone size are available for this unpublished study. Apart from abnormalities of ejaculation, treatment‐related adverse events did not occur more often in the alpha‐blocker group.
The most recently published paper describes favourable effects of tamsulosin reported by Ye and colleagues for the largest trial yet (Ye 2017). This double‐blind, placebo‐controlled study, which included distal stones, demonstrated specific benefit of tamsulosin for larger stones (> 5 mm), although trial authors reported no results from testing for interaction. Data show no differences in the incidence of adverse events between the two study groups, and investigators reported no serious adverse events. These findings are supported by a unique feature of this study, in that participants underwent weekly non‐contrast computed tomography scans.
Authors' conclusions
Implications for practice.
Results of both the main analysis and a predefined subgroup analysis of placebo‐controlled studies indicate that alpha‐blockers improve stone clearance but may slightly increase the risk of major adverse events. Use of alpha‐blockers may also improve several other patient‐important outcomes. This information should provide valuable guidance to clinicians, patients, and guideline developers for decision‐making about the use of alpha‐blockers.
For clinical settings in which stone size is known, evidence from this review suggests that the effectiveness of alpha‐blockers is most often proportionate to stone size. Patients with larger stones (>5mm) will benefit most from an alpha‐blocker, because smaller stones often tend to pass spontaneously even without the use of an alpha‐blocker. This may have important implications for consideration of selective use of alpha‐blockers if, for example, stone size has been determined accurately by imaging. Meanwhile, we based on the test of interaction, we cannot confirm that stone clearance differs by stone location. Furthermore, we found no evidence that the effectiveness of alpha‐blockers on investigated outcomes differs by the type of alpha‐blocker used.
Implications for research.
Whereas most trials on medical expulsive therapy with alpha‐blockers were of low methodological quality, prompting us to downgrade analyses for study limitations, we have identified a subset of higher‐quality, placebo‐controlled studies. Through this approach, we found evidence of at least moderate quality for the outcome stone clearance. For these outcomes, future trials appear unlikely to change our understanding of the effects of alpha‐blockers and may not be needed.
Data show less certainty with regards to other outcomes. Moderate quality of evidence was found for the primary outcome major adverse events with conflicting results when selecting placebo‐controlled studies only (more adverse events in alpha‐blocker group) or high‐quality studies only (no difference in adverse events between both groups). Results on stone expulsion time, the need for pain medication, hospitalisation and need for surgical intervention are secondary outcomes with low to moderate quality of evidence. These outcomes should be investigated in future trials to further explore the effectiveness of alpha‐blockers on these parameters. In addition, some uncertainty surrounds the relative effectiveness of alpha‐blockers in terms of stone location. Future studies should be placebo‐controlled and should implement adequate allocation concealment, blinding of all relevant parties (participants, personnel, and outcome assessors), and complete or near‐complete follow‐up. In addition, studies should be stratified and adequately powered for any planned secondary analyses such as those related to stone size and location.
What's new
| Date | Event | Description |
|---|---|---|
| 18 November 2017 | New search has been performed | In this update we have added 35 new studies. GRADE approach has been applied to assess the quality of evidence. |
| 18 November 2017 | New citation required and conclusions have changed | In this update we have added 35 new studies. GRADE approach has been applied to assess the quality of evidence.The conclusion of the review has changed. |
History
Protocol first published: Issue 5, 2010 Review first published: Issue 4, 2014
| Date | Event | Description |
|---|---|---|
| 2 April 2014 | Amended | First published |
| 9 July 2012 | New search has been performed | Assessed as up‐to‐date |
Acknowledgements
The review authors thank Cochrane Urology and the referees for feedback and advice provided during preparation of this review. We also thank the authors of the included studies who responded to our written correspondence.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| Embase |
|
| ClinicalTrials.gov |
|
| Conference proceedings | 1. Proceedings of major (mainly renal and stone‐disease) conferences from 2005 to March 2016 2. EAU (European Association of Urology) 3. AUA (American Urological Association) 4. ESD (Experts in Stone Disease) 5. WCE (World Congress of Endourology) 6. SIU (Société Internationale d'Urologie) |
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomization method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomization; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomization stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomization; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse event); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Alpha‐blocker versus standard therapy or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stone clearance | 67 | 10509 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.36, 1.55] |
| 2 Major adverse events | 18 | 3124 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.80, 1.96] |
| 3 Stone expulsion time | 37 | 6031 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐4.17, ‐2.63] |
| 4 Pain episodes | 15 | 1363 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.91, ‐0.42] |
| 5 Dose of diclofenac | 14 | 4373 | Mean Difference (IV, Random, 95% CI) | ‐82.41 [‐122.51, ‐42.31] |
| 6 Hospitalisation | 13 | 1876 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34, 0.77] |
| 7 Surgical intervention | 19 | 3292 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.02] |
Comparison 2. Subgroup analysis 1: stones measuring 5 mm or less versus stones measuring 6 to 10 mm.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stone clearance | 16 | 5509 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.08, 1.27] |
| 1.1 Stone size ≤ 5 mm | 14 | 2622 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.15] |
| 1.2 Stone size 6‐10 mm | 10 | 2887 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.22, 1.72] |
| 2 Stone expulsion time | 6 | 3148 | Mean Difference (IV, Random, 95% CI) | ‐3.39 [‐5.82, ‐0.97] |
| 2.1 Stone size ≤ 5 mm | 6 | 1264 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.99, ‐0.15] |
| 2.2 Stone size 6‐10 mm | 4 | 1884 | Mean Difference (IV, Random, 95% CI) | ‐5.99 [‐7.16, ‐4.82] |
Comparison 3. Subgroup analysis 2: distal ureter versus mid or proximal ureter stones.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stone clearance | 60 | 9370 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.34, 1.54] |
| 1.1 Distal ureter | 57 | 8576 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.36, 1.57] |
| 1.2 Mid or proximal ureter | 9 | 794 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.99, 1.66] |
| 2 Stone expulsion time | 32 | 5599 | Mean Difference (IV, Random, 95% CI) | ‐3.67 [‐4.50, ‐2.85] |
| 2.1 Distal ureter | 32 | 5453 | Mean Difference (IV, Random, 95% CI) | ‐3.43 [‐4.26, ‐2.60] |
| 2.2 Mid or proximal ureter | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐8.64 [‐19.75, 2.48] |
Comparison 4. Subgroup analysis 3: type of alpha‐blocker.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stone clearance | 67 | 10897 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.39, 1.58] |
| 1.1 Tamsulosin | 54 | 8896 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.37, 1.59] |
| 1.2 Alfuzosin | 6 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.14, 2.01] |
| 1.3 Doxazosin | 7 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.29, 1.72] |
| 1.4 Terazosin | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.10, 1.89] |
| 1.5 Naftopidil | 3 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.01, 4.03] |
| 1.6 Silodosin | 6 | 780 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.20, 1.61] |
| 2 Major adverse events | 18 | 3003 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.12, 3.48] |
| 2.1 Tamsulosin | 13 | 2062 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.62, 2.47] |
| 2.2 Alfuzosin | 5 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 5.51 [1.46, 20.83] |
| 2.3 Doxazosin | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 3.84 [0.47, 31.11] |
| 2.4 Silodosin | 3 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 6.25 [0.74, 52.57] |
| 2.5 Naftopidil | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Stone expulsion time | 35 | 6212 | Mean Difference (IV, Random, 95% CI) | ‐3.56 [‐4.25, ‐2.87] |
| 3.1 Tamsulosin | 30 | 5052 | Mean Difference (IV, Random, 95% CI) | ‐3.07 [‐4.04, ‐2.09] |
| 3.2 Alfuzosin | 3 | 180 | Mean Difference (IV, Random, 95% CI) | ‐4.75 [‐6.63, ‐2.88] |
| 3.3 Doxazosin | 4 | 263 | Mean Difference (IV, Random, 95% CI) | ‐4.73 [‐6.15, ‐3.32] |
| 3.4 Terazosin | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐4.42 [‐5.36, ‐3.48] |
| 3.5 Naftopidil | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐2.80, ‐0.80] |
| 3.6 Silodosin | 4 | 512 | Mean Difference (IV, Random, 95% CI) | ‐4.98 [‐6.43, ‐3.52] |
| 4 Pain episodes | 15 | 1595 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐0.89, ‐0.52] |
| 4.1 Tamsulosin | 13 | 992 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.98, ‐0.41] |
| 4.2 Alfuzosin | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.82, 0.18] |
| 4.3 Doxazosin | 3 | 190 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐0.92, ‐0.60] |
| 4.4 Terazosin | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.16, ‐0.17] |
| 4.5 Naftopidil | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.23, ‐0.37] |
| 4.6 Silodosin | 2 | 150 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐2.05, 0.26] |
| 5 Dose of diclofenac [mg] | 14 | 4429 | Mean Difference (IV, Random, 95% CI) | ‐78.11 [‐113.25, ‐42.97] |
| 5.1 Tamsulosin | 12 | 4135 | Mean Difference (IV, Random, 95% CI) | ‐87.60 [‐131.98, ‐43.21] |
| 5.2 Doxazosin | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐63.46 [‐72.88, ‐54.04] |
| 5.3 Terazosin | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐64.29 [‐74.17, ‐54.41] |
| 5.4 Silodosin | 2 | 181 | Mean Difference (IV, Random, 95% CI) | ‐54.72 [‐89.91, ‐19.53] |
| 6 Hospitalisation | 13 | 2028 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.29, 0.66] |
| 6.1 Tamsulosin | 11 | 1606 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.86] |
| 6.2 Alfuzosin | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.09, 1.45] |
| 6.3 Doxazosin | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.05] |
| 6.4 Silodosin | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.12, 0.55] |
| 7 Surgical intervention | 19 | 3404 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 0.99] |
| 7.1 Tamsulosin | 16 | 2820 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.59, 1.13] |
| 7.2 Alfuzosin | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.09, 2.35] |
| 7.3 Doxazosin | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.15, 1.11] |
| 7.4 Silodosin | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.31] |
Comparison 5. Sensitivity analysis 1: alpha‐blocker versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stone clearance | 15 | 5787 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.07, 1.25] |
| 2 Major adverse events | 10 | 1650 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.13, 3.86] |
| 3 Stone expulsion time | 7 | 3240 | Mean Difference (IV, Random, 95% CI) | ‐1.98 [‐3.71, ‐0.24] |
| 4 Pain episodes | 2 | 215 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐1.07, 0.29] |
| 5 Dose of diclofenac | 4 | 3576 | Mean Difference (IV, Random, 95% CI) | ‐126.32 [‐181.73, ‐70.90] |
| 6 Hospitalisation | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.48, 1.47] |
| 7 Surgical intervention | 5 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.24] |
Comparison 6. Sensitivity analysis 2: high‐quality studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stone clearance | 5 | 4133 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.06, 1.13] |
| 2 Major adverse events | 2 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.51, 1.72] |
| 3 Stone expulsion time | 3 | 2891 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐5.13, 1.68] |
| 4 Pain episodes | 1 | 119 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.72, 0.72] |
| 5 Dose of diclofenac | 2 | 3386 | Mean Difference (IV, Random, 95% CI) | ‐173.28 [‐277.60, ‐68.95] |
| 6 Hospitalisation | 1 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.49, 1.52] |
| 7 Surgical intervention | 3 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.38, 2.32] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Meguid 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Baseline assessment included non‐contrast spiral CT and furthermore weekly follow‐ups with KUB and US. NCCT was repeated at end of study to confirm stone status. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated randomly in 1:1 ratio into either group." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Using sealed envelopes." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both treating physicians and patients were blinded to randomization." Comment: double‐blind and therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of outcome assessments described. Comment: No description was available; therefore this method of outcome assessment was considered to have unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "17 patients were excluded due to not accepting randomization (6) and loss to follow‐up during study period (4 in group A and 7 in group B)." Comment: Owing to relatively high numbers of exclusions and losses to follow‐up, incomplete outcome data were stated and risk of bias was unclear. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias therefore was considered to be low. |
| Other bias | Low risk | No quotes available. The study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Agrawal 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
All groups received 75 mg diclofenac intramuscularly and were advised to drink at least 3 L of fluids. |
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Follow‐up weekly with KUB and US. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomized to 3 groups." Comment: Randomisation was stated, but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, risk of allocation concealment was considered to be unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Spontaneous stone expulsion was observed in 28 of 34 patients (82.3%) in group 1, 24 of 34 (70.5%) in group 2, and 12 of 34 (35.2%) patients in group 3.” Comment: Stone expulsion rate was reported for all participants; therefore, probably none were lost to follow‐up and accordingly risk of bias was considered low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. The study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Ahmad 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Placebo group
