Abstract
Background
Appendectomy, the surgical removal of the appendix, is performed primarily for acute appendicitis. Patients who undergo appendectomy for complicated appendicitis, defined as gangrenous or perforated appendicitis, are more likely to suffer from postoperative complications. The routine use of abdominal drainage to reduce postoperative complications after appendectomy for complicated appendicitis is controversial. This is an update of the review first published in 2015.
Objectives
To assess the safety and efficacy of abdominal drainage to prevent intra‐peritoneal abscess after open appendectomy for complicated appendicitis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2017, Issue 6), Ovid MEDLINE (1946 to 30 June 2017), Ovid Embase (1974 to 30 June 2017), Science Citation Index Expanded (1900 to 30 June 2017), World Health Organization International Clinical Trials Registry Platform (30 June 2017), ClinicalTrials.gov (30 June 2017) and Chinese Biomedical Literature Database (CBM) (1978 to 30 June 2017).
Selection criteria
We included all randomised controlled trials (RCTs) that compared abdominal drainage and no drainage in people undergoing emergency open appendectomy for complicated appendicitis.
Data collection and analysis
Two review authors identified the trials for inclusion, collected the data, and assessed the risk of bias independently. We performed the meta‐analyses using Review Manager 5. We calculated the risk ratio (RR) for dichotomous outcomes (or a Peto odds ratio for very rare outcomes), and the mean difference (MD) for continuous outcomes with 95% confidence intervals (CI). We used GRADE to rate the quality of evidence.
Main results
We included six RCTs (521 participants), comparing abdominal drainage and no drainage in patients undergoing emergency open appendectomy for complicated appendicitis. The studies were conducted in North America, Asia and Africa. The majority of the participants had perforated appendicitis with local or general peritonitis. All participants received antibiotic regimens after open appendectomy. None of the trials was at low risk of bias.
There was insufficient evidence to determine the effects of abdominal drainage and no drainage on intra‐peritoneal abscess at 14 days (RR 1.23, 95% CI 0.47 to 3.21; 5 RCTs; 453 participants; very low‐quality evidence) or for wound infection at 14 days (RR 2.01, 95% CI 0.88 to 4.56; 5 RCTs; 478 participants; very low‐quality evidence). The increased risk of 30‐day overall complication rate (morbidity) in the drainage group was rated as very low‐quality evidence (RR 6.67, 95% CI 2.13 to 20.87; 1 RCT; 90 participants). There were seven deaths in the drainage group (N = 183) compared to one in the no drainage group (N = 180), equating to an increase in the risk of 30‐day mortality from 0.6% to 2.7% (Peto odds ratio (OR) 4.88, 95% CI 1.18 to 20.09; 4 RCTs; 363 participants; moderate‐quality evidence). There is 'very low‐quality' evidence that drainage increases hospital stay compared to the no drainage group by 2.17 days (95% CI 1.76 to 2.58; 3 RCTs; 298 participants). Other outlined outcomes, hospital costs, pain, and quality of life, were not reported in any of the included studies.
Authors' conclusions
The quality of the current evidence is very low. The effect of abdominal drainage on the prevention of intra‐peritoneal abscess or wound infection after open appendectomy is uncertain for patients with complicated appendicitis. The increased rates for overall complication rate and hospital stay for the drainage group compared to no drainage group is also subject to great uncertainty. Thus, there is no evidence for any clinical improvement by using abdominal drainage in patients undergoing open appendectomy for complicated appendicitis. The increased risk of mortality with drainage comes from eight deaths observed in just under 400 people recruited to the studies. Larger studies are needed to determine the effects of drainage on morbidity and mortality outcomes more reliably.
Plain language summary
Drain use after an open appendectomy for complicated appendicitis
We asked
Is drainage able to reduce the incidence of intra‐peritoneal abscess (a localised collection of pus in the abdomen or pelvis) after an open appendectomy (removal of the appendix through a large incision in the lower abdomen, known as laparotomy) for complicated appendicitis?
Background
Appendicitis refers to inflammation of the appendix. Appendectomy, the surgical removal of the appendix, is performed primarily in individuals who have acute appendicitis. Individuals undergoing an appendectomy for complicated appendicitis, which is defined as gangrenous (soft‐tissue death) or perforated (burst) appendicitis, are more likely to suffer from postoperative complications. The routine placement of a surgical drain to prevent intra‐peritoneal abscess after an appendectomy for complicated appendicitis is controversial and has been questioned.
Study characteristics
We searched for all relevant studies up to 30 June 2017. We identified six clinical studies involving a total of 521 participants. All six studies compared drain use versus no drain use in individuals having an emergency open appendectomy for complicated appendicitis. Studies were conducted in the USA, India, Kenya, Pakistan, and Turkey. The age of the individuals in the trials ranged from 0 years to 82 years.
Key results
The analyses were unable to show a difference in the number of individuals with intra‐peritoneal abscess or wound infection when comparing drain use with no drain use. The death rate was higher in the drainage group than in the no drainage group. The hospital stay was longer (about two days ‐ an 43.5% increase on an 'average' stay) in the drain group than in the no drain group. None of the studies reported the costs, pain, and quality of life. Overall, there is no evidence for any clinical improvement by using abdominal drainage in individuals undergoing open appendectomy for complicated appendicitis.
Quality of the evidence
All of the included studies had shortcomings in terms of methodological quality or reporting of outcomes. Overall, the quality of the current evidence is judged to be very low.
Summary of findings
Summary of findings for the main comparison. Drainage compared to no drainage for complicated appendicitis.
| Abdominal drainage to prevent intra‐peritoneal abscess after open appendectomy for complicated appendicitis | ||||||
| Patient or population: people undergoing emergency open appendectomy for complicated appendicitis Setting: hospital Intervention: drainage Comparison: no drainage | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no drain use | Risk with drain use | |||||
|
Intra‐peritoneal abscess Follow‐up: 14 days |
107 per 1000 | 131 per 1000 (50 to 342) | RR 1.23 (0.47 to 3.21) | 453 (5 studies) | ⊕⊝⊝⊝ Very lowa,b,c | |
|
Wound infection Follow‐up: 30 days |
254 per 1000 | 511 per 1000 (224 to 1000) | RR 2.01 (0.88 to 4.56) | 478 (5 studies) | ⊕⊝⊝⊝ Very lowa,b,c | |
|
Morbidity Follow‐up: 30 days |
67 per 1000 | 445 per 1000 (142 to 1000) | RR 6.67 (2.13 to 20.87) | 90 (1 study) | ⊕⊝⊝⊝ Very lowa,c | |
|
Mortality Follow‐up: 30 days month |
6 per 1000 | 27 per 1000 (7 to 101) | Peto OR 4.88 (1.18 to 20.09) | 363 (4 studies) | ⊕⊕⊕⊝ Moderatec | |
| Hospital stay (days) | The mean hospital stay in the control groups was 4.60 days | The mean hospital stay in the intervention groups was 2.17 days higher (1.76 days to 2.58 days higher) | MD 2.17 days higher (1.76 higher to 2.58 higher) | 298 (3 studies) | ⊕⊝⊝⊝ Very lowa,d | |
| Hospital cost | Not reported | |||||
| Pain | Not reported | |||||
| Quality of life | Not reported | |||||
| *The basis for the assumed risk is the mean comparison group proportion in the studies. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; Peto OR: Peto odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded two levels for very serious risk of bias.
b Downgraded one level for severe inconsistency (substantial heterogeneity as indicated by the I2 statistic).
c Downgraded one level for serious imprecision. For abscess, morbidity and infection, the confidence interval includes appreciable benefit and harm, and the sample size is small. For mortality, there are few events (8 deaths in total)
d Downgraded one level for serious imprecision (small sample size).
Background
Description of the condition
Appendicitis refers to inflammation of the appendix. Appendectomy, the surgical removal of the appendix, is performed primarily as an emergency procedure to treat acute appendicitis (Andersen 2005). Acute appendicitis is the most common cause of acute abdominal pain (Andersen 2005; Rehman 2011; Sauerland 2010; Wilms 2011). The overall incidence of acute appendicitis varies between 76 and 227 cases per 100,000 population per year in different countries (Addiss 1990; Andersson 1994; Andreu‐Ballester 2009; Buckius 2012; Ferris 2017; Körner 1997; Lee 2010; Pieper 1982; Williams 1998). The overall lifetime risk for acute appendicitis is approximately 7% to 8% in the USA, but as high as 16% in South Korea (Addiss 1990; Lee 2010). It affects all age groups, with the highest incidence in individuals 10 to 20 years of age (Addiss 1990; Wilms 2011).
The cause of acute appendicitis is an issue of considerable debate (Andersen 2005; Rehman 2011; Sauerland 2010; Wilms 2011). Acute appendicitis may be associated with obstruction of the appendix lumen (the inside space of the appendix), which could result in increased intraluminal pressure with transmural tissue necrosis (Andersen 2005; Rehman 2011; Sauerland 2010; Wilms 2011). Tissue necrosis is followed by bacterial invasion, which leads to inflammation of the appendix (Andersen 2005; Rehman 2011; Sauerland 2010; Wilms 2011).
