Abstract
Background
Postoperative delirium (POD) and postoperative cognitive dysfunction (POCD) may complicate a patient's postoperative recovery in several ways. Monitoring of processed electroencephalogram (EEG) or evoked potential (EP) indices may prevent or minimize POD and POCD, probably through optimization of anaesthetic doses.
Objectives
To assess whether the use of processed EEG or auditory evoked potential (AEP) indices (bispectral index (BIS), narcotrend index, cerebral state index, state entropy and response entropy, patient state index, index of consciousness, A‐line autoregressive index, and auditory evoked potentials (AEP index)) as guides to anaesthetic delivery can reduce the risk of POD and POCD in non‐cardiac surgical or non‐neurosurgical adult patients undergoing general anaesthesia compared with standard practice where only clinical signs are used.
Search methods
We searched CENTRAL, MEDLINE, Embase and clinical trial registry databases up to 28 March 2017. We updated this search in February 2018, but these results have not been incorporated in the review.
Selection criteria
We included randomized or quasi‐randomized controlled trials comparing any method of processed EEG or evoked potential techniques (entropy, BIS, AEP etc.) against a control group where clinical signs were used to guide doses of anaesthetics in adults aged 18 years or over undergoing general anaesthesia for non‐cardiac or non‐neurosurgical elective operations.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcomes were: occurrence of POD; and occurrence of POCD. Secondary outcomes included: all‐cause mortality; any postoperative complications; and postoperative length of stay. We used GRADE to assess the quality of evidence for each outcome.
Main results
We included six randomized controlled trials (RCTs) with 2929 participants comparing processed EEG or EP indices‐guided anaesthesia with clinical signs‐guided anaesthesia. There are five ongoing studies and one study awaiting classification.
Anaesthesia administration guided by the indices from a processed EEG (bispectral index) probably reduces the risk of POD within seven days after surgery with risk ratio (RR) of 0.71 (95% CI 0.59 to 0.85; number needed to treat for an additional beneficial outcome (NNTB) of 17, 95% CI 11 to 34; 2197 participants; 3 RCTs; moderate quality of evidence). Three trials also showed the lower rate of POCD at 12 weeks after surgery (RR 0.71, 95% CI 0.53 to 0.96; NNTB 38, 95% CI 21 to 289; 2051 participants; moderate‐quality evidence), but it is uncertain whether processed EEG indices reduce POCD at one week (RR 0.84, 95% CI 0.69 to 1.02; 3 trials; 1989 participants; moderate‐quality evidence), and at 52 weeks (RR 0.30, 95% CI 0.05 to 1.80; 1 trial; 59 participants; very low quality of evidence). There may be little or no effect on all‐cause mortality (RR 1.01, 95% CI 0.62 to 1.64; 1 trial; 1155 participants; low‐quality evidence). One trial suggested a lower risk of any postoperative complications with processed EEG (RR 0.51, 95% CI 0.37 to 0.71; 902 participants, moderate‐quality evidence). There may be little or no effect on reduced postoperative length of stay (mean difference −0.2 days, 95% CI −2.02 to 1.62; 1155 participants; low‐quality evidence).
Authors' conclusions
There is moderate‐quality evidence that optimized anaesthesia guided by processed EEG indices could reduce the risk of postoperative delirium in patients aged 60 years or over undergoing non‐cardiac surgical and non‐neurosurgical procedures. We found moderate‐quality evidence that postoperative cognitive dysfunction at three months could be reduced in these patients. The effect on POCD at one week and over one year after surgery is uncertain. There are no data available for patients under 60 years. Further blinded randomized controlled trials are needed to elucidate strategies for the amelioration of postoperative delirium and postoperative cognitive dysfunction, and their consequences such as dementia (including Alzheimer's disease (AD)) in both non‐elderly (below 60 years) and elderly (60 years or over) adult patients. The one study awaiting classification and five ongoing studies may alter the conclusions of the review once assessed.
Plain language summary
Optimized anaesthesia depth guided by brain electrical activity for protection against postoperative delirium and cognitive dysfunction in adults
Review question
We wanted to discover whether using brain electrical activity monitoring to guide doses of anaesthetics can reduce the risk of postoperative delirium (POD) and cognitive dysfunction (POCD) in adults undergoing general anaesthesia for non‐cardiac and non‐neurological surgical procedures. Background
Postoperative delirium (POD) is a disturbed state of mind which occurs a few days after surgery. POD involves a fluctuating course of confusion and disorganized behaviour. Postoperative cognitive dysfunction (POCD) is a decline in the ability of a person to think clearly after an operation. This may persist for weeks or months. POCD can affect a person's concentration, attention, memory, learning, and the speed of their movement and mental responses. POD and POCD can complicate the quality of a person's recovery from anaesthesia, as well as the quality of their life after surgery.
Processed electroencephalogram (EEG) monitors generate numerical values of brain electrical activity. The number provides information about the depth of anaesthesia during surgery, and is used to guide the dose of anaesthetic given. This is to prevent someone receiving either too small or too large a dose of anaesthetics.
Search date
The evidence is current to March 2017. We found six completed studies, five ongoing studies, and one awaiting classification.
Study characteristics
All six completed studies were randomized controlled trials (RCT) conducted in 2929 male or female participants undergoing a surgical procedure and aged 60 years or over. An RCT is a study (or trial) which aims to reduce bias when testing a new treatment. The people taking part in the trial are randomly allocated to either the group receiving the treatment under investigation, or to a group receiving standard treatment (or placebo treatment) as the control. RCTs provide the most reliable evidence.
Key results
Results from three studies (2529 participants) indicate that using the processed EEG to help deliver the optimal depth of anaesthesia could reduce the incidence of POD from 21.3% to 15.2%. Results from three studies (2051 participants) indicate that this could also reduce the incidence of POCD at three months from 9.1% to 6.4%.
Quality of the evidence
Our review provides moderate‐quality evidence that anaesthesia guided by processed EEG indices could reduce the risk of postoperative delirium in patients aged 60 years or over undergoing non‐cardiac and non‐neurological surgical procedures. We found some moderate‐quality evidence that postoperative cognitive dysfunction at three months could be reduced in these participants. There is insufficient evidence supporting the effect on POCD at one week and over one year after surgery or in younger patients.
Summary of findings
Summary of findings for the main comparison. Processed EEG or EP indices guided anaesthesia for patients undergoing general anaesthesia for surgical procedures.
| Does use of a processed EEG or EP indices to control anaesthetic depth, ameliorate postoperative delirium or postoperative cognitive dysfunction in patients undergoing general anaesthesia for surgical procedures | |||||||
|
Patient or population: adults aged 18 years or over undergoing general anaesthesia for non‐cardiac or non‐neurosurgical elective operations
Settings: University Hospitals in Germany, Hong Kong, Thailand, and the UK
Intervention: processed EEG or EP indices guided anaesthesia Control: clinical signs guided anaesthesia | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Clinical signs guided anaesthesia | Processed EEG versus clinical signs guided anaesthesia (available data analysis) | ||||||
|
Postoperative delirium (POD) within 7 days (As assessed by any method such as the Mini‐Mental State Examination (MMSE) and Confusion Assessment Method (CAM) in the first week after operation) |
213 per 1000 | 151 per 1000 (126 to 181) | RR 0.71 (0.59 to 0.85) | 2197 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
|
Postoperative cognitive dysfunction (POCD) (As determined by any method of assessing POCD including a battery of neuropsychological tests according to the criteria described by the International Study of Postoperative Cognitive Dysfunction (ISPOCD)) |
one week | 188 per 1000 | 158 per 1000 (130 to 192) | RR 0.84 (0.69 to 1.02) | 1989 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| 12 weeks | 91 per 1000 | 64 per 1000 (48 to 87) | RR 0.71 (0.53 to 0.96) | 2051 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 52 weeks | 125 per 1000 | 38 per 1000 (5 to 311) | RR 0.30 (0.05 to 1.80) | 59 (1 study) | ⊕⊝⊝⊝ very low3 | ||
| All‐cause mortality | 53 per 1000 | 54 per 1000 (33 to 88) | RR 1.01 (0.62 to 1.64) | 1155 (1 study) | ⊕⊕⊝⊝ low2 | ||
| Any postoperative complication | 208 per 1000 | 106 per 1000 (77 to 148) | RR 0.51 (0.37 to 0.71) | 902 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| Postoperative length of stay (days) | The mean postoperative length of stay in the control group was 15.9 | The mean postoperative length of stay in the intervention groups was 0.2 lower (2.02 lower to 1.62 higher) | 1155 (1 study) | ⊕⊕⊝⊝ low2 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval;EEG: electroencephalogram; EP: evoked potential; RR: Risk ratio | |||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||||
1 downgraded one level due to concern about biases due to unblinded anaesthesia providers and incomplete outcome data across the three studies 2 downgraded two levels due to concerns about 1) biases due to unblinded anaesthesia providers and incomplete outcome data across the three studies and 2) imprecision (wide confidence interval from only one study) 3 downgraded three levels due to concerns about 1) bias due to unblinded anaesthesia providers and incomplete outcome data) and 2) serious imprecision (very wide confidence interval from only one study with small sample size)
Background
Description of the condition
Neuropsychological disorders following anaesthesia and surgery commonly manifest in two forms, namely delirium and cognitive dysfunction. Postoperative delirium (POD) is a transient form of neuropsychological disturbance which occurs suddenly after surgery in a fluctuating course of confusion and disorganization of behaviours. Postoperative cognitive dysfunction (POCD) is a form of cognitive deterioration following surgery which is defined and quantified by a battery of neuropsychological tests as a gradual and continual loss of “memory, attention, concentration, learning, and speed of motor and mental response” (Moller 1997). The incidence of POD is 5% to 15% and it usually occurs between the first and third postoperative day. The incidence of POCD varies and depends on the methods used for detection (Newman 2007). The reported incidence of POCD could be as high as 19.2% at one week after major non‐cardiac surgery and decreases to 7% after three months (Johnson 2002). The incidences at one week (early POCD) and after three months (late POCD) seem to be higher in the elderly than in middle‐aged patients (26% versus 19.2% and 10% versus 6.2%, respectively) (Johnson 2002; Moller 1998). POD and early POCD may complicate a patient's postoperative recovery in several ways, such as delayed motor and mental responses resulting in postponement of discharge from hospital and delayed return to work. Furthermore, persistent postoperative dysfunction can diminish a person's quality of life (Newman 2001). The International Study of Postoperative Cognitive Dysfunction (ISPOCD) group conducted a long‐term follow‐up study and found that POCD after non‐cardiac surgery was associated not only with an increased risk of mortality but also with a risk of socioeconomic problems such as being dependent on social transfer payments due to leaving the labour market too early (Steinmetz 2009). The pathophysiology of POD and POCD remains unclear. It may be related to inflammation (Vacas 2013), release of stress hormones, ischaemia or hypoxaemia. It is unclear whether POCD is related to anaesthetic technique or depth of anaesthesia (Mason 2010). High doses of anaesthetics can impair neurological function, which may persist into the postoperative period. On the other hand inadequate doses of anaesthetics may preclude patients from the beneficial effect of anaesthetics in terms of neurological protection. This leads to the postulate that optimized doses of anaesthetics may prevent or minimize POD or POCD. The traditional approach of using clinical signs such as changes in blood pressure or heart rate are not reliable for titrating anaesthetic doses in order to provide adequate depth of anaesthesia (Punjasawadwong 2014). Hence, a measure to quantify anaesthetic level for optimizing doses of anaesthetics is required.
Description of the intervention
The term 'general anaesthesia' implies that the individual is unconscious and unaware of his or her surroundings, including any painful or other sensory input. The accurate estimation of the 'depth of anaesthesia' at any point in time remains an active field of investigation. Some methods that are in use include the monitoring of clinical signs and the processing of cerebral electrical activity using the electroencephalograph (EEG), electromyograph (EMG) and auditory evoked potentials (AEP). Commonly, the analysis of electrical activity is processed to produce the 'Anaesthesia Index', a dimensionless number derived from cerebral electrical activity, including EEG, EMG, or AEP, to represent the sedative and hypnotic component of anaesthesia (Schneider 2010). The index values are numbers in a range between 0 to 100, where 0 reflects deep coma state and 100 reflects awake state. Basically, processed EEG indices are derived from signal analysis of the EEG (Rampil 1998), while processed AEP indices are from auditory evoked potentials (Rinaldi 2005). There are various commercially available anaesthesia depth monitors to measure processed EEG or AEP indices, such as bispectral index (BIS); narcotrend index (Kreuer 2003); cerebral state index (CSI) (Delfino 2009); state entropy (SE) and response entropy (RE) (Aime 2006); patient state index (PSI), index of consciousness (IoC), A‐line autoregressive index (AAI) (Bruhn 2005); and AEP‐Index (AEP‐index); (see Appendix 1).
An optimal range of depth of anaesthesia is defined as the range of anaesthesia index that is neither too deep nor too light. The recommended ranges for adequate depth of anaesthesia, as measured by processed EEG or AEP indices, vary and depend on the algorithms used to develop the indices. For example, the recommended ranges of BIS, CSI, SE and RE are between 40 and 60 while the range of narcotrend is 35 to 64 and that for AAI is between 15 and 25. The values below these ranges indicate too deep anaesthesia and the values above the ranges indicate anaesthesia that is too light.
How the intervention might work
The use of processed EEG indices to guide doses of anaesthetics in order to provide an optimum depth of anaesthesia could avoid unnecessary high or low doses of anaesthetics (Punjasawadwong 2014). Excessive doses of anaesthetics can damage the brain by either direct neurotoxicity (Culley 2007; Perouansky 2009; Wan 2010), or enhancing neuroinflammatory responses to surgical trauma leading to postoperative neurological disturbances such as delirium or cognitive decline (Vacas 2013). Rasmussen 2000 reported the elevation of S‐100B protein, a biological marker for brain damage, in patients with delirium after abdominal surgery. Recently Ballard and colleagues reported a correlation between S‐100B and duration of BIS outside the optimal BIS range (below 40 or above 60) (Ballard 2012). This may indirectly indicate that adequate depth of anaesthesia may protect against brain damage (Sanders 2005).
Why it is important to do this review
While sufficient evidence from previous studies supports the utility of anaesthesia depth monitors for improving anaesthetic delivery and recovery (Gan 1977; Lui 2004; Punjasawadwong 2014; Song 1997), as well as for the prevention of intraoperative recall (awareness) (Myles 2004), more evidence is required to support the positive impact of anaesthesia depth monitors on the prevention of POD and POCD and their consequences. Recently there have indeed been a number of publications in this area (Ballard 2012; Farag 2006; Jildenstål 2011; Santarpino 2011; Wong 2002), hence this systematic review is pertinent at this time.
Objectives
To assess whether the use of processed EEG or auditory evoked potential (AEP) indices (bispectral index (BIS), narcotrend index, cerebral state index, state entropy and response entropy, patient state index, index of consciousness, A‐line autoregressive index, and auditory evoked potentials (AEP index)) as guides to anaesthetic delivery can reduce the risk of POD and POCD in non‐cardiac surgical or non‐neurosurgical adult patients undergoing general anaesthesia compared with standard practice where only clinical signs are used.