Both groups were given tab diclofenac sodium 50 mg, 1 tab 8 hourly for pain control on required basis. |
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Participants were evaluated with plain X‐ray KUB after 2 weeks and 4 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned into one of the two groups." Comment: Randomisation was stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, risk of allocation concealment was considered to be unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Three patients lost to follow up, therefore 97 out of 100 patients were evaluated.” Comment: Small number of participants were lost to follow‐up; accordingly, risk of bias was considered low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered low. |
Ahmed 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
All groups received 50 mg diclofenac every 12 hours for 1 week, then 75 mg diclofenac intramuscularly as needed up to 2 times per day. |
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Follow‐up visits on a weekly basis with urine analysis, serum creatinine measurement, plain X‐ray KUB, and abdominal ultrasonography. Abdominal CT was performed for participants with radiolucent stones if the stone was not expulsed by the end of study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A total of 90 patients with distal ureteral stones ≤10 mm in diameter were randomly divided into 3 equal groups and given medications for 30 days." Comment: Randomisation was stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No quotes available. No intention‐to‐treat analysis. Comment: Owing to lack of intention‐to‐treat analysis, risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Al Ansari 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Sample size calculated. Weekly follow‐up with KUB, US, and urine analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized between study and placebo medications using a computer‐generated random number assignment, adjusted at a ratio of 1:1." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization data were kept strictly confidential, in sealed envelops, accessible only to the pharmacist at the end of the study." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigators and patients were masked to the type of the treatment throughout the study." Comment: double‐blind; therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The investigators and patients were masked to the type of the treatment throughout the study.” Comment: Personnel responsible for outcome assessments were blinded; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “Four patients in the placebo group were lost to follow‐up.” Comment: Four participants in the placebo group were lost to follow‐up and were excluded from analysis. It is unclear whether this had a clinically relevant impact on the intervention effect estimate; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias therefore was considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered low. |
Albert 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Follow‐up visits on a weekly basis with urine analysis, serum creatinine measurement, plain X‐ray KUB, and abdominal ultrasonography. Abdominal CT was performed for all participants by end of study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quote available. Comment: Randomisation was stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Out of the 120 patients included in the study, patients were divided randomly into three groups of 40 patients." Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Blood pressure was not reported in the Results section (as a secondary outcome measurement); therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered low. |
Aldemir 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Participants in groups 1 and 2 received diclofenac when needed (100 mg once daily). |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Missing data on stone expulsion rate. Follow‐up was 10 days, then again KUB, US, or CT. All participants were suggested to drink at least 2 L of drinking water daily. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomized in 3 groups.” Comment: Randomisation was stated, but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: No predefined endpoints were mentioned; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered low. |
Alizadeh 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Control group
Treatment group
Researchers emphasised that all participants should drink 2 L of water daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up was every 2 weeks with KUB, US, or CT. Not all abbreviations were explained in the text. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomly assigned to a control group (n = 46) and study group (n = 50).” Comment: Randomisation was stated but no information on method used was available; therefore selection bias was considered to have unclear risk. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No quotes available. No intention‐to‐treat analysis. Comment: Owing to lack of intention‐to‐treat analysis, risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Results on pain episodes were not reported; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore we considered risk of other bias to be low. |
Arrabal‐Martin 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Control group
Treatment group
Researchers emphasised that all participants should drink 2 L of water daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Assessment of trial results was conducted at 10 days and 30 days. On day 30, stone expulsion was assessed by plain X‐ray, ultrasonography, or urography. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were divided randomly into 2 treatment groups.” Comment: Randomisation was stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Autorino 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Aescin = standard therapy at this centre. Included participants from the study by Autorino et al 2005. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization, not blinded, was performed using a stratified permuted randomization algorithm.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: All participants were included in the analysis; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Ayubov 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “were randomly divided into.” Comment: Randomisation was stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Unclear risk | No quotes available. Baseline data are missing. Comment: Owing to lack of information at baseline, risk of other bias was considered to be unclear. |
Bajwa 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients satisfying the inclusion criteria were randomly divided in to 2 groups by random number table.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Primary outcomes were not prespecified; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Balci 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
All participants were encouraged to maintain water intake of 2‐2.5 L/d. |
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed using the Power Analysis & Sample Size Software (PASS_ ) for Windows (NCSS Inc., Kaysville, UT). According to the software, the patients were randomly assigned to one of the following three groups.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Bayraktar 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "randomly divided into three groups according to the order of arrival (1:1:1 ratio)." Comment: This method of random sequence generation was considered to have high risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all tomography and direct urinary system graphy of the patients were analyzed and confirmed by a urologist blinded to the group of the patients." Comment: Risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 13 patients, 6 in group 1, 4 in group 2, and 3 in group 3, were excluded from the study because they were lost in follow‐up. 5 patients in group 1 and 3 patients in group 3 were also excluded from the study because they declared more than one sexual intercourse or masturbation per week." Comment: 21/211 participants were lost to follow‐up and were equally divided over the 3 groups; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | High risk | No quotes available. Only males were included in the study. Comment: Potential selection bias; therefore high risk of other bias. |
Berger 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Placebo group
|
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "using a convenience sample." Comment: This method of random sequence generation was considered to have high risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blinded, placebo‐controlled." Comment: double‐blind; therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 127 patients enrolled during this study, 15 were lost to follow‐up, and 12 received surgical intervention before the 7‐day mark, leaving 100 patients for analysis." Comment: Owing to a reasonable number of participants lost to follow‐up, risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Cervenakov 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
All participants were made to keep a drinking regimen of at least 2.5 L of water or weak tea daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Study authors stated the study model was a double blind RCT but described no blinding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "we have applied a double blind randomized study." Comment: Randomisation was stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "As two pts. were excluded from group “A” because during treatment they developed acute obstruction pyelonephritis, both groups consisted of a rather unusual identical number of experimental and standardly treated pts. (51)." Comment: In the light of the small number of participants lost to follow‐up, risk of attrition bias was stated as low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Cha 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | All participants received trospium chloride (50 mg 3 times daily). All participants received initial treatment of 90 mg diclofenac by intramuscular injection and 5 mg cimetropium bromide by intravenous injection, with a second dose after 30 minutes or 1 hour if necessary. Treatment group 1
Treatment group 2
Treatment group 3
Control group
|
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomization list was generated by using the permuted block method.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: “... was concealed from the patient‐enrolling investigators (confined with a doctor assisting in the procedure but not participating in the study).” Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Cho 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Placebo group
|
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Participants were followed up at days 14 (visit 1), 28 (visit 2), 60 (visit 3), and 90 (visit 4) after initiation of medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out by the Medical Research Collaboration Center of Seoul National University Bundang Hospital using random permuted blocks of different sizes. The size of the next block was randomly chosen from the available block sizes." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "The size of the next block was randomly chosen from the available block sizes. Randomization was stratified by each recruiting study site." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "One person packed the 14‐day supply of tablets for each patient. All study staff at all hospitals were blinded to treatment allocation and remained blind until the end of the trial." Comment: double‐blind; therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Figure 1 shows a detailed flow diagram of participant follow‐up, including information regarding loss to follow‐up, exclusion due to adverse events, or need for intervention. Comment: Detailed information on participant follow‐up was available; risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Information on primary and secondary outcomes was presented in detail in the protocol at www.clinicaltrials.gov. Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Doluoglu 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Follow‐up visits on a weekly basis with urine analysis, plain X‐ray KUB, and abdominal ultrasonography. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients were randomly divided into 3 groups with the random number table envelope method.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: “The names of the groups were written on small papers with the same size, they were folded, put in an envelope, and drawn by the patients.“ Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quote available. Participants were blinded, and blinding of doctors was not described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “On follow‐up, plain urinary tract x‐ray images of the patients were seen and analyzed by an urologist (MFK) blinded to the group of the patients.“ Comment: Risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No quote available. 13/57 participants lost to follow‐up equally distributed among control and treatment groups. Comment: Owing to a reasonable number of participants lost to follow‐up (> 20%), risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | High risk | No quote available. No number of pain episodes described in the Results section (although this was a secondary outcome measurement). Furthermore adverse effects were predefined in Methods section and were not described in Results section. Comment: Owing to inconsistent reporting, risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Dong 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group (group 3)
Control group (group 4)
Four treatment arms, of which the first 2 underwent ESWL (groups 1 and 2); groups 3 and 4 did not want to undergo ESWL. |
|
| Outcomes |
|
|
| Funding sources | Study was supported by research funds from Astellas Phama Korea, Inc. | |
| Declarations of interest | None stated. | |
| Notes | Dong Il Kang; only the abstract was written in English and therefore was interpretable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No quotes available. Comment: Participants were those who did not want to undergo ESWL; therefore risk of selection bias was considered to be high. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, risk of allocation concealment was considered to be unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