Acute appendicitis can be divided into two subgroups: simple appendicitis (e.g. early appendicitis, uncomplicated appendicitis) and complicated appendicitis (e.g. gangrenous appendicitis, perforated appendix without phlegmon or abscess, perforated appendicitis with phlegmon or abscess) (Andersen 2005; Cheng 2017; Simillis 2010). The proportion of complicated appendicitis varies between 15% and 35% in different case series (Andersson 1994; Boomer 2010; Cheng 2017; Cueto 2006; Körner 1997; Livingston 2007; Oliak 2000).
Description of the intervention
Patients with complicated appendicitis usually require appendectomy to relieve symptoms and avoid complications (Andersson 1994; Santacroce 2017). Appendectomy is one of the most common emergency surgical procedures worldwide (Andersen 2005; Rehman 2011; Santacroce 2017; Sauerland 2010; Wilms 2011). There are two types of appendectomy: open appendectomy (removal of the appendix by laparotomy) and laparoscopic appendectomy (removal of the appendix by key‐hole surgery) (Cheng 2012b; Cheng 2015; Yu 2017; Santacroce 2017; Sauerland 2010). Approximately 300,000 appendectomies are performed each year in the USA alone (Hall 2010).
The prognosis of complicated appendicitis is good (Santacroce 2017). The overall mortality rate of complicated appendicitis following appendectomy is less than 1% (Markides 2010; Santacroce 2017). The most common complication after an appendectomy for complicated appendicitis is surgical site infection (e.g. wound infection, intra‐peritoneal abscess) (Andersen 2005; Cueto 2006). Patients with complicated appendicitis are more likely to suffer from surgical site infections than those with simple appendicitis (Markides 2010). Recent published reviews have reported an approximately 10% incidence of surgical site infection (Markides 2010; Santacroce 2017). Patients with surgical site infections usually present with a mild fever, abdominal pain, and bowel dysfunction (e.g. diarrhoea, constipation) (Santacroce 2017). Surgical site infections are associated with increased hospital stays and costs (Horan 1992; Mangram 1999).
Various methods for the prevention of surgical site infections have been suggested, including antibiotic regimens, delayed wound closure, and the use of laparoscopic appendectomy (Andersen 2005; Duttaroy 2009; Markides 2010; Sauerland 2010). One of the most common and convenient interventions might be the application of surgical drains after appendectomy for patients with complicated appendicitis (Petrowsky 2004).
Surgical drains are used to remove blood, pus, and other body fluids from wounds (Durai 2009). There are two primary types of surgical drains: open and closed. An open drain is not air tight (Durai 2009; Gurusamy 2007a; Samraj 2007; Wang 2015). A closed drain consists of a tube that drains into a bag or bottle, the contents of which is air tight (Durai 2009; Gurusamy 2007a; Samraj 2007; Wang 2015).
How the intervention might work
The primary reasons for placing an abdominal drain after an appendectomy are as follows: (i) drainage of established intra‐peritoneal collection; (ii) prevention of further fluid accumulation; (iii) identification and drainage of faecal fistula (Allemann 2011; Gurusamy 2007a; Jani 2011).
The use of abdominal drainage can avoid the accumulation of intra‐peritoneal dirty collections, thereby reducing bacterial contamination of the surgical site (Greenall 1978; Jani 2011; Stone 1978; Tander 2003). Theoretically, abdominal drainage has the potential to reduce the rate of surgical site infection (Greenall 1978; Jani 2011; Stone 1978; Tander 2003).
However, abdominal drainage may fail to prevent intra‐peritoneal abscess because a drain may become blocked and thus ineffective within a few hours after appendectomy (Greenall 1978; Haller 1973; Jani 2011; Magarey 1971). Additionally, the drain itself may act as a foreign body, which interferes with wound healing and increases the risk of surgical site infection (Jani 2011; Stone 1978; Magarey 1971). The use of a drain may also increase the length of the patient's hospital stay (Allemann 2011; Jani 2011; Stone 1978; Tander 2003).
Why it is important to do this review
The use of abdominal drainage after open appendectomy for complicated appendicitis is controversial (Narci 2007; Petrowsky 2004; Piper 2011). It may potentially decrease the risk of surgical site infection following open appendectomy, but it is also possible that it may have no therapeutic benefit and may be associated with negative outcomes (Jani 2011; Mustafa 2016; Petrowsky 2004). The first version of this review was published in 2015 (Cheng 2015). Further randomised controlled trials (RCTs) evaluating the role of abdominal drainage after open appendectomy for complicated appendicitis have been published since the review, and these studies have now been assessed for inclusion and presented in this update.
Objectives
To assess the safety and efficacy of abdominal drainage to prevent intra‐peritoneal abscess after open appendectomy for complicated appendicitis.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) (irrespective of sample size, language, or publication status) that compared drain use and no drain use in patients undergoing open appendectomy for complicated appendicitis. Quasi‐randomised controlled trials (in which the allocation was performed on the basis of a pseudo‐random sequence, e.g. odd/even hospital number or date of birth, alternation) were also included (Chapter 16 in Cochrane Handbook for Systematic Reviews of Interventions,Higgins 2011a).
Types of participants
We included all persons (irrespective of age, sex, or race) who underwent emergency open appendectomy for complicated appendicitis (irrespective of gangrenous appendicitis, perforated appendix without phlegmon or abscess, or perforated appendicitis with phlegmon or abscess), and receiving antibiotic regimens after open appendectomy.
Types of interventions
Use of drain (irrespective of type or material) versus no drain.
Types of outcome measures
Primary outcomes
Intra‐peritoneal abscess (e.g. intra‐abdominal abscess, pelvic abscess) (14 days).
Secondary outcomes
Wound infection (14 days).
Morbidity (overall complication rate; graded by the Clavien‐Dindo complications classification system) (30 days).
Mortality (30 days).
Hospital stay (days).
Hospital costs.
Pain (30 days, any validated score).
Quality of life (30 days, any validated score).
Morbidity was defined by review authors and graded according to the Clavien‐Dindo classification of surgical complications (Clavien 2009). Surgical site infection has been defined and classified as superficial incisional, deep incisional, and organ/space surgical site infection by the Centers for Disease Control and Prevention (CDC) (Anderson 2014; Horan 1992; Mangram 1999). We included intra‐peritoneal abscess (organ/space surgical site infection) as the primary outcome. Wound infection (either superficial or deep incisional surgical site infection) as defined by the study authors.
Search methods for identification of studies
We designed the search strategy with the help of Sys Johnsen (Cochrane Information Specialist of the Cochrane Colorectal Cancer Group). Searches were conducted 30 June 2017 irrespective of language, year, or publication status.
Electronic searches
We searched the following electronic databases with no language or date of publication restrictions:
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library) (2017, Issue 6) (Appendix 1);
MEDLINE (Ovid) (1946 to 30 June 2017) (Appendix 2);
Embase (Ovid) (1974 to 30 June 2017) (Appendix 3);
Science Citation Index Expanded (Web of Science) (1900 to 30 June 2017) (Appendix 4);
World Health Organization International Trials Registry Platform search portal (apps.who.int/trialsearch/) (30 June 2017);
ClinicalTrials.gov (www.clinicaltrials.gov/) (30 June 2017);
Chinese Biomedical Literature Database (CBM) (1978 to 30 June 2017).
Searching other resources
Furthermore, we also searched the following databases 30 June 2017:
Current Controlled Trials (www.controlled‐trials.com/);
Chinese Clinical Trial Register (www.chictr.org/);
EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
We also searched the reference lists of identified studies and meeting abstracts via the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) (www.sages.org/) and Conference Proceedings Citation Index to explore further relevant clinical trials. We planned to communicate with the authors of RCTs that were included for further information in the review.
Data collection and analysis
We conducted this systematic review according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), and the Cochrane Colorectal Cancer Group Module (Andersen 2016).
Selection of studies
After completing the searches, we merged the search results using the software package Endnote X7 (reference management software) and removed duplicate records. Two review authors (CY, CN) independently scanned the title and abstract of every record identified by the search for inclusion. We retrieved the full text for further assessment if the inclusion criteria were unclear from the abstract. We included eligible studies irrespective of whether they reported the measured outcome data. We detected duplicate publications by identifying common authors, centres, details of the interventions, numbers of participants, and baseline data (Chapter 7, the Cochrane Handbook for Systematic Reviews of Interventions,Higgins 2011c). We excluded papers that did not meet the inclusion criteria and listed the reasons for their exclusion. A third review author (DY) resolved any discrepancy between the two review authors by discussion.
Data extraction and management
We used a standard data collection form for study characteristics and outcome data, which had been piloted on at least one study in the review. Two review authors (LZ, ZL) extracted the following study characteristics from included studies:
methods: study design, total duration study and run in, number of study centres and location, study setting, withdrawals, date of study;
participants: number of participants, mean age, age range, gender, severity of condition, diagnostic criteria, inclusion criteria, exclusion criteria;
interventions: intervention, comparison;
outcomes: primary and secondary outcomes specified and collected, time points reported;
notes: funding for trial, notable conflicts of interest of trial authors.
