Abstract
Background
Although complementary feeding is a universal practice, the methods and manner in which it is practiced vary between cultures, individuals and socioeconomic classes. The period of complementary feeding is a critical time of transition in the life of an infant, and inappropriate complementary feeding practices, with their associated adverse health consequences, remain a significant global public health problem. Educational interventions are widely acknowledged as effective in promoting public health strategy, and those aimed at improving complementary feeding practices provide information about proper complementary feeding practices to caregivers of infants/children. It is therefore important to summarise evidence on the effectiveness of educational interventions to improve the complementary feeding practices of caregivers of infants.
Objectives
To assess the effectiveness of educational interventions for improving the complementary feeding (weaning) practices of primary caregivers of children of complementary feeding age, and related health and growth outcomes in infants.
Search methods
In November 2017, we searched CENTRAL, MEDLINE, Embase, 10 other databases and two trials registers. We also searched the reference lists of relevant studies and reviews to identify any additional studies. We did not limit the searches by date, language or publication status.
Selection criteria
Randomised controlled trials (RCTs), comparing educational interventions to no intervention, usual practice, or educational interventions provided in conjunction with another intervention, so long as the educational intervention was only available in the experimental group and the adjunctive intervention was available to the control group. Study participants included caregivers of infants aged 4 to 24 months undergoing complementary feeding. Pregnant women who were expected to give birth and commence complementary feeding during the period of the study were also included.
Data collection and analysis
Two review authors independently extracted data on participants, settings, interventions, methodology and outcomes using a specifically‐developed and piloted data extraction form. We calculated risk ratios (RR) and 95% confidence intervals (CIs) for dichotomous data, and mean differences (MD) and 95% CIs for continuous data. Where data permitted, we conducted a meta‐analysis using a random‐effects model. We assessed the included studies for risk of bias and also assessed the quality of evidence using the GRADE approach.
Main results
We included 23 studies (from 35 reports) with a total of 11,170 caregiver‐infant pairs who were randomly assigned to receive an educational intervention delivered to the caregiver or usual care. Nineteen of the included studies were community‐based studies while four were facility‐based studies. In addition, 13 of the included studies were cluster‐randomised while the others were individually randomised. Generally, the interventions were focused on the introduction of complementary feeding at the appropriate time, the types and amount of complementary foods to be fed to infants, and hygiene. Using the GRADE criteria, we assessed the quality of the evidence as moderate, mostly due to inadequate allocation concealment and insufficient blinding.
Educational interventions led to improvements in complementary feeding practices for age at introduction of complementary foods (average RR 0.88, 95% CI 0.83 to 0.94; 4 studies, 1738 children; moderate‐quality evidence) and hygiene practices (average RR 1.38, 95% CI 1.23 to 1.55; 4 studies, 2029 participants; moderate‐quality evidence). For duration of exclusive breastfeeding, pooled results were compatible with both a reduction and an increase in the outcome (average RR 1.58, 95% CI 0.77 to 3.22; 3 studies, 1544 children; very low‐quality evidence). There was limited (low to very low‐quality) evidence of an effect for all growth outcomes.
Quality of evidence
There is moderate to very low‐quality evidence that educational interventions can improve complementary feeding practices but insufficient evidence to conclude that it impacts growth outcomes.
Authors' conclusions
Overall, we found evidence that education improves complementary feeding practices.
Plain language summary
Educational interventions for improving complementary feeding practices
Background
Complementary feeding is the period when an infant moves from taking only breast milk or breast‐milk substitutes (such as infant formula) to family food. It is a critical period in the life of an infant. Inappropriate complementary feeding practices, with their associated adverse health consequences, remain a significant global public health problem. This is because inappropriate complementary feeding practices, such as introduction of semi‐solid foods too early (before six months of age), poor hygiene or giving foods that do not contain adequate nutrients, are all major causes of illness. Such illnesses include malnutrition, diarrhoea, poor growth, infections and poor mental development of children. Education has been proposed as an effective means of improving complementary feeding practices.
Review question
Does education improve complementary feeding practices of caregivers of infants as well as the health and growth of the infants?
Study characteristics
We searched for randomised controlled trials (a type of experiment in which people are randomly allocated to one or more treatment groups) up until November 2017. The search identified 23 studies involving a total of 11,170 caregivers and their children. The ages of the children ranged from birth to 24 months. The caregivers received educational interventions alone while the control group received no intervention, usual care or any other non‐educational intervention. The educational methods included printed materials such as leaflets, counselling, teaching sessions, peer support, videos and practical demonstrations. Generally, the education messages were focused on the introduction of semi‐solid foods at the appropriate age, the types and amount of complementary foods to be fed to infants, and hygiene.
Key results
Education reduced the number of caregivers that introduced semi‐solid foods to their infants before six months of age by up to 12% (moderate‐quality evidence). Hygiene practices of caregivers who received education also showed some improvement compared to those that did not (moderate‐quality evidence). In studies conducted in the community, education increased the duration of exclusive breastfeeding, but not in studies conducted in health facilities. There was no convincing evidence of an effect of education on the growth of children (low to very low‐quality evidence). We could not combine the results from different studies for diarrhoea, knowledge of caregivers and adequacy of complementary food. However, from the individual reports of the study authors, education led to a reduction in diarrhoea and an improvement in the knowledge of caregivers. It also led to improvement in the quality and quantity of complementary foods fed to infants.
Overall, we found evidence that education improves complementary feeding practices.
Summary of findings
Background
Description of the condition
Complementary feeding is defined as, "the process starting when breast milk alone or infant formula alone is no longer sufficient to meet the nutritional requirements of infants, and therefore, other foods and liquids are needed, along with breast milk or a breast‐milk substitute" (WHO 2008, p v). It is the period of transition from breast milk or breast‐milk substitute to family foods, and entails, "introducing a range of foods gradually until the baby is eating the same foods as the rest of the family" (UNICEF 2008, p 3; WHO 2015a).
Although complementary feeding is a universal practice, the methods and manners in which it is practiced vary between cultures, individuals, and socioeconomic classes. For example, although the recommended time for initiation of complementary foods is six months of age (World Health Assembly 2001), when breast milk alone is insufficient for the infant, some caregivers may initiate complementary feeding before this time for personal or cultural reasons. Alternatively, some caregivers may give teas or sugary drinks to infants based on personal reasons or the influence of family members or peers (Black 2001). Therefore, although complementary feeding may be defined in different ways based on these variances, for the purpose of this review we will adopt the WHO 2008 definition of complementary feeding stated above.
Most babies at the age of six months are developmentally prepared for the consumption of other foods. As this period is usually characterised by increases in the nutritional needs of the infants for growth and physiological development, and as breast milk alone or breast‐milk substitute alone are insufficient for meeting these requirements, complementary feeding is needed (World Health Assembly 2001).
Complementary foods are, ''any food or liquids, whether manufactured or locally prepared, suitable as a complement to breast milk or to a breast‐milk substitute, fed to infants during the complementary feeding period" (WHO 2008, p v). This should not include drinks and beverages that are low in nutrient content, like coffee, teas, and sugary drinks like soda. Coffee and teas also contain compounds that can inhibit the absorption of iron (PAHO/WHO 2003). Proper complementary feeding is essential for healthy growth, survival and the attainment of a child's human potential (PAHO/WHO 2003). The introduction of complementary foods should be timely and adequate in nutritional content, tailored to meet the age‐specific needs of the infant, and should provide all the micronutrients and vitamins needed by infants for adequate growth and cognitive development. In settings where complementary foods lack basic micronutrients, there may be a need for food fortification and micronutrient supplementation to boost the dietary content of these foods (Lutter 2003; PAHO/WHO 2003). Vitamin supplements given to babies as part of recommended public health interventions are not considered part of complementary feeding.
The period starting from birth to two years of age has been identified as a critical period in the life of infants for the promotion of optimal growth, health and development (Shrimpton 2001; Victora 2008), and poor nutrition at this stage will result in malnutrition in many infants (WHO 2008). Most incidents of stunting occur in the first two years of life when there is increased demand for adequate nutrition to fuel infant growth and physiological development (Shrimpton 2001). Inappropriate complementary feeding practices during this period, such as early onset of complementary foods, inadequate nutritional content of complementary foods and poor hygiene behaviours, have been identified as the leading causes of undernutrition, growth faltering, diarrhoea, increased rate of infections, vitamin‐mineral deficiency, poor cognitive development and increased mortality among children (Motarjemi 1993; WHO 2012a; WHO 2015a). Undernutrition results from poor dietary intake and repeated infections and, “occurs when infants do not eat (or absorb) enough nutrients to cover their needs for energy and growth, or to maintain a healthy immune system” (Burgess 2012, p 1). An undernourished infant, "can no longer maintain natural bodily capacities, such as growth, resisting infections and recovering from disease" (UNICEF 2006, p 1). Undernutrition can have far‐reaching implications for the infant that can persist throughout his or her lifespan. Stunting that occurs during the first two to three years of a child's life is irreversible (Martorell 1994; Shrimpton 2001), and chances are high that a malnourished girl child would give birth to a malnourished and low‐birth‐weight infant (PAHO/WHO 2003). Malnutrition is responsible directly or indirectly for over half of all childhood deaths globally (WHO 2012a), with 45% of childhood deaths associated with undernutrition. More than two‐thirds of undernutrition‐associated deaths happen in the first year of life, and are usually correlated with poor complementary feeding practices (WHO 2003). A number of epidemiological studies have traced a nexus between poor complementary feeding practices, malnutrition and stunting in young children (Arimond 2004; Black 2008; Philips 2000; Shrimpton 2001). Black 2008 identifies suboptimum complementary feeding to be a causal factor of stunting and states categorically that, "even with optimum breastfeeding children will become stunted if they do not receive an adequate quantity and quality of complementary foods after 6 months of age" (p 251). Also, many studies have reported that the incidence of diarrhoeal disease is especially high after complementary feeding is initiated due to bacterial contamination (Black 1982; Henry 1990; Motarjemi 1993; Sheth 2006). Bacterial contamination can result from complementary foods of poor quality and improper food handling practices, which include unhygienic preparation, storage and preservation of complementary foods Motarjemi 1993.
In 2016, about 155 million children under five years of age were estimated to be stunted while 52 million children were estimated to be wasted (WHO 2018). It is reported that two out of five children in low‐income countries are stunted, "while 50‐70% of the burden of diarrhoeal diseases, measles, malaria and lower respiratory tract infections in childhood is attributable to undernutrition" (WHO 2003, p v). Diarrhoeal disease, which is the second‐leading cause of death in children aged from birth to 59 months, accounts for about 760,000 deaths in children under five years of age annually (Fischer Walker 2012; Fischer Walker 2013; Kosek 2003; WHO 2013a).
A number of factors have been identified to influence complementary feeding practices. Studies conducted in Bangladesh (Kabir 2012), Ireland (Tarrant 2010), and Tanzania (Victor 2014), found that the socioeconomic status of caregivers, maternal education level and age, opinions of family and friends, traditional feeding practices, influence of social network, father's occupation, postnatal care, and lack of professional advice influence complementary feeding practices. Some of the problems commonly associated with complementary feeding include starting complementary feeding too early, poor nutrient content of complementary foods, inadequate feed rations, insufficient breastfeeding, poor feeding practices, poor hygiene, and bacterial contamination of complementary foods and feeding utensils. Studies show that about 20% of mothers in the USA and Ireland introduce solid foods to their infants before four months of age (Fein 2008; Tarrant 2010). Recent studies from Nepal (Khanal 2013) and Tanzania (Victor 2014) report that an average of about 35% of complementary foods fed to infants in both countries met the minimum requirement for dietary diversity.
These variations or problems associated with complementary feeding, and the need to make safe the period of complementary feeding for the infant, necessitated the development of evidence‐informed guidelines for complementary feeding by the World Health Organization (WHO) and appropriate indicators to evaluate the process of complementary feeding (PAHO/WHO 2003). Caregivers need skilled support to provide adequate nutrition for their infants (WHO 2015a), and educational interventions to improve the timing and process of complementary feeding may be believed to be helpful in ensuring safe complementary feeding for infants. It is therefore necessary to evaluate the effects of educational interventions on the complementary feeding practices of caregivers of children of complementary feeding age.
Description of the intervention
In this review, educational interventions refer to health education interventions. Health education is defined by the WHO as, "consciously constructed opportunities for learning involving some form of communication designed to improve health literacy, including improving knowledge, and developing life skills, which are conducive to individual and community health" (WHO 1998, p 4). The Committee on Health Education and Promotion defines health education as, "any combination of planned learning experiences based on sound theories that provide individuals, groups and communities the opportunity to acquire information and the skills needed to make quality health decisions" (Gold 2002, p 3).
Health education interventions can be delivered to individuals or groups, face to face or by telephone in communities, hospitals, homes, schools, or organisations. They may be delivered by verbal, written or audiovisual means such as printed materials, multimedia (video messages, PowerPoint presentations), counselling sessions, practical demonstrations, lectures, and role plays (Ciciriello 2013; ILEP 1998; Nkhoma 2013). Within this review, we define educational interventions as consciously planned interventions that seek to communicate information (verbal, written or audiovisual) to individuals, groups or communities, with the aim of improving their knowledge and life skills to enable them to make quality health decisions. These interventions are usually consciously planned and constructed based on sound theories.
Educational interventions are widely acknowledged as effective in promoting public health strategy (Brunello 2012; Higgins 2008; Shah 2009). They have been used to prevent diseases; help patients or their caregivers to effectively manage health conditions; and improve or encourage adoption of healthy lifestyles, practices, and behaviours in individuals and the community (Darity 1997; Fredericks 2013; Hunter 2010; Ofotokun 2010; Saunders 1986). Educational interventions for improving weaning practices provide information about proper weaning practices (proper timing for initiation of complementary feeding; continuation of breastfeeding after introduction of semisolid foods; hygiene; composition, amount, consistency, and frequency of complementary food; and feeding of the infant during or after illness; to caregivers of infants/children (PAHO/WHO 2003). (We define caregivers as mothers, guardians or other family members responsible for caring for and feeding the infant, and personnel charged with the responsibility of looking after infants in childcare centres).
A number of studies suggest that educational interventions can be used to improve complementary feeding practices (Monte 1997; Roy 2007). Guldan 2000 and Kilaru 2005 reported that counselling sessions on appropriate complementary feeding practices improved outcomes such as growth of infants, infant feeding practices, and knowledge of mothers. Studies by Hotz 2005 and Saleem 2014 found that lectures or nutritional messages delivered to caregivers of infants were effective in improving energy intake and growth of infants. In Black 2001, an educational videotape intervention integrated into home visits improved time of initiating complementary feeding among adolescent mothers, while in Guldan 2000 and Yin 2009, lectures and counselling improved nutritional knowledge of caregivers. Nutrition education through focus group discussions have also been reported to be effective in preventing malnutrition and growth faltering in children under two years of age (Roy 2007).
How the intervention might work
Educational interventions essentially seek to achieve change in knowledge, attitudes, and behaviours by providing information, opportunities, or both, for participants to acquire or improve the skills required for the desired change. The scientific rigour and potential effectiveness of health promotion interventions depend on the availability of an evidence‐informed theoretical framework that can inform their design and implementation. Research suggests that health promotion and public health interventions built on social behavioural theories, such as the theory of planned behaviour, the health belief model, social cognitive theory, social ecological model, amongst others, are likely to be more effective than those that do not have strong theoretical foundations (Bluethmann 2017; Davis 2015; Glanz 2010; NCI 2005). This is more so if the theoretical models used include appropriate explanatory as well as action models, and provide a broad framework that addresses interpersonal, organisational, and environmental factors that influence health behaviour and not just the individual (Glanz 2014).
According to McLeroy's ecological model for health promotion, health behaviour is said to be influenced by five major factors or processes, namely intrapersonal, interpersonal, institutional (or organisational), community, and public policy factors (McLeroy 1988). Institutional, community, and public policy factors together constitute environmental factors (WHO 2012b). Intrapersonal factors include the attitudes, beliefs, skills, self‐efficacy and self‐concept of the individual. Interpersonal factors that influence health behaviour comprise the formal and informal social networks and support systems of an individual such as family members, peers or friends, or work group. Organisational or institutional factors include social institutions or organisations that provide formal (and informal) rules and regulations for operation, while community factors include social networks or norms (formal and informal) among individuals, groups or organisations. Public policy factors are local, state and federal laws and policies that promote healthy behaviours.
Educational interventions, which are expected to be effective in promoting health behaviours, must therefore seek to address not only intrapersonal factors, such as knowledge, attitudes and beliefs of individuals, but must also take cognisance of interpersonal and environmental factors. The way the intervention works can be explained using the theory of planned behaviour, which states that the likelihood that an individual will adopt a new behaviour is determined by his or her 'intention' to perform that behaviour, which in turn is influenced by his or her attitude, subjective norms and perceived behavioural controls (Ajzen 1991). Attitudes refer to an individual's positive or negative attitudes towards the desired behaviour. Subjective norms are the social pressures the individual experiences to adopt or avoid the desired behaviour (that is, how others view the behaviour). Perceived behavioural controls are a person’s perception of their ability to perform a given behaviour. Interventions that seek to improve complementary feeding practices are likely to focus on inducing and sustaining behaviour change that will minimise the risk of undernutrition and diarrhoea, which have been identified as the key morbidity consequences of poor complementary feeding practice. As a first step, these interventions may involve interfacing with communities to identify the common challenges associated with complementary feeding, which may include understanding their perceptions and constraints in adopting adequate complementary feeding practices (USAID 2011). The outcome of this often reveals knowledge gaps and deficiencies in practice, which are usually amenable to educational interventions specifically tailored to address the knowledge gaps and complementary feeding problems that have been identified (Gibbons 1984). The explanatory model would therefore be expected to explain the mechanisms and steps through which known undesirable behaviours (inappropriate complementary feeding practices) cause undernutrition, diarrhoea and other childhood problems, and also provide unambiguous information on the benefits of appropriate complementary feeding practices, which is expected to stimulate the adoption of appropriate complementary feeding practices. On the other hand, the action model would show how the proposed interventions would eliminate barriers or induce positive actions that would reverse or prevent the mechanisms that lead to diarrhoea or undernutrition during complementary feeding. Critical appraisal of studies included in this review will extract and report information on the use and appropriateness of theoretical models based on these basic constructs.
Educational interventions to improve complementary feeding practices that provide knowledge alone, without addressing barriers as a result of social norms and perceived behavioural controls, may not be effective in improving complementary feeding practices. Interventions may therefore seek to address social norms, such as cultural practices, which may pose as barriers to adopting recommended complementary feeding practices, and to improve self‐efficacy of caregivers by boosting their confidence and improving their skills to take action and, if need be, change their physical and social environments to aid behaviour change (USAID 2011).
In line with the theory of planned behaviour, a number of empirical studies have shown that attitudes, normative influences, and perceived behavioural controls influence breastfeeding and complementary feeding practices of caregivers (Hamilton 2011; Swanson 2005; Walingo 2014; Zhang 2009). The theory of planned behaviour agrees with McLeroy's ecological model for health promotion in that it proposes that the individual's intention to perform a health behaviour is determined by attitudes of the individual (intrapersonal factors), social norms, and perceived behavioural controls (interpersonal and environmental factors).
We have presented an example of a logic model or theory of change in Figure 1, which illustrates educational interventions to improve complementary feeding practice based on the health belief model. The health belief model hypothesises that a person’s decision to take a recommended health action is determined by their perceived susceptibility to the health problem, perceived severity of problem, perceived benefits of the health action, and perceived barriers to adopting the recommended action, as well as cues to action and self‐efficacy (Janz 1984; Rosenstock 1974). According to this model, knowledge about dangers or benefits (or both) of a health action (in this case proper complementary feeding practices), as well as self‐efficacy, determine a person’s decision to take the recommended action.
1.
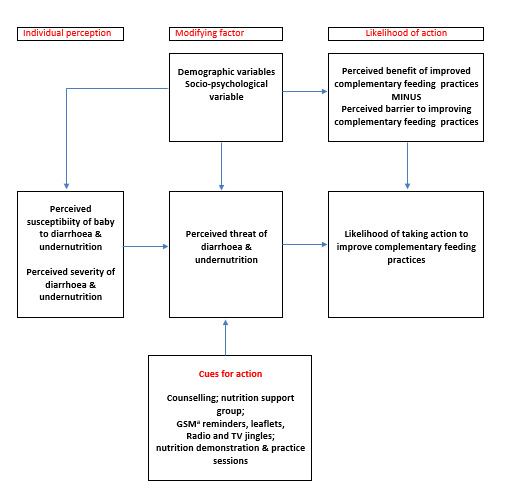
Theoretical model: educational interventions for improving complementary feeding practices
Footnotes aGSM: global system for mobile communication.
Caregivers with improved knowledge, skills, and self‐efficacy are more likely to practice better hygiene in food preparation, as well as ensure proper composition of complementary diets. Improved complementary foods will lead to reduced incidence of undernutrition, diarrhoea, and growth faltering (Monte 1997; Shi 2011).
Why it is important to do this review
The period of complementary feeding is a critical time of transition in the life of an infant, and inappropriate complementary feeding practices, with their associated adverse health consequences, remain a significant, global public health problem. A recent review of the epidemiology of global nutrition identified poor complementary feeding practices as major contributors to undernutrition and increased rates of infections in children under five years of age, and has proposed improvement in complementary feeding practices along with promotion of breastfeeding and micronutrient supplementation as strategies for combating undernutrition (Bhutta 2012). We can therefore expect that educational interventions aimed at improving complementary feeding practices would reduce the risk of malnutrition and food‐borne infections, especially diarrhoeal diseases.
A number of reviews have been conducted to evaluate the effectiveness of complementary feeding interventions, but none have been conducted to evaluate the effectiveness of educational interventions in promoting appropriate or recommended complementary feeding practices. Dewey 2008 conducted a non‐Cochrane systematic review on ‘The efficacy and effectiveness of complementary feeding interventions in developing countries'. This study did not focus on educational interventions, but looked broadly at different types of complementary feeding strategies. In addition, the authors only included studies conducted between 1996 and 2006 in the review, and they have not updated it to include studies from 2007 to date. Imdad 2011 and Lassi 2013 conducted two other non‐Cochrane systematic reviews assessing the impact of education and the provision of complementary feeding on growth and morbidity in children. Although the studies included children under two years of age, they were limited to low‐ and middle‐income countries and were not based strictly on randomised studies. Shi 2011 conducted a literature review on ‘Recent evidence of the effectiveness of educational interventions for improving complementary feeding practices in developing countries’ from 1998 onward. The systematic reviews listed above focused on growth and morbidity (stunting), but did not assess the effects of these interventions on behavioural outcomes and changes in knowledge of infant caregivers.
This Cochrane Review aims to summarise evidence on the effectiveness of educational interventions to improve complementary feeding practices of caregivers of infants. We will not limit the review to studies from low‐ and middle‐income countries alone, but will also include studies from high‐income countries. In addition to growth and morbidity outcomes, we will assess a number of other key outcomes, including changes in complementary feeding behaviour and knowledge of caregivers. This review will provide useful information on which educational intervention approaches are effective for promoting recommended complementary feeding practices.
Objectives
To assess the effectiveness of educational interventions for improving the complementary feeding (weaning) practices of primary caregivers of children of complementary feeding age, and related health and growth outcomes in infants.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), including cluster‐RCTs.
Types of participants
Study participants comprised caregivers of infants aged 4 to 24 months undergoing complementary feeding. Pregnant women who were expected to give birth and commence complementary feeding during the period of the study were also included.
Caregivers were defined as mothers, guardians, or other family members responsible for caring for and feeding the infant.
Types of interventions
We included studies that compared:
educational intervention to no intervention or usual practice (e.g. usual weaning or child care practice); and
educational interventions provided in conjunction with another intervention (e.g. provision of complementary food), so long as the educational intervention was only available in the experimental group and the adjunctive intervention was available to the control group.
We defined educational interventions as comprising one or more of the following, delivered in any setting: multimedia, lectures, workshops, practical demonstrations, printed materials, skills training, counselling, campaigns, or other instructional methods (written, verbal, or audiovisual).
Types of outcome measures
Primary outcomes
-
Improved complementary feeding practices (measured as a continuous outcome or dichotomous outcome), of the following:
age at introduction of complementary foods;
duration of exclusive breastfeeding;
adequacy of complementary foods (measured by number of children fed with adequate amount and consistency of complementary foods, children fed with at least five different classes of food, consisting mainly of protein, carbohydrate, vegetable, fats and oils, fruits; vitamin supplementation (for infant and mother); energy density of complementary foods; and meal frequency (number of times children are fed in a day); or based on the WHO minimum acceptable diet, minimum dietary diversity, minimum meal frequency or as assessed by study authors); and
hygiene practices: safe preparation and storage of complementary foods (measured by handwashing practices (washing of caregiver's and child's hands with soap before cooking, feeding, or eating); water sanitation practices; food preparation and storage practices; serving foods immediately after preparation; using clean utensils, plates, pots, etc. for preparing or serving food and for feeding the child; and avoiding the use of feeding bottles).
Adverse events (as defined by study authors). For example, overburdening of personnel delivering the intervention who were also responsible for other tasks in the health facility, stress on caregivers.
Secondary outcomes
Growth (measured by weight, height/length, head circumference, mid upper‐arm circumference (MUAC), weight‐for‐age (WAZ), height/length‐for‐age (H/LAZ), weight‐for‐height/length (WH/LZ) z scores, etc.)
Incidence of malnutrition among participants (as defined by WHO guidelines: WHO 2013b)
Morbidity (measured by episodes of diarrhoea)
Mortality (indicated by all‐cause mortality, diarrhoea‐specific mortality, malnutrition‐associated mortality)
Hospitalisation (indicated by the number hospitalised, length or duration of hospital stay)
Change in knowledge (measured by a difference in the pre‐test (baseline) and post‐test (postintervention) results in the intervention and control arms)
We presented our primary outcomes in Table 1, and our secondary outcomes in Table 2.
Summary of findings for the main comparison. Educational intervention versus no educational intervention for improving complementary feeding practices.
| Educational intervention versus no educational intervention for improving complementary feeding practices | ||||||
|
Patient or population: children of complementary feeding age
Settings: community and facility
Intervention: educational intervention Comparison: no educational intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No educational intervention | Educational intervention (ICC = 0.02) | |||||
| Age at introduction of complementary foods Measurement: proportion participants with event Follow‐up: 4 to 16 months | Study population | RR 0.88 (0.83 to 0.94) | 1738 (4 studies) | ⊕⊕⊕⊝ Moderatea | ‐ | |
| 661 per 1000 | 581 per 1000 (548 to 621) | |||||
| Moderate | ||||||
| 746 per 1000 | 656 per 1000 (619 to 701) | |||||
| Duration of exclusive breastfeeding (≥ 4 months of age) Measurement: proportion of participants with event Follow‐up: 1 to 36 months | Study population | RR 1.58 (0.77 to 3.22) | 1544 (3 studies) | ⊕⊝⊝⊝ Very lowa,b,c | ‐ | |
| 129 per 1000 | 204 per 1000 (100 to 416) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Duration of exclusive breastfeeding (≥ 4 months of age): community‐based intervention Measurement: proportion of participants with event Follow‐up: 1 to 36 months | Study population | RR 2.32 (1.45 to 3.73) | 1167 (2 studies) | ⊕⊕⊝⊝ Lowa,c | ‐ | |
| 40 per 1000 | 92 per 1000 (58 to 148) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Duration of exclusive breastfeeding (≥ 4 months of age): facility‐based intervention Measurement: proportion of participants with event Follow‐up: mean 18 months | Study population | RR 0.95 (0.70 to 1.29) | 377 (1 studies) | ⊕⊕⊝⊝ Lowa,c | ‐ | |
| 426 per 1000 | 405 per 1000 (298 to 550) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Hygiene practices: community‐based intervention Measurement: proportion of participants with event Follow‐up: 6 to 18 months | Study population | RR 1.38 (1.23 to 1.55) | 2029 (4 studies) | ⊕⊕⊕⊝ Moderatea | ‐ | |
| 546 per 1000 | 754 per 1000 (672 to 847) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aWe downgraded the quality of the evidence by one level due to serious risks of bias; the method of sequence generation, allocation concealment and blinding of outcome assessors was unclear or not undertaken in some of the studies bWe downgraded the quality of the evidence by one level due to serious inconsistency; I2 = 80% cWe downgraded the quality of the evidence by one level due to serious imprecision; the CI crossed the line of no effect
Summary of findings 2. Educational intervention versus no educational intervention for improving complementary feeding practices: growth outcomes.
| Educational intervention versus no educational intervention for improving complementary feeding practices: growth outcomes | ||||||
|
Patient or population: children of complementary feeding age
Settings: community and facility
Intervention: educational Intervention Comparison: no educational intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No educational intervention | Educational intervention (ICC = 0.05) | |||||
| Weight (at 6 months of age) Measurement: kg (mean and standard deviation) Follow‐up: 9 to 12 months | ‐ | The mean weight at 6 months of age in the intervention groups was 0.03 kg higher (0.10 lower to 0.17 higher) | ‐ | 1221 (3 studies) | ⊕⊝⊝⊝ Very lowa,b | ‐ |
| Weight (at 12 months of age) Measurement: kg (mean and standard deviation) Follow‐up: 9 to 18 months | ‐ | The mean weight at 12 months of age in the intervention groups was 0.06 kg higher (0.04 lower to 0.15 higher) | ‐ | 2464 (5 studies) | ⊕⊝⊝⊝ Very lowa,b | ‐ |
| Height/length (at 6 months of age) Measurement: cm (mean and standard deviation) Follow‐up: 9 to 12 months | ‐ | The mean height/length at 6 months of age in the intervention groups was 0.16 cm higher (0.21 lower to 0.52 higher) | ‐ | 1221 (3 studies) | ⊕⊝⊝⊝ Very lowa,b | ‐ |
| Height/length (at 12 months of age) Measurement: cm (mean and standard deviation) Follow‐up: 9 to 18 months | ‐ | The mean height/length at 12 months of age in the intervention groups was 0.32 cm higher (0.11 to 0.52 higher) | ‐ | 2464 (5 studies) | ⊕⊕⊝⊝ Lowa | ‐ |
| Nutritional status: stunting (H/LAZ ≤ −2 SD) Measurement: proportion of participants with events Follow‐up: 6 to 24 months | 199 per 1000 | 177 per 1000 (147 to 211) | RR 0.89 (0.74 to 1.06) | 3487 (5 studies) | ⊕⊕⊝⊝ Lowa,b | ‐ |
| Nutritional status: wasting (WH/LZ ≤ −2 SD) Measurement: proportion of participants with event Follow‐up: 4 to 12 months | 400 per 1000 | 316 per 1000 (192 to 520) | RR 0.79 (0.48 to 1.30) | 2000 (2 studies) | ⊕⊕⊝⊝ Lowa,b | ‐ |
| Nutritional status: underweight (WAZ ≤ −2 SD) Measurement: proportion of participants with event Follow‐up: 6 to 18 months | 138 per 1000 | 136 per 1000 (94 to 198) | RR 0.99 (0.68 to 1.44) | 2900 (3 studies) | ⊕⊕⊝⊝ Lowa,b | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; ICC: intra‐class correlation coefficient; H/LAZ: height/length‐for‐age z‐score; RR: risk ratio; SD: standard deviation; WAZ: weight‐for‐age z‐score; WH/LZ: weight‐for‐height/length z‐score | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aWe downgraded the quality of the evidence by two levels due to very serious risks of bias; the method of sequence generation, allocation concealment and blinding of outcome assessors was unclear or not undertaken in most of the studies bWe downgraded the quality of the evidence by one level due to serious imprecision; the CI crossed the line of no effect
Search methods for identification of studies
Electronic searches
In November 2017, we searched the following electronic databases and trials registers from inception onwards. We did not limit our searches by date, language or publication status. All of the search strategies are reported in Appendix 1.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 10) in the Cochrane Library, and which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 6 November 2017)
MEDLINE Ovid (1946 to October week 4 2017)
MEDLINE In‐process and Other Non‐indexed Citations Ovid (3 November 2017)
MEDLINE Epub Ahead of Print Ovid (3 November 2017)
Embase Ovid (1974 to 2017 week 45)
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 6 November 2017)
Science Citation Index Web of Science: Clarivate Analytics (SCI; 1970 to 6 November 2017)
Social Sciences Citation Index Web of Science: Clarivate Analytics (SSCI; 1970 to 6 November 2017)
Conference Proceedings Citation Index ‐ Science Web of Science: Clarivate Analytics (CPCI‐S; 1990 to 6 November 2017)
Conference Proceedings Citation Index ‐ Social Science & Humanities Web of Science: Clarivate Analytics (CPCI‐SS&H; 1990 to 6 November 2017)
Cochrane Database of Systematic Reviews (CDSR; 2017, Issue 11) part of the Cochrane Library (searched 6 November 2017)
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2 of 4; final issue) part of the Cochrane Library (last searched 1 July 2015)
LILACS (Latin American and Caribbean Health Science Information database; lilacs.bvsalud.org/en; searched 7 November 2017)
ClinicalTrials.gov (clinicaltrials.gov; searched 7 November 2017)
WHO International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch; searched 7 November 2017)
Searching other resources
We checked the reference lists of relevant studies and reviews identified by the electronic searches to identify any additional studies. In addition, we contacted relevant individuals and organisations for information about any ongoing or unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (DA, MTC) independently screened titles and abstracts for eligibility, and obtained the full reports of any potentially relevant studies. The same review authors independently applied the inclusion criteria to the full reports using an eligibility form and scrutinised publications to ensure that we included each study in the review only once. We also contacted study authors for clarification if eligibility was unclear, and resolved disagreements through discussion with a third review author (EE or FO).
We listed studies that were excluded after their full‐texts were assessed and the reasons for their exclusion in Characteristics of excluded studies tables.
We recorded our decisions in a PRISMA study flow diagram (Moher 2009).
Data extraction and management
Two review authors (DA, MTC) independently extracted data on the following, using a specifically developed and piloted data extraction form.
General information about the study
Study characteristics, including study settings and characteristics of the participants
Methods and quality of the study, including duration of the study, study design, type of randomisation employed, inclusion and exclusion criteria, details of the control and comparison groups, description and number of participants, duration of follow‐up
Details of the intervention
How information was collected and outcome measures assessed
Results
Both review authors (DA, MTC) compared the extracted data for discrepancies and resolved any disagreements through discussion with all review authors. Where information was unclear or data were missing, we contacted the corresponding authors of identified publications (see section on Dealing with missing data).
DA entered relevant data into Cochrane's statistical software: Review Manager 5 (RevMan 5) (Review Manager 2014).
Assessment of risk of bias in included studies
Using the Cochrane 'Risk of bias' tool (Higgins 2017, Section 8.5, Table 8.5a), two review authors (DA, MC) independently assessed the risks of bias of each included study across the domains described below.
Sequence generation
Description: we examined the method used to generate the allocation sequence in sufficient detail to assess whether it would produce comparable groups.
Review authors' judgement: what is the risk of selection bias due to inadequate generation of a randomised sequence?
Allocation concealment
Description: we described the method used to conceal the allocation sequence in sufficient detail in order to assess whether intervention allocation schedules could have been foreseen in advance of, or during, recruitment.
Review authors' judgement: what is the risk of selection bias due to inadequate concealment of allocations prior to assignments?
Blinding of participants and personnel
Description: we examined the measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received and any information as to whether the intended blinding was effective.
Review authors' judgement: what is the risk of performance bias due to knowledge of the allocated interventions by participants and personnel during the study?
Blinding of outcome assessment
Description: we examined the measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received and any information as to whether the intended blinding was effective.
Review authors' judgement: what is the risk of detection bias due to knowledge of the allocated interventions by outcome assessors?
Incomplete outcome data
Description: we examined the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis, and if attrition and exclusions were reported. We also examined if the reasons for the attrition and exclusion, numbers in each intervention and control group, and any re‐inclusions in the analyses performed by the review authors were reported.
Review authors' judgement: what is the risk of attrition bias due to the amount, nature and handling of incomplete outcome data?
Selective outcome reporting
Description: we assessed how the study authors examined the possibility of selective outcome reporting and their findings.
Review authors' judgement: what is the risk of reporting bias due to selective outcome reporting?
Other bias
Description: we examined other sources of bias not covered by the 'Risk of bias' tool.
Review authors' judgement: what is the risk of bias due to issues not addressed in the other domains of the 'Risk of bias' tool?
We assigned ratings of low, high, or unclear risk of bias to each of the domains for each included study and recorded these ratings in the 'Risk of bias' tables (beneath the Characteristics of included studies tables). We assigned a low risk of bias to studies that provided adequate information to ascertain that the investigators used the appropriate methods to successfully reduce bias. We assigned a high risk of bias to studies that provided adequate information to ascertain that investigators did not use appropriate methods to reduce bias, and we assigned an unclear risk of bias to studies that did not provide adequate information to ascertain whether or not investigators used the appropriate methods to reduce bias (Higgins 2017, Section 8.5, Table 8.5d). We resolved any differences by discussion with all review authors.
We presented our judgements about each 'Risk of bias' item as percentages across all included studies (Figure 2), and summarised our assessment in a 'Risk of bias' summary graph (Figure 3).
2.