El Said 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group (group 2)
Control group (group 1)
All participants were asked to drink > 2 L of water daily and received diclofenac 75 mg IM on demand. |
|
| Outcomes |
|
|
| Funding sources | Welcome Trust: Howard Hughes Medical Institute and others. | |
| Declarations of interest | None. | |
| Notes | Article 2015. Conference abstract 2014. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomly assigned to either the control group or the alfuzosin group based on a computer‐generated random table.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “This was a prospective, randomized, open–label, controlled study.” Comment: open label trial; therefore risk of performance bias was considered to be high. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “This was a prospective, randomized, open–label, controlled study.“ Comment: open label trial; therefore risk of detection bias was considered to be high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quote available. Comment: All participants were included in the analysis; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
El‐Gamal 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1: placebo group. Group 2: tamsulosin group. Group 3: Uralyt group. Group 4: Uralyt and tamsulosin group. All participants were advised to increase fluid intake to more than 2 L per day and to receive IM injection of 75 mg of diclofenac sodium. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Non‐enhanced spiral CT was done at the end of study period for all participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were prospectively randomized through a computer generated randomization process into four equal groups.” Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: “The investigators and the patients were blinded to the treatment given, until the end of the study.” Comment: Randomisation method was described and would not allow investigator/participant to know or influence the intervention group before eligible participant entered into the study. Therefore this method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The investigators and the patients were blinded to the treatment given, until the end of the study.” Comment: double‐blind; therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “5 cases were lost to follow‐up (2 in the control group and one in each of the other three groups).” Comment: Owing to the small number of participants lost to follow‐up, risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Primary outcomes were not prespecified; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
Erkan 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Group 1: tamsulosin group. Group 2: corticosteroid group. Group 3: tamsulosin and corticosteroid group. Group 4: control group. Data for groups 1 and 4 were used for the review. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. Follow‐up on weekly basis with imaging (not discussed in detail). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “A total of 134 patients with distal ureteral stone of 4‐10 mm were randomized into 4 groups.” Comment: Randomisation stated but no information on method used was available; therefore selection bias was considered to be at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No quotes available. 6 patients in control group lost to follow‐up. Comment: losses to follow‐up not balanced between treatment groups; therefore risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
Erturhan 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1:
Treatment group 2:
Treatment group 3:
Control group:
All participants were treated with prophylactic antibiotic therapy (cefuroxime axetil 250 mg (once a day)) and received 2500 mL hydration daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Weekly checkups and follow‐up with renal ultrasonography, complete urinalysis, serum urea creatinine measurements, and direct urinary system graphics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After randomization to one of four groups, the patients received treatment.” Comment: Owing to insufficient information, random sequence generation was considered to be at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. 5 participants were lost to follow‐up; they were included in the analysis. Comment: Owing to the small number of participants lost to follow‐up, risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Eryildirim 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Control group
Tamsulosin group
|
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “simple randomization method by generating a random digit (0–60 in each group) has been used within each group.” Comment: This method of random sequence generation was considered to be at low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, risk of allocation concealment was considered to be unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No quotes available. Comment: Losses to follow‐up were not clearly described; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Ferre 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Funding sources | This study was funded by an academic grant from the Maine Medical Center Mentored Research Committee. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up with all participants at 2, 5, and 14 days post discharge from the ED. Sample size calculated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was accomplished by using a table of random numbers.” Comment: This method of random sequence generation was considered to be at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: “The information on group assignment was contained in a sealed envelope within each study packet, and the envelopes were clearly labelled “do not open until informed consent is obtained.” Comment: This method of allocation concealment was considered to be at low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Participants lost to follow‐up (4), discontinued intervention (2), excluded because the stone was located in the proximal ureter (1). Comment: Losses to follow‐up were balanced across treatment groups; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Furyk 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Placebo group
Analgesia was given at the discretion of the treating physician. |
|
| Outcomes |
|
|
| Funding sources | Grant from the Queensland Emergency Medicine Research Foundation. | |
| Declarations of interest | None stated. | |
| Notes | Power calculation was performed. Intention‐to‐treat‐principle. Telephone contacts at 7, 14, 21, and 28 days with pelvic non‐contrast CT at day 28. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was produced with a computer‐generated program in permuted blocks of random lengths stratified by hospital and stone size." Comment: This method of random sequence generation was considered to be at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Once informed consent was obtained and patients were deemed appropriate for discharge, they were allocated to the next sequentially numbered study medication pack." Comment: This method of allocation concealment was considered to be at low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Investigators, the treating physician, and patients were blinded to the allocation for the duration of the study and data analysis." Comment: double‐blind; therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Investigators, the treating physician, and patients were blinded to the allocation for the duration of the study and data analysis.” Comment: Personnel responsible for outcome assessments were blinded; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Similar proportions of patients with missing and nonmissing outcome data." Comment: Missing outcome data were balanced in numbers across both groups, and reasons for missing data were similar; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Georgescu 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up at 14 ± 2 days with urinalysis, KUB, and ultrasonography. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization process was achieved by means of sealed envelopes equally nominating one of the three treatment alternative." Comment: This method of random sequence generation was considered to be at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was performed using the SNOSE method (sequentially‐numbered, opaque, sealed envelopes)." Comment: This method of allocation concealment was considered to be at low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Griwan 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Successful results were defined as complete stone passage; failure was considered if:
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Follow‐up weekly with KUB. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... were divided randomly." Comment: Randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: All participants completed the study; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Outcomes were not prespecified; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Han 2006a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
All participants were allowed 30 mg ketorolac IM injections on demand. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Only English abstract available for judgement. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No quotes available. Comment: Losses to follow‐up were not clearly described; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
Hermanns 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Sample size calculated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Enrolled patients underwent randomisation in a 1:1 fashion in blocks of 10 to receive either a daily single dose of tamsulosin (0.4 mg) or placebo. The sequence of randomisation was computer generated and was performed by the university hospital pharmacy using DatInf Randlist software v.1.0 (DatInf GmbH, Tübingen, Germany)." Comment: This method of random sequence generation was considered to be at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation data were kept strictly confidential in sealed envelopes, accessible only to the primary and senior investigator." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patient, the attending urologist were not aware of study arm assignments until the final assessment of outcome." Comment: double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Personnel responsible for outcome assessments were blinded." Comment: double‐blind; therefore low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Patients were included in the final analysis on an intention‐to‐treat basis. Patients who experienced stone expulsion before first medication, who withdrew their consent, or who were lost to follow‐up were excluded from the analysis." Comment: missing outcome data and losses to follow‐up balanced in numbers across both groups; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Hong 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. Follow‐up time: 2 weeks, visits at 1st and 2nd weeks after medication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No quotes available. 61 patients excluded from the study; no details available. Comment: large number of patients excluded from the study without clarification. Therefore, risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Unclear risk | No quotes available. No information on baseline data. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
Ibrahim 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Control group/Study group I
Treatment group 1/Study group II
Treatment group 2/Study group III
|
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Follow‐up weekly for 4 weeks; every visit focussed history, physical examination, and urinary US. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised systematically at a ratio of 1:1." Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No quotes available. Comment: Numbers of participants lost to follow‐up in the different study groups were not equally distributed; therefore risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Islam 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
All participants received 2500 mL hydration daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up weekly with renal ultrasonography, X‐ray KUB, urinalysis, and serum creatinine measurements. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: Low percentage of loss to follow‐up in each group (< 5%); therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Mean diclofenac sodium dosage and drug adverse events were not described as outcome parameters in the Methods section; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Itoh 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Control group
Treatment group
All participants were instructed to drink 2 L of water daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No quotes available. Comment: Loss to follow‐up was not described; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | High risk | No quotes available. Need for analgesics was one of the outcome parameters but was not described in Results section. Adverse events were described in Results section but were not stated as one of the outcome parameters. Comment: Owing to inconsistency in describing outcome parameters, risk of reporting bias was considered to be high. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
Itoh 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Study group A/Control group
Study group B/Treatment group
All participants were instructed to drink 2 L of water daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None. | |
| Notes | Diagnostics for follow‐up were not described. Conference abstract in 2015. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly divided into two groups by using a random number table envelope method." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: 1 participant lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | High risk | No quotes available. Stone size was significantly different between the 2 study groups. Furthermore, only men were included in the study. Comment: Owing to potential selection bias, we considered judgement as having high risk of bias. |
Kaneko 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
50 mg diclofenac suppository on demand for all participants. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up: maximum of 4 weeks, weekly or biweekly X‐ray and US. Sample size was arbitrarily determined and was not based on statistical calculation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated into two treatment groups by using a random number table." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: 6 participants were excluded from the study and were balanced between intervention groups; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Kim 2007b.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