Two review authors (LZ, ZL) independently extracted outcome data from included studies. We resolved disagreements by consensus or by involving a third review author (DY). One review author (LZ) copied across the data from the data collection form into Review Manager 5 (version 5.3, RevMan 2014). We double‐checked that the data were entered correctly by comparing the study reports with how the data were presented in the systematic review. A second review author (ZL) cross‐checked study characteristics for accuracy against the trial reports.
Assessment of risk of bias in included studies
Two review authors (CY, CN) independently assessed the risk of bias in the included trials, using the Cochrane 'Risk of bias' tool (Chapter 8, the Cochrane Handbook for Systematic Reviews of Interventions,Higgins 2011d). We assessed risk of bias for the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective reporting bias;
other sources of bias (baseline imbalances).
We judged each domain as low risk, high risk, or unclear risk of bias according to the criteria used in the Cochrane 'Risk of bias' tool (see Appendix 5) (Chapter 8.5.d, Higgins 2011d). We considered a trial to be at low risk of bias if we assessed the trial as at low risk of bias across all domains. Otherwise, we considered trials at unclear risk of bias or at high risk of bias regarding one or more domains as at high risk of bias. We resolved any difference in opinion by discussion. In case of disagreements, consensus was reached by discussion with a third review author (DY).
We presented the results of the risk of bias in two figures (a 'Risk of bias' graph and a 'Risk of bias' summary) generated by Review Manager 5 (RevMan 2014).
Measures of treatment effect
We performed the meta‐analyses using the software package Review Manager 5.3 (RevMan 2014). For dichotomous outcomes, we calculated the risk ratio (RR) with 95% confidence interval (Deeks 2011). In the case of rare events (e.g. mortality), we calculated the Peto odds ratio (Peto OR) (Deeks 2011). For continuous outcomes, we calculated the mean difference (MD) with 95% confidence interval (Deeks 2011).
Unit of analysis issues
The unit of analysis was the individual patient. We did not encounter any cluster‐randomised trials.
Dealing with missing data
We contacted the original investigators to request further information in case of missing data. However, there was no reply. Thus, we used only the available data in the analyses.
Assessment of heterogeneity
We described heterogeneity in the data using the Chi2 test (Deeks 2011). We considered a P value less than 0.05 to be statistically significant heterogeneity (Deeks 2011). We also used the I2 statistic to measure the quantity of heterogeneity. In case of statistical heterogeneity or clinical heterogeneity (or both), we performed the meta‐analysis but interpreted the result cautiously and planned to investigate potential sources of the heterogeneity.
Assessment of reporting biases
We planned to perform and examine a funnel plot to explore possible publication biases. However, as the number of trials included was less than 10, we did not produce any funnel plots as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 10 (Sterne 2011).
Data synthesis
We performed the meta‐analyses using the Review Manager 5 software provided by Cochrane (version 5.3, RevMan 2014). For all analyses, we employed the random‐effects model for conservative estimation, except for the Peto OR which only has a fixed‐effect method. We considered a P value less than 0.05 to be statistically significant.
Subgroup analysis and investigation of heterogeneity
We did not perform any planned subgroup analysis because too few trials were included in this review.
Sensitivity analysis
We performed a sensitivity analysis for the primary outcome Intra‐peritoneal abscess by excluding quasi‐randomised controlled trials to determine whether the conclusions were robust to the decisions made during the review process.
Trial sequential analysis
We performed trial sequential analysis (TSA) for the primary outcome if possible. TSA aims to reduce the risk of random error in the setting of repetitive testing of accumulating data, thereby improving the reliability of conclusions (Brok 2008; Wetterslev 2008; Wetterslev 2009). The required information size was calculated on the basis of a risk ratio reduction (RRR) of 20% (Brok 2008; Wetterslev 2008; Wetterslev 2009). The results of the trials were presented as a cumulative Z‐curve. The trial sequential monitoring boundaries were constructed and the diversity‐adjusted required information size calculated with a type 1 error of 5% and a type 2 error of 20% (Brok 2008; Wetterslev 2008; Wetterslev 2009). The results were presented as a graph with the cumulative meta‐analysis results entered. The TSA shows firm evidence of intervention effects (or no intervention effects) if the cumulative Z‐curve crosses the monitoring boundaries; it also shows that additional trials may be needed if the boundaries are not crossed (Brok 2008; Wetterslev 2008; Wetterslev 2009). TSA was performed using Trial Sequential Analysis software (TSA 2011).
'Summary of findings' tables
We evaluated the quality of evidence using the GRADE (Schünemann 2009) approach for each outcome, including any subgroup analysis or sensitivity analysis.
We presented the quality of evidence in 'Summary of finding' table for the following comparison:
drainage versus no drainage.
We justified judgements about the quality of the evidence (high, moderate, low, or very low), documented, and incorporated into the reporting of results for each outcome. The quality of evidence could be downgraded by one level (serious concern) or two levels (very serious concerns) applying to each of the following five reasons listed: risk of bias; inconsistency (unexplained heterogeneity, inconsistency of results); indirectness (indirect population, intervention, control, outcomes); imprecision (wide CIs, single trials); and publication bias.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
Date of search 30 June 2017.
In this updated review, we identified 746 records through the electronic searches of the Cochrane Library (46 records), MEDLINE (Ovid) (65 records), Embase (Ovid) (102 records), Science Citation Index Expanded (Web of Science) (231 records), and Chinese Biomedical Literature Database (CBM) (302 records). We identified two records through scanning reference lists of the identified randomised controlled trials (Haller 1973; Johnson 1993). Of the 748 records, 677 records had already been assessed for the first version of this updated review (648 records prior to 2014 and 29 duplicates). Of the remaining 71 records, we excluded 68 clearly irrelevant records after reading titles and abstracts. The remaining three records were retrieved in full for further assessment (Mustafa 2016; Beek 2015; Song 2015). Two of these, Beek 2015 and Song 2015, were excluded being non‐randomised studies. The study by Mustafa 2016 was included in this updated review. We identified six trials ( with a total of 521 participants) comparing drainage with no drainage for persons undergoing appendectomy for complicated appendicitis (Dandapat 1992; Haller 1973; Jani 2011; Mustafa 2016; Stone 1978; Tander 2003). Two hundred and sixty‐two persons were randomised to the drainage group and 259 persons to the no drainage group. With so few participants, all of the analyses were underpowered.The study flow diagram is shown in Figure 1.
1.
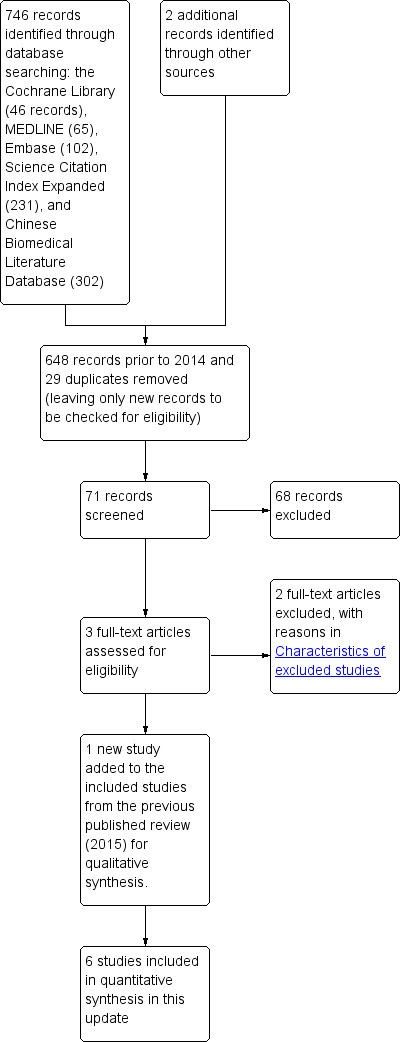
Study flow diagram.
Included studies
In the first published version of this review from 2015 (Cheng 2015), we included five trials, published between 1973 and 2003 (Dandapat 1992; Haller 1973; Jani 2011; Stone 1978; Tander 2003). In this update, we identified one recent trial (Mustafa 2016), to a total of six included trials (including 521 participants). All the six trials were completed trials and all of these provided data for the analyses. Details of the trials are shown in the table "Characteristics of included studies". Four trials were randomised controlled trials (Dandapat 1992; Jani 2011; Mustafa 2016; Tander 2003), and two trials were quasi‐randomised controlled trials (Haller 1973; Stone 1978). All six trials compared drain use with no drain use for patients undergoing open appendectomy. Studies were conducted in the USA (Haller 1973; Stone 1978), India (Dandapat 1992), Kenya (Jani 2011), Pakistan (Mustafa 2016), and Turkey (Tander 2003). The age of the individuals in the trials varied between 0 years and 82 years. The mean proportion of females varied between 19% and 44%. There was no difference in the characteristics of patients in the intervention group or control in any of the trials. Overall, 32 (6.1%) participants had gangrenous appendicitis, 11 (2.1%) participants had appendiceal abscess, and 478 (91.8%) participants had perforated appendicitis in the trials. All of the participants received antibiotic regimens after open appendectomy. The outcomes measured were intra‐peritoneal abscess, wound infection, morbidity, mortality, and hospital stay.