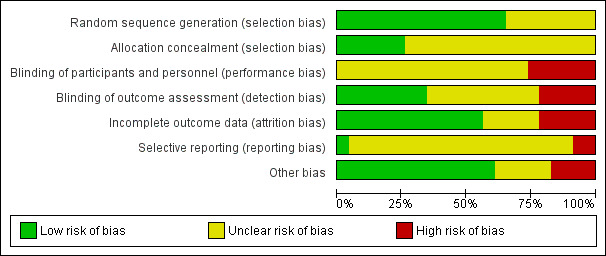
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
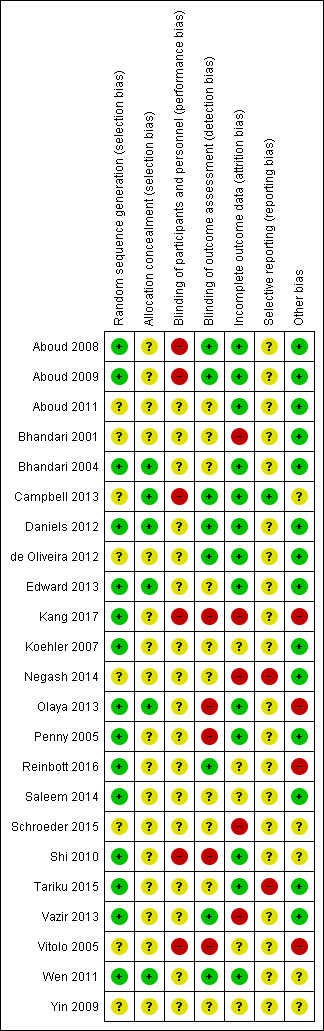
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Measures of treatment effect
Dichotomous outcomes
We calculated risk ratios (RR) and 95% confidence intervals (CIs) for dichotomous outcomes, such as adequate hygiene (handwashing).
Continuous outcomes
We calculated mean differences (MD) and 95% CIs for continuous data measured using the same scale (e.g. kilograms (kg)). We did not calculate a standardised mean difference since outcomes were reported using the same scale.
See Arikpo 2015 and Table 3.
1. Additional methods.
| Measures of treatment effect |
Event rate outcomes In this review, it is possible that some outcomes (e.g. diarrhoea, hospitalisation, malnutrition) may have been recorded as counts where the event can occur multiple times to the same participant. Where study data allow (i.e. data are available on both events and person‐years at risk), we will calculate rate ratios for count outcomes. However, study authors can report count data in a number of ways. As such, our strategy will be to extract count data in the form as reported by the original authors. For example, if study authors have reported the outcome using a rate ratio, we will extract it as such. If study authors have reported the outcome as dichotomous, we will extract it as a dichotomous outcome, noting the potential disadvantages of doing so. Multiple outcome data It is possible that studies will summarise outcomes in several ways, for example, both as a continuous and dichotomous measure. For the primary outcomes, if person‐years at risk are available, our preference will be to analyse count data as a rate ratio. However, if sufficient information is not available, and the event is common, we will analyse count data as if it were continuous. We consider the continuous measure to be clinically reasonable and preferable to dichotomising the primary outcomes. If neither of these approaches is suitable, we will extract the data as if it were dichotomous, ensuring that we classify all participants into one of two possible groups only. |
| Unit of analysis issues |
Multiple intervention groups Studies with more than two intervention arms can pose analytical problems in a meta‐analysis. For example, it is important to avoid 'double‐counting' of participants. Where studies may have two or more active arms to be compared against a control, or two control conditions versus an experimental condition, we will combine similar interventions to generate a single pair‐wise comparison for the meta‐analysis. If interventions are not similar, we will split the 'shared' comparator into two groups and include as two comparisons. |
| Dealing with missing data | If we are unable to retrieve missing dichotomous data, we will conduct an available‐case analysis. We plan to undertake a sensitivity analysis assuming that participants who withdrew from either arm after randomisation experienced a negative event. In common with many public health educational interventions, dropouts are often due to perceived difficulties with the intervention or information contradictory to existing beliefs or community norms (among other reasons). As such, it is not realistic to consider a 'best case' sensitivity analysis where all dropouts successfully adhered to the intervention, for weaning practice. We will analyse missing continuous data on a completers basis, including only those participants with a final assessment. Where we are unable to obtain the missing SDs from the study authors, we will calculate them from P values, t values, confidence intervals, or standard errors, where these have been reported. If this is not possible, and only a minority of studies are missing SDs, we will impute the SD using other studies in the meta‐analysis. We will also report the extent of the missing data, describe the attrition for each study in the 'Risk of bias' tables, and discuss the possible impact of this missing data on the results of the review. We will perform a sensitivity analysis to assess the impact of the inclusion of studies with missing data on the findings of the review (Deeks 2017, Section 9.7). |
| Assessment of reporting biases | We will try to minimise publication bias by doing a comprehensive search of multiple sources and databases, and by including studies of good methodological quality and data from unpublished and ongoing studies (Sterne 2017, Section 10.3). If we have a sufficient number of included studies (at least 10), we will use outcome data to run a funnel plot regression to investigate the possibility of publication bias (Sterne 2017, Section 10.4). Funnel plot asymmetry could be due to publication bias, poor methodological quality, true heterogeneity, or a real relationship between study size and effect size or chance. We will further investigate publication bias by comparing the data extracted from published and unpublished studies in a sensitivity analysis (Sterne 2017, Section 10.4.4) |
| Subgroup analysis and investigation of heterogeneity |
|
| Sensitivity analysis | We will conduct a sensitivity analysis in order to detect the effect of excluding studies with missing data, unpublished studies, and studies with high risk of bias (judged using Cochrane’s tool for assessing risk of bias (Higgins 2017)) on the overall results of the meta‐analysis. In this analysis, we will explore the possible effects of marked differences between included studies. We will also undertake a fixed‐effect meta‐analysis to determine the robustness of the results from the random‐effects meta‐analysis |
SD: standard deviation.
Unit of analysis issues
Multiple intervention groups
For studies with two or more intervention arms, we included only the intervention arm of interest (the arm that received educational interventions alone or educational interventions provided in conjunction with another intervention, so long as the educational intervention was only available in the experimental group and the adjunctive intervention was available to the control group) and the control arm.
Cluster‐randomised studies
For appropriately analysed studies, where the analysis was adjusted for clustering, we extracted data for the estimates of treatment effect, as reported by the study authors, to use directly in the meta‐analysis. However, for the majority of studies that reported results at the individual level without explicitly accounting for clustering, we followed the guidance on inflating the standard error for incorporating cluster‐randomised studies in meta‐analyses, as reported in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011, Section 16.3). In order to calculate the design effect, we used the original randomised sample at baseline for both dichotomous and continuous outcomes. Where the study investigators did not report the intra‐cluster correlation coefficient (ICC), number of clusters, or mean cluster size, we contacted them in the first instance to request the additional information. If the ICC was not available, we used estimates from similar studies included in the review or appropriate external studies. We considered sensitivity analyses for a range of ICCs (see Sensitivity analysis section). If information on cluster size was unavailable, we excluded the study from the meta‐analysis.
Dealing with missing data
We contacted the contact authors of included studies to retrieve missing data needed for analysis up to three times, and included the data in the analyses. We describe the attrition for each study in the Characteristics of included studies and 'Risk of bias' tables.
We included dichotomous outcomes in the main analysis on an intent‐to‐treat basis, where we assumed missing participants did not experience the event. However, we examine this assumption in a best‐worse case sensitivity analysis (see Sensitivity analysis section). For continuous outcomes we analysed data for completers only.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by examining study characteristics such as design; setting; participant; intervention; follow‐up; outcome measures; method of randomisation; sequence generation; allocation concealment; and blinding of outcome assessors, interventions, or outcome measures. The similarities and differences between included studies in terms of these study characteristics are discussed in the Results section. Due to concerns regarding the low power of the Chi2 test, we also report the Tau2 and I2 statistics in the main text. Tau2 provides an estimate of the between‐study variance in a random effects meta‐analysis. I2 describes the proportion of variation in the estimates of intervention effect that is attributable to heterogeneity, rather than sampling error (Higgins 2003). We had planned to use the guideline ranges reported in the Cochrane Handbook for Systematic Reviews of Interventions for the interpretation of the I2 (Deeks 2017), where a I2 value of 0% to 40% may indicate non‐important heterogeneity, 30% to 60% may indicate moderate heterogeneity, 50% to 90% may indicate substantial heterogeneity, and 75% to 100% may indicate considerable heterogeneity (Section 9.5.2). However, having too few studies in a meta‐analysis can present challenges for the estimation of heterogeneity, which may not be reliable when only two or three studies are available. As such, we did not apply the I2 ranges as specified in the protocol (Arikpo 2015). Where heterogeneity was observed (e.g. I2 greater than 50%, with consideration of the direction of effects and strength of evidence for heterogeneity (P value)), we had also planned to conduct a subgroup analysis to investigate possible explanations (see Subgroup analysis and investigation of heterogeneity section). However, as few studies were available for meta‐analysis, we report subgroup analyses for illustrative purposes only.
Assessment of reporting biases
We were unable to assess reporting bias using a funnel plot analysis as planned, due to the insufficient numbers of studies included in each category of the meta‐analyses. Our strategy for assessing reporting biases in future updates of this review is documented in our protocol (Arikpo 2015) and also presented in Table 3.
Data synthesis
We performed a meta‐analysis to obtain the overall estimate of the effect of educational interventions when more than one study was sufficiently comparable in terms of methodology, population and outcomes. We compared the information extracted for each study in the Characteristics of included studies tables to determine whether the quantitative combination of studies was appropriate. Where data were from individually‐randomised, parallel‐group studies, we conducted the meta‐analysis using RevMan 5 (Review Manager 2014), employing the random‐effects model, since we had anticipated the possibility of substantial clinical heterogeneity, given the nature of educational interventions. We used the Mantel‐Haenszel method for dichotomous outcomes, and the inverse variance method for continuous outcomes. However, where we needed to account for clustering in studies (Unit of analysis issues), we followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011, section 16.3.6), and combined studies using the generic inverse variance approach in RevMan 5 (Review Manager 2014).
We provided a narrative summary for outcomes where a meta‐analysis was not feasible. This was for two reasons:
either insufficient statistics were reported/provided by an individual study to enable a calculation of an effect estimate; or
the study‐reported outcome was incompatible with the others in the meta‐analysis.
In both cases, we report the fullest information possible as extracted from the individual study report, that is, where an effect estimate was not provided or was possible to calculate, we state this in the text. We also clearly annotate extracted metrics as 'study author‐reported'.
Asessment of the quality of evidence
Using the GRADE approach, we assessed the quality of evidence for each outcome pooled in the meta‐analysis, according to the presence of the following five factors: risk of bias, consistency, directness, precision, and publication bias (Guyatt 2008). We exported data from RevMan 5 (Review Manager 2014) to GRADEprofiler GDT (GRADEpro GDT 2015) to produce 'Summary of findings' tables for the comparisons: educational intervention versus no educational intervention for improving complementary feeding practices and educational intervention versus no educational intervention for improving complementary feeding practices: growth outcomes. We included the following outcomes in these tables.
Table 1: Improved complementary feeding practices
Age at introduction of complementary food
Duration of exclusive breastfeeding
Hygiene practices
Table 2: growth outcomes
Weight at 6 month
Weight at 12 months
Height/length at 6 months
Height/length at 12 months
Nutritional status: stunting
Nutritional status: wasting
Nutritional status: underweight
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analysis for the study setting.
Setting: community‐based studies and facility‐based studies
There were insufficient studies to perform a subgroup analysis for educational intervention delivery strategy. We were also unable to conduct subgroup analyses for educational intervention focus/message because the intervention focus/messages of the studies overlapped with the different aspects of complementary feeding. These analyses have been archived for use in future updates of this review (see Arikpo 2015; Table 3).
Sensitivity analysis
Due to the limited number of studies we were able to include in our meta‐analyses, we did not conduct the planned sensitivity analyses to detect the effect of excluding studies with missing data, unpublished studies, and studies with high risk of bias (judged using Cochrane’s tool for assessing risk of bias; Higgins 2017) on the overall results of the meta‐analysis. These have been archived for use in future updates of this review (see Arikpo 2015; Table 3).
We conducted sensitivity analyses for the primary outcomes only, to investigate the impact of assuming an alternative ICC on the summary effect estimates.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies.
Results of the search
The search strategy identified 11,079 records while our search of other sources yielded 11 records for possible inclusion. We identified 10,880 records for further consideration after removing 210 duplicates. After screening titles and available abstracts, we excluded 10,766 records and assessed 114 full‐text reports for eligibility. Three of these full‐text reports were published in other languages (Koehler 2007; Vitolo 2005; Yin 2009), and were translated to English for data extraction. We included 23 studies from 35 reports, excluded 51 studies from 57 full‐text reports with reasons (Excluded studies), categorised 10 other studies (from 12 reports) as awaiting classification because we were unable to obtain their full‐text reports, and identified 10 ongoing studies. See Figure 4.
4.
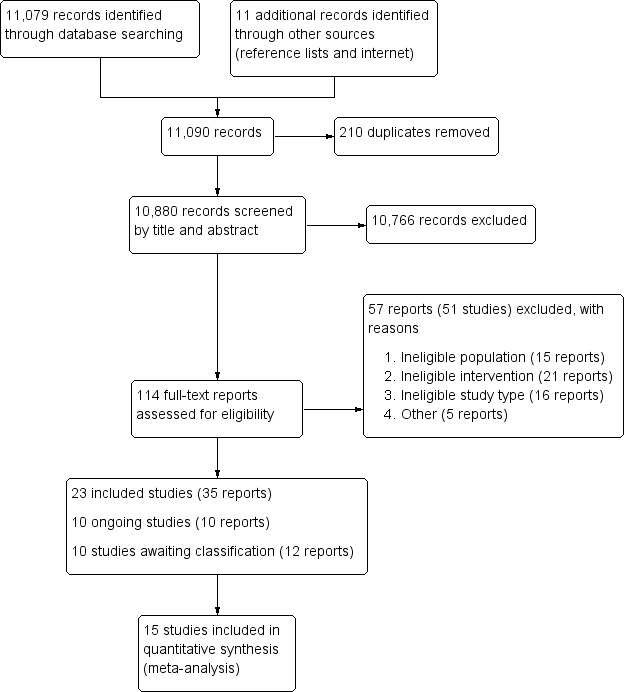
Study flow diagram
Included studies
Details of the 23 included studies are summarised in the Characteristics of included studies tables.
Design
Of the 23 studies that met the inclusion criteria, 13 were cluster‐RCTs (Aboud 2008; Aboud 2009; Aboud 2011; Bhandari 2004; Campbell 2013; Kang 2017; Penny 2005; Reinbott 2016; Saleem 2014; Schroeder 2015; Shi 2010; Tariku 2015; Vazir 2013), while 10 were individually randomised (Bhandari 2001; Daniels 2012; de Oliveira 2012; Edward 2013; Koehler 2007; Negash 2014; Olaya 2013; Vitolo 2005; Wen 2011; Yin 2009).
Ten of the cluster‐RCTs reported using appropriate statistical approaches to allow for clustering in the analysis (Aboud 2008; Aboud 2009; Aboud 2011; Bhandari 2004; Campbell 2013; Kang 2017; Reinbott 2016; Saleem 2014; Tariku 2015; Vazir 2013). However, not all outcomes from these studies were reported as having allowed for the effect of clustering. One study did not appear to have adjusted for clustering (Schroeder 2015). One study reported that they omitted the ICC in the final analyses as it did not impact on results (Shi 2010), while another study stated that the outcomes were reported at an individual level and not at the cluster level (Penny 2005). In order to include these three studies in our analyses (Schroeder 2015; Shi 2010; Penny 2005), we calculated effective sample sizes and inflated the standard errors in accordance with the approximate approach outlined in section 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Settings
Five studies were conducted in high‐income countries: Australia (Campbell 2013; Daniels 2012; Wen 2011), Germany (Koehler 2007) and the USA (Schroeder 2015). Six studies were conducted in upper‐middle‐income countries: Brazil (de Oliveira 2012; Vitolo 2005), China (Shi 2010; Yin 2009), Colombia (Olaya 2013), and Peru (Penny 2005). Eight studies were conducted in lower‐middle‐income countries: Bangladesh (Aboud 2008; Aboud 2009; Aboud 2011), Cambodia (Reinbott 2016), India (Bhandari 2001; Bhandari 2004; Vazir 2013), and Pakistan (Saleem 2014). Three studies were conducted in a low‐income country: Ethiopia (Kang 2017; Negash 2014; Tariku 2015). The location of one study was not explicitly stated in the study report (Edward 2013).
Of these studies, 19 were community‐based (Aboud 2008; Aboud 2009; Aboud 2011; Bhandari 2001; Bhandari 2004; Campbell 2013; Daniels 2012; de Oliveira 2012; Edward 2013; Kang 2017; Negash 2014; Reinbott 2016; Saleem 2014; Shi 2010; Tariku 2015; Vazir 2013; Vitolo 2005; Wen 2011; Yin 2009), while four studies were facility‐based (Koehler 2007; Olaya 2013; Penny 2005; Schroeder 2015).
Eight studies were conducted in urban settings (Daniels 2012; de Oliveira 2012; Edward 2013; Koehler 2007; Olaya 2013; Schroeder 2015; Vitolo 2005; Wen 2011), two in peri‐urban settings (Penny 2005; Saleem 2014), one in an urban slum (Bhandari 2001), one in local government areas (Campbell 2013), and 11 in rural settings (Aboud 2008; Aboud 2009; Aboud 2011; Bhandari 2004; Kang 2017; Negash 2014; Reinbott 2016; Shi 2010; Tariku 2015; Vazir 2013; Yin 2009).
Participants
Twenty‐three studies, including 11,170 caregiver‐child pairs met the inclusion criteria (Criteria for considering studies for this review). Nineteen studies included mother/caregiver‐child pairs, three studies enrolled pregnant women (Edward 2013; Penny 2005; Vazir 2013), and one study enrolled first‐time mothers (Wen 2011). The range of the sample size was 85 to 2064 caregivers, while that of the cluster size was 4 to 60 clusters.
All outcomes were assessed in children except for adverse events, which were assessed in both children and caregivers, and knowledge outcomes, which were assessed in caregivers. The ages of the children ranged from birth to 24 months with 10 studies including newborn infants.
Interventions
See Table 4 and Table 5 for details of the educational interventions.
2. Description of educational interventions: community‐based interventions.
| Study | Promotional activity | Message content | Ways information was collected/outcome measure assessed | Intervention providers | Delivery (e.g. mechanism, medium, intensity, fidelity) |
| Aboud 2008 |
|
|
|
Peer educators | During weekly group sessions |
| Aboud 2009 |
|
|
|
Peer educators | Group training sessions held weekly |
| Aboud 2011 |
|
|
|
Peer educators | Group training sessions held weekly |
| Bhandari 2001 | Counselling sessions using a nutritional counselling guide book | Not described |
|
Trained nutritionists | Monthly counselling sessions |
| Bhandari 2004 |
|
|
|
|
Counselling on complementary feeding conducted as follows:
|
| Campbell 2013 |
|
Intervention materials incorporated 6 purpose‐designed key messages (for example, “Color Every Meal With Fruit and Veg,” “Eat Together, Play Together,” “Off and Running”) within a purpose‐designed DVD and written materials |
|
Dietician | 6 x 2‐h sessions delivered quarterly at first‐time parents’ group regular meeting |
| Daniels 2012 |
|
Messages in:
|
|
|
Interactive group sessions at a choice of days and times, and at the same child health centres as those used for measurements |
| de Oliveira 2012 |
|
|
|
|
The counselling sessions occurred in the maternity ward close to the time for hospital discharge and at 7, 15, 30, 60, and 120 days after the birth at the mother's home |
| Edward 2013 |
|
Doulas discouraged the introduction of solid food during the early months of life for both breast‐fed and formula‐fed infant |
|
Doulas |
|
| Kang 2017 |
|
Mothers discussed messages around:
with the operators |
|
Female operators | During group nutrition education sessions |
| Negash 2014 |
|
|
|
|
The counselling was carried out during education sessions in the community |
| Reinbott 2016 |
|
|
|
Trained community nutrition promoter (CNP) together with local NGO conducted the nutrition education sessions | The 7 nutrition education sessions were held 2–4 hours weekly or biweekly depending on the availability of the participants |
| Saleem 2014 |
|
|
Unclear | 2 female research assistants (with at least 14 years of schooling) and 2 female community health workers (with at least 10 years of schooling) | Interventions were offered in participants' homes |
| Shi 2010 |
|
Not described |
|
Healthcare providers in the intervention areas |
|
| Tariku 2015 |
|
|
Interviews using questionnaires |
|
|
| Vazir 2013 |
|
|
|
High‐school‐educated village women who were themselves mothers | Home visits |
| Vitolo 2005 |
|
Intervention messages were based on the “Ten steps for healthy feeding for Brazilian children from birth to 2 years of age” |
|
Trained field workers who were undergraduate students in nutritional sciences | Home visits |
| Wen 2011 | Counselling sessions on infant feeding practices, infant nutrition and active play, family physical activity and nutrition, as well as social support |
|
|
Trained research nurses | Home visits |
| Yin 2009 |
|
Mothers were educated with feeding guideline on infants and young children
|
‐ | Experts in maternal and child nutrition | ‐ |
NGO: non‐governmental organisation; TN: study number
3. Description of educational interventions: facility‐based interventions.
| Study | Promotional activity | Message content | Ways information was collected/outcome measure assessed | Intervention providers | Delivery (e.g. mechanism, medium, intensity, fidelity) |
| Koehler 2007 |
|
Nutrition counselling was based on the Dietary Schedule for the First Year of Life (Dietary Schedule) recommended by the Nutrition Committee of the German Pediatric Society. Recommendations of the schedule include:
|
|
Counsellors | Telephone calls and printed materials |
| Olaya 2013 |
|
Guidelines focused on the following 3 main messages that were emphasised at all study visits:
Mothers were offered specific advice on the number of portions of meat that should be given; mothers were also advised to include chicken liver and heart as affordable forms of meat, and suggestions were given for the preparation of recommended foods. Mothers were also advised to give fruit and vegetables daily |
|
Researchers | Clinic visits |
| Penny 2005 |
|
|
|
Health workers | Health facility |
| Schroeder 2015 |
|
The intervention was based on the modules of Growing Leaps and Bounds, a set of educational materials developed by a group of experts and funded by the Dannon Institute. These materials aim at:
While the brochures emphasise a few key points, they also provide detailed advice on infant feeding practices, physical activity, and developmental milestones related to eating patterns |
|
|
Paediatric visits at 1, 2, 4, 6, 9, 12, 15, 18, and 24 months of age and at annual visits thereafter up to 5 years of age |
S/N: study number
Five studies had multiple intervention arms. Aboud 2011 was a three‐arm study in which intervention group one received six‐weekly sessions on responsive parenting (feeding and stimulation) in addition to the regular programme, intervention group two received six‐weekly sessions on responsive parenting (feeding and stimulation) in addition to the regular programme and six months of a food powder fortified with minerals and vitamins, and the control group continued with the regular programme (standard care). We considered group one (weekly sessions on responsive feeding and parenting) versus standard care in this review. Vazir 2013 was also a three‐arm study where intervention group one (complementary feeding group) received the WHO recommendations on breastfeeding and complementary foods in addition to routine integrated child development services, intervention group two (responsive complementary feeding and play group) received the same intervention as the complementary feeding group plus skills for responsive feeding and psychosocial stimulation, and the control group received the routine Integrated Child Development Services (ICDS) ‐ standard care. In this review we considered group one versus standard care only. Bhandari 2001 was a four‐arm study where intervention group one received a milk‐based cereal and nutritional counselling, intervention group two received monthly nutritional counselling alone, intervention group three was the visitation group which received home visits for morbidity assessment only (used as the control group in the study), while the no‐intervention group were contacted at three time points for anthropometric measurements and dietary assessment. We considered intervention group two versus intervention group three for morbidity outcomes and intervention group two versus the control group for growth and dietary outcomes. Koehler 2007 was also a four‐arm study and had three intervention arms and one control arm. All of the intervention arms received nutritional counselling via telephone but the interventions were slightly varied among the intervention groups. Intervention group one received the intervention by means of a telephone hotline, which was accessible for two hours each, three times per week. Intervention group two received, "additional written information on the dietary schedule distributed in 3 parts, each dealing with the diet in the coming period" (p 107). Intervention group three received additional personal telephone counselling while the control group received no intervention. Tariku 2015 also had two intervention arms with one of the arms receiving educational interventions in line with the constructs of the health belief model, while the other group received educational intervention via the traditional (didactic) method. The control group was without intervention. We discussed the results of Koehler 2007 and Tariku 2015 narratively since all of the intervention arms received educational interventions. Details of these interventions are reported in Table 6.
4. Studies with multiple interventions arms and adjunctive interventions.
| Study | Interventions |
| Aboud 2011 | Intervention group 1 (RFS): 6 weekly sessions on responsive parenting (feeding and stimulation) in addition to the regular programme Intervention group 2 (RFS plus Sprinkles): 6 weekly sessions on responsive parenting (feeding and stimulation) in addition to the regular programme and 6 months of a food powder fortified with minerals and vitamins Control: regular programme |
| Bhandari 2001 | Intervention group 1: received a milk‐based cereal and nutritional counselling Intervention group 2: monthly nutritional counselling alone Intervention group 3: visitation group (used as the control group in the study) Control: no intervention |
| Koehler 2007 | Intervention group 1: were offered a telephone hotline 3 times per week, open for 2 hours each time Intervention group 2: received additional written information on the Dietary Schedule distributed in 3 parts, each dealing with the diet in the coming period Intervention group 3: were offered additional personal telephone counselling |
| Vazir 2013 | Intervention group 1: the complementary feeding group (CFG) received the integrated child development services plus the World Health Organization recommendations on breastfeeding and complementary foods Intervention group 2: the responsive complementary feeding and play group received the same intervention as the CFG plus skills for responsive feeding and psychosocial stimulation Control: routine Integrated Child Development Services ‐ standard of care |
RFS: responsive feeding and stimulation
All other studies were two‐arm studies with the intervention arms receiving educational interventions or nutritional counselling and the control groups receiving routine services (usual care) or no intervention or an agriculture intervention. The control group intervention was not described in detail in two studies (de Oliveira 2012; Penny 2005).
In the study by Reinbott 2016, the intervention group received nutrition education plus agriculture intervention, while the control group received agriculture intervention alone.
One study stratified the participating mothers into two groups, namely co‐habiting with the grandmother and not co‐habiting with the grandmother, before randomising into intervention or control arms (de Oliveira 2012).
The educational interventions' promotion activities included: group education or counselling sessions, demonstration or practical sessions and role plays (Aboud 2008; Aboud 2009); stories (Aboud 2008; Aboud 2009); use of posters (Aboud 2009; Bhandari 2004; Negash 2014; Reinbott 2016); flip charts (de Oliveira 2012; Penny 2005; Vazir 2013); work books (Daniels 2012); booklets and picture books (Aboud 2008; Aboud 2009; Bhandari 2004; de Oliveira 2012; Saleem 2014; Shi 2010; Vazir 2013; Vitolo 2005); flyers and leaflets (Olaya 2013; Penny 2005); brochures and post cards (Schroeder 2015); peer support (Aboud 2011; Campbell 2013); women's group meetings (Bhandari 2004); sharing meetings (Reinbott 2016); village rallies (Bhandari 2004); feeding recommendation cards (Bhandari 2004); video tapes (Campbell 2013; Edward 2013); telephone counselling (Edward 2013; Koehler 2007; Schroeder 2015); text messaging and mail outs (Campbell 2013). With the exception of two studies (Wen 2011; Yin 2009), all of the included studies used multiple promotion activities.
Intervention messages were centred on the appropriate time to introduce complementary foods; specific foods to be offered or avoided and how to offer them; meal frequencies; amounts of complementary foods to be fed to infants at different ages while continuing breastfeeding; offering a variety of foods from different food groups; family nutrition; health seeking; child nutrition during illness; hand washing at critical points; reading infant's signals by watching, listening and interpreting them, and being responsive to infant cues; and using or enriching locally available foods for complementary feeding.
The common sources of intervention information included messages developed by the implementing organization or researchers (Aboud 2008; Aboud 2009; Aboud 2011; Olaya 2013; Penny 2005), WHO/UNICEF (Saleem 2014), Dietary Schedule for the First Year of Life recommended by the Nutrition Committee of the German Pediatric Society (Koehler 2007), the Alive and Thrive programme (Negash 2014), Modules of Growing Leaps and Bounds (Schroeder 2015), Ten Steps to Healthy Feeding (Vitolo 2005), National Nutrition Programme and UNICEF in Cambodia (Reinbott 2016), and the Integrated Management of Childhood Illnesses training manual on nutrition counselling (Bhandari 2004).
Seven studies' reports stated explicitly that their respective studies were theory based. The theories deployed in these studies included social cognitive learning theory (Aboud 2008; Aboud 2009; Aboud 2011; Campbell 2013), the health belief model (Tariku 2015), the positive deviance approach (Kang 2017), and the cognitive behavioural approach (Daniels 2012). Other study reports did not specify whether or not they were theory‐based.
Comparators
The control arms in all of the included studies did not receive the educational intervention but rather continued with the routine care or regular programme or an agriculture intervention (one study, Reinbott 2016). This was also applicable to studies with more than one intervention arm.
Duration of the intervention
The duration of the interventions ranged from four to nine months (Aboud 2008; Aboud 2009; Bhandari 2001; Edward 2013; Negash 2014; Saleem 2014; Tariku 2015; Yin 2009), 10 to 20 months (Bhandari 2004; Campbell 2013; Kang 2017; Koehler 2007; Vazir 2013), two years (Penny 2005; Reinbott 2016), and eight years and four years respectively (Vitolo 2005; Wen 2011). It was unclear in six studies (Aboud 2011; Daniels 2012; de Oliveira 2012; Olaya 2013; Schroeder 2015; Shi 2010).
Outcomes and method of assessment
Outcomes commonly reported across the studies include the following.
Primary outcomes
Age at introduction of complementary foods: seven studies reported this outcome (Daniels 2012; de Oliveira 2012; Edward 2013; Reinbott 2016; Schroeder 2015; Vitolo 2005; Wen 2011). This outcome was assessed by information provided by the mothers/caregivers (self‐report) during home or hospital visits.
Duration of exclusive breastfeeding: four studies reported this outcome (de Oliveira 2012; Penny 2005; Vitolo 2005; Wen 2011). This outcome was assessed by information provided by caregivers (self‐report) during home or hospital visits.
Adequacy of complementary foods: 17 studies reported this outcome (Aboud 2008; Aboud 2009; Aboud 2011; Bhandari 2001; Bhandari 2004; Campbell 2013; Daniels 2012; Koehler 2007; Negash 2014; Olaya 2013; Penny 2005; Reinbott 2016; Schroeder 2015; Shi 2010; Tariku 2015; Vazir 2013; Vitolo 2005). This outcome was assessed by information on the types of foods fed to infants, mouthfuls consumed, energy intakes, diet scores, consistency of foods fed to infants, and dietary diversity. This information was usually provided by caregivers (self‐report) during home or hospital visits, dietary recalls, or records based on the observations of research assistants or field workers.
Hygiene practices: six studies reported this outcome (Aboud 2009; Aboud 2011; Bhandari 2004; Negash 2014; Shi 2010, Tariku 2015). This outcome was assessed by information provided by caregivers (self‐report) during home or hospital visits, and observations by research assistants or field workers during home visits.
Secondary outcomes
Growth: 12 studies reported this outcome (Aboud 2008; Aboud 2009; Bhandari 2001; Bhandari 2004; Campbell 2013; Daniels 2012; Olaya 2013; Penny 2005; Saleem 2014; Schroeder 2015; Vazir 2013; Vitolo 2005). This outcome was commonly assessed by anthropometric measurements during home or clinic visits.
Diarrhoea: four studies reported this outcome (Bhandari 2001; Bhandari 2004; Reinbott 2016; Vitolo 2005). This outcome was assessed by information provided by mothers/caregivers (self‐report) during home or hospital visits.
Hospitalisation: one study reported this outcome (Vitolo 2005). This outcome was assessed by information by provided by mothers/caregivers (self‐report) during home visits and medical/hospital records.
Knowledge: seven studies reported this outcome (Aboud 2008; Aboud 2009; Aboud 2011; Negash 2014; Penny 2005; Vazir 2013; Yin 2009). This outcome was assessed by messages recalled by caregivers, change in knowledge scores, and change in knowledge, attitude and practice scores.
In general, outcomes were commonly assessed across the studies via information provided by caregivers (self‐report) and observations by research assistants or field workers during home or hospital visits. Data collection methods included: records taken during home visits; use of questionnaires; structured face‐to‐face interviews during home or hospital visits; data retrieval from medical or hospital records; dietary recalls; anthropometric measurements during home or clinic visits; and observations by research assistants or field workers.
Anthropometric measurements were usually carried out by trained data collectors or by clinic or hospital staff. In addition to these methods, some studies also used telephone calls and standardised telephone interviews to collect data on outcomes of interest (Campbell 2013; de Oliveira 2012; Koehler 2007; Wen 2011).
Excluded studies
We excluded 51 studies (from 57 reports) after assessing the full‐text reports. These studies were mainly excluded on the basis of having an ineligible population, intervention or design. The excluded studies and the reasons for their exclusion are found in the Characteristics of excluded studies.
Studies awaiting classification
We grouped 10 studies as awaiting classification because we were unable to obtain their full‐text reports (Dunlevy 2010; Dunlvey 2012; Guan 2016; Jordan 2015; Palacios 2017; Paul 2011; Rabadi 2013; Savage 2010; Shafique 2013; Toure 2016). From their abstracts, the studies included mothers of infants from birth to two months of age; mother‐infant dyads; full‐term, low birth‐weight infants; and rural women who were pregnant or had a child under two years of age. Common interventions included nutrition education, nutrition, health and hygiene education, soothe and sleep interventions, messages for improving feeding practices delivered via short mobile messages (SMS), and infant weaning talks. Some of these interventions were delivered with additional interventions such as agricultural interventions, home gardening, provision of hand sanitisers, provision of micronutrient powders and gender sensitisation. Details of these studies can be found in the Characteristics of studies awaiting classification tables.
Ongoing studies
We identified 10 ongoing studies that are likely to meet our inclusion criteria (Campbell 2016; Cloutier 2015; Helle 2017; Hernes 2013; Horodynski 2011; Horodynski 2015; Kimani‐Murage 2013; Kulwa 2014; SHINE Team 2015; Wasser 2015). These studies were either cluster‐RCTs or RCTs. Some of these studies included first‐time parents of infants less than two years of age or infants less than two years of age and their mothers or caregivers, while others included pregnant women in their last trimester. The common interventions in these studies included educational interventions delivered via web‐based materials, written sources, telephone contacts, face‐to‐face sessions, home visits, skill‐set training, personalised home‐based counselling, cooking courses, etc. The interventions were delivered by dieticians or community health workers. All the control arms received usual care except one study that had an attention control that received safety education. Details of these studies can be found in the Characteristics of ongoing studies tables.
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of the 'Risk of bias' assessment of all included studies.
Allocation
Random sequence generation
Community‐based studies
Twelve of the 19 community‐based studies used appropriate methods to generate the random sequence (Aboud 2008; Aboud 2009; Bhandari 2004; Daniels 2012; Edward 2013; Kang 2017; Reinbott 2016; Saleem 2014; Shi 2010; Tariku 2015; Vazir 2013; Wen 2011). The method of random sequence generation was unclear in seven studies (Aboud 2011; Bhandari 2001; Campbell 2013; de Oliveira 2012; Negash 2014; Vitolo 2005; Yin 2009).
Facility‐based studies
Three of the four facility‐based studies used appropriate methods to generate the random sequence (Koehler 2007; Olaya 2013; Penny 2005), while the remaining study was unclear (Schroeder 2015).
Allocation concealment
Community‐based studies
The allocation sequence was adequately concealed in five studies (Bhandari 2004; Campbell 2013; Daniels 2012; Edward 2013; Wen 2011), but was unclear in 14 studies (Aboud 2008; Aboud 2009; Aboud 2011; Bhandari 2001; de Oliveira 2012; Kang 2017; Negash 2014; Reinbott 2016; Saleem 2014; Shi 2010; Tariku 2015; Vazir 2013; Vitolo 2005; Yin 2009).
Facility‐based studies
The allocation sequence was adequately concealed in one study (Olaya 2013) but unclear in three studies (Koehler 2007; Penny 2005; Schroeder 2015).
Blinding
Blinding of participants and personnel (performance bias)
Community‐based studies
Blinding of participants and personnel was unclear in 13 of the 19 community‐based studies (Aboud 2011; Bhandari 2001; Bhandari 2004; Daniels 2012; de Oliveira 2012; Edward 2013; Negash 2014; Reinbott 2016; Saleem 2014; Tariku 2015; Vazir 2013; Wen 2011; Yin 2009), and judged to be at high risk of bias in six studies (Aboud 2008; Aboud 2009; Campbell 2013; Kang 2017; Shi 2010; Vitolo 2005).
Facility‐based studies
All of the four included facility‐based studies were unclear on blinding of participants and personnel (Koehler 2007; Olaya 2013; Penny 2005; Schroeder 2015).