All participants were instructed to ingest at least 2 L of fluids daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No quotes available. Comment: Loss to follow‐up was not described; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
Kupeli 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group (group 2)
Control group (group 1)
Four‐arm study, of which groups 3 and 4 underwent SWL. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up only at the 15th day (last day of study) with plain abdominal X‐ray and helical CT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the coin method." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patient follow‐up examinations were performed by two of us who were unaware of the treatment received." Comment: Investigators evaluating follow‐up examinations were blinded; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: No participants were lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Lee 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Study group A/Control group
Study group B/Treatment group
All participants were asked to drink 2 L water daily. |
|
| Outcomes |
|
|
| Funding sources | None. | |
| Declarations of interest | None. | |
| Notes | Sample size calculation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A predefined randomization sequence was created by a computer random number generator using a block size of 4." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This prospective, randomized, open‐label, multicenter trial." Comment: Open‐label trial is considered to have high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No quotes available. Comment: significant number of participants lost to follow‐up. Loss to follow‐up was not balanced between intervention groups; therefore risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Liatsikos 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Control group 1
Treatment group 2
Control group 2
All participants were advised to consume at least 2 L of water daily and were allowed to use symptomatic therapy of 75 mg of diclofenac on demand. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The type of therapy (doxazosin or not) was determined according to the day the patient presented to the hospital, with every patient who presented on odd‐numbered days being assigned to take doxazosin." Comment: This method of random sequence generation was considered to have high risk of bias. |
| Allocation concealment (selection bias) | High risk | No quotes available. Open random allocation schedule. Comment: This method of allocation concealment was considered to have high risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "unblinded." Comment: no blinding; therefore risk of performance bias was considered to be high. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Although mentioned as outcome measurement in the Methods section, total dosage of diclofenac use was not described in the Results section; therefore risk of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Lojanapiwat 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
If participants developed renal colic during treatment, they were given an IM injection of 75 mg diclofenac in the emergency department. |
|
| Outcomes |
|
|
| Funding sources | Astellas Pharma (Thailand). | |
| Declarations of interest | None. | |
| Notes | Sample size calculated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Block randomized in three groups by the assistant nurse." Comment: This method of random sequence generation was considered to have unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No quote available. Comment: no blinding; therefore risk of performance bias was considered to be high. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: No participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Lojanapiwat 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Control group/Study group I
Tamsulosin group/Study group 2
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. Data on stone clearance and stone relocation rate at 4 weeks were not described separately. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No quotes available. Comment: Loss to follow‐up was not described; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
Maitra 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1/tamsulosin group
Treatment group 2/tamsulosin + nifedipine group
Placebo group
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None. | |
| Notes | Follow‐up weekly with KUB and US. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Occurrence of adverse effects was not predefined as outcome measurement. Time to stone expulsion was not measured with SDs. Number of colic episodes was not specified. Analgesic use was not described in detail (no SDs given). Comment: inconsistency in describing outcome measurements; therefore risk of bias was considered to be high. |
| Other bias | High risk | No quotes available. Comment: questionable whether this study was placebo‐controlled based on information provided in the Methods section; therefore risk of other sources of bias was considered to be unclear. |
Meltzer 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1/tamsulosin group
Placebo group. |
|
| Outcomes |
|
|
| Funding sources | Research reported in this abstract was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number 3 U01 DK 096037. Content is solely the responsibility of the trial authors and does not necessarily represent the official views of the National Institutes of Health. |
|
| Declarations of interest | None. | |
| Notes | 5 telephone calls during trial period (day 2, day 7, day 15, day 20, day 29). Follow‐up CT in a certain group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No quotes available. Blinding of both participants and personnel. Comment: Double‐blinding was performed; therefore risk of performance bias was considered to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: Only a small number of participants were lost to follow‐up with clear description; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
Mshemish 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1/tamsulosin group
Treatment group 2/doxazosin group
Control group
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up weekly with urine analysis, serum creatinine measurement, KUB, and US NCCT at the end of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. Blinding was not described in detail. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No quotes available. Comment: no intention‐to‐treat analysis used and 5 participants lost to follow‐up; therefore risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | High risk | No quotes available. Comment: Owing to incorrect data for stone clearance stratified for stone size, information is not interpretable and risk of other sources of bias was considered to be high. |
Mukhtarov 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Control group 1
Treatment group 2
Control group 2
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. Maximum follow‐up in group 1: 28 days. Weekly re‐evaluation with X‐ray and US. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: No participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered to be unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
Ochoa‐Gomez 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Study group A/Treatment group
Study group B/placebo group
All participants were instructed to drink at least 2 L of water per day and to carry out normal activities. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up every 14 days with plain abdominal film and abdominal ultrasonogram. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No quotes available. Blinding of both participants and personnel. Comment: Double‐blinding was performed; therefore risk of performance bias was considered to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. No imaging at the end of follow‐up was done to evaluate stone clearance. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Park 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Study group A/control group
Study group B/treatment group
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up weekly with KUB or CT. Primary endpoint was cumulative stone passage rate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Not all participants received CT scan at the end of the trial period. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No quotes available. Comment: a significant number of participants lost to follow‐up (20%‐30%); therefore risk of attrition bias was considered to be high. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
Pedro 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Funding sources | Sanofi‐Aventis Pharmaceuticals. | |
| Declarations of interest | None stated. | |
| Notes | Sample size calculated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... using a random number assignment. A computerized random number generator was used." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | No quotes available. Comment: Allocation was concealed; therefore the allocation method was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Investigators were blinded to the randomization scheme and patients and investigators were blinded to medication until termination of the study.” Comment: Double‐blinding was performed; therefore risk of performance bias was considered to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. 7 patients excluded: stone passage before first dose in 4. and withdrawal of consent in 3. Comment: small number of participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Pickard 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1/tamsulosin group
Treatment group 2/nifedipine group
Control group
Standard pain medication ‐ not described in detail. |
|
| Outcomes | Primary outcome measurements
Secondary outcome measurements
|
|
| Funding sources | UK National Institute for Health Research Health Technology Assessment Programme. | |
| Declarations of interest | Trial author JN is a member of the HTA commissioning board and the NIHR HTA and Efficacy and Mechanism Evaluation editorial board. All other trial authors declare no competing interests. | |
| Notes | Sample size calculation. Follow‐up at 4 and 12 weeks with questionnaires, case report forms during clinical visits, or telephone contact. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... by a remote randomisation system." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "... supplied by an independent Source." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, clinicians, and trial personnel remained unaware of the allocated group." Comment: Double‐blinding was performed; therefore risk of performance bias was considered to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No quotes available. Comment: Personnel responsible for outcome assessments were blinded; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: Small number of participants lost to follow‐up equally balanced between treatment groups; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Unclear risk | No quotes available. Comment: Time to stone passage was evaluated in only a small portion of the study group (potential selection bias); therefore risk of other bias was considered to be unclear. |
Porpiglia 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
All participants received first treatment with 500 mL saline and analgesic (diclofenac or tramadol). All participants were allowed to use symptomatic therapy with IM injections of diclofenac (on demand) and were instructed to drink a minimum of 2 L of water daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Treatment on an "intention‐to‐treat" basis. Sample size calculated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Not all participants received CT scan at the end of the trial period. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: small number (3) of participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Porpiglia 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
All participants were allowed to use therapy to alleviate symptoms, that is, IM injections with 75 mg diclofenac on demand, and were required to drink ≥ 2 L of water daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None. | |
| Notes | Sample size calculated. Follow‐up only at the end of the study (11th day). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Rahim 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Follow‐up weekly with US. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using random number table." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: No participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Total dosage of diclofenac used was not described in Results section, although it was mentioned as an outcome measurement in the Methods section. Owing to this inconsistency in describing outcome measurements, this type of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Sayed 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Every week, a clinical examination was performed and KUB X‐rays and abdominal US were obtained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, risk of allocation concealment was considered to be unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: No participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Sen 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None. | |
| Notes | Weekly follow‐up urine analysis and radiological assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed with the MedCalc statistical software." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: No participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Stone clearance rate and stone clearance time were not predefined explicitly in the Methods section. Owing to this inconsistency in describing outcome measurements, this type of reporting bias was considered to be unclear. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Sun 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
All participants were instructed to drink a minimum of 2 L water daily. An indomethacin suppository was used to control acute episodes of ureteral colic, if present. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Naftopidil = selective alpha‐1D. Ultrasonography and KUB were performed on days 7 and 14. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | High risk | No quotes available. Comment: Number of pain episodes and requirement for pain medication were not reported explicitly. Owing to this inconsistency in describing outcome measurements, risk of this type of reporting bias was considered to be high. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Sur 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Placebo group
All participants received pain medication (oxycodone 5 mg). Other medication that would not confound study results was permitted and documented. |
|
| Outcomes | Primary outcome variables
Secondary outcome variables
|
|
| Funding sources | Actavis Inc. supported the study. | |
| Declarations of interest | None stated. | |