Methods
Criteria for considering studies for this review
Types of studies
We searched for all randomized or quasi‐randomized controlled trials, irrespective of language and publication status. The studies that we considered as 'quasi‐randomized' included studies where the allocation was performed on the basis of a pseudo‐random sequence such as odd or even hospital number or date of birth, and alternation.
We searched for all randomized or quasi‐randomized controlled trials with two or more arms and at least two arms compared any method using processed EEG or AEP indices against a control group
We also looked for cluster randomized controlled trials.
We excluded non‐randomized studies.
Types of participants
We included studies of adults aged 18 years or over undergoing general anaesthesia for non‐cardiac or non‐neurosurgical elective operations.
Types of interventions
We included trials comparing any method using processed EEG or AEP indices (entropy, BIS, AEP etc.) against a control group where clinical signs were monitored for this purpose.
Types of outcome measures
Primary outcomes
Occurrence of postoperative delirium (POD) as assessed by any method such as the Mini‐Mental State Examination (MMSE) and Confusion Assessment Method (CAM) in the first week after operation.
Occurrence of postoperative cognitive dysfunction (POCD) at one week (immediate), 12 weeks or three months (intermediate) and 52 weeks or over one year (long term) as determined by any method of assessing POCD including a battery of neuropsychological tests according to the criteria described by the ISPOCD group (Moller 1998).
Secondary outcomes
All‐cause mortality
Postoperative any complications
Postoperative length of stay
Other consequences of cognitive disturbances such as dementia (including Alzheimer's disease (AD)), which was described as deterioration in memory, thinking, behaviour and the ability to perform daily activities
Occurrence of intraoperative awareness, which was defined as the unexpected and explicit recall by patients of events occurring during anaesthesia
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4) in the Cochrane Library (searched 28 March 2017); MEDLINE OvidSP (1990 to 28 March 2017); and Embase OvidSP (1990 to 28 March 2017).
We performed a further search in February 2018. This result has been added to 'Studies awaiting classification' and will be incorporated into the review at the next update.
We restricted our searches to 1990 onwards because processed EEG was not used earlier than 1990.
We identified randomized controlled trials (RCTs) using search strategies based on the search terms modified from the search strategies in a previous systematic review ( Punjasawadwong 2014) as shown in Appendix 2 (CENTRAL), Appendix 3 (MEDLINE Silver Platter) and Appendix 4 (Embase Silver Platter).
Searching other resources
We searched the reference lists of retrieved trial reports and review articles for additional studies.
We reviewed PubMed's 'related articles' feature for all eligible trials and reviews.
We reviewed trial registry databases, such as ClinicalTrials.gov and other national or international clinical trials registry platforms, up to March 2017, to identify trials still being carried out.
We communicated with experts in this field in order to identify unpublished or ongoing research.
Data collection and analysis
Selection of studies
Two authors (YP, WC) independently examined all studies to identify which were to be included in the review. Furthermore, we identified and excluded any duplicate studies. We independently recorded the reason for exclusion of any trial.
We resolved disagreements by a consensus meeting between three authors (YP, WC, SP).
Data extraction and management
Three authors (YP, WC, PP) independently extracted and collected data on a paper data extraction form (see Appendix 5). We dealt with any discrepancies in the data that were extracted by discussion. We resolved disagreements by a consensus meeting between the three authors (YP, WC, PP).
We collected details regarding the study method, country of investigation, number of participants, demographic characteristics, treatment groups, types of surgery, details of anaesthesia management, experience of the anaesthesiologists, types of processed EEG or AEP monitoring, the depth of anaesthesia as determined by the indices of the processed EEG or AEP monitoring, sponsors, psychological tests and any other relevant outcomes.
Assessment of risk of bias in included studies
Three authors (YP, WC, PP) independently examined the methodological quality of the included trials. We dealt with disagreements by a consensus meeting between the three authors (YP, WC, PP).
We assessed risk of bias separately for different domains, namely:
random sequence generation;
allocation concealment;
blinding of participants, personnel and outcome assessors;
blinding of outcome assessment;
incomplete outcome data; and
other biases such as reporting bias.
We classified the bias risk as: low risk of bias, high risk of bias, and unclear. We used the criteria and guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We displayed the results by creating a risk of bias graph and a risk of bias summary figure using Review Manager 2014 software, We present the risks of bias in the results section. We provide summary assessments of the risk of bias for each outcome within and across studies.
Measures of treatment effect
We used risk ratios (RRs) with 95% confidence intervals (CI) for all dichotomous data. If data were available, we used mean difference (MD) to demonstrate the effect measure for continuous variables having the same unit across the studies, and standardized mean difference for variables with different scales of measurement. To solve the potential methodological problems regarding the combined results from different anaesthetic depth monitors, initially we examined the results from different technologies. We combined the results if, after examination of heterogeneity, they were consistent. Otherwise we reported the results separately.
Unit of analysis issues
We analysed data based on two parallel groups in an RCT. We did not use other methods as described in the protocol for multi‐arm trials or cluster randomized trials because trials with these types of designs were not available for inclusion.
Dealing with missing data
The process for obtaining missing data from trialists was unattainable. Therefore, we performed the available case analysis and imputed the missing events by using the suggested approach in the Cochrane Handbook for Systematic Reviews of Interventions in section 16.2.2 (Higgins 2011), for the best‐ and worst‐case scenario assumptions as follows.
For the best‐case scenario, we imputed the missing events of any POD or POCD outcomes in the processed EEG group as the lower limit of confidence interval for the group event rate; and we imputed the missing events in the clinical signs group as the upper limit of confidence interval for the group event rate. We used the reverse direction for the worst‐case scenario; (see Appendix 6).
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a meaningful summary. We explored the clinical heterogeneity across studies based on patient characteristics such as age and sex, type of operation and type of anaesthetic. In addition, we looked for diversity across studies regarding methods of monitoring depth of anaesthesia in the intervention group, the levels of depth of anaesthesia in both the intervention and control groups, and methods of assessing POD or POCD. Statistically, we tested for heterogeneity with the Chi² test and by calculating the I² statistic. We considered heterogeneity to be problematic when the I² value was 50% or more and carefully considered the data before reporting any pooled results (Higgins 2011). If we had detected substantial heterogeneity, we would have explored possible explanations in subgroup analyses.
Assessment of reporting biases
We assessed the included studies to determine whether there was a tendency for reporting bias based on the direction of the results by examining whether all results were reported, irrespective of whether they are positive or negative.
We planned to create a funnel plot if more than 10 studies had been included, to determine the small‐studies' effect, including publication bias and other sources of bias.
Data synthesis
If considered appropriate, we pooled the results of comparable groups of trials (Review Manager 2014). Generally we used the fixed‐effect model and 95% CIs for those outcomes with unimportant heterogeneity (I² < 50%). We considered using the random‐effects model, especially where there was unexplained heterogeneity as assessed using the I² statistic. If there had been substantial heterogeneity (I² = 75% to 100%), we would have checked that the data entry was correct for the studies involved. If so, we considered using a random‐effects model if the 95% CIs were generally overlapping. If not, we undertook sensitivity and subgroup analyses to consider whether pooling of data was appropriate. For those outcomes with moderate heterogeneity (I² = 30% to 60%) we considered using either a random‐effects model or a fixed‐effect model depending on the degree of overlap of the 95% CIs. If meta‐analysis could not be undertaken, for example because of incompatible or incomplete data from comparable studies, we used systematic approaches to report the results of the included studies in the text and tables.
For any outcomes in a single study (such as POCD at 52 weeks), we used a standard non‐meta‐analytic software, i.e. Stata software(STATA 10.1), to calculate RR and its 95% CI.
Subgroup analysis and investigation of heterogeneity
We analysed the occurrence of POCD in three periods after the surgery: early (at seven days), intermediate (at three months) and long term (over one year).
However, we did not perform the subgroup analyses as mentioned in the protocol (Punjasawadwong 2014a), because the number of included studies was too small to be categorized. Furthermore, we found no significance of heterogeneity across included studies in the meta‐analysis.
Sensitivity analysis
We determined the effect of methodological quality on the results by performing sensitivity analyses excluding trials at high risk of bias, such as quasi‐randomized trials. We planned sensitivity analyses to assess the effect of any missing data using best‐case versus worst‐case scenarios for missing data (best case: the best possible outcome in a given situation; worst case: the worst possible outcome in a given situation), and sensitivity analysis based on the statistical models used for combining the effect sizes across the studies.
'Summary of findings' table and GRADE
We constructed a 'Summary of findings' (SoF) table using the GRADEpro software GRADEpro GDT, to compare the effect of using processed EEG indices versus clinical signs to guide doses of anaesthetics on the incidences of POD, POCD at one week, 12 weeks or three months, and 52 weeks or over one year; all‐cause mortality; postoperative any complications; and postoperative length of stay (Table 1).
We used the GRADE approach to appraise the quality of a body of evidence regarding the study risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias (Guyatt 2008).
Results
Description of studies
Results of the search
We identified 5609 possible studies from the initial search. From those 5609 possible studies we identified 28 potentially relevant studies and retrieved them for further assessment. We excluded 16 studies for the reasons explained in the table Characteristics of excluded studies. As a result, 12 studies met the inclusion criteria and five of those studies were ongoing studies. One study report from an updated search in February 2018 was added to Studies awaiting classification. Finally, we included six studies in the review (see Figure 1).
1.
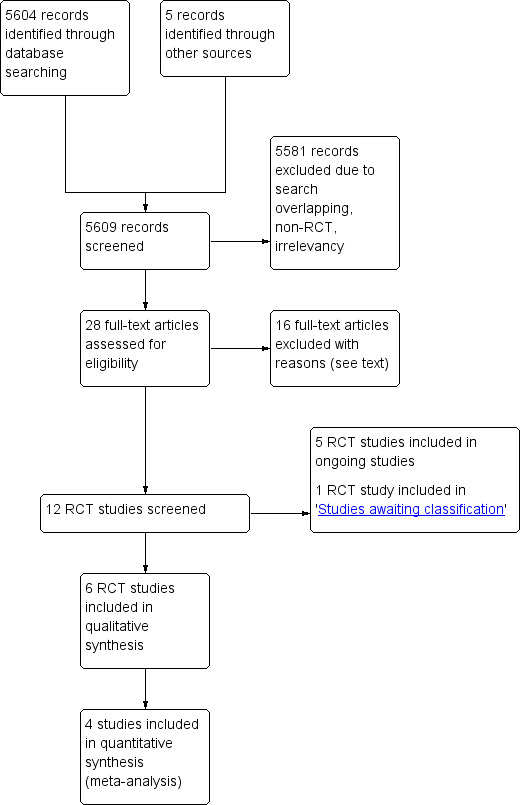
Study flow diagram.
Included studies
We included six studies which fulfilled the inclusion criteria by comparing the use of processed EEG indices with clinical signs (CS group) in guiding doses of currently used anaesthetics (propofol, desflurane, sevoflurane or isoflurane) in this review (Ballard 2012; Chan 2013; Jildenstål 2011; Punjasawadwong 2016; Radtke 2013; Wong 2002). See the table Characteristics of included studies for further details. Five of them were published in English. Only one was not published but provided full reported text in Thai (Punjasawadwong 2016).
Type of study, setting, and number of participants
All of the six included studies were RCTs conducted in University Hospitals in Canada (Wong 2002); Germany (Radtke 2013); Hong Kong (Chan 2013); Sweden (Jildenstål 2011); Thailand (Punjasawadwong 2016); and the UK (Ballard 2012). The total number of participants included in the six studies was 2929.
We did not find relevant studies with multi‐arm trials or cluster randomized controlled trials.
Intervention
Five of the six included studies used BIS as a guide to deliver anaesthetics (Ballard 2012; Chan 2013; Punjasawadwong 2016; Radtke 2013; Wong 2002). Only one study used an autoregressive AEP index (AAI) to guide dosage of an anaesthetic (Jildenstål 2011).
Participants
All of the six included studies were conducted in either male or female participants undergoing surgery, aged 60 years or above and American Society of Anesthesiologists Physical Status (ASA PS) I to III or IV. We did not find any trials conducted in participants aged younger than 60 years.
Type of surgery
Four studies were conducted in elective surgical procedures (Ballard 2012; Chan 2013; Punjasawadwong 2016; Radtke 2013), including interventions in general (Punjasawadwong 2016; Radtke 2013); abdominal (Ballard 2012; Punjasawadwong 2016; Radtke 2013); thoracic (Radtke 2013); vascular (Radtke 2013); orthopaedic (Ballard 2012; Punjasawadwong 2016; Radtke 2013); otorhinolaryngological, oral and maxillofacial (Punjasawadwong 2016; Radtke 2013); gynaecological (Punjasawadwong 2016; Radtke 2013); and urologic (Radtke 2013). These surgeries were expected to last at least one hour (Radtke 2013), one and a half hours (Ballard 2012; Punjasawadwong 2016), or two hours (Chan 2013).
One study focused on only orthopaedic surgery, i.e. knee or hip replacement surgery which lasted about one to two hours (Wong 2002).
Only one study was conducted in eye surgery, including anterior or posterior segment ophthalmic surgery (Jildenstål 2011).
Premedication
Midazolam was used for premedication in two studies (Jildenstål 2011; Radtke 2013).
One study did not give any premedication drug (Wong 2002).
The other three studies did not mention regarding premedication (Ballard 2012; Chan 2013; Punjasawadwong 2016).
Anaesthesia
Anaesthesia was induced with thiopentone (Radtke 2013), propofol (Ballard 2012; Chan 2013; Jildenstål 2011; Punjasawadwong 2016; Radtke 2013; Wong 2002), or etomidate (Radtke 2013); and maintained with intravenous propofol (Radtke 2013), or volatile anaesthetics including desflurane (Jildenstål 2011; Radtke 2013), isoflurane (Ballard 2012; Radtke 2013; Wong 2002), or sevoflurane (Punjasawadwong 2016; Radtke 2013), together with an opioid (fentanyl or remifentanil) and a muscle relaxant. Despite using volatile anaesthetics in 90% of participants, Chan 2013 did not specify the volatile anaesthetics used.
Two studies mentioned the experience of the anaesthesia providers: about four years in Radtke 2013, and greater than five years in Wong 2002.
Detection of POD and POCD
Three studies contributed data to POD (Chan 2013; Punjasawadwong 2016; Radtke 2013). Chan and colleagues used the Confusion Assessment Method criteria (CAM, Inouye 1990), to assess delirium on the morning after surgery (Chan 2013). Punjasawadwong and colleagues used the Confusion Assessment Method algorithm (CAM Algorithms, Wongpakaran 2011), and the Confusion Assessment Method for Intensive Care Units (CAM‐ICU, Pipanmekaporn 2014), to assess delirium at 2 hours, 24 hours, 48 hours, and 72 hours after surgery (Punjasawadwong 2016). Radtke and colleagues assessed the delirium twice daily (morning and night) using criteria based on the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV, American Psychiatric Association 1994), (Radtke 2013).