Excluded studies
One randomised controlled trial was excluded because it focused on extraperitoneal wound drainage (Everson 1977). Another randomised controlled trial was excluded because it compared peritoneal lavage with abdominal drainage (Toki 1995). Two randomised controlled trials were excluded because the antibiotic regimens were used in a non‐random manner (several participants received antibiotic regimens after appendectomy, whereas other participants did not) (Greenall 1978; Magarey 1971). None of the other excluded studies were randomised controlled trials (Allemann 2011; Al‐Shahwany 2012; Beek 2015; Ezer 2010; Johnson 1993; Narci 2007; Piper 2011; Song 2015).
Risk of bias in included studies
The risk of bias of the included studies is shown in Figure 2 and Figure 3. None of the included trials were judged to be of low risk of bias.
2.
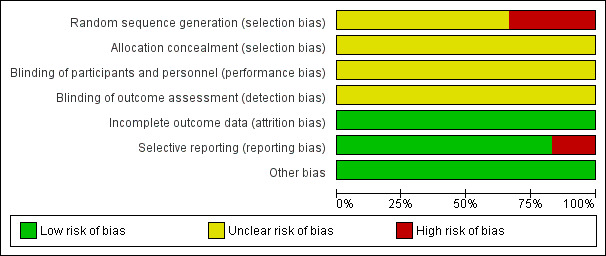
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
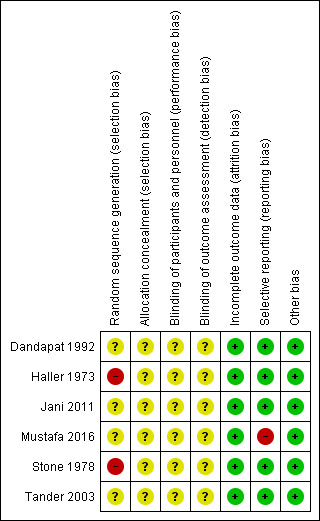
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was at unclear risk of bias in four trials (Dandapat 1992; Jani 2011; Mustafa 2016; Tander 2003) and high risk of bias in two trials where participants were randomised using pseudo‐random sequences (odd/even hospital number) (Haller 1973; Stone 1978). Allocation concealment was at an unclear risk of bias in all six trials (Dandapat 1992; Haller 1973; Jani 2011; Mustafa 2016; Stone 1978; Tander 2003).
Blinding
Blinding of participants and personnel was at an unclear risk of bias in all six trials (Dandapat 1992; Haller 1973; Jani 2011; Mustafa 2016; Stone 1978; Tander 2003). Blinding of outcome assessment was also of unclear risk of bias in all six trials (Dandapat 1992; Haller 1973; Jani 2011; Mustafa 2016; Stone 1978; Tander 2003).
Incomplete outcome data
There was a low risk of bias for incomplete outcome data in all six trials (Dandapat 1992; Haller 1973; Jani 2011; Mustafa 2016; Stone 1978; Tander 2003).
Selective reporting
The trial protocols were not available for any of the included trials. Five trials reported the primary outcomes of this review (Dandapat 1992; Haller 1973; Jani 2011; Stone 1978; Tander 2003). There may have been some selective outcome reporting in the secondary outcomes (secondary outcomes of this review were not reported), but the review authors considered these five trials to be free of selective reporting for the primary outcomes. One trial was at high risk of selective reporting bias as the primary outcome of the review was not reported (Mustafa 2016).
Other potential sources of bias
No baseline imbalances were observed, therefore this was at a low risk of bias in all six trials (Dandapat 1992; Haller 1973; Jani 2011; Mustafa 2016; Stone 1978; Tander 2003).
Effects of interventions
See: Table 1
Primary outcome
Intra‐peritoneal abscess
We identified five trials (453 participants) reporting intra‐peritoneal abscess. The rate of intra‐peritoneal abscess was 15.8% in the drainage group and 10.7% in the no drainage group. There was no evidence of a difference in the rate of intra‐peritoneal abscess (including intra‐abdominal abscess and pelvic abscess) between the groups (risk ratio (RR) 1.23, 95% confidence interval (CI) 0.47 to 3.21; P = 0.67; Analysis 1.1). We downgraded the quality of the evidence to very low due to risk of bias, serious imprecision, and serious inconsistency.
1.1. Analysis.
Comparison 1 Drain use versus no drain use, Outcome 1 Intra‐peritoneal abscess.
The Trials Sequential Analysis graph showed that the cumulative Z‐curve did not cross the trial sequential boundaries (Figure 4). The analysis showed a diversity‐adjusted required information size of 2570 participants (the number of participants needed to reach firm evidence of an intervention effect of 20% RRR). The number of participants included corresponded to only a small fraction (20.3%) of the diversity‐adjusted required information size. Accordingly, we lack evidence to support or refute an intervention effect as data were too few.
4.
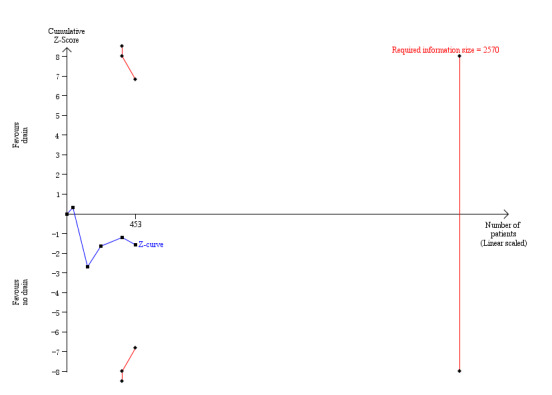
Trial sequential analysis of drain use versus no drain use for intra‐peritoneal abscess. Analysis was performed with an event rate of 10.7% (Pc) in the control group, a risk ratio reduction of 20%, alpha 5%, beta 20%, and observed diversity 63%. The cumulative Z‐curve did not cross the trial sequential boundaries (inward sloping etched lines). The results showed that the observed diversity‐adjusted required information size was 2,570 participants, corresponding to 20.3% of the total sample size in the included trials. Accordingly, the meta‐analysis did not support or refute an intervention effect as data were too few.
Secondary outcomes
Wound infection
We identified 5 trials (478 participants) reporting wound infection. The rate of wound infection was 37.0% in the drainage group and 25.4% in the no drainage group. There was no evidence of a difference in the wound infection rate between the groups (RR 2.01, 95% CI 0.88 to 4.56; P = 0.10; Analysis 1.2). We downgraded the quality of the evidence to very low due to risk of bias, serious imprecision, and serious inconsistency.
1.2. Analysis.
Comparison 1 Drain use versus no drain use, Outcome 2 Wound infection.
Morbidity
We identified one trial (90 participants) reporting overall complication rate (morbidity). The overall complication rate defined according to the Clavien‐Dindo classification was 44.4% in the drainage group and 6.7% in the no drainage group. The overall morbidity was higher in the drainage group than in the no drainage group (RR 6.67, 95% CI 2.13 to 20.87; P = 0.001; Analysis 1.3). We downgraded the quality of the evidence to very low due to risk of bias and serious imprecision.
1.3. Analysis.
Comparison 1 Drain use versus no drain use, Outcome 3 Morbidity.
Mortality
We identified four trials (363 participants) reporting mortality. The death rate was 3.8% in the drainage group and 0.6% in the no drainage group. Mortality was higher in the drainage group than in the no drainage group (Peto odds ratio (OR) 4.88, 95% CI 1.18 to 20.09; P = 0.03; Analysis 1.4). We downgraded the quality of the evidence to moderate due to serious imprecision.
1.4. Analysis.
Comparison 1 Drain use versus no drain use, Outcome 4 Mortality.
Hospital stay
We identified three trials (298 participants) reporting hospital stay. The mean length of hospital stay was 6.6 days in the drainage group and 4.6 days in the no drainage group. The hospital stay was longer in the drainage group than in the no drainage group [mean difference (MD) 2.17 days (43.5% increase of an 'average' hospital stay); 95% CI 1.76 to 2.58; P < 0.001; Analysis 1.5). We downgraded the quality of the evidence to very low due to risk of bias and serious imprecision.
1.5. Analysis.
Comparison 1 Drain use versus no drain use, Outcome 5 Hospital stay.
Hospital costs
None of the trials reported this outcome.
Pain
None of the trials reported this outcome.
Quality of life
None of the trials reported this outcome.
Reporting biases
We did not create funnel plots to assess reporting biases because the number of trials included was fewer than 10 (Sterne 2011).
Sensitivity analysis
We observed no change in the intra‐peritoneal abscess outcome by excluding two quasi‐randomised controlled trials (Haller 1973; Stone 1978) (Analysis 2.1).
2.1. Analysis.
Comparison 2 Drain use versus no drain use (sensitivity analyses by excluding quasi‐randomised trials), Outcome 1 Intra‐peritoneal abscess.
Discussion
Summary of main results
Six studies with 521 people contributed data to the primary outcome of this review, and showed no evidence of difference in the incidence of intra‐peritoneal abscess between the drainage group and the no drainage group. Very low‐quality trials furthermore showed that abdominal drainage increased rate of wound infection, overall morbidity, mortality, and length of hospital stay.