| Notes | Power calculation, intention‐to‐treat‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... using a randomization schedule prepared by a nonstudy statistician." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Each kit had a unique sequential number." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigator, patient, and study team were blinded to treatment assignment throughout the study." Comment: Double‐blinding was performed; therefore risk of performance bias was considered to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No quotes available. Comment: Personnel responsible for outcome assessments were blinded; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: Numbers of participants lost to follow‐up in the different study groups were equally distributed; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Taghavi 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Conference abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No quotes available. Comment: Loss to follow‐up was not described; therefore risk of attrition bias was considered to be unclear. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of reporting bias was considered unclear. |
| Other bias | Unclear risk | No quotes available. Comment: Owing to insufficient information to permit judgement, risk of other sources of bias was considered to be unclear. |
Vincendeau 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups received ketoprofen 50 mg, 3 capsules daily, and phloroglucinol 80 mg, 6 tablets daily, for 5 days. All participants were told to drink at least 2 L water daily and to filter urine. |
|
| Outcomes |
|
|
| Funding sources | Financed by the French Ministry of Health and Yamanouchi Pharmaceutical Co Ltd. | |
| Declarations of interest | Dr. Vincendeau is an investigator for Astellas Pharma Inc, AstraZeneca, Beckman‐Coulter/Hybritech Inc, and Pfizer Incorporated. | |
| Notes | Follow‐up of 42 days. Evaluation with X‐ray and US on days 1, 14, 28, and 42. Telephone contact on days 21 and 35. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was centrally performed, concealed, and stratified by center in blocks of 4 according to a computer generated random number table." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "... sequentially numbered boxes containing the whole treatment for each patient following the order of the randomization list." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All patients, health medical and nursing staffs, and pharmacists remained masked throughout the study period." Comment: Double‐blinding was performed; therefore risk of performance bias was considered to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No quotes available. Comment: Personnel responsible for outcome assessments were blinded; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: missing outcome data balanced in numbers across both groups, with similar reasons for missing data across groups; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Wang 2008a.
| Methods |
|
|
| Participants |
|
|
| Interventions |
All participants were prescribed ketorolac 10 mg, 3 times/d, allowed to use sublingual buprenorphine 0.3 mg as needed, and were required to drink a minimum of 2 L water/d. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up with KUB and abdominal US. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... randomly divided into three groups using the statistical software programs Plus 1.0 and Plus 2.10." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Ye 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Placebo group
|
|
| Outcomes | Primary endpoints
Secondary endpoints
|
|
| Funding sources | Supported by health industry special scientific research projects, Ministry of Health of China (201002010). Astellas Pharma supported this study and was involved in preparation of the manuscript. No financial disclosures. |
|
| Declarations of interest | None. | |
| Notes | Weekly follow‐up with CT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization and double blinding were performed according to the randomization sequence, which was produced with a computer‐generated program." Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered study e‐packs were securely stored at each study center using a password‐protected computer database and were known only to the trial designer and statistician." Comment: This method of allocation concealment was considered to have low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The investigator, participants, care providers, and those assessing outcomes were blinded to treatment assignment throughout the trial, as the randomization list was generated by an independent statistician and was not available until the study analysis." Comment: double‐blind; therefore low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The investigator, participants, care providers, and those assessing outcomes were blinded to treatment assignment throughout the trial, as the randomization list was generated by an independent statistician and was not available until the study analysis." Comment: blinding of outcome assessment; therefore risk of detection bias was considered to be low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Figure 1 shows the trial profile with a detailed schedule of participant follow‐up including information regarding loss to follow‐up, withdrawal percentage, etc. Intention‐to‐treat analysis was used. Comment: Detailed information on participant follow‐up was available; risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Yencilek 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group/group 1
All participants were instructed for adequate hydration. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Follow‐up of 4 weeks. Evaluation with X‐ray, US, or NCCT when stone passage occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Yilmaz 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
All participants were allowed to use symptomatic therapy with injections of 75 mg diclofenac on demand and were required to consume a minimum of 2 L of water daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Each participant was controlled with KUB and US every week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Yuksel 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups were also advised to remain active and to drink at least 2 L of water daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None. | |
| Notes | Follow‐up weekly visits with X‐ray or low‐dose CT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore selection bias was considered to have unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Unclear risk | No quotes available. Comment: Adverse effects were not described in the Results section, although they were stated as secondary outcomes in the Methods section; therefore risk of reporting bias was considered to be unclear. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Zehri 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups received 50 mg diclofenac twice daily for a maximum of 10 days and were allowed to drink 2 L of fluids daily. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None stated. | |
| Notes | Weekly follow‐up with KUB or US. Sample size was calculated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Randomization was done by assigning consecutive patients to alternate groups." Comment: This method of random sequence generation was considered to have high risk of bias. |
| Allocation concealment (selection bias) | High risk | No quotes available. Comment: The type of allocation concealment used in this study was considered to have high risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label." No blinding performed. Comment: Risk of performance bias was considered to be high. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: one exclusion; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
Zhou 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Both groups were also advised to drink at least 2 L of fluids daily. An indomethacin suppository was recommended for routine use during pain episodes. |
|
| Outcomes |
|
|
| Funding sources | None stated. | |
| Declarations of interest | None. | |
| Notes | Follow‐up weekly with KUB and ultrasonography. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No quotes available. Comment: randomisation stated but no information on method used was available; therefore risk of selection bias was considered to be unclear. |
| Allocation concealment (selection bias) | Unclear risk | No quotes available. Insufficient information to permit judgement. Comment: Owing to insufficient information, allocation concealment was considered to have unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No quotes available. No blinding described. Comment: Owing to insufficient information, risk of performance bias was considered to be unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No quotes available. No blinding of outcome assessments described. Comment: Owing to insufficient information, risk of detection bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No quotes available. Comment: no participants lost to follow‐up; therefore risk of attrition bias was considered to be low. |
| Selective reporting (reporting bias) | Low risk | No quotes available. Expected outcomes were reported according to objectives. Comment: Risk of reporting bias was therefore considered to be low. |
| Other bias | Low risk | No quotes available. Study appears to be free of other sources of bias. Comment: No other sources of bias could be found; therefore risk of other bias was considered to be low. |
AKI: acute kidney injury; CCB: calcium channel blocker; CKD: chronic kidney disease; CT: computed tomography; DB: double‐blind; ED: emergency department; eGFR: estimated glomerular filtration rate; ESWL: extracorporeal shockwave lithotripsy; EuroQOL: EuroQOL Group quaity of life questionnaire; GFR: glomerular filtration rate: HRQOL: health‐related quality of life; HTA: health technology assessment; HU: Hounsfield unit; IM: intramuscular; IQR: interquartile ratio; IV: intravenous; IVU: Intravenous urography; KUB: kidney, ureter, and bladder plain x‐ray; MET: medical expulsive therapy; NCCT: non‐contrast computed tomography; NHS: National Health Service; NIHR: National Institute for Health Research; NS: not stated; NSAID: non‐steroidal anti‐inflammatory drug; PDE5: phosphodiesterase type 5; QALY: quality‐adjusted life‐year; QOL: quality of life; RCT: randomised controlled trial; SCr: serum creatinine; SD: standard deviation; SWL: shockwave lithotripsy; US: ultrasound; UTI: urinary tract infection; VAS: visual analogue scale; WBC: white blood cell.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2009 | MET adjunct therapy to ESWL. |
| Aravinthan 2012 | Stones larger than 10 mm were included, and data could not be separated for adequate analysis of stones measuring 10 mm or less. |
| Avdoshin 2005 | Stones larger than 10 mm were included, and separate data were not available for stones measuring 10 mm or less. |
| Aydogdu 2009 | Participants were children. |
| Bak 2007 | No extracted data were available. |
| Bhat 2015 | Prospective study, but unclear whether this study was randomised. |
| Borghi 1994 | Intervention was calcium channel blocker. |
| Brausi 2015 | No randomisation was performed. |
| Chau 2011 | Patients with pyelonephritis were included. |
| Cooper 2000 | Intervention was calcium channel blocker. |
| Cuni 2013 | Use of Rowatinex/Terpenes vs tamsulosin without control or placebo group. |
| Damiano 2008 | Participants were patients who had undergone ureteral stent positioning. |
| Deliveliotis 2006 | Participants were patients with stone‐related hydronephrosis who had opted for conservative management with insertion of a double‐J ureteral stent. |
| Dellabella 2003 | Stones larger than 10 mm were included, and separate data were not available for stones measuring 10 mm or less. |
| Dellabella 2005 | Stones larger than 10 mm were included, and separate data were not available for stones measuring 10 mm or less. |
| Dellabella 2005a | The intervention comprised corticosteroids added to 1 of 2 tamsulosin groups. |
| Gandhi 2013 | Stones larger than 10 mm were included, and separate data were not available for stones measuring 10 mm or less. |
| Gupta 2013 | Tamsulosin vs silodosin. No placebo or control group available for comparison. |
| Gupta Shyam 2014 | Tamsulosin vs herbal preparation. No placebo or control group available for comparison. |
| Gurbuz 2011 | Hyoscine‐N‐butylbromide (antispasmodic drug) vs alfuzosin vs doxazosin vs terazosin. HBB was not given to alpha‐blocker groups. No placebo or control group available for comparison. |
| Haxhiu 2014 | Unclear Results section. Data not interpretable. |
| Hwang 2012 | Retrospective study. |
| Imperatore 2014 | Tamsulosin vs silodosin. No placebo or control group available for comparison. |
| ISRCTN24675122 | Study closed in January 2011 owing to insufficient recruitment. |
| Itano 2012 | Retrospective study. |
| Jayant 2014 | Tamsulosin vs tamsulosin and tadalafil. No placebo or control group available for comparison. |
| John 2010 | Participants were patients with large renal or ureteral calculi who underwent ureteroscopic laser lithotripsy. |
| Khawaja 2005 | Patients with multiple stones were included. |
| Kumar 2014 | Tamsulosin vs tamsulosin and tadalafil. No placebo or control group available for comparison. |
| Kumar 2015 | Tamsulosin and prednisolone vs naftopidil and prednisolone vs watchful waiting (no prednisolone). Therapy groups cannot be compared with watchful waiting group because prednisolone was used by treatment groups. Every positive effect reported in the therapy group could be due to use of prednisolone instead of the alpha‐blocker. |
| Loftus 2015 | Retrospective study. |
| Lu 2012 | Naftopidil vs naftopidil and tolterodine vs tolterodine. No placebo or control group available for comparison. |
| Mohseni 2006 | Stones larger than 10 mm were included, and separate data were not available for stones measuring 10 mm or less. |
| Moon 2015 | Retrospective study. |
| Morozumi 2013 | Naftopidil vs buscopan group. No placebo or control group available for comparison. |
| Multescu 2014 | Retrospective study. Not an RCT. |
| Nasim 2014 | Retrospective study. |
| Ohgaki 2010 | Data on primary and secondary outcomes not available. |
| Porpiglia 2000 | Intervention was calcium channel blocker. |
| Ramesh 2015 | No information on inclusion and exclusion criteria available. Stones measuring > 7 mm were included; maximum diameter was unknown. |
| Reddy 2016 | Study method is cohort; not an RCT. |
| Resim 2005 | Stones larger than 10 mm were included; separate data were not available for stones measuring 10 mm or less. |
| Resorlu 2011 | Investigators included no control group. |
| Salem 2015 | Silodosin vs tamsulosin. No placebo or control group was available for comparison. |
| Sameer 2014 | Children were included in the study (8 years old). |