Blinding of outcome assessment (detection bias)
Community‐based studies
We assessed eight of the 19 community‐based studies as having low risk of detection bias (Aboud 2008; Aboud 2009; Campbell 2013; Daniels 2012; de Oliveira 2012; Reinbott 2016; Vazir 2013; Wen 2011), while blinding of outcome assessment was unclear in eight studies (Aboud 2011; Bhandari 2001; Bhandari 2004; Edward 2013; Negash 2014; Saleem 2014; Tariku 2015; Yin 2009). We considered three studies to be at high risk of detection bias (Kang 2017; Shi 2010; Vitolo 2005).
Facility‐based studies
Blinding of outcome assessors was unclear in two of the four facility‐based studies (Koehler 2007; Schroeder 2015) and judged to be at high risk of bias in the other two studies (Olaya 2013; Penny 2005).
Incomplete outcome data
Community‐based studies
We assessed 11 studies as having low attrition bias because they met at least one of the following criteria: the losses were similar across intervention and control groups; study authors accounted for losses to follow‐up and also used appropriate statistical analysis methods to make up for the losses to follow‐up (Aboud 2008; Aboud 2009; Aboud 2011; Bhandari 2004; Campbell 2013; Daniels 2012; de Oliveira 2012; Edward 2013; Shi 2010; Tariku 2015; Wen 2011). In four studies the risk of attrition bias was unclear (Reinbott 2016; Saleem 2014; Vitolo 2005; Yin 2009). We assessed four studies at high risk of attrition bias (Bhandari 2001; Kang 2017; Negash 2014; Vazir 2013). In Bhandari 2001 and Vazir 2013 the attrition rates were reported to be 12% and 15% respectively, although the reasons for attrition were provided for all participants in both studies, while the attrition rates in Kang 2017 and Negash 2014 were about 18% and 20%.
Facility‐based studies
We assessed two studies at low risk of bias as the losses were balanced across groups, study authors accounted for losses to follow‐up and also used appropriate statistical analysis methods to make up for the losses to follow‐up (Olaya 2013; Penny 2005). We rated one study at unclear risk of bias as there was no information on total number of participants lost to follow‐up (Koehler 2007). We rated one study at high risk of bias as the attrition rate was high (21%) and no reason was given for the losses to follow‐up (Schroeder 2015).
Selective reporting
Community‐based studies
We assessed one study as having low risk of reporting bias (Campbell 2013). The study protocol was available for assessment and study authors reported on all outcomes listed in the Methods section of the study reports. We assessed 16 other studies as having unclear risk of reporting bias in this domain because the study protocols were not available for assessment (Aboud 2008; Aboud 2009; Aboud 2011; Bhandari 2001; Bhandari 2004; Daniels 2012; de Oliveira 2012; Edward 2013; Kang 2017; Reinbott 2016; Saleem 2014; Shi 2010; Vazir 2013; Vitolo 2005; Wen 2011; Yin 2009), and one of which, Yin 2009, was originally published in Chinese and we were limited by the translated study. We assessed two studies as having high risk of reporting bias (Negash 2014; Tariku 2015). Negash 2014 did not report the results of the anthropometric measurements although the authors reported that the measurements were taken, while Tariku 2015 did not clearly present data for some outcomes.
Facility‐based studies
We assessed the four facility‐based studies as having unclear risk of reporting bias (Koehler 2007; Olaya 2013; Penny 2005; Schroeder 2015). The studies reported on all outcomes listed in the Methods section of the study reports but study protocols were unavailable for assessment.
Other potential sources of bias
Community‐based studies
We assessed 12 studies at low risk of other biases (Aboud 2008; Aboud 2009; Aboud 2011; Bhandari 2001; Bhandari 2004; Daniels 2012; de Oliveira 2012; Edward 2013; Negash 2014; Saleem 2014; Tariku 2015; Vazir 2013). We assessed four studies at unclear risk of other biases (Campbell 2013; Shi 2010; Wen 2011; Yin 2009), two of which reported baseline imbalances (Shi 2010; Wen 2011). We were unable to assess Yin 2009 since it was originally published in Chinese and we were limited by the translated study report. We considered three studies as having high risk of other biases (Kang 2017; Reinbott 2016; Vitolo 2005).
Facility‐based studies
We judged two studies, which reported adequate comparability between study arms at baseline, at low risk of bias (Koehler 2007; Penny 2005). We judged Schroeder 2015 at unclear risk of bias. Although it reported baseline imbalances in the ethnicity, employment, household income, education, home ownership, usage of food stamps, usage of WIC (women, infants and children) program services and breastfeeding rates, we were not sure how this affected the results following the intervention, since it was a facility‐based study and participants would have been exposed to the same conditions. We judged Olaya 2013 at high risk of bias due to baseline differences in child growth indices.
Effects of interventions
Primary outcomes
1a. Age at introduction of complementary foods
Community‐based studies
Pooled results
Six, individually‐randomised, community‐based studies reported the effect of educational intervention on age at introduction of complementary foods. Four studies reported data suitable for quantitative analysis (de Oliveira 2012; Edward 2013; Vitolo 2005; Wen 2011). The pooled effect estimate suggests that, compared to standard care, educational intervention reduces the risk of early introduction of complementary food (before four to six months of age) by 12% (average RR 0.88, 95% CI 0.83 to 0.94; 4 studies, 1738 children; Tau2 = 0.00, I2 = 0%; moderate‐quality evidence; Analysis 1.1; Table 1). Studies used intervention delivery strategies that ranged from counselling sessions to the use of printed materials (booklets, brochures, leaflets), flip charts and videos, with some studies using a combination of at least two of the listed delivery strategies.
1.1. Analysis.
Comparison 1 Educational intervention versus no educational intervention for improving complementary feeding practices (ICC = 0.02), Outcome 1 Complementary food introduced at appropriate age.
Single study results
Two community‐based studies were not included in the meta‐analysis. Daniels 2012 reported a difference in mean age of complementary food introduction (intervention mean age 22.8 (± 4.4) weeks versus control mean age 22.7 (± 4.9) weeks; study author‐reported P = 0.85). Reinbott 2016 reported the proportion of children introduced to semi‐solid/soft foods between the WHO recommended ages of six to eight months (intervention 88.1% versus control 92.6%; study author‐reported P = 0.349). Insufficient information was reported by Reinbott 2016 to estimate an intervention effect and the study could not be included in the meta‐analysis. (See Table 7).
5. Morbidity (diarrhoea).
| Study | Result |
| Bhandari 2001 | The incidence and prevalence of diarrhoea and ALRI were not significantly affected by either intervention Nutritional counselling group: episodes per child 6.9 (± 3.2), prevalence per 100: d 14.6 (± 12.0) Visitation group: episodes per child 6.7 (± 3.4), prevalence per 100: d 13.2 (± 9.8) |
| Bhandari 2004 | The reported prevalences of common illnesses in the previous 7 days did not differ in the 2 groups at 9, 12, 15, and 18 months of age At 12 months of age, the prevalence of diarrhoea was 16.8 vs 13.1% (P = 0.174) |
| Reinbott 2016 | Diarrhoeal illness in the past 2 weeks (%) Baseline: intervention = 36.9%, control = 41.6% Impact: intervention = 27.9%, control = 26.2% |
| Vitolo 2005 | Number with event: intervention = 46, control = 98 |
ALRI: acute lower respiratory infection.
Facility‐based studies
One facility‐based study, Schroeder 2015, reported insufficient information to estimate an intervention effect and therefore was not included in Analysis 1.1 above. Schroeder 2015 narratively reported that mothers in the intervention arm delayed the introduction of complementary foods compared with mothers in the control arm (study author‐reported P < 0.051). This study used intervention delivery strategies that included printed materials (educational brochures and reminder postcards containing intervention messages) and telephone calls.
1b. Duration of exclusive breastfeeding
Four studies measured the effect of educational intervention on the duration of exclusive breastfeeding (de Oliveira 2012; Penny 2005; Vitolo 2005; Wen 2011), of which three reported sufficient data for inclusion in a meta‐analysis (Penny 2005; Vitolo 2005; Wen 2011). We conducted the analysis for duration of exclusive breastfeeding (≥ four months of age) using the generic inverse variance approach in RevMan 5 (Review Manager 2014), to allow for inflating the standard error of Penny 2005 (see below). The average RR, pooled across both community‐ and facility‐based studies was RR 1.58 (95% CI 0.77 to 3.22; 3 studies, 1544 children; Tau2 = 0.30, I2 = 80%; very low‐quality evidence; Analysis 1.2; Table 1). We further investigated the impact of the ICC value on the pooled intervention effect in a sensitivity analysis (See Sensitivity analysis and Figure 5).
1.2. Analysis.
Comparison 1 Educational intervention versus no educational intervention for improving complementary feeding practices (ICC = 0.02), Outcome 2 Duration of exclusive breastfeeding (≥ 4 months old).
5.
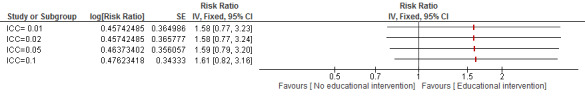
Sensitivity analysis 3. Comparison of different ICC (primary outcomes), outcome: 3.1 duration of exclusive breastfeeding (≥ 4 months of age)
The intervention delivery strategy in Wen 2011 was counselling and social support, Vitolo 2005 included dietary counselling, printed materials (brochures with key messages; simple, coloured leaflet with food pictures depicting a healthful meal), while that of de Oliveira 2012 included counselling sessions and promotional materials like booklets and flip charts. In Penny 2005 the intervention involved group sessions for caregivers of children of similar ages, demonstrations of the preparation of complementary foods, the use of flip charts and single‐page recipe fliers.
Community‐based studies
Pooled results
Three studies examined community‐based educational intervention (de Oliveira 2012; Vitolo 2005; Wen 2011). Only two studies reported data that could be combined in a meta‐analysis (Vitolo 2005; Wen 2011). The pooled estimate of effect suggests that educational intervention increased the duration of exclusive breastfeeding by 132% (average RR 2.32, 95% CI 1.45 to 3.73; 2 studies, 1167 children; Tau2 = 0.00, I2 = 0%; low‐quality evidence; Analysis 1.2; Table 1).
Individual study results
de Oliveira 2012 reported insufficient information to be included in the meta‐analysis. The authors reported the median duration of exclusive breastfeeding: 2.9 months (interquartile range 1.0 to 4.7) in the intervention arm and 1.3 (interquartile range 0.6 to 3.0) in the control arm, (study author‐reported P = 0.001, no further detail available).
Facility‐based studies
Only one facility‐based study reported the effect of educational intervention on the duration of exclusive breastfeeding (Penny 2005). After we retrospectively accounted for clustering (using the approximate approach outlined above in the Unit of analysis issues section), and assuming an ICC of 0.02, the estimate of intervention effect was compatible with both a decrease and an increase in the duration of exclusive breastfeeding (RR 0.95, 95% CI 0.70 to 1.29; 1 study, 377 children; low‐quality evidence; Analysis 1.2; Table 1).
1c. Adequacy of complementary foods
Eighteen studies reported the outcome of adequacy of complementary foods. However, the types of foods, measures and methods of assessment reported were too diverse to be combined in a meta‐analysis. Several studies reported a dietary diversity score or infant/child feeding index, or both, but it was not sufficiently clear from the reports whether they were based on comparable criteria or food groups and so we considered it inappropriate to combine them in a meta‐analysis. We provide a narrative summary of the individual study findings below.
Community‐based studies
Thirteen community‐based studies reported findings for the outcome of adequacy of complementary foods fed to children (Aboud 2008; Aboud 2009; Aboud 2011; Bhandari 2001; Bhandari 2004; Campbell 2013; Daniels 2012; Negash 2014; Reinbott 2016; Shi 2010; Tariku 2015; Vazir 2013; Vitolo 2005). We categorised outcomes into those that focused on the adequacy of nutrient intake/diversity of complementary food (i.e. quality), and the volume and frequency of adequate complementary food (i.e. quantity).
Adequacy of nutrient intake/diversity of complementary food
Eleven community‐based studies reported an outcome related to the adequacy of nutrient intake/diversity of complementary food (Aboud 2008; Aboud 2009; Aboud 2011; Bhandari 2001; Bhandari 2004; Campbell 2013; Negash 2014; Reinbott 2016; Shi 2010; Vazir 2013; Vitolo 2005). One study reported energy intake only (Bhandari 2001), and one study reported details for responsive feeding only (Daniels 2012).
Although we were unable to combine the studies in a meta‐analysis, due to the manner in which the results were reported, 10 of the 11 study authors reported intervention effect estimates or sufficient details of at least one relevant outcome. One study reported insufficient detail (Bhandari 2001).
Aboud 2008 reported the mean number of times specific foods were eaten (in 24 hours) for separate foods and asserts, "eggs, fruit, vegetables and carbohydrates were more often reportedly given to the children of caregivers in the complementary feeding intervention, and biscuits/sugar more often given to controls" (p 282). Intervention effect estimates at follow‐up could be calculated from Table 3 of their report for consumption of: rice (MD 0.07, 95% CI −0.12 to 0.26); dal (MD −0.12, 95% CI −0.31 to 0.07); fish (MD −0.15, 95% CI −0.42 to 0.12); egg (MD 0.19, 95% CI 0.07 to 0.31); fruit (MD 0.28, 95% CI 0.06 to 0.50); vegetables (MD 0.57, 95% CI 0.26 to 0.88), cows' milk (MD 0.12, 95% CI −0.19 to 0.43); carbohydrate (MD 0.32, 95% CI 0.08 to 0.56); and biscuits (MD −0.30, 95% CI −0.60 to 0.00). All food types are study author‐reported.
Aboud 2009 also reported the mean number of times specific foods were eaten (in 24 hours) for separate foods; rice (MD −0.11, 95% CI −0.32 to 0.10); dal (MD 0.09, 95% CI −0.05 to 0.23); fish (MD 0.07, 95% CI −0.27 to 0.41); egg (MD 0.06, 95% CI −0.07 to 0.19); fruit (MD 0.22, 95% CI 0.10 to 0.34); vegetables (MD −0.42, 95% CI −0.86 to 0.02); cows' milk (MD 0.09, 95% CI −0.14 to 0.32); carbohydrate (MD −0.21, 95% CI −0.44 to 0.02); and biscuits (MD 0.10, 95% CI −0.24 to 0.44). All food types are study author‐reported. Aboud 2009 also reported a mean dietary diversity score for each group, which can be used to calculate an unadjusted difference in means; MD 0.32 (95% CI 0.05 to 0.59) in favour of the complementary‐food intervention group.
Aboud 2011 did not provide sufficient information to estimate an intervention effect for the adequacy of nutrient intake/diversity of complementary food. Study author‐reported findings stated that, "of the 7 critical food categories, 20 control children ate a mean of 2.96 foods and the children in the intervention group ate 3.07 foods" (p e1195). In addition, they stated that group differences were non significant at postintervention and follow‐up. The study authors also reported that dietary diversity scores increased for all groups from pre‐testing (mean = 2.61) to follow‐up (mean = 3.03) (study author‐reported P < 0.001). No further information was reported to allow estimation of relative effect.
Bhandari 2004 reported energy intake (Kj/24 hours) from all foods at nine months of age (MD 531.00 Kj/24 hours, 95% CI 398.24 to 663.76) and at 18 months (MD 1230.00 Kj/24 hours, 95% CI 1052.50 to 1407.50).
The types of food consumed (24‐hour recall) were also reported at nine months of age and 18 months of age. At nine months of age foods consumed were: commercially‐available bread (RR 6.77, 95% CI 3.11 to 14.71); home‐made bread (RR 1.03, 95% CI 0.93 to 1.14); rice (RR 3.08, 95% CI 1.48 to 6.39); potatoes (RR 2.40, 95% CI 1.44 to 3.99); legumes (RR 2.68, 95% CI 1.77 to 4.06); any milk (i.e. breastmilk or non‐breastmilk) (RR 1.11, 95% CI 1.05 to 1.17); meat or egg (RR 4.47, 95% CI 0.22 to 92.81); vegetables (RR 3.35, 95% CI 1.55 to 7.22); fruits (RR 1.36, 95% CI 0.97 to 1.91). At 18 months of age foods consumed were: commercially‐available bread (RR 2.16, 95% CI 1.54 to 3.01); home‐made bread (RR 0.95, 95% CI 0.90 to 1.01); rice (RR 1.09, 95% CI 0.68 to 1.73); potatoes (RR 1.31, 95% CI 1.04 to 1.66); legumes (RR 1.24, 95% CI 0.99 to 1.56); any milk (i.e. breastmilk or non‐breastmilk) (RR 1.03, 95% CI 1.00 to 1.05); meat or egg (RR 4.53, 95% CI 0.22 to 94.07); vegetables (RR 1.08, 95% CI 0.85 to1.36); and fruits (RR 1.11, 95% CI 0.95 to 1.30). All food types are study author‐reported.
Intakes of cereal legume gruels or mixes (RR 3.52, 95% CI 2.44 to 5.06), milk cereal gruels or milk cereal mixes (RR 3.20, 95% CI 2.36 to 4.32), undiluted milk (RR 3.02, 95% CI 2.42 to 3.78), addition of butter/oil (RR 17.42, 95% CI 4.23, 71.70), and recommended snacks (RR 1.31, 95% CI 1.15 to 1.49) were also reported by study authors to be higher in nine‐month‐old children in the educational intervention communities. Similar patterns were seen at 18 months of age, but the study authors reported that differences between the two groups were less pronounced for cereal legume gruels than those at nine months of age, possibly because these foods are commonly given at this age. Estimates are based on raw means, SDs and percentages, as reported in the original paper.
Campbell 2013 reported a 24‐hour dietary recall outcome at postintervention for average daily consumption of: fruits (MD 10.99, 95% CI −6.09 to 28.06); vegetables (MD 4.53, 95% CI −4.38 to 13.43); non‐core drinks (MD −2.21, 95% CI −13.71 to 9.30); non‐core sweet foods such as chocolate, candy and cakes (MD −3.69, 95% CI −6.41 to 20.96); non‐core savoury foods such as crisps and savoury biscuits (MD −1.01, 95% CI −2.82 to 0.80); and water consumption (MD 24.17, 95% CI −9.85 to 58.20). All food types, effect estimates and 95% CIs are study author‐reported.
Negash 2014 reported the raw mean dietary energy intake (kcal) at postintervention, from which we calculated the MD with 95% CIs (MD 160.00, 95% CI −24.31 to 344.31). They also reported mean protein intake (g) for each intervention group (MD 7.10 g, 95% CI 1.56 to 12.64); mean fat intake (g) (MD −0.60 g, 95% CI −10.35 to 9.15); carbohydrate intake (g) (MD 32.00 g, 95% CI 3.18 to 60.82); and iron intake (mg) (MD 9.70 mg, 95% CI 4.19 to 15.21). It is not stated whether nutrient intakes are based on a 24‐hour recall.
Reinbott 2016 assessed dietary diversity (RR 1.16, 95% CI 1.04 to 1.30), and minimum acceptable diet (RR 1.26, 95% CI 1.07 to 1.48). They also reported that a 24‐hour dietary diversity score was calculated using a seven‐food‐group score, the child dietary diversity score (RR 0.20, 95% CI 0.00 to 0.40), and reported individually for: grains (RR 1.01, 95% CI 0.99 to 1.03); roots and white tubers (RR 1.87, 95% CI 1.50 to 2.34); legumes, nuts and seeds (RR 1.03, 95% CI 0.86 to 1.24); dairy products (RR 0.75, 95% CI 0.57 to 0.98); flesh foods namely meat, poultry, fish and offal (RR 1.02, 95% CI 0.95 to 1.09); eggs (RR 1.28, 95% CI 1.09 to 1.50); pro‐vitamin‐A‐rich foods such as yellow‐ and orange‐fleshed roots and tubers, orange‐fleshed fruits, and dark green leafy vegetables (RR 1.17, 95% CI 1.03 to 1.33); other fruits and vegetables (RR 1.12, 95% CI 1.01 to 1.25); fats and oils (RR 1.02, 95% CI 0.92 to 1.14); and sugary foods and crisps (RR 0.93, 95% CI 0.86 to 1.00). All food types are study author‐reported.
Shi 2010 reported findings for diversity of complementary foods at three time points: when child was six months, nine months and 12 months of age. RR greater than 1 suggested the educational intervention increased the consumption of the food. They reported whether the child had ever been fed at six months: bread, rice or noodles (RR 1.37, 95% CI 1.22 to 1.54); roots or tubers (RR 1.06, 95% CI 0.78 to 1.43); yellow or orange foods (RR 1.25, 95% CI 1.02 to 1.53); green leafy vegetables (RR 1.78, 95% CI 1.33 to 2.38); beans, peas or lentils (RR 2.22, 95% CI 1.61 to 3.04); fruits (RR 1.16, 95% CI 1.05 to 1.28); eggs (RR 1.27, 95% CI 1.14 to 1.41); meats (RR 2.84, 95% CI 1.91, 4.21); and cooking oils/fats (RR 1.92, 95% CI 1.40 to 2.63).
At nine months the findings were: bread, rice or noodles (RR 1.03, 95% CI 1.00 to 1.06); roots or tubers (RR 1.10, 95% CI 1.00 to 1.21); yellow or orange foods (RR 1.15, 95% CI 1.07 to 1.24); green leafy vegetables (RR 1.20, 95% CI 1.12 to 1.30); beans, peas or lentils (RR 1.43, 95% CI 1.28 to 1.59); fruit (RR 1.04, 95% CI 1.01 to 1.07); eggs (RR 1.04, 95% CI 1.00 to 1.07); meats (RR 1.61, 95% CI 1.44 to 1.81); and cooking oils/fats (RR 1.19, 95% CI 1.09 to 1.29).
At 12 months the findings were: bread, rice or noodles (RR 1.01, 95% CI 0.99 to 1.04); roots or tubers (RR 1.23, 95% CI 1.13 to 1.34); yellow or orange foods (RR 1.27, 95% CI 1.18 to 1.37); green leafy vegetables (RR 1.11, 95% CI 1.05 to 1.17); beans, peas or lentils (RR 1.37, 95% CI 1.24 to 1.51); fruits (RR 1.03, 95% CI 1.00 to 1.06); eggs (RR 1.08, 95% CI 1.03 to 1.12); meats (RR 1.67, 95% CI 1.49 to 1.86); and cooking oils/fats (RR 1.21, 95% CI 1.13 to1.30).
Vazir 2013 reported the percentage of each group who consumed the following foods, at nine and 15 months: rice (9 months: RR 1.17, 95% CI 1.09 to 1.27; 15 months: RR 2.92, 95% CI 1.89 to 4.51); goat's liver (9 months: RR 13.42, 95% CI 4.97 to 36.27; 15 months: RR 2.92, 95% CI 1.89 to 4.51); goat's meat (9 months: RR 4.85, 95% CI 2.33 to 10.07; 15 months: RR 1.33, 95% CI 1.01 to 1.75); poultry (9 months: RR 2.65, 95% CI 0.72 to 9.83; 15 months: RR 1.98, 95% CI 1.37 to 2.85); banana (9 months: RR 1.58, 95% CI 1.27 to 1.97; 15 months: RR 1.28, 95% CI 1.11 to 1.48); buffalo milk (9 months: RR 0.99, 95% CI 0.97 to 1.01; 15 months: RR 1.13, 95% CI 1.00 to 1.27); egg (9 months: RR 3.14, 95% CI 2.22 to 4.44; 15 months: RR 1.37, 95% CI 1.16 to 1.61); spinach (9 months: RR 17.90, 95% CI 4.38 to 73.20; 15 months: RR 1.42, 95% CI 1.06 to 1.90); pulses (9 months: RR 1.01, 95% CI 0.98 to 1.03; 15 months: RR 1.25, 95% CI 1.12 to 1.40); and added fat (9 months: RR 2.10, 95% CI 1.56 to 2.83; 15 months: RR 1.42, 95% CI 1.06 to 1.90). Median nutrient and energy intakes were also reported.
Vitolo 2005 reported the relative effect of caregiver educational intervention on the consumption of energy‐dense food at 12 to 16 months of age. RR less than 1 suggested the educational intervention reduced the consumption of energy‐dense food; candies (RR 0.85, 95% CI 0.74 to 0.98); soft drinks (RR 0.88, 95% CI 0.79 to 0.99); table sugar (RR 0.98, 95% CI 0.93 to 1.03); honey (RR 0.65 95% CI 0.50 to 0.84); cookies (RR 0.79, 95% CI 0.71 to 0.89); chocolate (RR 0.72, 95% CI 0.60 to 0.86); salty snacks (RR 0.86, 95% CI 0.76 to 0.97); lipid‐dense foods group (RR 0.62, 95% CI 0.49 to 0.80); and sugar‐dense foods group (RR 0.46, 95% CI 0.31 to 0.68). The effect estimates are study author‐reported (Vitolo 2012 in Vitolo 2005). At two to three years' follow‐up (when children were aged three to four years old), the study authors also reported a Health Eating Index score (MD 3.52, 95% CI 1.18 to 5.88) (Vitolo 2010 in Vitolo 2005). For the outcome of 'good diet' (Healthy Eating Index score > 80), the study‐author‐reported RR was 2.12 (95% CI 1.09 to 4.12).
With regards to consumption of specific foods and nutrients at the two‐to‐three‐year follow‐up time point, the study authors reported the following MDs for the following food types: grains (MD ‐0.11, 95% CI 0.60 to 0.38); meats (MD 0.10, 95% CI −0.56 to 0.75); vegetables (MD 0.53, 95% CI 0.10 to 0.95); fruits (MD 0.87, 95% CI 0.15 to 1.59); milk (MD 0.34, 95% CI −0.20 to 0.88); total fat* (MD 0.07, 95% CI −0.32 to 0.46); sodium* (MD 0.91, 95% CI 0.15 to 1.66); cholesterol* (MD −0.31, 95% CI −0.69 to 0.07); saturated fat* (MD 0.33, 95% CI −0.43 to 1.09). (*Lower scores indicate a greater intake.)
Volume and frequency of adequate complementary food
Seven community‐based studies reported outcomes related to the volume and frequency of adequate complementary food (quantity). Intervention effect estimates were either reported by study authors or could be estimated by the review authors in all of these studies.
For the outcome 'total mouthfuls' for Aboud 2008, we calculated an unadjusted MD of 1.45 (95% CI −0.74 to 3.64). For the outcome percentage child self‐fed mouthfuls, we calculated a follow‐up MD of 16.42 (95% CI 3.32 to 29.52).
Aboud 2009 also reported that the mean number of mouthfuls per meal consumed by children at follow‐up did not differ, with an overall MD of −0.39 (95% CI −4.62 to 3.84). The mean number of self‐fed mouthfuls as a percentage of total mouthfuls was 47.8 (± 42.4) in the intervention group compared with 32.2 (± 41.0) in the control group (study author‐reported); MD 15.60 (95% CI 3.83 to 27.37). The results of the ANCOVA, as reported by study authors, was d = 0.37 P = 0.01.
Aboud 2011 reported mean number of mouthfuls per meal for control and two active intervention groups. Here, we combined the two active arms of the intervention (it was a three‐armed study) to allow this comparison to be made; MD 5.76, 95% CI 2.10 to 9.42. Also reported was the mean number of self‐fed mouthfuls which, as a percentage of the total for each group, favoured the intervention: MD 10.19 (95% CI −0.20 to 20.58).
Bhandari 2004 reported mean meal frequency within a 24‐hour period at nine months of age (MD 0.50, 95% CI 0.31 to 0.69) and 18 months of age (MD 0.50, 95% CI 0.33 to 0.67) in favour of the intervention. Study author‐reported P values for the comparisons were < 0.01.
Campbell 2013 also reported the effect of the educational intervention on prevalence of any (versus none) non‐core food and drink consumption at postintervention (mean child age = 18 months). For non‐core drink intake the study authors reported an odds ratio (OR) of 0.81 (95% CI 0.51 to 1.30), for sweet snack intake an OR of 0.69 (95% CI 0.43 to 1.10), and for savoury snack intake an OR of 1.25 (95% CI 0.87 to 1.81). These effect estimates are not adjusted for covariates.
Reinbott 2016 reported the minimum meal frequency (as defined by WHO) as a RR of 1.04 (95% CI 0.98 to 1.10). The study authors also reported the following results from a linear regression of seven‐day food frequency, adjusted for age of child, wealth and maternal education: fish (B (beta) = 0.73, SE (standard error)(B) = 0.36, 95% CI 0.02 to 1.44, P = 0.05), pro‐vitamin‐A‐rich roots and tubers (B = 1.11, SE(B) = 0.25, 95% CI 0.62 to 1.60, P < 0.001), and dark green leafy vegetables (B = 1.15, SE(B) = 0.33, 95% CI 0.51 to 1.80, P = 0.001). Other categories of food frequencies were not reported.
Shi 2010 reported meal frequency (semi‐solid or solid foods) at three time points: six, nine and 12 months of age. At six months of age the MD was 0.57 (95% CI 0.34 to 0.80) and at nine months of age the MD was 2.72 (95% CI 2.35 to 3.09). Incomplete data were reported for the 12‐month outcome and we were unable to calculate an effect estimate for this time point.
Facility‐based studies
Amongst the facility‐based studies, Koehler 2007 reported compliance with food‐based recommendations and standardised daily nutrition scores. It was not possible to estimate an intervention effect from the published paper.
Olaya 2013 assessed the frequency and number of portions of each food consumed. Study author‐reported findings for the mean number of portions (per week) of each food consumed were reported in box and whisker plots for meat, red meat, vegetables, fruit, follow‐on formula milk, cows' milk, legumes, and sugar and sweetened foods (frequency). We have not extracted effect estimates from this plot. Olaya 2013 also reports the proportion of infants consuming recommended food groups, at the recommended frequency per week for the following food groups: meat (all types) (RR 1.65, 95% CI 1.10 to 2.46); red meat (RR 1.48, 95% CI 1.17 to 1.87); vegetables (RR 2.45, 95% CI 1.43 to 4.20); fruit (RR 1.59, 95% CI 1.19 to 2.12); and legumes (RR 1.44, 95% CI 0.91 to 2.26). Study authors also reported the MD for iron and zinc status between the intervention and control groups at six and 12 months of age (six months: ferritin = MD 24.69, 95% CI 221.8 to 12.4 mg/L; zinc = MD 3.65, 95% CI 28.8 to 16.0 mg/dL. 12 months: ferritin = MD 6.31, 95% CI 2.7 to 15.4 mg/dL; zinc = MD 24.23, 95% CI 217.9 to 9.4 mg/dL).
Adequacy of complementary food outcomes reported in Penny 2005 included eating nutrient‐dense, thick foods at lunch (a recommended complementary feeding practice) (six months: intervention 48 (31%) of 157 versus control 29 (20%) of 147; difference between groups 19 (11%), P = 0·03); achieving dietary requirements for energy (8 months: intervention 30 (18%) of 170 versus control 45 (27%) of 167, P = 0·04; 12 months: 64 (38%) of 168 versus 82 (49%) of 167, P = 0·043); dietary iron intake from complementary foods (8 months: intervention 155 (91%) of 170 versus control 161 (96%) of 168, P = 0.047; 9 months: 152 (93%) of 163 versus 165 (99%) of 167, P = 0.003); and dietary zinc intake from complementary foods (9 months: intervention 125 (77%) of 163 versus control 145 (87%) of 167, P = 0·012). Effect estimates and P values are as reported by Penny 2005. Unadjusted mean energy and nutrient intakes from complementary foods (24‐hour recall) were reported in a figure, but we were not able to estimate an intervention effect for the outcomes
It was not possible to estimate intervention effect estimates from Schroeder 2015. The study authors reported that the "intervention group was less likely to use infant cereal (P < 0.001) or stage 1 vegetables (P < 0.05) as the first complementary food. Also, the intervention group offered significantly less soda (P < 0.006), sweetened tea (P < 0.01), punch (P < 0.02), or cows' milk (P < 0.001) than the control group" (p 3). A comparison between six and 24 months indicated that the control group increased consumption of unsweetened drinks (P < 0.04) and of vitamin supplements (P < 0.04) relative to the intervention group, as reported by study authors. Parents in the intervention group exerted more dietary restriction on their child (P < 0.01) and were more active in monitoring child feeding (P < 0.05) than those in the control group.
1d. Hygiene practices
Six community‐based studies reported the impact of educational interventions on hygiene practices (Aboud 2009; Aboud 2011; Bhandari 2004; Negash 2014; Shi 2010; Tariku 2015), of which only one was an individually‐randomised study (Negash 2014) and five were cluster‐randomised studies.
There was considerable variation in the definition of the outcome of hygiene practices across studies; for example, washing a child's hands before feeding (Aboud 2009; Bhandari 2004; Shi 2010), washing a child's hands with soap (Aboud 2011; Tariku 2015), washing of the caregivers' hands before feeding or food preparation (Bhandari 2004; Negash 2014; Shi 2010; Tariku 2015), and handwashing after defecation (Negash 2014). Where a study reported more than one handwashing outcome, we chose the outcome relating to handwashing before feeding and prioritised caregiver handwashing for the meta‐analysis. The intervention delivery strategies included group education sessions, demonstrations/practicals of meal preparation, role play with infants, use of printed materials (posters, flip books, feeding‐recommendation cards, picture books), home visits, women's group meetings, village rallies, debates, side plays and nutrition fairs.
Community‐based studies
Pooled results
Four studies provided sufficient data for inclusion in a meta‐analysis, having retrospectively accounted for clustering (assuming an ICC of 0.02) (Aboud 2009; Aboud 2011; Bhandari 2004; Shi 2010). We conducted a random‐effects meta‐analysis using the generic inverse variance approach in RevMan 5 (Review Manager 2014), and explored the impact of the ICC in the sensitivity analyses in Figure 6. Having accounted for clustering, there was moderate‐quality evidence that educational intervention increased caregiver‐reported handwashing before feeding by an average of 38% (Analysis 1.3: average RR 1.38, 95% CI 1.23 to 1.55; 4 studies, 2029 participants; Tau2 = 0.00, I2 = 0%; Table 1).
6.
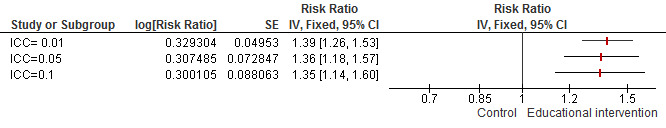
Sensitivity analysis 3. Comparison of different ICC (primary outcomes), outcome: 3.2 hygiene: handwashing before feeding
1.3. Analysis.
Comparison 1 Educational intervention versus no educational intervention for improving complementary feeding practices (ICC = 0.02), Outcome 3 Hygiene practices: community‐based intervention.
Single study results
Two studies were not included in the meta‐analysis (Negash 2014; Tariku 2015), as neither reported sufficient information to calculate an intervention effect estimate. Negash 2014 narratively reported that handwashing before feeding and after defecation had decreased in the intervention group but remained unchanged in the control group. We could not calculate an effect estimate for either study, due to a lack of clarity around the numbers randomised. Tariku 2015 reported, "regarding to the hand washing practice, the proportion of mothers who would wash their hands after intervention significantly increased for all Kebeles [administrative district] compared to pre‐intervention, but no significant differences were found in the proportion of hand washing practices. For the use of soap to wash their child’s hand, there were significant difference between the Traditional intervention and Control Kebeles (p = .005); and between the Health Belief Model intervention and Control Kebeles (p = .001)" (p 8). Note, the study authors reported P values only, and we were unable to estimate an intervention effect estimate due to insufficient information reported in the paper.
Facility‐based studies
None of the facility‐based studies reported the effect of educational intervention on hygiene practices.
2. Adverse events
One study investigated the compliance with, and acceptability of, the intervention (Olaya 2013). They reported a 74% compliance rate with the recommendations of the intervention. Only one out of the 38 mothers felt that the recommendations were not helpful. On the affordability of recommended complementary foods, 83.8% of the mothers could afford the recommended complementary food while six mothers found the foods too expensive. The recommended complementary food was tolerated by all infants in the study and there were no reported adverse effects.
Secondary outcomes
1. Growth
Fourteen studies reported growth outcomes (Aboud 2008; Aboud 2009; Bhandari 2001; Bhandari 2004; Campbell 2013; Daniels 2012; Negash 2014; Olaya 2013; Penny 2005; Reinbott 2016; Saleem 2014; Schroeder 2015; Shi 2010; Vazir 2013). Of these, we were able to combine eight quantitatively in at least one of the growth meta‐analyses. Four studies were not included in the meta‐analyses because they included age ranges or reported growth data at time points that were insufficiently similar to other studies (Aboud 2008; Aboud 2009; Negash 2014; Saleem 2014). They are reported below under the heading 'Individual study results'. Campbell 2013 was not included in the meta analysis because the study reported body mass index (BMI) only and we could not combine this with other measures of growth. Reinbott 2016 reported mean height‐for‐age (HAZ) and mean weight‐for‐age (WAZ) z scores, rather than stunting, wasting or underweight outcomes. The results from Campbell 2013 and Reinbott 2016 are also reported below.
The 14 studies moreover reported growth outcomes at various time points. However, we had a priori selected time points of six and 12 months of age because these mark the half and first year of an infant's life respectively. Thereafter, we chose to analyse growth parameters at six‐monthly intervals (18 and 24 months of age), since the rate of growth reduces after infancy.
Pooled analysis results
We conducted the meta‐analysis using the generic inverse variance approach, to allow for inflating the standard error of Penny 2005, Schroeder 2015 and Shi 2010. For all growth outcomes, we assumed an ICC = 0.05. Overall, the body of evidence for all growth outcomes was considered low quality. See Table 2.