Three studies contributed data to POCD which was detected by using the Mini‐Mental State Exam (MMSE, (Folstein 1975)), and a battery of neuropsychological tests. They defined POCD as a decline in the POCD Z score of greater than 1.96 SD in at least two domains (Ballard 2012; Chan 2013; Radtke 2013).
One study used the Mini‐Mental Test (MMT, Rakusan 2006), and the Cognitive Failure Questionnaire (CFQ, Broadbent 1982), to detect POCD; and defined POCD as a value of MMT below 25 at day one, and below 16 at week one and week four (Jildenstål 2011).
Another study conducted the MMSE, Trieger dot test (Letourneau 1983), and Digit Symbol Substitution Test (DSST, Lichtenberger 2009), and obtained symptoms reported from nurses, family members, research assistant, or participant to diagnose POCD (Wong 2002).
Financial support
Four of the included studies were supported in part by a manufacturing company (Ballard 2012; Jildenstål 2011; Radtke 2013; Wong 2002).
One study was supported by National Research Council of Thailand (NRCT) and the faculty research fund of the Faculty of Medicine, Chiang Mai University (Punjasawadwong 2016).
Excluded studies
We excluded 16 studies because they did not meet the inclusion criteria (Farag 2006; Fernandes 2007; McDonagh 2012; Meineke 2014; Ovezov 2012; Plaschke 2010; Santarpino 2011; Seo 2014; Short 2015; Shu 2015; Soehle 2015; Steinmetz 2010; Trafidło 2015; Whitlock 2014; Zhang 2015; Zywiel 2014). For detailed reasons for their exclusion see the table Characteristics of excluded studies.
Ongoing studies
There are five ongoing RCT studies (NCT02382445; NCT02604459 ; NCT02692300; NCT02698982; NCT02841423). See Characteristics of ongoing studies for more details.
Studies awaiting classification
There is one study awaiting classification (Goyal 2017). See Characteristics of studies awaiting classification for more details.
Risk of bias in included studies
Figure 2 and Figure 3 summarize the risks of bias, which have been described in the 'Risk of bias' table for each study.
2.
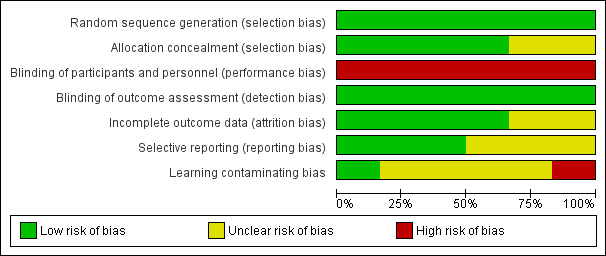
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
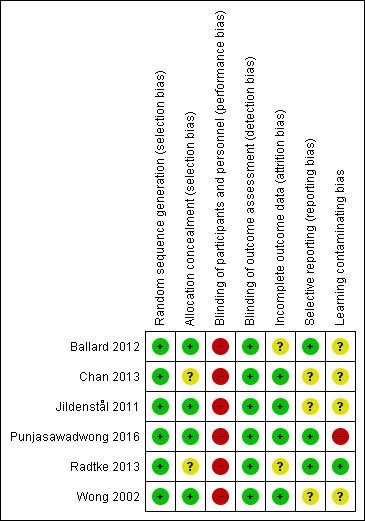
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Punjasawadwong 2016 was authored by an author of this Cochrane Review. Two independent authors of this Cochrane Review (WC, PP), who were not involved in Punjasawadwong 2016, assessed the risk of biases and performed data extraction in this trial.
Random sequence generation (selection bias)
We classified all included studies as having a low risk of bias with regard to the randomization method used.
Allocation
We classified allocation concealment as 'low risk of bias' in four of the included studies (Ballard 2012; Jildenstål 2011; Punjasawadwong 2016; Wong 2002). The remaining two studies did not mention allocation concealment: we therefore classified them as 'unclear risk of bias' (Chan 2013; Radtke 2013).
Blinding
Performance bias
In all six included studies, the anaesthesiologists could not be blinded to the assigned groups. We therefore graded them as 'high risk of performance bias'.
This could introduce a 'learning contamination' bias, which involves changing clinical practice in the parallel control or unmonitored group by using the information from the BIS group (Roizen 1994). However, one study tried to minimize the 'learning contamination' bias by assigning anaesthesiologists who had never used BIS to provide anaesthesia in the control or non‐BIS group (Radtke 2013).
Detection bias
All of the six included studies mentioned blinding the outcome assessors to the assigned groups. We therefore graded them 'low risk of bias'
Incomplete outcome data
Regarding bias relating to incomplete outcome data, we classified four studies as 'low risk of bias' (Chan 2013; Jildenstål 2011; Punjasawadwong 2016; Wong 2002). The remaining two studies were classified as 'unclear' due to uncertainty about how missing outcome data could affect the observed effect size (Ballard 2012; Radtke 2013).
Selective reporting
We classified three studies as being 'low risk of bias' from selective reporting because they reported all outcomes as indicated in their trial registrations (Ballard 2012; Punjasawadwong 2016; Radtke 2013). We did not find any information regarding the trial registration in the other three studies, therefore we classified them as being 'unclear risk of bias' (Chan 2013; Jildenstål 2011; Wong 2002).
Effects of interventions
See: Table 1
See Table 1
Primary outcomes
1. Postoperative delirium (POD) according to the Confusion Assessment Method (CAM) criteria within seven days
Three trials contributed data to this outcome (Chan 2013; Punjasawadwong 2016; Radtke 2013). These trials included 2197 participants in the analysis, which accounted for 94% of the total 2338 enrolled participants. Radtke 2013 contributed 52.7% of the weight in this analysis. There was a lower proportion of participants with POD among those who were monitored with a processed EEG (166/1095; 15.2%) than with the use of clinical signs (235/1102; 21.3%) (RR 0.71, 95% CI 0.59 to 0.85; I² = 0%; 2197 participants; Analysis 1.1; Figure 4). The overall risk difference (RD) was −0.06 (95% CI −0.09 to −0.03) and the number needed to treat for an additional beneficial outcome (NNTB) was 17 (95% CI 11 to 34).
1.1. Analysis.
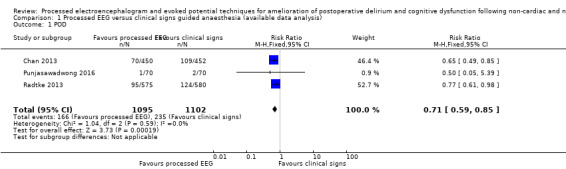
Comparison 1 Processed EEG versus clinical signs guided anaesthesia (available data analysis), Outcome 1 POD.
4.
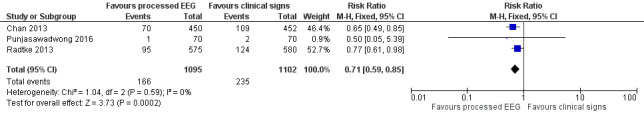
Forest plot of comparison: 1 Processed EEG versus clinical signs guided anaesthesia, outcome: 1.1 POD.
The power of analysis for this outcome was 96%.
Data were missing in 75 out of 1100 participants (6.8%) in the processed EEG group and 66 out of 1098 participants (6.0%) in the clinical signs group. Sensitivity analysis under the best‐ and worst‐case approaches for this missing data suggested similar results to that found in the available data analysis. Details are presented in Analysis 2.1
2.1. Analysis.
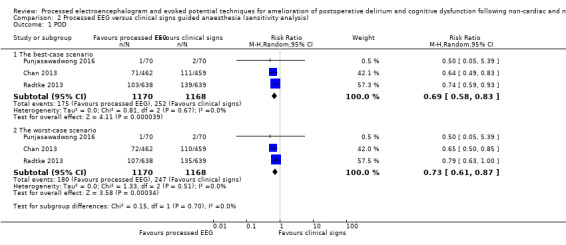
Comparison 2 Processed EEG versus clinical signs guided anaesthesia (sensitivity analysis), Outcome 1 POD.
We downgraded the evidence for this outcome to moderate quality because of biases due to unblinded anaesthesia providers and incomplete outcome data across the three studies.
2. Postoperative cognitive dysfunction (POCD) according to the International Study of Postoperative Cognitive Dysfunction (ISPOCD) criteria
Five trials reported this outcome (Ballard 2012; Chan 2013; Jildenstål 2011; Radtke 2013; Wong 2002); but two of these studies did not use compatible definitions and could not be included in our pooled analysis (Jildenstål 2011; Wong 2002). The results of these two trials are reported separately below.
The remaining three trials contributed to this analysis (Ballard 2012; Chan 2013; Radtke 2013). These trials enrolled 2270 participants with 1134 (50.0%) of the participants allocated to use a processed EEG to monitor anaesthetic depth and 1136 (50.05%) to clinical signs. We performed the analyses for this outcome at three follow‐up periods after surgery—that is POCD at one week, POCD at 12 weeks and POCD at 52 weeks—as follows.
Risk of postoperative cognitive dysfunction at one week
Three trials contributed data to this outcome (Ballard 2012; Chan 2013; Radtke 2013). We included the available data from 1989 participants (87.6% of the total enrolled participants) for this analysis. Two of the studies contributed more than 96% of the weight to the analysis (Chan 2013; Radtke 2013). There was a lower proportion of participants with POCD at one week among those who were monitored with a processed EEG (154/979; 15.7%) than with the use of clinical signs (190/1010; 18.8%); however the risk reduction is not important (RR 0.84, 95% CI 0.69 to 1.02; I² = 33%; 1989 participants; Analysis 1.2; Figure 5). The overall risk difference (RD) was −0.03 (95% CI −0.06 to 0.00) and NNTB was 33 (95% CI −15.6 to 422.5).
1.2. Analysis.
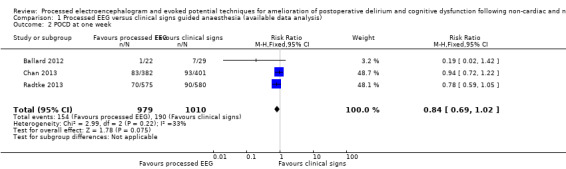
Comparison 1 Processed EEG versus clinical signs guided anaesthesia (available data analysis), Outcome 2 POCD at one week.
5.
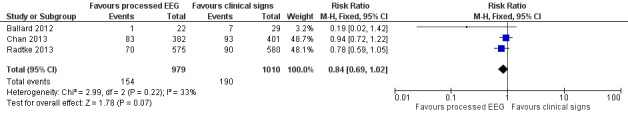
Forest plot of comparison: 1 Processed EEG versus clinical signs guided anaesthesia, outcome: 1.2 POCD at one week.
The calculated power for this analysis was 44%.
Among 2270 enrolled participants, data were missing in 155 out of 1134 participants (13.66%) in the processed EEG group and 126 out of 1136 participants (11.09%) in the clinical signs group. Sensitivity analysis under the best‐ and worst‐case approaches for this missing data suggested similar results to that found in the available data analysis. Details are presented in Analysis 2.2.
2.2. Analysis.
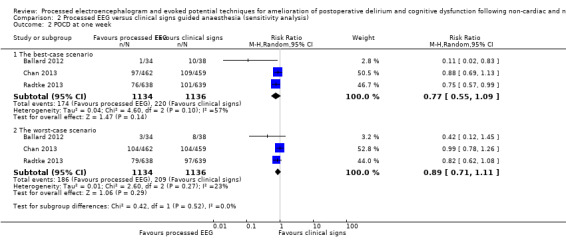
Comparison 2 Processed EEG versus clinical signs guided anaesthesia (sensitivity analysis), Outcome 2 POCD at one week.
We downgraded the evidence for this outcome to moderate quality because of biases due to unblinded anaesthesia providers and incomplete outcome data across the three studies.
Risk of postoperative cognitive dysfunction at 12 weeks
We included available data from 2051 participants (90.4%) of the total enrolled participants who completed the protocol for this analysis. Two studies contributed more than 96% of the weight to the analysis (Chan 2013; Radtke 2013). There was a lower proportion of participants with POCD among those who were monitored with a processed EEG (65/1014; 6.4%) than with the use of clinical signs (94/1037; 9.1%). The combined result of the three studies, using a fixed‐effect model, demonstrated the effect of the processed EEG monitoring on a reduction in the rate of POCD at 12 weeks after surgery (RR 0.71, 95% CI 0.53 to 0.96; I² = 0; 2051 participants; Analysis 1.3; Figure 6). The overall risk difference (RD) was −0.03 (95% CI −0.05 to −0.00) and the NNTB was 38 (95% CI 21 to 289).
1.3. Analysis.

Comparison 1 Processed EEG versus clinical signs guided anaesthesia (available data analysis), Outcome 3 POCD at 12 weeks.
6.
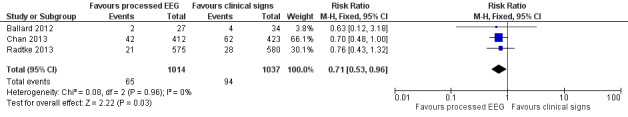
Forest plot of comparison: 1 Processed EEG versus clinical signs guided anaesthesia, outcome: 1.3 POCD at 12 weeks.
The calculated power for analysis for this outcome was 62%.
Among 2270 enrolled participants, data were missing in 120 out of 1134 participants (10.6%) in the processed EEG group and 99 out of 1136 participants (8.7%) in the clinical signs group. Sensitivity analysis under the best‐ and worst‐case approaches for this missing data suggested similar results to that found in the available data analysis. Details are presented in Analysis 2.3.
2.3. Analysis.
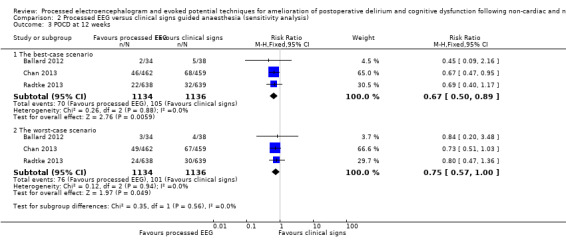
Comparison 2 Processed EEG versus clinical signs guided anaesthesia (sensitivity analysis), Outcome 3 POCD at 12 weeks.
We downgraded the evidence for this outcome to moderate quality because of biases due to unblinded anaesthesia providers and incomplete outcome data across the three studies.
Risk of postoperative cognitive dysfunction (POCD) at 52 weeks
There was only one study reporting the occurrence of POCD at 52 weeks after surgery (Ballard 2012). One out of 27 (4%) in the processed EEG group and four out of 32 (13%) participants in the clinical signs group developed POCD. The analysis by using a standard non‐meta‐analysis software failed to reliably demonstrate a protective effect of the processed EEG monitoring on POCD at 52 weeks after surgery, with RR of 0.30 (95% CI 0.05 to 1.8; 59 participants).
We downgraded the evidence for this outcome to very low quality because of:
biases due to unblinded anaesthesia providers and incomplete outcome data); and
serious imprecision (very wide confidence interval from only one study with small sample size).