The primary reason for the placement of a drain after appendectomy is to prevent intra‐peritoneal abscess (e.g. intra‐abdominal abscess, pelvic abscess). We found that the routine use of abdominal drainage after complicated appendectomy did not reduce the incidence of intra‐peritoneal abscess. The possible reasons for the failure to decrease the incidence of intra‐peritoneal abscess are as follows. First, surgical drains may become blocked by blood or fibrin clots (Schein 2008; Yates 1905). Additionally, surgical drains cannot drain the entire abdominal cavity. Abdominal collections or abscesses can occur despite abdominal drainage (Schein 2008; Yates 1905). Moreover, this review included six trials with only 262 participants undergoing abdominal drainage. This review may not have the statistical power to detect the any clinically meaningful difference between abdominal drainage and no drainage for the prevention of intra‐peritoneal abscess even if such a difference was present. In addition, where differences were detected, confidence in these results is low, as the small numbers means they could be spurious results, and the impact of a bias can be exacerbated in underpowered analyses. Thus, it is not clear whether routine abdominal drainage has any effect on the prevention of intra‐peritoneal abscess in patients undergoing open appendectomy for complicated appendicitis.
Overall completeness and applicability of evidence
All of the trials included patients undergoing emergency open appendectomy for complicated appendicitis (e.g. gangrenous appendicitis and perforated appendicitis). The majority (91.8%) of the patients had perforated appendicitis with local or general peritonitis. Thus, the results of this review are applicable to patients undergoing emergency open appendectomy for perforated appendicitis.
Quality of the evidence
None of the trials were at a low risk of bias. The trials included under each comparison were too few to assess inconsistency and publication bias. Direct comparisons of different types of drain were not available, only comparisons for each type of drain with no drain. In theory, these trials could allow indirect comparisons of the effect of different types of drain, however, small sample sizes and methodological flaws of the trials preclude such indirect comparison. The confidence intervals for the majority of outcomes were wide, indicating that the estimates of effect obtained are imprecise. Overall, we considered the quality of the evidence to be very low (Table 1).
Potential biases in the review process
There were several potential biases of note in the review process. First, there were only six trials with 521 patients included in the review; thus, there was a lack of data on this topic to date. Second, we did not create funnel plots to assess potential publication bias due to the small number of included trials. Third, we did not perform any planned subgroup analyses to assess heterogeneity because of the small number of trials included for each outcome. Additionally, patient selection processes and blinding were unclear for most of the studies. We contacted the original investigators to request further information. However, we did not receive any replies. Moreover, an important source of bias in the included studies was the use of antibiotic regimens (Andersen 2005). For example, the type and length of antibiotic therapy were important confounding factors for various outcomes (e.g. intra‐peritoneal abscess, wound infection) (Andersen 2005). However, the type and length of antibiotic therapy varied a great deal in different trials. It was difficult to perform subgroup analyses to assess the heterogeneity. A final point is that the imputation of the standard deviation for hospital stay from the range might also introduce bias into this review.
Agreements and disagreements with other studies or reviews
There is increasing evidence in Cochrane Reviews that routine abdominal drainage after various abdominal operations is not essential (Cheng 2016; de Jesus 2004; Gurusamy 2007a; Gurusamy 2013; Gurusamy 2007b; Wang 2015). The routine use of surgical drains has been questioned in other areas, including thyroid, gynaecological, and orthopaedic surgeries (Charoenkwan 2017; Gates 2013; Parker 2007; Samraj 2007).
The systematic review by Petrowsky and colleagues (Petrowsky 2004) included five trials comparing drain use with no drain use in patients undergoing appendectomy for complicated appendicitis (Dandapat 1992; Greenall 1978; Haller 1973; Magarey 1971; Stone 1978). Two of these five trials, in which antibiotic regimens were used in a non‐random manner (some participants received antibiotic regimens after appendectomy, whereas other participants did not), were not included in this review (Greenall 1978; Magarey 1971). Petrowsky and colleagues concluded that abdominal drainage did not reduce postoperative complications and appeared harmful with respect to the development of faecal fistula (Petrowsky 2004). Thus, these authors recommended that abdominal drainage should be avoided at any stage of appendicitis (Petrowsky 2004). This review has not reached definitive conclusions, in part because the number of participants included is low for detecting a benefit on intra‐peritoneal abscess. A sample size of 2570 would be required to detect an absolute reduction in the intra‐peritoneal abscess rate of 2% (from 10% to 8%) at 80% power and an alpha‐error set at 0.05.
Authors' conclusions
Implications for practice.
The effect of abdominal drainage for prevention of intra‐peritoneal abscess after open appendectomy is uncertain for patients with complicated appendicitis due to the very low quality of evidence. The effect on wound infection after open appendectomy is also uncertain for patients with complicated appendicitis. The increased rates for overall complication rate and hospital stay for the drainage group compared to no drainage group is also subject to great uncertainty. The excess mortality in the drainage group was observed from eight deaths in total across the studies. There is no evidence for any clinical improvement by using abdominal drainage in patients undergoing open appendectomy for complicated appendicitis.
Implications for research.
High‐quality trials are urgently required. Investigators should employ adequate methods of randomisation, allocation concealment and blinding of outcome assessors to reduce the risk of bias. Studies with larger sample sizes will help to ensure that there is adequate power to detect effects on morbidity outcomes, and to provide further information on mortality. Future trials should specify a set of criteria for antibiotics use and define all of the patient‐important outcomes (e.g. pain, quality of life) more accurately and report them in accordance with validated criteria.
What's new
| Date | Event | Description |
|---|---|---|
| 31 August 2017 | New citation required but conclusions have not changed | Searches updated 30 June, 2017. One new RCT identified and included in analyses. |
| 31 August 2017 | New search has been performed | Searches updated 30 June, 2017, and review updated accordingly with one new randomised controlled trial included. Author byline changed. This updated review furthermore included trial sequential analysis (TSA) for the primary outcome, aiming to reduce the risk of random error in the setting of repetitive testing of accumulating data, thereby improving the reliability of conclusions. |
History
Protocol first published: Issue 10, 2012 Review first published: Issue 2, 2015
| Date | Event | Description |
|---|---|---|
| 11 December 2014 | Amended | The authors changed the title in the review stage. |
Acknowledgements
We would like to thank Cochrane Colorectal Cancer editorial office, Dr. Henning Keinke Andersen and Dr. Anne Sofie Christensen, who assisted in the development of the review, and Dr. Sys Johnsen and Dr. Sara Hallum, who developed the search strategy and ran the literature search. We would also like to thank editors and peer referees for valuable comments to this updated review Finally, we would like to thank the contribution of authors of the previous version of this review: including Dr. Shiyi Zhou, Dr. Rongxing Zhou, Dr. Jiong Lu, Dr. Sijia Wu, Dr. Xianze Xiong, Dr. Hui Ye, Dr. Yixin Lin and Dr. Taixiang Wu.
Appendices
Appendix 1. Search strategy for CENTRAL (2017, Issue 6)
#1 MeSH descriptor: [Appendectomy] explode all trees
#2 MeSH descriptor: [Appendicitis] explode all trees
#3 appendectom* or appendic*:ti,ab,kw
#4 (#1 or #2 or #3)
#5 MeSH descriptor: [Drainage] explode all trees
#6 MeSH descriptor: [Suction] explode all trees
#7 MeSH descriptor: [Negative‐Pressure Wound Therapy] explode all trees
#8 ((negative pressure or negative‐pressure) near/3 (dressing* or therap*)):ti,ab,kw
#9 ((vacuum‐assisted or vacuum assisted) near/3 closure*):ti,ab,kw
#10 (drain* or suction*):ti,ab,kw
#11 (#5 or #6 or #7 or #8 or #9 or #10)
#12 (#4 and #11)
Appendix 2. Search strategy for MEDLINE (Ovid) (1946 to 30 June 2017)
1. exp Appendectomy/
2. exp Appendicitis/
3. (appendectom* or appendic*).mp.
4. 1 or 2 or 3
5. exp Drainage/
6. exp Negative‐Pressure Wound Therapy/
7. exp Suction/
8. ((negative pressure or negative‐pressure) adj3 (dressing* or therap*)).mp.
9. ((vacuum‐assisted or vacuum assisted) adj closure*).mp.
10. (drain* or suction*).mp.
11. 5 or 6 or 7 or 8 or 9 or 10
12. 4 and 11
13. randomized controlled trial.pt.
14. controlled clinical trial.pt.
15. randomized.ab.
16. placebo.ab.
17. clinical trials as topic.sh.
18. randomly.ab.
19. trial.ti.
20. 13 or 14 or 15 or 16 or 17 or 18 or 19
21. exp animals/ not humans.sh.
22. 20 not 21
23. 12 and 22
Appendix 3. Search strategy for Embase (Ovid) (1974 to 30 June 2017)
1. exp appendectomy/
2. exp acute appendicitis/
3. exp appendicitis/
4. (appendectom* or appendic*).mp.
5. 1 or 2 or 3 or 4
6. exp drain/
7. exp abscess drainage/
8. exp abdominal drainage/
9. exp wound drainage/
10. exp surgical drainage/
11. exp vacuum assisted closure/
12. exp suction/
13. ((negative pressure or negative‐pressure) adj3 (dressing* or therap*)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
14. ((vacuum‐assisted or vacuum assisted) adj closure*).mp.