For attained weight (kg), the pooled results for the three studies that recruited women during pregnancy (Bhandari 2001; Shi 2010;Vazir 2013) are compatible with both a reduction and an increase in attained weight at six months of age, relative to control (MD 0.03 kg, 95% CI −0.10 to 0.17; 3 studies, 1221 children; Tau2 = 0.00, I2 = 0%; very low‐quality evidence). This was also observed at 12 months of age (MD 0.06 kg, 95% CI −0.04 to 0.15; 5 studies, 2464 children; Tau2 = 0.00, I2 = 0%; very low‐quality evidence), 18 months of age (MD 0.10 kg, 95% CI −0.14 to 0.35; 2 studies, 1402 children; Tau2 = 0.02, I2 = 52%; very low‐quality evidence), and at 24 months of age (MD −0.14 kg, 95% CI −0.36 to 0.08; 2 studies, 920 children; Tau2 = 0.00, I2 = 0%; low‐quality evidence). See Analysis 2.1.
2.1. Analysis.
Comparison 2 Educational intervention versus no educational intervention for improving complementary feeding practices: growth outcomes (ICC = 0.05), Outcome 1 Weight.
For the outcome of mean height/length (cm), findings from the meta‐analysis are indicative of both a harm and a benefit of educational intervention relative to the control intervention, at all four time points assessed (see Analysis 2.2). Summary effect estimates were similar at six months of age (MD 0.16 cm, 95% CI −0.21 to 0.52; 3 studies, 1221 children; Tau2 = 0.00, I2 = 0%; very low‐quality evidence), 12 months of age (MD 0.32 cm, 95% CI 0.11 to 0.52; 5 studies, 2464 children; Tau2 = 0.00, I2 = 0%; low‐quality evidence), 18 months of age (MD 0.58 cm, 95% CI −0.22 to 1.38; 2 studies, 1402 children; Tau2 = 0.21, I2 = 61%; very low‐quality evidence), and 24 months of age (MD −0.13 cm, 95% CI −0.58 to 0.32; 2 studies, 920 children; Tau2 = 0.00, I2 = 0%; low‐quality evidence).
2.2. Analysis.
Comparison 2 Educational intervention versus no educational intervention for improving complementary feeding practices: growth outcomes (ICC = 0.05), Outcome 2 Height/length.
Individual study results
Six studies could not be included in the meta analyses (Aboud 2008; Aboud 2009; Campbell 2013; Negash 2014; Reinbott 2016; Saleem 2014).
Aboud 2008 reported mean attained weight (kg) at five months postintervention in each group (MD 0.46 kg, 95% CI 0.07 to 0.85) and weight gain (kg) (MD 0.34 kg, 95% CI 0.12 to 0.56). Aboud 2008 also reported effect sizes for weight (d = 0.28) and weight gain (d = 0.48). It was not feasible to combine this study in the meta‐analysis due to the different age groups studied (aged 12 to 24 months at baseline).
Aboud 2009 reported two growth outcomes: WAZ (MD 0.01, 95% CI −0.24 to −0.26) and child's attained weight (kg) (MD 0.01 kg, 95% CI −0.29 to 0.31). Again, it was not feasible to combine this outcome due to the different age groups studied (aged 8 to 20 months at baseline).
It was not possible to calculate intervention effect estimates for Negash 2014. The only information available was study author‐reported, "control and intervention children had similar gains in weight (˜ 0.9 kg) and height (˜ 4 cm)" (p 483).
Saleem 2014 measured the following infant growth outcomes: weight, length, mid upper‐arm circumference (MUAC), stunting, wasting, and underweight at four time points. They reported weight, length and MUAC at follow‐up in a figure, all of which favoured the intervention group (P values = 0.001, 0.002 and 0.001 respectively). We have not extracted effect estimates from this plot. They also reported the reduction of stunting and underweight as OR 8.36 (95% CI 5.6 to 12.42) and OR 0.75 (95% CI 0.4 to1.79), favouring the intervention compared to the control group (adjusted OR).
2. Incidence of malnutrition among participants
Pooled analysis results
We report the findings of the meta‐analyses for the outcome of nutritional status measures in Analysis 2.3. Five studies reported stunting, defined as HAZ ≤ −2 SD (Bhandari 2001; Bhandari 2004; Kang 2017; Olaya 2013; Penny 2005). Two studies reported usable data for wasting, defined as WHZ ≤ −2 SD (Bhandari 2001; Kang 2017). Three studies reported usable data for the outcome of underweight, defined as WAZ ≤ −2 SD (Bhandari 2004; Kang 2017; Olaya 2013). For the outcome of stunting, the 95% CIs for the effect estimate are suggestive of both a harm and a benefit of educational intervention, relative to the control intervention (average RR 0.89, 95% CI 0.74 to 1.06; 5 studies, 3487 children; Tau2 = 0.00, I2 = 0%; low‐quality evidence). For the outcome of wasting, 95% CI are again suggestive of both a benefit and harm of the complementary feeding intervention relative to control (average RR 0.79, 95% CI 0.48 to 1.30; 2 studies, 2000 children; Tau2 = 0.00, I2 = 0%; low‐quality evidence). Three studies were included in the analysis for underweight (Bhandari 2004; Kang 2017; Olaya 2013). Again, 95% CIs for the average RR were compatible with both an increase and decrease in the outcome (average RR 0.99, 95% CI 0.68 to 1.44; 3 studies, 2900 children; Tau2 = 0.00, I2 = 0%; low‐quality evidence).
2.3. Analysis.
Comparison 2 Educational intervention versus no educational intervention for improving complementary feeding practices: growth outcomes (ICC = 0.05), Outcome 3 Nutritional status (underweight, stunting, wasting).
Individual results for studies that could not be included in the meta‐analyses are presented below.
Individual study results
Daniels 2012 reported HAZ (MD −0.02, 95% CI −0.19 to 0.15), WAZ (MD −0.13, 95% CI 0.27 to 0.01). They also reported rapid weight gain (OR 1.5, CI 95% 1.1 to 2.1) (control put on more weight, more rapidly).
Saleem 2014 reported MUAC, stunting, wasting, and underweight at four time points. They reported weight, length and MUAC at follow‐up in a figure, all of which favoured the intervention group (P values = 0.001, 0.002 and 0.001 respectively). We have not extracted effect estimates from this plot. They also reported the reduction of stunting and underweight as OR 8.36 (95% CI 5.6 to 12.42) and OR 0.75 (95% CI 0.4 to1.79), favouring the intervention group compared to the control group (adjusted OR).
Reinbott 2016 reported unadjusted means for the following nutritional status outcomes: HAZ (MD −0.06, 95% CI −0.20 to 0.08), WHZ (MD 0.00, 95% CI −0.13 to 0.13) and WAZ (MD −0.02, 95% CI −0.15 to 0.11).
3. Morbidity
Morbidity was measured by episodes of diarrhoea. We were unable to conduct a meta‐analysis for this outcome due to differences in the ways it was measured and reported. Four studies evaluated the effect of educational intervention on diarrhoea (Bhandari 2001; Bhandari 2004; Reinbott 2016; Vitolo 2005). Vitolo 2005 reported a beneficial effect of educational intervention on the incidence of diarrhoea, with the number of events reported as 46 in the intervention arm and 98 in the control arm. Numbers were not provided.
Bhandari 2001 reported that the intervention had no effect on diarrhoea episodes and prevalence: nutritional counselling group (study author‐reported episodes per child in the intervention group = 6.9 (± 3.2), prevalence per 100 d 14.6 (± 12.0); episodes per child in the visitation/control group = 6.7 (± 3.4), prevalence per 100 d 13.2 (± 9.8)). Diarrhoea prevalence at 12 months of age as reported by Bhandari 2004 was 16.8 in the intervention arm versus 13.1% in the control arm (study author‐reported P = 0.174).
Reinbott 2016 reported a decrease in the prevalence of diarrhoea in the past two weeks in the intervention and control groups between the baseline (control 41.6%, intervention 36.9%) and impact survey (control 26.2%, intervention 27.9%).
See Table 7 for details of the effect of the intervention on diarrhoea as reported by the study authors.
4. Mortality
None of the included studies reported or evaluated the effects of educational intervention on infant/child mortality.
5. Hospitalization
Only one, community‐based study measured the effect of educational intervention on hospitalisation (Vitolo 2005). The study reported that the number of days spent hospitalised was nine days in the intervention arm and 15 days in the control group.
See Table 8 for details of the effect of the intervention on hospitalisation as reported by the study authors.
6. Hospitalisation (days spent).
| Study | Result |
| Vitolo 2005 | Intervention = 9 days, control = 15 days |
6. Change in knowledge
Eight of the included studies reported positive outcomes of the intervention on the knowledge of caregivers (Aboud 2008; Aboud 2009; Aboud 2011; Negash 2014; Penny 2005; Shi 2010; Vazir 2013; Yin 2009). More intervention mothers recalled the intervention messages at follow‐up, could recall the recommended feeding practices and messages accurately, gave correct responses to questions on complementary feeding practices, and had higher knowledge scores. We were unable to combine the results in a meta‐analysis due to differences in the measures of knowledge that were used in the various studies. We present the study authors' report on the effect of the intervention on knowledge outcomes in Table 9.
7. Change in knowledge.
| Study | Result (trial authors' judgement) |
| Aboud 2008 | More intervention mothers recalled messages (5 out of 8 message categories P < 0.0001), especially hygiene (washing hands before eating), responsive feeding and talking to the child during the meal |
| Aboud 2009 | More intervention mothers recalled messages at follow‐up |
| Aboud 2011 | Mothers in the intervention group recalled more messages at follow‐up, especially pertaining to hygiene, self‐feeding, responding, stimulating, and foods to feed. Of 8 messages, control mothers recalled a mean of 0.59 (SD 1.0) and mothers in the intervention group recalled a mean of 2.37 (SD 1.5) |
| Negash 2014 | Knowledge of complementary feeding in the intervention group rose from 5.8 (± 2.1) at baseline to 7.1 (± 1.0) at end line (P < 0.001), whereas scores for the control group stayed unchanged at 6.3 (± 1.6) at both time points |
| Penny 2005 | Caregivers in the intervention group were more knowledgeable of key feeding practices and messages. |
| Shi 2010 | At 6, 9, 12 and 18 months of age, after the implementation of the intervention, more caregivers in the intervention group responded correctly to the questions on feeding practices than those in the control group (statistically significant results for all questions) |
| Vazir 2013 | Educational messages to the intervention groups were significantly associated with changed maternal knowledge/beliefs about foods that are good for infants at ages 9 and 15 months. The percentage of mothers who had more knowledge regarding recommended foods from animal sources, such as egg and liver, and responded positively on selected appropriate foods to be given to infants, was higher, both at 9 and 15 months, in the intervention groups but this was not seen in the control group |
| Yin 2009 | After being educated with feeding guideline on infants and young children, the knowledge of infants' mothers was greatly improved and KAP scores of the mothers after intervention were higher than at baseline (F = 183.556, P = 0.006); the percentage of correct answers on nutrition knowledge in the intervention groups was significantly higher than that of the control group. At six months of intervention, the KAP scores of intervention group 1 (12.0) and intervention group 2 (11.6) were higher than that of the control group (10.5) (least significant difference? (LSD) t = 5.96, P < 0.001; LSD t = 4.25, P < 0.001) |
KAP: knowledge, attitude and practice; SD: standard deviation
Sensitivity analyses
We conducted sensitivity analyses for the primary outcomes only. We re‐ran all analyses assuming a fixed‐effect model. The conclusions remained unchanged.
We investigated the impact of assuming an alternative ICC on the summary effect estimates for the following primary outcomes: duration of exclusive breastfeeding (≥ four months of age) and hygiene practices (predominantly defined as washing hands before feeding). For both outcomes we compared the impact on the pooled summary estimates using ICCs of 0.01, 0.05 and 0.10. For the outcome of duration of exclusive breastfeeding, only three studies were included in the meta‐analysis, and a single study was adjusted (Penny 2005). Increasing the ICC to 0.10 did not impact the results for this outcome (see Analysis 3.1). For the outcome of hygiene practices (handwashing before feeding), results remained in favour of educational intervention (see Analysis 3.2).
3.1. Analysis.
Comparison 3 Sensitivity analyses for dropouts (primary outcomes), Outcome 1 Sensitivity analysis: introduction of complementary food.
3.2. Analysis.
Comparison 3 Sensitivity analyses for dropouts (primary outcomes), Outcome 2 Sensitivity analysis: duration of exclusive breastfeeding (dropouts as responders).
For the main analyses, we included studies according to intention‐to‐treat principles for dichotomous outcomes, and assumed that all study dropouts (regardless of allocation) had not experienced the 'event'. For complementary food introduced before four to six months, 149 participants dropped out of the intervention arms and 184 dropped out from the control arms. In the main analysis, we assumed that these participants had not introduced complementary foods. In the sensitivity analysis, therefore, we examined the impact of assuming dropouts had introduced complementary food before six months. The pooled average RR and 95% CI are very slightly attenuated towards the null (RR 0.89, 95% CI 0.81 to 0.97; Analysis 3.1), however, conclusions remained unchanged.
For duration of exclusive breastfeeding, 122 participants dropped out of the intervention arms and 160 dropped out from the control arms. In the main analysis it was assumed that these participants had not exclusively breastfed for at least four months. In the sensitivity analysis, we assumed that dropouts had been exclusively breastfed for four months or longer. The pooled average RR and 95% CI are attenuated towards the null (RR 1.00, 95% CI 0.85 to 1.18; Analysis 3.2). However, due to the extent of the uncertainty in the main analysis (RR 1.58, 95% CI 0.77 to 3.22), our conclusions for this outcome remain unchanged.
For improved hygiene practices (handwashing before feeding), 181 participants dropped out of the intervention arms and 150 dropped out from the control arms. (Note, for Shi 2010, we assumed the 110 dropouts had occurred equally between the control and intervention arms.) In the sensitivity analysis, we assumed that dropouts used appropriate hygiene practices before feeding their infant. Conclusions for this outcome also remain unchanged (RR 1.30, 95% CI 1.17 to 1.46; Analysis 3.3).
3.3. Analysis.
Comparison 3 Sensitivity analyses for dropouts (primary outcomes), Outcome 3 Sensitivity analysis: hygiene practice (dropouts as responders).
Discussion
Summary of main results
The review sought to assess the effectiveness of educational interventions for improving complementary feeding practices and other related health and growth outcomes in young children. We identified a total of 23 studies, 19 of which were community‐based studies and four were facility‐based studies. Overall, the evidence available suggests that educational interventions improve complementary feeding practices marginally; there was little evidence of an effect for growth patterns or nutritional status.
Effect of educational intervention on complementary feeding practices
There was a small positive effect of educational interventions on the time of commencement of complementary feeding by the caregivers of the children. However, the studies that were included in the meta‐analysis were all conducted in high‐income and lower‐ and upper‐ middle‐income countries (de Oliveira 2012; Edward 2013; Vitolo 2005; Wen 2011). The studies conducted in the lower‐middle‐ and low‐income countries did not report on time of commencement of complementary feeding hence there was no information to report. Edward 2013 showed greater benefit of the intervention in delaying the onset of complementary feeding than other studies. This may have been due to the mentorship model employed in the study as community doulas were used to deliver the educational intervention to adolescent mothers. These doulas had also been teenage mothers and were sufficiently familiar with the ethos and environment of the participants.
The focus of most of the included studies seemed to be on the adequacy (quality and quantity) of complementary foods fed to infants. Eighteen of the 23 included studies reported on this outcome in ways that were too varied to be combined for any form of analysis. All of the studies, however, reported improvements in the quality and quantity of complementary foods as indicated by the conclusions of the study authors. This showed that most caregivers in the intervention arms complied with the intervention messages irrespective of the fact that the studies did not provide complementary foods as part of the interventions. A possible explanation for this improvement is that most of the studies were conducted after undertaking formative research to identify gaps and resources available in these locations. This made the intervention messages culturally appropriate and enhanced the acceptability or affordability (or both) of the interventions, since most of the recommended foods were readily available in the intervention settings. This strengthens the evidence that educational interventions without the provision of foods are effective in improving complementary feeding practices. Although standard measures for accessing infant and young child feeding have been developed (e.g. the WHO minimum acceptable diet, minimum dietary diversity, minimum meal frequency), only one, recently conducted study put them to use (Reinbott 2016). This made it difficult to assess the adequacy of foods fed to infants using these indicators in a meta‐analysis and, as such, in this review we assessed adequacy of food fed to children based on results reported in the individual studies and the study authors' conclusions.
Educational interventions showed positive effect on the duration of exclusive breastfeeding for studies conducted in the community (Vitolo 2005; Wen 2011), but showed no effect on the studies conducted in health facilities (Penny 2005). The test for subgroup differences between community and facility‐based studies suggested a difference in treatment effect by setting.
Analysis of the (mostly community‐based) studies that reported hygienic practices showed that educational interventions had a weak positive effect on hygiene practices (Aboud 2009; Aboud 2011; Bhandari 2004; Shi 2010). One study conducted in sub‐Saharan Africa reported that educational intervention had a negative effect on hygiene practices (not included in meta‐analysis; Negash 2014). Although the study authors did not report on water availability in the study area, it is well established that this can threaten compliance with recommended hygiene practices. Interestingly, all of the studies that reported on hygiene practices were conducted in the community.
The effect of educational interventions in preventing diarrhoea showed mixed results. Four community‐based studies reported this outcome and only one study recorded a clearly beneficial effect of educational interventions in reducing the episodes of diarrhoea in the intervention group. The other three studies found no clear effect on the incidence and prevalence of diarrhoea.
Educational interventions were effective in reducing the days spent in the hospital in one community‐based study. Other studies did not report this outcome.
Educational interventions were also effective in improving the knowledge of caregivers in all of the included studies. Although we were unable to pool the results in a meta‐analysis, the study authors reported that caregivers in the intervention groups were able to recall the intervention messages at follow‐up, recall recommended feeding practices and messages accurately, and had higher knowledge scores.
None of the studies reported any clear adverse effects of the interventions.
Effect of educational intervention on growth
The studies included in the meta‐analyses did not show an effect of educational intervention on growth parameters. The test for differences in the weight of the children taken at baseline and at 6, 12, 18 and 24 months did not show any statistical difference. The analysis showed similar findings for height/length and for underweight, stunting and wasting.
Of the studies not included in the quantitative analysis, three showed a positive effect of educational intervention on growth parameters, while the other two did not suggest a positive effect of educational intervention.
Although the study authors measured growth parameters at various time points, we only included growth parameters at 6, 12, 18 and 24 months of age in the meta‐analysis. This is because 6 and 12 months of age mark the half and first year of an infant's life respectively, and since the rate of growth reduces after infancy, we choose a six‐monthly interval thereafter (18 and 24 months of age).
We found no studies evaluating or reporting the effects of educational interventions on mortality.
Overall completeness and applicability of evidence
Of the 23 studies included in this review, five were conducted in high‐income countries: Australia (Campbell 2013; Daniels 2012; Wen 2011), Germany (Koehler 2007) and the USA (Schroeder 2015). Six were conducted in upper‐middle‐income countries: Brazil (de Oliveira 2012; Vitolo 2005), China (Shi 2010; Yin 2009), Colombia (Olaya 2013), and Peru (Penny 2005). Eight were conducted in lower‐middle‐income countries, including Bangladesh (Aboud 2008; Aboud 2009; Aboud 2011), Cambodia (Reinbott 2016), India (Bhandari 2001; Bhandari 2004; Vazir 2013), and Pakistan (Saleem 2014). Three studies were conducted in a low‐income country: Ethiopia in tropical Africa ((Kang 2017; Negash 2014; Tariku 2015). The location of one study was not stated in the study report (Edward 2013).
Eight of the 23 studies were conducted in urban settings (Daniels 2012; de Oliveira 2012; Edward 2013; Koehler 2007; Olaya 2013; Schroeder 2015; Vitolo 2005; Wen 2011), two in peri‐urban settings (Penny 2005; Saleem 2014), one in an urban slum (Bhandari 2001), and 11 in rural settings (Aboud 2008; Aboud 2009; Aboud 2011; Bhandari 2004; Kang 2017; Negash 2014; Reinbott 2016; Shi 2010; Tariku 2015; Vazir 2013; Yin 2009). One study report stated that the study was conducted in local government areas but did not state clearly whether the setting was urban, semi‐urban or rural (Campbell 2013). Community‐based studies were well distributed among the high‐ and middle‐income countries but health facility‐based studies were conducted mainly in the high‐ and upper‐middle‐income countries.
The findings of these studies could be applied across the social groups because the studies were conducted in high‐, upper‐middle‐ and lower‐middle‐income countries. However, it is important to note that the studies from low‐income settings were all from the same country in sub‐Saharan Africa (Ethiopia), consequently while the findings of this study could be applied in the high‐, lower‐upper‐ and lower‐middle‐income countries, the same cannot be said of the low‐income countries where the three studies in this classification were conducted in the same country (Ethiopia).
The participants included in the studies, mother/caregiver‐child pairs, were also properly suitable for the review since the children included in the studies ranged from birth to 24 months of age and this age bracket includes the time frame for the onset of complementary feeding. Most of the outcomes were measured on children while mothers/caregivers received the educational intervention. The intervention delivery mechanisms and promotional activities are also assessed as applicable across settings since they generally included group sessions/meetings, demonstration and practical sessions, the use of flip charts, picture books and brochures. These strategies are easily reproducible across settings irrespective of income classification or development rating.
The intervention messages were also culturally appropriate and incorporated locally available foods in recommendations on the types of foods and food groups to be fed to children of complementary feeding age. This encouraged the mothers/caregivers to use resources locally available to them and increased the acceptability of the intervention. This was evident by the rate of compliance and, in one of the studies, the mothers contributed the cooking materials used in the nutrition sessions. The messages also included key aspects of adequate complementary feeding such as recommendations on the duration of breastfeeding, continued breastfeeding in addition to complementary foods, dietary diversity, consistency of complementary foods, hygiene and feeding based on satiety cues.
In general, the majority of the interventions were delivered to groups of women (typically the mothers) or caregivers in their own homes. Interventions used a mixture of interactive sessions, demonstrations of correct practice, imitation, role plays, group discussions, peer support, story telling, picture books and village rallies amongst others. Reporting of exact intervention content was mostly poor; for example, replication of interventions from reported detail may not be possible. In the same vein, an appraisal of the educational approaches used in the studies is most likely not feasible. Nothwithstanding that this review did not set out to evaluate the education models/approaches used in implementing the studies, participatory approaches, such as the 'Trials of Improved Practices' (TIPs) and other formative research procedures, are believed to yield higher levels of acceptability for the interventions being implemented.
The studies also measured key child‐feeding indicators and outcomes, which are generally measurable across settings and, as such, can be easily applied and replicated.
Quality of the evidence
We assessed the quality of evidence using the GRADE approach (Guyatt 2008). The evidence that educational interventions improve complementary feeding practices (time of introduction of complementary foods) is considered to be of moderate quality (Table 1), while that of growth outcomes is considered to be of low to very low quality (Table 2). Most of the studies were at unclear risk of selection bias due to unclear allocation concealment. In addition, some of the studies were at high or unclear risk of performance and detection bias since they did not blind or describe the blinding of participants, personnel and outcome assessors. Most of the studies favoured the intervention arms, although the results of the meta analysis showed some imprecision.
Consequently, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate for improved complementary feeding practices. We are very uncertain about the estimates of effects for the growth outcomes, which indicate that evidence is insufficient to confirm that education is an effective intervention for improving the growth of infants, while further research is very likely to have an important impact on our confidence in the estimate of effect for nutritional status and is likely to change the estimate.
Potential biases in the review process
This review attempted to assess the effect of educational interventions on a broad spectrum of topical aspects of complementary feeding. It is the only Cochrane Review that has evaluated the effectiveness of education on four key aspects of complementary feeding across the globe. Other non‐Cochrane reviews have assessed the effectiveness of education and other complementary feeding interventions on complementary feeding and growth in low‐income countries (Imdad 2011; Lassi 2013; Shi 2011), while Dewey 2008 assessed the effectiveness of complementary feeding interventions in general in low‐income countries. Our search strategy was highly sensitive and we did not apply any language restrictions. We also included published data and contacted study authors for unpublished data.
As shown in the 'Risk of bias' assessment, one potential bias in the review process was that a number of included studies were unable to blind participants and personnel, as such we cannot rule out the possibility of detection bias and its effect on the results in the intervention groups. We were also unable to retrieve the full texts of 10 studies we believe might qualify for inclusion in this review (see Studies awaiting classification). Due to the limited number of studies we were able to include in our meta‐analyses, we did not conduct the planned sensitivity analyses to detect the effect of excluding studies with missing data, unpublished studies, and studies with high risk of bias on the overall results of the meta‐analysis.
Some studies in our analysis either did not account for the effect of clustering in their analysis, or reported raw (unadjusted) estimates. As such, we followed section 16.3.4 and 16.3.5 of the Cochrane Handbook for Systematic Reviews of Interventions for calculating the effective sample size and incorporating cluster studies in the meta‐analysis (Higgins 2011). These are approximate methods and results should be interpreted accordingly.
Agreements and disagreements with other studies or reviews
The effectiveness of educational interventions for improving complementary feeding practices in low‐income countries has been previously studied by Shi 2011. The findings of this review agree with that of Shi 2011, although it was limited to low‐income countries. On the effect of educational interventions on growth, the findings of this review are similar to those of Imdad 2011 and Lassi 2013, notwithstanding that the studies were also undertaken in low‐income countries. In general, the review by Dewey 2008 found educational interventions to be an effective strategy for promoting appropriate complementary feeding in low‐income countries.
Authors' conclusions
Implications for practice.
Overall, educational interventions led to improvement in complementary feeding practices. It delayed the early onset of complementary feeding, increased the duration of exclusive breastfeeding, enhanced the adequacy of complementary foods in both settings and improved hygiene practices in community‐based settings. The weight of evidence from the community‐based studies (four of five included studies) was in favour of educational interventions as a promoter of hygienic practices.
The facility‐based studies did not assess hygiene practices. Community‐based studies are preferred in assessing hygiene practices of caregivers as the facility‐based studies are conducted in an 'ideal' condition hence hygiene of the environment is taken care of by the study team and not the caregivers. The improvement in hygiene practices was mainly due to improved practice of handwashing by caregivers before feeding of children. No information was available on water sanitation practices and food preparation and storage properties. This review showed that educational interventions without the provision of complementary foods were effective in improving complementary feeding practices. This may have been accounted for by the formative research undertaken by most of the studies before the commencement of the intervention, making the interventions culturally appropriate and acceptable.
Implications for research.
The findings of this review point to the need for further research of high methodological quality to determine the effectiveness of educational interventions for improving complementary feeding practices. There is a need for studies with adequate concealment of allocation sequence and studies that blind outcome assessors.
Also, structured methods or metrics for assessing and reporting complementary feeding practices are needed for accurate judgement of the complementary feeding practices. We observed that study authors used highly subjective methods that made it impossible to conduct meta‐analysis. This also has implications on our confidence in the outcomes of the interventions given the high rate of self‐reporting since there is the tendency for caregivers to report socially desirable behaviours. This may have accounted for the little or nonexistent effect of the intervention on growth outcomes, which were measured objectively, despite reports of high compliance with the interventions, and is contrary to the clear effects of the intervention on complementary feeding practices mostly self‐reported by caregivers.
None of the included studies reported the effect of educational interventions on the storage and preservation of complementary foods by mothers/caregivers of the children as well as on mortality. Well‐conducted research, which assesses these outcomes, is therefore necessary to fill this gap. There is also a need for more studies that deploy participatory approaches and other formative research in order to boost the acceptability and sustainability of the interventions and newly imbibed practices at the end of the studies.
Furthermore, there is need for more studies to be conducted in African and other low‐income countries to make the conclusions on the effectiveness of the intervention more robust across the various settings.
Acknowledgements
The review authors are grateful to the external reviewers and statisticians for their comments, and also wish to thank the Cochrane Developmental, Psychosocial and Learning Problems Editorial Team: Professor Geraldine Macdonald (Co‐ordinating Editor), Dr Joanne Duffield (Managing Editor), Gemma O’Loughlin (former Assistant Managing Editor), and Margaret Anderson (Information Specialist) for their expert advice during the development of this review.
We thank Ingrid Toews, Carolina de Oliveira Cruz‐Latorraca, and Heng‐Lien Lo for their kind assistance with the translations of some of the included studies. We also appreciate the immense contributions of Dr Olabisi Oduwole to the completion of this review.
Dachi Arikpo was awarded a Reviews for Africa Programme (RAP) Fellowship. The protocol for this review was developed during this Fellowship Programme, organised by Cochrane Nigeria, March 2014. The UK Department for International Development (DFID) supported this programme through UKAid funding provided to the Effective Health Care Research Consortium (EHCRC) at Liverpool School of Tropical Medicine (LSTM), a project for the benefit of low‐ and middle‐income countries. This document is an output from this project. The views expressed herein are those of the authors and not necessarily those of UKAid or the Department for International Development (DFID).
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library
#1[mh ^"Infant Nutritional Physiological Phenomena"] #2[mh ^" Infant Nutrition Disorders"] #3[mh ^"Infant Food"] #4[mh ^Weaning] #5wean*:ti,ab #6((compl*ment* or supplement*) near/3 (food* or feed* or nutrition*)):ti,ab #7[mh "Breast feeding"] or [mh "Bottle Feeding"] #8(breast* near/1 (duration or exclusiv* or optimal*)):ti,ab #9((substitut* or stop* or ceas* or cessation or partial*) near/1 breast*):ti,ab #10(bottle next fed or formula next fed) or (bottle next feed* or formula next feed*):ti,ab #11(infant next formula or formula next milk):ti,ab #12((fortif* near/1 food*) and (baby or babies or infant*)):ti,ab #13(((solid* or semi‐solid* or soft) near/3 (food* or feed* or diet*)) and (baby or babies or infant*)):ti,ab #14((introduc* near/3 (solid* or semi‐solid)) and (baby or babies or infant*)):ti,ab #15{or #1‐#14} #16[mh ^Education] #17[mh "Health Education"] #18[mh ^"Health promotion"] #19[mh Counseling] #20[mh /ED] #21[mh ^"Health Knowledge Attitudes Practice"] #22(class* or counsel* or educat* or instruct* or program* or teach* or train*):ti,ab188259 #23{or #16‐#22} #24#15 and #23
MEDLINE Ovid
1 Infant Nutritional Physiological Phenomena/ 2 Child Nutrition Sciences/ 3 Infant Nutrition Disorders/ 4 Infant Food/ 5 (infant$ adj1 (food or feeding or nutrition$)).tw. 6 Weaning/ 7 wean$.tw. 8 ((compl#mentary or supplementary) adj3 (food$ or feed$ or nutrition$)).tw. 9 Breast feeding/ 10 (breast$ adj1 (duration or exclusiv$ or optimal$)).tw. 11 ((Stop$ or cease or cessation or partial) adj1 breast$).tw. 12 (breast$ adj1 substitut$).tw. 13 Bottle Feeding/ 14 (bottle fe?d$ or formula milk or infant formula).tw. 15 (fortif$ adj1 food$).tw. 16 ((solid$ or semi‐solid$ or soft) adj3 (food$ or feed$ or diet$)).tw. 17 (introduc$ adj3 (solid$ or semi‐solid)).tw. 18 or/1‐17 19 Education/ 20 Health Education/ 21 Health Promotion/ 22 Counseling/ (28833) 23 ed.fs. 24 Health Knowledge, Attitudes, Practice/ 25 (class$ or counsel$ or demonstrat$ or educat$ or instruct$ or intervention$ or program$ or teach$ or train$).tw. 26 or/19‐25 27 randomized controlled trial.pt. 28 controlled clinical trial.pt. 29 randomi#ed.ab. 30 placebo.ab. 31 clinical trials as topic.sh. 32 randomly.ab. 33 trial.ti. 34 or/27‐33 35 exp animals/ not humans.sh. 36 34 not 35 37 18 and 26 and 36 38 remove duplicates from 37
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid
1 (infant$ adj1 (food or feeding or nutrition$)).tw. 2 wean$.tw. 3 ((compl#mentary or supplementary) adj3 (food$ or feed$ or nutrition$)).tw. 4 (breast$ adj1 (duration or exclusiv$ or optimal$)).tw. 5 ((Stop$ or cease or cessation or partial) adj1 breast$).tw. 6 (breast$ adj1 substitut$).tw. 7 (bottle fe?d$ or formula milk or infant formula).tw. 8 (fortif$ adj1 food$).tw. 9 ((solid$ or semi‐solid$ or soft) adj3 (food$ or feed$ or diet$)).tw. 10 (introduc$ adj3 (solid$ or semi‐solid)).tw. 11 or/1‐10 12 (class$ or counsel$ or demonstrat$ or educat$ or instruct$ or intervention$ or program$ or teach$ or train$).tw. 13 (random$ or trial$ or control$ or group$ or placebo$).tw. 14 11 and 12 and 13
MEDLINE E‐Pub Ahead of Print Ovid
1 (infant$ adj1 (food or feeding or nutrition$)).tw. 2 wean$.tw. 3 ((compl#mentary or supplementary) adj3 (food$ or feed$ or nutrition$)).tw. 4 (breast$ adj1 (duration or exclusiv$ or optimal$)).tw. 5 ((Stop$ or cease or cessation or partial) adj1 breast$).tw. 6 (breast$ adj1 substitut$).tw. 7 (bottle fe?d$ or formula milk or infant formula).tw. 8 (fortif$ adj1 food$).tw. 9 ((solid$ or semi‐solid$ or soft) adj3 (food$ or feed$ or diet$)).tw. 10 (introduc$ adj3 (solid$ or semi‐solid)).tw. 11 or/1‐10 12 (class$ or counsel$ or demonstrat$ or educat$ or instruct$ or intervention$ or program$ or teach$ or train$).tw. 13 (random$ or trial$ or control$ or group$ or placebo$).tw. 14 11 and 12 and 13
Embase Ovid
1 infant nutrition/ 2 child nutrition/ 3 baby food/ 4 breast feeding/ 5 bottle feeding/ 6 (infant$ adj1 (food or feeding or nutrition$)).tw. 7 (breast$ adj1 (duration or exclusiv$ or optimal$)).tw. 8 ((stop$ or cease or cessation or partial) adj1 breast$).tw. 9 (breast$ adj1 substitut$).tw. 10 (bottle fe?d$ or formula milk or infant formula).tw. 11 or/1‐10 12 weaning/ 13 wean$.tw. 14 ((compl#ment$ or supplement$) adj3 (food$ or feed$ or nutrition$)).tw. 15 (fortif$ adj1 food$).tw. 16 ((solid$ or semi‐solid$ or soft) adj3 (food$ or feed$ or diet$)).tw. 17 (introduc$ adj3 (solid$ or semi‐solid)).tw. 18 or/12‐17 19 exp child/ 20 (baby or babies or infant$ or child$).tw. 21 19 or 20 22 18 and 21 23 11 or 22 24 exp health education/ 25 education/ 26 education program/ 27 health promotion/ 28 counseling/ 29 nutritional counseling/ 30 (class$ or counsel$ or educat$ or instruct$ or program$ or teach$ or train$).tw. 31 or/24‐30 32 Randomized controlled trial/ 33 controlled clinical trial/ 34 Single blind procedure/ 35 Double blind procedure/ 36 triple blind procedure/ 37 Crossover procedure/ 38 (crossover or cross‐over).tw. 39 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 40 Placebo/ 41 placebo.tw. 42 prospective.tw. 43 factorial$.tw. 44 random$.tw. 45 assign$.ab. 46 allocat$.tw. 47 volunteer$.ab. 48 (control$ adj3 (group or participant$ or population)).ab. 49 or/32‐48 50 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 51 human/ or normal human/ or human cell/ 52 50 and 51 53 50 not 52 54 49 not 53 55 23 and 31 and 54 56 remove duplicates from 55
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S44 S25 AND S43 S43 S40 OR S41 OR S42 S42 (MH "Treatment Outcomes") S41 (MH "Program Evaluation") S40 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 S39 TI (evaluat* study or evaluat* research) or AB (evaluate* study or evaluat* research) or TI (effectiv* study or effectiv* research) or AB(effectiv* study or effectiv* research) S38 TI (prospectiv* study or prospectiv* research) or AB(prospectiv* study or prospectiv* research) S37 TI ("follow‐up study" or "follow‐up research") or AB ("follow‐up study" or "follow‐up research") S36 AB("cross over") S35 (MH "Crossover Design") S34 AB((tripl* N3 mask*) or (tripl* N3 blind*)) S33 AB((trebl* N3 mask*) or (trebl* N3 blind*)) S32 AB ((doubl* N3 mask*) or (doubl* N3 blind*)) S31 AB ((singl* N3 mask*) or(singl* N3 blind*)) S30 AB ((clinical trial*) or(control* trial*)) S29 AB((random* N3 allocat* ) or(random* N3 assign*)) S28 (MH "Meta Analysis") S27 MH random assignment S26 (MH "Clinical Trials+") S25 S17 AND S24 S24 S18 OR S19 OR S20 OR S21 OR S22 OR S23 S23 (class* or counsel* or educat* or instruct* or program* or teach* or train*) S22 (MH "Nutritional Counseling") S21 (MH "Counseling") S20 (MH "Health Promotion") S19 (MH "Health Education") S18 (MH "Education") S17 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 S16 (introduc* N3 (solid* or semi‐solid)) S15 (solid* or semi‐solid* or soft) N3 (food* or feed* or diet*)) S14 (fortif* N1 food*) S13 (bottle fed or bottle feed* or formula milk or infant formula) S12 (breast* N1 substitut*) S11 ((Stop* or cease or cessation or partial) N1 breast*) S10 (breast* N1 (duration or exclusiv* or optimal*)) S9 ((compl*mentary or supplement*) N3 (food* or feed* or nutrition*)) S8 wean* S7 (MH "Bottle Feeding") OR (MH "Breast Feeding") S6 (MH "Weaning") S5 (infant* N1 (food or feeding or nutrition*)) S4 (MH "Child Nutrition Disorders") S3 (MH "Infant Nutrition Disorders") S2 (MH "Infant Food") S1 (MH "Infant Nutrition")
Science Citation Index (SCI), Social Sciences Citation Index (SSCI), Conference Proceedings Citation Indexes ‐ Science, Conference Proceedings Citation Indexes ‐ Social Science & Humanities (CPCI‐SS&H) Clarivate Analytics
#7 #6 AND #5 DocType=All document types; Language=All languages; #6 TS=(random* or group* or trial* or control* or prospectiv* ) DocType=All document types; Language=All languages; #5 #4 AND #3 DocType=All document types; Language=All languages; #4 TS=(class* or counsel* or educat* or instruct*or program* or teach* or train*) DocType=All document types; Language=All languages; #3 #2 and #1 DocType=All document types; Language=All languages; #2 TS=( (infant* or baby or babies or child*) NEAR/3 ( food* or feed* or nutrition)) DocType=All document types; Language=All languages; #1 TS=(wean* or complementary or supplement* or solid* or semi‐solid* or soft) DocType=All document types; Language=All languages;
Cochrane Database of Systematic Reviews (CDSR) part of the Cochrane Library
#1[mh ^Weaning] #2wean*:ti,ab #3((compl*ment* or supplement*) near/3 (food* or feed* or nutrition*)):ti,ab #4{or #1‐#3} #5[mh ^Education] #6[mh "Health Education"] #7[mh ^"Health promotion"] #8[mh Counseling] #9[mh /ED] #10(class* or counsel* or educat* or instruct* or program* or teach* or train*):ti,ab #11{or #5‐#10} #12#4 and #11 #13(baby or babies or infant* or child*):ti #14#12 and #13
Database of Abstracts of Reviews of Effects (DARE) part of the Cochrane Library
#1[mh ^Weaning] #2wean*:ti,ab #3((compl*ment* or supplement*) near/3 (food* or feed* or nutrition*)):ti,ab #4{or #1‐#3} #5[mh ^Education] #6[mh "Health Education"] #7[mh ^"Health promotion"] #8[mh Counseling] #9[mh /ED] #10(class* or counsel* or educat* or instruct* or program* or teach* or train*):ti,ab #11{or #5‐#10} #12#4 and #11 #13(baby or babies or infant* or child*):ti #14#12 and #13
LILACS (Latin American and Caribbean Health Science Information database; search.bvsalud.org/portal/?lang=en)
tw:((tw:(complementary feed* OR complementary food* OR supplement* feed* OR supplement* food*)) OR (tw:(infant feed* OR infant food* OR infant nutrition*)) OR (tw:(wean* AND (infant* OR child* OR baby OR babies))) AND (tw:((class* OR counsel* OR educat* OR instruct* OR program* OR teach* OR train*)))) AND (instance:"regional") AND ( type_of_study:("clinical_trials"))
Clinicaltrials.gov (clinicaltrials.gov)
Search terms: infant feeding OR infant nutrition OR complementary feeding OR weaning AND Intervention : education OR counselling OR teaching OR classes
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch/AdvSearch.aspx)
4 separate search strings were run and records exported to Excel and duplicates removed.
infant feeding AND counseling OR infant nutrition AND counseling OR complementary feeding AND counseling OR weaning AND counseling [8 records] infant feeding AND education OR infant nutrition AND education OR complementary feeding AND education OR weaning AND education [23 records] infant feeding AND teaching OR infant nutrition AND teaching OR complementary feeding AND teaching OR weaning AND teaching [5 records] infant feeding AND classes OR infant nutrition AND classes OR complementary feeding AND classes OR weaning AND classes [3 records]
Data and analyses
Comparison 1. Educational intervention versus no educational intervention for improving complementary feeding practices (ICC = 0.02).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complementary food introduced at appropriate age | 4 | 1738 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.83, 0.94] |
| 1.1 Community intervention (≥ 6 months old) | 3 | 1490 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.80, 0.93] |
| 1.2 Community intervention (≥ 4 months old) | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.83, 1.02] |
| 2 Duration of exclusive breastfeeding (≥ 4 months old) | 3 | 1544 | Risk Ratio (Random, 95% CI) | 1.58 [0.77, 3.22] |
| 2.1 Community‐based intervention | 2 | 1167 | Risk Ratio (Random, 95% CI) | 2.32 [1.45, 3.73] |
| 2.2 Facility‐based intervention | 1 | 377 | Risk Ratio (Random, 95% CI) | 0.95 [0.70, 1.29] |
| 3 Hygiene practices: community‐based intervention | 4 | 2029 | Risk Ratio (Random, 95% CI) | 1.38 [1.23, 1.55] |
| 4 Knowledge | 2 | 399 | Mean Difference (IV, Random, 95% CI) | 1.29 [0.33, 2.25] |
1.4. Analysis.
Comparison 1 Educational intervention versus no educational intervention for improving complementary feeding practices (ICC = 0.02), Outcome 4 Knowledge.