POCD in studies not included in the meta‐analysis
We did not include the results of two studies in the above meta‐analysis because the criteria used to detect POCD in their studies were different from those using the definition of a decline in POCD Z score from a baseline greater than 1.96 SDs (Jildenstål 2011; Wong 2002; see table 'Characteristics of included studies').
Jildenstål 2011 used a Mini‐Mental Test (MMT) score. An MMT value below 25 was regarded as POCD at day 1 and a value below 16 was regarded as POCD at week one and week four after surgery. They found POCD at day one in 16 out of 226 participants in the control group and two out of 225 participants in the study group. The proportion was lower in the study group with an RR of 0.13 (95% CI 0.03 to 0.54; 451 participants). No participants in either group developed POCD at weeks one and four after surgery.
Wong 2002 used clinical symptoms reported from nurses, family members, research assistants or participants to diagnose POCD and found only one participant in the control group whose clinical symptoms of POCD were recorded in the nursing notes on postoperative days two and three.
Secondary outcomes
1. All‐cause mortality
Radtke 2013 reported no important difference in proportion between the two groups regarding death at three months after operation (5.4% versus 5.3%). Jildenstål 2011 reported no death at one year after operation in both groups. No other studies reported mortality.
We downgraded the evidence for this outcome to low quality because of:
biases due to unblinded anaesthesia providers and incomplete outcome data across the three studies; and
imprecision (wide confidence interval from only one study).
2. Postoperative any complications.
Chan 2013 reported a lower proportion of postoperative 'any complications' in the processed EEG group compared to the clinical signs group (10.7% versus 20.8%) with an RR of 0.51 (95% CI 0.37 to 0.71; 902 participants).
We downgraded the evidence for this outcome to moderate quality because of biases due to unblinded anaesthesia providers and incomplete outcome data across the three studies.
3. Postoperative length of stay
Radtke 2013 reported no important difference in length of hospital stay between the two groups (15.7 ± 16.9 versus 15.9 ± 14.6 days) with the mean difference of −0.2 (95% CI −2.02 to 1.62; participants 1155). No other studies reported on any other morbidity outcomes.
We downgraded the evidence for this outcome to low quality because of:
biases due to unblinded anaesthesia providers and incomplete outcome data across the three studies; and
imprecision (wide confidence interval from only one study).
4. Other consequences of cognitive disturbances
None of the five included studies reported other potential consequences of cognitive disturbances such as dementia and Alzheimer's disease.
5. Intraoperative recall awareness
None of the five included studies reported the incidence of intraoperative recall or awareness.
Discussion
Summary of main results
We have found that anaesthesia guided by processed EEG indices could reduce the risk of postoperative delirium (POD) within seven days after surgery with risk ratio (RR) of 0.71 (95% CI 0.59 to 0.85; I² statistic = 0%; number needed to treat for an additional beneficial outcome (NNTB) of 17 (95% CI 11 to 34); fixed‐effect model; three trials; 2197 participants; moderate quality of evidence). We found that use of the processed EEG indices could reduce the risk of severe postoperative cognitive dysfunction (POCD) at 12 weeks after surgery with RR of 0.71 (95% CI 0.53 to 0.96; I² statistic = 0%; NNTB of 38 (95% CI 21 to 289); fixed‐effect model; 3 trials; 2051 participants; moderate quality of evidence). However, we have not been able to confirm any protective effect of the processed EEG effect on POCD at one week with RR of 0.84 (95% CI 0.69 to 1.02; I² statistic = 45%; fixed‐effect model; three trials; 1989 participants; moderate quality of evidence), and one year after operation with RR of 0.30 (95% CI 0.05 to 1.8; 1 trial; 59 participants; very low quality of evidence). Furthermore, we could not demonstrate any protective effect of the processed EEG on all‐cause mortality from a single study with RR of 1.01 (95% CI 0.62 to 1.64; one trial; 1155 participants; low quality of evidence). We found one study which suggested a protective effect of the processed EEG on postoperative complications with RR of 0.51 (95% CI 0.37 to 0.71; 902 participants; moderate quality of evidence). A result from a single study could not show the impact of the processed EEG on the postoperative length of stay with the mean difference of −0.2 days (95% CI −2.02 to 1.62; 1155 participants; low quality of evidence). (See Table 1).
Overall completeness and applicability of evidence
Based on our protocol (Punjasawadwong 2014a), we intended to find evidence supporting or refuting the use of the processed EEG or evoked potential techniques to optimize doses of anaesthetics in order to prevent POD and POCD in non‐cardiac surgical or non‐neurosurgical adult patients undergoing general anaesthesia.
There was insufficient evidence available to be conclusive about the efficacy and safety of these devices for reducing POCD at one week or one year although the evidence was more complete for reducing POD and POCD at three months. One explanation could be that there were more missing data due to either refusal to be assessed or unfitness for neuropsychological tests for POCD at one week after surgery than the assessment at three months. The other explanation is that there was only one small study contributing data from POCD assessment at one year. Furthermore, there were few studies concerning complications and cost.
We only found evidence supporting the use of the processed EEG 'Bispectral Index (BIS)' in patients aged 60 years or over. It is likely that all of the studies included this age group specifically because they were more at risk of POD or POCD than groups of younger adult patients. Postoperative confusion in older patients, in a form of either delirium (POD) or postoperative cognition, has become increasingly recognized as clinically important because of an increase in the population age. This review could provide evidence supporting use of this device for this specific group of patients. However, the evidence for supporting its use in surgical patients aged below 60 years is still lacking.
Furthermore, despite providing some evidence supporting the advantages of processed EEG‐guided anaesthesia in terms of reduced risk of POD or POCD, few included studies reported any effects on other secondary outcomes such as dementia, awareness, morbidity and mortality. Moreover, most studies did not report other adverse effects of too light or too deep depth of anaesthesia such as unacceptably high blood pressure or too low blood pressure.
There are five ongoing studies that need to be followed to obtain more complete evidence addressing the question posed in our protocol (Punjasawadwong 2014a).
Quality of the evidence
In this review we assessed the quality of evidence using the GRADE approach. Although all of the included studies were RCTs, we downgraded the evidence for each outcome by at least one level. We downgraded quality of the evidence one level (to moderate quality) for the outcomes POD, POCD at week one and POCD at 12 weeks because of concern about biases due to unblinded anaesthesia providers and incomplete outcome data across the three studies. Unblinded anaesthesia providers could lead to a "learning contamination bias" as described in Roizen 1994. A learning contamination bias could reduce the point estimate of an intervention's effectiveness because unblinded anaesthesia providers might learn about the dose of anaesthetics guided by processed EEG or evoked potential indices in the intervention group and apply it in the control group.
The incomplete outcome data could affect the power of analysis, determined as 96% for POD, 44% for POCD at one week and 62% for POCD at 12 weeks.
We further downgraded the quality of the evidence by two levels (to low quality) for the outcomes 'all‐cause mortality' and 'length of postoperative stay'. This is because we were concerned about biases due to both unblinded anaesthesia providers and to imprecision of the result with wide confidence intervals.
We found only one study reporting the POCD at one year after surgery and downgraded the quality of the evidence for this outcome by three levels (to very low quality) for potential bias due to unblinded assessment, imprecision and incompleteness of the data (see Table 1).
Potential biases in the review process
We followed the search strategies as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Five out of the six included studies were published in medical indexed journals. However, we did not perform a funnel plot because of the small number of studies for each outcome. Therefore, we were not able to assess any publication bias in this review.
Only one out of the six included studies contributed unpublished data for POD. It was a small study, in which one author of this review was involved (Punjasawadwong 2016). We asked the other two authors of the Cochrane Review (WC, PP), who had not been involved in the included study, to assess the risk of biases and perform data extraction. Moreover, we performed sensitivity analysis by excluding and including Punjasawadwong 2016 in the meta‐analysis and found no effect on the conclusion regarding the effect of BIS on POD.
Four of the included studies were sponsored in part by manufacturing companies, though they claimed that there was no involvement of the companies in designing and conducting the research (Ballard 2012; Jildenstål 2011; Radtke 2013; Wong 2002).
We attempted to conduct a comprehensive search for studies, but the fact that one study has not yet been incorporated may be a source of potential bias.
Agreements and disagreements with other studies or reviews
An earlier systematic review focused on orthopaedic surgery provided limited evidence supporting the influence of optimised anaesthetic depth on POCD or POD from either randomized controlled or non‐randomized controlled trials (Zywiel 2014), and was therefore in broad agreement with our findings. Similarly, the finding in our review is consistent with the finding in a systematic review conducted to find out interventions for preventing delirium in hospitalized non‐ICU (non‐intensive care unit) patients (Siddiqi 2016). Furthermore, our findings are in accordance with the findings in a recent published systematic review reporting a reduction of 3% in the risk of cognitive impairment at three months postoperatively and 6% reduction in the risk of postoperative delirium in patients monitored with bispectral index (Oliveira 2017).
The findings of our current review are complementary to the findings in our previous systematic review which reported the effect of BIS‐guided anaesthesia on improvement in drug delivery and recovery and reduction of intraoperative awareness (Punjasawadwong 2014).
Authors' conclusions
Implications for practice.
Our review provides moderate‐quality evidence that optimized anaesthesia guided by processed EEG indices probably reduces the risk of postoperative delirium (POD) in patients aged 60 years or over undergoing non‐cardiac surgical and non‐neurosurgical procedures. We found moderate‐quality evidence that postoperative cognitive dysfunction at three months could be reduced in these patients. The effect on POCD at one week and over one year after surgery is uncertain. There is no data available for patients under 60 years. The one study awaiting classification and five ongoing studies may alter the conclusions of the review once assessed.
Implications for research.
Further blinded randomized controlled trials are needed to elucidate strategies for the amelioration of postoperative delirium and postoperative cognitive dysfunction, and their consequences such as dementia (including Alzheimer's disease (AD)) in both non‐elderly (below 60) and elderly (60 years or over) adult patients.
What's new
| Date | Event | Description |
|---|---|---|
| 4 October 2018 | Amended | Acknowledgement section amended to include Co‐ordinating Editor |
Acknowledgements
We would like to thank Mike Bennett (content editor), Jing Xie (statistical editor), Tim Short and Jan G Jakobsson (peer reviewers), Patricia Tong (consumer referee), and Andrew Smith (Co‐ordinating Editor), for their help and editorial advice during the preparation of this systematic review.
We would also like to thank Mike Bennett (content editor), Nathan Pace (statistical editor), and Tong J Gan and Tim Short (peer reviewers) for their help and editorial advice during the preparation of the protocol for the systematic review (Punjasawadwong 2014a).
Furthermore, we would like to express many thanks to Janne Vendt, a Cochrane Information Specialist, and Chompunuch Saravudecha, a librarian of Chaing Mai University, for kindly helping with electronic searching.
Lastly, we would like to express our sincere thanks to Jane Cracknell, Managing Editor of the Cochrane Anaesthesia, Critical and Emergency Care Group, for her advice during the preparation and conducting of this systematic review.
Appendices
Appendix 1. Glossary: processed EEG indices
Bispectral index (BIS) is a dimensionless numerical scale derived from cerebral electrical activity (an electroencephalogram (EEG)) for measuring brain electrical activity. Its value is a number within a range between 0 to 100, where 0 represents 'no detectable brain electrical activity' and 100 represents 'awake state'.
Narcotrend index is a dimensionless numerical scale derived from algorithm pattern recognition of the raw electroencephalogram (EEG) for measuring the depth of anaesthesia. Its value is a number within a range between 0 to 100, where 0 represents 'electrical silence' and 100 represents 'awake state'.
Cerebral state index (CSI) is a dimensionless numerical scale passively derived from electroencephalogram for measuring cerebral electrical activity. Its value is a number between 0 and 100, where 0 represents 'electrical silence' and 100 represents 'awake state'.
State entropy (SE) and Response entropy (RE) are two dimensionless numerical scales derived from algorithm based on degree of irregularity of electroencephalogram and frontal electromyography for measuring depth of anaesthesia. The state entropy is a fast‐reacting parameter based on both EEG and FEMG signals, and is sensitive to facial muscle activation while response entropy is a stable parameter based on EEG intended to assess the hypnotic effect of anaesthetics on the brain. The RE scale ranges from 0 (no brain activity) to 100 (fully awake) and the SE scale ranges from 0 (no brain activity) to 91 (fully awake).
Patient state index (PSI) is a quantitative EEG index derived from a retrospective exploration of the multivariate changes in brain electrical activity observed from loss to return of consciousness for assessing the hypnotic effect of anaesthetics on the brain.
Index of consciousness (IoC) is a numerical value derived from EEG spectrum and symbolic dynamics through a fuzzy inference system for assessing the hypnotic effect of anaesthetics on the brain.
A‐line autoregressive index (AAI) is numerical value derived from auditory evoked potentials for assessing the hypnotic effect of anaesthetics on the brain.