| Shabana 2015 | Tamsulosin vs alfuzosin. No placebo or control group was available for comparison. |
| Shah 2013 | "Random base"; not an RCT. |
| Skrekas 2003 | Intervention was calcium channel blocker. |
| Su 2016 | Data were not consistent throughout the article. Participant numbers were different in tables as compared with the flow chart and text. |
| Sumer 2012 | Primary outcome was resolution of colicky pain. Stone clearance rate was not reported. |
| Tchey 2011 | Retrospective study. |
| Tsuzaka 2011 | Naftopidil vs silodosin. No placebo or control group available for comparison. |
| Vavassori 2012 | No randomisation performed. |
| Wang 2009c | Patients underwent ESWL before study entry. |
ESWL: extracorporeal shockwave lithotripsy; HBB: hyoscine‐N‐butylbromide; MET: medical expulsive therapy; RCT: randomised controlled trial.
Differences between protocol and review
This Review is based on a published protocol (Zhu 2010). It provides an update of the published review (Campschroer 2014).
Major differences between the previous review and the update include the following.
'Major adverse events' has been added as a co‐primary outcome.
Need for surgical intervention has been added as a secondary outcome.
The comparison of alpha‐blockers versus calcium channel blockers has been omitted in light of readability and clinical applicability.
A new type of alpha‐blocker (silodosin) has been added as an intervention.
GRADE approach has been applied to assess the quality of evidence.
Contributions of authors
Draft the protocol: XZ, TL.
Select studies: TC, XZ.
Extract data from studies: TC, XZ, RV.
Enter data into RevMan: TC, XZ, RV.
Carry out the analysis: TC, XZ, RV.
Interpret the analysis: TC, XZ, RV.
Draft the final review: TC, XZ, TL.
Resolve disagreements: TL.
Update the review: TC, XZ, RV, TL.
Sources of support
Internal sources
Department of Urology, Rijnstate, Arnhem, Netherlands.
Department of Urology, University Medical Center Utrecht, Utrecht, Netherlands.
Department of Research, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, Netherlands.
External sources
No sources of support supplied
Declarations of interest
Thijs Campschroer: none. Xiaoye Zhu: none. Robin WM Vernooij: none. MTW Tycho Lock: none.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abdel‐Meguid 2010 {published data only}
- Abdel‐Meguid TA, Tayib A, Al‐Sayyad A. Tamsulosin to treat uncomplicated distal ureteral calculi: a double blind randomized placebo‐controlled trial. Canadian Journal of Urology 2010 Jun;17(3):5178‐83. [MEDLINE: ] [PubMed] [Google Scholar]
Agrawal 2009 {published data only}
- Agrawal M, Gupta M, Gupta A, Agrawal A, Sarkari A, Lavania P. Prospective randomized trial comparing efficacy of alfuzosin and tamsulosin in management of lower ureteral stones. Urology 2009;73(4):706‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ahmad 2015 {published data only}
- Ahmad H, Azim W, Akmal M, Murtaza B, Mahmood A, Nadim A, et al. Medical expulsive treatment of distal ureteral stone using tamsulosin. Journal of Ayub Medical College Abbottabad 2015;27(1):48‐50. [PubMed] [Google Scholar]
Ahmed 2010 {published data only}
- Ahmed AA. Tamsulosin versus alfuzosin in the treatment of patients with distal ureteral stones: prospective, randomized, comparative study. Korean Journal of Urology 2010;51:193‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Al Ansari 2010 {published data only}
- Al‐Ansari A, Al‐Naimi A, Alobaidy A, Assadiq K, Azmi MD, Shokeir AA. Efficacy of tamsulosin in the management of lower ureteral stones: a randomized double‐blind placebo‐controlled study of 100 patients. Urology 2010;75(1):4‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Albert 2016 {published data only}
- Albert AS, Pillai SR, Mary A, Aravindakshan R. Efficacy of tamsulosin and silodosin as medical expulsive therapy in the management of distal ureteral stones: a randomized controlled study. International Surgery Journal 2016;3(2):578‐81. [DOI: ] [Google Scholar]
Aldemir 2011 {published data only}
- Aldemir M, Ucgul YE, Kayigil O. Evaluation of the efficiency of tamsulosin and Rowatinex in patients with distal ureteral stones: a prospective, randomized, controlled study. International Urology & Nephrology 2011;43(1):79‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alizadeh 2014 {published data only}
- Alizadeh M, Magsudi M. The effect of tamsulosin in the medical treatment of distal ureteral stones. Global Journal of Health Science 2014;6(7):44‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arrabal‐Martin 2010 {published data only}
- Arrabal‐Martin M, Valle‐Diaz de la Guardia F, Arrabal‐Polo MA, Palao‐Yago F, Mijan‐Ortiz JL, Zuluaga‐Gomez A. Treatment of ureteral lithiasis with tamsulosin: literature review and meta‐analysis. Urologia Internalis 2010;84:254‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Autorino 2005 {published data only}
- Autorino R, Sio M, Damiano R, Lorenzo G, Perdona S, Russo A, et al. The use of tamsulosin in the medical treatment of ureteral calculi: where do we stand?. Urological Research 2005;33(6):460‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sio M, Autorino R, Lorenzo G, Damiano R, Giordano D, Cosentino L, et al. Medical expulsive treatment of distal‐ureteral stones using tamsulosin: a single‐center experience. Journal of Endourology 2006;20(1):12‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ayubov 2007 {published data only}
- Ayubov B, Arustamov D, Mukhtarov S. Efficacy of doxazosin in the management of ureteral stones [abstract no: 368]. European Urology Supplements 2007;6(2):114. [CENTRAL: CN‐00793464] [Google Scholar]
Bajwa 2013 {published data only}
- Bajwa SA, Rahim J, Rahim J, Saeed Q, Hussain K, Ashraf S, et al. Efficacy of tamsulosin for clearance of lower ureteric stones. Pakistan Journal of Medical and Health Sciences 2013;7(3):769‐72. [Google Scholar]
Balci 2014 {published data only}
- Balci M, Tuncel A, Aydin O, Aslan Y, Guzel O, Toprak U, et al. Tamsulosin versus nifedipine in medical expulsive therapy for distal ureteral stones and the predictive value of Hounsfield unit in stone expulsion. Renal Failure 2014;36(10):1541‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bayraktar 2017 {published data only}
- Bayraktar Z, Albayrak S. Sexual intercourse as a new option in the medical expulsive therapy of distal ureteral stones in males: a prospective, randomized, controlled study. International Urology and Nephrology 2017;49:1941‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berger 2015 {published data only}
- Berger DA, Ross MA, Hollander JB, Ziadeh J, Chen C, Jackson RE, et al. Tamsulosin does not increase 1‐week passage rate of ureteral stones in ED patients. American Journal of Emergency Medicine 2015;33(12):1721‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cervenakov 2002 {published data only}
- Cervenakov I, Fillo J, Mardiak J, Kopecny M, Smirala J, Lepies P. Speedy elimination of ureterolithiasis in lower part of ureters with the alpha 1‐blocker ‐ tamsulosin. International Urology and Nephrology 2002;34(1):25‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cha 2012 {published data only}
- Cha WH, Choi JD, Kim KH, Seo YJ, Lee K. Comparison and efficacy of low‐dose and standard‐dose tamsulosin and alfuzosin in medical expulsive therapy for lower ureteral calculi: prospective, randomized, comparative study. Korean Journal of Urology 2012;53:349‐54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2017 {published data only}
- Cho SY, Na W, Lee SW, Cho MC, Oh JJ, Lee S, et al. Medical expulsive therapy for ureter stone using naftopidil: a multicenter, randomized, double‐blind, and placebo‐controlled trial. Plos One 2017;12(4):e0174962. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doluoglu 2015 {published data only}
- Doluoglu OG, Demirbas A, Kilinc MF, Karakan T, Kabar M, Bozkurt S, et al. Can sexual intercourse be an alternative therapy for distal ureteral stones? A prospective randomized, controlled study. Urology 2015;86:19‐24. [DOI: ] [DOI] [PubMed] [Google Scholar]
Dong 2009 {published data only}
- Dong IK, Won YC, Tae HK, Jae MC, Park J, Jang HY, et al. Effect of tamsulosin 0.2 mg on the short‐term treatment of urinary stones: multicenter, prospective, randomized study. Korean Journal of Urology 2009;50(6):586‐90. [EMBASE: 2009369223] [Google Scholar]
El‐Gamal 2012 {published data only}
- El‐Gamal O, El‐Bendary M, Ragab M, Rasheed M. Role of combined use of potassium citrate and tamsulosin in the management of uric acid distal ureteral calculi. Urological Research 2012;40:219‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
El Said 2015 {published data only}
- Said NO, Wakeel L, Kamal KM, Reheem Morad A. Alfuzosin treatment improves the rate and time for stone expulsion in patients with distal ureteral stones: a prospective randomized controlled study. Pharmacotherapy 2015;35(5):470‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Erkan 2011 {published data only}
- Erkan E, Toktas G, Kocaslan R, Sacak V, Toker A, Unluer E. Medical expulsive therapy reduces the need for surgery in the distal management of distal ureteral stones. Urology (Suppl 3A) 2011;78:S28‐S29. [Google Scholar]
Erturhan 2007 {published data only}
- Erturhan S, Erbagci A, Yagci F, Celik M, Solakhan M, Sarica K. Comparative evaluation of efficacy of use of tamsulosin and/or tolterodine for medical treatment of distal ureteral stones. Urology 2007;69(4):633‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eryildirim 2015 {published data only}
- Eryildirim B, Sahin C, Tuncer M, Sabuncu K, Cetinel C, Tarhan F, et al. Effect of medical expulsive therapy on the health‐related quality of life of patients with ureteral stones: a critical evaluation. International Urology and Nephrology 2015;47:1271‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferre 2009 {published data only}
- Ferre RM, Wasielewski JN, Strout TD, Perron AD. Tamsulosin for ureteral stones in the emergency department: a randomized, controlled trial. Annals of Emergency Medicine 2009;54(3):432‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Furyk 2016 {published data only}
- Furyk JS, Chu K, Banks C, Greenslade J, Keijzers G, Thom O, et al. Distal ureteric stones and tamsulosin: a double‐blind placebo‐controlled, randomized, multicenter trial. Annals of Emergency Medicine 2016;67(1):86‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Georgescu 2015 {published data only}
- Georgescu D, Ionita‐Radu F, Multescu R, Dragutescu M, Geavlete B, Geavlete P, et al. The role of a1‐blockers in the medical expulsive therapy for ureteral calculi ‐ a prospective controlled randomized study comparing tamsulosin and silodosin. Farmacia 2015;63(2):184‐8. [Google Scholar]
Griwan 2010 {published data only}
- Griwan MS, Singh SK, Paul H, Pahar DS, Verma M. The efficacy of tamsulosin in lower ureteral calculi. Urology Annals 2010;2(2):63‐6. [10.4103/0974‐7796.65110] [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2006a {published data only}
- Han MC, Park YY, Shim BS. Effect of tamsulosin on the expectant treatment of lower ureteral stones. Korean Journal of Urology 2006;47(7):708‐11. [EMBASE: 2006517991] [Google Scholar]
Hermanns 2009 {published data only}
- Hermanns T, Sauermann P, Rufibach K, Frauenfelder T, Sulser T, Strebel RT. Is there a role for tamsulosin in the treatment of distal ureteral stones of 7 mm or less? Results of a randomised, double‐blind, placebo‐controlled trial. European Urology 2009;56(3):407‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hong 2008 {published data only}
- Hong YK, Jang WK, Lee YK, Park DS. Efficacy of a furosemide‐based medical expulsive therapy with tamsulosin‐deflazacort for a symptomatic distal ureter stone [abstract no: 1492]. Journal of Urology 2008;179(4 Suppl 1):508‐9. [CENTRAL: CN‐00783144] [Google Scholar]
Ibrahim 2013 {published data only}
- Ibrahim AK, Mahmood IH, Mahmood NS. Efficacy and safety of tamsulosin vs. alfuzosin as medical expulsive therapy for ureteric stones. Arab Journal of Urology 2013;11:142‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Islam 2010 {published data only}
- Islam MS, Islam MW, Hooda MN, Alam AKMK, Chowdhury GM, Shameem IA. The comparison and efficacy of nifedipine and tamsulosin for the management of lower ureteric stones. Bangladesh Journal of Urology 2010;13(1):5‐9. [Google Scholar]
Itoh 2011 {published data only}
- Itoh Y, Okada A, Yasui T, Taguchi K, Niimi K, Fujii Y, et al. Efficacy of a selective alpha 1A adrenoceptor antagonist silodosin in the medical expulsive therapy for ureteral stones. International Journal of Urology 2011;18:672‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Itoh 2013 {published data only}
- Itoh Y, Okada A, Taguchi K, Hirose Y, Fujii Y, Kobayashi T, et al. Administration of the selective alpha 1A adrenoceptor antagonist silodosin facilitates expulsion of size 5‐10 mm distal ureteral stones, as compared to control. Journal of Urology (Suppl) 2015;193(4S):e502. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Okada A, Yasui T, Ando R, Tozawa K, Sasaki S, et al. Administration of the selective alpha 1A‐adrenoceptor antagonist silodosin facilitates expulsion of size 5‐10 mm distal ureteral stones, as compared to control. Internation Urology and Nephrology 2013;45:675‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Itoh Y, Taguchi K, Fujii Y, Hirose Y, Usami M, Hamamoto S, et al. Silodosin, the selective alpha 1A adrenoceptor antagonist, facilitates expulsion of size 5–10 mm distal ureteral stones. Urolithiasis. 2016; Vol. 44 (Suppl 1):S1‐S66. [DOI: 10.1007/s00240-016-0883-8] [DOI]
Kaneko 2010 {published data only}
- Kaneko T, Matsushima H, Morimoto H, Tsuzaka Y, Homma Y. Efficacy of low dose tamsulosin in medical expulsive therapy for ureteral stones in Japanese male patients: a randomized controlled study. International Journal of Urology 2010;17(5):462‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2007b {published data only}
- Kim JW, Cho DY, Lee JG. Effect of tamsulosin on the expected treatment of upper and lower ureteral stones. Korean Journal of Urology 2007;48(7):724‐30. [EMBASE: 2007375242] [Google Scholar]
Kupeli 2004 {published data only}
- Kupeli B, Irkilata L, Gurocak S, Tunc L, Kirac M, Karaoglan U, et al. Does tamsulosin enhance lower ureteral stone clearance with or without shock wave lithotripsy?. Urology 2004;64(6):1111‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee SW, Woo SH, Yoo D‐S, Park J. Effect of tamsulosin on stone expulsion in proximal ureteral calculi: an open‐label randomized controlled trial. International Journal of Clinical Practice 2014;68(2):216‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liatsikos 2007 {published data only}
- Liatsikos E, Voudoukis T, Katsakiori P, Asimakopoulos K, Athanasopoulos A, Perimenis P, et al. Doxazosin for the management of distal ureteral stones [abstract no: 51]. European Urology Supplements 2006;5(2):35. [CENTRAL: CN‐00653793] [DOI] [PubMed] [Google Scholar]