Comparison 2. Educational intervention versus no educational intervention for improving complementary feeding practices: growth outcomes (ICC = 0.05).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight | 7 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Mean weight (kg) at 6 months old | 3 | 1221 | Mean Difference (Random, 95% CI) | 0.03 [‐0.10, 0.17] |
| 1.2 Mean weight (kg) at 12 months old | 5 | 2464 | Mean Difference (Random, 95% CI) | 0.06 [‐0.04, 0.15] |
| 1.3 Mean weight (kg) at 18 months old | 2 | 1402 | Mean Difference (Random, 95% CI) | 0.10 [‐0.14, 0.35] |
| 1.4 Mean weight (kg) at 24 months old | 2 | 920 | Mean Difference (Random, 95% CI) | ‐0.14 [‐0.36, 0.08] |
| 2 Height/length | 7 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 Height/length (cm) at 6 months old | 3 | 1221 | Mean Difference (Random, 95% CI) | 0.16 [‐0.21, 0.52] |
| 2.2 Height/length (cm) at 12 months old | 5 | 2464 | Mean Difference (Random, 95% CI) | 0.32 [0.11, 0.52] |
| 2.3 Height/length (cm) at 18 months old | 2 | 1402 | Mean Difference (Random, 95% CI) | 0.58 [‐0.22, 1.38] |
| 2.4 Height/length (cm) at 24 months old | 2 | 920 | Mean Difference (Random, 95% CI) | ‐0.13 [‐0.58, 0.32] |
| 3 Nutritional status (underweight, stunting, wasting) | 5 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Stunting (HAZ ≤ ‐2 SD) | 5 | 3487 | Risk Ratio (Random, 95% CI) | 0.89 [0.74, 1.06] |
| 3.2 Wasting (WHZ ≤ ‐2 SD) | 2 | 2000 | Risk Ratio (Random, 95% CI) | 0.79 [0.48, 1.30] |
| 3.3 Underweight (WAZ ≤ ‐2 SD) | 3 | 2900 | Risk Ratio (Random, 95% CI) | 0.99 [0.68, 1.44] |
Comparison 3. Sensitivity analyses for dropouts (primary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis: introduction of complementary food | 4 | 1738 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.97] |
| 2 Sensitivity analysis: duration of exclusive breastfeeding (dropouts as responders) | 3 | 1544 | Risk Ratio (Random, 95% CI) | 1.00 [0.85, 1.18] |
| 3 Sensitivity analysis: hygiene practice (dropouts as responders) | 4 | 2029 | Risk Ratio (Random, 95% CI) | 1.30 [1.17, 1.46] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aboud 2008.
| Methods | Design: cluster‐RCT Unit of randomisation: village clusters Intention to treat: yes Adjustment for clustering: yes |
|
| Participants | Total number randomised: intervention: 16 villages with 102 mother‐child pairs; control: 16 villages with 100 mother‐child pairs Inclusion criteria: children aged of 12‐24 months at pre‐test Exclusion criteria: child is physically or mentally handicapped or not yet started on complementary foods Age: children aged 12‐24 months at pre‐test Gender: intervention: 38.2% male, 61.8% female; control: 55% male, 45% female Ethnicity: not reported Settings: rural subdistrict of Sripur, in the district of Gazipur, Bangladesh, 60 km north of the capital Dhaka Country: Bangladesh Attrition: intervention: 9/102 (8.8%); control: 9/100 (9%) |
|
| Interventions | Intervention (see Table 4 for detailed description): 6 sessions on responsive feeding added on to the regular programme Control: regular weekly sessions on nutrition (regular programme) Duration of each intervention session: not reported |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Time points reported: 2 weeks after the sessions ended (post‐test) and at 5‐month follow‐up |
|
| Notes | Study start and end dates: "study took place between March and November 2006" (quote, p 277) Study duration: 9 months Conflict of interest: "none declared" (quote, p 286) Source of funding: "funding was provided by the UK Department for International Development, Bangladesh with additional amounts from Plan International, Bangladesh and BRAC University’s Institute of Educational Development. The pilot study was funded by Concordia University’s Human Development Research Center grant from FQRSC." (quote, p 285‐6) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: random number table (see p 278) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on whether allocation was concealed from study personnel. Study authors comment in paper that "mothers were told they could participate in the group sessions even if they did not want to be involved in the research. Thus, allocation to the intervention group was concealed during recruitment" (quote, p 278). However, "mothers were informed that they would receive nutrition education, and signed their consent to participate in data collection" (quote, p 277) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Peer educators implementing the responsive feeding intervention received extra training and knew that they were participating in a non‐regular programme" but "eight research assistants, blind to the group assignment, recruited mothers to the study" (p 278) Comment: in addition, it is unlikely that participants could be blinded to receiving the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the research team’s independence from the implementation of sessions was maintained; research assistants were not present in the area when the intervention was being implemented. To assess the continued blindness of research assistants, after follow‐up we asked them what parenting programmes the mothers had received. They assumed all had received messages about responsive feeding, and were unaware that there were two programmes. No one noticed special feeding messages or materials in the homes they visited" (p 278) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "...analysis was based on intention to treat" (p 281) |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed, but no protocol available |
| Other bias | Low risk | Comment: none observed |
Aboud 2009.
| Methods | Design: cluster‐RCT Unit of randomisation: village clusters Intention to treat: yes Adjustment for clustering: design effect calculated and incorporated in sample size calculations |
|
| Participants | Total number randomised: mothers and children from 37 village groups (intervention: 19 clusters (108 mother‐child pairs); control: 18 clusters (95 mother‐child pairs)) Inclusion criteria: children aged 8‐20 months at pre‐test Exclusion criteria: not stated Age: children aged 8‐20 months at pre‐test Gender: intervention: 61.1% male, 38.9% female; control: 49.5% male, 50.5% female Ethnicity: not reported Settings: rural subdistrict of Jaldhaka, in the district of Nilphamari, Bangladesh, 650 km north of the capital Dhaka Country: Bangladesh Attrition: intervention: 2/108 (1.85%); control: 7/95 (7.36%) |
|
| Interventions | Intervention (see Table 4 for detailed description): 6 educational sessions on responsive feeding in addition to the regular programme Control: regular programme Duration of each intervention session: not reported |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Time points reported: 2 weeks after the sessions ended (post‐test), and at follow‐up 5 months after the sessions ended, and 6 weeks after the booster |
|
| Notes | Study start and end dates: "study took place between April to December 2007" (quote, p 1739) Study duration: 9 months Conflicts of interest: "no conflicts of interest" (quote, p 1738) Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: random number table (see p 1739) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described, probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Peer educators implementing the responsive feeding intervention received extra training and knew that they were participating in an atypical program. Mothers’ awareness of different programs was not assessed" (p 1739) Quote: "Mothers were informed that they would receive nutrition education and signed consent forms to participate in data collection" (p 1739) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "eight research assistants who were not aware of group assignment visited mothers at home and recruited them into the study during May. They recruited all eligible mothers from the organization’s ongoing health and nutrition program. The research team’s independence from the implementation of sessions was maintained; research assistants were not present in the area when the intervention was being implemented. After follow‐up they were still unaware that there were 2 distinct programs" (p 1739) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "approximately 5% of the sample was lost to follow‐up, 7% of control mothers and 2% of intervention mothers. Also, analysis was by intention to treat" (p 1740) |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed. No protocol was available for assessment |
| Other bias | Low risk | Comment: none observed |
Aboud 2011.
| Methods | Design: cluster‐RCT Unit of randomisation: village clusters Intention to treat: yes Adjustment for clustering: design effect calculated and incorporated in sample size calculations |
|
| Participants | Total number randomised: 302 mother‐child pairs in 45 village groups (intervention 1 (RFS): 15 village clusters; intervention 2 (RFS plus Sprinkles) 14 village clusters; control: 16 village clusters)
Inclusion criteria: mothers with children aged 8‐20 months Exclusion criteria: disabled children and those who had not started complementary feeding Age: mothers and their children aged 8‐20 months at pre‐test Gender: intervention 1: 46% male, 54% female; intervention 2: 43% male, 57% female; control: 51% male, 49% female Ethnicity: not reported Settings: Khansama subdistrict of northern Bangladesh Country: Bangladesh Attrition: intervention 1: 7/92 (7.6%); intervention 2: 1/100 (1%); control: 9/110 (8.18%) |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control: regular programme For the purpose of comparison, we considered intervention group 1 and the control arm Duration of each intervention session: not reported |
|
| Outcomes |
Time points reported: 2 weeks after the RFS sessions ended (post‐test), and at follow‐up |
|
| Notes | Study start and end dates: unclear Study duration: unclear Conflicts of interest: "the authors have indicated they have no financial relationships relevant to this article to disclose" (quote, p e1191) Source of funding: "this research was supported by a grant from the Social Sciences and Humanities Research Council of Canada" (quote, p e1197) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: study was cluster‐randomised field study but not described (p e1192) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described, probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "ten research assistants who were kept unaware of group assignment throughout the study visited mothers and recruited them" (p e1192) Comment: it is unlikely that participants could be blinded to fact that they were receiving an intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described, probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "approximately 5.6% of the sample was lost to follow‐up: 8% of control mothers, 7% of mothers in the RFS group, and 1% of mothers in the RFS group" (p e1194) Comment: also, analysis was by intention to treat |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed. No protocol available |
| Other bias | Low risk | Comment: none observed |
Bhandari 2001.
| Methods | Design: RCT Unit of randomisation: children Intention to treat: no Adjustment for clustering: N/A. Parallel‐group study |
|
| Participants | Total number randomised: 418 children. Intervention group 1 (food supplementation group ‐ 104), intervention group 2 (nutritional counselling group ‐104), intervention group 3 (visitation group ‐ 104), control group (no intervention ‐ 106) Inclusion criteria: infants enrolled as they reached the age of 4 months if written informed consent was available Exclusion criteria: infants of families likely to emigrate during the study and with major congenital malformations Age: infants enrolled at 4 months of age and followed up until 12 months of age Gender: food supplementation: 54% male, 46% female; nutritional counselling: 43.3% male, 56.7% female; no intervention: 41.9% male, 58.1% female; visitation (control): 48.4% male, 51.6% female Ethnicity: not reported Settings: South Delhi, the urban slum of Nehru place, India Country: India Attrition: food supplementation: 17/104 (16.3%); nutritional counselling: 7/104 (6.7%); no intervention: 13/106 (12.2%); visitation (control): 13/104 (12.5%) |
|
| Interventions | Intervention (see for Table 4 detailed description):
Control: no intervention For the purpose of comparison we considered intervention group 2 and intervention group 3 Duration of each intervention session: not reported |
|
| Outcomes |
Time points reported: 26, 38, 52 weeks |
|
| Notes | Study start and end dates: not stated Study duration: 8 months (infants were followed from 4 months to 12 months of age) Conflict of interest: not stated Source of funding: "supported by United Nations Children’s Fund, Delhi" (quote, p 1946) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described, probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described, probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described, probably not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "four hundred and eighteen children were randomised and end study weight was available in 368 (88%). The common reasons for missing anthropometry were non availability of the family (72%), emigration (8%) and refusal to participate in the study after an initial consent (8%). Six infants died during the study, two each in the counselling and no intervention groups and one each in the food supplementation and visitation group" (p 1948) Comment: analyses not by intention to treat |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed, but no protocol available for thorough assessment |
| Other bias | Low risk | Comment: none observed |
Bhandari 2004.
| Methods | Design: cluster‐RCT Unit of randomisation: communities Intention to treat: yes Adjustment for clustering: yes. Quote: "All results reported are adjusted for cluster randomisation (using the “cluster” option of the “regress” command)" (p 2344) |
|
| Participants | Number: 8 communities with 1025 newborn infants (intervention: 552; control: 473) Inclusion criteria: newborns enrolled if they were local residents and informed written consent was obtained Exclusion criteria: not stated Age: newborns enrolled and followed up every 3 months up to the age of 18 months Gender: intervention: 52.2% male, 47.8% female; control: 53.5% male, 46.5% female Ethnicity: not reported Settings: State of Haryana Country: India Attrition: intervention: 117/552 (21.2%); control: 79/473 (16.7%) |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control: treatment as usual (routine services) Duration of each intervention session: not reported |
|
| Outcomes |
Not used in this review:
Time points reported: weights and lengths at 6, 12 and 18 months, and complementary feeding practices at 9 and 18 months |
|
| Notes | Study start and end dates: not reported Study duration: 18 months Conflict of interest: not stated Source of funding: "supported by the Department of Child and Adolescent Health and Development, World Health Organization, Geneva, Switzerland" (quote, p 2342) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: sequence was generated using random numbers table (see p 2344) |
| Allocation concealment (selection bias) | Low risk | Quote: "a statistician, not involved with the study, generated 4 single‐digit random numbers using a random numbers table" (p 2344) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described, probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described, probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: more than 10% loss (196/1025) but "all analyses were by intention to treat" (quote, p 2344) |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed. No protocol available |
| Other bias | Low risk | Comment: none observed |
Campbell 2013.
| Methods | Design: cluster‐RCT Unit of randomisation: clusters Intention to treat: yes Adjustment for clustering: yes |
|
| Participants | Total number randomised: 542 mother/infant pairs (271 children in the intervention and 271 children in the control group) Inclusion criteria:
Exclusion criteria: not explicitly stated Gender: intervention: 51.7% male, 48.3 female; control: 53.5% male, 46.5 % female Ethnicity: not reported Settings: 14 LGAs randomly selected from the 28 eligible LGAs located within a 60 km radius of the research centre, situated within the major metropolitan city of Melbourne Country: Australia Attrition: 10%, intervention: 21/241; control: 27/239 |
|
| Interventions | Intervention (see Table 4 for detailed description): 6 x 2‐h dietitian‐delivered sessions, DVD and written resources for infant aged 4‐15 months Control: parents received usual care Duration: each session lasted 2 h |
|
| Outcomes |
Time points reported: 4, 9 and 20 months of age |
|
| Notes | Study start and end dates: June 2008 and February 2010 Study duration: 20 months Conflict of interest/financial disclosure: "Drs Campbell and Crawford are supported by fellowships from the Victorian Health Promotion Foundation; Dr Hesketh is supported by a National Heart Foundation of Australia Career Development Award; Dr Lioret is supported by a Deakin University Alfred Deakin Postdoctoral Fellowship; Dr McNaughton is supported by an Australian Research Council Future Fellowship; Dr Cameron is supported by a fellowship from the Australian National Health and Medical Research Council; Dr Ball is supported by a Senior Research Fellowship from the National Health and Medical Research Council. Dr Salmon is supported by a National Health and Medical Research Council Principal Research Fellowship (APP1026216); Dr Ukoumunne is supported by the UK National Institute for Health Research funded Peninsula Collaboration for Leadership in Applied Health Research and Care; Ms Hnatiuk is supported by a Deakin International Postgraduate Research Scholarship; the other authors have indicated they have no financial relationships relevant to this article to disclose" (quote, p 660) Source of funding: "supported by the National Health and Medical Research Council (grant 425801). Additional funds were supplied by the Heart Foundation Victoria and Deakin University" (quote, p 660) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not enough information is provided. The study authors only state that "This study was a cluster RCT with balanced (1:1) randomisation. Fourteen local government areas (LGAs) were randomly selected from the 28 eligible LGAs located within a 60‐km radius of the research center, situated within the major metropolitan city of Melbourne,Australia " (quote, p 653) "Randomization (stratified by LGA) was conducted by an independent statistician" (quote, p 653) |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization of first‐time parents’ groups (clusters) occurred after recruitment to avoid selection bias" (p 653) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "although parents were not blinded to allocation, they were not informed of the study aims or hypotheses. Staff measuring height and weight were not blinded to intervention status because they also delivered the intervention. Participants were not blinded so may have revealed their group allocation to outcome assessors" (p 653) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all dietary recalls, data entry, and analyses were conducted with staff blinded to participant’s group allocation" (p 653) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 10% (21/241 in the intervention arm and 27/239 in the control arm) (see p 656) Comment: missing data were accounted for, see Figure 1. In addition analysis was on intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Comment: none observed. Protocol assessed and all outcomes stated in methods were reported |
| Other bias | Unclear risk | Comment: duplicate report but protocol is available |
Daniels 2012.
| Methods | Design: RCT Unit of randomisation: mother‐infant dyads Intention to treat: yes Adjustment for clustering: N/A. Parallel‐group study |
|
| Participants | Total number randomised: 698 mothers (intervention: 352; control: 346) with healthy infants Inclusion criteria: first‐time mothers (≥ 18 years) who had delivered a healthy‐term infant (> 35 weeks, > 2500 g), had no documented history of domestic violence or intravenous drug use, had no self‐reported eating or psychiatric disorder, had facility with written and spoken English, had an ability to attend group sessions, were still living locally (that is, could attend intervention sessions), had no serious infant health problems, had a maternal score on the Kessler 10 Psychological Distress Scale < 30 (not indicative of high maternal psychological distress) Exclusion criteria: not stated explicitly Age: newborn infants but intervention commenced at 4‐6 months of age and infants were followed‐up to 2 years of age Gender: intervention: 49% male, 51% female; control: 50% male, 50% female Ethnicity: not reported Settings: 2 Australian states, Brisbane and Adelaide Country: Australia Attrition: intervention: 65/346 (18.7%); control: 92/352 (26.1%) |
|
| Interventions | Intervention (see Table 4 for detailed description): comprehensive skills‐based programme, which focused on feeding and parenting practices that mediate children's early feeding experiences. It comprised 2 group education modules of 6, fortnightly group sessions (10–15 mothers per group), each of 1–1.5 h duration Control: self‐directed access to usual, community, child health services Duration: each session lasted 1‐1.5 h |
|
| Outcomes |
Time points reported: 9 months from baseline (infants aged 13–15 months, 6 months after completion of the first and immediately before commencement of the second module) and 18 months from baseline (children aged 2 years, 6 months after the second module) |
|
| Notes | Study authors provided additional data Study start and end dates: not reported Study duration: not reported Conflict of interest: "the authors declare no conflict of interest" (quote, p 1298) Source of funding: "NOURISH was funded 2008–2010 by the Australian National Health and Medical Research Council (Grant 426704). Additional funding was provided by HJ Heinz (postdoctoral fellowship KM), Meat and Livestock Australia (MLA), Department of Health South Australia, Food Standards Australia New Zealand (FSANZ), Queensland University of Technology, and NHMRC Career Development Award 390136 (JMN)" (quote, p 1298) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: sequence was generated using a permutated‐block schedule (see p 1293) |
| Allocation concealment (selection bias) | Low risk | Quote: "individual dyads were allocated randomly to the intervention or control group by a statistician external to the study" (p 1293) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described. Probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "anthropometric measurements were undertaken by trained study staff blinded to participant allocation status and not involved in intervention delivery" (p 1293) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: total attrition was at 18 months was 22% and 14% at 9 months but "analysis was by intention to treat" (quote, p 1294) |
| Selective reporting (reporting bias) | Unclear risk | Comment: duplicate publication and total attrition in Daniels 2012 was reported at 9 months (14%) instead of at 18 months (22%) |
| Other bias | Low risk | Quote: "despite our rigorous sampling strategy and strong retention, there is evidence of selection and retention bias" (p 1297) Quote: "however, these biases do not compromise the internal validity of the study" (p e116) |
de Oliveira 2012.
| Methods | Design: RCT Unit of randomisation: adolescent mothers Intention to treat: yes Adjustment for clustering: N/A. Parallel‐group study |
|
| Participants | Total number randomised: 323 mother‐child pairs (intervention: 163; control: 160) Inclusion criteria: adolescent mothers, infants, and maternal grandmothers living in the city of Porto Alegre, with healthy non‐twin newborn infants, in the rooming‐in ward, having started breastfeeding, with infant birth weight ≥ to 2500 g Exclusion criteria:
Age: newborn infants followed up to 6 months of age Gender: intervention: 46.6.% male, 53.4% female; control: 55 male, 45% female Ethnicity: not reported but skin colour reported as white (intervention: 63.8%; control: 61.9%) Settings: Porto Alegre Country: Brazil Attrition: intervention: 28/163 (17.2%); control: 35/160 (21.9%) |
|
| Interventions | Intervention (see Table 4 for detailed description) (2 arms): counselling sessions on breastfeeding and complementary feeding Control: (2 arms): not described Duration of each intervention session: not reported |
|
| Outcomes |
Time points reported: 4 and 6 months of infant's age |
|
| Notes | Study start and end dates: May 2006. End date not reported Study duration: unclear Conflict of interest: the study authors reported no conflict of interest Source of funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were assigned to the study groups by block random allocation in groups of two" (p 358) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "two spheres of similar texture and size, one bearing the word “Yes” (assignment to intervention group) and the other bearing the word “No” (assignment to control group) were drawn from a dark bag and subjects allocated to the study groups accordingly" (p 358) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described. Probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the interviewers were blind to the group to which the mothers belonged" (p 358) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: about 20% loss to follow‐up. Study reported "data were analysed according to intention to treat" (quote, p 358) |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed, but no protocol available for assessment |
| Other bias | Low risk | Comment: none observed |
Edward 2013.
| Methods | Design: RCT Unit of randomisation: mothers Intention to treat: yes Adjustment for clustering: N/A. Parallel‐group study |
|
| Participants | Total number randomised: 248 pregnant women (intervention‐ doula group: 124; control: 124)
Inclusion criteria: women who were < 34 weeks pregnant, under 21 years of age, and planning to deliver at the affiliated hospital
Exclusion criteria: mothers who were aware at the time of recruitment that they would require a surgical delivery, who planned to move from the area, or who planned to give up custody of the infant
Age: newborn infants enrolled and followed up to age 4 months Gender: not reported Ethnicity: young, African‐American mothers Setting: a major urban university hospital and community Country: unclear Attrition: intervention: 16/124 (12.9%); control: 11/124 (8.9%) |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control: treatment as usual Duration of each intervention session: not reported |
|
| Outcomes |
Time points reported: 4 months |
|
| Notes | Study start and end dates: unclear Study duration: unclear Conflict of interest: the study authors indicated they had no potential conflicts of interest to disclose Source of funding: "all phases of the research study reported in this paper were supported by the Maternal and Child Health Bureau Research Program, HRSA, DHHS, grant R40 MC 00203. The intervention implementation was funded by grants from the Irving B. Harris Foundation, the Blowitz‐Ridgeway Foundation, the Prince Charitable Trusts, the Visiting Nurses Association Foundation, and the Michael Reese Health Trust." (quote, p s160) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation took place in blocks of 4, 6, or 8, with equal numbers assigned to the intervention and control groups within each block" (p s162) |
| Allocation concealment (selection bias) | Low risk | Quote: "a biostatistician prepared a set of opaque envelopes, each labelled with a subject ID number and containing a group assignment" (p s162) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described. Probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described. Probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: overall attrition was low about 11%. All participants lost to follow‐up were accounted with reasons. 12.9% from intervention and 8.9% from control group Quote: "all analyses were by intent‐to‐treat" (p s163) |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed, but no protocol available for assessment |
| Other bias | Low risk | Comment: none observed |
Kang 2017.
| Methods | Design: cluster‐RCT Unit of randomisation: village clusters Intention to treat: no Adjustment for clustering: design effect calculated and incorporated in sample size calculations |
|
| Participants | Total number randomised: mothers and children from 12 village groups (intervention: 6 areas (1032 mother‐child pairs); control: 6 areas (1032 mother‐child pairs)) Inclusion criteria: all children aged 6‐12 months residing in the two districts Exclusion criteria: not explicitly stated Age: 6‐12 months Gender: intervention: 53.4.% male, 46.6% female; control: 51.5% male, 48.5% female Ethnicity: not reported Settings: rural Kebeles (the smallest administrative unit) in Habro and Melka Bello Country: Ethiopia Attrition: out of the 2064 children randomly selected from the roster, 876 children from the intervention areas and 914 children from the control areas were enrolled in the study. Exclusions were related to not finding children/refusal (intervention: 89; control: 14) or age criteria not being met (intervention: 67; control: 104). Thus, a total of 1790 child and mother pairs were enrolled at visit 1 and followed up every 3 months Quote: "out of 1790 subject children, 750 (82.1%, n = 914 in control area) and 725 (82.8%, n = 876 in intervention area) were included in the longitudinal analysis, who had at least two measures at different time points." (p 7) |
|
| Interventions | Intervention (see Table 4 for detailed description): 12‐day group nutrition sessions in addition to the ongoing routine Essential Nutrition Action (ENA) programme and the Community‐based Management of Acute Malnutrition (CMAM) programme in both study areas Control: ongoing routine ENA programme and the CMAM programme in both study areas Duration of each intervention session: not reported |
|
| Outcomes | Primary outcomes:
Time points reported: cohort of children aged 6‐12 months: at enrolment (visit 1), 3 months (visit 2), 6 months (visit 3), 9 months (visit 4), and at the 12‐month follow‐up (visit 5) |
|
| Notes | Study start and end dates: August 2012 and August 2013 Study duration: 12 months Conflict of interest: "Yunhee Kang and Parul Christian had no conflict of interest related to the study. Sungtae Kim is an employee of World Vision Korea. Sisay Sinamo is an employee of World Vision International" (quote, p 13) Source of funding: "this project was supported by World Vision Korea (project # E197814) and Korea International Cooperation Agency (KOICA). The funding agencies had no role in the design of the study, data collection and analysis, or presentation of the results" (quote, p 13) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "intervention allocation was decided by tossing a coin in the presence of the local authorities" (p 3) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "intervention allocation was not blinded among study subjects and community members because of the public nature of the intervention" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the intervention allocation and data collection procedures were not blinded to subject mothers and interviewers by the nature of the intervention of the CPNP. Some mothers knew of the existence of the CPNP programme in their community, but they still did not know that the purpose of this study was to evaluate the intervention impact" (p 12) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the intervention allocation and data collection procedures were not blinded to subject mothers and interviewers by the nature of the intervention of the CPNP. Some mothers knew of the existence of the CPNP programme in their community, but they still did not know that the purpose of this study was to evaluate the intervention impact" (p 12) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: about 17.2% in the intervention area and 17.9% in the control area Quote: "out of 1790 subject children, 750 (82.1%, n = 914 in control area) and 725 (82.8%, n = 876 in intervention area) were included in the longitudinal analysis, who had at least two measures at different time points." (p 7) Comment: analysis was not by intention to treat |
| Selective reporting (reporting bias) | Unclear risk | Comment: all primary outcomes indicated in the study protocol were reported. Results of the secondary outcomes listed in the study protocol (complementary feeding practices such as dietary diversity and feeding frequency, and hand washing practices) although measured were not reported |
| Other bias | High risk | Comment:
|
Koehler 2007.
| Methods | Design: RCT Unit of randomisation: mothers Intention to treat: no Adjustment for clustering: N/A. Parallel‐group study |
|
| Participants | Total number randomised: 183 (intervention 1: 55; intervention 2: 40; intervention 3: 47; intervention 0 (control): 41) Inclusion criteria:
Exclusion criteria: not described Age: newborn infants. Intervention commenced when the infant reached 2 months of age and lasted until the infant was 12 months old Gender: male (control 24.4%, intervention 75.6%), female (control 20.4%, intervention 79.6%) Ethnicity: not reported but inclusion criteria stated that the mothers speak German Settings: Dortmund Country: Germany Attrition: not reported |
|
| Interventions | Intervention (see Table 5 for detailed description): nutritional counselling
Control: no intervention Duration: mean duration of personal telephone counselling was 14 min |
|
| Outcomes |
Time points reported: 4, 6, 9 and 12 months |
|
| Notes | Study start and end dates: unclear Study duration: 10 months Conflict of interest: not reported Source of funding: unclear but study authors report that the study was "supported by NOVITAS Vereinigte BKK, Duisburg, Germany" (quote, p 106), a nationwide compulsory health insurance company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "infants were randomly assigned to the study groups by random numbers generated with the RANUNI function" (p 108) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information on total number of participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: all measures discussed in the methods section of the article were reported in the results, but no protocol available for assessment |
| Other bias | Low risk | Comment: none observed |
Negash 2014.
| Methods | Design: RCT Unit of randomisation: mother‐child pairs Intention to treat: no Adjustment for clustering: no. Reported as a parallel‐group study, but possibly a cluster study |
|
| Participants | Number: 197 caregivers (intervention: 100; control: 97) Inclusion criteria: caregivers who had been residents of the study area for > 6 months and who gave consent Exclusion criteria: children who had signs of illness, such as persistent vomiting, coughing, diarrhoea or fever, or acute signs such as runny nose, watery eyes, itchy eyes, red eyes, or redness around the lips and swollen lips Age: children aged 6‐23 months at baseline Gender: not reported Ethnicity: not reported Settings: 2 Kebeles (Titicha and Debicha) of Hula Woreda, Southern Nations Nationalities and Peoples’ Region (SNNPR) Country: Ethiopia Attrition: not reported |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control: no intervention Duration of each intervention session: 2 h |
|
| Outcomes |
Time points reported: baseline and end‐line |
|
| Notes | Study start and end dates: September 2012 and March 2013 Study duration: 6 months Conflict of interest: not reported Source of funding: "financial support was provided by the Canadian Department of Foreign Affairs, Trade and Development, International Development Research Centre (IDRC) Canadian International Food Security Research Fund (CIFSRF)" (quote, p 485) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: loss to follow‐up not reported in the study but table 3 shows an attrition rate of almost 20% at end line (see p 484). Analyses not by intention to treat |
| Selective reporting (reporting bias) | High risk | Quote: "by the end of the intervention period, physical growth assessment was completed for 78.5% of the children in both groups, and 24‐hour recall was completed for 85% of the study participants in both groups" (p 482) Comment: the results of the anthropometric measurements were not reported in the study although the study authors reported that "the limitations of our study included a large age range (6 of 23 months) of the children enrolled at baseline and the fact that older children were outside this range after 6 months of follow‐up. This made analysis of changes in growth parameters difficult to evaluate" (quote, p 485) |
| Other bias | Low risk | Comment: none observed |
Olaya 2013.
| Methods | Design: RCT Unit of randomisation: individual Intention to treat: no Adjustment for clustering: N/A. Parallel‐group study |
|
| Participants | Total number randomised: 85 children (intervention: 42; control: 43) Inclusion criteria: mothers of term infants with a birth weight > 2500 g who were still being breastfed at 6 months of age Exclusion criteria: not meeting above criteria or infants with a haemoglobin concentration of 11 g/dL (the cutoff used to define anaemia in Colombia) Age: 6 months and followed up to 12 months of age Gender: intervention 50% male, 50% female; control 50% male, 50% female Ethnicity: not reported Settings: 2 hospitals in Bogota, Colombia, that serve populations with low socioeconomic status Country: Colombia Attrition: intervention: 4/42 (9.5%); control: 5/43 (11.6%) |
|
| Interventions | Intervention (see Table 5 for detailed description): nutrition counselling with face‐to‐face sessions and detailed verbal and written guidance from researchers (new guideline group, NGG) Control: standard advice on complementary feeding from healthcare professionals in the growth monitoring programme (control group‐CG) Duration: each session lasted ˜ 45 min |
|
| Outcomes |
Time points reported: 6 and 12 months of age |
|
| Notes |
Study authors provided additional data Study start and end dates: unclear Study duration: unclear Conflict of interest: "none of the authors declared a conflict of interest following the guidelines of the International Committee of Medical Journal Editors" (quote, p 992) Source of funding: "supported by the Childhood Nutrition Research Centre, University College London Institute of Child Health, and Pontificia Universidad Javeriana. Tommee Tippee (United Kingdom) donated the feeding spoons, cups, and beakers used in the study" (quote, p 983) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization assignments were prepared by using randomised blocks of permuted length..." (p 984) |
| Allocation concealment (selection bias) | Low risk | Quote: "... by a member of the team who had no contact with study subjects and were stored in sealed opaque envelopes" (p 984) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described. Probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "it was not possible to blind researchers who collected anthropometric and food‐intake data, but laboratory measurements were blinded" (p 984) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants lost to follow‐up (4 in the intervention arm and 5 in the control arm) were accounted for (see p 985) |
| Selective reporting (reporting bias) | Unclear risk | Comment: all measures discussed in the methods section of the article were reported in the results, but no protocol available for assessment |
| Other bias | High risk | Quote: "for all infants randomly assigned, those in the CG were significantly heavier with higher mid upper arm circumference (MUAC), weight‐for‐age z score (WAZ), weight‐for‐length z score (WLZ), and MUAC z score (MUACZ) at baseline (6 mo of age); for infants with data at 12 mo of age, CG infants were also heavier with higher MUAC at baseline" (p 897) |
Penny 2005.