Audio evoked potential ‐Index (AEP‐index) is a numerical value derived from auditory evoked potentials
Appendix 2. CENTRAL
#1 MeSH descriptor: [Electroencephalography] explode all trees #2 MeSH descriptor: [Monitoring, Physiologic] explode all trees #3 ((intraoperative monitoring) or (intraoperative near (patient* or monitoring)) or BIS or bispectral* or (bispectral near index*) or bispectral index* or electroencephalograph*):ti,ab #4 #1 or #2 or #3 #5 MeSH descriptor: [Anesthesia and Analgesia] explode all trees #6 MeSH descriptor: [Postoperative Period] explode all trees #7 (anaesth* or anesth*):ti,ab #8 #5 or #6 or #7 #9 #4 and #8
Appendix 3. MEDLINE (OvidSP) search strategy
#1 exp Electroencephalography/ or Monitoring‐Physiologic/ or ((intra?operative* or intra operative*) adj5 (monitoring or patient)).mp. or (BIS or bispectral*).ti,ab. or (electro?encephalograph* or electro encephalograph*).ti,ab. #2 Anesthesia‐and‐Analgesia/ or Anesthesia/ or exp Anesthetics‐General/ or exp Anesthesia‐General/ or Postoperative‐Period/ or an?esth*.ti,ab. #3 (randomised controlled trial.pt. or controlled clinical trial.pt.or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals.sh not (humans.sh and animals.sh)) #4 1 and 2 and 3
Appendix 4. Embase (OvidSP) search strategy
#1 exp electroencephalography/ or physiologic monitoring/ or ((intra?operative* or intra operative*) adj5 (monitoring or patient)).mp. or (BIS or bispectral*).ti,ab. or (electro?encephalograph* or electro encephalograph*).ti,ab. #2 anesthesiological procedure/ or anaesthesia/ or exp anaesthetic agent/ or exp general anaesthesia/ or an?esth*.ti,ab or postoperative period/ #3 (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animal not (human and animal)).sh. #4 1 and 2 and 3
Appendix 5. Data extraction form
Checklists for selection of study
| "Study ID" | |
| "Reviewer" | |
| "Study Title" | |
| "Source of data base" | "MEDLINE EMBASE CENTRAL Handsearch" |
| "The study is published" "Not published" "Is the topic relevant?" "Is the study randomised /quasi‐ randomized?" "Are the participant adults (> 18 years)?" |
"Yes/No Yes/NO Yes/No/Unclear Yes/No/Unclear Yes/No/Unclear" |
| "Is the surgery under general anaesthesia?" | "Yes/No/Unclear" |
| "Did the study group use processed EEG indices guiding the dose of anaesthetics?" | "Yes/No/Unclear" |
| "Did the control group use clinical signs guiding the dose of anaesthetics? | "Yes/No/Unclear" |
| "Does the study fulfil the inclusion criteria? If no, state why?" |
"Yes/No/Unclear" |
DATA EXTRACTION FORM
| Study ID | |
| Authors | |
| MEDLINE Journal ID | |
| Year of Publication | |
| Language | |
| "Type of study" | "RCT Quasi‐RCT Non‐ RCT" |
| "Comments on study design" | |
| "Does the study compare the use of processed EEG indices and the use of clinical signs (SP group) in guiding doses of anaesthetics?" "What type of the processed EEG indices ?" |
|
| "Was the assignment of subjects to treatment groups randomised?" | |
| "Was there blinding? If so, who was blinded" | "Subject ‐Blinded? Yes/No/Unclear Anaesthesiologist Blinded? Yes/No/Unclear Outcome assessor blinded? Yes/No/Unclear" |
| "Were the processes EEG monitoring and SP groups similar at the start of the trial?" | |
| "Apart from the treatment under investigation, were the groups treated equally?" | |
| "Are all relevant outcomes measured in a standard, valid and reliable way?" | |
| "What percentage of the individuals or clusters recruited into the study are included in the analysis?" | |
| "Were all the subjects analysed in the groups to which they were randomly allocated?" |
QUALITY OF CONCEALMENT OF ALLOCATION
| "Was an adequate concealment method used?" | "A = (adequate) if the allocation concealment was described as central randomizations; serially numbered; opaque; or sealed envelopes B = (uncertain) if there was no mention about the allocation concealment C = (inadequate) if the allocation concealment was not used D = the randomizations was not used" |
| PARTICIPANTS How many patients participated in the study? Overall number, and in each arm of the study. |
Total number: Number in each arm of study: Withdrawals: Yes/No/Unclear Number of withdrawals in each arm: |
| "What are the characteristics of the study population? E.g. age range, sex, and disease characteristics of the population, disease prevalence." | EEG group CS group Age Sex ASA Operation" |
| "What are the characteristics of the study setting? E.g. rural, urban, hospital inpatient or outpatient, general practice, community." | |
| "How many groups/sites are there in the study? If the study is carried out on more than one group of patients, or at more than one site, indicate how many are involved." | |
| "Are there any specific issues raised by this study? Make any general comments on the study results and their implications" | |
| "INTERVENTION: What interventions are evaluated in this study? |
Outcome
| Outcomes | Groups | |||
| Processed EEG Indices guided | Clinical signs guided | |||
| Cn | n | Cn | n | |
| POCD ( 1 week ) | ||||
| POCD ( 3 months) | ||||
| POCD ( > 12 months) | ||||
| Delirium ( 3 days ) | ||||
| Delirium ( 1 week ) | ||||
POCD = postoperative decline/dysfunction
Cn = number of event
n = total number of participants in each arm
"CONTACT WITH AUTHOR:.....................................................................
REMARKS:.............................................................................................
REVIEWER .............................................................................................
Date........................................................................................................ "
Appendix 6. Imputing outcomes for the missing data
Best cases scenario assumption: the missing events of any POD or POCD outcomes in the processed EEG group were imputed as the lower limit of confidence interval for the group event rate and the missing events in the clinical signs group were imputed as the upper limit of confidence interval for the group event rate.
| Outcomes | Study | Processed EEG | Imputed events | |||||
| Observed events |
Missing participants |
Total | Observed proportion of events | |||||
| POD | lower 95% CI | Upper 95%CI | ||||||
| Chan 2013 | 70 | 12 | 462 | 0.16 | 0.12 | 0.19 | 1 | |
| Radtke 2013 | 95 | 63 | 638 | 0.17 | 0.13 | 0.20 | 8 | |
| POCD at one week | ||||||||
| Ballard 2012 | 1 | 12 | 34 | 0.05 | ‐0.04 | 0.13 | 0 | |
| Chan 2013 | 83 | 80 | 462 | 0.22 | 0.18 | 0.26 | 14 | |
| Radtke 2013 | 70 | 63 | 638 | 0.12 | 0.10 | 0.15 | 6 | |
| POCD at 12 weeks | ||||||||
| Ballard 2012 | 2 | 7 | 34 | 0.07 | ‐0.02 | 0.17 | 0 | |
| Chan 2013 | 42 | 50 | 462 | 0.10 | 0.07 | 0.13 | 4 | |
| Radtke 2013 | 21 | 63 | 638 | 0.04 | 0.02 | 0.05 | 1 | |
| Outcomes | Study | Clinical signs | ||||||
| Observed POD |
Missing participants |
Total | Observed proportion of POD | Imputed events | ||||
| POD | lower 95% CI | Upper 95%CI | ||||||
| Chan 2013 | 109 | 7 | 459 | 0.24 | 0.20 | 0.28 | 2 | |
| Radtke 2013 | 124 | 59 | 639 | 0.21 | 0.18 | 0.25 | 15 | |
| POCD at one week | ||||||||
| Ballard 2012 | 7 | 7 | 38 | 0.23 | 0.08 | 0.37 | 3 | |
| Chan 2013 | 93 | 58 | 459 | 0.23 | 0.19 | 0.27 | 16 | |
| Radtke 2013 | 90 | 59 | 639 | 0.16 | 0.13 | 0.18 | 11 | |
| POCD at 12 weeks | ||||||||
| Ballard 2012 | 4 | 4 | 38 | 0.12 | 0.01 | 0.23 | 1 | |
| Chan 2013 | 62 | 36 | 459 | 0.15 | 0.11 | 0.18 | 6 | |
| Radtke 2013 | 28 | 59 | 639 | 0.05 | 0.03 | 0.07 | 4 | |
Worst cases scenario assumption: the missing events of any POD or POCD outcomes in the processed EEG group were imputed as the upper limit of confidence interval for the group event rate and the missing events in the clinical signs group were imputed as the lower limit of confidence interval for the group event rate.
| Outcomes | Study | Processed EEG | Imputed events | |||||
| Observed events |
Missing participants |
Total | Observed proportion of events | |||||
| POD | lower 95% CI | Upper 95%CI | ||||||
| Chan 2013 | 70 | 12 | 462 | 0.16 | 0.12 | 0.19 | 2 | |
| Radtke 2013 | 95 | 63 | 638 | 0.17 | 0.13 | 0.20 | 12 | |
| POCD at one week | ||||||||
| Ballard 2012 | 1 | 12 | 34 | 0.05 | ‐0.04 | 0.13 | 2 | |
| Chan 2013 | 83 | 80 | 462 | 0.22 | 0.18 | 0.26 | 21 | |
| Radtke 2013 | 70 | 63 | 638 | 0.12 | 0.10 | 0.15 | 9 | |
| POCD at 12 weeks | ||||||||
| Ballard 2012 | 2 | 7 | 34 | 0.07 | ‐0.02 | 0.17 | 1 | |
| Chan 2013 | 42 | 50 | 462 | 0.10 | 0.07 | 0.13 | 7 | |
| Radtke 2013 | 21 | 63 | 638 | 0.04 | 0.02 | 0.05 | 3 | |
| Outcomes | Study | Clinical signs | ||||||
| Observed POD |
Missing participants |
Total | Observed proportion of POD | Imputed events | ||||
| POD | lower 95% CI | Upper 95%CI | ||||||
| Chan 2013 | 109 | 7 | 459 | 0.24 | 0.20 | 0.28 | 1 | |
| Radtke 2013 | 124 | 59 | 639 | 0.21 | 0.18 | 0.25 | 11 | |
| POCD at one week | ||||||||
| Ballard 2012 | 7 | 7 | 38 | 0.23 | 0.08 | 0.37 | 1 | |
| Chan 2013 | 93 | 58 | 459 | 0.23 | 0.19 | 0.27 | 11 | |
| Radtke 2013 | 90 | 59 | 639 | 0.16 | 0.13 | 0.18 | 7 | |
| POCD at 12 weeks | ||||||||
| Ballard 2012 | 4 | 4 | 38 | 0.12 | 0.01 | 0.23 | 0 | |
| Chan 2013 | 62 | 36 | 459 | 0.15 | 0.11 | 0.18 | 5 | |
| Radtke 2013 | 28 | 59 | 639 | 0.05 | 0.03 | 0.07 | 2 | |
EEG:electroencephalogram; POCD: postoperative cognitive dysfunction; POD: postoperative delirium
Data and analyses
Comparison 1. Processed EEG versus clinical signs guided anaesthesia (available data analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 POD | 3 | 2197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.59, 0.85] |
| 2 POCD at one week | 3 | 1989 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.69, 1.02] |
| 3 POCD at 12 weeks | 3 | 2051 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.53, 0.96] |
Comparison 2. Processed EEG versus clinical signs guided anaesthesia (sensitivity analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 POD | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 The best‐case scenario | 3 | 2338 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.58, 0.83] |
| 1.2 The worst‐case scenario | 3 | 2338 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.61, 0.87] |
| 2 POCD at one week | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 The best‐case scenario | 3 | 2270 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.55, 1.09] |
| 2.2 The worst‐case scenario | 3 | 2270 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.71, 1.11] |
| 3 POCD at 12 weeks | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 The best‐case scenario | 3 | 2270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.89] |
| 3.2 The worst‐case scenario | 3 | 2270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.57, 1.00] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ballard 2012.
| Methods | RCT, multicentre Country: United Kingdom (UK) Setting: two teaching hospitals Conducting dates: June 2007 to January 2010 |
|
| Participants | Number of participants (N) = 72 Age, years (mean ± S.D.): 76.69 ± 7.4 in the BIS group, 75.16 ± 6.51 in the Control group Sex: female, n (%): 24 (71%) in the BIS group, 26 (68%) in the Control group ASA PS class: ASA III or less MMSE score: equal to or greater than 23 English literacy Surgery Type: elective surgery Orthopaedic surgery, n (%): 28 (82%) in the BIS group, 32 (84%) in the Control group Major abdominal surgery, n (%): 6 (18%) in the BIS group, 6 (16%) in the Control group Duration of surgery: 108 to 150 min in the BIS group, 110 to 159 min in the Control group Anaesthesia Premedication: not stated Induction: propofol Maintenance: isoflurane Exclusion criteria Unable to complete the outcome measures Alzheimer’s disease or other dementia Surgical procedures under regional anaesthesia, or delirium at one week post surgery |
|
| Interventions |
|
|
| Outcomes | Postoperative cognitive decline was classified at three levels — mild, moderate and severe — based on the change of ISPOCD Z score of individual participants from the baseline, as follows. Mild POCD was defined as a decline in performance in at least one of the seven cognitive domains by greater than one standard deviation (SD); Moderate POCD required an additional decline of at least 1.5 SD in an additional domain; and Severe POCD was defined as a decline of ISPOCD Z score greater than 1.96 SD in at least two domains. Note: the following seven cognitive measures were used to analyse the level of cognitive decline: MMSE, simple reaction time, digit vigilance accuracy, digit vigilance reaction time, choice reaction time accuracy, choice reaction time and cognitive reaction time. |
|
| Notes | The study was supported by the BUPA foundation. One of the authors received honoraria and expenses for meetings organized by Covidien Inc, manufacturers of the BIS and Invos monitors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomised according to randomizations lists, generated by the study statistician in the statistical program package R, which were stratified by age group (65 to 70, 70 to 75 and over 75)." |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes containing the randomizations codes were delivered to operating theatres, and an envelope selected randomly by the anaesthetist." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the nested RCT trial was double‐blinded; patients and researchers collecting outcome data were blind to treatment allocation. Only the anaesthetist delivering the intervention was aware of the treatment condition." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the nested RCT trial was double‐blinded; patients and researchers collecting outcome data were blind to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome assessments in the BIS versus (vs.) Control groups as follows: 35.29% vs. 23.68% at week one, 20.58% vs. 10.52% at week twelve, and 17.64% vs. 15.78% at week 52. |
| Selective reporting (reporting bias) | Low risk | They reported all outcomes as indicated in the trial registration. |
| Learning contaminating bias | Unclear risk | The anaesthetist delivering the intervention was aware of the treatment condition. |
Chan 2013.
| Methods | RCT, single centre Country: Hong Kong Setting: a university hospital Conducting dates: January 2007 to December 2009 |
|
| Participants | Number of participants (N) = 921 Age, years (mean±S.D.): 68.1 ± 8.2 in the BIS group, 67.6 ± 8.3 in the Control group Sex Male, n (%): 280 (60.2%) in the BIS group, 283 (60.4%) in the Control group ASA PS Class ASA I to II, n (%): 373 (82.8%) in the BIS group, 382 (84.5%) in the Control group MMSE score: 24 to 30 in the BIS group, 24 to 30 in the Control group Chinese literacy Surgery Type of surgery: an elective major surgery expected to last for two hours or longer with an anticipated hospital stay of at least four days Surgery for cancer, n (%): 338 (75.1%) in the BIS group, 352 (77.9%) in the Control group Anaesthesia Premedication: not stated Propofol dose (mg.): 136 ± 30 in the BIS group, 148 ± 33 in the Control group Midazolam use, n (%): 33 ( 7.3%) in the BIS group, 27 ( 5.9%) in the Control group Fentanyl use, n (%): 400 (88.8%) in the BIS group, 411 (90.9%) in the Control group Morphine use, n (%): 373 (83.0%) in the BIS group, 411 (84.4%) in the Control group Nitrous oxide use n (%): 241 (53.5%) in the BIS group, 259 (57.4%) in the Control group Propofol TCI use, n (%): 45 (10.0%) in the BIS group, 54 (11.9%) in the Control group Effect site propofol concentration, µg/ml: 2.7 ± 0.9 in the BIS group, 3.3 ± 1.1 in the Control group Volatile anaesthetics use, n (%): 405 (90.0%) in the BIS group, 398 (88.1%) in the Control group End‐tidal volatile concentration, MAC equivalents:0.57 ± 0.29 in the BIS group, 0.93 ± 0.34 in the Control group Clinically significant hypotension, n (%): 53 (11.7%) in the BIS group, 61 (14%) in the Control group Exclusion criteria Participants not expected to be available for, or cooperate with, postoperative review Participants with illiteracy, language difficulties, or significant hearing or visual impairment Participants with major psychosis taking tranquillizer or antidepressants; or participants with diseases of the central nervous system, suspected dementia or memory impairment with a score on their Mini‐Mental State examination (MMSE) of 23 or less |
|
| Interventions |
|
|
| Outcomes |
Primary outcomes POCD at 3 months after surgery. POCD was defined as two or more Z scores of a battery of the three neuropsychological tests (i.e. a verbal fluency test, a Chinese auditory verbal learning test and a colour trial making test) were 1.96 or greater Secondary outcomes POCD at 1 week Delirium in hospital as measured by using the Confusion Assessment Method criteria, and rate and quality of recovery after anaesthesia and surgery (QoR) using the Chinese QoR score. Other outcomes: major postoperative complications up to 3 months after surgery, as follows.