- Liatsikos EN, Katsakiori PF, Assimakopoulos K, Voudoukis T, Kallidonis P, Constantinides C, et al. Doxazosin for the management of distal‐ureteral stones. Journal of Endourology 2007;21(5):538‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lojanapiwat 2008 {published data only}
- Lojanapiwat B, Kochakarn W, Suparatchatpan N, Lertwuttichaikul K. Effectiveness of low‐dose and standard‐dose tamsulosin in the treatment of distal ureteric stones: a randomized controlled study. Journal of International Medical Research 2008;36(3):529‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lojanapiwat 2012 {published data only}
- Lojanapiwat B. Role of tamsulosin as medical expulsive therapy for proximal ureteral calculi: a randomized controlled study. Urology (Suppl 3A) 2012;80:S176. [Google Scholar]
Maitra 2012 {published data only}
- Maitra T. Prospective randomized trial comparing the efficacy of tamsulosin and tamsulosin combined with nifedipine for the management of lower ureteral stones. Turkish Journal of Urology 2012;38(1):32‐5. [10.5152/tud.2012.007] [Google Scholar]
Meltzer 2017 {published data only}
- Meltzer AC, Burrows PK, Hollander JE, Wolfson AA, Kurz MM, Richards LM, et al. Study of Tamsulosin for Urolithiasis In The Emergency Department – STONE. AUA 2017. 14 May 2017.
Mshemish 2012 {published data only}
- Mshemish BA. A comparative study between tamsulosin and doxazosin for management of lower ureteral stones. Iraqi Journal of Community Medicine 2012;2:153‐7. [Google Scholar]
Mukhtarov 2007 {published data only}
- Mukhtarov S, Turdiev A, Fozilov A, Arustamov D, Ayubov B. Using doxazosin for distal ureteral stone clearance with or without shock wave lithotripsy [abstract no: 774]. European Urology Supplements 2007;6(2):216. [CN‐00797078] [Google Scholar]
Ochoa‐Gomez 2011 {published data only}
- Ochoa‐Gomez R, Prieto‐Diaz‐Chavez E, Trujillo‐Hernandez B, Vasquez C. Tamsulosin does not have greater efficacy than conventional treatment for distal ureteral stone expulsion in Mexican patients. Urological Research 2011;39:491‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Park 2012 {published data only}
- Park J, Joo HT, Whang HW, Lee SW. Effect of tamsulosin for stone expulsion and patient's quality of life in proximal ureteral calculi. Journal of Endourology 2012;26(Suppl 1):178. [Google Scholar]
Pedro 2008 {published data only}
- Pedro RN, Hinck B, Hendlin K, Feia K, Canales BK, Monga M. Alfuzosin stone expulsion therapy for distal ureteral calculi: a double‐blind, placebo controlled study. Journal of Urology 2008;179(6):2244‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pickard 2015 {published data only}
- Pickard R, Starr K, MacLennan G, Lam T, Thomas R, Burr J, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo‐controlled trial. Lancet 2015;386:341‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Porpiglia 2006 {published data only}
- Porpiglia F, Vaccino D, Billia M, Renard J, Cracco C, Ghignone G, et al. Corticosteroids and tamsulosin in the medical expulsive therapy for symptomatic distal ureter stones: single drug or association?. European Urology 2006;50(2):339‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Porpiglia 2009 {published data only}
- Porpiglia F, Fiori C, Ghignone G, Vaccino D, Billia M, Morra I, et al. A second cycle of tamsulosin in patients with distal ureteric stones: a prospective randomized trial. BJU International 2009;103(12):1700‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Porpiglia F, Vaccino D, Billia M, Grande S, Ghignone G, Cossu M, et al. Second cycle of medical expulsive therapy with tamsulosin in patients non responders to a first cycle for distal ureteral stones: results of a prospective randomised trial [abstract no: 314]. European Urology Supplements 2008;7(3):149. [CENTRAL: CN‐00795753] [Google Scholar]
Rahim 2012 {published data only}
- Rahim J, Mahmood A, Ashraf S, Tahir MM, Khan MU. Efficacy of tamsulosin spontaneous expulsion in the treatment of distal ureteric stones. Pakistan Journal of Medical and Health Sciences 2012;6(1):191‐4. [Google Scholar]
Sayed 2008 {published data only}
- Sayed MA, Abolyosr A, Abdalla MA, El‐Azab AS. Efficacy of tamsulosin in medical expulsive therapy for distal ureteral calculi. Scandinavian Journal of Urology and Nephrology 2008;42(1):59‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sen 2017 {published data only}
- Sen H, Erturhan S, Sadioglu E, Bayrak O, Seckiner I. A comparison of efficacy of doxazosin 4 and 8 mg in medical expulsive therapy of distal ureteral stones: a prospective randomized clinical trial. Urolithiasis 2017;45(5):461‐4. [DOI: 10.1007/s00240-016-0927-0] [DOI] [PubMed] [Google Scholar]
Sun 2009 {published data only}
- Sun X, He L, Ge W, Lv J. Efficacy of selective alpha1D‐blocker naftopidil as medical expulsive therapy for distal ureteral stones. Journal of Urology 2009;181(4):1716‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sur 2015 {published data only}
- Sur RL, Shore N, L'Esperance J, Knudsen B, Gupta M, Olsen S, et al. Silodosin to facilitate passage of ureteral stones: a multi‐institutional, randomized, double‐blinded, placebo‐controlled trial. European Urology 2015;67:959‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taghavi 2005 {published data only}
- Taghavi R, Darabi MR, Tavakoli K, Keshvari M. Survey of the effect of tamsulosin and nifedipine on facilitating juxtavesical ureteral stone passage [abstract no: MP03‐06]. Journal of Endourology 2005;19(Suppl 1):A9. [CENTRAL: CN‐00653798] [Google Scholar]
Vincendeau 2010 {published data only}
- Vincendeau S, Bellissant E, Bensalah K, Houlgatte A, Dore B, Bruyere F, et al. Lack of efficacy of tamsulosin in the treatment of distal ureteral stones [abstract no: 1491]. Journal of Urology 2008;179(4 Suppl 1):508. [Google Scholar]
- Vincendeau S, Bellissant E, Bensalah K, Houlgatte A, Dore B, Bruyere F, et al. Lack of efficacy of tamsulosin in the treatment of distal ureteral stones [abstract no: 313]. European Urology Supplements 2008;7(3):149. [CN‐00794344] [Google Scholar]
- Vincendeau S, Bellissant E, Houlgatte A, Dore B, Bruyere F, Renault A, et al. Tamsulosin hydrochloride vs placebo for management of distal ureteral stones: a multicentric, randomized, double‐blind trial. Archives of Internal Medicine 2010;170(22):2021‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2008a {published data only}
- Wang CJ, Huang SW, Chang CH. Efficacy of an alpha1 blocker in expulsive therapy of lower ureteral stones. Journal of Endourology 2008;22(1):41‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ye 2017 {published data only}
- Ye Z, Zeng G, Yang H, Tang K, Zhang X, Li H, et al. Efficacy and safety of tamsulosin in medical expulsive therapy for distal ureteral stones with renal colic: a multicenter, randomized, double‐blind, placebo‐controlled trial. European Urology 12 Nov 2017;Epub ahead of print(3):385‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yencilek 2010 {published data only}
- Yencilek F, Erturhan S, Canguven O, Koyuncu H, Erol B, Sarica K. Does tamsulosin change the management of proximally located ureteral stones?. Urological Research 2010;38(3):195‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yilmaz 2005 {published data only}
- Yilmaz E, Batislam E, Basar MM, Tuglu D, Ferhat M, Basar H. The comparison and efficacy of 3 different alpha1‐adrenergic blockers for distal ureteral stones. Journal of Urology 2005;173(6):2010‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yuksel 2015 {published data only}
- Yuksel M, Yilmaz S, Tokgoz H, Yalcinkaya S, Bas S, Ipekci T, et al. Efficacy of silodosin in the treatment of distal ureteral stones 4 to 10 mm in diameter. International Journal of Clinical and Experimental Medicine 2015;8(10):19086‐92. [PMC free article] [PubMed] [Google Scholar]
Zehri 2010 {published data only}
- Zehri AA, Ather MH, Abbas F, Biyabani SR. Preliminary study of efficacy of doxazosin as a medical expulsive therapy of distal ureteric stones in a randomized clinical trial. Urology 2010;75(6):1285‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhou 2011 {published data only}
- Zhou SG, Lu JL, Hui JH. Comparing efficacy of a1D‐receptor antagonist naftopidil and a1A/D‐receptor antagonist tamsulosin in management of distal ureteral stones. World Journal of Urology 2011;29:767‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agarwal 2009 {published data only}
- Agarwal MM, Naja V, Singh SK, Mavuduru R, Mete UK, Kumar S, et al. Is there an adjunctive role of tamsulosin to extracorporeal shockwave lithotripsy for upper ureteric stones: results of an open label randomized nonplacebo controlled study. Urology 2009;74(5):989‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Aravinthan 2012 {published data only}
- Aravinthan T. The efficacy of tamsulosin in lower ureteral calculi. International Journal of Urology 2012;19(Suppl 1):64. [Google Scholar]
Avdoshin 2005 {published data only}
- Avdoshin VP, Andriukhin MI, Barabash MI, Taskinen I, Ol'shanskaia EV, Motin PI, et al. Tamsulosin in the treatment of patients with ureteroliths of the lower third of the ureter clinical and pharmacoeconomic grounds. Urologiia (Moscow, Russia) 2005, (4):36‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Aydogdu 2009 {published data only}
- Aydogdu O, Burgu B, Gucuk A, Suer E, Soygur T. Effectiveness of doxazosin in treatment of distal ureteral stones in children. Journal of Urology 2009;182(6):2880‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bak 2007 {published data only}
- Bak CW, Yoon SJ, Chung H. Effects of an alpha‐blocker and terpene mixture for pain control and spontaneous expulsion of ureter stone. Korean Journal of Urology 2007;48:517‐21. [Google Scholar]
Bhat 2015 {published data only}
- Bhat SK, Bhushan R, Chakarbarty N. Role of alfuzosin in spontaneous passage of distal ureteric calculi. Journal of Medical Science and Clinical Research 2015;3(11):8157‐60. [Google Scholar]
Borghi 1994 {published data only}
- Borghi L, Meschi T, Amato F, Novarini A, Giannini A, Quarantelli C, et al. Nifedipine and methylprednisolone in facilitating ureteral stone passage: a randomized, double‐blind, placebo‐controlled study. Journal of Urology 1994;152(4):1095‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brausi 2015 {published data only}
- Brausi M, Peracchia G, Luca G, Viola M, Giliberto GL. The efficacy of tamsulosin (tams) alone vs corticosteroids alone vs tamsulosin + corticosteroids in determining the spontaneous passage of distal ureteral stones: results of a prospective study. European Urology Supplements 2015;14(2):e389. [Google Scholar]
Chau 2011 {published data only}
- Chau LH, Tai DC, Fung BT, Li JC, Fan CW, Li MK. Medical expulsive therapy using alfuzosin for patient presenting with ureteral stone less than 10mm: a prospective randomized controlled trial. International Journal of Urology 2011;18(7):510‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cooper 2000 {published data only}
- Cooper JT, Stack GM, Cooper TP. Intensive medical management of ureteral calculi. Urology 2000;56(4):575‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cuni 2013 {published data only}
- Cuni Xh, Haxhiu I, Xhani M, Cuni H, Haxhiu A, Frangu B, et al. The efficacy of Rowatinex and tamsulosin in patients with selected ureteral stones: a controlled study. European Urology Supplements 2013;12:e1330. [Google Scholar]
Damiano 2008 {published data only}
- Damiano R, Autorino R, Sio M, Giacobbe A, Palumbo IM, D'Armiento M. Effect of tamsulosin in preventing ureteral stent‐related morbidity: a prospective study. Journal of Endourology 2008;22(4):651‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deliveliotis 2006 {published data only}
- Deliveliotis C, Chrisofos M, Gougousis E, Papatsoris A, Dellis A, Varkarakis IM. Is there a role for alpha1‐blockers in treating double‐J stent‐related symptoms?. Urology 2006;67(1):35‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dellabella 2003 {published data only}
- Dellabella M, Milanese G, Muzzonigro G. Efficacy of tamsulosin in the medical management of juxtavesical ureteral stones. Journal of Urology 2003;170(6 Pt 1):2202‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dellabella 2005 {published data only}
- Dellabella M, Milanese G, Muzzonigro G. Randomized trial of the efficacy of tamsulosin, nifedipine and phloroglucinol in medical expulsive therapy for distal ureteral calculi. Journal of Urology 2005;174(1):167‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dellabella 2005a {published data only}
- Dellabella M, Milanese G, Muzzonigro G. Medical‐expulsive therapy for distal ureterolithiasis: randomized prospective study on role of corticosteroids used in combination with tamsulosin‐simplified treatment regimen and health‐related quality of life. Urology 2005;66(4):712‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gandhi 2013 {published data only}
- Gandhi HR, Agrawal C. The efficacy of tamsulosin vs. nifedipine for the medical expulsive therapy of distal ureteric stones: a randomised clinical trial. Arab Journal of Urology 2013;11:405‐10. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2013 {published data only}
- Gupta S, Lodh B, Singh AK, Somarendra K, Meitei KS, Singh SR. Comparing the efficacy of tamsulosin and silodosin in the medical expulsion therapy for ureteral calculi. Journal of Clinical and Diagnostic Research 2013;7(8):1672‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta Shyam 2014 {published data only}
- Shyam KG, Vishal K, Geetanjali G, Ankush B, Anjali S. Clinical evaluation of 'suspend' trial with herbal preparation and tamsulosin: a medical expulsive therapy for urolithiasis. International Research Journal of Pharmacy 2014;5(12):915‐7. [Google Scholar]
Gurbuz 2011 {published data only}
- Gurbuz MC, Polat H, Canat L, Kilic M, Caskurlu T. Efficacy of three different alpha 1‐adrenergic blockers and hyoscine N‐butylbromide for distal ureteral stones. International Brazilian Journal of Urology 2011;37(2):195‐202. [DOI] [PubMed] [Google Scholar]
Haxhiu 2014 {published data only}
- Haxhiu I, Quni X, Haxhiu A, Arifi H, Haxhiu E, Ahmeti H, et al. Our experience in the treatment of urolithiasis with tamsulosin. Urolithiasis 2014;42:490‐1. [Google Scholar]
Hwang 2012 {published data only}
- Hwang EC, Hwang IS, Yu HS, Kim S‐O, Jung SI, Kang TW, et al. Effects of alfuzosin with methylprednisolone for spontaneous expulsion and pain control of lower ureteral stone. Urological Research 2012;40:605‐9. [DOI] [PubMed] [Google Scholar]
Imperatore 2014 {published data only}
- Imperatore V, Fusco F, Creta M, Meo S, Buonopane R, Longo N, et al. Medical expulsive therapy for distal ureteric stones: tamsulosin versus silodosin. Archivio Italiano di Urologia e Andrologia 2014;86(2):103‐7. [DOI] [PubMed] [Google Scholar]
ISRCTN24675122 {published data only}
- Davenport K. A randomised control trial comparing spontaneous ureteric stone passage rates with tamsulosin versus placebo in the management of acute renal colic. http://www.controlled‐trials.com [accessed on 18 November 2017] 2003.