| Methods | Design: cluster‐RCT Unit of randomisation: health facilities Intention to treat: yes Adjustment for clustering: no |
|
| Participants | Total number randomised: 12 health facilities (intervention: 6 facilities with 187 babies; control: 6 facilities with 190 babies) Inclusion criteria: newborns who were found at home, who were aged ≤ 10 days, who had no known congenital malformation or chronic condition that could affect growth, and whose parents gave written informed consent Exclusion criteria: the main reasons for infants not being enrolled were that the needed sample size had been achieved or that the baby had been born before predicted and was outside the age criterion. Also excluded congenital malformation or chronic conditions that could affect growth of the baby. Health facilities excluded if the randomisation resulted in a control site being directly adjacent to an intervention site Age: newborn infants enrolled and followed up from birth to 18 months of age Gender: intervention: 54% male, 46% female; control: 48% male, 52% female Ethnicity: not reported Setting: health facilities in Trujillo, a poor peri‐urban area (i.e. shanty town) of Peru Country: Peru Attrition: intervention: 16/187 (8.5 %); control: 23/190 (12.1%) |
|
| Interventions | Intervention (see Table 5 for detailed description): nutrition advice based on recommended complementary feeding practices Control: not described Duration of each intervention session: not reported |
|
| Outcomes |
Time points reported: 3, 4, 6, 8, 9, 12, 15, 18 months |
|
| Notes | Study authors provided additional data Study start and end dates: 13 August 1999. End date unclear Study duration: 2 years Conflict of interest: "we declare that we have no conflict of interest" (quote, p 1871) Source of funding: "this project was supported by the Family Health and Child Survival Cooperative Agreement between the United States Agency for International Development and Department of International Health, Johns Hopkins Bloomberg School of Public Health, MD, USA" (quote, p 1871) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: sequence was generated by tossing a coin (see p 1864) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: participants were blinded but it is not feasible to blind the personnel who delivered intervention Quote: "families were not told whether they were in the intervention or control group" (p 1865) Comment: study authors did not describe the control intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the study could not be blinded, which could have led to bias. However, data collection was standardised, interviews were structured, and interviewers rotated between intervention and control areas to limit any bias that might result from the same team always interviewing intervention or control families. Nevertheless, knowledge of the group could have influenced data collectors’ interpretation of responses or the recording of dietary‐recall data, but this knowledge is unlikely to have affected weight or height measurements" (p 1870) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: number lost to follow‐up reported with reasons (10%). Also analysis was by intention to treat (see p 866) |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed, but no protocol available for assessment |
| Other bias | Low risk | Comment: none observed |
Reinbott 2016.
| Methods | Design: cluster‐RCT Unit of randomisation: communes Intention to treat: no Adjustment for clustering: yes. Design effect reported |
|
| Participants | Number: intervention: 10 communes with 510 caregiver‐child pairs; control: 5 communes with 233 caregiver‐child pairs Inclusion criteria: for the nutrition education programme, caregivers with a child aged 5–18 months were recruited on the basis of their interest in participating; priority was given to caregiver–child pairs from households already participating in a farmer field or farmer business school Exclusion criteria: children with missing birth certificates, vaccination cards or where the month of birth of the child could not be estimated and/or the primary caregiver was not available Age: children aged from birth to 23 months at baseline Gender: intervention: 56.9% male, 43.1% female; control: 51.5% male, 48.5% female Ethnicity: not reported Settings: Preah Vihear and Oddar Meanchey in rural Cambodia Country: Cambodia Attrition: not reported |
|
| Interventions | Intervention (see Table 2 for detailed description): households had access to farmer field/business school training (agricultural intervention) and nutrition education by the 'Improving market linkages for smallholder farmers' (MALIS) project Control: households had access to MALIS farmer field/business school training (agricultural intervention) only Duration: nutrition education sessions were conducted 2–4 h weekly or biweekly depending on the availability of the participants |
|
| Outcomes |
Time points reported: baseline and impact |
|
| Notes |
Study authors provided additional data Study start and end dates: 2012 and 2014 Study duration: 2 years Conflict of Interest: not reported Source of funding: "the research was funded by the FAO with support of the German Federal Ministry of Food and Agriculture. FAO supported the research team in providing office space at the project sites and information about the intervention at all stages of the project, but neither the project staff nor the project management at country level participated in the study design, data collection, analysis or interpretation of the results. FAO headquarters staff were aware of the research design while designing and implementing the nutrition education intervention to allow the rigorous research design" (quote, p 1467) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "intervention and comparison areas were identified using the software package ‘Experiment’ and the operation ‘randomise’. The ‘Experiment’ package is a software extension to the statistical software R©. The restricted randomisation was used to identify ten intervention and five comparison communes out of the sixteen surveyed communes." (p 1459) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described, probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described, probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "at impact, enumerators were blind to group assignment" (p 1461) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unclear. Not described |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed. No protocol available |
| Other bias | High risk | Comment:
|
Saleem 2014.
| Methods | Design: cluster‐RCT Unit of randomisation: geographically distinct areas Intention to treat: no Adjustment for clustering: yes. Design effect reported |
|
| Participants | Total number randomised: 10 clusters with 212 infants (intervention: 118; control: 94) Inclusion criteria: infants aged 10‐20 weeks, who were either exclusively or partially breastfed but had not started complementary feeding or had recently started (< 1 week prior to enrolment), and lived in the study area Exclusion criteria: infants already below the 5th percentile in WHO growth charts on weight‐for‐age at baseline, had a history of ≥ 2 hospital admissions at the time of enrolment (each hospital stay > 7 days), had serious congenital anomalies (cleft palate, congenital heart disease, neural tube defect), other chronic conditions impairing feeding (e.g. cerebral palsy), or the presence of acute illness or severe anaemia (or both), which required urgent hospitalisation at the time of enrolment Age: infants aged 10‐20 weeks Gender: intervention: 59% male, 41% female; control: 64% male, 36% female Ethnicity: not stated Settings: Bhains Colony (Cattle Colony), a peri‐urban setting of Karachi located in Bin Qasim Town, Karachi Country: Pakistan Attrition: intervention: 8/118 (6.8%); control: 10/94 (10.6%) |
|
| Interventions | Intervention (see Table 4 for detailed description): education sessions on breastfeeding and complementary feeding using 10 key messages developed based on recommended practices (WHO/UNICEF 2000 and 2006)
Control: advice about breastfeeding according to national guidelines (usual care) Duration: each teaching session lasted an average of 15‐20 min |
|
| Outcomes |
Time points reported: baseline, visit 2 (10 weeks), visit 3 (20 weeks), visit 4 (30 weeks) |
|
| Notes | Design effect of 1.25 Study start and end dates: unclear Study duration: 30 weeks Conflct of interest: not reported Source of funding: "this study was funded by Aga Khan University Research Council and NIH‐Fogarty research training fund. Dr Ali Faisal Saleem received research training support from the Fogarty International Center (1 D43 TW007585‐01) of the National Institutes of Health, USA." (quote, p 631) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: sequence generated using random number table (see p 624) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "a total of 212 infants (118 in the intervention and 94 in the control clusters) were recruited in the study. One hundred and ninety‐four infants (intervention 110 and control 84) were considered in the final statistical analysis. Overall, there were 95 remaining infants in the intervention, and 75 in the control cluster at the end of the study (fourth visit)" (p 626) Quote: "we used a mixed model approach for analysis that deals with the missing values in the data" (p 630) |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed, but no protocol available for assessment |
| Other bias | Low risk | Quote: "in order to minimize the bias, educational session was conducted, and infants’ anthropometric measurements were taken by different teams and on different days" (p 625) |
Schroeder 2015.
| Methods | Design: cluster‐RCT Unit of randomisation: health centres Intention to treat: no Adjustment for clustering: no |
|
| Participants | Total number randomised: 4 clinics with 292 infants (intervention: unclear; control: unclear) but final analyses (intervention: 112; control: 110) Inclusion criteria: all healthy newborns with ≥ 2000 g body weight not requiring specialised medical or nutritional care and discharged home within 5 days after birth Exclusion criteria: not described Age: newborns followed up to 2 years of age Gender: centre 1: 31 male, 32 female (n = 63); centre 2: 18 male, 31 female (n = 49); centre 3: 31 male, 26 female (n = 57); centre 4: 28 male, 25 female (n = 53); all at final analysis Ethnicity: black 48%, white 35%, Asian 2%, Hispanic 2%, Indian 0%, multiracial 0%, others 6%, and unknown 7% Settings: health centres from the Johns Hopkins Community Physicians (JHCP) network in Maryland Country: USA Attrition: 60/292 (20.55%) |
|
| Interventions | Intervention (see Table 5 for detailed description): educational sessions based on the modules of Growing Leaps and Bounds (GLB), a set of educational materials developed by a group of experts and funded by the Dannon Institute Control: no intervention Duration: the GLB programme was designed to be presented in about 5 min, focusing on ≤ 3 items at each visit and including a printed brochure as a permanent record of each mini session |
|
| Outcomes |
Time points reported: anthropometry at baseline, 12 months, 24 months; child feeding practices at 24 months |
|
| Notes | Participating paediatricians signed a memorandum of agreement and received compensation of USD 150 per infant enrolled Study start and end dates: not stated Study duration: not stated Conflict of interest: "the authors have no conflict of interests to disclose. The authors have no financial relationships relevant to this paper to disclose" (quote, p 6) Source of funding: "this study was funded by a competitive grant from the Dannon Institute (USA)" (quote, p 6) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described, probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described, probably not done even though most important outcomes were measured objectively so not blinding would not likely affect them |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: probably not done. While there may be bias towards subjective outcomes, anthropometric outcomes are unlikely to be affected Quote: "all staff were trained on how to complete the various measurements and followed up with a gold standard check where one staff member completed a re measure of the infant to check for agreement. This was completed approximately once a quarter. Two repeat measures were completed if the initial two measurements were more than a set amount apart" (p 2) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: attrition rate was high (21%) and no reason was given for the loss to follow‐up Quote: "a total of 292 infants were enrolled and 232 completed the study. This was consistent with our predicted attrition rate of 20%. All clinics but one had retention rates above 80%" (p 2) |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed, but no protocol available for assessment |
| Other bias | Unclear risk | Comment: the intervention group had higher number of African‐American caregivers, higher unemployment rate, lower household income, lower completed education level, and less home ownership than the control group. The intervention group also used more food stamps and more WIC programme services and had lower rates of breastfeeding (see p 2 & 3) |
Shi 2010.
| Methods | Design: cluster‐RCT Unit of randomisation: townships Intention to treat: yes Adjustment for clustering: no |
|
| Participants | Total number randomised: 599 infants (intervention: 294; control: 305) in 8 townships (intervention: 4; control: 4) Inclusion criteria: all infants in the selected townships who were full term (gestational age > 37 weeks), singletons, without major birth defects, and aged 2–4 months at the time of the baseline survey were eligible for the study. 8 townships were selected that each had at least 2 primary healthcare providers who could provide intervention and evaluation for the study. Townships were paired based on population, geographic type and economic condition Exclusion criteria: not stated Age: infants aged 2‐4 months and followed up until 1 year of age Gender: intervention: 48.3% male, 51.7% female; control: 53.1% male, 46.9% female Ethnicity: Han and other minorities Settings: Laishui County of Hebei Province in the north west Country: China Attrition: 72 (12%) at 6 months; 127 (21%) at 9 months; 110 (18%) at 12 months |
|
| Interventions | Intervention: (see Table 4 for detailed description): educational messages and enhanced home‐prepared recipes disseminated to caregivers through group training and home visits Control: "standard package of child health care from the township hospitals, which included breast‐feeding counselling but did not contain other than standard counselling on complementary feeding" (quote, p 557) Duration of each intervention session: not reported |
|
| Outcomes |
Time points reported: 6, 9, 12, 15 and 18 months |
|
| Notes | Study start and end dates: April 2006. End date not clear Study duration: unclear Conflict of interest: "the authors do not have any financial, personal or professional conflicts of interest" (quote, p 564) Source of funding: "the study was funded by the Proctor & Gamble Fellowship provided through the Johns Hopkins Bloomberg School of Public Health. The funding source had no role in the study design, data analysis, interpretation of data, writing of the report, or in the decision to submit the paper for publication" (quote, p 564) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the paired townships were listed alphabetically in blocks of two and assigned randomly to be intervention or control sites" (p 557) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "due to the shortage of health‐care staff, those people conducting questionnaire survey and anthropometric measurement were the same ones who delivered the intervention, and they were aware of the treatment assignment. The study participants were also aware of their treatment as it was clearly stated in the consent procedure. However, we believe that this should not have introduced information bias because the anthropometric outcomes were objective." (p 563) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "due to the shortage of health‐care staff, those people conducting questionnaire survey and anthropometric measurement were the same ones who delivered the intervention, and they were aware of the treatment assignment. The study participants were also aware of their treatment as it was clearly stated in the consent procedure. However, we believe that this should not have introduced information bias because the anthropometric outcomes were objective. In addition, we implemented strict training, supervision and quality control measures" (p 563) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition rates (at 6 months ‐72 (12%); at 9 months ‐127 (21%); at 12 months ‐110 (18%). Analysis was by intention to treat. (p 558) |
| Selective reporting (reporting bias) | Unclear risk | Comment: duplicate publication. Not all outcomes measured at time points covered in the original report were reported in the original study report but reported as in a different report |
| Other bias | Unclear risk | Comment: baseline differences in mother’s and father's employment: "more mothers at intervention sites than controls engaged in agriculture work (57.1% vs 49.8%, P < 0.05) and more fathers at intervention sites than controls were migrant labourers who worked temporarily in cities (67.3% vs 55.7%, P < 0.05)" (quote, p 558), but study reports that "the intervention group did not differ significantly from controls with respect to infant gender, age, birth weight and length, parents’ age, ethnicity, education, number of siblings, household possessions, as well as parents’ weight and height (Table 1)" (quote, p 558) |
Tariku 2015.
| Methods | Design: cluster‐RCT Unit of randomisation: Kebeles Intention to treat: no Adjustment for clustering: yes. Design effect reported |
|
| Participants | Total number randomised: 180 households with children 6–18 months of age. 60 households per group (intervention group 1: 60 children; intervention group 2: 60 children; control: 60 children)
Inclusion criteria: being resident in the Kebele and likely to be resident for the entire 3‐month intervention period. The child must have been breastfed during the pre‐intervention (baseline) data collection period Exclusion criteria: children without a mother and those with serious congenital anomalies Age: children 6–18 months of age Gender: 76 boys (45.8%). Number in intervention and control arms unclear Ethnicity: not reported Settings: rural‐Dore Bafano district, a district of the Sidama Zone in the Southern Nations, Nationalities, and People’s Region (SNNPR) of Ethiopia. Country: Ethiopia Attrition: 14 households out of 180 households |
|
| Interventions | Intervention: (see Table 4 for detailed description):
Control: no education (routine activities) Duration of each intervention session: not reported |
|
| Outcomes |
Time points reported: pre‐intervention and postintervention |
|
| Notes | Study start and end dates: April 2012 and July 2012 Study duration: 4 months Conflict of interest: not reported Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using the lottery method, one group of matched Kebeles was selected to comprise each study group: one allocated to the HBM intervention (Jara Gelelcha), one to the Traditional education (Udo Wotate), and the third one as Control (Doyo Chale); again allocated to the intervention group by lottery method." (p 3) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described, probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described, probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described, probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 14 (7.8%) out of 180 households. All missing data were accounted for with reasons "of households, 14 were lost to follow‐up; 5 households later refused to participate in the nutrition education and after a repeated attempt, a further 9 were not at their home during the post‐intervention data collection" (quote, p 5) |
| Selective reporting (reporting bias) | High risk | Comment: data for some outcomes were not clearly presented e.g. data for Hygiene (handwashing) Quote: "for example, regarding to the hand washing practice, the proportion of mothers who would wash their hands after intervention significantly increased for all Kebeles compared to pre‐intervention, but no significant differences were found in the proportion of hand washing practices. For the use of soap to wash their child’s hand, there were significant difference between TM and Control Kebeles (p = .005); and HBM and Control Kebeles (p = .001).’’ |
| Other bias | Low risk | Comment: none observed |
Vazir 2013.
| Methods | Design: cluster‐RCT Unit of randomisation: clusters Intention to treat: no Adjustment for clustering: yes. Cluster‐adjustment method. All results reported were adjusted for cluster randomisation using mixed models for continuous variables |
|
| Participants | Total number randomised: 60 village clusters randomised into 3 groups with 20 clusters per group and 200 mother‐infant dyads in each group Inclusion criteria: pregnant women in their third trimester in Integrated Child Development Services (ICDS) programme areas Exclusion criteria: not described Age: 3‐month old infants followed up for 12 months Gender: intervention group 1: male 48.3%, female 51.7%; intervention group 2: male 49.0%, female 51%; control: male 50.8%, female 49.2% Ethnicity: scheduled castes, scheduled tribes, other backward castes, other castes Settings: rural Andhra Pradesh, India Country: India Attrition: actual loss to follow‐up was 15% |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control: standard of care‐ the Integrated Child Development Services (ICDS) programme For the purpose of comparison we considered intervention group 1 and the control arm Duration of each intervention session: not reported |
|
| Outcomes |
Time points reported: 6, 9, 12 and 15 months of infants’ age |
|
| Notes | Study start and end date: unclear Study duration: about 15 months Conflict of interest: "the authors declare that they have no conflicts of interest" (quote, p 115) Source of funding: "Indian Council of Medical Research, India and the NIH/NICHD (5 R01 HD042219‐S1); additional funding from UNICEF, New York" (quote, p 115) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random allocation using a random number generator" (p 101) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described, probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described, probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the assessment teams (psychologists and nutritionists) were blinded to the intervention and had no interaction with the VW" (p 104) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 15% attrition after 12 months of intervention Quote: "all 60 clusters remained in the study. Loss to follow‐up was greater in the RCF&PG (22%) compared with the CG (9%) and CFG (16%) although this difference was not statistically significant (see Fig. 1 for full details of attrition)" (p 106) Comment: reasons for attrition provided for all participants (see p 102). Analyses not by intention to treat |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed, but no protocol available for assessment |
| Other bias | Low risk | Comment: none observed |
Vitolo 2005.
| Methods | Design: RCT Unit of randomisation: mothers Intention to treat: unclear Adjustment for clustering: N/A. Parallel‐group study |
|
| Participants | Total number randomised: 500 (intervention: 200; control: 300) Inclusion criteria: newborns weighing > 2.500 kg and > 37 weeks' gestation age. Child‐birth by the public system Exclusion criteria: HIV‐positive mothers, need for the intensive care unit, twins, congenital malformation Age: newborn infants, followed up to 16 months of age Gender: intervention: 57.1% male, 42.9% female; control: 55.5% male, 44.5% female Ethnicity: not reported Settings: City of São Leopoldo, in Rio Grande do Sul Country: Brazil Attrition: intervention: 37/200 (18.5%); control: 66/300 (22%) |
|
| Interventions | Intervention (see Table 4 for detailed description): dietary guidance based on Ten Steps to Healthy Feeding: A Nutritional Guide for Children under Two (Dez Passos para uma Alimentação Saudável: Guia Alimentar para Crianças Menores de Dois Anos). Mothers were given a simplified illustrated folder on the Ten Steps and a printed sheet with 4 recipes providing examples of food groups and meal preparation Control: 2 visits at 6 and 12 months old to collect anthropometric, feeding, social, demographic and health data Duration: each dietary counselling session lasted 30 to 40 minutes |
|
| Outcomes |
Time points reported: 3 months, 12 to 16 months, 3 to 4 years and 7 to 8 years |
|
| Notes | Randomised study with parallel design taken from Vitolo 2005 (translated into English) Study start and end dates: unclear Study duration: 8 years Conflict of interest: "no conflicts of interest declared concerning the publication of this article" (p 33) Source of funding: "Supported by the Brazil CNPq (National Funding for Research) and Capes Foundation, Ministry of Education (M.R.V. Postdoctoral Fellowship, No. 2080/09‐5)" (p 2002) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: block randomisation. One researcher that was not directly involved with the sample selection was responsible for the randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: one researcher that was not directly involved with the sample selection was responsible for the randomisation. No further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: team, participants and evaluators were not blinded. The study authors report that there was one limitation of this study. They say that in studies about feeding behaviour it is impossible to blind the participants and evaluators: "em estudos de intervenção sobre comportamento alimentar, não é possível cegar os indivíduos e entrevistadores" (quote, p 1455) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: team, patients and evaluators were not blinded. The study authors report that there was one limitation of this study. They say that in studies about feeding behaviour it is impossible to blind the participants and evaluators: "em estudos de intervenção sobre comportamento alimentar, não é possível cegar os indivíduos e entrevistadores" (quote, p 1455) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: study authors do not clearly describe how they handled participants who withdrew or who were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed, but no protocol available for assessment |
| Other bias | High risk | Comment: study has multiple publications reporting different outcomes and different time points. Bortolini 2012, Louzada 2012, Vitolo 2010, Vitolo 2012 |
Wen 2011.
| Methods | Design: RCT
Unit of randomisation: individuals Intention to treat: yes Adjustment for clustering: N/A. Parallel‐group study |
|
| Participants | Total number randomised: 667 first‐time mothers (intervention: 337; control: 330)
Inclusion criteria: women were eligible for the study if they were aged ≥ 16 years, were expecting their first child, were between weeks 24 and 34 of pregnancy, were able to communicate in English, and lived in the local area
Exclusion criteria: women were excluded from the study if they had a severe medical condition as evaluated by their physicians Age: newborn infants followed up to 12 months of age Gender: not stated Ethnicity: not stated Settings: socially and economically disadvantaged areas of southwest Sydney Country: Australia Attrition: intervention: 69/337 (20.4%); control: 71/330 (21.5%) |
|
| Interventions | Intervention (see Table 4 for detailed description): counselling on infant feeding practices, infant nutrition and active play, family physical activity and nutrition, as well as social support Control: families in the control group received the usual childhood nursing service |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Time points reported: 6 and 12 months |
|
| Notes | Study start and end dates: 1 January 2007 and 31 December 2010 Study duration: 4 years Conflict of interest: "none reported" (quote, p 706) Source of funding: "this study is part of the Healthy Beginnings Trial funded by the Australian National Health and Medical Research Council (ID number: 393112)" (quote, p 706) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "group allocation was determined by a computer‐generated random number. Randomization was stratified by hospital, with a block size of 50" (p 702) |
| Allocation concealment (selection bias) | Low risk | Quote: "random allocation was concealed by sequentially numbered, sealed, opaque envelopes containing the group allocation, which was determined by a computer‐generated random number. Randomization was stratified by hospital, with a block size of 50. A research assistant who had no direct contact with participating mothers was responsible for generating the random numbers and preparing the envelopes" (p 702) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the data collectors and the research staff who dealt with data entry and analysis were masked to treatment allocation" (p 702) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: a total of 106 participating mothers were lost to follow‐up at 6 months and an additional 34 at 12 months. Comment: all losses to follow‐up were accounted for and were similar across both arms (69 in intervention group, 71 in control group) (p 703) |
| Selective reporting (reporting bias) | Unclear risk | Comment: none observed, but no protocol available for assessment |
| Other bias | Unclear risk | Quote: "those lost to follow‐up at 12 months were significantly younger and less educated and were more likely to be unemployed or have low income" (p 703) |
Yin 2009.
| Methods | Design: RCT Unit of randomisation: individuals Intention to treat: yes Adjustment for clustering: N/A. Parallel‐group study |
|
| Participants | Total number randomised: 515 mother‐infant pairs (intervention 1: 160; intervention 2: 180; control: 175) Inclusion criteria: mothers who had infants aged 4‐6 months Exclusion criteria: premature birth, low birth weight, asphyxia, newborn with chronic disease or congenital disease Age: infants aged 4‐6 months Gender: all participants were female Ethnicity: not stated Settings: rural areas of Tianjin municipality Country: China Attrition: not reported |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control: mothers in the control group received routine guidance at the local health station |
|
| Outcomes |
Time points reported: before intervention (baseline), 3 months after intervention, 6 months after intervention |
|
| Notes | Study start and end dates: March 2007 and September 2007 Study duration: 6 months Conflict of interest: unclear Source of funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described (allocations were firstly stratified according to local health station, then simple randomisation was applied) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the study authors did not mention how they dealt with those lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: not described |
| Other bias | Unclear risk | Comment: not described |
BMI: body mass index; DHHS: Department of Health and Human Services; FAO: Food and Agriculture Organization of the United Nations; FQRSC: Fonds de recherche du Québec – Société et culture; HRSA: Health Resources & Services Administration; Kebele: small administrative area in Ethiopa; LAZ: length‐for‐age z‐score; LGA: local government area; N/A: not applicable; NIH: National Institutes of Health; NICHD: National Institute of Child Health and Human Development; RCT: randomised controlled trial; RFS: responsive feeding and stimulation; WAZ: weight‐for‐age z‐score; WHO: World Health Organization; WLZ: weight‐for‐length z‐score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arimond 2017 | Evaluation study of 4 RCTs |
| Arpadi 2009 | Control group not without intervention. The control group participated in a programme that encouraged continued exclusive breastfeeding to 6 months of age with gradual introduction of complementary foods. Participants were HIV‐infected mothers |
| Black 2001 | The definition of optimal feeding (assumed to be adequate complementary feeding) at 3 months of age included sugar, which is not compliant with the WHO's definition and time of onset of complementary feeding |
| Brown 1992 | Study not randomised |
| Cameron 2013 | No educational intervention on complementary feeding |
| Clark 2009 | Participants were childcare providers from childcare centres who were asked to assess an infant‐feeding website |
| Dumaguing 2015 | Study not randomised but a longitudinal prospective study |
| Faerber 2017 | Intervention and control arms both received educational interventions |
| Fangupo 2015 | 2 of the 3 intervention arms did not receive educational intervention alone, also the intervention message was not on complementary feeding alone and the results were not stratified according to the arms but according to the main interventions |
| Fernald 2016 | All arms, including the control arm, received educational intervention |
| Fildes 2015 | Trial did not assess complementary feeding practices per se but infants' consumption of a novel vegetable and their liking of this vegetable |
| Ford 2009 | Before and after study where participants were pregnant and postpartum women, not infant of complementary feeding age, and also did not assess complementary feeding practices at all |
| Guldan 2000 | Study not randomised |
| Haider 2013 | Study not randomised |
| Hotz 2005 | Study not randomised |
| Jakobsen 2008 | Study had no component on complementary feeding. It was focused on breastfeeding only |
| Kabahenda 2011 | The children included in the study were aged 6‐48 months, which did not meet the inclusion criteria of 4‐24 months of age. Also, the results reported were not stratified by age |
| Kapur 2003 | Age of children included in the trial does not met the eligibility criteria |
| Kilaru 2005 | Study not randomised |
| Kim 2016 | The study design is a before and after study not a RCT |
| Klingberg 2017 | Study not randomised |
| Kuchenbecker 2017 | Although it described itself as a randomised trial, the approach taken made it difficult to extract reliable sample size and number randomised (n/Ns). Baseline measurements were not taken on the same cohort of caregivers/infants as those at follow‐up |
| Maslowsky 2016 | Outcomes measured at 3 months of age, which is not compliant with the WHO's definition and time of onset of complementary feeding considered in this review |
| Menon 2016 | Control arm received information on IYCF. They also received mass media campaigns on various aspects of IYCF targeted at mothers, family members and health workers |
| Mulualem 2016 | Quasi‐experimental study promoting a particular complementary food |
| Nair 2017 | The intervention objective was to evaluate the effectiveness of a new strategy proposed by the government involving the engagement of a new community health worker for conducting home visits and participatory women’s group meetings. The control arm, in addition to routine care, also participated in meetings targeted at strengthening the capacity of village health sanitation and nutrition committees to assess community health needs, prepare and implement village health plans, and monitor the provision of local health and nutrition services |
| Neyzi 1991 | Study had no component on complementary feeding. It was focused on breastfeeding only |
| Nikiema 2017 | Intervention was targeted at health workers with the objective of improving health providers’ skills in: 1. providing appropriate feeding counselling; 2. assessing child nutritional status and feeding problems; and 3. making recommendations. Particular attention was paid to imparting communication skills to the health providers |
| Olney 2015 | The intervention groups did not receive educational interventions alone |
| Owais 2017 | Study not randomised |
| Pachon 2002 | Study area was commune hamlets with the highest levels of malnutrition |
| Pant 1996 | Intervention was not on complementary feeding practices and participants included children up to 10 years of age |
| Pelto 2004 | Participants were health workers (doctors) |
| Reich 2010 | The intervention message was not on complementary feeding alone but included other aspects such as infant physical, cognitive and emotional development; safety practices inside and outside of the home and in the car; maternal self‐care; benefits of breastfeeding; discipline strategies; and nutrition recommendations. In addition, the results were not stratified according to the intervention message |
| Reinsma 2016 | Study not randomised |
| Robling 2016 | The intervention did not include education on complementary feeding and study did not measure outcomes of interest |
| Roset‐Salla 2016 | Intervention was aimed at promoting adherence of the consumption of the Mediterranean diet and not complementary feeding in general and included children aged 1 year and above |
| Roy 2005 | Participants were moderately‐malnourished children |
| Roy 2007 | Participants were well‐nourished or mildly malnourished children and the results were not separated for each category |
| Salehi 2004 | Age of children included in the trial does not meet the eligibility criteria |
| Santos 2001 | Participants were doctors and not caregivers of children of complementary feeding age |
| Savage 2016 | Study focused on responsive parenting for preventing obesity |
| Spigelblatt 1991 | The study aimed to delay the introduction of solids to infants until 2 months of age, which is at variance with WHO guideline on complementary feeding |
| Taylor 2017 | Intervention was aimed at promoting a baby‐led, infant self‐feeding approach for reducing the risk of overweight by making infants have a greater control over their eating rather than the conventional spoon feeding of infants by their caregivers |
| Thompson 2012 | Age of children included in the trial does not meet the eligibility criteria |
| Vitolo 2014 | Participants were primary healthcare professionals and the objective of the trial was to assess the impact of a child feeding training programme for primary healthcare professionals about breastfeeding and complementary feeding practices |
| Wambach 2011 | Study had no component on complementary feeding. It was focused on breastfeeding only |
| Waswa 2015 | Although it described itself as a randomised trial, the approach taken made it difficult to extract reliable sample size and number randomised. Baseline measurements were not taken on the same cohort of caregivers/infants as those at follow‐up, but on a different, randomly selected group of women |
| Yousafzai 2016 | Intervention group 1 received nutrition education and an adjunctive intervention (multiple micronutrient powder), which was not administered to the control group |
| Zaman 2008 | Participants were health workers and the objective of the study was to determine the efficacy of training health workers in nutrition counselling in enhancing their communication skills and performance, and improving feeding practices |
| Zhang 2016 | The main intervention was a daily complementary food supplement for children aged 6‐23 months in addition to complementary feeding counselling |
IYCF: Infant and young child feeding; n/N: sample size; RCT: randomised controlled trial; WHO: World Health Organization
Characteristics of studies awaiting assessment [ordered by study ID]
Dunlevy 2010.
| Methods | RCT |
| Participants | Pregnant women |
| Interventions | The participants were invited to attend and evaluate a weaning talk during their third trimester and complete a questionnaire on their planned time to wean |
| Outcomes | Planned time to wean and parents' evaluation of the antenatal intervention talk |
| Notes |
Dunlvey 2012.
| Methods | RCT |
| Participants | Pregnant women and their partners |
| Interventions | In the 3rd trimester the intervention group (group 1) and their partners were invited to attend an educational infant weaning talk |
| Outcomes | Timing of introduction of nutrient‐specific weaning foods |
| Notes |
Guan 2016.
| Methods | Cluster‐RCT |
| Participants | Caregivers with children aged 6‐11 months |
| Interventions | Nutrition education based on 6 locally adapted lessons for complementary feeding practices and behaviours comprising group training and cooking demonstrations were conducted monthly over a period of 6 months in village health facility |
| Outcomes | Haemoglobin levels and complementary feeding behaviours score |
| Notes |
Jordan 2015.
| Methods | Cluster‐RCT |
| Participants | Children < years and their primary caregivers |
| Interventions | Agriculture interventions were carried out in both arms, intervention and control, whereas nutrition education was carried out in the intervention arm only |
| Outcomes | Changes in children's dietary diversity |
| Notes |
Palacios 2017.
| Methods | RCT |
| Participants | Mothers of infants aged from birth to 2 months participating in the women, infants and children programme |
| Interventions | Participants were randomised to receive short mobile messages (SMS) about general infant's health issues (control) or SMS for improving feeding practices (intervention) for 4 months |
| Outcomes | Infant feeding practices |
| Notes | Conference abstract |
Paul 2011.
| Methods | RCT |
| Participants | 160 mother‐infant dyads |
| Interventions | 1 of 4 treatment cells. The first intervention (“Soothe/Sleep”) instructed parents on discriminating between hunger and other sources of infant distress. Soothing strategies were taught to minimise feeding for non‐hunger‐related fussiness and to prolong sleep duration, particularly at night; the second intervention (“Introduction of Solids”) taught parents about hunger and satiety cues, the timing for the introduction of solid foods, and how to overcome infants’ initial rejection of healthy foods through repeated exposure; to receive both; or no interventions delivered at 2 nurse home visits |
| Outcomes | Weight‐for‐length percentile at 1 year of age, conditional weight gain score |
| Notes |
Rabadi 2013.
| Methods | RCT |
| Participants | 118 mother‐child pairs |
| Interventions | The intervention group received key messages and support for positive infant feeding practices during home‐visits throughout the 16 months. The comparison group were not exposed to any messages but were visited only for data collection such as disease incidence |
| Outcomes | Infant feeding practices; exclusive breastfeeding, duration of breastfeeding above 1 year, timely introduction of the complementary meals and minimum meal diversity |
| Notes |
Savage 2010.
| Methods | RCT |
| Participants | 110 mother‐infant dyads |
| Interventions | The intervention group received an intervention that taught parents about the timing and methods for the introduction of solid foods and how to overcome food neophobia, using repeated exposure to improve liking and acceptance of unfamiliar foods such as vegetables |
| Outcomes | Timing of introduction of complementary foods, infant feeding practices |
| Notes |
Shafique 2013.
| Methods | Cluster‐RCT |
| Participants | Full‐term, low‐birth‐weight infants |
| Interventions |
|
| Outcomes | Growth, morbidity |
| Notes |
Toure 2016.
| Methods | Cluster‐RCT |
| Participants | Rural women who were pregnant or had a child < 2 years |
| Interventions | Multi‐faceted intervention (home gardening, gender sensitisation), with and without nutrition education |
| Outcomes | Maternal self‐efficacy in complementary feeding |
| Notes | Conference abstract |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Campbell 2016.
| Trial name or title | The extended Infant Feeding, Activity and Nutrition Trial (InFANT Extend) program: a cluster‐randomized controlled trial of an early intervention to prevent childhood obesity |
| Methods | Cluster‐RCT |
| Participants | First time parents of children (aged 3 months at baseline) |
| Interventions | Intervention: 6 x 2‐h, dietitian‐delivered sessions; web‐based materials; Facebook® engagement and written resources Control: usual care |
| Outcomes |
|
| Starting date | Not stated |
| Contact information | Karen Campbell Deakin University, Centre for Physical Activity and Nutrition Research, School of Exercise and Nutrition Sciences, Faculty of Health Email: karen.campbell@deakin.edu.au |
| Notes | ANZCTR ACTRN12611000386932 Conflict of interest: "The authors declare that they have no competing interests" (quote, p 174) Source of funding: "This project was funded by a World Cancer Research Fund grant (no. 2010/244)" (quote, p 175) |
Cloutier 2015.
| Trial name or title | The Early Childhood Obesity Prevention Program (ECHO): an ecologically‐based intervention delivered by home visitors for newborns and their mothers |
| Methods | RCT |
| Participants | Pregnant women or women who had just delivered a baby |
| Interventions | Intervention: enhanced Nurturing Family Network (NFN) home programme (education and skill‐set training with materials to implement the behaviours recommended. Using a motivational interviewing framework, intervention participants will receive dietary and activity counselling, develop a Family Wellness Plan and will be linked to community resources) Control: usual care (NFN home visitation) |
| Outcomes | Number of months of breastfeeding |
| Starting date | June 2013 |
| Contact information | Michelle M Cloutier Department of Pediatrics, University of Connecticut Health Center Email: mclouti@connecticutchildrens.org |
| Notes | ClinicalTrials.gov NCT02052518 Conflict of interest: not reported Source of funding: Connecticut Children's Medical Center,University of Connecticut, UConn Health |
Helle 2017.