|
|
| Notes | The study was supported by the Competitive Earmarked Research Grant, Research Grants Council of Hong Kong, and Health and Health Services Research Fund The authors had no conflicts of interest to disclose The study started in January 2007 and ended in December 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "after enrolment and immediately before induction of anaesthesia, the attending anaesthesiologist randomised the patient according to a computer‐generated random group assignment, accessed through an intranet system". |
| Allocation concealment (selection bias) | Unclear risk | The authors did not mention clearly regarding the allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, surgeons, and all research staff, including those responsible for postoperative data collection and outcome assessment, were blinded to the treatment identity. However, the authors did not mention about the blinding of the attending anaesthesiologists. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...those responsible for postoperative data collection and outcome assessment, were blinded to the treatment identity..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 85.0% and 90.7% of participants completed the one‐week and three‐month assessments, respectively. |
| Selective reporting (reporting bias) | Unclear risk | Detail regarding the trial registration was not found. |
| Learning contaminating bias | Unclear risk | The authors did not mention about the blinding of the attending anaesthesiologists. |
Jildenstål 2011.
| Methods | RCT, single centre Country: Sweden Setting: a university hospital Conducting dates: January 2005 to April 2008 |
|
| Participants | Number of participants (N) = 451 Age, years (mean±S.D.): 77.3 ± 14.2 in the BIS group, 79.7 ± 14.7 in the Control group Sex Male, n (%): 128 (57.14%) in the BIS group, 138 (61.06%) in the Control group ASA PS Class ASA I to II, n (%): 198 (88.39%) in the BIS group, 203 (89.82%) in the Control group Surgery: anterior or posterior segment ophthalmic surgery Anaesthesia Premedication: oral midazolam 0.1 mg/kg and oral paracetamol 1 g Induction: fentanyl 1.5 mg/kg, propofol 1 mg/kg, and additional propofol 0.3 mg/kg Intubation: atracurium 0.3 mg/kg Maintenance: nitrous oxide and desflurane in oxygen combined with sub‐Tenon’s block with 2 ml bupivacaine 0.5% (10 mg) Recovery: droperidol 10 mg/kg 30 min prior to extubation Duration of anaesthesia: not stated but similar between the two groups Exclusion criteria Pregnancy Inability to fulfil investigational procedures due to mental disabilities Hearing impairment or any form of substance abuse Participants operated on out of hours, and those who could not fulfil the perioperative protocol |
|
| Interventions |
|
|
| Outcomes | Cognitive dysfunction at one day after surgery measured by Mini Mental Test (MMT), Cognitive Failure Questionnaire (CFQ) and activities of daily living (ADL) Cognitive dysfunction test performed at one week and one month after surgery was done via telephone interview An MMT value below 25 was regarded as POCD at day 1 and a value below 16 was regarded as POCD at 1 week and 1 month |
|
| Notes | This study received funding from Danmeter‐Denmark for AEP 2 silver electrodes. The sponsor did not participate in generation of hypothesis, data collection, analysis or writing of the article. The study was sponsored by the scientific committee of Örebro county council |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "an independent person not involved in the study performed the computerised randomisation procedure..." |
| Allocation concealment (selection bias) | Low risk | Quote: "assigning each patient a specific study number and group allocation, which were then enclosed in envelopes and sealed" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants blinding ‒ unclear. Anaesthesia personnel blinding ‒ unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the post‐operative personnel were blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant in the intervention group was excluded due to protocol violation. |
| Selective reporting (reporting bias) | Unclear risk | Detail regarding the trial registration was not found. |
| Learning contaminating bias | Unclear risk | The study did not mention regarding the blinding of anaesthesia providers who delivered the anaesthetic management |
Punjasawadwong 2016.
| Methods | RCT, single centre Country: Thailand Setting: a university hospital Conducting dates: December 2013 to November 2014 |
|
| Participants | Number of participants (N) = 140 Age, years , (mean±S.D.): 69.7 ± 5.3 in the BIS group, 70.5 ± 6.2 in the Control group Sex Female, n (%): in 35 (50.0%) the BIS group, 38 (54.3%) in the Control group ASA PS Class ASA I to II, n (%): 68 (97.1%) in the BIS group, 66 (94.3%) in the Control group Surgery Type of surgery: elective General surgery, n (%): 42 (60.0%) in the BIS group, 40 (57.1%) in the Control group Orthopaedic surgery, n (%): 8 (11.4%) in the BIS group, 7 (10.0%) in the Control group Gynaecologic surgery, n (%): 8 (11.4%): in the BIS group, 18 (25.7%) in the Control group Ear, nose, and throat surgery, n (%): 12 (17.2%) in the BIS group, 5 (7.2%) in the Control group Duration of surgery, min, median (interquartile range): 150 (105 to 190) in the BIS group; 152.5 (105 to 200) in the Control group Anaesthesia Anaesthetic technique: Premedication: not mentioned Induction: propofol + fentanyl Intubation: a neuromuscular blocking agent Maintenance: sevoflurane, in combination with fentanyl and muscle relaxants with or without nitrous oxide Exclusion criteria Cognitive impairment characterized by Mini‐Mental State Examination (MMSE) of 24, or having a history of neurologic deficits (e.g. stroke, history of seizures, etc.) Eye surgery |
|
| Interventions |
|
|
| Outcomes |
Primary outcome Number of participants have postoperative delirium as assessed daily by Confusion Assessment Method (CAM, Thai version) (Time frame: participants will be followed during the duration of hospital stay (3 to 7 days) Time to recovery (time frame: the end of surgery) |
|
| Notes | The study was funded by National Research Council of Thailand and the faculty research fund of Faculty of Medicine, Chiang Mai University. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly allocated into two groups based on randomization numbers kept in a sealed opaque envelope. |
| Allocation concealment (selection bias) | Low risk | The participants were randomly allocated into two groups based on randomization numbers kept in a sealed opaque envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The anaesthesia providers were not blinded to the allocated group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome was assessed by the outcome assessor who was unaware of the allocated groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants in each arm completed the study. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes mentioned in the trial registration were reported. |
| Learning contaminating bias | High risk | The anaesthesia providers were not blinded to the allocated groups. |
Radtke 2013.
| Methods | RCT, single centre Country: Germany Setting: a university hospital Starting dates: March 2009 to August 2010 |
|
| Participants | Number of participants (N) = 1277 Age, years , (mean ± S.D.): 69.7 ±6 .3 in the BIS group, 70.1 ± 6.5 in the Control group Sex Female, n (%): 257 (44.7%) in the BIS group, 276 (47.6%) in the Control group ASA PS Class ASA I to II, n (%): 305 (53.0%) in the BIS group, 300 (51.7%) in the Control group Surgery Type of surgery: elective General surgery, n (%): 275 (47.8%) in the BIS group, 284 (49.0%) in the Control group Orthopaedic surgery, n (%): 182 (31.7%) in the BIS group, 153 (26.4%) in the Control group Gynaecologic surgery, n (%): 64 (11.1%) in the BIS group, 61 (10.5%) in the Control group Urologic surgery, n (%): 40 (7.0%) in the BIS group, 63 (10.9%) in the Control group Other surgery, n (%): 14 (2.4%) in the BIS group, 19 (3.3%) in the Control group Duration of surgery, min: 164 ± 98 in the BIS group, 175 ± 105 in the Control group Anaesthesia Anaesthetic technique
Premedication: oral midazolam 0.1 mg/kg adjusted to the patient condition Induction: thiopental, propofol, or etomidate (depending on the indications) + fentanyl or remifentanil Intubation: a neuromuscular blocking agent Maintenance: volatile anaesthetics or total intravenous propofol in combination with opioids and muscle relaxants with or without nitrous oxide Remifentanil use, n (%): 166 (28.9%) in the BIS group, 189 (32.6%) in the Control group Fentanyl use, n (%): 409 (71.1%) in the BIS group, 391 (67.4%) in the Control group Intraoperative non‐opioids (paracetamol or metamizole) with or without opioids (morphine or piritramid) 30 min before the end of surgery for postoperative pain management Exclusion criteria Cognitive impairment characterized by Mini‐Mental State Examination (MMSE) of 24, or having a history of neurologic deficits (e.g. stroke, history of seizures, etc.) Participation in a pharmaceutical study Not planned for general anaesthesia Not speaking the local language, or Unable to provide written informed consent |
|
| Interventions |
|
|
| Outcomes | Delirium assessed twice daily (morning and night) according to the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) during postoperative days 1 to 7. POCD at one week and 12 weeks after surgery by the Neuropsychological Test Battery consisting of tests provided by CANTAB® (Cantab Cognition, Cambridge, UK) which included a Motor Screening Test, two tests of visual memory (pattern recognition memory and spatial recognition memory) and a test of attention (choice reaction time) POCD was defined as in an individual when the RCI score was −1.96 on ≥ 2 tests, the combined Z score was < −1.96, or both Other outcomes: mortality at three months |
|
| Notes | This work was supported by Charité–Universitätsmedizin
Berlin with additional funding provided by Aspect Medical Systems, now Covidien The authors had no conflicts of interest to disclose The study started in March 2009 and ended early in May 2010 due to limited funds |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients were consecutively recruited and after stratification according to American Society of Anesthesiologists’ physical status (ASA PS; I or II vs III or IV) and electronically randomised into two study groups" |
| Allocation concealment (selection bias) | Unclear risk | The authors did not mention regarding the allocation concealment method. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Binding of participants; yes. Blinding of anaesthesiologists: no |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment: yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In the BIS group (n = 638), 45 did not receive assigned BIS‐guided anaesthesia and 18 were lost to follow‐up and discontinued intervention. In the control group (n = 639), 39 did not receive assigned BIS‐blinded anaesthesia and 20 were lost to follow‐up/dropouts and discontinued intervention. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes mentioned in the trial registration were reported |
| Learning contaminating bias | Low risk | The anaesthetists in the intervention group always used BIS but in the control group never used BIS. |
Wong 2002.
| Methods | RCT, single centre Country: Canada Setting: a university hospital Starting dates: not stated. |
|
| Participants | Number of participants (N) = 68 Age, years (mean ± S.D.): 71 ± 15 in the BIS group, 70 ± 6 in the Control group Sex Male, n (%): 19 (65.51%) in the BIS group, 21 (67.74%) in the Control group ASA PS Class ASA I to II, n (%): 26 (89.65%) in the BIS group, 30 (96.77%) in the Control group Surgery Type of surgery: elective orthopaedic surgery Knee replacement, n (%): 14 (48.28%) in the BIS group, 10 (32.26%) in the Control group Hip replacement, n (%): 15 (51.72%) in the BIS group, 21 (67.74%) in the Control group Duration of surgery, min: 90 ± 16 in the BIS group, 92 ± 16 in the Control group Anaesthesia Anaesthetic technique: volatile (isoflurane) anaesthesia Premedication: none Induction: propofol 1 to 2 mg/kg, fentanyl 2 µg/kg to 3 µg/kg, and midazolam 1 mg. Intubation: rocuronium: 0.6 mg/kg Maintenance: isoflurane in combination with nitrous oxide, fentanyl, and rocuronium. Intraoperative drug usage: Propofol, mg: 146 ± 44 in the BIS group, 141 ± 43 in the Control group Fentanyl, µg: 307 ± 64 in the BIS group, 310 ± 95 in the control group Isoflurane, mL: 5.6 ± 2.6 in the BIS group, 7.7 ± 3.4 in the Control group Duration of anaesthesia, min: 120 ± 17 in the BIS group, 121 ± 17 in the Control group Exclusion criteria Significant cardiopulmonary diseases or other end‐organ disease Depression or psychiatric disorders, dementia previous CVA, head trauma Inadequate command of English Drug or alcohol abuse Preoperative baseline of Mini Mental State Examination (MMSE) < 24 |
|
| Interventions |
|
|
| Outcomes | Time to orientation to person, place and time (main outcome) End tidal concentration (%) Consumption of isoflurane (ml) Time to awakening (eye opening to verbal commands) Time to extubation Time to readiness for transfer to PACU Time to readiness for discharge from PACU (Aldrete score > 9) Symptoms of postoperative cognitive dysfunction reported from nurses, family members, research assistant, or the participant Recall awareness of intraoperative events | |
| Notes | The study was supported in part by a grant from Aspect Medical, Newton, MA, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block randomization with concealed varying block sizes was performed with computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Block randomization with concealed varying block sizes was performed with computer‐generated random numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants blinding ‒ unclear. Anaesthesia personnel blinding ‒ no |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "symptoms of clinical cognitive dysfunction (i.e., reduced ability to maintain attention to external stimuli, disorganized thinking, disorientation, memory impairment, etc.) reported from nurses, family members, research assistant, or patient (all blinded to group assignment) were recorded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "....., eight patients (three from the SP group, and five from the BIS group) were excluded from the analysis for protocol violations." The missing outcome data seem to balance across intervention group. The plausible effect size (difference in mean) among missing outcome probably not enough to have a clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Unclear risk | Detail regarding the trial registration was not found. |
| Learning contaminating bias | Unclear risk | The unblinded anaesthesiologist could potentially lead to 'learning contamination bias' |
AEP: Auditory Evoked Potential; AAI: A‐line autoregressive index; ASA PS: American Society of Anesthesiologists Physical Status; CVA: cerebrovascular accident; MMSE: Mini Mental State Examination; MMT: Mental State test; ISPOCD: International Study of Postoperative Cognitive Dysfunction; TCI: Target Controlled Infusion; MAC equivalent: Minimal Alveolar Concentration equivalent; N: total number of participants in each trial; n: number of participants in each arm after random allocation; mg/kg:milligramme per kilogramme; g: gramme; ml: millilitre; min: minute; mm.Hg: millimetre of mercury; PACU: post anaesthesia care unit; S.D: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Farag 2006 | It did not address the objectives of our study. |
| Fernandes 2007 | It did not address the objectives of our study. |
| McDonagh 2012 | It did not address the objectives of our study. |
| Meineke 2014 | It did not address the objectives of our study. |
| Ovezov 2012 | It did not address the objectives of our study. |
| Plaschke 2010 | It was not an RCT. |
| Santarpino 2011 | It was not an RCT. |
| Seo 2014 | It was not an RCT. |
| Short 2015 | It did not address the objectives of our study. |
| Shu 2015 | It did not address the objectives of our study. |
| Soehle 2015 | It was not an RCT. |
| Steinmetz 2010 | It was not an RCT. |
| Trafidło 2015 | It did not address the objectives of our study. |
| Whitlock 2014 | It did not address the objectives of our study. |
| Zhang 2015 | It was not an RCT. |
| Zywiel 2014 | It was not an RCT. |
BAG‐RECALL: BIS or anaesthesia gas to reduce explicit recall; BIS: bispectral index; ETAC: end‐tidal anaesthetic concentration; iv: intravenous; POCD: postoperative cognitive dysfunction; POD: postoperative delirium; RCT: randomized controlled trial; TIVA: total intravenous anaesthesia
Characteristics of studies awaiting assessment [ordered by study ID]
Goyal 2017.