15. (drain* or suction*).mp.
16. 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
17. 5 and 16
18. CROSSOVER PROCEDURE.sh.
19. DOUBLE‐BLIND PROCEDURE.sh.
20. SINGLE‐BLIND PROCEDURE.sh.
21. (crossover* or cross over*).ti,ab.
22. placebo*.ti,ab.
23. (doubl* adj blind*).ti,ab.
24. allocat*.ti,ab.
25. trial.ti.
26. RANDOMIZED CONTROLLED TRIAL.sh.
27. random*.ti,ab.
28. 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
29. (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.)
30. 28 not 29
31. 17 and 30
Appendix 4. Search strategy for Science Citation Index Expanded (1900 to 30 June 2017)
#1 Topic=(appendectom* OR appendic*)
#2 Topic=(drain* OR suction* OR negative pressure wound therap* OR negative‐pressure wound therap* OR vacuum‐assisted closure OR vacuum assisted closure*)
#3 Topic=(random* OR control* OR RCT* OR placebo OR trial* OR group*)
#4 (#1 AND #2 AND #3)
Appendix 5. Criteria for judging risk of bias in the 'Risk of bias' assessment tool
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. | |
| Criteria for a judgement of 'Low risk' of bias. | The investigators described a random component in the sequence generation process such as:
*Minimisation may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for the judgement of 'High risk' of bias. | The investigators described a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, e.g.:
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, e.g.:
|
| Criteria for the judgement of 'Unclear risk' of bias. | Insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk.' |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. | |
| Criteria for a judgement of 'Low risk' of bias. | Participants and investigators enrolling participants could not have foreseen assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| Criteria for the judgement of 'High risk' of bias. | Participants or investigators enrolling participants could possibly have foreseen assignments and thus introduced selection bias, such as allocation based on:
|
| Criteria for the judgement of 'Unclear risk' of bias. | Insufficient information to permit judgement of 'Low risk' or 'High risk.' This is usually the case if the method of concealment was not described or not described in sufficient detail to allow a definite judgement; e.g. if the use of assignment envelopes was described, but it remained unclear whether envelopes were sequentially numbered, opaque and sealed. |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. | |
| Criteria for a judgement of 'Low risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'High risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'Unclear risk' of bias. | Any one of the following:
|
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. | |
| Criteria for a judgement of 'Low risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'High risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'Unclear risk' of bias. | Any one of the following:
|
|
Incomplete outcome data Attrition bias due to amount, nature, or handling of incomplete outcome data. | |
| Criteria for a judgement of 'Low risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'High risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'Unclear risk' of bias. | Any one of the following:
|
|
Selective reporting Reporting bias due to selective outcome reporting. | |
| Criteria for a judgement of 'Low risk' of bias. | Any of the following:
|
| Criteria for the judgement of 'High risk' of bias. | Any one of the following:
|
| Criteria for the judgement of 'Unclear risk' of bias. | Insufficient information to permit judgement of 'Low risk' or 'High risk.' It is likely that the majority of studies will fall into this category. |
|
Other bias Bias due to problems not covered elsewhere in the table. | |
| Criteria for a judgement of 'Low risk' of bias. | Study appeared to be free of other sources of bias. |
| Criteria for the judgement of 'High risk' of bias. | There was ≥ 1 important risk of bias; e.g. the study:
|
| Criteria for the judgement of 'Unclear risk' of bias. | There may be a risk of bias, but there was either:
|
Data and analyses
Comparison 1. Drain use versus no drain use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intra‐peritoneal abscess | 5 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.47, 3.21] |
| 2 Wound infection | 5 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.88, 4.56] |
| 3 Morbidity | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 6.67 [2.13, 20.87] |
| 4 Mortality | 4 | 363 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.88 [1.18, 20.09] |
| 5 Hospital stay | 3 | 298 | Mean Difference (IV, Random, 95% CI) | 2.17 [1.76, 2.58] |
Comparison 2. Drain use versus no drain use (sensitivity analyses by excluding quasi‐randomised trials).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intra‐peritoneal abscess | 3 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.28, 2.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dandapat 1992.
| Methods | Randomised controlled trial | |
| Participants | Country: India
Number randomised: 86
Post‐randomisation dropout: 0 (0%)
Children: 16 (19%) Adults: 70 (81%) Females: 16 (19%) Normal appendix: 0 (0%) Simple appendicitis: 0 (0%) Gangrenous appendicitis: 0 (0%) Perforated appendicitis: 86 (100%) Appendiceal phlegmon or abscess: 0 (0%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants with complicated appendicitis (n = 86) were randomly assigned to 2 groups Group 1: drainage (n = 40) Group 2: no drainage (n = 46) | |
| Outcomes | The outcomes reported were wound infection, intra‐peritoneal abscess, duration of postoperative fever, postoperative complications, mortality, and hospital stay | |
| Notes | The drainage tube (corrugated rubber drain) was placed down into the right iliac fossa | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all of the primary outcomes were reported. There was some selective outcome reporting in the secondary outcomes, but the review authors considered this trial to be free of selective reporting for the primary outcomes |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Haller 1973.
| Methods | Quasi‐randomised controlled trial | |
| Participants | Country:USA
Number randomised: 43
Post‐randomisation dropout: 0 (0%)
Children (0 ‐ 14 years): 43 (100%) Adults: 0 (0%) Females: 10 (23%) Normal appendix: 0 (0%) Simple appendicitis: 0 (0%) Gangrenous appendicitis: 0 (0%) Perforated appendicitis: 43 (100%) Appendiceal phlegmon or abscess: 0 (0%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants with complicated appendicitis (n = 43) were randomly assigned to 2 groups Group 1: drainage (n = 24) Group 2: no drainage (n = 19) | |
| Outcomes | The outcomes reported were intra‐peritoneal abscess, postoperative complications, mortality, and hospital stay | |
| Notes | The drainage tube (Penrose drain) was placed through the wound down into the right iliac fossa and pelvis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Transperitoneal drainage was used in children with even hospital numbers and no drainage or wound drainage alone was used in children with odd hospital numbers" Comment: the allocation was performed on the basis of a pseudo‐random sequence (odd/even hospital number) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all of the primary outcomes were reported. There was some selective outcome reporting in the secondary outcomes, but the review authors considered this trial to be free of selective reporting for the primary outcomes |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Jani 2011.
| Methods | Randomised controlled trial | |
| Participants | Country: Kenya
Number randomised: 90
Post‐randomisation dropout: 0 (0%)
Age (13 to 26): 44 (49%) Age (27 to 54): 46 (51%) Females: 40 (44%) Normal appendix: 0 Simple appendicitis: 0 Gangrenous appendicitis: 0 (0%) Perforated appendicitis: 79 (87.8%) Appendiceal phlegmon or abscess: 11 (12.2%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants with complicated appendicitis (n = 90) were randomly assigned to 2 groups Group 1: drainage (n = 45) Group 2: no drainage (n = 45) | |
| Outcomes | The outcomes reported were wound infection, intra‐peritoneal abscess, morbidity, postoperative complications, hospital stay, and duration of antibiotic use | |
| Notes | The drainage tube (PVC suction catheter) was placed through a separate incision down into the right iliac fossa | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all of the primary outcomes were reported. There was some selective outcome reporting in the secondary outcomes, but the review authors considered this trial to be free of selective reporting for the primary outcomes |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Mustafa 2016.
| Methods | Randomised controlled trial | |
| Participants | Country: Pakistan
Number randomised: 68
Post‐randomisation dropout: 0 (0%)
Age (18 to 25): 31 (46%) Age (26 to 33): 26 (38%) Age (34 to 39): 11 (16%) Females: 32 (47%) Normal appendix: 0 Simple appendicitis: 0 Gangrenous appendicitis: 0 (0%) Perforated appendicitis: 68 (100%) Appendiceal phlegmon or abscess: 0 (0%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants with complicated appendicitis (n = 68) were randomly assigned to 2 groups Group 1: drainage (n = 34) Group 2: no drainage (n = 34) | |
| Outcomes | The outcomes reported were wound infection and hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "These patients were randomly allocated into 2 treatment groups using lottery method" Comment: no information provided about the method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | High risk | Comment: the primary outcome was not reported |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Stone 1978.
| Methods | Quasi‐randomised controlled trial | |
| Participants | Country: USA
Number randomised: 283
Post‐randomisation dropout: 0 (0%)
Children: not mentioned Adults: not mentioned Females: 124 (44%) Normal appendix: 0 (0%) Simple appendicitis: 66 (23%) Suppurative appendicitis: 123 (44%) Gangrenous appendicitis: 32 (11%) Perforated appendicitis: 62 (22%) Appendiceal phlegmon or abscess: 0 (0%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants with complicated appendicitis (n = 94) were randomly assigned to 2 groups Group 1: drainage (n = 49) Group 2: no drainage (n = 45) | |
| Outcomes | The outcomes reported were wound infection, intra‐peritoneal abscess, postoperative complications, and mortality | |
| Notes | The drainage tube (Penrose drain) was placed through a separate incision down into the right iliac fossa | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "A Penrose drain was inserted through a separate stab wound if the final digit of the patient's hospital number was an odd figure. An even final digit dictated exclusion of any form of peritoneal drainage". Comment: the allocation was performed on the basis of a pseudo‐random sequence (odd/even hospital number) |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all of the primary outcomes were reported. There was some selective outcome reporting in the secondary outcomes, but the review authors considered this trial to be free of selective reporting for the primary outcomes |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Tander 2003.