| Trial name or title | Early food for future health: a randomized controlled trial evaluating the effect of an eHealth intervention aiming to promote healthy food habits from early childhood |
| Methods | RCT |
| Participants | Parents of infants |
| Interventions | Intervention: parents receive monthly emails with links to age‐appropriate website when child between 6 and 12 months of age Control: receive ordinary care from child health centres |
| Outcomes | Infant primary outcome measures:
Parent primary outcome measures:
Secondary outcomes:
|
| Starting date | 1 February 2015 |
| Contact information | Christine Helle Department of Public Health, Sport and Nutrition, Faculty of Health and Sport Sciences, University of Agder, PO Box 422, 4604 Kristiansand, Norway Email: christine.helle@uia.no |
| Notes | ISRCTN registry ISRCTN13601567 Conflict of interest: "The authors declare that they have no competing interests" (quote, p 39) Source of funding: "The study is funded by the University of Agder, with financial support from the Eckbo Foundation, Norway. The financial contributors were not involved in designing the study, collection, analyses and interpretation of data or in writing the manuscript." (quote, p 39) |
Hernes 2013.
| Trial name or title | First food for infants |
| Methods | RCT |
| Participants | Parents of infants |
| Interventions | Intervention: parents participate in 2 cooking courses on how to prepare a variety of baby food Control: given a brochure about infant nutrition only |
| Outcomes | The project will show whether a practical cooking course to parents will increase homemade food practice resulting in a greater variety in food intake, reduce prevalence of neophobia and reduce risk of obesity at toddler's age |
| Starting date | Autumn 2011 |
| Contact information | S Hernes Department of Public Health, Sport and Nutrition, University of Agder, Kristiansand, Norway |
| Notes | onlinelibrary.wiley.com/o/cochrane/clcentral/articles/796/CN‐01006796/frame.html Conflict of interest: not reported Source of funding: not reported |
Horodynski 2011.
| Trial name or title | Healthy babies through infant‐centered feeding protocol: an intervention targeting early childhood obesity in vulnerable populations |
| Methods | RCT |
| Participants | 372 economically and educationally disadvantaged African American, Hispanic, and white mothers with infants |
| Interventions | Intervention: 6 in‐home visits by a trained paraprofessional instructor, followed by 3 reinforcement telephone contacts when the baby is 6, 8, and 10 months old Control: usual care |
| Outcomes | Main maternal outcomes include:
Main infant outcome: infant growth pattern |
| Starting date | February 2010 |
| Contact information | Mildred A Horodynski College of Nursing, Michigan State University, 1355 Bogue Street, Bott, Nursing Building, East Lansing, MI 48824, USA Email: millie@msu.edu |
| Notes | ClinicalTrials.gov NCT01816516 Conflict of interest: "The authors declare that they have no competing interests" (quote, p 874) Source of funding: "This project is funded by the United States Department of Agriculture, National Institute of Food and Agriculture No. 2009‐55215‐05220" (quote, p 874) |
Horodynski 2015.
| Trial name or title | Tools for teen moms to reduce infant obesity: a randomised clinical trial |
| Methods | RCT |
| Participants | 100 low‐income African‐American and white adolescents, first‐time mothers of infants |
| Interventions | Intervention: provides infant feeding information to mothers via a web‐based application, and includes daily behavioural challenges, text message reminders, discussion forums, and website information as a comprehensive social media strategy over 6 weeks. Participants continue to receive usual care during the intervention Control: usual care |
| Outcomes | Main maternal outcomes include:
Primary infant outcome: infant weight |
| Starting date | June 2014 |
| Contact information | Mildred A Horodynski College of Nursing, Michigan State University, 1355 Bogue Street, Bott, Nursing Building, East Lansing, MI 48824, USA Email: millie@msu.edu |
| Notes | ClinicalTrials.gov NCT02244424 Conflict of interest: "The authors declare they have no competing interests" (quote, p 28) Source of funding: "The National Institute of Child Health and Development funds this trial (NIH grant number 1R21HDO75974‐OIAL)" (quote, p 28) |
Kimani‐Murage 2013.
| Trial name or title | Effectiveness of personalised, home‐based nutritional counselling on infant feeding practices, morbidity and nutritional outcomes among infants in Nairobi slums: study protocol for a cluster randomised controlled trial |
| Methods | Cluster‐RCT |
| Participants | 780 mother‐child pairs |
| Interventions | Intervention: mothers will receive regular, personalised, home‐based counselling by trained community health workers on maternal, infant and young child nutrition (MIYCN) Control: usual care |
| Outcomes |
|
| Starting date | March 2012 |
| Contact information | Elizabeth Kimani‐Murage African Population and Health Research Center (APHRC), PO 10787, 00100, Nairobi, Kenya Email: ekimani@aphrc.org |
| Notes | ISRCTN registry ISRCTN83692672 Conflict of interest: "The authors declare that they have no competing interests" (quote, p 455) Source of funding: "This study is funded by the Wellcome Trust, Grant # 097146/Z/11/Z. We also acknowledge core funding for APHRC from The William and Flora Hewlett Foundation and the Swedish International Cooperation Agency (SIDA); and funding for the NUHDSS from the Bill and Melinda Gates Foundation" (quote, p 455) |
Kulwa 2014.
| Trial name or title | Effectiveness of a nutrition education package in improving feeding practices, dietary adequacy and growth of infants and young children in rural Tanzania: rationale, design and methods of a cluster randomised trial |
| Methods | Parallel, cluster‐RCT |
| Participants | Infants aged 6 months |
| Interventions | Intervention: nutrition education package in addition to routine health education Control: routine health education offered monthly by health staff at health facilities |
| Outcomes | Primary outcome: linear growth as length‐for‐age z‐scores Secondary outcomes:
Assessed at baseline and ages 9, 12 and 15 months |
| Starting date | September 2014 |
| Contact information | KBM Kulwa Department of Food Science and Technology, Sokoine University of Agriculture, P,O, Box 3006, Chuo Kikuu, Morogoro, Tanzania kissakulwa@yahoo.com. |
| Notes | ClinicalTrials.gov NCT02249754 Conflict of interest: the study authors declare they have no competing interests Source of funding: "Funding was provided at different phases by Schlumberger Foundation’s Faculty for the Future Programme, Nestle Foundation for the Study of Problems of Nutrition in the World, Belgian Development Agency and Nutrition Third World. The views expressed are those of the author(s) and not necessarily those of the funding organisations. The funding bodies had no role in the design, data collection and analysis and interpretation of results." (quote, p 1092) |
SHINE Team 2015.
| Trial name or title | Sanitation, Hygiene, Infant Nutrition Efficacy (SHINE) project |
| Methods | RCT |
| Participants | Pregnant women |
| Interventions | Intervention: 3 groups
Control: standard of care |
| Outcomes |
|
| Starting date | November 2012 |
| Contact information | Professor Jean Humphrey Johns Hopkins Bloomberg School of Public Health Email: not provided |
| Notes | ClinicalTrials.gov NCT01824940 Conflict of interest: not reported Source of funding: Johns Hopkins Bloomberg School of Public Health |
Wasser 2015.
| Trial name or title | Mothers and others: designing a randomised trial to prevent obesity among infants and toddlers |
| Methods | RCT |
| Participants | Mothers recruited through antenatal clinics |
| Interventions | Intervention: multi‐component obesity prevention intervention in promoting healthy weight gain patterns among African‐American (AA) infants. Delivery channels include face‐to‐face peer counselling through 6 home visits, support from a lactation consultant, 6 newsletters, and twice‐weekly text messages Control: attention control (child safety) |
| Outcomes | Main outcome: weight‐for‐length z‐scores at 18 months Secondary outcomes:
Formative feedback was generally positive, with target participants also requesting information on postpartum weight loss, depression, maternal sleep, father‐infant bonding and maintaining intimate relationships |
| Starting date | October 2013 |
| Contact information | Margaret Bently Nutrition University of North Carolina, Chapel Hill (NC), United States |
| Notes | ClinicalTrials.gov NCT01938118 Conflict of interest: not reported Source of funding: University of North Carolina, Chapel Hill |
BMI: body mass index; RCT: randomised controlled trial
Differences between protocol and review
Title
We changed the title from 'Educational interventions for improving complementary feeding practices' to 'Educational interventions for improving primary caregiver complementary feeding practices for children aged 24 months and under', following the editor's advice to include the target population.
Primary outcomes
See Primary outcomes. We revised primary outcome 1 (shown below), to make for easier data analyses.
-
"Improved complementary feeding practices (measured as a continuous outcome or dichotomous outcome), for change from baseline values of the following:
duration of exclusive breastfeeding and age of introduction of complementary foods as measured by length of exclusive breastfeeding; timely introduction of complementary foods;
adequacy of complementary foods as measured by number of children fed with adequate amount and consistency of complementary foods (e.g. thick gruels); number of times children were fed in a day; meal frequency (e.g. children fed with at least five different classes of food, consisting mainly of protein, carbohydrate, vegetable, oil and fat, fruits); vitamin supplementation (for infant and mother); and energy density of complementary foods; and
safe preparation and storage of complementary foods as measured by handwashing practices (washing of caregiver’s and child’s hands with soap before cooking, feeding, or eating); water sanitation practices; food preparation and storage practices; serving foods immediately after preparation; using clean utensils, plates, pots, etc. for preparing/serving food and for feeding the child; and avoiding the use of feeding bottles".
Specifically, we broke 1.a. "duration of exclusive breastfeeding and age of introduction of complementary foods as measured by length of exclusive breastfeeding" into two categories, rather than having them as one category, as specified in our protocol (Arikpo 2015, p 6). In addition, we added the WHO's minimum acceptable diet, minimum dietary diversity, minimum meal frequency to primary outcome 1.b. ("adequacy of complementary foods"), and we renamed primary outcome 1.c. (" safe preparation and storage of complementary foods as measured by handwashing practices") as "hygiene practices". Primary outcome 1. now states:
-
"Improved complementary feeding practices (measured as a continuous outcome or dichotomous outcome), of the following:
age at introduction of complementary foods;
duration of exclusive breastfeeding;
adequacy of complementary foods (measured by number of children fed with adequate amount and consistency of complementary foods, children fed with at least five different classes of food, consisting mainly of protein, carbohydrate, vegetable, fats and oils, fruits; vitamin supplementation (for infant and mother); energy density of complementary foods; and meal frequency (number of times children are fed in a day); or based on the WHO minimum acceptable diet, minimum dietary diversity, minimum meal frequency or as assessed by study authors); and
hygiene practices: safe preparation and storage of complementary foods (measured by handwashing practices (washing of caregiver's and child's hands with soap before cooking, feeding, or eating); water sanitation practices; food preparation and storage practices; serving foods immediately after preparation; using clean utensils, plates, pots, etc. for preparing/serving food and for feeding the child; and avoiding the use of feeding bottles).
Furthermore, we added an example to our second primary outcome, "Adverse events (as defined by study authors)", so it now reads, "Adverse events (as defined by study authors). For example, overburdening of personnel delivering the intervention who were also responsible for other tasks in the health facility, stress on caregivers".
Contributions of authors
DA, ESE and MTC contributed to screening of studies and extraction of data.
DA retrieved study reports, entered data into Review Manager 2014, analysed data, wrote to study authors for additional data and drafted the review.
DA, ESE, MTC and DMC contributed to appraising the risk of bias in the papers for this review.
FO drafted and commented on the review.
DMC revised the statistical aspects of the review and analysed data.
DMC and FO interpreted the data for this review.
MTC and DMC edited the review.
All review authors read and approved the final manuscript.
DA has overall responsibility for this review.
Sources of support
Internal sources
-
Cochrane Nigeria, Institute of Tropical Diseases Research and Prevention, University of Calabar Teaching Hospital, Calabar, Nigeria.
Dachi Arikpo was awarded a Reviews for Africa Programme (RAP) Fellowship. The protocol for this review was developed during this Fellowship Programme, organised by Cochrane Nigeria, March 2014.
-
Effective Health Care Research Consortium (EHCRC), Liverpool School of Tropical Medicine, UK.
Supported the Reviews for Africa Programme, organised by Cochrane Nigeria, March 2014.
External sources
-
Medical Research Council, UK.
Population Health Scientist Fellowship award (G0902118) awarded to Deborah M Caldwell
-
Department for International Development (DFID), UK.
Supported the Reviews for Africa Programme through UKAid funding provided to the Effective Health Care Research Consortium at the Liverpool School of Tropical Medicine.
Declarations of interest
Dachi Arikpo ‐ none known Ededet Sewanu Edet ‐ none known Moriam T Chibuzor ‐ none known Friday Odey ‐ none known Deborah M Caldwell received a Population Health Scientist Fellowship from the Medical Research Council (MRC), UK. The views expressed herein are those of the author and not necessarily those of the MRC.
New
References
References to studies included in this review
Aboud 2008 {published data only}
- Aboud FE, Moore AC, Akhter S. Effectiveness of a community‐based responsive feeding programme in rural Bangladesh: a cluster randomised field trial. Maternal and Child Nutrition 2008;4(4):275‐86. [DOI: 10.1111/j.1740-8709.2008.00146.x; PUBMED: 18811792 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aboud 2009 {published data only}
- Aboud FE, Shafique S, Akhter S. A responsive feeding intervention increases children's self‐feeding and maternal responsiveness but not weight gain. The Journal of Nutrition 2009;139(9):1738‐43. [DOI: 10.3945/jn.109.104885; PUBMED: 19587124] [DOI] [PubMed] [Google Scholar]
Aboud 2011 {published data only}
- Aboud FE, Akhter S. A cluster‐randomised evaluation of a responsive stimulation and feeding intervention in Bangladesh. Pediatrics 2011;127(5):e1191‐7. [DOI: 10.1542/peds.2010-2160; PUBMED: 21502222] [DOI] [PubMed] [Google Scholar]
Bhandari 2001 {published data only}
- Bhandari N, Bahl R, Nayyar B, Khokhar P, Rohde JE, Bhan MK. Food supplementation with encouragement to feed it to infants from 4 to 12 months of age has a small impact on weight gain. The Journal of Nutrition 2001;131(7):1946‐51. [PUBMED: 11435512] [DOI] [PubMed] [Google Scholar]
Bhandari 2004 {published data only}
- Bhandari N, Mazumder S, Bahl R, Martines J, Black RE, Bhan MK, et al. An educational intervention to promote appropriate complementary feeding practices and physical growth in infants and young children in rural Haryana, India. The Journal of Nutrition 2004;134(9):2342‐8. [PUBMED: 15333726] [DOI] [PubMed] [Google Scholar]
- Bhandari N, Mazumder S, Bahl R, Martines J, Black RE, Bhan MK, et al. Use of multiple opportunities for improving feeding practices in under‐twos within child health programmes. Health Policy and Planning 2005;20(5):328‐36. [DOI: 10.1093/heapol/czi039; PUBMED: 16113403] [DOI] [PubMed] [Google Scholar]
Campbell 2013 {published data only}
- Cameron AJ, Ball K, Hesketh KD, McNaughton SA, Salmon J, Crawford DA, et al. Variation in outcomes of the Melbourne Infant, Feeding, Activity and Nutrition Trial (InFANT) Program according to maternal education and age. Preventive Medicine 2014;58:58‐63. [DOI: 10.1016/j.ypmed.2013.10.021; PUBMED: 24201090] [DOI] [PubMed] [Google Scholar]
- Campbell KJ, Lioret S, McNaughton SA, Crawford DA, Salmon J, Ball K, et al. A parent‐focused intervention to reduce infant obesity risk behaviors: a randomized trial. Pediatrics 2013;131(4):652‐60. [DOI: 10.1542/peds.2012-2576; PUBMED: 23460688] [DOI] [PubMed] [Google Scholar]
Daniels 2012 {published and unpublished data}
- Daniels L. Australia, actual number of children with the event in the intervention & control arms [personal communication]. Email to: D Arikpo 29 August 2016.
- Daniels LA, Mallan KM, Battistutta D, Nicholson JM, Perry R, Magarey A. Evaluation of an intervention to promote protective infant feeding practices to prevent childhood obesity: outcomes of the NOURISH RCT at 14 months of age and 6 months post the first of two intervention modules. International Journal of Obesity 2012;36(10):1292‐8. [DOI: 10.1038/ijo.2012.96; PUBMED: 22710926] [DOI] [PubMed] [Google Scholar]
- Daniels LA, Mallan KM, Nicholson JM, Battistutta D, Magarey A. Outcomes of an early feeding practices intervention to prevent childhood obesity. Pediatrics 2013;132(1):e109‐18. [DOI: 10.1542/peds.2012-2882; PUBMED: 23753098] [DOI] [PubMed] [Google Scholar]
de Oliveira 2012 {published data only}
- Nunes LM, Vigo Á, Oliveira LD, Giugliani ERJ. Effect of a healthy eating intervention on compliance with dietary recommendations in the first year of life: a randomized clinical trial with adolescent mothers and maternal grandmothers [Efeito de intervenção no cumprimento das recomendações alimentares no primeiro ano de vida: ensaio clínico randomizado com mães adolescentes e avós maternas]. Cadernos de Saude Publica 2017;33(6):e00205615. [DOI: 10.1590/0102-311X00205615; PUBMED: 28678940] [DOI] [PubMed] [Google Scholar]
- Soldateli B, Vigo A, Giugliani ERJ. Adherence to dietary recommendations for preschoolers: clinical trial with teenage mothers. Revista De Saúde Pública 2016;50:83. [DOI: 10.1590/S1518-8787.2016050006622; PMC5167099; PUBMED: 28099665] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira LD, Giugliani ER, Santo LC, Nunes LM. Impact of a strategy to prevent the introduction of non‐breast milk and complementary foods during the first 6 months of life: a randomised clinical trial with adolescent mothers and grandmothers. Early Human Development 2012;88(6):357‐61. [DOI: 10.1016/j.earlhumdev.2011.09.010; NCT00910377; PUBMED: 22001312] [DOI] [PubMed] [Google Scholar]
Edward 2013 {published data only}
- Edwards RC, Thullen MJ, Korfmacher J, Lantos JD, Henson LG, Hans SL. Breastfeeding and complementary food: randomized trial of community doula home visiting. Pediatrics 2013;132(Suppl 2):S160‐6. [DOI: 10.1542/peds.2013-1021P; NCT01925664; PUBMED: 24187119] [DOI] [PubMed] [Google Scholar]
Kang 2017 {published data only}
- Kang Y, Kim S, Sinamo S, Christian P. Effectiveness of a community‐based nutrition programme to improve child growth in rural Ethiopia: a cluster‐randomized trial. Maternal and Child Nutrition 2017;13(1):e12349. [DOI: 10.1111/mcn.12349; PUBMED: 27549570] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koehler 2007 {published data only}
- Koehler S, Sichert‐Hellert W, Kersting M. Measuring the effects of nutritional counseling on total infant diet in a randomised controlled intervention trial. Journal of Pediatric Gastroenterology and Nutrition 2007;45(1):106‐13. [DOI: 10.1097/MPG.0b013e3180331e2a; PUBMED: 17592372] [DOI] [PubMed] [Google Scholar]
Negash 2014 {published data only}
- Negash C, Belachew T, Henry CJ, Kebebu A, Abegaz K, Whiting SJ. Nutrition education and introduction of broad bean‐based complementary food improves knowledge and dietary practices of caregivers and nutritional status of their young children in Hula, Ethiopia. Food and Nutrition Bulletin 2014;35(4):480‐6. [DOI: 10.1177/156482651403500409; 25639132] [DOI] [PubMed] [Google Scholar]
Olaya 2013 {published and unpublished data}
- Fewtrell M. Colombia, actual number of children with the event in the intervention & control arms [personal communication]. Email to: D Arikpo 6 October 2016.
- Olaya GA, Lawson M, Fewtrell MS. Efficacy and safety of new complementary feeding guidelines with an emphasis on red meat consumption: a randomised trial in Bogota, Colombia. The American Journal of Clinical Nutrition 2013;98(4):983‐93. [DOI: 10.3945/ajcn.112.053595; ISRCTN57733004; PUBMED: 23945724] [DOI] [PubMed] [Google Scholar]
Penny 2005 {published data only}
- Penny ME, Creed‐Kanashiro HM, Robert RC, Narro MR, Caulfield LE, Black RE. Effectiveness of an educational intervention delivered through the health services to improve nutrition in young children: a cluster‐randomised controlled trial. Lancet 2005;365(9474):1863‐72. [DOI: 10.1016/S0140-6736(05)66426-4; PUBMED: 15924983] [DOI] [PubMed] [Google Scholar]
- Robert RC. Australia, actual number of children with the event in the intervention & control arms and time points growth was measured [personal communication]. Email to: D Arikpo 27 December 2016.
Reinbott 2016 {published data only}
- Reinbott A. Germany, actual number of children with the event in the intervention & control arms [personal communication]. Email to: D Arikpo 20 April 2017.
- Reinbott A, Schelling A, Kuchenbecker J, Jeremias T, Russell I, Kevanna O, et al. Nutrition education linked to agricultural interventions improved child dietary diversity in rural Cambodia. British Journal of Nutrition 2016;116(8):1457‐68. [DOI: 10.1017/S0007114516003433; PMC5082286; PUBMED: 27702425] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saleem 2014 {published data only}
- Saleem AF, Mahmud S, Baig‐Ansari N, Zaidi AKM. Impact of maternal education about complementary feeding on their infants' nutritional outcomes in low‐ and middle‐income households: a community‐based randomised interventional study in Karachi, Pakistan. Journal of Health, Population, and Nutrition 2014;32(4):623‐33. [PMC4438693] [PMC free article] [PubMed] [Google Scholar]
Schroeder 2015 {published data only}
- Schroeder N, Rushovich B, Bartlett E, Gittelsohn J, Caballero B. Early obesity prevention: a randomized trial of a practice‐based intervention in 0‐24 months infants. The FASEB Journal 2012;26(1 Suppl):374.3. [DOI: 10.1096/fasebj.26.1_supplement.374.3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder N, Rushovich B, Bartlett E, Sharma S, Gittelsohn J, Caballero B. Early obesity prevention: a randomised trial of a practice‐based intervention in 0‐24‐month infants. Journal of Obesity 2015;2015:795859. [DOI: 10.1155/2015/795859; PMC4442409; PUBMED: 26078877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2010 {published data only}
- Shi L, Zhang J, Wang Y, Caulfield LE, Guyer B. Effectiveness of an educational intervention on complementary feeding practices and growth in rural China: a cluster randomised controlled trial. Public Health Nutrition 2010;13(4):556‐65. [DOI: 10.1017/S1368980009991364; PUBMED: 19706219] [DOI] [PubMed] [Google Scholar]
- Zhang J, Shi L, Chen DF, Wang J, Wang Y. Effectiveness of an educational intervention to improve child feeding practices and growth in rural China: updated results at 18 months of age. Maternal and Child Nutrition 2013;9(1):118‐29. [DOI: 10.1111/j.1740-8709.2012.00447.x; PUBMED: 23020102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tariku 2015 {published data only}
- Tariku B, Whiting SJ, Mulualem D, Singh P. Application of the Health Belief Model to teach complementary feeding messages in Ethiopia. Ecology of Food and Nutrition 2015;54(5):572‐82. [DOI: 10.1080/03670244.2015.1049344; PUBMED: 26075935] [DOI] [PubMed] [Google Scholar]
Vazir 2013 {published data only}
- Bentley ME, Vazir S, Engle P, Balakrishna N, Johnson S, Creed H, et al. A home‐based educational intervention to caregivers in South India to improve complementary feeding and responsive feeding, and psychosocial stimulation increases dietary intake, growth and development of infants. The FASEB Journal 2010;24(1 Suppl):564.14. [DOI: 10.1096/fasebj.24.1_supplement.564.14] [DOI] [Google Scholar]
- Vazir S, Engle P, Balakrishna N, Griffiths PL, Johnson SL, Creed‐Kanashiro H, et al. Cluster‐randomized trial on complementary and responsive feeding education to caregivers found improved dietary intake, growth and development among rural Indian toddlers. Maternal and Child Nutrition 2013;9(1):99‐117. [DOI: 10.1111/j.1740-8709.2012.00413.x; PMC3434308; PUBMED: 22625182] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vitolo 2005 {published data only}
- Bortolini GA, Vitolo MR. The impact of systematic dietary counseling during the first year of life on prevalence rates of anemia and iron deficiency at 12‐16 months. Jornal de Pediatria 2012;88(1):33‐9. [DOI: 10.2223/JPED.2156; NCT00629629; PUBMED: 22159301] [DOI] [PubMed] [Google Scholar]
- Louzada ML, Campagnolo PD, Rauber F, Vitolo MR. Long‐term effectiveness of maternal dietary counseling in a low‐income population: a randomized field trial. Pediatrics 2012;129(6):e1477‐84. [DOI: 10.1542/peds.2011-3063; NCT00629629; PUBMED: 22566413] [DOI] [PubMed] [Google Scholar]
- Vitolo MR, Bortolini GA, Campagnolo PD, Hoffman DJ. Maternal dietary counseling reduces consumption of energy‐dense foods among infants: a randomized controlled trial. Journal of Nutrition Education and Behavior 2012;44(2):140‐7. [DOI: 10.1016/j.jneb.2011.06.012; PUBMED: 22189004] [DOI] [PubMed] [Google Scholar]
- Vitolo MR, Bortolini GA, Feldens CA, Drachler ML. Impacts of the 10 Steps to Healthy Feeding in Infants: a randomized field trial [Impactos da implementação dos dez passos da alimentação saudável para crianças: ensaio de campo randomizado]. Cadernos de Saude Publica 2005;21(5):1448‐57. [DOI: ; PUBMED: 16158151] [DOI] [PubMed] [Google Scholar]
- Vitolo MR, Rauber F, Campagnolo PD, Feldens CA, Hoffman DJ. Maternal dietary counseling in the first year of life is associated with a higher healthy eating index in childhood. The Journal of Nutrition 2010;140(11):2002‐7. [DOI: 10.3945/jn.110.125211; NCT00629629; PUBMED: 20844187] [DOI] [PubMed] [Google Scholar]
Wen 2011 {published data only}
- Wen LM, Baur LA, Simpson JM, Rissel C, Flood VM. Effectiveness of an early intervention on infant feeding practices and "tummy time": a randomized controlled trial. Archives of Pediatrics and Adolescent Medicine 2011;165(8):701‐7. [DOI: 10.1001/archpediatrics.2011.115; ACTRN012607000168459; PUBMED: 21810633] [DOI] [PubMed] [Google Scholar]
Yin 2009 {published data only}
- Yin SA, Li N, Yan ZY, Pan L, Lai JQ, Zhao XF. Effects of nutritional education on improvement of nutritional knowledge of infant's mothers in rural area in China. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine] 2009;43(2):103‐7. [PUBMED: 19534900] [PubMed] [Google Scholar]
References to studies excluded from this review
Arimond 2017 {published data only}
- Arimond M, Abbeddou S, Kumwenda C, Okronipa H, Hemsworth J, Jimenez EY, et al. Impact of small quantity lipid‐based nutrient supplements on infant and young child feeding practices at 18 months of age: results from four randomized controlled trials in Africa. Maternal and Child Nutrition 2017;13(3):e12377. [DOI: 10.1111/mcn.12377; PMC5516197; PUBMED: 27910260] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arpadi 2009 {published data only}
- Arpadi S, Fawzy A, Aldrovandi GM, Kankasa C, Sinkala M, Mwiya M, et al. Growth faltering due to breastfeeding cessation in uninfected children born to HIV‐infected mothers in Zambia. The American Journal of Clinical Nutrition 2009;90(2):344‐53. [DOI: 10.3945/ajcn.2009.27745.; NCT00310726; PMC2709311; PUBMED: 19553300] [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 2001 {published data only}
- Black MM, Siegel EH, Abel Y, Bentley ME. Home and videotape intervention delays early complementary feeding among adolescent mothers. Pediatrics 2001;107(5):e67. [PUBMED: 11331717] [DOI] [PubMed] [Google Scholar]
Brown 1992 {published data only}
- Brown LV, Zeitlin MF, Peterson KE, Chowdhury AMR, Rogers BL, Weld LH, et al. Evaluation of the impact of weaning food messages on infant feeding practices and child growth in rural Bangladesh. The American Journal of Clinical Nutrition 1992;56(6):994‐1003. [PUBMED: 1442668] [DOI] [PubMed] [Google Scholar]
Cameron 2013 {published data only}
- Cameron SL, Taylor RW, Gray AR, Taylor BJ, Heath A‐LM. Exclusive breastfeeding to six months: results from a randomised controlled trial including lactation consultant support. The FASEB Journal 2013;27(1 Suppl):lb345. [DOI: 10.1096/fasebj.27.1_supplement.lb345] [DOI] [Google Scholar]
Clark 2009 {published data only}
- Clark A, Anderson, J, Adams E, Baker S, Barrett K. Assessing an infant feeding web site as a nutrition education tool for child care providers. Journal of Nutrition Education and Behavior 2009;41(1):41‐6. [DOI: 10.1016/j.jneb.2007.12.007; PUBMED: 19161919] [DOI] [PubMed] [Google Scholar]
Dumaguing 2015 {published data only}
- Dumaguing NV, Hurtada WA, Yee MG. Complementary feeding counselling promotes physical growth of infant and young children in rural villages in Leyte, Philippines. Journal of Society and Technology 2015;5(1):1‐15. [Available at: www.jst‐online.org/index.php/JST/article/view/39] [Google Scholar]
Faerber 2017 {published data only}
- Faerber EC, Ko J, Weiss J, Girard AW. Evaluation of an innovative feeding toolkit to improve complementary feeding and child growth in rural Malawi. The FASEB Journal 2017;31(1 Suppl):165.2. [DOI: 10.1096/fasebj.31.1_supplement.165.2] [DOI] [Google Scholar]
Fangupo 2015 {published data only}
- Cameron SL, Heath AL, Gray AR, Churcher B, Davies RS, Newlands A, et al. Lactation consultant support from late pregnancy with an educational intervention at 4 months of age delays the introduction of complementary foods in a randomized controlled trial. The Journal of Nutrition 2015;145(7):1481‐90. [DOI: 10.3945/jn.114.202689; NCT00892983; PUBMED: 25995280] [DOI] [PubMed] [Google Scholar]
- Fangupo LJ, Heath AL, Williams SM, Somerville MR, Lawrence JA, Gray AR, et al. Impact of an early‐life intervention on the nutrition behaviors of 2‐y‐old children: a randomized controlled trial. The American Journal of Clinical Nutrition 2015;102(3):704‐12. [DOI: 10.3945/ajcn.115.111823; NCT00892983; PUBMED: 26224299] [DOI] [PubMed] [Google Scholar]
Fernald 2016 {published data only}
- Fernald LCH, Galasso E, Qamruddin J, Ranaivoso C, Ratsifandrihamanana L, Stewart CP, et al. A cluster‐randomized, controlled trial of nutritional supplementation and promotion of responsive parenting in Madagascar: the MAHAY study design and rationale. BMC Public Health 2016;16:466. [DOI: 10.1186/s12889-016-3097-7; ISRCTN14393738; PMC4891833; PUBMED: 27255923] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fildes 2015 {published data only}
- Fildes A, Lopes C, Moreira P, Moschonis G, Oliveira A, Mavrogianni C, et al. An exploratory trial of parental advice for increasing vegetable acceptance in infancy. The British Journal of Nutrition 2015;114(2):328‐36. [DOI: 10.1017/S0007114515001695; PUBMED: 26063588] [DOI] [PubMed] [Google Scholar]
Ford 2009 {published data only}
- Ford FA, Mouratidou T, Wademan SE, Fraser RB. Effect of the introduction of 'Healthy Start' on dietary behaviour during and after pregnancy: early results from the 'before and after' Sheffield study. The British Journal of Nutrition 2009;101(12):1828‐36. [DOI: 10.1017/S0007114508135899; PUBMED: 19017424] [DOI] [PubMed] [Google Scholar]
Guldan 2000 {published data only}
- Guldan GS, Fan HC, Ma X, Ni ZZ, Xiang X, Tang MZ. Culturally appropriate nutrition education improves infant feeding and growth in rural Sichuan, China. The Journal of Nutrition 2000;130(5):1204‐11. [PUBMED: 10801920] [DOI] [PubMed] [Google Scholar]
Haider 2013 {published data only}
- Haider R. Challenges of a community‐based peer counselling programme to promote and support appropriate complementary feeding in Bangladesh. Annals of Nutrition and Metabolism 2013;63(Suppl 1):659. [DOI: 10.1159/000354245; PO831] [DOI] [Google Scholar]
Hotz 2005 {published data only}
- Hotz C, Gibson RS. Participatory nutrition education and adoption of new feeding practices are associated with improved adequacy of complementary diets among rural Malawian children: a pilot study. European Journal of Clinical Nutrition 2005;59(2):226‐37. [DOI: 10.1038/sj.ejcn.1602063; PUBMED: 15483634] [DOI] [PubMed] [Google Scholar]
Jakobsen 2008 {published data only}
- Jakobsen MS, Sodemann M, Biai S, Nielsen J, Aaby P. Promotion of exclusive breastfeeding is not likely to be cost effective in West Africa. A randomised intervention study from Guinea‐Bissau. Acta Paediatrica 2008;97(1):68‐75. [DOI: 10.1111/j.1651-2227.2007.00532.x; PUBMED: 18053000 ] [DOI] [PubMed] [Google Scholar]
Kabahenda 2011 {published data only}
- Kabahenda M, Mullis RM, Erhardt JG, Northrop‐Clewes C, Nickols SY. Nutrition education to improve dietary intake and micronutrient nutriture among children in less‐resourced areas: a randomised controlled intervention in Kabarole district, Western Uganda. South African Journal of Clinical Nutrition 2011;24(2):83‐8. [DOI: 10.1080/16070658.2011.11734355] [DOI] [Google Scholar]
Kapur 2003 {published data only}
- Kapur D, Sharma S, Agarwal KN. Effectiveness of nutrition education, iron supplementation or both on iron status in children. Indian Pediatrics 2003;40(12):1131‐44. [PUBMED: 14722364] [PubMed] [Google Scholar]
Kilaru 2005 {published data only}
- Kilaru A, Griffiths PL, Ganapathy S, Ghosh S. Community‐based nutrition education for improving infant growth in rural Karnataka. Indian Pediatrics 2005;42(5):425‐32. [PUBMED: 15923688] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Kim SS, Rawat R, Mwangi EM, Tesfaye R, Abebe Y, Baker J, et al. Exposure to large‐scale social and behavior change communication interventions is associated with improvements in infant and young child feeding practices in Ethiopia. Plos One 2016;11(10):e0164800. [DOI: 10.1371/journal.pone.0164800; PMC5068829; PUBMED: 27755586] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klingberg 2017 {published data only}
- Klingberg S, Ludvigsson J, Brekke HK. Introduction of complementary foods in Sweden and impact of maternal education on feeding practices. Public Health Nutrition 2017;20(6):1054‐62. [DOI: 10.1017/S1368980016003104; PUBMED: 27917749] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuchenbecker 2017 {published data only}
- Kuchenbecker J, Reinbott A, Mtimuni B, Krawinkel M, Jordan I. Nutrition education improves dietary diversity of children 6‐23 months at community‐level: results from a cluster randomized controlled trial in Malawi. PLoS One 2017;12(4):e0175216. [DOI: 10.1371/journal.pone.0175216; PMC5398527; PUBMED: 28426678] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maslowsky 2016 {published data only}
- Maslowsky J, Frost S, Hendrick CE, Trujillo Cruz FO, Merajver SD. Effects of postpartum mobile phone‐based education on maternal and infant health in Ecuador. International Journal of Gynaecology and Obstetrics 2016;134(1):93‐8. [DOI: 10.1016/j.ijgo.2015.12.008; PUBMED: 27126905 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Menon 2016 {published data only}
- Menon P, Nguyen PH, Saha KK, Khaled A, Sanghvi T, Baker J, at al. Combining intensive counseling by frontline workers with a nationwide mass media campaign has large differential impacts on complementary feeding practices but not on child growth: results of a cluster‐randomized program evaluation in Bangladesh. The Journal of Nutrition 2016;146(10):2075‐84. [DOI: 10.3945/jn.116.232314; NCT01678716; PMC5037872; PUBMED: 27581575] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulualem 2016 {published data only}