| Methods | RCT, single centre Country: India |
| Participants | 60 participants between the age of 20 and 60 years, belonging to American Society of Anesthesiologists‐Physical Status PS I and II, body mass index 18 to 25 kg/m2, undergoing elective abdominal hysterectomy, requiring administration of GA using endotracheal tube |
| Interventions | Entropy group: isoflurane titrated to entropy values between 40 and 60 Clinical practice group: isoflurane titrated by clinical parameters and MAC values |
| Outcomes | Isoflurane consumption Recovery profile Intraoperative recall awareness |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
NCT02382445.
| Trial name or title | Anesthesia depth increases the degree of postoperative dementia, delirium, and cognitive dysfunction (BIS & dementia) |
| Methods | A randomized controlled trial |
| Participants | 260 |
| Interventions | Anaesthesia depth monitor at an anaesthesia level of BIS 50 to 60 titrating propofol and sevoflurane versus standard care |
| Outcomes | Postoperative cognition and mental capacity including postoperative cognitive dysfunction (POCD) Incidence of mental and other complications including delirium |
| Starting date | January 2014 |
| Contact information | Thomas Frietsch, Prof, MD, MBA; Universitätsmedizin Mannheim; Germany. E‐mail:Frietsch@me.com |
| Notes |
NCT02604459.
| Trial name or title | Does optimized general anesthesia care reduce postoperative delirium? (OPCare) |
| Methods | A randomized controlled trial |
| Participants | 160 participants aged 65 years or older for hip fracture surgery |
| Interventions | 1. Optimal care ‐ the intraoperative depth of anaesthesia will be directed using a BIS monitor, blood pressure will be maintained within 20% of preoperative levels, and cerebral oxygenation will be maintained > 60% during anaesthesia 2. Usual care ‐ the anaesthetic management will be at the discretion of the anaesthesia provider |
| Outcomes | Primary outcome
Secondary outcome
|
| Starting date | June 2015 |
| Contact information | Terri G Monk, monkt@health.missouri.edu Adam Zino, zioa@helath.missouri.edu |
| Notes |
NCT02692300.
| Trial name or title | Protocol for the electroencephalography guidance of anesthesia to alleviate geriatric syndromes |
| Methods | A randomized controlled trial |
| Participants | 1200 |
| Interventions | Bispectral Index (BIS)‐Guided Group versus standard anaesthesia control group |
| Outcomes | Incidence of postoperative delirium Incidence of delirium |
| Starting date | 2016 |
| Contact information | Eric Jacobsohn, MBChB FRCPC, University of Manitoba , Canada. E‐mail: ejacobsohn@hsc.mb.ca Michael Avidan, MBBCh FCASA, E‐mail: avidanm@anest.wustl.edu |
| Notes | A full protocol has been published in Wildes 2016 |
NCT02698982.
| Trial name or title | Impact of tight Intraoperative blood pressure and depth of anesthesia control on the incidence of postoperative cognitive impairment in elderly patients |
| Methods | A randomized controlled trial |
| Participants | 41 participants aged 70 years or older undergoing elective moderate risk surgery requiring a general anaesthesia |
| Interventions | Device: depth of anaesthesia monitoring (BIS, Covidien USA) and continuous non‐invasive blood pressure monitoring (Clearsight, Edwards LifeScience, USA) Procedure |
| Outcomes | Postoperative delirium detected by the Confusion Assessment Method (CAM) (time frame: 3 day postoperative) Postoperative change in cognitive function detected by a battery of neuropsychological test; (time frame: baseline and 1 week and 3 month postoperative). |
| Starting date | May 2016 |
| Contact information | Luc Van Obbergh and Céline Boudart, Erasme University Hospital, Brussels, Belgium, 1070 |
| Notes |
NCT02841423.
| Trial name or title | Postoperative cognitive dysfunction after propofol anesthesia for noncardiac surgery (POCD ELA) |
| Methods | A randomized controlled trial |
| Participants | 204 participants aged 55 years or older receiving non‐cardiac surgery |
| Interventions | Closed‐loop Coadministration of Propofol and Remifentanil Guided by Bispectral Index versus Intravenous anaesthesia |
| Outcomes | The incidence of early POCD (time frame: 3 days) |
| Starting date | 2013 |
| Contact information | E Samain, PR Lucie Vettoretti, lvettoretti@chu‐besancon.fr |
| Notes |
BIS: bispectral index
Differences between protocol and review
We made the following changes to the published protocol (Punjasawadwong 2014a).
We altered the title slightly by adding 'evoked potential techniques' as follows: 'Processed electroencephalogram and evoked potential techniques for amelioration of postoperative delirium and cognitive dysfunction following non‐cardiac and non‐neurosurgical procedures in adults'
We altered the objective slightly by adding 'audio evoked potential' as follows: "To assess whether the use of processed EEG or auditory evoked potential (AEP) indices (bispectral index ..."
-
We added multi‐arm trials and cluster randomized controlled trials in the Types of studies section as follows:
"We searched for all randomized or quasi‐randomized controlled trials with two or more arms and at least two arms compared any method using processed EEG or AEP indices against a control group.
We also looked for cluster randomized trials."
In the Unit of analysis issues section we replaced the original paragraph in the protocol "For studies with three (or more) arms that have the same control, we will use appropriate proportions of the control group as the comparator for each of the included intervention groups. For cluster randomized trials, we will reduce the size of each trial to its 'effective sample size' by dividing the original sample size with a quantity called 'the design effect'. The design effect is calculated from 1 + (M ‐ 1) x ICC, where M is the average cluster size and ICC is the intracluster correlation coefficient (Higgins 2011)" with a new one as ' We did not use other methods as described in the protocol, for multi‐arm trials or cluster randomized trials because trials with these types of study designs were not available for the inclusion'
-
In 'Subgroup analysis and investigation of heterogeneity' section, we did not perform the subgroup analyses as mentioned in the protocol (Punjasawadwong 2014a), because the number of the included studies were too small to be categorized. Furthermore, we found no significance of heterogeneity across included studies in the meta‐analysis. We originally planned to perform the following subgroup analyses based on the following categories.
Age groups (> 65 versus < 65 years old).
Anaesthetics (volatile versus intravenous anaesthetics).
Depth of anaesthesia ('within' versus 'below' or 'above' the recommended ranges of the processed EEG indices) in the control group.
Participants at low risk for intraoperative awareness versus participants at high risk for intraoperative awareness’.
Hospital admitted versus ambulatory surgery.
-
We modified the sub‐heading Dealing with missing data in the Methods section as follows.
The process for obtaining missing data from trialists was unattainable. Therefore, we performed the available case analysis and imputed the missing events by adapting the suggested approach in the Cochrane Handbook for Systematic Reviews of Interventions in section 16.2.2 (Higgins 2011), for the best‐ and worst‐case scenario assumptions as following:
Under the best case scenario, the missing events of any POD or POCD outcomes in the processed EEG group were imputed from the lower limit of confidence interval for the group event rate and the missing events in the clinical signs group were imputed from the upper limit of confidence interval for the group event rate. The reverse direction was adapted for the worst‐case scenario.
We included the following outcomes in the 'Summary of findings' table: all‐cause mortality, postoperative any complications and postoperative length of stay.
-
We have changed order of the secondary outcomes in the method section as follows.
All‐cause mortality.
Postoperative any complications.
Postoperative length of stay.
Other consequences of cognitive disturbances such as dementia (including Alzheimer's disease (AD)), which was described as deterioration in memory, thinking, behaviour and the ability to perform daily activities.
Occurrence of intraoperative awareness, which was defined as the unexpected and explicit recall by patients of events occurring during anaesthesia.
Contributions of authors
Yodying Punjasawadwong (YP), Waraporn Chau‐in (WC), Malinee Laopaiboon (ML), Sirivimol Punjasawadwong (SP), Pathomporn Pin‐on (PP).
Conceiving the review: YP, WC, ML, SP
Co‐ordinating the review: YP
Undertaking manual searches: YP, WC, SP
Screening search results: YP, WC, SP
Organizing retrieval of papers: YP, SP
Screening retrieved papers against inclusion criteria: YP, WC, SP
Appraising quality of papers: YP, WC, PP
Abstracting data from papers: YP, WC, PP
Writing to authors of papers for additional information: YP
Providing additional data about papers: YP
Obtaining and screening data on unpublished studies: YP, SP
Data management for the review: YP, ML
Entering data into Review Manager 5 (Review Manager 2014): YP, SP
RevMan statistical data: YP, ML
Other statistical analysis not using RevMan 5: ML
Interpretation of data: YP, WC, ML
Statistical inferences: ML
Writing the review: YP, WC, ML
Securing funding for the review: YP
Performing previous work that was the foundation of the present study: YP
Guarantor for the review (one author): YP
Persons responsible for reading and checking review before submission: YP, ML
Sources of support
Internal sources
Faculty of Medicine, Chiang Mai University, Thailand.
External sources
No sources of support supplied
Declarations of interest
Yodying Punjasawadwong was involved in an included study (Punjasawadwong 2016) as a primary investigator. Dr Punjasawadwong did not assess the risk of bias and perform data extraction in this trial.
Waraporn Chau‐in: none known
Malinee Laopaiboon: none known
Sirivimol Punjasawadwong: none known
Pathomporn Pin‐on: none known
Edited (no change to conclusions)
References
References to studies included in this review
Ballard 2012 {published data only}
- Ballard C, Jones E, Gauge N, Aarsland D, Nilsen OB, Saxby BK, et al. Optimised anaesthesia to reduce post operative cognitive decline ( POCD) in older patients undergoing elective surgery, a randomised controlled trial. PLoS ONE 2012;7(6):1‐8. [Control‐Triasl.com: ISRCTN39503939; DOI: 10.1371/journal.pone.0037410; DOI: 10.1371/journal.pone.0037410; PUBMED: 22719840] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013 {published data only}
- Chan MT, Cheng BC, Lee TM, Gin T, CODA Trial Group. BIS‐guided anesthesia decreases postoperative delirium and cognitive decline. Journal of Neurosurgical Anesthesiology 2013;25(1):33‐42. [PUBMED: 23027226] [DOI] [PubMed] [Google Scholar]
Jildenstål 2011 {published data only}
- Jildenstål PK, Hallén JL, Rawal N, Gupta A, Berggren L. Effect of auditory evoked potential‐guided anaesthesia on consumption of anaesthetics and early postoperative cognitive dysfunction: a randomised controlled trial. European Journal of Anaesthesiology 2011;28(3):213‐9. [PUBMED: 21088592] [DOI] [PubMed] [Google Scholar]
Punjasawadwong 2016 {unpublished data only}
- Punjasawadwong Y, Pipanmekaporn T, Wongpakaran N. Optimized anesthesia to reduce incidence of postoperative delirium in elderly undergoing elective, non‐cardiac surgery: a randomized controlled trial. Anesthesia and Analgesia 2016; Vol. 123, issue 3S_Suppl:211. [ClinicalTrials.gov.: NCT02133430]
Radtke 2013 {published data only}
- Radtke FM, Franck M, Lendner J, Krüger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomised trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. British Journal of Anaesthesia 2013;110(Suppl 1):i98‐105. [PUBMED: 23539235] [DOI] [PubMed] [Google Scholar]
Wong 2002 {published data only}
- Wong J, Song D, Blanshard H, Grady D, Chung F. Titration of isoflurane using BIS index improves early recovery of elderly patients undergoing orthopedic surgeries. Canadian Journal of Anaesthesia 2002;49(1):13‐8. [PUBMED: 11782323] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Farag 2006 {published data only}
- Farag E, Chelune GJ, Schubert A, Mascha EJ. Is depth of anaesthesia, as assessed by the Bispectral Index, related to postoperative cognitive dysfunction and recovery?. Anesthesia and Analgesia 2006;103(3):633‐40. [PUBMED: 16931673] [DOI] [PubMed] [Google Scholar]
Fernandes 2007 {published data only}
- Fernandes CR, Gomes JM, Cordeiro RA, Pereira Kde S. Assessment of the cognitive effects of inhalational induction with sevoflurane associated or not with nitrous oxide: a comparative study in adult volunteers. Revista Brasileira de Anestesiologia 2007;57(3):237‐46. [PUBMED: 19466359] [DOI] [PubMed] [Google Scholar]
McDonagh 2012 {unpublished data only}
- McDonagh D, Attix D, Pieper C, Martin G, Monk T. Preoperative cognitive status, not type of general anaesthesia, predicts early postoperative cognitive dysfunction. 40th Annual Meeting of the Society for Neuroscience in Anesthesiology and Critical Care. Lippincott Williams and Wilkins, 2012.
Meineke 2014 {published data only}
- Meineke M, Applegate RL, Rasmussen T, Anderson D, Azer S, Mehdizadeh A, et al. Cognitive dysfunction following desflurane versus sevoflurane general anesthesia in elderly patients: A randomized controlled trial. Medical Gas Research 2014;4(1):6. [DOI: 10.1186/2045-9912-4-6; PUBMED: 24666542] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ovezov 2012 {published data only}
- Ovezov A, Lobov M, Knyazev A, Nad'kina E, Bragina S, Myatchin P, et al. Neuroprotective prevention of early postoperative cognitive dysfunction in adults. 16th Congress of the European Federation of Neurological Societies. Blackwell Publishing Ltd, 2012.