| Methods | Randomised controlled trial | |
| Participants | Country: Turkey Number randomised: 140 Post‐randomisation dropout: 0 (0%) Children (0 ‐ 11 years): 140 (100%) Adults: 0 (0%) Females: 38 (27%) Normal appendix: 0 (0%) Simple appendicitis: 0 (0%) Gangrenous appendicitis: 0 (0%) Perforated appendicitis: 140 (100%) Appendiceal phlegmon or abscess: 0 (0%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants with complicated appendicitis (n = 140) were randomly assigned to 2 groups Group 1: drainage (n = 70) Group 2: no drainage (n = 70) | |
| Outcomes | The outcomes reported were wound infection, intra‐peritoneal abscess, postoperative complications, mortality, hospital stay, and duration before oral intake | |
| Notes | 2 drainage tubes (Penrose drains) were placed through the wound down into the right iliac fossa and pelvis, respectively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all of the primary outcomes were reported. There was some selective outcome reporting in the secondary outcomes, but the review authors considered this trial to be free of selective reporting for the primary outcomes |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Shahwany 2012 | A non‐randomised study |
| Allemann 2011 | A non‐randomised study |
| Beek 2015 | A non‐randomised study |
| Everson 1977 | Randomised controlled trial about extraperitoneal wound drainage (wound drainage versus no wound drainage) |
| Ezer 2010 | A non‐randomised study |
| Greenall 1978 | Randomised controlled trial in which antibiotic regimens were used in a non‐random manner |
| Johnson 1993 | A non‐randomised study |
| Magarey 1971 | Randomised controlled trial in which antibiotic regimens were used in a non‐random manner |
| Narci 2007 | A non‐randomised study |
| Piper 2011 | A non‐randomised study |
| Song 2015 | A non‐randomised study |
| Toki 1995 | Randomised controlled trial about peritoneal lavage versus abdominal drainage |
Differences between protocol and review
We changed the title to reflect the primary outcome and open appendectomy because none of the trials included patients undergoing laparoscopic appendectomy. We included two quasi‐RCTs and performed a sensitivity analysis by excluding the two trials according to the suggestions of the editors and reviewers. None of the included RCTs compared open drain with closed drain (tubes), or early drain removal with late drain removal. Thus, we did not consider these two types of interventions. We added that all patients received similar antibiotic regimens after open appendectomy in the intervention; studies that included participants who did not receive prophylactic antibiotics were excluded, because the use of antibiotic regimens after open appendectomy was found to have a positive effect on clinically relevant outcomes by another Cochrane Review (Andersen 2005). The trials did not report infection at 14 days, therefore the data for 30 days were reported as these data were still considered to be clinically relevant. We also performed trial sequential analysis (TSA) for the primary outcome intra‐peritoneal abscess. TSA aims to reduce the risk of random error in the setting of repetitive testing of accumulating data, thereby improving the reliability of conclusions.
Contributions of authors
Li Z: drafted the final review, extracted data from the trials, entered data into RevMan, and carried out the analysis.
Zhao L: extracted data from the trials and entered data into RevMan.
Cheng Y: drafted the protocol, selected which trials to include, and assessed the risk of bias of the trials.
Cheng N: selected which trials to include and assessed the risk of bias of the trials.
Deng Y: revised the final review and secured funding for the review.
Li Z, Zhao L, and Cheng Y: contributed equally to developing the review.
Declarations of interest
Li Z: None known
Zhao L: None known
Cheng Y: None known
Cheng N: None known
Deng Y: None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Dandapat 1992 {published data only}
- Dandapat MC, Panda C. A perforated appendix: should we drain?. Journal of the Indian Medical Association 1992;90(6):147‐8. [PubMed] [Google Scholar]
Haller 1973 {published data only}
- Haller JA Jr, Shaker IJ, Donahoo JS, Schnaufer L, White JJ. Peritoneal drainage versus non‐drainage for generalized peritonitis from ruptured appendicitis in children: a prospective study. Annals of Surgery 1973;177(5):595‐600. [PMC free article] [PubMed] [Google Scholar]
Jani 2011 {published data only}
- Jani PG, Nyaga PN. Peritoneal drains in perforated appendicitis without peritonitis: a prospective randomized controlled study. East and Central African Journal of Surgery 2011;16(2):62‐71. [Google Scholar]
Mustafa 2016 {published data only}
- Mustafa MIT, Chaudhry SM, Mustafa RIT. Comparison of early outcome between patients of open appendectomy with and without drain for perforated appendicitis. Pakistan Journal of Medical and Health Sciences 2016;10(3):890‐3. [Google Scholar]
Stone 1978 {published data only}
- Stone HH, Hooper CA, Millikan WJ Jr. Abdominal drainage following appendectomy and cholecystectomy. Annals of Surgery 1978;187(6):606‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tander 2003 {published data only}
- Tander B, Pektas O, Bulut M. The utility of peritoneal drains in children with uncomplicated perforated appendicitis. Pediatric Surgery International 2003;19(7):548‐50. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allemann 2011 {published data only}
- Allemann P, Probst H, Demartines N, Schäfer M. Prevention of infectious complications after laparoscopic appendectomy for complicated acute appendicitis‐‐the role of routine abdominal drainage. Langenbeck's Archives of Surgery 2011;396(1):63‐8. [DOI] [PubMed] [Google Scholar]
Al‐Shahwany 2012 {published data only}
- Al‐Shahwany IW, Hindoosh LN, Rassam R, Al‐Qadhi A. Drain or not to drain in appendectomy for perforated appendicitis. Iraqi Postgraduate Medical Journal 2012;11(3):349‐52. [Google Scholar]
Beek 2015 {published data only}
- Beek MA, Jansen TS, Raats JW, Twiss ELL, Gobardhan PD, Kloot EJHV. The utility of peritoneal drains in patients with perforated appendicitis. Springerplus 2015;4(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Everson 1977 {published data only}
- Everson NW, Fossard DP, Nash JR, Macdonald RC. Wound infection following appendicectomy: the effect of extraperitoneal wound drainage and systemic antibiotic prophylaxis. British Journal of Surgery 1977;64(4):236‐8. [DOI] [PubMed] [Google Scholar]
Ezer 2010 {published data only}
- Ezer A, Törer N, Calışkan K, Colakoğlu T, Parlakgümüş A, Belli S, et al. Use of drainage in surgery for perforated appendicitis: the effect on complications. Turkish Journal of Trauma & Emergency Surgery 2010;16(5):427‐32. [PubMed] [Google Scholar]
Greenall 1978 {published data only}
- Greenall MJ, Evans M, Pollock AV. Should you drain a perforated appendix?. British Journal of Surgery 1978;65(12):880‐2. [DOI] [PubMed] [Google Scholar]
Johnson 1993 {published data only}
- Johnson DA, Kosloske AM, Macarthur C. Perforated appendicitis in children: to drain or not to drain?. Pediatric Surgery International 1993;8(5):402‐5. [Google Scholar]
Magarey 1971 {published data only}
- Magarey CJ, Chant AD, Rickford CR, Margarey JR. Peritoneal drainage and systemic antibiotics after appendicectomy. A prospective trial. Lancet 1971;2(7717):179‐82. [DOI] [PubMed] [Google Scholar]
Narci 2007 {published data only}
- Narci A, Karaman I, Karaman A, Erdoğan D, Cavuşoğlu YH, Aslan MK, et al. Is peritoneal drainage necessary in childhood perforated appendicitis?‐‐a comparative study. Journal of Pediatric Surgery 2007;42(11):1864‐8. [DOI] [PubMed] [Google Scholar]
Piper 2011 {published data only}
- Piper HG, Derinkuyu B, Koral K, Perez EA, Murphy JT. Is it necessary to drain all postoperative fluid collections after appendectomy for perforated appendicitis?. Journal of Pediatric Surgery 2011;46(6):1126‐30. [DOI] [PubMed] [Google Scholar]
Song 2015 {published data only}
- Song RY, Jung K. Drain insertion after appendectomy in children with perforated appendicitis based on a single‐center experience. Annals of Surgical Treatment and Research 2015;88(6):341‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toki 1995 {published data only}
- Toki A, Ogura K, Horimi T, Tokuoka H, Todani T, Watanabe Y, et al. Peritoneal lavage versus drainage for perforated appendicitis in children. Surgery Today 1995;25(3):207‐10. [DOI] [PubMed] [Google Scholar]
Additional references
Addiss 1990
- Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. American Journal of Epidemiology 1990;132(5):910‐25. [DOI] [PubMed] [Google Scholar]
Andersen 2005
- Andersen BR, Kallehave FL, Andersen HK. Antibiotics versus placebo for prevention of postoperative infection after appendicectomy. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD001439.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersen 2016
- Andersen HK. Cochrane Colorectal Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2016, Issue 4. Art. No.: COLOCA.