Itano 2012 {published data only}
- Itano N, Ferlic E, Nunez‐Nateras R, Humphreys MR. Medical expulsive therapy in a tertiary care emergency department. Urology 2012;79:1242‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jayant 2014 {published data only}
- Jayant K, Agrawal R, Agrawal S. Tamsulosin versus tamsulosin plus tadalafil as medical expulsive therapy for lower ureteric stones: a randomized controlled trial. International Journal of Urology 2014;21:1012‐5. [DOI] [PubMed] [Google Scholar]
John 2010 {published data only}
- John TT, Razdan S. Adjunctive tamsulosin improves stone free rate after ureteroscopic lithotripsy of large renal and ureteric calculi: a prospective randomized study. Urology 2010;75(5):1040‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- John TT, Razdan S. Adjunctive tamsulosin improves stone free rate after ureteroscopic lithotripsy of large renal and ureteric calculi: a prospective randomized study [abstract no: 1460]. Journal of Urology 2008;179(4 Suppl 1):498. [DOI] [PubMed] [Google Scholar]
Khawaja 2005 {published data only}
- Khawaja AA, Ahmed R, Anjum R, Zaidi AI. Role of alpha blockers in the management of lower ureteric stones. Medical Forum Monthly 2005;16(1):7‐11. [EMBASE: 2007147954] [Google Scholar]
Kumar 2014 {published data only}
- Kumar S, Kurdia KC, Ganesamoni R, Singh SK, Nanjappa B. Randomized controlled trial to compare the safety and efficacy of naftopidil and tamsulosin as medical expulsive therapy in combination with prednisolone for distal ureteral stones. Korean Journal of Urology 2014;54:311‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2015 {published data only}
- Kumar S, Jayant K, Agrawal MM, Singh SK, Agrawal S, Parmar KM. Role of tamsulosin, tadalafil, and silodosin as the medical expulsive therapy in lower ureteric stone: a randomized trial (a pilot study). Urology 2015;85:59‐63. [DOI] [PubMed] [Google Scholar]
Loftus 2015 {published data only}
- Loftus C, Nyame Y, Hinck B, Greene D, Chaparala H, Alazem K, et al. Medical expulsive therapy is underused for the management of renal colic in the emergency setting. Journal of Urology 2015;E‐pub, ahead of print(doi: 10.1016/j.juro.2015.11.026):987–91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lu 2012 {published data only}
- Lu JL, Tang QL, Liu F, Hui JH. Naftopidil and tolterodine in the medical expulsive therapy for intramural ureteral stones: a prospective randomized study. Urological Research 2012;40:757‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mohseni 2006 {published data only}
- Mohseni MG, Hosseini SR, Alizadeh F. Efficacy of terazosin as a facilitator agent for expulsion of the lower ureteral stones. Saudi Medical Journal 2006;27(6):838‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Moon 2015 {published data only}
- Moon YJ, Kim HW, Kim JB, Kim HJ, Chang YS. Distribution of ureteral stones and factors affecting their location and expulsion in patients with renal colic. Korean Journal of Urology 2015;56(10):717‐21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morozumi 2013 {published data only}
- Morozumi M, Ishii N, Cho E, Sakaguchi K, Yano A, Okada Y, et al. Effects of naftopidil or butylscopolamine for the ureteral stone events. European Urology Supplements 2013;12:58‐9. [Google Scholar]
Multescu 2014 {published data only}
- Multescu R, Georgescu D, Satalan R, Geavlete B, Geavlete P. Conservative treatment in patients with ureteral stones and renal colic. Urology 2014;84(4 Suppl 1):UP575, unmoderated poster session. [Google Scholar]
Nasim 2014 {published data only}
- Nasim I, Ather H. Predictors for spontaneous stone passage in patients with ureteral calculi at emergency department. Urolithiasis 2014;42:475‐508. [Google Scholar]
Ohgaki 2010 {published data only}
- Ohgaki K, Horiuchi K, Hikima N, Kondo Y. Facilitation of expulsion of ureteral stones by addition of alpha1‐blockers to conservative therapy. Scandinavian Journal of Urology and Nephrology 2010;44(6):420‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Porpiglia 2000 {published data only}
- Porpiglia F, Destefanis P, Fiori C, Fontana D. Effectiveness of nifedipine and deflazacort in the management of distal ureter stones. Urology 2000;56(4):579‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ramesh 2015 {published data only}
- Ramesh O, Rani BS, Prabhu GR. Medical expulsive therapy of ureteric calculi ‐ our experience. Journal of Evidence Based Medicine and Healthcare 2015;2(40):6612‐8. [DOI: 10.18410/jebmh/2015/902] [DOI] [Google Scholar]
Reddy 2016 {published data only}
- Reddy RK, Reddy SA. The efficacy of alpha‐blockers for expulsion of distal ureteral stones. International Surgery Journal 2016;3(1):336‐40. [DOI: ] [Google Scholar]
Resim 2005 {published data only}
- Resim S, Ekerbicer H, Ciftci A. Effect of tamsulosin on the number and intensity of ureteral colic in patients with lower ureteral calculus. International Journal of Urology 2005;12(7):615‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Resorlu 2011 {published data only}
- Resorlu B, Bozkurt OF, Senocak C, Unsal A. Effectiveness of doxazosin in the management of lower ureteral stones in male and female patients. International Urology and Nephrology 2011;43:645‐9. [DOI: 10.1007/s11255-010-9867-8] [DOI] [PubMed] [Google Scholar]
Salem 2015 {published data only}
- Salem EAH, Kamel M, Sakr AMN, Fawzy A, Shahin A. Silodosin versus tamsulosin in the management of distal ureteric stones. European Urology Supplements 2015;14(2):e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sameer 2014 {published data only}
- Sameer, Lal S, Charak KS, Chakravarti S, Kohli S, Ahmad S. Efficacy of nifedipine and alfuzosin in the management of distal ureteric stones: a randomized, controlled study. Indian Journal of Urology 2014;30(4):387‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shabana 2015 {published data only}
- Shabana W, Teleb M, Dawod T, Eladl M. A prospective randomized study to evaluate the outcome of alpha blockers and the combination with methylprednisolone in medical expulsive therapy for lower ureteral stones. Journal of Urology 2015;193(4S):e503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2013 {published data only}
- Shah YD, Thekdi PI, Patel KG. Medical expulsive therapy for the management of ureteric calculi. International Journal of Research in Medical Sciences 2013;1(3):267‐70. [DOI: 10.5455/2320-6012.ijrms20130821] [DOI] [Google Scholar]
Skrekas 2003 {published data only}
- Skrekas T, Liapis D, Kalantzis A, Argyropoulos A, Doumas K, Lycourinas M. Increasing the success rate of medical therapy for expulsion of distal ureteral stones using adjunctive treatment with calcium channel blocker [abstract no: 317]. European Urology Supplements 2003;2(1):82. [CENTRAL: CN‐00653802] [Google Scholar]
Su 2016 {published data only}
- Su YC, Wang CJ, Chang CH, Chen HW, Tsai PC. Efficacy of a highly selective α‐1 adrenoceptor antagonist in expulsive therapy of distal ureteral stones: a randomized controlled study. Academia Journal of Biotechnology 2016;4(6):241‐6. [DOI: 10.15413/ajb.2016.0103] [DOI] [Google Scholar]
Sumer 2012 {published data only}
- Sumer A, Kaynar M, Topbas E, Hassan MA, Gurbuz R. Comparison of the therapeutic effects of diclofenac sodium,prednisolone and an alpha blocker for the treatment of renal colic [Akut renal kolik tedavisinde diklofenak sodyum, metilprednizolon ve alfa blokörtedavilerinin karşılaştırılması]. Turkish Journal of Urology 2012;38(1):23‐8. [DOI: 10.5152/tud.2012.005] [DOI] [Google Scholar]
Tchey 2011 {published data only}
- Tchey DU, Ha YS, Kim WT, Yun SJ, Lee SC, Kim WJ. Expectant management of ureter stones: outcome and clinical factors of spontaneous passage in a single institution's experience. Korean Journal of Urology 2011;52(12):847‐51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsuzaka 2011 {published data only}
- Tsuzaka Y, Matsushima H, Kaneko T, Yamaguchi T, Homma Y. Naftopidil vs silodosin in medical expulsive therapy for ureteral stones: a randomized controlled study in Japanese male patients. International Journal of Urology 2011;18:792‐5. [DOI] [PubMed] [Google Scholar]
Vavassori 2012 {published data only}
- Vavassori I, Giliberto GL, Cau L, Brausi M, Vecchia S. The comparison between efficacy of tamsulosin alone or in combination with corticosteroids vs corticosteroids alone vs analgesics in spontaneous passage of distal ureteral stones: results of a prospective study. Journal of Endourology 2012;26(Suppl 1):178. [Google Scholar]
Wang 2009c {published data only}
- Wang CJ, Huang SW, Chang CH. Adjunctive medical therapy with an alpha‐1A‐specific blocker after shock wave lithotripsy of lower ureteral stones. Urologia Internationalis 2009;82(2):166‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Endnote 2016 [Computer program]
- Clarivate Analytics. EndNote. Version 7.5. Clarivate Analytics, 2016.
Fan 2013
- Fan B, Yang D, Wang J, Che X, Li X, Wang L, et al. Can tamsulosin facilitate expulsion of ureteral stones? A meta‐analysis of randomized controlled trials. Internation Journal of Urology 2013;20(8):818‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ghani 2014
- Ghani KR, Roghmann F, Sammon JD, Trudeau V, Sukumar S, Rahbar H, et al. Emergency department visits in the United States for upper urinary tract stones: trends in hospitalization and charges. Journal of Urology 2014;191(1):90‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Spiegelhalter DJ. Being sceptical about meta‐analyses: a Bayesian perspective on magnesium trials in myocardial infarction. International Journal of Epidemiology 2002;31:96‐104. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollingsworth 2006
- Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, et al. Medical therapy to facilitate urinary stone passage: a meta‐analysis. Lancet 2006;368(9542):1171‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hollingsworth 2016
- Hollingsworth JM, Canales BK, Rogers MAM, Sukumar S, Yan P, Kuntz GM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta‐analysis. BMJ 2016;355:i6112. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2015
- Huang W, Xue P, Zong H, Zhang Y. Efficacy and safety of silodosin in the medical expulsion therapy for distal ureteral calculi: a systematic review and meta‐analysis. British Journal of Clinical Pharmacology 2016;81(1):13‐22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2015
- Liu C, Zeng G, Kang R, Wu W, Li J, Chen K, et al. Efficacy and safety of alfuzosin as medical expulsive therapy for ureteral stones: a systematic review and meta‐analysis. PLoS One 2015;10(8):e0134589. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matlaga 2007
- Matlaga BR, Coe FL, Evan AP, Lingeman JE. The role of Randall's plaques in the pathogenesis of calcium stones. Journal of Urology 2007;177(1):31‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Michel 2006
- Michel MC, Rosette JJ. Alpha‐blocker treatment of urolithiasis. European Urology 2006;50(2):213‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miller 1999
- Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: a guide for patient education. Journal of Urology 1999;162(3 Pt 1):688‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morita 1987
- Morita T, Wada I, Saeki H, Tsuchida S, Weiss RM. Ureteral urine transport: changes in bolus volume, peristaltic frequency, intraluminal pressure and volume of flow resulting from autonomic drugs. Journal of Urology 1987;137(1):132‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakada 2008
- Nakada SY. Tamsulosin: ureteric motility. BJU International 2008;101(9):1061‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pearle 2005
- Pearle MS, Calhoun EA, Curhan GC, Urologic Diseases of America Project. Urologic Diseases in America Project: Urolithiasis. Journal of Urology 2005;173(3):848‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pearle 2007
- Pearle MS, Lotan Y. Urinary lithiasis: etiology, epidemiology, and pathogenesis. In: Campbell MF, Wein AJ, Kavoussi LR editor(s). Campbell‐Walsh Urology. 9th Edition. Philadelphia: Saunders Elsevier, 2007. [Google Scholar]
Preminger 2007
- Preminger GM, Tiselius HG, Assimos DG, Alken P, Buck AC, Gallucci M, et al. 2007 guideline for the management of ureteral calculi. Journal of Urology 2007;178(6):2418‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ramello 2000
- Ramello A, Vitale C, Marangella M. Epidemiology of nephrolithiasis. Journal of Nephrology 2000;13 Suppl 3:S45‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2006
- Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El‐Solh AA, Ernst A, et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006;174:605‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singh 2007
- Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy for facilitate passage of ureteral calculi. Annals of Emergency Medicine 2007;50(5):552‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54:1046‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sterrett 2008
- Sterrett SP, Nakada SY. Medical expulsive therapy. Current Opinion in Urology 2008;18(2):210‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tiselius 2003
- Tiselius HG. Epidemiology and medical management of stone disease. BJU International 2003;91(8):758‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Turney 2011
- Turney BW, Reynard JM, Noble JG, Keoghane SR. Trends in urological stone disease. BJU International 2011;109:1082‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
UDA 2012
- Litwin MS, Saigal CS (editors). Table 14.47. Economic impact of urological disease. In: Urologic Diseases in America. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office, 2012; NIH Publication No. 12‐7865; pp. 486. http://www.udaonline.net (accessed 21 March 2014).
References to other published versions of this review
Campschroer 2014
- Campschroer T, Zhu Y, Duijvesz D, Grobbee DE, Lock MTWT. Alpha‐blockers as medical expulsive therapy for ureteral stones. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD008509.pub2] [DOI] [PubMed] [Google Scholar]
Zhu 2010
- Zhu Y, Rovers MM, Duijvesz D, Lock MT. Alpha‐blockers as medical expulsive therapy for ureteral stones. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD008509] [DOI] [PubMed] [Google Scholar]