Plaschke 2010 {published data only}
- Plaschke K, Fichtenkamm P, Schramm C, Hauth S, Martin E, Verch M, et al. Early postoperative delirium after open‐heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin‐6. Intensive Care Medicine 2010;36(12):2081‐9. [PUBMED: 20689917 ] [DOI] [PubMed] [Google Scholar]
Santarpino 2011 {published data only}
- Santarpino G, Fasol R, Sirch J, Ackermann B, Pfeiffer S, Fischlein T. Impact of bispectral index monitoring on postoperative delirium in patients undergoing aortic surgery. HSR proceedings in intensive care & cardiovascular anesthesia 2011;3(1):47‐58. [PUBMED: 23440016 ] [PMC free article] [PubMed] [Google Scholar]
Seo 2014 {published data only}
- Seo JS, Park SW, Lee YS, Chung C, Kim YB. Risk factors for delirium after spine surgery in elderly patients. Journal of Korean Neurosurgical Society 2014;56(1):28‐33. [PUBMED: 25289122] [DOI] [PMC free article] [PubMed] [Google Scholar]
Short 2015 {published data only}
- Short TG, Leslie K, Chan MT, Campbell D, Frampton C, Myles P. Rationale and design of the balanced anesthesia study: a prospective randomized clinical trial of two levels of anesthetic depth on patient outcome after major surgery. Anesthesia and Analgesia 2015;121(2):357‐65. [PUBMED: 25993386 ] [DOI] [PubMed] [Google Scholar]
Shu 2015 {published data only}
- Shu AH, Wang Q, Chen XB. Effect of different depths of anaesthesia on postoperative cognitive function in laparoscopic patients: a randomised clinical trial. Current Medical Research and Opinion 2015;31(10):1883‐7. [PUBMED: 26202165 ] [DOI] [PubMed] [Google Scholar]
Soehle 2015 {published data only}
- Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiology 2015;15:61. [DOI: 10.1186/s12871-015-0051-7; PUBMED: 25928189] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinmetz 2010 {published data only}
- Steinmetz J, Funder KS, Dahl BT, Rasmussen LS. Depth of anaesthesia and post‐operative cognitive dysfunction. Acta Anaesthesiologica Scandinavica 2010;54(2):162‐8. [10.1111/j.1399‐6576.2009.02098.x. Epub 2009 Sep 1; PUBMED: 19764909] [DOI] [PubMed] [Google Scholar]
Trafidło 2015 {published data only}
- Trafidło T, Gaszyński T, Gaszyński W, Nowakowska‐Domagała K. Intraoperative monitoring of cerebral NIRS oximetry leads to better postoperative cognitive performance: A pilot study. International Journal of Surgery 2015;16(Pt A):23‐30. [10.1016/j.ijsu.2015.02.009. Epub 2015 Feb 18; PUBMED: 25701620] [DOI] [PubMed] [Google Scholar]
Whitlock 2014 {published data only}
- Whitlock EL, Torres BA, Lin N, Helsten DL, Nadelson MR, Mashour GA, Avidan MS. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG‐RECALL clinical trial. Anesthesia and Analgesia 2014;118(4):809‐17. [PUBMED: 24413548 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2015 {unpublished data only}
- Zhang X, Rizvi B, Shalabi A, Leung JM. The association between intraoperative patient state index and postoperative cognitive outcomes. Anesthesia and Analgesia 2015;120(3):S257. [CENTRAL: 01128948] [Google Scholar]
Zywiel 2014 {published data only}
- Zywiel MG, Prabhu A, Perruccio AV, Gandhi R. The influence of anaesthesia and pain management on cognitive dysfunction after joint arthroplasty: a systematic review. Clinical Orthopaedics and Related Research 2014;472(5):1453‐66. [DOI: 10.1007/s11999-013-3363-2; PUBMED: 24186470 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Goyal 2017 {published data only}
- Goyal KA, Nileshwar A, Budania LS, Gaude Y, Mathew S, Vaidya S. Evaluation of effect of entropy monitoring on isoflurane consumption and recovery from anesthesia. Journal of Anaesthesiology Clinical Pharmacology 2017;33(4):529‐33. [PUBMED: PMID: 29416249 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02382445 {unpublished data only}
- NCT02382445. Anesthesia depth increases the degree of postoperative dementia, delirium, and cognitive dysfunction (BIS & dementia). https://clinicaltrials.gov/ct2/show/NCT02382445 first received 6 March 2015.
NCT02604459 {published and unpublished data}
- NCT02604459. Does optimized general anesthesia care reduce postoperative delirium? (OPCare). https://clinicaltrials.gov/ct2/show/NCT02604459 first received 13 November 2015.
NCT02692300 {unpublished data only}
- NCT02692300. EEG guidance of anesthesia (ENGAGES‐CANADA) (ENGAGES). https://clinicaltrials.gov/ct2/show/NCT02692300 first received 26 February 2016.
NCT02698982 {published data only}
- NCT02698982. Impact of tight intraoperative blood pressure and depth of anesthesia control on the incidence of postoperative cognitive impairment in elderly patients. https://clinicaltrials.gov/ct2/show/NCT02698982 first received March 4, 2016.
NCT02841423 {published and unpublished data}
- NCT02841423. Postoperative cognitive dysfunction after propofol anesthesia for noncardiac Surgery (POCD ELA). https://clinicaltrials.gov/ct2/show/NCT02841423 first received July 22, 2016.
Additional references
Aime 2006
- Aime I, Verroust N, Masson‐Lefoll C, Taylor G, Laloe P, Liu N, et al. Does monitoring bispectral index or spectral entropy reduce sevoflurane use?. Anesthesia and Analgesia 2006;103(6):1469‐77. [PUBMED: 17959961] [DOI] [PubMed] [Google Scholar]
American Psychiatric Association 1994
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th Edition. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
Broadbent 1982
- Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The cognitive failures questionnaire (CFQ) and its correlates. British Journal of Clinical Psychology 1982;21(part1):1‐16. [PUBMED: 7126941] [DOI] [PubMed] [Google Scholar]
Bruhn 2005
- Bruhn J, Kreuer S, Bischoff P, Kessler P, Schmidt GN, Grzesiak A, et al. Bispectral index and A‐line AAI index as guidance for desflurane‐remifentanil anaesthesia compared with a standard practice group: a multicentre study. British Journal of Anaesthesia 2005;94(1):63‐9. [PUBMED: 15516347] [DOI] [PubMed] [Google Scholar]
Culley 2007
- Culley DJ, Xie Z, Crosby G. General anesthetic‐induced neurotoxicity: an emerging problem for the young and old?. Current Opinion in Anaesthesiology 2007;20:408‐13. [PUBMED: 17873593] [DOI] [PubMed] [Google Scholar]
Delfino 2009
- Delfino AE, Cortinez LI, Munoz HR. Propofol consumption and recovery times after bispectral index or cerebral state index guidance of anaesthesia. British Journal of Anaesthesia 2009;103(2):255‐9. [PUBMED: 19502288] [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein M, Folstein SE, McHugh PR. " Mini‐Mental State". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189‐98. [PUBMED: 1202204] [DOI] [PubMed] [Google Scholar]
Gan 1977
- Gan TJ, Glass PS, Windsor A, Payne F, Rosow C, Sebel P, et al. Bispectral Index monitoring allows faster emergence and improved recovery from propofol, alfentanil and nitrous oxide anaesthesia. BIS Utility Study Group. Anesthesiology 1997;87(4):808‐15. [PUBMED: 9357882] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 6 September 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Inouye 1990
- Inouye SK, Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Annals of Internal Medicine 1990;113:941–8. [PUBMED: 2240918] [DOI] [PubMed] [Google Scholar]
Johnson 2002
- Johnson T, Monk T, Rasmussen LS, Abildstrom H, Houx P, Korttila K, et al. Postoperative cognitive dysfunction in middle‐aged patients. Anesthesiology 2002;96:1351‐7. [PUBMED: 12170047] [DOI] [PubMed] [Google Scholar]
Kreuer 2003
- Kreuer S, Biedler A, Larsen R, Altmann S, Wilhelm W. Narcotrend monitoring allows faster emergence and a reduction of drug consumption in propofol‐remifentanil anaesthesia. Anesthesiology 2003;99(1):34‐41. [PUBMED: 12826839] [DOI] [PubMed] [Google Scholar]
Letourneau 1983
- Letourneau JE, Denis R. The reliability and validity of the Trieger tests as a measure of recovery from general anaesthesia in a day‐care surgery unit. Anesthesia Progress 1983;5:152‐5. [PMC free article] [PubMed] [Google Scholar]
Lichtenberger 2009
- Lichtenberger EO, Kaufman AS. Essentials of WAIS‐IV assessement. Essentials of Psychological Assessment. Hoboken, N.J: Wiley, 2009:27. [Google Scholar]
Lui 2004
- Liu SS. Effects of Bispectral Index monitoring on ambulatory anaesthesia: a meta‐analysis of randomised controlled trials and a cost analysis. Anesthesiology 2004;101(2):311‐5. [PUBMED: 15277912] [DOI] [PubMed] [Google Scholar]
Mason 2010
- Mason SE, Noel‐Storr A, Ritchie CW. The impact of general and regional anaesthesia on the incidence of post‐operative cognitive dysfunction and post‐operative delirium: a systematic review with meta‐analysis. Journal of Alzheimers Disease 2010;22 Suppl 3:67‐9. [DOI: 10.3233/JAD-2010-101086; PUBMED: 20858956 ] [DOI] [PubMed] [Google Scholar]
Moller 1997
- Moller TJ. Cerebral dysfunction after anaesthesia. Acta Anaesthesiologica Scandinavica 1997;110:13‐6. [PUBMED: 9248516] [DOI] [PubMed] [Google Scholar]
Moller 1998
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long‐term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post‐operative cognitive dysfunction. Lancet 1998;351:857‐61. [PUBMED: 9525362] [DOI] [PubMed] [Google Scholar]
Myles 2004
- Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: the B‐Aware randomised controlled trial. Lancet 2004;363(9423):1757‐63. [PUBMED: 15172773] [DOI] [PubMed] [Google Scholar]
Newman 2001
- Newman MF, Grocott HP, Mathew JP, White WD, Landolfp K, Reves JG, et al. Report of the sub study assessing the impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke 2001;32:2874‐81. [PUBMED: 11739990] [DOI] [PubMed] [Google Scholar]
Newman 2007
- Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology 2007;106:572‐90. [PUBMED: 17325617] [DOI] [PubMed] [Google Scholar]
Oliveira 2017
- Oliveira CR, Bernardo WM, Nunes VM. Benefit of general anesthesia monitored by bispectral index compared with monitoring guided only by clinical parameters. Systematic review and meta‐analysis [bispectralBenefício da anestesia geral com monitorac¸ão do índice bispectral em comparac¸ãocom o monitoramento guiado apenas por parâmetros clínicos. Revisão sistemática emetanáliseResumoJustificativa]. REVISTABRASILEIRA DEANESTESIOLOGIA 2017;67(1):72‐84. [DOI: 10.1016/j.bjane.2015.09.001; PUBMED: PMID: 28017174] [DOI] [PubMed] [Google Scholar]
Perouansky 2009
- Perouansky M, Hemmings HC. Neurotoxicity of general anesthetics: cause for concern?. Anesthesiology 2009;111:1365‐71. [PUBMED: 1993488] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pipanmekaporn 2014
- Pipanmekaporn T, Wongpakaran N, Mueankwan S, Dendumrongkul P, Chittawatanarat K, Khongpheng N, et al. Validity and reliability of the Thai version of the confusion assessment method for the intensive care unit (CAM‐ICU). Clinical Interventions in Aging 2014;9:879‐885. [DOI: 10.2147/CIA.S62660; PUBMED: 24904208 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Punjasawadwong 2014
- Punjasawadwong Y, Boonjeungmonkol N, Phongchiewboon A. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD003843.pub3; PUBMED: 24937564] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rakusan 2006
- Rakusa M, Granda G, Kogoj A, Mlakar J, Vodusek DB. Mini‐mental state examination:standardization and validation for the elderly Slovenian population. European Journal of Neurology 2006;13:141‐5. [PUBMED: 16490044] [DOI] [PubMed] [Google Scholar]
Rampil 1998
- Rampil IJ. A primer for EEG signal processing in anaesthesia. Anesthesiology 1998;89(4):980‐1002. [PUBMED: 9778016] [DOI] [PubMed] [Google Scholar]
Rasmussen 2000
- Rasmussen LS, Christiansen M, Rasmussen H, Kristensen PA, Moller JT. Do blood concentrations of neuron specific enolase and S‐100 beta protein reflect cognitive dysfunction after abdominal surgery? ISPOCD Group. British Journal of Anaesthesia 2000;84(2):242‐4. [PUBMED: 10743460] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rinaldi 2005
- Rinaldi S, Consales G, Gallerani E, Ortolani O, Gaudio AR. A‐line autoregression index monitoring to titrate inhalational anaesthesia: effects on sevoflurane consumption, emergence time and memory. Acta Anaesthesiologica Scandinavica 2005;49(5):692‐7. [PUBMED: 15836686] [DOI] [PubMed] [Google Scholar]
Roizen 1994
- Roizen MF, Toledano A. Technology assessment and the ‘learning contamination’ bias. Anesthesia and Analgesia 1994;79:410‐2. [PUBMED: 8067542] [DOI] [PubMed] [Google Scholar]
Sanders 2005
- Sanders RD, Ma D, Maze M. Anaesthesia induced neuroprotection. Best Practice & Research. Clinical Anaesthesiology 2005;19:461‐74. [PUBMED: 16013194] [DOI] [PubMed] [Google Scholar]
Schneider 2010
- Schneider G. Monitoring anaesthetic depth. In: Mashour GA editor(s). Consciousness, Awareness, and Anesthesia. 1st Edition. Cambridge University Press, 2010:122‐30. [Google Scholar]
Siddiqi 2016
- Siddiqi N, Harrison JK, Clegg A, Teale EA, Young J, Taylor J, et al. Interventions for preventing delirium in hospitalised non‐ICU patients. Cochrane Database of Systematic Reviews 2016, Issue 3. [CENTRAL: CD005563; DOI: 10.1002/14651858.CD005563.pub3; PUBMED: 26967259] [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 1997
- Song D, Joshi GP, White PF. Titration of volatile anesthetics using bispectral index facilitates recovery after ambulatory anaesthesia. Anesthesiology 1977;87(4):842‐8. [PUBMED: 9357886] [DOI] [PubMed] [Google Scholar]
STATA 10.1 [Computer program]
- Stata Corp LP. Stata Statistical Software (STATA). Version 10.1. College Station: Stata Corp LP, 2007.
Steinmetz 2009
- Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, the ISPOCD Group. Long‐term consequences of postoperative cognitive dysfunction. Anesthesiology 2009;110:548‐55. [PUBMED: 19225398] [DOI] [PubMed] [Google Scholar]
Vacas 2013
- Vacas S, Degos V, Feng X, Maze M. The neuroinflammatory response of postoperative cognitive decline. British Medical Bulletin 2013;106:1‐18. [PUBMED: 23558082] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2010
- Wan Y, Xu J, Meng F, Bao Y, Ge Y, Lobo N, et al. Cognitive decline following surgery is associated with gliosis, beta‐amyloid accumulation, and tau phosphorylation in old mice. Critical Care Medicine 2010;38:2190‐8. [PUBMED: 20711073] [DOI] [PubMed] [Google Scholar]
Wildes 2016
- Wildes TS, Winter AC, Maybrier HR, Mickle AM, Lenze EJ, Stark S, et al. Protocol for the electroencephalography guidance of anesthesia to alleviate Geriatric Syndromes (ENGAGES) study: a pragmatic, randomised clinical trial. BMJ Open 2016;6:e011505. [DOI: 10.1136/bmjopen-2016011505] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wongpakaran 2011
- Wongpakaran N, Wongpakaran T, Bookamana P, Pinyopornpanish M, Maneeton B, Lerttrakarnnon P, et al. Diagnosing delirium in elderly Thai patients: utilization of the CAM algorithm. BioMed Central Family Practice 2011;12:65. [DOI: 10.1186/1471-2296-12-65; PUBMED: 21722373] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Punjasawadwong 2014a
- Punjasawadwong Y, Chau‐in W, Laopaiboon M, Punjasawadwong S. Processed electroencephalogram indices for amelioration of postoperative delirium and cognitive dysfunction following non‐cardiac and non‐neurosurgical procedures. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD011283] [DOI] [PMC free article] [PubMed] [Google Scholar]