Anderson 2014
- Anderson DJ, Podgorny K, Berríos‐Torres SI, Bratzler DW, Dellinger EP, Greene L, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infection Control and Hospital Epidemiology 2014;35(6):605‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersson 1994
- Andersson R, Hugander A, Thulin A, Nyström PO, Olaison G. Indications for operation in suspected appendicitis and incidence of perforation. BMJ 1994;308(6921):107‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreu‐Ballester 2009
- Andreu‐Ballester JC, González‐Sánchez A, Ballester F, Almela‐Quilis A, Cano‐Cano MJ, Millan‐Scheiding M, et al. Epidemiology of appendectomy and appendicitis in the Valencian community (Spain), 1998‐2007. Digestive Surgery 2009;26(5):406‐12. [DOI] [PubMed] [Google Scholar]
Boomer 2010
- Boomer L, Freeman J, Landrito E, Feliz A. Perforation in adults with acute appendicitis linked to insurance status, not ethnicity. Journal of Surgical Research 2010;163(2):221‐4. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI] [PubMed] [Google Scholar]
Buckius 2012
- Buckius MT, McGrath B, Monk J, Grim R, Bell T, Ahuja V. Changing epidemiology of acute appendicitis in the United States: study period 1993–2008. Journal of Surgical Research 2012;175(2):185‐90. [DOI] [PubMed] [Google Scholar]
Charoenkwan 2017
- Charoenkwan K, Kietpeerakool C. Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy for the prevention of lymphocyst formation in women with gynaecological malignancies. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD007387.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2012b
- Cheng Y, Xiong XZ, Wu SJ, Lin YX, Cheng NS. Laparoscopic vs. open cholecystectomy for cirrhotic patients: a systematic review and meta‐analysis. Hepatogastroenterology 2012;59(118):1727‐34. [DOI] [PubMed] [Google Scholar]
Cheng 2016
- Cheng Y, Xia J, Lai M, Cheng N, He S. Prophylactic abdominal drainage for pancreatic surgery. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD010583.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2017
- Cheng Y, Xiong X, Lu J, Wu S, Zhou R, Cheng N. Early versus delayed appendicectomy for appendiceal phlegmon or abscess. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD011670.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clavien 2009
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Annals of Surgery 2009;250(2):187‐96. [DOI] [PubMed] [Google Scholar]
Cueto 2006
- Cueto J, D'Allemagne B, Vázquez‐Frias JA, Gomez S, Delgado F, Trullenque L, et al. Morbidity of laparoscopic surgery for complicated appendicitis: an international study. Surgical Endoscopy 2006;20(5):717‐20. [DOI] [PubMed] [Google Scholar]
de Jesus 2004
- Jesus EC, Karliczek A, Matos D, Castro AA, Atallah ÁN. Prophylactic anastomotic drainage for colorectal surgery. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD002100.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Durai 2009
- Durai R, Mownah A, Ng PC. Use of drains in surgery: a review. Journal of Perioperative Practice 2009;19(6):180‐6. [DOI] [PubMed] [Google Scholar]
Duttaroy 2009
- Duttaroy DD, Jitendra J, Duttaroy B, Bansal U, Dhameja P, Patel G, et al. Management strategy for dirty abdominal incisions: primary or delayed primary closure? A randomized trial. Surgical Infections 2009;10(2):129‐36. [DOI] [PubMed] [Google Scholar]
Ferris 2017
- Ferris M, Quan S, Kaplan BS, Molodecky N, Ball CG, Chernoff GW, et al. The global incidence of appendicitis: a systematic review of population‐based studies. Annals of Surgery 2017;266(2):237‐41. [DOI] [PubMed] [Google Scholar]
Gates 2013
- Gates S, Anderson ER. Wound drainage for caesarean section. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD004549.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2007a
- Gurusamy KS, Samraj K, Davidson BR. Routine abdominal drainage for uncomplicated liver resection. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006232.pub2] [DOI] [PubMed] [Google Scholar]
Gurusamy 2007b
- Gurusamy KS, Samraj K. Routine abdominal drainage for uncomplicated open cholecystectomy. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006003.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2013
- Gurusamy KS, Koti R, Davidson BR. Routine abdominal drainage versus no abdominal drainage for uncomplicated laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD006004.pub4] [DOI] [PubMed] [Google Scholar]
Hall 2010
- Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. National Health Statistics Reports 2010;26(29):1‐20, 24. [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011d
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horan 1992
- Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infection Control and Hospital Epidemiology 1992;13(10):606‐8. [PubMed] [Google Scholar]
Körner 1997
- Körner H, Söndenaa K, Söreide JA, Andersen E, Nysted A, Lende TH, et al. Incidence of acute nonperforated and perforated appendicitis: age‐specific and sex‐specific analysis. World Journal of Surgery 1997;21(3):313‐7. [DOI] [PubMed] [Google Scholar]
Lee 2010
- Lee JH, Park YS, Choi JS. The epidemiology of appendicitis and appendectomy in South Korea: national registry data. Journal of Epidemiology 2010;20(2):97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Livingston 2007
- Livingston EH, Woodward WA, Sarosi GA, Haley RW. Disconnect between incidence of nonperforated and perforated appendicitis: implications for pathophysiology and management. Annals of Surgery 2007;245(6):886‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mangram 1999
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infection Control and Hospital Epidemiology 1999;20(4):250‐78. [DOI] [PubMed] [Google Scholar]
Markides 2010
- Markides G, Subar D, Riyad K. Laparoscopic versus open appendectomy in adults with complicated appendicitis: systematic review and meta‐analysis. World Journal of Surgery 2010;34(9):2026‐40. [DOI] [PubMed] [Google Scholar]
Oliak 2000
- Oliak D, Yamini D, Udani VM, Lewis RJ, Vargas H, Arnell T, et al. Can perforated appendicitis be diagnosed preoperatively based on admission factors?. Journal of Gastrointestinal Surgery 2000;4(5):470‐4. [DOI] [PubMed] [Google Scholar]
Parker 2007
- Parker MJ, Livingstone V, Clifton R, McKee A. Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD001825.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrowsky 2004
- Petrowsky H, Demartines N, Rousson V, Clavien PA. Evidence‐based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta‐analyses. Annals of Surgery 2004;240(6):1074‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pieper 1982
- Pieper R, Kager L. The incidence of acute appendicitis and appendectomy. An epidemiological study of 971 cases. Acta Chirurgica Scandinavica 1982;148(1):45‐9. [PubMed] [Google Scholar]
Rehman 2011
- Rehman H, Rao AM, Ahmed I. Single incision versus conventional multi‐incision appendicectomy for suspected appendicitis. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009022.pub2] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.3. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Samraj 2007
- Samraj K, Gurusamy KS. Wound drains following thyroid surgery. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006099.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santacroce 2017
- Santacroce L, Geibel J, Ochoa JB, Hines OJ, Talavera F. Appendectomy. http://emedicine.medscape.com/article/195778‐overview 2017 (accessed 16 July 2017).
Sauerland 2010
- Sauerland S, Jaschinski T, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD001546.pub3] [DOI] [PubMed] [Google Scholar]
Schein 2008
- Schein M. To drain or not to drain? The role of drainage in the contaminated and infected abdomen: an international and personal perspective. World Journal of Surgery 2008;32(2):312‐21. [DOI] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann H, Brozek J, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Version 3.2 (updated March 2009). The GRADE Working Group, 2009. Available from www.cc‐ims.net/gradepro.
Simillis 2010
- Simillis C, Symeonides P, Shorthouse AJ, Tekkis PP. A meta‐analysis comparing conservative treatment versus acute appendectomy for complicated appendicitis (abscess or phlegmon). Surgery 2010;147(6):818‐29. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9.5.5 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Wang 2015
- Wang Z, Chen J, Su K, Dong Z. Abdominal drainage versus no drainage post‐gastrectomy for gastric cancer. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD008788.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1998
- Williams NM, Jackson D, Everson NW, Johnstone JM. Is the incidence of acute appendicitis really falling?. Annals of The Royal College of Surgeons of England 1998;80(2):122‐4. [PMC free article] [PubMed] [Google Scholar]
Wilms 2011
- Wilms IM, Hoog DE, Visser DC, Janzing HM. Appendectomy versus antibiotic treatment for acute appendicitis. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD008359.pub2] [DOI] [PubMed] [Google Scholar]
Yates 1905
- Yates JL. An experimental study of the local effects of peritoneal drainage. Surgery, Gynecology and Obstetrics 1905; 1:473‐92. [Google Scholar]
Yu 2017
- Yu T, Cheng Y, Wang X, Tu B, Cheng N, Gong J, et al. Gases for establishing pneumoperitoneum during laparoscopic abdominal surgery. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD009569.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cheng 2012a
- Cheng Y, Zhou R, Wu S, Lu J, Xiong X, Lin Y, et al. Abdominal drainage after appendectomy for complicated appendicitis. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD010168] [DOI] [PubMed] [Google Scholar]
Cheng 2015
- Cheng Y, Zhou S, Zhou R, Lu J, Wu S, Xiong X, et al. Abdominal drainage to prevent intra‐peritoneal abscess after open appendectomy for complicated appendicitis. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD010168.pub2] [DOI] [PubMed] [Google Scholar]