- Mulualem D, Henry CJ, Berhanu G, Whiting SJ. The effectiveness of nutrition education: applying the Health Belief Model in child‐feeding practices to use pulses for complementary feeding in Southern Ethiopia. Ecology of Food and Nutrition 2016;55(3):308‐23. [DOI: 10.1080/03670244.2016.1161617; PUBMED: 27065308 ] [DOI] [PubMed] [Google Scholar]
Nair 2017 {published data only}
- Nair N, Tripathy P, Sachdev HS, Pradhan H, Bhattacharyya S, Gope R, et al. Effect of participatory women's groups and counselling through home visits on children's linear growth in rural eastern India (CARING trial): a cluster‐randomised controlled trial. Lancet, Global Health 2017;5(10):e1004‐16. [DOI: 10.1016/S2214-109X(17)30339-X; PMC5640793; PUBMED: 28911749] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neyzi 1991 {published data only}
- Neyzi O, Güleçyüz M, Dinçer Z, Olgun P, Kutluay T, Uzel N, et al. An educational intervention on promotion of breast feeding complemented by continuing support. Paediatric and Perinatal Epidemiology 1991;5(3):299‐303. [PUBMED: 1881840] [DOI] [PubMed] [Google Scholar]
Nikiema 2017 {published data only}
- Nikièma L, Huybregts L, Martin‐Prevel Y, Donnen P, Lanou H, Grosemans J, et al. Effectiveness of facility‐based personalized maternal nutrition counseling in improving child growth and morbidity up to 18 months: a cluster‐randomized controlled trial in rural Burkina Faso. PLoS One 2017;12(5):e0177839. [DOI: 10.1371/journal.pone.0177839; PMC5444625; PUBMED: 28542391] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olney 2015 {published data only}
- Olney DK, Pedehombga A, Ruel MT, Dillon A. A 2‐year integrated agriculture and nutrition and health behavior change communication program targeted to women in Burkina Faso reduces anemia, wasting, and diarrhea in children 3‐12.9 months of age at baseline: a cluster‐randomized controlled trial. The Journal of Nutrition 2015;145(6):1317‐24. [DOI: 10.3945/jn.114.203539; NCT01825226; PUBMED: 25904734] [DOI] [PubMed] [Google Scholar]
Owais 2017 {published data only}
- Owais A, Schwartz B, Kleinbaum DG, Suchdev PS, Faruque ASG, Das SK, et al. A nutrition education program in rural Bangladesh was associated with improved feeding practices but not with child growth. The Journal of Nutrition 2017;147(5):948‐54. [DOI: 10.3945/jn.116.243956; PUBMED: 28298543] [DOI] [PubMed] [Google Scholar]
Pachon 2002 {published data only}
- Marsh DR, Pachón H, Schroeder DG, Ha TT, Dearden K, Lang TT, et al. Design of a prospective, randomised evaluation of an integrated nutrition program in rural Viet Nam. Food and Nutrition Bulletin 2002;23(4 Suppl):36‐47. [PUBMED: 12503230] [PubMed] [Google Scholar]
- Pachón H, Schroeder DG, Marsh DR, Dearden KA, Ha TT, Lang TT. Effect of an integrated child nutrition intervention on the complementary food intake of young children in rural north Viet Nam. Food and Nutrition Bulletin 2002;23(4 Suppl):62‐9. [PUBMED: 12503233] [PubMed] [Google Scholar]
- Schroeder DG, Pachón H, Dearden KA, Kwon CB, Ha TT, Lang TT, et al. An integrated child nutrition intervention improved growth of younger, more malnourished children in northern Viet Nam. Food and Nutrition Bulletin 2002;23(4 Suppl):53‐61. [PUBMED: 12503232] [PubMed] [Google Scholar]
Pant 1996 {published data only}
- Pant CR, Pokharel GP, Curtale F, Pokhrel RP, Grosse RN, Lepkowski J, et al. Impact of nutrition education and mega‐dose vitamin A supplementation on the health of children in Nepal. Bulletin of the World Health Organization 1996;74(5):533‐45. [PMC2486860; PUBMED: 9002334] [PMC free article] [PubMed] [Google Scholar]
Pelto 2004 {published data only}
- Pelto GH, Santos I, Gonçalves H, Victora C, Martines J, Habicht JP. Nutrition counseling training changes physician behavior and improves caregiver knowledge acquisition. The Journal of Nutrition 2004;134(2):357‐62. [PUBMED: 14747672] [DOI] [PubMed] [Google Scholar]
Reich 2010 {published data only}
- Reich SM, Bickman L, Saville BR, Alvarez J. The effectiveness of baby books for providing paediatric anticipatory guidance to new mothers. Pediatrics 2010;125(5):997‐1002. [DOI: 10.1542/peds.2009-2728; NIHMS191726; PMC2875122] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinsma 2016 {published data only}
- Reinsma K, Nkuoh G, Nshom E. The potential effectiveness of the nutrition improvement program on infant and young child feeding and nutritional status in the Northwest and Southwest regions of Cameroon, Central Africa. BMC Health Services Research 2016;16(1):654. [DOI: 10.1186/s12913-016-1899-z; PMC5109805; PUBMED: 27846828] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robling 2016 {published data only}
- Robling M, Bekkers MJ, Bell K, Butler CC, Cannings‐John R, Channon S, et al. Effectiveness of a nurse‐led intensive home‐visitation programme for first‐time teenage mothers (Building Blocks): a pragmatic randomised controlled trial. Lancet 2016;387(10014):146‐55. [DOI: 10.1016/S0140-6736(15)00392-X; ISRCTN23019866; PMC4707160; PUBMED: 26474809] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roset‐Salla 2016 {published data only}
- Roset‐Salla M, Ramon‐Cabot J, Salabarnada‐Torras J, Pera G, Dalmau A. Educational intervention to improve adherence to the Mediterranean diet among parents and their children aged 1‐2 years. EniM clinical trial. Public Health Nutrition 2016;19(6):1131‐44. [DOI: 10.1017/S1368980015002219; PUBMED: 26258462] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roy 2005 {published data only}
- Roy SK, Fuchs GJ, Mahmud Z, Ara G, Islam S, Shafique S, et al. Intensive nutrition education with or without supplementary feeding improves the nutritional status of moderately‐malnourished children in Bangladesh. Journal of Health, Population, and Nutrition 2005;23(4):320‐30. [PUBMED: 16599102] [PubMed] [Google Scholar]
Roy 2007 {published data only}
- Roy SK, Jolly SP, Shafique S, Fuchs GJ, Mahmud Z, Chakraborty B, at al. Prevention of malnutrition among young children in rural Bangladesh by a food‐health‐care educational intervention: a randomised, controlled trial. Food and Nutrition Bulletin 2007;28(4):375‐83. [DOI: 10.1177/156482650702800401; PUBMED: 18274163 ] [DOI] [PubMed] [Google Scholar]
Salehi 2004 {published data only}
- Salehi M, Kimiagar SM, Shahbazi M, Mehrabi Y, Kolahi AA. Assessing the impact of nutrition education on growth indices of Iranian nomadic children: an application of a modified beliefs, attitudes, subjective‐norms and enabling‐factors model. The British Journal of Nutrition 2004;91(5):779‐87. [DOI: 10.1079/BJN20041099; PUBMED: 15137930] [DOI] [PubMed] [Google Scholar]
Santos 2001 {published data only}
- Santos I, Victora CG, Martines J, Gonçalves H, Gigante DP, Valle NJ, et al. Nutrition counseling increases weight gain among Brazilian children. The Journal of Nutrition 2001;131(11):2866‐73. [PUBMED: 11694610] [DOI] [PubMed] [Google Scholar]
Savage 2016 {published data only}
- Hohman EE, Paul IM, Birch LL, Savage JS. INSIGHT responsive parenting intervention is associated with healthier patterns of dietary exposures in infants. Obesity 2017;25(1):185‐91. [DOI: 10.1002/oby.21705; PMC5189916; PUBMED: 28008749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman EE, Savage JS, Paul IM, Birch LL. INSIGHT study parenting intervention to prevent childhood obesity improves patterns of dietary exposures in infants. The FASEB Journal 2016;30(1 Suppl):295.2. [DOI: 10.1096/fasebj.30.1_supplement.295.2] [DOI] [Google Scholar]
- Savage JS, Birch LL, Marini M, Anzman‐Frasca S, Paul IM. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year: a randomized clinical trial. JAMA Pediatrics 2016;170(8):742‐9. [DOI: 10.1001/jamapediatrics.2016.0445; NCT01167270; PMC4969142; PUBMED: 27271455] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spigelblatt 1991 {published data only}
- Spigelblatt L, Lainé‐Ammara G, Arsenault L, Zvagulis I, Pless IB. Influence of follow‐up education of mothers about too early introduction of solid foods to infants [Influence d'une education suivie aupres des meres sur l'introduction trop rapede des solides dans l'alimentation du nourrisson]. Pediatrie 1991;46(5):475‐9. [PUBMED: 1663244] [PubMed] [Google Scholar]
Taylor 2017 {published data only}
- Taylor RW, Williams SM, Fangupo LJ, Wheeler BJ, Taylor BJ, Daniels L, et al. Effect of a baby‐led approach to complementary feeding on infant growth and overweight: a randomized clinical trial. JAMA Pediatrics 2017;171(9):838‐46. [DOI: 10.1001/jamapediatrics.2017.1284; ACTRN12612001133820; PMC5710413 [Available on 05‐09‐2018] ; PUBMED: 28692728] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2012 {published data only}
- Thompson DA, Joshi A, Hernandez RG, Bair‐Merritt MH, Arora M, Luna R, et al. Nutrition education via a touchscreen: a randomised controlled trial in Latino immigrant parents of infants and toddlers. Academic Pediatrics 2012;12(5):412‐9. [DOI: 10.1016/j.acap.2012.03.020; NCT01272492; PUBMED: 22682718] [DOI] [PubMed] [Google Scholar]
Vitolo 2014 {published data only}
- Vitolo MR, Louzada ML, Rauber F. Positive impact of child feeding training program for primary care health professionals: a cluster randomized field trial. Revista Brasileira de Epidemiologia 2014;17(4):873‐86. [DOI: 10.1590/1809-4503201400040007; PUBMED: 25388488] [DOI] [PubMed] [Google Scholar]
- Vitolo MR, Louzada ML, Rauber F, Grechi P, Gama CM. The impact of health workers' training on breastfeeding and complementary feeding practices [Impacto da atualização de profissionais de saúde sobre as práticas de amamentação e alimentação complementar]. Cadernos de Saude Publica 2014;30(8):1695‐707. [DOI: 10.1590/0102-311X00186913; PUBMED: 25210909] [DOI] [PubMed] [Google Scholar]
Wambach 2011 {published data only}
- Wambach KA, Aaronson L, Breedlove G, Domian EW, Rojjanasrirat W, Yeh HW. A randomized controlled trial of breastfeeding support and education for adolescent mothers. Western Journal of Nursing Research 2011;33(4):486‐505. [DOI: 10.1177/0193945910380408; PUBMED: 20876551 ] [DOI] [PubMed] [Google Scholar]
Waswa 2015 {published data only}
- Waswa LM, Jordan I, Herrmann J, Krawinkel MB, Keding GB. Community‐based educational intervention improved the diversity of complementary diets in western Kenya: results from a randomized controlled trial. Public Health Nutrition 2015;18(18):3406‐19. [DOI: 10.1017/S1368980015000920; PUBMED: 25857703] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yousafzai 2016 {published data only}
- Yousafzai AK, Obradović J, Rasheed MA, Rizvi A, Portilla XA, Tirado‐Strayer N, et al. Effects of responsive stimulation and nutrition interventions on children's development and growth at age 4 years in a disadvantaged population in Pakistan: a longitudinal follow‐up of a cluster‐randomised factorial effectiveness trial. Lancet, Global Health 2016;4(8):e548‐58. [DOI: 10.1016/S2214-109X(16)30100-0; NCT00715936; PUBMED: 27342433] [DOI] [PubMed] [Google Scholar]
Zaman 2008 {published data only}
- Zaman S, Ashraf RN, Martines J. Training in complementary feeding counselling of healthcare workers and its influence on maternal behaviours and child growth: a cluster‐randomised controlled trial in Lahore, Pakistan. Journal of Health, Population, and Nutrition 2008;26(2):210‐22. [PMC2740673; PUBMED: 18686554] [PMC free article] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang Y, Wu Q, Wang W, Velthoven MH, Chang S, Han H, et al. Effectiveness of complementary food supplements and dietary counselling on anaemia and stunting in children aged 6‐23 months in poor areas of Qinghai Province, China: a controlled interventional study. BMJ Open 2016;6(10):e011234. [DOI: 10.1136/bmjopen-2016-011234; ChiCTRPRC12002444; PMC5093399; PUBMED: 27799239] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dunlevy 2010 {published data only}
- Dunlevy F, Sheridan‐Pereira M, Koornneef E. Can antenatal education alter the preferred time of weaning?. Journal of Pediatric Gastroenterology and Nutrition 2010;50(Suppl 2):e40‐41. [PD‐N‐099; journals.lww.com/jpgn/toc/2010/06002] [Google Scholar]
Dunlvey 2012 {published data only}
- Dunlevy FB, Sheridan‐Pereira M. Improved timing of fish introduction in infancy. Clinical Nutrition Supplements 2012;7(1):128. [DOI: 10.1016/S1744-1161(12)70312-5; PP261‐SUN] [DOI] [Google Scholar]
Guan 2016 {published data only}
- Guan H. Community‐based educational intervention improved caregivers feeding behaviors and the nutritional anemia in infant aged 6‐11 months: a randomized controlled trial in rural China. European Journal of Pediatrics 2016;175(11):1487‐88. [DOI: 10.1007/s00431-016-2785-8; 283] [DOI] [Google Scholar]
Jordan 2015 {published data only}
- Jordan I, Kuchenbecker J, Phiri GC, Mühlhoff E, Herrmann J, Krawinkel MB. Food based nutrition education improved complementary feeding practices and nutritional status of children below 2 years in Malawi. Annals of Nutrition and Metabolism 2015;67(Suppl 1):471‐2. [DOI: 10.1159/000440895; 149/1396] [DOI] [Google Scholar]
Palacios 2017 {published data only}
- Palacios C, Campos M, Gibby C, Banna J. Multi‐site trial using short mobile messages (SMS) to improve infant feeding practices among participants in the WIC program. The FASEB Journal 2017;31(1 Suppl):959.8. [DOI: 10.1096/fasebj.31.1_supplement.959.8] [DOI] [Google Scholar]
Paul 2011 {published data only}
- Paul IM, Savage JS, Anzman SL, Beiler JS, Marini ME, Stokes JL, et al. Preventing obesity during infancy: a pilot study. Obesity 2011;19(2):353‐61. [DOI: 10.1038/oby.2010.182; NCT00359242; PMC3477360; PUBMED: 20725058] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabadi 2013 {published data only}
- Rabadi H, Al Sharif N. Community based intervention improves infant feeding practices in Bethlehem villages. Annals of Nutrition and Metabolism 2013;63(Suppl 1):401. [DOI: 10.1159/000354245; P0302] [DOI] [Google Scholar]
Savage 2010 {published data only}
- Savage JS, Paul IM, Marini ME, Birch LL. Pilot intervention promoting responsive feeding, the division of feeding responsibility, and healthy dietary choices during infancy. Appetite 2010;54(3):673. [DOI: 10.1016/j.appet.2010.04.178] [DOI] [Google Scholar]
Shafique 2013 {published data only}
- Shafique S, Chowdhury J, Jolly S, Shikder H, Sellen D, Zlotkin S. Prevention of linear growth faltering among low birth weight infants in rural Bangladesh: a community‐based cluster randomised trial. Annals of Nutrition and Metabolism 2013;63(Suppl 1):1127‐8. [DOI: 10.1159/000354245; P03332] [DOI] [Google Scholar]
- Shafique S, Jalal CSB, Jolly SP, Shikder H, Sellen DW, Zlotkin S. Effects of water‐based hand sanitizers and micronutrient powders along with nutrition and hygiene education to prevent infections and linear growth faltering among low birth weight infants in Bangladesh. The FASEB Journal 2013;27(1 Suppl):243.1. [DOI: 10.1096/fasebj.27.1_supplement.243.1] [DOI] [Google Scholar]
- Shafique S, Singla D, Aboud F, Jolly S, Zlotkin S. Multiple micronutrient powders reduce stunting and anaemia and improve language development among full‐term low birth weight children in Bangladesh. The FASEB Journal 2014;28(1 Suppl):389.3. [DOI: 10.1096/fasebj.28.1_supplement.389.3] [DOI] [Google Scholar]
Toure 2016 {published data only}
- Toure D, Rawat R, Stoltzfus RJ, Harvey D, Mwanamwenge M, Pelletier DL. The effects of a nutrition‐sensitive agricultural intervention on social support, food security and maternal self‐efficacy in complementary feeding. The FASEB Journal 2016;30(1 Suppl):247.4. [DOI: 10.1096/fasebj.30.1_supplement.274.4] [DOI] [Google Scholar]
References to ongoing studies
Campbell 2016 {published data only}
- Campbell KJ, Hesketh KD, McNaughton SA, Ball K, McCallum Z, Lynch J, et al. The Extended Infant Feeding, Activity and Nutrition Trial (InFANT Extend) Program: a cluster‐randomized controlled trial of an early intervention to prevent childhood obesity. BMC Public Health 2016;16:166. [DOI: 10.1186/s12889-016-2836-0; ACTRN12611000386932; PMC4758178; PUBMED: 26888759] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cloutier 2015 {published data only}
- Cloutier MM, Wiley J, Wang Z, Grant A, Gorin AA. The Early Childhood Obesity Prevention Program (ECHO): an ecologically‐based intervention delivered by home visitors for newborns and their mothers. BMC Public Health 2015;15:584. [DOI: 10.1186/s12889-015-1897-9; NCT02052518; PUBMED: 26104068] [DOI] [PMC free article] [PubMed] [Google Scholar]
Helle 2017 {published data only}
- Helle C, Hillesund ER, Omholt ML, Øverby NC. Early food for future health: a randomized controlled trial evaluating the effect of an eHealth intervention aiming to promote healthy food habits from early childhood. BMC Public Health 2017;17(1):729. [DOI: 10.1186/s12889-017-4731-8; PMC5607575; PUBMED: 28931384] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernes 2013 {published data only}
- Hernes S, Haugen M, Øverby N. First food for infants. Annals of Nutrition and Metabolism 2013;63(Suppl 1):326‐7. [DOI: 10.1159/000354245; P0148] [DOI] [Google Scholar]
Horodynski 2011 {published data only}
- Horodynski MA, Olson B, Baker S, Brophy‐Herb H, Auld G, Egeren L, et al. Healthy babies through infant‐centred feeding protocol: an intervention targeting early childhood obesity in vulnerable populations. BMC Public Health 2011;11:868. [DOI: 10.1186/1471-2458-11-868; ACTRN126100000415000; PMC3339510; PUBMED: 22085421] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horodynski 2015 {published data only}
- Horodynski MA, Silk K, Hsieh G, Hoffman A, Robson M. Tools for teen moms to reduce infant obesity: a randomised clinical trial. BMC Public Health 2015;15:22. [DOI: 10.1186/s12889-015-1345-x; NCT02244424; PMC4308927; PUBMED: 5604090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimani‐Murage 2013 {published data only}
- Kimani‐Murage EW, Kyobutungi C, Ezeh AC, Wekesah F, Wanjohi M, Muriuki P, et al. Effectiveness of personalised, home‐based nutritional counselling on infant feeding practices, morbidity and nutritional outcomes among infants in Nairobi slums: study protocol for a cluster randomised controlled trial. Trials 2013;14:445. [DOI: 10.1186/1745-6215-14-445; ISRCTN83692672; PUBMED: 24370263] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kulwa 2014 {published data only}
- Kulwa KB, Verstraeten R, Bouckaert KP, Mamiro PS, Kolsteren PW, Lachat C. Effectiveness of a nutrition education package in improving feeding practices, dietary adequacy and growth of infants and young children in rural Tanzania: rationale, design and methods of a cluster randomised trial. BMC Public Health 2014;14:1077. [DOI: 10.1186/1471-2458-14-1077; NCT02249754; PMC4216379; PUBMED: 25318980] [DOI] [PMC free article] [PubMed] [Google Scholar]
SHINE Team 2015 {published data only}
- Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial Team, Humphrey JH, Jones AD, Manges A, Mangwadu G, Maluccio JA, et al. The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) trial: rationale, design, and methods. Clinical Infectious Diseases 2015;61(Suppl 7):S685‐702. [DOI: 10.1093/cid/civ844; NCT01824940; PMC4657589; PUBMED: 26602296] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wasser 2015 {published data only}
- Wasser H, Bentley M. Mothers and others: designing a randomized trial to prevent obesity among infants and toddlers. FASEB Journal 29 2015; Vol. 29, issue 1 Suppl:584.16. [DOI: 10.1096/fasebj.29.1_supplement.584.16] [DOI]
Additional references
Ajzen 1991
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes 1991;50(2):179‐211. [Google Scholar]
Arimond 2004
- Arimond M, Ruel M. Dietary diversity is associated with child nutritional status: evidence from 11 demographic and health surveys. Journal of Nutrition 2004;134(10):2579–85. [PUBMED: 15465751] [DOI] [PubMed] [Google Scholar]
Bhutta 2012
- Bhutta ZA, Salam RA. Global nutrition epidemiology and trends. Annals of Nutrition & Metabolism 2012;61(Suppl 1):19‐27. [DOI: 10.1159/000345167; PUBMED: 23343944] [DOI] [PubMed] [Google Scholar]
Black 1982
- Black RE, Brown KH, Becker S, Alim ARMA, Merson MH. Contamination of weaning foods and transmission of enterotoxigenic Escherichia coli diarrhoea in children in rural Bangladesh. Transactions of the Royal Society of Tropical Medicine and Hygiene 1982;76(2):259‐64. [PUBMED: 7048652] [DOI] [PubMed] [Google Scholar]
Black 2008
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243‐60. [DOI: 10.1016/S0140-6736(07)61690-0; PUBMED: 18207566] [DOI] [PubMed] [Google Scholar]
Bluethmann 2017
- Bluethmann SM, Bartholomew LK, Murphy CC, Vernon SW. Use of theory in behavior change interventions: an analysis of programs to increase physical activity in posttreatment breast cancer survivors. Health Education & Behavior 2017;44(2):245‐53. [DOI: 10.1177/1090198116647712; NIHMS872708; PMC5503486] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brunello 2012
- Brunello G, Fort M, Schneeweis N, Winter‐Ebmer R. The causal effect of education on health: what is the role of health behaviours? Working Paper Series 08‐2012. www.share‐project.org/uploads/tx_sharepublications/WP_08_2012_Brunello_Fort_Schneeweis_Winter‐Ebmer_01.pdf (accessed 26 January 2018). [DOI] [PubMed]
Burgess 2012
- Burgess A, Danga L. Undernutrition in adults and children: causes, consequences and what we can do. www.ajol.info/index.php/ssmj/article/view/132347/121948 (accessed 14 February 2015).
Ciciriello 2013
- Ciciriello S, Johnston RV, Osborne RH, Wicks I, DeKroo T, Clerehan R, et al. Multimedia educational interventions for consumers about prescribed and over‐the‐counter medications. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008416.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Darity 1997
- Darity WA, Chen TTL, Tuthill RW, Buchanan DR, Winder AE, Stanek E, et al. A multi‐city community based smoking research intervention project in the African‐American population. International Quarterly of Community Health Education 1997;17(2):117‐30. [DOI: 10.2190/CEXY-WG7C-GL3E-A2BP; PUBMED: 20841058] [DOI] [PubMed] [Google Scholar]
Davis 2015
- Davis R, Campbell R, Hildon Z, Hobbs L, Michie S. Theories of behaviour and behaviour change across the social and behavioural sciences: a scoping review. Health Psychology Review 2015;9(No. 3):323–44. [DOI: 10.1080/17437199.2014.941722; 25104107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston M, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Dewey 2008
- Dewey KG, Adu‐Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal and Child Nutrition 2008;4(Suppl 1):24–85. [DOI: 10.1111/j.1740-8709; PUBMED: 18289157] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fein 2008
- Fein SB, Labiner‐Wolfe J, Scanlon KS, Grummer‐Strawn LM. Selected complementary feeding practices and their association with maternal education. Pediatrics 2008;122(Suppl 2):S91–7. [DOI: 10.1542/peds.2008-1315l; PUBMED: 18829837] [DOI] [PubMed] [Google Scholar]
Fischer Walker 2012
- Fischer Walker CL, Perin J, Aryee M, Boschi‐Pinto C, Black RE. Diarrhea incidence in low‐and middle‐income countries in 1990 and 2010: a systematic review. BMC Public Health 2012;12:220. [DOI: 10.1186/1471-2458-12-220; PMC3323412; PUBMED: 22436130] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer Walker 2013
- Fischer Walker CL, Rudan I, Liu L, Nair H, Theodoratu E, Bhutta Z, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381(9875):1405‐16. [DOI: 10.1016/S0140-6736(13)60222-6; PUBMED: 23582727 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fredericks 2013
- Fredericks S, Yau T. Educational intervention reduces complications and re‐hospitalizations after heart surgery. Western Journal of Nursing Research 2013;35(10):1251‐65. [DOI: 10.1177/0193945913490081; PUBMED: 23720096] [DOI] [PubMed] [Google Scholar]
Gibbons 1984
- Gibbons G, Griffiths M. Program Activities for Improving Weaning Practices. Geneva (CH): World Federation of Public Health Associations, 1984. [Google Scholar]
Glanz 2010
- Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annual Review of Public Health 2010;31:399–418. [DOI: 10.1146/annurev.publhealth.012809.103604; PUBMED: 20070207] [DOI] [PubMed] [Google Scholar]
Glanz 2014
- Glanz K. E‐source: the authority on behavioral and social science research. www.esourceresearch.org (accessed 6 July 2014).
Gold 2002
- Gold RS, Miner KR, 2000 Joint Committee on Health Education and Promotion Terminology. Report of the 2000 Joint Committee on Health Education and Promotion Terminology. The Journal of School Health 2002;72(1):3‐7. [PUBMED: 11865796] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 19 January 2015. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 2011
- Hamilton K, Daniels L, White KM, Murray N, Walsh A. Predicting mothers’ decisions to introduce complementary feeding at 6 months. An investigation using an extended theory of planned behaviour. Appetite 2011;56(3):674‐81. [DOI: 10.1016/j.appet.2011.02.002; PUBMED: 21316413] [DOI] [PubMed] [Google Scholar]
Henry 1990
- Henry FJ, Patwary Y, Huttly SR, Aziz KM. Bacterial contamination of weaning foods and drinking water in rural Bangladesh. Epidemiology and Infection 1990;104(1):79‐85. [PMC2271730; PUBMED: 2307187] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins C, Lavin T, Metcalfe O. Health Impacts of Education: A Review. Belfast/Dublin (IE): Institute of Public Health, 2008. [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston M, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hunter 2010
- Hunter PR, Ramírez Toro GI, Minnigh HA. Impact on diarrhoeal illness of a community educational intervention to improve drinking water quality in rural communities in Puerto Rico. BMC Public Health 2010;10:219. [DOI: 10.1186/1471-2458-10-219; PMC2876105; PUBMED: 20426831] [DOI] [PMC free article] [PubMed] [Google Scholar]
ILEP 1998
- ILEP Medico‐Social Commission. Planning health education interventions. Technical Bulletin 13. www.leprosy‐information.org/files/ILEP%20Techn%20Bulletin%2013%20‐%20Planning%20health%20education%20interventions.pdf (accessed 13 Feb 2015).
Imdad 2011
- Imdad A, Yakoob MY, Bhutta ZA. Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public Health 2011;11(Suppl 3):S25. [DOI: 10.1186/1471-2458-11-S3-S25; PMC3231899; PUBMED: 21501443] [DOI] [PMC free article] [PubMed] [Google Scholar]
Janz 1984
- Janz NK, Becker MH. The Health Belief Model: a decade later. Health Education Quarterly 1984;11(1):1‐47. [DOI: 10.1177/109019818401100101; PUBMED: 6392204] [DOI] [PubMed] [Google Scholar]
Kabir 2012
- Kabir I, Khanam M, Agho KE, Mihrshahi S, Dibley MJ, Roy SK. Determinants of inappropriate complementary feeding practices in infant and young children in Bangladesh: secondary data analysis of Demographic Health Survey 2007. Maternal and Child Nutrition 2012;8(Suppl 1):11‐27. [DOI: 10.1111/j.1740-8709.2011.00379.x; PUBMED: 22168516 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khanal 2013
- Khanal V, Sauer K, Zhao Y. Determinants of complementary feeding practices among Nepalese children aged 6–23 months: findings from Demographic and Health Survey 2011. BMC Pediatrics 2013;13:131. [DOI: 10.1186/1471-2431-13-131; PMC3766108 ; PUBMED: 23981670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kosek 2003
- Kosek M, Caryn B, Guerrant R. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bulletin of the World Health Organization 2003;81(3):197‐204. [PMC2572419; PUBMED: 12764516] [PMC free article] [PubMed] [Google Scholar]
Lassi 2013
- Lassi ZS, Das JK, Zahid G, Imdad A, Bhutta ZA. Impact of education and provision of complementary feeding on growth and morbidity in children less than 2 years of age in developing countries: a systematic review. BMC Public Health 2013;13(Suppl 3):S13. [DOI: 10.1186/1471-2458-13-S3-S13; PMC3847349; PUBMED: 24564534] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lutter 2003
- Lutter CK, Rivera JA. Nutritional status of infants and young children and characteristics of their diets. The Journal of Nutrition 2003;133(9):2941S‐9S. [PUBMED: 12949391] [DOI] [PubMed] [Google Scholar]
Martorell 1994
- Martorell R, Khan KL, Schroeder DG. Reversibility of stunting: epidemiological findings in children from developing countries. European Journal of Clinical Nutrition 1994;48(Suppl 1):S45‐57. [PUBMED: 8005090] [PubMed] [Google Scholar]
McLeroy 1988
- McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Education Quarterly 1988;15(4):351‐77. [PUBMED: 3068205] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599 ; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Monte 1997
- Monte CM, Ashworth A, Nations MK, Lima AA, Barreto A, Huttly SR. Designing educational messages to improve weaning food hygiene practices of families living in poverty. Social Science and Medicine 1997;44(10):1453‐64. [PUBMED: 9160436] [DOI] [PubMed] [Google Scholar]
Motarjemi 1993
- Motarjemi Y, Käferstein F, Moy G, Quevedo F. Contaminated weaning food: a major risk factor for diarrhoea and associated malnutrition. Bulletin of the World Health Organization 1993;71(1):79‐92. [PMC2393433; PUBMED: 8440042] [PMC free article] [PubMed] [Google Scholar]
NCI 2005
- National Cancer Institute. Theory at a Glance. A Guide for Health Promotion Practice. 2nd Edition. Bethesda (MD): US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute, 2005. [Google Scholar]
Nkhoma 2013
- Nkhoma K, Seymour J, Arthur A. An educational intervention to reduce pain and improve pain management for Malawian people living with HIV/AIDS and their family carers: study protocol for a randomised controlled trial. Trials 2013;14:216. [DOI: 10.1186/1745-6215-14-216; ISRCTN72861423; PMC3717041; PUBMED: 23849502] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ofotokun 2010
- Ofotokun I, Binongo JNG, Rosenberg ES, Kane M, Ifland R, Lennox JL, et al. Culturally‐adapted and audio‐technology assisted HIV/AIDS awareness and education program in rural Nigeria: a cohort study. BMC International Health and Human Rights 2010;10:2. [DOI: 10.1186/1472-698X-10-2; PMC2834647; PUBMED: 20181073] [DOI] [PMC free article] [PubMed] [Google Scholar]
PAHO/WHO 2003
- Pan American Health Organization, World Health Organization. Guiding principles for complementary feeding of the breastfed child. www.who.int/nutrition/publications/guiding_principles_compfeeding_breastfed.pdf (accessed 29 January 2014).
Philips 2000
- Philips N, Chirmulay D, Engle P, Houser RF, Bhagwat IP, Levinson FJ. Does timely introduction of complementary foods lead to improved nutritional status? Analysis of data from Maharashtra, India. nutrition.tufts.edu/sites/default/files/fpan/wp22‐comp_foods_india.pdf (accessed 5 February 2015).
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenstock 1974
- Rosenstock IM. Historical origins of the health belief model. Health Education Monographs 1974;2(4):328‐35. [DOI: 10.1177/109019817400200403] [DOI] [PubMed] [Google Scholar]
Saunders 1986
- Saunders S, Pine J. Seat belt education program‐‐a model for public health settings. Health Education Quarterly 1986;13(3):243‐7. [PUBMED: 3759477] [DOI] [PubMed] [Google Scholar]
Shah 2009
- Shah A, Blackhall K, Ker K, Patel D. Educational interventions for the prevention of eye injuries. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006527.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheth 2006
- Sheth M, Dwivedi R. Complementary foods associated diarrhoea. Indian Journal of Pediatrics 2006;73(1):61‐4. [PUBMED: 16444063] [DOI] [PubMed] [Google Scholar]
Shi 2011
- Shi L, Zhang J. Recent evidence of the effectiveness of educational interventions for improving complementary feeding practices in developing countries. Journal of Tropical Pediatrics 2011;57(2):91‐8. [DOI: 10.1093/tropej/fmq053] [DOI] [PubMed] [Google Scholar]
Shrimpton 2001
- Shrimpton R, Victora CG, Onis M, Lima RC, Blössner M, Clugston G. Worldwide timing of growth faltering: implications for nutritional interventions. Pediatrics 2001;107(5):e75. [PUBMED: 11331725] [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JAC, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Swanson 2005
- Swanson V, Power K. Initiation and continuation of breastfeeding: theory of planned behaviour. Journal of Advanced Nursing 2005;50(3):272‐82. [DOI: 10.1111/j.1365-2648.2005.03390.x; PUBMED: 15811106] [DOI] [PubMed] [Google Scholar]
Tarrant 2010
- Tarrant RC, Younger KM, Sheridan‐Pereira M, White MJ, Kearney JM. Factors associated with weaning practices in term infants: a prospective observational study in Ireland. The British Journal of Nutrition 2010;104(10):1544‐54. [DOI: 10.1017/S0007114510002412; PUBMED: 20598218] [DOI] [PubMed] [Google Scholar]
UNICEF 2006
- United Nations Children's Fund. Progress for children: a report card on nutrition. www.unicef.org/progressforchildren/2006n4/index_undernutrition.html (accessed 29 January 2015).
UNICEF 2008
- United Nations Children's Fund. Introducing solid foods: giving your baby a better start in life. www.unicef.org.uk/babyfriendly/wp‐content/uploads/sites/2/2008/02/Start4Life‐Introducing‐Solid‐Foods‐2015.pdf (accessed 21 October 2014).
USAID 2011
- United States Agency for International Development. Behavior change interventions and child nutritional status: evidence from the promotion of improved complementary feeding practices. www.iycn.org/files/IYCN_comp_feeding_lit_review_062711.pdf (accessed 12 June 2015).
Victor 2014
- Victor R, Baines SK, Agho KE, Dibley MJ. Factors associated with inappropriate complementary feeding practices among children aged 6‐23 months in Tanzania. Maternal and Child Nutrition 2014;10(4):545‐61. [DOI: 10.1111/j.1740-8709.2012.00435.x; PUBMED: 22925557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Victora 2008
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;371(9609):340‐57. [DOI: 10.1016/S0140-6736(07)61692-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walingo 2014
- Walingo MK, Mutuli LA. Influence of maternal beliefs, attitude, perceived behavior on breast‐feeding among post partum mothers in Western Kenya. Pakistan Journal of Nutrition 2014;13(5):250‐4. [DOI: 10.3923/pjn.2014.250.254] [DOI] [Google Scholar]
WHO 1998
- World Health Organization. Health promotion glossary. www.who.int/healthpromotion/about/HPR%20Glossary%201998.pdf?ua=1 (accessed 13 February 2015).
WHO 2003
- World Health Organization. Global strategy for infant and young child feeding. www.who.int/nutrition/publications/gs_infant_feeding_text_eng.pdf (accessed 18 January 2015).
WHO 2008
- World Health Organization, UNICEF. Strengthening action to improve feeding of infants and young children 6‐23 months of age in nutrition and child health programmes: Geneva, 6‐9 October 2008. Report of proceedings. apps.who.int/iris/bitstream/10665/44034/1/9789241597890_eng.pdf (accessed 29 January 2015).
WHO 2012a
- World Health Organization. Complementary feeding. Report of global consultation, Geneva, 10‐13 December 2011. Summary of guiding principles. www.who.int/nutrition/publications/Complementary_Feeding.pdf (accessed 29 January 2015).
WHO 2012b
- World Health Organization. Health education: theoretical concepts, effective strategies and core competencies. apps.who.int/iris/bitstream/10665/119953/1/EMRPUB_2012_EN_1362.pdf?ua=1 (accessed 1 May 2015).
WHO 2013a
- World Health Organization. Diarrhoeal disease. www.who.int/mediacentre/factsheets/fs330/en (accessed 5 February 2015).
WHO 2013b
- World Health Organization. Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children. Geneva (CH): World Health Organization, 2013. [PubMed] [Google Scholar]
WHO 2015a
- World Health Organization. Complementary feeding. www.who.int/nutrition/topics/complementary_feeding/en (accessed 1 February 2015).
WHO 2018
- World Health Organization. Infant and young child feeding. http://www.who.int/news‐room/fact‐sheets/detail/infant‐and‐young‐child‐feeding (accessed 13 Jan 2018).
World Health Assembly 2001
- World Health Assembly. Infant and young child nutrition. www.who.int/nutrition/topics/WHA54.2_iycn_en.pdf (accessed 3 March 2014).
Zhang 2009
- Zhang J, Shi L, Chen D, Wang J, Wang Y. Using the theory of planned behavior to examine effectiveness of an educational intervention on infant feeding in China. Preventive Medicine 2009;49(6):529‐34. [DOI: 10.1016/j.ypmed.2009.10.002; PUBMED: 19850063] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Arikpo 2015
- Arikpo D, Edet ES, Chibuzor MT, Odey F, Caldwell DM. Educational interventions for improving complementary feeding practices. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011768] [DOI] [PMC free article] [PubMed] [Google Scholar]


